
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 10 March 2025
Sec. Cancer Immunity and Immunotherapy
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1528932
This article is part of the Research TopicApplication of Enzyme Biotechnology in Food and Health ProductsView all articles
Phosphodiesterase 4 (PDE4) is an enzyme that specifically hydrolyzes the second messenger cAMP and has a critical role in the regulation of a variety of cellular functions. In recent years, PDE4 has attracted great interest in cancer research, and its role in tumorigenesis and development has been gradually elucidated. Research indicates that abnormal expression or heightened activity of PDE4 is associated with the initiation and progression of multiple cancers, including lung, colorectal, and hematological cancers, by facilitating cell proliferation, migration, invasion, and anti-apoptosis. Moreover, PDE4 also influences the tumor immune microenvironment, significantly immune evasion by suppressing anti-tumor immune responses, reducing T-cell activation, and promoting the polarization of tumor-associated macrophages toward a pro-tumorigenic phenotype. However, the PDE4 family may have both oncogenic and tumor-suppressive effects, which could depend on the specific type and grade of the tumor. PDE4 inhibitors have garnered substantial interest as potential anti-cancer therapeutics, directly inhibiting tumor cell growth and restoring immune surveillance capabilities to enhance the clearance of tumor cells. Several PDE4 inhibitors are currently under investigation with the aim of exploring their potential in cancer therapy, particularly in combination strategies with immune checkpoint inhibitors, to improve therapeutic efficacy and mitigate the side effects of conventional chemotherapy. This review provides an overview of PDE4 in tumorigenesis, drug resistance, immunotherapy, and the anti-tumor actions of its inhibitors, intending to guide the exploration of PDE4 as a new target in tumor therapy.
Phosphodiesterases (PDEs) are a class of enzymes that catalyze the cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) (1). The PDE superfamily is divided into 11 families (PDE1-PDE11), classified by structure and enzymatic properties (2). Serving as a secondary messenger, cAMP plays a crucial role in regulating cell proliferation (3–5), differentiation (6, 7), apoptosis (8–11), and immune responses (9, 12, 13), by influencing the development of both physiological and pathological processes. The PDE4 family is arguably the most studied of all the PDE families. However, as an important PDE subtype that catalyzes the hydrolysis of cAMP, PDE4 has gradually attracted researchers’ attention for its role in modulating a variety of pathological processes. The PDE4 family comprises several subtypes, notably including PDE4A, PDE4B, PDE4C, and PDE4D. These subtypes are respectively located on different chromosomes: PDE4A at 19p13.2, PDE4B at 1p31, PDE4C at 19p13.11, and PDE4D at 5q12 (14). Each subtype can be divided into long, short, and ultrashort forms based on molecular weight. This diversity underpins the specific functions of PDE4 in various cell types and tissues (15). These subtypes exhibit differential cellular distributions and carry out unique roles in both physiological and pathophysiological processes. For example, PDE4B is highly expressed in neutrophils and other immune cells (16–18), closely related to inflammatory responses. Whereas PDE4D is abundant in the heart (19, 20) and brain (21–23), contributing to cardiovascular function and memory formation. The specific expression patterns and functions of PDE4 subtypes offer possibilities for developing targeted therapies for specific diseases.
Growing evidence highlights the complex relationships between PDE4 and various aspects of cancer. By regulating cAMP levels, PDE4 subfamilies were involved in tumorigenesis (24–28), metastasis (29–31), drug resistance (32, 33), and immune microenvironment (34–37). However, due to the PDE4 family having multiple subtypes and the heterogeneity of tumor cells, the role of PDE4 in tumor progression is also complex and varied. For instance, PDE4A appears to act as an oncogene in hepatocellular carcinoma cell line Huh7 (38), whereas it exhibits tumor-suppressive effects in HepG2 and BEL7402 cells (39). Thus, the PDE4/cAMP signaling pathway has emerged as a promising but extremely challenging target for drug development due to its strong association with tumor progression.
Advancements in understanding the structure and function of PDE4, have propelled the development of small molecule inhibitors targeting PDE4. Several PDE4-selective inhibitors have successfully reached the market, targeting diseases such as psoriasis, atopic dermatitis, chronic obstructive pulmonary disease (COPD), asthma and alleviating pain caused by smooth muscle spasm (Table 1). More recent work is examining PDE4 in the context of cancer, including B-cell lymphomas (40), triple negative breast cancer (41), and colorectal cancer (42). These studies offer promising insights into the potential of PDE4 inhibitors to target tumors.
This review aims to provide a comprehensive overview of the latest progress in understanding the relationship between PDE4 and cancer, examining its molecular mechanisms in tumorigenesis, drug resistance, and immunotherapy, summarizing the status and challenges of PDE4 inhibitors in tumor treatment, and prospecting the future research directions.
Various patterns of expression and localization within cells and tissues significantly influence the specific roles and functions of PDE4 subfamilies and isoforms (15). Additionally, researchers have found that the expression of different PDE4 subtypes in tumor cells varies significantly (Figure 1). It suggests that PDE4 could play either a promotional or inhibitory role in the development and progression of tumors, depending on the type of disease and its stage of advancement.
PDE4A is one of the PDE4 subfamilies. Malfunction or altered regulation of PDE4A has been connected to a multitude of diseases ranging from neurological disorders like Alzheimer’s and Parkinson’s diseases to psychiatric conditions such as depression and anxiety (50, 51). Furthermore, it also implicated in chronic inflammatory diseases including asthma and COPD (52). Recently, accumulating researches suggest a potential role for PDE4A in cancer development, highlighting its complex influence across physiological process (38). PDE4A has been found to be expressed in medulloblastomas, glioblastomas, oligodendrogliomas, ependymomas, and meningiomas (53), and may be a new target for treating brain tumors. Additionally, PDE4 inhibitor not only directly inhibits the proliferation of glioma cells (54), but also combination with first-line therapy for malignant gliomas (such as temozolomide), enhances the survival rate of mice with intracranial implants of U87 glioblastoma cells (53).
However, PDE4A may be protumorigenic or antitumorigenic, depending on the type of tumor or the type of tumor cells. It is reported that most patients with hepatocellular carcinoma (HCC) show elevated PDE4A expression in tumor tissues relative to corresponding adjacent liver tissues (38). Furthermore, increased PDE4A expression in tumor tissues is positively correlated with hepatitis B virus (HBV) infection, liver cirrhosis, higher levels of serum alpha-fetoprotein (AFP), advanced TNM staging, the presence of vascular emboli, intrahepatic metastasis, and portal vein tumor thrombus (PVTT) (38). Knockout of PDE4A in Huh7 cells inhibited cell migration and epithelial-mesenchymal transition (EMT) (Figure 2). Nevertheless, research also shows that PDE4A protects against EMT in HepG2 and BEL7402 cells (39). Autophagy triggers increased TGF-β1 expression and induces EMT in HepG2 and BEL7402 cells which occurs through the cAMP/PKA/CREB signaling pathway. Notably, this activation is facilitated by autophagy-mediated degradation of PDE4A (39) (Figure 2). The phenomenon of PDE4A exerting differential impacts on EMT across various HCC cells might be ground in the heterogeneous characteristics of the cells. This highlights the necessity for in-depth investigation into the precise mechanisms by which PDE4A modulates EMT in HCC.
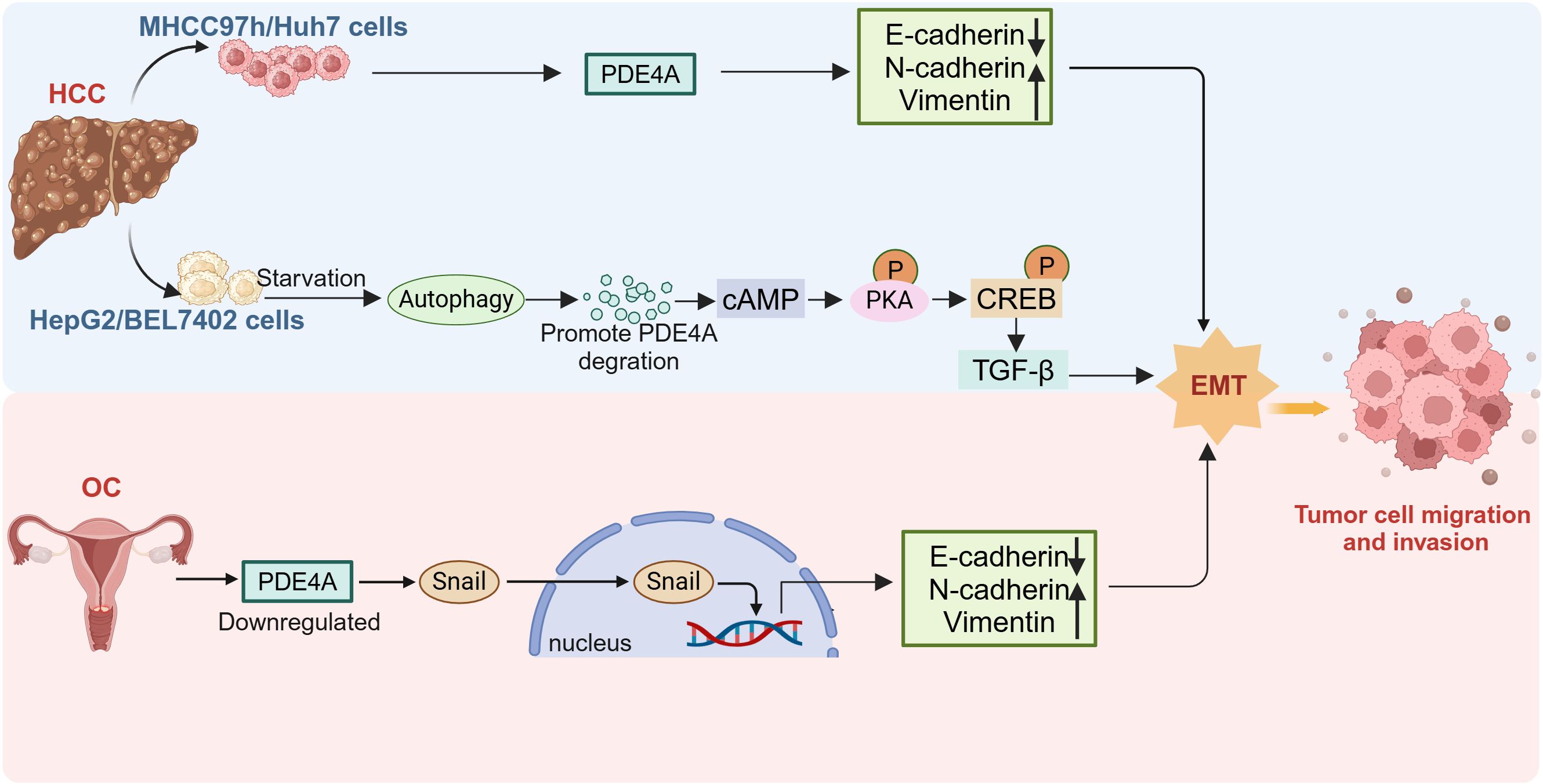
Figure 2. The different role of PDE4A in tumor EMT. The expression of PDE4A is upregulated in MHCC97H and Huh7 cell, and promote the EMT. PDE4A is degraded by starvation induced autophagy in HepG2 cells, thus promoting EMT conversion. Downregulation of PDE4A expression in ovarian cancer induce EMT and nuclear translocation of Snail. HCC, hepatocellular carcinoma; OC, ovarian cancer; EMT, epithelial-mesenchymal transition (This figure was created with Biorender.com).
PDE4A is significantly upregulated in several hematologic tumors (55), stomach adenocarcinoma, and cholangiocarcinoma, and is associated with cell proliferation (56). Conversely, although PDE4A expression is also elevated in estrogen receptor-positive and progesterone receptor-positive breast cancer patients, patients with high PDE4A expression have longer progression-free survival (PFS) and overall survival (OS) (57). Similarly, low expression of PDE4A has been proved to promote the progression of ovarian cancer (OC) by inducing snail nuclear translocation (27) (Figure 2). These findings indicate that PDE4A may exert different roles depending on the tumor type.
During the process of tumorigenesis, multiple factors were reported to modulate the expression or activity of PDE4A. In non-small cell lung cancer (NSCLC) tissues, there is a reduction in PDE4A protein expression, while mRNA level exhibits an inverse trend (56). This indicates the potential presence of post-translational modifications of PDE4A in NSCLC. Hypoxia is a consequence of excessive oxygen consumption by tumor cells and insufficient oxygen supply owing to a compromised vasculature. In A549 adenocarcinoma cells, the expression of PDE4A and PDE4D increase under hypoxic conditions and enhance HIF signaling, which promotes the proliferation of tumor cells (58). Chewing betel nut is closely related to oral submucous fibrosis, considered a precancerous condition. Arecoline enhances TGF-β-induced buccal mucosal fibroblast (BMF) activation by modulating the activity of PDE4A without altering the expression of PDE4A (54). PDE4A showed a critical role in the progression of esophageal squamous cell carcinoma (ESCC) through its regulation by lncRNA HCP5. This interaction also activates the PI3K/AKT/mTOR signaling pathway and provided insights that may enhance therapeutic strategies for ESCC (59). In summary, PDE4A not only acts directly on tumor cells to promote their growth but also influences the tumor microenvironment. Further research is needed in the future to clearly define the specific role of PDE4A in tumor development and its potential therapeutic implications.
