
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 31 March 2023
Sec. Marine Megafauna
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1144398
 Jianqing Lin
Jianqing Lin Yan Liang
Yan Liang Hancheng Zhao
Hancheng Zhao Qilin Gutang
Qilin Gutang Zonghuan Wu
Zonghuan Wu Yan Gao
Yan Gao Sailan Liu
Sailan Liu Kunhuan Li
Kunhuan Li Yinglin Wu
Yinglin Wu Zonghang Zhang
Zonghang Zhang Ping Li
Ping Li Wenhua Liu*
Wenhua Liu*Introduction: Overfishing and climate change have combined to cause fishery stocks to decline and fish community composition to change, further threatening the predation and nutritional health of marine mammals.
Methods: In this study, we collected potential prey fishes catched by fishermen in six habitats of Indo-Pacific humpback dolphins and analyzed their proximate composition (moisture, water, fat and protein), the fatty acid composition and the amino acid composition to evaluate the possible health effect on humpback dolphins.
Results: The results showed that the nutritional composition varied significantly with species and locations. Fishes in the families Sciaenidae and Engraulidae displayed richer fatty acid composition, while those in the family Clupeidae had the highest value of amino acid quality index. In Zhuhai, home to the largest Indo-Pacific humpback dolphin population, pelagic/neritic prey fishes possessed lower energy density, PUFA content, PUFA/SFA ratio, DHA content, and EAA content compared to demersal fish, suggesting nutritional stress when there is a dietary switch from demersal to pelagic/neritic fishes in Zhuhai population.
Discussion: Our study provided a framework, with energy density and fatty acid composition as its most important indicator, for assessment of the marine top predators based on the nutritional composition of their prey fishes and revealed the potential threats. Data here is expected to facilitate the development of scientific programs for successful conservation of not only the Indo-Pacific humpback dolphins, but also other marine top predators, possibly through reconstructing their prey fish’s quantity and quality.
The rapid development of coastal economy and human disturbances pose severe effects on the inshore and estuarine environment (Cheung et al., 2013; Breitburg et al., 2018; He and Silliman, 2019), leading to not only the collapse of fishery resources (Myers and Worm, 2003; Mullon et al., 2005; Dadswell et al., 2021) but also the alteration of fishery structure (Perry et al., 2005; Essington et al., 2006), with a gradual transition from long-lived, high trophic level, piscivorous demersal fish toward short-lived, low trophic level planktivorous pelagic fish (Christensen, 2015). These could further threaten the predation and nutritional health of marine mammals (Avila et al., 2018). Mammals suffering from nutritional stress typically exhibit reduced body size, reduced productivity, high mortality in pups and juveniles, altered blood chemistry, and specific behavioral modifications, because of less fishery resources and the relatively low quality of available prey (Trites and Donnelly, 2003; Österblom et al., 2008).
The Indo-Pacific humpback dolphin (Sousa chinensis) is a coastal mammal that primarily inhabits in warm-temperate estuaries. It is assessed as Vulnerable on the IUCN Red List, where the sum of available abundance estimates adds up to about 5,700 individuals worldwide, with a declining population trend (Jefferson and Smith, 2016; Jefferson et al., 2017). There are around 4000 individuals (70%) inhabited in Chinese waters, with apparently fragmented distribution ranging from Taiwan Strait to Beibu Gulf (Jiulongjiang estuary in Xiamen, Hanjiang River-Rongjiang River estuary in Shantou, the Pearl River estuary (PRE), Dajin Island area in Jiangmen, Jianjiang estuary in Zhanjiang and Sanniang Bay in Qinzhou).
The Indo-Pacific humpback dolphin appears to be opportunistic feeders, consuming a wide variety of nearshore, estuarine, and reef fishes, with preference for shoaling and meaty fishes, such as the spiny head croaker (Collichthys lucida), the flathead grey mullet (Mugil cephalus), croaker (Johnius spp), and anchovies (Thryssa spp) (Jefferson and Smith, 2016; Lin et al., 2021). Most of the humpback dolphin’s prey fish are commercial fish and have been overfished. The total amount of marine fishing in Guangdong, home to the largest population of humpback dolphins, showed a downward trend from 2003 to 2020, according to China Fisheries Statistical Yearbook. While the total number of spiny croakers has increased slightly, the populations of some of the humpback’s other prey fish, including mullet, clupeoid and anchovy, have declined over the last 20 years (Figure S1). In most of the humpback dolphin’s habitat, heavy fishing activity and climate change have combined to cause the fishery resources collapse and fish community composition alteration (Teh et al., 2019; Wang et al., 2022; Yuan et al., 2022). Stomach samples taken from stranded humpback dolphin carcasses covering Lingding Bay and the western Pearl River Delta during 2003 and 2017 indicate not only a prey spectrum wider than those previously reported, but also a dietary switch from primarily demersal to greater intake of neritic and pelagic fish (Lin et al., 2021). Therefore, it is critical to assess the health status of humpback dolphins based on the nutritional status of their prey.
In this study, we analyzed the proximate composition (moisture, water, fat and protein), the fatty acids composition and the amino acid composition of the prey fishes of humpback dolphins and evaluated their nutritional status. The results may facilitate the conservation of this marine mammal through management of their habitat and food.
A total of 14 species of Indo-Pacific humpback dolphins’ prey fishes were collected from the fishermen in six sampling sites between August 11, 2013, and December 15, 2013. We first acquired their fishing route and area to make sure the fish were caught in the corresponding offshore waters. The 14 fish species studied were of 4 family: Sciaenidae (Pseudosciaena crocea, Johnius amblycephalus, Johnius fasciatus, Johnius grypotus, Johnius belangerii, Collichthys lucidus, Argyrosomus argentatus, Pennahia anea), Mugilidae (Mugilcephalus linnaeus, Liza affinis, Liza dussumieri), Clupeidae (Clupanodon thrissa, Konosirus punctatus) and Engraulidae (Coilia mystus). The 14 fish species was classified into three groups: pelagic/neritic, benthopelagic, and demersal fishes (Sanganyado et al., 2018; Yu et al., 2020) (Table 1). Six sampling sites were assigned in the South China Sea coastal waters including Xiamen, Shantou, Zhuhai, Jiangmen, Zhanjiang, and Qinzhou (Figure 1), which have been identified as the major habitats for humpback dolphins (Jefferson and Smith, 2016).
At least four different fish species (n = 5) were collected from each sampling site with a total of 165 fish samples. The samples were stored in an ice chest before being transported to the laboratory for further study. Fish samples were identified, and then weighted and measured. After rinsed with ultrapure water, the samples were stored at -80°C until further treatment.
Moisture determination. The moisture content of the fish was determined through drying the sample at a lyophilizer for nine days to a constant weight, according to the national standard of China GB/T 5009.3 - 2010. The difference in weight before and after drying was divided by the initial weight of the fish sample.
The ash content was determined following the GB/T 5009.4-2010 national standard of China. Each homogenized sample (about 0.1 g) was weighed in a well-dried porcelain basin. The porcelain basin with fish sample was placed into a muffle furnace with temperature of 200°C for one hour until carbonized without smoke. The temperature was raised to 550°C and kept for five hours until there were no carbon granules. When it cools to 200°C, remove the crucible and the sample from the muffle furnace to the desiccator and cool to room temperature. The amount of ash was calculated considering the difference of weight after and before this procedure.
The crude protein was determined using the Kjeldahl method according to the national standard of China GB/T 5009.5 - 2010. Each homogenized sample (about 0.1 g) was heated to 400 °C with 0.7-0.9 g CuSO4 and 10 ml 98% H2SO4 in a digestive tube for 3-4 h. After cooling for 15-20 min, the digestive tube with completely digested sample was subjected to FOSS 1035 automatic nitrogen analyzer. The results were multiplied by the coefficient 6.25.
