- 1Department of Obstetrics and Gynecology, PGIMER, Chandigarh, India
- 2Department of Hepatology, PGIMER, Chandigarh, India
- 3Department of Transfusion Medicine, PGIMER, Chandigarh, India
- 4Department of Medicine, Microbiology and Infectious Diseases, University of Calgary, Calgary, AB, Canada
- 5Department of Hepatology, PGIMER, Chandigarh, India
- 6Department of Community Medicine, All India Institute of Medical Sciences, Nagpur, India
Introduction: Acute fatty liver of pregnancy (AFLP) is a fatal disease occurring in 3rd trimester. The safety and efficacy of plasmapheresis/plasma exchange (PP/PE) as an adjunctive treatment in patients of AFLP has been studied. We performed systematic review and meta-analysis to estimate the clinical parameters that included mortality rates and improvement of the biochemical parameters including Liver and Renal function enzymes, coagulopathy factors of AFLP patients.
Methods: We searched PubMed, Ovid MEDLINE, Cochrane, CINAHL and Scopus, ClinicalTrials.gov. RevMan statistical software was used for meta-analysis.
Results: Pooled survival proportion for AFLP patients treated with PP/PE was 87.74% (95% CI: 82.84 to 91.65). Efficacy of PP/PE was studied by its effect on mortality. PE/PP was associated with the reduction in the mortality with pooled odds ratio of 0.51 (95% CI: 0.08 to 3.09) with I2 = 86%. Sensitivity analysis after excluding outlier study, yielded a pooled odds ratio of 0.19 (95% CI: 0.02 to 1.52) with reduced heterogeneity (I2 = 63%). Biochemical parameter analysis demonstrated significant improvement post-PP/PE treatment, including decreased bilirubin (MD: 8.30, 95% CI: 6.75 to 9.84), AST (MD: 107.25, 95% CI: 52.45 to 162.06), ALT (MD: 111.08, 95% CI: 27.18 to 194.97), creatinine (MD: 1.66, 95% CI: 1.39 to 1.93), and Prothrombin time (MD: 5.08, 95% CI: 2.93 to 7.22).
Discussion: Despite some heterogeneity, PP/PE shows promise in improving biochemical parameters in AFLP patients. PE can serve as a therapeutic approach for AFLP particularly in severe or refractory cases. PE provides the time for organ to recover and helps in creating a homeostatic environment for liver. Large RCTs and propensity matched studies are needed to better understand the safety and efficacy of the treatment.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022315698.
1 Introduction
Acute fatty liver of Pregnancy (AFLP) is infrequent fatal hepatic dysfunction that is seen in early postpartum or 3rd trimester of pregnancy. Its reported prevalence is 1/7000 to 1/20,000. It is associated with maternal mortality of 10–15% and perinatal mortality of 7–85% (1, 2). It was first described by Sheehan in 1940, as ‘acute yellow atrophy of the liver’ for AFLP (3). The prevalence in Indian population is not known (1). Though the exact cause of the AFLP is not yet clear, it may be related to the deficiency of the fatty acid oxidation enzymes in the fetus which may include SCAD (short chain acyl CoA dehydrogenase), LCHAD enzyme (Long chain hydroxy acyl-CoA dehydrogenase), MCAD (Medium Chain Acyl Dehydrogenase) and MTP (Mitochondrial trifunctional protein) which causes mitochondrial dysfunction leading to oxidative stress, in women developing of the AFLP.
AFLP is associated with clinical features like first pregnancy, male fetus, multiple pregnancies, preeclampsia, fetal fatty acid oxidation defects. AFLP often progresses to liver and renal failure, coagulopathy and metabolic dysfunction. The Swansea criteria are most widely used to diagnose AFLP (4). Timely termination of pregnancy and intensive medical support are necessary to ensure good maternal and foetal outcome. Intensive standard medical support usually includes transfusions for anaemia and coagulation deficiencies, hypoglycaemia and electrolyte correction and broad-spectrum antibiotic treatment. Recovery is slow especially with developing complications. For early diagnosis of AFLP, Goel et al. (5) proposed a ‘simple criterion’ to diagnose AFLP, i.e., Women in late pregnancy (Second or third trimester) with no explained cause of acute liver failure (i.e., jaundice in addition to coagulopathy and/or encephalopathy and/or hypoglycaemia).
Considering the high mortality, morbidity, liver and renal dysfunction, various supportive techniques have been used to offer interim support to allow liver recovery, to reduce hospital stay and thus improve the prognosis. These include Artificial liver support therapy (ALST) or Blood Purification Techniques. These modalities have shown significant efficacy as a bridge therapy, either aiding in spontaneous recovery or preparing patients for a liver transplant. ALST includes therapeutic plasma exchange (PE), Plasma Perfusion (PP), hemoperfusion and continuous renal replacement therapy (CRRT). Apheresis is the extracorporeal removal of blood constituents. Plasmapheresis or Plasma exchange is the apheresis technique in which plasma is removed from blood and remainder is returned to the body with the replacement fluid such as Albumin/Fresh Frozen Plasma conducted with clear therapeutic purpose by selectively eliminating or modifying the particular components present in the plasma. Replacement fluid is carefully chosen to address the underlying medical condition. Plasma perfusion is incorporating fresh frozen plasma in the body. CRRT is extracorporeal blood purification technique which aims to remove the excess fluid and blood solutes to treat Acute Kidney Injury.
Different modalities of CRRT are Slow continuous ultrafiltration, continuous veno-venous hemofiltration, continuous veno-venous haemodialysis, continuous veno-venous hemodiafiltration (6). In a systematic review by Tan et al. (7) use of PE in patients of acute liver failure improves survival and biochemical improvement. First performed by Russian physicians Vadim A Yurevich and Nikolay Konstantinovich Rosenberg (8) in 1913, Therapeutic plasma exchange (TPE) is carried out using two types of systems: membrane-based TPE (mTPE) and centrifugal-based TPE (cTPE). Membrane based TPE is based on molecular size and involves separating blood plasma from cellular components with the use of filter which removes the blood plasma and retains the cellular components. Heparin, an anticoagulant, is typically added to the blood before it is pumped through the filter. On the other hand, cTPE is based on molecular density and utilizes centrifugation to separate incoming whole blood into plasma, red and white blood cell components. Prior to centrifugation, citrate, an anticoagulant, is usually added. In both procedures, the remaining blood rich in cells is mixed with a replacement fluid (such as albumin or fresh frozen plasma) and returned to the patient to prevent hypovolemia (9). Membrane based TPE systems require calibration and thus more setup and priming time in contrast to cTPE (10–13). mTPE require high blood flow rate which may cause hemodynamic fluxes that could worsen the perfusion in the weakened hepatic microcirculation. mTPE is associated with higher incidence of clotting and cellular components loss due to limited pore diameter in the filter.
In this systematic review we aim to determine the safety and efficacy of PP/PE as an adjunctive treatment in acute fatty liver of pregnancy. Our objectives include effect of PE/PP on
1. Clinical parameters, i.e., mortality rates and length of the hospital stay of AFLP patients,
2. Improvement of the biochemical parameters including Liver function enzymes, renal function enzymes and coagulopathy factors.
2 Methods
2.1 Protocol registration
The protocol for this systematic review is registered with PROSPERO (International Prospective Register of Systematic Reviews; no. CRD42022315698.
2.2 Eligibility criteria
All the articles till September 2023 were included. As per the PICO, we included studies that reported patients of acute fatty liver of pregnancy (Population) who received plasmapheresis/PE as the treatment. Safety and efficacy of plasmapheresis was assessed by noting clinical outcomes like maternal mortality and hospital stay and biochemical parameters, i.e., The pre and post treatment change in biochemical profiles of the AFLP patients.
2.3 Information sources and literature search strategy
A systematic literature search was done from different databases such as PubMed, Ovid MEDLINE, Cochrane, CINAHL and Scopus, ClinicalTrials.gov. The search strategy included the terms (“plasmapheresis OR therapeutic plasma exchange/TPE, PLEX and acute fatty liver of pregnancy/AFLP”) and (Efficacy OR “effects”). Randomized control trials, observational studies, case series and case reports published in English language were included. Review articles and only abstracts were excluded during initial screening.
2.4 Study selection
Two reviewers independently screened the titles and abstracts of the retrieved records for eligibility (BK and SK). Any discrepancies were resolved by discussion or consultation with a third reviewer (SS). The full texts of potentially eligible records were obtained and assessed data extracted by BK and SK. Reasons for exclusion were recorded and reported.
2.5 Data extraction
Data extraction was done in structured manner to derive the following information: study author, year of publication, country of origin, study participants, clinical presentation (of subjects, age, gravida parity, gestational age, mode of delivery, foetal outcome, no. of days spent in hospital and ICU, biochemical profiling (haemoglobin, platelets, creatinine, AST, ALT, total bilirubin, PT) and plasma exchange procedure, indications for the plasma exchange, complications during the procedure etc. For comparison, the data was converted into same units. The studies were assessed for the quality and heterogeneity.
2.6 Quality assessment
Eligible studies included non-randomized controlled trials, prospective and retrospective cohort studies, case series, case reports that evaluated the use of plasmapheresis in the treatment of acute fatty liver of pregnancy. Each study’s quality was evaluated by quality assessment tools. ROBINS-1 tool (“Risk of bias in non-randomized controlled studies-of interventions”) was used for non-randomized controlled studies, i.e., case control studies. The tool assesses the studies in seven domains-confounding, selection, intervention classification, intervention deviation, missing data, outcome measurement and selective reporting. On the basis of these domains, studies are labelled as having low, moderate, serious, or critical risk of bias depending on the outcome studied (14). NIH quality assessment tool was used for observational studies (prospective or retrospective studies) (15). Quality assessment of the case reports was done by JBI critical appraisal tool (16). Authors assessed the risk of bias independently for each of the studies included. Disagreements were resolved among authors by a consensus.
2.7 Statistical analysis
Review Manager Software (RevMan 5.4,Cochrane Collaboration, Oxford, UK) and MedCalc statistical software was used for statistical analysis. Meta-analysis of the outcome variables was done to estimate the effect of PE/PP on maternal mortality. Odds Ratios and 95% CI were calculated. Random and fixed effects models were used to consider heterogeneity as applicable and the tests for heterogeneity of the studies was assessed using the I2 test. Sensitivity Analysis was conducted by removing the study with a high influence on the pooled estimate.
To study the pre and post intervention effect, Mean difference (MD) and 95% confidence interval (CI) was calculated. Random and fixed effects models were used to consider heterogeneity as applicable and the tests for heterogeneity of the studies was assessed using the I2 test (17). An I2 value of 0–39% was considered as non-significant heterogeneity; 40–75% as moderate heterogeneity; and 76–100% as considerable heterogeneity. A p-value > 0.05 was considered to reject the null hypothesis that the studies were heterogeneous. The studies with patient number (n) less than 2 were not included in the meta-analysis.
