- 1Aquaculture Research Division, National Institute of Fisheries Science (NIFS), Busan, South Korea
- 2Major in Aquaculture and Applied Life Sciences, Pukyong National University, Busan, South Korea
- 3Department of Animal Biotechnology, Jeju International Animal Research Center (JIA) and Sustainable Agriculture Research Institute (SARI), Jeju National University, Jeju, South Korea
- 4Feeds and Foods Nutrition Research Center, Pukyong National University, Busan, South Korea
- 5Aquafeed Research Center, National Institute of Fisheries Science, Pohang, South Korea
We investigated the nine experimental diets containing fish meal (FM) and/or fish meal analog (FMA) as the major source of animal protein to determine the optimum FMA level as the substitute of FM protein in the diet of juvenile Japanese eel. In addition, two natural feed additives such as Song-Gang stone (SG) and Yucca meal (YM) were supplemented in the diet to evaluate their efficacy as the immunostimulants. The diets are as follows: 100% FM + 0% FMA in diet (FMA0), 90% FM + 10% FMA in diet (FMA10), 80% FM + 20% FMA in diet (FMA20), 70% FM + 30% FMA in diet (FMA30), 60% FM + 40% FMA in diet (FMA40), FMA0 + 0.4% SG (FMA0SG), FMA0 + 0.1% YM (FMA0YM), FMA20 + 0.4% SG (FMA20SG), and FMA20 + 0.1% YM (FMA20YM). Nine groups of Japanese eel each with three replicates were distributed (initial weight of 9 ± 0.2 g) in rectangular tanks receiving flow through water. Each group of the treatment consisted with 15 fish and fed one of the diets for 8 weeks. At the end of the feeding trial, fish fed with the FMA0 and FMA10 diets showed no significant differences in weight gain (WG), specific growth rate (SGR), feed efficiency (FE), and protein efficiency ratio (PER). Meanwhile, fish fed with FMA20, FMA30, and FMA40 diets showed significantly lower WG, SGR, FE, and PER than the fish fed with the FMA0 (control) diet. In addition, there were no significant differences among fish fed with the SG- and YM-supplemented diet groups. However, lysozyme activities in fish fed with the FMA10, FMA20, FMA30, and FMA40 were significantly lower than the fish fed with the FMA0SG, FMA0YM, FMA20SG, and FMA20YM diets. After 7 days of injection with V. Anguillarum, cumulative survival rates of fish fed with the FMA0SG and FMA0YM diets were significantly higher than the FMA0 diet group. The results revealed that the FMA could replace up to 10% of FM as a protein source in the diet of Japanese eel and both of the natural feed additives (SG and YM) could improve replacing rates of FMA from 10% to 20% without compromising growth and health status of fish.
Introduction
It has been predicted that world population would increase up to nearly 10 billion by 2050 (FAO, 2016). This means a 33% rise in demands for varieties of healthy and desirable animal protein sources. Whereas aquaculture is increasingly satisfying the growing demand, further progress of other animal protein production sectors does not seem to be feasible due to environmental and economic barriers (FAO, 2016; UNSD, 2016). However, aquaculture is also facing some major challenges associated with social, financial, and ecological sustainability such as the excesses use of fish meal (FM) due to increased production of aquafeeds (FAO, 2016; Moutinho et al., 2016). Therefore, lowering the inclusion level of FM in aquafeeds could significantly drop expenses and have meaningful effect on reducing the pressure of wild stock overexploitation (Naylor et al., 2009; Kokou et al., 2015).
FM is the most relevant and reliable protein source used extensively in commercial aquafeeds, especially for carnivorous aquatic species. FM is constituted with high-profile biochemical amino acids, high acceptability, and bioavailability (Zhou et al., 2004; Glencross et al., 2007; Kokou et al., 2015). Numerous research works have reported on the replacement of FM as the valuable ingredient in the diets of fish and shellfish through the use of less expensive alternatives such as bacteria (Aas et al., 2006), algae (Kiron, 2012), plants (Gatlin et al., 2007), invertebrates (Barrows and Frost, 2014), and by-products (Fowler, 1991; Tung and Alfaro, 2012). However, plant-derived protein sources such as soybean meal, wheat gluten meal, and corn gluten meal contain some anti-nutritional factors (ANFs) that negatively influence the digestibility of fish (Krogdahl et al., 2010; Oliva-Teles et al., 2015). Whereas, by-products from terrestrial animals are considered as potent alternatives for FM (Tacon, 1993; Moutinho et al., 2016). Fish meal analog (FMA) is a premix of animal by-products and plant ingredients including leather meal, poultry by-product, feather meal, blood meal, squid liver powder, tuna by-product, and soybean meal (Damusaru et al., 2019). With high crude protein, low ANFs (e.g., proteinase inhibitors, lectins, and phytic acid), acceptable palatability (because of tuna by-product and squid liver powder), and rich amino acid profile (by addition of methionine and lysine), FMA could be considered as a qualified candidate for substitution of FM (Jo et al., 2017; Damusaru et al., 2019). In a recent finding, we reported that 15.39% to 20% of dietary FM could replace with FMA in the diets of growing Japanese eel without affecting the growth and health status of the fish.
Feed additives are familiar as supplemental and nutritional/non-nutritional ingredients that added to the formulated diets to increase physical characteristics of feed and/or to enhance biological performance of aquatic animals (Bai et al., 2015). A variety of feed additives such as feeding stimulants and palatability enhancers, antimicrobial agents, antioxidants, binders, pigmentation agents, organic acids, enzymes, immunostimulants, and hormones are used in aquafeeds (NRC, 2011). Song-Gang stone (SG) powder is a natural mineral compound that could found in Republic of Korea. It consists mainly of SiO2, Al2O3, K2O, Na2O, Fe2O3, and other trace elements (Won et al., 2017). The SG is considered as an immunostimulant that could enhance performance and health status of fish (Choi et al., 2004; Won et al., 2017). Yucca meal (YM) is a naturally manufactured powder derived from a flowering plant known as Yucca schidigera (Cheeke et al., 2006; Njagi et al., 2017). YM can act as an antioxidant, antistress, and antimicrobial agent that can enhance growth, immunity, and appetite of target species (Kelly and Kohler, 2003; Piacente et al., 2005; Gaber, 2006; Citarasu, 2010; Güroy et al., 2014; Amoah et al., 2017; Bae et al., 2020 ). Both of the SG and YM additives show their potentials as immunostimulants in fish in terms of improving chemical composition of diet and boost the nutritional quality of aquafeeds, which ultimately improved the growth and immune performance in fish (Won et al., 2017; Bae et al., 2020 ).
Japanese eel, Anguilla japonica, is an expensive and important freshwater fish species with high medicinal value cultured in several Asian countries especially in China, Japan, and Republic of Korea (Jo et al., 2017; Damusaru et al., 2019). The species is already enlisted as endangered by the International Union for Conservation of Nature and Natural Resources (Jacoby and Gollock, 2014), meaning that special attention should be directed its aquaculture and nutrition. Therefore, as the price of FM increasing and supply is squeezing around the world, it is imperative to find alternatives of FM for highly carnivorous aquaculture fish species like Japanese eel. For this, the aim of the present study was to evaluate the influence of FM replacement by graded levels of FMA with or without YM and SG supplementations in terms of growth, whole-body proximate composition, hematological indexes, and non-specific immune responses of juvenile Japanese eel. In addition, we also conducted a challenge test with Vibrio anguillarum, a globally distributed bacterial pathogen that causes severe hemorrhagic disease in aquaculture farms including Japanese eel (Frans et al., 2011).
Materials and methods
Ethical statement
The experiment was followed under the guidelines of Institutional Animal Care and Use Committee Regulations, No. 554, issued by the Pukyong National University, Busan, Republic of Korea. Every effort was taken to minimize fish suffering.
