- 1Department of Hepatobiliary Surgery, the First Affiliated Hospital of Shenzhen University, Health Science Center, Shenzhen Second People’s Hospital, Shenzhen, China
- 2Guangxi University of Chinese Medicine, Nanning, China
- 3Department of Clinical Medicine, Guizhou Medical University, Guiyang, China
- 4Department of Physiology, School of Medicine and State Key Laboratory of Bioactive Molecules and Druggability Assessment, Jinan University, Guangzhou, China
- 5Institute of Biopharmaceutical and Health Engineering, Shenzhen Key Laboratory of Gene and Antibody Therapy, State Key Laboratory of Chemical Oncogenomics, Tsinghua University Shenzhen International Graduate School, Guangdong, China
The liver is a vital organ responsible for numerous metabolic processes in the human body, including the metabolism of drugs and nutrients. After liver damage, the organ can rapidly return to its original size if the causative factor is promptly eliminated. However, when the harmful stimulus persists, the liver’s regenerative capacity becomes compromised. Substantial theoretical feasibility has been demonstrated at the levels of gene expression, molecular interactions, and intercellular dynamics, complemented by numerous successful animal studies. However, a robust model and carrier that closely resemble human physiology are still lacking for translating these theories into practice. The potential for liver regeneration has been a central focus of ongoing research. Over the past decade, the advent of organoid technology has provided improved models and materials for advancing research efforts. Liver organoid technology represents a novel in vitro culture system. After several years of refinement, human liver organoids can now accurately replicate the liver’s morphological structure, nutrient and drug metabolism, gene expression, and secretory functions, providing a robust model for liver disease research. Regenerative medicine aims to replicate human organ or tissue functions to repair or replace damaged tissues, restore their structure or function, or stimulate the regeneration of tissues or organs within the body. Liver organoids possess the same structure and function as liver tissue, offering the potential to serve as a viable replacement for the liver, aligning with the goals of regenerative medicine. This review examines the role of liver organoids in regenerative medicine.
Introduction
The liver organoids are laboratory-engineered models that replicate the liver’s structure and functions using three-dimensional culture and stem cell technologies. Despite their promising potential in medical research and clinical applications, liver organoids face several challenges. First, current liver organoids models often fail to fully replicate the structural and functional complexity of the adult liver, limiting their application in long-term drug testing and disease research. Second, improving the maturity and functionality of liver organoids—particularly their metabolic capacity and immune response—remains a critical challenge. Additionally, challenges such as large-scale production, standardized culture conditions, and the adaptability of liver organoids to individual human variations must be addressed for future clinical applications. In view of the above issues, this review discusses the future development and application prospects of liver organoids in experimental regenerative medicine.
The liver is a vital organ in the human body that is responsible for detoxification, digestion, and nutrient metabolism. Common liver diseases include viral and nonviral hepatitis, hepatic hemangioma, cirrhosis/fibrosis, traumatic liver injury, and liver cancer (1). The adult liver possesses a robust regenerative capacity, allowing it to rapidly restore function following damage (2). Even when a portion of the liver is removed, the remaining liver cells can swiftly proliferate to restore the organ’s volume and function (3). In mammals, when 75 to 85% of the liver is surgically removed, the remaining portion cannot compensate for systemic metabolism, often resulting in fatal posthepatectomy liver failure (PHLF) (4, 5). Chronic damage to the liver typically reduces its regenerative capacity over time (6, 7). Organoid technology has garnered significant attention since its initial discovery (8). Liver organoids, which are three-dimensional cell clusters that mimic the structure and function of the liver, have become a focal point in drug development, metabolism studies, safety assessments, and precision medicine (9–11). Regenerative medicine involves the development of functional tissues and materials to repair or replace damaged organs and tissues or to promote the regeneration of tissues and organs through biological, chemical, and physical means (12). Undoubtedly, the emergence of liver organoids represents a powerful experimental model and a potential tool in regenerative medicine.
The research model in liver injury
Liver injury can be categorized as either acute or chronic. Acute liver injury often results from traumatic liver ruptures or nontraumatic causes, such as drug-induced liver injury and acute hepatitis A. Chronic liver injury is predominantly caused by hepatitis viruses or autoimmune diseases (13). Traumatic liver injury is relatively common, with numerous cases reported annually (14). Due to the liver’s abundant blood supply and bile secretion, liver injury can lead to shock and acute peritonitis (15). Timely blood volume support and surgical intervention are effective treatments for this type of injury (16). Nontraumatic liver injury often occurs after the consumption of hepatotoxic substances or drugs or following hepatitis A virus infection within a short period (17, 18). Clinically, nontraumatic liver injury is characterized by upper abdominal pain, gastrointestinal symptoms, such as nausea and vomiting, and elevated transaminase and bilirubin levels in biochemical tests (19). Most individuals achieve a favorable prognosis by avoiding hepatotoxic agents and using hepatoprotective drugs, such as glutathione.
The hepatitis B virus (HBV) is the most common cause of chronic liver injury (20). The prevailing view is that long-term HBV infection inhibits virus-specific T-cell function and downregulates innate immune signaling pathways, enabling the virus to evade immune surveillance (21). However, high-level viral replication and the production of subviral hepatitis B surface antigen (HBsAg) particles play crucial roles in promoting liver damage (22). Chronic liver damage leads to edema and fatty degeneration of hepatocytes, triggering persistent low-grade inflammation (23). This is often accompanied by transient high-grade inflammation and the activation of fibrogenic processes, ultimately resulting in viral liver fibrosis (24). Currently, the primary method for preventing hepatitis B virus infection is the administration of the hepatitis B vaccine. Clinical treatment primarily involves the use of interferons and antiviral drugs, although a standardized treatment protocol has not yet been established.
As liver fibrosis progresses, the liver’s basic structure is gradually compromised, eventually leading to irreversible cirrhosis (25). Cirrhosis is a prevalent global disease with common causes including hepatitis B virus infection, excessive fat and lipid intake, and autoimmune hepatitis (26). The main symptoms include liver dysfunctions, such as portal hypertension, ascites, and hypoalbuminemia (27). Treatment focuses on addressing the underlying cause and managing complications, with liver transplantation a viable option in certain cases (27, 28). As cirrhosis advances, regenerative disorders may arise, potentially progressing into hepatocellular carcinoma (29, 30), which is the eighth most prevalent cancer and the third leading cause of cancer-related deaths globally (31).
In the early stages of liver disease, symptoms may be subtle or entirely absent. Space-occupying liver lesions are frequently discovered incidentally during routine physical examinations. Diagnosis typically involves a combination of blood alpha-fetoprotein (AFP) levels, enhanced computed tomography (CT), Magnetic resonance imaging (MRI), and ultrasound angiography (32, 33). Treatment options, depending on the stage of the disease and the patient’s specific condition, include surgical resection, local ablation, interventional therapy, pharmacotherapy, or liver transplantation (34). Unfortunately, the prognosis remains poor even with these treatments (35). Hepatitis B virus infection, cirrhosis, and hepatocellular carcinoma are closely interconnected, forming a critical disease (36). Hepatitis B virus infection is a leading cause of cirrhosis and hepatocellular carcinoma, particularly in Asia (37). Cirrhosis represents a critical intermediate stage in the progression to hepatocellular carcinoma, which is the most severe outcome for patients with cirrhosis (36). For patients with advanced cirrhosis and hepatocellular carcinoma, liver transplantation is a viable treatment option that can significantly enhance survival and quality of life (27, 38, 39).
Liver damage may also result from immune-mediated and genetic disorders. Immune-mediated liver damage, such as autoimmune hepatitis, occurs when the immune system mistakenly targets liver cells, potentially leading to chronic inflammation and significant hepatic injury (40). Primary biliary cholangitis and primary sclerosing cholangitis are additional immune-mediated liver diseases that affect the bile ducts, potentially causing cholestasis and hepatic damage (41). Conversely, hereditary liver diseases, such as hemochromatosis, Wilson’s disease, and α1-antitrypsin deficiency, result from genetic mutations inherited from parents (42, 43). These genetic mutations impair the liver’s ability to metabolize specific substances, leading to their accumulation and subsequent liver damage. For instance, Wilson’s disease involves defective copper metabolism, whereas α1-antitrypsin deficiency impacts protease activity (42, 44). Despite their relative rarity, these disorders significantly affect patient health (45). Additional causes of liver damage include alcoholic liver damage, nonalcoholic fatty liver disease (NAFLD) due to fat accumulation, and drug-induced liver injury (Figure 1). As of 2023, liver diseases have resulted in over 2 million deaths globally, with an increasing trend (46). All of these conditions, with the exception of traumatic liver damage (which involves fewer affected areas), contribute to a reduction in liver regenerative capacity (47).
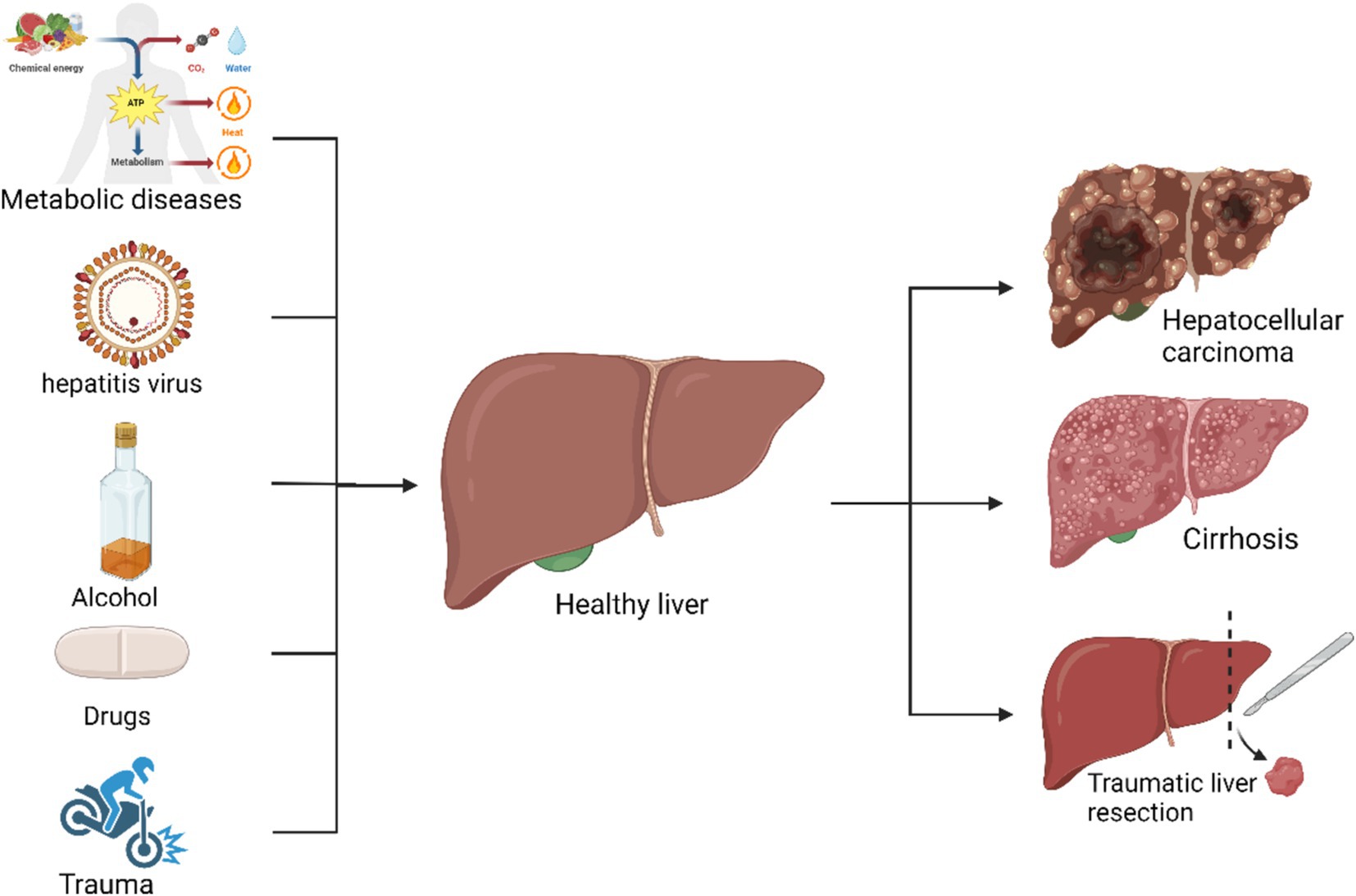
Figure 1. Common factors contributing to liver injury and their consequences: autoimmune diseases, viral hepatitis, alcohol, drugs, and trauma can cause cirrhosis, hepatocellular carcinoma, and acute liver failure.
