- 1Chengdu University of Technology Logistics Service Group, Chengdu, China
- 2Department of Internal Medicine, the Hospital of Chengdu University of Technology, Chengdu, China
Background: The characteristics of blood lipid and metabolic indicators were analyzed in patients with subclinical hypothyroidism (SCH), and the effect of type 2 diabetes mellitus (T2DM) on SCH patients was determined.
Methods: The physical examination data of 2,119 residents in a university community were retrospectively divided into 2 groups (SCH and non-SCH groups). Furthermore, the SCH group was divided into SCH-T2DM and SCH–non-T2DM subgroups, and the data between the groups were analyzed.
Results: The SCH group had significantly higher levels of triglycerides (p = 0.044), total cholesterol (p = 0.001), low-density lipoprotein cholesterol (p = 0.019), very low-density lipoprotein cholesterol (p = 0.044), 2-h plasma glucose (p = 0.023), and globulin (p = 0.000) compared to the non-SCH group. The SCH–T2DM group was older (p < 0.001), had a greater BMI (p = 0.028), a more rapid heart rate (p = 0.025), and a greater waist circumference (p < 0.001) than the SCH–non-T2DM group based on the subgroup analysis. The SCH–T2DM group had significantly higher dyslipidemia and dysglycemia levels than the SCH–non-T2DM group.
Conclusion: Patients with SCH with or without T2DM may have dyslipidemia and dysglycemia and should be evaluated accordingly.
1 Introduction
Subclinical hypothyroidism (SCH) is a medical condition in which thyroid-stimulating hormone (TSH) levels are slightly elevated, while thyroid hormone levels (FT3 and FT4) remain within the normal range (1). SCH correlates with long-term metabolic disorders and is related to congestive heart failure, coronary artery disease events, and fatal stroke (1, 2). SCH is a significant public health concern. The prevalence of SCH is 3–15%, depending on the population (1). The Universal Salt Iodization (USI) program was implemented in China in 1995 and has successfully lowered the prevalence of SCH in 10 cities from 16.7% in 1999 to 3.22% in 2016 (1, 3). The prevalence of SCH in Jiangxi Province, China, was reported to be 7.89% in 2023 (4). SCH is more common in women, the elderly, and individuals with a family history of diabetes and thyroid diseases (1, 5–8). A survey among women in northeast China showed that SCH patients had significantly higher triglyceride (TG) levels (1.69 ± 1.9 vs. 1.45 ± 1.4) than the healthy population (9). Another study by Sindhu and Vijay (10) suggested that SCH patients had more significant dyslipidemia than the euthyroid population, including total cholesterol (TC), very low-density lipoprotein cholesterol (VLDL-C), and low-density lipoprotein cholesterol (LDL-C). Given the potential health consequences of SCH and the correlation with metabolic disorders and other diseases (1–10), there is a need to determine the changes in blood lipid levels and other metabolic indicators in patients with SCH.
Moreover, a positive correlation has been shown between poor glycemic control and a high TSH level in type 2 diabetes mellitus (T2DM) patients and mouse models of T2DM (11). SCH increases insulin resistance in people with normal glucose levels (12). Han et al. (13) reported that T2DM patients are more likely to have SCH than the non-T2DM population, and SCH may be associated with increased diabetic complications and T2DM progression than the non-SCH population. Alharbi et al. (14) showed no association between SCH and T2DM development. Based on the collective findings from these studies, SCH patients with T2DM should receive special attention.
The association between SCH and T2DM development has received wide attention. We have focused our attention on the association between SCH and T2DM in a population group in southwest China. The current study was conducted in southwest China, the purpose of which was to determine blood lipid characteristics and metabolic indicators in patients with SCH in a university community. Moreover, the current study compared SCH patients with or without T2DM to determine the effect of T2DM on SCH patients. This study focused on diverse populations and may be helpful in further understanding SCH patients.
2 Patient and methods
2.1 Participants
This study was approved by the Ethics Committee of the Hospital of Chengdu University of Technology (No. 2020001). All university community residents who underwent physical examinations at the hospital from March to December 2019 were screened for eligibility.
2.2 Diagnostic criteria
Thyroid dysfunction was diagnosed using the standards established by the 2007 Chinese Guidelines for Diagnosis and Treatment of Thyroid Diseases - Hyperthyroidism (15) and the 2017 Guidelines for Diagnosis and Treatment of Adult Hypothyroidism (16). The usual reference value range for thyroid-related hormones in our institution is as follows: TSH, 0.27–4.0 mIU/ml; FT4, 12–22 pmol/L; and FT3, 3.1–6.8 pmoL/L. The TSH level was elevated, and the FT4 and FT3 levels were normal, which were consistent with SCH.
