- 1Key Laboratory of Tropical Translational Medicine of Ministry of Education, School of Pharmacy, Hainan Medical University, Haikou, China
- 2College of Science, Hainan University, Haikou, China
- 3College of Life Science, Hunan Normal University, Changsha, China
- 4Institute of Edible and Medicinal Fungi, College of Life Sciences, Zhejiang University, Hangzhou, China
- 5School of Pharmaceutical Sciences and Yunnan Key Laboratory of Pharmacology for Natural Products, Kunming Medical University, Kunming, China
- 6Yinggeling Substation, Hainan Tropical Rainforest National Park, Baisha, China
Cantharellus, one of the main genera of Hydnaceae (Cantharellales), is both ecologically and economically important. Although many studies have focused on this genus in China, the taxonomy should be further updated. In the present study, Cantharellus subgenera Afrocantharellus and Magni were investigated based on morphology and molecular phylogenetic analyses with new collections from China. Five phylogenetic species were recognized among the studied collections, three of which were described as new: C. bellus, C. cineraceus, and C. laevigatus; one was previously described taxon: C. hygrophoroides; and the remaining species was not defined due to the paucity of the materials. Among the four described species, both C. bellus and C. laevigatus are members of subgen. Magni, whereas C. cineraceus and C. hygrophoroides belong to subgen. Afrocantharellus.
Introduction
Cantharellus Adans. ex Fr. (Hydnaceae, Cantharellales) is interesting and important in forestry for its mycorrhizal properties, medicinal values, and edibility of many species (Pilz et al., 2003; Yun and Hall, 2004; Zhang M. et al., 2022). Recently, the genus has been divided into seven subgenera: Afrocantharellus Eyssart. & Buyck, Cantharellus Adans. ex Fr., Cinnabarini Buyck & V. Hofst., Magni T. Cao & H.S. Yuan, Parvocantharellus Eyssart. & Buyck, Pseudocantharellus Eyssart. & Buyck, and Rubrini Eyssart. & Buyck (Buyck et al., 2014; Cao et al., 2021). In China, researchers paid much attention to subgenera Cantharellus, Cinnabarini, and Parvocantharellus (An et al., 2017; Zhang M. et al., 2021; Zhang Y. Z. et al., 2021; Zhang M. et al., 2022; Zhang Y. Z. et al., 2022), whereas subgenera Afrocantharellus and Magni received little attention.
Subgen. Afrocantharellus, typified by C. symoensii Heinem., was proposed in 2001 (Eyssartier and Buyck, 2001), and then, Tibuhwa et al. (2012) elevated the subgenus to genus rank. However, further molecular-based infrageneric classification of Cantharellus did not support subgen. Afrocantharellus as a separate genus (Buyck et al., 2013, 2014), and thus, the subgenus rank of Afrocantharellus was redefined, which includes two sections, viz., Afrocantharellus Buyck & V. Hofstetter and Cutirellus Corner. It is characterized by a high proportion of four-spored basidia, an absence of clamp connections, and thin-walled hyphal extremities at the cap surface (Buyck et al., 2014). Subgen. Magni, a monotypic subgenus typified by C. magnus T. Cao & H. S. Yuan, was erected in 2021, which is characterized by a large basidioma, smooth, azonate, deep yellow to deep orange pileal surface, almost perfectly smooth hymenophore, thin- to slightly thick-walled terminal cells of pileipellis hyphae, and a presence of clamp connections (Cao et al., 2021).
In China, only two taxa of subgen. Afrocantharellus, viz., C. cerinoalbus Eyssart. & Walleyn and C. hygrophoroides S.C. Shao, Buyck & F.Q. Yu, and one species of subgen. Magni, viz., C. magnus, have been described/reported in previous studies (Shao et al., 2014; Song et al., 2017; Zeng and Jiang, 2020; Cao et al., 2021). Recently, several specimens of subgen. Afrocantharellus and Magni were collected in China, and they were studied using morphological and molecular phylogenetic analyses, aiming to update the taxonomy of the two subgenera.
Materials and methods
Morphological studies
The studied specimens were collected from Hainan, Fujian, Hunan, and Zhejiang Provinces of China and deposited in the Fungal Herbarium of Hainan Medical University (FHMU), Haikou city, Hainan Province of China. Based on detailed notes and photographs taken from fresh basidiomata, we obtained the macroscopic descriptions. Color documentation of fresh materials follows Kornerup and Wanscher (1981). Observations and measurements of microscopic features were made in 5% KOH solution and stained with 1% Congo Red (Zhang Y. Z. et al., 2022). The sections of the pileipellis were taken from the pileus between the center and the margin. The number of measured basidiospores is given as n/m/p, where “n” represents the total number of basidiospores measured from “m” basidiomata of “p” collections. The dimensions of basidiospores are presented in the form (a–)b–e–c(−d), where the range b–c contains at least of 90% of the measured values (5th to 95th percentile), “a” and “d” are the extreme values, and “e” refers to the average length/width of basidiospores. “Q” refers to the length/width ratio of basidiospores and “Qm” refers to the average Q of basidiospores and is given with standard deviation. For basidiospore shape, Qm = 1.15–1.3 describes “broadly ellipsoid,” Qm = 1.3–1.6 “ellipsoid,” and Qm = 1.6–2.0 “elongate” (Yang, 2005). The terms referring to the size of basidioma are based on Bas (1969).
Molecular procedures
The total genomic DNA was extracted from collections and dried with silica gel using the Plant Genomic DNA Kit (CWBIO, Beijing, China) according to the manufacturer’s instructions. Primer pairs used for amplification were nuc 28S rDNA D1-D2 domains (28S) with LR0R/LR5 (Vilgalys and Hester, 1990; James et al., 2006) and the translation elongation factor 1-α gene (TEF1) with EF1-α-F/EF1-α-R (Mikheyev et al., 2006). PCR conditions followed the program of Zhang Y. Z. et al. (2021). PCR products were checked in 1% (w/v) agarose gels. The PCR amplification products were sequenced using an ABI 3730 DNA Analyzer (Guangzhou Branch of BGI, China) with the same primers. DNA sequences were compiled with SeqMan (DNASTAR Lasergene 9) or BioEdit (Hall, 1999) and then deposited in GenBank.1
Dataset assembly
Forty DNA sequences (22 of 28S and 18 of TEF1) from 23 collections were newly generated. The GenBank accession numbers are listed in Table 1. For the concatenated dataset, the sequences of 28S and TEF1 from new collections were aligned with sequences of taxa of subgenera Afrocantharellus and Magni, and representative species of other subgenera of Cantharellus from previous studies and GenBank (Table 1). Craterellus badiogriseus T. Cao & H.S. Yuan was chosen as out-group inferred from Cao et al. (2021). To test for phylogenetic conflict among 28S and TEF1, single-gene phylogenetic trees based on each of these two fragments were analyzed. A conflict was assumed to be significant if two different relationships for the same set of taxa (one being monophyletic and the other non-monophyletic) were observed in rival trees (Vadthanarat et al., 2021). The results of analyses showed that 28S and TEF1 were not in conflict. Thus, the datasets (28S and TEF1) were aligned with MUSCLE v3.6 (Edgar, 2004) and concatenated using Phyutility v2.2 for further analyses (Smith and Dunn, 2008).
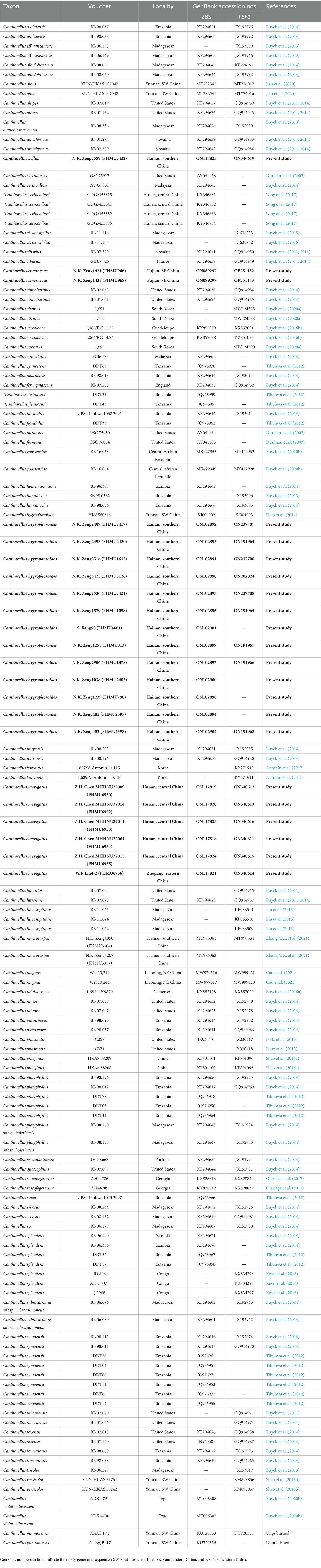
Table 1. Taxa, vouchers, locations, and GenBank accession numbers of DNA sequences used in phylogenetic analyses.
