
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Mar. Sci., 28 January 2025
Sec. Marine Biotechnology and Bioproducts
Volume 11 - 2024 | https://doi.org/10.3389/fmars.2024.1523246
With an estimated global prevalence of 32.4%, non-alcoholic fatty liver disease (NAFLD) is currently the most prevalent chronic liver condition. The marine ecosystem, distinguished by its distinctive environmental characteristics, is a treasure trove of novel lead compounds possessing unique chemical structures, offering promising avenues for the development of new therapeutic agents or dietary supplement targeting NAFLD. Marine bioactive substances from natural products, such as polysaccharides, polyphenols, polyunsaturated fatty acids, and peptides, have been shown to benefit liver health by alleviating metabolic dysfunction through multiple mechanisms. This paper reviews the effects of marine bioactive substances from various marine entities, including marine fauna, flora, and microorganisms, on the regulation of NAFLD. A brief overview of the predominant pathogenic mechanisms underlying the disease is also provided, thereby establishing a critical link between the therapeutic potential of marine bioactive substances and the management of NAFLD.
The liver is the body’s largest metabolic organ, and it plays essential roles in regulating glucose and lipid metabolism (Wang et al., 2021a). It has been reported that liver disease causes more than 2 million deaths per year (including but not limited to cirrhosis, viral hepatitis, liver cancer, and their medical complications), which accounts for 4% of all deaths worldwide (Devarbhavi et al., 2023). Liver diseases encompass a broad spectrum of conditions, including genetic, autoimmune, infectious, neoplastic, vascular, and metabolic diseases. Chronic liver disease, in particular, is a significant public health challenge, with over 1 billion cases reported globally (Devarbhavi et al., 2023; Effandie and Gupte, 2023). Chronic injury to the liver and the progressive accumulation of fibrous extracellular matrix proteins cause fibrosis of the liver, which may subsequently progress to hepatocellular carcinoma (Gutierrez-Reyes et al., 2007). If left untreated, this leads to irreversible consequences. Historically, viral hepatitis was the leading cause of chronic liver disease. However, improvements in prevention strategies, such as hepatitis B vaccination and advancements in treatment options for hepatitis C, have marked a positive shift in managing chronic liver conditions (Cheemerla and Balakrishnan, 2021). Currently, non-alcoholic fatty liver disease (NAFLD) has become the leading chronic liver disease worldwide (Wang et al., 2014; Loomba et al., 2021; Zhai et al., 2021), according to its alarming prevalence obtained from epidemiological studies (Le et al., 2022) and its interweaving with other liver diseases or complications. In the past 2 years, there have been some debates among scientists regarding the ambiguity of NAFLD’s naming, and relevant scholars have jointly issued a consensus on a new naming: metabolic dysfunction-associated fatty liver disease (MAFLD)/metabolic dysfunction-associated steatotic liver disease (MASLD) (Eslam et al., 2020; Rinella et al., 2023). In this paper, we still refer to it as NAFLD despite the change of name, as MAFLD/MASLD has not yet obtained an International Classification of Diseases code, not to mention its impact on the clinical trials currently underway (Hsu and Loomba, 2024).
NAFLD, characterized by the abnormal accumulation of fat within liver cells, is a condition that is primarily linked to metabolic factors and is distinct from alcohol-related liver diseases or injury caused by agents. It is currently the most serious non-communicable liver disease, with a global prevalence of 25.2% in 2015 (Younossi et al., 2016), which further escalated to a combined rate of 32.4% by 2021 (Le et al., 2022).
Currently, potential drugs for the treatment of NAFLD have different targets, including those that inhibit de novo lipogenesis (DNL); relieve oxidative stress, inflammation, and regulatory-related pathways; activate peroxisome proliferator-activated receptors (PPARs); and modulate the farnesoid X receptor (FXR) axis. Additionally, anti-obesity, hypoglycemic, and hypolipidemic drugs such as orlistat, biguanides, and statins are also part of the treatment mix. However, the side effects of these drugs are also significant, including nausea, vomiting, diminished appetite, and gastrointestinal complications (Rotman and Sanyal, 2017). As of May 2024, only one medicine, Rezdiffra—a thyroid hormone receptor-beta (THR-β) agonist—has been approved by the US Food and Drug Administration (FDA) to be marketed for the treatment of non-alcoholic steatohepatitis (NASH), intended to be used in conjunction with lifestyle modifications, such as diet and exercise (The Lancet Gastroenterology Hepatology, 2024). NAFLD’s close relationship with obesity and type 2 diabetes is well-documented, reinforcing the criticality of weight loss as a primary and effective intervention strategy (Andersen et al., 1991). However, the reality of maintaining a healthy lifestyle through exercise and dietary restrictions, while ideal, is often easier said than done. This reality underscores the necessity for supportive pharmacological interventions and dietary supplements to aid in weight management.
These challenges highlight the pressing need for the development of novel therapeutic programs that can more effectively and safely address the multifaceted challenges posed by NAFLD.
The ocean covers more than 70% of the earth’s surface and consists of a huge diversity of species. After terrestrial flora and non-marine microorganisms, marine organisms are an emerging treasure trove of bioactive natural products, with an average of more than 1,000 new compounds of marine origin being discovered each year in recent years (Carroll et al., 2022, 2023, 2024), making marine natural products a likely source of bioactive substances with hepatoprotective effects. The term “natural product” is generally used to refer to the chemical entities derived from nature (Newman et al., 2000; Rasul et al., 2013). Commonly, it is considered the other name for secondary metabolites (Abegaz and Kinfe, 2019). Botanical (plant-based) natural product is defined as the substance produced by a variety of natural sources, which can be either a complex mixture extracted from raw material or a single compound (Kellogg et al., 2019). Therefore, in this paper, we refer to the botanical (plant-based) natural product definition to describe marine natural products (MNPs) from a certain perspective. MNPs mentioned in this paper may contain certain mixtures, not only compounds. Marine-derived peptides (by hydrolysate, water extraction, or other methods) are also widely used in NAFLD research; we have also mentioned them in this article.
Marine biodiversity encompasses a wide taxonomic range, including organisms such as fish, mollusks, crustaceans, and algae (Costa et al., 2019; Masoudian Khouzani et al., 2019; Wu et al., 2023b), and fungi (Song et al., 2013) and bacteria that reside in sediments or other biotic environments (Sowunmi et al., 2018; Santos et al., 2019). This rich diversity serves as a fertile ground for the exploration and discovery of bioactive substances with significant pharmacological potential. Due to their special living environments, such as high pressure and high salt, they produce compounds with unique structural features and exhibit various types of biological activities: anti-aging (Vladkova et al., 2022), anti-tumor, anti-cancer, and amelioration of neurodegenerative diseases (Zhao et al., 2018), and more.
Several marine-derived agents have been approved as medications in earlier years including saccharides and glycosides (Kramer et al., 2021), peptides (Williams et al., 2008), phenylpropanoids, quinones, flavonoids, terpenoids, steroids, alkaloids (Larsen et al., 2016), phenolics (D’Incalci and Zambelli, 2016), acids, and aldehydes. These drugs, which are derived from a variety of marine organisms, each play a distinct role in the treatment of different conditions, as detailed in Table 1. Given the varied and specialized functions of these marine-derived medications, there is a growing anticipation and urgent demand for the identification of new therapeutic agents that can effectively target chronic liver diseases.

Table 1. Some well-known marine-derived medications that have been approved for marketing or are in different clinical phases.
Because of its special taste, marine food has always been loved by most people, such as sea fish and marine algae. Combined with its active ingredients to alleviate chronic liver disease, it will be a new direction for the alleviation or prevention of chronic liver disease with functional food prepared from marine products in a special way. This is also to alleviate the adverse consequences caused by dietary restriction or drug intervention.
In this review, we will discuss the relationship between marine bioactive substances and their association with NAFLD or its more severe form, NASH, exploring their potential as novel treatment or compensation options for these increasingly prevalent liver conditions.
NAFLD encompasses a broad range of conditions, from the relatively benign simple steatosis, also known as non-alcoholic fatty liver (NAFL), to more severe stages such as NASH, liver fibrosis, cirrhosis, and ultimately hepatocellular carcinoma (HCC). These conditions represent a progressive disease spectrum that evolves through a continuous process. The disease progression of NAFLD is briefly illustrated in Figure 1.
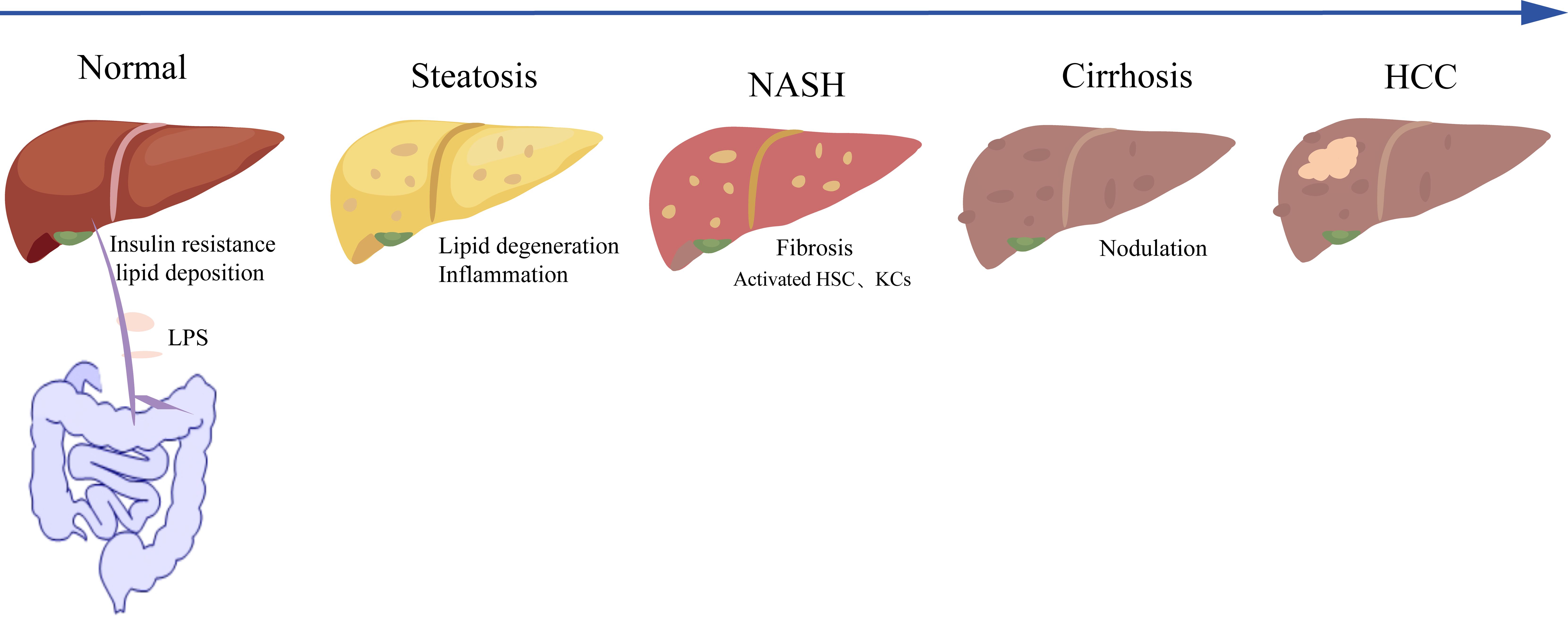
Figure 1. Disease progression of NAFLD. NASH, non-alcoholic steatohepatitis; HCC, hepatocellular carcinoma; HSC, hepatic stellate cell; KC, Kupffer cell; LPS, lipopolysaccharide.
Several studies have shed light on the factors contributing to the progression of NAFLD. For instance, some studies have identified an increase in mortality factor 4-like protein 1 (MORF4L1, also called MRG15) level (Wei et al., 2020; Tian et al., 2022), a lower level of Myc interacting zinc finger protein 1 (Miz-1) (Jin et al., 2023), and the upregulation of X-box binding protein-1 (XBP-1) (Wang et al., 2022), and a decrease in CASP8 and FADD-like apoptosis regulator (CFLAR) level (Wang et al., 2017b) promotes the progression of NAFLD.
Among them, MRG15 is considered to be an epigenetic regulatory factor localized in the nucleus. During NASH development, inflammatory factors enhance the protein stability of MRG15, and MRG15 promotes the degradation of Tu translation elongation factor, mitochondrial (TUFM) by mitochondrial ClpXP proteasome. The decrease in TUFM in the liver leads to inhibition of mitochondrial autophagy, increased oxidative stress, and activation of NOD-like receptor family pyrin domain containing 3 (NLRP3), which aggravates inflammation and fibrosis (Wei et al., 2020; Tian et al., 2022). Miz1 is a transcription factor that can limit inflammation of the liver cells drive (Zhang et al., 2021b) and promote mitochondrial autophagy (Jin et al., 2023), thus inhibiting the development of NASH, the precursor of HCC. The transcription factor XBP1 is abnormally elevated in NASH patients. Specifically, the knockdown of XBP1 inhibits the expression of NLRP3 and secretion of pro-inflammatory factors, which alleviates steatohepatitis by mediating the polarization of M2 macrophages. CFLAR is an apoptotic regulatory protein involved in innate immune regulation, which has a key inhibitory effect on inflammation, fibrosis, insulin resistance (IR), and lipid accumulation in the liver of NASH. CFLAR also blocks the activation of apoptosis signal-regulating kinase 1 (ASK1) by inhibiting the formation of N-terminal dimerization of ASK1 to improve and reverse the course of NASH (Wang et al., 2017c).
Not only are the proteins listed above important targets involved in the progression of NAFLD, but so are many other proteins, such as nuclear pore protein 85 (NUP85) (Wu et al., 2024b) and ring finger protein 13 (RNF13) (Lin et al., 2023). The spectrum of NAFLD is complex and multi-factorial, and more targets and mechanisms need to be further investigated.
The overall pathogenesis and course of NAFLD can be described as a “multiple-hit” process (Buzzetti et al., 2016), which encompasses not only IR and oxidative stress but also hormones secreted by adipose tissues; nutrient factors, the influence from the gut microbiota (GM), and the interplay of genetic and epigenetic factors also play key roles in the pathogenesis of NAFLD (Buzzetti et al., 2016; Juanola et al., 2021).
There is a well-established link between NAFLD and IR (Bugianesi et al., 2010; Hurjui et al., 2012). IR is a key contributor to postprandial hyperglycemia and elevated insulin levels, which in turn stimulates the breakdown of lipids in the adipose tissue, releasing free fatty acids (FFAs). These fatty acids are taken up by hepatocytes through various fatty acid transport proteins, such as the fatty acid translocase/cluster of differentiation 36 (FAT/CD36) family, fatty acid transport protein (FATP) family, and liver fatty acid-binding protein (L-FABP), and are converted into triglycerides (TG), leading to lipid accumulation and degeneration (Loomba et al., 2021). The mechanism of hepatic insulin resistance (HIR) has also been tentatively explained (Lyu et al., 2020). TANK binding kinase 1 (TBK1) is another crucial player in the regulation of hepatic lipid metabolism. TBK1 is necessary for the catabolism of fatty acids and can be induced in the liver, thereby modulating fatty acid oxidation. The activity of acyl-coenzyme A synthetases (ACS), including long-chain acyl-coenzyme A synthetase 1 (ACSL1), is often diminished in insulin-resistant states (Poppelreuther et al., 2023). TBK1 serves as a scaffolding protein that interacts with ACSL1, influencing the mitochondrial localization of ACSL1 and, consequently, the regulation of fatty acid oxidation in hepatocytes (Huh et al., 2020). Therefore, HIR is intricately connected to the pathogenesis of NAFLD through its impact on lipid metabolism, glucose homeostasis, and the interplay of various proteins and enzymes involved in these processes.
The excessive influx of FFAs into the liver leads to hepatocyte mitochondria impairment and subsequent dysfunction. The compromised mitochondria trigger an upsurge in the generation of reactive oxygen species and reactive nitrogen species. When these reactive species exceed the cell’s antioxidant defenses, they inflict damage upon vital cellular constituents, including lipids, proteins, and critically, nucleic acids, particularly mitochondrial DNA. This damage culminates in a state of oxidative stress and ultimately propels the cell toward apoptosis (Nassir and Ibdah, 2014). Oxidative stress is a pivotal contributor to the pathogenesis of hepatic lipid peroxidation, a hallmark of deteriorating liver health (Huang et al., 2018a). Moreover, the interplay between oxidative stress and endoplasmic reticulum stress (ERS) has been implicated in the complex mechanisms underlying NAFLD. ERS, triggered by the accumulation of misfolded proteins in the endoplasmic reticulum, further exacerbates the oxidative environment and contributes to the inflammatory response and cellular damage observed in NAFLD (Wang et al., 2020a). These interconnected biochemical reactions create a vicious cycle that synergistically drives the progression of NAFLD.
