- 1Department of Biological Sciences, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia
- 2Research Center, King Fahad Medical City, Riyadh Second Health Cluster, Riyadh, Saudi Arabia
- 3Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Saud University, Riyadh, Saudi Arabia
- 4Riyadh Regional Laboratory, Ministry of Health, Riyadh, Saudi Arabia
The pandemic potential of the Middle East Respiratory Syndrome Coronavirus (MERS-CoV) highlights the critical need for effective vaccines due to its high fatality rate of around 36%. In this review, we identified a variety of immunotherapeutic molecules and diagnostic biomarkers that could be used in MERS vaccine development as human-derived adjuvants. We identified immune molecules that have been incorporated into standard clinical diagnostics such as CXCL10/IP10, CXCL8/IL-8, CCL5/RANTES, IL-6, and the complement proteins Ca3 and Ca5. Utilization of different human monoclonal antibodies in the treatment of MERS-CoV patients demonstrates promising outcomes in combatting MERS-CoV infections in vivo, such as hMS-1, 4C2H, 3B11-N, NBMS10-FC, HR2P-M2, SAB-301, M336, LCA60, REGN3051, REGN3048, MCA1, MERs-4, MERs-27, MERs-gd27, and MERs-gd33. Host-derived adjuvants such as CCL28, CCL27, RANTES, TCA3, and GM-CSF have shown significant improvements in immune responses, underscoring their potential to bolster both systemic and mucosal immunity. In conclusion, we believe that host-derived adjuvants like HBD-2, CD40L, and LL-37 offer significant advantages over synthetic options in vaccine development, underscoring the need for clinical trials to validate their efficacy.
Introduction
Middle East respiratory syndrome (MERS), a zoonotic disease caused by a member of the Coronaviridae family, was discovered in 2012 in Jeddah, Saudi Arabia (1, 2). This disease primarily targets the lower respiratory tract, eliciting host responses ranging from asymptomatic to severe acute respiratory syndrome, and may also impair other tissues, such as the kidneys (3, 4). Camels serve as the main reservoir for the virus and bats are considered the initial reservoir (5). Transmission to humans occurs through direct contact with infected camels or the consumption of their products (6). Between April 2012 and April 2024, the World Health Organization (WHO) recorded 2613 laboratory-confirmed cases from 27 countries, with approximately 36% (943 cases) resulting in mortality. Most of these cases - approximately 2204 occurrences with 862 deaths, representing a mortality rate of 39% - were documented in Saudi Arabia (7). Adults aged 50–59 exhibited the highest vulnerability to initial infection, whereas those aged 30–39 had the greatest risk for secondary infection (7). The case fatality rate (CFR) is highest among individuals aged 70–79 years, regardless of whether the infection was new or recurring (7).
The mean incubation period for MERS-CoV is approximately five days - although variations from 2–14 days occur (8, 9) - during which the host exhibits no symptoms of infection (9). Clinical manifestations of the illness vary widely, from mild symptoms such as cough, fever, and muscular discomfort, to severe conditions including pneumonitis, acute respiratory distress syndrome (ARDS), and respiratory failure (10). ARDS can result from cytokine release syndrome (CRS), which is characterized by an uncontrolled release of multiple proinflammatory cytokines due to an excessive immunological response by the host (11). To effectively understand the immunopathology of MERS-CoV, particularly MERS-CoV-induced CRS, acknowledgment of the potential overlap in the presentation and progression of severe MERS-CoV infections, as well as the lack of effective treatment options, is crucial.
COVID-19 pandemic has fast-forward the development of next generation vaccines. mRNA vaccines, like those developed by Pfizer-BioNTech and Moderna for COVID-19, use lipid nanoparticles to deliver genetic instructions for viral proteins, allowing for swift production and potent immune stimulation (12). Viral vector platforms, exemplified by AstraZeneca’s adenovirus-based vaccine, introduce genetic material to trigger immunity. Progress in structural vaccinology and nanoparticle engineering, as seen in Novavax’s SARS-CoV-2 vaccine, improves antigen presentation and durability (13). These innovations offer the potential for faster development, wider pathogen coverage, and enhanced thermostability, although expanding production and ensuring fair global distribution remain significant challenges. In contrast to SARS-CoV-2, MERS-CoV lacks approved preventive or therapeutic interventions, leaving supportive care as the only option. A vaccine could potentially curb transmission in high-risk regions, protect healthcare personnel, and mitigate pandemic risks associated with viral evolution or increased human-animal interactions. Moreover, lessons from COVID-19 emphasize the importance of proactive vaccine platforms against coronaviruses, which could be adapted for emerging variants.
This review aimed to explore the inflammatory biomarkers associated with MERS-CoV to ascertain whether MERS-CoV is linked to a unique inflammatory profile. A variety of immunotherapeutic molecules and diagnostic biomarkers that could be used in MERS vaccine development as human-derived adjuvants have been identified. The review also explores the possibility of identifying therapeutic agents and diagnostic markers targeting MERS-CoV, and contributes significantly to the fields of vaccinology and immunology by discussing the role of host-derived adjuvants in vaccine formulation.
Diagnostic biomarkers
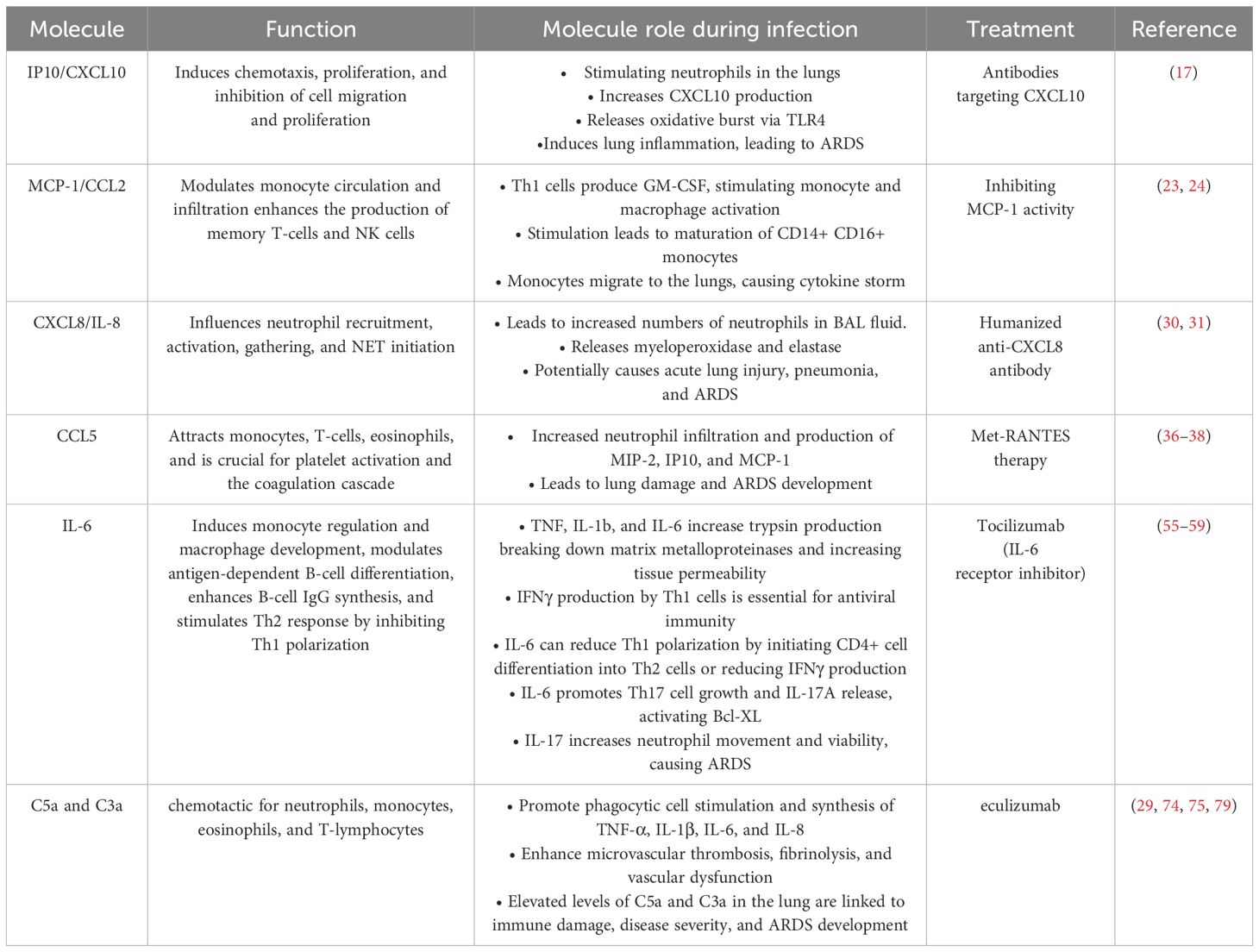
Addressing clinical MERS-CoV infections poses significant challenges, given the severity of the symptoms (14). Identifying a biomarker indicative of disease progression is crucial for diagnostic kit development. Cytokines and chemokine molecules can help to predict disease severity. The most prevalent cytokines and chemokines that could be diagnostic biomarkers for MERS (Table 1) are reviewed.
Interferon gamma-induced protein 10 (IP10/CXCL10) has been suggested as a biomarker for severe MERS-CoV infection. Kim et al. reported that CXCL10 levels were highest in patients during the second and third weeks of onset with severe MERS (13), compared with those with mild disease. Hong et al. indicated that CXCL10/IP10 concentrations were significantly elevated in patients who did not survive compared with those in surviving patients with MERS (15). Min et al. observed that patients who developed pneumonia during MERS infection exhibited high IP10/CXCL10 levels, which often decreased during the therapy phase in individuals who successfully recovered from pneumonia (16). The main role of CXCL10 are to mediate chemotaxis, and to inhibit cell migration and proliferation (17). CXCL10 plays a crucial function in stimulating migration, and infiltrating certain subsets of T lymphocytes at the infection sites during a viral infection (18). Elevated CXCL10 concentration has been associated with lung injury, as it promotes neutrophil infiltration into the lungs, leading to increased CXCL10 production and the release of oxidative bursts by neutrophils through Toll-like receptor 4 (TLR4) activation, resulting in ARDS (17). The role of this chemokine in viral infection can be protective or pathogenic, depending on host immunity and the type of virus (17). Considering its increased expression in previous research, CXCL10 appears to play a pathogenic role in MERS infection. Consequently, the development of antibodies targeting CXCL10 might offer a promising therapeutic strategy for treating ARDS, as demonstrated in the H1N1 mouse model of influenza A virus (19).
Monocyte chemoattractant protein-1 (MCP-1/CCL2) has been identified as a diagnostic marker for MERS-CoV progression. Alhetheel et al. reported that patients with symptomatic MERS who did not survive exhibited higher MCP-1 levels than those who recovered (2139 ± 548.2 vs. 776.5 ± 165.3 pg/mL; p < 0.004) (20). Furthermore, Hong et al. found that MCP-1 levels were significantly upregulated in patients with MERS who did not survive compared with levels in those who survived (15). Shin et al. demonstrated that plasma MCP-1 concentration was elevated fourfold in patients with severe and moderate disease (21). CCL2/MCP-1 modulates the circulation and infiltration of monocytes, memory T-lymphocytes, and natural killer (NK) cells, promoting inflammatory activities in tissues, particularly in the lungs (22). The upregulation of MCP-1 may activate T helper-1 (Th1) cell responses (23). Th1 cells produce granulocyte-macrophage colony-stimulating factor (GM-CSF), which may stimulate monocyte and macrophage activation. In individuals with coronavirus disease of 2019 (COVID-19), this stimulation leads to the maturation of CD14+ CD16+ monocytes, which release interleukin 6 (IL-6) (24). After migrating to the lungs, these monocytes exacerbate the cytokine storm, damaging the lungs (25). Therefore, inhibiting MCP-1 activity could be a therapeutic approach for treating MERS severity. Chirathaworn et al. demonstrated that MCP-1 is a potential biomarker implicated in immunopathological processes induced by Chikungunya virus, and is viewed as a possible therapeutic target (26). The severity of COVID-19 and potential mortality risk in patients can be predicted by biomarkers IP-10 and MCP-1, which serve as indicators of disease progression (22, 27). In addition, Tsaur et al. found that during the development of prostate cancer, chemokines undergo substantial alterations, with CCL2 emerging as a potential diagnostic indicator (28).
Chemokines such as CXCL8/IL-8 have been proposed as biomarkers for the severity of MERS infection. Patients with MERS-CoV who did not survive exhibited significantly higher levels of CXCL8 compared with those who survived (29). Alosaimi et al. demonstrated a significant correlation between the mortality rate of individuals with MERS-CoV and elevated levels of CXCL8 expression, compared to healthy controls (30). The chemokine CXCL8 influences key mechanisms, including neutrophil recruitment, activation, and aggregation, as well as the initiation of neutrophil extracellular traps (NETs) (30). Increased levels of CXCL8 leads to a higher concentration of neutrophils in the bronchoalveolar lavage (BAL) fluid, resulting in the release of myeloperoxidase and elastase. These compounds have the potential to cause acute lung injury, potentially progressing to pneumonia and ARDS (31). Additionally, CXCL8 enhances the production of CD4+ molecules and the activity of T helper cells during MERS infection (32). Consequently, humanized anti-CXCL8 antibody treatment has been shown to prevent lung neutrophil infiltration and alleviate acute lung injury syndrome, as demonstrated in rabbit models (33).
RANTES (CCL5) is another chemokine suggested as a diagnostic marker of the severity of MERS-CoV infection. Patients with MERS-CoV exhibited upregulated expression of CCL5, associated with disease severity (29). CCL5 effectively attracts monocytes, T-cells, and eosinophils (34). It is pivotal in activating platelets and initiating coagulation cascade (35). However, two different studies reported that CCL5 levels were significantly higher in recovered patients with MERS than in those with mild or severe disease (16, 21). The elevated RANTES levels may be linked to the release of this chemokine by activated virus-responsive T-cells (21). Elevated CCL5 levels in the lungs have been associated with increased neutrophil infiltration and the production of MIP-2, IP10, and MCP-1 in transgenic mice, leading to lung damage and ARDS development (36, 37). Additionally, CCL5 was elevated in RSV-infected and eosinophilic disease-sensitized mice. Met-RANTES therapy reduced inflammatory cell recruitment and local cytokine production (38).
CXCL10 and CXCL8 and CCL-5 are proinflammatory chemokines that play critical roles in the pathogenesis of infection, and function as prognostic indicators of coronaviruses severity (30, 39–43). CXCL10 is secreted by various cells, including monocytes, endothelial cells, and fibroblasts, in response to IFN-γ (44). CXCL8 is also secreted by numerous cell types in response to IL-6 and TNF-mediated cytokines, while antigen-presenting cells and activated T lymphocytes produce and release CCL5 (45–47). The concentration of CXCL10 in blood serum could serve as a potential indicator for identifying severe cases of Mycoplasma pneumoniae pneumonia in pediatric patients (48). CXCL10 has been found to be the most promising indicator for detecting acute Zika virus infection in potential clinical applications (49). CXCL10 and CXCL8 may serve as serum biomarkers for predicting liver injury induced by hepatitis B virus (HBV) infection (50). Gastric cancer progression can be predicted by using CXCL8 as a potential biological marker (51). Hu et al. found that concentrations of CCL5 in blood serum proved effective in distinguishing cirrhosis from chronic hepatitis B (CHB), with CCL5 emerging as the most dependable indicator (52). Moreover, CCL5 was initially recognized as an immunological and prognostic biomarker for cancer patients (53).
