
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 13 July 2023
Sec. Ophthalmology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1130117
 Yuanfang Yang1,2,3,4
Yuanfang Yang1,2,3,4 Qinghui Wu5
Qinghui Wu5 Yao Tang2
Yao Tang2 Haoran Wu2
Haoran Wu2 Zhiwei Luo2
Zhiwei Luo2 Wenyu Gao2
Wenyu Gao2 Ziqi Hu2
Ziqi Hu2 Lijun Hou2
Lijun Hou2 Min Wang6
Min Wang6 Zhikuan Yang1,2,3,4,5
Zhikuan Yang1,2,3,4,5 Xiaoning Li2,3,4,5,7*
Xiaoning Li2,3,4,5,7*Purpose: This aim of this study was to evaluate the effect of 3% Diquafosol Ophthalmic Solution (DQS) on children with dry eye from wearing overnight orthokeratology (OrthoK) lenses.
Methods: Myopic children aged 8–18 years with dry eye syndrome were enrolled in this prospective observational study, and they were grouped according to their OrthoK treatment history for at least 1 year. All participants received DQS 4 times per day for 1 month. The following indicators were measured at baseline 1 month after treatment: the Dry Eye Questionnaire-5 (DEQ-5), non-invasive tear meniscus height (TMH), non-invasive tear film break-up time (first and average, NIBUT-F and NIBUT-A), meibomian gland score (MG score), conjunctival hyperemia redness score (R-scan), and blink pattern analysis.
Results: A total of 104 participants (189 eyes) including 40 OrthoK wearers (72 eyes) and 64 Orthok candidates (117 eyes) completed the study. Of all, after DQS treatment for 1 month, DEQ-5 scores reduced from 5.54 ± 3.25 to 3.85 ± 2.98 (t = −3.36, p = 0.00). TMH increased from 0.20 ± 0.05 mm to 0.21 ± 0.05 mm (t = 2.59, p = 0.01), NIBUT-F and NIBUT-A were prolonged from 6.67 ± 4.71 s to 10.32 ± 6.19 s and from 8.86 ± 5.25 s to 13.30 ± 6.03 s (all p = 0.00), respectively. R-scan decreased from 0.69 ± 0.28 to 0.50 ± 0.25 (t = −9.01, p = 0.00). Upper MG scores decreased from 1.04 ± 0.32 to 0.97 ± 0.36 (t = −2.14, p = 0.03). Lower MG scores, partial blink rate, partial blinks, and total blinks did not change significantly. Both break-up time (BUT) and R-scan improved significantly after DQS treatment for 1 month (all p = 0.00) in OrthoK candidates and OrthoK wearers. Among the OrthoK wearers, TMH and dry eye symptoms increased significantly (all p = 0.00) but did not increase in OrthoK candidates (p > 0.05). There were no adverse events related to DQS.
Conclusion: Diquafosol Ophthalmic Solution was effective for children wearing overnight orthokeratology in relieving dry eye symptoms and improving ocular surface parameters, which may help improve children's OrthoK wearing tolerance and compliance.
The prevalence of myopia in children has increased markedly worldwide, especially in East and Southeast Asia (1, 2). The burden of pathological consequences caused by myopia lead to irreversible blindness, such as myopic maculopathy and high myopia-associated optic neuropathy (3). Orthokeratology (OrthoK) is effective in controlling myopia progression in children (4, 5), which has gained increasingly wide application (6–8). The safety and comfort of OrthoK wearing have become a primary concern and are important for maintaining corneal morphology (9) to ensure the effect and safety of myopia control (10). Contact lens discomfort, especially contact lens-related dry eye (CLADE), is a major cause of the discontinuation of contact lens wear (11). According to the Tear Film and Ocular Surface Society Dry Eye Workshop II, even 33.33% of “healthy” children had a dry eye disease (12). Besides, contact lens wearing is believed to be one of the main causes of dry eye (13). Ocular surface problems of dry eyes in children wearing OrthoK have raised increasing attention from physicians (14).
Studies have highlighted that there is an interplay between tear film stability and effect, that is, the safety of OrthoK wearing. The hydrostatic pressure or negative pressure generated by the accumulation of tears in the reversing arc area of the OrthoK lens forms a strong positive pressure around the base arc area, while the tears in the surrounding arc are continuously sucked into the reversing arc area by a similar siphon effect to supplement the tear loss caused by the positive pressure, forming a circulating system (15). The quantity and the quality of the tear may affect the prescription, effect, and safety of OrthoK fitting and wearing, for example, they affect the measurement of corneal curvature (16), the repeatability of axial measurement (17), and the speed and amplitude of OrthoK in controlling myopia (10). Meanwhile, OrthoK wearing may affect tear stability and ocular surface health. Previous studies have found that OrthoK use may damage the ocular surface in adolescents. Some adolescents experience ocular discomfort symptoms and tear film instability (18), corneal staining (19), or even meibomian gland atrophy (20). Tear-related visual function parameters were correlated with ocular discomfort, while 40% of the patients reported dry eye or itch about 1–2 times per week during OrthoK wear at night (21). Dry eye discomfort symptoms can offset the visual benefits although current studies suggest that satisfaction with OrthoK is positive (22). However, rare studies pay attention to the diagnosis and treatment of dry eye syndrome in children. While previous dry eye treatment options for children are similar to those for adults; the initial treatment consists of artificial tear eye drops and environmental recommendations (23). Owing to the popularization and widespread usage of OrthoK, it is urgent to improve the level of treatment of dry eye in children through OrthoK use.