The PDE4B gene resides on chromosome 1p31, with enlightening single nucleotide polymorphisms (SNPs) clustering in the region encoding PDE4B predominantly situated at the 5′-terminals (50). Furthermore, the PDE4B gene also encodes PDE4B monomers known as PDE4B2 and PDE4B5 (71). Altered expression of PDE4B is involved in a wide range of disease progression, including Parkinson’s disease (60), asthma (61), hematologic malignancies (62), and other cancer diseases (24, 63).
Melanoma is a highly malignant skin tumor with a significant propensity for metastasis. Given its propensity for metastasis, the manner and site of melanoma spread significantly influence patient prognoses (64). PDE4B was identified as a prognostic gene in metastatic melanoma by multiple bioinformatics approaches analysis (65). In addition, the expression of PDE4B was also associated with the prognosis of uveal melanoma (66). Silencing PDE4B or PDE4D significantly reduced PDE4 activity in melanoma cells, whereas PDE4A had no such effect (67), suggesting that PDE4B and PDE4D play crucial roles in regulating PDE4 activity in melanoma. Further investigation revealed that PDE4B2 is required for the oncogenic Ras-driven melanoma (67).
Patients with advanced urinary bladder cancer (UBC) exhibit increased levels of PDE4B mRNA (68) which predicts poor prognosis in UBC Patients. Mechanistically, PDE4B is negatively regulated by chromobox protein homolog 7 (CBX7) in a PRC1-dependent manner in UBC cells. However, the role of PDE4B in prostate cancer appears to differ from that in UBC. Alterations in PDE sequences are closely associated with the development of prostate cancer. In the early stages, the proliferation of prostate cancer cells relies on androgens, and androgen deprivation therapy can suppress the growth of prostate cancer cells (69, 70). However, most prostate cancers progress to a castration-resistant phenotype, where treatments based on androgen docetaxel often prove minimally effective. It was found that oxidative stress inhibits PDE4B expression during this process, which activates PKA and promotes cell proliferation (71). This study demonstrates that PDE4B may perform a tumor suppressor role in advanced prostate cancer, but further studies are needed to confirm this role.
Initial evidence implicating a connection between PDE4B and B-cell lymphoma emerged from comprehensive genomic profiling analyses (72). These studies indicate that PDE4B expression is upregulated in patients with diffuse large B-cell lymphoma (DLBCL) who have developed resistance to chemotherapy (72). The following studies have demonstrated that cAMP promoted DLBCL cells apoptosis by inhibiting the PI3K/AKT pathway. Notably, this effect is suppressed when cAMP is degraded via the upregulated of PDE4B (73) (Figure 3). Furthermore, the cAMP-PDE4B axis is also linked to the tumor microenvironment of DLBCL (74, 75). Angiogenesis plays a critical role in the prognosis of DLBCL, and there is evidence that PDE4B influence this process. Mechanistically, PDE4B enhances hypoxia-induced VEGF production by catalyzing cAMP hydrolysis, which regulates the tumor microenvironment and promotes angiogenesis (74) (Figure 3). Extensive research indicates that B-cell lymphomas with enhanced Myc expression are associated with an aggressive phenotype and poor prognosis (76, 77). While, there may exist a Myc-PDE4B positive feedback loop which cooperatively enhance the proliferation of DLBCL cells (78) (Figure 3). In cells with high PDE4B expression, PDE4 inhibitors augment the JQ1-mediated cell death, whereas this synergistic action is not observed in cells with low PDE4B expression (78). This indicates that B-cell lymphomas might necessitate stratification into PDE4B-high and PDE4B-low expression groups to facilitate personalized combination therapies incorporating PDE4 inhibitors.
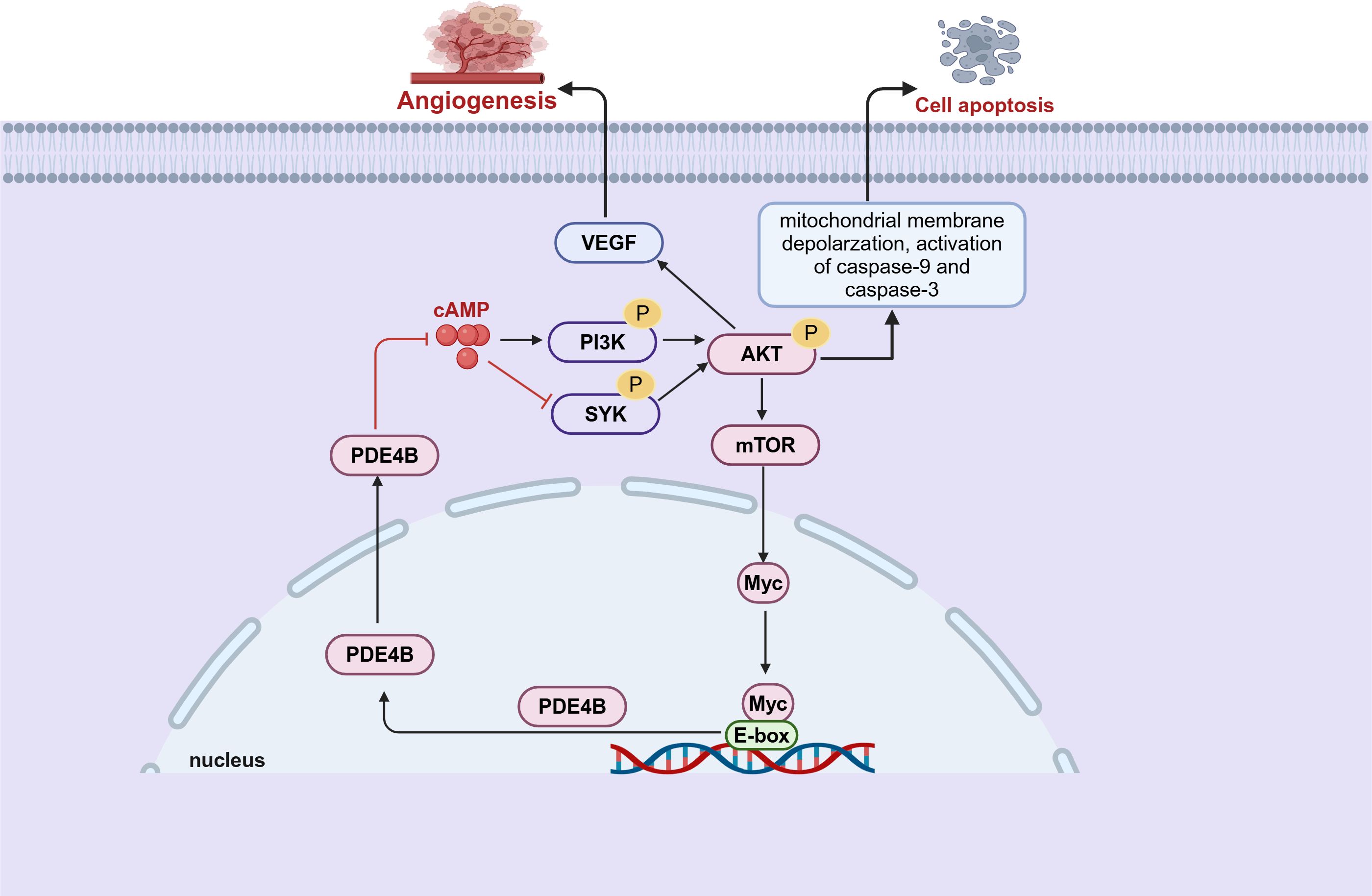
Figure 3. PDE4B regulates tumor angiogenesis and apoptosis through multiple pathways in B-cell lymphoma. There is a pre-feedback path between Myc and PDE4B. PDE4B regulates Myc expression through the SYK/AKT/mTOR pathway. Meanwhile, Myc regulates the expression of PDE4B by directly binding to the E-box of the PDE4B promoter (This figure was created with Biorender.com).
Colorectal cancer (CRC) is regarded as the most commonly diagnosed malignancy within the gastrointestinal tract. It lacks homogeneity and can be categorized into various subtypes characterized by specific molecular and morphological changes. Research has revealed that PDE4B is conserved across three mammalian species—mouse, rat, and human—and exhibits differences between colorectal adenomas and normal tissue (79). Loss of PDE4B function in the ApcMin/+ mouse, a spontaneous pre-cancerous colonic lesion model mouse, results in a significant increase in the number of colonic adenomas (79). This finding implies that PDE4B can prevent ApcMin-induced adenoma formation and protect against the early stages of colon cancer in the mouse. However, the study revealed that while the expression of PDE4B is elevated in patients with advanced colorectal cancer, its functional activity is conversely diminished. Similarly, another research showed the highest PDE4B expression in the control tissue and Dukes’ A stage (80). However, in patients with advanced colon cancer, PDE4B may become inactivated through epigenetic suppression (80). PDE4B is also excessively expressed as a dysfunctional protein in non-neoplastic appearing colonic mucosa from patients with colorectal neoplasia (81). Oncogenic KRAS specifically upregulates PDE4B2 in a three-dimensional (3-D) colonic crypt model (82). Suppression of PDE4 catalytic activity can induce epithelial cell polarity and apoptosis within the luminal space in CRC (82).
In contrast with other PDE4 subtypes, current evidence indicates that PDE4C gene expression yields exclusively long-form enzyme isoforms. Consequently, every PDE4C enzyme features a distinct N-terminal segment, paired with two invariant regulatory segments (designated UCR1 and UCR2), a conserved catalytic region, and a C-terminal tail characteristic to its subfamily, adhering to the overarching modular architecture described for PDE4 enzymes (83). Mounting evidences suggest that the PDE4C is associated with a variety of diseases, including cancer (83). The Human Protein Atlas (HPA) provides comprehensive and significant insights into the expression of PDE4C across different types of cancer (84). As described in HPA, PDE4C exhibited harmful effects in five types of cancer: renal carcinoma, glioblastoma, pancreatic carcinoma, melanoma, and breast cancer (84). Instead, it shows a protective role in four cancers: head and neck carcinoma, urothelial carcinoma, gastric carcinoma, and cervical cancer (84). Compared to other PDE4 subtypes, PDE4C tends to exhibit lower expression levels in majority of tumors. However, PDE4C emerged as the sole prominent member of the PDE4 family in glioma and pancreatic cancer specifically. Notably, elevated PDE4C expression is strongly associated with lower survival and poorer treatment outcomes in a variety of malignant tumors, such as myelodysplastic syndromes (MDS), pancreatic cancer, and lung cancer (55, 85, 86).
In patients with thyroid carcinoma (THCA) across various stages, the expression of PDE4A, PDE4B, and PDE4D showed a decrease; conversely, PDE4C expression was notably elevated (87). Furthermore, THCA patients with higher expression levels of PDE4C experienced a shorter progression-free survival than those with lower PDE4C expression (87). Heightened promoter methylation of PDE4C coupled with a reduction in PDE4C protein expression was inversely correlated with overall patient survival rates in brain cancer (88) (Figure 4). However, this is inconsistent with the data from the HPA (55). This is potentially due to the HPA’s lack of consideration for the staging of gliomas. Another study has revealed an association in glioma patients between reduced expression of PDE4C and downregulation of apoptotic pathways coupled with upregulation of cell migration pathways by transcriptomic analysis (88). It was found that there was a protective relationship between PDE4C and p53 (88, 89). In cancer cell with p53 mutations, which impede its ability to govern transcription of target genes, transcriptomic profiling consistently pointed to reduced expression of PDE4C (89). Additionally, the p53 regulatory sequence within the PDE4C gene’s promoter zone implies that functional wild-type p53 enhances the expression of the PDE4C gene. These findings indicate a reciprocal regulatory relationship between PDE4C and p53 and disruptions to this protective interplay might foster oncogenesis.