Interleukin-6 (IL-6) could help to predict disease progression in MERS-CoV-infected patients. Kim et al. revealed a significant increase in IL-6 levels in patients with severe MERS up to the third week after symptom onset (54). In another study, plasma IL-6 concentration was considerably elevated and was correlated with MERS infection severity (21). Hong et al. showed that IL-6 levels were highly upregulated in patients who did not survive compared to those who survived (15). IL-6 regulates multiple immune-stimulating pathways, which in turn influence the host defense. These pathways include: the regulation of monocytes and their development into macrophages, modulation of antigen-dependent B-cell differentiation, enhanced IgG synthesis by B-cells, and stimulation of Th2 response via Th1 polarization inhibition (55). IL-6 levels have been shown to be associated with the severity of lung inflammation in a study of influenza virus (56). IFNγ produced by Th1 cells is crucial for a successful antiviral immune response. IL-6 hinders Th1 polarization via the stimulation of CD4+ cells to transform into Th2 cells or by decreasing IFNγ production (57). IL-6 also enhances Th17 development and stimulates the release of IL-17A, which in turn activates antiapoptotic molecules such as Bcl-XL. This supports the survival of cells that have been infected by a virus (58). Simultaneously, IL-17 enhances the movement and viability of neutrophils, which are involved in the development of ARDS in patients with COVID-19 (55, 59). Hence, treating patients who have increased IL-6 levels with tocilizumab, an IL-6 receptor inhibitor, could be effective against severe MERS cases, and has also provided therapeutic advantages in treating COVID-19 (60, 61). This treatment is now considered one of the most promising options available (62).
IL-6, a proinflammatory cytokine, has been found to have increased expression in various conditions, including respiratory ailments, cancer and viral infections, such as HIV and HCV. Significantly elevated levels of IL-6 have been observed in patients with severe cases of severe acute respiratory syndrome (SARS), MERS, and COVID-19 compared to milder cases (15, 54, 63–71) and is considered as an indicator for MERS progression. Santa Cruz A et al. demonstrated that IL-6 serves as a valuable instrument for assessing prognosis, particularly in predicting patient outcomes (72). In addition, IL-6 has been recommended to be a diagnostic biomarker for gastric cancer (73).
Complement anaphylatoxins, such as C5a and C3a, can be used as markers for predicting the progression of MERS-CoV infection. Hamed et al. revealed that MERS-CoV-infected patients had elevated levels of C5a and C3a, which were positively associated with severity and mortality rates (29). C5a is a chemotactic agent for neutrophils, monocytes, eosinophils, and T-lymphocytes (74). Complement anaphylatoxins C3a and C5a are formed, following the overactivation of the pulmonary and systemic complement systems, in turn causing inflammation, endothelial cell damage, thrombus formation, intravascular coagulation, and, ultimately, death due to multiple organ failure (74–76). Following infection, complement anaphylatoxins promote the stimulation of phagocytic cells and the synthesis of TNF-α, IL‐1β, IL‐6, IL‐8, granular enzymes, and free radicals. These substances enhance the development of microvascular thrombosis, fibrinolysis, and vascular dysfunction (75–78). Elevated levels of C5a and C3a in the lung have been suggested in contributing to immune-related damage, disease severity, ARDS development, and higher mortality rates in MERS-CoV-infected patients (29). Patients with high levels of complement anaphylatoxins could be treated therefore with eculizumab, which is a human monoclonal antibody (hmAb) with a significant affinity for the complement protein C5 (79). This antibody blocks the separation of C5a and C5b and stops the production of the cell-destroying C5b-9 complement complex (80). Inhibiting the C5a-C5aR pathway in MERS-CoV infected hDPP4 transgenic mice led to a decrease in the extent of infection-induced tissue damage (81). Patients with COVID-19 demonstrated a rapid, significant, and evident response to eculizumab, resulting in complete recovery, despite severe lung injury (79).
Complement proteins C3a and C5a have been found to be biomarkers of MERS and COVID-19 severity. C5a serves as a potent chemoattractant, facilitating the recruitment of inflammatory cells (neutrophils, eosinophils, monocytes, and T lymphocytes), induces the activation of phagocytic cells, and elicits the release of granule enzymes and oxidants (82). C3a effectively activates eosinophils, inducing granule release, reactive oxygen intermediate generation, and chemotaxis in in-vitro assays (83). A study by Alosaimi et al. demonstrated that C5a and C3a can be prognostic biomarkers of COVID 19 severity (84). In addition, C5a has been considered to be a potential marker of severity in patients with myasthenia gravis (85). C3a could serve as an indicator for early identification of hepatitis C virus-associated hepatocellular carcinoma (86).
Human immunotherapeutic molecules
Currently, MERS is the most fatal human coronavirus-related disease, with a mortality rate exceeding 35% (14, 62), with no verified antiviral treatments available. Identifying markers that enhance the effectiveness of treatment is crucial. Our study investigates the most common human derived molecules that could aid in treating MERS (Table 2).
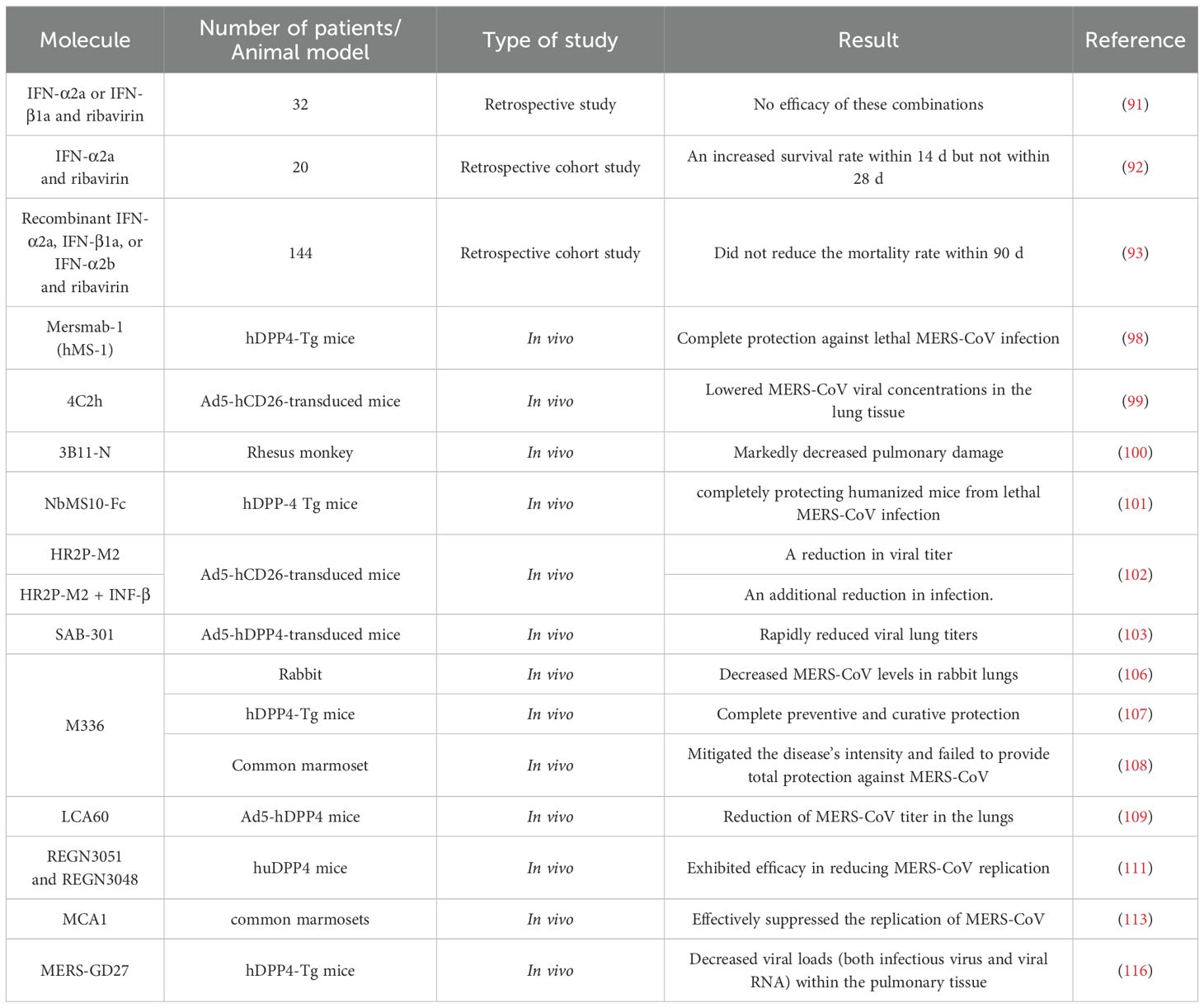
Interferon (IFN) has been used for viral treatment. Type I interferon (IFN-I) is the first cytokine upregulated after infection, activating approximately 300 genes involved in immunomodulation and antiviral defense (87, 88). Falzarano et al. demonstrated that administering IFN-α2b and ribavirin within 8 hours of viral exposure effectively reduced lung damage and decreased viral load in the lungs (89). However, this combination treatment provided no benefit when administered to severely ill patients with multiple comorbidities (90). A retrospective study involving 32 patients revealed no efficacy in treating MERS with IFN-α2a or IFN-β1a combined with ribavirin (91). In another retrospective cohort study, 20 patients with severe MERS-CoV infection were treated with IFN-α2a and ribavirin; this resulted in an increased survival rate within 14 days but not within 28 days (92). Arabi et al. conducted a retrospective cohort study involving 144 critical patients with MERS and treated with recombinant IFN-α2a, IFN-β1a, or IFN-α2b and ribavirin; however, no reduction was observed in the 90-day mortality rate (93). These combinations may be more effective in the early stages of the disease. Additionally, marmosets infected with MERS and treated with IFN-β1b exhibited less severe illness and lower than average viral loads in the lungs and extrapulmonary organs during necropsy compared with those in untreated animals (94). INF-I used on SARS patients showed no effective results. A study by Wu et al. demonstrated that INF-α could potentially help reduce the duration of the clinical course (95). Loutfy et al. revealed that the combination of interferon alfacon-1 and corticosteroids was linked to several positive outcomes: a decrease in oxygen saturation impairment caused by the disease, faster improvement of lung abnormalities visible on radiographs, and reduced levels of creatine kinase (96). However, Zhao et al. found that administering both interferon and high doses of immunoglobulins yielded no significant results in combatting SARS infection (97).
Human immunotherapeutic agents have been tested against MERS-CoV infection. Mersmab-1 (hMS-1) is a neutralizing monoclonal antibody that specifically targets the MERS-CoV receptor-binding domain (RBD) with strong affinity. A study by Qiu et al. concluded that a single administration of hMS-1 effectively impeded MERS-CoV RBD from binding to its viral receptor. This intervention offered complete protection against lethal MERS-CoV infection in genetically modified mice that expressed human dipeptidyl peptidase 4 (hDPP4-Tg) (98).
A neutralizing monoclonal antibody named 4C2h was developed to target the receptor binding domain of MERS spike protein and inhibit viral entry. In their study, Li et al. showed that 4C2h effectively lowered MERS-CoV viral concentrations in the lung tissue of mice that were genetically modified with Ad5-hCD26 and later infected (99).
3B11-N, a human anti-MERS monoclonal antibody, has been tested against MERS-CoV infection in vivo. 3B11-N did not show any escape mutants during the initial characterization, demonstrated the highest virus neutralization ability, and was determined to be suitable for mass production, potentially providing significant therapeutic advantages (100). Johnson et al. illustrated that MERS-infected rhesus monkeys treated with 3B11-N exhibited markedly decreased pulmonary damage compared to infected individuals who received no treatment, suggesting that this antibody could be an effective therapy for MERS-CoV infection (100).
NbMS10-Fc, a neutralizing nanobody and its human-Fc-fused version, is a protective treatment against MERS-CoV. NbMS10 exhibited strong binding affinity to the MERS-CoV RBD and inhibited interaction between RBD and DPP4 (101). A study by Zhao, et al. showed that administering a single dose of NbMS10-Fc exhibited exceptional prophylactic and therapeutic efficacy, completely protecting humanized mice from lethal MERS-CoV infection (101).
The peptide MERS-CoV fusion inhibitor HR2P-M2, which specifically targets the S protein HR1 domain, demonstrates significant efficacy in suppressing both in vitro and in vivo infections caused by various strains of MERS-CoV (102). Intranasal administration of HR2P-M2 protected mice expressing human dipeptidyl peptidase 4 via adenovirus serotype-5 from MERS-CoV infection, and was effective against viral strains with and without HR1 region mutations in the S protein (102). The protective effect was enhanced when combined with INF-β, which indicates promising prospects for its advancement as a preventive measure, and highlights its potential application as a treatment option for patients infected with MERS-CoV (102).
SAB-301 is a trans-chromosomic human IgG immunoglobulin (Tc hIgG), derived from purified Al-Hasa strain MERS-CoV spike protein nanoparticles. Single doses of SAB-301 administered to Ad5-hDPP4 receptor–transduced mice before or after MERS-CoV infection rapidly reduced viral lung titers (103). A clinical trial, registered with ClinicalTrials.gov (number NCT02788188), was conducted to evaluate SAB-301 safety and tolerability. It indicated that SAB-301 exhibits safety and tolerability at 50 mg/kg, which may be therapeutically effective (104).
M336 are human monoclonal antibodies that target the RBD of the MERS-CoV spike glycoprotein and interact with CD26/DPP4 (105). Research conducted in vivo revealed that preventive treatment with m336 decreased MERS-CoV levels in rabbit lungs (106). M336 also offered complete preventive and curative protection against MERS-CoV in genetically modified mice expressing human DPP4 (107). However, A separate investigation involving a non-human primate - the common marmoset - indicated that m336 only mitigated the disease’s intensity and failed to provide total protection against MERS-CoV (108).
LCA60 is an additional human neutralizing monoclonal antibody developed to combat MERS-CoV. This antibody was generated by isolating IgG memory B cells from an individual infected with MERS and then immortalizing these cells through the use of the Epstein-Barr virus (109). The antibody LCA60 demonstrates efficacy in neutralizing MERS-CoV infection in cellular models and offers both preventive and therapeutic protection in BALB/c mice that have been modified with adenoviral vectors to express hDPP4 (110). In a more challenging model using IFN-α/β receptor-deficient mice expressing hDPP4, LCA60 treatment led to a substantial decrease in viral load within the lungs (109). This reduction occurred more rapidly compared to BALB/c mice, with a three-log decrease observed in just one day, as opposed to the three days required in BALB/c mice (109).
