
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 28 January 2025
Sec. Global Change and the Future Ocean
Volume 12 - 2025 | https://doi.org/10.3389/fmars.2025.1539865
This article is part of the Research TopicImpacts of Climate Change on SeaweedsView all 10 articles
Coral reef algae serve many important ecological functions, from primary production to nutrient uptake and reef stabilization, but our knowledge of longer-term effects of thermal stress on algae in situ is limited. While ocean warming can facilitate proliferation of algae and potential phase shifts from coral to macroalgal-dominated states, algal responses may vary by species, genus, functional group, or type (e.g., calcareous vs. fleshy). We used 11 years of annual monitoring data (2009-2019) that spans two El Niño-associated heatwaves to examine benthic algal community dynamics on Palmyra Atoll in the central Pacific Ocean. We quantified the percent cover of algal taxa via image analysis of permanent benthic photoquadrats from two habitats on Palmyra: the deeper, wave-exposed fore reef (10 m depth) and the shallower, wave-sheltered reef terrace (5 m depth). Each habitat was characterized by distinct algal communities: predominantly calcareous taxa on the fore reef and predominantly fleshy taxa on the reef terrace. Patterns in abundance fluctuated over time and/or in response to thermal anomalies in 2009 and 2015. Fleshy algae generally increased in cover post-warming, which coincided with large declines of the calcified macroalgae, Halimeda spp. Long-term monitoring of coral reef algal communities is critical for understanding their differential responses to thermal stress and can improve projections of ecosystem functioning in the context of global change.
Benthic algae are key components of coral reef ecosystems, where they contribute to primary production and reef building as well as sand, sediment, and carbonate production. The dominance of one functional group or taxon over another has implications for coral reef functioning and the ecological services they provide (Woodhead et al., 2019). Although many coral reefs across the globe are shifting from coral to algal dominance (Pandolfi et al., 2003; McManus and Polsenberg, 2004; Hughes et al., 2010, 2017), algae are inherently a natural component of healthy coral reefs. Despite their functional, morphological, and taxonomic diversity (Fong and Paul, 2011), reef algae remain understudied relative to other reef taxa. Aside from some short-term laboratory studies, little is known about how individual algal taxa or functional groups respond to a combination of stressors in nature (Wernberg et al., 2012). Thus, in situ studies integrating natural environmental conditions with longer-term benthic algal community dynamics are essential for revealing possible reef community trajectories in the coming decades.
Algae on coral reefs are often classified into functional groups (e.g., turf, crustose coralline algae, and macroalgae), based on the underlying assumption that shared traits correspond to similar ecological roles, functions, or processes. Algal functional groups have previously been defined by their susceptibility to herbivory (Steneck and Watling, 1982), their nutrient uptake, productivity, and turnover rates (Littler and Littler, 1980; Littler et al., 1983), or their morphology, internal anatomy (e.g., cortication), thallus structure, and branching pattern (Steneck and Dethier, 1994; Balata et al., 2011). However, there is still a potential for variable responses to environmental conditions within functional groups, particularly following disturbance events (Phillips et al., 1997). Moreover, calcareous algal taxa (in which photosynthesis is coupled with the deposition of calcium carbonate) and non-calcareous (i.e., fleshy) taxa are differentially affected by environmental stressors (Johnson et al., 2014). While the functional group approach (when based on morphological traits) can sometimes predict community assemblage (Stelling-Wood et al., 2020), these traits may not accurately represent functional identity (Mauffrey et al., 2020) and individual genus and/or species variability must be considered (Fong and Fong, 2014; Ryznar et al., 2021).
Two algal functional groups that are sometimes pooled in reef benthic studies, yet have distinct ecological roles, are the crustose coralline algae (CCA) and the algal turfs. CCA are encrusting, calcifying red algae that stabilize the reef framework and support structural complexity (Teichert et al., 2020; Littler and Littler, 2013; Steneck, 1986). They also contribute to carbonate production, possibly more so than reef-building corals (Cornwall et al., 2023). By releasing chemical cues that induce settlement in coral larvae (Harrington et al., 2004), CCA further promote reef growth and resilience. The ecological contributions of CCA on coral reefs are threatened by environmental change, as they are sensitive to thermal stress in both experimental and field settings (Martin and Gattuso, 2009; Short et al., 2015). “Turf algae” (algal turfs) refers to a mixed assemblage of largely fleshy filamentous algae, juvenile macroalgae, and/or cyanobacteria less than 2 cm tall (Adey and Steneck, 1985). Algal turfs are opportunistic and rapid colonizers of open space after coral bleaching or disease outbreaks (Diaz-Pulido and McCook, 2002). They are a main food source for herbivorous grazers (Carpenter, 1986), but can have negative effects on reefs by inhibiting coral recruitment (Birrell et al., 2008) or harboring pathogenic microbes that compromise coral health (Pratte et al., 2018). Despite occupying much of the benthos on today’s reefs (Wismer et al., 2009), they are often miscategorized as “bare space” and, thus, grossly underestimated in surveys of benthic community coverage. Turfs thrive under conditions that threaten corals, including nutrient pollution (Smith et al., 2010), warming (Johnson et al., 2017), ocean acidification (Falkenberg et al., 2013), and sedimentation (Birrell et al., 2005), which suggests that their abundance on reefs will continue to increase with the progression of climate change (Harris et al., 2015; Tebbett and Bellwood, 2019).
Another distinction lost with the typical categorization of algae is the presence or absence of a calcium carbonate skeleton (i.e., calcification). The relative balance of fleshy to calcareous or reef-building taxa may be indicative of more degraded vs. “healthier” coral reefs (Smith et al., 2016), and thus tracking the abundance of calcareous and fleshy algal taxa is useful for assessing ecosystem status. Moreover, fleshy and calcareous taxa have different ecological functions, whether beneficial or detrimental. Fleshy macroalgae typically grow faster than calcareous macroalgae and are generally more edible to herbivores. However, fleshy macroalgae can harm corals directly through abrasion, or indirectly by releasing toxic allelochemicals (Rasher and Hay, 2010), causing hypoxia and physiological stress (Barott et al., 2012) by limiting photosynthetic activity and depleting the corals of energy (Titlyanov et al., 2007). Calcareous algae are generally more benign competitors with corals than fleshy algae (Barott et al., 2012; but see: Keats et al., 1997a and Longo and Hay, 2015, where corals frequently experienced damage from contact with calcareous algae), although their competitive ability may be influenced by seasonality (Brown et al., 2020). Therefore, to holistically evaluate the ecological implications of stressors such as warming, it is informative to look not only at variability across individual algal taxa or functional groups, but also between fleshy and calcareous algae.
For algae and other primary producers, temperature is expected to increase metabolic and photosynthetic rates until a thermal tolerance limit is exceeded (Davison, 1991). Calcification in calcareous algae may initially benefit from warmer temperatures until prolonged exposure leads to mortality or reduction in productivity, as seen in experimental studies (Martin and Gattuso, 2009; Page et al., 2021; but see: Krieger et al., 2023). In contrast, fleshy algae have been found to respond positively to thermal stress in field studies (McClanahan et al., 2001; Burt et al., 2013; Graham et al., 2015). The combined effects of temperature and other stressors can be synergistic (Ellis et al., 2019) or antagonistic (Darling et al., 2010). For example, ocean acidification has been found to cause net negative or species-specific effects on tropical calcareous algae while stimulating growth in some fleshy algae (Johnson et al., 2014), but when combined with warming, effects can be more complex or interactive (Diaz-Pulido et al., 2012; Kram et al., 2016; Johnson et al., 2017).
The calcareous macroalgal genus Halimeda is a group of siphonous green algae that contribute significantly to productivity and calcification on coral reefs (Hillis-Colinvaux, 1980), and can cover up to 20% of the benthos (Perry et al., 2020). Halimeda is one of the most ubiquitous tropical algal genera with representative species occurring on reefs around the world. Indeed, Halimeda spp. may contribute more to tropical carbonate budgets than corals (Rees et al., 2007) due to their fast growth and high turnover rates (Vroom et al., 2003; Smith et al., 2004). Most species of Halimeda are holocarpic and as such, when they reproduce they die and their calcified segments break down into sand (Harney and Fletcher, 2003). Halimeda spp. are synchronous spawners that release all of their gametes simultaneously, leading to complete adult mortality (Hay, 1997), although the exact mechanisms that trigger their reproduction are unknown (Clifton and Clifton, 1999; Clifton, 2013). Considering the high abundance, cosmopolitan distribution, and ecological significance of Halimeda spp., it is important to monitor their cover on a consistent basis as well as before, during, and after thermal anomalies. Few studies have examined the long-term changes in cover of Halimeda spp. in situ (but see: Lambo and Ormond, 2006, where Halimeda cover decreased in Kenya at the time of the 1998 coral bleaching event but increased drastically by 2004).
Here, we measured benthic algal cover over an 11-year time series of permanent benthic photoquadrats from two reef habitats on Palmyra Atoll. Thermal anomalies occurred in both 2009 and 2015 (Williams et al., 2010; Fox et al., 2019), which allowed us to explore how temperature may influence algal community dynamics. Our objectives were to (i) describe benthic algal community composition on the fore reef and reef terrace habitats, (ii) quantify the abundance of individual algal taxa or functional groups, (iii) compare fleshy (turf and fleshy macroalgae) vs. calcareous (CCA and calcareous macroalgae) cover, and (iv) determine whether benthic algal cover varied over time, with temperature, and/or by habitat. Additionally, for the major calcareous macroalgal genus, Halimeda, we measured yearly changes in benthic cover by habitat and site to validate our hypothesis that Halimeda spp. may be temperature-sensitive and negatively affected by warm-water events.