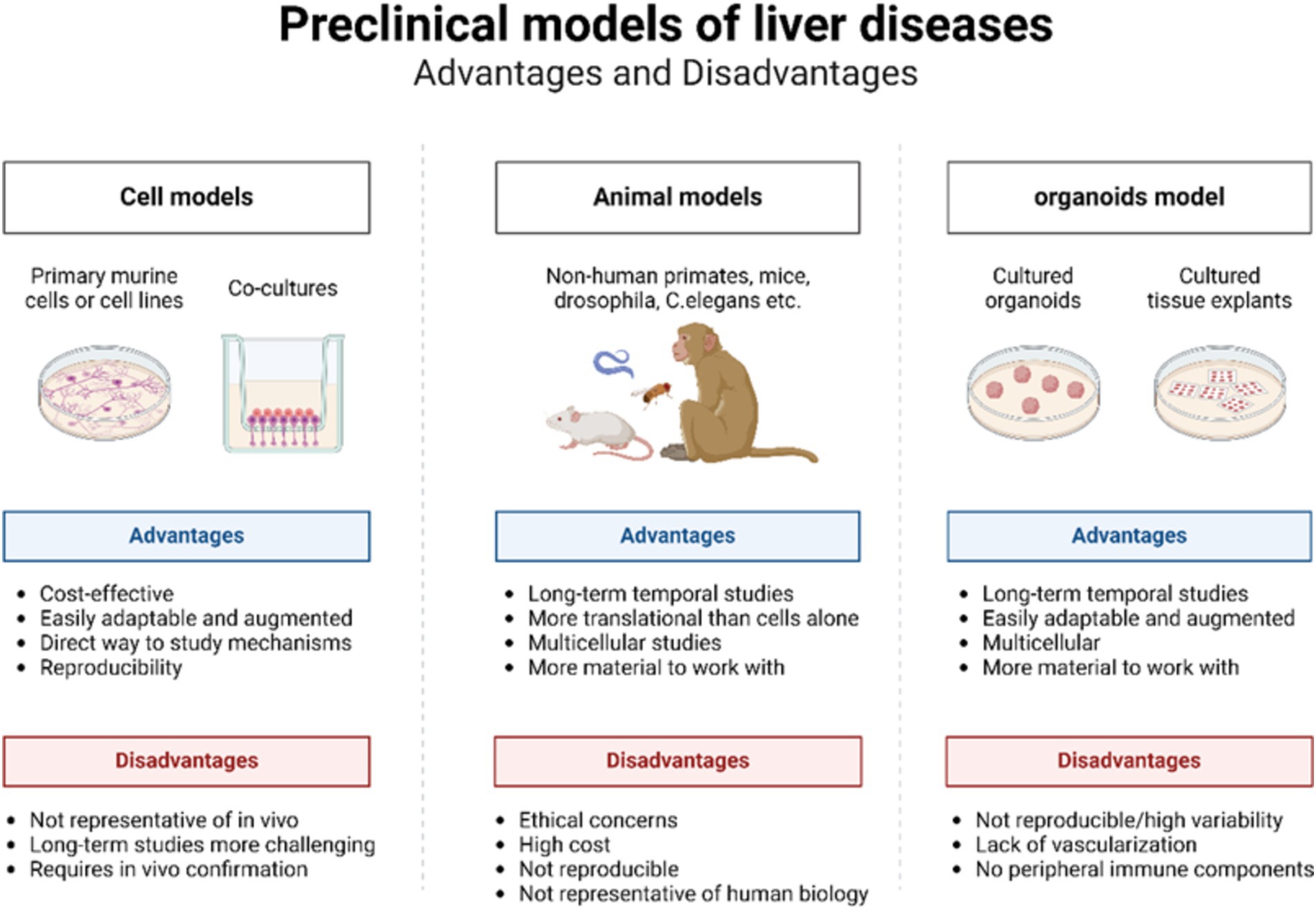
Traditionally, animal models and in vitro cell models have been extensively utilized to investigate liver diseases (Figure 2) (48). Animal models closely mirror the physiological metabolic processes, tissues, and microenvironment of the human body (49). These models aid in elucidating the pathogenesis of liver diseases, assessing drug efficacy, and investigating novel treatments. Among the various models used for liver diseases, the mouse model is the most prevalent. Models of cirrhosis and fibrosis are typically induced through bile duct ligation, thioacetamide administration, or high-concentration alcohol infusion into the hepatic artery (28, 50). Liver cancer models are commonly established using methods such as chemical induction, dietary modification, genetic engineering, heterotopic transplantation, and humanized mouse systems (51). Numerous construction methods and animal models exist, although deviations are common due to species differences and variations in disease progression (52). The long duration, high cost, and ethical constraints associated with animal models limit the feasibility of short-term, homogeneous, and high-throughput experiments (48).
Traditional 2D cell models, which involve culturing single layers of cells, are straightforward to culture and grow rapidly (53, 54). However, they are confined to a 2D structure consisting of a single layer of cells adhering to a culture medium. These models lack the matrix and immune cells essential for regulating cell behaviors, such as differentiation, survival, proliferation, and migration, making it impossible to accurately simulate the organ structure (55). Consequently, 2D models fail to provide the varied levels of oxygen and nutrients required for multidimensional cell behavior (52). Furthermore, repeated subcultures often lead to the loss of the original cell characteristics (56, 57). As a central organ for food, drug, and energy metabolism, the liver is characterized by a complex and multilayered structure, thus posing significant challenges for 2D cell models. While 2D models can offer valuable insights, they cannot fully replicate the complexity and multidimensional nature of human liver disease.
The advances in liver organoids
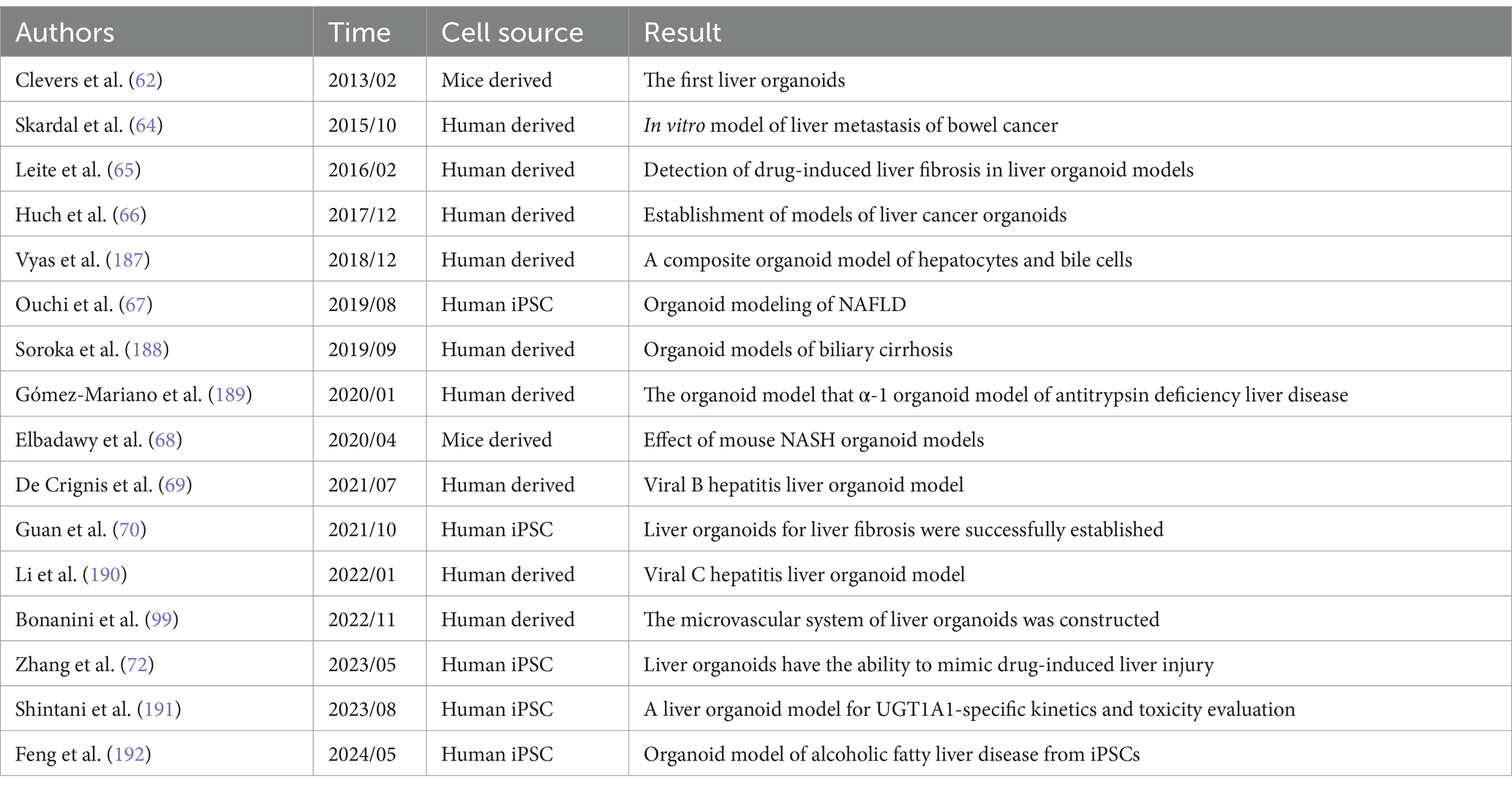
The organoids represent a novel technology that has emerged over the past decade (58). These models exhibit a three-dimensional tissue structure and retain functions and characteristics similar to those of the original tissue (59). The development history of liver organoids is summarized in Table 1 and Figure 3. Clevers et al. (60) successfully cultured intestinal organoids from Lgr+ intestinal stem cells. The authors utilized pluripotent adult stem cells with high plasticity and self-renewal capacity to explore methods for simulating natural growth conditions, precisely controlling growth factors and the cellular microenvironment and guiding stem cells to differentiate along specific pathways to form organoid structures (61). Huch et al. (62) cultured liver organoids from damaged Lgr5+ bile duct cells and demonstrated that these organoids maintained the original characteristics of the liver even after long-term proliferation. A subsequent study indicated that liver organoids preserve most of their characteristics even during extended proliferation and passage (63). Skardal et al. (64) developed an in vitro model of colorectal cancer liver metastasis. Leite et al. (65) established an organoid model of liver fibrosis. In 2017, they successfully established primary liver tumor (PLC) organoids and showed that these organoids retained the characteristics and heterogeneity of liver cancer (66). Ouchi et al. (67) developed an organoid model of nonalcoholic fatty liver disease (NAFLD) in 2019. In the subsequent year, Elbadawy et al. (68) created a mouse-derived organoid model for nonalcoholic steatohepatitis (NASH). Later, De Crignis et al. (69) successfully cultured liver organoids infected with the hepatitis virus. Organoid models for various liver diseases, such as liver fibrosis, drug-induced liver injury, and glycogen storage disease type Ia (GSD1a), have now been developed (70–72). Based on the number of passages, organoids are classified into short-term and long-term. Short-term organoids are cultured for up to 30 generations or 3 months and are primarily used for low-throughput drug screening, biomarker identification, and the exploration of drug sensitivity and resistance mechanisms (73). In contrast, long-term organoids are suited for high-throughput drug screening and the development of organoid databases (74, 75).
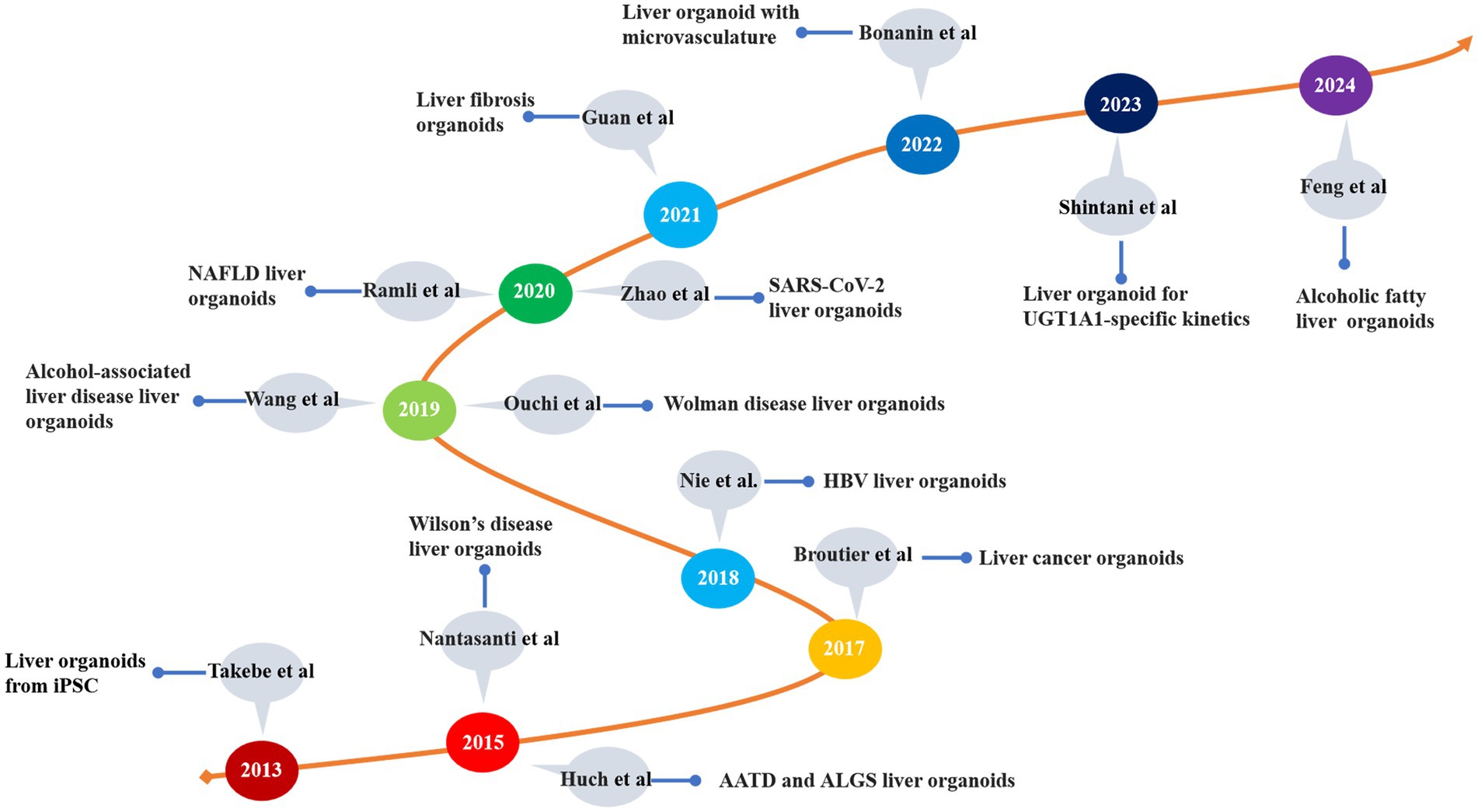
Figure 3. The development of liver disease models. iPSC, induced pluripotent stem cell; AATD, alpha-1 antitrypsin deficiency; ALGS, Alagille syndrome; HBV, hepatitis B virus; NAFLD, nonalcoholic fatty liver disease; UGTIA1, UDP glucuronosyltransferase family 1 member A1.
Organoids are categorized based on their cell sources into two main types: tissue-derived organoids and pluripotent stem cell-derived organoids, including adult stem cells (ASCs), pluripotent stem cells (PSCs), and patient-derived organoids (PDOs). PSCs are subclassified into embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) (Figure 4) (76). Patient-derived organoids (PDOs) are sourced from mature tissues and preserve the morphological, physiological, and genetic characteristics of the original tissues (77). These organoids are relatively straightforward and require less time to culture and exhibit high cell maturity (78, 79). In 2017, three types of liver tumor organoids—hepatocellular carcinoma, cholangiocarcinoma, and mixed-cell carcinoma—were established from eight liver tumor patients, retaining the morphological and physiological characteristics of the original tumors (66). The following year, Nuciforo et al. (80) utilized tumor needle biopsy technology to generate liver tumor organoids from biopsy samples of hepatocellular carcinoma (HCC) patients, preserving the histological, transcriptomic, and genetic characteristics of the original tumors. While Wu et al. (81) expanded circulating tumor cells (CTCs) from 41 liquid biopsy samples of 31 pancreatic ductal adenocarcinoma (PDAC) patients to develop CTC-derived organoids in vitro, there are currently no reports of successfully inducing malignant liver tumor organoids from CTCs. Nevertheless, mature liver tissue can still be used to establish liver organoids, even after extended cryopreservation (82).
