- 1Hospital Universitario de Gran Canaria Dr. Negrín, Respiratory Service, Las Palmas de Gran Canaria, Spain
- 2Hospital Universitario de Gran Canaria Dr. Negrín, Research Unit, Las Palmas de Gran Canaria, Spain
- 3Universidad de La Laguna, Research Unit, Santa Cruz de Tenerife, Spain
- 4Hospital Universitario de Gran Canaria Dr. Negrín, Hematology Service, Las Palmas de Gran Canaria, Spain
Eosinophils are polymorphonuclear cells that have progressively gained attention due to their involvement in multiple diseases and, more recently, in various homeostatic processes. Their well-known roles range from asthma and parasitic infections to less prevalent diseases such as eosinophilic granulomatosis with polyangiitis, eosinophilic esophagitis, and hypereosinophilic syndrome. In recent years, various biological therapies targeting these cells have been developed, altering the course of eosinophilic pathologies. Recent research has demonstrated differences in eosinophil subtypes and their functions. The presence of distinct classes of eosinophils has led to the theory of resident eosinophils (rEos) and inflammatory eosinophils (iEos). Subtype differences are determined by the pattern of protein expression on the cell membrane and the localization of eosinophils. Most of this research has been conducted in murine models, but several studies confirm these findings in peripheral blood and tissue. The objective of this review is to provide a comprehensive analysis of eosinophils, by recent findings that divide this cell line into two distinct populations with different functions and purposes.
Introduction
Jones (1) and Brewer (2) first described granular cells, but without appropriate staining techniques, they were unable to correctly characterize or evaluate the various types of granulocytes. Paul Ehrlich, in 1846, using his blood staining technique with coal tar dyes (eosin), successfully described eosinophils based on their strong affinity for this marker (3). In addition to describing the staining properties of their granules, Ehrlich studied their distribution in various species and tissues, concluding that they likely developed in the bone marrow. The discovery of the eosinophil precursor cell took much longer. The higher density of these cells in the bone marrow was demonstrated in 1960 by Rytomaa, but it was not until 1984 that Fischkoff et al. (4) showed that eosinophils and neutrophils share the same precursor: the promyelocytic cell line HL-60. In 1998, it was discovered that the gene EOS47, specifically expressed in bone marrow eosinophils, has a promoter region with binding sites for the transcription factors Myb-Ets, c/EBP, and GATA (5), which are responsible for lineage commitment. Subsequent studies have shown that eliminating the high-affinity GATA-1 binding site in the GATA-1 gene promoter results in the loss of the eosinophil lineage (6).
Another significant finding relates to eosinopoiesis and its regulation. Boyer et al. (7) and Basten and Beeson (8) demonstrated that immunocompetent lymphocytes are responsible for the increased number of eosinophils in peripheral blood during parasitic infections. A few years later, interleukin 5 (IL-5) was isolated as the main protein associated with terminal differentiation, eosinophil production in the bone marrow, their growth, activation, and inhibition of apoptosis (9).
Granules and degranulation
Eosinophils contain numerous cytoplasmic granules that include specific eosinophilic proteins, cytokines, chemokines, enzymes, and lipid mediators contributing to their function (10). These granules contain four specific proteins stored in secondary granules (major basic proteins, MBP1 and MBP2; eosinophil peroxidase, EPO, eosinophil cationic protein, ECP; and eosinophil-derived neurotoxin, EDN), which can induce tissue damage and dysfunction (11). ECP and EDN are ribonucleases with antiviral activity, with ECP creating voltage-insensitive toxic ion pores in target cell membranes, potentially facilitating the entry of other cytotoxic molecules (12–15). ECP also has additional non-cytotoxic activities, including suppressing T cell proliferative responses, inhibiting immunoglobulin synthesis by B cells, inducing mast cell degranulation, and stimulating airway mucus secretion and glycosaminoglycan production by human fibroblasts (16). MBP directly alters smooth muscle contraction responses by dysregulating M2 and M3 vagal muscarinic receptor function and inducing mast cell and basophil degranulation (17–19). EPO, comprising approximately 25% of the total specific granule protein mass, catalyzes the oxidation of pseudohalides and nitric oxide to form highly reactive oxygen species (hypohalous acids) and reactive nitrogen metabolites (peroxynitrite), which oxidize nucleophilic targets in proteins, promoting oxidative stress and subsequent cell death via apoptosis and necrosis (20–22).
Eosinophils degranulate through four mechanisms: classical exocytosis, compound exocytosis, piecemeal degranulation (regulated), and cytolysis (necrosis). Classical exocytosis refers to the process by which secretory granules release their complete contents into the extracellular space following the fusion of the granule membrane with the plasma membrane. This process encompasses compound exocytosis, which additionally involves the fusion of intracellular granules prior to the subsequent release of their contents into the extracellular environment (23). Piecemeal degranulation (PMD) is a process characterized by the secretion of substances from intracellular granules, facilitated by the transport of vesicles (23). This mechanism allows for the gradual release of granule contents, enabling precise regulation of cellular functions and responses. Cytolysis, the release of granule contents due to cell rupture, involves chromatolysis (disintegration of nuclear chromatin) followed by the rupture of the cell’s plasma membrane. This process leads to the release of membrane-bound eosinophilic granules (FEGs) (24) and is often associated with the formation of eosinophil extracellular traps (EETs) (25).
EETs consist of DNA fibers embedded with granule proteins, such as MBP and ECP (25), or associated with FEGs (23) and eosinophil sombrero vesicles EoSVs (24). The release of EETs has been observed from both live eosinophils and those undergoing cell lysis (EETosis) (26). In recent years, EETosis has gained more attention (25, 27), it drives the release of EETs in tissues and the secretion during several inflammatory diseases (26), playing a critical role in the pathophysiology of severe asthma (28). External stimuli have been suggested to influence EET release, and the extent of release appears to be time-dependent based on exposure duration (25). However, the molecular mechanisms underlying this process remain poorly understood (25). The process of EET formation is associated with the development of Charcot-Leyden crystals (CLCs), which are composed of the protein galectin-10. These crystals serve as a biomarker of eosinophil involvement in conditions such as asthma, allergic rhinitis, and other forms of eosinophilic inflammation (26, 29).
In areas of eosinophilic inflammation characterized by the presence of FEGs and occasionally CLCs, EoSVs are often observed near or intermingled with extracellular, expanded, and highly decondensed chromatin (24). This represents an ultrastructural hallmark of the late stage of EETosis (26). EoSVs are thought to be crucial intermediaries in this process. The total number of EoSVs increases when eosinophils are exposed to inflammatory stimuli in activated eosinophils both in vitro and in vivo (24). In tissues affected by eosinophilic cytolytic inflammation, extracellular EoSVs are present; however, their clinical significance in eosinophil-associated diseases remains unclear (24).
Different cytokines have distinct effects on eosinophil degranulation, influencing both the type and extent of granule release (27, 30, 31). The nature and extent of eosinophil degranulation can vary depending on the specific cytokine stimulation the cell receives (32, 33). For example, TNF-α is a potent pro-inflammatory cytokine that induces oxidative stress and membrane destabilization in eosinophils, promoting cytolysis (23). However, it is hypothesized that each degranulation form corresponds to the specific function the eosinophil is performing. For instance, during PMD, eosinophils selectively release components of their specific granules (34). IFN-γ is associated with Th1 responses and can modulate eosinophil degranulation in a more controlled manner. It often acts as a suppressor of eosinophil degranulation, particularly in allergic inflammation (32, 35). However, human eosinophil activation by IFN-γ promotes the mobilization of RANTES (CCL5) derived from granules to the cell periphery without releasing cationic proteins (36, 37). Regulated exocytosis occurs through the formation of a docking complex composed of soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAP receptors or SNARE) located on the vesicle (v-SNARE) and the target membrane (t-SNARE) (38).
Migration
Under normal conditions, eosinophils migrate from the bone marrow to specific organs, primarily the gastrointestinal system. Most eosinophils reside in the non-esophageal portion of the intestine. Other target organs include the uterus and mammary glands of young women, the thymus, adipose tissue, and the lungs.
Traditionally, eosinophils have been associated with the inflammatory response to helminth infections and allergic diseases. However, it is now recognized that they have more varied functions depending on the tissue in which they are found. Studies in mice have shown that, under stable conditions, eosinophils play a homeostatic role in these tissues. In the intestine, they are involved in the IgA response and mucus production (39); in the mammary glands, they seem to play a role in development (40), while in adipose tissue, they are associated with insulin sensitivity and the transition to brown fat (39).
Eosinophils are tissue cells, therefore typically constitute less than 5% of the total leukocytes in the blood (39) (Figure 1A). In vivo studies have shown that the residence time of eosinophils in the bloodstream is quite short, approximately 8–10 h, although the range can vary from 3 to 24 h (41–43). In contrast, their persistence in tissues is longer, with a half-life of 36 h in the lung and up to 6 days in the intestine, thymus, and uterus (41). The tissue longevity of eosinophils appears to be related to the expression of CD11c, which is expressed by eosinophils in the thymus, uterus, and intestine but not by those in the blood and lung. This longevity also depends on the inhibition of apoptosis mediated by IL-5 (41, 44, 45).
