- 1Department of Marine Biomedicine and Polar Medicine, Naval Medical Center of PLA, Naval Medical University, Shanghai, China
- 2Department of Biochemistry and Molecular Biology, College of Basic Medical Sciences, Naval Medical University, Shanghai, China
- 3School of Traditional Chinese Medicine, Naval Medical University, Shanghai, China
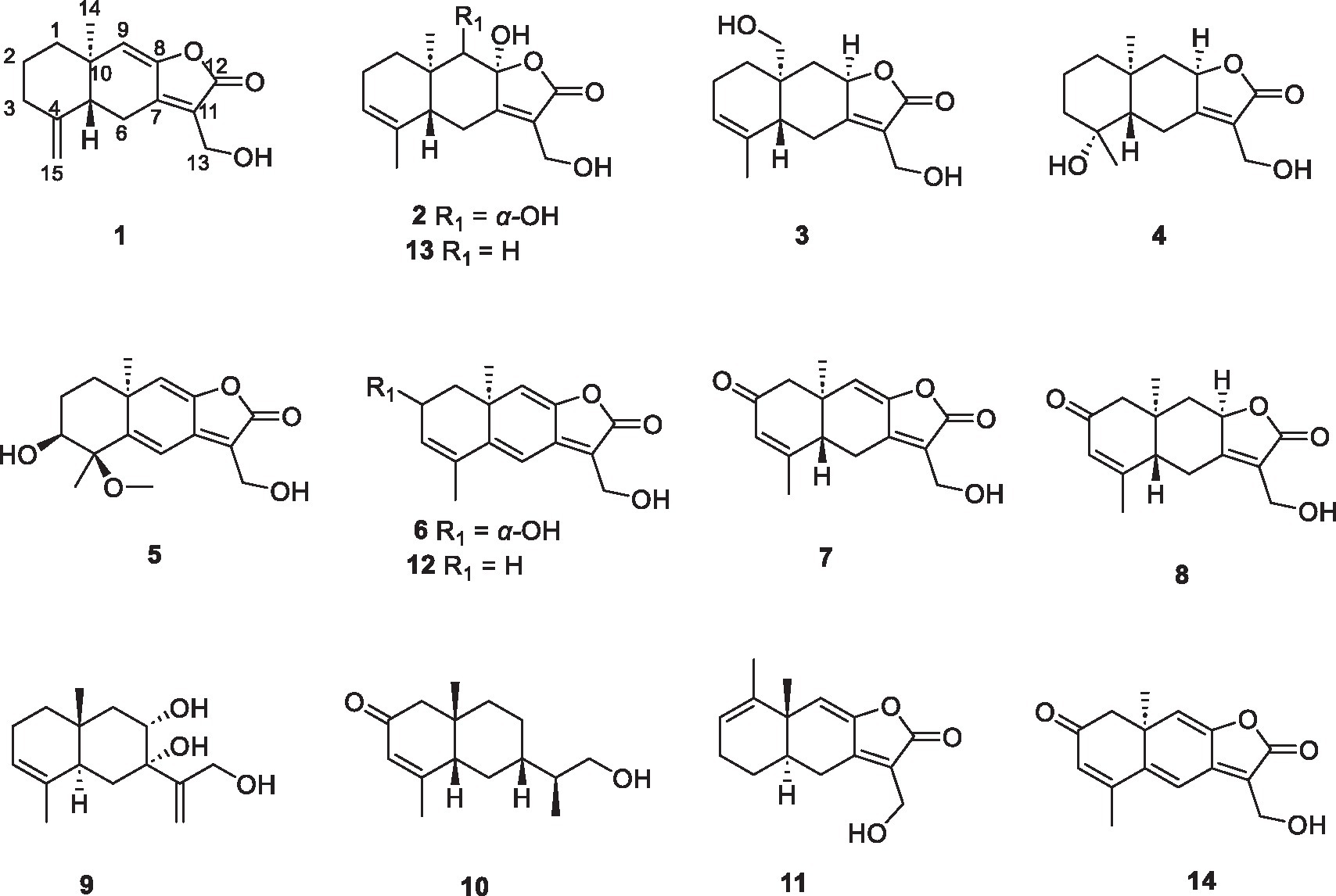
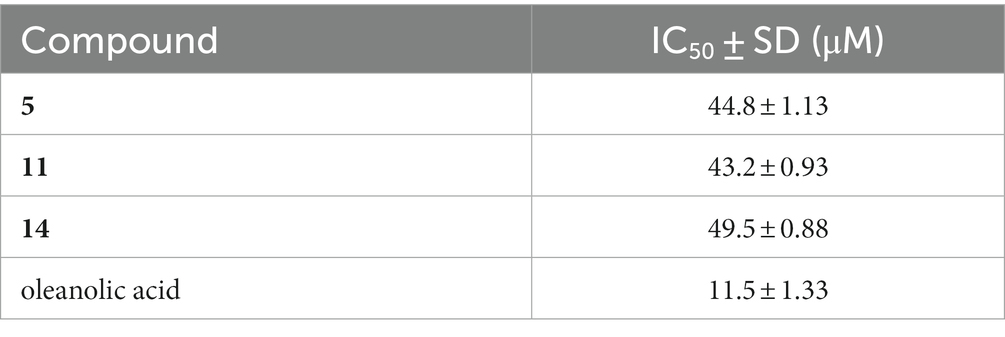
Eight new 12,8-eudesmanolide sesquiterpenes, eutypellaolides A–H (1–8), and two new eudesmane-type sesquiterpenes, eutypellaolides I–J (9–10), along with four known 12,8-eudesmanolide compounds 11–14, were isolated from the culture extract of the polar fungus Eutypella sp. D-1 by one strain many compounds (OSMAC) approach. The structures of these compounds were determined through comprehensive spectroscopic data and experimental and calculated ECD analysis. Antibacterial, immunosuppressive, and PTP1B inhibition activities of these compounds were evaluated. Compounds 1 and 11 exhibited strong inhibitory activities against Bacillus subtilis and Staphylococcus aureus, with each showing an MIC value of 2 μg/mL. Compound 9 displayed weak immunosuppressive activity against ConA-induced T-cell proliferation with an inhibitory rate of 61.7% at a concentration of 19.8 μM. Compounds 5, 11, and 14 exhibited weak PTP1B inhibition activities with IC50 values of 44.8, 43.2, and 49.5 μM, respectively.
1 Introduction
The polar regions are renowned for their harsh environmental conditions, characterized by extremely low temperatures, hurricanes, and intense ultraviolet radiation. These extreme conditions contribute to the development of unique physiological adaptations in microorganisms that inhabit the polar regions, leading to a diversity of microbial secondary metabolites (Santiago et al., 2015). Due to the exceptional conditions and abundant microbiological resources, polar regions have been an increasing interest in human activities and scientific research (Lu et al., 2014; Tian et al., 2017). However, compared with the vast number of natural products reported in tropical regions, compounds from the polar regions have been relatively limited, with only over one hundred new compounds being reported in recent years (Dos Santos et al., 2021). Consequently, polar microbiology has gained recognition as a crucial source of bioactive natural products.
The fungi of Eutypella genus have been extensively investigated for decades due to the respected biological and pharmacological activities of their secondary metabolites (Pongcharoen et al., 2006; Ning et al., 2023). The polar fungus Eutypella sp. D-1 has been discovered to have the ability to produce a variety of structurally distinct and biologically active secondary metabolites, such as cytochalasins, pimarane diterpenes, cytosporins, and sesquiterpenes, with significant antimicrobial and cytotoxic activities (Liu et al., 2014; Zhou et al., 2017; Wang et al., 2018; Yu et al., 2018a,b; Zhang et al., 2019). Previous research on Eutypella sp. D-1 has discovered one sesquiterpene, eut-Guaiane (11), with significant antibacterial activity (Zhou et al., 2017). The OSMAC (One Strain Many Compounds) approach has been extensively employed for the identification of novel metabolites from microorganisms. To enhance the sesquiterpene chemical diversity of Eutypella sp. D-1, drawing inspiration from the OSMAC strategy, we utilized different culture conditions. Subsequently, the EtOAc extracts of the different fermentation broths were subjected to HPLC analysis, and a large number of sesquiterpene analogs were found in solid defined medium compared with PDB medium (Supplementary Figure S110). A follow-up chemical investigation led to the isolation of ten new sesquiterpenes, eutypellaolides A–J (1–10), and four known related compounds 11–14 (Figure 1). Herein, we present the isolation, structure elucidation, and bioactive evaluation of these compounds.
