
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 24 February 2025
Sec. Cancer Immunity and Immunotherapy
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1515718
This article is part of the Research Topic Community Series in Novel Biomarkers for Predicting Response to Cancer Immunotherapy: Volume III View all 7 articles
Interferon-Induced Protein with Tetratricopeptide Repeats 3 (IFIT3) plays a dual role in innate immunity and tumor immunity, functioning as both a viral defense molecule and a regulator of tumor progression. This review explores the mechanisms through which IFIT3 modulates immune responses, including interferon signaling, RIG-I-like receptors, and the NF-κB pathway. IFIT3 facilitates immune evasion and promotes inflammation-mediated tumor growth by regulating immune checkpoints and the tumor microenvironment, its emerging role as a target for cancer immunotherapy opens new avenues for therapeutic strategies. Finally, this paper underscores IFIT3’s potential clinical applications in the modulation of tumor immunity, highlighting the need for further research on IFIT3-targeted therapies.
Cancer remains one of the foremost causes of death globally (1). Despite significant advancements in treatment, the complex biology of tumors and their immune escape mechanisms continue to present substantial challenges in cancer therapy. Studies have shown that tumorigenesis and progression depend on the intrinsic properties of tumor cells and are also profoundly influenced by the host immune system. In particular, there is a tight and complex network of interactions between cancer and innate immunity (2). Innate immunity is the first line of host defense against pathogen invasion (3, 4). Among interferon-inducible proteins, IFIT3 has garnered significant attention in recent years due to its dual role in innate immunity and tumor regulation (5). As a key member of the interferon-inducible protein family, IFIT3 is especially critical in the regulation of viral infections and tumor development. Recent studies have demonstrated that IFIT3 plays a crucial role in antiviral immunity, tumor microenvironment regulation, immune evasion, and inflammatory responses. Given its diverse biological functions, IFIT3 has emerged as a promising target in cancer immunotherapy (6, 7). This paper will explore the molecular mechanisms of IFIT3 across various cancers, as well as its emerging role in tumor immunity, providing a theoretical basis for future research and clinical applications.
Beyond its crucial role in cancer progression, IFIT3 is intricately involved in key immune pathways, making it a critical player in both antiviral defense and tumor-immune dynamics.
IFIT3 belongs to the IFIT family of proteins in the cytoplasm and has been extensively studied for its antiviral properties. The IFIT family consists of four members, IFIT1, IFIT2, IFIT3, and IFIT5, which are clustered on human chromosome 10q23.31 (8). These proteins have no known enzymatic activity, but all of them contain unique structural patterns known as tetratricopeptide repeats (TPRs). TPRs are structural components of IFIT proteins. These TPRs consist of 3 to 16 repeated and modified tandem sequences containing 34 amino acids each. These TPRs are organized into helix-turn-helix configurations that promote their participation in protein-protein interactions leading to several protein complexes that play important roles in various biological processes in the cell (6). All four IFIT proteins have conserved structures in the N-terminal region containing the first three TPR structural domains. However, sequence conservation among IFIT proteins progressively declines towards the C-terminus, leading to increased structural diversity (9). IFIT proteins are involved in a variety of biological processes, such as cell proliferation, migration, virus-induced translation initiation, replication, and double-stranded RNA signaling. The transcription of IFIT genes can be rapidly induced by interferon (IFN) therapy and viral infection (6). The C-terminus of IFIT3 binds to the mitochondrial antiviral signaling complex and connects to NF-κB-binding kinase, which leads to the phosphorylation of IRF3 and triggers the early production of IFN-β in response to intracellular RNA viruses (9).
IFIT3, an integral member of the IFIT family, occupies a pivotal position in the innate immune system. It is integrated into multiple key signaling pathways, including the JAK-STAT, IFN (interferon), and Toll-like receptor (TLR)-mediated recognition pathways, the IFN (interferon) signaling pathway, and the Toll-like receptor (TLR)-mediated recognition pathway, and thus deeply participates in and strengthens the host defense mechanism. Through these pathways, IFIT3 enhances immune sensitivity and facilitates precise pathogen recognition. It participates in host defense mechanisms and plays an important role in pathogen recognition and clearance (5).
For a visual representation of these signaling pathways and IFIT3’s role within them, see (Figure 1). The figure illustrates how IFIT3 interacts with different immune pathways, reinforcing its central role in immune response activation through interferon signaling, innate immune receptor pathways, and inflammatory modulation.

Figure 1. The regulatory mechanism of IFIT3 in innate immune signaling pathways. IFIT3 plays a crucial role in key immune pathways, including IFNAR, RIG-I-like receptors (RLRs), Toll-like receptors (TLRs), and cGAS-STING. IFIT3 regulates antiviral immune responses by interacting with essential signaling molecules, enhancing antigen presentation by dendritic cells, facilitating T-cell activation, and amplifying antiviral cytokine production. Additionally, IFIT3 promotes macrophage polarization and antiviral capacity, ultimately contributing to immune system regulation.
The IFN signaling pathway is a critical component of the innate immune system, playing a pivotal role in protecting against viral infections and regulating immune responses (10). IFN-α/β and IFN-λ initiate the expression of downstream effector genes such as IFIT3 via the JAK-STAT signaling pathway. During viral infection, IFN-α/β binds to its receptor (IFNAR) on the cell surface, activating JAK1 and TYK2 kinases. This triggers the phosphorylation and dimerization of STAT1 and STAT2. The phosphorylated STAT1/STAT2 complex then associates with IRF9, forming the ISGF3 complex, which translocates to the nucleus to drive the expression of interferon-stimulated genes (ISGs), including IFIT3 (11–13). Recent studies suggest that IFIT3 may further modulate the ISGF3 complex by influencing STAT1 phosphorylation dynamics, reinforcing the interferon response. IFIT3 is considered an early responder to viral infections (14).
Moreover, IFIT3 functions not only as a target of interferon induction but also as a regulator of interferon signaling. It has been shown that IFIT3 enhances the sustained expression of type I interferons by interacting with transcription factors, including IRF3 and IRF7, thereby amplifying the antiviral immune response (15). Through this positive feedback loop, IFIT3 enhances the interferon response, strengthening the host’s early antiviral defense mechanisms.
The RIG-I-like receptors (RLRs)—including RIG-I and MDA5—are key elements of the innate immune system responsible for recognizing viral RNA and triggering downstream antiviral signaling pathways (16). IFIT3 enhances the responsiveness of these receptors to viral RNA by interacting directly with RLRs. For example, IFIT3 binding induces structural rearrangements in RIG-I, enhancing viral RNA recognition and expediting the activation of downstream interferon signaling cascades (17). Recent studies suggest that IFIT3 stabilizes MAVS interactions with TBK1, prolonging antiviral signaling and enhancing IFN-β production.
Upon recognizing viral double-stranded RNA (dsRNA), RLRs oligomerize and form active complexes, which subsequently interact with the mitochondrial antiviral signaling protein (MAVS). MAVS functions as a hub, activating TANK-binding kinase 1 (TBK1) and IκB kinase (IKK), which in turn initiate the nuclear translocation of IRF3/7 along with transcription factors such as NF-κB. These factors regulate the expression of type I interferons and pro-inflammatory cytokines, reinforcing the antiviral state of infected and neighboring cells. IFIT3, an ISG, plays a critical role in amplifying this response by interacting with key proteins in the antiviral immune network (18, 19), Additionally, IFIT3 has been implicated in fine-tuning NF-κB activity, potentially modulating the balance between antiviral immunity and inflammatory responses.
