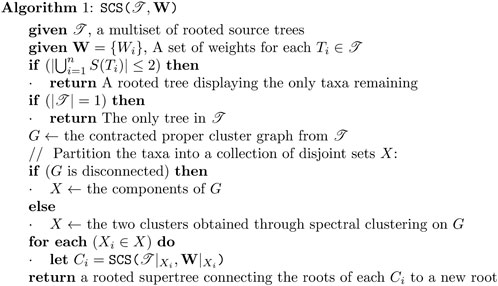
- 1Research School of Biology, The Australian National University, Canberra, ACT, Australia
- 2School of Computing, The Australian National University, Canberra, ACT, Australia
- 3School of Natural Sciences, University of Tasmania, Hobart, TAS, Australia
The algorithms for phylogenetic reconstruction are central to computational molecular evolution. The relentless pace of data acquisition has exposed their poor scalability and the conclusion that the conventional application of these methods is impractical and not justifiable from an energy usage perspective. Furthermore, the drive to improve the statistical performance of phylogenetic methods produces increasingly parameter-rich models of sequence evolution, which worsens the computational performance. Established theoretical and algorithmic results identify supertree methods as critical to divide-and-conquer strategies for improving scalability of phylogenetic reconstruction. Of particular importance is the ability to explicitly accommodate rooted topologies. These can arise from the more biologically plausible non-stationary models of sequence evolution. We make a contribution to addressing this challenge with Spectral Cluster Supertree, a novel supertree method for merging a set of overlapping rooted phylogenetic trees. It offers significant improvements over Min-Cut supertree and previous state-of-the-art methods in terms of both time complexity and overall topological accuracy, particularly for problems of large size. We perform comparisons against Min-Cut supertree and Bad Clade Deletion. Leveraging two tree topology distance metrics, we demonstrate that while Bad Clade Deletion generates more correct clades in its resulting supertree, Spectral Cluster Supertree’s generated tree is generally more topologically close to the true model tree. Over large datasets containing 10,000 taxa and
1 Introduction
The relentless pace of DNA sequence data acquisition has exposed the poor scalability of phylogenetic reconstruction algorithms, establishing that new scalable and accurate algorithms are required for phylogenetic analysis. The well known sensitivity of phylogenetic results to data features that violate models of sequence evolution is increasing the interest in applying non-stationary models to phylogenetic inference (e.g., Dang et al., 2022; Kaehler et al., 2015; Sumner et al., 2012) including estimation of rooted phylogenies (Kaehler, 2017; Yap and Speed, 2005). However, such models increase the number of free parameters and thus worsen computational performance. Theoretical results confirming the statistical consistency of the divide-and-conquer Disk-Covering Method (DCM) (Huson et al., 1999) identified this as a promising candidate for overcoming the robustness versus performance trade-off, illustrated more recently by DACTAL (Nelesen et al., 2012). DCM algorithms fundamentally rely on merging phylogenies estimated from overlapping subsets of the full data. While most prior DCM work has been focused on unrooted trees, rooted topologies can also be handled. Given these considerations, we have sought to resolve one important bottleneck affecting the generalisation of DCM by developing a rooted supertree method that is statistically robust and scalable.
Algorithms for merging phylogenies into a single phylogenetic tree can largely be divided into two categories - supertree methods and consensus tree methods. Consensus tree methods assume the sets of taxa in each of the phylogenies are identical. Supertree methods solve the more general problem where the sets of taxa are not necessarily the same but are overlapping (Steel and Bocker, 2000). A spectral clustering based consensus tree method has been developed for clustering gene trees for multilocus phylogenetic analysis (Yoshida et al., 2019). We will show how spectral clustering can be applied as part of an efficient and statistically robust supertree method.
In this work, we introduce Spectral Cluster Supertree (SCS) for the case of rooted trees. SCS is a method which recursively partitions the set of all taxa in the source trees until a rooted supertree is generated. SCS derives its origins from Min-Cut Supertree (Semple and Steel, 2000), replacing the min-cut step with a substantially more scalable spectral clustering approach (Shi and Malik, 2000) to partition an internal graph. We also extend the method to make use of information such as branch lengths and the depths of taxa in the source trees. These modifications lead to a much more efficient and accurate method, capable of solving problems with hundreds of source trees and ten thousand taxa in the order of seconds.
1.1 Related work
Fleischauer and Böcker (2017) performed an extensive systematic comparison among supertree methods in their paper that introduced Bad Clade Deletion (BCD). The comparison evaluated the running time and accuracy of both rooted and unrooted supertree methods. The accuracy over generated datasets was measured by counting the number of splits in the generated supertree that were “true positives” (splits in the generated supertree that should occur), “false positives” (splits in the generated supertree that should not occur) and “false negatives” (splits in the generated supertree that should occur but do not). These values can be used to calculate the well-known
The methods that were evaluated by the comparison included the Greedy Strict Consensus Merger (GSCM) (Roshan et al., 2003); FastRFS (Vachaspati and Warnow, 2017); Matrix Representation with Parsimony (MRP) (Baum, 1992; Ragan, 1992); SuperFine (Swenson et al., 2012); Combined Analysis using Maximum Likelihood (CA-ML) with RAxML (Stamatakis, 2006); and their own method BCD (Fleischauer and Böcker, 2017). A rooted variant of GSCM exists that generates a supertree only containing clades that are compatible with all of the source trees (Fleischauer and Böcker, 2016). FastRFS (Vachaspati and Warnow, 2017) generates an unrooted supertree optimising the Robinson-Foulds distance (Robinson and Foulds, 1981) to the source trees under a constrained search space using dynamic programming. MRP generates a rooted supertree by performing parsimony analysis on a Baum-Ragan encoding of the source trees (Baum, 1992; Ragan, 1992). Superfine (Swenson et al., 2012) is a meta-method which combines the Strict Consensus Merger (Huson et al., 1999) to merge the source trees together, with another supertree method to resolve polytomies (MRP yielded the best performance); it generates unrooted trees. CA-ML concatenates the sequences used to generate each of the source trees and creates a supertree using maximum likelihood analysis; it has the potential to generate rooted trees depending on the chosen substitution model (e.g., using a strand-symmetric model with IQ-TREE, Minh et al., 2020). The methods were evaluated with dataset sizes varying from 100 to
Of the methods evaluated, CA-ML consistently performed the best in terms of topological accuracy of the constructed trees according to the
Spectral Cluster Supertree was created out of the desire for a rooted supertree algorithm that was capable of resolving a supertree containing thousands of taxa in both a fast and topologically accurate manner. Of the rooted supertree algorithms, based on the comparisons by Fleischauer and Böcker (2017), BCD is clearly the most efficient and accurate. We thus restrict our main comparisons in this paper to be between Spectral Cluster Supertree, and Bad Clade Deletion.
