- 1Third Hospital of Shanxi Medical University, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital, Taiyuan, China
- 2First Clinical Medical College, Shanxi Medical University, Taiyuan, China
- 3Department of Nuclear Medicine, Nanyang First People’s Hospital, Nanyang, Henan, China
- 4Department of biliary and Pancreatic Surgery, First Hospital of Shanxi Medical University, Taiyuan, China
- 5Department of Hepatobiliary Surgery, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Third Hospital of Shanxi Medical University, Tongji Shanxi Hospital, Taiyuan, China
Cholangiocarcinoma is the second most common primary liver cancer, and its global incidence has increased in recent years. Radical surgical resection and systemic chemotherapy have traditionally been the standard treatment options. However, the complexity of cholangiocarcinoma subtypes often presents a challenge for early diagnosis. Additionally, high recurrence rates following radical treatment and resistance to late-stage chemotherapy limit the benefits for patients. Immunotherapy has emerged as an effective strategy for treating various types of cancer, and has shown efficacy when combined with chemotherapy for cholangiocarcinoma. Current immunotherapies targeting cholangiocarcinoma have predominantly focused on T lymphocytes within the tumor microenvironment, and new immunotherapies have yielded unsatisfactory results in clinical trials. Therefore, it is essential to achieve a comprehensive understanding of the unique tumor microenvironment of cholangiocarcinoma and the pivotal role of T lymphocytes within it. In this review, we describe the heterogeneous immune landscape and intercellular communication in cholangiocarcinoma and summarize the specific distribution of T lymphocytes. Finally, we review potential immune checkpoints in cholangiocarcinoma.
1 Introduction
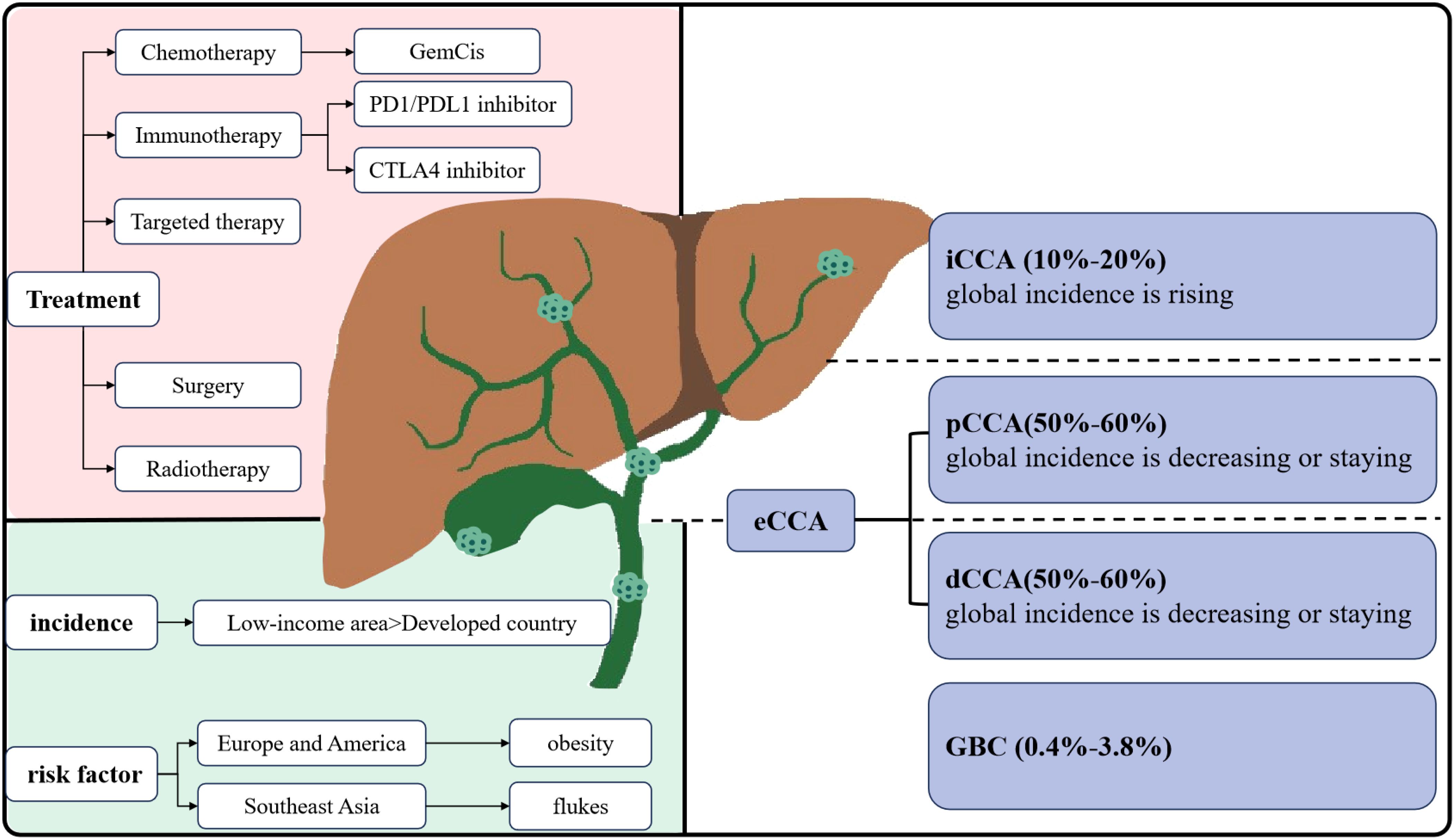
Biliary tract cancers (BTC) include cholangiocarcinoma (CCA) and gallbladder carcinoma (GBC) (1). CCA is further categorized into intrahepatic (iCCA), perihilar (pCCA), and distal (dCCA) cholangiocarcinoma based on its anatomical location within the biliary tree. Among these subtypes, pCCA is the most prevalent, accounting for 50–60% of CCA, followed by dCCA at 20–30%, and iCCA at 10–20% (2) (Figure 1).