2 Materials and methods
2.1 General experimental procedures
Optical rotations were obtained on the Shanghai Shenguang SGWzz-2 model automatic polarimeter (Shanghai Shenguang Instrument and Meter Co., Ltd., Shanghai, China). IR spectra of all compounds were recorded on Bruker’s VERTEX 70v FT-IR Spectrometer (Bruker Biospin Corp., Billerica, Mass., USA). UV and CD spectra were measured on a JASCO-810 model spectrometer (Jasco Inc., Tokyo, Japan). HRESIMS data were recorded on an Agilent 6,520 AccuTOF LC-plus 4G instrument (JEOL., Tokyo, Japan). The 1D and 2D spectral data were acquired on Bruker AMX-500 and Bruker AVANCE-600 instruments (Bruker Biospin Corp., Billerica, Mass., USA). Semi-performance liquid chromatography was performed on a Waters 1,525 separation module (Waters Corp., Milford, Mass., USA), with YMC-PackPro C18 RS (5 μm) columns and octadecyl silyl silica gel (50 μm, YMC Co. Ltd., Kyoto, Japan). Column chromatographic purifications were performed on silica gel 60 (200–300 mesh, Qingdao Ocean Chemical Co., Qingdao, China), ODS (50 μm, YMC Co. Ltd., Kyoto, Japan).
2.2 Fungal material
The strain Eutypella sp. D-1 was collected near the Ny-Ålesund District in the London Island of Kongsfjorden of the Arctic. It was purified at 20°C by using potato dextrose agar (PDA) medium and identified as Eutypella sp. through 18S rDNA gene sequence analysis (GenBank Accession number FJ430580). At present, the strain of Eutypella sp. D-1 was deposited at the Naval Medical University, Shanghai, China.
2.3 Fermentation, extraction, and isolation
The fungal strain Eutypella sp. D-1 was cultured on the PDB medium at 28°C for 3 days to activate the strain, and then, 5% of the activated strain was cultivated into 250 mL Erlenmeyer flasks, each containing 100 mL of seed medium (glucose 12.5%, NaNO3 0.33%, MgSO4·7H2O 0.04%, K2HPO4·3H2O 0.007%, KCl 0.0625%, yeast extract 0.07%, Lornithine hydrochloride 1.5%, and microelement including FeSO4·7H2O 1.875‰, CoCl2·6H2O, 0.3125‰, CaCl2 0.65‰). After 2 days of incubation at 28°C on a rotary shaker at 180 r/min, each containing 200 μL of activated liquid strain cultures was transferred on the modified solid defined medium (sucrose 5.14%, NaNO3 0.33%, MgSO4·7H2O 0.04%, K2HPO4·3H2O 0.007%, KCl 0.0625%, yeast extract 0.07%, CaCl2 0.65%, agar powder 2%), with total 40 pieces (φ20 × 20 cm). The modified solid cultivation was kept for 45 days at 20°C.
After subjecting the solid defined medium to 1 hour of ultrasonic treatment with a mixture of CH2Cl2/CH3OH (v/v, 1:1), it was subsequently extracted three times using the same mixture. The CH2Cl2/CH3OH solution was evaporated under reduced pressure to obtain an aqueous solution and then extracted with EtOAc three times under reduced pressure at 40°C to yield a dark brown gum (6.86 g).
The crude extract was subjected to silica gel column chromatography using petroleum ether (PE)/EtOAc in a gradient elution (v/v, 100:0, 100:1, 80:1, 50:1, 30:1, 15:1, 10:1, 5:1, 2:1, 1:1, and 0:1) to afford twenty fractions (A − T). Fr. K was purified by HPLC (65% CH3OH/H2O, 2.0 mL/min) and detected at the wavelength of 300 nm, to yield 1 (tR = 37.4 min, 2.0 mg), 11 (tR = 40.1 min, 234.4 mg), and 12 (tR = 27.2 min, 147.6 mg). Fr. O was separated on an ODS (50 μm) column followed by gradient elution with MeOH/H2O (from 50 to 100%) to give eight subfractions (O1–O8). Fr. O3 was detected at the wavelength of 300 nm to yield 5 (tR = 32.5 min, 3.5 mg) by further purifying using HPLC (25% CH3CN /H2O, 2.0 mL/min). Fr. O5 was isolated by HPLC with elution of 35% CH3CN detected at the wavelength of 240 nm to afford 13 (tR = 33.6 min, 18.6 mg). Fr. P was separated by ODS (50 μm) using MeOH/H2O (from 60 to 100%) to give five subfractions (P1–P5). Compound 2 (tR = 21.8 min, 1.5 mg) was purified from Fr. P2 by HPLC using 30% CH3CN/H2O at the wavelength of 222 nm. Compound 9 (tR = 18.4 min, 3.0 mg) was purified from Fr. P4 by HPLC using 40% CH3CN/H2O at the wavelength of 222 nm. Compound 10 (tR = 29.4 min, 4.1 mg) was purified from Fr. P3 by HPLC using 40% CH3CN/H2O at the wavelength of 222 nm. Fr. Q was separated by ODS (50 μm) using MeOH/H2O (from 60 to 100%) to give six subfractions (Q1 − Q6). Compounds 6 (tR = 47.2 min, 9.4 mg) and 14 (tR = 66.9 min, 19.8 mg) were isolated from Fr. Q1 by HPLC using 20% CH3CN/H2O at the wavelength of 240 nm. Fr. R was separated by ODS (50 μm) using MeOH/H2O (from 30 to 80%) to give five subfractions (R1–R5). Fr. R1 was separated by HPLC using 20% CH3CN/H2O to afford 7 (tR = 33.7 min, 8.0 mg) at the wavelength of 220 nm. Fr. R2 was further separated using silica gel with a PE/EtOAc gradient elution (v/v, 10:1, 5:1, 3:1, 2:1, 1:1, and 0:1) to give five subfractions (R2a–R2e). Compounds 3 (tR = 69.1 min, 3.3 mg) and 4 (tR = 73.1 min, 2.9 mg) were obtained from Fr. R2d by HPLC using 37% MeOH/H2O at the wavelength of 220 nm. Fr. S was separated by ODS using MeOH/H2O (from 20 to 60%) to give three subfractions (S1–S3). Compound 8 (tR = 32.0 min, 8.7 mg) was obtained from Fr. S1 by HPLC using 18% CH3CN/H2O at the wavelength of 245 nm.
