- Molecular Synthesis Laboratory, School of Pharmacy, Institute of Clinical Sciences, University of Birmingham, Edgbaston, United Kingdom
The treatment of common steroids: estrone, estradiol, cortisol, and pregnenolone with tributylsulfoammonium betaine (TBSAB) provides a convenient chemoselective conversion of the steroids alcohol/phenol moiety to the corresponding steroidal organosulfate. An important feature of the disclosed methodology is the millimolar scale of the reaction, and the isolation of the corresponding steroid sulfates as their biologically relevant sodium salts without the need for ion-exchange chromatography. The scope of the method was further explored in the estradiol and pregnanediol steroid systems with the bis-sulfated derivatives. Ultimately, a method to install an isotopic label, deuterium (2H) combined with estrone sulfation is a valuable tool for its mass-spectrometric quantification in biological studies.
Introduction
The preparation of authentic reference samples of sulfated steroids with either regioselective mono or di-sulfation patterns, (Lightning et al., 2021) combined with methods to isotopically label the resulting sulfated steroids is an ongoing challenge to their biological study. The resulting authentic sulfated steroids are key reference standards of paramount importance to the understanding of sulfatases (Mueller et al., 2015), (Günal et al., 2019), (Foster and Mueller, 2018), the role of steroid sulfation in diseases (Mueller et al., 2021) and the fields of detection of steroids, whether in abuse (Waller and McLeod, 2014) or in the environment, (Petrie et al., 2013) using spectroscopic techniques (Hill et al., 2019). Furthermore, the development of improved sulfation methods can be applied to both sulfated steroid containing natural products synthesis and structural elucidation studies (Hoye et al., 2007).
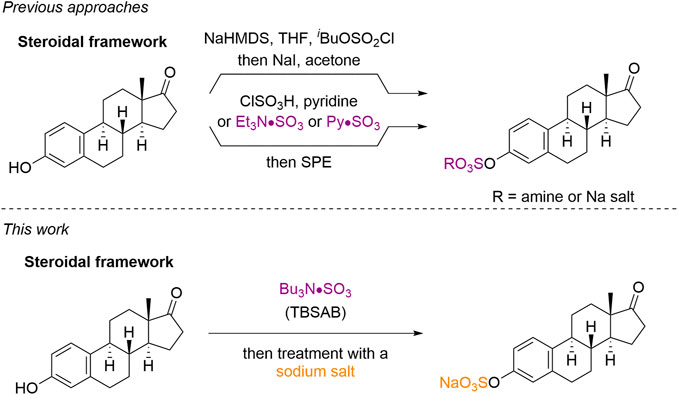
Current methods to sulfate steroids fall into two main categories (Chart 1). The use of a protected sulfate group (e.g., isobutyl protected sulfate esters) with subsequent deprotection (Simpson and Widlanski, 2006), or the use of a sulfur trioxide equivalent (e.g., chlorosulfonic acid or pyridine-sulfur trioxide complex) (Waller and McLeod, 2014), (Hungerford et al., 2006). Although these methods are effective, they suffer from the additional steps of deprotection and/or purification cascades. Issues with toxicity regarding pyridine contamination from the use of pyridine-sulfur trioxide complex in related carbohydrate scaffolds (Gabriel et al., 2020), (Vo et al., 2021) requires either an exceptionally vigilant isolation and analysis; or an improved overall method for steroid sulfation.
Our own current interest in the sulfation field derives from the development of tributylsulfoammonium betaine (TBSAB) (Gill et al., 2019a), (Jones, 2021) as a convenient one-pot method for the sulfation of heteroatom containing bioactive molecules. (Benedetti et al., 2020), (Alshehri et al., 2020) This was inititated due to challenges encountered with the purification of sulfated small molecule heparin sulfate glycomimetics (Gill et al., 2021), (Gill et al., 2019b), (Mahmoud et al., 2019), (Langford-Smith et al., 2019), (Mahmoud et al., 2017) with conventional, pre-existing sulfation methods. A key advantage of TBSAB over similar amine containing-sulfur trioxide complexes (e.g., triethylamine-sulfur trioxide) is the lipophilic nature of the counterion avoiding the need for ion-exchange chromatography. Herein we report our findings on the use of TBSAB as a general, scalable and regioselective sulfating reagent for steroids, and the application of TBSAB in conjugation with isotopic labelling for steroidal-organosulfate reference standards.