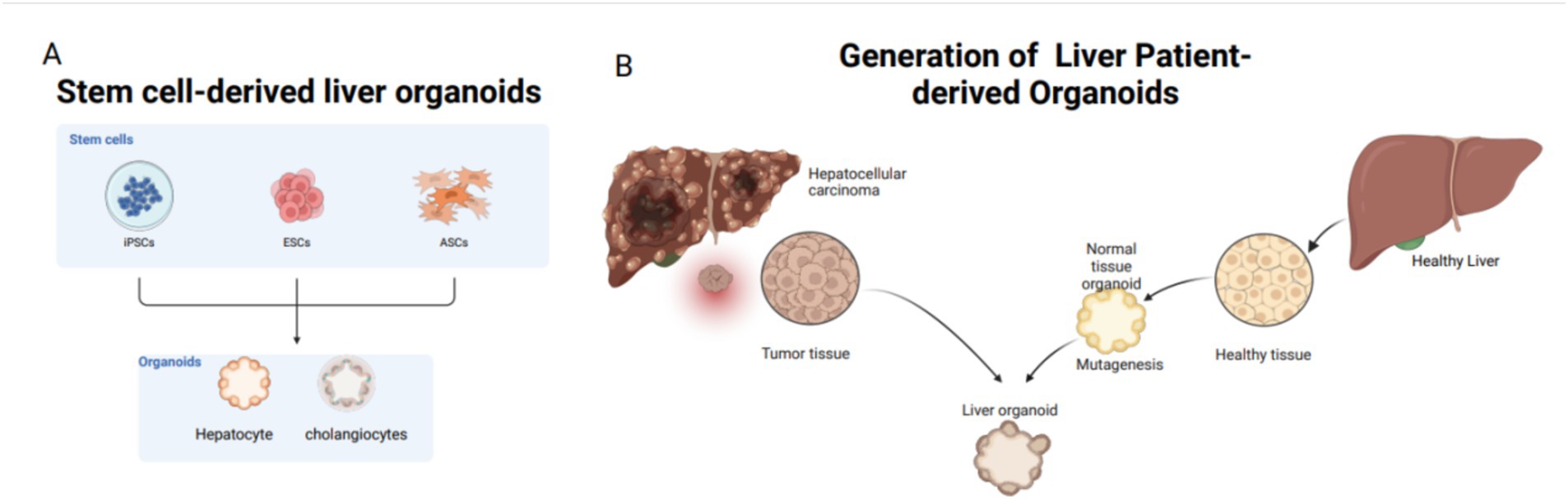
Figure 4. Common tissue sources for liver organoids: stem cells and adult cells. Stem cells include iPSCs, ESCs, and ASCs; adult cells mostly come from patients. (A) Sources of stem cells for liver organoids: from ECS, iPSC, ASCs. (B) Patient derived, mainly from liver tissue removed after clinical surgery.
PSC-derived organoids originate from ESCs or iPSCs. Cells such as skin fibroblasts are first reprogrammed into pluripotent stem cells in a culture medium and subsequently differentiated into liver cells for organoid culture (83). Since the first iPSCs were generated from adult mouse cells in 2006 (84), subsequent studies have demonstrated the applicability of iPSC technology to human cells (85). CRISPR/Cas9 technology has been employed to overexpress the c-Myc gene, successfully inducing HCC organoids from human liver cell organoids (86). Adult cells from human sources are reprogrammed into iPSCs through the knockout of specific transcription factors, followed by differentiation into liver cells. Although the process is time-consuming, the resulting organoids possess a complex array of cellular components, including mesenchymal, epithelial, and potentially endothelial cells (87). Compared to patient-derived organoids, PSC-derived organoids are particularly useful for studying the early stages of human organ development (88). Additionally, organoids can be derived from human tissues that difficulty to obtain, such as the brain, heart, and bone marrow. Currently, the availability of tissues for establishing liver organoids is increasing, and the associated materials are becoming more accessible.
The technology in liver organoids
The production of liver organoids has become relatively advanced. The main steps are as follows: tissue is digested into single cells using digestive enzymes (e.g., collagenase, DNAse, and dispase) filtered through a sieve and mixed with matrix gel to form microspheres. Subsequently, signal molecules and biological agents that simulate stem cell growth factors required by stem cells are added. For instance, hepatocyte growth factor (HGF), epidermal growth factor (EGF), fibroblast growth factor (FGF), and Wnt family member 3A (Wnt3A) promote cell proliferation, while dexamethasone, bone morphogenetic protein (BMP), and other cytokines induce hepatocyte maturation. Additionally, mTeSR facilitates the differentiation of bile duct cells (62, 89). In recent years, advancements across multiple disciplines have driven innovation in organoid technology.
Microfluidics is a technology that utilizes microchannels ranging from tens to hundreds of micrometers to process and manipulate small volumes of fluid. This technology can precisely control the cellular environment in fields such as biology and biomedical engineering (90). Microfluidic technology allows for precise control over organoids and provides dynamic physical conditions that facilitate the production of high-throughput, uniform organoid microspheres (91). Organoid microspheres produced using this technology more accurately replicate the in vivo liver microenvironment and the interactions between the liver organ model and other tissues and organs (92). This approach holds significant potential for disease simulation, personalized medicine, drug screening, and regenerative medicine (93). Organ-on-a-chip technology employs microfabrication techniques to create a micromodel of a biological organ on a chip-sized device (94). The integration of organoid and organ-on-a-chip technologies results in organoid chips (organoids-on-a-chip). By incorporating microfluidic technology, these chips enable the construction of more precise and controllable organoid models that simulate the microenvironment, vascularization, and tissue interactions (95). Microfluidic liver organoid chips are designed to simulate the in vivo liver sinusoid structure and the liver microphysiological environment. This approach partially compensates for the limitations in the structure, composition, and mechanical microenvironment observed in conventional liver organoids (96–98). Co-culturing vascular endothelial cells with liver cells on the chip simulates a three-dimensional vascular network, potentially offering an in vitro alternative to animal models and conventional transplantation (99).
3D bioprinting is a branch of 3D printing that uses cells and an extracellular matrix (ECM) as raw materials to construct the desired biological structures (100). This technology can directly print complex tissue or organ structures and has garnered significant attention across various medical fields (101). The primary methods of 3D bioprinting include inkjet, extrusion, laser-assisted bioprinting (LAB), and acoustic-assisted bioprinting (102). This technology has been employed to print various tissues, including skin, heart, bone, cartilage, liver, lung, nerve, and pancreatic tissues (103). 3D bioprinting plays a crucial role in drug screening, disease modeling, and liver regenerative medicine (104, 105). Organoids possess complex biological components and structures, including inherent 3D architecture (59). The synergy between organoids and 3D bioprinting represents a highly promising approach. Currently, the requirements for bioink composition are more stringent than those for 2D cell cultures. Bioink is a specialized material used in bioprinting that supports living cells and other biological components, providing a matrix for cell growth and organization (106, 107). Liver endothelial cells can be layered and printed into a composite organoid model, demonstrating a well-developed vascular and bile duct system, thereby maximizing liver function (108). Using multicellular 3D droplets, HepaRG/HUVECs/LX-2 liver organoids with biomimetic lobule structures have been constructed, effectively simulating cell heterogeneity, spatial organization, and ECM characteristics (109). Current methods for constructing liver organoids still face challenges related to cell heterogeneity, tissue structure, and the integrity of functional components. Therefore, developing liver organoids using integrated design strategies and multidisciplinary engineering methods is paramount.
The application of liver organoids in liver regeneration
The regenerative capacity of a healthy liver is robust. Even after a major hepatectomy, it can regenerate to its original size within a relatively short period (110). The number of hepatocytes and their sizes both increase, while the number of hepatic lobules decreases (3). In chronic liver injury, the liver’s regenerative capacity becomes unbalanced due to cell damage, repair processes, extracellular matrix deposition, and subsequent structural alterations (111, 112). In viral hepatitis and immune-mediated liver disease, chronic injury is characterized by ongoing repair and regeneration and leads to an imbalance between these two processes over time (113). Ultimately, fibrous tissue repair compresses hepatocyte space, inhibits liver regeneration, and results in cirrhosis characterized by diffuse liver fibrosis, pseudolobules, and regenerative nodules (114). This progression can lead to end-stage liver diseases, including portal hypertension and hepatocellular carcinoma (115, 116).
Regenerative medicine has been a field of exploration for many years. It has evolved from a search for regenerative tissues or organs to methods that stimulate the regeneration of an individual’s own tissues or organs (12). Bone and tooth regeneration techniques are currently well developed (117, 118). However, progress in liver regeneration remains relatively slow. Liver organoids hold a significant position and offer substantial potential in regenerative medicine (Table 2).
When the liver is injured or a significant portion is removed, the remaining liver may temporarily be unable to compensate, potentially leading to post-hepatectomy liver failure (PHLF), a serious complication and cause of death following liver surgery (119). Clinicians use artificial liver blood purification systems (ALBPS) as extracorporeal temporary replacement therapy, analogous to extracorporeal membrane oxygenation (ECMO) systems. These systems provide time for the remaining liver to regenerate; however, their treatment efficacy remains limited (120). Artificial livers are extracorporeal devices designed for detoxification and metabolic functions (121). They play a pivotal role in treating liver failure and are recommended as a primary option for patients with this condition (122). These devices can be broadly categorized into two types, nonbioartificial livers (NBALs) and bioartificial livers (BALs), which can also be combined to form hybrid artificial livers (HALs) (123). Nonbioartificial livers operate primarily by purifying blood through techniques such as plasma exchange (PE), hemodialysis (HD), hemofiltration (HF), and hemoperfusion (HP)/plasma perfusion (PP).
These devices focus on the detoxification and filtration of metabolic wastes using systems such as the molecular adsorption recirculating system (MARS), fractionated plasma separation and adsorption, and therapeutic plasma exchange (TPE) (124). A bioartificial liver primarily consists of functional hepatocytes within an in vitro bioreactor. Hepatocytes perform metabolism, synthesis, and secretion either through blood flow across a semipermeable membrane or by direct contact. These devices are typically used for short-term support (125). Compared to NBALs, BALs are composed of functional hepatocytes that provide essential liver functions, including detoxification, metabolite synthesis, and biotransformation (126). Hybrid artificial livers integrate both approaches: the nonbiological component handles liver detoxification, while the bioartificial component performs detoxification, synthesis, and biotransformation. This combination offers complementary benefits and holds promise for future advancements. Addressing the challenges of stability and long-term functionality in bioartificial livers could potentially be enhanced by integrating liver organoids.
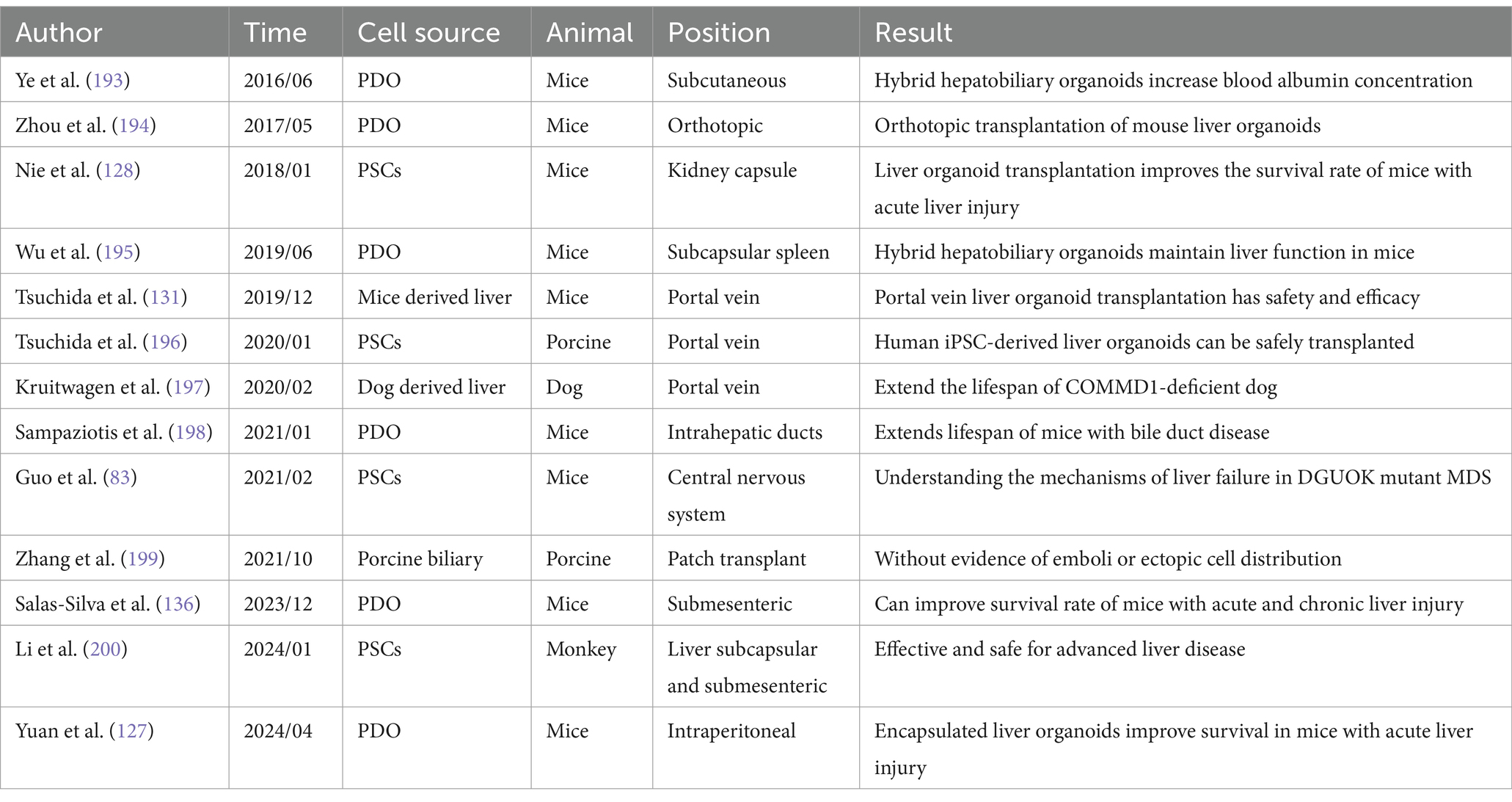
Organoid transplantation has emerged as a prominent topic in regenerative medicine and has shown promising results in animal experiments (Table 2). Yuan et al. (127) transplanted proliferating human hepatocytes (ProliHHs) encapsulated in organoids into the peritoneal cavity of mice with PHLF. This approach aimed to enhance liver regeneration, reduce endotoxins and hyperammonemia, alleviate hypoglycemia, and improve overall survival (127). Functional liver organoid (LO) tissue generated from the endoderm, endothelial cells (EC), and mesenchymal cells (MC) derived from human-induced pluripotent stem cells (hiPSCs) from a single donor can significantly enhance the survival rate and liver function of mice with acute liver injury (128). Such liver organoid grafts need to survive for approximately 1 week to allow the liver to regenerate sufficiently to support systemic metabolic functions. To address this need, Labour et al. (129) developed a structurally adjustable, animal-free, three-dimensional porous scaffold that evenly distributes cells and supports their survival for over 7 days. When combined with other advancements, this scaffold has potential for clinical application.
