- 1Division of Family Medicine, Department of Family and Community Medicine, Tri-Service General Hospital, School of Medicine, Taipei, Taiwan
- 2National Defense Medical Center, School of Medicine, Taipei, Taiwan
- 3Division of Geriatric Medicine, Department of Family and Community Medicine, Tri-Service General Hospital Taipei, Taipei, Taiwan
- 4Department of Medical Education, Taipei Veterans General Hospital, Taipei, Taiwan
- 5Department of Biochemistry, National Defense Medical Center, Taipei, Taiwan
Aim: This investigation explored the relationship between oral bacteria and metabolic syndrome (METS).
Materials and Methods: There were 4,882 subjects enrolled in this cross-sectional study from the NHANES III database. The severity of periodontitis was classified into mild, moderate and severe. We measured oral bacterial antibodies. We examined the relationship between serum immunoglobulin G (IgG) antibodies of oral bacteria and METS via performing multivariate regression analysis. Mediation analysis of oral bacteria on the correlation between periodontitis and METS was also executed.
Results: After adjusting for covariates, the serum IgG antibodies of P. nigrescens, E. corrodens, and E. nodatum were associated with the presence of METS (p = 0.006, p = 0.014 and p = 0.018, respectively). Furthermore, serum IgG antibodies of P. intermedia, T. forsythia and V. parvula were positively associated with the presence of METS (p = 0.001, p = 0.011, and p = 0.002, respectively) and ≥4 features of METS (p = 0.019, p = 0.025, and p = 0.02, respectively). P. intermedia IgG mediated 11.2% of the relationship between periodontitis and METS.
Conclusion: Serological markers of oral pathogens were correlated with the presence and the number of METS features after multivariable adjustment. Oral bacteria acted as a mediator of the correlation between periodontitis and METS. Our study provided a biologically plausible explanation for the association between periodontitis and METS, which provides a comprehensive evaluation of periodontitis.
Introduction
Periodontitis, a chronic inflammatory disease, affects 10–15% of the global population and results in gingival recession, alveolar bone destruction, and tooth loss (1). Dental plaque biofilms formed by periodontal microorganisms are possibly the major etiologic factors (2). Numerous periodontopathic bacteria, rather than a single periodontal pathogen, may contribute to periodontitis (2). Particular periodontal bacteria, such as Porphyromonas gingivalis, Prevotella intermedia, Tannerella forsythia, Aggregatibacter actinomycetemcomitans, and Treponema denticola, are crucial causative pathogens (3). Periodontal bacterial infection may elicit a serum immunoglobulin G (IgG) antibody response. The presence of antibodies to specific periodontal species may represent the clinical periodontal status (4).
Metabolic syndrome (METS) is an emerging public health disorder worldwide (5). Multiple factors such as sociodemographic, environmental, and genetic features influence METS (6). It is well-established that METS is associated with increased odds of cardiovascular disease, diabetes, cancer, and all-cause mortality (7). Several hypotheses, including endothelial disruption, chronic inflammation, visceral fat accumulation, and insulin resistance have been proposed as possible mechanisms (8). Endothelial dysfunction was observed in Zucker obese rats, a model of METS (9). Excessive inflammatory biomarkers were linked to the development of METS (10). Tumor necrosis factor alpha (TNF-α) may interfere with insulin signaling in human skeletal muscle (11). A case control study revealed higher interleukin-6 (IL-6) levels in subjects with METS (12). Adipokine derangement and adipocyte inflammation were implicated in the pathogenesis of METS (13). Thus, METS is considered as a chronic low-grade inflammatory status (14).
Studies reveal that periodontitis is correlated with obesity, hypertension, diabetes mellitus, and cardiovascular diseases (15–17). Several possible pathways, including endothelial dysfunction, an imbalanced immune response and oxidative stress have been presented (18, 19). Epidemiological research has examined the relationship between periodontitis and METS (20). Previous investigations indicate that periodontitis and METS are both associated with systemic inflammation, suggesting a similar pathophysiological mechanism linking these two diseases (15). However, few reports have conducted a comprehensive analysis between oral bacteria and METS. This study's objectives were to investigate the correlation between the serologic markers of 19 oral bacteria and METS and to ascertain the mediation effect of oral bacteria on the correlation between periodontitis and METS.
Methods
Study design and participants
Data were retrieved from the Third National Health and Nutrition Examination Survey (NHANES III) and examined for this cross-sectional study. NHANES III, an observational survey of a non-institutionalized population from the United States, was conducted by the Centers for Disease Control and Prevention and the National Center for Health Statistics between October of 1988 and October of 1994. The survey consisted of sociodemographic information, a clinical examination, medical history and laboratory data. The NHANES III survey had National Center for Health Statistics Institutional Review Board approval and details of its study protocols and consent documents were available on the NHANES website. We excluded subjects without complete information regarding the severity of periodontitis, serum IgG antibody titers against the 19 oral pathogens, the number of METS features, laboratory data, and past history. A total of 4,882 suitable subjects were initially recruited in our study.
