
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 31 January 2025
Sec. Inflammation
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1518886
Scoparone (SCO), also known as 6,7-Dimethoxycoumarin, is a naturally occurring bioactive ingredient originally derived from Chinese herb Artemisiae Scopariae Herba (Yin-Chen-Hao). Previous studies have shown that it is effective in treating some of the liver diseases. Beyond its hepatoprotective effects, an expanding body of research has underscored the immunoregulatory properties of SCO, indicating its potential therapeutic benefits for autoimmune and other inflammatory diseases. Over the past decade, significant advances have been made in understanding the mechanistic insights into its effects on immune-mediated diseases as well as liver diseases. SCO has an impact on various immune cells, including mast cells, monocytes, macrophages, neutrophils and T cells, and affects a broad range of intracellular signaling pathways, including TLR4/Myd88/NFκB, TGFβR/Smad3 and JNK/Sab/SHP-1 etc. Therefore, this review not only summarizes the immunomodulatory and therapeutic effects of SCO on immune-based inflammatory diseases (IMIDs), such as inflammatory bowel disease, osteoarthritis, allergic rhinitis, acute lung injury, type 1 diabetes and neuroinflammatory diseases etc., but also provides a comprehensive summary of its therapeutic effects on hepatic diseases, including non-alcoholic steatohepatitis, fulminant hepatic failure and hepatic fibrosis. In this review, we also include the broad impacts of SCO on intracellular signaling pathways, such as TLR4/Myd88/NFκB, TGFβR/Smad3, Nrf2/P38, JAK2/STAT3 and JNK/Sab/SHP-1 etc. Further researches on SCO may help understand its in-depth mechanisms of action and pave the way for the development of novel drugs to prevent and treat various immune-mediated inflammatory disorders as well as hepatic diseases, thereby significantly advancing its innovations and pharmaceutical applications.
Scoparone (6,7-Dimethoxycoumarin, SCO), a naturally occurring bioactive ingredient originally derived from Chinese herb Artemisiae Scopariae Herba (Yin-Chen-Hao), possesses the diverse pharmacological properties, including anti-inflammatory, antioxidant and anti-cholestatic effects. Yin-Chen-Hao has been commonly used in the treatment of liver and bile disorders, such as acute jaundice, hepatitis and cholestasis disorders (1). Notably, SCO serves as a principal active component in the traditional formulation of Yin-Chen-Hao (YCH) and Yinzhihuang decoctions (YZHDs), which have been clinically administered to treat liver and cholestasis ailments (2, 3). Recent studies have made significant progresses in understanding the mechanisms by which SCO, or in combination with other pharmaceutical ingredients, exerts its anti-inflammatory effects.
Immune-mediated inflammatory diseases (IMIDs) include a broad condition of organ/tissue inflammation characterized by dysregulated immune responses, resulting in inflammation and the subsequent damage to target organs (4, 5). Previous studies have reported the effects of SCO on IMIDs, which are mostly autoimmune and allergic diseases, such as inflammatory bowel disease, osteoarthritis, allergic rhinitis, type 1 diabetes and neuroinflammatory diseases etc. Researchers have also demonstrated its therapeutic effects on hepatic diseases, including non-alcoholic steatohepatitis, fulminant hepatic failure and hepatic fibrosis (6). In this review, we have summarized recent studies exploring the immunomodulatory and anti-inflammatory effects of SCO on immune-related inflammatory and liver diseases, as well as the cellular and molecular mechanisms underlying its immunoregulatory and anti-inflammatory effects (Table 1). We also provided potential research prospects for the treatment of various IMIDs using SCO, thus helping lay the foundation for its clinical trials to treat various inflammatory diseases, especially autoimmune diseases.
Immune cells play important roles in the development and progression of immune-mediated inflammatory diseases (IMIDs). SCO alleviates the deterioration of IMIDs by regulating immune responses as well as the expression of numerous cytokines/chemokines in various immune cells (Figure 1).
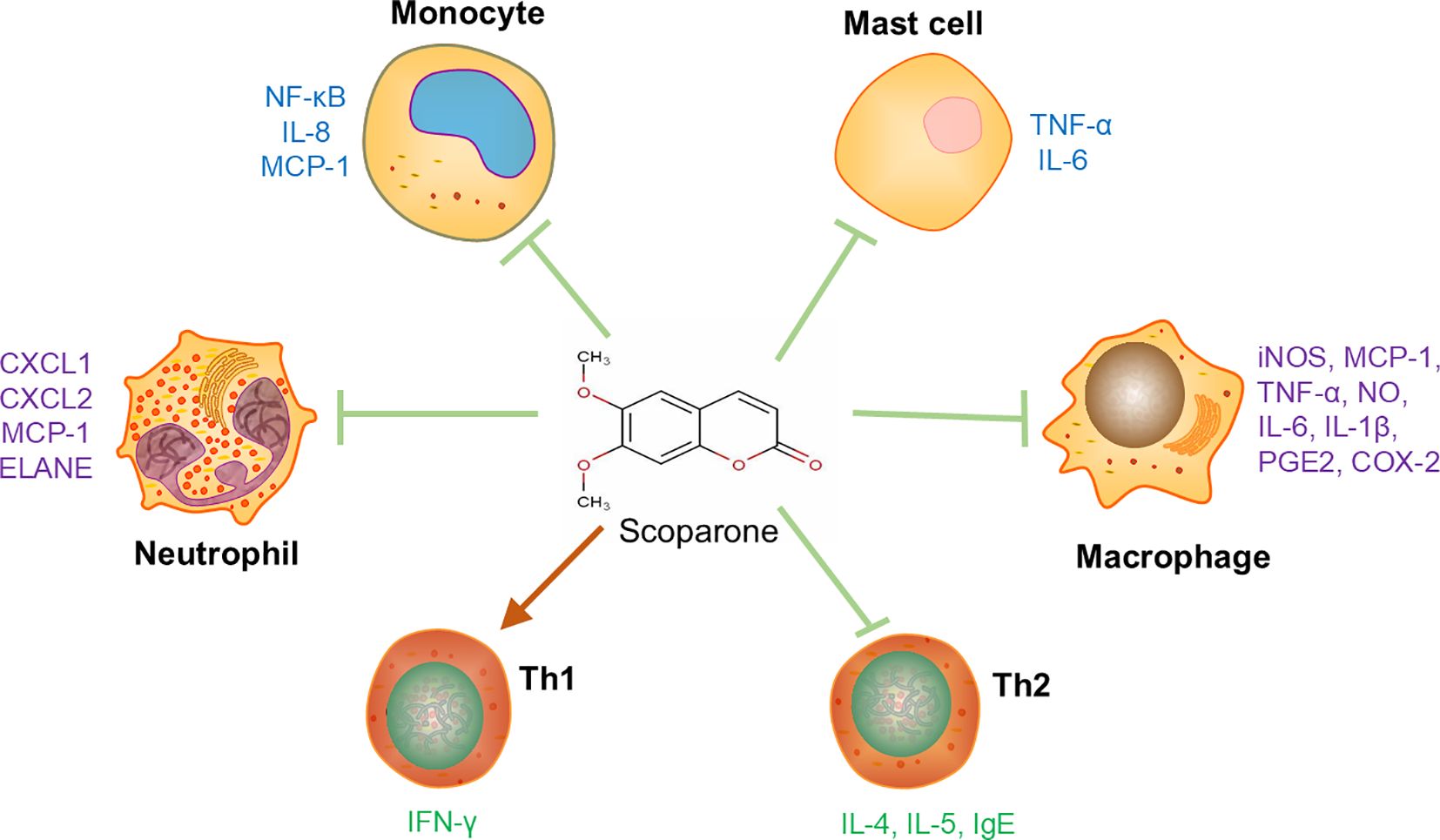
Figure 1. The effects of scoparone on adaptive and innate immune cells. Scoparone can regulate the cytokine expression in T helper cells, macrophages, neutrophils, mast cells and monocytes. Arrows “↓” mean “enhancing” while “⊥” means “inhibiting”. Th1, T helper cell 1; Th2, T helper cell 2; CXCL1, C-X-C motif chemokine ligand 1; CXCL2, C-X-C motif chemokine ligand 2; COX-2, cyclooxygenase 2; ELANE, elastase, neutrophil-expressed; PGE2, prostaglandin E2.
