
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 15 June 2023
Sec. Microbial Symbioses
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1175880
This article is part of the Research Topic Rumen Microbiome: Interacting with Host Genetics, Dietary Nutrients Metabolism, Animal Production, and Environment View all 15 articles
 Fei Jiang1
Fei Jiang1 Yanhua Gao1,2,3,4*
Yanhua Gao1,2,3,4* Zhongli Peng1,2,3,4
Zhongli Peng1,2,3,4 Xiulian Ma1
Xiulian Ma1 Yinjie You1
Yinjie You1 Zhibin Hu1
Zhibin Hu1 Anxiang He5
Anxiang He5 Yupeng Liao6
Yupeng Liao6Introduction: This study was conducted to assess the effect of mixed isoacid (MI) supplementation on fermentation characteristics, nutrient apparent digestibility, growth performance, and rumen bacterial community in yaks.
Methods: A 72-h in vitro fermentation experiment was performed on an ANKOM RF gas production system. MI was added to five treatments at doses of 0, 0.1%, 0.2%, 0.3%, 0.4%, and 0.5% on the dry matter (DM) basis of substrates using a total of 26 bottles (4 bottles per treatment and 2 bottles as the blank). Cumulative gas production was measured at 4, 8, 16, 24, 36, 48, and 72 h. Fermentation characteristics including pH, the concentration of volatile fatty acids (VFAs), ammonia nitrogen (NH3-N), microbial proteins (MCP), and the disappearance rate of dry matter (DMD), neutral detergent fiber (NDFD), and acid detergent fiber (ADFD) were measured after a 72-h in vitro fermentation to determine an optimal MI dose. Fourteen Maiwa male yaks (180–220 kg, 3–4 years old of age) were randomly assigned to the control group (without MI, n = 7) and the supplemented MI group (n = 7, supplemented with 0.3% MI on DM basis) for the 85-d animal experiment. Growth performance, nutrient apparent digestibility, rumen fermentation parameters, and rumen bacterial diversity were measured.
Results: Supplementation with 0.3% MI achieved the greatest propionate and butyrate content, NDFD and ADFD compared with other groups (P < 0.05). Therefore, 0.3% was used for the animal experiment. Supplementation with 0.3% MI significantly increased the apparent digestibility of NDF and ADF (P < 0.05), and the average daily weight gain of yaks (P < 0.05) without affecting the ruminal concentration of NH3-N, MCP, and VFAs. 0.3% MI induced rumen bacteria to form significantly different communities when compared to the control group (P < 0.05). g__norank_f__Bacteroidales_BS11_gut_group, g__norank_f__Muribaculaceae, g__Veillonellaceae_UCG-001, g__Ruminococcus_gauvreauii_group, g__norank_f__norank_o__RF39 and g__Flexilinea were identified as the biomarker taxa in responding to supplementation with 0.3% MI. Meanwhile, the abundance of g__Flexilinea and g__norank_f__norank_o__RF39 were significantly positively correlated with the NDF digestibility (P < 0.05).
Conclusion: In conclusion, supplementation with 0.3% MI improved the in vitro rumen fermentation characteristics, feed fiber digestibility, and growth performance in yaks, which was associated with changes of the abundance of g__Flexilinea and g__norank_f__norank_o__RF39.
Yaks (Bos grunniens), a large ruminant, have adapted well to the high-altitude, low oxygen, and cold environment of the Qinghai-Tibet Plateau, and provide milk, meat, wool, leather, and labor for local residents (Jing et al., 2022). The adaptation of yaks to the highlands is closely related to their unique characteristics in terms of digestion, physiology, rumen fermentation, and rumen microbiota composition. It has been found that yaks have a larger heart and lung volume, a higher capacity to sense the environment, and a higher energy metabolism than cattle (Wang et al., 2011). In addition, yaks are more tolerant to roughage than cattle (Shao et al., 2010), e.g., they digest neutral detergent fiber (NDF) better than cattle and they use nitrogen more efficiently (Zhou et al., 2017, 2018). Yaks are mainly reared on natural pastures, but due to the harsh climatic conditions in the winter season, this feeding pattern cannot sustain adequate nutrient and energy intake for yaks throughout the year, resulting in a retarded growth rate and even weight loss (Long et al., 2005; Ding et al., 2012). Therefore, barn- or feedlot-feeding was introduced to conserve natural pastures, improve the growth rate, and shorten the feeding period of yaks (Kang et al., 2020; Du et al., 2021; Hu et al., 2021).
Similar to other ruminants, yaks rely on rumen microorganisms to break down dietary nutrient components. Differences in physiological characteristics and digestibility suggest that the rumen microbiome composition of yaks may differ from that of other ruminants (Xin et al., 2019). Compared with cattle, yaks show a higher abundance of Firmicutes in the rumen (Xue et al., 2018). A representative genus is Clostridium, which can degrade cellulose, xylan or pectin, and other substances in the rumen (Cholewińska et al., 2020). The ruminal bacterial community of yaks also showed better dietary fiber degradation features compared with the normal cattle or hybrid cattle-yak (Dai et al., 2021; Liu et al., 2022). This may contribute to improving the rumen fermentation characteristics of yaks, for example, yaks showed greater ruminal production of VFA but achieved lower methane emission when compared with cattle and sheep (Zhang et al., 2016). Therefore, altering the composition and structure of the bacterial community may improve the rumen fermentation characteristics, which is beneficial for the growth performance of yaks.
Isoacids, a term referring to the branched-chain volatile fatty acids (BCVFAs), namely, isovaleric acid (isovalerate), isobutyric acid (isobutyrate), and 2-methylbutyric acid (2-methybutyrate), and the straight-chain valeric acid (valerate), are the microbial metabolites of amino acids, including valine, leucine, isoleucine, and proline (Andries et al., 1987; Apajalahti et al., 2019). Previous studies have shown that the addition of isoacids to ruminant diets, alone or in combination, favors increased enzymatic activities and proliferation of ruminal fiber-degrading bacteria, and promotes crude fiber digestibility in vivo (Liu et al., 2014, 2020; Wang et al., 2015, 2018a; Liu C. S. et al., 2016). Furthermore, in an in vitro continuous fermentation model, supplementation of a mixture of BCVFAs showed improved NDF digestibility compared with the addition of individual isoacids (Roman-Garcia et al., 2021a,b) and stimulated the relative abundance of fiber-degrading bacteria, such as Fibrobacter and Treponema (Roman-Garcia et al., 2021c). Given these beneficial effects of isoacids on rumen fiber degradation, we hypothesize that supplementation with isoacids could be an effective option to promote feed fiber utilization in barn-feeding yaks. Therefore, the objective of this study was to evaluate the effects of supplementing a mixture of isoacids on growth performance, rumen fermentation characteristics, and nutrient digestibility in yaks. Considering the metabolic process of isoacids by microorganisms in the rumen, we evaluated the changes in the ruminal bacterial community in response to isoacid supplementation using 16s rRNA gene sequencing and correlated the ruminal microbiome with fermentation metabolites and fiber digestibility in yaks.
The supplemental mixture of isoacids used in this study is a commercially available feed additive product (Yangleyou, provided by Si Chuan Action Biotech Co., Ltd), which consisted of a mixture of isobutyrate, isovalerate, valerate, and 2-methylbutyrate. The effective content of mixed isoacid (MI) was 63.6 g/100 g. The liquid mixture of isoacids was absorbed using silica as the carrier in a ratio of 64:36.
An in vitro fermentation experiment was conducted to evaluate the role of different supplemental levels of MI on the fiber disappearance rate and the fermentation characteristics. The treatment groups included a non-supplementation control group (CON) and five MI-supplemented groups supplemented with MI on the dry matter (DM) basis of the substrate, namely: 0.1% MI (treatment group 1, T1), 0.2% MI (treatment group 2, T2), 0.3% MI (treatment group 3, T3), 0.4% MI (treatment group 4, T4), and 0.5% MI (treatment group 5, T5). Each treatment group contained four replicates.
The in vitro fermentation experiment was performed using the ANKOM RF Gas Production Systems (ANKOM), which is designed to automatically measure the kinetics of a microbial fermentation by monitoring the gas pressure within multiple modules equipped with temperature sensors and remotely recording the data in computer spreadsheets. The modules can communicate information to a computer using radio frequency (RF) transmission. Numerous variables can be operated from the computer interface such as data recording intervals and the automatic pressure release through internal valves in each module. A run was performed with a total of 24 bottles assigned to 6 groups and 2 bottles as blank. A total of 30 ml of rumen fluid, 120 ml of artificial saliva, and 1.00 g of substrate were added to a glass fermentation bottle and covered with the ANKOM module. A zero-reference module was used to measure ambient pressure. Two additional bottles incubated with rumen fluid and artificial saliva only (no substrate) were used as a blank control to correct for gas production. Briefly, fresh rumen fluid was collected from four male Maiwa yaks through a stainless-steel stomach tube attached to a rumen vacuum sampler as previously reported (Wang et al., 2016a). Yaks were fed the same regime as described in Table 1. Feed particles were discarded by filtering the liquid collection through four layers of sterile gauze. The filtered solutions were then transferred to a vacuum thermobottle, which was pre-incubated at 37°C. Carbon dioxide (CO2) was continuously injected into the rumen fluid to maintain an anaerobic atmosphere during all of the processes. Artificial saliva solution was prepared according to the previous procedure (Menke et al., 1979) and well mixed with rumen fluid in a ratio of 1:4 while simultaneously streaming with CO2. The fermentation bottles and modules were stabilized at 39°C for 1 h, then added with substrates and 150 ml of the mixing liquids, and incubated at 39°C, 110 rpm/min for 72 h. Real-time absolute gas pressure and temperature of each module were recorded every 15 min during the 72 h of fermentation. The cumulative gas production at 4, 8, 16, 24, 48, and 72 h was converted according to the ANKOM manual using the following equation:
where Vx = gas production volume, Vj = volume of space above the liquid surface inside the bottle, and Ppsi = gas pressure, recorded by the ANKOM GPM software (1 psi≈ 6.89 kPa).