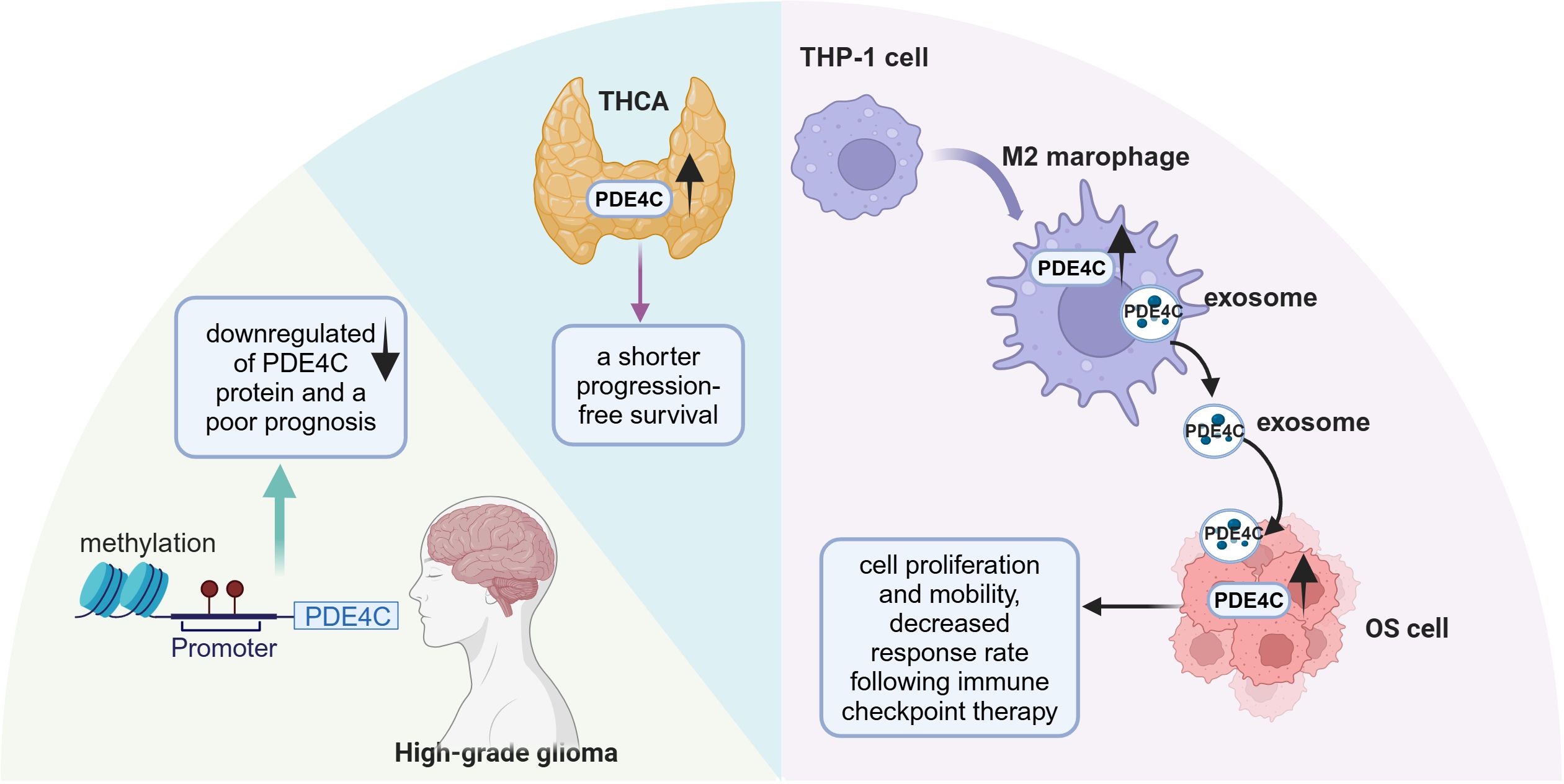
Figure 4. The role of PDE4C in different tumor progression. PDE4C had significant promoter methylation and lower expression in high-grade glioma. PDE4C expression was elevated in THCA and is associated with poor prognosis. The expression of PDE4C was observed to increase during the conversion process of THP-1 cells to M2 macrophage, which transferred the PDE4C mRNA to OS cells through exosome approach. TCHA, thyroid carcinoma; OS, osteosarcoma (This figure was created with Biorender.com).
Furthermore, PDE4C has been identified as a crucial intermediary in communication between tumor cells and immune cells. Tumor-associated macrophages (TAMs), including M1 and M2 phenotypes, play a pivotal role in influencing the progression of osteosarcoma (OS) and promoting immunosuppressive environments (90). Increased expression of PDE4C observed in OS tissues, coinciding with heightened levels of M2 macrophages, an unfavorable prognosis, and the presence of metastasis (91) (Figure 4). PDE4C secreted by M2 macrophages may promote the proliferation and migratory capabilities of OS cells through elevated collagen production. This suggests that PDE4C might serve as a potential biomarker for predicting prognosis and therapeutic response in OS. In summary, the role of PDE4C in tumors appears to be highly heterogeneous, and further research is warranted to clarify its potential as both a tumor biomarker and a therapeutic target.
Multiple protein variants of PDE4D (PDE4D1 through PDE4D11) result from complex arrangements and splicing of PDE4D locus, with nine variants identified in humans and eleven in mice (92). These isoforms generate proteins with diverse lengths, containing or lacking domains vital for their functional roles. They divide into three classifications: extended isoforms such as PDE4D3, PDE4D5-10 in humans and PDE4D11 in mice, compact isoforms represented by PDE4D1, and ultra-compact isoforms observed in mice, specifically PDE4D2 and PDE4D6 (92). Expression patterns of these variants exhibit varying degrees of tissue specificity; notably, PDE4D4 and PDE4D6 are predominantly confined to the brain, while PDE4D8 is found in tissues beyond the confines of the central nervous system (93). Recently, it has been reported that PDE4D gives rise to a notably stable, predominantly localized in the cytoplasm, circular RNA, designated as circPDE4D (94). This circRNA originates from the exon 2 to 5 region of the PDE4D gene through a process of exon circularization (94) and the functional role of circPDE4D remains largely to be determined. Furthermore, extensive studies have been conducted to reveal the role of PDE4D in regulating tumor cell growth, angiogenesis, and responses to immune therapy (Figure 5). However, the role of PDE4D in tumor progression is multifaceted, potentially exhibiting both oncogenic and tumor-suppressive activities, which may be associated with the type and stage of the tumor.
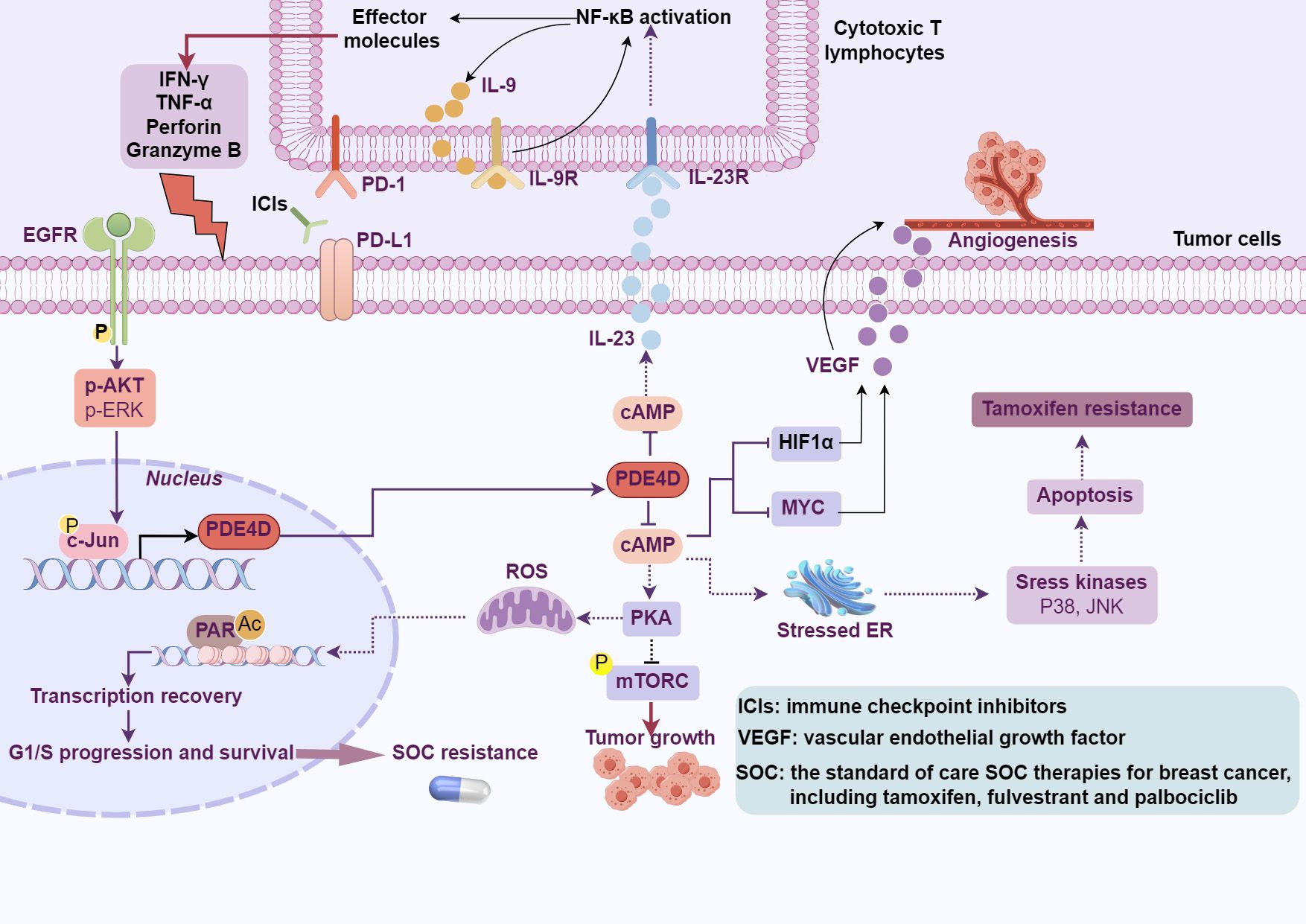
Figure 5. The effects of PDE4D-cAMP signaling on cancer. PDE4D modulates tumor cell growth, angiogenesis, resistance to tamoxifen and SOC treatments, and influences the response to cancer immunotherapy through the hydrolysis of cAMP (This figure was created with Biorender.com).
Studies found that the PDE4D gene in approximately 20% of prostate tumors (95), and is closely related to tumor progression. In prostates, PDE4D is expressed in both stromal and epithelial compartments, with its hydrolytic activity confined to cytoplasmic compartments (96). However, the role of PDE4D7 in prostate cancer appears distinct from that of PDE4D. PDE4D7 is localized to the submembrane region of prostate cancer cells (97). In addition, the expression of PDE4D7 decreased as the disease advanced from an androgen-sensitive (AS) localized prostate cancer to a castration-resistant form, suggesting its involvement in fostering a more aggressive disease manifestation (97). The differential roles of PDE4D and PDE4D7 in prostate cancer indicate the complex involvement of PDE4D subtypes in the development of prostate cancer, highlighting the need for further research to elucidate the specific functions of each subtype.
Growing evidence has focused on the role of PDE4D in CRC progression, but the results were not consistent. Studies showed that PDE4D acted as an oncogenic protein involved in the development of CRC (28, 98). Inhibition of PDE4D suppresses the malignant properties of DLD-1 cells by inhibiting the AKT/mTOR/Myc signaling pathway (99). Around 5% of CRC cases harbor mutations in the GNAS (guanine nucleotide binding protein, alpha stimulating) gene, resulting in the stimulation of cAMP-mediated signaling pathways and a poor prognosis (27). RNA sequencing results revealed that the most upregulated gene in GNAS-mutated (GNASmt) cells was PDE4D. Notably, the PDE4 inhibitor Ro 20-1724 and PDE4D selective inhibitor GEBR-7b suppressed the proliferation of GNASmt cells while not affecting parental cells. This provides evidence for targeting PDE4D in the treatment of CRC with GNAS mutations. However, one study also found that the PDE4D mRNA was down-regulated in CRC compared with normal colon as revealed by oncomine data-mining analysis and knockdown of PDE4D increase the cell proliferation (100). Some studies suggest that a hallmark of disease progression is the overall downregulation of the long isoforms of PDE4D, whereas the short isoforms (PDE4D1/2) appear to be relatively unaffected (101). Thus, the data indicated that compared to benign tissue, the long isoforms of PDE4D may be altered in primary human CRC.
For metastasis of choriocarcinoma cells, PDE4D was found to be predominantly expressed among all PDE4 isoforms (30). PDE4D inhibitor suppressed migration and invasion of human choriocarcinoma cells in vitro via suppressing EMT (30). Conversely, downregulation of PDE4D expression was observed in esophageal cancer tissues, and it was found to bind with lncRNA MANCR promoting esophageal cancer cell proliferation and inhibiting cell apoptosis (102). By interacting with other proteins and signaling pathways, cAMP effectively regulates the status of diverse cell types, including HCC (103). Decreased intracellular levels or inhibition of PDE4D hinders the proliferation of HCC cells, exhibiting tumor-suppressive properties (104). Consistent with this finding, our previous research has established that PDE4D is overexpressed in HCC tissues and synergistically promotes tumor progression with YAP (105).
Concurrently, PDE4D has been reported to impact the therapeutic efficacy of drugs. By genome-wide screening, elevated levels of PDE4D correlated with diminished responsiveness to docetaxel (TXT) therapy, whereas reduced expression enhanced the efficacy of TXT (106) in hypopharyngeal cancers. Conversely, low PDE4D expression was associated with poor outcomes in bladder cancer patients (107). Furthermore, the downregulation of intracellular cAMP through PDE4D enhanced the efficacy of IFN-α against bladder cancer in both in vitro and in vivo (107). Interestingly, study revealed that roflumilast-induced upregulation of PDE4D expression works in concert with IFN-α, and amplifying the antiproliferative action of IFN-α on bladder cancer cells in vitro and vivo (107). Relative to all other PDE4 subtypes, PDE4D exhibits significantly higher expression in metastatic Caki-1 cells (108). PDE4D knockout augmented the sorafenib-induced apoptosis in Caki-1 cells. These studies highlight the substantial potential of PDE4D in the efficacy of anticancer therapies, necessitating further research to unravel its exact role.