2.4 Compound characterization data
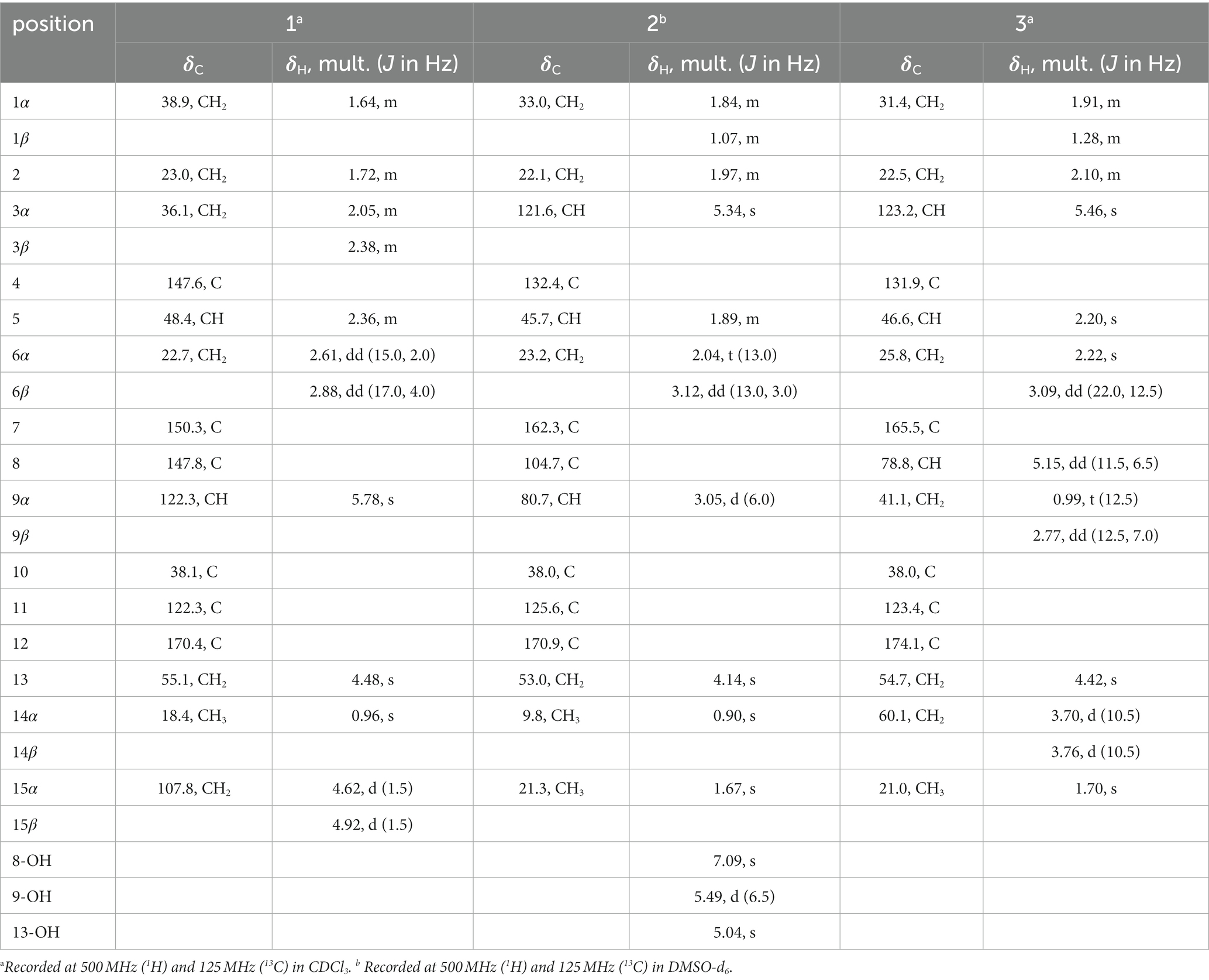
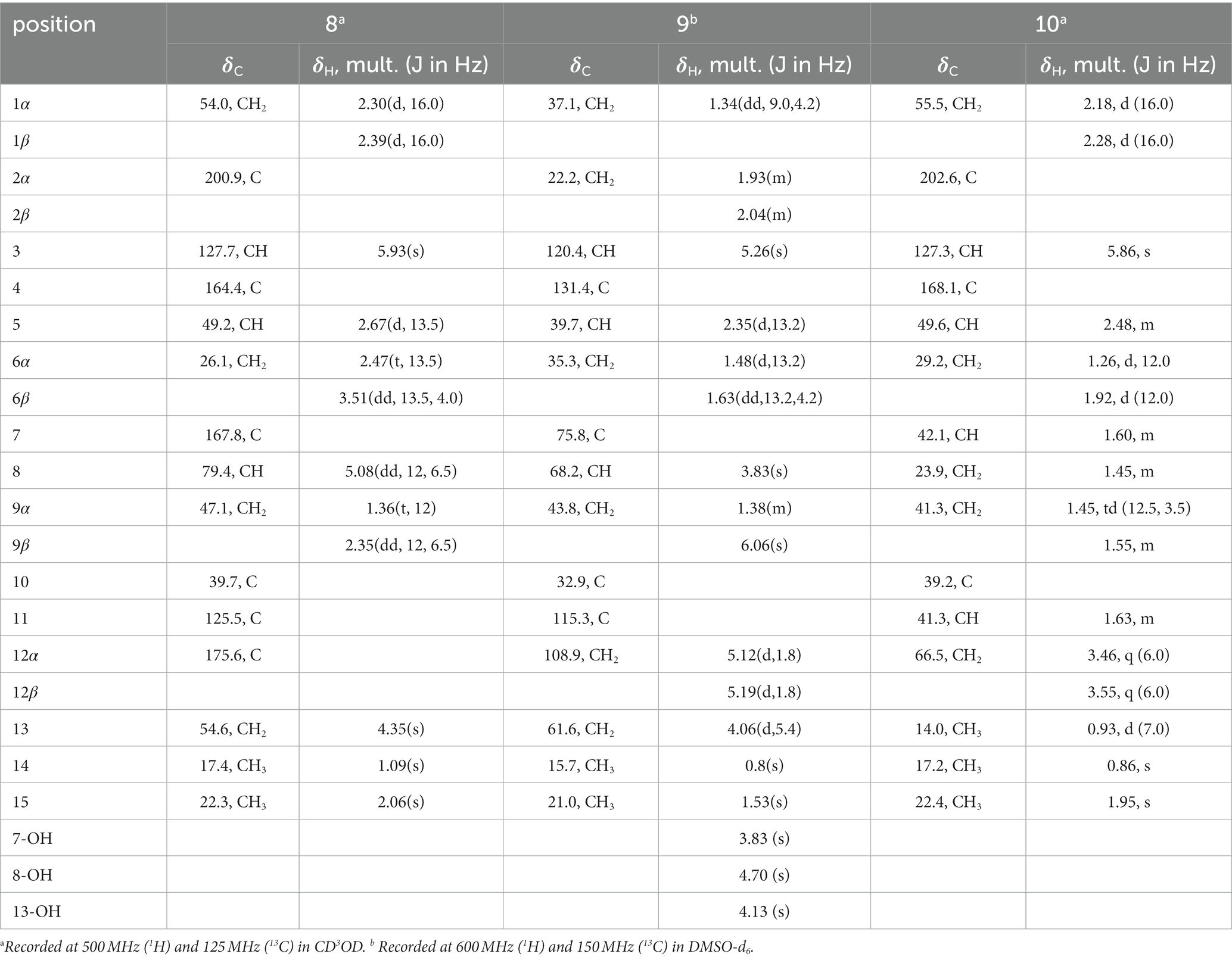
Eutypellaolide A (1): yellowish oil; −27.9 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 278 (3.98) nm; IRνmax 3,444, 2,929, 2,851, 1757, 1,645, 1,260, 1,091, 1,006, 891, 795, 735 cm−1; 1H and 13C NMR date, table 1; HRESIMS m/z 269.1153 [M + Na]+ (calcd for C15H18O3 Na, 269.1148).
Eutypellaolide B (2): yellowish oil; −15.5 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 222 (3.64) nm; IR νmax 3,323, 2,924, 2,854, 1742, 1,587, 1,381, 1,204, 1,125, 1,024, 950, 880, 794, 625 cm−1; 1H and 13C NMR date, table 2; HRESIMS m/z 303.1203 [M + Na]+ (calcd for C15H20O5Na, 303.1203).
Eutypellaolide C (3): yellowish oil; −31.9 (c 0.5, MeOH); UV (MeOH) λmax (log ε) 218 (4.24), 276 (2.98) nm; IR νmax 3,386, 2,927, 1731, 1,675, 1,600, 1,442, 1,345, 1,219, 1,069, 1,014, 956, 798, 702, 617 cm−1; 1H and 13C NMR data, table 3; HRESIMS m/z 287.1266 [M + Na]+ (calcd for C15H20O4Na, 287.1254).
