- 1Lupus Center, Division of Rheumatology, Columbia University Medical Center, New York, NY, United States
- 2Lupus Clinic, Division of Rheumatology, University of California, San Francisco, CA, United States
- 3Nephrology Division, Wexler Medical Center, The Ohio State University, Columbus, OH, United States
Lupus nephritis (LN) is a common and serious manifestation of systemic lupus erythematosus and is a major cause of mortality and morbidity. The current standard-of-care treatment for LN include conventional immunosuppressive treatments such as mycophenolate mofetil, cyclophosphamide, or azathioprine, combined with glucocorticoids. However, this treatment approach has several unmet needs, such as achieving only modest remission rates, potential toxicities, and prolonged cumulative steroid exposure, resulting in suboptimal patient outcomes. The LN treatment landscape is evolving rapidly to meet these unmet needs, with belimumab and voclosporin being the first drugs approved specifically for treatment of LN in 2020 and 2021, respectively. Here, we review the likely roles in LN therapy for several targeted therapies, including select therapies under investigation, and interventions in early development such as therapies targeting B cells (obinutuzumab, atacicept, ianalumab, and CD19 chimeric antigen T-cell therapy), inflammatory cytokines (secukinumab and anifrolumab), and the immunoproteasome (zetomipzomib); we also review treatment strategies designed to minimize steroid exposure. Treatments in development have demonstrated encouraging short- and long-term efficacy and steroid-sparing potential, potentially paving the way for improved treatment regimens and patient outcomes in LN.
1 Introduction
Systemic lupus erythematosus (SLE) is a multisystem autoimmune disease with a relapsing and remitting course (1). Lupus nephritis (LN) is a common and serious manifestation of SLE, occurring in ≤50% of patients (2–4), and remains a major cause of mortality and morbidity for patients with SLE (5, 6). Approximately 10%–30% of patients with LN will progress to end-stage kidney disease (ESKD) (7–11). Death directly attributable to kidney disease will occur in 5%–25% of patients within 5 years (8). Active LN is correlated with worse health-related quality-of-life compared with inactive LN or SLE without kidney involvement, as well as higher direct healthcare costs and indirect healthcare costs due to loss of productivity (12–15).
2 Management of LN
Guidelines recommend that patients with SLE and kidney involvement (glomerular hematuria and/or cellular casts, proteinuria >0.5 g/day, spot urine protein-creatine ratio [UPCR] >500 mg/g, or unexplained decrease in estimated glomerular filtration rate [eGFR]) should have a kidney biopsy performed (16, 17). More recent data, which will be reviewed in this publication, suggest that a broader indication for kidney biopsy should be recommended, such as the presence of glomerular hematuria accompanied by any degree of proteinuria (18–20). The 2003 International Society of Nephrology/Renal Pathology Society (ISN/RPS) classification represents the gold standard for assessment of kidney biopsies in LN, affirming the diagnosis of LN and guiding treatment decisions (16, 17, 21). The 2018 update to the ISN/RPS classification include an increased emphasis on the National Institutes of Health activity index and chronicity index (22, 23).
Complete clinical remission is the main treatment goal in LN due to its strong association with outcomes such as kidney survival at 10 years: 94% for patients with complete remission, 45% for patients with partial remission, and 19% for patients without remission (7). There is a lack of consensus on definitions of complete and partial clinical remission following treatment, although the criteria usually involve clinical markers of proteinuria and serum creatinine (24). Clinical guidelines have defined complete clinical remission as a reduction in proteinuria to <0.5–0.7 g/day within 6–12 months of starting or escalating therapy; partial clinical remission is defined as ≥50% reduction in proteinuria or UPCR to <3 in the same time period (16, 17).
The terms induction and maintenance, which have traditionally been used to characterize the treatment of LN, fail to capture evolution in the understanding of LN management; the terms initial and subsequent have been proposed instead (25). Because there is no consensus on the best terminology, we used initial or induction and subsequent or maintenance to describe LN treatment in this publication.
Based on the recent Kidney Disease: Improving Global Outcomes (KDIGO) 2021 glomerular diseases guidelines and the European League Against Rheumatism/European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) 2019 guidelines, treatment for class III or IV LN includes an initial or induction treatment of glucocorticoids, combined with either mycophenolate mofetil (MMF) or cyclophosphamide (CYC) followed by maintenance or subsequent therapy with either MMF or azathioprine (AZA) combined with glucocorticoids (8, 16, 17). Glucocorticoids improve mortality rates in patients with LN and have been the mainstay of LN treatment since the 1960s (26). Landmark trials in the 1970s and 1980s reported that the addition of CYC to glucocorticoids resulted in better preservation of long-term kidney function compared with glucocorticoids alone (27, 28); subsequent data showed that patients receiving either MMF or CYC had similar response rates if they were also receiving glucocorticoids (29), indicating that either MMF or CYC were reasonable options for initial or induction treatment (16, 17).
In recent years, belimumab (2020) and voclosporin (2021) became the first two drugs to be approved specifically for the treatment of LN; these approvals could potentially have significant implications for the current standard of care (SoC) in LN (30, 31). There is increasing support for early use of belimumab and voclosporin in combination with standard therapy, as proposed in the KDIGO 2024 guidelines for the management of LN and the 2023 update of the EULAR SLE recommendations (32, 33).
The optimal treatment duration for LN is not clearly defined; early withdrawal of treatment leads to relapses, whereas prolonged treatment exposure increases the risk of toxicity. The KDIGO 2024 guidelines recommend that the combined duration of initial or induction therapy and subsequent or maintenance therapy for proliferative LN should be ≥36 months (33), whereas the EULAR/ERA-EDTA 2019 guidelines recommend that immunosuppression treatment should continue for at least 3–5 years after achieving complete clinical remission; duration should be individualized according to the timing and magnitude of response, duration of flare-free maintenance, extrarenal SLE activity, and patient preferences (17). In the updated EULAR 2023 recommendations, the duration of treatment was revised to ≥3 years following renal response (32).
2.1 Unmet needs in current management of LN
A systematic review of over 18,000 patients found that the 5-year risk of ESKD in patients with LN in 1970 to 1979 was 16%; subsequently, the risk declined gradually until the mid-1990s, when it plateaued at 11%, then increased in the late 2000s (34). This fluctuation highlights the limitations of conventional therapies and the need for new therapies to improve patient outcomes. Repeat biopsy studies have demonstrated that conventional therapies are ineffective at preventing accrual of kidney damage despite good clinical response (35, 36). Remission rates for LN are modest, with <60% of patients achieving complete clinical remission after 2 years (37–39); among those who have achieved clinical remission, kidney flares still occur in 27% to 66% (7, 40, 41). It is worth noting that recent retrospective studies conducted in Europe have reported complete response rates of up to 72% at 18 months (42, 43), highlighting the heterogeneity of treatment response across populations.
Additionally, standard immunosuppressive treatments are linked to significant toxicities, including myelosuppression and infections (44). The use of glucocorticoids has greatly improved survival in patients with LN; however, long-term use is associated with safety concerns, including infections, elevated risk of cardiovascular disease, metabolic side effects (45), and accrual of damage from SLE, which are major causes of morbidity and mortality in SLE (5, 46–49). Hence, treatment strategies that aim to minimize glucocorticoid exposure while maintaining treatment efficacy have substantial clinical value (45).
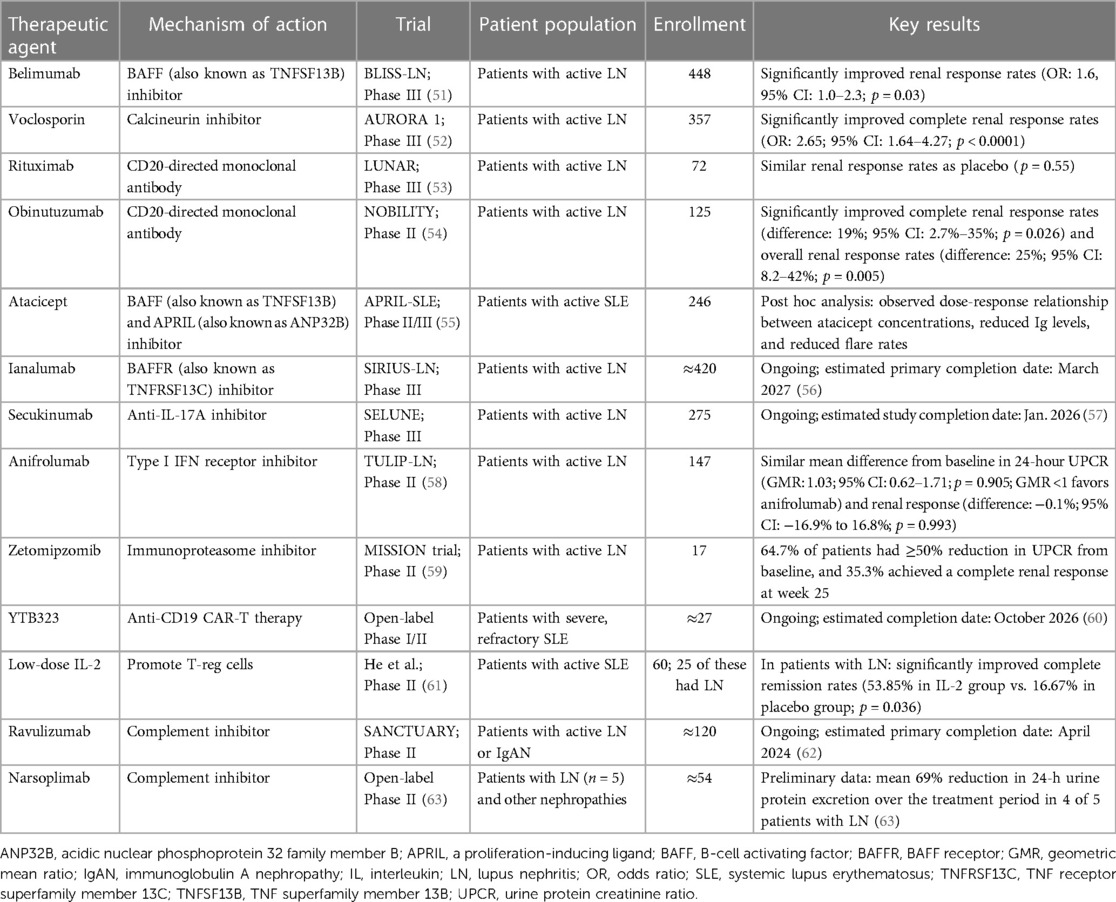
The LN therapeutic landscape is evolving rapidly, and investigations of novel therapies are ongoing to address the unmet needs of current treatment strategies. Here, we provide an overview of approved targeted therapies that have the potential to address these unmet needs, select treatments that are being investigated in late-stage clinical trials, and promising new treatments in early developmental stages that offer the possibility of glucocorticoid-free regimens. The mechanisms of action of these therapies are illustrated in Figure 1, and data from key clinical trials are detailed in Table 1.