The crude fat content was estimated using a Soxhlet extraction method based on the national standard of China GB/T 14772 - 2008. Each homogenized sample was placed in an extraction cylinder added with petroleum ether. The extraction cylinder was incubated at 65°C until the sample was completely extracted. Then, the extraction cylinder was put into a rotary evaporator with temperature at 50°C to volatilize organic reagents and dried at 105°C to constant weight. The crude fat content was calculated by dividing the initial weight of the fish sample by the difference in weight after and before this procedure.
The energy densities of prey fishes were based on protein and fat content, since the carbohydrates are considered negligible in fish. Energy density (kJ/g) = (protein content × 5.96 + fat content ×9.50) ×4.2 (Payne et al., 1999).
The lipid extraction was carried out using the method of Folch et al. (Folch et al., 1957). In brief, about 0.1 g (w0) homogenized fish sample was added to 4 mL chloroform: methanol (C-M) (2:1, v/v) in tube A. The tube A was settled at rest at 4°C for 48 h. After centrifugation at 11000 rpm and 4°C for 10 min, the supernatant was transferred to tube B (w1). After 2 μL C-M was added to the residue of tube A, and tube A was centrifuged at 11000 rpm and 4°C for 10 min and the supernatant was transferred to tube B. Then, 1.2 mL of 1.6% CaCl2 was added to the supernatant and mixed. After settling at rest for more than 12 h, the upper phase was removed from tube B. The lower phase of tube B was dried under pure nitrogen flux. The dried tube A was evaporated at 75°C and reweighed (w2). Therefore, the fat content of the sample (%) is calculated as (w2−w1)/w0×100.
Fatty acid methyl esters (FAME) were prepared using boron trifluoride (BF3)-catalyzed methylation method (Ichihara and Fukubayashi, 2010). Firstly, 2 mL of NaOH methanol solution (0.5 M) was added to the pure lipid and the mixture was bathed at 68°C for about 1 h until the oil globule disappeared, then 2 mL methanolic boron trifluoride (15%) was added to the mixture, and bathed at 72 °C for 30 min. Four mL n-hexane was added immediately followed by vigorous shaking for 30 s and bathed for 1 min. Lastly, 1 mL saturated NaCl solution was settled at rest; after stratification, the upper isooctane layer was filtered at 0.45 μm membrane filters, and then diluted with n-hexane to 500 μL.
The fatty acid compositions were determined by gas chromatography (Shimadzu GC-2010) using a flame ionization detector (FID) and a HP-88 column (60 m × 0.25 mm × 0.25 μm). Nitrogen was used as the carrier gas at a flow rate of 30 mL/min. Sample was injected (1 μL) with a split mode (ratio 10:1). Injector temperature and detector temperature were set at 250 and 280°C, respectively. The oven temperature increased from 50°C (1 min) to 175°C (5 min) at a rate of 40°C/min and was further increased at a rate of 3°C/min to a final temperature of 230°C (1 min). Fatty acids were identified with retention time obtained from commercial FAME standards (Sigma Chemical, St. Louis, MO). The relative amount of each fatty acid was calculated from the integrated area of each peak and expressed as the percentage of the total area of all peaks.
The amino acid analysis was performed according to the method of national standard of China GB/T 18246-2000. Frozen-dried samples (0.05 g) were hydrolyzed with 10 mL of 6 M HCl under reduced pressure at 110°C for 24 h. The hydrolysates were cooled to room temperature and pipetted into 10 mL volumetric flask and diluted to 10 mL using 0.02 M HCl. Two milliliters of hydrolysates were pipetted into a 5 mL beaker and heated until dry. Two milliliters of sodium eluent (NA-740) were added. After filtering, the eluent as subjected to a SYKAM 433-D amino acid analyzer. Amino acid contents were expressed as g per 100 g dry sample.
The protein quality indices, such as amino acid score (AAS), chemical score (CS), and essential amino acid index (EAAI), were determined as follows (Wang et al., 2021):
where n is the number of essential amino acids compared, A-I represents the essential amino acid content in fish protein, and AE-IE indicates the amino acids content of chicken egg protein.
The generalized linear model (GLM) was used to test the difference among multiple groups. Duncan’s Multiple-Range Test of a one-way analysis of variance (ANOVA) was used to test the differences between each two groups when the data meet the assumption of no difference in variance for ANOVA, otherwise, nonparametric test (Kruskal-Wallis test) was used.The level of significance was set at p< 0.05. The results are presented as mean values with their standard errors (n = 5), and all above statistical analyses were performed using IBM SPSS 26.0 Statistics (IBM, USA).
The analysis of similarities (ANOSIM) was used to test the difference of community structure of potential prey fishes among provinces (Guangdong, Guangxi and Fujian provinces) and stages (2018-2020, 2012-2014 and 2006-2008). The fishing amount data was collected from China Fisheries Statistical Yearbook published in corresponding year. The level of significance was set at p< 0.05 and R>0.75. The ANOSIM analyses were performed using the R package vagen.
In this study, a total of 14 prey fish species of Indo-Pacific humpback dolphins were collected from their major habitats in the South China Sea (Figure 1; Table 1) and the proximate composition of fishes was determined (Table S1).
The moisture contents of prey fishes varied significantly with species (p< 0.001, GLM). For example, in Zhuhai, the spiny head croaker (Collichthys lucidus) had the highest moisture contents (82.28 ± 0.70%), which was significantly higher than those of other prey fish species with lowest moisture content recorded for the Keled mullet (Liza affinis) (65.32 ± 1.25%). Although the generalized linear model showed that in general the moisture contents of prey fishes were not significantly affected by their habitats (p = 0.2593, GLM), some specific species showed significantly different moisture contents in different habitats. For example, the moisture contents of large yellow croaker (Pseudosciaena crocea) from Shantou (77.76 ± 0.90%) are significantly higher than those from other habitats, especially Jiangmen (63.73 ± 1.09%) (Table S1).
The crude protein and fatty content of prey fishes were also significantly influenced by species and location (p< 0.001, GLM). Taking the large yellow croaker for the example again, the crude protein of the large yellow croaker from Xiamen (12.88 ± 0.48%) were lower than those of not only other species collected in the same habitat but also the large yellow croaker from other sites, especially Shantou (15.89 ± 0.20%) (Table S1). For the crude fat, the content of Keled mullet from Xiamen reached as high as 20.69 ± 1.33% but that from Qinzhou was significantly much lower (3.42 ± 0.60%). The crude fat content of large yellow croaker from Jiangmen (15.49 ± 0.99%) and Zhanjiang (12.98 ± 0.84%) is much higher than those from Xiamen (4.72 ± 0.77%), Shantou (4.82 ± 0.35%) and Zhuhai (6.41 ± 1.17%) (Table S1).
Comparison of the proximal composition among them in Zhuhai (Lingding Bay) and Jiangmen (western PRE) showed that the patterns of fish proximal composition are different between Zhuhai and Jiangmen. In Zhuhai, the crude protein content showed of demersal and benthopelagic fishes were significantly higher than pelagic/neritic fishes (p< 0.05, Kruskal-Wallis test). (Figure 2A), while there was no significant difference in fat contents among species niche group (p > 0.05, one-way ANOVA). Evaluation of the energy density of prey fish based on protein and fat content reversed that the demersal fishes had the highest energy density while the pelagic/neritic displayed the lowest one (Figure 2A). The fishes in Jiangmen had higher crude protein contents than those in Zhuhai, especially those of pelagic/neritic fishes. Unlike the prey fish in Zhuhai, the pelagic/neritic fish in Jiangmen had significantly higher (p< 0.05, Kruskal-Wallis test) fat content and energy density compared to the demersal and benthopelagic groups (Figure 2B).