Fish meal analog
FMA consisted of 15% leather meal (The Feed Co., Seoul, Republic of Korea), 15% poultry by-product meal (Cherry Buro Co., Chungcheongbuk-do, Republic of Korea), 25% feather meal (Cherry Buro Co., Chungcheongbuk-do, Republic of Korea), 2% tuna by-product (Woonam Fish Co., Seoul, Republic of Korea), 24% blood meal (The Feed Co., Seoul, Republic of Korea), 1% squid liver powder (The Feed Co., Seoul, Republic of Korea), 15% soybean meal (The Feed Co., Seoul, Republic of Korea), 2% lysine (The Feed Co., Seoul, Republic of Korea), and 1% methionine (The Feed Co., Seoul, Republic of Korea). Here, lysine and methionine were supplemented in the FMA based on our previous findings (Damusaru et al., 2019).
Experimental design and diets
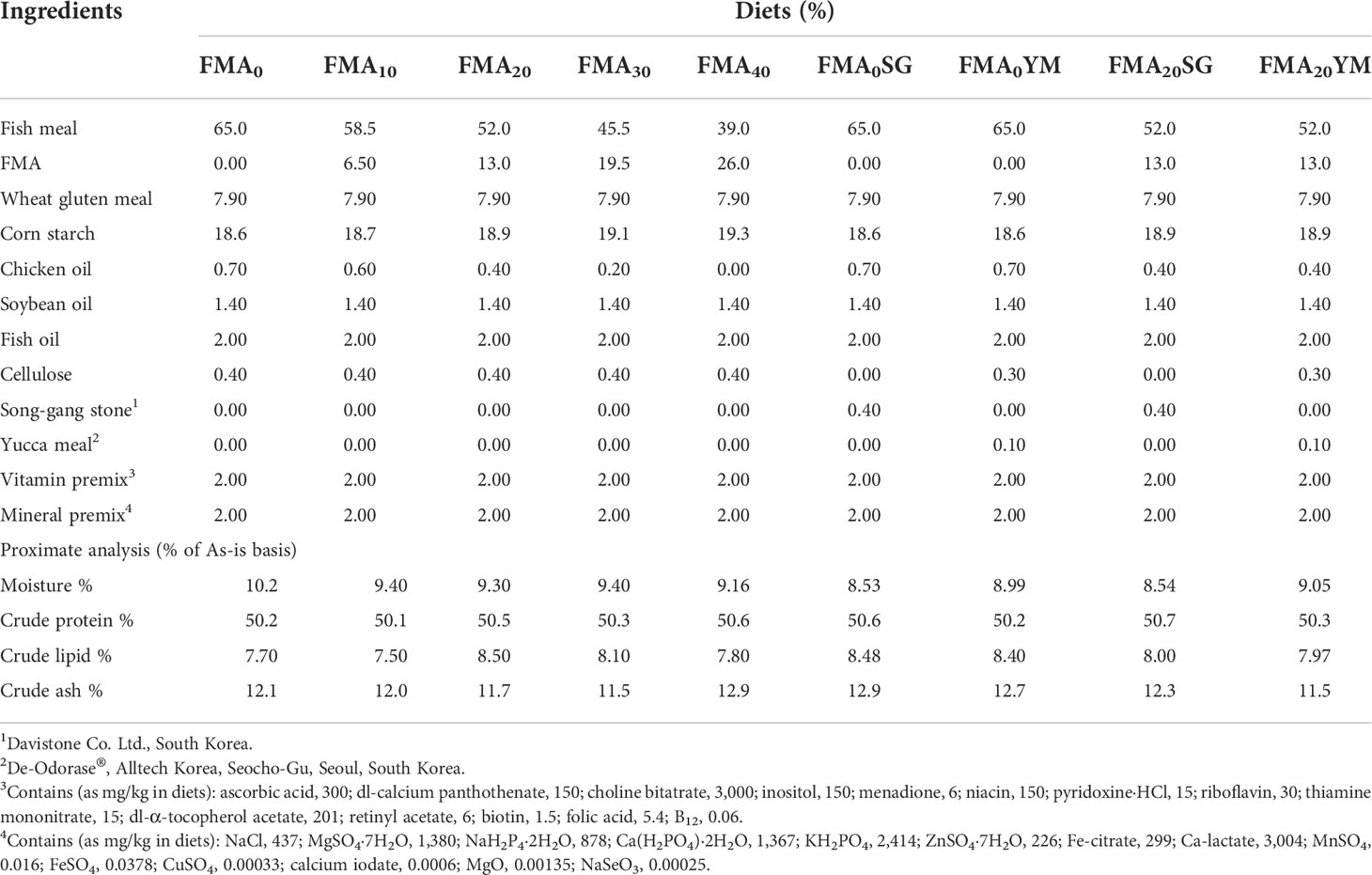
A total of nine experimental diets were formulated (Table 1) to replace FM with FMA at 0% (FMA0), 10% (FMA10), 20% (FMA20), 30% (FMA30), 40% (FMA40), FMA0 + SG (FMA0SG), FMA0 + YM (FMA0YM), FMA20 + 0.4% SG (FMA20SG0.4), and FMA20 + 0.1% YM (FMA20YM0.1). The supplementation of SG, SG (Davistone Co. Ltd., Busan, Republic of Korea) and YM, and YM (as De-Odorase, Alltech Korea, Seoul, Republic of Korea) was based on our recent studies reported (Won et al., 2017; Amoah et al., 2017; Bae et al., 2020). The experimental diets were iso-nitrogenic at 50% crude protein. The FM, wheat gluten meal, and FMA were main sources of protein in the experimental diets. Soybean oil, fish oil, and chicken oil were main sources of lipid. Dietary corn starch was mainly used as energy source, and cellulose was used as binder in the diets. At first, all dry ingredients were mixed using a mechanical mixing machine (HYVM-1214, Hanyoung Food Machinery, Republic of Korea) thoroughly. Then, filtered fresh water (300 ml kg−1 diet) was added simultaneously with fish oil, soybean oil, and chicken oil in the diets. The experimental diets were pelleted using a screw-type pelleting machine (SFD-GT, Shinsung, Republic of Korea) and stored at −20°C until use.
Experimental fish and feeding trial
Juvenile Japanese eel Anguilla japonica were purchased from Nonsan fish farm (Nonsan city, Republic Korea) and distributed in 250-L fiberglass tanks belongs to Feed & Foods Nutrition Research Center (FFNRC, Pukyong National University, Busan, Republic of Korea). The fish were adapted to the experimental environment for 4 weeks and fed with the control diet two times per day. After this step, 405 fish with average initial body weight of 9 ± 0.2 g (mean ± SD) were randomly distributed into the 27 glass aquarium tanks (15 fish per tank). The flow of filtered freshwater was maintained in the glass tanks (40-liter capacity) at a rate of 2 L/min from the semi-circulating central tank during the experimental period. The 50% of freshwater in the experimental tanks was daily renewed through clean water that was continuous filtrated and aerated to ensure the high saturation of dissolved oxygen (10 ppm) level. A photoperiod of 12-h light:12-h dark was used throughout the experimental period. The water heater was used to maintain the water temperature at 27.0 ± 0.5°C during the experimental period. Fish were fed with one of the nine experimental diets two times per day (10:00 and 18:00) in triplicate at the rate of 1.5% to 2% of wet body weight for 8 weeks. Fish tanks were daily siphoned at 15:00 pm to clean up the fecal matters and uneaten feeds. Mortality of all treatment groups was checked every day during the experimental period. Dead fish were immediately removed from the tanks and weighed to keep record, if there any died. Total fish weight in each tank was recorded at the end of the fourth week and at the end of the eight week, and the amount of feeds fed to the fish was calculated accordingly. Before each sampling, feeding was stopped and the fish were starved for 24 h to prevent any intervention of additional measurement of ingested feeds by the fish during fish weight sampling as well as to reduce stress in fish.
