
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 06 November 2023
Sec. Hepatobiliary Diseases
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1294425
Background: Metabolic dysfunction-associated steatotic liver disease (MASLD) and type 2 diabetes frequently co-occur, imposing a tremendous medical burden. A convenient and effective MASLD indicator will be beneficial to the early diagnosis of disease. In the clinical laboratory, the neutrophil-to-lymphocyte ratio (NLR) is a readily accessible hematological marker. This study designed to determine the relation between the NLR and MASLD in type 2 diabetes patients.
Methods: Data from 1,151 type 2 diabetes inpatients without infections, malignancy or hematological diseases who were recruited from 2016 through 2022 were analyzed in the retrospective study. The patients were stratified into NLR tertiles (total population: high NLR level > 2.18; middle NLR level: 1.58–2.18; low NLR level < 1.58), with additional subgroup stratification by sex (men: high NLR level > 2.21; middle NLR level: 1.60–2.21; and low NLR level < 1.60; women: high NLR level > 2.12; middle NLR level: 1.53–2.12; and low NLR level < 1.53). After adjusting for confounders (age, sex, weight, Glu, ALT and TG) associated with MASLD, the odds ratio (OR) and the corresponding 95% confidence interval (CI) of the NLR were obtained by using a binary logistic regression analysis to verify the correlation between the NLR and MASLD.
Results: Compared to non-MASLD patients, MASLD patients had higher weight, blood glucose, insulin and C-peptide, worse liver function (higher ALT and GGT), lower HDL (all p < 0.05), and lower NLR (p < 0.001). The prevalence of MASLD was 43.75% (high NLR level), 55.21% (middle NLR level) and 52.22% (low NLR level) (p < 0.05). Compared to those of the high NLR level, the adjusted ORs and 95% CIs of the middle and low NLR levels were 1.624 (95% CI: 1.141–2.311) and 1.456 (95% CI: 1.025–2.068), for all subjects, while they were 1.640 (95% CI: 1.000–2.689) and 1.685 (95% CI: 1.026–2.766), for men.
Conclusion: A low NLR is associated with a greater risk of MASLD.
Recently, a multi-society Delphi consensus statement published in 2023 that proposed the new term: metabolic dysfunction-associated steatotic liver disease (MASLD). MASLD describes liver disease associated with metabolic abnormalities, which is based on hepatic steatosis, and one of the five criteria, BMI ≥25 kg/m2 (≥23 kg/m2 in Asian) or waist circumference > 94 cm in men, >80 cm in women, or ethnicity adjusted; Fasting serum glucose ≥100 mg/dL (≥5.6 mmol/L) or 2-h post-load glucose level ≥ 140 mg/dL (≥7.8 mmol/L) or HbA1c ≥5.7% or on specific drug treatment; Blood pressure ≥ 130/85 mmHg or specific drug treatment; Plasma triglycerides ≥150 mg/dL (≥1.70 mmol/L) or specific drug treatment; or Plasma HDL cholesterol <40 mg/dL (<1.0 mmol/L) for men and < 50 mg/dL (<1.3 mmol/L) for women or specific drug treatment (1). MASLD is widely recognized as the most prevalent chronic liver disease, which affects around 30% of the global population (2). The prevalence of MASLD in type 2 diabetes patients is approximately 65% (3). Additionally, previous researches have demonstrated that the prevalence of MASLD shows a remarkable sex disparity, with higher risk among men (4). The diagnostic methods of MASLD are liver biopsy, imaging examination and additional tests (5, 6), but these detection methods suffer from certain imperfections, such as higher price, exposure to trauma, significant complications and dependence on the operator. Ultrasound diagnosis sensitivity may be limited in mild steatosis but is considered adequate for moderate–severe steatosis (7, 8). The quantification accuracy of Controlled Attenuation Parameter (CAP) is limited (9, 10). Magnetic resonance (MR) demonstrates higher accuracy in identifying and quantifying intrahepatic fat but is generally more expensive (7, 11). Although the fatty liver index (FLI) and hepatic steatosis index (HSI) exhibit reasonable sensitivity and specificity, their use as diagnostic methods in clinical practice is not recommended at the present time (7, 12, 13). There is still a lack of convenient and useful markers to help people identify MASLD.
MASLD is a systemic metabolic disease with hepatic and systemic inflammation (5, 14, 15). Currently, there are few proven biological indicators associated with MASLD. Levels of alanine aminotransferase (ALT) are usually seen as a simple indictor for assessing the inflammation of liver. However, previous studies demonstrated that normal ALT levels do not guarantee absence of inflammatory damage to liver tissue, and elevated ALT levels do not necessarily indicate steatohepatitis (16, 17). Therefore, we wanted to explore new biomarkers related to MASLD.