Drug resistance is the main cause of tumor treatment failure and recurrence. The causes of tumor cell resistance are multiple, including reduced intracellular drug accumulation (109), increased drug efflux (109), alterations in DNA repair mechanisms (110), cancer stem cells (CSC) (111), and modifications in cell death pathways (112). PDE4 subtypes are abnormally expressed in cancer stem cells from various tumors, including breast cancer (111), glioma (37, 113), prostate cancer (114), and leukemia (115). For example, the expression of the PDE4A1 protein is increased in breast CSCs with reduced levels of cAMP (111). The combination of rolipram with paclitaxel demonstrated a synergistic effect and effectively inhibiting the growth of CSCs. Tamoxifen is widely used as the standard first-line adjuvant therapy for estrogen receptor (ER)-positive breast cancer patients, particularly in premenopausal women (116). These studies have demonstrated that tamoxifen administered as adjuvant therapy for at least five years reduces the recurrence rate and mortality of breast cancer by roughly half and one-third, respectively (117). Nevertheless, despite its clinical successes, tamoxifen encounters a significant challenge in that approximately 20%-30% of high-risk, late-stage ER-positive breast cancer patients develop either de novo or acquired resistance to the drug (33, 118). The mechanism of tamoxifen resistance is partially understood. By whole-transcriptome sequencing, PDE4D was identified as a potential mediator of tamoxifen resistance (33) (Figure 5). Moreover, an elevation in PDE4D expression has been observed in tamoxifen-resistant cells and breast cancer tissues. Resistance to tamoxifen treatment was reversed by PDE4D siRNA or cAMP analogs, which restore sensitivity by activating JNK and P38 pathways and concurrently suppressing AKT activation. Interestingly, aspirin was identified to influence cAMP levels both in vitro and in vivo by modulating PDE4D activity, and overcoming tamoxifen resistance (33). Acquired resistance to standard-of-care (SOC) treatments for estrogen receptor-positive (ER+) breast cancer, including endocrine therapy and CDK4/6 inhibitors, significantly diminishes patient survival (119, 120). In non-resistant cells, SOC treatment downregulates PDE4D, resulting in elevated cAMP levels, which prompts PKA-dependent phosphorylation of mitochondrial COXIV-I, the generation of reactive oxygen species (ROS), and DNA damage (121). However, during the development of SOC resistance, an ER-to-EGFR switch activates c-Jun, which in turn induces overexpression of PDE4D. Of note, combining SOC with inhibitors of PDE4D, EGFR, or PARP1 has been shown to overcome SOC resistance (121) (Figure 5). As a rare and highly aggressive form, inflammatory breast cancer (IBC) presents distinctive clinical features with a low survival rate (122). At the same time, PDE4A exhibited a special role in paclitaxel-resistant IBC cells (123). An increase in AMP, cAMP, and PDE4A expression is observed in paclitaxel-resistant cells, suggesting a potential upregulation of the entire cAMP pathway (123). However, the knockdown of PDE4A has a minimal impact on paclitaxel sensitivity, which may be attributed to the activation of other PDE4 subtypes. Further investigation revealed that pSTAT3 regulates the expression of genes associated with inflammation, EMT, and PDE4A in resistant cells, contributing to paclitaxel therapeutic resistance.
Studies have found that miR-494 is significantly downregulated in gastric cancer cells compared to normal gastric epithelial cells. In addition, its expression is reduced in doxorubicin-resistant gastric cancer cells (AGS/dox) compared to parental cells (32). The increased expression of miR-494 inhibits the expression of PDE4D mRNA and protein in gastric cancer cells. Luciferase assays show that miR-494 directly targets the 3’ untranslated region (3’UTR) of PDE4D. Additionally, restoration of PDE4D reverse the drug sensitivity of in gastric cancer cells with miR-494 overexpression (32). Studies have confirmed that PDE4D is the predominant subtype of PDE4 in either normal or neoplastic renal cells (108). Knockdown of PDE4D or treatment with PDE4 inhibitors enhances the inhibition of sorafenib (108). Similarly, PDE4 inhibitors have also promising synergistic anti-tumor effects in B-cell lymphoma (124, 125).
Cancer immunotherapy represents a revolutionary approach in cancer (126, 127). However, despite remarkable progress and groundbreaking successes, especially with checkpoint inhibitors (128–130) and CAR T-cell therapies (131–133), not all patients respond equally, and many patients become resistant to them. PD-L1 (Programmed Death-Ligand 1) serves as a target for immune checkpoint inhibition in cancer therapy, preventing cancer cells from evading immune system attacks by binding to the PD-1 receptor on T cells. Given the role of PD-L1 in suppressing anti-tumor T cell activity, considerable efforts have been made to identify regulators of PD-L1 expression. In immune system cells, the PDE4 family is responsible for a major portion of cAMP hydrolysis (134). In DLBCL cells, the cAMP effectors, specifically PKA and CREB, stimulate the transcription and secretion of IL-10, IL-8, and IL-6. This initiates an autocrine feedback loop, which in turn activates the JAK/STAT signaling pathway, ultimately resulting in elevated expression of PD-L1 on the cell surface (35). Among the members of the PDE4 family, PDE4B stands out as a pivotal regulator of cAMP levels in lymphocytes (135, 136). In PDE4B knockout mice, the proportion of B cells and T cells expressing PD-L1 is significantly higher than in wild-type mice (35). Crucially, despite its broad immunosuppressive properties, the PDE4 inhibitor roflumilast did not diminish the clinical activity of checkpoint inhibitors in a B-cell lymphoma mouse model. Indeed, PDE4 inhibition led to the emergence of a favorable anti-tumor immune profile, including a relative increase in CD8+ cytotoxic T cells (35). Similarly, the PDE4/cAMP pathway may also play a crucial role in regulating PD-L1 and immune infiltration in LUAD (137). Tumor mutation burden (TMB), defined as the number of somatic mutations in a tumor genome after excluding germline mutations, is widely regarded as a potential predictor of immune responsiveness. In a study, bioinformatic screening of LUAD cases with varying TMB values identified PDE4D as a gene associated with the efficacy of immunotherapy (34). High expression of PDE4D correlates with reduced infiltration of CD8+ T cells, suggesting a relative inhibition of the tumor immune microenvironment in LUAD. Moreover, increased PDE4D expression in LUAD patients who were insensitive to PD-1 therapy was consistent with reduced infiltration of CD8+ T cells. Mechanistically, PDE4D negatively regulates the expression and secretion of IL-23 in LUAD cells through cAMP. IL-23 derived from tumor cells fosters a self-amplifying loop of IL-9 secretion through an NF-κB-dependent mechanism, thereby enhancing the cytotoxic function of cytotoxic T lymphocytes (34) (Figure 5). These research findings may help to inform the clinical application of FDA-approved PDE4 inhibitors.
Glioblastoma (GBM) is often referred to as “immune cold” characterized by low T cell infiltration. This is not only attributed to the low expression of tumor antigens and a highly immunosuppressive tumor microenvironment but also to the tightly regulated blood-brain barrier that hinders the infiltration of immune cells into the tumor (138). Endothelial cells derived from glioblastomas (GdECs) are thought to arise from glioblastoma stem cells (GSCs) and participate in the formation of vasculogenic mimicry, which facilitates the growth and invasion of cancer (139). Through a rigorous drug screening approach, two cAMP activators have been identified as effectively inhibiting tubule structure formation in human GSCs (140). Indeed, combining cAMP activators with PD-1 blockade therapy resulted in a significant extension of survival in mice, accompanied by the observation of a robust memory immune response (141). Unstable responses to bevacizumab pose a major challenge in the antiangiogenic treatment of high-grade gliomas, which appears to be associated with increased levels of HIF1α and activation of AKT within tumors following treatment (142, 143). Studies show that there is an interaction between PDE4A and HIF1α in tumor cells (58). Selectively inhibiting PDE4 not only blocks this interaction but also suppresses angiogenesis and enhances the efficacy of bevacizumab in an orthotopic glioma model, opening new directions for anti-angiogenic treatment in malignant gliomas (36).
Rolipram and Ro 20-1724 were first identified as high-selectivity PDE4 inhibitors (144). Subsequently, many specific PDE4 inhibitors have been found and are considered anti-inflammatory agents for asthma and COPD, as they can reduce oxidative stress, TNF-α production, and cytokine generation. Roflumilast, marketed as Daxas®, was the first PDE4 inhibitor developed for the treatment of COPD (145). Additional indications are also being explored for roflumilast. Phase IV studies have shown that roflumilast reduce fat mass, leading to weight loss in women with obese polycystic ovary syndrome (PCOS); However, these reductions are less pronounced than those induced by liraglutide (146, 147). Roflumilast has also been tested for its potential to enhance cognition and information processing abilities in healthy individuals, with promising results observed (148). Apremilast, a third-generation PDE4 inhibitor, was approved in 2014 for the treatment of adult patients with psoriatic arthritis (PsA) and moderate-to-severe plaque psoriasis. A significant amount of preclinical data has validated the potential of PDE4 inhibitors as therapeutic agents in the treatment of schizophrenia and cognitive dysfunctions (149–152). Based on preclinical findings, the novel PDE4 inhibitor ASP9831 was tested in Phase I and Phase II trials for nonalcoholic steatohepatitis (NASH), but failed to improve biochemical biomarkers of the disease (153).
Furthermore, a multitude of preclinical and clinical investigations has substantiated the critical role of PDE4 inhibitors in cancer treatments (Table 2). Preclinical studies and clinical data collectively indicate that roflumilast demonstrate antitumor activity in B cell lymphomas (40, 125). In conjunction with cAMP-enhancing agents, the PDE4 inhibitor rolipram has exhibited the ability to inhibit triple-negative breast cancer in vitro and vivo (41). Likewise, apremilast prompted tumor regression in murine models of colorectal cancer (42). Possibly even more significantly, precise inhibition of PDE4D, whether through genetic approaches or by employing the PDE4D inhibitor Gebr-7b, re-established chemosensitivity in estrogen receptor-positive breast cancer cells that had become resistant to chemotherapy (33). Furthermore, studies have shown that rolipram enhance the therapeutic effect of bevacizumab on gliomas in vitro (36, 37). A clinical case report describes the use of apremilast in managing pustular psoriasis that occurred concurrently in a patient with Ewing’s sarcoma, following chemotherapy with Ifosfamide and Etoposide (154). This suggests that apremilast may offer a novel therapeutic strategy, particularly in the management of cutaneous side effects associated with chemotherapy.
In pharmacotherapy, a critical challenge lies in determining the minimum effective dose, which ensures therapeutic efficacy while minimizing drug utilization as much as possible. In other words, a central issue in drug development and clinical practice is how to administer the smallest dose of medication while maintaining its therapeutic effect. It was observed that in MCF-7 cells, which are responsive to hormonal stimuli, chylomicrons demonstrated superior performance over liposomes. Additionally, when rolipram was encapsulated within these vesicles, its IC50 value decreased fourfold relative to the administration of free rolipram (165). Conversely, the IC50 value for Rolipram encapsulated in liposomes was notably higher (165). The underlying mechanisms behind these observations can potentially be better understood through detailed molecular docking investigation. This provides significant guidance for achieving more effective drug delivery.
However, the clinical use of PDE4 inhibitors has faced challenges due to their associated side effects. For instance, although rolipram holds potential pharmacological effects, it has a considerably narrow therapeutic window. In clinical trials, adverse reactions such as nausea, vomiting, and headaches frequently occurred. These adverse effects of rolipram have significantly limited its clinical application (174). While roflumilast demonstrated superior performance to rolipram in clinical trials, gastrointestinal adverse reactions still occurred in 9.5% of cases, characterized by symptoms such as diarrhea, nausea, headache, weight loss, urinary tract infections, and psychiatric disorders (175). Nevertheless, patients may experience adverse reactions such as weight loss (10%), nausea (8.9%), diarrhea (7.7%), nasopharyngitis (2.6%), and upper respiratory tract infection (3.9%) (175). Several hypotheses have been proposed regarding the mechanisms behind PDE4-induced side effects (such as hypothermia, dizziness, diarrhea, nausea, vomiting, etc.), including subclass selectivity, partial inhibition, subcellular compartmentalization, and tissue-specific distribution, but none of these hypotheses has been fully substantiated (176). To overcome the adverse effects induced by PDE4 inhibitors, there is a need for more refined target specificity and a deeper exploration of PDE4 subtype particularities. This involves identifying and leveraging those subtypes that do not contribute to adverse reactions as new targets for drug development. It will require researchers to intricately decipher the functional differences between subtypes at the molecular level and to develop next-generation PDE4 inhibitors that can precisely act on the subtypes relevant to therapy without affecting others that may be implicated in side effects.
Emerging evidences shed light on the role of PDE4 and PDE4 inhibitors in cancer. However, the exact function of PDE4 isoforms and inhibitors in tumor development, drug resistance and immunotherapy remain debatable. Because of the ubiquitous distribution of PDE4, this superfamily of enzymes plays a role in a wide variety of biological processes. Nevertheless, the biological roles played by specific PDE4 isozymes vary due to their unique expression patterns at the level of tissues/organs, cell types and subcellular compartments. PDE4 are not only responsible for regulating the total cellular levels of cyclic nucleotides; instead, they generate distinct pockets or nanodomains of cyclic nucleotide signaling. This subcellular compartmentalization of cyclic nucleotide signaling allows a single cell to selectively respond to various extracellular and intracellular signals (15). In addition, different PDE4 inhibitors have different inhibitory effects on PDE4 subtypes, which leads to different effects of PDE4 inhibitors in the treatment of different tumors. For example, zardaverine has shown inhibitory effects on HCC both in vivo and in vitro (177). However, the inhibitory effect of zardaverine on cells appears to be cell-specific, as zardaverine is just not sensitive to cells, including HepG2 cells. More interestingly, the PDE4 inhibitor rolipram failed to mimic this effect, although it also elevated intracellular cAMP levels. Notably, the PDE4 inhibitor rolipram did not have a significant growth inhibitory effect on the zardaverine-sensitive HCC cell line SMMC-7721, which has a relatively low level of PDE4D expression. Consistent with our findings (105), roflumilast inhibited the growth of HepG2 that highly expressed PDE4D. Thus, these HCC cell lines may have different levels of PDE4D expression, and PDE4D may be responsible for cell proliferation during HCC pathology. Again, a similar situation may exist in other tumors. Alternatively, the regulation of the cAMP pathway by PDE4 inhibitors may depend on cell type and conditions. PDE4 inhibitors have the ability to induce the expression of PDE4 isoforms within endothelial and human airway epithelial cells (178, 179). These findings reinforce the notion that specific PDE4 isoforms are segregated by distinct signalosome complexes that regulate local cAMP signaling and confer distinct functional roles.
