
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol. , 29 January 2025
Sec. Microbiotechnology
Volume 15 - 2024 | https://doi.org/10.3389/fmicb.2024.1457908
 Yuhui Wang1,2,3,4†
Yuhui Wang1,2,3,4† Haodi Liu1,2,3,4†
Haodi Liu1,2,3,4† Baoying Wang1,2,3,4
Baoying Wang1,2,3,4 Gülzire Gheyret5
Gülzire Gheyret5 Jingliang Qin6
Jingliang Qin6 Hanlin Wang1,2,3,4
Hanlin Wang1,2,3,4 Yuhan Di1,2,3,4
Yuhan Di1,2,3,4 Yanling Wang6
Yanling Wang6 Juan Wang7*
Juan Wang7* Haining Tan1,2,3,4*
Haining Tan1,2,3,4*Glycoconjugate vaccines are a vital category of effective and safe commercial vaccines that have significantly reduced the global prevalence of drug-resistant bacterial infections. These vaccines are synthesized by covalently linking bacterial polysaccharide antigens to a carrier protein. Given that they produce a stronger and longer-lasting immune response than pure polysaccharides that activate only B cells, glycoconjugate vaccines have become one of the most promising vaccine types. However, the chemical synthesis of glycoconjugate vaccines is complex, costly, and labor-intensive. Therefore, the efficient preparation of biosynthetic glycoconjugates using microbial cell factories has emerged as a highly desirable manufacturing alternative. This review focuses on advancements in the recombinant microbial biosynthesis of glycoconjugate vaccines and summarizes various strategies to optimize their production. It is based on three key aspects: the selection of oligosaccharyltransferase (OST), the use of different vaccine carrier proteins, and the enhancement of key concentrations in the uridine diphosphate (UDP)-sugar supply. Finally, the review highlights technical challenges and discusses future directions for the recombinant synthesis of glycoconjugate vaccines.
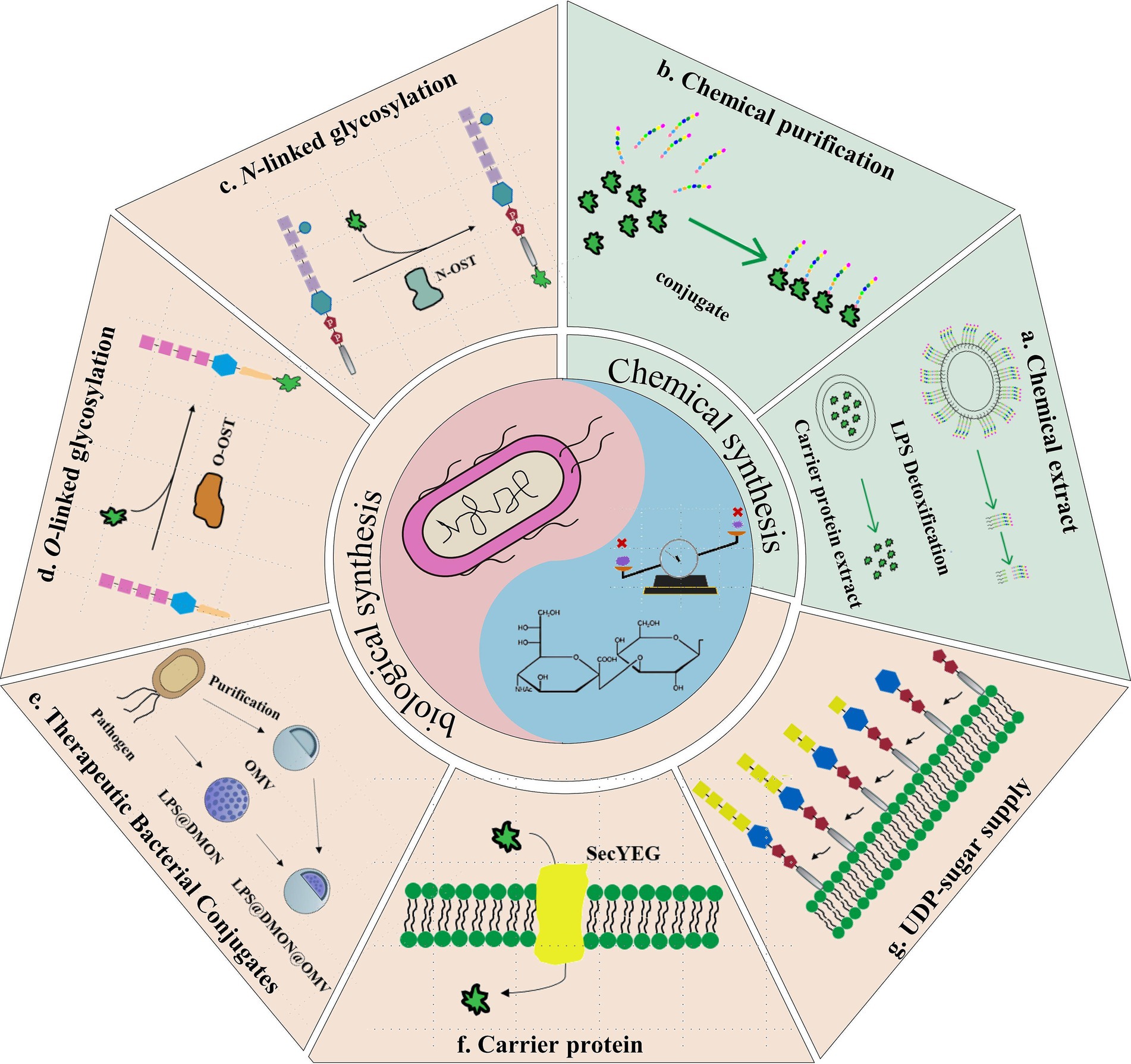
Graphical Abstract. Overview of the glycoconjugate vaccine synthesis technologies. a,b. Chemical approach for synthesizing glycoconjugate vaccines. a. Extraction and purification of the LPS/glycan and protein backbone from the bacterium; b. Chemical linkage of the OPS to the protein backbone; c–g. Biosynthesis of glycoconjugate vaccines. c. Biosynthesis of glycoconjugate vaccines using N-linked glycosylation; d. Biosynthesis of glycoconjugate vaccines using O-linked glycosylation; e. Alternative therapeutic conjugates targeting bacterial pathogens; f. Application of different carrier proteins in glycoconjugate vaccines; g. Strategies to optimize the UDP-sugar supply.
Drug-resistant bacteria are on the rise and pose a major threat, highlighting the urgent need for effective vaccines to prevent infections and save lives (O’Neil, 2014; Pai and Memish, 2016; Micoli et al., 2021; Zhou et al., 2023). Capsular polysaccharides (CPS) or O-antigen polysaccharides (OPSs) are key components of bacterial cells and play significant roles in various biological processes, including inflammation, cellular adhesion, molecular recognition, catalysis, pathogenic infections, and signal transduction events (Figure 1). Given their prominent biological roles, bacterial polysaccharides are promising candidates for use in vaccines. However, pure polysaccharide vaccines can only induce B cells to produce low-affinity IgM, thereby making them ineffective in infants and elderly individuals with immunodeficiencies (Pon and Jennings, 2009; Avci et al., 2011). Glycoconjugate vaccines link a glycan to a protein, resulting in multiple immune system triggers that create long-term immunological memory and increase vaccine stability (Pace, 2013; Micoli et al., 2018b; Xu et al., 2019). In particular, the implementation of fully licensed glycoconjugate vaccines for Haemophilus influenzae type b (Hib) (Ladhani, 2012; Perrett et al., 2013), Neisseria meningitidis (McCarthy et al., 2018), and some strains of Streptococcus pneumoniae (Grijalva et al., 2007) has significantly reduced the occurrence of bacterial meningitis and pneumonia worldwide. In addition, they have contributed to a decrease in the prevalence of antibiotic-resistant infections. Glycoconjugate vaccines provide a significant benefit because they can be effectively and safely administered to a wide range of age groups, including infants and the elderly (Rappuoli, 2018). As a result of the increasing demand for such versatile vaccines, the global glycoconjugate vaccine market was projected to reach approximately US$10 billion by 2020 (Kay et al., 2019).
Global vaccination rates for conjugate vaccines in children are still approximately 30%, with limited access and insufficient immunization coverage contributing to most of the ongoing disease burdens (Wahl et al., 2018). In recent years, the demand for therapeutic and diagnostic glycoconjugates—such as those based on polysaccharides used for pneumonia and meningitis—has significantly increased. However, progress in their development and distribution has been slow due to the complex and expensive nature of their production. The conventional process for producing conjugate vaccines involves chemically linking carrier proteins to polysaccharide antigens, which are extracted from extensive cultures of pathogenic bacteria. The production of OPS-based glycoconjugates involves several detailed steps (Wang et al., 2023b): (i) extraction of both the LPS/glycan and the protein backbone from the bacterial source; (ii) thorough purification of the protein backbone alongside the LPS; (iii) detoxification of the LPS through the chemical removal of lipid A, isolating the OPS; and (iv) chemical conjugation of the isolated OPS to the protein backbone. However, there are several drawbacks to large-scale fermentative production. The isolation of polysaccharides from the corresponding pathogenic bacterial serovars always involves safety concerns. Each step of the process incurs considerable losses and is time-consuming, which greatly increases the cost of glycoconjugates and limits their application in developing countries. Moreover, each glycoconjugate synthesis presents unique challenges, requiring a specific conjugation method and an individually designed synthetic scheme for each glycoconjugate.
Following the discovery of glycoconjugate synthesis in bacteria and the successful transfer of glycosylation pathways across species, Escherichia coli (E. coli) has emerged as a practical model for exploring glycosylation, decoding the glycan structures of living cells, and producing therapeutic glycoconjugates (Merritt et al., 2013). The use of recombinant E. coli as a host for glycoconjugate production has shown considerable promise, with significant developments (Jaffé et al., 2014). Therefore, the biosynthesis of glycoconjugate vaccines is often of interest to synthetic biologists.
Here, we review the promising field of biosynthetic glycoconjugate vaccines, focusing on optimizing strategies for the production of polysaccharide-based glycoconjugate vaccines.
In recent years, there has been a growing interest in developing bacterial species as hosts for glycoengineering applications involving the biosynthesis of structurally diverse polysaccharides, which can be produced as free glycans or as conjugates to carrier proteins (Reid and Szymanski, 2010; Kightlinger et al., 2020). The most obvious advantage of this approach is the much simpler and cheaper culturing conditions required for the maintenance of bacterial cells compared to eukaryotic cell cultures (Schmidt, 2004; Waegeman and Soetaert, 2011; Guarino, 2013). Bacteria carry N- and O-glycosylation systems that are mediated by oligosaccharyltransferase (OST). In OST-dependent glycosylation mechanisms, an oligosaccharide is synthesized on a lipid carrier and subsequently transferred to proteins en bloc by OST. Multiple proteins are glycosylated using this mechanism (Eichwald, 1865; O’Connor and Imperiali, 1996; Wacker et al., 2002). Some unconjugated polysaccharides and glycoconjugates are being biosynthesized as vaccines using microbial cell factories and are currently in the clinical trial phase (Riddle et al., 2016; Huttner et al., 2017). Figure 2 shows the key steps in the history of vaccine technologies and their evolution.
OST selection is a critical consideration in glycosylation, particularly when designing and producing glycoconjugate vaccines and other (Szymanski et al., 1999; Schwarz and Aebi, 2011; Harding and Feldman, 2019; Yakovlieva et al., 2021; Bagdonaite et al., 2022). OST is an enzyme complex responsible for transferring a pre-assembled glycan to specific amino acid residues of nascent proteins (Iwashkiw et al., 2013; Valguarnera et al., 2016). The integration of prokaryotic OST-catalyzed in vivo glycosylation into the production pipeline of glycoconjugate vaccines represents a powerful tool for facilitating a critical step in the pathway to generate more effective and accessible vaccines (Figure 3).
