- 1Department of Surgery and Cancer, Faculty of Medicine, Imperial College London, London, United Kingdom
- 2Cancer Research UK Convergence Science Centre at The Institute of Cancer Research, London, and Imperial College London, London, United Kingdom
- 3Royal National Orthopaedic Hospital NHS Trust, London, United Kingdom
- 4Wolfson Institute of Population Health, Queen Mary University of London, London, United Kingdom
- 5Academic Centre for Healthy Ageing, Barts Health NHS Trust, London, United Kingdom
Background: The Value Proposition (VP) in diagnostic technology serves as a “positioning statement” outlining the unique benefits, costs, and differentiation an innovation under development offers to healthcare organizations and its ability to effectively deliver these advantages in comparison to current interventions in the market. Despite its significance however, VP lacks a universally accepted definition, which is compounded by the diversity of technologies, their applications, and the varying needs of stakeholders. This paper aims to address this gap by offering a detailed conceptual analysis, revised definition of VP, and actionable recommendations for advancing VP development.
Methodology: We conducted a targeted narrative review, focusing on literature explicitly defining VPs in diagnostic technologies. Using Ovid’s Medline and Embase databases, we identified 19 relevant papers, of which only 5 provided explicit VP definitions. Our analysis incorporated principles of team science, encompassing reflective and thematic analyses of (1) interdisciplinary co-author discussions enabling us to weave together diverse insights into a cohesive exploration of the topic, and (2) MTech’s publicly available set of anonymised responses from NHS Associates, to capture the perspectives of the decision-makers and further enhance depth and breadth of our discourse.
Results and discussion: Our findings highlight the multifaceted nature of VP and its primary hurdles: inadequate identification of unmet needs and insufficient recognition of key stakeholders. We synthesized the evolution of VP definitions and explored the importance of unmet needs in their development, guided by frameworks, such as the Health Technology Navigation Pathway Tool, to ensure VPs meet both the pragmatic and aspirational goals of the healthcare. Thematic insights revealed opportunities for addressing these barriers through implementation science and collaborative strategies. This multi-perspective approach provided a conceptual examination of VP, enabling integration of varied viewpoints and insights.
Conclusion: By employing team science principles and reflective analysis, we introduced a revised definition of VP and a set of actionable recommendations to guide VP development in diagnostics. These findings highlight the importance of addressing stakeholder diversity, unmet needs, and the intricacies of blending interdisciplinary perspectives to advance the field.
Introduction
A Value Proposition (VP) in healthcare is considered a “positioning statement” that outlines the unique benefits, costs, and differentiation an innovation under development offers (in terms of improved patient-centered care, quality, and effectiveness) to healthcare organizations and its ability to effectively deliver these advantages in comparison to current interventions in the market. As innovations evolve, their corresponding VPs must also adapt to meet the needs of increasingly diverse stakeholders. This adaptability is often a key prerequisite for securing funding, investment, and support for researchers, SMEs, and MedTech developers. Despite its significance however, the concept of VP lacks a universally accepted definition in the context of diagnostic technologies. This ambiguity is further compounded by the diversity of technologies available, their applications, and the varying needs of stakeholders (1, 2).
This manuscript explores the broader category of diagnostic technologies, encompassing both traditional and digitally enabled tools, to address the challenge of defining VPs. The focus on diagnostics reflects their unique role in VP development, particularly their primary role in supporting clinical decision-making and care pathways, with their contributions to health outcomes often being indirect or mediated through subsequent clinical actions. Clarifying these conceptual foundations is essential to establishing a stronger basis for the development of operational models in the future.
While productivity, health outcomes, and care efficiency are critical considerations in applied VP frameworks, this manuscript focuses on conceptual elements foundational to defining VPs, rather than presenting a comprehensive evaluative tool. This focus allows for the identification of how VPs are described and understood across stakeholder groups, serving as a precursor to formalized operational frameworks.
Although this manuscript references UK-based frameworks such as the NHS Health Technology Navigation Pathway (HTNP) and the NHS Accelerated Access Collaborative, these models are provided as illustrative examples due to their structured approaches to VP development. The insights presented aim to be conceptually transferable across multiple healthcare systems where stakeholder engagement and value-based decision-making influence the adoption of diagnostic technologies.
A clearer definition of VPs in diagnostic technologies is expected to provide clarity and a standardized understanding that can streamline innovation development, improve stakeholder alignment, and ensure more effective adoption of diagnostic solutions to meet clinical, operational and economic needs.
Our aim was to offer a comprehensive and reflective analysis of VP development focused exclusively on diagnostics and paying special attention to the diverse challenges and potential opportunities presented from multiple stakeholder viewpoints. Our objectives were to:
O1. Examine the evolving definitions of VP in diagnostic technology.
O2. Identify and address unmet needs crucial for effective VP understanding and development.
O3. Synthesize diverse viewpoints from stakeholders, including NHS clinicians, industry experts, and patient representatives.
O4. Explore challenges and opportunities in VP understanding and development for diagnostic technologies.
O5. Provide actionable insights and future directions for advancing VP understanding and development, focusing on stakeholder collaboration and adaptability.
Examining definitions of VP in diagnostics (O1)
We conducted a targeted narrative review to identify papers defining VP in the context of diagnostic technologies. Addressing the lack of a universally accepted definition is critical, as this gap poses challenges for innovators, healthcare providers, and other stakeholders when attempting to advance VP concepts and ensure their practical applicability.
To meet this goal, studies were included if they clearly defined or elaborated on a definition of a VP for diagnostic technologies. Papers that discussed VPs without providing a standalone definition, or those focused solely on broader frameworks rather than explicit definitions, were excluded. This approach ensured that the results offered a robust conceptual foundation for understanding and developing VPs in diagnostics.
Using Ovid’s Medline and Embase databases, we combined the search terms “value propos*” (858 hits) and “diagnos*” (6,340,821 hits) to ensure direct relevance of VPs to diagnostic technologies. This combined search yielded 138 results, subsequently narrowed to 132 English-language publications. After removing duplicates, 129 papers remained, of which five explicitly defined VP (3–7), and two discussed challenges in developing a VP (1, 2). An additional 12 papers elaborated on a VP for a diagnostic technology (8–19). Thus, among these 19 VP-focused papers, only five offered explicit definitions (see Table 1) (3–7). The remaining publications were excluded for not meeting the specified criteria.
We selected Medline and Embase for their strong biomedical and healthcare focus. PubMed’s content largely overlaps with Medline, and Embase complements this coverage by capturing European and industry-related research. Omitting broader interdisciplinary databases like Scopus or Web of Science maintained the diagnostic focus, ensuring a targeted and conceptually pertinent dataset.
Drawing on the selected papers, we identified following key insights. The concept of VP was initially defined by Lanning and Michaels in 1988 (3) and has since been adopted in the commercial sector to “deliver better value.” Table 1 outlines the definitions of VP within the domains of laboratory medicine and medical devices. The authors concurred on the concept’s multifaceted nature, highlighting elements such as clarity, specificity, complexity, and the incorporation of multiple stakeholder perspectives (4–7). They pointed out that at the core of VP should be the benefits—both tangible and intangible—that stem from the adoption of new technology. Furthermore, VP is articulated in ways that support decision-making in clinical care (4, 5), moving beyond merely a product-centric view to highlight the extensive influence of laboratory medicine on the healthcare ecosystem.
Various authors (1, 2), address the complexities involved in crafting a successful VP, despite not defining VP directly. They stress the importance of thoroughly investigating the issues and needs of potential customers and involving a wide array of stakeholders in the process. Furthermore, they assert that identifying unmet needs is crucial for determining the added value brought by innovation in diagnostics (1, 2). Similarly, Rodriguez-Manzano et al. (78) highlight regulatory hurdles and the need to navigate evolving policy landscapes, further illustrating the multifaceted challenges that innovators face in developing effective VPs.
We identified 12 papers (8–19) discussing VPs for a variety of diagnostic technologies. A consensus emerged on the necessity to articulate unmet needs and define clinical, operational, and economic outcomes, as well as highlighting the technology’s more obvious benefits. Three studies (17–19) mention VPs without adhering to a particular framework or covering only selected VP aspects. While these contributions may not encompass all VP development stages, they offer valuable insights and a foundation for further exploration into VPs, as suggested by the authors of these studies (17–19).
While we identified that the primary challenges in developing a successful VP stem from inadequately addressing a clear unmet need and a failure to identify key stakeholders, these barriers can also be viewed as facilitators. In implementation science, it is understood that identifying and understanding barriers to implementation provides an opportunity to develop targeted strategies that can facilitate successful implementation (20–22). One systematic review (20) highlighted the importance of understanding both barriers and facilitators for successful innovation delivery, while another (22) emphasized the importance of context-dependent nature of these factors in the implementation process. By aligning our strategies accordingly, we can convert the challenges into opportunities for VP development, leading to user-centered diagnostics with a higher chance of successful implementation and sustainment.
