
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Immunol., 26 February 2025
Sec. Viral Immunology
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1521029
This article is part of the Research TopicLong COVID and Brain Inflammation: Unravelling Mechanisms and Potential TherapiesView all 10 articles
Animal models are indispensable for unraveling the mechanisms underlying post-acute sequelae of COVID-19 (PASC). This review evaluates recent research on PASC-related perturbations in animal models, drawing comparisons with clinical findings. Despite the limited number of studies on post-COVID conditions, particularly those extending beyond three months, these studies provide valuable insights. Three hallmark features of PASC—lung fibrosis, hyperglycemia, and neurological sequelae—have been successfully replicated in animal models, paving the way for mechanistic discoveries and future medical interventions. Although most studies have reported post-COVID conditions within 14–60 days post-infection, they still offer critical reference for future long-term research. This review also explores potential mechanisms of persisting immune misfiring, a key factor in the chronicity of PASC symptoms. Moreover, challenges in modeling PASC are also discussed, including the limited genetic diversity in inbred animal strains and difficulties in accurately identifying PASC-affected individuals. To address these issues, we propose methodological improvements, such as comparing individual animal parameters with control averages and incorporating genetically diverse populations like collaborative cross models. These strategies will enhance the identification and characterization of PASC endotypes in animal studies. By integrating findings from animal models with clinical manifestations of PASC, future research can provide more valuable insights into its mechanisms and support the development of effective therapeutic strategies. Finally, we emphasize the urgent need for longitudinal studies in animal models to fully uncover the mechanisms driving PASC and guide interventions to mitigate its public health impact.
Microbial infections can induce long-term consequences beyond acute diseases or chronic infections. In particular, repeated viral exposure plays a key role in neurodegenerative diseases (1–3). COVID-19 patients not only suffered from acute- phase disease, but also experienced highly heterogeneous post-COVID conditions (4). Furthermore, SARS-CoV-2 infection has been associated with an increased risk of developing other non-infectious diseases such as diabetes (5), cardiovascular problems (6), autoimmune diseases (7), and neurological or psychiatric disorders (8). These findings reinforce this correlation. In the post-COVID era, PASC triggered by repeated infections from new variants of SARS-CoV-2, will continue to pose a significant threat to public health. Therefore, understanding the mechanisms behind long COVID and advancing therapeutic innovations remain crucial. However, currently, several limitations hinder the identification of a clear, reproducible, and generalizable long COVID/PASC signature, including sample accessibility, disease heterogeneity, inconsistent PASC definitions, variations in sample collection timing and methodologies, and uncontrollable factors such as infection severity, reinfection, co-infection, and subsequent infections. Therefore, the development and careful characterization of relevant animal models, which can can offer better control over certain factors, are crucial for revealing the underlying mechanisms of PASC.
Animal models are powerful tools for elucidating disease pathomechanisms. While post-infection perturbations and dyshomeostasis have been widely reported in COVID-19 models (9), research specifically focused on PASC is far less extensive. A key challenge is identifying PASC in animal models and appropriate control groups, often limiting comparisons to infected versus uninfected, vaccinated, or influenza-infected animals (10, 11). Ideally, more rigorous comparisons should be made between infected animals with and without PASC (12). Furthermore, the limited intraspecific diversity of most inbred animal strains may prevent them from fully exhibiting the heterogeneous symptoms observed in humans. Although animal models may not fully recapitulate the clinical manifestations of PASC, animal models offer several advantages for PASC studies, as long as findings from animal models are reproducible. 1. Systematic analysis can be conducted in animal models at serial time points, such as systematic histopathology analysis, multiple omics analysis, and viral detection. 2. Consistent pathogenic features in animal models can be profiled and reevaluated for further research. 3. Engineered animal models or experimental interventions can be applied for PASC research. Here, we systematically reviewed recently published research (including preprints) on PASC or coronavirus infection induced long-term perturbations, and present the similarities and differences by comparing these models with clinical findings (Table 1). Most importantly, these studies offer a valuable reference for future PASC research. Applying improved methodologies for identifying PASC in animals will offer more valuable insights though rigorous comparisons between infected animals with and without PASC.
Due to the very few animal studies revealed that long-term sequelae of COVID-19 beyond three months, we employed a less stringent criteria for selecting studies investigating post-COVID conditions (9). According to the current consensus definition of PASC, only five animal studies qualify as PASC research. Three of these studies reported the fibrotic changes in rodent lungs (13–15), one reported a neurological sequelae in mice (16), and another reported hyperglycemia in a non-human primate model (17). Apart from the post-COVID studies, four studies revealed sequelae post mouse hepatic virus (MHV) infection were also included, including two studies on central nerve system impacts (18, 19) and two studies reflecting MHV-induced histopathological changes lasting for one year (20, 21). The MHV model represents a natural coronavirus infection process in mice. Although it may trigger different immune response compared with SARS-CoV-2, it may help to reveal some shared mechanisms of viral infection induced neurodegenerative diseases. Moreover, extensive longitudinal systematic histopathology examination is recommended in future PASC studies (20, 21).
While most included studies had durations of less than three months, many observed signs of exacerbation or permanent injury initiated during the acute phase of infection. These observations suggest that these sequelae may extend beyond the study durations, thus providing valuable references for future research. However, some short-term sequelae, such as olfactory dysfunction, testicular damage, and muscle atrophy (22–24), are likely to resolve within one or two regenerative cycles. Despite this tendency toward ceasing, it remains possible for individuals to experience long-term impacts, such as persistent muscle weakness or anosmia. Therefore, we compiled the main organ manifestations observed in these studies, along with their similarities to clinical findings and PASC sequelae at the study endpoints, in Table 1. Future research should prioritize monitoring these manifestations over extended timeframes to reveal the mechanisms underlying PASC. Findings from these animal models substantiate three pillars underlying the PASC (Figures 1A, B): 1. Damage caused during the acute infection phase that does not fully recover after viral resolution. 2. Subsequent and persistent damage caused by immune perturbations or other dyshomeostasis, with or without viral persistence. 3. Maladaptive tissue repair leading to conditions like interstitial pulmonary fibrosis and alveolar bronchiolization.