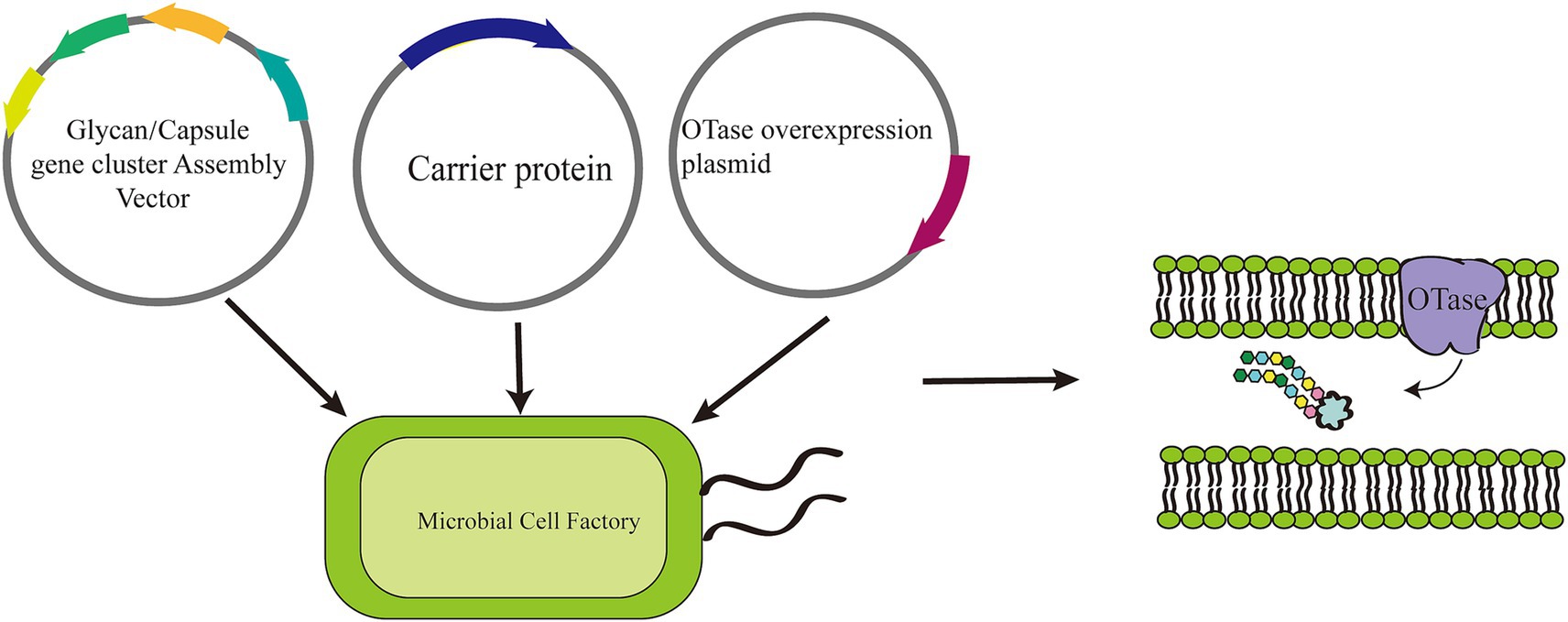
Figure 3. Glycoengineering approach to the production of glycoconjugate vaccines. An E. coli cell is engineered with three plasmids to generate glycoconjugate vaccines through a process called Protein Glycan Coupling Technology (PGCT), which unfolds in three distinct stages: polysaccharide expression, carrier protein design and expression, and coupling. Initially, the polysaccharide is synthesized on an undecaprenol pyrophosphate lipid anchor (represented by a blue/black circle) in the cytoplasm. It is then transported to the periplasmic space, where the enzyme PglB recognizes the lipid-linked reducing-end sugar. PglB then transfers the polysaccharide en bloc to an acceptor sequon (D/E-X-N-X-S/T) located on the carrier protein, culminating in the production of the glycoconjugate vaccines. IM refers to the inner membrane, and OM to the outer membrane.
For many years, it was believed that protein N-glycosylation occurred exclusively in eukaryotic systems. However, this perception shifted in 1999, when it was discovered that Campylobacter jejuni (C.jejuni), a Gram-negative bacterium and a pathogen in the human gut mucosa, has a protein N-glycosylation apparatus. Subsequent studies found that an OST named CjPglB (PglB from C. jejuni) was responsible for glycan transfer to the asparagine side chain in a consensus N-X-S/T sequence of the acceptor protein (Szymanski et al., 1999; Szymanski et al., 2003; Larsen et al., 2004). Notably, CjPglB, a single-subunit protein, was found to be homologous to STT3, the catalytic domain of the multi-subunit eukaryotic OST (Matsumoto et al., 2012).
In 2002, Aebi et al. first reported a bottom-up glycoengineering method using PglB-catalyzed glycosylation to produce glycoconjugate vaccines in E. coli (Wacker et al., 2002). Following this concept, several bacterial glycoconjugate vaccines have been biosynthesized using the N-linked glycosylation system in E. coli, and some of these vaccines have been successfully applied in clinical trials. Table 1 summarizes the glycoconjugate vaccine candidates generated and tested to date. In 2010, Ihssen et al. designed a glycoconjugate vaccine against Shigella dysenteriae, which was recently applied in a phase I clinical trial (Ihssen et al., 2010). Urinary tract infections (UTIs) are among the most common bacterial infections in humans. In over 80% of acute, uncomplicated cystitis cases, uropathogenic E. coli (UPEC) is the responsible pathogen (Kot, 2019). Indeed, the generation of antibodies targeting the O-antigen has proven to be effective in providing protection against recurrent UTIs caused by E. coli. Consequently, a vaccine targeting this antigen is promising due to its demonstrated safety and effectiveness. The promising glycoconjugate vaccine ExPEC4V, which contains O-antigens from UPEC serotypes O1A, O2, O6A, and O25B, was produced and showed positive results in phase II human clinical trials (Huttner et al., 2017). The EXPEC9V vaccine, another conjugate vaccine currently in a phase 3 clinical trial, has also shown promise against UPEC (Saade et al., 2020). In addition, the decavalent conjugate vaccine known as EXPEC10V, which targets a broad spectrum of serotypes (O1, O2, O4, O6, O8, O15, O16, O18, O25B, and O75), demonstrated high effectiveness against invasive extraintestinal E. coli in phase 1 clinical trials (Fierro et al., 2023). Hence, recombinant production of glycoconjugates in E. coli appears to be a promising alternative to traditional methods used for biomanufacturing conjugate vaccines. Although bacterial-linked OST can transfer a broader array of glycan structures, they still require acetylation at the C2 position of the reducing sugar, which limits the transfer of some glycans (Izquierdo et al., 2009; Ramírez et al., 2017; Napiórkowska et al., 2018).
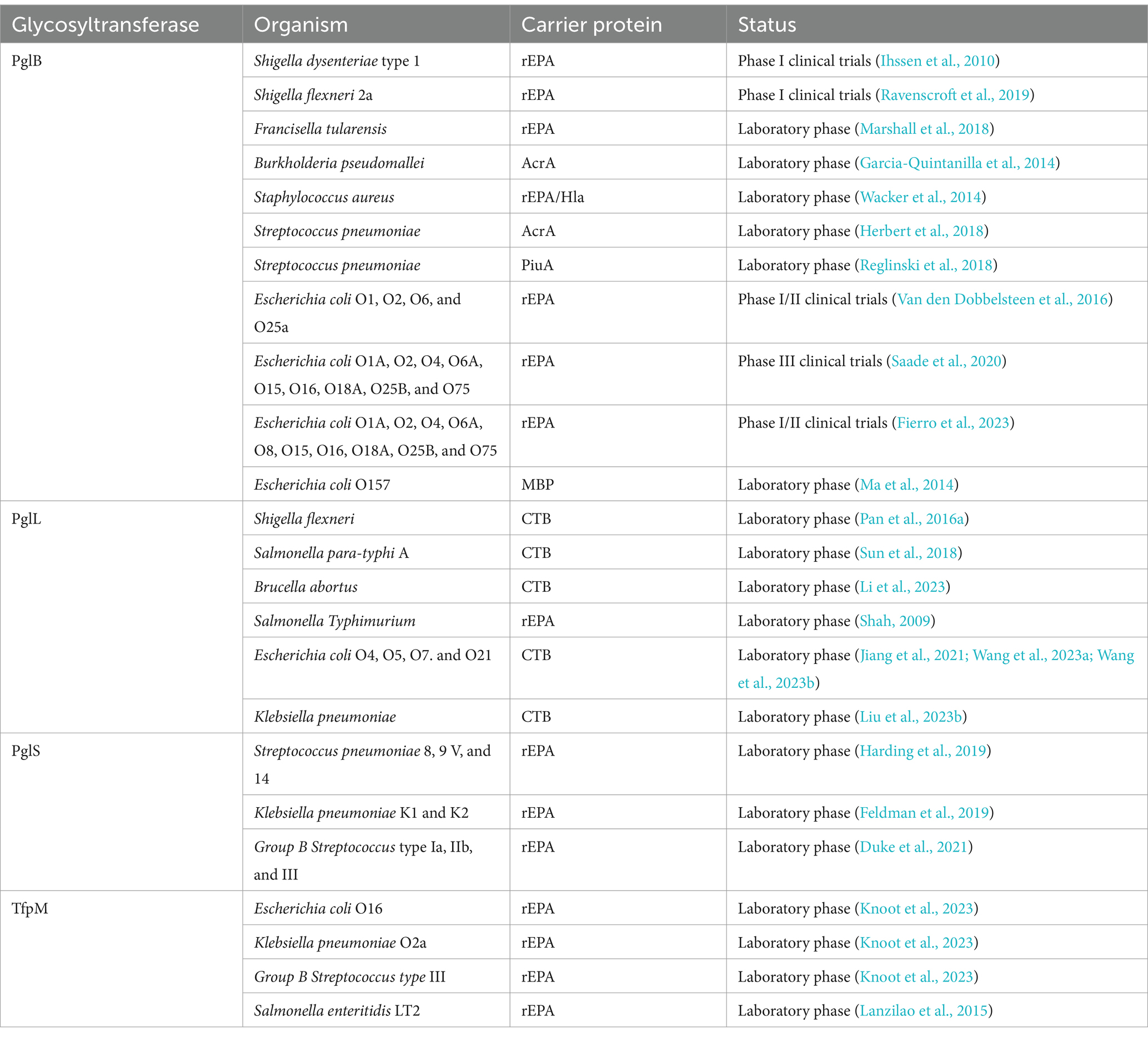
Table 1. Summary of biosynthetic vaccine candidates using various glycosyltransferases in this review, with the potential to prevent bacterial infectious diseases.
Over the last decade, in addition to the bacterial N-glycosylation mechanism mentioned above, O-linked glycosylation that led to the modification of serine or threonine residues has been identified in several bacterial species (Iwashkiw et al., 2013). In contrast to the N-linked oligosaccharyltransferase (OST), the O-linked OST typically demonstrates more relaxed specificities for glycans while maintaining stricter specificities for acceptor molecules. Four types of bacterial O-linked OST such as PilO, PglL, PglS, and TfpM have been utilized in glycobiology. These were first identified in Pseudomonas aeruginosa, Neisseria meningitidis, Acinetobacter baylyi, and Moraxella osloensis, respectively (Iwashkiw et al., 2013; Harding and Feldman, 2019; Knoot et al., 2023). In P. aeruginosa, PilA has been identified as being modified with a glycan, a modification catalyzed by the glycosyltransferase PilO (Castric, 1995). A similar machinery was found in N. meningitidis, where PglL was responsible for the attachment of a carbohydrate moiety to the protein PilE, generating a glycoconjugate (Power et al., 2006). Both PilO and PglL proteins can recognize Und-PP-linked glycans as substrate and tag proteins, demonstrating a promising application of these proteins in the development of glycoconjugate vaccines containing O-linked sugars (Table 2).
