
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 08 January 2024
Sec. Intensive Care Medicine and Anesthesiology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1278879
 Lu Wang1†
Lu Wang1† Wen Dai2†
Wen Dai2† Ruiyao Zhu1,3†
Ruiyao Zhu1,3† Tingting Long2
Tingting Long2 Zhaocai Zhang4
Zhaocai Zhang4 Zhenju Song5
Zhenju Song5 Sucheng Mu5
Sucheng Mu5 Shasha Wang1
Shasha Wang1 Huijuan Wang1
Huijuan Wang1 Jiaxi Lei1
Jiaxi Lei1 Jing Zhang1
Jing Zhang1 Wenfang Xia1
Wenfang Xia1 Guang Li1
Guang Li1 Wenwei Gao1
Wenwei Gao1 Handong Zou1
Handong Zou1 Yan Li2
Yan Li2 Liying Zhan1*
Liying Zhan1*Sepsis-induced cardiomyopathy (SIC) is characterized by high mortality and poor outcomes. This study aimed to explore the relationship between testosterone and soluble ST2 (sST2) and all-cause mortality in patients with SIC. Clinical data from SIC patients at Renmin Hospital of Wuhan University from January 2021 and March 2023 were reviewed. Serum testosterone and sST2 were measured at admission. Kaplan–Meier analysis and receiver operative characteristic curve (ROC) were used to estimate the predictive values of testosterone and sST2 on 28 days and 90 days mortality of SIC. A total of 327 male subjects with SIC were enrolled in this study. During the 28 days and 90 days follow-up, 87 (26.6%) and 103 deaths (31.5%) occurred, respectively. Kaplan–Meier analysis showed significantly higher 28 days and 90 days survival in patients with higher testosterone and decreased sST2 levels (p < 0.001). Testosterone, sST2, and N-terminal pro-B-type natriuretic peptide (NT-proBNP) were significantly associated with 28 days and 90 days mortality (p < 0.05). Partial correlation analysis showed strong positive correlation between testosterone and left ventricular ejection fraction (LVEF) (p < 0.001), and negative correlation between testosterone and sST2 (p < 0.001), high-sensitivity troponin I (hs-TnI) levels (p < 0.001) and smoke history (p < 0.01). The concentrations of sST2 were positively related with E/e′ ratio (p < 0.001), and negatively correlated with TAPSE (p < 0.001). The combination of testosterone and sST2 enhanced the prediction of both 28 days [area under the ROC curve (AUC), 0.805] and 90 days mortality (AUC, 0.833). Early serum testosterone and sST2 levels could predict mortality of SIC independently and jointly. Further research is needed to determine the utility of biochemical markers in identifying high-risk patients with SIC.
Sepsis remains the leading cause of mortality in intensive care units (ICUs) globally, resulting in 5 million deaths annually (1). Sepsis-induced cardiomyopathy (SIC) is a common and life-threatening complication of sepsis and manifests as left ventricular dysfunction, impaired ejection fraction and heart failure (2). The published incidence rate of SIC among septic patients is 28.0%–38.8% (3, 4). Despite the standardization of sepsis management, 70%–90% of mortality was reported in patients with SIC (5). Risk factors for developing SIC include elderly age, male sex, pre-existing heart failure, decreased systolic blood pressure, and elevated N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels (6–8). The underlying mechanisms of SIC may involve myocardial suppression factors, abnormal calcium regulation, mitochondrial injury, and production of reactive oxygen species (9).
Studies showed that the incidence and mortality in male patients were higher than those in female patients (10, 11). Disruption of testosterone levels has been linked to worse prognosis in critically ill patients (11). Lazzerini et al. reported testosterone levels during inflammatory activation in men may increase the risk of Torsades de Pointes (TdP) by contributing to the overall prolonging effect of inflammation on QTc, possibly due to increasing conversion of testosterone to estrogen (12). The deficiency of testosterone was considered a risk factor for adverse cardiovascular events (13). However, data evaluating the prognostic predictive values of testosterone in patients with SIC are limited, and large-scale multicenter analysis is necessary.
sST2 is a novel biomarker for risk stratification, prognosis assessment, and therapeutic guidance in heart failure over the past decades (14). Previous data indicate that sST2 levels correlated with severity and mortality of sepsis, especially in patients with greater cardiovascular impairment (15). Compared with NT-proBNP and troponin T, sST2 levels may be a stronger independent predictor to evaluate the severity of inflammatory cardiomyopathy (16, 17). However, findings on sST2 in sepsis have been ambiguous. The study of Yang et al. reported that sST2 levels were not associated with cardiac function in septic patients (18). Studies determining the relationship between sST2 and SIC to predict prognosis in affected patients should be conducted.
Therefore, this prospective study aimed to measure the potential value of serum testosterone and sST2 concentrations for mortality prediction and risk evaluation of mortality in patients with SIC.
This prospective study enrolled 1,083 patients with suspected sepsis in the Department of Critical Care Medicine (n = 736), General ICU in East Campus (n = 128), Cardiovascular ICU (n = 96), Cardiovascular surgical ICU (n = 101), and Emergency ICU (n = 22) of Renmin Hospital of Wuhan University between January 2021 and March 2023. After excluding 756 patients, 327 male patients were diagnosed with SIC. The patients were followed up until 90 days after ICU admission. The investigators collected data, then blinded statisticians analyzed the data. The study protocol was approved by the Ethics Committee of Renmin Hospital of Wuhan University (Study No. WDRY2021-K094). This study was conducted following the International Conference on Harmonization Good Clinical Practice guideline. All patients or their authorized representatives provided written informed consent.
