- 1Department of Internal Medicine and Clinical Immunology, University Hospital Mohammed VI, Tangier, Morocco
- 2Neurology and Neurophysiology Department, University Hospital Mohammed VI, Tangier, Morocco
We present the rare case of a 33-year-old pregnant woman who developed severe ataxic sensory neuronopathy associated with isolated anti-Ro/SS-A antibodies without other features of Sjögren's syndrome. Her symptoms, which began with numbness and paresthesia, progressed to severe ataxia and sensory impairment, complicated by preeclampsia and intrauterine fetal death. Despite treatment with intravenous immunoglobulin, azathioprine, and steroids, she remains disabled. This case raises awareness for anti-Ro/SS-A antibodies in pregnant women and underscores the importance of early intervention and a multidisciplinary approach to prevent severe neurological disability.
Introduction
Sensory neuronopathies (SNNs), or ganglionopathies, are rare, specific subgroups of neuropathies characterized by primary and selective degeneration of sensory neurons in the root dorsal ganglia (DRG), leading to severe ataxia and asymmetric, non-length-dependent sensory symptoms responsible for significant disability (1, 2). In contrast to classical polyneuropathies, they are more frequently associated with immune-related disease, neoplastic origins, and toxic agents (3).
The most dysimmune-related diseases associated with SNNs are Sjogren's syndrome, coeliac disease, and autoimmune hepatitis (4). In Sjogren's syndrome, SNNs can precede the onset of sicca symptoms and be the first presentation. In such cases, the presence of anti Ro/SS-A antibodies can be a highly valuable diagnosis.
During pregnancy, anti-Ro/SSA and anti-La/SSB antibodies have been associated with a risk for neonatal lupus and congenital heart block (CHB). But there were no adverse maternal outcomes (5).
Here, we report a rare case of a pregnant African young woman who experienced severe onset ataxic sensory neuronopathy associated with isolated anti Ro/SS-A antibodies without other features of Sjogren's syndrome, leading to intrauterine fetal death, pre-eclampsia, and neurologic disability despite intravenous immunoglobulin, azathioprine, and steroid treatment.
Case description
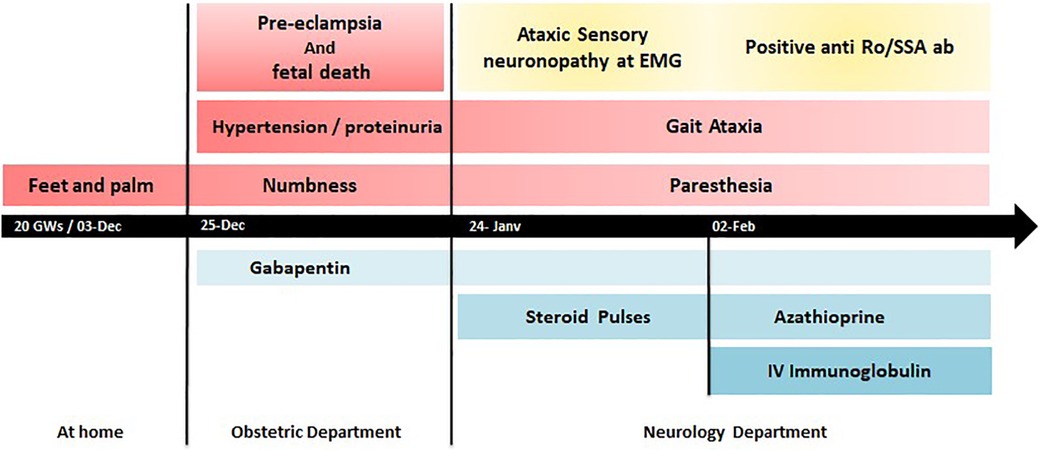
A 33-year-old African G2P0 woman, with a history of unexplained second-trimester pregnancy loss and a family history of undifferentiated connective tissue disease in her sister, presented at 20 weeks gestation with subacute numbness and paresthesia in her feet and palms. Three weeks later, she was admitted to the gynecology department for headaches, hypertension, and proteinuria. She was diagnosed with pre-eclampsia, complicated by intrauterine fetal death, on ultrasonography.