Importance of identifying unmet needs in the development of VP (O1, O2)
Some authors (5) emphasized the importance of understanding unmet needs as foundational to defining the VP. Here, we delve deeper into how their identification and understanding are crucial for developing effective VPs that meet both practical and aspirational healthcare goals. Three categories of unmet needs are identified in the literature: clinical, operational, and economic/efficiency (8–19). Recognizing and conceptualizing these needs are essential steps that precede the crafting of the VP and analysis of the care pathway, even when some evidence (e.g., economic and operational unmet needs) is not available during the creation phase (6).
In England, the recently developed Health Technology Navigation Pathway (HTNP) Tool (23), (Figure 1), an initiative of the NHS Accelerated Access Collaborative is designed to guide health technology innovators in integrating new technologies into the NHS framework. As part of the Accelerated Access Collaborative Pathway, it focuses on “affordable products which can dramatically improve efficiency, fill an unmet need or make a step change in patient outcomes” (23). Therefore, the identification of an unmet need is a first step; as well as a recognized key challenge in the formulation of VP (3–5).
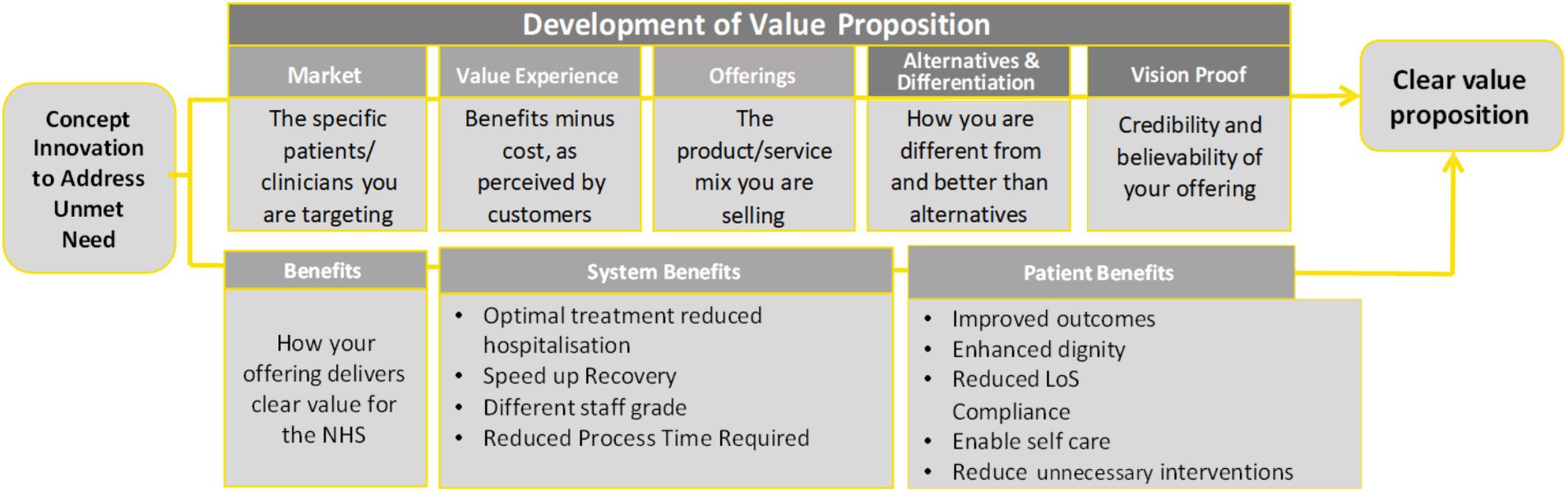
Figure 1. Value proposition analysis based on the Health Technology Navigation Pathway tool, developed by the NHS England Accelerated Access Collaborative (23).
Figure 1 illustrates the development of the VP and its elements within the HTNP tool, including system and patient benefits, which are recognized as elements of value in various Value Assessment Frameworks (VAFs).
Enhancing diagnostic equity: navigating clinical, operational, and economic challenges
The development and integration of health technologies, as exemplified by the HTNP Tool (Figure 1) (23), demonstrates the potential of diagnostics to significantly impact patient care by addressing unmet needs. However, despite widespread recognition of their importance, diagnostics often receive insufficient appreciation in terms of reimbursement and health expenditure. Recent work by Rodriguez-Manzano et al. (78) emphasizes that these systemic challenges are compounded by complex regulatory frameworks, pointing to the critical role of policy alignment and streamlined pathways for the successful integration of diagnostics into healthcare systems. This disparity is a key factor contributing to limited access to diagnostic services (24). The 76th World Health Assembly on strengthening diagnostics capacity (25), identified an urgent need “to consider health technology assessment systems for the systematic evaluation of the effectiveness and cost-effectiveness of diagnostics to support decision-making for the selection of diagnostics for interventions for universal health coverage.” However, the economic assessment of diagnostics within HTA has been less comprehensive compared to therapeutics, particularly in measuring value. As of February 2024, the Cost-Effectiveness Analysis (CEA) Registry (26) recorded 5,573 articles on pharmaceutical interventions but only 877 on diagnostics, highlighting this discrepancy.
A recent systematic literature review summarized evidence on 57 VAFs (27). Notably, the ISPOR Special Task Force (STF) on US Value Assessment Frameworks (28), agreed on elements of value measured in conventional CEA (survival, HRQoL, net costs) and other novel elements of value such as those related to uncertainty, in particular, insurance value, real option value, the value of knowing, and the value of hope (29). These key uncertainty-related elements were included as elements of value of complementary diagnostics (30), and recognized as the first version of the ‘ISPOR value flower’ (31). The review of HTA consideration of elements of value for complementary diagnostics was presented in Zamora et al. (32), including the English Diagnostic Assessment Program (DAP) (33). Zamora et al. (32) highlighted that the NICE DAP acknowledged a broad concept of the novel element “value of knowing,” and how incremental innovation in diagnostics for cancer biomarkers generated “scientific spillovers” as a novel element of value.
The most distinctive novel element of value for diagnostics is the “value of knowing” generated by prognostic and predictive diagnostic information that reduces uncertainty for the patient. The updated NICE manual (34) implicitly recognizes the value of knowing as a clinical outcome, by adopting the DAP broader definition of diagnostic outcomes: “Relevant outcomes include any health outcomes resulting directly or indirectly from any technologies being evaluated. They may also include informational outcomes of value to the patient for the relief, or imposition, of anxiety or for personal planning” (34). A review (35) of 53 HTA guidelines across the world reported that value of knowing (reduction of uncertainty) was only included by HTA bodies in 3 countries: Australia, Canada, and Denmark. The recent VAF for In-Vitro Diagnostics in Asia Pacific (36), mentions that the Australia’s Medical Services Advisory Committee (MSAC) recommended funding genetic testing for cardiomyopathies considering this novel element of value. In parallel, the London School of Economics collaborative value framework, discussed in Augustovki et al. (76) targets NGS/CGP diagnostics, reinforcing the trend toward comprehensive frameworks that inform VP development by integrating multiple elements of value across diverse healthcare contexts.
Since HTA for diagnostics is mostly limited to measuring short-term health outcomes and costs, innovators may not have incentives to include novel elements of value, such as value of knowing. The relevant health outcomes resulting directly from the innovation are less recognized for diagnostics than for therapies as noted in a recent Augustovski’s et al. systematic review: “It is difficult in these technologies to translate the evidence into the decision” (37). Notably, the framework developed by Augustovski et al. (37) emerged from a Latin American context and integrated a multistakeholder deliberative process, emphasizing that defining value for diagnostics may require a different set of metrics than those used for therapeutics. This VAF aligns with other evolving frameworks that collectively push the conceptual boundaries of value assessment for diagnostics beyond traditional cost-effectiveness measures.
The wider value of diagnostic information is initiated in the test and channeled to societal benefit through changes in decision-making for the clinical pathway, with impact on clinicians, carers, and broader society and environment (36, 37, 38). As sustainability and carbon footprint become increasingly important in healthcare decision-making – aligning with Environmental, Societal and Governance (ESG) principles – the potential for diagnostics to contribute to environmental stewardship is gaining attention. Recognizing this dimension expands the assessment of value beyond patient and health system benefits to include the environmental impact of diagnostic development, use and disposal. Recent discussions, as noted by Augustovski et al. (76), emphasize incorporating sustainability metrics into VPs, ensuring that environmental value – such as reduced carbon footprints, resource conservation, and eco-friendly manufacturing processes – is transparently considered.
