
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 05 December 2019
Sec. Nephrology
Volume 6 - 2019 | https://doi.org/10.3389/fmed.2019.00288
This article is part of the Research Topic Renal Function in Acute and Chronic Kidney Diseases View all 31 articles
 Chih-Yen Hsiao1,2
Chih-Yen Hsiao1,2 Tsung-Hsien Chen1
Tsung-Hsien Chen1 Yi-Chien Lee3,4
Yi-Chien Lee3,4 Meng-Chang Hsiao5
Meng-Chang Hsiao5 Peir-Haur Hung1,6
Peir-Haur Hung1,6 Yih-Yuan Chen7
Yih-Yuan Chen7 Ming-Cheng Wang8*
Ming-Cheng Wang8*Urinary tract infection (UTI) is a common complication in patients with urolithiasis. This study aimed to compare clinical manifestations and treatment outcomes among UTI patients with or without urolithiasis. It also focused on identifying relationships among urolithiasis, uroseptic shock, and acute kidney injury (AKI). This retrospective study enrolled hospitalized UTI patients who underwent imaging in an acute care setting from January 2006 to March 2015. Of 662 participants enrolled, 113 (17.1%) had urolithiasis, 107 (16.2%) developed uroseptic shock, and 184 (27.8%) developed AKI. A multivariate logistic regression analysis showed that in UTI patients, urolithiasis is associated with an increased risk of uroseptic shock (OR 1.80, 95% CI: 1.08–3.02, P = 0.025), AKI (OR 1.95, 95% CI: 1.22–3.12, P = 0.005), and bacteremia (OR 1.68, 95% CI: 1.08–2.64, P = 0.022). Urolithiasis is common in UTI patients and is associated with an increased risk of uroseptic shock and AKI.
Urolithiasis is a global problem with an increasing incidence and prevalence rate (1, 2). The global prevalence of urolithiasis ranges from 2 to 20% and varies based on geographical locations and socioeconomic conditions of different populations (3, 4). Urolithiasis is a well-known risk factor of urinary tract infection (UTI), and a vicious cycle leading to several clinical consequences is observed seen as follows: stones → obstruction → stasis → infection → stones (5). Furthermore, urolithiasis is associated with an increased risk of chronic kidney disease (CKD), hypertension, and myocardial infarction (6–8).
Urolithiasis can lead to urinary stasis, which enables bacteria to adhere to the urothelium and multiply, thereby causing UTI (9). Complicated UTI related to obstructive uropathy can progress to urosepsis and may cause septic shock and disseminated intravascular coagulopathy (10). However, only a few studies have demonstrated the correlation among urolithiasis, uroseptic shock, and acute kidney injury (AKI) in UTI patients. Hence, we conducted a study to investigate whether urolithiasis is an important risk factor for uroseptic shock and AKI in UTI patients.
The data for this retrospective observational study were collected between January 2006 and March 2015 from patients visiting Chiayi Christian Hospital in southern Taiwan, which consists of 1,077 inpatient beds and an outpatient department serving ~4,110 patients per day. This study was conducted after obtaining ethical approval from the Institutional Review Board of Chiayi Christian Hospital (approval no. CYCH-IRB-100015).
In total, 662 consecutively hospitalized UTI patients without any other concurrent infectious disease were enrolled in the study. All patients were adults and presented with UTI symptoms such as pain on urination, lumbago, or fever with bacterial isolation of >105 colony-forming units/mL from a urine specimen. All patients underwent imaging studies, such as ultrasonography, intravenous urography (IVU), or computed tomography (CT), to identify urolithiasis and urinary tract obstruction. The patients were divided into two groups based on the presence or absence of urolithiasis. The clinical characteristics and laboratory data were collected using a standard form for further analysis.
Data on demographic characteristics (age and sex), comorbidities [diabetes mellitus (DM), hypertension, coronary artery disease, congestive heart failure (CHF), stroke, and long-term indwelling Foley catheter], vital signs (blood pressure and temperature), laboratory results [white blood cell (WBC) count, platelet count, serum creatinine, and estimated glomerular filtration rate (eGFR) at baseline and after hospitalization], causative microorganisms and antimicrobial resistance pattern, and imaging of patients were collected and analyzed.