Diquafosol, a P2Y2 receptor agonist, stimulate both water secretion from conjunctival epithelial cells and mucin secretion from conjunctival goblet cells (24, 25). It can improve tear secretion and prevent corneal epithelial damage in dry eye animal models of rabbits (26) and rats (27). Besides, studies have also shown that diquafosol may induce the release of total cholesterol from rabbit blephomain cells by P2Y2 purine receptor signal transduction. This reveals that diquafosol may have a positive effect on the three-layered tear structure (28). Approximately 3% Diquafosol Ophthalmic Solution (DQS®) exhibited effects similar to and superior to those of sodium hyaluronate in the treatment of adults with dry eyes (29–32). Real-world clinical practices also confirmed that the topical application of DQS could be an effective and safe treatment for children with dry eyes (33, 34). For persistent dry eye patients after LASIK (35, 36) and cataract surgery (37, 38), DQS improved tear film stability and dry eye symptoms. In addition, DQS is safe for patients with contact lens-related dry eye, and it also mitigates ocular surface damage and subjective symptoms (39, 40).
As dry eye in children has its own characteristics, we lack relevant information on the treatment and effectiveness of dry eyes in children. Moreover, it is unknown whether the efficacy of DQS treatment for dry eye is associated with OrthoK wearing in children. Thus, we conducted this study to evaluate the efficacy and safety of DQS for OrthoK candidates and OrthoK wearers with dry eyes based on keratograph and LipiView tests.
This prospective open-label study was conducted at Changsha Aier Eye Hospital between February 2022 and July 2022. All participants were provided with a full explanation of the study and provided their written informed consent. The Institutional Ethics Committee of Changsha Aier Eye Hospital approved the study (approval No.: KYPJ002, 2022). All procedures were conducted following the principles of the Declaration of Helsinki.
The inclusion criteria were as follows: (1) age between 8 years and 18 years; (2) myopia from −1.00 Diopter to −5.50 D, with-the-rule astigmatism of up to −1.75 D or against-the-rule astigmatism of less than −0.75 D with keratometry from 41.00 to 46.00 D; (3) participants were first-time users of OrthoK or had worn OrthoK for at least 1 year; (4) participants with a potential risk of mild-to-moderate dry eye based on TMH <0.20 mm or BUT <10 s from Keratograph 5M examinations adopted by the Dry Eye Workshop (41); and (5) participants cooperated with eye drop usage as required, completed examinations, and went back to the hospital for follow-up examinations within the specified time. The exclusion criteria were as follows: (1) participants with allergic or autoimmune diseases associated with dry eye are not suitable for OrthoK lenses; (2) participants had pathological changes of the lid margin, cornea, uvea, retina, and other systemic diseases that may influence the ocular surface, for example, serious ocular surface disease (e.g., Sjögren syndrome, allergic conjunctivitis, ocular pemphigoid, conjunctivochalasis, conjunctival scarring, and chemical injury); (3) participants had received any dry eye treatment within 14 days before the start date of this study or continued to use other topical ophthalmic solutions that can affect the study results; and (4) participants received other ocular treatments or surgeries.
Participants were divided into two groups depending on their history of wearing OrthoK, namely, the OrthoK wearers group (who had worn OrthoK lenses for at least 1 year) and the OrthoK candidates group (new wearers without a history of using any contact lens). All participants received DQS® (3% Diquafosol Ophthalmic Solution; Santen Pharmaceutical Co. Ltd., Osaka, Japan) 4 times per day for 1 month. The usage frequency of DQS (4 times daily) and follow-up time (1 month) in this study were based on the studies conducted by Hwang et al. (42) and Holland et al. (43). It is more feasible and rigorous to observe its effect and safety on adolescents with 4 times daily for 1 month. We also recorded age, sex, habit outdoors, the screen using time, daily circumstances, and slit-lamp examination. We administered Questionnaire-5 (DEQ-5) and measured non-invasive keratograph tear film break-up time (NIBUT, first and average, BUT-F BUT-A), non-invasive tear meniscus height (TMH), conjunctival hyperemia redness score (R-scan), upper and lower meibomian gland scores (MG scores), and blink pattern. All these dry eye-related indicators were evaluated at baseline and 1 month after DQS treatment. Two skilled physicians performed baseline and follow-up examinations for all participants without awareness of the participants' medication use and OrthoK lens wear history to ensure the reliability of the examination results.
Subjective symptoms were assessed using Dry Eye Questionnaire-5 (DEQ-5) (44). DEQ-5 was used to compare the dry eye-related symptoms at baseline and 1 month after DQS treatment, and a total score greater than 6 indicated dry eye symptoms. The questionnaire consisted of the following five questions:
Q1: During a typical day in the past month, how often did your eyes feel discomfort?
Q2: When your eyes felt discomfort, how intense was this feeling of discomfort at the end of the day, within 2 h of going to bed?
Q3: During a typical day in the past month, how often did your eyes feel dry?
Q4: When your eyes felt dry, how intense was this feeling of discomfort at the end of the day, within 2 h of going to bed?
Q5: During a typical day in the past month, how often did your eyes feel excessively watery?
TMH, BUT-F, BUT-A, R-scan, and MG score were conducted with the Keratograph 5M (Oculus GmbH, Wetzlar, Germany) to evaluate the ocular surface status and tear film stability at baseline and 1 month after DQS treatment. Participants were instructed to look straight ahead, and TMH, R-scan, and MG scores were measured in TMH quantitative photography mode, R-scan quantitative photography mode, and MG photography mode, respectively. Meibomian glands loss scores were graded as 0 (no loss), grade 1 (dropout <1/3), grade 2 (dropout 1/3–2/3), and grade 3 (dropout >2/3). BUT was generated by automatic detection and calculation as follows: (1) BUT-F, the time at which the first distortion in the reflected Placido ring occurred and (2) BUT-A, related to the localized TBUTs and calculated based on the average time of all detected perturbations.
Partial blink rate (PBR), partial blinks, and total blinks were recorded using LipiView Ocular Surface Interferometer (Johnson & Johnson, USA). During the examination, the patients were asked to look at the front lightspot to ensure that their pupils were directly in the center of the interferometer camera with natural blinking, and all the measurements were conducted by the same experienced examiner. Participants with an outcome conformance factor of < 0.8 were asked to repeat the measurement.
At every visit, the occurrence of systemic adverse events was checked. If adverse events were found, the findings were reported.