Growth parameters
At the end of the 8-week feeding trial, weight gain (WG), specific growth rate (SGR), feed efficiency (FE), protein efficiency ratio (PER), and survival rate (SR) of experimental treatments were calculated. Three fish per tank randomly were collected and the weight and length of fish were measured to obtain condition factor (CF), and then, the fish were dissected to measure weight of different organs for organosomatic indices such as weight of visceral mass for viscerosomatic index (VSI) and weight of liver for hepatosomatic index (HSI) in relation to the fish weight. Equations of each variable are as follows:
Proximate composition analysis
The proximate compositions of diets and fish were determined according to standard procedures of AOAC (2000). For this, three additional fish per tank were collected according to the experimental group to analyze the whole-body proximate composition. The fresh fish samples were minced and stored in −20°C according to the dietary treatments until further analyses. Samples of the diets and minced fish were dried to a constant weight (1 g for each sample) at 105°C to determine the moisture contents in experimental diets and fish. For the analysis of ash contents, samples (1 g for each sample) were incinerated in a muffle furnace at 550°C for 3 h. For crude lipid analysis, samples (1 g for each sample) were extracted by ethyl-ether to obtain fat contents using a Soxhlet extraction unit (Soxtec system 1046, Tecator AB, Foss, Hoganas, Sweden). The crude protein contents in the diets and fish samples were analyzed using the Kjeldahl method (N × 6.25) after the determination of nitrogen (N) content through acid digestion, distillation, and titration of the samples.
Serum biochemical and non-specific immune responses analyses
Blood samples were collected from the same fish (three fish per tank) after measuring weight and length of fish but before dissecting the fish for organosomatic indices. The whole blood was withdrawn from the randomly collected three fish per tank and then pooled for the analyses of serum biochemical parameters and non-specific immune responses. For this, fish from the each tank were anesthetized with ethylene glycol phenyl ether (200 mg/L), and blood samples were collected through the puncturing of caudal vein using 1 ml of non-heparinized syringe. Serum was separated from the blood by centrifugation at 5,000 × g for 10 min, and then, the transparent supernatant (serum) was stored at −70°C freezer for the biochemical analysis such as glutamic pyruvic transaminase (GPT), glutamic oxaloacetic transaminase (GOT), glucose, and total protein (TP) and non-specific immune response enzymes such as lysozyme and superoxide dismutase (SOD) activities.
For the analysis of serum biochemical parameters in fish (0.1 ml for each sample), different kits for GPT, GOT, TP, and glucose analyses (Fuji Photo Film Ltd., Tokyo, Japan) were used through an automatic biochemical analyzer (Fuji DRI-CHEM 3500i, Fuji Photo Film, Ltd., Tokyo, Japan) according to the manufacturer’s protocols.
For the analyses of non-specific immune responses in fish, turbidometric assay was used according to Hultmark et al. (1980) for serum lysozyme activity with slight modifications. Briefly, serum samples (0.2 ml each) were added to bacterial solution, Micrococcus lysodeikticus (0.75 mg/ml) with 0.1 M sodium phosphate buffer (pH 6.4) based on the dietary treatments and according to the manufacturers’ protocols. The enzymatic reactions were taken place in a 96-well microplate at 20°C and an absorbance at 570 nm between 0 and 30 min using a microplate reader (TECAN M200 Plate Reader, Tecan Trading AG, Männedorf, Switzerland). A reduction in the absorbance of 0.001/min was considered as one unit of lysozyme activity. The lysozyme activity was analyzed using the following formula:
Furthermore, SOD activity of serum was determined by the percentage inhibitory reaction of enzyme with WST-1 (water-soluble tetrazolium dye) substrate and xanthine oxidase using a SOD assay kit (Enzo ADI-900-157, Enzo Life Sciences, Inc., Farmingdale, NY, USA) according to the manufacturer’s instructions. The absorbance was read at 450 nm, and each point was monitored after incubation of the samples for 20 min at 37°C (the absorbance wavelength for the colored product of WST-1 reaction with superoxide) using the microplate reader (TECAN M200 Plate Reader, Tecan Trading AG, Männedorf, Switzerland). The inhibition percentage was normalized by milligram (mg) protein and presented as SOD enzyme activity unit.
Challenge test against Vibrio anguillarum
At the end of 8-week feeding trial, a 7-day long bacterial challenge test was conducted to check the efficacy of experimental diets against disease resistance in fish infected against the pathogenic bacterium, Vibrio anguillarum. The pathogenic bacterium, V. anguillarum (KCCM 40293, Korean Culture Center of Microorganisms, Seoul, Republic of Korea), was obtained from the Department of Biotechnology, Pukyong National University, Busan, Korea. The bacteria originally sourced from infected Japanese eel with Vibrio anguillarum was cultured in tryptic soy broth (tryptic soy agar, Sigma-Aldrich, USA) at 26°C for 24 h and stored with 20% glycerol at −80°C for further use in challenge test. For the challenge test program, fish were re-accommodated in the experimental tanks according to the diet groups, and five fish for each tank were intraperitoneally (i.p.) injected with 0.1 ml per fish at a suitable concentration of 5 × 107 CFU/ml of the pathogenic bacterium based on the study of Lee et al. (2018). The water temperature in the experimental tanks was maintained at 27 ± 0.5°C throughout the challenge test. The water in the experimental tanks was unchanged, and no water circulation is provided in the tanks during challenge test. Everyday mortality of fish was checked and recorded accordingly. If there were any dead fish, then they were immediately removed from the tanks until 7 days. The pathological samples such as skin mucus, liver, gill, and kidney from immediately removed dead fish were analyzed by an API 20 E pathogenic bacteria identification kit (BioMerieux, Durham, NC, USA) to ensure the pathogenicity of V. anguillarum in dead fish.
Statistical analysis
The mean values of the fish tanks (n = 3) were subjected to statistical analysis. The Shapiro–Wilk and O’Brien tests were used for normality and homogeneity of variance, respectively. Data were statistically presented using one-way analysis of variance (ANOVA) to verify the effects of the experimental treatments. For the comparison of the experimental treatment means, a Fisher’s protected least significant difference (LSD) post hoc test was used to elucidate the significant effects of the dietary treatments. The dietary effects were based on the significant level at P< 0.05. The statistical analyses were performed using a SAS version 9.1 analytical software (SAS Institute, Cary, NC, USA). According to Kaplan and Meier (1958), a survivability curve for the challenge test was used to represent the cumulative survival rate in fish fed with the experimental diets.
Results
Growth performance
Growth performance data of juvenile Japanese eel, Anguilla japonica, fed with the dietary treatments for 8 weeks are presented in Table 2. At the end of the feeding trial, WG, SGR, FE, and PER of fish fed with the FMA0 were significantly higher than fish fed with the FMA20 diet. However, there were no significant differences among fish fed with the FMA0, FMA10, FMA0SG, FMA0YM, FMA20SG, and FMA20YM diets. Moreover, no significant differences were observed in WG and SGR of fish fed with the FMA10, FMA20, FMA20SG, and FMA20YM diets. HSI of fish fed with the FMA0 diet was significantly higher than fish fed with the FMA20 and FMA40 diets. However, there were no significant differences in HSI among fish fed with the FMA0, FMA10, FMA30, FMA0SG, FMA0YM, FMA20SG, and FMA20YM. VSI of fish fed with the FMA0 diet was significantly higher than fish fed with the FMA40 diet. However, there were no significant differences in VSI among fish fed with the FMA0, FMA10, FMA20, FMA30, FMA0SG, FMA0YM, FMA20SG, and FMA20YM diets. There were no significant differences in CF and survival among fish fed with all the experimental diets.