Phylogenetic analyses
The combined nuclear dataset (28S + TEF1) was analyzed using maximum likelihood (ML) and Bayesian inference (BI). For ML, tree generation and bootstrap analyses were performed with RAxML7.2.6 (Stamatakis, 2006) running 1,000 replicates combined with an ML search. For BI, the best-fit model of substitution among those implementable in MrBayes was estimated separately for each character set using jModelTest (Darriba et al., 2012) on the CIPRES portal, based on the Bayesian information criterion. The best-fit likelihood models for 28S and TEF1 were GTR + I + G and SYM + I + G, respectively. BI was conducted in MrBayes v3.1 (Huelsenbeck and Ronquist, 2005) on the CIPRES Science Gateway portal (Miller et al., 2011). For the BI analyses, four chains were processed with the generation set as 10 million using the selected model for each gene. The trees were sampled every 100 generations. Other parameters were kept at their default setting. The chain convergence was determined using Tracer v 1.52 to ensure sufficiently large ESS values. The stop rule was used when parallel MCMC runs converged (ESS value > 200). Finally, 6.5 million generations were taken to reach the convergence, and the average deviation of split frequencies was 0.009922. The trees were summarized, and statistical values were obtained using the sump and sumt commands with burn-ins (i.e., the first 25% of the samples) discarded. In addition, phylogenetic distances of some Cantharellus species were calculated using MEGA 11 (Tamura et al., 2021).
Results
Molecular data
The combined dataset (28S + TEF1) consisted of 139 taxa and 1862 nucleotide sites (986 of 28S and 876 of TEF1). The phylogram with branch lengths generated from RAxML and support values (BS and PP) is shown in Figure 1. The topologies of phylogenetic trees generated from ML and BI analyses were identical, although statistical support for some branches showed slight differences.
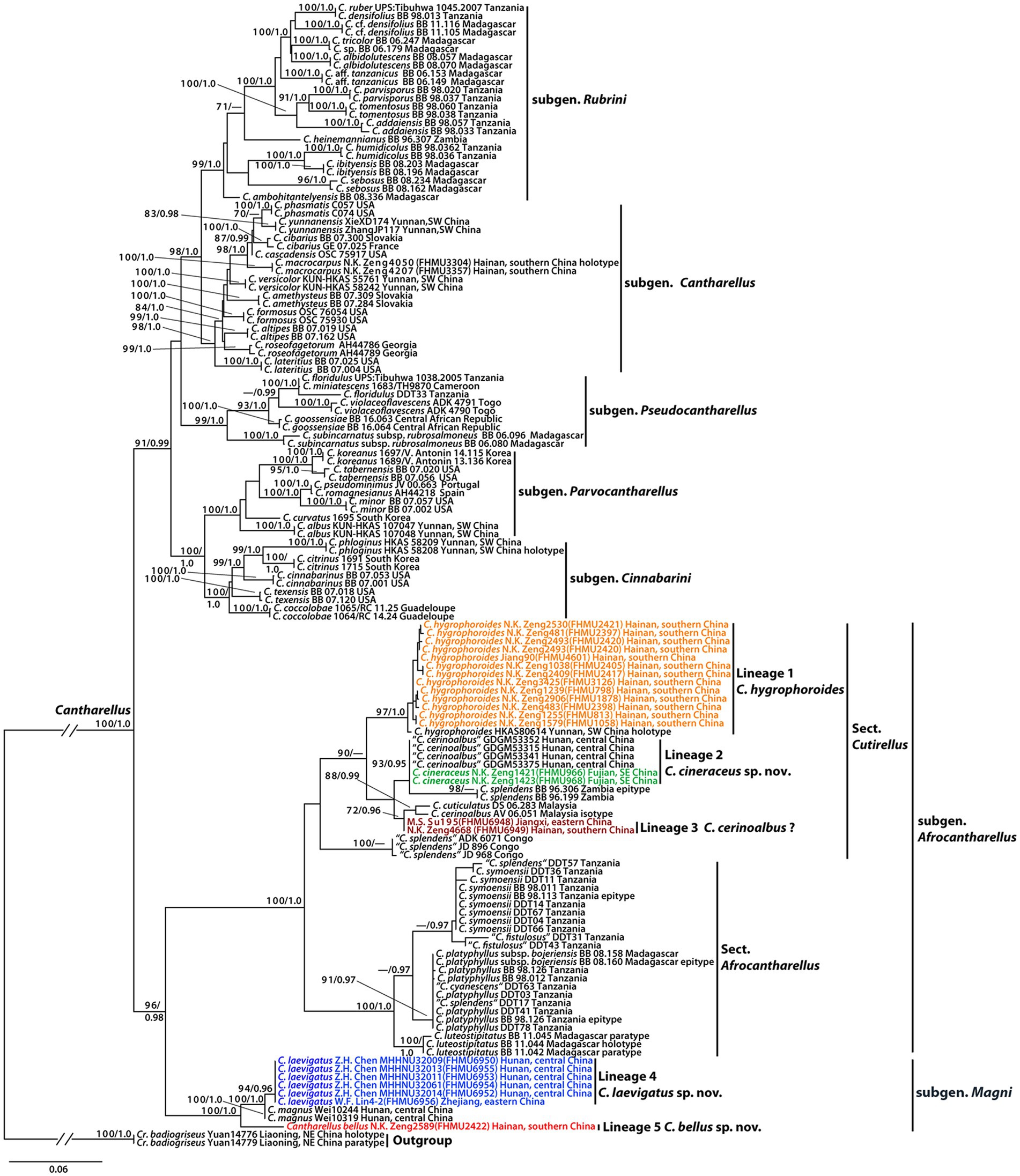
Figure 1. Phylogram of genus Cantharellus, with emphasis on subgenera Afrocantharellus and Magni, inferred from a two-locus (rDNA 28S, TEF1) dataset using RAxML. BS (≥70%) and PP (≥0.95) are indicated above the branches.
The present molecular data indicated that the Chinese collections of subgenera Afrocantharellus and Magni were grouped into five independent lineages (Figure 1). Lineage 1, with strong statistical support (BS = 97%, PP = 1.0), included the holotype of C. hygrophoroides (HKAS80614) and 13 new collections (FHMU798, FHMU813, FHMU1058, FHMU1635, FHMU1878, FHMU2397, FHMU2398, FHMU2405, FHMU2417, FHMU2420, FHMU2421, FHMU3126, and FHMU4601) from southern China; lineage 2, with high statistical support (BS = 93%, PP = 0.95), included two new specimens (FHMU966 and FHMU968) from southeastern China and four collections labeled as C. cerinoalbus from central China; lineage 3, with weak statistical support, was comprised of two new specimens (FHMU6948 and FHMU6949) from eastern China and southern China, respectively; lineage 4, with strong statistical support (BS = 94%, PP = 0.96), was comprised of five new collections (FHMU6950, FHMU6952, FHMU6953, FHMU6954, and FHMU6955) from central China and one new specimen (FHMU6956) from eastern China; lineage 5, only included one new collection (FHMU2422) from southern China (Figure 1).
Taxonomy
Cantharellus bellus N.K. Zeng, Y.Z. Zhang & Zhi Q. Liang, sp. nov.
Figures 2A,B, 3.

Figure 2. Basidiomata of Cantharellus subg. Afrocantharellus species. (A,B) Cantharellus bellus (FHMU2422, holotype); (C,D) Cantharellus cineraceus (C) FHMU968, holotype; (D) FHMU966; (E,F) Cantharellus hygrophoroides (E) FHMU2421; (F) FHMU1635; (G,H) Cantharellus laevigatus (G) FHMU6955, holotype; (H) FHMU6952; Photographs: (A–F) N. K. Zeng; (G,H) Z. H. Chen.