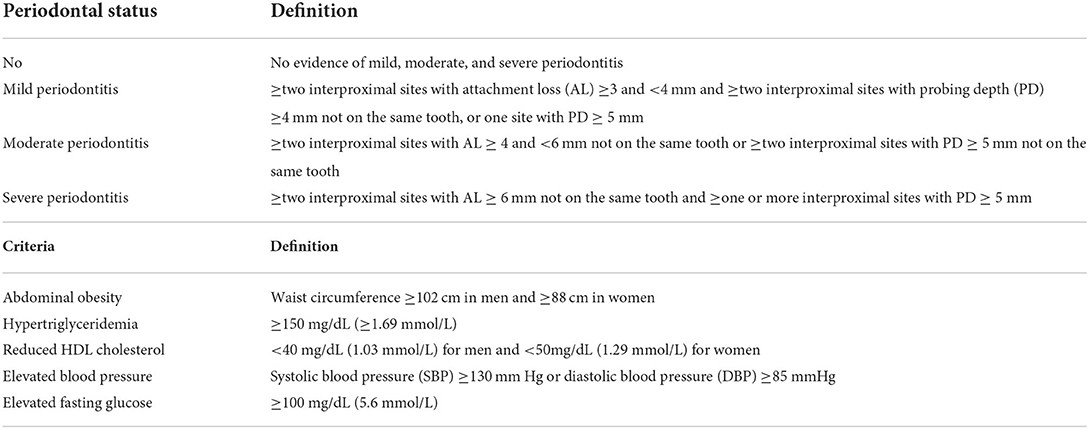
Definition of periodontitis
The definition and severity of periodontitis were assessed by the pocket depth and attachment loss (21). Table 1 shows the four categories of periodontitis severity.
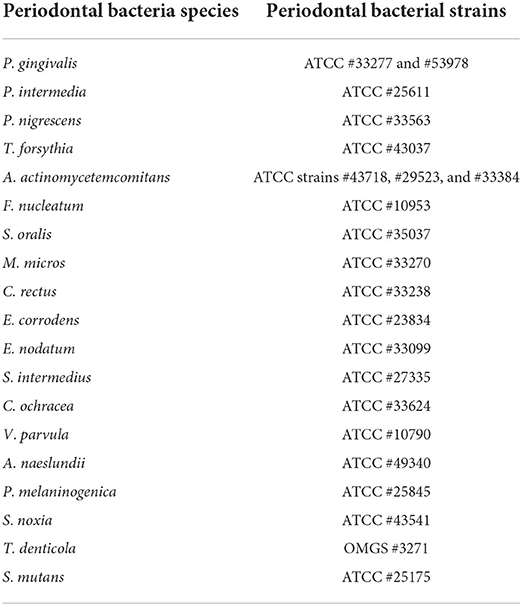
Serum IgG antibody measurement of oral pathogens
The level of serum IgG antibodies of 19 oral pathogens was determined using a rapid checkerboard immunoblotting technique (22). Table 2 shows the 19 oral bacterial species and strains. The bacterial IgG titers are presented as Elisa Units (EU).
Definition of METS
The presence of METS was defined by the revised National Cholesterol Education Program's Adult Treatment Panel III (23). Table 1 lists the five components of METS. The participants were considered to have METS if they had three or more of the components.
Covariates
Individual characteristics, including age, sex, race/ethnicity, education, smoking history, and medical records, were collected from a self-reported questionnaire. Subjects were categorized as smokers if they had smoked at least 100 cigarettes in their lifetime. We also recorded diabetes mellitus diagnosed by a doctor and measured BMI as the body weight in kilograms divided by the square of the body height in meters (kg/m2). Waist circumference was measured to the nearest 0.1 cm around the horizontal line at the high point of the iliac crest. We performed blood pressure measurements using a mercury sphygmomanometer. The fasting blood sugar level was quantified by a modified enzymatic reaction. The serum total cholesterol level and serum HDL cholesterol level were quantified by a Hitachi 737 analyzer (Boehringer-Mannheim Diagnostics, Indianapolis, IN). The data collection and laboratory procedures followed standardized guidelines and protocols.
Statistical analysis
We classified the subjects based on periodontitis severity. Socio-demographic characteristics, laboratory variables, and other co-variables were compared between subjects using the one-way ANOVA and chi-square test. We examined the association between periodontitis and the number of METS features by performing multivariate regression analysis. In addition, the relationship between the IgG antibody of the oral bacteria and the number of METS features was also assessed by multivariate regression analysis. Covariates including age, sex, race/ethnicity, BMI, education, smoking, periodontitis, and diabetes mellitus, were adjusted.
We performed a mediation analysis to assess which oral bacteria mediate the relationship between periodontitis (independent variable) and METS (outcome variable) (24). Bootstrapping with 1,000 replicates was performed to calculate the medication effects without making assumptions about the normality of the sample distribution (25). The direct and indirect (mediation) effects and 95% CIs were calculated using the bias-corrected bootstrap method. If the 95% bootstrap CIs did not include zero, the indirect effect of the independent variable on the outcome variable as mediated through the intermediary was considered significant. The proportion of the indirect effect was quantified by the following formula: ORdirect effect (ORindirect effect-1)*100/(ORdirect effect × ORindirect effect-1) (26). All statistical analyses and data management were executed using SPSS version 18 (SPSS Inc., Chicago, IL, USA).