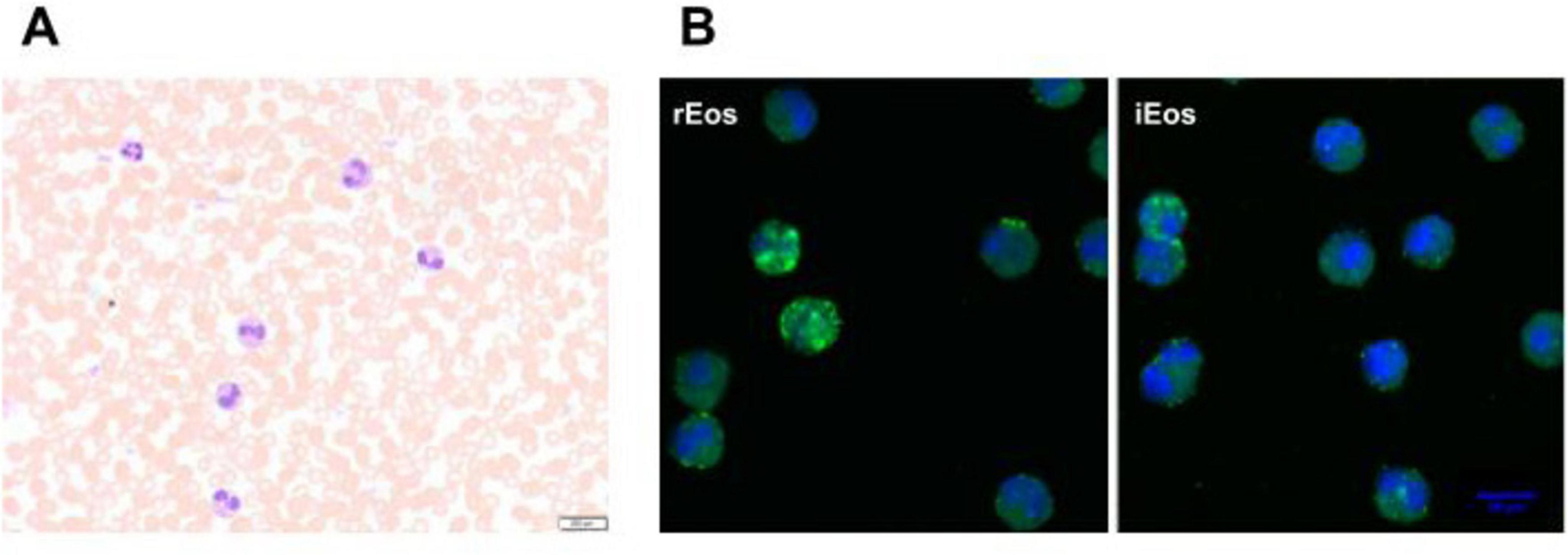
Figure 1. Human eosinophils in peripheral blood from an asthma patient. (A) Blood eosinophils were directly stained with Hematoxylin-eosin before sorting (40× magnification). (B) Representative confocal microscopy photographs of blood rEos and iEos after FACS sorting, following the gating strategy of Cabrera López et al. (93). Eosinophils were stained for CD62L (green) and DAPI (blue, nucleus). DAPI, 4′,6-diamidino-2-phenylindole; 63× magnification. rEos: resident eosinophils, iEos: inflammatory eosinophils.
Cytokines and chemokines
The recruitment of eosinophils into tissues is driven by the eotaxin family, primarily eotaxin-1 (CCL11), a chemokine produced mainly by epithelial cells, endothelial cells, fibroblasts, and monocytes in response to inflammatory signals such as IL-4, IL-13, and TNF-α (46–49). The differential chemotactic potential and expression profiles of CCL11, eotaxin-2 (CCL24), and eotaxin-3 (CCL26) suggest that they play distinct roles over time. CCL11 is the most potent eosinophil chemoattractant of the three (50). CCL11 may act early in an inflammatory response to recruit eosinophils quickly, while CCL24 and CCL26 might sustain eosinophil accumulation and inflammation over longer periods. Its strong affinity for CCR3 and the efficient signaling it induces lead to rapid and robust eosinophil migration (51). The loss of CCR3, the principal receptor for eotaxin-1 (52, 53), results in defective localization of eosinophils in tissues, particularly in the intestine, but does not affect the cell count in the lung or thymus (54), suggesting that eotaxin-1 may act through alternative receptors such as CCR5 (55). Interleukins, IL-5 and IL-13, released by T lymphocytes and type 2 innate lymphoid cells (ILC2) (9, 56–59), can also promote eosinophil trafficking under normal conditions (60, 61), albeit to a lesser extent than eotaxin-1. IL-13 enhances the production of eotaxin-3 (56), while IL-5 promotes eosinophil generation from bone marrow progenitors, increases their sensitivity to eotaxin-1, and maintains their survival (9, 57, 58).
IL-5, the cytokine most specific to the eosinophil lineage, is essential for eosinophil production in steady-state conditions. The recruitment of resident eosinophils to tissues is independent in the lungs, partially dependent in the gastrointestinal tract and uterus, and completely dependent in adipose tissue on local IL-5 production (59–63).
In allergic and reactive diseases, eosinophils have been identified as significant sources of IL-5, IL-13, IL-25, and CCL26, contributing to the Th2-skewed immune response and subsequent eosinophilic inflammation (64). Among the eotaxins, CCL26 displays the weakest chemotactic activity, despite also binding to CCR3 with lower affinity and eliciting a reduced chemotactic response. CCL26 is upregulated by IL-13 and is predominantly expressed in airway epithelial cells during allergic inflammation. Although its role in eosinophil recruitment is more limited compared to CCL11 and CCL24, it is thought to play a role in asthma (65). In the lung, IL-4 and IL-13 secreted locally are responsible for increasing endothelial adhesiveness by upregulating VCAM-1 (66) and inducing CCL11 secretion by bronchial epithelial cells (50), which promotes greater eosinophil recruitment into the tissue.
The enhanced tissue survival of eosinophils is mediated by IL-5, IL-3 and granulocyte-macrophage colony-stimulating factor (GM-CSF). These cytokines are essential hematopoietic signals that regulate eosinophil development and differentiation within the bone marrow (10, 67). IL-3 is primarily involved in the early expansion of eosinophil progenitor cells, while IL-5 is crucial for the terminal differentiation of these cells (56). GM-CSF further supports the maturation and survival of both progenitors and mature eosinophils. IL-3 signals through the IL-3 receptor (IL-3R), composed of a specific α-subunit (IL-3Rα) and a shared β-common chain (βc), the latter of which is also utilized by GM-CSF and IL-5 (67). Upon ligand binding, IL-3R activates several intracellular pathways, including JAK/STAT, MAPK, and PI3K, which act in synergy with IL-5 and GM-CSF (68). Dysregulation of IL-3 and GM-CSF signaling pathways is implicated in eosinophilic disorders, contributing to excessive eosinophil survival, tissue damage, and chronic inflammation (67, 69).
Eosinophils promote humoral immunity by priming B cells (39) and play a central role in type 2 immunity, including antigen presentation to CD4+ T cells and secretion of granular contents containing type 2 mediators, such as IL-4, IL-5, and IL-13 (39), thereby closely regulating Th1 and Th2 immunity (70).
Eosinophil activation
Eosinophils are terminal effector cells that degranulate and release highly cytotoxic substances when activated. In the case of infection, these granular proteins act directly against parasites; however, in allergic situations, they contribute to tissue destruction, as seen in patients with atopic asthma, where the number of eosinophils in the bronchi correlates with lung epithelial damage (71, 72). As mentioned above, fully activated eosinophils can also expel EET composed of mitochondrial DNA and granular proteins, which are destructive to tissues (73). In this way, eosinophils, like neutrophils, can trap and kill other types of microorganisms.
However, this cytotoxic reaction occurs only under inflammatory conditions when eosinophils are highly stimulated by cytokines such as interferon gamma (IFN-γ) and require high levels of IL-5 to induce the formation of a DNA net (73). EET formation has been observed to be triggered by eosinophil activation through IL-5 and thymic stromal lymphopoietin (TSLP) (74). Future studies are needed to better understand how the molecular mechanisms of EET production are regulated (28).
Besides these inflammatory functions, eosinophils also play a beneficial role in regulating and modulating immune responses, partly by synthesizing and secreting a wide array of cytokines and immune mediators (75). They do this, at least in part, by synthesizing and secreting a surprisingly broad spectrum of different cytokines and immune mediators (10).
The actions of eosinophils go beyond the secretion of toxic proteins. Eosinophil activation promotes the secretion of various pro-inflammatory cytokines (IL-2, IL-4, IL-5, IL-10, IL-12, IL-13, IL-16, IL-18, and TGF-α/β), chemokines (RANTES and eotaxin-1), and lipid mediators (platelet-activating factor and leukotriene C4, LTC4) (76). These molecules have pro-inflammatory effects, positively regulating adhesion systems, modulating cell trafficking, activating and regulating vascular permeability, mucus secretion, and smooth muscle constriction. Eosinophils can initiate antigen-specific immune responses by acting as antigen-presenting cells (APC) to major histocompatibility complex class II and co-stimulatory molecules (CD40, CD80, CD86).
Eosinophils are also activated by epithelial-derived innate cytokines (TSLP and IL-33), promoting their recruitment by amplifying Th2 responses and stimulating ILC2 cells to secrete IL-5, IL-4, and IL-13, as well as by stimulating T lymphocytes. In addition to promoting Th2 responses, TSLP and IL-33 act directly on eosinophils, preventing apoptosis through direct activation of the TSLP receptor (TSLPR) present on eosinophils (77, 78).
