
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 08 September 2022
Sec. Microbe and Virus Interactions with Plants
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.1006140
This article is part of the Research Topic Crop-Microorganisms Interactions: Diseases and Symbioses View all 9 articles
 Rui-Ting Sun1
Rui-Ting Sun1 Ze-Zhi Zhang2
Ze-Zhi Zhang2 Ming-Yang Liu1
Ming-Yang Liu1 Xiang-Cao Feng1
Xiang-Cao Feng1 Nong Zhou3
Nong Zhou3 Hai-Dong Feng2
Hai-Dong Feng2 Abeer Hashem4
Abeer Hashem4 Elsayed Fathi Abd_Allah5
Elsayed Fathi Abd_Allah5 Wiwiek Harsonowati6
Wiwiek Harsonowati6 Qiang-Sheng Wu1*
Qiang-Sheng Wu1*The medicinal plant Polygonum cuspidatum Sieb. Et Zucc is rich in stilbenes (e.g., polygonin and resveratrol) and anthraquinones (e.g., emodin) for the therapy of human diseases, while how to increase the growth and medicinal composition concentrations of P. cuspidatum has become an urgent issue. The aim of the present study was to evaluate the effects of inoculation with an arbuscular mycorrhizal (AM) fungus, Funneliformis mosseae, on plant growth, phosphorus (P) acquisition, medicinal component concentrations, and expressions of resveratrol synthesis-associated enzyme genes of P. cuspidatum at two P levels (0 M and 0.2 M). P supply (0.2 M) stimulated root AM fungal colonization rate. F. mosseae inoculation significantly improved growth performance (height, diameter, and biomass) and root morphology (diameter, length, and projected area), irrespectively of substrate P levels. P supply and F. mosseae distinctly increased soil acid and neutral phosphatase activities, as well as root P concentrations. P supply increased root physcion and resveratrol concentrations in inoculated and uninoculated plants, along with up-regulated expressions of PcCHS1, PcCRS1, PcRS11, and PcSTS. AM plants represented significantly higher root aloe-emodin, chrysophanol, emodin, physcion, polydatin, and resveratrol concentrations than non-AM plants irrespective of P levels, coupled with up-regulated expressions of PcCHS1, PcCHS2, PcRS11, PcRS, and PcSTS. It is concluded that 0.2 M P supply and F. mosseae inoculation promoted chrysophanol, physcion, polydatin, and resveratrol concentrations of P. cuspidatum, with the increase in resveratrol associated with up-regulated expressions of related genes.
Polygonum cuspidatum Sieb. Et Zucc is a medicinal plant, whose active ingredients contain stilbene compounds (e.g., resveratrol) and anthraquinones (e.g., emodin) (Kong et al., 2020). In East Asia, P. cuspidatum is widely used in the therapy of hepatitis, cough, jaundice, and other diseases (Zhang et al., 2013). Resveratrol, emodin, physcion, and chrysophanol of P. cuspidatum have been used as monomeric ingredients in pharmaceutical, chemical, and food applications, and thus P. cuspidatum becomes the raw material for these monomeric ingredients (Sun, 2007). Among them, resveratrol can not only improve the disease resistance of plants, but also have antioxidant and anti-tumor effects, along with great demand market (Wu et al., 2019); emodin has the functions of lowering blood pressure, protecting liver and anti-tumor, and immunomodulatory effects (Wu et al., 2022). Of all reported plant species, P. cuspidatum has much greater resveratrol concentrations than other plants (Wu et al., 2019).
Arbuscular mycorrhizal (AM) fungi are beneficial fungi in soil that colonize the roots of approximately 72% of terrestrial plants, thus establishing a reciprocal symbiosis (Genre et al., 2020). AM fungi affect primary metabolic processes of plants and also change secondary metabolites in various medicinal plants (Zeng et al., 2014; Sun et al., 2021). In Glycyrrhiza uralensis plants, AM fungi distinctly raised concentrations of glycyrrhizic acid, and the increase became greater with the prolongation of inoculated time (Liu et al., 2007). In Angelica dahurica plants, AM fungi also dramatically induced the increase in total coumarin and imperatorin concentrations (Zhao and He, 2011). However, in Ocimum basilicum plants, Glomus intraradices triggered an increase in anthocyanin levels, but it did not affect concentrations of polyphenolic substances (Lee and Scagel, 2009). These results imply the potential of AM fungi in increasing specific medicinal ingredients in certain medicinal plants.
Substrate phosphorus (P) levels have an impact on the colonization response of AM fungi (Campos et al., 2018; Cao et al., 2021). High levels of substrate P inhibit AM fungal colonization (Breuillin et al., 2010), and low levels of P and AM fungi have improved effects on the accumulation of secondary metabolites in plants (Zeng et al., 2014). Inoculation with G. mosseae promoted artemisinin content in the medicinal plant Artemisia annua under low P conditions (40 mg/kg), while high P (120 mg/kg) significantly decreased artemisinin content by 42.5% (Tan et al., 2013). P fertilizer supply could achieve similar results to AM fungal inoculation in promoting the content of active ingredients in medicinal plants, such as castanospermine levels in Castanospermum austral plants and essential oils in Foeniculum vulgare plants (Kapoor et al., 2004; Bastami and Majidian, 2016). In Anadenanthera colubrina seedlings, AM fungi had an increased effect on the content of total phenols, total flavonoids, and total tannins in leaves, even under high P supply (30 and 50 mg/dm3) (Pedone-Bonfim et al., 2013). Interestingly, G. mosseae inoculation, but not P fertilization application, accelerated the production of essential oils in oregano plants (Khaosaad et al., 2006), suggesting different potential mechanisms for P fertilization and AM fungi to affect secondary metabolism.
Polygonum cuspidatum is mostly planted in mountainous areas, where the soil is poor, especially with low levels of P (Yu, 2016). An AM fungus, Funneliformis mosseae, could establish mycorrhizal symbionts in roots of P. cuspidatum and improve plant growth and root development, coupled with the increase in root medicinal components (Sun et al., 2022a). However, it is not clear whether the AM fungus promotes these medicinal components of P. cuspidatum under different P levels and what the underlying molecular mechanisms are involved in the promotion of medicinal components. The purpose of the present study was to assess the effects of F. mosseae on plant growth, P acquisition, soil phosphatase activity, concentrations of medicinal ingredients, and relative expressions of related genes in potted P. cuspidatum under low and appropriate P conditions.
Seeds of P. cuspidatum (identification: WUK 0310891)1 (provided by the Shiyan Academy of Agricultural Sciences) were disinfected in 75% alcohol solutions for 8 min and sown in 2.4 l plastic pots supplied with 4 mm sieved autoclaved (0.11 Mpa, 2 h) soil. After 4 weeks, seedlings with four leaves were selected for transplanting and were also inoculated with an AM fungus at the same time. The AM fungal strain used was F. mosseae (BGC XZ02A), whose origin and propagation were reported by Sun et al. (2022a). The plastic pot for transplanting had the size of 21 cm × 18 cm × 12 cm, together with 2050 g of pre-acid-eluted autoclaved river (Φ < 4 mm) sand. AM fungal inoculation received 150 g of AM fungal inoculum (2,850 spores), while non-inoculation with AM fungus also supplied 150 g of autoclaved mycorrhizal inoculum plus 2 ml 30 μm filtrates of the inoculum, aiming to maintain consistent microbes except for this AM fungus. After transplanting, the treated seedlings were allowed to grow indoors for 4 days and then transferred to a controlled greenhouse for growth, whose environmental conditions have been described in detail by Sun et al. (2022a).