Results and Discussion
Our initial exploration of the method builds upon early screening results of TBSAB, including a single example on β-estradiol (1) (Gill et al., 2019a). We firstly sought to demonstrate the reproducibility of this method on a 1.0 mmol scale, thus taking commercially available β-estradiol (1) and treating it with TBSAB resulted in exclusive C (17), secondary alcohol, sulfation (2). Furthermore the same conditions using an excess of TBSAB resulted in both C (17) sulfation and C (3), phenol, sulfation of (4) presumably occurs via initial C (17) alcohol sulfation in a stepwise installation. In both cases, a work-up using sodium iodide isolated the mono (3) and double (5) sulfated steroids as their sodium salts, in good yields without the risk of pyridinium ion contamination (Scheme 1).
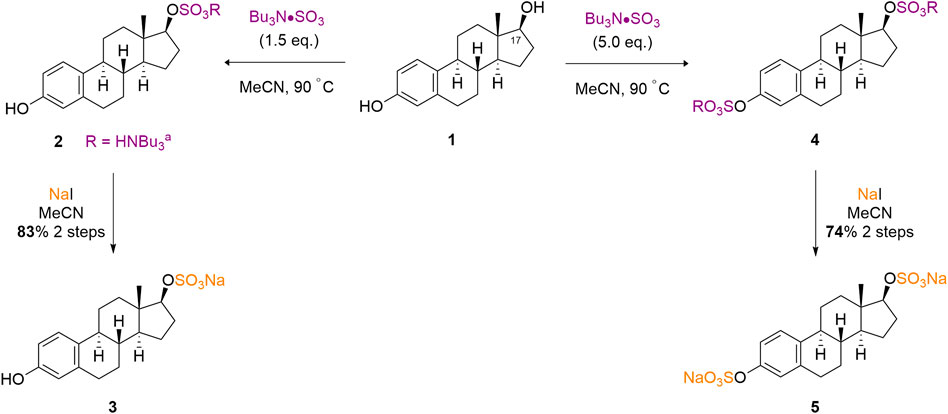
Scheme 1. TBSAB mediated regioselective sulfation of β-estradiol (1) affords the mono- or double sulfated estradiols as their sodium salts.
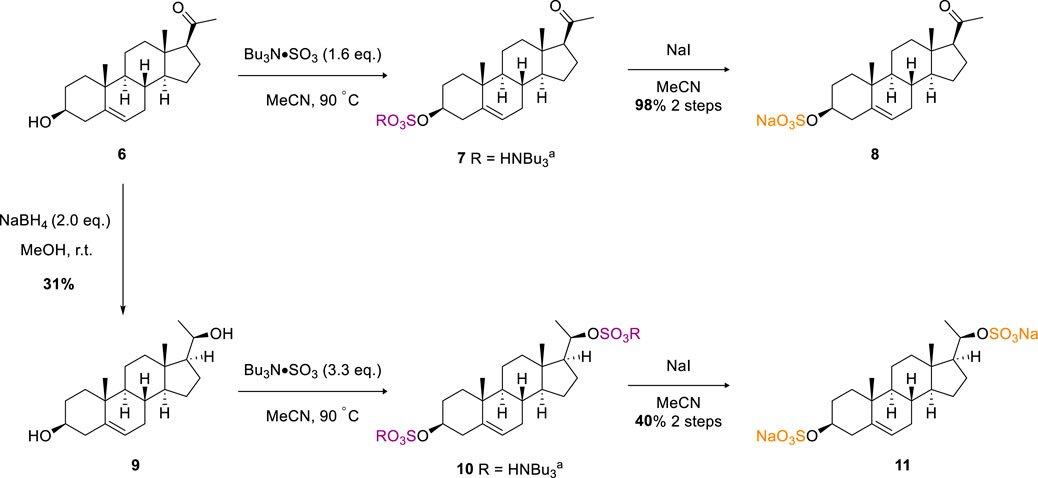
Next, we considered sulfation of a more challenging biologically active substrate, pregnenolone (6). (Harteneck, 2013). Under analogous conditions to the β-estradiol examples, and on a 0.3 mmol scale, steroidal sulfate 8 was afforded after sodium exchange in an excellent 98% isolated yield (Scheme 2). Diastereoselective reduction of the ketone moiety of pregnenolone using sodium borohydride afforded pregnanediol in 31% yield (9). Crystallographic data of the bulk material from d6-DMSO crystallisation supports the assignment of the major diastereomer as R at the newly set stereocentre (Supplementary Figure S3) (2120263 contain, 2120). As 9 contains two secondary alcohol motifs, treatment with TBSAB afforded the double sulfated pregnanediol (11) in a modest 40% isolated yield on a 0.6 mmol scale.