Eosinophil subtypes
In recent years, several publications have classified eosinophils into different subtypes. It remains unclear whether these represent the same cell line at different activation stages or, as occurs with Th1 or Th2 lymphocytes, distinct cells with different properties secreted from the bone marrow. A pivotal study by Mesnil et al. (79) using an asthmatic murine model delineates the distinction between resident, homeostatic or physiological eosinophils (rEos) and inflammatory eosinophils (iEos). This research conducted multiple experiments in the lungs and blood of mice, revealing clear differences between populations in different models (allergic asthmatic mice vs. healthy mice). In mice, this differentiation is characterized by nuclear shape, membrane proteins, and cell localization. rEos exhibit most typical eosinophil features, including red-staining granules containing specific proteins (e.g., MBP, EPO) and combined expression of CCR3, Siglec-F, and CD125 (the IL-5 receptor α subunit) (39, 45, 80). They can also express CD11b (intestine, thymus, and adipose tissue), F4/80 (mammary glands, lung, and adipose tissue), CD69 and CD44 (intestine and thymus) (45, 79–85). Most tissue rEos have a segmented nucleus and express CD11c (12, 75, 82–86).
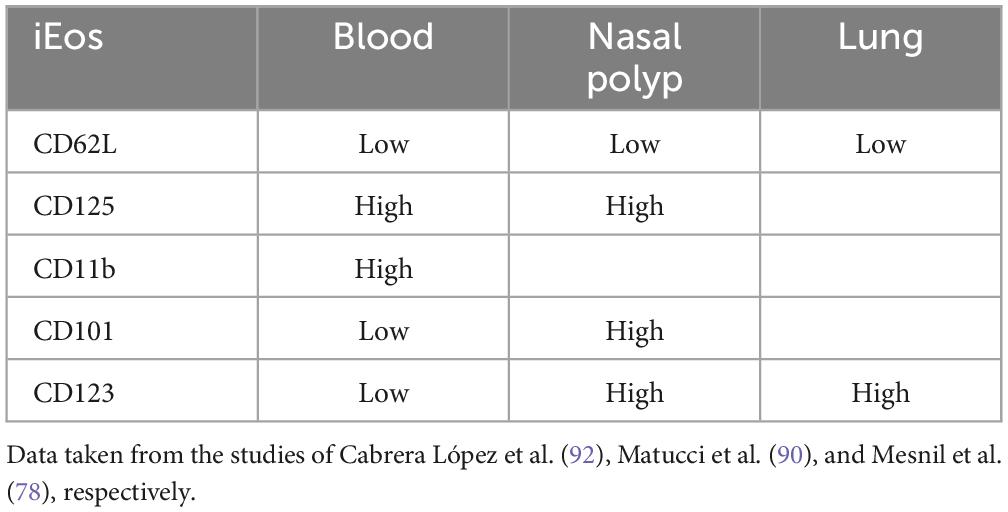
Lung mice rEos are an exception and resemble resting blood eosinophils with a ring-shaped nucleus, express CD62L, show intermediate Siglec-F levels, and are CD11c negative (6, 79, 82, 85–87). In mice, such characteristics, especially the ring-shaped nucleus, indicate cellular immaturity (88, 89), suggesting that lung rEos retain an immature phenotype upon dissemination to the lungs. These eosinophils undergo gradual degranulation and are capable of phagocytosis, demonstrating their functionality.
Interestingly, the number, localization, and morphological, phenotypic, and transcriptomic characteristics of lung rEos remain unchanged and differ from iEos during allergic airway inflammation. iEos, abundantly recruited to the lungs during allergen exposure episodes, are defined as SiglecFhiCD62L–CD101hi cells with a segmented nucleus (CD101 is an iEos marker not expressed in lung rEos). These observations support the theory of rEos and iEos, suggesting that similar subsets exist in the blood of asthmatic mice, indicating differentiation occurs even before tissue recruitment. This study also conducted a human experiment comparing lung tissue from healthy individuals with sputum from asthmatic patients (79). The results showed that parenchymal rEos in non-asthmatic human lungs (Siglec-8+CD62LhiIL-3Rlo cells) are phenotypically distinct from iEos isolated from asthmatic patient sputum (Siglec-8+CD62LloIL-3Rhi cells), confirming mouse findings in humans (Figure 2).
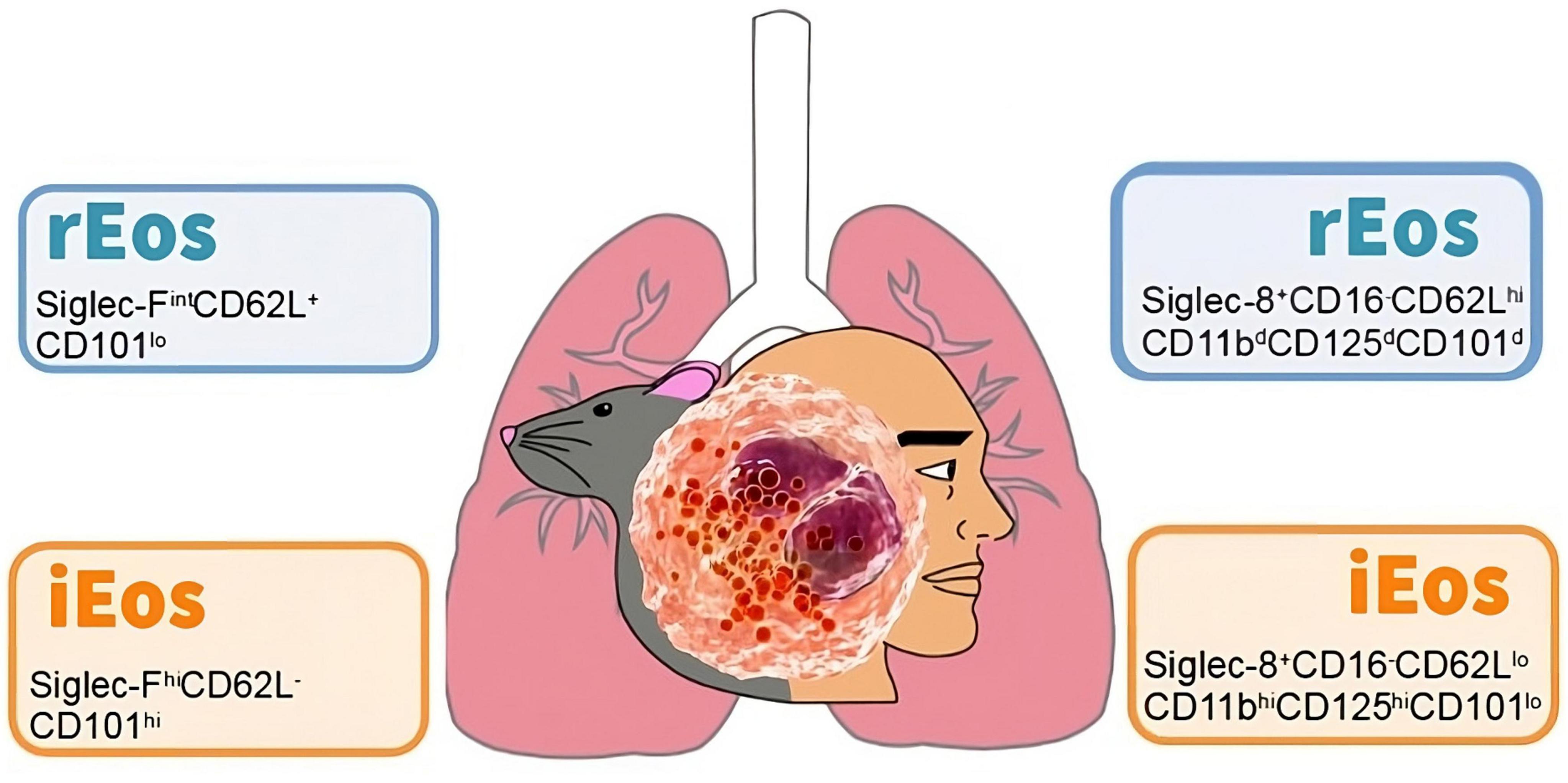
Figure 2. Cell phenotyping of blood eosinophils subtypes in mouse and human. Data taken from the studies of Mesnil et al. (79) (mouse) and Cabrera López et al. (93) (human). rEos: resident eosinophils, iEos: inflammatory eosinophils.
