
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol., 17 January 2025
Sec. Microbe and Virus Interactions with Plants
Volume 15 - 2024 | https://doi.org/10.3389/fmicb.2024.1510036
Plant-parasitic nematodes (PPNs), including root-knot nematodes (Meloidogyne spp.), cyst nematodes (Heterodera and Globodera spp.), and other economically significant nematode species, pose severe threats to global agriculture. These nematodes employ diverse survival strategies, such as dormancy in cysts or robust infective juvenile stages. Consequently, their management is challenging. Traditional control methods, such as the use of chemical nematicides, are increasingly scrutinized because of environmental and health concerns. This review focuses on the specific mechanisms employed by Bacillus spp., including nematicidal compound production, systemic resistance induction, and cuticle degradation, to target root-knot and cyst nematodes. These mechanisms offer sustainable solutions for managing nematodes and promoting soil health by enhancing microbial diversity and nutrient cycling. An integrated approach leveraging Bacillus-based biocontrol is proposed to maximize efficacy and agricultural sustainability.
Nematode infestations significantly threaten global agriculture, causing substantial economic losses of over USD 80 billion annually (Nicol et al., 2011; Abd-Elgawad, 2024). Plant-parasitic nematodes (PPNs) are highly diverse and include various species, such as root-knot nematodes (Meloidogyne spp.), cyst nematodes (Heterodera and Globodera spp.), lesion nematodes (Pratylenchus spp.), and reniform nematodes (Rotylenchulus reniformis). These nematodes exhibit unique parasitic mechanisms. Hence, their management in agricultural systems is challenging.
Root-knot nematodes invade root tissues and induce the formation of specialized feeding structures called giant cells, diverting host resources and stunting plant growth. Bacillus subtilis produces nematicidal enzymes, such as proteases, which degrade nematode cuticles, reducing mobility and infectivity. Secondary metabolites, such as fengycin and surfactin, exhibit potent activity by disrupting nematode cell membranes, causing cell lysis and death (Jiang et al., 2021). Moreover, these metabolites inhibit egg hatching and juvenile development, effectively suppressing the nematode life cycle. On the other hand, cyst nematodes form syncytia in root tissues, resulting in long-term nutrient extraction. Lesion nematodes produce migratory lesions that compromise root integrity and increase susceptibility to secondary infections (Gupta et al., 2023). These adaptations result in yield losses, with root-knot nematodes alone accounting for an estimated loss of over 5% globally. Their adaptability and multiple life cycles in warm climates exacerbate this damage (Subbotin et al., 2021). Similarly, cyst nematodes survive under unfavorable conditions by forming resilient cysts containing eggs, enabling extended dormancy in the soil (Moens et al., 2018). B. amyloliquefaciens plays a crucial role in managing cyst nematodes by inducing systemic resistance in plants, thereby suppressing the formation of syncytia within root tissues. This bacterium also produces chitinases to degrade cyst shells, preventing hatching and subsequent infestations (Ngalimat et al., 2021). Given these functions of Bacillus spp. and their role in improving plant vigor, they are effective against cyst nematodes in diverse agricultural systems.
The survival strategies of nematodes demand tailored management approaches that account for the distinct biological traits of each group. For instance, root-knot nematodes secrete effector proteins that suppress key host plant defense pathways, such as those mediated by jasmonic acid (JA) and salicylic acid (SA), while cyst nematodes release effector proteins that alter root architecture to facilitate syncytium formation (Ahmad et al., 2021). Moreover, lesion nematodes disrupt cell walls enzymatically, contributing to extensive root decay. Understanding these intricate molecular interactions is crucial for devising effective and sustainable management strategies.
Traditional control methods, such as crop rotation, the use of resistant cultivars, and the use of chemical nematicides, are limited by the biological versatility of nematodes and the environmental concerns associated with chemical usage. The ability of root-knot nematodes to overcome resistant cultivars further complicates breeding efforts (Pradhan et al., 2023). Moreover, although chemical nematicides are initially effective, they pose risks to nontarget organisms and contribute to environmental degradation (Kumar et al., 2017). These limitations underscore the need for safer, eco-friendly alternatives.
Recent advances in biocontrol have demonstrated the potential of Bacillus spp. in combating specific PPNs. Bacillus spp. employ various mechanisms, such as the production of nematicidal metabolites (e.g., lipopeptides and proteases), the induction of systemic resistance in plants, and competition with nematodes for resources (Patil et al., 2019; Jiang et al., 2021). For instance, B. subtilis produces fengycin and surfactin lipopeptides, which disrupt root-knot nematode cuticles, while B. amyloliquefaciens induces systemic resistance in plants, enhancing defenses against cyst nematodes (Lin et al., 2020). Understanding the mechanisms underlying these distinct interactions is crucial for optimizing their applications in nematode management programs and ensuring that they also contribute positively to soil health. This review emphasizes the targeted use of Bacillus spp. against root-knot and cyst nematodes, detailing their distinct survival strategies and biocontrol mechanisms.
Given the diversity of PPNs and the limitations of conventional management strategies, this review focuses on Bacillus spp. as biocontrol agents, discussing their mechanisms, efficacy, and potential for integration into sustainable nematode management programs. The discussion covers multiple PPNs, focusing on crop nematodes, especially root-knot, cyst, lesion, and reniform nematodes. The literature is sourced from reputable databases, including Elsevier, Springer, and MDPI, ensuring the inclusion of high-quality and relevant studies.
Phytopathogenic nematodes pose a significant threat to global agriculture. They impact a wide range of crops by feeding on plant roots, disrupting nutrient uptake, and serving as vectors for other pathogens. The most harmful genera include Meloidogyne, Heterodera, Globodera, Pratylenchus, Radopholus, Rotylenchulus, Ditylenchus, and Bursaphelenchus, each exhibiting unique life cycles, modes of action, and seasonal habitats that contribute to pathogenicity (Mesa-Valle et al., 2020; Palomares-Rius et al., 2020).
Root-knot nematodes (Meloidogyne spp.), including M. incognita, M. javanica, and M. arenaria, are particularly damaging. Their life cycles progress from eggs to infective juveniles and adults, with juveniles primarily causing damage by penetrating plant roots. These nematodes thrive in warm climates and cause peak damage during spring and summer, contributing to significant yield losses in various crops, such as tomatoes, soybeans, and cotton in Brazil, China, and other regions (Blouin et al., 1998; Subbotin et al., 2021). Cyst nematodes (Heterodera and Globodera spp.) pose unique challenges because of their ability to form cysts containing eggs. Consequently, they can survive for long durations under adverse conditions. The soybean cyst nematode H. glycines and the golden potato cyst nematode G. rostochiensis cause substantial crop losses, particularly in temperate regions. Their dormant cysts hatch under favorable environmental conditions, typically in spring, aligning with the planting season (He et al., 2022). Lesion nematodes (Pratylenchus spp.) are migratory endoparasites that create lesions in root tissues as they feed, significantly impairing plant health. These nematodes are active throughout the year in warm, moist environments, such as those in tropical agricultural regions, causing severe yield losses in various crops, such as banana, coffee, and soybean (Saikai and MacGuidwin, 2022; Riascos-Ortiz et al., 2022). Similarly, burrowing nematodes (Radopholus similis) and stem nematodes (Ditylenchus dipsaci) exhibit seasonal activity, with the former thriving in wet tropical climates and the latter affecting bulbous plants in cooler climates (Mathew and Opperman, 2019; Sturhan and Brzeski, 2020). The global burden of these nematodes is substantial. Hence, there is an urgent need for sustainable, effective management strategies to mitigate the impact of these nematodes on global food security.
Traditional nematode management approaches, including cultural practices, biocontrol methods, and chemical treatments, have been widely implemented to mitigate the detrimental effects of nematodes and maintain crop health and productivity (Elango et al., 2020). Cultural methods, such as crop rotation, soil solarization, and sanitation, aim to interrupt the life cycle of nematodes, thereby diminishing their populations in the soil (Oka, 2010). Biocontrol methods leverage natural predators and antagonistic plants to maintain the ecological balance of nematode populations (El-Saadony et al., 2021). Chemical treatments, which involve the application of nematicides, can directly target nematodes and rapidly reduce their populations.
Despite their extensive use, these conventional methods have several limitations that undermine their long-term efficacy and sustainability (Sikora and Roberts, 2018). Although cultural practices, such as crop rotation, are theoretically effective, they require extensive knowledge and labor and can yield inconsistent results because of environmental variations (Grubišić et al., 2018). Biocontrol methods, including the use of antagonistic plants, such as marigold (Tagetes spp.) and neem (Azadirachta indica), offer environmentally friendly alternatives; however, they often fail to exhibit adequate suppressive effects and may require considerable time to be effective (Waller and Thamsborg, 2004). Moreover, the efficacy of biocontrol methods can significantly vary depending on the species involved and the environmental conditions.
Although chemical treatments provide rapid and effective nematode control, they pose significant risks to human health, nontarget organisms, and the environment. The persistent use of nematicides has led to the emergence of resistant nematode strains, thereby diminishing their long-term effectiveness (Timper, 2014). The regulatory restrictions posed on many effective nematicides because of their adverse environmental impacts have further limited their availability and use (Grubišić et al., 2018).
These inherent limitations of traditional nematode control methods highlight the need for innovative and sustainable approaches. Integrated pest management (IPM) strategies that combine traditional practices with modern technological advancements present a promising solution. These strategies aim to enhance the effectiveness of nematode control while minimizing the associated environmental and health risks.
Biocontrol strategies are being recognized as sustainable and environmentally friendly alternatives to chemical nematicides for managing nematode infestations. Various microbial agents and botanical extracts have shown potential for reducing nematode populations. For instance, fungal strains, such as Auxarthron reticulatum DY-2, Verticillium saksenae A-1, Lecanicillium psalliotae A-1, and L. antillanum B-3, have been explored for their effectiveness in parasitizing and reducing nematode populations (Oh et al., 2014a,b; Nguyen et al., 2014). Additionally, extracts of Cinnamomum cassia bark and C. aromaticum have demonstrated enzyme-inhibitory and nematicidal properties, thereby serving as potential agents for botanical interventions (Nguyen et al., 2009, 2012; Nguyen and Jung, 2014). Nguyen et al. (2011) demonstrated that treatment with C. cassia crude extracts significantly reduced gall formation and nematode growth in a dose-dependent manner in root-knot nematode-infested cucumber plants. This treatment also enhanced the activities of antioxidative enzymes, such as SOD, CAT, and APX, in cucumber leaves, indicating a strengthened defense response against the nematode. Furthermore, bark extracts of Terminalia nigrovenulosa and related compounds have been found to disrupt nematode life cycles (Seo et al., 2013).
In addition to fungi and botanical extracts, entomopathogenic nematodes (EPNs), such as Steinernema and Heterorhabditis spp., are known for their ability to release symbiotic bacteria (e.g., Xenorhabdus and Photorhabdus spp.) that produce toxins lethal to nematodes (El Aimani et al., 2022). Furthermore, predatory fungi, such as Paecilomyces and Arthrobotrys spp., can trap and digest nematodes, while endophytic fungi, such as Trichoderma spp., can colonize plant roots and produce enzymes and metabolites that can inhibit nematode activity and enhance plant resistance (Singh et al., 2019). The incorporation of organic amendments, such as compost and green manure, into the soil can also boost the populations of beneficial microbes that compete with or directly antagonize nematodes. These biocontrol strategies can not only reduce the reliance on chemical nematicides but also promote sustainable agricultural practices by enhancing soil health and biodiversity. The schematic representation of comparison of chemical pesticide-based nematode management with Bacillus-based biocontrol approaches, showcasing differences in mode of action, scalability, production costs, environmental impacts, non-target species effects, soil health, economic value, and sustainability was displayed (Figure 1).
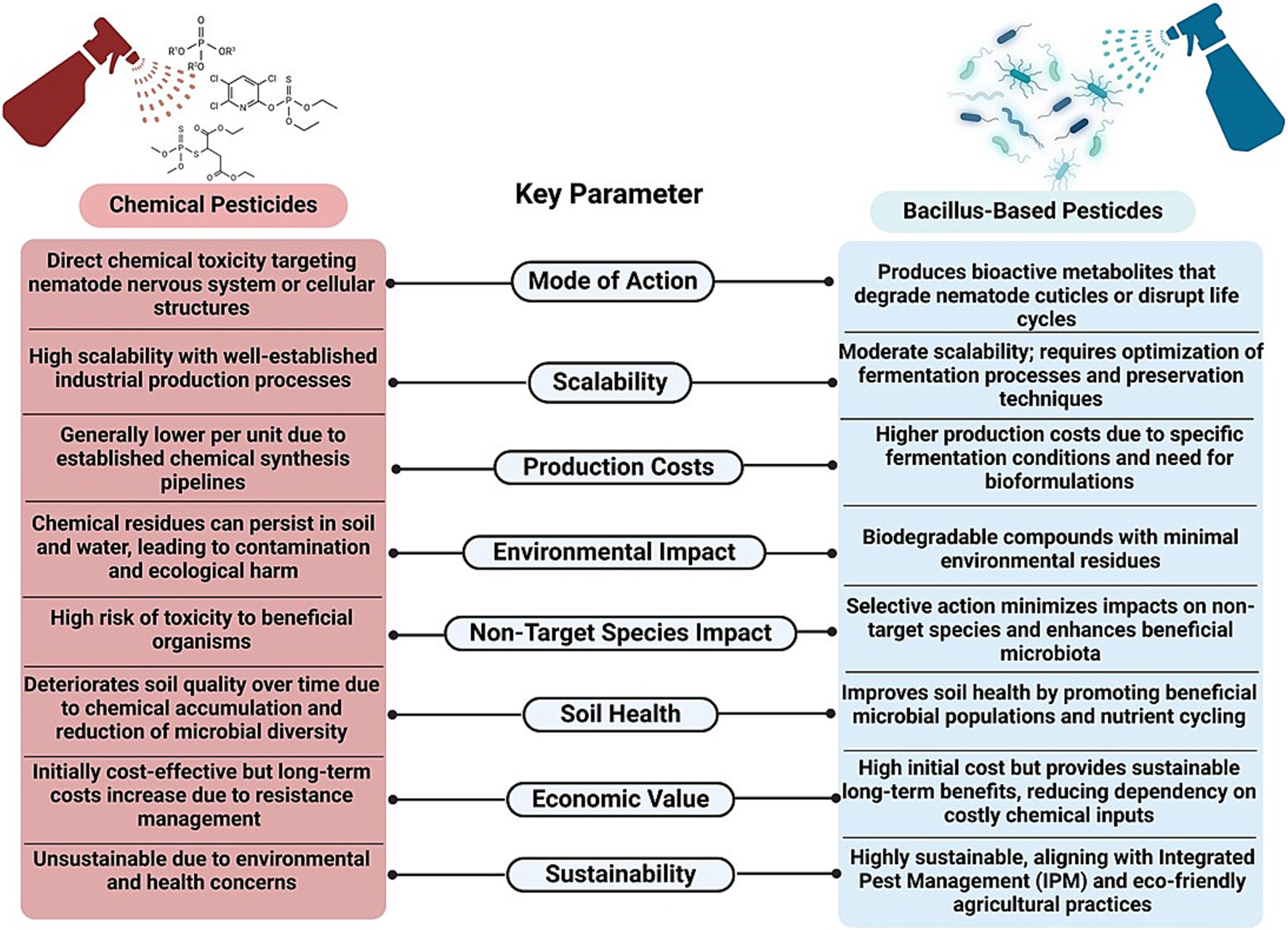
Figure 1. Schematic representation of comparison of conventional and Bacillus-based nematode management strategies.
Bacterial antagonists are among the most promising biocontrol agents. They suppress PPNs through multiple mechanisms, including the production of nematicidal lipopeptides, such as surfactin and fengycin, which disrupt nematode cuticles and membranes. Bacillus spp. produce various enzymes, such as chitinases and proteases, which degrade nematode eggshells and cuticles, effectively reducing juvenile development and reproduction (Yang et al., 2013). In particular, B. subtilis triggers systemic resistance in plants by activating JA and SA signaling pathways, thereby enhancing the natural defenses of plants against nematode attacks (Chowdhury et al., 2015). The antagonistic effects of Paenibacillus elgii HOA73 and P. illinoisensis KJA-424 were evaluated through in vitro nematicidal assays and greenhouse experiments. Key methodologies included assessing nematode motility and mortality using bacterial supernatants and evaluating the activity of enzymes, such as chitinases and proteases. Greenhouse trials confirmed reductions in nematode gall formation and reproduction in infested tomato plants (Jung et al., 2002; Nguyen et al., 2013). Bacillus spp., in particular, are a diverse group of gram-positive, rod-shaped, endospore-forming bacteria commonly found in soil and plant environments. They can produce various bioactive compounds, including enzymes, antibiotics, and toxins, which enhance their effectiveness in controlling plant pathogens and promoting plant health (El Aimani et al., 2022). Some Bacillus spp. are notably effective against nematodes and other plant pathogens, making them valuable for sustainable agricultural practices.
Bacillus spp. produce various nematicidal compounds, including lipopeptides, proteases, and chitinases, which target nematodes at various life stages (Tran et al., 2019). These soil-dwelling bacteria produce spores that can endure extreme environmental conditions, making them ideal candidates for sustainable nematode management (Singh et al., 2019). They can directly antagonize nematodes by producing toxins, enzymes, and other bioactive compounds that impact nematode mobility, development, and reproduction (Migunova and Sasanelli, 2021). Bacillus spp., such as B. thuringiensis (Bt) and B. firmus, have been extensively studied for their nematicidal activities (Zuckerman et al., 1993). For instance, Bt produces crystal (Cry) proteins that are toxic to a broad range of nematodes and can cause cell lysis and death upon ingestion (Forghani and Hajihassani, 2020). Similarly, B. firmus produces enzymes and secondary metabolites that degrade the nematode cuticle and interfere with physiological processes. The use of Bacillus spp. not only reduces the reliance on chemical nematicides, thereby mitigating environmental impacts, but also promotes soil health by maintaining beneficial microbial populations (Tran et al., 2019).
Bacillus spp. can effectively manage PPN infestations through various biocontrol strategies (Tian et al., 2007; Gamalero and Glick, 2020; Diyapoglu et al., 2022). The nematicidal activity of B. subtilis was assessed through in vitro bioassays focusing on lipopeptides, such as surfactin and fengycin, which can cause significant disruption of nematode cell membranes, resulting in mortality (El Aimani et al., 2022). Similarly, studies on B. amyloliquefaciens have revealed its efficacy in IPM programs. By producing antifungal and antibacterial metabolites, the bacterium could exhibit dual efficacy against PPNs and secondary infections in plants under controlled and field conditions (Cetintas et al., 2018). These strategies highlight the versatility of Bacillus spp. as biocontrol agents through multiple mechanisms, including direct toxicity, the inhibition of nematode development, and the enhancement of plant resistance. These bacteria also induce systemic resistance in plants, enhancing their defensive capabilities against nematode attacks (Yang et al., 2022). They produce chitinase and other enzymes that can degrade nematode eggshells, thereby reducing hatching rates and subsequent infection levels. Field trials have also revealed that formulations containing Bacillus spp. can significantly reduce root galling and improve plant health, demonstrating their practical applicability in agricultural settings (Forghani and Hajihassani, 2020).
In summary, Bacillus spp. employ various proteins and secondary metabolites to exhibit nematicidal effects. The key proteins include Cry proteins from Bt, which act by forming pores in the gut cells of nematodes, causing cell lysis and death (Forghani and Hajihassani, 2020; Diyapoglu et al., 2022). B. firmus produces chitinase, an enzyme that breaks down chitin in nematode eggshells, thereby preventing hatching and reducing nematode populations (Tran et al., 2019). Additionally, B. subtilis produces lipopeptides, such as surfactin and fengycin, which disrupt nematode cell membranes, causing the loss of cell integrity and cell death (El Aimani et al., 2022). B. amyloliquefaciens produces proteases, which degrade nematode cuticles and interfere with their physiological processes, resulting in reduced viability and reproduction (Cetintas et al., 2018). The primary modes of action through which Bacillus spp. target nematodes include direct toxicity by producing toxins and enzymes, the inhibition of egg hatching and juvenile development, the induction of systemic resistance in plants, and the disruption of physiological processes by degrading structural components (e.g., cuticles) and interfering with metabolic pathways essential for nematode survival (Shafi et al., 2017). The detailed mechanisms of action underlying the efficacy of Bacillus spp. against PPNs are presented in Figure 2.
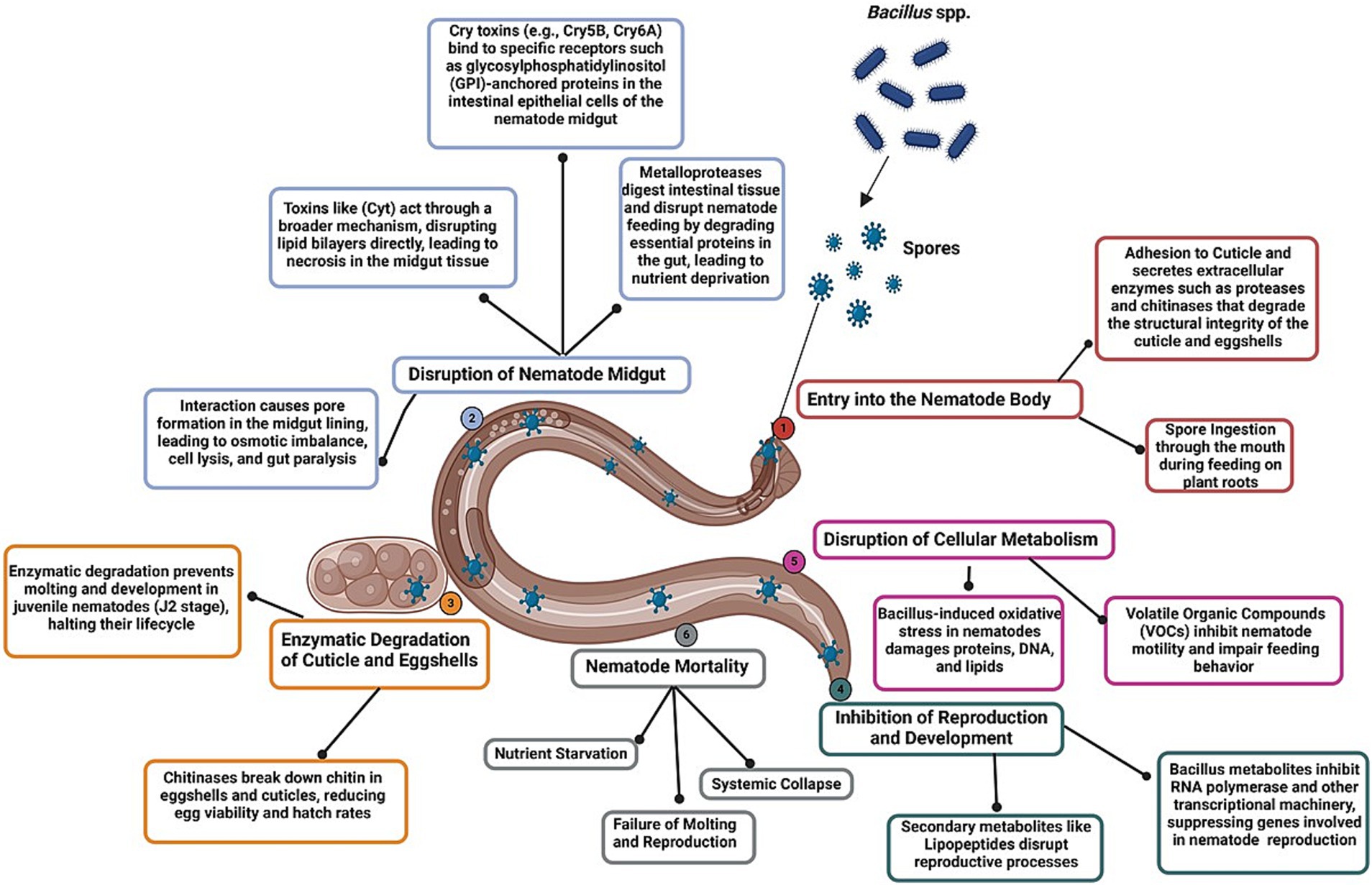
Figure 2. Mode of action of Bacillus spp. against plant-parasitic nematodes. The figure illustrates the sequential mechanisms of Bacillus species, including the entry of spores into the nematode body via ingestion or adhesion to the cuticle, enzymatic degradation of structural components (such as cuticles and eggshells), and disruption of intestinal cells through Cry and Cyt toxin-induced pore formation. The figure also highlights the inhibition of nematode reproduction, the disruption of cellular metabolism, and systemic physiological collapse, ultimately resulting in nematode mortality.