Whole-body proximate analysis
Whole-body proximate compositions of juvenile Japanese eel fed with the experimental diets are presented in Table 3. There were no significant differences (P< 0.05) in whole-body crude protein, crude lipid, moisture, and ash contents among fish fed with all the experimental diets after the feeding trial.
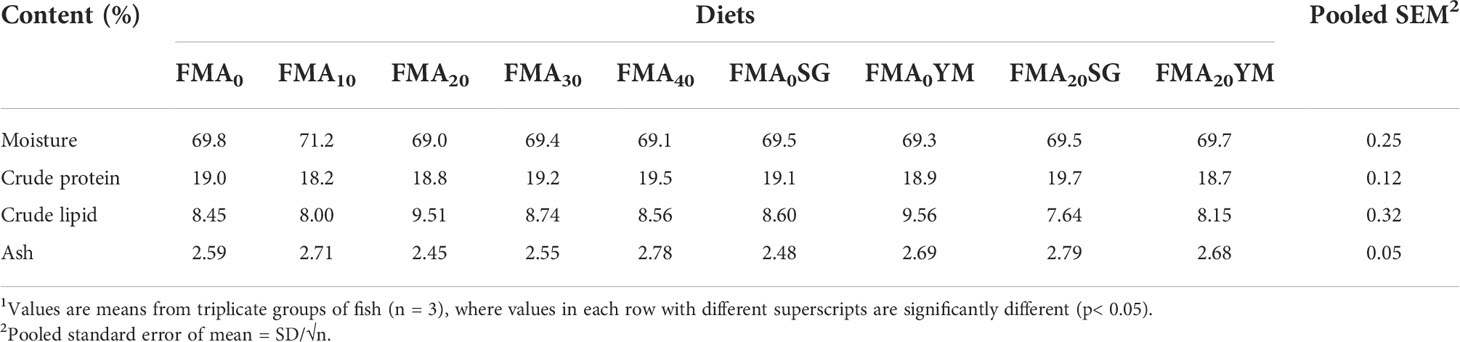
Table 3 Whole-body proximate composition of juvenile Japanese eel fed with the experimental diets for 8 weeks (% dry matter basis)1.
Non-specific immune responses
Non-specific immune responses of juvenile Japanese eel fed with the experimental diets are summarized in Table 4. Lysozyme activities in fish fed with the FMA10, FMA20, FMA30, and FMA40 diets were significantly lower than fish fed with the FMA0SG, FMA0YM, FMA20SG, and FMA20YM diets. However, there were no significant differences in lysozyme activities among the fish fed with the FMA0, FMA10, FMA20, FMA30, and FMA40 diets as well as among the fish fed with the FMA0SG, FMA0YM, FMA20SG, and FMA20YM diets. There were no significant differences (P< 0.05) in SOD activities among fish fed with all the experimental diets

Table 4 Non-specific immune responses in juvenile Japanese eel fed with the experimental diets for 8 weeks1.
Hematological indices
Serum biochemical parameters of juvenile Japanese eel fed with the experimental diets are summarized in Table 5. There were no significant differences (P< 0.05) in serum glucose, TP, GOT, and GPT among fish fed with all the experimental diets at the end of 8 weeks feeding trial.
Challenge test
Cumulative survival rate of juvenile Japanese eel challenged with V. Anguillarum for 7 days is shown in Figure 1. During the challenge test, the first mortality in fish was recorded on the second day, and it was prominent after the third day of intraperitoneal injection in fish. At the end of 7 days of challenge test program, cumulative survival rates of fish fed with the FMA0SG and FMA0YM diets were significantly higher than fish fed with the FMA0 diet (P< 0.05). However, cumulative survival rate of fish fed with the FMA20SG, FMA20YM, and FMA0 diets showed no significant differences (P< 0.05).
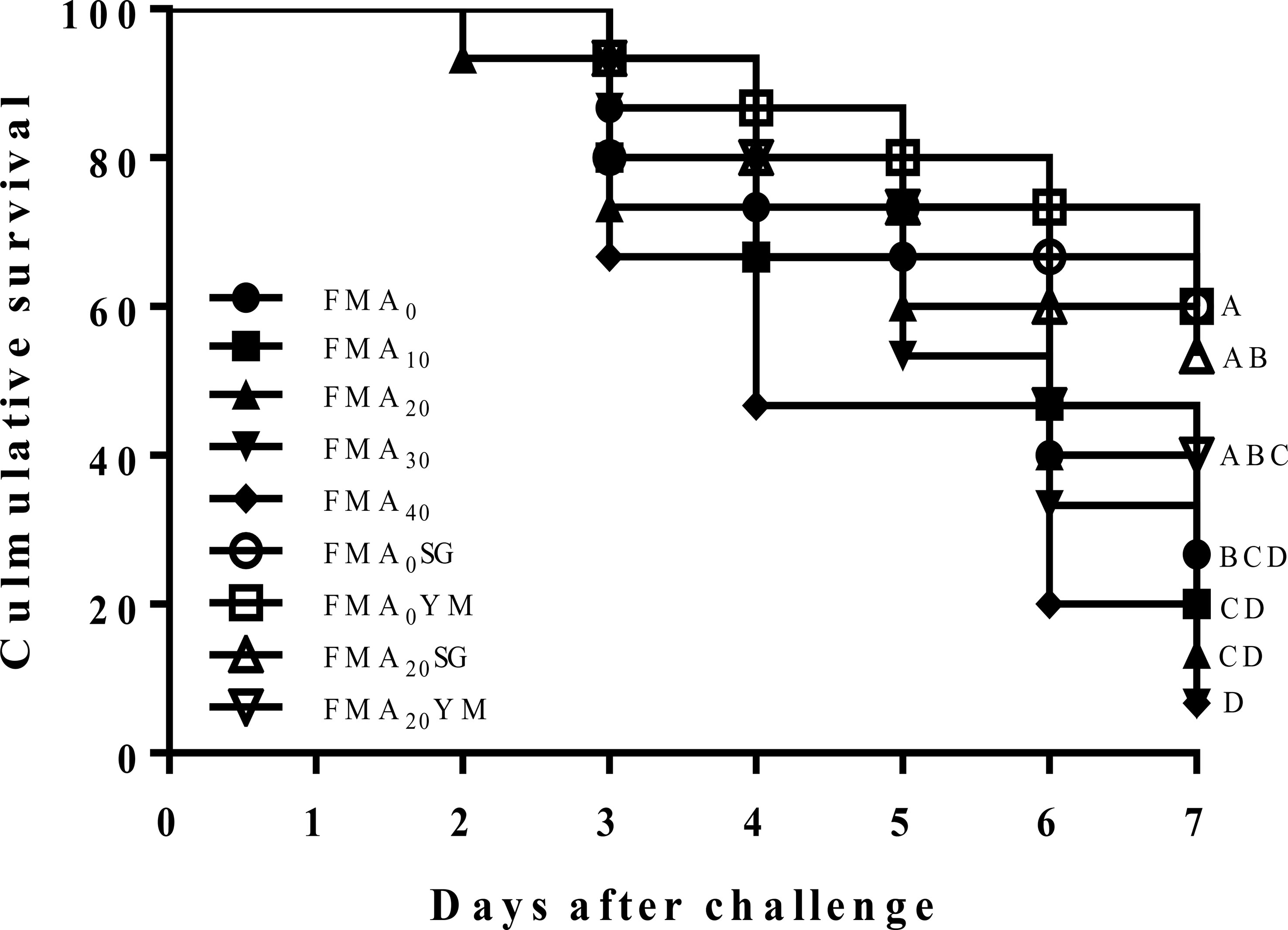
Figure 1 Cumulative survival rate after challenged with V. anguillarum for 7 days in Japanese eel fed with the nine experimental diets after 8 weeks.
