
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 07 February 2020
Sec. Plant Pathogen Interactions
Volume 11 - 2020 | https://doi.org/10.3389/fmicb.2020.00091
Phosphoglycerate kinase (Pgk), catalyzing the reversible conversions between glycerate-1.3-2P and glycerate-3P, plays an important role in carbohydrate metabolism. Here, we show that a Pgk-deficient mutant (NΔpgk) of Xanthomonas axonopodis pv. glycines (Xag) could grow in medium with glucose, galactose, fructose, mannose, or sucrose, as the sole carbon source, suggesting that Xag may employ Entner-Doudoroff (ED) and pentose phosphate pathway (PPP), but not glycolysis, to catabolize glucose. NΔpgk could not utilize pyruvate, suggesting that Pgk might be essential for gluconeogenesis. Mutation in pgk led to a reduction of extracellular polysaccharide (EPS) biosynthesis, cell motility, and intracellular ATP. As a result, the virulence of NΔpgk was significantly compromised in soybean. NΔpgk could be fully complemented by the wild-type pgk, but not by clp (encoding Crp-like protein). qRT-PCR analyses demonstrated that pgk is regulated by the HrpG/HrpX cascade, but not by Clp. These results suggest that Pgk is involved in carbohydrate utilization, EPS biosynthesis, and cell motility of Xag independent of Clp.
Xanthomonas axonopodis pv. glycines (Xag) is widely distributed in soybean-producing areas throughout the world (Chatnaparat et al., 2012). The pathogen enters soybean leaves through stomata or wounds, and reproduces within the intercellular spaces of the spongy mesophyll, resulting in erumpent pustules surrounded by yellow halos (Guo et al., 2019). Xag is a quarantine pathogen that can be spread by rain droplets during the growing season and by seed transportation (Liu et al., 2016). Bacterial pustule, caused by Xag, is an important bacterial disease on soybean, resulting in premature defoliation and decreasing yield and seed quality (Thowthampitak et al., 2008; Athinuwat et al., 2009).
Over the past two decades, significant progress has been made on elucidating the association of carbon metabolism to virulence and quorum sensing (QS) in Xanthomonas spp., such as in X. oryzae pv. oryzicola (Xoc), which infects rice (Guo et al., 2017). Results of these studies have shown that carbohydrate acquisition is essential for the pathogen to grow and establish a successful infection within the hosts (Tang et al., 2005; Mellgren et al., 2009). When the pathogen gains entry into the host plant, it first propagates in the apoplastic space until reaching a density threshold, followed by the expression of lots of virulence-related genes and secretion of cell-wall degrading enzymes through the diffusible signal factor (DSF)-mediated QS mechanism (Büttner and Bonas, 2010). The pathogen overcomes host defenses, adapts to the hostile environment, and ultimately degrades the plant parenchyma cells to gain access to its nutritional reservoirs (Tamir-Ariel et al., 2007; Zhou et al., 2013).
Xanthomonas spp. carry out catabolic processes, such as glycolysis, Entner-Doudoroff (ED), pentose phosphate pathway (PPP), and the tricarboxylic acid (TCA) cycle, to catabolize glucose and other simple sugars (sucrose, fructose, mannose, and galactose) (Lu et al., 2009; Kim et al., 2010). These metabolic pathways provide nutrition for growth and propagation of pathogen and supply energy that drives energy-requiring activities such as extracellular polysaccharide (EPS) production and cell motility (Mellgren et al., 2009; Guo et al., 2017). Phosphoglycerate kinase (Pgk), reversibly converting glycerate-1.3-2P to glycerate-3P, is involved in glycolysis, ED, PPP, and gluconeogenesis in Xag. However, little is known about the biological processes in which Pgk participates.
Acetyl-CoA and amino acids, produced by catabolic processes, act as precursor molecules for synthesizing DSF signals (Zhou et al., 2015). Crp-like protein (Clp), located at the end of the DSF signaling pathway, is the global regulator in Xanthomonas spp. In addition to DSF signals, Clp could also converge on low-oxygen signals transduced by the RavS/RavR two-component regulatory system (He et al., 2009) and environmental signals transduced by the HrpG/HrpX cascade (Wengelnik and Bonas, 1996; Wengelnik et al., 1996). As a consequence, the activated Clp directly or indirectly regulates the expression of downstream genes and modulates numerous biological properties, such as carbohydrate utilization, cell motility, EPS production, and synthesis of extracellular enzymes (Guo et al., 2019), which are all essential for virulence and adaptation of Xanthomonas spp. in host plant. However, it is unknown whether Clp could completely or partially complement the impaired properties in the pgk mutant. In addition, the inherent relationship between Clp and Pgk is unclear.
In this study, we aimed to explore the biological properties of Pgk and to reveal its association with Clp. Here, we present evidence that Pgk is involved in carbohydrate utilization, cell motility, and EPS biosynthesis independent of Clp in Xag. In addition, the expression of pgk is regulated by the HrpG/HrpX cascade, but not by DSF signals or Clp.
Strains and plasmids used in this study are listed in Supplementary Table S1. Unless otherwise specified, Xag NEAU001 and its derivative strains were grown at 28°C in NYG (5 g L–1 polypeptone, 3 g L–1 yeast extract, 20 g L–1glycerol), NY (NYG without glycerol), or NCM (2 g L–1 (NH4)2SO4, 0.2 g L–1 MgSO4⋅7H2O, 4 g L–1 K2HPO4, 6 g L–1 KH2PO4) media (Liu et al., 2019). Escherichia coli strains were routinely grown at 37°C in LB (10 g L–1 tryptone, 10 g L–1 NaCl, 5 g L–1 yeast extract) medium. Antibiotics were added at the following concentrations: ampicillin, 50 μg mL–1; kanamycin, 25 μg mL–1; spectinomycin, 100 μg mL–1; and carbenicillin, 50 μg mL–1.
The in-frame deletion mutant of pgk in Xag was generated by using homologs recombination as described by Li et al. (2011), using pKMS1 as a suicide vector and the primers listed in Supplementary Table S2. The obtained mutant was named NΔpgk. The complemented and by-path complemented strains were also constructed by separately introducing the recombinant plasmids pCpgk and pCclp into NΔpgk (Li et al., 2011). The resulting complemented and by-path complemented strains were designated as CNΔpgk and NΔpgk(clp), respectively.
Carbohydrate utilization and EPS production assays were measured as previously described by Lu et al. (2009). Each experiment was repeated at least in triplicate.
Cell motility of Xag strains was investigated on plates of semi-solid NY medium with 0.3% agar as described by Long et al. (2018). The exoenzyme activities of Xag strains in the supernatants of the cultures were analyzed on NY plates supplemented with different substrates (such as skimmed milk for protease, carboxymethyl cellulose for carboxymethylcellulase, soluble starch for α-amylase, and locust bean gum for endo-β-mannanase) following the method described previously by Thowthampitak et al. (2008). The experiment was repeated independently in triplicate, and five replicate plates were conducted for each treatment.
Intracellular ATP levels were determined by using an ATP Assay Kit (Beyotime), following the manufacturer’s protocols. All samples were measured at least in triplicate.
H2O2 resistance assays were performed as described previously by Wang et al. (2013). Briefly, the fresh Xag strains were grown in NY medium at 28°C until the exponential growth phase was reached (OD600 ≈ 1.0). Then, the semi-solid NY plates mixed with Xag were prepared. After solidification, the saturated filter papers with 0.5 or 1.0 mM H2O2 were placed on the center of the plates. These bioassay plates were then incubated at 28°C for 2 days. H2O2 resistance was indicated by measuring the diameter of the zone of inhibition. The same experiment was repeated at least three times.
In addition to pgk-related strains, the following strains were used in the qRT-PCR: the deletion mutants of rpfF (responsible for the synthesis of DSF), rpfC, rpfG (the RpfC/RpfG two-component system is involved in sensing and transduction of DSF) (Cai et al., 2017), rpfB (required for DSF signals turnover) (Wang et al., 2016), rpfS (a second sensor for DSF), rpfR (putative interplay with rpfS) (An et al., 2014), and clp (Guo et al., 2019) in the DSF signaling pathway; the deletion mutants ravS and ravR (the RavS/RavR two-component system is involved in sensing and transduction of low-oxygen signals) (He et al., 2009); and the deletion mutants of hrpX, hrpG, and their upstream regulatory genes, such as rsmA (Andrade et al., 2014), zur (Huang et al., 2009), trh (Li et al., 2011), and xopL (also called lrpX) (Islam et al., 2009), which are all involved in regulating the mRNA level of clp in Xag (Guo et al., 2019).
All tested strains were incubated in NY medium until OD600 ≈ 2.0. Total RNA was extracted using the Trizol reagent (Invitrogen), following the manufacturer’s protocol. cDNA synthesis was conducted with a PrimeScriptTM RT reagent Kit (TaKaRa). The transcriptional levels of tested genes were determined by qRT-PCR using the primers listed in Supplementary Table S2. qRT-PCR was performed on the Applied BiosystemsTM 7500 Real-Time PCR System using SYBR Premix ExTaqTM (TaKaRa). The transcriptional level of integration host factor A (ihfA) was used as a reference (Thowthampitak et al., 2008). All qRT-PCR analyses were performed in two independent experiments.
The virulence of Xag strains was assessed as described previously (Chatnaparat et al., 2012). Briefly, all tested strains were grown in NY medium with shaking until the exponential phase was reached. Bacterial cells were then harvested, washed twice, and re-suspended to OD600 ≈ 0.2. Then bacterial cells were high-pressure sprayed into soybean leaves (Glycine max cv. Zhoudou63; susceptible to bacterial pustule, 6 weeks old). Phenotypes were scored 15 days post-inoculation. The experiments were repeated at least in triplicate.
Bacterial cell suspensions at OD600 ≈ 0.1 were infiltrated into the intercellular spaces of leaves with needleless syringes. Bacterial growth within soybean leaves was assessed as previously described by Liu et al. (2014). The experiments were repeated at least in triplicate.