The neutrophil-to-lymphocyte ratio (NLR) is a major inflammatory marker that receiving more and more attention globally, and it has the advantage of being inexpensive and easily accessible over other methods. The NLR is a sensitive indicator of the body’s inflammatory status (18, 19), and numerous studies have suggested that the NLR is correlated with the prognosis of lung cancer (20), hepatocellular carcinoma (21) and other tumors (22), the occurrence of myocardial infarction (23) and sepsis (24), and the severity of COVID-19 (18, 25, 26). Furthermore, there is a sex difference in the NLR values among Chinese adults (27, 28). However, it has not yet been substantiated that the association between the NLR and MASLD.
Increasing researches have demonstrated that MASLD is linked with multiple metabolic disorders, including insulin resistance, obesity and abnormal glucose metabolism (29). MASLD and type 2 diabetes frequently occur together (3). Therefore, our study was to explore the relation between peripheral NLR values and MASLD in Chinese type 2 diabetes patients.
We set up a database of type 2 diabetes inpatients at the Shandong Provincial Hospital Affiliated to Shandong First Medical University who were recruited between January 1, 2016, and December 31, 2022. All subjects in this study were type 2 diabetes patients. MASLD was identified by ultrasonographic confirmation of hepatic steatosis, which was based on a multi-society Delphi consensus statement (1). The exclusion criteria are mentioned below: 1. Patients aged below 18 years or above 80 years; 2. Patients with concomitant other liver disease, including chronic viral hepatitis, hepatocellular carcinoma, drug-induced liver injury and autoimmune liver disease; 3. Patients with a history of malignancy or hematological diseases before the study; 4. Patients with acute or chronic infections; 5. Patients with history of severe renal insufficiency; 6. Patients whose clinical and laboratory data are insufficient. Finally, 1,151 patients were eligible for enrollment.
The protocol was approved by the Ethics Committee of the Shandong Provincial Hospital Affiliated to Shandong First Medical University (SWYX: NO. 2023–230) and was designed in accordance with the Helsinki Declaration. No informed consent was needed owing to the retrospective noninterventional study design.
The study parameters included age, diastolic blood pressure (DBP), systolic blood pressure (SBP), weight, BMI, and waist circumference (WC). We also collected the laboratory test indicators: Glu (glucose), Ins (insulin), C-Peptide (C-P), glycated hemoglobin (HbA1c), alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transpeptidase (GGT), serum creatinine (SCr), serum uric acid (SUA), white blood cell (WBC), red blood cell (RBC), lymphocyte (L), monocyte (M), neutrophil (N), NLR (the NLR was the number of neutrophils divided by the number of lymphocytes), hemoglobin (Hb), platelet (PLT), blood lipid indicators: total cholesterol (TC), triglycerides (TG), high-density lipoprotein-cholesterol (HDL-c), low-density lipoprotein-cholesterol (LDL-c), and admission reasons. Body mass index (BMI) was the weight in kilograms divided by height in meters squared.
Hepatic steatosis was diagnosed by ultrasonic imaging. Ultrasound liver testing was carried out by experienced radiologists. The standard of hepatic steatosis by abdominal ultrasound referred to the standardized criteria established by the Chinese Society of Hepatology, Chinese Medical Association (a 2018 update): diffuse enhancement of near-field echo in the liver, gradual attenuation of far-field echo and intrahepatic ductal structure blurring (30). According to the hepatic steatosis grading proposed by the Chinese Society of Hepatology (31), the attenuation degree of echo attenuation in the posterior field, the intensities of hepatic dotted echoes, and the clarity of intrahepatic portal vein into I (low), II (intermediate), and III (high). The posterior-field echo attenuation in fatty liver patients was further graded: degree I, attenuated by <1/3; degree II, attenuated by 1/3–2/3; and degree III, attenuated by >2/3.
Continuous variables were represented as the mean ± standard deviation (SD) if normal distributed, and nonnormally distributed continuous variables were represented by the median (IQR). Categorical variables are presented with frequency distributions (n, %). For the comparison between normal and MASLD groups, we used Kruskal–Wallis analysis or chi-square tests. Participants were classified into NLR tertiles for the total study population (high NLR level > 2.18; middle NLR level: 1.58–2.18; low NLR level < 1.58), for men (high NLR level > 2.21; middle NLR level: 1.60–2.21; low NLR level < 1.60) and for women (high NLR level > 2.12; middle NLR level: 1.53–2.12; low NLR level < 1.53), with the first tertile representing the highest NLR values and the third tertile representing the lowest NLR values. Logistic regression was employed to identify the relation between the risk of MASLD and NLR values. The high NLR level served as the reference category. Both unadjusted and adjusted models were analyzed. Statistical analyses were performed using SPSS version 25.0.
The study population contained 1,151 hospitalized type 2 diabetes patients, including 634 men (55.08%) and 517 women (44.92%). See Figure 1 for the study flow diagram. Among the patients, there were 580 MASLD patients and 571 non-MASLD patients. Table 1 lists baseline characteristics. Compared to non-MASLD patients, MASLD patients showed higher weight, blood glucose, insulin and C-peptide levels, worse liver function (higher ALT and GGT), and lower HDL (all p < 0.05). Additionally, the NLR in the MASLD group (1.98 ± 0.92) was lower than that in the non-MASLD group (2.26 ± 1.53) (p < 0.001). The reasons for hospitalizations in our cohort were type 2 diabetes (82.62%), coronary heart disease (1.48%), cerebral infarction (1.30%), osteoporosis (1.22%), hypertension (0.96%) and others (12.42%). The level of NLR stratification in type 2 diabetes patients is presented in Table 2. The prevalence of MASLD was 43.75% (high NLR level), 55.21% (middle NLR level) and 52.22% (low NLR level) (p < 0.05). The NLR in the middle and low NLR levels were significantly higher than those in the high NLR level.