Also, the value of unmet needs for diagnostics can be considered as included in the novel element of value “equity” Is included as a novel element of value (31), environmental sustainability can also be integrated into VAFs. This ensures that diagnostic innovations are evaluated not only on their clinical, operational, and economic merits but also on their capacity to align with ESG goals, ultimately promoting a more holistic assessment of their value.
Frameworks for assessing unmet needs
To our knowledge, there is not an established “unmet need framework,” but we use this terminology to refer to broader studies on evidence requirements to support diagnostics development and implementation, even if these studies define elements beyond unmet needs. We have selected and reviewed three “unmet need frameworks” recognized by diagnostic organizations. Two of them include an analysis of interviews with stakeholders across the healthcare ecosystem.
The framework, POCKET (39), points out that: “[…] establishing whether there is a clinical need for a test to be available at the point of care should ideally precede device development to maximize the chances of success.” POCKET presents a checklist that goes beyond unmet needs to all evidence generation needed for implementation, which must be evaluated along the clinical pathway. The second framework from the Working Group of the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM TE-WG) (40, 41) highlights wider aspects of unmet need taking into account the impact on clinical, operational and economic outcomes, similarly to the VP framework for laboratory medicine (5, 15). The third and most recent framework focused on clinical needs due to lack of approved devices or by offering a clinically meaningful advantage over existing approved devices (42). In recognizing that the adoption of innovations relies on the support of all stakeholders, we suggest considering ‘stakeholder buy-in’ as an additional unmet need or outcome in VPs. For instance, equitable healthcare provision and patient access alone do not ensure patient uptake.
Unmet needs considered relevant by stakeholders
The POCKET checklist (39) has been developed and consulted with four group of stakeholders: clinicians, commissioners and regulators, methodologists and industry. A study on unmet medical device needs for patients with rare diseases (42) surveyed clinicians, mostly physicians and associated FDA advisory committees on rare diseases, separating diagnostics from therapeutic devices. In both, the POCKET and the FDA checklists, the items related to the evidence requirements/unmet needs linked to the clinical pathway overlap. In particular, the definition of evidence requirements to define clinical needs related to the indication, population, and setting, are considered relevant by all stakeholders. POCKET also adds some dimensions related to the clinical pathways that require evidence on consequences - advantages and disadvantages - for the patient and at an institutional or regional level. The consequences for the patient are considered relevant for all stakeholders, but methodologists do not require evidence at institutional or regional levels. The FDA unmet needs study for rare diseases acknowledges barriers in government regulations as a dimension of need, necessary to tackle which is related to the evidence requirement on consequences at institutional and regional levels, as included in the POCKET clinical pathway dimension.
The POCKET (39) and the FDA (42) unmet needs frameworks also consider economic/efficiency needs, although the requirements for a full cost-effectiveness model are only considered necessary for commissioners and regulators in POCKET, whereas all stakeholders consider the information on costs relevant. Profitability for the industry and costs of development are also considered in the FDA unmet needs framework. Of note, all stakeholders consider the required evidence on health outcomes as consequences for the patient. Therefore, some form of health economic evaluation can include costs and health outcomes. For instance, a cost-consequences model can demonstrate that the diagnostic device has the potential to be cost effective even if not aggregating outcomes as quality-adjusted life years (QALYs).
Lastly, the operational unmet needs identified by the laboratory medicine working groups, including those outlined in the most recent toolkit (43), include maintenance, staff training, reassignment costs, system integration, and interoperability. While the FDA does not define such needs, it covers some of their limitations (e.g., turnaround time: “takes too long”) (42).
Value proposition through the lens of different stakeholder perspectives (O3)
The literature (1, 7), and recent discussions in the field, such as Powell and Hannah (77), emphasize the importance of identifying and understanding of stakeholders’ perspectives as foundational to defining the VP, including, clinical, patient and public, industry and human factors. To begin unpacking these perspectives, we formed an expert group of co-authors who are working in the fields of MedTech/Med devices and come from diverse disciplinary backgrounds, including, clinical, patient and public, industry and human factors. To enhance our interdisciplinary collaboration and dialogue, we embraced the science of team science (SciTS) principles (44, 45), aligning our approach with established big-team science models (46, 47). This was particularly useful in tackling the complex and interdisciplinary nature of VP, enabling us to weave together diverse insights into a cohesive exploration of the topic. To streamline the convergence of our diverse interdisciplinary viewpoints and manage the intricacies involved in blending such varied perspectives (48, 49), we used reflective analysis in, and thematic analysis of our own discussions, geared toward a conceptual examination of VP in diagnostics in terms of what value means and what are the challenges faced.
To enrich the depth and breadth of our discourse, we utilized and thematically summarized publicly available (50) anonymised survey responses from the NHS Associates (e.g., directors, nurses, managers, public health consultants), which explored what value means to NHS decision-makers. These were part of MTech’s (50) engagement strategy aimed at understanding NHS customers’ challenges and decision drivers, crucial for developing effective market access strategies and VPs in the UK healthcare sector.
In what follows, we provide a summary of common concerns and stakeholders’ perspectives, identified through our reflective and thematic exploration, further illustrating how diverse viewpoints shape VP development (77). Their overview is presented in Figure and Table 2, with a description of each perspective provided in Supplementary File 1.
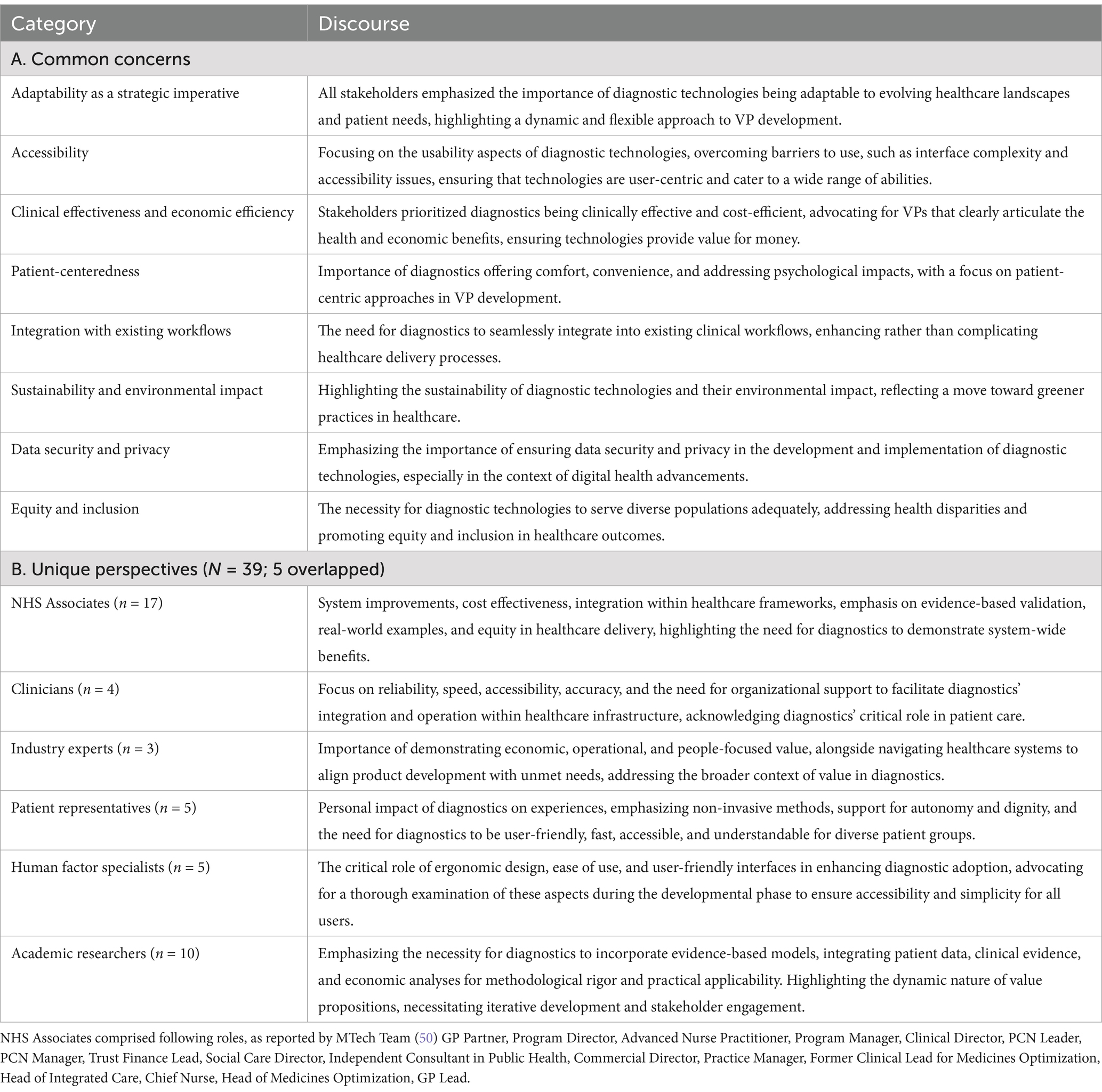
Table 2. Common concerns and unique perspectives of stakeholders on value propositions in diagnostic technologies.