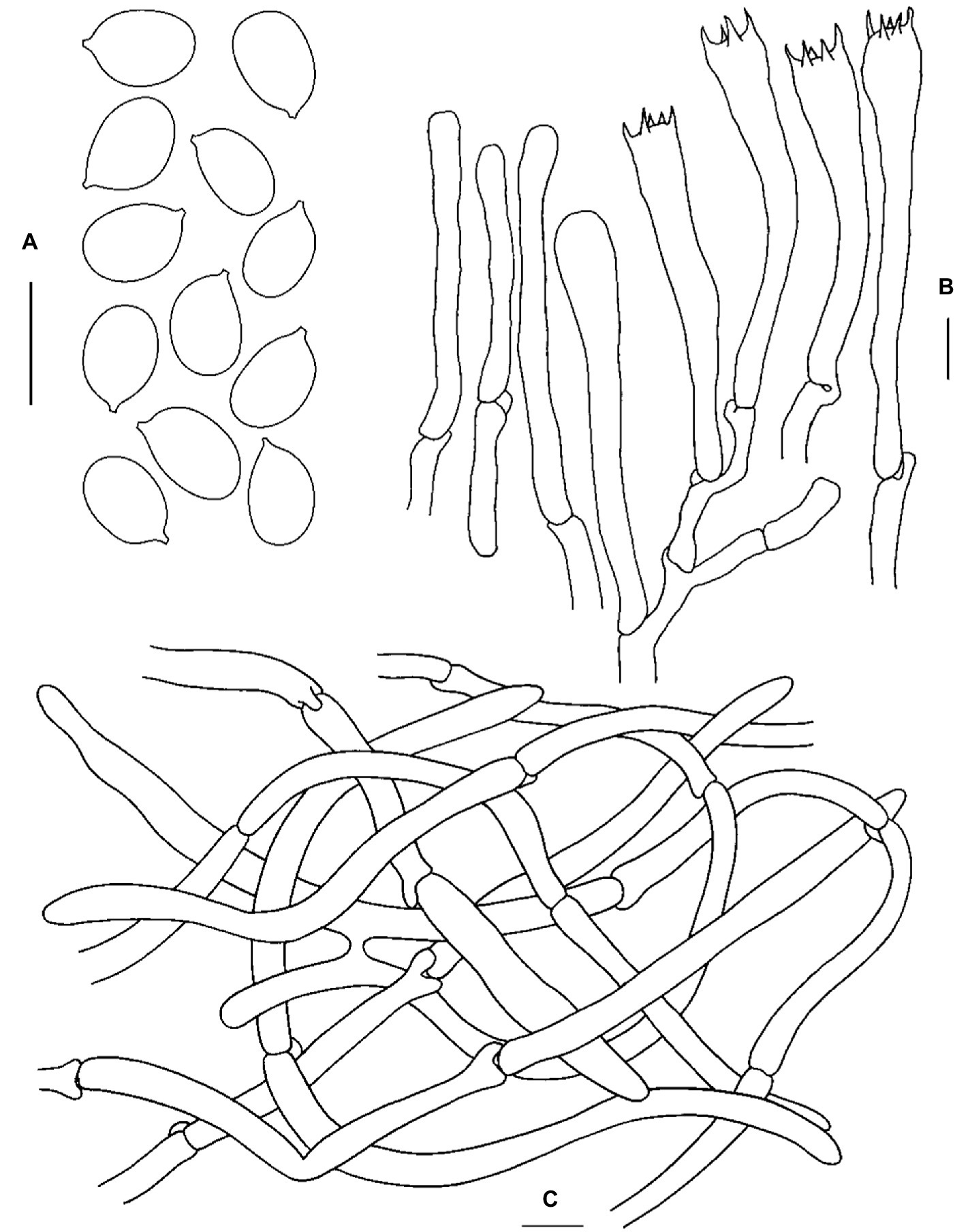
Figure 3. Microscopic features of Cantharellus bellus (FHMU2422, holotype). (A) Basidiospores. (B) Basidia. (C) Pileipellis. Scale bars = 10 μm. Drawings by Y. Z. Zhang.
MycoBank: MB845017.
Diagnosis: Differs from other species of Cantharellus subgen. Magni by a bright orange-yellow pileus, a well-developed hymenophore, and an intricate trichodermal pileipellis.
Etymology: The specific epithet “bellus” refers to the beautiful basidioma of the new species.
Holotype: China. Hainan Province: Yinggeling of Hainan Tropical Rainforest National Park, elev. 650 m, 5 August 2015, N. K. Zeng2589 (FHMU2422). GenBank accession number: 28S = ON117825, TEF1 = ON340619.
Basidiomata small-sized. Pileus 2.5–5 cm in diameter, plano-convex to infundibuliform, margin irregularly wavy; surface smooth, bright orange-yellow (5A6), shiny; context above stipe about 0.2 cm in thickness, yellowish (3A1), unchanging in color when injured. Hymenophore composed of well-developed, well-spaced, decurrent, unequal and forked gill folds, commonly anastomosing; folds about 0.15 cm broad, pale yellow (3A3). Stipe 2.5–3.7 × 0.45–0.6 cm, subcylindrical, central, solid, hollow when old; surface dry, grayish yellow (4A2) to fulvous (2B2); context yellowish (1A2). Taste and Odor not distinctive. Spore print not obtained.
Basidiospores [40/2/1] (7–)7.5–8.7–9.5(−10) × 5.5–6.3–6.5(−7) μm, Q = (1.08–)1.15–1.55(−1.73), Qm = 1.39 ± 0.12, broadly ellipsoid to ellipsoid, slightly thick-walled (up to 0.5 μm), smooth, yellowish in KOH. Basidia 55–83 × 8–10.5 μm, narrow, subcylindric, slightly thick-walled (0.5–0.7 μm), 3–5–spored, yellowish in KOH; sterigmata 5.5–7.5 μm in length. Cystidia absent. Pileipellis an intricate trichoderm composed of cylindrical, 4–7.5 μm wide, slightly thick-walled (up to 0.5 μm) hyphae, faintly pale yellow in KOH; terminal cells 20–100 × 4.5–7 μm, slightly thick-walled (up to 0.5 μm), subcylindrical to subclavate, with obtuse apex. Clamp connections present in all tissues.
Habitat: Solitary on the ground of forests dominated by Castanopsis kawakamii Hayata.
Known distribution: Southern China (Hainan Province).
Notes: Phylogenetically, C. bellus is closely related to C. magnus (Figure 1), also a member of subgen. Magni; however, the latter has a larger basidioma (pileus up to 20 cm in diameter), a smooth hymenophore, and larger basidiospores measuring (8.5–)9–11(−11.5) × (6.5–)6.8–7.5(−8.0) μm (Cao et al., 2021). Cantharellus chuiweifanii N.K. Zeng, Y.Z. Zhang, and Zhi Q. Liang, C. cibarius Fr., C. pallens Pilát, and C. yunnanensis W.F. Chiu are morphologically similar to C. bellus. However, the four species are all members of subgen. Cantharellus (Zhang Y. Z. et al., 2022).
Cantharellus cineraceus N.K. Zeng, Y.Z. Zhang & Zhi Q. Liang, sp. nov.
Figures 2C,D, 4.
