
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 10 October 2024
Sec. Dermatology
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1445288
Background: Psoriasis is closely associated with cardiovascular disease (CVD). However, the current evidence on the correlation between Life’s Essential 8 and Psoriasis is insufficient. Our aim was to investigate the relationship between Life’s Essential 8 (LE8), a measure of cardiovascular health (CVH), and psoriasis.
Objective: This study aimed to clarify the impact of Life’s Essential 8 on Psoriasis and explore its implications.
Methods: This population-based cross-sectional study included 9,876 US adults aged 20 to 59 years from the National Health and Nutrition Examination Survey (NHANES) 2003–2006 and 2009–2014 cycles. The LE8 score comprises 8 metrics and was categorized into low, moderate, and high CVH. Logistic regression and restricted cubic splines (RCS) were used to assess the association between LE8 score and psoriasis.
Results: Among the 9,876 participants, those with moderate and high CVH had higher risks of psoriasis compared to low CVH. Additionally, every 10-point increase in the LE8 score was associated with a 10% reduced risk of psoriasis. Interaction was observed between gender, age, education level, race/ethnicity, marital status, and PIR.
Conclusion: LE8 and its subscale scores were strongly negatively related to the risk of psoriasis. Encouraging optimal CVH levels may be advantageous in reducing the incidence of psoriasis.
Psoriasis, a chronic immune-mediated systemic disease, is characterized by red patches covered with silvery scales, often causing itching, pain, and in severe cases, joint involvement. It is associated with cardiovascular diseases, diabetes, and metabolic syndrome, significantly impacting patients’ quality of life. Early identification and treatment of cardiovascular complications are crucial for improving patient outcomes (1).
The Life’s Essential 8 (LE8), an algorithm developed by the American Heart Association, assesses cardiovascular health through diet, physical activity, smoking habits, sleep patterns, body mass index, lipid status, blood glucose levels, and blood pressure. Understanding the relationship between LE8 and psoriasis is critical in healthcare and public health (2). This study aims to quantify the association between cardiovascular health levels, as measured by LE8 scores, and the prevalence of psoriasis among US adults.
Existing research indicates that psoriasis patients with cardiovascular risk factors have a higher prevalence of traditional cardiovascular risk factors, such as type 2 diabetes, hypertension, lipid abnormalities, and obesity (3), with obesity being an independent factor in psoriasis (3). The systemic inflammation caused by psoriasis is a key factor in increased cardiovascular risk (4), making cardiovascular disease a leading cause of mortality among psoriasis patients (5). Furthermore, psoriasis patients are at an increased risk of subclinical atherosclerosis and endothelial dysfunction. For those with more severe and/or prolonged disease, there should be a specific focus on screening for cardiovascular disease risk (6). The correlation between psoriasis and atherosclerotic plaques involves similar inflammatory pathways related to T helper cells, impaired angiogenesis, and endothelial dysfunction (7). The overall relationship between LE8 scores and the eight factors covered by LE8 and psoriasis requires further investigation.
This cross-sectional study used data from the National Health and Nutrition Examination Survey (NHANES) to examine the potential relationship between LE8 and psoriasis among the US adult population, offering new strategies for the prevention and management of psoriasis in clinical practice.
The study employed data from the National Health and Nutrition Examination Survey conducted between 2003–2006 and 2009–2014. Multivariable logistic regression and restricted cubic spline models were utilized to assess the relationships, revealing linear dose–response relationships. Subgroup analyses based on age, sex, poverty income ratio, education levels, and marital status demonstrated inverse associations between LE8 and psoriasis. All data are publicly accessible and can be obtained from the NHANES website.1
NHANES aims to assess the prevalence of primary illnesses and disease-specific risk factors in the United States, and a comprehensive overview of the survey can be found at http://www.cdc.gov/nchs/nhanes.htm. The survey employed sophisticated multiperiod probability-based sampling methods to obtain nationally representative samples. The NCHS Institutional Review Board approved the NHANES research protocol, and participants provided written informed consent at the time of enrollment.2 As this study is based on publicly available de-identified data, no ethical approval or consent is required.
This cross-sectional study utilized NHANES data from 2003 to 2006 and 2009 to 2014, adhering to the standards for Strengthening the Reporting of Observational Studies in Epidemiology. The investigation focused on adults aged 20 years and older with available LE8 score and psoriasis data. Exclusion criteria included missing demographic data (including educational level and marital status) (n = 13) and unavailable hypertension data (n = 11). Ultimately, 9,876 participants aged 20 years and older were included from a total of 26,043 participants in the 2003–2006 and 2009–2014 NHANES cycles. The flowchart for participant enrollment is depicted in Figure 1.