Table 3 presents the outcomes of the logistic regression. Compared with the high NLR level, the prevalence of MASLD was grossly elevated in the middle and low NLR levels. Compared to the high NLR level, the ORs and 95% CIs of the middle and low NLR levels were 1.585 (95% CI: 1.192–2.107) and 1.405 (95% CI: 1.057–1.867). After adjusting for the clinical variables (age, sex, weight, Glu, ALT, TG) which were demonstrated to be related to MASLD in prior studies (32–34), the NLR remained an independent risk factor for MASLD, and decreased NLR values were related to a higher risk of MASLD. The adjusted ORs and 95% CIs of the middle and low NLR levels vs. the high NLR level were1.624 (95% CI: 1.141–2.311) and 1.456 (95% CI: 1.025–2.068). This suggested that the risk of MASLD in the middle and low NLR levels was 1.624 and 1.456 times higher than that in the high NLR level.
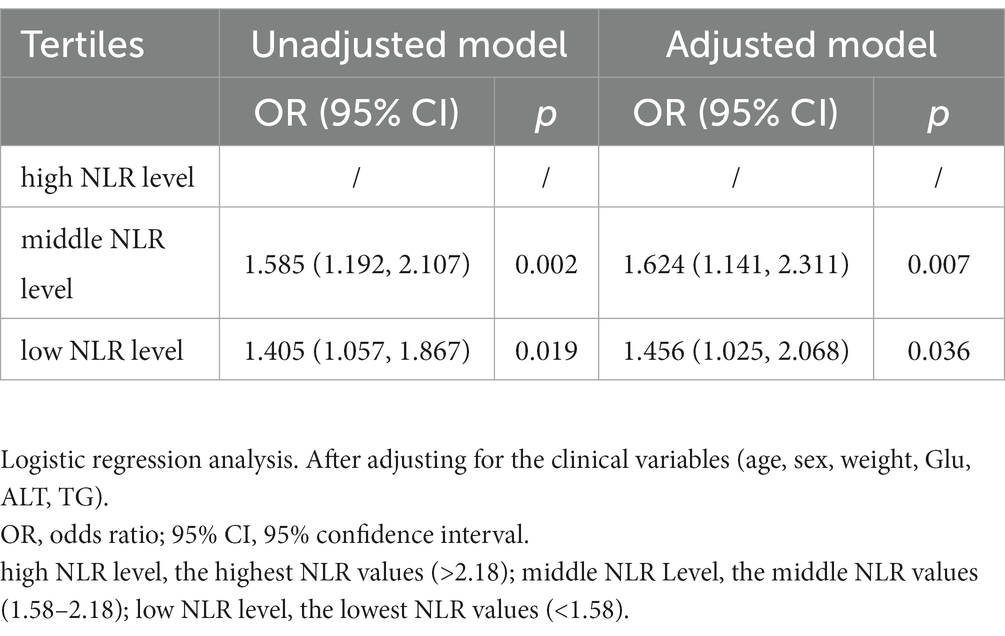
Table 3. Unadjusted and adjusted odds ratios of the NLR tertiles for the risk of MASLD in participants.
To verify whether sex differences in the correlation between NLR and MASLD, we further investigated a subgroup analysis by sex (Table 4). The NLR values in the separate MASLD groups of men and women were both higher than those in the non-MASLD group (p < 0.05). The stratification of the NLR in men and women type 2 diabetes patients is presented in Table 5. The prevalence rate of MASLD showed a significantly increasing trend in men: 45.02% (high NLR level), 56.60% (middle NLR level), and 57.82% (low NLR level), (p < 0.05). No significant increase in women [43.60% (high NLR level), 51.45% (middle NLR level), and 45.93% (low NLR level) (p = 0.326)] was found. The outcomes of the logistic regression in men and women are displayed in Table 6. Compared to men with a high NLR, the prevalence of MASLD was significantly elevated in men with a middle or low NLR. Compared with the high NLR level, the ORs and 95% CIs of the middle and low NLR levels in men were 1.593 (95% CI: 1.085–2.338) and 1.674 (95% CI: 1.139–2.460). After adjusting for the clinical variables (age, weight, Glu, ALT, TG), the NLR remained an independent risk factor for MASLD in men. The adjusted ORs and 95% CIs of the middle and low NLR levels vs. those of the high NLR level were 1.640 (95% CI: 1.000–2.689) and 1.685 (95% CI: 1.026–2.766). This suggested that the risk of MASLD in the middle and low NLR levels was 1.640 and 1.685 times higher than that in the high NLR level. The NLR was not an independent risk factor for MASLD in women.