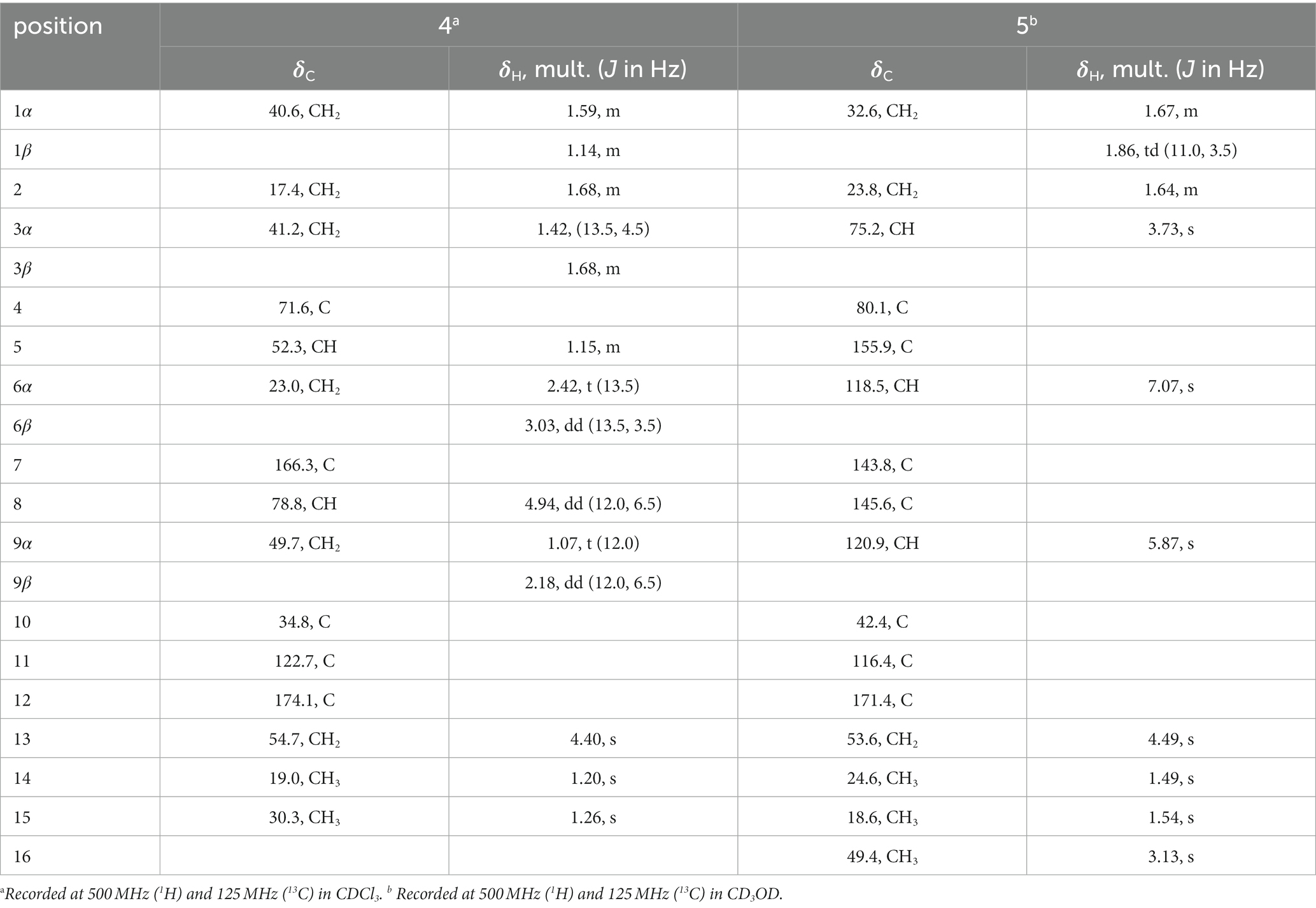
Eutypellaolide D (4): yellowish oil; −47.2 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 238 (4.16), 278 (4.12) nm; IR νmax 3,420, 2,929, 2,865, 1733, 1,674, 1,600, 1,457, 1,381, 1,351, 1,220, 1,135, 1,078, 1,009, 919, 838, 696 cm−1; 1H and 13C NMR data, table 4; HRESIMS m/z 289.1401 [M + Na]+ (calcd for C15H22O4Na, 289.1410).
Eutypellaolide E (5): yellowish oil; −44.0 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 302 (4.17) nm; IR νmax 3,424, 2,930, 1748, 1,692, 1,633, 1,597, 1,450, 1,371, 1,312, 1,205, 1,162, 1,096, 1,069, 984, 867, 669 cm−1; 1H and 13C NMR data, table 5; HRESIMS m/z 315.1204[M + Na]+ (calcd for C16H20O5Na, 315.1203).
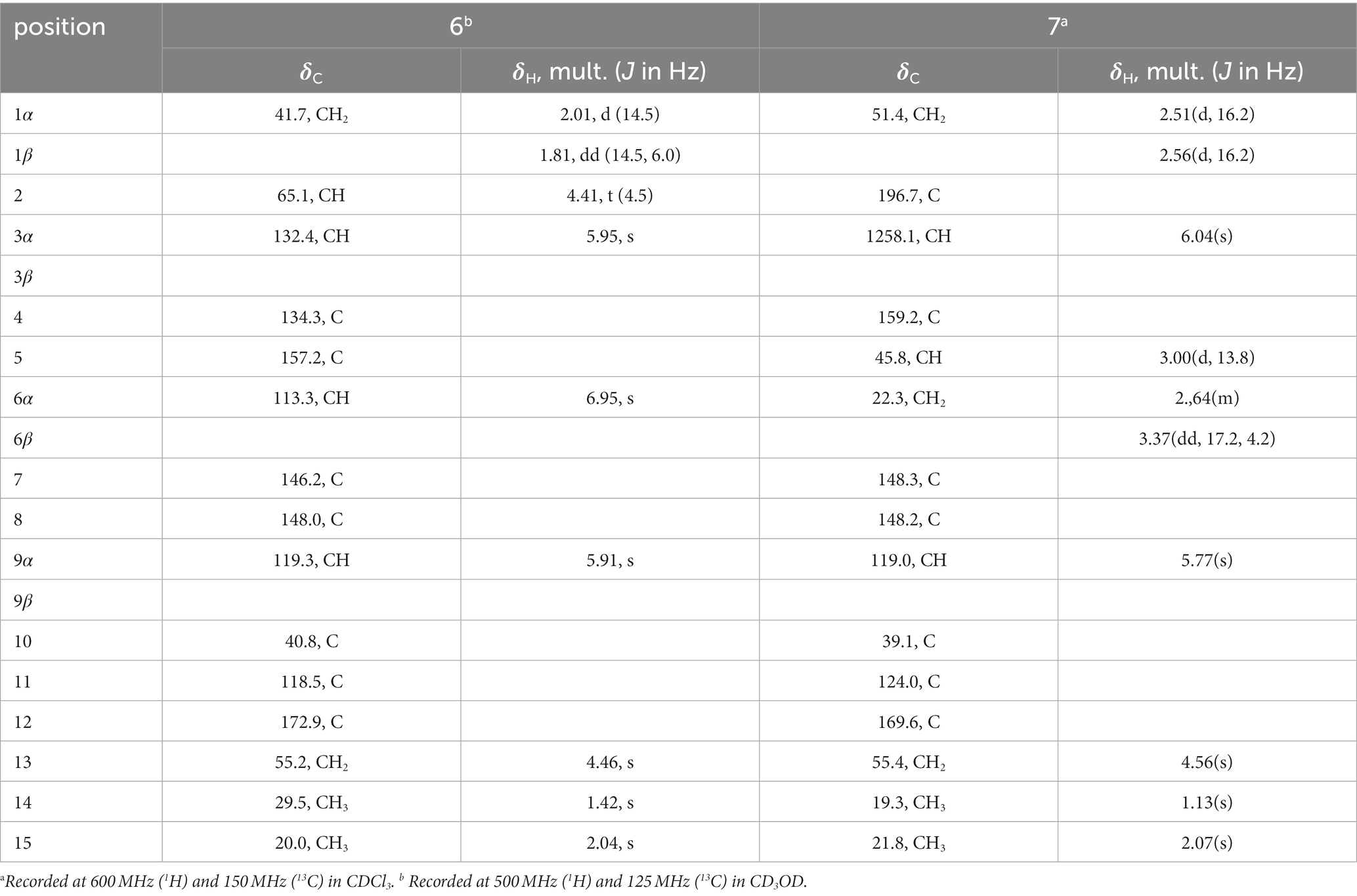
Eutypellaolide F (6): yellowish oil; +125.7 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 218 (4.17), 325 (4.27) nm; IR νmax 3,396, 2,922, 2,853, 1744, 1,619, 1,452, 1,329, 1,213, 1,071, 996, 958, 885, 788 cm−1; 1H and 13C NMR data, table 6; HRESIMS 283.0942 m/z [M + Na]+ (calcd for C15H16O4Na, 283.0941).
Eutypellaolide G (7): yellowish oil; −9.3 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 220 (3.99) nm; IR νmax 3,375, 2,925, 2,857, 1743, 1,686, 1,619, 1,453, 1,377, 1,330, 1,214, 1,127, 1,071, 997, 959, 886, 827, 749 cm−1; 1H and 13C NMR data, table 8; HRESIMS m/z 283.0947 [M + Na]+ (calcd for C15H16O4Na, 283.0941).
Eutypellaolide H (8): yellowish oil; −91.2 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 225 (4.27) nm; IR νmax 3,373, 2,925, 1740, 1,649, 1,434, 1,380, 1,333, 1,078, 1,013, 621 cm−1; 1H and 13C NMR data, table 9; HRESIMS m/z 285.1103 [M + Na]+ (calcd for C15H18O4Na, 285.1097).
Eutypellaolide I (9): white powder; +0.6 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 194 (3.40) nm; IR νmax 3,251, 2,915, 1,639, 1,434, 1,378, 1,272, 1,215, 1,062, 1,031, 1,021, 900, 795, 674 cm−1; 1H and 13C NMR data, table 10; HRESIMS m/z 275.1620 [M + Na]+ (calcd for C15H24O3Na, 275.1618).