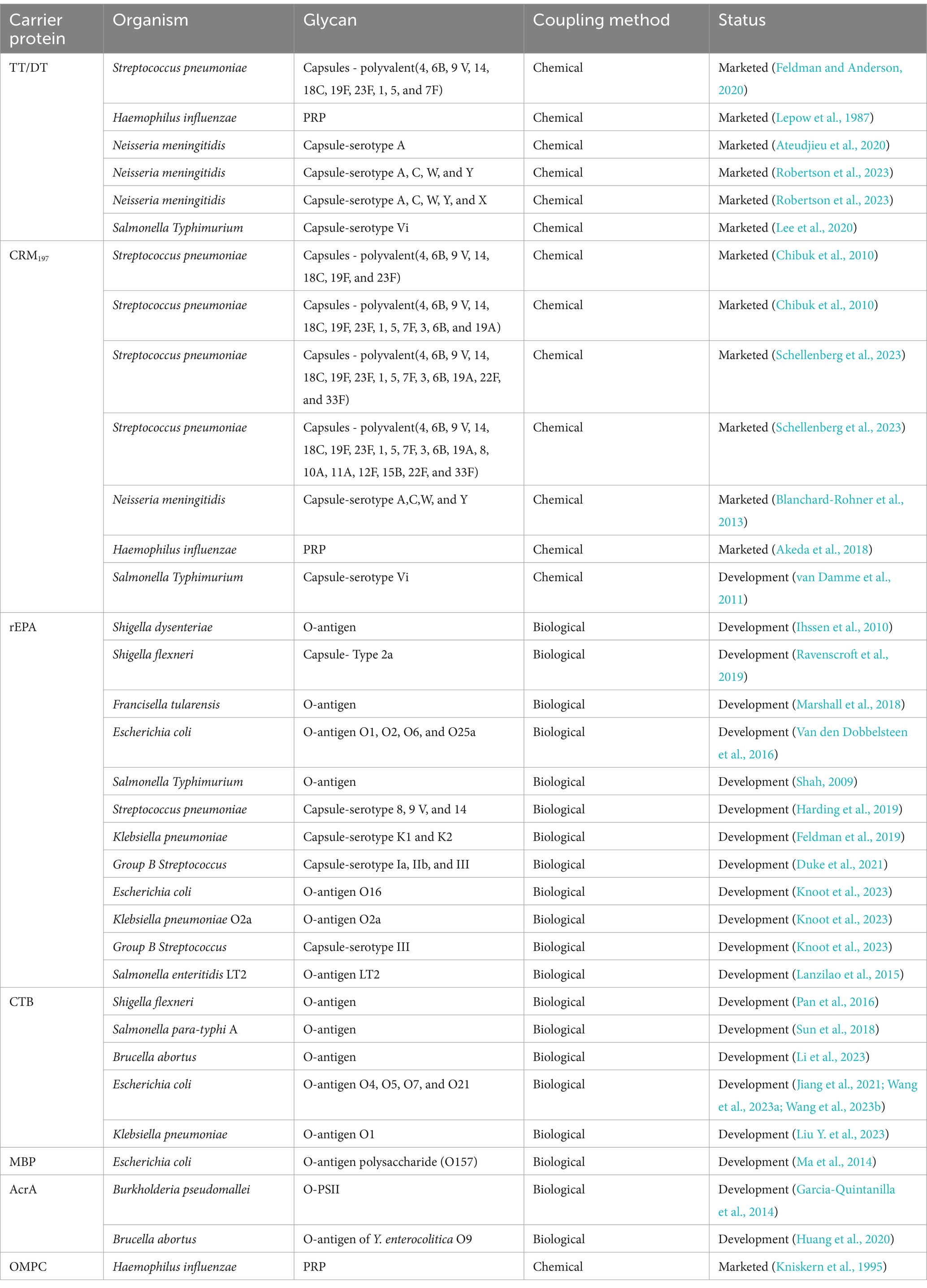
Table 2. Summary of polysaccharide-based glycoconjugate vaccine candidates using various carrier proteins.
However, both native PilO and PglL proteins were found to transfer only a single O-antigen subunit rather than longer polysaccharides, which limited their further application. This issue appears to have been recently solved by Pan et al., who elucidated and optimized an O-linked “glycosylation tag” as a recognition motif, known as MOOR, for the O-glycosyltransferase PglL (Pan et al., 2016). In their research, this recognition motif was successfully fused to both the N-terminus and C-terminus of different potential carrier proteins, generating glycoconjugate vaccines against S. typhimurium and S. flexneri 2a pathogen infections, respectively. Inspired by these technological advances, we also added a peptide fragment (45SAVTEYYLNHGEWPGNNTSAGVATSSEIK73) to the C-terminus of the carrier protein cholera toxin B subunit (CTB) using the PglL-dependent O-glycosylation system to generate OPS-based glycoconjugate vaccines against UPEC (Wang et al., 2023a).
Although both the N-OST PglB and the O-OST PglL exhibit remarkable versatility toward glycan substrates, neither enzyme has been experimentally proven to conjugate glycans containing a glucose residue at the reducing end. However, in approximately 75% of S. pneumoniae and many other pathogenic bacteria, CPSs contain glucose as the reducing-end monosaccharide. This indicates that these types of OST are not suitable for the biosynthesis of glycoconjugate vaccines, thereby limiting further application. Nonetheless, the two types of OST, PglS and Tfpm, are now known to transfer glycans with glucose at the reducing end (Harding et al., 2019). PglS was first discovered in Acinetobacter baylyi ADP1 and is capable of transferring a diverse array of polysaccharides, including those with glucose as the reducing-end sugar (Harding et al., 2015). Furthermore, Feldman et al. engineered a polyvalent pneumococcal glycoconjugate vaccine using the natural acceptor protein ComP as a vaccine carrier (Feldman et al., 2019; Harding et al., 2019). Several antimicrobial glycoconjugate vaccines using the conventional vaccine carrier Pseudomonas aeruginosa exotoxin A protein are already in the clinical trial phase (Porstendörfer et al., 2000; Schulz et al., 2013).
Harding et al. (2019) explored the recognition motif of PglS by fusing a peptide fragment from ComP to the N-terminus and C-terminus of two vaccine carrier proteins. These proteins included the detoxified variant of diphtheria toxin, CRM197, and recombinant ExoProtein A (rEPA) (Knoot et al., 2021; Knoot et al., 2023). As a result, both proteins were glycosylated. Recombinant O-glycoconjugate vaccines were produced with PglS-dependent O-glycosylation against a variety of pathogens, such as Streptococcus mastitis and Klebsiella pneumoniae (Geno et al., 2015; Pan et al., 2015; Carboni et al., 2017).
In 2023, Harding et al. identified a novel type of O-OST, termed TfpM, from Moraxella bacteria (Knoot et al., 2023). TfpM proteins are similar in size and sequence to PilO enzymes; however, these proteins can transfer long-chain polysaccharides to acceptor proteins. Furthermore, one of the glycosylation sites on pilin-like proteins is serine (Ser). The ability to tag proteins for TfpM-dependent O-glycosylation expands the potential biotechnological applications of this enzyme family. Utilizing this system, they engineered a variety of glycoconjugate vaccines against bacterial infections.
Although protein glycoconjugation is the most widely studied approach in vaccine research, researchers in the field of bacterial glycobiology are exploring alternative approaches to boost the immunogenicity of carbohydrate epitopes. Nearly all Gram-negative bacteria and some Gram-positive bacteria release outer membrane vesicles (OMVs) during their life cycles (Schwechheimer and Kuehn, 2015). These vesicles are usually nanosized proteoliposomes (ranging in size from 20 to 250 nm) with bilayer membranes that are mainly composed of virulence-associated components (e.g., membrane proteins, CPS, and LPS) (Tan et al., 2018). In light of their immunogenic capacities and high built-in adjuvanticity, OMVs have become promising vaccine candidate antigens (Lei et al., 2019). An OMV-based vaccine derived directly from N. meningitidis was developed as a licensed vaccine termed Bexsero® (GlaxoSmithKline), which has proven to be an effective vaccine against serogroup B meningococcal infections (Gorringe and Pajón, 2012). Compared to traditional subunit vaccines, OMV vaccines have numerous advantages: (i) OMVs carry significant amounts of virulence-associated pathogen-associated molecular patterns (PAMPs), which play an essential role in inducing an immune response; (ii) OMVs, as nanoscale particles, enhance the accumulation of antigens in lymph nodes, thereby boosting immunogenicity; and (iii) nanocarriers provide efficient adjuvanticity and stimulate antigen-presenting cell activation to elicit robust immune responses.
A novel bacterial glycoengineering approach to develop OMV-based nanovaccines was reported (Morelli et al., 2021; Long et al., 2022). Inspired by these technological advances, a series of E. coli-derived glycosylated OMVs (glycOMVs) were generated (Valguarnera and Feldman, 2017; Xie et al., 2022). These glycOMVs, carrying O-antigens from eight bacterial species, including F. tularensis and the CPS of S. pneumoniae serotype 14 (CPS14), were shown to elicit significant serum titers of class-switched, glycan-specific IgG antibodies in mice (Price et al., 2016). Notably, mice immunized with glycOMVs decorated with the CPS14 of S. pneumoniae elicited the same level of antigen-specific serum titers as mice vaccinated with the commercially licensed glycoconjugate vaccine Prevnar13®. These results indicate that the use of bacterial OMVs decorated with heterologous antigens holds great potential in the design of effective antibacterial vaccines.
In another investigation, a nanoconjugate vaccine was generated using a nano-B5 self-assembly system that carries the O-polysaccharide from K. pneumoniae (Pan et al., 2020). This nanovaccine has been shown to effectively boost antigen uptake by antigen-presenting cells and provoke a humoral immune response against K. pneumoniae. The designed nano-B5 self-assembly system in this study can effectively integrate various modular components and antigen cargos to efficiently create a potentially vast array of nanovaccine structures using multiple bacterial species.
Furthermore,to explore new areas within the structural domain of glycans and proteins in E. coli, Tytgat et al. (2019) engineered a cytoplasmic glycoengineering system to generate a nanoscale glycoconjugate. In their work, the shift from en bloc glycosylation to sequential glycosylation was a significant change in methodology. Sequential glycosylation in the cytoplasm allows for a more tailored, stepwise addition of glycan moieties directly to proteins. Moreover, the glycoengineering process occurred in the cytoplasm, marking a groundbreaking approach to protein glycosylation. This innovative approach could potentially enable new functionalities in proteins, enhance the stability and efficacy of therapeutic proteins, and allow for the production of glycoconjugates for diverse future biomedical applications.
Four carrier proteins have been used in licensed bacterial vaccines that promote a T cell-dependent (TD) immune response: tetanus toxoid (TT), diphtheria toxoid (DT), Cross Reactive Material 197 (CRM197), and Haemophilus protein D (PD) (Giannini et al., 1984; Micoli et al., 2018; David et al., 2019; Ravenscroft et al., 2019; Del et al., 2022). Diphtheria and tetanus toxoids were initially selected as carrier proteins for Hib conjugate vaccines because of their long history of safety and efficacy (Prymula et al., 2006; Forsgren et al., 2008). Immunization of mice with DT/TT/CRM197 prior to CRM197-conjugated N. meningitidis serogroup A and C polysaccharides was found to significantly improve anti-polysaccharide IgG titers (Terra et al., 2012; Moeller, 2022). Additional experiments showed that the activation of carrier protein-specific T helper cells could result in more effective activation of glycan-specific B cells, with carrier-derived fragments presented on their surface (Adamo et al., 2012; Oleksiewicz et al., 2012; Saggy et al., 2012; Zhang et al., 2013).
In addition to the carrier proteins already used in licensed commercial glycoconjugate vaccines, many others have been tested in preclinical studies and clinical trials with significant results. The recombinant protein rEPA has been engineered as a carrier to chemically conjugate with Shigella O-antigens, Staphylococcus aureus CPS5 and CPS8, and Salmonella Typhi Vi antigen (Szu et al., 1987; Brakke, 1992; Fattom et al., 1993; Cohen et al., 1997; Kossaczka et al., 1999). These glycoconjugate vaccines have been shown to boost vaccine efficacy. The cholera toxin B subunit (CTB) is a non-toxic pentameric moiety of cholera toxin (CT) and can be safely administered through various routes to humans (Hol et al., 1995; Sanchez and Holmgren, 2008; Baldauf et al., 2015). It has the capacity to induce an antigen-specific serum IgG response, along with toxin-neutralizing immunity. Recently, the CTB has been successfully used as a carrier protein by conjugating antigens to induce immune responses against several pathogens (such as C. trachomatis, H. pylori, S. paratyphi A) (McKenzie and Halsey, 1984; Vempati, 2014; He et al., 2022). Therefore, the CTB is a promising carrier that can be utilized in the development of glycoprotein vaccines.