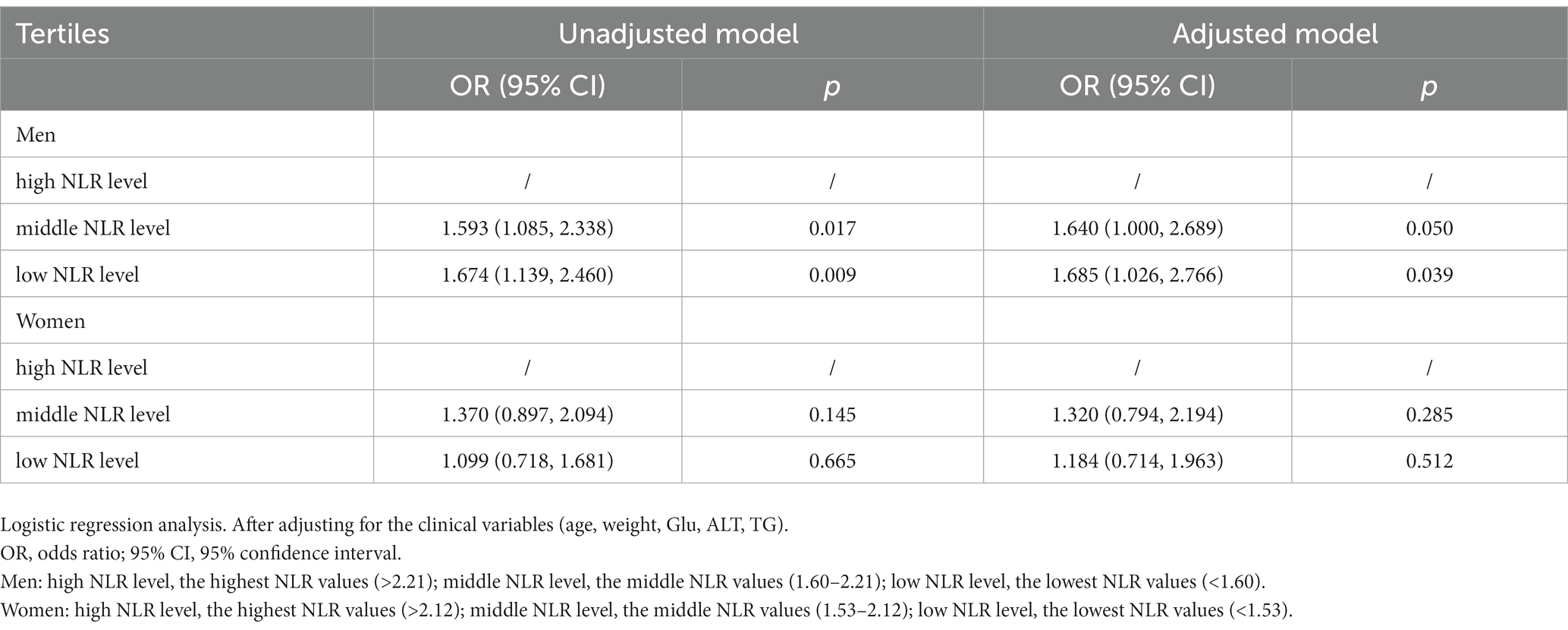
Table 6. Unadjusted and adjusted odds ratios of the NLR tertiles for the risk of MASLD among men and women.
This retrospective cross-sectional study showed that the NLR was independently, significantly and inversely related to the prevalence of MASLD in type 2 diabetes patients. The study is the first to reveal the relation between the NLR and the risk of MASLD in type 2 diabetes patients.
MASLD is frequently accompanied by increased inflammation (14). Generally, the NLR increases with the initiation and progression of inflammation (19). However, a low NLR was associated with MASLD in the present study. Several possible mechanistic reasons are provided below. First, the low level of neutrophils might be a major factor in oxidative stress in MASLD. Recent animal experimental studies have shown that the inhibition of myeloperoxidase (MPO) can induce oxidative stress (35). Myeloperoxidase is present in primary azurophilic granules of neutrophils (36). Oliviero et al. confirmed a significant positive association between neutrophil proportion and myeloperoxidase in certain types of patients (children with gastroesophageal reflux and asthma-like symptoms) (37). This may suggest that the low level of neutrophils may be accompanied by low MPO expression. Therefore, the low level of neutrophils may play a key role in oxidative stress. Oxidative stress can consume energy and break down DNA, lipids and proteins by impairing mitochondrial function, leading to hepatic inflammation and fibrosis (38). One of the key mechanisms of MASLD is oxidative stress (39), and the adaptive immune reactions induced by oxidative stress play relevant roles in the evolution of MASLD and other diseases toward fibrosis (40). Therefore, a low level of neutrophils has been implicated in the pathogenesis of MASLD. Second, B and T lymphocytes induce the development and progression of MASLD. New evidence suggests that obesity-induced inflammation of visceral adipose tissue can cause glucose intolerance and systemic insulin resistance, and B and T lymphocytes are involved in this process (41, 42). B lymphocytes have a direct effect on the activation of hepatic macrophages and hepatic stellate cells. B lymphocytes stimulate inflammation and fibrosis by multiple interactions with T lymphocytes and hematopoietic stem cells, suggesting that the accumulation of B and T lymphocytes is related to more severe lobular inflammation and enhanced fibrosis (40, 43, 44). Since insulin resistance, inflammation and liver fibrosis are related to the occurrence and development of MASLD (45, 46), we believe that the increase in B and T lymphocytes is related to the onset of MASLD. Third, neutrophils can interact with all kinds of surrounding cell types in the later stage of inflammation and then produce anti-inflammatory lipid mediators, such as lipoxins and resolvins. These lipid mediators inhibit neutrophil activation and recruitment (47). Therefore, we believe that the anti-inflammatory ability of the body may decrease when the NLR is low.
