- 1Third Institute of Oceanography, Ministry of Natural Resources, Xiamen, China
- 2Key Laboratory of Global Change and Marine-Atmospheric Chemistry, Ministry of Natural Resources, Xiamen, China
- 3School of Life Sciences, Xiamen University, Xiamen, China
- 4School of Marine Biology, Xiamen Ocean Vocational College, Xiamen, China
Due to the carrier’s role of microplastics, attached microalgae may be transported further, posing a threat to marine ecosystems, especially those red tide species. By combining the investigated results of Dongshan Bay and Quanzhou Bay with the simulation of transport trajectories using the Lagrangian particle tracking model, this study systematically investigated the characteristics and transport trajectories of epimicroplastic red tide species. Based on the investigations of Dongshan Bay and Quanzhou Bay respectively in summer of 2022, the characteristics of epimicroplastic red tide species were learned. Results showed that totally 13 red tide species were found in two bays, with 6 species in Dinophyta, 5 species in Diatom, 1 species in Ochrophyta and 1 species in Cyanophyta respectively. Also, the potential transport trajectories of epimicroplastic species were simulated to study their effect to the ecological environment of the surrounding waters. According to the simulated transport trajectories, those species could be transported further by microplastics while some particles would be obstructed during these three-month processes. During the transport processes, epimicroplastic red tide species from two bays would influence three provinces, which have high records of red tide outbreak in China. This study firstly combined models to investigate the potential transport trajectory of epimicroplastic red tide species, providing insights into the mechanisms of red tide outbreak.
1 Introduction
Red tide (also known as harmful algal blooms, HABs) recorded with those proliferations of algae that can cause fish kills, or contaminate seafood with toxins, or form unsightly scums, or detrimentally alter ecosystem function have been increasing in frequency, magnitude, and duration worldwide (Glibert et al., 2014). There was a global increase in the frequency, magnitude, and geographic extent of HAB events over the preceding decades (Anderson et al., 2012). Generally, the expansion of red tide is used considered to be caused by ballast water transport, climate change and eutrophication (Anderson et al., 2012). In addition to eutrophication of seawater, there are many factors that affect the occurrence of red tide (Gao et al., 2024). Regions of convergence resulting from dynamic processes like tidal fronts, Langmuir circulation, and thermal convection can agglomerate organisms, significantly increasing plankton concentrations beyond those in surrounding waters (Gao et al., 2024). These physical mechanisms engender a transport impact on red tide organisms. In instances of vigorous wind and ocean current interplay, they also function as diffusion mechanisms (Steidinger and Haddad, 1981; Gao et al., 2024). To date, some researchers have suggested that floating plastics may play potential roles on the expansion of HABs (Wang et al., 2022; Do Prado Leite et al., 2022). Microplastics (MPs) are plastic particles smaller than 5 mm in size (Thompson et al., 2004; Rocha-Santos, 2018), which contaminate marine habitats across the globe (Eriksen et al., 2014) and are recognized as a significant environmental challenge requiring urgent management (UNEP, 2016; Green et al., 2016). Previous studies have proposed that MPs can affect the algal growth, photosynthesis, antioxidant system (Su et al., 2020; You et al., 2021; Zhang J. et al., 2022), and further influence the phytoplankton community and marine ecosystem. However, through exposure experiment, Zhang J. et al. (2022) found that the effects of MPs on microalgae will likely not be substantial in future warming scenarios if MPs concentrations are controlled at a certain level. Therefore, the carrier effect of MPs to red tide species should be pay more attention to in the future study. Naik et al. (2019) indicated that MPs in ballast water could be an emerging source and vectors for harmful chemicals, antibiotics, metals, bacterial pathogens and red tide species.
MPs, as novel substrata for microbial colonization within aquatic ecosystems, are a matter of growing concern due to their potential to propagate foreign or invasive species across different environments (Song et al., 2022). Xianbiao et al. (2023) defined microalgae colonizing on MPs as epimicroplastic microalgae (EMP-MA). Meanwhile, harmful species have been found on EMP-MA community in many bays worldwide (Masó et al., 2003; Wang et al., 2022). Because MPs persist longer than other natural substrates such as feathers, wood, and macroalgae, it can traverse significant distances, and it has been shown to transport invasive species (Senderovich et al., 2010; Zettler et al., 2013). These invasive species may proliferate rapidly and even develop into HABs in suitable environment (Wang et al., 2022). An illustrative example of such transportation is the Great East Japan Tsunami of March 2011, which carried vast amounts of plastic debris into the North Pacific Ocean (González-Ortegón et al., 2024). In the following years, numerous coastal species from Japan were found on polyethylene, polystyrene, polyvinyl chloride, and fiberglass materials in North America and Hawaii (Haram et al., 2023; González-Ortegón et al., 2024).
Previous studies focused on the modeling transport process of microplastics or red tide species, by combining hydrodynamic model and particle tracking model, to assess their respective negative effects to marine ecosystem. Many studies indicated that while algae continually grow and perish during their diffusion, they generally move as a cohesive unit, allowing particle tracking methods to be utilized for simulating HABs trajectories (Yamamoto and Okai, 2000; Chen et al., 2007; Kuang et al., 2016). While red tide species transporting with microplastics, a stable substrate, their impact on the marine ecosystem as a whole is more long-lasting and wide-ranging.
In this study, the potential transport effects of epimicroplastic red tide species in marine environment were learned. Based on the survey data and current reanalysis data of epimicroplastic red tide species in Dongshan Bay and Quanzhou Bay, located on the west side of the Taiwan Strait of China in 2022, this paper investigates the characteristics and transport trajectories of epimicroplastic red tide species under the ocean currents by using the Lagrangian particle tracking model. This study firstly combined models to investigate the potential transport trajectory of epimicroplastic red tide species, providing new insights into the mechanisms of red tide.
2 Materials and method
2.1 Study area
Dongshan Bay and Quanzhou Bay, both are typical bays around west of the Taiwan Strait, playing important roles in the socio-economic development of coastal areas in China (Figure 1). The Taiwan Strait connected the South China Sea and the East China Sea, is an important pathway for the water and materials, and the main navigation channel (Hong et al., 2011; Yang, 2021). Dongshan Bay, located along the southwestern coast of the Taiwan Strait, is an important area for mariculture and seawater fishing in China (Zheng, 2019; Wang, 2023). With unique location advance and rich fishery resource, Quanzhou Bay is an inner bay of Fujian Province, surrounding by Meizhou Bay, Weitou Bay and the Taiwan Strait (Xie, 2021).