Discussion
The high survival rate recorded during this study could be attributed to the high acceptability of the experimental diets by the juvenile Japanese eel. In the present study, WG, SGR, and FE of fish fed with 20%, 30%, and 40% of FMA were significantly lower than those of control (FMA0) group. Wang et al. (2013) reported similar findings while replacing FM by dietary poultry by-product meal for Japanese sea bass, Lateolabrax japonicas. However, there is scarce information on the replacement of FM by FMA in the diets of any type of fish species. In a study, it was demonstrated that inclusion of 25% of feather meal in the diet of juvenile tench, Tinca tinca, significantly lowered the growth performance (total length, total weight, SGR, and FCR) compared with the control treatment without feather meal (González-Rodríguez et al., 2016). One of the main ingredients of FMA is feather meal; thus, our results are in consistent with the findings by González-Rodríguez et al. (2016). It is reported that animal protein sources such as meat and bone meal, feather meal, blood meal, and poultry by-product meal can replace FM up to 50% in salmonid feeds (Lee et al., 2001). Fowler (1991) found that a practical diet consist of 20% of poultry by-product meal could reduce 50% of FM in the diet of chinook salmon without hampering the growth and FE in fish. In another study, MAB (mixture of animal by-product; mixture of leather meal, meat and bone meal, feather meal, squid liver powder, poultry by-product meal, and blood meal) was used as a fishmeal replacer in the diet of juvenile rainbow trout, and lower WG, SGR, FE, and PER were reported for fish fed with 20% of FM replacement level (Lee et al., 2001). To the best of our knowledge, the results are the first to use six animal protein sources in combined form as FMA to replace FM in the diet of a fish species (Lee et al., 2001). Feather meal as an alternative animal protein source was found to be slight replacer for FM in the diets for coho salmon (Higgs et al., 1979), chinook salmon (Fowler, 1991), and Nile tilapia (Bishop et al., 1995). Blood meal was also evaluated as a FM replacer in the diets for rainbow trout (Luzier et al., 1995), eel (Lee and Bai, 1997a), and tilapia (Lee and Bai, 1997b). Ma et al. (2014) indicated that more than 21% of FM can be replaced by poultry by-product meal in the diets for golden pompano. Likewise, the present study demonstrated that the dietary FMA with addition of additives produced similar responses in terms of growth increment, feed utilization, and immunity in juvenile Japanese eel. In this study, the results demonstrated a more positive response of juvenile Japanese eel to dietary SG and YM supplementations compared with the control diet. Fish fed with the FMA0SG, FMA0YM, FMA20SG, and FMA20YM diets exhibited significantly higher WG, SGR, FE, and PER compared with fish fed with the FMA20 diet but they were not significantly different among FMA0, FMA0SG, FMA0YM, FMA20SG, and FMA20YM diet groups. The results indicate the potential of the supplementation of natural feed additives in replacing FM in the diet of juvenile Japanese eel. In consistent with the present study, growth enhancement by Yucca extract additives have been reported previously in several fish species such as channel catfish, tilapia, and striped catfish (Kelly and Kohler, 2003; Gaber, 2006; Güroy et al., 2014). Francis et al. (2001) reported that supplementation of Quillaja saponin at 150 mg/kg diet can increase the growth performance of Nile tilapia. Likewise, it is reported that dietary additive, Quillaja saponin, at the same inclusion level (150 mg/kg) could increase 18% of average weight of common carp. In another study, Güroy et al. (2014) found that a diet containing 0.15% of Yucca schidigera extract could improve the zootechnical parameters in striped catfish (Pangasianodon hypophthalmus). Moreover, supplementation of Yucca in the diet of Nile tilapia could increase the feed utilization, apparent protein digestibility coefficient, and whole-body protein content of the fish (Gaber, 2006). The observations were similar to the findings by Won et al. (2017); Amoah et al. (2017), and Bae et al. (2020) with yellow loess, SG, and YM in rainbow trout, Amur catfish, and olive flounder fish. It is widely reported that dietary plant extract together with minerals can enhance the growth performance in fish through lipid metabolism by the breakdown of fatty acids and production of energy (Ji et al., 2007). According to our results, more than 10% replacement of FM resulted in reduction of growth, which is probably due to the inferior quality of the animal protein sources, where leather meal and squid liver powder were found to be effective in the diets of freshwater fish species (Higgs et al., 1979). Animal by-products are important source of minerals especially calcium (Ca), iron (Fe), and manganese (Mn) that may exert the antagonistic behavior of Ca with other minerals during the mineral absorption in fish (Sugiura et al., 2000). When FM is replaced with FMA that constitute with blended ingredients from animal by-products, it is difficult to interpret the results in nutrient metabolism due to the presence of minerals in the diets (Jamil et al., 2007). Consequently, the findings of the present study suggest up to 10% of FM can be replaced by FMA with the addition of YM and SG as natural mineral additives (Won et al., 2017) in the diets of juvenile Japanese eel diets, which ultimately improved the growth and feed utilization in fish.
CF, HSI, and VSI are important measurement tools in terms of assessing food value in an animal and its health status and general welfare (Ighwela et al., 2014). In the present study, there were significant differences in HSI and VSI between fish fed with the control and FMA40 diet groups, which can be attributed to the reduced growth, feed utilization, and health status in fish at higher replacement level of FM with FMA in diet. Abdel-Warith et al. (2001) reported the alterations of liver tissues in terms of degeneration in liver structures in African catfish fed with high inclusion levels of poultry by-product meal in relation to FM in the diets. Therefore, it has utmost importance to examine the liver and gastrointestinal tract (GIT) of fish in replacing FM with the rendered animal protein ingredients in the fish diets. In this study, insignificant and high survival rate of fish might be due to the high acceptability of the experimental diets by the juvenile Japanese eel.
Whole-body proximate composition in fish fed with the experimental diets was not significantly affected after chemical analyses. Gaber (2006) reported higher body protein and low body lipid levels in Nile tilapia fed Yucca-supplemented diets in comparison with the basal diet. In a related study, Yilmaz et al. (2012) also found lower crude lipid levels in sea bass, Dicentrarchus labrax, fed with the herbal extracts compared with the control diet. According to these studies, plant extracts could reduce the crude lipid contents in cultured fish. However, the observations were on contrary to our results and the reason could be related to different diet compositions and culture conditions. In agreement with the present study, Damusaru et al. (2019) did not find any significant effect of different levels of FMA replacing FM on proximate composition of growing Japanese eel.
Non-specific immune response is a primary or basic defense system in fish which also plays an important function in the specific or secondary immune system and body homeostasis through a system of receptor proteins (Halver and Hardy, 2002; Uribe et al., 2011; Reverter et al., 2014; Srivastava and Pandey, 2015). Lysozyme is produced mainly by macrophages in response to microbial components and many other immune stimulants (Siwicki and Anderson, 1993; Ringø et al., 2012), and it is a preferred marker of the immune response due to its close association with leucocytes (Kiron, 2012). On the other hand, SOD is a metallo-enzyme that catalyzes the dismutation of the superoxide radical (O2) into hydrogen peroxide (H2O2) and molecular oxygen (O2) (Radi et al., 1985), providing an important defense against oxidative damage. In the present study, no significant differences for serum SOD enzyme were observed in fish fed with the experimental diets. However, serum lysozyme activities of fish fed with the diets consisting of SG and YM were significantly higher than other diets, which indicate the enhanced immunity function in fish due to the supplementation of natural minerals (SG and YM) in the diets. Improved immunity has been reported in cultured fish and shellfish fed with several feed additives such as mushroom extract (Chelladurai and Maran, 2019), yellow loess (Won et al., 2017), SG (Won et al., 2017; Bae et al., 2020), and YM (Njagi et al., 2017; Bae et al., 2020). Ji et al. (2015) demonstrated that 80% replacement of FM by silkworm pupae meal in the diet of juvenile Jian carp (Cyprinus carpio) significantly decreased hepatic SOD activities. These results are partly comparable with our results showing the beneficial effects of SG and YM for improving non-specific immune responses.