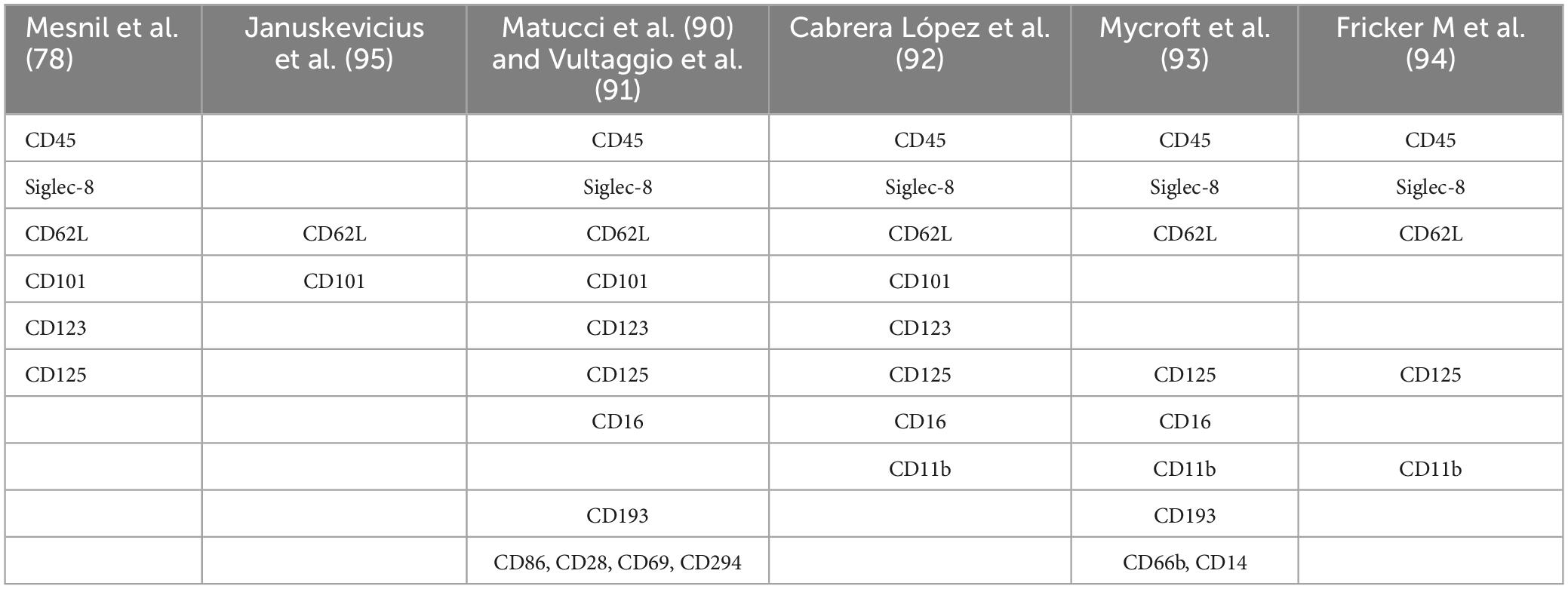
Other studies have validated Mesnil et al. (79) proposed pattern in horses (90) and humans (91–95) (Table 1). Matucci et al. (91) focused on different eosinophil subpopulations in peripheral blood and nasal polyps in patients with severe eosinophilic asthma (SEA) with chronic rhinosinusitis with nasal polyps (CRSwNP). They recruited 23 SEA patients (14 with CRSwNP), comparing them with 15 non-severe asthma patients (NSEA), 15 allergic rhinitis without asthma patients, and 15 healthy volunteers. They also studied eotaxin-3 and eotaxin-1 expression in nasal polyps. They observed an increase in peripheral blood eosinophils in SEA patients (Siglec8+CD45+CD16–), revealing two eosinophil subtypes based on CD62L expression across all groups. There was a higher number of CD62Llo eosinophils in SEA patients compared to controls, expressing high CCR3, CD69, and low CD125 (IL-5R), CRTH2, CD86, CD28, CD101, and VLA-4 levels. Nasal polyps had a higher proportion of CD62Llo eosinophils than peripheral blood. Surface expression of IL-3R, IL-5R, CD69, and CD86 was significantly higher in CD62Llo eosinophils from nasal polyps compared to blood. Further, eotaxin-3 expression correlated with the percentage of CD62Llo eosinophils in nasal polyps. In relation to what was previously published, CD62Llo was associated with iEos and CD62Lbright with rEos (79, 96).
The Vultaggio et al. (92), study is notable for correlating iEos presence with clinical outcomes, is undoubtedly one of the most interesting published so far. It examined the relationship between iEos (characterized by CD62Llo) in blood and the severity of severe eosinophilic asthma, evaluating the impact of mepolizumab on iEos (92). They recruited 112 patients: 51 naive and 61 previously treated with biologics. They analyzed 19 naive patients before and after 100 mg SC mepolizumab/4 weeks treatment, and 23 patients already on mepolizumab at study start. In vitro effects of IL-5 and mepolizumab on CD62L expression were also evaluated. There was a significant correlation between CD62Llo cells and better ACT scores in asthma, lower SNOT-22 scores in nasal polyposis (better asthma and nasal polyposis control in patients with low CD62Llo eosinophils), as well as exacerbations in untreated patients. The Naive group showed a reduction in CD62Llo with an increase in CD62bright proportion after mepolizumab treatment, associating with improved asthma control, resembling healthy volunteer rEos/iEos proportions. In vitro, IL-5 and anti-IL-5 regulated CD62L expression on eosinophils. IL-3, GM-CSF, IL-33, TSLP, and TNF-α modulated CD62L expression, but not IL-4.
Fricker et al. (95) analyzed eosinophil subpopulations in patients with severe asthma, finding results similar to those of Matucci et al. (91) and Cabrera López et al. (93). Additionally, a longitudinal analysis was conducted in patients undergoing treatment (n = 30) at two timepoints (4–24 weeks) post-initiation of mepolizumab (n = 20) or benralizumab (n = 10). Similar to Vultaggio’s findings, both mepolizumab and benralizumab effectively iEos to a comparable extent. Mepolizumab, however, specifically depleted iEos while preserving a residual population of rEos in patients with severe asthma, whereas benralizumab depleted both subtypes. This confirms that an increase in the proportion of circulating iEos is associated with poorer asthma control (95).
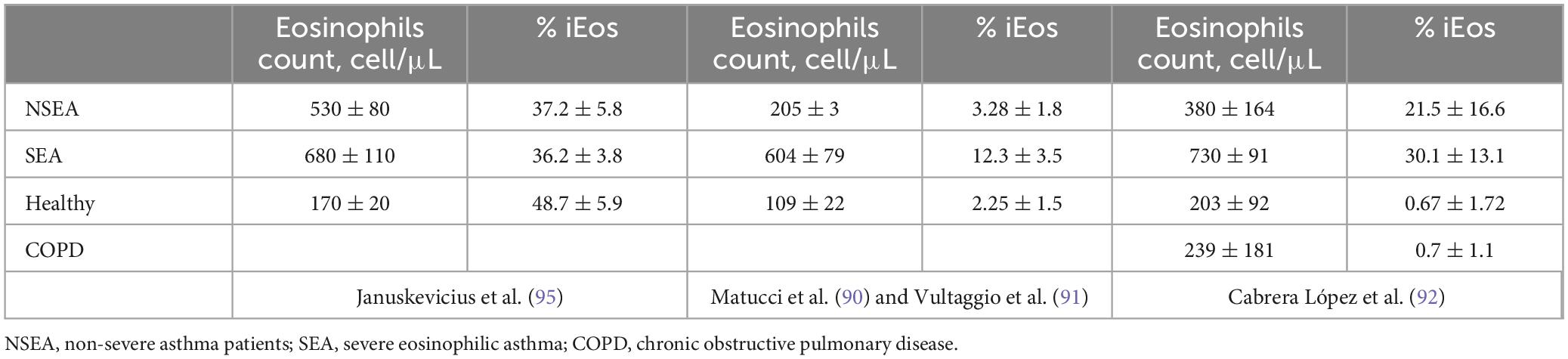
The Cabrera López et al. (93), study highlights the presence of iEos in asthmatic patients (over 20% of the total count) with minimal percentages (less than 1%) in healthy subjects, smokers without chronic obstructive pulmonary disease (COPD), and COPD subjects. In this study, it was observed that iEos are independent of disease severity, treatment, and exacerbations in patients with COPD. Additionally, the proportion of iEos in asthmatic subjects is independent of the total blood eosinophil count. For instance, patients with only 250 eosinophils per microliter can have up to 45% iEos. This finding may explain the discrepancy between the number of eosinophils in the blood and in the tissues.
Cabrera López et al. (93) analyzed freshly unfractionated blood (100 μl) from 10 stable subjects of four groups: (COPD), asthma, smokers without COPD, and healthy volunteers; data were validated in 59 patients with COPD and in 17 patients with asthma. Cell phenotyping was according to the Mesnil criteria and other crucial proteins as CD125 and CD11b (Table 2). iEos were identified following the algorithm: CD45+Siglec8+CD16–CD62LloCD11bhiCD125hiCD101lo and rEos were identified as CD45+Siglec8+CD16–CD62LhiCD11bdCD125d CD101d by flow cytometry and confocal microscopy (Figure 1B).