3 Results
3.1 Study selection
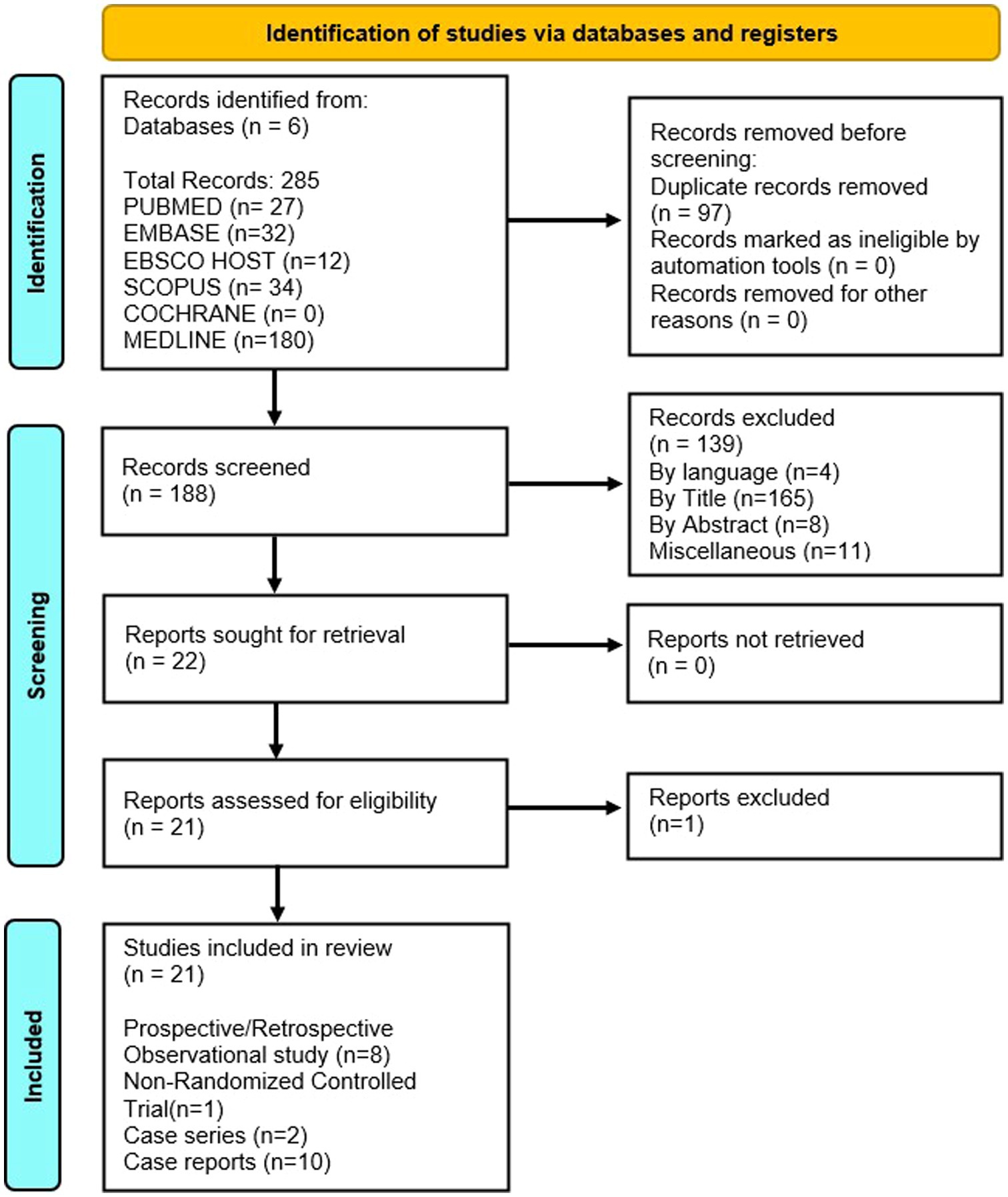
The literature search (detailed in Figure 1) resulted in identification of 285 published studies using the online database search of which 21 studies were included. Ten case reports, 2 case series, 8 observational studies and 1 non randomized control trial were assessed and finalized for eligibility (18–37). Sixteen were selected for the qualitative synthesis and 9 for quantitative synthesis (metanalysis; Figure 1). Searched articles were reported using the PRISMA checklist to ensure scientific precision. The PRISMA flow chart provides overview of the article selection process as shown in Figure 1. The meta-analysis contains 5 observational cohort studies and 1 case series. The studies included 10 studies from China (18, 19, 21–24, 33, 35–37), 2 studies from Japan (25, 26), 3 studies from USA (20, 34, 38), 1 study from North Africa (27), 2 studies from Iran (29, 32) and 1 study from India (31) and 2 from Morocco (28, 30) (Table 1).
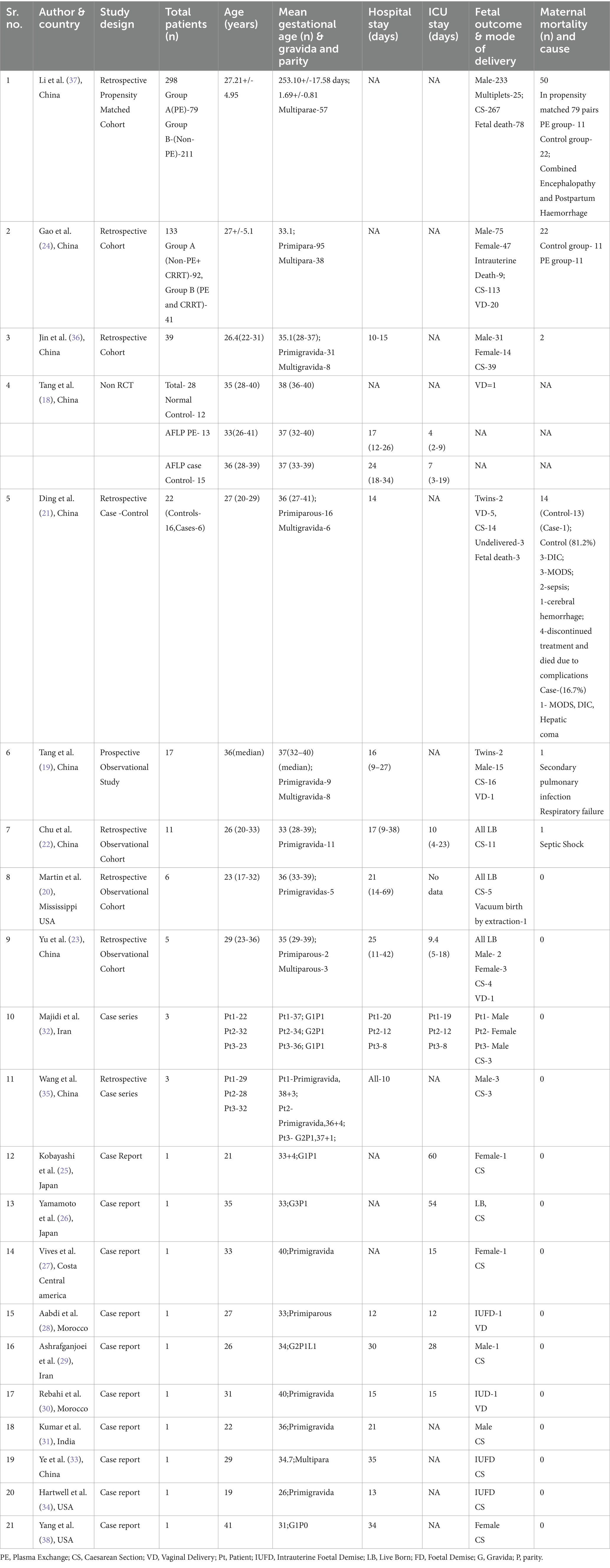
Table 1. Characteristics of the included studies [author, year of publication,country of origin, total patients (n), age (years), mean gestational age (weeks), hospital and icu stay (days), maternal and fetal outcomes, maternal mortality (percentage and reason).
3.2 Risk of Bias assessment
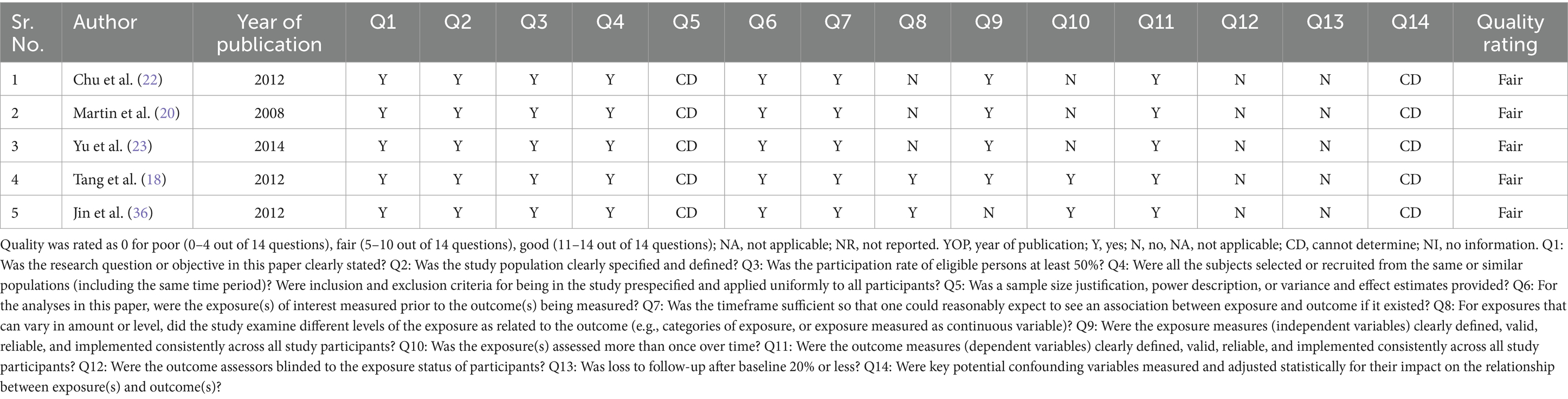
The primary outcome of our study was safety of Plasmapheresis/Plasma exchange (PP/PE) with or without other liver support therapy in acute fatty liver of pregnancy patients. Quantitative data of mortality in the case control studies was collected and assessed using ROBINS-I tool (14). The risk of bias was variable among included studies. Three studies (18, 21, 37) were at moderate risk of bias and one study (24) was at serious risk of bias. Different biases of all the included non-randomized studies are depicted by the traffic light plot and weighted plot using the ROBINS (visualization tool) web application (39). They are shown in Figures 2, 3 respectively. The overall judgement on the risk of bias assessment for each domain in the included studies has been found to have moderate to serious risk. The secondary outcome was biochemical improvement with PE/PP. For the same, NIH quality assessment tool was used. Five observational studies (19, 20, 22, 23, 36) were assessed and all studies were of fair quality (Table 2). Quality Assessment of the case reports were from low to moderate (Supplementary Table 2).