The diagnosis of urolithiasis was based on imaging studies. Urolithiasis was defined as the presence of any stone formation in the urinary tract that was detected during imaging studies such as ultrasonography, CT with or without enhancement, or IVU. Uroseptic shock was defined as sepsis-induced hypotension [systolic blood pressure (SBP) <90 mmHg or mean arterial pressure <70 mmHg or a decrease in SBP by >40 mmHg and lasting for at least 1 h, despite adequate fluid resuscitation and in the absence of other causes of hypotension] (11). eGFR was determined using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation (12, 13). For patients without recognized CKD, the estimation of baseline creatinine level was performed using the CKD-EPI creatinine equation assuming a GFR of 75 ml/min/1.73 m2 (14). AKI was defined as an increase in serum creatinine level to ≥1.5 times the baseline values according to the KDIGO Clinical Practice Guideline criteria for serum creatinine value for AKI stages 1, 2, and 3 (15). Afebrile status was defined as a body temperature of ≤ 38.3°C (101°F) during hospitalization. Multiple drug resistance (MDR) was defined as non-susceptibility of the isolates to at least one agent in three or more antimicrobial categories (16).
The patients were grouped into two categories: those with or without urolithiasis. The continuous variables were expressed as mean ± SD, and the categorical variables were expressed as number (percentage). The data were analyzed using Student's t-test or chi-square test depending on the type of variables. Multivariate logistic regression analyses were performed to identify factors associated with urolithiasis, septic shock, and AKI post-admission. Only variables significant at the 0.15 level in the univariate analysis were selected for consecutive multivariate analyses. The goodness-of-fit of the logistic regression model was assessed using the Cox and Snell test, and the explanatory power was reported using Nagelkerke's pseudo-R-square. A P-value of <0.05 was considered statistically significant. All analyses were performed using SPSS software for Windows (SPSS Science, v. 17.0, Chicago, IL).
The demographic and clinical characteristics of 662 hospitalized UTI patients are shown in Table 1. The mean age on admission was 67 ± 17 years. A majority of the patients [468 (70.7%)] were females, and 229 (34.6%) had a prior history of UTI. There were 107 patients (16.2%) with uroseptic shock and 184 patients (27.8%) with AKI during hospitalization. The overall mortality rate was 0.45% (3/662). Among UTI patients, 113 (17.1%) had urolithiasis and 41 had ureteral stone with urinary tract obstruction. The ureteral stones were located at the upper ureter or ureteropelvic junction in 18 patients (43.9%), mid ureter in 5 (12.2%), and distal ureter and ureterovesical junction in 18 (43.9%). Thirty (16.3%) and 81 patients (16.9%) underwent radiologic examinations using contrast media (IVU or CT) in the AKI and non-AKI groups, respectively. The prevalence of male sex (40.7 vs. 27.0%, P = 0.003), bacteremia (57.5 vs. 40.3%, P = 0.001), uroseptic shock (26.5 vs. 14.0%, P = 0.001), AKI (40.7 vs. 25.1%, P = 0.001) and Proteus spp. isolates (10.6 vs. 2.6%, P < 0.001) was higher, while Escherichia coli isolates (61.1 vs. 75.8%, P = 0.002) was lower in patients with urolithiasis than in those without. Among UTI patients with urolithiasis, the incidences of AKI were 60.0 and 33.7%, respectively, in patients with and without uroseptic shock. The top five bacteria identified in UTI patients with urolithiasis were E. coli (61.1%), Proteus spp. (10.6%), Klebsiella spp. (8.8%), Pseudomonas spp. (8.0%), and Enterococcus spp. (3.5%). The top five bacteria identified in UTI patients without urolithiasis were E. coli (75.8%), Klebsiella spp. (7.7%), Pseudomonas spp. (7.1%), Enterococcus spp. (4.6%), and Proteus spp. (2.6%).