P treatments were carried out after 3 weeks. P levels of 0.2 M (pH 6.5) and 0 M were selected by varying the level of P in the Hoagland nutrient solution (Hoagland, 1950) with 70 ml/pot. P supply was performed at 2-day intervals, and a total of 17 supplies of P were made. This experiment was conducted in a completely randomized block design with inoculation and non-inoculation of F. mosseae and two P levels, including low (0 M; P0) and appropriate (0.2 M; P0.2) P treatments (Song et al., 2006). As a result, the experiment consisted of four treatments, each of which was replicated eight times, with a total of 32 pots. The experiment ended after 12 weeks.
Plant growth traits (height, diameter, and leaf number) were determined directly before harvesting. After harvesting, plants were split into shoots and roots and then weighed individually. The complete root system was scanned using a scanner (J221A, EPSON, Jakarta Selatan, Indonesia), and the root images obtained were used to analyze root morphological parameters as per a WinRHIZO software (Regent Instruments Inc., Quebec, Canada), including length, area, volume, and diameter.
After roots were scanned, fresh root segments were cleared by 10% potassium hydroxide solution at 95°C for 2 h, bleached with 30% hydrogen peroxide solution for 15 min, and stained with 0.05% trypan blue in lactic acid for 30 s for microscopic observation (Phillips and Hayman, 1970). The estimation of the AM fungal colonization rate was performed using the protocol outlined by Zou et al. (2021).
The content of P in roots was determined using the colorimetric method outlined by Cavell (1955), after dried root samples were digested with H2SO4-H2O2. Soil phosphatase activity was assayed using the procedure outlined by Wu et al. (2015a), in which acid, neutral, and alkaline phosphatases were extracted with acetate buffer (pH 5.0), citrate–phosphate buffer (pH 7.0), and borate buffer (pH 10.0), respectively.
The extraction of root medicinal ingredients (aloe-emodin, chrysophanol, emodin, physcion, polydatin, and resveratrol) was performed by sonicating 10 ml of 80% methanol with 0.20 of dried root samples (Φ < 4 mm) for 30 min, followed by centrifugation at 4000 ×g for 10 min. The supernatant was filtered through a 0.22 μm filter membrane and assayed by high-performance liquid chromatography (HPLC) (LC-20A, Shimadzu, Japan). The chromatographic condition was performed according to the protocol reported by Sun et al. (2022a).
Total RNA in roots was extracted using a Quick RNA Isolation Kit (Huayueyang), referring to the user manual. The RNA was reversely transcribed into cDNA using the TRUE 1st Strand cDNA Synthesis Kit with gDNA Eraser kit (Aidlab). The six genes associated with the synthesis of medicinal ingredients were screened in the NCBI database, and then Primer Premier 5.0 was used to design primer sequences (Supplementary Table S1) for selected genes. qRT-PCR was conducted according to the protocol of Sun et al. (2022b). Each gene had three biological replicates, and each biological replicate contained three technical replicates. The relative expression of genes was calculated by the 2-ΔΔCt method (Livak and Schmittgen, 2001), normalized to the treatment with non-AM fungal colonization under P0 levels.
Before data analysis, homogeneity of variance was performed according to Levine’s test. Subsequently, two-way analysis of variance was performed on the experimental data using the SAS® software, and Duncan’s Multiple Range test was performed to compare significant differences (p < 0.05). Figures were made using SigmaPlot software and Adobe Photoshop CS6.
AM fungal colonization was found in F. mosseae-inoculated roots of P. cuspidatum (Figure 1B), regardless of substrate P levels, accompanied by 62.5–73.2% of root AM fungal colonization rate. Among them, P supply significantly increased root AM fungal colonization rate by 17.1% (Figure 1C). No significant interaction between AM fungal inoculation and P supply was observed in root AM fungal colonization (Table 1).
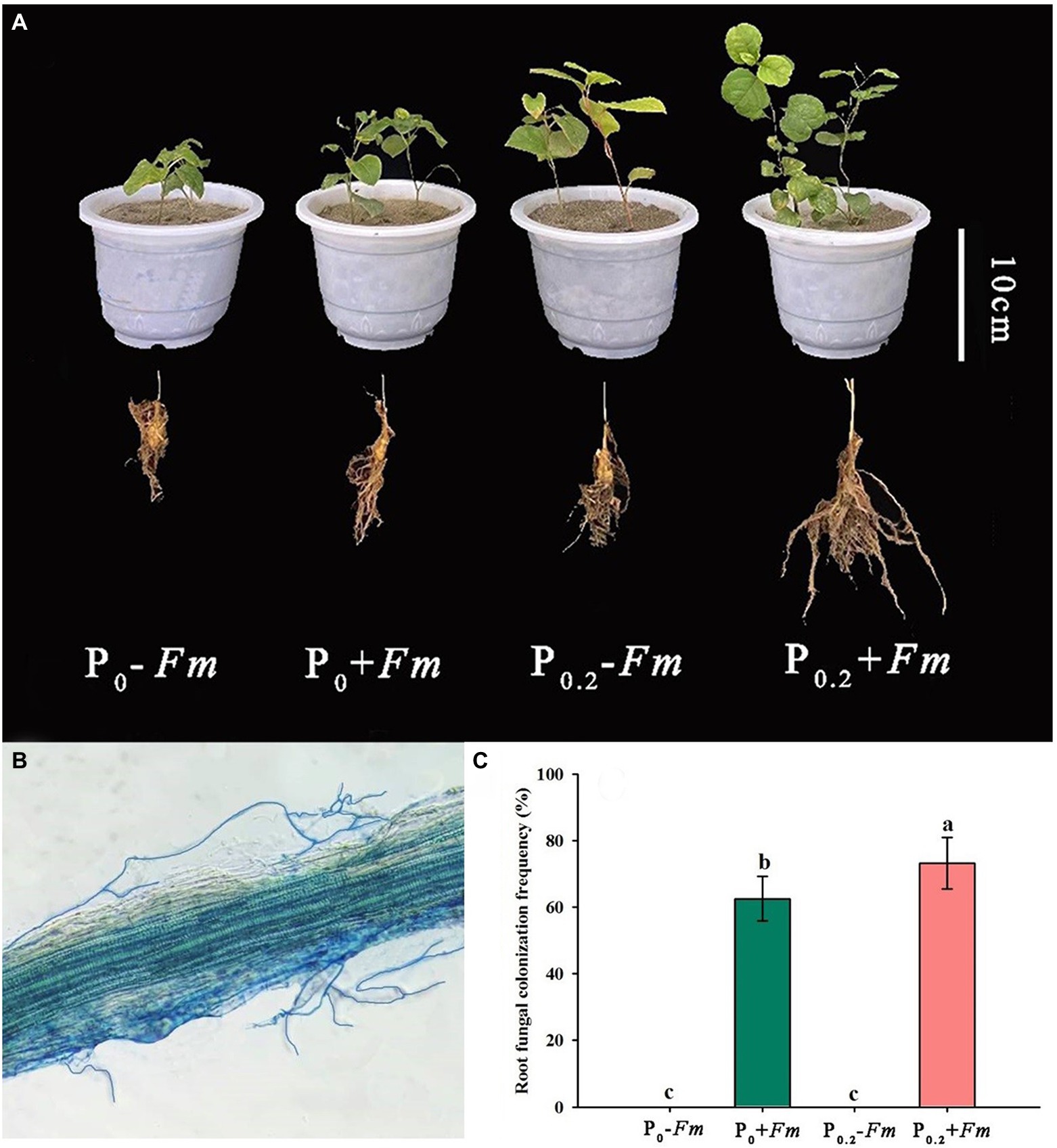
Figure 1. Plant growth responses (A), root fungal colonization (B), and changes in root fungal colonization rate (C) of Polygonum cuspidatum to Funneliformis mosseae and P supply. Data (means ± SD, n = 4)followed by different letters above the bars indicate significant (p < 0.05) differences among treatments. +Fm, inoculation with F. mosseae; −Fm, inoculation without F. mosseae; P0, 0 M P; P0.2, 0.2 M P; P, phosphorus.
