
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med. , 26 March 2025
Sec. Translational Medicine
Volume 12 - 2025 | https://doi.org/10.3389/fmed.2025.1566080
This article is part of the Research Topic 25 Years of 21st Century Medicine View all articles
Despite the annual rise in patients with end-stage diseases necessitating organ transplantation, the scarcity of high-quality grafts constrains the further development of transplantation. The primary causes of the graft shortage are the scarcity of standard criteria donors, unsatisfactory organ preservation strategies, and mismatching issues. Organ preservation strategies are intimately related to pre-transplant graft viability and the incidence of adverse clinical outcomes. Static cold storage (SCS) is the current standard practice of organ preservation, characterized by its cost-effectiveness, ease of transport, and excellent clinical outcomes. However, cold-induced injury during static cold preservation, toxicity of organ preservation solution components, and post-transplantation reperfusion injury could further exacerbate graft damage. Long-term ex vivo dynamic machine perfusion (MP) preserves grafts in a near-physiological condition, evaluates graft viability, and cures damage to grafts, hence enhancing the usage and survival rates of marginal organs. With the increased use of extended criteria donors (ECD) and advancements in machine perfusion technology, static cold storage is being gradually replaced by machine perfusion. This review encapsulates the latest developments in cryopreservation, subzero non-freezing storage, static cold storage, and machine perfusion. The emphasis is on the injury mechanisms linked to static cold storage and optimization strategies, which may serve as references for the optimization of machine perfusion techniques.
Donation after brain death (DBD), living donation, and extended criteria donors (ECD) constitute the primary sources of donor grafts. Despite the annual rise in patients with end-stage diseases necessitating organ transplantation worldwide, the total number of DBD and living organ donors remains mostly unchanged (1). The scarcity of high-quality donor organs has constrained the development of organ transplantation, making the enhancement of utilization and survival rates of ECD a pressing concern.
In recent decades, the development and application of various organ preservation solutions have established static cold storage (SCS) as the standard preservation practice for donated organs. This organ preservation technique is secure, simple, and easily transportable, while ex vivo dynamic machine perfusion (MP) was previously overlooked. Current SCS techniques are well established. However, SCS techniques and some components in organ preservation solutions may be hazardous (2, 3). Numerous current research studies reported the addition of diverse protective agents into existing organ preservation solutions, with the minimization or substitution of harmful constituents, to enhance the protective effects of organ preservation solutions. The literature mostly concerns a modified variant of the histidine-tryptophan-ketoglutarate (HTK) solution, known as the HTK-N solution. Preclinical evidence indicates that the HTK-N solution outperforms the HTK solution in organ preservation. The effectiveness and safety of HTK-N solution have been confirmed by a pilot randomized controlled clinical phase II trial in living donor transplantation of human kidney preservation, and the outcomes of a prospective, randomized, single-blind, multicenter, phase III study on kidney, liver, and pancreatic transplantation are currently unavailable (4, 5). Despite the fact that existing organ preservation solutions are being gradually improved, the absence of donor organ viability assessment, tissue damage from prolonged hypothermia, limited cold storage duration, and the ischemia–reperfusion injury (IRI) that inevitably occurs during transplantation have constrained the clinical application of ECD grafts (6–8). Contemporary technologies of cryopreservation and subzero non-freezing preservation remain significantly underdeveloped. This has led to a resurgence of interest in MP preservation.
Ex vivo dynamic MP facilitates the evaluation of graft viability and functional repair, hence alleviating IRI and reducing the risk of primary non-function and delayed graft function (DGF) (1, 9). Despite the high cost and technical complexity of MP preservation for transplants, along with the risk of organ waste in the event of preservation failure, this technology creates the conditions for the successful clinical application of ECD grafts. This review describes several preservation strategies, including cryopreservation, subzero non-freezing preservation, SCS, and MP, to enhance the reader’s comprehensive understanding of the diverse preservation alternatives for grafts. Furthermore, we emphasize the constraints of SCS and its related damage mechanisms, which may offer insights into the optimization of MP approaches.
For decades, cryopreservation solutions have been widely utilized to freeze cells in liquid nitrogen or at −80°C refrigerators. To further diminish the toxicity of cryopreservation solutions and the possibility of pathogenic bacterial contamination, an increasing number of cryopreservation solutions are devoid of serum and dimethyl sulfoxide. While cell cryopreservation technology is secure and well-established, research on whole-organ cryopreservation remains in its nascent stages. The cryopreservation of kidneys and hearts has been extensively explored; nevertheless, organ function cannot be reinstated following cryopreservation at temperatures below −45°C (10). The primary causes of cryopreservation failure are the cytotoxic effects of cryoprotective agents (CPAs) and the formation of sharp ice crystals, which result in tissue matrix fragmentation and endothelial damage, leading to microvascular dysfunction, significant metabolic disturbances, and acute immune rejection (11, 12). Recent innovations in the composition of cryopreservation solutions and freezing and rewarming technologies have significantly enhanced the potential for the preservation of human whole organs.
Hydrogel encapsulation provides a non-toxic alternative to conventional cryopreservation (13, 14). Alginate is a non-toxic, biocompatible polymer, and alginate encapsulation is being used for both cryopreservation and non-cryopreservation of higher plants, animals, and human cells (15, 16). The thicknesses of 3% alginate or 5% gelatin-methacryloyl hydrogel encapsulation optimally preserved mouse testicular tissues by inhibiting ice crystal formation, minimizing basement membrane contraction, improving cell morphology, and augmenting mitochondrial activity (14). Vitrification is the rapid cooling of an organ to a stable, ice-free, glassy state, preventing solid ice injury to tissues; however, vitrification necessitates the application of highly concentrated and possibly hazardous CPAs (17–19). High-quality CPAs should possess excellent water solubility, membrane permeability, and little harmful effects at lower temperatures and higher concentrations (20, 21). Using higher concentrations of cryoprotectants, reducing solution volumes to lessen toxicity and osmotic stresses, and implementing innovative technologies like radiofrequency heating or nano-heating to fast and evenly rewarm are all likely to minimize freezing injury further (17, 22, 23). Cryopreservation by vitrification has effectively preserved heart valves, liver and kidney tissue sections, corneas, blood vessels, embryonic tiny structural tissues, and whole organs such as the liver and kidneys. In the cryopreservation of solid organs, the grafts were initially perfused with standard organ preservation solutions (e.g., UW solution) and subsequently stored at low temperatures (approximately 4℃) for preservation. Once the equipment was ready, the organs were connected to a specialized perfusion system, where they were first rinsed with a diluted carrier solution, with careful adjustments made to the pressure or flow rate. After attaining initial osmotic equilibrium, the organ was perfused with a full-strength carrier solution, supplemented with specialized nanoparticles, and subsequently placed in a controlled-rate freezer for vitrification. The vitrification techniques enabled the cryopreservation of rat kidneys for a duration of up to 100 days, with the ability for the gradual restoration of kidney function following nano-heated thawing and allografting (17). The same technique was applied to freeze and revive rat liver, successfully retaining the liver tissue architecture and vascular endothelial cells, enabling the liver to absorb indocyanine green and generate bile during reperfusion (19). The unsatisfactory results of vitrification-based cryopreservation for the heart may be attributed to the loss of myocardial tone caused by uneven freezing and rewarming, which inhibits the myocardium’s ability to pump blood. Inspired by the cryo-tolerance characteristics of the North American wood frog, the intracellular delivery of non-coding RNA not only activates the intrinsic antioxidant system but also enables cells to develop cold tolerance and reduce graft freezing damage (24). This technique optimizes the preservation of vascular endothelial cell integrity in the donor organs and mitigates IRI and immune rejection.
Considering the difficulties and risks linked to cryopreservation protocols, the subzero non-freezing preservation protocol, also known as the supercooling preservation protocol, has been explored concurrently. This technique enables the preservation of organs without freezing at subzero temperatures by employing organ preservation solutions enriched with high concentrations of osmotic cryoprotectants to perfuse and submerge the organs, regulating the height of the gas-liquid interface, while simultaneously monitoring and regulating the preservation temperature in real-time. A newly developed organ preservation system based on isochoric supercooling facilitates real-time monitoring of the preservation process, maintains the stability of the preservation solution, and prevents the formation of ice nuclei at subzero temperatures without the addition of CPAs, thereby enabling the extended preservation of large grafts (25). The subzero non-freezing preservation protocol has been validated in models of heart, liver, and kidney transplantation, extending organ preservation time by a minimum of 48 h (26, 27). Prevalent CPAs presently accessible include glycerol, methanol, ethanol, propanediol, ethylene glycol, butylene glycol, dimethyl sulfoxide, polyethylene glycol, 3-O-methyl-glucose, and antifreeze proteins (12, 26–28). Trehalose is a disaccharide commonly present in plants, animals, and microbes in nature. Rat lungs were preserved using ET-Kyoto solution with trehalose at 4°C and −2°C for 17 h, followed by perfusion with low-oxygenated blood. It was observed that lungs preserved at −2°C exhibited reduced arterial pressure, higher tidal volume and arterial partial pressure of oxygen, decreased endothelial cell damage, and increased intracellular adenosine triphosphate (ATP) levels (28). The superior protective effect may be associated with trehalose in the ET-Kyoto solution. A human liver transplantation model was used to verify the protective effect of the University of Wisconsin (UW) solution with glycerol and trehalose as adjuvants. This approach safely preserves the liver at −4°C without freezing for 33–42 h, retaining its viability after subnormothermic machine perfusion (SNMP) (29). It is important to emphasize that the livers in this experiment were not subjected to in vivo transplantation or later functional analyses. The use of 3-O-methyl-glucose and polyethylene glycol as CPAs prevented intra- and extracellular freezing at −6°C and maintained rat liver viability for 96 h. Following rewarming and homotransplantation, liver survival rates were 100 and 58% for 72 and 96 h of preservation, respectively. However, recipient rats exhibited delayed recovery of consciousness and movement throughout the initial postoperative week, with some experiencing weight loss (30). Porcine kidneys can be securely preserved at −5°C for 120 h with a peptoid-based preservation solution, which has demonstrated superior effectiveness compared to the UW solution for urogenesis, blood flow, and oxygen consumption following hypothermic machine perfusion (HMP) (31). The subzero non-freezing preservation protocol presents a promising alternative for organ preservation; nevertheless, these advantages have yet to be substantiated in large animal models. Moreover, the stability and safety of the subzero non-freezing preservation protocol are suboptimal, as random icing influenced by the volume of supercooled preservation solutions and the freezing characteristics following vibration could hinder the transport of donor organs. The toxicity of CPAs, osmotic stress, and IRI may also adversely impact the organ’s functional recovery.
SCS is now the standard practice for organ preservation, and the development of organ preservation solutions has been crucial in reducing graft injury during cold storage. The preservation process is straightforward, necessitating that the organ be submerged in the same solution at around 4℃ after being perfused with the organ preservation solution. For kidney preservation, Celsior solution, HTK solution, and UW solution are more favorable than Eurocollins solution in terms of the incidence of DGF (31). Currently, the most commonly used lung preservation solution is Perfadex solution, which contains glucose and the colloidal osmotic agent dextran and can prevent cell swelling and endothelial cell damage and reduce the incidence of primary graft dysfunction (32, 33). The selection between UW and HTK solutions for static liver preservation remains controversial. Despite the International Liver Transplantation Society’s disapproval of HTK solution for preserving donation after circulatory death (DCD) livers, Foley et al. utilized a comprehensive national database to compare the two preservation options, revealing that HTK solution exhibited an equivalent protective effect to UW solution in the preservation of DCD livers (34, 35). Given that various organs exhibit distinct tolerances to ischemia and hypothermia and that the composition of organ preservation solutions influences organ viability and clinical outcomes, it is essential to customize preservation strategies for individual organs. Table 1 summarizes the composition of widely used SCS solutions.
Donor organs are underutilized because of geographic limitations on donor-recipient matching and the restricted in vitro preservation duration. The application of organ preservation solutions at around 4°C for perfusion and immersion of grafts could inhibit cell metabolism and sustain organ viability for 4–6 h (36). Donor heart storage longer than 4 h is related to primary graft dysfunction (37). An optimal long-term survival rate was observed in pancreatic grafts with cold ischemia durations under 12 h. The incidence of graft failure increased by 1.2–1.4 times when pancreatic cold ischemia time ranged from 12 to 24 h (38). Previously, a duration of 6 h was considered the maximum permissible cold ischemia time for lung transplantation (39). Nonetheless, extensive distribution of donor lungs frequently requires the utilization of allografts with ischemia times exceeding 6 h. There is a dispute concerning the maximum duration of SCS of the lungs. Some reports indicate that a storage duration exceeding 6 or 8 h does not influence grade 3 primary graft dysfunction within 72 h after transplantation, reintubation, extracorporeal membrane oxygenation post-transplantation, acute rejection within 30 days, duration of hospital stay, and 5-year survival rates (39, 40). Unfortunately, the optimization of various existing organ preservation solutions cannot attain a significant breakthrough in SCS duration due to the inherent limitations of SCS technology. Contemporary biochemical and basic molecular biology techniques have facilitated a deeper comprehension of the injury mechanisms behind SCS (Figure 1).
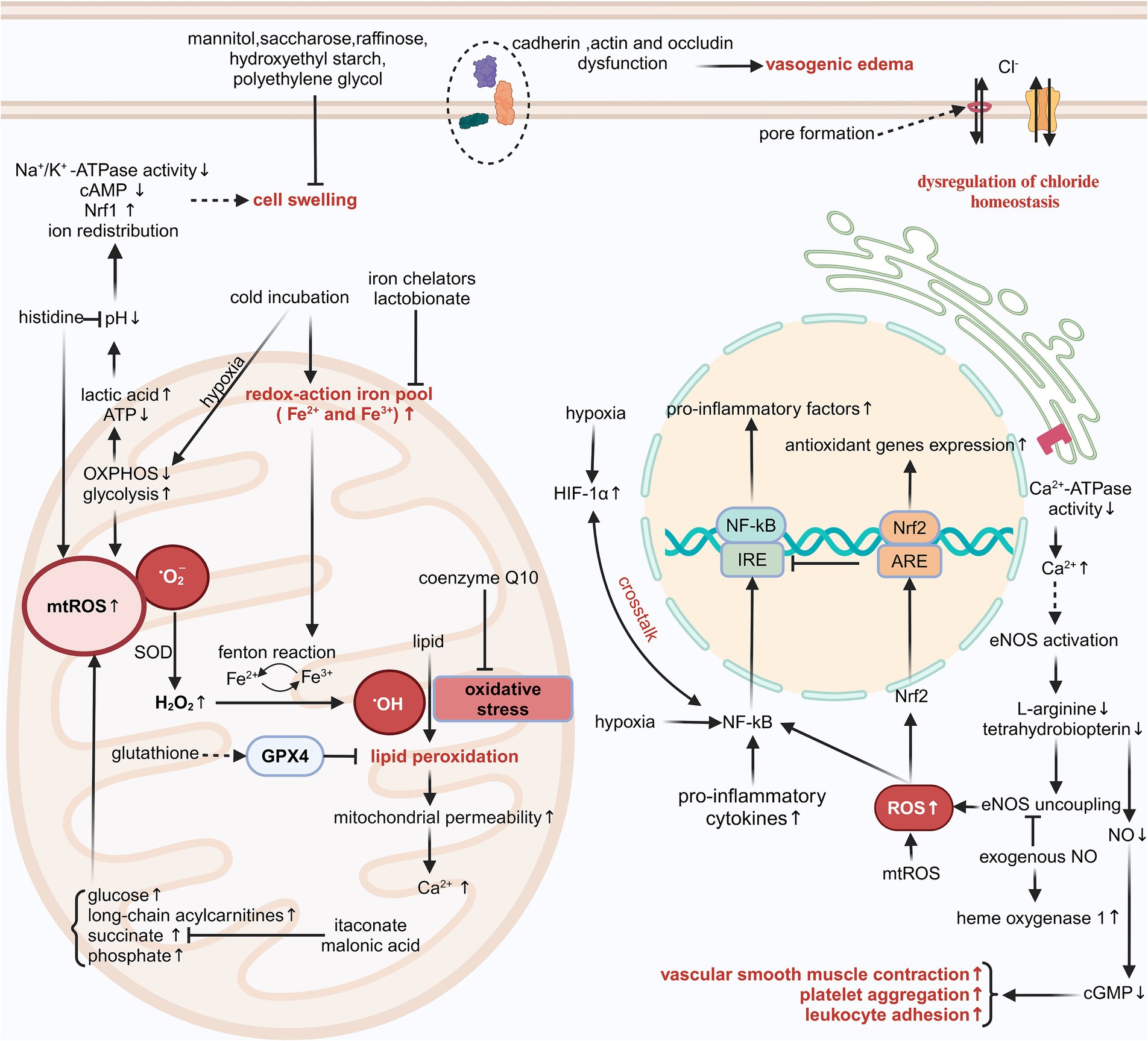
Figure 1. The injury mechanisms associated with SCS. cGMP, cyclic guanosine monophosphate; ARE, antioxidant responsive element; mtROS, mitochondrial ROS; Nrf2, nuclear factor-erythroid 2-related factor 2; cAMP, cyclic adenosine monophosphate; H2O2, hydrogen peroxide. Created in BioRender. Ran, Q. (2025) https://BioRender.com/z95k029, licensed under Academic License.