Palmyra Atoll (5.89 °N, 162.08 °W), U.S. Minor Outlying Islands, is located in the Northern Line Islands, central Pacific. Palmyra was designated as a National Wildlife Refuge in 2001 and this protection was further expanded in 2009 as part of the Pacific Remote Islands Marine National Monument. The Atoll was temporarily occupied by the U.S. military during World War II but is currently uninhabited aside from a small field research station. Thus, its reefs are considered quasi-pristine (Sandin et al., 2008) and relatively undisturbed from localized human impacts such as fishing or pollution, yet are still susceptible to global climate change. Palmyra’s benthic communities are dominated by reef-builders such as hard corals and CCA, with remaining surfaces covered by turf algae, macroalgae, soft corals, and other invertebrates (Braun et al., 2009; Williams et al., 2013; Khen et al., 2022).
In September 2009, permanent monitoring plots were established in the two major reef habitats on Palmyra: the wave-exposed fore reef (FR) at 10 m depth and the wave-sheltered reef terrace (RT) at 5 m depth, with four sites per habitat and ten replicate plots (90 cm x 60 cm) per site (Supplementary Figure 1), for a total surveyed area of 21.6 m2 at each habitat. Replicate plots were 5 m apart along a 50 m transect perpendicular to shore, marked by stainless steel eye bolts in opposing corners that were secured to the benthos with marine epoxy. At least once a year from 2009 to 2019, usually in the late summer or early fall, plots were photographed by SCUBA divers with a Canon G-series camera attached to a PVC frame that maintained a fixed distance from the substrate. All images were digitized (i.e., manually traced) in Adobe Photoshop (Creative Cloud) to quantify abundance of algal taxa in terms of planar areas or percent cover at the functional group level for CCA and turf, family-level for peyssonnelioids, and genus or species-level for other macroalgae. Algae were identified visually by morphology, and taxa were grouped as either calcareous (CCA, Halimeda spp., Galaxaura rugosa, and Peyssonneliaceae sp.) or fleshy (Avrainvillea sp., Lobophora sp., Dictyosphaeria spp., Caulerpa serrulata, and turf) based on the presence or absence of biogenic calcium carbonate structures. Palmyra’s thermal history was obtained from a revised percentile-based method of estimating Degree Heating Weeks (DHW; Liu et al., 2006) developed by Mollica et al. (2019), which more accurately captures the degree of accumulated thermal stress experienced by central equatorial Pacific reefs than traditional DHW (Fox et al., 2021).
All analyses were conducted in R software version 3.6.3 (R Core Team, 2018). First, using only annual time points taken during the late summer or fall (excluding irregular time points to minimize the effect of seasonal variation), we constructed a non-metric multidimensional scaling (nMDS, via metaMDS in vegan for R; Oksanen et al., 2019) ordination plot visualizing the trajectory of algal community composition through time at each habitat. This nMDS was based on Bray-Curtis dissimilarity measures for square-root-transformed algal percent cover data (Anderson, 2001). We applied a square-root transformation to balance the effect of disproportionately-abundant taxa. We tested the effects of habitat, year, and/or their interaction by conducting a three-way permutational multivariate analysis of variance (PERMANOVA with 9999 permutations via adonis in vegan; Anderson, 2001; Oksanen et al., 2019) on the same Bray-Curtis distance matrix. We did not include site as a nested factor because not all algal taxa were present at each site within a habitat. To identify which algal taxa were the main contributors to differences among habitats, we ran a SIMPER or “similarity percentages” analysis (via simper in vegan; Clarke, 1993; Oksanen et al., 2019).
To test whether percent cover of fleshy or calcareous algae varied by habitat and/or over time (only for consistent annual time points), we ran two-way analyses of variance (ANOVAs) with Type-II sum of squares. Assumptions of normality and homogeneity of variance were checked through visual inspection of the residuals. We did not incorporate repeated measures and instead treated years independently because different algal populations were sampled each year rather than the same individuals. Post-hoc letter groupings were assigned via Tukey’s multiple comparisons using multcomp (Hothorn et al., 2008).
Next we explored possible effects of temperature on a single taxon of interest, Halimeda, through an analysis of covariance (ANCOVA) with Type-II sum of squares. Habitat was considered a fixed factor and temperature (in terms of percentile-based DHW values during the week of sampling) was considered a continuous factor. We also examined the relationship between accumulated thermal stress and Halimeda cover using Pearson’s correlation. To further investigate patterns in abundance for this genus, we plotted its percent cover within each quadrat, by site, over time. Lines were smoothed by locally-weighted regression (i.e., LOESS in ggplot2; Wickham, 2016). Finally, we calculated the difference in mean percent cover of Halimeda by site (with quadrats as replicates) between consecutive years. Two-tailed t-tests were used to determine which sites experienced significant changes not overlapping zero (e.g., an increase or decrease in percent cover one year later).
The benthic algal community on Palmyra’s fore reef was calcifier-dominated compared to the fleshy-dominated reef terrace (Figure 1; Supplementary Figure 1). Certain taxa were only present in either habitat: G. rugosa on the reef terrace and Avrainvillea sp. on the fore reef. Across both habitats, the most abundant algal taxa or groups on Palmyra included CCA (exhibiting a percent cover range of 0 to 87.6% of the benthos within a single quadrat, average = 20.2 ± 17.4% SD), turf (percent cover = 0 to 88.3%, average = 16.7 ± 17.6%), and Halimeda (percent cover = 0 to 92.3%, average = 8.4 ± 12.4%). The least abundant algal genera were Avrainvillea (percent cover = 0 to 1.7%, average = 0 ± 0.1%), Dictyosphaeria (percent cover = 0 to 27.1%, average = 0.4 ± 1.7%), and Caulerpa (percent cover = 0 to 46.6%, average = 0.6 ± 3.3%). Distinct yearly trajectories of algal community composition were seen in each habitat (Figure 2).
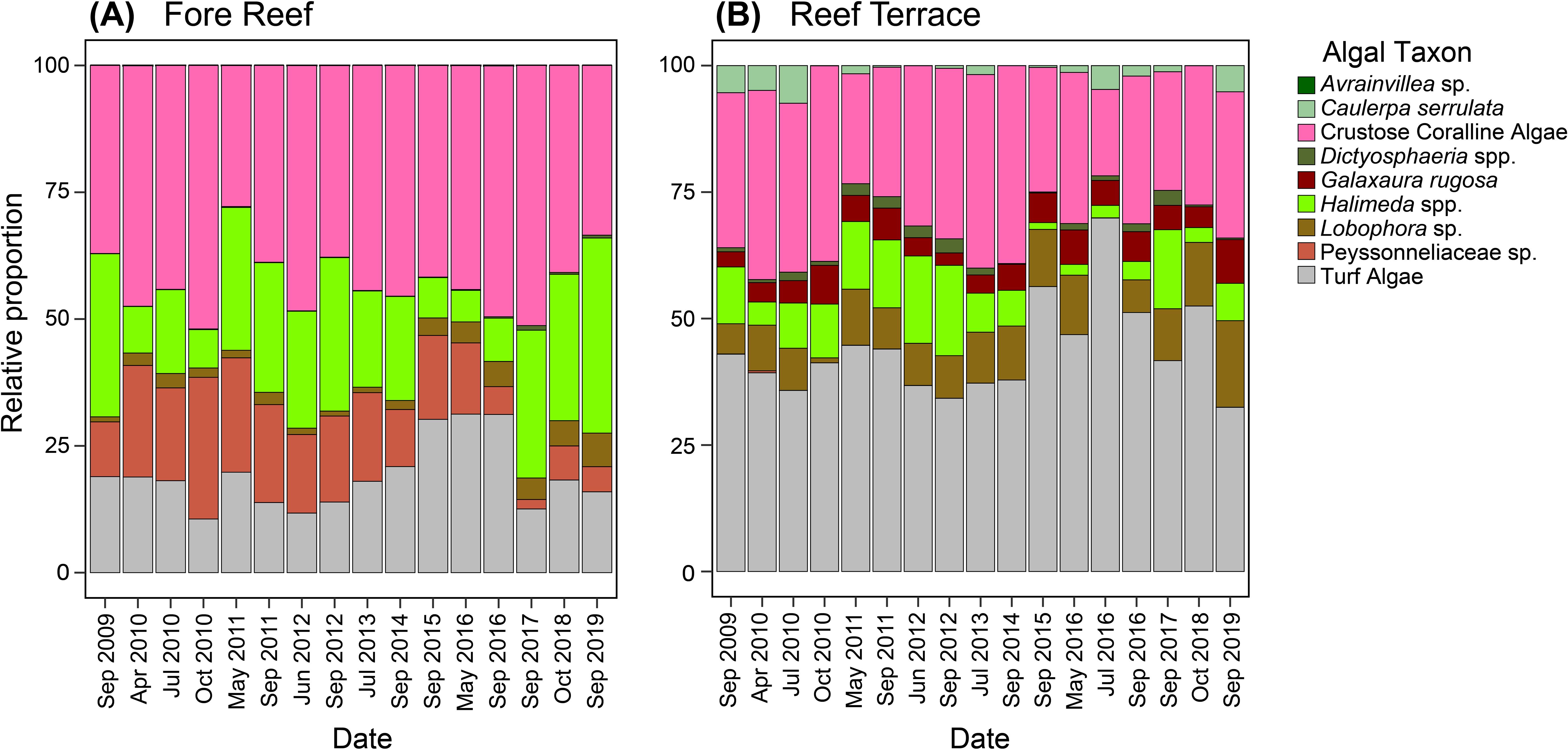
Figure 1. Benthic algal community composition over time on Palmyra from 2009 to 2019 at the (A) Fore Reef and (B) Reef Terrace habitats in terms of relative proportions of each taxon or functional group.