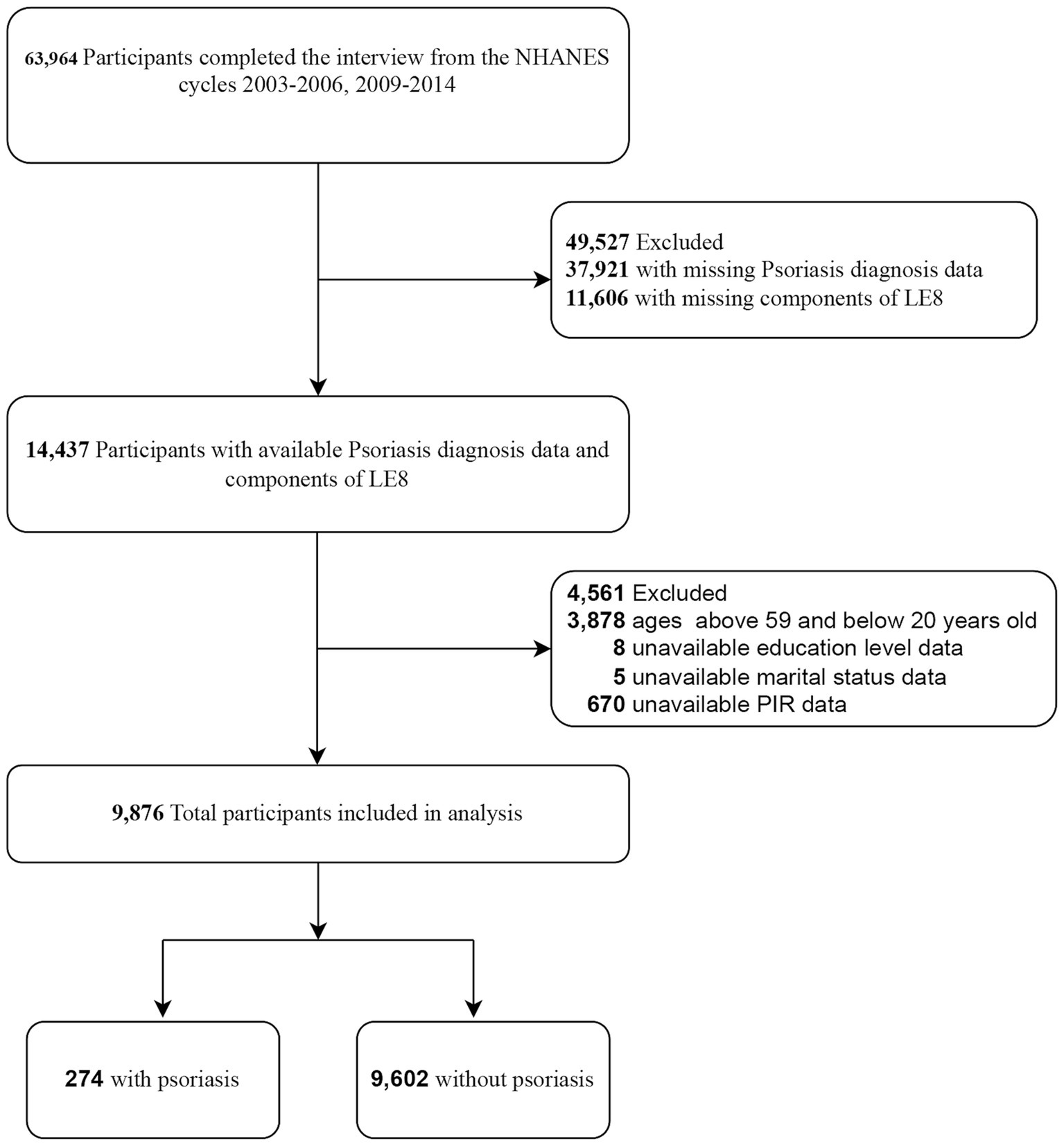
Figure 1. Selection of participants in the study. LE8, Life’s Essential 8; NHANES, national health and nutrition examination survey. PIR, family poverty income ratio.
The American Heart Association recently updated Life’s Simple 7 to LE8 in order to quantify cardiovascular health (CVH), encompassing 4 health behaviors and 4 health factors. This enhancement aims to provide more effective guidance for improving CVH in the general population. The detailed calculation of scores for each LE8 metric using NHANES data can be found in Supplementary Table S1. Each of the LE8 indicators was rated on a scale of 0 to 100, and the unweighted mean of these 8 indicators was used to calculate the total LE8 score. The American Heart Association suggested that participants with LE8 scores ≥80 be classified as having high CVH, those with scores of 50 to 79 as having moderate CVH, and those with scores <50 as having low CVH (2).
The Healthy Eating Index–2015 was employed to assess the diet metric. Supplementary Table S2 outlines the components and criteria for scoring the Healthy Eating Index–2015. The Healthy Eating Index–2015 score was calculated using information from the first 24-h diet recall interview conducted during the NHANES mobile examination center visit. If two 24-h recalls were available, the first one providing diet data was used. Information on physical activity, nicotine exposure, sleep health, diabetes, and medication history was obtained through self-report questionnaires. During the physical examination, participants’ blood pressure, height, and weight were professionally measured. Blood samples were collected for analysis of blood lipids, plasma glucose, and glycated hemoglobin at central laboratories.
Health behaviors encompass four factors: diet, physical activity, nicotine exposure, and sleep duration. The health behaviors score is the total score of these four factors divided by 4.
Health factors include four metrics: body mass index (BMI), non-high-density-lipoprotein cholesterol, blood glucose, and blood pressure. The health factors score is the total score of these four metrics divided by 4.
Psoriasis was defined as an affirmative response to the question, “Have you ever been told by a health care provider that you had psoriasis?”
Based on the literature, the following covariates were considered: age, sex, race and ethnicity, educational level, family income, marital status, diabetes, smoking status, alcohol drinking status, fasting total cholesterol, HDL cholesterol, diabetes history, hypertension history, physical activity, and sleep duration. In NHANES, self-reported race and ethnicity information were obtained from responses to survey questions on race and Hispanic ethnicity. As per NHANES categorization, participants were grouped into the following 4 races and ethnicities: non-Hispanic White, non-Hispanic Black, Mexican American, and Other (including non-Hispanic Black and multiracial). Educational level was classified into 3 levels (high school or less, some college, and college graduate or higher). For reporting NHANES dietary and health data, family income was categorized into 3 levels based on the family poverty income ratio: low income (≤1.3), medium income (>1.3 to 3.5), and high income (>3.5). Marital status was divided into 2 groups: married or living with partners and living alone. Diabetes history was determined by the survey question, “Has a doctor told you that you have diabetes?” Participants who answered “yes” were classified as having diabetes. Hypertension history was established by the survey question, “Have you ever been told that you have high blood pressure?” Participants who answered “yes” were classified as having hypertension. Smoking status was categorized into 3 groups: never smoker (or smoked <100 cigarettes), former smoker (smoked at least 100 cigarettes but has quit), and current smoker. Alcohol drinking status was determined by the survey question, “In any 1 year, have you had at least 12 drinks of any type of alcoholic beverage?” Participants who answered “yes” were classified as alcohol drinkers, while those who answered “no” were classified as non-alcohol drinkers.