The results demonstrate that in the general population, and particularly in men, a decreased NLR is a risk factor for MASLD, but this association is not significant in women. These effects have been possibly attributed to the protective effects of estrogen: a meta-analysis revealed that the risk of developing MASLD was lower for women than for men (4, 48, 49). Estrogen seems to possess antiadipogenic, antioxidant and antifibrotic properties in the liver. Estrogen increases the expression of miRNA-29a and decreases CCL4 induction in the liver, which may inhibit hepatic steatosis and hepatic fibrosis (50). From experiments with animal models, it is known that estrogen can inhibit astrocyte activation and the formation of fibers (51). Estradiol is an endogenous inhibitor of fibrinolysis that explains sex-related differences in the development of cirrhosis from hepatic fibrosis, and it retards the progression of disease in women (51). Estradiol may also reduce the production of proinflammatory cytokines and prevent macrophage accumulation; therefore, it has anti-inflammatory and antioxidative stress effects (52). Up to this point, we are still not sure of the reasons for this discrepancy, but it is probably due to the smaller sample size of this study, and all patients with type 2 diabetes may conceal some of the evidence.
In addition, this study has several limitations. Firstly, the research was a single-center, cross-sectional study using retrospective data collection. The findings of this study might not be representative of other regions. Therefore, multicenter large-scale prospective studies are required to verify the correlation between the peripheral NLR and MASLD in type 2 diabetes patients. Secondly, the mechanism of the relationship between a decreased NLR and MASLD remains unclear, and we cannot exclude other possible confounders that can result in a decreased NLR. Thirdly, the inflammatory markers, phenotyping of patients and steatosis stratification were not collected for this study, but we will take those variables into account in future studies. Fourthly, this study used abdominal ultrasonography rather than liver biopsy to determine hepatic steatosis. At present, liver ultrasound is still the first-choice imaging diagnostic tool for hepatic steatosis (8, 53). It has a high sensitivity (85%) and specificity (93%) for the diagnosis of moderate-to-severe hepatic steatosis (53). When considering all degrees of steatosis, sensitivity ranges from53.3 to 66.6% and specificity ranges from 77.0 to 93.1% (54).
This study demonstrates that a low NLR is related to the risk of MASLD. This means that a low NLR in type 2 diabetes patients is a potential clinical indicator of MASLD. The NLR is an inexpensive, easily available biomarker that is convenient to promote even in remote regions and is not dependent on the operator, and the NLR can help identify MASLD when people are checking routine blood tests and making interventions. We will investigate the association between the NLR and MASLD in larger population and cohort studies in the near future.
In conclusion, based on our retrospective cross-sectional study, a low NLR may portend increased susceptibility in MASLD patients. The independent association between the NLR and MASLD was proven by a binary logistic regression model. The NLR appears to be a potentially reliable and inexpensive biomarker for the identification of MASLD.
The data analyzed in this study is subject to the following licenses/restrictions: The datasets that support the findings of the current study are available from the corresponding author upon reasonable request. Requests to access these datasets should be directed to aGFuanVubWluZ0BzZGZtdS5lZHUuY24=.
The studies involving humans were approved by the Ethics Committee of the Shandong Provincial Hospital Affiliated to Shandong First Medical University (SWYX: NO. 2023-230). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because no informed consent was needed because of the retrospective noninterventional study design.
NZ: Writing – original draft, Conceptualization, Methodology. YS: Supervision, Writing – review & editing. CZ: Writing – original draft. KW: Writing – original draft. JH: Conceptualization, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (grant number 82270922), Natural Science Foundation of Shandong Province (grant number ZR2020ZD14, ZR202211160178) and National Key Research and Development Program of China (grant number 2022YFA0806100).