The neurologic problem was also noted and worsened with the onset of balance disturbances and acroataxia in the lower limbs, symptomatically treated with gabapentin. After managing the pre-eclampsia and fetal expulsion, she was referred to the neurology and internal medicine departments for further investigations.
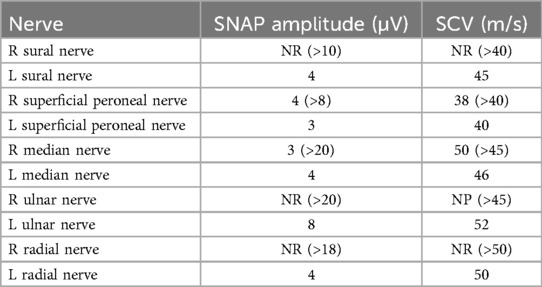
The clinical exam revealed normal muscle strength using the Medical Research Council (MRC) scale, proprioceptive gait ataxia, pseudoathetotic movements of the fingers, a positive Romberg sign, and a generalized absence of deep tendon reflexes. The arms and legs exhibited hypoesthesia for touch, and all four limbs exhibited hypoesthesia for vibration and proprioception. There was no purpura, skin rash, Raynaud phenomenon, malar rash, arthralgia, arthritis, parotidomegaly, or adenomegaly. Electromyography showed normal nerve motor conduction, while sensory nerve conduction demonstrated an absence of sensory response and a negative H reflex consistent with sensory neuronopathy (Table 1).
Her inflammatory markers revealed an accelerated sedimentation rate of 50 mm in the first hour, C-reactive protein at 14 mg/L, and hypergammaglobulinemia at 18 g/L without monoclonal gammopathy at immunoelectrophoresis.
Initial workups for vitamins B6 and B12 showed normal results. Negative results were obtained for tests conducted on antinuclear antibody, anti–double-stranded DNA (dsDNA) antibodies, antiphospholipid lupus anticoagulant, anticardiolipin, Beta-2-glycoprotein 1, anti-neutrophil cytoplasmic antibody (ANCA), cryoglobulin, rheumatoid factor, and anti-citrullinated protein antibodies (ACPA). Complements C3 and C4 were within the normal range. In addition, the results of thyroid tests, celiac disease antibody tests, and serial neuronal antibody tests were all within normal.
Interestingly, anti-Ro/SS-A antibodies were positive at 86 U/ml. The patient denied having Sicca syndrome. Further investigation of salivary accessory gland histology was normal; Schirmer's test and ophthalmic exam were normal. A CT scan of the chest, abdomen, and pelvis revealed no adenopathy, pulmonary involvement, or tumoral lesions. Serological tests for HIV, HCV, and HBV were negative.
A diagnosis of autoimmune sensory neuronopathy (SNN) was considered probable based on the pattern of her neuropathy, with a Camdessanche's score >6.5 and the presence of anti-Ro/SS-A antibodies. She was initially treated with IV methylprednisolone (1 g/day for 3 days) with oral tapering. In the second week, due to lack of improvement, intravenous immunoglobulin at doses of 0.4 g/kg/day for 5 days was initiated, along with azathioprine 2 mg/kg/day (Figure 1). She noticed stabilization and mild improvement in sensory function, a decrease in numbness and paresthesia, and a partial return of deep tendon reflexes. The treatment was well tolerated, and the patient reported clinical improvement in her symptoms. However, she remained disabled at the 3-month follow-up with a modified Rankin Scale (mRS) score of 4.