The SCS of grafts reduces ATP consumption and extends preservation, but the low temperature may induce additional injury (2). The cold-induced injury occurs independently of hypoxia/reoxygenation injury and is linked to increased chelatable iron pool and disruption of chloride (Cl−) homeostasis, ultimately resulting in apoptosis (41–44).
The majority of intracellular iron is tightly linked to proteins, with just a tiny percentage (0.2–3%) constituting the chelatable iron pool (45). The intracellular pool of redox-active iron (Fe2+/Fe3+) increased swiftly following the initiation of cold incubation at 4°C; however, this augmentation was reversible upon rewarming after a brief cold incubation duration (2, 43, 45, 46). Cells incubated with HTK solution, histidine-lactobionate solution, and CE solution at 21°C and 37°C, respectively, still exhibited cytotoxicity. This damage was associated with significant lipid peroxidation, which could be mitigated by the antioxidants trolox, butylated hydroxytoluene, N-acetylcysteine, and membrane-permeable iron chelators such as 2,2′-dipyridyl, 1,10-phenanthroline, LK614 LK616, and deferoxamine. The testing of individual components of different organ preservation solutions, utilizing a modified Krebs–Henseleit buffer, indicated that the cytotoxicity of these solutions originates from histidine and phosphate (47, 48). The two substances are closely linked to the production of iron-dependent reactive oxygen species (ROS).
Oxidative stress refers to the imbalance between ROS and antioxidants within cells. Low-reactivity ROS, such as hydrogen peroxide, can interact with redox-active iron and generate highly reactive species, including iron-oxygen species and hydroxyl radicals, in a process referred to as the Fenton reaction (2). The hydroxyl radical induces various forms of damage, including lipid peroxidation, alterations in mitochondrial permeability, and a loss in mitochondrial membrane potential, particularly observed in hepatocytes and vascular endothelial cells (45, 46). Modified TiProtec solution and HTK-N solution contain the iron chelators desferrioxamine, which is hydrophilic and exhibits poor membrane permeability, and LK614, which is lipophilic and demonstrates good membrane permeability. Additionally, these solutions partially or completely substitute histidine with N-acetyl-L-histidine, effectively inhibiting the generation of hydroxyl radicals (4, 5, 49). Lactobionate in UW solution, CE solution, and Institut Georges Lopez-1 (IGL-1) solution also chelates ferric iron and mitigates oxidative damage during SCS (50). These organ preservation solutions also contain the antioxidant glutathione. Glutathione peroxidase 4 utilizes the tripeptide glutathione as a cofactor and is essential in mitigating lipid peroxidation (51). Coenzyme Q10 also functions as an effective antioxidant; however, its poor water solubility and stability present challenges for adding it into organ preservation solutions as a pharmaceutical agent.
A decrease in serum Cl− concentration will increase mortality risk in individuals with hypertension, heart failure, or myocardial infarction (52, 53). In recent years, there has been growing evidence that intra- and extracellular Cl− concentrations and Cl− channels are intimately related to IRI (54, 55). The decrease in intracellular Cl− concentration can inhibit the formation of the nicotinamide adenine dinucleotide phosphate oxidase complex and promote the combination of vascular endothelial growth factor receptor 2 with protein tyrosine phosphatase 1B, thus preventing the activation of oxidase-mediated signaling of vascular endothelial growth factor receptor 2 and ultimately suppressing angiogenesis (56). In a recent multicenter, double-blind, randomized, controlled trial of DCD kidney transplantation, the administration of a balanced low-Cl− crystalloid solution (Plasma-Lyte 148) in place of a 0.9% normal saline solution for the maintenance of blood volume was associated with a decreased incidence of dialysis treatment within 7 days post-transplantation (57). Consequently, the balanced crystalloid solution is recommended as the gold standard method of intravenous infusion for DCD kidney transplantation.
Maintaining appropriate intra- and extracellular Cl− concentrations is crucial not only during the reperfusion process following transplantation but also during the SCS of grafts. A comparative analysis of cold incubation of hepatocytes utilizing modified Krebs–Henseleit buffer and various common organ preservation solutions indicated that cold-induced iron-nondependent injury stemmed from the higher Cl− concentration in the preservation solutions; however, this damage was relatively mild (43). The incorporation of L-arginine into the HTK solution, the partial substitution of histidine with N-acetyl-L-histidine, and the reduction of Cl− concentration led to a modified HTK fluid. This Cl−-deficient HTK solution improved myocardial systolic and diastolic force post-heart transplantation in rats; nevertheless, this advantageous effect could not be definitively ascribed to Cl− deficiency (58). To clarify the impact of Cl− concentration in preservation solutions on preservation effectiveness, Wu et al. conducted a comparison between a new HTK solution with low Cl− concentration and one with high Cl− concentration in a mouse heart transplantation model. Their findings indicated that the preservation solution with high Cl− concentration significantly decreased myocardial injury and resulted in a higher graft survival rate, but this model cannot differentiate whether the benefits of HTK solution variants with high Cl− concentration are due to positive effects on cardiac cells or endothelial cells (59). Comparable research protocols and outcomes of experiments have also been validated in a rat liver transplantation model, with Cl− toxicity manifesting only when concentrations exceed 40 to 50 mmol/L (43, 60). Consequently, we reach a preliminary conclusion that the extent of iron-nondependent injury associated with SCS has a U-shaped correlation with the Cl− concentration in the storage solution.
The mechanism by which extracellular Cl− concentration induces cell death at low temperatures remains ambiguous, while alterations in intracellular pH and the activation of the early intrinsic apoptosis pathway might relate to this phenomenon. The ATP-gated P2X7 receptor is a plasma membrane receptor that is part of the P2X purinoceptor family. The P2X7 receptor agonist 2′-3′-O-(4-benzoylbenzoyl)-ATP can elicit membrane potential depolarization, pore formation, cell shrinkage, and lactate dehydrogenase release. Replacement of Cl− in the culture medium with gluconate inhibits cell contraction and the release of lactate dehydrogenase mediated by the P2X7 receptor, suggesting that pore formation and extracellular Cl− inflow are crucial in P2X7 receptor-induced apoptosis (61). Apoptosis is differentiated from necrosis by a characteristic volume loss or apoptotic volume decrease, which is attributed to ion redistribution. The UV-C light-induced changes in intracellular Cl− concentration in Jurkat T cells influenced the activation of the mitogen-activated protein kinase signaling pathway at an early stage of the apoptotic signaling cascade. This apoptotic feature could be inhibited by the Cl− channel inhibitor disodium 4-acetamido-4-isothiocyanato-stilbene-2,2-disulfonate (62). The Cl− channels in the cell membrane exhibit a lack of specificity regarding the transport of Cl−. Alongside Cl−, these channels can also transport other anions such as halides, pseudohalides, and bicarbonates, exhibiting a higher transport efficiency than Cl−. Members of the dimorphic chloride intracellular channels (CLICs) family are extensively distributed across various intracellular compartments and exhibit unique characteristics, including a single transmembrane domain and a dimorphic existence as either soluble or membranous forms. They play a significant role in membrane trafficking, cytoskeletal function, apoptosis, cell cycle regulation, and vascular endothelial growth factor-mediated angiogenesis (63). CLIC1, CLIC4, and CLIC5 are abundant in rodent hearts, with CLIC4 localized to the mitochondrial outer membrane and CLIC5 found in the mitochondrial inner membrane (64). An acidic pH facilitates the conversion of CLICs into a membrane-associated conformation, enhancing the exposure of the hydrophobic inter-domain interface. Additionally, they found that purified CLIC5 can function as a fusogen, interacting with membranes to induce fusion, as evidenced by the increased diameter of liposomes and the mixing of lipids and contents between liposomes (65). Despite extensive research over several decades, definitive evidence supporting the function of CLICs as Cl− channels remains absent, hindering a comprehensive understanding of their physiological role.
The coronary circulation resembles the renal circulation and exhibits strong auto-regulation capabilities (66–69). Microvascular resistance is crucial for blood perfusion within a specific microzone (70). Utilizing organ preservation solutions at 4–5°C for the perfusion and immersion of donor organs effectively lowers cell metabolism and prolongs storage duration. However, low-temperature liquid immersion significantly affects blood flow. The contraction of microvessels results in inadequate perfusion and supply of energy precursors in specific regions. Additionally, the buffering capacity against metabolic acidosis is compromised (71). The dysfunction of coronary vasodilatory functions is a significant, independent predictor of cardiac mortality in patients not having coronary artery disease (72). The activation of the sympathetic nervous system induced by cold exposure does not significantly affect blood flow in transplanted denervated human hearts, suggesting impaired vasodilatory functions of coronary arteries (73). Successive warm and cold ischemia also impairs the vasodilation of the DCD kidneys. Unfortunately, the vasodilatory functions cannot be improved by the constituents of the UW solution or IGL-1 solution (74). The rapid function deterioration of vascular smooth muscle cells may be intricately linked to the intimal hyperplasia observed in late graft failure.
Nitric oxide (NO) is a gaseous, lipophilic nitrogen oxide. The diminished NO bioavailability is a prevalent characteristic of endothelial dysfunction in cardiovascular disease. Low quantities of NO exhibit a significant anti-apoptotic effect in healthy organisms and can also mitigate cold-induced injury in isolated grafts (75). During SCS, the NO produced by endothelial nitric oxide synthase (eNOS) decreases with the consumption of L-arginine and tetrahydrobiopterin. Following reperfusion, most of the eNOS is suddenly activated by an influx of extracellular calcium. The deficiency of L-arginine and tetrahydrobiopterin results in increased superoxide generation by eNOS, further exacerbating oxidative stress. This procedure is known as the “uncoupling” of eNOS (76, 77). Prior research indicates that exogenous NO not only inhibits platelet aggregation and leukocyte adhesion and diminishes the inflammatory response, but also activates guanylate cyclase and enhances the synthesis of cyclic guanosine monophosphate, which induces myosin dephosphorylation in vascular smooth muscle and ultimately facilitates vascular smooth muscle relaxation and augments organ perfusion and energy reserves (78–80). Moreover, exogenous NO enhances both systolic and diastolic cardiac performance post-reperfusion, inhibits eNOS uncoupling, and increases the production of the antioxidant enzyme heme oxygenase-1, hence diminishing superoxide generation and apoptosis (75, 81). Some works of literature have reported the direct sources of NO or substrates of NO synthase, including nitroglycerin, S-nitroso human serum albumin, nitrosothiols, and L-arginine, as adjuvants in organ preservation solutions (82–85). These substances exhibit superior protective effects in improving the microvascular function of the liver, kidneys, and heart.
The limited ATP generated by anaerobic metabolism permits the grafts to survive just a brief duration of ischemia. A shortage of cellular ATP inhibits the activity of endoplasmic reticulum calcium-ATPase in renal tubular cells, hepatocytes, and cardiomyocytes, resulting in elevated cytosolic calcium levels. The opening of the mitochondrial membrane permeability transition pore during ischemia may subsequently result in mitochondrial calcium overload (2). Calcium-dependent proteases, including phospholipase A2 and calpain, are rendered inactive in acidic environments. Following reperfusion, the restoration of intra- and extracellular pH, along with mitochondrial calcium overload, activates these proteases, resulting in cell damage (86). The direct administration of ATP disodium into the coronary arteries during percutaneous coronary intervention has demonstrated enhancement of ventricular wall motion function, reduction of no-reflow rates, and mitigation of reperfusion injury (87, 88). Nonetheless, the poor membrane permeability of ATP challenges its direct application as an energy source for donor organs. The research employed adenosine, a metabolite of ATP, as its focal point. Adenosine is presently utilized as a substrate for the synthesis of ATP in UW, IGL-1, and Institut Georges Lopez-2 (IGL-2) solutions (89, 90). Aspartate can undergo transamination to produce oxaloacetate, which functions as a substrate in the tricarboxylic acid cycle and facilitates ATP production. Prior research indicates that a preservation solution supplemented with aspartate and α-ketoglutarate enables renal proximal tubules to generate ATP via anaerobic metabolism and sustain mitochondrial membrane potential, thereby mitigating hypoxia-reoxygenation mitochondrial injury (91). In recent years, modified HTK-N solutions have been developed by supplementing aspartate and α-ketoglutarate (already present in the HTK solution) to provide a tricarboxylic acid cycle substrate. This preservation solution ameliorates the deficiency of energy precursors during the SCS of grafts (82, 86, 92, 93). CE solutions utilize glutamate as a precursor for the synthesis of ATP (94). The addition of adequate energy precursors to organ preservation solutions not only creates a modest quantity of ATP but also moderately slows the accumulation of intermediates from the tricarboxylic acid cycle.
Increased mitochondrial ROS production can frequently be observed in diseases characterized by mitochondrial dysfunction, and an important hallmark of these diseases is reduced or impaired oxidative phosphorylation and enhanced glycolysis enzyme activity, indicating that mitochondrial ROS production is closely linked to glycolysis processes (5, 95). ROS-induced glucose uptake can, in turn, influence ROS generation and elimination. Elevated extracellular glucose concentrations can promote spontaneous glucose auto-oxidation and the synthesis of advanced glycation end products, along with collateral glucose metabolism, which involves hexosamine, protein kinase C, and polyol pathways (96). These processes collaborate to cause increased oxidative stress and damage to cell structures. When the delicate balance between glucose-induced ROS formation and the intrinsic antioxidant system as well as glucose-mediated ROS scavenging is disrupted, intracellular ROS and glucose levels will increase in a vicious cycle, ultimately resulting in cell death. Long-chain acylcarnitines are intermediates of detrimental long-chain fatty acids. In ischemic myocardium, the activity of carnitine palmitoyltransferase 1 is elevated while that of carnitine palmitoyltransferase 2 is diminished, resulting in the progressive accumulation of long-chain acylcarnitines within the intermembrane space of mitochondria. This accumulation causes hyperpolarization of mitochondrial membrane potential, inhibition of oxidative phosphorylation, and excessive generation of ROS (97).