P supply and AM fungal inoculation significantly improved plant growth performance (Figure 1A; Table 2). Under P0 conditions, F. mosseae significantly increased height, stem diameter, number of leaves, shoot biomass, and root biomass by 18.5, 17.0, 31.8, 42.6, and 11.6%, respectively; under P0.2 conditions, F. mosseae significantly increased height, stem diameter, shoot biomass and root biomass by 18.8, 24.2, 45.7 and 93.8%, respectively (Table 2). A significant interaction was observed in root biomass. The combination of P supply and F. mosseae inoculation represented greater plant growth traits than other treatments.
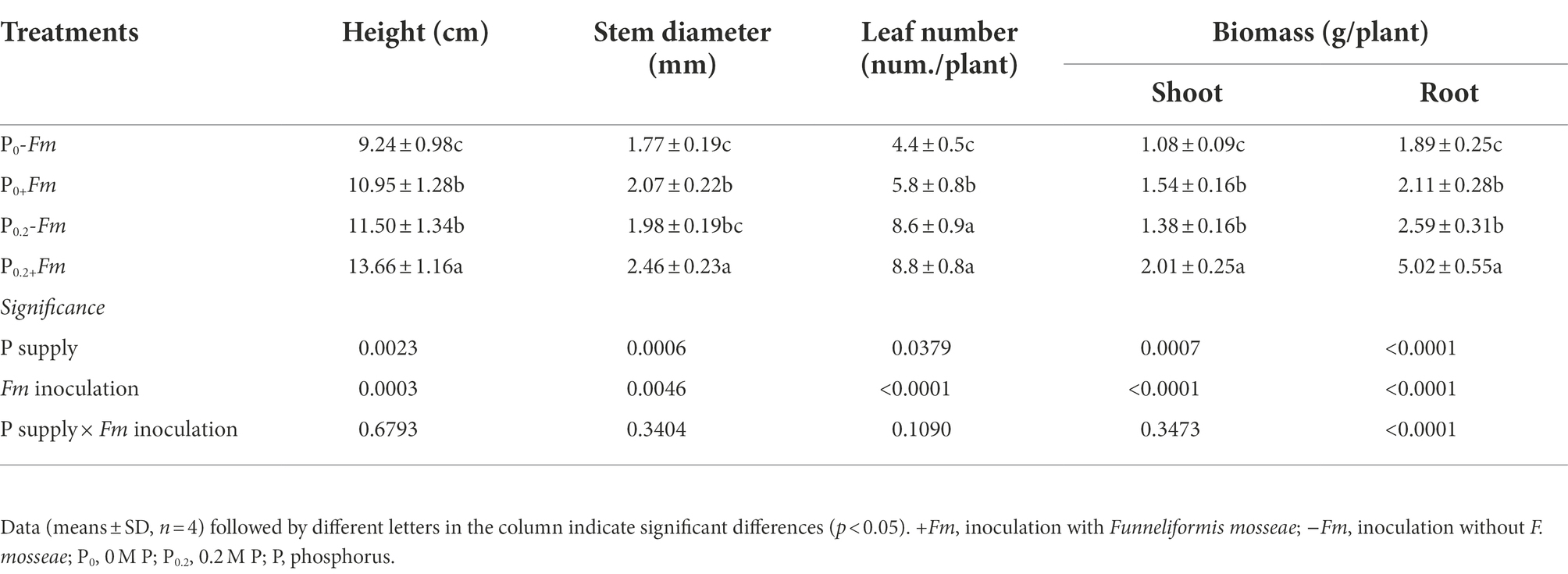
Table 2. Effects of Funneliformis mosseae and P supply on plant growth performance of Polygonum cuspidatum Sieb. et Zucc.
P supply and AM fungal inoculation significantly promoted root morphological architecture, and P supply into AM plants exhibited greater positive effects than non-AM plants (Table 3). F. mosseae significantly increased root average diameter, total length, and projected area by 18.1, 22.3, and 11.2% under P0 conditions and 26.4, 84.4, and 62.8% under P0.2 conditions. However, F. mosseae did not significantly improve root surface area and volume, independent of substrate P levels. There were significant interactions between AM fungal inoculation and P supply in root total length and projected area.
P supply dramatically increased root P levels, irrespective of AM fungal inoculation or not (Figure 2). Funneliformis mosseae inoculation significantly raised root P levels by 57.1 and 40.5% under P0 and P0.2 levels conditions, respectively. P supply and AM fungal inoculation, to a certain extent, improved soil acid, neutral and alkaline phosphatase activities (Figure 3). Under P0 conditions, F. mosseae significantly increased soil acid, neutral and alkaline phosphatase activities by 17.3, 38.9, and 18.2%, respectively; under P0.2 conditions, F. mosseae significantly elevated soil acid and neutral phosphatase activities by 21.1 and 26.1%, respectively. There was a significant interaction in root total length and projected area (Table 1). It indicated that dual application of P supply and F. mosseae exhibited greater increased magnitude that single application.
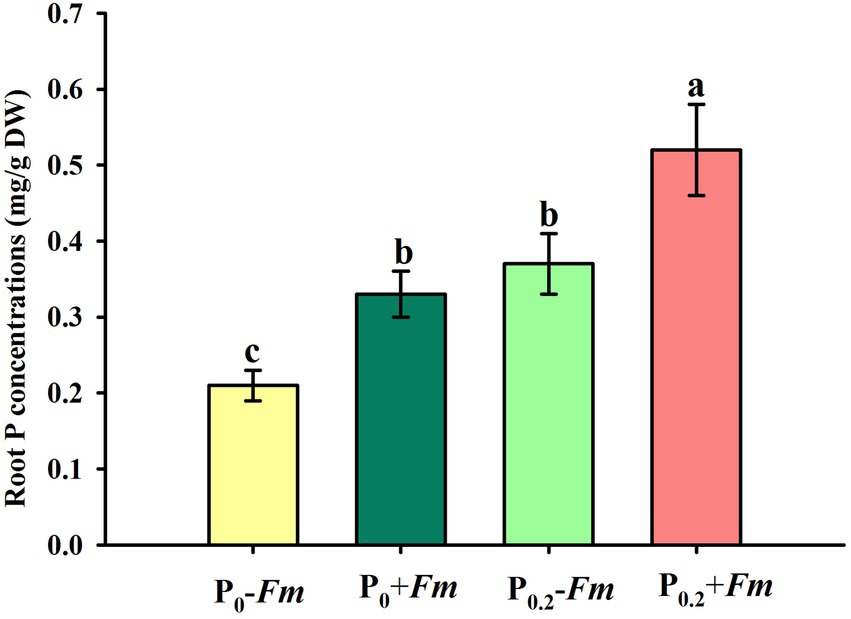
Figure 2. Changes in root P concentration of Polygonum cuspidatum by Funneliformis mosseae and P supply. Data (means ± SD, n = 4) followed by different letters above the bars indicate significant (p < 0.05) differences among treatments. +Fm, inoculation with F. mosseae; −Fm, inoculation without F. mosseae; P0, 0 M P; P0.2, 0.2 M P; P, phosphorus.