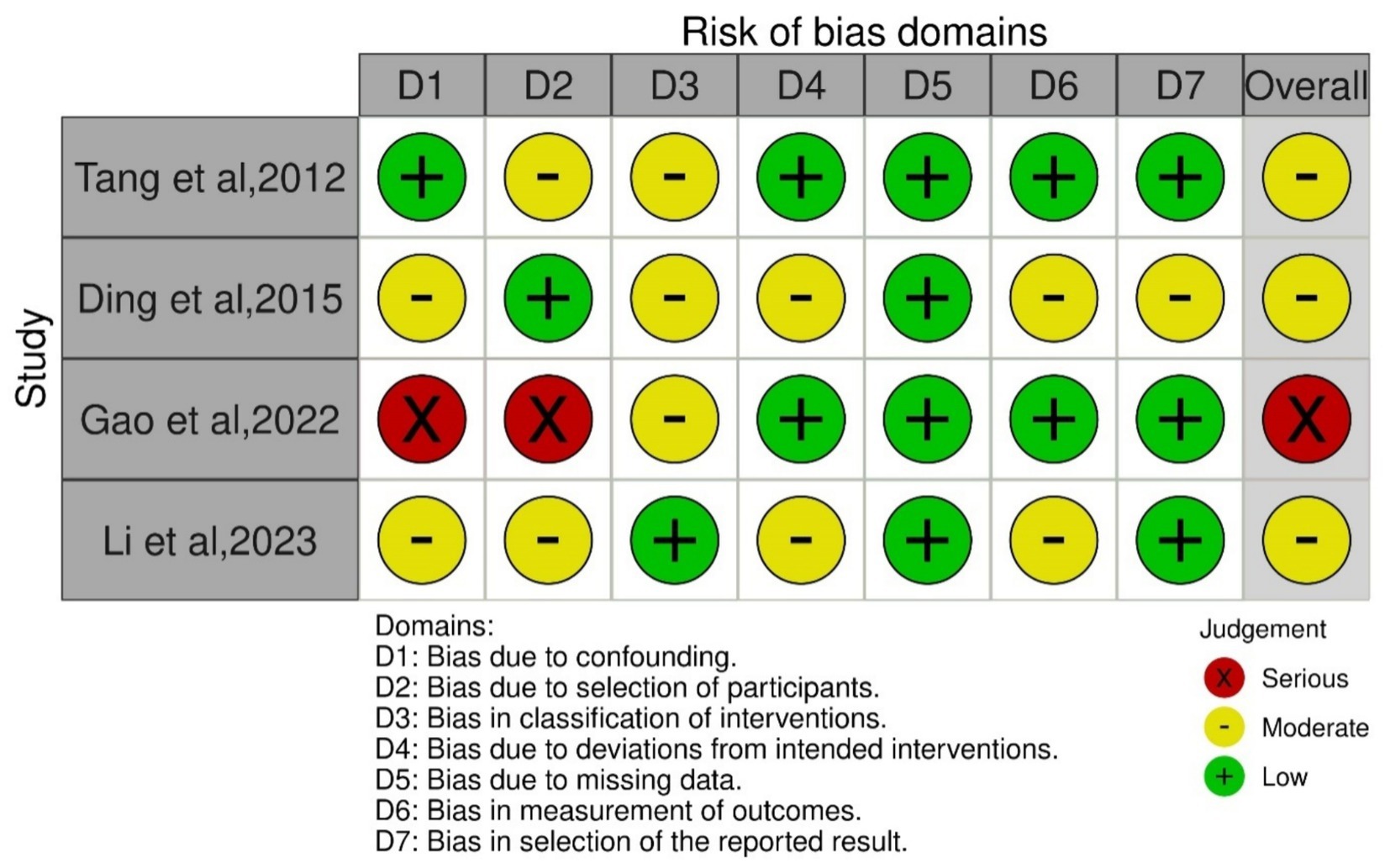
Figure 2. Graph of risk of bias assessment of the included case control studies using ROBINS-1 tool.
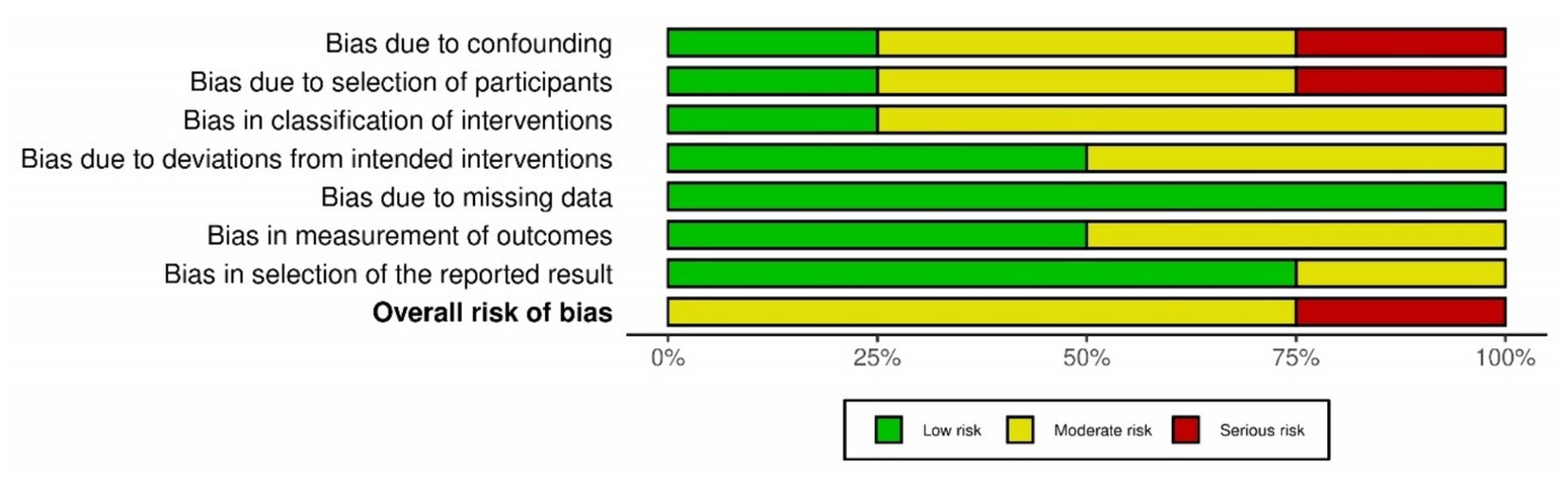
Figure 3. Summary of the risk of bias assessment of the included case control studies using ROBINS-1 tool.
3.3 Study characteristics
The key characteristics of the included studies are summarized in Tables 1, 3.
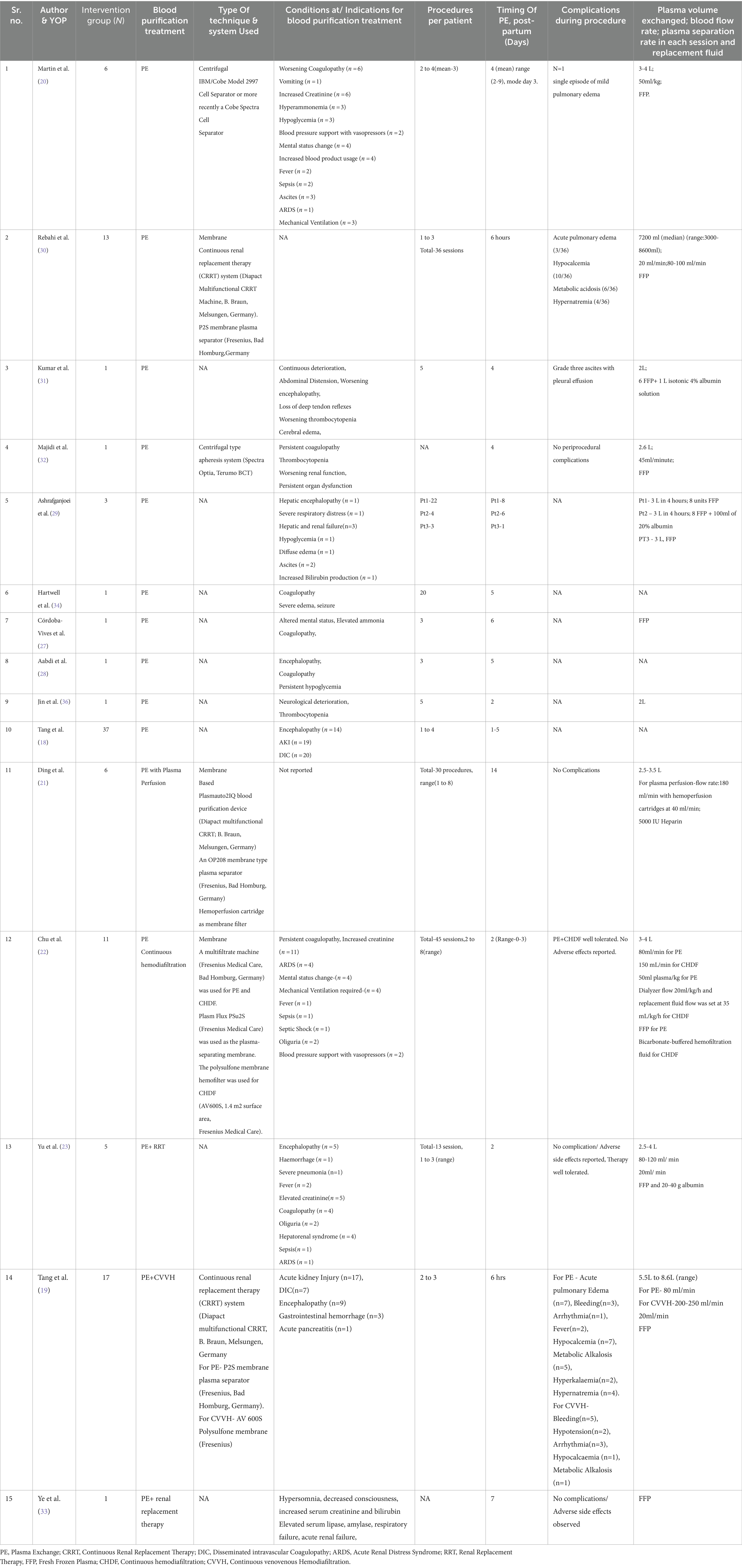
Table 3. Characteristics of the included studies [intervention group (N), blood purification treatment, type of technique & system used, indications for plex, procedures per patient, timing of pe, postpartum (days), complication during procedure, plasma volume exchanged; blood flow rate; plasma separation rate in each session and replacement fluid].
3.3.1 Obstetrical information
In the 21 included studies, total cases of AFLP Patients were 575. The age range of the women was 19–41 years and the gestational age ranged from 26–40 weeks. Swansea score and simplified criteria were used to diagnose the AFLP patients.
3.3.2 Foetal outcomes
Mode of delivery in most of studies was caesarean with notable more than 480 cases. From available data, 30 vaginal deliveries were reported in 6 studies (19, 21, 23, 24, 28, 30) while 94 cases of intrauterine fetal demise or fetal death were reported in 7 studies (21, 24, 28, 30, 33, 34, 37).