The accumulation of succinate in mitochondria is a prevalent metabolic characteristic of ischemic tissues. The synergistic effect of fumarate overflow resulting from purine nucleotide decomposition and the partial reversal of the malate/aspartate shuttle induces a reversal of succinate dehydrogenase, finally resulting in the accumulation of mitochondrial succinate (98). Following reperfusion, succinate accumulated in the mitochondria is swiftly re-oxidized by succinate dehydrogenase; meanwhile, reverse electron transport through mitochondrial complex I facilitates the rapid and substantial production of ROS. The ROS pulse operates together with a series of damaging events following reperfusion, ultimately resulting in cell death (99). Malonic acid serves as a competitive inhibitor of succinate dehydrogenase. Dimethyl malonate, a prodrug of malonic acid, can mitigate IRI when administered before and during ischemia (99). Itaconate, a metabolite of cis-aconitate that accumulates concurrently with succinate during ischemia, can also function as a competitive inhibitor of succinate dehydrogenase. It serves as a regulator to modulate mitochondrial redox metabolism, inhibiting succinate accumulation and suppressing ROS generation (100–102). Experiments on large animals such as rabbits, dogs, and pigs have demonstrated that lungs preserved at 10°C have less mitochondrial damage and can withstand cold ischemia for a longer time. This protective effect may be due to the fact that lung tissue produces more itaconate at 10°C (100, 101, 103). So far, lungs preserved at 10°C have achieved a total clinical preservation time of up to 24 h without negative effects on organ function or short-term outcomes following transplantation. As a result, the appropriate temperature for donor lung preservation has been re-evaluated, and 10°C may become the standard criteria (104). Interestingly, most HMP is performed at temperatures ranging from 6 to 10°C, and this higher temperature may be another explanation for its superiority compared to SCS (4°C). The immune response gene 1 (IRG1) encodes an enzyme responsible for the production of itaconate. The histone deacetylase inhibitor valproic acid can induce the transcription of IRG1, which in turn promotes the nuclear translocation of nuclear factor erythroid 2-related factor 2 and the synthesis of heme oxygenase-1 as well as superoxide dismutase 1 in mouse cardiomyocytes. This process improves the utilization of itaconate and diminishes the accumulation of succinate during cold storage (37). The IRG1/itaconate pathway similarly activates nuclear factor erythroid 2-related factor 2-mediated antioxidant responses in hepatocytes and attenuates hepatic IRI (102). Targeting metabolic pathways to modulate metabolite concentrations is an attractive therapeutic option during SCS.
Cell damage resulting from hypoxia and edema is both progressive and cumulative, with hypoxia-induced cell swelling inflicting greater damage than either condition independently. The extent of swelling correlates with the duration of SCS and the precise composition of the organ preservation solution selected (105). Under hypothermic hypoxic conditions, cell swelling is attributed to altered intra- and extracellular ion concentrations (driven by the Na+/H+ exchanger, Na+/ symporter, and Na+-K+-2Cl− cotransporter), reduced levels of cyclic adenosine monophosphate, decreased Na+/K+-ATPase activity, and upregulated expression of nuclear respiratory factor 1, ultimately resulting in inadequate organ perfusion (86, 106–108). Pathological pore formation in cell membranes under hypoxic conditions can also facilitate the permeation of Na+ and Cl−, resulting in cell swelling, and this process can be prevented by glycine and alanine in HTK-N solution (3, 61). Moreover, the disruption of endothelial cell occludin, VE-cadherin, and F-actin results in increased capillary permeability, which subsequently promotes plasma leakage following reperfusion and induces vasogenic edema (106, 109). This implies that alterations in tight junctions and adherens junctions may be the fundamental cause of vasogenic edema linked to SCS. Injury to vascular endothelial cells predisposes the graft to acute rejection, resulting in primary graft dysfunction.
Maintaining moderate crystalloid or colloid osmotic pressure of organ preservation solutions helps to reduce microcirculatory disturbances. Mannitol has been mixed into HTK, CE, IGL-1, and IGL-2 solutions to alleviate hypoxia-induced cell swelling; nonetheless, mannitol can permeate the hepatocyte membrane, resulting in hepatocyte edema (110). Due to the inability of sucrose molecules to traverse cell membranes, the modified HTK-N solution utilizes sucrose in place of mannitol within the HTK solution (111). UW solutions utilize the relatively high molecular weight of anions, raffinose, lactobionate, and hydroxyethyl starch to alleviate SCS-induced cell swelling, demonstrating superior efficacy in reducing cardiac and pancreatic endothelial cell injury. However, certain studies indicate that high-viscosity hydroxyethyl starch could trigger acute kidney injury and elevate mortality risk (50, 112, 113). Although sucrose and raffinose cause cell shrinkage, moderate cell shrinkage seemingly does not impact cell function as well as cell membrane integrity (110). Low potassium dextran glucose solution uses dextran to sustain osmotic pressure in the solution and demonstrates superiority in lung preservation (114). Polyethylene glycol 35 is a non-immunogenic, non-toxic, water-soluble polyether compound that improves the activity of the vasodilator NO and the mitochondrial enzyme aldehyde dehydrogenase 2, regulates immunogenicity and ameliorates microcirculatory abnormalities following SCS and reperfusion (115, 116). It serves as a colloidal osmotic agent for IGL-1 and IGL-2 solutions, presenting superior protection for fatty liver grafts during SCS and oxygenated hypothermic machine perfusion (HMPox) (90). Clarysse et al. have demonstrated that polyethylene glycol 35 can stabilize the endothelial glycocalyx in a rat intestinal IRI model, consequently restricting reperfusion-mediated cell swelling, bacterial translocation, and inflammatory responses (117). Hyperbranched polyglycerol, a compact branched polymer characterized by low viscosity, is a promising colloid for the preservation of donor organs. In a mouse cardiac allograft transplantation model, hyperbranched polyglycerol decreased neutrophil infiltration and markedly improved cardiac function and tissue integrity (118).
Hypoxia is believed to be the fundamental cause of inflammation and immunological response (119). Hypoxia can also interact with the inflammatory response, causing further deterioration of organ function and, in particular, promoting the advancement of inflammatory diseases (120). A common hallmark of perioperative organ failure in surgical patients is a hypoxia-induced inflammatory response, suggesting that hypoxia itself is an inflammatory stimulation (121, 122). Hypoxia and inflammation are related to the activation of the hypoxia-inducible factor (HIF) and the nuclear factor κB (NF-κB) transcription factor family, respectively. HIF and NF-κB co-regulate the effector functions of immune cells and increase susceptibility to hypoxia (123, 124). HIF is crucial for the activation and increased phagocytosis of macrophages and neutrophils, while NF-kB is involved in the proliferation of several immune cells, including T cells, B cells, and dendritic cells (125). The expression of the endotoxin receptor Toll-like receptor 4 and the conduction of NF-κB-mediated inflammatory signaling during kidney transplantation increased progressively with the prolongation of SCS. Research utilizing Toll-like receptor 4 knockout mice observed diminished renal IRI, implying that prolonged ischemia and hypoxia of donor organs amplify inflammatory responses through the Toll-like receptor 4 signaling pathway (126). Active inflammation also causes tissue hypoxia, which results from the concentration of inflammatory cells (including neutrophils, eosinophils, and monocytes), higher rates of cellular oxidative metabolism, and increased activity of various oxygen-consuming enzymes (119, 121).
There is extensive crosstalk between the two molecular signaling pathways, HIF and NF-κB, as they share a number of activating stimulus sources, modulators, and targets (120, 125). Hypoxia has been demonstrated to stimulate the co-activation of the HIF and NF-κB signaling pathways in a manner dependent on IκB kinase and transforming growth factor β-activated kinase 1 (127, 128). Elevated NF-κB activity stimulated by inflammatory cytokines promotes the transcription of messenger RNA of HIF-1α, and the negative feedback of HIF-1α can also regulate NF-κB transcriptional activity under inflammatory conditions (129, 130). Signaling mediators, including S-2-hydroxyglutarate, hydrogen sulfide, and ROS, activated by tissue inflammation, also play a role in the regulation of HIF activity in immune cells (131). There is also reciprocal feedback between oxidative stress and the inflammatory response, which leads to further damage of grafts. ROS are powerful inflammatory initiators that promote the synthesis and release of many pro-inflammatory mediators, including prostaglandin E2, leukotriene B4, tumor necrosis factor-α, interleukin-1β (IL-1β), and IL-6 (132, 133). The metabolite itaconate, which accumulates during SCS, not only inhibits succinate accumulation but also exhibits anti-inflammatory properties. The knockdown of IRG1 intensified injury to grafts and systemic inflammatory responses, while the metabolic reprogramming of itaconate by upregulating the expression of IRG1, which encodes the enzyme responsible for itaconate production, improved cardiac and hepatic function after extended SCS (37, 38, 102). Targeting immunometabolic pathways could be a promising method for improving graft outcomes.
Prolonged ischemia results in irreversible organ damage; however, tissue damage persists and exacerbates following reperfusion. This phenomenon is known as IRI (134). Prior research indicates that IRI encompasses multiple pathological mechanisms, including apoptosis, necrosis, ferroptosis, calcium overload, oxidative stress, no-reflow phenomenon, inflammatory response, protease activation, subcellular remodeling, cell hypertrophy, extracellular matrix remodeling, and fibrosis, among others (51, 135–137). The mechanisms of IRI partially overlap with those previously discussed and will not be described in detail here. It is crucial to stress that the ischemia typically described in these publications is warm ischemia. The cascade pathways of injury associated with prolonged cold ischemia of grafts partially differ from those of warm ischemia; for instance, in porcine DCD kidneys, warm ischemia preferentially activates caspase-1, while cold ischemia significantly elevates caspase-3 activity and triggers tubular apoptosis (219). Multiple studies have documented the application of caspase inhibitors aimed at the apoptotic pathway as a prospective strategy to enhance graft performance. While caspase inhibition mitigated the severity of post-transplantation injury, it failed to avert cellular damage entirely (220–222). This indicates a simultaneous activation of alternative damage pathways. In recent years, controlled reperfusion approaches, also known as ischemic postconditioning, which regulate blood pressure, acid-base balance, oxygen saturation, and ion concentrations during reperfusion, continue to be investigated to alleviate reperfusion injury (223). However, each variable section in the ischemia-reperfusion process further complicates these damage mechanisms. During hibernation, animals decrease their basal metabolic rate, and comprehending the mechanisms of organ protection during this state may elucidate the pathophysiology of DGF. However, animal organs do not experience true ischemia during hibernation, which more accurately parallels the HMPox protocol (224, 225). Additional advancements in the protective benefits of SCS seem challenging to attain. Ischemia and reperfusion are two consecutive processes, and IRI inevitably occurs when grafts are preserved by SCS, thus making dynamic MP an attractive field of research.
The requirement to improve post-transplantation survival of ECD (which includes DCD donors) grafts and recent advances in MP technology have resulted in the development of clinically effective ex vivo MP devices for liver, heart, lung, and kidney grafts that maintain organ viability for up to 24 h. MP entails linking the organ’s own blood vessels to a perfusion system that continuously supplies the isolated organ with a perfusate containing oxygen, nutrients, and therapeutic agents during the preservation and transportation phase. Long-term ex vivo dynamic MP not only maintains microvascular tone, enhances aerobic metabolism, and excretes toxic metabolites, but also repairs ECD grafts and evaluates graft viability (8, 9). The MP also aids in diminishing the occurrence of DGF, even when utilized to preserve grafts from standard criteria donors (138). Currently, MP technology encompasses multiple aspects like perfusion temperature, oxygen saturation, selection of perfusion solution, continuous versus pulsatile perfusion, duration of perfusion, evaluation of organ viability, and organ repair. Owing to the varied types of grafts and their functional conditions, a standardized MP technique is presently unavailable.
The efficiency of cell energy metabolism correlates directly with ambient temperature. Based on the perfusion temperature, the MP strategies are categorized into HMP, SNMP, controlled oxygenation rewarming, and normothermic machine perfusion (NMP). The nonoxygenated HMP (0–8°C) protocol initially perfuses the graft directly using an existing organ preservation solution. This approach enables the organ to achieve a lower metabolism, sustain intra- and extracellular pH levels, and mitigate cell swelling (1). The advantages and disadvantages of HMP protocols and SCS protocols for the preservation of DCD kidneys have been controversial. The latest data from six organ procurement facilities in the United States indicate that the SCS protocol is less effective than the nonoxygenated HMP protocol in reducing the DGF of DBD kidneys (139). Oxygenation of the perfusate promotes intracellular ATP synthesis and delays ischemic injury, which is known as the HMPox protocol (1, 140, 141). Multicenter clinical controlled trials have demonstrated that HMPox-preserved livers have a lower risk of primary nonfunction and nonanastomotic biliary strictures compared to conventional SCS protocols (142, 143). The SNMP protocol (12–34°C) is currently in an awkward situation. Although this protocol not only reduces cell energy metabolism but also allows for organ repair/recovery, experimental animal studies have shown mixed results. In an in vitro preservation experiment of DCD kidneys, the SNMP protocol markedly increased blood flow, urine output, and creatinine clearance and maintained renal structural integrity in comparison to HMPox and SCS. Nonetheless, in this research, the preservation effect of SNMP was not compared with NMP. In fact, the NMP protocol appears to be more advantageous than SNTM for kidney preservation (144). Research on liver transplantation indicates that fatty livers produce more ATP during SNMP compared to NMP; however, glutathione is significantly depleted in hepatocytes during SNMP, which is probably attributed to the hepatocytes’ inability to overcome the threshold energy necessary for glutathione synthesis under subnormothermic conditions (145). Controlled oxygenation rewarming (8–20°C) is the relay process of SCS and HMP, enabling a slow and precisely controlled rewarming of grafts to prevent mitochondrial malfunction and the activation of apoptotic signaling pathways resulting from excessively rapid rewarming (146). NMP (35–38°C) adopts a membrane oxygenator to oxygenate erythrocytes or other oxygen carriers within the perfusate. An adequate supply of oxygen and nutrients can restore the grafts to a physiological metabolic state, allowing the functional assessment and repair of ECD grafts (8, 147, 148). This approach effectively prevents cell injury resulting from hypothermia and the rewarming process, offering substantial benefits in suppressing vasospasm, safeguarding vascular endothelial cells, and suppressing immune rejection (7, 149). NMP can restore ATP depleted during ischemia in isolated porcine kidneys, enabling metabolic recovery, and is capable of maintaining the equilibrium of acid–base and the integrity of renal tubular structure compared to SCS, HMP, and SNMP (144, 150). Several studies have demonstrated that normothermic conditions (about 36°C) are the optimal temperature for the preservation of liver grafts. NMP can lower lactate levels during hepatic perfusion in vitro, maintain hepatic metabolic activity, and lower the risk of early allograft dysfunction (151, 152). As a result, the NMP technique has been gradually applied to clinical liver transplantation.