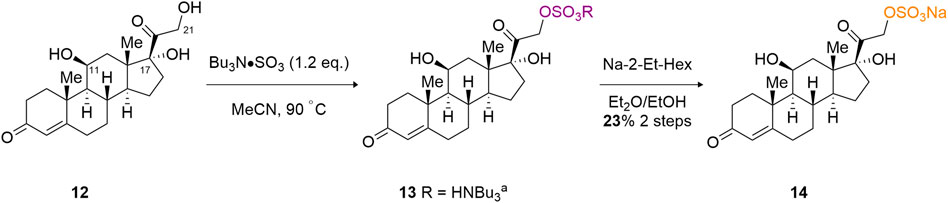
The ultimate test of the TBSAB method, in relation to regioselective sulfation, is the complex triol, cortisol (12) (Scheme 3). Cortisol contains three potentially reactive hydroxyl motifs at the C (11), C (17) and C (21) positions. It was anticipated that a regioselective sulfation of the primary C (21) alcohol would result over the C (11), secondary, or C (17), tertiary, alcohol moieties, despite the presence of the α-ketone affecting the reactivity of the C (21)-OH. To our delight, a microscale (8 mg) treatment of cortisol with TBSAB afforded the C (21) organosulfate in a modest 17% overall yield (23% based on recovered starting material) as the sodium salt (14). Furthermore, no unwanted C (11) or indeed C (17) sulfate ester formation was observed.
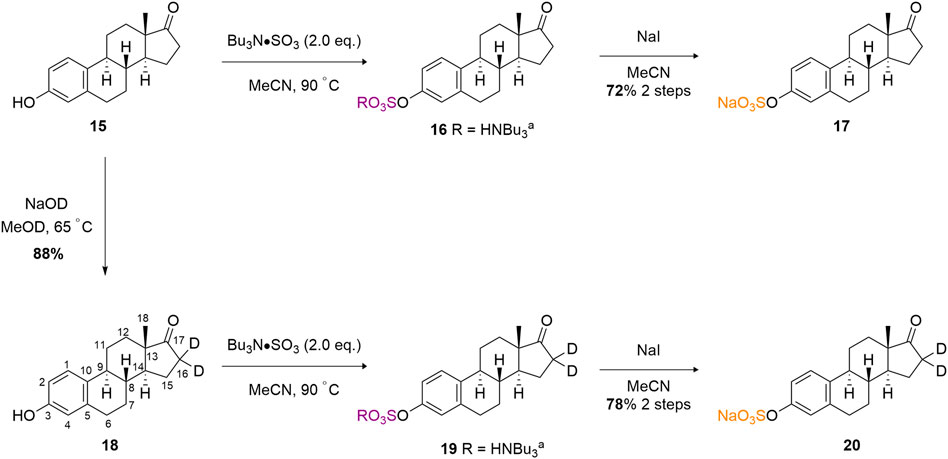
Finally, we sought to develop a proof-of-concept isotopic labelling-chemoselective sulfation method for the estrone scaffold (15) (Scheme 4). Prior to developing a deuterium labelling method at the C (16) methylene position, a model non-deuterated estrone was sulfated at the C (3) phenolic position in good 72% isolated yield as the sodium salt (17). A higher equivalence of TBSAB (2.0 eq) was used to ensure complete sulfation at the sole reactive C (3) phenolic centre. This provided confidence that sulfation should occur readily at the C (3) position using TBSAB on the deuterium labeled substrate.
