- 1Department of Nephrology, Arterial Hypertension, Dialysis and Transplantation, University Hospital Center Zagreb, Zagreb, Croatia
- 2Department of Gastroenterology, University Hospital Center Zagreb, Zagreb, Croatia
- 3School of Medicine, University of Zagreb, Zagreb, Croatia
- 4Department of Abdominal Surgery, University Hospital Center Zagreb, Zagreb, Croatia
- 5Department of Pathology, University Hospital Center Zagreb, Zagreb, Croatia
Chronic kidney disease (CKD) is a very common chronic non-communicable disease. Phosphate and calcium metabolism disorders are one of the most common features of CKD. Sevelamer carbonate is the most widely used non-calcium phosphate binder. Gastrointestinal (GI) injury associated with sevelamer use is a documented adverse effect but is underrecognized as a cause of gastrointestinal symptoms in patients with CKD. We report a case of a 74-year-old woman taking low-dose sevelamer with serious gastrointestinal adverse effects causing colon rupture and severe gastrointestinal bleeding.
1. Introduction
Sevelamer is an anion exchange resin used to lower hyperphosphatemia in CKD. Originally approved in 1998 as sevelamer hydrochloride, it has been largely replaced by sevelamer carbonate. Although both medications have similar efficacy, sevelamer carbonate has a lower risk of metabolic acidosis (1). Treatment of hyperphosphatemia is crucial in CKD patients, given its well-known association with vascular and endothelial damage, as well as increased mortality (2–4). Sevelamer is a calcium-free phosphate binder, thus avoiding the positive calcium balance induced by calcium-based binders, which is associated with increased mortality in CKD patients (5). It has also been suggested that sevelamer has beneficial pleiotropic effects (6). Sevelamer is a non-absorbable resin able to bind the phosphate in the gastrointestinal tract. Although side effects reported in patients receiving sevelamer include nausea, diarrhea, vomiting, dyspepsia, abdominal pain, flatulence, and constipation, there is little research to determine the mechanism of gastrointestinal symptoms (7). Sevelamer can also cause gastrointestinal tract motility disorders, which is mostly attributable to sevelamer hydrochloride (7).
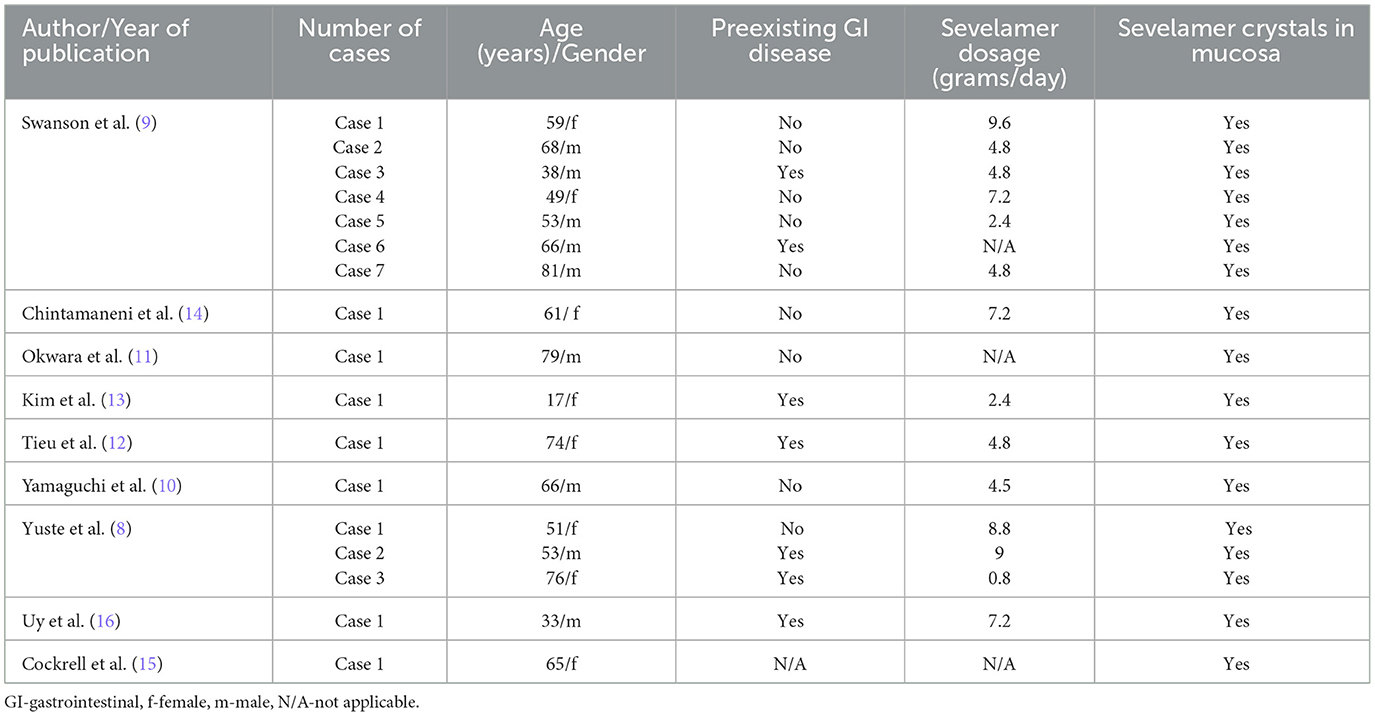
