- Molecular Synthesis Laboratory, School of Pharmacy, Institute of Clinical Sciences, University of Birmingham, Edgbaston, United Kingdom
A Corrigendum on
A sulfuryl group transfer strategy to selectively prepare sulfated steroids and isotopically labelled derivatives
by Alshehri JA, Gill DM and Jones AM (2021). Front. Mol. Biosci. 8:776900. doi: 10.3389/fmolb.2021.776900
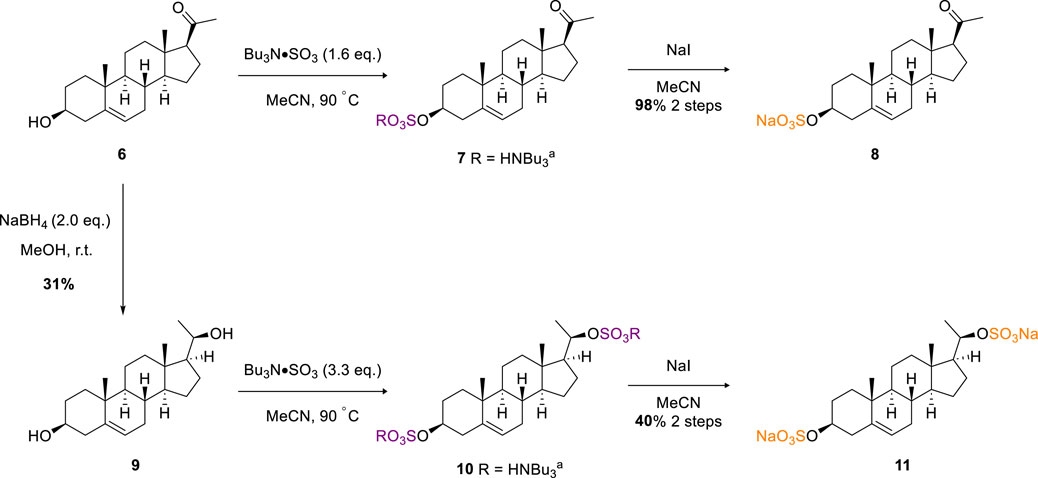
In the published article, there was an error in Scheme 2 as published. The stereogenic center in compounds 9-11 was incorrectly depicted. The corrected Scheme 2 and its caption appear below.
In the published article, there was an error in Supplementary Data Sheet 1, section Compound characterisation. The name of the fourth compound was incorrectly captured as “Pregnanediol or (3S,8S,9S,10R,13S,14S,17S)-17-((S)-1-hydroxyethyl)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-ol (9).”
The correct name appears below:
“Pregnanediol or (3S,8S,9S,10R,13S,14S,17S)-17-((R)-1-hydroxyethyl)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-ol (9).”
In the published article, there was an error in Supplementary Data Sheet 1, section Compound characterisation. The name of the fifth compound was incorrectly captured as “Disodium-3,17-pregnanediol disulfate or sodium (S)-1-((3S,8S,9S,10R,13S,14S,17S)-10,13-dimethyl-3-(sulfonatooxy)-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)ethyl sulfate (11).”
The correct name appears below:
“Disodium-3,17-pregnanediol disulfate or sodium (3S,8S,9S,10R,13S,14S,17S)-10,13-dimethyl-17-((R)-1-(sulfonatooxy)ethyl)-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl sulfate (11).”
The authors apologize for these errors and state that these do not change the scientific conclusions of the article in any way. The original article has been updated.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: sulfation, selectivity, isotopic labelling, sulfuryl transfer, TBSAB
Citation: Alshehri JA, Gill DM and Jones AM (2024) Corrigendum: A sulfuryl group transfer strategy to selectively prepare sulfated steroids and isotopically labelled derivatives. Front. Mol. Biosci. 11:1504226. doi: 10.3389/fmolb.2024.1504226
Received: 30 September 2024; Accepted: 25 November 2024;
Published: 19 December 2024.
Edited and reviewed by:
Graça Soveral, University of Lisbon, PortugalCopyright © 2024 Alshehri, Gill and Jones. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alan M. Jones, YS5tLmpvbmVzLjJAYmhhbS5hYy51aw==
 Jaber A. Alshehri
Jaber A. Alshehri Daniel M. Gill
Daniel M. Gill Alan M. Jones
Alan M. Jones