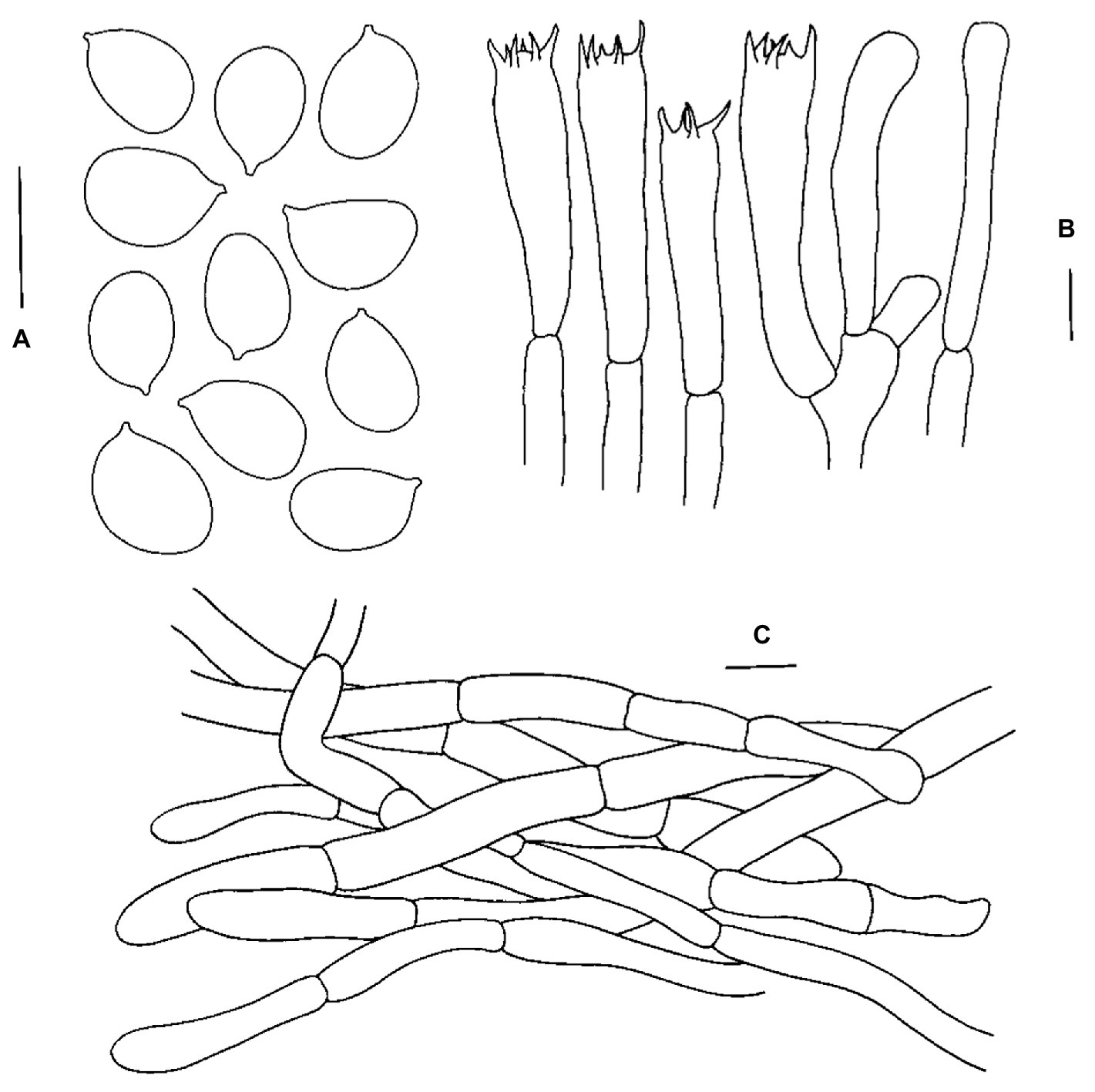
Figure 4. Microscopic features of Cantharellus cineraceus (FHMU968, holotype). (A) Basidiospores. (B) Basidia. (C) Pileipellis. Scale bars = 10 μm. Drawings by Y. Z. Zhang.
MycoBank: MB845018.
Diagnosis: Differs from other species of Cantharellus subgen. Afrocantharellus by a yellowish orange pileus, grayish white stipe and context, and it is associated with fagaceous trees.
Etymology: The specific epithet “cineraceus” refers to the grayish-white context of the new species.
Holotype: China. Fujian Province: Zhangping city, Xinqiao town, Chengkou village, elev. 350 m, 17 August 2013, N. K. Zeng1423 (FHMU968). GenBank accession number: 28S = ON089298, TEF1 = OP251153.
Basidiomata small-sized. Pileus 3–4.5 cm in diameter, central depression to broadly infundibuliform, margin slightly incurved, irregular, often wavy and lobed; surface smooth, yellowish orange (6B6); context above stipe 0.2–0.3 cm in thickness, yellowish (4A2), unchanging in color when injured. Hymenophore composed of well-developed, well-spaced, decurrent, unequal, occasionally to commonly forked gill folds, mostly anastomosing near the cap margin; folds 0.05–0.1 cm broad, white (2A1), pale orange-ochre (4A3) to pale ochre-yellow (2A4). Stipe 2.5–3.5 × 0.5–0.8 cm, subcylindrical, central, solid, hollow when old; surface dry, grayish white (2A1); context grayish white (3A1). Taste and Odor not distinctive. Spore print not obtained.
Basidiospores [79/9/2] 8–9.3–10(−11.5) × (5–)5.5–6.4–7 μm, Q = 1.23–1.73(−1.9), Qm = 1.46 ± 0.14, broadly ellipsoid to ellipsoid, slightly thick-walled (up to 0.5 μm), smooth, yellowish in KOH. Basidia 56–98 × 8–11 μm, long, narrow, subcylindric, slightly thick-walled (up to 0.5 μm), 3–6–spored, yellowish in KOH; sterigmata 6–8 μm in length. Cystidia absent. Pileipellis a cutis composed of cylindrical, 5.5–10 μm wide, slightly thick-walled (0.5–0.8 μm) hyphae, faintly pale yellow in KOH; terminal cells 24–36 × 5–8.5 μm, thin- to slightly thick-walled (up to 0.5 μm), subcylindrical to subclavate, with obtuse apex. Clamp connections absent in all tissues.
Habitat: Gregarious on the ground in forests dominated by Castanopsis kawakamii Hayata.
Known distribution: Southeastern and central China (Fujian and Hunan Provinces).
Additional specimen examined: China. Fujian Province: Zhangping city, Xinqiao town Chengkou village, elev. 350 m, 17 August 2013, N. K. Zeng1421 (FHMU966).
Notes: Although the values of phylogenetic distances among C. cineraceus, C. cerinoalbus Eyssart. & Walleyn, and C. cuticulatus Corner seem to be not high (Figure 1) for only 28S sequences of the three taxa were obtained, C. cerinoalbus and C. cuticulatus are morphologically different with C. cineraceus. Malaysian C. cerinoalbus has a yellow cap often with olivaceous or grayish tinges, smaller basidiospores measuring 7.5–9(−10) × 5–5.75(−6) μm, and it is associated with trees of Dipterocarpaceae (Eyssartier et al., 2009); C. cuticulatus, originally described from Malaysia, has a larger basidioma (pileus up to 7.5 cm in diameter), a stipe tinged with yellow, and a trichodermal structure of the pileipellis (Corner, 1966; Buyck et al., 2014). In future, more interesting information will be provided with more collections made and more genes investigated.
Cantharellus splendens Buyck is also morphologically similar to C. cineraceus. However, the former has narrower basidiospores measuring 8–9.5–11 × 5–5.25–6 μm, a trichodermal structure of the pileipellis, and it is distributed in Africa (Buyck, 2012; Buyck et al., 2014).
Cantharellus hygrophoroides S.C. Shao, Buyck & F.Q. Yu, Cryptog. Mycol. 35(3): 287, 2014.
Figures 2E,F, 5.
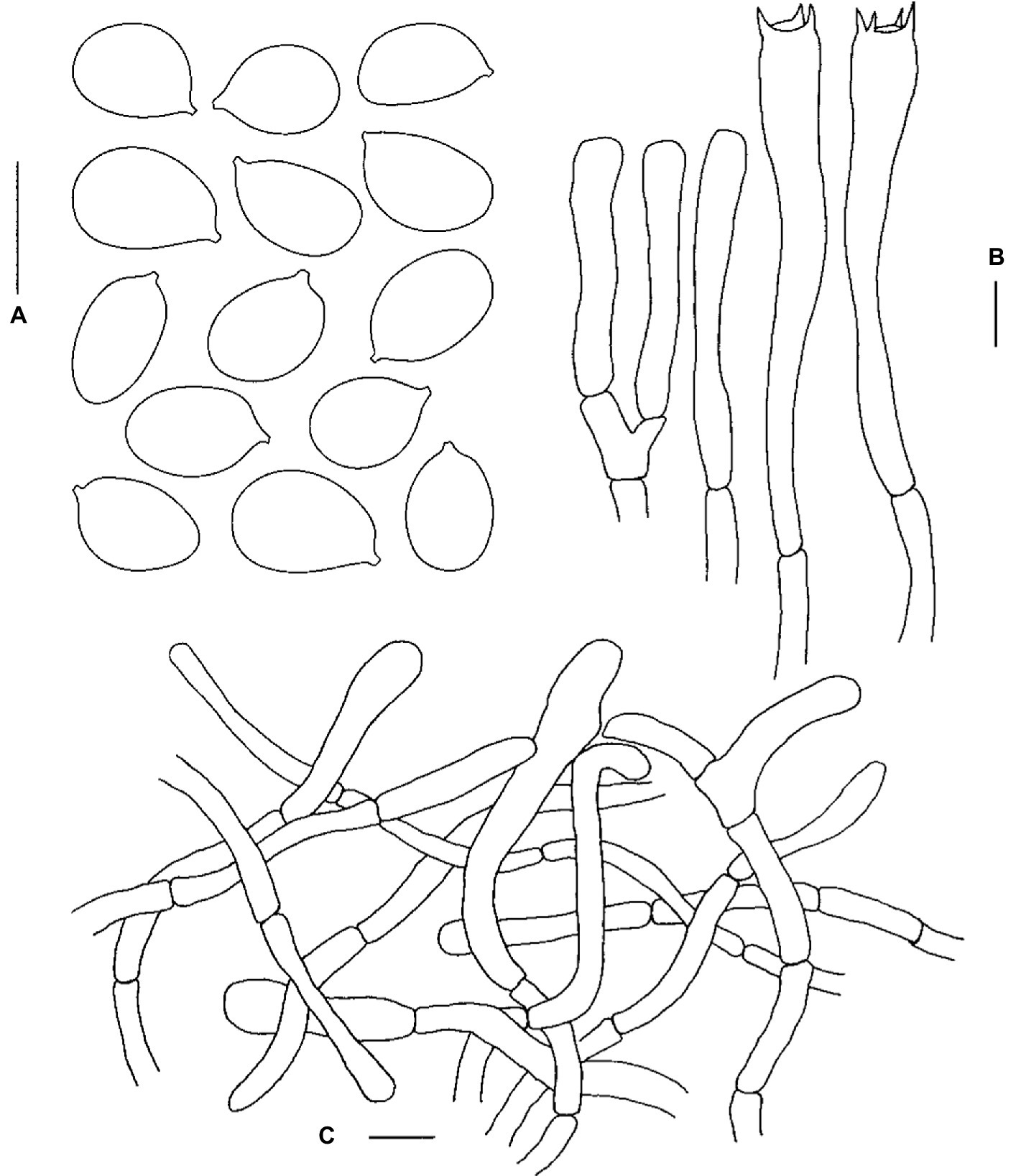
Figure 5. Microscopic features of Cantharellus hygrophoroides (FHMU1635). (A) Basidiospores. (B) Basidia. (C) Pileipellis. Scale bars = 10 μm. Drawings by Y. Z. Zhang.