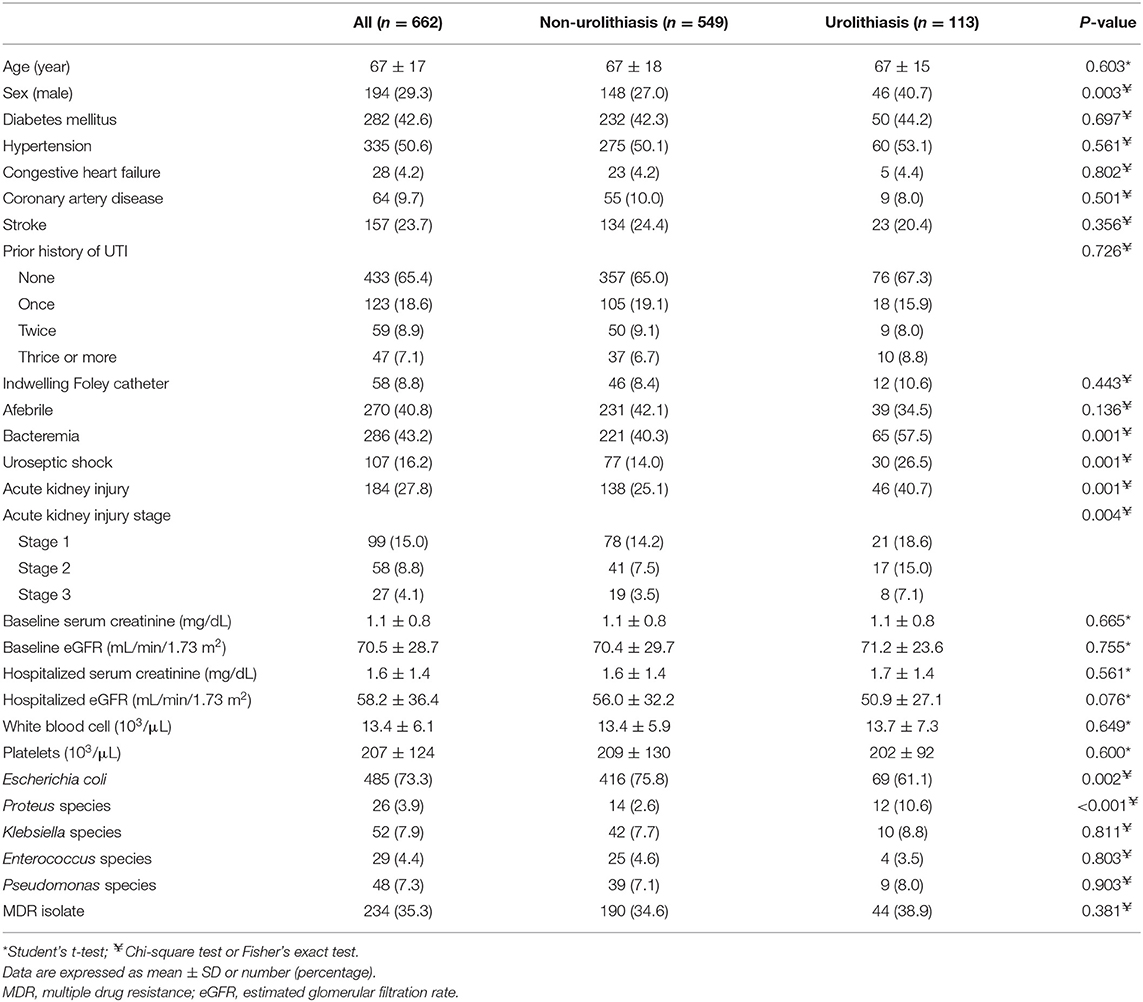
Table 1. Characteristics of hospitalized patients with urinary tract infection with respect to urolithiasis.
CHF (OR 2.43, 95% CI: 1.03–5.77, P = 0.043), urolithiasis (OR 1.80, 95% CI: 1.08–3.02, P = 0.025), and AKI (OR 2.30, 95% CI: 1.45–3.65, P < 0.001) were independently associated with an increased risk of uroseptic shock (Table 2). Patients with DM (OR 1.78, 95% CI: 1.21–2.60, P = 0.003), afebrile during hospitalization (OR 1.63, 95% CI: 1.08–2.44, P = 0.019), bacteremia (OR 2.19, 95% CI: 1.47–3.26, P < 0.001), uroseptic shock (OR 2.44, 95% CI: 1.51–3.93, P < 0.001), urolithiasis (OR 1.95, 95% CI: 1.22–3.12, P = 0.005), and higher WBC count (OR 1.04, 95% CI: 1.00–1.07, P = 0.025) were independently associated with an increased risk of AKI. Conversely, higher eGFR (OR 0.99, 95% CI: 0.98–0.99, P < 0.001) was independently associated with a decreased risk of AKI in UTI patients (Table 3). Figure 1 shows a reduction in eGFR in UTI patients with uroseptic shock and urolithiasis. The reduction in eGFR values in uroseptic shock patients with or without urolithiasis, and UTI patients with or without urolithiasis were 28.35 (95% CI: 19.23–37.46), 20.78 (95% CI: 16.58–24.97), 17.05 (95% CI: 13.72–20.39), and 13.28 (95% CI: 12.05–14.50) mL/min/1.73 m2, respectively. Significant differences were observed between uroseptic shock patients with urolithiasis and UTI patients with urolithiasis, uroseptic shock patients without urolithiasis and UTI patients without urolithiasis, uroseptic patients with urolithiasis and UTI patients without urolithiasis, and UTI patients with urolithiasis and UTI patients without urolithiasis. Urolithiasis (OR 1.68, 95% CI: 1.08–2.64, P = 0.022), AKI (OR 1.87, 95% CI: 1.26–2.78, P = 0.002), and higher WBC count (OR 1.04, 95% CI: 1.01–1.07, P = 0.006) were independently associated with an increased risk of bacteremia, while afebrile status (OR 0.32, 95% CI: 0.22–0.46, P < 0.001) and higher platelet count (OR 1.00, 95% CI: 0.99–1.00, P < 0.001) were independently associated with a decreased risk of bacteremia in UTI patients (Table 4).