Nonetheless, we acknowledge a limitation: while laboratory technicians are important end-users of diagnostic technologies, their perspectives were not captured by either our interdisciplinary co-authors’ reflections or the NHS Associates’ survey data. Consequently, their viewpoint does not appear in Figure 2. Future explorations should explicitly include laboratory technicians’ insights to further enrich the conceptual landscape of VPs in diagnostic technologies.

Figure 2. Diagram representing key words and reflections from diverse stakeholders on what value means to them. The themes presented were derived from stakeholder perspectives and do not represent a formalized framework for value proposition development.
Adaptability as a strategic imperative
At the heart of discourse emerged the principle of adaptability. The emergence of diagnostic technologies is a critical factor in advancing healthcare outcomes, necessitating a flexible approach to their integration within the existing healthcare infrastructure (51). However, the rapid pace of change, both in terms of disease profiles and healthcare delivery models, requires diagnostic solutions that are not just reactive but anticipatory. This adaptability is not a technical requirement but a strategic imperative, enabling healthcare systems to remain resilient in the face of emerging challenges and opportunities (52). Hence, the value of diagnostic technologies extends beyond basic functionality, to improving how patients feel about their care, and to enhancing system delivery and sustainability through adaptability. The dynamic nature of diagnostic technologies is a testament to the sector’s commitment to evolving alongside the healthcare needs of the population, ensuring that advancements in diagnostics are both reflective of and responsive to the shifting landscape of healthcare demands.
Accessibility
In addressing the critical aspect of usability in diagnostic technology development, a nuanced understanding of specific challenges such as interface complexity and accessibility issues emerged as essential. Methods such as participatory design, where end-users are involved in the development process, and agile development frameworks that allow for rapid iteration based on user feedback, have proven effective in overcoming these hurdles. Such strategies ensure that technologies are not only technically proficient but also user-centric, catering to a wide range of abilities and reducing barriers to effective use (19, 39).
Balancing clinical effectiveness with economic efficiency
The dialogue around clinical effectiveness and economic efficiency encapsulated a dual focus that is critical in the context of constrained healthcare budgets and the imperative for high-quality care. The nuanced balance between cost and benefit, articulated through frameworks such as those employed by NICE (34), highlights a sophisticated understanding of value that extends beyond monetary metrics to encompass health outcomes and quality of life (37). This perspective represents a maturing healthcare ecosystem that seeks to optimize resource allocation, ensuring that the adoption of diagnostic technologies is both a clinically and economically judicious decision.
Patient-centeredness
In the development and implementation of diagnostic technologies, patient-centeredness was fundamental, representing a shift toward more holistic and empathetic healthcare delivery (53). The emphasis on patient experience—comfort, convenience, and psychological impact—signals a move away from a one-size-fits-all approach to diagnostics, toward solutions that are tailored to the diverse needs and preferences of patients. This shift not only enhances the diagnostic technologies but also reinforces the centrality of the patient in healthcare decision-making processes.
Integration with existing workflows
It was clear that the importance of diagnostics seamlessly integrating into existing clinical workflows cannot be overstated, because it is crucial for enhancing rather than complicating healthcare delivery processes (54). Successful adoption hinges not only on clinical efficacy and cost-effectiveness (24) but also on the technology’s compatibility with healthcare professionals’ established routines (36). Minimizing disruption, maximizing user acceptance, and fully realizing the benefits of these technologies in practice are essential (52). Effective strategies for achieving this integration encompass the development of intuitive interfaces (29), comprehensive training for healthcare staff (43), and ensuring new technologies align with existing protocols and systems (55). By focusing on the seamless integration of diagnostic technologies into clinical workflows, improvements can be achieved in operational efficiency, patient outcomes, and creating a more resilient system (53).
Sustainability and data security
Emerging themes such as the sustainability of diagnostic technologies and the imperative for data security reflect an expanding understanding of value that incorporates environmental awareness and the ethical use of patient data (11, 43). These considerations, alongside the foundational principles of equity and inclusion, highlight the multifaceted nature of value in diagnostics, and an interplay between technological advancement and societal good.
Equity and inclusion
Accessibility emerged as a critical axis around which the discourse on diagnostic technologies orbits. Specifically, the equitable distribution of healthcare resources, including diagnostics, is a barometer for the inclusivity of healthcare systems (24). In bridging the gap between advanced healthcare settings and remote or underserved areas, diagnostic technologies act as a lever for equity, ensuring that every individual, irrespective of geography or socioeconomic status, has access to the benefits of medical advancements. This focus on accessibility not only addresses the practical challenges of healthcare delivery but also has a broader commitment to universal health coverage, a cornerstone of global health initiatives (36).
Navigating unique stakeholder perspectives for shared value
The dialogue around VP development in diagnostics was further enriched by the unique perspectives of various stakeholders. From NHS associates’ (50) focus on system-wide benefits and integrated care to clinicians’ emphasis on reliability and accuracy, each perspective contributed to a composite view of value that is as diverse as the ecosystem it seeks to serve (18, 56). Industry experts navigate the complexities of market access and product development, while patient representatives and human factors specialists advocate for diagnostics that are not only effective but also humane, accessible and inclusive (1, 8, 39).
Exploring the intersections and potential conflicts between stakeholder perspectives reveals a complex mix of challenges in aligning around a shared definition of value. For instance, the industry’s drive for innovation and market penetration can sometimes clash with healthcare providers’ focus on cost-effectiveness and clinical utility. Similarly, the push for state-of-the-art diagnostic solutions may inadvertently sideline user-friendly design principles, highlighting the tension between technological advancement and accessibility. Navigating these complexities requires a concerted effort to foster dialogue and compromise, ensuring that the development and implementation of diagnostic technologies truly reflect the collective interests and values of all stakeholders (43, 56).
In synthesizing these diverse perspectives, it becomes clear that the future of diagnostics lies not just in their technical capabilities but in their integration within a broader healthcare, societal, and ethical framework. As we look toward the future, the challenge and opportunity for stakeholders across the healthcare spectrum is to collaboratively navigate this complex landscape, highlighting the power of diagnostic technologies to not only advance healthcare outcomes but also to embody the values of adaptability, accessibility, efficacy, inclusivity, security and sustainability.
Discussion
Navigating complexities and enhancing measurements (O4)
Advancing the VP in diagnostics presents exciting opportunities for innovation. For example, precision and personalized medicine, supported by genomics and advanced technologies, tailors diagnostics to individual needs (57). Digital health platform integration enhances monitoring and remote access (58), while AI/ML contribute toward improved diagnostic accuracy and offer predictive analytics for proactive healthcare (59). Point-of-care testing delivers crucial rapid results in remote or underserved areas (60), and multi-modal diagnostics combine imaging, lab tests, and clinical data for comprehensive health assessments (61).
However, VPs, being dynamic and context-dependent, require an inclusive, collaborative development approach that aligns with existing frameworks and involves extensive stakeholder engagement, beyond the immediate team (62, 63). A patient-centered diagnostic approach, in particular, can broaden the focus to include satisfaction, usability, access, and its role in the care continuum, treating healthcare value as a complex construct (64). An effective VP harmonizes diverse, sometimes conflicting, stakeholder priorities, crafting diagnostics that are clinically and economically sound, patient-focused, ethical, and well-integrated into the healthcare system (65).
Recognizing diagnostics’ multidimensional nature—clinical, economic, technological, and ethical—further highlights the need to address stakeholder values comprehensively including those of patients, providers, insurers, and technology manufacturers (55, 64, 65). Evidence-based models that incorporate patient data, clinical evidence, and economic analyses are essential in ensuring methodological rigor and applicability, supported by empirical research and stakeholder feedback for continuous VP refinement (31). VP needs to evolve with changing stakeholder needs and technological advancements, maintaining their relevance and effectiveness, with clear articulation of the proposition providing balance between value demonstration and proprietary information (54).
Core to this strategy are psychometrically robust measurement systems, providing comprehensive value assessments with both quantitative and qualitative insights into stakeholder perspectives (66), embracing SciTS principles for enhanced interdisciplinary collaboration (44, 45). Applying implementation science methods further enhances VP development and refinement by identifying adoption barriers and ensuring diagnostics’ sustainability and adaptability meet evolving healthcare needs (67). Such psychometrically sound system is also essential for accurately assessing VP success, while addressing the broad needs and expectations to enable more precise diagnostic innovation evaluation (66, 68, 69). It is important to adapt across diseases and innovations to provide a consistent value evaluation method in varied contexts, aiding decision-making and resource allocation by highlighting VP similarities and differences across diseases and innovations (70). This structured system should also prioritize patient-centered outcomes (e.g., quality of life and accessibility), reflecting the broad spectrum of stakeholder priorities and enhancing the framework’s overall impact (71).