We conducted a descriptive analysis for all participants. Categorical variables were presented as numbers and percentages, while continuous variables were expressed as mean and standard deviation (SD) for normally distributed data, or median and interquartile range for skewed distributions. We employed the chi-square test, T-test, and Kruskal-Wallis test to compare categorical variables, normally distributed continuous variables, and non-normally distributed continuous variables, respectively.
The correlation between covariates and psoriasis status was explored using univariate multinomial logistic regression. To assess the relationship between dietary LE8, health behaviors score, health factors score, and psoriasis, we utilized multivariate multinomial logistic regression models. We calculated odds ratios (ORs) and 95% confidence intervals (CIs). We established three models: the crude model did not adjust any confounders; Model 1 was adjusted for sociodemographic factors such as age (as a continuous variable), sex, and race/ethnicity; Model 2 was the fully adjusted model, which included adjustments for all factors in Model 1 plus poverty ratio (as a continuous variable), education levels, and marital status. To confirm the findings of LE8 score, health behaviors score, and health factors score as continuous variables and to test for nonlinearity, we categorized the above three scores according to the literature and calculated the p for trend (8).
To further explore nonlinearity, we conducted restricted cubic spline (RCS) regression with the continuous variables of LE8 score, health behaviors score, and health factors score to examine the dose–response relationship between these scores and psoriasis (6).
Furthermore, we investigated the associations between LE8 and psoriasis in different populations through subgroup analysis based on age strata, sex, PIR levels, education levels, and marital status. The significance of interactions was assessed using p values for the interaction coefficients between LE8 and subgroup populations.
All analyses were performed using the statistical software packages R 3.3.2 (http://www.R-project.org, The R Foundation) and Free Statistics software versions 1.9.2 (9). We conducted two-tailed tests, and p < 0.05 was considered statistically significant.
The study involved 9,876 participants, with an average (SD) age of 39.3 (11.3) years, of whom 5,084 (51.5%) were female. Among the total population, 44.6% were non-Hispanic White, 20.4% were non-Hispanic Black, and 16.0% were Mexican American; 59.1% had a college degree or higher. The percentage of married or cohabiting individuals was 61.0%, which is higher than those living alone. Table 1 presents the characteristics of the participants categorized by CVH groups. The prevalence of psoriasis was found to be 2.8% (95% CI, 5.26–6.56%). Participants in the high CVH group were typically younger, female, with higher income, college graduates or with higher education, and non-Hispanic white. They had a slim figure, drank alcohol, never smoked, had low levels of total cholesterol, high levels of HDL cholesterol, and no history of diabetes or hypertension. They spent significantly less time engaging in physical activity each day and considerably more time sleeping, as indicated in Table 1 (unweighted data used to describe the baseline characteristics of the patients).
Table 2 displays the association between each component of LE8, health behaviors, and health factors, and psoriasis in univariate logistic regression. The findings suggest that a high health factors score, particularly lower BMI (as indicated by the elevated body mass index score within the LE8 component), might have potential benefits in lowering the risk of psoriasis. The other scores do not demonstrate statistical significance.
Multivariable logistic regression models and smooth curve fitting were employed to investigate the connections between LE8 score, health behaviors score, health factors score, and psoriasis. We observed that the relationship between LE8 score, health behaviors score, health factors score, and risk of psoriasis was linear and negative (Figure 2). In the two-piecewise regression models, model 1 remained unadjusted for any covariates, while model 2 included adjustments for age (as a continuous variable), sex, and race/ethnicity. Model 3 underwent further adjustments for poverty ratio (as a continuous variable), education levels, and marital status. Table 3 delineated the associations between LE8 score, health behaviors score, health factors score, and psoriasis. In the unadjusted model, each 10-point increase in the LE8 score was linked to a reduced prevalence of psoriasis (OR: 0.88, 95% CI: 0.82–0.96). Regarding the categorical variable, compared to the low CVH level, the moderate CVH level (OR: 0.65, 95% CI: 0.46–0.92) and high CVH level (OR: 0.47, 95% CI: 0.31–0.71) exhibited a lower prevalence of psoriasis in the unadjusted model. This association remained significant and independent of latent confounders. The trend for health factors score mirrored that of the LE8 score. However, there was no significant association observed between health behaviors score and psoriasis.