In chronic liver damage, such as viral hepatitis, advanced disease stages involve concurrent damage, repair, and regeneration. The resulting fibrous tissue compresses functional liver cells, leading to liver failure and symptoms such as ascites and spider nevi (130). In a rat model of chronic liver injury, liver organoid transplantation via the portal vein significantly improved bile reconstruction rates and the replacement of damaged liver tissue. Additionally, ductal reactions were reduced, and precancerous lesions marked by placental glutathione S-transferase (GST-p) were diminished (131). In cases of portal hypertension caused by cirrhosis, transjugular intrahepatic portosystemic shunt (TIPS) and portal vein shunt surgeries can alleviate symptoms such as ascites and hematemesis (132). However, these treatments are ineffective against hepatic encephalopathy caused by hyperammonemia, which can only be managed by restricting protein intake and using medications (133). Combining 3D printing technology with liver organoids to replace liver cell functions can facilitate the degradation of toxic substances, thereby alleviating hepatic encephalopathy (134). Compared to hepatocyte-derived artificial livers, liver organoids offer more precise regulation of biomarkers such as Aspartate aminotransferase (AST), Alanine transaminase (ALT), and blood ammonia (135, 136).
Due to trauma, the liver sustains significant damage. Following emergency surgery, the remaining liver tissue is insufficient to meet the metabolic demands of the body. In such cases, the primary clinical treatment involves providing artificial liver support while waiting for the liver to regenerate to a level that meets the body’s minimum requirements (137). Liver organoids may assist in repairing damaged liver tissue through transplantation or cell therapy. Transplanting liver organoids, or mature hepatocytes derived from them, into the liver of patients with traumatic liver injury can accelerate liver repair by promoting hepatocyte proliferation, differentiation, and tissue regeneration.
Hemochromatosis is an abnormality in iron metabolism that results in excessive iron accumulation in the blood (138). This surplus iron is deposited in various organs, with the liver being the primary site of storage. As a result, liver lesions are a major cause of complications such as unexplained cirrhosis, bronze skin, and diabetes, with an increased risk of subsequent hepatocellular carcinoma (139). In clinical practice, diagnosis is confirmed through a positive genetic test (140). Treatment is primarily aimed at reducing blood iron levels through methods such as phlebotomy and iron chelation therapy. Patients with hemochromatosis often experience pathological changes in the liver, including damage, inflammation, and fibrosis, which can progress to liver failure (138). In this case, liver organoids offer a valuable tool for simulating and evaluating the effects of iron overload on hepatocyte function, as well as exploring potential restorative therapies through cell-based approaches.
Wilson’s disease is a genetic disorder characterized by impaired copper metabolism, leading to the accumulation of copper in the body (44). This copper overload can cause damage to the liver, nervous system, and other organs, with the liver being the most significantly affected organ in patients (44). Chronic copper accumulation results in liver damage, inflammation, fibrosis, liver failure, and even hepatocellular carcinoma (141, 142). The primary treatments for Wilson’s disease include pharmacological interventions, such as copper chelators, and liver transplantation (143). However, these therapies do not fully restore liver function and may be associated with side effects. In cases of advanced liver damage or liver failure, liver transplantation is often the only viable treatment option (144). Liver organoids hold significant potential in regenerative medicine, offering a promising approach to repair liver damage. These organoids can expand and differentiate into large numbers of functional hepatocytes in vitro, making them a potential alternative to liver transplantation or a “biological patch” for partially damaged liver tissue. Furthermore, the strong differentiation and proliferative capacity of organoids provide a novel strategy for promoting liver repair and regeneration in patients with Wilson’s disease.
Autoimmune hepatitis (AIH) is a chronic inflammatory liver disease caused by an abnormal immune response, characterized by immune cell attacks (especially T cells) on the liver, leading to liver damage, inflammation, and fibrosis (145). If untreated, AIH can progress to liver failure, cirrhosis, and even hepatocellular carcinoma (40). AIH is strongly associated with human leukocyte antigen (HLA) genes. The primary susceptible genotype in the European population is HLA-DRB10301, while the secondary susceptible genotype is HLA-DRB10401 (146). The pathophysiology of AIH involves an autoimmune attack on liver cells triggered by T cell activation (145). As a result, prednisone and azathioprine are commonly recommended as first-line treatments (147). In the chronic stage of AIH, the liver may develop pathological changes, including fibrosis and connective tissue hyperplasia, which can ultimately lead to liver failure (40). The regenerative potential of liver organoids offers a promising new avenue for treating AIH. By transplanting liver organoids derived from the patient or a donor, or by inducing the proliferation of liver organoids in vitro and directing them to the site of liver damage, liver tissue regeneration can be promoted, potentially restoring liver function. Additionally, liver stem cells or differentiated hepatocytes within the organoids can contribute to tissue repair and mitigate the progression of fibrosis.
Drugs are absorbed from the gastrointestinal tract, enter the liver through the portal vein, and are metabolized by liver enzymes (148). Unmetabolized drugs are then transported throughout the body via the bloodstream, exerting their effects on target organs (149). In recent years, the incidence of drug-induced liver injury (DILI) has gradually increased, partly due to the diminished efficacy of drugs caused by immune detection inhibitors, chemotherapy agents, polypharmacy, and adverse reactions resulting from interactions during drug metabolism (18). The Roussel Uclaf Causality Assessment Method (RUCAM) is widely employed to assess the causality of DILI, and it is crucial to discontinue the causative drug (150). Common drugs associated with DILI include acetaminophen and carbon tetrachloride, while specific drugs such as isoniazid, itraconazole, and oral contraceptives are also implicated. A small subset of DILI cases may result in chronic liver damage even after discontinuation of the causative drug (151). Autoantibodies, such as antinuclear antibodies (ANA), may become positive during DILI, complicating the diagnosis as they can overlap with AIH (152). Both conditions may present with positive ANA, elevated immunoglobulin G, and similar histopathological findings (153). The key to differentiating DILI from AIH lies in whether liver inflammation resolves after stopping the causative drug (153). In DILI, treatment primarily involves cessation of the offending drug, with supportive therapies such as ursodeoxycholic acid (UDCA) to promote bile secretion, enhance antioxidant activity, and protect the liver (154). Zhang et al. (72) demonstrated that dispersed human liver organoids (HLOs) derived from the iPSC system exhibit DILI prediction capabilities comparable to intact HLOs, with the ability to measure IC50 values for compound cytotoxicity. In regenerative medicine, liver replacement therapy becomes crucial when acute liver damage caused by DILI leads to liver failure. As a cell model with a high degree of self-organization, liver organoids can stimulate the proliferation and regeneration of hepatocytes through specific growth factors and signaling pathways. Transplanting liver organoids or their derivatives could potentially restore damaged liver function, offering a promising treatment option for severe drug-induced liver injury.
For patients with advanced liver cancer, liver transplantation remains the preferred treatment option (155). Current transplantation methods are categorized into orthotopic liver transplantation, heterotopic liver transplantation, and various forms of cell therapy. Cell therapy itself is further divided into hepatocyte transplantation (HTx), nonparenchymal hepatocyte transplantation, hepatic progenitor cell (HPC) transplantation, and bioartificial liver (BAL) trials (156). However, the global shortage of liver donors and the high cost of transplantation mean that many patients either cannot access a liver transplant or cannot afford the procedure, often resulting in death (155). HTx faces challenges due to issues with engraftment and host immune responses (157). Nonparenchymal cell transplantation, while used to regulate immune function and hepatocyte survival, has not yet demonstrated substantial clinical progress (89). The safety and efficacy of HPC transplantation are controversial, partly due to the role of HPCs in liver cancer development (158). Although effective in short-term liver support and detoxification, BAL devices have serious adverse reactions and do not improve long-term survival rates, limiting their application (159). Liver organoids can be transplanted into various sites, including the liver, mesentery, renal capsule, and omentum, with common methods being spleen injection, portal vein injection, and liver implantation (62, 131, 156, 160). In this context, combining liver organoids with 3D printing technology and biomaterials to create hybrid artificial livers holds promise as a potential alternative to traditional liver transplantation (161).
Liver cell transplantation necessitates long-term proliferation, sustained liver function activity, cell maturation, angiogenesis, and minimal immune rejection. Sgodda et al. (162) reported a scalable three-dimensional suspension culture system that allowed PSC-derived liver organoid cells to maintain a stable gene expression profile and metabolic characteristics for up to 3 weeks. By 2018, researchers demonstrated that human hepatocyte-derived liver organoids could proliferate for several months and exhibited successful outcomes when transplanted into mice undergoing liver resection (74). Further advancements in 2019 saw Mun et al. (163) culture liver organoids with stable liver function and long-term proliferation using PSCs. Regarding cell activity and functional stability, liver organoids composed of hepatocytes and bile duct cells generated using honeycomb micropores based on polydimethylsiloxane showed significant improvements in cell albumin secretion, liver marker expression, cytochrome P450-mediated metabolism, and bile transport function (164). Reza et al. (165) produced liver organoids that enhanced bilirubin metabolism, and portal vein transplantation in rats alleviated symptoms in a Crigler–Najjar syndrome model.
In general, iPSC-derived liver organoids are less mature than adult liver cells (166). To address this issue, liver bud (LB) cells were exposed to dexamethasone, which stimulated the BMP, FGF, HGF, and Wnt signaling pathways while inhibiting the TGFβ pathway, leading to the development of mature hepatocyte-like cells (HLCs). These cells were then used to model mature liver organoids in vitro and further matured into functional hepatocytes and bile ducts (167). Incorporating 3D printing technology, hiPSC-derived hepatoblasts were encapsulated in preformed aggregates of alginate beads to create human-engineered livers that closely mimic human liver function (127). This approach generates mature, functional hepatocytes and offers a permanent, unlimited source of liver cells (168). In liver organoid transplantation, the formation of blood vessels is crucial for ensuring an energy supply and metabolic circulation. Microfluidic chips have been used to simulate conventional liver transplantation within microvascular beds cultured in vitro l (99). Following the transplantation of liver microtissues, these microvascular beds were successfully anastomosed to establish a stable, perfusable vascular network (99). Additionally, Takebe et al. (169) developed vascularized and functional human livers from human iPSCs (iPSC-LBs) and implanted them into a mouse model of lethal liver failure. The liver buds integrated with the host blood vessels within 48 h, resulting in significant improvements in mouse survival (169). Therefore, the application of liver organoids in regenerative medicine holds tremendous promise.
Challenges ad future of liver organoids
For liver organoids, the absence of an immune microenvironment, large blood vessels, and nerves remains a significant barrier to their broader application. In addition to these, we also need to pay attention to the standardized generation process of liver organoids, preclinical validation and regulations, interdisciplinary cooperation in approval, and industrial support. Immune rejection is a major challenge in organ transplantation, particularly in liver and kidney transplants (170). When rejection occurs, the recipient’s immune system identifies the transplanted organ as foreign and mounts an attack against it (171). To mitigate this risk, immunosuppressants are used to dampen the recipient’s immune response (172), but this approach increases susceptibility to infections and other complications (173). Thus, achieving a balance between effective immunosuppression and minimizing rejection is crucial for successful posttransplant management.
Advances in human leukocyte antigen (HLA) matching technology and transplant immunology have significantly improved the prognoses of transplant recipients (174). Although excellent HLA matching can substantially reduce rejection rates, it cannot entirely eliminate the risk (174). In organoid cultures, constructing blood vessels is a key challenge. Blood vessels are essential not only for material transport but also for maintaining tissue structure and function (175). The absence of large blood vessels can result in an inadequate nutrient supply to the organoid’s central areas, adversely impacting its development, functional expression, and long-term viability (176, 177). The absence of innervation significantly impacts liver regulation, an aspect that remains challenging within the scope of current orthotopic liver transplantation techniques. The autonomic nerves and sensory fibers within the liver’s nervous system are crucial for regulating liver function, regeneration, and disease (178). The absence or dysfunction of these nerves can significantly disrupt normal liver regulation, potentially affecting its metabolic function, regenerative capacity, and disease resistance (179). The neural activity of the liver is influenced by factors such as food intake, emotional states, and physical activity. Hepatic encephalopathy, a neurological disorder resulting from liver dysfunction, can be triggered by various conditions, including infection, gastrointestinal bleeding, electrolyte and acid–base imbalances, and drug use (180). Inflammatory responses, alterations in ammonia and lactate levels, changes in neurotransmitter concentrations, and modifications in metabolic pathways can impact nervous system function (181).
The liver communicates with the central nervous system through the autonomic nervous system, with nerve fibers playing a role in regulating liver function and response. The liver may influence the regulation of food intake and other physiological behaviors. Following liver organoid transplantation, it is imperative to simulate the electrical signals of both the autonomic and sensory nerves to maintain the body’s fluid balance. The liver nervous system is integral to liver development, regeneration, and disease processes. Strategies to enhance liver organoid function post-transplantation through the simulation of liver nervous system electrical signals—particularly regarding fluid and immune regulation—warrant further investigation.