The in vitro fermentation experiment was terminated in an ice-water bath after 72 h. The pH value of each fermentation fluid was immediately measured using a pH meter. The fermentation fluid was collected to determine the rumen fermentation characteristics, including the concentration of NH3-N, MCP, and VFAs. Briefly, the fermentation fluid was centrifuged at 3,000 rpm for 10 min, and an aliquot of the supernatant was used to determine the concentration of NH3-N using the micro-Kjeldahl method (AOAC, 2001). Another aliquot of the supernatant was transferred to a 10-ml centrifuge tube, ultrasonicated (ultrasonic probe = 2 mm, 350 W, repeated 3 times, 30 s each time, and interval = 30 s), and centrifuged at 3,000 rpm at 4°C for 10 min. Briefly, 1 ml of the supernatant was centrifuged at 10,000 rpm at 4°C for 20 min. The precipitate was rinsed twice with 1 ml of saline and then re-suspended with 1 ml of distilled water to determine the concentration of MCP according to the manufacturer's instructions of the BCA kits (Beijing Solarbio Science & Technology Co. Ltd, Beijing, China).
The concentration of VFAs in the fermentation fluid was measured according to the method described by Wang et al. (2014). Briefly, 1.0 ml of the supernatant was acidified with 200 μL metaphosphoric acid (25%) overnight at 4°C. The samples were analyzed on a gas chromatograph (7890B, Agilent Technology Inc., Santa Clara, CA) with a chromatographic column (HP INNOwax - 19091N, 30 m long, 0.32 mm ID, 0.50 m film) according to the manufacturer's protocol. The VFAs content was determined by comparison with the linear retention times of known standards (Shanghai Anpel Experimental Technology Co., LTD, Shanghai, China).
DM, NDF, and acid detergent fiber (ADF) degradability (DMD, NDFD, and ADFD) of the fermentation residue were measured after 72 h of incubation. The DM content was determined according to the AOAC method (AOAC, 2001), and the contents of NDF and ADF were analyzed using a fiber analyzer (automatic fiber determination analyzer, Gernardt F12) as described by Van Soest et al. (1991). Finally, they were calculated using the following equations:
All animal experimental procedures were approved by the Animal Care and Use Committee of Southwest Minzu University (approval number P20210510-2). The animal experiment was conducted at Seda Niuduoduo Yak Breeding Co. LTD (Ganzi, China, 30°055′ N and 101°969′ E). A total of fourteen Maiwa male yaks (body weight of 180–220 kg and 3–4 years of age) were randomly divided into two groups (n = 7 per group), tied and fed individually, and provided with a basal diet (control group; CON) or the basal diet supplemented with 0.3% MI. The Total Mixed Ration (TMR) diet was formulated according to the Feeding Standards of Beef Cattle of China (NY/T 815-2004, Ministry of Agriculture of the People's Republic of China, 2004). The ingredients and nutrient compositions are listed in Table 1. During the 95-day experimental period, the yaks had free access to water and were offered diets ad libitum twice a day (at 08:00–09:00 and 17:30–18:00). After 10-days of acclimatization, average dry matter intake (ADMI) was recorded before morning feeding. Animal body weight (BW) was recorded at the beginning and the end of the experiment to calculate the average daily gain (ADG) and feed conversion ratio (FCR).
Feed samples were collected once every 2 weeks and stored at −20°C for nutritional evaluation. Total fecal samples were collected three times daily and pooled on the last 3 days of the experiment. In total, 200 g of total fecal sample was collected from each yak, sampled, and stored at −20°C until analysis. Briefly, the contents of DM, crude protein (CP), acid insoluble ash (AIA), and ether extract (EE), in the diet and fecal samples, were analyzed according to the standard methods described by AOAC (2001). Calcium (Ca) content was measured by EDTA complexometric titration, and total phosphorus (P) content was determined using a spectrophotometer (the People's Republic of China National Standard, 2002a,b). NDF and ADF were analyzed as described in 2.3. AIA was used as an internal marker to evaluate the apparent digestibility of nutrients, which was calculated using the following equation:
where Rn= nutrient content in the feed, Mn= nutrient content in the feces, RAIA= hydrochloric acid insoluble ash content in the feed, and MAIA=hydrochloric acid insoluble ash content in the feces (Cheng et al., 2014).
Rumen fluid was collected through a stainless-steel stomach tube at the end of the experiment after overnight fasting. The first two tubes of ruminal fluid were discarded to avoid saliva contamination. The rumen fluid was then divided into two aliquots. One aliquot (5 ml) was transferred to a cryogenic vial and immediately frozen in liquid nitrogen for 16S rRNA sequencing. The other aliquot (30 ml) was transferred to a centrifuge tube and stored at −80°C for chemical determination of VFAs, NH3-N, and MCP concentrations using the same methods described in Section 2.2. Blood samples were collected from the jugular vein of yaks and anticoagulated with heparin sodium. Plasma was collected after centrifugation at 4,000 rpm for 10 min and stored at −80°C for further analysis of biochemical and hormonal parameters.
Total microbial genomic DNA from the rumen fluid was extracted using a FastDNA® Spin Kit for soil (MP Biomedicals, USA), according to the standard operating procedures. The concentration, quality, and completeness of the extracted DNA were determined using a spectrophotometer (NanoDrop2000, Thermo Fisher, USA) and 1.0% agarose gel electrophoresis (Biowest Agarose, Biowest, Spain), and then the DNA was stored at −80°C for further use. The hypervariable region V3–V4 of the bacterial 16S rRNA gene was amplified with primer pairs 338F (5′ ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) using an ABI GeneAmp® 9700 PCR thermocycler (ABI, CA, USA; Liu Q. et al., 2016). A 20-μL reaction system contained 4 μL of 5 × Fast Pfu buffer, 2 μL of 2.5 mM dNTPs, 0.8 μL of each primer (5 μM), 0.4 μL of Fast Pfu polymerase, 10 ng of template DNA, and ddH2O to a final volume of 20 μL. The temperature program was performed as follows: 95°C for 3 min, followed by 27 cycles of denaturation at 95°C for 30 s, 55°C for 30 s, and 72°C for 45 s, a single extension at 72°C for 10 min, and ending at 4°C. Purification and quantification of the amplification product were performed using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Axygen, USA) and the Quantus™ Fluorometer (Promega, USA).
The amplicons were sequenced on an Illumina MiSeq PE300 platform (Illumina, San Diego, CA, USA) at Majorbio (Shanghai, China). Raw sequencing reads were de-multiplexed using an in-house Perl script, quality-filtered using FASTP (v 0.19.6; Chen et al., 2018), and merged using FLASH (v1.2.11; Magoc and Salzberg, 2011). Although chloroplast DNA was not removed during the quality control process, this had a negligible effect on the analysis results. Paired-end reads were merged into a sequence with a minimum overlap length of 10 bp and a maximum mismatch ratio of 0.2 according to the overlapping relationships. The optimized sequences were clustered into operational taxonomic units (OTUs) based on a similarity >97% by using UPARSE 11 (Stackebrandt and Goebel, 1994; Edgar, 2013). To minimize the effect of sequencing depth on the bacterial diversity measure, the number of 16S rRNA gene sequences from each sample was rarefied to 32095, which still resulted in an average Good's coverage of 99.06 ± 0.044%.
The data were analyzed on the online platform of Majorbio Cloud Platform (www.majorbio.com) according to the description found in the study by Ren et al. (2022). The Mothur software programs (version v.1.30.2; Schloss et al., 2009) were applied to calculate the alpha diversity of the bacterial community, including the Sobs, Shannon, Simpson, and Chao indices. Principal coordinates analysis (PCoA) was used to determine the similarity among the microbial communities in different samples based on the Bray-Curtis distance metric using the Vegan v2.5-3 package of R (version 3.3.1). The bacterial community composition and sample-to-taxa relationship were visualized using Circos-0.67-7 (http://circos.ca/). The most discriminative abundant bacterial taxa (from phyla to genera) between groups were identified using a linear discriminant analysis (LDA) effect size (LEfSe; LDA score>3, P < 0.05; Segata et al., 2011). The association between the biomarker taxa and the phenotypic parameters was analyzed using Spearman's correlation and visualized using the Pheatmap package of R (version 3.3.1).