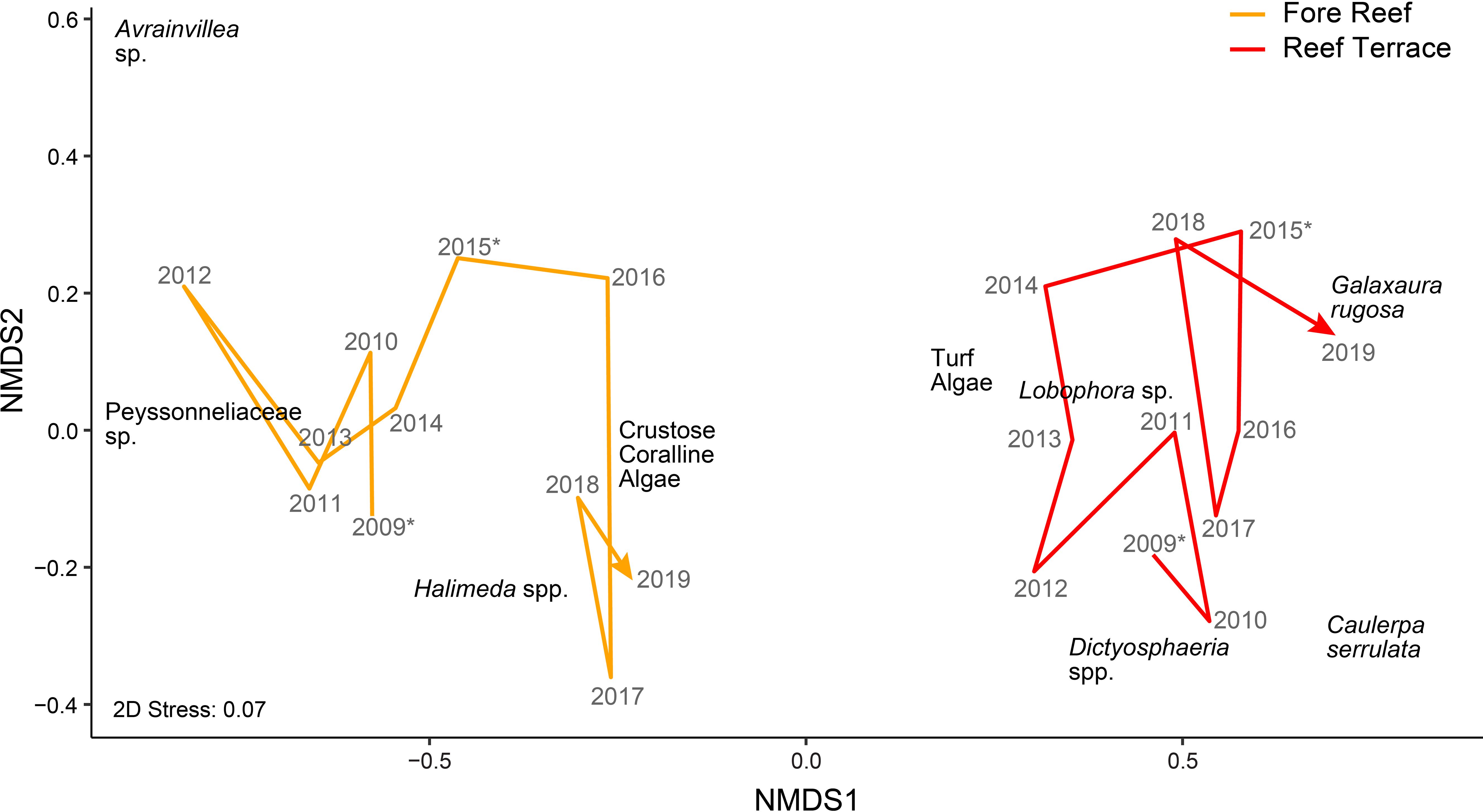
Figure 2. Non-metric multidimensional scaling (nMDS) based on Bray-Curtis dissimilarity measures of benthic algal community composition by taxon (in terms of square-root-transformed percent cover data). Lines terminating in an arrowhead represent the yearly trajectory of each habitat (Fore Reef in orange, Reef Terrace in red) from 2009 to 2019. Asterisks denote thermal anomalies in 2009 and 2015.
Benthic algal community composition on Palmyra varied significantly by habitat (p <0.001) and year (p <0.001), with an interaction indicating that habitats changed differently across years (p <0.001; Supplementary Table 1). There was more year-to-year variation in algal community composition on the fore reef compared to the reef terrace, particularly after the second thermal anomaly in 2015. However, habitat was a better predictor for algal community composition than year, explaining 11.6% of the variation (R2 = 0.116; Supplementary Table 1) compared to 4.3%. A SIMPER analysis revealed that the taxa contributing most to habitat differences were CCA, turf algae, and Halimeda (Supplementary Table 2). Calcareous algae (particularly CCA, Halimeda spp., and Peyssonneliaceae sp.) were more abundant on the fore reef whereas fleshy algae (turf, Lobophora sp., C. serrulata, and Dictyosphaeria spp.) were more abundant on the reef terrace.
Overall, CCA were more abundant on the fore reef than the reef terrace, covering 25.3 ± 0.8% (mean ± SE) and 15.4 ± 0.8% of the total benthos, respectively (Figure 3B). In contrast, turf algae were more abundant on the reef terrace than the fore reef at 21.3 ± 1.0% and 11.5 ± 0.5% cover, respectively (Figure 3H). Between fall 2014 and fall 2015 on the reef terrace, there was a decline in CCA from 20.0 ± 3.2% to 12.7 ± 2.9% and a concomitant rise in turf algae from 19.6 ± 3.5% to 28.6 ± 3.7%; the increase in turf at the time of the second thermal anomaly was seen to a lesser extent on the fore reef. However, by fall 2017, turf and CCA cover were restored to pre-disturbance levels in both habitats. Other algal groups were far less abundant than turf and CCA. Benthic cover of C. serrulata, found almost exclusively on the reef terrace, was highest in the fall of 2010 and 2019 at 3.6 ± 1.5%, but dropped to undetectable levels in fall 2012, 2014, and 2018 (Figure 3A). Similarly, also on the reef terrace, Dictyosphaeria spp. (D. cavernosa and D. versluysii) comprised up to 1.5% total cover but were nearly negligible in the fall of 2014, 2015, 2018, and 2019 (Figure 3C). The reef terrace had 4.3 ± 0.6% cover of G. rugosa in fall 2019 but was typically around 2.5% (Figure 3D). There was consistently higher cover of Lobophora sp. on the reef terrace (5.0 ± 0.5%) compared to the fore reef (1.9 ± 0.2%; Figure 3F). Cover of Peyssonneliaceae sp., found mainly at the fore reef, was lowest in the fall of 2017 at 1.2 ± 0.3% yet reached up to 10-15% of the benthos every fall between 2011 and 2014 (Figure 3G). Avrainvillea sp. was not plotted because it occupied less than 0.01% of the benthos. Halimeda spp. (primarily H. opuntia with minor coverage by H. taenicola and H. fragilis) were more abundant on the fore reef, at 14.2 ± 0.8% cover throughout the time series compared to 4.4 ± 0.3% on the reef terrace (Figure 3E).
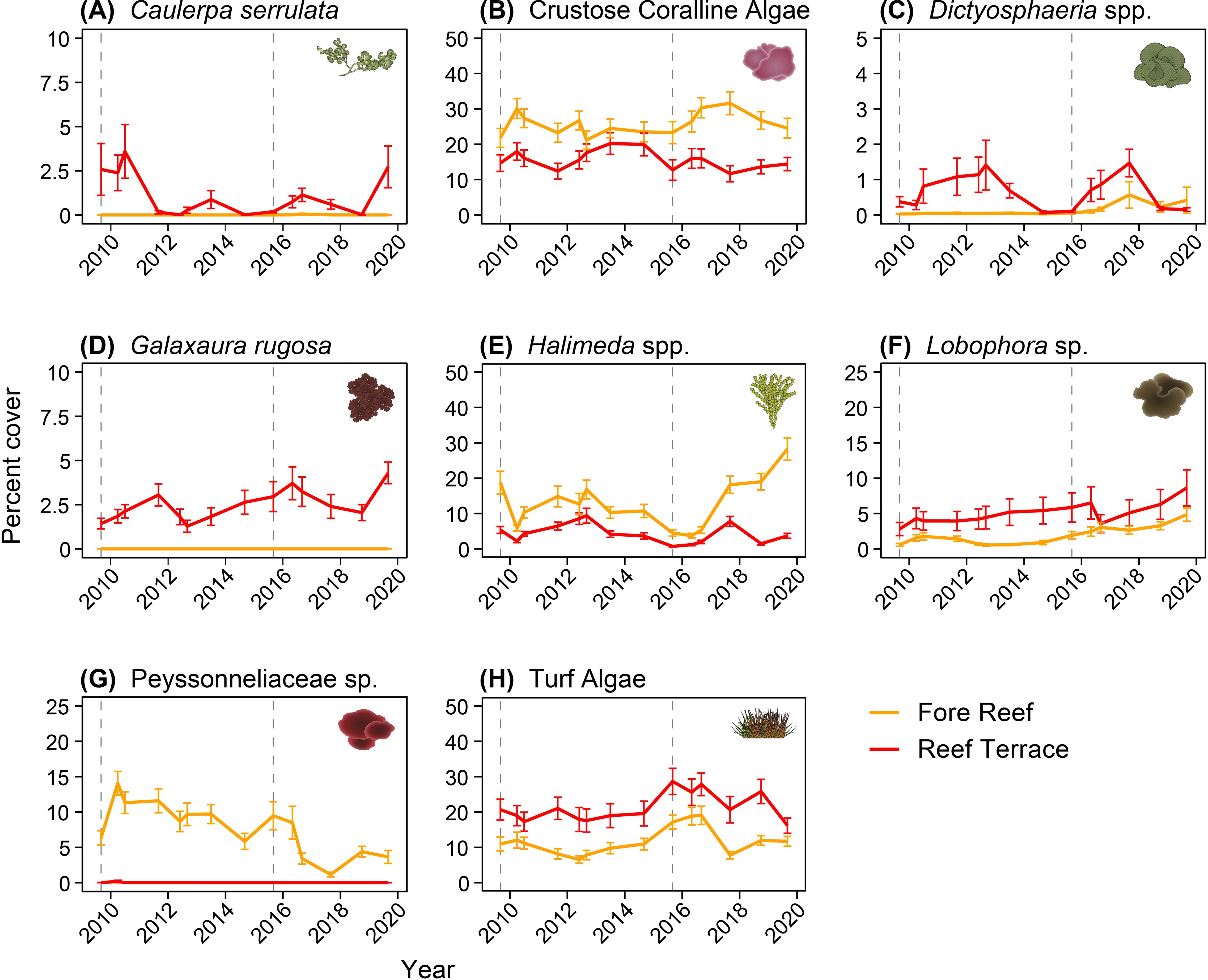
Figure 3. Percent cover (mean ± SE) of (A) Caulerpa serrulata, (B) Crustose Coralline Algae, (C) Dictyosphaeria spp., (D) Galaxaura rugosa, (E) Halimeda spp., (F) Lobophora sp., (G) Peyssonneliaceae sp., and (H) Turf Algae, by habitat (Fore Reef in orange, Reef Terrace in red). Dashed vertical lines indicate thermal anomalies in 2009 and 2015.