Sevelamer-associated gastrointestinal injury presents a diagnostic challenge and is probably underrecognized cause of gastrointestinal symptoms in patients with CKD. Sevelamer has been shown to have mucosal deposits throughout the gastrointestinal tract with great variability in associated symptoms. However, the most common clinical presentation of sevelamer-induced GI lesions is GI bleeding, followed by acute abdomen and GI discomfort (8). Diabetics seem to be more prone to develop sevelamer-associated GI lesions (8). Mucosal ulceration including wall perforation and post-inflammatory stricture formation are among the serious complications (10–16). There is also one report describing sevelamer induce colitis presenting as pseudotumor (11). Some patients with sevelamer-induced GI lesions had previous GI diseases (dyspepsia, peptic ulcer, diabetic gastroparesis, ulcerative colitis), GI infection caused by Clostridium difficile, or major abdominal surgery (8–16). Concomitant anticoagulant and/or antiplatelet therapy may also have influenced the clinical presentation (8–16). The average daily dose of sevelamer taken by the patients with GI lesions is 4.8 g (range 0.8–9.6 g), but the association between the sevelamer dosage and the severity of GI lesions was not clearly established (8). There is also no clear association between the form of the drug (powder or tablet) or anion content (carbonate or hydrochloride) and the quantity or severity of GI lesions (8). The summary of previous reports of gastrointestinal lesions induced by sevelamer is presented in Table 1.
Notable mucosal abnormalities found in patients with sevelamer-associated GI lesions include chronic mucosal damage, acute inflammation, inflammatory polyps, extensive ulceration, ischemia, and necrosis (9). Histological features of sevelamer crystals include irregularly arranged “fish-scale” crystals found within the GI mucosa with different colors during in vitro and in vivo studies (9). Patients with sevelamer deposits in the GI tract can also be asymptomatic (8).
As mentioned above, there is little information documenting serious gastrointestinal bleeding or colon rupture with sevelamer as a cause. Here we describe a case of sevelamer-induced various life-threatening gastrointestinal complications, namely bleeding, and pseudotumor, in a single patient on a low dose of sevelamer.
2. Case report
A 74-year-old woman with several comorbidities (arterial hypertension, diabetes mellitus, valvular heart disease, and chronic kidney disease) was admitted to the hospital due to lower gastrointestinal bleeding resulting in hemodynamic instability and shock.