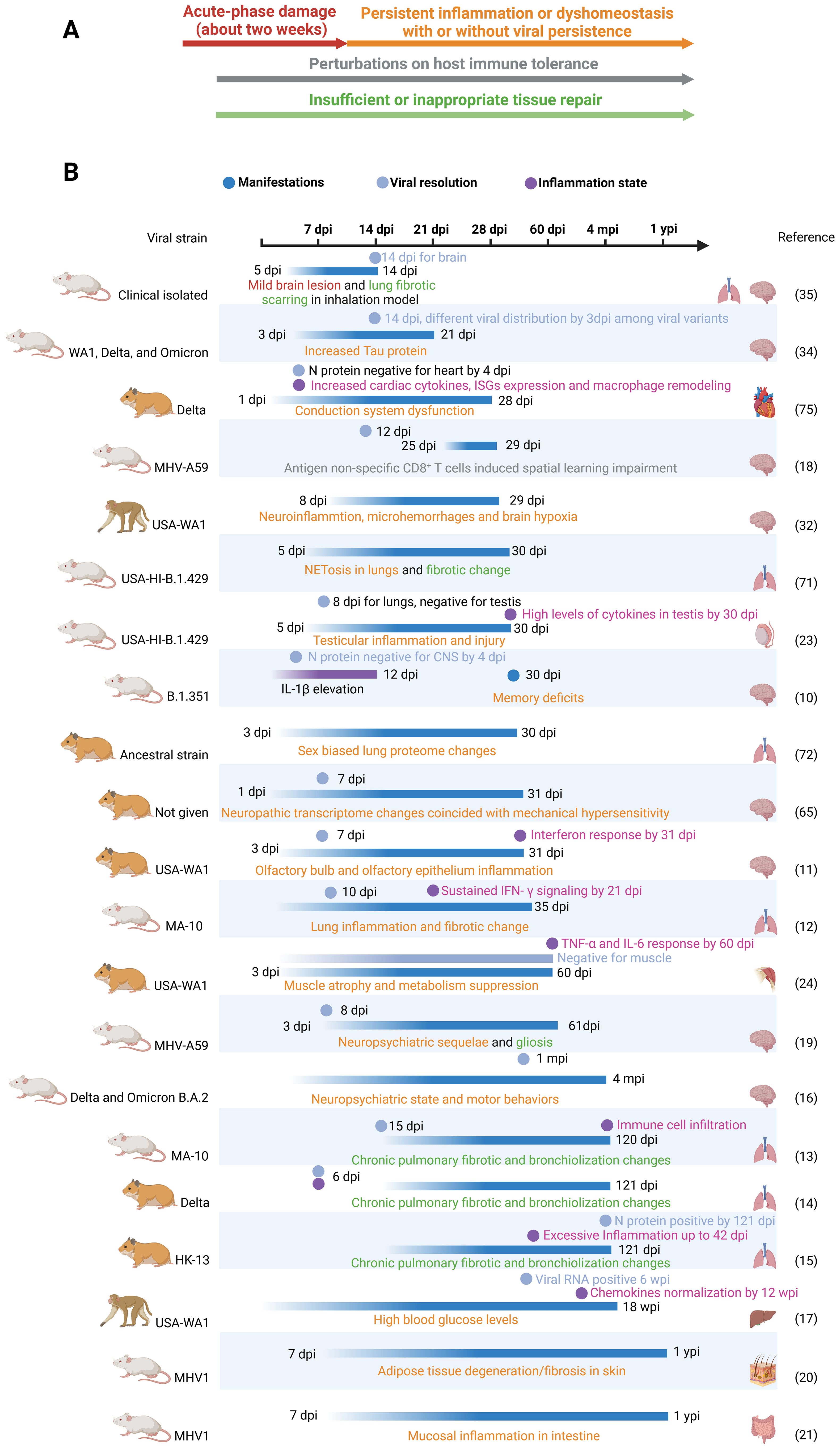
Figure 1. (A) Three pillars underlie long-COVID: acute phase damage, persistent inflammation or perturbations and maladaptive repair. Persistent inflammation can be caused by perturbations in immune tolerance (acquired immunity) and persisting activation of innate immunity by unknown reasons. In Figure (A), four classes are represented by distinct colors. These colors correspond to the same classes depicted in (B). (B) Timeframes for long-COVID animal models. In addition to only recording organ manifestations in long-COVID models, viral clearance and inflammation resolution can provide valuable insights into the underlying mechanisms. We put only one study with a duration of less than 2 weeks’ here to address the importance of acute damage. Dpi, wpi, mpi and ypi represent days, weeks, months and years post infection respectively. .
While most of the included post-COVID studies reported resolution of coronavirus infection within the acute phase, two PASC studies documented prolonged viral replication: one lasting 121 dpi (15) and the other persisting for 6 weeks post-infection (dpw) (17). However, recent research suggests that viral remnants, rather than actively replicating virus, may be responsible for the neurological sequelae of COVID-19 (25). Therefore, future PASC research employing small-micelle-mediated organ-efficient clearing and labeling techniques could be crucial in determining the duration of viral remnant persistence and their presence in relevant animal models.