Means and standard deviations (SD) of experimental data were calculated using Microsoft Office Excel. Statistical analyses were performed using a Student’s t-test. ∗Indicates significance at P < 0.05; ∗∗indicate significance at P < 0.01.
Genomic sequence analysis showed that only one open reading frame, in the genome of Xag strain NEAU001, is annotated to encode Pgk. To facilitate the functional study of pgk, a non-polar deletion mutant was constructed by using homologous recombination and pKMS1 as a suicide vector. As expected, a 2,095 bp fragment was amplified from the wild-type Xag strain with the primer pairs 1F/2R, whereas only a 923 bp fragment was amplified in the pgk mutant because of an in-frame deletion of 1,172 bp from pgk (Figure 1). The in-frame deletion was further verified by a nested PCR using the primer pairs 3F/3R (Figure 1). The deletion mutant, designated NΔpgk, was used in our studies.
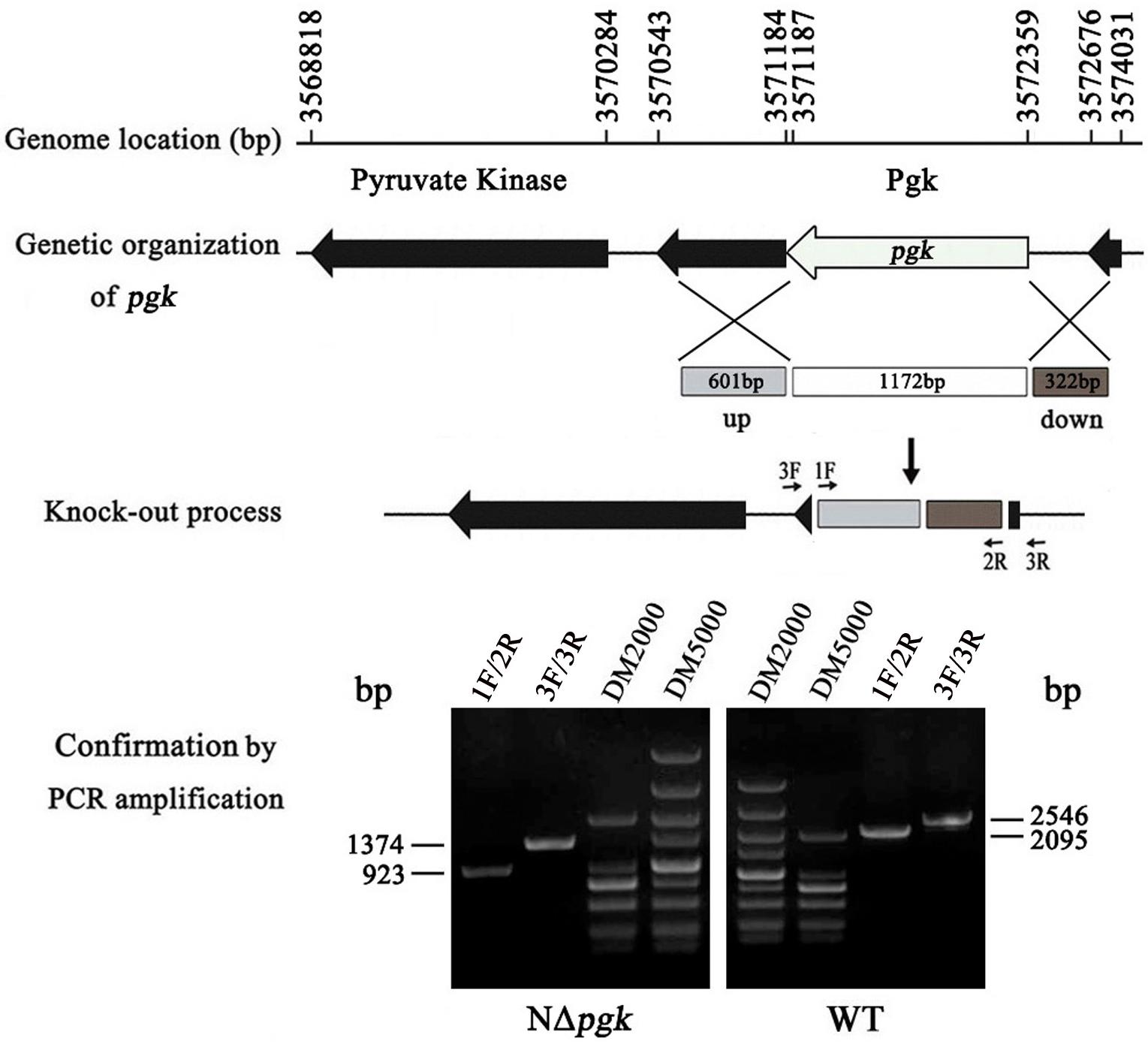
Figure 1. Schematic map and molecular analysis of pgk mutation in X. axonopodis pv. glycines (Xag). The positions and orientations of pgk, encoding phosphoglycerate kinase (Pgk), and other adjacent open reading frames (ORFs) are shown using the genome sequence of Xag 12-2 strain as a reference. Numbers and arrows respectively represent the locations and orientations of the ORFs; lines indicate intergenic sequences. A non-polar construction of the pgk deletion mutant was sketched. The gray and brown box indicated where the left and right flanks targeted pgk. The white box shows the 1,172 bp deletion of pgk. pgk was knocked out after two homologous crossover events occurred, and then was verified by nested PCR with two primer pairs 1F/2R and 3F/3R.
Phosphoglycerate kinase is an indispensable component of glycolysis, ED, PPP, and gluconeogenesis (Kim et al., 2010); therefore, we measured the growth of NΔpgk to determine whether Pgk is involved in carbohydrate metabolism in Xag. We found that NΔpgk has a similar growth pattern to that of the wild-type Xag when grown in NY medium (Supplementary Figure S1), indicating that NΔpgk was not auxotrophic. To qualitatively assess the effect of Pgk on carbohydrate utilization, we used NCM medium, which is similar to the conditions encountered in the plant apoplast, to investigate the growth of NΔpgk. When glucose, galactose, fructose, mannose, sucrose, or pyruvate was used as the sole carbon source, the pgk mutant displayed smaller colonies than the wild-type strain (Supplementary Figure S2), suggesting that mutation in pgk diminishes the ability of Xag to utilize carbohydrates.
To quantitatively evaluate the contribution of Pgk to carbohydrate utilization, the growth of NΔpgk, the complemented strain CNΔpgk, the by-path complemented strain NΔpgk(clp), and the wild-type strain was tested in NCM liquid medium supplemented with different carbon sources. When glucose, galactose, fructose, mannose, or sucrose was used as the sole carbon source, NΔpgk exhibited significantly slower growth compared to the wild-type strain (P < 0.01), and NΔpgk harboring the pgk in trans completely restored the ability to acquire these sugars (Figure 2). Compared with the growth of NΔpgk, the growth rate of NΔpgk(clp) was lower (Figure 2), indicating a reduced ability to acquire sugars supplemented and an optimal level of Clp is required for Xag to utilize carbohydrates. When pyruvate was used as the sole carbon source, the growths of both NΔpgk and NΔpgk(clp) were similarly limited (Figure 2). These results revealed that Pgk is required for Xag to utilize glucose, galactose, fructose, mannose, sucrose, and especially pyruvate.
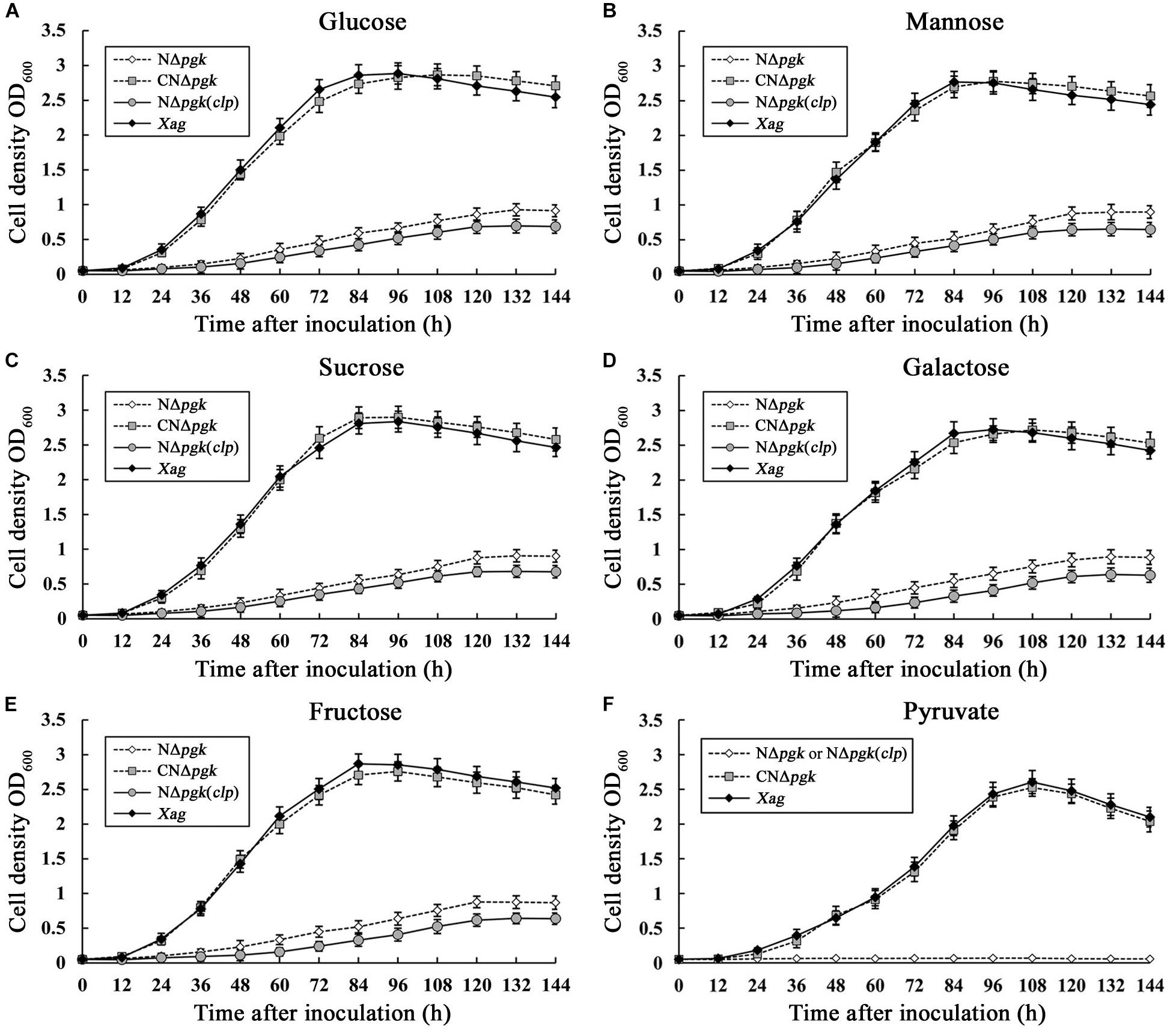
Figure 2. Phosphoglycerate kinase is involved in the utilization of glucose (A), mannose (B), sucrose (C), galactose (D), fructose (E), and pyruvate (F) in Xag independent of Clp. Aliquots were taken in triplicate at intervals of 144 h after incubation at 28°C, and bacterial growth was determined by measuring OD600 against the medium blank. Data are means ± standard deviations (SD) from three repeats.