We sincerely appreciate Yidu Cloud (Beijing) Technology Co. Ltd., China for providing data and for training in the use of the datasets.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Rinella, ME, Lazarus, JV, Ratziu, V, Francque, SM, Sanyal, AJ, Kanwal, F, et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. (2023) 101133. doi: 10.1016/j.jhep.2023.06.003
2. Chan, WK, Chuah, KH, Rajaram, RB, Lim, LL, Ratnasingam, J, and Vethakkan, SR. Metabolic dysfunction-associated Steatotic Liver disease (MASLD): a state-of-the-art review. J Obes Metab Syndr. (2023) 32:197–213. doi: 10.7570/jomes23052
3. Forlano, R, Stanic, T, Jayawardana, S, Mullish, BH, Yee, M, Mossialos, E, et al. A prospective study on the prevalence of MASLD in people with type-2 diabetes in the community. Cost effectiveness of screening strategies. Liver Int. (2023) 1–11. doi: 10.1111/liv.15730
4. Riazi, K, Azhari, H, Charette, JH, Underwood, FE, King, JA, Afshar, EE, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. (2022) 7:851–61. doi: 10.1016/S2468-1253(22)00165-0
5. Eslam, M, Newsome, PN, Sarin, SK, Anstee, QM, Targher, G, Romero-Gomez, M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. (2020) 73:202–9. doi: 10.1016/j.jhep.2020.03.039
6. Kopec, KL, and Burns, D. Nonalcoholic fatty liver disease: a review of the spectrum of disease, diagnosis, and therapy. Nutr Clin Pract. (2011) 26:565–76. doi: 10.1177/0884533611419668
7. Ciardullo, S, Vergani, M, and Perseghin, G. Nonalcoholic fatty Liver disease in patients with type 2 diabetes: screening, diagnosis, and treatment. J Clin Med. (2023) 12:12175597. doi: 10.3390/jcm12175597
8. Liver EAftSot . EASL clinical practice guidelines on non-invasive tests for evaluation of liver disease severity and prognosis −2021 update. J Hepatol. (2021) 75:659–89. doi: 10.1016/j.jhep.2021.05.025
9. Caussy, C, Alquiraish, MH, Nguyen, P, Hernandez, C, Cepin, S, Fortney, LE, et al. Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology. (2018) 67:1348–59. doi: 10.1002/hep.29639
10. Rinella, ME, Neuschwander-Tetri, BA, Siddiqui, MS, Abdelmalek, MF, Caldwell, S, Barb, D, et al. AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. (2023) 77:1797–835. doi: 10.1097/hep.0000000000000323
11. Ajmera, V, and Loomba, R. Imaging biomarkers of NAFLD, NASH, and fibrosis. Mol Metab. (2021) 50:101167. doi: 10.1016/j.molmet.2021.101167
12. Bedogni, G, Bellentani, S, Miglioli, L, Masutti, F, Passalacqua, M, Castiglione, A, et al. The fatty Liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. (2006) 6:33. doi: 10.1186/1471-230x-6-33
13. Lee, JH, Kim, D, Kim, HJ, Lee, CH, Yang, JI, Kim, W, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. (2010) 42:503–8. doi: 10.1016/j.dld.2009.08.002
14. Tilg, H, and Effenberger, M. From NAFLD to MAFLD: when pathophysiology succeeds. Nat Rev Gastroenterol Hepatol. (2020) 17:387–8. doi: 10.1038/s41575-020-0316-6
15. Grander, C, Grabherr, F, and Tilg, H. Non-alcoholic fatty liver disease: pathophysiological concepts and treatment options. Cardiovasc Res. (2023) 119:1787–98. doi: 10.1093/cvr/cvad095
16. Verma, S, Jensen, D, Hart, J, and Mohanty, SR. Predictive value of ALT levels for non-alcoholic steatohepatitis (NASH) and advanced fibrosis in non-alcoholic fatty liver disease (NAFLD). Liver Int. (2013) 33:1398–405. doi: 10.1111/liv.12226
17. Senior, JR . Alanine aminotransferase: a clinical and regulatory tool for detecting liver injury-past, present, and future. Clin Pharmacol Ther. (2012) 92:332–9. doi: 10.1038/clpt.2012.108
18. Toori, KU, Qureshi, MA, Chaudhry, A, and Safdar, MF. Neutrophil to lymphocyte ratio (NLR) in COVID-19: a cheap prognostic marker in a resource constraint setting. Pak J Med Sci. (2021) 37:1435–9. doi: 10.12669/pjms.37.5.4194
19. Huang, Z, Fu, Z, Huang, W, and Huang, K. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: a meta-analysis. Am J Emerg Med. (2020) 38:641–7. doi: 10.1016/j.ajem.2019.10.023
20. Mandaliya, H, Jones, M, Oldmeadow, C, and Nordman, II. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl Lung Cancer Res. (2019) 8:886–94. doi: 10.21037/tlcr.2019.11.16