In the context of the posttransplantation responses of liver organoids, CRISPR/Cas9 technology has been employed to create 3D biomanufactured liver structures from HLA-edited hiPSCs. This manipulation of HLA molecules results in immune-tolerant or customized liver tissues, enhancing donor-recipient compatibility and reducing the risk of acute and chronic rejection reactions (182). The rapid advancement of biomaterials and 3D printing technology is poised to revolutionize the medical field, particularly in the fabrication of artificial organs. These technologies have enabled the creation of artificial liver models with intricate vascular structures (183).
Researchers at Peking Union Medical College Hospital have successfully developed a novel artificial liver featuring a hepatic venous structure, utilizing suspension printing technology and holographic lattice acoustic tweezers (184). This development opens new avenues for alternative sources in liver transplantation (184). This model not only replicates the structural features of the liver but also demonstrates significant functional potential. Additionally, it offers a reference method for constructing an artificial liver vascular system integrated with organoids.
Integrating liver organoids into a bioartificial liver involves replacing liver cells in an artificial liver device with liver organoids and combining these with the filtering, adsorption, detoxification, and other functions of a nonbiological artificial liver (185). This hybrid approach aims to leverage the advantages of liver organoids to simulate the liver’s natural functions, thereby supporting or replacing the functions of a damaged liver. This in vitro device mimics the liver’s metabolic and detoxification functions by processing nutrients and metabolic waste from the gastrointestinal tract and venous blood through a semipermeable membrane, ultimately returning these substances safely to the patient’s body. It functions similarly to the in vitro hemodialysis equipment used for renal failure. The advantage of using liver organoids lies in their ability to replicate the cellular heterogeneity, structural functionality, and microenvironment of in vivo liver tissue. This maximizes the restoration of authentic liver function and helps prevent rejection and vascular embolisms associated with foreign body implantations. Another advantage is the monitorability and maintainability of function: unlike traditional transplanted organs, the activity and functionality of artificial organoid livers can be rigorously assessed before and after transplantation to ensure that they are in optimal condition prior to patient implantation. This monitoring includes evaluating the metabolic activity, protein expression, and cell signaling of liver cells to confirm proper functionality posttransplantation.
Surgery is crucial in treating liver malignancies; however, advanced-stage liver disease often necessitates the removal of a substantial portion of the liver due to systemic intolerance, severe liver damage, or a large tumor size. In such cases, the remaining liver may temporarily be insufficient to support the body’s metabolic functions postsurgery, necessitating additional supportive care to maintain vital signs. Liver organ transplantation is an effective treatment for end-stage liver disease; however, it carries risks, including immune rejection and vascular embolism (186). To mitigate these issues, artificial liver organoids and liver integrants are anticipated to replace traditional transplantation methods. These can be selected and customized based on the patient’s specific needs to minimize immune rejection.
The advancement of CRISPR/Cas9 technology offers a novel approach by enabling precise editing and customization of liver organoid cells at the molecular level, providing more targeted treatment options. Utilizing CRISPR/Cas9 technology allows for the correction of genetic defects at the cellular level or the introduction of specific functional genes. These engineered livers can more effectively adapt to the patient’s physiological environment, thereby reducing the likelihood of rejection. This technology maybe can also enhance the liver’s regenerative capacity and reduce its reliance on prolonged proliferation. Consequently, gene-edited liver cells can be expanded ex vivo and then transplanted into the patient, where they can continue to grow and function without eliciting an excessive immune response.
Currently, liver organoids production is typically conducted in small-scale laboratories, while clinical translation necessitates standardized, large-scale production technologies, including automated culture systems, culture medium optimization, and organoid quality control. A quality control system must be established for liver organoids to ensure that their functionality, structure, and stability meet the standards required for clinical application. To ensure the safety and efficacy of liver organoids in clinical applications, extensive preclinical validation is required. Such studies include the use of animal models, long-term safety testing, and comparative analysis of traditional models. Clinical application of liver organoids must comply with local ethical and legal requirements. This process requires enhanced collaboration with regulatory agencies, as well as the pre-design of clinical trial pathways and data collection methods to ensure the smooth approval of relevant procedures.
Summary and outlook
The research and application of liver organoids represent a cutting-edge frontier in regenerative medicine. This technology offers novel possibilities for treating liver diseases by replicating the structure and function of the human liver. The development of liver organoids marks a significant advancement in liver transplantation technology. Traditional transplantation faces challenges such as donor shortages and rejection. Liver organoid technology offers a potential solution by reducing reliance on donor livers. Advances in biomaterials and 3D bioprinting technology are expected to enhance the structural and functional complexity of future liver organoids, bringing them closer to authentic human livers. The integration of these technologies will not only enhance the maturity and functionality of organoids but also facilitate the rapid and efficient establishment of comprehensive liver organoid models in vitro. This advancement has significant implications for treating end-stage liver disease, studying liver pathogenic mechanisms, and conducting drug screening. In clinical applications, liver organoid technology offers physicians a broader range of treatment options. These organoids can reconstruct the hepatobiliary system both in vivo and in vitro, demonstrating significant potential for liver function replacement and tissue regeneration. Particularly in in vivo transplantation, liver organoids have shown the potential for safe and effective implantation, with demonstrated therapeutic effects. Moreover, combining liver organoids with other tissue-engineering materials may further enhance their therapeutic efficacy.
In summary, liver organoid technology has broad application prospects in regenerative medicine. Although liver organoid transplantation has shown promising results at the laboratory stage, its efficacy has been demonstrated in mice, pigs, and other animal models. This technology holds significant potential. However, the clinical application of liver organoid transplantation has not yet been reported, and crucial evidence-based clinical research remains insufficient. As related technologies continue to advance, more personalized and precise treatment plans for liver disease are anticipated, offering renewed hope to patients. Progress in this field could not only enhance the quality of life for patients with liver disease but may also fundamentally transform our understanding and treatment approaches for liver disease.
Author contributions
DG: Data curation, Formal analysis, Investigation, Resources, Software, Validation, Visualization, Writing – original draft. JM: Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft. MZ: Investigation, Resources, Writing – review & editing. FZ: Investigation, Resources, Validation, Writing – review & editing. GW: Funding acquisition, Investigation, Resources, Writing – review & editing. SM: Conceptualization, Methodology, Resources, Writing – review & editing. XDa: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing. XDe: Conceptualization, Funding acquisition, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by Guangdong Basic and Applied Basic Research Foundation (2023B1515120025), Shenzhen Municipal Science and Technology Foundation (No. JCYJ20240813141046059), China Postdoctoral Science Foundation (No. 2023M741983), Shenzhen Health Talent Program (2022).
Acknowledgments
The authors would like to thank Synorg Biotech for providing the concepts and support for the engineered organoid technologies involved in this review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bhalla, S, McQuillen, B, Cay, E, and Reau, N. Preoperative risk evaluation and optimization for patients with liver disease. Gastroenterol Rep. (2024) 12:goae071. doi: 10.1093/gastro/goae071
2. Michalopoulos, GK, and DeFrances, MC. Liver regeneration. Science. (1997) 276:60–6. doi: 10.1126/science.276.5309.60
3. Michalopoulos, GK. Liver regeneration. J Cell Physiol. (2007) 213:286–300. doi: 10.1002/jcp.21172
4. Court, FG, Laws, PE, Morrison, CP, Teague, BD, Metcalfe, MS, Wemyss-Holden, SA, et al. Subtotal hepatectomy: a porcine model for the study of liver regeneration. J Surg Res. (2004) 116:181–6. doi: 10.1016/j.jss.2003.08.007
5. Duncan, AW, Dorrell, C, and Grompe, M. Stem cells and liver regeneration. Gastroenterology. (2009) 137:466–81. doi: 10.1053/j.gastro.2009.05.044
6. Sakamoto, T, Liu, Z, Murase, N, Ezure, T, Yokomuro, S, Poli, V, et al. Mitosis and apoptosis in the liver of interleukin-6-deficient mice after partial hepatectomy. Hepatology. (1999) 29:403–11. doi: 10.1002/hep.510290244
7. Thomas, H. Senescence prevents regeneration after acute liver injury. Nat Rev Gastroenterol Hepatol. (2018) 15:582. doi: 10.1038/s41575-018-0068-8
8. Yu, JH, and Ma, S. Organoids as research models for hepatocellular carcinoma. Exp Cell Res. (2022) 411:112987. doi: 10.1016/j.yexcr.2021.112987
9. Chen, Y, Liu, Y, Chen, S, Zhang, L, Rao, J, Lu, X, et al. Liver organoids: a promising three-dimensional model for insights and innovations in tumor progression and precision medicine of liver cancer. Front Immunol. (2023) 14:1180184. doi: 10.3389/fimmu.2023.1180184
10. Obeid, DA, Mir, TA, Alzhrani, A, Altuhami, A, Shamma, T, Ahmed, S, et al. Using liver organoids as models to study the pathobiology of rare liver diseases. Biomedicines. (2024) 12:446. doi: 10.3390/biomedicines12020446
11. Palasantzas, V, Tamargo-Rubio, I, Le, K, Slager, J, Wijmenga, C, Jonkers, IH, et al. iPSC-derived organ-on-a-chip models for personalized human genetics and pharmacogenomics studies. Trends Genet. (2023) 39:268–84. doi: 10.1016/j.tig.2023.01.002
12. Polak, DJ. Regenerative medicine. Opportunities and challenges: a brief overview. J R Soc Interface. (2010) 7:S777–81. doi: 10.1098/rsif.2010.0362.focus
13. Songtanin, B, Chaisrimaneepan, N, Mendóza, R, and Nugent, K. Burden, outcome, and comorbidities of extrahepatic manifestations in hepatitis B virus infections. Viruses. (2024) 16:618. doi: 10.3390/v16040618
14. Brillantino, A, Iacobellis, F, Festa, P, Mottola, A, Acampora, C, Corvino, F, et al. Non-operative management of blunt liver trauma: safety, efficacy and complications of a standardized treatment protocol. Bull Emerg Trauma. (2019) 7:49–54. doi: 10.29252/beat-070107
15. Hetherington, A, Cardoso, FS, Lester, ELW, and Karvellas, CJ. Liver trauma in the intensive care unit. Curr Opin Crit Care. (2022) 28:184–9. doi: 10.1097/mcc.0000000000000928
16. Coccolini, F, Coimbra, R, Ordonez, C, Kluger, Y, Vega, F, Moore, EE, et al. Liver trauma: WSES 2020 guidelines. World J Emerg Surg. (2020) 15:24. doi: 10.1186/s13017-020-00302-7
17. Lanini, S, Ustianowski, A, Pisapia, R, Zumla, A, and Ippolito, G. Viral hepatitis: etiology, epidemiology, transmission, diagnostics, treatment, and prevention. Infect Dis Clin N Am. (2019) 33:1045–62. doi: 10.1016/j.idc.2019.08.004
18. Li, X, Tang, J, and Mao, Y. Incidence and risk factors of drug-induced liver injury. Liver Int. (2022) 42:1999–2014. doi: 10.1111/liv.15262