Other human neutralizing monoclonal antibodies were developed to protect and treat MERS-CoV infection: REGN3051 and REGN3048. REGN3051 and REGN3048 were produced by immunizing humanized transgenic mice (VelocImmune mice) with DNA encoding the MERS-CoV S protein to engineer hybridoma B cells that produce neutralizing monoclonal antibodies (111). A study by Pascal et al. conducted in vivo revealed that REGN3051 and REGN3048 inhibited MERS-CoV multiplication in mice with humanized DPP4, both as a preventive measure and as a treatment (111). However, when tested in common marmosets, these monoclonal antibodies appeared to be more efficient in preventing MERS-CoV infection, rather than treating it once established (112).
A human monoclonal antibody, MCA1, was identified by isolating B cells from a patient who had previously overcome MERS, targeting the receptor-binding domain of the MERS-CoV S glycoprotein (113). MCA1 demonstrated strong neutralizing activity against MERS-CoV in cell entry assessments. In vivo, MCA1 effectively suppressed the replication of MERS-CoV in common marmosets when given as a preventive or therapeutic treatment (113).
Two strong human neutralizing monoclonal antibodies, MERS-4 and MERS-27, were tested against MERS-CoV infection. MERS-4 and MERS-27 were derived from a non-immune human yeast display antibody library generated using polyadenylated RNA sourced from the spleen and lymph nodes of regular individuals (114). Both MERS-4 and MERS-27 effectively inhibited pseudovirus and live MERS-CoV from entering cells. The combined use of MERS-4 and MERS-27 demonstrated a synergistic effect on pseudotyped MERS-CoV. The primary approach to neutralizing MERS-4 and MERS-27 is by inhibiting the attachment of the RBD to DPP4 (114).
MERS-GD27 and MERS-GD33 are human neutralizing monoclonal antibodies that are produced from the whole blood of a MERS patient (115). MERS-GD27 and MERS-GD33 demonstrated the most potent neutralizing activity against pseudotyped and live MERS-CoV in vitro. Analysis of mutagenesis showed that MERS-GD27 and MERS-GD33 focused on distinct areas in the S glycoproteins. The synergy of the two monoclonal antibodies effectively neutralized pseudotyped MERS-CoV (115). A study conducted in vivo highlighted the prophylactic and therapeutic advantages of MERS-GD27 in protecting HDPP4-transgenic mice against MERS-CoV infection (116).
The neutralizing monoclonal antibody S309, isolated from the peripheral blood mononuclear cells of a patient infected with SARS-CoV in 2003, was tested against MERS-CoV infection. S309 demonstrated strong binding affinity for both SARS-CoV and SARS-CoV-2 (117). In vivo, the monoclonal antibody CR9114 exhibits neutralizing capabilities against both influenza A and B types, and CR6261 has shown the ability to lower mortality rates in mice infected with H1N1 and H5N1 influenza A subtypes (118, 119). Additionally, monoclonal antibodies 70-1F02 and 9-3A01 have demonstrated the capacity to inhibit infections caused by two H1N1/H1N5 influenza A subtypes (120, 121).
Host-derived adjuvants in vaccine development
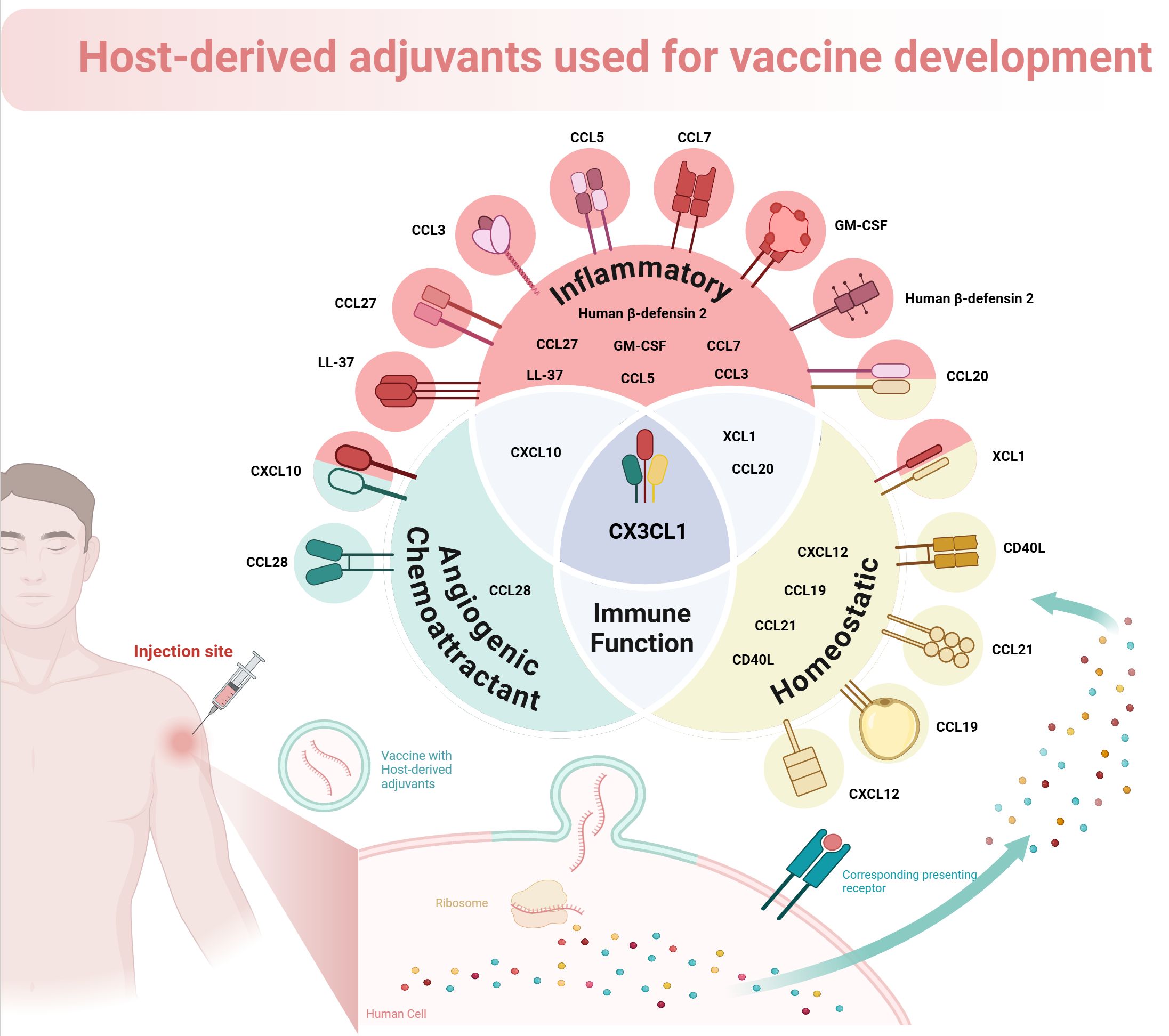
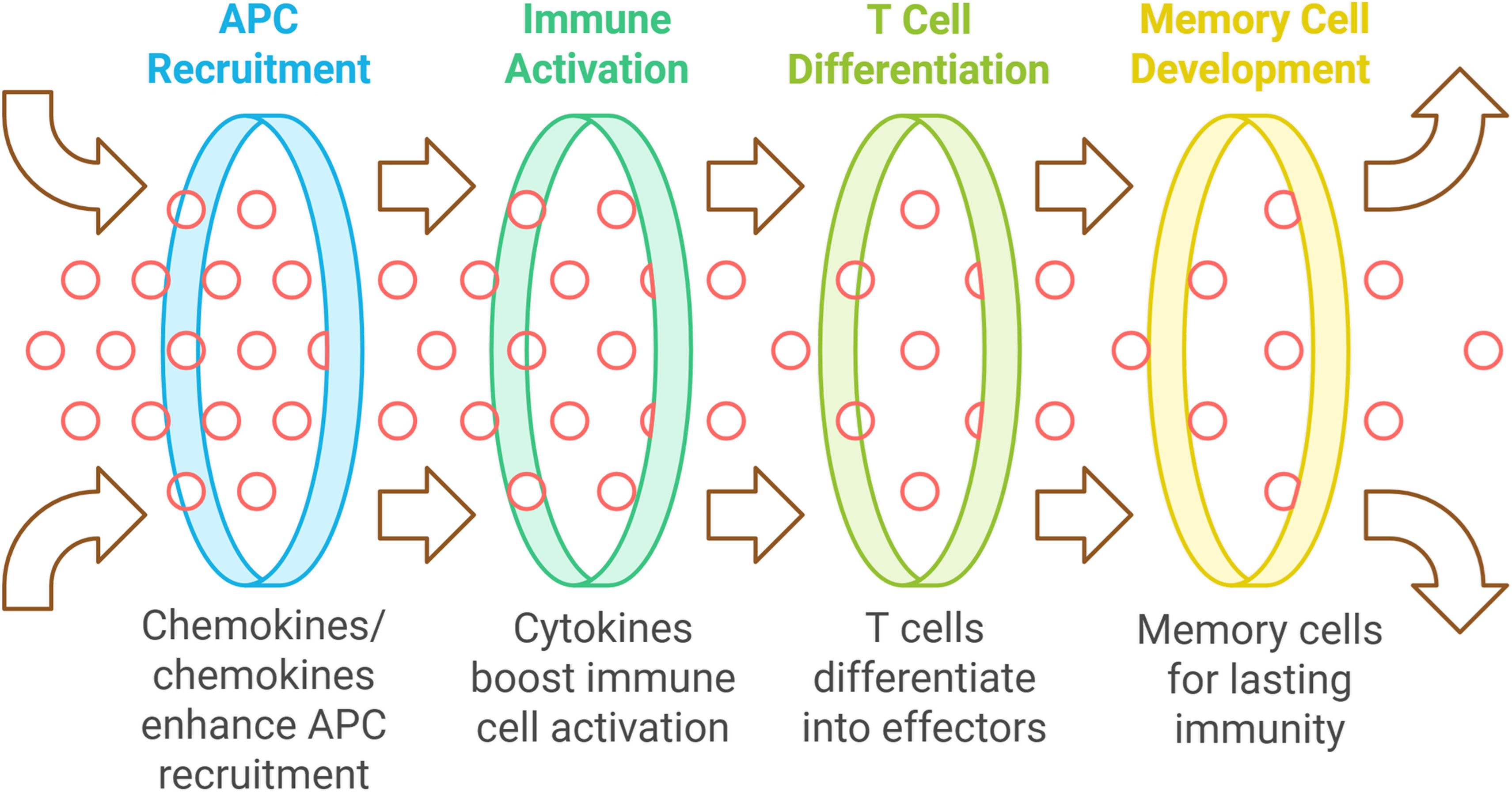
Inducing a robust memory response from T- and B-cells targeted toward the specific pathogen, along with the presence of durable plasma cells, is the primary objective of an effective immunization strategy against infectious diseases. The unavailability of vaccines specifically targeting MERS-CoV highlights the urgent need for targeted immune responses against the virus. Various strategies have been employed to develop a MERS-CoV vaccine. This study reviews the strategies used to generate human-derived vaccine adjuvants (Table 3).
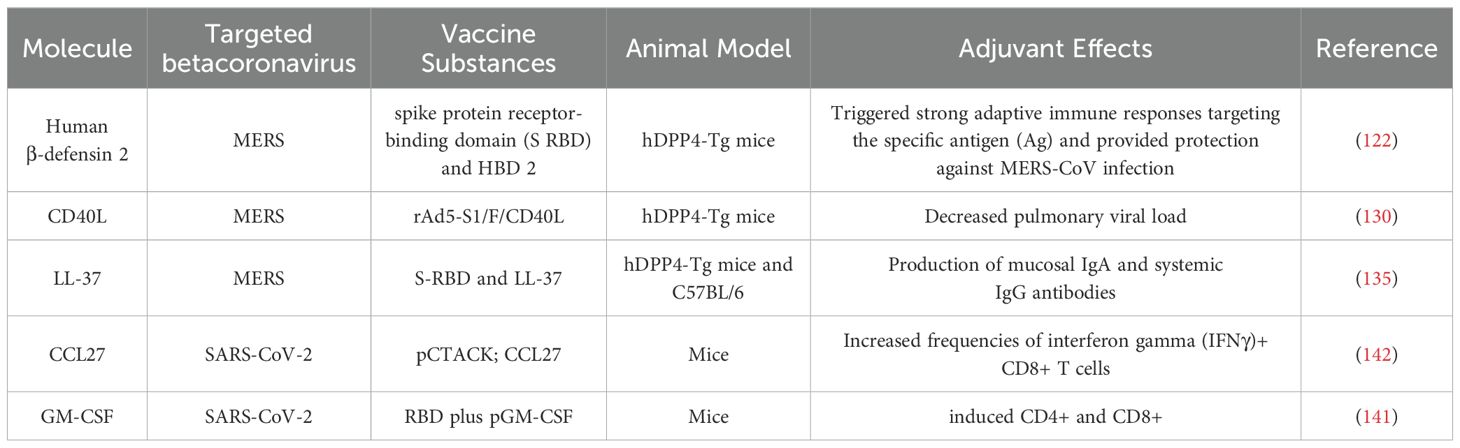
Human β-defensin-2 (HBD-2) has been used as a vaccine adjuvant against MERS-CoV. Human β-defensins (HBDs) are short host defense peptides produced by epithelial cells to create mucosal barriers that protect against different types of infectious agents (122). HBDs play a crucial role in connecting the activation of pathogen-specific innate and adaptive immunity by recruiting and activating different types of leukocytes such as macrophages, dendritic cells (DCs), and T cells (123–125). Kim et al. demonstrated that immunization of hDPP4-Tg with a fusion of spike protein receptor-binding domain S RBD and HBD 2 (S RBD-HBD 2) induced robust antigen-specific adaptive immune responses and conferred protection against MERS-CoV infection. Additionally, S RBD-HBD 2 immunization reduced the progression of pulmonary fibrosis in the lungs of MERS-CoV-infected hDPP4-Tg mice and suppressed the activation of endoplasmic reticulum stress signaling following viral infection (122).
Another human derived molecule that can be utilized as vaccine adjuvant for MERS-CoV is CD40L. CD40L, a membrane protein of type II, serves a critical function as a co-stimulatory molecule and essential regulator of immune function (126). The primary expression occurs temporarily on activated CD4+ T cells (127). The interaction between CD40L and its receptor CD40, found on all antigen-presenting cells (APCs), plays a crucial role in connecting innate and adaptive immune responses (128, 129). Research conducted by Hashem et al. demonstrated that hDPP4-Tg mice inoculated with a combination of non-replicating recombinant adenovirus 5 (rAd5), MERS-CoV S1 protein, and murine CD40L (rAd5-S1/F/CD40L), provided complete protection against MERS-CoV, as demonstrated by the significantly decreased pulmonary viral load (130).
LL-37, a human antimicrobial peptide, exhibits chemotactic properties and modulate the activities of various immune cells, including dendritic cells (131). During infection, LL-37 functions as an alarm signal, linking the innate and adaptive immune systems by attracting immune cells to the infection site (132). LL-37 has the potential to exhibit antiviral activity and regulate the delicate balance between pro- and anti-inflammatory responses by modulating inflammatory cytokine expression; therefore, these peptides may serve as effective vaccine adjuvants (133, 134). In their study, Kim et al. found that immunized mice with a combination of S-RBD and LL-37 (S-RBD-LL-37) stimulated the production of mucosal IgA and systemic IgG antibodies, which demonstrated virus-neutralizing capabilities (135).