4 Results of the individual studies
4.1 Plasmapheresis/plasma exchange
16 studies detailed the information about the plasmapheresis/PE treatment. PE alone was given in 10 studies while PE with other Blood purification (BP) techniques like renal replacement therapy, hemofiltration, plasma perfusion was given in 6 studies. Six studies mentioned the techniques they used for the plasma exchange. 2 studies reported using centrifugal based plasma exchange system while 4 studies reported membrane-based plasma exchange system.
In the included studies, on average, plasmapheresis/PE was initiated within 4–8 days of the hospital admission. Procedures per patient varied depending upon the severity of the disease. Mostly, fresh frozen plasma was used as replacement fluid in PE (details in Table 3).
4.2 Indications for plasmapheresis/plasma exchange in AFLP patients
The most frequent reasons for initiating plasma exchange reported were abnormalities such as changes in sensorium and coma, persistent coagulopathy, advanced renal dysfunction and hepatic failure, fluid management issues such as significant ascites, oedema, anuria/oliguria, and/or fluid overload.
4.3 Meta-analysis results
4.3.1 Maternal mortality
The main outcome of our study was to assess the safety and efficacy of PP/PE in terms of reducing mortality in AFLP patients. Survival with PE/PP as Adjunctive treatment were studied in 11 studies (18–24, 32, 35–37) with 223 AFLP patients. The studies with n < 2 were excluded in survival proportion analysis as they had no control arm. Metanalysis of the case reports and case series cannot be done as there are no comparable groups. There were 10 case reports and 1 case series. Five observational studies (19, 20, 22, 23, 36) had no control arm.
Pooled survival estimate was calculated. Pooled survival proportion for 223 patients with PE/PP as adjunctive treatment was 87.74% (95% CI: 82.84 to 91.65) under fixed effect model with I2 = 25.57% (95%CI: 0.00 to 63.11).
A meta-analysis was conducted for four studies to assess the mortality on the use of Plasmapheresis/Plasma exchange (PP/PE) as adjunctive therapy on AFLP patients. Mortality data of the case control studies in the PE/PP group (Intervention) in comparison to control group is mentioned in Table 4. In the four included studies (18, 21, 24, 37), Pooled odds ratio and 95% confidence interval (CI) found that there is association between mortality and PP/PE as the adjuvant treatment. (Figure 2) PE/PP was associated with the reduction in the mortality with pooled odds ratio of 0.51(95% CI: 0.08 to 3.09) under random effect model. There is high heterogeneity among studies with Tau2 estimated as 2.03; Chi2 test estimated to be 14.03 with degree of freedom of 2 (p = 0.0009); I2 test estimated to be 86% as compared to no PP/PE as treatment (Figure 4).
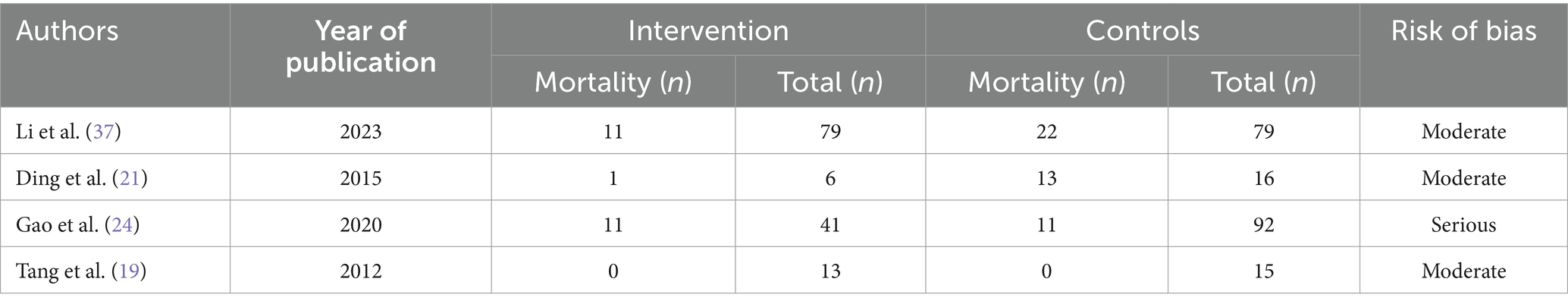
Table 4. Mortality data of the case control studies in the PE/PP group (Intervention) vs. Control group.
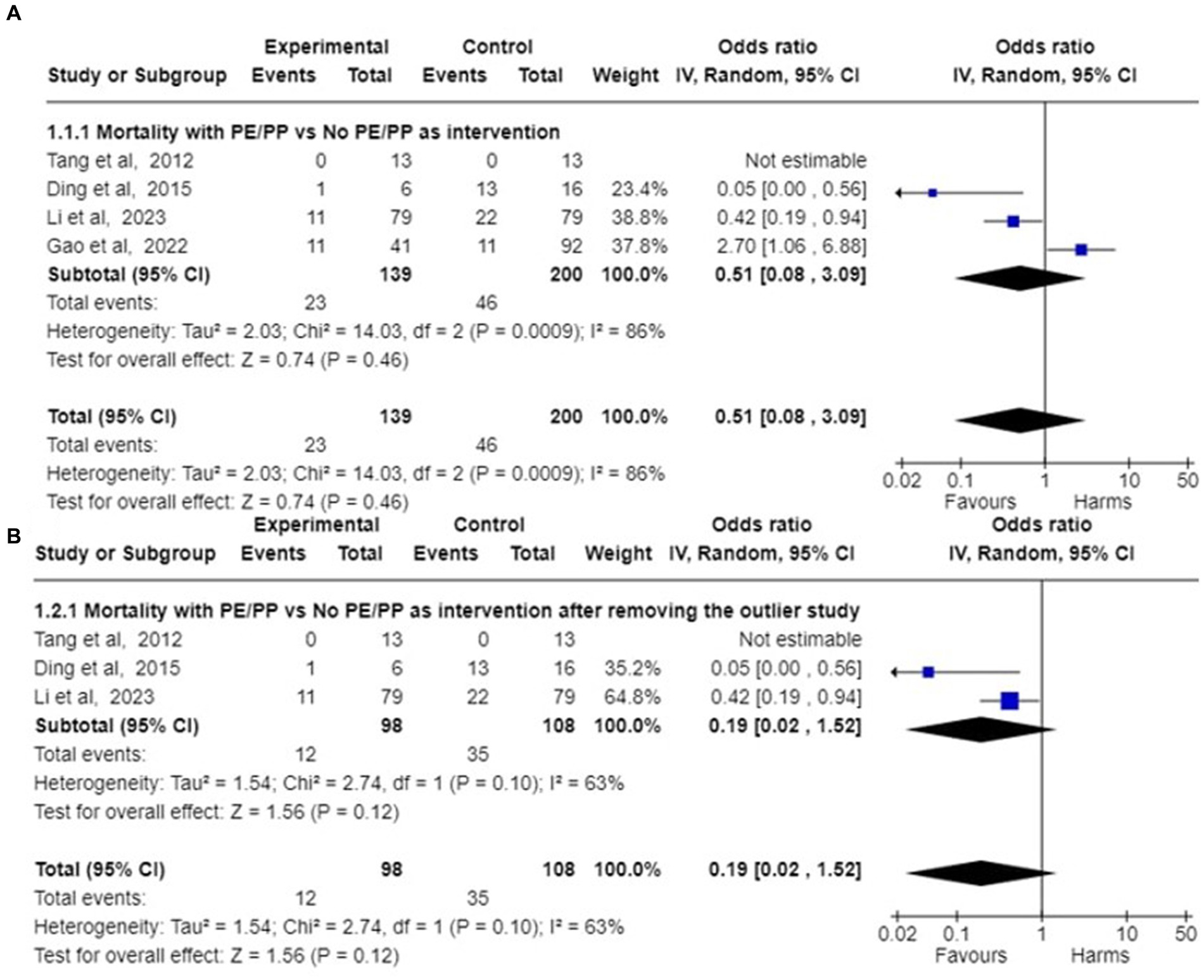
Figure 4. Forest plot of pooled odds ratio of mortality and their 95% confidence intervals (CI) and weights for individual studies. (A) Pooled Odds ratio of the mortality among AFLP patients given plasmapheresis/PE with or without other Blood Purification Technique as Intervention among 4 case control studies are depicted. (B) Pooled odds ratios of mortality among 3 case control studies are depicted after removing the outlier study (24).
4.3.2 Cause of mortality
Causes of mortality are described Table 1. Tang et al. and Chu et al. reported the causes of mortality as septic shock and pulmonary failure, respectively. In study by Li et al., combined encephalopathy and post-partum haemorrhage were associated with maternal mortality (37). While Ding et al. observed multiorgan dysfunction and disseminated intravascular coagulation, sepsis, cerebral hemorrhage, hepatic coma associated with maternal mortality (21).
4.3.3 Sensitivity analysis
A sensitivity analysis was performed to view any changes by omitting the outlier study. This was conducted by removing the outlier, i.e., Gao et al. (24) in which patients taken in PE group and non PE/PP were not comparable. In the study by Gao et al., patients treated with PE/PP had numerous poor prognostic factors, thus leading to higher maternal mortality as compared to the control group.
After removing the outlier study by Gao et al. (24), pooled survival proportion for 182 patients with PE/PP as adjunctive treatment was 90.37% (95% CI: 85.29 to 94.14) under fixed effect model with I2 = 0.00% (95%CI:0.00 to 42.09). Studies of Li et al., Ding et al. and Tang et al. (18, 21, 37) were analysed to assess the mortality in AFLP patients after the use of Plasmapheresis/Plasma exchange (PP/PE) as adjunctive therapy. The pooled odds ratio calculated came out to be 0.19 (95% CI: 0.02 to 1.52) under random effects model. There was still heterogeneity among studies with Tau2 estimated to be 1.54; Chi2 test estimated to be 2.74 with degree of freedom of 1 (p = 0.10); I2 test estimated the heterogeneity to be 63% (Figure 4).
4.4 Changes in biochemical parameters after plasmapheresis/plasma exchange
A meta-analysis was conducted to assess the effect of Plasmapheresis/Plasma exchange on acute fatty liver of pregnancy patients in terms of improvement in the biochemical profile. Table 5 mentions the quantitative values of the variables studied in the biochemical profile. Although included in the systematic review, the study by Tang et al. (19) was not included in the formal meta-analysis of change in biochemical parameters as data about standard deviation or error of relevant parameters were not reported in the study. Pooled standardized mean difference for different biochemical outcomes are as follows.
1. Bilirubin-Six studies reported the change in the bilirubin values after treatment. There was significant decrease in pooled bilirubin values after plasmapheresis treatment with mean difference (MD) of 8.30 (95% Cl: 6.75 to 9.84) using random effects model. There was no observed heterogeneity between studies with Tau2 estimated to be 0.00; Chi2 test estimated to be 1.95 with degree of freedom of 5 (p = 0.86) and I2 test estimated the heterogeneity to be 0% (Figure 5).