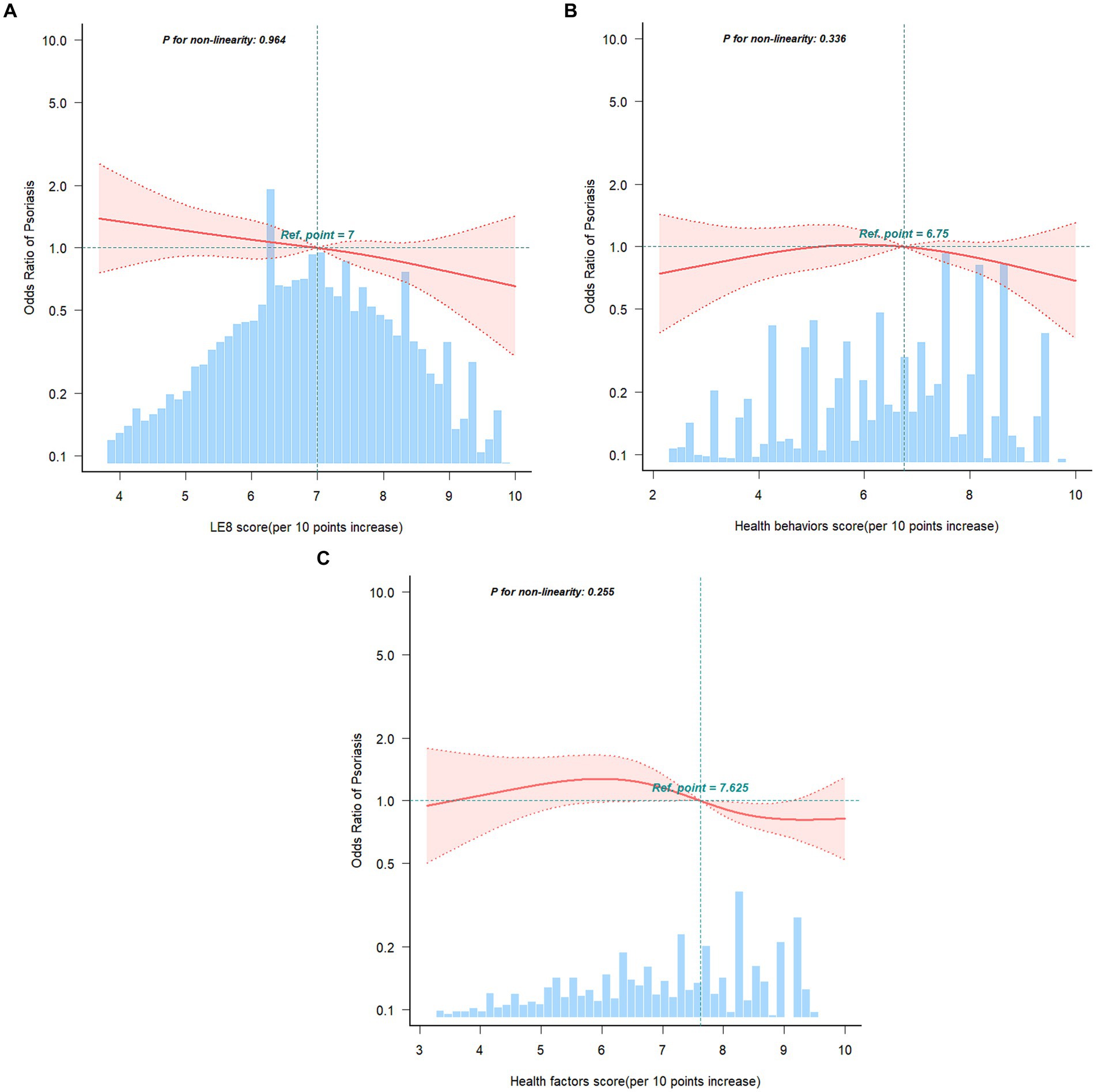
Figure 2. Restricted cubic spline fitting for the association between LE8 score (A), health behaviors score (B), health factors score (C) with psoriasis. Adjusted for age (as a continuous variable), sex, race/ethnicity, poverty ratio (as a continuous variable), education levels, and marital status. The red line and pink area in distribution histograms represent the estimated values and their corresponding 95% confidence intervals, respectively.
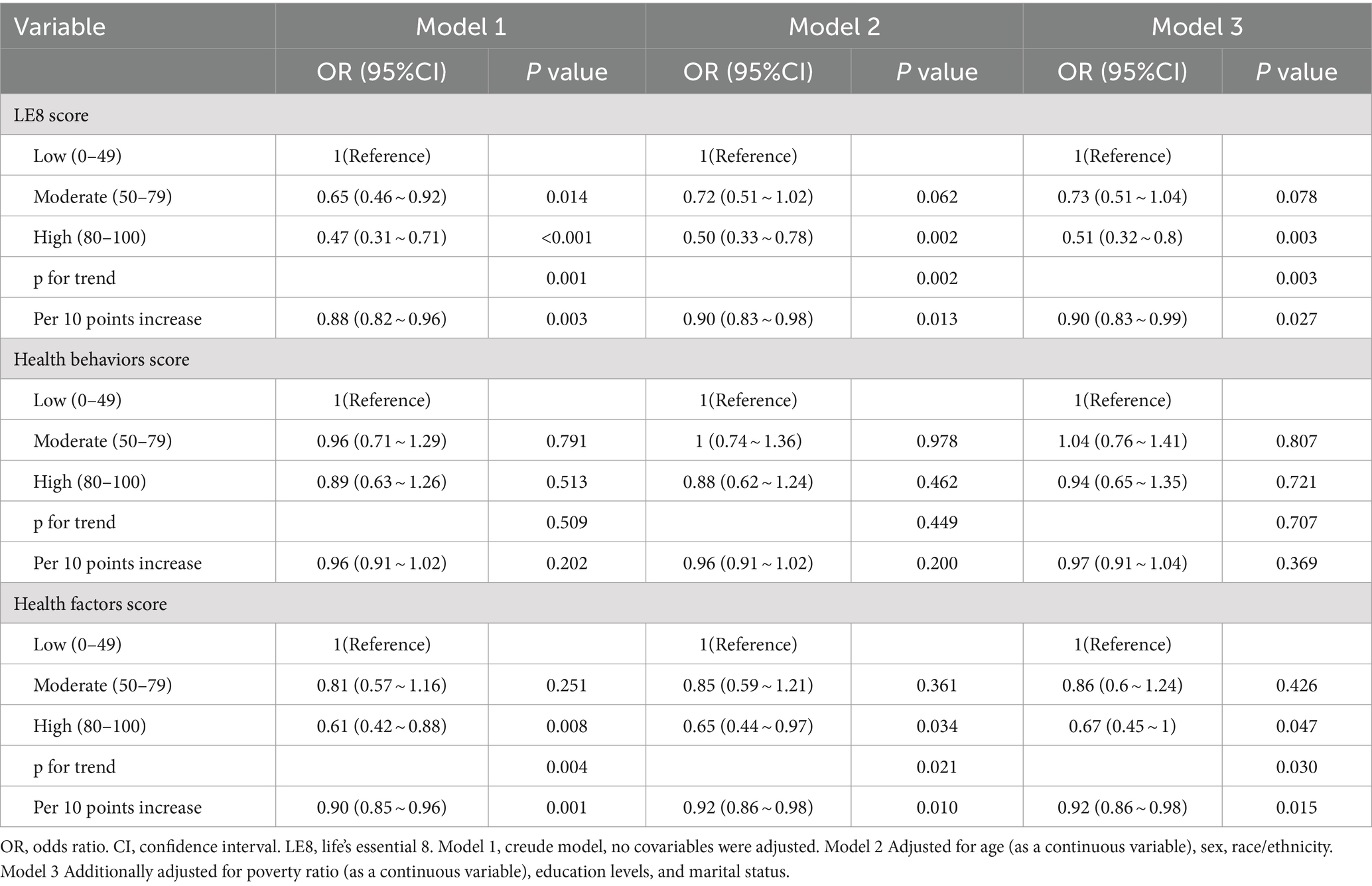
Table 3. Association of the Life’s Essential 8 scores, health behaviors score, health factors score with psoriasis.