Lung fibrosis, a consequence of maladaptive repair mechanisms following lung damage, is a key feature of PASC, which is significantly influenced by age (26). Two studies reported fibrosis and alveolar-bronchiolization in aged rodents over a three-month period, supporting this age-related predisposition (13, 14). Notably, in BALB/c mice, while similar mortality rates and subpleural opacities were observed in both young and aged animals, aged mice exhibited slower recovery and tendency toward fibrotic changes and chronic inflammation (13). Another study demonstrated that mild treadmill exercise at 21 dpi after the Delta variant infection re-induced lung dysfunction and fibrotic changes after apparent recovery (14). Moreover, long-term fibrosis can also be triggered by chronic viral infection in young hamsters (15). Notably, shared mechanisms underlying post-COVID sequelae in lungs between young and aged hamsters involve the CK14+ cells’ proliferation and differentiation into SCGB1A+ club cells, leading to fibrosis and alveolar bronchiolization (14, 15). Mechanistically, dysregulated lung regeneration has been correlated with increased Notch3 and Hes1 protein expression (15). Given that Notch4 has been shown to hinder regulatory T-cell-mediated tissue repair and induce severe inflammation in an influenza model (27), these findings suggest that Notch signaling may disrupt normal tissue repair following viral infections.
Heterogeneity in damage resolution is observed both within the same strain and between different mouse strains, such as BALB/c and C57BL/6J (13), after MA10 infection. The resolution of fibrosis in some individuals highlights host-specific differences in damage repair. Recent research has identified IFNγ as a key factor in post-COVID respiratory sequelae. By comparing single-cell RNA sequencing data from bronchoalveolar lavage fluid of convalescent donors with and without persistent respiratory complications, researchers found that IFNγ secreted by lung-resident T cells recruits pro-fibrotic monocyte-derived macrophages, contributing to fibrosis (12). Interestingly, blocking IFNγ alleviates fibrosis only in C57BL/6J mice and has no effect in BALB/c mice following MA10 infection (12). These findings emphasize the distinct underlying mechanisms driving similar long-term pathological changes in these genetically different mouse strains.
A study reveals SARS-CoV-2 infection induces a lasting hyperglycemia for 18 weeks in African green monkeys without detectable virus or inflammation in the liver and pancreas, along with a dysregulated blood chemokine signature (14). Increased liver glycogen was found in this model, and an enhanced gluconeogenesis was thought to be a main cause of hyperglycemia. This research presents a lasting stress response in glycometabolism post-infection and highlights a link between immune response and metabolism. Apart from the previously reported pancreatitis from the same task group (28), this research provides insights into and may open a new avenue for immunometabolism perturbations after infection. Moreover, an increased risk of Type 2 diabetes is also found in children after COVID-19 (29). Future research on how an infection can induce a disorder in glycogen metabolism as well as insulin resistance is required.
A four-month longitudinal study extensively monitoring mouse behavior after severe SARS-CoV-2 infection revealed a broad spectrum of neurological abnormalities in neuropsychiatric state, motor behavior, autonomic function, and reflex and sensory function (16). Minimal astrocyte activation and mild microglial activation persisted in the brain, accompanied by transcriptomic changes related to complement activation, phagocytosis, and humoral immune response and gene expression levels associated with ataxia telangiectasia, impaired cognitive function and memory recall, and neuronal dysfunction and degeneration. Notably, lingering complement activation is also revealed in PASC patients, which is correlated with the persistence of autoantibodies and antibodies against herpesviruses (30). This convergence of findings suggests a potential link between complement activation and neuropathological changes in both animal models and human PASC. Therefore, future research should focus on identifying the specific antibodies or other pathways triggering complement activation and mediating these neuropathological changes. The autoimmunity resulting from SARS-CoV-2 infection and its impact on immune tolerance will be discussed in the “Immune Tolerance” section.
Interestingly, although mild brain inflammation persisted throughout the study, some abnormalities in gait, whisker response, ear twitch, and palpebral reflex abnormalities, decreased over time. Others, including body position, grip strength, touch escape, and reach touch, remained relatively constant. Conversely, abnormalities in spontaneous activity, tail position, and tremor increased over the four-month period. These results suggest that the impact on the brain may stem from three distinct types of damage: permanent damage incurred during the acute phase, reversible damage incurred during the acute phase, and subsequent damage that gradually develops after the acute phase. The latter on aligns with the gradual exacerbation of cognitive deficits in some asymptomatic patients (31) highlighting the need for future PASC models induced by less severe infections.
The most consistent finding across these studies is the persistence of neuroinflammation, primarily characterized by microglial activation (10, 11, 32), elevated levels of Iba1, elevated pro-inflammatory cytokines, such as IL-1β (10), IL-6 (16, 19), TNF-α (18), IFN-γ (18), and chemokines such as CCL5 and CXCL10 (11). While microglial activation is a normal response to infection or injury, its persistence can have detrimental effects on neuronal function and contribute to neurodegeneration. The prolonged elevation of these inflammatory mediators can disrupt neuronal signaling, impair synaptic plasticity, and promote neuronal damage or death. Notably, a research revealed gliosis in the mouse brain after MHV infection (19). Gliosis, as an end stage of microglial activation, is thought to be a main feature and driver for persistent depressive and cognitive symptoms in patients (33). In some cases, astrocyte activation (16, 32), tau pathology (34), and perivascular lymphocyte/blood cuffing (32, 35) can be salient in some animal models.
Several studies provide evidence of neuronal damage and dysfunction (32), including neuronal cell death (18, 32), impaired neurogenesis (36), and reduced synapses (10). These findings indicate that coronavirus infection can have a direct and lasting impact on neuronal function. Notably, one study revealed the neuronal apoptosis gradually increase over time in primates, and is absent in the acute phase (32), suggesting different damage in separate phases.
Aged animals often exhibit more severe and prolonged effects due to a heighten tone of basal inflammation (18), and female-specific neuropathological changes (19), which both mirroring the clinical findings. These findings highlight the importance of considering individual variability when studying the neurological impact of coronavirus infection.