In addition, the effect of PDE4 inhibitors on tumor still needs more research to confirm. In the future, therapeutic strategies involving PDE4 inhibitors will place a higher value on subtype selectivity and tissue specificity to maximize anti-tumor effects while minimizing side effects. Identifying the patient populations most likely to benefit from PDE4-targeted therapy through precision medicine approaches is critical to improving treatment outcome. Furthermore, combining PDE4 inhibitors with chemotherapy, targeted therapy or immunotherapy will be the focus of research to inhibit tumor growth and restore and enhance the body’s anti-tumor immune response via multiple pathways.
As research progresses and technology advances, our understanding of the functional roles and molecular interactions of each PDE4 isoform and how their function and/or localization is altered in specific diseases grows, it will become more promising to see how precisely we can target PDE4 isoforms to achieve therapeutic efficacy while minimizing side effects. Also, the refinement of these studies is an important basis for the use of PDE4 as a therapeutic target for tumors.
H-LR: Writing – original draft, Writing – review & editing. S-HZ: Writing – original draft. P-YL: Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by supported by Hainan Province Science and Technology Special Fund (No. ZDYF2022SHFZ057), Hainan Provincial Natural Science Foundation of China (No. 821RC736) and National Natural Science Foundation of China (No. 82404019).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Delhaye S, Bardoni B. Role of phosphodiesterases in the pathophysiology of neurodevelopmental disorders. Mol Psychiatry. (2021) 26:4570–82. doi: 10.1038/s41380-020-00997-9
2. Keravis T, Lugnier C. Cyclic nucleotide phosphodiesterase (Pde) isozymes as targets of the intracellular signalling network: benefits of pde inhibitors in various diseases and perspectives for future therapeutic developments. Br J Pharmacol. (2012) 165:1288–305. doi: 10.1111/j.1476-5381.2011.01729.x
3. Zhang H, Liu Y, Liu J, Chen J, Wang J, Hua H, et al. Camp-pka/epac signaling and cancer: the interplay in tumor microenvironment. J Hematol Oncol. (2024) 17:5. doi: 10.1186/s13045-024-01524-x
4. Zhang L, Liu W, Li S, Wang J, Sun D, Li H, et al. Astragaloside iv alleviates renal fibrosis by inhibiting renal tubular epithelial cell pyroptosis induced by urotensin ii through regulating the camp/pka signaling pathway. PloS One. (2024) 19:e0304365. doi: 10.1371/journal.pone.0304365
5. Lu P, Fan J, Li B, Wang X, Song M. A novel protein encoded by circlarp1b promotes the proliferation and migration of vascular smooth muscle cells by suppressing camp signaling. Atherosclerosis. (2024) 395:117575. doi: 10.1016/j.atherosclerosis.2024.117575
6. Zhang Y, Yuan M, Cai W, Sun W, Shi X, Liu D, et al. Prostaglandin I(2) signaling prevents angiotensin ii-induced atrial remodeling and vulnerability to atrial fibrillation in mice. Cell Mol Life Sci. (2024) 81:264. doi: 10.1007/s00018-024-05259-3
7. Sampei C, Kato K, Arasaki Y, Kimura Y, Konno T, Otsuka K, et al. Gprc5a is a novel parathyroid hormone-inducible gene and negatively regulates osteoblast proliferation and differentiation. J Cell Physiol. (2024) 239:e31297. doi: 10.1002/jcp.31297
8. Jiang G, Zhou X, Hu Y, Tan X, Wang D, Yang L, et al. The antipsychotic drug pimozide promotes apoptosis through the raf/erk pathway and enhances autophagy in breast cancer cells. Cancer Biol Ther. (2024) 25:2302413. doi: 10.1080/15384047.2024.2302413
9. Zhuang L, Jia N, Zhang L, Zhang Q, Antwi SO, Sartorius K, et al. Gpbar-1/camp/pka signaling mitigates macrophage-mediated acute cholestatic liver injury via antagonizing nlrp3-asc inflammasome. Biochim Biophys Acta Mol Basis Dis. (2024) 1870:167266. doi: 10.1016/j.bbadis.2024.167266
10. Zhang S, Hu Y, Zhao Y, Feng Y, Wang X, Miao M, et al. Molecular mechanism of chang shen hua volatile oil modulating brain camp-pka-creb pathway to improve depression-like behavior in rats. Phytomedicine. (2024) 130:155729. doi: 10.1016/j.phymed.2024.155729
11. Ahmed MA, Kamel EO, Abd-Eldayem AM. Role of camp/pcreb and gsk-3beta/nf-kappab P65 signaling pathways in the renoprotective effect of mirabegron against renal ischemia-reperfusion injury in rats. Eur J Pharmacol. (2024) 974:176617. doi: 10.1016/j.ejphar.2024.176617
12. Zhao R, Ran L, Yao H, He Y, Lu X, Zhu W, et al. Moxibustion ameliorates ovarian function in premature ovarian insufficiency rats by activating camp/pka/creb to promote steroidogenesis in ovarian granulosa cells. J Steroid Biochem Mol Biol. (2024) 242:106547. doi: 10.1016/j.jsbmb.2024.106547
13. Ednacot EMQ, Nabhani A, Dinh DM, Morehouse BR. Pharmacological potential of cyclic nucleotide signaling in immunity. Pharmacol Ther. (2024) 258:108653. doi: 10.1016/j.pharmthera.2024.108653
14. Wei X, Yu G, Shen H, Luo Y, Shang T, Shen R, et al. Targeting phosphodiesterase 4 as a therapeutic strategy for cognitive improvement. Bioorg Chem. (2023) 130:106278. doi: 10.1016/j.bioorg.2022.106278
15. Baillie GS, Tejeda GS, Kelly MP. Therapeutic targeting of 3’,5’-cyclic nucleotide phosphodiesterases: inhibition and beyond. Nat Rev Drug Discovery. (2019) 18:770–96. doi: 10.1038/s41573-019-0033-4
16. Wan Q, Xu C, Zhu L, Zhang Y, Peng Z, Chen H, et al. Targeting pde4b (Phosphodiesterase-4 subtype B) for cardioprotection in acute myocardial infarction via neutrophils and microcirculation. Circ Res. (2022) 131:442–55. doi: 10.1161/CIRCRESAHA.122.321365
17. Rochford I, Joshi JC, Rayees S, Anwar M, Akhter MZ, Yalagala L, et al. Evidence for reprogramming of monocytes into reparative alveolar macrophages in vivo by targeting pde4b. Am J Physiol Lung Cell Mol Physiol. (2021) 321:L686–702. doi: 10.1152/ajplung.00145.2021
18. Suzuki O, Goto T, Yoshino T, Nakamura S, Maeda H. The role of phosphodiesterase 4b in il-8/ltb4-induced human neutrophil chemotaxis evaluated with a phosphodiesterase 4b inhibitor. Acta Pharm. (2015) 65:191–7. doi: 10.1515/acph-2015-0016
19. Gao R, Guo W, Fan T, Pang J, Hou Y, Feng X, et al. Phosphodiesterase 4d contributes to angiotensin ii-induced abdominal aortic aneurysm through smooth muscle cell apoptosis. Exp Mol Med. (2022) 54:1201–13. doi: 10.1038/s12276-022-00815-y
20. Xu R, Fu J, Hu Y, Yang X, Tao X, Chen L, et al. Roflumilast-mediated phosphodiesterase 4d inhibition reverses diabetes-associated cardiac dysfunction and remodeling: effects beyond glucose lowering. Diabetes. (2022) 71:1660–78. doi: 10.2337/db21-0898
21. Jino K, Miyamoto K, Kanbara T, Unemura C, Horiguchi N, Ago Y. Allosteric inhibition of phosphodiesterase 4d induces biphasic memory-enhancing effects associated with learning-activated signaling pathways. Psychopharmacol (Berl). (2024) 241:805–16. doi: 10.1007/s00213-023-06510-8
22. Shi Y, Lv J, Chen L, Luo G, Tao M, Pan J, et al. Phosphodiesterase-4d knockdown in the prefrontal cortex alleviates memory deficits and synaptic failure in mouse model of alzheimer’s disease. Front Aging Neurosci. (2021) 13:722580. doi: 10.3389/fnagi.2021.722580
23. Kozak BE, Rosch J. Curved guide wire for percutaneous pulmonary angiography. Radiology. (1988) 167:864–5. doi: 10.1148/radiology.167.3.3363155
24. Kim DU, Kwak B, Kim SW. Phosphodiesterase 4b is an effective therapeutic target in colorectal cancer. Biochem Biophys Res Commun. (2019) 508:825–31. doi: 10.1016/j.bbrc.2018.12.004
25. Bolger GB, Bizzi MF, Pinheiro SV, Trivellin G, Smoot L, Accavitti MA, et al. Camp-specific pde4 phosphodiesterases and aip in the pathogenesis of pituitary tumors. Endocr Relat Cancer. (2016) 23:419–31. doi: 10.1530/ERC-15-0205
26. Warrington NM, Gianino SM, Jackson E, Goldhoff P, Garbow JR, Piwnica-Worms D, et al. Cyclic amp suppression is sufficient to induce gliomagenesis in a mouse model of neurofibromatosis-1. Cancer Res. (2010) 70:5717–27. doi: 10.1158/0008-5472.CAN-09-3769
27. Wang J, Gu Q, Liu Y, Huang X, Zhang J, Liu B, et al. Low pde4a expression promoted the progression of ovarian cancer by inducing snail nuclear translocation. Exp Cell Res. (2024) 439:114100. doi: 10.1016/j.yexcr.2024.114100
28. Nummela P, Zafar S, Veikkolainen E, Ukkola I, Cinella V, Ayo A, et al. Gnas mutation inhibits growth and induces phosphodiesterase 4d expression in colorectal cancer cell lines. Int J Cancer. (2024) 154:1987–98. doi: 10.1002/ijc.34865
29. Zhang S, Yun D, Yang H, Eckstein M, Elbait GD, Zhou Y, et al. Roflumilast inhibits tumor growth and migration in stk11/lkb1 deficient pancreatic cancer. Cell Death Discovery. (2024) 10:124. doi: 10.1038/s41420-024-01890-y
30. Huang Y, Zheng Y, Wang Q, Qi C. Rolipram suppresses migration and invasion of human choriocarcinoma cells by inhibiting phosphodiesterase 4-mediated epithelial-mesenchymal transition. J Biochem Mol Toxicol. (2023) 37:e23363. doi: 10.1002/jbt.23363
31. Clarysse L, Gueguinou M, Potier-Cartereau M, Vandecasteele G, Bougnoux P, Chevalier S, et al. Camp-pka inhibition of sk3 channel reduced both ca2+ Entry and cancer cell migration by regulation of sk3-orai1 complex. Pflugers Arch. (2014) 466:1921–32. doi: 10.1007/s00424-013-1435-5
32. Peng QP, Du DB, Ming Q, Hu F, Wu ZB, Qiu S. Microrna 494 increases chemosensitivity to doxorubicin in gastric cancer cells by targeting phosphodiesterases 4d. Cell Mol Biol (Noisy-le-grand). (2018) 64:62–6. doi: 10.14715/cmb/2017.64.15.10
33. Mishra RR, Belder N, Ansari SA, Kayhan M, Bal H, Raza U, et al. Reactivation of camp pathway by pde4d inhibition represents a novel druggable axis for overcoming tamoxifen resistance in er-positive breast cancer. Clin Cancer Res. (2018) 24:1987–2001. doi: 10.1158/1078-0432.CCR-17-2776
34. Feng B, Pan B, Huang J, Du Y, Wang X, Wu J, et al. Pde4d/camp/il-23 axis determines the immunotherapy efficacy of lung adenocarcinoma via activating the il-9 autocrine loop of cytotoxic T lymphocytes. Cancer Lett. (2023) 565:216224. doi: 10.1016/j.canlet.2023.216224
35. Sasi B, Ethiraj P, Myers J, Lin AP, Jiang S, Qiu Z, et al. Regulation of pd-L1 expression is a novel facet of cyclic-amp-mediated immunosuppression. Leukemia. (2021) 35:1990–2001. doi: 10.1038/s41375-020-01105-0
36. Ramezani S, Vousooghi N, Ramezani Kapourchali F, Yousefzadeh-Chabok S, Reihanian Z, Alizadeh AM, et al. Rolipram optimizes therapeutic effect of bevacizumab by enhancing proapoptotic, antiproliferative signals in a glioblastoma heterotopic model. Life Sci. (2019) 239:116880. doi: 10.1016/j.lfs.2019.116880
37. Ramezani S, Vousooghi N, Kapourchali FR, Hadjighasem M, Hayat P, Amini N, et al. Rolipram potentiates bevacizumab-induced cell death in human glioblastoma stem-like cells. Life Sci. (2017) 173:11–9. doi: 10.1016/j.lfs.2017.02.005
38. Peng Y, Li Y, Tian Y, Ao G. Pde4a predicts poor prognosis and promotes metastasis by inducing epithelial-mesenchymal transition in hepatocellular carcinoma. J Cancer. (2018) 9:2389–96. doi: 10.7150/jca.24079
39. Hu S, Wang L, Zhang X, Wu Y, Yang J, Li J. Autophagy induces transforming growth factor-beta-dependent epithelial-mesenchymal transition in hepatocarcinoma cells through camp response element binding signalling. J Cell Mol Med. (2018) 22:5518–32. doi: 10.1111/jcmm.13825
40. Cooney JD, Aguiar RC. Phosphodiesterase 4 inhibitors have wide-ranging activity in B-cell Malignancies. Blood. (2016) 128:2886–90. doi: 10.1182/blood-2016-09-737676
41. Wang W, Li Y, Zhu JY, Fang D, Ding HF, Dong Z, et al. Triple negative breast cancer development can be selectively suppressed by sustaining an elevated level of cellular cyclic amp through simultaneously blocking its efflux and decomposition. Oncotarget. (2016) 7:87232–45. doi: 10.18632/oncotarget.13601
42. Nishi K, Luo H, Ishikura S, Doi K, Iwaihara Y, Wills L, et al. Apremilast induces apoptosis of human colorectal cancer cells with mutant kras. Anticancer Res. (2017) 37:3833–9. doi: 10.21873/anticanres.11762
43. Nejman-Gryz P, Grubek-Jaworska H, Glapinski J, Hoser G, Chazan R. Effects of the phosphodiestrase-4 inhibitor rolipram on lung resistance and inflammatory reaction in experimental asthma. J Physiol Pharmacol. (2006) 57 Suppl 4:229–39.