Throughout the time series, the fore reef had higher cover of calcareous algae than fleshy algae, at 46.5 ± 0.8% (mean ± SE) and 13.4 ± 0.5%, respectively (Figure 4A), whereas the reef terrace had similar cover of calcareous and fleshy algae, at 22.1 ± 0.8% and 28.0 ± 0.9%, respectively (Figure 4B). Percent cover of fleshy algae varied by habitat (p <0.001) and year (p <0.001) with no significant interaction (Supplementary Table 3). Percent cover of calcareous algae also varied by habitat (p <0.001) and year (p = 0.004), with habitats changing differently over time (p = 0.011). On the reef terrace, the cover of calcareous algae remained consistent through time whereas on the fore reef, calcareous algae were replaced by fleshy algae at the time of the second thermal anomaly in 2015 but re-stabilized by fall 2017. A similar yet less pronounced response was observed on the reef terrace.
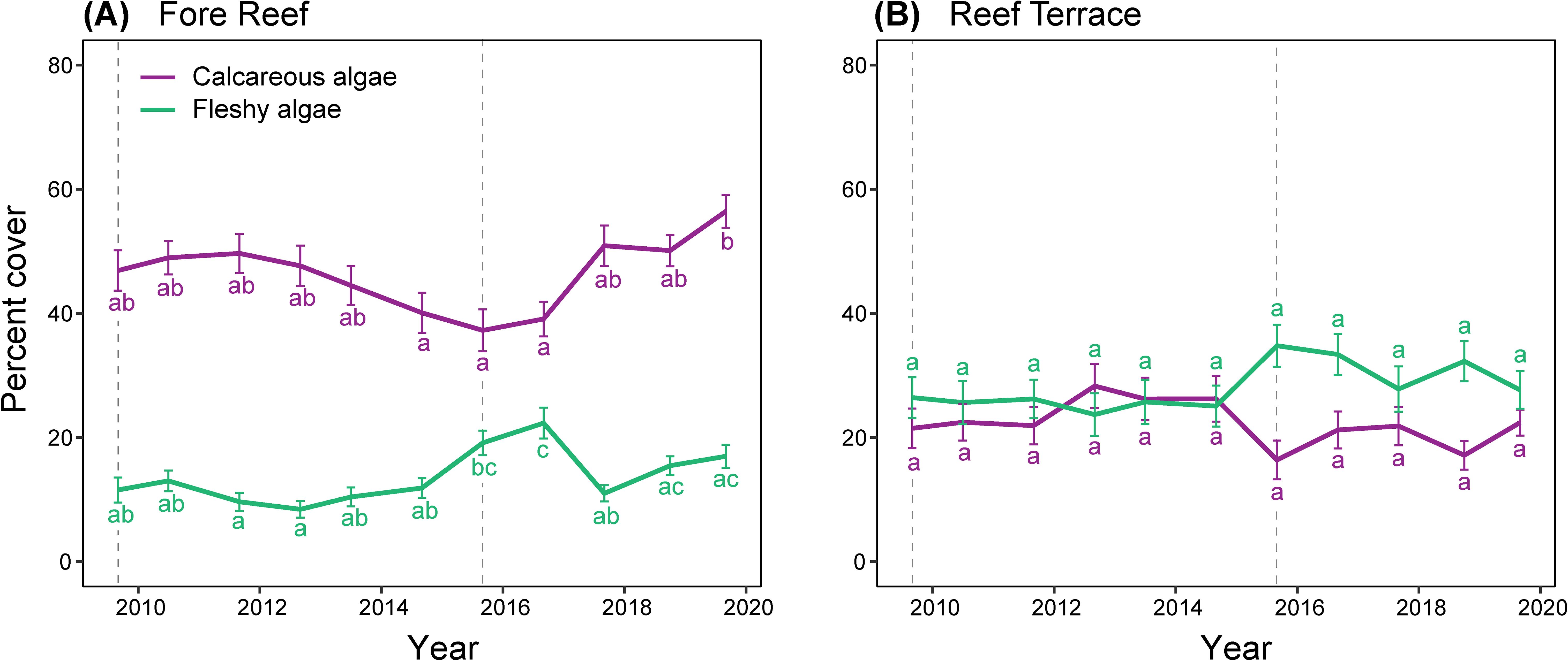
Figure 4. Percent cover (mean ± SE) of calcareous (in purple) and fleshy algae (in green) on Palmyra at the (A) Fore Reef and (B) Reef Terrace habitats, along with post-hoc letter groupings for significant (α = 0.01) differences among years. Dashed vertical lines indicate thermal anomalies in 2009 and 2015.
Several months after the first thermal anomaly, Halimeda cover dropped from 18.8 ± 3.2% (mean ± SE) in fall 2009 to 5.8 ± 0.8% in spring 2010 on the fore reef and 5.4 ± 1.0% to 2.2 ± 0.4% on the reef terrace (Figure 3E). By late summer 2010, Halimeda cover had decreased significantly at four out of eight sites (Supplementary Table 5) but increased in subsequent years. Between fall 2014 and fall 2015, Halimeda cover decreased again at all sites; its cover during the second thermal anomaly was among its lowest throughout the time series, at 4.5 ± 0.9% on the fore reef and 0.7 ± 0.2% on the reef terrace. Regardless of the amount of Halimeda within each quadrat or site, its abundance followed a similar trajectory with sharp declines by 2015, and growth or no change thereafter (Supplementary Figure 2). Between fall 2016 and fall 2017, Halimeda cover increased significantly at six out of eight sites by up to 20% (Supplementary Table 5). Thus, in all cases where significant differences were detected, the sites that changed did so in the same direction. There were significant effects of percentile-based DHW (p = 0.023) and habitat (p <0.001) on Halimeda cover (Supplementary Table 4). A negative relationship between Halimeda cover and accumulated thermal stress was seen (Figure 5), with a linear correlation on the reef terrace (Pearson’s r = -0.65, p = 0.03) but not on the fore reef (Pearson’s r = -0.03, p = 0.92).