Toll-like receptors (TLRs) are essential in pathogen recognition and innate immune activation (20). They detect pathogen-associated molecular patterns (PAMPs), such as viral RNA or bacterial lipopolysaccharides (LPS), and initiate downstream signaling through adaptor proteins like MyD88 or TRIF. This, in turn, activates transcription factors such as NF-κB and IRF3, leading to the induction of inflammatory cytokines and interferons, respectively (21). IFIT3, a key effector molecule in the interferon signaling pathway, not only amplifies IFN-mediated antiviral responses but also modulates TLR signaling by interacting with key adaptor proteins such as MyD88 and TRIF.
When double-stranded RNA (dsRNA) binds to TLR3, it triggers the production of interferons, initiating a cascade that includes the phosphorylation of STAT1, amplifying the expression of ISGs, including IFIT3 (14). Notably, activation of both TLR3 and TLR4 significantly upregulates IFIT3 expression, enhancing the antiviral response (14, 22). This demonstrates IFIT3’s broad role in reinforcing the cellular defense mechanisms against viral infections.
The cGAS-STING pathway plays a vital role in defending against DNA viruses and certain RNA viruses. In this pathway, cyclic GMP-AMP synthase (cGAS) detects viral DNA within the cytoplasm and catalyzes the production of cyclic GMP-AMP (cGAMP), which activates the stimulator of interferon genes (STING). STING, in turn, triggers the phosphorylation of TBK1 and IRF3, leading to the robust expression of type I interferons (23, 24). IFIT3, an important effector in the type I interferon signaling pathway, enhances antiviral immunity by curbing viral replication and transmission.
Research has shown that IFIT3 is significantly elevated in monocytes from systemic lupus erythematosus (SLE) patients, where it is positively correlated with cGAS-STING pathway activity, highlighting its role in amplifying antiviral responses (25).
NF-κB is a family of transcription factors central to regulating inflammation, immune responses, and cell survival. It controls the expression of various inflammatory cytokines (e.g., IL-1, TNF-α, IL-6), chemokines, and immunoregulatory molecules (26, 27). IFIT3 has been shown to enhance immune responses through dual mechanisms. First, it promotes the expression of cytokines mediated by NF-κB, including TNF-α, IL-6, and IL-1β (28). IFIT3 participates in the activation of the NF-κB pathway, which subsequently contributes to STAT1 activation, thereby enhancing immune responses and promoting pro-inflammatory cytokine release (29). However, evidence suggests that IFIT3 is not the sole regulator of this process. A study showed that while IFIT3 knock-down reduces cytokine secretion, IFIT3 knock-out does not completely abolish pro-inflammatory cytokine release, indicating the involvement of additional pathways in this response (30).
In addition, the critical role of IFIT3 in the NF-κB signaling pathway is also notable in its complex interaction mechanism with pattern recognition receptors (PRRs). Specifically, RIG-I-like receptors (RLRs) and Toll-like receptors (TLRs), as key upstream regulatory elements of NF-κB activation, significantly enhance the transduction efficiency of the NF-κB signaling pathway through their interaction with IFIT3 (5). A study in HIV and HCV mono-infected patients revealed that IFIT3 expression was significantly upregulated in CD8 T cells from these patients, accompanied by a simultaneous upregulation of “cytokine-cytokine receptor interactions” and “NF-kappa B signaling pathway”. This was accompanied by a simultaneous upregulation of “cytokine-cytokine receptor interactions” and “NF-kappa B signaling pathway”, a chain reaction that in turn contributed to a sustained state of immune activation (31).
Dendritic cells (DCs), as a class of highly specialized antigen-presenting cells (APCs), occupy an important position in the regulation of adaptive immune responses, and they play an indispensable role in both the maintenance of physiological homeostasis and the modulation of immune responses in pathological states (32, 33). In a recent study, it was shown that type I interferon (IFN-I) is essential for the immune response induced in dendritic cells, and its regulated markers (IRF7, SIGLEC1), as well as induced biomarkers (IFI27, IFIT3, etc.), were validated in vitro and in vivo, which work together to build a robust immune defense system (34). Furthermore, interferon regulatory factor 4 (IRF4), a key transcription factor with hematopoietic cell specificity, significantly influences the maturation and differentiation process of immune cells. Strikingly, IRF4 can induce the expression of a specific subset of interferon-stimulated genes (ISGs) directly in epithelial cells and B-cell lines, which includes the IFIT3 gene with antiviral activity, in the absence of dependence on the type I interferon (IFN-I) signaling pathway (35). Macrophages are immune cells that receive signals from pathogens and activate the innate immune response by reprogramming gene expression (36). Overexpression of a splicing regulator called SRSF7 (formerly known as 9G8) in macrophages has been reported to result in increased IFIT3 abundance in macrophages and enhanced resistance to VSV (vesicular stomatitis virus) viral replication (37). In addition, IFIT3 was found to be included among 36 candidate genes associated with ARDS severity and also involved in M1 polarization of macrophages in one study (38). It can be seen that IFIT3, as a key antiviral protein, exhibits antiviral efficacy through its regulatory role in immune cells. IFIT3 not only inhibits the replication and transmission of viruses; but also participates in the regulation of immune cells, thereby synergistically enhancing the overall antiviral immune response of the body.
A key mechanism by which IFIT3 modulates tumor immunity is through its interaction with immune checkpoint molecules, particularly programmed death ligand 1 (PD-L1). Strong evidence indicates that IFIT3 plays a pivotal role in regulating PD-L1 expression in cancers such as non-small cell lung cancer (NSCLC) and head and neck cancer (HNC). By upregulating PD-L1, IFIT3 facilitates tumor immune evasion by suppressing cytotoxic T-cell function, thereby impairing anti-tumor immune responses (39–41). This regulation is likely mediated through the NF-κB signaling pathway, as studies have demonstrated that NF-κB activation enhances PD-L1 expression (42). Thus, targeting IFIT3 or its downstream effectors may serve as a therapeutic strategy to restore anti-tumor immunity and enhance the efficacy of immune checkpoint inhibitors, making IFIT3 a promising target for cancer immunotherapy.
The tumor microenvironment (TME), which consists of cancer cells, immune cells, stromal cells, and cytokines, plays a central role in both tumor growth and immune evasion (43). IFIT3 acts as a significant regulator of the TME, influencing immune cell infiltration and the inflammatory environment within tumors. Research has shown that IFIT3 promotes the recruitment of tumor environment cells, particularly regulatory T cells (Tregs), which dampen anti-tumor immune responses (44, 45), Moreover, IFIT3 influences the polarization of tumor-associated macrophages (TAMs), promoting an M2 phenotype, which further enhances a tumor environment TME (9). Both are associated with immunosuppression in the tumor microenvironment (46, 47). These actions help foster a tumor-supportive environment by inhibiting dendritic cell maturation and reducing antigen presentation. Thus, targeting IFIT3 within the TME may offer novel strategies to reprogram the immune landscape toward tumor clearance.
In addition to regulating immune cell infiltration and polarization, IFIT3 is also involved in mediating the inflammatory responses within the TME, which play a key role in tumor progression.
Chronic inflammation is a well-recognized factor contributing to cancer development and progression during tumorigenesis (48). Notably, in pancreatic ductal adenocarcinoma (PDAC), high IFIT3 expression is closely linked to elevated inflammatory markers and poor clinical outcomes. IFIT3 specifically promotes pancreatic cancer cell metastasis by inhibiting IFIT2’s pro-apoptotic effects and upregulating vascular endothelial growth factor (VEGF) and interleukin-6 (IL-6) secretion (49, 50). VEGF enhances tumor vascularization, while IL-6 fosters an inflammatory environment conducive to tumor growth. Furthermore, IFIT3 regulates intratumoral inflammatory responses by modulating key inflammatory pathways, including NF-κB and interferon regulatory factors (IRF). In particular, IFIT3 enhances NF-κB activity, which subsequently promotes the secretion of inflammatory mediators such as IL-6, TNF-α, and COX-2, driving tumor cell proliferation, resistance to apoptosis, and angiogenesis (50, 51). In addition, IFIT3 modulates pro-inflammatory macrophages, amplifying the expression of pro-inflammatory cytokines and exacerbating inflammation, which fosters a microenvironment favorable to tumor growth (52). This dual regulatory role of IFIT3 in both inflammation and tumor progression underscores its complex functions in tumor immunomodulation.