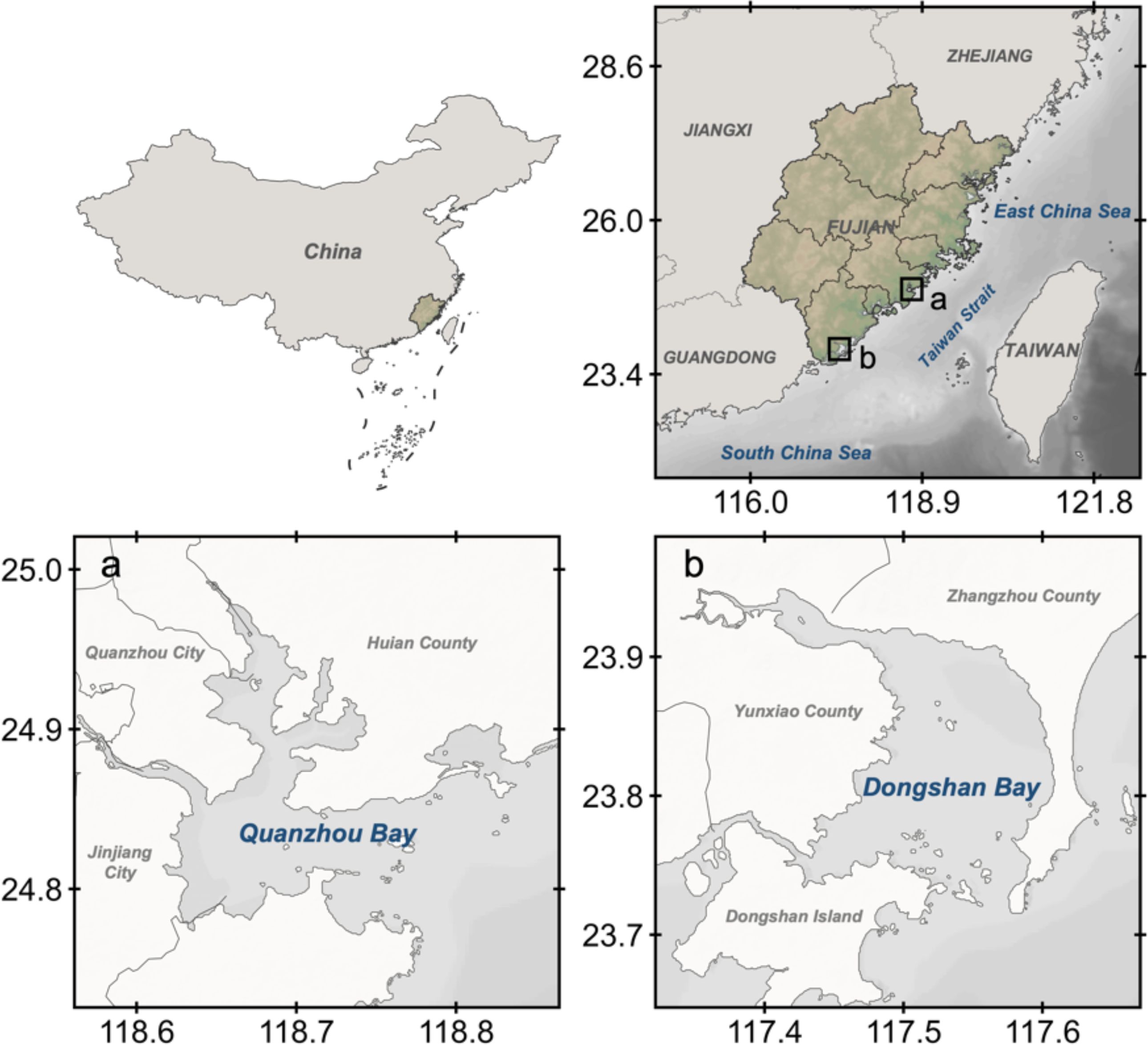
Figure 1. Geographic location of the investigated areas in this study. (a) Quanzhou Bay; (b) DongshanBay.
2.2 Sample collection and analysis
Surveys were conducted on April 22, 2022 in Dongshan Bay and on May 17, 2022 in Quanzhou Bay, respectively. The procedure of sample collection was according to Wang et al. (2022). Floating microplastics were collected by a 330 μm manta trawl and rinsed gently with sterile artificial seawater (salinity = 30) for three times, then transferred into 10 mL glass vials containing 2–5 mL of sterile artificial seawater and 1 mL of formaldehyde solution (Wang et al., 2022). Epimicroplastic red tide species were separated by ultrasonic cleaner at 300 W for 25 s and identified under a research microscope (Leica DMi8A) (Wang et al., 2022).
2.3 Modelling approach
2.3.1 HYCOM
The three-month (90-day) potential transport trajectories of these particles were calculated with the 3 hourly 1/12° flow fields from the Hybrid Coordinate Ocean Model (HYCOM, https://www.hycom.org, accessed 3 January 2024) Global Ocean Forecast System (GOFS) 3.1 output. HYCOM simulations include the Navy Coupled Ocean Data Assimilation data (NCODA) (Cummings, 2006; Cummings and Smedstad, 2013; Allende-Arandía et al., 2023). We used the eastward (u-component) and northward (v-component) surface current velocity from the HYCOM + NCODA GOFS 3.1 reanalysis from April to August, 2022.
2.3.2 Particle tracking
Using the Lagrangian particle tracking model to develop the potential transport process of epimicroplastic red tide species from Dongshan Bay and Quanzhou Bay. Following a Lagrangian framework, microplastics were assimilated to point-like particles whose passive movement is completely consistent with that of surface water masses (Guerrini et al., 2021). Studies on the plastisphere have shown strong shifts of distinct communities throughout the early stage of colonization (Dey et al., 2022). However, in mature biofilms, microbial communities are converged over time and remained stable (Dey et al., 2022). Cultivation experiments have demonstrated little difference in epimicroplastic microalgae community structure over a 7-month period, and no significant community succession characteristics have emerged (Wang et al., summited)1. Therefore, any internal or external factors affecting community succession or structure variation were not considered in the simulated processes. 1,000 particles were placed randomly and uniformly within a radius of 10 km in the mouth of two bays and those on land were removed. Therefore, the number of particles in the actual tracking simulation of Dongshan Bay and Quanzhou Bay were 897 and 876, respectively (Table 1).
In the model, microplastics in water bodies were treated as discrete particles to simulated their transport processes in the water. There were three assumptions in this model: (1) though the potential transport trajectory of red tide species was discussed, the microplastics, as a vector, do not have the ability to move autonomously, so the particles in the model only transport passively with the ocean currents; (2) the density of microplastics is relatively small, and these samples in this study were collected from the surface water, so the transport trajectory was only simulated in horizontal direction, which may cause the estimated trajectory longer than the actual; (3) the particles in the model are conservative particles and do not participate in biochemical reactions. The changes of their positions are described by the following equations:
In the above equation, X(t) is the position of the particles at time t, and X0 is the initial position of the particle. The migration rate is represented by the surface current, with a time step of 3 hours.
2.4 Data analysis
Statistics and calculation were performed in Microsoft Excel 2021 (Microsoft Corporation, Washington, DC, USA). Lagrangian particle tracking model was run by MatLab and the figures of the transport trajectory was drawn with Qgis (3.32).
3 Result
3.1 Biodiversity of epimicroplastic red tide species in two bays
In this study, 1022 and 433 items of microplastics particles were collected and processed for the identification of epimicroplastic red tide species in Dongshan Bay and Quanzhou Bay, respectively. A total of 13 epimicroplastic red tide species from 4 phyla (Diatom, Dinophyta, Ochrophyta and Cyanophyta) were observed by optic microscope, with 5 species in Diatom and 6 species in Dinophyta, which contributed 38.5% and 46.2% of the species richness, respectively (Table 2). However, the composition and diversity of those epimicroplastic species between two bays were different. There were 4 species shared in these two bays, and 1 and 8 unique species were observed in Dongshan Bay and Quanzhou Bay, respectively. Some typical red tide species, such as Prorocentrum donghaiense from Dinophyta and Trichodesmium erythraeum from Cyanophyta, were only found in Quanzhou Bay. The unique species in Dongshan Bay was Pseudo-nitzschia pungens, a potentially toxic species from Diatom. Another toxic species was Chattonella marina from Ochrophyta, occurring in both two bays.