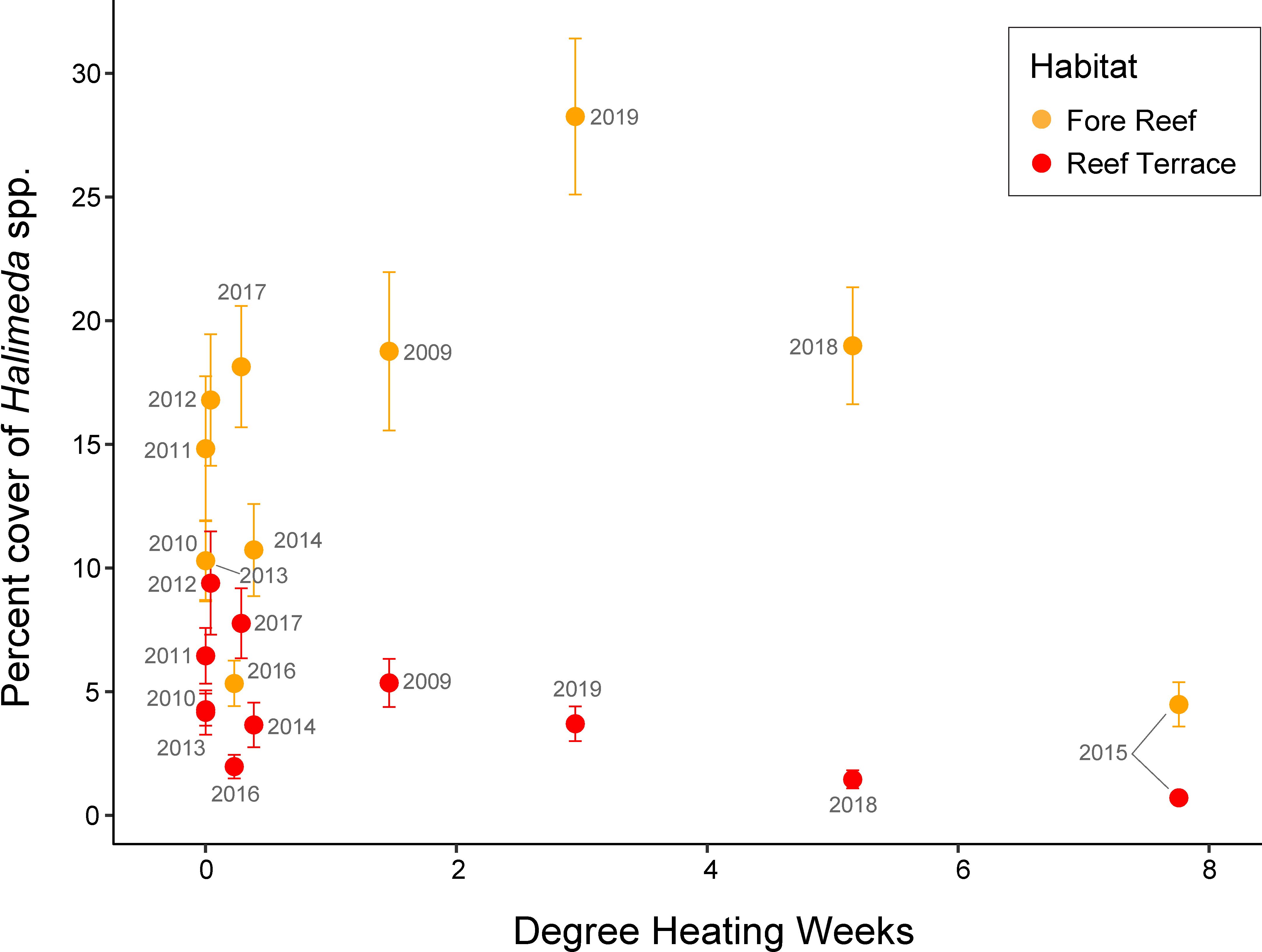
Figure 5. Percent cover of Halimeda spp. (mean ± SE) by habitat (Fore Reef in orange, Reef Terrace in red) corresponding to the percentile-based Degree Heating Weeks (DHW) at each observation time point, labeled by year.
As corals suffer widespread declines due to climate change, there has been a corresponding rise in the abundance of algae on reefs worldwide (Pandolfi et al., 2003; Hughes et al., 2017; Reverter et al., 2021). However, “algae” encompass a heterogenous group of functionally, phylogenetically, morphologically, and taxonomically distinct taxa (Fong and Paul, 2011). While short-term changes in macroalgal abundance on coral reefs, including seasonality, have been well-documented (Aguila Ramírez et al., 2003; Ateweberhan et al., 2006; Lefèvre and Bellwood, 2010), longer-term dynamics of benthic algae at the community, functional group, or species level remain poorly characterized. Here, we present results of an 11-year time series from Palmyra Atoll in the central Pacific Ocean. From 2009 to 2019, the cover of fleshy and calcareous algae was more stable at the reef terrace but fluctuated at the fore reef. At the time of the second, more-severe thermal anomaly in 2015, there was a general decrease in calcareous algae at both habitats accompanied by an increase in fleshy algae which was restored within two years. Given Palmyra’s remote location and high level of federal protection, such data sets can provide baseline information on coral reef algal communities in the context of global stressors.
Long-term ecological monitoring is necessary for detecting trends in species abundance and distribution through time. Prior to this study, the latest comprehensive analysis of Palmyra’s benthic algal community composition was based on summary data from surveys conducted sporadically between 2004 to 2008 (Braun et al., 2009). Before that, knowledge of algal diversity on Palmyra was limited to early explorers’ species lists (Rock, 1916; Dawson et al., 1955; Dawson, 1959). In 2008, the most abundant macroalgal genera on Palmyra were Halimeda, Lobophora, Galaxaura, and Dictyosphaeria (Braun et al., 2009). This remained consistent through 2019, although we also identified C. serrulata as a common macroalgal taxon on the reef terrace (Supplementary Table 2). Additionally, Braun et al. (2009) mentioned high cover of the red alga Dichotomaria marginata near a shipwrecked longliner vessel which was removed in 2013. Dichotomaria was absent from our analyses, although not all of the same reef habitats or sites were represented here, and our study involved small-scale photoquadrats as opposed to large spatial scale surveys. Braun et al. (2009) found algal communities to be relatively similar across sites from the reef terrace and fore reef habitats across the atoll, whereas in the present study, algal communities showed significant differences by habitat and time, with more overall stability at the reef terrace. Calcareous algal cover was consistently higher at the fore reef, although it is worthwhile to note that Palmyra’s reef terrace is largely occupied (up to 50%) by hard corals (Fox et al., 2019; Khen et al., 2022, 2024). Overall, fleshy algal abundance on Palmyra (average percent cover = 20.8%) was low in comparison to reefs with local human populations (average percent cover = 59.3% according to Smith et al., 2016) whereas calcareous algal abundance (average percent cover = 34.4%) was much higher than that of inhabited islands (average percent cover = 16.9%; Smith et al., 2016).
Ecological succession and community structure can be shaped by physical forces such as light and sediment transport (Glynn, 1976), irradiance and water motion (Done, 1982), and wave energy (Dollar, 1982). On Palmyra, local environmental factors likely contributed to the spatial variability in benthic algal communities by habitat. The shallower, wave-sheltered reef terrace, which receives more light, solar irradiance (Hamilton et al., 2014), and an influx of nutrients and sediments from the nearby lagoon (Rogers et al., 2017), had a higher relative abundance of turf and other fleshy algae throughout the study (Figure 1; Supplementary Figure 1). The fore reef, which is subject to more wave action and water motion (Williams et al., 2013; Hamilton et al., 2014; Gove et al., 2015), had a higher relative abundance of calcareous algae. Calcified crusts such as CCA and peyssonnelioid taxa are resistant to high wave energy, which may explain their dominance at this habitat, as has been seen elsewhere in the tropical Pacific (Page-Albins et al., 2012). Coralline algae can also shed their epithallial cells to prevent fouling by fleshy organisms and reinforce their foundation in wave-exposed habitats (Keats et al., 1997b). Articulated algal morphologies such as Halimeda are more vulnerable to dislodgement by waves (Steneck and Dethier, 1994), but nutrients supplied from upwelling and internal tides on the fore reef (Williams et al., 2018) may have promoted their growth (Smith et al., 2004). While temperature could be expected to differ by habitat, our observations were limited to 10 m depth and upwelling-induced cooling on Palmyra has only been found to occur below 15 m (Fox et al., 2023).
Although we did not quantify herbivore abundance in this study, given that Palmyra has very high fish biomass (Williams et al., 2011; Edwards et al., 2014) and that grazing pressure drives algal succession (Carpenter, 1986; Hixon and Brostoff, 1996), biological factors such as grazing may have further contributed to differences in algal community structure. In our photoquadrat time series, algal turfs often appeared cropped (pers. obs.), indicative of grazing. Herbivores can help control fleshy algal cover (Littler et al., 2006; Burkepile and Hay, 2009) and their presence is associated with higher cover of corals and CCA (Smith et al., 2010). With herbivores now being used as a restoration tool to reverse coral-algal phase shifts on degraded reefs (Mumby, 2014; Ladd and Shantz, 2020), Palmyra exemplifies the role of herbivory in maintaining a “healthy” calcifier-dominated reef. Palmyra’s reef system is dominated by top predators and larger-bodied grazers (e.g., parrotfish and surgeonfish) as opposed to small planktivores or echinoids (Sandin et al., 2008). Hamilton et al. (2014) found that Palmyra’s reef terrace had a higher density of herbivorous fish and higher grazing intensity (in terms of bite rates) than the fore reef. Most herbivorous fish on Palmyra feed preferentially on algal turfs (Hamilton et al., 2014), which are more abundant on the reef terrace (although parrotfish bite scars are also seen frequently on CCA on the fore reef; see Charendoff et al., 2023), suggesting that habitat-specific differences in algal and herbivore assemblages are interrelated.
Our study also provides observational evidence that the calcareous macroalgal genus, Halimeda, may be sensitive to warming. At both habitats on Palmyra, benthic cover of Halimeda was among its lowest in 2015 (Figure 5), when percentile-based DHWs reached a value of 7.76 (or a monthly mean sea surface temperature of 29.8 °C; National Oceanic and Atmospheric Administration’s Coral Reef Watch). Perhaps if temperatures on Palmyra had reached a more extreme upper limit, this would have had a more measurable impact on Halimeda cover across the atoll. It has previously been proposed that Halimeda growth and calcification could benefit from seawater temperatures ranging from 24 to 32 °C, but that temperatures above 34 °C will have consequences that may become lethal at 36 °C (Wei et al., 2020). Other experimental studies have shown that exposure to elevated temperatures can either inhibit (Sinutok et al., 2011) or enhance (Campbell et al., 2016) photosynthetic efficiency, calcification, and growth in Halimeda spp., indicating that results may be context-dependent or species-specific (Schubert et al., 2023). Given their role in both primary and calcium carbonate production on reefs (Rees et al., 2007), and as a preferred food source to many reef fishes (Mantyka and Bellwood, 2007; Hamilton et al., 2014), refining the thermal sensitivity limits of Halimeda by species (while also taking into account accumulated thermal stress) and identifying the mechanisms behind this observed phenomenon will be ecologically relevant in the face of global climate change.