Discussion
This patient demonstrates a rare and challenging case of sensory neuronopathy onset in a pregnant woman with isolated anti-Ro/SS-A antibodies, emphasizing the importance of a thorough early workup for neuronopathy in pregnancy, prompt treatment of this rare and severe neuropathy subset, which is typically less responsive to classic treatments, and vigilant monitoring of pregnancy outcomes in the presence of anti-Ro/SS-A antibodies.
Sensory neuronopathies, also known as ganglionopathies, are a rare and specific subgroup of neuropathies characterized by degeneration of sensory neurons in the dorsal root ganglia (DRG); they impair both central and peripheral sensory pathways (1). The DRGs are particularly vulnerable to cytotoxic T cells because of their permeable blood-nerve barrier, leading to paraneoplastic sensory ganglionopathy when tumor antigens cross-react with sensory neurons (6). Similarly, Sjögren's syndrome and idiopathic cases are mediated by CD8 cytotoxic T cells (3, 6).
Compared to classical polyneuropathies, SNNs are more commonly associated with immune-related diseases, neoplastic origins, and toxic agents (1). SNNs autoimmune-related diseases include Sjögren's syndrome, coeliac disease, and autoimmune hepatitis (4, 7). Sjögren's syndrome is the most common autoimmune disease related to SNNs. It primarily affects exocrine glands through epithelial lymphoid infiltration, leading to ocular and oral dryness. However, one-third of patients have systemic extra-glandular manifestations, including pulmonary, renal, vascular, or neurologic involvement (8). Among these, neurological manifestations affect the peripheral nervous system in 20%–25% of patients (9); sensory axonal neuropathy and small fiber neuropathy are the most frequent; sensory neuronopathy is less frequent but the most severe form, affecting up to 5% and leading to major disability (10). SNNs can precede the onset of sicca symptoms, making diagnosis difficult. In such cases, the presence of focal sialadenitis with focus score ≥1 focus/4 mm2 of glandular tissue and anti-Ro/SS-A, anti-La/SS-B antibodies is highly valuable for diagnosis. Mostly found in systemic lupus (30%–40%) and Sjogren's syndrome (50%–90%), anti Ro/SS-A antibodies are also present in other autoimmune diseases (11).
Pregnancy may reveal preexisting autoimmune disorders or exacerbate symptoms in existing conditions such as lupus erythematosus. The presence of anti-Ro/SS-A antibodies raises concerns due to the potential adverse outcomes, especially in associated Sjögren's syndrome with systemic lupus erythematosus or antiphospholipid syndrome (12, 13).
This patient's neurological examination revealed prominent gait ataxia, pseudoathetotic movements of the fingers, positive Romberg sign, and generalized absence of deep tendon reflexes. Sensory examination showed hypoesthesia to touch, vibration, and proprioception in all four limbs, with no significant muscle weakness. These clinical signs were suggestive of the SNNs diagnosis, which was confirmed by primary sensory neuropathy on electromyography (EMG) with a Camdessanche's score of >6.5 points (14).
Laboratory investigations showed elevated inflammatory markers, including an accelerated sedimentation rate, elevated C-reactive protein, and hypergammaglobulinemia suggestive of dysimmunity. Despite extensive investigations for paraneoplastic syndrome, notably with negative CT imaging, serial onconeuronal antibody, vitamins, and coeliac antibody tests, only the anti Ro/SS-A antibodies were positive, with negative antinuclear antibodies in indirect immunofluorescence. Isolated anti-Ro/SS-A antibodies are mainly associated with Sjögren's syndrome and systemic lupus erythematosus. Salivary gland histology and Shirmer's test were normal, antinuclear antibody, anti-ENA and anti-dsDNA were negative. Despite the absence of classic Sjögren's features, this patient's positive SNNs-associated anti-Ro/SS-A antibodies indicate an autoimmune origin, possibly Sjögren's syndrome.