Perturbation of immune tolerance can lead to an autoimmune response. Immune tolerance serves as a supervisor in determining what our immune response should react to and what it should not react to. Perturbations of immune tolerance encompass two key concepts: failure to respond to a substance that should elicit an immune response, and responding to a substance that should be be indifferent to. During the pathogen infection, immune tolerance is expected to protect our normal tissue from immune misfiring and not to favor pathogen replication by tolerating pathogen. However, in the SARS-CoV-2 infection, it seem immune tolerance turn into malfunctioning mode to cause damage to innocent tissue, and lead to viral persistence. The process of PASC is a long-term immune misfiring of both innate and adaptive immune response triggered by infection.
For pathogens like viruses, the best way to evade our immunity is to mimic host molecular traits. Several studies reveal that SARS-CoV-2 can trigger self-reactive antibodies and T cells due to molecular mimicry between host proteins and virus (37, 38), which can trigger a post-infection malaise, such as multisystem inflammatory syndrome in children (39). Apart from the mimicry strategies, SARS-CoV-2 infection is known to manipulate (40) or relax (41–43) our immune tolerance and to trigger an autoimmunity (44, 45), resulting in both acute symptoms and post-COVID sequelae. For instance, autoantibodies in cerebrospinal fluid against brain antigens are found in COVID-19 patients with neurological symptoms (46). Moreover, disruption of peripheral tolerance in the pancreas caused by SARS-CoV-2 infection is thought to be a key driver of type 1 diabetes (5). Thus, within the large infected population, a subset of critical COVID-19 patients may have a previous immune tolerance dysregulation, resulting in the production of autoantibodies against immunomodulatory proteins (47) and interferons (48). This dysregulation can ensue from genetic deficiency in the NF-κB pathway (49) or age induced (50, 51) tolerance loss. Individuals in this group are more susceptible to SARS-CoV-2 infection and more likely to develop a lasting self-reactive acquired immune response, leading to immune misfiring (43). If these immune misfirings cannot be eliminated by central or peripheral tolerance, they may lead to long-term conditions.
Circulating autoantibodies have been linked to PASC, and their levels can predict PASC symptoms (46, 52). Recently, a study demonstrated that transferring IgGs from stratified PASC subgroups based on Glial Fibrillary Acidic Protein and type-I interferon expression can lead to sensory hypersensitivity or reduced locomotor activity in mouse models, depending on IgG cohorts (53). Even though the mechanisms by which these two cohorts of antibodies can trigger different symptoms in mice remain elusive, this research does necessitate the stratification of PASC patients and highlights the diverse mechanisms underlying PASC. Indeed, another research reveal similar finding that passive transfer of IgG from long-COVID patients with neurocognitive and neurological symptoms can cause increased sensitivity and pain (54). They further demonstrate these antibodies target mouse sciatic nerves, spinal cord, and meninges, which can lead to loss of balance and coordination in mice. Similar findings also reported in clinical that bispecific antibodies targeted on both spike protein and neural tissue were found in patients with neurological symptoms (55). In a mouse model, SARS-CoV-2 infection can also trigger anti-platelet factor-4 antibody production, causing coagulopathy (56). Apart from the interfering self-antigens’ function, the activation of complement system is also a key driver of PASC (30). Further screening of human extracellular and secreted proteins will help to reveal what self-antigens are the antibodies from PASC patients react to (47), similar methods can be applied in animal models with PASC.
Similar to B cell tolerance, T cell dysfunction is also considered a significant driver of PASC. A recent study found that PASC patients can maintain robust SARS-CoV-2-specific T cells for two years compared to non-PASC controls (57). However, there are no report of immune misfiring caused by autoreactive cytotoxic CD8+ T cells. Only one research revealed children with multisystem inflammatory syndrome had both anti-SNX8 autoantibodies and cross-reactive T cells engaged both the SNX8 and the SARS-CoV-2 nucleocapsid protein epitopes (39). Future screening of the T cells function may offer a clearer vision of the mechanisms of PASC (36).
In a MHV post infection model, activated spike protein non-specific CD8+ cells were increased in the brains of aged mice and correlated with neuronal death, further leading to cognitive decline (18). The authors further revealed these CD8+ cells can induce primary neuronal cell death in vitro. Therefore, we may have underestimated the dysregulated T cell in PASC (58). The correlation of IFN-γ released by activated CD8+ cells with PASC patients is also reported in clinical investigations (59, 60). Similarly, these CD8+ were also found in an EBV infection-induced Alzheimer model (61). Moreover, expanded activated CD8+ cells are found to be a key signature of Alzheimer’s disease (61, 62). Thus, dysregulated T cells play a key role in dementia caused by different viral exposures. Furthermore, more research reflecting how these T cell responses leave a long-term impact on health is needed. Unlike the cytotoxic T cells, their functions relied on the target that react to. These by stander T cells in PASC can be evaluated by their populations. Therefore, future studies monitoring these by stander T cells in SARS-CoV-2 infection are required.
Two main obstacles hinder the study of PASC in animal models: the limited heterogeneity of PASC symptoms due to the relatively low genetic diversity of inbred animals, and the difficulty in accurately identifying PASC-affected animals. To address the first issue, using collaborative cross populations in future PASC studies could increase genetic diversity, facilitating the identification of genetic associations with PASC. However, the challenge of distinguishing PASC-affected animals remains. Simply comparing infected animals with uninfected controls or animals infected with other viruses may cover individuals with PASC and PASC phenotypes. For instance, female mice are predisposed to developing post-coronavirus syndrome. Therefore, to better discriminate PASC phenotypes, comparing individual animal parameters with the average of the control group is necessary (16). Furthermore, comparing each individual’s pre-infection and post-infection parameters is also crucial (17). By combining these approaches with reference from human post-COVID sequelae, researchers can make more robust comparisons between animals with and without PASC, leading to valuable insights into PASC mechanisms and, ultimately, promoting the development of effective therapies.
1000 articles were scraped from Google scholar after searching for animal models of long-COVID and manually screened for eligibility.