In conclusion, more species-specific studies on the thermal tolerance of benthic algae are needed in order to better understand current and potential impacts of climate change on coral reefs. Additionally, comparing calcareous vs. fleshy responses of benthic algae in situ will be useful for assessing ecosystem status in the context of rising seawater temperatures. Long-term monitoring in relatively unimpacted locations, such as Palmyra Atoll, allows us to track baseline algal community dynamics over time. To strengthen the value and resolution of these ecological data sets, future efforts should consider larger-scale surveys with higher sampling frequency. Although Palmyra’s reefs have remained calcifier-dominated as of 2019, successional trajectories from Palmyra could inform mitigation strategies at more degraded reefs shifting toward fleshy algal dominance.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://github.com/akhen1/palmyra-algae.
AK: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Visualization, Writing – original draft, Writing – review & editing, Methodology. MJ: Conceptualization, Investigation, Methodology, Writing – review & editing. MF: Conceptualization, Formal Analysis, Investigation, Methodology, Writing – review & editing. JS: Conceptualization, Funding acquisition, Investigation, Resources, Supervision, Writing – review & editing, Methodology, Project administration.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. AK was supported by the National Science Foundation Graduate Research Fellowship (Award No. 1650112) and the Beyster Family Fellowship in Conservation and Biodiversity. Funding for this work was generously provided by the Scripps Family Foundation, the Bohn Family, and the Gordon and Betty Moore Foundation.
We thank the staff of The Nature Conservancy, U.S. Fish and Wildlife Service, and the Palmyra Atoll Research Consortium (PARC) for their logistical support and access to the refuge. This publication is PARC contribution #168. We thank Gareth Williams, Brian Zgliczynski, Clinton Edwards, Amanda Carter, Samantha Clements, and Stuart Sandin for their assistance with fieldwork. We thank Karina Arzuyan, Marie Diaz, Sarah Romero, Kyle Conner, Shelley Hazen, and Kailey Ramsing for their help with image digitization.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1539865/full#supplementary-material
Adey W. H., Steneck R. S. (1985). Highly productive eastern Caribbean reefs: Synergistic effects of biological, chemical, physical, and geological factors. Ecol. Coral Reefs 3, 163–187.
Aguila Ramírez R., Valdez M. C., García S. O., López R. N., Ayala M. C. (2003). Spatial and seasonal variation of macroalgal biomass in Laguna Ojo de Liebre, Baja California Sur, Mexico. Hydrobiologia 501, 207–214. doi: 10.1023/A:1026210312362
Anderson M. J. (2001). A new method for non-parametric multivariate analysis of variance. Austral Ecol. 26, 32–46. doi: 10.1111/j.1442-9993.2001.01070.pp.x
Ateweberhan M., Bruggemann J., Breeman A. (2006). Effects of extreme seasonality on community structure and functional group dynamics of coral reef algae in the southern Red Sea (Eritrea). Coral Reefs 25, 391–406. doi: 10.1007/s00338-006-0109-6
Balata D., Piazzi L., Rindi F. (2011). Testing a new classification of morphological functional groups of marine macroalgae for the detection of responses to stress. Mar. Biol. 158, 2459–2469. doi: 10.1007/s00227-011-1747-y
Barott K. L., Williams G. J., Vermeij M. J., Harris J., Smith J. E., Rohwer F. L., et al. (2012). Natural history of coral–algae competition across a gradient of human activity in the Line Islands. Mar. Ecol. Prog. Ser. 460, 1–12. doi: 10.3354/meps09874
Birrell C. L., Mccook L. J., Willis B. L. (2005). Effects of algal turfs and sediment on coral settlement. Mar. pollut. Bull. 51, 408–414. doi: 10.1016/j.marpolbul.2004.10.022
Birrell C. L., Mccook L. J., Willis B. L., Diaz-Pulido G. A. (2008). Effects of benthic algae on the replenishment of corals and the implications for the resilience of coral reefs. Oceanogr. Mar. Biol. Annu. Rev. 46, 25–63. doi: 10.1201/9781420065756-4
Braun C., Smith J., Vroom P. (2009). “Examination of algal diversity and benthic community structure at Palmyra Atoll, US Line Islands,” in Proc 11th Coral Reef Symp, Ft. Lauderdale, Florida. 865–869.
Brown K. T., Bender-Champ D., Hoegh-Guldberg O., Dove S. (2020). Seasonal shifts in the competitive ability of macroalgae influence the outcomes of coral–algal competition. R. Soc. Open Sci. 7, 201797. doi: 10.1098/rsos.201797
Burkepile D. E., Hay M. E. (2009). Nutrient versus herbivore control of macroalgal community development and coral growth on a Caribbean reef. Mar. Ecol. Prog. Ser. 389, 71–84. doi: 10.3354/meps08142
Burt J. A., Al-Khalifa K., Khalaf E., Alshuwaikh B., Abdulwahab A. (2013). The continuing decline of coral reefs in Bahrain. Mar. pollut. Bull. 72, 357–363. doi: 10.1016/j.marpolbul.2012.08.022
Campbell J. E., Fisch J., Langdon C., Paul V. J. (2016). Increased temperature mitigates the effects of ocean acidification in calcified green algae (Halimeda spp.). Coral Reefs 35, 357–368. doi: 10.1007/s00338-015-1377-9
Carpenter R. C. (1986). Partitioning herbivory and its effects on coral reef algal communities. Ecol. Monogr. 56, 345–364. doi: 10.2307/1942551
Charendoff J. A., Edwards C. B., Pedersen N. E., Petrovic V., Zgliczynski B., Sandin S. A., et al. (2023). Variability in composition of parrotfish bite scars across space and over time on a central Pacific atoll. Coral Reefs 42, 905–918. doi: 10.1007/s00338-023-02392-6
Clarke K. R. (1993). Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 18, 117–143. doi: 10.1111/j.1442-9993.1993.tb00438.x
Clifton K. E. (2013). The ecological significance of sexual reproduction by tropical green algae. Smithsonian Contributions to the Marine Sciences 39, 219–228.
Clifton K. E., Clifton L. M. (1999). The phenology of sexual reproduction by green algae (Bryopsidales) on Caribbean coral reefs. J. Phycol. 35, 24–34. doi: 10.1046/j.1529-8817.1999.3510024.x
Cornwall C. E., Carlot J., Branson O., Courtney T. A., Harvey B. P., Perry C. T., et al. (2023). Crustose coralline algae can contribute more than corals to coral reef carbonate production. Commun. Earth Environ. 4, 105. doi: 10.1038/s43247-023-00766-w
Darling E. S., McClanahan T. R., Côté I. M. (2010). Combined effects of two stressors on Kenyan coral reefs are additive or antagonistic, not synergistic. Conserv. Lett. 3, 122–130. doi: 10.1111/j.1755-263X.2009.00089.x
Davison I. R. (1991). Environmental effects on algal photosynthesis: Temperature. J. Phycol. 27, 2–8. doi: 10.1111/j.0022-3646.1991.00002.x
Dawson E. (1959). Changes in Palmyra Atoll and its vegetation through the activities of man 1913-1958. Pacif. Naturalist 1, 1–51.
Dawson E. Y., Aleem A. A., Halstead B. W. (1955). Marine algae from Palmyra Island with special reference to the feeding habits and toxicology of reef fishes. Occ. Pap. Allan Hancock Fdn. 17, 1–39.
Diaz-Pulido G., Anthony K., Kline D., Dove S., Hoegh-Guldberg O. (2012). Interactions between ocean acidification and warming on the mortality and dissolution of coralline algae. J. Phycol. 48, 32–39. doi: 10.1111/j.1529-8817.2011.01084.x
Diaz-Pulido G., McCook L. J. (2002). The fate of bleached corals: Patterns and dynamics of algal recruitment. Mar. Ecol. Prog. Ser. 232, 115–128. doi: 10.3354/meps232115
Dollar S. (1982). Wave stress and coral community structure in Hawaii. Coral Reefs 1, 71–81. doi: 10.1007/BF00301688
Done T. (1982). Patterns in the distribution of coral communities across the central Great Barrier Reef. Coral Reefs 1, 95–107. doi: 10.1007/BF00301691
Edwards C. B., Friedlander A., Green A., Hardt M., Sala E., Sweatman H., et al. (2014). Global assessment of the status of coral reef herbivorous fishes: Evidence for fishing effects. Proc. R. Soc. B: Biol. Sci. 281, 20131835. doi: 10.1098/rspb.2013.1835
Ellis J. I., Jamil T., Anlauf H., Coker D. J., Curdia J., Hewitt J., et al. (2019). Multiple stressor effects on coral reef ecosystems. Global Change Biol. 25, 4131–4146. doi: 10.1111/gcb.14819
Falkenberg L. J., Russell B. D., Connell S. D. (2013). Contrasting resource limitations of marine primary producers: Implications for competitive interactions under enriched CO2 and nutrient regimes. Oecologia 172, 575–583. doi: 10.1007/s00442-012-2507-5
Fong C. R., Fong P. (2014). Why species matter: an experimental assessment of assumptions and predictive ability of two functional-group models. Ecology 95, 2055–2061. doi: 10.1890/13-1557.1
Fong P., Paul V. J. (2011). “Coral reef algae,” in Coral Reefs: An Ecosystem in Transition (Dordrecht: Springer), 241–272.