While primary Sjögren's syndrome is associated with a lower risk of obstetric complications compared to associated lupus or antiphospholipid syndrome, the presence of isolated anti-Ro/SS-A antibodies raises pregnancy risks for congenital complete atrioventricular block (13), especially in severe cases with non-glandular manifestations. Although anti-Ro/SS-A antibodies and primary Sjögren's syndrome do not usually increase the risk of pre-eclampsia (15), in our case, management of the pre-eclampsia and intrauterine fetal death complicated delayed explorations and treatment initiation. The risk of obstetric complications in the presence of isolated anti-Ro/SS-A antibodies should not be underestimated.
Several treatments have been tested on small groups of patients with autoimmune sensory neuropathies (SNNs), including steroids, intravenous immunoglobulin (IVIG), plasma exchange, azathioprine, rituximab, and cyclophosphamide (6, 16). These treatments have shown varied efficacy, with patients experiencing a 20% improvement and 12% stabilization in symptoms (16). A retrospective study of 13 people with SNNs connected to Sjögren's syndrome found that the best treatment was a mix of steroids and immunosuppressants, especially mycophenolate mofetil (MMF). However, intravenous immunoglobulins produced disappointing findings (17). Moreover, not all of these treatments are safe during pregnancy.
In our case, the delayed identification of anti-Ro/SS-A antibodies resulted in a delay in initiating steroid pulse therapy. The patient's clinical symptoms improved after receiving intravenous immunoglobulin and azathioprine, but she remains disabled. Cyclophosphamide was avoided due to the risk of infertility, and plasmapheresis was not available.
Currently, there is no consensus on treating SNNs with anti-Ro/SS-A antibodies in pregnancy due to a lack of high-quality evidence on the best treatment strategy. This highlights the crucial role of multidisciplinary team management, including obstetricians, neurologists, and rheumatologists, along with the shared decision-making process.
Conclusion
This case emphasizes the necessity of conducting anti-Ro/SS-A antibodies screening in individuals with neuropathy symptoms, rheumatic disease, a previous occurrence of fetal loss, intrauterine fetal death, or fetal heart block, regardless of the absence of classical signs of Sjögren's syndrome. Early diagnosis and aggressive treatment with immunomodulatory therapies can prevent irreversible neuronal damage and enhance outcomes for SNNs patients. Further trials and research are needed to understand the pathophysiological mechanisms and the best treatment strategy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
RS: Investigation, Methodology, Writing – original draft, Writing – review & editing. ZF: Data curation, Investigation, Methodology, Writing – review & editing. SE: Investigation, Writing – review & editing. MB: Investigation, Methodology, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Griffin JW, Cornblath DR, Alexander E, Campbell J, Low PA, Bird S, et al. Ataxic sensory neuropathy and dorsal root ganglionitis associated with Sjögren's syndrome. Ann Neurol. (1990) 27(3):304–15. doi: 10.1002/ana.410270313
3. Sheikh SI, Amato AA. The dorsal root ganglion under attack: the acquired sensory ganglionopathies. Pract Neurol. (2010) 10(6):326–34. doi: 10.1136/jnnp.2010.230532
4. Martinez AR, Nunes MB, Nucci A, França MC Jr. Sensory neuronopathy and autoimmune diseases. Autoimmune Dis. (2012) 2012:873587. doi: 10.1155/2012/873587