Hematological and serum parameters are good indicators of the health status of an organism, and they can vary with season, temperature, and nutritional status (Blaxhall, 1972). Bae et al. (2020) suggested that any unhealthy condition caused by poor nutrition could affect the hematological characteristics of fish. Glucose is one of the stress indicators in fish. Stress hormones in conjunction with cortisol mobilize and elevate glucose production in fish to cope with the energy demand of a stressor (Aedo et al., 2019). Total plasma protein is considered a strong innate response in fish (Reverter et al., 2014), and measurements can reflect nutritional status (Acharya and Mohanty, 2014). It is the protein component of the blood, which increases with starvation or any other stress. Plasma GOT and GPT, on the other hand, are proteins or enzymes found mainly in liver cells and elevated levels in the blood indicate an inflammation or damage of liver cells (Giannini et al., 2005). In the present study, no significant differences were recorded in all serum parameters in juvenile Japanese eel fed with the experimental diets, indicating good health status of the fish. In the present study, challenge test supports the results of the immune responses such as lysozyme activities in fish fed with the experimental diets.
Conclusions
In summary, the results indicated that WG, SGR, FE, and PER were significantly different in fish fed with the FMA0 (control diet) diet compared with fish fed with the FMA20, FMA30, and FMA40 diets. Whereas no significant differences were observed among fish fed with the FMA0, FMA10, FMA0SG, FMA0YM, FMA20SG, and FMA20YM diets. Whole-body proximate composition of fish did not show significant differences among treatment groups. Fish fed with the diets consisting of SG and YM showed a higher lysozyme activity than all other diets that replaced FM with FMA. In addition, there were no significant differences in the hematological indexes among fish fed with the experimental diets. Therefore, on the basis of the results for WG, SGR, FE, PER, lysozyme, SOD, and hematological indexes, FMA could replace up to 10% of FM in the diets of juvenile Japanese eel. However, considering the natural minerals as additives, supplementation of dietary SG and YM in FMA could replace up to 20% of FM in the diets of juvenile Japanese eel.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by Institutional Animal Care and Use Committee Regulations, No. 554, issued by the Pukyong National University, Busan, South Korea.
Author contributions
JB has planned the experiment; determined the growth, somatic indices, non-specific immune enzyme activities, and hematology; and drafted the final article. SgL did supervision, statistical analyses, and final drafting of the manuscript. MM analyzed the statistical data and finalized the manuscript. AH, WC, and SnL determined the growth, somatic indices, non-specific immune enzyme activities, and hematology. TM helped in final drafting and checking grammar of the manuscript. S-KK and SB critically supervised and helped in experimental planning with the addition of manuscript drafting and finalizing. The authors read and approved the final manuscript.
Funding
This work was supported by a grant from the National Institute of Fisheries Science (NIFS), Republic of Korea (R2022016).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
MM would like to acknowledge the National Research Foundation of Korea (NRF) for providing the postdoctoral research fellowship under the Brain Pool Program (Grant No. 2019H1D3A1A01101555) funded by the Ministry of Science, ICT, and Future Planning.
References
Aas T. S., Helland B. G., Terjesen B. F., Helland S. J. (2006). Improved growth and nutrient utilisation in Atlantic salmon (Salmo salar) fed diets containing a bacterial protein meal. Aquaculture 259, 1–4. doi: 10.1016/j.aquaculture.2006.05.032
Abdel-Warith A. A., Russel P. M., Davies S. J. (2001). Inclusion of a commercial poultry by-product meal as a protein replacement of fish meal in practical diets for African catfish Clarias gariepinus (Burchell 1822). Aquac. Res. 32, 296–305. doi: 10.1046/j.1355-557x.2001.00053.x
Acharya G., Mohanty P. K. (2014). Comparative Haematological and serum biochemical analysis of catfishes clarias batrachus (Linnaeus 1758) and Heteropneustes fossilis (Bloch 1794) with respect to sex. J. Entomol. Zool. Stud. 2, 191–197. doi: 10.22271/j.ento
Aedo J. E., Ruiz-Jarabo I., Martinez-Rodriguez G., Boltana S., Molina A., Valdes J. A., et al. (2019). Contribution of non-canonical cortisol actions in the early modulation of glucose metabolism of gilthead Sea bream (Sparus aurata). Front. Endocrinol. 10, 779. doi: 10.3389/fendo.2019.00779
Amoah Y. T., Moniruzzaman M., Lee S. H., Bae J. H., Won S. H., Seong M., et al. (2017). Evaluation of different dietary additives based on growth performance, innate immunity and disease resistance in juvenile amur catfish, Silurus asotus. Int. Aquat. Res. 9, 351–360. doi: 10.1007/s40071-017-0181-2
AOAC (2000). Official methods of analysis of the association of official analytical chemists (Arlington, VA: Association of Official Analytical Chemists).
Bae J., Hamidoghli A., Won S., Choi W., Lim S. G., Kim K. W., et al. (2020). Evaluation of seven different functional feed additives in a low fish meal diet for olive flounder, Paralichthys olivaceus. Aquaculture 525, 735333. doi: 10.1016/j.aquaculture.2020.735333
Bai S. C., Katya K., Yun H. (2015). “Additives in aquafeed: an overview,” in Feed and feeding practices in aquaculture. Ed. Allen D. D. (Kidlington, UK: Woodhead publishing, Elsevier), 170–202.
Barrows F. T., Frost J. B. (2014). Evaluation of the nutritional quality of co-products from the nut industry, algae and an invertebrate meal for rainbow trout, Oncorhynchus mykiss. Aquaculture 434, 315–324. doi: 10.1016/j.aquaculture.2014.08.037
Bishop C. D., Angus R. A., Watts A. (1995). The use of feather meal as a replacement for fishmeal in the diet of Oreochromis niloticus fry. Bioresour. Technol. 54, 291–295. doi: 10.1016/0960-8524(95)00146-8
Blaxhall P. C. (1972). The haematological assessment of the health of freshwater fish. J. Fish Biol. 4, 593–604. doi: 10.1111/j.1095-8649.1972.tb05704.x
Cheeke P. R., Piacente S., Oleszek W. (2006). Anti-inflammatory and anti-arthritic effects of Uucca schidigera: A review. J. Inflamm. 3, 6. doi: 10.1186/1476-9255-3-6
Chelladurai G., Maran B. A. V. (2019). Dietary supplementation of mushroom extract enhances growth and antioxidant levels of Babylonia spirata (Mollusca: Gastropoda). Aquac. Rep. 15, 100218. doi: 10.1016/j.aqrep.2019.100218
Choi S., Ko S., Park G., Lim S., Yu G., Lee J., et al. (2004). Utilization of song-gang stone as the dietary additive in juvenile olive flounder, Paralichthys olivaceus. J. Aquac 17, 39–45.
Citarasu T. (2010). Herbal biomedicines: A new opportunity to aquaculture industry. Aquacult. Int. 18, 403–414. doi: 10.1007/s10499-009-9253-7
Damusaru J. H., Moniruzzaman M., Park Y., Seong M., Jung J. Y., Kim D. J., et al. (2019). Evaluation of fish meal analogue as partial fish meal replacement in the diet of growing Japanese eel, Anguilla japonica. Anim. Feed Sci. Technol. 247, 41–52. doi: 10.1016/j.anifeedsci.2018.10.018
FAO (Food and Agriculture Organization) (2016). The state of world fisheries and aquaculture (Rome: Food and Agriculture Organization of the United Nations).