The BCD (Fleischauer and Böcker, 2017) algorithm seeks to minimise the number of deleted characters in a Baum-Ragan (Baum, 1992; Ragan, 1992) matrix encoding of the source trees so that a consistent supertree is formed. In the matrix, rows represent the taxa, and columns represent the clades, or equivalently internal nodes, of a phylogenetic tree. An entry in the matrix is “1” if the taxon is in the clade, “0” if it is in the tree for the clade but not in the clade, and “?” otherwise. BCD at each iteration aims to delete a locally minimum number of columns from this matrix to yield a supertree.
From the matrix representation, BCD constructs a graph with an edge connecting clade
BCD also introduces a number of additional strategies to improve the accuracy of the generated supertrees and the time it takes to construct them. This includes a number of weighting strategies for the clades during the min-cut step, which can take into account information including branch lengths and bootstrap values. There is also an optional preprocessing step. A rooted variant of the Greedy Strict Consensus Merger algorithm is used to collect clades that do not contradict with any of the source trees and ensure they are never cut (Fleischauer and Böcker, 2016; Roshan et al., 2003). This step is later referred to as GSCM preprocessing. There is an additional step which reduces the problem size by merging identical clades in the matrix representation. Parallelisation is additionally exploited when finding min-cuts and over the different partitions of the problem.
1.2 Using more statistically robust distance measures
It is conventional to use the Robinson-Foulds distance measure (Robinson and Foulds, 1981) to compare tree topologies. In the case of two rooted trees, the measure is determined from the number of clades that do not match between them. The Robinson-Foulds measure is known to exhibit poor statistical behaviour (Böcker et al., 2013; Lin et al., 2011), for instance, moving a single leaf in a caterpillar tree can maximise the metric (Lin et al., 2011). We will later show in the definitions and methods a mapping between the Robinson-Foulds distance, and a rooted variant of the
2 Definitions and methods
2.1 Preliminaries
A phylogenetic tree,
A taxon,
Two taxa
For a graph
We say that a tree,
Let T be a phylogenetic tree and X be a set of taxa. The induced subtree
A rooted supertree algorithm is one which takes as its input a multiset of rooted trees
2.2 Min-Cut supertree
Min-Cut Supertree is a well-studied polynomial-time algorithm for merging rooted phylogenetic trees (Semple and Steel, 2000). It works by recursively partitioning the set of taxa until a rooted tree is formed. There are proven properties of the algorithm, including that if all of the source trees are compatible, then the returned tree displays all of them, and that any triple that is displayed by all of the source trees is displayed in the supertree.
2.2.1 Proper cluster graph
Given a multiset of rooted source trees,
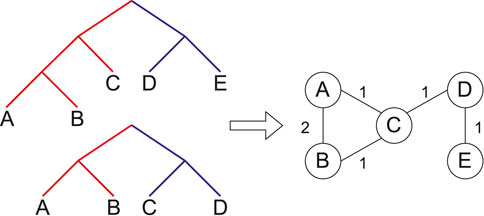
Figure 1. A proper cluster graph (right) for an example set of two source trees (left). Same coloured edges identify taxa belonging to a proper cluster. Here, the trees have unit weights. That is, each edge in the proper cluster graph is weighted by the number of source trees in which the taxa form a proper cluster.
If there are any edges in the proper cluster graph
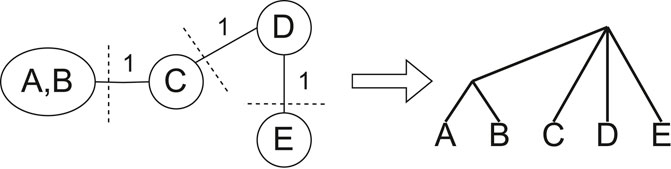
Figure 2. Graphical representation of the partitioning and result merging processes. After contraction, any edge on any min-cut of the proper cluster graph (left) is removed. Min-Cut Supertree is recursively called on the induced subtrees of the components of the proper cluster graph, making the roots of the resulting trees adjacent to a new root vertex (right).
2.2.2 Finding the best partition
If the resulting proper cluster graph is disconnected, then the taxa in each of the components of the graph are always split over the roots of the source trees. Taxa in these components should thus be separated over the root of the supertree. Otherwise, if the proper cluster graph is connected, there is no partition of the taxa for the root of the supertree satisfying all of the source trees. To resolve these cases, every edge that lies on any min-cut of the proper cluster graph is removed. This is analogous to removing groupings of taxa with the weakest support in the source trees so that the graph can be partitioned.
The components of this disconnected graph partition the taxa into
If there are two or fewer taxa remaining when Min-Cut Supertree is called, the method immediately returns the phylogenetic tree containing those taxa. Once the trees generated from the recursive calls are returned, Min-Cut Supertree combines the results by connecting the roots of these trees to a new root vertex. This thus allows Min-Cut supertree to recursively create a complete rooted phylogenetic tree. Figure 2 demonstrates this, continuing the example in Figure 1.
2.3 Spectral clustering
Spectral clustering (Ng et al., 2001; Shi and Malik, 2000) is a clustering technique that can be applied to partition a graph over a “bottleneck”. We explain how spectral clustering works from two perspectives.
2.3.1 Random walk perspective
We adapt this point of view from a tutorial on spectral clustering by Von Luxburg (2007). Consider an agent performing a random walk along a graph with
As
All eigenvalues of
2.3.2 A normalised cut perspective
Another perspective for looking at this problem is from the normalised cut perspective. Let
The normalised cut (Shi and Malik, 2000) of
The solution to this optimisation problem essentially separates the vertices into the two most densely connected regions of the graph across a bottleneck. Minimising this value has been shown to be NP-complete (Shi and Malik, 2000). However, a relaxation of the problem can be solved in polynomial time. The solution to the relaxation is the solution obtained through spectral clustering, yielding an efficient solution relying on eigensolvers (Ng et al., 2001; Von Luxburg, 2007).
2.4 Proposed algorithm: spectral cluster supertree
Spectral Cluster Supertree is a supertree method derived from Min-Cut Supertree. Through analysis of the Min-Cut Supertree algorithm, it was determined that the most time-consuming operation was the min-cut step. In particular, the need to remove every edge on any min-cut of the proper cluster graph. The original paper (Semple and Steel, 2000) proposed a test for determining if an edge was in any min-cut of the proper cluster graph. For each edge, if the edge was deleted and the weight of the min-cut of the new graph was equal to the weight of the min-cut of the original graph minus the weight of the edge, then the edge is on a min-cut of the graph. Page (2002) proposed an improvement to this method, instead using Picard and Queryanne’s algorithm (Picard and Queyranne, 1980) to explicitly find all minimum cuts of the graph. However, in practice, finding even a single arbitrary min-cut of the proper cluster graph in this algorithm was too computationally expensive.