Basidiomata medium-sized. Pileus 5–12 cm in diameter, convex with central depression at first, becoming infundibuliform at maturity, margin inrolled and slight lobed; surface smooth, orange-yellow (3A6); context above stipe 0.3 cm in thickness, white (3A1), unchanging in color when injured. Hymenophore composed of well-developed, well-spaced, decurrent, unequal, occasionally to commonly forked gill folds, mostly anastomosing near the cap margin; folds about 0.5 cm broad, pale yellow (3A4). Stipe 4.5–9 × 0.9–1.5 cm, subcylindrical, central, solid, hollow when old; surface dry, lemon yellow (1A5) to yellowish (2A1). Taste and Odor not distinctive. Spore print not obtained.
Basidiospores [254/18/13] (8–)9–9.92–11(−12) × (5.5–)6–6.87–8(−9) μm, Q = (1.19–)1.25–1.67(−1.83), Qm = 1.45 ± 0.12, broadly ellipsoid to ellipsoid to elongate, slightly thick-walled (up to 0.5 μm), smooth, yellowish in KOH. Basidia 65–82 × 9–12 μm, narrowly clavate, thin-walled to slightly thick-walled (0.4–0.5 μm), 3–5–spored, yellowish in KOH; sterigmata 3.5–5 μm in length. Cystidia absent. Pileipellis an intricate trichoderm composed of cylindrical, 4–9 μm wide, slightly thick-walled (0.5–0.8 μm) hyphae, faintly pale yellow in KOH; terminal cells 17–54 × 3.5–8.5 μm, thin- to slightly thick-walled (up to 0.5 μm), subcylindrical to subclavate, with obtuse apex. Clamp connections absent in all tissues.
Habitat: Solitary, scattered, or gregarious on the ground of forests dominated by fagaceous trees such as Castanopsis fissa (Champion ex Bentham) Rehder et E. H. Wilson.
Known distribution: Southwestern China (Yunnan Province; Shao et al., 2014) and southern China (Hainan Province).
Specimens examined: China. Hainan Province: Yinggeling of Hainan Tropical Rainforest National Park, elev. 650 m, 17 June 2013, N. K. Zeng1239 (FHMU798); same location, elev. 650 m, 31 July 2015, N. K. Zeng2409 (FHMU2417); same location, elev. 650 m, 3 August 2015, N. K. Zeng2493 (FHMU2420); same location, elev. 650 m, 4 August 2015, N. K. Zeng2530 (FHMU2421); same location, elev. 650 m, 9 September 2016, N. K. Zeng2906 (FHMU1878); same location, elev. 650 m, 26 July 2017, S. Jiang90 (FHMU4601); Jianfengling of Hainan Tropical Rainforest National Park, elev. 850 m, 6 August 2009, N. K. Zeng481 (FHMU2397); same location, elev. 850 m, 6 August 2009, N. K. Zeng483 (FHMU2398); same location, elev. 850 m, 3 July 2012, N. K. Zeng1038 (FHMU2405); same location, elev. 850 m, 9 July 2013, N. K. Zeng1255 (FHMU813); same location, elev. 850 m, 4 July 2014, N. K. Zeng1579 (FHMU1058); same location, elev. 850 m, 27 June 2018, N. K. Zeng3425 (FHMU3126).
Notes: Our recent collections and the holotype of C. hygrophoroides phylogenetically group together with high statistical support are presented in Figure 1. Moreover, the new specimens match well with the protologue of C. hygrophoroides, except that the color of the pileal surface is described as “bright red” by Shao et al. (2014) whereas that of our new specimens is distinctly orange-yellow. It is worth noting the color of Cantharellus species sometimes is not constant (Buyck et al., 2016c; Olariaga et al., 2017); thus, we suggest these new specimens are C. hygrophoroides although the color of the pileal surface between our new collections and the holotype of C. hygrophoroides is slightly different.
Cantharellus laevigatus N.K. Zeng, Y.Z. Zhang, Z.H. Chen & W.F. Lin, sp. nov.
Figures 2G,H, 6.
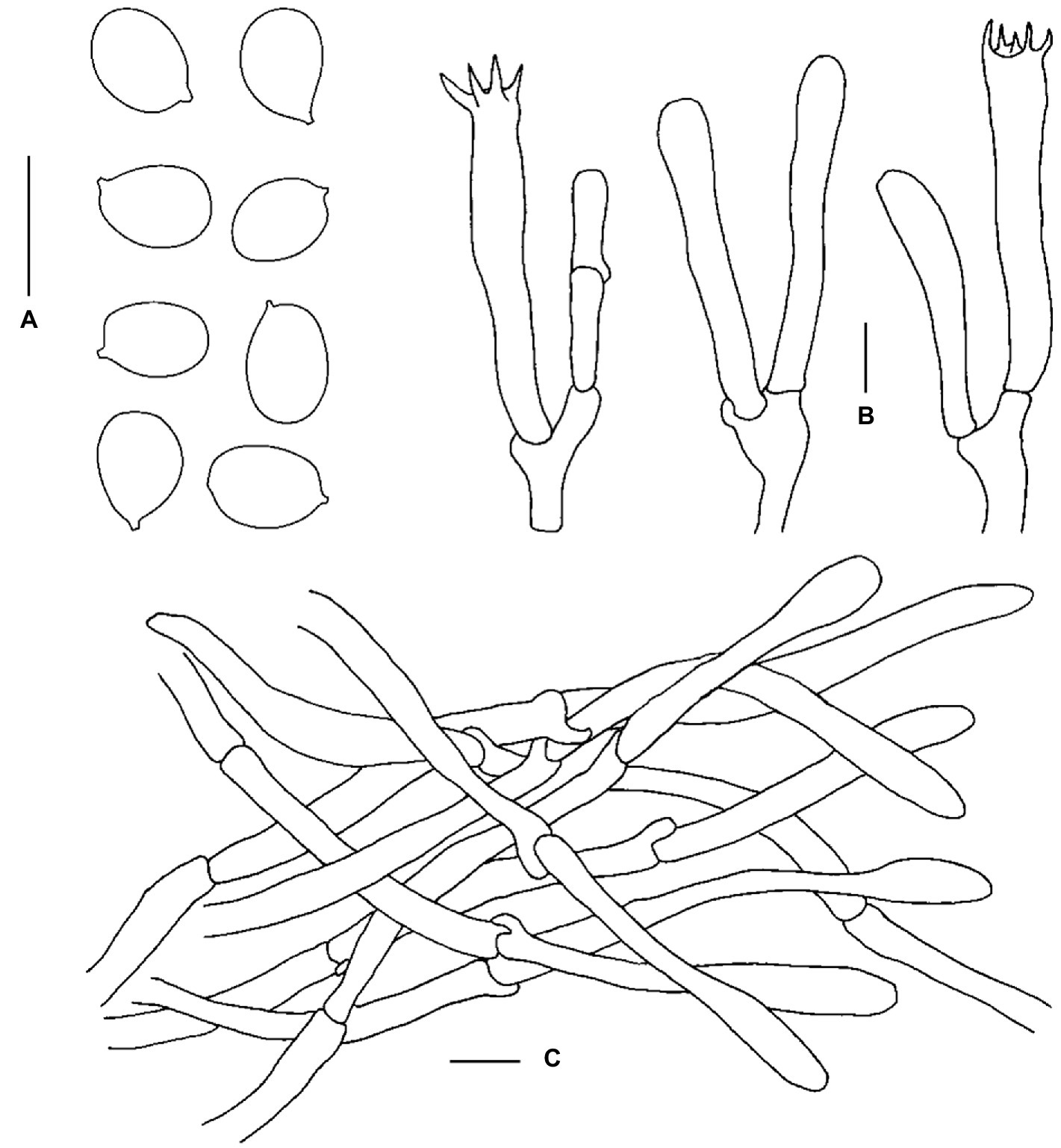
Figure 6. Microscopic features of Cantharellus laevigatus (FHMU6951, holotype). (A) Basidiospores. (B) Basidia. (C) Pileipellis. Scale bars = 10 μm. Drawings by Y. Z. Zhang.