The inclusion criteria were (1) diagnosis of SIC based on a previous study (19): meeting the diagnostic definition of sepsis-3 (1); (2) LVEF <50%, or plasma hs-TnI > 0.78 ng/mL (normal range, 0–0.78 ng/mL) or NT-proBNP >500 pg/mL; (3) male sex; and (4) age between 18 and 80 years. The exclusion criteria were (1) previous diagnosis of cancer; (2) previously existing grade III-IV heart failure (New York Heart Association Classification); (3) dropped out during the 90 days follow-up, excluding death; (4) patients with missing data.
Clinical information, including age, sex, body mass index (BMI), past medical history, including systolic (SBP) and diastolic blood pressure (DBP), were obtained from the electronic medical records. The values of white blood cells, serum creatinine, total bilirubin, NT-proBNP, hs-TnI, procalcitonin (PCT), C-reaction protein (CRP), serum amyloid A (SAA), and lactate were obtained from the laboratory reporting system. Furthermore, the scores of acute physiology and chronic health evaluation (APACHE) II and sequential organ failure assessment (SOFA), indicating the severity of illness and organ dysfunction/failure, were developed based on the above data.
After ICU admission, serum samples were immediately obtained and centrifuged, followed by storage at −80°C and thawed rapidly before test. Testosterone and sST2 levels in the baseline samples were measured using chemiluminescence (Siemens Atellica IM1600, Germany) and the immunofluorescence dry quantitative method i-CHROMATM (BodiTech, Guangxi, China), respectively.
Based on the distribution type of the measured variables, continuous variables were reported as mean and standard deviation (SD) or median and interquartile ranges (IQRs). Categorical variables were presented as frequencies and percentages. The Kolmogorov–Smirnov test was utilized to evaluate normally distributed values. Following the grouping method of Bidadkosh et al. (20), we grouped the whole population into T1 (lowest), T2 (middle) and T3 (highest) group based on tertiles of the values of testosterone and sST2. The chi-square test was utilized to compare dichotomous and categorical variables. The student’s t-test and Mann–Whitney U-test were employed to compare continuous data between groups, as appropriate. Moreover, the Kaplan–Meier method was used to calculate the survival time between groups. Univariate and multivariate Cox proportional hazards analysis was performed to evaluate the hazard ratio (HR) and 95% confidence intervals (CIs) for the association between testosterone or sST2 and mortality. To evaluate the relationship between testosterone and sST2 and clinical and echocardiographic parameters, Spearman’s rank correlation analyses were conducted with the “metadar” package in R statistical software. Receiver operating curve (ROC) analysis was performed to evaluate the sensitivity and specificity of testosterone and sST2 at different cut-off values, and the Youden index showed optimal cut-off values from the ROC for prognostic determination. A two-tailed p < 0.05 indicated statistical significance. SPSS software version 22.0, GraphPad Prism software version 9.0.0 and R version 4.2.3 statistical software were used for data filing and statistical analysis.
The study flowchart are shown in Figure 1. The clinical and demographic characteristics of all study participants are listed in Table 1; Supplementary Table S1. A total of 327 male SIC patients were identified. The median age and BMI of the patients were 61 years and 21.7 kg/m2, respectively. Additionally, 98 patients were smokers (30.0%), and 56 patients had previous myocardial infarction (MI) history (17.1%). Furthermore, 26.6% (n = 87) and 31.5% (n = 103) of the patients died during the 28 days and 90 days follow-up (Table 1). The median serum testosterone and sST2 levels were 133.9 (IQR, 69.3–200.0) ng/dL and 153.2 (IQR, 63.0–405.8) ng/mL, respectively. The participants were grouped based on their serum testosterone and sST2 levels. Specifically, lower testosterone levels were related to a higher proportion of smoking habits, 28 days mortality, and 90 days mortality. Moreover, serum testosterone levels were correlated with SBP, DBP, LVEF, hs-TnI, PCT, CRP, SAA, lactate, and sST2, as well as APACHE II and SOFA scores (all p < 0.01). Meanwhile, sST2 concentration was also significantly associated with 28 days and 90 days mortality, SOFA score, NT-proBNP, hs-TnI, PCT, SAA, and lactate levels (all p < 0.05).
The Kaplan–Meier analysis showed that the survival curves differed between groups. During the 28-day and 90-day follow-ups, patients with lower testosterone levels had significantly lower survival (p < 0.001, Figure 2A). The survival rate during the 28-day and 90-day follow-up was higher in the low tertiles of the sST2 group than in the middle and high tertiles of sST2 values (both p < 0.001, Figure 2B). Moreover, the univariate analyses showed that testosterone (HR, 0.994; 95% CI, 0.991–0.991, p < 0.001), sST2 (HR, 1.003; 95% CI, 1.002–1.004, p < 0.001), and NT-proBNP (HR, 1.000; 95% CI, 1.000–1.000; p < 0.001) levels were significantly associated with 28 days mortality (Table 2). In addition, BMI (HR, 1.084; 95% CI, 1.001–1.175, p = 0.048), testosterone (HR, 0.992; 95% CI, 0.989–0.995; p < 0.001), sST2 (HR 1.003; 95%CI, 1.002–1.003, p < 0.001), and NT-proBNP (HR, 1.000; 95%CI, 1.000–1.000, p < 0.001) were related with 90 days mortality (Table 2). The multivariate analysis showed that testosterone, sST2 and NT-proBNP levels were independently associated with 28-day and 90-day mortality (Table 2).