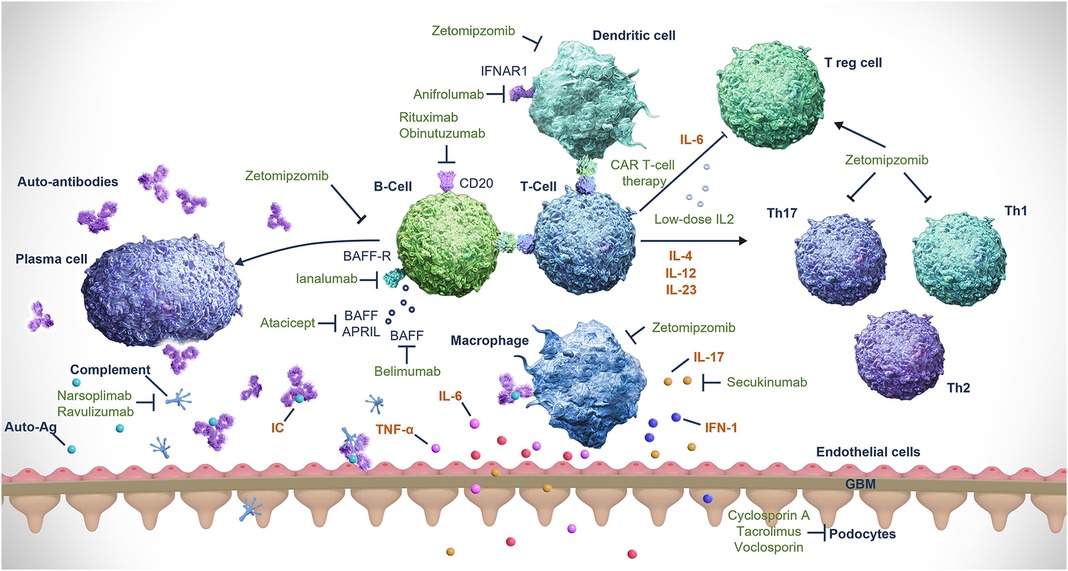
Figure 1. Mechanisms of action of treatments for LN. Ag, antigen; APRIL, a proliferation-inducing ligand (also known as ANP32B); ANP32B, acidic nuclear phosphoprotein 32 family member B; BAFF, B-cell activating factor (also known as TNFSF13B); BAFFR, B-cell activating factor receptor (also known as TNFRSF13C); CAR, chimeric antigen receptor; GBM, glomerular basement membrane; IFN, interferon; IFNAR1, interferon alfa and beta receptor subunit 1; IL, interleukin; LN, lupus nephritis; Th, T helper; TNF, tumor necrosis factor; TNFRSF13C, TNF receptor superfamily member 13C; TNFSF13B, TNF superfamily member 13B; T-reg cell, regulatory T-cell. Reprinted from Obrișcă B, et al. (50). Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/).
2.2 Novel approved targeted therapies
2.2.1 Belimumab
B cells play a significant role in the pathogenesis of SLE, acting directly via autoantibody production and indirectly via antigen-presenting activity to promote T-cell activation and production of inflammatory cytokines (64, 65). Belimumab is a fully human monoclonal antibody (mAb) that inhibits the soluble form of B-cell activating factor [BAFF; also known as TNFSF13B (TNF superfamily member 13b)], thereby inhibiting B-cell survival and reducing the differentiation of B cells into immunoglobulin-producing plasma cells (65, 66).
In the Phase III BLISS-LN trial, 448 patients with LN were randomized to either belimumab or placebo in combination with standard induction therapy. Over 104 weeks, the belimumab group demonstrated a significantly higher renal response rate vs. placebo [43% vs. 32%, respectively; odds ratio (OR), 1.6; 95% CI, 1.0–2.3; p = 0.03] and a significantly lower risk of kidney-related events or death [hazard ratio (HR), 0.51; 95% CI, 0.34–0.77; p = 0.001]; the safety profile was similar between both groups (51). Based on the positive trial results, both KDIGO 2024 LN guidelines and the updated 2023 EULAR SLE guidelines now state that initial combination treatment with belimumab may be considered alongside standard therapy with MMF or CYC and glucocorticoids (32, 33).
Two additional Phase III clinical trials are ongoing and will evaluate the safety and efficacy of belimumab in LN, including long-term data, across different patient populations (NCT03370263 and NCT05863936) (67, 68). Data from the long-term extension of the extrarenal belimumab studies are reassuring and show no increased risk of side effects with up to 11 years of treatment (69).
2.2.2 Voclosporin
Calcineurin inhibitors (CNIs) such as voclosporin, tacrolimus, and cyclosporine exert immunomodulatory effects on T cells, leading to reduction of lymphocyte proliferation and T-cell–mediated responses, as well as reduction of proteinuria via stabilization of podocytes (70, 71). Due to the unpredictable and complex pharmacokinetics (PK) profile of conventional CNIs (tacrolimus and cyclosporine), therapeutic drug monitoring is required to ensure efficacy and minimize toxicity (72). Voclosporin, a newer CNI and an analog of cyclosporine, has higher potency, a more favorable metabolic profile, and a more consistent PK profile compared with conventional CNIs due to altered binding of the voclosporin-cyclophilin complex to calcineurin and a reduced drug and metabolite load (16, 72); hence, therapeutic drug monitoring is not needed (73). In patients with refractory disease, multitarget therapy (i.e., a combination of CNI and MMF) has shown reasonable efficacy and safety in small-scale observational studies and can be considered an option in this patient population (74–77). However, the initial data on the efficacy of multitarget therapy as initial or induction treatment in LN did not include multiethnic populations and were of low quality, leading to limited generalizability to other populations (16, 17, 71, 78, 79). Newer data evaluating the novel CNI voclosporin support the use of multitarget therapy as initial or induction treatment across multiethnic populations (52, 80–82).
In the Phase II AURA-LV trial, 265 patients were randomized to receive either low-dose voclosporin, high-dose voclosporin, or placebo as initial LN treatment. All treatments were combined with MMF 2 g/day and low-dose glucocorticoids (80). Significantly higher complete renal remission rates were observed in patients receiving either low-dose or high-dose voclosporin after 48 weeks compared with placebo at 23.9% (with low-dose voclosporin, 49.4%; OR, 3.21; 95% CI, 1.68–6.13; p < 0.001; and with high-dose voclosporin, 27.3%; OR, 2.10; 95% CI, 1.09–4.02; p = 0.026) (80). The voclosporin groups had higher rates of serious adverse events (low-dose voclosporin, 28.1%; high-dose voclosporin, 25.0%; placebo, 15.9%), and the low-dose voclosporin group had higher rates of death compared with the other two groups (low-dose voclosporin, 11.2%; high-dose voclosporin, 2.3%; placebo, 1.1%) (80). However, this higher rate of death was not observed in the high-dose voclosporin group, nor was it replicated in the Phase III trial; therefore, it is likely attributable to causes beyond the addition of voclosporin to the treatment regimen (80). The authors of the AURA-LV study explained that the uneven distribution of deaths was likely associated with study-site characteristics rather than the doses of voclosporin received (80).
The subsequent Phase III AURORA 1 trial randomized 357 patients to either voclosporin (using the low dose in the AURA-LV trial) or placebo (52). The primary endpoint of complete renal response was defined as a composite endpoint of UPCR ≤0.5 mg/g, eGFR 60 ml/min or no confirmed increase of eGFR >20% from baseline, and no more than 10 mg/day of prednisolone for 3 consecutive days or for ≥7 days during Week 44 through 52 (52). The voclosporin group had significantly improved complete renal response rates compared with placebo at week 52 (41% vs. 23%, respectively; OR, 2.65; 95% CI, 1.64–4.27; p < 0.0001) (52). Unlike the AURA-LV trial, the AURORA 1 trial had similar safety profiles in both groups, and the rates of death were not imbalanced (<1% in the voclosporin group vs. 3% in the placebo group) (52). A pooled post hoc analysis of the AURA-LV and AURORA 1 studies (N = 534) showed that significantly more patients in the pooled voclosporin groups achieved a complete renal response at 1 year compared with those in the control groups (43.7% vs. 23.3%, respectively; OR, 2.76; 95% CI, 1.88–4.05; p< 0.0001) (81). Patients who completed the AURORA 1 trial were enrolled into a follow-up AURORA 2 trial, continuing the same blinded randomized treatment for an additional 2 years (83). The mean reductions in UPCR observed in patients treated with voclosporin in AURORA 1 were maintained in AURORA 2, with no increase in UPCR noted at the follow-up visit 4 weeks after study-drug discontinuation (83).
Based on these positive data, both the KDIGO 2024 LN guidelines and the EULAR 2023 SLE guidelines recommend adding voclosporin to MMF and glucocorticoids as an initial or induction treatment option for patients with LN; use of voclosporin has not been evaluated in combination with CYC or in patients with eGFR <45 ml/min/1.73 m2 (32, 33). However, because CNIs decrease proteinuria through additional nonimmune mechanisms, clinical trials that use a clinical remission criterion based mainly on reduction in proteinuria must be interpreted cautiously (84). As the AURORA studies used a composite endpoint that assessed improvements in UPCR, eGFR, and steroid use (52), the renal efficacy results of voclosporin are considered robust.
2.3 Unapproved SOC treatment
2.3.1 Rituximab
Rituximab is a type I CD20-directed mAb that mediates B-cell lysis by complement-dependent cytotoxicity (CDC), antibody-dependent cell-mediated cytotoxicity (ADCC), and direct cell death (85, 86). Data from studies evaluating the efficacy of rituximab in patients with SLE and LN have been mixed (53, 87, 88). The Phase III LUNAR trial found that the addition of rituximab to standard induction treatment (MMF and glucocorticoids) did not significantly improve renal response rates in patients with Class III or IV LN after 1 year of treatment compared with the placebo group (p = 0.55) (53). However, a post hoc analysis of the LUNAR trial demonstrated that patients with complete peripheral B-cell depletion had significantly increased odds of having a complete response (unadjusted OR, 5.8; 95% CI, 1.2–2.8; p = 0.03), supporting B-cell depletion as a mechanism of action for LN treatment (89). Accordingly, a systematic review and meta-analysis of 31 studies of refractory SLE/LN patients found the global, complete, and partial response rates of rituximab were 70%, 51%, and 27%, respectively; use of rituximab significantly decreased prednisone dose (mean difference, −12.50 mg/day; 95% CI, −6.36 to −18.64; p < 0.001) and demonstrated a numerical but statistically nonsignificant decrease in proteinuria (mean difference, −2.52 g/day; 95% CI, 0.22 to −5.27; p = 0.07) (90). Multiple uncontrolled studies have shown that rituximab produced favorable outcomes in patients with refractory LN, including improvements in renal response rates, proteinuria, repeat biopsies, serum autoantibody levels, and disease activity scores (91–94). Despite only low-grade evidence for its use in LN, both the EULAR 2023 SLE guidelines and KDIGO 2024 guidelines recommend off-label use of rituximab in refractory LN (32, 33), as approved treatment options for refractory LN remain scarce.
2.4 Select targeted therapies under investigation
2.4.1 Therapies targeting B cells
2.4.1.1 Obinutuzumab
Obinutuzumab is a novel humanized anti-CD20 type II mAb that was developed to increase B-cell depletion peripherally and in lymph nodes, including in key B-cell subsets that are considered resistant to conventional B-cell–targeted agents, particularly memory B-cells and proliferating tissue plasmablasts (95, 96). Compared with rituximab, obinutuzumab induces better ADCC, less CDC, and higher tissue B-cell depletion in vitro (86, 95, 97) and was more effective in improving SLE in murine models (98).