The composition of the perfusate is essential for grafts during in vitro MP preservation, similar to SCS. The ideal perfusate should include oxygen carriers, suitable concentrations of diverse ions or colloidal osmotic agents, acid–base buffering agents, energy precursors, and growth factors (8). Currently, perfusates are mainly classified as banked blood (BB), plasma-free red cell-based solutions, and acellular perfusates. The oxygenation of the crystalloid or colloid organ preservation solution, commonly applied in clinical practice, supplies dissolved oxygen that may only satisfy the metabolic requirements of the grafts during HMP and SNMP at temperatures below 20°C (141). So far, BB has been successfully used as a perfusate for the extended preservation of the lungs, liver, kidneys, and heart (153–155). Nevertheless, conflicting evidence persists about the application of BB in cardiac NMP. The TransMedics OCS Heart advocates for using BB to perfuse the heart; however, clinical trials conducted by Chew et al. indicate that BB is inappropriate as a perfusate for donor hearts (156). The intricacies of BB and the potential hazards of immunological responses, thrombosis, hemolysis, and infectious disease transmission have resulted in the progressive substitution of blood products with novel perfusates featuring diverse, precisely defined compositions (157). The plasma-free red cell-based solutions comprise physiological oxygen carriers and establish an anti-inflammatory milieu free of platelets and leukocytes, presenting advantages over BB (149). Prolonged NMP following the oxygenation of a plasma-free red cell-based solution could progressively rehabilitate the impaired function in marginal liver grafts. However, when the perfusion duration surpasses 24 h, the iron in hemoglobin will gradually oxidize, resulting in an accumulation of methemoglobin (158). The incorporation of the antioxidant N-acetylcysteine into plasma-free red cell-based solutions can efficiently inhibit the synthesis and accumulation of methemoglobin (159). Furthermore, the oxygenator applied during extended MP can trigger platelet dysfunction and damage the integrity of red blood cells, resulting in increased hemolysis in the perfusate (158). Comparatively, membrane oxygenators are more effective than other types of oxygenators in reducing hemolysis.
Considering the hemolysis of erythrocytes, a variety of artificial oxygen carriers have been manufactured (Table 2). These oxygen carriers exhibit superior oxygen-transport capabilities; nonetheless, certain kinds have been documented to possess nephrotoxic, ophthalmotoxic, or induce systemic vasoconstriction (160). Erythrocruorin, a giant metalloprotein in invertebrates like annelids and crustaceans, is comparatively safe. It not only facilitates effective oxygen delivery but also possesses anti-inflammatory qualities (161). However, there is currently no human red blood cell substitute acknowledged by the United States Food and Drug Administration. The search for a suitable oxygen carrier remains an appealing research direction since it would significantly reduce the waste of BB and allow for mass production of perfusates, finally resulting in cost savings.
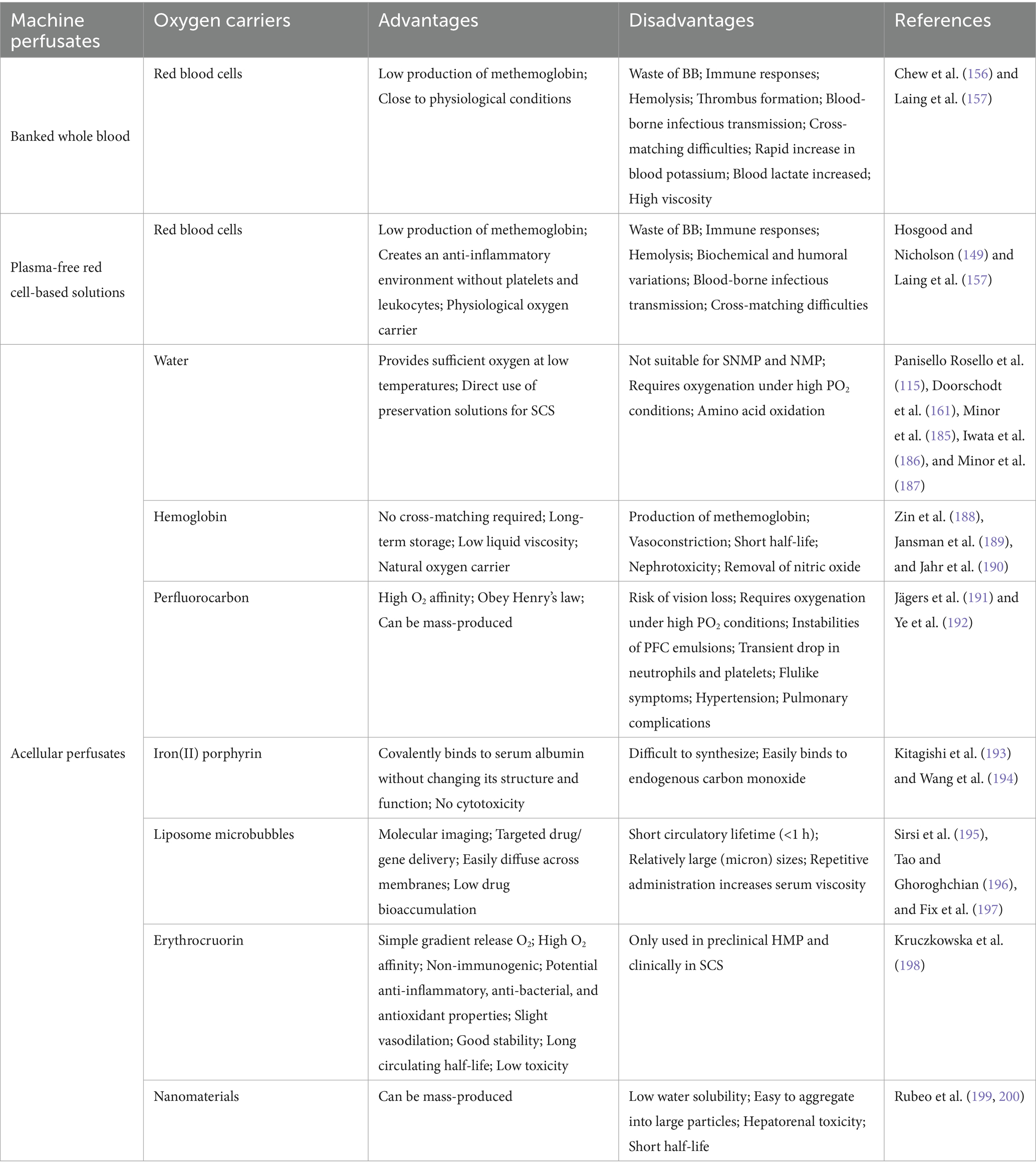
Table 2. Overview of the advantages and disadvantages of different artificial oxygen carriers in machine perfusates.
The viability evaluation and screening of grafts before transplantation can decrease the risk of adverse events post-transplantation. The MP parameters and the analysis of perfusate composition are frequently employed to evaluate graft activity; nevertheless, these data typically exhibit constrained prediction accuracy. The MP parameters frequently employ hemodynamic data as a reference, including vascular resistance (the ratio of perfusion pressure to blood flow) and hemodynamic alterations following the administration of vasoactive drugs (162–165). Functional parameters obtained by left ventricular catheterization offer an effective assessment of myocardial performance during MP of cardiac grafts (165). A study published over a decade ago provided a comprehensive review of contrast-enhanced ultrasound, three-dimensional ultrasound, and magnetic resonance spectrometry techniques for assessing renal perfusion and ATP levels preoperatively (166, 167). The resonance Raman spectroscopy technique is a novel, real-time, non-invasive method for assessing graft function which uses a 441 nm laser and high-resolution spectroscopy to quantify the oxidative status of mitochondrial cytochromes during perfusion. This approach elucidates mitochondrial function and the degree of graft damage, enabling the customization of optimal reoxygenation strategies (168). Evaluating the extent of mitochondrial damage in DCD livers during HMP by analyzing flavin mononucleotide and nicotinamide adenine dinucleotide levels in the perfusates through mass spectrometry and fluorimetry can forecast liver function (169). Indocyanine green fluorescence imaging during HMP of liver grafts may also provide valuable insights into the functional evaluation and selection of marginal livers following the damage-repair process (170). These non-invasive techniques provide rapid access to test results in a sterile operating environment and present benefits over conventional assessment methods.
Components of the perfusate applicable for evaluating graft injury include organ damage markers (such as cardiac enzymes), activated immune cells, lipid peroxidation products, lactate, electrolytes, oxygen partial pressure of perfusate, and both anti-inflammatory and pro-inflammatory cytokines (171, 172). The concentration of bilirubin, creatinine, and urea nitrogen in the perfusate, together with urine and bile output, are critical parameters for evaluating the vitality of renal tubular cells and hepatocytes. Variations in pH, bicarbonate, and glucose levels between MP perfusate and bile have also been demonstrated to be reliable indicators of bile duct cell functionality (165, 173). The extracellular vesicles originating from the donor grafts can be released into the blood and urine, which could reflect the graft’s functional condition. The analysis of nanoparticles, including extracellular vesicles, in perfusate and urine during NMP can evaluate renal quality before and during transplantation (174, 175). Cell-free microRNAs serve as sensitive biomarkers for early tissue damage. The quantitative reverse transcriptase polymerase chain reaction analysis of microRNAs within perfusate and bile indicated that the quantities of hepatocyte-derived microRNA-122 and cholangiocyte-derived microRNA-222 could indicate bile secretion function and the severity of cholangiocyte injury after 6 h of NMP (176, 177). Future research may focus on transcriptomic analysis of inflammation-related gene expression during MP to evaluate graft function.
Graft viability is closely linked to the occurrence of primary nonfunction and DGF following transplantation (172). In comparison with grafts from DBD and living donation, ECD grafts suffer more severe IRI and exhibit a higher incidence of post-transplant acute rejection, graft insufficiency, or graft loss, hence adversely impacting the long-term survival of the grafts (178–180). Although HMPox can increase ATP levels and decrease mitochondrial ROS formation during reperfusion, the practical application of this technique for organ repair remains debatable (1, 145, 181). The warm, oxygen- and nutrient-rich perfusate sustains the graft in a near-physiological metabolic condition, enabling both the evaluation of graft function and the targeted administration of therapeutic agents in an isolated environment, thereby offering a chance for functional repair of marginal organs and cell regeneration. This comparatively isolated environment not only blocks inflammatory cell infiltration and immunological rejection but also mitigates the adverse consequences of systemic administration of drugs. Prolonging the ex-vivo dynamic MP preservation of grafts beyond 24 h could theoretically facilitate marginal organ repair and cell regeneration while eliminating metabolically detrimental waste products; however, this depends on the further development of more technologically sophisticated perfusion equipment (182, 183). Prevalent techniques for graft repair encompass anti-inflammatory therapy, stem cell therapy, RNA interference therapy, and nutrient delivery (9, 184) (Table 3). These techniques could improve organ function and augment graft survival via multiple mechanisms. The successful salvage of marginal organs helps mitigate the imbalance between the supply and demand for grafts.
Organ preservation solutions are one of the most significant instruments in organ transplantation, and lowering cell metabolism or minimizing the graft’s ischemia duration in vitro is a prerequisite for maintaining graft viability. Cryopreservation, subzero non-freezing preservation, and SCS adopt low temperatures to diminish cell metabolism, hence improving graft ischemic tolerance. With the growing demand for organ transplants, doctors are compelled to use grafts from ECD donors; nonetheless, these grafts are more susceptible to harm, and conventional static preservation techniques might further worsen the grafts. The elimination or partial substitution of potentially toxic substances in organ preservation solutions serves as an important modification strategy for these solutions. In other words, the high levels of hazardous CPAs will inevitably restrict the clinical application of cryopreservation and subzero non-freezing preservation protocols. Despite the costly expense and technical complexity of long-term ex vivo dynamic MP, which increases the discard of grafts in the event of preservation failure, it offers multiple benefits over conventional static storage methods, particularly in enabling the dynamic assessment of organ viability and facilitating organ repair and regeneration. The successful repair and regeneration of grafts relies mainly on the prolonged duration of ex vivo MP as well as the development and utilization of safe and effective artificial oxygen carriers and therapeutic agents. Interestingly, several energy precursors utilized in organ preservation solutions for SCS, together with therapeutic medicines documented in the literature, are probably compatible for direct incorporation into machine perfusates. The spiral development of organ preservation strategies necessitates reliance on established research findings and the emergence of innovative technologies. Consequently, artificial oxygen carriers and organ repair/regeneration techniques require further investigation. This review offers a systematic overview of advancements in diverse organ preservation techniques, which we believe will contribute to the optimization and innovation of organ preservation strategies.
QR: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – original draft. JiaZ: Conceptualization, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft. JisZ: Writing – original draft. JL: Writing – original draft. SZ: Writing – original draft. GL: Writing – original draft. BY: Conceptualization, Formal analysis, Project administration, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bon, D, Delpech, P-O, Chatauret, N, Hauet, T, Badet, L, and Barrou, B. Does machine perfusion decrease ischemia reperfusion injury? Prog Urol. (2014) 24:S44–50. doi: 10.1016/S1166-7087(14)70063-6
2. Rauen, U, and De Groot, H. New insights into the cellular and molecular mechanisms of cold storage injury. J Investig Med. (2004) 52:299–309. doi: 10.1136/jim-52-05-29
3. Bahde, R, Palmes, D, Gemsa, O, Minin, E, Stratmann, U, De Groot, H, et al. Attenuated cold storage injury of rat livers using a modified HTK solution. J Surg Res. (2008) 146:49–56. doi: 10.1016/j.jss.2007.08.011
4. Hoyer, DP, Benkö, T, Gallinat, A, Lefering, R, Kaths, M, Kribben, A, et al. HTK-N as a new preservation solution for human kidney preservation: results of a pilot randomized controlled clinical phase II trial in living donor transplantation. Clin Transpl. (2022) 36:e14543. doi: 10.1111/ctr.14543
5. Kniepeiss, D, Houben, P, Stiegler, P, Berghold, A, Riedl, R, Kahn, J, et al. A prospective, randomized, single-blind, multicentre, phase III study on organ preservation with Custodiol-N solution compared with Custodiol® solution in organ transplantation (kidney, liver and pancreas). Trials. (2020) 21:62. doi: 10.1186/s13063-019-3823-4
6. Griffiths, C, Scott, WE, Ali, S, and Fisher, AJ. Maximizing organs for donation: the potential for ex situ normothermic machine perfusion. QJM Int J Med. (2023) 116:650–7. doi: 10.1093/qjmed/hcz321
7. Hosgood, SA, Callaghan, CJ, Wilson, CH, Smith, L, Mullings, J, Mehew, J, et al. Normothermic machine perfusion versus static cold storage in donation after circulatory death kidney transplantation: a randomized controlled trial. Nat Med. (2023) 29:1511–9. doi: 10.1038/s41591-023-02376-7
8. Jing, L, Yao, L, Zhao, M, Peng, L, and Liu, M. Organ preservation: from the past to the future. Acta Pharmacol Sin. (2018) 39:845–57. doi: 10.1038/aps.2017.182
9. Broere, R, Luijmes, SH, De Jonge, J, and Porte, RJ. Graft repair during machine perfusion: a current overview of strategies. Curr Opin Organ Transplant. (2024) 29:248–54. doi: 10.1097/MOT.0000000000001151
10. Regner, KR, Nilakantan, V, Ryan, RP, Mortensen, J, White, SM, Shames, BD, et al. Protective effect of Lifor solution in experimental renal ischemia-reperfusion injury. J Surg Res. (2010) 164:e291–7. doi: 10.1016/j.jss.2010.08.033
11. Liu, Z, Zheng, X, and Wang, J. Bioinspired ice-binding materials for tissue and organ cryopreservation. J Am Chem Soc. (2022) 144:5685–701. doi: 10.1021/jacs.2c00203
12. Bruinsma, BG, and Uygun, K. Subzero organ preservation: the dawn of a new ice age? Curr Opin Organ Transplant. (2017) 22:281–6. doi: 10.1097/MOT.0000000000000403
13. Zhao, G, Liu, X, Zhu, K, and He, X. Hydrogel encapsulation facilitates rapid-cooling cryopreservation of stem cell-laden Core–Shell microcapsules as cell–biomaterial constructs. Adv Healthc Mater. (2017) 6:1700988. doi: 10.1002/adhm.201700988
14. Tan, J, Jia, S, Xu, Q, Lin, C, Cao, Y, Shen, J, et al. Hydrogel encapsulation facilitates a low-concentration cryoprotectant for cryopreservation of mouse testicular tissue. Colloids Surf B Biointerfaces. (2024) 242:114096. doi: 10.1016/j.colsurfb.2024.114096
15. Benson, EE, Harding, K, Ryan, M, Petrenko, A, Petrenko, Y, and Fuller, B. Alginate encapsulation to enhance biopreservation scope and success: a multidisciplinary review of current ideas and applications in cryopreservation and non-freezing storage. Cryo Letters. (2018) 39:14–38.