The expression of pgk was assessed using qRT-PCR after the wild-type was grown in NY medium supplemented with glucose, galactose, fructose, mannose, sucrose, or pyruvate. We found that pgk was strongly induced by these carbohydrates, with a minimum of 10 times higher than that in NY medium not supplemented with any sugar (Figure 3). These results indicate that the expression of pgk was significantly enhanced by carbohydrates involved in the carbon metabolic pathways, further supporting that Pgk plays a crucial role in carbohydrate metabolism.
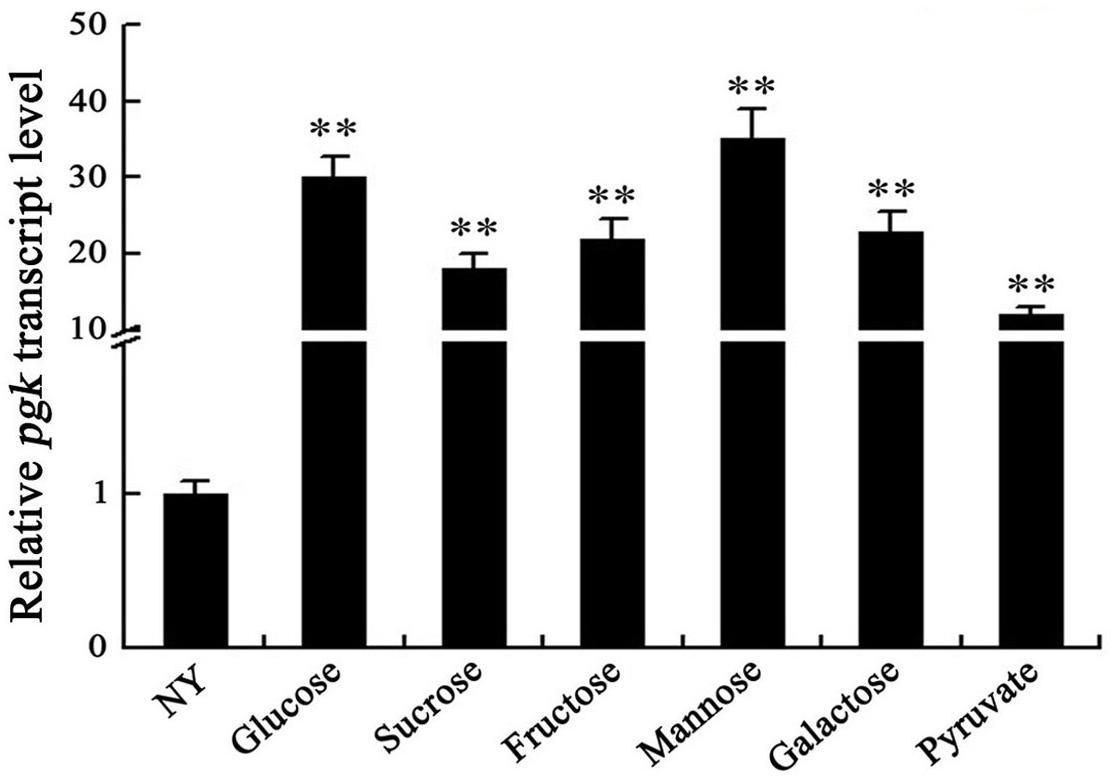
Figure 3. Carbohydrates induce the expression of pgk in Xag. ihfA was used as an internal control for data analyses. Data are means ± SD from three replicates. The asterisks in each horizontal column indicate significant differences at P < 0.01.
Because Pgk is essential for glycolysis, ED, PPP, and gluconeogenesis (Kim et al., 2010), we further explored whether a mutation in pgk has any effect on EPS biosynthesis in Xag. All tested strains were grown on NY plates supplemented with 2% glucose, galactose, fructose, mannose, sucrose, or pyruvate. NΔpgk had smaller colonies than the wild-type strain on all plates tested (Figure 4A), indicating that Pgk might be involved in the biosynthesis of EPS in Xag.
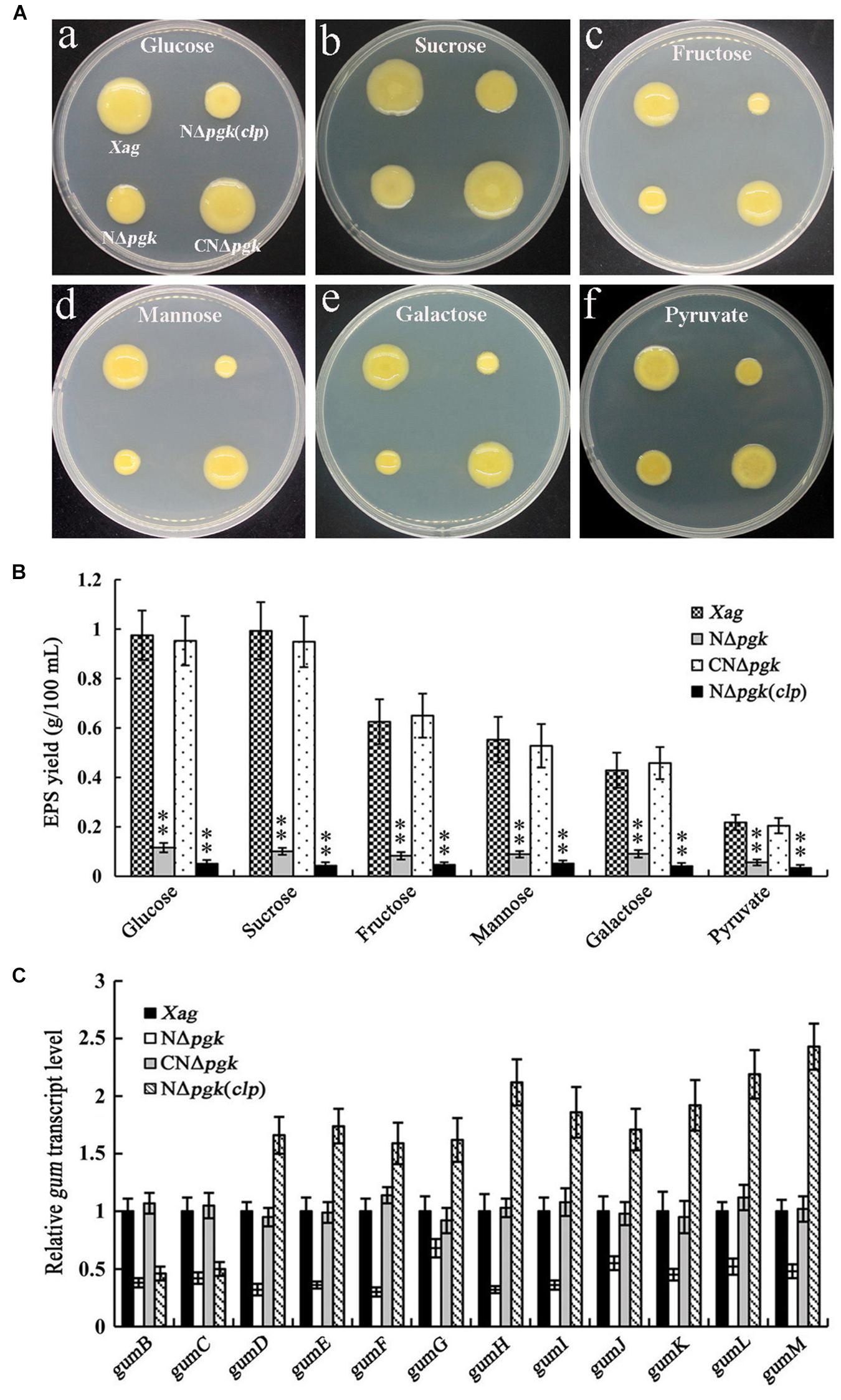
Figure 4. Phosphoglycerate kinase is involved in the biosynthesis of EPS in Xag independent of Clp. EPS productions of the pgk mutant (NΔpgk), the NΔpgk complemented strain (CNΔpgk), and the by-path complemented strain [NΔpgk(clp)] in NY medium supplemented with 2% glucose, galactose, fructose, mannose, sucrose, or pyruvate were determined by qualitative (A) and quantitative (B) methods. (C) The transcriptional levels of gum genes in NΔpgk, CNΔpgk, and NΔpgk(clp) were detected by qRT-PCR. Data are means ± SD from three repeats. The asterisks in each horizontal column indicate significant differences at P < 0.01.