Given IFIT3’s central role in both immune evasion and inflammation-mediated tumor progression, it emerges as an attractive candidate for targeted cancer immunotherapy approaches.
Studies on the involvement of IFIT3 in immune escape, tumor microenvironment regulation, and inflammation are gradually revealing its potential as a target for cancer immunotherapy (Figure 2). This figure illustrates how IFIT3 influences tumor progression through NF-κB activation, PD-L1 regulation, inflammatory cytokine secretion, and immune cell modulation, ultimately shaping an immunosuppressive microenvironment. Strategies aimed at inhibiting IFIT3 function or blocking its interaction with key immunoregulatory pathways, such as NF-κB and PD-L1, may enhance anti-tumor immune responses (39, 41). It has been reported in the literature that Radiotherapy, RT, is associated with a strong up-regulation of interferon-responsive genes, including IFIT3, in macrophages and dendritic cells. An in-depth study also found differences in the expression of immune checkpoints in tumors treated with RT versus those not treated with RT (53). In summary, combining IFIT3-targeted therapies with existing immunotherapies (e.g., immune checkpoint inhibitors or radiotherapy in combination with immunotherapy) may provide a synergistic approach to overcoming tumor immune resistance. Future studies should focus on validating these therapeutic strategies and evaluating their efficacy in clinical trials.
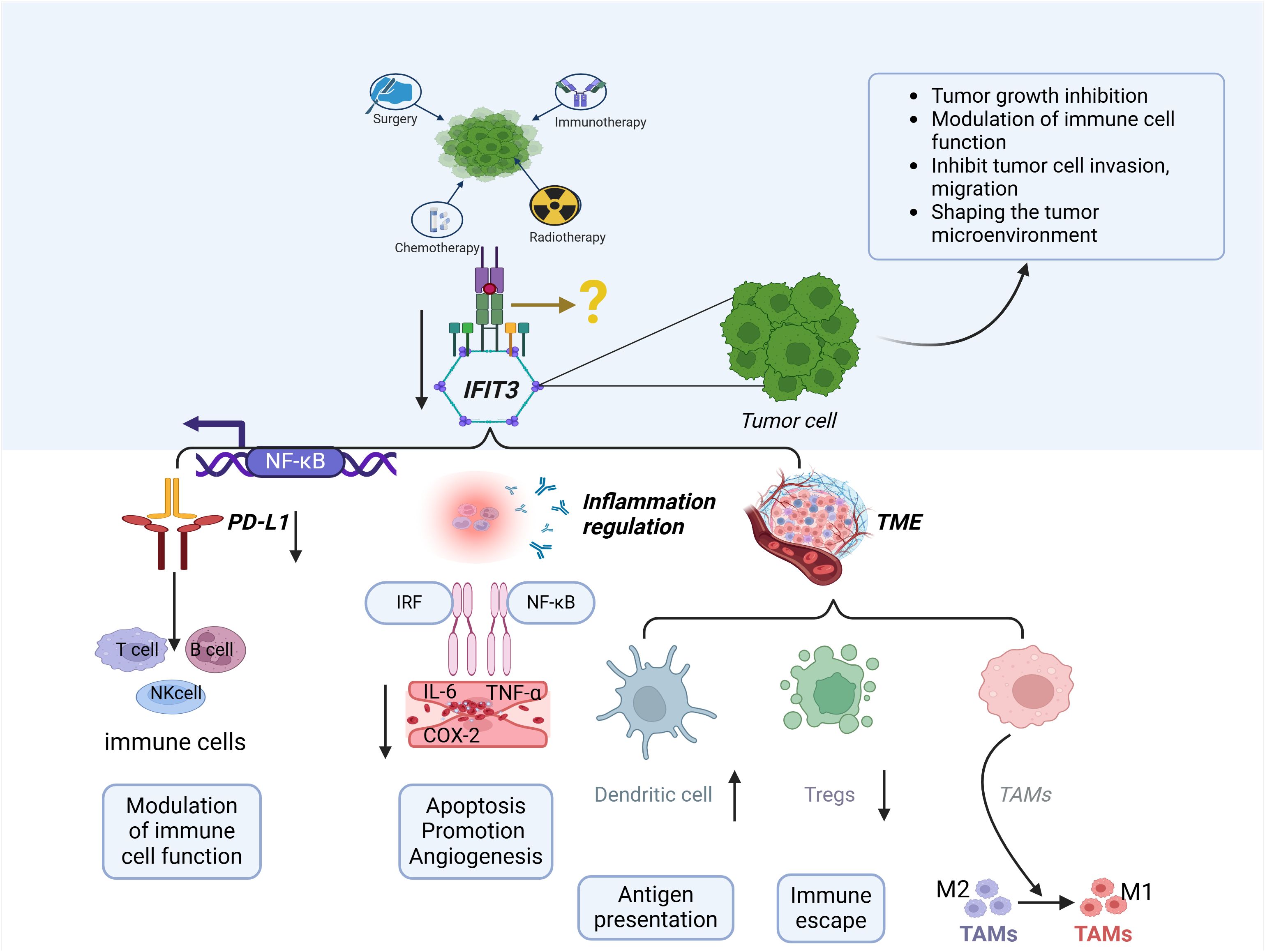
Figure 2. The role of IFIT3 in tumor immunity and the tumor microenvironment (TME). IFIT3 regulates tumor progression and immune evasion through multiple mechanisms. IFIT3 expression is influenced by various cancer treatments, including chemotherapy, radiotherapy, immunotherapy, and personalized therapy. Through NF-κB activation, IFIT3 upregulates PD-L1 expression, suppressing T-cell, B-cell, and NK-cell functions, thereby promoting immune evasion. In the TME, IFIT3 modulates immune cell infiltration, enhances regulatory T cell (Treg) expansion, and skews tumor-associated macrophages (TAMs) toward an M2 phenotype, fostering an immunosuppressive environment. Additionally, IFIT3 promotes inflammation via NF-κB and IRF signaling, increasing pro-inflammatory cytokines (IL-6, TNF-α, and COX-2), leading to enhanced tumor angiogenesis, apoptosis resistance, and immune escape. These findings suggest IFIT3 is a potential target for cancer immunotherapy by reshaping the immune landscape of the TME.
The IFIT3 gene exhibits significant expression variations across a wide range of cancer types, playing a crucial biological role. It has a profound impact on tumor initiation, progression, and patient prognosis by modulating immune response-related signaling pathways, regulating chemokine expression levels, and deeply participating in key cellular processes such as apoptosis and autophagy.
A summary of the tumor-specific mechanisms involving IFIT3 is provided in (Table 1), highlighting its role in different cancer types.