Statistical analysis was performed with IBM SPSS Statistics 26.0 (IBM SPSS, Inc., USA). The continuous measurement data subjected to normal distribution were presented as the mean ± SD, and the enumeration data were presented as the ratio (%). The missing data incurred during examinations in this study were at random, and the proportion was very small (≤7%); in addition, relevant missing data were not included in the statistics. The chi-squared test was used for baseline enumeration of variables such as sex, parental smoking, daily outdoors (h), and daily electronic screen time (h). Baseline continuous data, such as DEQ-5 scores, TMH, NIBUT, R-scan, MG Scores, PBR, partial blinks, and total blinks, were compared between the two groups using the independent samples t-test. We used the paired t-test to obtain overall and intergroup differences of continuous data between, before, and after DQS treatment responses throughout the study period. The significance level was set as α = 0.05.
A total of 104 participants (189 eyes) completed the Keratograph 5M and LipiView examination at baseline and 1 month after DQS treatment. The OrthoK wearers group consisted of 40 participants (72 eyes), and the OrthoK candidates group consisted of 64 participants (117 eyes). The baseline demographic data are summarized in Table 1. There was no significant difference in sex (p = 0.26), parental smoking (p = 0.68), daily outdoors (p = 0.33), and daily electronic screen time (p = 0.62) between OrthoK candidates and OrthoK wearers, except for age (11.49 ± 2.29 vs. 12.58 ± 2.16, p = 0.00).
The baseline characteristics of the ocular evaluation are summarized in Table 2. There was no significant difference in DEQ-5 scores (p = 0.06), TMH (p = 0.07), BUT-F (p = 0.84), BUT-A (p = 0.50), and R-scan (p = 0.13), Upper MG Scores (p=0.88), and total blinks (p = 0.62) between OrthoK candidates and OrthoK wearers at baseline. However, there were significant differences in lower MG scores (1.08 ± 0.40 vs. 1.00 ± 0.00, p = 0.04), PBR (0.63 ± 0.35 vs. 0.82 ± 0.28, p = 0.00), and partial blinks (4.06 ± 3.26 vs. 5.78 ± 3.78, p = 0.00) between OrthoK candidates and OrthoK wearers at baseline.
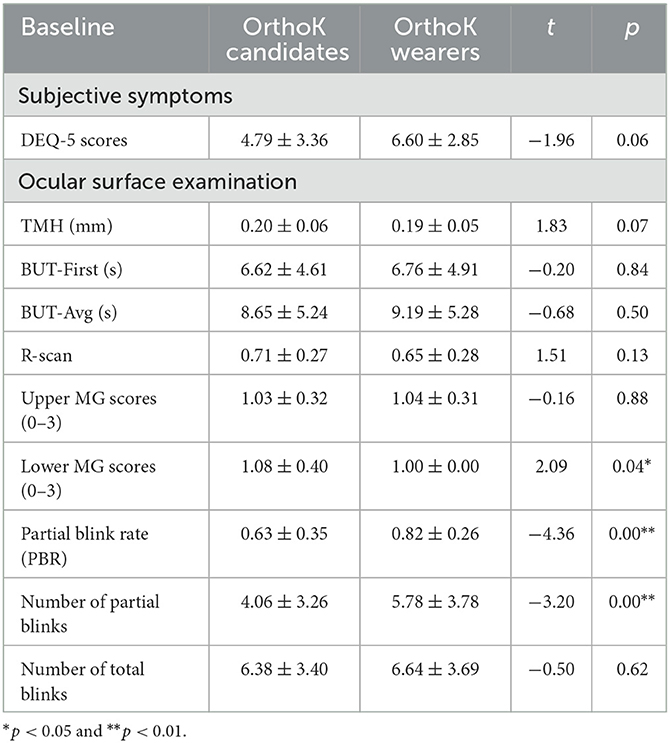
Table 2. Baseline characteristics of dry eye symptoms and ocular surface of OrthoK candidates and OrthoK wearers.
Figure 1 shows the changes in subjective symptoms from baseline to 1 month after DQS treatment. Among all participants, compared with the baseline, the DEQ-5 scores decreased (5.54 ± 3.25 vs. 3.85 ± 2.98, t = −3.36, p = 0.00). Among OrthoK wearers, the DEQ-5 scores (6.60 ± 2.85 vs. 4.40 ± 2.52, t = −4.22, p = 0.00), Q1 (1.75 ± 0.79 vs. 1.20 ± 0.83, t = −2.98, p = 0.01), Q2 (0.90 ± 0.64 vs. 0.60 ± 0.60, t = −2.35, p = 0.03), and Q4 (1.05 ± 0.76 vs. 0.45 ± 0.60, t = −3.27, p = 0.00) decreased from baseline to 1 month, whereas among OrthoK candidates, there was a significant decrease only in Q5 (0.88 ± 0.99 vs. 0.54 ± 0.81, t = −2.37, p = 0.03).