19. Embade, N, and Millet, O. Molecular determinants of chronic liver disease as studied by NMR-metabolomics. Curr Top Med Chem. (2017) 17:2752–66. doi: 10.2174/1568026617666170707124539
20. Zhang, YY, and Hu, KQ. Rethinking the pathogenesis of hepatitis B virus (HBV) infection. J Med Virol. (2015) 87:1989–99. doi: 10.1002/jmv.24270
21. Yuen, MF, Chen, DS, Dusheiko, GM, Janssen, HLA, Lau, DTY, Locarnini, SA, et al. Hepatitis B virus infection. Nat Rev Dis Primers. (2018) 4:18035. doi: 10.1038/nrdp.2018.35
22. Summers, J, Smith, PM, and Horwich, AL. Hepadnavirus envelope proteins regulate covalently closed circular DNA amplification. J Virol. (1990) 64:2819–24. doi: 10.1128/jvi.64.6.2819-2824.1990
23. De Siervi, S, Cannito, S, and Turato, C. Chronic liver disease: latest research in pathogenesis, detection and treatment. Int J Mol Sci. (2023) 24:10633. doi: 10.3390/ijms241310633
24. Parola, M, and Pinzani, M. Liver fibrosis: pathophysiology, pathogenetic targets and clinical issues. Mol Asp Med. (2019) 65:37–55. doi: 10.1016/j.mam.2018.09.002
25. Lurie, Y, Webb, M, Cytter-Kuint, R, Shteingart, S, and Lederkremer, GZ. Non-invasive diagnosis of liver fibrosis and cirrhosis. World J Gastroenterol. (2015) 21:11567–83. doi: 10.3748/wjg.v21.i41.11567
26. Hsu, YC, Huang, DQ, and Nguyen, MH. Global burden of hepatitis B virus: current status, missed opportunities and a call for action. Nat Rev Gastroenterol Hepatol. (2023) 20:524–37. doi: 10.1038/s41575-023-00760-9
27. Ginès, P, Krag, A, Abraldes, JG, Solà, E, Fabrellas, N, and Kamath, PS. Liver cirrhosis. Lancet. (2021) 398:1359–76. doi: 10.1016/s0140-6736(21)01374-x
28. Zhou, WC, Zhang, QB, and Qiao, L. Pathogenesis of liver cirrhosis. World J Gastroenterol. (2014) 20:7312–24. doi: 10.3748/wjg.v20.i23.7312
29. Cacoub, P, Comarmond, C, Domont, F, Savey, L, Desbois, AC, and Saadoun, D. Extrahepatic manifestations of chronic hepatitis C virus infection. Ther Adv Infect Dis. (2016) 3:3–14. doi: 10.1177/2049936115585942
30. Ganem, D, and Prince, AM. Hepatitis B virus infection—natural history and clinical consequences. N Engl J Med. (2004) 350:1118–29. doi: 10.1056/NEJMra031087
31. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
32. Cannella, R, Zins, M, and Brancatelli, G. ESR essentials: diagnosis of hepatocellular carcinoma-practice recommendations by ESGAR. Eur Radiol. (2024) 34:2127–39. doi: 10.1007/s00330-024-10606-w
33. Yeo, YH, Lee, YT, Tseng, HR, Zhu, Y, You, S, Agopian, VG, et al. Alpha-fetoprotein: past, present, and future. Hepatol Commun. (2024) 8:e0422. doi: 10.1097/hc9.0000000000000422
34. Couri, T, and Pillai, A. Goals and targets for personalized therapy for HCC. Hepatol Int. (2019) 13:125–37. doi: 10.1007/s12072-018-9919-1
35. Toh, MR, Wong, EYT, Wong, SH, Ng, AWT, Loo, LH, Chow, PK, et al. Global epidemiology and genetics of hepatocellular carcinoma. Gastroenterology. (2023) 164:766–82. doi: 10.1053/j.gastro.2023.01.033
36. Fattovich, G, Stroffolini, T, Zagni, I, and Donato, F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. (2004) 127:S35–50. doi: 10.1053/j.gastro.2004.09.014
37. Zhang, CH, Cheng, Y, Zhang, S, Fan, J, and Gao, Q. Changing epidemiology of hepatocellular carcinoma in Asia. Liver Int. (2022) 42:2029–41. doi: 10.1111/liv.15251
38. Chu, KK, Wong, KH, and Chok, KS. Expanding indications for liver transplant: tumor and patient factors. Gut Liver. (2021) 15:19–30. doi: 10.5009/gnl19265
39. D'Souza, S, Lau, KC, Coffin, CS, and Patel, TR. Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma. World J Gastroenterol. (2020) 26:5759–83. doi: 10.3748/wjg.v26.i38.5759
40. Yuming, Z, Ruqi, T, Gershwin, ME, and Xiong, M. Autoimmune hepatitis: pathophysiology. Clin Liver Dis. (2024) 28:15–35. doi: 10.1016/j.cld.2023.06.003
41. Reshetnyak, VI, and Maev, IV. New insights into the pathogenesis of primary biliary cholangitis asymptomatic stage. World J Gastroenterol. (2023) 29:5292–304. doi: 10.3748/wjg.v29.i37.5292
42. Dasí, F. Alpha-1 antitrypsin deficiency. Med Clin. (2024) 162:336–42. doi: 10.1016/j.medcli.2023.10.014
43. Merle, U, and Mohr, I. Hereditary liver diseases: Wilson’s disease and hemochromatosis. Dtsch Med Wochenschr. (2023) 148:836–43. doi: 10.1055/a-1871-6393
44. Lucena-Valera, A, Ruz-Zafra, P, and Ampuero, J. Wilson’s disease: overview. Med Clin. (2023) 160:261–7. doi: 10.1016/j.medcli.2022.12.016
45. Pedersen, MR, and Mayo, MJ. Advances in the evaluation and treatment of autoimmune hepatitis. Curr Opin Gastroenterol. (2024) 40:126–33. doi: 10.1097/mog.0000000000001014
46. Devarbhavi, H, Asrani, SK, Arab, JP, Nartey, YA, Pose, E, and Kamath, PS. Global burden of liver disease: 2023 update. J Hepatol. (2023) 79:516–37. doi: 10.1016/j.jhep.2023.03.017
47. Campana, L, Esser, H, Huch, M, and Forbes, S. Liver regeneration and inflammation: from fundamental science to clinical applications. Nat Rev Mol Cell Biol. (2021) 22:608–24. doi: 10.1038/s41580-021-00373-7
48. Massa, A, Varamo, C, Vita, F, Tavolari, S, Peraldo-Neia, C, Brandi, G, et al. Evolution of the experimental models of cholangiocarcinoma. Cancers. (2020) 12:2308. doi: 10.3390/cancers12082308
49. Khan, HA, Ahmad, MZ, Khan, JA, and Arshad, MI. Crosstalk of liver immune cells and cell death mechanisms in different murine models of liver injury and its clinical relevance. Hepatobiliary Pancreat Dis Int. (2017) 16:245–56. doi: 10.1016/s1499-3872(17)60014-6
50. Wang, L, He, FL, Liu, FQ, Yue, ZD, and Zhao, HW. Establishment of a hepatic cirrhosis and portal hypertension model by hepatic arterial perfusion with 80% alcohol. World J Gastroenterol. (2015) 21:9544–53. doi: 10.3748/wjg.v21.i32.9544
51. Romualdo, GR, Leroy, K, Costa, CJS, Prata, GB, Vanderborght, B, da Silva, TC, et al. In vivo and in vitro models of hepatocellular carcinoma: current strategies for translational modeling. Cancers. (2021) 13:5583. doi: 10.3390/cancers13215583
52. Storey, J, Gobbetti, T, Olzinski, A, and Berridge, BR. A structured approach to optimizing animal model selection for human translation: the animal model quality assessment. ILAR J. (2021) 62:66–76. doi: 10.1093/ilar/ilac004
53. Aldarmahi, A. Establishment and characterization of female reproductive tract epithelial cell culture. J Microsc Ultrastruct. (2017) 5:105–10. doi: 10.1016/j.jmau.2016.07.004
54. Hirsch, C, and Schildknecht, S. In vitro research reproducibility: keeping up high standards. Front Pharmacol. (2019) 10:1484. doi: 10.3389/fphar.2019.01484
55. Yang, B, Wei, K, Loebel, C, Zhang, K, Feng, Q, Li, R, et al. Enhanced mechanosensing of cells in synthetic 3D matrix with controlled biophysical dynamics. Nat Commun. (2021) 12:3514. doi: 10.1038/s41467-021-23120-0
56. Bell, CC, Dankers, ACA, Lauschke, VM, Sison-Young, R, Jenkins, R, Rowe, C, et al. Comparison of hepatic 2D Sandwich cultures and 3D spheroids for long-term toxicity applications: a multicenter study. Toxicol Sci. (2018) 162:655–66. doi: 10.1093/toxsci/kfx289
57. Lee, TK, Guan, XY, and Ma, S. Cancer stem cells in hepatocellular carcinoma—from origin to clinical implications. Nat Rev Gastroenterol Hepatol. (2022) 19:26–44. doi: 10.1038/s41575-021-00508-3
58. Gu, Y, Zhang, W, Wu, X, Zhang, Y, Xu, K, and Su, J. Organoid assessment technologies. Clin Transl Med. (2023) 13:e1499. doi: 10.1002/ctm2.1499
59. Rossi, G, Manfrin, A, and Lutolf, MP. Progress and potential in organoid research. Nat Rev Genet. (2018) 19:671–87. doi: 10.1038/s41576-018-0051-9
60. Sato, T, Vries, RG, Snippert, HJ, van de Wetering, M, Barker, N, Stange, DE, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. (2009) 459:262–5. doi: 10.1038/nature07935
61. Clevers, H. The intestinal crypt, a prototype stem cell compartment. Cell. (2013) 154:274–84. doi: 10.1016/j.cell.2013.07.004
62. Huch, M, Dorrell, C, Boj, SF, van Es, JH, Li, VS, van de Wetering, M, et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. (2013) 494:247–50. doi: 10.1038/nature11826
63. Huch, M, Gehart, H, van Boxtel, R, Hamer, K, Blokzijl, F, Verstegen, MM, et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. (2015) 160:299–312. doi: 10.1016/j.cell.2014.11.050
64. Skardal, A, Devarasetty, M, Rodman, C, Atala, A, and Soker, S. Liver-tumor hybrid organoids for modeling tumor growth and drug response in vitro. Ann Biomed Eng. (2015) 43:2361–73. doi: 10.1007/s10439-015-1298-3
65. Leite, SB, Roosens, T, El Taghdouini, A, Mannaerts, I, Smout, AJ, Najimi, M, et al. Novel human hepatic organoid model enables testing of drug-induced liver fibrosis in vitro. Biomaterials. (2016) 78:1–10. doi: 10.1016/j.biomaterials.2015.11.026
66. Broutier, L, Mastrogiovanni, G, Verstegen, MM, Francies, HE, Gavarró, LM, Bradshaw, CR, et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med. (2017) 23:1424–35. doi: 10.1038/nm.4438
67. Ouchi, R, Togo, S, Kimura, M, Shinozawa, T, Koido, M, Koike, H, et al. Modeling steatohepatitis in humans with pluripotent stem cell-derived organoids. Cell Metab. (2019) 30:374–384.e6. doi: 10.1016/j.cmet.2019.05.007
68. Elbadawy, M, Yamanaka, M, Goto, Y, Hayashi, K, Tsunedomi, R, Hazama, S, et al. Efficacy of primary liver organoid culture from different stages of non-alcoholic steatohepatitis (NASH) mouse model. Biomaterials. (2020) 237:119823. doi: 10.1016/j.biomaterials.2020.119823
69. De Crignis, E, Hossain, T, Romal, S, Carofiglio, F, Moulos, P, Khalid, MM, et al. Application of human liver organoids as a patient-derived primary model for HBV infection and related hepatocellular carcinoma. eLife. (2021) 10:e60747. doi: 10.7554/eLife.60747
70. Guan, Y, Enejder, A, Wang, M, Fang, Z, Cui, L, Chen, SY, et al. A human multi-lineage hepatic organoid model for liver fibrosis. Nat Commun. (2021) 12:6138. doi: 10.1038/s41467-021-26410-9
71. Mun, SJ, Hong, YH, Shin, Y, Lee, J, Cho, HS, Kim, DS, et al. Efficient and reproducible generation of human induced pluripotent stem cell-derived expandable liver organoids for disease modeling. Sci Rep. (2023) 13:22935. doi: 10.1038/s41598-023-50250-w
72. Zhang, CJ, Meyer, SR, O'Meara, MJ, Huang, S, Capeling, MM, Ferrer-Torres, D, et al. A human liver organoid screening platform for DILI risk prediction. J Hepatol. (2023) 78:998–1006. doi: 10.1016/j.jhep.2023.01.019
73. Shi, R, Radulovich, N, Ng, C, Liu, N, Notsuda, H, Cabanero, M, et al. Organoid cultures as preclinical models of non-small cell lung cancer. Clin Cancer Res. (2020) 26:1162–74. doi: 10.1158/1078-0432.Ccr-19-1376
74. Hu, H, Gehart, H, Artegiani, B, Löpez-Iglesias, C, Dekkers, F, Basak, O, et al. Long-term expansion of functional mouse and human hepatocytes as 3D organoids. Cell. (2018) 175:1591–1606.e19. doi: 10.1016/j.cell.2018.11.013
75. Yang, H, Cheng, J, Zhuang, H, Xu, H, Wang, Y, Zhang, T, et al. Pharmacogenomic profiling of intra-tumor heterogeneity using a large organoid biobank of liver cancer. Cancer Cell. (2024) 42:535–551.e8. doi: 10.1016/j.ccell.2024.03.004
76. Clevers, H. Modeling development and disease with organoids. Cell. (2016) 165:1586–97. doi: 10.1016/j.cell.2016.05.082
77. Xian, L, Zhao, P, Chen, X, Wei, Z, Ji, H, Zhao, J, et al. Heterogeneity, inherent and acquired drug resistance in patient-derived organoid models of primary liver cancer. Cell Oncol. (2022) 45:1019–36. doi: 10.1007/s13402-022-00707-3
78. Brassard, JA, and Lutolf, MP. Engineering stem cell self-organization to build better organoids. Cell Stem Cell. (2019) 24:860–76. doi: 10.1016/j.stem.2019.05.005
79. Schutgens, F, and Clevers, H. Human organoids: tools for understanding biology and treating diseases. Annu Rev Pathol. (2020) 15:211–34. doi: 10.1146/annurev-pathmechdis-012419-032611
80. Nuciforo, S, Fofana, I, Matter, MS, Blumer, T, Calabrese, D, Boldanova, T, et al. Organoid models of human liver cancers derived from tumor needle biopsies. Cell Rep. (2018) 24:1363–76. doi: 10.1016/j.celrep.2018.07.001
81. Wu, YH, Hung, YP, Chiu, NC, Lee, RC, Li, CP, Chao, Y, et al. Correlation between drug sensitivity profiles of circulating tumour cell-derived organoids and clinical treatment response in patients with pancreatic ductal adenocarcinoma. Eur J Cancer. (2022) 166:208–18. doi: 10.1016/j.ejca.2022.01.030
82. Nantasanti, S, Spee, B, Kruitwagen, HS, Chen, C, Geijsen, N, Oosterhoff, LA, et al. Disease modeling and gene therapy of copper storage disease in canine hepatic organoids. Stem Cell Rep. (2015) 5:895–907. doi: 10.1016/j.stemcr.2015.09.002
83. Guo, J, Duan, L, He, X, Li, S, Wu, Y, Xiang, G, et al. A combined model of human iPSC-derived liver organoids and hepatocytes reveals ferroptosis in DGUOK mutant mtDNA depletion syndrome. Adv Sci. (2021) 8:2004680. doi: 10.1002/advs.202004680