Oral squamous cell carcinoma (OSCC) accounts for more than 90% of all oral cancer cases, with a five-year survival rate of less than 50%, ranking 16th in global cancer mortality (81, 82). Notably, a previous study has reported that the expression levels of IFIT3 are significantly upregulated in OSCC (54). IFIT3 overexpression has been identified as a major contributor to epithelial-mesenchymal transition (EMT) in OSCC, where it promotes tumor invasiveness. Inhibition of this pathway has shown potential therapeutic benefits, making IFIT3 a viable target for future drug development (55). A significant positive correlation between LOXL2 expression and the overexpression of IFIT1 and IFIT3 was observed in human OSCC tissues. This suggests that LOXL2 may regulate the expression of IFIT3, which has important implications for tumor progression. Later in the paragraph, LOXL2’s role in modulating the tumor microenvironment is discussed in more detail. Additional studies revealed that Lysyl oxidase-like 2 (LOXL2) expression in OSCC tissues was significantly correlated with tumor clinical stage, and lymph node metastasis patient overall survival. Human OSCC TW2.6 (TW2.6/LOXL2) cells overexpressing LOXL2 exhibit enhanced migration, invasion, epithelial-mesenchymal transition (EMT), and cancer stem cell (CSC) phenotypes. Notably, in LOXL2-overexpressing cells, LOXL2 increased the levels of interferon-inducible proteins IFIT1 and IFIT3, which are key downstream components involved in the migration, invasion, EMT, and CSC phenotypes of TW2.6 cells (56).
IFIT3 overexpression has been identified as a major contributor to epithelial-mesenchymal transition (EMT) in OSCC, where it promotes tumor invasiveness. Inhibition of this pathway has shown potential therapeutic benefits, making IFIT3 a viable target for future drug development. In summary, IFIT3 overexpression plays a crucial role in OSCC progression.
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal malignant tumors worldwide (83). with recent research revealing the significantly high expression of IFIT3 in aggressive PDAC cells. This abnormally high expression not only strengthens the anti-apoptotic ability of PDAC cells but also significantly increases their resistance to chemotherapeutic drugs, directly correlating with shorter patient survival (57). IFIT3 plays a key role in the regulation of mitochondria-mediated apoptosis during chemotherapy. Knockdown of IFIT3 expression effectively weakened PDAC cells’ resistance to a range of chemotherapeutic agents, including gemcitabine, paclitaxel, and FOLFIRINOX, whereas overexpression of IFIT3 significantly promoted the development of resistance. Immunoprecipitation studies revealed a direct interaction between IFIT3 and mitochondrial voltage-dependent anion channel protein 2 (VDAC2), a key regulator of the mitochondria-associated apoptosis pathway. IFIT3 forms a protective barrier against chemotherapy-induced apoptotic signals in PDAC cells by stabilizing the binding of VDAC2 to O-GlcNAc transferase (49). Additionally, studies showed that N-myc and STAT interactor (NMI) promoted IFIT3 expression and accelerated tumor growth and migration by activating the STAT3-IFIT3 signaling pathway. NMI-mediated upregulation of IFIT3 plays a central role in PDAC cell resistance to chemotherapeutic drugs such as gemcitabine, providing a potential target for novel therapeutic strategies (58).
Hepatocellular carcinoma (HCC) is one of the most prevalent malignant tumors, accounting for approximately 90% of liver cancer cases and representing a leading cause of cancer-related deaths globally (84, 85). Research into the molecular mechanisms underlying HCC has shown that the expression of CXCL11 in cancer-associated fibroblasts (CAFs) is significantly upregulated compared to other molecules, a trend that is also evident in both cirrhotic and HCC tissues when compared to normal liver tissues. Moreover, analysis of non-metastatic and metastatic HCC tissue samples has revealed markedly elevated mRNA levels of IFIT1 and IFIT3 in comparison to paraneoplastic tissues (59).
CircUBAP2, a circular RNA, is highly upregulated in the majority of HCC tissues and is associated with poor patient prognosis. HCC patients exhibiting high levels of circUBAP2 expression tend to have greater vascular invasion and worse differentiation (86). CircUBAP2 has been identified as a key regulatory molecule that modulates the expression of IFIT1 and IFIT3. Acting as a competitive endogenous RNA (ceRNA), it sponges miR-4756, thereby preventing the inhibition of IFIT1 and IFIT3 expression. This regulatory axis is critical in HCC, as circUBAP2 influences both immune responses and tumor progression (59).
Additionally, studies have demonstrated that silencing IFIT1 and IFIT3 significantly downregulates IL-17 and IL-1β expression, impairing the migration and invasiveness of HCC cells (59). Notably, the ubiquitin-binding enzyme UBE2O, which is strongly correlated with HCC prognosis, plays an essential role in the ubiquitination of IFIT3. Findings indicate that HCC cells with high levels of UBE2O expression and low levels of IFIT3 expression exhibit strong resistance to interferon α treatment, whereas those with low UBE2O expression and high IFIT3 expression display heightened sensitivity to interferon α (60).
Colorectal cancer (CRC), the third most prevalent type of cancer globally, accounts for approximately 1.9 million new cases annually, constituting one-tenth of all new cancer diagnoses (87). Recent studies have uncovered the pivotal role of ETS variant transcription factor 7 (ETV7) in CRC pathogenesis. ETV7 expression is significantly upregulated in CRC tissues and cells, promoting abnormal proliferation, migration, and cell cycle acceleration while inhibiting the normal apoptotic processes of CRC cells. Notably, ETV7 expression levels in CRC patients were positively correlated with IFIT3, with ETV7 enhancing IFIT3 transcriptional activity, mRNA levels, and protein expression in CRC cells (61).
Head and neck squamous cell carcinoma (HNSCC), the most prevalent malignant tumor of the upper respiratory and digestive tracts, accounts for the vast majority of head and neck cancer (HNC) cases and ranks as the seventh most common cancer worldwide. It is characterized by high aggressiveness, significant metastasis propensity, and a high recurrence rate (88). Recent bioinformatics analyses, cellular experiments, and animal models have revealed the critical role of IFIT3 in HNSCC. IFIT3 is highly expressed in HNSCC tissues, and its abnormal overexpression is directly correlated with poor prognosis in patients with clinical stage IV or pathological grade 3, as reflected by significantly reduced survival rates. Additional studies have investigated the mechanisms through which IFIT3 promotes malignant progression in HNSCC. IFIT3 specifically targets programmed death ligand 1 (PD-L1) expression by activating the PI3K/AKT signaling pathway, thereby regulating the epithelial-mesenchymal transition (EMT) and cancer stem cell (CSC) activity. These molecular cascade reactions represent a key mechanism through which IFIT3 drives tumor progression and metastasis in HNSCC (39).
Lung cancer has one of the highest incidence and mortality rates globally. In 2023, the American Cancer Society estimated more than 1.8 million new cases and 1.6 million deaths worldwide (89) Non-small cell lung cancer (NSCLC) constitutes the majority of lung cancer cases (85% of patients) (90).
The epidermal growth factor receptor (EGFR) signaling pathway plays a crucial role in lung cancer development, with EGFR mutations and overexpression being key features of NSCLC. This signaling influences several biological processes, including cell proliferation, differentiation, and survival (91). Some studies have shown that the knockdown of IFIT1 or IFIT3 inhibits NSCLC cell proliferation and invasion and promotes apoptosis, suggesting that IFIT3 acts as an oncogene in NSCLC progression. Furthermore, IFIT3 overexpression significantly enhances the phosphorylation of EGFR and AKT, regulating multiple effector molecules in the EGFR pathway, and thus plays a multilevel role in cell proliferation and survival (40). A recent study on lung adenocarcinoma (LUAD) identified cyclic RNA Circ_BBS9 as a tumor suppressor. Overexpression of circ_BBS9 inhibited LUAD cell proliferation and promoted ferroptosis. IFIT3, which directly interacts with circ_BBS9, is involved in immune infiltration and the formation of the immune microenvironment. It may serve as a diagnostic biomarker by regulating ferroptosis and the immune microenvironment through competitive binding to miR-7150 (44).