Future needs in the field and recommendations (O5)
The diagnostic landscape within the UK’s NHS has undergone significant transformations, shaped by a complex interplay of fiscal constraints, resource allocation, and shifting healthcare priorities (51). These transformations have variably affected different medical conditions, emphasizing the inherent complexities and disparities within diagnostics (56). The integration of novel technologies and evolving policy frameworks has further complicated this landscape, leading to an uneven distribution of diagnostic resources that often disproportionately benefit certain conditions, such as cancer, compared to others, such as mental health (72).
For example, the UK’s Innovative Devices Access Pathway (IDAP) pilot phase aims to support innovative technologies and solutions to address unmet clinical needs (73). It narrows indications and target population to potentially life-threatening or seriously debilitating conditions, but the criteria require evidence on system wide benefit, including for adoption and sustainability and cost-effectiveness. Therefore, any medical device applying to enter this pilot phase must formulate a VP to cover an unmet clinical need.
Furthermore, it is arguable that stakeholders buy-in need should be considered as a relevant dimension not recognized before, as preceding VP. In the unmet needs frameworks (39–42), patients were not among the stakeholders consulted, and only the POCKET framework (39) included needs that are key to achieve uptake of diagnostics: acceptability as “their attitudes to test (including how this was determined).” The FDA framework (42) included a limitation of a diagnostics test as “invasive, cumbersome, painful and/or inconvenient.” Only the POCKET (39) included all stakeholders’ views as an evidence requirement in the form of stakeholder analysis (identification of individuals/groups likely to be affected by test adoption, the impact of adoption and their attitudes). This evidence requirement or unmet need by an existing diagnostic must be also demonstrated by any innovator to fulfill the IDAP criterion, i.e., “The product will be widely adopted and is sustainable” (73).
Central to the effectiveness of a VP in diagnostics is its dual capacity to address current unmet needs while also anticipating future healthcare demands. This deep understanding is vital for aligning VPs with both the practical realities of healthcare delivery and aspirational goals of enhanced patient care and diagnostic innovation (73). In developing these propositions, ensuring their validity and reliability to reflect the diverse values and emerging trends across stakeholder groups is crucial. Such a robust VP not only meets technical and psychometric standards but also embodies empathy and foresight, effectively bridging the gap between today’s service provisions and tomorrow’s healthcare aspirations (53).
Addressing the present gaps in diagnostic VPs necessitates a focused trajectory in future research. There is an urgent need for greater inclusivity in stakeholder representation and for VPs to adapt to the dynamic healthcare landscape. Future research should focus on creating dynamic, yet psychometrically robust, VPs that can accommodate shifts in healthcare priorities and technological advancements, utilizing implementation science. Enhancing stakeholder engagement methods (e.g., with SciTS principles) and integrating advanced data analytics and AI into the VP development process are critical areas warranting attention (52). This advancement will enable the development of effective VPs in diagnostics, meeting the healthcare system’s diverse needs (74, 75).
Building upon this article’s insights, we propose eight actionable recommendations for developing comprehensive and strategic VPs for diagnostic technologies, aligned with NICE’s aims and approach (34), addressing both the immediate and long-term needs of the healthcare system (Table 3). These recommendations span from identifying unmet needs to ensuring sustainability and impact, culminating in our refined VP definition:
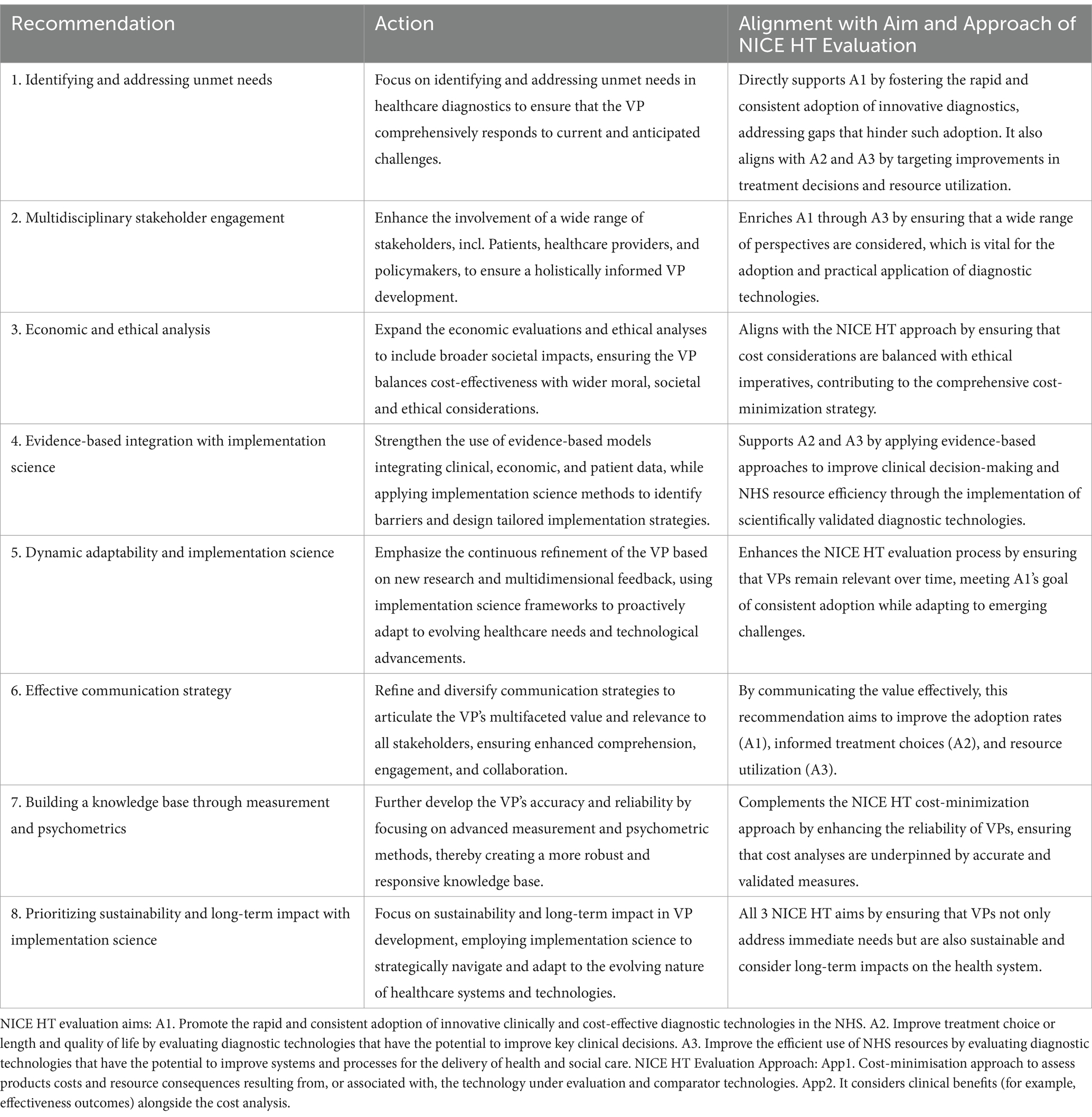
Table 3. Actionable recommendations for value proposition development in healthcare diagnostics aligned with aims and approach of NICE HT evaluation.
‘A VP in diagnostics is a dynamic, evidence-based statement that articulates the specific and measurable benefits a diagnostic technology offers all healthcare stakeholders. It reflects a comprehensive understanding of unmet needs, aligning with both operational efficiencies and economic value. It adapts to healthcare advancements and market demands, addressing the requirements of varied healthcare systems while responding to multifaceted challenges. It highlights the innovation’s value in improving clinical outcomes, system efficiency, and stakeholder benefits in a complex ecosystem.’
This definition encapsulates the essence of the article’s insights and recommendations, serving as a guiding roadmap for advancing VPs in diagnostics. However, it is pertinent to acknowledge that while this article explores various perspectives, the exploration’s breadth and depth have limitations. The breadth of exploration could benefit from the inclusion of more professional groups (e.g., commissioners) across the national spectrum to capture a wider array of experiences and viewpoints. Similarly, by engaging with a larger number of stakeholders, a deeper, more nuanced understanding of the ecosystem could be achieved. Recognizing the potential to expand our insights through broader and deeper engagement highlights the foundational nature of our current work, helping unveil layers of insight that might otherwise remain obscured. Such endeavors not only enrich the discourse on VPs but also pave the path for future directions, enhancing the scope and effectiveness of diagnostic technologies in the healthcare ecosystem.