Instead of relying on the (in practice) slow min-cut algorithm to partition the proper cluster graph, we use spectral clustering to efficiently separate the graph into two densely connected components. The remainder of the process is mostly identical to that of Semple and Steel (2000), using these components to induce the new trees for the recursive call. We also include an optimisation from Page’s modified Min-Cut Supertree Page (2002) whereby if only one tree is present on a recursive call, it is returned early and grafted onto the growing tree. We modify the contraction condition for the proper cluster graph to further decrease the problem size. Where Min-Cut Supertree contracted edges representing proper clusters which appeared in every source tree, we instead contract edges representing proper clusters which appear only in every source tree where either of the two taxa are present. An overview of the algorithm is presented in Algorithm 1; Figure 3. We now present additional modifications that allow the method to make use of additional information provided by the source trees.
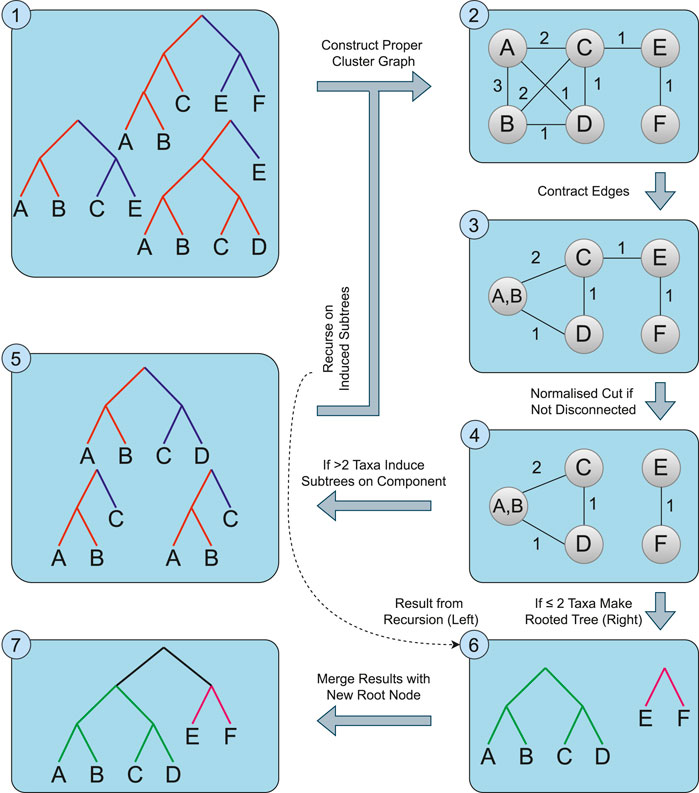
Figure 3. An overview of the Spectral Cluster Supertree Algorithm. The source trees (1) are used to construct the proper cluster graph (2). Edges for proper clusters that only appear together in the source trees are contracted (3). If the graph is not disconnected, spectral clustering is used to take a normalised cut of the graph (4). For any component of the graph with more than two taxa, the source trees are induced on the taxa set and the algorithm recurses on the induced trees (5); otherwise a rooted tree containing the one or two taxa is formed (6). The rooted trees obtained from this step are merged together, connecting the roots of the trees to a new root node (7).
2.5 Weighting strategies
BCD (Fleischauer and Böcker, 2017) introduced weighting strategies over the clades of the source trees to enhance the accuracy of their algorithm. Making use of the additional information that could be gleaned from the source trees allowed for more accurate supertree generation. To boost the topological performance of SCS, we outline similar weighting strategies gathered from information of the proper clusters in the source trees to weight the proper cluster graph.
For this part, recall that
2.5.1 Depth weighting
SCS works by recursively splitting taxa over a root. The further the lowest common ancestor of a proper cluster is from the root of a source tree, the more likely that proper cluster is to be correct in the true tree. Conversely, the closer the lowest common ancestor of a proper cluster is to the root of a source tree, the weaker the proper cluster is. That is, it is more probable that the two taxa were incorrectly misplaced together on the same side of the root.
Depth weighting can be used when the source trees are provided without branch lengths, or when there is other reason for branch lengths to be ignored. Let
2.5.2 Branch length weighting
Branch length weighting works under the same motivation as depth weighting, making use of the branch lengths of the source trees. Let
2.6 Implementation
We provide an implementation of our algorithm on GitHub under an open-source license. A Zenodo archive of the repository is available (https://doi.org/10.5281/zenodo.11118433). The algorithm was implemented using Python 3 and is installable as a package, sc-supertree, on PyPI. The Spectral Clustering step of the algorithm was implemented using scikit-learn (Pedregosa et al., 2011). The algorithm can be invoked through the command line using the scs command in its installed environment, taking as input a file name containing line-separated Newick-formatted source trees. The algorithm can also be utilised as a library with the construct_supertree function, taking as input a sequence of cogent3 tree objects (Knight et al., 2007). We also provide a load_trees function to convert a line separated Newick-formatted source tree file into a list of cogent3 trees. Further details on usage, including specifying the weighting strategy, are available in the project’s README.
3 Experimental design
We perform extensive comparisons between SCS and BCD to evaluate their statistical and computational performance. Comparisons were made over a number of datasets, both existing and new. Our new dataset differs from those previously studied as they are generated without relying on an outgroup, and in that it aims to mimic what may be encountered during the last stage of divide-and-conquer algorithms such as DACTAL (Nelesen et al., 2012). This also corresponds to the practical application of inferring the tree root without prior knowledge of the phylogeny. An extended description of the datasets is presented below and summarised in Table 1.
3.1 Datasets
3.1.1 SMIDGenOG
The SMIDGenOG dataset was generated by Fleischauer and Böcker (2016) using the SMIDGen protocol (Swenson et al., 2010) in a rooted context using outgroup rooting. The SMIDGen protocol emulates data collection processes typically used by systematists, having many densely-sampled clade-based source trees, and a more widely sampled scaffold tree representing relations at a higher taxonomic level. Each model tree was paired with a single such scaffold tree and a number of densely sampled clade-based source trees. The scaffold tree samples a percentage of taxa, the scaffold-factor, uniformly at random over the entire tree. Five “universal” genes (which originate at the root and do not become extinct) were simulated to estimate maximum-likelihood scaffold trees for the dataset. For the clade-based trees, a gene birth-death process (Swenson et al., 2010) was used to select 200 subtrees to simulate non-universal genes. All genes were simulated under a GTR + Gamma + Invariant sites process. Alignments for each of the clade-based trees were created by selecting a clade of interest (under a birth node process according to the SMIDGen protocol), and concatenating three non-universal gene sequences with the highest taxa coverage. An outgroup with all three non-universal genes present was also added to the alignment. Maximum likelihood trees were estimated over the alignments with RAxML (Stamatakis, 2006). All source trees were rooted with the outgroup, and the outgroup was subsequently removed. There are 30 model trees with corresponding source trees for every combination of 100, 500, and 1,000 taxa with a scaffold factor of 20%, 50%, 75% and 100%.
3.1.2 SMIDGenOG-5500
The SMIDGenOG-5500 dataset was generated by Fleischauer and Böcker (2017) as a large-scale variant of the SMIDGenOG dataset. It was created using a similar methodology to the SMIDGenOG dataset with 10 model trees with an average of 5,500 taxa. Each model tree was paired with 500 densely sampled clade-based source trees with 75–125 taxa. Due to the size of the dataset (to ensure the maximum likelihood process could run in a reasonable time frame), each model tree was also paired with five sparsely-sampled scaffold source trees with 100 randomly selected taxa. This differs to SMIDGenOG where a single scaffold tree with varied scaffold factors were used (Fleischauer and Böcker, 2016).