Interestingly, a study found that Rig-G (an alias of IFIT3) expression was often downregulated in lung cancer, and its low levels were strongly associated with poor prognosis (63). Further exploration revealed that Rig-G overexpression effectively inhibited tumor growth and migration in lung cancer cells and animal models, highlighting its potential as a tumor suppressor capable of significantly slowing lung cancer progression and metastasis. In A549 lung cancer cells, Rig-G overexpression significantly suppressed p53 downstream genes. Upregulation of Rig-G promoted the expression of E-cadherin and p21, enhancing cell adhesion and growth inhibition; while suppressing the expression of vimentin, a key EMT marker. Interestingly, however, the intervention of the p53 inhibitor Pifithrin-α (PFTα) significantly attenuated the inhibitory effect of Rig-G on p53 and EMT pathways in lung cancer cells. Overall, Rig-G exerts its tumor suppressor effects in lung cancer through p53-dependent pathways (63).
Prostate cancer (PrCa) is one of the leading causes of cancer morbidity and mortality in men worldwide (92). It is estimated that 288,300 new cases of prostate cancer (PrCa) will be diagnosed in the U.S. in 2023, accounting for 29% of new cancer cases in men. It is the most common cancer among men in the United States, and the current lifetime risk of prostate cancer in men is 1 in 8 (93). In prostate cancer (PrCa) study, downregulation of the β6 integrin subunit was found to significantly promote IFIT3 expression in PrCa cells and their released small extracellular vesicles (sEVs), suggesting that IFIT3 may be negatively regulated by β6 integrin. Meanwhile, there was a complex interaction between IFIT3 and STAT1, and although both were highly expressed in PrCa cells, IFIT3 was the only factor secreted into sEVs. Further, the reduction of IFIT3 resulted in STAT1 enrichment in sEVs and decreased intracellular STAT1 levels, revealing a critical role of IFIT3 in regulating STAT1 distribution. These findings emphasize the importance of IFIT3 in PrCa progression and intercellular communication (64).
With ongoing research, the role of IFIT3 in cancer has become increasingly prominent. In addition to the known fields, the critical role of IFIT3 in a variety of cancer types, including but not limited to esophageal squamous cell carcinoma (45, 65), myeloma, leukemia (73, 74), breast cancer (66), nasopharyngeal carcinoma (80), bladder carcinoma (69), thyroid carcinoma (72), and melanoma (70), has been further revealed utilizing comprehensive bioinformatic analyses and other means. These studies have shown that IFIT3 can significantly regulate the proliferation rate, migration and invasion potential, and the dynamic balance of immune responses of tumor cells, as well as affect the sensitivity of cancer cells to chemotherapeutic agents, and thus it is considered as an indispensable key regulator in the mechanism of the occurrence and development of these cancers.
As a key immunoregulatory molecule, IFIT3 has demonstrated its important role in tumorigenesis, development, and immune escape, and has become a hotspot of tumor immunity research in recent years. This review provides a comprehensive overview of IFIT3’s multifaceted roles in cancer progression, immune evasion, and drug resistance. By regulating key processes such as cell proliferation, invasion, immune checkpoint modulation, and inflammation, IFIT3 has emerged as a critical player in tumor immunity. Its involvement in pathways such as interferon signaling, RIG-I-like receptors, Toll-like receptors, cGAS-STING, and NF-κB highlights its potential as a target for cancer immunotherapy. Future research should focus on uncovering IFIT3’s interactions with additional immune checkpoints across various cancer types and investigating the efficacy of IFIT3-targeted therapies in clinical settings.
Of interest is the unique role of IFIT3 in the remodeling of the tumor microenvironment, the regulation of inflammatory response, and the modulation of immune checkpoint molecules, suggesting that it has an important potential for clinical application in tumor immunotherapy. Future studies should explore more deeply the regulatory role of IFIT3 in other immune checkpoint molecules, especially the differential expression in different cancer types. Meanwhile, it will be clinically important to evaluate the effect of IFIT3 inhibitors or targeted modulation strategies against tumors in conjunction with the latest advances in immunotherapy. Integrating IFIT3-targeted therapies with established immunotherapies, such as PD-1/PD-L1 inhibitors, holds promise for novel cancer treatment strategies. In addition, the cross-regulation of IFIT3 with signaling pathways such as NF-κB and cGAS-STING should be the focus of future studies to understand its multiple roles in tumors fully.
RW: Conceptualization, Writing – original draft, Methodology, Investigation. HY: Methodology, Supervision, Validation, Resources, Writing – original draft, Writing – review & editing, Formal analysis, Funding acquisition. CL: Methodology, Conceptualization, Investigation, Funding acquisition, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Clinical medical research and clinical new technology promotion projects of Inner Mongolia Autonomous Region Health Commission (YSXH2024KYF065), Central guiding local technology development projects (2024ZY0152), Zhi Yuan Talent Projects of Inner Mongolia Medical University (ZY20241213), Science and Technology Program of the Joint Fund of Scientific Research for the Public Hospitals of Inner Mongolia Academy of Medical Sciences (2023GLLHO136, 2023GLLH0142, 2023GLLH0139), Public hospital reform and high-quality development demonstration project research fund, gastrointestinal tumors (2023SGGZ114), Key Laboratoy of Radiation Physics and Biology of Inner Mongolia Medical University (PIKY2023030). This study was supported by the Talent Project of the Chinese PLA General Hospital.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
2. Maiorino L, Daßler-Plenker J, Sun L, Egeblad M. Innate immunity and cancer pathophysiology. Annu Rev Pathology: Mech Dis. (2022) 17:425–57. doi: 10.1146/annurev-pathmechdis-032221-115501
3. Savan R, Gale M. Innate immunity and interferon in SARS-CoV-2 infection outcome. Immunity. (2023) 56:1443–50. doi: 10.1016/j.immuni.2023.06.018
4. Pradeu T, Thomma BPHJ, Girardin SE, Lemaitre B. The conceptual foundations of innate immunity: Taking stock 30 years later. Immunity. (2024) 57:613–31. doi: 10.1016/j.immuni.2024.03.007
5. Zhang W, Li Y, Xin S, Yang L, Jiang M, Xin Y, et al. The emerging roles of IFIT3 in antiviral innate immunity and cellular biology. J Med Virol. (2023) 95:e28259. doi: 10.1002/jmv.28259
6. Pidugu VK, Pidugu HB, Wu MM, Liu CJ, Lee TC. Emerging functions of human IFIT proteins in cancer. Front Mol Biosci. (2019) 6. doi: 10.3389/fmolb.2019.00148
7. Wu YY, Xing J, Li XF, Yang YL, Shao H, Li J. Roles of interferon induced protein with tetratricopeptide repeats (IFIT) family in autoimmune disease. Autoimmun Rev. (2023) 22:103453. doi: 10.1016/j.autrev.2023.103453
8. Varela M, Diaz-Rosales P, Pereiro P, Forn-Cuní G, Costa MM, Dios S, et al. Interferon-induced genes of the expanded IFIT family show conserved antiviral activities in non-mammalian species. PloS One. (2014) 9:e100015. doi: 10.1371/journal.pone.0100015
9. Tan XF, Chen Q, Hua SH, Yip GW. Roles of interferon induced protein with tetratricopeptide repeats (IFIT) family in cancer. Curr Medicinal Chem. (2021) 28:5034–47. doi: 10.2174/0929867328666210617105209
10. Walter MR. The role of structure in the biology of interferon signaling. Front Immunol. (2020) 11:606489. doi: 10.3389/fimmu.2020.606489
11. Dowling JW, Forero A. Beyond good and evil: molecular mechanisms of type I and III IFN functions. J Immunol. (2022) 208:247–56. doi: 10.4049/jimmunol.2100707
12. Quarleri J, Delpino MV. Type I and III IFN-mediated antiviral actions counteracted by SARS-CoV-2 proteins and host inherited factors. Cytokine Growth Factor Rev. (2021) 58:55–65. doi: 10.1016/j.cytogfr.2021.01.003
13. Husain M. Influenza virus host restriction factors: the ISGs and non-ISGs. Pathogens. (2024) 13:127. doi: 10.3390/pathogens13020127
14. Imaizumi T, Yoshida H, Hayakari R, Xing F, Wang L, Matsumiya T, et al. Interferon-stimulated gene (ISG) 60, as well as ISG56 and ISG54, positively regulates TLR3/IFN-β/STAT1 axis in U373MG human astrocytoma cells. Neurosci Res. (2016) 105:35–41. doi: 10.1016/j.neures.2015.09.002
15. Zhang W, Jiang M, Liao X, Li Y, Xin S, Yang L, et al. IFIT3 inhibits Epstein-Barr virus reactivation via upregulating innate immunity. J Med Virol. (2023) 95:e29237. doi: 10.1002/jmv.v95.11
16. Rehwinkel J, Gack MU. RIG-I-like receptors: their regulation and roles in RNA sensing. Nature Reviews. Immunology. (2020) 20:537–51.