Chemokines enhance the recruitment of antigen-presenting cells (APCs) to vaccination sites, improving antigen uptake and T cell presentation, which is vital for a strong adaptive immune response (136–138). Cytokines directly boost immune cell activation and proliferation, aiding the differentiation of naive T cells into effector T cells necessary for infection clearance. They also help to develop memory T and B cells for lasting immunity post-vaccination (139) (see Figure 1). Host-derived cytokines and chemokines are generally better tolerated than synthetic adjuvants, which can trigger adverse immune reactions. Using the body’s own signaling molecules can optimize immune responses, and these substances are versatile for various vaccine types, including protein subunit, DNA, and viral vector vaccines (138).
Host-derived cytokines and chemokines are being explored as vaccine adjuvants to enhance immune responses by utilizing the body’s own signaling molecules. These proteins can modulate immune responses, potentially offering a safer and more effective alternative to synthetic adjuvants. Various cytokines, including interleukins and interferons, have shown promise in promoting antigen-specific immune responses when used with vaccines. Notable examples include CXCL10, CXCL12, CCL19, CCL5, CCL3, CX3CL1, IL-1, and INF-α, which have been tested in both murine and human studies. The selection of these adjuvants can either be homeostatic or inflammatory, influencing their effects on immunity (136, 139).
Previous studies have highlighted the effectiveness of cytokines like CCL28, GM-CSF, IL-2, IL-12, IL-15, IL-21, and IL-33 in enhancing immune responses to various vaccines (140, 141). Innovative approaches using host-derived cytokines have demonstrated improved systemic and mucosal immunity post-vaccination. For instance, the mucosal chemokine pCTACK (CCL27) has been shown to enhance vaccine responses to SARS-CoV-2, while GM-CSF (pGM-CSF) has been effective in DNA vaccinations against the virus by promoting antigen expression and immune cell recruitment (140–143).
Human derived adjuvants used in clinical and pre-clinical studies
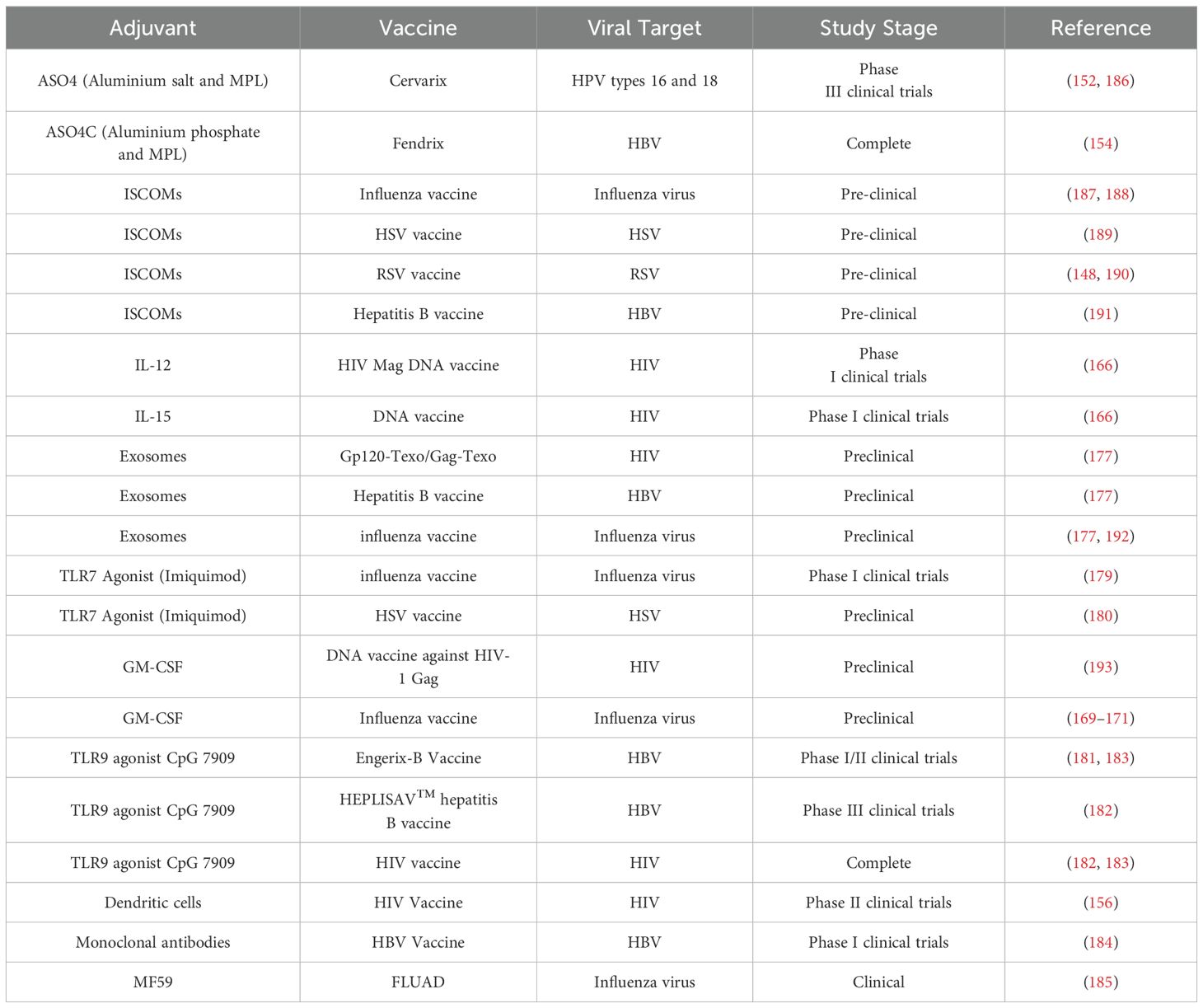
Adjuvants derived from human sources such as immune cells, cytokines, and proteins improve the efficacy of viral vaccines by boosting innate immunity, increasing antigen presentation, and enhancing overall immune responses. These adjuvants are primarily being evaluated for safety, efficiency and widespread applicability in clinical and preclinical research. Current research on notable human-derived adjuvants includes immuno-stimulating complexes (ISCOMs), aluminium salts, granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukins (IL-12, IL-15), exosomes, dendritic cells (DCs), and monoclonal antibodies (mAb). Furthermore, Toll-like receptor (TLR) agonists (e.g., Imiquimod, CpG 7909) and MF59 (an oil-in-water emulsion) exhibit the potential to augment responses to vaccines (Table 4).
ISCOMs, consisting of saponin, phospholipids, cholesterol, and antigens such as Quil A (144), serve as strong adjuvants for hydrophobic antigens, particularly those derived from enveloped viruses (145). The saponin-cholesterol matrix reduces toxicity and hemolytic activity (144), demonstrating robust cellular and humoral responses in both animal and human trials (144, 146). ISCOMs also induce strong mucosal and systemic immunity (147), rendering them interesting candidates for nasal vaccinations, including those for influenza (148).
Monophosphoryl lipid A (MPL), derived from salmonella minnesota in detoxified form, stimulates TLR4 on DCs, thereby augmenting innate immunity (149) and priming CD4+ and CD8+ T-cell responses to establish adaptive immunity (150) and immunological memory (151). MPL is utilized in Adjuvant System 4 (AS04) with aluminium salt, in HPV (Cervarix™) (152, 153) and HBV (Fendrix) vaccinations (154, 155). Published clinical trials indicate that DC immunotherapy in HIV-1 infection can provoke HIV-specific immune responses (156).
Type I interferons (IFNs) facilitate the maturation of DCs, hence augmenting the formation of antigen-specific CD8+ T lymphocytes for tumor suppression. Employing IFNs as adjuvants to vaccination may represent a promising strategy. IFNs possess a brief half-life but albumin conjugated to a protein will extend the half-life of the associated protein (157).
Cytokine adjuvants like IL-12 and IL-15 (158–160) boost immune responses. IL-12, produced by DCs and monocytes, is crucial for cellular immunity (161), where defects increase susceptibility to intracellular pathogens (162, 163). IL-15, produced by DCs, monocytes and epithelial cells, supports proliferation of B and T cells, activation of NK cells, and long-term memory cell responses (164, 165). Early clinical trials combining IL-12 or IL-15 with an HIV DNA vaccine show their potential as adjuvants (166).
GM-CSF improves vaccine effectiveness by stimulating DCs. GM-CSF genes (codon optimized) enhance protein expression and immunological responses, particularly against HIV-1 Gag (167). GM-CSF produces enhanced antibody responses to influenza vaccines (168) and demonstrates potential as an effective adjuvant in clinical trials (169–171).
Exosomes originating from infected cells can transmit viral components to adjacent cells, thereby eliciting antiviral immunity (172). The evolutionary parallels between viruses and exosomes indicate that exosomes may serve as viable vaccine platforms (173). Exosome-based HIV vaccines, such as Gag-Texo and Gp120-Texo, have demonstrated robust, tailored immune responses (174). Moreover, modified Nefmut-exosomes proficiently stimulate CTL responses against HIV and other viruses, including Ebola, HBV, and influenza (175, 176). Preliminary research indicates that exosomes may serve as adjuvants for influenza and HBV vaccines, augmenting immune responses and protection, hence reinforcing their potential as effective vaccine adjuvants (177).
TLR7 agonist Imiquimod augments vaccine immunogenicity by facilitating DC maturation and eliciting a Th1 response (178). Research in humans and animals demonstrates that it enhances and extends immune responses, especially in influenza and HSV vaccinations, affirming its efficacy as an adjuvant (179, 180). Similarly, TLR9 agonist CpG oligodeoxynucleotides (ODNs) stimulate plasmacytoid DCs and B cells, promoting Th1 and proinflammatory responses. As adjuvants, they augment antigen-presenting cell function, thereby fortifying humoral and cellular immunity. Preclinical and clinical experiments demonstrate that CpG ODNs enhance the efficiency of HIV and HBV vaccines (181, 182), with CpG 7909 being effective for immunocompromised patients (182, 183).
Creating HBV-specific neutralizing mAbs may facilitate the elimination of surplus viral proteins, perhaps reinstating adaptive immunity and augmenting the efficacy of antiviral medications. Fully human mAbs from individuals vaccinated against HBV and those who have recovered demonstrate potential as adjunctive therapies to diminish viral protein levels and enhance immunological recovery, hence improving the results of antiviral treatments (184). Immunosenescence results in diminished antibody responses to inactivated influenza vaccine (IIV) in elderly persons. To resolve this, adjuvants such as MF59, an oil-in-water emulsion, have been included to improve vaccine efficacy. Since 1997, MF59-adjuvanted IIV3 (FLUAD) has been authorized for older patients in Europe and exhibits superior immunogenicity compared to nonadjuvanted IIV, underscoring its significance in enhancing vaccine responses in the elderly (185).
Conclusion
The Middle East Respiratory Syndrome Coronavirus (MERS-CoV) remains a significant global health threat. This review emphasized critical biomarkers linked to MERS-CoV infection. These biomarkers could improve clinical diagnostics, therapeutic interventions and vaccine development for MERS-CoV. The benefits of using host-derived adjuvants in vaccine development were also highlighted, focusing on their safety and effectiveness in enhancing immune responses. Disease progression of MERS-CoV can be estimated by assessing the levels of certain molecules, including CXCL10/IP10, CXCL8/IL-8, CCL5/RANTES, IL-6, and the complement proteins Ca3 and Ca5. However, further studies must be conducted to measure the level of cytokines and chemokines at different time points during the infection. Despite investigations into several therapeutic agents, such as interferons, their efficacy has proven inadequate. In vivo studies, various human monoclonal antibodies showed substantial benefits in fighting MERS-CoV infection. The antibodies tested include hMS-1, 4C2h, 3B11-N, NbMS10-Fc, HR2P-M2, SAB-301, M336, LCA60, REGN3051, REGN3048, MCA1, MERS-4, MERS-27, MERS-GD27, and MERS-GD33. This highlights the urgent need for ongoing clinical trials to discover more effective treatment options. Additionally, exploring vaccine adjuvants is crucial for advancing immunization strategies against MERS-CoV. MERS infections may be prevented by designing a vaccine containing human-derived molecules that includes one or more adjuvants, such as HBD-2, CD40L and LL-37. The potential of host-derived adjuvants, particularly cytokines and chemokines, offers a promising direction for enhancing vaccine effectiveness. These natural signaling molecules not only improve the recruitment of antigen-presenting cells (APCs) to vaccination sites but also promote robust activation and differentiation of T cells. By harnessing the body’s own immune mediators, adaptive immune responses can be optimized while minimizing the adverse effects commonly associated with synthetic adjuvants.
Evidence from both murine and human studies supports the use of various cytokines, including interleukins and interferons, as effective adjuvants that enhance antigen-specific immunity across diverse vaccine platforms, including protein subunit, DNA, and viral vector vaccines. Host-derived adjuvants such as CCL28, CCL27, RANTES, TCA3, and GM-CSF have shown significant improvements in immune responses, highlighting their potential to bolster both systemic and mucosal immunity. This underscores the importance of host-derived adjuvants in vaccine development and their advantages over traditional synthetic options. In addition, while these adjuvants offer numerous advantages, including improved compatibility, precise immune activation, and the ability to mimic natural immune responses, the study emphasizes that diagnostic biomarker molecules may not be suitable as adjuvants due to their proinflammatory activity during MERS-CoV infection.
As research progresses, the integration of host-derived adjuvants into vaccine formulations could lead to safer and more effective immunization strategies, ultimately enhancing protection against infectious diseases. Future studies should prioritize optimizing the delivery and combination of these adjuvants to maximize their immunological benefits, paving the way for innovative vaccine development.