MycoBank: MB845019.
Diagnosis: Differs from other species of Cantharellus subgen. Magni by a small- to medium-sized basidioma, an orange-yellow pileus, a smooth hymenophore, and smaller basidiospores.
Etymology: Latin “laevigatus” refers to the smooth hymenophore of the new species.
Holotype: China. Hunan Province: Sangzhi county, Badagong Mountain, Tianping Mountain, elev. 1,478 m, 31 July 2020, Z. H. Chen MHHNU32013 (FHMU6955). GenBank accession number: 28S = ON117824, TEF1 = ON340615.
Basidiomata small- to medium-sized. Pileus 2.3–5.2 cm in diameter, plano-convex to infundibuliform, margin slightly incurved, irregular, often wavy and lobed; surface dry, egg-yolk yellow (3A6) to orange-yellow (4A5), shiny; context above stipe about 0.2 cm in thickness, yellowish (4A2), unchanging in color when injured. Hymenophore smooth, decurrent, lemon yellow (1A6). Stipe 2.3–4.2 × 0.5–0.8 cm, subcylindrical, central, solid, hollow when old; surface dry, white (2A1); context yellowish (3A2). Taste and Odor not distinctive. Spore print not obtained.
Basidiospores [120/10/6] 6.5–7.9–8.5(−9) × 5–5.6–6(−7.5) μm, Q = (1.17–)1.23–1.6(−1.64), Qm = 1.42 ± 0.11, broadly ellipsoid to ellipsoid, thin- to slightly thick-walled (up to 0.5 μm), smooth, yellowish in KOH. Basidia 42–64 × 6.5–9 μm, narrowly clavate, thin-walled, 4–5–spored, yellowish in KOH; sterigmata 6.5–7 μm in length. Cystidia absent. Pileipellis a cutis composed of cylindrical, 4.5–11 μm wide, slightly thick-walled (0.5–0.8 μm) hyphae, faintly pale yellow in KOH; terminal cells 45–128 × 5–10 μm, thin- to slightly thick-walled (up to 0.5 μm), subcylindrical to subclavate, with obtuse apex. Clamp connections few in all tissues.
Habitat: Scattered or gregarious on the ground in forests dominated by fagaceous trees.
Known distribution: Central and eastern China (Hunan and Zhejiang Provinces).
Additional specimens examined: China. Zhejiang Province: Hangzhou city, Tianmushan Nature Reserve, elev. 1,100 m, 22 July 2020, W. F. Lin4-1, W. F. Lin4-2 (FHMU6951 and FHMU6956). Hunan Province: Sangzhi county, Badagong Mountain, Tianping Mountain, elev. 1,456 m, 31 July 2020, Z. H. Chen MHHNU32011 (FHMU6953); same location, 31 July 2020, Z. H. Chen MHHNU32014, Z. H. Chen MHHNU32061, Z. H. Chen MHHNU32009 (FHMU6952, FHMU6954, and FHMU6950).
Notes: Phylogenetically speaking, C. laevigatus is closely related to C. magnus, also a species of subgen. Magni. Although the value of phylogenetic distance between the two taxa is not high (Figure 1), which was also observed between C. cibarius and C. roseocanus (Redhead, Norvell & Danell) Redhead, Norvell & Moncalvo (Foltz et al., 2013), C. magnus featured by a larger basidioma (pileus up to 20 cm in diameter) and larger basidiospores measuring (8.5–)9–11(−11.5) × (6.5–)6.8–7.5(−8.0) μm (Cao et al., 2021) is morphologically different from C. laevigatus. Thus, we proposed the new taxon “C. laevigatus.”
In China, C. laevigatus was misidentified as C. hainanensis N.K. Zeng, Zhi Q. Liang & S. Jiang (Chen and Zhang, 2019), a species also has a smooth hymenophore (An et al., 2017). However, the latter is a member of subgen. Cantharellus. Cantharellus cibarioides (Heinem.) Buyck, C. eccentricus Buyck, V. Hofst. & Eyssart., C. flavolateritius Buyck & V. Hofst., C. incrassatus Buyck & V. Hofst., C. lateritius (Berk.) Singer, C. laevihymeninus T. Cao & H.S. Yuan, C. sebosus Buyck, Randrianj. & V. Hofst., C. sublaevis Buyck & Eyssart., and C. vaginatus S.C. Shao, X.F. Tian & P.G. Liu also have smooth hymenophores (Shao et al., 2011; Buyck, 2014; Buyck et al., 2016c; Cao et al., 2021). However, C. cibarioides, C. sublaevis, and C. sebosus belong to subgen. Rubrini Eyssart. & Buyck (Buyck, 2014; Buyck et al., 2014); C. eccentricus, C. flavolateritius, C. incrassatus, C. laevihymeninus, C. lateritius, and C. vaginatus also are members of subgen. Cantharellus (Buyck, 2014; Buyck et al., 2014). In addition, C. neocaledoniensis Buyck, V. Hofst., Eyssart. & Ducousso and C. solidus De Kesel, Yorou & Buyck, two species waiting to be defined in the subgeneric ranking, are characterized by smooth hymenophores either (Buyck, 2014; Buyck et al., 2014). However, C. neocaledoniensis has narrower basidiospores measuring (6.2–)6.6–7.33–8(−8.5) × (4.2–)4.5–4.96–5.4(−6) μm and abundant clamp connections in all tissues. It is distributed in New Caledonia, associating with Melaleuca L./Acacia Mill (Buyck, 2014); C. solidus has larger basidiospores measuring (8.3–)8.4–10.2–12(−12.5) × (6.3–)6.6–8.1–9.5(−9.6) μm, two-spored basidia, and it grows under the West African humid gallery forest (De Kesel, 2011; Buyck, 2014).
Discussion
In the present study, five phylogenetic species of Cantharellus were recognized (Figure 1), three lineages were described as new species: C. bellus, C. cineraceus, and C. laevigatus, one was previously described taxon: C. hygrophoroides, and the remaining one was not defined due to the paucity of the materials. Cantharellus bellus and C. laevigatus are both members of subgen. Magni, whereas C. cineraceus and C. hygrophoroides belong to subgen. Afrocantharellus. As mentioned earlier, subgen. Afrocantharellus has been divided into two sections (Buyck et al., 2014). Our molecular data indicated C. cineraceus and C. hygrophoroides are members of sect. Cutirellus (Figure 1).
In addition to the four described taxa of subgenera Afrocantharellus and Magni, C. cerinoalbus and C. magnus were also described/reported in previous studies (Song et al., 2017; Cao et al., 2021). It is worth noting that the Chinese collections labeled as C. cerinoalbus and our new species C. cineraceus grouped together with high statistical support (Figure 1); moreover, judging from the descriptions of Chinese specimens identified as C. cerinoalbus (Song et al., 2017), they match well with those of C. cineraceus. Thus, we are sure the specimens identified as C. cerinoalbus from China are really C. cineraceus. Interestingly, the other two new collections (FHMU6948 and FHMU6949) also from the south of China are probably the true C. cerinoalbus for they grouped with the isotype of the Malaysian species with statistical support (Figure 1). Unfortunately, due to the paucity of the two materials, they were not studied thoroughly. Thus, the occurrence of C. cerinoalbus in China will be confirmed with more collections made and more DNA sequences obtained in future.