In some cases, prior or simultaneous exposure to a protein can lead to vaccine interference, thereby decreasing glycoconjugate efficacy (Dagan et al., 2010; Borrow et al., 2011). To overcome unwanted vaccine interference, new carrier candidates from different pathogens have been researched at the preclinical level (Micoli et al., 2019; Gebre et al., 2021). Some protein carriers serve a dual role of both a carrier and a protective antigen to elicit or enhance immune responses. Group B Streptococcus (GBS) pili proteins GBS80 and GBS67, previously selected as pathogen-derived protein carriers and shown to confer protection, were conjugated to capsular PS type II and V, respectively (Singh and Srivastava, 2011; Moeller et al., 2021; Micoli et al., 2023). Furthermore, the recombinant protein termed TcdB_GT from Clostridium difficile was conjugated to its polysaccharide II (PSII) and induced similar anti-PSII IgG levels in mice, comparable to those induced by a CRM197-PSII conjugate. Simon et al. also proposed using the flagellin protein of Salmonella enteritidis as a carrier to conjugate with its OPS, thereby achieving enhanced protection through the additive effect of anti-O-antigen and anti-flagellin immune responses (Simon et al., 2011). Despite the fact that each new carrier protein needs to undergo testing for safety and efficacy, their development as scaffolds for next-generation glycoconjugates appears promising.
Uridine diphosphate (UDP)-sugars, such as UDP-glucose (UDPG), are crucial sugar precursors for the biosynthesis of important sugar-containing compounds, such as polysaccharides, glycoproteins, and glycolipids. These compounds are critical for cell growth and survival and are often limiting during recombinant biosynthesis (Feng et al., 2020). Therefore, it is crucial to ensure that their supply is sufficient in vivo. To address this issue, several regulatory schemes have been developed to improve the accumulation of endogenous UDP-sugars, such as the inhibition or knockout of non-essential pathways that consume UDP-sugars (Zhuang et al., 2017) and the fine-tuning of gene expression (Lv et al., 2019).
As key precursors, UDP-sugars, especially UDPG, are involved in many cellular activities in E. coli, which can reduce their availability for OPS-based glycoconjugates biosynthesis (Verstrepen et al., 2004). Earlier studies have shown that supplementing large amounts of carbon sources, such as glucose, in the medium can alleviate the limitation of insufficient supply of UDPG (Liu S. et al., 2023). However, an excessive carbon source during the fermentation process can lead to overproduction of the acetic acid byproduct, which can ultimately lead to metabolic imbalance and inhibit the expression of recombinant enzymes (Pei et al., 2019). The main glucose-consuming pathways in E. coli are glycolysis and the PPP (Feng et al., 2020). Therefore, inhibiting multiple genes involved in these glucose-consuming pathways may have a positive effect on the production of OPS-based glycoconjugates (Simkhada et al., 2010; Pandey et al., 2013). Meanwhile, some studies have focused on using a mixed carbon source during the fermentation process, aiming to separate glycoside biosynthesis and cell growth (Soellner et al., 2013; Pei et al., 2019). This strategy was found to improve the overall titer, yield, and productivity of isoorientin generation (Wu et al., 2017; Tang et al., 2020).
To biosynthesize OPS-based glycoconjugates with high efficiency, the glycoengineering chassis was optimized by redirecting the carbon flux toward the biosynthesis of the required precursors (Wang et al., 2023a). To this end, E. coli K12 MG1655 was selected as the original strain, and multiple gene deletions were engineered in the genome to prevent carbon leakage from the pathway, thereby increasing the carbon flux toward OPS biosynthesis (Gleizer et al., 2019). Herein, Liu et al. established a synergistic glucose–glycerol co-feeding system to improve OPS accumulation by separating bacterial growth from polysaccharide biosynthesis. Specifically, pfkA/B, zwf, nagB, and pykA/F were blocked to inhibit or knockout non-essential pathways, such as the E. coli Embden-Meyerhof-Parnas pathway and the PPP that consume UDP-sugars (Jiang et al., 2015; Gleizer et al., 2019). Moreover, genes involved in the synthesis of ECA and the incomplete O16-specific OPS in E. coli MG1655 were also deleted to avoid interference with OPS production or to inhibit the consumption of the pool of essential substrates (Datsenko and Wanner, 2000; Yates et al., 2019). To enhance the glycerol consumption pathway and alleviate carbon catabolite repression, the gene gldA, encoding glycerol dehydrogenase, was also disrupted (Soellner et al., 2013). Overall, such a strategy can directly improve the reserve of UDP-sugar precursors and further increase OPS synthesis.
Efficient protein glycosylation of glycoconjugates in E. coli requires sufficient availability of polysaccharide precursors, prior to their transfer by OST to engineered carrier proteins (Ihssen et al., 2010). The most common strategy is to enhance the expression levels of native biosynthesis pathway genes for NDP-sugars or dNDP-sugars that can channel more glucose into these NDP-sugars or dNDP-sugars due to the elevated production of pathway enzymes (Hernández-Montalvo et al., 2003). The first step is to clone the gene cluster that expresses O-antigen by PCR into E. coli and further ensure the correct assembly of the glycan (Liu et al., 2017). Some studies have reported that the overexpression of the genes pgm and galU1, both of which are essential for UDPG biosynthesis, resulted in improved glucoside production (Weyler and Heinzle, 2015). Wang et al. (2023a) applied this strategy to significantly boost the levels of glycosyl donors (UDP-Glc, UDP-Gal, and UDP-GlcNAc) for monosaccharide building blocks present in the OPS of UPEC O21 cells (Figure 4). In their study, the genes pgm, galU, and galE, which are involved in the biosynthesis of UDP-Gal and UDP-Glc, were overexpressed. Furthermore, the genes glmS, glmM, and glmU were also overexpressed to boost the glycosyl donor UDP-GlcNAc (Deng et al., 2006). In such a system, this approach boosted the availability of UDP-sugars and glycosylation in the glycoengineering strain MGD15.
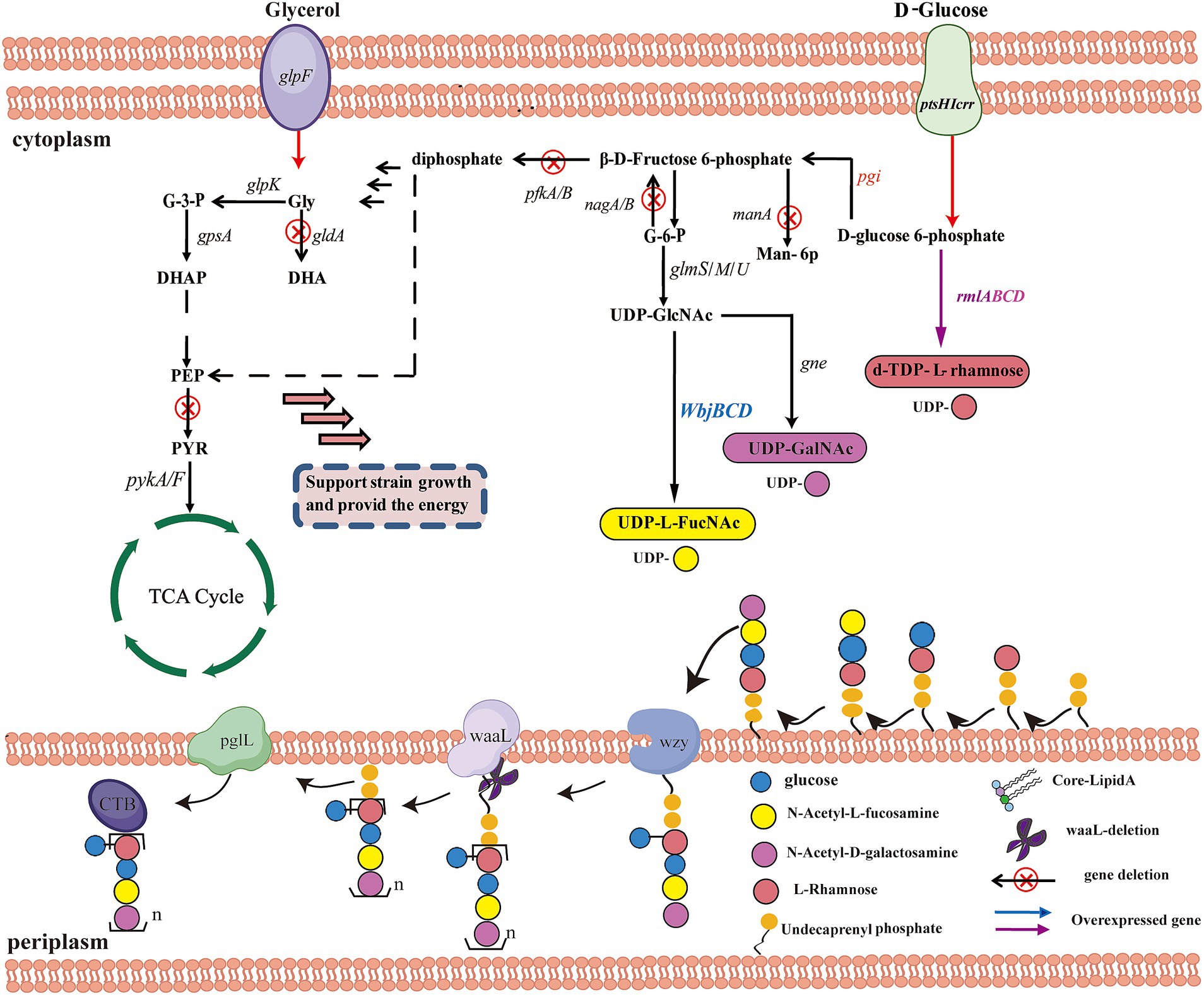
Figure 4. Schematic representation of a system in E. coli for dual-carbon utilization and orthogonal glycoprotein biosynthesis engineering. ptsHICrr, encoding phosphotransferase system (PTS); PEP, phosphoenolpyruvate; PYR, pyruvate; pgi, glucose-6-phosphate isomerase; pgm, phosphoglucomutase; glmS, glucosamine 6-phosphate synthase; glmM, phosphoglucosamine mutase; glmU, glucosamine 1-phosphate acetyltransferase/N-acetylglucosamine 1-phosphate uridyl transferase; UDP-GlcNAc, UDP-N-acetyl--D-glucosamine; manA, mannose-6-phosphate isomerase; Man-6P, D-mannose 6-phosphate; pfkA, 6-phosphofructokinase I; pfkB, 6-phosphofructokinase II; nagB, glucosamine 6-phosphate deaminase; glpK, glycerol kinase; gldA, glycerol dehydrogenase; gpsA, glycerol-3-phosphate dehydrogenase; G3P, glycerol 3-phosphate; DHAP, glycerone phosphate; pykA/F, pyruvate kinase II/I; ppsA, phosphoenolpyruvate synthetase; TCA cycle, Tricarboxylic acid cycle.
In another case, gene fine-tuning strategies were employed to promote OPS4 accumulation. Optimization of the pathway for enhancing dTDP-L-Rha and UDP-L-FucNAc synthesis can be targeted to improve glycosylation performance (Cress et al., 2014; Keinhörster et al., 2019). To identify enzymes with high catalytic activity, the biosynthetic pathways of dTDP-L-Rha and UDP-L-FucNAc from different bacterial sources were evaluated for efficient precursor production. Moreover, modular optimization was employed in this study by codon optimization (Alper et al., 2005). The biosynthetic pathways of dTDP-L-Rha and UDP-L-FucNAc from different bacterial sources were screened to identify enzymes with high catalytic activities to facilitate efficient precursor production (Ajikumar et al., 2010). Codon-optimized genes involved in the biosynthetic pathways of dTDP-L-Rha from Mycobacterium tuberculosis and E. coli, as well as those genes involved in the biosynthetic pathways of UDP-L-FucNAc from P. aeruginosa, have been studied (Sharon et al., 2012).