Mast cells play a key role in anaphylaxis by releasing a variety of pro-inflammatory mediators and cytokines. It was found that activated mast cells produced tumor necrosis factor (TNF-α) and interleukin (IL)-6, which played a central role in triggering and maintaining allergic inflammation (7). Previous studies also demonstrated that SCO inhibited the expression of TNF-α and IL-6 proteins and the activation of p38 MAPK and NF-kB signaling pathways through prohibiting the uptake of calcium by mast cells, thereby alleviating IgE-mediated mast cell hypersensitivity (8). Thus, SCO may be used to treat allergic diseases.
Mononuclear cells normally maintain the inflammatory balance and immune tolerance in a physiological condition (9). Previous results showed that SCO inhibited the proliferation of mononuclear cells in a dose-dependent manner in mixed lymphocyte responses (10, 11). SCO antagonized the effects of the diabetogenic drug alloxan on mononuclear cells and increased the levels of PGE2, PGF2α, leukotriene B4 and 2,3-dinor-thromboxane B2 in phytohemagglutinin (PHA)-stimulated monocyte culture (10). Moreover, SCO inhibited the response of mononuclear cells to PHA, mixed lymphocytes, mitogen and alloantigen, while it played a role in relaxing blood vessels and suppressing immunity (11). Another study also reported that SCO suppressed NF-κB activation in U937 human monocytes activated by phorbol 12-myristate 13-acetate (PMA) and reduced PMA-induced toxicity and release of IL-8 and MCP-1 proteins (12).
Macrophages process antigens and carry them to the lymph nodes, while also secreting pro-inflammatory factors (13). Yeh et al. found that SCO not only inhibited M1 macrophage markers, such as iNOS, IL-6 and CCL2, but also significantly reduced the protein level of TNF-α in LPS-treated macrophages (14). In addition, they reported that SCO significantly increased the gene expression of M2 macrophage markers and the protein level of Arg1, thus promoting the differentiation of macrophages towards anti-inflammatory M2 phenotype. Similarly, Liu et al. demonstrated that SCO suppressed macrophage autophagy and M1 polarization (15). Previous studies also showed that SCO inhibited the activation of NLRP3 inflammasome in LPS-induced murine macrophages and mouse models of bacterial enteritis and septic shock, and suppressed the production of TNFα, IL-6, NO and PGE2 in IFN-gamma/LPS stimulated RAW 264.7 cells (16, 17). Liu et al. proved that SCO mitigated macrophage responses induced by NASH and LPS via blocking the TLR-4/NF-κB signaling pathway (18). Invasive liver macrophages led to chronic liver injury and fibrosis through HSC transdifferentiation and proliferation, while SCO inhibited HSC activation, downregulated macrophage infiltration, suppressed the secretion of NO, PGE2, iNOS and COX-2 in RAW 264.7 cells, and reduced the production of TNF-α, IL-1β and IL-6 in RAW264.7 cells stimulated by LPS (17). Similarly, Niu et al. found that SCO inhibited NF-κB activation and TLR4 expression as well as the production of TNF-α, IL-6 and IL-1β in LPS-induced alveolar macrophages in vitro (19). Thus, SCO suppressed the expression of pro-inflammatory cytokines in macrophages and promoted their polarization towards M2 phenotype, suggesting that SCO may be effective in treating macrophage-mediated inflammatory diseases.
Neutrophils play a pivotal role in the innate immune responsiveness and mainly exert pro-inflammatory effects, causing severe tissue inflammation (20). Experiments by Niu et al. demonstrated that SCO significantly diminished neutrophil numbers and downregulated the expression of CXCL1, CXCL2 and CCL2, thereby mitigating inflammation (19). Studies by Chan et al. showed that SCO (6,7-dimethoxycoumarin) exhibited significant inhibition of superoxide anion generation and the release of elastase by human neutrophils in vitro (21). These findings indicate that SCO can exert anti-inflammatory effects.
T cells are a core component of adaptive immunity, mainly including type 1 helper T cells (Th1), type 2 helper T cells (Th2) and Th17 cells classified under CD4+ T cells (22). The immunological basis of allergic rhinitis (AR) was closely related to the imbalance between Th1 and Th2 cells (23). Th2 cytokines IL-4 and IL-5 were involved in AR allergic inflammation (24). SCO inhibited the synthesis of IgE, upregulated the level of Th1 cytokines in serum, reduced the level of Th2 cytokines, and restored the balance between Th1 and Th2 cells, thus improving the symptoms of allergic rhinitis in rats (25).
SCO has been shown to treat various hepatic diseases in animal models (6), including immune-associated inflammatory liver diseases. It also has been used to treat many of the other IMIDs in pre-clinical studies. In fact, SCO has demonstrated the efficacy in treating various IMIDs, including the intestinal disorders, respiratory ailments and osteoarthritis etc. IMIDs encompass a spectrum of conditions characterized by dysregulated immune responses, leading to inflammation and subsequent damage to target organs (4, 5). Some of the prevalent IMIDs are classified as autoimmune diseases, in which the immune system mistakenly targets and attacks the self organ/tissue. These conditions are often accompanied with a range of comorbidities, including cardiovascular diseases, liver diseases, metabolic disorders, skeletal abnormalities and cognitive impairments (26, 27).
Non-alcoholic steatohepatitis(NASH), a progressive form of non-alcoholic fatty liver disease and a prevalent chronic liver condition, is characterized by inflammation without or with fibrosis in addition to the hepatic steatosis (28). Previous studies have shown that NASH is caused by lipotoxic liver injury, in which excess lipid accumulation promotes insulin resistance, oxidative stress, mitochondrial dysfunction and endoplasmic reticulum stress, leading to apoptosis, inflammation and liver tissue fibrosis (29). Study by Jiang et al. showed that the activation of JNK/Sab signaling pathway induced by palmitic acid was blocked by SCO treatment, with a decrease in the blood lipid and aminotransferase, therefore improving liver histopathological conditions via restoration of mitochondrial function and reversal of hepatic steatosis in mice (30). Liu et al. found that SCO also improved liver steatosis, apoptosis, inflammation and fibrosis in a mouse model of MCD diet-induced NASH. Additionally, they highlighted capacity of SCO to modulate immune responses in LPS-induced RAW264.7 macrophages through the inhibition of TLR-4/NF-κB signaling pathway (18). Wei et al. revealed that SCO could reduce murine liver injury, oxidative stress and inflammation by suppressing lipid accumulation and improving alcohol metabolism (31). SCO also was shown to decrease the expression of TNF-α, IL-6, IL-1β and Rela, increase the expression of krueppel-like factor 10 (Klf10), and attenuate lipid metabolism dysfunction and inflammation by activating the peroxisome proliferator-activated receptor α (PPARα) signaling pathway (32). Zhao et al. showed that SCO alone reduced the expression of apoptosis-associated speck-like protein containing a CARD (ASC), IL-1β, TNF-α and IL-6, inhibited the activation of NLRP3 inflammasome in the liver, and increased the levels of the antioxidants CAT and SOD, thus ameliorating liver inflammation (33). Finally, scoparone treatment improved glycerophospholipid metabolism and liver histopathology in a murine NASH model (34). Collectively, those findings underscored the potential protective effects of SCO on NASH and its hepatic pathology.