Interactions between the environment and a susceptible polygenic host background determine the phenotype and influences the progression of NAFLD. Notably, the patatin-like phospholipase domain-containing protein 3 (PNPLA3) gene variant has been identified as the major common genetic determinant of NAFLD (Romeo et al., 2008). A possible beneficial effect of downregulation of the PNPLA3 I148M mutated form in individuals at risk of NAFLD (Valenti and Dongiovanni, 2017). Variants with moderate effect size in transmembrane 6 superfamily member 2 (TM6SF2), membrane-bound O-acyltransferase domain-containing 7 (MBOAT7), and glucokinase regulator (GCKR) gene have also been shown to have a significant contribution (Eslam et al., 2018). The same gene may act differently in different environments; for example, GCKR-rs1260326-T allele elevates disease severity only under diabetic states but protects from fibrosis under non-diabetic states (Kimura et al., 2022). Additional large candidate gene studies are required to enrich our understanding of the genetic basis and determine the more genome-wide associations of NAFLD.
NAFLD development and progression are also modulated by epigenetic factors, including alterations in DNA methylation, modifications to histone proteins, and the remodeling of chromatin, and RNA-based mechanisms, such as non-coding RNAs. Micro-RNAs (miRNA), in particular, control many complementary target mRNAs at the post-transcriptional level and whose dysregulation has been shown to have high prognostic and predictive value in NAFLD (Dongiovanni et al., 2018). Among those that have been demonstrated are miR-21 (Calo et al., 2016), miR-34a (Xu et al., 2015b), and especially miR-122 (Cheung et al., 2008).
In addition to the exploration of NAFLD mechanisms from the genetic and epigenetic level, the relationship between metabolic disorders, the gut microbiome, and immunity are also something we need to continue to focus on (Tilg et al., 2021). Unhealthy eating and lifestyle habit choices will cause various metabolic disorders, establishing changes in the gut microbiome, which then activates the human immune response, including innate immunity and adaptive immunity to promote liver inflammation, resulting in further liver damage.
The study of gut microbes is also a popular topic of interest in recent years. Marshall proposed the concept of the “gut–liver axis” in 1998 (Marshall, 1998), which describes the mutual regulation and influence of substances and cytokines between the liver and the intestine through the portal system (Pabst et al., 2023). Intestinal microorganisms affect the homeostasis of hepatic triglyceride metabolism by increasing endotoxin levels, affecting nutrient absorption, and changing the types and contents of metabolites such as amino acids, fatty acids, and bile acids in the body (Loomba et al., 2021). In recent years, there has been a burgeoning interest in the intestinal dimension of liver disease management (Ni et al., 2023; Yang et al., 2023). Research into pharmaceuticals and functional food components that bolster the stability of the gut microecosystem and contribute to the amelioration of hepatic steatosis is increasingly prevalent (Cheng et al., 2022). These studies underscore the potential of modulating the GM as a novel therapeutic strategy for NAFLD. Subsequently, it is imperative that research explores the complex interconnection between key gut microbial species and their metabolites and the pathogenesis of NAFLD they involved (Llorente and Schnabl, 2015; Saini and Keum, 2018).
In this narrative review study, an electronic search was performed using the Web of Science and PubMed databases to identify relevant studies published between January 2000 and May 2024. Keywords in this review include the following: “non-alcoholic fatty liver disease,” “marine unsaturated fatty acid,” “marine polysaccharide,” “marine polyphenol,” “marine polypeptide,” “terpenoid,” and “marine vitamin and mineral.” During the search, we combined marine bioactive substances with NAFLD. Searches were limited to English language studies.
Unsaturated fatty acids (UFAs) are essential components of body fat and play crucial roles in various physiological processes. For example, it maintains the fluidity of cell membranes; regulates blood lipids, blood pressure, and hormone synthesis; and promotes neurodevelopment (Tvrzicka et al., 2011). These fatty acids are classified based on the number of double bonds present in their molecular structure into monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs) and further categorized into n-6 and n-3 series according to the position of the initial double bond from the methyl end of the carbon chain. The n-3 series of fatty acids, represented by docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), have received great attention, as they are associated with anti-tumor activities and regulation of lipid metabolism (Della Corte et al., 2016), glucose metabolism (Zhang et al., 2019), and promotion of neurodevelopment (Guxens et al., 2011).
DHA and EPA are predominantly found in deep-sea marine fish and in phytoplankton and zooplankton, with comparatively lower concentrations in terrestrial organisms. These PUFAs predominantly exist in the form of TG, with phospholipids (PLs; such as lecithin), diglycerides, cholesteryl esters, and fat-soluble vitamin esters (for example, retinyl palmitate and tocopherol acetate) also present. Among these forms, PLs are recognized for their superior bioavailability (Zhang et al., 2019). DHA-PL and EPA-PL can effectively mitigate obesity-related metabolic disorders (Liu et al., 2014), which have been shown to enhance insulin sensitivity within the adipose tissue and suppress hepatic sterol regulatory element-binding protein-1c (SREBP-1c)-mediated adipogenesis in NAFLD mice. Furthermore, PLs have been found to significantly activate PPAR-mediated fatty acid oxidation in the liver. This is due to DHA being a natural endogenous ligand for PPARs, which can potentially reduce the accumulation of lipids in hepatocytes, thereby aiding in the management and improvement of NAFLD symptoms.
The important effects of PUFAs on the liver have also been demonstrated in clinical studies (Vell et al., 2023). DHA plus EPA treatment for 15–18 months in patients with NAFLD would reduce hepatic fat, and erythrocyte DHA enrichment with DHA plus EPA treatment was linearly associated with decreased liver fat (Scorletti et al., 2014; Hodson et al., 2017). Valerio Nobili extended this research to pediatric populations, utilizing DHA supplementation in 60 children diagnosed with NAFLD through liver biopsy (Nobili et al., 2011). The findings indicated that DHA supplementation led to improvements in hepatic steatosis and insulin sensitivity among these children, further highlighting the therapeutic potential of DHA in NAFLD.
In addition, DHA plays an important role in preventing fructose-induced hepatic steatosis by activating deacetylase Sirtuin 1 (SIRT1) (Luo et al., 2020) and alleviating endoplasmic reticulum (ER) stress (Zheng et al., 2016) response. IR is recognized as a key contributor to the development of NAFLD (Krebs et al., 2002; Lyu et al., 2020; Shama and Liu, 2020). A suggestion was proposed to appropriately increase the DHA ratio (1.5:1) in DHA : EPA supplements to prevent IR in NAFLD (Yu et al., 2023). This suggestion is supported by evidence indicating that an increased intake of DHA, as compared to EPA, may be more efficacious in alleviating the symptoms of NAFLD (Kelley, 2016).
Although PUFAs from marine fish and shrimp have been widely developed and utilized, due to pollution of marine ecosystems, toxic dioxins, polychlorinated biphenyls (PCBs), heavy metals, and organochlorine pesticides will accumulate in fish in large quantities (Vandermeersch et al., 2015). It may be solved by artificial cultivation of algal source DHA or using an optimization model with constraints to calculate optimum seafood cluster consumption levels (Sirot et al., 2012). This should also be considered when preparing prebiotics or mixed crude extracts from marine organisms.
Polysaccharides represent a diverse class of carbohydrates characterized by glycosidic linkages, with common types including α-1,4, β-1,4, and α-1,6 glycosidic bonds (Gupta et al., 2016). There is a wide distribution of polysaccharides in land and marine life. Compared to polysaccharides from other sources, most polysaccharides originating from marine foods contain modifying groups such as sulfate, amide, and amino groups. These unique structural features endow marine polysaccharides with a range of physiological functions that are not commonly found in other polysaccharides, including anticoagulant, lipid-lowering, immune-enhancing, anti-aging, anti-tumor (Liu et al., 2022a), anti-cancer (Jin et al., 2022a), anti-inflammatory, anti-osteoporosis, and immunomodulatory effects (Yang et al., 2021b).
According to their sources, marine polysaccharides can be categorized as algal (classified as brown, red, and green algae according to pigment deposition, classified as microalgae and macroalgae based on morphology and size), marine animal, and marine microbial polysaccharides. Algal polysaccharides have been extensively researched.
Both in vitro and in vivo studies have illuminated the beneficial properties of algal polysaccharides, which include their impact on satiety, caloric intake, fat absorption, and the regulation of GM. A study has demonstrated that low molecular weight fucoidan (LMWF) can mitigate the accumulation of hepatic TG and total cholesterol (TC), alleviate hepatic oxidative stress and lipid peroxidation, and exert significant anti-inflammatory effects (Zheng et al., 2018). For example, soluble polysaccharides derived from Laminaria japonica (Zhang et al., 2021c) alleviate NAFLD by regulating GM.
The Ulva polysaccharides, sourced from the green seaweed Ulva stramonium, have garnered attention for their potential anticancer properties (Zhao et al., 2020; Jin et al., 2022a), with particular implications for liver cancer (Xu et al., 2024). Ulva polysaccharides containing highly sulfated derivatives reduced TC, TG, LDL-C, and AST levels in mice with high-fat diet induced NAFLD. Additionally, there was a decrease in lipid droplets within the liver and a suppression of the abnormal enlargement of epididymal fat cells (Wan et al., 2022). Porphyran-derived oligosaccharides from Porphyra yezoensis (PYOs) activate the IRS-1/AKT/GSK-3β signaling pathway and the AMP-activated protein kinase (AMPK) pathway, resulting in the decrease in lipid accumulation within the livers of mice with NAFLD (Wang et al., 2021b). Additionally, PYO treatment downregulates TGF-β and its associated proteins and alleviates dysbiosis of the cecal microbiota, oxidative stress, inflammation, and lipid metabolism, providing a degree of liver protection. Sulfated polysaccharides from Enteromorpha prolifera (EP) suppress SREBP-2 and HMG-CoA reductase expression (Ren et al., 2017) and increase hydrogen sulfide(H2S) production (Ren et al., 2018) to alleviate NAFLD.
Such reassuring finds are not limited to seaweed-sourced polysaccharides. Polysaccharides of marine animal origins such as Tegillarca granosa polysaccharide (TGP) (Yang et al., 2024) and Chitosan oligosaccharide (COS/COSM) (Qian et al., 2019; Feng et al., 2022) also alleviate NAFLD by regulating GM. However, only a few studies have examined marine microbial polysaccharides’ ability to reduce lipids in the liver, which makes them a significant area for future studies.
Polyphenols are described as secondary metabolites that are found abundantly in plants and small quantities in microorganisms. Polyphenols contain multiple polyphenol hydroxyl structures and therefore have powerful antioxidant activities (Wang et al., 2023). Phenolics can be subdivided into two main groups: flavonoids (e.g., anthocyanins, flavanols, flavanones, flavanols, and isoflavones) and non-flavonoid (e.g., phenolic acids, xanthones, stilbenes, lignans, and tannins) polyphenols (Rathod et al., 2023). Relationships between polyphenol intake and human health have been progressively identified, particularly in conditions such as cardiovascular disease, hypertension, diabetes, metabolic syndrome, obesity, and cancer (Murray et al., 2018; Zheng et al., 2022).
The antioxidant, anti-inflammatory, antifibrotic, and antilipemic properties of polyphenols confer them great potential as a strategy for preventing the progression of NAFLD (Pisonero-Vaquero et al., 2015), and their ability to modulate the expression of genes primarily involved in DNL and fatty acid oxidation contributes to their lipid-lowering effects in the liver (Rodriguez-Ramiro et al., 2016). A compilation of these findings, including the specific effects of various polyphenols on hepatic health, has been summarized in Table 2.
Peptides are common active substances involved in various biological reactions in the body. In recent years, the study of active peptides in marine foods has attracted widespread attention due to their antioxidant, anti-aging, anti-hypertensive, anti-diabetic, anti-obesity, and many other health-benefiting effects. Many active peptides with hypolipidemic physiological functions have been obtained from marine foods by extraction or enzymatic methods. Marine-derived bioactive peptides are a class of naturally occurring polyphenolic peptide compounds. Mainly isolated from deep-sea bacteria, fungi, microalgae, and marine mollusks, these active peptides have complex and diverse structures involved in important life activities of living organisms, and the available evidence supports the use of bioactive peptides derived from marine organisms to alleviate metabolic syndrome (Wang et al., 2023).
Arthrospira platensis phycobiliprotein peptide extract (PPE) supplementation intervention helps in ameliorating NAFLD through the modulation of hepatic lipid profile and the reinforcement of the fat mobilization of intestinal metabolites (Liu et al., 2023b). Animal-derived polypeptide Alaska pollock (Theragra chalcogramma) protein (APP) partially attenuated liver steatosis in part through the enhancement and suppression of gene expression involved in liver fatty acid oxidation and synthesis while increasing the abundance of beneficial GM and acetic acid content in ob/ob mice (Maeda et al., 2020). At the same time, APP reduces the excessive accumulation of lipids in rat liver by affecting the FXR/small heterodimer chaperon-dependent pathway, activating liver receptor homologous-1 (LRH-1), and increasing binding to CYP7A1 promoter (Hosomi et al., 2009). The mitigation effects of related peptides extracted from other marine organisms on NAFLD have been listed in Table 2.
Some of the peptides currently used to treat obesity or type 2 diabetes such as the GLP-1 receptor agonist semaglutide (Niu et al., 2022) and liraglutide (Moreira et al., 2018) have also been recently discovered to have NAFLD-alleviating properties.
The powerful efficacy of marine peptides has been demonstrated by Ziconotide for pain relief (Williams et al., 2008), anti-tumor (Brodowicz, 2014), and more. Therefore, we are eagerly looking forward to the discovery of other compounds with better effects on the field of chronic liver diseases.
Terpenoids are compounds derived from mevalonate having the isoprene unit (C5 unit) as the basic structural unit of the molecular skeleton. Terpenoids are widely found in nature, especially in marine organisms such as algae, sponges, cnidarians, and mollusks. Over 400 types of marine sesquiterpenoids have been identified to date.
Marine terpenoids often contain unique functional groups, such as halogens, isocyanides, and furan rings, which endow them with special activities, including anticancer and antibacterial properties. It is encouraging to note that a diterpenoid compound derived from gorgonian corals has been identified, which exhibits an inhibitory effect on the hepatitis B virus (Li et al., 2020). Therefore, there is hope that more marine-derived terpenoids will be found to have effects on hepatic diseases.
In this context, we introduce several carotenoid tetraterpenes that are currently of research interest, primarily focusing on compounds sourced from marine algae and crustaceans, such as fucoxanthin and astaxanthin.
The main mechanisms of carotenoids, astaxanthin, and fucoxanthin in the prevention and treatment of NASH are antioxidative stress, inhibition of inflammation and fibrosis, promotion of M2 macrophage polarization, and improvement of mitochondrial oxidative respiration and IR (Ni et al., 2016). One study has shown that 200 μM of astaxanthin can significantly downregulate key lipogenic enzymes such as fatty acid synthetase (FAS), SREGP-1C, and acetyl-CoA carboxylase (ACC) in oleic and palmitic-acid-induced HepG2 cells and upregulate Nrf2 (nuclear factor erythro2-related factor 2) and AMPK. Meanwhile, 60 mg/kg of astaxanthin alleviates HFD-induced hepatic lipid metabolism disorder and oxidative stress imbalance in mice. Overall, astaxanthin alleviates NAFLD by regulating GM and targets the AMPK/Nrf2 signaling axis (Li et al., 2022). It is demonstrated that astaxanthin attenuates hepatic injury and mitochondrial dysfunction in NAFLD mice by upregulating the FGF21/PGC-1α pathway. Astaxanthin may be a promising drug candidate for the treatment or alleviation of NAFLD, and its related therapeutic targets and molecular mechanisms would need to be further explored (Wu et al., 2020). Fucoxanthin, an orange-red carotenoid, can be isolated from brown algae (Ye et al., 2022b), and its association with NAFLD has also been reviewed (Sayuti et al., 2023). Previous studies have shown that fucoxanthin-containing microalgae extracts can alleviate NAFLD by alleviating liver lipid and oxidative stress in mouse models and HepG2 cells (Guo et al., 2021). Related signaling pathways have also been studied, such as AMPK/Nrf2/TLR4 (Ye et al., 2022b). Different delivery modes affect the efficiency of drug utilization, and it has been shown that fucoidan loaded through extracellular vesicles had a stronger effect on NAFLD alleviation than fucoidan delivery alone (Wu et al., 2024a).
Adequate daily mineral intake is essential for the prevention of chronic nutrition-related and degenerative diseases, including cancer, cardiovascular disease, and obesity. Perturbations in micronutrient homeostasis have also been found to be associated with NAFLD (Clemente et al., 2016). Alexandra Feldman described the critical role that iron and copper homeostasis play in the progression of NAFLD (Feldman et al., 2015). Blood selenium levels have also been linked to liver fibrosis (Liu et al., 2022c). A large number of trace elements essential to human health are present in the oceans. This is well represented in seaweeds, rich in both macronutrients and micronutrients, with a mineral content of at least 10 times that of terrestrial plants, amounting to 20%–50% of their dry weight (Lozano Muñoz and Díaz, 2022). Dietary supplements of magnesium-rich marine mineral mixtures can enhance the diversity of microbiota in the gastrointestinal tract to promote digestive-health (Crowley et al., 2018). Additionally, a study has analyzed the micronutrient composition of 35 species of edible fish from India and their significance in human nutrition, emphasizing that fish are rich in all fat-soluble vitamins. Results obtained in this study provide a basis for considering marine-derived organisms as potential sources of essential minerals for functional foods, dietary supplements, and nutraceuticals in the future (Mohanty et al., 2016). It is suggested that future research should elucidate the potential contribution of trace elements, vitamins, and undesirable substances present in seafood (Liaset et al., 2019).