Limitations
This review employed a targeted narrative approach, focusing on literature that explicitly defined VPs in diagnostic technologies. While this provided conceptual clarity, it narrowed the scope of our findings. By prioritizing explicit definitions over broader thematic inquiry, we may have excluded potentially informative perspectives or indirectly relevant frameworks that could offer additional context. Future research could adopt a more expansive approach, incorporating a wider range of methodologies and literature sources.
Furthermore, the stakeholder perspectives captured in our thematic and reflective analyses were drawn from our interdisciplinary team of co-authors and publicly available NHS Associates’ survey responses. While this approach aligned with the principles of team science and facilitated the integration of multiple viewpoints, it inadvertently omitted certain key stakeholders, notably laboratory technicians. Their exclusion limits insight into operational and technical considerations vital to VP implementation. Future efforts should incorporate laboratory technicians to further enrich the conceptual landscape of VPs in diagnostics.
The NHS frameworks cited in this manuscript, including the HTNP and Accelerated Access Collaborative, serve primarily as illustrative models rather than prescriptive frameworks. While these tools are referenced to demonstrate structured Value Assessment Frameworks approaches, with the VP at the creation phase, the insights presented aim to be conceptually transferable across multiple healthcare systems where stakeholder engagement and value-based decision-making influence the adoption of diagnostic technologies.
Additionally, this manuscript does not include real-world examples of diagnostics with established VPs. This decision was intentional, as the primary focus is on advancing conceptual clarity around the foundational elements of VPs rather than assessing applied models. While constructs such as productivity, health outcomes, and care efficiency are critical components in practical VP models, many cannot be included in the VP creation phase until more evidence is generated. The primary aim was to clarify definitional elements rather than prescribe an exhaustive set of criteria for VP assessment. Future work could extend this conceptual foundation by exploring how clearly defined VPs influence the adoption and sustainability of diagnostic technologies in applied contexts, where metrics such as productivity, health outcomes, and care efficiency could be more systematically incorporated and evaluated along evidence generation.
Despite these limitations, our application of team science principles demonstrates a replicable and adaptable methodology that can be extended to larger, more diverse stakeholder cohorts. By doing so, future investigations can achieve a more nuanced, equitable, and comprehensive understanding of the multifaceted elements shaping VPs in diagnostics.
Conclusion
We embarked on an analytical journey to unravel the complex dynamics of VP development in diagnostics, shedding light on the nuanced challenges and opportunities that define this process. Drawing on a literature search, while utilizing the principles of team science, we highlighted the importance of meeting unmet needs and fostering engagement across varied stakeholders. The focus on diagnostic technologies in this manuscript was intentional, providing foundational clarity on how VPs are described, which may inform broader operational models for both diagnostic and other health technologies in future work. Our findings advocate for a flexible and refined approach to VP formulation, one that is in harmony with the fast pace of innovation and transformation in the healthcare and technological sectors. Through this lens, our paper serves as a cornerstone for future endeavors aimed at optimizing the integration and impact of diagnostic innovations, ultimately contributing to the advancement of patient care and system efficiency.
Author contributions
TS: Writing – original draft, Writing – review & editing. BZ-T: Writing – original draft, Writing – review & editing. SB: Writing – original draft, Writing – review & editing. RL: Writing – original draft, Writing – review & editing. PK: Writing – original draft, Writing – review & editing. OB: Writing – original draft, Writing – review & editing. HK-L: Writing – original draft, Writing – review & editing. K-VS: Writing - original draft, Writing - review & editing. MM: Writing – original draft, Writing – review & editing. SZ: Writing – original draft, Writing – review & editing. SN: Writing – original draft, Writing – review & editing. SW: Writing – original draft, Writing – review & editing. CP: Writing – original draft, Writing – review & editing. AG: Writing – original draft, Writing – review & editing. MN: Writing – original draft, Writing – review & editing. PB: Writing – original draft, Writing – review & editing. GH: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Infrastructure support for this research was provided by the NIHR London HealthTech Research Centre in In-Vitro Diagnostics, the NIHR Imperial Biomedical Research Centre, and the NIHR Imperial Clinical Research Facility. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. Prof Adam Gordon is an NIHR Senior Investigator. Dr Patrick Kierkegaard is supported by the CRUK Convergence Science Centre at The Institute of Cancer Research, London, and Imperial College London (A26234).
Acknowledgments
The collaborating authors for the NIHR HRC IVD PPIE Team are Rashmi Kumar, Diane Eaton, Shamil Al-Ameen, and Rebecca Harmston (London, UK).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AHSN, Academic Health Science Networks; AI, Artificial Intelligence; CEA, Cost-Effectiveness Analysis; DAP, Diagnostic Assessment Program; EFLM TE-WG, Working Group of the European Federation of Clinical Chemistry and Laboratory Medicine; FDA, Food and Drug Administration; GP, General Practitioner; HCP, Healthcare Professional; HT, Health Technology; HTA, Health Technology Assessment; HTNP, Health Technology Navigation Pathway; IDAP, Innovative Devices Access Pathway; ISPOR, International Society for Pharmacoeconomics and Outcomes Research; MDT, Medical Doctor Trainee; ML, Machine Learning; MSAC, Medical Services Advisory Committee; MTech, Medical Technology; NHS, National Health Service; NICE, National Institute for Health and Care Excellence; PCN, Primary Care Network; POCKET, Point-of-Care Key Evidence Tool; QALY, Quality-Adjusted Life Year; SciTS, Science of Team Science; SME, Small and Medium-sized Enterprises; STF, Special Task Force; UK, United Kingdom; VAF, Value Assessment Framework; VP, Value Proposition.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1498618/full#supplementary-material
References
1. Bobelyn, A, Unteregger, R, Hoffken, JI, and Reymen, I. Problem exploration for creating value propositions when developing point-of-care solutions. Biosens Bioelectron. (2023) 241:115636. doi: 10.1016/j.bios.2023.115636
2. Metcalfe, TA. Development of novel IVD assays: a manufacturer's perspective. Scand J Clin Lab Invest. (2010) 242:23–6. doi: 10.3109/00365513.2010.493361