Fulminant hepatic failure (FHF) is a severe clinical syndrome with extensive liver cell damage. Toxic hepatitis and acute viral hepatitis represent the most prevalent etiologies of FHF (35). Proinflammatory cytokines and chemokines promote oxidative stress in the damage caused by toxic substances, leading to the massive infiltration of proinflammatory cells and eventually the formation of severe hepatitis (36). Kang et al. demonstrated that the pathogenesis of FHF was related to the upregulation of MyD88 and TRF-dependent signaling pathways in the TLR system. Their results showed that pretreatment with SCO inhibited the expression of TLR4/Myd88 and suppressed the phosphorylation of p38, ERK1/2 and cJun N-terminal kinase (JNK) as well as the activation of NF-κB in mice with D-galactosamine(D-GalN)/LPS-induced FHF (37). Zhang et al. used STRING analyses to map the protein interaction networks and found that the liver protective effects of SCO on acute liver injury in rats were associated with the expression of six proteins, including Ig kappa chain C, zinc finger protein 407, prothrombin, haptoglobin, alpha-1-antitrypsin and transthyretin, thus providing new insights into the mechanisms responsible for its liver protection (38).
Hepatic fibrosis is a chronic liver disease characterized by excessive production and deposition of extracellular matrix (ECM) in the liver. The disease mainly develops from chronic liver inflammation caused by viral hepatitis, alcoholism, metabolic drugs and steatohepatitis, etc (39). The activation of hepatic stellate cells (HSC) is a main mechanism underlying the chronic hepatic fibrosis (40). Experiments by Liu et al. demonstrated that SCO significantly reduced the expression of NADPH oxidase (NOX), production of ROS, and Smad3 phosphorylation in TGF-β1-stimulated HSC T6 cells in vitro (41), suggesting that SCO likely alleviates liver fibrosis, which is usually caused by chronic inflammation.
Inflammatory bowel disease (IBD), including Crohn’s disease and ulcerative colitis, represents a chronic, recurrent and immune-mediated condition that inflicts damage on the gastrointestinal tracts and significantly impairs patients’ quality of life (42, 43). Oxidative stress induced by free radicals and reactive oxygen species is strongly implicated in the pathogenesis of IBD, which is characterized by diminished endogenous antioxidants and heightened oxidative stress biomarkers (44, 45). Witaicenis et al. previously reported that administration of SCO significantly reduced the incidence of diarrhea, injury score and colon weight in a mouse model of trinitrobenzene sulfonic acid (TNBS)-induced colitis (46). Besides, they also observed that SCO decreased the activity of alkaline phosphatase (AP), a sensitive marker of intestinal inflammation (46), and carbon tetrachloride-induced oxidative stress but enhanced the expression of gamma-glutamyl-cysteinyl-glycine (GSH), which acted as an endogenous reactive oxygen scavenger (47). These findings imply that the protective effects of SCO on intestinal inflammation are associated with its antioxidant property.
Osteoarthritis (OA) is a progressive joint ailment characterized by joint swelling, pain and functional impairment due to the damage to cartilage, bone and the synovial cavity (48). Previous investigations have revealed that synovial inflammation in OA involves the infiltration of macrophages and T cells, with increased levels of Th1, Th9 and Th17 cells in OA joint fluid (49). A pro-inflammatory cytokine interleukin-1β (IL-1β) was implicated in OA pathogenesis, and capable of inducing chondrocyte senescence (50). Treatment with SCO weakened the effects of IL-1β on chondrocyte viability by decreasing the expression of NO, PGE2, MMP-3, MMP-13, ADAMTS-4, ADAMTS-5, iNOS and COX-2 in a dose-dependent manner (3). Meanwhile, SCO also interfered with the development of OA by regulating the PI3K/Akt/NF-κB signaling pathway (3). These findings indicate that SCO is potentially a therapeutic agent for OA management and treatment.
Acute lung injury (ALI) is an acute inflammatory disease that commonly occurs in clinical settings with a high mortality rate, posing a significant threat to patients’ life. It was reported that LPS induced the production of pro-inflammatory cytokines TNF-α, IL-6, IL-1β and IFNγ etc. (51), thereby accelerating the pathophysiological process of endotoxin-induced ALI. Previous studies showed that LPS, as a key risk factor for ALI, stimulated alveolar macrophages to activate Toll-like receptor 4 (TLR4) signaling (52), while LPS could also upregulate the expression of chemokines CXCL1, CXCL2 and CCL2 on the margins of macrophages and neutrophils in the lung (53).
Study performed by Niu et al. demonstrated that SCO exerted a protective effect on LPS-induced ALI. They used a mouse model of acute lung injury induced by LPS through nasal gavage and found that treatment with SCO inhibited the accumulation of pulmonary neutrophils and macrophages and suppressed myeloperoxidase activity and expression of CXCL1, CXCL2, CCL2, TNF-α, IL-6 and IL-1β in vivo, resulting in the mitigation of pulmonary edema and damage. Additionally, SCO was found to inhibit TLR4-mediated NF-κB signaling pathway and the expression of TLR4, TNF-α, IL-6 and IL-1β in LPS-stimulated alveolar macrophages, underscoring its efficacy in alleviating LPS-induced ALI (19). Thus, SCO may be implicated for the treatment of lung diseases that cause an ALI condition.
Allergic rhinitis (AR) is a type 1 allergic disease mediated by IgE (54). Experiments performed by Yuan et al. showed that SCO ameliorated AR in rats by regulating the balance of Th1 and Th2 immune responses via inhibiting TLR4/NF-κB signaling pathway. They reported that SCO treatment led to a reduction in rhinitis symptom scores and decrease in serum levels of IgE, IL-4 and IL-5, with an increase in IFN-γ level (55). Similar results were observed by Cheng et al. showing that the pro-inflammatory cells of mice with AR were significantly reduced after SCO treatment, with the mucosal structure returning to normal. Compared with AR group, the serum level of IFN-γ was increased, while that of IL-4, IL-5 or IgE was significantly decreased in SCO treatment group (25). Therefore, these results suggest that SCO can inhibit or eliminate symptoms of AR by improving the imbalance of Th1/Th2 immune responses.
Type 1 diabetes, commonly referred to as autoimmune diabetes, arises from the autoimmunity-mediated destruction of insulin-producing β cells within the pancreas and is influenced by genetic predisposition and potential environmental triggers (56). Cytokines produced by immune cells infiltrating pancreatic islets serve as pivotal mediators in the destruction of β cells, leading to insulin-dependent diabetes mellitus. Kim et al. demonstrated, for the first time, that SCO treatment not only protected rat insulinoma cells stimulated by IL-1β and IFN-γ, but also maintained the secretion of insulin by islets stimulated by glucose, while SCO also inhibited NF-κB nuclear translocation, thereby suppressing the expression of cytokine-induced iNOS (57). These findings have underscored the therapeutic potential of SCO for halting the progression of type 1 diabetes.
Neuroinflammation is an inflammatory reaction in the brain or spinal cord, which is mediated by cytokines, chemokines, reactive oxygen species, etc. (58). Activation of microglia leads to elevated levels of the neurotoxic and pro-inflammatory mediators, resulting in severe damage to brain cells and occurrence of various neuroinflammatory diseases, including Alzheimer’s disease (AD) and epilepsy (59). Thus, modulation of microglial activation emerges as a pivotal strategy for averting diverse neuroinflammatory diseases.
SCO is gaining prominence in neurotherapeutics owing to its low toxicity and ability to inhibit microglial activation. Santibanez et al. employed HPLC-DAD-UV bioassays to elucidate the temporal concentration profile of each coumarin in a LPS-induced neuroinflammation model. They revealed that SCO maintained elevated tissue concentrations compared to other compounds, exhibiting notable bioavailability in both brain tissue and plasma. This finding demonstrated the capacity of SCO to traverse the blood-brain barrier, indicating its potential advantage in treating various neuroinflammatory diseases (60).