3.1.3 SuperTriplets
The SuperTriplets dataset was generated by Ranwez et al. (2010) to explore the effect of both the size and number of source trees on supertree construction. It contains 100 model trees with 101 taxa each (including an outgroup). The source trees are divided into a number of deletion rates
3.1.4 SCS datasets
We generated the SCS datasets to evaluate supertrees in a setting as may be encountered by rooted variants of divide-and-conquer methods for phylogenetic reconstruction (e.g., Nelesen et al., 2012). In this case, the data generation process does not necessarily rely on an outgroup.
Ten model trees (with 500, 1,000, 2000, 5,000 and 10,000 taxa) were generated following a birth-death process with a birth rate of 1.0 and a death rate of 0.2. The rooted ultrametric trees were initially scaled such that every tip was of distance one from the root. Similar to the SMIDGen protocol (Swenson et al., 2010), branch lengths were then scaled by a random scaling factor. At the root, the scaling factor was 1.0 and the scaling factor evolved down different parts of the tree by adding a number from a normal distribution with a mean of 0 and a standard deviation of 0.05. The scaling factor was additionally bounded in this process between 0.05 and 8.0 to guard against excessively long or short branch lengths following the SMIDGen protocol.
For each of the model trees, cogent3 (Huttley, 2020) was used to simulate a sequence alignment of length 10,000 under a strand-symmetric general nucleotide Markov substitution process under which the root is identifiable (Kaehler, 2017). The alignment length was chosen to allow for a sufficient level of accuracy when estimating the source trees. The parameters for the process were estimated from a sequence alignment of three bacterial species (Kaehler et al., 2015).
Rec-I-DCM3 (Roshan et al., 2004) is a method that can be used to decompose a tree into overlapping subsets of taxa. We have made an implementation of this algorithm in Python publicly available (https://doi.org/10.5281/zenodo.11118313). We used Rec-I-DCM3 to partition each model tree into overlapping subsets of taxa of maximal sizes 50 and 100. We extracted the subtrees for these partitions from the model tree to form a collection of source trees. This forms a simple dataset we call the “SCS-Exact” dataset, from which all methods should always be able to reconstruct the true tree.
For the more interesting second dataset, for each subset in the partition of taxa formed by Rec-I-DCM3, the corresponding simulated sequences were extracted. IQ-TREE 2 (Minh et al., 2020), under a strand symmetric model, was used to fit a rooted tree to these sequences. The set of trees generated for a partition formed the source trees for the “SCS-DCM-IQ” dataset.
3.2 Distance measures
We measure the topological accuracy of supertrees generated by the evaluated methods using the standard Robinson-Foulds distance (Robinson and Foulds, 1981), but also the more statistically robust Matching Cluster distance (Bogdanowicz and Giaro, 2013). For this paper, we use a definition of Robinson-Foulds that is the cardinality of the set containing the symmetric difference of the clades between the two compared trees. Let
The Robinson-Foulds distance, while quick to compute, is known to exhibit poor statistical behaviours (Lin et al., 2011; Böcker et al., 2013). For instance, it is known to saturate quickly. This means similar topologies differing by only a single leaf can maximise this metric, as illustrated by Figure 4.
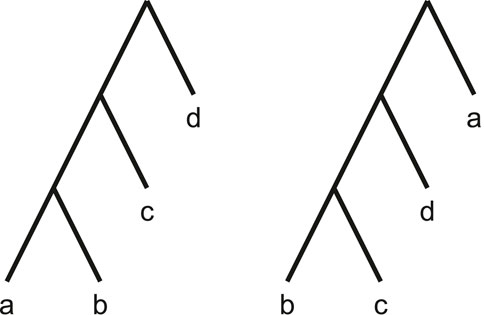
Figure 4. By moving a single leaf (a) to the root of the tree, the Robinson-Foulds distance is maximised.
Consider the
Further, the number of non-root internal vertices in the supertree is equal to the sum of the number of true positive and false positive clades. In the case of a fully resolved tree with
We also include the more statistically robust Matching Cluster distance in our comparisons. The Matching Cluster distance (Bogdanowicz and Giaro, 2013) is similar to the
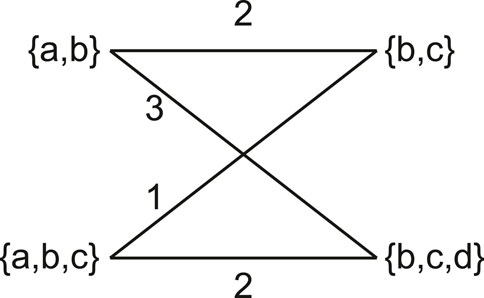
Figure 5. The Matching Cluster distance is calculated by solving the min-weight matching problem of a bipartite graph. Each side of the bipartite graph contains the clusters of the respective trees. Edges are weighted by the cardinality of the symmetric distance of the sets it connects.
When considering multifurcating trees, one tree may have fewer clades than the other. This could mean one side of the graph in the min-weight matching problem could have fewer vertices than the other. The Matching Cluster distance generalises to such scenarios by adding empty sets to the side with fewer vertices until the number of vertices on each side are equal.
The Matching Cluster distance has been shown as more statistically robust than the Robinson-Foulds distance for general tree topologies (Bogdanowicz and Giaro, 2013). That said, for bifurcating trees, the Robinson-Foulds distance and Matching Cluster distance are equal if the weights of the edges for clusters are replaced by 0 if they do match, and two if they do not. See Smith (2020) for further reading on distance measures.
3.3 Experiments
Experiments were performed using the Australian NCI’s Gadi (https://nci.org.au), running on a single core of an Intel(R) Xeon(R) Platinum 8268 CPU @ 2.90 GHz with 16 GB of RAM. SCS, BCD with and without the Greedy Strict Consensus Merger (GSCM) preprocessing, and Min-Cut Supertree (MCS) were evaluated over all of the datasets. We used our own Python implementation for SCS and MCS, and the Java implementation of BCD by Fleischauer and Böcker (2017). A modified version of Min-Cut Supertree was used that included our weighting strategies for the proper cluster graph. We also allowed MCS to find only a single arbitrary min-cut of the proper cluster graph, rather than all min-cuts, so the problems could be solved within a reasonable time. Branch length weighting was utilised across all methods where possible (except for the SuperTriplets dataset). Otherwise, depth weighting for our method, or unit weighting for BCD, was used. The CPU time to resolve each supertree was recorded, as well as the Robinson-Foulds Distance and Matching Cluster Distance when compared to the model tree (
4 Results
4.1 Spectral cluster supertree vs. Min-Cut supertree
Here, we show how SCS is a significant improvement over Min-Cut Supertree across all measures. Figure 6 displays the difference in the recorded measures between the two methods over the largest dataset where Min-Cut Supertree resolved at least some problems (4/10 for each of the maximum subproblem sizes). Where SCS takes a matter of seconds to solve a problem, Min-Cut Supertree can take from 2 hours to close to a full day. SCS additionally outperforms Min-Cut Supertree in every metric—consistently producing more correct clades in the generated supertree (from the Robinson-Foulds Distance), and markedly superior general topological accuracy from the Matching Cluster Distance.