17. Lozhkov AA, Plotnikova MA, Egorova MA, Baranovskaya IL, Elpaeva EA, Klotchenko SA, et al. Simultaneous detection of RIG-1, MDA5, and IFIT-1 expression is a convenient tool for evaluation of the interferon-mediated response. Viruses. (2022) 14:2090. doi: 10.3390/v14102090
18. Zhang L, Chen WQ, Hu YW, Wu XM, Nie P, Chang MX. TBK1-like transcript negatively regulates the production of IFN and IFN-stimulated genes through RLRs-MAVS-TBK1 pathway. Fish Shellfish Immunol. (2016) 54:135–43. doi: 10.1016/j.fsi.2016.04.002
19. Avila-Bonilla RG, Macias S. The molecular language of RNA 5′ ends: guardians of RNA identity and immunity. RNA. (2024) 30:327–36. doi: 10.1261/rna.079942.124
20. Duan T, Du Y, Xing C, Wang HY, Wang RF. Toll-like receptor signaling and its role in cell-mediated immunity. Front Immunol. (2022) 13. doi: 10.3389/fimmu.2022.812774
21. Pereira M, Durso DF, Bryant CE, Kurt-Jones EA, Silverman N, Golenbock DT, et al. The IRAK4 scaffold integrates TLR4-driven TRIF and MYD88 signaling pathways. Cell Rep. (2022) 40:111225. doi: 10.1016/j.celrep.2022.111225
22. Li YX, Liu T, Liang YW, Huang JJ, Huang JS, Liu XG, et al. Integrative analysis of long non-coding RNA and messenger RNA expression in toll-like receptor 4-primed mesenchymal stem cells of ankylosing spondylitis. Ann Trans Med. (2021) 9:1563–3. doi: 10.21037/atm-21-5020
23. Liu N, Pang X, Zhang H, Ji P. The cGAS-STING pathway in bacterial infection and bacterial immunity. Front Immunol. (2022) 12. doi: 10.3389/fimmu.2021.814709
24. Ghosh M, Saha S, Bettke J, Nagar R, Parrales A, Iwakuma T, et al. Mutant p53 suppresses innate immune signaling to promote tumorigenesis. Cancer Cell. (2021) 39:494–508.e5. doi: 10.1016/j.ccell.2021.01.003
25. Wang J, Dai M, Cui Y, Hou G, Deng J, Gao X, et al. Association of abnormal elevations in IFIT3 with overactive cyclic GMP-AMP synthase/stimulator of interferon genes signaling in human systemic lupus erythematosus monocytes. Arthritis Rheumatol (Hoboken N.J.). (2018) 70:2036–45. doi: 10.1002/art.2018.70.issue-12
26. Ilchovska DD, Barrow DM. An Overview of the NF-kB mechanism of pathophysiology in rheumatoid arthritis, investigation of the NF-kB ligand RANKL and related nutritional interventions. Autoimmun Rev. (2021) 20:102741. doi: 10.1016/j.autrev.2020.102741
27. Zinatizadeh MR, Schock B, Chalbatani GM, Zarandi PK, Jalali SA, Miri SR. (NF-kB) signaling in cancer development and immune diseases. Genes Dis. (2021) 8:287–97. doi: 10.1016/j.gendis.2020.06.005
28. Sun J, Zhang Q, Liu X, Shang X. Downregulation of interferon-induced protein with tetratricopeptide repeats 3 relieves the inflammatory response and myocardial fibrosis of mice with myocardial infarction and improves their cardiac function. Exp Anim. (2021) 70:522–31. doi: 10.1538/expanim.21-0060
29. Sun R, Wang YF, Yang X. Knockdown of IFIT3 ameliorates multiple sclerosis via selectively regulating M1 polarization of microglia in an experimental autoimmune encephalomyelitis model. Int Immunopharmacol. (2024) 128:111501. doi: 10.1016/j.intimp.2024.111501
30. Hsu YL, Shi SF, Wu WL, Ho LJ, Lai JH. Protective roles of interferon-induced protein with tetratricopeptide repeats 3 (IFIT3) in dengue virus infection of human lung epithelial cells. PloS One. (2013) 8:e79518. doi: 10.1371/journal.pone.0079518
31. Li SY, Zhang ZN, Jiang Y, Fu Y, Shang H. Transcriptional insights into the CD8+ T cell response in mono-HIV and HCV infection. J Trans Med. (2020) 18:96. doi: 10.1186/s12967-020-02252-9
32. Liu Z, Wang H, Li Z, Dress RJ, Zhu Y, Zhang S, et al. Dendritic cell type 3 arises from Ly6C+ monocyte-dendritic cell progenitors. Immunity. (2023) 56:1761–1777.e6. doi: 10.1016/j.immuni.2023.07.001
33. Møller SH, Wang L, Ho PC. Metabolic programming in dendritic cells tailors immune responses and homeostasis. Cell Mol Immunol. (2022) 19:370–83. doi: 10.1038/s41423-021-00753-1
34. Ye L, Li P, Wang M, Wu F, Han S, Ma L. Profiling of early immune responses to vaccination using THP-1-derived dendritic cells. Int J Mol Sci. (2024) 25:5509. doi: 10.3390/ijms25105509
35. Forero A, Moore PS, Sarkar SN. Role of IRF4 in IFN-stimulated gene induction and maintenance of Kaposi sarcoma-associated herpesvirus latency in primary effusion lymphoma cells. J Immunol (Baltimore Md.: 1950). (2013) 191:1476–85. doi: 10.4049/jimmunol.1202514
36. Rodríguez-Morales P, Franklin RA. Macrophage phenotypes and functions: resolving inflammation and restoring homeostasis. Trends Immunol. (2023) 44:986–98. doi: 10.1016/j.it.2023.10.004
37. Hm S, Mh S, Ak C, Ks A, Mj C, Sl A, et al. Serine/arginine-rich splicing factor 7 promotes the type I interferon response by activating Irf7 transcription. Cell Rep. (2024) 43. doi: 10.1016/j.celrep.2024.113816
38. Zhang S, Chu C, Wu Z, Liu F, Xie J, Yang Y, et al. IFIH1 contributes to M1 macrophage polarization in ARDS. Front Immunol. (2020) 11:580838. doi: 10.3389/fimmu.2020.580838
39. Liu P, Kong X, Yi S, Chen Y, Luo W. IFIT3 accelerates the progression of head and neck squamous cell carcinoma by targeting PD-L1 to activate PI3K/AKT signaling pathway. World J Surg Oncol. (2024) 22:34. doi: 10.1186/s12957-023-03274-5
40. Zan X, Li S, Wei S, Gao L, Zhao L, Yan X, et al. COL8A1 promotes NSCLC progression through IFIT1/IFIT3-mediated EGFR activation. Front Oncol. (2022) 12. doi: 10.3389/fonc.2022.707525
41. Tang N, Yang Y, Xie Y, Yang G, Wang Q, Li C, et al. CD274 (PD-L1) negatively regulates M1 macrophage polarization in ALI/ARDS. Front Immunol. (2024) 15. doi: 10.3389/fimmu.2024.1344805
42. Antonganeli F, Natalini A, Garassino MC, Sica A, Santoni A, Di Rosa F. Regulation of PD-L1 expression by NF-κB in cancer. Front Immunol. (2020) 11. doi: 10.3389/fimmu.2020.584626
43. Peng C, Xu Y, Wu J, Wu D, Zhou L, Xia X. TME-related biomimetic strategies against cancer. Int J Nanomedicine. (2024) 19:109–35. doi: 10.2147/IJN.S441135