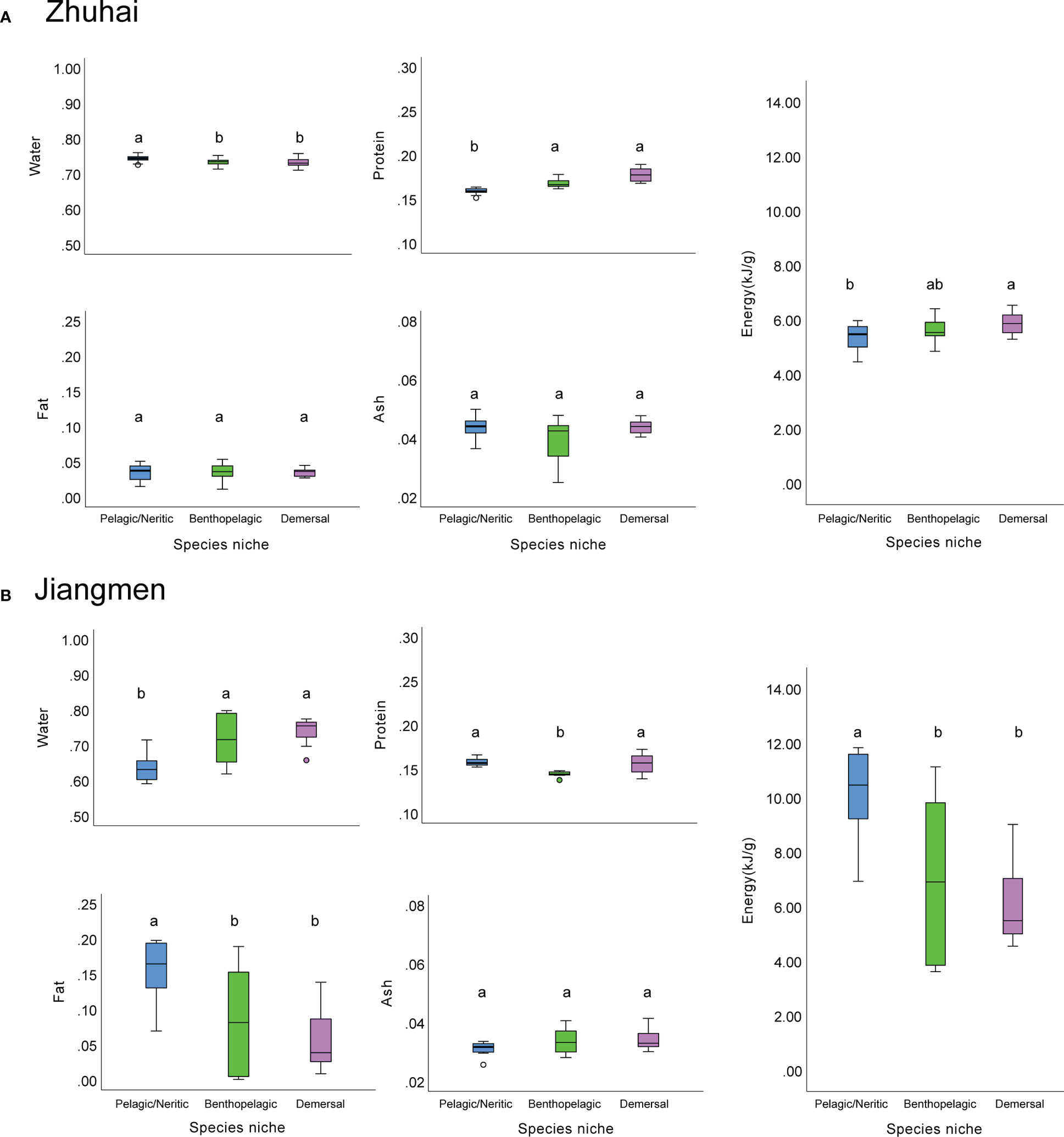
Figure 2 The box plots of proximal composition and energy density of fishes in different species niches from Zhuhai (A) and Jiangmen (B). Different lower-case letters indicate a significant difference among groups.
A total of 23 kinds of the fatty acid were detected from 14 prey fish species of Indo-Pacific humpback dolphins, which included 12 saturated fatty acids (SFA), 9 monounsaturated fatty acids (MUFA) and 11 polyunsaturated fatty acids (PUFA) (Table S2).
Seven dominant fatty acids (more than 1% of total fatty acids) in the prey fishes were found: 14:0, 15:0, 16:0, 17:0 and 18:0 as SFA; 16:1, 17:1, C18:1n-9cis as MUFA; C18:2n-6cis, C20:3n-3, C20:5n-3 (EPA), C22:6n-3 (DHA) as PFA. The average content of SFA, MUFA and PFA is 57.09%, 33.49% and 8.71%, respectively (Table S2). The SFA, MUFA and PFA contents of prey fishes varied significantly with species and location (p< 0.001). Among the 14 species of prey fish in this study, the fishes of Sciaenidae and Engraulidae family displayed significantly higher (p< 0.05, one-way ANOVA/Kruskal-Wallis test) PUFA, EPA, DHA content and PUFA/SFA ratio, and significantly lower (p< 0.05, one-way ANOVA) MUFA content than those of Clupeidae and Mugilidae family (Figure 3). In particular, the spiny head croaker of Sciaenidae family had the highest PUFA, EPA, DHA contents and PUFA/SFA ratio among the studied species (Table S2). Our results suggested that prey fishes in Sciaenidae and Engraulidae family are the most important PUFA resource.
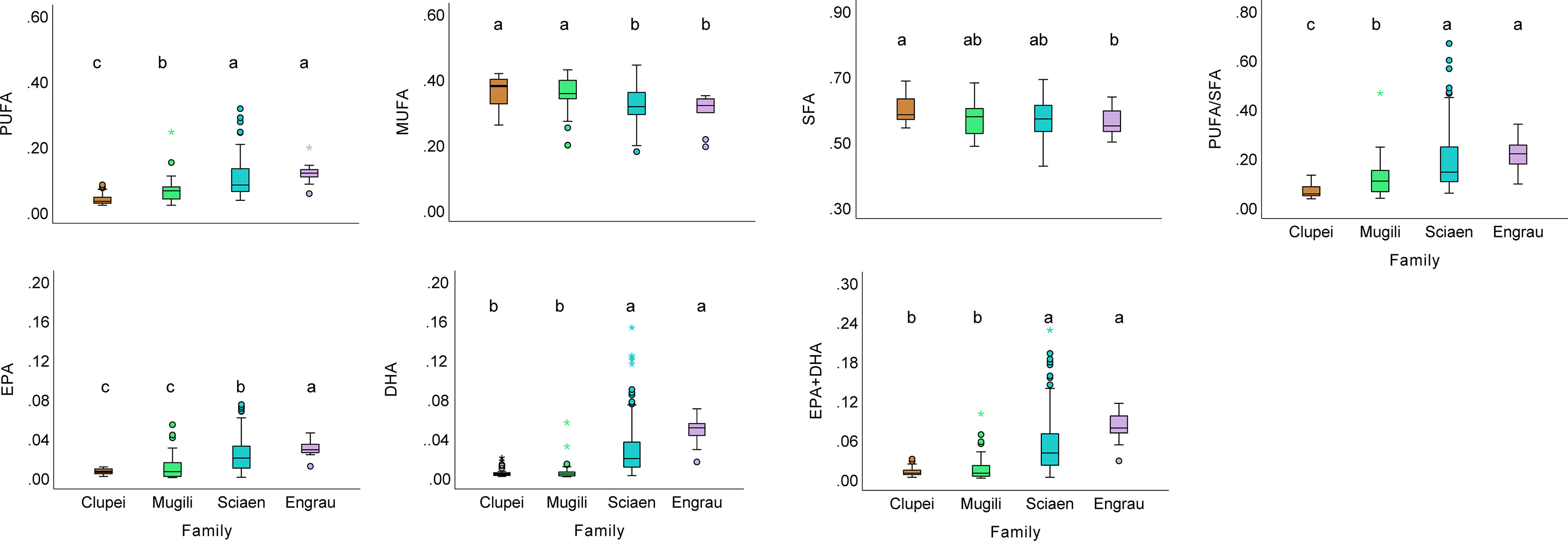
Figure 3 The box plots of fatty acids composition of fishes of different families. Different lower-case letters indicate a significant difference among groups.