The historical development of Bacillus spp. as biocontrol agents against plant pathogens, particularly nematodes, highlights significant advancements in scientific understanding and practical applications. Bacillus spp. were first identified by Ferdinand Cohn in the late 19th century. Early research highlighted their roles in improving soil health and promoting plant growth through the production of nematicidal compounds, such as enzymes and secondary metabolites (Brzezinska et al., 2020).
The mid-20th century marked a pivotal advancement with the discovery of Bt and its insecticidal Cry proteins, forming the foundation for experimental biocontrol applications (Sanahuja et al., 2011). A timeline highlighting significant milestones in the development of Bacillus species as biocontrol agents, from their initial discovery to advancements in genetic engineering and sustainable agricultural practices, emphasizing their expanding role in integrated pest management, is presented (Figure 3). Initial studies on nematode management focused on nematicidal compounds, such as chitinases and proteases, (Bacon et al., 2006). By the 1970s and 1980s, researchers identified specific toxins and enzymes produced by Bacillus spp., revealing their targeted actions against nematodes (Van Frankenhuyzen, 2009, 2013). Field trials in the 1990s evaluated the efficacy of Bacillus-based biocontrol agents under various environmental and agronomic conditions. These studies highlighted the importance of application methods, soil properties, and microbial interactions in achieving consistent nematode suppression (Etesami et al., 2023; Serrão et al., 2024). With advancements in genomic technologies, researchers unraveled genes and regulatory pathways responsible for the biocontrol properties of Bacillus spp. in the early 21st century. This enabled the development of genetically enhanced strains with improved efficacy and environmental resilience (Carmona-Hernandez et al., 2019). Given the commercial success of Bacillus-based products, these biocontrol agents were further integrated into IPM systems, offering sustainable alternatives to chemical nematicides (Castillo et al., 2013). Current research underscores the role of Bacillus spp. in promoting soil biodiversity and enhancing plant microbiomes, which contribute to long-term nematode suppression (Calvo et al., 2010). Biotechnological advances, including CRISPR and synthetic biology, have further expanded the potential of Bacillus spp., enhancing their stability, specificity, and ability to produce nematicidal compounds (Baptista et al., 2022). Key Bacillus spp., including Bt, B. subtilis, and B. cereus, are crucial because they produce diverse nematicidal compounds, such as Cry proteins, chitinases, and lipopeptides, which exhibit broad-spectrum activity against nematodes (Jouzani et al., 2017; Saxena et al., 2020; Ahmad et al., 2021). Comparative studies have demonstrated the unique strengths of Bacillus spp., providing insights into their compatibility with specific crops and soil environments. For example, B. subtilis induces systemic resistance in plants, Bt acts through direct toxin-mediated gut disruption, and B. cereus enhances soil health through microbial synergism (Diyapoglu et al., 2022; Tran et al., 2019). This historical trajectory highlights the evolution of Bacillus spp. from their initial discovery to becoming cornerstones of sustainable agriculture. The roles of Bacillus spp. in nematode biocontrol highlight their potential as integral components of IPM strategies, addressing key challenges in plant health management (Sanahuja et al., 2011; Raymond and Federici, 2017).
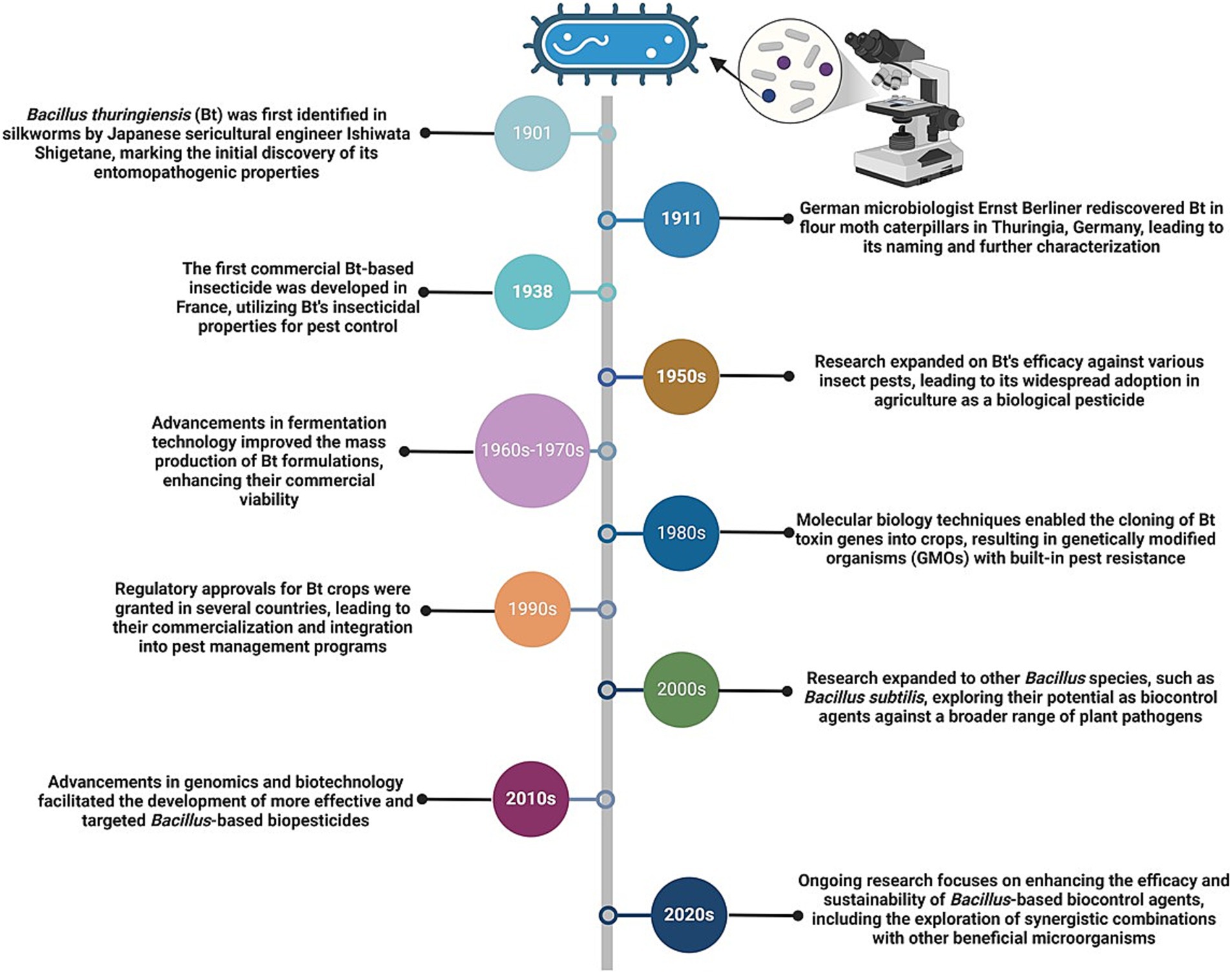
Figure 3. Timeline of Bacillus species development as biocontrol agents. This timeline highlights significant milestones in the development of Bacillus species as biocontrol agents, from their initial discovery to advancements in genetic engineering and sustainable agricultural practices.
Bt is widely recognized for its potent nematicidal activity, primarily mediated by the production of insecticidal Cry and cytolytic (Cyt) proteins. These proteins, synthesized as protoxins during sporulation, exhibit significant efficacy against various PPNs, including Meloidogyne and Heterodera spp. (Verduzco-Rosas et al., 2021; Kahn et al., 2021). Experimental studies on the efficacy of Bt toxins generally utilize nematode bioassays, in which second-stage juveniles (J2) of Meloidogyne spp. are exposed to varying concentrations of Cry and Cyt proteins under controlled environmental conditions. Mortality, hatching inhibition, and mobility reduction are the commonly measured endpoints in such studies. Upon ingestion, the alkaline gut environment of nematodes solubilizes these protoxins, which are then activated by specific gut proteases. The activated Cry proteins bind to gut epithelial receptors, such as cadherin-like proteins, aminopeptidases, and alkaline phosphatases, inducing structural changes that facilitate membrane insertion and pore formation (Griffitts et al., 2005; Schnepf et al., 1998). This pore formation disrupts osmotic balance, causing cell lysis, gut paralysis, and eventual nematode death due to starvation or secondary infections (Bravo et al., 2007). Cyt proteins complement Cry proteins by targeting the lipid components of nematode cell membranes, thereby inducing cell lysis through distinct pore-forming mechanisms (Gill et al., 1992; Wei et al., 2003). In laboratory assays, Cry5B has been found to interact with glycosylphosphatidylinositol-anchored proteins in the gut cells of M. incognita, causing cell swelling and epithelial rupture. Cry6A specifically targets aspartyl protease and alkaline phosphatase receptors, initiating apoptosis and disrupting gut integrity (Barros dos Santos et al., 2022; Shi et al., 2020). These experiments typically involve histological analysis of nematode midgut tissues and the use of advanced imaging techniques to confirm receptor interactions and cellular damage. The specificity and effectiveness of Bt toxins vary among nematode species because of differences in gut receptor structures and proteolytic activation. Nematodes can use innate defenses, such as enzyme detoxification and pH modulation, to mitigate Bt toxicity, highlighting the complexity of host–pathogen interactions (Zhang et al., 2012). These interactions underscore the versatility and adaptability of Bt in managing diverse nematode infestations. Advances in molecular biology have facilitated the engineering of transgenic crops expressing Cry proteins, conferring continuous protection against nematodes. For example, in field trials, transgenic rice expressing Cry6A exhibited significant resistance to M. graminicola, with the nematode populations decreasing by 80% and yield improving by 30% (Lilley et al., 2011; Berlitz et al., 2014). Such experiments typically involve randomized field plots, and the efficacy of treatments is compared with those of chemical nematicides and untreated controls. Nematode population dynamics and yield data are analyzed to assess efficacy. The integration of Bt formulations with organic amendments, such as chitin or neem extracts, can further enhance their efficacy through synergistic effects (Chen et al., 2000; Radwan, 2007). Field applications of Bt-based biopesticides can be evaluated using standardized protocols. For instance, Cry55A-containing formulations have shown notable efficacy in reducing M. incognita populations under greenhouse and field conditions, with Cry55A-treated soil exhibiting 70% lower nematode gall formation than untreated controls. These findings highlight the potential of Cry55A as a soil inoculant (Manivannan et al., 2019; Ramalakshmi et al., 2020). Innovative delivery systems, such as seed treatments and soil inoculants, ensure early and sustained activity throughout the growing season (Etesami et al., 2023). These advancements align with sustainable agricultural practices, offering an eco-friendly alternative to chemical nematicides (Hui et al., 2012; Chen et al., 2024). Given its robust mechanisms of action, adaptability to various nematode species, and compatibility with sustainable practices, Bt plays a crucial role in modern nematode management frameworks. Comparative insights across species and delivery systems underscore its effectiveness as a cornerstone of nematode biocontrol strategies.
B. subtilis, a versatile PGPR, exhibits remarkable efficacy against PPNs through diverse mechanisms. This bacterium produces lipopeptides, such as surfactins, fengycins, and iturins, which disrupt nematode cell membranes, causing cell lysis and death (Heerklotz and Seelig, 2007; Henry et al., 2011). In vitro studies can confirm these effects by exposing Meloidogyne juveniles to purified lipopeptides and assessing mortality through microscopic observations and viability staining. Additionally, B. subtilis secretes hydrolytic enzymes, such as chitinases and proteases, which degrade nematode eggshells and cuticles, thereby inhibiting juvenile emergence and reproduction (Hu et al., 2007; Huang et al., 2008). Enzymatic activity is often assessed using substrate degradation assays, in which enzymatic activity is correlated with nematode population decline. B. subtilis also induces systemic resistance in plants by activating JA and SA pathways, thereby enhancing the production of phenolics and defense proteins that limit nematode penetration (Adiwena et al., 2023). In greenhouse studies, RT-qPCR and phenolic quantification assays can be used to validate these responses. Volatile organic compounds (VOCs), such as 2,3-butanediol and acetoin, further suppress nematode motility and reproduction while promoting rhizosphere health (Henry et al., 2011). These VOCs can be identified through GC–MS analysis, and their inhibitory effects can be confirmed by performing bioassays. The applications of B. subtilis include seed treatments, soil drenching, and foliar sprays. Seed treatments ensure early root colonization, while soil drenching targets root zones for sustained nematode suppression. Foliar sprays activate induced systemic resistance (ISR) pathways, indirectly reducing nematode infestations (Barnawal et al., 2017; Basiouny and Abo-Zaid, 2018). In field trials, these methods can be assessed through randomized designs to monitor nematode levels and yield improvements. When integrated into IPM frameworks, B. subtilis performs synergistically with organic amendments and other biocontrol agents, enhancing efficacy and promoting soil health (Cavalcanti et al., 2024). These combined strategies can maximize nematicidal potential and support sustainable agriculture. The multifaceted actions of B. subtilis highlight its pivotal role in reducing nematode infestations and promoting eco-friendly pest management practices.
B. cereus exhibits robust nematicidal activity against PPNs through diverse mechanisms. It secretes metalloproteinases, such as neutral protease (Npr) and bacillolysin (BlyA), which degrade nematode cuticle proteins, thereby causing structural collapse and death (Yin et al., 2021a,b; Kulkova et al., 2023). Enzyme assays have confirmed the degradation of nematode cuticles, correlating enzymatic activity with nematode mortality. Lipopeptides, such as surfactin and fengycin, disrupt nematode cell membranes via pore formation, causing cell leakage and lysis (Tong-Jian et al., 2013; Hu et al., 2020). Fluorescent dyes have been used to validate membrane disruption.
B. cereus also produces siderophores, such as bacillibactin, which can chelate iron, thereby depriving nematodes of essential nutrients (Köhl et al., 2019). Furthermore, they produce bacteriocins, such as cerein, which can act as antibiotics and target nematode cellular processes. Bioassays have confirmed nutrient depletion and reduced viability in treated nematodes. Nano-bioformulations have further improved the stability and bioavailability of these bioactive compounds, ensuring prolonged nematode suppression in diverse soils (Kumar et al., 2021). Field trials have highlighted their extended activity and reduced application frequencies. Optimized delivery methods include soil drenching, seed treatments, and foliar sprays. Soil drenching ensures uniform root-zone colonization, while seed treatments enable early protection during crucial growth stages (Ahmed et al., 2019). Randomized trials have revealed significant reductions in M. incognita populations and improvements in yield. When combined with mycorrhizal fungi, B. cereus exhibits synergistic effects, enhancing soil microbial diversity and plant resilience (Hu et al., 2017). Genetic engineering approaches, including CRISPR, are being used to enhance the production of bioactive compounds and target-specific nematicidal properties (Mohamed et al., 2021). Through its diverse mechanisms of action, including enzyme secretion, nutrient competition, and direct nematode disruption, B. cereus offers a sustainable biocontrol option for PPN management. Its integration into IPM strategies and compatibility with sustainable agriculture highlight its crucial role in reducing chemical nematicide usage while improving crop health and productivity.
B. megaterium is a robust biocontrol agent that has been proven to be effective against PPNs by producing various bioactive compounds and enzymes. It secretes proteases, such as neutral and serine proteases, which degrade structural proteins in nematode cuticles, causing severe damage and death (Padgham and Sikora, 2007). Lipopeptides, such as surfactin and iturin, disrupt nematode cell membranes through pore formation, causing cell leakage and lysis (Pueyo et al., 2009). Additionally, B. megaterium synthesizes siderophores, such as bacillibactin, which can chelate iron in the rhizosphere, thereby depriving nematodes of vital nutrients and suppressing their populations while promoting a balanced microbial community. These processes have been validated through enzyme assays, correlating siderophore activity with nematode suppression (Huang et al., 2010). Nano-bioformulations have further enhanced the stability and bioavailability of B. megaterium metabolites, ensuring prolonged nematode suppression and reduced application frequency (Kumar et al., 2021). Various application techniques, including soil drenching and seed treatments, have been optimized for efficient delivery. Soil drenching ensures deep root penetration, while seed treatments facilitate early root colonization, offering sustained protection during crucial growth stages (Padgham and Sikora, 2007; Raza et al., 2024). These strategies have been effective against root-knot nematodes, such as M. incognita, significantly improving plant health and yields in field trials (Mostafa et al., 2018). Genetic engineering approaches, such as the overexpression of genes responsible for lipopeptide synthesis and VOC production, have been employed to enhance nematicidal efficacy. These efforts have shown promise in increasing activity against nematodes while maintaining environmental safety (Grage et al., 2017; Hartz et al., 2021). Through its multifaceted nematicidal mechanisms, B. megaterium serves as an eco-friendly alternative to chemical nematicides. Its adaptability and integration into IPM strategies make it a cornerstone of sustainable pest management. It can support agricultural productivity while minimizing environmental impacts.
B. pumilus employs diverse nematicidal mechanisms, making it a powerful biocontrol agent against PPNs. It acts by secreting proteolytic enzymes, such as subtilisin, which can degrade nematode cuticle proteins, causing osmotic imbalance and eventual death (Ramezani Moghaddam et al., 2014). Lipopeptides, such as pumilacidin and bacilysin, disrupt nematode cell membranes and induce pore formation, ion leakage, and cytoplasmic efflux, thereby causing rapid cell lysis (Dobrzyński et al., 2023). B. pumilus also synthesizes siderophores, such as bacillibactin, which can chelate iron and other essential nutrients, depriving nematodes of crucial resources and fostering beneficial microbial competition in the rhizosphere (Lee et al., 2016). Additionally, B. pumilus produces antimicrobial compounds, including bacteriocins, which disrupt nematode metabolic pathways. A guanidine compound from B. pumilus strain LYMC-3 exhibited potent activity against Bursaphelenchus xylophilus; the LC50 values were 113.5 and 62.5 mg/L after 24 and 48 h, respectively, highlighting its targeted efficacy (Li et al., 2018). Nano-bioformulations have improved the stability and bioavailability of B. pumilus metabolites, ensuring consistent nematode suppression in different agricultural conditions (Mahmoud et al., 2016). B. pumilus differentiates itself by integrating siderophore-mediated nutrient deprivation with enzymatic and antimicrobial strategies, unlike Bt (which relies on Cry proteins) or B. cereus (which relies on lipopeptides). Its compatibility with agronomic practices, such as seed treatments and soil drenching, facilitates early root colonization and uniform metabolite distribution, enhancing field performance. Furthermore, its synergy with mycorrhizal fungi and other beneficial microbes enhances nutrient cycling and plant resilience, creating a holistic defense against nematodes (Carriel and Soto, 2022). Through its multifaceted actions and adaptability, B. pumilus exhibits significant potential for integration into IPM strategies. Further research on genetic optimization, delivery systems, and formulations is warranted to sustainably maximize its agricultural impact.
B. licheniformis employs diverse mechanisms, including enzymatic degradation, antimicrobial activity, and soil microbiome modulation, to manage PPNs. Its nematicidal activity is attributed to the secretion of hydrolytic enzymes, such as proteases and chitinases, which target the cuticles and eggshells of nematodes, impairing their mobility, reproduction, and viability (Park et al., 2015). For example, strain MH48 effectively degrades nematode structures, particularly in B. xylophilus (Jeong et al., 2015). Additionally, B. licheniformis produces lipopeptides, such as bacillomycin and fengycin, which disrupt nematode and fungal cell membranes, causing ion leakage and cytoplasmic loss. Thus, it exhibits dual functionality as a biocontrol agent (Stoica et al., 2019). B. licheniformis strains, such as strain XF32, have exhibited enhanced production of fengycin through genetic modifications, highlighting their potential for agricultural and industrial applications (Zhaojian et al., 2021). Furthermore, strain JF-22 was found to reduce M. incognita populations and enrich beneficial microbial communities in tomato rhizospheres, promoting soil health and plant resilience (Du et al., 2022). Unlike Bt, which relies on Cry proteins, or B. pumilus, which relies on nutrient deprivation, B. licheniformis integrates enzymatic lysis with microbiome enhancement to suppress nematodes. It also supports plant defenses indirectly. Studies have indicated its ability to bolster the resistance of C. elegans to bacterial infections through hormonal signaling pathways, such as those involving serotonin, suggesting its potential for inducing systemic resistance in plants (Yun et al., 2014). Advances in genetic engineering, such as promoter and ribosome binding site engineering, have increased the capacity of B. licheniformis to produce antimicrobial compounds and enzymes, enhancing its biocontrol potential (Xiao et al., 2024). Field trials have highlighted its dual role in managing nematodes and promoting plant growth. For instance, strain MH48 was found to reduce fungal infections and improve nutrient availability in pine seedlings (Won et al., 2018). The synergy of B. licheniformis with other biocontrol agents further enhances its effectiveness in IPM strategies.
B. firmus exhibits remarkable versatility in suppressing nematode populations and enhancing plant growth. As an alkaliphilic, endospore-forming bacterium, it thrives in various soil environments, making it suitable for diverse agricultural systems (Settu et al., 2024). It is distinguished from other Bacillus spp. by its ability to colonize plant roots and induce systemic resistance, exhibiting both direct nematicidal effects and indirect plant-protective effects (Huang et al., 2021). A primary mode of action of B. firmus involves the production of lytic enzymes, such as chitinases and proteases. These enzymes target the structural integrity of nematode eggshells and cuticles, resulting in the degradation and reduced viability of eggs and juveniles. Genomic studies on B. firmus strains, such as strain TNAU1, have identified genes like chiA and chiB, which are involved in the synthesis of chitinase, an enzyme crucial for breaking down the chitinous components of nematode structures (Settu et al., 2024). This enzymatic degradation not only disrupts nematode development but also facilitates nutrient recycling in the rhizosphere, indirectly benefiting plant health. Moreover, B. firmus produces antimicrobial peptides, including surfactin and fengycin, which disrupt nematode cell membranes. These lipopeptides interact with membrane lipids, forming pores that cause ion imbalance, cytoplasmic leakage, and eventual nematode death (Daulagala, 2021). For example, strain YBf-10 can significantly reduce M. incognita populations by producing these bioactive compounds, effectively suppressing nematode-induced damage, such as gall formation and egg mass production (Xiong et al., 2015). Among Bacillus spp., B. firmus is distinguished by its efficacy in reducing nematode reproductive potential. Strain I-1582, widely studied for its nematicidal efficacy, can suppress egg hatching and juvenile viability by producing proteases and secondary metabolites. These metabolites interfere with nematode signaling pathways essential for reproduction and development, offering a comprehensive mechanism for population control (Huang et al., 2021). Furthermore, B. firmus promotes plant growth by enhancing nutrient uptake and root colonization, thereby effectively mitigating the damage caused by nematode infestations. Comparative analyses have revealed that B. firmus differentiates itself from other Bacillus spp. through its robust adaptability to diverse soil pH levels and its ability to induce systemic resistance. Unlike Bt, which relies on Cry proteins for specific gut receptor targeting, or B. subtilis, which is known for its VOC-mediated effects, B. firmus integrates multiple mechanisms, including enzymatic degradation, lipopeptide production, and systemic resistance induction, to combat nematodes and support plant health. The dual role of B. firmus in nematode suppression and plant growth promotion highlights its suitability for sustainable agricultural practices. Recent advancements in genomic studies have further elucidated the biocontrol potential of B. firmus. For instance, strain TNAU1 harbors genes encoding nematode-virulent proteases and other antimicrobial compounds, which can enhance its specificity and efficacy against PPNs. Additionally, B. firmus YBf-10 can modulate microbial communities in the rhizosphere, enriching beneficial microbes and suppressing harmful pathogens. Thus, it can play a role in IPM strategies (Marin-Bruzos et al., 2021). Field applications of B. firmus include soil drenching and seed treatments, which ensure effective delivery of bioactive compounds to nematode hotspots. Pot experiments using soil-drenched YBf-10 revealed substantial reductions in nematode populations and an increase in overall plant growth, showcasing its practical applicability in real-world agricultural systems (Xiong et al., 2015). B. firmus employs a multifaceted approach involving enzymatic lysis, antimicrobial activity, and systemic resistance induction for managing nematodes. Its ability to thrive in diverse soil environments, its biocontrol efficacy, and its plant growth-promoting properties underscore its potential as a key agent in sustainable nematode management and IPM strategies.