JD: Writing – original draft, Conceptualization, Data curation, Supervision, Writing – review & editing, Funding acquisition, Resources, Visualization, Validation. FH: Data curation, Formal analysis, Software, Visualization, Writing – original draft, Writing – review & editing, Methodology. QC: Conceptualization, Data curation, Formal analysis, Software, Visualization, Writing – original draft, Writing – review & editing, Investigation. QL: Data curation, Formal analysis, Validation, Writing – review & editing, Conceptualization. LZ: Data curation, Formal analysis, Validation, Writing – review & editing, Investigation, Methodology. YD: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – review & editing, Writing – original draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The work was supported by the Yunnan Key Research and Development Program (202303AC100026), the Project of Health Science and Technology Talents Ten Hundred Thousand in Kunming 2021-SW(DAITOU)-06, the Scientific Research Fund Project of the Yunnan Provincial Education Department (2023J0918).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Blackhurst BM, Funk KE. Viral pathogens increase risk of neurodegenerative disease. Nat Rev Neurol. (2023) 19:259–60. doi: 10.1038/s41582-023-00790-6
2. Levine KS, Leonard HL, Blauwendraat C, Iwaki H, Johnson N, Bandres-Ciga S, et al. Virus exposure and neurodegenerative disease risk across national biobanks. Neuron. (2023) 111:1086–93.e2. doi: 10.1016/j.neuron.2022.12.029
3. Bjornevik K, Cortese M, Healy BC, Kuhle J, Mina MJ, Leng Y, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. (2022) 375:296–301. doi: 10.1126/science.abj8222
4. Greenhalgh T, Sivan M, Perlowski A, Nikolich JŽ. Long COVID: a clinical update. Lancet. (2024) 404:707–24. doi: 10.1016/s0140-6736(24)01136-x
5. Debuysschere C, Nekoua MP, Alidjinou EK, Hober D. The relationship between SARS-CoV-2 infection and type 1 diabetes mellitus. Nat Rev Endocrinol. (2024) 20:588–99. doi: 10.1038/s41574-024-01004-9
6. Eberhardt N, Noval MG, Kaur R, Amadori L, Gildea M, Sajja S, et al. SARS-CoV-2 infection triggers pro-atherogenic inflammatory responses in human coronary vessels. Nat Cardiovasc Res. (2023) 2:899–916. doi: 10.1038/s44161-023-00336-5
7. Sharma C, Bayry J. High risk of autoimmune diseases after COVID-19. Nat Rev Rheumatol. (2023) 19:399–400. doi: 10.1038/s41584-023-00964-y
8. Taquet M, Sillett R, Zhu L, Mendel J, Camplisson I, Dercon Q, et al. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients. Lancet Psychiatry. (2022) 9:815–27. doi: 10.1016/S2215-0366(22)00260-7
9. Usai C, Mateu L, Brander C, Vergara-Alert J, Segalés J. Animal models to study the neurological manifestations of the post-COVID-19 condition. Lab Animal. (2023) 52:202–10. doi: 10.1038/s41684-023-01231-z
10. Vanderheiden A, Hill JD, Jiang X, Deppen B, Bamunuarachchi G, Soudani N, et al. Vaccination reduces central nervous system IL-1β and memory deficits after COVID-19 in mice. Nat Immunol. (2024) 25:1158–71. doi: 10.1038/s41590-024-01868-z
11. Frere JJ, Serafini RA, Pryce KD, Zazhytska M, Oishi K, Golynker I, et al. SARS-CoV-2 infection in hamsters and humans results in lasting and unique systemic perturbations after recovery. Sci Trans Med. (2022) 14:eabq3059. doi: 10.1126/scitranslmed.abq3059
12. Li C, Qian W, Wei X, Narasimhan H, Wu Y, Arish M, et al. Comparative single-cell analysis reveals IFN-gamma as a driver of respiratory sequelae after acute COVID-19. Sci Transl Med. (2024) 16:eadn0136. doi: 10.1126/scitranslmed.adn0136
13. Dinnon KH, Leist SR, Okuda K, Dang H, Fritch EJ, Gully KL, et al. SARS-CoV-2 infection produces chronic pulmonary epithelial and immune cell dysfunction with fibrosis in mice. Sci Transl Med. (2022) 14:eabo5070. doi: 10.1126/scitranslmed.abo5070
14. Baumgärtner W, Heydemann L, Ciurkiewicz M, Störk T, Zdora I, Hülskötter K, et al. Persistent alveolar bronchiolization, interstitial fibrosis and impaired lung function post-exercise are features of long-COVID in SARS-CoV-2-Delta variant infected aged hamsters. Research Square [Preprint] (2024) Available at: https://www.researchsquare.com/article/rs-4681343/v1.
15. Li C, Xiao N, Song W, Lam AH, Liu F, Cui X, et al. Chronic lung inflammation and CK14+ basal cell proliferation induce persistent alveolar-bronchiolization in SARS-CoV-2-infected hamsters. EBioMedicine. (2024) 108:105363. doi: 10.1016/j.ebiom.2024.105363
16. Singh A, Adam A, Aditi, Peng B-H, Yu X, Zou J, et al. A murine model of post-acute neurological sequelae following SARS-CoV-2 variant infection. Front Immunol. (2024) 15:1384516. doi: 10.3389/fimmu.2024.1384516
17. Palmer CS, Perdios C, Abdel-Mohsen M, Mudd J, Datta PK, Maness NJ, et al. Non-human primate model of long-COVID identifies immune associates of hyperglycemia. Nat Commun. (2024) 15:6664. doi: 10.1038/s41467-024-50339-4
18. Reagin KL, Lee R-L, Cocciolone L, Funk KE. Antigen non-specific CD8+ T cells accelerate cognitive decline in aged mice following respiratory coronavirus infection. bioRxiv [Preprint] (2024). doi: 10.1101/2024.01.02.573675
19. Pimenta JC, Beltrami VA, Oliveira BDS, Queiroz-Junior CM, Barsalini J, Teixeira DC, et al. Neuropsychiatric sequelae in an experimental model of post-COVID syndrome in mice. bioRxiv [Preprint]. (2024). Available online at: https://www.biorxiv.org/content/10.1101/2024.01.10.575003v1.