2. Alanine Transferase (ALT)- Six studies reported the change in the ALT levels after the treatment A decrease in pooled ALT values with MD of 111.08 (95% CI:27.18 to 194.97) under random effects model. There was heterogeneity among studies with Tau2 estimated to be 6326.64; Chi2 test estimated to be 21.72 with degree of freedom of 5 (p = 0.0006 and I2 test estimated the heterogeneity to be 77% (Figure 6).
3. Aspartate Transferase (AST)- Analysis of six studies reporting the change in the AST levels after the treatment demonstrated a substantial decrease in pooled AST values after plasmapheresis treatment with MD of 107.25 (95% CI:52.45 to 162.06) under fixed effects model. There was heterogeneity among studies with Tau2 estimated to be 2047.66; Chi2 test estimated to be 10.65 with degree of freedom of 5 (p = 0.06 and I2 test estimated the heterogeneity to be 53% (Figure 6).
4. Creatinine-Pooled Creatinine values also showed improvement with the plasmapheresis with MD of 1.66 (95% CI: 1.39 to 1.93) under random effect model. There was heterogeneity among studies with Tau2 estimated to be 0.00; Chi2 test estimated to be 3.99 with degree of freedom of 5 (p = 0.55) and I2 test estimated the heterogeneity to be 0% (Figure 7).
5. Prothrombin Time (PT)- Five studies reported the change in the prothrombin time levels after the treatment. Pooled Prothrombin Time showed some improvement after the plasmapheresis with MD of 5.08 (95% CI: 2.93 to 7.22) under random effects model. There was heterogeneity among studies with Tau2 estimated to be 2.69; Chi2 test estimated to be 7.87 with degree of freedom of 4 (p = 0.10) and I2 test estimated the heterogeneity to be 49% (Figure 8).
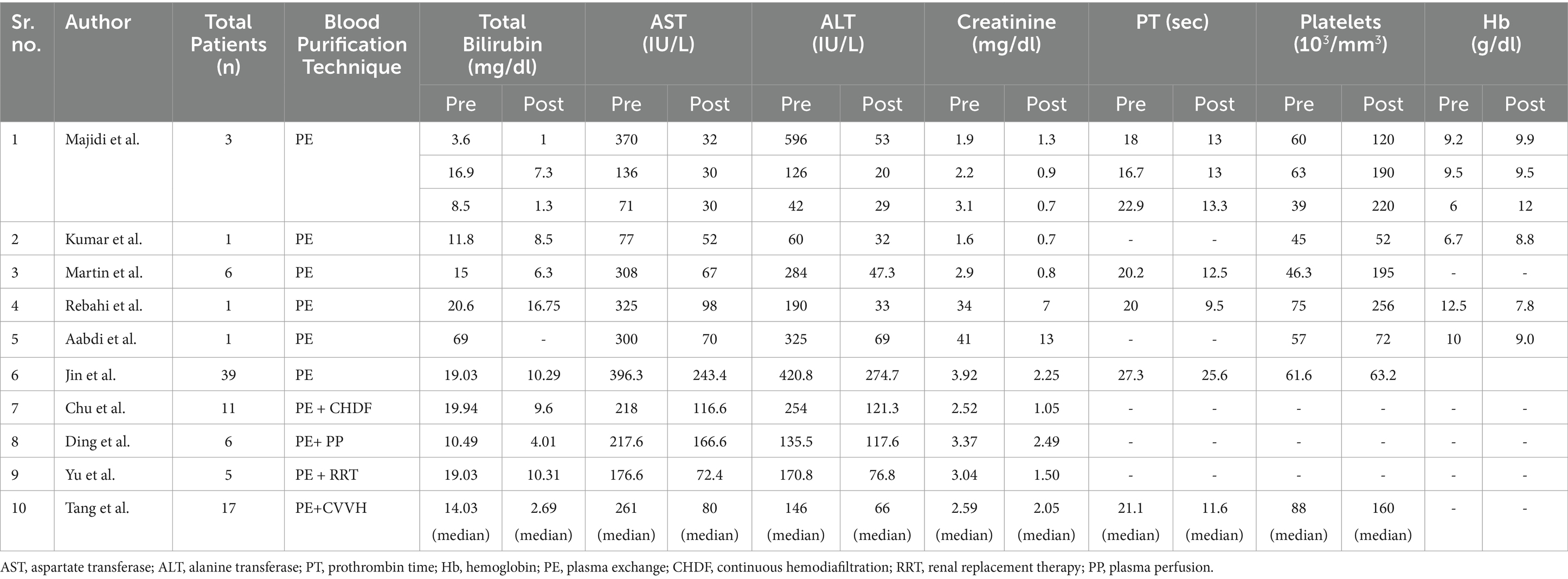
Table 5. Comparison of clinical parameters pre-plasma exchange vs. post-plasma exchange data with/without another blood purification technique as intervention in acute fatty liver of pregnancy (AFLP) patients during hospital stay.
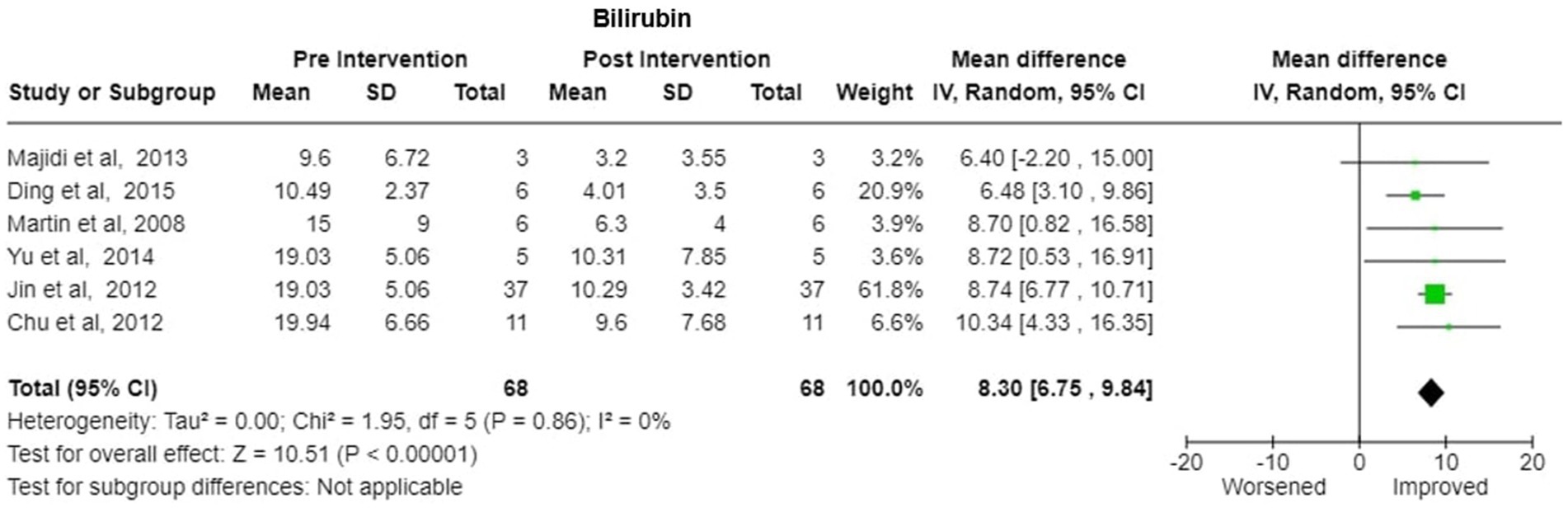
Figure 5. Bilirubin—forest plot showing mean difference (MD) and their 95% confidence interval (CI) and weights for individual studies from Pre and Post Intervention (plasmapheresis/PE with or without other blood purification techniques) obtained from six studies. MD values are depicted by green squares for each study with positive value indicating positive effect of the Intervention and diamond depicts pooled MD.
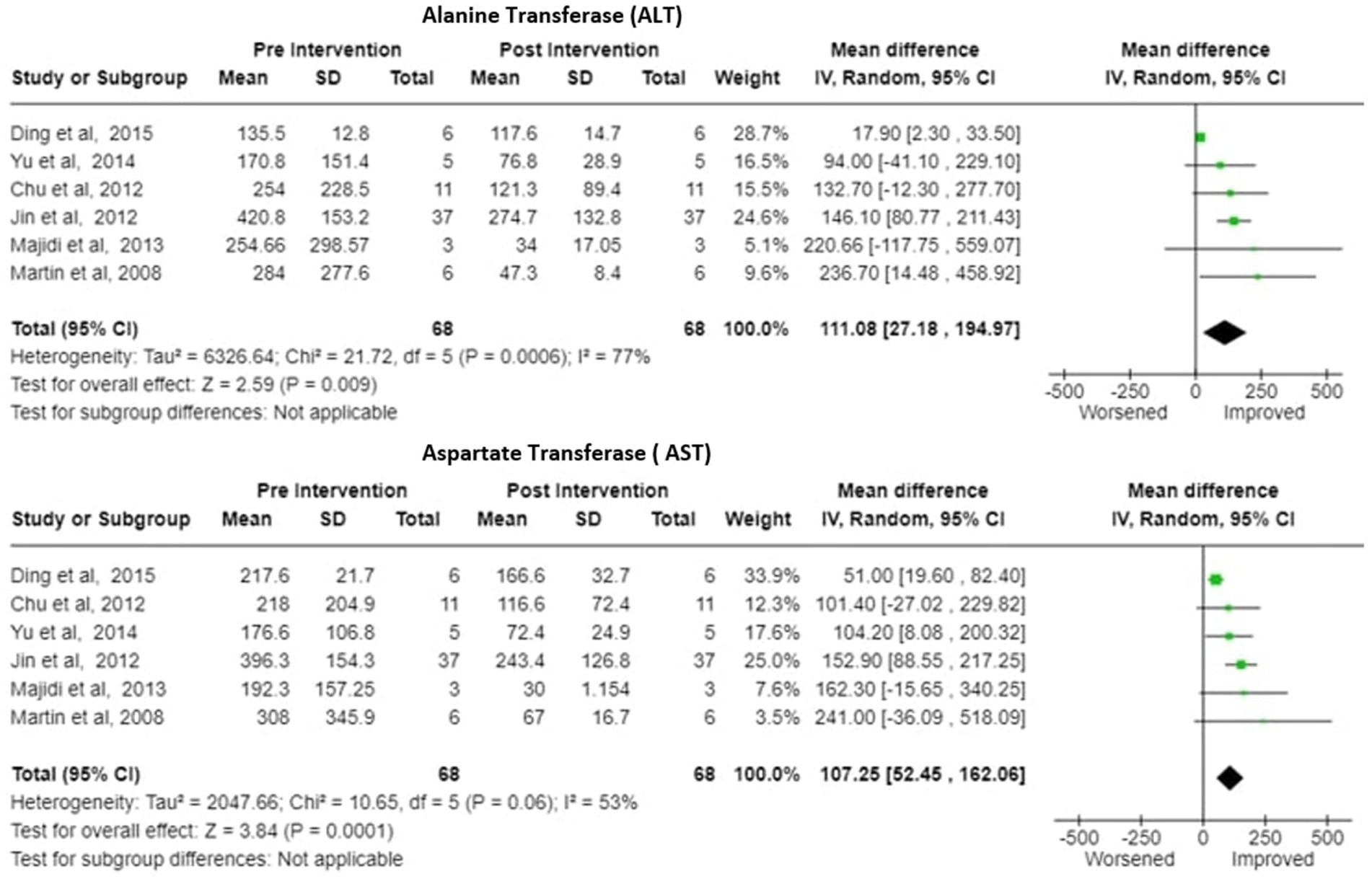
Figure 6. ALT (alanine transferase) and AST (aspartate transferase)—Forest plot showing pooled mean difference (MD) and their 95% confidence interval (CI) and weights for individual studies)from Pre and Post Intervention (plasmapheresis/PE with or without other blood purification techniques) obtained from six studies. MD values are depicted by green squares for each study with positive value indicating positive effect of the Intervention and diamond depicts pooled MD.