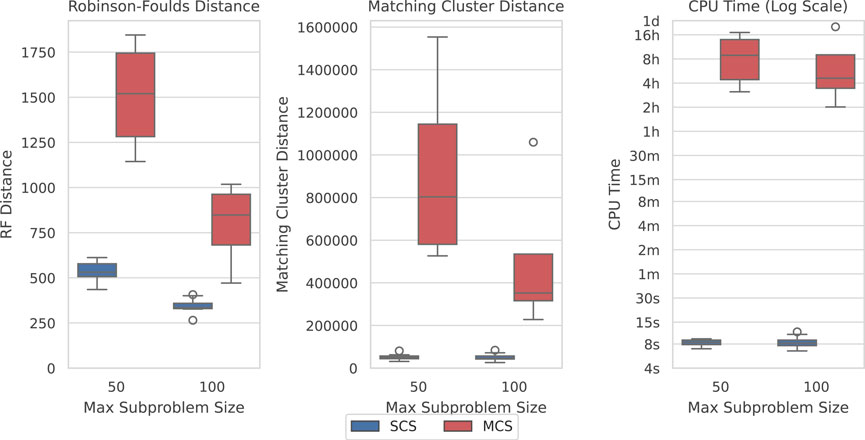
Figure 6. SCS outperforms MCS on the SCS-DCM-IQ dataset with 5,000 taxa. Lower is better for all graphs. Time results are shown on a log scale. Each parameterisation of the dataset contains 10 problems. While SCS solved all problems, MCS only solved 4/10 within the time limit for each of the maximum subproblem sizes.
The results shown in Figure 6 are similar across most datasets, though not necessarily to the same extent with regard to time on the smaller datasets. MCS only outperformed SCS with respect to time on the SCS-Exact dataset, where importantly no min-cut or spectral clustering calls are required, and all methods recovered the true tree. On the largest SCS-Exact problems, both methods took around 6–10 s. This illustrates the extent at which the min-cut component of the MCS impacts its computational efficiency. SCS always dominated MCS with respect to topological accuracy under both distance metrics on all other datasets. Going forward, we thus only compare the performance of SCS to BCD.
4.2 Spectral cluster supertree vs. Bad Clade Deletion
All methods could construct the correct supertree in the SCS-Exact dataset, though GSCM preprocessing made BCD sometimes take hours for the largest problems. Without GSCM, BCD finished in a few seconds. Note that this is contrary to the time results for the other datasets shown in the figures. We now show the results for SCS compared with BCD with and without GSCM preprocessing over each of the datasets.
Figure 7 shows the results over the SCS-DCM-IQ datasets with 10,000 taxa. Results for the other taxa counts follow the same pattern (see Supplementary Figures S2–S5). The figure shows that with GSCM preprocessing, BCD outperforms SCS in terms of the Robinson-Foulds distance. This metric measures the number of clades that are different (no matter how similar) between the model tree and generated supertree. SCS however outperforms BCD under the Matching Cluster distance, which measures more generally how similar the overall topologies of the tree are—taking into account the degree of similarity/dissimilarity in the clades. SCS is also vastly superior here compared to BCD in terms of the time taken to solve the problems. Where BCD takes multiple hours per problem instance, SCS can solve these problems in less than a minute—usually in under 20 seconds.
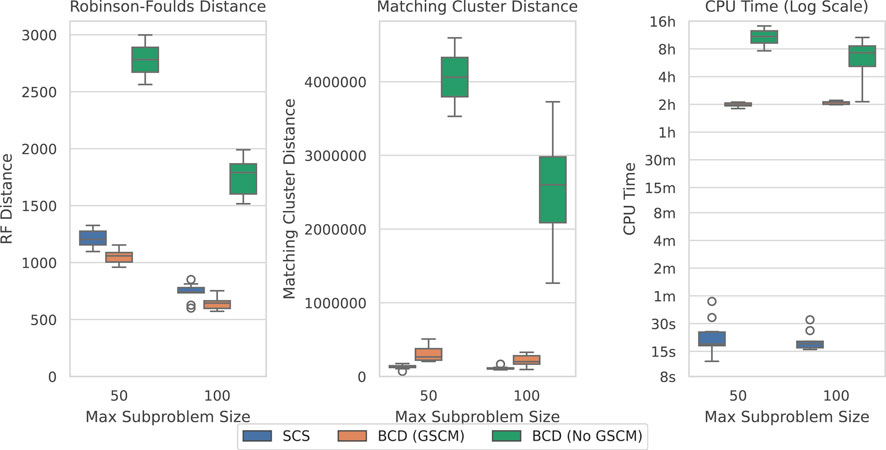
Figure 7. SCS vs. BCD on the SCS-DCM-IQ dataset with 10,000 taxa. The dataset emulates what may be encountered when using divide-and-conquer algorithms for phylogenetic reconstruction. Each parameterisation of the dataset contains 10 problems. BCD without GSCM processing only solved the first two and six problems within the timeout for the 50 and 100 max subproblem sizes respectively. The other methods solved all ten problems.
Figure 8 compares the results of SCS to BCD under the large SMIDGenOG-5500 dataset. The results follow a similar pattern to the SCS-DCM-IQ dataset. BCD still outperforms SCS in terms of the Robinson-Foulds Distance, though the difference is less pronounced. SCS again outperforms BCD in terms of the Matching Cluster distance, which compares the tree topologies more generally. SCS is still a vast improvement over BCD in terms of the time required to solve the problems, taking minutes, where the others can take multiple hours.
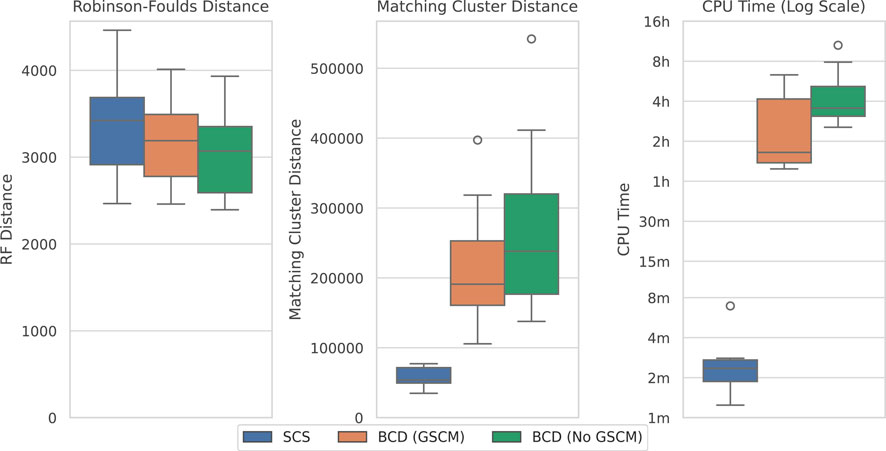
Figure 8. SCS vs. BCD on the SMIDGenOG-5500 dataset. The dataset applies the SMIDGen protocol to mimic what may be encountered by systematists at a large scale. As there is no parameterisation for this dataset, no x-axis is displayed. All methods solved all ten problem instances within the timeout.