The contribution of Pgk to EPS production was quantitatively examined by culturing all strains in NY liquid medium supplemented with 2% carbohydrates for 5 days. NΔpgk produced approximately 35–85% less EPS than the wild-type when cultured in glucose-, galactose-, fructose-, mannose-, sucrose-, or pyruvate-containing medium (Figure 4B). In addition, the EPS yield of CNΔpgk was fully restored to the wild-type level (Figure 4B). These results demonstrate that Pgk is involved in the biosynthesis of EPS in Xag.
We next determined whether Clp could, either completely or partially, restore EPS production in NΔpgk. Our results showed that by-path complementation of clp in NΔpgk did not restore EPS biosynthesis, on the contrary, it showed weakened EPS biosynthesis (Figures 4A,B). To further understand how Pgk is involved in the biosynthesis of EPS in Xag, qRT-PCRs were performed to explore the transcriptional levels of gum and xan genes. As shown in Figure 4C, the expression of 12 gum genes in NΔpgk was significantly lower than in CNΔpgk and the wild-type strain, indicating that the mutation in pgk negatively regulates the expression of gum genes. The transcriptional levels of gumB and gumC in NΔpgk(clp) was significantly lower than that in the wild-type strain, while the transcriptional levels of gumD-M in NΔpgk(clp) was significantly higher than that in the wild-type strain, suggesting that by-path complementation of clp up-regulates the expression of gum genes except for gumB and gumC, which play a critical role in the biosynthesis of EPS in Xag. However, a minimal change in the expression level of xan genes was observed in NΔpgk or NΔpgk(clp) (Supplementary Figure S3). These results demonstrate that Pgk is involved in the biosynthesis of EPS in Xag independent of Clp, possibly through partially inhibiting the expression of the gum gene cluster.
We explored whether a mutation in pgk affects the cell motility of Xag. Our results showed that there was little visible difference in cell motility among NΔpgk, CNΔpgk, NΔpgk(clp), and the wild-type strain on NY plates (Figure 5A). However, on NY plates supplemented with 0.5% glucose, NΔpgk displayed severely reduced cell motility compared with the wild-type strain (Figure 5A). The diameter of the colonies resulting from migration away from the inoculation sites on the surface of the plate was approximately 0.72 cm for NΔpgk, 2.83 cm for CNΔpgk, 1.21 cm for NΔpgk(clp), and 2.74 cm for the wild-type strain (Figure 5B). A t-test indicated that the mean diameter of NΔpgk was significantly smaller than that of the wild-type strain (P < 0.01), and the cell motility of CNΔpgk was fully restored to the wild-type level.
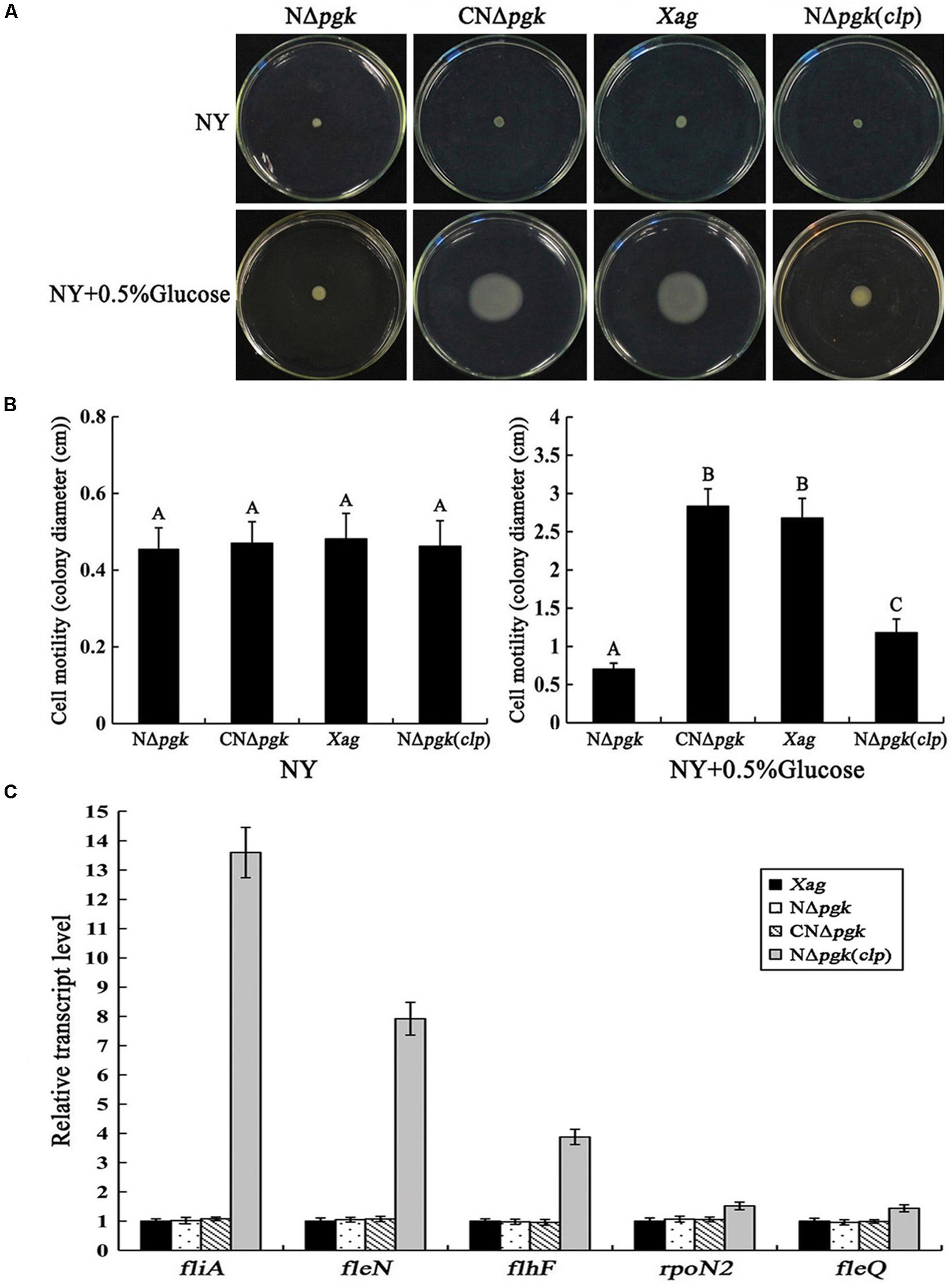
Figure 5. Phosphoglycerate kinase is involved in cell motility of Xag independent of Clp. (A) Cell motility of NΔpgk, CNΔpgk, and NΔpgk(clp) was detected on NY plates or NY plates supplemented with 0.5% glucose and photographed after 1–2 days of incubation at 28°C. (B) Swimming diameter of NΔpgk, CNΔpgk, and NΔpgk(clp) on plates was measured. (C) The transcriptional levels of the flagellar-associated genes in NΔpgk, CNΔpgk, and NΔpgk(clp) were examined by qRT-PCR. Data are means ± SD from three replicates. The different letters above the horizontal columns represent significant differences at P < 0.01.
We then investigated whether Clp could, either partially or completely, restore cell motility of NΔpgk. Interestingly, by-path complementation of clp in NΔpgk only slightly enhanced the cell motility of NΔpgk compared to that of the wild-type (Figure 5B). To further understand how Pgk is involved in cell motility, qRT-PCRs were performed to explore the expression of fliA, fleN, flhF, rpoN2, and fleQ, which have been shown crucial for cell motility (Guo et al., 2019). As shown in Figure 5C, no change in the expression of these five genes was observed in NΔpgk, CNΔpgk, or the wild-type strain. However, the transcription of these five genes, especially fliA and fleN, was significantly increased in NΔpgk(clp) (P < 0.01) (Figure 5C). As reported previously, the overexpression of clp in the wild-type background also results in the increased expressions of these five genes and slightly enhanced cell motility compared to the wild-type (Guo et al., 2019). Therefore, our results suggest that the by-path complementation of clp could promote cell motility, rather than restore the impaired motility of NΔpgk. Further, Pgk is involved in the cell motility of Xag independent of Clp.
Phosphoglycerate kinase is involved in energy-requiring activities, such as EPS biosynthesis and cell motility; therefore, we determine whether Pgk affects intracellular energy production in Xag. Our results showed that intracellular ATP of NΔpgk was 83.6 and 72.8% of the wild-type level when cultured for 12 and 24 h, respectively, in NY medium supplemented with 2% glucose (Figure 6). The ATP levels in CNΔpgk were completely restored to the wild-type level at all incubation times, indicating that pgk is required for producing intracellular ATP in Xag. Interestingly, by-path complementation of clp in NΔpgk did not increase or maintain intracellular ATP levels of NΔpgk, instead, it slightly decreased the ATP levels (Figure 6). Overall, these results reveal that Pgk is required for the production of ATP in Xag.