Vitamin D is a group of structurally similar steroid-derived compounds; well-known examples of vitamin D are vitamin D2 and D3 (Bartolini et al., 2023). The human body synthesizes over 80% of its vitamin D3 through sunlight exposure, with the remainder obtained from dietary sources (Hernigou et al., 2019). Vitamin D3 is found in abundance in the livers of marine animals and certain algal substances. In recent years, the close relationship between vitamin D3 and NAFLD has been increasingly recognized (Pacifico et al., 2019); Liu et al. have reviewed the research progress and mechanisms of vitamin D3 action in NAFLD (Liu et al., 2022b). Studies have also explored the use of vitamin D3 alone (Geier et al., 2018) or in combination with other marine bioactive substances to alleviate NAFLD (Guo et al., 2022; Reda et al., 2023). A study has demonstrated that vitamin D3 alleviates non-alcoholic fatty liver disease in rats by inhibiting liver oxidative stress and inflammation through the SREBP-1c/PPARα-NF-κB/IR-S2 signaling pathway (Reda et al., 2023). Similar findings have been reported by Du et al. (2023), noting that vitamin D improves hepatic steatosis in NAFLD by modulating fatty acid uptake and β-oxidation. Vitamin E, also known as tocopherol, is well-recognized for its significant antioxidant capabilities. The relationship between vitamin E and NAFLD has been reviewed (Nagashimada and Ota, 2019; Sumida et al., 2021; Vogli et al., 2023). Marine-derived tocopherols have been shown to treat metabolic diseases by alleviating inflammatory responses (Beppu et al., 2020).
It is hoped that future research will uncover more benefits of marine-derived vitamins and minerals for chronic liver diseases and other chronic conditions.
Xyloketal B (Xyl-B) is a novel marine compound with unique chemical structure isolated from the mangrove fungus Xylaria species found in the South China Sea (Lin et al., 2001; Gong et al., 2022). Tong et al. conducted experiments using Xyl-B on NAFLD mice and found that it reverses nutritional hepatic steatosis, steatohepatitis, and hepatic fibrosis by activating the PPARα/PGC1α signaling pathway, making it an extremely effective potential natural product for the treatment of NAFLD (Tong et al., 2022). Candidusin A (CHNQD-0803), an AMPK activator, reduces lipid synthesis by inhibiting the expression of adipose production genes, decreases fat deposition, negatively regulates the NF-κB-TNF-α inflammatory axis to suppress inflammation, and ameliorates liver injury and fibrosis (Chen et al., 2023). Ikarugamycin (IKA), extracted from a marine-derived bacterial strain SNB-040 isolated from a sediment sample collected from Sweetings Cay in the Bahamas, is a TFEB agonist that provides hepatoprotection against diet-induced steatosis in a mouse model (Wang et al., 2017a). The structural formula of some common marine bioactive substances has been summarized in Figure 2, including DHA (A), EPA (B), high-sulfated Ulva pertusa polysaccharide (HU) (C), dieckol (D), diphlorethohydroxycarmalol (DPHC) (E), phloroglucinol (F), astaxanthin (G), fucoxanthin (H), cyclo (-Pro-Tyr) (I), vitamin D3 (J), marine-derived tocopherol (MDT) (K), Xyl-B (L), CHNQD-0803 (M), and HN-001 (N).
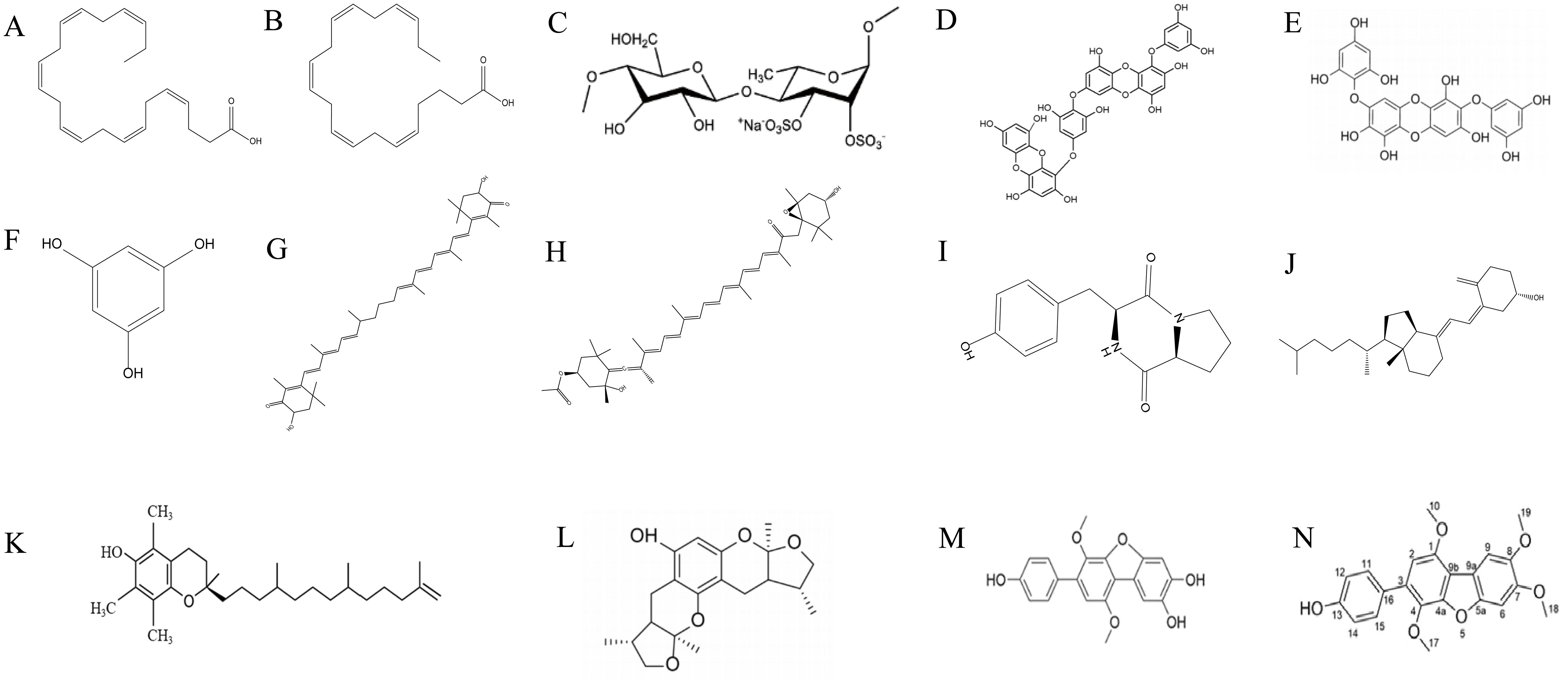
Figure 2. Some structures of MNPs are mentioned in Table 2 and the content. (A) DHA, (B) EPA, (C) high-sulfated Ulva pertusa polysaccharide (HU), (D) dieckol, (E) diphlorethohydroxycarmalol (DPHC), (F) phloroglucinol, (G) astaxanthin, (H) fucoxanthin, (I) cyclo (-Pro-Tyr), (J) vitamin D3, (K) marine-derived tocopherol (MDT), (L) Xyl-B, (M) CHNQD-0803, and (N) HN-001.
Marine bioactive substances can improve serum transaminase levels, liver antioxidant enzyme activities, and liver inflammation pathological characteristics in animal models of NAFLD. In addition, oxidative stress, lipid peroxidation, liver inflammation, and changes in gut microbiota are considered to be important pathological processes in NAFLD. Therefore, marine bioactive substances can prevent or treat NAFLD by antioxidative, anti-inflammatory, inhibiting DNL, enhancing fatty acid β-oxidation and alleviating the gut microenvironment, as shown in Figure 3.
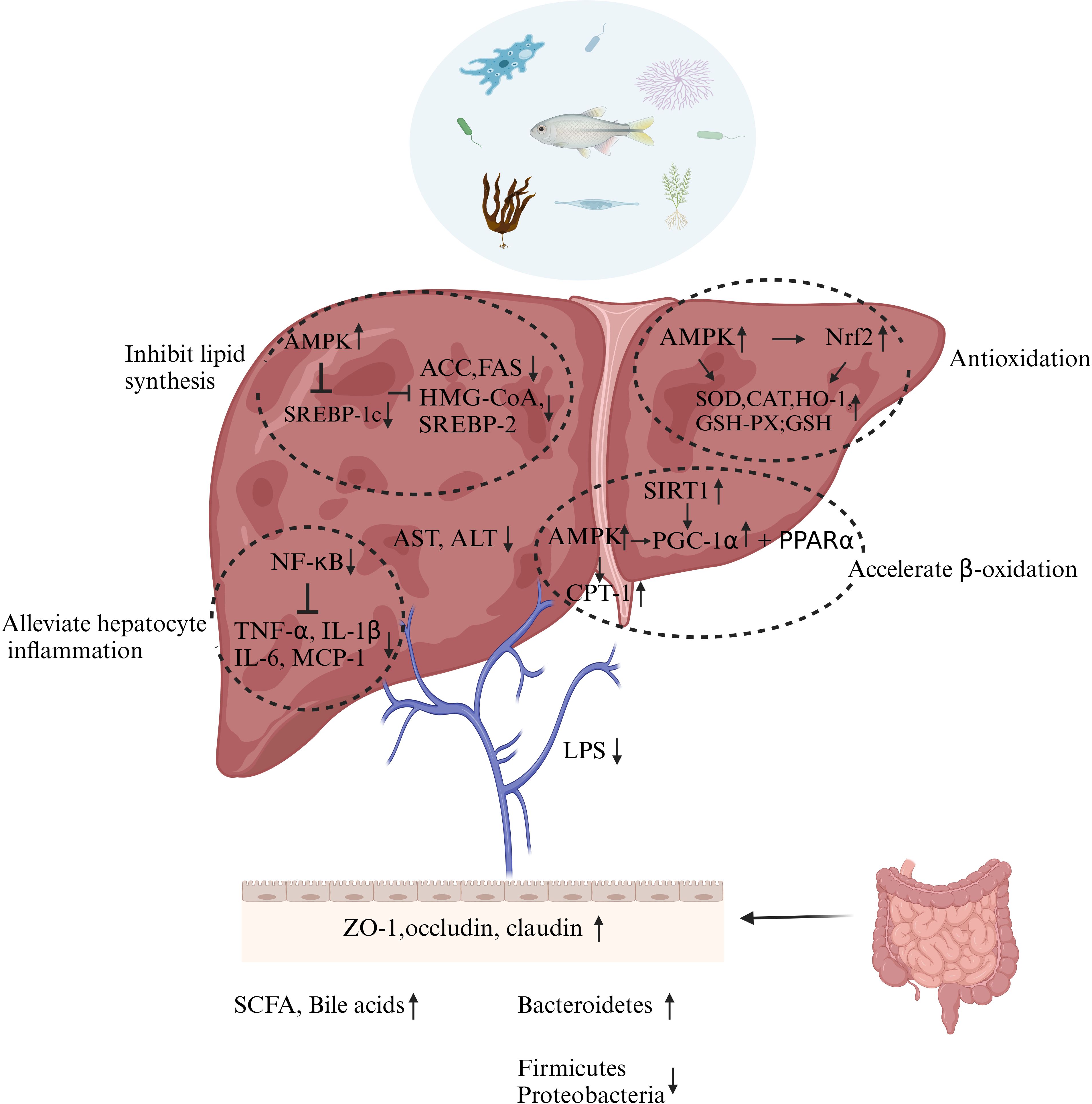
Figure 3. Possible mechanisms by marine bioactive substances involved in the prevention or treatment of NAFLD. ↑: Elevated level, activate or cause. ↓: Decrease in level, downward adjustment or cause. →: Cause. ⊥: Suppression. Figure is created using Biorender®.
Lipid metabolism consists of multiple processes, including fatty acids and cholesterol’s uptake, synthesis, transport, and efflux. The inhibitory pathway of lipid synthesis has been extensively studied; on the other hand, other pathways have been studied less. As a key regulator of lipid homeostasis, SREBP-1c overexpression can regulate lipogenic enzymes involved in fatty acid synthesis, such as ACC, FAS, stearate-CoA desaturase-1 (SCD-1), and ATP citrate lyase (ACLY), and triacylglycerols synthesis, diacylglycerol acyltransferase (DGAT). AMPK is known to be an important regulator of lipid metabolism that reduces the transcriptional and translational levels of SREBP-1c. AMPK senses the energy status of the cell and is activated when the intracellular AMP/ATP (adenosine monophosphate/adenosine triphosphate) ratio is elevated, i.e., when the cellular energy level decreases. Marine bioactive substances can alleviate NAFLD by inhibiting lipase expression, for example DHA (Luo et al., 2020), astaxanthin (Li et al., 2022), EPA-PC from Cucumaria frondose (Liu et al., 2016), and polyphenol-rich fraction of Ecklonia cava (Park et al., 2015). HMG-CoA reductase is a rate-limiting enzyme for cholesterol synthesis, and SREBP-2 is a transcription factor that plays a key role in cholesterol regulation; both expressions can be suppressed by sulfated polysaccharides from Enteromorpha prolifera (EP) (Ren et al., 2017).
Lipid accumulation can trigger ERS in hepatocytes and, by promoting ROS production, leads to oxidative stress and activation of the NF-κB signaling pathway. NF-κB is a key transcription factor for the transcription of many inflammatory genes. Normally, NF-κB exists in the cytoplasm inactive in combination with its inhibitory protein (IκB). When there is lipid accumulation in hepatocytes, IκB is phosphorylated, causing nuclear transfer of NF-κB to activate cytokines, chemokines, and gene expression of icosyl-like metabolic enzymes that synthesize inflammatory lipid mediators (COX-2). Among them, pro-inflammatory cytokines are thought to initiate inflammatory cascade, and chemokines are thought to promote inflammation by recruiting neutrophils and monocytes. Janus kinase (JAK)–signal transducer and activator of transcription (STAT) signaling pathway is an important intracellular signaling pathway that is commonly activated in inflammatory settings. Cytokines such as IL-6 bind to receptors on the cell membrane and activate JAK, which phosphorylates and recruits STAT proteins, which are phosphorylated to form a dimer that enters the nucleus and binds to specific regions of target genes to regulate gene transcription. In the inflammatory response, this pathway promotes the expression of inflammation-related genes. Marine fungi-derived HN-001 treatment blocked palmitic-acid-induced JNK pathway activation, alleviated ER-stress-mediated lipotoxicity, and suppressed liver inflammation in NAFLD mice (Rao et al., 2024).
Polyphenol-rich fraction of Ecklonia cava treatment of high-fat fed NAFLD mouse models can significantly reduce the levels of inflammatory markers such as TNF-α, IL-1β, and MCP-1 (Park et al., 2015). Many studies have shown that marine bioactive substances can reduce related inflammatory factors (Feng et al., 2022).
The ectopic accumulation of lipids in the liver increases the production of ROS, including superoxide, hydroxyl radicals, and hydrogen peroxide, which requires enzyme [superoxide dismutase (SOD, catalase (CAT), and GSH-Px] and non-enzymatic (GSH) antioxidant defense systems in the liver to clear it. CAT and SOD activities in the liver of db/db mice were also significantly increased after treatments of LMWF from Laminaria japonica Areschoug (Zheng et al., 2018). Oligopeptide extracted from Meretrix meretrix (MMO) significantly increased the level of GSH-Px and SOD activity in the liver and decreased the level of MDA in high-fat fed mice (Huang et al., 2018b). It is worth mentioning that MMO has also demonstrated abilities to alleviate early apoptosis in NAFLD mice.
AMPK phosphorylation promotes nuclear factor erythroid-2 related factor 2 (Nrf2) activation (Park et al., 2016). The cellular antioxidative stress defense system is significantly regulated by Nrf2. Under normal conditions, Nrf2 binds to Kelch-like epichlorohydrin-associated protein 1 (Keap1) and exists in the cytoplasm in an inactive form. When cells are stimulated by internal or external stimuli, Nrf2 dissociates from Keap1, binds to antioxidant response elements (AREs), and stimulates the transcription of downstream antioxidant genes such as hemeoxygenase-1 (HO-1), CAT, and SOD. The study by Li has shown that astaxanthin alleviates NAFLD by regulating the AMPK/Nrf2 signal axis (Li et al., 2022). This supports the idea that marine bioactive substances pose properties for antioxidation and are worth additional research.