Firstly, we adapted the method of Rudqvist for C (19) deuteration (Rudqvist, 1983). Treatment of the estrone with NaOD in MeOD resulted in estrone-d2 formation (18). The C (16)-H2 protons were selectively deuterated by enolate formation with sodium deutroxide and resultant deuterium incorporation by quenching the enolate with methanol-D (CH3OD). This was confirmed via comparative 2D-NMR spectroscopic studies (see Supporting Information) but the key disappearance of the C (16) protons can be clearly observed in the 1H-NMR spectral overlay (Figure 1). It should be noted that deuteration next to a carbonyl group is not usually recommended for applied quantification studies as the deuterium label could readily back exchange through a keto-enol tautomerisation leading to a loss of the label (Wudy, 1990). In our system we observed, a decline of deuterium label in solution based mass-spectrometry studies (Supplementary Figures S1, S2).
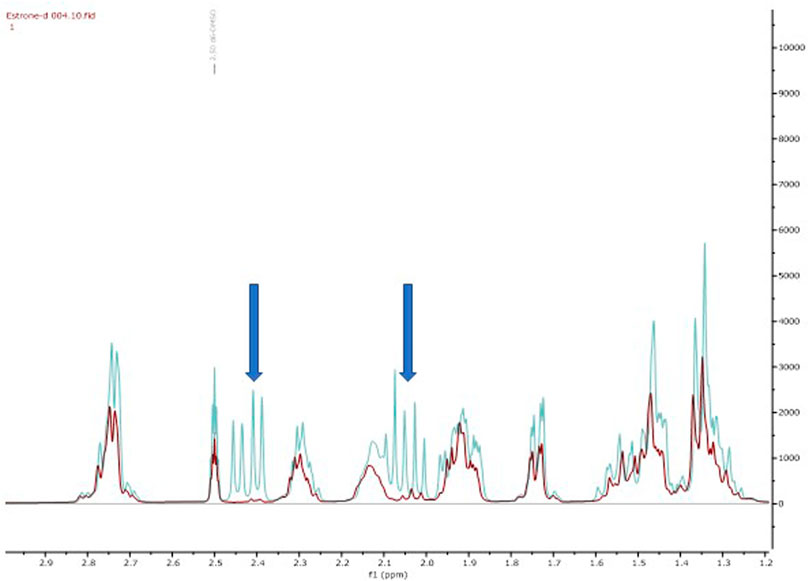
Figure 1. Overlay of 1H NMR spectra of estrone (blue, 15) and estrone-d2 (red, 18) shows the diagnostic reduction of the diastereotopic C (16) protons.
Finally, the treatment of estrone-d2 with TBSAB afforded the sulfated and isotopically labelled estrone-d2 sulfate in 78% isolated yield and 67% incorportation of the deuterium label (20).
Conclusion
In summary, we have demonstrated a general method for the synthesis of mono- or di-sulfated steroidal skeletons of importance to the fields of biology and spectroscopmetric detection. We have showcased chemo-selective sulfation within a variety of complex structures, such as cortisol, and developed a simplified deuterium labeling-sulfation strategy for estrone. Overall, these approaches provide tractable routes on preparative scales to multiple sulfated steroid classes as reference compounds for detection of substances of abuse through to cancer diagnosis applications.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author fid files can be found at the following doi: https://doi.org/10.25500/edata.bham.00000720.
Author Contributions
Conducted experiments, spectral analysis, and revised manuscript (JA and DG); provided materials and supervision, analysed results, drafted and revised manuscript (AJ). All authors agree to be accountable for the content of the work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank the above funding bodies for supporting our research programme. The authors thank Louise Male for X-ray crystallography, Allen Bowden for HPLC analysis, Chris Williams for mass-spectroscopic studies, and Cecile Le Duff for NMR assistance. Requests for milligram samples will be considered by the authors until samples are exhausted.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmolb.2021.776900/full#supplementary-material
References
Alshehri, J. A., Benedetti, A. M., and Jones, A. M. (2020). A Novel Exchange Method to Access Sulfated Molecules. Sci. Rep. 10, 16559. doi:10.1038/s41598-020-72500-x
Benedetti, A. M., Gill, D. M., Tsang, C. W., and Jones, A. M. (2020). Chemical Methods for N- and O-Sulfation of Small Molecules, Amino Acids and Peptides. ChemBioChem 21, 938–942. doi:10.1002/cbic.201900673
CCDC 2120263 Contains the Supplementary Crystallographic Data for This Paper. These Data Can Be Obtained Free of Charge from the Cambridge Crystallographic Data Centre. Available at: www.ccdc.cam.ac.uk/data_request/cif.