21. Mouchli, M, Reddy, S, Gerrard, M, Boardman, L, and Rubio, M. Usefulness of neutrophil-to-lymphocyte ratio (NLR) as a prognostic predictor after treatment of hepatocellular carcinoma. Review article Annals hepatol. (2021) 22:100249. doi: 10.1016/j.aohep.2020.08.067
22. Guthrie, GJ, Charles, KA, Roxburgh, CS, Horgan, PG, McMillan, DC, and Clarke, SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. (2013) 88:218–30. doi: 10.1016/j.critrevonc.2013.03.010
23. Azab, B, Zaher, M, Weiserbs, KF, Torbey, E, Lacossiere, K, Gaddam, S, et al. Usefulness of neutrophil to lymphocyte ratio in predicting short– and long-term mortality after non-ST-elevation myocardial infarction. Am J Cardiol. (2010) 106:470–6. doi: 10.1016/j.amjcard.2010.03.062
24. Liu, X, Shen, Y, Wang, H, Ge, Q, Fei, A, and Pan, S. Prognostic significance of neutrophil-to-lymphocyte ratio in patients with Sepsis: a prospective observational study. Mediat Inflamm. (2016) 2016:8191254–8. doi: 10.1155/2016/8191254
25. Li, X, Liu, C, Mao, Z, Xiao, M, Wang, L, Qi, S, et al. Predictive values of neutrophil-to-lymphocyte ratio on disease severity and mortality in COVID-19 patients: a systematic review and meta-analysis. Crit Care. (2020) 24:647. doi: 10.1186/s13054-020-03374-8
26. Yang, AP, Liu, JP, Tao, WQ, and Li, HM. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. (2020) 84:106504. doi: 10.1016/j.intimp.2020.106504
27. Wang, J, Zhang, F, Jiang, F, Hu, L, Chen, J, and Wang, Y. Distribution and reference interval establishment of neutral-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), and platelet-to-lymphocyte ratio (PLR) in Chinese healthy adults. J Clin Lab Anal. (2021) 35:e23935. doi: 10.1002/jcla.23935
28. Luo, H, He, L, Zhang, G, Yu, J, Chen, Y, Yin, H, et al. Normal reference intervals of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, and systemic immune inflammation index in healthy adults: a large multi-center study from Western China. Clin Lab. (2019) 65:180715. doi: 10.7754/Clin.Lab.2018.180715
29. Heeren, J, and Scheja, L. Metabolic-associated fatty liver disease and lipoprotein metabolism. Mol Metab. (2021) 50:101238. doi: 10.1016/j.molmet.2021.101238
30. Klevens, RM, Morrison, MA, Nadle, J, Petit, S, Gershman, K, Ray, S, et al. National Workshop on fatty Liver and alcoholic Liver disease CSoH, Chinese Medical Association, fatty Liver expert committee, Chinese medical doctor association. Guidelines of prevention and treatment for nonalcoholic fatty liver disease: a 2018 update. Zhonghua Gan Zang Bing Za Zhi. (2018) 26:195–203. doi: 10.3760/cma.j.issn.1007-3418.2018.03.008
31. Huimin, X, Qingyu, Z, Mailin, C, Jianwei, H, Jianxin, L, Manwei, H, et al. An easy way to estimate the disgree of homogeneous fatty liver with ultrasonography. Chinese J Interventional Imaging and Therapy. (2008) 5:148–51. doi: 10.13929/j.1672-8475.2008.02.008
32. Gerges, SH, Wahdan, SA, Elsherbiny, DA, and El-Demerdash, E. Non-alcoholic fatty liver disease: an overview of risk factors, pathophysiological mechanisms, diagnostic procedures, and therapeutic interventions. Life Sci. (2021) 271:119220. doi: 10.1016/j.lfs.2021.119220
33. Juanola, O, Martinez-Lopez, S, Frances, R, and Gomez-Hurtado, I. Non-alcoholic fatty Liver disease: metabolic, genetic, epigenetic and environmental risk factors. Int J Environ Res Public Health. (2021) 18:18105227. doi: 10.3390/ijerph18105227
34. Amarapurkar, DN, Amarapurkar, AD, Patel, ND, Agal, S, Baigal, R, Gupte, P, et al. Nonalcoholic steatohepatitis (NASH) with diabetes: predictors of liver fibrosis. Ann Hepatol. (2006) 5:30–3. doi: 10.1016/s1665-2681(19)32036-8
35. Tavasoli, S, Eghtesadi, S, Vafa, M, Moradi-Lakeh, M, Sadeghipour, A, and Zarnani, AH. High dose pomegranate extract suppresses neutrophil myeloperoxidase and induces oxidative stress in a rat model of Sepsis. Int J Vitam Nutr Res. (2019) 89:271–84. doi: 10.1024/0300-9831/a000563
36. Aratani, Y . Myeloperoxidase: its role for host defense, inflammation, and neutrophil function. Arch Biochem Biophys. (2018) 640:47–52. doi: 10.1016/j.abb.2018.01.004
37. Sacco, O, Silvestri, M, Sabatini, F, Sale, R, Moscato, G, Pignatti, P, et al. IL-8 and airway neutrophilia in children with gastroesophageal reflux and asthma-like symptoms. Respir Med. (2006) 100:307–15. doi: 10.1016/j.rmed.2005.05.011