The initial event of lower gastrointestinal bleeding was documented during the hospitalization 7 months earlier. At that point, the patient presented with rectorrhagia and worsening renal function. A multi-slice computed tomography scan scan revealed a thickened wall of the ascending colon and hepatic flexure with no other significant pathology. Lower endoscopy showed inflamed and friable mucosa of the entire circumference of the transverse colon, splenic flexure, and part of descending colon with normal rectal mucosa. Taking into account the typical endoscopic appearance, the patient was diagnosed with ischemic colitis. Her diabetes was well controlled with dietary measures. She had high C-reactive protein levels and was treated with antibiotics including ciprofloxacin and metronidazole which resulted in complete clinical and laboratory normalization (Table 2). Stool cultures were negative. Due to unspecific inflammation in the colon mesalazine was added (3 g/day). At discharge, the treatment with sevelamer carbonate was induced (1.6 g /day) due to worsening of the kidney function and hyperphosphatemia. Importantly, the patient was not taking sodium polystyrene sulfonate or any other cation exchange resin. Mesalazine therapy was stopped after 3 months since the patient had no GI symptoms.
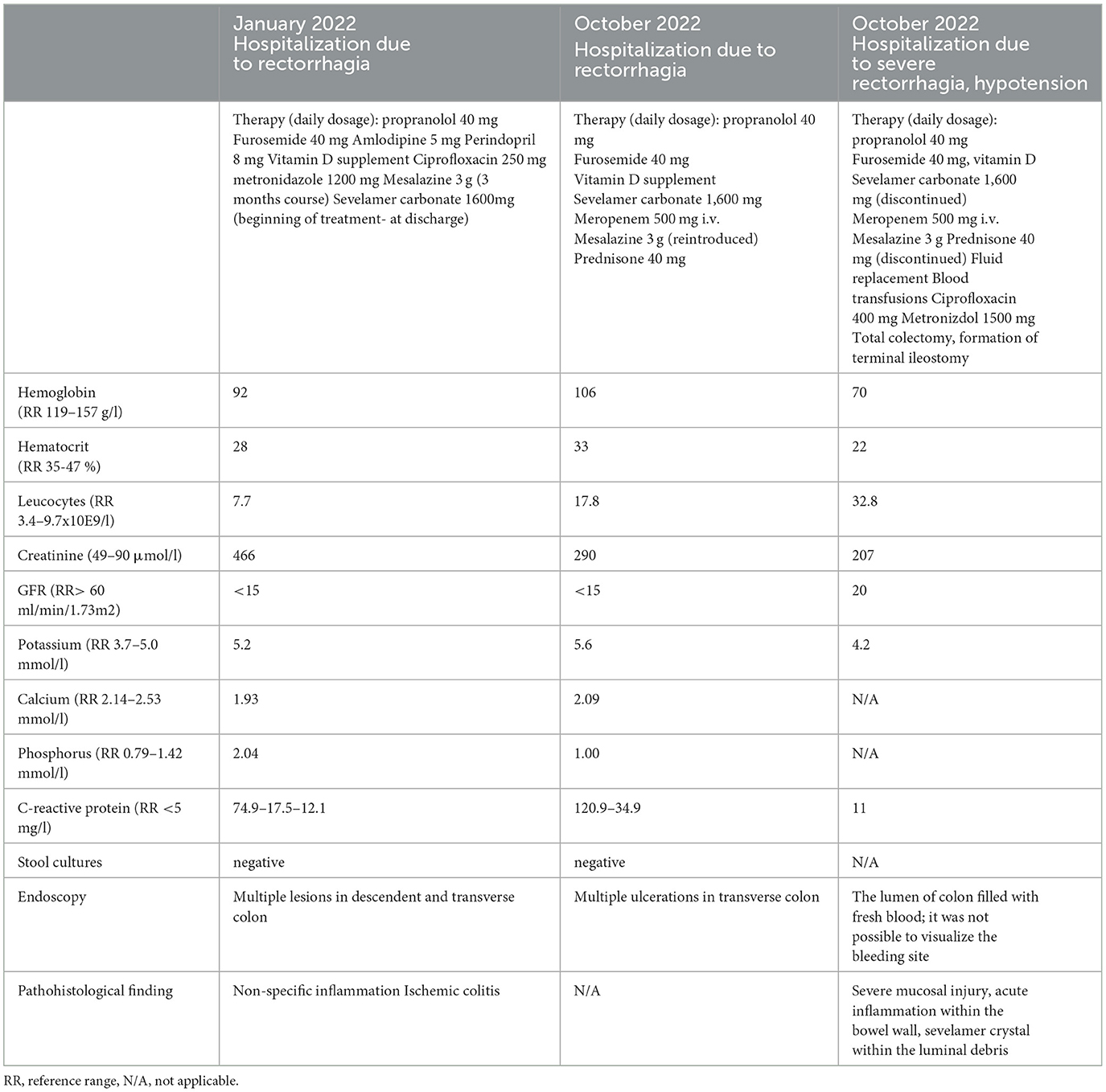
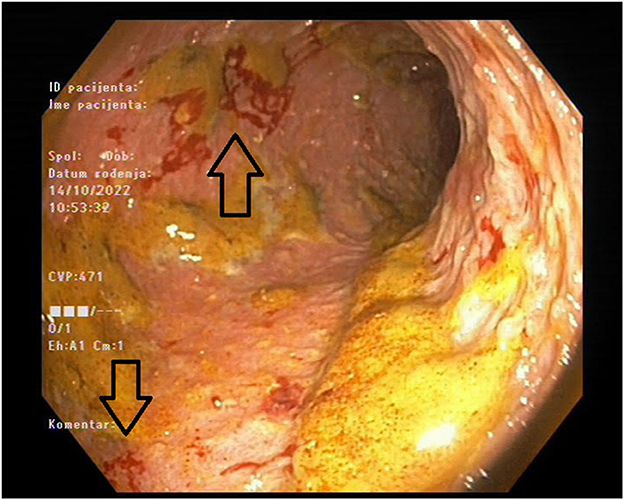
The patient was readmitted after 7 months and presented with diarrhea and abdominal pain. Her laboratory results showed again significantly increased inflammatory markers, but with negative stool cultures. Her kidney function was stable (Table 2). Endoscopy was performed and revealed a large ulceration in the transverse colon encompassing the whole circumference of the colon (Figure 1). A multi-slice computed tomography scan showed a significantly thickened wall of the cecum, ascending and transversal colon.