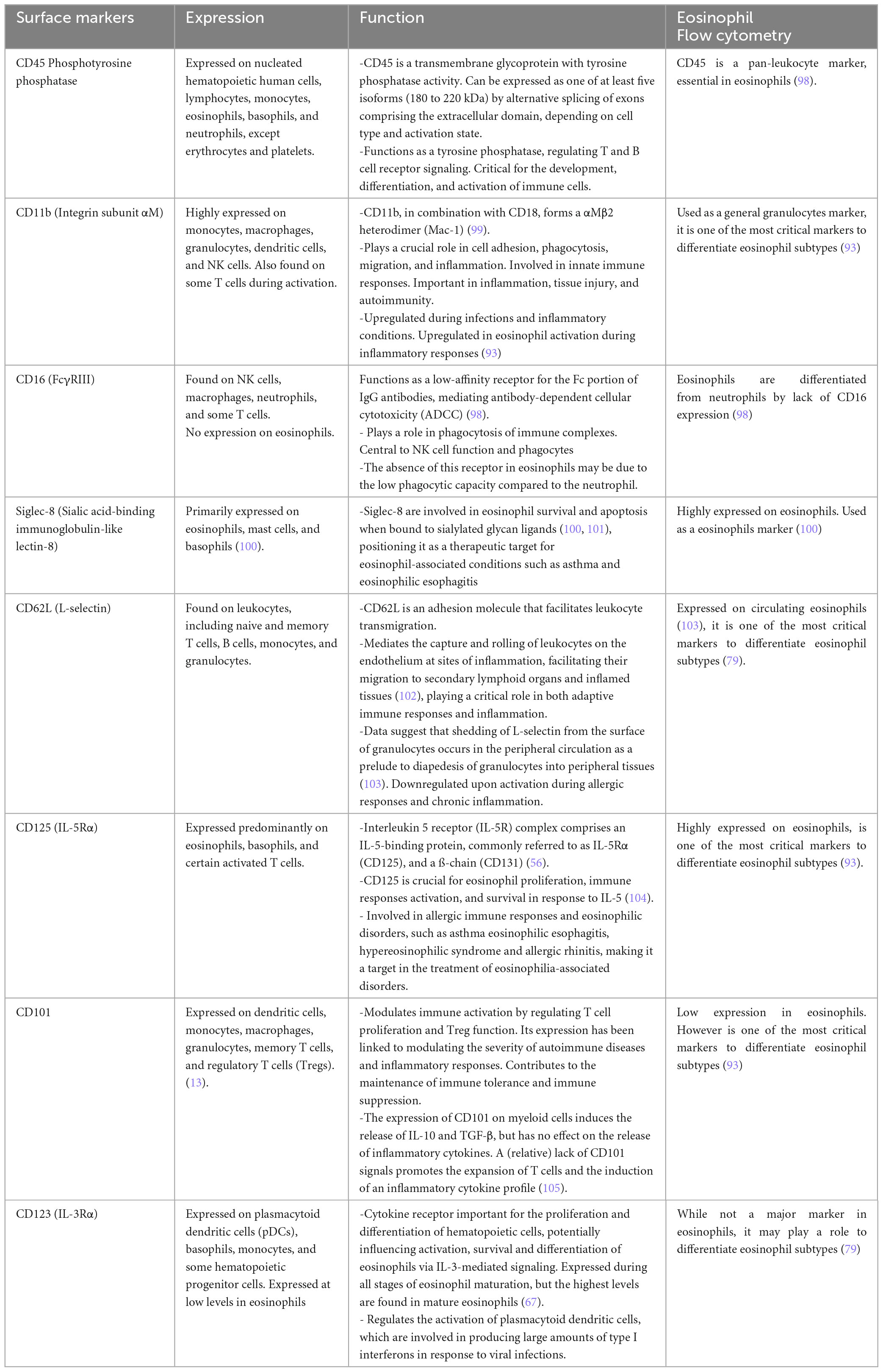
Table 2. Expression and function of the crucial surface markers of the eosinophils and their importance in flow cytometry.
For the purposes of this review, these asthmatic patients were divided into SEA and NSEA. The observed variations in the proportions of human blood eosinophils subpopulations may be attributed to divergent processing methods of the samples in the different studies. Specifically, Januskevicius et al. (96) demonstrated higher levels of iEos in SEA patients (Table 3), likely due to the magnetic selection of eosinophils using CD62L as a marker. This selection process may lead to the downregulation of the protein following interaction with the magnetic beads.
Conversely, the proportions reported by Matucci et al. (91), Vultaggio et al. (92), and Cabrera López et al. (93) exhibit greater similarity. However, discrepancies persist that could be linked to different methods employed. Matucci et al. (91) and Vultaggio et al. (92) observed a lower percentage of iEos in their studies compared to Cabrera López et al. (93) (Tables 3, 4); a difference that cannot be explained by the analysis algorithm alone. It is possible that there could be a loss of cellularity due to the methodology employed: Ficoll (91, 92) vs. lysis (93), under the hypothesis that the latter method subjects eosinophils to less stress than the Ficoll method, resulting in less loss of eos especially iEos. Furthermore, it is known that in the Canary Islands there is an increase in the number of eosinophils in the blood due to their weather conditions, which could explain the differences found between iEos in SEA and NSEA with other studies.
Possible implications in asthma and COPD of the different eosinophil’s subpopulations
The concept of iEos and rEos is novel. There is limited evidence regarding the mechanisms and roles these cells play in various diseases. No studies have been conducted on these cellular phenotypes in exacerbations or in patients treated with monoclonal antibodies other than mepolizumab and benralizumab. Furthermore, their functional roles have not been published, and their potential contributions remain speculative. This has been highlighted by the EAACI task force paper on new molecular insights and clinical functions of eosinophils states (97), which calls for research in this topic for the next years. However, several thoughts arise when addressing the possible role of the eosinophil’s subpopulations. One possibility is that identifying a threshold of iEos (probably a 8–10% would be adequate) may be sufficient to categorize an asthmatic patient as having a Th2-high endotype. It is well established that approximately 20% of patients with severe asthma exhibit discordance between blood and tissue eosinophils. This discrepancy might be explained by different eosinophil subpopulations. As exposed previously, asthmatic patients can have a low blood eosinophil count but a high proportion of them can be iEos. This could be the case of the iEos found in non-eosinophilic asthma patients in the study of Fricker et al. (95). We speculate that iEos, due to their molecular surface markers, are the ones driven to the inflammation site. iEos in the blood may serve as a surrogate marker of a Th2 signal, even when the total blood eosinophil count does not exceed 250 cells/mm3. This could allow iEos to endotype severe asthma patients, pointing out candidates for biological therapy with anti-IL-5/IL-5R agents even though they do not have a high eosinophil count in peripheral blood. This might also explain why Tezepelumab is effective in non-Th2 asthma. Asthma has traditionally been classified as Th2-high based on blood eosinophil counts rather than tissue eosinophils. There could be a subset of patients with low blood eosinophil counts but elevated eosinophil levels in the bronchi, who may respond well to treatments like Tezepelumab. However, this could represent only part of the explanation, as Tezepelumab affects multiple cell types and mechanisms beyond IL-5 inhibition. According to data from Vultaggio et al. (92) these eosinophil subpopulations may be more predictive of symptom control (asthma and nasal polyposis) than the total blood eosinophil count and could maybe serve as a biomarker of control in patients treated with mepolizumab.
Studies of biological therapies have been disappointing in COPD. Only dupilumab can reduce exacerbations so far and the population where it works better is in those who have high eosinophil blood count, high FENO and IgE. It is necessary to define accurately the COPD patient suitable for monoclonal antibodies in order to achieve a good therapeutic response. Eosinophil’s subpopulations may help identify those who are suitable candidates for anti-eosinophilic treatments. In the study by Cabrera López et al. (93) we found that COPD patients, even those with elevated eosinophil blood counts, had less than 1% of iEos. Identifying COPD patients with a significant percentage of iEos could be highly useful for selecting those who are more likely to respond to monoclonal antibody therapies.
Another gap is if the proportion of iEos and rEos could vary between stable states and exacerbations. iEos could potentially increase during an exacerbation both in asthma and COPD. Such findings could also have important implications for identifying candidates for biological therapies.
Conclusion
Eosinophils are granulocytic cells historically viewed as purely inflammatory and defensive, often associated with pathological conditions. In recent years, this perception has evolved as research has uncovered their homeostatic roles and synergistic interactions with other immune cells. Recent advancements have demonstrated the existence of different eosinophil subtypes and their potential association with disease severity. However, several questions remain unanswered: Are these true subtypes or merely activated cells? Are they generated in this form in the bone marrow, or do they differentiate later? Can they serve as biomarkers for the use of monoclonal antibodies in asthma and COPD? Do they function similarly when stimulated? Is their genetics similar? Most studies on eosinophils have treated them as a homogeneous population without distinguishing subtypes. To address these questions, it is essential to conduct subtype-specific investigations, as previous studies that did not differentiate subtypes are less comparable. Future research should focus on resolving these issues, which could significantly improve the characterization of patients with eosinophilia and facilitate the development of personalized medicine.
Author contributions
AS: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review and editing. IS: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review and editing. HG: Investigation, Methodology, Writing – original draft, Writing – review and editing. SC: Conceptualization, Investigation, Writing – original draft. AL: Conceptualization, Formal analysis, Investigation, Supervision, Writing – original draft, Writing – review and editing. CC: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review and editing.
Funding
The authors declare that financial support was received for the research, authorship, and/or publication of this article. Sara Cazorla Rivero was a recipient of a Margarita Salas postdoctoral grant (Ministerio de Universidades grant UNI/551/2021; Fondos Next Generation EU; University of La Laguna).
Acknowledgments
We thank the Advanced Confocal and Electron Microscopy Research Service (SIMACE) University of Las Palmas de Gran Canaria; and Valeria A. Espinoza Sánchez for their help with the design of the images.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
COPD, chronic obstructive pulmonary disease; ECP, eosinophil cationic protein; EDN, eosinophil-derived neurotoxin; MBP, major basic proteins; rEos, resident eosinophils; iEos, inflammatory eosinophils; SEA, Severe eosinophilic asthma; NSEA, non-severe eosinophilic asthma.
