
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 18 February 2025
Sec. Molecular Innate Immunity
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1540774
Mitochondrial antiviral signaling (MAVS) was first discovered as an activator of NF-κB and IRF3 in response to viral infection in 2005. As a key innate immune adapter that acts as an ‘on/off’ switch in immune signaling against most RNA viruses. Upon interaction with RIG-I, MAVS aggregates to activate downstream signaling pathway. The MAVS gene, located on chromosome 20p13, encodes a 540-amino acid protein that located in the outer membrane of mitochondria. MAVS protein was ubiquitously expressed with higher levels in heart, skeletal muscle, liver, placenta and peripheral blood leukocytes. Recent studies have reported MAVS to be associated with various conditions including cancers, systemic lupus erythematosus, kidney disease, and cardiovascular disease. This article provides a comprehensive summary and description of MAVS research in cardiac disease, encompassing structure, expression, protein-protein interactions, modifications, as well as the role of MAVS in heart disease. It is aimed to establish a scientific foundation for the identification of potential therapeutic target.
The mitochondrial antiviral signaling protein (MAVS) was initially discovered as a crucial molecule of antiviral innate immunity and is also referred to as IPS-1, VISA and Cardif (1–4) in 2005. It contains an N-terminal caspase activation and recruitment domain (CARD), a central proline-rich region (PRR) and a C-terminal transmembrane domain (TM). It plays a central role in regulating the complex processes that result in antiviral or inflammatory responses (5). Located ubiquitously on the outer mitochondrial membrane, peroxisomes and endoplasmic reticulum (6). MAVS acts as an articulatory protein. Retinoic acid-inducible gene I (RIG-I, also known as DExD/H-box helicase 58, DDX58, belonging to the RLRs family, detects exogenous RNAs, including viral RNAs. During viral infection, RIG-I identifies viral RNA, triggering the association between the CARD domains of MAVS and RIG, ultimately leading to the formation of MAVS aggregates. Subsequently, MAVS interacts with TNF receptor-associated factor 3 (TRAF 3) to recruit downstream IRF 3 and NF-κB activated kinases, triggering the innate immune response (5). MAVS has been recognized as a pivotal regulatory target for viruses and hosts, due to its dual function in immune homeostasis and antiviral signaling. Beyond its well-established role in antiviral defense, MAVS has emerged as a key effector in various physiological and metabolic processes. Recent studies demonstrate that MAVS is implicated in responses to bacterial and parasitic infections, autoimmune diseases, cancer advancement, kidney diseases and cardiovascular diseases (7–10). While MAVS is well-known for its role in antiviral immunity and various other immune responses, some studies have documented its involvement in viral myocarditis, cardiac insufficiency and CVD (11–13). The pathophysiological mechanisms of cardiovascular disease are complex and involve many pathological processes, including endothelial dysfunction, imbalance of calcium regulatory homeostasis, abnormal cardiac autophagy, autonomic dysfunction, metabolic reprogramming, iron imbalance, oxidative stress, inflammation, impaired mitochondrial dynamics, impaired mitochondrial autophagy, imbalance of NO synthesis. In the study of the molecular mechanisms of CVD shows a growing focus on the role of MAVS, crucial for the spontaneous high basal expression of IFN-β in the heart (14). Research has revealed that both partial or complete MAVS deficiency in mice leads to decreased cardiac function and enlarged hearts in mice due to disruptions in mitochondrial function, energy production, and lipid metabolism (11). Therefore, this article offers a brief summary of the molecular biology, protein interactions, modifications and research progress of MAVS and elucidating role of MAVS in cardiac disease to establish a scientific basis for therapeutic intervention.
The human mavs gene locates on chromosome 20 and shares approximately half amino acid identity with its mouse counterpart (15). The full−length of mavs mRNA consists of 2912 bp. According to the Human Protein Atlas (http://www.protein-atlas.org/), the highest mRNA levels of MAVS are found in the skeletal muscle, tongue and heart muscle, whereas the lowest levels are found in choroid plexus, gallbladder and testis. The mRNA level of MAVS in different human tissues, is illustrated in Figure 1A. We furtherly analyzed the expression of MAVS mRNA levels in different single cell type of heart muscle in the website (http://www.protein-atlas.org/), and found that the highest MAVS mRNA levels were in cardiomyocytes and lowest levels in endothelial cells (Figure 1B).
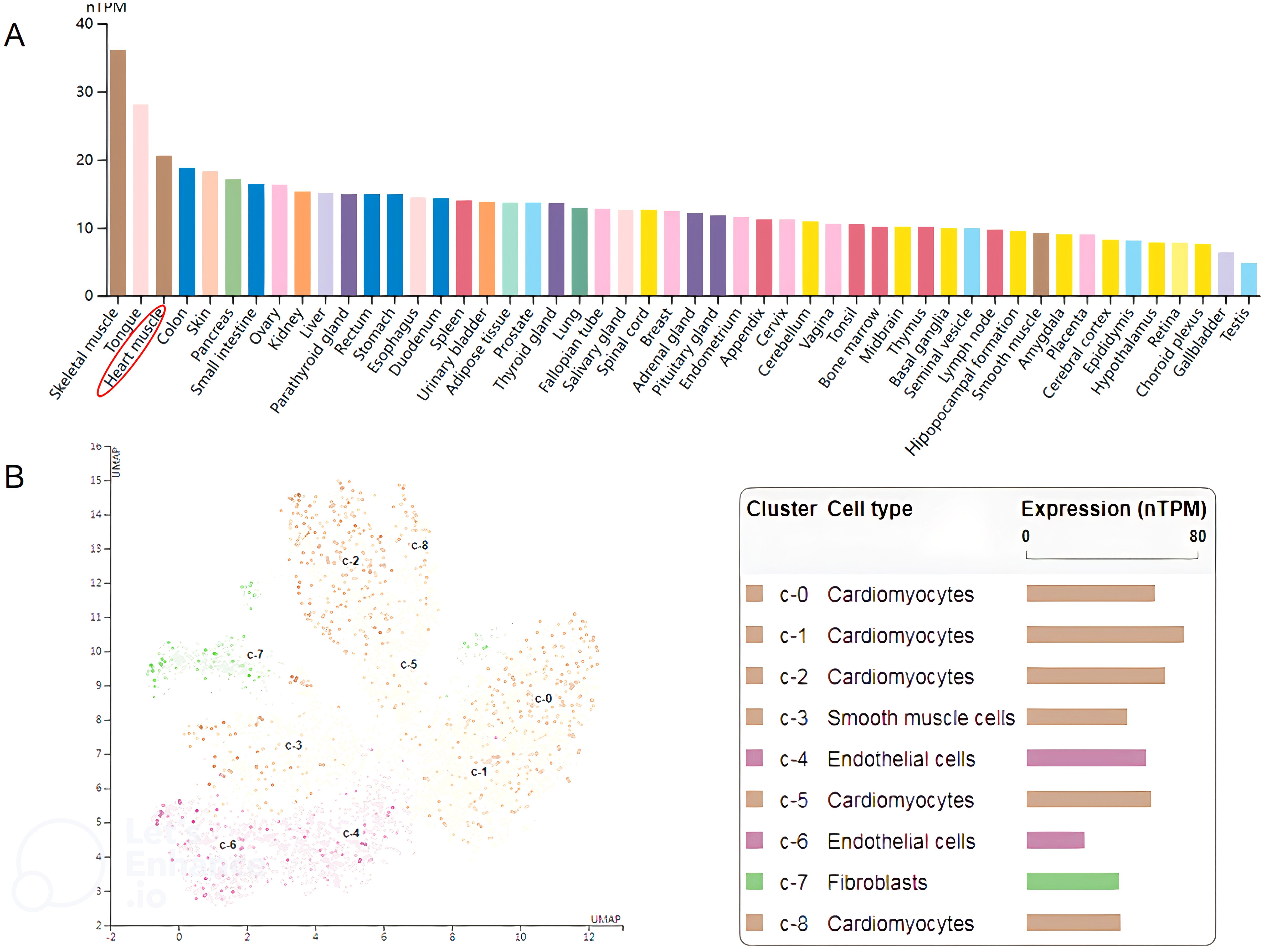
Figure 1. (A) Relative MAVS expression at the mRNA level in different human tissues. The data shown were derived from The Human Protein Atlas (http://www.proteinatlas.org/). (B) The expression of MAVS is enriched in cardiomyocytes. The data shown were derived from The Human Protein Atlas (http://www.proteinatlas.org/).