44. Halpin DMG, Criner GJ, Papi A, Singh D, Anzueto A, Martinez FJ, et al. Global initiative for the diagnosis, management, and prevention of chronic obstructive lung disease. The 2020 gold science committee report on covid-19 and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2021) 203:24–36. doi: 10.1164/rccm.202009-3533SO
45. Garnock-Jones KP. Roflumilast: A review in copd. Drugs. (2015) 75:1645–56. doi: 10.1007/s40265-015-0463-1
46. Freitas E, Gooderham M, Torres T. New topical therapies in development for atopic dermatitis. Drugs. (2022) 82:843–53. doi: 10.1007/s40265-022-01722-2
47. Martina SD, Ismail MS, Vesta KS. Cilomilast: orally active selective phosphodiesterase-4 inhibitor for treatment of chronic obstructive pulmonary disease. Ann Pharmacother. (2006) 40:1822–8. doi: 10.1345/aph.1H049
48. Beeh KM, Beier J, Lerch C, Schulz AK, Buhl R. Effects of piclamilast, a selective phosphodiesterase-4 inhibitor, on oxidative burst of sputum cells from mild asthmatics and stable copd patients. Lung. (2004) 182:369–77. doi: 10.1007/s00408-004-2518-z
49. Poole RM, Ballantyne AD. Apremilast: first global approval. Drugs. (2014) 74:825–37. doi: 10.1007/s40265-014-0218-4
50. Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. (2006) 58:488–520. doi: 10.1124/pr.58.3.5
51. Maurice DH, Ke H, Ahmad F, Wang Y, Chung J, Manganiello VC. Advances in targeting cyclic nucleotide phosphodiesterases. Nat Rev Drug Discovery. (2014) 13:290–314. doi: 10.1038/nrd4228
52. Beghe B, Rabe KF, Fabbri LM. Phosphodiesterase-4 inhibitor therapy for lung diseases. Am J Respir Crit Care Med. (2013) 188:271–8. doi: 10.1164/rccm.201301-0021PP
53. Goldhoff P, Warrington NM, Limbrick DD Jr., Hope A, Woerner BM, Jackson E, et al. Targeted inhibition of cyclic amp phosphodiesterase-4 promotes brain tumor regression. Clin Cancer Res. (2008) 14:7717–25. doi: 10.1158/1078-0432.CCR-08-0827
54. Chen TC, Wadsten P, Su S, Rawlinson N, Hofman FM, Hill CK, et al. The type iv phosphodiesterase inhibitor rolipram induces expression of the cell cycle inhibitors P21(Cip1) and P27(Kip1), resulting in growth inhibition, increased differentiation, and subsequent apoptosis of Malignant a-172 glioma cells. Cancer Biol Ther. (2002) 1:268–76. doi: 10.4161/cbt.80
55. Chamseddine AN, Cabrero M, Wei Y, Ganan-Gomez I, Colla S, Takahashi K, et al. Pde4 differential expression is a potential prognostic factor and therapeutic target in patients with myelodysplastic syndrome and chronic myelomonocytic leukemia. Clin Lymphoma Myeloma Leuk. (2016) 16 Suppl:S67–73. doi: 10.1016/j.clml.2016.02.026
56. Yang J, Hu X, Wang Y, Liu W, Zhang M, Zhang A, et al. Identification of the shared gene signatures and molecular mechanisms between multiple sclerosis and non-small cell lung cancer. Front Immunol. (2023) 14:1180449. doi: 10.3389/fimmu.2023.1180449
57. Wittliff JL, Sereff SB, Daniels MW. Expression of genes for methylxanthine pathway-associated enzymes accompanied by sex steroid receptor status impacts breast carcinoma progression. Horm Cancer. (2017) 8:298–313. doi: 10.1007/s12672-017-0309-2
58. Pullamsetti SS, Banat GA, Schmall A, Szibor M, Pomagruk D, Hanze J, et al. Phosphodiesterase-4 promotes proliferation and angiogenesis of lung cancer by crosstalk with hif. Oncogene. (2013) 32:1121–34. doi: 10.1038/onc.2012.136
59. Xu J, Ma J, Guan B, Li J, Wang Y, Hu S. Lncrna hcp5 promotes Malignant cell behaviors in esophageal squamous cell carcinoma via the pi3k/akt/mtor signaling. Cell Cycle. (2021) 20:1374–88. doi: 10.1080/15384101.2021.1944512
60. Chen MY, Fan K, Zhao LJ, Wei JM, Gao JX, Li ZF. Long non-coding rna nuclear enriched abundant transcript 1 (Neat1) sponges microrna-124-3p to up-regulate phosphodiesterase 4b (Pde4b) to accelerate the progression of parkinson’s disease. Bioengineered. (2021) 12:708–19. doi: 10.1080/21655979.2021.1883279
61. Chinn AM, Salmeron C, Lee J, Sriram K, Raz E, Insel PA. Pde4b is a homeostatic regulator of cyclic amp in dendritic cells. Front Pharmacol. (2022) 13:833832. doi: 10.3389/fphar.2022.833832
62. Bolger GB. The pde4 camp-specific phosphodiesterases: targets for drugs with antidepressant and memory-enhancing action. Adv Neurobiol. (2017) 17:63–102. doi: 10.1007/978-3-319-58811-7_4
63. Miao Y, Peng L, Chen Z, Hu Y, Tao L, Yao Y, et al. Recent advances of phosphodiesterase 4b in cancer. Expert Opin Ther Targets. (2023) 27:121–32. doi: 10.1080/14728222.2023.2183496
64. Salinas MP, Sepulveda J, Hidalgo L, Peirano D, Morel M, Uribe P, et al. A systematic review and meta-analysis of artificial intelligence versus clinicians for skin cancer diagnosis. NPJ Digit Med. (2024) 7:125. doi: 10.1038/s41746-024-01103-x
65. Li Z, Zhang X, Jin Q, Zhang Q, Yue Q, Fujimoto M, et al. Development of a macrophage-related risk model for metastatic melanoma. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms241813752
66. Du Y, Jiang X, Zhang Y, Ying J, Yi Q. Integrating single-cell and bulk rna-seq to construct a metastasis-related model for evaluating immunotherapy and chemotherapy in uveal melanoma. Curr Med Chem. (2024) 31:7030–42. doi: 10.2174/0109298673286355231222054226
67. Marquette A, Andre J, Bagot M, Bensussan A, Dumaz N. Erk and pde4 cooperate to induce raf isoform switching in melanoma. Nat Struct Mol Biol. (2011) 18:584–91. doi: 10.1038/nsmb.2022
68. Huang Z, Liu J, Yang J, Yan Y, Yang C, He X, et al. Pde4b induces epithelial-to-mesenchymal transition in bladder cancer cells and is transcriptionally suppressed by cbx7. Front Cell Dev Biol. (2021) 9:783050. doi: 10.3389/fcell.2021.783050
69. Shiota M, Endo S, Tsukahara S, Tanegashima T, Kobayashi S, Matsumoto T, et al. Importance of 3beta-hydroxysteroid dehydrogenases and their clinical use in prostate cancer. Endocr Relat Cancer. (2024) 31. doi: 10.1530/ERC-24-0023
70. Gebrael G, Hage Chehade C, Sayegh N, Tripathi N, Chigarira B, Goel D, et al. Natural course of metastatic castration-resistant prostate cancer in the era of intensified androgen deprivation therapy in the hormone-sensitive setting. Prostate. (2024) 84:888–92. doi: 10.1002/pros.24696
71. Kashiwagi E, Shiota M, Yokomizo A, Itsumi M, Inokuchi J, Uchiumi T, et al. Downregulation of phosphodiesterase 4b (Pde4b) activates protein kinase a and contributes to the progression of prostate cancer. Prostate. (2012) 72:741–51. doi: 10.1002/pros.21478
72. Shipp MA, Ross KN, Tamayo P, Weng AP, Kutok JL, Aguiar RC, et al. Diffuse large B-cell lymphoma outcome prediction by gene-expression profiling and supervised machine learning. Nat Med. (2002) 8:68–74. doi: 10.1038/nm0102-68
73. Smith PG, Wang F, Wilkinson KN, Savage KJ, Klein U, Neuberg DS, et al. The phosphodiesterase pde4b limits camp-associated pi3k/akt-dependent apoptosis in diffuse large B-cell lymphoma. Blood. (2005) 105:308–16. doi: 10.1182/blood-2004-01-0240
74. Ethiraj P, Sasi B, Holder KN, Lin AP, Qiu Z, Jaafar C, et al. Cyclic-amp signalling, myc and hypoxia-inducible factor 1alpha intersect to regulate angiogenesis in B-cell lymphoma. Br J Haematol. (2022) 198:349–59. doi: 10.1111/bjh.18196
75. Suhasini AN, Wang L, Holder KN, Lin AP, Bhatnagar H, Kim SW, et al. A phosphodiesterase 4b-dependent interplay between tumor cells and the microenvironment regulates angiogenesis in B-cell lymphoma. Leukemia. (2016) 30:617–26. doi: 10.1038/leu.2015.302
76. Lin C, Kuma L, Shen L. High-grade B-cell lymphoma with myc and bcl2 rearrangements presenting as a cervical mass. Pathology. (2024) 56:588–90. doi: 10.1016/j.pathol.2023.08.012
77. Hilton LK, Collinge B, Ben-Neriah S, Alduaij W, Shaalan H, Weng AP, et al. Motive and opportunity: myc rearrangements in high-grade B-cell lymphoma with myc and bcl2 rearrangements (an llmpp study). Blood. (2024) 144:525–40. doi: 10.1182/blood.2024024251
78. Nam J, Kim DU, Kim E, Kwak B, Ko MJ, Oh AY, et al. Disruption of the myc-pde4b regulatory circuitry impairs B-cell lymphoma survival. Leukemia. (2019) 33:2912–23. doi: 10.1038/s41375-019-0492-y
79. Pleiman JK, Irving AA, Wang Z, Toraason E, Clipson L, Dove WF, et al. The conserved protective cyclic amp-phosphodiesterase function pde4b is expressed in the adenoma and adjacent normal colonic epithelium of mammals and silenced in colorectal cancer. PloS Genet. (2018) 14:e1007611. doi: 10.1371/journal.pgen.1007611
80. Bevanda M, Kelam N, Racetin A, Filipovic N, Bevanda Glibo D, Bevanda I, et al. Expression pattern of pde4b, pde4d, and sfrp5 markers in colorectal cancer. Med (Kaunas). (2024) 60. doi: 10.3390/medicina60081202
81. Mahmood B, Damm MM, Jensen TS, Backe MB, Dahllof MS, Poulsen SS, et al. Phosphodiesterases in non-neoplastic appearing colonic mucosa from patients with colorectal neoplasia. BMC Cancer. (2016) 16:938. doi: 10.1186/s12885-016-2980-z
82. Tsunoda T, Ota T, Fujimoto T, Doi K, Tanaka Y, Yoshida Y, et al. Inhibition of phosphodiesterase-4 (Pde4) activity triggers luminal apoptosis and akt dephosphorylation in a 3-D colonic-crypt model. Mol Cancer. (2012) 11:46. doi: 10.1186/1476-4598-11-46
83. Wright TA, Gemmell AO, Tejeda GS, Blair CM, Baillie GS. Cancer: phosphodiesterase type 4c (Pde4c), the forgotten subfamily as a therapeutic target. Int J Biochem Cell Biol. (2023) 162:106453. doi: 10.1016/j.biocel.2023.106453
84. Uhlen M, Zhang C, Lee S, Sjostedt E, Fagerberg L, Bidkhori G, et al. A pathology atlas of the human cancer transcriptome. Science. (2017) 357. doi: 10.1126/science.aan2507
85. Chen L, Zhang X, Zhang Q, Zhang T, Xie J, Wei W, et al. A necroptosis related prognostic model of pancreatic cancer based on single cell sequencing analysis and transcriptome analysis. Front Immunol. (2022) 13:1022420. doi: 10.3389/fimmu.2022.1022420
86. He RQ, Li XJ, Liang L, Xie Y, Luo DZ, Ma J, et al. The suppressive role of mir-542-5p in nsclc: the evidence from clinical data and in vivo validation using a chick chorioallantoic membrane model. BMC Cancer. (2017) 17:655. doi: 10.1186/s12885-017-3646-1
87. Wang Y, Zhang Y, Li Y, Huang J. Elevated pde4c level serves as a candidate diagnostic biomarker and correlates with poor survival in thyroid carcinoma. Sci Rep. (2024) 14:6813. doi: 10.1038/s41598-024-57533-w
88. Bao Z, Feng Y, Wang H, Zhang C, Sun L, Yan Z, et al. Integrated analysis using methylation and gene expression microarrays reveals pde4c as a prognostic biomarker in human glioma. Oncol Rep. (2014) 32:250–60. doi: 10.3892/or.2014.3176
89. Garritano S, Inga A, Gemignani F, Landi S. More targets, more pathways and more clues for mutant P53. Oncogenesis. (2013) 2:e54. doi: 10.1038/oncsis.2013.15
90. Huang Q, Liang X, Ren T, Huang Y, Zhang H, Yu Y, et al. The role of tumor-associated macrophages in osteosarcoma progression - therapeutic implications. Cell Oncol (Dordr). (2021) 44:525–39. doi: 10.1007/s13402-021-00598-w
91. Pan F, Pan R, Hu R, Zhang H, Lei S, Zhang L, et al. Analysis of the effects of M2 macrophage-derived pde4c on the prognosis, metastasis and immunotherapy benefit of osteosarcoma. J Cell Mol Med. (2024) 28:e18395. doi: 10.1111/jcmm.18395
92. Paes D, Schepers M, Rombaut B, van den Hove D, Vanmierlo T, Prickaerts J. The molecular biology of phosphodiesterase 4 enzymes as pharmacological targets: an interplay of isoforms, conformational states, and inhibitors. Pharmacol Rev. (2021) 73:1016–49. doi: 10.1124/pharmrev.120.000273
93. Houslay MD, Adams DR. Pde4 camp phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem J. (2003) 370:1–18. doi: 10.1042/BJ20021698
94. Wu Y, Hong Z, Xu W, Chen J, Wang Q, Chen J, et al. Circular rna circpde4d protects against osteoarthritis by binding to mir-103a-3p and regulating fgf18. Mol Ther. (2021) 29:308–23. doi: 10.1016/j.ymthe.2020.09.002
95. Baca SC, Prandi D, Lawrence MS, Mosquera JM, Romanel A, Drier Y, et al. Punctuated evolution of prostate cancer genomes. Cell. (2013) 153:666–77. doi: 10.1016/j.cell.2013.03.021
96. Uckert S, Oelke M, Stief CG, Andersson KE, Jonas U, Hedlund P. Immunohistochemical distribution of camp- and cgmp-phosphodiesterase (Pde) isoenzymes in the human prostate. Eur Urol. (2006) 49:740–5. doi: 10.1016/j.eururo.2005.12.050
97. Henderson DJ, Byrne A, Dulla K, Jenster G, Hoffmann R, Baillie GS, et al. The camp phosphodiesterase-4d7 (Pde4d7) is downregulated in androgen-independent prostate cancer cells and mediates proliferation by compartmentalising camp at the plasma membrane of vcap prostate cancer cells. Br J Cancer. (2014) 110:1278–87. doi: 10.1038/bjc.2014.22
98. Cao B, Wang K, Liao JM, Zhou X, Liao P, Zeng SX, et al. Inactivation of oncogenic camp-specific phosphodiesterase 4d by mir-139-5p in response to P53 activation. Elife. (2016) 5. doi: 10.7554/eLife.15978
99. Kim DU, Nam J, Cha MD, Kim SW. Inhibition of phosphodiesterase 4d decreases the Malignant properties of dld-1 colorectal cancer cells by repressing the akt/mtor/myc signaling pathway. Oncol Lett. (2019) 17:3589–98. doi: 10.3892/ol.2019.9996
100. Chen L, Gao H, Liang J, Qiao J, Duan J, Shi H, et al. Mir-203a-3p promotes colorectal cancer proliferation and migration by targeting pde4d. Am J Cancer Res. (2018) 8:2387–401.