3. Lanning, MJ, and Michaels, EG. A business is a value delivery system. New York: McKinsey Staff Paper (1988).
4. Price, CP, and St, JA. Anatomy of a value proposition for laboratory medicine. Clin Chem Acta. (2014) 436:104–11. doi: 10.1016/j.cca.2014.05.017
5. Price, CP, St John, A, Christenson, R, Scharnhorst, V, Oellerich, M, Jones, P, et al. Leveraging the real value of laboratory medicine with the value proposition. Clin Chim Acta. (2016) 462:183–6. doi: 10.1016/j.cca.2016.09.006
6. Graziadio, S, Winter, A, Lendrem, BC, Suklan, J, Jones, WS, Urwin, SG, et al. How to ease the pain of taking a diagnostic point of care test to the market: a framework for evidence development. Micromachines. (2020) 11:30291. doi: 10.3390/mi11030291
7. Lehoux, P, Hivon, M, Williams-Jones, B, Miller, FA, and Urbach, DR. How do medical device manufacturers' websites frame the value of health innovation? An empirical ethics analysis of five Canadian innovations. Med Health Care Philos. (2012) 15:61–77. doi: 10.1007/s11019-011-9312-5
8. Fuller, SS, Clarke, E, and Harding-Esch, EM. Molecular chlamydia and gonorrhoea point of care tests implemented into routine practice: systematic review and value proposition development. PLoS One. (2021) 16:e0259593. doi: 10.1371/journal.pone.0259593
9. Haselmann, V, Hedtke, M, and Neumaier, M. Liquid profiling for cancer patient stratification in precision medicine-current status and challenges for successful implementation in standard care. Diagnostics. (2022) 12:748. doi: 10.3390/diagnostics12030748
10. Lam, WKJ. Circulating tumor DNA in cancer management: a value proposition. J Appl Lab Med. (2020) 5:1017–26. doi: 10.1093/jalm/jfaa112
11. Oellerich, M, Christenson, RH, Beck, J, and Walson, PD. Plasma EGFR mutation testing in non-small cell lung cancer: a value proposition. Clin Chem Acta. (2019) 495:481–6. doi: 10.1016/j.cca.2019.05.019
12. Oellerich, M, Christenson, RH, Beck, J, Schutz, E, Sherwood, K, Price, CP, et al. Donor-derived cell-free DNA testing in solid organ transplantation: a value proposition. J Appl Lab Med. (2020) 5:993–1004. doi: 10.1093/jalm/jfaa062
13. O'Kane, M, Porter, D, McCann, M, Julicher, P, Christenson, R, Oellerich, M, et al. A value proposition for natriuretic peptide measurement in the assessment of patients with suspected acute heart failure. Clin Chem Acta. (2020) 500:98–103. doi: 10.1016/j.cca.2019.09.023
14. Price, CP, and St, JA. The value proposition for point-of-care testing in healthcare: HbA1c for monitoring in diabetes management as an exemplar. Scand J Clin Lab Invest. (2019) 79:298–304. doi: 10.1080/00365513.2019.1614211
15. John, AS, Cullen, L, Julicher, P, and Price, CP. Developing a value proposition for high-sensitivity troponin testing. Clin Chem Acta. (2018) 477:154–9. doi: 10.1016/j.cca.2017.12.007
16. Wrede, C, Braakman-Jansen, A, and van Gemert-Pijnen, L. How to create value with unobtrusive monitoring technology in home-based dementia care: a multimethod study among key stakeholders. BMC Geriatr. (2022) 22:921. doi: 10.1186/s12877-022-03550-1
17. Froelich, MF, Capoluongo, E, Kovacs, Z, Patton, SJ, Lianidou, ES, and Haselmann, V. The value proposition of integrative diagnostics for (early) detection of cancer. Clin Chem Lab Med. (2022) 60:821–9. doi: 10.1515/cclm-2022-0129
18. Fritz, J, Kijowski, R, and Recht, MP. Artificial intelligence in musculoskeletal imaging: a perspective on value propositions, clinical use, and obstacles. Skeletal Radiol. (2021) 51:239–43. doi: 10.1007/s00256-021-03802-y
19. Hansen, GT. Point-of-care testing in microbiology: a mechanism for improving patient outcomes. Clin Chem. (2020) 66:124–37. doi: 10.1373/clinchem.2019.304782
20. Wu, S, Tannous, E, Haldane, V, Ellen, ME, and Wei, X. Barriers and facilitators to implementing evidence-based interventions among third sector organizations: a systematic review. Implement Sci. (2020) 15:62. doi: 10.1186/s13012-018-0789-7
21. Powell, BJ, Fernandez, ME, Williams, NJ, Aarons, GA, Beidas, RS, Lewis, CC, et al. Enhancing the impact of implementation strategies in healthcare: a research agenda. Front Public Health. (2020) 7:3. doi: 10.3389/fpubh.2019.00003
22. Waltz, TJ, Powell, BJ, Matthieu, MM, Damschroder, LJ, Chinman, MJ, Smith, JL, et al. Use of concept mapping to characterize relationships among implementation strategies and assess their feasibility and importance: results from the expert recommendations for implementing change (ERIC) study. Implement Sci. (2020) 10:109. doi: 10.1186/s13012-015-0295-0
23. NHS Accelerated Access Collaborative. Health Technology Navigation Pathway (HTNP) Tool. Available at: https://www.england.nhs.uk/aac/what-we-do/how-can-the-aac-help-me/the-medtech-funding-mandate/ (Accessed October 30, 2023).
24. Fleming, KA, Horton, S, Wilson, ML, Atun, R, DeStigter, K, Flanigan, J, et al. The lancet commission on diagnostics: transforming access to diagnostics. Lancet. (2021) 398:1997–2050. doi: 10.1016/S0140-6736(21)00673-5
25. World Health Organization. (2023). Strengthening diagnostics capacity. Seventy-sixth world health assembly. Available at: https://apps.who.int/gb/ebwha/pdf_files/WHA76/A76_R5-en.pdf (Accessed March 11, 2024).
26. Center for the Evaluation of Value and Risk in Health. (2024). The cost-effectiveness analysis registry. Tufts Medical Center. Available at: https://cevr.tuftsmedicalcenter.org/databases/cea-registry (Accessed February 12, 2024).
27. Zhang, M, Bao, Y, Lang, Y, Fu, S, Kimber, M, Levine, M, et al. What is value in health and healthcare? A systematic literature review of value assessment frameworks. Value Health. (2022) 25:302–17. doi: 10.1016/j.jval.2021.07.005
28. Neumann, PJ, Willke, RJ, and Garrison, LP Jr. A health economics approach to US value assessment frameworks—introduction: an ISPOR special task force report [1]. Value Health. (2018) 21:119–23. doi: 10.1016/j.jval.2017.12.012
29. Garrison, LP Jr, Zamora, B, Li, M, and Towse, A. Augmenting cost-effectiveness analysis for uncertainty: the implications for value assessment—rationale and empirical support. J Manag Care Spec Pharm. (2020) 26:400–6. doi: 10.18553/jmcp.2020.26.4.400
30. Garrison, L, Mestre-Ferrandiz, J, and Zamora, B. The value of knowing and knowing the value: Improving the health technology assessment of complementary diagnostics. London, UK: Office of Health Economics (2016).
31. Neumann, PJ, Garrison, LP Jr, and Willke, RJ. The history and future of the “ISPOR value flower”: addressing limitations of conventional cost-effectiveness analysis. Value Health. (2022) 25:558–65. doi: 10.1016/j.jval.2022.01.010
32. Zamora, B, Mestre-Ferrandiz, J, and Garrison, L. Landscape review of complementary diagnostics in Europe. London: Office of Health Economics and EPEMED (2016).
33. National Institute for Health and Care Excellence. (2011). Diagnostics assessment Programme manual. Available at: https://www.nice.org.uk/media/default/about/what-we-do/nice-guidance/nice-diagnostics-guidance/diagnostics-assessment-programme-manual.pdf (Accessed October 30, 2023).
34. National Institute for Health and Care Excellence. (2022). NICE health technology evaluations: the manual. Available at: https://www.nice.org.uk/process/pmg36/chapter/introduction-to-health-technology-evaluation (Accessed October 30, 2023).
35. Breslau, RM, Cohen, JT, Diaz, J, Malcolm, B, and Neumann, PJ. A review of HTA guidelines on societal and novel value elements. Int J Technol Assess Health Care. (2023) 39:e31. doi: 10.1017/S026646232300017X
36. Asia Pacific Medical Technology Association. (2023). Value of in-vitro diagnostics in APAC: value Assessment Framework with applications for Cardiovascular Diseases. Available at: https://apacmed.org/value-assessment-framework-with-applications-for-coronary-artery-disease-and-heart-failure/ (Accessed March 11, 2024).
37. Augustovski, F, Alfie, V, Alcaraz, A, Martí, SG, Drummond, MF, and Pichon-Riviere, A. A value framework for the assessment of diagnostic technologies: a proposal based on a targeted systematic review and a multistakeholder deliberative process in Latin America. Value Health. (2021) 24:486–96. doi: 10.1016/j.jval.2020.11.008
38. Wurcel, V, Cicchetti, A, Garrison, L, Kip, MM, Koffijberg, H, Kolbe, A, et al. The value of diagnostic information in personalised healthcare: a comprehensive concept to facilitate bringing this technology into healthcare systems. Public Health Genomics. (2019) 22:8–15. doi: 10.1159/000501832
39. Huddy, JR, Ni, M, Misra, S, Mavroveli, S, Barlow, J, and Hanna, GB. Development of the point-of-care key evidence tool (pocket): a checklist for multi-dimensional evidence generation in point-of-care tests. Clin Chem Lab Med. (2019) 57:845–55. doi: 10.1515/cclm-2018-1089
40. Monaghan, PJ, Lord, SJ, St John, A, Sandberg, S, Cobbaert, CM, Lennartz, L, et al. Biomarker development targeting unmet clinical needs. Clin Chim Acta. (2016) 460:211–9. doi: 10.1016/j.cca.2016.06.037
41. Monaghan, PJ, Robinson, S, Rajdl, D, Bossuyt, PM, Sandberg, S, St John, A, et al. Practical guide for identifying unmet clinical needs for biomarkers. EJIFCC. (2018) 29:129–37.
42. US Food and Drug Administration. (2021). Unmet medical device needs for patients with rare diseases. Available at: https://www.fda.gov/media/111309/download (Accessed March 11, 2024).