Recent studies have also unveiled some mechanisms underlying the pharmacological effects of SCO. Cho and colleagues found that SCO inhibited neuroinflammation by reducing ERK and the TRIF-dependent signaling molecule IRF3, rather than affecting the activation of NF-κB and MAPK in LPS-stimulated BV-2 microglia (61). Neuroinflammation mediated by microglia activation is a key factor in the onset of Alzheimer’s disease (62). As reported by Ibrahim et al., SCO promoted the polarization of microglia toward an M2 subtype by shutting down the TLR4/MyD88/TRAF-6/TAK-1/NF-κB axis and inhibiting the NLRP3 pathway, and effectively mitigated ovariectomy/D-galactose(OVX/D-Gal)-induced neuroinflammation and neuronal degeneration, leading to a reduction in neuroinflammation and neurodegeneration in OVX/D-Gal models of Alzheimer’s disease (63). Besides, SCO significantly reduced the expression of GFAP and Iba1 in the cortex of epileptic mice and inhibited the expression of CXCL-1, IL-1β, TNF-α, MCP-1, IL-6, HIF-1 and HMGB1, thereby protecting mice from pilocarpine-induced seizures by inhibiting Casapse-3 fragmentation, activation of astrocytes and microglia, inflammation and cell apoptosis through the TLR4/NF-κB pathway (64). These findings underscored the potential of SCO as an anti-neuroinflammatory agent and offered promising avenues for drug development in various neuroinflammatory diseases.
SCO regulates immune homeostasis and ameliorates inflammatory diseases through acting on the complex signaling networks. SCO modulates cellular responsiveness by altering many of the intracellular signaling pathways, such as TLR/NF-κB, PI3K-Akt, Nrf2, JNK/Sab, TGF−β/Smad, Nitric oxide (NO)-cGMP and JAK2-STAT3 signaling axes (Figure 2).
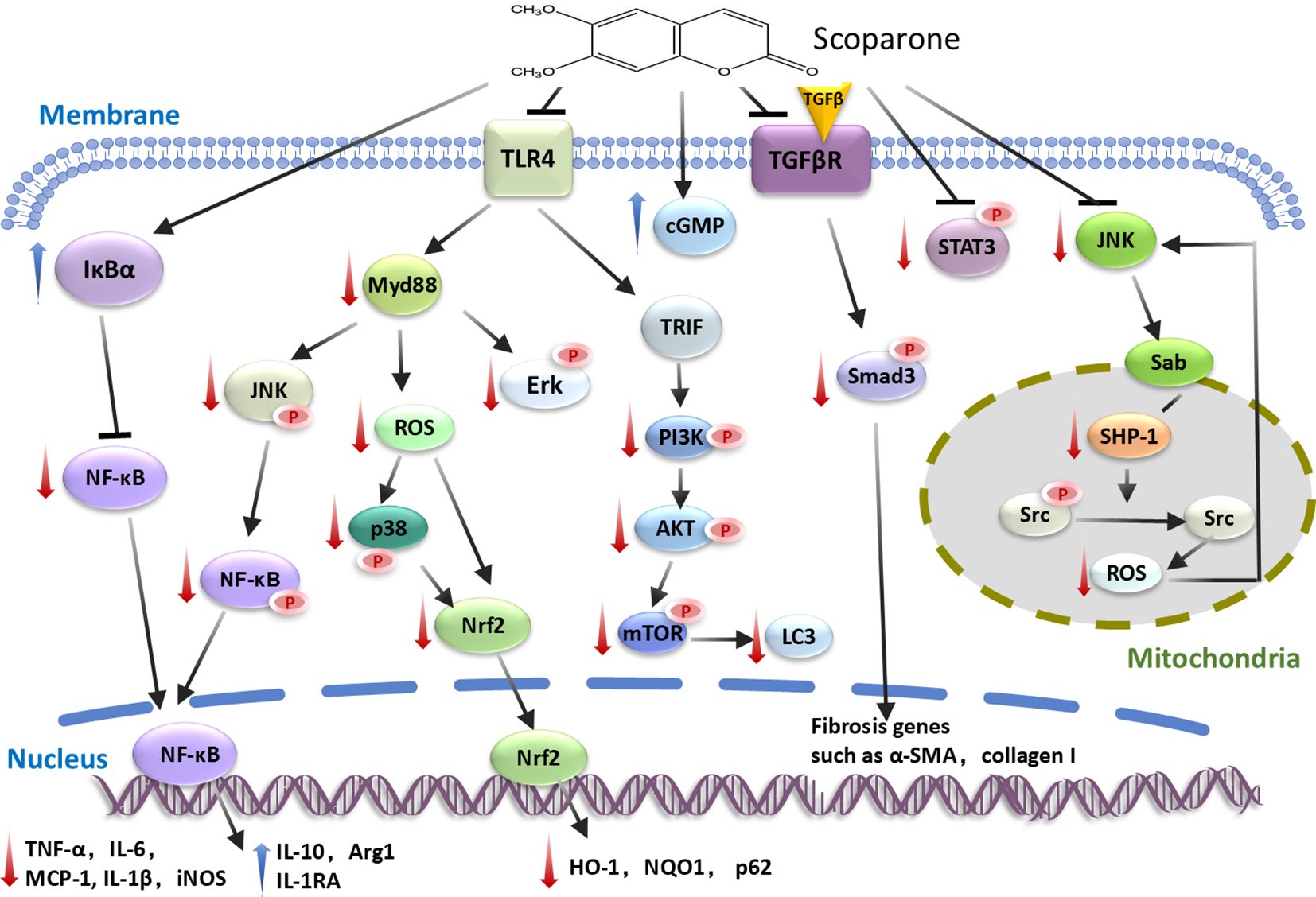
Figure 2. Signaling pathways regulated by scoparone. By interfering with TLR/NF-κB, PI3K-Akt, Nrf2, JNK/Sab, TGF-β/Smad, Nitric oxide (NO)-cGMP and JAK2-STAT3 signaling pathways, scoparone downregulates pro-inflammatory genes and upregulates anti-inflammatory and antioxidant genes. The downward red arrows indicate inhibition, while the upward blue arrows denote stimulation/increase. α-SMA, actin alpha 2; cGMP, cyclic guanosine monophosphate; HO-1, oxygenase 1; iNOS, inducible nitric oxide synthase; MCP-1, monocyte chemoattractant protein-1; NQO1, NADPH:quinone oxidoreductase 1; Nrf2, NFE2 like bZIP transcription factor 2; ROS, reactive oxygen species; SHP-1, SH2 domain-containing protein tyrosine phosphatase 1; Smad3, SMAD family member 3; Sab, SH3-domain binding protein 5; STAT3, signal transducer and activator of transcription 3; TLR4, Toll like receptor 4.
Nuclear factor-kappa B (NF-κB) signaling plays a vital role in both immunity and inflammation. It regulates the expression of various proinflammatory genes and serves as a critical mediator for inflammatory responses (65). The activation of NF-κB induces many of the pro-inflammatory mediators and molecules, leading to inflammatory responses caused by the activation of various immune cells (66). Thus, NF-κB is tightly regulated to maintain the immunological balance. Niu et al. used a murine model of acute lung injury induced by LPS via nasal gavage and found that SCO inhibited TLR4-mediated NF-κB activation and the production of proinflammatory cytokines, such as TNF-α, IL-1β and IL-6, thereby suppressing inflammation (19). Another study demonstrated that SCO blocked LPS-stimulated increases in the levels of TLR4/MyD88 proteins and downstream phosphorylated NF-κB activity in RAW264.7 cells (18). Kang et al. revealed that SCO attenuated IgE-mediated allergic and inflammatory responses in mast cells by inhibiting the overexpression of TLR4 and blocking NF-κB activation (37). Their findings showed that SCO reversed a LPS-induced reduction in IκB-α and an increase in the expression of MyD88, NF-κB/p65 and c-Jun proteins.