5. Brucato A, Doria A, Frassi M, Castellino G, Franceschini F, Faden D, et al. Pregnancy outcome in 100 women with autoimmune diseases and anti-Ro/SSA antibodies: a prospective controlled study. Lupus. (2002) 11(11):716–21. doi: 10.1191/0961203302lu252oa
6. Gwathmey KG. Key points for issue. Peripheral nerve and motor neuron disorders. Continuum (Minneap Minn). (2020) 26(5):1212–6. doi: 10.1212/01.CON.0000718940.59848.68
7. Camdessanché JP, Jousserand G, Franques J, Pouget J, Delmont E, Créange A, et al. A clinical pattern-based etiological diagnostic strategy for sensory neuronopathies: a French collaborative study. J Peripher Nerv Syst. (2012) 17(3):331–40. doi: 10.1111/j.1529-8027.2012.00411.x
8. Mariette X, Criswell LA. Primary Sjögren’s syndrome. N Engl J Med. (2018) 378(10):931–9. doi: 10.1056/NEJMcp1702514
9. Pavlakis PP, Alexopoulos H, Kosmidis ML, Stamboulis E, Routsias JG, Tzartos SJ, et al. Peripheral neuropathies in Sjogren syndrome: a new reappraisal. J Neurol Neurosurg Psychiatry. (2011) 82(7):798–802. doi: 10.1136/jnnp.2010.222109
10. Birnbaum J. Peripheral nervous system manifestations of Sjögren syndrome: clinical patterns, diagnostic paradigms, etiopathogenesis, and therapeutic strategies. Neurologist. (2010) 16(5):287–97. doi: 10.1097/NRL.0b013e3181ebe59f
11. Pasoto SG, Adriano de Oliveira Martins V, Bonfa E. Sjögren’s syndrome and systemic lupus erythematosus: links and risks. Open Access Rheumatol. (2019) 11:33–45. doi: 10.2147/OARRR.S167783
12. Miyasato-Isoda M, Waguri M, Yamada Y, Miyano A, Wada Y. Anti-Ro52 antibody level is an important marker of fetal congenital heart block risk in anti-Ro/SSA antibody positive pregnancy. Mod Rheumatol. (2018) 28(4):690–6. doi: 10.1080/14397595.2017.1374235
13. Erton ZB, Sevim E, de Jesús GR, Cervera R, Ji L, Pengo V, et al. Pregnancy outcomes in antiphospholipid antibody positive patients: prospective results from the AntiPhospholipid syndrome alliance for clinical trials and International networking (APS ACTION) clinical database and repository (“registry”). Lupus Sci Med. (2022) 9(1):e000633. doi: 10.1136/lupus-2021-000633
14. Camdessanché JP, Jousserand G, Ferraud K, Vial C, Petiot P, Honnorat J, et al. The pattern and diagnostic criteria of sensory neuronopathy: a case-control study. Brain. (2009) 132(Pt 7):1723–33. doi: 10.1093/brain/awp136
15. de Frémont GM, Costedoat-Chalumeau N, Lazaro E, Belkhir R, Guettrot-Imbert G, Morel N, et al. Pregnancy outcomes in women with primary Sjögren’s syndrome: an analysis of data from the multicentre, prospective, GR2 study. Lancet Rheumatol. (2023) 5(6):e330–40. doi: 10.1016/S2665-9913(23)00099-1
16. Antoine JC. Les neuronopathies sensitives dysimmunes: enjeux diagnostiques et thérapeutiques. Bull Acad Natl Med. (2021) 205(8):937–45. doi: 10.1016/j.banm.2021.05.017
Keywords: sensory neuronopathy, pregnancy, anti-Ro/SS-A antibodies, Sjogren’s syndrome, fetal death
Citation: Smaili R, Ferjouchia Z, Elbachiri S and Bourkia M (2024) Ataxic sensory neuronopathy with isolated anti-Ro/SS-A antibody during pregnancy: a case report of adverse outcomes. Front. Lupus 2:1461157. doi: 10.3389/flupu.2024.1461157
Received: 7 July 2024; Accepted: 27 August 2024;
Published: 25 September 2024.
Edited by:
Catherine Nelson-Piercy, Guy’s and St Thomas’ NHS Foundation Trust, United KingdomReviewed by:
Valerie Michelle Lewis, University of Oklahoma Health Sciences Center, United StatesAntonia Szanto, University of Debrecen, Hungary
Copyright: © 2024 Smaili, Ferjouchia, Elbachiri and Bourkia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rachid Smaili, c21haWxpLnJhY2hpZC5taWljQGdtYWlsLmNvbQ==
 Rachid Smaili
Rachid Smaili Zahra Ferjouchia2
Zahra Ferjouchia2