The Phase II NOBILITY trial randomized 125 patients with LN to receive either obinutuzumab or placebo in addition to MMF and glucocorticoids (54). After 52 weeks, a higher proportion of patients in the obinutuzumab group achieved the primary endpoint of complete renal response (35% with obinutuzumab vs. 23% with placebo; percentage difference, 12%; 95% CI, −3.4% to 28%; p = 0.115). Exploratory analyses at week 104 reported significantly higher complete renal response (41% with obinutuzumab vs. 23% with placebo; percentage difference, 19%; 95% CI, 2.7%–35%; p = 0.026) and overall renal response (54% with obinutuzumab vs. 29% with placebo; percentage difference, 25%; 95% CI, 8.2%–42%; p = 0.005) (54). In the obinutuzumab group, 98% of patients achieved B-cell depletion (defined as CD19 count ≤5 cells/µl) at week 2 after one infusion of obinutuzumab, and 94% had sustained depletion at week 52 (26 weeks after the last infusion) (54). In contrast, in the LUNAR study, only 12% of the patients had achieved complete B-cell depletion (defined as CD19 count of 0) at week 2 after one infusion of rituximab, and 78% had done so at week 52 (26 weeks after last infusion). The rates of adverse events and serious adverse events were similar between both groups in the NOBILITY trial (54).
While the NOBILITY trial was considered to meet its primary endpoint, results should be interpreted with caution, considering the high prespecified significance level of 0.2. Additional data from two ongoing Phase III studies of obinutuzumab in LN will be needed to provide more evidence to support its use. The global REGENCY study (NCT04221477) will further evaluate the use of obinutuzumab across a wider population (99). Another study (NCT04702256) will assess obinutuzumab as a replacement for oral glucocorticoids during induction treatment of LN (100); these results may provide further evidence of the potential for glucocorticoid-free management of LN.
2.4.1.2 Atacicept
Atacicept is a fully human recombinant fusion protein that inhibits soluble and membrane-bound BAFF and a proliferation-inducing ligand [APRIL; also known as ANP32B (acidic nuclear phosphoprotein 32 family member B)], resulting in reduced numbers of B-cells and plasma cells and, consequently, serum IgG, IgM, and IgA (101).
The Phase II/III APRIL-LN trial was to evaluate the use of atacicept in addition to MMF in LN, but it was prematurely terminated due to an unexpected decline in serum IgG levels and the occurrence of serious infections in patients receiving atacicept (102). Data from the Phase II JANUS study in IgA nephropathy were encouraging: atacicept demonstrated an acceptable safety profile, improved proteinuria, and stabilized kidney function (103). Subsequently, results of the Phase II/III APRIL-SLE trial, conducted in patients with non-renal SLE, suggested that patients with elevated levels of BAFF and APRIL had a greater response to atacicept, with no increased risk of infections (55). The Phase III COMPASS trial (NCT05609812) was initiated in November 2022 with the aim of evaluating the efficacy of atacicept compared with placebo, in combination with SoC treatment, in patients with LN; however, the trial had been suspended as of July 2023, although not as a result of regulatory or safety concerns (104).
2.4.1.3 Ianalumab
Ianalumab is a mAb that modulates B-cell survival via a dual mechanism: direct lysis of B-cells by ADCC as well as BAFF receptor blockade that interrupts BAFF-mediated signaling for B-cell maturation, proliferation, and survival (105). Studies suggest that elevated BAFF levels correlated with a shortened duration of B-cell depletion and contributed substantially to SLE flares after B-cell repopulation, paving the way for a dual-mechanism treatment involving B-cell depletion and BAFF blockade (106, 107).
In a Phase II trial in patients with primary Sjögren's syndrome, a disease in which B-cells play a central role, ianalumab showed a marked dose-response relationship with reduction in disease activity (108). Other mAbs targeting B-cells, such as rituximab and belimumab, did not show convincing efficacy in Sjögren's syndrome (109, 110); the efficacy demonstrated by ianalumab could be partially attributed to its dual target mechanism, providing an added blockade of BAFF receptors that could counteract the elevated BAFF levels seen after administration of rituximab (108). Investigations into whether the increased B-cell depletion of ianalumab will translate to better clinical outcomes in patients with SLE (NCT05639114; NCT05624749) and LN (NCT05126277) are currently ongoing (56, 111, 112).
2.4.2 Therapies targeting cytokines
2.4.2.1 Secukinumab
The interleukin (IL)-23/IL-17 axis and CD4+ T-helper (Th17) cells that produce IL-17 have recently emerged as mediators in the pathogenesis of SLE and LN (113, 114) through induction of vascular inflammation, leukocyte recruitment, B-cell activation, and autoantibody production (114). Patients with LN typically present with elevated IL-17 levels, which are independently associated with a worse prognosis (115).
Secukinumab is an anti–IL-17A inhibitor that blocks IL-17A cytokine interaction with the IL-17 receptor (116). To date, only case reports have been published on the use of secukinumab in treating refractory LN (117–119). The ongoing global Phase III SELUNE clinical trial (NCT04181762) on secukinumab and the Phase II ORCHID-LN trial (NCT04376827) on guselkumab, an IL-23 inhibitor, will shed light on the role in therapy for inhibitors of the IL-23/IL-17 axis (57, 120).
2.4.2.2 Anifrolumab
Type I interferons (IFNs) are a central factor in the pathophysiology of SLE (121, 122). In patients with LN, high type I IFN gene signatures are associated with active disease, proteinuria, and treatment failure (121, 122). Type I IFN promotes kidney fibrosis, scarring, and loss via formation of immune complexes, recruitment of leukocytes, and direct action on kidney cells (123).
Anifrolumab is a mAb that binds to the type I IFN receptor with high specificity and affinity, inhibiting type I IFN signaling (124). In the Phase III TULIP-2 trial, anifrolumab demonstrated a significant reduction in disease activity scores in patients with nonrenal SLE compared with placebo at week 52 (125), although patients with severe LN were excluded from the study. This finding led to the Phase II TULIP-LN trial, in which 147 patients with LN were randomized to receive either a basic regimen of anifrolumab, an intensified regimen of anifrolumab, or placebo; all three regimens were combined with MMF and glucocorticoids (58). The primary endpoint, which was the change in baseline 24-h UPCR at week 52 for combined anifrolumab vs. placebo groups, was not met [geometric mean ratio (GMR), 1.03; 95% CI, 0.62–1.71; p = 0.905; GMR <1 would have favored anifrolumab] (58). The complete renal response rates were also not statistically different between the combined anifrolumab groups and the placebo group (difference, −0.1%; 95% CI, −16.9% to 16.8%; p = 0.993), although a numerical improvement was noted in the intensified regimen group vs. the placebo group (difference, 14.3%; 95% CI, −5.8% to 34.5%; p = 0.162) (58). The lack of benefit observed in the study was partially attributed to the suboptimal exposure obtained with the basic regimen of anifrolumab, resulting in the anifrolumab exposure being approximately half that normally achieved in nonrenal SLE (58). The authors of the TULIP-LN trial suggested that suboptimal exposure with the basic regimen was likely due to increased clearance associated with proteinuria in LN. Only the intensified regimen of anifrolumab achieved serum exposure and pharmacodynamic neutralization levels similar to those in nonrenal SLE. The efficacy and safety of anifrolumab in LN will be further investigated in the Phase III IRIS trial (NCT05138133) (126).
2.4.3 Therapies targeting the immunoproteasome
2.4.3.1 Zetomipzomib
Proteasomes contribute to the degradation of intracellular proteins, providing dynamic control of key cell signaling components and the maintenance of overall cellular homeostasis (127). Proteasomes are expressed ubiquitously throughout the body, while immunoproteasomes, which are derived from constitutive proteasomes, are expressed primarily in immune cells (127, 128). Immunoproteasomes regulate multiple immune effector cell functions, including class I antigen presentation, cytokine expression, and plasma cell proliferation, migration, and adhesion (128, 129). Expression of immunoproteasomes is induced in nonimmune cells by inflammatory and autoimmune conditions (128); this process occurs in LN, and increased immunoproteasome expression has been seen in murine models of LN and in kidney cells of patients with LN (130). Selective inhibition of the immunoproteasome results in broad immunomodulatory activity across both the innate and adaptive immune systems without leading to the apoptosis or immunosuppression seen with dual proteasome inhibitors (129, 131, 132). In murine models, selective immunoproteasome inhibition blocked differentiation of inflammatory Th1 and Th17 cells, promoted differentiation of regulatory T cells (Tregs), and inhibited the release of proinflammatory cytokines, such as IFN-α, from dendritic cells (129, 131, 132). Hence, the immunoproteasome has emerged as an attractive therapeutic target in various inflammatory conditions, including LN.
Zetomipzomib is a first-in-class selective immunoproteasome inhibitor (133). In mouse models of LN, treatment with zetomipzomib ameliorated disease progression, resulting in resolution of proteinuria and marked decreases in the incidence of glomerular nephritis, glomerular sclerosis, and tubular changes (133). In these animal models, zetomipzomib did not affect normal immune-response mechanisms (133). In the Phase II part of the MISSION Phase 1b/II trial (n = 17), which evaluated the efficacy and safety of zetomipzomib in patients with LN, 64.7% of patients had ≥50% reduction in UPCR from baseline, and 35.3% had achieved a complete renal response at week 25; renal responses were sustained or improved until the end of study at week 37, 12 weeks after end of treatment (59). A reduction in daily steroid dose to 10 mg/day was achieved in 82.4% of patients at week 25, and doses of other background immunosuppressive therapies remained stable throughout the study (59). Further evaluation of zetomipzomib in active LN is ongoing in the Phase IIb PALIZADE trial (NCT05781750) (134).
2.5 Potential interventions in early development
2.5.1 Chimeric antigen T-cell (CAR-T) therapy
Based on clinical experience with B-cell therapy, a complete and sustained B-cell depletion may lead to better response in patients with SLE or LN. CAR-T therapy may induce more robust B-cell depletion, especially in tissues that are easier for engineered cells to access (135).
Case reports have suggested the potential efficacy of anti-CD19 CAR-T therapy in SLE and LN: in one case, a 20-year-old woman with severe refractory SLE and LN was prescribed anti-CD19 CAR-T and low-dose glucocorticoids and stopped all other therapy for LN (136). The patient demonstrated substantial improvements in serological markers and disease activity scores, and her proteinuria decreased from over 2000mg/g to <250 mg/ g (136). A subsequent case series similarly demonstrated the efficacy of anti-CD19 CAR-T therapy in refractory LN (137, 138). Larger clinical trials evaluating the long-term efficacy and safety of anti-CD19 CAR-T therapy are warranted (137). An open-label Phase I/II study to assess the safety, efficacy, and cellular kinetics of YTB323, an anti-CD19 CAR-T therapy, in refractory SLE and LN is ongoing (NCT05798117) (60).