Fowler L. G. (1991). Poultry by-product meal as a dietary protein source in fall chinook salmon diets. Aquaculture 99, 3–4. doi: 10.1016/0044-8486(91)90251-2
Francis G., Makkar H. P. S., Becker K. (2001). Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 199, 197–227. doi: 10.1016/S0044-8486(01)00526-9
Frans I., Michiels C. W., Bossier P., Willems K., Lievens B., Rediers H. (2011). Vibrio anguillarum as a fish pathogen: virulence factors, diagnosis and prevention. J. Fish Dis. 34, 643–661. doi: 10.1111/j.1365-2761.2011.01279.x
Gaber M. M. (2006). The effects of plant-protein-based diets supplemented with yucca on growth, digestibility, and chemical composition of Nile tilapia (Oreochromis niloticus, l) fingerlings. J. World Aquac. Soc 37, 74–81. doi: 10.1111/j.1749-7345.2006.00008.x
Gatlin D. M., Barrows F. T., Brown P., Dabrowski K., Gaylord T. G., Hardy R. W., et al. (2007). Expanding the utilization of sustainable plant products in aquafeeds: a review. Aquac. Res. 38, 551–579. doi: 10.1111/j.1365-2109.2007.01704.x
Giannini E. G., Testa R., Savarino V. (2005). Liver enzyme alteration: a guide for clinicians. Can. Med. Assoc. J. 172, 367–379. doi: 10.1503/cmaj.1040752
Glencross B. D., Booth M., Allan G. L. (2007). A feed is only as good as its ingredients – a review of ingredient evaluation strategies for aquaculture feeds. Aquac. Nutr. 13, 17–34. doi: 10.1111/j.1365-2095.2007.00450.x
González-Rodríguez A., Celada J. D., Carral J. M., Saez-Royuela M., Garcia V., Fuertes J. B. (2016). Evaluation of poultry by-product meal as partial replacement of fish meal in practical diets for juvenile tench (Tinca tinca l.). Aquac. Res. 47, 1612–1621. doi: 10.1111/are.12622
Güroy B., Mantoğlu S., Kayal S., Şahin İ. (2014). Effect of dietary yucca schidigera extract on growth, total ammonia-nitrogen excretion and haematological parameters of juvenile striped catfish pangasianodon hypophthalmus. Aquac. Res. 45, 647–654. doi: 10.1111/are.12001
Halver J. E., Hardy R. W. (2002). “Fish nutrition,” in The lipids, 3rd edition. Eds. Sargent J. R., Tocher D. R., Bell G. (California: Academic Press), 182–246.
Higgs D. A., Markert J. R., MacQuarrie D. W., McBride J. R., Dosanjh B. S., Nichols C., et al. (1979). “Development of practical dry diets for coho salmon using poultry-by-product meal, feather meal, soybean meal and rapeseed meal as major protein sources,” in Finfish nutrition and fish feed technology. Eds. Halver J. E., Tiews K. (Berlin: Heenemann Verlagsgesellschaft MbH), 3–68.
Hultmark D., Steiner H., Rasmuson T., Boman H. G. (1980). Insect immunity, purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of hyalophora cecropia. Eur. J. Biochem. 106, 7–16. doi: 10.1111/j.1432-1033.1980.tb05991.x
Ighwela K., Ahmad A., Abol-Munafi A. (2014). The selection of viscerosomatic and hepatosomatic indices for the measurement and analysis of Oreochromis niloticus condition fed with varying dietary maltose levels. Int. J. Fauna Biol. Stud. 1, 18–20. doi: 10.22271/23940522
Jacoby D., Gollock M. (2014). “Anguilla Anguilla,” in The IUCN red list of threatened species. version 2014.3. Available at: www.iucnredlist.org.
Jamil K., Abbas G., Akhtar R., Hong L., Zhenxing L. (2007). Effects of replacing fishmeal with animal by-products meal supplementation in diets on the growth and nutrient utilization of mangrove red snapper. J. Ocean Univ. China 6, 292–298. doi: 10.1007/s11802-007-0292-2
Ji S. C., Takaoka O., Jeong G. S., Lee S. W., Ishimaru K., Seoka M., et al. (2007). Dietary medicinal herbs improve growth and some non-specific immunity of red sea bream pagrus major. Fish. Sci. 73, 63–69. doi: 10.1111/j.1444-2906.2007.01302.x
Ji H., Zhang J. L., Huang J. Q., Cheng X. F., Liu C. (2015). Effect of replacement of dietary fish meal with silkworm pupae meal on growth performance, body composition, intestinal protease activity and health status in juvenile jian carp (Cyprinus carpio var. jian). Aquac. Res. 46, 1209–1221. doi: 10.1111/are.12276
Jo H., Lee S., Yun H., Hong J., Moniruzzaman M., Bai S. C., et al. (2017). Evaluation of dietary fishmeal analogue with addition of shrimp soluble extract on growth and nonspecific immune response of rainbow trout, Oncorhynchus mykiss. J. World Aquac. Soc 48, 583–591. doi: 10.1111/jwas.12355
Kaplan E. L., Meier P. (1958). Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 53, 457–481. doi: 10.1080/01621459.1958.10501452
Kelly A. M., Kohler C. C. (2003). Effects of yucca shidigera extract on growth, nitrogen retention, ammonia excretion, and toxicity in channel catfish ictalurus punctatus and hybrid tilapia Oreochromis mossambicus x O-niloticus. J. World Aquac. Soc 34, 156–161. doi: 10.1111/j.1749-7345.2003.tb00052.x
Kiron V. (2012). Fish immune system and its nutritional modulation for preventive health care. Anim. Feed Sci. Technol. 173, 111–133. doi: 10.1016/j.anifeedsci.2011.12.015
Kokou F., Sarropoulou E., Cotou E., Rigos G., Henry M., Alexis M., et al. (2015). Effects of fish meal replacement by a soybean protein on growth, histology, selected immune and oxidative status markers of gilthead Sea bream, Sparus aurata. J. World Aquac. Soc 46, 115–128. doi: 10.1111/jwas.12181
Krogdahl A., Penn M., Thorsen J., Refstie S., Bakke A. M. (2010). Important antinutrients in plant feedstuffs for aquaculture: an update on recent findings regarding responses in salmonids. Aquac. Res. 41, 333–344. doi: 10.1111/j.1365-2109.2009.02426.x
Lee K. J., Bai S. C. (1997a). Haemoglobin powder as a dietary fish meal replacer in juvenile Japanese eel, Anguilla japonica (Temminck et schlegel). Aquac. Res. 28, 509–516. doi: 10.1046/j.1365-2109.1997.00887.x
Lee K. J., Bai S. C. (1997b). Hemoglobin powder as a dietary animal protein source for juvenile Nile tilapia. Progress. Fish-Cult. 59, 266–271. doi: 10.1577/1548-8640(1997)059<0266:HPAADA>2.3.CO;2
Lee K., Dabrowski K., Blom J. H., Bai S. C. (2001). Replacement of fish meal by a mixture of animal by-products in juvenile rainbow trout diets. North Am. J. Aquac. 63, 109–117. doi: 10.1577/1548-8454(2001)063<0109:ROFMBA>2.0.CO;2
Lee S., Katya K., Hamidoghli A., Hong J., Kim D. J., Bai S. C. (2018). Synergistic effects of dietary supplementation of bacillus subtilis WB60 and mannanoligosaccharide (MOS) on growth performance, immunity and disease resistance in Japanese eel, Anguilla japonica. Fish Shellfish Immunol. 83, 283–291. doi: 10.1016/j.fsi.2018.09.031
Luzier J. M., Summerfelt R. C., Ketola H. G. (1995). Partial replacement of fish meal with spray-dried blood powder to reduce phosphorus concentrations in diets for juvenile rainbow trout, Oncorhynchus mykiss (Walbaum). Aquac. Res. 26, 577–587. doi: 10.1111/j.1365-2109.1995.tb00948.x
Ma Z., Guo H., Zheng P., Wang L., Jiang S., Qin J. G., et al. (2014). Ontogenetic development of diges-tive functionality in golden pompano trachinotus ovatus (Linnaeus 1758). Fish Physiol. Biochem. 40, 1157–1167. doi: 10.1007/s10695-014-9912-0
Moutinho S., Martínez-Llorens S., Tomás-Vidal A., Jover-Cerdá M., Oliva-Teles A., Peres H. (2016). Meat and bone meal as partial replacement for fish meal in diets for gilthead seabream (Sparus aurata) juveniles: Growth, feed efficiency, amino acid utilization, and economic efficiency. Aquaculture 468, 271–277. doi: 10.1016/j.aquaculture.2016.10.024
Naylor R. L., Hardy R. W., Bureau D. P., Chiu A., Elliott M. A., Farrell P., et al. (2009). Feeding aquaculture in an era of finite resources. Proc. Nat. Acad. Sci. 106, 15103–15110. doi: 10.1073/pnas.0905235106
Njagi G. W., Lee S. H., Won S. H., Hong J. W., Hamidoghli A., Bai S. C. (2017). Effects of dietary yucca meal on growth, haematology, non-specific immune responses and disease resistance of juvenile Nile tilapia Oreochromis niloticus. Aquac. Res. 48, 4399–4408. doi: 10.1111/are.13264
NRC (National Research council) (2011). Nutrient requirements of fish and shrimp (Washington, DC: The National Academic Press).