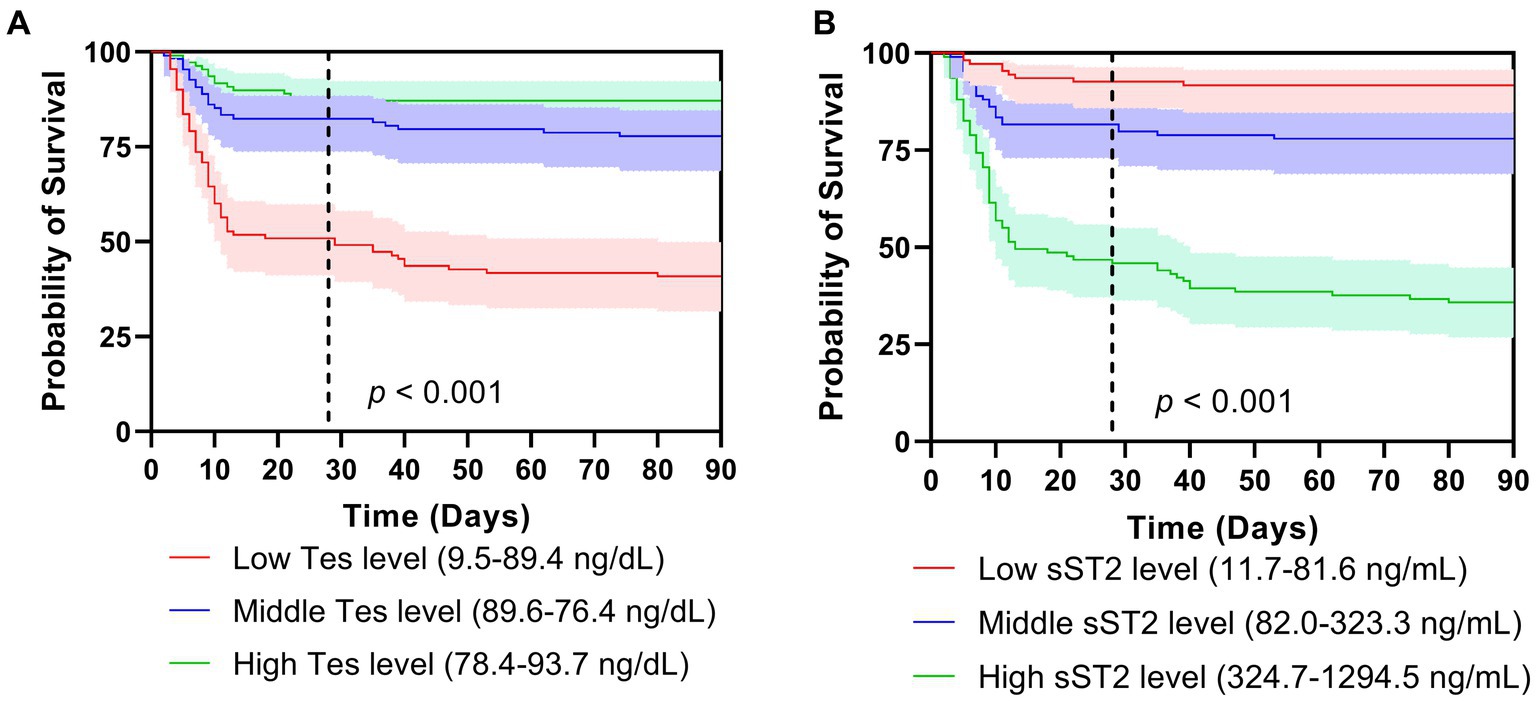
Figure 2. Survival curves of tertiles of testosterone (A) and sST2 (B) levels on mortality after 28 days (A) and 90 days follow-ups. Tes, testosterone.
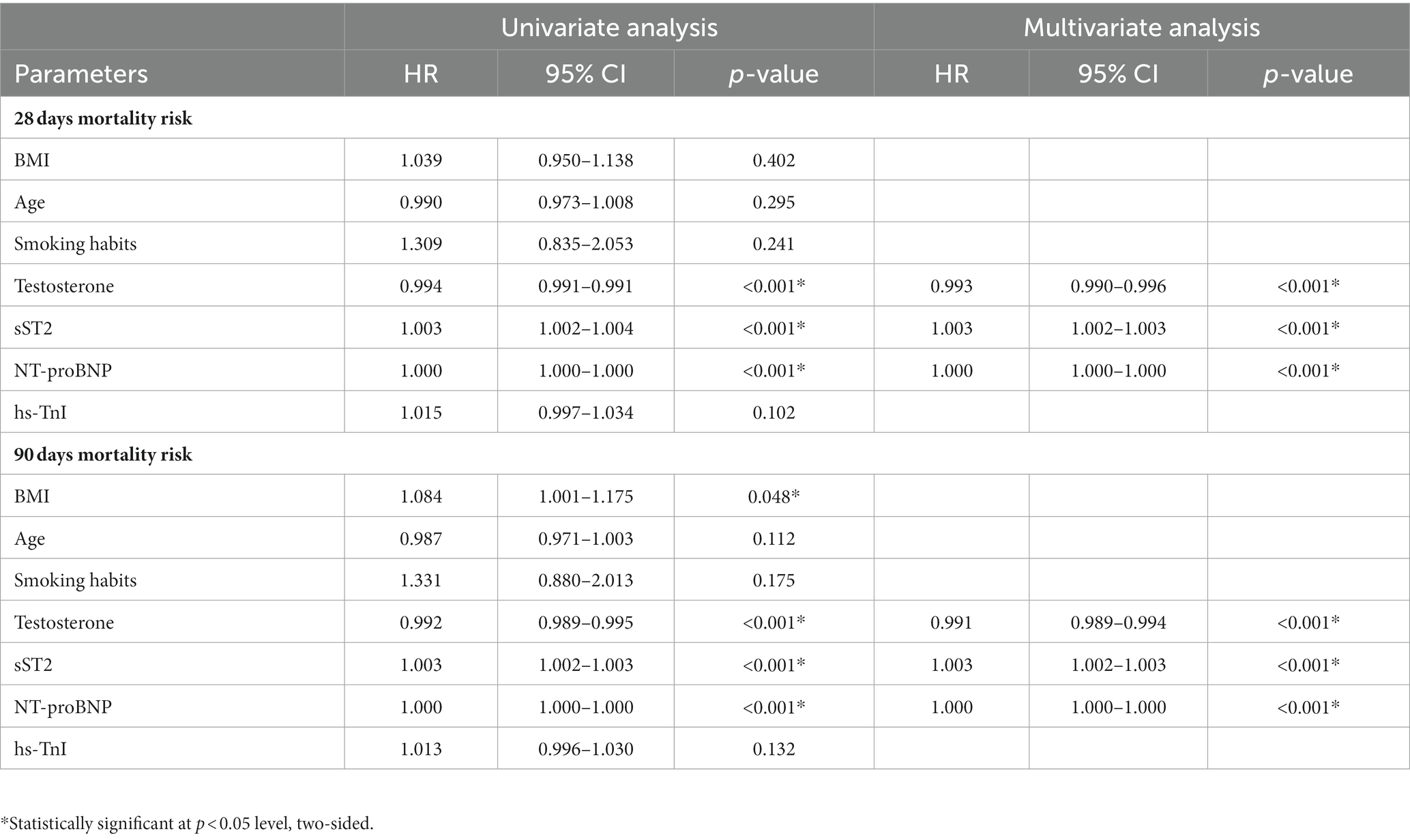
Table 2. Univariate and multivariate Cox regression model for 28 days and 90 days mortality in patients with SIC.