Promoting β-oxidation of fatty acids is also a key target for inhibiting liver fat accumulation and lipid degeneration. PPARs are a class of ligand-activated nuclear transcription factors that play a key role in lipid metabolism. PPARα activates the expression of genes involved in fatty acid uptake, transport, and β-oxidation, such as carnitine/organic cation transporter 2 (OCTN2) and fatty acid transporter protein (FATP), and promotes fatty acid metabolism and utilization, etc. PPARγ regulates adipocyte differentiation and lipid storage and also affects hepatic lipid metabolism. Its activation can increase the expression of fatty acid binding protein (FABP) and other genes, promoting fatty acid uptake and triglyceride synthesis. Dieckol-enriched extraction from Laminaria japonica and antioxidant peptides extracted from the swim bladder of Lophius litulon activate AMPK and PPARα to accelerate β-oxidation (Liu et al., 2019; Wu et al., 2023a). In addition to being a regulator of lipid metabolism, AMPK also regulates the activity of carnitine palmitoyl transferase 1 (CPT-1) and PPAR. Activation of AMPK reduces the level of malonyl-CoA (a precursor of fatty acid synthesis and an inhibitor of CPT1) by inhibiting ACC overexpression, thereby alleviating the inhibition of CPT-1 and accelerating the transport of fatty acids from the cytoplasm to mitochondria (the main site of fatty acid β-oxidation). Activation of AMPK can phosphorylate PPARγ coactivator 1α (PGC-1α), enhance its interaction with PPAR, and promote β-oxidation of fatty acids. PGC-1α can also be activated by Sirtuin1; thus, marine bioactive substances are also hypothesized to target SIRT1 to promote fatty acid β-oxidation (Luo et al., 2020).
NAFLD is often accompanied by damage to the intestinal barrier, including damage to the intestinal villi and increased intestinal permeability. A leaky gut caused by disruption of the intestinal barrier triggers lipopolysaccharide (LPS)-mediated endotoxemia. The integrity of the intestinal barrier is regulated by tight junction proteins between intestinal epithelial cells and mucin secreted by goblet cells. The use of COSM improved the intestinal wall barrier integrity and endotoxemia in NAFLD mouse model, which improved ZO-1, occludin, and claudin protein expression (Feng et al., 2022).
Gut-associated lymphoid tissue (GALT) is an important component of the intestinal immune barrier. It includes Peyer’s patches, mesenteric lymph nodes, and isolated lymphoid follicles. Immune cells in these lymphoid tissues, such as T cells, B cells, macrophages, and dendritic cells, recognize and remove pathogens from the intestine.
The Pyle’s lymph nodes are an important site of initiation of the intestinal immune response. When an antigen enters the intestine, the microfold cells (M cells) in the Pyle’s lymph nodes are able to take up the antigen and pass it on to the immune cells, thus triggering an immune response. At the same time, the intestinal epithelial cells themselves can express some immune-related molecules, which are involved in antigen recognition and immune regulation.
After entering the liver through the portal vein, LPS activates Kupffer cells through a TLR4/NF-κB-dependent mechanism, which initiates the innate immunity of the liver, triggers an inflammatory cascade, and releases inflammatory factors.
Each stage of NAFLD has a special gut microbiota signature. The severity of NAFLD has been associated with dysbiosis and loss of commensal bacterial metabolic functions (Aron-Wisnewsky et al., 2020). In NAFLD, at the bacterial phylum level, decreased levels of Bacteroidetes are reported, while levels of Firmicutes and Proteobacteria were increased. Sulfated polysaccharides (GLP) and their agaro-oligosaccharides (GLO) derived from Gracilaria lemaneiformis increased the abundance of Bacteroidetes and decreased the abundance of Firmicutes (Zhang et al., 2020b), which can also be accomplished through the use of PYOs (Wang et al., 2021b) and astaxanthin (Li et al., 2022). A study had reviewed the effects of marine polysaccharides (especially those derived from marine microorganisms) and their metabolites on attenuating metabolic syndrome (MetS). Although NAFLD is a hepatic manifestation of MetS, the microbial polysaccharides listed in the article did not improve lipid deposition in the liver (Wang et al., 2018).
LPS, secondary bile acids, short-chain fatty acid (SCFA) (Zhang et al., 2021a), and choline metabolites such as trimethylamine (TMA) and trimethylamine N-oxide (TMAO) have been found to be associated with NAFLD (Chen and Vitetta, 2020). GLP was shown to aid in cholesterol regulation and bile acid metabolism in high-fat diet mice. After undergoing GLP treatment, secondary BAs [including taurolithocholic acid (TLCA), glycodeoxycholic acid (GDCA), glycoursodeoxycholic acid (GUDCA), and tauroursodeoxycholic acid (TUDCA)] increased in high-fat mouse model (Huang et al., 2019). The levels of SCFAs, especially the butyrate (p < 0.05), were appreciably restored by mussel polysaccharide, α-D-glucan (MP-A), which was isolated from Mytilus coruscus (Wu et al., 2019).
Marine sponges have been considered a remarkable field for the discovery of bioactive natural products, being the most studied source of marine bioactive substances so far (Blunt et al., 2015; Máximo et al., 2016). An investigation of the activity of the total extract of the marine sponge Spongia irregularis and its different fractions against the hepatitis C virus (HCV) was pursued; the results revealed that the ethyl acetate fraction exhibited the highest anti-HCV activity, with an IC50 value of 12.6 ± 0.05 μg/ml (Abdelaleem et al., 2022b). The varied marine bioactive substances and their functions produced by the diverse marine sponge species are yet to be studied and warrant further exploration (Orfanoudaki et al., 2021; Abdelaleem et al., 2022a; Jin et al., 2022b).
Marine fish consumption accounts for 20% of people’s animal protein intake, and the related by-products are also being investigated (Roy et al., 2022; Liu et al., 2024; Yuan et al., 2024), for example, the finding of improving hepatic functions with salmon milt (Takahashi et al., 2022). Not only limited to fish, other marine animals and their related by-products are suggested to also be studied to find more active substances that can help alleviate NAFLD while avoiding waste.
Algae have been studied and utilized for centuries. For decades, algae have been widely cultivated in 61 countries and territories, contributing over 50% of global marine and coastal aquaculture production (Pomin, 2011; Wade et al., 2020; Xie et al., 2023). At present, there are many projects studying the implementation of seaweed components on multiple medical conditions. Algae polyphenols are utilized to target diabetes (Lopes et al., 2016), cardiovascular disease (Gómez-Guzmán et al., 2018), cancer (Zenthoefer et al., 2017), and neurodegenerative diseases (Lomartire and Gonçalves, 2023). Algal polysaccharides are used in antiviral treatments (Wei et al., 2022; Liyanage et al., 2023) and treatment of Alzheimer’s disease (Bauer et al., 2021), and algal fatty acids and carotenoids are used as anti-inflammatory, for lowering of blood lipid (Cherry et al., 2019), and for other aspects. Additionally, some seaweed polysaccharides mentioned above have been shown to have positive effects on NAFLD, including but not limited to LMWF, Ulva, and PYOs. Therefore, it is very promising and convenient to isolate various components from seaweed, prepare prebiotics, and manufacture some dietary supplements. Given the potential of these seaweed-derived compounds, hopefully, there will be more seaweed products on the market in the future, especially targeting NAFLD.
The hepatoprotective effects of microalgae, such as Chlorella (Chlorella vulgaris Beijer.) and spirulina (Arthrospira maxima and Arthrospira platensis), have also been well documented (Ferreira-Hermosillo et al., 2010; González-Arceo et al., 2023; Fakhoury-Sayegh et al., 2024). Although they are mostly distributed in alkaline lakes and estuaries, the presence of microalgae in marine environments warrants continued exploration (Shirouchi et al., 2023). Oral administration of Chaetoceros gracilis—a marine microalga—alleviates hepatic lipid accumulation in rats fed a high-sucrose and cholesterol-containing diet (Shirouchi et al., 2023). Two extracts (carotenoids and lipids) of Phaeodactylum tricornutum, a ubiquitous marine diatom, have been shown to have preventive effects on non-alcoholic fatty liver disease (Mayer et al., 2020). It has been shown that the ethanol extracts of microalgae Isochrysis zhanjiangensis, which are distributed in the sea with high transparency, have a protective effect against acute alcoholic liver injury (Wen et al., 2024). However, attention should be paid to the possible toxicity of cyanobacteriotoxins to the liver caused by cyanobacteria bloom (El-Shehawy et al., 2012; Sánchez-Parra et al., 2020).
Beyond algae, which is currently a popular topic of study, other plants with unique habitats in the ocean also deserve further investigation, such as Avicennia marina, Ceriops tagal, Ipomoea pes-caprae, Sonneratia apetala, and other marine halophytes (Murugesan et al., 2023).
Marine-derived fungi are a rich source of novel metabolites with unique structural features and bioactivities (Mohamed and Ibrahim, 2021). So far, only a handful of marine fungi have been studied (Amend et al., 2019). Various studies have been conducted on its activity with antibacterial properties being the main direction of research (Xu et al., 2015a; Qi et al., 2023). However, we think that under specific marine environments, these marine-derived fungi may be capable of producing compounds with activity against chronic metabolic diseases.
Dihydrotrichodimerol, purified from the marine fungus, Acremonium citrinum, has demonstrated positive effects on NAFLD prevention by targeting PPAR pathways (Liu et al., 2023a). In addition, phospholipase A2 (PLA2) inhibitor HN-001 was isolated from marine fungus Aspergillus sp. C1. Inhibition of PLA2 in hepatocytes or in mice decreases lysophosphatidylcholine (LPC) content, thus blocking the c-Jun N-terminal kinase (JNK) pathway and ameliorating hepatocyte apoptosis. On the other hand, a reduction in LPC inhibits inositol-requiring enzyme 1α (IRE-1α) activation, leading to XBP-1 splicing inhibition and transcriptional regulation blockade and ameliorating ER stress, hepatocyte apoptosis, and inflammation. As a result, NAFLD is alleviated (Rao et al., 2024). Both marine fungi were isolated from coastal intertidal zones, which are characterized by high salinity and abundance of organic material. These findings support our hypothesis that MNPs of marine fungi are beneficial to ameliorate chronic metabolic diseases.
Moreover, we suggest that perhaps harvesting fungi in special marine environments would bring greater value, such as from deep-sea hydrothermal vents, cold springs, and other extreme environments, as they have a higher possibility of producing novel active products with special structures (Burgaud et al., 2009).
In addition to marine fungi, marine bacteria are also an important source of active drug ingredients, with over 13,000 natural compounds endowed with different pharmacological properties have been isolated from different bacterial sources documented as of 2022 (Stincone and Brandelli, 2020; Zhang et al., 2020a; Mohan et al., 2022). Over the past two decades, studies of these compounds have been focused on their antibacterial and anticancer properties intensively (Stincone and Brandelli, 2020; Zhang et al., 2020a; Mohan et al., 2022). However, since GM plays a critical role in the amelioration of NAFLD, it has been found that probiotic supplementation can alleviate NAFLD through the influence of GM as well (Yao et al., 2021). This area of interest in the implementations of marine-derived probiotics on NAFLD is yet to be further expanded on.
To conclude, we hope that future researchers can continue to explore the hidden treasures of the ocean from different perspectives such as discovering probiotics and various marine bioactive substances. We hope that in the future, we can continue to explore the treasure house of the ocean from the perspective of finding probiotics. The discovery of MNPs from different sources in recent years has been shown in Figure 4 (Carroll et al., 2022, 2023, 2024).

Figure 4. Discovery of MNPs in recent years. (A) Number of MNPs from different marine sources in 2020, 2021, and 2022. (B) Proportion of MNPs from different sources in 2022.
NAFLD is emerging as a significant global health challenge, with its growing prevalence leading to an increasing burden on social healthcare systems. It is an integral component of the metabolic syndrome, and future research endeavors should aim to establish stronger connections between NAFLD and other related conditions, namely, obesity (Li et al., 2016), diabetes (Hazlehurst et al., 2016; Cernea, 2024), cardiovascular diseases (Targher et al., 2020), chronic kidney disease (Park et al., 2019), extrahepatic malignancies (Thomas et al., 2024), inflammatory bowel disease (Maresca et al., 2024), and even psoriasis (Costache et al., 2024). We therefore suggest that some of the marine bioactive substances used in the above disease studies might also be considered for their beneficial effects on NAFLD.
Researchers are progressively uncovering new mechanisms and therapeutic targets, for instance, reduced fatty acid uptake and lipid synthesis/accumulation, and interventions that decrease bacterial endotoxin production or enhance the production of certain enterobacterial metabolites such as bile acids and short-chain fatty acids. However, given that NAFLD has a wide range of pathogenic variables and numerous correlations with other illnesses, monotherapy may lead to undesirable side effects. Combination therapies therefore may be essential in the management of NAFLD.
Antibody–drug conjugates (ADCs) represent a therapeutic class that integrates the high specificity of monoclonal antibodies with the potent cytotoxicity of small molecule drugs, thereby enhancing the precision of cancer therapeutics and mitigating systemic toxicities. The concept of ADCs was introduced in the 1980s, and the first such conjugate received its approval from the FDA in 2000 for the treatment of acute myeloid leukemia. Unfortunately, this particular ADC was subsequently withdrawn from the market in 2010 due to its associated lethality (Fu et al., 2022). Nevertheless, the past decade has witnessed a resurgence of interest in ADCs research, attributable to their demonstrated efficacy and targeted therapeutic potential. Notably, unique cytotoxic agents derived from marine sources have been successfully incorporated into ADC formulations and are currently under evaluation in clinical trials, exemplified by agents such as Adcetris® and Blenrep™ (Table 1). A majority of these marine-derived ADCs are currently being investigated for their utility in the treatment of orphan diseases. It is advocated that future research endeavors in the ADC field should be expanded to include a greater focus on marine natural products, given their novel and potentially therapeutically relevant chemical structures.
Studies have shown that patients with NAFLD may have a higher risk of drug-induced liver injury (Lammert et al., 2019). The approval of Rezdiffra is a landmark event, calling an end to the absence of an FDA-approved drug for NAFLD in nearly four decades (Schaffner and Thaler, 1986; FDA approves first MASH drug, 2024). Rezdiffra, in combination with diet and exercise, is used for the treatment of moderate to advanced liver fibrosis in adults with non-cirrhotic NASH, an advanced form of NAFLD. However, it is important not to forget the possible side effects of Rezdiffra, such as diarrhea and nausea (Ledford, 2024). Hence, an urgent demand persists for the development of pharmaceuticals or dietary supplements that are both safe and efficacious, while exhibiting a diminished propensity for adverse side effects at the same time.
Diet, serving as a vital source of nutrients, exerts a profound influence on human health and disease progression. Dietary interventions have emerged as promising adjunctive treatment strategies (Xiao et al., 2024). The use of marine bioactive substances to prepare functional foods is a promising direction and low risk.
In light of previous studies and the rapid advancement of computer technology in recent years, the following points should be taken into consideration in future studies about MNPs:
(1) Compared to the numerous studies, including the ones listed above, which have explored the potential activity of MNPs through in vivo and in vitro models, only a few studies have investigated the bioavailability of these compounds. In the future, more research is required to better understand the bioavailability of these compounds, which would allow us to possibly alter the delivery methods or chemical structure of these drug candidates.
(2) After studies at the cellular level and the use of mouse models, additional testing should be considered by researchers, acknowledging the differences between rodents and primates and the limitations of the models used.
(3) Traditional drug screening has certain technical errors and scope. In future studies, computer platforms should be considered for the use of virtual screening with large capacity and small dosage to determine the approximate biological activity. At the same time, multiple omics methods such as transcriptome and metabolome were used to further identify the regulatory pathways.
(4) By reading searched literatures, we found that most of the studies on the alleviation of NAFLD by marine bioactive substances mainly focused on the phenotypes and related molecular mechanisms, but the structure–activity relationships of specific compounds were less discussed. For more NAFLD drug output, specific compounds and their structures must be studied. When extracting and preparing active molecules, after obtaining the initial extract, we must analyze its components by means of chromatography, mass spectrometry, nuclear magnetism, and more and study its specific composition, structural characterization, and structure–activity relationship to better explore its molecular mechanism and obtain drug lead molecules. At the same time, the extraction process was optimized, and synthetic chemistry was combined when necessary to solve the problem of fewer products from traditional extraction methods, laying the foundation for subsequent patent medicine and clinical treatment.
While the exploitation of terrestrial resources by the scientific community has been extensive, the marine environment, characterized by its vast expanse, profound depths, and unique ecological conditions, remains an untapped and potentially rich repository for “blue drugstore.” Given the high probability of identifying novel bioactive compounds within this marine milieu that are efficacious against chronic hepatic pathologies, there is a compelling need for an augmented research effort directed toward this largely uncharted domain to access more lead compounds for NAFLD treatment in the future.
Notably, natural products are generally more preferable to chemically synthesized drugs. It is also the main source of molecules for current drug development. After a series of extraction, screening, activity evaluation, and mechanism exploration based on the material of possible source, researchers also need to address the challenges of transitioning from small trials to factory production.