We then compared the fatty acid composition among pelagic/neritic, benthopelagic and demersal fishes in Zhuhai and Jiangmen. In Zhuhai, for the PUFA, DHA, DHA+EPA content and PUFA/SFA ratio, the contents of demersal fishes were significantly higher (p< 0.05, Kruskal-Wallis test) than those of benthopelagic and pelagic/neritic fishes (Figure 4A). However, in Jiangmen, the SFA, MUFA, PUFA, EPA and DHA contents, and PUFA/SFA ratio show little difference among the three species niches, especially between pelagic/neritic and demersal fishes (Figure 4B).
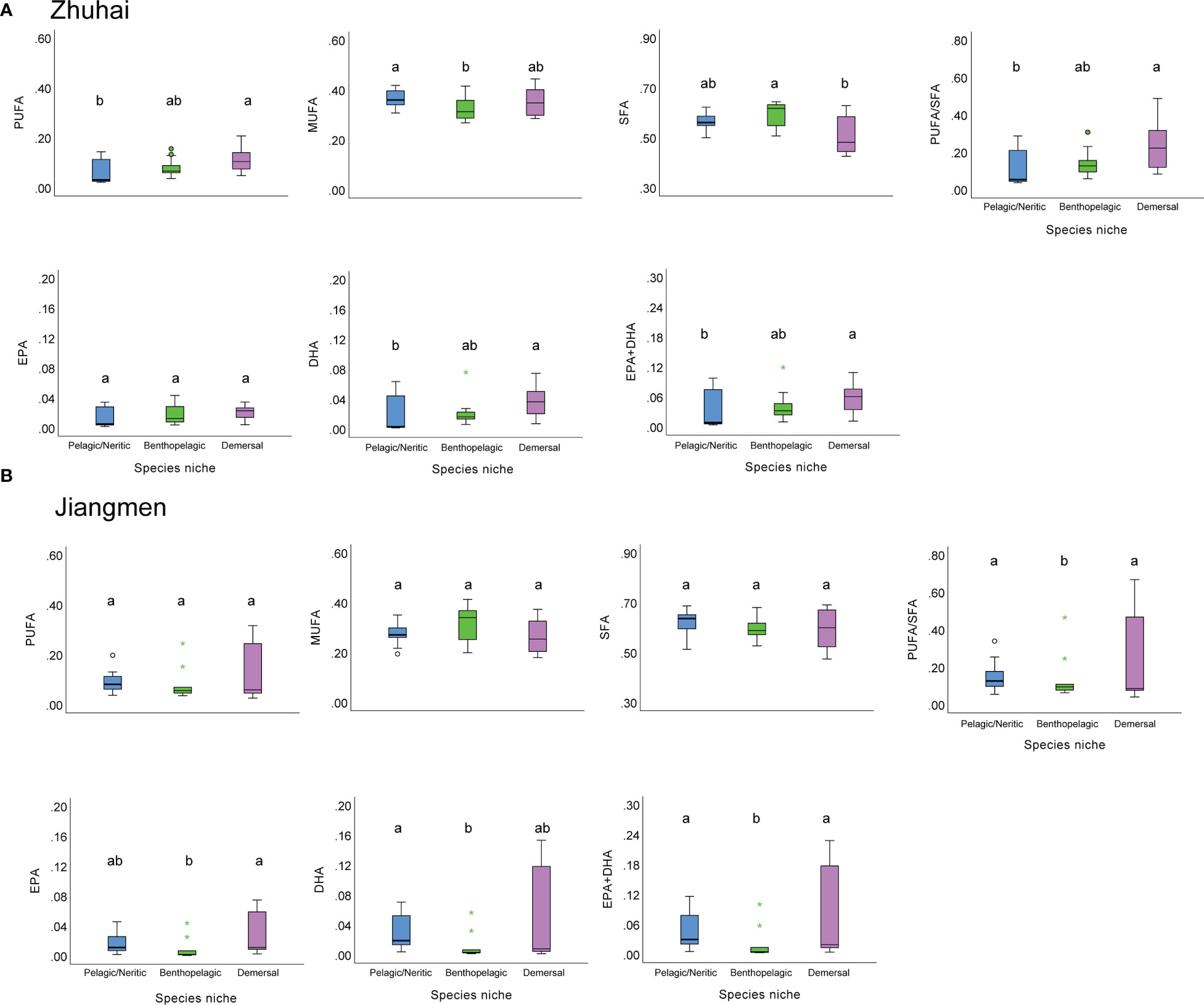
Figure 4 The box plots of fatty acids composition of fishes in different species niches from Zhuhai (A) and Jiangmen (B). Different lower-case letters indicate a significant difference among groups.
The amino acid compositions in the 14 prey fish species of humpback dolphins are listed in Table S3, where 9 essential amino acids and 8 nonessential amino acids were detected. Glutamine (Glu, 7.48 ± 1.76%) is the dominant amino acid, followed by aspartic acid (Asp, 4.84 ± 1.14%), lysine (Lys, 4.16 ± 0.97%), arginine (Arg, 4.02± 0.88%), glycine (Gly, 3.84 ± 0.86%), leucine (Leu, 3.77% ± 0.88) and alanine (Ala, 3.55 ± 0.75%). In general, the average concentration of essential amino acids (EAA) of all the samples was 23.63 ± 4.87%. Their essential amino acids/total amino acids (EAA/TAA) and essential amino acids/non-essential amino acids (EAA/NEAA) were 47.21 ± 2.00% and 89.70 ± 7.08%, respectively. The prey fishes were high-quality protein sources, according to the ideal FAO/WHO model which recommended that the EAA/TAA and EAA/NEAA ratios of high-quality protein should be around 40% and higher than 60%, respectively (Table S3) (WHO, 1991).
We also calculated the AAS, CS and EAAI of all the fishes studied (Tables S4–S6), and found that except for Met+Cys, which is the first limiting amino acid (Table S4), the other EAA contents are close to or higher than those of FAO/WHO reference protein, and lower than egg protein. All the fishes have EAAI higher than 60 (Table S6). These results again suggested that the prey fishes possessed rich and amino-acid-balanced high-quality protein.
Although good at protein quality in general, the EAA, NEAA, TAA contents, EAA/TAA, EAA/NEAA ratios of prey fishes varied significantly with species (p< 0.001, GLM). Compared with Engraulidae and Sciaenidae fishes, Clupeidae and Mugilidae fishes have higher (q< 0.05, Kruskal-Wallis test) EAA and total TAA contents but lower EAAI, suggesting discordance of EAA richness and EAA balance. However, Johnius fasciatus had the highest EAA content and EAAI, implying that this species could serve as the best of both options (Figure 5).