20. Hussain H, Paidas MJ, Rajalakshmi R, Fadel A, Ali M, Chen P, et al. Dermatologic changes in experimental model of long COVID. Microorganisms. (2024) 12:272. doi: 10.3390/microorganisms12020272
21. Hussain H, Elumalai N, Sampath N, Shamaladevi N, Hajjar R, Druyan BZ, et al. Acute and long COVID intestinal changes in an experimental model of coronavirus in mice. Viruses. (2024) 16:823. doi: 10.3390/v16060832
22. Tostanoski LH, Wegmann F, Martinot AJ, Loos C, McMahan K, Mercado NB, et al. Ad26 vaccine protects against SARS-CoV-2 severe clinical disease in hamsters. Nat Med. (2020) 26:1694–700. doi: 10.1038/s41591-020-1070-6
23. Giannakopoulos S, Pak J-H, Bakse J, Ward MA, Nerurkar V, Tallquist MD, et al. Sars-Cov-2-induced cytokine storm drives prolonged testicular injury and functional impairments in mice that are mitigated by dexamethasone. SSRN [Preprint] (2024) doi: 10.2139/ssrn.4901150
24. Homma ST, Wang X, Frere JJ, Gower AC, Zhou J, Lim JK, et al. Respiratory SARS-CoV-2 infection causes skeletal muscle atrophy and long-lasting energy metabolism suppression. Biomedicines. (2024) 12. doi: 10.3390/biomedicines12071443
25. Rong Z, Mai H, Ebert G, Kapoor S, Puelles VG, Czogalla J, et al. Persistence of spike protein at the skull-meninges-brain axis may contribute to the neurological sequelae of COVID-19. Cell Host Microbe. (2024) 32:2112–30.e10. doi: 10.1016/j.chom.2024.11.007
26. John AE, Joseph C, Jenkins G, Tatler AL. COVID-19 and pulmonary fibrosis: A potential role for lung epithelial cells and fibroblasts. Immunol Rev. (2021) 302:228–40. doi: 10.1111/imr.12977
27. Harb H, Benamar M, Lai PS, Contini P, Griffith JW, Crestani E, et al. Notch4 signaling limits regulatory T-cell-mediated tissue repair and promotes severe lung inflammation in viral infections. Immunity. (2021) 54:1186–99.e7. doi: 10.1016/j.immuni.2021.04.002
28. Qadir MMF, Bhondeley M, Beatty W, Gaupp DD, Doyle-Meyers LA, Fischer T, et al. SARS-CoV-2 infection of the pancreas promotes thrombofibrosis and is associated with new-onset diabetes. JCI Insight. (2021) 6. doi: 10.1172/jci.insight.151551
29. Miller MG, Terebuh P, Kaelber DC, Xu R, Davis PB. SARS-CoV-2 infection and new-onset type 2 diabetes among pediatric patients, 2020 to 2022. JAMA Netw Open. (2024) 7:e2439444. doi: 10.1001/jamanetworkopen.2024.39444
30. Cervia-Hasler C, Bruningk SC, Hoch T, Fan B, Muzio G, Thompson RC, et al. Persistent complement dysregulation with signs of thromboinflammation in active Long Covid. Science. (2024) 383:eadg7942. doi: 10.1126/science.adg7942
31. Trender W, Hellyer PJ, Killingley B, Kalinova M, Mann AJ, Catchpole AP, et al. Changes in memory and cognition during the SARS-CoV-2 human challenge study. eClinicalMedicine. (2024) 76:102842. doi: 10.1016/j.eclinm.2024.102842
32. Rutkai I, Mayer MG, Hellmers LM, Ning B, Huang Z, Monjure CJ, et al. Neuropathology and virus in brain of SARS-CoV-2 infected non-human primates. Nat Commun. (2022) 13:1745. doi: 10.1038/s41467-022-29440-z
33. Braga J, Lepra M, Kish SJ, Rusjan PM, Nasser Z, Verhoeff N, et al. Neuroinflammation after COVID-19 with persistent depressive and cognitive symptoms. JAMA Psychiatry. (2023) 80:787–95. doi: 10.1001/jamapsychiatry.2023.1321
34. Choi CY, Gadhave K, Villano J, Pekosz A, Mao X, Jia H. Generation and characterization of a humanized ACE2 mouse model to study long-term impacts of SARS-CoV-2 infection. J Med Virol. (2024) 96:e29349. doi: 10.1002/jmv.29349
35. Jeon D, Kim S-H, Kim J, Jeong H, Uhm C, Oh H, et al. Discovery of a new long COVID mouse model via systemic histopathological comparison of SARS-CoV-2 intranasal and inhalation infection. Biochim Biophys Acta (BBA) - Mol Basis Dis. (2024) 1870:167347. doi: 10.1016/j.bbadis.2024.167347
36. Woodbridge Y, Amit S, Huppert A, Kopelman NM. Viral load dynamics of SARS-CoV-2 Delta and Omicron variants following multiple vaccine doses and previous infection. Nat Commun. (2022) 13:6706. doi: 10.1038/s41467-022-33096-0
37. Khavinson V, Terekhov A, Kormilets D, Maryanovich A. Homology between SARS CoV-2 and human proteins. Sci Rep. (2021) 11:17199. doi: 10.1038/s41598-021-96233-7
38. Lake CM, Breen JJ. Sequence similarity between SARS-CoV-2 nucleocapsid and multiple sclerosis-associated proteins provides insight into viral neuropathogenesis following infection. Sci Rep. (2023) 13:389. doi: 10.1038/s41598-022-27348-8
39. Bodansky A, Mettelman RC, Sabatino JJ Jr., Vazquez SE, Chou J, Novak T, et al. Molecular mimicry in multisystem inflammatory syndrome in children. Nature. (2024) 632:622–9. doi: 10.1038/s41586-024-07722-4