84. Takahashi, K, and Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. (2006) 126:663–76. doi: 10.1016/j.cell.2006.07.024
85. Takahashi, K, Tanabe, K, Ohnuki, M, Narita, M, Ichisaka, T, Tomoda, K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. (2007) 131:861–72. doi: 10.1016/j.cell.2007.11.019
86. Sun, L, Wang, Y, Cen, J, Ma, X, Cui, L, Qiu, Z, et al. Modelling liver cancer initiation with organoids derived from directly reprogrammed human hepatocytes. Nat Cell Biol. (2019) 21:1015–26. doi: 10.1038/s41556-019-0359-5
87. Andrews, MG, and Kriegstein, AR. Challenges of organoid research. Annu Rev Neurosci. (2022) 45:23–39. doi: 10.1146/annurev-neuro-111020-090812
88. McCracken, KW, Aihara, E, Martin, B, Crawford, CM, Broda, T, Treguier, J, et al. Wnt/β-catenin promotes gastric fundus specification in mice and humans. Nature. (2017) 541:182–7. doi: 10.1038/nature21021
89. Yang, X, Meng, Y, Han, Z, Ye, F, Wei, L, and Zong, C. Mesenchymal stem cell therapy for liver disease: full of chances and challenges. Cell Biosci. (2020) 10:123. doi: 10.1186/s13578-020-00480-6
90. Sackmann, EK, Fulton, AL, and Beebe, DJ. The present and future role of microfluidics in biomedical research. Nature. (2014) 507:181–9. doi: 10.1038/nature13118
91. Brandenberg, N, Hoehnel, S, Kuttler, F, Homicsko, K, Ceroni, C, Ringel, T, et al. High-throughput automated organoid culture via stem-cell aggregation in microcavity arrays. Nat Biomed Eng. (2020) 4:863–74. doi: 10.1038/s41551-020-0565-2
92. Imparato, G, Urciuolo, F, and Netti, PA. Organ on chip technology to model cancer growth and metastasis. Bioengineering. (2022) 9:28. doi: 10.3390/bioengineering9010028
93. Kim, SK, Kim, YH, Park, S, and Cho, SW. Organoid engineering with microfluidics and biomaterials for liver, lung disease, and cancer modeling. Acta Biomater. (2021) 132:37–51. doi: 10.1016/j.actbio.2021.03.002
94. Koyilot, MC, Natarajan, P, Hunt, CR, Sivarajkumar, S, Roy, R, Joglekar, S, et al. Breakthroughs and applications of organ-on-a-chip technology. Cells. (2022) 11:1828. doi: 10.3390/cells11111828
95. Baptista, LS, Porrini, C, Kronemberger, GS, Kelly, DJ, and Perrault, CM. 3D organ-on-a-chip: the convergence of microphysiological systems and organoids. Front Cell Dev Biol. (2022) 10:1043117. doi: 10.3389/fcell.2022.1043117
96. Du, Y, Li, N, Yang, H, Luo, C, Gong, Y, Tong, C, et al. Mimicking liver sinusoidal structures and functions using a 3D-configured microfluidic chip. Lab Chip. (2017) 17:782–94. doi: 10.1039/c6lc01374k
97. Ma, C, Zhao, L, Zhou, EM, Xu, J, Shen, S, and Wang, J. On-chip construction of liver lobule-like microtissue and its application for adverse drug reaction assay. Anal Chem. (2016) 88:1719–27. doi: 10.1021/acs.analchem.5b03869
98. Ya, S, Ding, W, Li, S, Du, K, Zhang, Y, Li, C, et al. On-chip construction of liver lobules with self-assembled perfusable hepatic sinusoid networks. ACS Appl Mater Interfaces. (2021) 13:32640–52. doi: 10.1021/acsami.1c00794
99. Bonanini, F, Kurek, D, Previdi, S, Nicolas, A, Hendriks, D, de Ruiter, S, et al. In vitro grafting of hepatic spheroids and organoids on a microfluidic vascular bed. Angiogenesis. (2022) 25:455–70. doi: 10.1007/s10456-022-09842-9
100. Wu, Y, Wenger, A, Golzar, H, and Tang, XS. 3D bioprinting of bicellular liver lobule-mimetic structures via microextrusion of cellulose nanocrystal-incorporated shear-thinning bioink. Sci Rep. (2020) 10:20648. doi: 10.1038/s41598-020-77146-3
101. Chang, R, Emami, K, Wu, H, and Sun, W. Biofabrication of a three-dimensional liver micro-organ as an in vitro drug metabolism model. Biofabrication. (2010) 2:045004. doi: 10.1088/1758-5082/2/4/045004
102. Li, J, Chen, M, Fan, X, and Zhou, H. Recent advances in bioprinting techniques: approaches, applications and future prospects. J Transl Med. (2016) 14:271. doi: 10.1186/s12967-016-1028-0
103. Matai, I, Kaur, G, Seyedsalehi, A, McClinton, A, and Laurencin, CT. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials. (2020) 226:119536. doi: 10.1016/j.biomaterials.2019.119536
104. Cross-Najafi, AA, Farag, K, Chen, AM, Smith, LJ, Zhang, W, Li, P, et al. The long road to develop custom-built livers: current status of 3D liver bioprinting. Transplantation. (2024) 108:357–68. doi: 10.1097/tp.0000000000004668
105. Kim, D, Kim, M, Lee, J, and Jang, J. Review on multicomponent hydrogel bioinks based on natural biomaterials for bioprinting 3D liver tissues. Front Bioeng Biotechnol. (2022) 10:764682. doi: 10.3389/fbioe.2022.764682
106. Valot, L, Martinez, J, Mehdi, A, and Subra, G. Chemical insights into bioinks for 3D printing. Chem Soc Rev. (2019) 48:4049–86. doi: 10.1039/c7cs00718c
107. Wang, F, Song, P, Wang, J, Wang, S, Liu, Y, Bai, L, et al. Organoid bioinks: construction and application. Biofabrication. (2024) 16:032006. doi: 10.1088/1758-5090/ad467c
108. Lee, H, and Cho, DW. One-step fabrication of an organ-on-a-chip with spatial heterogeneity using a 3D bioprinting technology. Lab Chip. (2016) 16:2618–25. doi: 10.1039/c6lc00450d
109. Jian, H, Li, X, Dong, Q, Tian, S, and Bai, S. In vitro construction of liver organoids with biomimetic lobule structure by a multicellular 3D bioprinting strategy. Cell Prolif. (2023) 56:e13465. doi: 10.1111/cpr.13465
110. He, J, Lu, H, Zou, Q, and Luo, L. Regeneration of liver after extreme hepatocyte loss occurs mainly via biliary transdifferentiation in zebrafish. Gastroenterology. (2014) 146:789–800.e8. doi: 10.1053/j.gastro.2013.11.045
111. Friedman, SL, Roll, FJ, Boyles, J, and Bissell, DM. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci USA. (1985) 82:8681–5. doi: 10.1073/pnas.82.24.8681
112. Mederacke, I, Hsu, CC, Troeger, JS, Huebener, P, Mu, X, Dapito, DH, et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. (2013) 4:2823. doi: 10.1038/ncomms3823
113. Brenner, C, Galluzzi, L, Kepp, O, and Kroemer, G. Decoding cell death signals in liver inflammation. J Hepatol. (2013) 59:583–94. doi: 10.1016/j.jhep.2013.03.033
114. Kisseleva, T, and Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. (2021) 18:151–66. doi: 10.1038/s41575-020-00372-7
115. Ding, BS, Cao, Z, Lis, R, Nolan, DJ, Guo, P, Simons, M, et al. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature. (2014) 505:97–102. doi: 10.1038/nature12681
116. Tummala, KS, Brandt, M, Teijeiro, A, Graña, O, Schwabe, RF, Perna, C, et al. Hepatocellular carcinomas originate predominantly from hepatocytes and benign lesions from hepatic progenitor cells. Cell Rep. (2017) 19:584–600. doi: 10.1016/j.celrep.2017.03.059
117. Dai, K, Geng, Z, Zhang, W, Wei, X, Wang, J, Nie, G, et al. Biomaterial design for regenerating aged bone: materiobiological advances and paradigmatic shifts. Natl Sci Rev. (2024) 11:nwae076. doi: 10.1093/nsr/nwae076
118. Hermans, F, Hasevoets, S, Vankelecom, H, Bronckaers, A, and Lambrichts, I. From pluripotent stem cells to organoids and bioprinting: recent advances in dental epithelium and ameloblast models to study tooth biology and regeneration. Stem Cell Rev Rep. (2024) 20:1184–99. doi: 10.1007/s12015-024-10702-w
119. Bekheit, M, Grundy, L, Salih, AK, Bucur, P, Vibert, E, and Ghazanfar, M. Post-hepatectomy liver failure: a timeline centered review. Hepatobiliary Pancreat Dis Int. (2023) 22:554–69. doi: 10.1016/j.hbpd.2023.03.001
120. Søreide, JA, and Deshpande, R. Post hepatectomy liver failure (PHLF)—recent advances in prevention and clinical management. Eur J Surg Oncol. (2021) 47:216–24. doi: 10.1016/j.ejso.2020.09.001
121. Katarey, D, and Jalan, R. Update on extracorporeal liver support. Curr Opin Crit Care. (2020) 26:180–5. doi: 10.1097/mcc.0000000000000708
122. Saliba, F, Bañares, R, Larsen, FS, Wilmer, A, Parés, A, Mitzner, S, et al. Artificial liver support in patients with liver failure: a modified DELPHI consensus of international experts. Intensive Care Med. (2022) 48:1352–67. doi: 10.1007/s00134-022-06802-1
123. Struecker, B, Raschzok, N, and Sauer, IM. Liver support strategies: cutting-edge technologies. Nat Rev Gastroenterol Hepatol. (2014) 11:166–76. doi: 10.1038/nrgastro.2013.204
124. Larsen, FS. Artificial liver support in acute and acute-on-chronic liver failure. Curr Opin Crit Care. (2019) 25:187–91. doi: 10.1097/mcc.0000000000000584
125. Starokozhko, V, and Groothuis, GMM. Challenges on the road to a multicellular bioartificial liver. J Tissue Eng Regen Med. (2018) 12:e227–36. doi: 10.1002/term.2385
126. Tuerxun, K, He, J, Ibrahim, I, Yusupu, Z, Yasheng, A, Xu, Q, et al. Bioartificial livers: a review of their design and manufacture. Biofabrication. (2022) 14:032003. doi: 10.1088/1758-5090/ac6e86
127. Yuan, X, Wu, J, Sun, Z, Cen, J, Shu, Y, Wang, C, et al. Preclinical efficacy and safety of encapsulated proliferating human hepatocyte organoids in treating liver failure. Cell Stem Cell. (2024) 31:484–498.e5. doi: 10.1016/j.stem.2024.02.005
128. Nie, YZ, Zheng, YW, Ogawa, M, Miyagi, E, and Taniguchi, H. Human liver organoids generated with single donor-derived multiple cells rescue mice from acute liver failure. Stem Cell Res Ther. (2018) 9:5. doi: 10.1186/s13287-017-0749-1
129. Labour, MN, Le Guilcher, C, Aid-Launais, R, El Samad, N, Lanouar, S, Simon-Yarza, T, et al. Development of 3D hepatic constructs within polysaccharide-based scaffolds with tunable properties. Int J Mol Sci. (2020) 21:3644. doi: 10.3390/ijms21103644
130. Gao, Y, Yin, X, and Ren, X. Advance of mesenchymal stem cells in chronic end-stage liver disease control. Stem Cells Int. (2022) 2022:1526217–8. doi: 10.1155/2022/1526217
131. Tsuchida, T, Murata, S, Matsuki, K, Mori, A, Matsuo, M, Mikami, S, et al. The regenerative effect of portal vein injection of liver organoids by retrorsine/partial hepatectomy in rats. Int J Mol Sci. (2019) 21:178. doi: 10.3390/ijms21010178
132. Vizzutti, F, Schepis, F, Arena, U, Fanelli, F, Gitto, S, Aspite, S, et al. Transjugular intrahepatic portosystemic shunt (TIPS): current indications and strategies to improve the outcomes. Intern Emerg Med. (2020) 15:37–48. doi: 10.1007/s11739-019-02252-8
133. Guixé-Muntet, S, Quesada-Vázquez, S, and Gracia-Sancho, J. Pathophysiology and therapeutic options for cirrhotic portal hypertension. Lancet Gastroenterol Hepatol. (2024) 9:646–63. doi: 10.1016/s2468-1253(23)00438-7
134. Lee, H, Chae, S, Kim, JY, Han, W, Kim, J, Choi, Y, et al. Cell-printed 3D liver-on-a-chip possessing a liver microenvironment and biliary system. Biofabrication. (2019) 11:025001. doi: 10.1088/1758-5090/aaf9fa
135. Alwahsh, SM, Rashidi, H, and Hay, DC. Liver cell therapy: is this the end of the beginning? Cell Mol Life Sci. (2018) 75:1307–24. doi: 10.1007/s00018-017-2713-8
136. Salas-Silva, S, Kim, Y, Kim, TH, Kim, M, Seo, D, Choi, J, et al. Human chemically-derived hepatic progenitors (hCdHs) as a source of liver organoid generation: application in regenerative medicine, disease modeling, and toxicology testing. Biomaterials. (2023) 303:122360. doi: 10.1016/j.biomaterials.2023.122360
137. van As, AB, and Millar, AJ. Management of paediatric liver trauma. Pediatr Surg Int. (2017) 33:445–53. doi: 10.1007/s00383-016-4046-3
138. Adams, PC, Jeffrey, G, and Ryan, J. Haemochromatosis. Lancet. (2023) 401:1811–21. doi: 10.1016/s0140-6736(23)00287-8
139. Olynyk, JK, and Ramm, GA. Hemochromatosis. N Engl J Med. (2022) 387:2159–70. doi: 10.1056/NEJMra2119758
140. Walter, K. What is hereditary hemochromatosis? JAMA. (2022) 328:1879. doi: 10.1001/jama.2022.19462
141. Ohkoshi-Yamada, M, Kamimura, K, Kamimura, H, and Terai, S. Rare complication of hepatocellular carcinoma in Wilson’s disease. JGH Open. (2021) 5:1220–2. doi: 10.1002/jgh3.12648
142. Ryan, A, Twomey, PJ, and Cook, P. Wilson’s disease: best practice. J Clin Pathol. (2023) 76:435–41. doi: 10.1136/jcp-2022-208551
143. Roberts, EA, and Schilsky, ML. Current and emerging issues in Wilson’s disease. N Engl J Med. (2023) 389:922–38. doi: 10.1056/NEJMra1903585
144. Ferrarese, A, Cazzagon, N, and Burra, P. Liver transplantation for Wilson disease: current knowledge and future perspectives. Liver Transpl. (2024) 30:1289–303. doi: 10.1097/lvt.0000000000000422
145. Terziroli Beretta-Piccoli, B, Mieli-Vergani, G, and Vergani, D. Autoimmmune hepatitis. Cell Mol Immunol. (2022) 19:158–76. doi: 10.1038/s41423-021-00768-8
146. Sucher, E, Sucher, R, Gradistanac, T, Brandacher, G, Schneeberger, S, and Berg, T. Autoimmune hepatitis-immunologically triggered liver pathogenesis-diagnostic and therapeutic strategies. J Immunol Res. (2019) 2019:9437043. doi: 10.1155/2019/9437043