2.3 Data collection
A standard questionnaire was used to collect personal history, past medical history, family history, and other general information from all participants. General clinical indices, including systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), body mass index (BMI), waist circumference (W), and hip circumference (H), were measured in the morning. Fasting venous blood was collected, and electrochemiluminescence was used to determine the TSH, FT4, and FT3 levels. Automatic biochemical instruments were used to detect TG, TC, LDL-C, VLDL-C, high-density lipoprotein cholesterol (HDL-C), fasting blood glucose (FPG), urea nitrogen (BUN), creatinine (Scr), uric acid (UA), albumin (A), globulin (G), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), γ-glutamyltransferase (GGT), total bilirubin (STB), conjugated bilirubin (CB), and unconjugated bilirubin (UCB). The postprandial blood glucose (2hPG) level was measured 2 h after a standard steamed bread meal.
2.4 Grouping
Based on the findings of thyroid function tests, participants were split into two groups (SCH and non-SCH groups). The non-SCH group had normal thyroid function, and clinical hyperthyroidism, SCH, and hypothyroidism were excluded. The patients with familial dyslipidemia, arterial hypertension, morbid obesity, and nephrotic syndrome, as well as patients taking lipid-lowering treatments (fibrates and HMG-coA inhibitors), were excluded. The clinical baseline data, blood lipid levels, and other metabolic markers in the two groups were compared and examined.
To compare SCH patients with or without T2DM and to determine the effect of T2DM on SCH patients, the SCH patients were divided into SCH–T2DM and SCH–non-T2DM subgroups. The clinical baseline data, blood lipid levels, and other metabolic markers were compared and examined in the two subgroups.
2.5 Biochemical parameter assay methods
Biochemical indicators were detected using spectrophotometry (Roche Cobas c 501 automatic biochemical analyzer). TSH, FT4, and FT3 levels were determined using electrochemiluminescence (electrochemical luminescence) assay (Roche Cobas e 411 electrochemiluminescence automatic immunoassay).
2.6 Statistical processing
Data processing and statistical analysis were performed using SPSS 25.0. The measurement data were compared across groups using a t-test. A statistically significant difference was indicated by a significance level of p < 0.05.
3 Result
3.1 Patients included
Of 3,226 university community residents, 2,119 met the inclusion criteria for this study, including 1,040 males and 1,079 females 23–95 years of age. A total of 1,107 individuals were disqualified and not included in the final analysis. Specifically, 735 patients were excluded because of incomplete physical examination items, and 42 patients had clinical hyperthyroidism, subclinical hyperthyroidism, clinical hypothyroidism, a history of thyroid surgery, a pituitary tumor, or severe parathyroid disease. Additionally, 206 individuals had a history of diabetes, 5 had a recent history of acute and severe infection, 11 had a history of long-term use of glucocorticoids and other drugs that could affect thyroid function, and 12 had a history of malignant tumors. Moreover, 94 individuals had severe heart, liver, and kidney disease, while 3 lacked the ability to comprehend and cooperate with physicians for necessary examinations and treatments.
3.2 Comparison of general clinical indices between the SCH and non-SCH groups
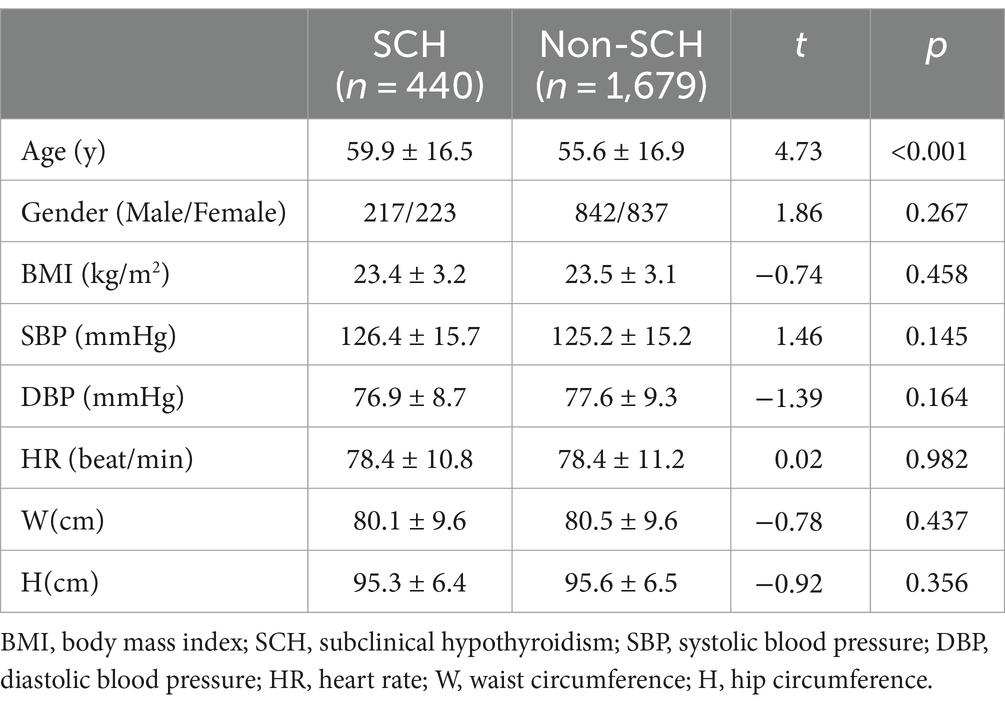
The SCH group consisted of 440 patients (20.56%) and the non-SCH group consisted of 1,679 individuals. The SCH group was significantly older than the non-SCH group (t = 4.73; p < 0.001). The BMI, gender, heart rate, blood pressure, W, and H were not significantly different between the SCH and non-SCH groups (p > 0.05). Table 1 provides a detailed presentation of the study results.