With the discovery of more therapeutic targets and the discovery and modification of active molecules, the prospects for the treatment of NAFLD are becoming brighter. The integration of diet, pharmacology, and prevention strategies is expected to provide a more comprehensive and effective approach to managing chronic liver disease and other complex diseases. We believe that MNPs will play an important role. The study of MNPs should not conclude here; we are eager to see future more studies on this topic.
MS: Visualization, Writing – original draft, Writing – review & editing. SC: Writing – review & editing. YF: Writing – review & editing. SW: Writing – review & editing. YX: Writing – review & editing. JH: Conceptualization, Funding acquisition, Project administration, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the General Project of Fujian Provincial Natural Science Foundation, grant number 2023J011376; Science and Technology Plan Program of Fujian Province, grant numbers 2024Y0077 and 2023N0035; the Scientific Research Foundation of Third Institute of Oceanography, Ministry of Natural Resources, grant number 2023009; Xiamen Natural Science Foundation, grant number 3502Z20227247; and Xiamen Oceanic Research & Development Institute Co-construction Project, grant number K230102.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdelaleem E. R., Samy M. N., Ahmed S. A., Aboulmagd A. M., Alhadrami A. H., Rateb M. E., et al. (2022a). The Red Sea marine sponge Spongia irregularis: metabolomic profiling and cytotoxic potential supported by in silico studies. Nat. Prod Res. 36, 6359–6363. doi: 10.1080/14786419.2022.2030328
Abdelaleem E. R., Samy M. N., Ali T. F. S., Mustafa M., Ibrahim M. A. A., Bringmann G., et al. (2022b). NS3 helicase inhibitory potential of the marine sponge Spongia irregularis. RSC Adv. 12, 2992–3002. doi: 10.1039/d1ra08321j
Abegaz B. M., Kinfe H. H. (2019). Secondary metabolites, their structural diversity, bioactivity, and ecological functions: An overview. Phys. Sci. Rev. 4, 20180100. doi: 10.1515/psr-2018-0100
Allavena P., Belgiovine C., Digifico E., Frapolli R., D’Incalci M. (2022). Effects of the anti-tumor agents trabectedin and lurbinectedin on immune cells of the tumor microenvironment. Front. Oncol. 12. doi: 10.3389/fonc.2022.851790
Alonso-Álvarez S., Pardal E., Sánchez-Nieto D., Navarro M., Caballero M. D., Mateos M. V., et al. (2017). Plitidepsin: design, development, and potential place in therapy. Drug Des. Devel Ther. 11, 253–264. doi: 10.2147/dddt.S94165
Al Rijjal D., Liu Y., Lai M., Song Y., Danaei Z., Wu A., et al. (2021). Vascepa protects against high-fat diet-induced glucose intolerance, insulin resistance, and impaired β-cell function. iScience 24, 102909. doi: 10.1016/j.isci.2021.102909
Amend A., Burgaud G., Cunliffe M., Edgcomb V. P., Ettinger C. L., Gutiérrez M. H., et al. (2019). Fungi in the marine environment: open questions and unsolved problems. mBio 10, e01189-18. doi: 10.1128/mBio.01189-18
Andersen T., Gluud C., Franzmann M.-B., Christoffersen P. (1991). Hepatic effects of dietary weight loss in morbidly obese subjects. J. Hepatol. 12, 224–229. doi: 10.1016/0168-8278(91)90942-5
Aron-Wisnewsky J., Vigliotti C., Witjes J., Le P., Holleboom A. G., Verheij J., et al. (2020). Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 17, 279–297. doi: 10.1038/s41575-020-0269-9
Baines A. C., Ershler R., Kanapuru B., Xu Q., Shen G., Li L., et al. (2022). FDA approval summary: belantamab mafodotin for patients with relapsed or refractory multiple myeloma. Clin. Cancer Res. 28, 4629–4633. doi: 10.1158/1078-0432.Ccr-22-0618
Baker W. J., Royer G. L. Jr., Weiss R. B. (1991). Cytarabine and neurologic toxicity. J. Clin. Oncol. 9, 679–693. doi: 10.1200/jco.1991.9.4.679
Bartolini D., Zatini L., Migni A., Frammartino T., Guerrini A., Garetto S., et al. (2023). Transcriptomics of natural and synthetic vitamin D in human hepatocyte lipotoxicity. J. Nutr. Biochem. 117, 109319. doi: 10.1016/j.jnutbio.2023.109319
Bauer S., Jin W., Zhang F., Linhardt R. J. (2021). The application of seaweed polysaccharides and their derived products with potential for the treatment of Alzheimer’s disease. Mar. Drugs 19, 89. doi: 10.3390/md19020089
Beppu F., Aida Y., Kaneko M., Kasatani S., Aoki Y., Gotoh N. (2020). Functional evaluation of marine-derived tocopherol, a minor homolog of vitamin E, on adipocyte differentiation and inflammation using 3T3-L1 and RAW264.7 cells. Fish. Sci. 86, 415–425. doi: 10.1007/s12562-020-01404-6
Bhatnagar D., Hussain F. (2007). Omega-3 fatty acid ethyl esters (Omacor®) for the treatment of hypertriglyceridemia. Future Lipidol. 2, 263–270. doi: 10.2217/17460875.2.3.263
Blunt J. W., Copp B. R., Keyzers R. A., Munro M. H., Prinsep M. R. (2015). Marine natural products. Nat. Prod Rep. 32, 116–211. doi: 10.1039/c4np00144c
Boccellato C., Kolbe E., Peters N., Juric V., Fullstone G., Verreault M., et al. (2021). Marizomib sensitizes primary glioma cells to apoptosis induced by a latest-generation TRAIL receptor agonist. Cell Death Dis. 12, 647. doi: 10.1038/s41419-021-03927-x
Brodowicz T. (2014). Trabectedin in soft tissue sarcomas. Future Oncol. 10, s1–s5. doi: 10.2217/fon.14.117
Bugianesi E., Moscatiello S., Ciaravella M. F., Marchesini G. (2010). Insulin resistance in nonalcoholic fatty liver disease. Curr. Pharm. Des. 16, 1941–1951. doi: 10.2174/138161210791208875
Burgaud G., Le Calvez T., Arzur D., Vandenkoornhuyse P., Barbier G. (2009). Diversity of culturable marine filamentous fungi from deep-sea hydrothermal vents. Environ. Microbiol. 11, 1588–1600. doi: 10.1111/j.1462-2920.2009.01886.x
Buzzetti E., Pinzani M., Tsochatzis E. A. (2016). The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 65, 1038–1048. doi: 10.1016/j.metabol.2015.12.012
Calo N., Ramadori P., Sobolewski C., Romero Y., Maeder C., Fournier M., et al. (2016). Stress-activated miR-21/miR-21* in hepatocytes promotes lipid and glucose metabolic disorders associated with high-fat diet consumption. Gut 65, 1871–1881. doi: 10.1136/gutjnl-2015-310822
Carroll A. R., Copp B. R., Davis R. A., Keyzers R. A., Prinsep M. R. (2022). Marine natural products. Nat. Prod Rep. 39, 1122–1171. doi: 10.1039/D1NP00076D
Carroll A. R., Copp B. R., Davis R. A., Keyzers R. A., Prinsep M. R. (2023). Marine natural products. Nat. Prod Rep. 40, 275–325. doi: 10.1039/D2NP00083K
Carroll A. R., Copp B. R., Grkovic T., Keyzers R. A., Prinsep M. R. (2024). Marine natural products. Nat. Prod Rep. 41, 162–207. doi: 10.1039/D3NP00061C
Cernea S. (2024). NAFLD fibrosis progression and type 2 diabetes: the hepatic–metabolic interplay. Life 14, 272. doi: 10.3390/life14020272
Cha S.-H., Hwang Y., Heo S.-J., Jun H.-S. (2018). Diphlorethohydroxycarmalol attenuates methylglyoxal-induced oxidative stress and advanced glycation end product formation in human kidney cells. Oxid. Med. Cell Longev 2018, 3654095. doi: 10.1155/2018/3654095
Cha S. H., Hwang Y., Heo S. J., Jun H. S. (2020). Diphlorethohydroxycarmalol attenuates palmitate-induced hepatic lipogenesis and inflammation. Mar. Drugs 18, 475. doi: 10.3390/md18090475
Cheemerla S., Balakrishnan M. (2021). Global epidemiology of chronic liver disease. Clin. Liver Dis. (Hoboken) 17, 365–370. doi: 10.1002/cld.1061
Chen J., Vitetta L. (2020). Gut microbiota metabolites in NAFLD pathogenesis and therapeutic implications. Int. J. Mol. Sci. 21, 5214. doi: 10.3390/ijms21155214
Chen J., Xu L., Zhang X. Q., Liu X., Zhang Z. X., Zhu Q. M., et al. (2023). Discovery of a natural small-molecule AMP-activated kinase activator that alleviates nonalcoholic steatohepatitis. Mar. Life Sci. Technol. 5, 196–210. doi: 10.1007/s42995-023-00168-z
Cheng R., Wang L., Le S., Yang Y., Zhao C., Zhang X., et al. (2022). A randomized controlled trial for response of microbiome network to exercise and diet intervention in patients with nonalcoholic fatty liver disease. Nat. Commun. 13, 2555. doi: 10.1038/s41467-022-29968-0
Cherry P., Yadav S., Strain C. R., Allsopp P. J., McSorley E. M., Ross R. P., et al. (2019). Prebiotics from seaweeds: an ocean of opportunity? Mar. Drugs 17, 327. doi: 10.3390/md17060327
Cheung O., Puri P., Eicken C., Contos M. J., Mirshahi F., Maher J. W., et al. (2008). Nonalcoholic steatohepatitis is associated with altered hepatic MicroRNA expression. Hepatology 48, 1810–1820. doi: 10.1002/hep.22569
Clemente M. G., Mandato C., Poeta M., Vajro P. (2016). Pediatric non-alcoholic fatty liver disease: Recent solutions, unresolved issues, and future research directions. World J. Gastroenterol. 22, 8078–8093. doi: 10.3748/wjg.v22.i36.8078
Costa M., Rosa F., Ribeiro T., Hernandez-Bautista R., Bonaldo M., Gonçalves Silva N., et al. (2019). Identification of cyanobacterial strains with potential for the treatment of obesity-related co-morbidities by bioactivity, toxicity evaluation and metabolite profiling. Mar. Drugs 17, 280. doi: 10.3390/md17050280
Costache D. O., Blejan H., Cojocaru D. L., Ioniţă G. A., Poenaru M., Constantin M. M., et al. (2024). Intersecting pathways: nonalcoholic fatty liver disease and psoriasis duet—A comprehensive review. Int. J. Mol. Sci. 25, 2660. doi: 10.3390/ijms25052660
Crowley E. K., Long-Smith C. M., Murphy A., Patterson E., Murphy K., O’Gorman D. M., et al. (2018). Dietary supplementation with a magnesium-rich marine mineral blend enhances the diversity of gastrointestinal microbiota. Mar. Drugs 16, 216. doi: 10.3390/md16060216
D’Incalci M., Zambelli A. (2016). Trabectedin for the treatment of breast cancer. Expert Opin. Investig. Drugs 25, 105–115. doi: 10.1517/13543784.2016.1124086
Della Corte C., Mosca A., Ionata A., Nobili V. (2016). Docosahexaenoic acid and its role in G-protein-coupled receptor 120 activation in children affected by nonalcoholic fatty liver disease. Endocr. Dev. 30, 29–36. doi: 10.1159/000439324
Devarbhavi H., Asrani S. K., Arab J. P., Nartey Y. A., Pose E., Kamath P. S. (2023). Global burden of liver disease: 2023 update. J. Hepatol. 79, 516–537. doi: 10.1016/j.jhep.2023.03.017
Dongiovanni P., Meroni M., Longo M., Fargion S., Fracanzani A. L. (2018). miRNA signature in NAFLD: A turning point for a non-invasive diagnosis. Int. J. Mol. Sci. 19, 3966. doi: 10.3390/ijms19123966
Du T., Xiang L., Zhang J., Yang C., Zhao W., Li J., et al. (2023). Vitamin D improves hepatic steatosis in NAFLD via regulation of fatty acid uptake and β-oxidation. Front. Endocrinol. (Lausanne) 14. doi: 10.3389/fendo.2023.1138078
Eccles R., Meier C., Jawad M., Weinmüllner R., Grassauer A., Prieschl-Grassauer E. (2010). Efficacy and safety of an antiviral Iota-Carrageenan nasal spray: a randomized, double-blind, placebo-controlled exploratory study in volunteers with early symptoms of the common cold. Respir. Res. 11, 108. doi: 10.1186/1465-9921-11-108
Effandie E., Gupte G. L. (2023). Chronic liver disease - what’s new? Indian J. Pediatr. 91, 391–397. doi: 10.1007/s12098-023-04819-y
El-Shehawy R., Gorokhova E., Fernández-Piñas F., del Campo F. F. (2012). Global warming and hepatotoxin production by cyanobacteria: What can we learn from experiments? Water Res. 46, 1420–1429. doi: 10.1016/j.watres.2011.11.021
Eslam M., Newsome P. N., Sarin S. K., Anstee Q. M., Targher G., Romero-Gomez M., et al. (2020). A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 73, 202–209. doi: 10.1016/j.jhep.2020.03.039
Eslam M., Valenti L., Romeo S. (2018). Genetics and epigenetics of NAFLD and NASH: Clinical impact. J. Hepatol. 68, 268–279. doi: 10.1016/j.jhep.2017.09.003
Fakhoury-Sayegh N., Hamdan A., Lebbos S., Itani T., Trak-Smayra V., Khazzaka A., et al. (2024). Spirulina (Arthrospira platensis) improved nonalcoholic fatty liver disease characteristics and microbiota and did not affect organ fibrosis induced by a fructose-enriched diet in wistar male rats. Nutrients 16, 1701. doi: 10.3390/nu16111701
Feldman A., Aigner E., Weghuber D., Paulmichl K. (2015). The potential role of iron and copper in pediatric obesity and nonalcoholic fatty liver disease. BioMed. Res. Int. 2015, 287401. doi: 10.1155/2015/287401
Feng J., Liu Y., Chen J., Bai Y., He J., Cao H., et al. (2022). Marine chitooligosaccharide alters intestinal flora structure and regulates hepatic inflammatory response to influence nonalcoholic fatty liver disease. Mar. Drugs 20, 383. doi: 10.3390/md20060383
Ferreira-Hermosillo A., Torres-Duran P. V., Juarez-Oropeza M. A. (2010). Hepatoprotective effects of Spirulina maxima in patients with non-alcoholic fatty liver disease: A case series. J. Med. Case Rep. 4, 103. doi: 10.1186/1752-1947-4-103
Fu Z., Li S., Han S., Shi C., Zhang Y. (2022). Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduct. Target Ther. 7, 93. doi: 10.1038/s41392-022-00947-7
Geier A., Eichinger M., Stirnimann G., Semela D., Tay F., Seifert B., et al. (2018). Treatment of non-alcoholic steatohepatitis patients with vitamin D: a double-blinded, randomized, placebo-controlled pilot study. Scand. J. Gastroenterol. 53, 1114–1120. doi: 10.1080/00365521.2018.1501091
Gómez-Guzmán M., Rodríguez-Nogales A., Algieri F., Gálvez J. (2018). Potential role of seaweed polyphenols in cardiovascular-associated disorders. Mar. Drugs 16, 250. doi: 10.3390/md16080250
Gong H., Bandura J., Wang G.-L., Feng Z.-P., Sun H.-S. (2022). Xyloketal B: A marine compound with medicinal potential. Pharmacol. Ther. 230, 107963. doi: 10.1016/j.pharmthera.2021.107963
González-Arceo M., Trepiana J., Aguirre L., Ibarruri J., Martínez-Sanz M., Cebrián M., et al. (2023). Anti-Steatotic Effects of Chlorella vulgaris, Nannochloropsis gaditana and Gracilaria vermiculophylla Algae Extracts in AML-12 Hepatocytes. Nutrients 15, 1960. doi: 10.3390/nu15081960
Gravanis I., Tzogani K., van Hennik P., de Graeff P., Schmitt P., Mueller-Berghaus J., et al. (2016). The European medicines agency review of brentuximab vedotin (Adcetris) for the treatment of adult patients with relapsed or refractory CD30+ Hodgkin lymphoma or systemic anaplastic large cell lymphoma: summary of the scientific assessment of the committee for medicinal products for human use. Oncologist 21, 102–109. doi: 10.1634/theoncologist.2015-0276
Guo X. F., Wang C., Yang T., Ma W. J., Zhai J., Zhao T., et al. (2022). The effects of fish oil plus vitamin D(3) intervention on non-alcoholic fatty liver disease: a randomized controlled trial. Eur. J. Nutr. 61, 1931–1942. doi: 10.1007/s00394-021-02772-0
Guo B., Zhou Y., Liu B., He Y., Chen F., Cheng K.-W. (2021). Lipid-lowering bioactivity of microalga nitzschia laevis extract containing fucoxanthin in murine model and carcinomic hepatocytes. Pharmaceuticals 14, 1004. doi: 10.3390/ph14101004
Gupta V. K., Kubicek C. P., Berrin J. G., Wilson D. W., Couturier M., Berlin A., et al. (2016). Fungal enzymes for bio-products from sustainable and waste biomass. Trends Biochem. Sci. 41, 633–645. doi: 10.1016/j.tibs.2016.04.006
Gutierrez-Reyes G., Gutierrez-Ruiz M. C., Kershenobich D. (2007). Liver fibrosis and chronic viral hepatitis. Arch. Med. Res. 38, 644–651. doi: 10.1016/j.arcmed.2006.10.001
Guxens M., Mendez M. A., Moltó-Puigmartí C., Julvez J., García-Esteban R., Forns J., et al. (2011). Breastfeeding, long-chain polyunsaturated fatty acids in colostrum, and infant mental development. Pediatrics 128, e880–e889. doi: 10.1542/peds.2010-1633
Hazlehurst J. M., Woods C., Marjot T., Cobbold J. F., Tomlinson J. W. (2016). Non-alcoholic fatty liver disease and diabetes. Metabolism 65, 1096–1108. doi: 10.1016/j.metabol.2016.01.001
Hernigou P., Sitbon J., Dubory A., Auregan J. C. (2019). Vitamin D history part III: the “modern times”-new questions for orthopaedic practice: deficiency, cell therapy, osteomalacia, fractures, supplementation, infections. Int. Orthop. 43, 1755–1771. doi: 10.1007/s00264-019-04334-w
Hodson L., Bhatia L., Scorletti E., Smith D. E., Jackson N. C., Shojaee-Moradie F., et al. (2017). Docosahexaenoic acid enrichment in NAFLD is associated with improvements in hepatic metabolism and hepatic insulin sensitivity: a pilot study. Eur. J. Clin. Nutr. 71, 973–979. doi: 10.1038/ejcn.2017.9
Hosomi R., Fukunaga K., Arai H., Nishiyama T., Yoshida M. (2009). Effects of dietary fish protein on serum and liver lipid concentrations in rats and the expression of hepatic genes involved in lipid metabolism. J. Agric. Food Chem. 57, 9256–9262. doi: 10.1021/jf901954r
Hsu C. L., Loomba R. (2024). From NAFLD to MASLD: implications of the new nomenclature for preclinical and clinical research. Nat. Metab. 6, 600–602. doi: 10.1038/s42255-024-00985-1
Huang B., Bao J., Cao Y. R., Gao H. F., Jin Y. (2018a). Cytochrome P450 1A1 (CYP1A1) catalyzes lipid peroxidation of oleic acid-induced hepG2 cells. Biochem. (Mosc) 83, 595–602. doi: 10.1134/s0006297918050127
Huang S., Pang D., Li X., You L., Zhao Z., Cheung P. C.-K., et al. (2019). A sulfated polysaccharide from Gracilaria Lemaneiformis regulates cholesterol and bile acid metabolism in high-fat diet mice. Food Funct. 10, 3224–3236. doi: 10.1039/C9FO00263D
Huang F., Wang J., Yu F., Tang Y., Ding G., Yang Z., et al. (2018b). Protective Effect of Meretrix meretrix Oligopeptides on High-Fat-Diet-Induced Non-Alcoholic Fatty Liver Disease in Mice. Mar. Drugs 16, 39. doi: 10.3390/md16020039
Huang F., Zhao S., Yu F., Yang Z., Ding G. (2017). Protective Effects and Mechanism of Meretrix meretrix Oligopeptides against Nonalcoholic Fatty Liver Disease. Mar. Drugs 15, 31. doi: 10.3390/md15020031
Huh J. Y., Reilly S. M., Abu-Odeh M., Murphy A. N., Mahata S. K., Zhang J., et al. (2020). TANK-binding kinase 1 regulates the localization of acyl-coA synthetase ACSL1 to control hepatic fatty acid oxidation. Cell Metab. 32, 1012–1027.e7. doi: 10.1016/j.cmet.2020.10.010
Hurjui D. M., Niţă O., Graur L. I., Mihalache L., Popescu D. S., Graur M. (2012). The central role of the non alcoholic fatty liver disease in metabolic syndrome. Rev. Med. Chir Soc. Med. Nat. Iasi 116, 425–431.