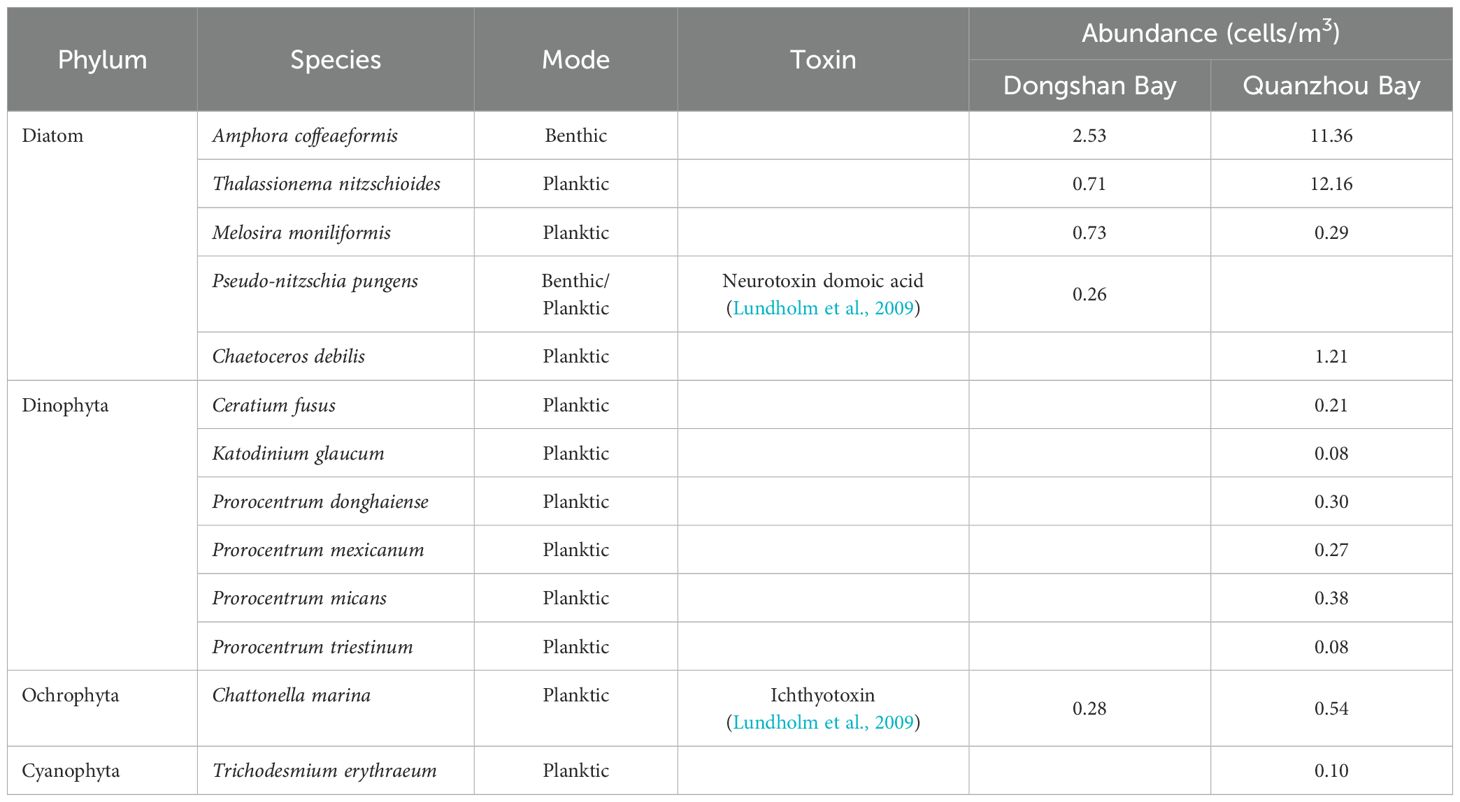
Table 2. Epimicroplastic red tide species in Dongshan Bay and Quanzhou Bay (Wang, 2024).
3.2 Potential transport trajectories of epimicroplastic red tide species
Three-month transport trajectories of epimicroplastic red tide species from Dongshan Bay were simulated (Figure 2). During the first-month transportation, the particles transported along the coastal water of Guangdong Province in a southwestward direction. About 50% particles had reached Mirs Bay and the surrounding waters areas of Hong Kong in Day 30. The particle started to transport in the opposite direction until they reached around the Pearl River Estuary at the beginning of the second month. Meanwhile, some particles started to be obstructed. According to the second month transport trajectories, the particles turned back to Fujian coastal water and entered the middle part of the Taiwan Strait. A total of 46.4% particles were obstructed in other sea areas during the second month, including 22.2% particles in West Lamma Channel, 19% particles in Daya Bay, 5.1% particles in Port Shelter and 0.1% particle in Mirs Bay. In the last month, some particles were dispersed in northern Fujian waters, like Pingtan sea area, and kept transporting in a northeastward direction. Meanwhile, 36.1% particles were obstructed, of which 24.7% particles in Mirs Bay. In these epimicroplastics red tide species transport trajectories from Dongshan Bay, about 82.5% particles were obstructed in the surrounding sea area of Guangdong Province and Hong Kong, while mainly obstructed in Daya Bay, Mirs Bay and West Lamma Channel.
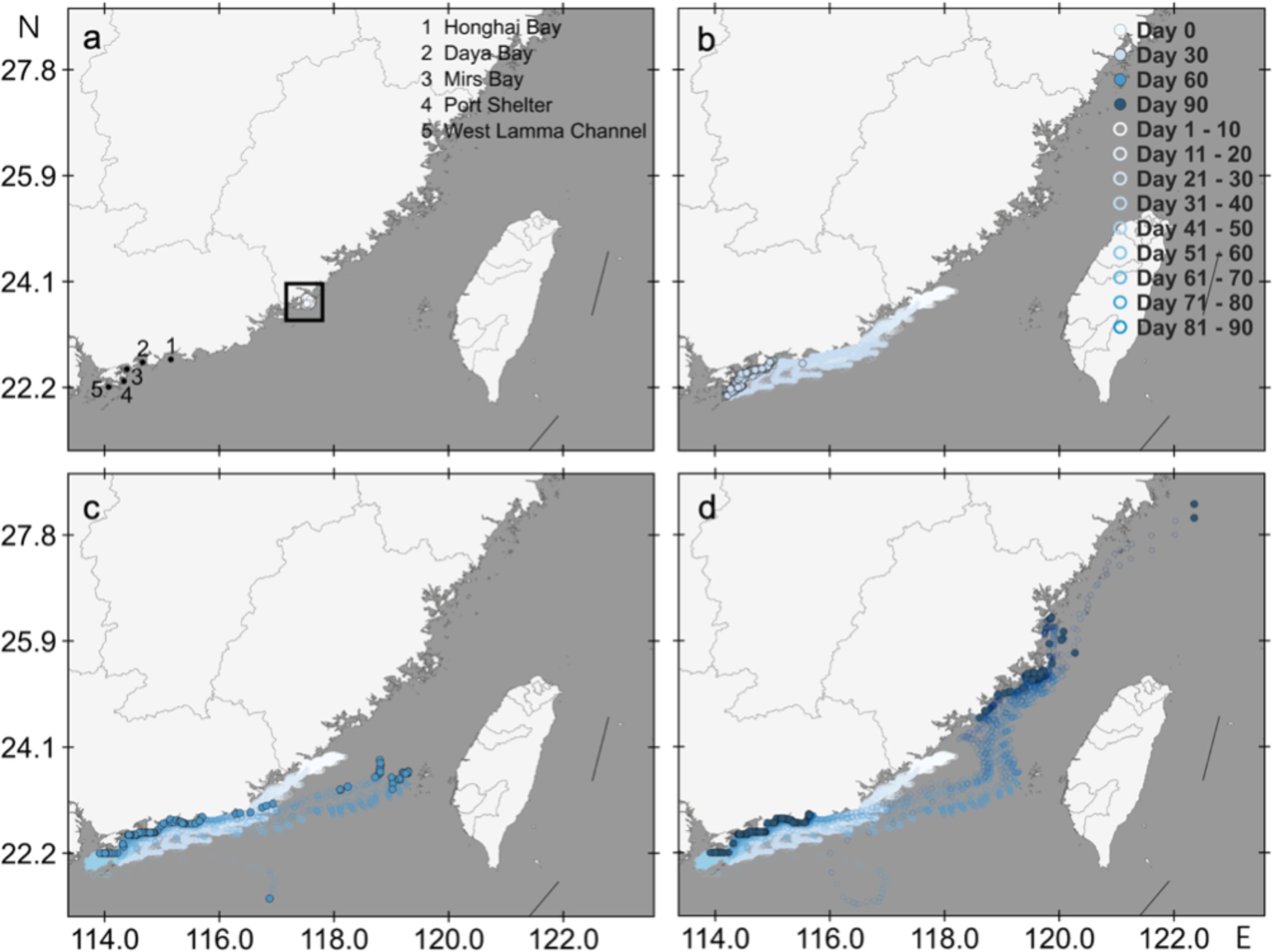
Figure 2. Three-month transport trajectory of epimicroplastic red tide species from Dongshan Bay. (a) Initial position of the released particle; (b) Potential transport trajectory within 30 days; (c) Potential transport trajectory within 60 days; (d) Potential transport trajectory within 90 days.
Three-month transport trajectories of epimicroplastic red tide species from Quanzhou Bay were simulated (Figure 3). During the first month, the particles transported in a southwestward direction, then turned back to the surrounding waters of Quanzhou Bay, and finally transported to other sea areas in a northeastward direction. According to the first-month transport trajectories, Xiamen Bay, Shenhu Bay and Weitou Bay were potentially affected by those red tide species. In the second month, the particles spread northeastward to Meizhou Bay and other waters, and reached as far as the coast of Zhejiang Province, but most of the particles always lingered around Quanzhou Bay. However, due to the influence of coastal topography and hydrological characteristics, some particles started to be obstructed in the second month, including 5.9% particles in Weitou Bay and 0.6% particles in Shenhu Bay. In the third month, 16.3% particles were obstructed, including 12.2% and 4.1% particles in Weitou Bay and Shenhu Bay, respectively. In these transport trajectories from Quanzhou Bay, only 2.2% particles moved into waters further, while other 97.8% particles still transported or be obstructed in the surrounding waters of Quanzhou Bay.