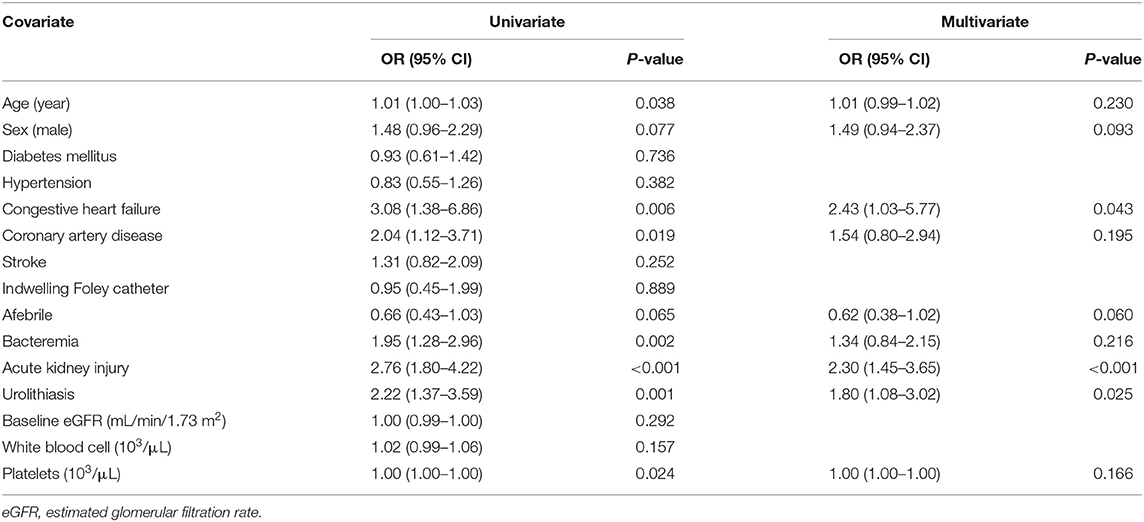
Table 2. Univariate and multivariate logistic regression analyses of factors related to uroseptic shock in patients with urinary tract infection.
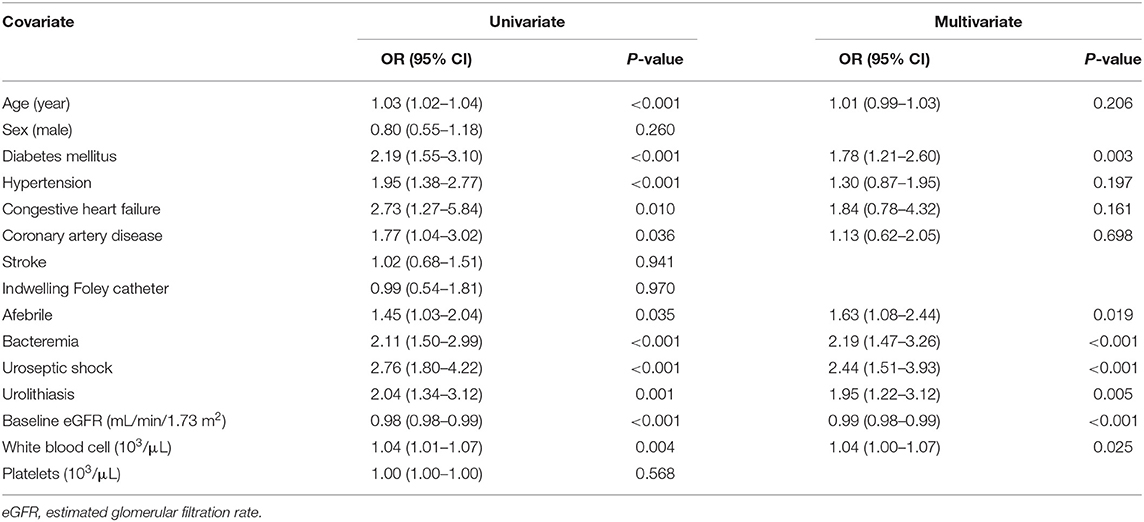
Table 3. Univariate and multivariate logistic regression analyses of factors related to acute kidney injury in patients with urinary tract infection.
