Policy Brief
Published on 03 Feb 2025
Survey of Notified Bodies reveals very limited use of conditional certification for high-risk medical devices
in Regulatory Affairs
- 568 views
Policy Brief
Published on 03 Feb 2025
in Regulatory Affairs
Review
Published on 16 Dec 2024
in Regulatory Affairs
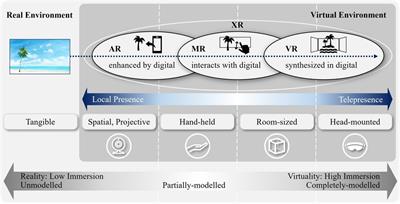
Mini Review
Published on 28 Nov 2024
in Regulatory Affairs
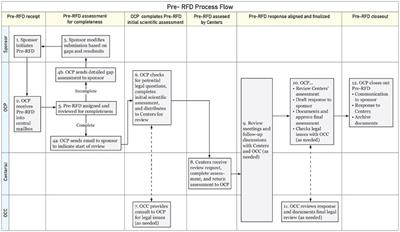
Perspective
Published on 12 Nov 2024
in Regulatory Affairs
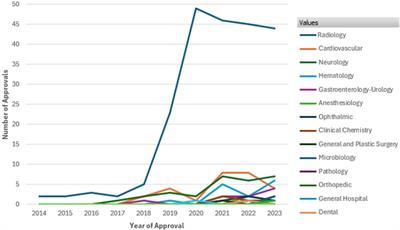
Original Research
Published on 06 Aug 2024
in Regulatory Affairs
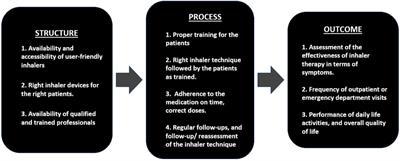
Review
Published on 10 Jul 2024
in Regulatory Affairs

Review
Published on 14 Jun 2024
in Regulatory Affairs

Original Research
Published on 05 Apr 2024
in Regulatory Affairs
Systematic Review
Published on 21 Mar 2024
in Regulatory Affairs
Editorial
Published on 04 Mar 2024
in Regulatory Affairs
Original Research
Published on 22 Feb 2024
in Regulatory Affairs
Brief Research Report
Published on 24 Nov 2023
in Regulatory Affairs
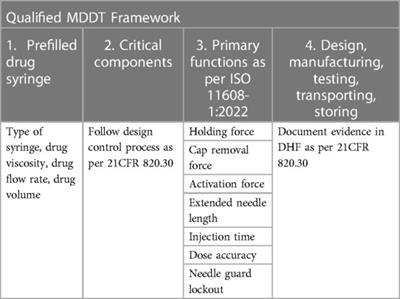
Community Case Study
Published on 20 Nov 2023
in Regulatory Affairs
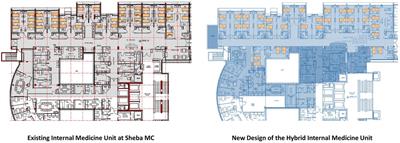
Original Research
Published on 23 Oct 2023
in Regulatory Affairs
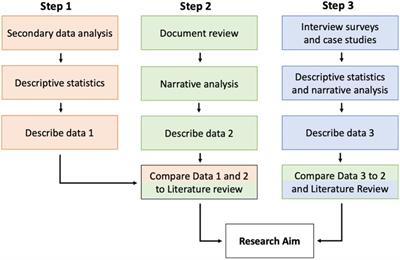
Perspective
Published on 02 Oct 2023
in Regulatory Affairs
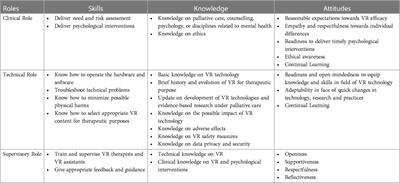
Hypothesis and Theory
Published on 20 Sep 2023
in Regulatory Affairs