Results
Characteristics of the study population
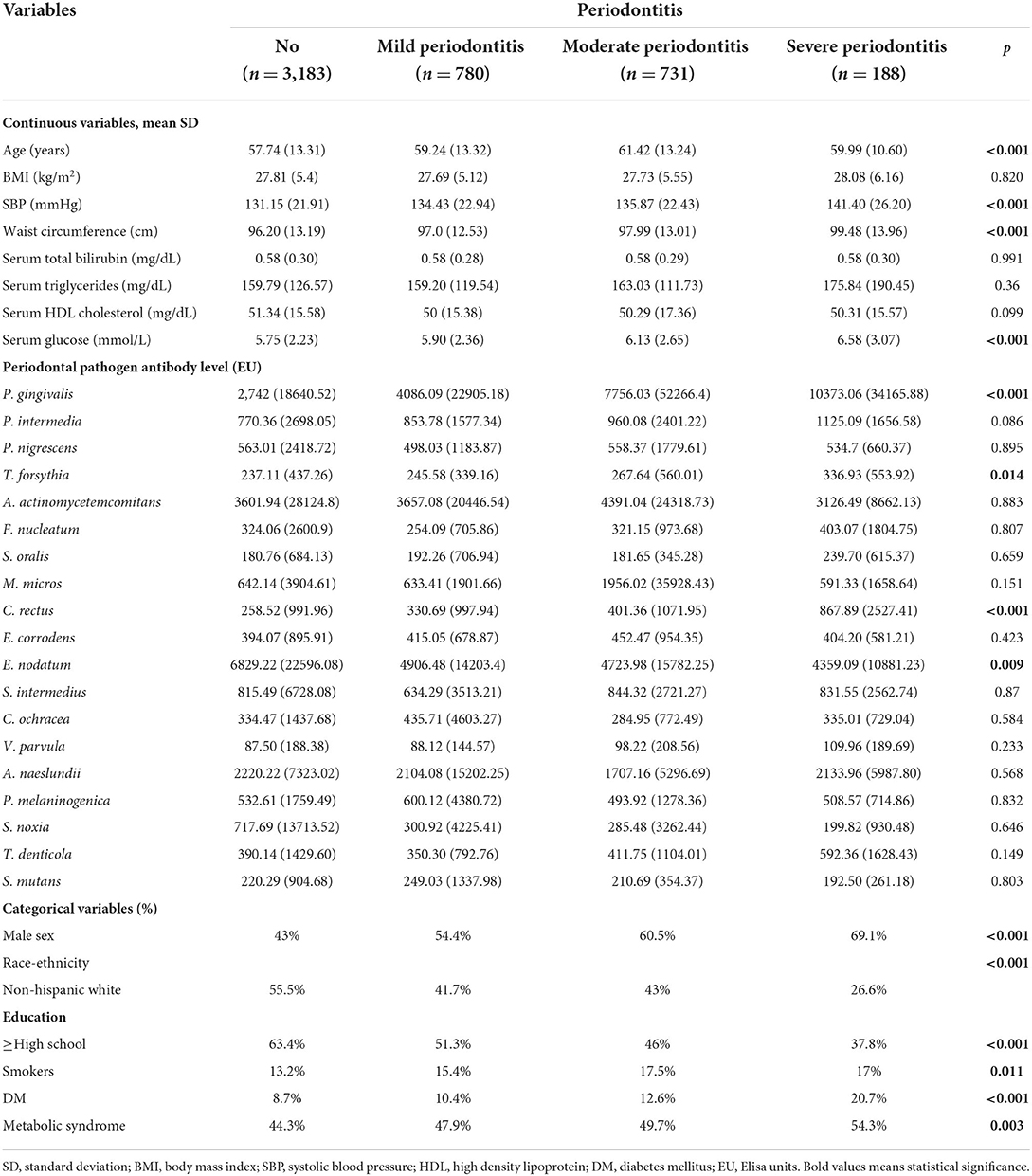
Table 3 presents the characteristics of the participants stratified by periodontitis severity. The average age of the subjects with no, mild, moderate and severe periodontitis was 57.74 ± 13.31, 59.24 ± 13.32, 61.42 ± 13.42, and 59.99 ± 10.60 years, respectively. The SBP of the participants with no, mild, moderate and severe periodontitis was 131.15 ± 21.91, 134.43 ± 22.94, 135.87 ± 22.43, and 141.40 ± 26.20 mmHg, respectively (p < 0.001). The waist circumference of the participants with no, mild, moderate and severe periodontitis was 96.20 ± 13.19, 97.0 ± 12.53, 97.99 ± 13.01, and 99.48 ± 13.96 cm, respectively (p < 0.001).
The serum glucose level of the participants with no, mild, moderate and severe periodontitis was 5.75 ± 2.23, 5.90 ± 2.36, 6.13 ± 2.65, and 6.58 ± 3.07 mmol/L, respectively (p < 0.001). The proportion of METS raised from 44.3% among subjects with no periodontitis to 47.9, 49.7, and 54.3% for participants with mild, moderate, and severe periodontitis, respectively (p = 0.003). The mean concentration of P. gingivalis IgG of the participants with no, mild, moderate, and severe periodontitis was 2,742 ± 18640.52, 4086.09 ± 22905.18, 7756.03 ± 52266.4, and 10373.06 ± 34165.88 EU, respectively (p < 0.001). The mean concentration of C. recuts IgG of the participants with no, mild, moderate and severe periodontitis was 258.52 ± 991.96, 330.69 ± 997.94, 401.36 ± 1071.95, and 867.89 ± 2527.41 EU, respectively (p < 0.001).