The results of the subgroup analysis in Figure 3 suggest a strong inverse relationship between LE8 components and psoriasis across different population subgroups, and the findings were dependable and in line with the initial results.
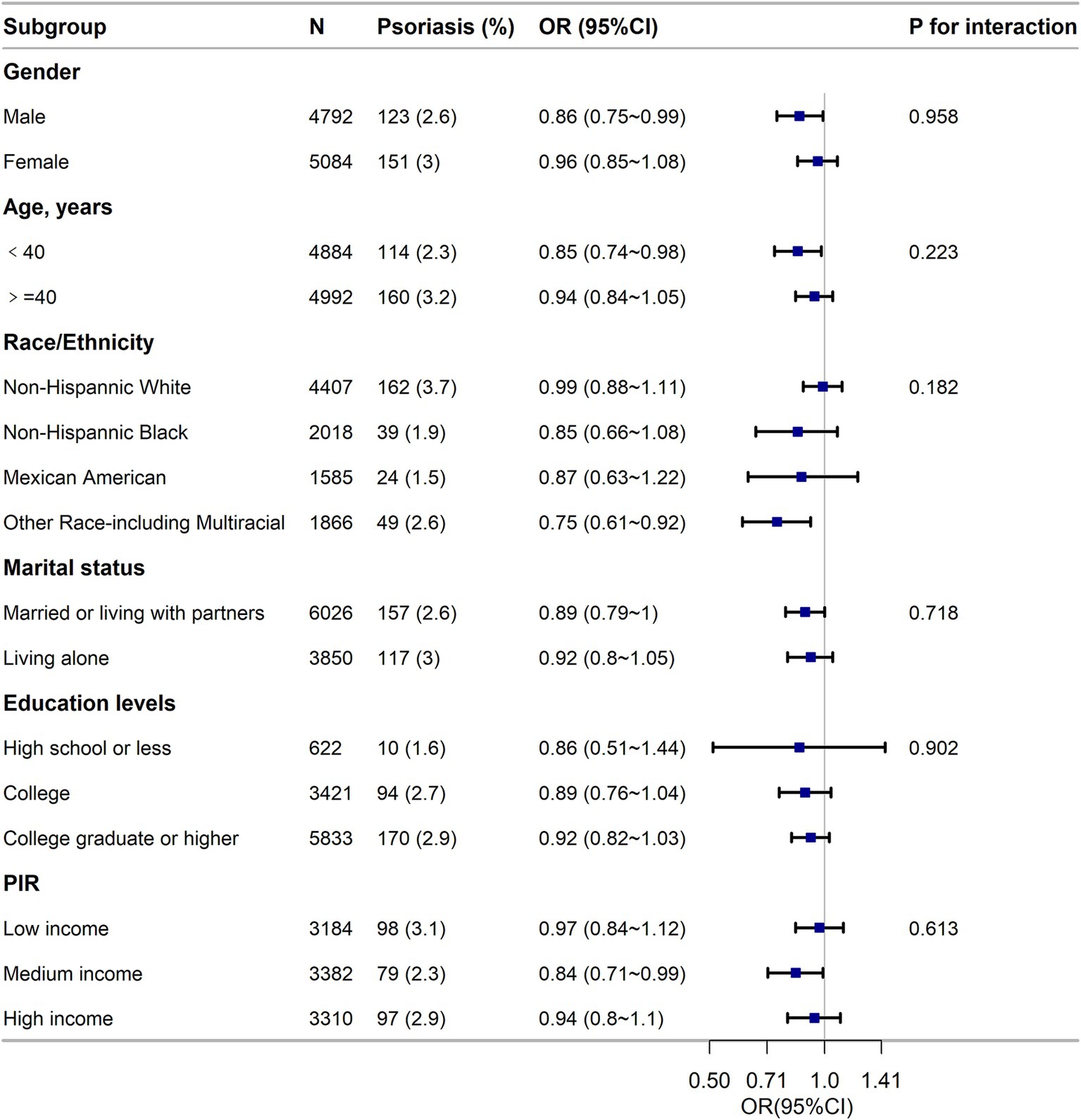
Figure 3. Subgroup analysis of the association of the Life’s Essential 8 scores and the risk of psoriasis. ORs were calculated as per score increase in LE8 total score. Each stratification was adjusted for gender, age (as a categorical variable), race/ethnicity, marital status, education level and family poverty income ratio (as a categorical variable). OR, odds ratio; CI, confidence interval; n, number; PIR, family poverty income ratio.