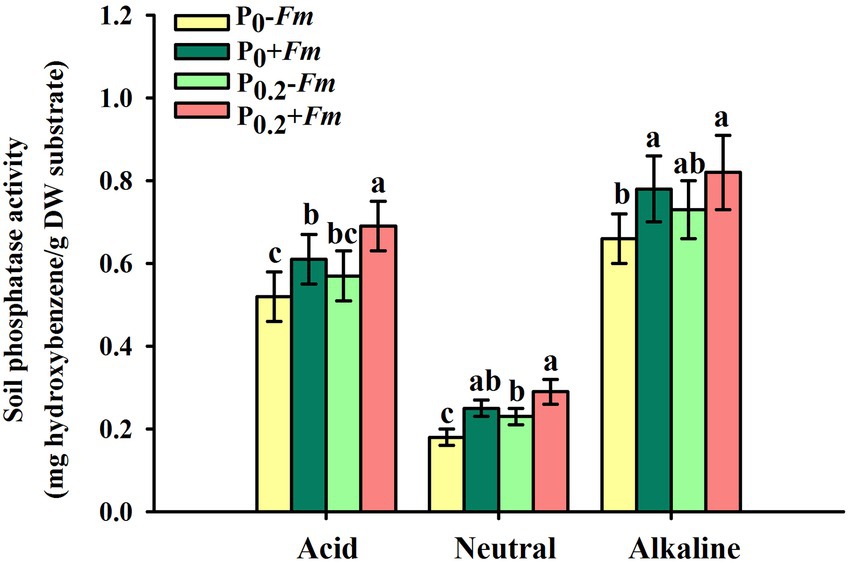
Figure 3. Changes in soil phosphatase activities of Polygonum cuspidatum by Funneliformis mosseae and P supply. Data (means ± SD, n = 4) followed by different letters above the bars indicate significant (p < 0.05) differences among treatments. +Fm, inoculation with F. mosseae; −Fm, inoculation without F. mosseae; P0, 0 M P; P0.2, 0.2 M P; P, phosphorus.
P supply dramatically increased root chrysophanol, physcion, polydatin, and resveratrol concentrations in AM plants, along with the increase in aloe-emodin, physcion, and resveratrol concentrations in non-AM plants (Figures 4A–F). Under P0 conditions, F. mosseae inoculation significantly raised root aloe-emodin, chrysophanol, emodin, physcion, polydatin, and resveratrol concentrations by 55.6, 26.5, 160.7, 622.2, 27.4, and 105.1%, respectively. Under P0.2 conditions, F. mosseae-inoculated plants exhibited greater chrysophanol, emodin, physcion, polydatin, and resveratrol concentrations by 42.6, 87.9, 252.3, 205.3, and 67.5%, respectively, compared with non-AM fungal plants. Aloe-emodin, emodin, physcion, polydatin, and resveratrol concentrations were significantly interacted by AM fungal inoculation and P supply (Table 1). It thus indicated that P supply and F. mosseae inoculation promoted chrysophanol, physcion, polydatin, and resveratrol concentrations of P. cuspidatum.
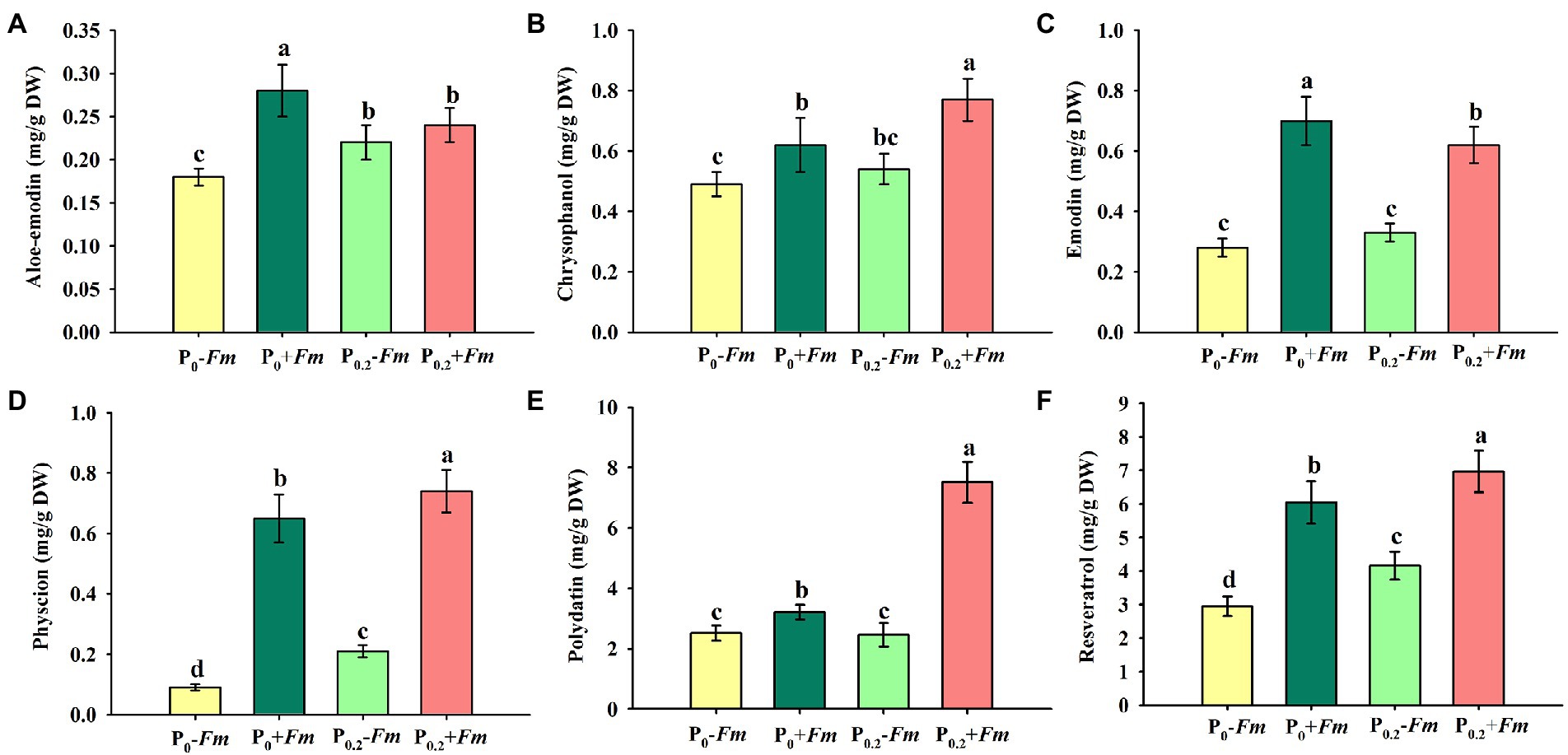
Figure 4. Changes in root aloe-emodin (A), chrysophanol (B), emodin (C), physcion (D), polydatin (E), and resveratrol (F) of Polygonum cuspidatum by Funneliformis mosseae and P supply. Data (means ± SD, n = 4) followed by different letters above the bars indicate significant (p < 0.05) differences among treatments. +Fm, inoculation with F. mosseae; −Fm, inoculation without F. mosseae; P0, 0 M P; P0.2, 0.2 M P; P, phosphorus.