Data were analyzed using the SPSS (Statistical software package, version 20.0, IBM, USA). A two-way ANOVA used a general linear model (GLM) to examine the effect of treatment on in vitro gas production. Duncan's method was used for multiple comparisons. A one-way ANOVA was used to analyze other parameters after data normality was tested using the Shapiro-Wilk test, and polynomial contrasts were generated using a curve estimation regression analysis. The data from the animal experiment were analyzed via the student's t-test. The student's t-test was used to determine the differential bacterial taxa. Significance was declared at P ≤ 0.05, and a significant trend was considered when 0.05 < P ≤ 0.10.
The raw sequencing reads of the bacterial 16S rRNA amplicon for all of the ruminal fluid samples are deposited in the genome sequence archive (GSA) database under the BioProject accession number PRJCA016021.
The addition of different levels of MI did not affect the cumulative in vitro gas production volumes, and no interaction between incubation time and treatment was observed (Supplementary Figure 1). Nevertheless, the inclusion of MI strongly affected the NDFD and ADFD (P = 0.002 and P = 0.004; Table 2). With increasing supplementation, the NDFD and ADFD showed significant quadratic trends (P < 0.01). Compared with the control and other supplementation levels, the supplementation with 0.3% MI achieved the greatest NDFD and ADFD (P < 0.05).

Table 2. Effects of mixed isoacids supplementation on DMD, NDFD, and ADFD of the in vitro fermentation experiment.
The effects of supplementing MI on the in vitro fermentation characteristics are presented in Table 3. The pH value of the fermentation fluid was strongly affected by MI (P < 0.001). When the addition levels were higher than 0.3%, the pH value of the fermentation fluid was significantly lower than that of the control group (P < 0.05). The addition of MI showed a quadratic effect on the concentration of NH3-N (P = 0.021), but not on the concentration of MCP (P = 0.685). Propionate and butyrate production displayed a clear quadratic effect in response to MI supplementation (P < 0.05). Supplementation with 0.3% MI achieved the highest levels of these two volatile metabolites compared with the control and other supplementation groups (P < 0.05). Moreover, the concentrations of total VFAs (TVFA) tended to show a quadratic increase tendency (P = 0.055). No marked differences were detected for the production of acetate, valerate, and the ratio of acetate to propionate among the groups (P > 0.05). No significant difference was observed in the molar percentage of any of these VFAs (P > 0.05).
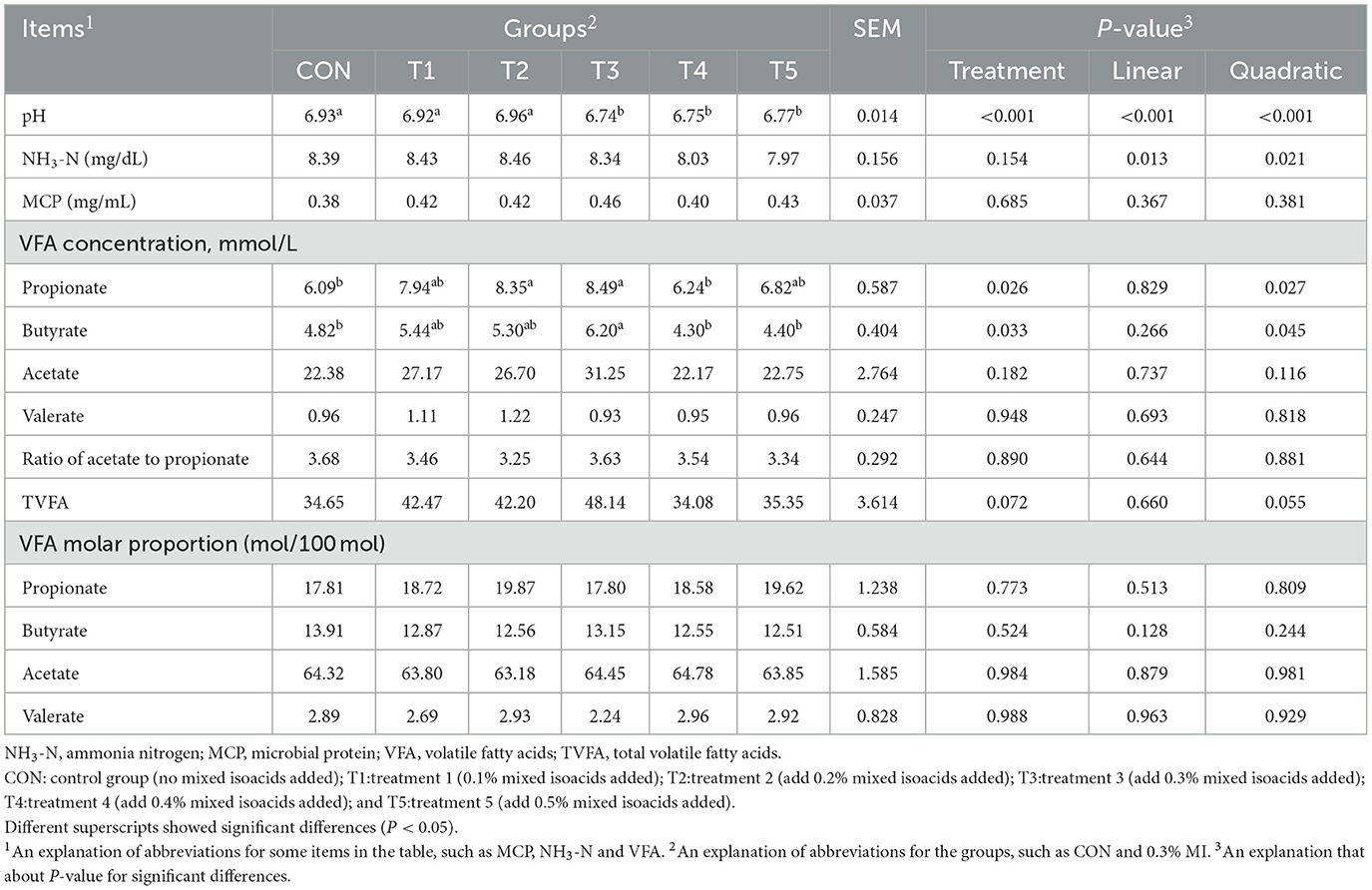
Table 3. Effects of mixed isoacids supplementation on ruminal fermentation characteristics of the in vitro fermentation experiment.
As shown in Table 4, supplementation with 0.3% MI significantly increased the ADG and ADMI when compared with the control (P < 0.05), but did not affect the FCR (P > 0.05). Yaks exhibited greater apparent digestibility of NDF and ADF in response to supplementation with 0.3% MI (P < 0.05). There was no significant difference in the apparent digestibility of EE, CP, Ca, and P between the treatments (P > 0.05). Meanwhile, supplementation with 0.3% MI had no effect on the concentration of plasma biochemical parameters and hormones (Supplementary Table 1), suggesting the nutrition metabolism of yaks themselves may not attribute to the growth-promoting effect of MI.
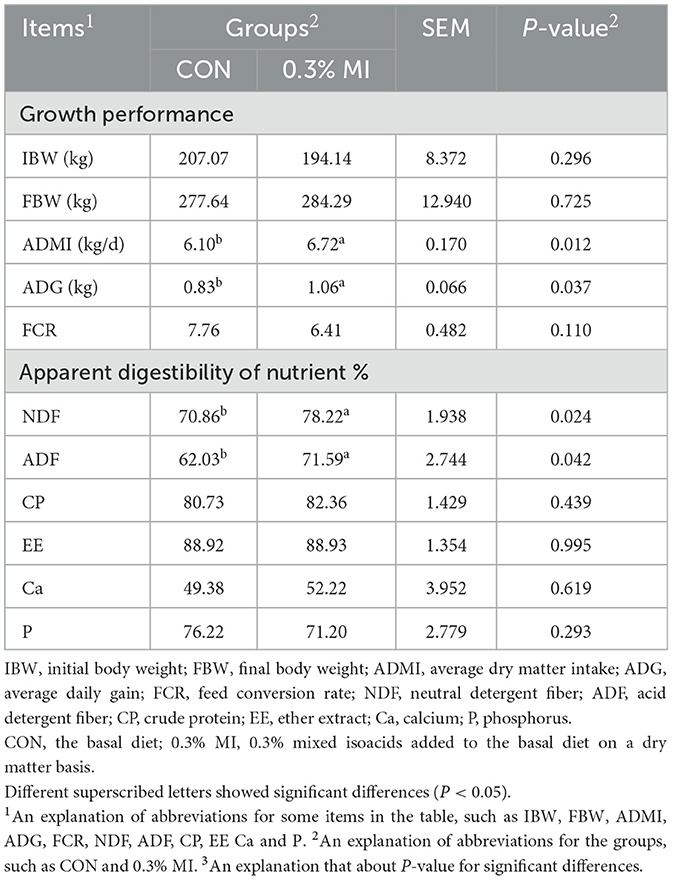
Table 4. Effects of mixed isoacids supplementation on the growth performance and apparent digestibility of nutrients in yaks.