Figure 9 shows the results over the largest original SMIDGenOG dataset with 1,000 taxa and different scaffold factors (the percentage of taxa sampled for the scaffold trees). This dataset shows the best results for BCD when compared to SCS. BCD outperforms SCS in terms of the Robinson-Foulds Distance. The results are roughly even in terms of Matching Cluster Distance for the smaller two scaffold factors, though BCD with GSCM preprocessing is slightly ahead with the higher two scaffold factors. SCS is slightly ahead with respect to time compared with BCD with GSCM preprocessing. However, problems here are being solved in the order of seconds and the difference is not significant for practical purposes. Results are similar for the 100 and 500 taxa counts (see Supplementary Figures S6, S7), with BCD being faster on the 100 taxa dataset.
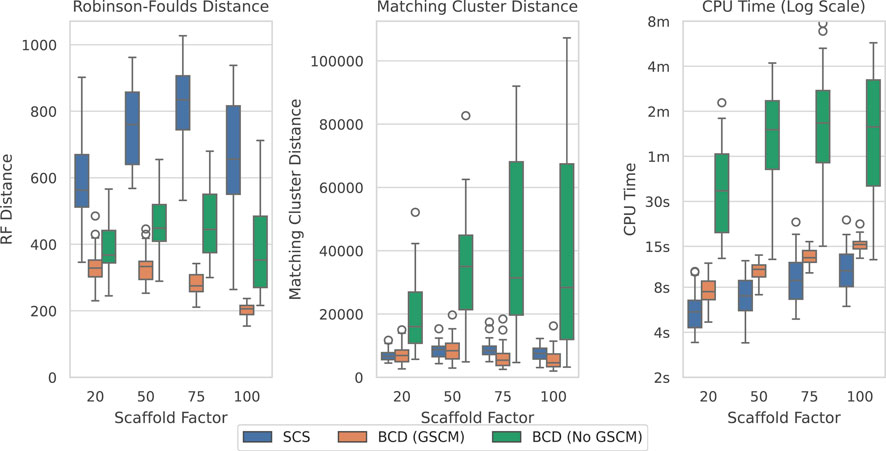
Figure 9. SCS vs. BCD on the SMIDGenOG dataset with 1,000 taxa. The dataset aims to imitate data curation processes of systematists. The SMIDGenOG dataset contains one scaffold tree sampling over “Scaffold Factor” percent of the taxa, as well as many densely sampled clade-based source trees. Each parameterisation of the dataset contains 30 problems.
Figure 10 shows the results over the SuperTriplets dataset with a 50% deletion rate (percentage of taxa removed when generating each source tree). The SuperTriplets dataset shows a significant amount of variance over the different deletion rates. With a 50% deletion rate, SCS generally performs worse with respect to the Robinson-Foulds Distance, but better in terms of Matching Cluster distance. With a 75% deletion rate (Supplementary Figure S9), SCS generally outperforms BCD in all distance metrics. For the 25% deletion rate (Supplementary Figure S8), BCD outperforms SCS in terms of the Robinson-Foulds distance. The central distribution of the Matching Cluster distance is roughly identical, with BCD achieving better minimum values, though SCS achieves better maximum values. The time results here are all low enough to not make much of a difference for practical purposes.
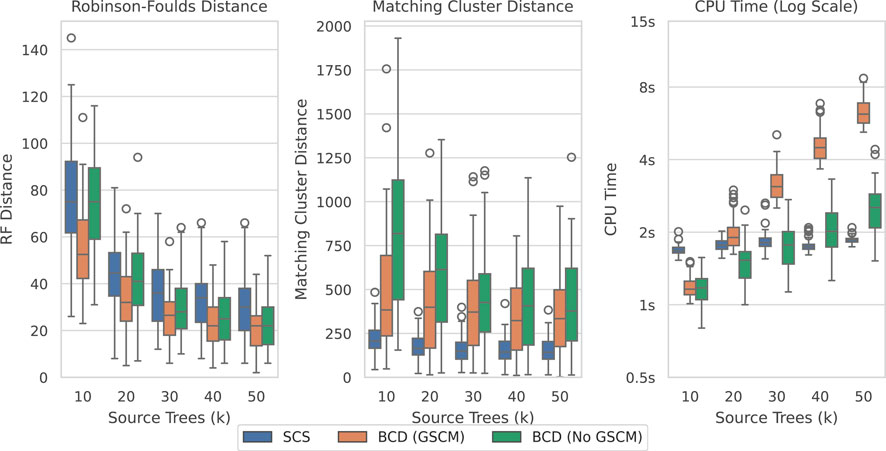
Figure 10. SCS vs. BCD on the SuperTriplets dataset with a deletion rate of 50%. The SuperTriplets dataset explores the effect of percentage of taxa in the source trees, and number of source trees, on supertree construction. Each parameterisation of the dataset contains 100 problems. Results are variable depending on the deletion rate, and results for the others are included in the appendix.
Full figures for the other parameterisations of the datasets are available in the appendix. The raw results have been archived on Zenodo (https://doi.org/10.5281/zenodo.11118313).
5 Discussion
The Spectral Cluster Supertree (SCS) algorithm for merging rooted phylogenetic trees exhibits comparable or markedly better statistical and computational performance than current approaches. While Min-Cut Supertree (MCS) was demonstrated impractical for modern sized data sets, Bad Clade Deletion (BCD) was far more efficient when used in conjunction with its GSCM pre-processing step. Under most conditions examined, particularly for large problem size, SCS was orders of magnitude faster than BCD. Comparison of the statistical performance of the algorithms was sensitive to the topology distance metric used. Thus, judgement of the statistical merits of SCS relative to competing approaches hinges on the properties of the Robinson-Foulds and Matching Cluster distance metrics.
5.1 SCS statistically outperforms BCD on most datasets
In terms of Robinson-Foulds distance, BCD with GSCM preprocessing almost always outperformed SCS or were otherwise roughly equivalent. However, the Robinson-Foulds distance metric suffers from poor statistical qualities. It can saturate quickly; it is possible for trees that are identical minus the placement of a single leaf to maximise this distance metric. It simply compares how many of the clades are an exact match between the model tree and supertree, without considering the degree of similarity or dissimilarity between the clades.