P supply induced expressions of PcCHS1, PcCHS2, PcCRS1, PcRS11, and PcSTS in non-AM plants, along with a decrease in PcRS (Figures 5A–F). Similarly, in AM plants, PcCHS1, PcCRS1, PcRS11, and PcSTS were up-regulated by P supply, coupled with no change in PcCHS2 and inhibited expression in PcRS. Under P0 conditions, F. mosseae inoculation significantly raised root PcCHS1, PcCHS2, PcRS11, PcRS, and PcSTS by 2.88-, 4.26-, 1.24-, 1.64, and 0.31-fold, respectively. Under P0.2 conditions, F. mosseae-inoculated plants exhibited greater PcCHS1, PcCHS2, PcCRS1, PcRS11, PcRS, and PcSTS expressions by 0.67-, 0.71-, 1.30-, 0.25-, 1.08-, and 2.31-fold, respectively, compared with non-AM fungal plants. A significant interaction was observed in PcCHS2, PcCRS1, PcRS, and PcSTS expressions (Table 1). It indicated that resveratrol-associated gene expressions were involved in resveratrol accumulation.
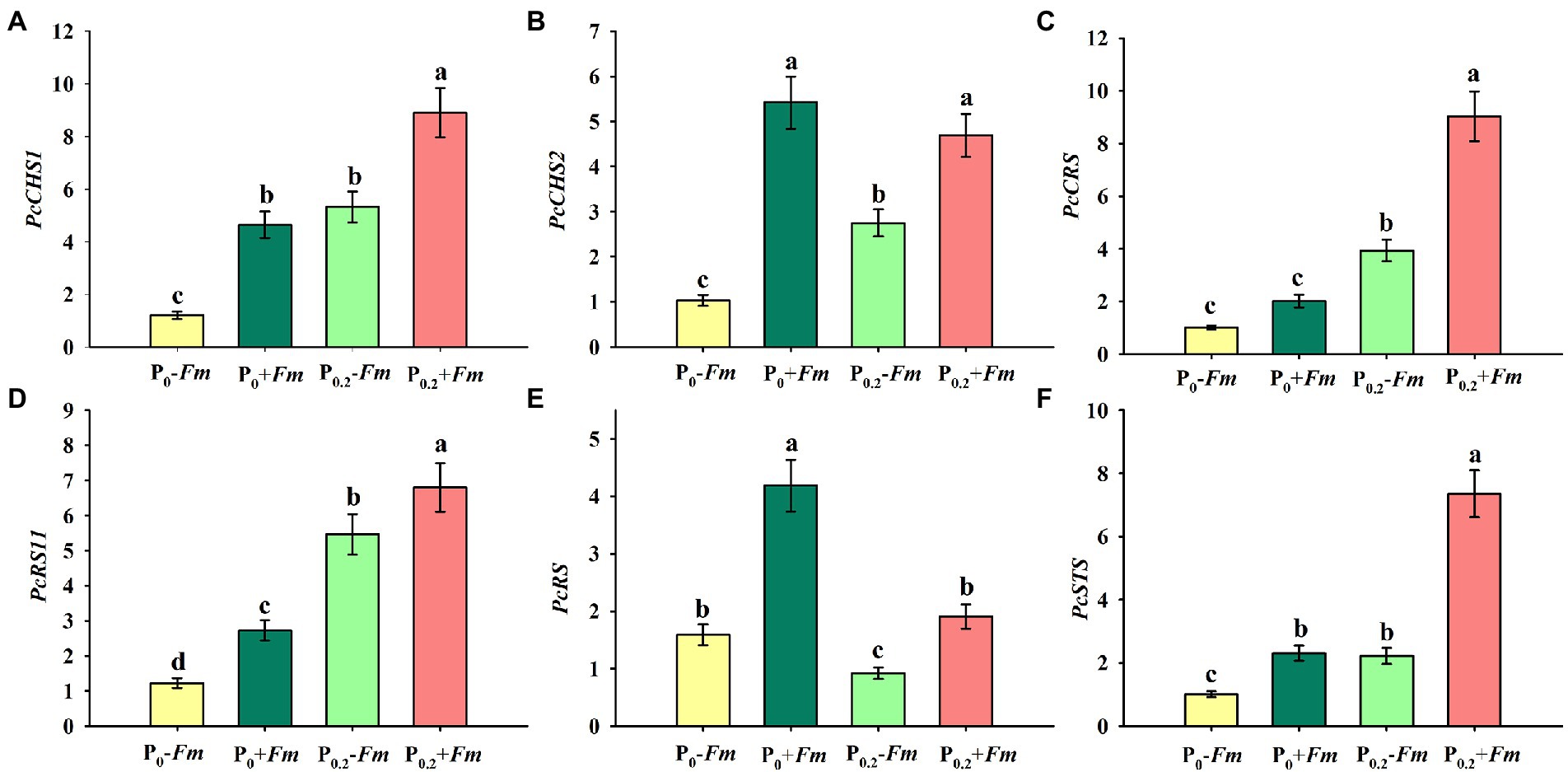
Figure 5. Changes in root PcCHS1 (A), PcCHS2 (B), PcCRS1 (C), PcRS11 (D), PcRS (E), and PcSTS (F) of Polygonum cuspidatum by Funneliformis mosseae and P supply. Data (means ± SD, n = 3) followed by different letters above the bars indicate significant (p < 0.05) differences among treatments. +Fm, inoculation with F. mosseae; −Fm, inoculation without F. mosseae; P0, 0 M P; P0.2, 0.2 M P; P, phosphorus; CHS, chalcone synthase; RS, resveratrol synthase; STS, stilbene synthase; CRS, supposed stilbene synthase; RS11, resveratrol-forming stilbene synthase 11.
Moderate P supply promoted root mycorrhizal colonization, thus promoting plant growth and root morphogenesis more prominently than single inoculation
AM fungal colonization in roots is often influenced by substrate P levels and root architecture (Wang et al., 2020). Low P levels favor root AM fungal colonization, but high P levels inhibit AM fungal colonization (Mora et al., 2008; Breuillin et al., 2010). The present study revealed that root mycorrhizal colonization rate was significantly lower at P0 than at P0.2, which is consistent with the findings of Shao et al. (2021), who reported that under P deficit conditions, P uptake by tea plants relied more on root hairs than on AM fungi, resulting in low AM fungal colonization under low P levels. Therefore, future work is needed to evaluate the response of root hairs and AM fungal colonization of P. cuspidatum to different P levels.
P supply and AM fungal inoculation collectively improved growth performance of P. cuspidatum, and the improvement was better at P0.2 than at P0. This results indicated that P is a key mineral element for vigor growth of P. cuspidatum, and P supply can promote their growth (Feng et al., 2019). On the other hand, AM symbiosis promoted the growth performance of P. cuspidatum, independent of substrate P levels, which is consistent with our earlier findings of inoculating P. cuspidatum with symbiotic fungi, including F. mosseae (Sun et al., 2022a). AM fungi also improved biomass production of Salvia miltiorrhiza and Artemisia annua plants (Huang et al., 2011; Jia et al., 2020). AM fungi can improve rhizospheric microenvironment and enhance nutrient uptake and utilization as well as water uptake by the plant after establishing a symbiosis, thus promoting plant growth (Xie et al., 2020).