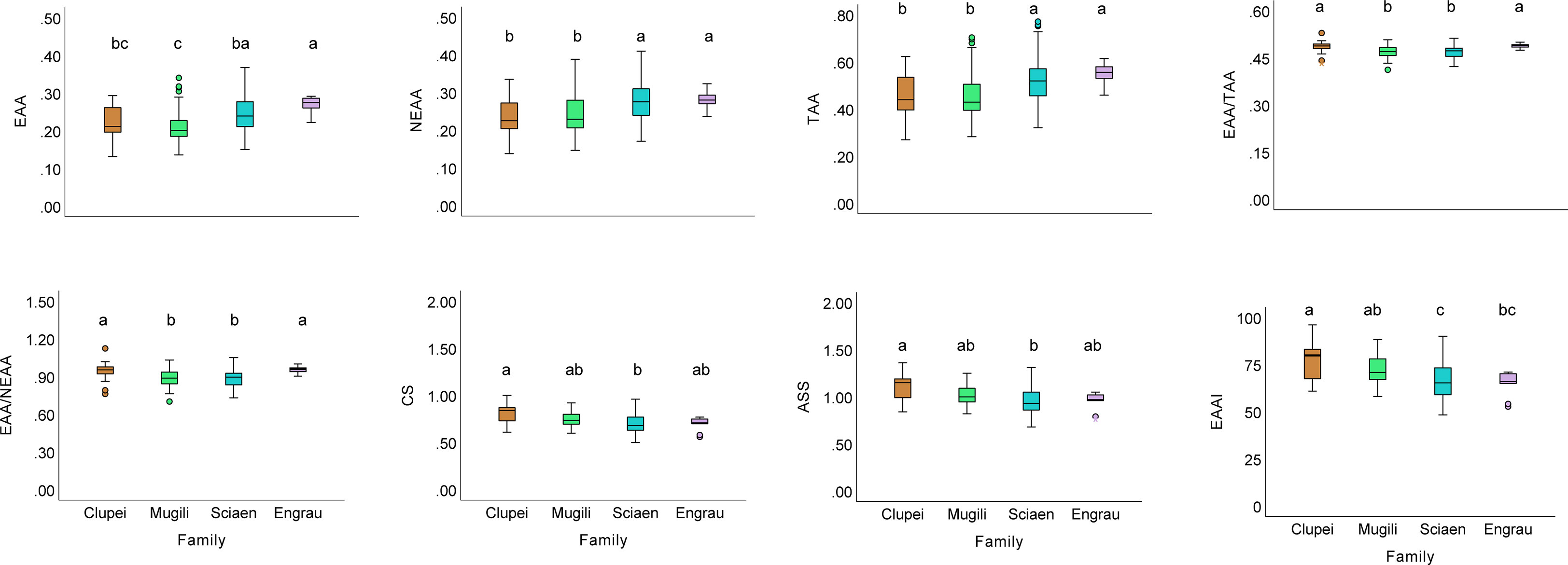
Figure 5 The box plots of amino acids composition of fishes of different families. Different lower-case letters indicate a significant difference among groups.
We then compared the amino acid composition among pelagic/neritic, benthopelagic and demersal fishes in Zhuhai and Jiangmen. In Zhuhai, the demersal fishes had significantly higher EAA and TAA content than pelagic/neritic and benthopelagic fishes (Figure 6A). While in Jiangmen, the highest EAA contents were recorded from the pelagic/neritic fishes and the lowest from benthopelagic fishes (Figure 6B). However, in both areas, EAA/TAA and EAAI did not show significant differences among the three species niches.
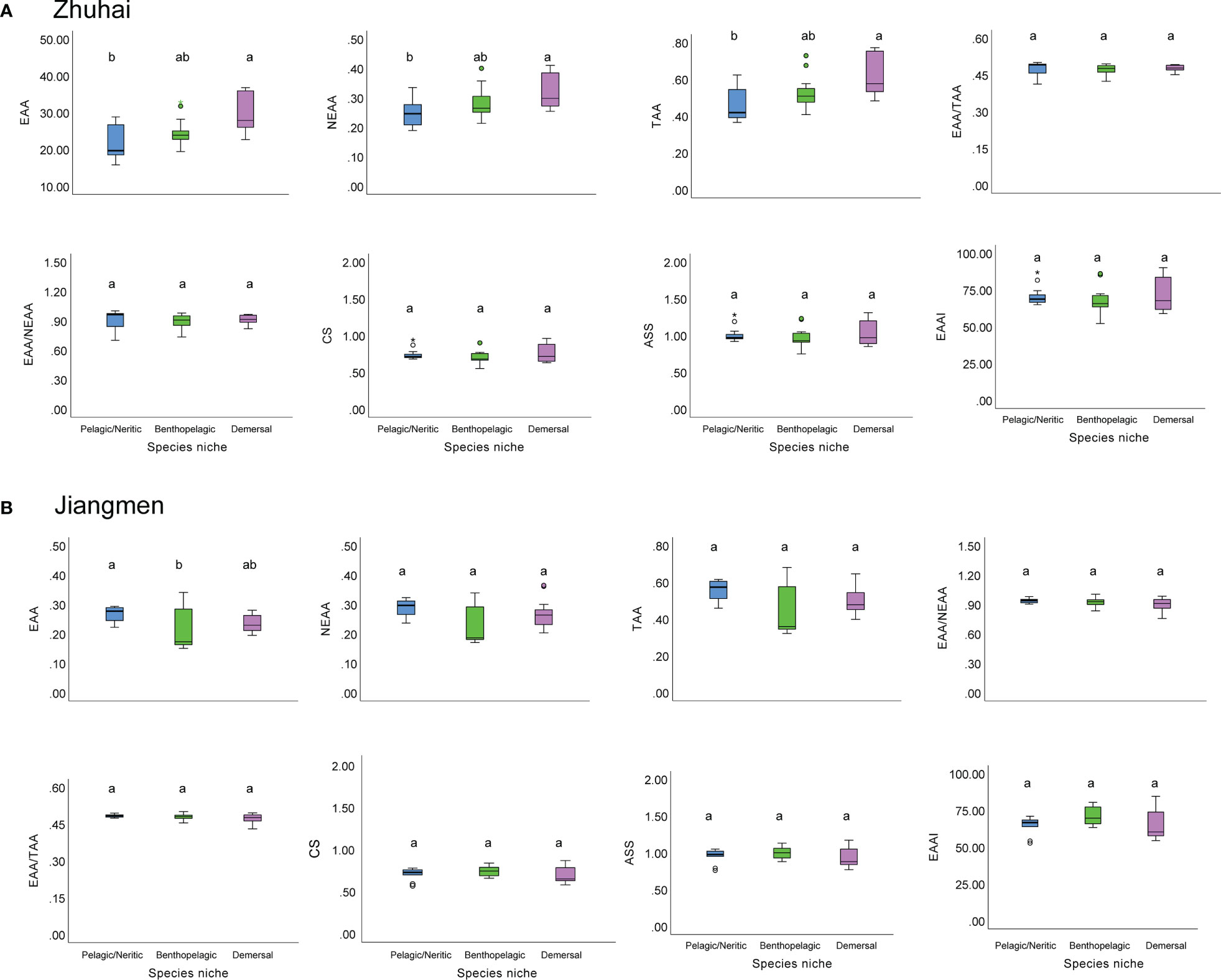
Figure 6 The box plots of amino acids composition of fishes in different species niches from Zhuhai (A) and Jiangmen (B). Different lower-case letters indicate a significant difference among groups.
Overall, our data provided the nutritional profiling of the prey fishes of Indo-Pacific humpback dolphins along the southern coastal areas of China. The results showed that the crude fat, protein content, fatty acids and amino acid composition varied significantly with species and locations. We specifically focused on the Pearl River Estuary (Zhuhai and Jiangmen), home to the largest Indo-Pacific humpback dolphin population, which has shown a dietary switch from primarily demersal to neritic and pelagic fish (Lin et al., 2021). In Zhuhai, the prey fishes were found to have lower energy densities, poorer fatty acid and amino acid quality indices (PUFA content, PUFA/SFA ratio, DHA content, EAA content) in pelagic/neritic fishes compared with demersal ones, suggesting that the largest humpback dolphin population would suffer from nutritional stress due to a dietary switch from higher nutritional quality demersal fishes to pelagic/neritic fishes (Figure 7).