The concentrations of NH3-N, MCP, and VFAs in the rumen fluid of yaks are shown in Table 5. No significant difference was observed in NH3-N, MCP, and VFAs between the two groups (P > 0.05). Among the VFAs, the concentrations of acetate, propionate, and butyrate tended to decrease in response to the supplementation with 0.3% MI (0.05 < P < 0.1).
Illumina Miseq 16S rRNA gene sequencing obtained 48,307 ± 2,147 high-quality trimmed sequences per sample from 14 rumen fluid samples, which belonged to 2,612 OTUs, with an average sequence length of 418 bp (Supplementary Table 2). The information on barcodes from 14 rumen fluid samples is provided in Supplementary Table 2. A total of 1,937 OTUs (74.16%) were commonly shared by the control and 0.3% MI groups, while 276 OTUs (10.57%) were specific to the control group and 399 OTUs (15.28%) were specific to the 0.3% MI group (Supplementary Figure 2). The composition of the ruminal bacterial community was visualized using the Circos plot (Supplementary Figure 3). Both groups displayed similar bacterial composition at the phylum level (Supplementary Figure 3A), family level (Supplementary Figure 3B), and genus level (Supplementary Figure 3C).
The rarefaction curves based on Sobs indices reached the saturation stage with the increasing number of sequenced reads (Figure 1A), suggesting that the majority of rumen bacterial members were captured from rumen fluid samples in this experiment. However, the bacterial community richness (Chao index, Figure 1B) and diversity (Shannon and Simpson indices, Figures 1C, D) did not differ between the control and 0.3% MI groups (P>0.05). Principal coordinates analysis (PCoA) based on the Bray-Curtis distance metric showed that supplementation with 0.3% MI induced the rumen bacteria to form significantly different communities when compared with the control group (PERMANOVA, R2 = 0.1098, P = 0.022, Figure 2A). A similarities analysis showed that the difference in bacterial communities between the groups was greater than the difference within a group (ANOSIM, R2 = 0.2765, P = 0.004, Figure 2B).
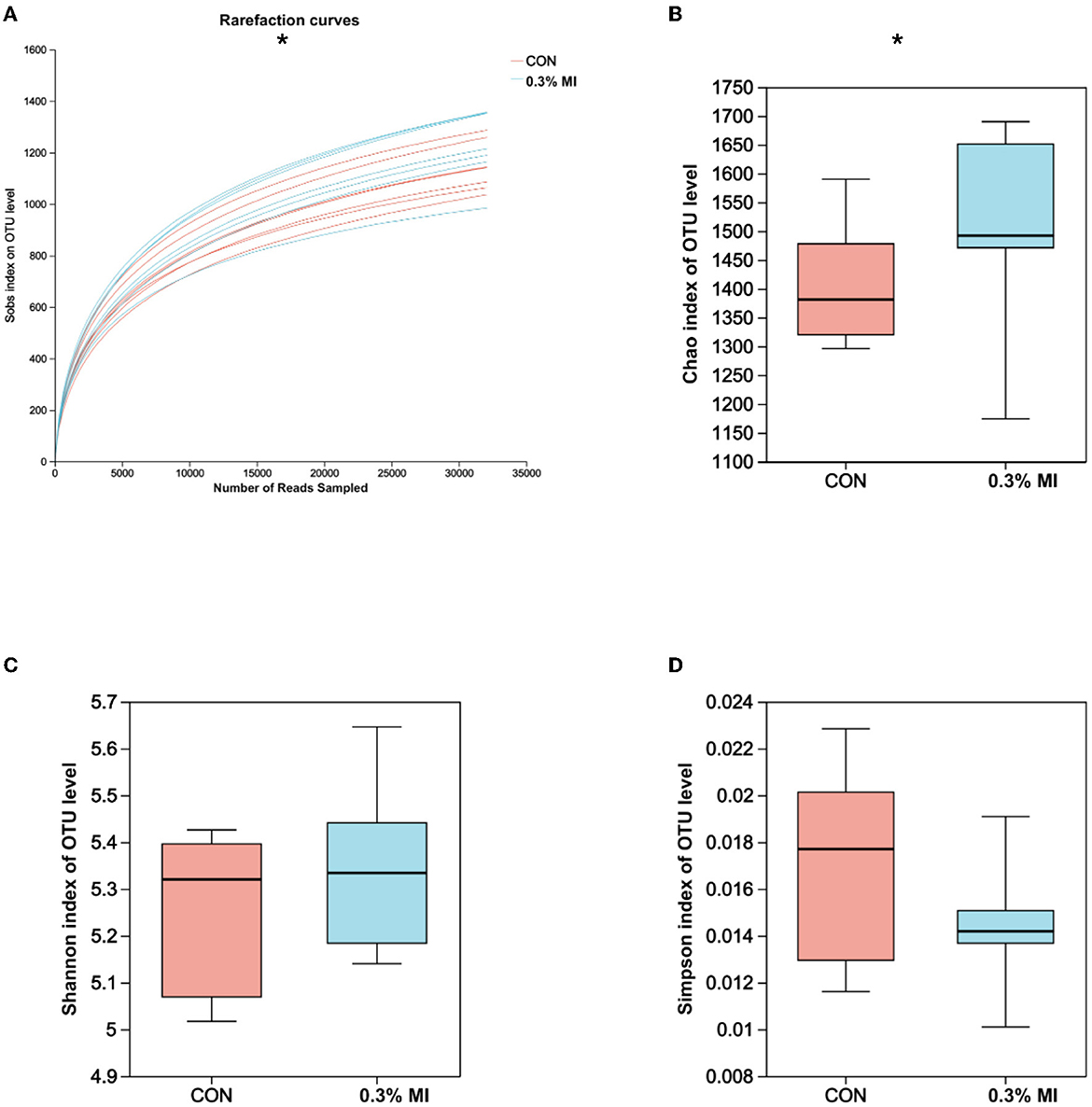
Figure 1. Effects of mixed isoacids supplementation on rumen bacterial richness and diversity in yaks. (A) Rarefaction curve based on Sobs indices; (B) Boxplot of Chao indices; (C) Boxplot of Shannon indices; (D) Boxplot of Simpson indices. CON, the basal diet; 0.3% MI, 0.3% mixed isoacids added to the basal diet on a dry matter basis. Significance was tested using the independent two-group Wilcoxon rank-sum tests. *P < 0.05, n = 7 per group.
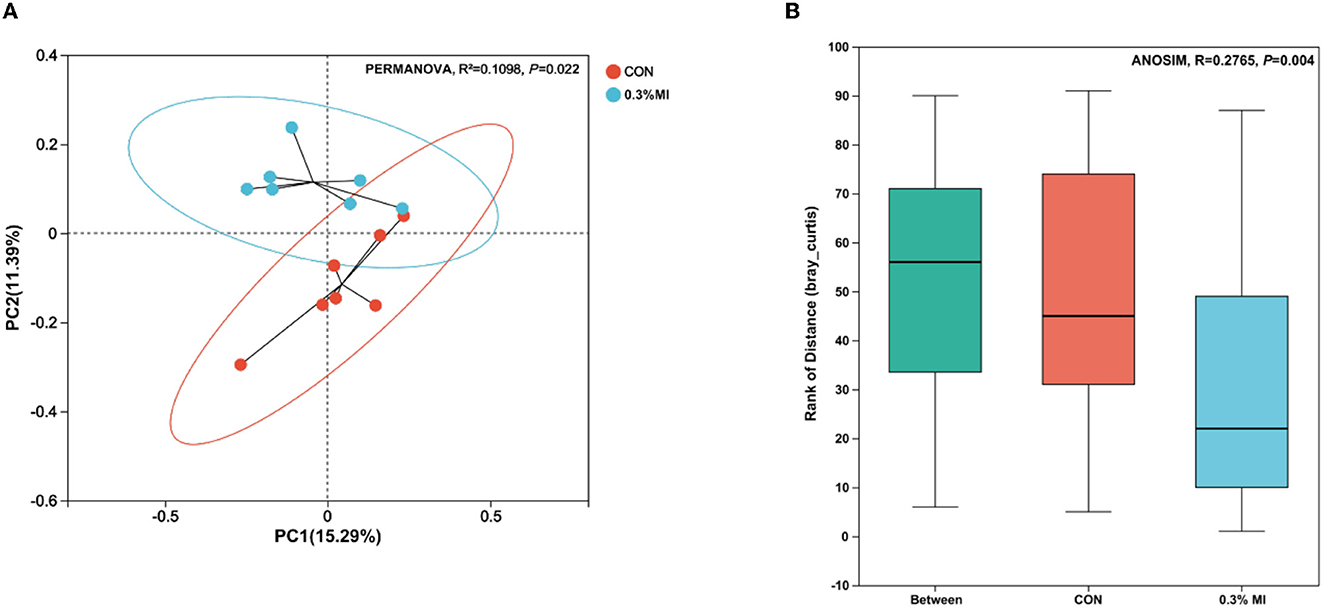
Figure 2. Effects of mixed isoacids supplementation on rumen bacterial community similarity. (A) Principal coordinates analysis (PCoA) plot based on the Bray-Curtis distance metric. Difference between clusters is measured by the permutational multivariate analysis of variance (PERMANOVA). (B) Distances box plot depicted the analysis of similarities (ANOSIM) between the CON group and the 0.3% MI group. CON, the basal diet; 0.3% MI, 0.3% mixed isoacids added to the basal diet on a dry matter basis.