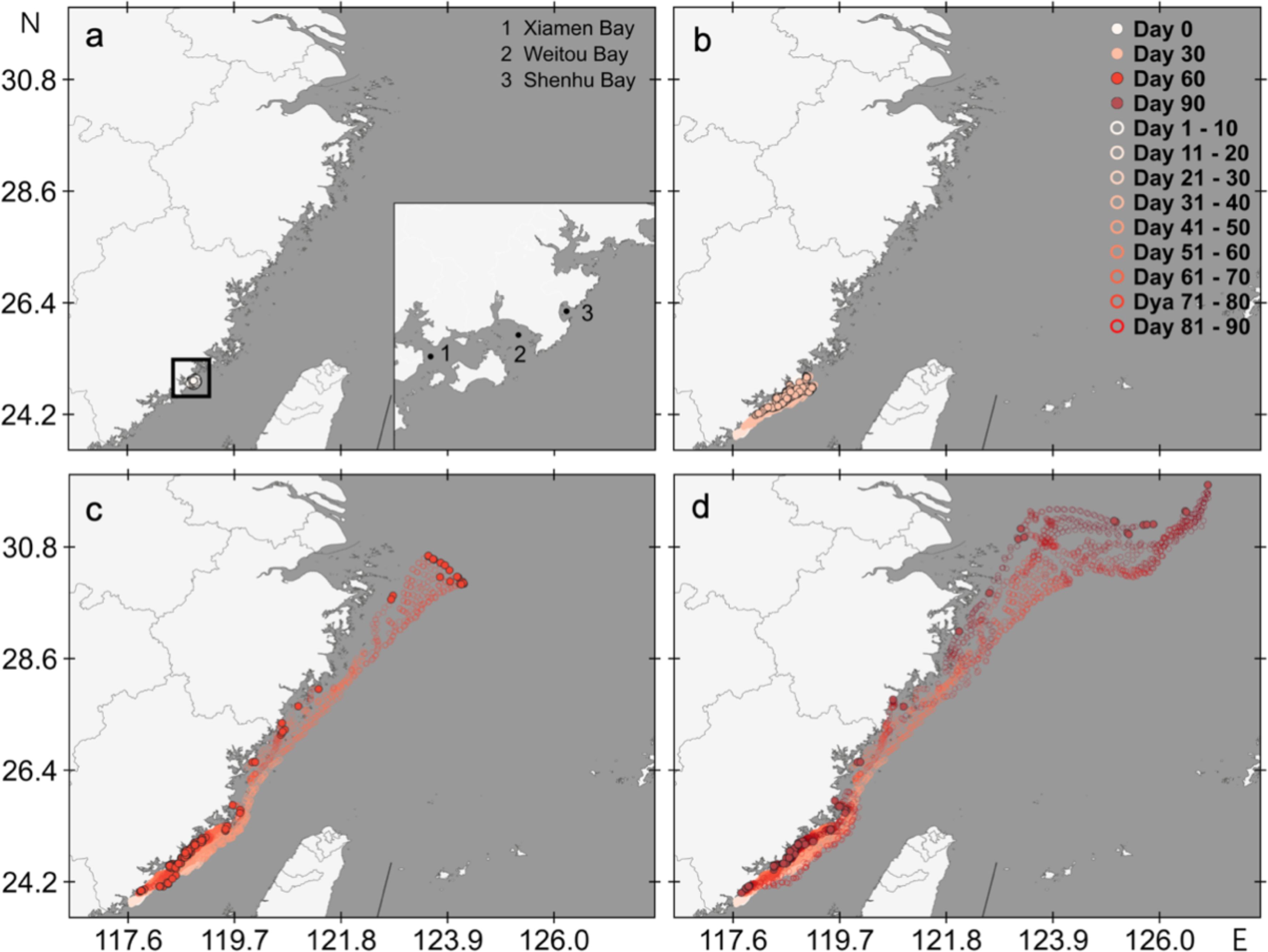
Figure 3. Three-month transport trajectories of epimicroplastic red tide species from Quanzhou Bay. (a) Initial position of the released particle; (b) Potential transport trajectory within 30 days; (c) Potential transport trajectory within 60 days; (d) Potential transport trajectory within 90 days.
4 Discussion
4.1 The potential effect of epimicroplastic red tide species to the marine environment
With an increasing frequency, red tide outbreak has posed a threat to the marine ecosystem (Yan, 2022), by changing the community structure of marine organisms (Yu et al., 2017), destroying the fisheries resources and harming human health (Song et al., 2018; Baohong et al., 2021). In the recent years, researchers have started to suggest that red tide species can transported by attaching to microplastic in addition to ballast water and ocean currents, which has been confirmed by the discovery of the red tide species in the EMP-MA community (Wang et al., 2021, 2022; Pasqualini et al., 2023). In this study, totally 13 epimicroplastic red tide species from 4 phyla were found, with 5 species in Diatom and 6 species in Dinophyta. Among them, two species have been identified as may contain algal toxins in previous studies, namely C. marina may produce Ichthyotoxin (Lundholm et al., 2009); and P. pungens may produce neurotoxin domoic acid (Lundholm et al., 2009). Additionally, A. coffeaeformis has been reported to have the ability to produce neurotoxin domoic acid (Shimizu et al., 1989), while this finding has been disputed (Bates, 2000; Zabaglo et al., 2016). In our simulated potential transport processes, epimicroplastic red tide species from Dongshan Bay and Quanzhou Bay would influence three provinces, including Guangdong Province, Fujian Province and Zhejiang Province, all of which have high records of red tide outbreak in China. T. nitzschioides and P. donghaiense, found in the epimicroplastic community in our study, were two of the main red tide species in these three provinces (Li et al., 2019; Shiyong et al., 2021; Zhang, 2022). The remaining 10 red tide species, except for A. coffeaeformis, also have outbreaks records in Chinese coastal waters (Chen and Zhang, 2021; Chen and Chen, 2021), indicating that they have the potential to become main causative species to the other provinces’ waters in the future. Previous studies about epimicroplastic microalgae, also identified as a part of “plastisphere” (Zettler et al., 2013) or biofilm, have proved their species specificity (Wang et al., 2022) and community stability (Dey et al., 2022) in natural waters. When we describe the transportation of red tide species by the vector of microplastics and further causing red tide outbreak in other waters, the ability of those species to detach from microplastics in the waters with suitable nutrient condition become a crucial node. A laboratory experiment has demonstrated that microalgae in the biofilm consortium could easily detach and develop in the pelagic phase by dispersal following shaking and movements (Binda et al., 2024). This does not mean, however, that the microplastics will be completely ‘washed’ by physical factors such as marine currents. Same experiment also showed that besides colonizing the pelagic environment, species composing in the biofilm further colonize the plastic surface (Binda et al., 2024). The epimicroplastic red tide species we found in this study were almost planktonic, and the species richness between Diatom and Dinophyta were almost the same. Most studies have proved that Diatom dominated in the epimicroplastic microalgae community, since these diatom species are benthic and capable of adhesion and motility on natural or artificial substrata (Chiovitti et al., 2006; Wang et al., 2022). Over the last decades, red tide outbreak associated with epiphytic dinoflagellates (BHABs) have also been reported more frequently, as most of their representatives are potential toxin producers (Wang et al., 2022). Do Prado Leite et al. (2022) suggested that plastics provides significant surfaces for potential colonization by planktonic and benthic harmful microalgae and for the adsorption of their toxins. Binda et al. (2024) has found that microplastic can be xenobiotic substrates for benthic species, leading to competition with the natural pelagic community and posing risks for the depletion of available nutrients. Those benthic microalgae can even form another biofilm community at the bottom of all microcosms while engaging in competition in the surface (Binda et al., 2024). This means that as long as those epimicroplastic benthic species turn over with the currents for a certain period of time in the waters, they have the potential to cause an outbreak event.