MAVS is composed of three primary structural domains: the N-terminal cysteine caspase recruitment and activation domain (CARD), the internal proline-rich region and the C-terminal transmembrane domain (TM) (16). The N-terminal CARD of MAVS plays a crucial role in facilitating protein-protein interactions, establishing a significant foundation for the assembly of signaling complexes crucial for antiviral response mechanisms. The CARD domain interacts with RIG-I and MDA5 during viral infection or upon exposure to exogenous nucleic acids. Subsequently, the activated MAVS complex recruits IKK and TBK1/IKKi complexes to induce transcriptional expression of type I interferon by facilitating the nuclear translocation of NF-κB and IRF3/IRF7 transcription factors. This process triggers innate antiviral responses (17). The deletion of the CARD-like structural domain in MAVS abolishes its signaling function, converting it into a dominant-negative mutant that inhibits interferon-induced responses (3). The C-terminal transmembrane domain of MAVS functions to anchor MAVS to the mitochondrial membrane, aiding in MAVS signaling and suggesting that mitochondria serve as a functional platform for innate antiviral signaling (3, 18, 19). PRR (Proline-rich domain) structural domains are proline-rich protein motifs that bind to members of the tumor necrosis factor receptor-associated factor (TRAF) family, including: TRAF2, TRAF3, TRAF5 and TRAF6, thereby mediating downstream signal transduction (20). We also presented the 3D structures of MAVS from PDB (representative) and AlphaFold (predicted) (Figure 2).
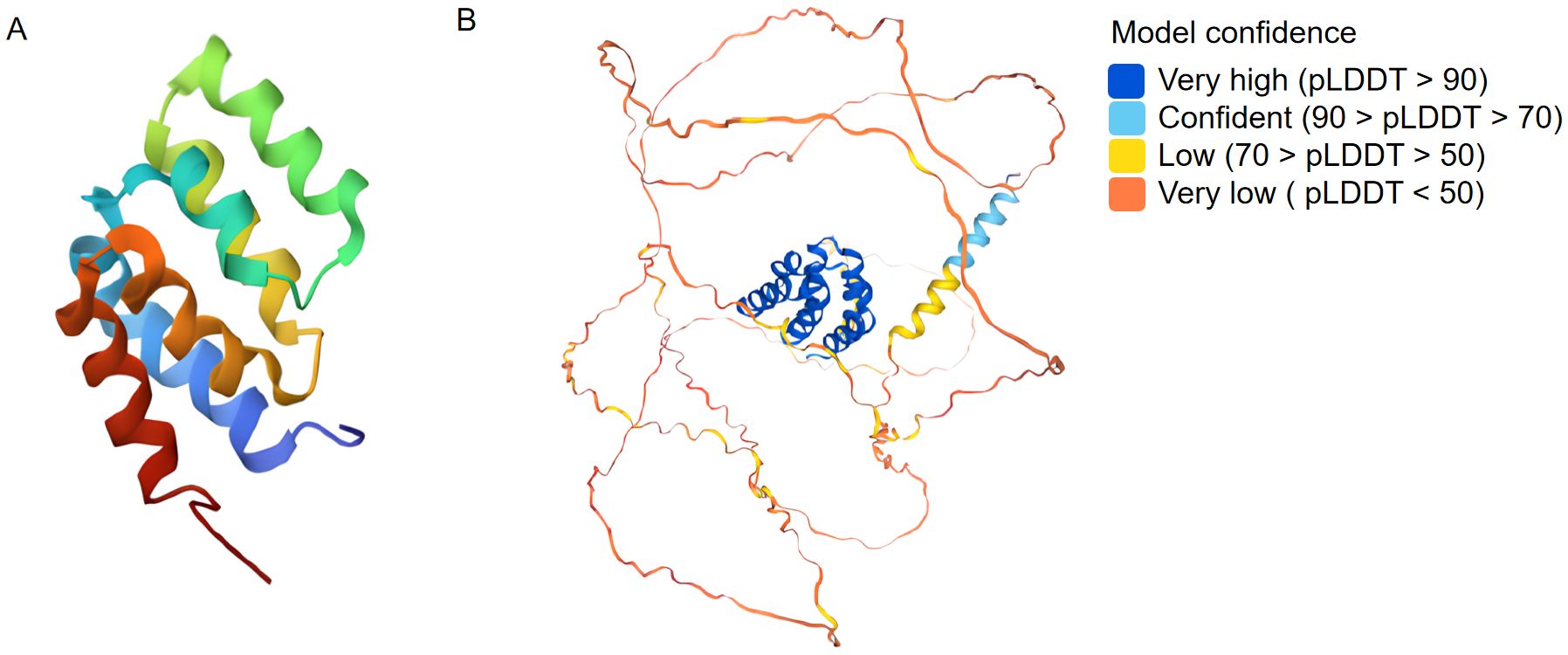
Figure 2. Three dimensional structures from PDB (A: representative) and AlphaFold (B: predicted) for MAVS.
Protein−protein interactions play vital roles in cellular biological processes. MAVS is known to interact with multiple proteins to activate downstream signaling pathways, including proteins in RIG-I/MAVS signaling and proteins that regulate RIG-I/MAVS signaling. The potential interacted proteins are shown in Figure 3. Modifications of MAVS include protein ubiquitination and phosphorylation. They are discussed below.
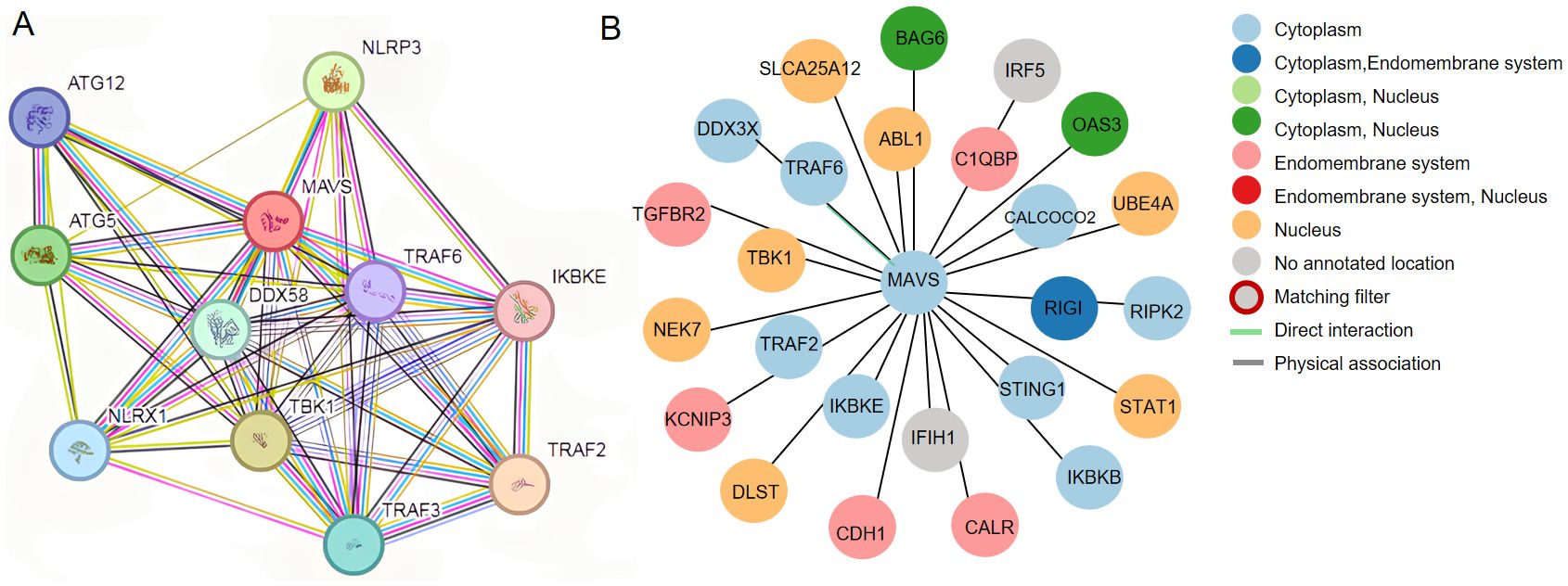
Figure 3. (A) Potential interplay between MAVS, DDX58(RIG-I) and NLRP3. The data shown were from the STRING database (www.string-db.org). (B) Prediction of protein interactions related to MAVS based on Human Protein Atlas (http://www.proteinatlas.org/).