The PI3K/AKT/mTOR pathway is thought to be a negative regulator of TLR4/NF-κB signaling pathway in macrophages, and it plays a specific role in the regulation of inflammatory responses (67). In a study performed by Liu et al., treatment with SCO downregulated LPS-induced increases in phosphorylated AKT and mTOR, reduced the accumulation of p62 and LC3 and rescued autophagy via blocking the PI3K/AKT/mTOR pathway in macrophages (15). Treatment with SCO also weakened the phosphorylation of PI3K and AKT in IL-1β-stimulated chondrocytes, suggesting that SCO has a protective effect on IL-1β-induced inflammatory responses in chondrocytes (3).
Transcription factor Nrf2 plays an important role in the antioxidant and detoxification responses, while its overactivation is harmful (68). Nrf2 is regulated by a high concentration of ROS and affects the transcription of p62 (69). Liu et al. found that SCO reduced Nrf2 protein level induced by the Methionine-Choline deficient (MCD) diet in mice, inhibited the production of a high concentration of ROS and decreased the levels of Nrf2 and phospho-p38 proteins as well as p62 target genes in LPS-stimulated RAW264.7 cells, indicating that SCO alleviates cell damage and inflammation by inhibiting ROS/P38/Nrf2 axis (15).
c-Jun N-terminal kinase (JNK) is a member of the mitogen-activated protein kinase (MAPK) family, and its continuous activation can impair the function of mitochondrial respiratory chains, leading to mitochondrial dysfunction (70). Studies have shown that JNK, when combined with Sab, which is a scaffold protein located in the outer membrane of mitochondria, triggers the destruction of mitochondrial electron transport chain and promotes the release of reactive oxygen species (ROS), ultimately resulting in cell death (71, 72). JNK binds to Sab and separates SHP-1 from Sab, and then the activated SHP-1 is transferred to the mitochondrial intima, thereby causing Src inactivation and mitochondrial dysfunction (73). In a study by Yu et al., it was found that SCO improved PA-induced reduction of mitochondrial membrane potential and ATP production in hepatocytes and downregulated ROS by inhibiting the activation of JNK and SHP-1 and preventing the inactivation of Src (30). Therefore, SCO can restore mitochondrial function by blocking the JNK/Sab activation.
TGF-β1, a key regulator of fibrosis in many organs (74), induces expression of NOXs and production of ROS, and is involved in regulating hepatic stellate cells (HSC) activation, which is a key step in initiating liver fibrosis (75). Liu et al. found that administration of SCO suppressed cell proliferation, Smad3 phosphorylation and extracellular matrix (ECM) expression in TGF-β1-induced HSC-T6 cells, accompanied by decreases in the expression of α-SMA, collagen I, collagen III, NOXs and ROS production (41). In addition, Xu et al. also demonstrated that SCO inhibited proliferation, fibrotic phenotype and oxidative stress level of the pancreatic stellate cells by downregulating the expression of α-SMA and type I collagen, and alleviated pancreatic fibrosis through TGF-β/Smad pathway (74). Taken together, SCO can inactivate TGF-β1/Smad3 signaling pathway, therefore improving the pathology and symptoms of fibrosis-related diseases.
NO activates cytoplasmic guanylate cyclase, which then increases the level of cGMP in smooth muscle cells, leading to relaxation of smooth muscle cells (76). Choi et al. demonstrated that treatment with SCO increased the level of cGMP in rabbits, resulting in the significant relaxation of their penile corpus cavernosum smooth muscle (PCCSM). This effect of SCO was weakened by blocking NO synthetase or guanylate cyclase using N-ω-nitro-l-arginine methyl ester hydrochloride (L-NAME) or 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), suggesting that SCO promotes penile erection by activating the NO-cGMP signaling pathway (77).
Janus Kinase 2 (JAK2) can induce the phosphorylation of signal transducer and activator of transcription 3 (STAT3) and is an important regulator of inflammatory responses as well as cell proliferation/differentiation (78). It was found that alterations in JAK2/STAT3 pathway affected the expression of many pro-inflammatory cytokines (79). SCO was reported to suppress the accumulation of STAT3 transported from the cytosol to the nucleus, resulting in the inhibition of vascular smooth muscle cell (VSMC) proliferation through G1 phase arrest and suppression of Rb phosphorylation (80). SCO also exerted antitumor effects on prostate cancer cells by inhibiting the transcription of STAT3 target genes, such as cyclin D1, c-Myc, survivin, Bcl-2 and Socs3, and decreasing the phosphorylation and nuclear accumulation of STAT3, but not JAK2 (81).
Currently, SCO is predominantly utilized as a component or ingredient of Artemisiae Scopariae Herba in compound formulations rather than as a standalone treatment. While animal and cellular experiments have provided valuable insights into the effects of SCO on immune cells and immune-based diseases, especially some autoimmune diseases, there is a lack of data concerning its toxicity, pharmacokinetics and side effects. Moreover, there is also a gap between preclinical findings and clinical data since SCO has not been studied in humans. In these aspects, both animal and clinical studies are warranted to ascertain its optimal dosages, efficacy and potential side effects in the treatment of IMIDs, in particular, common autoimmune diseases such as IBD, rheumatoid arthritis, osteoarthritis, lupus and type 1 diabetes. Although SCO holds much promise as an effective therapeutic agent for some IMIDs in preclinical studies, well-designed clinical trials are imperative to provide conclusive evidence and inform clinical practice for treating various autoimmune diseases as well as inflammatory hepatic diseases. Since SCO is not a powerful conventional immunosuppressant, however, it alone may not be sufficient to effectively inhibit inflammation in a clinical setting, which may pose a significant challenge to recruit patients for a large-scale clinical trial. Perhaps, a feasible clinical trial should start to explore the potentially synergistical effects of SCO on IMIDs with other immunomodulatory or anti-inflammatory agents. It’s also imperative to screen its derivatives for a better bioavailability, stability, efficacy or reduced toxicity. We should also carefully observe the potential side effects of SCO in preclinical studies even before clinical trials. Additionally, further studies are required to understand its drug metabolism and pharmacokinetics before the clinical application of SCO to optimize its dosages. Finally, as an additional limitation, previous studies have not identified the exact binding sites of various signaling molecules and pathways. It remains unclear how SCO interacts with each molecule in a specific intracellular signaling axis. Thus, warranted are in-depth studies on exact mechanisms of action underlying its effects, especially its binding sites of various intracellular signaling pathways, such as TLR4/Myd88/NFκB, TGFβR/Smad3 and JNK/Sab/SHP-1 axes.
Conventional immunosuppressive drugs often carry high costs and may increase the risk of infections and development of cancers. Therefore, it’s imperative to seek alternatives of moderate immunosuppressants with less side effects, such as SCO. Here we have compiled this review showcasing the efficacy of SCO in treating IMIDs, such as allergic rhinitis, osteoarthritis, type 1 diabetes, IBD and neuroinflammatory diseases, and inflammatory hepatic diseases. SCO modulates both adaptive and innate immune cells, including mast cells, monocytes, macrophages, neutrophils and T cells, primarily through regulating their secretion of proinflammatory or anti-inflammatory cytokines. It also has a broad impact on intracellular signal transduction pathways, mainly including TLR4/Myd88/NFκB, TGFβR/Smad3, Nrf2/P38, JAK2/STAT3 and JNK/Sab/SHP-1 etc. Future study should focus on clinical trials to evaluate the toxicity and efficacy of SCO in the treatment of various IMIDs.