3.3 Comparison of blood lipid and other biochemical indices between the SCH and non-SCH groups
The TG levels were higher in the SCH group than the non-SCH group (t = 2.02; p = 0.044), as were the TC (t = 3.22; p = 0.001), LDL-C (t = 2.35; p = 0.019), and VLDL-C levels (t = 2.01; p = 0.044). The SCH group had a lower HDL-C level than the non-SCH group but the difference was not statistically significant (t = 0.68; p = 0.496). Furthermore, the 2hPG level in the SCH group was significantly higher than in the non-SCH group (t = 2.28; p = 0.023). The TG level was also significantly higher in the SCH group than in the non-SCH group (t = 4.12; p < 0.001). No substantial differences were detected in other biochemical indexes between the two groups (p > 0.05). Table 2 presents a detailed summary of the results.
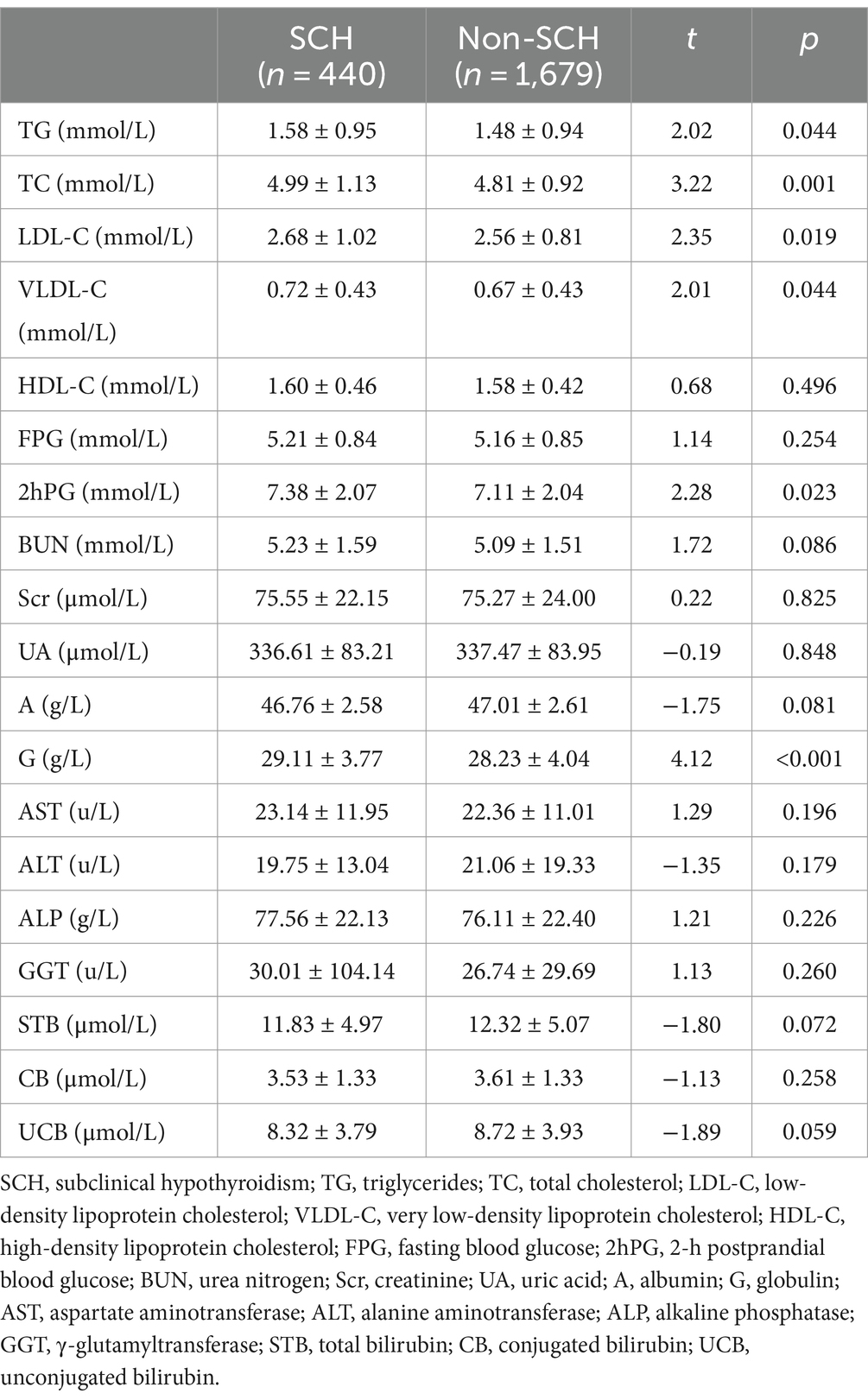
Table 2. Comparison of blood lipid and other biochemical indices between the SCH and non-SCH groups.