The patient was treated empirically with meropenem alongside other supportive treatments. Due to the presence of inflammatory changes in the colon, inflammatory bowel disease was suspected and, prednisone was started with rapid clinical and laboratory improvement. The patient was discharged after 10 days of hospitalization with normal bowel movements and no other symptoms. Two days after discharge, she was readmitted due to severe lower gastrointestinal bleeding with hemorrhagic shock. Despite the intensive treatment measures the patient remained unstable, and given the impossibility of definitive endoscopic hemostasis with hemodynamic instability patient was referred to urgent surgery.
Intraoperatively, the colon was described as malignantly altered, with the tumor-changed central part of the transverse colon, and the retained perforation. The total colectomy and terminal ileostomy were performed. The histopathological finding of the colon described severe mucosal injury with acute inflammation and visible characteristic “fish-scale” crystals of sevelamer within the luminal debris, accompanied by acute inflammation and focal necrosis of the bowel wall, with no signs of dysplasia or malignant alteration (Figures 2A, B). The conclusion of these findings was that the necrosis of the colonic wall was caused by sevelamer, and the drug was discontinued. Postsurgical recovery was complicated by an episode of cardiac decompensation which resolved with optimized diuretic treatment. The patient was discharged after 8 days of hospitalization in stable clinical condition.