Foster, P. A., and Mueller, J. W. (2018). SULFATION PATHWAYS: Insights into Steroid Sulfation and Desulfation Pathways. J. Mol. Endocrinol. 61, T271–T283. doi:10.1530/JME-18-0086
Gabriel, L., Günther, W., Pielenz, F., and Heinze, T. (2020). Determination of the Binding Situation of Pyridine in Xylan Sulfates by Means of Detailed NMR Studies. Macromol. Chem. Phys. 221, 1900327. doi:10.1002/macp.201900327
Gill, D. M., Male, L., and Jones, A. M. (2019). A Structure-Reactivity Relationship of the Tandem Asymmetric Dihydroxylation on a Biologically Relevant Diene: Influence of Remote Stereocenters on Diastereofacial Selectivity. Eur. J. Org. Chem. 2019, 7568–7577. doi:10.1002/ejoc.201901474
Gill, D. M., Male, L., and Jones, A. M. (2019). Sulfation Made Simple: a Strategy for Synthesising Sulfated Molecules. Chem. Commun. 55, 4319–4322. doi:10.1039/C9CC01057B
Gill, D. M., R. Povinelli, A. P., Zazeri, G., Shamir, S. A., Mahmoud, A. M., Wilkinson, F. L., et al. (2021). The Modulatory Role of Sulfated and Non-sulfated Small Molecule Heparan Sulfate-Glycomimetics in Endothelial Dysfunction: Absolute Structural Clarification, Molecular Docking and Simulated Dynamics, SAR Analyses and ADMET Studies. RSC Med. Chem. 12, 779–790. doi:10.1039/D0MD00366B
Günal, S., Hardman, R., Kopriva, S., and Mueller, J. W. (2019). Sulfation Pathways from Red to green. J. Biol. Chem. 294, 12293–12312. doi:10.1074/jbc.REV119.007422
Harteneck, C. (2013). Pregnenolone Sulfate: From Steroid Metabolite to TRP Channel Ligand. Molecules 18, 12012–12028. doi:10.3390/molecules181012012
Hill, M., Hána, V., Velíková, M., Pařízek, A., Kolátorová, L., Vítků, J., et al. (2019). A Method for Determination of One Hundred Endogenous Steroids in Human Serum by Gas Chromatography-Tandem Mass Spectrometry. Physiol. Res. 68, 179–207. doi:10.33549/physiolres.934124
Hoye, T. R., Dvornikovs, V., Fine, J. M., Anderson, K. R., Jeffrey, C. S., Muddiman, D. C., et al. (2007). Details of the Structure Determination of the Sulfated Steroids PSDS and PADS: New Components of the Sea Lamprey (Petromyzon marinus) Migratory Pheromone. J. Org. Chem. 72, 7544–7550. doi:10.1021/jo0709571
Hungerford, N. L., McKinney, A. R., Stenhouse, A. M., and McLeod, M. D. (2006). Selective Manipulation of Steroid Hydroxyl Groups with Boronate Esters: Efficient Access to Antigenic C-3 Linked Steroid-Protein Conjugates and Steroid Sulfate Standards for Drug Detection. Org. Biomol. Chem. 4, 3951–3959. doi:10.1039/b610499a
Jones, A. M. (2021). Tributylsulfoammonium Betaine. Encyclopaedia Reagents Org. Synth., 1–3. doi:10.1002/047084289X.rn02393
Langford-Smith, A. W. W., Hasan, A., Weston, R., Edwards, N., Jones, A. M., Boulton, A. J. M., et al. (2019). Diabetic Endothelial colony Forming Cells Have the Potential for Restoration with Glycomimetics. Sci. Rep. 9, 2309. doi:10.1038/s41598-019-38921-z
Lightning, T. A., Gesteira, T. F., and Mueller, J. W. (2021). Steroid Disulfates - Sulfation Double Trouble. Mol. Cell Endocrinol. 524, 111161. doi:10.1016/j.mce.2021.111161