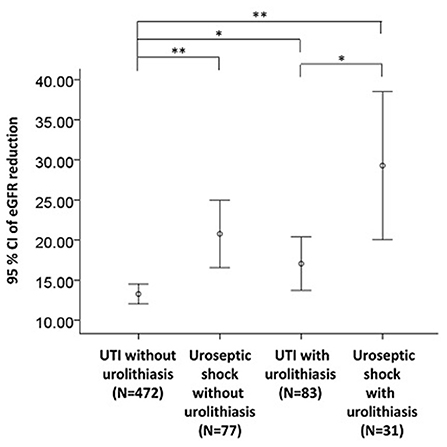
Figure 1. eGFR reduction in UTI patients with respect to uroseptic shock and urolithiasis. eGFR, estimated glomerular filtration rate; UTI, urinary tract infection; eGFR reduction, baseline minus worst value of eGFR. *p < 0.05, **p < 0.01.
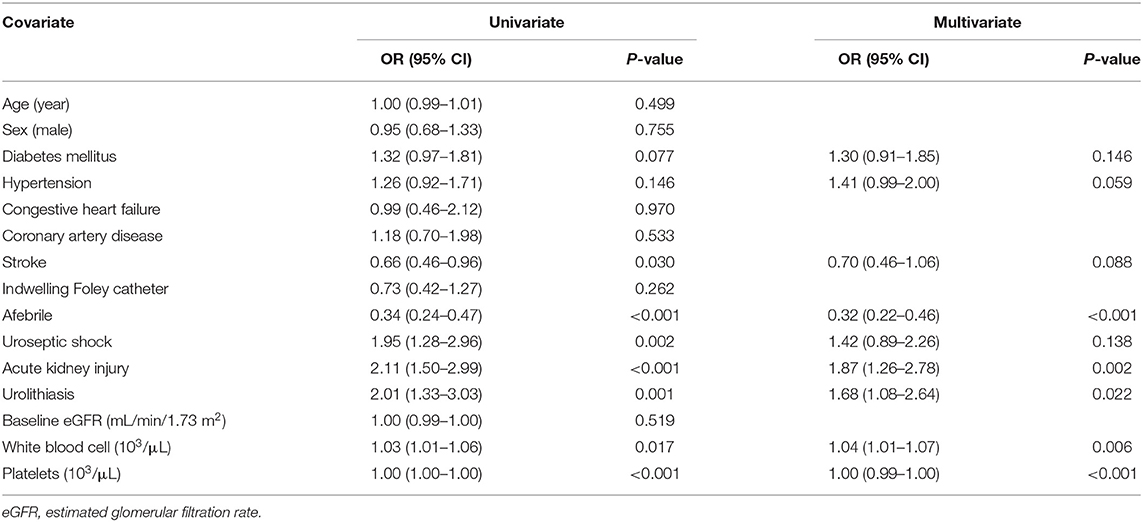
Table 4. Univariate and multivariate logistic regression analyses of factors related to bacteremia in patients with urinary tract infection.
The relationship between urolithiasis and UTI is complex. UTI is a common complication in patients with urolithiasis, and it promotes the formation of urolithiasis (5). Our study demonstrated a higher prevalence of urolithiasis in UTI patients than in the general population. The presence of urolithiasis was associated with worse clinical outcomes in UTI patients, including an increased risk of bacteremia, uroseptic shock, and AKI. To the best of our knowledge, this is the first study to demonstrate that urolithiasis is associated with an increased risk of uroseptic shock and AKI in UTI patients.
Patients with septic shock are in a critical situation that requires significant healthcare resources. It is estimated that UTI is the underlying cause in 30% of patients with severe sepsis and septic shock (17). The rate of developing septic shock in patients with UTI can range from 20.8 to 32.9% based on different underlying conditions (18–20). In the current study, the incidence of uroseptic shock in UTI patients that necessitated admission was 16.2%. Multiple factors attributing to septic shock have previously been reported in patients with UTI. Indwelling urinary catheter and increased C-reactive protein levels were risk factors of septic shock in UTI patients with or without bacteremia (18, 21). Our study showed that urolithiasis could be a good predictor of the development of uroseptic shock in UTI patients. Previous studies showed that the rate of uroseptic shock in calculous acute pyelonephritis (APN) ranged from 20.8 to 36.2% (22, 23). Here, we found a similar incidence (26.5%) of uroseptic shock in UTI patients with urolithiasis. UTI with urinary tract obstruction can lead to urosepsis in ~10% of cases (24), and the presence of urinary tract obstruction is a risk factor for septic shock in patients with bacteremic APN (19, 25). A lack of decompression leads to an increased risk of mortality for patients with sepsis and ureteral stone (26). It is important to decompress the renal collecting system by ureteral stent or pyelonephrostomy for UTI patients with severe urinary tract obstruction (27). Because urolithiasis is an independent risk factor for the development of septic shock in UTI patients, early imaging for sepsis patients with clinical suspicion of urinary source and decompressing the urinary collecting system for those with obstruction is recommended. For patients identifying severe sepsis or septic shock, administration of effective intravenous antibiotics within an hour is critical according to the recommendation of Surviving Sepsis Campaign guidelines (11).