The Matching Cluster distance is a more statistically robust distance measure when comparing tree topologies. It gathers the clades of the model tree and supertree, and measures the degree of dissimilarity between the clades of these two sets (based on the size symmetric difference of the clades, or in other words, how many taxa in the clades are different). Having found the optimal pairing of clades between these sets, the distance measure is calculated. By considering the degree of dissimilarity between the clades of the trees, rather than whether they match or not (as per Robinson-Foulds), it is more robust. If instead the degree of dissimilarity was measured as two if the clades do not match and 0 otherwise, the distance measure would become identical to Robinson-Foulds.
SCS frequently outperformed BCD with respect to the Matching Cluster distance on all datasets except the SMIDGenOG dataset, and SuperTriplets dataset with a deletion rate of
It is somewhat curious, on first consideration, the discrepancy between the SMIDGenOG and SMIDGenOG-5500 results given the underlying data was simulated under a similar protocol. The primary difference between the two datasets here is the number of taxa in the scaffold tree. Due to practical limitations, SMIDGenOG-5500 contains five scaffold trees with 100 taxa each (less than
The algorithmic properties of the two methods could potentially explain this relationship. BCD effectively aims to delete a minimum number of clades from the source trees when conflicts arise to construct a supertree. Importantly, every clade in the supertree must also be a clade in one of the source trees (which leads to lower in comparison Robinson-Foulds distances). Having a strong backbone with a widely sampled scaffold tree can support this. When conflicts arise in SCS, however, we consider the degree to which the source trees support taxa appearing on the same side of a prospective root. We partition the taxa, into two groups with maximal support within each group but minimal support between the two groups. This is clearly quite effective in practice, but it may not count as heavily, in comparison, the presence of a strong backbone. On further examination. we found that by increasing the tree weight associated with the scaffold tree in the SMIDGenOG dataset, the accuracy of the SCS method improved significantly (sometimes better than BCD). The improvement was particularly strong for high scaffold factors. This supports our hypothesis for the explanation of the relationship here. However, as BCD also supports tree weights and to avoid overfitting these extra parameters to the reported accuracy, we do not include these results in our comparisons.
When considering practical applications of the algorithms on large datasets, the choice of method is clear. For large phylogenetic reconstruction problems, the time required to perform a full maximum likelihood analysis over sequences of interest is far too computationally taxing. In these scenarios (as in the SMIDGenOG-5500 dataset), the presence of a large scaffold tree can potentially be infeasible. Divide-and-conquer methods relying on the Disk-Covering Method (Roshan et al., 2004), breaking down the problem into small overlapping subproblems, may thus be required to make computation possible. In these situations, it is clear SCS performs best with respect to topological accuracy, though BCD may still be useful for smaller problems with a strong scaffold tree.
5.2 SCS displays superior computational efficiency over BCD in serial
SCS is also vastly more efficient in terms of CPU Time compared to BCD. For the largest problems in the SCS-DCM-IQ dataset, SCS could solve problems (that took BCD roughly 2 h) in under a minute—usually in less than 20 s (median speedup of 386 and 409 times for maximum subproblem sizes of 50 and 100 respectively). For the SMIDGenOG-5500 dataset, where BCD took 1–8 h to solve each problem, SCS took only 1–8 min (median 58 times speedup). Ignoring problems which took both BCD (GSCM) and SCS under 15 seconds to solve, the median speedup over BCD under each combination of parameters of all the datasets ranged from 12 to 409 — the greatest speedups on the largest datasets. The only problems BCD beat SCS on speed, SCS still solved in only 1–2 s. SCS is clearly the superior choice in terms of time needed to fully resolve a supertree.
It must be noted that the results illustrated in the figures were obtained from running the experiments on a single CPU core. BCD has support for multi-threading, which is performed over both min-cut computations and recursive calls. In the current version of SCS, concurrency is only utilised within NumPy operations (Harris et al., 2020) and during a part of the spectral clustering step as implemented in scikit-learn (Pedregosa et al., 2011). There is room for further parallelisation in SCS, however. Similar to how BCD parallelises across independent recursive calls, the same can be done trivially for SCS. This parallelisation was trialled, but the improvement was minor on these problem sizes due to the associated overhead, and the time domination of performing the first and largest split. Parallelisation will likely have a much more beneficial effect for even larger problems than we have tested, and we accordingly leave a full comparison of the parallel versions for future work. Due to BCD’s parallelisation over solving min-cuts in addition to the subproblems, greater speedup may be obtained, particularly on these problem sizes. However, SCS improves on the time results of BCD over large problems sizes to such a significant extent that this difference may not matter. This is especially true when considering limitations such as Amdahl’s law (which gives a theoretical asymptotic limit to the speedup of a program as the number of processors are increased).
6 Conclusion
We presented a new algorithm, Spectral Cluster Supertree, for merging overlapping rooted phylogenetic trees. Our algorithm is significantly faster than Bad Clade Deletion on large problem sizes, taking on average 20 s where Bad Clade Deletion took
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://doi.org/10.5281/zenodo.11118022.
Author contributions
RM: Investigation, Methodology, Software, Writing–original draft, Writing–review and editing. AZ: Methodology, Supervision, Writing–review and editing. MC: Methodology, Writing–review and editing. YL: Conceptualization, Methodology. GH: Conceptualization, Investigation, Methodology, Project administration, Resources, Software, Supervision, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
This research was undertaken with the assistance of resources and services from the National Computational Infrastructure (NCI), which is supported by the Australian Government.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmolb.2024.1432495/full#supplementary-material
References
Baum, B. R. (1992). Combining trees as a way of combining data sets for phylogenetic inference, and the desirability of combining gene trees. Taxon 41, 3–10. doi:10.2307/1222480
Böcker, S., Canzar, S., and Klau, G. W. (2013). “The generalized robinson-foulds metric,” in Algorithms in bioinformatics: 13th international workshop, WABI 2013, sophia antipolis, France, september 2-4, 2013. Proceedings 13 (Springer), 156–169.