B. nematocida is a spore-forming bacterium with distinct nematicidal properties. Thus, it is a pivotal agent for managing PPNs. This bacterium is predominantly found in soil and plant rhizospheres. It utilizes a multifaceted approach involving enzymatic, biochemical, and molecular strategies, which collectively contribute to its efficacy (Huang et al., 2005). Its nematicidal action is attributed to its ability to secrete lytic enzymes, such as chitinases and proteases, which are encoded by genes like chiA, chiB, aprE, and nprB. These enzymes target and damage the structural integrity of nematode eggshells and cuticles, directly impairing nematode survival and reproduction. The breakdown of these protective structures not only suppresses nematode populations but also releases essential nutrients, thereby enhancing soil fertility (Sun et al., 2024). Moreover, B. nematocida produces antimicrobial lipopeptides, such as fengycin, surfactin, and bacillomycin. These bioactive metabolites disrupt nematode cell membranes by interfering with lipid bilayers, resulting in pore formation, ion leakage, and eventual mortality (Niu et al., 2006; Niu et al., 2011; Niu et al., 2016; Bo et al., 2022). This biochemical disruption demonstrates the potent antagonistic effects of the bacterium on nematode physiology. A unique aspect of the mode of action of B. nematocida is the synthesis of 2-heptanone, a volatile compound that acts as a nematode attractant. These chemical lures nematodes toward the bacterium, enhancing its ability to target and infect nematodes with high precision. This mechanism exemplifies an evolutionary adaptation for host–pathogen interactions, as highlighted by Zhu et al. (2019). Such attractant-based pathogenicity differentiates B. nematocida from other Bacillus spp., adding a layer of specificity to its biocontrol efficacy. Recent studies have identified adaptive molecular responses in B. nematocida under stress conditions. For example, Sun et al. (2018) reported that protein acetylation modulates the enzymatic activity of the bacterium, enhancing its nematicidal efficacy. This adaptive regulation reflects a dynamic interaction between B. nematocida and its nematode targets, showcasing the ability of the bacterium to respond to environmental stimuli. Comparative analyses have revealed that B. nematocida utilizes a highly specialized approach compared with other Bacillus spp. Unlike B. subtilis, which primarily induces systemic resistance in plants and produces VOCs, or Bt, which relies on Cry proteins for gut-specific toxicity, B. nematocida integrates enzymatic degradation, membrane disruption, and chemical attraction to exhibit nematicidal effects. This multipronged strategy underscores its effectiveness in managing PPNs while minimizing collateral effects on nontarget organisms. The practical application of B. nematocida has shown promising results in field trials, with its soil drench formulations and seed treatments effectively reducing nematode populations and enhancing plant growth. The specificity of B. nematocida for nematodes reduces the ecological risks often associated with broad-spectrum chemical nematicides. Furthermore, its potential for integration into IPM strategies highlights its role in promoting sustainable agriculture. B. nematocida is an advanced biocontrol agent characterized by enzymatic degradation, biochemical toxicity, and adaptive molecular interactions. Its unique mechanisms of action and its specificity for nematodes make it a promising alternative to chemical nematicides, contributing to environmentally sustainable agricultural practices.
B. amyloliquefaciens exhibits robust nematicidal activity. It is distinct from other Bacillus spp. because of the production of diverse enzymes and bioactive secondary metabolites. Its efficacy is mainly attributed to its ability to synthesize lipopeptides, such as fengycin and iturin, which disrupt nematode cell membranes. These lipopeptides interact with lipid bilayers and cause pore formation and subsequent cell lysis, resulting in nematode mortality (Ngalimat et al., 2021). Moreover, B. amyloliquefaciens secretes hydrolytic enzymes, such as chitinases and proteases, which enzymatically degrade nematode cuticles and eggshells, thereby inhibiting juvenile development and reducing nematode reproduction rates (Migunova and Sasanelli, 2021). Genomic studies have highlighted the roles of various genes, such as fenA and ituD, in the biosynthesis of these lipopeptides, underscoring the genetic adaptability of the bacterium for biocontrol applications (Luo et al., 2022). In addition to exhibiting direct nematicidal effects, B. amyloliquefaciens significantly contributes to soil health and plant growth. It stimulates plant development by producing phytohormones and promotes nutrient availability by altering the soil microbiome. For instance, VOCs produced by B. amyloliquefaciens not only suppress pathogens but also enhance root growth and nutrient uptake, reinforcing its dual role as a biocontrol agent and a growth promoter (Chowdhury et al., 2015). Strain FZB42 exhibits these attributes by inducing systemic resistance in plants. ISR is achieved through the activation of JA and ethylene (ET) signaling pathways, resulting in the increased production of defense-related enzymes and antimicrobial compounds that protect plants from nematodes and other pathogens (Chowdhury et al., 2015). The genetic manipulation of B. amyloliquefaciens has further enhanced its efficacy. For example, the fusion of B. amyloliquefaciens SA5 with Lysinibacillus sphaericus created a hybrid strain (Bas8) with elevated chitinase production. This strain exhibited significant nematicidal effects against M. incognita in controlled trials (Abdel-Salam et al., 2018). Similarly, Liu et al. (2013) demonstrated that the deletion of the gene RBAM_007470, responsible for the synthesis of plantazolicin, reduced the nematicidal efficacy of strain FZB42, highlighting the importance of specific metabolites in biocontrol strategies. Field and greenhouse trials have substantiated the biocontrol potential of B. amyloliquefaciens. For example, applications of this bacterium at varying concentrations (50–200%) effectively suppressed M. javanica in common beans by inhibiting juvenile hatching and reducing motility. These effects were observed both in vitro and in vivo, showcasing its adaptability across different environmental conditions (Messa et al., 2019). Furthermore, the spiral nematode Helicotylenchus dihystera was effectively controlled in soybean fields treated with B. amyloliquefaciens-based formulations, with the nematicidal effects being comparable to those of chemical nematicides, such as abamectin. Improvements were also noted in soybean yield and soil health (Camatti et al., 2023). Compared with other Bacillus spp., B. amyloliquefaciens uniquely combines potent direct nematicidal mechanisms with plant growth-promoting traits. While Bt primarily relies on Cry proteins for nematode control and B. subtilis relies on systemic resistance induction, B. amyloliquefaciens integrates membrane disruption, enzymatic degradation, and systemic resistance induction, making it a versatile and holistic agent for nematode management. Its ability to modulate the soil microbiome and enhance nutrient cycling further distinguishes it as an indispensable component of sustainable agricultural practices. Overall, B. amyloliquefaciens employs a synergistic blend of biochemical, enzymatic, and ecological strategies to control PPNs and enhance plant health. Continued research on its genetic pathways, interaction mechanisms, and field applications can further enhance its role in IPM and sustainable agriculture (Table 1).
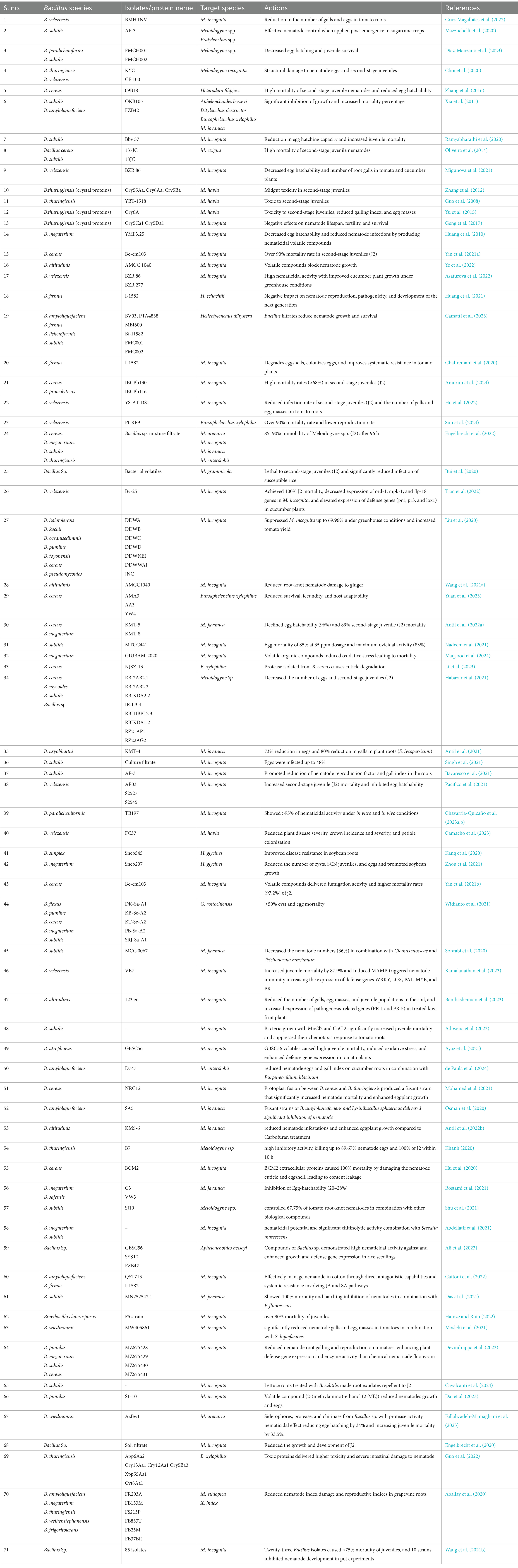
Table 1. Mode of actions of different isolates or proteins from Bacillus species against major pytopathogenic nematodes.
B. velezensis, a species closely related to B. amyloliquefaciens, exhibits substantial nematicidal activity by producing diverse bioactive compounds, making it a key player in sustainable agriculture. Its effects are mainly attributed to the production of lipopeptides (surfactin, fengycin, and iturin), polyketides, and siderophores, which collectively target PPNs and other phytopathogens (Rabbee et al., 2019, 2023). These compounds act by disrupting cell membranes, interfering with metabolic pathways, and creating a hostile environment for pathogens. Moreover, B. velezensis contributes to soil health by promoting beneficial microbial communities and enhancing nutrient cycling, making it a multifunctional agent in IPM systems. The nematicidal efficacy of B. velezensis has been well documented in controlled environments (Wu et al., 2023). For instance, strain YS-AT-DS1 was found to significantly reduce M. incognita infection rates in tomato plants by affecting water and solute transport mediated by TIP genes, without activating the JA or SA pathway (Hu et al., 2022). This finding highlights the unique mode of action of the strain compared with other Bacillus spp., which often rely heavily on ISR through JA/SA pathway activation. Another prominent strain, GB03, has been extensively studied for its ability to enhance plant growth and immunity by producing VOCs that prime plant defenses by inducing systemic resistance (Jang et al., 2023). Strain GB03 is recognized for its practical applications. It has also been validated by the U.S. EPA as an eco-friendly alternative to synthetic pesticides. Its ability to suppress nematodes, fungi, and bacteria while concurrently promoting plant health underscores its versatility. Genome sequencing of B. velezensis strains, such as strains Ag109 and FZB42, has provided a robust genetic basis for secondary metabolite production. The genome of these strains has been found to contain 13 gene clusters responsible for the synthesis of antimicrobial compounds (Borriss et al., 2019). These metabolites, including surfactin, bacillomycin, and fengycin, not only inhibit nematode activity but also suppress fungal pathogens, providing a comprehensive biocontrol solution. In one study, strain Ag109 was found to reduce M. javanica and P. brachyurus populations by 69 and 45%, respectively, while exhibiting notable antifungal properties (Mian et al., 2024). Greenhouse studies further validated the nematicidal potential of B. velezensis. Strains BMH and INV caused over 90% reductions in M. incognita gall formation and egg masses while concurrently enhancing tomato growth (Cruz‐Magalhães et al., 2022). However, combining these strains did not enhance efficacy, suggesting that competitive interactions among strains limit their synergistic potential. A novel approach combining B. velezensis with T. harzianum and gamma radiation-induced mutants caused significant reductions in M. javanica egg hatching (16–45%) and juvenile mortality (30–46%). This synergistic approach, when supplemented with chitosan, led to a 94% reduction in nematode reproduction factors under greenhouse conditions (Rostami et al., 2021, 2024). While B. velezensis has gained widespread recognition for its biocontrol properties, its dual nature requires careful management. Reports of pathogenicity in various crops, such as peaches, onions, and potatoes, necessitate stringent application strategies to avoid unintended consequences (Rabbee et al., 2019). Hence, understanding strain-specific interactions and environmental conditions is crucial to optimize its use. Compared with other Bacillus spp., B. velezensis has unique strengths, including its genetic diversity, robust secondary metabolite production ability, and ability to influence plant physiology through nontraditional ISR pathways. For its integration into sustainable agriculture, further research should be conducted on its ecological interactions and application methodologies to ensure that its potential is maximized and risks are minimized. The major Bacillus spp. and their diverse array of proteins and secondary metabolites against PPNs are schematically displayed in Figure 4.
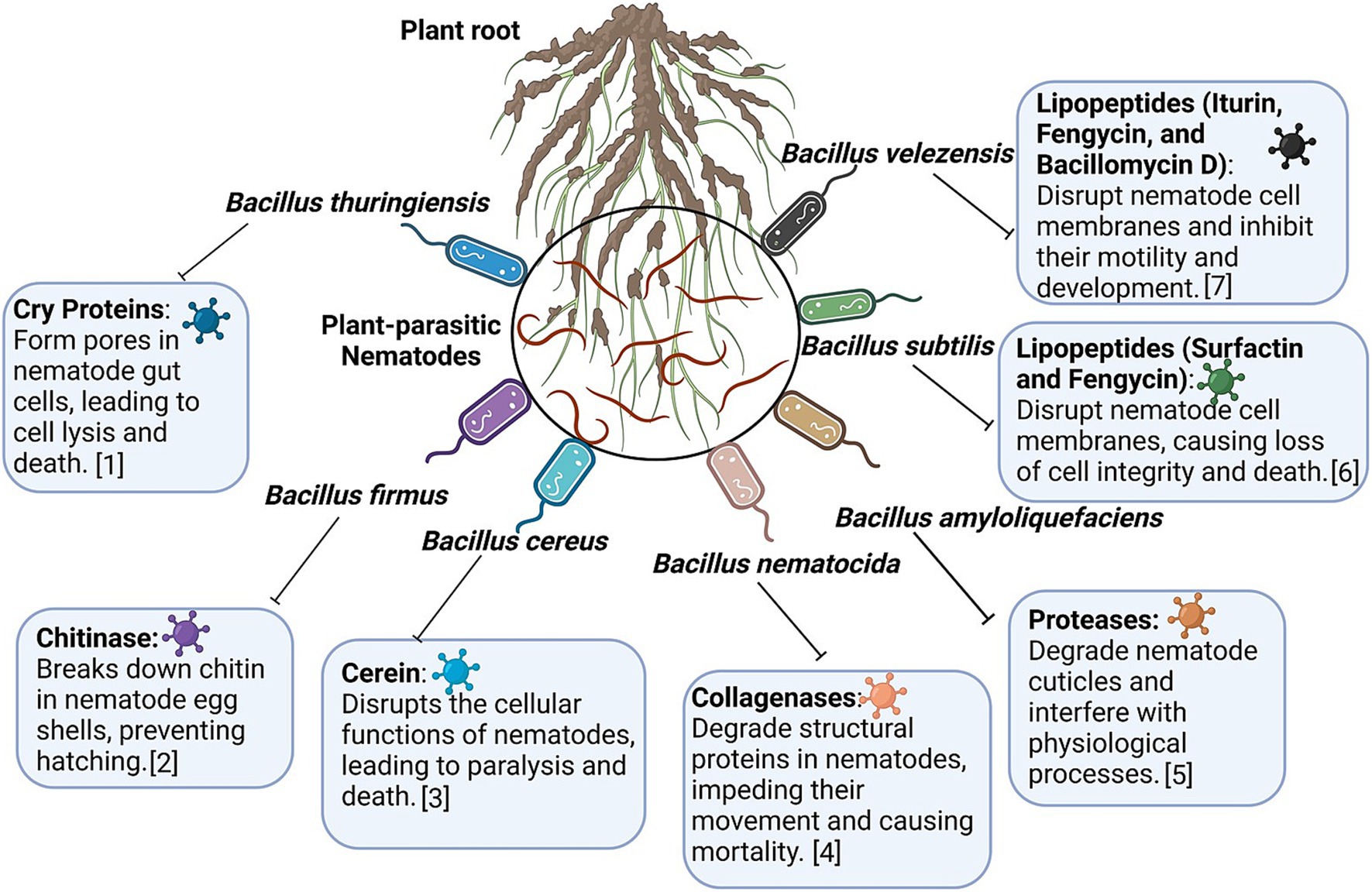
Figure 4. Major Bacillus species and their diverse array of proteins and secondary metabolites against the plant-parasitic nematodes. Information adapted from [1] Kahn et al. (2021), [2] Ghahremani et al. (2020), [3] Kulkova et al. (2023), [4] Niu et al. (2006), [5] Jamal et al. (2017), [6] Manju and Subramanian, 2017, and [7] Hu et al. (2022).
Various methods and strategies can be used for the application of Bacillus spp. to effectively manage phytopathogenic nematodes. A widely used approach is soil amendment, which involves mixing Bacillus inoculants with organic matter, such as compost or manure, to improve soil structure and health. This method indirectly suppresses nematode populations by fostering beneficial microbial communities and enhancing plant resilience (Fabiyi, 2024). Seed treatment is another effective strategy. It involves the coating of seeds with Bacillus spores before planting. This approach confers early protection to seedlings by colonizing the root zone and creating a hostile environment for nematodes. Additionally, foliar sprays with Bacillus formulations can induce systemic resistance in plants, thereby activating defense mechanisms that reduce nematode penetration and reproduction. Biofertilizers incorporating Bacillus strains can be directly applied to the soil or used for root drenching, thereby enhancing nutrient availability and promoting robust plant growth. This can help plants withstand nematode attacks.
In IPM programs, Bacillus strains are often combined with other biocontrol agents, chemical treatments, or cultural practices, providing a multifaceted approach for the management of nematodes. For instance, integrating B. subtilis with organic amendments and reducing the use of chemical nematicides have led to enhanced efficacy against root-knot nematodes, thereby lowering infestations and improving crop yields. Such synergistic approaches can reduce reliance on chemical inputs while maintaining nematode suppression. B. amyloliquefaciens formulations have exhibited notable efficacy in field trials by reducing cyst nematode populations and promoting plant health through the induction of systemic resistance. This approach reduces reliance on chemical nematicides and promotes sustainable agricultural practices. Bacillus strains are being increasingly recognized for their potential for managing PPNs because of their diverse modes of action and adaptability to different agricultural environments. They produce various secondary metabolites, such as lipopeptides, enzymes, and antibiotics, which directly inhibit nematodes through a process known as direct antagonism (Iftikhar et al., 2020). These metabolites disrupt nematode membranes, degrade their structural proteins, or interfere with their signaling pathways, resulting in reduced nematode viability and infectivity (Bhat et al., 2023). The detailed mechanisms of the different application strategies of Bacillus spp. for managing nematodes are outlined below and presented in Figure 5.
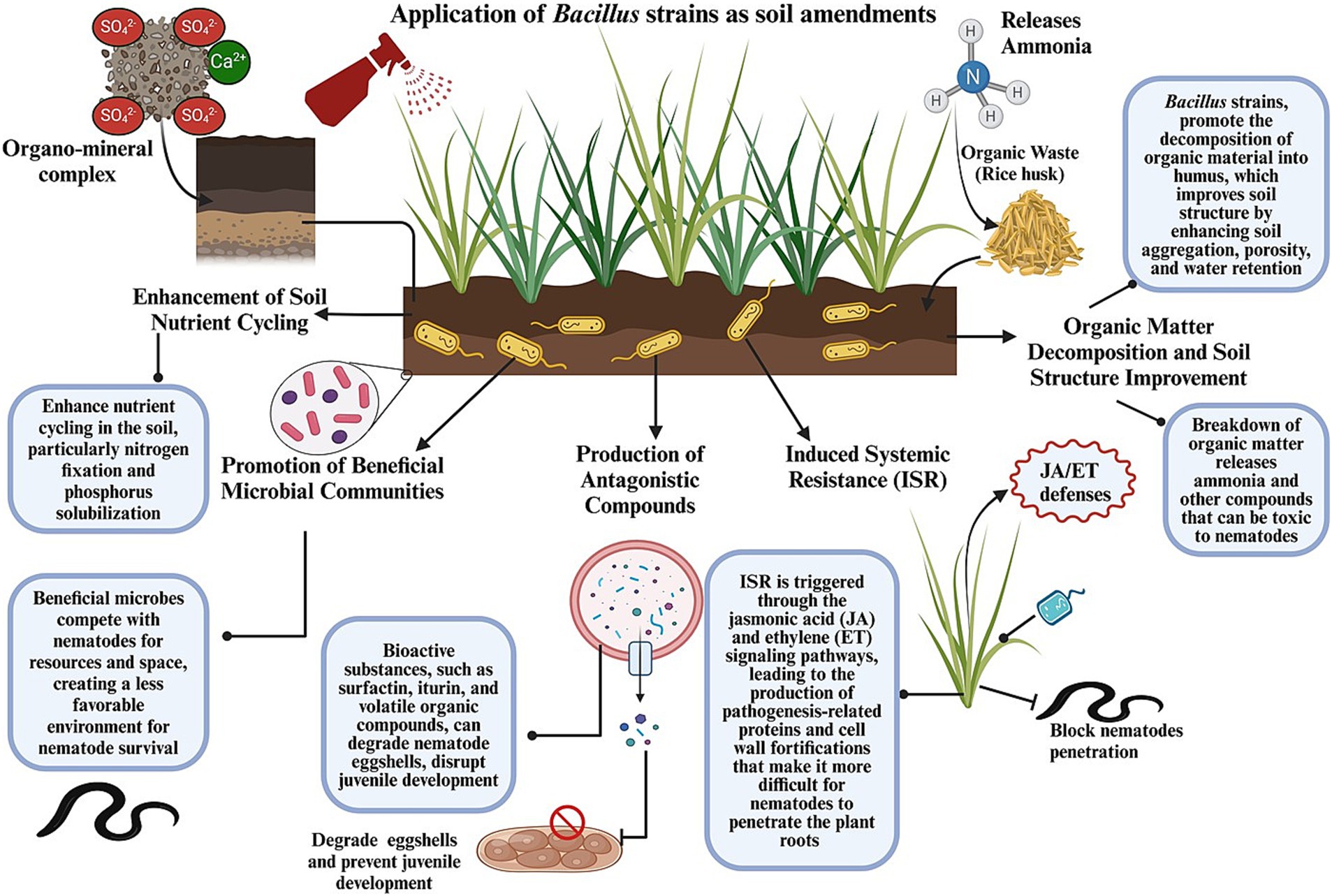
Figure 5. Graphical representation of how the application of Bacillus strains as soil amendments alone or in combination with organic matter enhances soil health and structure and reduces nematode proliferation through several interrelated mechanisms.