Eutypellaolide J (10): yellowish oil; −66.2 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 238 (3.90) nm; IR νmax 3,425, 2,928, 2,873, 1,655, 1,617, 1,438, 1,378, 1,347, 1,294, 1,260, 1,037, 985, 905, 869, 837 cm−1; 1H and 13C NMR data, table 10; HRESIMS m/z 237.1847 [M + H]+ (calcd for C15H25O2, 237.1849).
2.5 Biological assay
The antimicrobial activities of compounds 1–14 against (Bacillus subtilis) (ATCC 21951), (Staphylococcus aureus) (ATCC 27217), (Pseudomonas aeruginosa) (ATCC 27853), (Vibrio vulnificus) (ATCC 27562), and (Vibrio parahaemolyticus) (ATCC 17802) were evaluated as previously described (Liao et al., 2017), with levofloxacin positive control. The immunosuppressive activities of compounds 1–14 against ConA-induced T-cell proliferation were performed as previously described (Xu et al., 2021), with cyclosporin A used as a positive control. The inhibitory activity of all isolates against PTP1B was tested in 96-well microplates, as previously reported (Hou et al., 2019), with oleanolic acid used as a positive control. PTP1B was purchased from Sino Biological, Inc. (Beijing, China).
3 Results and discussion
Eutypellaolide A (1) was obtained as a yellowish oil. Its molecular formula C15H18O3 was established through HRESIMS (m/z 269.1153 [M + Na]+), indicating seven degrees of unsaturation. The IR absorption at 3444, 1757, and 1,645 cm−1 confirmed the existence of the α,β-unsaturated γ-lactone carbonyl group (Jang et al., 2017). The 1H NMR spectrum showed exocyclic double bond signals at δH 4.92 (1.5) and 4.62 (1.5), one olefinic singlet at δH 5.78, and one methyl singlet at δH 0.96 (Table 1). The 13C NMR date for 1 revealed the presence of one ester carbonyl at δC 170.4 and six olefinic carbons at δC 150.3, 147.8, 147.6, 122.3, 122.3, and 107.8. The NMR data accounted for four degrees of unsaturation, implying a tricyclic core structure in 1 (Tables 2−4).
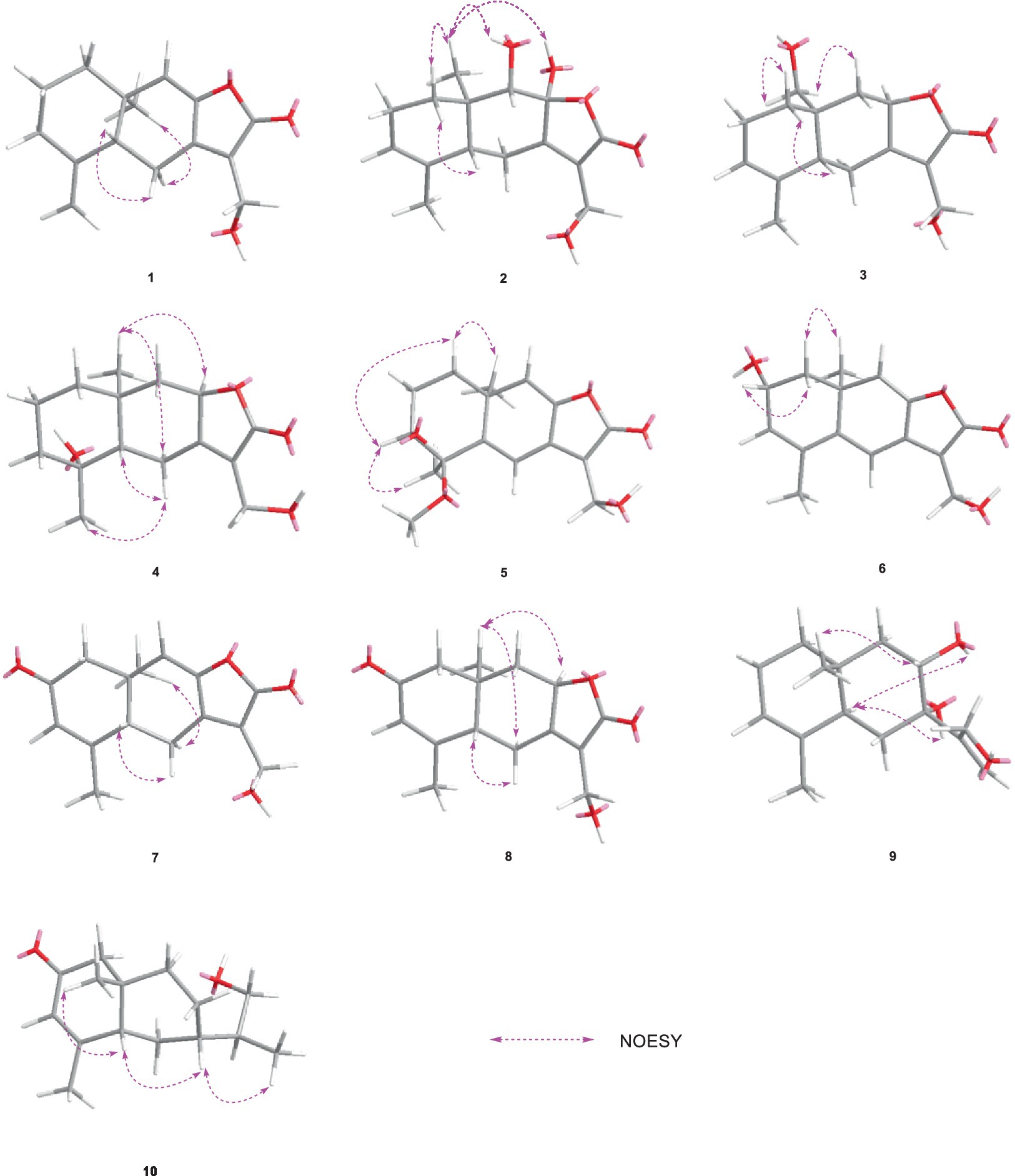
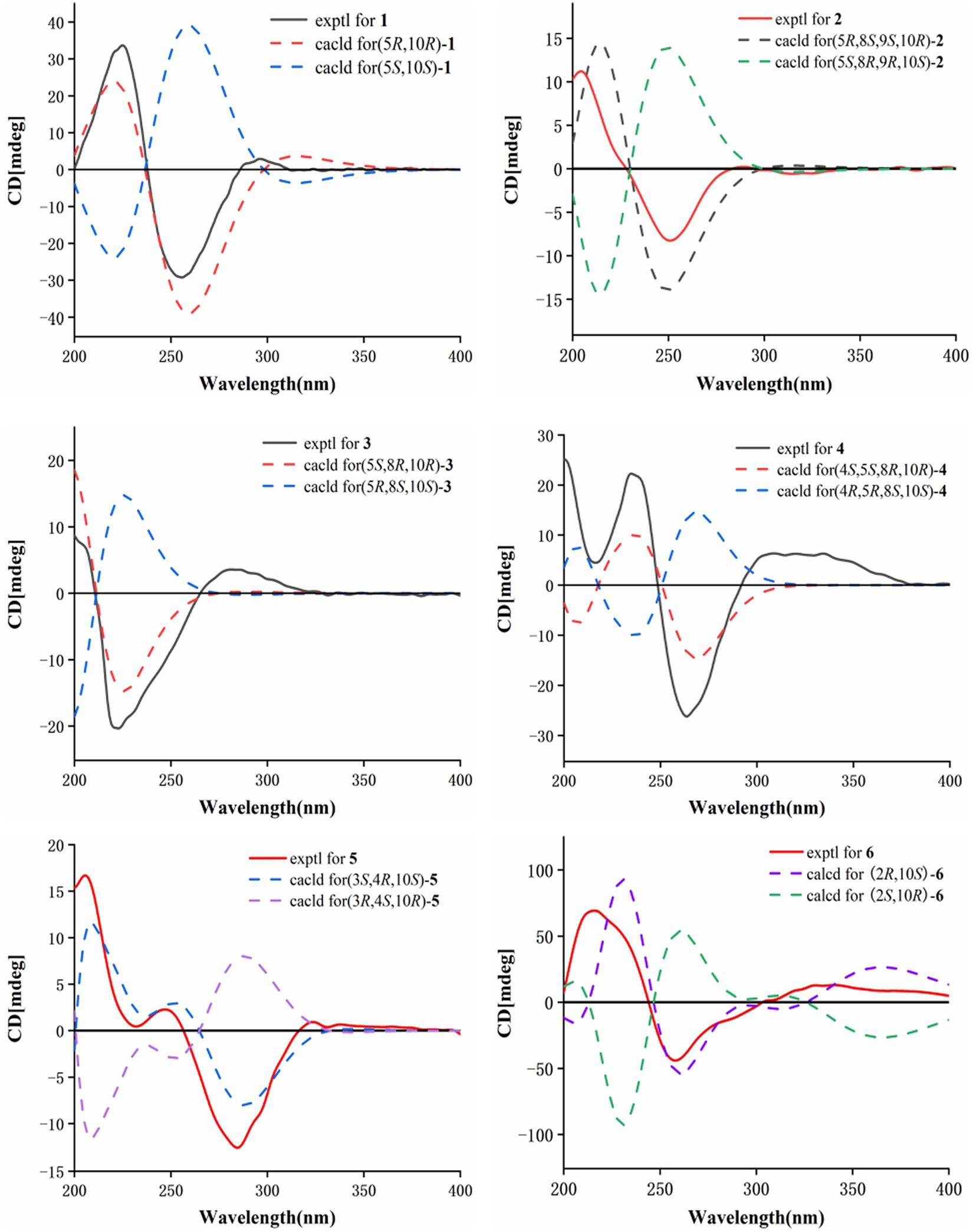
HMBC correlations from H2-6 to C-5, C-7 (δC 150.3), and C-10, and from H-9 to C-7, C-8 (δC 147.8), and C-10, H3-14 to C-1, C-9, and C-10, and from H3-15 to C-3, C-4, and C-5, together with the COSY correlations of H-1/H-2, H-2/H-3, and H-5/H2-6 (Figure 2), established the two six-membered rings A and B with the methyl group CH3-14 attached at C-10. Moreover, the HMBC correlations from H2-13 (δH 4.48) to C-7, C-11 (δC 122.3), and C-12 (δC 170.4) and the presence of downfield C-8 established a C ring. The relative configuration of 1 was deduced through NOESY correlations of H-6α (δH 2.61)/H3-14 (δH 0.96) and H-6β (δH 2.88)/H-5 (δH 2.36) (Figure 3). The absolute configuration of 1 was determined to be 5R,10R by comparing its specific rotation ( −27.9, c 0.1, MeOH) with the known compound atractylenolide II ( +22.2, c 1.05, MeOH) (Li and Yang, 2018). The similarity between the calculated and experimental ECD spectra further provided evidence for the determination of the absolute configuration of 1 (Figure 4).