To address the complexities, costs, and labor-intensive nature of traditional chemical and chemoenzymatic methods, the use of microbial cell factories has emerged as a promising alternative for the biosynthesis of OPS-based glycoconjugate vaccines (Weyant et al., 2018; Sorieul et al., 2023). However, there are several challenges that need to be addressed in the further application of microbial cell factories in synthesizing the desired glycoconjugate vaccines.
i. The generation of multivalent glycoconjugates using cytoplasmic glycoconjugates presents unique challenges and complexities (Frasch, 2009). Creating multivalent glycoconjugates requires precise control over the number and arrangement of glycan chains attached to the protein (Bernardi et al., 2013). This requires not only specific glycosyltransferases for heterologous substrates but also strategies to control the density and pattern of glycosylation, which can significantly impact the immunogenicity and biological function of the resulting multivalent glycostructures (Clomburg et al., 2017).
ii. Polysaccharide heterogeneity produced by microbial cell factories presents challenges in the application of glycoconjugate vaccines (Huang and Wu, 2010). The size, branching, and composition of polysaccharides, whose biosynthesis in microbial cell factories can vary, contribute to this heterogeneity. Since the immunogenicity of polysaccharide antigens can vary based on their molecular weight, branching, and sugar composition,the heterogeneity in polysaccharide structures can significantly affect the quality and efficacy of glycoconjugate vaccines (Anish et al., 2021). Controlling the uniformity and length of polysaccharide structures is crucial for ensuring consistent vaccine performance and regulatory approval.
iii. The lack of structural information about glycosyltransferases limits their application and modification. Glycosyltransferases are multi-transmembrane proteins, which makes resolving their structures challenging. However, with the development of cryo-electron microscopy techniques, it is likely that more glycosyltransferase structures will be clearly resolved. This will greatly enhance our understanding of the functions of different structural domains within glycosyltransferases, and it holds promise for the artificial design and reconstruction of these domains. Such advancements could enable engineered enzymes to possess a more relaxed and extensive recognition capability for polysaccharide structures, as well as more precise glycosylation motifs, thereby laying the foundation for the development of multivalent conjugate vaccines using sets of orthogonal glycosyltransferases.
iv. The biosynthesis of polysaccharide-conjugate vaccines relies heavily on the bioinformatic analysis of bacterial polysaccharide antigen synthesis gene clusters and the establishment of molecular serotyping (Hu et al., 2013). However, deciphering polysaccharide antigens and conducting serotyping take time, thereby delaying the development of polysaccharide-based glycoconjugate vaccines and hindering the timely prevention and control of epidemic diseases.
v. Most licensed glycoconjugate vaccines typically utilize traditional carrier proteins (Wilder-Smith, 2008; Micoli et al., 2018; Del Bino et al., 2022). Rational design and screening of novel carrier proteins are expected to further enhance the immunogenicity of glycoconjugate vaccines (Yue and Ma, 2015). The selection of new carrier proteins must adhere to some key principles for use in glycoconjugate vaccine development: a. The carrier protein should be produced in sufficient quantities, reliably and economically, with the appropriate degree of purity, to meet clinical requirements and allow for future commercial supply. b. It is essential for the carrier protein to be able to activate T-cells, thereby enhancing the overall immune response to the conjugate vaccine (Sun et al., 2019).
YW: Writing – original draft, Conceptualization. HL: Writing – review & editing, Investigation. BW: Writing – review & editing, Visualization. GG: Writing – review & editing, Visualization. JQ: Writing – original draft. HW: Writing – review & editing. YD: Writing – original draft. YW: Writing – original draft. JW: Writing – original draft, Funding acquisition. HT: Writing – review & editing, Funding acquisition.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Key Research and Development Program of China (2023YFF1103600) and Qingdao Natural Science Foundation(24-4-4-zrjj-32-jch).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adamo, R., Romano, M. R., Berti, F., Leuzzi, R., Tontini, M., Danieli, E., et al. (2012). Phosphorylation of the synthetic Hexasaccharide repeating unit is essential for the induction of antibodies to Clostridium difficilehf PSII cell wall polysaccharide. ACS Chem Biol 7, 1420–1428. doi: 10.1021/cb300221f
Ajikumar, P. K., Xiao, W. H., Tyo, K. E., Wang, Y., Simeon, F., Leonard, E., et al. (2010). Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli. Science 330, 70–74. doi: 10.1126/science.1191652
Akeda, Y., Koizumi, Y., Takanami, Y., Sumino, S., Hattori, Y., Sugizaki, K., et al. (2018). Comparison of serum bactericidal and antibody titers induced by two Haemophilus influenzae type b conjugate vaccines: A phase III randomized double-blind study. Vaccine 36, 1528–1532. doi: 10.1016/j.vaccine.2018.02.011
Alper, H., Fischer, C., Nevoigt, E., and Stephanopoulos, G. (2005). Tuning genetic control through promoter engineering. Proc. Natl. Acad. Sci. U. S. A. 102, 12678–12683. doi: 10.1073/pnas.0504604102
Anish, C., Beurret, M., and Poolman, J. (2021). Combined effects of glycan chain length and linkage type on the immunogenicity of glycoconjugate vaccines. NPJ Vaccines 6:150. doi: 10.1038/s41541-021-00409-1
Ateudjieu, J., Stoll, B., Bisseck, A. C., Tembei, A. M., and Genton, B. (2020). Safety profile of the meningococcal conjugate vaccine (Menafrivac™) in clinical trials and vaccination campaigns: a review of published studies. Hum. Vaccin. Immunother. 16, 1245–1259. doi: 10.1080/21645515.2019.1652041
Avci, F. Y., Li, X., Tsuji, M., and Kasper, D. L. (2011). A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat. Med. 17, 1602–1609. doi: 10.1038/nm.2535
Bagdonaite, I., Malaker, S. A., Polasky, D. A., Riley, N. M., Schjoldager, K., Vakhrushev, S. Y., et al. (2022). Glycoproteomics. Glycoproteomics 2:48. doi: 10.1038/s43586-022-00128-4
Baldauf, K. J., Royal, J. M., Hamorsky, K. T., and Matoba, N. (2015). Cholera toxin B: one subunit with many pharmaceutical applications. Toxins (Basel) 7, 974–996. doi: 10.3390/toxins7030974
Bernardi, A., Jiménez-Barbero, J., Casnati, A., De Castro, C., Darbre, T., Fieschi, F., et al. (2013). Multivalent glycoconjugates as anti-pathogenic agents. Chem. Soc. Rev. 42, 4709–4727. doi: 10.1039/c2cs35408j
Blanchard-Rohner, G., Snape, M. D., Kelly, D. F., O'Connor, D., John, T., Kibwana, E., et al. (2013). Seroprevalence and placental transmission of maternal antibodies specific for Neisseria meningitidis Serogroups A, C, Y and W135 and influence of maternal antibodies on the immune response to a primary course of men ACWY-CRM vaccine in the United Kingdom. Pediatr. Infect. Dis. J. 32, 768–776. doi: 10.1097/INF.0b013e318292f425
Borrow, R., Dagan, R., Zepp, F., Hallander, H., and Poolman, J. J. (2011). Glycoconjugate vaccines and immune interactions, and implications for vaccination schedules. Expert Rev. Vaccines 10, 1621–1631. doi: 10.1586/erv.11.142
Brakke, K. A. (1992). The surface evolver. Exp. Math. 1, 141–165. doi: 10.1080/10586458.1992.10504253
Carboni, F., Adamo, R., Fabbrini, M., De Ricco, R., Cattaneo, V., Brogioni, B., et al. (2017). Structure of a protective epitope of group B Streptococcus type III capsular polysaccharide. Proc. Natl. Acad. Sci. U. S. A. 114, 5017–5022. doi: 10.1073/pnas.1701885114
Castric, P. J. M. (1995). pilO, a gene required for glycosylation of Pseudomonas aeruginosa 1244 pilin. Microbiology (Reading) 141, 1247–1254. doi: 10.1099/13500872-141-5-1247
Chibuk, T. K., Robinson, J. L., and Hartfield, D. S. (2010). Pediatric complicated pneumonia and pneumococcal serotype replacement: trends in hospitalized children pre and post introduction of routine vaccination with pneumococcal conjugate vaccine (PCV7). Eur. J. Pediatr. 169, 1123–1128. doi: 10.1007/s00431-010-1195-6
Clomburg, J. M., Crumbley, A. M., and Gonzalez, R. (2017). Industrial biomanufacturing: the future of chemical production. Science 355:804. doi: 10.1126/science.aag0804
Cohen, D., Ashkenazi, S., Green, M. S., Gdalevich, M., Robin, G., Slepon, R., et al. (1997). Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet 349, 155–159. doi: 10.1016/S0140-6736(96)06255-1
Cress, B. F., Englaender, J. A., He, W., Kasper, D., Linhardt, R. J., and Koffas, M. A. (2014). Masquerading microbial pathogens: capsular polysaccharides mimic host-tissue molecules. FEMS Microbiol. Rev. 38, 660–697. doi: 10.1111/1574-6976.12056
Dagan, R., Poolman, J., and Siegrist, C. A. (2010). Glycoconjugate vaccines and immune interference: A review. Vaccine 28, 5513–5523. doi: 10.1016/j.vaccine.2010.06.026
Datsenko, K. A., and Wanner, B. L. (2000). One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97, 6640–6645. doi: 10.1073/pnas.120163297
David, S., Reuter, S., Harris, S. R., Glasner, C., Feltwell, T., Argimon, S., et al. (2019). Epidemic of carbapenem-resistant Klebsiella pneumoniae in Europe is driven by nosocomial spread. Nat. Microbiol. 4, 1919–1929. doi: 10.1038/s41564-019-0492-8
Del, L., Østerlid, K. E., Wu, D.-Y., Nonne, F., Romano, M. R., Codée, J., et al. (2022). Synthetic Glycans to improve current Glycoconjugate vaccines and fight antimicrobial resistance. Chem. Rev. 122, 15672–15716. doi: 10.1021/acs.chemrev.2c00021
Deng, M. D., Grund, A. D., Wassink, S. L., Peng, S. S., Nielsen, K. L., Huckins, B. D., et al. (2006). Directed evolution and characterization of Escherichia coli glucosamine synthase. Biochimie 88, 419–429. doi: 10.1016/j.biochi.2005.10.002
Duke, J. A., Paschall, A. V., Robinson, L. S., Knoot, C. J., Vinogradov, E., Scott, N. E., et al. (2021). Development and immunogenicity of a prototype multivalent group B Streptococcus bioconjugate vaccine. ACS Infect. Dis. 7, 3111–3123. doi: 10.1021/acsinfecdis.1c00415
Eichwald, E. G. (1865). Beiträge zur Chemie der gewebbildenden Substanzen und ihrer Abkömmlinge. I. Ueber das Mucin, besonders der Weinbergschnecke. Eur. J. Organ. Chem. 134, 177–211. doi: 10.1002/jlac.18651340207
Fattom, A., Schneerson, R., Watson, D., Karakawa, W., Fitzgerald, D., Pastan, I., et al. (1993). Laboratory and clinical evaluation of conjugate vaccines composed of Staphylococcus aureus type 5 and type 8 capsular polysaccharides bound to Pseudomonas aeruginosa recombinant exoprotein A. Eur. J. Organic Chem. 61, 1023–1032. doi: 10.1128/iai.61.3.1023-1032.1993
Feldman, C., and Anderson, R. (2020). Recent advances in the epidemiology and prevention of Streptococcus pneumoniae infections. F1000Res 9:22341. doi: 10.12688/f1000research.22341.1
Feldman, M. F., Mayer Bridwell, A. E., Scott, N. E., Vinogradov, E., McKee, S. R., Chavez, S. M., et al. (2019). A promising bioconjugate vaccine against hypervirulent Klebsiella pneumoniae. Proc. Natl. Acad. Sci. U. S. A. 116, 18655–18663. doi: 10.1073/pnas.1907833116
Feng, Y., Yao, M., Wang, Y., Ding, M., Zha, J., Xiao, W., et al. (2020). Advances in engineering UDP-sugar supply for recombinant biosynthesis of glycosides in microbes. Biotechnol. Adv. 41:107538. doi: 10.1016/j.biotechadv.2020.107538
Fierro, C. A., Sarnecki, M., Doua, J., Spiessens, B., Go, O., Davies, T. A., et al. (2023). Safety, Reactogenicity, immunogenicity, and dose selection of 10-Valent Extraintestinal pathogenic Escherichia coli bioconjugate vaccine (VAC52416) in adults aged 60-85 years in a randomized, multicenter, interventional, first-in-human, phase 1/2a study. Open forum. Infect. Dis. 10:ofad417. doi: 10.1093/ofid/ofad417
Forsgren, A., Riesbeck, K., and Janson, H. (2008). Protein D of Haemophilus influenzae: a protective nontypeable H. influenzae antigen and a carrier for pneumococcal conjugate vaccines. Clin. Infect. Dis. 46, 726–731. doi: 10.1086/527396
Frasch, C. E. (2009). Preparation of bacterial polysaccharide-protein conjugates: analytical and manufacturing challenges. Vaccine 27, 6468–6470. doi: 10.1016/j.vaccine.2009.06.013
Garcia-Quintanilla, F., Iwashkiw, J. A., Price, N. L., Stratilo, C., and Feldman, M. F. (2014). Production of a recombinant vaccine candidate against Burkholderia pseudomallei exploiting the bacterial N-glycosylation machinery. Front. Microbiol. 5:381. doi: 10.3389/fmicb.2014.00381
Gebre, M. S., Brito, L. A., Tostanoski, L. H., Edwards, D. K., Carfi, A., and Barouch, D. H. (2021). Novel approaches for vaccine development. Cell 184, 1589–1603. doi: 10.1016/j.cell.2021.02.030
Geno, K. A., Gilbert, G. L., Song, J. Y., Skovsted, I. C., Klugman, K. P., Jones, C., et al. (2015). Pneumococcal capsules and their types: past, present, and future. Clin. Microbiol. Rev. 28, 871–899. doi: 10.1128/CMR.00024-15
Giannini, G., Rappuoli, R., and Ratti, G. J. (1984). The amino-acid sequence of two non-toxic mutants of diphtheria toxin: CRM45 and CRM197. Nucleic Acids Res. 12, 4063–4069. doi: 10.1093/nar/12.10.4063
Gleizer, S., Ben-Nissan, R., Bar-On, Y. M., Antonovsky, N., Noor, E., Zohar, Y., et al. (2019). Conversion of Escherichia coli to generate all biomass carbon from CO2. Cell 179, 1255–1263.e12. doi: 10.1016/j.cell.2019.11.009
Gorringe, A. R., and Pajón, R. (2012). Bexsero: a multicomponent vaccine for prevention of meningococcal disease. Hum Vaccin Immunother 8, 174–183. doi: 10.4161/hv.18500
Grijalva, C. G., Nuorti, J. P., Arbogast, P. G., Martin, S. W., Edwards, K. M., and Griffin, M. R. (2007). Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet 369, 1179–1186. doi: 10.1016/S0140-6736(07)60564-9
Guarino, C. M. (2013). Investigating oligosaccharyltransferases of N-linked glycosylation using Escherichia coli. Ithaca, NY: Cornell University.