References
1. Jones TW. The blood-corpuscle considered in its different phases of development in the animal series. Memoir I.—Vertebrata. Philos Trans R Soc Lond. (1846) 136:63–87. doi: 10.1098/rstl.1846.0005
2. Brewer DB. Max schultze and the living, moving, phagocytosing leucocytes: 1865. Med Hist. (1994) 38:91–101.
3. Badillo CL, Mendoza D, López JGH. La Historia Del Eosinófilo, Su Papel Fisiopatológico y Manifestaciones Clínicas de La Eosinofilia. Rev. Alergia Asma Inmunol Pediátr. (2018). 27:79–93.
4. Fischkoff SA, Pollak A, Gleich GJ, Testa JR, Misawa S, Reber TJ. Eosinophilic differentiation of the human promyelocytic leukemia cell line, HL-60. J Exp Med. (1984) 160:179–96. doi: 10.1084/jem.160.1.179
5. McNagny KM. Regulation of eosinophil-specific gene expression by a C/EBP-Ets complex and GATA-1. EMBO J. (1998) 17:3669–80. doi: 10.1093/emboj/17.13.3669
6. Yu C, Cantor AB, Yang H, Browne C, Wells RA, Fujiwara Y, et al. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J Exp Med. (2002) 195:1387–95. doi: 10.1084/jem.20020656
7. Boyer MH, Basten A, Beeson PB. Mechanism of eosinophilia. 3. suppression of eosinophilia by agents known to modify immune responses. Blood. (1970) 36:458–69.
8. Basten A, Beeson PB. Mechanism of eosinophilia. J Exp Med. (1970) 131:1288–305. doi: 10.1084/jem.131.6.1288
9. Yamaguchi Y, Hayashi Y, Sugama Y, Miura Y, Kasahara T, Kitamura S, et al. Highly purified murine interleukin 5 (IL-5) stimulates eosinophil function and prolongs in vitro survival. IL-5 as an eosinophil chemotactic factor. J Exp Med. (1988) 167:1737–42. doi: 10.1084/jem.167.5.1737
10. Sastre B, Rodrigo-Muñoz J, Garcia-Sanchez D, Cañas J, Del Pozo V. Eosinophils: Old players in a new game. J Investig Allergol Clin Immunol. (2018) 28:289–304. doi: 10.18176/jiaci.0295
11. Gleich GJ, Adolphson CR. The eosinophilic leukocyte: Structure and function. Adv Immunol. (1986) 39:177–253. doi: 10.1016/S0065-2776(08)60351-X
12. Gleich GJ, Loegering DA, Bell MP, Checkel JL, Ackerman SJ, McKean DJ. Biochemical and functional similarities between human eosinophil-derived neurotoxin and eosinophil cationic protein: homology with ribonuclease. Proc Natl Acad Sci USA. (1986) 83:3146–50. doi: 10.1073/pnas.83.10.3146
13. Young JDE, Peterson CGB, Venge P, Cohn ZA. Mechanism of membrane damage mediated by human eosinophil cationic protein. Nature. (1986) 321:613–6. doi: 10.1038/321613a0
14. Slifman NR, Loegering DA, McKean DJ, Gleich GJ. Ribonuclease activity associated with human eosinophil-derived neurotoxin and eosinophil cationic protein. J Immunol Baltim Md 1950. (1986) 137:2913–7.
15. Rosenberg HF, Domachowske JB. Eosinophils, eosinophil ribonucleases, and their role in host defense against respiratory virus pathogens. J Leukoc Biol. (2001) 70:691–8.
16. Venge P, Byström J, Carlson M, Hâkansson L, Karawacjzyk M, Peterson C, et al. Eosinophil cationic protein (ECP): molecular and biological properties and the use of ECP as a marker of eosinophil activation in disease. Clin Exp Allergy. (1999) 29:1172–86. doi: 10.1046/j.1365-2222.1999.00542.x
17. Piliponsky AM, Pickholtz D, Gleich GJ, Levi-Schaffer F. Human eosinophils induce histamine release from antigen-activated rat peritoneal mast cells: A possible role for mast cells in late-phase allergic reactions. J Allergy Clin Immunol. (2001) 107:993–1000. doi: 10.1067/mai.2001.114656
18. Zheutlin LM, Ackerman SJ, Gleich GJ, Thomas LL. Stimulation of basophil and rat mast cell histamine release by eosinophil granule-derived cationic proteins. J Immunol Baltim Md 1950. (1984) 133:2180–5.
19. Jacoby DB, Costello RM, Fryer AD. Eosinophil recruitment to the airway nerves. J Allergy Clin Immunol. (2001) 107:211–8. doi: 10.1067/mai.2001.112940
20. Wu W, Chen Y, Hazen SL. Eosinophil peroxidase nitrates protein tyrosyl residues. J Biol Chem. (1999) 274:25933–44. doi: 10.1074/jbc.274.36.25933
21. Agosti JM, Altman LC, Ayars GH, Loegering DA, Gleich GJ, Klebanoff SJ. The injurious effect of eosinophil peroxidase, hydrogen peroxide, and halides on pneumocytes in vitro. J Allergy Clin Immunol. (1987) 79:496–504. doi: 10.1016/0091-6749(87)90368-X
22. MacPherson JC, Comhair SAA, Erzurum SC, Klein DF, Lipscomb MF, Kavuru MS, et al. Eosinophils are a major source of nitric oxide-derived oxidants in severe asthma: characterization of pathways available to eosinophils for generating reactive nitrogen species. J Immunol. (2001) 166:5763–72. doi: 10.4049/jimmunol.166.9.5763
23. Melo RCN, Spencer LA, Dvorak AM, Weller PF. Mechanisms of eosinophil secretion: large vesiculotubular carriers mediate transport and release of granule-derived cytokines and other proteins. J Leukoc Biol. (2008) 83:229–36. doi: 10.1189/jlb.0707503
24. Neves VH, Palazzi C, Malta KK, Bonjour K, Kneip F, Dias FF, et al. Extracellular sombrero vesicles are hallmarks of eosinophilic cytolytic degranulation in tissue sites of human diseases. J Leukoc Biol. (2024) 116:398–408. doi: 10.1093/jleuko/qiae079
25. Mukherjee M, Lacy P, Ueki S. Eosinophil extracellular traps and inflammatory pathologies-untangling the web! Front Immunol. (2018) 9:2763. doi: 10.3389/fimmu.2018.02763
26. Neves VH, Palazzi C, Bonjour K, Ueki S, Weller PF, Melo RCN. In vivo ETosis of human eosinophils: The ultrastructural signature captured by TEM in eosinophilic diseases. Front Immunol. (2022) 13:938691. doi: 10.3389/fimmu.2022.938691
27. Tomizawa H, Arima M, Miyabe Y, Furutani C, Kodama S, Ito K, et al. Characteristics and regulation of human eosinophil ETosis in vitro. Am J Respir Cell Mol Biol. (2024): doi: 10.1165/rcmb.2023-0438OC [Epub ahead of print].
28. Weihrauch T, Melo RCN, Gray N, Voehringer D, Weller PF, Raap U. Eosinophil extracellular vesicles and DNA traps in allergic inflammation. Front Allergy. (2024) 5:1448007. doi: 10.3389/falgy.2024.1448007
29. Ueki S, Tokunaga T, Melo RCN, Saito H, Honda K, Fukuchi M, et al. Charcot-Leyden crystal formation is closely associated with eosinophil extracellular trap cell death. Blood. (2018) 132:2183–7. doi: 10.1182/blood-2018-04-842260
30. Weiler CR, Kita H, Hukee M, Gleich GJ. Eosinophil viability during immunoglobulin-induced degranulation. J Leukoc Biol. (1996) 60:493–501. doi: 10.1002/jlb.60.4.493
31. Davoine F, Ferland C, Chakir J, Lee JE, Adamko DJ, Moqbel R, et al. Interleukin-12 inhibits eosinophil degranulation and migration but does not promote eosinophil apoptosis. Int Arch Allergy Immunol. (2006) 140:277–84. doi: 10.1159/000093705
32. Ueki S, Melo RCN, Ghiran I, Spencer LA, Dvorak AM, Weller PF. Eosinophil extracellular DNA trap cell death mediates lytic release of free secretion-competent eosinophil granules in humans. Blood. (2013) 121:2074–83. doi: 10.1182/blood-2012-05-432088
33. Kaneko M, Swanson MC, Gleich GJ, Kita H. Allergen-specific IgG1 and IgG3 through Fc gamma RII induce eosinophil degranulation. J Clin Invest. (1995) 95:2813–21. doi: 10.1172/JCI117986
34. Dvorak AM, Ackerman SJ, Furitsu T, Estrella P, Letourneau L, Ishizaka T. Mature eosinophils stimulated to develop in human-cord blood mononuclear cell cultures supplemented with recombinant human interleukin-5. II. Vesicular transport of specific granule matrix peroxidase, a mechanism for effecting piecemeal degranulation. Am J Pathol. (1992) 140:795–807.
35. Melo RCN, Weller PF. Vesicular trafficking of immune mediators in human eosinophils revealed by immunoelectron microscopy. Exp Cell Res. (2016) 347:385–90. doi: 10.1016/j.yexcr.2016.08.016
36. Lacy P, Mahmudi-Azer S, Bablitz B, Hagen SC, Velazquez JR, Man SF, et al. Rapid mobilization of intracellularly stored RANTES in response to interferon-gamma in human eosinophils. Blood. (1999) 94:23–32.