Ubiquitination plays a crucial role as a post-translational modification of host proteins, which is essential for establishing an effective antiviral response (21). Regulated post-translational modifications of host proteins by regulatory ubiquitin ligases and deubiquitinating enzymes play a role in the regulation of RLR/MAVS-mediated signaling (22). There are three ubiquitylation patterns of MAVS, including K27-linked ubiquitylation, K48-linked ubiquitylation, and K63-linked ubiquitylation. Some researchers report about K27-linked ubiquitylation of MAVS, which primarily functions in the autophagic degradation of MAVS. E3 ubiquitin ligase MARCH8, RNF34 and RNF5 are recruited to catalyze K27-linked ubiquitin chains on MAVS, resulting in the autophagic degradation of MAVS and inhibit the innate immune response (23–27). However, another E3 ubiquitin ligase TRIM21 interacts with MAVS and catalyzes K27-linked polyubiquitination, promotes the recruitment of TBK1 to MAVS and positively regulates innate immune response (28, 29). K48-linked ubiquitylation has been reported to mediate the degradation of MAVS and regulation of innate antiviral immunity. Most ubiquitination enzymes mediate the K48-linked ubiquitylation of MAVS, including TRIM25, TRIM28, MARCH5, RNF5, RNF90, RNF115, RNF146, AIP4 and SUMRF1/2. Most of them target MAVS through K48-linked polyubiquitination and negatively regulated the RLR signaling pathway by degrading MAVS (30–38). While TRIM25 activates the type-I interferon signaling pathway by degrading MAVS via K48-linked polyubiquitination (39). MAVS K63-linked ubiquitylation promotes the activation of the RIG-I/MAVS signaling by enhancing MAVS aggregation. MAVS K63-linked ubiquitylation promotes the interaction of RIG-I and MAVS though their CARD domains. TRIM31 is reported to interact with MAVS and catalyze the K63-linked polyubiquitination of Lys10, Lys311 and Lys461 on MAVS, leading to enhanced cellular antiviral response (40). N4BP3 facilitates the K63-linked ubiquitination modification of MAVS and mediates the innate immune response by accelerating the interaction of MAVS and TRAF2 (41).
Deubiquitination is the reverse reaction of the ubiquitination process. In addition to maintaining appropriate levels of MAVS protein, de-ubiquitylation also plays an important role in the innate immunological signaling. De-ubiquitinases involved in the regulation of MAVS are listed in Table 1, including TRIM44, USP19, USP25, OTUD3, OTUD4 and YOD1. Studies have reported that TRIM44 and OTUD4 can suppress the K48-linked polyubiquitylation of MAVS in response to virus infection (42, 43). USP19, USP25, OTUD3 and YOD1 are reported to interact with MAVS and deubiquitinates K63-linked ubiquitinated MAVS for negative regulation of type I IFN signaling (44–47).
Phosphorylation and dephosphorylation are equally critical in antiviral innate immunity.
Recent research has demonstrated that TBK1 directly targets MAVS, playing a crucial role in activating IRF3 (48). The non-receptor tyrosine kinase c-Abl positively regulates the RLR signaling pathway by phosphorylation of Y9, Y30 and Y71 in the CARD domain of MAVS (49). Studies have shown that Nemo-like kinase (NLK) interacts with MAVS during the latter stages of viral infection, resulting in MAVS phosphorylation, degradation, and subsequent inactivation of IRF3 (50). Conversely, dephosphorylation of MAVS can also act as a switch to deregulate the body’s antiviral signaling (51). Purified PPM1A completely eliminated phosphorylation on MAVS, indicating that PPM1A directly dephosphorylated phospho-MAVS Protein phosphatase 1A (Phospha-tase magnesium-dependent 1A, PPM1A) and protein phosphatase 1G (Phosphatase magnesium-dependent 1G, PPM1G) function as de-phosphatases, both of which can dephosphorylate MAVS and subsequently silence the RLR antiviral signaling pathway (52, 53). All the related modifications of MAVS are listed in Table 1.
Emerging evidence indicates that MAVS is intricately involved in the pathogenesis of heart diseases. This review highlights recent progress in understanding the contributions of MAVS in viral myocarditis, ischemic myocardial damage, hypertrophic cardiomyopathy and other cardiomyopathy. The roles of MAVS in heart diseases are summarized in Figure 4.
Myocarditis is defined as inflammation of cardiac tissue resulting from an inflammatory infiltrate with or without myocyte necrosis. There are experimental studies showing that activation of cardiac inflammation causes left ventricular remodeling and left ventricular dysfunction. Viral myocarditis presents a substantial risk of sudden death in young individuals due to the heart’s limited ability to regenerate damaged cardiomyocytes. The disease involves three stages: viral infection, autoimmune, and remodeling of dilated cardiopathy and then leads to cardiac failure (54, 55). Some studies show that type I interferon treatment plays an effective therapeutic for viral myocarditis, with cardiac myocytes expressing higher levels of type I interferon compared to cardiac fibroblasts (56–59). MAVS, as a critical adapter for type I interferon expression, has been reported to be essential for high levels of type I interferon expression in cardiac myocytes (14). Maria G. et al. furtherly finds that MAVS signaling is essential for cardiac clearance of the virus. In the absence of MAVS signaling, persistent infection leads to focal myocarditis and vasculitis. MAVS knockout mice were infected with Chikungunya virus (CHIKV) and found that the MAVS was essential for the clearance of CHIKV infection (12). Coxsackie B viruses (CVB) are enteroviruses commonly linked to myocarditis. Jennifer P et al. found that MAVS was critical for type I interferon responses to CVB, as the lack of MAVS results in the absence of type I interferon production and mortality in mice infected with CVB (60). Coxsackievirus B3 (CVB3) is a common enterovirus and CVB3 infection induces mitophagy and then suppresses IFN pathways, and MAVS is involved in this process (61). Moreover, TRIM18 and TRIM21 serve as regulator of IFN-β signaling by targeting MAVS during CVB3 infection. Loss of TRIM18 enhances production of type I IFN and shields mice from viral myocarditis. Mechanistically, TRIM18 recruits protein phosphatase 1A (PPM1A) to dephosphorylate TBK1, inhibiting the interaction of TBK1 with MAVS, thereby dampening antiviral signaling (13). However, TRIM21 catalyzes the K27-linked polyubiquitination of MAVS, and enhances type I interferon signaling and consequently reducing CVB3 viral replication (62). Encephalomyocarditis virus (EMCV) is a zoonotic pathogen known to causes myocarditis. A study found EMCV VP2 acted as a negative regulator of the IFN-β pathway, and the structural protein VP2 interacted with MAVS to block the type I interferon signaling (63). Another research found metalloproteinase domain 9 (ADAM9) bind to MDA5 and promoted (MAVS), and thereby induced type I interferon production during encephalomyocarditis virus infection, which provides a therapeutic target for viral myocarditis (64).