Bacillus strains can trigger plant defense mechanisms, enhancing the ability of plants to resist nematode infections (Choudhary and Johri, 2009). ISR is achieved through the upregulation of plant defense-related genes, resulting in the production of pathogenesis-related proteins and other defense-related compounds that inhibit nematode invasion and reproduction (Mahapatra et al., 2022). Bacillus strains produce specific elicitors, such as lipopeptides, VOCs, and secondary metabolites, which prime the plants to enhance defense responses. Upon nematode attacks, these primed plants exhibit accelerated production of pathogenesis-related proteins, oxidative enzymes, and secondary metabolites, thereby reducing nematode penetration, nematode reproduction, and overall damage. Adam et al. (2014) found that certain B. subtilis strains, known for their antifungal properties, can effectively reduce root-knot nematode infestations in tomatoes, primarily through ISR rather than direct antagonism. This demonstrates the potential of multipurpose bacteria for IPM in nematode–fungal disease complexes. Additionally, Xing et al. (2020) identified six ISR-active compounds from B. simplex Sneb545 that conferred resistance against the pathogen H. glycines in soybeans. Among these compounds, the cyclic dipeptide Val-Pro, tryptophan, and uracil were particularly effective in inducing defense-related gene expression in soybeans, offering potential novel agents for managing this destructive nematode.
The application of Bacillus strains as soil amendments alone or in combination with organic matter can significantly improve soil health and structure, creating an environment less conducive to nematode proliferation. Bacillus-based biofertilizers not only enhance plant growth but also foster beneficial microbial communities in the rhizosphere, in turn antagonizing nematodes (Fabiyi, 2024). For instance, Tong-Jian et al. (2013) demonstrated that the use of B. cereus strain X5 in combination with bio-organic fertilizers and biofumigation materials significantly improved plant biomass and reduced nematode infestation under greenhouse and field conditions. This suggests its potential for integrated nematode management in agricultural systems. Moreover, a consortium of three plant growth-promoting rhizobacteria—B. cereus (AR156), B. subtilis (SM21), and Serratia sp. (XY21)—was found to reduce root-knot nematode disease severity in cucumbers by up to 72%. This consortium not only enhanced yield and fruit quality but also improved soil properties by increasing the abundance of disease-suppressive bacterial genera in the rhizosphere. The resulting changes in the microbial community positively correlated with improvements in soil chemical properties, contributing to nematode suppression and overall plant health (Zhang et al., 2024). The several interrelated mechanisms through which Bacillus spp. improve soil health and reduce nematode proliferation when used as soil amendments alone or in combination with organic matter are illustrated in Figure 5.
Treating seeds with Bacillus spores confers early protection to seedlings against nematodes. As the seeds germinate, Bacillus spp. colonize the root system, forming a protective barrier that hinders nematode penetration and colonization (Diyapoglu et al., 2022). Seeds are treated with Bacillus strains using different methods, such as dry coating, wet coating, or pelletization, to ensure even distribution and firm adherence of the bacteria to the seeds. After coating, the seeds are carefully dried and packaged to preserve bacterial viability. Upon planting, Bacillus spores germinate alongside the seeds. They colonize the root zone and confer protection against nematodes while promoting plant growth and soil health (Migunova and Sasanelli, 2021).
Seed treatment with Bacillus strains can improve soil health and reduce nematode proliferation through several key mechanisms, including the colonization of the rhizosphere, induction of systemic resistance, enhancement of soil microbial communities, production of antimicrobial compounds, improvement of soil structure, and reduction of phytopathogens (Figure 6). When seeds are treated with Bacillus strains, these beneficial bacteria colonize the root zone as the plant germinates and grows. This early colonization creates a protective microbial shield around the roots, i.e., the rhizosphere, which acts as the first line of defense against nematode invasion. Bacillus strains occupy key ecological niches in the soil and outcompete nematodes for space and nutrients, thereby reducing the likelihood of nematode attachment and penetration into plant roots (Hu et al., 2017). Moreover, Bacillus strains induce systemic resistance in plants through seed treatment, priming the immune system of plants to respond more robustly to nematode attacks by activating JA and ET pathways (Choudhary and Johri, 2009). The introduction of Bacillus strains via seed treatment enriches the soil microbiome. These beneficial bacteria promote the growth of other advantageous microorganisms, such as mycorrhizal fungi and nitrogen-fixing bacteria, collectively improving soil health and structure. A rich and diverse microbial community enhances nutrient cycling, organic matter decomposition, and soil aggregation, creating a more stable and fertile soil environment that can support healthy plant growth and reduce nematode populations (Chernov and Semenov, 2021). Additionally, antimicrobial compounds produced by Bacillus strains can degrade nematode eggs, inhibit juvenile development, and reduce nematode motility, thereby limiting the ability of nematodes to infect plant roots. The persistence of these antimicrobial substances in the rhizosphere helps maintain a soil environment hostile to nematodes (Diyapoglu et al., 2022). Moreover, when applied to seeds, Bacillus strains colonize the rhizosphere—the area of soil directly affected by root exudates and associated soil microorganisms—and produce extracellular polymeric substances (EPS). These complex organic molecules are crucial for improving soil structure. EPS act as a natural adhesive and bind soil particles together to form stable aggregates, in turn enhancing soil porosity, promoting better air circulation, and improving water infiltration (O’Callaghan, 2016). Improved soil structure not only enhances root growth and plant vigor but also creates a less favorable environment for nematode movement and survival, as nematodes prefer compact, poorly aerated soils (Khan et al., 2022).
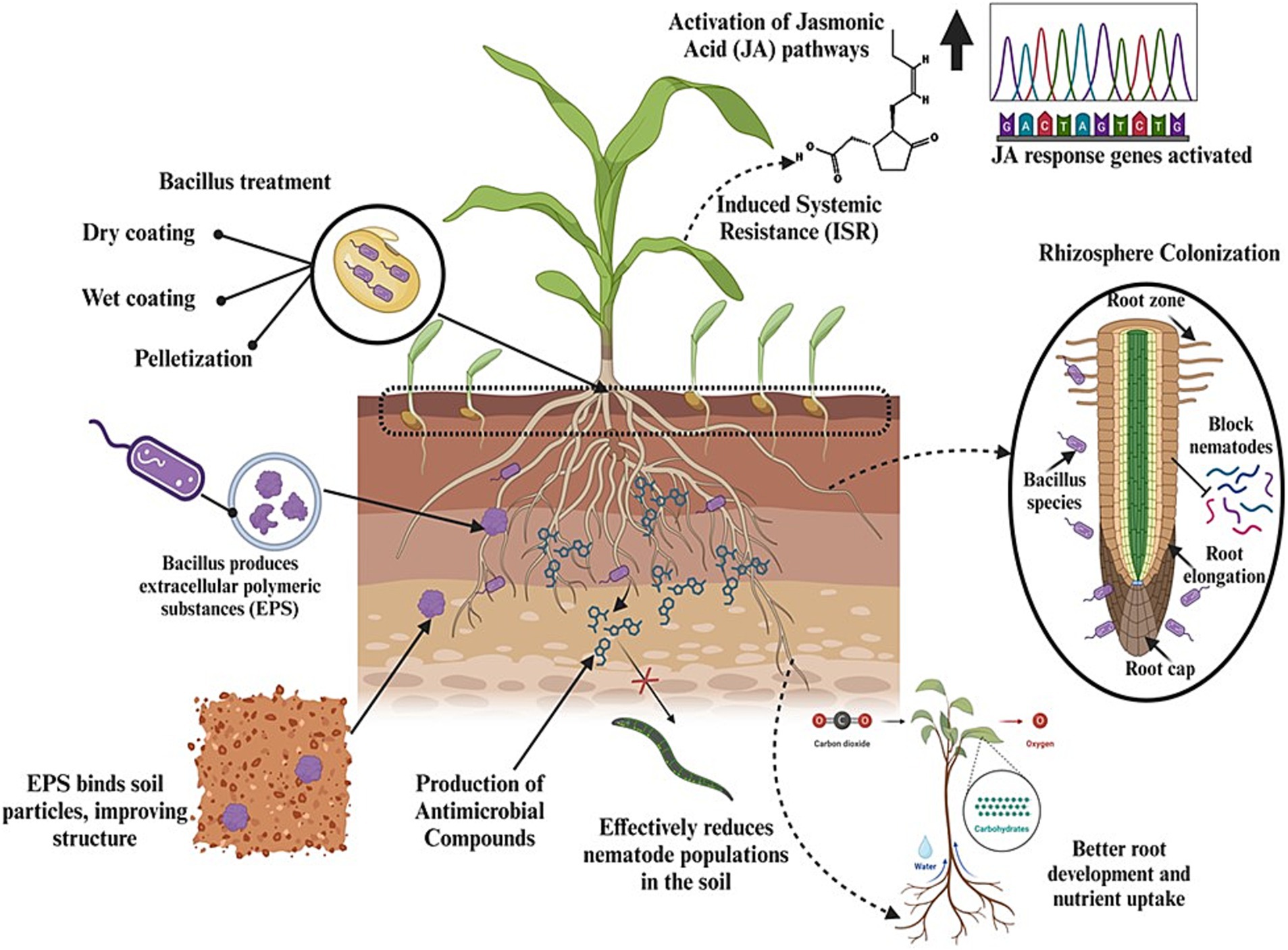
Figure 6. Graphical representation of how seed treatment with Bacillus spp. enhances plant growth, improves soil health, and reduces nematode populations.
Overall, seed treatment with Bacillus strains represents a multifaceted approach for the management of nematodes by enhancing soil health, improving plant resilience, and directly suppressing nematode populations. Thus, it is a more sustainable and effective method of nematode control (Zhang et al., 2009; O’Callaghan, 2016; Hsiao et al., 2023; Hayat et al., 2023).
Foliar application of Bacillus spp. is an effective biocontrol strategy for managing phytopathogenic nematodes (Shafi et al., 2017). This process involves culturing selected Bacillus strains and formulating them into a sprayable solution. Optimal timing is crucial for the success of this method, with applications typically performed during early plant growth stages under favorable environmental conditions to ensure effective colonization. Uniform application using sprayers ensures that the bacteria adhere well to plant surfaces, thereby inducing systemic resistance and protecting against nematode damage (Fu et al., 2020). This method has gained popularity in the U.S., China, India, Brazil, Spain, and South Africa, particularly for high-value crops in areas with substantial nematode pressure (Chien and Huang, 2020; Efthimiadou et al., 2020; Karačić et al., 2024). The effectiveness of foliar application is attributed to a combination of direct antagonism, ISR, and plant health enhancement, which collectively reduce nematode populations and improve crop growth and yield (Esitken et al., 2002; Ryu et al., 2011; El-Sawy et al., 2023).
However, the success of this approach hinges on optimizing the application techniques and timing and understanding the specific interactions between Bacillus spp., the host plant, and the target nematode species (Shafi et al., 2017). Despite the advantages, including reduced environmental impacts and improved plant vigor, various challenges need to be addressed; these include ensuring consistent root protection and managing environmental variables (Abd-Elgawad and Askary, 2020). Thus, continued research and field trials will be crucial for refining this strategy and integrating it into sustainable nematode management programs.
The application of Bacillus spp. as biocontrol agents provides multifaceted benefits beyond nematode suppression. Bacillus spp. significantly affect overall soil health through biochemical, microbial, and ecological interactions (Vasques et al., 2024). They enhance soil microbial diversity and activity by producing various secondary metabolites, such as lipopeptides, antibiotics, and VOCs, which act as antagonists to soilborne pathogens. These bioactive compounds disrupt the growth of phytopathogenic fungi, bacteria, and nematodes, thereby fostering a healthier and more balanced soil microbiome (Miljaković et al., 2020). Moreover, the metabolites released by Bacillus spp. often serve as signaling molecules, promoting beneficial microbial symbiosis and microbial niche differentiation within the rhizosphere. A crucial mechanism through which Bacillus spp. influence soil health is the decomposition of organic matter by secreting hydrolytic enzymes, such as cellulases, proteases, and chitinases. These enzymes accelerate the breakdown of complex organic materials into simpler compounds, improving soil organic carbon content and nutrient availability (Riseh et al., 2024). Bacillus spp. produce chitinases that degrade chitin-containing structures, such as nematode eggshells and fungal cell walls, thereby facilitating the recycling of essential elements, such as nitrogen and carbon, within soil ecosystems. This degradation process releases N-acetylglucosamine monomers, which serve as nutrient sources for various soil microorganisms, thereby enhancing nutrient cycling and soil fertility. The breakdown of these structures by Bacillus-derived chitinases also suppresses soilborne pathogens and pests, contributing to a healthier soil microbiome (Gomaa, 2021). Moreover, Bacillus spp. play a vital role in nutrient cycling, particularly in nitrogen fixation and phosphate solubilization. Certain strains, such as B. subtilis and B. megaterium, possess the genetic and enzymatic machinery required for solubilizing insoluble phosphates in the soil. They produce organic acids (e.g., gluconic acid and citric acid) and phosphatases and convert insoluble phosphates into plant-accessible forms, such as dihydrogen phosphate (Saeid et al., 2018). Several Bacillus spp., including Paenibacillus polymyxa and P. macerans, contain nitrogenase enzymes that enable them to fix atmospheric nitrogen into ammonia, thereby enhancing soil fertility and providing essential nutrients for plant growth. This biological nitrogen fixation facilitates sustainable agricultural practices by reducing the need for chemical nitrogen fertilizers. Studies have demonstrated the efficacy of these bacteria in promoting plant growth through nitrogen fixation (Li et al., 2022). Bacillus spp. can enhance soil structure by secreting EPS, which facilitate the aggregation of soil particles. This aggregation improves soil porosity, aeration, and water infiltration, thereby promoting plant root growth and nutrient uptake. Additionally, the production of EPS facilitates moisture retention and reduces soil erosion, thereby enhancing soil resilience under stress conditions. These benefits underscore the role of Bacillus spp. in sustainable soil management and plant health enhancement (Olagoke et al., 2022). Moreover, Bacillus spp. can induce systemic resistance in plants, indirectly influencing soil health by reducing pathogen pressure. Bacillus-treated plants exhibit enhanced production of antimicrobial compounds and defense-related enzymes through the activation of JA and SA pathways. This reduces the likelihood of pathogen colonization and minimizes disease-mediated disruptions to soil microbial dynamics (Kloepper et al., 2004). While Bacillus spp. offer numerous benefits as biocontrol agents, their application must be carefully managed to maintain ecological balance within the soil microbiome. Overapplication or improper use can result in the overdominance of Bacillus strains, potentially suppressing other beneficial microorganisms and disrupting microbial community structures. This imbalance may result in competition for resources, negatively impacting native microbial populations and overall soil health (Li et al., 2022). Therefore, it is crucial to monitor and regulate the use of Bacillus-based biocontrol agents in order to preserve the diversity and functionality of soil microbial communities. Sustainable management practices, including the rotation of microbial inoculants, integrated use of organic amendments, and minimal use of chemical treatments, can mitigate these risks and optimize the long-term benefits of Bacillus applications.
Incorporating Bacillus strains into IPM strategies offers an effective and sustainable approach for the management of phytopathogenic nematodes. Bacillus spp., such as B. subtilis and Bt, employ multiple mechanisms to suppress nematodes (Gassmann et al., 2008; Jaiswal et al., 2022). These strains not only produce nematicidal compounds but also promote plant growth by producing phytohormones and enhancing nutrient availability. This dual action improves crop health and resilience, further mitigating the impact of nematode infestations (Abd-Elgawad and Askary, 2018).
Within an IPM framework, Bacillus strains are most effective when used in combination with other biocontrol agents, chemical nematicides, and cultural practices. For instance, the application of B. firmus strain 1–1,582 in combination with chemical nematicides and organic amendments significantly enhanced tomato yield and effectively suppressed M. incognita and P. lycopersici populations under greenhouse conditions, particularly when environmental conditions were less favorable for nematode development. These findings underscore the potential of B. firmus as a viable component of IPM strategies during tomato cultivation (d'Errico et al., 2019).
A recent review by Paradva and Kalla (2023) highlighted the potential of microbial biocontrol agents, particularly Bacillus-based nanoparticles, as sustainable and eco-friendly alternatives to chemical pesticides for plant disease and pest management. The synergistic use of Bacillus strains with nematophagous fungi or predatory nematodes can confer multilevel protection by targeting different stages of the nematode life cycle (Gassmann et al., 2008; d'Errico et al., 2019).
Native Bacillus strains, such as B. marisflavi CRB2 and B. subtilis CRB7, which harbor multiple antimicrobial peptide genes, have been proven to be effective against M. incognita in okra. Within an IPM framework, these strains have caused significant reductions in nematode incidence and improvements in crop yields in laboratory, pot, and field trials (Gurikar et al., 2022). When applied with reduced doses of chemical nematicides, Bacillus strains can help lower the use of chemical treatments and maintain effective nematode control, thereby minimizing the potential for resistance development and environmental impacts (Ruiu, 2015). Moreover, cultural practices, such as crop rotation, cover cropping, and the use of organic soil amendments, enhance the efficacy of Bacillus applications by creating less favorable conditions for nematode proliferation and supporting a healthier soil microbiome (Singh et al., 2019). For instance, the integration of B. subtilis with cow manure resulted in a 54% reduction in PPN populations in common beans and preserved nematode biodiversity, thereby serving as a sustainable and effective pest management strategy (Wepuhkhulu et al., 2011). Furthermore, Rao et al. (2017) demonstrated that the application of B. subtilis IIHR BS-2 as a seed treatment in combination with a vermicompost-enriched soil application significantly reduced nematode populations by 69.3% and disease incidence by 70.2%, resulting in a 28.8% increase in carrot yield. This integrated approach outperformed chemical treatments, highlighting the efficacy of B. subtilis IIHR BS-2 in managing the M. incognita–Pectobacterium carotovorum disease complex in carrots.
Thus, the strategic incorporation of Bacillus strains into IPM programs has several advantages, including sustainable nematode management, enhanced efficacy through synergistic effects, and improved resistance management (Wepuhkhulu et al., 2011). Regular monitoring of nematode populations and crop health is crucial for optimizing the timing and application of Bacillus treatments to ensure the highest efficacy in conjunction with other control measures (Chinheya et al., 2017). By integrating Bacillus strains into a comprehensive IPM strategy, farmers can achieve long-term nematode suppression, reduce reliance on chemical pesticides, and ultimately improve crop productivity and sustainability in agricultural systems (Figure 7).
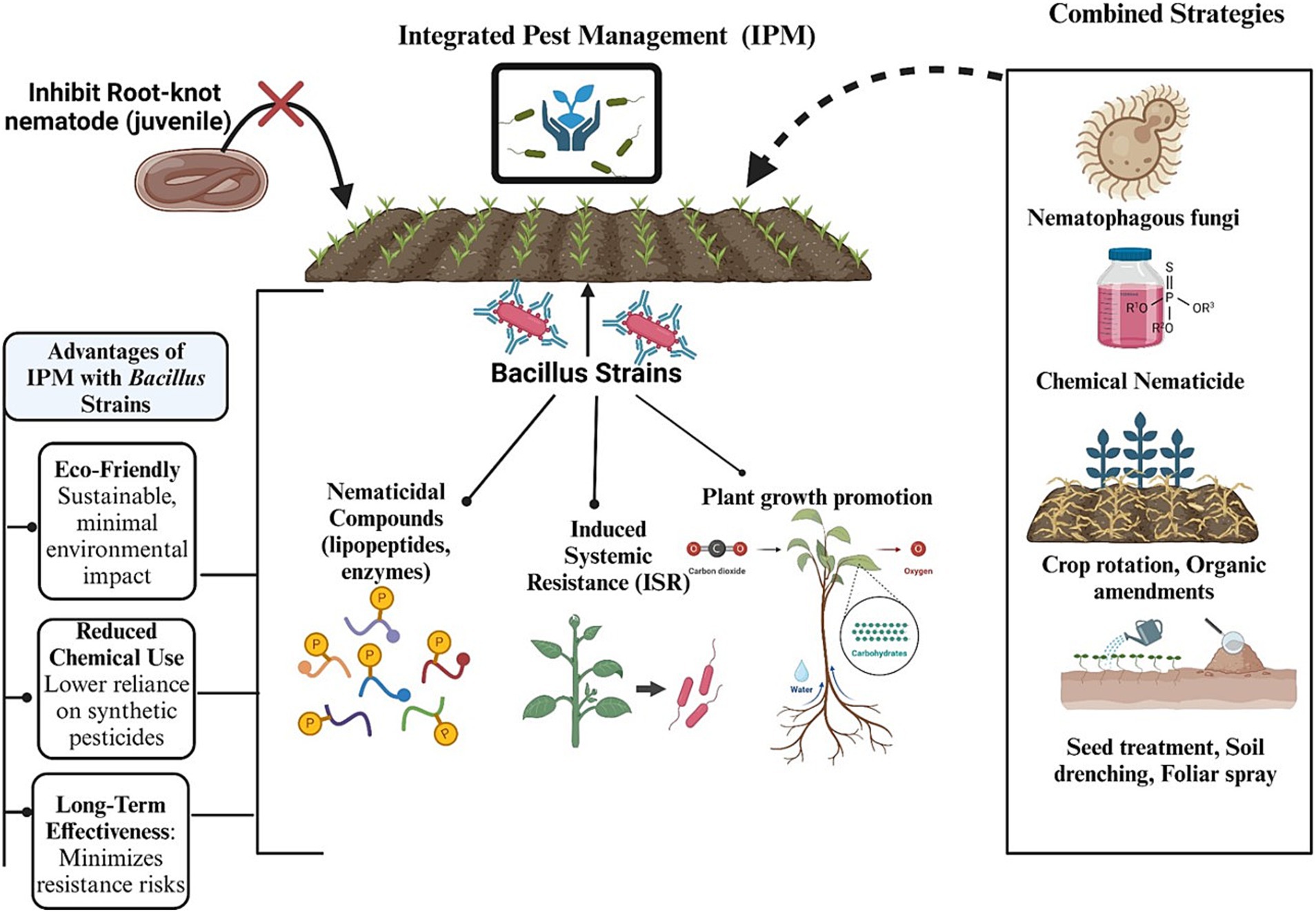
Figure 7. Graphical representation of integrated pest management strategies using Bacillus strains for nematode control.