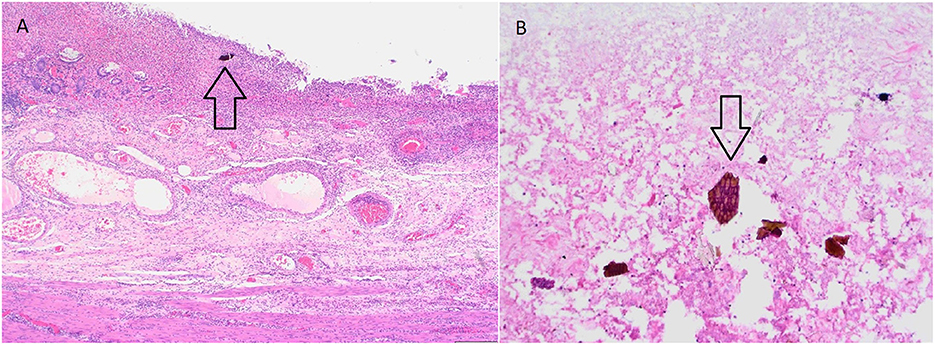
Figure 2. (A) Severe mucosal injury with acute inflammation within the bowel wall. Note the sevelamer crystal within the luminal debris. (H&E, 40x). (B) Sevelamer crystal within the luminal debris—characteristic fish scale appearance with orange and pinkish hue (H&E, 200x).
3. Discussion
Sevelamer is an anion-exchange resin designed for the treatment of hyperphosphatemia in patients with chronic renal disease. Its structure is made of a non-absorbed hydrogel with ammonia on the hydrochloride or the carbonate. Due to the acidic content of the stomach, the polymer dissociates from its anion and consequently is available to bind phosphate within the intestine (17). Sevelamer also causes dehydration in the intestinal tract, resulting in the formation of hard stool (7).
Despite the side effects reported in patients receiving sevelamer, e.g., nausea, diarrhea, vomiting, dyspepsia, abdominal pain, flatulence, and constipation, there is little research to determine the direct mechanism of sevelamer on the gastrointestinal tract (18–20). Post-marketing data show rare cases of ileus and intestinal obstruction, attributable mainly to sevelamer hydrochloride, but the frequency of severe intestinal adverse effects is unknown (7). The direct effect of hard stool and constipation following sevelamer administration may trigger perforation, but given the lack of investigation the potential cause remains unclear. Moreover, in patients undergoing hemodialysis, hypotension episodes and hypovolemia may cause mesenteric ischemia and intestinal necrosis, making the mucosa more vulnerable to other causes of injury (9, 10). Swanson et al. described the histopathology of sevelamer crystals in the GI tract, and their association with mucosal abnormalities which can be found in the esophagus, small bowel, and colon (9).
It has been reported that patients on hemodialysis have a high risk of colonic perforation even without sevelamer administration, and there were no significant changes in the incidence of bowel perforation after the approval of sevelamer (21, 22).
In our case, the patient had the serious adverse effects of sevelamer therapy presenting with life-threatening GI hemorrhage. Although our patient was diagnosed with ischemic colitis, she responded well to mesalazine therapy and had no GI symptoms. The sevelamer was induced after the first hospitalization when her GI symptoms resolved. Thus, we cannot conclude with certainty that our patient had an underlying GI disease, contrary to theother reports in the literature (8–16). On the other hand, our patient is diabetic, which makes her more prone to sevelamer-induced GI lesions (8). Our patient was treated with a relatively low dose of sevelamer (1.6 g/day), and the duration of therapy was only seven months. However, previous reports did not establish the association between the sevelamer dosage and the severity of GI lesions, which occurred also in patients taking 800 mg sevelamer daily (8). Moreover, our patient showed characteristic histopathological findings of sevelamer crystals in lesion biopsies and direct deposition of sevelamer crystals in areas of ulceration and pseudotumor formation was found. In conclusion, we believe that sevelamer use is the most likely etiology of pseudotumor formation and restrained rupture of the transverse colon in our patient. Sevelamer-induced GI lesions should always be considered in CKD patients treated with sevelamer who present with GI symptoms, especially lower GI bleeding, regardless of the sevelamer dosage. Sevelamer therapy should be prescribed with caution in patients with a history of major abdominal surgery, chronic GI disease, and diabetes given the high risk of serious GI complications in this patient population.