Ischemic myocardial injury is a common cardiovascular emergency and leads to higher morbidity and mortality. Despite therapeutic advancements, Ischemic cardiomyopathy remains a significant public health challenge, with 1-year mortality at 16% and 5-year mortality approaching 40% in the USA and Europe (65, 66). Increasing evidence showed that inflammasome activation and apoptosis were participated in the pathogenesis of ischemic myocardial injury. NLRP3 is a key component of the inflammasome. MAVS is essential for NLRP3 inflammasome activity (67). Following myocardial infarction, NLRP3 inflammatory vesicles are upregulated, potentially contributing to the progression of infarct size during ischemia-reperfusion (68). Study finds that inhibiting the NLRP3 inflammasome reduces infarct size and preserves cardiac function in an animal model of MI (69). Recent study found TAX1BP1 exerted cardioprotective effects in acute myocardial infarction by inhibiting inflammasome activation in an RNF34/MAVS-dependent mechanism (70). Another study showed that NLRX1 played a protectional role in myocardial ischemic injury by suppressing MAVS-dependent inflammation and apoptosis (71). Inflammasome activation and pyroptosis are reported to contribute to the pathogenesis of myocardial ischemia-reperfusion (I/R) injury. In the context of ischemia-reperfusion (I/R) injury, research demonstrated an increase in levels of the E3 ubiquitin ligase membrane-associated RING finger protein 2 (MARCH2) in ischemic hearts (72). Interestingly, the absence of MARCH2 worsened myocardial infarction and cardiac dysfunction. Moreover, MARCH2 played a protective role against cardiomyocyte pyroptosis and myocardial injury during ischemia-reperfusion by negatively regulating the PGAM5/MAVS/NLRP3 pathway (72). These findings align with Stefano et al.’s study, which showed that inhibiting NLRP3 pharmacologically in the hippocampus limited secondary inflammatory damage and reduced infarct size one hour after myocardial ischemia-reperfusion in mice (73).
Belonging to the nucleotide-binding oligomerization domain (NOD)-like receptor family, NLRP3 is associated with cardiac inflammation (74). MAVS, on the other hand, is essential for maximizing the function of NLRP3 inflammatory bodies and contributes significantly to regulating inflammation (48). In their study, Li et al. observed that the absence of NLRP3 hastened cardiac hypertrophy, fibrosis, inflammatory reactions, and worsened cardiac function in a mouse model of pressure overload-induced cardiac remodeling (75). These results suggest that targeting NLRP3 could hold therapeutic promise for managing cardiac remodeling and heart failure (76). Jing Zong et al. observed upregulated NOD2 expression in cardiomyocytes of aortic fasciculation-type hypertrophic mice (77). They also demonstrate that NOD2 inhibits myocardial hypertrophy and fibrosis in mice, counteracting hypertrophic stimuli by inhibiting TLR4 and TGF-b/Smad signaling pathways, while regulating pro-fibrotic cytokines and collagen content (77). However, recent studies have revealed that MAVS acts nucleotide-binding oligomeric structural domain-containing protein 1/receptor-interacting protein 2 (NOD1/RIP2) downstream to promote cardiac hypertrophy in response to transecting pressure overload induced by aortic constriction (TAC). Nod1-/- and RIP2-/- mice demonstrated better survival, enhanced cardiac function, and reduced cardiac hypertrophy during subjecting to TAC. This process critically involves MAVS regulation in the inflammatory response, and mitochondrial energy metabolism. The NOD1/RIP2/MAVS signaling complex effectively coordinates remodeling, inflammatory response and mitochondrial energy metabolism in stressed cardiomyocytes (78).
Mitochondria plays a crucial role in energy production. MAVS, located on the mitochondrial outer membrane, regulates mitochondrial dynamics, energetics and facilitates the interaction of RLR signaling and glucose metabolism (79). Recent research revealed that silencing MAVS mitigated the radiation-induced mitochondrial dysfunction (including mitochondrial membrane potential disruption and ATP production) (80). MAVS suppression affects both mitochondrial function and morphology in cardiomyocytes (78). Qian Wang et al. found that MAVS deficiency exacerbated the deterioration of cardiac insufficiency and cardiac dilation. Metabonomic suggested MAVS deletion disturbed energy metabolism, especially lipid metabolism. Knockout of MAVS induced the mitochondrial structure and function impairments, leading to elevated mitochondrial ROS levels (11).
Although the role of MAVS in cardiac diseases requires further explored, its significance and relevance to cardiac diseases are widely acknowledged. Consequently, it is imperative to explore and clarify its mechanism of action in cardiac diseases.
NLRP3 is a crucial component of the inflammasome, involved in regulating inflammatory responses within the immune system. Emerging evidence has indicated that the NLRP3 inflammasome plays an important role in cardiovascular diseases, such as atherosclerosis, ischemic heart disease, dilated cardiomyopathy, hypertensive heart disease, metabolic disorders and diabetic cardiomyopathy, cancer therapy-associated cardiac injury, myocarditis, and pericarditis (74). MAVS mediates recruitment of NLRP3 to mitochondria, and activates of the NLRP3 inflammasome in vivo (67). Peter Duewell et al. first demonstrated the role of the NLRP3 inflammasome in promoting atherosclerosis in western diet-fed LDL receptor-deficient mice (81). Negatively regulating the MAVS/NLRP3 pathway played a protective role against cardiomyocyte pyroptosis and myocardial injury during ischemia-reperfusion (70, 72). Studies reported that inhibiting NLRP3 and other inflammasome components in animal model of ischemic cardiac injury showed beneficial effects in terms of reduced infarct size and improved cardiac function (82, 83). Several NLRP3 inflammasome inhibitors are developing for CVD in preclinical and clinical stage (Table 2). Some of these inhibitors block the NLRP3 inflammasome, others block NLRP3 signaling. Overall, the investigation of the close association between MAVS and the NLRP3 inflammasome in cardiovascular diseases provides us with deeper insights into the immune mechanisms underlying these conditions, while also offering potential drugs targeted NLRP3 for future therapeutic strategies.
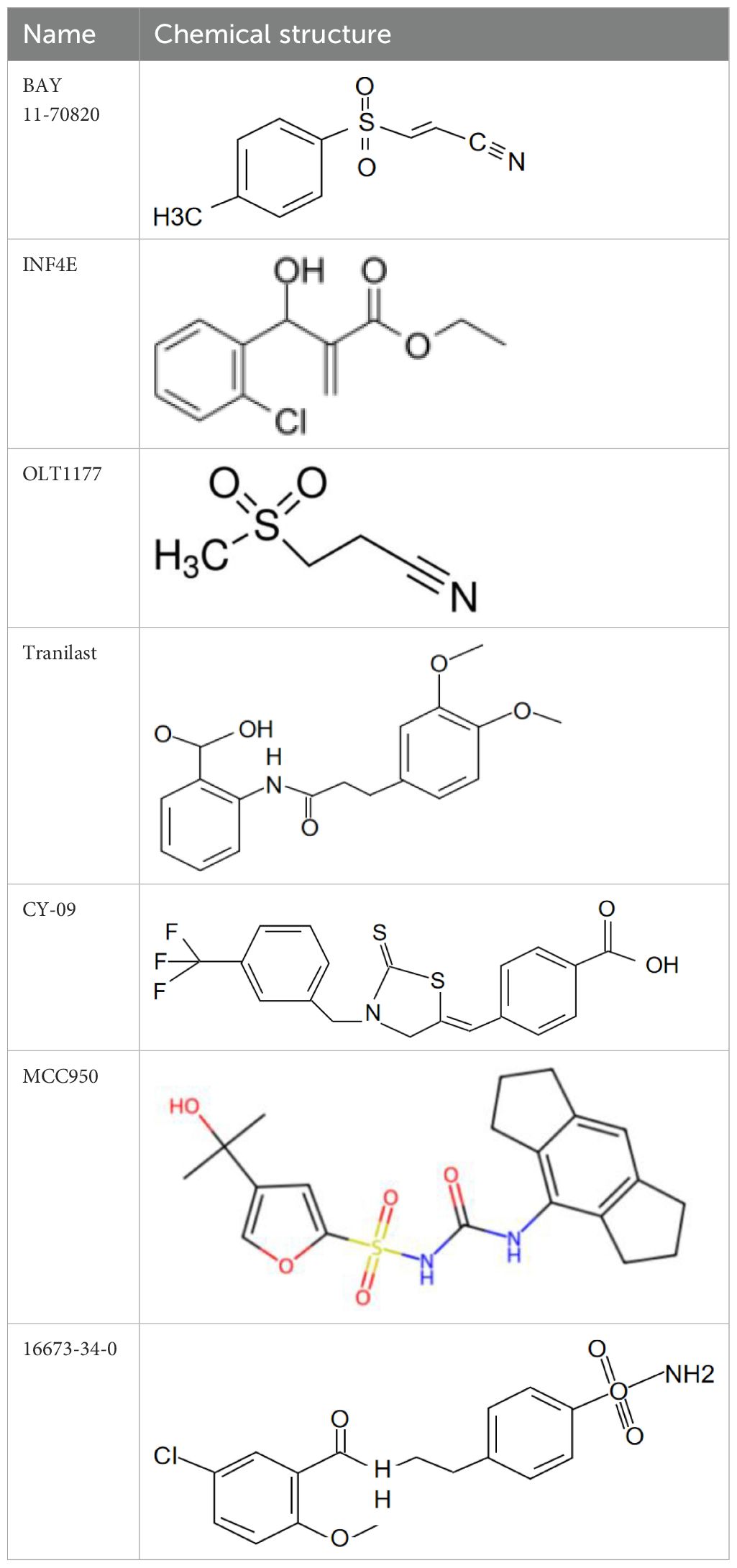
Table 2. NLRP3 inhibitors under clinical development in cardiovascular diseases: name and chemical structure.