Bacillus spp. are recognized for their scalability as biocontrol agents, primarily because of their ability to form resilient spores that can be produced on a large scale through cost-effective industrial fermentation processes (Serrão et al., 2024). These spore-based formulations exhibit extended shelf lives and require minimal storage conditions, thereby reducing logistical expenses for farmers, especially in resource-limited regions (Cho and Chung, 2020). Moreover, Bacillus formulations are compatible with existing agricultural practices, including seed treatments, soil amendments, and foliar sprays, facilitating their integration into IPM systems. Their synergistic interactions with organic amendments, such as compost, and microbial consortia further enhance their efficacy and cost-effectiveness (Asif et al., 2024). Economic analyses have indicated that Bacillus-based products can significantly reduce reliance on chemical nematicides and fertilizers, resulting in substantial cost savings. For instance, the application of B. subtilis during tomato cultivation has been shown to reduce nematode-induced losses by over 60%, resulting in notable yield improvements and financial benefits (Pontes et al., 2024). Case studies from countries like Brazil and India have demonstrated the successful large-scale application of Bacillus spp. in soybean and rice production systems, respectively (Galbieri et al., 2023; Pandey et al., 2024). Moreover, smallholder farmers in Africa have adopted these formulations because of their affordability and effectiveness across various crops, including maize and vegetables (Vasques et al., 2024). Cost comparisons have revealed that Bacillus-based biopesticides are approximately 30–50% less expensive than chemical alternatives, enhancing their appeal in low-income regions (Hezakiel et al., 2024). In addition to economic advantages, these biopesticides offer significant environmental benefits by reducing pollution and health risks associated with chemical nematicides, thereby contributing to global sustainability goals (Köhl et al., 2019). They also promote ecological balance by enhancing soil biodiversity and mitigating secondary pest outbreaks, further reinforcing their role in sustainable agriculture (Abd-Elgawad, 2024).
The application of Bacillus strains as biocontrol agents for managing nematodes in agricultural systems has several challenges and limitations. Environmental factors, such as soil type and climate, play crucial roles in determining the efficacy of these bacteria. Soil characteristics, including pH, organic matter content, and texture, can significantly influence the survival, colonization, and nematicidal activity of Bacillus strains. For instance, sandy soils may cause the bacteria to leach away, while heavy clay soils could limit bacterial distribution (Gurikar et al., 2022). Additionally, climatic conditions, particularly temperature and moisture levels, can significantly influence the efficacy of Bacillus spp. (Ayaz et al., 2023). Extreme temperatures can inhibit bacterial activity, while optimal moisture levels are necessary for the germination and functioning of bacterial spores. Furthermore, interactions with other soil microorganisms can limit the establishment of Bacillus strains because of competition for resources or antagonistic effects.
In addition to environmental factors, regulatory and safety concerns pose substantial barriers to the widespread use of Bacillus strains as biocontrol agents. The approval process for these biocontrol agents involves rigorous testing to ensure their safety for humans, animals, and the environment. This process can be time-consuming and expensive, particularly for smaller companies, thereby delaying the introduction of effective biocontrol products.
The efficacy of Bacillus strains in nematode control is significantly influenced by soil type, climatic conditions, and interactions with other soil microorganisms (Shafi et al., 2017; Singh et al., 2023). Bacillus strains often perform more consistently in controlled environments, such as greenhouses, where conditions are more predictable and manageable. However, translating the obtained results to field conditions can be challenging because of the variability in environmental factors across different geographical locations and crop systems (Ayaz et al., 2023).
Despite the promising potential of Bacillus spp. in managing PPNs, several challenges need to be overcome to ensure consistent efficacy under field conditions. Environmental factors, such as soil type, temperature, moisture level, and pH, can significantly impact the survival, colonization, and biocontrol activity of Bacillus strains (Shafi et al., 2017). Additionally, the presence of native soil microbiota can necessitate competitive interactions that may suppress the establishment and function of introduced Bacillus spp. Native microorganisms compete with introduced Bacillus strains for essential nutrients and ecological niches. This competition can limit the growth and activity of the biocontrol agents, thereby reducing their effectiveness against PPNs. For instance, indigenous soil bacteria may outcompete introduced Bacillus strains for carbon sources, thereby inhibiting their proliferation (Mawarda et al., 2022). Moreover, native microorganisms can form biofilms on root surfaces, creating physical barriers that can prevent Bacillus spp. from accessing plant roots and exhibiting their biocontrol effects. These biofilms can effectively exclude introduced bacteria from key interaction sites. For instance, biofilms formed by indigenous Pseudomonas spp. can inhibit the root colonization of introduced Bacillus strains (Steinberg et al., 2020). Additionally, field variability significantly influences the efficacy of Bacillus spp. as biocontrol agents, with the outcomes in controlled environments often differing from those in diverse agricultural settings. Environmental factors, such as soil type, pH, moisture level, temperature, and organic matter content, play crucial roles in the survival, colonization, and activity of introduced Bacillus strains (Serrão et al., 2024). To overcome these challenges, comprehensive field studies need to be conducted. Moreover, robust Bacillus formulations that can withstand environmental fluctuations and can be effectively integrated into existing soil microbial communities need to be developed.
For the commercialization and large-scale application of Bacillus strains, significant hurdles related to formulation, storage, and regulatory approval need to be overcome (Montesinos, 2003; Butu et al., 2022). The stability and shelf life of Bacillus products can be affected by formulation methods, storage environments, and shipment conditions. To ensure the success of Bacillus spp. as biocontrol agents, it is essential to enhance formulation technologies, extend product shelf life, and reduce production costs (Ortiz and Sansinenea, 2023). Ongoing efforts by researchers and industry partners are focused on optimizing microbial strains for large-scale applications, in addition to ensuring that these products meet rigorous environmental and human health safety standards (Hossain et al., 2023).
These challenges underscore the need for continued research and collaboration to effectively integrate Bacillus strains into sustainable agricultural practices. Safety evaluations must also ensure that Bacillus strains do not pose risks to nontarget organisms or the environment and do not have unintended ecological impacts, such as the disruption of soil microbial communities or induction of resistance in pest populations (Ayaz et al., 2023). Public perception and acceptance of microbial biocontrol agents further complicate their application, highlighting the need for better education and communication about their safety and benefits. Addressing these challenges is essential to fully harness the potential of Bacillus strains for sustainable nematode management (Hossain et al., 2023).
Targeted genome editing, particularly CRISPR/Cas9 technology, has revolutionized plant pathology by enabling precise genetic modifications to enhance disease resistance in crops. This technology is preferred for its simplicity, cost-effectiveness, and adaptability, offering a promising approach for the development of pest- and disease-resistant plants (Das et al., 2023; Yin et al., 2024). These genetic modifications often aim to increase the production of antimicrobial compounds, such as lipopeptides, enzymes, and VOCs, which are crucial for suppressing various plant pathogens (Rocha and Duggal, 2023; Maqsood et al., 2024). Additionally, genetic engineering has facilitated the introduction of novel traits, such as enhanced root colonization and rhizosphere persistence, ensuring that engineered Bacillus strains are more effective and resilient under diverse environmental conditions (Ramírez-Pool et al., 2024). A recent review by Khan et al. (2023) highlighted that advanced molecular strategies, including transcriptomics, RNA interference, and CRISPR/Cas9, are increasing our understanding of plant–nematode interactions and boosting plant resistance to root-knot nematodes. Engineered Bacillus strains exhibit improved activity against nematodes, offering a broad-spectrum biocontrol solution that is highly specific to target pests (Danilova et al., 2023).
Although genetic engineering has significant potential for enhancing the nematicidal efficacy of Bacillus strains, its use is associated with several biosafety concerns. Unintended ecological impacts, such as the disruption of native microbial communities or off-target effects on nontarget organisms, must be carefully evaluated (Samal et al., 2024). Horizontal gene transfer poses additional risks, potentially resulting in the spread of engineered traits to unintended microbial populations. Regulatory hurdles, including stringent testing for environmental and public health safety, also pose significant challenges. For example, the process of obtaining approval for genetically modified Bacillus strains varies across jurisdictions, with extensive environmental impact assessments required to ensure compliance with biosafety standards (Rozas et al., 2024).
Formulation improvements have been a major focus in the advancement of Bacillus-based biocontrol products (Tong-Jian et al., 2013; Umamaheswari et al., 2020). Innovations in this area include the development of more stable and effective formulations to maximize the viability and efficacy of Bacillus derivatives (Chavarria-Quicaño et al., 2023a,b). A significant advancement is microencapsulation. In this process, spores are enclosed within a protective matrix to shield them from environmental stressors while enabling controlled release (Gao et al., 2024). This technique has been crucial for maintaining the viability of spores over extended periods, thereby enhancing the shelf life and effectiveness of the product (Khullar et al., 2024).
Researchers are also exploring synergistic combinations of Bacillus strains with other biocontrol agents or biostimulants in order to create multifunctional formulations that can confer comprehensive plant protection and promote plant growth. Advances in delivery systems and increases in shelf life have further revolutionized the application of Bacillus-based biocontrol agents (Karačić et al., 2024). Novel delivery systems, such as nano-bioformulations and polymer-based carriers, are being developed to optimize the precision and efficacy of Bacillus applications (Behl et al., 2024). These systems are designed to optimize the release of active agents at the site of infection, thereby reducing the need for frequent applications and lowering the overall costs (Kumar et al., 2021). Moreover, improvements in storage technology, including the development of temperature-stable formulations and vacuum packaging techniques, have significantly extended the shelf life of Bacillus products (Gotor-Vila et al., 2019). These innovations not only ensure the long-term viability of biocontrol agents but also enhance their accessibility on a global scale, particularly in regions with challenging storage and transportation conditions.
Bacillus spp. have emerged as potent biocontrol agents against PPNs, offering a promising and sustainable alternative to traditional chemical treatments. Their effectiveness is attributed to their multifaceted mechanisms, including the production of nematicidal compounds, enhancement of plant resistance, and improvement of soil health. Thus, they play invaluable roles in IPM strategies. Recent advances in genetic engineering and formulation technologies have significantly bolstered the efficacy and reliability of Bacillus strains for agricultural applications. However, various challenges, such as environmental variability, regulatory hurdles, and the need for optimized application methods, persist. Overcoming these challenges is essential for maximizing the efficacy of Bacillus spp. in sustainable nematode management and ensuring global food security.
PV-S: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing. KP: Formal analysis, Investigation, Writing – review & editing. KK: Conceptualization, Formal analysis, Investigation, Writing – review & editing. W-JJ: Conceptualization, Investigation, Project administration, Resources, Supervision, Writing – review & editing. YH: Funding acquisition, Investigation, Project administration, Resources, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) through the Agricultural Machinery/Equipment Localization Technology Development Program funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (No. 122020–3) and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (Grant No. 2022R1A2C1013108).
KP was the CEO of Invirustech Co., Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aballay, E., Prodan, S., Correa, P., and Allende, J. (2020). Assessment of rhizobacterial consortia to manage plant parasitic nematodes of grapevine. Crop Prot. 131:105103. doi: 10.1016/j.cropro.2020.105103
Abd-Elgawad, M. M. (2024). Upgrading strategies for managing nematode pests on profitable crops. Plan. Theory 13:1558. doi: 10.3390/plants13111558
Abd-Elgawad, M. M., and Askary, T. H. (2018). Fungal and bacterial nematicides in integrated nematode management strategies. Egypt. J. Biol. Pest Control 28, 1–24. doi: 10.1186/s41938-018-0080-x
Abd-Elgawad, M. M. M., and Askary, T. H. (2020). Factors affecting success of biological agents used in controlling the plant-parasitic nematodes. Egypt. J. Biol. Pest Control 30:17. doi: 10.1186/s41938-020-00215-2
Abdellatif, A. A., Tahany, A. R., Sayed, M. A., Dina, I., and Elmaghraby, M. M. K. (2021). Activity of Serratia spp. and Bacillus spp. as biocontrol agents against Meloidogyne incognita infecting tomato. Pakist. J. Biotechnol. 18, 37–47. doi: 10.34016/pjbt.2021.18.2/3.37
Abdel-Salam, M. S., Ameen, H. H., Soliman, G. M., Elkelany, U. S., and Asar, A. M. (2018). Improving the nematicidal potential of Bacillus amyloliquefaciens and Lysinibacillus sphaericus against the root-knot nematode Meloidogyne incognita using protoplast fusion technique. Egypt. J. Biol. Pest Control 28, 1–6. doi: 10.1186/s41938-018-0034-3
Adam, M., Heuer, H., and Hallmann, J. (2014). Bacterial antagonists of fungal pathogens also control root-knot nematodes by induced systemic resistance of tomato plants. PLoS One 9:e90402. doi: 10.1371/journal.pone.0090402
Adiwena, M., Murtilaksono, A., Egra, S., Hoesain, M., Asyiah, I. N., Pradana, A. P., et al. (2023). The effects of micronutrient-enriched media on the efficacy of Bacillus subtilis as biological control agent against Meloidogyne incognita. Biodiv. J. Biol. Divers. 24, 33–39. doi: 10.13057/biodiv/d240105
Ahmad, G., Khan, A., Khan, A. A., Ali, A., and Mohhamad, H. I. (2021). Biological control: a novel strategy for the control of the plant parasitic nematodes. Antonie Van Leeuwenhoek 114, 885–912. doi: 10.1007/s10482-021-01577-9
Ahmed, S., Liu, Q., and Jian, H. (2019). Bacillus cereus a potential strain infested cereal cyst nematode (Heterodera avenae). Pak. J. Nematol. 37, 53–61. doi: 10.18681/pjn.v37.i01.p53-61
Ali, Q., Yu, C., Wang, Y., Sheng, T., Zhao, X., Wu, X., et al. (2023). High killing rate of nematode and promotion of rice growth by synthetic volatiles from Bacillus strains due to enhanced oxidative stress response. Physiol. Plant. 175:e13868. doi: 10.1111/ppl.13868
Amorim, D. J., Tsujimoto, T. F., Baldo, F. B., Leite, L. G., Harakava, R., Wilcken, S. R. S., et al. (2024). Bacillus, Pseudomonas and Serratia control Meloidogyne incognita (Rhabditida: Meloidogynidae) and promote the growth of tomato plants. Rhizosphere 31:100935. doi: 10.1016/j.rhisph.2024.100935
Antil, S., Kumar, R., Pathak, D. V., Kumar, A., Panwar, A., and Kumari, A. (2022a). Plant growth-promoting rhizobacteria-Bacillus cereus KMT-5 and B. megaterium KMT-8 effectively suppressed Meloidogyne javanica infection. Appl. Soil Ecol. 174:104419. doi: 10.1016/j.apsoil.2022.104419
Antil, S., Kumar, R., Pathak, D. V., Kumar, A., Panwar, A., Kumari, A., et al. (2021). On the potential of Bacillus aryabhattai KMT-4 against Meloidogyne javanica. Egypt. J. Biol. Pest Control 31, 1–9. doi: 10.1186/s41938-021-00417-2
Antil, S., Kumar, R., Pathak, D. V., Kumar, A., Panwar, A., Kumari, A., et al. (2022b). Potential of Bacillus altitudinis KMS-6 as a biocontrol agent of Meloidogyne javanica. J. Pest. Sci. 95, 1443–1452. doi: 10.1007/s10340-021-01469-x
Asaturova, A. M., Bugaeva, L. N., Homyak, A. I., Slobodyanyuk, G. A., Kashutina, E. V., Yasyuk, L. V., et al. (2022). Bacillus velezensis strains for protecting cucumber plants from root-knot nematode Meloidogyne incognita in a greenhouse. Plan. Theory 11:275. doi: 10.3390/plants11030275
Asif, K., Shabaan, M., Mahmood, W., Asghar, H. N., Zahir, Z. A., Zulfiqar, U., et al. (2024). Synergistic application of bacterial consortium and organic amendments improves the growth and seed quality of mash bean (Vigna Mungo L.). Soil Sci. Plant Nutr. 24, 6893–6905. doi: 10.1007/s42729-024-02012-4
Ayaz, M., Ali, Q., Farzand, A., Khan, A. R., Ling, H., and Gao, X. (2021). Nematicidal volatiles from Bacillus atrophaeus GBSC56 promote growth and stimulate induced systemic resistance in tomato against Meloidogyne incognita. Int. J. Mol. Sci. 22:5049. doi: 10.3390/ijms22095049
Ayaz, M., Li, C. H., Ali, Q., Zhao, W., Chi, Y. K., Shafiq, M., et al. (2023). Bacterial and fungal biocontrol agents for plant disease protection: journey from lab to field, current status, challenges, and global perspectives. Molecules 28:6735. doi: 10.3390/molecules28186735
Bacon, C. W., Hinton, D. M., and Hinton, A. Jr. (2006). Growth-inhibiting effects of concentrations of fusaric acid on the growth of Bacillus mojavensis and other biocontrol Bacillus species. J. Appl. Microbiol. 100, 185–194. doi: 10.1111/j.1365-2672.2005.02770.x
Banihashemian, S. N., Jamali, S., Golmohammadi, M., and Ghasemnezhad, M. (2023). Management of root-knot nematode in kiwifruit using resistance-inducing Bacillus altitudinis. Trop. Plant Pathol. 48, 443–451. doi: 10.1007/s40858-023-00573-w
Baptista, J. P., Teixeira, G. M., Jesus, M. L. A., Bertê, R., Higashi, A., Mosella, M., et al. (2022). Antifungal activity and genomic characterization of the isolate Bacillus velezensis CMRP 4489, a biocontrol agent for plant-pathogenic fungi. Res. Sq. doi: 10.21203/rs.3.rs-1619465/v1
Barnawal, D., Bharti, N., Pandey, S. S., Pandey, A., Chanotiya, C. S., and Kalra, A. (2017). Plant growth-promoting rhizobacteria enhance wheat salt and drought stress tolerance by altering endogenous phytohormone levels and TaCTR1/TaDREB2 expression. Physiol. Plant. 161, 502–514. doi: 10.1111/ppl.12614
Basiouny, A. G., and Abo-Zaid, G. A. (2018). Biocontrol of the root-knot nematode, Meloidogyne incognita, using an eco-friendly formulation from Bacillus subtilis, lab and greenhouse studies. Egypt. J. Biol. Pest Control 28:87. doi: 10.1186/s41938-018-0094-4
Bavaresco, L. G., Guaberto, L. M., and Araujo, F. F. (2021). Interaction of Bacillus subtilis with resistant and susceptible tomato (Solanum lycopersicum L.) in the control of Meloidogyne incognita. Arch. Phytopathol. Plant Protect. 54, 359–374. doi: 10.1080/03235408.2020.1833279
Behl, K., Jaiswal, P., and Pabbi, S. (2024). Recent advances in microbial and nano-formulations for effective delivery and agriculture sustainability. Biocatal. Agric. Biotechnol. 58:103180. doi: 10.1016/j.bcab.2024.103180
Berlitz, D. L., Knaak, N., Cassal, M. C., and Fiuza, L. M. (2014). “Bacillus and biopesticides in control of phytonematodes” in Basic and applied aspects of biopesticides. ed. K. Sahayaraj (New Delhi: Springer).
Bhat, A. A., Shakeel, A., Waqar, S., Handoo, Z. A., and Khan, A. A. (2023). Microbes vs. nematodes: insights into biocontrol through antagonistic organisms to control root-knot nematodes. Plan. Theory 12:451. doi: 10.3390/plants12030451
Blouin, M. S., Yowell, C. A., Courtney, C. H., and Dame, J. B. (1998). Substitution bias, rapid saturation, and the use of mtDNA for nematode systematics. Mol. Biol. Evol. 15, 1719–1727. doi: 10.1093/oxfordjournals.molbev.a025898
Bo, T., Kong, C., Zou, S., Mo, M., and Liu, Y. (2022). Bacillus nematocida B16 enhanced the rhizosphere colonization of Pochonia chlamydosporia ZK7 and controlled the efficacy of the root-knot nematode Meloidogyne incognita. Microorganisms 10:218. doi: 10.3390/microorganisms10020218
Borriss, R., Wu, H., and Gao, X. (2019). “Secondary metabolites of the plant growth promoting model rhizobacterium Bacillus velezensis FZB42 are involved in direct suppression of plant pathogens and in stimulation of plant-induced systemic resistance” in Secondary metabolites of plant growth promoting rhizomicroorganisms: discovery and applications. eds. H. Singh, C. Keswani, M. Reddy, E. Sansinenea, and C. García-Estrada. 147–168.
Bravo, A., Gill, S. S., and Soberón, M. (2007). Mode of action of Bacillus thuringiensis cry and Cyt toxins and their potential for insect control. Toxicon 49, 423–435. doi: 10.1016/j.toxicon.2006.11.022
Brzezinska, M. S., Kalwasińska, A., Świątczak, J., Żero, K., and Jankiewicz, U. (2020). Exploring the properties of chitinolytic Bacillus isolates for the pathogens biological control. Microb. Pathog. 148:104462. doi: 10.1016/j.micpath.2020.104462
Bui, H. X., Hadi, B. A., Oliva, R., and Schroeder, N. E. (2020). Beneficial bacterial volatile compounds for the control of root-knot nematode and bacterial leaf blight on rice. Crop Prot. 135:104792. doi: 10.1016/j.cropro.2019.04.016
Butu, M., Rodino, S., and Butu, A. (2022). Biopesticide formulations-current challenges and future perspectives. Biopesticides 2, 19–29. doi: 10.1016/B978-0-12-823355-9.00010-9
Calvo, P., Ormeño-Orrillo, E., Martínez-Romero, E., and Zúñiga, D. (2010). Characterization of Bacillus isolates of potato rhizosphere from Andean soils of Peru and their potential PGPR characteristics. Braz. J. Microbiol. 41, 899–906. doi: 10.1590/S1517-83822010000400008
Camacho, M., de Los Santos, B., Vela, M. D., and Talavera, M. (2023). Use of bacteria isolated from berry rhizospheres as biocontrol agents for charcoal rot and root-knot nematode strawberry diseases. Horticulturae 9:346. doi: 10.3390/horticulturae9030346
Camatti, G., dos Santos, F. M., Júnior, G. L. D. S. R., Camargo, D. P., Manfio, G. S., Santos, J. R. P., et al. (2023). Bacillus-and Trichoderma-based products control the spiral nematode Helicotylenchus dihystera in soybean. Rhizosphere 27:100717. doi: 10.1016/j.rhisph.2023.100717
Carmona-Hernandez, S., Reyes-Pérez, J. J., Chiquito-Contreras, R. G., Rincon-Enriquez, G., Cerdan-Cabrera, C. R., and Hernandez-Montiel, L. G. (2019). Biocontrol of postharvest fruit fungal diseases by bacterial antagonists: a review. Agronomy 9:121. doi: 10.3390/agronomy9030121
Carriel, C. B., and Soto, D. V. (2022). Persistence of Bacillus thuringiensis and Bacillus pumilus potential biological control agents of the coffee berry borer under field conditions of Puerto Rico. Sci. Agric. 19, 43–56. doi: 10.19053/01228420.v19.n3.2022.14685
Castillo, H. F., Reyes, C. F., Morales, G. G., Herrera, R. R., and Aguilar, C. (2013). “Biological control of root pathogens by plant growth promoting Bacillus spp” in Weed and pest control - conventional and new challenges. eds. S. Soloneski and M. L. Larramendy (Rijeka, Croatia: InTech), 79–103.