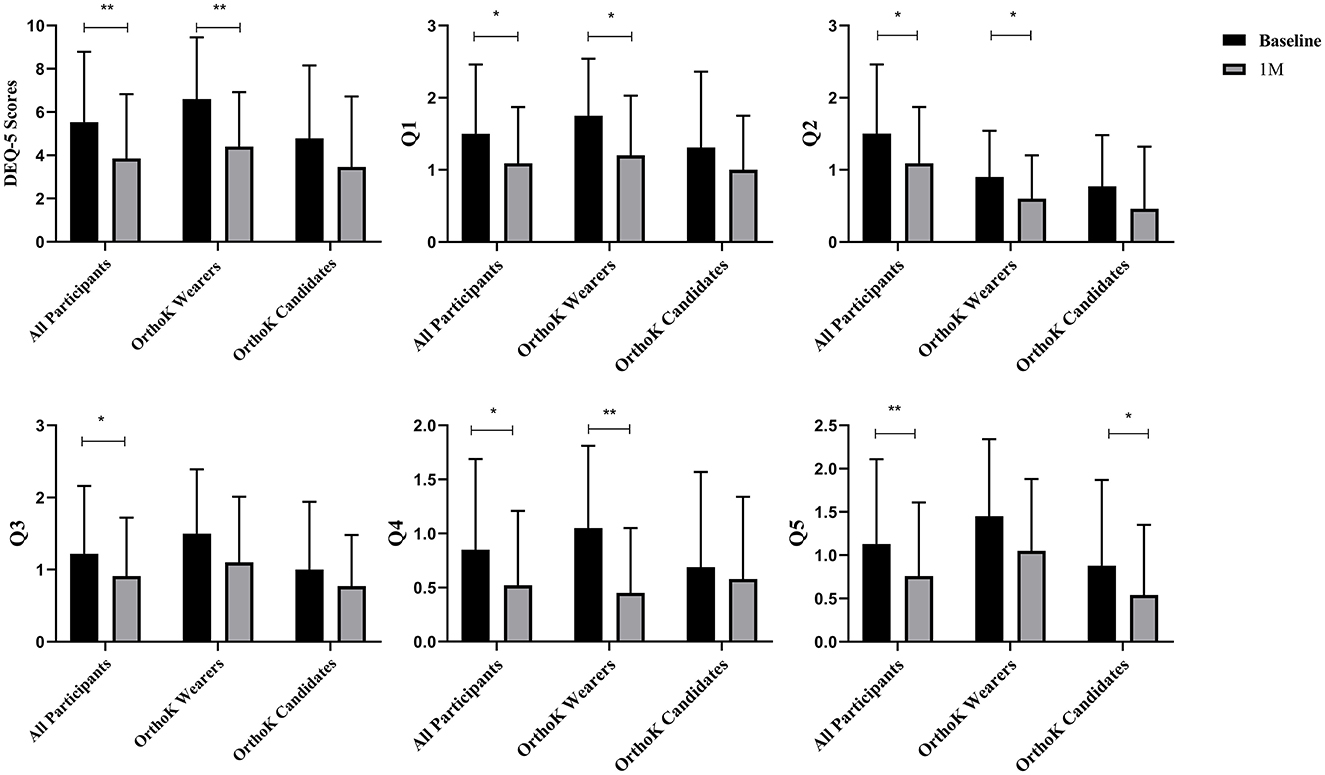
Figure 1. Subjective symptoms: changes in Dry Eye Questionnaire-5 (DEQ-5) scores before and after DQS treatment. Q1: During a typical day in the past month, how often did your eyes feel discomfort? Q2: When your eyes felt discomfort, how intense was this feeling of discomfort at the end of the day, within 2 h of going to bed? Q3: During a typical day in the past month, how often did your eyes feel dry? Q4: When your eyes felt dry, how intense was this feeling of discomfort at the end of the day, within 2 h of going to bed? Q5: During a typical day in the past month, how often did your eyes feel excessively watery? *p < 0.05 and **p < 0.01.
Figure 2 shows the changes in the ocular surface from baseline to 1 month after DQS treatment. Among all participants, compared with the baseline, TMH (0.20 ± 0.05 vs. 0.21 ± 0.05, t = 2.59, p = 0.01), BUT-F (6.67 ± 4.71 vs. 10.32 ± 6.19, t = 7.20, p = 0.00), BUT-A (8.86 ± 5.25 vs. 13.30 ± 6.03, t = 9.42, p = 0.00), R-scan (0.69 ± 0.28 vs. 0.50 ± 0.25, t = −9.01, p = 0.00), and upper MG scores (1.04 ± 0.32 vs. 0.97 ± 0.36, t = −2.14, p = 0.03) improved significantly after 1-month DQS treatment. However, lower MG scores did not change significantly (p = 0.10). Of OrthoK candidates and OrthoK wearers, compared with the baseline, BUT-F (6.62 ± 4.61 vs. 10.24 ± 6.66, t = 5.62, p = 0.00 and 6.76 ± 4.91 vs. 10.45 ± 5.38, t = 4.46, p = 0.00, respectively), BUT-A (8.65 ± 5.24 vs. 12.65 ± 6.33, t = 7.02, p = 0.00, and 9.19 ± 5.28 vs. 14.34 ± 5.37, t = 6.30, p = 0.00, respectively), and R-scan (0.71 ± 0.27 vs. 0.50 ± 0.23, t = −8.68, p = 0.00 and 0.65 ± 0.28 vs. 0.52 ± 0.28, t = −3.84, p = 0.00, respectively) improved after 1-month DQS treatment; however, Upper MG scores did not change significantly (p = 0.11, p = 0.17). Among the OrthoK wearers, TMH increased significantly (0.19 ± 0.05 vs. 0.21 ± 0.04, t = 3.18, p = 0.00), but not in OrthoK candidates (p = 0.32). However, among the OrthoK candidates, lower MG scores (1.08 ± 0.40 vs. 1.00 ± 0.16, t = −2.41, p = 0.02) decreased significantly but not in OrthoK wearers (p = 0.42).

Figure 2. Ocular surface examination: changes in TMH, BUT-F, BUT-A, R-scan, upper MG scores, and lower MG scores before and after DQS treatment in all participants, the OrthoK wearers group, and the OrthoK candidates group. *p < 0.05 and **p < 0.01.
Figure 3 shows the changes in the blink pattern from baseline to 1 month. Among all participants, compared with baseline, no significant changes were observed in PBR (0.70 ± 0.33 vs. 0.73 ± 0.31, t = 1.00, p = 0.31), partial blinks (4.72 ± 3.56 vs. 4.78 ± 3.18, t = 0.18, p = 0.86), and total blinks (6.48 ± 3.51 vs. 6.36 ± 2.85, t = −0.42, p = 0.68) after DQS treatment. Of OrthoK candidates and OrthoK wearers, no significant changes were observed in PBR (p = 0.11 and p = 0.42), partial blinks (p = 0.88 and p = 0.93), and total blinks (p = 0.15 and p = 0.26) from baseline to 1 month.