Cardiovascular disease (CVD) poses a substantial public health burden, and is currently a leading cause of disability and mortality among the elderly. The epidemiology of CVD has shifted from predominantly affecting developed countries to becoming a global disease, with the highest prevalence rate in our country. CVD and its associated complications are the primary cause of mortality in patients. MAVS, a crucial connector protein in the RLR signaling pathway, significantly contributes to the pathology of both innate immunity and cardiac diseases. Nevertheless, the regulatory mechanisms of MAVS-mediated antiviral signaling pathways in various animal organisms remain unclear to date. Studying the regulatory mechanisms of MAVS in cardiovascular diseases is anticipated to provide some insights for identifying relevant target drugs against this pathway and treating related diseases. Furthermore, MAVS plays a critical role in enhancing optimal NLRP3 inflammasome activation. Specifically, MAVS promotes NLRP3 oligomerization by recruiting it in proximity to mitochondrial ROS, a crucial element for NLRP 3 activation. Several studies suggest that MAVS and its downstream factor NLRP 3 may provide promising strategies for treating heart disease. Additionally, TRIM29 targets MAVS to negatively regulate the production of antiviral type I interferons and the activation of inflammasomes, thereby modulating the host immune response to viral infections (84, 85). Junying Wang et al. found that the deficiency of TRIM29 alleviated viral myocarditis (86). Similarly, some studies reported that TRIM18 regulates the TBK1 and MAVS signaling pathways by recruiting protein phosphatase 1A (PPM1A). Deletion of TRIM18 protects mice from viral myocarditis (13, 52). Therefore, targeting TRIM29 and TRIM18 may provide new therapeutic approaches for reducing myocardial inflammation and improving cardiac function by regulating the MAVS signaling pathway.
Cardiovascular diseases are closely associated with the immune system. Immune responses and microenvironment play a pivotal role in the initiation, progression, and prognosis of cardiovascular diseases. A recent study showed that M1-like pro-inflammatory macrophages also contributed to myocardial injury by secreting pro-inflammatory exosomes and pro-inflammatory miRNAs which inhibited angiogenesis and cardiac healing (87). Li Liu et al. found that neutrophils can release annexin A1 and lactoferrin, as well as engage in chemokine scavenging, thereby halting the migration of granulocytes into the infarcted myocardial tissue (88). Depletion of neutrophils was linked to an exacerbation of fibrosis and a deterioration in heart function in the chronic MI model (89). Moreover, proinflammatory cytokines play a critical role in the pathogenesis of heart failure. Douglas L. Mann proposed that inflammatory mediators such as TNF-α, IL-1β, and IL-6 are remarkable (90).
Recent studies have revealed the role of MAVS in immune cell infiltration and activation, particularly in macrophages, neutrophils, and T cells, which play key roles in the immune response in cardiovascular diseases. Macrophages, as important immune cells in cardiovascular diseases, directly influence disease progression through their polarization. Specifically, activation of MAVS can promote M1 macrophage polarization by enhancing the secretion of type I interferons, such as IFN-β. M1 macrophages not only amplify local inflammation by releasing pro-inflammatory cytokines such as TNF-α and IL-1β but also promote immune cell infiltration and tissue damage in cardiovascular pathologies such as atherosclerosis, myocardial infarction and cardiac remodeling (91, 92). Neutrophil infiltration and activation are also crucial in driving cardiac inflammation and damage. Freja et al. demonstrated that in RSV-infected mice, the MAVS signaling pathway is essential for neutrophil recruitment and activation through type I interferon production (93). Moreover, the involvement of T cells in chronic cardiovascular diseases has become increasingly significant. For instance, Huanle Luo et al. investigated the role of MAVS in regulating host immunity against the live attenuated West Nile virus (WNV) vaccine strain. They found that MAVS is critical for enhancing the primary CD4 T cell response during NS4B-P38G vaccination (94). In summary, the excessive activation and dysregulation of these immune cells drive the progression of inflammatory responses and exacerbate tissue damage in cardiovascular diseases. Future studies should further explore the specific mechanisms of MAVS as an immune modulator in cardiovascular diseases, providing new insights for immune-based therapies in cardiovascular disorders.
However, the existing body of literature primarily focuses on animal or cellular models of heart disease, neglecting the exploration of MAVS expression levels in clinical patients. Therefore, attaining a comprehensive understanding of the regulatory mechanisms involved in this pathway is of utmost importance as it could serve as a guiding principle for the development of innovative therapeutic strategies to address cardiac diseases in the future.