2.5.2 Low-dose Il-2
IL-2 is produced by activated T-cells and dendritic cells and is crucial for maintenance of T-cell–mediated self-tolerance (139). Decreased serum IL-2 levels in healthy mice led to a strong reduction in the number of CD4+ T-regs, progression of nephritis, and mortality (140). Lupus-prone mouse models and blood samples from patients with SLE revealed impaired IL-2 production (140, 141). Low-dose IL-2 treatment in patients with SLE selectively corrected T-reg defects and expanded the T-reg population (141), resulting in marked reductions in disease activity (142). In a randomized clinical trial evaluating the use of low-dose IL-2 compared with placebo, combined with standard treatment, complete remission was achieved in 7 of 13 patients (53.85%) with LN in the IL-2 group and 2 of 12 (16.67%) in the placebo group (61). Larger randomized controlled trials are warranted to further evaluate the efficacy of the IL-2 regimen across multiple patient cohorts (61).
2.5.3 Complement-targeting therapies
Dysregulated complement activation and complement deficiencies are associated with impaired processing of immune complexes and clearance of cellular debris (143). This process can contribute to kidney damage and LN flares and may result in development of SLE and LN (143).
Narsoplimab is a mAb that blocks mannan-binding lectin (MBL) associated serine protease 2 (MASP2), an effector enzyme that activates the lectin pathway of the complement system (144). Ravulizumab is a terminal complement inhibitor that binds to complement protein C5 with high affinity, inhibiting its cleavage to C5a and C5b and preventing the formation of the membrane attack complex (145). Phase II clinical trials on these two therapeutics in the treatment of LN (NCT02682407, NCT04564339) are ongoing (62, 146).
2.6 Future directions in the treatment of LN
2.6.1 Early diagnosis and treatment
In patients with SLE, guidelines recommend thresholds of proteinuria ≥0.5 g/day or UPCR ≥0.5 g/day as indication for a kidney biopsy (16, 17). However, proteinuria should not be the sole consideration for kidney involvement in SLE because many patients with low-grade proteinuria still present with early LN in their kidney biopsies (19, 20). A retrospective analysis of 222 patients with SLE and glomerular hematuria found that 85% of patients with proteinuria <0.5 g/day and 76% of patients with proteinuria <0.25 g/day had class III or IV LN (20); another retrospective analysis reported that 40 of 52 (76%) and 9 of 10 (90%) patients with proteinuria <1 g/day and <0.5 g/day, respectively, had LN detected in their biopsies (19). Studies in silent LN have shown that a substantial proportion of patients with proliferative LN have no urine abnormalities (147, 148). These data suggest that other parameters, such as glomerular hematuria, should be taken into account when deciding whether to perform a kidney biopsy. A broader indication for kidney biopsies, such as the presence of glomerular hematuria accompanied by any degree of proteinuria, could allow for earlier diagnosis and treatment of LN, which could potentially decrease the need for steroids with the current treatment regimens.
2.6.2 Decrease of glucocorticoid exposure and consideration of glucocorticoid-free regimens
We propose that the main treatment goal for LN is to achieve long-term complete clinical remission without the need for glucocorticoids in order to minimize the adverse effects from prolonged and/or cumulative exposure to glucocorticoids (45). Several glucocorticoid-free treatment protocols have been evaluated to date; one example is the Rituxilup protocol, which comprises two doses of rituximab and methylprednisolone followed by maintenance with MMF and without the use of oral glucocorticoids (149). In a prospective single-center cohort study, 45 of 50 patients (90%) achieved a renal response to treatment with the Rituxilup protocol after a median follow-up duration of 37 weeks (149).
Novel targeted therapies may facilitate a reduced dose and a faster taper of oral glucocorticoids in the management of LN. Recent clinical trials on novel therapies, such as zetomipzomib, have used glucocorticoid reduction as an outcome measure (59), while other clinical trials, such as the BLISS-LN and the AURORA 1 trials, have incorporated glucocorticoid target doses as part of the primary endpoint or treatment failure protocol (51, 52). Additionally, LN clinical trials are increasingly using accelerated glucocorticoid-tapering regimens in their treatment protocols, such as the AURORA 1 trial (which tapered to 2.5 mg/day at week 16) and the NOBILITY trial (which tapered to 7.5 mg/day by week 12) (52, 54). An ongoing Phase III study (OBILUP; NCT04702256) will evaluate the use of obinutuzumab with MMF as induction treatment; oral glucocorticoids will not be used except in cases of extrarenal involvement (100). Future clinical trials evaluating novel therapies should consider assessing their glucocorticoid-sparing effect in light of increasing recognition for the reduction of glucocorticoid exposure as an important outcome in LN management. Validated measures of glucocorticoid toxicity, such as the Glucocorticoid Toxicity Index, can possibly be incorporated as an endpoint in future clinical trials to objectively measure the impact of LN treatments on glucocorticoid-induced toxicity (150).
2.6.3 Combination therapies
Based on the latest clinical trial data and global guideline recommendations, a proposed treatment algorithm for patients with LN is outlined in Figure 2. We propose that the approved LN therapies (belimumab and voclosporin) should be used early within the treatment paradigm for LN. A more aggressive approach to initial or induction therapy, using combinations of either MMF/CYC plus belimumab or MMF plus voclosporin, is recommended to better preserve kidney function and allow for earlier tapering of oral glucocorticoids: belimumab may help reduce the risk of relapses, and voclosporin can lead to faster proteinuria reduction (52, 80, 151). Based on data from the BLISS-LN trial, belimumab can be used with either MMF or CYC; voclosporin should be used with MMF only because of a current lack of data on the use of voclosporin with CYC or AZA (51, 52). Additional studies of belimumab and voclosporin are needed to confirm their use in other combination regimens.
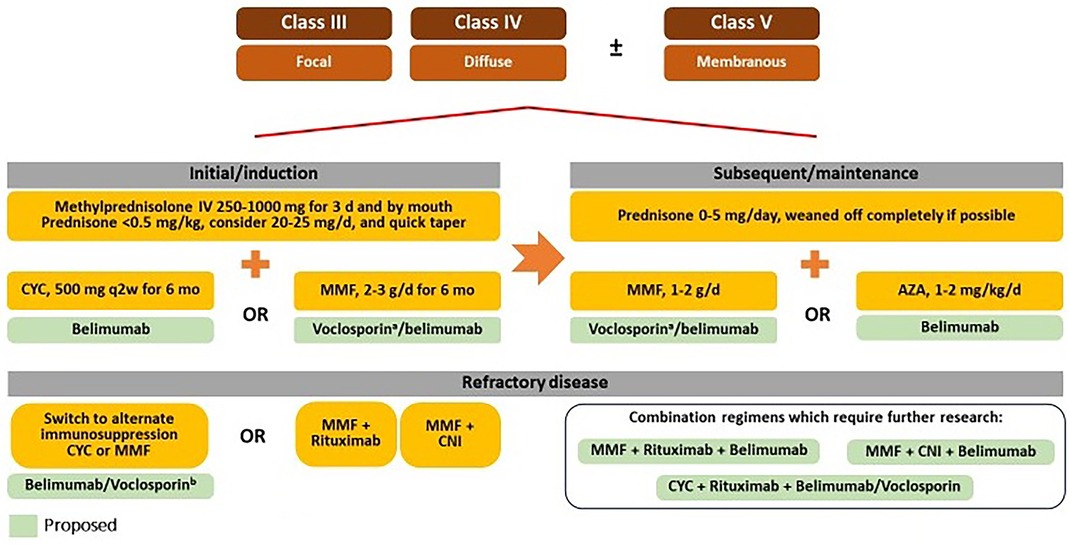
Figure 2. Proposed future treatment algorithm for LN. AZA, azathioprine; CNI, calcineurin inhibitor; CYC, cyclophosphamide; ESRD, end-stage renal disease; GFR, glomerular filtration rate; IV, intravenous; MMF, mycophenolate mofetil. aUse of voclosporin is not indicated in patients with GFR <45 ml/min. bVoclosporin should only be used in combination with MMF.
After completion of the initial or induction phase (which typically lasts approximately 3–6 months), subsequent or maintenance treatment options include either MMF plus belimumab or plus voclosporin, or AZA with belimumab; these combination regimens are based on data from the BLISS-LN and AURORA studies (51, 52, 82). We propose that the dose of prednisone should be tapered to a maximum of 5 mg/day and tapered to zero if possible.
2.6.4 Treatment of refractory disease
We consider complete renal response as UPCR ≤0.5 mg/ g and eGFR stabilization of within 10%–20% after 6–12 months of initiating therapy. In nonresponders or patients with refractory disease, observational data and guideline recommendations have suggested clinical benefit in switching to an alternative first-line immunosuppressive regimen (i.e., MMF to CYC or vice versa) (16, 152–154). The addition of rituximab to either MMF or CYC has demonstrated efficacy in refractory LN, including meaningful improvements in response rates, proteinuria, and disease activity scores (91–94).
The combination of rituximab, belimumab, and MMF demonstrated good renal response and sustained B-cell depletion in a small Phase II proof-of-concept study; MMF dose was tapered to avoid an excess of immunosuppression (155). In the CALIBRATE trial (N = 43), the add-on of belimumab to a combination of CYC and rituximab did not increase the frequency of adverse events among patients with recurrent or refractory LN, but there was no significant difference in complete response rates (156). Similarly, data from several studies have indicated the efficacy of multitarget immunosuppression (i.e., CNI with MMF) in refractory LN (157, 158). Minimal data exist for the use of CNIs with belimumab: in a small, single-center, retrospective analysis of 33 patients with SLE (including 11 patients with renal flares) treated with a combination of belimumab and tacrolimus, a state of low disease activity of lupus was achieved in 64.0% of patients at 52 weeks after initiation compared with 9.1% at initiation; the combination did not appear to increase the risk of infectious complications (159). A recent publication by Baum et al. discussed data from four patients with LN treated with a combination of both voclosporin and belimumab, including one patient treated with triple therapy (MMF plus voclosporin and belimumab) who achieved a reduction in proteinuria and glucocorticoid dose after 8–14 months without evidence of toxicity or intolerable side effects (160). Although initial data is promising, further research on combination therapies with belimumab and voclosporin is needed to establish their role in treatment of refractory LN.
2.6.5 Cessation of background immunosuppressive therapy
Newer agents in development for LN offer the exciting potential for achieving remission that would allow background immunosuppressive treatment to be discontinued or paused. The recent CAR-T data suggest that long-term drug-free remissions are possible for patients with LN (136–138). The opportunity to taper off background therapy is attractive to patients because it will simplify their treatment regiments and reduce potential toxicities from these medications.
3 Conclusion
Patients with LN face several unmet needs with current treatments, including unsatisfactory response rates, progression to ESKD, and adverse effects of treatment, especially with long-term use of glucocorticoids. The treatment landscape for LN is rapidly evolving; the development and evaluation of many new therapeutics with novel mechanisms have the potential to address these unmet needs to improve patient outcomes.
Author contributions
AA: Writing – original draft, Writing – review & editing. MD: Writing – original draft, Writing – review & editing. SA: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
Medical writing support for the manuscript was funded by Kezar Life Sciences, South San Franciso, CA (USA). Authors maintained full control, and the funder was not involved in the writing of this article or the decision to submit it for publication.
Acknowledgments
Medical writing support was provided by Zhi Yang Loh, and Lenka Yunk, Nucleus Global, in accordance with Good Publication Practice 2022 guidelines.