This study demonstrates a significant relationship between higher LE8 scores and lower prevalence of psoriasis, as well as a notable association between higher nicotine exposure scores, BMI, and lower psoriasis risk. Subgroup and sensitivity analyses indicate that the negative correlation between LE8 and psoriasis aligns with the overall findings.
Psoriasis, a chronic and recurrent immune-mediated inflammatory skin disease, varies in prevalence by country, can occur at any age (10), and is characterized by genetic susceptibility (11), adversely impacting the physical, social, psychological, and emotional well-being of patients (12). According to the methodology of the Global Burden of Disease Study 2019, there were an estimated 4,622,594 new cases of psoriasis worldwide in 2019. The age-standardized incidence rate in 2019 was 57.8 per 100,000 people (13). It represents a significant public health concern (14).
The typical skin manifestations of psoriasis include localized or widespread scaly erythematous plaques, with the removal of adherent scales resulting in pinpoint bleeding. Biologic therapies have become a pivotal point in the management of the disease, offering an effective and reasonably safe alternative for patients inadequately controlled by conventional therapies (15). Psoriasis is mediated by Th17 and Th23, leading to abnormal production of inflammatory mediators and excessive proliferation of keratinocytes. While Th-1 cells have long been a hallmark of psoriasis management (16), recent research has highlighted the prominence of Th17 cells, which are present in the dermis of psoriatic lesions and mediate skin inflammation upon recognition of self-lipid antigens presented by CD1a (17). The IL-23/Th-17 axis plays a crucial role in the exacerbation and treatment of psoriasis. In cardiovascular diseases (18), Th17 cells play a key role in thrombosis and atherosclerosis (19).
Previous studies have demonstrated that psoriasis patients exhibit higher cardiovascular prevalence factors, including metabolic syndrome, diabetes, hypertension, smoking, obesity, insulin resistance, and dyslipidemia (20–23). Research by Snekvik et al. (24) suggests a positive correlation between psoriasis and obesity indices and cardiovascular risk factors, particularly significant in moderate to severe psoriasis patients. Alexandre et al. (25) found that psoriasis patients exhibit lower levels of physical activity due to psychological and physiological reasons, potentially increasing their risk of cardiovascular diseases. Dyslipidemia, also a cardiovascular risk factor, is associated with psoriasis, although Ma’s et al. (26) research found no significant correlation between psoriasis and certain lipid level changes. Additionally, sleep deprivation is common among psoriasis patients, linked to psychological and physiological factors (27). Studies on smoking habits have revealed the following: one study found that psoriasis patients have a higher smoking rate compared to the control group, which may directly lead to or exacerbate chronic obstructive pulmonary disease, resulting in subclinical airway inflammation in patients (28). Another study (29, 30) found that psoriasis patients have underlying airway inflammation, so they tend to smoke more. Despite a significant global decrease in smoking rates from 1990 to 2019 (decreasing by 1.21% annually), the use of smokeless tobacco has not improved (actually increasing by 0.46% annually).
The meta-analysis also revealed a close association between psoriasis and cardiovascular disease. Liu et al.’s (31) study demonstrated the association of psoriasis with all adverse cardiovascular outcomes, particularly in severe patients, indicating psoriasis as an independent risk factor for adverse cardiovascular outcomes. Furthermore, Garshick et al. (32) indicated that patients with psoriasis have over a 50% increased risk of developing cardiovascular disease, with this cardiovascular risk increasing with the severity of skin involvement. Zhang et al.’s (33) research, combining Mendelian randomization, provided preliminary evidence suggesting a shared genetic origin between psoriasis and CVD, and targeted psoriasis treatments may improve cardiovascular outcomes. Additionally Zhang et al. (33), Galajda et al. (34) found that early use of TNF inhibitors may help reduce the cardiovascular disease risk in psoriasis patients.
While the underlying mechanisms between LE8 and psoriasis remain elusive, extensive research has revealed the critical roles of metabolic syndrome and lifestyle in the development of psoriasis, both of which are potential health factors and indicators of healthy behaviors in LE8 (35–37).
In conclusion, our study results indicate that maintaining optimal cardiovascular health can reduce the prevalence of developing psoriasis. These findings provide important guidance for the management and prevention of psoriasis.
However, some limitations should be considered in this study. Firstly, despite controlling for several potential confounding variables, the cross-sectional nature of the study precludes establishing a causal relationship between LE8 and psoriasis prevalence. Further research is needed to explore the longitudinal causal relationship between LE8 and psoriasis prevalence. Secondly, the assessment of some indicators in the LE8 assessment section was based on questionnaire surveys, which may lead to estimation errors. Thirdly, the impact of non-random missing data on the results cannot be ruled out due to baseline differences between included and excluded individuals. Finally, the generalizability of the correlations identified in this study to younger individuals or populations in other countries remains uncertain and requires further investigation.
The LE8 score may be negatively associated with the prevalence of psoriasis in the adult population in the United States. Additionally, higher scores in the assessment of nicotine exposure and BMI should be prioritized within the LE8 components. These findings suggest that LE8, as a practical and beneficial composite indicator for improving cardiovascular health (CVH), can be applied in clinical practice to aid in the early identification of psoriasis prevalence among patients and the general population, thereby minimizing the burden of psoriasis. Dermatologists should provide appropriate non-pharmacological advice to patients, including smoking cessation and weight management.
Publicly available datasets were analyzed in this study. This data can be found at: https://www.cdc.gov/nchs/nhanes/index.htm.