Results from the covariance analysis showing partial correlation coefficients between testosterone and sST2 and the clinical and echocardiographic parameters are presented in Figure 3. A strong positive correlation was found between testosterone and LVEF (p < 0.001), and a negative correlation between testosterone and sST2 (p < 0.001), hs-TnI levels (p < 0.001) and smoke history (p < 0.01). Meanwhile, the sST2 concentrations were positively related with E/e′ ratio (p < 0.001), and negatively correlated with TAPSE (p < 0.001).
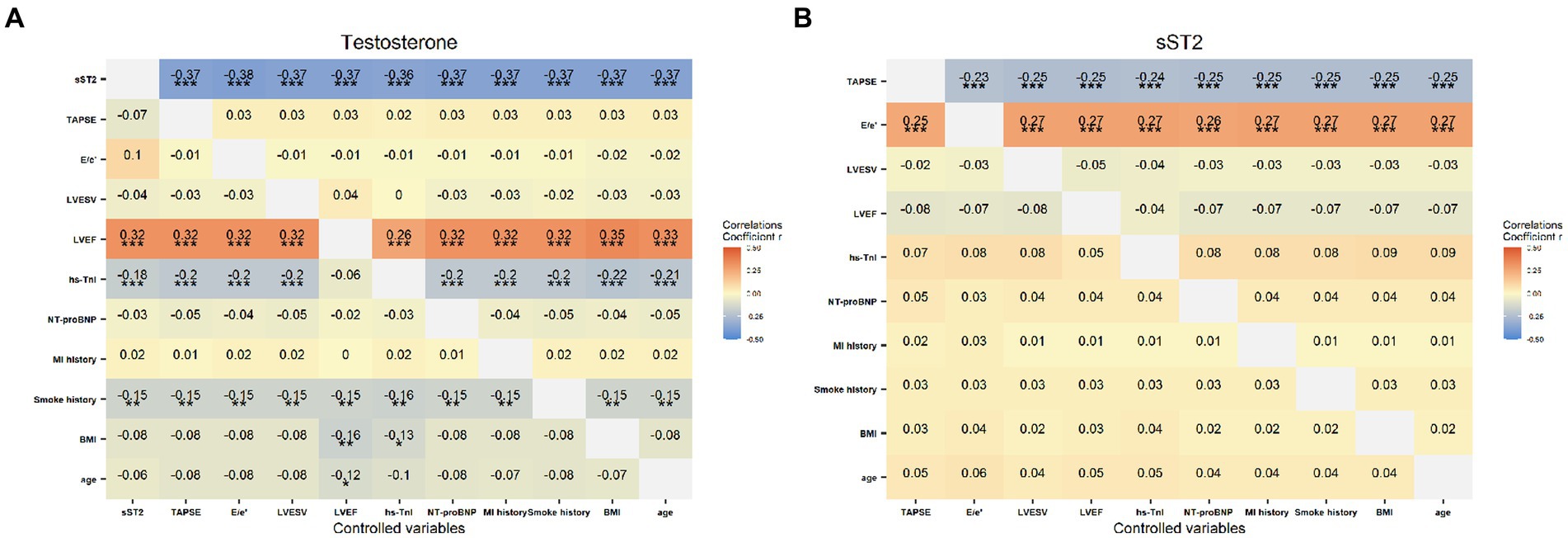
Figure 3. The correlation of the testosterone (A) and sST2 (B) levels with clinical characteristics (MI history, smoke history, BMI, age), laboratory (hs-TnI, NT-proBNP) and echocardiographic indicators (TAPSE, E/e’ ratio, LVEWV, LVEF), respectively. Color depth correspond to the strength of the correlation coefficients. *p < 0.05, **p < 0.01 and ***p < 0.001.
The ROC curves were plotted, and the AUCs were analyzed to further identify the mortality-predictive abilities of the two biomarkers. The AUCs of testosterone and sST2 were higher than NT-proBNP and hs-TnI for predicting 28 days and 90 days mortality in SIC (Figure 4). Moreover, the AUC of the combination of testosterone and sST2 for predicting 28 days and 90 days mortality were 0.805 (95% CI, 0.750–0.859) and 0.833 (95% CI, 0.785–0.882), respectively (Figure 4). The optimal cut-off values of testosterone and sST2 for predicting 28 days and 90 days mortality were 112.4 ng/dL (28 days mortality, AUC = 0.721; 90 days mortality, AUC = 0.758) and 168.9 ng/mL (28 days mortality, AUC = 0.797; 90 days mortality, AUC = 0.813), respectively (Figure 4 and Table 3).