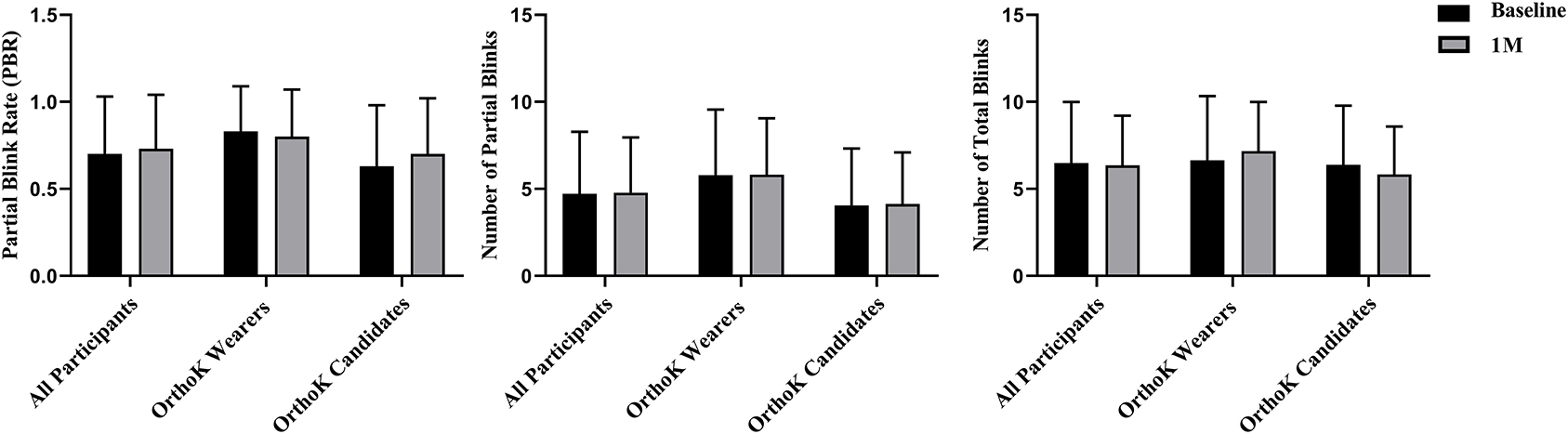
Figure 3. Blink pattern analysis. Changes in PBR, partial blinks, and total blinks before and after DQS use in both groups, the OrthoK wearers group, and OrthoK candidates group.
All participants adhered to the DQS treatment for 1 month, and no adverse reactions or events developed during the observation period.
We investigated the efficacy and safety of DQS on OrthoK lens-related dry eye symptoms and measured the ocular surface parameters in children with OrthoK wearing history or candidates for OrthoK. Owing to the poor coordination with the examination of children, we used non-invasive Keratograph 5M (45) and LipiView (46) in this study to obtain objective parameters. Besides, we balanced the effect of time spent outdoors, electronic screen use, and parental smoking that might affect tear quality (14).
At baseline, DEQ-5, TMH, BUT, R-scan, and total blinks were similar between OrthoK wearers and OrthoK candidates; however, the DEQ-5 score and TMH were slightly worse in OrthoK wearers, suggesting that wearing OrthoK lenses may impact a wearer's subjective symptoms as well as tear quantity. We also found that lower MG scores were higher in OrthoK candidates than OrthoK wearers at baseline, this may be due to meibomian gland growth with aging (47) considering the age difference between Orthok candidates and wearers. Similarly, OrthoK wearers had higher PBR and partial blinks than OrthoK candidates, but similar total blinks at baseline. To our knowledge, total blinks can promote reconstruction and generation of the lipid layer to sustain tear film stability of the ocular surface; however, partial blinking is detrimental (48, 49). OrthoK influences the tear film stability, which in turn alters the children's blinking habits. According to the results confirmed by Hui et al. (50), ocular surface and meibomian gland function did not change significantly although wearing OrthoK lenses may have aggravated dry eye symptoms. Children's dry eye subjective symptoms may precede objective examinations, and it is essential to explore the effectiveness and safety of treatment of the ocular surface in children with OrthoK. This may also indicate that, when OrthoK children have ocular surface examinations abnormality, there may already exist symptoms that require a physician's attention.
In this study, through subjective symptoms analysis, we found that the frequency of eye discomfort, the intensity of eye discomfort, and the intensity of dry eye sensation were diminished more significantly in OrthoK wearers than in OrthoK candidates. OrthoK wearers had significant improvement in TMH than OrthoK candidates. Ocular surface subjective parameters were significantly improved in children with topical DQS in this study, which is consistent with the results of Kojima et al. (51) for adult dry eye treatment. Besides, our study revealed that OrthoK wearers are more sensitive to DQS in promoting tear secretion and relieving contact lens-related discomfort than those without a contact lens-wearing history. We considered that OrthoK may disturb tear film stability and that DQS just compensates quite well for the negative influences. Of course, further long-term observation of DQS's sustained effect on OrthoK wearers is needed. TMH, NIBUT, DEQ-5 scores, R-scan, and upper MG scores of all participants also improved significantly after 1-month DQS treatment. As previous studies showed that DQS significantly improved BUT and subjective symptoms in soft contact lens-related dry eye (40) and also can stabilize tear film stability (31, 52). This study demonstrates the efficacy and safety of DQS on children and children with or without wearing OrthoK. In our study, we have proven that DQS could improve subjective and objective parameters in children with dry eyes. We believe that, although this is a short-term observation, it may inspire physicians to make clinical decisions when dealing with dry eye children before and during wearing OrthoK.