40. Geanes ES, McLennan R, Pierce SH, Menden HL, Paul O, Sampath V, et al. SARS-CoV-2 envelope protein regulates innate immune tolerance. iScience. (2024) 27:109975. doi: 10.1016/j.isci.2024.109975
41. Castleman MJ, Stumpf MM, Therrien NR, Smith MJ, Lesteberg KE, Palmer BE, et al. SARS-CoV-2 infection relaxes peripheral B cell tolerance. J Exp Med. (2022) 219:e20212553. doi: 10.1084/jem.20212553
42. Wallukat G, Wernike K, Bachamanda Somesh D, Mettenleiter TC, Muller J. Animals experimentally infected with SARS-CoV-2 generate functional autoantibodies against G-protein-coupled receptors. Biomedicines. (2023) 11:2668. doi: 10.3390/biomedicines11102668
43. Woodruff MC, Ramonell RP, Haddad NS, Anam FA, Rudolph ME, Walker TA, et al. Dysregulated naive B cells and de novo autoreactivity in severe COVID-19. Nature. (2022) 611:139–47. doi: 10.1038/s41586-022-05273-0
44. Macela A, Kubelkova K. Why does SARS-CoV-2 infection induce autoantibody production? Pathogens. (2021) 10. doi: 10.3390/pathogens10030380
45. Dotan A, Muller S, Kanduc D, David P, Halpert G, Shoenfeld Y. The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmune Rev. (2021) 20:102792. doi: 10.1016/j.autrev.2021.102792
46. Franke C, Ferse C, Kreye J, Reincke SM, Sanchez-Sendin E, Rocco A, et al. High frequency of cerebrospinal fluid autoantibodies in COVID-19 patients with neurological symptoms. Brain Behav Immun. (2021) 93:415–9. doi: 10.1016/j.bbi.2020.12.022
47. Wang EY, Mao T, Klein J, Dai Y, Huck JD, Jaycox JR, et al. Diverse functional autoantibodies in patients with COVID-19. Nature. (2021) 595:283–8. doi: 10.1038/s41586-021-03631-y
48. Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann HH, Zhang Y, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. (2020) 370:eabd4585. doi: 10.1126/science.abd4585
49. Le Voyer T, Parent AV, Liu X, Cederholm A, Gervais A, Rosain J, et al. Autoantibodies against type I IFNs in humans with alternative NF-kappaB pathway deficiency. Nature. (2023) 623:803–13. doi: 10.1038/s41586-023-06717-x
50. Fernbach S, Mair NK, Abela IA, Groen K, Kuratli R, Lork M, et al. Loss of tolerance precedes triggering and lifelong persistence of pathogenic type I interferon autoantibodies. J Exp Med. (2024) 221:e20240365. doi: 10.1084/jem.20240365
51. Manry J, Bastard P, Gervais A, Le Voyer T, Rosain J, Philippot Q, et al. The risk of COVID-19 death is much greater and age dependent with type I IFN autoantibodies. Proc Natl Acad Sci U S A. (2022) 119:e2200413119. doi: 10.1073/pnas.2200413119
52. Son K, Jamil R, Chowdhury A, Mukherjee M, Venegas C, Miyasaki K, et al. Circulating anti-nuclear autoantibodies in COVID-19 survivors predict long-COVID symptoms. Eur Respir J. (2022) 61:2200970. doi: 10.1183/13993003.00970-2022
53. Chen H-J, Appelman B, Willemen H, Bos A, Prado J, Geyer CE, et al. Transfer of IgG from Long COVID patients induces symptomology in mice. bioRxiv [Preprint] (2024). doi: 10.1101/2024.05.30.596590
54. Santos Guedes de Sa K, Silva J, Bayarri-Olmos R, Brinda R, Alec Rath Constable R, Colom Diaz PA, et al. A causal link between autoantibodies and neurological symptoms in long COVID. medRxiv. (2024). doi: 10.1101/2024.06.18.24309100
55. Song E, Bartley CM, Chow RD, Ngo TT, Jiang R, Zamecnik CR, et al. Divergent and self-reactive immune responses in the CNS of COVID-19 patients with neurological symptoms. Cell Rep Med. (2021) 2:100288. doi: 10.1016/j.xcrm.2021.100288
56. Drelich AK, Rayavara K, Hsu J, Saenkham-Huntsinger P, Judy BM, Tat V, et al. Characterization of unique pathological features of COVID-associated coagulopathy: studies with AC70 hACE2 transgenic mice highly permissive to SARS-CoV-2 infection. PLoS Pathog. (2024) 20:e1011777. doi: 10.1371/journal.ppat.1011777
57. Rowntree LC, Audsley J, Allen LF, McQuilten HA, Hagen RR, Chaurasia P, et al. SARS-CoV-2-specific CD8(+) T cells from people with long COVID establish and maintain effector phenotype and key TCR signatures over 2 years. Proc Natl Acad Sci U S A. (2024) 121:e2411428121. doi: 10.1073/pnas.2411428121
58. Yin K, Peluso MJ, Luo X, Thomas R, Shin MG, Neidleman J, et al. Long COVID manifests with T cell dysregulation, inflammation and an uncoordinated adaptive immune response to SARS-CoV-2. Nat Immunol. (2024) 25:218–25. doi: 10.1038/s41590-023-01724-6
59. Krishna BA, Lim EY, Metaxaki M, Jackson S, Mactavous L, Lyons PA, et al. Spontaneous, persistent, T cell–dependent IFN-γ release in patients who progress to Long Covid. Sci Adv. (2024) 10:eadi9379. doi: 10.1126/sciadv.adi9379
60. Martinez-Fleta P, Marcos MC, Jimenez-Carretero D, Galvan-Roman JM, Giron-Moreno RM, Calero-Garcia AA, et al. Imbalance of SARS-CoV-2-specific CCR6+ and CXCR3+ CD4+ T cells and IFN-gamma + CD8+ T cells in patients with Long-COVID. Clin Immunol. (2024) 264:110267. doi: 10.1016/j.clim.2024.110267