The primary mechanism of urolithiasis-associated AKI is obstructive nephropathy (28). However, urolithiasis is a rare cause of adult AKI, accounting for ~1–2% of all AKI events only (28). One of the common causes of AKI in critically ill patients is sepsis (29), and ~60% of patients with septic shock develop AKI (30). Urolithiasis is a risk factor for UTI, and UTI is one of the common causes of sepsis, which may lead to uroseptic shock and deterioration of renal function. Our study found a high incidence of AKI (46.7%) among the patients with uroseptic shock, and uroseptic shock was an independent risk factor for AKI. UTI patients with urolithiasis were more prone to develop uroseptic shock and tended to have a greater reduction in eGFR than those without urolithiasis, regardless of the presence of uroseptic shock. Because patients with urolithiasis or uroseptic shock are both predisposed to develop more severe AKI, nephrotoxic agents, including non-steroidal anti-inflammatory drugs, contrast media, and aminoglycosides should be avoided in patients with a high risk of AKI.
The presence of bacteremia in complicated APN patients results in an increased risk of developing severe sepsis or uroseptic shock (31). Previous studies have indicated that specific clinical features in UTI patients, such as old age, low SBP, high body temperature, and high procalcitonin levels, were significantly associated with bacteremia (32). Our study showed that UTI patients with urolithiasis, AKI, and higher WBC count were at a higher risk of developing bacteremia. Urolithiasis per se is an important source of secondary infection (33). A previous study reported that the incidence of bacteria isolated from urine and stone matrices of stone formers was 24 and 32%, respectively (34). Traditionally, UTI with urease-producing bacteria, in most cases belonging to Proteus spp., can split urinary urea and increase urinary pH, thus, promoting the precipitation and aggregation of struvite crystals to form infective urolithiasis (5). However, UTIs are frequently associated with kidney stones of different chemical compositions other than struvite (34). Tavichakorntrakool et al. reported that the three most common bacteria in urine samples of patients with renal stones were E. coli, Enterococcus spp., and Klebsiella/Enterobacter spp. (34). Our data showed that the three most common pathogens in UTI patients with urolithiasis were E. coli, Proteus spp., and Klebsiella spp. There were more isolates of Proteus spp. but fewer isolates of E. coli in UTI patients with urolithiasis than in those without urolithiasis. Third-generation cephalosporin is a reasonable empirical antibiotic for stable community-acquired UTI associated with urolithiasis, while broader spectrum antimicrobial coverage is needed for patients with severe sepsis or septic shock (18). In addition, urolithiasis contains bacteria, and the number of bacteria on the stone surface can increase despite antibiotic therapy. Thus, eradication of the associated UTI is only possible after complete removal of the stone (35).
Our study had several limitations. First, the retrospective design involved a potential bias in data collection. Although we collected data using a standard form to reduce bias, the data regarding prior history of urolithiasis, as well as the type and size of urolithiasis were absent. A prospective study is needed to collect the above information to determine the associations between urolithiasis burden and urosepsis, as well as uroseptic shock and AKI. Second, this was a single-center study involving hospitalized patients with UTI, which limits the generalizability of the results. A multicenter study with a larger sample size will be needed to confirm our results. Third, in this study, the patient number with urinary tract obstruction was insufficient to evaluate it as a risk factor for causing uroseptic shock and AKI in UTI patients with urolithiasis. These limitations will be addressed in our future study.
In conclusion, we demonstrated that UTI patients with urolithiasis have a higher risk of developing uroseptic shock and AKI than those without urolithiasis. Therefore, careful medical history and imaging studies for urolithiasis are important for physicians to prevent and manage acute and severe complications in UTI patients.
The datasets generated for this study are available on request to the corresponding author.
This study was conducted in concordance with institutional patient safety laws and has been approved by the Institutional Review Board of Chiayi Christian Hospital (approval no. CYCH-IRB-100015). This study was performed in accordance with the Declaration of Helsinki. The patients/participants provided their written informed consent to participate in this study.