Root morphology is plastic and can be influenced by various external factors (Wang et al., 2020). This study indicated that P supply distinctly promoted the establishment of root architecture, especially volume. Generally, P supply accelerates the formation and development of lateral roots to allow the root to penetrate into a larger soil volume to facilitate P acquisition (Williamson et al., 2001). Inoculation with AM fungi also dramatically improved root average diameter, total length, and projected area independent of P levels, being superior at P0.2 than at P0. Similar results were also observed in tea (Shao et al., 2021), walnut, and trifoliate orange exposed to P stress (Wu et al., 2015b; Huang et al., 2020). The improvement of root morphology triggered by AM fungi may be linked to the increase of root auxins and polyamine levels by AM fungi (Zhang et al., 2019; Zou et al., 2021).
AM fungi accelerated P acquisition of roots, associated with the release of acid and neutral phosphatase into soil
AM fungi play an essential role in nutrient acquisition by plants grown in nutrient-poor soil (Ren et al., 2022). In this study, AM fungi promoted the acquisition of root P in P. cuspidatum, accompanied by an increase in soil acid and neutral phosphatase activities, independent of substrate P levels. Phosphatases, which are P-solubilizing enzymes, are responsible for the mineralization of organic P to provide available inorganic P (Redel et al., 2019). AM soil possessed higher activities of acid and neutral phosphatase in P. cuspidatum, which can hydrolyze more organic Pi to enhance P acquisition (Hofmann et al., 2016). AM fungi secrete phosphatases and organic acids into mycorrhizosphere, and also possess phosphorus transport proteins that contribute to the uptake and transport of P, thereby enhancing P acquisition of plants (Bagyaraj et al., 2015; Yang et al., 2021).
AM fungi accelerated chrysophanol, emodin, physcion, polydatin, and resveratrol concentrations, with a higher promotion magnitude under P-deficient conditions than under adequate P conditions
In this study, HPLC was used to determine the medicinal components of P. cuspidatum, in which the concentrations of polydatin, resveratrol, and emodin were 1.89–8.64, 2.69–7.19 and 0.24–1.65 mg/g DW, respectively, which were much lower than those detected in polydatin (5.12–47.51 mg/g DW), resveratrol (3.73–15.16 mg/g DW), and emodin (2.23–17.33 mg/g DW) concentrations of P. cuspidatum by She et al. (2021) in Hubei, China. The difference could be attributed to different years of P. cuspidatum tested and different extraction methods. Future work should be made around extraction methods and gas chromatography–mass spectrometry assays. AM symbionts influence the production of secondary metabolites in various medicinal plants, and medicinal plants also regulate AM symbiosis through their secondary metabolites (Sun et al., 2021). Earlier studies also showed that Ocimum basilicum plants inoculated with Glomus caledonium had higher leaf rosmarinic and caffeic acid levels at three P levels (0.1, 0.2, and 0.3 g/kg of CaHPO4), as compared with uninoculated controls (Toussaint et al., 2007). G. mosseae at P levels of 40–80 mg/kg significantly promoted the accumulation of artemisinin in Artemisia annua plants, while high P (120 mg/kg) supply significantly reduced artemisinin content (Tan et al., 2013). In our study, P supply promoted the concentrations of aloe-emodin, physcion, and resveratrol in non-mycorrhizal plants as well as the concentrations of chrysophanol, physcion, polydatin, and resveratrol in mycorrhizal plants, implying that P is an important regulator of the synthesis of medicinal components. At P0 and P0.2 levels, AM fungi promoted chrysophanol, emodin, physcion, polydatin, and resveratrol accumulations, and the promote effect was better under P-deficient conditions than under adequate P conditions.
AM fungi up-regulated expressions of resveratrol-associated synthases, thereby promoting resveratrol production
AM fungi, after coexisting with host plants, are able to affect expressions of synthetic enzyme genes related to plant secondary metabolites (Zeng et al., 2013; Merlin et al., 2020; Oliveira et al., 2022; Sun et al., 2022a). The synthesis of resveratrol in P. cuspidatum is regulated by key enzymes such as resveratrol synthase (RS), stilbene synthase (STS), supposed stilbene synthase (CRS), and resveratrol-forming stilbene synthase (Zheng et al., 2021). In the present study, both AM fungi and P supply up-regulated PcCRS1, PcRS11, and PcSTS expression, except for down-regulation of PcRS expression, suggesting that both P supply and AM fungi were able to accelerate the expression of resveratrol-associated enzymes in P. cuspidatum, which, in turn, promoted the accumulation of resveratrol levels in roots. Although chalcone synthase (CS) is not directly involved in resveratrol biosynthesis, the enzyme can compete with RS for the same substrate (Flores-Sanchez and Verpoorte, 2009), which thus triggered down-regulated expression of PcRS and elevated expression of PcCHS1 and PcCHS2 by AM fungi and P supply. In Paris polyphylla var. yunnanensis plants, AM fungi also up-regulated PpSE expressions to accelerate polyphyllin accumulation (Li et al., 2021). Therefore, AM fungi as a biostimulator has the potential ability to regulate the expression of secondary metabolite-associated synthases, thereby promoting the level of secondary metabolites. Nevertheless, the underlying mechanisms of how AM fungi and P supply regulate the expression of these genes have yet to be studied.
In short, both P supply and AM fungal inoculation were able to dramatically enhance the concentration of root medicinal components (chrysophanol, physcion, polydatin, and resveratrol) of P. cuspidatum, together with up-regulated expression of associated synthase genes. Therefore, AM fungi as a biostimulator can be introduced or appropriate P fertilizer can be provided in P. cuspidatum cultivation to promote the production of medicinal components.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
R-TS and Q-SW designed the experiment. R-TS, Z-ZZ, and H-DF prepared the materials for the experiment. R-TS, M-YL, NZ, and X-CF conducted the experiment. R-TS, M-YL, and X-CF analyzed the data. R-TS wrote the manuscript. AH, EA, WH, and Q-SW revised the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the 2021 Undergraduate Innovation and Entrepreneurship Training Program of Yangtze University (Yz2021329). The authors extend their sincere appreciation to the Researchers Supporting Project Number (RSP-2021/134), King Saud University, Riyadh, Saudi Arabia.
The authors are grateful to their sincere appreciation to the Researchers Supporting Project Number (RSP-2021/134), King Saud University, Riyadh, Saudi Arabia.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.1006140/full#supplementary-material
Bagyaraj, D. J., Sharma, M. P., and Maiti, D. (2015). Phosphorus nutrition of crops through arbuscular mycorrhizal fungi. Curr. Sci. 108, 1288–1293.