Harding, C. M., and Feldman, M. F. (2019). Glycoengineering bioconjugate vaccines, therapeutics, and diagnostics in E. coli. Glycobiology 29, 519–529. doi: 10.1093/glycob/cwz031
Harding, C. M., Nasr, M. A., Kinsella, R. L., Scott, N. E., Foster, L. J., Weber, B. S., et al. (2015). Acinetobacter strains carry two functional oligosaccharyltransferases, one devoted exclusively to type IV pilin, and the other one dedicated to O-glycosylation of multiple proteins. Mol. Microbiol. 96, 1023–1041. doi: 10.1111/mmi.12986
Harding, C. M., Nasr, M. A., Scott, N. E., Goyette-Desjardins, G., Nothaft, H., Mayer, A. E., et al. (2019). A platform for glycoengineering a polyvalent pneumococcal bioconjugate vaccine using E. coli as a host. Nat. Commun. 10:891. doi: 10.1038/s41467-019-08869-9
He, X., Yang, J., Ji, M., Chen, Y., Chen, Y., Li, H., et al. (2022). A potential delivery system based on cholera toxin: a macromolecule carrier with multiple activities. J. Control Release 343, 551–563. doi: 10.1016/j.jconrel.2022.01.050
Herbert, J. A., Kay, E. J., Faustini, S. E., Richter, A., Abouelhadid, S., Cuccui, J., et al. (2018). Production and efficacy of a low-cost recombinant pneumococcal protein polysaccharide conjugate vaccine. Vaccine 36, 3809–3819. doi: 10.1016/j.vaccine.2018.05.036
Hernández-Montalvo, V., Martínez, A., Hernández-Chavez, G., Bolivar, F., Valle, F., and Gosset, G. (2003). Expression of galP and glk in a Escherichia coli PTS mutant restores glucose transport and increases glycolytic flux to fermentation products. Biotechnol. Bioeng. 83, 687–694. doi: 10.1002/bit.10702
Hol, W. G., Sixma, T. K., Merritt, E., and Marcel Dekker, I. (1995). Structure and function of E. coli heat-labile enterotoxin and cholera toxin B pentamer. New York, pp. 185–223.
Hu, D., Liu, B., Dijkshoorn, L., Wang, L., and Reeves, P. R. (2013). Diversity in the major polysaccharide antigen of Acinetobacter baumannii assessed by DNA sequencing, and development of a molecular serotyping scheme. PLoS One 8:e70329. doi: 10.1371/journal.pone.0070329
Huang, J., Pan, C., Sun, P., Feng, E., Wu, J., Zhu, L., et al. (2020). Application of an O-linked glycosylation system in Yersinia enterocolitica serotype O: 9 to generate a new candidate vaccine against Brucella abortus. Microorganisms 8:436. doi: 10.3390/microorganisms8030436
Huang, Y. L., and Wu, C. Y. (2010). Carbohydrate-based vaccines: challenges and opportunities. Expert Rev. Vaccines 9, 1257–1274. doi: 10.1586/erv.10.120
Huttner, A., Hatz, C., van den Dobbelsteen, G., Abbanat, D., Hornacek, A., Frölich, R., et al. (2017). Safety, immunogenicity, and preliminary clinical efficacy of a vaccine against extraintestinal pathogenic Escherichia coli in women with a history of recurrent urinary tract infection: a randomised, single-blind, placebo-controlled phase 1b trial. Lancet Infect. Dis. 17, 528–537. doi: 10.1016/S1473-3099(17)30108-1
Ihssen, J., Kowarik, M., Dilettoso, S., Tanner, C., Wacker, M., and Thöny-Meyer, L. (2010). Production of glycoprotein vaccines in Escherichia coli. Microb. Cell Factories 9:61. doi: 10.1186/1475-2859-9-61
Iwashkiw, J. A., Vozza, N. F., Kinsella, R. L., and Feldman, M. F. (2013). Pour some sugar on it: the expanding world of bacterial protein O-linked glycosylation. Mol. Microbiol. 89, 14–28. doi: 10.1111/mmi.12265
Izquierdo, L., Schulz, B. L., Rodrigues, J. A., Güther, M. L. S., Procter, J. B., Barton, G. J., et al. (2009). Distinct donor and acceptor specificities of Trypanosoma brucei oligosaccharyltransferases. EMBO J. 28, 2650–2661. doi: 10.1038/emboj.2009.203
Jaffé, S. R., Strutton, B., Levarski, Z., Pandhal, J., and Wright, P. C. (2014). Escherichia coli as a glycoprotein production host: recent developments and challenges. Curr. Opin. Biotechnol. 30, 205–210. doi: 10.1016/j.copbio.2014.07.006
Jiang, X., Bai, J., Yuan, J., Zhang, H., Lu, G., Wang, Y., et al. (2021). High efficiency biosynthesis of O-polysaccharide-based vaccines against extraintestinal pathogenic Escherichia coli. Carbohydr. Polym. 255:117475. doi: 10.1016/j.carbpol.2020.117475
Jiang, Y., Chen, B., Duan, C., Sun, B., Yang, J., and Yang, S. (2015). Multigene editing in the Escherichia coli genome via the CRISPR-Cas9 system. Appl. Environ. Microbiol. 81, 2506–2514. doi: 10.1128/aem.04023-14
Kay, E., Cuccui, J., and Wren, B. W. (2019). Recent advances in the production of recombinant glycoconjugate vaccines. NPJ Vaccines 4:16. doi: 10.1038/s41541-019-0110-z
Keinhörster, D., Salzer, A., Duque-Jaramillo, A., George, S. E., Marincola, G., Lee, J. C., et al. (2019). Revisiting the regulation of the capsular polysaccharide biosynthesis gene cluster in Staphylococcus aureus. Mol. Microbiol. 112, 1083–1099. doi: 10.1111/mmi.14347
Kightlinger, W., Warfel, K. F., DeLisa, M. P., and Jewett, M. C. (2020). Synthetic glycobiology: parts, systems, and applications. ACS Synth. Biol. 9, 1534–1562. doi: 10.1021/acssynbio.0c00210
Kniskern, P. J., Marburg, S., and Ellis, R. W. (1995). Haemophilus influenzae type b conjugate vaccines. Pharm. Biotechnol. 6, 673–694. doi: 10.1007/978-1-4615-1823-5_30
Knoot, C. J., Robinson, L. S., and Harding, C. M. J. G. (2021). A minimal sequon sufficient for O-linked glycosylation by the versatile oligosaccharyltransferase PglS. Glycobiology 31, 1192–1203. doi: 10.1093/glycob/cwab043
Knoot, C. J., Wantuch, P. L., Robinson, L. S., Rosen, D. A., Scott, N. E., and Harding, C. M. (2023). Discovery and characterization of a new class of O-linking oligosaccharyltransferases from the Moraxellaceae family. Glycobiology 33, 57–74. doi: 10.1093/glycob/cwac070
Kossaczka, Z., Lin, F.-Y. C., Ho, V. A., Thuy, N. T. T., Bay, P. V., Thanh, T. C., et al. (1999). Safety and immunogenicity of vi conjugate vaccines for typhoid fever in adults, teenagers, and 2- to 4-year-old children in Vietnam. Infect. Immun. 67, 5806–5810. doi: 10.1128/IAI.67.11.5806-5810.1999
Kot, B. (2019). Antibiotic resistance among uropathogenic Escherichia coli. Pol. J. Microbiol. 68, 403–415. doi: 10.33073/pjm-2019-048
Ladhani, S. N. (2012). Two decades of experience with the Haemophilus influenzae serotype b conjugate vaccine in the United Kingdom. Clin. Ther. 34, 385–399. doi: 10.1016/j.clinthera.2011.11.027
Lanzilao, L., Stefanetti, G., Saul, A., MacLennan, C. A., Micoli, F., and Rondini, S. (2015). Strain selection for generation of O-antigen-based Glycoconjugate vaccines against invasive Nontyphoidal Salmonella disease. PLoS One 10:e0139847. doi: 10.1371/journal.pone.0139847
Larsen, J. C., Szymanski, C., and Guerry, P. (2004). N-linked protein glycosylation is required for full competence in Campylobacter jejuni 81-176. J. Bacteriol. 186, 6508–6514. doi: 10.1128/JB.186.19.6508-6514.2004
Lee, E. Y., Park, J. Y., Kim, D. R., Song, M., Sahastrabuddhe, S., Kim, H., et al. (2020). Comparison of anti-vi IgG responses between two clinical studies of typhoid vi conjugate vaccines (vi-DT vs vi-TT). PLoS Negl. Trop. Dis. 14:e0008171. doi: 10.1371/journal.pntd.0008171
Lei, Y., Zhao, F., Shao, J., Li, Y., Li, S., Chang, H., et al. (2019). Application of built-in adjuvants for epitope-based vaccines. PeerJ 6:e6185. doi: 10.7717/peerj.6185
Lepow, M. L., Barkin, R. M., Berkowitz, C. D., Brunell, P. A., James, D., Meier, K., et al. (1987). Safety and immunogenicity of Haemophilus influenzae type b polysaccharide-diphtheria toxoid conjugate vaccine (PRP-D) in infants. J. Infect. Dis. 156, 591–596. doi: 10.1093/infdis/156.4.591
Li, S., Huang, J., Wang, K., Liu, Y., Guo, Y., Li, X., et al. (2023). A bioconjugate vaccine against Brucella abortus produced by engineered Escherichia coli. Front. Bioeng. Biotechnol. 11:1121074. doi: 10.3389/fbioe.2023.1121074
Liu, M. A., Kenyon, J. J., Lee, J., and Reeves, P. R. (2017). Rapid customised operon assembly by yeast recombinational cloning. Appl. Microbiol. Biotechnol. 101, 4569–4580. doi: 10.1007/s00253-017-8213-9
Liu, S., Li, D., Qin, Z., Zeng, W., and Zhou, J. (2023). Enhancing glycosylation of flavonoids by engineering the uridine diphosphate glucose supply in Escherichia coli. J. Agric. Food Chem. 71, 17842–17851. doi: 10.1021/acs.jafc.3c05264
Liu, Y., Pan, C., Wang, K., Guo, Y., Sun, Y., Li, X., et al. (2023). Preparation of a Klebsiella pneumoniae conjugate nanovaccine using glycol-engineered Escherichia coli. Microb. Cell Factories 22:95. doi: 10.1186/s12934-023-02099-x
Long, Q., Zheng, P., Zheng, X., Li, W., Hua, L., Yang, Z., et al. (2022). Engineered bacterial membrane vesicles are promising carriers for vaccine design and tumor immunotherapy. Adv. Drug Deliv. Rev. 186:114321. doi: 10.1016/j.addr.2022.114321
Lv, Y., Marsafari, M., Koffas, M., Zhou, J., and Xu, P. (2019). Optimizing oleaginous yeast cell factories for flavonoids and Hydroxylated flavonoids biosynthesis. ACS Synth. Biol. 8, 2514–2523. doi: 10.1021/acssynbio.9b00193
Ma, Z., Zhang, H., Shang, W., Zhu, F., Han, W., Zhao, X., et al. (2014). Glycoconjugate vaccine containing Escherichia coli O157: H7 O-antigen linked with maltose-binding protein elicits humoral and cellular responses. PLoS One 9:e105215. doi: 10.1371/journal.pone.0105215
Marshall, L. E., Nelson, M., Davies, C. H., Whelan, A. O., Jenner, D. C., Moule, M. G., et al. (2018). An O-antigen Glycoconjugate vaccine produced using protein glycan coupling technology is protective in an inhalational rat model of tularemia. J Immunol Res 2018, 8087916–8087912. doi: 10.1155/2018/8087916
Matsumoto, S., Igura, M., Nyirenda, J., Matsumoto, M., Yuzawa, S., Noda, N., et al. (2012). Crystal structure of the C-terminal globular domain of Oligosaccharyltransferase from Archaeoglobus fulgidus at 1.75 Å resolution. Biochemistry 51, 4157–4166. doi: 10.1021/bi300076u
McCarthy, P. C., Sharyan, A., and Sheikhi Moghaddam, L. J. V. (2018). Meningococcal vaccines: current status and emerging strategies. Vaccines (Basel) 6:12. doi: 10.3390/vaccines6010012
McKenzie, S. J., and Halsey, J. (1984). Cholera toxin B subunit as a carrier protein to stimulate a mucosal immune response. J. Immunol. 133, 1818–1824. doi: 10.4049/jimmunol.133.4.1818
Merritt, J. H., Ollis, A. A., Fisher, A. C., and DeLisa, M. P. (2013). Glycans‐by‐design: engineering bacteria for the biosynthesis of complex glycans and glycoconjugates. Biotechnol. Bioeng. 110, 1550–1564. doi: 10.1002/bit.24885
Micoli, F., Bagnoli, F., Rappuoli, R., and Serruto, D. (2021). The role of vaccines in combatting antimicrobial resistance. Nat. Rev. Microbiol. 19, 287–302. doi: 10.1038/s41579-020-00506-3
Micoli, F., Costantino, P., and Adamo, R. (2018). Potential targets for next generation antimicrobial glycoconjugate vaccines. FEMS Microbiol. Rev. 42, 388–423. doi: 10.1093/femsre/fuy011
Micoli, F., Del Bino, L., Alfini, R., Carboni, F., Romano, M. R., and Adamo, R. (2019). Glycoconjugate vaccines: current approaches towards faster vaccine design. Expert Rev. Vaccines 18, 881–895. doi: 10.1080/14760584.2019.1657012
Micoli, F., Stefanetti, G., and MacLennan, C. (2023). Exploring the variables influencing the immune response of traditional and innovative glycoconjugate vaccines. Front. Mol. Biosci. 10:1201693. doi: 10.3389/fmolb.2023.1201693
Moeller, T. D. (2022). Engineering the humoral response to generate antigen-specific antibodies. Ithaca, NY: Cornell University.