Associations of serum IgG antibodies of oral pathogens and the number of metabolic syndrome features
Table 4 illustrates the relationship between oral pathogens and the number of metabolic syndrome features using a multivariable model. P. nigrescens IgG was correlated with the presence of METS (β = 192.91, p = 0.006). E. corrodens IgG was correlated with the presence of METS (β = 69.66, p = 0.014). E. nodatum IgG was correlated with the presence of METS (β = 1565.63, p = 0.018). P. intermedia IgG was correlated with the presence of METS and ≥4 features of METS (β = 277.76, p = 0.001; β = 633.45, p = 0.019, respectively). T. forsythia IgG was correlated with the presence of METS and ≥4 features of METS (β = 37.22, p = 0.011; β = 109.71, p = 0.025, respectively). V. parvula IgG was correlated with the presence of METS and ≥4 features of METS (β = 18.66, p = 0.002; β = 47.23, p = 0.02, respectively).
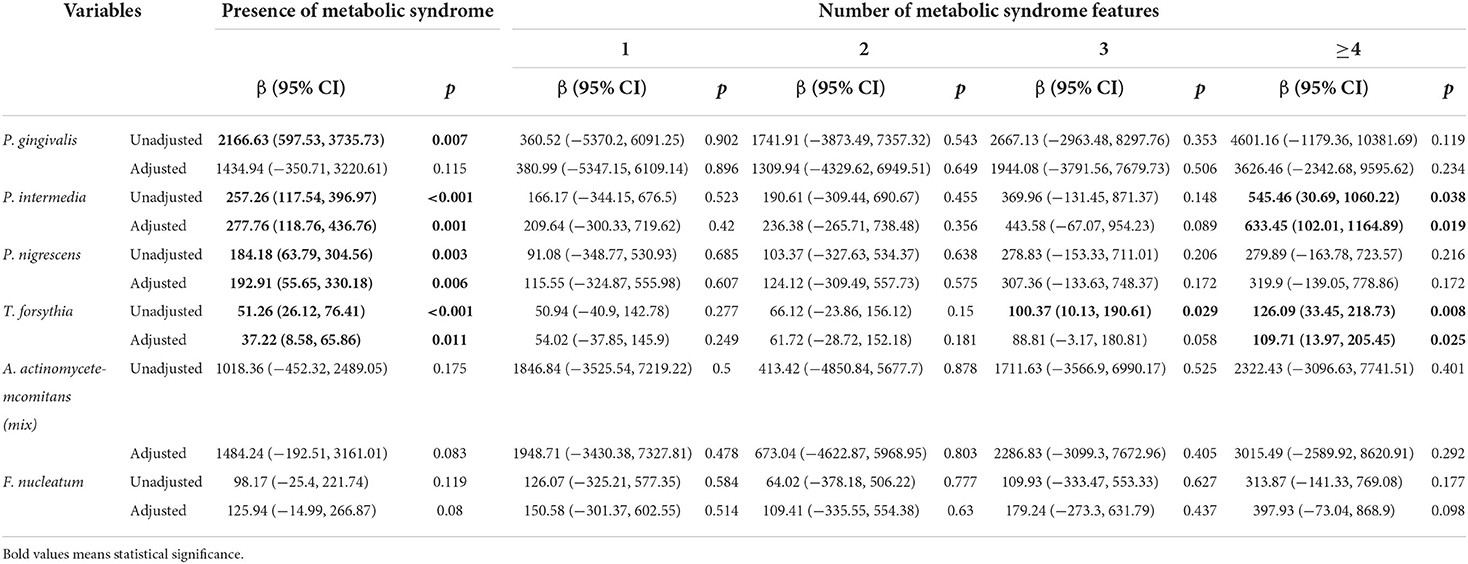
Table 4.1. Regression coefficients of the number of metabolic syndrome features for periodontal pathogens.
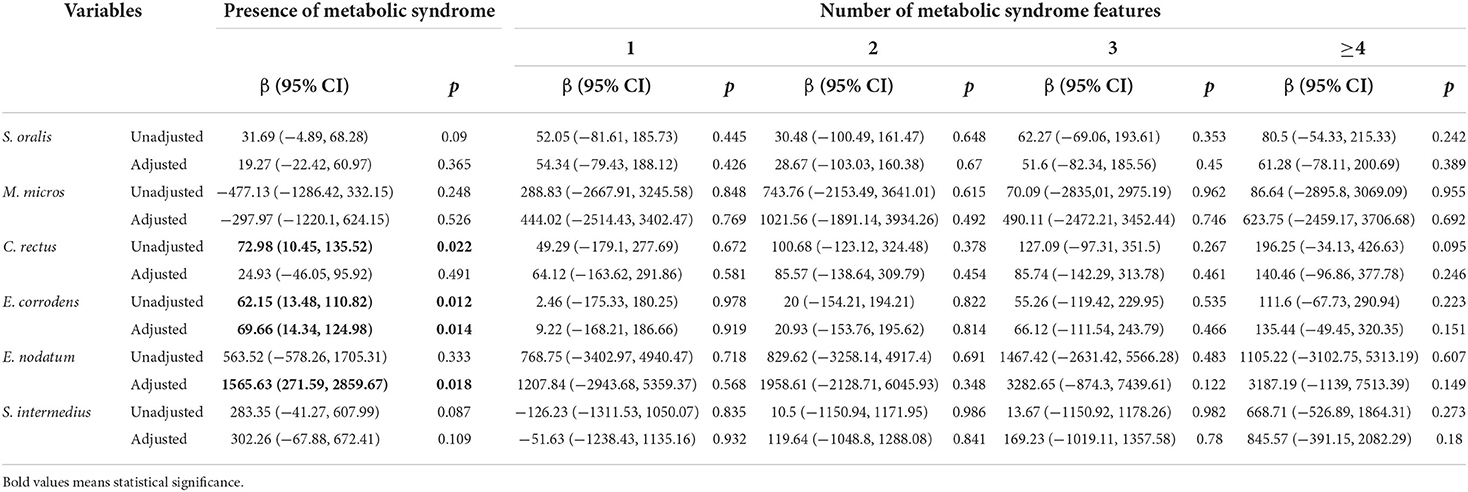
Table 4.2. Regression coefficients of the number of metabolic syndrome features for periodontal pathogens.