Earlier studies indicated the taxa of subgen. Afrocantharellus were all described from tropical areas of the world including Africa, Madagascar, and Malaysia (Corner, 1966; Buyck et al., 2014), while species of the subgenus, viz. C. hygrophoroides, was uncovered in tropical China (Shao et al., 2014). In the present study, C. cineraceus was described from subtropical China, which extends the range of distribution of subgen. Afrocantharellus.
It is noteworthy that collections identified as C. splendens appear in four parts of the tree; one of them (BB 96.306 and BB 96.199) corresponds to the true C. splendens for isotype of the taxon included in the lineage, the second (ADK 6071, JD 896, and JD968) awaits identification, the third (DDT57) seems to be C. symoensii Heinem. for the specimen and the epitype of C. symoensii group together with statistical support, and the fourth (DDT17) seems to be C. platyphyllus Heinem. for the collection clustered with the epitype of C. platyphyllus (Figure 1). Moreover, one specimen (DDT63) labeled as “C. cyanescens Buyck,” being also clustered with the epitype of C. platyphyllus, is most likely to be C. platyphyllus (Figure 1).
As already noted by previous studies, C. cuticulatus and C. splendens have trichodermal structures in the pileipellis (Corner, 1966; Buyck et al., 2014), and C. hygrophoroides were also observed to have intricate trichodermal pileipellis. In addition, the Chinese species classified in the subgen. Afrocantharellus lack bluish context, which was observed on African C. platyphyllus and its Malagasy subspecies bojeriensis (Buyck et al., 2014).
We also noted that most species of Cantharellus with smooth hymenophores belong to subgen. Cantharellus and subgen. Rubrinus, respectively (Shao et al., 2011; Buyck, 2014; Buyck et al., 2016c; An et al., 2017). The recently erected subgen. Magni was also reported to have a smooth hymenophore (Cao et al., 2021), and our new species C. laevigatus is the second species of the subgenus uncovered with a smooth hymenophore. Interestingly, a well-developed hymenophore was observed from our new species C. bellus, also a member of subgen. Magni, which indicates the diagnostic features of subgen. Magni should be revised.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material. The data presented in the study are deposited in the Genbank and MycoBank repository, Genbank accession number: ON089297–ON089298, ON102890–ON102902, ON117818–ON117821, ON117823–ON117825 (28S); ON191964–ON191968, ON202824, ON237706–ON237708, OP251152–OP251153, ON340611–ON340616, ON340619 (TEF1); MycoBank accession number: MB845017–MB845019.
Author contributions
Z-QL and N-KZ contributed to the conceptualization. Y-ZZ performed the methodology and conducted the formal analysis. The original draft preparation was written by Y-ZZ and H-ZQ. The experiment was performed by Y-ZZ. N-KZ, Z-HC, and W-FL. SJ carried out the resources. N-KZ and Z-QL wrote, reviewed, and edited the manuscript and directed the data. N-KZ was responsible for project management and funding access. All authors contributed to the article and approved the submitted version.
Funding
This study, including funds for open access publication fees, was supported by the National Natural Science Foundation of China (No. 32160001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
An, D. Y., Liang, Z. Q., Jiang, S., Su, M. S., and Zeng, N. K. (2017). Cantharellus hainanensis, a new species with a smooth hymenophore from tropical China. Mycoscience 58, 438–444. doi: 10.1016/j.myc.2017.06.004
Antonín, V., Hofstetter, V., Ryoo, R., Ka, K. H., and Buyck, B. (2017). New Cantharellus species from the Republic of Korea. Mycol. Prog. 16, 753–759. doi: 10.1007/s11557-017-1312-2
Bas, C. (1969). Morphology and subdivision of amanita and a monograph of its section Lepidella. Persoonia 5, 285–573.
Buyck, B. (2012). One neo-and four epitypifications for Cantharellus species from tropical African savannah woodlands. Cryptogam. Mycol. 33, 11–17. doi: 10.7872/crym.v33.iss1.2012.011
Buyck, B. (2014). Exploring the diversity of “smooth chanterelles” (Cantharellus, Cantharellales). Cryptogam. Mycol. 35, 23–40. doi: 10.7872/crym.v35.iss1.2014.23
Buyck, B., Cruaud, C., Couloux, A., and Hofstetter, V. (2011). Cantharellus texensis sp. nov. from Texas, a southern lookalike of C. cinnabarinus revealed by tef-1 sequence data. Mycologia 103, 1037–1046. doi: 10.3852/10-261
Buyck, B., Henkel, T. W., Dentinger, B. T. M., Séné, O., and Hofstetter, V. (2016a). Multigene sequencing provides a suitable epitype, barcode sequences and a precise systematic position for the enigmatic, African Cantharellus miniatescens. Cryptogam. Mycol. 37, 269–282. doi: 10.7872/crym/v37.iss3.2016.269
Buyck, B., Hofstetter, V., Ryoo, R., Ka, K. H., and Antonín, V. (2020a). New Cantharellus species from South Korea. MycoKeys 76, 31–47. doi: 10.3897/mycokeys.76.58179
Buyck, B., Kauff, F., Cruaud, C., and Hofstetter, V. (2013). Molecular evidence for novel Cantharellus (Cantharellales, Basidiomycota) from tropical African miombo woodland and a key to all tropical African chanterelles. Fungal Divers. 58, 281–298. doi: 10.1007/s13225-012-0215-4
Buyck, B., Kauff, F., Eyssartier, G., Couloux, A., and Hofstetter, V. (2014). A multilocus phylogeny for worldwide Cantharellus (Cantharellales, Agaricomycetidae). Fungal Divers. 64, 101–121. doi: 10.1007/s13225-013-0272-3
Buyck, B., Kauff, F., Randrianjohany, E., and Hofstetter, V. (2015). Sequence data reveal a high diversity of Cantharellus associated with endemic vegetation in Madagascar. Fungal Divers. 70, 189–208. doi: 10.1007/s13225-014-0314-5
Buyck, B., Moreau, P. A., Courtecuisse, R., Kong, A., Roy, M., and Hofstetter, V. (2016b). Cantharellus coccolobae sp. nov. and Cantharellus garnieri two tropical members of Cantharellus subg. Cinnabarinus. Cryptogam. Mycol. 37, 391–403. doi: 10.7872/crym/v37.iss3.2016.391
Buyck, B., Ndolo Ebika, S. T., De Kesel, A., and Hofstetter, V. (2020b). Tropical African Cantharellus Adans.: Fr. (Hydnaceae, Cantharellales) with lilac-purplish tinges revisited. Cryptogam. Mycol. 41, 161–177. doi: 10.5252/cryptogamie-mycologie2020v41a10
Buyck, B., Olariaga, I., Justice, J., Lewis, D., Roody, W., and Hofstetter, V. (2016c). The dilemma of species recognition in the field when sequence data are not in phase with phenotypic variability. Cryptogam. Mycol. 37, 367–389. doi: 10.7872/crym/v37.iss3.2016.367
Cao, T., Hu, Y. P., Yu, J. R., Wei, T. Z., and Yuan, H. S. (2021). A phylogenetic overview of the Hydnaceae (Cantharellales, Basidiomycota) with new taxa from China. Stud. Mycol. 99, 100–121. doi: 10.1016/j.simyco.2021.100121
Darriba, D., Taboada, G. L., Doallo, R., and Posada, D. (2012). jModelTest2: more models, new heuristics and parallel computing. Nat. Methods 9:772. doi: 10.1038/nmeth.2109
De Kesel, A. (2011). Cantharellus solidus, a new species from Benin (West-Africa) with a smooth hymenium. Cryptogam. Mycol. 32, 277–283. doi: 10.7872/crym.v32.iss3.2011.277
Dunham, S. M., O'dell, T. E., and Molina, R. (2003). Analysis of nrDNA sequences and microsatellite allele frequencies reveals a cryptic chanterelle species Cantharellus cascadensis sp. nov. from the American Pacific northwest. Mycol. Res. 107, 1163–1177. doi: 10.1017/S0953756203008475
Edgar, R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797. doi: 10.1093/nar/gkh340
Eyssartier, G., and Buyck, B. (2001). Note nomenclaturale et systématique sur le genre Cantharellus. Doc. Mycol. 31, 55–56.