FQ: Methodology, Writing – original draft. JL: Methodology, Writing – original draft. XH: Writing – review & editing. BY: Conceptualization, Writing – review & editing. WL: Supervision, Writing – review & editing. ZD: Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This review was supported by grants from National Natural Science Foundation of China (82302006), State Key Laboratory of Dampness Syndrome of Chinese Medicine (SZ2021ZZ18), and Guangzhou Municipal Science and Technology Bureau (2024A03J0127).
The authors declare that the review was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Cai Y, Zheng Q, Sun R, Wu J, Li X, Liu R. Recent progress in the study of Artemisiae Scopariae Herba (Yin Chen), a promising medicinal herb for liver diseases. BioMed Pharmacother. (2020) 130:110513. doi: 10.1016/j.biopha.2020.110513
2. Hung H-Y, Kuo S-C. Recent studies and progression of Yin Chen Hao (茵陳蒿 Yīn Chén Hāo), a long-term used traditional Chinese medicine. J Traditional Complementary Med. (2013) 3:2–6. doi: 10.4103/2225-4110.106533
3. Lu C, Li Y, Hu S, Cai Y, Yang Z, Peng K. Scoparone prevents IL-1β-induced inflammatory response in human osteoarthritis chondrocytes through the PI3K/Akt/NF-κB pathway. BioMed Pharmacother. (2018) 106:1169–74. doi: 10.1016/j.biopha.2018.07.062
4. David T, Ling SF, Barton A. Genetics of immune-mediated inflammatory diseases. Clin Exp Immunol. (2018) 193:3–12. doi: 10.1111/cei.13101
5. Gao Z, Feng Y, Xu J, Liang J. T-cell exhaustion in immune-mediated inflammatory diseases: New implications for immunotherapy. Front Immunol. (2022) 13:977394. doi: 10.3389/fimmu.2022.977394
6. Hui Y, Wang X, Yu Z, Fan X, Cui B, Zhao T, et al. Scoparone as a therapeutic drug in liver diseases: Pharmacology, pharmacokinetics and molecular mechanisms of action. Pharmacol Res. (2020) 160:105170. doi: 10.1016/j.phrs.2020.105170
7. Nigo YI, Yamashita M, Hirahara K, Shinnakasu R, Inami M, Kimura M, et al. Regulation of allergic airway inflammation through Toll-like receptor 4-mediated modification of mast cell function. Proc Natl Acad Sci U S A. (2006) 103:2286–91. doi: 10.1073/pnas.0510685103
8. Choi YH, Yan GH. Anti-allergic effects of scoparone on mast cell-mediated allergy model. Phytomedicine. (2009) 16:1089–94. doi: 10.1016/j.phymed.2009.05.003
9. Shi X, Zhao H, Kang Y, Dong X, Yu C, Xie Q, et al. The role of mononuclear phagocytes in the testes and epididymis. Int J Mol Sci. (2022) 24:53. doi: 10.3390/ijms24010053
10. Huang HC, Huang YL, Chang JH, Chen CC, Lee YT. Possible mechanism of immunosuppressive effect of scoparone (6,7-dimethoxycoumarin). Eur J Pharmacol. (1992) 217:143–8. doi: 10.1016/0014-2999(92)90835-r
11. Huang HC, Chu SH, Chao PD. Vasorelaxants from Chinese herbs, emodin and scoparone, possess immunosuppressive properties. Eur J Pharmacol. (1991) 198:211–3. doi: 10.1016/0014-2999(91)90624-y
12. Jang SI, Kim Y-J, Kim HJ, Lee J-C, Kim H-Y, Kim Y-C, et al. Scoparone inhibits PMA-induced IL-8 and MCP-1 production through suppression of NF-kappaB activation in U937 cells. Life Sci. (2006) 78:2937–43. doi: 10.1016/j.lfs.2005.11.020
13. Weiskopf D, Weinberger B, Grubeck-Loebenstein B. The aging of the immune system. Transplant Int. (2009) 22:1031–116. doi: 10.1111/j.1432-2277.2009.00927.x
14. Yeh Y-T, Hsu K-M, Chen H-J, Su N-W, Liao Y-C, Hsieh S-C. Identification of scoparone from Chinese olive fruit as a modulator of macrophage polarization. J Agric Food Chem. (2023) 71:5195–207. doi: 10.1021/acs.jafc.2c08132
15. Liu B, Deng X, Jiang Q, Li G, Zhang J, Zhang N, et al. Scoparone improves hepatic inflammation and autophagy in mice with nonalcoholic steatohepatitis by regulating the ROS/P38/Nrf2 axis and PI3K/AKT/mTOR pathway in macrophages. BioMed Pharmacother. (2020) 125:109895. doi: 10.1016/j.biopha.2020.109895
16. Feng W, Wang Y, Luo T, Jia X, Cheng C-Q, Wang H-J, et al. Scoparone suppresses mitophagy-mediated nlrp3 inflammasome activation in inflammatory diseases. Acta Pharmacol Sin. (2023) 44:1238–51. doi: 10.1038/s41401-022-01028-9
17. Jang SI, Kim Y-J, Lee W-Y, Kwak KC, Baek SH, Kwak GB, et al. Scoparone from Artemisia capillaris inhibits the release of inflammatory mediators in RAW 264.7 cells upon stimulation cells by interferon-gamma Plus LPS. Arch Pharm Res. (2005) 28:203–8. doi: 10.1007/BF02977716
18. Liu B, Deng X, Jiang Q, Li G, Zhang J, Zhang N, et al. Scoparone alleviates inflammation, apoptosis and fibrosis of non-alcoholic steatohepatitis by suppressing the TLR4/NF-κB signaling pathway in mice. Int Immunopharmacol. (2019) 75:105797. doi: 10.1016/j.intimp.2019.105797
19. Niu N, Li B, Hu Y, Li X, Li J, Zhang H. Protective effects of scoparone against lipopolysaccharide-induced acute lung injury. Int Immunopharmacol. (2014) 23:127–33. doi: 10.1016/j.intimp.2014.08.014
20. Liew PX, Kubes P. The neutrophil’s role during health and disease. Physiol Rev. (2019) 99:1223–48. doi: 10.1152/physrev.00012.2018
21. Chan Y-Y, Hwang T-L, Kuo P-C, Hung H-Y, Wu T-S. Constituents of the Fruits of Citrus medica L. var. sarcodactylis and the Effect of 6,7-Dimethoxy-coumarin on Superoxide Anion Formation and Elastase Release. Molecules. (2017) 22:1454. doi: 10.3390/molecules22091454
22. Sabat R, Wolk K, Loyal L, Döcke W-D, Ghoreschi K. T cell pathology in skin inflammation. Semin Immunopathol. (2019) 41:359–77. doi: 10.1007/s00281-019-00742-7
23. Haitchi HM, Holgate ST. New strategies in the treatment and prevention of allergic diseases. Expert Opin Investig Drugs. (2004) 13:107–24. doi: 10.1517/13543784.13.2.107
24. Ke X, Chen Z, Wang X, Kang H, Hong S. Quercetin improves the imbalance of Th1/Th2 cells and Treg/Th17 cells to attenuate allergic rhinitis. Autoimmunity. (2023) 56:2189133. doi: 10.1080/08916934.2023.2189133
25. Cheng L, Wang Z, Jiang C, Zhang S. Effect of Scoparone on Th1/Th2 cytokines and IgE in the experimental allergic rhinitis rats. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2013) 27:1310–2.