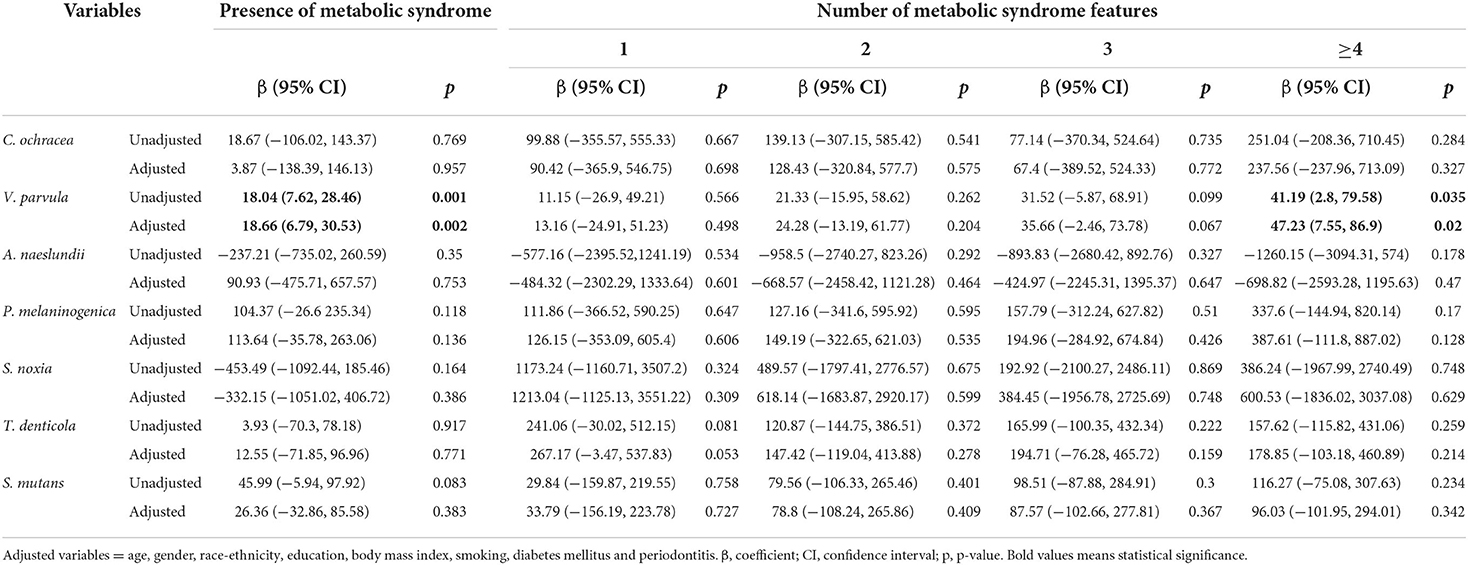
Table 4.3. Regression coefficients of the number of metabolic syndrome features for periodontal pathogens.
Mediation of serum IgG antibodies of oral pathogens for the association between periodontitis and METS
Table 5 presents the significant indirect effect of periodontitis on METS as only mediated through P. intermedia IgG (β = 0.003; 95% CI: 0.0007, 0.01). The proportion mediated through P. intermedia IgG was 11.2%. The indirect effects mediated through the other five oral bacterial antibodies were not significant, as their 95% CIs included zero.

Table 5. Mediation of serum IgG antibodies of oral bacteria for the association between periodontitis and metabolic syndrome.
Discussion
This study draws attention to the association between serum IgG antibodies of oral pathogens and the number of METS features. We observed that the antibodies of two periodontal bacterial (P. gingivalis and C. recuts) increased with the severity of periodontitis in a univariate analysis (Table 3). Participants with more severe periodontitis had a higher proportion of METS compared to those without periodontitis. We demonstrated a positive relationship between the antibodies of P. nigrescens, E. corrodens, E. nodatum, and the presence of METS. The antibodies of P. intermedia, T. forsythia, and V. parvula were positively correlated with the presence of METS and ≥4 features of METS. We observed an indirect effect between periodontitis and METS through P. intermedia IgG in the mediation analysis.
Previous literature revealed that periodontal bacteria was associated with the component of METS. A Japanese cross-sectional study reported that an elevated level of antibodies against P. gingivalis was observed in METS (27). Periodontal bacterial antibody (P. gingivalis and P. intermedia) titers were correlated with elevated blood glucose levels (28). The Oral Infections and Vascular Disease Epidemiology Study noted that compared with the lowest subgingival periodontal bacterial burden, subjects with the highest subgingival periodontal bacterial burden had a three times greater chance of having hypertension (29). An observational study from Columbia demonstrated antibodies of periodontopathic bacteria were associated with a reduced level of HDL cholesterol (30). In addition, serologic markers of periodontal pathogens are correlated with an elevated odd of stroke (31), type 2 diabetes mellitus (32), and coronary heart disease (33).