Future perspectives
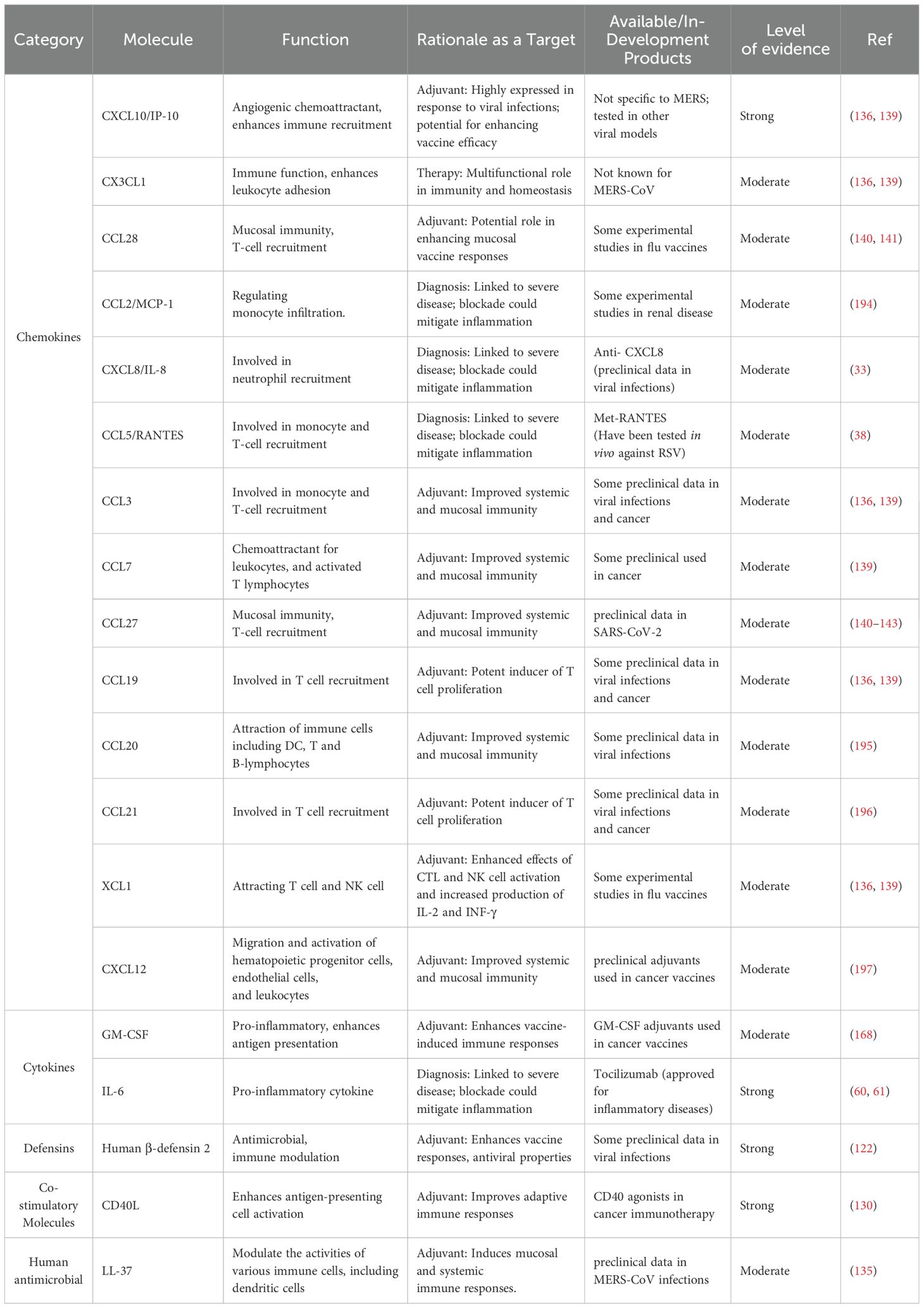
A structured framework has been implemented to categorize biomarkers by molecular type, function, and supporting evidence (Table 5), providing a clear hierarchy for MERS-CoV therapeutic development. Molecules are classified into diagnostic, therapeutic, and immunomodulatory roles, while host-derived adjuvants are grouped based on functional properties such as chemokines and cytokines.
Host-derived adjuvants is an area with much potential impact on vaccine development. The identified high-priority therapeutic targets including CXCL10/IP10 and IL-6 warrant monoclonal antibody development and clinical trials to reduce immunopathology and improve clinical outcomes. Chemokines such as CCL5, CCL27 and CXCL8 can be used as prognostic biomarkers. High-priority adjuvants such as CD40L, CXCL1, HBD-2, LL-37 and GM-CSF have higher criteria as immune adjuvants which can be a precise implementation of clinical trials. HBD-2 possesses multiple functions involved in determining innate and adaptive immunity: it has a direct antimicrobial function and can act against a broad range of pathogens by disrupting membrane integrity, acts as a chemotactic factor for neutrophils and T lymphocytes, promotes the maturation of dendritic cells for enhancing the presentation of antigens, modulates signaling pathways and inflammatory response, and also stimulates the production of pro-inflammatory cytokines for amplifying immune responses. On the other hand, CD40L (CD154) is a co-stimulatory protein expressed on activated T cells, and its interaction with CD40 receptors on antigen-presenting cells (APCs) stimulates them and increases their ability to present antigens. This interaction induces B cell proliferation and antibody production, dendritic cell maturation and secretions of cytokines IL-12, which is essential for T helper cell differentiation. Taken together, the unique mechanisms of HBD-2 and CD40L make them useful and excellent candidates as adjuvants in the design of safer and more effective MERS-CoV vaccine. Future studies should focus on clinical trials with adjuvants of human origin, and exploration of new biomarkers of disease progression that may help to elucidate the precise mechanisms of MERS-CoV immunity which can inform the rational development of vaccines utilizing human-derived adjuvants. These studies should focus on their ability to enhance both systemic and mucosal immunity.
Author contributions
AA: Data curation, Investigation, Software, Writing – original draft, Writing – review & editing. MA: Investigation, Supervision, Validation, Writing – original draft, Writing – review & editing. HA: Data curation, Investigation, Methodology, Writing – review & editing. MA: Data curation, Investigation, Resources, Writing – original draft. AA: Data curation, Investigation, Resources, Writing – original draft. IQ: Conceptualization, Project administration, Visualization, Writing – review & editing. BA: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to thank the Research Center at King Fahad Medical City for their valuable technical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus ADME, Fouchier RAM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. New Engl J Med. (2012) 367(19):1814–20. doi: 10.1056/NEJMoa1211721
2. Chan JF, Lau SK, To KK, Cheng VC, Woo PC, Yuen K-Y. Middle east respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin Microbiol Rev. (2015) 28(2):465–522. doi: 10.1128/CMR.00102-14
3. Cowling BJ, Park M, Fang VJ, Wu P, Leung GM, Wu JT. Preliminary epidemiological assessment of MERS-CoV outbreak in south korea, may to june 2015. Euro Surveill. (2015) 20(25):7–13. doi: 10.2807/1560-7917.ES2015.20.25.21163
4. Singh SK. Middle East respiratory syndrome virus pathogenesis. Semin Respir Crit Care Med. (2016) 37(04):572–7. doi: 10.1055/s-0036-1584796
5. Killerby ME, Biggs HM, Midgley CM, Gerber SI, Watson JT. Middle east respiratory syndrome coronavirus transmission. Emerg Infect Dis. (2020) 26(2):191–8. doi: 10.3201/eid2602.190697
6. Conzade R, Grant R, Malik MR, Elkholy A, Elhakim M, Samhouri D, et al. Reported direct and indirect contact with dromedary camels among laboratory-confirmed MERS-CoV cases. Viruses. (2018) 10(8):425. doi: 10.3390/v10080425
7. WHO. MERS outbreaks 2024. . Available at: https://www.emro.who.int/health-topics/mers-cov/mers-outbreaks.html (Accessed September 25, 2024).
8. Arabi YM, Balkhy HH, Hayden FG, Bouchama A, Luke T, Baillie JK, et al. Middle east respiratory syndrome. New Engl J Med. (2017) 376(6):584–94. doi: 10.1056/NEJMsr1408795
9. Choudhry H, Bakhrebah MA, Abdulaal WH, Zamzami MA, Baothman OA, Hassan MA, et al. Middle east respiratory syndrome: pathogenesis and therapeutic developments. Future Virology. (2019) 14(4):237–46. doi: 10.2217/fvl-2018-0201
10. Azhar EI, Hui DSC, Memish ZA, Drosten C, Zumla A. The middle east respiratory syndrome (MERS). Infect Dis Clinics North America. (2019) 33(4):891–905. doi: 10.1016/j.idc.2019.08.001
11. Lau SKP, Lau CCY, Chan K-H, Li CPY, Chen H, Jin D-Y, et al. Delayed induction of proinflammatory cytokines and suppression of innate antiviral response by the novel middle east respiratory syndrome coronavirus: implications for pathogenesis and treatment. J Gen Virology. (2013) 94(12):2679–90. doi: 10.1099/vir.0.055533-0
12. Krammer F. SARS-CoV-2 vaccines in development. Nature. (2020) 586(7830):516–27. doi: 10.1038/s41586-020-2798-3
13. Crank MC, Ruckwardt TJ, Chen M, Morabito KM, Phung E, Costner PJ, et al. A proof of concept for structure-based vaccine design targeting RSV in humans. Science. (2019) 365(6452):505–9. doi: 10.1126/science.aav9033
14. Muhammad L, Sahibzada Nawazash A, Zainab K, Iqra A, Sagar MG, Muhammad Shahid M. Toll-like receptors and emerging viral infections. In: Vijay K, editor. Thirty years since the discovery of toll-like receptors. Rijeka: IntechOpen (2024). p. Ch. 3.
15. Hong K-H, Choi J-P, Hong S-H, Lee J, Kwon J-S, Kim S-M, et al. Predictors of mortality in middle east respiratory syndrome (MERS). Thorax. (2018) 73(3):286. doi: 10.1136/thoraxjnl-2016-209313
16. Min C-K, Cheon S, Ha N-Y, Sohn KM, Kim Y, Aigerim A, et al. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci Rep. (2016) 6(1):25359. doi: 10.1038/srep25359
17. Elemam NM, Talaat IM, Maghazachi AA. CXCL10 chemokine: A critical player in RNA and DNA viral infections. Viruses. (2022) 14(11):2445. doi: 10.3390/v14112445
18. Trifilo MJ, Montalto-Morrison C, Stiles LN, Hurst KR, Hardison JL, Manning JE, et al. CXC chemokine ligand 10 controls viral infection in the central nervous system: evidence for a role in innate immune response through recruitment and activation of natural killer cells. J Virol. (2004) 78(2):585–94. doi: 10.1128/JVI.78.2.585-594.2004
19. Wang W, Yang P, Zhong Y, Zhao Z, Xing L, Zhao Y, et al. Monoclonal antibody against CXCL-10/IP-10 ameliorates influenza a (H1N1) virus induced acute lung injury. Cell Res. (2013) 23(4):577–80. doi: 10.1038/cr.2013.25
20. Alhetheel A, Albarrag A, Shakoor Z, Somily A, Barry M, Altalhi H, et al. Chemokine levels among patients with middle east respiratory syndrome coronavirus infection. Vaccines. (2023) 11(6):1048. doi: 10.3390/vaccines11061048
21. Shin H-S, Kim Y, Kim G, Lee JY, Jeong I, Joh J-S, et al. Immune responses to middle east respiratory syndrome coronavirus during the acute and convalescent phases of human infection. Clin Infect Diseases. (2019) 68(6):984–92. doi: 10.1093/cid/ciy595
22. Sierra B, Pérez AB, Aguirre E, Bracho C, Valdés O, Jimenez N, et al. Association of early nasopharyngeal immune markers with COVID-19 clinical outcome: Predictive value of CCL2/MCP-1. Open Forum Infect Dis. (2020) 7(10):ofaa407. doi: 10.1093/ofid/ofaa407
23. Singh S, Anshita D, Ravichandiran V. MCP-1: Function, regulation, and involvement in disease. Int Immunopharmacol. (2021) 101(Pt B):107598. doi: 10.1016/j.intimp.2021.107598
24. Zhang D, Guo R, Lei L, Liu H, Wang Y, Wang Y, et al. COVID-19 infection induces readily detectable morphological and inflammation-related phenotypic changes in peripheral blood monocytes, the severity of which correlate with patient outcome. medRxiv. (2020), 109(1):2020.03.24.20042655. doi: 10.1101/2020.03.24.20042655
25. Jafarzadeh A, Chauhan P, Saha B, Jafarzadeh S, Nemati M. Contribution of monocytes and macrophages to the local tissue inflammation and cytokine storm in COVID-19: Lessons from SARS and MERS, and potential therapeutic interventions. Life Sci. (2020) 257:118102. doi: 10.1016/j.lfs.2020.118102
26. Chirathaworn C, Poovorawan Y, Lertmaharit S, Wuttirattanakowit N. Cytokine levels in patients with chikungunya virus infection. Asian Pacific J Trop Med. (2013) 6(8):631–4. doi: 10.1016/S1995-7645(13)60108-X
27. Chen Y, Wang J, Liu C, Su L, Zhang D, Fan J, et al. IP-10 and MCP-1 as biomarkers associated with disease severity of COVID-19. Mol Med. (2020) 26(1):97. doi: 10.1186/s10020-020-00230-x
28. Tsaur I, Noack A, Makarevic J, Oppermann E, Waaga-Gasser AM, Gasser M, et al. CCL2 chemokine as a potential biomarker for prostate cancer: a pilot study. Cancer Res Treatment: Off J Korean Cancer Assoc. (2015) 47(2):306–12. doi: 10.4143/crt.2014.015
29. Hamed ME, Naeem A, Alkadi H, Alamri AA, AlYami AS, AlJuryyan A, et al. Elevated expression levels of lung complement anaphylatoxin, neutrophil chemoattractant chemokine IL-8, and RANTES in MERS-CoV-Infected patients: Predictive biomarkers for disease severity and mortality. J Clin Immunol. (2021) 41(7):1607–20. doi: 10.1007/s10875-021-01061-z
30. Alosaimi B, Hamed ME, Naeem A, Alsharef AA, AlQahtani SY, AlDosari KM, et al. MERS-CoV infection is associated with downregulation of genes encoding Th1 and Th2 cytokines/chemokines and elevated inflammatory innate immune response in the lower respiratory tract. Cytokine. (2020) 126:154895. doi: 10.1016/j.cyto.2019.154895
31. Khalil BA, Elemam NM, Maghazachi AA. Chemokines and chemokine receptors during COVID-19 infection. Comput Struct Biotechnol J. (2021) 19:976–88. doi: 10.1016/j.csbj.2021.01.034
32. Chu H, Zhou J, Wong BH-Y, Li C, Chan JF-W, Cheng Z-S, et al. Middle east respiratory syndrome coronavirus efficiently infects human primary t lymphocytes and activates the extrinsic and intrinsic apoptosis pathways. J Infect Diseases. (2016) 213(6):904–14. doi: 10.1093/infdis/jiv380
33. Bao Z, Ye Q, Gong W, Xiang Y, Wan H. Humanized monoclonal antibody against the chemokine CXCL-8 (IL-8) effectively prevents acute lung injury. Int Immunopharmacology. (2010) 10(2):259–63. doi: 10.1016/j.intimp.2009.11.005
34. Schall TJ, Bacon K, Toy KJ, Goeddel DV. Selective attraction of monocytes and t lymphocytes of the memory phenotype by cytokine RANTES. Nature. (1990) 347(6294):669–71. doi: 10.1038/347669a0
35. Machlus KR, Johnson KE, Kulenthirarajan R, Forward JA, Tippy MD, Soussou TS, et al. CCL5 derived from platelets increases megakaryocyte proplatelet formation. Blood J Am Soc Hematology. (2016) 127(7):921–6. doi: 10.1182/blood-2015-05-644583
36. Pan Z-Z, Parkyn L, Ray A, Ray P. Inducible lung-specific expression of RANTES: preferential recruitment of neutrophils. Am J Physiology-Lung Cell Mol Physiol. (2000) 279(4):L658–L66. doi: 10.1152/ajplung.2000.279.4.L658
37. Williams AE, Chambers RC. The mercurial nature of neutrophils: still an enigma in ARDS? Am J Physiol Lung Cell Mol Physiol. (2014) 306(3):L217–30. doi: 10.1152/ajplung.00311.2013
38. Culley FJ, Pennycook AMJ, Tregoning JS, Dodd JS, Walzl G, Wells TN, et al. Role of CCL5 (RANTES) in viral lung disease. J Virology. (2006) 80(16):8151–7. doi: 10.1128/JVI.00496-06
39. Zhou J, Chu H, Li C, Wong BH-Y, Cheng Z-S, Poon VK-M, et al. Active replication of middle east respiratory syndrome coronavirus and aberrant induction of inflammatory cytokines and chemokines in human macrophages: Implications for pathogenesis. J Infect Dis. (2013) 209(9):1331–42. doi: 10.1093/infdis/jit504
40. Cheung CY, Poon LL, Ng IH, Luk W, Sia S-F, Wu MH, et al. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J Virology. (2005) 79(12):7819–26. doi: 10.1128/JVI.79.12.7819-7826.2005