Cavalcanti, V. P., Terra, W. C., de Souza, J. T., Pacheco, P. V. M., de Sousa, L. F., Belizario, R. A., et al. (2024). A commercial formulation of Bacillus subtilis induces metabolomic changes in root exudates that invert the chemotactic responses of the nematode Meloidogyne incognita to host and non-host plants. J. Plant Dis. Protect. 131, 899–909. doi: 10.1007/s41348-024-00892-3
Cetintas, R., Kusek, M., and Fateh, S. A. (2018). Effect of some plant growth-promoting rhizobacteria strains on root-knot nematode, Meloidogyne incognita, on tomatoes. Egypt. J. Biol. Pest Control 28, 1–5. doi: 10.1186/s41938-017-0008-x
Chavarria-Quicaño, E., Contreras-Jácquez, V., Carrillo-Fasio, A., De la Torre-González, F., and Asaff-Torres, A. (2023a). Native Bacillus paralicheniformis isolate as a potential agent for phytopathogenic nematodes control. Front. Microbiol. 14:1213306. doi: 10.3389/fmicb.2023.1213306
Chavarria-Quicaño, E., De la Torre-González, F., González-Riojas, M., Rodríguez-González, J., and Asaff-Torres, A. (2023b). Nematicidal lipopeptides from Bacillus paralicheniformis and Bacillus subtilis: a comparative study. Appl. Microbiol. Biotechnol. 107, 1537–1549. doi: 10.1007/s00253-023-12391-w
Chen, J., Abawi, G. S., and Zuckerman, B. M. (2000). Efficacy of Bacillus thuringiensis, Paecilomyces marquandii, and Streptomyces costaricanus with and without organic amendments against Meloidogyne hapla infecting lettuce. J. Nematol. 32, 70–77 Available at: https://pubmed.ncbi.nlm.nih.gov/19270951/
Chen, L., Wang, Y., Zhu, L., Min, Y., Tian, Y., Gong, Y., et al. (2024). 3-(Methylthio) propionic acid from Bacillus thuringiensis Berliner exhibits high Nematicidal activity against the root knot nematode Meloidogyne incognita (Kofoid and white) Chitwood. Int. J. Mol. Sci. 25:1708. doi: 10.3390/ijms25031708
Chernov, T. I., and Semenov, M. V. (2021). Management of soil microbial communities: opportunities and prospects (a review). Eurasian Soil Sci. 54, 1888–1902. doi: 10.1134/S1064229321120024
Chien, Y. C., and Huang, C. H. (2020). Biocontrol of bacterial spot on tomato by foliar spray and growth medium application of Bacillus amyloliquefaciens and Trichoderma asperellum. Eur. J. Plant Pathol. 156, 995–1003. doi: 10.1007/s10658-020-01947-5
Chinheya, C. C., Yobo, K. S., and Laing, M. D. (2017). Biological control of the rootknot nematode, Meloidogyne javanica (Chitwood) using Bacillus isolates, on soybean. Biol. Control 109, 37–41. doi: 10.1016/j.biocontrol.2017.03.009
Cho, W. I., and Chung, M. S. (2020). Bacillus spores: A review of their properties and inactivation processing technologies. Food Sci. Biotechnol. 29, 1447–1461. doi: 10.1007/s10068-020-00809-4
Choi, T. G., Maung, C. E. H., Lee, D. R., Henry, A. B., Lee, Y. S., and Kim, K. Y. (2020). Role of bacterial antagonists of fungal pathogens, Bacillus thuringiensis KYC and Bacillus velezensis CE 100 in control of root-knot neatode, Meloidogyne incognita and subsequent growth promotion of tomato. Biocontrol Sci. Tech. 30, 685–700. doi: 10.1080/09583157.2020.1765980
Choudhary, D. K., and Johri, B. N. (2009). Interactions of Bacillus spp. and plants–with special reference to induced systemic resistance (ISR). Microbiol. Res. 164, 493–513. doi: 10.1016/j.micres.2008.08.007
Chowdhury, S. P., Hartmann, A., Gao, X., and Borriss, R. (2015). Biocontrol mechanism by root-associated Bacillus amyloliquefaciens FZB42–a review. Front. Microbiol. 6:780. doi: 10.3389/fmicb.2015.00780
Cruz‐Magalhães, V., Guimarães, R. A., Da Silva, J. C., de Faria, A. F., Pedroso, M. P., Campos, V. P., et al. (2022). The combination of two Bacillus strains suppresses Meloidogyne incognita and fungal pathogens, but does not enhance plant growth. Pest Manag. Sci. 78, 722–732. doi: 10.1002/ps.6685
Dai, M. M., Liu, R., Jiang, H., Zhang, X. P., Song, W. W., Zhang, J., et al. (2023). Volatile organic compounds of Bacillus pumilus strain S1-10 exhibit fumigant activity against Meloidogyne incognita. Plant Dis. 107, 3057–3063. doi: 10.1094/PDIS-10-22-2391-RE
Danilova, I. V., Vasileva, I. A., Gilmutdinova, A. I., Dyadkina, I. V., Khusnullina, L. K., Khasanov, D. I., et al. (2023). Characterization of Bacillus pumilus strains with targeted gene editing for antimicrobial peptides and sporulation factor. Microorganisms 11:1508. doi: 10.3390/microorganisms11061508
Das, K., Ayim, B. Y., Borodynko-Filas, N., Das, S. C., and Aminuzzaman, F. M. (2023). Genome editing (CRISPR/Cas9) in plant disease management: challenges and future prospects. J. Plant Protect. Res. 63, 159–172. doi: 10.24425/jppr.2023.145761
Das, S., Wadud, M. A., and Khokon, M. A. R. (2021). Functional evaluation of culture filtrates of Bacillus subtilis and Pseudomonas fluorescens on the mortality and hatching of Meloidogyne javanica. Saudi J. Biol. Sci. 28, 1318–1323. doi: 10.1016/j.sjbs.2020.11.055
Daulagala, P. W. H. K. P. (2021). Chitinolytic endophytic bacteria as biocontrol agents for phytopathogenic fungi and nematode pests: a review. Asian J. Res. Bot. 5, 14–24.
de Paula, L. L., Campos, V. P., Terra, W. C., de Brum, D., Jacobs, D. C., Bui, H. X., et al. (2024). The combination of Bacillus amyloliquefaciens and Purpureocillium lilacinum in the control of Meloidogyne enterolobii. Biol. Control 189:105438. doi: 10.1016/j.biocontrol.2023.105438
d'Errico, G., Marra, R., Crescenzi, A., Davino, S. W., Fanigliulo, A., Woo, S. L., et al. (2019). Integrated management strategies of Meloidogyne incognita and Pseudopyrenochaeta lycopersici on tomato using a Bacillus firmus-based product and two synthetic nematicides in two consecutive crop cycles in greenhouse. Crop Prot. 122, 159–164. doi: 10.1016/j.cropro.2019.05.004
Devindrappa, M., Kamra, A., Singh, D., Gawade, B., and Sirohi, A. (2023). Plant growth promoting Bacillus species elicit defense against Meloidogyne incognita infecting tomato in polyhouse. J. Basic Microbiol. 2023, 1–9. doi: 10.22541/au.168001566.62776546/v1
Díaz-Manzano, F. E., Amora, D. X., Martínez-Gómez, Á., Moelbak, L., and Escobar, C. (2023). Biocontrol of Meloidogyne spp. in Solanum lycopersicum using a dual combination of Bacillus strains. Front. Plant Sci. 13:1077062. doi: 10.3389/fpls.2022.1077062
Diyapoglu, A., Oner, M., and Meng, M. (2022). Application potential of bacterial volatile organic compounds in the control of root-knot nematodes. Molecules 27:4355. doi: 10.3390/molecules27144355
Dobrzyński, J., Jakubowska, Z., Kulkova, I., Kowalczyk, P., and Kramkowski, K. (2023). Biocontrol of fungal phytopathogens by Bacillus pumilus. Front. Microbiol. 14:1194606. doi: 10.3389/fmicb.2023.1194606
Du, J., Gao, Q., Ji, C., Song, X., Liu, Y., Li, H., et al. (2022). Bacillus licheniformis JF-22 to control Meloidogyne incognita and its effect on tomato rhizosphere microbial community. Front. Microbiol. 13:863341. doi: 10.3389/fmicb.2022.863341
Efthimiadou, A., Katsenios, N., Chanioti, S., Giannoglou, M., Djordjevic, N., and Katsaros, G. (2020). Effect of foliar and soil application of plant growth promoting bacteria on growth, physiology, yield and seed quality of maize under Mediterranean conditions. Sci. Rep. 10:21060. doi: 10.1038/s41598-020-78034-6
El Aimani, A., Houari, A., Laasli, S. E., Mentag, R., Iraqi, D., Diria, G., et al. (2022). Antagonistic potential of Moroccan entomopathogenic nematodes against root-knot nematodes, Meloidogyne javanica on tomato under greenhouse conditions. Sci. Rep. 12:2915. doi: 10.1038/s41598-022-07039-0
Elango, K., Sobhana, E., Sujithra, P., Bharath, D., and Ahuja, A. (2020). Traditional agricultural practices as a tool for management of insects and nematode pests of crops: an overview. J. Entomol. Zool. Stud. 8, 237–245.
El-Saadony, M. T., Abuljadayel, D. A., Shafi, M. E., Albaqami, N. M., Desoky, E. S. M., El-Tahan, A. M., et al. (2021). Control of foliar phytoparasitic nematodes through sustainable natural materials: current progress and challenges. Saudi J. Biol. Sci. 28, 7314–7326. doi: 10.1016/j.sjbs.2021.08.035
El-Sawy, S., El-Nagdi, W., Mohamed, S., Khalil, B., and Soliman, G. (2023). The efficiency of biofertilizer and bio-control on root-knot nematode, using bacterial strains, and its effect on tomato plant protein patterns, and improving yield under field conditions. Res. Sq., 2–37. doi: 10.21203/rs.3.rs-3475183/v1
Engelbrecht, G., Claassens, S., Mienie, C. M., and Fourie, H. (2022). Filtrates of mixed Bacillus spp inhibit second-stage juvenile motility of root-knot nematodes. Rhizosphere 22:100528. doi: 10.1016/j.rhisph.2022.100528
Engelbrecht, G., van Rensburg, P. J. J., Fourie, H., and Claassens, S. (2020). In vitro bioassays to determine the effect of Bacillus soli filtrates on the paralysis of Meloidogyne incognita second-stage juveniles. Nematology 22, 239–243. doi: 10.1163/15685411-00003345
Esitken, A. H. M. E. T., Karlidag, H. Ü. S. E. Y. İ. N., Ercisli, S. E. Z. A. İ., and Sahin, F. İ. K. R. E. T. T. İ. N. (2002). Effects of foliar application of Bacillus subtilis Osu-142 on the yield, growth and control of shot-hole disease (Coryneum blight) of apricot. Gartenbauwissenschaft 67, 139–142.
Etesami, H., Jeong, B. R., and Glick, B. R. (2023). Biocontrol of plant diseases by Bacillus Spp. Physiol. Mol. Plant Pathol. 126:102048. doi: 10.1016/j.pmpp.2023.102048
Fabiyi, O. A. (2024). “Application of Bacillus species in the Management of Meloidogyne incognita” in Sustainable Management of Nematodes in agriculture, role of microbes-assisted strategies, vol. 19 (Cham: Springer International Publishing), 249–264.
Fallahzadeh-Mamaghani, V., Shahbazi-Ezmareh, R., Shirzad, A., and Moslehi, S. (2023). Possible mechanisms of action of Bacillus wiedmannii AzBw1, a biocontrol agent of the root-knot nematode, Meloidogyne arenaria. Egypt. J. Biol. Pest Control 33:28. doi: 10.1186/s41938-023-00668-1
Forghani, F., and Hajihassani, A. (2020). Recent advances in the development of environmentally benign treatments to control root-knot nematodes. Front. Plant Sci. 11:1125. doi: 10.3389/fpls.2020.01125
Fu, H. Z., Marian, M., Enomoto, T., Hieno, A., Ina, H., Suga, H., et al. (2020). Biocontrol of tomato bacterial wilt by foliar spray application of a novel strain of endophytic Bacillus sp. Microbes Environ. 35:p.ME20078. doi: 10.1264/jsme2.ME20078
Galbieri, R., Oliveira, J. A. D., Negri, B. F., Boldt, A. S., Rizzi, U. D. S., and Belot, J. L. (2023). Bacillus subtilis as growth-promoting rhizobacteria co-inoculated on Bradyrhizobium-treated soybean seeds in the planting furrow. Rev. Ceres 70:e70601. doi: 10.1590/0034-737X202370060001
Gamalero, E., and Glick, B. R. (2020). The use of plant growth-promoting bacteria to prevent nematode damage to plants. Biology 9:381. doi: 10.3390/biology9110381
Gao, A., Zheng, L., Wang, S., Pan, H., and Zhang, H. (2024). Preparation of microcapsules and evaluation of their biocontrol efficacy. J. Biosci. Bioeng. 138, 328–337. doi: 10.1016/j.jbiosc.2024.05.007
Gassmann, A. J., Stock, S. P., Sisterson, M. S., Carrière, Y., and Tabashnik, B. E. (2008). Synergism between entomopathogenic nematodes and Bacillus thuringiensis crops: integrating biological control and resistance management. J. Appl. Ecol. 45, 957–966. doi: 10.1111/j.1365-2664.2008.01457.x
Gattoni, K. M., Park, S. W., and Lawrence, K. S. (2022). Evaluation of the mechanism of action of bacillus spp. to manage meloidogyne incognita with split root assay, RT-qPCR and qPCR. Front. Plant Sci. 13:1079109. doi: 10.3389/fpls.2022.1079109
Geng, C., Liu, Y., Li, M., Tang, Z., Muhammad, S., Zheng, J., et al. (2017). Dissimilar crystal proteins Cry5Ca1 and Cry5Da1 synergistically act against Meloidogyne incognita and delay Cry5Ba-based nematode resistance. Appl. Environ. Microbiol. 83, e03505–e03516. doi: 10.1128/AEM.03505-16
Ghahremani, Z., Escudero, N., Beltrán-Anadón, D., Saus, E., Cunquero, M., Andilla, J., et al. (2020). Bacillus firmus strain I-1582, a nematode antagonist by itself and through the plant. Front. Plant Sci. 11:796. doi: 10.3389/fpls.2020.00796
Gill, S. S., Cowles, E. A., and Pietrantonio, P. V. (1992). The mode of action of Bacillus thuringiensis endotoxins. Annu. Rev. Entomol. 37, 615–634. doi: 10.1146/annurev.en.37.010192.003151
Gomaa, E. Z. (2021). Microbial chitinases: properties, enhancement and potential applications. Protoplasma 258, 695–710. doi: 10.1007/s00709-021-01612-6
Gotor-Vila, A., Usall, J., Torres, R., Solsona, C., and Teixidó, N. (2019). Enhanced shelf-life of the formulated biocontrol agent Bacillus amyloliquefaciens CPA-8 combining diverse packaging strategies and storage conditions. Int. J. Food Microbiol. 290, 205–213. doi: 10.1016/j.ijfoodmicro.2018.10.013
Grage, K., McDermott, P., and Rehm, B. H. (2017). Engineering Bacillus megaterium for production of functional intracellular materials. Microb. Cell Factories 16, 211–212. doi: 10.1186/s12934-017-0823-5
Griffitts, J. S., Whitacre, J. L., Stevens, D. E., and Aroian, R. V. (2005). Bt toxin resistance from loss of a putative carbohydrate-modifying enzyme. Science 293, 860–864. doi: 10.1126/science.1062441
Grubišić, D., Uroić, G., Ivošević, A., and Grdiša, M. (2018). Nematode control by the use of antagonistic plants. Agric. Conspec. Sci. 83, 269–275. Available at: https://hrcak.srce.hr/207925
Guo, S., Liu, M., Peng, D., Ji, S., Wang, P., Yu, Z., et al. (2008). New strategy for isolating novel nematicidal crystal protein genes from Bacillus thuringiensis strain YBT-1518. Appl. Environ. Microbiol. 74, 6997–7001. doi: 10.1128/AEM.01346-08
Guo, Y., Weng, M., Sun, Y., Carballar-Lejarazú, R., Wu, S., and Lian, C. (2022). Bacillus thuringiensis toxins with nematocidal activity against the pinewood nematode Bursaphelenchus xylophilus. J. Invertebr. Pathol. 189:107726. doi: 10.1016/j.jip.2022.107726
Gupta, R., Mfarrej, M., Elnour, R., Hashem, M., and Ahmad, F. (2023). Defence response of host plants for cyst nematode: a review on parasitism and defence. Science 35:102829:102829. doi: 10.1016/j.jksus.2023.102829
Gurikar, C., Gowda, N. N., Hanumantharaju, K. N., and Netravati, B. P. (2022). “Role of Bacillus species in soil fertility with reference to rhizosphere engineering” in Rhizosphere engineering (Amsterdam, Netherlands: Elsevier), 65–76.
Habazar, T., Yanti, Y., Dani, M. R., and Monica, D. (2021). “Biocontrol of Meloidogyne sp. on tomato plants by selected Bacillus spp” in IOP Conference Series: Earth and Environmental Science (Bristol, United Kingdom: IOP Publishing). 757:012019.
Hamze, R., and Ruiu, L. (2022). Brevibacillus laterosporus as a natural biological control agent of soil-dwelling nematodes. Agronomy 12:2686. doi: 10.3390/agronomy12112686
Hartz, P., Gehl, M., König, L., Bernhardt, R., and Hannemann, F. (2021). Development and application of a highly efficient CRISPR-Cas9 system for genome engineering in Bacillus megaterium. J. Biotechnol. 329, 170–179. doi: 10.1016/j.jbiotec.2021.02.006
Hayat, H. S., Rehman, A. U., Farooq, S., Naveed, M., Ali, H. M., and Hussain, M. (2023). Boron seed coating combined with seed inoculation with boron tolerant bacteria (Bacillus sp. MN-54) and maize stalk biochar improved growth and productivity of maize (Zea mays L.) on saline soil. Heliyon 9:e22075. doi: 10.1016/j.heliyon.2023.e22075
He, Y., Wang, R., Zhao, H., Ren, Y., Agarwal, M., Zheng, D., et al. (2022). Predicting potential global distribution and risk regions for potato cyst nematodes (Globodera rostochiensis and Globodera pallida). Sci. Rep. 12:21843. doi: 10.1038/s41598-022-26443-0
Heerklotz, H., and Seelig, J. (2007). Leakage and lysis of lipid membranes induced by the lipopeptide surfactin. Eur. Biophys. J. 36, 305–314. doi: 10.1007/s00249-006-0091-5
Henry, G., Deleu, M., Jourdan, E., Thonart, P., and Ongena, M. (2011). The bacterial lipopeptide surfactin targets the lipid fraction of the plant plasma membrane to trigger immune-related responses. Cell. Microbiol. 13, 1824–1837. doi: 10.1111/j.1462-5822.2011.01664.x
Hezakiel, H. E., Thampi, M., Rebello, S., and Sheikhmoideen, J. M. (2024). Biopesticides: a green approach towards agricultural pests. Appl. Biochem. Biotechnol. 196, 5533–5562. doi: 10.1007/s12010-023-04765-7
Hossain, M. A., Hossain, M. S., and Akter, M. (2023). Challenges faced by plant growth-promoting bacteria in field-level applications and suggestions to overcome the barriers. Physiol. Mol. Plant Pathol. 126:102029. doi: 10.1016/j.pmpp.2023.102029
Hsiao, C. Y., Blanco, S. D., Peng, A. L., Fu, J. Y., Chen, B. W., Luo, M. C., et al. (2023). Seed treatment with calcium carbonate containing Bacillus amyloliquefaciens PMB05 powder is an efficient way to control black rot disease of cabbage. Agriculture 13:926. doi: 10.3390/agriculture13050926
Hu, H. J., Chen, Y. L., Wang, Y. F., Tang, Y. Y., Chen, S. L., and Yan, S. Z. (2017). Endophytic Bacillus cereus effectively controls Meloidogyne incognita on tomato plants through rapid rhizosphere occupation and repellent action. Plant Dis. 101, 448–455. doi: 10.1094/PDIS-06-16-0871-RE
Hu, H., Gao, Y., Li, X., Chen, S., Yan, S., and Tian, X. (2020). Identification and nematicidal characterization of proteases secreted by endophytic bacteria Bacillus cereus BCM2. Phytopathology 110, 336–344. doi: 10.1094/PHYTO-05-19-0164-R
Hu, L. B., Shi, Z. Q., Zhang, T., and Yang, Z. M. (2007). Fengycin antibiotics isolated from B-FS01 culture inhibit the growth of fusarium moniliforme Sheldon ATCC 38932. FEMS Microbiol. Lett. 272, 91–98. doi: 10.1111/j.1574-6968.2007.00743.x
Hu, Y., You, J., Wang, Y., Long, Y., Wang, S., Pan, F., et al. (2022). Biocontrol efficacy of Bacillus velezensis strain YS-AT-DS1 against the root-knot nematode Meloidogyne incognita in tomato plants. Front. Microbiol. 13:1035748. doi: 10.3389/fmicb.2022.1035748
Huang, M., Bulut, A., Shrestha, B., Matera, C., Grundler, F. M., and Schleker, A. S. S. (2021). Bacillus firmus I-1582 promotes plant growth and impairs infection and development of the cyst nematode Heterodera schachtii over two generations. Sci. Rep. 11:14114. doi: 10.1038/s41598-021-93567-0
Huang, X. W., Niu, Q. H., Zhou, W., and Zhang, K. Q. (2005). Bacillus nematocida sp. nov., a novel bacterial strain with nematotoxic activity isolated from soil in Yunnan, China. Syst. Appl. Microbiol. 28, 323–327. doi: 10.1016/j.syapm.2005.01.008
Huang, X., Wei, Z., Zhao, G., Gao, X., Yang, S., and Cui, Y. (2008). Optimization of sterilization of Escherichia coli in milk by surfactin and fengycin using a response surface method. Curr. Microbiol. 56, 376–381. doi: 10.1007/s00284-007-9066-8
Huang, Y., Xu, C., Ma, L., Zhang, K., Duan, C., and Mo, M. (2010). Characterisation of volatiles produced from Bacillus megaterium YFM3. 25 and their nematicidal activity against Meloidogyne incognita. Eur. J. Plant Pathol. 126, 417–422. doi: 10.1007/s10658-009-9550-z
Hui, F., Scheib, U., Hu, Y., Sommer, R. J., Aroian, R. V., and Ghosh, P. (2012). Structure and glycolipid binding properties of the nematicidal protein Cry5B. Biochemistry 51, 9911–9921. doi: 10.1021/bi301386q
Iftikhar, Y., Sajid, A., Shakeel, Q., Ahmad, Z., and Ul Haq, Z. (2020). “Biological antagonism: a safe and sustainable way to manage plant diseases” in Plant disease management strategies for sustainable agriculture through traditional and modern approaches: sustainability in plant and crop protection. eds. I. Ul Haq and S. Ijaz (Cham: Springer).