All authors: participated in the interpretation of the studies and analysis of the data and also reviewed and approved the final version of the manuscript. C-YH: protocol/project development. C-YH and Y-YC: data collection or management. C-YH and T-HC: manuscript writing/editing. T-HC and Y-CL: data analysis. M-CH, P-HH, and M-CW: manuscript review. M-CW: scientific advisor.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to acknowledge the support of Cheng-Lun Chiang.
1. Moe OW. Kidney stones: pathophysiology and medical management. Lancet. (2006) 367:333–44. doi: 10.1016/S0140-6736(06)68071-9
2. Scales CD Jr, Smith AC, Hanley JM, Saigal CS, Urologic Diseases in America P. Prevalence of kidney stones in the United States. Eur Urol. (2012) 62:160–5. doi: 10.1016/j.eururo.2012.03.052
3. Yongzhi L, Shi Y, Jia L, Yili L, Xingwang Z, Xue G. Risk factors for urinary tract infection in patients with urolithiasis-primary report of a single center cohort. BMC Urol. (2018) 18:45. doi: 10.1186/s12894-018-0359-y
4. Raheem OA, Khandwala YS, Sur RL, Ghani KR, Denstedt JD. Burden of urolithiasis: trends in prevalence, treatments, and costs. Eur Urol Focus. (2017) 3:18–26. doi: 10.1016/j.euf.2017.04.001
5. Borghi L, Nouvenne A, Meschi T. Nephrolithiasis and urinary tract infections: 'the chicken or the egg' dilemma? Nephrol Dial Transplant. (2012) 27:3982–4. doi: 10.1093/ndt/gfs395
6. Keddis MT, Rule AD. Nephrolithiasis and loss of kidney function. Curr Opin Nephrol Hypertens. (2013) 22:390–6. doi: 10.1097/MNH.0b013e32836214b9
7. Madore F, Stampfer MJ, Rimm EB, Curhan GC. Nephrolithiasis and risk of hypertension. Am J Hypertens. (1998) 11:46–53. doi: 10.1016/S0895-7061(97)00371-3
8. Rule AD, Roger VL, Melton LJ III, Bergstralh EJ, Li X, Peyser PA, et al. Kidney stones associate with increased risk for myocardial infarction. J Am Soc Nephrol. (2010) 21:1641–4. doi: 10.1681/ASN.2010030253
9. Choe HS, Lee SJ, Yang SS, Hamasuna R, Yamamoto S, Cho YH, et al. Summary of the UAA-AAUS guidelines for urinary tract infections. Int J Urol. (2018) 25:175–85. doi: 10.1111/iju.13493
10. Tambo M, Okegawa T, Shishido T, Higashihara E, Nutahara K. Predictors of septic shock in obstructive acute pyelonephritis. World J Urol. (2014) 32:803–11. doi: 10.1007/s00345-013-1166-4
11. Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. (2013) 41:580–637. doi: 10.1097/CCM.0b013e31827e83af
12. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
13. National Kidney F. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. (2002) 39:S1–266.
14. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. (2004) 8:R204–12. doi: 10.1186/cc2872
15. Kellum J, Lameire N, Aspelin P, MacLeod AM, Barsoum RS, Mehta RL, et al. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. (2012) 2:1–138. doi: 10.1038/kisup.2012.1
16. Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. (2012) 18:268–81. doi: 10.1111/j.1469-0691.2011.03570.x
17. Tandogdu Z, Johansen T, Bartoletti R, Florian Wagenlehner F. Management of the urologic sepsis syndrome. Eur Urol Suppl. (2016) 15:102–11. doi: 10.1016/j.eursup.2016.04.004
18. Shigemura K, Tanaka K, Osawa K, Arakawa S, Miyake H, Fujisawa M. Clinical factors associated with shock in bacteremic UTI. Int Urol Nephrol. (2013) 45:653–7. doi: 10.1007/s11255-013-0449-4
19. Lee JH, Lee YM, Cho JH. Risk factors of septic shock in bacteremic acute pyelonephritis patients admitted to an ER. J Infect Chemother. (2012) 18:130–3. doi: 10.1007/s10156-011-0289-z
20. Efstathiou SP, Pefanis AV, Tsioulos DI, Zacharos ID, Tsiakou AG, Mitromaras AG, et al. Acute pyelonephritis in adults: prediction of mortality and failure of treatment. Arch Intern Med. (2003) 163:1206–12. doi: 10.1001/archinte.163.10.1206