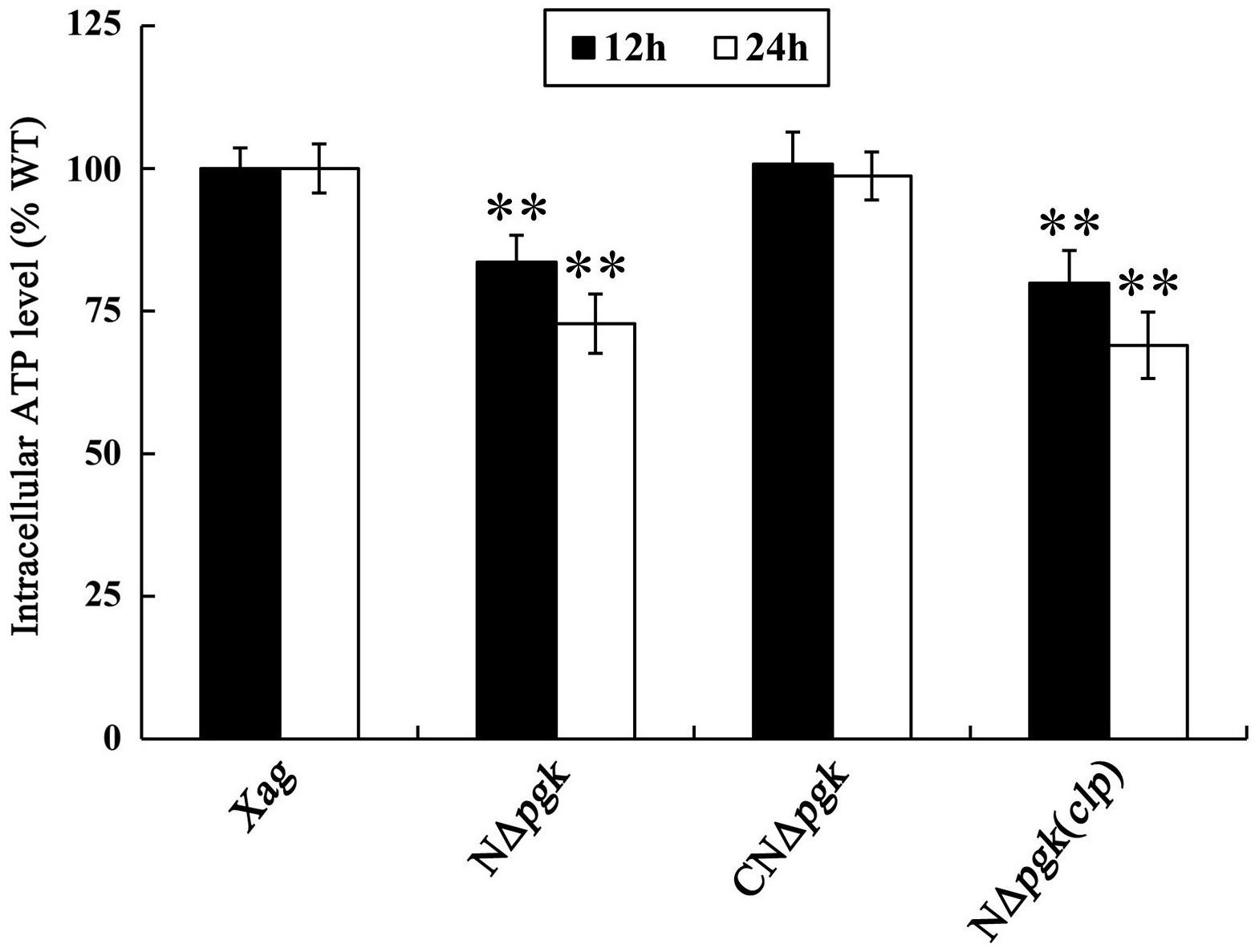
Figure 6. Phosphoglycerate kinase is required for producing intracellular ATP of Xag. Data are means ± SD from three replicates. The asterisks in each horizontal column indicate significant differences at P < 0.01.
We determined whether the mRNA level of pgk is regulated by the DSF signals, low-oxygen signals, and the HrpG/HrpX cascade in Xag. Our results showed that the expression of pgk in the deletion mutants of rpfG, rpfS, rpfR, ravS, and ravR was significantly lower than that in the wild-type, but the deletion mutants of rpfF, rpfB, rpfC, and clp had similar expression levels as the wild-type strain, implying that the expression of pgk is not regulated by DSF signals or the global regulator Clp in Xag (Figure 7A). Compared with the wild-type, the expression of pgk was reduced in the deletion mutants of hrpX, hrpG, trh, xopL, zur, and rsmA, indicating that pgk is positively regulated by the HrpG/HrpX cascade (Figure 7B).
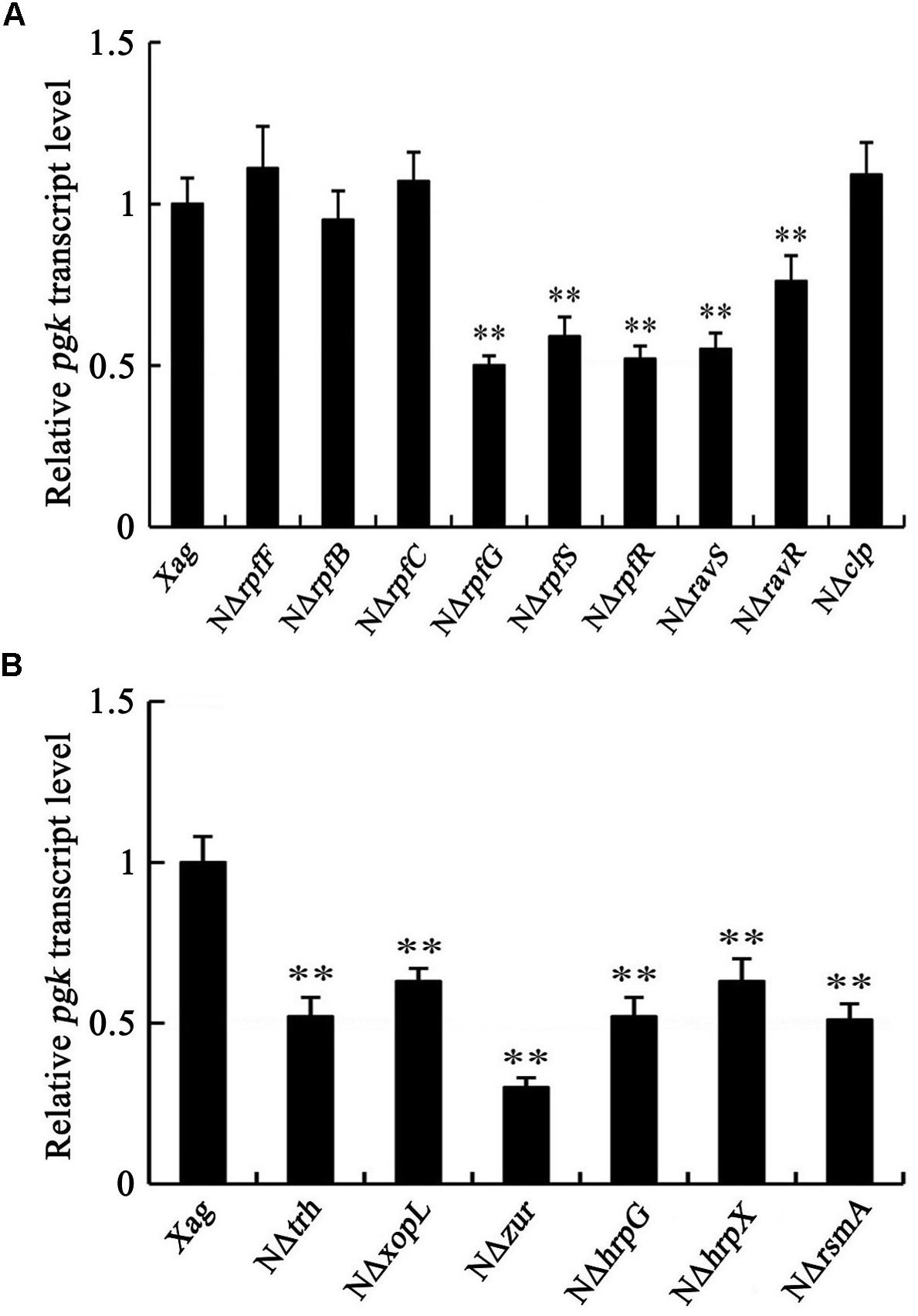
Figure 7. pgk is positively regulated by the HrpG/HrpX cascade (B), but not by DSF signals or Clp (A). Data are means ± SD from three replicates. The asterisks in each horizontal column indicate significant differences at P < 0.01.
Because pgk was positively regulated by Zur and RsmA, which are involved in regulating detoxification and exoenzymes production in Xanthomonas spp. (Büttner and Bonas, 2010), we examined the effect of Pgk in the exoenzymes production and in H2O2 resistance in Xag. We found that the deletion of pgk in Xag did not affect the production of exoenzymes, such as protease, α-amylase, carboxymethylcellulase, and endo-β-mannanase (Supplementary Figure S4). We have previously demonstrated that either deletion or over-expression of clp reduces the protease activity, and Clp positively regulates synthesis of α-amylase, endo-β-mannanase, and carboxymethylcellulase in Xag (Guo et al., 2019). Therefore, the halos produced by NΔpgk with by-path complementation clp looks smaller in protease assay and larger in α-amylase, carboxymethylcellulase, and endo-β-mannanase assays when compared with NΔpgk (Supplementary Figure S4). In addition, the deletion of pgk in Xag did not render more sensitive to H2O2 (Supplementary Figure S5). These results indicate that Pgk is not involved in the production of exoenzymes or in H2O2 resistance in Xag.
We determined whether Pgk plays a vital role in the virulence of Xag in soybean. Our findings indicated that NΔpgk exhibited significantly reduced virulence in soybean relative to the wild-type (Figure 8A), and CNΔpgk had similar virulence as the wild-type. Furthermore, NΔpgk(clp) showed increased virulence compared with NΔpgk, but less virulence compared with CNΔpgk. The T3SS deletion mutant NΔhrcC completely lost virulence on soybean (Figure 8A).