Mahmoud, A. M., Jones, A. M., Sidgwick, G., Arafat, A. M., Wilkinson, F. L., and Alexander, M. Y. (2019). Small Molecule Glycomimetics Inhibit Vascular Calcification via C-Met/Notch3/HES1 Signalling. Cell Physiol Biochem 53, 323–336. doi:10.33594/000000141
Mahmoud, A. M., Wilkinson, F. L., Jones, A. M., Wilkinson, J. A., Romero, M., Duarte, J., et al. (2017). A Novel Role for Small Molecule Glycomimetics in the protection against Lipid-Induced Endothelial Dysfunction: Involvement of Akt/eNOS and Nrf2/ARE Signaling. Biochim. Biophys. Acta (Bba) - Gen. Subjects 1861, 3311–3322. doi:10.1016/j.bbagen.2016.08.013
Mueller, J. W., Gilligan, L. C., Idkowiak, J., Arlt, W., and Foster, P. A. (2015). The Regulation of Steroid Action by Sulfation and Desulfation. Endocr. Rev. 36, 526–563. doi:10.1210/er.2015-1036
Mueller, J. W., Vogg, N., Lightning, T. A., Weigand, I., Ronchi, C. L., Foster, P. A., et al. (2021). Steroid Sulfation in Adrenal Tumors. J. Clin. Endocrinol. Metab., 106: Dgab182. doi:10.1210/clinem/dgab182
Petrie, B., McAdam, E. J., Richards, K. H., Lester, J. N., and Cartmell, E. (2013). Application of Ultra-performance Liquid Chromatography-Tandem Mass Spectrometry for the Determination of Steroid Oestrogens in Wastewaters. Int. J. Environ. Anal. Chem. 93 (13), 1343–1355. doi:10.1080/03067319.2012.717272
Rudqvist, U. (1983). Synthesis of C19 Steroid Monosulphates Labelled with Deuterium at Specific Positions. J. Label Compd. Radiopharm. 20, 1159–1170. doi:10.1002/jlcr.2580201005
Simpson, L. S., and Widlanski, T. S. (2006). A Comprehensive Approach to the Synthesis of Sulfate Esters. J. Am. Chem. Soc. 128, 1605–1610. doi:10.1021/ja056086j
Vo, Y., Schwartz, B. D., Onagi, H., Ward, J. S., Gardiner, M. G., Banwell, M. G., et al. (2021). A Rapid and Mild Sulfation Strategy Reveals Conformational Preferences in Therapeutically Relevant Sulfated Xylooligosaccharides. Chem. Eur. J. 27, 9830–9838. doi:10.1002/chem.202100527
Waller, C. C., and McLeod, M. D. (2014). A Simple Method for the Small Scale Synthesis and Solid-phase Extraction Purification of Steroid Sulfates. Steroids 92, 74–80. doi:10.1016/j.steroids.2014.09.006
Keywords: sulfation, selectivity, isotopic labelling, sulfuryl transfer, TBSAB
Citation: Alshehri JA, Gill DM and Jones AM (2021) A Sulfuryl Group Transfer Strategy to Selectively Prepare Sulfated Steroids and Isotopically Labelled Derivatives. Front. Mol. Biosci. 8:776900. doi: 10.3389/fmolb.2021.776900
Received: 14 September 2021; Accepted: 15 November 2021;
Published: 24 December 2021.
Edited by:
Tarsis G Ferreira, University of Houston, United StatesReviewed by:
Jozef Stec, Marshall B. Ketchum University, United StatesMalcolm McLeod, Australian National University, Australia
Fernando Ogata, Federal University of São Paulo, Brazil
Copyright © 2021 Alshehri, Gill and Jones. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alan M. Jones, YS5tLmpvbmVzLjJAYmhhbS5hYy51aw==
 Jaber A. Alshehri
Jaber A. Alshehri Daniel M. Gill
Daniel M. Gill Alan M. Jones
Alan M. Jones