21. Yamamichi F, Shigemura K, Kitagawa K, Takaba K, Tokimatsu I, Arakawa S, et al. Shock due to urosepsis: a multicentre study. Can Urol Assoc J. (2017) 11:E105–9. doi: 10.5489/cuaj.4097
22. Koga S, Arakaki Y, Matsuoka M, Ohyama C. Calculous pyelonephritis. Int Urol Nephrol. (1992) 24:109–12. doi: 10.1007/BF02549636
23. Kakinoki H, Tobu S, Kakinoki Y, Udo K, Uozumi J, Noguchi M. Risk factors for uroseptic shock in patients with urolithiasis-related acute pyelonephritis. Urol Int. (2018) 100:37–42. doi: 10.1159/000481801
24. Reyner K, Heffner AC, Karvetski CH. Urinary obstruction is an important complicating factor in patients with septic shock due to urinary infection. Am J Emerg Med. (2016) 34:694–6. doi: 10.1016/j.ajem.2015.12.068
25. Kalra OP, Raizada A. Approach to a patient with urosepsis. J Glob Infect Dis. (2009) 1:57–63. doi: 10.4103/0974-777X.52984
26. Borofsky MS, Walter D, Shah O, Goldfarb DS, Mues AC, Makarov DV. Surgical decompression is lifesaving for patients with sepsis and ureteral calculi. J Urol. (2013) 189:946–51. doi: 10.1016/j.juro.2012.09.088
27. Yamamichi F, Shigemura K, Kitagawa K, Fujisawa M. Comparison between non-septic and septic cases in stone-related obstructive acute pyelonephritis and risk factors for septic shock: a multi-center retrospective study. J Infect Chemother. (2018) 24:902–6. doi: 10.1016/j.jiac.2018.08.002
28. Tang X, Lieske JC. Acute and chronic kidney injury in nephrolithiasis. Curr Opin Nephrol Hypertens. (2014) 23:385–90. doi: 10.1097/01.mnh.0000447017.28852.52
29. Zarbock A, Gomez H, Kellum JA. Sepsis-induced acute kidney injury revisited: pathophysiology, prevention and future therapies. Curr Opin Crit Care. (2014) 20:588–95. doi: 10.1097/MCC.0000000000000153
30. Plataki M, Kashani K, Cabello-Garza J, Maldonado F, Kashyap R, Kor DJ, et al. Predictors of acute kidney injury in septic shock patients: an observational cohort study. Clin J Am Soc Nephrol. (2011) 6:1744–51. doi: 10.2215/CJN.05480610
31. Hsu CY, Fang HC, Chou KJ, Chen CL, Lee PT, Chung HM. The clinical impact of bacteremia in complicated acute pyelonephritis. Am J Med Sci. (2006) 332:175–80. doi: 10.1097/00000441-200610000-00004
32. Lee H, Lee YS, Jeong R, Kim YJ, Ahn S. Predictive factors of bacteremia in patients with febrile urinary tract infection: an experience at a tertiary care center. Infection. (2014) 42:669–74. doi: 10.1007/s15010-014-0615-3
33. Zanetti G, Paparella S, Trinchieri A, Prezioso D, Rocco F, Naber KG. Infections and urolithiasis: current clinical evidence in prophylaxis and antibiotic therapy. Arch Ital Urol Androl. (2008) 80:5–12.
34. Tavichakorntrakool R, Prasongwattana V, Sungkeeree S, Saisud P, Sribenjalux P, Pimratana C, et al. Extensive characterizations of bacteria isolated from catheterized urine and stone matrices in patients with nephrolithiasis. Nephrol Dial Transplant. (2012) 27:4125–30. doi: 10.1093/ndt/gfs057
Keywords: urolithiasis, urinary tract infection, uroseptic shock, acute kidney injury, bacteremia
Citation: Hsiao C-Y, Chen T-H, Lee Y-C, Hsiao M-C, Hung P-H, Chen Y-Y and Wang M-C (2019) Urolithiasis Is a Risk Factor for Uroseptic Shock and Acute Kidney Injury in Patients With Urinary Tract Infection. Front. Med. 6:288. doi: 10.3389/fmed.2019.00288
Received: 27 August 2019; Accepted: 22 November 2019;
Published: 05 December 2019.
Edited by:
John D. Imig, Medical College of Wisconsin, United StatesReviewed by:
Bassam G. Abu Jawdeh, University of Cincinnati, United StatesCopyright © 2019 Hsiao, Chen, Lee, Hsiao, Hung, Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming-Cheng Wang, dHN1dHN1Z2FtdXNoaTg5MDFAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.