YQ: Writing – original draft, Writing – review & editing. JY: Writing – original draft, Writing – review & editing. WX: Writing – original draft, Writing – review & editing. SY: Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The research conducted in this study received financial support from the National Natural Science Foundation of China (Grant No. 82100779, 82070760). This work was supported by the Medical Science and Technology Development Foundation of Jiangsu Commission of Health (ZD2021058), Research Project of Maternal and Child Health of Jiangsu Province (F202023), Medical Science and Technology Development Foundation of Nanjing Department of Health (ZKX24038).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. (2005) 6:981–8. doi: 10.1038/ni1243
2. Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. (2005) 437:1167–72. doi: 10.1038/nature04193
3. Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. (2005) 122:669–82. doi: 10.1016/j.cell.2005.08.012
4. Xu LG, Wang YY, Han KJ, Li LY, Zhai Z, Shu HB. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol Cell. (2005) 19:727–40. doi: 10.1016/j.molcel.2005.08.014
5. Belgnaoui SM, Paz S, Hiscott J. Orchestrating the interferon antiviral response through the mitochondrial antiviral signaling (MAVS) adapter. Curr Opin Immunol. (2011) 23:564–72. doi: 10.1016/j.coi.2011.08.001
6. Ablasser A, Hur S. Regulation of cGAS- and RLR-mediated immunity to nucleic acids. Nat Immunol. (2020) 21:17–29. doi: 10.1038/s41590-019-0556-1
7. Liehl P, Zuzarte-Luis V, Chan J, Zillinger T, Baptista F, Carapau D, et al. Host-cell sensors for Plasmodium activate innate immunity against liver-stage infection. Nat Med. (2014) 20:47–53. doi: 10.1038/nm.3424
8. Chao CC, Gutierrez-Vazquez C, Rothhammer V, Mayo L, Wheeler MA, Tjon EC, et al. Metabolic Control of Astrocyte Pathogenic Activity via cPLA2-MAVS. Cell. (2019) 179:1483–98.e22. doi: 10.1016/j.cell.2019.11.016
9. Gao L, Bird AK, Meednu N, Dauenhauer K, Liesveld J, Anolik J, et al. Bone marrow-derived mesenchymal stem cells from patients with systemic lupus erythematosus have a senescence-associated secretory phenotype mediated by a mitochondrial antiviral signaling protein-interferon-beta feedback loop. Arthritis Rheumatol. (2017) 69:1623–35. doi: 10.1002/art.40142
10. Zhang L, Xia Q, Li W, Peng Q, Yang H, Lu X, et al. The RIG-I pathway is involved in peripheral T cell lymphopenia in patients with dermatomyositis. Arthritis Res Ther. (2019) 21:131. doi: 10.1186/s13075-019-1905-z
11. Wang Q, Sun Z, Cao S, Lin X, Wu M, Li Y, et al. Reduced immunity regulator MAVS contributes to non-hypertrophic cardiac dysfunction by disturbing energy metabolism and mitochondrial homeostasis. Front Immunol. (2022) 13:919038. doi: 10.3389/fimmu.2022.919038
12. Noval MG, Spector SN, Bartnicki E, Izzo F, Narula N, Yeung ST, et al. MAVS signaling is required for preventing persistent chikungunya heart infection and chronic vascular tissue inflammation. Nat Commun. (2023) 14:4668. doi: 10.1038/s41467-023-40047-w
13. Fang M, Zhang A, Du Y, Lu W, Wang J, Minze LJ, et al. TRIM18 is a critical regulator of viral myocarditis and organ inflammation. J Biomed science. (2022) 29:55. doi: 10.1186/s12929-022-00840-z
14. Rivera-Serrano EE, DeAngelis N, Sherry B. Spontaneous activation of a MAVS-dependent antiviral signaling pathway determines high basal interferon-beta expression in cardiac myocytes. J Mol Cell Cardiol. (2017) 111:102–13. doi: 10.1016/j.yjmcc.2017.08.008
15. Hu Y, Dong X, He Z, Wu Y, Zhang S, Lin J, et al. Zika virus antagonizes interferon response in patients and disrupts RIG-I-MAVS interaction through its CARD-TM domains. Cell bioscience. (2019) 9:46. doi: 10.1186/s13578-019-0308-9
16. Yoneyama M, Fujita T. Recognition of viral nucleic acids in innate immunity. Rev Med virology. (2010) 20:4–22. doi: 10.1002/rmv.v20:1
17. Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, et al. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. (2003) 4:491–6. doi: 10.1038/ni921
18. Dixit E, Boulant S, Zhang Y, Lee AS, Odendall C, Shum B, et al. Peroxisomes are signaling platforms for antiviral innate immunity. Cell. (2010) 141:668–81. doi: 10.1016/j.cell.2010.04.018
19. Horner SM, Liu HM, Park HS, Briley J, Gale M Jr. Mitochondrial-associated endoplasmic reticulum membranes (MAM) form innate immune synapses and are targeted by hepatitis C virus. Proc Natl Acad Sci United States America. (2011) 108:14590–5. doi: 10.1073/pnas.1110133108
20. Liu S, Chen J, Cai X, Wu J, Chen X, Wu YT, et al. MAVS recruits multiple ubiquitin E3 ligases to activate antiviral signaling cascades. eLife. (2013) 2:e00785. doi: 10.7554/eLife.00785
21. Ribet D, Cossart P. Pathogen-mediated posttranslational modifications: A re-emerging field. Cell. (2010) 143:694–702. doi: 10.1016/j.cell.2010.11.019
22. Heaton SM, Borg NA, Dixit VM. Ubiquitin in the activation and attenuation of innate antiviral immunity. J Exp Med. (2016) 213:1–13. doi: 10.1084/jem.20151531
23. Jin S, Tian S, Luo M, Xie W, Liu T, Duan T, et al. Tetherin suppresses type I interferon signaling by targeting MAVS for NDP52-mediated selective autophagic degradation in human cells. Mol Cell. (2017) 68:308–22 e4. doi: 10.1016/j.molcel.2017.09.005
24. Jin S, Cui J. BST2 inhibits type I IFN (interferon) signaling by accelerating MAVS degradation through CALCOCO2-directed autophagy. Autophagy. (2018) 14:171–2. doi: 10.1080/15548627.2017.1393590
25. Wang P, Sun Y, Xu T. USP13 cooperates with MARCH8 to inhibit antiviral signaling by targeting MAVS for autophagic degradation in teleost. J Immunol. (2024) 212:801–12. doi: 10.4049/jimmunol.2300493
26. He X, Zhu Y, Zhang Y, Geng Y, Gong J, Geng J, et al. RNF34 functions in immunity and selective mitophagy by targeting MAVS for autophagic degradation. EMBO J. (2019) 38:e100978. doi: 10.15252/embj.2018100978
27. Zeng Y, Xu S, Wei Y, Zhang X, Wang Q, Jia Y, et al. The PB1 protein of influenza A virus inhibits the innate immune response by targeting MAVS for NBR1-mediated selective autophagic degradation. PloS pathogens. (2021) 17:e1009300. doi: 10.1371/journal.ppat.1009300
28. Xue B, Li H, Guo M, Wang J, Xu Y, Zou X, et al. TRIM21 promotes innate immune response to RNA viral infection through lys27-linked polyubiquitination of MAVS. J Virol. (2018) 92:10. doi: 10.1128/JVI.00321-18
29. Liu Q, Chen S, Tian R, Xue B, Li H, Guo M, et al. 3beta-hydroxysteroid-Delta24 reductase dampens anti-viral innate immune responses by targeting K27 ubiquitination of MAVS and STING. J virology. (2023) 97:e0151323. doi: 10.1128/JVI.00321-18
30. Chen YY, Ran XH, Ni RZ, Mu D. TRIM28 negatively regulates the RLR signaling pathway by targeting MAVS for degradation via K48-linked polyubiquitination. J Biol Chem. (2023) 299:104660. doi: 10.1016/j.jbc.2023.104660
31. Yoo YS, Park YY, Kim JH, Cho H, Kim SH, Lee HS, et al. The mitochondrial ubiquitin ligase MARCH5 resolves MAVS aggregates during antiviral signalling. Nat Commun. (2015) 6:7910. doi: 10.1038/ncomms8910
32. Zhong B, Zhang Y, Tan B, Liu TT, Wang YY, Shu HB. The E3 ubiquitin ligase RNF5 targets virus-induced signaling adaptor for ubiquitination and degradation. J Immunol. (2010) 184:6249–55. doi: 10.4049/jimmunol.0903748