16. Kumar Giri, T, Thakur, D, Ajazuddin, BH, and Krishna, TD. Alginate based hydrogel as a potential biopolymeric carrier for drug delivery and cell delivery systems: present status and applications. Curr Drug Deliv. (2012) 9:539–55. doi: 10.2174/156720112803529800
17. Han, Z, Rao, JS, Gangwar, L, Namsrai, B-E, Pasek-Allen, JL, Etheridge, ML, et al. Vitrification and nanowarming enable long-term organ cryopreservation and life-sustaining kidney transplantation in a rat model. Nat Commun. (2023) 14:3407. doi: 10.1038/s41467-023-38824-8
18. Finger, EB, and Bischof, JC. Cryopreservation by vitrification: a promising approach for transplant organ banking. Curr Opin Organ Transplant. (2018) 23:353–60. doi: 10.1097/MOT.0000000000000534
19. Han, Z, Rao, JS, Ramesh, S, Hergesell, J, Namsrai, B-E, Etheridge, ML, et al. Model-guided design and optimization of CPA perfusion protocols for whole organ cryopreservation. Ann Biomed Eng. (2023) 51:2216–28. doi: 10.1007/s10439-023-03255-5
20. Bojic, S, Murray, A, Bentley, BL, Spindler, R, Pawlik, P, Cordeiro, JL, et al. Winter is coming: the future of cryopreservation. BMC Biol. (2021) 19:56. doi: 10.1186/s12915-021-00976-8
21. Mashouf, P, Tabibzadeh, N, Kuraoka, S, Oishi, H, and Morizane, R. Cryopreservation of human kidney organoids. Cell Mol Life Sci. (2024) 81:306. doi: 10.1007/s00018-024-05352-7
22. Arav, A. Cryopreservation by directional freezing and Vitrification focusing on large tissues and organs. Cells. (2022) 11:1072. doi: 10.3390/cells11071072
23. Shah, AM. Reliable long-term organ cryopreservation makes donor organ bank a feasible reality. Artif Organs. (2023) 47:1421–2. doi: 10.1111/aor.14629
24. Luu, BE, and Storey, KB. Solving donor organ shortage with insights from freeze tolerance in nature: activating endogenous antioxidant systems with non-coding RNA to precondition donor organs. BioEssays. (2018) 40:e1800092. doi: 10.1002/bies.201800092
25. Năstase, G, Botea, F, Beșchea, G-A, Ștefan-I, C, Barcu, A, Neacșu, I, et al. Isochoric Supercooling organ preservation system. Bioengineering. (2023) 10:934. doi: 10.3390/bioengineering10080934
26. Yang, X, Zhu, Q, Layne, JR, Claydon, M, Hicks, GL, and Wang, T. Subzero nonfreezing storage of the mammalian cardiac explant. Cryobiology. (1993) 30:366–75. doi: 10.1006/cryo.1993.1036
27. Soltys, KA, Batta, AK, and Koneru, B. Successful nonfreezing, subzero preservation of rat liver with 2,3-Butanediol and type I antifreeze protein. J Surg Res. (2001) 96:30–4. doi: 10.1006/jsre.2000.6053
28. Okamoto, T, Nakamura, T, Zhang, J, Aoyama, A, Chen, F, Fujinaga, T, et al. Successful sub-zero non-freezing preservation of rat lungs at −2°C utilizing a new Supercooling technology. J Heart Lung Transplant. (2008) 27:1150–7. doi: 10.1016/j.healun.2008.07.008
29. De Vries, RJ, Tessier, SN, Banik, PD, Nagpal, S, Cronin, SEJ, Ozer, S, et al. Supercooling extends preservation time of human livers. Nat Biotechnol. (2019) 37:1131–6. doi: 10.1038/s41587-019-0223-y
30. Bruinsma, BG, Berendsen, TA, Izamis, M-L, Yeh, H, Yarmush, ML, and Uygun, K. Supercooling preservation and transplantation of the rat liver. Nat Protoc. (2015) 10:484–94. doi: 10.1038/nprot.2015.011
31. O’Callaghan, JM, Knight, SR, Morgan, RD, and Morris, PJ. Preservation solutions for static cold storage of kidney allografts: a systematic review and Meta-analysis. Am J Transplant. (2012) 12:896–906. doi: 10.1111/j.1600-6143.2011.03908.x
32. Bromberger, B, Brzezinski, M, and Kukreja, J. Lung preservation: from perfusion to temperature. Curr Opin Organ Transplant. (2023) 28:168–73. doi: 10.1097/MOT.0000000000001067
33. Keshavjee, SH, Yamazaki, F, Yokomise, H, Cardoso, PF, Mullen, JB, Slutsky, AS, et al. The role of dextran 40 and potassium in extended hypothermic lung preservation for transplantation. J Thorac Cardiovasc Surg. (1992) 103:314–25. doi: 10.1016/S0022-5223(19)35033-0
34. Foley, DP. Comparing preservation solutions for static cold storage in donation after circulatory death liver transplantation. Liver Transpl. (2022) 28:1423–4. doi: 10.1002/lt.26528
35. Parsons, RF, and Guarrera, JV. Preservation solutions for static cold storage of abdominal allografts: which is best? Curr Opin Organ Transplant. (2014) 19:100–7. doi: 10.1097/MOT.0000000000000063
36. Götzfried, J, Smirnova, NF, Morrone, C, Korkmaz, B, Yildirim, AÖ, Eickelberg, O, et al. Preservation with α1-antitrypsin improves primary graft function of murine lung transplants. J Heart Lung Transplant. (2018) 37:1021–8. doi: 10.1016/j.healun.2018.03.015
37. Lei, I, Huang, W, Noly, PE, Naik, S, Ghali, M, Liu, L, et al. Metabolic reprogramming by immune-responsive gene 1 up-regulation improves donor heart preservation and function. Sci Transl Med. (2023) 15:eade3782. doi: 10.1126/scitranslmed.ade3782
38. Rudolph, EN, Dunn, TB, Sutherland, DER, Kandaswamy, R, and Finger, EB. Optimizing outcomes in pancreas transplantation: impact of organ preservation time. Clin Transpl. (2017) 31:e13035. doi: 10.1111/ctr.13035
39. Halpern, SE, Au, S, Kesseli, SJ, Krischak, MK, Olaso, DG, Bottiger, BA, et al. Lung transplantation using allografts with more than 8 hours of ischemic time: a single-institution experience. J Heart Lung Transplant. (2021) 40:1463–71. doi: 10.1016/j.healun.2021.05.008
40. Grimm, JC, Valero, V, Kilic, A, Magruder, JT, Merlo, CA, Shah, PD, et al. Association between prolonged graft ischemia and primary graft failure or survival following lung transplantation. JAMA Surg. (2015) 150:547. doi: 10.1001/jamasurg.2015.12
41. Rauen, U, and De Groot, H. Cold-induced release of reactive oxygen species as a decisive mediator of hypothermia injury to cultured liver cells. Free Radic Biol Med. (1998) 24:1316–23. doi: 10.1016/S0891-5849(97)00456-5
42. Rauen, U, Elling, B, and De Groot, H. Injury to cultured liver endothelial cells after cold preservation: mediation by reactive oxygen species that are released independently of the known trigger hypoxia/Reoxygenation. Free Radic Biol Med. (1997) 23:392–400. doi: 10.1016/S0891-5849(96)00618-1
43. Rauen, U, Kerkweg, U, and De Groot, H. Iron-dependent vs. iron-independent cold-induced injury to cultured rat hepatocytes: a comparative study in physiological media and organ preservation solutions. Cryobiology. (2007) 54:77–86. doi: 10.1016/j.cryobiol.2006.11.008
44. Pless, G, Sauer, IM, and Rauen, U. Improvement of the cold storage of isolated human hepatocytes. Cell Transplant. (2012) 21:23–37. doi: 10.3727/096368911X580509
45. Rauen, U, Petrat, F, Li, T, and De Groot, H. Hypothermia injury/cold-induced apoptosis--evidence of an increase in chelatable iron causing oxidative injury in spite of low O2-/H2O2 formation. FASEB J. (2000) 14:1953–64. doi: 10.1096/fj.00-0071com
46. Rauen, U. Cold-induced apoptosis of hepatocytes: mitochondrial permeability transition triggered by nonmitochondrial chelatable iron. Free Radic Biol Med. (2003) 35:1664–78. doi: 10.1016/j.freeradbiomed.2003.09.018
47. Rauen, U, and De Groot, H. Inherent toxicity of organ preservation solutions to cultured hepatocytes. Cryobiology. (2008) 56:88–92. doi: 10.1016/j.cryobiol.2007.09.003
48. Rauen, U, Klempt, S, and De Groot, H. Histidine-induced injury to cultured liver cells, effects of histidine derivatives and of iron chelators. Cell Mol Life Sci. (2007) 64:192–205. doi: 10.1007/s00018-006-6456-1
49. Garbe, S, Zatschler, B, Müller, B, Dieterich, P, Ebner, A, Rauen, U, et al. Preservation of human artery function following prolonged cold storage with a new solution. J Vasc Surg. (2011) 53:1063–70. doi: 10.1016/j.jvs.2010.10.093
50. Isaacson, Y, Salem, O, Shepherd, RE, and Van Thiel, DH. Lactobionic acid as an iron chelator: a rationale for its effectiveness as an organ preservant. Life Sci. (1989) 45:2373–80. doi: 10.1016/0024-3205(89)90120-3
51. Liu, J, Kang, R, and Tang, D. Signaling pathways and defense mechanisms of ferroptosis. FEBS J. (2022) 289:7038–50. doi: 10.1111/febs.16059
52. Besnier, M, Galaup, A, Nicol, L, Henry, J, Coquerel, D, Gueret, A, et al. Enhanced angiogenesis and increased cardiac perfusion after myocardial infarction in protein tyrosine phosphatase 1B-deficient mice. FASEB J. (2014) 28:3351–61. doi: 10.1096/fj.13-245753
53. Ji, Y, and Li, L. Lower serum chloride concentrations are associated with increased risk of mortality in critically ill cirrhotic patients: an analysis of the MIMIC-III database. BMC Gastroenterol. (2021) 21:200. doi: 10.1186/s12876-021-01797-3
54. Ponnalagu, D, Antelo, D, Hussain, AT, Gao, E, Koch, W, and Singh, H. Cardioprotective role of inner membrane chloride channel, CLIC5 from ischemia-reperfusion injury. Biophys J. (2023) 122:97a. doi: 10.1016/j.bpj.2022.11.716
55. Yang, G, Tang, X, Tan, L, Nong, D, Yang, P, and Ning, H. Upregulation of miR-144-3p protects myocardial function from ischemia–reperfusion injury through inhibition of TMEM16A Ca2+−activated chloride channel. Hum Cell. (2021) 34:360–71. doi: 10.1007/s13577-020-00482-z
56. Li, K, Liu, Y, Lv, X, Lin, Z, Zhang, T, Zhang, F, et al. Reduced intracellular chloride concentration impairs angiogenesis by inhibiting oxidative stress-mediated VEGFR2 activation. Acta Pharmacol Sin. (2021) 42:560–72. doi: 10.1038/s41401-020-0458-7
57. Collins, MG, Fahim, MA, Pascoe, EM, Hawley, CM, Johnson, DW, Varghese, J, et al. Balanced crystalloid solution versus saline in deceased donor kidney transplantation (BEST-fluids): a pragmatic, double-blind, randomised, controlled trial. Lancet. (2023) 402:105–17. doi: 10.1016/S0140-6736(23)00642-6
58. Koch, A, Radovits, T, Loganathan, S, Sack, F-U, Karck, M, and Szabó, GB. Myocardial protection with the use of L-arginine and N-α-acetyl-histidine. Transplant Proc. (2009) 41:2592–4. doi: 10.1016/j.transproceed.2009.06.150
59. Wu, K, Turk, TR, Rauen, U, Su, S, Feldkamp, T, De Groot, H, et al. Prolonged cold storage using a new histidine-tryptophan-ketoglutarate-based preservation solution in isogeneic cardiac mouse grafts. Eur Heart J. (2011) 32:509–16. doi: 10.1093/eurheartj/ehq135
60. Fingas, CD, Wu, S, Gu, Y, Wohlschlaeger, J, Scherag, A, Dahmen, U, et al. Assessment of a chloride-poor versus a chloride-containing version of a modified histidine-tryptophan-ketoglutarate solution in a rat liver transplantation model. Liver Transpl. (2011) 17:650–60. doi: 10.1002/lt.22275
61. Tsukimoto, M, Harada, H, Ikari, A, and Takagi, K. Involvement of chloride in apoptotic cell death induced by activation of ATP-sensitive P2X7 Purinoceptor. J Biol Chem. (2005) 280:2653–8. doi: 10.1074/jbc.M411072200
62. Heimlich, G, and Cidlowski, JA. Selective role of intracellular chloride in the regulation of the intrinsic but not extrinsic pathway of apoptosis in Jurkat T-cells. J Biol Chem. (2006) 281:2232–41. doi: 10.1074/jbc.M507367200
63. Singh, H. Two decades with dimorphic chloride intracellular channels (CLICs). FEBS Lett. (2010) 584:2112–21. doi: 10.1016/j.febslet.2010.03.013
64. Ponnalagu, D, Gururaja Rao, S, Farber, J, Xin, W, Hussain, AT, Shah, K, et al. Molecular identity of cardiac mitochondrial chloride intracellular channel proteins. Mitochondrion. (2016) 27:6–14. doi: 10.1016/j.mito.2016.01.001
65. Manori, B, Vaknin, A, Vaňková, P, Nitzan, A, Zaidel-Bar, R, Man, P, et al. Chloride intracellular channel (CLIC) proteins function as fusogens. Nat Commun. (2024) 15:2085. doi: 10.1038/s41467-024-46301-z
66. Johnson, NP, Gould, KL, and De Bruyne, B. Autoregulation of coronary blood supply in response to demand. J Am Coll Cardiol. (2021) 77:2335–45. doi: 10.1016/j.jacc.2021.03.293
67. Tune, JD, Warne, CM, Essajee, SI, Tucker, SM, Figueroa, CA, Dick, GM, et al. Unraveling the Gordian knot of coronary pressure-flow autoregulation. J Mol Cell Cardiol. (2024) 190:82–91. doi: 10.1016/j.yjmcc.2024.04.008
68. Burke, M, Pabbidi, M, Farley, J, and Roman, R. Molecular mechanisms of renal blood flow autoregulation. Curr Vasc Pharmacol. (2014) 12:845–58. doi: 10.2174/15701611113116660149
69. Post, EH, and Vincent, J-L. Renal autoregulation and blood pressure management in circulatory shock. Crit Care. (2018) 22:81. doi: 10.1186/s13054-018-1962-8
70. Goodwill, AG, Dick, GM, Kiel, AM, and Tune, JD. Regulation of coronary blood flow. Compr Physiol. (2017) 7:321–82. doi: 10.1002/cphy.c160016
71. Mawhinney, C, Low, DA, Jones, H, Green, DJ, Costello, JT, and Gregson, W. Cold water mediates greater reductions in limb blood flow than whole body cryotherapy. Med Sci Sports Exerc. (2017) 49:1252–60. doi: 10.1249/MSS.0000000000001223