Microplastics in aquatic environments have been shown to absorb pollutants from surrounding water (Wang et al., 2021). Many studies have examined the absorption ability of various pollutants on microplastics (Guo and Wang, 2019; Guo et al., 2020) and thoroughly reviewed by several researches (Koelmans et al., 2016; Mei et al., 2020; Wang et al., 2021). Recently, the absorption of pollutants on biofilm-developed microplastics has been studied by in situ experiments and laboratory studies (Richard et al., 2019; Wang et al., 2020; Wang, 2024). Extracellular polymeric substances, natural dissolved organic matter, and other organic macromolecules can be absorbed onto the micro(nano)plastic surface to form the so-called eco-corona (Wei and Xinghui, 2024). The formation of biofilm on the surface of MPs changes its surface morphology, roughness, surface functional groups, and other physical and chemical properties (He et al., 2022; Tu et al., 2020; Yan et al., 2024), which further affect its adsorption behavior with environmental pollutants (Bhagwat et al., 2021; Xu et al., 2021). Numerous studies have demonstrated that biofilm formation increases the adsorption capacity of MPs for various environmental pollutants, such as organic pollutants (José and Jordao, 2022), heavy metals (Zhou et al., 2022), and radionuclides (Johansen et al., 2018; Yan et al., 2024). Microplastics with biofilm have higher affinities to pollutants than virgin microplastics, which may pose more serious consequences (Wang et al., 2021). Microplastics, with attaching red tide species and adsorbed pollutants, transport in the coast water, the harm they cause to the marine ecological environment will be aggravated.
4.2 Hydrodynamic conditions during the transport process of the epimicroplastic red tide species
Red tide outbreak event involves algal growth and migration (Chen, 2019). In addition to the effects of temperature (Singh and Singh, 2015), light (Edwards et al., 2015) and nutrients (Wang et al., 2016), hydrodynamic conditions are also considered to be essential factors affecting the growth and aggregation of algae in the waters (Chen et al., 2015; Zhong et al., 2024). Algal migration can lead to the aggregation of algae in the surface water within a short period of time, causing red tide outbreaking (Chen, 2019). Under certain conditions, algal aggregation is mainly affected by hydrodynamic conditions (Chen, 2019). Hydrodynamic conditions, including water velocity, changes in water flow and water level, directly affect the survival and growth of algae in the waters, which in turn play a crucial role in the occurrence and development of a red tide outbreak event (Zhong et al., 2024). The mechanism of hydrodynamic conditions on algal growth is mainly reflected in three aspects: (1) It affects the distribution of nutrient, regulating the growth and enrichment of algae; (2) It alters the transport process of nutrient, impacting the function of the algal cell; (3) It destroys the structural integrity of the algal cell structure of algae, rendering their inactive (Zhong et al., 2024). However, most red tide species possess a certain degree of autonomous motility, allowing them alter their movements in response to changes in current characteristics (Chen, 2019). For example, P. donghaiense, which allow the algal cells to move independently through the movement of these flagella (Chen, 2019). Motile algae exhibit distribution patterns different from scalar particles in various environments, such as phototaxis, thermotaxis, chemotaxis, and rheotaxis (Rusconi and Stocker, 2015; Pedley and Kessler, 1992). Water flow plays a role in carrying and transporting algae, thereby influencing their spatial distribution. When the transportation of red tide species in the waters carried by microplastics, the impact of the algal swimming ability may be negligible. Therefore, this study only considers the physical process of epimicroplastic species transportation with ocean currents, without taking into account the algal autonomous swimming ability. Additionally, this study does not consider the deposition caused by the weight of microplastics; the entire migration process occurs at the surface, so the only physical factors needed to consider are current velocity. The surface current velocity is generally greater than that at other depth levels in the vertical direction; therefore, this study only considers surface currents, resulting in a relatively larger simulated transport range.
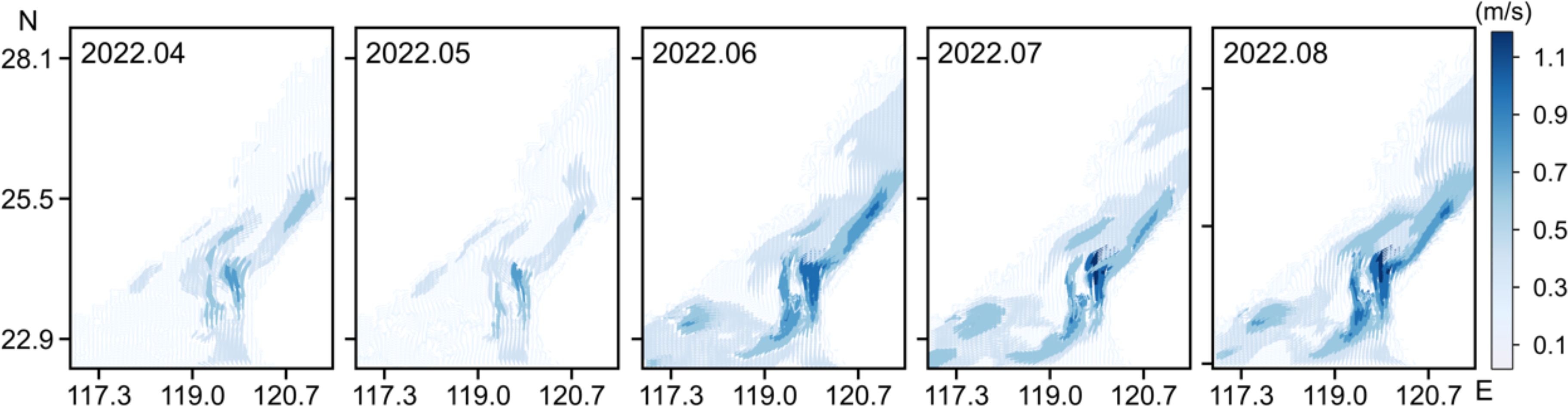
Simulating by Lagrangian particle tracking model, the transport trajectories of epimicroplastic red tide species from Dongshan Bay and Quanzhou Bay were learned in this study. Results showed that particles in those simulated processes of this study were crossing the South China Sea and the East China Sea, covering the coastal waters of three provinces – Guangdong, Fujian and Zhejiang through the Taiwan Strait. The duration of the simulation process is in summer, when the Taiwan Strait is mainly influenced by the wind field. Therefore, driven by the southwestern monsoon, a northeasterly flow in the same direction as the wind direction occurs along the western coast of the Taiwan Strait (Figure 4). Our study was conducted in the spring, which is the main red tide outbreak season in Fujian Province. Additionally, the ocean currents affecting the transport process are primarily influenced by the season and currents, so the simulated transport trajectory outside of spring would differ from the actual ones.
According to two simulated trajectories in our study, epimicroplastic red tide species were transported under the current velocities below 0.5m/s in the Taiwan Strait. Generally, hydrodynamic conditions affect the growth of algae mainly in the form of low flow rate to promote growth (Cao et al., 2008), medium intensity of disturbance will increase the nutrient uptake by algal cells and promote algal metabolism (Xiao et al., 2016); on the contrary, high intensity of disturbance inhibits algal growth, nutrient uptake and cellular metabolism (Tan et al., 2019; Zhang H. et al., 2022). However, the response of different algal species to flow velocity varies significantly in different water bodies (Zhong et al., 2024). When algal species are present in the water column in an epiphytic state, the effect of flow velocity on them is not that much. Similar with other biofilms, epimicroplastic community (“plastisphere”) generally involves microbial attachment, secretion of extracellular polymeric substances, and microbial proliferation (Zettler et al., 2013; Du et al., 2022). Biofilms often feature open-channel and pore structures, enhancing solute and microbial transport and promoting frequent cell-cell contacts (Flemming and Wingender, 2010; Wuertz et al., 2004; Dang and Lovell, 2016). Due to the close positioning of microorganisms, the protective nature of the EPS matrix, and the development of sensing, signaling, and regulatory mechanisms and social behaviors among different microorganisms in biofilms (Davey and O’Toole, 2000; Nadell et al., 2009; Dang and Lovell, 2016), the functional efficiency of biofilm microbial communities should be higher and more stable than those of planktonic microbial communities. biofilm-associated microbial communities may thrive in extreme or hostile environments where individual microorganisms would find the maintenance of activity and growth, even survival, challenging (Dang and Lovell, 2016). Meanwhile, hydrodynamic conditions still influence the epimicroplastic species. For example, in laminar flows, the biofilm is patchy and comprises round cells; in turbulent flows, it comprises wavy and elongated cells (Stoodley et al., 1998). The flow rate affects the density of the biofilm coverage on microplastics surfaces (Xiao et al., 2023; Qin et al., 2024).