Conflict of interest
AA: Investigator/Consultant: Abbvie, Amgen, AstraZeneca, Aurinia, BMS, Celgene, Eli Lilly, Idorsia, Janssen, Genentech, GSK, Mallinckrodt, Pfizer and UCB. MD: Investigator/Consultant: GSK, Aurinia, AstraZeneca, Genentech, Lilly, Biogen, Adicet Bio. SA: Consulting: Kezar, Aurnia, Amgen/Chemocentryx; Research support: Gilead.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ameer MA, Chaudhry H, Mushtaq J, Khan OS, Babar M, Hashim T, et al. An overview of systemic lupus erythematosus (sle) pathogenesis, classification, and management. Cureus. (2022) 14(10):e30330. doi: 10.7759/cureus.30330
2. Almaani S, Meara A, Rovin BH. Update on lupus nephritis. Clin J Am Soc Nephrol. (2017) 12(5):825–35. doi: 10.2215/CJN.05780616
3. El Hadidi KT, Medhat BM, Abdel Baki NM, Abdel Kafy H, Abdelrahaman W, Yousri AY, et al. Characteristics of systemic lupus erythematosus in a sample of the Egyptian population: a retrospective cohort of 1109 patients from a single center. Lupus. (2018) 27(6):1030–8. doi: 10.1177/0961203317751856
4. Hanly JG, O’Keeffe AG, Su L, Urowitz MB, Romero-Diaz J, Gordon C, et al. The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatology (Oxford). (2016) 55(2):252–62. doi: 10.1093/rheumatology/kev311
5. Yap DY, Tang CS, Ma MK, Lam MF, Chan TM. Survival analysis and causes of mortality in patients with lupus nephritis. Nephrol Dial Transplant. (2012) 27(8):3248–54. doi: 10.1093/ndt/gfs073
6. Reppe Moe SE, Molberg Ø, Strøm EH, Lerang K. Assessing the relative impact of lupus nephritis on mortality in a population-based systemic lupus erythematosus cohort. Lupus. (2019) 28(7):818–25. doi: 10.1177/0961203319847275
7. Chen YE, Korbet SM, Katz RS, Schwartz MM, Lewis EJ, Collaborative Study G. Value of a complete or partial remission in severe lupus nephritis. Clin J Am Soc Nephrol. (2008) 3(1):46–53. doi: 10.2215/CJN.03280807
8. Parikh SV, Almaani S, Brodsky S, Rovin BH. Update on lupus nephritis: core curriculum 2020. Am J Kidney Dis. (2020) 76(2):265–81. doi: 10.1053/j.ajkd.2019.10.017
9. Ferreira Braga FNH, das Chagas Medeiros MM, Viana AB Jr, Maia Barros LC, Pontes MX, de Sousa Lima ME, et al. Prognostic factors for chronic kidney disease and end-stage renal disease in patients with lupus nephritis: a retrospective cohort study. J Clin Nephrol. (2021) 5(1):034–41. doi: 10.29328/journal.jcn.1001071
10. Alarcon GS. Multiethnic lupus cohorts: what have they taught US? Reumatol Clin. (2011) 7(1):3–6. doi: 10.1016/j.reuma.2010.11.001
11. Korbet SM, Schwartz MM, Evans J, Lewis EJ, Collaborative Study G. Severe lupus nephritis: racial differences in presentation and outcome. J Am Soc Nephrol. (2007) 18(1):244–54. doi: 10.1681/ASN.2006090992
12. Jolly M, Toloza S, Goker B, Clarke AE, Navarra SV, Wallace D, et al. Disease-specific quality of life in patients with lupus nephritis. Lupus. (2018) 27(2):257–64. doi: 10.1177/0961203317717082
13. Aghdassi E, Zhang W, St-Pierre Y, Clarke AE, Morrison S, Peeva V, et al. Healthcare cost and loss of productivity in a Canadian population of patients with and without lupus nephritis. J Rheumatol. (2011) 38(4):658–66. doi: 10.3899/jrheum.100482
14. Abu Bakar F, Sazliyana Shaharir S, Mohd R, Mohamed Said MS, Rajalingham S, Wei Yen K. Burden of systemic lupus erythematosus on work productivity and daily living activity: a cross-sectional study among Malaysian multi-ethnic cohort. Arch Rheumatol. (2020) 35(2):205–13. doi: 10.46497/ArchRheumatol.2020.7405
15. Nieto R, Ferreira Borba E, Settecasse E, Fernandez-Avila D, Maurelli L, Gobbi C, et al. Impact of active lupus nephritis in patient-reported outcomes from a Latin American, multicenter lupus cohort. Arthritis Rheumatol. (2021) 73(Suppl 9):2644–7.
16. Kidney Disease: Improving Global Outcomes Glomerular Diseases Work G. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. (2021) 100(4S):S1–276. doi: 10.1016/j.kint.2021.05.021
17. Fanouriakis A, Kostopoulou M, Cheema K, Anders HJ, Aringer M, Bajema I, et al. 2019 update of the joint European league against rheumatism and European renal association-European dialysis and transplant association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis. (2020) 79(6):713–23. doi: 10.1136/annrheumdis-2020-216924
18. Wang S, Spielman A, Ginsberg M, Petri M, Rovin B, Buyon J, et al. Short- and long-term progression of kidney involvement in systemic lupus erythematosus patients with low-grade proteinuria. Arthritis Rheumatol. (2022) 74:1150–8. doi: 10.2215/CJN.01280122
19. Chedid A, Rossi GM, Peyronel F, Menez S, Atta MG, Bagnasco SM, et al. Low-level proteinuria in systemic lupus erythematosus. Kidney Int Rep. (2020) 5(12):2333–40. doi: 10.1016/j.ekir.2020.09.007
20. De Rosa M, Rocha AS, De Rosa G, Dubinsky D, Almaani SJ, Rovin BH. Low-grade proteinuria does not exclude significant kidney injury in lupus nephritis. Kidney Int Rep. (2020) 5(7):1066–8. doi: 10.1016/j.ekir.2020.04.005
21. Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. (2004) 65(2):521–30. doi: 10.1111/j.1523-1755.2004.00443.x
22. Bajema IM, Balow JE, Haas M, Jayne D, Lightstone L, Rovin BH, et al. Update on scoring and providing evidence basis for assessing pathology in lupus nephritis. Kidney Int. (2023) 103(5):813–6. doi: 10.1016/j.kint.2023.02.006
23. Bajema IM, Wilhelmus S, Alpers CE, Bruijn JA, Colvin RB, Cook HT, et al. Revision of the international society of nephrology/renal pathology society classification for lupus nephritis: clarification of definitions, and modified national institutes of health activity and chronicity indices. Kidney Int. (2018) 93(4):789–96. doi: 10.1016/j.kint.2017.11.023
24. Yo JH, Barbour TD, Nicholls K. Management of refractory lupus nephritis: challenges and solutions. Open Access Rheumatol. (2019) 11:179–88. doi: 10.2147/OARRR.S166303
25. Hans-Joachim A, Iva G, Liz L. A farewell to the concept of induction and maintenance therapy of lupus nephritis. Available at: https://www.era-online.org/wp-content/uploads/2023/05/A-farewell-to-the-concept-of-induction-and-maintenance-therapy-of-lupus-nephritis.pdf (Cited 2023 Oct 12).
26. Pollak VE, Pirani CL, Schwartz FD. The natural history of the renal manifestations of systemic lupus erythematosus. J Lab Clin Med. (1964) 63:537–50.14155443
27. Austin HA 3rd, Klippel JH, Balow JE, le Riche NG, Steinberg AD, Plotz PH, et al. Therapy of lupus nephritis. Controlled trial of prednisone and cytotoxic drugs. N Engl J Med (1986) 314(10):614–9. doi: 10.1056/NEJM198603063141004
28. Donadio JV Jr, Holley KE, Ferguson RH, Ilstrup DM. Treatment of diffuse proliferative lupus nephritis with prednisone and combined prednisone and cyclophosphamide. N Engl J Med. (1978) 299(21):1151–5. doi: 10.1056/nejm197811232992102
29. Appel GB, Contreras G, Dooley MA, Ginzler EM, Isenberg D, Jayne D, et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol. (2009) 20(5):1103–12. doi: 10.1681/ASN.2008101028
30. FDA approves Aurinia pharmaceuticals’ Lupkynis™ (voclosporin) for adult patients with active lupus nephritis Aurinia pharma. (2021). Available at: https://ir.auriniapharma.com/press-releases/detail/210/fda-approves-aurinia-pharmaceuticals-lupkynis (Accessed June 2023).
31. FDA approves GSK’s benlysta as the first medicine for adult patients with active lupus nephritis in the US GSK. (2020). Available at: https://www.gsk.com/en-gb/media/press-releases/fda-approves-gsk-s-benlysta-as-the-first-medicine-for-adult-patients-with-active-lupus-nephritis-in-the-us/ (Accessed June 2023).