44. Peng D, Liang M, Li L, Yang H, Fang D, Chen L, et al. Circ_BBS9 as an early diagnostic biomarker for lung adenocarcinoma: direct interaction with IFIT3 in the modulation of tumor immune microenvironment. Front Immunol. (2024) 15. doi: 10.3389/fimmu.2024.1344954
45. Jia Y, Zhang B, Zhang C, Kwong DL, Chang Z, Li S, et al. Single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in esophageal squamous cell carcinoma. Advanced Sci. (2023) 10:2204565. doi: 10.1002/advs.202204565
46. Shan F, Somasundaram A, Bruno TC, Workman CJ, Vignali DAA. Therapeutic targeting of regulatory T cells in cancer. Trends Cancer. (2022) 8:944–61. doi: 10.1016/j.trecan.2022.06.008
47. Wang Y, Lin YX, Qiao SL, Wang J, Wang H. Progress in tumor-associated macrophages: from bench to bedside. Advanced Biosyst. (2019) 3:e1800232. doi: 10.1002/adbi.201800232
48. Fernandes Q, Inchakalody VP, Bedhiafi T, Mestiri S, Taib N, Uddin S, et al. Chronic inflammation and cancer; the two sides of a coin. Life Sci. (2024) 338:122390. doi: 10.1016/j.lfs.2023.122390
49. Wang Z, Qin J, Zhao J, Li J, Li D, Popp M, et al. Inflammatory IFIT3 renders chemotherapy resistance by regulating post-translational modification of VDAC2 in pancreatic cancer. Theranostics. (2020) 10:7178–92. doi: 10.7150/thno.43093
50. Niess H, Camaj P, Mair R, Renner A, Zhao Y, Jäckel C, et al. Overexpression of IFN-induced protein with tetratricopeptide repeats 3 (IFIT3) in pancreatic cancer: cellular “pseudoinflammation” contributing to an aggressive phenotype. Oncotarget. (2015) 6:3306–18. doi: 10.18632/oncotarget.2494
51. Camaj P, Seeliger H, Ischenko I, Krebs S, Renner A, Jauch KW, et al. Abstract 3901: Sox9-associated overexpression of IFIT3 leads to pancreatic cancer progression by activation of “pseudoinflammatory” pathways. Cancer Res. (2010) 70:3901. doi: 10.1158/1538-7445.AM10-3901
52. Dos Santos CC, Walburg KV, van Veen S, Wilson LG, Trufen CEM, Nascimento IP, et al. Recombinant BCG-LTAK63 vaccine candidate for tuberculosis induces an inflammatory profile in human macrophages. Vaccines. (2022) 10:831. doi: 10.3390/vaccines10060831
53. Minns HE, Padilla O, Wei HJ, Webster-Carrion A, Tazhibi M, McQuillan N, et al. DIPG-45. Radiation induces a robust interferon response in Diffuse Midline Glioma (DMG), improving the potential for combination immunotherapy. Neuro-Oncology. (2022) 24:i28. doi: 10.1093/neuonc/noac079.102
54. Thakore VP, Patel KD, Vora HH, Patel PS, Jain NK. Up-regulation of extracellular-matrix and inflammation related genes in oral squamous cell carcinoma. Arch Oral Biol. (2024) 161:105925. doi: 10.1016/j.archoralbio.2024.105925
55. Pidugu VK, Wu MM, Yen AH, Pidugu HB, Chang KW, Liu CJ, et al. IFIT1 and IFIT3 promote oral squamous cell carcinoma metastasis and contribute to the anti-tumor effect of gefitinib via enhancing p-EGFR recycling. Oncogene. (2019) 38:3232–47. doi: 10.1038/s41388-018-0662-9
56. Lu YJ, Deng YT, Ko HH, Peng HH, Lee HC, Kuo MYP, et al. Lysyl oxidase-like 2 promotes stemness and enhances antitumor effects of gefitinib in head and neck cancer via IFIT1 and IFIT3. Cancer Sci. (2023) 114:3957–71. doi: 10.1111/cas.v114.10
57. Zhao Y, Altendorf-Hofmann A, Pozios I, Camaj P, Däberitz T, Wang X, et al. Elevated interferon-induced protein with tetratricopeptide repeats 3 (IFIT3) is a poor prognostic marker in pancreatic ductal adenocarcinoma. J Cancer Res Clin Oncol. (2017) 143:1061–8. doi: 10.1007/s00432-017-2351-4
58. Hu H, Li B, Chen H, Fan G, Ye Z, Ji S, et al. NMI promotes tumor progression and gemcitabine resistance in pancreatic cancer via STAT3-IFIT3 axis. Mol Carcinogenesis. (2024) 63:195–208. doi: 10.1002/mc.23645
59. Liu G, Sun J, Yang ZF, Zhou C, Zhou PY, Guan RY, et al. Cancer-associated fibroblast-derived CXCL11 modulates hepatocellular carcinoma cell migration and tumor metastasis through the circUBAP2/miR-4756/IFIT1/3 axis. Cell Death Dis. (2021) 12:260. doi: 10.1038/s41419-021-03545-7
60. Li H, Liu Y, Cheng C, Wu Y, Liang SH, Wu L, et al. UBE2O reduces the effectiveness of interferon-α via degradation of IFIT3 in hepatocellular carcinoma. Cell Death Dis. (2023) 14:1–13. doi: 10.1038/s41419-023-06369-9
61. Chai B, Li Y, Guo Y, Zhang Z, Jia K, Chai X, et al. ETV7 promotes colorectal cancer progression through upregulation of IFIT3. Funct Integr Genomics. (2024) 24:8. doi: 10.1007/s10142-023-01282-y
62. Tan H, Yu T, Liu C, Wang Y, Jing F, Ding Z, et al. Identifying tumor antigens and immuno-subtyping in colon adenocarcinoma to facilitate the development of mRNA vaccine. Cancer Med. (2024). doi: 10.1002/cam4.4846
63. Sun J, Wang X, Liu W, Ji P, Shang A, Wu J, et al. Novel evidence for Retinoic Acid-Induced G (Rig-G) as a tumor suppressor by activating p53 signaling pathway in lung cancer. FASEB journal : Off Publ Fed Am Societies Exp Biol. (2020) 34:11900–12. doi: 10.1096/fj.201903220R
64. Naranjo NM, Salem I, Harris MA, Languino LR. IFIT3 (interferon induced protein with tetratricopeptide repeats 3) modulates STAT1 expression in small extracellular vesicles. Biochem J. (2021) 478:3905–21. doi: 10.1042/BCJ20210580
65. Jiang S, Zhang Q, Su Y, Pan L. Network-based differential analysis to identify molecular features of tumorigenesis for esophageal squamous carcinoma. Molecules. (2018) 23:88. doi: 10.3390/molecules23010088
66. Nushtaeva AA, Stepanov GA, Semenov DV, Juravlev ES, Balahonova EA, Gerasimov AV, et al. Characterization of primary normal and Malignant breast cancer cell and their response to chemotherapy and immunostimulatory agents. BMC Cancer. (2018) 18:1–11. doi: 10.1186/s12885-018-4635-8
67. Mares-Quiñones MD, Galán-Vásquez E, Pérez-Rueda E, Pérez-Ishiwara DG, Medel-Flores MO, Gómez-García MDC. Identification of modules and key genes associated with breast cancer subtypes through network analysis. Sci Rep. (2024) 14:12350. doi: 10.1038/s41598-024-61908-4