3.4 Comparison of general clinical indices between the SCH–T2DM and SCH–non-T2DM subgroups
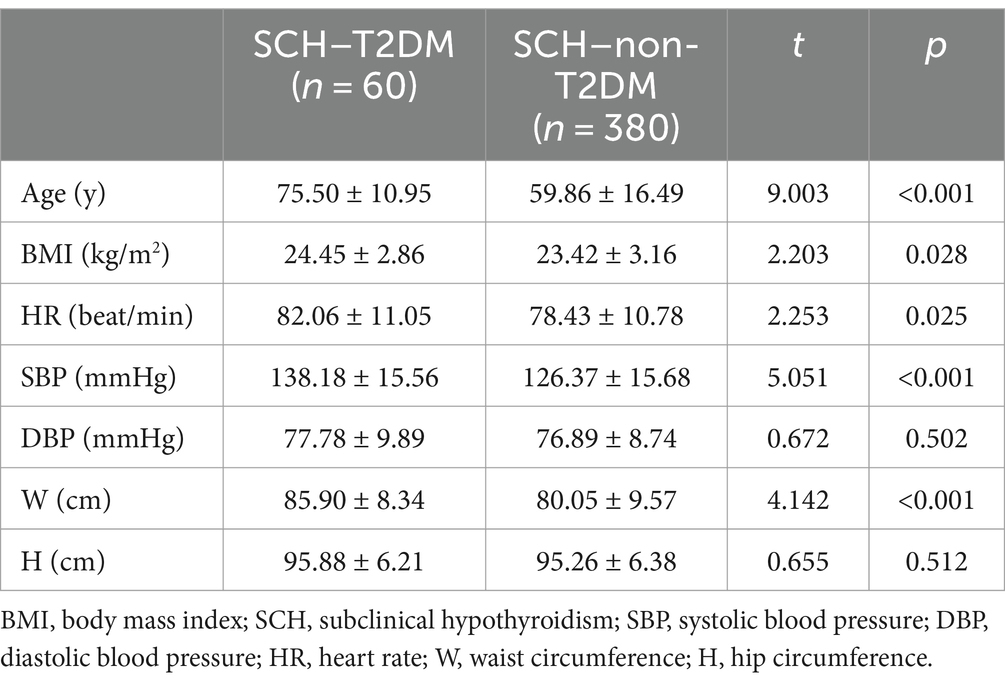
For further analysis, the SCH population was categorized into SCH–T2DM and SCH–non-T2DM groups. The SCH–T2DM group was older (t = 9.003; p < 0.001), the BMI was higher (t = 2.203; p = 0.028), the heart rate was more rapid (t = 2.253; p = 0.025), and the W was greater (t = 4.142; p < 0.001) than the SCH–non-T2DM group. The other indices we measured were not significantly different between the two groups (p > 0.05). Table 3 provides a detailed presentation of the study results.
3.5 Comparison of blood lipid and other biochemical indices between the SCH–T2DM and SCH–non-T2DM groups
Table 4 shows the comparisons of blood lipid and other biochemical indices between the SCH–T2DM and SCH–non-T2DM groups. The TG (t = 2.159; p = 0.036), TC (t = −2.147; p < 0.001), LDL-C (t = −2.541; p = 0.011), and VLDL-C levels (t = 2.158; p = 0.036) were significantly higher in the SCH–T2DM group than the SCH–non-T2DM group. The FPG (t = 8.886; p < 0.001), 2hPG (t = 6.725; p < 0.001), BUN (t = 3.236; p = 0.001), Scr (t = 2.170; p = 0.031), UA (t = 2.775; p = 0.006), A (t = −2.711; p = 0.007), and G levels (t = 3.696; p < 0.001) were also significantly different between the two groups.