32. Fanouriakis A, Kostopoulou M, Andersen J, Aringer M, Arnaud L, Bae SC, et al. EULAR recommendations for the management of systemic lupus erythematosus: 2023 update. Ann Rheum Dis. (2023) 83(1):15–29. doi: 10.1136/ard-2023-224762
33. Rovin BH, Ayoub IM, Chan TM, Liu Z-H, Mejía-Vilet JM, Floege J. KDIGO 2024 clinical practice guideline for the management of lupus nephritis. Kidney Int. (2024) 105(1):S1–69. doi: 10.1016/j.kint.2023.09.002
34. Tektonidou MG, Dasgupta A, Ward MM. Risk of end-stage renal disease in patients with lupus nephritis, 1971-2015: a systematic review and Bayesian meta-analysis. Arthritis Rheumatol. (2016) 68(6):1432–41. doi: 10.1002/art.39594
35. Malvar A, Pirruccio P, Alberton V, Lococo B, Recalde C, Fazini B, et al. Histologic versus clinical remission in proliferative lupus nephritis. Nephrol Dial Transplant. (2017) 32(8):1338–44. doi: 10.1093/ndt/gfv296
36. Zickert A, Sundelin B, Svenungsson E, Gunnarsson I. Role of early repeated renal biopsies in lupus nephritis. Lupus Sci Med. (2014) 1(1):e000018. doi: 10.1136/lupus-2014-000018
37. Davidson JE, Fu Q, Ji B, Rao S, Roth D, Magder LS, et al. Renal remission status and longterm renal survival in patients with lupus nephritis: a retrospective cohort analysis. J Rheumatol. (2018) 45(5):671–7. doi: 10.3899/jrheum.161554
38. Korbet SM, Lewis EJ, Collaborative Study G. Complete remission in severe lupus nephritis: assessing the rate of loss in proteinuria. Nephrol Dial Transplant. (2012) 27(7):2813–9. doi: 10.1093/ndt/gfr741
39. Ginzler EM, Dooley MA, Aranow C, Kim MY, Buyon J, Merrill JT, et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med. (2005) 353(21):2219–28. doi: 10.1056/NEJMoa043731
40. Sidiropoulos PI, Kritikos HD, Boumpas DT. Lupus nephritis flares. Lupus. (2005) 14(1):49–52. doi: 10.1191/0961203305lu2059oa
41. Sankhaanuruk A, Kasitanon N, Louthrenoo W. Incidence of lupus nephritis flares after complete response in a Thai population. Med Res Arch. (2022) 10(5). doi: 10.18103/mra.v10i5.2831
42. Kapsia E, Marinaki S, Michelakis I, Liapis G, Sfikakis PP, Boletis J, et al. Predictors of early response, flares, and long-term adverse renal outcomes in proliferative lupus nephritis: a 100-month median follow-up of an inception cohort. J Clin Med. (2022) 11(17):5017. doi: 10.3390/jcm11175017
43. Luis MSF, Bultink IEM, da Silva JAP, Voskuyl AE, Ines LS. Early predictors of renal outcome in patients with proliferative lupus nephritis: a 36-month cohort study. Rheumatology (Oxford). (2021) 60(11):5134–41. doi: 10.1093/rheumatology/keab126
44. Tesar V, Hruskova Z. Limitations of standard immunosuppressive treatment in ANCA-associated vasculitis and lupus nephritis. Nephron Clin Pract. (2014) 128(3-4):205–15. doi: 10.1159/000368569
45. Mejia-Vilet JM, Ayoub I. The use of glucocorticoids in lupus nephritis: new pathways for an old drug. Front Med (Lausanne). (2021) 8:622225. doi: 10.3389/fmed.2021.622225
46. Ward MM, Pyun E, Studenski S. Causes of death in systemic lupus erythematosus. Long-term followup of an inception cohort. Arthritis Rheum. (1995) 38(10):1492–9. doi: 10.1002/art.1780381016
47. Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, et al. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine (Baltimore). (2003) 82(5):299–308. doi: 10.1097/01.md.0000091181.93122.55
48. Terrell M, Morel L. The intersection of cellular and systemic metabolism: metabolic syndrome in systemic lupus erythematosus. Endocrinology. (2022) 163(7):bqac067. doi: 10.1210/endocr/bqac067
49. Gladman DD, Urowitz MB, Rahman P, Ibañez D, Tam L-S. Accrual of organ damage over time in patients with systemic lupus erythematosus. J Rheumatol. (2003) 30(9):1955–9.12966597
50. Obrișcă B, Sorohan B, Tuță L, Ismail G. Advances in lupus nephritis pathogenesis: from bench to bedside. Int J Mol Sci. (2021) 22:3766. doi: 10.3390/ijms22073766
51. Furie R, Rovin BH, Houssiau F, Malvar A, Teng YKO, Contreras G, et al. Two-year, randomized, controlled trial of belimumab in lupus nephritis. N Engl J Med. (2020) 383(12):1117–28. doi: 10.1056/NEJMoa2001180
52. Rovin BH, Teng YKO, Ginzler EM, Arriens C, Caster DJ, Romero-Diaz J, et al. Efficacy and safety of voclosporin versus placebo for lupus nephritis (Aurora 1): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. (2021) 397(10289):2070–80. doi: 10.1016/s0140-6736(21)00578-x
53. Rovin BH, Furie R, Latinis K, Looney RJ, Fervenza FC, Sanchez-Guerrero J, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the lupus nephritis assessment with rituximab study. Arthritis Rheum. (2012) 64(4):1215–26. doi: 10.1002/art.34359
54. Furie RA, Aroca G, Cascino MD, Garg JP, Rovin BH, Alvarez A, et al. B-cell depletion with obinutuzumab for the treatment of proliferative lupus nephritis: a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. (2022) 81(1):100–7. doi: 10.1136/annrheumdis-2021-220920
55. Gordon C, Wofsy D, Wax S, Li Y, Pena Rossi C, Isenberg D. Post hoc analysis of the phase II/III April-SLE study: association between response to atacicept and serum biomarkers including BLYS and April. Arthritis Rheumatol. (2017) 69(1):122–30. doi: 10.1002/art.39809
56. Clinicaltrials.Gov: safety, efficacy and tolerability of ianalumab versus placebo, combination with SOC therapy, in participants with active lupus nephritis (Sirius-LN) ClinicalTrials.gov. (2023). Available at: https://clinicaltrials.gov/ct2/show/NCT05126277 (Accessed June 2023).
57. Clinicaltrials.Gov: study of safety, efficacy and tolerability of secukinumab versus placebo, in combination with SOC therapy, in patients with active lupus nephritis (Selune) ClinicalTrials.gov. (2023). Available at: https://www.clinicaltrials.gov/ct2/show/NCT04181762 (Accessed June 2023).
58. Jayne D, Rovin B, Mysler EF, Furie RA, Houssiau FA, Trasieva T, et al. Phase II randomised trial of type I interferon inhibitor anifrolumab in patients with active lupus nephritis. Ann Rheum Dis. (2022) 81(4):496–506. doi: 10.1136/annrheumdis-2021-221478
59. Saxena A, Parikh S, Hua S, Leff R, Park E, Henig N. Pos1128 zetomipzomib (Kzr-616) treatment results in clinically meaningful renal responses in patients with lupus nephritis. Ann Rheum Dis (2023) 82(Suppl 1):891–2. doi: 10.1136/annrheumdis-2023-eular.2317
60. Clinicaltrials.Gov: an open-label, study to assess safety, efficacy and cellular kinetics of YTB323 in severe, refractory systemic lupus erythematosus ClinicalTrials.gov. (2023). Available at: https://clinicaltrials.gov/ct2/show/NCT05798117 (Accessed June 2023).
61. He J, Zhang R, Shao M, Zhao X, Miao M, Chen J, et al. Efficacy and safety of low-dose il-2 in the treatment of systemic lupus erythematosus: a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. (2020) 79(1):141–9. doi: 10.1136/annrheumdis-2019-215396
62. Clinicaltrials.Gov: study of ravulizumab in proliferative lupus nephritis (LN) or immunoglobulin a nephropathy (IGAN) (sanctuary) ClinicalTrials.gov. (2023). Available at: https://clinicaltrials.gov/ct2/show/NCT04564339 (Accessed June 2023).
63. Omeros reports more positive data in OMS721 phase 2 trial in renal diseases. Available at: https://investor.omeros.com/news-releases/news-release-details/omeros-reports-more-positive-data-oms721-phase-2-trial-renal: Omeros Corporation (Accessed March 30, 2017).
64. Dörner T, Giesecke C, Lipsky PE. Mechanisms of B cell autoimmunity in sle. Arthritis Res Ther. (2011) 13(5):243. doi: 10.1186/ar3433
65. Almaani S, Rovin BH. B-cell therapy in lupus nephritis: an overview. Nephrol Dial Transplant. (2019) 34(1):22–9. doi: 10.1093/ndt/gfy267
67. Clinicaltrials.Gov: Benlysta® special drug use investigation ClinicalTrials.gov. (2022). Available at: https://clinicaltrials.gov/ct2/show/NCT03370263 (Accessed June 2023).
68. Clinicaltrials.Gov: trial of belimumab combined with multi-target induction therapy in lupus nephritis (Beam) ClinicalTrials.gov. (2023). Available at: https://clinicaltrials.gov/ct2/show/NCT05863936 (Accessed June 2023).
69. Wallace DJ, Ginzler EM, Merrill JT, Furie RA, Stohl W, Chatham WW, et al. Safety and efficacy of belimumab plus standard therapy for up to thirteen years in patients with systemic lupus erythematosus. Arthritis Rheumatol. (2019) 71(7):1125–34. doi: 10.1002/art.40861
71. Peleg Y, Bomback AS, Radhakrishnan J. The evolving role of calcineurin inhibitors in treating lupus nephritis. Clin J Am Soc Nephrol. (2020) 15(7):1066–72. doi: 10.2215/CJN.13761119
72. van Gelder T, Lerma E, Engelke K, Huizinga RB. Voclosporin: a novel calcineurin inhibitor for the treatment of lupus nephritis. Expert Rev Clin Pharmacol. (2022) 15(5):515–29. doi: 10.1080/17512433.2022.2092470
73. van Gelder T, Huizinga RB, Noukens J, Lisk L, Solomons N. Use of therapeutic drug monitoring does not add clinical value for voclosporin in patients with lupus nephritis [abstract Po1918]. J Am Soc Nephrol. (2020) 31:592.