JZ: Conceptualization, Data curation, Methodology, Validation, Writing – original draft. CR: Data curation, Writing – original draft, Writing – review & editing. ZQ: Methodology, Software, Writing – review & editing. LZ: Data curation, Writing – review & editing. ZJ: Writing – review & editing. YY: Writing – review & editing. XP: Writing – original draft, Writing – review & editing. LL: Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We would like to extend our heartfelt appreciation to all the participants and the dedicated NHANES team for their hard work and unwavering commitment. We also want to sincerely thank Jie Liu from the Department of Vascular and Endovascular Surgery at the General Hospital of the Chinese People’s Liberation Army, as well as Haoxian Tang from Shantou University, for their contributions to statistical support and consultation on study design.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1445288/full#supplementary-material
1. Fernández-Armenteros, JM, Gómez-Arbonés, X, Buti-Soler, M, Betriu-Bars, A, Sanmartin-Novell, V, Ortega-Bravo, M, et al. Psoriasis, metabolic syndrome and cardiovascular risk factors. A population-based study. J Eur Acad Dermatol Venereol. (2019) 33:128–35. doi: 10.1111/jdv.15159
2. Allen, NB, Anderson, CAM, Black, T, Brewer, LC, Foraker, RE, Grandner, MA, et al. Life's essential 8: updating and enhancing the American Heart Association's construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. (2022) 146:e18–43. doi: 10.1161/CIR.0000000000001078
3. Gelfand, JM, Langan, SM, Mehta, NN, Ogdie, A, Takeshita, J, Van Voorhees, AS, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. (2017) 76:377–90. doi: 10.1016/j.jaad.2016.07.064
4. Berger, JS, Di Carli, M, Garshick, MS, Husni, ME, Merola, JF, Weber, B, et al. Psoriasis and cardiovascular disease: novel mechanisms and evolving therapeutics. Curr Atheroscler Rep. (2021) 23:67. doi: 10.1007/s11883-021-00963-y
5. Gniadecki, R, Kavanaugh, A, Lebwohl, MG, Merola, JF, and Wu, JJ. Psoriasis and metabolic syndrome: implications for the management and treatment of psoriasis. J Eur Acad Dermatol Venereol. (2022) 36:797–806. doi: 10.1111/jdv.18044
6. McEvoy, JW, Ali, SS, Blaha, MJ, Blumenthal, RS, Karim, A, Nasir, K, et al. Subclinical cardiovascular disease in plaque psoriasis: association or causal link? Atherosclerosis. (2014) 232:72–8. doi: 10.1016/j.atherosclerosis.2013.10.023
7. Kręcisz, B, Michalska, A, Sadowski, M, Siudak, Z, Stępień, R, Teichman, R, et al. Cardiovascular risk in patients with plaque psoriasis and psoriatic arthritis without a clinically overt cardiovascular disease: the role of endothelial progenitor cells. Postepy Dermatol Alergol. (2020) 37:299–305. doi: 10.5114/ada.2020.96085. Epub 2020 Jul 14
8. Shen, R, and Zou, T. The association between cardiovascular health and depression: results from the 2007-2020 NHANES. Psychiatry Res. (2024) 331:115663. doi: 10.1016/j.psychres.2023.115663
9. Yang, Q, Zheng, J, Chen, W, Chen, X, Wen, D, Chen, W, et al. Association between preadmission metformin use and outcomes in intensive care unit patients with sepsis and type 2 diabetes: a cohort study. Front Med. (2021) 8:640785. doi: 10.3389/fmed.2021.640785
10. Griffiths, CEM, Armstrong, AW, Gudjonsson, JE, and Barker, JNWN. Psoriasis. Lancet. (2021) 397:1301–15. doi: 10.1016/S0140-6736(20)32549-6
11. Tsoi, LC, Stuart, PE, Tian, C, Gudjonsson, JE, das, S, Zawistowski, M, et al. Large scale meta-analysis characterizes genetic architecture for common psoriasis associated variants. Nat Commun. (2017) 8:15382. doi: 10.1038/ncomms15382
12. Svoboda, SA, Ghamrawi, RI, Owusu, DA, and Feldman, SR. Treatment goals in psoriasis: which outcomes matter Most? Am J Clin Dermatol. (2020) 21:505–11. doi: 10.1007/s40257-020-00521-3
13. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
14. Masson, W, Lobo, M, and Molinero, G. Psoriasis and cardiovascular risk: a comprehensive review. Adv Ther. (2020) 37:2017–33. doi: 10.1007/s12325-020-01346-6
15. Furtunescu, AR, Georgescu, SR, Tampa, M, and Matei, C. Inhibition of the JAK-STAT pathway in the treatment of psoriasis: a review of the literature. Int J Mol Sci. (2024) 25:4681. doi: 10.3390/ijms25094681
16. Lew, W, Bowcock, AM, and Krueger, JG. Psoriasis vulgaris: cutaneous lymphoid tissue supports T-cell activation and "type 1" inflammatory gene expression. Trends Immunol. (2004) 25:295–305. doi: 10.1016/j.it.2004.03.006
17. Mills, KHG. IL-17 and IL-17-producing cells in protection versus pathology. Nat Rev Immunol. (2023) 23:38–54. doi: 10.1038/s41577-022-00746-9
18. Li, B, Huang, L, Lv, P, Li, X, Liu, G, Chen, Y, et al. The role of Th17 cells in psoriasis. Immunol Res. (2020) 68:296–309. doi: 10.1007/s12026-020-09149-1