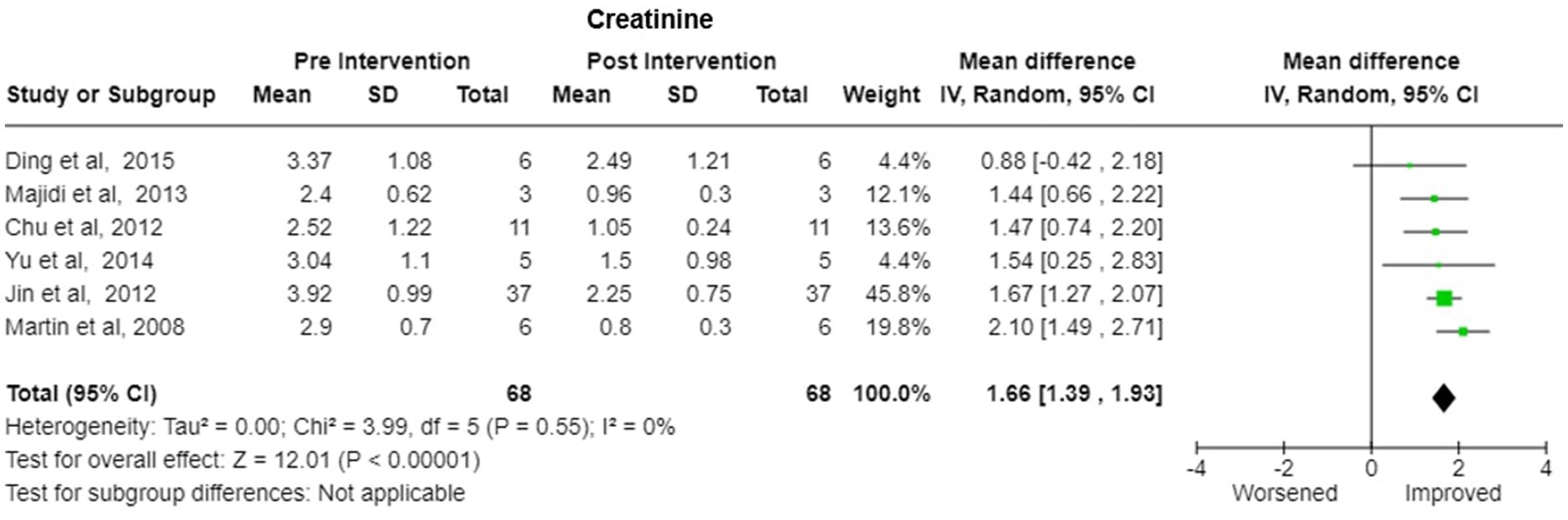
Figure 7. Creatinine—Forest plot showing pooled mean difference (MD) and their 95% confidence interval (CI) and weights for individual studies) from Pre and Post Intervention (plasmapheresis/PE with or without other blood purification techniques) obtained from six studies. MD values are depicted by green squares for each study with positive value indicating positive effect of the Intervention and diamond depicts pooled MD.
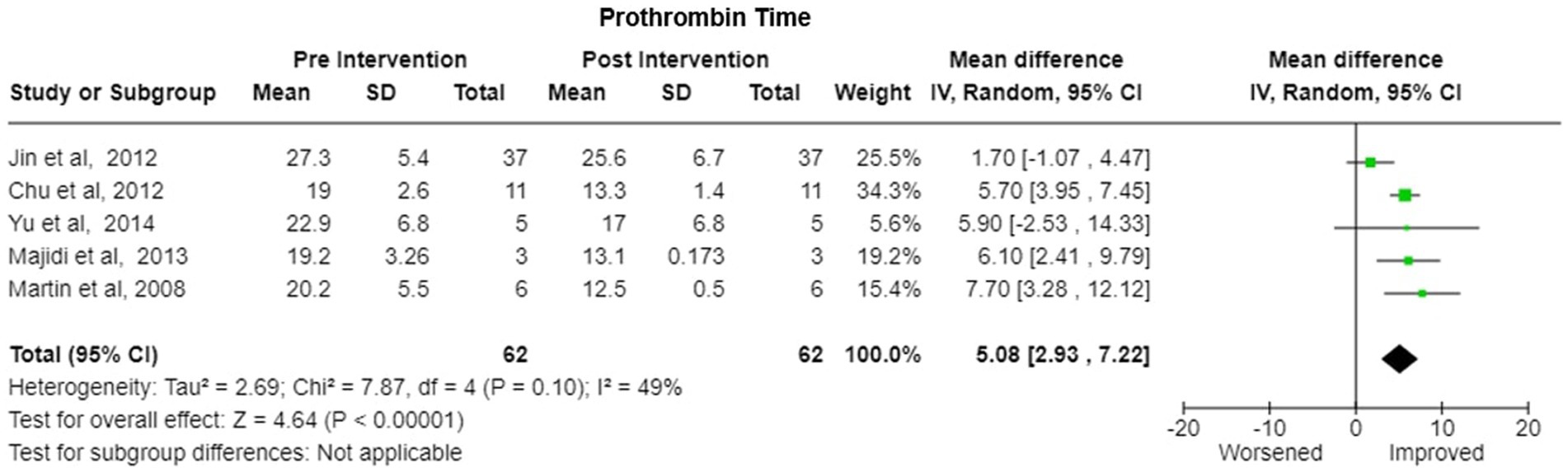
Figure 8. Prothrombin time—forest plot showing Pooled mean difference (MD) and their 95% confidence interval (CI) and weights for individual studies) from Pre and Post Intervention (plasmapheresis/PE with or without other blood purification techniques) obtained from six studies. MD values are depicted by green squares for each study with positive value indicating positive effect of the Intervention and diamond depicts pooled MD.
5 Complications during the plasma exchange/plasmapheresis
PE/PP appears to be generally well tolerated when used to treat with ALF and ACLF patients. The side effects of PE/PP may include sepsis, port-related infection, vein inflammation, bleeding, accidental arterial puncture, hypotensive and hypothermia and side effects by citrate anti-coagulation include hypocalecemia, muscle pain, arrythmia etc. Transfusion-related acute lung injury (TRALI) and acute pulmonary odema may arise due to FFP administered as a replacement during PP/PE. But the incidence of these pulmonary complications has decreased overtime due to female plasma being discarded as a risk reduction strategy or diverted for plasma fractionation (40, 41).
In this review, complications were studied/reported in four studies (18–20, 30). These included mild pulmonary oedema and grade 3 ascites with pleural effusion, hypocalcaemia, metabolic acidosis and hypernatremia which can be due to FFP.10 studies did not report complications or adverse side effects during procedures (21–23, 27–29, 31–34).
Tang et al. (19) compared various complications between PE and CVVH, respectively, (continuous venovenous hemofiltration) and reported a incidence of acute pulmonary oedema (7/17 vs. 0/17, p value of 0.007 and hypocalcaemia (7/17 vs. 1/17, p value-0.039) respectively. Other complications like bleeding, arrhythmia, metabolic acidosis, fever, hyperkalaemia, hypernatremia, hypotension was also reported but not statistically significant.
6 Discussion
The present systematic review demonstrates the potential role of plasma exchange in the treatment of acute fatty liver of pregnancy after delivery.
The main outcome of our study was safety and efficacy of the plasma exchange in reducing mortality of the patients. With advancement in the diagnosis and management of AFLP, the maternal mortality rate is now estimated to be 12.5 to 18% (42, 43).
Pooled survival proportion of patients (n = 223) from included studies was 87.741% and after removing the outlier study of Gao et al. (24), we observed an impressive pooled survival rate of >90% for AFLP patients (n = 182), despite the poor liver function status. Our metanalysis of pooled odds ratio suggests that there is reduction in the mortality with PP/PE treatment. But wide confidence interval and high heterogeneity among studies suggests variability in effect sizes across the studies. Even after removing the outlier study, there is still some degree of heterogeneity. So the findings should be interpreted cautiously. PE/PP has been widely used in the management of liver failure of other causes and can also improve the outcome of the AFLP patients.
Another outcome was change in the biochemical parameters with PP/PE as adjunctive treatment. In our meta-analysis, six studies were incorporated. Pooled mean difference (MD) between biochemical outcomes (Bilirubin and creatinine) showed significant decrease with no heterogeneity among studies. Pooled MD of other biochemical outcomes (AST, ALT, Prothrombin Time) also showed improvement but with some heterogeneity among studies. Plasma exchange (PE) can be beneficial for patients with severe illnesses as it promotes the faster normalization of liver and renal enzymes. However, for patients with less severe conditions, supportive therapy alone may be sufficient.
Prompt induction of labor and termination of the pregnancy is the definitive and vital in the obstetrical management. Though normal vaginal delivery is generally considered safer, Caesarean section usually reduces the time to deliver as compared to induction of labour and vaginal delivery. In their systematic review, Wang et al. studied the association between caesarean section and vaginal delivery and reported that maternal mortality was 44% lower (RR,0.56 [CI-0.41-0.76] in caesarean section although not statistically significant (44). AFLP is often complicated by hepatic encephalopathy, coagulopathy, multiorgan dysfunction syndrome, renal dysfunction, DIC, hypoglycaemia, septic shock, haemorrhage.
Guidelines by CSOG MFM Committee of China for clinical management of acute fatty liver of pregnancy includes the use of artificial liver treatment for patients with severe AFLP (45). Recently published guidelines by the European Association for the Study of Liver (EASL) and Japan Society of Blood Purification in Critical Care (JSBPCC), Indian National Association For Study Of The Liver (INASL) recommend using extracorporeal blood purification devices, including PE, for acute fulminant liver failure. However, the American Association for the Study of Liver Diseases (AASLD) has been more cautious and has not found enough solid evidence to routinely recommend the use of external artificial (sorbent-based) or bioartificial liver support systems (cell-based) in the management of acute liver failure (ALF) (46).
Tang’s study elaborates the effect of the PE on the molecular and cellular level and how PE can help lessen the hepatic injury in AFLP. Increased fatty acids in AFLP leads to excessive intake by hepatocytes, that stimulates the expression of reactive oxygen species, mitochondrial DNA mutations, and apoptosis. Dysfunction of the synthesis and detoxification function of liver leads to more accumulation of the toxic metabolites. PE significantly enhanced mitochondrial functionality by regulating mitochondrial membrane potential (MMP) and inhibiting oxidative stress responses and caspase-9 activation, resulting in a reduction in apoptosis in AFLP patients, the effect increasing, following several sessions (18).