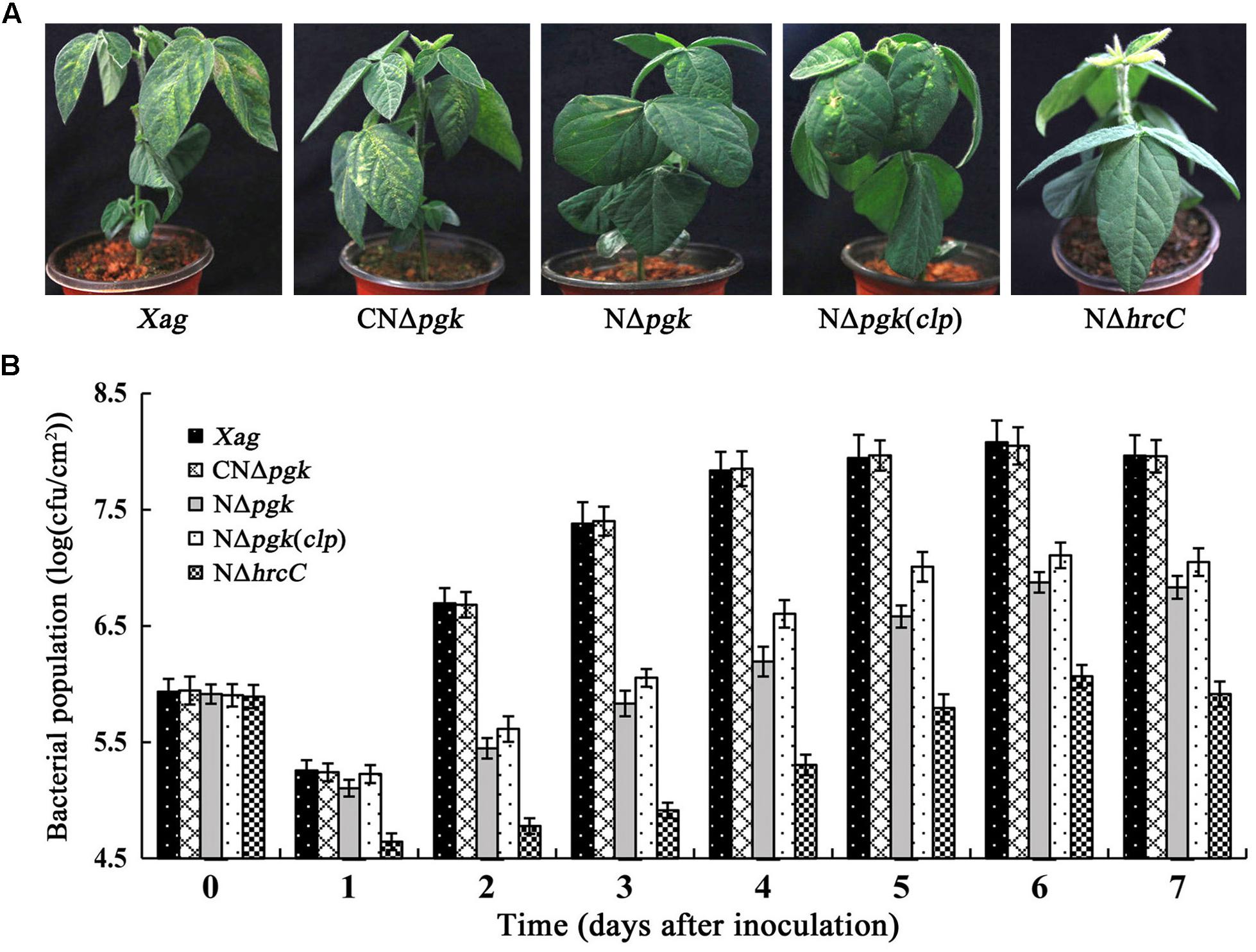
Figure 8. Phosphoglycerate kinase is essential for the growth and virulence of Xag in soybean. (A) Bacterial pustules caused by Xag strains on inoculated leaves of soybean seedlings. Photographs were taken 15 days post-inoculation. (B) Bacterial growth in inoculated leaves of soybean. Bacteria were recovered from the inoculated leaves every day for 7 days post-inoculation and homogenized in sterile water. The homogenates were diluted and plated on NY plates with appropriate antibiotics. Bacterial CFU was counted after incubation at 28°C for 3 days. Data are means ± SD from three repeats.
To determine whether the impaired virulence of NΔpgk is associated with decreased bacterial growth, we explored the growth of bacterial cells infiltrated into soybean leaves. The colonies of NΔpgk were significantly fewer than that of the wild-type strain at each of time points (P < 0.01). The growth of NΔpgk could be fully restored by pgk in trans, whereas NΔhrcC did not show increased growth in inoculated soybean tissue (Figure 8B). In addition, NΔpgk(clp) showed increased growth in soybean leaves compared with NΔpgk, but was less than that of CNΔpgk (Figure 8B). Taken together, these results indicate that pgk is required for virulence and growth of Xag, and by-path complementation of clp is beneficial to the virulence of NΔpgk in host soybean.
In this study, we found that Pgk plays an important role in the catabolism of glucose and other simple sugars in Xag. NΔpgk showed impaired growth when glucose, galactose, fructose, mannose, or sucrose was the sole carbon source (Figure 2). However, NΔpgk still showed certain level of growth in medium with these individual sugars as the sole carbon source, suggesting that Xag may employ ED and PPP, but not glycolysis, to utilize these sugars. This is consistent with the findings from previous studies conducted in the parenchyma pathogen Xoc (Guo et al., 2015), as well as the vascular pathogens X. campestris pv. campestris (Xcc) (Lu et al., 2009) and X. oryzae pv. oryzae (Xoo) (Kim et al., 2010), which lack phosphofructokinase activity required for glycolysis. In addition, NΔpgk shows no growth in medium with pyruvate as the sole carbon source (Figure 2), implying that inactivation of Pgk probably results in a severing of the gluconeogenic pathway. Similarly, this has also been observed in Xoc (Guo et al., 2017) and Xcc (Tang et al., 2005), in which the functional gluconeogenic pathway is necessary for the acquisition of pyruvate. Therefore, we deduce that Xag, probably similar to Xoc and Xcc, possesses an identical carbon metabolic pathway, using ED in conjunction with TCA to catabolize glucose. Interestingly, introduction of the recombinant plasmid pCclp into NΔpgk results in further impairment of the utilization of carbohydrates. This is consistent with results from a previous study on the overexpression of clp in Xag, which results in reduced utilizations of carbohydrates (Guo et al., 2019). Therefore, we speculate that overexpression or by-path complementation of clp may directly or indirectly inhibit the activity of a key metabolic enzyme from carbon metabolic pathways, leading to the limited acquisition of carbohydrates in Xag.
Our data showed that deletion of pgk in Xag resulted in significantly reduced EPS production (Figures 4A,B). This was further supported by the qRT-PCR results that showed that the expression of gum genes was significantly reduced in NΔpgk (Figure 4C). We also found that a mutation in pgk in Xag resulted in significantly reduced cell motility (Figure 5), which is similar to pgi (encoding glucose-6-phosphate isomerase) and zwf (encoding glucose-6-phosphate dehydrogenase) of Xoc (Guo et al., 2015, 2017). However, the decreased motility was not correlated with the expression of fliA, flhF, rpoN2, fleN, and fleQ (Figure 5C), which are required for cell motility (Tian et al., 2015). Pgk is involved in the reversible conversions between glycerate-1.3-2P and glycerate-3P in carbon metabolic pathways. Thus it is indicated that Pgk has a dual function in energy generation or consumption. Pgk is the producer of ATP in glycolytic pathway, but it is an ATP consumer in gluconeogenic pathway. In order to explore the possible reasons for the decrease of EPS synthesis and cell motility in NΔpgk, we investigated the intracellular ATP level, which provides energy to drive these activities (Chao et al., 2008). We found that mutation in pgk led to a reduced intracellular ATP level (Figure 6), which may result from the reduced acquisition of carbohydrates. Thus, we speculate that the reduced intracellular ATP level in NΔpgk may be one of the major causes that negatively affect EPS production and cell motility in this mutant strain.
Previous studies have shown that Clp directly or indirectly regulates diverse biological processes, including EPS production, cell motility, the TCA cycle, and the synthesis of extracellular enzymes (He et al., 2007; Guan et al., 2009). Recently, we also found that Clp functions as both an activator and/or a repressor in multiple biological processes, such as EPS production, cell motility, carbohydrate utilization, and extracellular enzyme activities (Guo et al., 2019). Thus, we further investigated the association between Pgk and Clp in these biological processes. However, by-path complementation of clp did not restore of the impaired properties caused by NΔpgk. Although pgk contains a putative Clp binding motif AGGCA-N6-TCACA in the promoter region (He et al., 2007), the transcriptional levels of pgk in the mutants of rpfF, and clp are equivalent to that in the wild-type (Figure 7A), indicating that pgk is not regulated by DSF signals/Clp regulon in Xag. Thus, we speculate that Pgk and Clp likely participate in diverse biological processes, such as carbohydrate utilization, cell motility, and EPS production, via different pathways.
A previous study has shown that the genes encoding catalytic enzymes in carbohydrate metabolic pathways could be regulated by the Hrp system in Xoc (Guo et al., 2017). Here, we found that pgk is also positively regulated by the HrpG/HrpX cascade and its upstream regulators RsmA and Zur (Figure 7B). Previous studies have reported that RsmA regulated EPS biosynthesis and cell motility, and Zur regulated EPS biosynthesis in Xanthomonas spp. (Chao et al., 2008; Huang et al., 2009; Zhu et al., 2011). Further studies should be conducted to investigate whether RsmA and Zur regulate EPS biosynthesis and cell motility partially through Pgk, or if both RsmA and Zur share the same signaling pathway as Pgk.
Our results showed that Pgk is essential for maintaining both virulence and growth of Xag in soybean (Figure 8). Attenuation in the virulence and growth of NΔpgk in host plant may have resulted, at least partially, from the combined effects of the compromised utilization of carbohydrates, the impaired production of EPS, and limited cell motility (Huang et al., 2009; Lu et al., 2009; Guo et al., 2019). Nutrient utilization is indispensable for pathogen to grow and colonize within host cells (Mellgren et al., 2009). Therefore, the reduced ability of NΔpgk to utilize carbohydrates in soybean reduces its growth and aggressiveness. In addition, previous studies have suggested that carbohydrates could induce metabolic changes, the secretion of extracellular enzymes, and the expression of virulence-related genes (Watt et al., 2009), which are crucial for the virulence of Xanthomonas spp. EPS can enhance susceptibility of host by repressing defense responses such as callose deposition (Yun et al., 2006), mask bacterium to prevent recognition by host (Lu et al., 2009), and contribute to biofilm formation (Dow et al., 2003), which play an important role during pathogen infection in Xanthomonas spp. Cell motility allows bacteria to obtain sufficiently nutritional sources, avoid unfavorable environments and disperse effectively, and seems to be required for the pathogenicity of the parenchyma pathogen Xanthomonas spp. (Malamud et al., 2011). Interestingly, by-path complementation of clp contributes to the virulence of NΔpgk to certain extent. The most likely reason is that significantly increased extracellular enzyme activity probably promotes the infection and colonization of pathogen in host soybean. Plant cell walls act as the first barrier of defense against bacterial invasion. Cell wall-degrading enzymes may facilitate pathogen invasion into host cells by digesting cell walls, thus contribute to disease development (Tayi et al., 2016).