Moeller, T. D., Weyant, K. B., and DeLisa, M. P. (2021). Interplay of carbohydrate and carrier in antibacterial glycoconjugate vaccines. Adv. Biochem. Eng. Biotechnol., 355–378. doi: 10.1007/10_2018_71
Morelli, L., Polito, L., Richichi, B., and Compostella, F. J. G. J. (2021). Glyconanoparticles as tools to prevent antimicrobial resistance. Glycoconj J. 38, 475–490. doi: 10.1007/s10719-021-09988-6
Napiórkowska, M., Boilevin, J., Darbre, T., Reymond, J.-L., and Locher, K. P. J. S. R. (2018). Structure of bacterial oligosaccharyltransferase PglB bound to a reactive LLO and an inhibitory peptide. Sci. Rep. 8:16297. doi: 10.1038/s41598-018-34534-0
O’Connor, S. E., and Imperiali, B. J. C. (1996). Modulation of protein structure and function by asparagine-linked glycosylation. Chem. Biol. 3, 803–812. doi: 10.1016/S1074-5521(96)90064-2
O’Neil, J. (2014). Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Review on antimicrobial resistance.
Oleksiewicz, M. B., Nagy, G., and Nagy, E. (2012). Anti-bacterial monoclonal antibodies: Back to the future? Arch. Biochem. Biophys. 526, 124–131. doi: 10.1016/j.abb.2012.06.001
Pace, D. J. (2013). Glycoconjugate vaccines. Expert Opin. Biol. Ther. 13, 11–33. doi: 10.1517/14712598.2012.725718
Pai, M., and Memish, Z. A. (2016). Antimicrobial resistance and the growing threat of drug-resistant tuberculosis. J. Epidemiol. Glob. Health 6:45. doi: 10.1016/j.jegh.2016.02.001
Pan, Y.-J., Lin, T.-L., Chen, C.-T., Chen, Y.-Y., Hsieh, P.-F., Hsu, C.-R., et al. (2015). Genetic analysis of capsular polysaccharide synthesis gene clusters in 79 capsular types of Klebsiella spp. Sci. Rep. 5:15573. doi: 10.1038/srep15573
Pan, C., Sun, P., Liu, B., Liang, H., Peng, Z., Dong, Y., et al. (2016). Biosynthesis of conjugate vaccines using an O-linked glycosylation system. MBio 7, e00443–e00416. doi: 10.1128/mBio.00443-16
Pan, C., Wu, J., Qing, S., Zhang, X., Zhang, L., Yue, H., et al. (2020). Biosynthesis of self‐assembled proteinaceous nanoparticles for vaccination. Adv Mater 32:2002940. doi: 10.1002/adma.202002940
Pandey, R. P., Malla, S., Simkhada, D., Kim, B. G., and Sohng, J. K. (2013). Production of 3-O-xylosyl quercetin in Escherichia coli. Appl. Microbiol. Biotechnol. 97, 1889–1901. doi: 10.1007/s00253-012-4438-9
Pei, J., Sun, Q., Zhao, L., Shi, H., Tang, F., and Cao, F. (2019). Efficient biotransformation of Luteolin to Isoorientin through adjusting induction strategy, controlling acetic acid, and increasing UDP-glucose supply in Escherichia coli. J. Agric. Food Chem. 67, 331–340. doi: 10.1021/acs.jafc.8b05958
Perrett, K. P., Nolan, T. M., and McVernon, J. (2013). A licensed combined Haemophilus influenzae type b-Serogroups C and Y meningococcal conjugate vaccine. Infect. Dis. Ther. 2, 1–13. doi: 10.1007/s40121-013-0007-5
Pon, R. A., and Jennings, H. J. (2009). Carbohydrate-based antibacterial vaccines. In: Z. Guo and G. J. Boons Carbohydrate-Based Vaccines and Immunotherapies. New York: John Wiley and Sons, pp. 117–166.
Porstendörfer, D., Gohl, O., Mayer, F., and Averhoff, B. (2000). ComP, a pilin-like protein essential for natural competence in Acinetobacter sp. J. Bacteriol. 182, 3673–3680. doi: 10.1128/JB.182.13.3673-3680.2000
Power, P. M., Seib, K. L., and Jennings, M. P. (2006). Pilin glycosylation in Neisseria meningitidis occurs by a similar pathway to wzy-dependent O-antigen biosynthesis in Escherichia coli. Biochem. Biophys. Res. Commun. 347, 904–908. doi: 10.1016/j.bbrc.2006.06.182
Price, N. L., Goyette-Desjardins, G., Nothaft, H., Valguarnera, E., Szymanski, C. M., Segura, M., et al. (2016). Glycoengineered outer membrane vesicles: A novel platform for bacterial vaccines. Sci. Rep. 6:24931. doi: 10.1038/srep24931
Prymula, R., Peeters, P., Chrobok, V., Kriz, P., Novakova, E., Kaliskova, E., et al. (2006). Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study 367, 740–748. doi: 10.1016/S0140-6736(06)68304-9
Ramírez, A. S., Boilevin, J., Biswas, R., Gan, B. H., Janser, D., Aebi, M., et al. (2017). Characterization of the single-subunit oligosaccharyltransferase STT3A from Trypanosoma brucei using synthetic peptides and lipid-linked oligosaccharide analogs. Glycobiology 27, 525–535. doi: 10.1093/glycob/cwx017
Rappuoli, R. (2018). Glycoconjugate vaccines: principles and mechanisms. Sci. Transl. Med. 10:eaat4615. doi: 10.1126/scitranslmed.aat4615
Ravenscroft, N., Braun, M., Schneider, J., Dreyer, A. M., Wetter, M., Haeuptle, M. A., et al. (2019). Characterization and immunogenicity of a Shigella flexneri 2a O-antigen bioconjugate vaccine candidate. Glycobiology 29, 669–680. doi: 10.1093/glycob/cwz044
Reglinski, M., Ercoli, G., Plumptre, C., Kay, E., Petersen, F. C., Paton, J. C., et al. (2018). A recombinant conjugated pneumococcal vaccine that protects against murine infections with a similar efficacy to Prevnar-13. NPJ Vaccines 3:53. doi: 10.1038/s41541-018-0090-4
Reid, A. N., and Szymanski, C. M. (2010). “Biosynthesis and assembly of capsular polysaccharides” in Microbial glycobiology. ed. A. N. Reid (Amsterdam, Netherlands: Elsevier), 351–373.
Riddle, M. S., Kaminski, R. W., di, C., Porter, C. K., Gutierrez, R. L., Clarkson, K. A., et al. (2016). Safety and immunogenicity of a candidate bioconjugate vaccine against Shigella flexneri 2a administered to healthy adults: a single-blind, randomized phase I study. mSphere 23, 908–917. doi: 10.1128/CVI.00224-16
Robertson, C. A., Jacqmein, J., Selmani, A., Galarza, K., and Oster, P. (2023). Immunogenicity and safety of a quadrivalent meningococcal conjugate vaccine (MenACYW-TT) administered as a booster to adults aged ≥59 years: A phase III randomized study. Hum. Vaccin. Immunother. 19:2160600. doi: 10.1080/21645515.2022.2160600
Saade, E., Gravenstein, S., Donskey, C. J., Wilson, B., Spiessens, B., Abbanat, D., et al. (2020). Characterization of Escherichia coli isolates potentially covered by ExPEC4V and ExPEC10V, that were collected from post-transrectal ultrasound-guided prostate needle biopsy invasive urinary tract and bloodstream infections. Vaccine 38, 5100–5104. doi: 10.1016/j.vaccine.2020.06.024
Saggy, I., Wine, Y., Shefet-Carasso, L., Nahary, L., Georgiou, G., Benhar, I., et al. (2012). Antibody isolation from immunized animals: comparison of phage display and antibody discovery via V gene repertoire mining. Protein Eng. Des. Sel. 25, 539–549. doi: 10.1093/protein/gzs060
Sanchez, J., and Holmgren, J. (2008). Cholera toxin structure, gene regulation and pathophysiological and immunological aspects. Cell. Mol. Life Sci 65, 1347–1360. doi: 10.1007/s00018-008-7496-5
Schellenberg, J. J., Adam, H. J., Baxter, M. R., Karlowsky, J. A., Golden, A. R., Martin, I., et al. (2023). Comparison of PCV10, PCV13, PCV15, PCV20 and PPSV23 vaccine coverage of invasive Streptococcus pneumoniae isolate serotypes in Canada: the SAVE study, 2011-20. J. Antimicrob. Chemother. 78, i37–i47. doi: 10.1093/jac/dkad068
Schmidt, F. R. (2004). Recombinant expression systems in the pharmaceutical industry. Appl. Microbiol. Biotechnol. 65, 363–372. doi: 10.1007/s00253-004-1656-9
Schulz, B. L., Jen, F. E., Power, P. M., Jones, C. E., Fox, K. L., Ku, S. C., et al. (2013). Identification of bacterial protein O-Oligosaccharyltransferases and their glycoprotein substrates. PLoS One 8:e62768. doi: 10.1371/journal.pone.0062768
Schwarz, F., and Aebi, M. (2011). Mechanisms and principles of N-linked protein glycosylation. Curr. Opin. Struct. Biol. 21, 576–582. doi: 10.1016/j.sbi.2011.08.005
Schwechheimer, C., and Kuehn, M. (2015). Outer-membrane vesicles from gram-negative bacteria: biogenesis and functions. Nat. Rev. Microbiol. 13, 605–619. doi: 10.1038/nrmicro3525
Shah, N. K. (2009). Indian conjugate vi typhoid vaccine: do we have enough evidence? Indian Pediatr. 46, 181–182
Sharon, E., Kalma, Y., Sharp, A., Raveh-Sadka, T., Levo, M., Zeevi, D., et al. (2012). Inferring gene regulatory logic from high-throughput measurements of thousands of systematically designed promoters. Nat. Biotechnol. 30, 521–530. doi: 10.1038/nbt.2205
Simkhada, D., Lee, H. C., and Sohng, J. K. (2010). Genetic engineering approach for the production of rhamnosyl and allosyl flavonoids from Escherichia coli. Biotechnol. Bioeng. 107, 154–162. doi: 10.1002/bit.22782
Simon, R., Tennant, S. M., Wang, J. Y., Schmidlein, P. J., Lees, A., Ernst, R. K., et al. (2011). Salmonella enterica serovar enteritidis core O polysaccharide conjugated to H: g, m flagellin as a candidate vaccine for protection against invasive infection with S. enteritidis. Infect. Immun. 79, 4240–4249. doi: 10.1128/IAI.05484-11
Singh, M., and Srivastava, I. K. (2011). Development of vaccines: From discovery to clinical testing. New York: John Wiley and Sons.