26. González-Serna D, Villanueva-Martin G, Acosta-Herrera M, Márquez A, Martín J. Approaching shared pathophysiology in immune-mediated diseases through functional genomics. Genes. (2020) 11:1482. doi: 10.3390/genes11121482
27. McInnes IB, Gravallese EM. Immune-mediated inflammatory disease therapeutics: past, present and future. Nat Rev Immunol. (2021) 21:680–6. doi: 10.1038/s41577-021-00603-1
28. Raza S, Rajak S, Upadhyay A, Tewari A, Anthony Sinha R. Current treatment paradigms and emerging therapies for NAFLD/NASH. Front Biosci (Landmark Ed). (2021) 26:206–37. doi: 10.2741/4892
29. Patel SS, Siddiqui MS. Current and emerging therapies for non-alcoholic fatty liver disease. Drugs. (2019) 79:75–84. doi: 10.1007/s40265-018-1040-1
30. Jiang Y, Xu J, Huang P, Yang L, Liu Y, Li Y, et al. Scoparone improves nonalcoholic steatohepatitis through alleviating JNK/Sab signaling pathway-mediated mitochondrial dysfunction. Front Pharmacol. (2022) 13:863756. doi: 10.3389/fphar.2022.863756
31. Wei M, Li T, Cao H, He H, Yang C, Yin Y, et al. The effects of scoparone on alcohol and high-fat diet-induced liver injury revealed by RNA sequencing. BioMed Pharmacother. (2022) 155:113770. doi: 10.1016/j.biopha.2022.113770
32. Huang P, Yang L, Liu T, Jiang Y, Chen Z, Song H, et al. Scoparone alleviates nonalcoholic fatty liver disease by modulating the PPARα signaling pathway. Eur J Pharmacol. (2024) 984:177033. doi: 10.1016/j.ejphar.2024.177033
33. Zhao T, Lun S, Yan M, Park J, Wang S, Chen C. 6,7-Dimethoxycoumarin, Gardenoside and Rhein combination improves non-alcoholic fatty liver disease in rats. J Ethnopharmacol. (2024) 322:117646. doi: 10.1016/j.jep.2023.117646
34. Song Q, Zhao Z, Liu H, Zhang J, Wang Z, Zhang Y, et al. Pseudotargeted lipidomics analysis of scoparone on glycerophospholipid metabolism in non-alcoholic steatohepatitis mice by LC-MRM-MS. PeerJ. (2024) 12:e17380. doi: 10.7717/peerj.17380
35. Gotthardt D, Riediger C, Weiss KH, Encke J, Schemmer P, Schmidt J, et al. Fulminant hepatic failure: etiology and indications for liver transplantation. Nephrol Dial Transplant. (2007) 22 Suppl 8:viii5–8. doi: 10.1093/ndt/gfm650
36. Malaguarnera G, Cataudella E, Giordano M, Nunnari G, Chisari G, Malaguarnera M. Toxic hepatitis in occupational exposure to solvents. World J Gastroenterol. (2012) 18:2756–66. doi: 10.3748/wjg.v18.i22.2756
37. Kang J-W, Kim D-W, Choi JS, Kim YS, Lee S-M. Scoparone attenuates D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure through inhibition of toll-like receptor 4 signaling in mice. Food Chem Toxicol. (2013) 57:132–9. doi: 10.1016/j.fct.2013.03.016
38. Zhang A, Sun H, Wu G, Sun W, Yuan Y, Wang X. Proteomics analysis of hepatoprotective effects for scoparone using MALDI-TOF/TOF mass spectrometry with bioinformatics. OMICS. (2013) 17:224–9. doi: 10.1089/omi.2012.0064
39. Friedman SL. Evolving challenges in hepatic fibrosis. Nat Rev Gastroenterol Hepatol. (2010) 7:425–36. doi: 10.1038/nrgastro.2010.97
40. Aydın MM, Akçalı KC. Liver fibrosis. Turk J Gastroenterol. (2018) 29:14–21. doi: 10.5152/tjg.2018.17330
41. Liu X, Zhao X. Scoparone attenuates hepatic stellate cell activation through inhibiting TGF-β/Smad signaling pathway. BioMed Pharmacother. (2017) 93:57–61. doi: 10.1016/j.biopha.2017.06.006
42. Qiu F, Liu J, Mo X, Liu H, Chen Y, Dai Z. Immunoregulation by artemisinin and its derivatives: A new role for old antimalarial drugs. Front Immunol. (2021) 12:751772. doi: 10.3389/fimmu.2021.751772
43. Bisgaard TH, Allin KH, Keefer L, Ananthakrishnan AN, Jess T. Depression and anxiety in inflammatory bowel disease: epidemiology, mechanisms and treatment. Nat Rev Gastroenterol Hepatol. (2022) 19:717–26. doi: 10.1038/s41575-022-00634-6
44. Kim YJ, Kim E-H, Hahm KB. Oxidative stress in inflammation-based gastrointestinal tract diseases: challenges and opportunities. J Gastroenterol Hepatol. (2012) 27:1004–10. doi: 10.1111/j.1440-1746.2012.07108.x
45. Zhu H, Li YR. Oxidative stress and redox signaling mechanisms of inflammatory bowel disease: updated experimental and clinical evidence. Exp Biol Med (Maywood). (2012) 237:474–80. doi: 10.1258/ebm.2011.011358
46. Witaicenis A, Seito LN, da Silveira Chagas A, de Almeida LD, Luchini AC, Rodrigues-Orsi P, et al. Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives. Phytomedicine. (2014) 21:240–6. doi: 10.1016/j.phymed.2013.09.001
47. Diaz-Vivancos P, de Simone A, Kiddle G, Foyer CH. Glutathione – linking cell proliferation to oxidative stress. Free Radical Biol Med. (2015) 89:1154–64. doi: 10.1016/j.freeradbiomed.2015.09.023
48. Glyn-Jones S, Palmer AJR, Agricola R, Price AJ, Vincent TL, Weinans H, et al. Osteoarthritis. Lancet. (2015) 386:376–87. doi: 10.1016/S0140-6736(14)60802-3
49. Li Y-S, Luo W, Zhu S-A, Lei G-H. T cells in osteoarthritis: alterations and beyond. Front Immunol. (2017) 8:356. doi: 10.3389/fimmu.2017.00356
50. Afonso V, Champy R, Mitrovic D, Collin P, Lomri A. Reactive oxygen species and superoxide dismutases: Role in joint diseases. Joint Bone Spine. (2007) 74:324–9. doi: 10.1016/j.jbspin.2007.02.002
51. Zhang C, Wang X, Wang C, He C, Ma Q, Li J, et al. Qingwenzhike prescription alleviates acute lung injury induced by LPS via inhibiting TLR4/NF-kB pathway and NLRP3 inflammasome activation. Front Pharmacol. (2021) 12:790072. doi: 10.3389/fphar.2021.790072
52. Tang J, Xu L, Zeng Y, Gong F. Effect of gut microbiota on LPS-induced acute lung injury by regulating the TLR4/NF-kB signaling pathway. Int Immunopharmacol. (2021) 91:107272. doi: 10.1016/j.intimp.2020.107272
53. Véliz Rodriguez T, Moalli F, Polentarutti N, Paroni M, Bonavita E, Anselmo A, et al. Role of Toll interleukin-1 receptor (IL-1R) 8, a negative regulator of IL-1R/Toll-like receptor signaling, in resistance to acute Pseudomonas aeruginosa lung infection. Infect Immun. (2012) 80:100–9. doi: 10.1128/IAI.05695-11
54. Bousquet J, Anto JM, Bachert C, Baiardini I, Bosnic-Anticevich S, Walter Canonica G, et al. Allergic rhinitis. Nat Rev Dis Primers. (2020) 6:95. doi: 10.1038/s41572-020-00227-0
55. Yuan B, Teng L, Zhang T, Cai W, Dong X, Zhang Z. Scoparone attenuates allergic rhinitis in rats through regulating Th1/Th2 imbalance and inhibiting TLR4/NF-κB signaling pathway. Latin Am J Pharm. (2020) 39:1834–9.