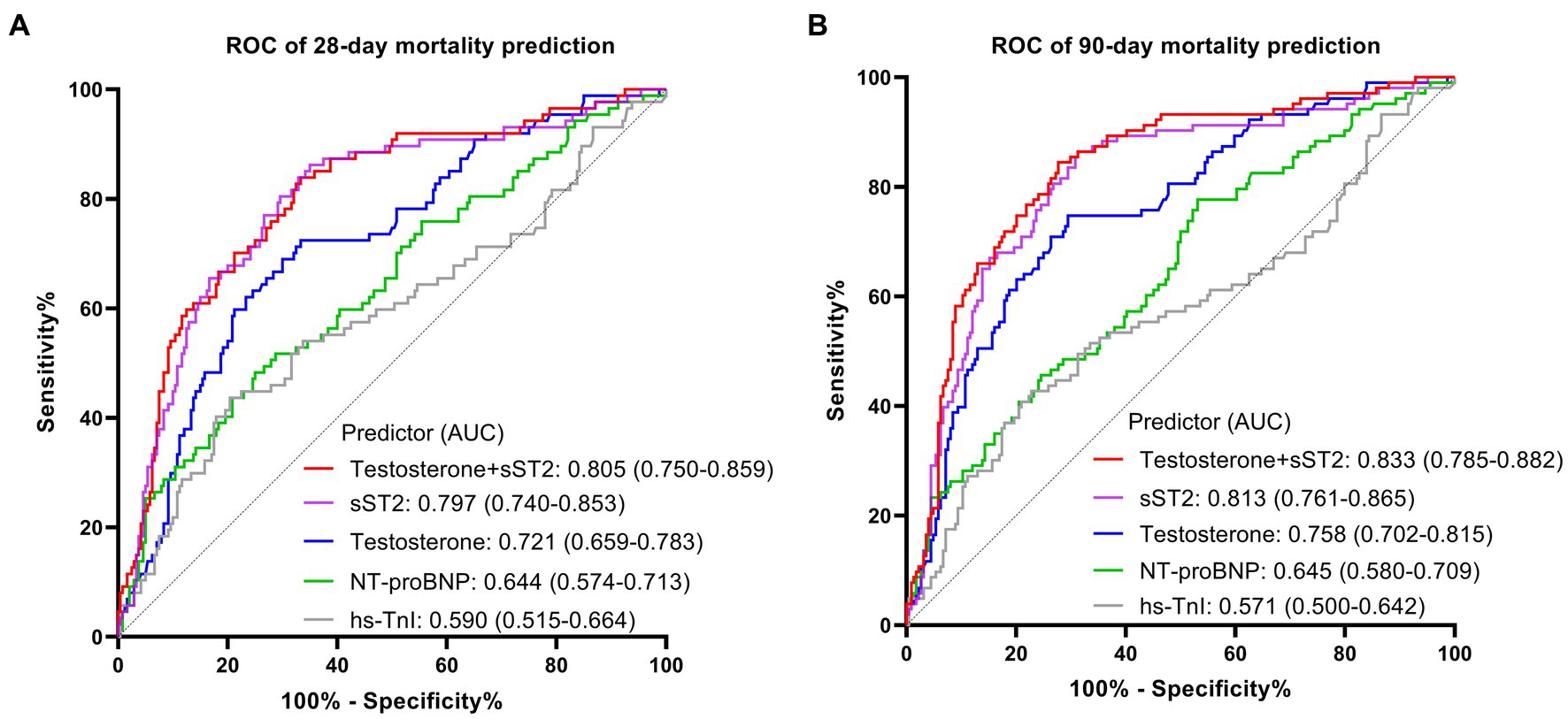
Figure 4. The ROC curve analysis of sST2, testosterone, hs-TnI, NT-proBNP for the prognostic value of 28-day mortality (A) and 90-day mortality (B) in patients with SIC. ROC, receiver operating characteristic; AUC, area under the curve; SIC, sepsis induced cardiomyopathy.
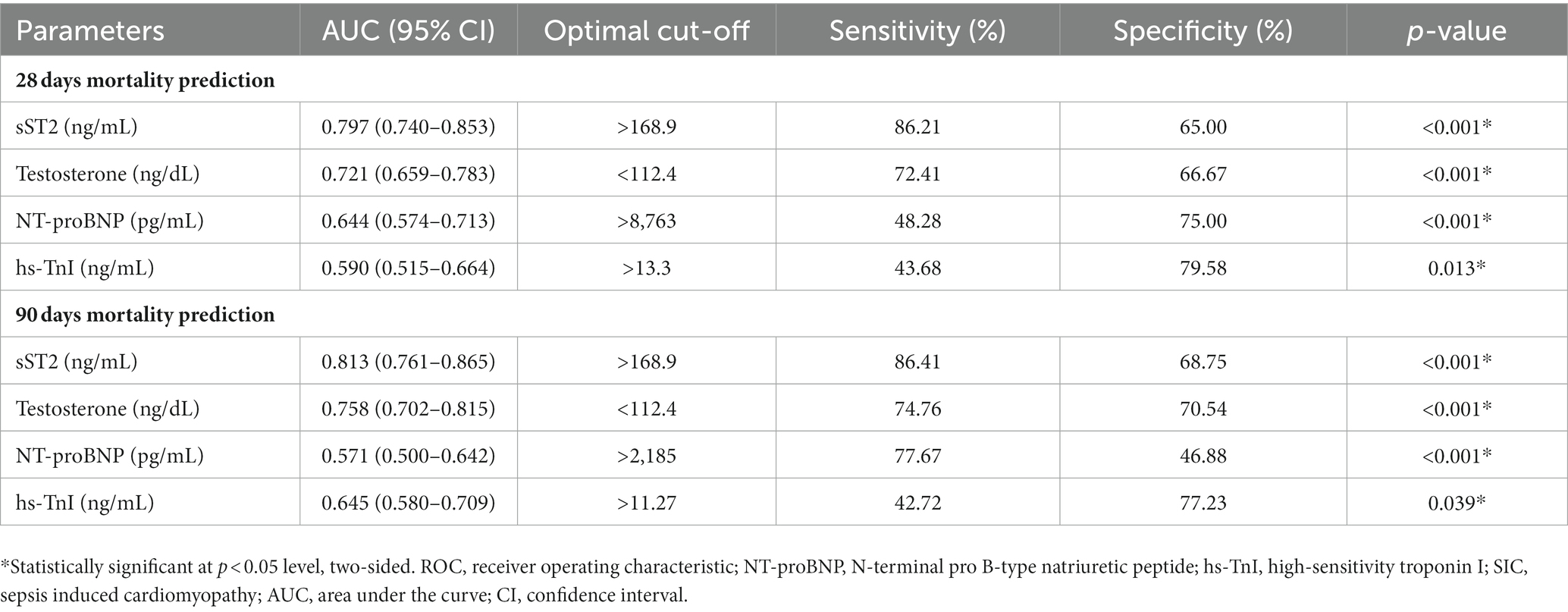
Table 3. The ROC curve analysis of sST2, testosterone, NT-proBNP, hs-TnI for the prognostic value of 28 days mortality and 90 days mortality in patients with SIC.