101. Bottcher R, Dulla K, van Strijp D, Dits N, Verhoef EI, Baillie GS, et al. Human pde4d isoform composition is deregulated in primary prostate cancer and indicative for disease progression and development of distant metastases. Oncotarget. (2016) 7:70669–84. doi: 10.18632/oncotarget.12204
102. Fan J, Wang F. Mancr drives esophageal carcinoma progression by targeting pde4d. J BUON. (2021) 26:1517–22.
103. Massimi M, Ragusa F, Cardarelli S, Giorgi M. Targeting cyclic amp signalling in hepatocellular carcinoma. Cells. (2019) 8. doi: 10.3390/cells8121511
104. Ragusa F, Panera N, Cardarelli S, Scarsella M, Bianchi M, Biagioni S, et al. Phosphodiesterase 4d depletion/inhibition exerts anti-oncogenic properties in hepatocellular carcinoma. Cancers (Basel). (2021) 13. doi: 10.3390/cancers13092182
105. Ren H, Chen Y, Ao Z, Cheng Q, Yang X, Tao H, et al. Pde4d binds and interacts with yap to cooperatively promote hcc progression. Cancer Lett. (2022) 541:215749. doi: 10.1016/j.canlet.2022.215749
106. Kawata-Shimamura Y, Eguchi H, Kawabata-Iwakawa R, Nakahira M, Okazaki Y, Yoda T, et al. Biomarker discovery for practice of precision medicine in hypopharyngeal cancer: A theranostic study on response prediction of the key therapeutic agents. BMC Cancer. (2022) 22:779. doi: 10.1186/s12885-022-09853-1
107. Qiang Z, Zhou ZY, Peng T, Jiang PZ, Shi N, Njoya EM, et al. Inhibition of tpl2 by interferon-alpha suppresses bladder cancer through activation of pde4d. J Exp Clin Cancer Res. (2018) 37:288. doi: 10.1186/s13046-018-0971-4
108. Cao M, Nawalaniec K, Ajay AK, Luo Y, Moench R, Jin Y, et al. Pde4d targeting enhances anti-tumor effects of sorafenib in clear cell renal cell carcinoma and attenuates mapk/erk signaling in a craf-dependent manner. Transl Oncol. (2022) 19:101377. doi: 10.1016/j.tranon.2022.101377
109. Shen DW, Pouliot LM, Hall MD, Gottesman MM. Cisplatin resistance: A cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol Rev. (2012) 64:706–21. doi: 10.1124/pr.111.005637
110. He W, Zhu H, Zhang S, Shu G, Lei H, Wang M, et al. Epigenetic editing of brca1 promoter increases cisplatin and olaparib sensitivity of ovarian cancer cells. Epigenetics. (2024) 19:2357518. doi: 10.1080/15592294.2024.2357518
111. Mukherjee P, Bagchi A, Banerjee A, Roy H, Bhattacharya A, Biswas A, et al. Pde4 inhibitor eliminates breast cancer stem cells via noncanonical activation of mtor. J Cell Biochem. (2022) 123:1980–96. doi: 10.1002/jcb.30325
112. Kapper C, Oppelt P, Arbeithuber B, Gyunesh AA, Vilusic I, Stelzl P, et al. Targeting ferroptosis in ovarian cancer: novel strategies to overcome chemotherapy resistance. Life Sci. (2024) 349:122720. doi: 10.1016/j.lfs.2024.122720
113. Dixit D, Prager BC, Gimple RC, Miller TE, Wu Q, Yomtoubian S, et al. Glioblastoma stem cells reprogram chromatin in vivo to generate selective therapeutic dependencies on dpy30 and phosphodiesterases. Sci Transl Med. (2022) 14:eabf3917. doi: 10.1126/scitranslmed.abf3917
114. Xie C, Lin PJ, Hao J. Eggmanone effectively overcomes prostate cancer cell chemoresistance. Biomedicines. (2021) 9. doi: 10.3390/biomedicines9050538
115. Copsel S, Bruzzone A, May M, Beyrath J, Wargon V, Cany J, et al. Multidrug resistance protein 4/atp binding cassette transporter 4: A new potential therapeutic target for acute myeloid leukemia. Oncotarget. (2014) 5:9308–21. doi: 10.18632/oncotarget.2425
116. Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Update of the national surgical adjuvant breast and bowel project study of tamoxifen and raloxifene (Star) P-2 trial: preventing breast cancer. Cancer Prev Res (Phila). (2010) 3:696–706. doi: 10.1158/1940-6207.CAPR-10-0076
117. Early Breast Cancer Trialists’ Collaborative G, Davies C, Godwin J, Gray R, Clarke M, Cutter D, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. (2011) 378:771–84. doi: 10.1016/S0140-6736(11)60993-8
118. Nass N, Kalinski T. Tamoxifen resistance: from cell culture experiments towards novel biomarkers. Pathol Res Pract. (2015) 211:189–97. doi: 10.1016/j.prp.2015.01.004
119. Herrera-Abreu MT, Palafox M, Asghar U, Rivas MA, Cutts RJ, Garcia-Murillas I, et al. Early adaptation and acquired resistance to cdk4/6 inhibition in estrogen receptor-positive breast cancer. Cancer Res. (2016) 76:2301–13. doi: 10.1158/0008-5472.CAN-15-0728
120. Lloyd MR, Spring LM, Bardia A, Wander SA. Mechanisms of resistance to cdk4/6 blockade in advanced hormone receptor-positive, her2-negative breast cancer and emerging therapeutic opportunities. Clin Cancer Res. (2022) 28:821–30. doi: 10.1158/1078-0432.CCR-21-2947
121. Saatci O, Cetin M, Uner M, Tokat UM, Chatzistamou I, Ersan PG, et al. Toxic parp trapping upon camp-induced DNA damage reinstates the efficacy of endocrine therapy and cdk4/6 inhibitors in treatment-refractory er+ Breast cancer. Nat Commun. (2023) 14:6997. doi: 10.1038/s41467-023-42736-y
122. Abraham HG, Xia Y, Mukherjee B, Merajver SD. Incidence and survival of inflammatory breast cancer between 1973 and 2015 in the seer database. Breast Cancer Res Treat. (2021) 185:229–38. doi: 10.1007/s10549-020-05938-2
123. Stevens LE, Peluffo G, Qiu X, Temko D, Fassl A, Li Z, et al. Jak-stat signaling in inflammatory breast cancer enables chemotherapy-resistant cell states. Cancer Res. (2023) 83:264–84. doi: 10.1158/0008-5472.CAN-22-0423
124. Cooney JD, Lin AP, Jiang D, Wang L, Suhasini AN, Myers J, et al. Synergistic targeting of the regulatory and catalytic subunits of pi3kdelta in mature B-cell Malignancies. Clin Cancer Res. (2018) 24:1103–13. doi: 10.1158/1078-0432.CCR-17-2218
125. Kim DY, Nam J, Chung JS, Kim SW, Shin HJ. Role of roflumilast combined with eshap chemotherapy in relapsed/refractory patients with diffuse large B-cell lymphoma. Cancer Res Treat. (2022) 54:301–13. doi: 10.4143/crt.2020.1371
126. Zhou L, Fan S, Zhang W, Wang D, Tang D. Microbes in the tumor microenvironment: new additions to break the tumor immunotherapy dilemma. Microbiol Res. (2024) 285:127777. doi: 10.1016/j.micres.2024.127777
127. Xiong H, Shen Z. Tissue-resident memory T cells in immunotherapy and immune-related adverse events by immune checkpoint inhibitor. Int J Cancer. (2024) 155:193–202. doi: 10.1002/ijc.34940
128. Fazio R, Audisio A, Dapra V, Conti C, Benhima N, Abbassi FZ, et al. Non-operative management after immune checkpoint inhibitors for early-stage, dmmr/msi-H gastrointestinal cancers. Cancer Treat Rev. (2024) 128:102752. doi: 10.1016/j.ctrv.2024.102752
129. Xu M, Li S. The opportunities and challenges of using pd-1/pd-L1 inhibitors for leukemia treatment. Cancer Lett. (2024) 593:216969. doi: 10.1016/j.canlet.2024.216969
130. Afshari AR, Sanati M, Ahmadi SS, Kesharwani P, Sahebkar A. Harnessing the capacity of phytochemicals to enhance immune checkpoint inhibitor therapy of cancers: A focus on brain Malignancies. Cancer Lett. (2024) 593:216955. doi: 10.1016/j.canlet.2024.216955
131. Boretti A. Improving chimeric antigen receptor T-cell therapies by using artificial intelligence and internet of things technologies: A narrative review. Eur J Pharmacol. (2024) 974:176618. doi: 10.1016/j.ejphar.2024.176618
132. Taherian MR, Azarbar P, Barkhordar M, Toufani S, Aliabadi LS, Bahri T, et al. Efficacy and safety of adoptive T-cell therapy in treating cytomegalovirus infections post-haematopoietic stem cell transplantation: A systematic review and meta-analysis. Rev Med Virol. (2024) 34:e2558. doi: 10.1002/rmv.2558
133. Ramapriyan R, Vykunta VS, Vandecandelaere G, Richardson LGK, Sun J, Curry WT, et al. Altered cancer metabolism and implications for next-generation car T-cell therapies. Pharmacol Ther. (2024) 259:108667. doi: 10.1016/j.pharmthera.2024.108667
134. Conti M, Richter W, Mehats C, Livera G, Park JY, Jin C. Cyclic amp-specific pde4 phosphodiesterases as critical components of cyclic amp signaling. J Biol Chem. (2003) 278:5493–6. doi: 10.1074/jbc.R200029200
135. Manning CD, Burman M, Christensen SB, Cieslinski LB, Essayan DM, Grous M, et al. Suppression of human inflammatory cell function by subtype-selective pde4 inhibitors correlates with inhibition of pde4a and pde4b. Br J Pharmacol. (1999) 128:1393–8. doi: 10.1038/sj.bjp.0702911
136. Gantner F, Gotz C, Gekeler V, Schudt C, Wendel A, Hatzelmann A. Phosphodiesterase profile of human B lymphocytes from normal and atopic donors and the effects of pde inhibition on B cell proliferation. Br J Pharmacol. (1998) 123:1031–8. doi: 10.1038/sj.bjp.0701688
137. Tong L, Shan M, Zou W, Liu X, Felsher DW, Wang J. Cyclic adenosine monophosphate/phosphodiesterase 4 pathway associated with immune infiltration and pd-L1 expression in lung adenocarcinoma cells. Front Oncol. (2022) 12:904969. doi: 10.3389/fonc.2022.904969
138. Perng P, Lim M. Immunosuppressive mechanisms of Malignant gliomas: parallels at non-cns sites. Front Oncol. (2015) 5:153. doi: 10.3389/fonc.2015.00153
139. Qin Z, Huang Y, Li Z, Pan G, Zheng L, Xiao X, et al. Glioblastoma vascular plasticity limits effector T-cell infiltration and is blocked by camp activation. Cancer Immunol Res. (2023) 11:1351–66. doi: 10.1158/2326-6066.CIR-22-0872
140. Garcia J, Hurwitz HI, Sandler AB, Miles D, Coleman RL, Deurloo R, et al. Bevacizumab (Avastin(R)) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat Rev. (2020) 86:102017. doi: 10.1016/j.ctrv.2020.102017
141. Lee J, Kay KE, Vogelbaum MA, Lathia JD. Let the guard down: camp activators can improve immunotherapy in gbm. Cancer Immunol Res. (2023) 11:1300–1. doi: 10.1158/2326-6066.CIR-23-0667
142. Hartwich J, Orr WS, Ng CY, Spence Y, Morton C, Davidoff AM. Hif-1alpha activation mediates resistance to anti-angiogenic therapy in neuroblastoma xenografts. J Pediatr Surg. (2013) 48:39–46. doi: 10.1016/j.jpedsurg.2012.10.016