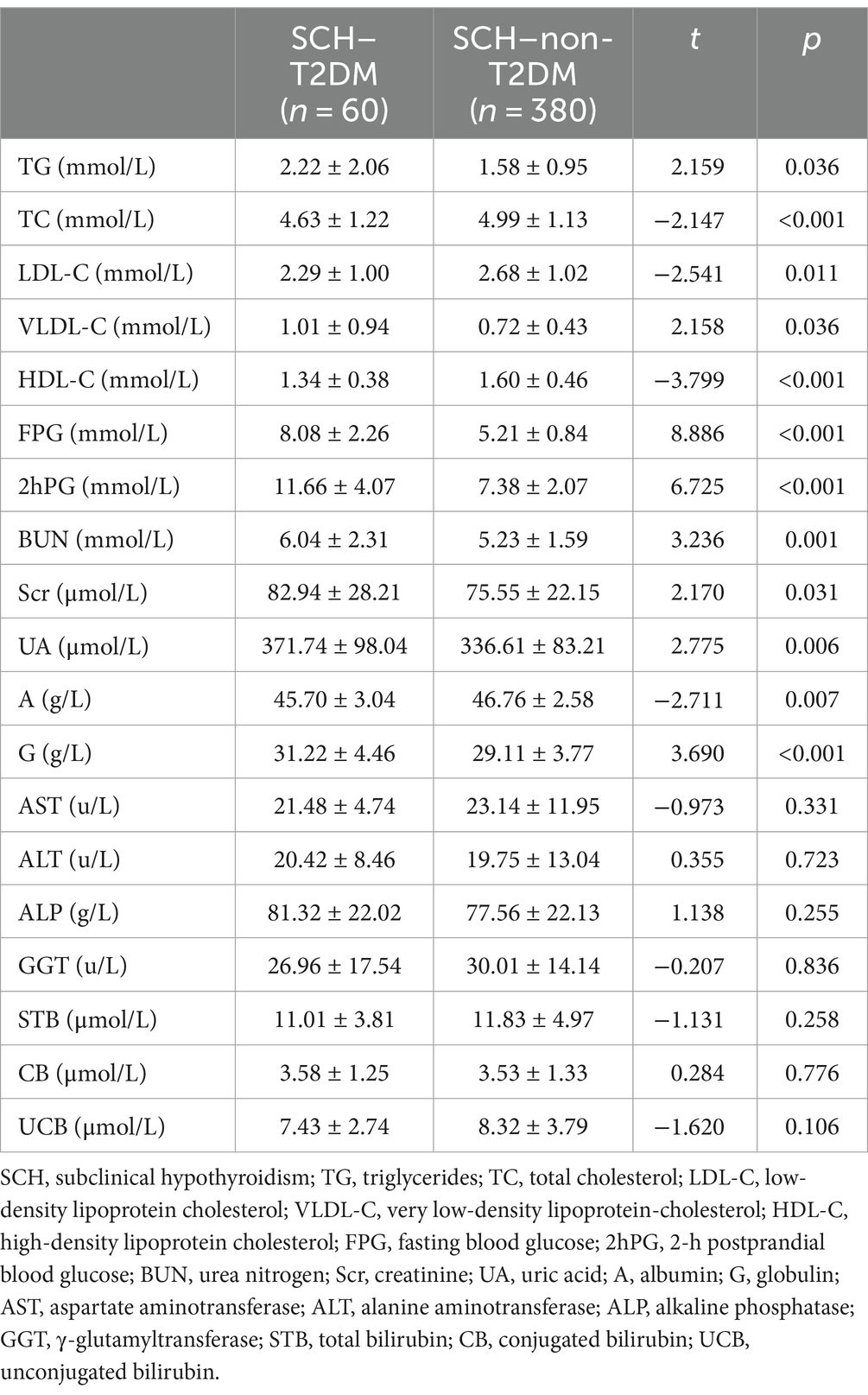
Table 4. Comparison of blood lipid and other biochemical indices between the SCH–T2DM and SCH–non-T2DM groups.
4 Discussion
SCH is the most prevalent type of thyroid dysfunction, as confirmed by numerous epidemiologic studies. SCH may be affected by geographic location, ethnicity, dietary patterns (e.g., iodine intake), age, and gender (1–10, 17). A community-based investigation in China showed that the population with SCH was older (17), which is consistent with the results of our study. This finding may be attributed to thyroid gland atrophy and fibrosis, reduced synthesis and release of thyroid hormones, and decreased levels of follicular epithelial cells, glia, and secretory granules in the follicles, especially among elderly individuals. Additionally, autoimmunity changes and a relative iodine deficiency in the elderly may also contribute to the decline in thyroid function (18). SCH usually affects the female population more than the male population (1–8). The number of males and females in the SCH group did not differ significantly, which may be due to the retrospective nature of the study based on a single-center community.