68. Hr Z, Yb W, Jq G, Xf L. Double-negative T cells with a distinct transcriptomic profile are abundant in the peripheral blood of patients with breast cancer. Breast Cancer Res Treat. (2024). doi: 10.1007/s10549-024-07477-6
69. Yao Z, Zhang H, Zhang X, Zhang Z, Jie J, Xie K, et al. Identification of tumor microenvironment-related signature for predicting prognosis and immunotherapy response in patients with bladder cancer. Front Genet. (2022) 13:923768. doi: 10.3389/fgene.2022.923768
70. Jiang Y, Zhang C, Zhang J, Han D, Shi X. Comprehensive analysis of the prognosis and biological significance for IFIT family in skin cutaneous melanoma. Int Immunopharmacol. (2021) 101:108344. doi: 10.1016/j.intimp.2021.108344
71. Mallardo D, Simeone E, Vanella V, Vitale MG, Palla M, Scarpato L, et al. Concomitant medication of cetirizine in advanced melanoma could enhance anti-PD-1 efficacy by promoting M1 macrophages polarization. J Trans Med. (2022) 20:436. doi: 10.1186/s12967-022-03643-w
72. Zhen J, Song Z, Su W, Zeng QC, Li J, Sun Q. Integrated analysis of RNA-binding proteins in thyroid cancer. PloS One. (2021) 16:e0247836. doi: 10.1371/journal.pone.0247836
73. Zhao YF, Zhang Y, Lu WY, Sun R, Guo RT, Cao XP, et al. The diagnostic/prognostic roles and biological function of the IFIT family members in acute myeloid leukemia. BMC Med Genomics. (2023) 16:296. doi: 10.1186/s12920-023-01735-0
74. He Y, Jiang S, Cui Y, Liang J, Zhong Y, Sun Y, et al. Induction of IFIT1/IFIT3 and inhibition of Bcl-2 orchestrate the treatment of myeloma and leukemia via pyroptosis. Cancer Lett. (2024) 588:216797. doi: 10.1016/j.canlet.2024.216797
75. Li M, Zhang D, Wang L, Yue C, Pang L, Guo Y, et al. Construction and validation of a SASP -related prognostic signature in patients with acute myeloid leukaemia. J Cell Mol Med. (2024) 28:e70017. doi: 10.1111/jcmm.v28.16
76. Wang F, Tian J, Pang L, Wu J, Quan W. Retinoic acid-induced gene G(RIG-G) as a novel monitoring biomarker in leukemia and its clinical applications. Genes. (2021) 12:1035. doi: 10.3390/genes12071035
77. Cheung CHY, Cheng CK, Leung KT, Zhang C, Ho CY, Luo X, et al. C-terminal binding protein 2 is a novel tumor suppressor targeting the MYC-IRF4 axis in multiple myeloma. Blood Adv. (2024) 8:2217–34. doi: 10.1182/bloodadvances.2023010218
78. Kulus M, Kranc W, Sujka-Kordowska P, Mozdziak P, Jankowski M, Konwerska A, et al. The processes of cellular growth, aging, and programmed cell death are involved in lifespan of ovarian granulosa cells during short-term IVC – Study based on animal model. Theriogenology. (2020) 148:76–88. doi: 10.1016/j.theriogenology.2020.02.044
79. Wu J, Jiang L, Wang S, Peng L, Zhang R, Liu Z. TGF β1 promotes the polarization of M2-type macrophages and activates PI3K/mTOR signaling pathway by inhibiting ISG20 to sensitize ovarian cancer to cisplatin. Int Immunopharmacol. (2024) 134:112235. doi: 10.1016/j.intimp.2024.112235
80. Zhong X, Shang J, Zhang R, Zhang X, Yu L, Niu H, et al. Explore the shared molecular mechanism between dermatomyositis and nasopharyngeal cancer by bioinformatic analysis. PloS One. (2024) 19:e0296034. doi: 10.1371/journal.pone.0296034
81. Chamoli A, Gosavi AS, Shirwadkar UP, Wangdale KV, Behera SK, Kurrey NK, et al. Overview of oral cavity squamous cell carcinoma: Risk factors, mechanisms, and diagnostics. Oral Oncol. (2021) 121:105451. doi: 10.1016/j.oraloncology.2021.105451
82. Alkhadar H, Macluskey M, White S, Ellis I. Perineural invasion in oral squamous cell carcinoma: Incidence, prognostic impact and molecular insight. J Oral Pathol Med. (2024). doi: 10.1111/jop.13069
83. Park W, Chawla A, O’Reilly EM. Pancreatic cancer: A review. JAMA. (2021) 326:851–62. doi: 10.1001/jama.2021.13027
84. Piñero F, Dirchwolf M, Pessôa MG. Biomarkers in hepatocellular carcinoma: diagnosis, prognosis and treatment response assessment. Cells. (2020) 9:1370. doi: 10.3390/cells9061370
85. Philips CA, Rajesh S, Nair DC, Ahamed R, Abduljaleel JK, Augustine P. Hepatocellular carcinoma in 2021: an exhaustive update. Cureus. (2021) 13:e19274. doi: 10.7759/cureus.19274
86. Yu MC, Ding GY, Fu PY, Ma P, Zhu XD, Cai JB, et al. CircRNA UBAP2 Serves as a Sponge of miR-1294 to Increase Tumorigenesis in Hepatocellular Carcinoma through Regulating c-Myc Expression. Res Square. (2020). doi: 10.21203/rs.3.rs-49281/v2
87. Klimeck L, Heisser T, Hoffmeister M, Brenner H. Colorectal cancer: A health and economic problem. Best Practice & Research. Clin Gastroenterol. (2023) 66:101839. doi: 10.1016/j.bpg.2023.101839
88. Basnayake BWMTJ, Leo P, Rao S, Vasani S, Kenny L, Haass NK, et al. Head and neck cancer patient-derived tumouroid cultures: opportunities and challenges. Br J Cancer. (2023) 128:1807–18. doi: 10.1038/s41416-023-02167-4
89. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA: A Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
90. Duma N, Santana-Davila R, Molina JR. Non–small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clinic Proc. (2019) 94:1623–40. doi: 10.1016/j.mayocp.2019.01.013
91. Aran V, Omerovic J. Current approaches in NSCLC targeting K-RAS and EGFR. Int J Mol Sci. (2019) 20:5701. doi: 10.3390/ijms20225701
92. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
Keywords: IFIT3, tumor immunity, cancer progression, innate immunity, immunotherapy
Citation: Wu R, Yang H and Liu C (2025) IFIT3: a crucial mediator in innate immunity and tumor progression with therapeutic implications. Front. Immunol. 16:1515718. doi: 10.3389/fimmu.2025.1515718
Received: 23 October 2024; Accepted: 30 January 2025;
Published: 24 February 2025.
Edited by:
Jian Song, University Hospital Münster, GermanyReviewed by:
Ivan Nombela, Spanish National Research Council (CSIC), SpainCopyright © 2025 Wu, Yang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Yang, aGFveWFuZzA1MDIwMUAxNjMuY29t; Chunlei Liu, Y2h1bmxlaWxpdTg3QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.