Fox M. D., Carter A. L., Edwards C. B., Takeshita Y., Johnson M. D., Petrovic V., et al. (2019). Limited coral mortality following acute thermal stress and widespread bleaching on Palmyra Atoll, central Pacific. Coral Reefs 38, 701–712. doi: 10.1007/s00338-019-01796-7
Fox M. D., Cohen A. L., Rotjan R. D., Mangubhai S., Sandin S. A., Smith J. E., et al. (2021). Increasing coral reef resilience through successive marine heatwaves. Geophysical Res. Lett. 48, e2021GL094128. doi: 10.1029/2021GL094128
Fox M. D., Guillaume-Castel R., Edwards C. B., Glanz J., Gove J. M., Green J. M., et al. (2023). Ocean currents magnify upwelling and deliver nutritional subsidies to reef-building corals during El Niño heatwaves. Sci. Adv. 9, eadd5032. doi: 10.1126/sciadv.add5032
Glynn P. W. (1976). Some physical and biological determinants of coral community structure in the eastern Pacific. Ecol. Monogr. 46, 431–456. doi: 10.2307/1942565
Gove J. M., Williams G. J., Mcmanus M. A., Clark S. J., Ehses J. S., Wedding L. M. (2015). Coral reef benthic regimes exhibit non-linear threshold responses to natural physical drivers. Mar. Ecol. Prog. Ser. 522, 33–48. doi: 10.3354/meps11118
Graham N. A., Jennings S., Macneil M. A., Mouillot D., Wilson S. K. (2015). Predicting climate-driven regime shifts versus rebound potential in coral reefs. Nature 518, 94–97. doi: 10.1038/nature14140
Hamilton S. L., Smith J. E., Price N. N., Sandin S. A. (2014). Quantifying patterns of fish herbivory on Palmyra Atoll (USA), an uninhabited predator-dominated central Pacific coral reef. Mar. Ecol. Prog. Ser. 501, 141–155. doi: 10.3354/meps10684
Harney J., Fletcher C. III. (2003). A budget of carbonate framework and sediment production, Kailua Bay, Oahu, Hawaii. J. Sedimentary Res. 73, 856–868. doi: 10.1306/051503730856
Harrington L., Fabricius K., De’ath G., Negri A. (2004). Recognition and selection of settlement substrata determine post-settlement survival in corals. Ecology 85, 3428–3437. doi: 10.1890/04-0298
Harris J. L., Lewis L., Smith J. (2015). Quantifying scales of spatial variability in algal turf assemblages on coral reefs. Mar. Ecol. Prog. Ser. 532, 41–57. doi: 10.3354/meps11344
Hay M. (1997). Synchronous spawning—When timing is everything. Science 275, 1080–1081. doi: 10.1126/science.275.5303.1080
Hillis-Colinvaux L. (1980). Ecology and taxonomy of Halimeda: Primary producer of coral reefs. Adv. Mar. Biol. 17, 1–327. doi: 10.1016/S0065-2881(08)60303-X
Hixon M. A., Brostoff W. N. (1996). Succession and herbivory: Effects of differential fish grazing on Hawaiian coral-reef algae. Ecol. Monogr. 66, 67–90. doi: 10.2307/2963481
Hothorn T., Bretz F., Westfall P. (2008). Simultaneous inference in general parametric models. Biometrical Journal: J. Math. Methods Biosci. 50, 346–363. doi: 10.1002/bimj.200810425
Hughes T. P., Barnes M. L., Bellwood D. R., Cinner J. E., Cumming G. S., Jackson J. B., et al. (2017). Coral reefs in the anthropocene. Nature 546, 82–90. doi: 10.1111/1365-2435.13247
Hughes T. P., Graham N. A., Jackson J. B., Mumby P. J., Steneck R. S. (2010). Rising to the challenge of sustaining coral reef resilience. Trends Ecol. Evol. 25, 633–642. doi: 10.1016/j.tree.2010.07.011
Johnson M. D., Comeau S., Lantz C. A., Smith J. E. (2017). Complex and interactive effects of ocean acidification and temperature on epilithic and endolithic coral-reef turf algal assemblages. Coral Reefs 36, 1059–1070. doi: 10.1007/s00338-017-1597-2
Johnson M. D., Price N. N., Smith J. E. (2014). Contrasting effects of ocean acidification on tropical fleshy and calcareous algae. PeerJ 2, e411. doi: 10.7717/peerj.411
Keats D., Chamberlain Y., Baba M. (1997a). Pneophyllum conicum (Dawson) comb. nov.(Rhodophyta, Corallinaceae), a widespread Indo-Pacific non-geniculate coralline alga that overgrows and kills live coral. Botanica Marina 40, 263–279. doi: 10.1515/botm.1997.40.1-6.263
Keats D., Knight M., Pueschel C. (1997b). Antifouling effects of epithallial shedding in three crustose coralline algae (Rhodophyta, Corallinales) on a coral reef. J. Exp. Mar. Biol. Ecol. 213, 281–293. doi: 10.1016/S0022-0981(96)02771-2
Khen A., Fox M. D., Johnson M. D., Wall C. B., Smith J. E. (2024). Inter-and intraspecific responses of coral colonies to thermal anomalies on Palmyra Atoll, central Pacific. PloS One 19, e0312409. doi: 10.1371/journal.pone.0312409
Khen A., Johnson M. D., Fox M. D., Clements S. M., Carter A. L., Smith J. E. (2022). Decadal stability of coral reef benthic communities on Palmyra Atoll, central Pacific, through two bleaching events. Coral Reefs 41, 1–13. doi: 10.1007/s00338-022-02271-6
Kram S., Price N., Donham E., Johnson M., Kelly E., Hamilton S., et al. (2016). Variable responses of temperate calcified and fleshy macroalgae to elevated pCO2 and warming. ICES J. Mar. Sci. 73, 693–703. doi: 10.1093/icesjms/fsv168
Krieger E. C., Taise A., Nelson W. A., Grand J., Le Ru E., Davy S. K., et al. (2023). Tolerance of coralline algae to ocean warming and marine heatwaves. PloS Climate 2, e0000092. doi: 10.1371/journal.pclm.0000092
Ladd M. C., Shantz A. A. (2020). Trophic interactions in coral reef restoration: A review. Food Webs 24, e00149. doi: 10.1016/j.fooweb.2020.e00149
Lambo A., Ormond R. (2006). Continued post-bleaching decline and changed benthic community of a Kenyan coral reef. Mar. pollut. Bull. 52, 1617–1624. doi: 10.1016/j.marpolbul.2006.05.028
Lefèvre C. D., Bellwood D. R. (2010). Seasonality and dynamics in coral reef macroalgae: Variation in condition and susceptibility to herbivory. Mar. Biol. 157, 955–965. doi: 10.1007/s00227-009-1376-x
Littler M. M., Littler D. S. (1980). The evolution of thallus form and survival strategies in benthic marine macroalgae: Field and laboratory tests of a functional form model. Am. Nat. 116, 25–44. doi: 10.1086/283610
Littler M. M., Littler D. S. (2013). The nature of crustose coralline algae and their interactions on reefs. Smithson. Contrib. Mar. Sci. 39, 199–212.
Littler M. M., Littler D. S., Brooks B. L. (2006). Harmful algae on tropical coral reefs: Bottom-up eutrophication and top-down herbivory. Harmful Algae 5, 565–585. doi: 10.1016/j.hal.2005.11.003
Littler M. M., Littler D. S., Taylor P. R. (1983). Evolutionary strategies in a tropical barrier reef system: Functional-form groups of marine macroalgae. J. Phycol. 19, 229–237. doi: 10.1111/j.0022-3646.1983.00229.x
Liu G., Strong A. E., Skirving W., Arzayus L. F. (2006). “Overview of NOAA coral reef watch program’s near-real time satellite global coral bleaching monitoring activities,” in Proc 10th Int Coral Reef Symp, Okinawa. 1783–1793.
Longo G., Hay M. (2015). Does seaweed–coral competition make seaweeds more palatable? Coral Reefs 34, 87–96. doi: 10.1007/s00338-014-1230-6
Mantyka C. S., Bellwood D. R. (2007). Macroalgal grazing selectivity among herbivorous coral reef fishes. Mar. Ecol. Prog. Ser. 352, 177–185. doi: 10.3354/meps07055
Martin S., Gattuso J.-P. (2009). Response of Mediterranean coralline algae to ocean acidification and elevated temperature. Global Change Biol. 15, 2089–2100. doi: 10.1111/j.1365-2486.2009.01874.x
Mauffrey A. R., Cappelatti L., Griffin J. N. (2020). Seaweed functional diversity revisited: Confronting traditional groups with quantitative traits. J. Ecol. 108, 2390–2405. doi: 10.1111/1365-2745.13460
McClanahan T., Muthiga N., Mangi S. (2001). Coral and algal changes after the 1998 coral bleaching: Interaction with reef management and herbivores on Kenyan reefs. Coral Reefs 19, 380–391. doi: 10.1007/s003380000133
McManus J. W., Polsenberg J. F. (2004). Coral–algal phase shifts on coral reefs: Ecological and environmental aspects. Prog. Oceanogr. 60, 263–279. doi: 10.1016/j.pocean.2004.02.014
Mollica N. R., Cohen A. L., Alpert A. E., Barkley H. C., Brainard R. E., Carilli J. E., et al. (2019). Skeletal records of bleaching reveal different thermal thresholds of Pacific coral reef assemblages. Coral Reefs 38, 743–757. doi: 10.1007/s00338-019-01803-x
Mumby P. J. (2014). Stratifying herbivore fisheries by habitat to avoid ecosystem overfishing of coral reefs. Fish Fisheries 17, 266–278. doi: 10.1111/faf.12078
Oksanen J., Blanchet F. G., Friendly M., Kindt R., Legendre P., Mcglinn D., et al. (2019). vegan: Community Ecology Package. R package version 2.5-6. Available online at: https://CRAN.R-project.org/package=vegan.