41. Yoshikawa T, Hill T, Li K, Peters CJ, Tseng C-TK. Severe acute respiratory syndrome (SARS) coronavirus-induced lung epithelial cytokines exacerbate SARS pathogenesis by modulating intrinsic functions of monocyte-derived macrophages and dendritic cells. J Virology. (2009) 83(7):3039–48. doi: 10.1128/JVI.01792-08
42. Tincati C, Cannizzo ES, Giacomelli M, Badolato R, d’Arminio Monforte A, Marchetti G. Heightened circulating interferon-inducible chemokines, and activated pro-cytolytic Th1-cell phenotype features covid-19 aggravation in the second week of illness. Front Immunol. (2020) 11:580987. doi: 10.3389/fimmu.2020.580987
43. Chen Y, Wang J, Liu C, Su L, Zhang D, Fan J, et al. IP-10 and MCP-1 as biomarkers associated with disease severity of COVID-19. Mol Med. (2020) 26:1–12. doi: 10.1186/s10020-020-00230-x
44. Bodnar RJ, Yates CC, Wells A. IP-10 blocks vascular endothelial growth factor-induced endothelial cell motility and tube formation via inhibition of calpain. Circ Res. (2006) 98(5):617–25. doi: 10.1161/01.RES.0000209968.66606.10
45. Romano M, Sironi M, Toniatti C, Polentarutti N, Fruscella P, Ghezzi P, et al. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. (1997) 6(3):315–25. doi: 10.1016/S1074-7613(00)80334-9
46. van den Borne P, Quax PH, Hoefer IE, Pasterkamp G. The multifaceted functions of CXCL10 in cardiovascular disease. BioMed Res Int. (2014) 2014(1):893106. doi: 10.1155/2014/893106
47. Pol JG, Workenhe ST, Konda P, Gujar S, Kroemer G. Cytokines in oncolytic virotherapy. Cytokine Growth Factor Rev. (2020) 56:4–27. doi: 10.1016/j.cytogfr.2020.10.007
48. Li M, Chen Y, Li H, Yang D, Zhou Y, Chen Z, et al. Serum CXCL10/IP-10 may be a potential biomarker for severe mycoplasma pneumoniae pneumonia in children. BMC Infect Diseases. (2021) 21(1):909. doi: 10.1186/s12879-021-06632-4
49. Naveca FG, Pontes GS, Chang AY-H, Silva GAVD, Nascimento VAD, Monteiro DCDS, et al. Analysis of the immunological biomarker profile during acute zika virus infection reveals the overexpression of CXCL10, a chemokine linked to neuronal damage. do Instituto Oswaldo Cruz. (2018) 113:e170542. doi: 10.1590/0074-02760170542
50. Yu X, Chen Y, Cui L, Yang K, Wang X, Lei L, et al. CXCL8, CXCL9, CXCL10, and CXCL11 as biomarkers of liver injury caused by chronic hepatitis b. Front Microbiol. (2022) 13. doi: 10.3389/fmicb.2022.1052917
51. Qi WQ, Zhang Q, Wang JB. CXCL8 is a potential biomarker for predicting disease progression in gastric carcinoma. Transl Cancer Res. (2020) 9(2):1053–62. doi: 10.21037/tcr.2019.12.52
52. Hu L, Zhu Y, Zhang J, Chen W, Li Z, Li L, et al. Potential circulating biomarkers of circulating chemokines CCL5, MIP-1β and HA as for early detection of cirrhosis related to chronic HBV (hepatitis B virus) infection. BMC Infect Diseases. (2019) 19(1):523. doi: 10.1186/s12879-019-4130-0
53. Huang Y, Wu L, Sun Y, Li J, Mao N, Yang Y, et al. CCL5 might be a prognostic biomarker and associated with immuno-therapeutic efficacy in cancers: A pan-cancer analysis. Heliyon. (2023) 9(7):e18215. doi: 10.1016/j.heliyon.2023.e18215
54. Kim ES, Choe PG, Park WB, Oh HS, Kim EJ, Nam EY, et al. Clinical progression and cytokine profiles of middle east respiratory syndrome coronavirus infection. J Korean Med Science. (2016) 31(11):1717. doi: 10.3346/jkms.2016.31.11.1717
55. Gubernatorova EO, Gorshkova EA, Polinova AI, Drutskaya MS. IL-6: Relevance for immunopathology of SARS-CoV-2. Cytokine Growth Factor Rev. (2020) 53:13–24. doi: 10.1016/j.cytogfr.2020.05.009
56. Indalao IL, Sawabuchi T, Takahashi E, Kido H. IL-1β is a key cytokine that induces trypsin upregulation in the influenza virus–cytokine–trypsin cycle. Arch Virology. (2017) 162:201–11. doi: 10.1007/s00705-016-3093-3
57. Velazquez-Salinas L, Verdugo-Rodriguez A, Rodriguez LL, Borca MV. The role of interleukin 6 during viral infections. Front Microbiol. (2019) 10:1057. doi: 10.3389/fmicb.2019.01057
58. Hou W, Kang HS, Kim BS. Th17 cells enhance viral persistence and inhibit T cell cytotoxicity in a model of chronic virus infection. J Exp Med. (2009) 206(2):313–28. doi: 10.1084/jem.20082030
59. Barnes BJ, Adrover JM, Baxter-Stoltzfus A, Borczuk A, Cools-Lartigue J, Crawford JM, et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med. (2020) 217(6):e20200652. doi: 10.1084/jem.20200652
60. Price CC, Altice FL, Shyr Y, Koff A, Pischel L, Goshua G, et al. Tocilizumab treatment for cytokine release syndrome in hospitalized patients with coronavirus disease 2019: Survival and clinical outcomes. Chest. (2020) 158(4):1397–408. doi: 10.1016/j.chest.2020.06.006
61. Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, Menozzi M, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. (2020) 2(8):e474–e84. doi: 10.1016/S2665-9913(20)30173-9
62. Zhang YY, Li BR, Ning BT. The comparative immunological characteristics of SARS-CoV, MERS-CoV, and SARS-CoV-2 coronavirus infections. Front Immunol. (2020) 11:2033. doi: 10.3389/fimmu.2020.02033
63. Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. (2020) 130(5):2620–9. doi: 10.1172/JCI137244
64. Zhang Y, Li J, Zhan Y, Wu L, Yu X, Zhang W, et al. Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infection Immunity. (2004) 72(8):4410–5. doi: 10.1128/IAI.72.8.4410-4415.2004
65. Han H, Ma Q, Li C, Liu R, Zhao L, Wang W, et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerging Microbes Infections. (2020) 9(1):1123–30. doi: 10.1080/22221751.2020.1770129
66. He S, Zhou C, Lu D, Yang H, Xu H, Wu G, et al. Relationship between chest CT manifestations and immune response in COVID-19 patients. Int J Infect Diseases. (2020) 98:125–9. doi: 10.1016/j.ijid.2020.06.059
67. Herold T, Jurinovic V, Arnreich C, Lipworth BJ, Hellmuth JC, von Bergwelt-Baildon M, et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. (2020) 146(1):128–36.e4. doi: 10.1016/j.jaci.2020.05.008
68. Liu Y, Liao W, Wan L, Xiang T, Zhang W. Correlation between relative nasopharyngeal virus RNA load and lymphocyte count disease severity in patients with COVID-19. Viral Immunol. (2021) 34(5):330–5. doi: 10.1089/vim.2020.0062
69. Luo M, Liu J, Jiang W, Yue S, Liu H, Wei S. IL-6 and CD8+ T cell counts combined are an early predictor of in-hospital mortality of patients with COVID-19. JCI Insight. (2020) 5(13):e139024. doi: 10.1172/jci.insight.139024
70. McElvaney OJ, McEvoy NL, McElvaney OF, Carroll TP, Murphy MP, Dunlea DM, et al. Characterization of the inflammatory response to severe COVID-19 illness. Am J Respir Crit Care Med. (2020) 202(6):812–21. doi: 10.1164/rccm.202005-1583OC
71. Chen R, Sang L, Jiang M, Yang Z, Jia N, Fu W, et al. Longitudinal hematologic and immunologic variations associated with the progression of COVID-19 patients in china. J Allergy Clin Immunol. (2020) 146(1):89–100. doi: 10.1016/j.jaci.2020.05.003
72. Santa Cruz A, Mendes-Frias A, Oliveira AI, Dias L, Matos AR, Carvalho A, et al. Interleukin-6 is a biomarker for the development of fatal severe acute respiratory syndrome coronavirus 2 pneumonia. Front Immunol. (2021) 12:613422. doi: 10.3389/fimmu.2021.613422
73. Wang X, Li J, Liu W, Zhang X, Xue L. The diagnostic value of interleukin 6 as a biomarker for gastric cancer: A meta-analysis and systematic review. Med (Baltimore). (2021) 100(47):e27945. doi: 10.1097/MD.0000000000027945
74. Wang R, Xiao H, Guo R, Li Y, Shen B. The role of C5a in acute lung injury induced by highly pathogenic viral infections. Emerging Microbes Infections. (2015) 4(1):1–7. doi: 10.1038/emi.2015.28
75. Noris M, Remuzzi G. Overview of complement activation and regulation. Semin Nephrol. (2013) 33(6):479–92. doi: 10.1016/j.semnephrol.2013.08.001
76. Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Trans Res. (2020) 220:1–13. doi: 10.1016/j.trsl.2020.04.007
77. Marchetti M. COVID-19-driven endothelial damage: complement, HIF-1, and ABL2 are potential pathways of damage and targets for cure. Ann Hematology. (2020) 99(8):1701–7. doi: 10.1007/s00277-020-04138-8
78. Riedl M, Fakhouri F, Le Quintrec M, Noone DG, Jungraithmayr TC, Fremeaux-Bacchi V, et al. Spectrum of complement-mediated thrombotic microangiopathies: Pathogenetic insights identifying novel treatment approaches. Semin Thromb Hemost. (2014) 40(04):444–64. doi: 10.1055/s-0034-1376153
79. Diurno F, Numis F, Porta G, Cirillo F, Maddaluno S, Ragozzino A, et al. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 nord experience. Eur Rev Med Pharmacol Sci. (2020) 24(7):4040–7. doi: 10.26355/eurrev_202004_20875
80. Jodele S, Medvedovic M, Luebbering N, Chen J, Dandoy CE, Laskin BL, et al. Interferon-complement loop in transplant-associated thrombotic microangiopathy. Blood Adv. (2020) 4(6):1166–77. doi: 10.1182/bloodadvances.2020001515
81. Jiang Y, Zhao G, Song N, Li P, Chen Y, Guo Y, et al. Blockade of the C5a-C5aR axis alleviates lung damage in hDPP4-transgenic mice infected with MERS-CoV. Emerg Microbes Infect. (2018) 7(1):77. doi: 10.1038/s41426-018-0063-8
82. Guo R-F, Ward PA. Role of C5a in inflammatory responses. Annu Rev Immunol. (2005) 23(1):821–52. doi: 10.1146/annurev.immunol.23.021704.115835
83. Daffern PJ, Pfeifer PH, Ember JA, Hugli TE. C3a is a chemotaxin for human eosinophils but not for neutrophils. I. C3a stimulation of neutrophils is secondary to eosinophil activation. J Exp Med. (1995) 181(6):2119–27.
84. Alosaimi B, Mubarak A, Hamed ME, Almutairi AZ, Alrashed AA, AlJuryyan A, et al. Complement anaphylatoxins and inflammatory cytokines as prognostic markers for COVID-19 severity and in-hospital mortality. Front Immunol. (2021) 12:668725. doi: 10.3389/fimmu.2021.668725
85. Aguirre F, Manin A, Fernandez VC, Justo ME, Leoni J, Paz ML, et al. C3, C5a and anti-acetylcholine receptor antibody as severity biomarkers in myasthenia gravis. Ther Adv Neurological Disord. (2020) 13:1756286420935697. doi: 10.1177/1756286420935697
86. Kanmura S, Uto H, Sato Y, Kumagai K, Sasaki F, Moriuchi A, et al. The complement component C3a fragment is a potential biomarker for hepatitis c virus-related hepatocellular carcinoma. J Gastroenterol. (2010) 45(4):459–67. doi: 10.1007/s00535-009-0160-5
87. Sadler AJ, Williams BR. Interferon-inducible antiviral effectors. Nat Rev Immunol. (2008) 8(7):559–68. doi: 10.1038/nri2314
88. Zielecki F, Weber M, Eickmann M, Spiegelberg L, Zaki AM, Matrosovich M, et al. Human cell tropism and innate immune system interactions of human respiratory coronavirus EMC compared to those of severe acute respiratory syndrome coronavirus. J Virol. (2013) 87(9):5300–4. doi: 10.1128/JVI.03496-12
89. Falzarano D, de Wit E, Rasmussen AL, Feldmann F, Okumura A, Scott DP, et al. Treatment with interferon-α2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat Med. (2013) 19(10):1313–7. doi: 10.1038/nm.3362
90. Al-Tawfiq JA, Momattin H, Dib J, Memish ZA. Ribavirin and interferon therapy in patients infected with the middle east respiratory syndrome coronavirus: an observational study. Int J Infect Diseases. (2014) 20:42–6. doi: 10.1016/j.ijid.2013.12.003
91. Shalhoub S, Farahat F, Al-Jiffri A, Simhairi R, Shamma O, Siddiqi N, et al. IFN-α2a or IFN-β1a in combination with ribavirin to treat middle east respiratory syndrome coronavirus pneumonia: a retrospective study. J Antimicrob Chemother. (2015) 70(7):2129–32. doi: 10.1093/jac/dkv085
92. Omrani AS, Saad MM, Baig K, Bahloul A, Abdul-Matin M, Alaidaroos AY, et al. Ribavirin and interferon alfa-2a for severe middle east respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis. (2014) 14(11):1090–5. doi: 10.1016/S1473-3099(14)70920-X
93. Arabi YM, Shalhoub S, Mandourah Y, Al-Hameed F, Al-Omari A, Al Qasim E, et al. Ribavirin and interferon therapy for critically ill patients with middle east respiratory syndrome: A multicenter observational study. Clin Infect Dis. (2020) 70(9):1837–44. doi: 10.1093/cid/ciz544
94. Chan JF-W, Yao Y, Yeung M-L, Deng W, Bao L, Jia L, et al. Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J Infect Diseases. (2015) 212(12):1904–13. doi: 10.1093/infdis/jiv392
95. Wu W, Wang JF, Liu PM, Chen WX, Yin SM, Jiang SP, et al. [Clinical features of 96 patients with severe acute respiratory syndrome from a hospital outbreak]. Zhonghua Nei Ke Za Zhi. (2003) 42(7):453–7.