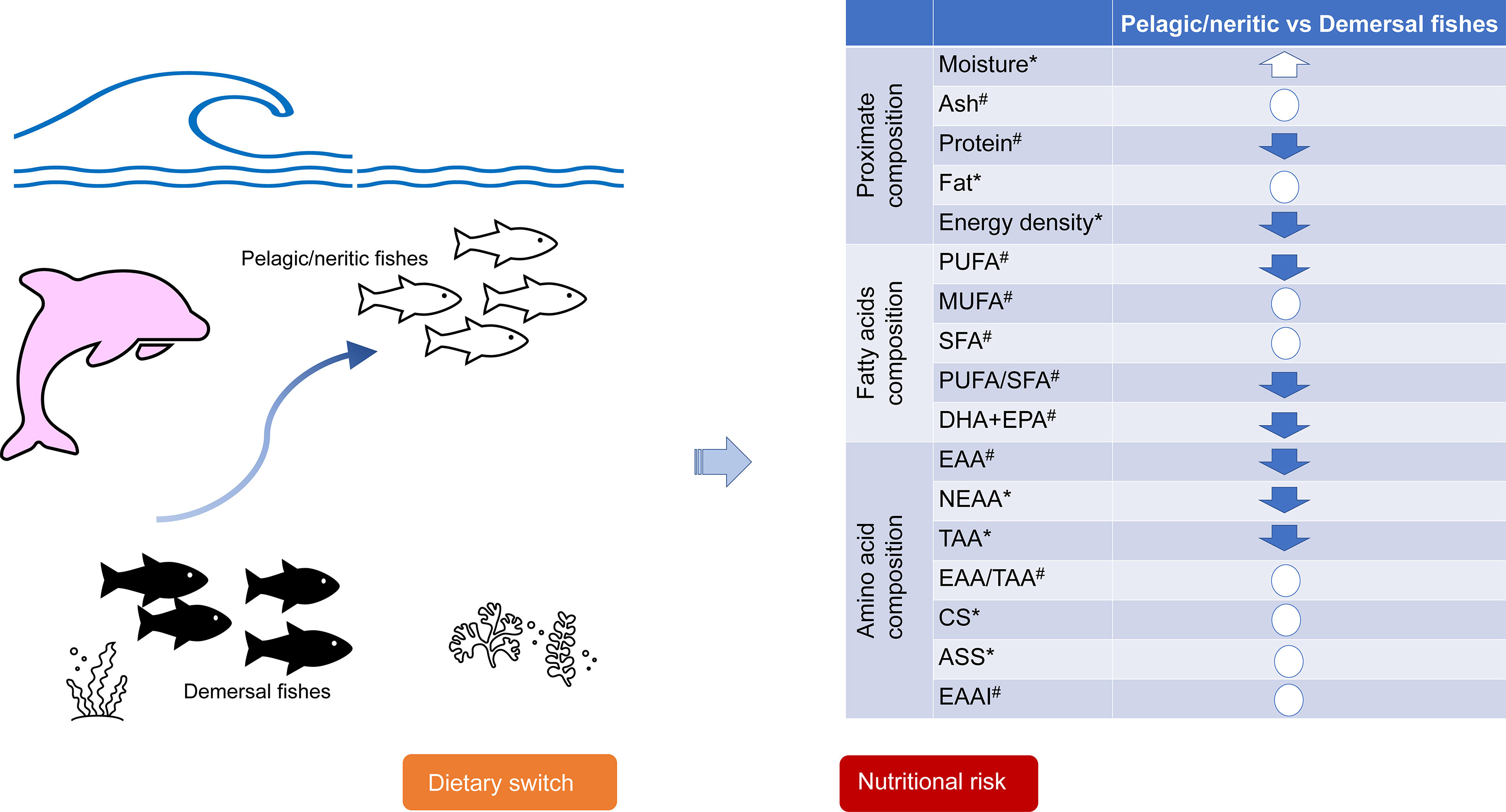
Figure 7 Summary of the dietary switch in the Zhuhai population of Indo-Pacific humpback dolphins and the potential nutritional risk. The arrows in the table indicate significantly higher (up-arrows) or lower (down-arrows) nutrition component in pelagic/neritic fishes compared with demersal fishes (p<0.05), while the circles indicate there is no significant difference. *: Duncan’s Multiple-Range Test of ANOVA; #: Kruskal-Wallis test. PUFA, Polyunsaturated fatty acids; MUFA, Monounsaturated fatty acids; SFA, Saturated fatty acids; DHA, Docosahexaenoic acid; EPA, Eicosapentaenoic acid; EAA, Essential amino acid; NEAA, Non-essential amino acids; TAA, Tatol amino acid; CS, Chemical score; ASS, Amino acid score; EAAI, Essential amino acid index.
Prey fishes are extremely important to the marine mammals by providing energy for basal metabolism, thermoregulation, swimming, osmoregulation and reproduction (Trites and Donnelly, 2003; Berta et al., 2015; IJsseldijk et al., 2021). Our results showed that the energy densities varied considerably with different species, with pelagic and neritic prey fishes having significantly lower energy densities compared to demersal fishes, which would affect the energy intake of dolphins, which depend on prey size and prey abundance (Baird and Dill, 1996; Emery, 2017). Facing the decline of prey resource, the humpback dolphins would try to adjust their foraging strategy by expand their targeting prey species to neritic and pelagic fish, however, as showed in Lin’s results, their overall foraging efficiency was still declining, not only in the prey availability and prey size (Lin et al., 2021), but also energy densities as revealed by our current study (Figure 2A).
Lipids are essential for all organisms, as they play a major role not only as fuel, but also in cell membrane structure and cellular signal transmission (Hulbert and Abbott, 2012). Like their terrestrial relatives, marine mammals do not possess the capability to synthesize all necessary fatty acids, particularly the polyunsaturated fatty acids (PUFA) which must be supplied by feeding on prey fishes (Dannenberger et al., 2020). PUFA, especially eicosapentaenoic acid (C20:5n-3, EPA) and docosahexaenoic acid (C22:6n-3, DHA), play a critical role in a variety of mammalian ontogenetic processes in modulating health status and the onset of chronic disease. Diets’ deficiency in PUFA can lead to improper development of the brain and visual systems (Powell et al., 2021; von Schacky, 2021), as well as damage to cardiovascular (Nordoy, 1999), immune (Swanson et al., 2012; Radzikowska et al., 2019), and reproduction functions (Maranesi et al., 2018) in mammals. Our results again pointed to a potential fatty acid crisis of the humpback dolphins in the water of Zhuhai resulting due to the dietary switch. Thereafter, prey fishes in the families Sciaenidae and Engraulidae, especially the demersal fishes in Zhuhai, should be carefully monitored and conserved as their population depression would increase the risk of lacking PUFA and affect the health of the humpback dolphins.
This crisis in terms of diet quality decline has already occurred in the Steller sea lion (Eumetopias jubatus) population in Alaska (Trites and Donnelly, 2003), which has shown reduced body size (Pitcher et al., 2000; Atkinson et al., 2008), reduced productivity (Pitcher et al., 1998), reduced pup and juvenile survival (Raum-Suryan et al., 2002), altered blood chemistry (Calkins and Goodwin, 1988), and eventually population decline (Calkins and Goodwin, 1988). Studies with regard to wild and captive seals suggest that this population depression is not due to an overall decline in fish abundance, but rather to a decline in the relative quality of prey fish. For example, seals’ body fatty content declined by 32% over 30 days while their body protein increased in proportion to protein intake when their prey fishes switched from Atlantic herring to Atlantic pollock (Kirsch et al., 2000), which is not a good phenomenon for the animal residing in cold environments and subject to periodic fasts between foraging bouts. Such a negative response to a dietary switch from high-energy forage fish (clupeids such as herring and sprat) to lean fish (such as hake and whiting) has also been documented in wild harbour seals in Scotland (Thompson et al., 1998). Because of the lack of direct evidence, there is still an unfilled gap between these relative qualitative defect of prey fish and the health of humpback dolphins. However, consider the multiple lines of evidence (declining prey availability, smaller prey size, lower energy densities, poorer fatty acid and amino acid quality indices) suggesting potentially adverse outcomes, and the lessons drawn from Steller sea lion and harbour seals’ example, we indeed raise concerns about the health of dolphins. In fact, our continuous field survey in the past fifteen years on humpback dolphin population in the PRE indicated a much less newborns and juveniles in the eastern PRE (Zhuhai area) than the western area (Jiangmen area) (unpublished data). This phenomenon may be at least in part due to the food quality in the Zhuhai area, such as less energy and PUFAs.
Amino acids are essential biomolecules, both as structural building blocks of proteins and as intermediates in a variety of metabolic pathways. Like EFA, essential amino acids (EAA) cannot be synthesized by mammals but should be obtained from proteins in diet. The quality of dietary protein is assessed from EAA to nonessential amino acid (NEAA) ratio (Mohanty et al., 2014). Our results indicate the EAA, NEAA, TAA contents, EAA/TAA, EAA/NEAA ratios of prey fishes varied significantly with species but in general the protein quality is good in humpback dolphins’ prey fishes. Our results suggested that the prey fishes possessed rich and amino-acid-balanced high-quality protein, although some of the indexes may vary among species and niche.