The most discriminative taxa responding to supplementation with 0.3% MI was identified using the linear discriminant analysis (LDA) effect size (LEfSe). Fifteen differentially abundant taxa from phylum to genus level were discovered as high-dimensional biomarkers for separating rumen bacterial communities between the two groups (Figure 3A). Nine of these taxa were higher, and six were lower in the 0.3% MI group than in the CON group (Figure 3A). Dietary supplementation with 0.3% MI showed a significantly higher abundance of f__Bacteroidales_BS11_gut_group, g__norank_f__Bacteroidales_BS11_gut_group, g__norank_f__Muribaculaceae, f__Muribaculaceae, g__Veillonellaceae_UCG-001, g__Ruminococcus_gauvreauii_group, g__norank_f__norank_o__RF39, f__norank_o__RF39, and o__RF39 (P < 0.05). In the CON group group, f__F082, g__norank_f__F082, f__Ruminococcaceae, p__Actinobacteriota, f__Eubacterium_coprostanoligenes_group, and g__norank_f__Eubacterium_coprostanoligenes_group were significantly enriched, from the family to the genus level (Figures 3A, B, P < 0.05). Besides, a Student's t-test was performed to detect all differentially abundant taxa in the top 100 (Supplementary Table 3).
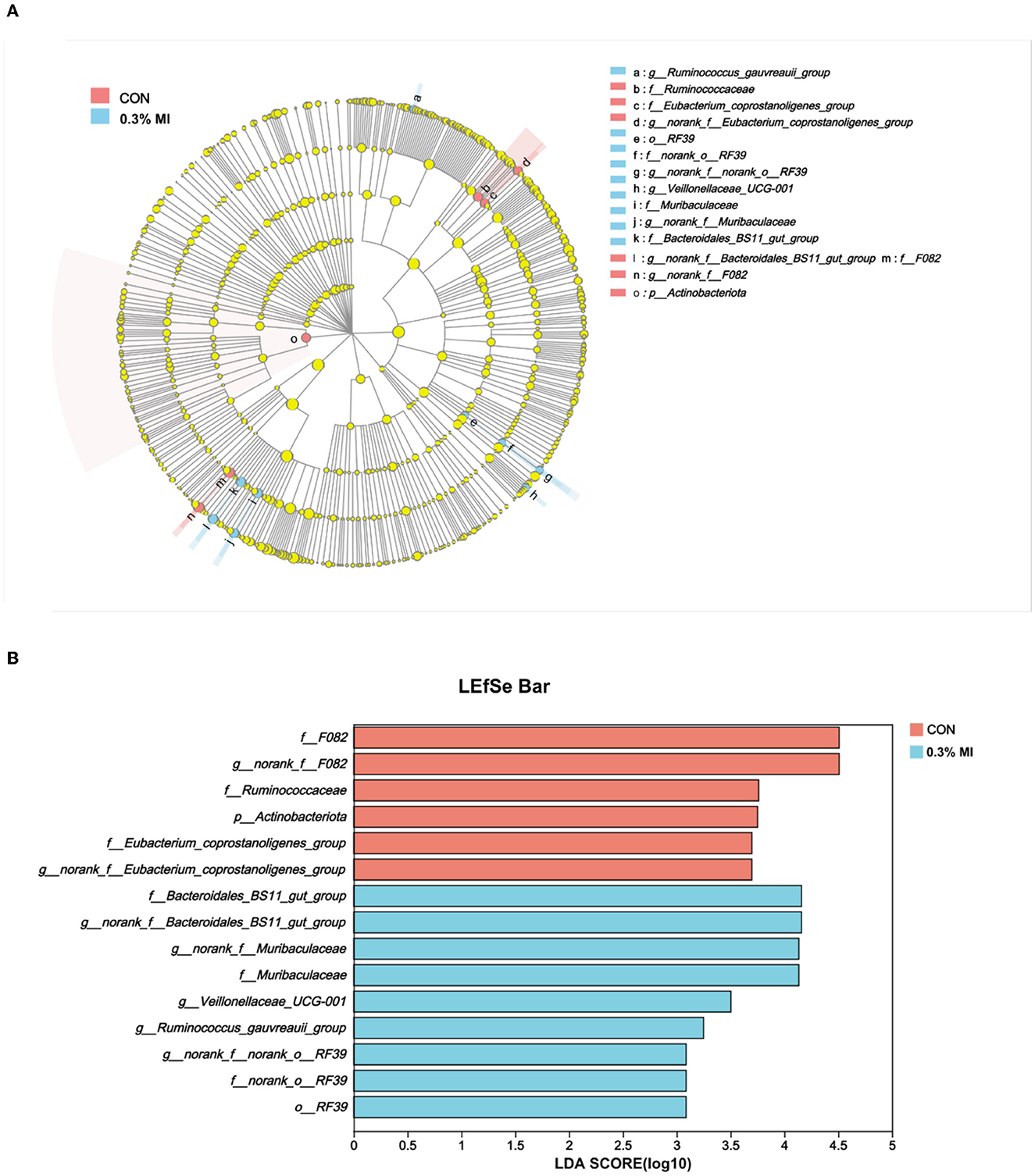
Figure 3. Effects of mixed isoacids supplementation on the most differentially abundant rumen bacterial taxa in yaks. (A) Cladogram plot of linear discriminant analysis (LDA) effect size (LEfSe) results from phylum to genus level. (B) Histogram of linear discriminant analysis (LDA) reveals the most differentially abundant taxa between the groups. CON, the basal diet; 0.3% MI, 0.3% mixed isoacids added to the basal diet on a dry matter basis.
Differentially abundant taxa at different levels, identified by the Student's t-test, were selected for the correlation analysis with apparent digestibility of nutrients. Spearman's correlation indicated that g__Flexilinea and g__norank_f__norank_o__RF39 were significantly positively correlated with the NDF digestibility (P < 0.05); and g__norank_f__norank_o__RF39 was significantly positively correlated with the ADF digestibility (P < 0.05). In addition, g__Ornithinimicrobium was significantly negatively correlated with the ADF and NDF digestibility (P < 0.05, Figure 4).

Figure 4. The correlations of feed digestibility with the bacterial community from phylum to genus level. The heatmap shows the absolute value of Spearman's correlation R > 0.1. CP, crude protein; NDF, neutral detergent fiber; ADF, acid detergent fiber. Asterisk indicates the significance of Spearman's correlation, *P < 0.05, **P < 0.01, n = 7 per group.
Improving the fiber utilization by yaks is important for highland yak farming in winter when forage is severely scarce (Dong et al., 2006). The rumen is well known as a natural bioreactor for its high efficiency of fiber degradation by rumen microbiota (Wang et al., 2020). Isoacids are a batch of amino acid-derived metabolites in the rumen that act as a nutrient factor for rumen fiber-degrading bacteria, thereby facilitating VFAs production and fiber utilization (Liu et al., 2009, 2014; Roman-Garcia et al., 2021a,c). Concentrations of butyrate and propionate, as well as the degradation of NDF and ADF, tended to increase and then decrease with increasing dose of MI in this study. These results are consistent with those of Wang et al. (2012), who obtained results from an in vitro fermentation assay supplemented with 2-methylbutyrate, suggesting that a higher dose of MI may dampen the in vitro fermentation characteristics. The supplementation level of 0.3% could be an optimal dose, as it achieved the highest propionate and butyrate content, as well as NDFD and ADFD, indicating that the effect of MI on rumen fermentation parameters in yaks is related to its supplementation level.
Supplementation with isoacids alone or in combination could improve feed fiber digestibility and average daily gain in beef cattle and dairy calves (Wang et al., 2012; Liu C. S. et al., 2016; Liu et al., 2018). Improved digestibility of NDF and NDF responsive to MI makes more fermentation metabolites, such as VFAs, available in the rumen. For example, Wang et al. (2018b) found that supplementation with 16.8 g of 2-methylbutyrate per steer per day increased the molar ratio of effectively degradable NDF and rumen acetate. Similar results were observed in this study, suggesting that supplementation with 0.3% MI could significantly improve the apparent digestibility of NDF and ADF, thereby increasing the ADG and ADMI in yaks. However, ruminal production of VFA was not affected by the addition of MI, which may be due to factors such as diet composition, rumen microbial species, pen temperature, and the amount or composition of MI (Wang et al., 2016a,b). NH3-N is a product of the fermentation and degradation of dietary proteins and is an essential component for microbial synthesis of MCP in the rumen (Garcia-Gonzalez et al., 2010; Thao et al., 2014; Lv et al., 2020). However, no difference in the response of NH3-N concentration to MI was observed in this study, which may be related to the amount of water consumed by the yaks or the amount or composition of the MI supplement.