Bastami, A., and Majidian, M. (2016). Comparison between mycorrhizal fungi, phosphate biofertilizer and manure application on growth parameters and dry weight of coriander (Coriandrum sativum L.). medicinal plant. J. Soil Plant Interact. Isfahan Univ. Technol. 7, 23–33. doi: 10.18869/acadpub.ejgcst.7.2.23
Breuillin, F., Schramm, J., Hajirezaei, M., Ahkami, A., Favre, P., Druege, U., et al. (2010). Phosphate systemically inhibits development of arbuscular mycorrhiza in Petunia hybrida and represses genes involved in mycorrhizal functioning. Plant J. 64, 1002–1017. doi: 10.1111/j.1365-313X.2010.04385.x
Campos, P., Borie, F., Cornejo, P., López-Ráez, J. A., López-García, Á., and Seguel, A. (2018). Phosphorus acquisition efficiency related to root traits: is mycorrhizal symbiosis a key factor to wheat and barley cropping? Front. Plant Sci. 9:752. doi: 10.3389/fpls.2018.00752
Cao, J. L., Shao, Y. D., Zou, Y. N., Wu, Q. S., Yang, T. Y., and Kuča, K. (2021). Inoculation with Clariodeoglomus etunicatum improves leaf food quality of tea exposed to P stress. Not. Bot. Horti Agrobo. 49:12166. doi: 10.15835/nbha49112166
Cavell, A. J. (1955). The colorimetric determination of phosphorus in plant materials. J. Sci. Food Agric. 6, 479–480. doi: 10.1002/jsfa.2740060814
Feng, H. D., Zhou, M., Li, K., Si, H. Q., Zhou, J., Guo, R., et al. (2019). Study on seedling technology of Polygonum cuspidatum seeds in field. Hubei Agric. Sci 58, 128–129. doi: 10.14088/j.cnki.issn0439-8114.2019.23.029
Flores-Sanchez, I. J., and Verpoorte, R. (2009). Plant polyketide synthases: a fascinating group of enzymes. Plant Physiol. Biochem. 47, 167–174. doi: 10.1016/j.plaphy.2008.11.005
Genre, A., Lanfranco, L., Perotto, S., and Bonfante, P. (2020). Unique and common traits in mycorrhizal symbioses. Nat. Rev. Microbiol. 18, 649–660. doi: 10.1038/s41579-020-0402-3
Hoagland, D. R. (1950). The water culture methods for growing plants without soils. Cal. Agric. Exp. St. Circ. 347, 1–32. doi: 10.1016/S0140-6736(00)73482-9
Hofmann, K., Heuck, C., and Spohn, M. (2016). Phosphorus resorption by young beech trees and soil phosphatase activity as dependent on phosphorus availability. Oecologia 181, 369–379. doi: 10.1007/s00442-016-3581-x
Huang, J. H., Tan, J. F., Jie, H. K., and Zeng, R. S. (2011). Effects of inoculating arbuscular mycorrhizal fungi on Artemisia annua growth and its officinal components. Chin. J. Appl. Ecol. 22, 1443–1449. doi: 10.3724/SP.J.1011.2011.00353
Huang, G. M., Zou, Y. N., Wu, Q. S., Xu, Y. J., and Kuča, K. (2020). Mycorrhizal roles in plant growth, gas exchange, root morphology, and nutrient uptake of walnuts. Plant Soil Environ. 66, 295–302. doi: 10.17221/240/2020-PSE
Jia, H. M., Fang, Q., Zhang, S. H., Yan, Z. Y., and Liu, M. (2020). Effects of AM fungi on growth and rhizosphere soil enzyme activities of salvia miltiorrhiza. Acta Prataculturae Sin. 29, 83–92. doi: 10.11686/cyxb2019494
Kapoor, R., Giri, B., and Mukerji, K. G. (2004). Improved growth and essential oil yield and quality in Foeniculum vulgare mill on mycorrhizal inoculation supplemented with P-fertilizer. Bioresour. Technol. 93, 307–311. doi: 10.1016/j.biortech.2003.10.028
Khaosaad, T., Vierheilig, H., Nell, M., Zitterl-Eglseer, K., and Novak, J. (2006). Arbuscular mycorrhiza alter the concentration of essential oils in oregano (Origanum sp., Lamiaceae). Mycorrhiza 16, 443–446. doi: 10.1007/s00572-006-0062-9
Kong, D. X., Zhu, Y. R., Zhu, W. R., Fu, M., and Wang, G. Z. (2020). Simultaneous determination of five active components in different parts of Polygonum cuspidatum root by HPLC. Mod. Chin. Med. 22, 53–57. doi: 10.13313/j.issn.1673-4890.20190401003
Lee, J., and Scagel, C. F. (2009). Chicoric acid found in basil (Ocimum basilicum L.) leaves. Food Chem. 115, 650–656. doi: 10.1016/j.foodchem.2008.12.075
Li, H. L., Xu, L. F., Li, Z. W., Zhao, S. X., Guo, D. Q., Rui, L., et al. (2021). Mycorrhizas affect polyphyllin accumulation of Paris polyphylla var. yunnanensis through promoting PpSE expression. Phyton Int. J. Exp. Bot. 90, 1535–1547. doi: 10.32604/phyton.2021.015697
Liu, J. N., Wu, L. J., Wei, S. L., Xiao, X., Su, C. X., Jiang, P., et al. (2007). Effects of arbuscular mycorrhizal fungi on the growth, nutrient uptake and glycyrrhizin production of licorice (Glycyrrhiza uralensis Fisch). Plant Growth Regul. 52, 29–39. doi: 10.1007/s10725-007-9174-2
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Merlin, E., Melato, E., Lourenço, E. L. B., Jacomassi, E., Gasparotto, J. A., Da Cruz, R. M. S., et al. (2020). Inoculation of arbuscular mycorrhizal fungi and phosphorus addition increase coarse mint (Plectranthus amboinicus Lour.) plant growth and essential oil content. Rhizosphere 15, 100217–100222. doi: 10.1016/j.rhisph.2020.100217
Mora, M. D. L. L., Pinilla, L., Rosas, A., and Cartes, P. (2008). Selenium uptake and its influence on the antioxidative system of white clover as affected by lime and phosphorus fertilization. Plant Soil 303, 139–149. doi: 10.1007/s11104-007-9494-z
Oliveira, J. S. D., Ramos, N. P., Júnior, J. L., Xavier, L. P., Andrade, E. H., Mello, A. H., et al. (2022). Secondary metabolism and plant growth of Piper divaricatum (piperaceae) inoculated with arbuscular mycorrhizal fungi and phosphorus supplementation. Agronomy 12:596. doi: 10.3390/agronomy12030596
Pedone-Bonfim, M. V., Lins, M. A., Coelho, I. R., Santana, A. S., Silva, F. S., and Maia, L. C. (2013). Mycorrhizal technology and phosphorus in the production of primary and secondary metabolites in cebil (Anadenanthera colubrina (Vell.) Brenan) seedlings. J. Sci. Food Agric. 93, 1479–1484. doi: 10.1002/jsfa.5919
Phillips, J. M., and Hayman, D. S. (1970). Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 55, 158–IN18. doi: 10.1016/S0007-1536(70)80110-3
Redel, Y., Staunton, S., Durán, P., Gianfreda, L., Rumpel, C., and Mora, M. (2019). Fertilizer P uptake determined by soil P fractionation and phosphatase activity. J. Soil Sci. Plant Nutr. 19, 166–174. doi: 10.1007/s42729-019-00024-z
Ren, W., Guo, Y., Han, X., Sun, Y., Li, Q., Wu, B., et al. (2022). Indigenous microorganisms offset arbuscular mycorrhizal fungi-induced plant growth and nutrient acquisition through negatively modulating the genes of phosphorus transport and nitrogen assimilation. Front. Plant Sci. 13:880181. doi: 10.3389/fpls.2022.880181
Shao, Y. D., Hu, X. C., Wu, Q. S., Yang, T. Y., Srivastava, A. K., Zhang, D. J., et al. (2021). Mycorrhizas promote P acquisition of tea plants through changes in root morphology and P transporter gene expression. S. Af. J. Bot. 137, 455–462. doi: 10.1016/j.sajb.2020.11.028
She, Y. Y., Liu, Z. H., Zhang, Y., Liu, Y. M., and Chen, Y. Z. (2021). Content analysis of main chemical components and its correlation with color difference of Polygonum cuspidatum in Hubei province. J. Food Saf. Quality 12, 960–967. doi: 10.19812/j.cnki.jfsq11-5956/ts.2021.03.026
Song, A. Q., Tong, F. P., Yi, A. Q., Ding, J., Li, G., and Huang, Z. (2006). Rooting out of bottles and fertilization on leaves experiment on tissue culture of Ploygonum cuspidatum sieb et zucc. Hunan For. Sci. Technol. 33, 27–30. doi: 10.3969/j.issn.1003-5710.2006.06.009
Sun, J. (2007). Separation and Analysis of Resveratrol and Volatile from Polygonum cuspidatum. Master’s Degree Thesis. Central South University, Changsha, China.