SIC, a frequent sepsis complication, results in left heart failure with decreased pumping ability and right heart failure with fluid overload. SIC is strongly associated with end-organ hypoperfusion and heart damage from inflammation (3). Despite receiving fluid resuscitation and standard care, SIC leads to poor outcomes and high mortality. Thus, exploring the potential risk biomarkers of male SIC patients is crucial. Endocrine dysfunction is related to the mortality and clinical outcomes in critically ill patients (21). Male patients showed higher mortality risk due to altered testosterone levels (10). The current analysis showed that decreased testosterone levels were related to severity and mortality in critically ill male patients with SIC. Furthermore, testosterone levels were associated with traditional cardiac biomarkers and echocardiography parameters in patients with SIC. To the best of our knowledge, this study contributes to the existing data by associating testosterone with clinical prognosis prediction among patients with SIC.
Previous research has demonstrated that testosterone plays a critical role in the preservation of cardiovascular homeostasis during inflammation. Castration-induced testosterone suppression in lipopolysaccharide-treated rats could lower blood pressure, raise neuronal inflammatory markers, and time- and frequency-domain measures of heart rate variability (22). On the other hand, testosterone supplementation mitigated superoxide-provoked apoptotic and necrotic cell death by upregulating the pro-survival Akt/NF-κB pathway and decreasing the pro-apoptotic enzyme caspase-3 in cardiac myocytes (23). In male patients with gastrointestinal infections, testosterone mitigates Th2-dominant responses and promotes Th1 immunity by modulating androgen receptor signaling and cytokine secretion in macrophages and lymphocytes (24). Temporarily suppressing inflammation leads to early hormone resistance, which makes testosterone deficiency a potential prognostic indicator of progressive sepsis and SIC.
Lower testosterone levels between 9.5 and 89.4 ng/dL were linked to greater inflammation and worse outcomes in septic cardiomyopathy, similar to coronavirus disease 2019 (COVID-19) (25). Earlier research has shown that decreased testosterone levels were associated with increased CRP, PCT, and interleukin (IL)-6 levels in patients with COVID-19 (25, 26). Furthermore, lower testosterone levels correlated with higher APACHE II and SOFA scores and independently predicted higher mortality in patients with SIC (27). Thus, declining testosterone levels could indicate worsening sepsis severity and increased mortality risk in male patients.
Moreover, conventional cardiac biomarkers in mortality prediction of SIC are not satisfactory (28). Chen et al. (29) reported the AUC for BNP and cTnI in SIC 28 days mortality predicting were 0.571, and 0.544, respectively. The current analysis found that the AUC for testosterone predicting 28 days mortality of SIC was 0.721, which was better than NT-proBNP (0.644) and hs-TnI (0.590). Thus, testosterone may have superior prognostic value compared with these established cardiac biomarkers for predicting SIC mortality.
Our analysis also found that sST2 level could serve as a potentially useful biomarker to predict outcomes in patients with SIC. sST2 inhibits immune responses and results in myocardial injury by targeting ST2L/IL-33 interaction (14). Although sST2 is an inflammation biomarker in other conditions (16, 17, 30, 31), its role in SIC is unclear. A previous study found sST2 elevation on days 1 and 3 of septic patients and correlated with lactate,which indicating illness severity (15). In patients with severe COVID-19, sST2 was also found to be upregulated and positively correlated with inflammatory markers (CRP and PCT), while negatively related to T lymphocyte counts (31, 32). Our results indicated that higher sST2 levels in patients with SIC positively correlate with E/e′ ratio and negatively correlate with TAPSE, suggesting that sST2 may be a potential biomarker reflecting cardiac injury in sepsis. ROC analysis has indicated that sST2 has a higher predictive value for mortality than traditional biomarkers. Thus, sST2 could enhance clinical predictive algorithms under heterogeneous sepsis conditions. Moreover, the combination of testosterone and sST2 improves mortality prediction in SIC patients.
However, our study has several limitations. Firstly, due to the lack of a universally accepted definition of SIC, the inclusion criteria for the current project referenced a study from Mayo Clinic (19), which may require further investigation to accurately identify the population affected by SIC. Secondly, biomarker values were measured at a single time point, limiting insights into the dynamic immune response in SIC over time. Moreover, altered biomarker levels may represent compensatory mechanisms early in SIC rather than a pathological effect. However,the cross-sectional design cannot prove a causal relationship between testosterone, sST2, and the outcomes. In summary, larger prospective multicenter studies measuring serial biomarker levels and long-term outcomes are warranted to validate these biomarkers as predictive tools.