Eutypellaolide B (2) was obtained as yellowish oil. The molecular formula of 2 was established as C15H20O5 by HRESIMS data (m/z 303.1203 [M + Na]+). The NMR data of 2 were almost identical to those of 13, except for the presence of the 9-OH proton resonance [δH 5.78 (d, 6.5)], which was supported by the HMBC correlations of 9-OH to C-8 (δC 104.7), C-9 (δC 80.7) and C-10 (δC 38.0). The relative configuration of H-5 and H-9 was determined to be β-oriented based on the NOESY correlation of H-1β/H-5 and H-9. Additionally, 8-OH, 9-OH, and H3-14 were established to be α-oriented based on NOESY correlations of H-1α/H3-14 and 8-OH/H3-14 in DMSO-d6. Furthermore, the similarity between the calculated and experimental ECD spectra further confirmed the absolute configurations of 2 as 5R,8S,9S,10R (Figure 4).
Eutypellaolide C (3) was obtained as a yellowish oil. The molecular formula was deduced as C15H20O4 based on HRESIMS (m/z 287.1266 [M + Na]+). The 1H and 13C NMR spectra of 3 displayed remarkable similarity to those of 13-hydroxy-3,7(11)-eudesmadien-2,8-olide (Wang et al., 2017), except for the presence of an additional hydroxy group at C-14 (δC 60.1). The methylene carbon C-14 was attached to C-10, which was supported by the HMBC correlations from H2-14 (δH, 3.73) to C-1 (δC 31.4), C-5 (δC 46.6), C-9 (δC 41.1), and C-10 (δC 38.0). Thus, the planar configuration of 3 is presented in Figure 2. The relative configuration of 3 was determined by the NOESY correlations of H2-14/H-1α, H2-14/H-8, and H-5/H-1β. The absolute configuration of 3 was subsequently determined to be 5S,8R,10R based on a comparison of its specific rotation ( –31.9, MeOH, c 0.1) with that of 13-hydroxy-3,7(11)-eudesmadien-12,8-olide ( –191.4, MeOH, c 0.5) (Wang et al., 2017). The validation of the absolute configuration of 3 was strengthened by the similarity observed between the calculated ECD spectrum and the experimental CD spectrum (Figure 4).
Eutypellaolide D (4) was obtained as a yellowish oil, with a molecular formula of C15H22O4 as determined by HRESIMS (m/z 289.1401 [M + Na]+). Comparison of the 1H and 13C NMR spectra of 4 with those of 4β-hydroxy-5α,8β(H)-eudesm-7(11)-en-8,12-olide (Zhang et al., 2012), with a difference in the presence of a hydroxyl group substituted at C-13 (δC 54.7) in 4, was supported by the HMBC correlations from H2-13 to C-7 (δC 166.3), C-11 (δC 122.7), and C-12 (δC 174.1). The relative configuration of 4 was established by NOESY correlations of H-5/H-6β, H3-15/H-6β, H-8/H3-14, and H-8/H-6α, which indicated that H-5 and H3-15 were in the same orientation, while H-8 and H3-14 had opposite orientation. The absolute configuration of 4 was established as 4S,5S,8R,10R by comparing its specific rotation data ( −47.2, MeOH, c 0.1) with that of 4β-hydroxy-5α,8β(H)-eudesm-7(11)-en-8,12-olide ( +20.0, c 0.57, MeOH) (Wang et al., 2017). The agreement between the calculated ECD spectrum and the experimental CD spectrum also supported the absolute configuration of 4 (Figure 4).
Eutypellaolide E (5) was isolated as a yellowish oil and had a molecular formula of C16H20O5 based on HRESIMS (m/z 315.1204 [M + Na]+). The NMR data of 5 closely resembled those of 12 (Wang et al., 2017), except for the presence of a hydroxy group at C-3 (δC 75.2) and methoxy group at C-4 (δC 80.1) in 5 and the absence of two olefinic carbons (δC 131.2 and δC 131.4) in 12. The C-3 was attached to C-16 (δC 49.4) via the C-4, which was confirmed by the HMBC correlation from H3-16 (δH, 3.13, s) to C-4 (δC 80.1). The NOESY correlations from H3-14 to H-1α and from H-3 to H-1α and H3-15 suggested that H-3/H3-14/H3-15 was located at the same orientation. Furthermore, a comparison between the calculated and the experimental ECD spectra confirmed the absolute configurations of 5 as 3S,4R,10S (Figure 4).
Eutypellaolide F (6), obtained as a yellowish oil, had the molecular formula of C15H16O4, according to its positive HRESIMS (m/z 283.0942 [M + Na]+). Comparative analysis of the data for 6 with that of 12 revealed a high degree of similarity (Wang et al., 2017), except for the hydroxy group substituted at C-2 (δC 65.1) in 6 instead of the H-2 in 12, which was confirmed by the COSY correlations between H-1/H-2/H-3, and the HMBC correlations from H-3 to C-1 (δC 41.7) and C-2 (δC 65.1). The same orientation of 2-OH and H3-14 was deduced from the NOESY correlation between H-1α [δH, 2.01 (d, 14.5 Hz)] and H3-14 and between H-1β [δH, 1.81 (dd, 14.5, 6.0 Hz)] and H-2 [δH, 4.41 (t, 4.5 Hz)]. A comparison between the calculated and experimental ECD spectrum of 6 determined its absolute configuration as 2R,10S (Figure 4).
Eutypellaolide G (7) was obtained as a yellowish oil, and its molecular formula was deduced as C15H16O4 due to its HRESIMS (m/z 283.0947 [M + Na]+), indicating eight degrees of unsaturation. Notably, the NMR data of 7 closely resembled that of chlorantholide A (Wang et al., 2012), except for the discernible hydroxy group at C-13 in 7, which was verified by the HRESIMS data and the HMBC correlations from H2-13 (δH 4.56) to C-7 (δC 148.3), C-11 (δC 124.0), and C-12 (δC 169.6). The NOESY correlations from H-6α (δH 2.64) to H3-14 and H-6β (δH 3.37) to H-5 confirmed that H3-14 and H-5 were in opposite orientations. The absolute configuration of 7 was determined by comparing its specific rotation with chlorantholide A. A comparison of the specific rotation between 7 ( −9.3, MeOH, c 0.1) and chlorantholide A (+5.0, MeOH, c 0.2) assigned the absolute configuration of 7 as 5R,10R. The comparison between the calculated and experimental ECD spectra further supported the absolute configuration of 7 (Figure 5).