Numerous studies have shown that SCH can lead to dyslipidemia, which is characterized by decreased levels of HDL-C and elevated levels of TG, TC, and LDL-C (19, 20). While the levels of FT3 and FT4 are negatively correlated with TG and HDL-C and positively correlated with TC and LDL-C, respectively, TSH is favorably correlated with TG and TC and negatively correlated with HDL-C (19, 20).
Inconsistent findings have been reported with respect to the impact of SCH on the blood lipid profile. Luo et al. (21) reported no conclusive correlation between SCH and TC, LDL-C, or HDL-C among >11,000 patients undergoing physical examinations. In a study involving 11,498 participants, Nakajima et al. (22) concluded that SCH was correlated with higher W, TG, and LDL-C levels in females. An increase in TC levels in females is likewise linked to the progression from euthyroidism to SCH (22). Khan et al. (23) showed that LDL-C, non-HDL-C, UA, and the albumin-to-creatinine ratio increased from euthyroidism to overt hypothyroidism, with more subtle changes observed in SCH. A meta-analysis revealed that individuals with SCH had notably higher levels of TC, TG, and LDL-C, but no significant difference was detected in the HDL-C level compared to individuals with normal thyroid function (24). Similarly, the findings in the current study showed that individuals with SCH had TG, TC, LDL-C, and VLDL-C levels that were considerably higher than among individuals in the non-SCH group. The difference was not statistically significant even though the HDL-C level was lower than the non-SCH group.
SCH is closely associated with impaired glucose metabolism. The prevalence of SCH in the T2DM group was 16.39% compared to 11.27% in the normoglycemic group (12). The SCH–T2DM patients had poor blood glucose control (25, 26), and there was a bidirectional relationship between the SCH and T2DM. The FBG, 2hPG, and HbA1c levels were significantly lower in hypothyroid patients than in healthy individuals in China (27). However, another study (28) showed that patients with SCH and T2DM had higher blood glucose levels than patients without hypothyroidism with relatively lower fasting blood glucose levels and a more significant difference in the 2-h postprandial blood glucose level. There were no appreciable differences between the population with SCH and the non-SCH group in the current study with respect to other metabolic markers. These metabolic indicators included fasting blood glucose, liver function, renal function, blood UA, SBP, DBP, heart rate, BMI, W, and H. The SCH patients combined with T2DM had significantly higher levels of dyslipidemia and dysglycemia than patients without T2DM, which is consistent with previous studies (27, 28). This finding indicates that SCH–T2DM patients may be at risk for severe complications and more obvious clinical symptoms. Therefore, healthcare providers should be vigilant and consider SCH as a potential diagnosis in T2DM patients.
Autoimmunity is considered the main etiology of SCH. Numerous studies have reported a high positivity rate of thyroid autoantibodies in patients with SCH (29, 30). Shi et al. (29) reported that 33.1% of SCH patients have autoimmune thyroid disease, with higher rates of thyroid peroxidase antibody and thyroglobulin antibody than the general population. Although no specific thyroid-related antibodies were detected in the current study, the SCH group exhibited a higher G level than the non-SCH group, which could be attributed to the increased presence of autoantibodies.
Yang et al. (12) reported significant differences in the BMI, HOMA-IR, and waist-to-hip ratio in the SCH–T2DM group compared to the SCH–non-T2DM group. This study also showed older age, a higher BMI, and a thicker W in the SCH–T2DM group than in the SCH–non-T2DM group. These results were similar to the study by Yang et al. (12).
In the future, it will be important to continue researching the mechanisms underlying the metabolic disorders associated with SCH to develop effective interventions and treatments. In addition, more research is needed to investigate the link between SCH and cardiovascular risk, as well as other potential health implications. The need to educate healthcare professionals and the general public about the dangers of SCH and the need for routine screening and monitoring is critical given the high incidence. Furthermore, because SCH is often asymptomatic, future research should focus on identifying biomarkers or other indicators that can help to identify individuals who are at risk of developing this condition and on developing targeted prevention strategies. Finally, the possible contribution of lifestyle variables, including food and exercise, to the therapy of metabolic diseases linked to SCH warrants further investigation.
This study was limited for the following reasons: First, this was a single-center community retrospective analysis. Second, confounding factors such as cigarette smoking, alcohol consumption, and physical activity, which may influence SCH and T2DM, were not analyzed. Third, this study only included the residents of a university community in southwest China. This population’s age, ethnicity, and education may have biased the results.
5 Conclusion
SCH patients in this community were shown to exhibit metabolic disorders (LDL-C, VLDL-C, TG, and elevated postprandial blood glucose). SCH patients with T2DM had significantly higher levels of dyslipidemia and dysglycemia than SCH patients without T2DM. Regular monitoring of this population’s blood lipid and glucose levels is advised for early intervention and prevention of complications.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the ethical committee of the Hospital of Chengdu University of Technology (No. 2020001). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the study is a retrospective study.