Decreased testosterone and elevated sST2 may predict prognosis in patients with SIC. These biomarkers could enable risk stratification and potentially guide treatment, leading to new immunomodulatory therapies. However, further studies are necessary to confirm their diagnostic and prognostic values before clinical implementation.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the Ethics Committee of Renmin Hospital of Wuhan University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
LW: Conceptualization, Formal analysis, Project administration, Resources, Writing – original draft. WD: Formal analysis, Writing – review & editing. RZ: Resources, Writing – original draft. TL: Formal analysis, Writing – review & editing. ZZ: Project administration, Writing – review & editing. ZS: Project administration, Writing – review & editing. SM: Project administration, Writing – review & editing. SW: Data curation, Investigation, Writing – review & editing. HW: Project administration, Formal analysis, Writing – review & editing. JL: Resources, Formal analysis, Writing – review & editing. JZ: Resources, Project administration, Writing – review & editing. WX: Data curation, Resources, Writing – review & editing. GL: Data curation, Resources, Writing – review & editing. WG: Data curation, Resources, Writing – review & editing. HZ: Resources, Data curation, Writing – review & editing. YL: Formal analysis, Project administration, Writing – review & editing. LZ: Formal analysis, Investigation, Project administration, Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the National Key Research and Development Program of China (2021YFC2501800) and National Natural Science Foundation of China (82102245). The funding institutions did not commit the design of study, option of enrolled patients, collect and analysis of data, written of manuscript, or final approval of manuscript.
The authors gratefully acknowledge the contributions of the following: the patients who participated in this study, the staff of the participating emergency departments and intensive care units, the research coordinators who facilitated this study, the laboratory technicians who processed the biological samples.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1278879/full#supplementary-material
1. Seymour, CW, Liu, VX, Iwashyna, TJ, Brunkhorst, FM, Rea, TD, Scherag, A, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. (2016) 315:762–74. doi: 10.1001/jama.2016.0288
2. Markwart, R, Saito, H, Harder, T, Tomczyk, S, Cassini, A, Fleischmann-Struzek, C, et al. Epidemiology and burden of sepsis acquired in hospitals and intensive care units: a systematic review and meta-analysis. Intensive Care Med. (2020) 46:1536–51. doi: 10.1007/s00134-020-06092-1
3. Lin, YM, Lee, MC, Toh, HS, Chang, WT, Chen, SY, Kuo, FH, et al. Association of sepsis-induced cardiomyopathy and mortality: a systematic review and meta-analysis. Ann Intensive Care. (2022) 12:112. doi: 10.1186/s13613-022-01089-3
4. Vieillard-Baron, A, Caille, V, Charron, C, Belliard, G, Page, B, and Jardin, F. Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit Care Med. (2008) 36:1701–6. doi: 10.1097/CCM.0b013e318174db05
5. L’Heureux, M, Sternberg, M, Brath, L, Turlington, J, and Kashiouris, MG. Sepsis-induced cardiomyopathy: a comprehensive review. Curr Cardiol Rep. (2020) 22:35. doi: 10.1007/s11886-020-01276-2
6. Liang, YW, Zhu, YF, Zhang, R, Zhang, M, Ye, XL, and Wei, JR. Incidence, prognosis, and risk factors of sepsis-induced cardiomyopathy. World J Clin Cases. (2021) 9:9452–68. doi: 10.12998/wjcc.v9.i31.9452
7. Jeong, HS, Lee, TH, Bang, CH, Kim, JH, and Hong, SJ. Risk factors and outcomes of sepsis-induced myocardial dysfunction and stress-induced cardiomyopathy in sepsis or septic shock: a comparative retrospective study. Medicine. (2018) 97:e0263. doi: 10.1097/MD.0000000000010263
8. Song, MJ, Lee, SH, Leem, AY, Kim, SY, Chung, KS, Kim, EY, et al. Predictors and outcomes of sepsis-induced cardiomyopathy in critically ill patients. Acute Crit Care. (2020) 35:67–76. doi: 10.4266/acc.2020.00024
9. Hollenberg, SM, and Singer, M. Pathophysiology of sepsis-induced cardiomyopathy. Nat Rev Cardiol. (2021) 18:424–34. doi: 10.1038/s41569-021-00541-4
10. Vázquez-Martínez, ER, García-Gómez, E, Camacho-Arroyo, I, and González-Pedrajo, B. Sexual dimorphism in bacterial infections. Biol Sex Differ. (2018) 9:27. doi: 10.1186/s13293-018-0186-9
11. Chicco, D, and Jurman, G. Survival prediction of patients with sepsis from age, sex, and septic episode number alone. Sci Rep. (2020) 10:17156. doi: 10.1038/s41598-020-74068-9
12. Lazzerini, PE, Cantara, S, Bertolozzi, I, Accioli, R, Salvini, V, Cartocci, A, et al. Transient hypogonadism is associated with heart rate-corrected QT prolongation and Torsades de Pointes risk during active systemic inflammation in men. J Am Heart Assoc. (2022) 11:e023371. doi: 10.1161/JAHA.121.023371
13. Boden, WE, Miller, MG, McBride, R, Harvey, C, Snabes, MC, Schmidt, J, et al. Testosterone concentrations and risk of cardiovascular events in androgen-deficient men with atherosclerotic cardiovascular disease. Am Heart J. (2020) 224:65–76. doi: 10.1016/j.ahj.2020.06.007