Page T. M., Bergstrom E., Diaz-Pulido G. (2021). Acclimation history of elevated temperature reduces the tolerance of coralline algae to additional acute thermal stress. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.660196
Page-Albins K. N., Vroom P. S., Hoeke R., Albins M. A., Smith C. M. (2012). Patterns in Benthic Coral Reef Communities at Pearl and Hermes Atoll along a Wave-Exposure Gradient. Pacific Sci. 66, 481–496. doi: 10.2984/66.4.6
Pandolfi J. M., Bradbury R. H., Sala E., Hughes T. P., Bjorndal K. A., Cooke R. G., et al. (2003). Global trajectories of the long-term decline of coral reef ecosystems. Science 301, 955–958. doi: 10.1126/science.1085706
Perry C. T., Morgan K. M., Lange I. D., Yarlett R. T. (2020). Bleaching-driven reef community shifts drive pulses of increased reef sediment generation. R. Soc. Open Sci. 7, 192153. doi: 10.1098/rsos.192153
Phillips J., Kendrick G., Lavery P. (1997). A test of a functional group approach to detecting shifts in macroalgal communities along a disturbance gradient. Mar. Ecol. Prog. Ser. 153, 125–138. doi: 10.3354/meps153125
Pratte Z. A., Longo G. O., Burns A. S., Hay M. E., Stewart F. J. (2018). Contact with turf algae alters the coral microbiome: Contact versus systemic impacts. Coral Reefs 37, 1–13. doi: 10.1007/s00338-017-1615-4
Rasher D. B., Hay M. E. (2010). Chemically rich seaweeds poison corals when not controlled by herbivores. Proc. Natl. Acad. Sci. 107, 9683–9688. doi: 10.1073/pnas.0912095107
R Core Team (2018). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing). Available at: https://www.R-project.org/.
Rees S., Opdyke B., Wilson P., Henstock T. (2007). Significance of Halimeda bioherms to the global carbonate budget based on a geological sediment budget for the Northern Great Barrier Reef, Australia. Coral Reefs 26, 177–188. doi: 10.1007/s00338-006-0166-x
Reverter M., Helber S. B., Rohde S., De Goeij J. M., Schupp P. J. (2021). Coral reef benthic community changes in the Anthropocene: Biogeographic heterogeneity, overlooked configurations, and methodology. Global Change Biol. 28, 1956–1971. doi: 10.1111/gcb.16034
Rock J. F. (1916). “Palmyra Island: With a Description of Its Flora.” Bull. Coll. Hawaii Publ. 4, 1–53.
Rogers J. S., Monismith S. G., Fringer O. B., Koweek D. A., Dunbar R. B. (2017). A coupled wave-hydrodynamic model of an atoll with high friction: Mechanisms for flow, connectivity, and ecological implications. Ocean Model. 110, 66–82. doi: 10.1016/j.ocemod.2016.12.012
Ryznar E. R., Fong P., Fong C. R. (2021). When form does not predict function: Empirical evidence violates functional form hypotheses for marine macroalgae. J. Ecol. 109, 833–846. doi: 10.1111/1365-2745.13509
Sandin S. A., Smith J. E., Demartini E. E., Dinsdale E. A., Donner S. D., Friedlander A. M., et al. (2008). Baselines and degradation of coral reefs in the Northern Line Islands. PloS One 3, e1548. doi: 10.1371/journal.pone.0001548
Schubert N., Alvarez-Filip L., Hofmann L. C. (2023). Systematic review and meta-analysis of ocean acidification effects in Halimeda: Implications for algal carbonate production. Climate Change Ecol. 4, 100059. doi: 10.1016/j.ecochg.2022.100059
Short J., Foster T., Falter J., Kendrick G. A., Mcculloch M. T. (2015). Crustose coralline algal growth, calcification and mortality following a marine heatwave in Western Australia. Continental Shelf Res. 106, 38–44. doi: 10.1016/j.csr.2015.07.003
Sinutok S., Hill R., Doblin M. A., Wuhrer R., Ralph P. J. (2011). Warmer more acidic conditions cause decreased productivity and calcification in subtropical coral reef sediment-dwelling calcifiers. Limnol. Oceanogr. 56, 1200–1212. doi: 10.4319/lo.2011.56.4.1200
Smith J. E., Brainard R., Carter A., Grillo S., Edwards C., Harris J., et al. (2016). Re-evaluating the health of coral reef communities: Baselines and evidence for human impacts across the central Pacific. Proc. R. Soc. B: Biol. Sci. 283, 20151985. doi: 10.1098/rspb.2015.1985
Smith J. E., Hunter C. L., Smith C. M. (2010). The effects of top–down versus bottom–up control on benthic coral reef community structure. Oecologia 163, 497–507. doi: 10.1007/s00442-009-1546-z
Smith J. E., Smith C. M., Vroom P. S., Beach K. L., Miller S. (2004). Nutrient and growth dynamics of Halimeda tuna on Conch Reef, Florida Keys: Possible influence of internal tides on nutrient status and physiology. Limnol. Oceanogr. 49, 1923–1936. doi: 10.4319/lo.2004.49.6.1923
Stelling-Wood T. P., Gribben P. E., Poore A. G. (2020). Habitat variability in an underwater forest: Using a trait-based approach to predict associated communities. Funct. Ecol. 34, 888–898. doi: 10.1111/1365-2435.13523
Steneck R. S. (1986). The ecology of coralline algal crusts: convergent patterns and adaptative strategies. Annu. Rev. Ecol. Systematics 71, 273–303. doi: 10.1146/annurev.es.17.110186.001421
Steneck R. S., Dethier M. N. (1994). A functional group approach to the structure of algal-dominated communities. Oikos 69, 476–498. doi: 10.2307/3545860
Steneck R. S., Watling L. (1982). Feeding capabilities and limitation of herbivorous mollusks: A functional group approach. Mar. Biol. 68, 299–319. doi: 10.1007/BF00409596
Tebbett S. B., Bellwood D. R. (2019). Algal turf sediments on coral reefs: What’s known and what’s next. Mar. pollut. Bull. 149, 110542. doi: 10.1016/j.marpolbul.2019.110542
Teichert S., Steinbauer M., Kiessling W. (2020). A possible link between coral reef success, crustose coralline algae and the evolution of herbivory. Sci. Rep. 10, 17748. doi: 10.1038/s41598-020-73900-9
Titlyanov E., Yakovleva I., Titlyanova T. (2007). Interaction between benthic algae (Lyngbya bouillonii, Dictyota dichotoma) and scleractinian coral Porites lutea in direct contact. J. Exp. Mar. Biol. Ecol. 342, 282–291. doi: 10.1007/bf00409596
Vroom P. S., Smith C. M., Coyer J. A., Walters L. J., Hunter C. L., Beach K. S., et al. (2003). Field biology of Halimeda tuna (Bryopsidales, Chlorophyta) across a depth gradient: Comparative growth, survivorship, recruitment, and reproduction. Hydrobiologia 501, 149–166. doi: 10.1023/A:1026287816324
Wei Z., Mo J., Huang R., Hu Q., Long C., Ding D., et al. (2020). Physiological performance of three calcifying green macroalgae Halimeda species in response to altered seawater temperatures. Acta Oceanologica Sin. 39, 89–100. doi: 10.1007/s13131-019-1471-3
Wernberg T., Smale D. A., Thomsen M. S. (2012). A decade of climate change experiments on marine organisms: Procedures, patterns and problems. Global Change Biol. 18, 1491–1498. doi: 10.1111/j.1365-2486.2012.02656.x
Williams G. J., Knapp I. S., Maragos J. E., Davy S. K. (2010). Modeling patterns of coral bleaching at a remote Central Pacific atoll. Mar. pollut. Bull. 60, 1467–1476. doi: 10.1016/j.marpolbul.2010.05.009
Williams I. D., Richards B. L., Sandin S. A., Baum J. K., Schroeder R. E., Nadon M. O., et al. (2011). Differences in reef fish assemblages between populated and remote reefs spanning multiple archipelagos across the central and western Pacific. J. Mar. Biol. 2011, 1–14. doi: 10.1155/2011/826234
Williams G. J., Sandin S. A., Zgliczynski B. J., Fox M. D., Gove J. M., Rogers J. S., et al. (2018). Biophysical drivers of coral trophic depth zonation. Mar. Biol. 165, 1–15. doi: 10.1007/s00227-018-3314-2
Williams G. J., Smith J. E., Conklin E. J., Gove J. M., Sala E., Sandin S. A. (2013). Benthic communities at two remote Pacific coral reefs: Effects of reef habitat, depth, and wave energy gradients on spatial patterns. PeerJ 1, e81. doi: 10.7717/peerj.81
Wismer S., Hoey A. S., Bellwood D. R. (2009). Cross-shelf benthic community structure on the Great Barrier Reef: Relationships between macroalgal cover and herbivore biomass. Mar. Ecol. Prog. Ser. 376, 45–54. doi: 10.3354/meps07790
Keywords: long-term monitoring, seaweed, macroalgae, Halimeda, community composition, thermal stress, coral reefs, climate change
Citation: Khen A, Johnson MD, Fox MD and Smith JE (2025) Benthic algal community dynamics on Palmyra Atoll throughout a decade with two thermal anomalies. Front. Mar. Sci. 12:1539865. doi: 10.3389/fmars.2025.1539865
Received: 04 December 2024; Accepted: 13 January 2025;
Published: 28 January 2025.
Edited by:
Guang Gao, Xiamen University, ChinaCopyright © 2025 Khen, Johnson, Fox and Smith. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adi Khen, YWtoZW5AdWNzZC5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.