96. Loutfy MR, Blatt LM, Siminovitch KA, Ward S, Wolff B, Lho H, et al. Interferon alfacon-1 plus corticosteroids in severe acute respiratory SyndromeA preliminary study. JAMA. (2003) 290(24):3222–8. doi: 10.1001/jama.290.24.3222
97. Zhao Z, Zhang F, Xu M, Huang K, Zhong W, Cai W, et al. Description and clinical treatment of an early outbreak of severe acute respiratory syndrome (SARS) in guangzhou, PR china. J Med Microbiol. (2003) 52(8):715–20. doi: 10.1099/jmm.0.05320-0
98. Qiu H, Sun S, Xiao H, Feng J, Guo Y, Tai W, et al. Single-dose treatment with a humanized neutralizing antibody affords full protection of a human transgenic mouse model from lethal middle east respiratory syndrome (MERS)-coronavirus infection. Antiviral Res. (2016) 132:141–8. doi: 10.1016/j.antiviral.2016.06.003
99. Li Y, Wan Y, Liu P, Zhao J, Lu G, Qi J, et al. A humanized neutralizing antibody against MERS-CoV targeting the receptor-binding domain of the spike protein. Cell Res. (2015) 25(11):1237–49. doi: 10.1038/cr.2015.113
100. Johnson RF, Bagci U, Keith L, Tang X, Mollura DJ, Zeitlin L, et al. 3B11-n, a monoclonal antibody against MERS-CoV, reduces lung pathology in rhesus monkeys following intratracheal inoculation of MERS-CoV jordan-n3/2012. Virology. (2016) 490:49–58. doi: 10.1016/j.virol.2016.01.004
101. Zhao G, He L, Sun S, Qiu H, Tai W, Chen J, et al. A novel nanobody targeting middle east respiratory syndrome coronavirus (MERS-CoV) receptor-binding domain has potent cross-neutralizing activity and protective efficacy against MERS-CoV. J Virol. (2018) 92(18):e00837–18. doi: 10.1128/JVI.00837-18
102. Channappanavar R, Lu L, Xia S, Du L, Meyerholz DK, Perlman S, et al. Protective effect of intranasal regimens containing peptidic middle east respiratory syndrome coronavirus fusion inhibitor against MERS-CoV infection. J Infect Dis. (2015) 212(12):1894–903. doi: 10.1093/infdis/jiv325
103. Luke T, Wu H, Zhao J, Channappanavar R, Coleman CM, Jiao J-A, et al. Human polyclonal immunoglobulin g from transchromosomic bovines inhibits MERS-CoV in vivo. Sci Trans Med. (2016) 8(326):326ra21–ra21. doi: 10.1126/scitranslmed.aaf1061
104. Beigel JH, Voell J, Kumar P, Raviprakash K, Wu H, Jiao JA, et al. Safety and tolerability of a novel, polyclonal human anti-MERS coronavirus antibody produced from transchromosomic cattle: a phase 1 randomised, double-blind, single-dose-escalation study. Lancet Infect Dis. (2018) 18(4):410–8. doi: 10.1016/S1473-3099(18)30002-1
105. Ying T, Du L, Ju TW, Prabakaran P, Lau CC, Lu L, et al. Exceptionally potent neutralization of middle east respiratory syndrome coronavirus by human monoclonal antibodies. J Virol. (2014) 88(14):7796–805. doi: 10.1128/JVI.00912-14
106. Houser KV, Gretebeck L, Ying T, Wang Y, Vogel L, Lamirande EW, et al. Prophylaxis with a middle east respiratory syndrome coronavirus (MERS-CoV)–specific human monoclonal antibody protects rabbits from MERS-CoV infection. J Infect Diseases. (2016) 213(10):1557–61. doi: 10.1093/infdis/jiw080
107. Agrawal AS, Ying T, Tao X, Garron T, Algaissi A, Wang Y, et al. Passive transfer of a germline-like neutralizing human monoclonal antibody protects transgenic mice against lethal middle east respiratory syndrome coronavirus infection. Sci Rep. (2016) 6(1):31629. doi: 10.1038/srep31629
108. van Doremalen N, Falzarano D, Ying T, de Wit E, Bushmaker T, Feldmann F, et al. Efficacy of antibody-based therapies against middle east respiratory syndrome coronavirus (MERS-CoV) in common marmosets. Antiviral Res. (2017) 143:30–7. doi: 10.1016/j.antiviral.2017.03.025
109. Corti D, Zhao J, Pedotti M, Simonelli L, Agnihothram S, Fett C, et al. Prophylactic and postexposure efficacy of a potent human monoclonal antibody against MERS coronavirus. Proc Natl Acad Sci USA. (2015) 112(33):10473–8. doi: 10.1073/pnas.1510199112
110. Zhao J, Li K, Wohlford-Lenane C, Agnihothram SS, Fett C, Gale MJ Jr., et al. Rapid generation of a mouse model for middle east respiratory syndrome. Proc Natl Acad Sci USA. (2014) 111(13):4970–5. doi: 10.1073/pnas.1323279111
111. Pascal KE, Coleman CM, Mujica AO, Kamat V, Badithe A, Fairhurst J, et al. Pre- and postexposure efficacy of fully human antibodies against spike protein in a novel humanized mouse model of MERS-CoV infection. Proc Natl Acad Sci USA. (2015) 112(28):8738–43. doi: 10.1073/pnas.1510830112
112. de Wit E, Feldmann F, Okumura A, Horne E, Haddock E, Saturday G, et al. Prophylactic and therapeutic efficacy of mAb treatment against MERS-CoV in common marmosets. Antiviral Res. (2018) 156:64–71. doi: 10.1016/j.antiviral.2018.06.006
113. Chen Z, Bao L, Chen C, Zou T, Xue Y, Li F, et al. Human neutralizing monoclonal antibody inhibition of middle east respiratory syndrome coronavirus replication in the common marmoset. J Infect Dis. (2017) 215(12):1807–15. doi: 10.1093/infdis/jix209
114. Jiang L, Wang N, Zuo T, Shi X, Poon K-MV, Wu Y, et al. Potent neutralization of MERS-CoV by human neutralizing monoclonal antibodies to the viral spike glycoprotein. Sci Trans Med. (2014) 6(234):234ra59–ra59. doi: 10.1126/scitranslmed.3008140
115. Niu P, Zhang S, Zhou P, Huang B, Deng Y, Qin K, et al. Ultrapotent human neutralizing antibody repertoires against middle east respiratory syndrome coronavirus from a recovered patient. J Infect Dis. (2018) 218(8):1249–60. doi: 10.1093/infdis/jiy311
116. Niu P, Zhao G, Deng Y, Sun S, Wang W, Zhou Y, et al. A novel human mAb (MERS-GD27) provides prophylactic and postexposure efficacy in MERS-CoV susceptible mice. Sci China Life Sci. (2018) 61(10):1280–2. doi: 10.1007/s11427-018-9343-8
117. Otsubo R, Yasui T. Monoclonal antibody therapeutics for infectious diseases: Beyond normal human immunoglobulin. Pharmacol Ther. (2022) 240:108233. doi: 10.1016/j.pharmthera.2022.108233
118. Dreyfus C, Laursen NS, Kwaks T, Zuijdgeest D, Khayat R, Ekiert DC, et al. Highly conserved protective epitopes on influenza B viruses. Science. (2012) 337(6100):1343–8. doi: 10.1126/science.1222908
119. Throsby M, van den Brink E, Jongeneelen M, Poon LL, Alard P, Cornelissen L, et al. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PloS One. (2008) 3(12):e3942. doi: 10.1371/journal.pone.0003942
120. Li GM, Chiu C, Wrammert J, McCausland M, Andrews SF, Zheng NY, et al. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci USA. (2012) 109(23):9047–52. doi: 10.1073/pnas.1118979109
121. Nachbagauer R, Shore D, Yang H, Johnson SK, Gabbard JD, Tompkins SM, et al. Broadly reactive human monoclonal antibodies elicited following pandemic H1N1 influenza virus exposure protect mice against highly pathogenic H5N1 challenge. J Virol. (2018) 92(16):e00949–18. doi: 10.1128/JVI.00949-18
122. Kim J, Yang YL, Jeong Y, Jang YS. Conjugation of human β-defensin 2 to spike protein receptor-binding domain induces antigen-specific protective immunity against middle east respiratory syndrome coronavirus infection in human dipeptidyl peptidase 4 transgenic mice. Vaccines (Basel). (2020) 8(4):635. doi: 10.3390/vaccines8040635
123. Yang D, Chertov O, Bykovskaia S, Chen Q, Buffo M, Shogan J, et al. β-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. (1999) 286(5439):525–8. doi: 10.1126/science.286.5439.525
124. Biragyn A, Ruffini PA, Leifer CA, Klyushnenkova E, Shakhov A, Chertov O, et al. Toll-like receptor 4-dependent activation of dendritic cells by β-defensin 2. Science. (2002) 298(5595):1025–9. doi: 10.1126/science.1075565
125. Biragyn A, Belyakov IM, Chow Y-H, Dimitrov DS, Berzofsky JA, Kwak LW. DNA vaccines encoding human immunodeficiency virus–1 glycoprotein 120 fusions with proinflammatory chemoattractants induce systemic and mucosal immune responses. Blood. (2002) 100(4):1153–9. doi: 10.1182/blood-2002-01-0086
126. Ma DY, Clark EA. The role of CD40 and CD154/CD40L in dendritic cells. Semin Immunol. (2009) 21(5):265–72. doi: 10.1016/j.smim.2009.05.010
127. van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukocyte Biol. (2000) 67(1):2–17. doi: 10.1002/jlb.67.1.2
128. Bishop GA, Hostager BS. The CD40–CD154 interaction in B cell–T cell liaisons. Cytokine Growth Factor Rev. (2003) 14(3):297–309. doi: 10.1016/S1359-6101(03)00024-8
129. Allen RC, Armitage RJ, Conley ME, Rosenblatt H, Jenkins NA, Copeland NG, et al. CD40 ligand gene defects responsible for x-linked hyper-IgM syndrome. Science. (1993) 259(5097):990–3. doi: 10.1126/science.7679801
130. Hashem AM, Algaissi A, Agrawal AS, Al-Amri SS, Alhabbab RY, Sohrab SS, et al. A highly immunogenic, protective, and safe adenovirus-based vaccine expressing middle east respiratory syndrome coronavirus S1-CD40L fusion protein in a transgenic human dipeptidyl peptidase 4 mouse model. J Infect Dis. (2019) 220(10):1558–67. doi: 10.1093/infdis/jiz137
131. Kim S-H, Yang I-Y, Kim J, Lee K-Y, Jang Y-S. Antimicrobial peptide LL-37 promotes antigen-specific immune responses in mice by enhancing Th17-skewed mucosal and systemic immunities. Eur J Immunol. (2015) 45(5):1402–13. doi: 10.1002/eji.201444988
132. Seil M, Nagant C, Dehaye J-P, Vandenbranden M, Lensink MF. Spotlight on human LL-37, an immunomodulatory peptide with promising cell-penetrating properties. Pharmaceuticals. (2010) 3(11):3435–60. doi: 10.3390/ph3113435
133. Nijnik A, Pistolic J, Wyatt A, Tam S, Hancock RE. Human cathelicidin peptide LL-37 modulates the effects of IFN-γ on APCs. J Immunol. (2009) 183(9):5788–98. doi: 10.4049/jimmunol.0901491
134. Reinholz M, Ruzicka T, Schauber J. Cathelicidin LL-37: an antimicrobial peptide with a role in inflammatory skin disease. Ann Dermatol. (2012) 24(2):126–35. doi: 10.5021/ad.2012.24.2.126
135. Kim J, Yang YL, Jeong Y, Jang YS. Application of antimicrobial peptide LL-37 as an adjuvant for middle east respiratory syndrome-coronavirus antigen induces an efficient protective immune response against viral infection after intranasal immunization. Immune Netw. (2022) 22(5):e41. doi: 10.4110/in.2022.22.e41
136. Egan MA, Israel ZR. The use of cytokines and chemokines as genetic adjuvants for plasmid DNA vaccines. Clin Appl Immunol Rev. (2002) 2(4):255–87. doi: 10.1016/S1529-1049(02)00051-X
137. Pulendran B S, Arunachalam P, O’Hagan DT. Emerging concepts in the science of vaccine adjuvants. Nat Rev Drug Discovery. (2021) 20(6):454–75. doi: 10.1038/s41573-021-00163-y
138. Facciolà A, Visalli G, Laganà A, Di Pietro A. An overview of vaccine adjuvants: Current evidence and future perspectives. Vaccines (Basel). (2022) 10(5):819. doi: 10.3390/vaccines10050819
139. Bobanga ID, Petrosiute A, Huang AY. Chemokines as cancer vaccine adjuvants. Vaccines (Basel). (2013) 1(4):444–62. doi: 10.3390/vaccines1040444
140. Gary EN, Kathuria N, Makurumidze G, Curatola A, Ramamurthi A, Bernui ME, et al. CCR10 expression is required for the adjuvant activity of the mucosal chemokine CCL28 when delivered in the context of an HIV-1 env DNA vaccine. Vaccine. (2020) 38(11):2626–35. doi: 10.1016/j.vaccine.2020.01.023
141. Liu C, Xue R-Y, Li G-C, Zhang Y, Wu W-Y, Liu J-Y, et al. pGM-CSF as an adjuvant in DNA vaccination against SARS-CoV-2. Int J Biol Macromolecules. (2024) 264:130660. doi: 10.1016/j.ijbiomac.2024.130660
142. Gary EN, Tursi NJ, Warner B, Parzych EM, Ali AR, Frase D, et al. Mucosal chemokine adjuvant enhances synDNA vaccine-mediated responses to SARS-CoV-2 and provides heterologous protection in vivo. Cell Rep Med. (2022) 3(7):100693. doi: 10.1016/j.xcrm.2022.100693
143. Kutzler MA, Kraynyak KA, Nagle SJ, Parkinson RM, Zharikova D, Chattergoon M, et al. Plasmids encoding the mucosal chemokines CCL27 and CCL28 are effective adjuvants in eliciting antigen-specific immunity in vivo. Gene Ther. (2010) 17(1):72–82. doi: 10.1038/gt.2009.112
144. Sun H-X, Xie Y, Ye Y-P. ISCOMs and ISCOMATRIX™. Vaccine. (2009) 27(33):4388–401. doi: 10.1016/j.vaccine.2009.05.032
145. Chen K, Wang N, Zhang X, Wang M, Liu Y, Shi Y. Potentials of saponins-based adjuvants for nasal vaccines. Front Immunol. (2023) 14:1153042. doi: 10.3389/fimmu.2023.1153042
146. Pearse MJ, Drane D. ISCOMATRIXTM adjuvant: a potent inducer of humoral and cellular immune responses. Vaccine. (2004) 22(19):2391–5. doi: 10.1016/j.vaccine.2003.12.031
147. Pabreja S, Garg T, Rath G, Goyal AK. Mucosal vaccination against tuberculosis using Ag85A-loaded immunostimulating complexes. Artif Cells Nanomedicine Biotechnol. (2016) 44(2):532–9. doi: 10.3109/21691401.2014.966195
148. Hu K-F, Lövgren-Bengtsson K, Morein B. Immunostimulating complexes (ISCOMs) for nasal vaccination. Advanced Drug delivery Rev. (2001) 51(1-3):149–59. doi: 10.1016/S0169-409X(01)00165-X
149. Ten Brinke A, Karsten ML, Dieker MC, Zwaginga JJ, Van Ham SM. The clinical grade maturation cocktail monophosphoryl lipid a plus IFNγ generates monocyte-derived dendritic cells with the capacity to migrate and induce Th1 polarization. Vaccine. (2007) 25(41):7145–52. doi: 10.1016/j.vaccine.2007.07.031
150. Thompson BS, Chilton PM, Ward JR, Evans JT, Mitchell TC. The low-toxicity versions of LPS, MPL® adjuvant and RC529, are efficient adjuvants for CD4+ T cells. J Leukocyte Biol. (2005) 78(6):1273–80. doi: 10.1189/jlb.0305172
151. Sun X, Hosomi K, Shimoyama A, Yoshii K, Saika A, Yamaura H, et al. Alcaligene s lipid a functions as a superior mucosal adjuvant to monophosphoryl lipid a via the recruitment and activation of CD11b+ dendritic cells in nasal tissue. Int Immunol. (2024) 36(1):33–43. doi: 10.1093/intimm/dxad045
152. Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. (2007) 369(9580):2161–70. doi: 10.1016/S0140-6736(07)60946-5
153. Monie A, Hung C-F, Roden R, Wu TC. Cervarix™: a vaccine for the prevention of HPV 16, 18-associated cervical cancer. Biologics: Targets Ther. (2008) 2(1):107–13.