Since the pre-mortem movement between sub-regions and post-mortem movement along with the drift is care in the PRD humpback dolphins, the dead dolphins in the study by Lin et al. (2021) represent the ration of the whole population. However, as the signifcantly difference of community structure of potential prey fishes among provinces (Guangdong, Guangxi and Fujian provinces) and stages (2018-2020, 2012-2014 and 2006-2008) (q<0.05, ANOSIM) (Figure S2), the variety of nutrition component among species and locations (Tables S1–S6), and the limitations of the stomach content data to Pearl River Estuary population, it is not rigorous to generalize our conclusion to all the populations of the humpback dolphins. Since we collected the samples in 2013, great changes have taken place in the marine habitat, which would lead to alteration in fish assemblages and nutritional contents in the Pearl River Estuary. Therefore, more continuous sample and data collection are necessary to obtain more robust results on current state. Despite these, our study indeed provided a framework, with energy density and fatty acid composition as its most important indicator, for assessment of not only the humpback dolphins but also the other marine top predators based on the nutritional composition of their prey fishes and revealed the potential threats.
In order to protect the humpback dolphins from such a “junk food” tragedy (Österblom et al., 2008), further action should be paid to prey fishes, in overall quantity and more importantly their nutritional quality. Bottom trawling is the most effective fishing practice for catching demersal fishery resources in China. According to the China Fishery Statistical Yearbook, Guangdong’s domestic trawlers landed 566,975 tons of fish in 2020, approximately half of the total reported marine domestic fish (BFMOA, 2021). As bottom trawling not only plunders the benthic fishery resources, but also imposes strong pressure on coastal marine ecosystems (Eigaard et al., 2017), it should be constrained through effective management in the Indo-Pacific humpback dolphin natural reserves and their adjacent sea area. In addition, as an effective management tool for fishery resources (Kitada, 2018), the artificial enhancement and release of nutrient-rich fish species, such as the spiny head croaker and Osbeck’s grenadier anchovy (Coilia mystus), would be helpful in restoring the dietary quantity and quality of humpback dolphins. Furthermore, novel high throughput technologies, such as targeted metabolomics, environmental DNA barcoding and genomic sequencing, should be used to assess the prey fish population and their nutritional quality as well as their habitat ecosystem (Valenzuela-Quinonez, 2016; Zhong et al., 2022).
Our study is expected to facilitate the development of scientific programs for the successful conservation of not only the Indo-Pacific humpback dolphin, but also other marine top predators, by conserving and reconstructing the quantity and quality of their prey fish.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by The Medical Animal Care & Welfare Committee of Shantou University.
JL: Investigation, formal analysis, funding acquisition, writing – original draft, writing – review & editing. YL, YW: Resources, methodology, investigation. HZ, QG, ZW, YG, SL, KL: Formal analysis, writing – original draft. ZZ, PL: Writing – review & editing. WL: Conceptualization, writing – review & editing, funding acquisition. All authors contributed to the article and approved the submitted version.
This work was supported by the Key Program of the National Natural Science Foundation of China (42230413), Key Program of Marine Economy Development (Six Marine Industries) Special Foundation of Department of Natural Resources of Guangdong Province (GDNRC[2022]48), and Scientific Research Foundation for Talents, STU (NTF21026).
We thank Xiaoqi Lin (Shantou University) for her help with data analysis and figure drawing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1144398/full#supplementary-material
Atkinson S., Calkins D., Burkanov V., Castellini M., Hennen D., Inglis S. (2008). Impact of changing diet regimes on steller sea lion body condition. Mar. Mamm. Sci. 24, 276–289. doi: 10.1111/j.1748-7692.2008.00188.x
Avila I. C., Kaschner K., Dormann C. F. (2018). Current global risks to marine mammals: Taking stock of the threats. Biol. Conserv. 221, 44–58. doi: 10.1016/j.biocon.2018.02.021
Baird R. W., Dill L. M. (1996). Ecological and social determinants of group size in transient killer whales. Behav. Ecol. 7, 408–416. doi: 10.1093/beheco/7.4.408
Berta A., Sumich J. L., Kovacs K. M. (2015). “Chapter 9 - energetics,” in Marine mammals, 3rd ed. Eds. Berta A., Sumich J. L., Kovacs K. M. (San Diego: Academic Press), 269–297.
BFMOA (2021). China Fishery statistical yearbook Vol. 2021 (Beijing, China: China Agriculture Press).
Breitburg D., Levin L. A., Oschlies A., Gregoire M., Chavez F. P., Conley D. J., et al. (2018). Declining oxygen in the global ocean and coastal waters. Science, 359, eaam7240. doi: 10.1126/science.aam7240
Calkins D., Goodwin E. (1988). Investigation of the declining Sea lion population in the gulf of Alaska (Anchorage, AK: Alaska Department of Fish and Game), 76.
Cheung W. W. L., Sarmiento J. L., Dunne J., Frolicher T. L., Lam V. W. Y., Palomares M. L. D., et al. (2013). Shrinking of fishes exacerbates impacts of global ocean changes on marine ecosystems. Nat. Clim. Change 3, 254–258. doi: 10.1038/nclimate1691
Christensen V. (2015). Fishing down through the food web. Fisheries 40, 370–372. doi: 10.1080/03632415.2015.1065155
Dadswell M., Spares A., Reader J., McLean M., McDermott T., Samways K., et al. (2021). The decline and impending collapse of the Atlantic salmon (Salmo salar) population in the north Atlantic ocean: A review of possible causes. Rev. Fish. Sci. Aquac. 30, 215–258. doi: 10.1080/23308249.2021.1937044
Dannenberger D., Moller R., Westphal L., Moritz T., Dahne M., Grunow B. (2020). Fatty acid composition in blubber, liver, and muscle of marine mammals in the southern Baltic Sea. Anim. (Basel) 10, 1509. doi: 10.3390/ani10091509
Eigaard O. R., Bastardie F., Hintzen N. T., Buhl-Mortensen L., Buhl-Mortensen P., Catarino R., et al. (2017). The footprint of bottom trawling in European waters: distribution, intensity, and seabed integrity. Ices. J. Mar. Sci. 74, 847–865. doi: 10.1093/icesjms/fsw194
Emery T. ,. M. (2017). Energetics of feeding, social behavior, and life history in non-human primates. Horm. Behav. 91, 84–96. doi: 10.1016/j.yhbeh.2016.08.009
Essington T. E., Beaudreau A. H., Wiedenmann J. (2006). Fishing through marine food webs. Proc. Natl. Acad. Sci. U. S. A. 103, 3171–3175. doi: 10.1073/pnas.0510964103
Folch J., Lees M., Sloane Stanley G. H. (1957). A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226, 497–509. doi: 10.1016/S0021-9258(18)64849-5
He Q., Silliman B. R. (2019). Climate change, human impacts, and coastal ecosystems in the anthropocene. Curr. Biol. 29, R1021–R1035. doi: 10.1016/j.cub.2019.08.042
Hulbert A. J., Abbott S. K. (2012). Nutritional ecology of essential fatty acids: an evolutionary perspective. Aust. J. Zool. 59, 369–379. doi: 10.1071/ZO11064
Ichihara K., Fukubayashi Y.. (2010). Preparation of fatty acid methyl esters for gas-liquid chromatography. J. Lipid Res. 51 (3), 635–640.