Jin T., Li P., Wang C., Tang X., Yu X., Sun F., et al. (2022b). Jellynolide A, pokepola esters, and sponalisolides from the aquaculture sponge Spongia officinalis L. Phytochemistry 194, 113006. doi: 10.1016/j.phytochem.2021.113006
Jin K., Shi Y., Zhang H., Zhangyuan G., Wang F., Li S., et al. (2023). A TNFα/Miz1-positive feedback loop inhibits mitophagy in hepatocytes and propagates non-alcoholic steatohepatitis. J. Hepatol. 79, 403–416. doi: 10.1016/j.jhep.2023.03.039
Jin J. O., Yadav D., Madhwani K., Puranik N., Chavda V., Song M. (2022a). Seaweeds in the oncology arena: anti-cancer potential of fucoidan as a drug-A review. Molecules 27, 6032. doi: 10.3390/molecules27186032
Juanola O., Martínez-López S., Francés R., Gómez-Hurtado I. (2021). Non-alcoholic fatty liver disease: metabolic, genetic, epigenetic and environmental risk factors. Int. J. Environ. Res. Public Health 18, 5227. doi: 10.3390/ijerph18105227
Karanam G., Arumugam M. K., Sirpu Natesh N. (2020). Anticancer effect of marine sponge-associated bacillus pumilus AMK1 derived dipeptide cyclo (-pro-tyr) in human liver cancer cell line through apoptosis and G2/M phase arrest. Int. J. Pept. Res. Ther. 26, 445–457. doi: 10.1007/s10989-019-09850-2
Kelley N. S. (2016). Treatment of nonalcoholic fatty liver disease with long-chain n-3 polyunsaturated fatty acids in humans. Metab. Syndr. Relat. Disord. 14, 417–430. doi: 10.1089/met.2016.0051
Kellogg J. J., Paine M. F., McCune J. S., Oberlies N. H., Cech N. B. (2019). Selection and characterization of botanical natural products for research studies: a NaPDI center recommended approach. Nat. Prod Rep. 36, 1196–1221. doi: 10.1039/C8NP00065D
Kimura M., Iguchi T., Iwasawa K., Dunn A., Thompson W. L., Yoneyama Y., et al. (2022). En masse organoid phenotyping informs metabolic-associated genetic susceptibility to NASH. Cell 185, 4216–4232.e16. doi: 10.1016/j.cell.2022.09.031
Kramer A., Eggers M., Hübner N. O., Walger P., Steinmann E., Exner M. (2021). Virucidal gargling and virucidal nasal spray. GMS Hyg Infect. Control 16, Doc02. doi: 10.3205/dgkh000373
Krebs M., Krssak M., Bernroider E., Anderwald C., Brehm A., Meyerspeer M., et al. (2002). Mechanism of amino acid-induced skeletal muscle insulin resistance in humans. Diabetes 51, 599–605. doi: 10.2337/diabetes.51.3.599
Lammert C., Imler T., Teal E., Chalasani N. (2019). Patients with chronic liver disease suggestive of nonalcoholic fatty liver disease may be at higher risk for drug-induced liver injury. Clin. Gastroenterol. Hepatol. 17, 2814–2815. doi: 10.1016/j.cgh.2018.12.013
Larsen A. K., Galmarini C. M., D’Incalci M. (2016). Unique features of trabectedin mechanism of action. Cancer Chemother. Pharmacol. 77, 663–671. doi: 10.1007/s00280-015-2918-1
Le M. H., Yeo Y. H., Li X., Li J., Zou B., Wu Y., et al. (2022). 2019 global NAFLD prevalence: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 20, 2809–2817.e28. doi: 10.1016/j.cgh.2021.12.002
Ledford H. (2024). First US drug approved for a liver disease surging around the world. Nature. doi: 10.1038/d41586-024-00747-9
Li X., Liu H., Cheng W., Wang J., Zhang H., Lu F., et al. (2020). Junceellolide B, a novel inhibitor of Hepatitis B virus. Bioorg. Med. Chem. 28, 115603. doi: 10.1016/j.bmc.2020.115603
Li L., Liu D. W., Yan H. Y., Wang Z. Y., Zhao S. H., Wang B. (2016). Obesity is an independent risk factor for non-alcoholic fatty liver disease: evidence from a meta-analysis of 21 cohort studies. Obes. Rev. 17, 510–519. doi: 10.1111/obr.12407
Li Y., Liu J., Ye B., Cui Y., Geng R., Liu S., et al. (2022). Astaxanthin alleviates nonalcoholic fatty liver disease by regulating the intestinal flora and targeting the AMPK/nrf2 signal axis. J. Agric. Food Chem. 70, 10620–10634. doi: 10.1021/acs.jafc.2c04476
Liaset B., Øyen J., Jacques H., Kristiansen K., Madsen L. (2019). Seafood intake and the development of obesity, insulin resistance and type 2 diabetes. Nutr. Res. Rev. 32, 146–167. doi: 10.1017/s0954422418000240
Lin Y., Wu X., Feng S., Jiang G., Luo J., Zhou S., et al. (2001). Five unique compounds: xyloketals from mangrove fungus xylaria sp. from the South China sea coast. J. Org Chem. 66, 6252–6256. doi: 10.1021/jo015522r
Lin Z., Yang P., Hu Y., Xu H., Duan J., He F., et al. (2023). RING finger protein 13 protects against nonalcoholic steatohepatitis by targeting STING-relayed signaling pathways. Nat. Commun. 14, 6635. doi: 10.1038/s41467-023-42420-1
Liu X., Cui J., Li Z., Xu J., Wang J., Xue C., et al. (2014). Comparative study of DHA-enriched phospholipids and EPA-enriched phospholipids on metabolic disorders in diet-induced-obese C57BL/6J mice. Eur. J. Lipid Sci. Technol. 116, 255–265. doi: 10.1002/ejlt.201300407
Liu J., Gao S., Zhou W., Chen Y., Wang Z., Zeng Z., et al. (2023a). Dihydrotrichodimerol purified from the marine fungus acremonium citrinum prevents NAFLD by targeting PPARα. J. Nat. Prod 86, 1189–1201. doi: 10.1021/acs.jnatprod.2c00990
Liu T., Li Q., Xu X., Li G., Tian C., Zhang T. (2022b). Molecular mechanisms of anti-cancer bioactivities of seaweed polysaccharides. Chin. Herb. Med. 14, 528–534. doi: 10.1016/j.chmed.2022.02.003
Liu Y., Meng-Ting Z., Xiao-Chen L., Hai-Yang Z., Hong-Mei H. (2022c). Research progress on the mechanism of vitamin D3 in the occurrence and development of nonalcoholic fatty liver disease. J. Biol. Regul. Homeost. Agents 36, 797–805. doi: 10.23812/j.biol.regul.homeost.agents.20223604.90
Liu D., Ren Y., Zhong S., Xu B. (2024). New Insight into Utilization of Fish By-Product Proteins and Their Skin Health Promoting Effects. Mar. Drugs 22, 215. doi: 10.3390/md22050215
Liu Y., Shi D., Liu Y., Zhao Y., Tian Y., Xu J., et al. (2016). Eicosapentaenoic acid-containing phosphatidylcholine alleviated lipid accumulation in orotic acid-induced non-alcoholic fatty liver. J. Funct. Foods 23, 294–305. doi: 10.1016/j.jff.2016.02.041
Liu J., Tan L., Liu Z., Shi R. (2022a). The association between non-alcoholic fatty liver disease (NAFLD) and advanced fibrosis with blood selenium level based on the NHANES 2017-2018. Ann. Med. 54, 2259–2268. doi: 10.1080/07853890.2022.2110277
Liu J., Wu H., Zhang Y., Hu C., Zhen D., Fu P., et al. (2023b). Phycobiliprotein Peptide Extracts from Arthrospira platensis Ameliorate Nonalcoholic Fatty Liver Disease by Modulating Hepatic Lipid Profile and Strengthening Fat Mobilization. Nutrients 15, 4573. doi: 10.3390/nu15214573
Liu Y., Zhang D., Liu G.-M., Chen Q., Lu Z. (2019). Ameliorative effect of dieckol-enriched extraction from Laminaria japonica on hepatic steatosis induced by a high-fat diet via β-oxidation pathway in ICR mice. J. Funct. Foods 58, 44–55. doi: 10.1016/j.jff.2019.04.051
Liyanage N. M., Nagahawatta D. P., Jayawardena T. U., Sanjeewa K. K. A., Jayawrdhana H., Kim J. I., et al. (2023). Sulfated polysaccharides from seaweeds: A promising strategy for combatting viral diseases-A review. Mar. Drugs 21, 461. doi: 10.3390/md21090461
Llorente C., Schnabl B. (2015). The gut microbiota and liver disease. Cell Mol. Gastroenterol. Hepatol. 1, 275–284. doi: 10.1016/j.jcmgh.2015.04.003
Lomartire S., Gonçalves A. M. M. (2023). Marine macroalgae polyphenols as potential neuroprotective antioxidants in neurodegenerative diseases. Mar. Drugs 21, 261. doi: 10.3390/md21050261
Loomba R., Friedman S. L., Shulman G. I. (2021). Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 184, 2537–2564. doi: 10.1016/j.cell.2021.04.015
Lopes G., Andrade P. B., Valentão P. (2016). Phlorotannins: towards new pharmacological interventions for diabetes mellitus type 2. Molecules 22, 56. doi: 10.3390/molecules22010056
Lozano Muñoz I., Díaz N. F. (2022). Minerals in edible seaweed: health benefits and food safety issues. Crit. Rev. Food Sci. Nutr. 62, 1592–1607. doi: 10.1080/10408398.2020.1844637
Ludwig M., Enzenhofer E., Schneider S., Rauch M., Bodenteich A., Neumann K., et al. (2013). Efficacy of a carrageenan nasal spray in patients with common cold: a randomized controlled trial. Respir. Res. 14, 124. doi: 10.1186/1465-9921-14-124
Luo X., He Z., Sun X., Gu X., Zhang W., Gao J., et al. (2020). DHA protects against hepatic steatosis by activating sirt1 in a high fat diet-induced nonalcoholic fatty liver disease mouse model. Diabetes Metab. Syndr. Obes. 13, 185–196. doi: 10.2147/dmso.S232279
Lyu K., Zhang Y., Zhang D., Kahn M., Ter Horst K. W., Rodrigues M. R. S., et al. (2020). A membrane-bound diacylglycerol species induces PKCϵ-mediated hepatic insulin resistance. Cell Metab. 32, 654–664.e5. doi: 10.1016/j.cmet.2020.08.001
Ma L., Diao A. (2015). Marizomib, a potent second generation proteasome inhibitor from natural origin. Anticancer Agents Med. Chem. 15, 298–306. doi: 10.2174/1871520614666141114202606
Maeda H., Hosomi R., Yokoyama T., Ikeda Y., Nishimoto A., Tanaka G., et al. (2020). Dietary Alaska pollock protein attenuates liver steatosis and alters gut microbiota in leptin-deficient ob/ob mice. J. Funct. Foods 75, 104266. doi: 10.1016/j.jff.2020.104266
Maresca R., Mignini I., Varca S., Calvez V., Termite F., Esposto G., et al. (2024). Inflammatory bowel diseases and non-alcoholic fatty liver disease: piecing a complex puzzle together. Int. J. Mol. Sci. 25, 3278. doi: 10.3390/ijms25063278
Markham A. (2020). Lurbinectedin: first approval. Drugs 80, 1345–1353. doi: 10.1007/s40265-020-01374-0
Marshall J. C. (1998). The gut as a potential trigger of exercise-induced inflammatory responses. Can. J. Physiol. Pharmacol. 76, 479–484. doi: 10.1139/y98-049
Martínez-Díez M., Guillén-Navarro M. J., Pera B., Bouchet B. P., Martínez-Leal J. F., Barasoain I., et al. (2014). PM060184, a new tubulin binding agent with potent antitumor activity including P-glycoprotein over-expressing tumors. Biochem. Pharmacol. 88, 291–302. doi: 10.1016/j.bcp.2014.01.026
Masoudian Khouzani M. R., Shushizadeh M. R., Kalantari H., Rajabzadeh Ghotrami E. (2019). Hepatoprotective effect of aqueous extract of Persian Gulf brown algae sargassum swartzii against acetaminophen-induced hepatotoxicity in mice. Jundishapur J. Nat. Pharm. Prod 14, e77168. doi: 10.5812/jjnpp.77168
Máximo P., Ferreira L. M., Branco P., Lima P., Lourenço A. (2016). The role of spongia sp. in the discovery of marine lead compounds. Mar. Drugs 14, 139. doi: 10.3390/md14080139
Mayer C., Côme M., Blanckaert V., Chini Zittelli G., Faraloni C., Nazih H., et al. (2020). Effect of carotenoids from phaeodactylum tricornutum on palmitate-treated hepG2 cells. Molecules 25, 2845. doi: 10.3390/molecules25122845
Mehta P. P., Dhapte-Pawar V. S. (2021). Novel and evolving therapies for COVID-19 related pulmonary complications(). Am. J. Med. Sci. 361, 557–566. doi: 10.1016/j.amjms.2021.02.019
Mohamed G. A., Ibrahim S. R. M. (2021). Untapped potential of marine-associated cladosporium species: an overview on secondary metabolites, biotechnological relevance, and biological activities. Mar. Drugs 19, 645. doi: 10.3390/md19110645
Mohan C. D., Rangappa S., Nayak S. C., Jadimurthy R., Wang L., Sethi G., et al. (2022). Bacteria as a treasure house of secondary metabolites with anticancer potential. Semin. Cancer Biol. 86, 998–1013. doi: 10.1016/j.semcancer.2021.05.006
Mohanty B. P., Sankar T. V., Ganguly S., Mahanty A., Anandan R., Chakraborty K., et al. (2016). Micronutrient composition of 35 food fishes from India and their significance in human nutrition. Biol. Trace Elem. Res. 174, 448–458. doi: 10.1007/s12011-016-0714-3
Moreira G. V., Azevedo F. F., Ribeiro L. M., Santos A., Guadagnini D., Gama P., et al. (2018). Liraglutide modulates gut microbiota and reduces NAFLD in obese mice. J. Nutr. Biochem. 62, 143–154. doi: 10.1016/j.jnutbio.2018.07.009
Murray M., Dordevic A. L., Bonham M. P., Ryan L. (2018). Do marine algal polyphenols have antidiabetic, antihyperlipidemic or anti-inflammatory effects in humans? A systematic review. Crit. Rev. Food Sci. Nutr. 58, 2039–2054. doi: 10.1080/10408398.2017.1301876
Murugesan M., Kandhavelu M., Thiyagarajan R., Natesan S., Rajendran P., Murugesan A. (2023). Marine halophyte derived polyphenols inhibit glioma cell growth through mitogen-activated protein kinase signaling pathway. BioMed. Pharmacother. 159, 114288. doi: 10.1016/j.biopha.2023.114288
Nagashimada M., Ota T. (2019). Role of vitamin E in nonalcoholic fatty liver disease. IUBMB Life 71, 516–522. doi: 10.1002/iub.1991
Nassir F., Ibdah J. A. (2014). Role of mitochondria in nonalcoholic fatty liver disease. Int. J. Mol. Sci. 15, 8713–8742. doi: 10.3390/ijms15058713
Newman D. J., Cragg G. M., Snader K. M. (2000). The influence of natural products upon drug discovery (Antiquity to late 1999). Nat. Prod Rep. 17, 215–234. doi: 10.1039/a902202c
Ni Y., Qian L., Siliceo S. L., Long X., Nychas E., Liu Y., et al. (2023). Resistant starch decreases intrahepatic triglycerides in patients with NAFLD via gut microbiome alterations. Cell Metab. 35, 1530–1547.e8. doi: 10.1016/j.cmet.2023.08.002