37. Bandeira-Melo C, Gillard G, Ghiran I, Weller PF. EliCell: a gel-phase dual antibody capture and detection assay to measure cytokine release from eosinophils. J Immunol Methods. (2000) 244:105–15. doi: 10.1016/S0022-1759(00)00264-7
38. Logan MR, Lacy P, Bablitz B, Moqbel R. Expression of eosinophil target SNAREs as potential cognate receptors for vesicle-associated membrane protein-2 in exocytosis. J Allergy Clin Immunol. (2002) 109:299–306. doi: 10.1067/mai.2002.121453
39. Rosenberg HF, Dyer KD, Foster PS. Eosinophils: Changing perspectives in health and disease. Nat Rev Immunol. (2013) 13:9–22. doi: 10.1038/nri3341
40. Marichal T, Mesnil C, Bureau F. Homeostatic eosinophils: Characteristics and functions. Front Med. (2017) 4:101. doi: 10.3389/fmed.2017.00101
41. Rytomaa T. Organ distribution and histochemical properties of eosinophil granulocytes in rat. Acta Pathol Microbiol Scand Suppl. (1960) 50(Suppl. 140):1–118.
42. Farahi N, Singh NR, Heard S, Loutsios C, Summers C, Solanki CK, et al. Use of 111-Indium–labeled autologous eosinophils to establish the in vivo kinetics of human eosinophils in healthy subjects. Blood. (2012) 120:4068–71. doi: 10.1182/blood-2012-07-443424
43. Wen T, Besse JA, Mingler MK, Fulkerson PC, Rothenberg ME. Eosinophil adoptive transfer system to directly evaluate pulmonary eosinophil trafficking in vivo. Proc Natl Acad Sci USA. (2013) 110:6067–72. doi: 10.1073/pnas.1220572110
44. Kim HJ, Alonzo ES, Dorothee G, Pollard JW, Sant’Angelo DB. Selective depletion of eosinophils or neutrophils in mice impacts the efficiency of apoptotic cell clearance in the thymus. PLoS One. (2010) 5:e11439. doi: 10.1371/journal.pone.0011439
45. Jung Y, Rothenberg ME. Roles and Regulation of Gastrointestinal Eosinophils in Immunity and Disease. J Immunol. (2014) 193:999–1005. doi: 10.4049/jimmunol.1400413
46. Mochizuki M, Bartels J, Mallet AI, Christophers E, Schröder JM. IL-4 induces eotaxin: a possible mechanism of selective eosinophil recruitment in helminth infection and atopy. J Immunol Baltim Md 1950. (1998) 160:60–8.
47. Rothenberg ME. Eotaxin: An Essential Mediator of Eosinophil Trafficking into Mucosal Tissues. Am J Respir Cell Mol Biol. (1999) 21:291–5. doi: 10.1165/ajrcmb.21.3.f160
48. Ahrens R, Waddell A, Seidu L, Blanchard C, Carey R, Forbes E, et al. Intestinal macrophage/epithelial cell-derived CCL11/eotaxin-1 mediates eosinophil recruitment and function in pediatric ulcerative colitis. J Immunol. (2008) 181:7390–9. doi: 10.4049/jimmunol.181.10.7390
49. Waddell A, Ahrens R, Steinbrecher K, Donovan B, Rothenberg ME, Munitz A, et al. Colonic eosinophilic inflammation in experimental colitis is mediated by Ly6Chigh CCR2+ inflammatory monocyte/macrophage-derived CCL11. J Immunol. (2011) 186:5993–6003. doi: 10.4049/jimmunol.1003844
50. Lilly CM, Nakamura H, Kesselman H, Nagler-Anderson C, Asano K, Garcia-Zepeda EA, et al. Expression of eotaxin by human lung epithelial cells: induction by cytokines and inhibition by glucocorticoids. J Clin Invest. (1997) 99:1767–73. doi: 10.1172/JCI119341
51. Provost V, Larose MC, Langlois A, Rola-Pleszczynski M, Flamand N, Laviolette M. CCL26/eotaxin-3 is more effective to induce the migration of eosinophils of asthmatics than CCL11/eotaxin-1 and CCL24/eotaxin-2. J Leukoc Biol. (2013) 94:213–22. doi: 10.1189/jlb.0212074
52. Ponath PD, Qin S, Post TW, Wang J, Wu L, Gerard NP, et al. Molecular cloning and characterization of a human eotaxin receptor expressed selectively on eosinophils. J Exp Med. (1996) 183:2437–48. doi: 10.1084/jem.183.6.2437
53. Daugherty BL, Siciliano SJ, DeMartino JA, Malkowitz L, Sirotina A, Springer MS. Cloning, expression, and characterization of the human eosinophil eotaxin receptor. J Exp Med. (1996) 183:2349–54. doi: 10.1084/jem.183.5.2349
54. Humbles AA, Lu B, Friend DS, Okinaga S, Lora J, Al-Garawi A, et al. The murine CCR3 receptor regulates both the role of eosinophils and mast cells in allergen-induced airway inflammation and hyperresponsiveness. Proc Natl Acad Sci USA. (2002) 99:1479–84. doi: 10.1073/pnas.261462598
55. Ogilvie P, Bardi G, Clark-Lewis I, Baggiolini M, Uguccioni M. Eotaxin is a natural antagonist for CCR2 and an agonist for CCR5. Blood. (2001) 97:1920–4. doi: 10.1182/blood.V97.7.1920
56. Clutterbuck EJ, Hirst EM, Sanderson CJ. Human interleukin-5 (IL-5) regulates the production of eosinophils in human bone marrow cultures: comparison and interaction with IL-1, IL-3, IL-6, and GMCSF. Blood. (1989) 73:1504–12.
57. Mould AW, Matthaei KI, Young IG, Foster PS. Relationship between interleukin-5 and eotaxin in regulating blood and tissue eosinophilia in mice. J Clin Invest. (1997) 99:1064–71. doi: 10.1172/JCI119234
58. Nussbaum JC, Van Dyken SJ, Von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. (2013) 502:245–8. doi: 10.1038/nature12526
59. Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. (2013) 210(3):535–49. doi: 10.1084/jem.20121964
60. Bettigole SE, Lis R, Adoro S, Lee AH, Spencer LA, Weller PF, et al. The transcription factor XBP1 is selectively required for eosinophil differentiation. Nat Immunol. (2015) 16:829–37. doi: 10.1038/ni.3225
61. Mishra A, Hogan SP, Lee JJ, Foster PS, Rothenberg ME. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J Clin Invest. (1999) 103:1719–27. doi: 10.1172/JCI6560
62. Kopf M, Brombacher F, Hodgkin PD, Ramsay AJ, Milbourne EA, Dai WJ, et al. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity. (1996) 4:15–24. doi: 10.1016/S1074-7613(00)80294-0
63. Robertson S, Mau V, Young I, Matthaei K. Uterine eosinophils and reproductive performance in interleukin 5-deficient mice. Reproduction. (2000) 120:423–32. doi: 10.1530/jrf.0.1200423
64. Roth N, Städler S, Lemann M, Hösli S, Simon H-U, Simon D. Distinct eosinophil cytokine expression patterns in skin diseases – the possible existence of functionally different eosinophil subpopulations. Allergy. (2011) 66:1477–86. doi: 10.1111/j.1398-9995.2011.02694.x
65. Larose MC, Chakir J, Archambault AS, Joubert P, Provost V, Laviolette M, et al. Correlation between CCL26 production by human bronchial epithelial cells and airway eosinophils: Involvement in patients with severe eosinophilic asthma. J Allergy Clin Immunol. (2015) 136:904–13. doi: 10.1016/j.jaci.2015.02.039
66. Wardlaw AJ. Molecular basis for selective eosinophil trafficking in asthma: A multistep paradigm✩✩✩. J Allergy Clin Immunol. (1999) 104:917–26. doi: 10.1016/S0091-6749(99)70069-2
67. Esnault S, Kelly EA. Essential mechanisms of differential activation of eosinophils by IL-3 compared to GM-CSF and IL-5. Crit Rev Immunol. (2016) 36:429–44. doi: 10.1615/CritRevImmunol.2017020172
68. Weller PF, Spencer LA. Functions of tissue-resident eosinophils. Nat Rev Immunol. (2017) 17:746–60. doi: 10.1038/nri.2017.95
69. Griseri T, Arnold IC, Pearson C, Krausgruber T, Schiering C, Franchini F, et al. Granulocyte macrophage colony-stimulating factor-activated eosinophils promote interleukin-23 driven chronic colitis. Immunity. (2015) 43:187–99. doi: 10.1016/j.immuni.2015.07.008
70. Spencer LA, Weller PF. Eosinophils and Th2 immunity: contemporary insights. Immunol Cell Biol. (2010) 88:250–6. doi: 10.1038/icb.2009.115
71. Bystrom J, Amin K, Bishop-Bailey D. Analysing the eosinophil cationic protein - a clue to the function of the eosinophil granulocyte. Respir Res. (2011) 12:10. doi: 10.1186/1465-9921-12-10
72. Amin K, Lúdvíksdóttir D, Janson C, Nettelbladt O, Björnsson E, Roomans GM, et al. Inflammation and structural changes in the airways of patients with atopic and nonatopic asthma. Am J Respir Crit Care Med. (2000) 162:2295–301. doi: 10.1164/ajrccm.162.6.9912001
73. Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. (2008) 14:949–53. doi: 10.1038/nm.1855
74. Morshed M, Yousefi S, Stöckle C, Simon HU, Simon D. Thymic stromal lymphopoietin stimulates the formation of eosinophil extracellular traps. Allergy. (2012) 67:1127–37. doi: 10.1111/j.1398-9995.2012.02868.x
75. Rothenberg ME, Hogan SP. The Eosinophil. Annu Rev Immunol. (2006) 24:147–74. doi: 10.1146/annurev.immunol.24.021605.090720
76. Kita H. The eosinophil: A cytokine-producing cell? J Allergy Clin Immunol. (1996) 97:889–92. doi: 10.1016/S0091-6749(96)80061-3
77. Schuijs MJ, Willart MA, Hammad H, Lambrecht BN. Cytokine targets in airway inflammation. Curr Opin Pharmacol. (2013) 13:351–61. doi: 10.1016/j.coph.2013.03.013
78. Mjösberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. (2011) 12:1055–62. doi: 10.1038/ni.2104
79. Mesnil C, Raulier S, Paulissen G, Xiao X, Birrell MA, Pirottin D, et al. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Invest. (2016) 126:3279–95. doi: 10.1172/JCI85664
80. Carlens J, Wahl B, Ballmaier M, Bulfone-Paus S, Förster R, Pabst O. Common γ-chain-dependent signals confer selective survival of eosinophils in the murine small intestine. J Immunol. (2009) 183:5600–7. doi: 10.4049/jimmunol.0801581
81. Chu DK, Jimenez-Saiz R, Verschoor CP, Walker TD, Goncharova S, Llop-Guevara A, et al. Indigenous enteric eosinophils control DCs to initiate a primary Th2 immune response in vivo. J Exp Med. (2014) 211:1657–72. doi: 10.1084/jem.20131800
82. Gouon-Evans V, Rothenberg ME, Pollard JW. Postnatal mammary gland development requires macrophages and eosinophils. Development. (2000) 127:2269–82. doi: 10.1242/dev.127.11.2269
83. Jung Y, Wen T, Mingler MK, Caldwell JM, Wang YH, Chaplin DD, et al. IL-1β in eosinophil-mediated small intestinal homeostasis and IgA production. Mucosal Immunol. (2015) 8:930–42. doi: 10.1038/mi.2014.123
84. Throsby M, Herbelin A, Pléau JM, Dardenne M. CD11c+ eosinophils in the murine thymus: developmental regulation and recruitment upon MHC Class I-restricted thymocyte deletion. J Immunol. (2000) 165:1965–75. doi: 10.4049/jimmunol.165.4.1965
85. Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. (2011) 332:243–7. doi: 10.1126/science.1201475
86. Matthews AN, Friend DS, Zimmermann N, Sarafi MN, Luster AD, Pearlman E, et al. Eotaxin is required for the baseline level of tissue eosinophils. Proc Natl Acad Sci USA. (1998) 95:6273–8. doi: 10.1073/pnas.95.11.6273
87. Abdala Valencia H, Loffredo LF, Misharin AV, Berdnikovs S. Phenotypic plasticity and targeting of S iglec- F high CD 11c low eosinophils to the airway in a murine model of asthma. Allergy. (2016) 71:267–71. doi: 10.1111/all.12776
88. el-Cheikh MC, Borojevic R. Extramedullar proliferation of eosinophil granulocytes in chronic schistosomiasis mansoni is mediated by a factor secreted by inflammatory macrophages. Infect Immun. (1990) 58:816–21. doi: 10.1128/iai.58.3.816-821.1990
89. Lee JJ, Jacobsen EA, Ochkur SI, McGarry MP, Condjella RM, Doyle AD, et al. Human versus mouse eosinophils: “That which we call an eosinophil, by any other name would stain as red.”. J Allergy Clin Immunol. (2012) 130:572–84. doi: 10.1016/j.jaci.2012.07.025
90. Pantelyushin S, Rhiner T, Jebbawi F, Sella F, Waldern N, Lam J, et al. Interleukin 5-dependent inflammatory eosinophil subtype involved in allergic insect bite hypersensitivity of horses. Allergy. (2023) 78:3020–3. doi: 10.1111/all.15859
91. Matucci A, Nencini F, Maggiore G, Chiccoli F, Accinno M, Vivarelli E, et al. High proportion of inflammatory CD62L low eosinophils in blood and nasal polyps of severe asthma patients. Clin Exp Allergy. (2023) 53:78–87. doi: 10.1111/cea.14153
92. Vultaggio A, Accinno M, Vivarelli E, Mecheri V, Maggiore G, Cosmi L, et al. Blood CD62L low inflammatory eosinophils are related to the severity of asthma and reduced by mepolizumab. Allergy. (2023) 78:3154–65. doi: 10.1111/all.15909
93. Cabrera López C, Sánchez Santos A, Lemes Castellano A, Cazorla Rivero S, Breña Atienza J, González Dávila E, et al. Eosinophil subtypes in adults with asthma and adults with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2023) 208:155–62. doi: 10.1164/rccm.202301-0149OC
94. Mycroft K, Paplińska-Goryca M, Proboszcz M, Nejman-Gryz P, Krenke R, Górska K. Blood and sputum eosinophils of COPD patients are differently polarized than in asthma. Cells. (2023) 12:1631. doi: 10.3390/cells12121631
95. Fricker M, Harrington J, Hiles SA, Gibson PG. Mepolizumab depletes inflammatory but preserves homeostatic eosinophils in severe asthma. Allergy (2024) 79:3118–28. doi: 10.1111/all.16267
96. Januskevicius A, Jurkeviciute E, Janulaityte I, Kalinauskaite-Zukauske V, Miliauskas S, Malakauskas K. Blood eosinophils subtypes and their survivability in asthma patients. Cells. (2020) 9:1248. doi: 10.3390/cells9051248
97. Jesenak M, Diamant Z, Simon D, Tufvesson E, Seys SF, Mukherjee M, et al. Eosinophils-from cradle to grave: An EAACI task force paper on new molecular insights and clinical functions of eosinophils and the clinical effects of targeted eosinophil depletion. Allergy. (2023) 78:3077–102. doi: 10.1111/all.15884
98. Thurau AM, Schylz U, Wolf V, Krug N, Schauer U. Identification of eosinophils by flow cytometry. Cytometry (1996) 23:150–8. doi: 10.1002/(SICI)1097-0320(19960201)23:23.0.CO;2-O
99. Gomułka K, Tota M, Brzda̧k K. Effect of VEGF stimulation on CD11b receptor on peripheral eosinophils in asthmatics. Int J Mol Sci. (2023) 24:8880. doi: 10.3390/ijms24108880
100. Johansson MW, Kelly EA, Nguyen CL, Jarjour NN, Bochner BS. Characterization of siglec-8 expression on lavage cells after segmental lung allergen challenge. Int Arch Allergy Immunol. (2018) 177:16–28. doi: 10.1159/000488951
101. O’Sullivan JA, Carroll DJ, Bochner BS. Glycobiology of eosinophilic inflammation: Contributions of siglecs, glycans, and other glycan-binding proteins. Front Med. (2017) 4:116. doi: 10.3389/fmed.2017.00116
102. Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: From mechanisms to disease. Annu Rev Immunol. (2012) 30:459–89. doi: 10.1146/annurev-immunol-020711-074942
103. Kuhns DB, Long Priel DA, Gallin JI. Loss of L-selectin (CD62L) on human neutrophils following exudation in vivo. Cell Immunol. (1995) 164:306–10. doi: 10.1006/cimm.1995.1174
104. Sato S, Katagiri T, Takaki S, Kikuchi Y, Hitoshi Y, Yonehara S, et al. IL-5 receptor-mediated tyrosine phosphorylation of SH2/SH3-containing proteins and activation of Bruton’s tyrosine and Janus 2 kinases. J Exp Med. (1994) 180:2101–11. doi: 10.1084/jem.180.6.2101
Keywords: asthma, COPD, eosinophils, subtypes, inflammation
Citation: Sanchez Santos A, Socorro Avila I, Galvan Fernandez H, Cazorla Rivero S, Lemes Castellano A and Cabrera Lopez C (2025) Eosinophils: old cells, new directions. Front. Med. 11:1470381. doi: 10.3389/fmed.2024.1470381
Received: 25 July 2024; Accepted: 20 December 2024;
Published: 16 January 2025.
Edited by:
Kian Fan Chung, Imperial College London, United KingdomReviewed by:
Manali Mukherjee, McMaster University, CanadaKatie Raby, Imperial College London, United Kingdom
Copyright © 2025 Sanchez Santos, Socorro Avila, Galvan Fernandez, Cazorla Rivero, Lemes Castellano and Cabrera Lopez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carlos Cabrera Lopez, Y2NhYmxvcG5AZ21haWwuY29t
†These authors have contributed equally to this work and share first authorship
 Alejandra Sanchez Santos1†
Alejandra Sanchez Santos1† Helena Galvan Fernandez
Helena Galvan Fernandez Sara Cazorla Rivero
Sara Cazorla Rivero Carlos Cabrera Lopez
Carlos Cabrera Lopez