After a long period of natural selection, the yak rumen microbiota has evolved a higher capacity for fiber degradation (Ma et al., 2020). Thus, the yak rumen may harbor a unique microbiome for efficient conversion of feed fiber. Isoacids improve the digestibility of feed fiber, mainly by increasing the activity of cellulase and cellulose-producing bacteria in the rumen. For example, Liu C. S. et al. (2016) reported that supplementation with 6.0 g of isovalerate per calf per day increased the activities of caboxymethylcellulase, cellobiase, xylanase and pectinase and the relative amounts of Butyrivibrio fibrisolvens, Ruminococcus albus, Fibrobacter succinogenes, and Ruminococcus flavefaciens in dairy calves at 90 days of age. Similarly, Wang et al. (2019) found that supplementation with 6 g of isobutyrate per calf per day increased the activities of cellobiase, xylanase, pectinase, b-amylase protease, and CMCase in post-weaned calves and the population of B. fibrisolvens in pre- and post-weaned calves. Rather than looking at changes in the rumen microbiota as a whole, these studies have focused on changes in the abundance of specific microbes. In this study, beta-diversity by PCoA showed that supplementation with 0.3% MI induced the rumen bacteria to form significantly different communities, indicating that 0.3% MI significantly altered the rumen microbial structure, which may be related to the fact that the microbial 16S rRNA gene was less diverse in the yak rumen than that in the bovine rumen (An et al., 2005). This resulted in a significant effect of 0.3% MI on some uncultured fiber-degrading bacteria in the yak rumen and ultimately caused a shift in the structure of the rumen microbiota.
The LEfSe analysis showed that some biomarker taxa such as norank_f__Bacteroidales_BS11_gut_group, norank_f__Muribaculaceae, Veillonella- ceae_UCG-001, and Ruminococcus_gauvreauii_group were found in 0.3% of the MI group. The Bacteroidales belong to the phyla of Bacteroidetes, which act mainly on steroids, polysaccharides, and bile acids, which contribute to the absorption of polysaccharides and proteins by the body (Backhed et al., 2005). Therefore, we can speculate that the norank_f__Bacteroidales_BS11_gut_group may be a fiber-degrading bacterium. One study found that norank_f__Muribaculaceae was positively correlated with milk yield (Sun et al., 2019), and some studies found that isoacids can increase milk production in dairy cows (Otterby et al., 1990). The higher relative expression of norank_f__Muribaculaceae in the 0.3% MI group suggests that 0.3% MI may increase milk production in yaks, but no studies have been reported on this aspect. In addition, Muribaculaceae can also degrade carbohydrates (He et al., 2022), which corresponds to a higher apparent digestibility of NDF and ADF in the 0.3% MI group than in the control group, suggesting that 0.3% MI can promote the growth of rumen fibrinolytic bacteria in yaks, which is consistent with other studies (Moharrery and Das, 2001; Firkins, 2010; Liu et al., 2014; Wang et al., 2015). Veillonellaceae_UCG-001 belong to the phylum Firmicutes, which contains many fibrolytic bacteria (Chen et al., 2022). According to a remarkably higher apparent digestibility of NDF and ADF in the 0.3% MI group than that in the control group. It is speculated that Veillonellaceae_UCG-001 may also be a type of fibrolytic bacteria in the yak rumen. Ruminococcus also belongs to the phylum Firmicutes, which can use cellulose and hemicellulose as substrates to produce VFA (Liu et al., 2019). The in vitro tests in this study showed a significant increase in propionate acid and butyrate in the 0.3% MI group and a trend toward higher TVFA, which may be related to the increased relative abundance of the Ruminococcus_gauvreauii_group. In addition, supplementation with 0.3% MI reduced the relative abundance of norank_f__F082 by 49.98%, which was reported to be negatively correlated with feed fiber concentration and digestibility in yaks (Yi et al., 2022), suggesting that supplementation with MI may increase the available fiber content and digestibility in the rumen of yaks.
The correlation analysis showed that g__Flexilinea and g__norank_f__norank_o__RF39 were positively correlated with the apparent digestibility of NDF. g__Flexilinea was reported as a filamentous strictly anaerobic Gram-negative bacterium which could digest all kinds of carbohydrates (Wang et al., 2021). In addition, one study has reported that the abundance of g__norank_f__norank_o__RF39 was increased with the increase of NDF digestibility (Liu et al., 2023). We speculate that supplementation with 0.3% MI significantly improved the apparent digestibility of feed fiber, which was associated with the change in the abundance of g__Flexilinea and g__norank_f__norank_o__RF39.
The optimum dose of MI was found to be 0.3% based on the in vitro experiment, which significantly reduced ruminal pH, increased NDFD, ADFD, propionate, and butyrate concentrations, and tended to increase the content of TVFA. Supplementation with 0.3% MI significantly improved the apparent digestibility of feed fiber and altered the ruminal bacterial diversity, which was positively correlated with the abundance of g__Flexilinea and g__norank_f__norank_o__RF39.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: NCBI - Genome Sequence Archive (GSA) database, and the BioProject accession number is PRJCA016021.
The animal study was reviewed and approved by Animal Care and Use Committee of Southwest Minzu University.
YG and ZP contributed to conception and design of the study. FJ and XM performed the in vitro fermentation experiment. FJ, YY, ZH, YL, and AH conducted the animal experiment. YG and FJ performed the statistical analysis and wrote the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
This work was funded by the Natural Science Foundation of Sichuan Province (Grant No. 2023NSFSC1148), the Sichuan Beef Cattle Innovation Team of China Agriculture Research System (Grant No. sccxtd-2020-13), the Sichuan Province Science and Technology Support Program (Grant No. GZ20220616), the Southwest Minzu University Fundamental Research Funds for the Central Universities (Grant No. ZYN2022106), and Southwest Minzu University Double World-Class Project (Grant No. XM2023010).
The authors thank Jianping Wu, Yao Pan, and Kanglin Zhang of Ganzi Prefectural Animal Science Research Institute, Zhongyun Zhi and Xi Xia of Seda County Agriculture, Animal Husbandry, Rural and Technology Bureau, and Lian Wang, Bo Chen, and Huajin Rong of Seda Niuduoduo Yak Breeding Co., LTD. for assisting with the animal experiment.
YL was employed by Si Chuan Action Biotech Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1175880/full#supplementary-material
An, D., Dong, X., and Dong, Z. (2005). Prokaryote diversity in the rumen of yak (Bos grunniens) and Jinnan cattle (Bos taurus) estimated by 16s rDNA homology analyses. Anaerobe. 11, 207–215. doi: 10.1016/j.anaerobe.2005.02.001
Andries, J. I., Buysse, F. X., Brabander, D., and Cottyn, B. G. (1987). Isoacids in ruminant nutrition: their role in ruminal and intermediary metabolism and possible influences on performances — a review. Anim. Feed. Sci. Tech. 18, 169–180. doi: 10.1016/0377-8401(87)90069-1
AOAC (2001). Official Methods of Analysis. Gaithersburg: Association of Official Analytical Chemists, Washington, DC.