33. Yang B, Zhang G, Qin X, Huang Y, Ren X, Sun J, et al. Negative regulation of RNF90 on RNA virus-triggered antiviral immune responses targeting MAVS. Front Immunol. (2021) 12:730483. doi: 10.3389/fimmu.2021.730483
34. Zhang ZD, Xiong TC, Yao SQ, Wei MC, Chen M, Lin D, et al. RNF115 plays dual roles in innate antiviral responses by catalyzing distinct ubiquitination of MAVS and MITA. Nat Commun. (2020) 11:5536. doi: 10.1038/s41467-020-19318-3
35. Xu YR, Shi ML, Zhang Y, Kong N, Wang C, Xiao YF, et al. Tankyrases inhibit innate antiviral response by PARylating VISA/MAVS and priming it for RNF146-mediated ubiquitination and degradation. Proc Natl Acad Sci United States America. (2022) 119:e2122805119. doi: 10.1073/pnas.2122805119
36. You F, Sun H, Zhou X, Sun W, Liang S, Zhai Z, et al. PCBP2 mediates degradation of the adaptor MAVS via the HECT ubiquitin ligase AIP4. Nat Immunol. (2009) 10:1300–8. doi: 10.1038/ni.1815
37. Wang Y, Tong X, Ye X. Ndfip1 negatively regulates RIG-I-dependent immune signaling by enhancing E3 ligase Smurf1-mediated MAVS degradation. J Immunol. (2012) 189:5304–13. doi: 10.4049/jimmunol.1201445
38. Pan Y, Li R, Meng JL, Mao HT, Zhang Y, Zhang J. Smurf2 negatively modulates RIG-I-dependent antiviral response by targeting VISA/MAVS for ubiquitination and degradation. J Immunol. (2014) 192:4758–64. doi: 10.4049/jimmunol.1302632
39. Castanier C, Zemirli N, Portier A, Garcin D, Bidere N, Vazquez A, et al. MAVS ubiquitination by the E3 ligase TRIM25 and degradation by the proteasome is involved in type I interferon production after activation of the antiviral RIG-I-like receptors. BMC Biol. (2012) 10:44. doi: 10.1186/1741-7007-10-44
40. Liu B, Zhang M, Chu H, Zhang H, Wu H, Song G, et al. The ubiquitin E3 ligase TRIM31 promotes aggregation and activation of the signaling adaptor MAVS through Lys63-linked polyubiquitination. Nat Immunol. (2017) 18:214–24. doi: 10.1038/ni.3641
41. Wang C, Ling T, Zhong N, Xu LG. N4BP3 regulates RIG-I-like receptor antiviral signaling positively by targeting mitochondrial antiviral signaling protein. Front Microbiol. (2021) 12:770600. doi: 10.3389/fmicb.2021.770600
42. Yang B, Wang J, Wang Y, Zhou H, Wu X, Tian Z, et al. Novel function of Trim44 promotes an antiviral response by stabilizing VISA. J Immunol. (2013) 190:3613–9. doi: 10.4049/jimmunol.1202507
43. Liuyu T, Yu K, Ye L, Zhang Z, Zhang M, Ren Y, et al. Induction of OTUD4 by viral infection promotes antiviral responses through deubiquitinating and stabilizing MAVS. Cell Res. (2019) 29:67–79. doi: 10.1038/s41422-018-0107-6
44. Weerawardhana A, Herath TUB, Gayan Chathuranga WA, Kim TH, Ekanayaka P, Chathuranga K, et al. SIAH1 modulates antiviral immune responses by targeting deubiquitinase USP19. J Med virology. (2024) 96:e29523. doi: 10.1002/jmv.29523
45. Xu S, Han L, Wei Y, Zhang B, Wang Q, Liu J, et al. MicroRNA-200c-targeted contactin 1 facilitates the replication of influenza A virus by accelerating the degradation of MAVS. PloS pathogens. (2022) 18:e1010299. doi: 10.1371/journal.ppat.1010299
46. Zhang Z, Fang X, Wu X, Ling L, Chu F, Li J, et al. Acetylation-dependent deubiquitinase OTUD3 controls MAVS activation in innate antiviral immunity. Mol Cell. (2020) 79:304–19.e7. doi: 10.1016/j.molcel.2020.06.020
47. Liu C, Huang S, Wang X, Wen M, Zheng J, Wang W, et al. The otubain YOD1 suppresses aggregation and activation of the signaling adaptor MAVS through lys63-linked deubiquitination. J Immunol. (2019) 202:2957–70. doi: 10.4049/jimmunol.1800656
48. Pythoud C, Rodrigo WW, Pasqual G, Rothenberger S, Martinez-Sobrido L, de la Torre JC, et al. Arenavirus nucleoprotein targets interferon regulatory factor-activating kinase IKKepsilon. J virology. (2012) 86:7728–38. doi: 10.1128/JVI.00187-12
49. Cheng J, Liao Y, Xiao L, Wu R, Zhao S, Chen H, et al. Autophagy regulates MAVS signaling activation in a phosphorylation-dependent manner in microglia. Cell Death differentiation. (2017) 24:276–87. doi: 10.1038/cdd.2016.121
50. Li SZ, Shu QP, Song Y, Zhang HH, Liu Y, Jin BX, et al. Phosphorylation of MAVS/VISA by Nemo-like kinase (NLK) for degradation regulates the antiviral innate immune response. Nat Commun. (2019) 10:3233. doi: 10.1038/s41467-019-11258-x
51. Xia Z, Xu G, Nie L, Liu L, Peng N, He Q, et al. NAC1 potentiates cellular antiviral signaling by bridging MAVS and TBK1. J Immunol. (2019) 203:1001–11. doi: 10.4049/jimmunol.1801110
52. Xiang W, Zhang Q, Lin X, Wu S, Zhou Y, Meng F, et al. PPM1A silences cytosolic RNA sensing and antiviral defense through direct dephosphorylation of MAVS and TBK1. Sci advances. (2016) 2:e1501889. doi: 10.1126/sciadv.1501889
53. Yu K, Tian H, Deng H. PPM1G restricts innate immune signaling mediated by STING and MAVS and is hijacked by KSHV for immune evasion. Sci Adv. (2020) 6:6. doi: 10.1126/sciadv.abd0276
54. Liu PP, Mason JW. Advances in the understanding of myocarditis. Circulation. (2001) 104:1076–82. doi: 10.1161/hc3401.095198
55. Klingel K, Hohenadl C, Canu A, Albrecht M, Seemann M, Mall G, et al. Ongoing enterovirus-induced myocarditis is associated with persistent heart muscle infection: quantitative analysis of virus replication, tissue damage, and inflammation. Proc Natl Acad Sci USA. (1992) 89:314–8. doi: 10.1073/pnas.89.1.314
56. Miric M, Miskovic A, Vasiljevic JD, Keserovic N, Pesic M. Interferon and thymic hormones in the therapy of human myocarditis and idiopathic dilated cardiomyopathy. Eur Heart J. (1995) 16 Suppl O:150–2. doi: 10.1093/eurheartj/16.suppl_O.150
57. Schultheiss HP, Piper C, Sowade O, Waagstein F, Kapp JF, Wegscheider K, et al. Betaferon in chronic viral cardiomyopathy (BICC) trial: Effects of interferon-beta treatment in patients with chronic viral cardiomyopathy. Clin Res cardiology: Off J German Cardiac Society. (2016) 105:763–73. doi: 10.1007/s00392-016-0986-9
58. Li L, Sherry B. IFN-alpha expression and antiviral effects are subtype and cell type specific in the cardiac response to viral infection. Virology. (2010) 396:59–68. doi: 10.1016/j.virol.2009.10.013
59. Stewart MJ, Smoak K, Blum MA, Sherry B. Basal and reovirus-induced beta interferon (IFN-beta) and IFN-beta-stimulated gene expression are cell type specific in the cardiac protective response. J virology. (2005) 79:2979–87. doi: 10.1128/JVI.79.5.2979-2987.2005
60. Wang JP, Cerny A, Asher DR, Kurt-Jones EA, Bronson RT, Finberg RW. MDA5 and MAVS mediate type I interferon responses to coxsackie B virus. J virology. (2010) 84:254–60. doi: 10.1128/JVI.00631-09
61. Oh SJ, Lim BK, Yun J, Shin OS. CVB3-mediated mitophagy plays an important role in viral replication via abrogation of interferon pathways. Front Cell infection Microbiol. (2021) 11:704494. doi: 10.3389/fcimb.2021.704494
62. Liu H, Li M, Song Y, Xu W. TRIM21 restricts coxsackievirus B3 replication, cardiac and pancreatic injury via interacting with MAVS and positively regulating IRF3-mediated type-I interferon production. Front Immunol. (2018) 9:2479. doi: 10.3389/fimmu.2018.02479
63. Han Y, Xie J, Xu S, Bi Y, Li X, Zhang H, et al. Encephalomyocarditis virus abrogates the interferon beta signaling pathway via its structural protein VP2. J Virol. (2021) 95:8–9. doi: 10.1128/JVI.01590-20