154. Wang Y-Q, Bazin-Lee H, Evans JT, Casella CR, Mitchell TC. MPL adjuvant contains competitive antagonists of human TLR4. Front Immunol. (2020) 11:577823. doi: 10.3389/fimmu.2020.577823
155. Mitchell TC, Casella CR. No pain no gain? adjuvant effects of alum and monophosphoryl lipid a in pertussis and HPV vaccines. Curr Opin Immunol. (2017) 47:17–25. doi: 10.1016/j.coi.2017.06.009
156. García F, Climent N, Assoumou L, Gil C, González N, Alcamí J, et al. A therapeutic dendritic cell-based vaccine for HIV-1 infection. J Infect Diseases. (2011) 203(4):473–8. doi: 10.1093/infdis/jiq077
157. Tseng S-H, Cheng MA, Farmer E, Ferrall L, Kung YJ, Lam B, et al. Albumin and interferon-β fusion protein serves as an effective vaccine adjuvant to enhance antigen-specific CD8+ T cell-mediated antitumor immunity. J Immunotherapy Cancer. (2022) 10(4):e004342. doi: 10.1136/jitc-2021-004342
158. Barouch DH, Santra S, Schmitz JE, Kuroda MJ, Fu T-M, Wagner W, et al. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science. (2000) 290(5491):486–92. doi: 10.1126/science.290.5491.486
159. Morrow MP, Pankhong P, Laddy DJ, Schoenly KA, Yan J, Cisper N, et al. Comparative ability of IL-12 and IL-28B to regulate treg populations and enhance adaptive cellular immunity. Blood J Am Soc Hematology. (2009) 113(23):5868–77. doi: 10.1182/blood-2008-11-190520
160. Kraynyak KA, Kutzler MA, Cisper NJ, Laddy DJ, Morrow MP, Waldmann TA, et al. Plasmid-encoded interleukin-15 receptor α enhances specific immune responses induced by a DNA vaccine in vivo. Hum Gene Ther. (2009) 20(10):1143–56. doi: 10.1089/hum.2009.025
161. Sartori A, Ma X, Gri G, Showe L, Benjamin D, Trinchieri G. Interleukin-12: an immunoregulatory cytokine produced by B cells and antigen-presenting cells. Methods. (1997) 11(1):116–27. doi: 10.1006/meth.1996.0395
162. Freeman AF, Holland SM. Persistent bacterial infections and primary immune disorders. Curr Opin Microbiol. (2007) 10(1):70–5. doi: 10.1016/j.mib.2006.11.005
163. Holland SM. Interferon gamma, IL-12, IL-12R and STAT-1 immunodeficiency diseases: disorders of the interface of innate and adaptive immunity. Immunologic Res. (2007) 38:342–6. doi: 10.1007/s12026-007-0045-8
164. Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by γc family cytokines. Nat Rev Immunol. (2009) 9(7):480–90. doi: 10.1038/nri2580
165. Wherry EJ, Teichgräber V, Becker TC, Masopust D, Kaech SM, Antia R, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. (2003) 4(3):225–34. doi: 10.1038/ni889
166. Kalams SA, Parker S, Jin X, Elizaga M, Metch B, Wang M, et al. Safety and immunogenicity of an HIV-1 gag DNA vaccine with or without IL-12 and/or IL-15 plasmid cytokine adjuvant in healthy, HIV-1 uninfected adults. PloS One. (2012) 7(1):e29231. doi: 10.1371/journal.pone.0029231
167. Yu T-W, Chueh H-Y, Tsai C-C, Lin C-T, Qiu JT. Novel GM-CSF-based vaccines: One small step in GM-CSF gene optimization, one giant leap for human vaccines. Hum Vaccines Immunotherapeutics. (2016) 12(12):3020–8. doi: 10.1080/21645515.2016.1221551
168. Operschall E, Schuh T, Heinzerling L, Pavlovic J, Moelling K. Enhanced protection against viral infection by co-administration of plasmid DNA coding for viral antigen and cytokines in mice. J Clin Virol. (1999) 13(1-2):17–27. doi: 10.1016/S1386-6532(99)00008-6
169. Condon C, Watkins SC, Celluzzi CM, Thompson K, Falo LD Jr.. DNA–based immunization by in vivo transfection of dendritic cells. Nat Med. (1996) 2(10):1122–8. doi: 10.1038/nm1096-1122
170. Loudon PT, Yager EJ, Lynch DT, Narendran A, Stagnar C, Franchini AM, et al. GM-CSF increases mucosal and systemic immunogenicity of an H1N1 influenza DNA vaccine administered into the epidermis of non-human primates. PloS One. (2010) 5(6):e11021. doi: 10.1371/journal.pone.0011021
171. Yager EJ, Dean HJ, Fuller DH. Prospects for developing an effective particle-mediated DNA vaccine against influenza. Expert Rev Vaccines. (2009) 8(9):1205–20. doi: 10.1586/erv.09.82
172. Martins S, Alves LR. Extracellular vesicles in viral infections: two sides of the same coin? FronT cell Infection Microbiol. (2020) 10:593170. doi: 10.3389/fcimb.2020.593170
173. Gould SJ, Booth AM, Hildreth JEK. The trojan exosome hypothesis. Proc Natl Acad Sci. (2003) 100(19):10592–7. doi: 10.1073/pnas.1831413100
174. Nanjundappa RH, Wang R, Xie Y, Umeshappa CS, Chibbar R, Wei Y, et al. GP120-specific exosome-targeted T cell-based vaccine capable of stimulating DC-and CD4+ t-independent CTL responses. Vaccine. (2011) 29(19):3538–47. doi: 10.1016/j.vaccine.2011.02.095
175. Chiozzini C, Manfredi F, Arenaccio C, Ferrantelli F, Leone P, Federico M. N-terminal fatty acids of NEFMUT are required for the CD8+ T-cell immunogenicity of In vivo engineered extracellular vesicles. Vaccines. (2020) 8(2):243. doi: 10.3390/vaccines8020243
176. Ferrantelli F, Manfredi F, Chiozzini C, Anticoli S, Olivetta E, Arenaccio C, et al. DNA vectors generating engineered exosomes potential CTL vaccine candidates against AIDS, hepatitis b, and tumors. Mol Biotechnol. (2018) 60:773–82. doi: 10.1007/s12033-018-0114-3
177. Santos P, Almeida F. Exosome-based vaccines: history, current state, and clinical trials. Front Immunol. (2021) 12:711565. doi: 10.3389/fimmu.2021.711565
178. Hung IFN, Zhang AJ, To KKW, Chan JFW, Li C, Zhu H-S, et al. Immunogenicity of intradermal trivalent influenza vaccine with topical imiquimod: a double blind randomized controlled trial. Clin Infect Diseases. (2014) 59(9):1246–55. doi: 10.1093/cid/ciu582
179. El Sahly HM, Atmar RL, Sendra E, Wegel A, Keitel WA. Topical imiquimod does not provide an adjuvant effect when administered with inactivated influenza A/H5N1 vaccine in healthy young adults. J Infect Diseases. (2021) 224(10):1712–9. doi: 10.1093/infdis/jiab206
180. Bernstein DI, Miller RL, Harrison CJ. Adjuvant effects of imiquimod on a herpes simplex virus type 2 glycoprotein vaccine in guinea pigs. J Infect Diseases. (1993) 167(3):731–5. doi: 10.1093/infdis/167.3.731
181. Bode C, Zhao G, Steinhagen F, Kinjo T, Klinman DM. CpG DNA as a vaccine adjuvant. Expert Rev Vaccines. (2011) 10(4):499–511. doi: 10.1586/erv.10.174
182. Duthie MS, Windish HP, Fox CB, Reed SG. Use of defined TLR ligands as adjuvants within human vaccines. Immunol Rev. (2011) 239(1):178–96. doi: 10.1111/j.1600-065X.2010.00978.x
183. Søgaard OS, Lohse N, Harboe ZB, Offersen R, Bukh AR, Davis HL, et al. Improving the immunogenicity of pneumococcal conjugate vaccine in HIV-infected adults with a toll-like receptor 9 agonist adjuvant: a randomized, controlled trial. Clin Infect Diseases. (2010) 51(1):42–50. doi: 10.1086/653112
184. Cerino A, Mantovani S, Mele D, Oliviero B, Varchetta S, Mondelli MU. Human monoclonal antibodies as adjuvant treatment of chronic hepatitis B virus infection. Front Immunol. (2019) 10:2290. doi: 10.3389/fimmu.2019.02290
185. Tsai TF. Fluad®-MF59®-adjuvanted influenza vaccine in older adults. Infection Chemotherapy. (2013) 45(2):159–74. doi: 10.3947/ic.2013.45.2.159
186. Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. New Engl J Med. (2007) 356(19):1928–43. doi: 10.1056/NEJMoa061760
187. Lövgren K. The serum antibody response distributed in subclasses and isotypes after intranasal and subcutaneous immunization with influenza virus immunostimulating complexes. Scandinavian J Immunol. (1988) 27(2):241–5. doi: 10.1111/j.1365-3083.1988.tb02343.x
188. Morein B, Hu K-F, Abusugra I. Current status and potential application of ISCOMs in veterinary medicine. Advanced Drug Delivery Rev. (2004) 56(10):1367–82. doi: 10.1016/j.addr.2004.02.004
189. Ott U. Intranasal immunization of mice with herpes simplex virus type 2 recombinant gD2: the effect of adjuvants on mucosal and serum antibody responses. Immunology. (1998) 93(4):563–71. doi: 10.1093/infdis/167.3.731
190. Hu KF, Ekström J, Merza M, Lövgren-Bengtsson K, Morein B. Induction of antibody responses in the common mucosal immune system by respiratory syncytical virus immunostimulating complexes. Med Microbiol Immunol. (1999) 187:191–8. doi: 10.1007/s004300050092
191. Pandey RS, Dixit VK. Evaluation of ISCOM vaccines for mucosal immunization against hepatitis b. J Drug Targeting. (2010) 18(4):282–91. doi: 10.3109/10611860903450015
192. Maemura T, Fukuyama S, Kawaoka Y. High levels of miR-483-3p are present in serum exosomes upon infection of mice with highly pathogenic avian influenza virus. Front Microbiol. (2020) 11:144. doi: 10.3389/fmicb.2020.00144
193. Qiu J-T, Chang T-C, Lin C-T, Chen Y-M, Li FQ, Soong Y-K, et al. Novel codon-optimized GM-CSF gene as an adjuvant to enhance the immunity of a DNA vaccine against HIV-1 gag. Vaccine. (2007) 25(2):253–63. doi: 10.1016/j.vaccine.2006.07.034
194. Watson D, Zheng G, Wu H, Wang YM, Wang Y, Harris DCH, et al. CCL2 DNA vaccine to treat renal disease. Int J Biochem Cell Biol. (2009) 41(4):729–32. doi: 10.1016/j.biocel.2008.04.028
195. Jayeshbhai C, Hajam IA, Verma AK, Bhanuprakash V, Kondabattula G, Kishore S. Chemokine CCL20 plasmid improves protective efficacy of the montanide ISA™ 206 adjuvanted foot-and-mouth disease vaccine in mice model. Vaccine. (2018) 36(35):5318–24. doi: 10.1016/j.vaccine.2018.07.003
196. Marsland BJ, Bättig P, Bauer M, Ruedl C, Lässing U, Beerli RR, et al. CCL19 and CCL21 induce a potent proinflammatory differentiation program in licensed dendritic cells. Immunity. (2005) 22(4):493–505. doi: 10.1016/j.immuni.2005.02.010
Keywords: MERS-CoV, immunotherapeutic molecules, human-derived adjuvants, diagnostic biomarkers, vaccine development
Citation: Alrasheed AR, Awadalla M, Alnajran H, Alammash MH, Almaqati AM, Qadri I and Alosaimi B (2025) Harnessing immunotherapeutic molecules and diagnostic biomarkers as human-derived adjuvants for MERS-CoV vaccine development. Front. Immunol. 16:1538301. doi: 10.3389/fimmu.2025.1538301
Received: 02 December 2024; Accepted: 20 February 2025;
Published: 13 March 2025.
Edited by:
Abel A. Ramos Vega, National Polytechnic Institute (IPN), MexicoReviewed by:
Lauren B. Reoma, National Institute of Neurological Disorders and Stroke (NIH), United StatesDania O. Govea-Alonso, Universidad Autónoma de Guadalajara, Mexico
Copyright © 2025 Alrasheed, Awadalla, Alnajran, Alammash, Almaqati, Qadri and Alosaimi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bandar Alosaimi, YmFsb3NhaW1pQGtmbWMubWVkLnNh
 Abdullah R. Alrasheed
Abdullah R. Alrasheed Maaweya Awadalla
Maaweya Awadalla Hadeel Alnajran
Hadeel Alnajran Mohammed H. Alammash
Mohammed H. Alammash Adil M. Almaqati4
Adil M. Almaqati4 Ishtiaq Qadri
Ishtiaq Qadri Bandar Alosaimi
Bandar Alosaimi