Jaiswal, D. K., Gawande, S. J., Soumia, P. S., Krishna, R., Vaishnav, A., and Ade, A. B. (2022). Biocontrol strategies: an eco-smart tool for integrated pest and diseases management. BMC Microbiol. 22:324. doi: 10.1186/s12866-022-02744-2
Jamal, Q., Cho, J. Y., Moon, J. H., Munir, S., Anees, M., and Kim, K. Y. (2017). Identification for the first time of Cyclo (d-pro-l-Leu) produced by Bacillus amyloliquefaciens Y1 as a Nematocide for control of Meloidogyne incognita. Molecules 22:1839. doi: 10.3390/molecules22111839
Jang, S., Choi, S. K., Zhang, H., Zhang, S., Ryu, C. M., and Kloepper, J. W. (2023). History of a model plant growth-promoting rhizobacterium, Bacillus velezensis GB03: from isolation to commercialization. Front. Plant Sci. 14:1279896. doi: 10.3389/fpls.2023.1279896
Jeong, M. H., Yang, S. Y., Lee, Y. S., Ahn, Y. S., Park, Y. S., Han, H. R., et al. (2015). Selection and characterization of Bacillus licheniformis MH48 for the biocontrol of pine wood nematode (Bursaphelenchus xylophilus). J. Korean Soc. Forest Sci. 104, 512–518. doi: 10.14578/jkfs.2015.104.3.512
Jiang, H., Tian, L., Bu, F., Sun, Q., Zhao, X., and Han, Y. (2021). RNA-seq-based identification of potential resistance genes against the soybean cyst nematode (Heterodera glycines) HG type 1.2.3.5.7 in ‘Dongnong L-10’. Physiol. Mol. Plant Pathol. 114:101627. doi: 10.1016/j.pmpp.2021.101627
Jouzani, G. S., Valijanian, E., and Sharafi, R. (2017). Bacillus thuringiensis: a successful insecticide with new environmental features and tidings. Appl. Microbiol. Biotechnol. 101, 2691–2711. doi: 10.1007/s00253-017-8175-y
Jung, W. J., Jung, S. J., An, K. N., Jin, Y. L., Park, R. D., Kim, K. Y., et al. (2002). Effect of chitinase-producing Paenibacillus illinoisensis KJA-424 on egg hatching of root-knot nematode (Meloidogyne incognita). J. Microbiol. Biotechnol. 12, 865–871. Available at: https://koreascience.kr/ksci/search/article/articleView.ksci?articleBean.atclMgntNo=E1MBA4_2002_v12n6_865
Kahn, T. W., Duck, N. B., McCarville, M. T., Schouten, L. C., Schweri, K., Zaitseva, J., et al. (2021). A Bacillus thuringiensis cry protein controls soybean cyst nematode in transgenic soybean plants. Nat. Commun. 12:3380. doi: 10.1038/s41467-021-23743-3
Kamalanathan, V., Sevugapperumal, N., and Nallusamy, S. (2023). Antagonistic bacteria Bacillus velezensis VB7 possess nematicidal action and induce an immune response to suppress the infection of root-knot nematode (RKN) in tomato. Genes 14:1335. doi: 10.3390/genes14071335
Kang, W. S., Chen, L. J., Wang, Y. Y., Zhu, X. F., Liu, X. Y., Fan, H. Y., et al. (2020). Bacillus simplex treatment promotes soybean defence against soybean cyst nematodes: a metabolomics study using GC-MS. PLoS One 15:e0237194. doi: 10.1371/journal.pone.0237194
Karačić, V., Miljaković, D., Marinković, J., Ignjatov, M., Milošević, D., Tamindžić, G., et al. (2024). Bacillus species: excellent biocontrol agents against tomato diseases. Microorganisms 12:457. doi: 10.3390/microorganisms12030457
Khan, A., Chen, S., Fatima, S., Ahamad, L., and Siddiqui, M. A. (2023). Biotechnological tools to elucidate the mechanism of plant and nematode interactions. Plan. Theory 12:2387. doi: 10.3390/plants12122387
Khan, A. R., Mustafa, A., Hyder, S., Valipour, M., Rizvi, Z. F., Gondal, A. S., et al. (2022). Bacillus spp. as bioagents: uses and application for sustainable agriculture. Biology 11:1763. doi: 10.3390/biology11121763
Khanh, T. L. V. (2020). Selection of Bacillus thuringiensis against pathogenic nematodes attacking pepper tree. Biotechnology 36, 57–62. doi: 10.21519/0234-2758-2020-36-3-57-62
Khullar, G., Karami, Z., and Prakitchaiwattana, C. (2024). Development of microencapsulated dried Bacillus sp. 63‐11 with enhanced shelf stability and bioactivity for use as a food supplement. Int. J. Food Sci. Technol. 59, 1291–1298. doi: 10.1111/ijfs.16853
Kloepper, J. W., Ryu, C. M., and Zhang, S. (2004). Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 94, 1259–1266. doi: 10.1094/PHYTO.2004.94.11.1259
Köhl, J., Kolnaar, R., and Ravensberg, W. J. (2019). Mode of action of microbial biological control agents against plant diseases: relevance beyond efficacy. Front. Plant Sci. 10:845. doi: 10.3389/fpls.2019.00845
Kulkova, I., Dobrzyński, J., Kowalczyk, P., Bełżecki, G., and Kramkowski, K. (2023). Plant growth promotion using Bacillus cereus. Int. J. Mol. Sci. 24:9759. doi: 10.3390/ijms24119759
Kumar, A., Kakrana, A., Sirohi, A., Subramaniam, K., Srinivasan, R., Abdin, M. Z., et al. (2017). Host-delivered RNAi-mediated root-knot nematode resistance in Arabidopsis by targeting splicing factor and integrase genes. J. Gen. Plant Pathol. 83, 91–97. doi: 10.1007/s10327-017-0701-3
Kumar, P., Pandhi, S., Mahato, D. K., Kamle, M., and Mishra, A. (2021). Bacillus-based nano-bioformulations for phytopathogens and insect–pest management. Egypt. J. Biol. Pest Control 31, 1–128. doi: 10.1186/s41938-021-00475-6
Lee, Y. S., Cho, J. Y., Moon, J. H., and Kim, K. Y. (2016). Identification of 2-methylbutyric acid as a Nematicidal metabolite, and biocontrol and biofertilization potentials of Bacillus pumilus L1. Korean J. Soil Sci. Fertil. 49, 401–408. doi: 10.7745/KJSSF.2016.49.4.401
Li, Q., Liu, S., Li, Y., Hao, T., and Chen, S. (2022). Nitrogen fixation by Paenibacillus polymyxa WLY78 is responsible for cucumber growth promotion. Plant Soil 473, 507–516. doi: 10.1007/s11104-022-05307-6
Li, L., Sun, Y., Chen, F., Hao, D., and Tan, J. (2023). An alkaline protease from Bacillus cereus NJSZ-13 can act as a pathogenicity factor in infection of pinewood nematode. BMC Microbiol. 23:10. doi: 10.1186/s12866-022-02752-2
Li, L., Tan, J., and Chen, F. (2018). Bacillus pumilus strain LYMC-3 shows nematicidal activity against Bursaphelenchus xylophilus via the production of a guanidine compound. Biocontrol Sci. Tech. 28, 1128–1139. doi: 10.1080/09583157.2018.1514587
Lilley, C. J., Kyndt, T., and Gheysen, G. (2011). “Nematode resistant GM crops in industrialised and developing countries” in Genomics and molecular genetics of plant-nematode interactions (Dordrecht: Springer), 17–541.
Lin, L. Z., Zheng, Q. W., Wei, T., Zhang, Z. Q., Zhao, C. F., Zhong, H., et al. (2020). Isolation and characterization of fengycins produced by Bacillus amyloliquefaciens JFL21 and its broad-spectrum antimicrobial potential against multidrug-resistant foodborne pathogens. Front. Microbiol. 11:579621. doi: 10.3389/fmicb.2020.579621
Liu, Z., Budiharjo, A., Wang, P., Shi, H., Fang, J., Borriss, R., et al. (2013). The highly modified microcin peptide plantazolicin is associated with nematicidal activity of Bacillus amyloliquefaciens FZB42. Appl. Microbiol. Biotechnol. 97, 10081–10090. doi: 10.1007/s00253-013-5247-5
Liu, G., Lin, X., Xu, S., Liu, G., Liu, F., and Mu, W. (2020). Screening, identification and application of soil bacteria with nematicidal activity against root‐knot nematode (Meloidogyne incognita) on tomato. Pest Manag. Sci. 76, 2217–2224. doi: 10.1002/ps.5759
Luo, L., Zhao, C., Wang, E., Raza, A., and Yin, C. (2022). Bacillus amyloliquefaciens as an excellent agent for biofertilizer and biocontrol in agriculture: an overview for its mechanisms. Microbiol. Res. 259:127016. doi: 10.1016/j.micres.2022.127016
Mahapatra, S., Chakraborty, S., Samanta, M., Das, S., and Islam, T. (2022). “Current understanding and future directions of biocontrol of plant diseases by Bacillus spp., with special reference to induced systemic resistance” in Bacilli in agrobiotechnology: plant stress tolerance, bioremediation, and bioprospecting (Cham: Springer International Publishing), 127–150.
Mahmoud, W. M., Abdelmoneim, T. S., and Elazzazy, A. M. (2016). The impact of silver nanoparticles produced by Bacillus pumilus as antimicrobial and nematicide. Front. Microbiol. 7:1746. doi: 10.3389/fmicb.2016.01746
Manivannan, A., Kumar, K. K., Varanavasiappan, S., Manimegalai, S., Poornima, K., Devrajan, B. C., et al. (2019). Expression, purification and bioassay of Cry55Aa protein against tomato root knot nematode, Meloidogyne incognita. Res. J. Pharmacogn. Phytochem. 8, 570–573. doi: 10.5958/0975-4385.2020.00004.7
Manju, P., and Subramanian, S. (2017). Iturin and Surfactin families of Lipopeptides as key factors in antagonism of Bacillus subtilis towards Meloidogyne incognita on Gerbera jamesonii. Indian J. Nematol. 47, 31–38.
Maqsood, A., Aslam, M. N., Khaliq, H., Shakeel, M. T., Wu, H., and Fahad, S. (2024). Endophytic Bacillus spp. mediated plant growth promotion of tomato seedlings and suppression of Meloidogyne incognita and fusarium oxysporum disease complex. J. Plant Growth Regul. 43, 2454–2469. doi: 10.1007/s00344-024-11279-x
Marin-Bruzos, M., Grayston, S. J., Forge, T., and Nelson, L. M. (2021). Isolation and characterization of streptomycetes and pseudomonad strains with antagonistic activity against the plant parasitic nematode Pratylenchus penetrans and fungi associated with replant disease. Biol. Control 158:104599. doi: 10.1016/j.biocontrol.2021.104599
Mathew, R., and Opperman, C. H. (2019). The genome of the migratory nematode, Radopholus similis, reveals signatures of close association to the sedentary cyst nematodes. PLoS One 14:e0224391. doi: 10.1371/journal.pone.0224391
Mawarda, P. C., Mallon, C. A., Le Roux, X., Van Elsas, J. D., and Salles, J. F. (2022). Interactions between bacterial inoculants and native soil bacterial community: the case of spore-forming Bacillus spp. FEMS Microbiol. Ecol. 98:fiac127. doi: 10.1093/femsec/fiac127
Mazzuchelli, R. D. C. L., Mazzuchelli, E. H. L., and de Araujo, F. F. (2020). Efficiency of Bacillus subtilis for root-knot and lesion nematodes management in sugarcane. Biol. Control 143:104185. doi: 10.1016/j.biocontrol.2020.104185
Mesa-Valle, C. M., Garrido-Cardenas, J. A., Cebrian-Carmona, J., Talavera, M., and Manzano-Agugliaro, F. (2020). Global research on plant nematodes. Agronomy 10:1148. doi: 10.3390/agronomy10081148
Messa, V., Nunes, J., and Mattei, D. (2019). Seed treatment with Bacillus amyloliquefaciens for the control of Meloidogyne javanica" in vivo" bean culture and its direct effect on the motility, mortality and hatching of M. javanica "in vitro". Agron. Sci. Biotechnol. 5:59. doi: 10.33158/ASB.2019v5i2p59
Mian, S., Machado, A. C. Z., Hoshino, R. T., Mosela, M., Higashi, A. Y., Shimizu, G. D., et al. (2024). Complete genome sequence of Bacillus velezensis strain Ag109, a biocontrol agent against plant-parasitic nematodes and Sclerotinia sclerotiorum. BMC Microbiol. 24:194. doi: 10.1186/s12866-024-03282-9
Migunova, V. D., and Sasanelli, N. (2021). Bacteria as biocontrol tool against phytoparasitic nematodes. Plan. Theory 10:389. doi: 10.3390/plants10020389
Migunova, V. D., Tomashevich, N. S., Konrat, A. N., Lychagina, S. V., Dubyaga, V. M., D’Addabbo, T., et al. (2021). Selection of bacterial strains for control of root-knot disease caused by Meloidogyne incognita. Microorganisms 9:1698. doi: 10.3390/microorganisms9081698
Miljaković, D., Marinković, J., and Balešević-Tubić, S. (2020). The significance of Bacillus spp. in disease suppression and growth promotion of field and vegetable crops. Microorganisms 8:1037. doi: 10.3390/microorganisms8071037
Moens, M., Perry, R. N., and Jones, J. T. (2018). “Cyst nematodes - life cycle and economic importance” in Cyst nematodes (Wallingford: CABI), 1–26.
Mohamed, S. A., El-Sayed, G. M., Elkelany, U. S., Youssef, M. M., El-Nagdi, W. M., and Soliman, G. M. (2021). A local Bacillus spp.: isolation, genetic improvement, nematode biocontrol, and nitrogen fixation. Egyptian. Pharm. J. 20, 352–363. doi: 10.4103/epj.epj_30_21
Montesinos, E. (2003). Development, registration and commercialization of microbial pesticides for plant protection. Int. Microbiol. 6, 245–252. doi: 10.1007/s10123-003-0144-x
Moslehi, S., Pourmehr, S., Shirzad, A., and Khakvar, R. (2021). Potential of some endophytic bacteria in biological control of root-knot nematode Meloidogyne incognita. Egypt. J. Biol. Pest Control 31, 1–11. doi: 10.1186/s41938-021-00396-4
Mostafa, F. A., Khalil, A. E., Nour, A., and Ibrahim, D. S. (2018). The role of Bacillus megaterium and other bio-agents in controlling root-knot nematodes infecting sugar beet under field conditions. Egypt. J. Biol. Pest Control 28, 1–6. doi: 10.1186/s41938-018-0068-6
Nadeem, H., Niazi, P., Asif, M., Kaskavalci, G., and Ahmad, F. (2021). Bacterial strains integrated with surfactin molecules of Bacillus subtilis MTCC441 enrich nematocidal activity against Meloidogyne incognita. Plant Biol. 23, 1027–1036. doi: 10.1111/plb.13301
Ngalimat, M. S., Yahaya, R. S. R., Baharudin, M. M. A. A., Yaminudin, S. M., Karim, M., Ahmad, S. A., et al. (2021). A review on the biotechnological applications of the operational group Bacillus amyloliquefaciens. Microorganisms 9:614. doi: 10.3390/microorganisms9030614
Nguyen, V. N., Ju, W. T., Kim, Y. J., Jung, W. J., Kim, K. Y., and Park, R. D. (2014). Suppression of cucumber root-knot nematode Meloidogyne incognita by chitinolytic fungi Lecanicillium pasalliotae A-1 and Lecanicillium antillanum B-3. J. Chitin Chitos. 19, 93–99.
Nguyen, D. M. C., and Jung, W. J. (2014). Nematicidal properties of crude extracts obtained from medicinal plants against root-lesion nematode Pratylenchus coffeae. J. Viet. Environ. 6, 264–269. doi: 10.13141/jve.vol6.no3.pp264-269
Nguyen, X. H., Naing, K. W., Lee, Y. S., Jung, W. J., Anees, M., and Kim, K. Y. (2013). Antagonistic potential of Paenibacillus elgii HOA73 against the root-knot nematode, Meloidogyne incognita. Nematology 15, 991–1000. doi: 10.1163/15685411-00002737
Nguyen, D. M. C., Seo, D. J., Kim, K. Y., Kim, T. H., and Jung, W. J. (2012). Nematode-antagonistic effects of Cinnamomum aromaticum extracts and a purified compound against Meloidogyne incognita. Nematology 14, 913–924. doi: 10.1163/156854112X634987
Nguyen, V. N., Seo, D. J., Park, R. D., and Jung, W. J. (2009). Nematicidal activity of compounds extracted from medicinal plants against the pine wood nematode Bursaphelenchus xylophilus. Nematology 11, 835–845. doi: 10.1163/156854109X424353
Nguyen, D. M. C., Seo, D. J., Park, R. D., Lee, B. R., and Jung, W. J. (2011). Changes in antioxidative enzyme activities in cucumber plants with regard to biological control of root-knot nematode, Meloidogyne incognita, with Cinnamomum cassia crude extracts. J. Korean Soc. Appl. Biol. Chem. 54, 507–514. doi: 10.3839/jksabc.2011.078
Nicol, J. M., Turner, S. J., Coyne, D. L., Nijs, L. D., Hockland, S., and Maafi, Z. T. (2011). “Current nematode threats to world agriculture” in Genomics and molecular genetics of plant-nematode interactions (Dordrecht: Springer), 21–43.
Niu, Q., Huang, X., Zhang, L., Li, Y., Li, J., Yang, J., et al. (2006). A neutral protease from Bacillus nematocida, another potential virulence factor in the infection against nematodes. Arch. Microbiol. 185, 439–448. doi: 10.1007/s00203-006-0112-x
Niu, Q., Tian, Y., Zhang, L., Xu, X. E., Niu, X., Xia, Z., et al. (2011). Overexpression of the key virulence proteases Bace16 and Bae16 in Bacillus nematocida B16 to improve its nematocidal activity. J. Mol. Microbiol. Biotechnol. 21, 130–137. doi: 10.1159/000332805
Niu, Q., Zhang, L., Zhang, K., Huang, X., Hui, F., Kan, Y., et al. (2016). Changes in intestinal microflora of Caenorhabditis elegans following Bacillus nematocida B16 infection. Sci. Rep. 6:20178. doi: 10.1038/srep20178
O’Callaghan, M. (2016). Microbial inoculation of seed for improved crop performance: issues and opportunities. Appl. Microbiol. Biotechnol. 100, 5729–5746. doi: 10.1007/s00253-016-7590-9
Oh, I. J., Ju, W. T., Kim, Y. J., Jung, W. J., Kim, K. Y., and Park, R. D. (2014a). Nematicidal activity of Auxarthron reticulatum DY-2 against the pine wood nematode Bursaphelenchus mucronatus. Nematology 16, 427–436. doi: 10.1163/15685411-00002775
Oh, I. J., Kim, Y. J., and Kim, K. Y. (2014b). Nematicidal activity of Verticillium saksenae A-1 against the pine wood nematode Bursaphelenchus mucronatus. J. Chitin Chitos. 19, 81–86.
Oka, Y. (2010). Mechanisms of nematode suppression by organic soil amendments—a review. Appl. Soil Ecol. 44, 101–115. doi: 10.1016/j.apsoil.2009.11.003
Olagoke, F. K., Bettermann, A., Nguyen, P. T. B., Redmile-Gordon, M., Babin, D., Smalla, K., et al. (2022). Importance of substrate quality and clay content on microbial extracellular polymeric substances production and aggregate stability in soils. Biol. Fertil. Soils 58, 435–457. doi: 10.1007/s00374-022-01632-1
Oliveira, D. F., Santos, H. M. D., Nunes, A. S., Campos, V. P., Pinho, R. S. D., and Gajo, G. C. (2014). Purification and identification of metabolites produced by Bacillus cereus and B. subtilis active against Meloidogyne exigua, and their in silico interaction with a putative phosphoribosyltransferase from M. incognita. An. Acad. Bras. Cienc. 86, 525–538. doi: 10.1590/0001-3765201402412
Ortiz, A., and Sansinenea, E. (2023). “Microbial-based biopesticides: commercialization and regulatory perspectives” in Development and commercialization of biopesticides (Cambridge, Massachusetts, USA: Academic Press), 103–118.
Osman, H. A., Ameen, H. H., Mohamed, M., and Elkelany, U. S. (2020). Efficacy of integrated microorganisms in controlling root-knot nematode Meloidogyne javanica infecting peanut plants under field conditions. Bull. Natl. Res. Cent. 44, 1–10. doi: 10.1186/s42269-020-00366-0
Pacifico, M. G., Eckstein, B., and Bettiol, W. (2021). Screening of Bacillus for the development of bioprotectants for the control of Fusarium oxysporum f. sp. vasinfectum and Meloidogye incognita. Biol. Control 164:104764. doi: 10.1016/j.biocontrol.2021.104764
Padgham, J. L., and Sikora, R. A. (2007). Biological control potential and modes of action of Bacillus megaterium against Meloidogyne graminicola on rice. Crop Prot. 26, 971–977. doi: 10.1016/j.cropro.2006.09.004
Palomares-Rius, J. E., Clavero-Camacho, I., Archidona-Yuste, A., Cantalapiedra-Navarrete, C., León-Ropero, G., Braun Miyara, S., et al. (2020). Global distribution of the reniform nematode genus Rotylenchulus with the synonymy of Rotylenchulus macrosoma with Rotylenchulus borealis. Plan. Theory 10:7. doi: 10.3390/plants10010007
Pandey, N., Vaishnav, R., Rajavat, A. S., Singh, A. N., Kumar, S., Tripathi, R. M., et al. (2024). Exploring the potential of Bacillus for crop productivity and sustainable solution for combating rice false smut disease. Front. Microbiol. 15:1405090. doi: 10.3389/fmicb.2024.1405090
Paradva, K. C., and Kalla, S. (2023). Nanopesticides: a review on current research and future perspective. Chem. Select 8:e202300756. doi: 10.1002/slct.202300756
Park, M. R., Oh, S., Son, S. J., Park, D. J., Oh, S., Kim, S. H., et al. (2015). Bacillus licheniformis isolated from traditional Korean food resources enhances the longevity of Caenorhabditis elegans through serotonin signaling. J. Agric. Food Chem. 63, 10227–10233. doi: 10.1021/acs.jafc.5b03730
Patil, G. B., Lakhssassi, N., Wan, J., Song, L., Zhou, Z., Klepadlo, M., et al. (2019). Whole‐genome re‐sequencing reveals the impact of the interaction of copy number variants of the rhg1 and Rhg4 genes on broad‐based resistance to soybean cyst nematode. Plant Biotechnol. J. 17, 1595–1611. doi: 10.1111/pbi.13086
Pontes, K. B., Machado, A. C. Z., Nogueira, A. F., Fagundes, D. F. V., de Lima Filho, R. B., Mosela, M., et al. (2024). Efficacy of microbiological nematicides in controlling root-knot nematodes in tomato. Front. Agron. 6:1462323. doi: 10.3389/fagro.2024.1462323
Pradhan, P., Naresh, P., Barik, S., Acharya, G. C., and Bastia, R. (2023). Adamala breeding for root-knot nematode resistance in fruiting Solanaceous vegetable crops: a review. Euphytica 219:71. doi: 10.1007/s10681-023-03204-2
Pueyo, M. T., Bloch, C., Carmona-Ribeiro, A. M., and Di Mascio, P. (2009). Lipopeptides produced by a soil Bacillus megaterium strain. Microb. Ecol. 57, 367–378. doi: 10.1007/s00248-008-9464-x
Rabbee, M. F., Ali, M. S., Choi, J., Hwang, B. S., Jeong, S. C., and Baek, K. H. (2019). Bacillus velezensis: a valuable member of bioactive molecules within plant microbiomes. Molecules 24:1046. doi: 10.3390/molecules24061046
Rabbee, M. F., Hwang, B. S., and Baek, K. H. (2023). Bacillus velezensis: a beneficial biocontrol agent or facultative phytopathogen for sustainable agriculture. Agronomy 13:840. doi: 10.3390/agronomy13030840
Radwan, M. A. (2007). Efficacy of Bacillus thuringiensis integrated with other non-chemical materials to control Meloidogyne incognita in tomato. Nematol. Mediterr. 35, 69–73.
Ramalakshmi, A., Sharmila, R., Iniyakumar, M., and Gomathi, V. (2020). Nematicidal activity of native Bacillus thuringiensis against the root knot nematode, Meloidogyne incognita (Kofoid and white). Egypt. J. Biol. Pest Control 30, 1–9. doi: 10.1186/s41938-020-00293-2
Ramezani Moghaddam, M., Mahdikhani Moghaddam, E., Baghaee Ravari, S., and Rouhani, H. (2014). The first report of Bacillus pumilus influence against Meloidogyne javanica in Iran. J. Crop Protect. 3, 105–112.