Author contributions
LZ: Conceptualization, Data curation, Formal analysis, Validation, Writing – original draft, Writing – review & editing. XJ: Conceptualization, Data curation, Formal analysis, Validation, Writing – original draft, Writing – review & editing, Project administration, Resources.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Shan, ZY, Chen, LL, Lian, XL, Liu, C, Shi, B, Shi, L, et al. Iodine status and prevalence of thyroid disorders after introduction of mandatory universal salt iodization for 16 years in China: a cross-sectional study in 10 cities. Thyroid. (2016) 26:1125–30. doi: 10.1089/thy.2015.0613
2. Hollowell, JG, Staehling, NW, Flanders, WD, Hannon, WH, Gunter, EW, Spencer, CA, et al. Serum TSH, T(4), and thyroid antibodies in the United States population(1988 to 1994): National Health and nutrition examination survey (NHANES III). J Clin Endocrinol Metab. (2002) 87:489–99. doi: 10.1210/jcem.87.2.8182
3. Lei, YL, Bao, T, Wang, T, and Tang, HR. The investigation of incidence of thyroid diseases in Chengdu. J North Sichuan Med Coll. (2017) 32:276–9. doi: 10.3969/j.issn.1005-3697.2017.02.038
4. Yan, DE, Hu, L, Shen, YF, Lai, XY, Zhang, MY, Zhou, M, et al. Iodine status and its association with prevalence of thyroid diseases in adults from Jiangxi Province, China. Endocrine. (2023) 82:335–42. doi: 10.1007/s12020-023-03413-8
5. Biondi, B, Kahaly, GJ, and Robertson, RP. Thyroid dysfunction and diabetes mellitus: two closely associated disorders. Endocr Rev. (2019) 40:789–824. doi: 10.1210/er.2018-00163
6. Handisurya, A, Pacini, G, Tura, A, Gessl, A, and Kautzky-Willer, A. Effects of T4 replacement therapy on glucose metabolism in subjects with subclinical (SH) and overt hypothyroidism (OH). Clin Endocrinol. (2008) 69:963–9. doi: 10.1111/j.1365-2265.2008.03280.x
7. Al Eidan, E, Ur Rahman, S, Al Qahtani, S, Al Farhan, AI, and Abdulmajeed, I. Prevalence of subclinical hypothyroidism in adults visiting primary health-care setting in Riyadh. J Community Hosp Intern Med Perspect. (2018) 8:11–5. doi: 10.1080/20009666.2017.1422672
8. Fatourechi, V. Subclinical hypothyroidism: an update for primary care physicians. Mayo Clin Proc. (2009) 84:65–71. doi: 10.4065/84.1.65
9. Gao, M, Cao, L, Wang, H, Peng, R, Xiao, X, Wang, G, et al. Correlation between subclinical hypothyroidism and dyslipidemia in women in Northeast China. Acta Endocrinol (Buchar). (2021) 17:282–5. doi: 10.4183/aeb.2021.282
10. Sindhu, S, and Vijay, MB. A study of lipid profile in patients with subclinical hypothyroidism. J Assoc Physicians India. (2022) 70:11–2.
11. Liu, Y, Li, X, Zhu, Y, Liu, J, and Liu, S. Subclinical hypothyroidism contributes to poor glycemic control in patients with type 2 diabetes mellitus, and ellagic acid attenuates methimazole-induced abnormal glucose metabolism in mice model. J Food Biochem. (2021) 45:e13753. doi: 10.1111/jfbc.13753
12. Yang, W, Jin, C, Wang, H, Lai, Y, Li, J, and Shan, Z. Subclinical hypothyroidism increases insulin resistance in normoglycemic people. Front Endocrinol (Lausanne). (2023) 14:1106968. doi: 10.3389/fendo.2023.1106968
13. Han, C, He, X, Xia, X, Li, Y, Shi, X, Shan, Z, et al. Subclinical hypothyroidism and type 2 diabetes: a systematic review and Meta-analysis. PLoS One. (2015) 10:e0135233. doi: 10.1371/journal.pone.0135233
14. Alharbi, M, Alsaleem, HN, Almuhaisni, R, Alzeadi, HS, Alsamani, RI, Alhammad, SI, et al. Association between subclinical hypothyroidism and the prognosis of diabetes mellitus and subsequent complications: a retrospective cohort study. Cureus. (2023) 15:e48329. doi: 10.7759/cureus.48329
15. Endocrine Society of the Chinese Medical Association. Chinese guidelines for diagnosis and treatment of thyroid diseases - hyperthyroidism. Chin J Intern Med. (2007) 46:876–82. doi: 10.3760/j.issn:0578-1426.2007.10.035
16. Endocrine Society of the Chinese Medical Association. Guidelines for diagnosis and treatment of adult hypothyroidism. Chin J Endocrinol Metabol. (2017) 33:167–80. doi: 10.3760/cma.j.issn.1000-6699.2017.02.018
17. Liu, HL, and Zhang, HM. The investigation of prevalence and risk factors of the thyroid disease for residents in Wuhan city. Mod Hosp. (2015) 15:150–3. doi: 10.3969/j.issn.1671-332X.2015.12.054