The results from this study, together with the results from our previous study (Guo et al., 2019), advance our understanding of the biological properties of Pgk, an enzyme which reversibly catalyzes the conversions between glycerate-1.3-2P and glycerate-3P, and enable us to propose a working model to depict the functional roles of Pgk and Clp, and understand how their gene expression is regulated in Xag. In this model (Figure 9), both pgk and clp are positively regulated by the HrpG/HrpX cascade, which transduces environmental signals. In addition, clp is also positively regulated by DSF signals. However, pgk is not regulated by either DSF signals or Clp. Even though Clp and Pgk contribute positively to carbohydrate utilization, EPS biosynthesis, and cell motility, they are not functionally interconnected. It is likely that Pgk contributes to carbohydrate utilization, EPS biosynthesis, and cell mobility through generating metabolic carbon products and/or altering the intracellular ATP levels either directly or indirectly by its enzymatic activities. It seems that the function of Pgk in these processes is totally independent of Clp, the global regulator in Xanthomonas spp.
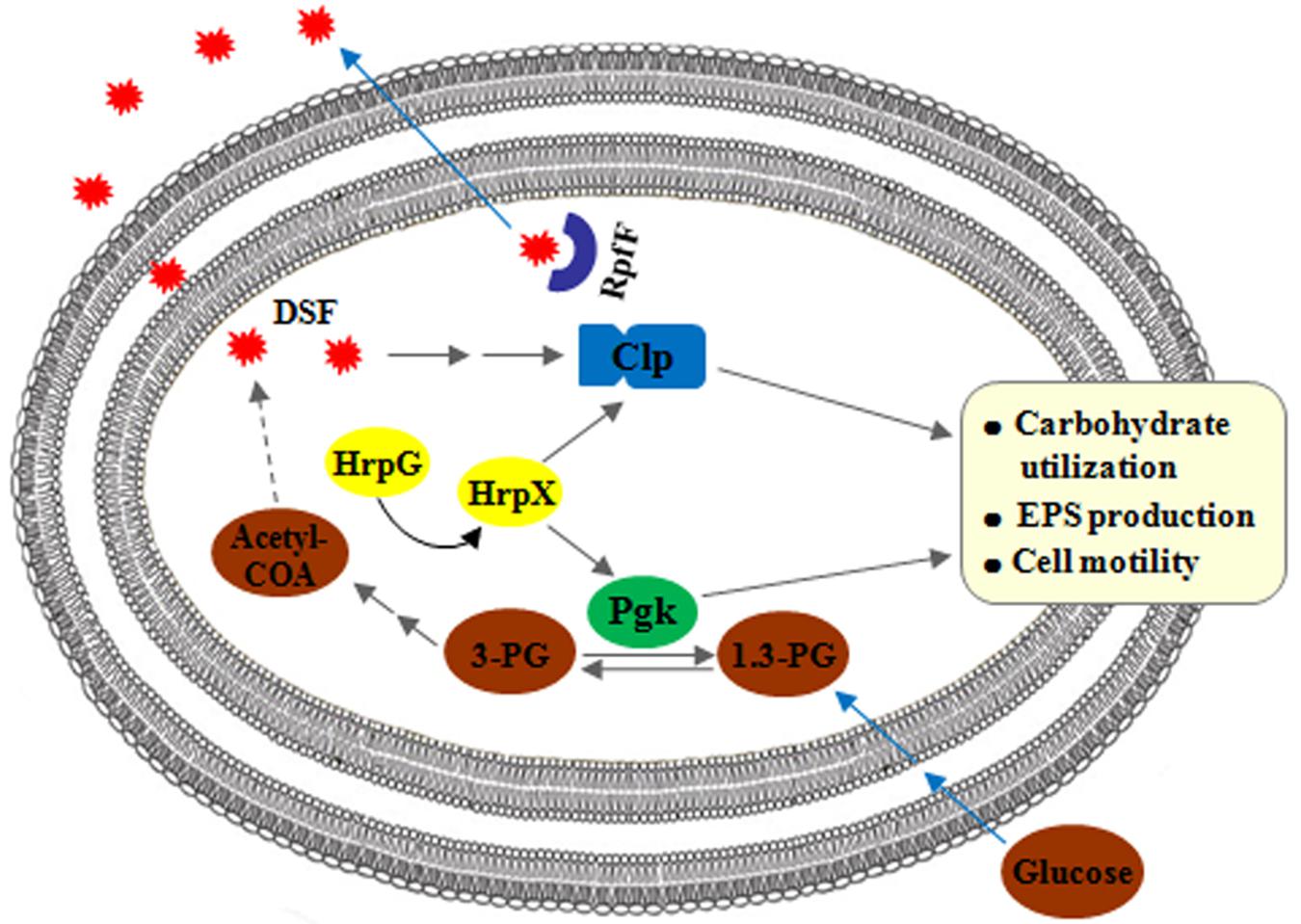
Figure 9. Working model to depict the association between Pgk and Clp in biological processes in Xag. Both pgk and clp, positively regulated by the HrpG/HrpX cascade, are involved in carbohydrate utilization, EPS biosynthesis, and cell motility of Xag. However, pgk is not regulated by either DSF signals or Clp, and is involved in the above biological properties independent of Clp.
All datasets generated for this study are included in the article/Supplementary Material.
WG, G-YC, and J-ZL conceived the study. WG, JG, H-JW, R-YS, C-YS, and S-HG performed the experiments. WG and J-ZL analyzed the data. WG wrote the manuscript. J-ZL and G-YC revised the manuscript.
This work was supported by the Zhejiang Provincial Natural Science Foundation of China (LY18C140004), the National Natural Science Foundation of China (31301633 and 31571423), and the National Innovation and Entrepreneurship Training Program for College Students (201910345031).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are very grateful to MogoEdit for critical editing of the manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2020.00091/full#supplementary-material
An, S. Q., Allan, J. H., McCarthy, Y., Febrer, M., Dow, J. M., and Ryan, R. P. (2014). The PAS domain-containing histidine kinase RpfS is a second sensor for the diffusible signal factor of Xanthomonas campestris. Mol. Microbiol. 92, 586–597. doi: 10.1111/mmi.12577
Andrade, M. O., Farah, C. S., and Wang, N. (2014). The post-transcriptional regulator rsmA/csrA activates T3SS by stabilizing the 5′ UTR of hrpG, the master regulator of hrp/hrc genes, in Xanthomonas. PLoS Pathog. 10:e1003945. doi: 10.1371/journal.ppat.1003945
Athinuwat, D., Prathuangwong, S., Cursino, L., and Burr, T. (2009). Xanthomonas axonopodis pv. glycines soybean cultivar virulence specificity is determined by avrBs3 homolog avrXg1. Phytopathology 99, 996–1004. doi: 10.1094/PHYTO-99-8-0996
Büttner, D., and Bonas, U. (2010). Regulation and secretion of Xanthomonas virulence factors. FEMS Microbiol. Rev. 34, 107–133. doi: 10.1111/j.1574-6976.2009.00192.x
Cai, Z., Yuan, Z. H., Zhang, H., Pan, Y., Wu, Y., Tian, X. Q., et al. (2017). Fatty acid DSF binds and allosterically activates histidine kinase RpfC of phytopathogenic bacterium Xanthomonas campestris pv. campestris to regulate quorum-sensing and virulence. PLoS Pathog. 13:e1006304. doi: 10.1371/journal.ppat.1006304
Chao, N. X., Wei, K., Chen, Q., Meng, Q. L., Tang, D. J., He, Y. Q., et al. (2008). The rsmA-like gene rsmAXcc of Xanthomonas campestris pv. campestris is involved in the control of various cellular processes, including pathogenesis. Mol. Plant Microbe Interact. 21, 411–423. doi: 10.1094/MPMI-21-4-0411
Chatnaparat, T., Prathuangwong, S., Ionescu, M., and Lindow, S. E. (2012). XagR, a LuxR homolog, contributes to the virulence of Xanthomonas axonopodis pv. glycines to soybean. Mol. Plant Microbe Interact. 25, 1104–1117. doi: 10.1094/MPMI-01-12-0008-R
Dow, J. M., Crossman, L., Findlay, K., He, Y. Q., Feng, J. X., and Tang, J. L. (2003). Biofilm dispersal in Xanthomonas campestris is controlled by cell-cell signaling and is required for full virulence to plants. Proc. Natl. Acad. Sci. U.S.A. 100, 10995–11000. doi: 10.1073/pnas.1833360100
Guan, W. J., Wu, M. S., and He, C. Y. (2009). Molecular identification and functional analysis of Clpxoo, a homologue to the nucleotide receptor protein in Xanthomonas oryzae pv. oryzae. Wei Sheng Wu Xue Bao 49, 32–37. doi: 10.13343/j.cnki.wsxb.2009.01.013
Guo, W., Gao, J., Chen, Q. S., Ma, B. J., Fang, Y., Liu, X., et al. (2019). Crp-like protein plays both positive and negative roles in regulating the pathogenicity of bacterial pustule pathogen Xanthomonas axonopodis pv. glycines. Phytopathology 109, 1171–1183. doi: 10.1094/PHYTO-07-18-0225-R
Guo, W., Zou, L. F., Cai, L. L., and Chen, G. Y. (2015). Glucose-6-phosphate dehydrogenase is required for extracellular polysaccharide production, cell motility and the full virulence of Xanthomonas oryzae pv. oryzicola. Microb. Pathog. 78, 87–94. doi: 10.1016/j.micpath.2014.11.007
Guo, W., Zou, L. F., Ji, Z. Y., Cai, L. L., and Chen, G. Y. (2017). Glucose 6-phosphate isomerase (Pgi) is required for extracellular polysaccharide biosynthesis, DSF signals production and full virulence of Xanthomonas oryzae pv. oryzicola in rice. Physiol. Mol. Plant 100, 209–219. doi: 10.1016/j.pmpp.2017.10.010
He, Y. W., Boon, C., Zhou, L., and Zhang, L. H. (2009). Co-regulation of Xanthomonas campestris virulence by quorum sensing and a novel two-component regulatory system RavS/RavR. Mol. Microbiol. 71, 1464–1476. doi: 10.1111/j.1365-2958.2009.06617.x
He, Y. W., Ng, A. Y., Xu, M., Lin, K., Wang, L. H., Dong, Y. H., et al. (2007). Xanthomonas campestris cell-cell communication involves a putative nucleotide receptor protein Clp and a hierarchical signalling network. Mol. Microbiol. 64, 281–292. doi: 10.1111/j.1365-2958.2007.05670.x
Huang, D. L., Tang, D. J., Liao, Q., Li, X. Q., He, Y. Q., Feng, J. X., et al. (2009). The Zur of Xanthomonas campestris is involved in hypersensitive response and positively regulates the expression of the hrp cluster via hrpX but not hrpG. Mol. Plant Microbe Interact. 22, 321–329. doi: 10.1094/MPMI-22-3-0321
Islam, M. R., Kabir, M. S., Hirata, H., Tsuge, S., and Tsuyumu, S. (2009). A leucine-rich protein, LrpX, is a new regulator of hrp genes in Xanthomonas oryzae pv. oryzae. J. Gen. Plant Pathol. 75, 66–71. doi: 10.1007/s10327-008-0124-2