Our study showed no significant difference in blink patterns, including PBR, partial blinks, and total blinks, after 1-month DQS treatment. However, we ascertained that the blink pattern was similar or slightly improved after DQS use for a month. However, in the baseline, the blink pattern was worse in the OrthoK wearers than in the OrthoK candidates. However, other investigators found that the topical application of DQS alleviated meibomian gland dysfunction (53, 54). We hypothesize that the blink pattern did not change due to the increase in tear film stabilization and meibomian gland dysfunction through using DQS. Blink patterns, partial blinks in specific, were important in assessing mild-to-moderate dry eyes and associate well with other ocular surface parameters (49). We also hypothesize that alleviation of dry eye symptoms is first manifested in tear film stability and meibomian gland function. While blink pattern improvement required additional long-term medication, some studies have reported that contact lens wearers with dry eyes benefit from increased blinking frequency, which may help them to reduce dry eye symptoms and to improve the ocular surface environment (55, 56). In this study, there was no change in blink patterns in children with or without a history of OrthoK after 1-month of DQS treatment, which demonstrated that DQS may have a stabilizing effect on blink patterns in children in short-term use.
This study inevitably has some limitations. First, it was a single-center, single-arm research project that lacks a control population, and a large sample randomized controlled trial is recommended. Second, this study was only a short-term observation, and long-term prospective studies need to be further explored. Meanwhile, further studies are needed to evaluate tear composition and distribution and how these factors are influenced by tear film stability and meibomian gland status.
Diquafosol Ophthalmic Solution was effective and safe for children wearing overnight orthokeratology in alleviating dry eye symptoms and ocular surface parameters, which may help improve children's OrthoK wearing tolerance and compliance.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Institutional Ethics Committee of Changsha Aier Eye Hospital. All procedures were conducted following the principles of the Declaration of Helsinki. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
XL, YY, QW, and ZY contributed to the study's conception and design. Material preparation and data collection were performed by YT, HW, ZL, WG, ZH, LH, and MW. Data analysis was performed by XL, YY, and ZY. The first draft of the manuscript was written by XL and YY. All authors commented on previous versions of the manuscript and read and approved the final manuscript.
This study was funded by the Science Research Foundation of Aier Eye Hospital Group (AF2003D7 and AC2009D2) and the Natural Science Foundation of Hunan Province (2021JJ40004).
We thank all participants for their involvement in the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Jonas JB, Ang M, Cho P, Guggenheim JA, He MG, Jong M, et al. IMI prevention of myopia and its progression. Invest Ophthalmol Vis Sci. (2021) 62:6. doi: 10.1167/iovs.62.5.6
2. Cho P, Tan Q. Myopia and orthokeratology for myopia control. Clin Exp Optom. (2019) 102:364–77. doi: 10.1111/cxo.12839
3. Saw SM, Matsumura S, Hoang QV. Prevention and management of myopia and myopic pathology. Invest Ophthalmol Vis Sci. (2019) 60:488–99. doi: 10.1167/iovs.18-25221
4. Cho P and Cheung SW, Retardation of myopia in Orthokeratology (ROMIO) study: a 2-year randomized clinical trial. Invest Ophthalmol Vis Sci. (2012) 53:7077–85. doi: 10.1167/iovs.12-10565
5. Hiraoka T, Kakita T, Okamoto F, Takahashi H, Oshika T. Long-term effect of overnight orthokeratology on axial length elongation in childhood myopia: a 5-year follow-up study. Invest Ophthalmol Vis Sci. (2012) 53:3913–9. doi: 10.1167/iovs.11-8453
6. Li Z, Hu Y, Cui D, Long W, He M, Yang X. Change in subfoveal choroidal thickness secondary to orthokeratology and its cessation: a predictor for the change in axial length. Acta Ophthalmol. (2019) 97:e454–9. doi: 10.1111/aos.13866
7. Lyu T, Wang L, Zhou L, Qin J, Ma H, Shi M. Regimen study of high myopia-partial reduction orthokeratology. Eye Contact Lens. (2020) 46:141–6. doi: 10.1097/ICL.0000000000000629
8. Wan K, Lau JK-K, Wan Cheung S, Cho P. Refractive and corneal responses of young myopic children to short-term orthokeratology treatment with different compression factors. Cont Lens Anterior Eye. (2020) 43:65–72. doi: 10.1016/j.clae.2019.10.134
9. Herbaut A, Liang H, Denoyer A, Baudouin C, Labbé A. Tear film analysis and evaluation of optical quality: a review of the literature. J Fr Ophtalmol. (2019) 42:e21–35. doi: 10.1016/j.jfo.2018.12.001
10. Fan L, Jun J, Jia Q, Wangqing J, Xinjie M, Yi S. Clinical study of orthokeratology in young myopic adolescents. Int Contact Lens Clin. (1999) 26:113–6. doi: 10.1016/S0892-8967(00)00032-8
11. Koh S. Contact lens wear and dry eye: beyond the known. Asia Pac J Ophthalmol (Phila). (2020) 9:498–504. doi: 10.1097/APO.0000000000000329
12. Rojas-Carabali W, Uribe-Reina P, Muñoz-Ortiz J, Terreros-Dorado JP, Ruiz-Botero ME, Torres-Arias N, et al. High prevalence of abnormal ocular surface tests in a healthy pediatric population. Clin Ophthalmol. (2020) 14:3427–38. doi: 10.2147/OPTH.S266261