Soellner, S., Rahnert, M., Siemann-Herzberg, M., Takors, R., and Altenbuchner, J. (2013). Evolution of pyruvate kinase-deficient Escherichia coli mutants enables glycerol-based cell growth and succinate production. J. Appl. Microbiol. 115, 1368–1378. doi: 10.1111/jam.12333
Sorieul, C., Dolce, M., Romano, M. R., Codée, J., and Adamo, R. J. (2023). Glycoconjugate vaccines against antimicrobial resistant pathogens. Expert. Rev. Vaccines 22, 1055–1078. doi: 10.1080/14760584.2023.2274955
Sun, P., Pan, C., Zeng, M., Liu, B., Liang, H., Wang, D., et al. (2018). Design and production of conjugate vaccines against S. paratyphi A using an O-linked glycosylation system in vivo. NPJ Vaccines 3:4. doi: 10.1038/s41541-017-0037-1
Sun, X., Stefanetti, G., Berti, F., and Kasper, D. L. (2019). Polysaccharide structure dictates mechanism of adaptive immune response to glycoconjugate vaccines. Proc. Natl. Acad. Sci. U. S. A. 116, 193–198. doi: 10.1073/pnas.1816401115
Szu, S. C., Stone, A. L., Robbins, J. D., Schneerson, R., and Robbins, J. B. (1987). Vi capsular polysaccharide-protein conjugates for prevention of typhoid fever. Preparation, characterization, and immunogenicity in laboratory animals. J. Exp. Med. 166, 1510–1524. doi: 10.1084/jem.166.5.1510
Szymanski, C. M., Logan, S. M., Linton, D., and Wren, B. W. (2003). Campylobacter – a tale of two protein glycosylation systems. Trends Microbiol. 11, 233–238. doi: 10.1016/S0966-842X(03)00079-9
Szymanski, C. M., Yao, R., Ewing, C. P., Trust, T. J., and Guerry, P. (1999). Evidence for a system of general protein glycosylation in Campylobacter jejuni. Mol. Microbiol. 32, 1022–1030. doi: 10.1046/j.1365-2958.1999.01415.x
Tan, K., Li, R., Huang, X., and Liu, Q. (2018). Outer membrane vesicles: current status and future direction of these novel vaccine adjuvants. Front. Microbiol. 9:344503. doi: 10.3389/fmicb.2018.00783
Tang, E., Shen, X., Wang, J., Sun, X., and Yuan, Q. (2020). Synergetic utilization of glucose and glycerol for efficient myo-inositol biosynthesis. Biotechnol. Bioeng. 117, 1247–1252. doi: 10.1002/bit.27263
Terra, V. S., Mills, D. C., Yates, L. E., Abouelhadid, S., Cuccui, J., and Wren, B. W. (2012). Recent developments in bacterial protein glycan coupling technology and glycoconjugate vaccine design. J. Med. Microbiol. 61, 919–926. doi: 10.1099/jmm.0.039438-0
Tytgat, H. L., Lin, C.-W., Levasseur, M. D., Tomek, M. B., Rutschmann, C., Mock, J., et al. (2019). Cytoplasmic glycoengineering enables biosynthesis of nanoscale glycoprotein assemblies. Nat. Commun. 10:5403. doi: 10.1038/s41467-019-13283-2
Valguarnera, E., and Feldman, M. F. (2017). Glycoengineered outer membrane vesicles as a platform for vaccine development. Methods Enzymol. 597, 285–310. doi: 10.1016/bs.mie.2017.06.032
Valguarnera, E., Kinsella, R. L., and Feldman, M. F. (2016). Sugar and spice make Bacteria not Nice: protein glycosylation and its influence in pathogenesis. J. Mol. Biol. 428, 3206–3220. doi: 10.1016/j.jmb.2016.04.013
Van Damme, P., Kafeja, F., Anemona, A., Basile, V., Hilbert, A. K., De Coster, I., et al. (2011). Safety, immunogenicity and dose ranging of a new vi-CRM₁₉₇ conjugate vaccine against typhoid fever: randomized clinical testing in healthy adults. PLoS One 6:e25398. doi: 10.1371/journal.pone.0025398
Van den Dobbelsteen, G. P., Faé, K. C., Serroyen, J., van den Nieuwenhof, I. M., Braun, M., Haeuptle, M. A., et al. (2016). Immunogenicity and safety of a tetravalent E. coli. O-antigen bioconjugate vaccine in animal models. Vaccine 34, 4152–4160. doi: 10.1016/j.vaccine.2016.06.067
Vempati, L. (2014). Construction and characterization of non-toxic bacterial enterotoxins as vaccine adjuvants. Boise State University Theses and Dissertations
Verstrepen, K. J., Iserentant, D., Malcorps, P., Derdelinckx, G., Van Dijck, P., Winderickx, J., et al. (2004). Glucose and sucrose: hazardous fast-food for industrial yeast? Trends Biotechnol. 22, 531–537. doi: 10.1016/j.tibtech.2004.08.001
Wacker, M., Linton, D., Hitchen, P. G., Nita-Lazar, M., Haslam, S. M., North, S. J., et al. (2002). N-linked glycosylation in Campylobacter jejuni and its functional transfer into E. coli. Science 298, 1790–1793. doi: 10.1126/science.298.5599.1790
Wacker, M., Wang, L., Kowarik, M., Dowd, M., Lipowsky, G., Faridmoayer, A., et al. (2014). Prevention of Staphylococcus aureus infections by glycoprotein vaccines synthesized in Escherichia coli. J. Infect. Dis. 209, 1551–1561. doi: 10.1093/infdis/jit800
Waegeman, H., and Soetaert, W. (2011). Increasing recombinant protein production in Escherichia coli through metabolic and genetic engineering. J. Ind. Microbiol. Biotechnol. 38, 1891–1910. doi: 10.1007/s10295-011-1034-4
Wahl, B., O'Brien, K. L., Greenbaum, A., Majumder, A., Liu, L., Chu, Y., et al. (2018). Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15 6, e744–e757. doi: 10.1016/S2214-109X(18)30247-X
Wang, Y., Perepelov, A. V., Senchenkova, S. N., Lu, G., Wang, X., Ma, G., et al. (2023a). Glycoengineering directs de novo biomanufacturing of UPEC O21 O-antigen polysaccharide based glycoprotein. Int. J. Biol. Macromol. 253:126993. doi: 10.1016/j.ijbiomac.2023.126993
Wang, Y., Wang, X., Ma, G., Xie, L., Liu, D., Wang, Y., et al. (2023b). Sustainable production of a polysaccharide-based glycoprotein by simultaneous conversion of glucose and glycerol in engineered Escherichia coli. Green Chem. 25, 4818–4832. doi: 10.1039/D3GC01279D
Weyant, K. B., Mills, D. C., and DeLisa, M. P. (2018). Engineering a new generation of carbohydrate-based vaccines. Curr. Opin. Chem. Eng. 19, 77–85. doi: 10.1016/j.coche.2017.12.009
Weyler, C., and Heinzle, E. (2015). Multistep synthesis of UDP-glucose using tailored, permeabilized cells of E. coli. Appl. Biochem. Biotechnol. 175, 3729–3736. doi: 10.1007/s12010-015-1540-3
Wilder-Smith, A. (2008). Meningococcal disease: risk for international travellers and vaccine strategies. Travel Med. Infect. Dis. 6, 182–186. doi: 10.1016/j.tmaid.2007.10.002
Wu, Y., Sun, X., Lin, Y., Shen, X., Yang, Y., Jain, R., et al. (2017). Establishing a synergetic carbon utilization mechanism for non-catabolic use of glucose in microbial synthesis of trehalose. Metab. Eng. 39, 1–8. doi: 10.1016/j.ymben.2016.11.001
Xie, J., Li, Q., Haesebrouck, F., Van Hoecke, L., and Vandenbroucke, R. E. (2022). The tremendous biomedical potential of bacterial extracellular vesicles. Trends Biotechnol. 40, 1173–1194. doi: 10.1016/j.tibtech.2022.03.005
Xu, L., Li, Z., Su, Z., Yang, Y., Ma, G., Yu, R., et al. (2019). Development of meningococcal polysaccharide conjugate vaccine that can elicit long-lasting and strong cellular immune response with hepatitis B core antigen virus-like particles as a novel carrier protein. Vaccine 37, 956–964. doi: 10.1016/j.vaccine.2018.12.073
Yakovlieva, L., Fülleborn, J. A., and Walvoort, M. (2021). Opportunities and challenges of bacterial glycosylation for the development of novel antibacterial strategies. Front Microbiol 12:745702. doi: 10.3389/fmicb.2021.745702
Yates, L. E., Natarajan, A., Li, M., Hale, M. E., Mills, D. C., and DeLisa, M. P. (2019). Glyco-recoded Escherichia coli: Recombineering-based genome editing of native polysaccharide biosynthesis gene clusters. Metab. Eng. 53, 59–68. doi: 10.1016/j.ymben.2019.02.002
Yue, H., and Ma, G. (2015). Polymeric micro/nanoparticles: particle design and potential vaccine delivery applications. Vaccine 33, 5927–5936. doi: 10.1016/j.vaccine.2015.07.100
Zhang, F., Lu, Y.-J., and Malley, R. (2013). Multiple antigen-presenting system (MAPS) to induce comprehensive B- and T-cell immunity. Proc. Natl. Acad. Sci. U. S. A. 110, 13564–13569. doi: 10.1073/pnas.1307228110
Zhou, Y., Zhou, Z., Zheng, L., Gong, Z., Li, Y., Jin, Y., et al. (2023). Urinary tract infections caused by uropathogenic Escherichia coli: mechanisms of infection and treatment options. Int. J. Mol. Sci. 24. doi: 10.3390/ijms241310537
Keywords: glycoconjugate vaccine, biosynthesis, optimization method, glycosyltransferase, glycoengineering
Citation: Wang Y, Liu H, Wang B, Gheyret G, Qin J, Wang H, Di Y, Wang Y, Wang J and Tan H (2025) Recent advances in the biosynthesis of polysaccharide-based antimicrobial glycoconjugate vaccines. Front. Microbiol. 15:1457908. doi: 10.3389/fmicb.2024.1457908
Received: 01 July 2024; Accepted: 12 December 2024;
Published: 29 January 2025.
Edited by:
Jose Ruben Morones-Ramirez, Autonomous University of Nuevo León, MexicoReviewed by:
Yajie Wang, Westlake University, ChinaCopyright © 2025 Wang, Liu, Wang, Gheyret, Qin, Wang, Di, Wang, Wang and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan Wang, c21hcnRqd3dAMTI2LmNvbQ==; Haining Tan, aGFpbmluZ3RhbkBzZHUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.