Oliva-Teles A., Enes P., Peres H. (2015). “Replacing fishmeal and fish oil in industrial aquafeeds for carnivorous fish,” in Feed and feeding practices in aquaculture. Ed. Davis D. A. (Oxford: Woodhead Publishing), 203–233.
Piacente S., Pizza C., Oleszek W. (2005). Saponins and phenolics of yucca schidigera roezl: Chemistry and bioactivity. Phytochem. Rev. 4, 177–190. doi: 10.1007/s11101-005-1234-5
Radi A. A. R., Hai D. Q., Matkovics B., Gabrielak T. (1985). Comparative antioxidant enzyme study in freshwater fish with different types of feeding behavior. Comp. Biochem. Physiol. Part C: Comp. Pharmacol. 81, 395–399. doi: 10.1016/0742-8413(85)90026-x
Reverter M., Bontemps N., Lecchini D., Banaigs B., Sasal P. (2014). Use of plant extracts in fish aquaculture as an alternative to chemotherapy: current status and future perspectives. Aquaculture 433, 50–61. doi: 10.1016/j.aquaculture.2014.05.048
Ringø E., Olsen R. E., Gonzales Vecino J. L., Wadsworth S., Song S. K. (2012). Use of immunostimulants and nucleotides in aquaculture: a review. J. Mar. Sci. Res. Dev. 2, 104. doi: 10.4172/2155-9910.1000104
Siwicki A. K., Anderson D. P. (1993). “Nonspecific defence mechanisms assay in fish. II. potential killing activity of neutrophils and macrophages, lysozyme activity in serum and organs and total immunoglobulin (T- ig) levels in serum,” in Fish diseases diagnosis and prevention’s methods (Poland:FAO-Project GCP/INT/526/JPN, IFI Olsztyn), 105–112.
Srivastava P. K., Pandey A. K. (2015). Role of immunostimulants in immune responses of fish and shellfish. Biochem. Cell Arch. 15, 47–73.
Sugiura S. H., Babbitt J. K., Dong F. M., Hardy R. W. (2000). Utilization of fish and animal by-product meals in low-pollution feeds for rainbow trout Oncorhynchus mykiss (Walbaum). Aquac. Res. 31, 585–593. doi: 10.1046/j.1365-2109.2000.00476.x
Tacon A. G. J. (1993). Feed ingredients for warm water fish, fish meal and other processed feedstuffs. FAO Fis. Circ. 64, 856 (FAO:Rome, Italy).
Tung C., Alfaro A. C. (2012). Alternative protein sources in artificial diets for new zealand's black-footed abalone, haliotis iris, martyn 1784, juveniles. J. World Aquac. Soc 43, 1–29. doi: 10.1111/j.1749-7345.2011.00545.x
UNSD (United Nations Statistical Division) (2016). Population and vital statistics report. series a vol. LXVIII (New York, USA:United Nations).
Uribe C., Folch H., Enriquez R., Moran G. (2011). Innate and adaptive immunity in teleost fish: a review. Veterinarni Medicina 56, 486–503. doi: 10.17221/3294-VETMED
Wang Y., Wang F., Ji W. X., Han H., Li P. (2013). Optimizing dietary protein sources for Japanese sea bass (Lateolabrax japonicus) with an emphasis on using poultry by-product meal to substitute fish meal. Aquac. Res. 46, 874–883. doi: 10.1111/are.12242
Won S., Moniruzzaman M., Lee S., Hong J. W., Park J. K., Kim S., et al. (2017). Evaluation of dietary natural mineral materials as an antibiotic replacer on growth performance, non-specific immune responses and disease resistance in rainbow trout, Oncorhynchus mykiss. Aquac. Res. 48, 4735–4747. doi: 10.1111/are.13295
Yilmaz S., Ergun S., Celik E. S. (2012). Effects of herbal supplements on growth performance of sea bass (Dicentrarchus labrax): Change in body composition and some blood parameters. J. Biosci. Biotech. 1, 217–222.
Keywords: fish meal, fish meal analog, feed additives, growth, hematology, innate immunity, challenge test, Japanese eel
Citation: Bae J, Lee S, Moniruzzaman M, Hamidoghli A, Choi W, Lee S, Min T, Kim S-K and Bai SC (2022) Evaluation of dietary fish meal analog with or without supplementation of natural feed additives as the substitute of fish meal in juvenile Japanese eel, Anguilla japonica. Front. Mar. Sci. 9:931940. doi: 10.3389/fmars.2022.931940
Received: 04 May 2022; Accepted: 28 June 2022;
Published: 22 July 2022.
Edited by:
Hüseyin Sevgili, Isparta University of Applied Sciences, TurkeyReviewed by:
Yu San Han, National Taiwan University, TaiwanAlessandra Roncarati, University of Camerino, Italy
Copyright © 2022 Bae, Lee, Moniruzzaman, Hamidoghli, Choi, Lee, Min, Kim and Bai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shin-Kwon Kim, a3NrNDExNkBrb3JlYS5rcg==; Sungchul C. Bai, c2NiYWlAcGtudS5hYy5rcg==
†These authors have contributed equally to this work
‡ORCID: Jinho Bae, https://orcid.org/0000-0001-9006-6844
Mohammad Moniruzzaman, https://orcid.org/0000-0002-5266-2087
Taesun Min, https://orcid.org/0000-0002-3998-7493
Shin-Kwon Kim, https://orcid.org/0000-0003-4508-9064
Sungchul C. Bai, https://orcid.org/0000-0001-7984-2267
 Jinho Bae
Jinho Bae Seunghyung Lee
Seunghyung Lee Mohammad Moniruzzaman
Mohammad Moniruzzaman Ali Hamidoghli
Ali Hamidoghli Wonsuk Choi4
Wonsuk Choi4 Taesun Min
Taesun Min Sungchul C. Bai
Sungchul C. Bai