4. Patient perspective
Our patient was hospitalized twice in a short period of time due to abdominal pain and bloody stools. Her appetite was poor and she was feeling ill, but responded well to treatment. A few days after being discharged, she was again hospitalized due to bloody stools and abdominal pain. She felt very weak. After the surgery, she had breathing difficulties, which resolved after therapy. She was discharged from the hospital and is currently in-home care.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MF, MJ, MB, DG, and IR: substantial contribution to the conception of the work, interpretation of findings, drafting the work, and final approval of the report. ZM: analysis of kidney biopsy material, interpretation of findings, critical revising of data for the work, and final approval of the report. EI: substantial contribution to the conception of the work, interpretation of data, critical revising of the work, and final approval of the report. BJ, IV, and ZK: interpretation of data, critical revising of the work, and final approval of the report. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pai AB, Shepler BM. Comparison of sevelamer hydrochloride and sevelamer carbonate: risk of metabolic acidosis and clinical implications. Pharmacotherapy. (2009) 29:554–61. doi: 10.1592/phco.29.5.554
2. Shanahan CM, Crouthamel MH, Kapustin A, Giachelli CM. Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ Res. (2011) 109:697–711. doi: 10.1161/CIRCRESAHA.110.234914
3. Abbasian N, Burton JO, Herbert KE, Tregunna BE, Brown JR, Ghaderi-Najafabadi M. Hyperphosphataemia, phosphoprotein phosphatases, and microparticle release in vascular endothelial cells. J Am Soc Nephrol. (2015) 26:2152–62. doi: 10.1681/ASN.2014070642
4. Floege J. Phosphate binders in chronic kidney disease: a systematic review of recent data. J Nephrol. (2016) 29:329–40. doi: 10.1007/s40620-016-0266-9
5. Patel L, Bernard LM, Elder GJ. Sevelamer vs. calcium-based binders for treatment of hyperphosphatemia in CKD: a meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol. (2016) 11:232–44. doi: 10.2215/CJN.06800615
6. Cozzolino M, Rizzo MA, Stucchi A, Cusi D, Gallieni M. Sevelamer for hyperphosphataemia in kidney failure: controversy and perspective. Ther Adv Chronic Dis. (2012) 3:59–68. doi: 10.1177/2040622311433771
8. Yuste C, Mérida E, Hernández E, García-Santiago A, Rodríguez Y, Muñoz T, et al. Gastrointestinal complications induced by sevelamer crystals. Clin Kidney J. (2017) 10:539–44. doi: 10.1093/ckj/sfx013
9. Swanson BJ, Limketkai BN, Liu TC, Montgomery E, Nazari K, Park JY. Sevelamer crystals in the gastrointestinal tract (GIT). Am J Surg Pathol. (2013) 37:1686–93. doi: 10.1097/PAS.0b013e3182999d8d
10. Yamaguchi T, Ohyama S, Furukawa H, Sato N, Ohnishi I, Kasashima S, et al. Sigmoid colon diverticula perforation associated with sevelamer hydrochloride administration: A case report. Ann Med Surg. (2016) 10:57–60. doi: 10.1016/j.amsu.2016.07.020
11. Okwara C, Choi C, Park JY. Sevelamer-induced colitis presenting as a pseudotumour. Clin Gastroenterol Hepatol. (2015) 13:A39–40. doi: 10.1016/j.cgh.2015.02.015