13. The epidemiology of dry eye disease. Report of the epidemiology subcommittee of the international dry eye workShop. Ocul Surf . (2007) 5:93–107. doi: 10.1016/S1542-0124(12)70082-4
14. Tao Z, Wang J, Zhu M, Lin Z, Zhao J, Tang Y, et al. Does orthokeratology wearing affect the tear quality of children? Front Pediatr. (2021) 9:773484. doi: 10.3389/fped.2021.773484
15. Vincent SJ, Cho P, Chan KY, Fadel D, Ghorbani-Mojarrad N, et al. CLEAR—orthokeratology. Cont Lens Anterior Eye. (2021) 44:240–69. doi: 10.1016/j.clae.2021.02.003
16. Röggla V, Leydolt C, Schartmüller D, Schwarzenbacher L, Meyer E, Abela-Formanek C, et al. Influence of artificial tears on keratometric measurements in cataract patients. Am J Ophthalmol. (2021) 221:1–8. doi: 10.1016/j.ajo.2020.08.024
17. Epitropoulos AT, Matossian C, Berdy GJ, Malhotra RP, Potvin R. Effect of tear osmolarity on repeatability of keratometry for cataract surgery planning. J Cataract Refract Surg. (2015) 41:1672–7. doi: 10.1016/j.jcrs.2015.01.016
18. Xie C, and Wei R. Long-term changes in the ocular surface during orthokeratology lens wear and their correlations with ocular discomfort symptoms. Cont Lens Anterior Eye. (2023) 46:101757. doi: 10.1016/j.clae.2022.101757
19. Miao CX, Xu XY, Zhang H. [Analysis of corneal complications in children wearing orthokeratology lenses at night]. Zhonghua Yan Ke Za Zhi. (2017) 53:198–202. doi: 10.3760/cma.j.issn.0412-4081.2017.03.010
20. Na K-S, Yoo Y-S, Hwang HS, Mok JW, Kim HS, Joo C-K. The influence of overnight orthokeratology on ocular surface and meibomian glands in children and adolescents. Eye Contact Lens. (2016) 42:68–73. doi: 10.1097/ICL.0000000000000196
21. Yang B, Ma X, Liu L, Cho P. Vision-related quality of life of Chinese children undergoing orthokeratology treatment compared to single vision spectacles. Cont Lens Anterior Eye. (2021) 44:101350. doi: 10.1016/j.clae.2020.07.001
22. Mohd-Ali B, Low YC, Shahimin MM, Arif N, Abdul-Hamid H, Wan Abdul-Halim WH, et al. Comparison of vision-related quality of life between wearing orthokeratology lenses and spectacles in myopic children living in kuala lumpur. Cont Lens Anterior Eye. (2023) 46:101774. doi: 10.1016/j.clae.2022.101774
23. Alves M, Dias AC, Rocha EM. Dry eye in childhood: epidemiological and clinical aspects. Ocul Surf. (2008) 6:44–51. doi: 10.1016/S1542-0124(12)70104-0
24. Li Y, Kuang K, Yerxa B, Wen Q, Rosskothen H, Fischbarg J. Rabbit conjunctival epithelium transports fluid, and P2Y2(2) receptor agonists stimulate Cl(-) and fluid secretion. Am J Physiol Cell Physiol. (2001) 281:C595–602. doi: 10.1152/ajpcell.2001.281.2.C595
25. Murakami T, Fujihara T, Horibe Y, Nakamura M. Diquafosol elicits increases in net Cl- transport through P2Y2 receptor stimulation in rabbit conjunctiva. Ophthalmic Res. (2004) 36:89–93. doi: 10.1159/000076887
26. Fujihara T, Murakami T, Nagano T, Nakamura M, Nakata K. INS365 suppresses loss of corneal epithelial integrity by secretion of mucin-like glycoprotein in a rabbit short-term dry eye model. J Ocul Pharmacol Ther. (2002) 18:363–70. doi: 10.1089/10807680260218524
27. Fujihara T, Murakami T, Fujita H, Nakamura M, Nakata K. Improvement of corneal barrier function by the P2Y(2) agonist INS365 in a rat dry eye model. Invest Ophthalmol Vis Sci. (2001) 42:96–100.
28. Endo KI, Sakamoto A, Fujisawa K. Diquafosol tetrasodium elicits total cholesterol release from rabbit meibomian gland cells via P2Y(2) purinergic receptor signalling. Sci Rep. (2021) 11:6989. doi: 10.1038/s41598-021-86433-6
29. Takamura E, Tsubota K, Watanabe H, Ohashi Y, A. randomised, double-masked comparison study of diquafosol vs. sodium hyaluronate ophthalmic solutions in dry eye patients. Br J Ophthalmol. (2012) 96:1310–5. doi: 10.1136/bjophthalmol-2011-301448
30. Matsumoto Y, Ohashi Y, Watanabe H, Tsubota K. Group DOSP2S Efficacy and safety of diquafosol ophthalmic solution in patients with dry eye syndrome: a Japanese phase 2 clinical trial. Ophthalmology. (2012) 119:1954–60. doi: 10.1016/j.ophtha.2012.04.010
31. Shimazaki-Den S, Iseda H, Dogru M, Shimazaki J. Effects of diquafosol sodium eye drops on tear film stability in short BUT type of dry eye. Cornea. (2013) 32:1120–5. doi: 10.1097/ICO.0b013e3182930b1d
32. Gong L, Sun X, Ma Z, Wang Q, Xu X, Chen X, et al. A randomised, parallel-group comparison study of diquafosol ophthalmic solution in patients with dry eye in China and Singapore. Br J Ophthalmol. (2015) 99:903–8. doi: 10.1136/bjophthalmol-2014-306084
33. Yamaguchi M, Nishijima T, Shimazaki J, Takamura E, Yokoi N, Watanabe H, et al. Clinical usefulness of diquafosol for real-world dry eye patients: a prospective, open-label, non-interventional, observational study. Adv Ther. (2014) 31:1169–81. doi: 10.1007/s12325-014-0162-4
34. Ohashi Y, Munesue M, Shimazaki J, Takamura E, Yokoi N, Watanabe H, et al. Long-term safety and effectiveness of Diquafosol for the treatment of dry eye in a real-world setting: a prospective observational study. Adv Ther. (2020) 37:707–17. doi: 10.1007/s12325-019-01188-x
35. Mori Y, Nejima R, Masuda A, Maruyama Y, Minami K, Miyata K, et al. Effect of diquafosol tetrasodium eye drop for persistent dry eye after laser in situ keratomileusis. Cornea. (2014) 33:659–62. doi: 10.1097/ICO.0000000000000136