Sun, R. T., Feng, X. C., Zhang, Z. Z., Zhou, N., Feng, H. D., Liu, Y. M., et al. (2022a). Root endophytic fungi regulate changes in sugar and emdicinal compositions of Polygonum cuspidatum. Front. Plant Sci. 13:818909. doi: 10.3389/fpls.2022.818909
Sun, R. T., Zhang, Z. Z., Feng, X. C., Zhou, N., Feng, H. D., Liu, Y. M., et al. (2022b). Endophytic fungi accelerate leaf physiological activity and resveratrol accumulation in Polygonum cuspidatum by up-regulating expression of associated genes. Agronomy 12:1220. doi: 10.3390/agronomy12051220
Sun, R. T., Zhang, Z. Z., Zhou, N., Srivastava, A. K., Kuča, K., Abd, A. E. F., et al. (2021). A review of the interaction of medicinal plants and arbuscular mycorrhizal fungi in the rhizosphere. Not. Bot. Horti Agrobo. 49:12454. doi: 10.15835/nbha49312454
Tan, W. D., Shen, M. J., Qiu, H. J., Zeng, F. L., Huang, J. H., Huang, R. S., et al. (2013). Effects of different phosphorus treatments on arbuscular mycorrhizal formation, growth and artemisinin content of Artemisia annua. J. South. Agric. 44, 1303–1307. doi: 10.3969/j:issn.2095-1191.2013.8.1303
Toussaint, J. P., Smith, F. A., and Smith, S. E. (2007). Arbuscular mycorrhizal fungi can induce the production of phytochemicals in sweet basil irrespective of phosphorus nutrition. Mycorrhiza 17, 291–297. doi: 10.1007/s00572-006-0104-3
Wang, X. X., Li, H., Chu, Q., Feng, G., Kuyper, T. W., and Rengel, Z. (2020). Mycorrhizal impacts on root trait plasticity of six maize varieties along a phosphorus supply gradient. Plant Soil 448, 71–86. doi: 10.1007/s11104-019-04396-0
Williamson, L. C., Ribrioux, S. P. C. P., Fitter, A. H., and Ottoline Leyser, H. M. (2001). Phosphate availability regulates root system architecture in Arabidopsis. Plant Physiol. 126, 875–882. doi: 10.1104/pp.126.2.875
Wu, Q. S., Li, Y., Zou, Y. N., and He, X. H. (2015a). Arbuscular mycorrhiza mediates glomalin-related soil protein production and soil enzyme activities in the rhizosphere of trifoliate orange grown under different P levels. Mycorrhiza 25, 121–130. doi: 10.1007/s00572-014-0594-3
Wu, Q. S., Srivastava, A. K., and Li, Y. (2015b). Effect of mycorrhizal symbiosis on growth behavior and carbohdyrate metabolism of trifoliate orange under different substrate P levels. J. Plant Growth Regul. 34, 499–508. doi: 10.1007/s00344-015-9485-x
Wu, Z., Wang, X., Chen, M., Hu, H., Cao, J., Chai, T., et al. (2019). Study on tissue- specific metabolite variations in Polygonum cuspidatum by high-resolution mass spectrometry-based metabolic profiling. Molecules 24:1058. doi: 10.3390/molecules24061058
Wu, G. L., Zhou, L., and Deng, W. X. (2022). Research progress on extraction methods of effective components of Polygonum cuspidatum. Cereals Oils 35, 16–18.
Xie, M. M., Zou, Y. N., Wu, Q. S., Zhang, Z. Z., and Kuča, K. (2020). Single or dual inoculation of arbuscular mycorrhizal fungi and rhizobia regulates plant growth and nitrogen acquisition in white clover. Plant Soil Environ. 66, 287–294. doi: 10.17221/234/2020-PSE
Yang, L., Zou, Y. N., Tian, Z. H., Wu, Q. S., and Kuča, K. (2021). Effects of beneficial endophytic fungal inoculants on plant growth and nutrient absorption of trifoliate orange seedlings. Sci. Hortic. 277:109815. doi: 10.1016/j.scienta.2020.109815
Yu, Z.F. (2016). Study on Mechanism of Quality Formated by Photosynthetic Characteristics and Regularity of Absorbing Fertilizer of Polygonum cuspidatum Sicb et Zucc. Master’s Thesis. Chengdu University of Traditional Chinese Medicine, Chengdu, China.
Zeng, Y., Guo, L. P., Chen, B. D., Hao, Z. P., Wang, J. Y., Huang, L. Q., et al. (2013). Arbuscular mycorrhizal symbiosis and active ingredients of medicinal plants: current research status and prospectives. Mycorrhiza 23, 253–265. doi: 10.1007/s00572-013-0484-0
Zeng, L., Wang, M. Y., Li, F. J., and Zheng, W. J. (2014). Mechanism and effects of AM fungi on medicinal plants. J. Anhui Agric. Sci. 42, 4231–4233. doi: 10.13989/j.cnki.0517-6611.2014.14.080
Zhang, H., Li, C., Kwok, S. T., Zhang, Q. W., and Chan, S. W. (2013). A review of the pharmacological effects of the dried root of Polygonum cuspidatum (Hu Zhang) and its constituents. Evid. Based Complement. Altern. Med. 2013:208349. doi: 10.1155/2013/208349
Zhang, F., Wang, P., Zou, Y. N., Wu, Q. S., and Kuča, K. (2019). Effects of mycorrhizal fungi on root-hair growth and hormone levels of taproot and lateral roots in trifoliate orange under drought stress. Arch. Agron. Soil Sci. 65, 1316–1330. doi: 10.1080/03650340.2018.1563780
Zhao, J. L., and He, X. L. (2011). Effects of AM fungi on drought resistance and content of chemical components in Angelica dahurica. Acta Agr. Bor. Occi. Sin. 19, 110–119. doi: 10.3724/SP.J.1011.2011.00110
Zheng, L., Zhou, C., Li, T., Yuan, Z., Zhou, H., Tamada, Y., et al. (2021). Global transcriptome analysis reveals dynamic gene expression profiling and provides insights into biosynthesis of resveratrol and anthraquinones in a medicinal plant Polygonum cuspidatum. Ind. Crop. Prod. 171:113919. doi: 10.1016/j.indcrop.2021.113919
Keywords: arbuscular mycorrhiza, medicinal plant, metabolite, P stress, resveratrol
Citation: Sun R-T, Zhang Z-Z, Liu M-Y, Feng X-C, Zhou N, Feng H-D, Hashem A, Abd_Allah EF, Harsonowati W and Wu Q-S (2022) Arbuscular mycorrhizal fungi and phosphorus supply accelerate main medicinal component production of Polygonum cuspidatum. Front. Microbiol. 13:1006140. doi: 10.3389/fmicb.2022.1006140
Received: 29 July 2022; Accepted: 23 August 2022;
Published: 08 September 2022.
Edited by:
Xiancan Zhu, Anhui Normal University, ChinaReviewed by:
Anthonymuthu Selvaraj, University of California, Irvine, United StatesCopyright © 2022 Sun, Zhang, Liu, Feng, Zhou, Feng, Hashem, Abd_Allah, Harsonowati and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang-Sheng Wu, d3VxaWFuZ3NoQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.