Ramírez-Pool, J. A., Calderón-Pérez, B., Ruiz-Medrano, R., Ortiz-Castro, R., and Xoconostle-Cazares, B. (2024). Bacillus strains as effective biocontrol agents against Phytopathogenic Bacteria and promoters of plant growth. Microb. Ecol. 87:76. doi: 10.1007/s00248-024-02384-1
Ramyabharathi, S. A., Meena, K. S., Rajendran, L., Raguchander, T., and Jonathan, E. I. (2020). Potential of a rhizobacterium Bacillus subtilis (Bbv 57) on fusarium oxysporum f. sp. gerberae and Meloidogyne incognita infecting Gerbera grown in protected cultivation. Eur. J. Plant Pathol. 158, 615–632. doi: 10.1007/s10658-020-02087-6
Rao, M. S., Kamalnath, M., Umamaheswari, R., Rajinikanth, R., Prabu, P., Priti, K., et al. (2017). Bacillus subtilis IIHR BS-2 enriched vermicompost controls root knot nematode and soft rot disease complex in carrot. Sci. Hortic. 218, 56–62. doi: 10.1016/j.scienta.2017.01.051
Raymond, B., and Federici, B. A. (2017). In defence of Bacillus thuringiensis, the safest and most successful microbial insecticide available to humanity - a response to EFSA. FEMS Microbiol. Ecol. 93. doi: 10.1093/femsec/fix084
Raza, A., Hassan, A., Akram, W., Anjum, T., and Ali, B. (2024). Seed coating with the synthetic consortium of beneficial Bacillus microbes improves seedling growth and manages fusarium wilt disease. Sci. Hortic. 325:112645. doi: 10.1016/j.scienta.2023.112645
Riascos-Ortiz, D., Mosquera-Espinosa, A. T., Varón de Agudelo, F., Oliveira, C. M. G., and Muñoz Flórez, J. E. (2022). “Non-conventional management of plant-parasitic nematodes in musaceas crops” in Sustainable management of nematodes in agriculture, Vol. 1: organic management (Cham: Springer International Publishing), 381–422.
Riseh, R. S., Vatankhah, M., Hassanisaadi, M., and Barka, E. A. (2024). Unveiling the role of hydrolytic enzymes from soil biocontrol Bacteria in sustainable Phytopathogen management. Front. Biosci. 29:105. doi: 10.31083/j.fbl2903105
Rocha, L. F., and Duggal, P. (2023). “Management of Cyst-Forming Nematodes in agricultural crops through novel biological and genetic engineering technologies” in Novel biological and biotechnological applications in plant nematode management (Singapore: Springer Nature), 313–339.
Rostami, M., Karegar, A., and Taghavi, S. M. (2021). Biocontrol potential of bacterial isolates from vermicompost and earthworm against the root-knot nematode Meloidogyne javanica infecting tomato plants. Egypt. J. Biol. Pest Control 31:36. doi: 10.1186/s41938-021-00383-9
Rostami, M., Shahbazi, S., Soleimani, R., and Ghorbani, A. (2024). Optimizing sustainable control of Meloidogyne javanica in tomato plants through gamma radiation-induced mutants of Trichoderma harzianum and Bacillus velezensis. Sci. Rep. 14:17774. doi: 10.1038/s41598-024-68365-z
Rozas, E. E., Dias, M., Acosta, A. M. L., Custódio, M. R., do, C., and Mendes, M. (2024). Proteomic characterization of metal recovery process realized by marine bacteria bacillus subtilis Hyhel1expossed to bioleaching liquor. Braz. J. Chem. Eng. 41, 865–874. doi: 10.1007/s43153-023-00350-x
Ruiu, L. (2015). Insect pathogenic bacteria in integrated pest management. Insects 6, 352–367. doi: 10.3390/insects6020352
Ryu, C. M., Shin, J. N., Qi, W., Ruhong, M., Kim, E. J., and Pan, J. G. (2011). Potential for augmentation of fruit quality by foliar application of bacilli spores on apple tree. Plant Pathol. J. 27, 164–169. doi: 10.5423/PPJ.2011.27.2.164
Saeid, A., Prochownik, E., and Dobrowolska-Iwanek, J. (2018). Phosphorus solubilization by Bacillus species. Molecules 23:2897. doi: 10.3390/molecules23112897
Saikai, K., and MacGuidwin, A. E. (2022). Impact of Pratylenchus penetrans on soybean grown in Wisconsin, USA. Plant Dis. 106, 2904–2910. doi: 10.1094/PDIS-09-21-1888-RE
Samal, I., Bhoi, T. K., Mahanta, D. K., Komal, J., and Singh, S. (2024). Chapter 3 biorational pest management: potentials, unintended consequences, and future concerns. In: R. Kumar, M. Oliveirade, E. Aguiar Andradede, D. Suyal, and R. Soni, eds. Biorationals and biopesticides: pest management, Berlin, Boston: De Gruyter 47–76.
Sanahuja, G., Banakar, R., Twyman, R. M., Capell, T., and Christou, P. (2011). Bacillus thuringiensis: a century of research, development and commercial applications. Plant Biotechnol. J. 9, 283–300. doi: 10.1111/j.1467-7652.2011.00595.x
Santos, J., Silva, A., Queiroz, P., Eckstein, B., and Monnerat, R. (2022). Selection of Bacillus thuringiensis strains toxic to Meloidogyne incognita. Anais Escol. Agron. Veter. 52:e73070. doi: 10.1590/1983-40632022v5273070
Saxena, A. K., Kumar, M., Chakdar, H., Anuroopa, N., and Bagyaraj, D. J. (2020). Bacillus species in soil as a natural resource for plant health and nutrition. J. Appl. Microbiol. 128, 1583–1594. doi: 10.1111/jam.14506
Schnepf, E., Crickmore, N., Van Rie, J., Lereclus, D., Baum, J., Feitelson, J., et al. (1998). Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 62, 775–806. doi: 10.1128/mmbr.62.3.775-806.1998
Seo, D. J., Nguyen, V. N., Kim, K. Y., Park, R. D., and Jung, W. J. (2013). Nematicidal activity of gallic acid purified from Terminalia nigrovenulosa bark against the root-knot nematode Meloidogyne incognita. Nematology 15, 507–518. doi: 10.1163/15685411-00002696
Serrão, C. P., Ortega, J. C. G., Rodrigues, P. C., and de Souza, C. R. B. (2024). Bacillus species as tools for biocontrol of plant diseases: a meta-analysis of twenty-two years of research, 2000–2021. World J. Microbiol. Biotechnol. 40:110. doi: 10.1007/s11274-024-03935-x
Settu, V., Annaiyan, S., and Mannu, J. (2024). Revealing the genetic arsenal of Bacillus firmus TNAU1: unleashing nematicidal and plant growth promotion traits. Physiol. Mol. Plant Pathol. 129:102177. doi: 10.1016/j.pmpp.2023.102177
Shafi, J., Tian, H., and Ji, M. (2017). Bacillus species as versatile weapons for plant pathogens: a review. Biotechnol. Biotechnol. Equip. 31, 446–459. doi: 10.1080/13102818.2017.1286950
Shi, J., Peng, D., Zhang, F., Ruan, L., and Sun, M. (2020). The Caenorhabditis elegans CUB-like-domain containing protein RBT-1 functions as a receptor for Bacillus thuringiensis Cry6Aa toxin. PLoS Pathog. 16:e1008501. doi: 10.1371/journal.ppat.1008501
Shu, J., Zhang, R. J., Liang, Y. C., Chen, Y. Q., Zhang, J., Guo, J., et al. (2021). Control of root-knot nematode disease by compounding biological agents from plant and microorganisms. Biotechnol. Bull. 37, 164–174. doi: 10.13560/j.cnki.biotech.bull.1985.2021-0408
Sikora, R. A., and Roberts, P. A. (2018). “Management practices: an overview of integrated nematode management technologies,” Plant Parasit. Nemat. Subtrop. Trop. Agric. eds. R. A. Sikora, D. Coyne, J. Hallmann, and P. Timper (Wallingford, UK: CABI), 2nd Edition. 795–838.
Singh, S., Balodi, R., Meena, P. N., and Singhal, S. (2021). Biocontrol activity of Trichoderma harzianum, Bacillus subtilis and Pseudomonas fluorescens against Meloidogyne incognita, fusarium oxysporum and Rhizoctonia solani. Indian Phytopathol. 74, 703–714. doi: 10.1007/s42360-021-00368-6
Singh, B. K., Delgado-Baquerizo, M., Egidi, E., Guirado, E., Leach, J. E., Liu, H., et al. (2023). Climate change impacts on plant pathogens, food security and paths forward. Nat. Rev. Microbiol. 21, 640–656. doi: 10.1038/s41579-023-00900-7
Singh, A., Sharma, P., Kumari, A., Kumar, R., and Pathak, D. V. (2019). “Management of Root-Knot Nematode in different crops using microorganisms” in Plant biotic interactions. eds. A. Varma, S. Tripathi, and R. Prasad (Cham: Springer), 85–99.
Sohrabi, F., Sheikholeslami, M., Heydari, R., Rezaee, S., and Sharifi, R. (2020). Investigating the effect of Glomus mosseae, Bacillus subtilis and Trichoderma harzianum on plant growth and controlling Meloidogyne javanica in tomato. Indian Phytopathol. 73, 293–300. doi: 10.1007/s42360-020-00227-w
Steinberg, N., Keren-Paz, A., Hou, Q., Doron, S., Yanuka-Golub, K., Olender, T., et al. (2020). The extracellular matrix protein TasA is a developmental cue that maintains a motile subpopulation within Bacillus subtilis biofilms. Sci. Signal. 13:eaaw8905. doi: 10.1126/scisignal.aaw8905
Stoica, R. M., Moscovici, M. I. Ș. U., Tomulescu, C. A. T. E. R. I. N. A., Cășărică, A. N. G. E. L. A., Băbeanu, N. A. R. C. I. S. A., Popa, O. V. I. D. I. U., et al. (2019). Antimicrobial compounds of the genus Bacillus: a review. Rom. Biotechnol. Lett. 24, 1111–1119. doi: 10.25083/rbl/24.6/1111.1119
Sturhan, D. I. E. T. E. R., and Brzeski, M. W. (2020). “Stem and bulb nematodes, Ditylenchus spp” in Manual of agricultural nematology (Boca Raton, Florida, USA: CRC Press), 423–464.
Subbotin, S. A., Rius, J. E. P., and Castillo, P. (2021). Systematics of root-knot nematodes (Nematoda: Meloidogynidae) : Brill Available at: https://Iccn.loc.gov/2021030916.
Sun, M., Liang, C., Fu, X., Liu, G., Zhong, Y., Wang, T., et al. (2024). Nematocidal activity and biocontrol efficacy of endophytic Bacillus velezensis Pt-RP9 from Pinus tabuliformis against pine wilt disease caused by Bursaphelenchus xylophilus. Biol. Control 196:105579. doi: 10.1016/j.biocontrol.2024.105579
Sun, X. L., Yang, Y. H., Zhu, L., Liu, F. Y., Xu, J. P., Huang, X. W., et al. (2018). The lysine acetylome of the nematocidal bacterium Bacillus nematocida and impact of nematode on the acetylome. J. Proteome 177, 31–39. doi: 10.1016/j.jprot.2018.02.005
Tian, B., Yang, J., and Zhang, K. Q. (2007). Bacteria used in the biological control of plant-parasitic nematodes: populations, mechanisms of action, and future prospects. FEMS Microbiol. Ecol. 61, 197–213. doi: 10.1111/j.1574-6941.2007.00349.x
Tian, X. L., Zhao, X. M., Zhao, S. Y., Zhao, J. L., and Mao, Z. C. (2022). The biocontrol functions of Bacillus velezensis strain Bv-25 against Meloidogyne incognita. Front. Microbiol. 13:843041. doi: 10.3389/fmicb.2022.843041
Tong-Jian, X. I. A. O., Fang, C. H. E. N., Chao, G. A. O., Qing-Yun, Z. H. A. O., Qi-Rong, S. H. E. N., and Wei, R. A. N. (2013). Bacillus cereus X5 enhanced bio-organic fertilizers effectively control root-knot nematodes (Meloidogyne sp.). Pedosphere 23, 160–168. doi: 10.1016/S1002-0160(13)60003-X
Tran, T. P. H., Wang, S. L., Nguyen, V. B., Tran, D. M., Nguyen, D. S., and Nguyen, A. D. (2019). Study of novel endophytic bacteria for biocontrol of black pepper root-knot nematodes in the central highlands of Vietnam. Agronomy 9:714. doi: 10.3390/agronomy9110714
Umamaheswari, R., Rao, M. S., Chaya, M. K., Sowmyavani, M., Navyashree, R. K., and Kavya, B. M. (2020). Bio-efficacy of liquid formulations of Bacillus subtilis IIHR Bs-2 (1% AS) and Bacillus amyloliquefaciens IIHR Ba-2 (1% AS) in the management of Meloidogyne incognita infecting tomato. Pest Manag. Horticul. Ecosyst. 26, 262–268.
Van Frankenhuyzen, K. (2009). Insecticidal activity of Bacillus thuringiensis crystal proteins. J. Invertebr. Pathol. 101, 1–16. doi: 10.1016/j.jip.2009.02.009
Van Frankenhuyzen, K. (2013). Cross-order and cross-phylum activity of Bacillus thuringiensis pesticidal proteins. J. Invertebr. Pathol. 114, 76–85. doi: 10.1016/j.jip.2013.05.010
Vasques, N. C., Nogueira, M. A., and Hungria, M. (2024). Increasing application of multifunctional Bacillus for biocontrol of pests and diseases and plant growth promotion: lessons from Brazil. Agronomy 14:1654. doi: 10.3390/agronomy14081654
Verduzco-Rosas, L. A., García-Suárez, R., López-Tlacomulco, J. J., and Ibarra, J. E. (2021). Selection and characterization of two Bacillus thuringiensis strains showing nematicidal activity against Caenorhabditis elegans and Meloidogyne incognita. FEMS Microbiol. Lett. 368:fnaa186. doi: 10.1093/femsle/fnaa186
Waller, P. J., and Thamsborg, S. M. (2004). Nematode control in ‘green’ ruminant production systems. Trends Parasitol. 20:493. doi: 10.1016/j.pt.2004.07.012
Wang, J. Y., Guo, C., Zhao, P., Yu, F. Y., Su, Y., Qu, J. P., et al. (2021a). Biocontrol potential of Bacillus altitudinis AMCC1040 against root-knot nematode disease of ginger and its impact on rhizosphere microbial community. Biol. Control 158:104598:104598. doi: 10.1016/j.biocontrol.2021.104598
Wang, J. Y., Zhang, X. C., Guo, C., Li, P. G., Yu, F. Y., Zhao, P., et al. (2021b). Diversity and nematocidal activity of culturable bacteria from suppressive soils in Shandong Province, China. Biocontrol Sci. Tech. 31, 387–399. doi: 10.1080/09583157.2020.1854176
Wei, J. Z., Hale, K., Carta, L., Platzer, E., Wong, C., Fang, S. C., et al. (2003). Bacillus thuringiensis crystal proteins that target nematodes. Proc. Natl. Acad. Sci. 100, 2760–2765. doi: 10.1073/pnas.0538072100
Wepuhkhulu, M., Kimenju, J., Anyango, B., Wachira, P., and Kyallo, G. (2011). Effect of soil fertility management practices and Bacillus subtilis on plant parasitic nematodes associated with common bean, Phaseolus vulgaris. Trop. Subtrop. Agroecosyst. 13, 27–34.
Widianto, D., Pramita, A. D., Kurniasari, I., Arofatullah, N. A., Prijambada, I. D., Widada, J., et al. (2021). Bacillus is one of the most potential genus as a biocontrol agent of golden cyst nematode (Globodera rostochiensis). Arch. Phytopathol. Plant Protect. 54, 2191–2205. doi: 10.1080/03235408.2021.1925501
Won, S. J., Choub, V., Kwon, J. H., Kim, D. H., and Ahn, Y. S. (2018). The control of fusarium root rot and development of coastal pine (Pinus thunbergii Parl.) seedlings in a container nursery by use of Bacillus licheniformis MH48. Forests 10:6. doi: 10.3390/f10010006
Wu, W., Zeng, Y., Yan, X., Wang, Z., Guo, L., Zhu, Y., et al. (2023). Volatile organic compounds of Bacillus velezensis GJ-7 against Meloidogyne hapla through multiple prevention and control modes. Molecules 28:3182. doi: 10.3390/molecules28073182
Xia, Y., Xie, S., Ma, X., Wu, H., Wang, X., and Gao, X. (2011). The purL gene of Bacillus subtilis is associated with nematicidal activity. FEMS Microbiol. Lett. 322, 99–107. doi: 10.1111/j.1574-6968.2011.02336.x
Xiao, F., Zhang, Y., Zhang, L., Li, S., Chen, W., Shi, G., et al. (2024). Advancing Bacillus licheniformis as a superior expression platform through promoter engineering. Microorganisms 12:1693. doi: 10.3390/microorganisms12081693
Xing, Z., Wu, X., Zhao, J., Zhao, X., Zhu, X., Wang, Y., et al. (2020). Isolation and identification of induced systemic resistance determinants from Bacillus simplex Sneb545 against Heterodera glycines. Sci. Rep. 10:11586. doi: 10.1038/s41598-020-68548-4
Xiong, J., Zhou, Q., Luo, H., Xia, L., Li, L., Sun, M., et al. (2015). Systemic nematicidal activity and biocontrol efficacy of Bacillus firmus against the root-knot nematode Meloidogyne incognita. World J. Microbiol. Biotechnol. 31, 661–667. doi: 10.1007/s11274-015-1820-7
Yang, J., Liang, L., Li, J., and Zhang, K. (2013). Nematicidal enzymes from microorganisms and their applications. Appl. Microbiol. Biotechnol. 97, 7081–7095. doi: 10.1007/s00253-013-5045-0
Yang, T., Xin, Y., Liu, T., Li, Z., Liu, X., Wu, Y., et al. (2022). Bacterial volatile-mediated suppression of root-knot nematode (Meloidogyne incognita). Plant Dis. 106, 1358–1365. doi: 10.1094/PDIS-06-21-1139-RE
Ye, L., Wang, J. Y., Liu, X. F., Guan, Q., Dou, N. X., Li, J., et al. (2022). Nematicidal activity of volatile organic compounds produced by Bacillus altitudinis AMCC 1040 against Meloidogyne incognita. Arch. Microbiol. 204:521. doi: 10.1007/s00203-022-03024-3
Yin, N., Liu, R., Zhao, J. L., Khan, R. A. A., Li, Y., Ling, J., et al. (2021a). Volatile organic compounds of Bacillus cereus strain Bc-cm103 exhibit fumigation activity against Meloidogyne incognita. Plant Dis. 105, 904–911. doi: 10.1094/PDIS-04-20-0783-RE
Yin, Y., Wang, P., Wang, X., and Wen, J. (2024). Construction of Bacillus subtilis for efficient production of fengycin from xylose through CRISPR-Cas9. Front. Microbiol. 14:1342199. doi: 10.3389/fmicb.2023.1342199
Yin, N., Zhao, J. L., Liu, R., Li, Y., Ling, J., Yang, Y. H., et al. (2021b). Biocontrol efficacy of Bacillus cereus strain Bc-cm103 against Meloidogyne incognita. Plant Dis. 105, 2061–2070. doi: 10.1094/PDIS-03-20-0648-RE
Yu, Z., Xiong, J., Zhou, Q., Luo, H., Hu, S., Xia, L., et al. (2015). The diverse nematicidal properties and biocontrol efficacy of Bacillus thuringiensis Cry6A against the root-knot nematode Meloidogyne hapla. J. Invertebr. Pathol. 125, 73–80. doi: 10.1016/j.jip.2014.12.011
Yuan, Y., Yan, Z., Chen, Y., Ye, J., and Tan, J. (2023). Effects of Bacillus cereus on survival, fecundity, and host adaptability of pine wood nematode. Diversity 15:566. doi: 10.3390/d15040566
Yun, H. S., Heo, J. H., Son, S. J., Park, M. R., Oh, S., Song, M. H., et al. (2014). Bacillus licheniformis isolated from Korean traditional food sources enhances the resistance of Caenorhabditis elegans to infection by Staphylococcus aureus. J. Microbiol. Biotechnol. 24, 1105–1108. doi: 10.4014/jmb.1406.06008
Zhang, L. N., Jiang, C. H., Si, F., Song, N., Yang, W., Zhu, Y., et al. (2024). Long-term field application of a plant growth-promoting rhizobacterial consortium suppressed root-knot disease by shaping the rhizosphere microbiota. Plant Dis. 108, 94–103. doi: 10.1094/PDIS-09-22-2196-RE
Zhang, J., Li, Y., Yuan, H., Sun, B., and Li, H. (2016). Biological control of the cereal cyst nematode (Heterodera filipjevi) by Achromobacter xylosoxidans isolate 09X01 and Bacillus cereus isolate 09B18. Biol. Control 92, 1–6. doi: 10.1016/j.biocontrol.2015.08.004
Zhang, F., Peng, D., Ye, X., Yu, Z., Hu, Z., Ruan, L., et al. (2012). In vitro uptake of 140 kDa Bacillus thuringiensis nematicidal crystal proteins by the second stage juvenile of Meloidogyne hapla. PLoS One 7:e38534. doi: 10.1371/journal.pone.0038534
Zhang, J. X., Xue, A. G., and Tambong, J. T. (2009). Evaluation of seed and soil treatments with novel Bacillus subtilis strains for control of soybean root rot caused by fusarium oxysporum and F. Graminearum. Plant Dis. 93, 1317–1323. doi: 10.1094/PDIS-93-12-1317
Zhaojian, G., Qiufen, W., Feihong, D., Xiang, X., Yifeng, Z., Wei, J., et al. (2021). Screening and mutagenesis of broad-spectrum antagonistic Bacillus licheniformis and purification and identification of antimicrobial substances produced by its mutant. Food Sci. 42, 143–150. doi: 10.7506/spkx1002-6630-20191112-161
Zhou, Y., Chen, J., Zhu, X., Wang, Y., Liu, X., Fan, H., et al. (2021). Efficacy of Bacillus megaterium strain Sneb207 against soybean cyst nematode (Heterodera glycines) in soybean. Pest Manag. Sci. 77, 568–576. doi: 10.1002/ps.6057
Zhu, M., Xu, X. E., Li, Y., Wang, P., Niu, S., Zhang, K., et al. (2019). Biosynthesis of the nematode attractant 2-Heptanone and its co-evolution between the pathogenic bacterium Bacillus nematocida and non-pathogenic bacterium Bacillus subtilis. Front. Microbiol. 10:1489. doi: 10.3389/fmicb.2019.01489
Keywords: plant-parasitic nematodes, biocontrol, Bacillus spp., nematicidal compounds, integrated pest management
Citation: Vasantha-Srinivasan P, Park KB, Kim KY, Jung W-J and Han YS (2025) The role of Bacillus species in the management of plant-parasitic nematodes. Front. Microbiol. 15:1510036. doi: 10.3389/fmicb.2024.1510036
Received: 12 October 2024; Accepted: 18 December 2024;
Published: 17 January 2025.
Edited by:
Pramod Kumar Sahu, National Bureau of Agriculturally Important Microorganisms (ICAR), IndiaReviewed by:
Kgabo Martha Pofu, University of Limpopo, South AfricaCopyright © 2025 Vasantha-Srinivasan, Park, Kim, Jung and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yeon Soo Han, aGFueXNAam51LmFjLmty; Woo-Jin Jung, d29vanVuZ0BqbnUuYWMua3I=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.