74. Kasitanon N, Boripatkosol P, Louthrenoo W. Response to combination of mycophenolate mofetil, cyclosporin a and corticosteroid treatment in lupus nephritis patients with persistent proteinuria. Int J Rheum Dis. (2018) 21(1):200–7. doi: 10.1111/1756-185x.13152
75. Mok C, To C, Yu K, Ho L. Combined low-dose mycophenolate mofetil and tacrolimus for lupus nephritis with suboptimal response to standard therapy: a 12-month prospective study. Lupus. (2013) 22(11):1135–41. doi: 10.1177/0961203313502864
76. Sheikholeslami M, Hajialilo M, Rasi Hashemi SS, Malek Mahdavi A, Gojazadeh M, Khabbazi A. Low dose cyclosporine a in the treatment of resistant proliferative lupus nephritis. Mod Rheumatol. (2018) 28(3):523–9. doi: 10.1080/14397595.2017.1352479
77. Fei Y, Wu Q, Zhang W, Chen H, Hou Y, Xu D, et al. Low-dose tacrolimus in treating lupus nephritis refractory to cyclophosphamide: a prospective cohort study. Clin Exp Rheumatol. (2013) 31(1):62–8.22935463
78. Liu Z, Zhang H, Liu Z, Xing C, Fu P, Ni Z, et al. Multitarget therapy for induction treatment of lupus nephritis: a randomized trial. Ann Intern Med. (2015) 162(1):18–26. doi: 10.7326/m14-1030
79. Sakai R, Kurasawa T, Nishi E, Kondo T, Okada Y, Shibata A, et al. Efficacy and safety of multitarget therapy with cyclophosphamide and tacrolimus for lupus nephritis: a prospective, single-arm, single-centre, open label pilot study in Japan. Lupus. (2018) 27(2):273–82. doi: 10.1177/0961203317719148
80. Rovin BH, Solomons N, Pendergraft WF 3rd, Dooley MA, Tumlin J, Romero-Diaz J, et al. A randomized, controlled double-blind study comparing the efficacy and safety of dose-ranging voclosporin with placebo in achieving remission in patients with active lupus nephritis. Kidney Int (2019) 95(1):219–31. doi: 10.1016/j.kint.2018.08.025
81. Arriens C, Teng YKO, Ginzler EM, Parikh SV, Askanase AD, Saxena A, et al. Update on the efficacy and safety profile of voclosporin: an integrated analysis of clinical trials in lupus nephritis. Arthritis Care Res (Hoboken). (2023) 75(7):1399–408. doi: 10.1002/acr.25007
82. Saxena A, Teng O, Collins C, England N, Leher H. 1202 voclosporin for lupus nephritis: results of the two-year Aurora 2 continuation study. Lupus Sci Med. (2022) 9(Suppl 3):A85–6. doi: 10.1136/lupus-2022-lupus21century.84
83. Saxena A, Ginzler EM, Gibson K, Satirapoj B, Santillan AEZ, Levchenko O, et al. Safety and efficacy of long-term voclosporin treatment for lupus nephritis in the phase 3 Aurora 2 clinical trial. Arthritis Rheumatol. (2023) 76(1):59–67. doi: 10.1002/art.42657
84. Ayoub I, Rovin BH. Calcineurin inhibitors in the treatment of lupus nephritis: a hare versus turtle story? J Am Soc Nephrol. (2017) 28(12):3435–7. doi: 10.1681/ASN.2017080830
86. Reddy V, Cambridge G, Isenberg DA, Glennie MJ, Cragg MS, Leandro M. Internalization of rituximab and the efficiency of B cell depletion in rheumatoid arthritis and systemic lupus erythematosus. Arthritis Rheumatol. (2015) 67(8):2046–55. doi: 10.1002/art.39167
87. Terrier B, Amoura Z, Ravaud P, Hachulla E, Jouenne R, Combe B, et al. Safety and efficacy of rituximab in systemic lupus erythematosus: results from 136 patients from the French autoimmunity and rituximab registry. Arthritis Rheum. (2010) 62(8):2458–66. doi: 10.1002/art.27541
88. Merrill JT, Neuwelt CM, Wallace DJ, Shanahan JC, Latinis KM, Oates JC, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. (2010) 62(1):222–33. doi: 10.1002/art.27233
89. Gomez Mendez LM, Cascino MD, Garg J, Katsumoto TR, Brakeman P, Dall’Era M, et al. Peripheral blood B cell depletion after rituximab and complete response in lupus nephritis. Clin J Am Soc Nephrol. (2018) 13(10):1502–9. doi: 10.2215/CJN.01070118
90. Alshaiki F, Obaid E, Almuallim A, Taha R, El-Haddad H, Almoallim H. Outcomes of rituximab therapy in refractory lupus: a meta-analysis. Eur J Rheumatol. (2018) 5(2):118–26. doi: 10.5152/eurjrheum.2018.17096
91. Weidenbusch M, Rommele C, Schrottle A, Anders HJ. Beyond the lunar trial. Efficacy of rituximab in refractory lupus nephritis. Nephrol Dial Transplant. (2013) 28(1):106–11. doi: 10.1093/ndt/gfs285
92. Davies RJ, Sangle SR, Jordan NP, Aslam L, Lewis MJ, Wedgwood R, et al. Rituximab in the treatment of resistant lupus nephritis: therapy failure in rapidly progressive crescentic lupus nephritis. Lupus. (2013) 22(6):574–82. doi: 10.1177/0961203313483376
93. Jonsdottir T, Zickert A, Sundelin B, Henriksson EW, van Vollenhoven RF, Gunnarsson I. Long-term follow-up in lupus nephritis patients treated with rituximab–clinical and histopathological response. Rheumatology (Oxford). (2013) 52(5):847–55. doi: 10.1093/rheumatology/kes348
94. Zhang J, Zhao Z, Hu X. Effect of rituximab on serum levels of anti-C1Q and antineutrophil cytoplasmic autoantibodies in refractory severe lupus nephritis. Cell Biochem Biophys. (2015) 72(1):197–201. doi: 10.1007/s12013-014-0437-z
95. Mossner E, Brunker P, Moser S, Puntener U, Schmidt C, Herter S, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. (2010) 115(22):4393–402. doi: 10.1182/blood-2009-06-225979
96. Looney CM, Schroeder A, Tavares E, Garg J, Schindler T, Vincenti F, et al. Obinutuzumab effectively depletes key B-cell subsets in blood and tissue in end-stage renal disease patients. Transplant Direct. (2023) 9(2):e1436. doi: 10.1097/TXD.0000000000001436
97. Reddy V, Klein C, Isenberg DA, Glennie MJ, Cambridge G, Cragg MS, et al. Obinutuzumab induces superior B-cell cytotoxicity to rituximab in rheumatoid arthritis and systemic lupus erythematosus patient samples. Rheumatology (Oxford). (2017) 56(7):1227–37. doi: 10.1093/rheumatology/kex067
98. Marinov AD, Wang H, Bastacky SI, van Puijenbroek E, Schindler T, Speziale D, et al. The type II anti-CD20 antibody obinutuzumab (GA101) is more effective than rituximab at depleting B cells and treating disease in a murine lupus model. Arthritis Rheumatol. (2021) 73(5):826–36. doi: 10.1002/art.41608
99. Clinicaltrials.Gov: a study to evaluate the efficacy and safety of obinutuzumab in patients with ISN/RPS 2003 class III or IV lupus nephritis (Regency) ClinicalTrials.gov. (2023). Available at: https://clinicaltrials.gov/ct2/show/NCT04221477?term=Obinutuzumab&phase=2&draw=2&rank=7 (Accessed June 2023).
100. Clinicaltrials.Gov: induction therapy for lupus nephritis with no added oral steroids: a trial comparing oral corticosteroids plus mycophenolate mofetil (MMF) versus obinutuzumab and MMF (Obilup) ClinicalTrials.gov. (2023). Available at: https://clinicaltrials.gov/ct2/show/NCT04702256?term=Obinutuzumab&phase=2&draw=2&rank=1 (Accessed June 2023).
101. Kaegi C, Steiner UC, Wuest B, Crowley C, Boyman O. Systematic review of safety and efficacy of atacicept in treating immune-mediated disorders. Front Immunol. (2020) 11:433. doi: 10.3389/fimmu.2020.00433
102. Ginzler EM, Wax S, Rajeswaran A, Copt S, Hillson J, Ramos E, et al. Atacicept in combination with MMF and corticosteroids in lupus nephritis: results of a prematurely terminated trial. Arthritis Res Ther. (2012) 14(1):R33. doi: 10.1186/ar3738
103. Barratt J, Tumlin J, Suzuki Y, Kao A, Aydemir A, Pudota K, et al. Randomized phase II Janus study of atacicept in patients with IGA nephropathy and persistent proteinuria. Kidney Int Rep. (2022) 7(8):1831–41. doi: 10.1016/j.ekir.2022.05.017
104. Clinicaltrials.Gov: atacicept in subjects with active lupus nephritis (compass) ClinicalTrials.gov. (2023). Available at: https://www.clinicaltrials.gov/study/NCT05609812 (Updated July 2023, October 2023).
105. McWilliams EM, Lucas CR, Chen T, Harrington BK, Wasmuth R, Campbell A, et al. Anti-BAFF-R antibody VAY-736 demonstrates promising preclinical activity in CLL and enhances effectiveness of ibrutinib. Blood Adv. (2019) 3(3):447–60. doi: 10.1182/bloodadvances.2018025684
106. Carter LM, Isenberg DA, Ehrenstein MR. Elevated serum BAFF levels are associated with rising anti-double-stranded DNA antibody levels and disease flare following B cell depletion therapy in systemic lupus erythematosus. Arthritis Rheum. (2013) 65(10):2672–9. doi: 10.1002/art.38074
107. Pers JO, Devauchelle V, Daridon C, Bendaoud B, Le Berre R, Bordron A, et al. BAFF-modulated repopulation of B lymphocytes in the blood and salivary glands of rituximab-treated patients with Sjögren’s syndrome. Arthritis Rheum. (2007) 56(5):1464–77. doi: 10.1002/art.22603
108. Bowman SJ, Fox R, Dorner T, Mariette X, Papas A, Grader-Beck T, et al. Safety and efficacy of subcutaneous ianalumab (VAY736) in patients with primary Sjogren’s syndrome: a randomised, double-blind, placebo-controlled, phase 2B dose-finding trial. Lancet. (2022) 399(10320):161–71. doi: 10.1016/S0140-6736(21)02251-0
109. Vita SD, Quartuccio L, Seror R, Salvin S, Ravaud P, Fabris M, et al. Thu0392 efficacy and safety of belimumab given for 12 months in primary Sjögren’s syndrome: the BELISS open-label phase II study. Ann Rheum Dis. (2015) 74(Suppl 2):338–9. doi: 10.1136/annrheumdis-2015-eular.3101
110. Devauchelle-Pensec V, Mariette X, Jousse-Joulin S, Berthelot JM, Perdriger A, Puéchal X, et al. Treatment of primary Sjögren syndrome with rituximab: a randomized trial. Ann Intern Med. (2014) 160(4):233–42. doi: 10.7326/m13-1085
111. Clinicaltrials.Gov: phase 3 study to evaluate two regimens of ianalumab on top of standard-of-care therapy in patients with systemic lupus erythematosus (Sirius-SLE 1) ClinicalTrials.gov. (2023). Available at: https://clinicaltrials.gov/ct2/show/NCT05639114?term=Ianalumab&draw=2&rank=1 (Accessed June 2023).
112. Clinicaltrials.Gov: phase 3 study to evaluate ianalumab on top of standard-of-care therapy in patients with systemic lupus erythematosus (Sirius-Sle 2) ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/show/NCT05624749?term=Ianalumab&draw=2&rank=4 (Accessed June 2023).
113. Farah Izati A, Wong KK, Che Maraina CH. Il-23/Il-17 axis in the pathogenesis and treatment of systemic lupus erythematosus and rheumatoid arthritis. Malays J Pathol. (2020) 42(3):333–47.33361714
114. Larosa M, Zen M, Gatto M, Jesus D, Zanatta E, Iaccarino L, et al. Il-12 and Il-23/Th17 axis in systemic lupus erythematosus. Exp Biol Med (Maywood). (2019) 244(1):42–51. doi: 10.1177/1535370218824547
115. Cheng Y, Yang X, Zhang X, An Z. Analysis of expression levels of Il-17 and Il-34 and influencing factors for prognosis in patients with lupus nephritis. Exp Ther Med. (2019) 17(3):2279–83. doi: 10.3892/etm.2019.7168
117. Costa R, Antunes P, Salvador P, Oliveira P, Marinho A. Secukinumab on refractory lupus nephritis. Cureus. (2021) 13(8):e17198. doi: 10.7759/cureus.17198
118. Yoshinari H, Nakano K, Nakayamada S, Iwata S, Kubo S, Miyagawa I, et al. 110 successful treatment of refractory lupus nephritis with secukinumab in a patient complicated with psoriasis vulgaris. Lupus Sci Med. (2017) 4(Suppl 1):A47–8. doi: 10.1136/lupus-2017-000215.110
119. Satoh Y, Nakano K, Yoshinari H, Nakayamada S, Iwata S, Kubo S, et al. A case of refractory lupus nephritis complicated by psoriasis vulgaris that was controlled with secukinumab. Lupus. (2018) 27(7):1202–6. doi: 10.1177/0961203318762598
120. Clinicaltrials.Gov: a study of guselkumab in participants with active lupus nephritis (Orchid-Ln) ClinicalTrials.gov. (2023). Available at: https://classic.clinicaltrials.gov/ct2/show/NCT04376827 (Updated March 15, 2023, October 2023).