61. Reagin KL, Funk KE. The role of antiviral CD8(+) T cells in cognitive impairment. Curr Opin Neurobiol. (2022) 76:102603. doi: 10.1016/j.conb.2022.102603
62. Gate D, Saligrama N, Leventhal O, Yang AC, Unger MS, Middeldorp J, et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer's disease. Nature. (2020) 577:399–404. doi: 10.1038/s41586-019-1895-7
63. Doty RL. Olfactory dysfunction in COVID-19: pathology and long-term implications for brain health. Trends Mol Med. (2022) 28:781–94. doi: 10.1016/j.molmed.2022.06.005
64. Matschke J, Lutgehetmann M, Hagel C, Sperhake JP, Schroder AS, Edler C, et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. (2020) 19:919–29. doi: 10.1016/S1474-4422(20)30308-2
65. Serafini RA, Frere JJ, Zimering J, Giosan IM, Pryce KD, Golynker I, et al. SARS-CoV-2 airway infection results in the development of somatosensory abnormalities in a hamster model. Sci Signaling. (2023) 16:eade4984. doi: 10.1126/scisignal.ade4984
66. Gressett TE, Leist SR, Ismael S, Talkington G, Dinnon KH, Baric RS, et al. Mouse adapted SARS-CoV-2 model induces "Long-COVID" Neuropathology in BALB/c mice. bioRxiv [Preprint]. (2023). Available at: https://www.biorxiv.org/content/10.1101/2023.03.18.533204v1.
67. Hamlin RE, Pienkos SM, Chan L, Stabile MA, Pinedo K, Rao M, et al. Sex differences and immune correlates of Long Covid development, symptom persistence, and resolution. Sci Transl Med. (2024) 16:eadr1032. doi: 10.1126/scitranslmed.adr1032
68. Lee B, Choi HN, Che YH, Ko M, Seong HM, Jo MG, et al. SARS-CoV-2 infection exacerbates the cellular pathology of Parkinson’s disease in human dopaminergic neurons and a mouse model. Cell Rep Med. (2024) 5:101570. doi: 10.1016/j.xcrm.2024.101570
69. Amruta N, Ismael S, Leist SR, Gressett TE, Srivastava A, Dinnon KH 3rd, et al. Mouse adapted SARS-CoV-2 (MA10) viral infection induces neuroinflammation in standard laboratory mice. Viruses. (2022) 15:114. doi: 10.3390/v15010114
70. Fontes-Dantas FL, Fernandes GG, Gutman EG, De Lima EV, Antonio LS, Hammerle MB, et al. SARS-CoV-2 Spike protein induces TLR4-mediated long-term cognitive dysfunction recapitulating post-COVID-19 syndrome in mice. Cell Rep. (2023) 42:112189. doi: 10.1016/j.celrep.2023.112189
71. Giannakopoulos S, Park J, Pak J, Tallquist MD, Verma S. Post-COVID pulmonary injury in K18-hACE2 mice shows persistent neutrophils and neutrophil extracellular trap formation. Immunity Inflammation Disease. (2024) 12:e1343. doi: 10.1002/iid3.1343
72. Boese AS, Warner BM, McQueen P, Vendramelli R, Tailor N, Griffin BD, et al. SARS-CoV-2 infection results in a unique lung proteome long after virus resolution in the hamster. NPJ Viruses. (2024) 2:40. doi: 10.1038/s44298-024-00049-x
73. Heydemann L, Ciurkiewicz M, Beythien G, Becker K, Schughart K, Stanelle-Bertram S, et al. Hamster model for post-COVID-19 alveolar regeneration offers an opportunity to understand post-acute sequelae of SARS-CoV-2. Nat Commun. (2023) 14:3267. doi: 10.1038/s41467-023-39049-5
74. Cao X, Nguyen V, Tsai J, Gao C, Tian Y, Zhang Y, et al. The SARS-CoV-2 spike protein induces long-term transcriptional perturbations of mitochondrial metabolic genes, causes cardiac fibrosis, and reduces myocardial contractile in obese mice. Mol Metab. (2023) 74:101756. doi: 10.1016/j.molmet.2023.101756
75. Ashok D, Liu T, Criscione J, Prakash M, Kim B, Chow J, et al. Innate immune activation and mitochondrial ROS invoke persistent cardiac conduction system dysfunction after COVID-19. bioRxiv [Preprint]. (2024). Available at: https://www.biorxiv.org/content/10.1101/2024.01.05.574280v1.
76. Cannarella R, Marino M, Crafa A, Bagnara V, La Vignera S, Condorelli RA, et al. Impact of COVID-19 on testicular function: a systematic review and meta-analysis. Endocrine. (2024) 85:44–66. doi: 10.1007/s12020-024-03705-7
Keywords: post-acute COVID-19 syndrome, animal models, long-term perturbations post coronavirus infection, immune tolerance, organ manifestations
Citation: Dai J, He F, Chen Q, Li Q, Zhao L and Du Y (2025) Animal models of post-acute COVID-19 syndrome: a call for longitudinal animal studies. Front. Immunol. 16:1521029. doi: 10.3389/fimmu.2025.1521029
Received: 01 November 2024; Accepted: 29 January 2025;
Published: 26 February 2025.
Edited by:
Fatemeh Saheb Sharif-Askari, University of Sharjah, United Arab EmiratesReviewed by:
Tom Aschman, Charité University Medicine Berlin, GermanyCopyright © 2025 Dai, He, Chen, Li, Zhao and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingrong Du, ZHlyX2ttQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.