143. Masoud GN, Li W. Hif-1alpha pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. (2015) 5:378–89. doi: 10.1016/j.apsb.2015.05.007
144. Lugnier C. Cyclic nucleotide phosphodiesterase (Pde) superfamily: A new target for the development of specific therapeutic agents. Pharmacol Ther. (2006) 109:366–98. doi: 10.1016/j.pharmthera.2005.07.003
145. Hatzelmann A, Morcillo EJ, Lungarella G, Adnot S, Sanjar S, Beume R, et al. The preclinical pharmacology of roflumilast–a selective, oral phosphodiesterase 4 inhibitor in development for chronic obstructive pulmonary disease. Pulm Pharmacol Ther. (2010) 23:235–56. doi: 10.1016/j.pupt.2010.03.011
146. Jensterle M, Kocjan T, Janez A. Phosphodiesterase 4 inhibition as a potential new therapeutic target in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. (2014) 99:E1476–81. doi: 10.1210/jc.2014-1430
147. Jensterle M, Salamun V, Kocjan T, Vrtacnik Bokal E, Janez A. Short term monotherapy with glp-1 receptor agonist liraglutide or pde 4 inhibitor roflumilast is superior to metformin in weight loss in obese pcos women: A pilot randomized study. J Ovarian Res. (2015) 8:32. doi: 10.1186/s13048-015-0161-3
148. Van Duinen MA, Sambeth A, Heckman PRA, Smit S, Tsai M, Lahu G, et al. Acute administration of roflumilast enhances immediate recall of verbal word memory in healthy young adults. Neuropharmacology. (2018) 131:31–8. doi: 10.1016/j.neuropharm.2017.12.019
149. Kanes SJ, Tokarczyk J, Siegel SJ, Bilker W, Abel T, Kelly MP. Rolipram: A specific phosphodiesterase 4 inhibitor with potential antipsychotic activity. Neuroscience. (2007) 144:239–46. doi: 10.1016/j.neuroscience.2006.09.026
150. Kelly MP, Isiegas C, Cheung YF, Tokarczyk J, Yang X, Esposito MF, et al. Constitutive activation of galphas within forebrain neurons causes deficits in sensorimotor gating because of pka-dependent decreases in camp. Neuropsychopharmacology. (2007) 32:577–88. doi: 10.1038/sj.npp.1301099
151. Kelly MP, Stein JM, Vecsey CG, Favilla C, Yang X, Bizily SF, et al. Developmental etiology for neuroanatomical and cognitive deficits in mice overexpressing galphas, a G-protein subunit genetically linked to schizophrenia. Mol Psychiatry. (2009) 14:398–415, 347. doi: 10.1038/mp.2008.124
152. Rodefer JS, Saland SK, Eckrich SJ. Selective phosphodiesterase inhibitors improve performance on the ed/id cognitive task in rats. Neuropharmacology. (2012) 62:1182–90. doi: 10.1016/j.neuropharm.2011.08.008
153. Ratziu V, Bedossa P, Francque SM, Larrey D, Aithal GP, Serfaty L, et al. Lack of efficacy of an inhibitor of pde4 in phase 1 and 2 trials of patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. (2014) 12:1724–30 e5. doi: 10.1016/j.cgh.2014.01.040
154. Valenti M, Gargiulo L, Ibba L, Bertuzzi A, Manara S, Toso F, et al. Apremilast for the treatment of pustular psoriasis induced by neoadjuvant ifosfamide + Etoposide chemotherapy for ewing sarcoma: A case report. J Dermatol Treat. (2024) 35:2319303. doi: 10.1080/09546634.2024.2319303
155. Powers GL, Hammer KD, Domenech M, Frantskevich K, Malinowski RL, Bushman W, et al. Phosphodiesterase 4d inhibitors limit prostate cancer growth potential. Mol Cancer Res. (2015) 13:149–60. doi: 10.1158/1541-7786.MCR-14-0110
156. Abusnina A, Keravis T, Zhou Q, Justiniano H, Lobstein A, Lugnier C. Tumour growth inhibition and anti-angiogenic effects using curcumin correspond to combined pde2 and pde4 inhibition. Thromb Haemost. (2015) 113:319–28. doi: 10.1160/TH14-05-0454
157. Yougbare I, Belemnaba L, Morin C, Abusnina A, Senouvo YF, Keravis T, et al. Ncs 613, a potent pde4 inhibitor, displays anti-inflammatory and anti-proliferative properties on A549 lung epithelial cells and human lung adenocarcinoma explants. Front Pharmacol. (2020) 11:1266. doi: 10.3389/fphar.2020.01266
158. Tinsley HN, Gary BD, Keeton AB, Zhang W, Abadi AH, Reynolds RC, et al. Sulindac sulfide selectively inhibits growth and induces apoptosis of human breast tumor cells by phosphodiesterase 5 inhibition, elevation of cyclic gmp, and activation of protein kinase G. Mol Cancer Ther. (2009) 8:3331–40. doi: 10.1158/1535-7163.MCT-09-0758
159. Ou M, Cho HY, Fu J, Thein TZ, Wang W, Swenson SD, et al. Inhibition of autophagy and induction of glioblastoma cell death by neo214, a perillyl alcohol-rolipram conjugate. Autophagy. (2023) 19:3169–88. doi: 10.1080/15548627.2023.2242696
160. Chen TC, Chan N, Labib S, Yu J, Cho HY, Hofman FM, et al. Induction of pro-apoptotic endoplasmic reticulum stress in multiple myeloma cells by neo214, perillyl alcohol conjugated to rolipram. Int J Mol Sci. (2018) 19. doi: 10.3390/ijms19010277
161. Kelly K, Mejia A, Suhasini AN, Lin AP, Kuhn J, Karnad AB, et al. Safety and pharmacodynamics of the pde4 inhibitor roflumilast in advanced B-cell Malignancies. Clin Cancer Res. (2017) 23:1186–92. doi: 10.1158/1078-0432.CCR-16-1207
162. Abdel-Wahab BA, Walbi IA, Albarqi HA, Ali FEM, Hassanein EHM. Roflumilast protects from cisplatin-induced testicular toxicity in male rats and enhances its cytotoxicity in prostate cancer cell line. Role of nf-kappab-P65, camp/pka and nrf2/ho-1, nqo1 signaling. Food Chem Toxicol. (2021) 151:112133. doi: 10.1016/j.fct.2021.112133
163. Gong S, Chen Y, Meng F, Zhang Y, Wu H, Wu F. Roflumilast restores camp/pka/creb signaling axis for ftmt-mediated tumor inhibition of ovarian cancer. Oncotarget. (2017) 8:112341–53. doi: 10.18632/oncotarget.22866
164. Yeo CD, Kim YA, Lee HY, Kim JW, Kim SJ, Lee SH, et al. Roflumilast treatment inhibits lung carcinogenesis in benzo(a)Pyrene-induced murine lung cancer model. Eur J Pharmacol. (2017) 812:189–95. doi: 10.1016/j.ejphar.2017.07.004
165. Mondal D, Bagchi A, Biswas S, Dagar T, Biswas A, Bagchi A, et al. Vesicle-encapsulated rolipram (Pde4 inhibitor) and its anticancer activity. ACS Appl Bio Mater. (2024) 7:369–78. doi: 10.1021/acsabm.3c00961
166. Ramezani S, Hadjighassem M, Vousooghi N, Parvaresh M, Arbabi F, Amini N, et al. The role of protein kinase B signaling pathway in anti-cancer effect of rolipram on glioblastoma multiforme: an in vitro study. Basic Clin Neurosci. (2017) 8:325–36. doi: 10.18869/nirp.bcn.8.4.325
167. Moon EY, Lee GH, Lee MS, Kim HM, Lee JW. Phosphodiesterase inhibitors control A172 human glioblastoma cell death through camp-mediated activation of protein kinase a and epac1/rap1 pathways. Life Sci. (2012) 90:373–80. doi: 10.1016/j.lfs.2011.12.010
168. Massimi M, Cardarelli S, Galli F, Giardi MF, Ragusa F, Panera N, et al. Increase of intracellular cyclic amp by pde4 inhibitors affects hepg2 cell cycle progression and survival. J Cell Biochem. (2017) 118:1401–11. doi: 10.1002/jcb.25798
169. Tan Y, Watkins AA, Freeman BB, Meyers JA, Rifkin IR, Lerner A. Inhibition of type 4 cyclic nucleotide phosphodiesterase blocks intracellular tlr signaling in chronic lymphocytic leukemia and normal hematopoietic cells. J Immunol. (2015) 194:101–12. doi: 10.4049/jimmunol.1401854
170. Copsel S, Garcia C, Diez F, Vermeulem M, Baldi A, Bianciotti LG, et al. Multidrug resistance protein 4 (Mrp4/abcc4) regulates camp cellular levels and controls human leukemia cell proliferation and differentiation. J Biol Chem. (2011) 286:6979–88. doi: 10.1074/jbc.M110.166868
171. Narita M, Murata T, Shimizu K, Nakagawa T, Sugiyama T, Inui M, et al. A role for cyclic nucleotide phosphodiesterase 4 in regulation of the growth of human Malignant melanoma cells. Oncol Rep. (2007) 17:1133–9. doi: 10.3892/or.17.5.1133
172. Mao P, Huang C, Li Y, Zhao Y, Zhou S, Zhao Z, et al. Pharmacological targeting of type phosphodiesterase 4 inhibits the development of acute myeloid leukemia by impairing mitochondrial function through the wnt/beta-catenin pathway. BioMed Pharmacother. (2023) 157:114027. doi: 10.1016/j.biopha.2022.114027
173. Liang L, Chen H, Mao P, Li Y, Xu L, He Y, et al. Zl-N-91, a specific phosphodiesterase-4 inhibitor, suppresses the growth of triple-negative breast cancer. Invest New Drugs. (2022) 40:875–83. doi: 10.1007/s10637-022-01258-y
174. Crocetti L, Floresta G, Cilibrizzi A, Giovannoni MP. An overview of pde4 inhibitors in clinical trials: 2010 to early 2022. Molecules. (2022) 27. doi: 10.3390/molecules27154964
175. Oba Y, Lone NA. Efficacy and safety of roflumilast in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. Ther Adv Respir Dis. (2013) 7:13–24. doi: 10.1177/1753465812466167
176. Du B, Luo M, Ren C, Zhang J. Pde4 inhibitors for disease therapy: advances and future perspective. Future Med Chem. (2023) 15:1185–207. doi: 10.4155/fmc-2023-0101
177. Sun L, Quan H, Xie C, Wang L, Hu Y, Lou L. Phosphodiesterase 3/4 inhibitor zardaverine exhibits potent and selective antitumor activity against hepatocellular carcinoma both in vitro and in vivo independently of phosphodiesterase inhibition. PloS One. (2014) 9:e90627. doi: 10.1371/journal.pone.0090627
178. Campos-Toimil M, Keravis T, Orallo F, Takeda K, Lugnier C. Short-term or long-term treatments with a phosphodiesterase-4 (Pde4) inhibitor result in opposing agonist-induced ca(2+) responses in endothelial cells. Br J Pharmacol. (2008) 154:82–92. doi: 10.1038/bjp.2008.56
Keywords: phosphodiesterase 4 (PDE4), tumorigenesis, drug resistance, tumor immunity, PDE4 inhibitors
Citation: Ren H-l, Zhang S-h and Li P-y (2025) The multifaceted role of phosphodiesterase 4 in tumor: from tumorigenesis to immunotherapy. Front. Immunol. 16:1528932. doi: 10.3389/fimmu.2025.1528932
Received: 15 November 2024; Accepted: 24 February 2025;
Published: 10 March 2025.
Edited by:
Li Yuan, Xinjiang University, ChinaReviewed by:
Ann Richmond, Vanderbilt University, United StatesCopyright © 2025 Ren, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pei-Yuan Li, cHlsaUB0amgudGptdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.