Studies have suggested that periodontal bacteria may induce an immune response and inflammatory processes (34). The serum IgG antibodies against P. gingivalis, P. intermedia, and E. corrodens are linked to oxidative stress (35). The local infections caused by periodontal bacteria were associated with systemic inflammatory markers including C-reactive protein (CRP), IL-6, and TNF-α (36, 37). Several plausible mechanisms have been proposed. Lipopolysaccharide, a constituent of the outer membrane of periodontopathogenic bacteria such as P. intermedia, T. forsythia, and V. parvula has been indicated to stimulate the production of various proinflammatory mediators (38–40). In an experimental study by Sun et al. (41), lipopolysaccharides elicited the release of inflammatory cytokines through toll-like receptors 2 and 4. Furthermore, complement system activation and neuropeptide modulation are linked to inflammation (42). Complement receptor 3 (CR3) activation by periodontal bacterial fimbriae and C5a accumulation may result in increased inflammation (43). Substance P, a neuropeptide, is involved in periodontal inflammation process (44). Collectively, these results suggest that periodontal bacterial infection could cause immune dysregulation (45) and result in systemic inflammation (1). Systemic inflammation plays an essential role in the pathogenesis of METS (46). Oxidative stress was also recognized as a potential pathophysiological link to the association between METS and periodontitis (47). Although the mechanism underlying the relationship between periodontal bacteria and METS remains unclear, the abovementioned investigations indicate that periodontal infection and subsequent inflammation might increase the risk of METS.
In this study, we discovered a mediation effect of P. intermedia on the association between periodontitis and METS. Our analyses imply that periodontal bacteria antibodies play a modest role in the mediation analysis. Prior reports have indicated the mediation effect of systemic inflammation regarding periodontitis. Demmer et al. (48) reported that inflammatory markers act as a mediator in the association between periodontal infection and insulin resistance. A cross-sectional study from Thailand revealed CRP (5.2%) and white blood count (19.1%) are mediators of the relationship between periodontitis and impaired fasting glucose (49). The significant indirect effect of periodontal infection on hypertension mediated through CRP was determined via two national databases (50).
There are several limitations in the present investigation. The cross-sectional analysis restricts the causal relationship between oral bacteria and METS. Our data were retrieved from a single database, and the study population was mainly Caucasian. Thus, the generalization of our results is limited. Lastly, because of the nature of observational studies, our results will inevitably be influenced by residual confounding factors due to unmeasured covariates.
Conclusion
Our study extended the examinations of the commonly reported relationship between P. gingivalis and P. intermedia to 19 oral bacterial antibodies. The serum IgG antibodies of oral pathogens were correlated with the presence and the number of METS features. We also conducted a mediation analysis, which revealed that P. intermedia acts as a mediator in the correlation between periodontitis and METS. An experimental model and prospective study are warranted to investigate the mechanisms underlying the observed correlations and to explore the potential median effect of oral bacteria.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by the National Center for Health Statistics Research Ethics Review Board. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
Z-YY: conceptualization, investigation, visualization, and writing—original draft preparation. W-HF: methodology, formal analysis, and supervision. C-CK: investigation and writing—original draft preparation. W-LC: conceptualization, data curation, methodology, formal analysis, supervision, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
METS, metabolic syndrome; BMI, body mass index; CRP, c-reactive protein; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; IgG, immunoglobulin G; CR3, complement receptor 3; EU, elisa units; NCHS, National Center for Health Statistics; NHANES III, the third National Health and Nutrition Examination Survey; HDL, high density lipoprotein; SBP, systolic blood pressure.
References
1. Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. (2017) 3:17038. doi: 10.1038/nrdp.2017.38
2. Harvey JD. Periodontal microbiology. Dent Clin North Am. (2017) 61:253–69. doi: 10.1016/j.cden.2016.11.005
3. Socransky SS, Haffajee AD. Dental biofilms: difficult therapeutic targets. Periodontol 2000. (2002) 28:12–55. doi: 10.1034/j.1600-0757.2002.280102.x
4. Dye BA, Herrera-Abreu M, Lerche-Sehm J, Vlachojannis C, Pikdoken L, Pretzl B, et al. Serum antibodies to periodontal bacteria as diagnostic markers of periodontitis. J Periodontol. (2009) 80:634–47. doi: 10.1902/jop.2009.080474
5. Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. (2018) 20:12. doi: 10.1007/s11906-018-0812-z
6. Bovolini A, Garcia J, Andrade MA, Duarte JA. Metabolic syndrome pathophysiology and predisposing factors. Int J Sports Med. (2021) 42:199–214. doi: 10.1055/a-1263-0898
7. O'Neill S, O'Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. (2015) 16:1–12. doi: 10.1111/obr.12229
8. Rochlani Y, Pothineni NV, Kovelamudi S, Mehta JL. Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Ther Adv Cardiovasc Dis. (2017) 11:215–25. doi: 10.1177/1753944717711379
9. Picchi A, Gao X, Belmadani S, Potter BJ, Focardi M, Chilian WM, et al. Tumor necrosis factor-alpha induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ Res. (2006) 99:69–77. doi: 10.1161/01.RES.0000229685.37402.80
10. Koh KK, Han SH, Quon MJ. Inflammatory markers and the metabolic syndrome. J Am Coll Cardiol. (2005) 46:1978–85. doi: 10.1016/j.jacc.2005.06.082
11. Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK. Tumor necrosis factor-α induces skeletal muscle insulin resistance in healthy human subjects via inhibition of AKT substrate 160 phosphorylation. Diabetes. (2005) 54:2939. doi: 10.2337/diabetes.54.10.2939
12. Mohammadi M, Gozashti MH, Aghadavood M, Mehdizadeh MR, Hayatbakhsh MM. Clinical significance of serum IL-6 and TNF-α levels in patients with metabolic syndrome. Rep Biochem Mol Biol. (2017) 6:74–9.
13. Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. (2010) 314:1–16. doi: 10.1016/j.mce.2009.07.031
14. Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm. (2010) 2010:289645. doi: 10.1155/2010/289645
15. Jepsen S, Suvan J, Deschner J. The association of periodontal diseases with metabolic syndrome and obesity. Periodontology 2000. (2020) 83:125–53. doi: 10.1111/prd.12326
16. Liccardo D, Cannavo A, Spagnuolo G, Ferrara N, Cittadini A, Rengo C, et al. Periodontal disease: a risk factor for diabetes and cardiovascular disease. Int J Mol Sci. (2019) 20:1414. doi: 10.3390/ijms20061414
17. Muñoz Aguilera E, Suvan J, Buti J, Czesnikiewicz-Guzik M, Barbosa Ribeiro A, Orlandi M, et al. Periodontitis is associated with hypertension: a systematic review and meta-analysis. Cardiovasc Res. (2019) 116:28–39. doi: 10.1093/cvr/cvz201
18. Zardawi F, Gul S, Abdulkareem A, Sha A, Yates J. Association between periodontal disease and atherosclerotic cardiovascular diseases: revisited. Front Cardiovasc Med. (2021) 7:625579. doi: 10.3389/fcvm.2020.625579
19. Polak D, Shapira L. An update on the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J Clin Periodontol. (2018) 45:150–66. doi: 10.1111/jcpe.12803
20. Nibali L, Tatarakis N, Needleman I, Tu Y-K, D'Aiuto F, Rizzo M, et al. Association between metabolic syndrome and periodontitis: a systematic review and meta-analysis. J Clin Endocrinol Metab. (2013) 98:913–20. doi: 10.1210/jc.2012-3552
21. Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. (2012) 83:1449–54. doi: 10.1902/jop.2012.110664
22. Papapanou PN, Neiderud AM, Sandros J, Dahlén G. Checkerboard assessments of serum antibodies to oral microbiota as surrogate markers of clinical periodontal status. J Clin Periodontol. (2001) 28:103–6. doi: 10.1034/j.1600-051x.2001.280116.x
23. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. (2005) 112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404
24. MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. (2007) 58:593–614. doi: 10.1146/annurev.psych.58.110405.085542
25. Hayes AF, Rockwood NJ. Regression-based statistical mediation and moderation analysis in clinical research: observations, recommendations, and implementation. Behav Res Ther. (2017) 98:39–57. doi: 10.1016/j.brat.2016.11.001
26. VanderWeele TJ, Vansteelandt S. Odds ratios for mediation analysis for a dichotomous outcome. Am J Epidemiol. (2010) 172:1339–48. doi: 10.1093/aje/kwq332
27. Iwasaki M, Minagawa K, Sato M, Kaneko N, Imai S, Yoshihara A, et al. Serum antibody to porphyromonas gingivalis in metabolic syndrome among an older Japanese population. Gerodontology. (2016) 33:193–200. doi: 10.1111/ger.12135
28. Merchant AT, Shrestha D, Chaisson C, Choi YH, Hazlett LJ, Zhang J. Association between serum antibodies to oral microorganisms and hyperglycemia in adults. J Dent Res. (2014) 93:752–9. doi: 10.1177/0022034514538451
29. Desvarieux M, Demmer RT, Jacobs DR Jr, Rundek T, Boden-Albala B, Sacco RL, et al. Periodontal bacteria and hypertension: the oral infections and vascular disease epidemiology study (invest). J Hypertens. (2010) 28:1413–21. doi: 10.1097/HJH.0b013e328338cd36
30. Jaramillo A, Lafaurie GI, Millán LV, Ardila CM, Duque A, Novoa C, et al. Association between periodontal disease and plasma levels of cholesterol and triglycerides. Colomb Med. (2013) 44:80–6. doi: 10.25100/cm.v44i2.1123
31. Pussinen PJ, Alfthan G, Rissanen H, Reunanen A, Asikainen S, Knekt P. Antibodies to periodontal pathogens and stroke risk. Stroke. (2004) 35:2020–3. doi: 10.1161/01.STR.0000136148.29490.fe
32. Ebersole JL, Holt SC, Hansard R, Novak MJ. Microbiologic and immunologic characteristics of periodontal disease in hispanic americans with type 2 diabetes. J Periodontol. (2008) 79:637–46. doi: 10.1902/jop.2008.070455
33. Pussinen PJ, Jousilahti P, Alfthan G, Palosuo T, Asikainen S, Salomaa V. Antibodies to periodontal pathogens are associated with coronary heart disease. Arterioscler Thromb Vasc Biol. (2003) 23:1250–4. doi: 10.1161/01.ATV.0000072969.71452.87
34. Oppermann RV, Weidlich P, Musskopf ML. Periodontal disease and systemic complications. Braz Oral Res. (2012) 26(Suppl. 1):39–47. doi: 10.1590/S1806-83242012000700007