Apajalahti, J., Vienola, K., Raatikainen, K., Holder, V., and Moran, C. A. (2019). Conversion of branched-chain amino acids to corresponding isoacids - an in vitro tool for estimating ruminal protein degradability. Front. Vet. Sci. 6, 311. doi: 10.3389/fvets.2019.00311
Backhed, F., Ley, R. E., Sonnenburg, J. L., Peterson, D. A., and Gordon, J. I. (2005). Host-bacterial mutualism in the human intestine. Science. 307, 1915–1920. doi: 10.1126/science.1104816
Chen, S. F., Zhou, Y. Q., Chen, Y. R., and Gu, J. (2018). Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 34, 884–890. doi: 10.1093/bioinformatics/bty560
Chen, X. D., Yan, F., Liu, T., Zhang, Y. L., Li, X. Y., Wang, M. Y., et al. (2022). Ruminal microbiota determines the high-fiber utilization of ruminants: evidence from the ruminal microbiota transplant. Microbiol spectr. 10, e00446–e00422. doi: 10.1128/spectrum.00446-22
Cheng, J. B., Bu, D. P., Wang, J. Q., Sun, X. Z., Pan, L., Zhou, L. Y., et al. (2014). Effects of rumen-protected γ-aminobutyric acid on performance and nutrient digestibility in heat-stressed dairy cows. J. Dairy Sci. 97, 5599–5607. doi: 10.3168/jds.2013-6797
Cholewińska, P., Czyż, K., Nowakowski, P., and Wyrostek, A. (2020). The microbiome of the digestive system of ruminants – a review. Anim. Health Res. Rev. 21, 3–14. doi: 10.1017/S1466252319000069
Dai, Q. D., Ma, J., Cao, G., Hu, R., Zhu, Y. X., Li, G. Y., et al. (2021). Comparative study of growth performance, nutrient digestibility, and ruminal and fecal bacterial community between yaks and cattle-yaks raised by stall-feeding. AMB Express. 11, 98. doi: 10.1186/s13568-021-01259-9
Ding, X. Z., Guo, X., Yan, P., Liang, C. N., Bao, P. J., and Chu, M. (2012). Seasonal and nutrients intake regulation of lipoprotein lipase (LPL) activity in grazing yak (Bos grunniens) in the alpine regions around Qinghai Lake. Livest. Sci. 143, 29–34. doi: 10.1016/j.livsci.2011.08.004
Dong, Q. M., Zhao, X. Q., Ma, Y. S., Xu, S. X., and Li, Q. Y. (2006). Live-weight gain, apparent digestibility, and economic benefits of yaks fed different diets during winter on the Tibetan Plateau. Livest. Sci. 101, 199–207. doi: 10.1016/j.livprodsci.2005.11.009
Du, M., Yang, C., Liang, Z. Y., Zhang, J. B., Yang, Y. Y., Ahmad, A. A., et al. (2021). Dietary energy levels affect carbohydrate metabolism-related bacteria and improve meat quality in the longissimus thoracis muscle of yak (Bos grunniens). Front. Vet. Sci. 8, 718036. doi: 10.3389/fvets.2021.718036
Edgar, R. C. (2013). Uparse: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods. 10, 996. doi: 10.1038/nmeth.2604
Firkins, J. L. (2010). Reconsidering rumen microbial consortia to enhance feed efficiency and reduce environmental impact of ruminant livestock production systems. Rev. Bra. Zootecn. 39, 445–457. doi: 10.1590/S1516-35982010001300049
Garcia-Gonzalez, R., Gonzalez, J. S., and Lopez, S. (2010). Decrease of ruminal methane production in rusitec fermenters through the addition of plant material from rhubarb (Rheum spp.) and alder buckthorn (Frangula alnus). J. Dairy Sci. 93, 3755–3763. doi: 10.3168/jds.2010-3107
He, Q. D., Guo, J. J., Zhang, Q., Yau, Y. M., Yu, Y., Zhong, Z. H., et al. (2022). Effects of electroacupuncture on the gut microbiome in cisplatin-induced premature ovarian failure mice. Evid.-based Compl. Alt. 2022, 9352833. doi: 10.1155/2022/9352833
Hu, C. S., Ding, L. M., Jiang, C. X., Ma, C. F., Liu, B. T., Li, D. L., et al. (2021). Effects of management, dietary intake, and genotype on rumen morphology, fermentation, and microbiota, and on meat quality in yaks and cattle. Front Nutr. 8, 755255. doi: 10.3389/fnut.2021.755255
Jing, X., Ding, L., Zhou, J., Huang, X., Degen, A., and Long, R. (2022). The adaptive strategies of yaks to live in the Asian highlands. Anim Nutr. 9, 249–258. doi: 10.1016/j.aninu.2022.02.002
Kang, K., Ma, J., Wang, H., Wang, Z., Peng, Q., Hu, R., et al. (2020). High-energy diet improves growth performance, meat quality and gene expression related to intramuscular fat deposition in finishing yaks raised by barn feeding. Vet. Med. Sci. 6, 755–765. doi: 10.1002/vms3.306
Liu, C. S., Zhao, D. F., Ma, W. J., Guo, Y. D., Wang, A. J., Wang, Q. L., et al. (2016). Denitrifying sulfide removal process on high-salinity wastewaters in the presence of Halomonas sp. Appl. Microbiol. Biot. 100, 1421–1426. doi: 10.1007/s00253-015-7039-6
Liu, Q., Wang, C., Guo, G., Huo, W. J., Zhang, Y. L., Pei, C. X., et al. (2018). Effects of branched-chain volatile fatty acids supplementation on growth performance, ruminal fermentation, nutrient digestibility, hepatic lipid content and gene expression of dairy calves. Anim. Feed Sci. Tech. 237, 27–34. doi: 10.1016/j.anifeedsci.2018.01.006
Liu, Q., Wang, C., Pei, C. X., Li, H. Y., Wang, Y. X., Zhang, S. L., et al. (2014). Effects of isovalerate supplementation on microbial status and rumen enzyme profile in steers fed on corn stover based diet. Livest. Sci. 161, 60–68. doi: 10.1016/j.livsci.2013.12.034
Liu, Q., Wang, C., Yang, W. Z., Zhang, B., Yang, X. M., He, D. C., et al. (2009). Effects of isobutyrate on rumen fermentation, lactation performance and plasma characteristics in dairy cows. Anim. Feed Sci. Tech. 154, 58–67. doi: 10.1016/j.anifeedsci.2009.08.001
Liu, Q., Wang, C., Zhang, Y. L., Pei, C. X., Zhang, S. L., Wang, Y. X., et al. (2016). Effects of isovalerate supplementation on growth performance and ruminal fermentation in pre- and post-weaning dairy calves. J. Agr. Sci. 154, 1499–1508. doi: 10.1017/S0021859616000630
Liu, S. Q., Shah, A. M., Yuan, M., Kang, K., Wang, Z. S., Wang, L. Z., et al. (2022). Effects of dry yeast supplementation on growth performance, rumen fermentation characteristics, slaughter performance and microbial communities in beef cattle. Anim. Biotechnol. 33, 1150–1160. doi: 10.1080/10495398.2021.1878204
Liu, Y. F., Hu, J. M., Li, M. M., Zhao, G. Y., et al. (2023). Effects of taurine on rumen fermentation, nutrient digestion, rumen bacterial community and metabolomics and nitrogen metabolism in beef steers. J. Sci. Food. Agr. doi: 10.1002/jsfa.12474
Liu, Y. R., Wang, C., Liu, Q., Guo, G., Huo, W. J., Zhang, Y. L., et al. (2020). Effects of branched-chain volatile fatty acids and fibrolytic enzyme on rumen development in pre- and post-weaned Holstein dairy calves. Anim. Biotechnol. 31, 512–519. doi: 10.1080/10495398.2019.1633340
Liu, Y. Z., Chen, X., Zhao, W., Lang, M., Zhang, X. F., Wang, T., et al. (2019). Effects of yeast culture supplementation and the ratio of non-structural carbohydrate to fat on rumen fermentation parameters and bacterial-community composition in sheep. Anim. Feed Sci. Tech. 249, 62–75. doi: 10.1016/j.anifeedsci.2019.02.003
Long, R. J., Dong, S. K., Wei, X. H., and Pu, X. P. (2005). The effect of supplementary feeds on the bodyweight of yaks in cold season. Livest. Prod. Sci. 93, 197–204. doi: 10.1016/j.livprodsci.2004.08.016
Lv, F., Wang, X. J., Pang, X., and Liu, G. H. (2020). Effects of supplementary feeding on the rumen morphology and bacterial diversity in lambs. PeerJ. 8, 9353. doi: 10.7717/peerj.9353
Ma, J. A., Zhu, Y. X., Wang, Z. S., Yu, X., Hu, R., Wang, X. Y., et al. (2020). Comparing the bacterial community in the gastrointestinal tracts between growth-retarded and normal yaks on the Qinghai-Tibetan Plateau. Front. Microbiol. 11, 600516. doi: 10.3389/fmicb.2020.600516
Magoc, T., and Salzberg, S. L. (2011). Flash: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 27, 2957–2963. doi: 10.1093/bioinformatics/btr507
Menke, K. H., Raab, L., Salewski, A., Steingass, H., Fritz, D., and Schneider, W. (1979). The estimation of the digestibility and metabolizable energy content of ruminant feedingstuffs from the gas production when they are incubated with rumen liquor in vitro. J. Agr. Sci. 93, 217–222. doi: 10.1017/S0021859600086305
Ministry of Agriculture of the People's Republic of China (2004). NY/T 815–2004 Beef Cattle Raising Standard. Beijing: China Agricultural Press.
Moharrery, A., and Das, T. K. (2001). Correlation between microbial enzyme activities in the rumen fluid of sheep under different treatments. Reprod. Nutr. Dev. 41, 513–529. doi: 10.1051/rnd:2001106
Otterby, D. E., Johnson, D. G., Towns, R., Cook, R. M., Erdman, R. A., Van Horn, H. H., et al. (1990). Dose response of dairy cows to ammonium salts of volatile fatty acids. J. Dairy Sci. 73, 2168–2178. doi: 10.3168/jds.S0022-0302(90)78897-2
People's Republic of China National Standard (2002a). GB/T 6436–2002 Determination of Total Phosphorus in Feed - Spectrophotometric Method. Beijing: China Standard Press.
People's Republic of China National Standard (2002b). GB/T 6437–2002 Determination of Total Phosphorus in Feed - Spectrophotometric Method. Beijing: China Standard Press.