121. Feng X, Wu H, Grossman JM, Hanvivadhanakul P, FitzGerald JD, Park GS, et al. Association of increased interferon-inducible gene expression with disease activity and lupus nephritis in patients with systemic lupus erythematosus. Arthritis Rheum. (2006) 54(9):2951–62. doi: 10.1002/art.22044
122. Der E, Suryawanshi H, Morozov P, Kustagi M, Goilav B, Ranabothu S, et al. Tubular cell and keratinocyte single-cell transcriptomics applied to lupus nephritis reveal type I IFN and fibrosis relevant pathways. Nat Immunol. (2019) 20(7):915–27. doi: 10.1038/s41590-019-0386-1
123. Ding X, Ren Y, He X. IFN-I mediates lupus nephritis from the beginning to renal fibrosis. Front Immunol. (2021) 12:676082. doi: 10.3389/fimmu.2021.676082
125. Morand EF, Furie R, Tanaka Y, Bruce IN, Askanase AD, Richez C, et al. Trial of anifrolumab in active systemic lupus erythematosus. N Engl J Med. (2020) 382(3):211–21. doi: 10.1056/NEJMoa1912196
126. Clinicaltrials.Gov: phase 3 study of anifrolumab in adult patients with active proliferative lupus nephritis (Iris) ClinicalTrials.gov. (2023). Available at: https://clinicaltrials.gov/ct2/show/NCT05138133?term=anifrolumab&draw=2&rank=5 (Accessed June 2023).
127. Wang J, Fang Y, Fan RA, Kirk CJ. Proteasome inhibitors and their pharmacokinetics, pharmacodynamics, and metabolism. Int J Mol Sci. (2021) 22(21):11595. doi: 10.3390/ijms222111595
128. Kimura H, Caturegli P, Takahashi M, Suzuki K. New insights into the function of the immunoproteasome in immune and nonimmune cells. J Immunol Res. (2015) 2015:541984. doi: 10.1155/2015/541984
129. Kirk CJ, Muchamuel T, Wang J, Fan RA. Discovery and early clinical development of selective immunoproteasome inhibitors. Cells. (2021) 11(1):9. doi: 10.3390/cells11010009
130. Berthier CC, Bethunaickan R, Gonzalez-Rivera T, Nair V, Ramanujam M, Zhang W, et al. Cross-species transcriptional network analysis defines shared inflammatory responses in murine and human lupus nephritis. J Immunol. (2012) 189(2):988–1001. doi: 10.4049/jimmunol.1103031
131. Ichikawa HT, Conley T, Muchamuel T, Jiang J, Lee S, Owen T, et al. Beneficial effect of novel proteasome inhibitors in murine lupus via dual inhibition of type I interferon and autoantibody-secreting cells. Arthritis Rheum. (2012) 64(2):493–503. doi: 10.1002/art.33333
132. Kalim KW, Basler M, Kirk CJ, Groettrup M. Immunoproteasome subunit LMP7 deficiency and inhibition suppresses TH1 and TH17 but enhances regulatory T cell differentiation. J Immunol. (2012) 189(8):4182–93. doi: 10.4049/jimmunol.1201183
133. Muchamuel T, Fan RA, Anderl JL, Bomba DJ, Johnson HWB, Lowe E, et al. Zetomipzomib (KZR-616) attenuates lupus in mice via modulation of innate and adaptive immune responses. Front Immunol. (2023) 14:1043680. doi: 10.3389/fimmu.2023.1043680
134. Clinicaltrials.Gov: a study of zetomipzomib (KZR-616) in patients with active lupus nephritis (Palizade) ClinicalTrials.gov. (2023). Available at: https://clinicaltrials.gov/ct2/show/NCT05781750 (Accessed June 2023).
135. Doglio M, Alexander T, Del Papa N, Snowden JA, Greco R, et al. New insights in systemic lupus erythematosus: from regulatory T cells to car-T-cell strategies. J Allergy Clin Immunol. (2022) 150(6):1289–301. doi: 10.1016/j.jaci.2022.08.003
136. Mougiakakos D, Krönke G, Völkl S, Kretschmann S, Aigner M, Kharboutli S, et al. CD19-targeted car T cells in refractory systemic lupus erythematosus. N Engl J Med. (2021) 385(6):567–9. doi: 10.1056/NEJMc2107725
137. Mackensen A, Muller F, Mougiakakos D, Boltz S, Wilhelm A, Aigner M, et al. Anti-CD19 car T cell therapy for refractory systemic lupus erythematosus. Nat Med. (2022) 28(10):2124–32. doi: 10.1038/s41591-022-02017-5
138. Schett G, Boeltz S, Müller F, Kleyer A, Völkl S, Aigner M, et al. OP0279 CAR-T cell treatment of refractory systemic lupus erythematosus—safety and preliminary efficacy data from the first four patients. Ann Rheum Dis. (2022) 81(Suppl 1):185. doi: 10.1136/annrheumdis-2022-eular.1120
139. Mizui M, Koga T, Lieberman LA, Beltran J, Yoshida N, Johnson MC, et al. Il-2 protects lupus-prone mice from multiple End-organ damage by limiting CD4-CD8- Il-17-producing T cells. J Immunol. (2014) 193(5):2168–77. doi: 10.4049/jimmunol.1400977
140. Humrich JY, Morbach H, Undeutsch R, Enghard P, Rosenberger S, Weigert O, et al. Homeostatic imbalance of regulatory and effector T cells due to Il-2 deprivation amplifies murine lupus. Proc Natl Acad Sci U S A. (2010) 107(1):204–9. doi: 10.1073/pnas.0903158107
141. Spee-Mayer CV, Siegert E, Abdirama D, Rose A, Klaus A, Alexander T, et al. Low-dose interleukin-2 selectively corrects regulatory T cell defects in patients with systemic lupus erythematosus. Ann Rheum Dis. (2016) 75(7):1407–15. doi: 10.1136/annrheumdis-2015-207776
142. He J, Zhang X, Wei Y, Sun X, Chen Y, Deng J, et al. Low-dose interleukin-2 treatment selectively modulates CD4+ T cell subsets in patients with systemic lupus erythematosus. Nat Med. (2016) 22(9):991–3. doi: 10.1038/nm.4148
143. Li NL, Birmingham DJ, Rovin BH. Expanding the role of complement therapies: the case for lupus nephritis. J Clin Med. (2021) 10(4):626. doi: 10.3390/jcm10040626
144. Khaled SK, Claes K, Goh YT, Kwong YL, Leung N, Mendrek W, et al. Narsoplimab, a mannan-binding lectin-associated serine protease-2 inhibitor, for the treatment of adult hematopoietic stem-cell transplantation-associated thrombotic microangiopathy. J Clin Oncol. (2022) 40(22):2447–57. doi: 10.1200/jco.21.02389
146. Clinicaltrials.Gov: safety study of IGAN, LN, MN, & C3 glomerulopathy including dense deposit disease treated with OMS721 ClinicalTrials.gov. (2020). Available at: https://clinicaltrials.gov/ct2/show/NCT02682407 (Accessed June 2023).
147. Wakasugi D, Gono T, Kawaguchi Y, Hara M, Koseki Y, Katsumata Y, et al. Frequency of class III and IV nephritis in systemic lupus erythematosus without clinical renal involvement: an analysis of predictive measures. J Rheumatol. (2012) 39(1):79–85. doi: 10.3899/jrheum.110532
148. Zabaleta-Lanz ME, Muñoz LE, Tapanes FJ, Vargas-Arenas RE, Daboin I, Barrios Y, et al. Further description of early clinically silent lupus nephritis. Lupus. (2006) 15(12):845–51. doi: 10.1177/0961203306070002
149. Condon MB, Ashby D, Pepper RJ, Cook HT, Levy JB, Griffith M, et al. Prospective observational single-centre cohort study to evaluate the effectiveness of treating lupus nephritis with rituximab and mycophenolate mofetil but No oral steroids. Ann Rheum Dis. (2013) 72(8):1280–6. doi: 10.1136/annrheumdis-2012-202844
150. Stone JH, McDowell PJ, Jayne DRW, Merkel PA, Robson J, Patel NJ, et al. The glucocorticoid toxicity index: measuring change in glucocorticoid toxicity over time. Semin Arthritis Rheum. (2022) 55:152010. doi: 10.1016/j.semarthrit.2022.152010
151. Zhang H, Chen J, Zhang Y, Zhao N, Xu D. Efficacy and safety of belimumab therapy in lupus nephritis: a systematic review and meta-analysis. Ren Fail. (2023) 45(1):2207671. doi: 10.1080/0886022X.2023.2207671
152. Hahn BH, McMahon MA, Wilkinson A, Wallace WD, Daikh DI, Fitzgerald JD, et al. American College of rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken). (2012) 64(6):797–808. doi: 10.1002/acr.21664
153. Dooley MA, Cosio FG, Nachman PH, Falkenhain ME, Hogan SL, Falk RJ, et al. Mycophenolate mofetil therapy in lupus nephritis: clinical observations. J Am Soc Nephrol. (1999) 10(4):833–9. doi: 10.1681/asn.V104833
154. Rivera F, Mérida E, Illescas ML, López-Rubio E, Frutos MA, García-Frías P, et al. Mycophenolate in refractory and relapsing lupus nephritis. Am J Nephrol. (2014) 40(2):105–12. doi: 10.1159/000365256
155. Kraaij T, Arends EJ, van Dam LS, Kamerling SWA, van Daele PLA, Bredewold OW, et al. Long-term effects of combined B-cell immunomodulation with rituximab and belimumab in severe, refractory systemic lupus erythematosus: 2-year results. Nephrol Dial Transplant. (2021) 36(8):1474–83. doi: 10.1093/ndt/gfaa117
156. Atisha-Fregoso Y, Malkiel S, Harris KM, Byron M, Ding L, Kanaparthi S, et al. Phase II randomized trial of rituximab plus cyclophosphamide followed by belimumab for the treatment of lupus nephritis. Arthritis Rheumatol. (2021) 73(1):121–31. doi: 10.1002/art.41466
157. Choi C-B, Won S, Bae S-C. Outcomes of multitarget therapy using mycophenolate mofetil and tacrolimus for refractory or relapsing lupus nephritis. Lupus. (2018) 27(6):1007–11. doi: 10.1177/0961203318758505
158. Jesus D, Rodrigues M, da Silva JAP, Inês L. Multitarget therapy of mycophenolate mofetil and cyclosporine a for induction treatment of refractory lupus nephritis. Lupus. (2018) 27(8):1358–62. doi: 10.1177/0961203318758508
159. Nakai T, Fukui S, Kidoguchi G, Ikeda Y, Kitada A, Nomura A, et al. Effect and safety profile of belimumab and tacrolimus combination therapy in thirty-three patients with systemic lupus erythematosus. Clin Rheumatol. (2022) 41(12):3735–45. doi: 10.1007/s10067-022-06325-6
Keywords: lupus nephritis, glucocorticoids, steroids, immunoproteasome, targeted therapy
Citation: Askanase AD, Dall’Era M and Almaani S (2024) Insights into future management of lupus nephritis. Front. Lupus 2:1334932. doi: 10.3389/flupu.2024.1334932
Received: 8 November 2023; Accepted: 22 January 2024;
Published: 6 February 2024.
Edited by:
Seerapani Gopaluni, University of Cambridge, United KingdomReviewed by:
Aggelos Banos, Biomedical Research Foundation of the Academy of Athens (BRFAA), GreeceLuís Pedro Sousa Inês, Coimbra Hospital and University Center, Portugal
© 2024 Askanase, Dall'Era and Almaani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anca D. Askanase YWRhMjBAY3VtYy5jb2x1bWJpYS5lZHU=
†These authors have contributed equally to this work
 Anca D. Askanase
Anca D. Askanase Maria Dall’Era2,†
Maria Dall’Era2,† Salem Almaani
Salem Almaani