147. Muratori, L, Lohse, AW, and Lenzi, M. Diagnosis and management of autoimmune hepatitis. BMJ. (2023) 380:e070201. doi: 10.1136/bmj-2022-070201
148. Almazroo, OA, Miah, MK, and Venkataramanan, R. Drug metabolism in the liver. Clin Liver Dis. (2017) 21:1–20. doi: 10.1016/j.cld.2016.08.001
149. van den Anker, J, Reed, MD, Allegaert, K, and Kearns, GL. Developmental changes in pharmacokinetics and pharmacodynamics. J Clin Pharmacol. (2018) 58:S10–s25. doi: 10.1002/jcph.1284
150. Teschke, R, Larrey, D, Melchart, D, and Danan, G. Traditional Chinese medicine (TCM) and herbal hepatotoxicity: RUCAM and the role of novel diagnostic biomarkers such as microRNAs. Medicines. (2016) 19:18. doi: 10.3390/medicines3030018
151. Hosack, T, Damry, D, and Biswas, S. Drug-induced liver injury: a comprehensive review. Ther Adv Gastroenterol. (2023) 16:17562848231163410. doi: 10.1177/17562848231163410
152. Björnsson, ES. The epidemiology of newly recognized causes of drug-induced liver injury: an update. Pharmaceuticals. (2024) 17:520. doi: 10.3390/ph17040520
153. Andrade, RJ, Aithal, GP, de Boer, YS, Liberal, R, Gerbes, A, Regev, A, et al. Nomenclature, diagnosis and management of drug-induced autoimmune-like hepatitis (DI-ALH): an expert opinion meeting report. J Hepatol. (2023) 79:853–66. doi: 10.1016/j.jhep.2023.04.033
154. Bessone, F, Hillotte, GL, Ahumada, N, Jaureguizahar, F, Medeot, AC, and Roma, MG. UDCA for drug-induced liver disease: clinical and pathophysiological basis. Semin Liver Dis. (2024) 44:001–22. doi: 10.1055/s-0044-1779520
155. Antarianto, RD, Mahmood, A, Giselvania, A, Asri Dewi, AAP, Gustinanda, J, and Pawitan, JA. Inventing engineered organoids for end-stage liver failure patients. J Mol Histol. (2022) 53:611–21. doi: 10.1007/s10735-022-10085-7
156. Reza, HA, Okabe, R, and Takebe, T. Organoid transplant approaches for the liver. Transpl Int. (2021) 34:2031–45. doi: 10.1111/tri.14128
157. Iansante, V, Mitry, RR, Filippi, C, Fitzpatrick, E, and Dhawan, A. Human hepatocyte transplantation for liver disease: current status and future perspectives. Pediatr Res. (2018) 83:232–40. doi: 10.1038/pr.2017.284
158. Lo, RC, and Ng, IO. Hepatic progenitor cells: their role and functional significance in the new classification of primary liver cancers. Liver Cancer. (2013) 2:84–92. doi: 10.1159/000343844
159. Chamuleau, RA, Poyck, PP, and van de Kerkhove, MP. Bioartificial liver: its pros and cons. Ther Apher Dial. (2006) 10:168–74. doi: 10.1111/j.1744-9987.2006.00359.x
160. Stevens, KR, Scull, MA, Ramanan, V, Fortin, CL, Chaturvedi, RR, Knouse, KA, et al. In situ expansion of engineered human liver tissue in a mouse model of chronic liver disease. Sci Transl Med. (2017) 9. doi: 10.1126/scitranslmed.aah5505
161. Davoodi, P, Rezaei, N, Hassan, M, Hay, DC, and Vosough, M. Bioengineering vascularized liver tissue for biomedical research and application. Scand J Gastroenterol. (2024) 59:623–9. doi: 10.1080/00365521.2024.2310172
162. Sgodda, M, Dai, Z, Zweigerdt, R, Sharma, AD, Ott, M, and Cantz, T. A scalable approach for the generation of human pluripotent stem cell-derived hepatic organoids with sensitive hepatotoxicity features. Stem Cells Dev. (2017) 26:1490–504. doi: 10.1089/scd.2017.0023
163. Mun, SJ, Ryu, JS, Lee, MO, Son, YS, Oh, SJ, Cho, HS, et al. Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids. J Hepatol. (2019) 71:970–85. doi: 10.1016/j.jhep.2019.06.030
164. Utami, T, Danoy, M, Khadim, RR, Tokito, F, Arakawa, H, Kato, Y, et al. A highly efficient cell culture method using oxygen-permeable PDMS-based honeycomb microwells produces functional liver organoids from human induced pluripotent stem cell-derived carboxypeptidase M liver progenitor cells. Biotechnol Bioeng. (2024) 121:1178–90. doi: 10.1002/bit.28640
165. Reza, HA, Farooqui, Z, Reza, AA, Conroy, C, Iwasawa, K, Ogura, Y, et al. Synthetic augmentation of bilirubin metabolism in human pluripotent stem cell-derived liver organoids. Stem Cell Rep. (2023) 18:2071–83. doi: 10.1016/j.stemcr.2023.09.006
166. Nuciforo, S, and Heim, MH. Organoids to model liver disease. JHEP Rep. (2021) 3:100198. doi: 10.1016/j.jhepr.2020.100198
167. Raggi, C, M’Callum, MA, Pham, QT, Gaub, P, Selleri, S, Baratang, NV, et al. Leveraging interacting signaling pathways to robustly improve the quality and yield of human pluripotent stem cell-derived hepatoblasts and hepatocytes. Stem Cell Rep. (2022) 17:584–98. doi: 10.1016/j.stemcr.2022.01.003
168. Hussein, M, Pasqua, M, Pereira, U, Benzoubir, N, Duclos-Vallée, JC, Dubart-Kupperschmitt, A, et al. Microencapsulated hepatocytes differentiated from human induced pluripotent stem cells: optimizing 3D culture for tissue engineering applications. Cells. (2023) 12:865. doi: 10.3390/cells12060865
169. Takebe, T, Sekine, K, Enomura, M, Koike, H, Kimura, M, Ogaeri, T, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. (2013) 499:481–4. doi: 10.1038/nature12271
170. Maddur, H, Wilson, N, Patil, P, and Asrani, S. Rejection in liver transplantation recipients. J Clin Exp Hepatol. (2024) 14:101363. doi: 10.1016/j.jceh.2024.101363
171. Li, Q, and Lan, P. Activation of immune signals during organ transplantation. Signal Transduct Target Ther. (2023) 8:110. doi: 10.1038/s41392-023-01377-9
172. Montano-Loza, AJ, Rodríguez-Perálvarez, ML, Pageaux, GP, Sanchez-Fueyo, A, and Feng, S. Liver transplantation immunology: immunosuppression, rejection, and immunomodulation. J Hepatol. (2023) 78:1199–215. doi: 10.1016/j.jhep.2023.01.030
173. Watt, KD, Pedersen, RA, Kremers, WK, Heimbach, JK, and Charlton, MR. Evolution of causes and risk factors for mortality post-liver transplant: results of the NIDDK long-term follow-up study. Am J Transplant. (2010) 10:1420–7. doi: 10.1111/j.1600-6143.2010.03126.x
174. Le Floc'h, B, Costet, N, Vu, N, Bernabeu-Gentey, P, Pronier, C, Houssel-Debry, P, et al. Involvement of circulating soluble HLA-G after liver transplantation in the low immunogenicity of hepatic allograft. PLoS One. (2023) 18:e0282736. doi: 10.1371/journal.pone.0282736
175. Eelen, G, Treps, L, Li, X, and Carmeliet, P. Basic and therapeutic aspects of angiogenesis updated. Circ Res. (2020) 127:310–29. doi: 10.1161/circresaha.120.316851
176. Naderi-Meshkin, H, Cornelius, VA, Eleftheriadou, M, Potel, KN, Setyaningsih, WAW, and Margariti, A. Vascular organoids: unveiling advantages, applications, challenges, and disease modelling strategies. Stem Cell Res Ther. (2023) 14:292. doi: 10.1186/s13287-023-03521-2
177. Salewskij, K, and Penninger, JM. Blood vessel organoids for development and disease. Circ Res. (2023) 132:498–510. doi: 10.1161/circresaha.122.321768
178. Miller, BM, Oderberg, IM, and Goessling, W. Hepatic nervous system in development, regeneration, and disease. Hepatology. (2021) 74:3513–22. doi: 10.1002/hep.32055
179. Yang, K, Huang, Z, Wang, S, Zhao, Z, Yi, P, Chen, Y, et al. The hepatic nerves regulated inflammatory effect in the process of liver injury: is nerve the key treating target for liver inflammation? Inflammation. (2023) 46:1602–11. doi: 10.1007/s10753-023-01854-x
180. Rose, CF, Amodio, P, Bajaj, JS, Dhiman, RK, Montagnese, S, Taylor-Robinson, SD, et al. Hepatic encephalopathy: novel insights into classification, pathophysiology and therapy. J Hepatol. (2020) 73:1526–47. doi: 10.1016/j.jhep.2020.07.013
181. Coltart, I, Tranah, TH, and Shawcross, DL. Inflammation and hepatic encephalopathy. Arch Biochem Biophys. (2013) 536:189–96. doi: 10.1016/j.abb.2013.03.016
182. Artegiani, B, Hendriks, D, Beumer, J, Kok, R, Zheng, X, Joore, I, et al. Fast and efficient generation of knock-in human organoids using homology-independent CRISPR-Cas9 precision genome editing. Nat Cell Biol. (2020) 22:321–31. doi: 10.1038/s41556-020-0472-5
183. Gold, KA, Saha, B, Rajeeva Pandian, NK, Walther, BK, Palma, JA, Jo, J, et al. 3D bioprinted multicellular vascular models. Adv Healthc Mater. (2021) 10:e2101141. doi: 10.1002/adhm.202101141
184. Jiang, Z, Jin, B, Liang, Z, Wang, Y, Ren, S, Huang, Y, et al. Liver bioprinting within a novel support medium with functionalized spheroids, hepatic vein structures, and enhanced post-transplantation vascularization. Biomaterials. (2024) 311:122681. doi: 10.1016/j.biomaterials.2024.122681
185. Messina, A, Luce, E, Hussein, M, and Dubart-Kupperschmitt, A. Pluripotent-stem-cell-derived hepatic cells: hepatocytes and organoids for liver therapy and regeneration. Cells. (2020) 9:420. doi: 10.3390/cells9020420
186. Yagi, S, Hirata, M, Miyachi, Y, and Uemoto, S. Liver regeneration after hepatectomy and partial liver transplantation. Int J Mol Sci. (2020) 21:8414. doi: 10.3390/ijms21218414
187. Vyas, D, Baptista, PM, Brovold, M, Moran, E, Gaston, B, Booth, C, et al. Self-assembled liver organoids recapitulate hepatobiliary organogenesis in vitro. Hepatology. (2018) 67:750–61. doi: 10.1002/hep.29483
188. Soroka, CJ, Assis, DN, Alrabadi, LS, Roberts, S, Cusack, L, Jaffe, AB, et al. Bile-derived organoids from patients with primary sclerosing cholangitis recapitulate their inflammatory immune profile. Hepatology. (2019) 70:871–82. doi: 10.1002/hep.30470
189. Gómez-Mariano, G, Matamala, N, Martínez, S, Justo, I, Marcacuzco, A, Jimenez, C, et al. Liver organoids reproduce alpha-1 antitrypsin deficiency-related liver disease. Hepatol Int. (2020) 14:127–37. doi: 10.1007/s12072-019-10007-y
190. Li, P, Li, Y, Wang, Y, Liu, J, Lavrijsen, M, Li, Y, et al. Recapitulating hepatitis E virus-host interactions and facilitating antiviral drug discovery in human liver-derived organoids. Sci Adv. (2022) 8:eabj5908. doi: 10.1126/sciadv.abj5908
191. Shintani, T, Imamura, C, Ueyama-Toba, Y, Inui, J, Watanabe, A, and Mizuguchi, H. Establishment of UGT1A1-knockout human iPS-derived hepatic organoids for UGT1A1-specific kinetics and toxicity evaluation. Mol Ther Methods Clin Dev. (2023) 30:429–42. doi: 10.1016/j.omtm.2023.08.003
192. Feng, Z, Zhou, B, Shuai, Q, Wei, Y, Jin, N, Wang, X, et al. Development of an alcoholic liver disease model for drug evaluation from human induced pluripotent stem cell-derived liver organoids. Acta Biochim Biophys Sin. (2024) 56:1460–72. doi: 10.3724/abbs.2024074
193. Ye, J, Shirakigawa, N, and Ijima, H. Fetal liver cell-containing hybrid organoids improve cell viability and albumin production upon transplantation. J Biosci Bioeng. (2016) 121:701–8. doi: 10.1016/j.jbiosc.2015.11.006
194. Zhou, VX, Lolas, M, and Chang, TT. Direct orthotopic implantation of hepatic organoids. J Surg Res. (2017) 211:251–60. doi: 10.1016/j.jss.2016.12.028
195. Wu, F, Wu, D, Ren, Y, Huang, Y, Feng, B, Zhao, N, et al. Generation of hepatobiliary organoids from human induced pluripotent stem cells. J Hepatol. (2019) 70:1145–58. doi: 10.1016/j.jhep.2018.12.028
196. Tsuchida, T, Murata, S, Hasegawa, S, Mikami, S, Enosawa, S, Hsu, HC, et al. Investigation of clinical safety of human iPS cell-derived liver organoid transplantation to infantile patients in porcine model. Cell Transplant. (2020) 29:096368972096438. doi: 10.1177/0963689720964384
197. Kruitwagen, HS, Oosterhoff, LA, van Wolferen, ME, Chen, C, Nantasanti Assawarachan, S, Schneeberger, K, et al. Long-term survival of transplanted autologous canine liver organoids in a COMMD1-deficient dog model of metabolic liver disease. Cells. (2020) 9:410. doi: 10.3390/cells9020410
198. Sampaziotis, F, Muraro, D, Tysoe, OC, Sawiak, S, Beach, TE, Godfrey, EM, et al. Cholangiocyte organoids can repair bile ducts after transplantation in the human liver. Science. (2021) 371:839–46. doi: 10.1126/science.aaz6964
199. Zhang, W, Lanzoni, G, Hani, H, Overi, D, Cardinale, V, Simpson, S, et al. Patch grafting, strategies for transplantation of organoids into solid organs such as liver. Biomaterials. (2021) 277:121067. doi: 10.1016/j.biomaterials.2021.121067
Keywords: liver organoids, regenerative medicine, liver injury, liver research model, microfluidic and 3D print, organoids-on-a-chip
Citation: Gong D, Mo J, Zhai M, Zhou F, Wang G, Ma S, Dai X and Deng X (2025) Advances, challenges and future applications of liver organoids in experimental regenerative medicine. Front. Med. 11:1521851. doi: 10.3389/fmed.2024.1521851
Edited by:
Aleksander Skardal, The Ohio State University, United StatesCopyright © 2025 Gong, Mo, Zhai, Zhou, Wang, Ma, Dai and Deng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyong Dai, ZGFpLXh5MThAdHNpbmdodWEub3JnLmNu; Xuesong Deng, ZGVuZ3h1ZXNvbmdAMjFjbi5jb20=
†These authors have contributed equally to this work and share first authorship
 Da Gong
Da Gong Jiaye Mo1,2†
Jiaye Mo1,2† Guocai Wang
Guocai Wang Shaohua Ma
Shaohua Ma Xiaoyong Dai
Xiaoyong Dai