Eyssartier, G., Stubbe, D., Walleyn, R., and Verbeken, A. (2009). New records of Cantharellus species (Basidiomycota, Cantharellaceae) from Malaysian dipterocarp rainforest. Fungal Divers. 26, 349–353. doi: 10.1002/yea.1667
Foltz, M. J., Perez, K. E., and Volk, T. J. (2013). Molecular phylogeny and morphology reveal three new species of Cantharellus within 20 m of one another in western Wisconsin, USA. Mycologia 105, 447–461. doi: 10.3852/12-181
Hall, T. A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analyses program for windows 95/98/NT. Nucleic Acids Symp. Ser. 9, 77–96. doi: 10.1016/S1468-1641(10)60416-1
Huelsenbeck, J. P., and Ronquist, F. (2005). “Bayesian analysis of molecular evolution using MrBayes” in Statistical methods in molecular evolution. ed. R. Nielsen (New York, NY: Springer), 183–226.
James, T. Y., Kauff, F., Schoch, C., Matheny, P. B., Hofstetter, V., Cox, C., et al. (2006). Reconstructing the early evolution of the fungi using a six gene phylogeny. Nature 443, 818–822. doi: 10.1038/nature05110
Jian, S. P., Dai, R., Gao, J. U. N., and Feng, B. (2020). Cantharellus albus, a striking new species from Southwest China. Phytotaxa 470, 133–144. doi: 10.11646/phytotaxa.470.2.2
Kesel, A. D., Amalfi, M., Ngoy, B. K. W., Yorou, N. S., Raspé, O., Degreef, J., et al. (2016). New and interesting Cantharellus from tropical Africa. Cryptogam. Mycol. 37, 283–327. doi: 10.7872/crym/v37.iss3.2016.283
Kornerup, A., and Wanscher, J. H. (1981). Taschenlexikon der Farben, 3. Göttingen: Muster-Schmidt Verlag.
Liu, J. K., Hyde, K. D., Jones, E. B., Ariyawansa, H. A., Bhat, D. J., Boonmee, S., et al. (2015). Fungal diversity notes 1–110: taxonomic and phylogenetic contributions to fungal species. Fungal Divers. 72, 1–197. doi: 10.1007/s13225-015-0324-y
Mikheyev, A. S., Mueller, U. G., and Abbot, P. (2006). Cryptic sex and many-to-one coevolution in the fungus-growing ant symbiosis. Proc. Natl. Acad. Sci. U. S. A. 103, 10702–10706. doi: 10.1073/pnas.0601441103
Miller, M. A., Pfeiffer, W., and Schwartz, T. (2011). “The CIPRES science gateway: a community resource for phylogenetic analyses,” in Proceedings of the 2011 TeraGrid Conference: extreme Digital Discovery, (New York, NY: ACM), 41.
Olariaga, I., Moreno, G., Manjón, J. L., Salcedo, I., Hofstetter, V., Rodríguez, D., et al. (2017). Cantharellus (Cantharellales, Basidiomycota) revisited in Europe through a multigene phylogeny. Fungal Divers. 83, 263–292. doi: 10.1007/s13225-016-0376-7
Pilz, D., Norvell, L., Danell, E., and Molina, R. (2003). Ecology and management of commercially harvested chanterelle mushrooms. Washington: US Department of Agriculture Pacific Northwest Research Station.
Shao, S. C., Buyck, B., Hofstetter, V., Tian, X. F., Geng, Y. H., Yu, F. Q., et al. (2014). Cantharellus hygrophorus, a new species in subgenus Afrocantharellus from tropical southwestern China. Cryptogam. Mycol. 35, 283–291. doi: 10.7872/crym.v35.iss3.2014.283
Shao, S. C., Buyck, B., Tian, X. F., Liu, P. G., and Geng, Y. H. (2016a). Cantharellus phloginus, a new pink-colored species from southwestern China. Mycoscience 57, 144–149. doi: 10.1016/j.myc.2015.12.004
Shao, S. C., Liu, P. G., Tian, X. F., Buyck, B., and Geng, Y. H. (2016b). A new species of Cantharellus (Cantharellales, Basidiomycota, fungi) from subalpine forest in Yunnan, China. Phytotaxa 252, 273–279. doi: 10.11646/phytotaxa.252.4.3
Shao, S. C., Tian, X. F., and Liu, P. G. (2011). Cantharellus in southwestern China: a new species and a new record. Mycotaxon 116, 437–446. doi: 10.5248/116.437
Smith, S. A., and Dunn, C. W. (2008). Phyutility: a phyloinformatics tool for trees, alignments andmolecular data. Bioinformatics 24, 715–716. doi: 10.1093/bioinformatics/btm619
Song, Z. P., Zhang, M., and Li, T. H. (2017). Cantharellus cerinoalbus—an edible fungus newly recorded in China. Acta Edulis Fungi 24, 98–103. doi: 10.16488/j.cnki.1005—9873.2017.01.018
Stamatakis, A. (2006). RAxML-VI-HPC: maximum likelihoodbased phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690. doi: 10.1093/bioinformatics/btl446
Tamura, K., Stecher, G., and Kumar, S. (2021). MEGA11: molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 38, 3022–3027. doi: 10.1093/molbev/msab120
Tibuhwa, D. D., Saviae, S., Tibell, L., and Kivaisi, A. K. (2012). Afrocantharellus gen. stat. nov. is part of a rich diversity of African Cantharellaceae. IMA Fungus 3, 25–38. doi: 10.5598/imafungus.2012.03.01.04
Vadthanarat, S., Halling, R. E., Amalfi, M., Lumyong, S., and Raspé, O. (2021). An unexpectedly high number of new Sutorius (Boletaceae) species from northern and northeastern Thailand. Front. Microbiol. 12:643505. doi: 10.3389/fmicb.2021.643505
Vilgalys, R., and Hester, M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 172, 4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990
Yun, W., and Hall, I. R. (2004). Edible ectomycorrhizal mushrooms: challenges and achievements. Can. J. Bot. 82, 1063–1073. doi: 10.1139/b04-051
Zeng, N. K., and Jiang, S. (2020). Atlas of macrofungi from Yinggeling of Hainan, China. Hainan: Nanhai Publishing House.
Zhang, Y. Z., Liang, Z. Q., Xie, H. J., Wu, L. L., Xue, R., and Zeng, N. K. (2021). Cantharellus macrocarpus (Cantharellaceae, Cantharellales), a new species from tropical China. Phytotaxa 484, 170–180. doi: 10.11646/phytotaxa.484.2.2
Zhang, Y. Z., Lin, W. F., Buyck, B., Liang, Z. Q., Su, M. S., Chen, Z. H., et al. (2022). Morphological and phylogenetic evidences reveal four new species of Cantharellus subgenus Cantharellus (Hydnaceae, Cantharellales) from China. Front. Microbiol. 13:900329. doi: 10.3389/fmicb.2022.900329
Zhang, M., Wang, C. Q., Buyck, B., Deng, W. Q., and Li, T. H. (2021). Multigene phylogeny and morphology reveal unexpectedly high number of new species of Cantharellus subgenus Parvocantharellus (Hydnaceae, Cantharellales) in China. J. Fungi 7:919. doi: 10.3390/jof7110919
Keywords: chanterelle, molecular phylogeny, morphology, new taxa, taxonomy
Citation: Zhang Y-Z, Qin H-Z, Chen Z-H, Lin W-F, Liang Z-Q, Jiang S and Zeng N-K (2023) Updated taxonomy of Chinese Cantharellus subgenera Afrocantharellus and Magni (Hydnaceae, Cantharellales): Three new taxa and amended descriptions of one previous species. Front. Microbiol. 14:1109831. doi: 10.3389/fmicb.2023.1109831
Edited by:
Xiang-Yu Zeng, Guizhou University, ChinaReviewed by:
Junfeng Liang, Chinese Academy of Forestry, ChinaMing Zhang, Guangdong Academy of Science, China
Valérie Hofstetter, Agroscope, Switzerland
Copyright © 2023 Zhang, Qin, Chen, Lin, Liang, Jiang and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi-Qun Liang, bGl6aHF1MTk4MEAxMjYuY29t; Nian-Kai Zeng, bmlhbmthaXpAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Yu-Zhuo Zhang
Yu-Zhuo Zhang Hua-Zhi Qin
Hua-Zhi Qin Zuo-Hong Chen3
Zuo-Hong Chen3 Shuai Jiang
Shuai Jiang Nian-Kai Zeng
Nian-Kai Zeng