19. Calabresi, P, and McDonald, CJ. Thromboembolic disorders associated with psoriasis. JAMA Dermatol. (1973) 107:918. doi: 10.1001/archderm.1973.01620210078025
20. Wild, J, Schüler, R, Knopp, T, Molitor, M, Kossmann, S, Münzel, T, et al. Telmisartan lowers elevated blood pressure in psoriatic mice without attenuating vascular dysfunction and inflammation. Int J Mol Sci. (2019) 20:4261. doi: 10.3390/ijms20174261
21. Chen, W, Hu, MY, Yang, Q, and Zheng, J. The association of psoriasis and hypertension: focusing on anti-inflammatory therapies and immunological mechanisms. Clin Exp Dermatol. (2020) 45:836–40. doi: 10.1111/ced.14327
22. Bohjanen, KA, Ingraham, SJ, Leon, AS, and Wilson, PB. Psoriasis and physical activity: a review. J Eur Acad Dermatol Venereol. (2012) 26:1345–53. doi: 10.1111/j.1468-3083.2012.04494.x
23. Millsop, JW, Bhatia, BK, Debbaneh, M, Koo, J, and Liao, W. Diet and psoriasis, part III: role of nutritional supplements. J Am Acad Dermatol. (2014) 71:561–9. doi: 10.1016/j.jaad.2014.03.016
24. Snekvik, I, Nilsen, TIL, Romundstad, PR, and Saunes, M. Psoriasis and cardiovascular disease risk factors: the HUNT Study, Norway. JEADV Clin Pract. (2018) 32:776–82. doi: 10.1111/jdv.14835
25. Alexandre, JM, Mendonça, D, Selores, M, Silva, BM, and Torres, T. Levels of physical activity in patients with severe psoriasis: a cross-sectional questionnaire study. Am J Clin Dermatol. (2014) 15:129–35. doi: 10.1007/s40257-014-0061-0
26. Ma, C, Schupp, CW, Armstrong, EJ, and Armstrong, AW. Psoriasis and dyslipidemia: a population-based study analyzing the National Health and nutrition examination survey (NHANES). J Eur Acad Dermatol Venereol. (2014) 28:1109–12. doi: 10.1111/jdv.12232
27. Bundy, C, Chisholm, A, Griffiths, CEM, Henry, AL, and Kyle, SD. A cross-sectional survey of the nature and correlates of sleep disturbance in people with psoriasis. Br J Dermatol. (2017) 177:1052–9. doi: 10.1111/bjd.15469
28. Buja, A, Miatton, A, Cozzolino, C, Brazzale, AR, Lo Bue, R, Mercuri, SR, et al. The prevalent Comorbidome at the onset of psoriasis diagnosis. Dermatol Ther. (2023) 13:2093–105. doi: 10.1007/s13555-023-00986-0
29. Damiani, G, Radaeli, A, Olivini, A, Calvara-Pinton, P, and Malerba, M. Increased airway inflammation in patients with psoriasis. Br J Dermatol. (2016) 175:797–9. doi: 10.1111/bjd.14546
30. GBD 2019 Chewing Tobacco Collaborators. Spatial, temporal, and demographic patterns in prevalence of chewing tobacco use in 204 countries and territories, 1990-2019: a systematic analysis from the global burden of disease study 2019. Lancet Public Health. (2021) 6:e482–99. doi: 10.1016/S2468-2667(21)00065-7
31. Liu, L, Cui, S, Liu, M, Huo, X, Zhang, G, Wang, N, et al. Psoriasis increased the risk of adverse cardiovascular outcomes: a new systematic review and Meta-analysis of cohort study. Front Cardiovasc Med. (2022) 9:829709. doi: 10.3389/fcvm.2022.829709
32. Garshick, MS, Ward, NL, Krueger, JG, and Berger, JS. Cardiovascular risk in patients with psoriasis: JACC review topic of the week. J Am Coll Cardiol. (2021) 77:1670–80. doi: 10.1016/j.jacc.2021.02.009
33. Zhang, L, Wang, Y, Qiu, L, Wu, J, et al. Psoriasis and cardiovascular disease risk in European and east Asian populations: evidence from meta-analysis and Mendelian randomization analysis. BMC Med. (2022) 20:421. doi: 10.1186/s12916-022-02617-5
34. Galajda, NÁ, Meznerics, FA, Mátrai, P, Fehérvári, P, Lengyel, AS, Kolonics, MV, et al. Reducing cardiovascular risk in immune-mediated inflammatory diseases: tumour necrosis factor inhibitors compared to conventional therapies-a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. (2024) 38:1070–88. doi: 10.1111/jdv.19900
35. Kamiya, K, Kishimoto, M, Komine, M, Ohtsuki, M, and Sugai, J. Risk factors for the development of psoriasis. Int J Mol Sci. (2019) 20:4347. doi: 10.3390/ijms20184347
36. Friis, NU, Gyldenløve, M, Hoffmann, N, Knop, FK, Skov, L, Storgaard, H, et al. Glucose metabolism in patients with psoriasis. Br J Dermatol. (2019) 180:264–71. doi: 10.1111/bjd.17349
Keywords: psoriasis, Life’s Essential 8, cardiovascular health, NHANES, risk factors
Citation: Zhang J, Ren C, Qin Z, Zhu L, Jin Z, Yan Y, Pan X and Luan L (2024) Association between Life’s Essential 8 and psoriasis in US adults: a cross-sectional study. Front. Med. 11:1445288. doi: 10.3389/fmed.2024.1445288
Received: 27 June 2024; Accepted: 25 September 2024;
Published: 10 October 2024.
Edited by:
Giovanni Damiani, University of Milan, ItalyReviewed by:
Thomas Graier, Medical University of Graz, AustriaCopyright © 2024 Zhang, Ren, Qin, Zhu, Jin, Yan, Pan and Luan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinghe Pan, MTUzODQ3MDg4ODhAMTYzLmNvbQ==; Lan Luan, bHVhbmxhbjMzQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.