It should be noted that most of the included cohort studies are retrospective in nature. Jin et al. treated 39 AFLP patients with PE and it was noted that the patient’s general condition improved after the first PE session (36). Additionally, Kumar et al. observed that PE led to significant improvements in renal and liver biomarkers (31).
Safety of PE also needs to be studied. PE is associated with complications during treatment like hypocalcaemia and metabolic acidosis, ascites, hypernatremia, bleeding, arrhythmia, fever, hyperkalaemia and complications like acute pulmonary oedema/TRALI due to FFP transfusion during PE/PP. But the incidence of these pulmonary complications has decreased overtime due to female plasma being discarded as a risk reduction strategy or diverted for plasma fractionation (40, 41). Tang et al. (19) study observes that the use of PE alone can induce pulmonary oedema, secondary to the substantial requirement of the fresh frozen plasma. A major concern associated with PE is the administration of large doses of citrate anticoagulants, which can lead to hypernatremia, hypocalcaemia, and metabolic alkalosis. Hypocalcaemia can be prevented during PE by prophylactic calcium administration and calcium monitoring (47). When treated with low-volume PE, citrate toxicity is generally seen less due to the low volumes of plasma exchanged (thus using less citrate) and the low rates of flow of the processed blood (using PE/PP centrifugal technique) in liver failure patients (48). In several reports, hemodynamic instability was seen as a contraindication to using PE/PP (49, 50). PE/PP in hemodynamically unstable patients raises concerns that it could exacerbate and have negative impact on patient outcomes. Active sepsis is regarded as a PE contraindication. It’s probable that PE’s suppression of the immune system’s overreaction will make sepsis worse (48). Optimal situation for starting PE may be in the golden window of sterile inflammation (51). PLEX use to treat liver failure is contraindicated if there has recently been a gastrointestinal bleeding (50). In the RCT by Larsen et al. for ALF patients (n = 92) there was no statistical difference between the complications who received high volume plasma exchange vs. standard medical treatment. PE/PP can be considered safe and tolerable for acute liver failure patients. Choice of the treatment depends on the severity of the condition (52). There is not much evidence to opine on whether normal volume or high volume exchange benefits more in AFLP patients. Plasmapheresis combined with other blood purification techniques have also been attempted in studies. Ding et al. observed that kidney and liver biochemical functions significantly enhanced when PE and Plasma perfusion were given for 2 weeks. The combination of plasma exchange (PE) and plasma perfusion (PP) in patients with liver disease enables the efficient removal of a significant quantity of toxic substances, as well as the improvement of clotting factors and albumin levels (21). Yu et al. used PE with renal replacement therapy such as continuous venovenous hemofiltration, continuous venovenous hemodiafiltration or continuous venovenous haemodialysis once on every other day. For severe AFLP patients with potentially fatal illness, plasma exchange and renal replacement therapy can be used for treatment of patients that do not respond to that conventional therapy (23). In the study by Tang et al. combining PE and continuous venovenous hemofiltration (CVVH), PE clears the bilirubin significantly than CVVH (p = 0.000). Plasmapheresis (PE) effectively eliminates circulating endotoxins and facilitates the replacement of coagulation factors and proteins, thereby correcting hepatic encephalopathy (HE). CVVH improves the renal functions by clearance of creatinine, inflammatory mediators, nitrogenous metabolic waste etc. (19). Yamamoto et al. and Ye et al. also endorse the same (26, 33).
Furthermore, it is worth noting that initiating plasmapheresis earlier appears to enhance its effectiveness and reduce the number of required sessions. Overall, these studies collectively suggest that plasma exchange, particularly when initiated promptly, can be effective in improving the clinical outcomes of severely ill AFLP patients, leading to favourable changes in various biochemical markers.
7 Limitations
Our study is limited by the quality of studies and heterogeneity in reporting. Published data on the patient outcomes with AFLP are mostly case reports, with no RCTs. Larger prospective studies may elucidate the impact of plasma exchange on the maternal survival. Clinical heterogeneity was a notable challenge. There were limited available studies on the topic and variations in study designs. MELD score for degree of liver failure is also not mentioned. The lack of standardized PE protocols, data collection methods and the absence of consistent reporting of treatment outcomes, use of different statistical measures made it challenging to ascertain the precise differences in treatment effects. While some case studies did provide pre-and post-plasma exchange data, it is crucial to consider that combining data from case reports may introduce biases and limitations, compromising the overall validity of the systematic review. The majority of studies lacked control groups. In order to address these limitations, the systematic review had to carefully consider the available evidence, focusing on the similarities and differences in study design, patient characteristics, and reported outcomes, while acknowledging the inherent limitations and potential biases associated with the included studies.
8 Conclusion
To conclude, emerging evidence suggests that PE can serve as a therapeutic approach for acute fatty liver of pregnancy (AFLP), particularly in severe or refractory cases. PE provides the organ with an opportunity to recover by ameliorating liver injury and creating a homeostatic environment conducive to hepatocyte regeneration. Better designed and larger randomized controlled trials or at the very least propensity matched retrospective or prospective cohort studies are the need of the hour, to gain granular understanding about the efficacy and patient selection for plasmapheresis/plasma exchange in acute fatty liver of pregnancy patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
SS: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Data curation, Resources, Software, Validation, Visualization. AD: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Supervision, Writing – original draft. BK: Data curation, Formal analysis, Methodology, Software, Validation, Writing – original draft, Investigation, Project administration. DL: Formal analysis, Methodology, Supervision, Validation, Writing – review & editing. SK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – review & editing. VS: Writing – review & editing, Conceptualization, Funding acquisition, Supervision. AP: Writing – review & editing, Formal analysis, Methodology.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. We would like to acknowledge the Indian Council of Medical Education and Research, New Delhi, for providing the funds for the project and publication charges (Project ID:9733).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1433324/full#supplementary-material
Abbreviations
AFLP, Acute fatty liver of pregnancy; TPE, Therapeutic plasma exchange; PE, Plasma exchange; PP, Plasmapheresis; MELD, Model for end stage liver disease; RCT, Randomized controlled trial; HE, Hepatic encephalopathy; AST, Aspartate transferase; ALT, Alanine transferase; PLEX, Plasma exchange; ACLF, Acute on chronic Liver Failure; PRISMA, Preferred reporting items for systematic reviews and meta-analysis; NIH, National Institute of Health; JBI, Joanna Briggs Institute; CSOG MFM Committee, Society of Obstetrics and Gynecology of Chinese Medical Association, Maternal Feotal Medicine Committee.
References
1. Ko, H, and Yoshida, EM. Acute fatty liver of pregnancy. Can J Gastroenterol. (2006) 20:25–30. doi: 10.1155/2006/638131
2. Fesenmeier, MF, Coppage, KH, Lambers, DS, Barton, JR, and Sibai, BM. Acute fatty liver of pregnancy in 3 tertiary care centers. Am J Obstet Gynecol. (2005) 192:1416–9. doi: 10.1016/j.ajog.2004.12.035
3. Sheehan, H. The pathology of acute yellow atrophy and delayed chloroform poisoning. BJOG Int J Obstet Gynaecol. (1940) 47:49–62. doi: 10.1111/j.1471-0528.1940.tb14731.x
4. Morton, A, and Laurie, J. Physiological changes of pregnancy and the Swansea criteria in diagnosing acute fatty liver of pregnancy. Obstet Med. (2018) 11:126–31. doi: 10.1177/1753495X18759353
5. Goel, A, Jamwal, KD, Ramachandran, A, Balasubramanian, KA, and Eapen, CE. Pregnancy-related liver disorders. J Clin Exp Hepatol. (2014) 4:151–62. doi: 10.1016/j.jceh.2013.03.220
6. Tandukar, S, and Palevsky, PM. Continuous renal replacement therapy: who, when, why, and how. Chest. (2019) 155:626–38. doi: 10.1016/j.chest.2018.09.004
7. Tan, EX-X, Wang, M-X, Pang, J, and Lee, G-H. Plasma exchange in patients with acute and acute-on-chronic liver failure: a systematic review. World J Gastroenterol. (2020) 26:219–45. doi: 10.3748/wjg.v26.i2.219
8. Sokolov, AA, and Solovyev, AG. Russian pioneers of therapeutic hemapheresis and extracorporeal hemocorrection: 100-year anniversary of the world’s first successful plasmapheresis. Ther Apher Dial. (2014) 18:117–21. doi: 10.1111/1744-9987.12067
9. Kielstein, JT, Hafer, C, Zimbudzi, E, and Hawes, S. A change for better exchange–from membrane therapeutic plasma exchange to centrifugal therapeutic plasma exchange. EMJ Nephrol. (2020) 8:2–10. doi: 10.33590/emjnephrol/20-0001
10. Puppe, B, and Kingdon, EJ. Membrane and centrifugal therapeutic plasma exchange: practical difficulties in anticoagulating the extracorporeal circuit. Clin Kidney J. (2014) 7:201–5. doi: 10.1093/ckj/sft163
11. Hafer, C, Golla, P, Gericke, M, Eden, G, Beutel, G, Schmidt, JJ, et al. Membrane versus centrifuge-based therapeutic plasma exchange: a randomized prospective crossover study. Int Urol Nephrol. (2016) 48:133–8. doi: 10.1007/s11255-015-1137-3
12. Kes, P, Janssens, ME, Bašić-Jukić, N, and Kljak, M. A randomized crossover study comparing membrane and centrifugal therapeutic plasma exchange procedures. Transfusion (Paris). (2016) 56:3065–72. doi: 10.1111/trf.13850
13. Lambert, C, Gericke, M, Smith, R, and Hermans, C. Plasma extraction rate and collection efficiency during therapeutic plasma exchange with spectra Optia in comparison with Haemonetics MCS+. J Clin Apher. (2011) 26:17–22. doi: 10.1002/jca.20271
14. Sterne, JAC, Hernán, MA, Reeves, BC, Savović, J, Berkman, ND, Viswanathan, M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. (2016) 355:i4919. doi: 10.1136/bmj.i4919
15. NIH (2024). Study Quality Assessment Tools. NHLBI. Available at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (Accessed July 3, 2024).
16. JBI (2024). JBI Critical Appraisal Tools. Available at: https://jbi.global/critical-appraisal-tools (Accessed July 3, 2024).