Eutypellaolide H (8), obtained as a yellowish oil, had the molecular formula of C15H18O4 based on the positive HRESIMS (m/z 285.1103 [M + Na]+). The overall NMR data of 8 indicated a structure similar to 7, with a notable difference in the absence of the double bond between C-8 (δC 148.2) and C-9 (δC 119.0) in 7, confirmed by the HMBC correlations from H-8 (δH 5.08, dd, 12.0, 6.5 Hz) to C-7(δC 167.8), C-9 (δC 47.1), C-11 (δC 125.5), and C-12 (δC 175.6) and COSY correlations from H-8 to H-9. The detectable NOESY correlations of H-8/H-6α, H-8/H3-14, and H-6β/H-5 confirmed that H-8 and H3-14 are oriented in the same way, while H-5 are oriented in an opposite direction. The absolute configuration of 8 could be assigned as 5S,8R,10R by a comparison of the specific rotation ( −91.2, MeOH, c 0.1) with that of chlorantholide B ( +74.7, MeOH, c 0.1) (Wang et al., 2012). The similarity between the calculated ECD spectrum and the experiment further supported the absolute configuration of 8 (Figure 5).
Eutypellaolide I (9) was isolated as a white powder, with a molecular formula of C15H24O3 as determined by HRESIMS and NMR data, indicating the presence of four degrees of unsaturation. The 1H NMR spectrum showed terminal methylene singlet at δH 5.29 and δH 5.36 and exocyclic methylene at δH 4.20 and δH 4.30. The 13C NMR spectrum for 9 revealed the presence of four olefinic carbons at δC 153.5, 133.9, 121.3, and 114.8, one oxygenated quaternary carbon at δC 76.9, one oxymethine carbon at δC 70.4, one oxygenated methylene at δC 64.7, and two methyl carbons at δC 15.9 and δC 21.2, and the remaining two degrees of unsaturation implied that 9 was likely to be dicyclic sesquiterpenes. The HMBC correlations from H3-15 to C-3 and C-4, from H3-14 to C-1, C-5, C-9, and C-10, from H2-6 to C-4, C-7, C-8, and C-10, and from H2-9 to C-5, C-7, C-8, and C-10, together with the COSY correlations between H-1/H-2/H-3, H-5/H2-6, and H-8/H2-9, confirmed the presence of a six-membered ring. Additional HMBC correlations from H-12a/12b to C-7, C-11, and C-12 and from H-13 to C-7 suggested direct linkages between C-7 and C-11. The NOESY correlations from H3-14 to H-8 indicated that these protons are on the same side, and those from H-5 to 7-OH and 8-OH confirmed that they were in opposite orientations. The absolute configuration of 9 was subsequently determined to be 5R,7S,8R,10S based on a comparison of the specific rotation ( +0.6, MeOH, c 0.1) with that of rhaponticol ( +33.3, MeOH, c 0.12) (Cheng et al., 1995), following the method as the cases of compounds 1, 3, 4, 7, and 8.
Eutypellaolide J (10) was a colorless oil and exhibited a planar structure identical to that of thomimarine E (Afiyatullov et al., 2017). However, the chemical shift of C-11 (δC 41.3) in 10 was different from C-11 (δC 33.1) in thomimarine E, confirming that 10 and thomimarine E have different configurations, which was supported by the NOESY spectrum correlations from H-5 (δH 2.48) to H-7 (δH 1.60) and H3-14 (δH 0.86) and from H-7 to H3-13 (δH 0.93), indicating that these protons were on the same side. The absolute configuration of compound 10 was established as 5S,7S,10S,11S by a comparison between experimental and calculated ECD (Figure 5).
The known compounds eut-Guaiane (11), 13-hydroxy-3,5,8,7(11)-eudesmatetraen-12,8-olide (12), 8,13-dihydroxy-3,7(11)-eudesmadien-12,8-olide (13), and 2-one-13hydroxy-3,5,8,7(11)-eudesmatetraen-12,8-olide (14) were also isolated from Eutypella sp. D-1 and were completely characterized by comparison of their NMR data with that previously reported (Wang et al., 2017; Zhou et al., 2017).
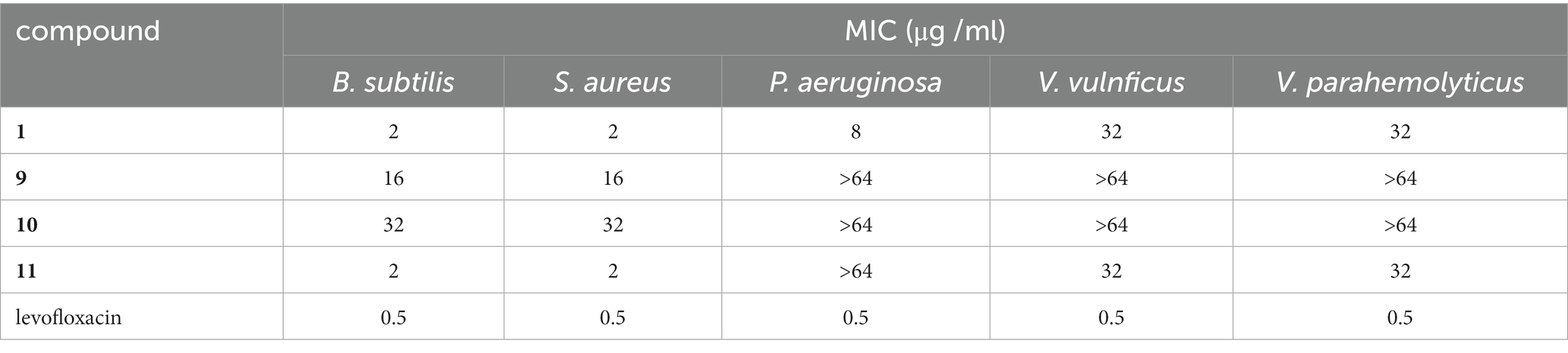
All the isolated compounds 1–14 were screened for their antibacterial activity against Staphylococcus aureus (ATCC 27217), Bacillus subtilis (ATCC 21951), Pseudomonas aeruginosa (ATCC 27853), Vibrio vulnificus (ATCC 27562), and Vibrio parahaemolyticus (ATCC 17802). Among them, compounds 1 and 11 displayed potent activity against B. subtilis and S. aureus, with MIC values of 2 μg/mL for both strains (Table 5). Additional immunosuppressive activity against ConA-induced T-cell proliferation for 1–14 was also tested. Among them, only 9 exhibited immunosuppressive activity, with inhibitory rates of 61.7% observed at a concentration of 19.8 μM. Compounds 1–14 were subjected to preliminary screening for their inhibitory activity against PTP1B. However, only compounds 5, 11, and 14 exhibited moderate activity, with IC50 values of 44.8, 43.2, and 49.5 μM, respectively (Table 6).