Ren, Y., Yu, G., Shi, C., Liu, L., Guo, Q., Han, C., et al. (2022). Majorbio Cloud: a one-stop, comprehensive bioinformatic platform for multiomics analyses. iMeta. 1, e12. doi: 10.1002/imt2.12
Roman-Garcia, Y., Denton, B. L., Mitchell, K. E., Lee, C., Socha, M. T., and Firkins, J. L. (2021a). Conditions stimulating neutral detergent fiber degradation by dosing branched-chain volatile fatty acids. I: Comparison with branched-chain amino acids and forage source in ruminal batch cultures. J. Dairy Sci. 104, 6739–6755. doi: 10.3168/jds.2020-20054
Roman-Garcia, Y., Mitchell, K. E., Denton, B. L., Lee, C., Socha, M. T., Wenner, B. A., et al. (2021b). Conditions stimulating neutral detergent fiber degradation by dosing branched-chain volatile fatty acids. II: Relation with solid passage rate and pH on neutral detergent fiber degradation and microbial function in continuous culture. J. Dairy Sci. 104, 9853–9867. doi: 10.3168/jds.2021-20335
Roman-Garcia, Y., Mitchell, K. E., Lee, C., Socha, M. T., Park, T., Wenner, B. A., et al. (2021c). Conditions stimulating neutral detergent fiber degradation by dosing branched-chain volatile fatty acids. III: Relation with solid passage rate and ph on prokaryotic fatty acid profile and community in continuous culture. J. Dairy Sci. 104, 9868–9885. doi: 10.3168/jds.2021-20336
Schloss, P. D., Westcott, S. L., Ryabin, T., Hall, J. R., Hartmann, M., Hollister, E. B., et al. (2009). Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microb. 75, 7537–7541. doi: 10.1128/AEM.01541-09
Segata, N., Izard, J., Waldron, L., Gevers, D., Miropolsky, L., Garrett, W. S., et al. (2011). Metagenomic biomarker discovery and explanation. Genome Biol. 12, r60. doi: 10.1186/gb-2011-12-6-r60
Shao, B., Long, R., Ding, Y., Wang, J., Ding, L., and Wang, H. (2010). Morphological adaptations of yak (Bos grunniens) tongue to the foraging environment of the Qinghai-Tibetan Plateau. J. Anim. Sci. 88, 2594–2603. doi: 10.2527/jas.2009-2398
Stackebrandt, E., and Goebel, B. M. (1994). Taxonomic note: a place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Bacterio. 44, 846–849. doi: 10.1099/00207713-44-4-846
Sun, Z. Q., Yu, Z., and Wang, B. (2019). Perilla frutescens leaf alters the rumen microbial community of lactating dairy cows. Microorganisms. 7, 562. doi: 10.3390/microorganisms7110562
Thao, N. T., Wanapat, M., Cherdthong, A., and Kang, S. (2014). Effects of eucalyptus crude oils supplementation on rumen fermentation, microorganism and nutrient digestibility in swamp buffaloes. Asian Austral. J. Anim. 27, 46–54. doi: 10.5713/ajas.2013.13301
Van Soest, P. J., Robertson, J. B., and Lewis, B. A. (1991). Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74, 3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2
Wang, C., Liu, Q., Guo, G., Huo, W. J., Pei, C. X., Zhang, S. L., et al. (2018a). Effects of concentrate-to-forage ratios and 2-methylbutyrate supplementation on ruminal fermentation, bacteria abundance and urinary excretion of purine derivatives in Chinese Simmental steers. J. Anim. Physiol. An. N. 102, 901–909. doi: 10.1111/jpn.12915
Wang, C., Liu, Q., Guo, G., Huo, W. J., Pei, C. X., Zhang, S. L., et al. (2018b). Effects of dietary protein levels and 2-methylbutyrate on ruminal fermentation, nutrient degradability, bacterial populations and urinary purine derivatives in Simmental steers. J. Anim. Physiol. An. N. 102, 611–619. doi: 10.1111/jpn.12797
Wang, C., Liu, Q., Guo, G., Huo, W. J., Wang, Y. X., Zhang, Y. L., et al. (2019). Effects of fibrolytic enzymes and isobutyrate on ruminal fermentation, microbial enzyme activity and cellulolytic bacteria in pre- and post-weaning dairy calves. Anim. Prod. Sci. 59, 471–478. doi: 10.1071/AN17270
Wang, C., Liu, Q., Pei, C. X., Li, H. Y., Wang, Y. X., Wang, H., et al. (2012). Effects of 2-methylbutyrate on rumen fermentation, ruminal enzyme activities, urinary excretion of purine derivatives and feed digestibility in steers. Livest. Sci. 145, 160–166. doi: 10.1016/j.livsci.2012.01.013
Wang, C., Liu, Q., Zhang, Y. L., Pei, C. X., Zhang, S. L., Wang, Y. X., et al. (2015). Effects of isobutyrate supplementation on ruminal microflora, rumen enzyme activities and methane emissions in Simmental steers. J. Anim. Physiol. An. N. 99, 123–131. doi: 10.1111/jpn.12191
Wang, H., Long, R., Liang, J. B., Guo, X., Ding, L., and Shang, Z. (2011). Comparison of nitrogen metabolism in yak (Bos grunniens) and indigenous cattle (Bos taurus) on the Qinghai-Tibetan Plateau. Asian Austral. J. Anim. 24, 766–773. doi: 10.5713/ajas.2011.10350
Wang, L. J., Zhang, G. N., Li, Y., and Zhang, Y. G. (2020). Effects of high forage/concentrate diet on volatile fatty acid production and the microorganisms involved in VFA production in cow rumen. Animals. 10, 223. doi: 10.3390/ani10020223
Wang, M., Sun, X. Z., Janssen, P. H., Tang, S. X., and Tan, Z. L. (2014). Responses of methane production and fermentation pathways to the increased dissolved hydrogen concentration generated by eight substrates in in vitro ruminal cultures. Anim. Feed Sci. Tech. 194, 1–11. doi: 10.1016/j.anifeedsci.2014.04.012
Wang, M., Wang, R., Janssen, P. H., Zhang, X. M., Sun, X. Z., Pacheco, D., et al. (2016a). Sampling procedure for the measurement of dissolved hydrogen and volatile fatty acids in the rumen of dairy cows. J. Anim. Sci. 94, 1159–1169. doi: 10.2527/jas.2015-9658
Wang, M., Wang, R., Xie, T. Y., Janssen, P. H., Sun, X. Z., Beauchemin, K. A., et al. (2016b). Shifts in rumen fermentation and microbiota are associated with dissolved ruminal hydrogen concentrations in lactating dairy cows fed different types of carbohydrates. J. Nutr. 146, 1714–1721. doi: 10.3945/jn.116.232462
Wang, Z., Yang, D. S., Li, X. Y., Yu, L. Y., Zhang, P. H., He, H. J., et al. (2021). Modulation of rumen fermentation and microbial community through increasing dietary cation–anion difference in Chinese Holstein dairy cows under heat stress conditions. J. Appl. Microbiol. 130, 722–735. doi: 10.1111/jam.14812
Xin, J., Chai, Z., Zhang, C., Zhang, Q., Zhu, Y., Cao, H., et al. (2019). Comparing the microbial community in four stomach of dairy cattle, yellow cattle and three yak herds in Qinghai-Tibetan Plateau. Front. Microbiol. 10, 1547. doi: 10.3389/fmicb.2019.01547
Xue, D., Chen, H., Luo, X., Guan, J., He, Y., and Zhao, X. (2018). Microbial diversity in the rumen, reticulum, omasum, and abomasum of yak on a rapid fattening regime in an agro-pastoral transition zone. J. Microbiol. 56, 734–743. doi: 10.1007/s12275-018-8133-0
Yi, S. M., Dai, D. W., Wu, H., Chai, S. T., Liu, S. J., Meng, Q. X., et al. (2022). Dietary concentrate-to-forage ratio affects rumen bacterial community composition and metabolome of yaks. Front Nutr. 9, 1459. doi: 10.3389/fnut.2022.927206
Zhang, Z., Xu, D., Wang, L., Hao, J., Wang, J., Zhou, X., et al. (2016). Convergent evolution of rumen microbiomes in high-altitude mammals. Curr. Biol. 26, 1873–1879. doi: 10.1016/j.cub.2016.05.012
Zhou, J. W., Liu, H., Zhong, C. L., Degen, A. A., Yang, G., Zhang, Y., et al. (2018). Apparent digestibility, rumen fermentation, digestive enzymes and urinary purine derivatives in yaks and Qaidam cattle offered forage-concentrate diets differing in nitrogen concentration. Livest. Sci. 208, 14–21. doi: 10.1016/j.livsci.2017.11.020
Keywords: yak (Bos grunniens), isoacids, growth performance, fiber digestibility, ruminal bacterial community, 16S rRNA gene sequencing
Citation: Jiang F, Gao Y, Peng Z, Ma X, You Y, Hu Z, He A and Liao Y (2023) Isoacids supplementation improves growth performance and feed fiber digestibility associated with ruminal bacterial community in yaks. Front. Microbiol. 14:1175880. doi: 10.3389/fmicb.2023.1175880
Received: 28 February 2023; Accepted: 26 May 2023;
Published: 15 June 2023.
Edited by:
Diego P. Morgavi, INRAE Clermont-Auvergne-Rhône-Alpes, FranceReviewed by:
Zuo Wang, Hunan Agricultural University, ChinaCopyright © 2023 Jiang, Gao, Peng, Ma, You, Hu, He and Liao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanhua Gao, Z2FveWFuaHVhQHN3dW4uZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.