Kim, S. Y., Lee, B. M., and Cho, J. Y. (2010). Relationship between glucose catabolism and xanthan production in Xanthomonas oryzae pv. oryzae. Biotechnol. Lett. 32, 527–531. doi: 10.1007/s10529-009-0193-0
Li, Y. R., Zou, H. S., Che, Y. Z., Cui, Y. P., Guo, W., Zou, L. F., et al. (2011). A novel regulatory role of HrpD6 in regulating hrp-hrc-hpa genes in Xanthomonas oryzae pv. oryzicola. Mol. Plant Microbe Interact. 24, 1086–1101. doi: 10.1094/MPMI-09-10-0205
Liu, L., Ma, W. X., Zou, L. F., Chen, X. B., and Chen, G. Y. (2016). Comparative analysis of type-III secretion system and T3SS-secreted effectors among seven quarantine Xanthomonas pathovars. Acta Phytopathol. Sin. 46, 37–46. doi: 10.13926/j.cnki.apps.2016.01.005
Liu, X., Lu, P. T., Shi, Y. S., Chen, Y. C., Wang, C. N., and Guo, W. (2019). Functional analysis of xanthomonappepsin precursor from soybean pathogen Xanthomonas axonopodis pv. glycines. Acta Phytopathol. Sin. 49, 174–182. doi: 10.13926/j.cnki.apps.000200
Liu, Z. Y., Zou, L. F., Xue, X. B., Cai, L. L., Ma, W. X., Xiong, L., et al. (2014). HrcT is a key component of the type III secretion system in Xanthomonas spp. and also regulates the expression of the key hrp transcriptional activator HrpX. Appl. Environ. Microbiol. 80, 3908–3919. doi: 10.1128/AEM.00308-14
Long, J. Y., Song, K. L., He, X., Zhang, B., Cui, X. F., and Song, C. F. (2018). Mutagenesis of phaR, a regulator gene of polyhydroxyalkanoate biosynthesis of Xanthomonas oryzae pv. oryzae caused pleiotropic phenotype changes. Front. Microbiol. 9:3046. doi: 10.3389/fmicb.2018.03046
Lu, G. T., Xie, J. R., Chen, L., Hu, J. R., An, S. Q., Su, H. Z., et al. (2009). Glyceraldehyde-3-phosphate dehydrogenase of Xanthomonas campestris pv. campestris is required for extracellular polysaccharide production and full virulence. Microbiology 155, 1602–1612. doi: 10.1099/mic.0.023762-0
Malamud, F., Torres, P. S., Roeschlin, R., Rigano, L. A., Enrique, R., Bonomi, H. R., et al. (2011). The Xanthomonas axonopodis pv. citri flagellum is required for mature biofilm and canker development. Microbiology 157, 819–829. doi: 10.1099/mic.0.044255-0
Mellgren, E. M., Kloek, A. P., and Kunkel, B. N. (2009). Mqo, a tricarboxylic acid cycle enzyme, is required for virulence of Pseudomonas syringae pv. tomato strain DC3000 on Arabidopsis thaliana. J. Bacteriol. 191, 3132–3141. doi: 10.1128/JB.01570-08
Tamir-Ariel, D., Navon, N., and Burdman, S. (2007). Identification of genes in Xanthomonas campestris pv. vesicatoria induced during its interaction with tomato. J. Bacteriol. 189, 6359–6371. doi: 10.1128/JB.00320-07
Tang, D. J., He, Y. Q., Feng, J. X., He, B. R., Jiang, B. L., Lu, G. T., et al. (2005). Xanthomonas campestris pv. campestris possesses a single gluconeogenic pathway that is required for virulence. J. Bacteriol. 187, 6231–6237. doi: 10.1128/JB.187.17.6231-6237.2005
Tayi, L., Maku, R., Patel, H. K., and Sonti, R. V. (2016). Action of multiple cell wall-degrading enzymes is required for elicitation of innate immune responses during Xanthomonas oryzae pv. oryzae infection in rice. Mol. Plant Microbe Interact. 29, 599–608. doi: 10.1094/MPMI-02-16-0039-R
Thowthampitak, J., Shaffer, B. T., Prathuangwong, S., and Loper, J. E. (2008). Role of rpfF in virulence and exoenzyme production of Xanthomonas axonopodis pv. glycines, the causal agent of bacterial pustule of soybean. Phytopathology 98, 1252–1260. doi: 10.1094/PHYTO-98-12-1252
Tian, F., Yu, C., Li, H. Y., Wu, X. L., Li, B., Chen, H. M., et al. (2015). Alternative sigma factor RpoN2 is required for flagellar motility and full virulence of Xanthomonas oryzae pv. oryzae. Microbiol. Res. 170, 177–183. doi: 10.1016/j.micres.2014.07.002
Wang, N., Wu, M. S., Tian, F., Chen, H. M., and He, C. Y. (2013). Effects of gene deletion of katExoo encoding putative catalase on resistance to hydrogen peroxide and pathogenicity of Xanthomonas oryzae pv. oryzae. Acta Phytopathol. Sin. 43, 615–621.
Wang, X. Y., Zhou, L., Yang, J., Ji, G. H., and He, Y. W. (2016). The RpfB-dependent quorum sensing signal turnover system is required for adaptation and virulence in rice bacterial blight pathogen Xanthomonas oryzae pv. oryzae. Mol. Plant Microbe Interact. 29, 220–230. doi: 10.1094/MPMI-09-15-0206-R
Watt, T. F., Vucur, M., Baumgarth, B., Watt, S. A., and Niehaus, K. (2009). Low molecular weight plant extract induces metabolic changes and the secretion of extracellular enzymes, but has a negative effect on the expression of the type-III secretion system in Xanthomonas campestris pv. campestris. J. Biotechnol. 140, 59–67. doi: 10.1016/j.jbiotec.2008.12.003
Wengelnik, K., and Bonas, U. (1996). HrpXv, an AraC-type regulator, activates expression of five of the six loci in the hrp cluster of Xanthomonas campestris pv. vesicatoria. J. Bacteriol. 178, 3462–3469. doi: 10.1128/jb.178.12.3462-3469.1996
Wengelnik, K., Van den Ackerveken, G., and Bonas, U. (1996). HrpG, a key hrp regulatory protein of Xanthomonas campestris pv. vesicatoria is homologous to two-component response regulators. Mol. Plant Microbe Interact. 9, 704–712. doi: 10.1094/mpmi-9-0704
Yun, M. H., Torres, P. S., El Oirdi, M., Rigano, L. A., Gonzalez-Lamothe, R., Marano, M. R., et al. (2006). Xanthan induces plant susceptibility by suppressing callose deposition. Plant Physiol. 141, 178–187. doi: 10.1104/pp.105.074542
Zhou, L., Wang, X. Y., and He, Y. W. (2013). DSF signal-dependent quorum sensing in plant pathogenic bacteria Xanthomonas. Sci. Agric. Sin. 46, 2910–2922. doi: 10.3864/j.issn.0578-1752.2013.14.007
Zhou, L., Yu, Y., Chen, X., Diab, A. A., Ruan, L., He, J., et al. (2015). The multiple DSF-family QS signals are synthesized from carbohydrate and branched-chain amino acids via the FAS elongation cycle. Sci. Rep. 5:13294. doi: 10.1038/srep13294
Keywords: Xanthomonas axonopodis pv. glycines, phosphoglycerate kinase, Crp-like protein, carbohydrate utilization, extracellular polysaccharide, cell motility
Citation: Guo W, Gao J, Wang H-J, Su R-Y, Sun C-Y, Gao S-H, Liu J-Z and Chen G-Y (2020) Phosphoglycerate Kinase Is Involved in Carbohydrate Utilization, Extracellular Polysaccharide Biosynthesis, and Cell Motility of Xanthomonas axonopodis pv. glycines Independent of Clp. Front. Microbiol. 11:91. doi: 10.3389/fmicb.2020.00091
Received: 13 November 2019; Accepted: 15 January 2020;
Published: 07 February 2020.
Edited by:
Zuhua He, Center for Excellence in Molecular Plant Sciences (CAS), ChinaReviewed by:
Yong-Qiang He, Guangxi University, ChinaCopyright © 2020 Guo, Gao, Wang, Su, Sun, Gao, Liu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Guo, d2VpZ3VvODE3QHpqbnUuY24=; Jian-Zhong Liu, anpsaXVAempudS5jbg==; Gong-You Chen, Z3lvdWNoZW5Ac2p0dS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.