5 Conclusion
By attached on the surface of microplastics, those red tide species may be transported further and posing a threat to marine ecosystems. Combined the investigated data of Dongshan Bay and Quanzhou Bay and the simulated transported trajectories, the characteristics and potential transport trajectory of epimicroplastic red tide species were learned in this study. 13 red tide species were totally found in two bays, with 6 species in Dinophyta and 5 species in Diatom. According to the simulated transport trajectories, epimicroplastic species could be transported further by microplastics while some particles would be obstructed during this three-month process. In these simulated trajectories from two bays, 82.50% particles from Dongshan Bay were obstructed in the surrounding waters of Guangdong Province and Hong Kong. However, 97.8% particles from Quanzhou Bay would be transported or be obstructed in the surrounding waters of Quanzhou Bay. During the transport processes, epimicroplastic red tide species from two bays would affect the surrounding waters of three provinces, which all have high records of red tide outbreak in China. This study firstly combined models to investigate the potential transport effect of epimicroplastic red tide species, providing insights into the mechanisms of red tide outbreak.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
CP: Formal analysis, Methodology, Software, Writing – original draft. KW: Conceptualization, Formal analysis, Investigation, Resources, Writing – original draft. HL: Conceptualization, Funding acquisition, Project administration, Writing – review & editing. SC: Resources, Validation, Writing – review & editing. XD: Resources, Supervision, Writing – review & editing. YG: Conceptualization, Project administration, Writing – review & editing. BC: Conceptualization, Funding acquisition, Methodology, Project administration, Writing – review & editing. FK: Methodology, Resources, Software, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was financially supported by the Natural Science Foundation of Fujian Province (2024J01184, 2023J011380), the Joint Funds of the National Natural Science Foundation of China (U24A20607), the Fund of the Key Laboratory of Global Change and Marine-Atmospheric Chemistry of the Ministry of Natural Resources (GCMAC2209).
Acknowledgments
This study was financially supported by the Natural Science Foundation of Fujian Province (2024J01184, 2023J011380), the Joint Funds of the National Natural Science Foundation of China (U24A20607), the Fund of the Key Laboratory of Global Change and Marine-Atmospheric Chemistry of the Ministry of Natural Resources (GCMAC2209), The authors are grateful to three reviewers for their valuable comments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
- ^ Wang, K., Peng, C. H., Lin, H., Dong, X., Lin, H. N., Chen, B. H., et al. Epimicroplastic harmful algae in Fujian coastal waters of China. (Submitted).
References
Allende-Arandía M., Duran R., Sanvicente-Añorve L., Appendini C. M. (2023). Lagrangian characterization of surface transport from the equatorial Atlantic to the Caribbean sea using climatological lagrangian coherent structures and self-organizing maps. J. Geophys. Res.-Oceans 128, e2023JC019894. doi: 10.1029/2023JC019894
Anderson D. M., Cembella A. D., Hallegraeff G. M. (2012). Progress in understanding harmful algal blooms: paradigm shifts and new technologies for research, monitoring, and management. Ann. Rev. Mar. Sci. 4, 143–176. doi: 10.1146/annurev-marine-120308-081121
Baohong C., Kang W., Xu D., Hui L. (2021). Long-term changes in red tide outbreaks in Xiamen Bay in China from 1986 to 2017. Estuar. Coast. Shelf S. 249, 107095. doi: 10.1016/j.ecss.2020.107095
Bates S. S. (2000). Domoic-acid-producing diatoms: another genus added! J. Phycol. 36, 978. doi: 10.1046/j.1529-8817.2000.03661.x
Bhagwat G., Tran T. K. A., Lamb D., Senathirajah K., Grainge I., O’Connor W., et al. (2021). Biofilms enhance the adsorption of toxic contaminants on plastic microfibers under environmentally relevant conditions. Environ. Sci. Technol. 55, 8877–8887. doi: 10.1021/acs.est.1c02012
Binda G., Carnati S., Costa M., Hostyeva V., Leu E., Skjelbred B., et al. (2024). The interaction between plastics and microalgae affects community assembly and nutrient availability. Commun. Earth Environ. 5, 545–545. doi: 10.1038/s43247-024-01706-y
Cao Q. L., Huang Y. L., Chen M. X. (2008). Experimental research on hydro-dynamic simulation of cyanobateria bloom outbreak and extinction. Pearl River 29, 8–10.
Chen X. (2019). Hydrodynamic mechanism of accumulation for typicalalgae in red tide in a complex flow.
Chen N. S., Chen Y. (2021). Advances in the study of biodiversity of phytoplankton and red tide species in China (II): The East China Sea. Oceanol. Limnol. Sin. 52, 363–384.
Chen R. H., Li F. P., Zhang H. P., Chen L., Zhao J. F., Huang Z. H. (2015). Conceptual mechanism of hydrodynamic impacts on freshwater algae for flow management. J. Lake Sci. 27, 24–30. doi: 10.18307/2015.0103
Chen N. S., Zhang M. J. (2021). Advances in the study of biodiversity of phytoplankton and red tide species in China (III): The South China Sea. Oceanol. Limnol. Sin. 52, 385–401.
Chen X. H., Zhu L. S., Zhang H. S. (2007). Numerical simulation of summer circulation in the East China Sea and its application in estimating the sources of red tides in the Yangtze River estuary and adjacent sea areas. J. Hydro. 19, 272–281. doi: 10.1016/S1001-6058(07)60059-6
Chiovitti T., Dugdale T. M., Wetherbee R. (2006). “Diatom adhesives: molecular and mechanical properties,” in Biological Adhesives. Eds. Smith A. M., Callow J. A. (Springer, Berlin, Germany), 79–103.
Cummings J. A. (2006). Operational multivariate ocean data assimilation. Q. J. R. Meteor. Soc 131, 3583–3604. doi: 10.1256/qj.05.105
Cummings J. A., Smedstad O. M. (2013). “Variational data assimilation for the global ocean,” in Data assimilation for atmospheric, oceanic and hydrologic applications, vol. II. , 303–343. doi: 10.1007/978-3-642-35088-7_13
Dang H., Lovell C. R. (2016). Microbial surface colonization and biofilm development in marine environments. Microbiol. Mol. Biol. R. 80, 91–138. doi: 10.1128/MMBR.00037-15
Davey M. E., O’Toole G. A. (2000). Microbial biofilms: from ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 64, 847–867. doi: 10.1128/MMBR.64.4.847-867.2000
Dey S., Rout A. K., Behera B. K., Ghosh K. (2022). Plastisphere community assemblage of aquatic environment: plastic-microbe interaction, role in degradation and characterization technologies. Environ. Microb. 17, 1–21. doi: 10.1186/s40793-022-00430-4
Do Prado Leite I., Menegotto A., Da Cunha Lana P., Júnior L. L. M. (2022). A new look at the potential role of marine plastic debris as a global vector of toxic benthic algae. Sci. Total Environ. 838, 156262. doi: 10.1016/j.scitotenv.2022.156262
Du Y., Liu X., Dong X., Yin Z. (2022). A review on marine plastisphere: biodiversity, formation, and role in degradation. Comput. Struct. Biotec. 20, 975–988. doi: 10.1016/j.csbj.2022.02.008
Edwards K. F., Thomas M. K., Klausmeier C. A., Litchman E. (2015). Light and growth in marine phytoplankton: allometric, taxonomic, and environmental variation. Limnol. Oceanogr. 60, 540–552. doi: 10.1002/lno.10033
Eriksen M., Lebreton L. C. M., Carson H. S., Thiel M., Moore C. J., Borerro J. C., et al. (2014). Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PloS One 9, e11191310. doi: 10.1371/journal.pone.0111913
Flemming H. C., Wingender J. (2010). The biofilm matrix. Nat. Rev. Microbiol. 8, 623–633. doi: 10.1038/nrmicro2415
Gao Y., Hao P., Wei Z., Li S., Song J., Yu C. (2024). Dynamic causes contribute to the increasing trend of red tides in the east China sea during 2020–2022. Mar. Enviro. Res. 198, 106521. doi: 10.1016/j.marenvres.2024.106521
Glibert P. M., Icarus Allen J., Artioli Y., Beusen A., Bouwman L., Harle J., et al. (2014). Vulnerability of coastal ecosystems to changes in harmful algal bloom distribution in response to climate change: projections based on model analysis. Global Change Biol. 20, 3845–3858. doi: 10.1111/gcb.2014.20.issue-12