72. Murthy, VL, Naya, M, Foster, CR, Gaber, M, Hainer, J, Klein, J, et al. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. (2012) 126:1858–68. doi: 10.1161/CIRCULATIONAHA.112.120402
73. Benvenuti, C, Aptecar, E, Mazzucotelli, J-P, Jouannot, P, Loisance, D, and Nitenberg, A. Coronary artery response to cold-pressor test is impaired early after operation in heart transplant recipients. J Am Coll Cardiol. (1995) 26:446–51. doi: 10.1016/0735-1097(95)80021-8
74. Maathuis, M-HJ, De Groot, M, Ploeg, RJ, and Leuvenink, HGD. Deterioration of endothelial and smooth muscle cell function in DCD kidneys after static cold storage in IGL-1 or UW. J Surg Res. (2009) 152:231–7. doi: 10.1016/j.jss.2008.02.055
75. Ishima, Y, Shinagawa, T, Yoneshige, S, Kragh-Hansen, U, Ohya, Y, Inomata, Y, et al. UW solution improved with high anti-apoptotic activity by S-nitrosated human serum albumin. Nitric Oxide. (2013) 30:36–42. doi: 10.1016/j.niox.2013.01.004
76. Janaszak-Jasiecka, A, Płoska, A, Wierońska, JM, Dobrucki, LW, and Kalinowski, L. Endothelial dysfunction due to eNOS uncoupling: molecular mechanisms as potential therapeutic targets. Cell Mol Biol Lett. (2023) 28:21. doi: 10.1186/s11658-023-00423-2
77. Karbach, S, Wenzel, P, Waisman, A, Munzel, T, and Daiber, A. eNOS uncoupling in cardiovascular diseases - the role of oxidative stress and inflammation. Curr Pharm Des. (2014) 20:3579–94. doi: 10.2174/13816128113196660748
78. Wanstall, J, Homer, K, and Doggrell, S. Evidence for, and importance of, cGMP-independent mechanisms with NO and NO donors on blood vessels and platelets. Curr Vasc Pharmacol. (2005) 3:41–53. doi: 10.2174/1570161052773933
79. Twiner, MJ, Hennessy, J, Wein, R, and Levy, PD. Nitroglycerin use in the emergency department: current perspectives. Open Access Emerg Med. (2022) 14:327–33. doi: 10.2147/OAEM.S340513
80. Sidhu, M, Boden, WE, Padala, SK, Cabral, K, and Buschmann, I. Role of short-acting nitroglycerin in the management of ischemic heart disease. Drug Des Devel Ther. (2015) 9:4793–805. doi: 10.2147/DDDT.S79116
81. Hallstrom, S, Franz, M, Gasser, H, Vodrazka, M, Semsroth, S, Losert, UM, et al. S-nitroso human serum albumin reduces ischaemia/reperfusion injury in the pig heart after unprotected warm ischaemia. Cardiovasc Res. (2007) 77:506–14. doi: 10.1093/cvr/cvm052
82. Mohr, A, Brockmann, JG, and Becker, F. HTK-N: modified histidine-tryptophan-ketoglutarate solution—a promising new tool in solid organ preservation. Int J Mol Sci. (2020) 21:6468. doi: 10.3390/ijms21186468
83. Schaefer, A-K, Kiss, A, Oszwald, A, Nagel, F, Acar, E, Aliabadi-Zuckermann, A, et al. Single donor infusion of S-Nitroso-human-serum-albumin attenuates cardiac Isograft fibrosis and preserves myocardial Micro-RNA-126-3p in a murine heterotopic heart transplant model. Transpl Int. (2022) 35:10057. doi: 10.3389/ti.2022.10057
84. Chatterji, A, and Sengupta, R. Stability of S-nitrosothiols and S-nitrosylated proteins: a struggle for cellular existence! J Cell Biochem. (2021) 122:1579–93. doi: 10.1002/jcb.30139
85. Baxter, K, Howden, B, Saunder, A, and Jablonski, P. Improved cardiac preservation by the addition of nitroglycerine to colloid-free University of Wisconsin solution (MUW). J Heart Lung Transplant. (1999) 18:769–74. doi: 10.1016/S1053-2498(99)00005-4
86. Kahn, J, and Schemmer, P. Comprehensive review on Custodiol-N (HTK-N) and its molecular side of Action for organ preservation. Curr Pharm Biotechnol. (2018) 18:1237–48. doi: 10.2174/1389201019666180409165154
87. Sakuma, T, Motoda, C, Tokuyama, T, Oka, T, Tamekiyo, H, Okada, T, et al. Exogenous adenosine triphosphate disodium administration during primary percutaneous coronary intervention reduces no-reflow and preserves left ventricular function in patients with acute anterior myocardial infarction: a study using myocardial contrast echocardiography. Int J Cardiol. (2010) 140:200–9. doi: 10.1016/j.ijcard.2008.11.041
88. Tokuyama, T, Sakuma, T, Motoda, C, Kawase, T, Takeda, R, Mito, S, et al. Intravenous administration of adenosine triphosphate disodium during primary percutaneous coronary intervention attenuates the transient rapid improvement of myocardial wall motion, not myocardial stunning, shortly after recanalization in acute anterior myocardial infarction. J Cardiol. (2009) 54:289–96. doi: 10.1016/j.jjcc.2009.06.002
89. Martins, PN, Berendsen, TA, Yeh, H, Bruinsma, BG, Izamis, M-L, Op Den Dries, S, et al. Oxygenated UW solution decreases ATP decay and improves survival after transplantation of DCD liver grafts. Transplantation. (2019) 103:363–70. doi: 10.1097/TP.0000000000002530
90. Bardallo, RG, Da Silva, RT, Carbonell, T, Folch-Puy, E, Palmeira, C, Roselló-Catafau, J, et al. Role of PEG35, mitochondrial ALDH2, and glutathione in cold fatty liver graft preservation: an IGL-2 approach. Int J Mol Sci. (2021) 22:5332. doi: 10.3390/ijms22105332
91. Weinberg, JM, Venkatachalam, MA, Roeser, NF, Saikumar, P, Dong, Z, Senter, RA, et al. Anaerobic and aerobic pathways for salvage of proximal tubules from hypoxia-induced mitochondrial injury. Am J Physiol Ren Physiol. (2000) 279:F927–43. doi: 10.1152/ajprenal.2000.279.5.F927
92. Saemann, L, Wächter, K, Gharpure, N, Pohl, S, Hoorn, F, Korkmaz-Icöz, S, et al. HTK vs. HTK-N for coronary endothelial protection during hypothermic, oxygenated perfusion of hearts donated after circulatory death. Int J Mol Sci. (2024) 25:2262. doi: 10.3390/ijms25042262
93. Loganathan, S, Radovits, T, Hirschberg, K, Korkmaz, S, Koch, A, Karck, M, et al. Effects of Custodiol-N, a novel organ preservation solution, on ischemia/reperfusion injury. J Thorac Cardiovasc Surg. (2010) 139:1048–56. doi: 10.1016/j.jtcvs.2009.09.034
94. Wittwer, T, Wahlers, T, Cornelius, JF, Elki, S, and Haverich, A. Celsior solution for improvement of currently used clinical standards of lung preservation in an ex vivo rat model1. Eur J Cardiothorac Surg. (1999) 15:667–71. doi: 10.1016/S1010-7940(99)00046-9
95. Ostróżka-Cieślik, A. The effect of antioxidant added to preservation solution on the protection of kidneys before transplantation. Int J Mol Sci. (2022) 23:3141. doi: 10.3390/ijms23063141
96. Liemburg-Apers, DC, Willems, PHGM, Koopman, WJH, and Grefte, S. Interactions between mitochondrial reactive oxygen species and cellular glucose metabolism. Arch Toxicol. (2015) 89:1209–1226. doi: 10.1007/s00204-015-1520-y
97. Liepinsh, E, Makrecka-Kuka, M, Volska, K, Kuka, J, Makarova, E, Antone, U, et al. Long-chain acylcarnitines determine ischaemia/reperfusion-induced damage in heart mitochondria. Biochem J. (2016) 473:1191–202. doi: 10.1042/BCJ20160164
98. Chouchani, ET, Pell, VR, Gaude, E, Aksentijević, D, Sundier, SY, Robb, EL, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. (2014) 515:431–5. doi: 10.1038/nature13909
99. Kula-Alwar, D, Prag, HA, and Krieg, T. Targeting succinate metabolism in ischemia/reperfusion injury. Circulation. (2019) 140:1968–70. doi: 10.1161/CIRCULATIONAHA.119.042791
100. Martins, PN, Schlegel, A, and Ghinolfi, D. Cold or not so cold?—static organ preservation at 10 °C may prolong organ preservation and facilitate transplant logistics. Transplantation. (2022) 106:427–9. doi: 10.1097/TP.0000000000004035
101. Ali, A, Wang, A, Ribeiro, RVP, Beroncal, EL, Baciu, C, Galasso, M, et al. Static lung storage at 10°C maintains mitochondrial health and preserves donor organ function. Sci Transl Med. (2021) 13:eabf7601. doi: 10.1126/scitranslmed.abf7601
102. Yi, Z, Deng, M, Scott, MJ, Fu, G, Loughran, PA, Lei, Z, et al. Immune-responsive gene 1/Itaconate activates nuclear factor erythroid 2–related factor 2 in hepatocytes to protect against liver ischemia–reperfusion injury. Hepatology. (2020) 72:1394–411. doi: 10.1002/hep.31147
103. Cenik, I, Van Slambrouck, J, Provoost, A-L, Barbarossa, A, Vanluyten, C, Boelhouwer, C, et al. Controlled hypothermic storage for lung preservation: leaving the ice age behind. Transpl Int. (2024) 37:12601. doi: 10.3389/ti.2024.12601
104. Hoetzenecker, K, Benazzo, A, Schwarz, S, Keshavjee, S, and Cypel, M. The advent of semi-elective lung transplantation—prolonged static cold storage at 10°C. Transpl Int. (2024) 37:12310. doi: 10.3389/ti.2024.12310
105. Jamart, J, and Lambotte, L. Differential effect of swelling and anoxia on kidney function and its consequences on the mechanism of action of intracellular organ preservation solutions. Transplantation. (1982) 34:176–82. doi: 10.1097/00007890-198210000-00004
106. Xue, Y, Wang, X, Wan, B, Wang, D, Li, M, Cheng, K, et al. Caveolin-1 accelerates hypoxia-induced endothelial dysfunction in high-altitude cerebral edema. Cell Commun Signal. (2022) 20:160. doi: 10.1186/s12964-022-00976-3
107. Yan, SF, Ogawa, S, Stern, DM, and Pinsky, DJ. Hypoxia-induced modulation of endothelial cell properties: regulation of barrier function and expression of interleukin-6. Kidney Int. (1997) 51:419–25. doi: 10.1038/ki.1997.56
108. El Alam, S, Pena, E, Aguilera, D, Siques, P, and Brito, J. Inflammation in pulmonary hypertension and edema induced by hypobaric hypoxia exposure. Int J Mol Sci. (2022) 23:12656. doi: 10.3390/ijms232012656
109. Trocha, SD, Kevil, CG, Mancini, MC, and Alexander, JS. Organ preservation solutions increase endothelial permeability and promote loss of junctional proteins. Ann Surg. (1999) 230:105–13. doi: 10.1097/00000658-199907000-00015
110. Klöppel, K, Gerlach, J, and Neuhaus, P. Addition of an osmotic agent to liver preservative solutions in a model of in vitro preservation of hepatocytes. Langenbecks Arch Chir. (1994) 379:329–34. doi: 10.1007/BF00191578
111. Türk, TR, Su, S, Rauen, U, Feldkamp, T, De Groot, H, Kribben, A, et al. Reduction of chronic graft injury with a new HTK-based preservation solution in a murine heart transplantation model. Cryobiology. (2012) 64:273–8. doi: 10.1016/j.cryobiol.2012.02.011
112. Zarychanski, R, Abou-Setta, AM, Turgeon, AF, Houston, BL, McIntyre, L, Marshall, JC, et al. Association of Hydroxyethyl Starch Administration with Mortality and Acute Kidney Injury in critically ill patients requiring volume resuscitation: a systematic review and Meta-analysis. JAMA. (2013) 309:678–88. doi: 10.1001/jama.2013.430
113. Mutter, TC, Ruth, CA, and Dart, AB. Hydroxyethyl starch (HES) versus other fluid therapies: effects on kidney function. Cochrane Database Syst Rev. (2013) 2013:CD007594. doi: 10.1002/14651858.CD007594.pub3
114. Fischer, S, Hopkinson, D, Liu, M, MacLean, AA, Edwards, V, Cutz, E, et al. Raffinose improves 24-hour lung preservation in low potassium dextran glucose solution: a histologic and ultrastructural analysis. Ann Thorac Surg. (2001) 71:1140–5. doi: 10.1016/S0003-4975(01)02426-2
115. Panisello Rosello, A, Teixeira Da Silva, R, Castro, C, Ardallo, GR, Calvo, M, Folch-Puy, E, et al. Polyethylene glycol 35 as a Perfusate additive for mitochondrial and Glycocalyx protection in HOPE liver preservation. Int J Mol Sci. (2020) 21:5703. doi: 10.3390/ijms21165703
116. Killinger, WA, Dorofi, DB, Tinsley, EA, Keagy, BA, and Johnson, G. Flow cytometric analysis of organ preservation-induced endothelial cell membrane damage. Ann Thorac Surg. (1992) 53:472–6. doi: 10.1016/0003-4975(92)90271-5
117. Clarysse, M, Accarie, A, Panisello-Roselló, A, Farré, R, Canovai, E, Monbaliu, D, et al. Intravenous polyethylene glycol alleviates intestinal ischemia-reperfusion injury in a rodent model. Int J Mol Sci. (2023) 24:10775. doi: 10.3390/ijms241310775
118. Gao, S, Guan, Q, Chafeeva, I, Brooks, DE, Nguan, CYC, Kizhakkedathu, JN, et al. Hyperbranched polyglycerol as a colloid in cold organ preservation solutions. PLoS One. (2015) 10:e0116595. doi: 10.1371/journal.pone.0116595
119. Colgan, SP, Furuta, GT, and Taylor, CT. Hypoxia and innate immunity: keeping up with the HIFsters. Annu Rev Immunol. (2020) 38:341–63. doi: 10.1146/annurev-immunol-100819-121537
120. Biddlestone, J, Bandarra, D, and Rocha, S. The role of hypoxia in inflammatory disease (review). Int J Mol Med. (2015) 35:859–69. doi: 10.3892/ijmm.2015.2079
121. Eltzschig, HK. Targeting hypoxia-induced inflammation. Anesthesiology. (2011) 114:239–42. doi: 10.1097/ALN.0b013e3182070c66
122. Colgan, SP, and Taylor, CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol. (2010) 7:281–7. doi: 10.1038/nrgastro.2010.39
123. Taylor, CT. Interdependent roles for hypoxia inducible factor and nuclear factor-κB in hypoxic inflammation. J Physiol. (2008) 586:4055–9. doi: 10.1113/jphysiol.2008.157669