17. Gandhi, AP, Shamim, MA, and Padhi, BK. Steps in undertaking meta-analysis and addressing heterogeneity in meta-analysis. Evidence. (2023) 1:78–92. doi: 10.61505/evidence.2023.1.1.7
18. Tang, W, Huang, Z, Wang, Y, Bo, H, and Fu, P. Effect of plasma exchange on hepatocyte oxidative stress, mitochondria function, and apoptosis in patients with acute fatty liver of pregnancy: effect of pe on hepatocyte in patients with AFLP. Artif Organs. (2012) 36:E39–47. doi: 10.1111/j.1525-1594.2011.01417.x
19. Tang, WX, Huang, ZY, Chen, ZJ, Cui, TL, Zhang, L, and Fu, P. Combined blood purification for treating acute fatty liver of pregnancy complicated by acute kidney injury: a case series. J Artif Organs. (2012) 15:176–84. doi: 10.1007/s10047-011-0621-5
20. Martin, JN, Briery, CM, Rose, CH, Owens, MT, Bofill, JA, and Files, JC. Postpartum plasma exchange as adjunctive therapy for severe acute fatty liver of pregnancy. J Clin Apher. (2008) 23:138–43. doi: 10.1002/jca.20168
21. Ding, J, Han, L-P, Lou, X-P, Geng, L-N, Liu, D, Yang, Q, et al. Effectiveness of combining plasma exchange with plasma perfusion in acute fatty liver of pregnancy: a retrospective analysis. Gynecol Obstet Investig. (2015) 79:97–100. doi: 10.1159/000368752
22. Chu, Y-F, Meng, M, Zeng, J, Zhou, H-Y, Jiang, J-J, Ren, H-S, et al. Effectiveness of combining plasma exchange with continuous Hemodiafiltration on acute fatty liver of pregnancy complicated by multiple organ dysfunction. Artif Organs. (2012) 36:530–4. doi: 10.1111/j.1525-1594.2011.01424.x
23. Yu, C-B, Chen, J-J, Du, W-B, Chen, P, Huang, J-R, Chen, Y-M, et al. Effects of plasma exchange combined with continuous renal replacement therapy on acute fatty liver of pregnancy. Hepatobiliary Pancreat Dis Int. (2014) 13:179–83. doi: 10.1016/S1499-3872(14)60028-X
24. Gao, Q, Ma, Y, Zhang, J, Chen, X, Liu, F, Tian, S, et al. Risk factors assessment in patients with acute fatty liver of pregnancy treated without plasma exchange or renal replacement therapy. J Matern Fetal Neonatal Med. (2022) 35:2036–40. doi: 10.1080/14767058.2020.1777267
25. Kobayashi, T, Minami, S, Mitani, A, Tanizaki, Y, Booka, M, Okutani, T, et al. Acute fatty liver of pregnancy associated with fetal mitochondrial trifunctional protein deficiency. J Obstet Gynaecol Res. (2015) 41:799–802. doi: 10.1111/jog.12609
26. Yamamoto, K, Suzuki, A, Shimaoka, M, Yo, Y, Mandai, M, and Matsumura, N. Severe fatty liver of pregnancy requiring an extremely large amount of blood transfusion, surgery and transarterial embolization: a case report. Case Rep Womens Health. (2019) 23:e00130. doi: 10.1016/j.crwh.2019.e00130
27. Córdoba-Vives, S, Pérez-Rodríguez, P, Marín-Delgado, A, Matus-Vargas, M, and Arias-González, A. AO Awonuga, editor. A rare disease presenting postpartum: acute fatty liver of pregnancy. Case Rep Obstet Gynecol. (2021); 2021:1–4. doi: 10.1155/2021/1143470
28. Aabdi, M, Mellagui, Y, Ouachaou, J, Ounci, E, Bkiyar, H, and Housni, B. Plasma exchange as treatment for acute fatty liver disease of pregnancy. Clin Case Reports. (2021) 9:1594–7. doi: 10.1002/ccr3.3845
29. Ashrafganjoei, T, Mirreza, S, Eftekhariyazdi, M, and Mortazavi, F. Plasmapheresis in the treatment of fatty liver of pregnancy: a case report. Nurs Pract Today. (2019) 6:49–54. doi: 10.18502/npt.v6i2.908
30. Rebahi, H, Still, ME, and El Adib, AR. A successful use of therapeutic plasma exchange in a fulminant form of acute fatty liver of pregnancy. J Gynecol Obstet Hum Reprod. (2019) 48:133–7. doi: 10.1016/j.jogoh.2018.10.001
31. Kumar, A, Sharma, A, Mohan, Y, Patnaik, I, Kumar, A, Khoiwal, K, et al. Therapeutic plasma exchange in acute fatty liver of pregnancy: a case report and literature review. Pan Afr Med J. (2021) 40:220. doi: 10.11604/pamj.2021.40.220.31324
32. Seyyed Majidi, MR, and Vafaeimanesh, J. Plasmapheresis in acute fatty liver of pregnancy: an effective treatment. Case Rep Obstet Gynecol. (2013) 2013:1–5. doi: 10.1155/2013/615975
33. Ye, R, Mai, Z, Pan, X, Cai, S, and Deng, L. Acute fatty liver of pregnancy causes severe acute pancreatitis and stillborn fetus: a case report. Medicine (Baltimore). (2021) 100:e25524. doi: 10.1097/MD.0000000000025524
34. Hartwell, L, and Ma, T. Acute fatty liver of pregnancy treated with plasma exchange. Dig Dis Sci. (2014) 59:2076–80. doi: 10.1007/s10620-014-3328-7
35. Wang, L, Gan, Q, Du, S, Zhao, Y, Sun, G, Lin, Y, et al. Acute fatty liver of pregnancy cases in a maternal and child health hospital of China: three case reports. Medicine (Baltimore). (2020) 99:e21110. doi: 10.1097/MD.0000000000021110
36. Jin, F, Cao, M, Bai, Y, Zhang, Y, Yang, Y, and Zhang, B. Therapeutic effects of plasma exchange for the treatment of 39 patients with acute fatty liver of pregnancy. Discov Med. (2012) 13:369–73. doi: 10.2337/dc12-0723
37. Li, L, Huang, D, Xu, J, Li, M, Zhao, J, Shi, Q, et al. The assessment in patients with acute fatty liver of pregnancy (AFLP) treated with plasma exchange: a cohort study of 298 patients. BMC Pregnancy Childbirth. (2023) 23:171. doi: 10.1186/s12884-023-05503-x
38. Yang, P, Sikachi, R, Gerasimov, M, Aronsohn, J, and Palleschi, G. Acute fatty liver of pregnancy leading to a delayed hepatic failure necessitating liver transplantation: a case report. J Obstet Anaesth Crit Care. (2021) 11:124–6. doi: 10.4103/JOACC.JOACC_16_21
39. McGuinness, LA, and Higgins, JPT. Risk-of-bias VISualization (robvis): An R package and shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. (2020) 12:55–61. doi: 10.1002/jrsm.1411
40. Kent, J, and Farrell, A-M. Risky bodies in the plasma bioeconomy: a feminist analysis. Body Soc. (2015) 21:29–57. doi: 10.1177/1357034X13520331
41. Sivakaanthan, A, Swain, F, Pahn, G, Goodison, K, Gutta, N, Holdsworth, R, et al. Transfusion-related acute lung injury (TRALI): a retrospective review of reported cases in Queensland, Australia over 20 years. Blood Transfus. (2022) 20:454–64. doi: 10.2450/2022.0020-22
42. Hepburn, IS. Pregnancy-associated liver disorders. Dig Dis Sci. (2008) 53:2334–58. doi: 10.1007/s10620-007-0167-9
43. Rajasri, A, Srestha, R, and Mitchell, J. Acute fatty liver of pregnancy (AFLP)–an overview. J Obstet Gynaecol. (2007) 27:237–40. doi: 10.1080/01443610701194705
44. Wang, H-Y, Jiang, Q, Shi, H, Xu, Y-Q, Shi, A-C, Sun, Y-L, et al. Effect of caesarean section on maternal and foetal outcomes in acute fatty liver of pregnancy: a systematic review and meta-analysis. Sci Rep. (2016) 6:28826. doi: 10.1038/srep28826
45. Maternal-Fetal Medicine Committee CS of O and GLi, P, Chen, Y, Zhang, W, and Yang, H. CSOG MFM committee guideline: clinical management guidelines for acute fatty liver of pregnancy in China (2021). Matern-fetal med. Chin Med J. (2021) 3:238–45. doi: 10.1097/FM9.0000000000000121
46. Hassanein, TI, Schade, RR, and Hepburn, IS. Acute-on-chronic liver failure: extracorporeal liver assist devices. Curr Opin Crit Care. (2011) 17:195–203. doi: 10.1097/MCC.0b013e328344b3aa
47. Krishnan, RG, and Coulthard, MG. Minimising changes in plasma calcium and magnesium concentrations during plasmapheresis. Pediatr Nephrol. (2007) 22:1763–6. doi: 10.1007/s00467-007-0549-4
48. Goel, A, Zachariah, U, Daniel, D, and Eapen, CE. Growing evidence for survival benefit with plasma exchange to treat liver failure. J Clin Exp Hepatol. (2023) 13:1061–73. doi: 10.1016/j.jceh.2023.06.002
49. Thomas, L, Chandran, J, Goel, A, Jacob, E, Chacko, B, Subramani, K, et al. Improving transplant-free survival with low-volume plasma exchange to treat children with rodenticide induced hepatotoxicity. J Clin Exp Hepatol. (2023) 13:252–8. doi: 10.1016/j.jceh.2022.10.013
50. Kumar, SE, Goel, A, Zachariah, U, Nair, SC, David, VG, Varughese, S, et al. Low volume plasma exchange and low dose steroid improve survival in patients with alcohol-related acute on chronic liver failure and severe alcoholic hepatitis-preliminary experience. J Clin Exp Hepatol. (2022) 12:372–8. doi: 10.1016/j.jceh.2021.07.010
51. Choudhury, A, Kumar, M, Sharma, BC, Maiwall, R, Pamecha, V, Moreau, R, et al. Systemic inflammatory response syndrome in acute-on-chronic liver failure: relevance of “golden window”: a prospective study. J Gastroenterol Hepatol. (2017) 32:1989–97. doi: 10.1111/jgh.13799
Keywords: acute fatty liver of pregnancy, plasmapheresis, plasma exchange, safety, efficacy, mortality, meta-analysis, biochemical improvement
Citation: Siwatch S, De A, Kaur B, Lamba DS, Kaur S, Singh V and Periyasamy AG (2024) Safety and efficacy of plasmapheresis in treatment of acute fatty liver of pregnancy—a systematic review and meta-analysis. Front. Med. 11:1433324. doi: 10.3389/fmed.2024.1433324
Edited by:
Vijeta Sharma, Hackensack Meridian Health, United StatesReviewed by:
Virginia Sedeno Monge, Popular Autonomous University of the State of Puebla, MexicoSoumya Jaladi, Texas Tech University Health Sciences Center El Paso, United States
Copyright © 2024 Siwatch, De, Kaur, Lamba, Kaur, Singh and Periyasamy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sujata Siwatch, c2l3YXRjaDFAeWFob28uY29t
 Sujata Siwatch
Sujata Siwatch Arka De
Arka De Bandhanjot Kaur
Bandhanjot Kaur Divjot Singh Lamba
Divjot Singh Lamba Simarpreet Kaur4
Simarpreet Kaur4 Aravind Gandhi Periyasamy
Aravind Gandhi Periyasamy