38. Chen, Z, Tian, R, She, Z, Cai, J, and Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic Biol Med. (2020) 152:116–41. doi: 10.1016/j.freeradbiomed.2020.02.025
39. Clare, K, Dillon, JF, and Brennan, PN. Reactive oxygen species and oxidative stress in the pathogenesis of MAFLD. J Clin Transl Hepatol. (2022) 10:939–46. doi: 10.14218/jcth.2022.00067
40. Bruzzi, S, Sutti, S, Giudici, G, Burlone, ME, Ramavath, NN, Toscani, A, et al. B2-lymphocyte responses to oxidative stress-derived antigens contribute to the evolution of nonalcoholic fatty liver disease (NAFLD). Free Radic Biol Med. (2018) 124:249–59. doi: 10.1016/j.freeradbiomed.2018.06.015
41. Osborn, O, and Olefsky, JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. (2012) 18:363–74. doi: 10.1038/nm.2627
42. Chatzigeorgiou, A, Karalis, KP, Bornstein, SR, and Chavakis, T. Lymphocytes in obesity-related adipose tissue inflammation. Diabetologia. (2012) 55:2583–92. doi: 10.1007/s00125-012-2607-0
43. Novobrantseva, TI, Majeau, GR, Amatucci, A, Kogan, S, Brenner, I, Casola, S, et al. Attenuated liver fibrosis in the absence of B cells. J Clin Invest. (2005) 115:3072–82. doi: 10.1172/JCI24798
44. Thapa, M, Chinnadurai, R, Velazquez, VM, Tedesco, D, Elrod, E, Han, JH, et al. Liver fibrosis occurs through dysregulation of MyD88-dependent innate B-cell activity. Hepatology. (2015) 61:2067–79. doi: 10.1002/hep.27761
45. Sakurai, Y, Kubota, N, Yamauchi, T, and Kadowaki, T. Role of insulin resistance in MAFLD. Int J Mol Sci. (2021) 22:22084156. doi: 10.3390/ijms22084156
46. Torre, P, Motta, BM, Sciorio, R, Masarone, M, and Persico, M. Inflammation and Fibrogenesis in MAFLD: role of the hepatic immune system. Front Med (Lausanne). (2021) 8:781567. doi: 10.3389/fmed.2021.781567
47. Wang, J, and Arase, H. Regulation of immune responses by neutrophils. Ann N Y Acad Sci. (2014) 1319:66–81. doi: 10.1111/nyas.12445
48. Zhou, YJ, Li, YY, Nie, YQ, Ma, JX, Lu, LG, Shi, SL, et al. Prevalence of fatty liver disease and its risk factors in the population of South China. World J Gastroenterol. (2007) 13:6419–24. doi: 10.3748/wjg.v13.i47.6419
49. Balakrishnan, M, Patel, P, Dunn-Valadez, S, Dao, C, Khan, V, Ali, H, et al. Women have a lower risk of nonalcoholic fatty Liver disease but a higher risk of progression vs men: a systematic review and Meta-analysis. Clin Gastroenterol Hepatol. (2021) 19:61–71.e15. doi: 10.1016/j.cgh.2020.04.067
50. Zhang, Y, Wu, L, Wang, Y, Zhang, M, Li, L, Zhu, D, et al. Protective role of estrogen-induced mi RNA-29 expression in carbon tetrachloride-induced mouse liver injury. J Biol Chem. (2012) 287:14851–62. doi: 10.1074/jbc.M111.314922
51. Yasuda, M, Shimizu, I, Shiba, M, and Ito, S. Suppressive effects of estradiol on dimethylnitrosamine-induced fibrosis of the liver in rats. Hepatology. (1999) 29:719–27. doi: 10.1002/hep.510290307
52. Shimizu, I, Kohno, N, Tamaki, K, Shono, M, Huang, HW, He, JH, et al. Female hepatology: favorable role of estrogen in chronic liver disease with hepatitis B virus infection. World J Gastroenterol. (2007) 13:4295–305. doi: 10.3748/wjg.v13.i32.4295
53. European Association for the Study of the L, European Association for the Study of D, European Association for the Study of O . EASL-EASD-EASO clinical practice guidelines for the Management of non-Alcoholic Fatty Liver Disease. Obes Facts. (2016) 9:65–90. doi: 10.1159/000443344
Keywords: metabolic dysfunction-associated steatotic liver disease, type 2 diabetes, neutrophil-to-lymphocyte ratio, logistic regression analysis, risk factor
Citation: Zhu N, Song Y, Zhang C, Wang K and Han J (2023) Association between the peripheral neutrophil-to-lymphocyte ratio and metabolic dysfunction-associated steatotic liver disease in patients with type 2 diabetes. Front. Med. 10:1294425. doi: 10.3389/fmed.2023.1294425
Received: 14 September 2023; Accepted: 25 October 2023;
Published: 06 November 2023.
Edited by:
Roberto Gramignoli, Karolinska Institutet (KI), SwedenReviewed by:
Lubomir Skladany, F. D. Roosevelt Teaching Hospital, SlovakiaCopyright © 2023 Zhu, Song, Zhang, Wang and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junming Han, aGFuanVubWluZ0BzZGZtdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.