Ni Y., Zhuge F., Nagashimada M., Ota T. (2016). Novel action of carotenoids on non-alcoholic fatty liver disease: macrophage polarization and liver homeostasis. Nutrients 8, 391. doi: 10.3390/nu8070391
Niu S., Chen S., Chen X., Ren Q., Yue L., Pan X., et al. (2022). Semaglutide ameliorates metabolism and hepatic outcomes in an NAFLD mouse model. Front. Endocrinol. (Lausanne) 13. doi: 10.3389/fendo.2022.1046130
Nobili V., Bedogni G., Alisi A., Pietrobattista A., Risé P., Galli C., et al. (2011). Docosahexaenoic acid supplementation decreases liver fat content in children with non-alcoholic fatty liver disease: double-blind randomised controlled clinical trial. Arch. Dis. Child 96, 350–353. doi: 10.1136/adc.2010.192401
Orfanoudaki M., Hartmann A., Alilou M., Mehic N., Kwiatkowski M., Jöhrer K., et al. (2021). Cytotoxic Compounds of Two Demosponges (Aplysina aerophoba and Spongia sp.) from the Aegean Sea. Biomolecules 11, 723. doi: 10.3390/biom11050723
Pabst O., Hornef M. W., Schaap F. G., Cerovic V., Clavel T., Bruns T. (2023). Gut-liver axis: barriers and functional circuits. Nat. Rev. Gastroenterol. Hepatol. 20, 447–461. doi: 10.1038/s41575-023-00771-6
Pacifico L., Osborn J. F., Bonci E., Pierimarchi P., Chiesa C. (2019). Association between vitamin D levels and nonalcoholic fatty liver disease: potential confounding variables. Mini Rev. Med. Chem. 19, 310–332. doi: 10.2174/1389557518666181025153712
Park E. Y., Choi H., Yoon J. Y., Lee I. Y., Seo Y., Moon H. S., et al. (2015). Polyphenol-rich fraction of ecklonia cava improves nonalcoholic fatty liver disease in high fat diet-fed mice. Mar. Drugs 13, 6866–6883. doi: 10.3390/md13116866
Park H., Dawwas G. K., Liu X., Nguyen M. H. (2019). Nonalcoholic fatty liver disease increases risk of incident advanced chronic kidney disease: a propensity-matched cohort study. J. Intern. Med. 286, 711–722. doi: 10.1111/joim.12964
Park S. Y., Jin M. L., Ko M. J., Park G., Choi Y.-W. (2016). Anti-neuroinflammatory effect of emodin in LPS-stimulated microglia: involvement of AMPK/nrf2 activation. Neurochem. Res. 41, 2981–2992. doi: 10.1007/s11064-016-2018-6
Pisonero-Vaquero S., González-Gallego J., Sánchez-Campos S., García-Mediavilla M. V. (2015). Flavonoids and related compounds in non-alcoholic fatty liver disease therapy. Curr. Med. Chem. 22, 2991–3012. doi: 10.2174/0929867322666150805094940
Pomin V. (2011). Seaweed: Ecology, Nutrient Composition and Medicinal Uses (New York: Nova Science).
Poppelreuther M., Lundsgaard A. M., Mensberg P., Sjøberg K., Vilsbøll T., Kiens B., et al. (2023). Acyl-CoA synthetase expression in human skeletal muscle is reduced in obesity and insulin resistance. Physiol. Rep. 11, e15817. doi: 10.14814/phy2.15817
Qi L., Du H. F., Sun T. T., Li L., Zhang Y. H., Liu Y. F., et al. (2023). Natural products from marine fungi as a source against agricultural pathogenic fungi. Appl. Microbiol. Biotechnol. 107, 5003–5017. doi: 10.1007/s00253-023-12657-3
Qian M., Lyu Q., Liu Y., Hu H., Wang S., Pan C., et al. (2019). Chitosan oligosaccharide ameliorates nonalcoholic fatty liver disease (NAFLD) in diet-induced obese mice. Mar. Drugs 17, 391. doi: 10.3390/md17070391
Rajan D. K., Mohan K., Zhang S., Ganesan A. R. (2021). Dieckol: a brown algal phlorotannin with biological potential. Biomed. Pharmacother. 142, 111988. doi: 10.1016/j.biopha.2021.111988
Rao Y., Su R., Wu C., Chai X., Li J., Yang G., et al. (2024). Identification of a natural PLA2 inhibitor from the marine fungus Aspergillus sp. c1 for MAFLD treatment that suppressed lipotoxicity by inhibiting the IRE-1α/XBP-1s axis and JNK signaling. Acta Pharm. Sin. B 14, 304–318. doi: 10.1016/j.apsb.2023.08.032
Rasul A., Millimouno F. M., Ali Eltayb W., Ali M., Li J., Li X. (2013). Pinocembrin: A novel natural compound with versatile pharmacological and biological activities. BioMed. Res. Int. 2013, 1–9. doi: 10.1155/2013/379850
Rathod N. B., Elabed N., Punia S., Ozogul F., Kim S. K., Rocha J. M. (2023). Recent developments in polyphenol applications on human health: A review with current knowledge. Plants (Basel) 12, 1217. doi: 10.3390/plants12061217
Reda D., Elshopakey G. E., Albukhari T. A., Almehmadi S. J., Refaat B., Risha E. F., et al. (2023). Vitamin D3 alleviates nonalcoholic fatty liver disease in rats by inhibiting hepatic oxidative stress and inflammation via the SREBP-1-c/PPARα-NF-κB/IR-S2 signaling pathway. Front. Pharmacol. 14. doi: 10.3389/fphar.2023.1164512
Ren R., Gong J., Zhao Y., Zhuang X., Ye Y., Lin W. (2017). Sulfated polysaccharides from Enteromorpha prolifera suppress SREBP-2 and HMG-CoA reductase expression and attenuate non-alcoholic fatty liver disease induced by a high-fat diet. Food Funct. 8, 1899–1904. doi: 10.1039/C7FO00103G
Ren R., Yang Z., Zhao A., Huang Y., Lin S., Gong J., et al. (2018). Sulfated polysaccharide from Enteromorpha prolifera increases hydrogen sulfide production and attenuates non-alcoholic fatty liver disease in high-fat diet rats. Food Funct. 9, 4376–4383. doi: 10.1039/c8fo00518d
Rinella M. E., Lazarus J. V., Ratziu V., Francque S. M., Sanyal A. J., Kanwal F., et al. (2023). A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 79, 1542–1556. doi: 10.1016/j.jhep.2023.06.003
Rodriguez-Ramiro I., Vauzour D., Minihane A. M. (2016). Polyphenols and non-alcoholic fatty liver disease: impact and mechanisms. Proc. Nutr. Soc. 75, 47–60. doi: 10.1017/s0029665115004218
Romeo S., Kozlitina J., Xing C., Pertsemlidis A., Cox D., Pennacchio L. A., et al. (2008). Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 40, 1461–1465. doi: 10.1038/ng.257
Rotman Y., Sanyal A. J. (2017). Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut 66, 180–190. doi: 10.1136/gutjnl-2016-312431
Roy V. C., Park J.-S., Ho T. C., Chun B.-S. (2022). Lipid indexes and quality evaluation of omega-3 rich oil from the waste of Japanese Spanish mackerel extracted by supercritical CO2. Mar. Drugs 20, 70. doi: 10.3390/md20010070
Saini R. K., Keum Y. S. (2018). Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance - A review. Life Sci. 203, 255–267. doi: 10.1016/j.lfs.2018.04.049
Sánchez-Parra E., Boutarfa S., Aboal M. (2020). Are cyanotoxins the only toxic compound potentially present in microalgae supplements? Results from a study of ecological and non-ecological products. Toxins (Basel) 12, 552. doi: 10.3390/toxins12090552
Santos J. D., Vitorino I., de la Cruz M., Díaz C., Cautain B., Annang F., et al. (2019). Bioactivities and extract dereplication of actinomycetales isolated from marine sponges. Front. Microbiol. 10. doi: 10.3389/fmicb.2019.00727
Sayuti N. H., Muhammad Nawawi K. N., Goon J. A., Mokhtar N. M., Makpol S., Tan J. K. (2023). A review of the effects of fucoxanthin on NAFLD. Nutrients 15, 1954. doi: 10.3390/nu15081954
Scorletti E., Bhatia L., McCormick K. G., Clough G. F., Nash K., Hodson L., et al. (2014). Effects of purified eicosapentaenoic and docosahexaenoic acids in nonalcoholic fatty liver disease: results from the Welcome* study. Hepatology 60, 1211–1221. doi: 10.1002/hep.27289
Senter P. D., Sievers E. L. (2012). The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nat. Biotechnol. 30, 631–637. doi: 10.1038/nbt.2289
Shama S., Liu W. (2020). Omega-3 fatty acids and gut microbiota: A reciprocal interaction in nonalcoholic fatty liver disease. Dig. Dis. Sci. 65, 906–910. doi: 10.1007/s10620-020-06117-5
Shirouchi B., Kawahara Y., Kutsuna Y., Higuchi M., Okumura M., Mitsuta S., et al. (2023). Oral administration of chaetoceros gracilis—A marine microalga—Alleviates hepatic lipid accumulation in rats fed a high-sucrose and cholesterol-containing diet. Metabolites 13, 436. doi: 10.3390/metabo13030436
Sirot V., Leblanc J. C., Margaritis I. (2012). A risk-benefit analysis approach to seafood intake to determine optimal consumption. Br. J. Nutr. 107, 1812–1822. doi: 10.1017/s0007114511005010
Song Y., Dou H., Gong W., Liu X., Yu Z., Li E., et al. (2013). Bis-N-norgliovictin, a small-molecule compound from marine fungus, inhibits LPS-induced inflammation in macrophages and improves survival in sepsis. Eur. J. Pharmacol. 705, 49–60. doi: 10.1016/j.ejphar.2013.02.008
Sowunmi A. R., Folayan C. O., Anafi F. O., Ajayi O. A., Omisanya N. O., Obada D. O., et al. (2018). Dataset on the comparison of synthesized and commercial zeolites for potential solar adsorption refrigerating system. Data Brief 20, 90–95. doi: 10.1016/j.dib.2018.07.040
Stincone P., Brandelli A. (2020). Marine bacteria as source of antimicrobial compounds. Crit. Rev. Biotechnol. 40, 306–319. doi: 10.1080/07388551.2019.1710457
Stone R. M., Manley P. W., Larson R. A., Capdeville R. (2018). Midostaurin: its odyssey from discovery to approval for treating acute myeloid leukemia and advanced systemic mastocytosis. Blood Adv. 2, 444–453. doi: 10.1182/bloodadvances.2017011080
Sumida Y., Yoneda M., Seko Y., Takahashi H., Hara N., Fujii H., et al. (2021). Role of vitamin E in the treatment of non-alcoholic steatohepatitis. Free Radic. Biol. Med. 177, 391–403. doi: 10.1016/j.freeradbiomed.2021.10.017
Takahashi Y., Konishi T., Nishimura M., Nishihira J. (2022). Evaluation of the efficacy and safety of chum salmon milt deoxyribonucleic acid for improvement of hepatic functions: a placebo-controlled, randomised, double-blind, and parallel-group, pilot clinical trial. Food Funct. 13, 9372–9382. doi: 10.1039/d2fo01145j
Targher G., Byrne C. D., Tilg H. (2020). NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut 69, 1691–1705. doi: 10.1136/gutjnl-2020-320622
The Lancet Gastroenterology Hepatology (2024). Resmetirom for NASH: balancing promise and prudence. Lancet Gastroenterol. Hepatol. 9, 273. doi: 10.1016/s2468-1253(24)00049-9
Thomas J. A., Kendall B. J., El-Serag H. B., Thrift A. P., Macdonald G. A. (2024). Hepatocellular and extrahepatic cancer risk in people with non-alcoholic fatty liver disease. Lancet Gastroenterol. Hepatol. 9, 159–169. doi: 10.1016/S2468-1253(23)00275-3
Tian C., Min X., Zhao Y., Wang Y., Wu X., Liu S., et al. (2022). MRG15 aggravates non-alcoholic steatohepatitis progression by regulating the mitochondrial proteolytic degradation of TUFM. J. Hepatol. 77, 1491–1503. doi: 10.1016/j.jhep.2022.07.017
Tilg H., Adolph T. E., Dudek M., Knolle P. (2021). Non-alcoholic fatty liver disease: the interplay between metabolism, microbes and immunity. Nat. Metab. 3, 1596–1607. doi: 10.1038/s42255-021-00501-9
Tong Y., Zhu W., Wen T., Mukhamejanova Z., Xu F., Xiang Q., et al. (2022). Xyloketal B reverses nutritional hepatic steatosis, steatohepatitis, and liver fibrosis through activation of the PPARα/PGC1α Signaling pathway. J. Nat. Prod 85, 1738–1750. doi: 10.1021/acs.jnatprod.2c00259
Tvrzicka E., Kremmyda L. S., Stankova B., Zak A. (2011). Fatty acids as biocompounds: their role in human metabolism, health and disease–a review. Part 1: classification, dietary sources and biological functions. BioMed. Pap Med. Fac Univ. Palacky Olomouc Czech Repub 155, 117–130. doi: 10.5507/bp.2011.038
Valenti L., Dongiovanni P. (2017). Mutant PNPLA3 I148M protein as pharmacological target for liver disease. Hepatology 66, 1026–1028. doi: 10.1002/hep.29298
Van Den Bent M., Eoli M., Sepulveda J. M., Smits M., Walenkamp A., Frenel J. S., et al. (2020). INTELLANCE 2/EORTC 1410 randomized phase II study of Depatux-M alone and with temozolomide vs temozolomide or lomustine in recurrent EGFR amplified glioblastoma. Neuro Oncol. 22, 684–693. doi: 10.1093/neuonc/noz222
Vandermeersch G., Lourenço H. M., Alvarez-Muñoz D., Cunha S., Diogène J., Cano-Sancho G., et al. (2015). Environmental contaminants of emerging concern in seafood–European database on contaminant levels. Environ. Res. 143, 29–45. doi: 10.1016/j.envres.2015.06.011
Vell M. S., Creasy K. T., Scorletti E., Seeling K. S., Hehl L., Rendel M. D., et al. (2023). Omega-3 intake is associated with liver disease protection. Front. Public Health 11. doi: 10.3389/fpubh.2023.1192099
Vladkova T., Georgieva N., Staneva A., Gospodinova D. (2022). Recent progress in antioxidant active substances from marine biota. Antioxid. (Basel) 11, 439. doi: 10.3390/antiox11030439
Vogli S., Naska A., Marinos G., Kasdagli M. I., Orfanos P. (2023). The effect of vitamin E supplementation on serum aminotransferases in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis. Nutrients 15, 3733. doi: 10.3390/nu15173733
Wade R., Augyte S., Harden M., Nuzhdin S., Yarish C., Alberto F. (2020). Macroalgal germplasm banking for conservation, food security, and industry. PloS Biol. 18, e3000641. doi: 10.1371/journal.pbio.3000641
Wan Y., Liu L., Zhang B., Wang S., Wang X., Chen K., et al. (2022). Structural characterization and anti-nonalcoholic fatty liver effect of high-sulfated ulva pertusa polysaccharide. Pharm. (Basel) 16, 62. doi: 10.3390/ph16010062