56. Gillespie KM. Type 1 diabetes: pathogenesis and prevention. CMAJ. (2006) 175:165–70. doi: 10.1503/cmaj.060244
57. Kim EK, Kwon KB, Lee JH, Park BH, Park JW, Lee HK, et al. Inhibition of cytokine-mediated nitric oxide synthase expression in rat insulinoma cells by scoparone. Biol Pharm Bull. (2007) 30:242–6. doi: 10.1248/bpb.30.242
58. DiSabato DJ, Quan N, Godbout JP. Neuroinflammation: the devil is in the details. J Neurochem. (2016) 139 Suppl 2:136–53. doi: 10.1111/jnc.13607
59. Kim B-W, Koppula S, Kumar H, Park J-Y, Kim I-W, More SV, et al. [amp]]alpha;-Asarone attenuates microglia-mediated neuroinflammation by inhibiting NF kappa B activation and mitigates MPTP-induced behavioral deficits in a mouse model of Parkinson’s disease. Neuropharmacology. (2015) 97:46–57. doi: 10.1016/j.neuropharm.2015.04.037
60. Santibáñez A, Herrera-Ruiz M, González-Cortazar M, Nicasio-Torres P, Sharma A, Jiménez-Ferrer E. Pharmacokinetics and tissue distribution of coumarins from tagetes lucida in an LPS-induced neuroinflammation model. Plants (Basel). (2022) 11:2805. doi: 10.3390/plants11212805
61. Cho D-Y, Ko HM, Kim J, Kim B-W, Yun Y-S, Park J-I, et al. Scoparone inhibits LPS-simulated inflammatory response by suppressing IRF3 and ERK in BV-2 microglial cells. Molecules. (2016) 21:1718. doi: 10.3390/molecules21121718
62. Block ML, Hong J-S. Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism. Prog Neurobiol. (2005) 76:77–98. doi: 10.1016/j.pneurobio.2005.06.004
63. Ibrahim WW, Skalicka-Woźniak K, Budzyńska B, El Sayed NS. NLRP3 inflammasome inhibition and M1-to-M2 microglial polarization shifting via scoparone-inhibited TLR4 axis in ovariectomy/D-galactose Alzheimer’s disease rat model. Int Immunopharmacol. (2023) 119:110239. doi: 10.1016/j.intimp.2023.110239
64. Xia J, Li C-Y, Wang H, Zhang Q-M, Han Z-M. Therapeutic effects of scoparone on pilocarpine (Pilo)-induced seizures in mice. BioMed Pharmacother. (2018) 97:1501–13. doi: 10.1016/j.biopha.2017.09.127
65. Yu H, Lin L, Zhang Z, Zhang H, Hu H. Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct Target Ther. (2020) 5:209. doi: 10.1038/s41392-020-00312-6
66. Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. (2010) 140:805–20. doi: 10.1016/j.cell.2010.01.022
67. Vergadi E, Ieronymaki E, Lyroni K, Vaporidi K, Tsatsanis C. Akt signaling pathway in macrophage activation and M1/M2 polarization. J Immunol. (2017) 198:1006–14. doi: 10.4049/jimmunol.1601515
68. Dodson M, Redmann M, Rajasekaran NS, Darley-Usmar V, Zhang J. KEAP1–NRF2 signalling and autophagy in protection against oxidative and reductive proteotoxicity. Biochem J. (2015) 469:347–55. doi: 10.1042/BJ20150568
69. Pall ML, Levine S. Nrf2, a master regulator of detoxification and also antioxidant, anti-inflammatory and other cytoprotective mechanisms, is raised by health promoting factors. Sheng Li Xue Bao. (2015) 67:1–18.
70. Gan LT, Van Rooyen DM, Koina ME, McCuskey RS, Teoh NC, Farrell GC. Hepatocyte free cholesterol lipotoxicity results from JNK1-mediated mitochondrial injury and is HMGB1 and TLR4-dependent. J Hepatol. (2014) 61:1376–84. doi: 10.1016/j.jhep.2014.07.024
71. Takeshita Y, Hashimoto Y, Nawa M, Uchino H, Matsuoka M. SH3-binding protein 5 mediates the neuroprotective effect of the secreted bioactive peptide humanin by inhibiting c-Jun NH2-terminal kinase*. J Biol Chem. (2013) 288:24691–704. doi: 10.1074/jbc.M113.469692
72. Win S, Than TA, Min RWM, Aghajan M, Kaplowitz N. c-Jun N-terminal kinase mediates mouse liver injury through a novel Sab (SH3BP5)-dependent pathway leading to inactivation of intramitochondrial Src. Hepatology. (2016) 63:1987–2003. doi: 10.1002/hep.28486
73. Win S, Than TA, Le BHA, García-Ruiz C, Fernandez-Checa JC, Kaplowitz N. Sab (Sh3bp5) dependence of JNK mediated inhibition of mitochondrial respiration in palmitic acid induced hepatocyte lipotoxicity. J Hepatol. (2015) 62:1367–74. doi: 10.1016/j.jhep.2015.01.032
74. Xu M, Cai J, Wei H, Zhou M, Xu P, Huang H, et al. Scoparone protects against pancreatic fibrosis via TGF-β/Smad signaling in rats. Cell Physiol Biochem. (2016) 40:277–86. doi: 10.1159/000452544
75. Aoyama T, Paik Y-H, Watanabe S, Laleu B, Gaggini F, Fioraso-Cartier L, et al. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology. (2012) 56:2316–27. doi: 10.1002/hep.25938
76. Chuang AT, Strauss JD, Murphy RA, Steers WD. Sildenafil, a type-5 CGMP phosphodiesterase inhibitor, specifically amplifies endogenous cGMP-dependent relaxation in rabbit corpus cavernosum smooth muscle in vitro. J Urol. (1998) 160:257–61. doi: 10.1016/S0022-5347(01)63100-8
77. Choi BR, Kim HK, Park JK. Penile Erection Induced by Scoparone from Artemisia capillaris through the Nitric Oxide-Cyclic Guanosine Monophosphate Signaling Pathway. World J Mens Health. (2017) 35:196–204. doi: 10.5534/wjmh.17023
78. Kiu H, Nicholson SE. Biology and significance of the JAK/STAT signalling pathways. Growth Factors. (2012) 30:88–106. doi: 10.3109/08977194.2012.660936
79. Zhu H, Jian Z, Zhong Y, Ye Y, Zhang Y, Hu X, et al. Janus kinase inhibition ameliorates ischemic stroke injury and neuroinflammation through reducing NLRP3 inflammasome activation via JAK2/STAT3 pathway inhibition. Front Immunol. (2021) 12:714943. doi: 10.3389/fimmu.2021.714943
80. Park S, Kim J-K, Oh CJ, Choi SH, Jeon J-H, Lee I-K. Scoparone interferes with STAT3-induced proliferation of vascular smooth muscle cells. Exp Mol Med. (2015) 47:e145. doi: 10.1038/emm.2014.113
Keywords: inflammatory disease, immunoregulation, liver disease, scoparone, signaling pathway
Citation: Qiu F, Lin J, Huang X, Yang B, Lu W and Dai Z (2025) The immunoregulatory effects of scoparone on immune-mediated inflammatory diseases. Front. Immunol. 16:1518886. doi: 10.3389/fimmu.2025.1518886
Received: 29 October 2024; Accepted: 15 January 2025;
Published: 31 January 2025.
Edited by:
Xing Li, Third Affiliated Hospital of Sun Yat-sen University, ChinaReviewed by:
Cheng Yang, Fudan University, ChinaCopyright © 2025 Qiu, Lin, Huang, Yang, Lu and Dai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weihui Lu, d2VpaHVpLmx1QGd6dWNtLmVkdS5jbg==; Zhenhua Dai, RGFpemhlbmh1YUBnenVjbS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.