4 Conclusion
In summary, the OSMAC approach effectively induced chemical diversities of the polar fungus Eutypella sp. D-1, using a modified solid nutrient medium, to produce fourteen sesquiterpene compounds. Among them, there were ten new sesquiterpenes eutypellaolides A − J (1–10) and four known 12,8-eudesmanolide compounds 11–14. Fortunately, the production of compound 11, which exhibits excellent antibacterial activity, increased sharply. Interestingly, these new metabolites were only detected in the solid nutrient medium and were not produced when the fungus was cultivated in potato dextrose broth (PDB) or other liquid media (Lu et al., 2014; Zhou et al., 2017; Wang et al., 2018). Therefore, it could be concluded that the OSMAC approach should be a feasible and effective strategy to trigger the production of bioactive secondary metabolites from the polar fungi. Compounds 5, 11, and 14 possess an α,β-unsaturated γ-lactone structure, which serves as a crucial pharmacophore for significant PTP1B inhibitory activity, and this characteristic was shared with sesterterpene phyllofolactones A and phyllofolactones F, as reported in the literature (Abdjul et al., 2015). The findings from this study contribute to the expanding knowledge of natural products from polar fungi and their potential for discovery as new drug leads.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
ZN: Data curation, Investigation, Methodology, Writing – original draft. BH: Supervision, Validation, Writing – original draft. Y-YS: Investigation, Writing – review & editing. J-FD: Software, Writing – review & editing. X-YH: Data curation, Formal Analysis, Writing – review & editing. X-LL: Project administration, Writing – review & editing. Z-FY: Visualization, Writing – review & editing. YH: Conceptualization, Writing – review & editing. B-HJ: Resources, Visualization, Writing – review & editing. H-BY: Conceptualization, Funding acquisition, Resources, Writing – review & editing. X-YL: Conceptualization, Funding acquisition, Methodology, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was financially supported by the National Key Research and Development Project (Nos. 2022YFC2804500 and 2022YFC2804105).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2024.1349151/full#supplementary-material
References
Abdjul, D. B., Yamazaki, H., Takahashi, O., Kirikoshi, R., Mangindaan, R. E. P., and Namikoshi, M. (2015). Two new protein tyrosine phosphatase 1B inhibitors, hyattellactones a and B, from the Indonesian marine sponge Hyattella sp. Bioorg. Med. Chem. Lett. 25, 904–907. doi: 10.1016/j.bmcl.2014.12.058
Afiyatullov, S. S., Leshchenko, E. V., Sobolevskaya, M. P., Antonov, A. S., Denisenko, V. A., Popov, R. S., et al. (2017). New Thomimarine E from marine isolate of the fungus Penicillium thomii. Chem. Nat. Compd. 53, 290–294. doi: 10.1007/s10600-017-1972-9
Cheng, D. L., Wei, H. X., and Cheng, J. K. (1995). A new eudesmane sesquiterpenes from the root of rhaponticum uniflorum. Chin. Chem. Lett. 6, 111–112.
Dos Santos, G. S., Teixeira, T. R., Colepicolo, P., and Debonsi, H. M. (2021). Natural products from the poles: structural diversity and biological activities. Rev. Bras 31, 531–560. doi: 10.1007/s43450-021-00203-z
Hou, J. Q., Fan, C. L., Pei, X., Zhang, P. L., Deng, F., Jiang, W. Q., et al. (2019). Psiguadiols A–J, rearranged Meroterpenoids as potent PTP1B inhibitors from Psidium guajava. J. Nat. Prod. 82, 3267–3278. doi: 10.1021/acs.jnatprod.9b00333
Jang, H. J., Lee, S., Lee, S. J., Lim, H. J., Jung, K., Kim, Y. H., et al. (2017). Anti-inflammatory activity of eudesmane-type sesquiterpenoids from Salvia plebeia. J. Nat. Prod. 80, 2666–2676. doi: 10.1021/acs.jnatprod.7b00326
Li, Y., and Yang, X. W. (2018). Chemical constituents of rhizomes of Atractylodes macrocephala. Mod. Chin. Med. 20, 382–386. doi: 10.13313/j.issn.1673-4890.20171029001
Liao, H. X., Sun, D. W., Zheng, C. J., and Wang, C. Y. (2017). A new hexahydrobenzopyran derivative from the gorgonian-derived fungus Eutypella sp. Nat. Prod. Res. 31, 1640–1646. doi: 10.1080/14786419.2017.1285301
Liu, J. T., Hu, B., Gao, Y., Zhang, J. P., Jiao, B. H., Lu, X. L., et al. (2014). Bioactive tyrosine-derived cytochalasins from fungus Eutypella sp. D-1. Chem. Biodivers. 11, 800–806. doi: 10.1002/cbdv.201300218
Lu, X. L., Liu, J. T., Liu, X. Y., Gao, Y., Zhang, J., Jiao, B. H., et al. (2014). Pimarane diterpenes from the arctic fungus Eutypella sp. D-1. J. Antibiot. (Tokyo) 67, 171–174. doi: 10.1038/ja.2013.104
Ning, Y. D., Zhang, S., Zheng, T., Xu, Y., Li, S., Zhang, J. P., et al. (2023). Pimarane-type Diterpenes with anti-inflammatory activity from Arctic-derived fungus Eutypella sp. D-1. Mar. Drugs 21:541. doi: 10.3390/md21100541
Pongcharoen, W., Rukachaisirikul, V., Phongpaichit, S., Rungjindamai, N., and Sakayaroj, J. (2006). Pimarane diterpene and cytochalasin derivatives from the endophytic fungus Eutypella scoparia PSU-D44. J. Nat. Prod. 69, 856–858. doi: 10.1021/np0600649
Santiago, I. F., Soares, M. A., Rosa, C. A., and Rosa, L. H. (2015). Lichensphere: a protected natural microhabitat of the non-lichenised fungal communities living in extreme environments of Antarctica. Extremophiles 19, 1087–1097. doi: 10.1007/s00792-015-0781-y
Tian, Y., Li, Y. L., and Zhao, F. C. (2017). Secondary metabolites from polar organisms. Mar. Drugs 15:28. doi: 10.3390/md15030028
Wang, X. L., Sun, K. L., and Wang, B. (2018). Bioactive pimarane diterpenes from the arctic fungus Eutypella sp. D-1. Chem. Biodivers. 15:e1700501. doi: 10.1002/cbdv.201700501
Wang, Y. Z., Wang, Y., Wu, A. A., Zhang, L., Hu, Z. Y., Huang, H. Y., et al. (2017). New 12,8-Eudesmanolides from Eutypella sp. 1–15. J. Antibiot. 70, 1029–1032. doi: 10.1038/ja.2017.89
Wang, F., Zhou, D. S., Wei, G. Z., Ren, F. C., and Liu, J. K. (2012). Chlorantholides A–F, eudesmane-type sesquiterpene lactones from Chloranthus elatior. Phytochemistry 77, 312–317. doi: 10.1016/j.phytochem.2012.02.008
Xu, J., Zhang, X. X., Huang, F. L., Li, G., and Leadlay, P. F. (2021). Efophylins a and B, two C2-asymmetric Macrodiolide Immunosuppres-sants from Streptomyces malaysiensis. J. Nat. Prod. 84, 1579–1586. doi: 10.1021/acs.jnatprod.1c00118
Yu, H. B., Wang, X. L., Xu, W. H., Zhang, Y. X., Qian, Y. S., Zhang, J. P., et al. (2018a). Eutypellenoids A–C, new Pimarane Diterpenes from the Arctic fungus Eutypella sp. D-1. Mar. Drugs 16:284. doi: 10.3390/md16080284
Yu, H. B., Wang, X. L., Zhang, Y. X., Xu, W. H., Zhang, J. P., Zhou, X. Y., et al. (2018b). Libertellenones O–S and Eutypellenones a and B, Pimarane Diterpene derivatives from the Arctic fungus Eutypella sp. D-1. J. Nat. Prod. 81, 1553–1560. doi: 10.1021/acs.jnatprod.8b00039
Zhang, M., Iinuma, M., Wang, J. S., Oyama, M., Ito, T., and Kong, L. Y. (2012). Terpenoids from Chloranthus serratus and their anti-inflammatory activities. J. Nat. Prod. 75, 694–698. doi: 10.1021/np200968p
Zhang, Y. X., Yu, H. B., Xu, W. H., Hu, B., Guild, A., Zhang, J. P., et al. (2019). Eutypellacytosporins A–D, Meroterpenoids from the Arctic fungus Eutypella sp. D-1. J. Nat. Prod. 82, 3089–3095. doi: 10.1021/acs.jnatprod.9b00700
Keywords: sesquiterpene, polar fungus, Eutypella sp., OSMAC approach, PTP1B inhibition activity
Citation: Ning Z, Hu B, Sun Y-Y, Ding J-F, Han X-Y, Lu X-L, Yin Z-F, He Y, Jiao B-H, Yu H-B and Liu X-Y (2024) Eutypellaolides A–J, Sesquiterpene diversity expansion of the polar fungus Eutypella sp. D-1. Front. Microbiol. 15:1349151. doi: 10.3389/fmicb.2024.1349151
Edited by:
Sílvia A. Sousa, Institute for Bioengineering and Biosciences, PortugalReviewed by:
Fengyu Du, Qingdao Agricultural University, ChinaGuangtao Zhang, Binzhou Medical University, China
Copyright © 2024 Ning, Hu, Sun, Ding, Han, Lu, Yin, He, Jiao, Yu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao-Bing Yu, eXVoYW9iaW5nMTk4NkAxMjYuY29t; Xiao-Yu Liu, YmlvbHh5QDE2My5jb20=
†These authors have contributed equally to this work
 Zhe Ning
Zhe Ning Bo Hu1†
Bo Hu1† Hao-Bing Yu
Hao-Bing Yu