Wang X. C., Gusdon A. M., Liu H., Qu S. (2014). Effects of glucagon-like peptide-1 receptor agonists on non-alcoholic fatty liver disease and inflammation. World J. Gastroenterol. 20, 14821–14830. doi: 10.3748/wjg.v20.i40.14821
Wang J., He W., Tsai P. J., Chen P. H., Ye M., Guo J., et al. (2020a). Mutual interaction between endoplasmic reticulum and mitochondria in nonalcoholic fatty liver disease. Lipids Health Dis. 19, 72. doi: 10.1186/s12944-020-01210-0
Wang P. X., Ji Y. X., Zhang X. J., Zhao L. P., Yan Z. Z., Zhang P., et al. (2017b). Targeting CASP8 and FADD-like apoptosis regulator ameliorates nonalcoholic steatohepatitis in mice and nonhuman primates. Nat. Med. 23, 439–449. doi: 10.1038/nm.4290
Wang P.-X., Ji Y.-X., Zhang X.-J., Zhao L.-P., Yan Z.-Z., Zhang P., et al. (2017c). Correction: Corrigendum: Targeting CASP8 and FADD-like apoptosis regulator ameliorates nonalcoholic steatohepatitis in mice and nonhuman primates. Nat. Med. 23, 1241. doi: 10.1038/nm1017-1241b
Wang X., Liu D., Wang Z., Cai C., Jiang H., Yu G. (2021b). Porphyran-derived oligosaccharides alleviate NAFLD and related cecal microbiota dysbiosis in mice. FASEB J. 35, e21458. doi: 10.1096/fj.202000763RRR
Wang C., Niederstrasser H., Douglas P. M., Lin R., Jaramillo J., Li Y., et al. (2017a). Small-molecule TFEB pathway agonists that ameliorate metabolic syndrome in mice and extend C. elegans lifespan. Nat. Commun. 8, 2270. doi: 10.1038/s41467-017-02332-3
Wang F., So K. F., Xiao J., Wang H. (2021a). Organ-organ communication: The liver’s perspective. Theranostics 11, 3317–3330. doi: 10.7150/thno.55795
Wang X., Wang X., Jiang H., Cai C., Li G., Hao J., et al. (2018). Marine polysaccharides attenuate metabolic syndrome by fermentation products and altering gut microbiota: An overview. Carbohydr. Polym. 195, 601–612. doi: 10.1016/j.carbpol.2018.05.003
Wang Y., Wozniak A., Wellens J., Gebreyohannes Y. K., Guillén M. J., Avilés P. M., et al. (2020b). Plocabulin, a novel tubulin inhibitor, has potent antitumor activity in patient-derived xenograft models of gastrointestinal stromal tumors. Transl. Oncol. 13, 100832. doi: 10.1016/j.tranon.2020.100832
Wang W., Xu C., Wang Q., Hussain M. A., Wang C., Hou J., et al. (2023). Protective effect of polyphenols, protein, peptides, and polysaccharides on alcoholic liver disease: A review of research status and molecular mechanisms. J. Agric. Food Chem. 71, 5861–5883. doi: 10.1021/acs.jafc.2c07081
Wang Z., Zang R., Niu Z., Wang W., Wang X., Tang Y. (2021c). Synthesis and antiviral effect of phosphamide modified vidarabine for treating HSV 1 infections. Bioorg. Med. Chem. Lett. 52, 128405. doi: 10.1016/j.bmcl.2021.128405
Wang Q., Zhou H., Bu Q., Wei S., Li L., Zhou J., et al. (2022). Role of XBP1 in regulating the progression of non-alcoholic steatohepatitis. J. Hepatol. 77, 312–325. doi: 10.1016/j.jhep.2022.02.031
Wei Q., Fu G., Wang K., Yang Q., Zhao J., Wang Y., et al. (2022). Advances in research on antiviral activities of sulfated polysaccharides from seaweeds. Pharm. (Basel) 15, 581. doi: 10.3390/ph15050581
Wei Y., Tian C., Zhao Y., Liu X., Liu F., Li S., et al. (2020). MRG15 orchestrates rhythmic epigenomic remodelling and controls hepatic lipid metabolism. Nat. Metab. 2, 447–460. doi: 10.1038/s42255-020-0203-z
Wen Y., Zhou Y., Tian L., He Y. (2024). Ethanol extracts of Isochrysis zhanjiangensis alleviate acute alcoholic liver injury and modulate intestinal bacteria dysbiosis in mice. J. Sci. Food Agric. 104, 4354–4362. doi: 10.1002/jsfa.13321
Williams J. A., Day M., Heavner J. E. (2008). Ziconotide: an update and review. Expert Opin. Pharmacother. 9, 1575–1583. doi: 10.1517/14656566.9.9.1575
Wu Y., Jin X., Zhang Y., Liu J., Wu M., Tong H. (2023b). Bioactive compounds from brown algae alleviate nonalcoholic fatty liver disease: an extensive review. J. Agric. Food Chem. 71, 1771–1787. doi: 10.1021/acs.jafc.2c06578
Wu L., Mo W., Feng J., Li J., Yu Q., Li S., et al. (2020). Astaxanthin attenuates hepatic damage and mitochondrial dysfunction in non-alcoholic fatty liver disease by up-regulating the FGF21/PGC-1α pathway. Br. J. Pharmacol. 177, 3760–3777. doi: 10.1111/bph.15099
Wu J., Shao H., Zhang J., Ying Y., Cheng Y., Zhao D., et al. (2019). Mussel polysaccharide α-D-glucan (MP-A) protects against non-alcoholic fatty liver disease via maintaining the homeostasis of gut microbiota and regulating related gut-liver axis signaling pathways. Int. J. Biol. Macromol. 130, 68–78. doi: 10.1016/j.ijbiomac.2019.02.097
Wu M.-F., Xi Q.-H., Sheng Y., Wang Y.-M., Wang W.-Y., Chi C.-F., et al. (2023a). Antioxidant Peptides from Monkfish Swim Bladders: Ameliorating NAFLD In Vitro by Suppressing Lipid Accumulation and Oxidative Stress via Regulating AMPK/Nrf2 Pathway. Mar. Drugs 21, 360. doi: 10.3390/md21060360.
Wu C., Xiang S., Wang H., Zhang X., Tian X., Tan M., et al. (2024a). Orally deliverable sequence-targeted fucoxanthin-loaded biomimetic extracellular vesicles for alleviation of nonalcoholic fatty liver disease. ACS Appl. Mater Interf. 16, 9854–9867. doi: 10.1021/acsami.3c18029
Wu Y., Yan Q., Yue S., Pan L., Yang D., Tao L., et al. (2024b). NUP85 alleviates lipid metabolism and inflammation by regulating PI3K/AKT signaling pathway in nonalcoholic fatty liver disease. Int. J. Biol. Sci. 20, 2219–2235. doi: 10.7150/ijbs.92337
Xiao Y. L., Gong Y., Qi Y. J., Shao Z. M., Jiang Y. Z. (2024). Effects of dietary intervention on human diseases: molecular mechanisms and therapeutic potential. Signal Transduct. Target Ther. 9, 581. doi: 10.1038/s41392-024-01771-x
Xie C., Lee Z. J., Ye S., Barrow C. J., Dunshea F. R., Suleria H. A. R. (2023). A review on seaweeds and seaweed-derived polysaccharides: nutrition, chemistry, bioactivities, and applications. Food Rev. Int. 1–36, 1312–1347. doi: 10.1080/87559129.2023.2212055
Xu J., Liao W., Yang S., Liu J., Jiang S., Liu Y., et al. (2024). Ulva lactuca polysaccharide inhibits hepatocellular carcinoma growth by induces the expression of CD5L and activates complement cascade. J. Funct. Foods 112, 105994. doi: 10.1016/j.jff.2023.105994
Xu L., Meng W., Cao C., Wang J., Shan W., Wang Q. (2015a). Antibacterial and antifungal compounds from marine fungi. Mar. Drugs 13, 3479–3513. doi: 10.3390/md13063479
Xu Y., Zalzala M., Xu J., Li Y., Yin L., Zhang Y. (2015b). A metabolic stress-inducible miR-34a-HNF4α pathway regulates lipid and lipoprotein metabolism. Nat. Commun. 6, 7466. doi: 10.1038/ncomms8466
Yanagita T., Tsuge K., Koga M., Inoue N., Nagao K. (2020). Eicosapentaenoic acid-containing polar lipids from seaweed Susabinori (Pyropia yezoensis) alleviate hepatic steatosis in obese db/db mice. Arch. Biochem. Biophys. 691, 108486. doi: 10.1016/j.abb.2020.108486
Yang X., Li A., Li D., Guo Y., Sun L. (2021b). Applications of mixed polysaccharide-protein systems in fabricating multi-structures of binary food gels—A review. Trends Food Sci. Technol. 109, 197–210. doi: 10.1016/j.tifs.2021.01.002
Yang M., Qi X., Li N., Kaifi J. T., Chen S., Wheeler A. A., et al. (2023). Western diet contributes to the pathogenesis of non-alcoholic steatohepatitis in male mice via remodeling gut microbiota and increasing production of 2-oleoylglycerol. Nat. Commun. 14, 228. doi: 10.1038/s41467-023-35861-1
Yang J., Sáinz N., Félix-Soriano E., Gil-Iturbe E., Castilla-Madrigal R., Fernández-Galilea M., et al. (2021a). Effects of long-term DHA supplementation and physical exercise on non-alcoholic fatty liver development in obese aged female mice. Nutrients 13, 501. doi: 10.3390/nu13020501
Yang X., Yao S., Jiang Q., Chen H., Liu S., Shen G., et al. (2024). Exploring the regulatory effect of tegillarca granosa polysaccharide on high-fat diet-induced non-alcoholic fatty liver disease in mice based on intestinal flora. Mol. Nutr. Food Res. 68, 2300453. doi: 10.1002/mnfr.202300453
Yao M., Qv L., Lu Y., Wang B., Berglund B., Li L. (2021). An update on the efficacy and functionality of probiotics for the treatment of non-alcoholic fatty liver disease. Engineering 7, 679–686. doi: 10.1016/j.eng.2020.01.017
Ye J., Tian X., Wang Q., Zheng J., Yang Y., Xu B., et al. (2022a). Monkfish peptides mitigate high fat diet-induced hepatic steatosis in mice. Mar. Drugs 20, 312. doi: 10.3390/md20050312
Ye J., Zheng J., Tian X., Xu B., Yuan F., Wang B., et al. (2022b). Fucoxanthin attenuates free fatty acid-induced nonalcoholic fatty liver disease by regulating lipid metabolism/oxidative stress/inflammation via the AMPK/nrf2/TLR4 signaling pathway. Mar. Drugs 20, 225. doi: 10.3390/md20040225
Younossi Z. M., Koenig A. B., Abdelatif D., Fazel Y., Henry L., Wymer M. (2016). Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64, 73–84. doi: 10.1002/hep.28431
Yu S., Xie Q., Tan W., Hu M., Xu G., Zhang X., et al. (2023). Different ratios of DHA/EPA reverses insulin resistance by improving adipocyte dysfunction and lipid disorders in HFD-induced IR mice. Food Funct. 14, 1179–1197. doi: 10.1039/d2fo02686d
Yuan Z., Ye X., Hou Z., Chen S. (2024). Sustainable utilization of proteins from fish processing by-products: Extraction, biological activities and applications. Trends Food Sci. Technol. 143, 104276. doi: 10.1016/j.tifs.2023.104276
Zenthoefer M., Geisen U., Hofmann-Peiker K., Fuhrmann M., Kerber J., Kirchhöfer R., et al. (2017). Isolation of polyphenols with anticancer activity from the Baltic Sea brown seaweed Fucus vesiculosus using bioassay-guided fractionation. J. Appl. Phycol 29, 2021–2037. doi: 10.1007/s10811-017-1080-z
Zhai M., Liu Z., Long J., Zhou Q., Yang L., Zhou Q., et al. (2021). The incidence trends of liver cirrhosis caused by nonalcoholic steatohepatitis via the GBD study 2017. Sci. Rep. 11, 5195. doi: 10.1038/s41598-021-84577-z
Zhang X., Aweya J. J., Huang Z. X., Kang Z. Y., Bai Z. H., Li K. H., et al. (2020b). In vitro fermentation of Gracilaria lemaneiformis sulfated polysaccharides and its agaro-oligosaccharides by human fecal inocula and its impact on microbiota. Carbohydr. Polym. 234, 115894. doi: 10.1016/j.carbpol.2020.115894
Zhang T. T., Xu J., Wang Y. M., Xue C. H. (2019). Health benefits of dietary marine DHA/EPA-enriched glycerophospholipids. Prog. Lipid Res. 75, 100997. doi: 10.1016/j.plipres.2019.100997
Zhang Y., Yang L., Zhao N., Hong Z., Cai B., Le Q., et al. (2021c). Soluble polysaccharide derived from laminaria japonica attenuates obesity-related nonalcoholic fatty liver disease associated with gut microbiota regulation. Mar. Drugs 19, 699. doi: 10.3390/md19120699
Zhang W., Zhangyuan G., Wang F., Jin K., Shen H., Zhang L., et al. (2021b). The zinc finger protein Miz1 suppresses liver tumorigenesis by restricting hepatocyte-driven macrophage activation and inflammation. Immunity 54, 1168–1185.e8. doi: 10.1016/j.immuni.2021.04.027
Zhang F., Zhao M., Braun D. R., Ericksen S. S., Piotrowski J. S., Nelson J., et al. (2020a). A marine microbiome antifungal targets urgent-threat drug-resistant fungi. Sci. (1979) 370, 974–978. doi: 10.1126/science.abd6919
Zhang S., Zhao J., Xie F., He H., Johnston L. J., Dai X., et al. (2021a). Dietary fiber-derived short-chain fatty acids: A potential therapeutic target to alleviate obesity-related nonalcoholic fatty liver disease. Obes. Rev. 22, e13316. doi: 10.1111/obr.13316
Zhao L. Y., Li J., Huang X. Q., Wang G. H., Lv X. F., Meng W. F., et al. (2018). Xyloketal B exerts antihypertensive effect in renovascular hypertensive rats via the NO-sGC-cGMP pathway and calcium signaling. Acta Pharmacol. Sin. 39, 875–884. doi: 10.1038/aps.2018.12
Zhao C., Lin G., Wu D., Liu D., You L., Högger P., et al. (2020). The algal polysaccharide ulvan suppresses growth of hepatoma cells. Food Front. 1, 83–101. doi: 10.1002/fft2.13
Zheng Y., Liu T., Wang Z., Xu Y., Zhang Q., Luo D. (2018). Low molecular weight fucoidan attenuates liver injury via SIRT1/AMPK/PGC1α axis in db/db mice. Int. J. Biol. Macromol. 112, 929–936. doi: 10.1016/j.ijbiomac.2018.02.072
Zheng J., Peng C., Ai Y., Wang H., Xiao X., Li J. (2016). Docosahexaenoic acid ameliorates fructose-induced hepatic steatosis involving ER stress response in primary mouse hepatocytes. Nutrients 8, 55. doi: 10.3390/nu8010055
Zheng H., Zhao Y., Guo L. (2022). A bioactive substance derived from brown seaweeds: phlorotannins. Mar. Drugs 20, 742. doi: 10.3390/md20120742
Keywords: metabolic disorders, chronic liver disease, lipids, lead compounds, dietary supplements
Citation: Shi M, Chen S, Feng Y, Wang S, Xia Y and He J (2025) Marine natural products as an important source of bioactive substances for non-alcoholic fatty liver disease management. Front. Mar. Sci. 11:1523246. doi: 10.3389/fmars.2024.1523246
Received: 05 November 2024; Accepted: 31 December 2024;
Published: 28 January 2025.
Edited by:
Zhongshan Zhang, Huzhou University, ChinaReviewed by:
Zhong-Ji Qian, Guangdong Ocean University, ChinaCopyright © 2025 Shi, Chen, Feng, Wang, Xia and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianlin He, amxoZUB0aW8ub3JnLmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.