18. Gosi, SKY, and Garla, VV. Subclinical hypothyroidism. Treasure Island, FL: StatPearls Publishing (2023).
19. Jiao, J, Li, Y, Wang, JW, Xiao, F, Huang, YC, Wang, X, et al. Clinical study on blood biochemistry, bone metabolism and bone mineral density in patients with abnormal thyroid function. Chin J Osteopor. (2017) 23:1600–1602, 1659. doi: 10.3969/j.issn.1006-7108.2017.12.013
20. Tarboush, F, Alsultan, M, and Alourfi, Z. The correlation of lipid profile with subclinical and overt hypothyroidism: a cross-sectional study from Syria. Medicine (Baltimore). (2023) 102:e34959. doi: 10.1097/MD.0000000000034959
21. Luo, Y, Wu, F, Huang, Z, Gong, Y, and Zheng, Y. Assessment of the relationship between subclinical hypothyroidism and blood lipid profile: reliable or not? Lipids Health Dis. (2022) 21:137. doi: 10.1186/s12944-022-01749-0
22. Nakajima, Y, Yamada, M, Akuzawa, M, Ishii, S, Masamura, Y, Satoh, T, et al. Subclinical hypothyroidism and indices for metabolic syndrome in Japanese women: one-year follow-up study. J Clin Endocrinol Metab. (2013) 98:3280–7. doi: 10.1210/jc.2013-1353
23. Khan, SH, Manzoor, SM, Niazi, NK, Asif, N, Ijaz, A, and Fazal, N. Association of metabolic risks with subclinical hypothyroidism: a cross-sectional analysis. Pak J Med Sci. (2018) 34:357–62. doi: 10.12669/pjms.342.13873
24. Liu, XL, He, S, Zhang, SF, Wang, J, Sun, XF, Gong, CM, et al. Alteration of lipid profile in subclinical hypothyroidism: a Meta-analysis. Med Sci Monit. (2014) 20:1432–41. doi: 10.12659/MSM.891163
25. Li, H, Zhang, LH, Hao, WQ, Du, YH, Yang, WJ, and Tian, ZF. Analysis of clinical characteristics of patients with type 2 diabetes with subclinical hypothyroidism. Shaanxi Medical J. (2018) 47:461–463, 481. doi: 10.3969/j.issn.1000-7377.2018.04.017
26. Cho, JH, Kim, HJ, Lee, JH, Park, IR, Moon, JS, Yoon, JS, et al. Poor glycemic control is associated with the risk of subclinical hypothyroidism in patients with type 2 diabetes mellitus. Korean J Intern Med. (2016) 31:703–11. doi: 10.3904/kjim.2015.198
27. Li, L, Ou, YJ, Pi, YZ, Wang, HJ, Hu, L, Zhao, JJ, et al. Effect of subclinical hypothyroidism on glucose metabolism of type 2 diabetic patients. China J Pharm Econ. (2014) 12:56–8.
28. Wang, XQ, and Wang, W. Effects of subclinical hypothyroidism on blood glucose, lipid and uric acid levels in diabetic patients. J Prev Med Chin People’s Liberat Army. (2019) 37:47–8.
29. Shi, XG, Chong, W, Teng, WP, Shan, ZY, Li, YS, Guan, HX, et al. A prospective study of 93 patients with subclinical hypothyroidism. Chin J Endocrinol Metabol. (2003) 19:86–8. doi: 10.3760/j.issn:1000-6699.2003.02.003
Keywords: blood lipid, dysglycemia, free tetraiodothyronine, free triiodothyronine, subclinical hypothyroidism, thyroid-stimulating hormone
Citation: Zhu L and Jiang X (2024) Characteristics of blood lipid and metabolic indicators in subclinical hypothyroidism patients: a retrospective study. Front. Med. 11:1439626. doi: 10.3389/fmed.2024.1439626
Edited by:
Randal Westrick, Oakland University, United StatesReviewed by:
Anthony Martin Gerdes, New York Institute of Technology, United StatesEleonore Fröhlich, Medical University of Graz, Austria
Copyright © 2024 Zhu and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohong Jiang, amlhbmd4aWFvaG9uZ2NkQDE2My5jb20=
 Lili Zhu1
Lili Zhu1 Xiaohong Jiang
Xiaohong Jiang