124. Korbecki, J, Simińska, D, Gąssowska-Dobrowolska, M, Listos, J, Gutowska, I, Chlubek, D, et al. Chronic and cycling hypoxia: drivers of Cancer chronic inflammation through HIF-1 and NF-κB activation: a review of the molecular mechanisms. Int J Mol Sci. (2021) 22:10701. doi: 10.3390/ijms221910701
125. D’Ignazio, L, Bandarra, D, and Rocha, S. NF -κB and HIF crosstalk in immune responses. FEBS J. (2016) 283:413–24. doi: 10.1111/febs.13578
126. O’Neill, S, Ross, JA, Wigmore, SJ, and Harrison, EM. The role of heat shock protein 90 in modulating ischemia–reperfusion injury in the kidney. Expert Opin Investig Drugs. (2012) 21:1535–48. doi: 10.1517/13543784.2012.713939
127. Wang, G, Wang, J, Li, X, Wu, Q, Yao, R, and Luo, X. Hypoxia and TNF-α synergistically induce expression of IL-6 and IL-8 in human fibroblast-like Synoviocytes via enhancing TAK1/NF-κB/HIF-1α signaling. Inflammation. (2023) 46:912–24. doi: 10.1007/s10753-022-01779-x
128. Maimaitiaili, N, Zeng, Y, Ju, P, Zhakeer, GEG, Yao, H, Shi, Y, et al. NLRC3 deficiency promotes hypoxia-induced pulmonary hypertension development via IKK/NF-κB p65/HIF-1α pathway. Exp Cell Res. (2023) 431:113755. doi: 10.1016/j.yexcr.2023.113755
129. Bandarra, D, Biddlestone, J, Mudie, S, Muller, HA, and Rocha, S. HIF-1α restricts NF-κB dependent gene expression to control innate immunity signals. Dis Model Mech. (2014) 8:169–81. doi: 10.1242/dmm.017285
130. van Uden, P, Kenneth, NS, and Rocha, S. Regulation of hypoxia-inducible factor-1α by NF-κB. Biochem J. (2008) 412:477–84. doi: 10.1042/BJ20080476
131. Taylor, CT, and Colgan, SP. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat Rev Immunol. (2017) 17:774–85. doi: 10.1038/nri.2017.103
132. Fujiwara, N, and Kobayashi, K. Macrophages in inflammation. Curr Drug Target Inflamm Allergy. (2005) 4:281–6. doi: 10.2174/1568010054022024
133. Brüne, B, Dehne, N, Grossmann, N, Jung, M, Namgaladze, D, Schmid, T, et al. Redox control of inflammation in macrophages. Antioxid Redox Signal. (2013) 19:595–637. doi: 10.1089/ars.2012.4785
134. Eltzschig, HK, and Eckle, T. Ischemia and reperfusion—from mechanism to translation. Nat Med. (2011) 17:1391–401. doi: 10.1038/nm.2507
135. Zhang, M, Liu, Q, Meng, H, Duan, H, Liu, X, Wu, J, et al. Ischemia-reperfusion injury: molecular mechanisms and therapeutic targets. Signal Transduct Target Ther. (2024) 9:12. doi: 10.1038/s41392-023-01688-x
136. Algoet, M, Janssens, S, Himmelreich, U, Gsell, W, Pusovnik, M, Van Den Eynde, J, et al. Myocardial ischemia-reperfusion injury and the influence of inflammation. Trends Cardiovasc Med. (2023) 33:357–66. doi: 10.1016/j.tcm.2022.02.005
137. Anghileri, LJ, Maincent, P, and Thouvenot, P. Role of lipid peroxidation in iron-induced cellular calcium overload. Biol Trace Elem Res. (1996) 52:163–9. doi: 10.1007/BF02789458
138. Cannon, RM, Brock, GN, Garrison, RN, Marvin, MR, Franklin, GA, and Davis, EG. Machine perfusion: not just for marginal kidney donors. Am Surg. (2015) 81:550–6. doi: 10.1177/000313481508100616
139. Malinoski, D, Saunders, C, Swain, S, Groat, T, Wood, PR, Reese, J, et al. Hypothermia or machine perfusion in kidney donors. N Engl J Med. (2023) 388:418–26. doi: 10.1056/NEJMoa2118265
140. Tingle, SJ, Thompson, ER, Figueiredo, RS, Moir, JA, Goodfellow, M, Talbot, D, et al. Normothermic and hypothermic machine perfusion preservation versus static cold storage for deceased donor kidney transplantation. Cochrane Database Syst Rev. (2024) 2024:CD011671. doi: 10.1002/14651858.CD011671.pub3
141. Bodewes, SB, Van Leeuwen, OB, Thorne, AM, Lascaris, B, Ubbink, R, Lisman, T, et al. Oxygen transport during ex situ machine perfusion of donor livers using red blood cells or artificial oxygen carriers. Int J Mol Sci. (2020) 22:235. doi: 10.3390/ijms22010235
142. Van Rijn, R, Schurink, IJ, De Vries, Y, Van Den Berg, AP, Cortes Cerisuelo, M, Darwish Murad, S, et al. Hypothermic machine perfusion in liver transplantation — a randomized trial. N Engl J Med. (2021) 384:1391–401. doi: 10.1056/NEJMoa2031532
143. Panayotova, GG, Lunsford, KE, Quillin, RC, Rana, A, Agopian, VG, Lee-Riddle, GS, et al. Portable hypothermic oxygenated machine perfusion for organ preservation in liver transplantation: a randomized, open-label, clinical trial. Hepatology. (2024) 79:1033–47. doi: 10.1097/HEP.0000000000000715
144. Adams, TD, Patel, M, Hosgood, SA, and Nicholson, ML. Lowering Perfusate temperature from 37°C to 32°C diminishes function in a porcine model of ex vivo kidney perfusion. Transplant Direct. (2017) 3:e140. doi: 10.1097/TXD.0000000000000655
145. Karimian, N, Raigani, S, Huang, V, Nagpal, S, Hafiz, EOA, Beijert, I, et al. Subnormothermic machine perfusion of Steatotic livers results in increased energy charge at the cost of anti-oxidant capacity compared to Normothermic perfusion. Meta. (2019) 9:246. doi: 10.3390/metabo9110246
146. Schopp, I, Reissberg, E, Lüer, B, Efferz, P, and Minor, T. Controlled rewarming after hypothermia: adding a new principle to renal preservation. Clin Transl Sci. (2015) 8:475–8. doi: 10.1111/cts.12295
147. Vogel, T, Brockmann, JG, Coussios, C, and Friend, PJ. The role of normothermic extracorporeal perfusion in minimizing ischemia reperfusion injury. Transplant Rev. (2012) 26:156–62. doi: 10.1016/j.trre.2011.02.004
148. Nassar, A, Liu, Q, Farias, K, D’Amico, G, Tom, C, Grady, P, et al. Ex vivo Normothermic machine perfusion is safe, simple, and reliable: results from a large animal model. Surg Innov. (2015) 22:61–9. doi: 10.1177/1553350614528383
149. Hosgood, SA, and Nicholson, ML. First in Man renal transplantation after ex vivo Normothermic perfusion. Transplantation. (2011) 92:735–8. doi: 10.1097/TP.0b013e31822d4e04
150. Hosgood, SA, Patel, M, and Nicholson, ML. The conditioning effect of ex vivo normothermic perfusion in an experimental kidney model. J Surg Res. (2013) 182:153–60. doi: 10.1016/j.jss.2012.08.001
151. for the Consortium for Organ Preservation in EuropeNasralla, D, Coussios, CC, Mergental, H, Akhtar, MZ, Butler, AJ, et al. A randomized trial of normothermic preservation in liver transplantation. Nature. (2018) 557:50–6. doi: 10.1038/s41586-018-0047-9
152. Yoshida, K, Nakamura, S, Sakamoto, H, Kondo, M, Chouno, T, Ikegami, Y, et al. Normothermic machine perfusion system satisfying oxygen demand of liver could maintain liver function more than subnormothermic machine perfusion. J Biosci Bioeng. (2021) 131:107–13. doi: 10.1016/j.jbiosc.2020.08.011
153. Loor, G, Howard, BT, Spratt, JR, Mattison, LM, Panoskaltsis-Mortari, A, Brown, RZ, et al. Prolonged EVLP using OCS lung: cellular and acellular Perfusates. Transplantation. (2017) 101:2303–11. doi: 10.1097/TP.0000000000001616
154. Czigany, Z, Lurje, I, Tolba, RH, Neumann, UP, Tacke, F, and Lurje, G. Machine perfusion for liver transplantation in the era of marginal organs—new kids on the block. Liver Int. (2019) 39:228–49. doi: 10.1111/liv.13946
155. Hosgood, SA, Saeb-Parsy, K, Wilson, C, Callaghan, C, Collett, D, and Nicholson, ML. Protocol of a randomised controlled, open-label trial of ex vivo normothermic perfusion versus static cold storage in donation after circulatory death renal transplantation. BMJ Open. (2017) 7:e012237. doi: 10.1136/bmjopen-2016-012237
156. Chew, HC, Scheuer, S, Dhital, K, and Macdonald, P. Banked blood for normothermic machine perfusion of the donor heart: a clinical perspective. J Heart Lung Transplant. (2019) 38:1322. doi: 10.1016/j.healun.2019.09.001
157. Laing, RW, Bhogal, RH, Wallace, L, Boteon, Y, Neil, DAH, Smith, A, et al. The use of an acellular oxygen carrier in a human liver model of Normothermic machine perfusion. Transplantation. (2017) 101:2746–56. doi: 10.1097/TP.0000000000001821
158. Benedetti, M, De Caterina, R, Bionda, A, Gardinali, M, Cicardi, M, Maffei, S, et al. Blood - artificial surface interactions during cardiopulmonary bypass: a comparative study of four oxygenators. Int J Artif Organs. (1990) 13:488–97. doi: 10.1177/039139889001300808
159. Clarke, G, Mao, J, Fan, Y, Hann, A, Gupta, A, Nutu, A, et al. N-acetylcysteine: a novel approach to methaemoglobinaemia in normothermic liver machine perfusion. Sci Rep. (2023) 13:19022. doi: 10.1038/s41598-023-45206-z
160. Krezdorn, N, Tasigiorgos, S, Wo, L, Turk, M, Lopdrup, R, Kiwanuka, H, et al. Tissue conservation for transplantation. Innov Surg Sci. (2017) 2:171–87. doi: 10.1515/iss-2017-0010
161. Doorschodt, BM, Schreinemachers, M-CJM, Florquin, S, Lai, W, Sitzia, M, Zernecke, A, et al. Evaluation of a novel system for hypothermic oxygenated pulsatile perfusion preservation. Int J Artif Organs. (2009) 32:728–38. doi: 10.1177/039139880903201004
162. Angelico, R, Perera, MTPR, Ravikumar, R, Holroyd, D, Coussios, C, Mergental, H, et al. Normothermic machine perfusion of deceased donor liver grafts is associated with improved Postreperfusion hemodynamics. Transplant Direct. (2016) 2:e97. doi: 10.1097/TXD.0000000000000611
163. Op Den Dries, S, Karimian, N, Westerkamp, AC, Sutton, ME, Kuipers, M, Wiersema-Buist, J, et al. Normothermic machine perfusion reduces bile duct injury and improves biliary epithelial function in rat donor livers. Liver Transpl. (2016) 22:994–1005. doi: 10.1002/lt.24436
164. Jochmans, I, Moers, C, Smits, JM, Leuvenink, HGD, Treckmann, J, Paul, A, et al. The prognostic value of renal resistance during hypothermic machine perfusion of deceased donor kidneys. Am J Transplant. (2011) 11:2214–20. doi: 10.1111/j.1600-6143.2011.03685.x
165. Li, J, Lu, H, Zhang, J, Li, Y, and Zhao, Q. Comprehensive approach to assessment of liver viability during Normothermic machine perfusion. J Clin Transl Hepatol. (2022) 11:466–79. doi: 10.14218/JCTH.2022.00130
166. Stenberg, B, Talbot, D, Khurram, M, Kanwar, A, Ray, C, Mownah, O, et al. A new technique for assessing renal transplant perfusion preoperatively using contrast-enhanced ultrasound (CEUS) and three-dimensional ultrasound (3DUS) – a porcine model pilot study. Ultraschall Med Eur J Ultrasound. (2011) 32:E8–E13. doi: 10.1055/s-0031-1281650
167. Lazeyras, F, Buhler, L, Vallee, J-P, Hergt, M, Nastasi, A, Ruttimann, R, et al. Detection of ATP by “in line” 31P magnetic resonance spectroscopy during oxygenated hypothermic pulsatile perfusion of pigs’ kidneys. Magn Reson Mater Phys Biol Med. (2012) 25:391–9. doi: 10.1007/s10334-012-0319-6
168. Jain, R, Ajenu, EO, Lopera Higuita, M, Hafiz, EOA, Muzikansky, A, Romfh, P, et al. Real-time monitoring of mitochondrial oxygenation during machine perfusion using resonance Raman spectroscopy predicts organ function. Sci Rep. (2024) 14:7328. doi: 10.1038/s41598-024-57773-w
169. Schlegel, A, Muller, X, Mueller, M, Stepanova, A, Kron, P, De Rougemont, O, et al. Hypothermic oxygenated perfusion protects from mitochondrial injury before liver transplantation. EBioMedicine. (2020) 60:103014. doi: 10.1016/j.ebiom.2020.103014
170. Goto, T, Noguchi, Y, Linares, I, Mazilescu, L, Nogueira, E, Hobeika, C, et al. Indocyanine green fluorescence quantification during normothermic ex situ perfusion for the assessment of porcine liver grafts after circulatory death. Liver Transpl. (2024) 30:907–17. doi: 10.1097/LVT.0000000000000416
171. Selten, J, Schlegel, A, De Jonge, J, and Dutkowski, P. Hypo- and normothermic perfusion of the liver: which way to go? Best Pract Res Clin Gastroenterol. (2017) 31:171–9. doi: 10.1016/j.bpg.2017.04.001
172. Van Smaalen, TC, Hoogland, ERP, and Van Heurn, LWE. Machine perfusion viability testing. Curr Opin Organ Transplant. (2013) 18:168–73. doi: 10.1097/MOT.0b013e32835e2a1b
173. Groen, PC, Van Leeuwen, OB, De Jonge, J, and Porte, RJ. Viability assessment of the liver during ex-situ machine perfusion prior to transplantation. Curr Opin Organ Transplant. (2024) 29:239–47. doi: 10.1097/MOT.0000000000001152
174. Morelli, AE. Exosomes: from cell debris to potential biomarkers in transplantation. Transplantation. (2017) 101:2275–6. doi: 10.1097/TP.0000000000001856
175. Woud, WW, Merino, A, Hoogduijn, MJ, Boer, K, Van Den Hoogen, MWF, Baan, CC, et al. Nanoparticle release by extended criteria donor kidneys during Normothermic machine perfusion. Transplantation. (2019) 103:e110–1. doi: 10.1097/TP.0000000000002642
176. Nakayama, T, and Sasaki, K. Advanced viability assessment in machine perfusion: what lies ahead? EBioMedicine. (2024) 108:105351. doi: 10.1016/j.ebiom.2024.105351
177. Matton, APM, Selten, JW, Roest, HP, De Jonge, J, JNM, I, De Meijer, VE, et al. Cell-free microRNAs as early predictors of graft viability during ex vivo normothermic machine perfusion of human donor livers. Clin Transpl. (2020) 34:e13790. doi: 10.1111/ctr.13790