IJsseldijk L. L., Hessing S., Mairo A., Ten Doeschate M. T., Treep J., van den Broek J., et al. (2021). Nutritional status and prey energy density govern reproductive success in a small cetacean. Sci. Rep. 11, 1–13. doi: 10.1038/s41598-021-98629-x
Jefferson T. A., Smith B. D. (2016). Re-assessment of the conservation status of the indo-pacific humpback dolphin (Sousa chinensis) using the IUCN red list criteria. Adv. Mar. Biol. 73, 1–26. In Humpback Dolphins. doi: 10.1016/bs.amb.2015.04.002
Jefferson T. A., Smith B. D., Braulik G. T., Perrin W. (2017). Sousa Chinensis. the IUCN red list of threatened species. (Switzerland: IUCN) doi: 10.2305/IUCN.UK.2017-3.RLTS.T82031425A50372332.en
Kirsch P. E., Iverson S. J., Bowen W. D. (2000). Effect of a low-fat diet on body composition and blubber fatty acids of captive juvenile harp seals (Phoca groenlandica). Physiol. Biochem. Zool. 73, 45–59. doi: 10.1086/316723
Kitada S. (2018). Economic, ecological and genetic impacts of marine stock enhancement and sea ranching: A systematic review. Fish. Fish. 19, 511–532. doi: 10.1111/faf.12271
Lin W., Karczmarski L., Zhou R., Mo Y., Guo L., Yiu S. K. F., et al. (2021). Prey decline leads to diet shift in the largest population of indo-pacific humpback dolphins? Integr. Zool. 16, 548–574. doi: 10.1111/1749-4877.12548
Maranesi M., Castellini C., Dall'Aglio C., Petrucci L., Mattioli S., Boiti C., et al. (2018). Effects of PUFAs on animal reproduction: male and female performances and endocrine mechanisms. Phytochem. Rev. 17, 801–814. doi: 10.1007/s11101-018-9559-z
Mohanty B., Mahanty A., Ganguly S., Sankar T., Chakraborty K., Rangasamy A., et al. (2014). Amino acid compositions of 27 food fishes and their importance in clinical nutrition. J. Amino. Acids 2014, 269797. doi: 10.1155/2014/269797
Mullon C., Freon P., Cury P. (2005). The dynamics of collapse in world fisheries. Fish. Fish. 6, 111–120. doi: 10.1111/j.1467-2979.2005.00181.x
Myers R. A., Worm B. (2003). Rapid worldwide depletion of predatory fish communities. Nature 423, 280–283. doi: 10.1038/nature01610
Nordoy A. (1999). Dietary fatty acids and coronary heart disease. Lipids 34 (Suppl), S19–S22. doi: 10.1007/BF02562223
Österblom H., Olsson O., Blenckner T., Furness R. W. (2008). Junk-food in marine ecosystems. Oikos 117, 967–977. doi: 10.1111/j.0030-1299.2008.16501.x
Payne S. A., Johnson B. A., Otto R. S. (1999). Proximate composition of some north-eastern pacific forage fish species. Fish. Oceanogr. 8 (3), 159–177. doi: 10.1046/j.1365-2419.1999.00097.x
Perry A. L., Low P. J., Ellis J. R., Reynolds J. D. (2005). Climate change and distribution shifts in marine fishes. Science 308, 1912–1915. doi: 10.1126/science.1111322
Pitcher K. W., Calkins D. G., Pendleton G. W. (1998). Reproductive performances of female steller sea lions from the gulf of Alaska: An energetics-based reproductive strategy. Can. J. Zoo. 76, 2075–2083. doi: 10.1139/z98-149
Pitcher K. W., Calkins D. G., Pendleton G. W. (2000). Steller sea lion body condition indices. Mar. Mamm Sci. 16, 427–436. doi: 10.1111/j.1748-7692.2000.tb00934.x
Powell N., Chaudhary S., Zaidi A. (2021). It is time for an oil change: Polyunsaturated fatty acids and human health. Mo. Med. 118, 426–430.
Radzikowska U., Rinaldi A. O., Celebi Sozener Z., Karaguzel D., Wojcik M., Cypryk K., et al. (2019). The influence of dietary fatty acids on immune responses. Nutrients 11, 2990. doi: 10.3390/nu11122990
Raum-Suryan K. L., Pitcher K. W., Calkins D. G., Sease J. L., Loughlin T. R. (2002). Dispersal, rookery fidelity, and metapopulation structure of steller sea lions (Eumetopias jubatus) in an increasing and a decreasing population in alaska. Mar. Mamm. Sci. 18, 746-764. doi: 10.1111/j.1748-7692.2002.tb01071.x
Sanganyado E., Rajput I. R., Liu W. (2018). Bioaccumulation of organic pollutants in indo-pacific humpback dolphin: A review on current knowledge and future prospects. Environ. pollut. 237, 111–125. doi: 10.1016/j.envpol.2018.01.055
Swanson D., Block R., Mousa S. A. (2012). Omega-3 fatty acids EPA and DHA: health benefits throughout life. Adv. Nutr. 3, 1–7. doi: 10.3945/an.111.000893
Teh L. S., Cashion T., Alava J. J., Cheung W. W., Sumaila U. R. (2019). Status, trends, and the future of fisheries in the East and south China seas. Fish. Cent. Res. Rep. 27 (1), 101.
Thompson P. M., Corpe H. M., Reid R. J. (1998). Prevalence and intensity of the ectoparasite echinophthirius horridus on harbour seals (Phoca vitulina): effects of host age and inter-annual variability in host food availability. Parasitology 117, 393–403. doi: 10.1017/S0031182098003072
Trites A. W., Donnelly C. P. (2003). The decline of steller sea lions eumetopias jubatus in Alaska: a review of the nutritional stress hypothesis. Mamm. Rev. 33, 3–28. doi: 10.1046/j.1365-2907.2003.00009.x
Valenzuela-Quinonez F. (2016). How fisheries management can benefit from genomics? Brief. Funct. Genomics 15, 352–357. doi: 10.1093/bfgp/elw006
von Schacky C. (2021). Importance of EPA and DHA blood levels in brain structure and function. Nutrients 13, 1074. doi: 10.3390/nu13041074
Wang F. T., He J., Jiang S. T., Lin L., Lu J. F. (2021). Comparison of nutritional quality and nutrient compositions of three edible tissues from different sourced cultured female mud crabs (Scylla paramamosain). J. Food. Compost. Anal. 104, 104163. doi: 10.1016/j.jfca.2021.104163
Wang Y., Zhou X., Chen J., Xie B., Huang L. (2022). Climate-induced habitat suitability changes intensify fishing impacts on the life history of large yellow croaker (Larimichthys crocea). Ecol. Evol. 12, e9342. doi: 10.1002/ece3.9342
WHO (1991). Protein quality evaluation Vol. 51 (Bethesda, Md., USA: Report of the Joint FAO/WHO Expert Consultation).
Yu X. X., He Q. Y., Sanganyado E., Liang Y., Bi R., Li P., et al. (2020). Chlorinated organic contaminants in fish from the south China Sea: Assessing risk to indo-pacific humpback dolphin. Environ. pollut. 263, 114346. doi: 10.1016/j.envpol.2020.114346
Yuan H. R., Chen P. M., Yu J., Li X. G. (2022). Assessment of quality of fishery resources in the northeastern south China Sea. J. Mar. Sci. Eng. 10, 930. doi: 10.3390/jmse10070930
Keywords: Indo-Pacific humpback dolphin, prey fish, nutritional composition, fatty acid, amino acid
Citation: Lin J, Liang Y, Zhao H, Gutang Q, Wu Z, Gao Y, Liu S, Li K, Wu Y, Zhang Z, Li P and Liu W (2023) Better or worse food: Nutrition value of the prey fishes and the potential health implications for Indo-Pacific humpback dolphins. Front. Mar. Sci. 10:1144398. doi: 10.3389/fmars.2023.1144398
Received: 14 January 2023; Accepted: 20 March 2023;
Published: 31 March 2023.
Edited by:
Xuelei Zhang, Ministry of Natural Resources, ChinaReviewed by:
Dmitry Lajus, Independent Researcher, Saint Petersburg, RussiaCopyright © 2023 Lin, Liang, Zhao, Gutang, Wu, Gao, Liu, Li, Wu, Zhang, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenhua Liu, d2hsaXVAc3R1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.