González-Ortegón E., Demmer J., Robins P., Jenkins S. (2024). Floating plastics as a potential dispersal vector for rafting marine non-native species. Mar. pollut. Bull. 207, 116919. doi: 10.1016/j.marpolbul.2024.116919
Green D. S., Boots B., O’Connor N. E., Thompson R. C. (2016). Microplastics affect the ecological functioning of an important biogenic habitat. Environ. Sci. Technol. 51, 68–77. doi: 10.1021/acs.est.6b04496
Guerrini F., Mari L., Casagrandi R. (2021). The dynamics of microplastics and associated contaminants: Data-driven Lagrangian and Eulerian modelling approaches in the Mediterranean Sea. Sci. Total Environ. 777, 145944. doi: 10.1016/j.scitotenv.2021.145944
Guo X., Liu Y., Wang J. L. (2020). Equilibrium, kinetics and molecular dynamic modeling of Sr2+ sorption onto microplastics. J. Hazard. Mater. 400, 123324. doi: 10.1016/j.jhazmat.2020.123324
Guo X., Wang J. L. (2019). The phenomenological mass transfer kinetics model for Sr2+ sorption onto spheroids primary microplastics. Environ. pollut. 250, 737–745. doi: 10.1016/j.envpol.2019.04.091
Haram L. E., Carlton J. T., Centurioni L., Choong H., Cornwell B., Crowley M., et al. (2023). Extent and reproduction of coastal species on plastic debris in the North Pacific Subtropical Gyre. Nat. Ecol. Evol. 7, 687–697. doi: 10.1038/s41559-023-01997-y
He S., Jia M., Xiang Y., Song B., Xiong W., Cao J., et al. (2022). Biofilm on microplastics in aqueous environment: Physicochemical properties and environmental implications. J. Hazard. Mater. 424, 127286. doi: 10.1016/j.jhazmat.2021.127286
Hong H., Chai F., Zhang C., Huang B., Jiang Y., Hu J. (2011). An overview of physical and biogeochemical processes and ecosystem dynamics in the Taiwan Strait. Cont. Shelf Res. 31, S3–S12. doi: 10.1016/j.csr.2011.02.002
Johansen M. P., Prentice E., Cresswell T., Howell N. (2018). Initial data on adsorption of Cs and Sr to the surfaces of microplastics with biofilm. J. Environ. Radioact. 190, 130–133. doi: 10.1016/j.jenvrad.2018.05.001
José S., Jordao L. (2020). Exploring the interaction between microplastics, polycyclic aromatic hydrocarbons and biofilms in freshwater. Polycycl. Aromat. Compd. 42, 2210–2221. doi: 10.1080/10406638.2020.1830809
Koelmans A. A., Bakir A., Burton G. A., Janssen C. R. (2016). Microplastic as a vector for chemicals in the aquatic environment: critical review and model-supported reinterpretation of empirical studies. Environ. Sci. Technol. 50, 3315–3326. doi: 10.1021/acs.est.5b06069
Kuang C., Xie H., Su P., Gu J., Map X. (2016). Tracking migration and diffusion of red tides in Qinhuangdao coastal water based on FBM method. China Environ. Sci. 36, 2505–2515.
Li L., Lü S., Cen J. (2019). Spatio-temporal variations of Harmful algal blooms along the coast of Guangdong, Southern China during 1980–2016. J. Ocean. Limnol. 37, 535–551. doi: 10.1007/s00343-019-8088-y
Lundholm N., Churro C., Escalera L., Fraga S., Hoppenrath M., Iwataki M., et al. (2009). IOC-UNESCO Taxonomic Reference List of Harmful Micro Algae. Available online at: https://www.marinespecies.org/hab (Accessed September 1, 2024).
Masó M., Garcés E., Pagés F., Camp J. (2003). Drifting plastic debris as a potential vector for dispersing Harmful Algal Bloom (HAB) species. Sci. Mar. 67, 107–111. doi: 10.3989/scimar.2003.67n1107
Mei W., Chen G., Bao J., Song M., Li Y., Luo C. (2020). Interactions between microplastics and organic compounds in aquatic environments: A mini review. Sci. Total Environ. 736, 139472. doi: 10.1016/j.scitotenv.2020.139472
Nadell C. D., Xavier J. B., Foster K. R. (2009). The sociobiology of biofilms. FEMS Microbiol. Rev. 33, 206–224. doi: 10.1111/j.1574-6976.2008.00150.x
Naik R. K., Naik M. M., D’Costa P. M., Shaikh F. (2019). Microplastics in ballast water as an emerging source and vector for harmful chemicals, antibiotics, metals, bacterial pathogens and HAB species: A potential risk to the marine environment and human health. Mar. pollut. Bull. 149, 110525. doi: 10.1016/j.marpolbul.2019.110525
Pasqualini V., Garrido M., Cecchi P., Connès C., Couté A., Rakwe M. E., et al. (2023). Harmful algae and pathogens on plastics in three mediterranean coastal lagoons. Heliyon 9, e13654. doi: 10.1016/j.heliyon.2023.e13654
Pedley T. J., Kessler J. O. (1992). Hydrodynamic phenomena in suspensions of swimming microorganisms. Annu. Rev. Fluid Mech. 24, 313–358. doi: 10.1146/annurev.fl.24.010192.001525
Qin Y., Tu Y., Chen C., Wang F., Yang Y., Hu Y. (2024). Biofilms on microplastic surfaces and their effect on pollutant adsorption in the aquatic environment. J. Mater. Cycles Waste 26, 3303–3323. doi: 10.1007/s10163-024-02066-7
Richard H., Carpenter E. J., Komada T., Palmer P. T., Rochman C. M. (2019). Biofilm facilitates metal accumulation onto microplastics in estuarine waters. Sci. Total Environ. 683, 600–608. doi: 10.1016/j.scitotenv.2019.04.331
Rocha-Santos T. A. (2018). Editorial overview: micro and nano-plastics. Curr. Opin. Environ. Sci. Hl. 1, 52–54. doi: 10.1016/j.coesh.2018.01.003
Rusconi R., Stocker R. (2015). Microbes in flow. Curr. Opin. Microbiol. 25, 1–8. doi: 10.1016/j.mib.2015.03.003
Senderovich Y., Izhaki I., Halpern M. (2010). Fish as reservoirs and vectors of Vibrio cholerae. PloS One 5, e8607. doi: 10.1371/journal.pone.0008607
Shimizu Y., Gupta S., Masuda K., Maranda L., Walker C. K., Wang R. (1989). Dinoflagellate and other microalgal toxins: chemistry and biochemistry. Pure Appl. Chem. 61, 513–516. doi: 10.1351/pac198961030513
Shiyong W., Tianli S., Zizhu W., Fei L., Dongmei L., Na L. (2021). Spatial and temporal characteristics of harmful algal blooms in Zhejiang Province waters during 1933–2018. Mar. Biol. Res. 17, 545–553. doi: 10.1080/17451000.2021.2009874
Singh S. P., Singh P. (2015). Effect of temperature and light on the growth of algae species: A review. Renew. Sust. Energ. Rev. 50, 431–444. doi: 10.1016/j.rser.2015.05.024
Song J., Beule L., Jongmans-Hochschulz E., Wichels A., Gerdts G. (2022). The travelling particles: community dynamics of biofilms on microplastics transferred along a salinity gradient. ISME Commun. 2, 35. doi: 10.1038/s43705-022-00117-4
Song N., Wang N., Wu N., Lin W. N. (2018). Temporal and spatial distribution of harmful algal blooms in the Bohai Sea during 1952-2016 based on GIS. China Environ. Sci. 38, 1142–1148. doi: 10.19674/j.cnki.issn1000-6923.2018.0136
Steidinger K. A., Haddad K. (1981). Biologic and hydrographic aspects of red tides. BioScience 31, 814–819. doi: 10.2307/1308678
Stoodley P., Dodds I., Boyle J. D., Lappin-Scott H. M. (1998). Influence of hydrodynamics and nutrients on biofilm structure. J. Appl. Microbiol. 85, 19S–28S. doi: 10.1111/j.1365-2672.1998.tb05279.x
Su Y., Zhang K., Zhou Z., Wang J., Yang X., Tang J., et al. (2020). Microplastic exposure represses the growth of endosymbiotic dinoflagellate Cladocopium goreaui in culture through affecting its apoptosis and metabolism. Chemosphere 244, 125485. doi: 10.1016/j.chemosphere.2019.125485
Tan Y., Li J., Zhang L., Chen M., Zhang Y., An R. (2019). Mechanism underlying flow velocity and its corresponding influence on the growth of euglena gracilis, a dominant bloom species in reservoirs. Int. J. Env. Res. Pub. He. 16, 4641. doi: 10.3390/ijerph16234641
Thompson R. C., Olsen Y., Mitchell R. P., Davis A., Rowland S. J., John A. W., et al. (2004). Lost at sea: where is all the plastic? Science 304, 838–838. doi: 10.1126/science.1094559
Tu C., Chen T., Zhou Q., Liu Y., Wei J., Waniek J. J., et al. (2020). Biofilm formation and its influences on the properties of microplastics as affected by exposure time and depth in the seawater. Sci. Total Environ. 734, 139237. doi: 10.1016/j.scitotenv.2020.139237
UNEP (2016). GRID-Arendal. Marine Litter Vital Graphics (Nairobi and Arendal: United Nations Environment Programme and GRID-Arendal). Available at: www.unep.org (Accessed September 1, 2024).