43. Greaves, R, Kricka, L, Gruson, D, Ferrari, M, Martin, H, Loh, TP, et al. IFCC emerging technologies division. Toolkit for emerging technologies in laboratory medicine. Clin Chem Lab Med. (2023) 61:2102–14. doi: 10.1515/cclm-2023-0571
44. Huang, Y, Liu, X, Li, R, and Zhang, L. The science of team science (SciTS): an emerging and evolving field of interdisciplinary collaboration. Prof Inform. (2023) 32:4. doi: 10.3145/epi.2023.mar.04
45. Hall, KL, Vogel, AL, Huang, GC, Serrano, KJ, Rice, EL, Tsakraklides, S, et al. The science of team science: a review of the empirical evidence and research gaps on collaboration in science. Am Psychol. (2018) 73:532–48. doi: 10.1037/amp0000319
46. Diffendorfer, J, Drum, R, Mitchell, G, Rendón-Salinas, E, Sánchez-Cordero, V, Semmens, D, et al. The benefits of big-team science for conservation: lessons learned from trinational monarch butterfly collaborations. Front Environ Sci. (2023) 11:11. doi: 10.3389/fenvs.2023.1079025
47. Strekalova, YA, Kamp, K, Hildago, R, et al. Strategic team science: Scaffolded training for research self-efficacy, interdisciplinarity, diversity, equity, and inclusive excellence in biomedical research. J Clin Transl Sci. (2021) 5:e59. doi: 10.1017/cts.2021.810
48. Specht, A, and Crowston, K. The impact of team diversity on scientific output: publishing in and citing more diverse sources, but at a cost to team satisfaction. PLoS One. (2022) 17:e0278043. doi: 10.1371/journal.pone.0278043
49. Love, H, Fosdick, BK, Cross, J, Suter, M, Egan, D, Tofany, E, et al. Team science principles enhance cancer care delivery quality improvement: interdisciplinary implementation of breast cancer screening shared decision making. JCO Oncol Pract. (2022) 19:e1–7. doi: 10.1200/OP.22.00355
50. Mtech Team. (2022). What does 'Value' mean to NHS decision-makers? Mtech access. Available at: https://mtechaccess.co.uk/what-does-value-mean-to-nhs-decision-makers/ (Accessed March 11, 2024).
51. Appleby, J, and Galea, A. (2014). The NHS productivity challenge. The King's Fund. Available at: https://www.kingsfund.org.uk/publications/nhs-productivity-challenge (Accessed March 11, 2024).
52. Bates, DW, and Singh, H. Two decades since to err is human: an assessment of progress and emerging priorities in patient safety. Health Aff. (2018) 37:1736–43. doi: 10.1377/hlthaff.2018.0738
53. Halpern, J. Empathy and patient-physician conflicts. J Gen Intern Med. (2007) 22:696–700. doi: 10.1007/s11606-006-0102-3
54. Gagnon, MP, Desmartis, M, Labrecque, M, Car, J, Pagliari, C, Pluye, P, et al. Systematic review of factors influencing the adoption of information and communication technologies by healthcare professionals. J Med Syst. (2012) 36:241–77. doi: 10.1007/s10916-010-9473-4
55. Greenhalgh, T, and Swinglehurst, D. Studying technology use as social practice: the untapped potential of ethnography. BMC Med. (2011) 9:45. doi: 10.1186/1741-7015-9-45
56. Dixon-Woods, M, and Martin, GP. Does quality improvement improve quality? Future Hosp J. (2016) 3:191–4. doi: 10.7861/futurehosp.3-3-191
57. Ho, C, Chee, D, and Chai, J. Precision and personalized medicine: how genomic approach improves the Management of Cardiovascular and Neurodegenerative Disease. Genes. (2020) 11:747. doi: 10.3390/genes11070747
58. Kang, M, Park, E, Cho, BH, and Lee, K-S. Recent patient health monitoring platforms incorporating internet of things-enabled smart devices. Int Neurourol J. (2018) 22:S76–82. doi: 10.5213/inj.1836144.072
59. Christou, C, and Tsoulfas, G. Challenges and opportunities in the application of artificial intelligence in gastroenterology and hepatology. World J Gastroenterol. (2021) 27:6191–223. doi: 10.3748/wjg.v27.i37.6191
60. Herd, GCE, and Musaad, SMA. Point-of-care testing in rural and remote settings to improve access and improve outcomes: a snapshot of the New Zealand experience. Arch Pathol Lab Med. (2021) 145:327–35. doi: 10.5858/arpa.2020-0104-RA
61. Kaur, C, Mohammed Al-Ansari, AR, Gongada, TN, Saravanan, KA, Rao, DS, Cosio Borda, RF, et al. Integrating transfer learning and deep neural networks for accurate medical disease diagnosis from multi-modal data. Int J Adv Comput Sci Appl. (2023) 14:857. doi: 10.14569/IJACSA.2023.0140857
62. Elwyn, G, Frosch, D, and Rollnick, S. Dual equipoise shared decision making: definitions for decision and behaviour support interventions. Implement Sci. (2009) 4:75. doi: 10.1186/1748-5908-4-75
63. Ovretveit, J, Bate, P, Cleary, P, Cretin, S, Gustafson, D, McInnes, K, et al. Quality collaboratives: lessons from research. Qual Saf Health Care. (2002) 11:345–51. doi: 10.1136/qhc.11.4.345
64. Porter, ME. What is value in health care? N Engl J Med. (2010) 363:2477–81. doi: 10.1056/NEJMp1011024
65. Hwang, J, and Christensen, CM. Disruptive innovation in health care delivery: a framework for business-model innovation. Health Aff. (2008) 27:1329–35. doi: 10.1377/hlthaff.27.5.1329
66. Wijsen, LD, Borsboom, D, and Alexandrova, A. Values in psychometrics. Perspect Psychol Sci. (2021) 17:788–804. doi: 10.1177/17456916211014183
67. Eccles, MP. Mittman BS welcome to implementation science. Implement Sci. (2006) 1:1–3. doi: 10.1186/1748-5908-1-1
68. Fong, PSW, Shen, Q, and Cheng, EWL. A framework for benchmarking the value management process. Benchmarking Int J. (2001) 8:306–16. doi: 10.1108/14635770110403800
69. Angelis, A, Lange, A, and Kanavos, P. Using health technology assessment to assess the value of new medicines: results of a systematic review and expert consultation across eight European countries. Eur J Health Econ. (2017) 19:123–52. doi: 10.1007/s10198-017-0871-0
70. Angelis, A, and Kanavos, P. Multiple criteria decision analysis (MCDA) for evaluating new medicines in health technology assessment and beyond: the advance value framework. Soc Sci Med. (2017) 188:137–56. doi: 10.1016/j.socscimed.2017.06.024
71. Rathert, C, Wyrwich, MD, and Boren, SA. Patient-centered care and outcomes: a systematic review of the literature. Med Care Res Rev. (2013) 70:351–79. doi: 10.1177/1077558712465774
72. Greenhalgh, T, Robert, G, Macfarlane, F, Bate, P, and Kyriakidou, O. Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Q. (2004) 82:581–629. doi: 10.1111/j.0887-378X.2004.00325.x
73. Kaplan, RS, and Porter, ME. How to solve the cost crisis in health care. Harv Bus Rev. (2011) 89:46–64.
74. Topol, EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. (2019) 25:44–56. doi: 10.1038/s41591-018-0300-7
75. Medicines and Healthcare products Regulatory Agency. (2023). The innovative devices access pathway (IDAP) - pilot phase. Available at: https://www.gov.uk/government/publications/the-innovative-devices-access-pathway-idap (Accessed March 11, 2024).
76. Augustovski, F, Colaci, C, Mills, M, Chavez, D, Argento, F, Alfie, V, et al. A systematic review of value criteria for next-generation sequencing/comprehensive genomic profiling to inform value framework development. Value Health. (2024) 27:670–85. doi: 10.1016/j.jval.2024.02.002
77. Powell, D, and Hannah, A. The dichotomy of diagnostics: exploring the value for consumers, clinicians, and care pathways. NPJ Digit Med. (2024) 7:101. doi: 10.1038/s41746-024-01087-8
Keywords: value proposition, diagnostics, digital health, stakeholder perspectives, conceptual framework
Citation: Soukup T, Zamora-Talaya B, Bahadori S, Luxardo R, Kierkegaard P, Butt O, Kettley-Linsell H, Savva K-V, Micocci M, Zhou S, Newman S, Walne S, Peters CJ, Gordon A, Ni M, Buckle P, Hanna GB and NIHR HRC IVD PPIE Team (2025) Defining the value proposition in diagnostic technology: challenges and opportunities for its understanding and development – a review with a multiperspective reflective analysis. Front. Med. 12:1498618. doi: 10.3389/fmed.2025.1498618
Edited by:
Hosna Salmani, Iran University of Medical Sciences, IranReviewed by:
Prakamya Gupta, National Health Systems Resource Center, IndiaJuli Goldstein, Digital Diagnostics Inc., United States
Copyright © 2025 Soukup, Zamora-Talaya, Bahadori, Luxardo, Kierkegaard, Butt, Kettley-Linsell, Savva, Micocci, Zhou, Newman, Walne, Peters, Gordon, Ni, Buckle, Hanna and NIHR HRC IVD PPIE Team. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tayana Soukup, dC5zb3VrdXBAaWMuYWMudWs=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share second authorship
 Tayana Soukup
Tayana Soukup Bernarda Zamora-Talaya
Bernarda Zamora-Talaya Shayan Bahadori1‡
Shayan Bahadori1‡ Patrick Kierkegaard
Patrick Kierkegaard Hannah Kettley-Linsell
Hannah Kettley-Linsell Melody Ni
Melody Ni