14. Lotierzo, M, Dupuy, AM, Kalmanovich, E, Roubille, F, and Cristol, JP. sST2 as a value-added biomarker in heart failure. Clin Chim Acta. (2020) 501:120–30. doi: 10.1016/j.cca.2019.10.029
15. Hur, M, Kim, H, Kim, HJ, Yang, HS, Magrini, L, Marino, R, et al. Soluble ST2 has a prognostic role in patients with suspected sepsis. Ann Lab Med. (2015) 35:570–7. doi: 10.3343/alm.2015.35.6.570
16. Obradovic, DM, Büttner, P, Rommel, KP, Blazek, S, Loncar, G, von Haehling, S, et al. Soluble ST2 receptor: biomarker of left ventricular impairment and functional status in patients with inflammatory cardiomyopathy. Cells. (2022) 11:414. doi: 10.3390/cells11030414
17. Coronado, MJ, Bruno, KA, Blauwet, LA, Tschöpe, C, Cunningham, MW, Pankuweit, S, et al. Elevated sera sST2 is associated with heart failure in men ≤50 years old with myocarditis. J Am Heart Assoc. (2019) 8:e008968. doi: 10.1161/JAHA.118.008968
18. Yang, HS, Hur, M, Kim, H, Magrini, L, Marino, R, Di Somma, S, et al. Soluble suppression of tumorigenicity 2 and echocardiography in sepsis. Ann Lab Med. (2016) 36:590–4. doi: 10.3343/alm.2016.36.6.590
19. Wang, L, Xie, W, Li, G, Hu, B, Wu, W, Zhan, L, et al. Lipocalin 10 as a new prognostic biomarker in sepsis-induced myocardial dysfunction and mortality: a pilot study. Mediat Inflamm. (2021) 2021:6616270. doi: 10.1155/2021/6616270
20. Bidadkosh, A, Lambooy, SPH, Heerspink, HJ, Pena, MJ, Henning, RH, Buikema, H, et al. Predictive properties of biomarkers GDF-15, NTproBNP, and hs-TnT for morbidity and mortality in patients with type 2 diabetes with nephropathy. Diabetes Care. (2017) 40:784–92. doi: 10.2337/dc16-2175
21. Zhang, MQ, Macala, KF, Fox-Robichaud, A, Mendelson, AA, and Lalu, MM. Sepsis Canada National Preclinical Sepsis Platform. Sex- and gender-dependent differences in clinical and preclinical sepsis. Shock. (2021) 56:298–307. doi: 10.1097/SHK.0000000000001708
22. Sallam, MY, El-Gowilly, SM, and El-Mas, MM. Cardiac and brainstem neuroinflammatory pathways account for androgenic incitement of cardiovascular and autonomic manifestations in endotoxic male rats. J Cardiovasc Pharmacol. (2021) 77:632–41. doi: 10.1097/FJC.0000000000000993
23. Xiao, FY, Nheu, L, Komesaroff, P, and Ling, S. Testosterone protects cardiac myocytes from superoxide injury via NF-κB signalling pathways. Life Sci. (2015) 133:45–52. doi: 10.1016/j.lfs.2015.05.009
24. Takahashi, T, and Iwasaki, A. Sex differences in immune responses. Science. (2021) 371:347–8. doi: 10.1126/science.abe7199
25. Salciccia, S, Del Giudice, F, Eisenberg, ML, Mastroianni, CM, De Berardinis, E, Ricciuti, GP, et al. Testosterone target therapy: focus on immune response, controversies and clinical implications in patients with COVID-19 infection. Ther Adv Endocrinol Metab. (2021) 12:20420188211010105. doi: 10.1177/20420188211010105
26. Vena, W, Pizzocaro, A, Maida, G, Amer, M, Voza, A, Di Pasquale, A, et al. Low testosterone predicts hypoxemic respiratory insufficiency and mortality in patients with COVID-19 disease: another piece in the COVID puzzle. J Endocrinol Investig. (2022) 45:753–62. doi: 10.1007/s40618-021-01700-7
27. Birtolo, MF, Vena, W, Pizzocaro, A, Lavezzi, E, Brunetti, A, Jaafar, S, et al. Serum testosterone mirrors inflammation parameters in females hospitalized with COVID-19. J Endocrinol Investig. (2023) 46:939–45. doi: 10.1007/s40618-022-01957-6
28. Méndez Hernández, R, and Ramasco, RF. Biomarkers as prognostic predictors and therapeutic guide in critically ill patients: clinical evidence. J Pers Med. (2023) 13:333. doi: 10.3390/jpm13020333
29. Chen, FC, Xu, YC, and Zhang, ZC. Multi-biomarker strategy for prediction of myocardial dysfunction and mortality in sepsis. J Zhejiang Univ Sci B. (2020) 21:537–48. doi: 10.1631/jzus.B2000049
30. Van Nynatten, LR, Slessarev, M, Martin, CM, Leligdowicz, A, Miller, MR, Patel, MA, et al. Novel plasma protein biomarkers from critically ill sepsis patients. Clin Proteomics. (2022) 19:50. doi: 10.1186/s12014-022-09389-3
31. Hoogerwerf, JJ, Tanck, MW, van Zoelen, MA, Wittebole, X, Laterre, PF, and van der Poll, T. Soluble ST2 plasma concentrations predict mortality in severe sepsis. Intensive Care Med. (2010) 36:630–7. doi: 10.1007/s00134-010-1773-0
Keywords: sepsis-induced cardiomyopathy, male, testosterone, sST2, mortality prediction, biomarkers
Citation: Wang L, Dai W, Zhu R, Long T, Zhang Z, Song Z, Mu S, Wang S, Wang H, Lei J, Zhang J, Xia W, Li G, Gao W, Zou H, Li Y and Zhan L (2024) Testosterone and soluble ST2 as mortality predictive biomarkers in male patients with sepsis-induced cardiomyopathy. Front. Med. 10:1278879. doi: 10.3389/fmed.2023.1278879
Received: 17 August 2023; Accepted: 15 December 2023;
Published: 08 January 2024.
Edited by:
Bryan D. Kraft, Washington University in St. Louis, United StatesReviewed by:
John Grotberg, Washington University in St. Louis, United StatesCopyright © 2024 Wang, Dai, Zhu, Long, Zhang, Song, Mu, Wang, Wang, Lei, Zhang, Xia, Li, Gao, Zou, Li and Zhan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liying Zhan, emhhbmxpeWluZ0B3aHUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.