Wang C. (2023). Research on the Risk Assessment of Storm Surge Disaster in the Gulf Based on Machine Learning Algorithm (Xiamen: Third Institute of Oceanography, Ministry of Natural Research).
Wang K. (2024). Community characteristics of epimicroplastic microalgaeand their interaction with microplastics in typical bays (Xiamen: Xiamen University).
Wang J., Guo X., Xue J. (2021). Biofilm-developed microplastics as vectors of pollutants in aquatic environments. Environ. Sci. Technol. 55, 12780–12790. doi: 10.1021/acs.est.1c04466
Wang K., Lin H., Wang S., Dong X., Sun L., Zhou Q., et al. (2022). Species diversity and community structure of microalgae living on microplastics in Luoyuan Bay, China. Mar. pollut. Bull. 180, 113809. doi: 10.1016/j.marpolbul.2022.113809
Wang Y., Wang X., Li Y., Li J., Wang F., Xia X., et al. (2020). Biofilm alters tetracycline and copper adsorption behaviors onto polyethylene microplastics. Chem. Eng. J. 392, 123808. doi: 10.1016/j.cej.2019.123808
Wang C., Wang Z., Wang P., Zhang S. (2016). Multiple effects of environmental factors on algal growth and nutrient thresholds for harmful algal blooms: application of response surface methodology. Environ. Model. Assess. 21, 247–259. doi: 10.1007/s10666-015-9481-3
Wei H., Xinghui X. (2024). Element cycling with micro(nano)plastics. Science 385, 933–935. doi: 10.1126/science.adk9505
Wuertz S., Okabe S., Hausner M. (2004). Microbial communities and their interactions in biofilm systems: an overview. Water Sci. Technol. 49, 327–336. doi: 10.2166/wst.2004.0873
Xianbiao J., Baohong C., Kang W., Conghui P., Yahui G., Hui L. (2023). A new microalgae community — Epimicroplastic microalgae (EMP-MA). Algal Res. 71, 103059. doi: 10.1016/j.algal.2023.103059
Xiao Y., Li Z., Li C., Zhang Z., Guo J. (2016). Effect of small-scale turbulence on the physiology and morphology of two bloom-forming cyanobacteria. PloS One 11, e0168925. doi: 10.1371/journal.pone.0168925
Xiao C., Lian X., Wang Y., Chen S., Sun Y., Tao G., et al. (2023). Impacts of hydraulic conditions on microplastics biofilm development, shear stresses distribution, and microbial community structures in drinking water distribution pipes. J. Environ. Manage. 325, 116510. doi: 10.1016/j.jenvman.2022.116510
Xie Y. H. (2021). Numerical simulation of hydrodynamics and ecological water quality in Quanzhou Bay based on FVCOM (Fujian China: Fuzhou University).
Xu L., Li H., Han L., Zou G., Chen Y., Liu D., et al. (2021). Research progress on the adsorption and desorption of typical pollutants on microplastics. Chin. J. Eco-Agr. 29, 961–969.
Yamamoto T., Okai M. (2000). Effects of diffusion and upwelling on the formation of red tides. J. Plankton Res. 22, 363–380. doi: 10.1093/plankt/22.2.363
Yan T. (2022). The “harmful algae and algal toxins in coastal waters of China: investigation and database” project. J. Ocean. Limnol. 40, 2081–2093. doi: 10.1007/s00343-022-2165-3
Yan X., Chio C., Li H., Zhu Y., Chen X., Qin W. (2024). Colonization characteristics and surface effects of microplastic biofilms: Implications for environmental behavior of typical pollutants. Sci. Total Environ. 937, 173141–173141. doi: 10.1016/j.scitotenv.2024.173141
Yang Y. (2021). Distribution characteristics of emerging contaminants in the water of the western Taiwan Strait and Dongshan Bay (Xiamen Uniersity, Xiamen, China).
You X., Cao X., Zhang X., Guo J., Sun W. (2021). Unraveling individual and combined toxicity of nano/microplastics and ciprofloxacin to Synechocystis sp. at the cellular and molecular levels. Environ. Int. 157, 106842. doi: 10.1016/j.envint.2021.106842
Yu R., Zhang Q., Kong F., Zhou Z., Chen Z., Zhao Y., et al. (2017). Status, impacts and long changes of harmful algae blooms in the sea area adjacent to the Changjiang River estuary. Oceanol. Limnol. Sin. 48, 1178–1186.
Zabaglo K., Chrapusta E., Bober B., Kaminski A., Adamski M., Bialczyk J. (2016). Environmental roles and biological activity of domoic acid: A review. Algal Res. 13, 94–101. doi: 10.1016/j.algal.2015.11.020
Zettler E. R., Mincer T. J., Amaral-Zettler L. A. (2013). Life in the “plastisphere”: microbial communities on plastic marine debris. Environ. Sci. Technol. 47, 713746. doi: 10.1021/es401288x
Zhang C. (2022). Interannual and decadal changes in harmful algal blooms in the coastal waters of Fujian, China. Toxins 14, 578. doi: 10.3390/toxins14090578
Zhang J., Kong L., Zhao Y., Lin Q., Huang S., Jin Y., et al. (2022). Antagonistic and synergistic effects of warming and microplastics on microalgae: Case study of the red tide species Prorocentrum donghaiense. Environ. pollut. 307, 119515. doi: 10.1016/j.envpol.2022.119515
Zhang H., Wang N., Zong R., Huang T., Miao Y., Shi Y., et al. (2022). Research progress on influence of hydrodynamic conditions on algal physiology and ecology. Res. Environ. Sci. 35, 181–190. doi: 10.13198/j.issn.1001-6929.2021.10.20
Zheng H. D. (2019). Constructing and evaluating an index system for aquaculture carrying capacity in Dongshan Bay. J Fujian Fish. 41, 393–398. doi: 10.14012/j.cnki.fjsc.2019.05.005
Zhong B., Wang Z., Feng W., Huang B. (2024). Effects of hydrodynamic conditions on algal organism growth. Am. J. Biochem. Biot. 20, 292–301. doi: 10.3844/ajbbsp.2024.292.301
Keywords: microplastics, red tide species, particle tracking model, the Taiwan strait, transport trajectory
Citation: Peng C, Wang K, Lin H, Chen S, Dong X, Gao Y, Chen B and Kuang F (2025) The characteristics and potential transport trajectory of epimicroplastic red tide species in the Taiwan Strait. Front. Mar. Sci. 12:1547278. doi: 10.3389/fmars.2025.1547278
Received: 18 December 2024; Accepted: 23 January 2025;
Published: 26 February 2025.
Edited by:
Ram Kumar, Central University of Bihar, IndiaReviewed by:
Naga Radha Srinivas Tanuku, Council of Scientific and Industrial Research (CSIR), IndiaXiang Yu, Shandong Marine Resource and Environment Research Institute, China
Malayaj Rai, University of Hawaii at Manoa, United States
Copyright © 2025 Peng, Wang, Lin, Chen, Dong, Gao, Chen and Kuang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baohong Chen, Y2hlbmJhb2hvbmdAdGlvLm9yZy5jbg==; Fangfang Kuang, a3VhbmdmYW5nZmFuZ0B0aW8ub3JnLmNu
 Conghui Peng
Conghui Peng Kang Wang1,2,3
Kang Wang1,2,3 Hui Lin
Hui Lin Shunyang Chen
Shunyang Chen Xu Dong
Xu Dong Yahui Gao
Yahui Gao Baohong Chen
Baohong Chen Fangfang Kuang
Fangfang Kuang