36. Toda I, Ide T, Fukumoto T, Ichihashi Y, Tsubota K. Combination therapy with diquafosol tetrasodium and sodium hyaluronate in patients with dry eye after laser in situ keratomileusis. Am J Ophthalmol. (2014) 157:616–22. doi: 10.1016/j.ajo.2013.11.017
37. Park DH, Chung JK, Seo DR, Lee SJ. Clinical effects and safety of 3% diquafosol ophthalmic solution for patients with dry eye after cataract surgery: a randomized controlled trial. Am J Ophthalmol. (2016) 163:122–31. doi: 10.1016/j.ajo.2015.12.002
38. Yamazaki K, Yoneyama J, Kimoto R, Shibata Y, Mimura T. Prevention of surgery-induced dry eye by diquafosol eyedrops after femtosecond laser-assisted cataract surgery. J Clin Med. (2022) 11:19. doi: 10.3390/jcm11195757
39. Ogami T, Asano H, Hiraoka T, Yamada Y, Oshika T. The effect of diquafosol ophthalmic solution on clinical parameters and visual function in soft contact lens-related dry Eye. Adv Ther. (2021) 38:5534–47. doi: 10.1007/s12325-021-01910-8
40. Shigeyasu C, Yamada M, Akune Y, Fukui M. Diquafosol for soft contact lens dryness: clinical evaluation and tear analysis. Optom Vis Sci. (2016) 93:973–8. doi: 10.1097/OPX.0000000000000877
41. Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo C-K, et al. TFOS DEWS II definition and classification report. Ocul Surf. (2017) 15:276–83. doi: 10.1016/j.jtos.2017.05.008
42. Hwang HS, Sung Y-M, Lee WS, Kim EC. Additive Effect of preservative-free sodium hyaluronate 01% in treatment of dry eye syndrome with diquafosol 3% eye drops. Cornea. (2014) 33:935–41. doi: 10.1097/ICO.0000000000000213
43. Holland EJ, Darvish M, Nichols KK, Jones L, Karpecki PM. Efficacy of topical ophthalmic drugs in the treatment of dry eye disease: a systematic literature review. Ocul Surf. (2019) 17:412–23. doi: 10.1016/j.jtos.2019.02.012
44. Chalmers RL, Begley CG, Caffery B. Validation of the 5-Item Dry Eye Questionnaire (DEQ-5): Discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Cont Lens Anterior Eye. (2010) 33:55–60. doi: 10.1016/j.clae.2009.12.010
45. García-Marqués JV, Martínez-Albert N, Talens-Estarelles C, García-Lázaro S, Cerviño A. Repeatability of Non-invasive Keratograph Break-Up Time measurements obtained using Oculus Keratograph 5M. Int Ophthalmol. (2021) 41:2473–83. doi: 10.1007/s10792-021-01802-4
46. Lee JM, Jeon YJ, Kim KY, Hwang K-Y, Kwon Y-A, Koh K. Ocular surface analysis: a comparison between the LipiView(®) II and IDRA(®). Eur J Ophthalmol. (2021) 31:2300–6. doi: 10.1177/1120672120969035
47. Arita R, Itoh K, Inoue K, Amano S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology. (2008) 115:911–5. doi: 10.1016/j.ophtha.2007.06.031
48. Jeon YJ, Song MY, Kim KY, Hwang K-Y, Kwon Y-A, Koh K. Relationship between the partial blink rate and ocular surface parameters. Int Ophthalmol. (2021) 41:2601–8. doi: 10.1007/s10792-021-01819-9
49. Jie Y, Sella R, Feng J, Gomez ML, Afshari NA. Evaluation of incomplete blinking as a measurement of dry eye disease. Ocul Surf. (2019) 17:440–6. doi: 10.1016/j.jtos.2019.05.007
50. Hui W, Xiao-Feng H, Song-Guo L, Jing-Jing W, Xuan H, Yong T. Application of orthokeratology on myopia control and its effect on ocular surface and meibomian gland function in Chinese myopic adolescents. Front Med (Lausanne). (2022) 9:979334. doi: 10.3389/fmed.2022.979334
51. Kojima T. Contact lens-associated dry eye disease: recent advances worldwide and in Japan. Invest Ophthalmol Vis Sci. (2018) 59:Des102-des108. doi: 10.1167/iovs.17-23685
52. Kaido M, Kawashima M, Shigeno Y, Yamada Y, Tsubota K. Randomized controlled study to investigate the effect of topical diquafosol tetrasodium on corneal sensitivity in short tear break-up time dry eye. Adv Ther. (2018) 35:697–706. doi: 10.1007/s12325-018-0685-1
53. Amano S, and Inoue K. Effect of topical 3% diquafosol sodium on eyes with dry eye disease and meibomian gland dysfunction. Clin Ophthalmol. (2017) 11:1677–82. doi: 10.2147/OPTH.S148167
54. Ikeda K, Simsek C, Kojima T, Higa K, Kawashima M, Dogru M, et al. The effects of 3% diquafosol sodium eye drop application on meibomian gland and ocular surface alterations in the Cu, Zn-superoxide dismutase-1 (Sod1) knockout mice. Graefes Arch Clin Exp Ophthalmol. (2018) 256:739–50. doi: 10.1007/s00417-018-3932-x
55. Martín-Montañez V, López-de la Rosa A, López-Miguel A, Pinto-Fraga J, González-Méijome JM, González-García MJ. End-of-day dryness, corneal sensitivity and blink rate in contact lens wearers. Cont Lens Anterior Eye. (2015) 38:148–51. doi: 10.1016/j.clae.2015.01.003
Keywords: diquafosol, ocular surface, discomfort, tear film stability, orthokeratology
Citation: Yang Y, Wu Q, Tang Y, Wu H, Luo Z, Gao W, Hu Z, Hou L, Wang M, Yang Z and Li X (2023) Short-term application of diquafosol ophthalmic solution benefits children with dry eye wearing orthokeratology lens. Front. Med. 10:1130117. doi: 10.3389/fmed.2023.1130117
Received: 09 January 2023; Accepted: 20 June 2023;
Published: 13 July 2023.
Edited by:
Gerami Seitzman, University of California, San Francisco, United StatesReviewed by:
Wanqing Jin, Wenzhou Medical University, ChinaCopyright © 2023 Yang, Wu, Tang, Wu, Luo, Gao, Hu, Hou, Wang, Yang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoning Li, bGl4aWFvbmluZ0BhaWVyY2hpbmEuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.