12. Tieu C, Moreira RK, Song LM, Majumder S, Papadakis KA, Hogan MC. A case report of sevelamer-associated recto-sigmoid ulcers. BMC Gastroenterol. (2016) 16:20. doi: 10.1186/s12876-016-0441-4
13. Kim J, Olson K, Butani L. Sevelamer crystals in the mucosa of the gastrointestinal tract in a teenager with end-stage renal disease. Pediatr Nephrol. (2016) 31:339–41. doi: 10.1007/s00467-015-3269-1
14. Chintamaneni P, Das R, Kuan SF, Kermanshahi TR, Hashash JG. Hematochezia associated with sevalamer-induced mucosal injury. ACG Case Rep J. (2014) 1:145–7. doi: 10.14309/crj.2014.32
15. Cockrell HC, Cottrell-Cumber S, Brown K, Murphy JG. Sevelamer crystals-an unusual case of large bowel obstruction. J Surg Case Rep. (2021) 6:1–2. doi: 10.1093/jscr/rjab228
16. Uy PP, Vinsard DG, Hafeez S. Sevelamer-associated rectosigmoid ulcers in an end-stage renal disease patient. ACG Case Rep J. (2018) 28:5. doi: 10.14309/02075970-201805000-00083
17. Barna M, Kapoian T. O'Mara. Sevelamer carbonate. Ann Pharmacother. (2010) 44:127–34. doi: 10.1345/aph.1M291
18. Tonelli M, Pannu N, Manns B. Oral phosphate binders in patients with kidney failure. N Engl J Med. (2010) 362:1312–24. doi: 10.1056/NEJMra0912522
19. Navaneethan SD, Palmer SC, Craig JC, Elder GJ, Strippoli GFM. Benefits and harms of phosphate binders in CKD: a systematic review of randomized controlled trials. Am J Kidney Dis. (2009) 54:619–37. doi: 10.1053/j.ajkd.2009.06.004
20. Evenepoel P, Selgas R, Caputo F, Foggensteiner L, Heaf JG, Ortiz A, et al. Efficacy and safety of sevelamer hydrochloride and calcium acetate in patients on peritoneal dialysis. Nephrol Dial Transpl. (2009) 24:278–85. doi: 10.1093/ndt/gfn488
21. Nozoe T, Matsumata T, Sugimachi K. Surgical strategy to save patients with colon perforation with chronic renal failure on long-term hemodialysis. Hepato-gastroenterol. (2002) 50:385–7.
Keywords: gastrointestinal lesion, gastrointestinal bleeding, colon rupture, chronic kidney disease, sevelamer carbonate, case report
Citation: Fistrek Prlic M, Jelakovic M, Brinar M, Grgic D, Romic I, Marusic Z, Ivandic E, Jelakovic B, Vukovic Brinar I and Krznaric Z (2023) Case report: Sevelamer-associated colitis—a cause of pseudotumor formation with colon perforation and life-threatening bleeding. Front. Med. 10:1097469. doi: 10.3389/fmed.2023.1097469
Received: 13 November 2022; Accepted: 07 April 2023;
Published: 27 April 2023.
Edited by:
Sree Bhushan Raju, Nizam's Institute of Medical Sciences, IndiaReviewed by:
Jung Eun Lee, Yonsei University, Republic of KoreaLin-Lin Li, Henan Provincial People's Hospital, China
Copyright © 2023 Fistrek Prlic, Jelakovic, Brinar, Grgic, Romic, Marusic, Ivandic, Jelakovic, Vukovic Brinar and Krznaric. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Margareta Fistrek Prlic, bWFyZ2FyZXRhLmZpc3RyZWtAZ21haWwuY29t
†These authors have contributed equally to this work and share first authorship
 Margareta Fistrek Prlic
Margareta Fistrek Prlic Mislav Jelakovic2†
Mislav Jelakovic2† Dora Grgic
Dora Grgic Ivan Romic
Ivan Romic Zlatko Marusic
Zlatko Marusic Ema Ivandic
Ema Ivandic Bojan Jelakovic
Bojan Jelakovic Ivana Vukovic Brinar
Ivana Vukovic Brinar