64. Bazzone LE, Zhu J, King M, Liu G, Guo Z, MacKay CR, et al. ADAM9 promotes type I interferon-mediated innate immunity during encephalomyocarditis virus infection. Nat Commun. (2024) 15:4153. doi: 10.1038/s41467-024-48524-6
65. Pastena P, Frye JT, Ho C, Goldschmidt ME, Kalogeropoulos AP. Ischemic cardiomyopathy: epidemiology, pathophysiology, outcomes, and therapeutic options. Heart failure Rev. (2024) 29:287–99. doi: 10.1007/s10741-023-10377-4
66. Lincoff AM, Bhasin S, Flevaris P, Mitchell LM, Basaria S, Boden WE, et al. Cardiovascular safety of testosterone-replacement therapy. New Engl J Med. (2023) 389:107–17. doi: 10.1056/NEJMoa2215025
67. Subramanian N, Natarajan K, Clatworthy MR, Wang Z, Germain RN. The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation. Cell. (2013) 153:348–61. doi: 10.1016/j.cell.2013.02.054
68. Sandanger O, Ranheim T, Vinge LE, Bliksoen M, Alfsnes K, Finsen AV, et al. The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia-reperfusion injury. Cardiovasc Res. (2013) 99:164–74. doi: 10.1093/cvr/cvt091
69. van Hout GP, Bosch L, Ellenbroek GH, de Haan JJ, van Solinge WW, Cooper MA, et al. The selective NLRP3-inflammasome inhibitor MCC950 reduces infarct size and preserves cardiac function in a pig model of myocardial infarction. Eur Heart J. (2017) 38:828–36. doi: 10.1093/eurheartj/ehw247
70. Xu H, Yu W, Sun S, Li C, Ren J, Zhang Y. TAX1BP1 protects against myocardial infarction-associated cardiac anomalies through inhibition of inflammasomes in a RNF34/MAVS/NLRP3-dependent manner. Sci bulletin. (2021) 66:1669–83. doi: 10.1016/j.scib.2021.01.030
71. Li H, Zhang S, Li F, Qin L. NLRX1 attenuates apoptosis and inflammatory responses in myocardial ischemia by inhibiting MAVS-dependent NLRP3 inflammasome activation. Mol Immunol. (2016) 76:90–7. doi: 10.1016/j.molimm.2016.06.013
72. Liu S, Bi Y, Han T, Li YE, Wang Q, Wu NN, et al. The E3 ubiquitin ligase MARCH2 protects against myocardial ischemia-reperfusion injury through inhibiting pyroptosis via negative regulation of PGAM5/MAVS/NLRP3 axis. Cell discovery. (2024) 10:24. doi: 10.1038/s41421-023-00622-3
73. Toldo S, Marchetti C, Mauro AG, Chojnacki J, Mezzaroma E, Carbone S, et al. Inhibition of the NLRP3 inflammasome limits the inflammatory injury following myocardial ischemia-reperfusion in the mouse. Int J Cardiol. (2016) 209:215–20. doi: 10.1016/j.ijcard.2016.02.043
74. Toldo S, Mezzaroma E, Buckley LF, Potere N, Di Nisio M, Biondi-Zoccai G, et al. Targeting the NLRP3 inflammasome in cardiovascular diseases. Pharmacol Ther. (2022) 236:108053. doi: 10.1016/j.pharmthera.2021.108053
75. Liao LZ, Chen ZC, Wang SS, Liu WB, Zhao CL, Zhuang XD. NLRP3 inflammasome activation contributes to the pathogenesis of cardiocytes aging. Aging. (2021) 13:20534–51. doi: 10.18632/aging.203435
76. Li F, Zhang H, Yang L, Yong H, Qin Q, Tan M, et al. NLRP3 deficiency accelerates pressure overload-induced cardiac remodeling via increased TLR4 expression. J Mol Med. (2018) 96:1189–202. doi: 10.1007/s00109-018-1691-0
77. Zong J, Salim M, Zhou H, Bian ZY, Dai J, Yuan Y, et al. NOD2 deletion promotes cardiac hypertrophy and fibrosis induced by pressure overload. Lab investigation; J Tech Methods pathology. (2013) 93:1128–36. doi: 10.1038/labinvest.2013.99
78. Lin HB, Naito K, Oh Y, Farber G, Kanaan G, Valaperti A, et al. Innate immune nod1/RIP2 signaling is essential for cardiac hypertrophy but requires mitochondrial antiviral signaling protein for signal transductions and energy balance. Circulation. (2020) 142:2240–58. doi: 10.1161/CIRCULATIONAHA.119.041213
79. He QQ, Huang Y, Nie L, Ren S, Xu G, Deng F, et al. MAVS integrates glucose metabolism and RIG-I-like receptor signaling. Nat Commun. (2023) 14:5343. doi: 10.1038/s41467-023-41028-9
80. Du Y, Pan D, Jia R, Chen Y, Jia C, Wang J, et al. The reduced oligomerization of MAVS mediated by ROS enhances the cellular radioresistance. Oxid Med Cell longevity. (2020) 2020:2167129. doi: 10.1155/2020/2167129
81. Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. (2010) 464:1357–61. doi: 10.1038/nature08938
82. Mezzaroma E, Toldo S, Farkas D, Seropian IM, Van Tassell BW, Salloum FN, et al. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci United States America. (2011) 108:19725–30. doi: 10.1073/pnas.1108586108
83. Liu Y, Lian K, Zhang L, Wang R, Yi F, Gao C, et al. TXNIP mediates NLRP3 inflammasome activation in cardiac microvascular endothelial cells as a novel mechanism in myocardial ischemia/reperfusion injury. Basic Res Cardiol. (2014) 109:415. doi: 10.1007/s00395-014-0415-z
84. Xing J, Zhang A, Minze LJ, Li XC, Zhang Z. TRIM29 negatively regulates the type I IFN production in response to RNA virus. J Immunol. (2018) 201:183–92. doi: 10.4049/jimmunol.1701569
85. Xing J, Weng L, Yuan B, Wang Z, Jia L, Jin R, et al. Identification of a role for TRIM29 in the control of innate immunity in the respiratory tract. Nat Immunol. (2016) 17:1373–80. doi: 10.1038/ni.3580
86. Wang J, Lu W, Zhang J, Du Y, Fang M, Zhang A, et al. Loss of TRIM29 mitigates viral myocarditis by attenuating PERK-driven ER stress response in male mice. Nat Commun. (2024) 15:3481. doi: 10.1038/s41467-024-44745-x
87. Liu S, Chen J, Shi J, Zhou W, Wang L, Fang W, et al. M1-like macrophage-derived exosomes suppress angiogenesis and exacerbate cardiac dysfunction in a myocardial infarction microenvironment. Basic Res Cardiol. (2020) 115:22. doi: 10.1007/s00395-020-0781-7
88. Liu L, Wang Y, Cao ZY, Wang MM, Liu XM, Gao T, et al. Up-regulated TLR4 in cardiomyocytes exacerbates heart failure after long-term myocardial infarction. J Cell Mol Med. (2015) 19:2728–40. doi: 10.1111/jcmm.2015.19.issue-12
89. Horckmans M, Ring L, Duchene J, Santovito D, Schloss MJ, Drechsler M, et al. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur Heart J. (2017) 38:187–97. doi: 10.1161/01.res.0000043825.01705.1b
90. Mann DL. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res. (2002) 91:988–98. doi: 10.1161/01.RES.0000043825.01705.1B
91. Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. (2007) 204:3037–47. doi: 10.1084/jem.20070885
92. Li Z, Liu X, Zhang X, Zhang W, Gong M, Qin X, et al. TRIM21 aggravates cardiac injury after myocardial infarction by promoting M1 macrophage polarization. Front Immunol. (2022) 13:1053171. doi: 10.3389/fimmu.2022.1053171
93. Kirsebom FCM, Kausar F, Nuriev R, Makris S, Johansson C. Neutrophil recruitment and activation are differentially dependent on MyD88/TRIF and MAVS signaling during RSV infection. Mucosal Immunol. (2019) 12:1244–55. doi: 10.1038/s41385-019-0190-0
Keywords: MAVS, NLRP3, heart disease, innate immune, inflammation
Citation: Qi Y, Yin J, Xia W and Yang S (2025) Exploring the role of mitochondrial antiviral signaling protein in cardiac diseases. Front. Immunol. 16:1540774. doi: 10.3389/fimmu.2025.1540774
Received: 06 December 2024; Accepted: 31 January 2025;
Published: 18 February 2025.
Edited by:
Junji Xing, Houston Methodist Research Institute, United StatesReviewed by:
Pan Ma, Washington University in St. Louis, United StatesCopyright © 2025 Qi, Yin, Xia and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiwei Xia, eGlhd3dwa3VAMTYzLmNvbQ==; Shiwei Yang, anJkb2N0b3J5YW5nQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.