35. Tamaki N, Hayashida H, Fukui M, Kitamura M, Kawasaki K, Nakazato M, et al. Oxidative stress and antibody levels to periodontal bacteria in adults: the Nagasaki islands study. Oral Dis. (2014) 20:e49–56. doi: 10.1111/odi.12127
36. Bretz WA, Weyant RJ, Corby PM, Ren D, Weissfeld L, Kritchevsky SB, et al. Systemic inflammatory markers, periodontal diseases, and periodontal infections in an elderly population. J Am Geriatr Soc. (2005) 53:1532–7. doi: 10.1111/j.1532-5415.2005.53468.x
37. D'Aiuto F, Nibali L, Parkar M, Patel K, Suvan J, Donos N. Oxidative stress, systemic inflammation, and severe periodontitis. J Dent Res. (2010) 89:1241–6. doi: 10.1177/0022034510375830
38. Bodet C, Grenier D. Synergistic effects of lipopolysaccharides from periodontopathic bacteria on pro-inflammatory cytokine production in an ex vivo whole blood model. Mol Oral Microbiol. (2010) 25:102–11. doi: 10.1111/j.2041-1014.2010.00566.x
39. Kim S-J, Choi E-Y, Kim EG, Shin S-H, Lee J-Y, Choi J-I, et al. Prevotella intermedia lipopolysaccharide stimulates release of tumor necrosis factor-α through mitogen-activated protein kinase signaling pathways in monocyte-derived macrophages. FEMS Immunol Med Microbiol. (2007) 51:407–13. doi: 10.1111/j.1574-695X.2007.00318.x
40. Matera G, Muto V, Vinci M, Zicca E, Abdollahi-Roodsaz S, van de Veerdonk FL, et al. Receptor recognition of and immune intracellular pathways for veillonella parvula lipopolysaccharide. Clin Vaccine Immunol. (2009) 16:1804–9. doi: 10.1128/CVI.00310-09
41. Sun Y, Shu R, Li CL, Zhang MZ. Gram-negative periodontal bacteria induce the activation of toll-like receptors 2 and 4, and cytokine production in human periodontal ligament cells. J Periodontol. (2010) 81:1488–96. doi: 10.1902/jop.2010.100004
42. Cekici A, Kantarci A, Hasturk H, Van Dyke TE. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontology 2000. (2014) 64:57–80. doi: 10.1111/prd.12002
43. Hussain M, Stover CM, Dupont A.P Gingivalis in periodontal disease and atherosclerosis - scenes of action for antimicrobial peptides and complement. Front Immunol. (2015) 6:45. doi: 10.3389/fimmu.2015.00045
44. de Avila ED, de Molon RS, de Godoi Gonçalves DA, Camparis CM. Relationship between levels of neuropeptide substance P in periodontal disease and chronic pain: a literature review. J Invest Clin Dent. (2014) 5:91–7. doi: 10.1111/jicd.12087
45. Slocum C, Kramer C, Genco CA. Immune dysregulation mediated by the oral microbiome: potential link to chronic inflammation and atherosclerosis. J Intern Med. (2016) 280:114–28. doi: 10.1111/joim.12476
46. Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. (2019) 25:1822–32. doi: 10.1038/s41591-019-0675-0
47. Bullon P, Morillo JM, Ramirez-Tortosa MC, Quiles JL, Newman HN, Battino M. Metabolic syndrome and periodontitis: is oxidative stress a common link? J Dent Res. (2009) 88:503–18. doi: 10.1177/0022034509337479
48. Demmer RT, Squillaro A, Papapanou PN, Rosenbaum M, Friedewald WT, Jacobs DR Jr, et al. Periodontal infection, systemic inflammation, and insulin resistance: results from the continuous national health and nutrition examination survey (NHANES) 1999-2004. Diabetes Care. (2012) 35:2235–42. doi: 10.2337/dc12-0072
49. Torrungruang K, Ongphiphadhanakul B, Jitpakdeebordin S, Sarujikumjornwatana S. Mediation analysis of systemic inflammation on the association between periodontitis and glycaemic status. J Clin Periodontol. (2018) 45:548–56. doi: 10.1111/jcpe.12884
Keywords: periodontitis, periodontal bacteria, metabolic syndrome, EPI—epidemiology, multivariate analysis
Citation: Yang Z-Y, Fang W-H, Kao C-C and Chen W-L (2022) Examining the association between serum IgG of oral bacteria and metabolic syndrome. Front. Med. 9:899063. doi: 10.3389/fmed.2022.899063
Received: 18 March 2022; Accepted: 30 June 2022;
Published: 22 July 2022.
Edited by:
Francisco Mesa, University of Granada, SpainReviewed by:
Vittoria D'Esposito, Istituto per l'Endocrinologia e l'oncologia “Gaetano Salvatore (CNR), ItalyRubén León, Dentaid S. L, Spain
Copyright © 2022 Yang, Fang, Kao and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei-Liang Chen, d2VpbGlhbmcwNTA4QGdtYWlsLmNvbQ==
 Zhe-Yu Yang1,2
Zhe-Yu Yang1,2 Wen-Hui Fang
Wen-Hui Fang Wei-Liang Chen
Wei-Liang Chen