178. Yarlagadda, SG, Coca, SG, Formica, RN, Poggio, ED, and Parikh, CR. Association between delayed graft function and allograft and patient survival: a systematic review and meta-analysis. Nephrol Dial Transplant. (2008) 24:1039–47. doi: 10.1093/ndt/gfn667
179. Perico, N, Cattaneo, D, Sayegh, MH, and Remuzzi, G. Delayed graft function in kidney transplantation. Lancet. (2004) 364:1814–27. doi: 10.1016/S0140-6736(04)17406-0
180. Nemes, B, Gámán, G, Polak, WG, Gelley, F, Hara, T, Ono, S, et al. Extended-criteria donors in liver transplantation part II: reviewing the impact of extended-criteria donors on the complications and outcomes of liver transplantation. Expert Rev Gastroenterol Hepatol. (2016) 10:841–59. doi: 10.1586/17474124.2016.1149062
181. Schlegel, A, Porte, RJ, and Dutkowski, P. Protective mechanisms and current clinical evidence of hypothermic oxygenated machine perfusion (HOPE) in preventing post-transplant cholangiopathy. J Hepatol. (2022) 76:1330–47. doi: 10.1016/j.jhep.2022.01.024
182. Lascaris, B, Thorne, AM, Lisman, T, Nijsten, MWN, Porte, RJ, and De Meijer, VE. Long-term normothermic machine preservation of human livers: what is needed to succeed? Am J Physiol Gastrointest Liver Physiol. (2022) 322:G183–200. doi: 10.1152/ajpgi.00257.2021
183. Groen, PC, De Jonge, J, and Porte, RJ. Prolonged Normothermic machine perfusion: buying more time for liver graft assessment and repair. Transplantation. (2023) 107:1221–2. doi: 10.1097/TP.0000000000004553
184. DiRito, JR, Hosgood, SA, Tietjen, GT, and Nicholson, ML. The future of marginal kidney repair in the context of normothermic machine perfusion. Am J Transplant. (2018) 18:2400–8. doi: 10.1111/ajt.14963
185. Minor, T, Sitzia, M, and Dombrowski, F. Kidney transplantation from non-heart-beating donors after oxygenated low-flow machine perfusion preservation with histidine?Tryptophan?Ketoglutarate solution. Transpl Int. (2005) 17:707–12. doi: 10.1007/s00147-004-0795-3
186. Iwata, H, Matsuno, N, Ishii, D, Toriumi, A, Otani, M, Ohara, M, et al. Applicability of the histidine–tryptophan–ketoglutarate solution as a machine perfusion solution for marginal liver grafts. J Gastroenterol Hepatol. (2023) 38:783–90. doi: 10.1111/jgh.16140
187. Minor, T, Paul, A, Efferz, P, Wohlschlaeger, J, Rauen, U, and Gallinat, A. Kidney transplantation after oxygenated machine perfusion preservation with Custodiol-N solution. Transpl Int. (2015) 28:1102–8. doi: 10.1111/tri.12593
188. Zin, NKM, Bochimoto, H, Kondoh, D, Ishihara, Y, Iwata, H, Shonaka, T, et al. Machine perfusion preservation with hemoglobin based oxygen vesicles alleviate ultrastructural damages in porcine liver donated after cardiac death. Microsc Res Tech. (2023) 86:1725–32. doi: 10.1002/jemt.24405
189. Jansman, MMT, and Hosta-Rigau, L. Recent and prominent examples of nano- and microarchitectures as hemoglobin-based oxygen carriers. Adv Colloid Interf Sci. (2018) 260:65–84. doi: 10.1016/j.cis.2018.08.006
190. Jahr, JS, Moallempour, M, and Lim, JC. HBOC-201, hemoglobin glutamer-250 (bovine), Hemopure ® (biopure corporation). Expert Opin Biol Ther. (2008) 8:1425–33. doi: 10.1517/14712598.8.9.1425
191. Jägers, J, Wrobeln, A, and Ferenz, KB. Perfluorocarbon-based oxygen carriers: from physics to physiology. Pflüg Arch Eur J Physiol. (2021) 473:139–50. doi: 10.1007/s00424-020-02482-2
192. Ye, Q, Zheng, D, Chen, K, and Wu, J. Research Progress in oxygen carrier design and application. Mol Pharm. (2023) 20:4373–86. doi: 10.1021/acs.molpharmaceut.3c00289
193. Kitagishi, H, Mao, Q, Kitamura, N, and Kita, T. HemoCD as a Totally Synthetic artificial oxygen carrier: improvements in the synthesis and O 2 /CO discrimination: HemoCD AS a TOTALLY SYNTHETIC AOC. Artif Organs. (2017) 41:372–80. doi: 10.1111/aor.12870
194. Wang, R-M, Komatsu, T, Nakagawa, A, and Tsuchida, E. Human serum albumin bearing covalently attached Iron(II) porphyrins as O 2 -coordination sites. Bioconjug Chem. (2005) 16:23–6. doi: 10.1021/bc049859m
195. Sirsi, SR, and Borden, MA. Microbubble compositions, properties and biomedical applications. Bubble Sci Eng Technol. (2009) 1:3–17. doi: 10.1179/175889709X446507
196. Tao, Z, and Ghoroghchian, PP. Microparticle, nanoparticle, and stem cell-based oxygen carriers as advanced blood substitutes. Trends Biotechnol. (2014) 32:466–73. doi: 10.1016/j.tibtech.2014.05.001
197. Fix, SM, Borden, MA, and Dayton, PA. Therapeutic gas delivery via microbubbles and liposomes. J Control Release. (2015) 209:139–49. doi: 10.1016/j.jconrel.2015.04.027
198. Kruczkowska, W, Kciuk, M, Pasieka, Z, Kłosiński, K, Płuciennik, E, Elmer, J, et al. The artificial oxygen carrier erythrocruorin—characteristics and potential significance in medicine. J Mol Med. (2023) 101:961–72. doi: 10.1007/s00109-023-02350-3
199. Rubeo, C, Hoti, G, Giordano, M, Molinar, C, Aragno, M, Mantuano, B, et al. Enhancing heart transplantation: utilizing gas-loaded Nanocarriers to mitigate cold/hypoxia stress. Int J Mol Sci. (2024) 25:5685. doi: 10.3390/ijms25115685
200. Rubeo, C, Giordano, M, Tannous, M, Duràn, AM, Comità, S, Landolfi, V, et al. Gas-loaded nanocarriers to improve cardioprotection by cardioplegic solution. Vasc Pharmacol. (2024) 155:107346. doi: 10.1016/j.vph.2024.107346
201. Yu, Y, Cheng, Y, Pan, Q, Zhang, Y-J, Jia, D-G, and Liu, Y-F. Effect of the selective NLRP3 Inflammasome inhibitor mcc950 on transplantation outcome in a pig liver transplantation model with organs from donors after circulatory death preserved by hypothermic machine perfusion. Transplantation. (2019) 103:353–62. doi: 10.1097/TP.0000000000002461
202. Weinberg, EM, Curry, MP, Frenette, CT, Regenstein, FG, Schiff, ER, Goodman, ZD, et al. Multicenter, double-blind, randomized trial of Emricasan in hepatitis C–treated liver transplant recipients with residual fibrosis or cirrhosis. Liver Transpl. (2021) 27:568–79. doi: 10.1002/lt.25934
203. Arni, S, Necati, C, Maeyashiki, T, Opitz, I, and Inci, I. Perfluorocarbon-based oxygen carriers and Subnormothermic lung machine perfusion decrease production of pro-inflammatory mediators. Cells. (2021) 10:2249. doi: 10.3390/cells10092249
204. Ferdinand, JR, Hosgood, SA, Moore, T, Ferro, A, Ward, CJ, Castro-Dopico, T, et al. Cytokine absorption during human kidney perfusion reduces delayed graft function–associated inflammatory gene signature. Am J Transplant. (2021) 21:2188–99. doi: 10.1111/ajt.16371
205. Ishigooka, H, Katsumata, H, Saiga, K, Tokita, D, Motoi, S, Matsui, C, et al. Novel complement C5 small-interfering RNA lipid nanoparticle prolongs graft survival in a Hypersensitized rat kidney transplant model. Transplantation. (2022) 106:2338–47. doi: 10.1097/TP.0000000000004207
206. Yuzefovych, Y, Valdivia, E, Rong, S, Hack, F, Rother, T, Schmitz, J, et al. Genetic engineering of the kidney to permanently silence MHC transcripts during ex vivo organ perfusion. Front Immunol. (2020) 11:265. doi: 10.3389/fimmu.2020.00265
207. Dong, B, Stewart, PW, and Egan, TM. Postmortem and ex vivo carbon monoxide ventilation reduces injury in rat lungs transplanted from non–heart-beating donors. J Thorac Cardiovasc Surg. (2013) 146:429–436.e1. doi: 10.1016/j.jtcvs.2012.11.005
208. Gage, FA, and Vodovotz, Y. Normalization of nitric oxide flux improves physiological parameters of porcine kidneys maintained on pulsatile perfusion. Nitric Oxide. (2003) 9:141–7. doi: 10.1016/j.niox.2003.10.001
209. Maassen, H, Hendriks, KDW, Venema, LH, Henning, RH, Hofker, SH, Van Goor, H, et al. Hydrogen sulphide-induced hypometabolism in human-sized porcine kidneys. PLoS One. (2019) 14:e0225152. doi: 10.1371/journal.pone.0225152
210. Lohmann, S, Pool, MBF, Rozenberg, KM, Keller, AK, Moers, C, Møldrup, U, et al. Mesenchymal stromal cell treatment of donor kidneys during ex vivo normothermic machine perfusion: a porcine renal autotransplantation study. Am J Transplant. (2021) 21:2348–59. doi: 10.1111/ajt.16473
211. Laing, RW, Stubblefield, S, Wallace, L, Roobrouck, VD, Bhogal, RH, Schlegel, A, et al. The delivery of multipotent adult progenitor cells to extended criteria human donor livers using Normothermic machine perfusion. Front Immunol. (2020) 11:1226. doi: 10.3389/fimmu.2020.01226
212. Lebreton, F, Hanna, R, Wassmer, CH, Bellofatto, K, Perez, L, Othenin-Girard, V, et al. Mechanisms of immunomodulation and Cytoprotection conferred to pancreatic islet by human amniotic epithelial cells. Stem Cell Rev Rep. (2022) 18:346–59. doi: 10.1007/s12015-021-10269-w
213. Masaoka, H, Yamamoto, Y, Uchiyama, M, Iguchi, K, Nakamura, M, Yagita, H, et al. Graft protective and intercellular immunomodulatory effects by adoptive transfer of an agonistic anti-BTLA mAb (3C10) induced CD4+CD25+ regulatory T cells in murine cardiac allograft transplant model. Transplant Proc. (2024) 56:692–700. doi: 10.1016/j.transproceed.2024.01.015
214. Rampino, T, Gregorini, M, Germinario, G, Pattonieri, EF, Erasmi, F, Grignano, MA, et al. Extracellular vesicles derived from mesenchymal stromal cells delivered during hypothermic oxygenated machine perfusion repair ischemic/reperfusion damage of kidneys from extended criteria donors. Biology. (2022) 11:350. doi: 10.3390/biology11030350
215. De Haan, MJA, Jacobs, ME, Witjas, FMR, De Graaf, AMA, Sánchez-López, E, Kostidis, S, et al. A cell-free nutrient-supplemented perfusate allows four-day ex vivo metabolic preservation of human kidneys. Nat Commun. (2024) 15:3818. doi: 10.1038/s41467-024-47106-w
216. Nalesso, F, Bertacco, A, Bettin, E, Cacciapuoti, M, Bogo, M, Cattarin, L, et al. The rationale for combining Normothermic liver machine perfusion with continuous renal replacement therapy to maintain physiological Perfusate during ex vivo organ perfusion. J Clin Med. (2024) 13:5214. doi: 10.3390/jcm13175214
217. Del Turco, S, Cappello, V, Tapeinos, C, Moscardini, A, Sabatino, L, Battaglini, M, et al. Cerium oxide nanoparticles administration during machine perfusion of discarded human livers: a pilot study. Liver Transpl. (2022) 28:1173–85. doi: 10.1002/lt.26421
218. Zhang, Q, Tong, J, Zhou, W, Zhong, Z, Hu, Q, Ma, Q, et al. Antibacterial and antioxidant chitosan nanoparticles improve the preservation effect for donor kidneys in vitro. Carbohydr Polym. (2022) 287:119326. doi: 10.1016/j.carbpol.2022.119326
219. Jani, A, Zimmerman, M, Martin, J, Lu, L, Turkmen, K, Ravichandran, K, et al. Perfusion storage reduces apoptosis in a porcine kidney model of donation after cardiac death. Transplant. (2011) 91:169–75. doi: 10.1097/TP.0b013e3182013753
220. Jani, A, Ljubanovic, D, Faubel, S, Kim, J, Mischak, R, and Edelstein, CL. Caspase inhibition prevents the increase in Caspase-3, -2, -8 and -9 activity and apoptosis in the cold ischemic mouse kidney. Am J Transplant. (2004) 4:1246–254. doi: 10.1111/j.1600-6143.2004.00498.x
221. Nydam, TL, Plenter, R, Jain, S, Lucia, S, and Jani, A. Caspase inhibition during cold storage improves graft function and histology in a murine kidney transplant model. Transplant. (2018) 102:1487–495. doi: 10.1097/TP.0000000000002218
222. Mueller, THJ, Kienle, K, Beham, A, Geissler, EK, Jauch, KW, and Rentsch, M. Caspase 3 inhibition improves survival and reduces early graft injury after ischemia and reperfusion in rat liver transplantation. Transplant. (2004) 78:1267–273. doi: 10.1097/01.TP.0000141095.06273.10
223. Vinten-Johansen, J. Postconditioning and controlled reperfusion: the nerve of it all. Anesthesiology. (2009) 111:1177–179. doi: 10.1097/ALN.0b013e3181bf1dac
224. Jain, S, Keys, D, Martin, S, Edelstein, CL, and Jani, A. Protection from apoptotic cell death during cold storage followed by rewarming in 13-Lined ground squirrel tubular cells: the role of prosurvival factors X-Linked inhibitor of apoptosis and phosphoAkt. Transplant. (2016) 100:538–45. doi: 10.1097/TP.0000000000000937
Keywords: organ preservation, transplantation, static cold storage, machine perfusion, graft quality, organ repair
Citation: Ran Q, Zhang J, Zhong J, Lin J, Zhang S, Li G and You B (2025) Organ preservation: current limitations and optimization approaches. Front. Med. 12:1566080. doi: 10.3389/fmed.2025.1566080
Received: 24 January 2025; Accepted: 28 February 2025;
Published: 26 March 2025.
Edited by:
Mack Sheraton, University of Texas Health Science Center at Houston, United StatesReviewed by:
Naoaki Sakata, Fukuoka University, JapanCopyright © 2025 Ran, Zhang, Zhong, Lin, Zhang, Li and You. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin You, ZHJfeW91YmluQHZpcC5zaW5hLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.