Bogdanowicz, D., and Giaro, K. (2013). On a matching distance between rooted phylogenetic trees. Int. J. Appl. Math. Comput. Sci. 23, 669–684. doi:10.2478/amcs-2013-0050
Boorman, S. A., and Olivier, D. C. (1973). Metrics on spaces of finite trees. J. Math. Psychol. 10, 26–59. doi:10.1016/0022-2496(73)90003-5
Dang, C. C., Minh, B. Q., McShea, H., Masel, J., James, J. E., Vinh, L. S., et al. (2022). nQMaker: estimating time nonreversible amino acid substitution models. Syst. Biol. 71, 1110–1123. doi:10.1093/sysbio/syac007
Fleischauer, M., and Böcker, S. (2016). Collecting reliable clades using the greedy strict consensus merger. PeerJ 4, e2172. doi:10.7717/peerj.2172
Fleischauer, M., and Böcker, S. (2017). Bad clade deletion supertrees: a fast and accurate supertree algorithm. Mol. Biol. Evol. 34, 2408–2421. doi:10.1093/molbev/msx191
Harris, C. R., Millman, K. J., van der Walt, S. J., Gommers, R., Virtanen, P., Cournapeau, D., et al. (2020). Array programming with NumPy. Nature 585, 357–362. doi:10.1038/s41586-020-2649-2
Huson, D. H., Nettles, S. M., and Warnow, T. J. (1999). Disk-covering, a fast-converging method for phylogenetic tree reconstruction. J. Comput. Biol. 6, 369–386. doi:10.1089/106652799318337
Kaehler, B. D. (2017). Full reconstruction of non-stationary strand-symmetric models on rooted phylogenies. J. Theor. Biol. 420, 144–151. doi:10.1016/j.jtbi.2017.03.007
Kaehler, B. D., Yap, V. B., Zhang, R., and Huttley, G. A. (2015). Genetic distance for a general non-stationary markov substitution process. Syst. Biol. 64, 281–293. doi:10.1093/sysbio/syu106
Knight, R., Maxwell, P., Birmingham, A., Carnes, J., Caporaso, J. G., Easton, B. C., et al. (2007). Pycogent: a toolkit for making sense from sequence. Genome Biol. 8, 1–16. doi:10.1186/gb-2007-8-8-r171
Lin, Y., Rajan, V., and Moret, B. M. (2011). A metric for phylogenetic trees based on matching. IEEE/ACM Trans. Comput. Biol. Bioinforma. 9, 1014–1022. doi:10.1109/TCBB.2011.157
Meilă, M., and Shi, J. (2001). “A random walks view of spectral segmentation,” in International workshop on artificial intelligence and statistics (Key West, Florida, USA: PMLR), 203–208.
Minh, B. Q., Schmidt, H. A., Chernomor, O., Schrempf, D., Woodhams, M. D., Von Haeseler, A., et al. (2020). Iq-tree 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 37, 1530–1534. doi:10.1093/molbev/msaa015
Nelesen, S., Liu, K., Wang, L.-S., Linder, C. R., and Warnow, T. (2012). Dactal: divide-and-conquer trees (almost) without alignments. Bioinformatics 28, i274–i282. doi:10.1093/bioinformatics/bts218
Ng, A., Jordan, M., and Weiss, Y. (2001). On spectral clustering: analysis and an algorithm. Adv. neural Inf. Process. Syst. 14.
Page, R. D. (2002). “Modified mincut supertrees,” in International workshop on algorithms in bioinformatics (Springer), 537–551.
Pedregosa, F., Varoquaux, G., Gramfort, A., Michel, V., Thirion, B., Grisel, O., et al. (2011). Scikit-learn: machine learning in Python. J. Mach. Learn. Res. 12, 2825–2830.
Picard, J.-C., and Queyranne, M. (1980). On the structure of all minimum cuts in a network and applications. Springer.
Ragan, M. A. (1992). Phylogenetic inference based on matrix representation of trees. Mol. phylogenetics Evol. 1, 53–58. doi:10.1016/1055-7903(92)90035-f
Ranwez, V., Criscuolo, A., and Douzery, E. J. (2010). Supertriplets: a triplet-based supertree approach to phylogenomics. Bioinformatics 26, i115–i123. doi:10.1093/bioinformatics/btq196
Robinson, D. F., and Foulds, L. R. (1981). Comparison of phylogenetic trees. Math. Biosci. 53, 131–147. doi:10.1016/0025-5564(81)90043-2
Roshan, U. W., Moret, B. M., Warnow, T., and Williams, T. L. (2003). Greedy strict-consensus merger: a new method to combine multiple phylogenetic trees (Citeseer)
Roshan, U. W., Warnow, T., Moret, B. M., and Williams, T. L. (2004). “Rec-i-dcm3: a fast algorithmic technique for reconstructing phylogenetic trees,” in Proceedings. 2004 IEEE computational systems bioinformatics conference, 2004 (IEEE), 98–109.
Semple, C., and Steel, M. (2000). A supertree method for rooted trees. Discrete Appl. Math. 105, 147–158. doi:10.1016/s0166-218x(00)00202-x
Shi, J., and Malik, J. (2000). Normalized cuts and image segmentation. IEEE Trans. pattern analysis Mach. Intell. 22, 888–905. doi:10.1109/34.868688
Smith, M. R. (2020). Information theoretic generalized robinson–foulds metrics for comparing phylogenetic trees. Bioinformatics 36, 5007–5013. doi:10.1093/bioinformatics/btaa614
Stamatakis, A. (2006). Raxml-vi-hpc: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690. doi:10.1093/bioinformatics/btl446
Steel, M., and Bocker, S. (2000). Simple but fundamental limitations on supertree and consensus tree methods. Syst. Biol. 49, 363–368. doi:10.1093/sysbio/49.2.363
Sumner, J., Fernández-Sánchez, J., and Jarvis, P. (2012). Lie markov models. J. Theor. Biol. 298, 16–31. doi:10.1016/j.jtbi.2011.12.017
Swenson, M. S., Barbançon, F., Warnow, T., and Linder, C. R. (2010). A simulation study comparing supertree and combined analysis methods using smidgen. Algorithms Mol. Biol. 5, 8–16. doi:10.1186/1748-7188-5-8
Swenson, M. S., Suri, R., Linder, C. R., and Warnow, T. (2012). Superfine: fast and accurate supertree estimation. Syst. Biol. 61, 214–227. doi:10.1093/sysbio/syr092
Vachaspati, P., and Warnow, T. (2017). Fastrfs: fast and accurate robinson-foulds supertrees using constrained exact optimization. Bioinformatics 33, 631–639. doi:10.1093/bioinformatics/btw600
Von Luxburg, U. (2007). A tutorial on spectral clustering. Statistics Comput. 17, 395–416. doi:10.1007/s11222-007-9033-z
Yap, V. B., and Speed, T. (2005). Rooting a phylogenetic tree with nonreversible substitution models. BMC Evol. Biol. 5 (2), 2. doi:10.1186/1471-2148-5-2
Keywords: supertree, spectral clustering, rooted phylogenetic trees, phylogenetics, molecular evolution
Citation: McArthur RN, Zehmakan AN, Charleston MA, Lin Y and Huttley G (2024) Spectral cluster supertree: fast and statistically robust merging of rooted phylogenetic trees. Front. Mol. Biosci. 11:1432495. doi: 10.3389/fmolb.2024.1432495
Received: 14 May 2024; Accepted: 24 September 2024;
Published: 30 October 2024.
Edited by:
Matthew Wakefield, The University of Melbourne, AustraliaReviewed by:
Ira Cooke, James Cook University, AustraliaDavid Richard Powell, Monash University, Australia
Copyright © 2024 McArthur, Zehmakan, Charleston, Lin and Huttley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robert N. McArthur, cm9iZXJ0Lm1jYXJ0aHVyQGFudS5lZHUuYXU=
†Deceased
 Robert N. McArthur
Robert N. McArthur Ahad N. Zehmakan
Ahad N. Zehmakan Michael A. Charleston
Michael A. Charleston Yu Lin2†
Yu Lin2† Gavin Huttley
Gavin Huttley