CCA is acknowledged as a relatively rare form of cancer, comprising less than 2% of all cancers (3). A epidemiological analysis in the United States indicated that the incidence of CCA, iCCA, and extrahepatic cholangiocarcinoma (eCCA) has increased by 43.8%, 148.8%, and 7.5%, respectively (4). A study conducted in European countries revealed that mortality rates due to iCCA are rising at a higher rate than those due to eCCA (5). Additionally, while increasing mortality from iCCA has been observed globally, mortality from eCCA has either leveled off or decreased (6). CCA accounts for nearly 15% of all primary hepatic carcinomas and 3% of gastrointestinal cancers (5). The incidence and etiology of CCA subtypes vary across different regions. Developed regions exhibit low incidences of CCA, with rates falling below 2 per 100,000 (7). In the Western world, primary sclerosing cholangitis, metabolic syndrome, and nonalcoholic fatty liver disease are recognized risk factors for iCCA (8). In contrast, low-income countries in Southeast Asia report significantly higher incidences of CCA, up to 40 times that of Western countries. Infections with specific flukes have been identified as the primary cause of CCA in endemic regions, such as Thailand and China (9). In conclusion, the complex etiology and regional variations in incidence are closely linked to the highly heterogeneous subtypes of CCA.
Multiple diagnoses and distinct therapies for CCA are contingent on specific genetic aberrations and the primary site of the disease (10). Most CCAs are asymptomatic, with diagnosis is usually made at more advanced stages. Regional lymph node invasion is present in nearly half of patients, and distant metastases affect approximately one-quarter (11). This contributes to poor prognosis, high mortality rates, and limited treatment options, with a 5-year mortality rate of 80% (12). Surgical resection remains the preferred treatment for localized disease. Unfortunately, due to delayed diagnosis and locally advanced situation, curative resection is possible in less than 30% of patients (13). Even patients who undergo potentially curative surgical resection experience a high rate of recurrence and early local or distant metastases. Currently, the combination of gemcitabine and cisplatin (GemCis) is considered the standard treatment for unresectable or metastatic CCA (14), and ongoing clinical trials are investigating various targeted therapies. Nevertheless, dismal survival rates and adverse side effects following chemotherapy significantly affect patients’ quality of life. In summary, overall survival and genuine benefit of surgical resection and adjuvant therapy for CCA remain suboptimal (15, 16).
Immunotherapy has demonstrated significant potential in the treatment of solid tumors by effectively enhancing antitumor immunity through the modulation of immune checkpoints (17). The TOPAZ-1 trial, which evaluated the combination of GemCis and durvalumab in cholangiocarcinoma patients, has shown a notable improvement in overall survival (OS) and progression-free survival (PFS) for those with advanced cholangiocarcinoma (18). However, grade 3-4 adverse events were reported in three-quarters of patients, and the overall health of the patients demonstrated a prolonged trend of deterioration. Additionally, the study also did not reveal the influence of PD-L1 expression, primary tumor site, disease state, or geographic region on the findings. The positive results of the KEYNOTE-966 trial, a Phase III clinical study assessing Pembrolizumab in combination with chemotherapy for advanced biliary tract tumors, provided additional evidence supporting the incorporation of immune checkpoint inhibitors into standard chemotherapy regimens (19). Based on these two trials, the role of immune checkpoint inhibitors (ICIs) in the first-line treatment of advanced CCA is firmly established. However, the efficacy of ICIs in unselected groups of patients with advanced CCA is limited. Therefore, identifying predictive biomarkers for patients and understanding their resistance mechanisms are critical (20).
2 The characteristics of tumor microenvironment in cholangiocarcinoma
The tumor microenvironment (TME) constitutes a complex microecosystem surrounding a developing and progressing tumor. It includes not only the tumor cells themselves but also cancer-associated fibroblasts, vascular endothelial cells, and immunocytes from both innate and adaptive immune systems. Additionally, the TME comprises an extracellular matrix rich in various proteins such as collagen, laminin, and proteoglycan complexes (21, 22). The formation of this highly dynamic, multicellular functional compartment in conjunction with tumor growth is a hallmark feature of numerous epithelial cancers, which are often characterized by significant invasiveness and limited therapeutic options (23).
2.1 The phenotypic conversion and functional changes of tumor-infiltrating cells
In this internal environment, cancer-associated fibroblasts (CAFs) are the primary cells that contribute to tumorigenesis and are likely involved in tumor progression (24). CAFs may induce immune exclusion by overproducing aberrant extracellular matrix (ECM) (25), thereby affecting the immune microenvironment and delivery of chemotherapy drugs (26). As cancer advances, all cells undergo phenotypic conversion and functional changes (27). In innate immune cells, tumor-associated macrophages (TAMs) consistently tend to differentiate into the M2 phenotype, which possesses protumorigenic characteristics. The current conflicting findings in studies evaluating TAMs and patient outcomes in CCA suggest the need for further exploration of the relationship between TAMs and mechanisms underlying CCA progression (28, 29). Tumor-associated neutrophils (TANs) are inflammatory during the early stages of tumor development but adopt an immunosuppressive phenotype as the tumor progresses (30). Natural killer cells (NKs) are recognized for their potent cytotoxic effector functions, their ability to eliminate malignant cells and limit tumor metastasis is constrained within the TME (31). Some studies have indicated that the low cytotoxic activity of NKs is associated with an increased risk of cancer (32). Dendritic cells play a crucial role in maintaining communication between adaptive and innate immune cells, and are essential for orchestrating specific antitumor immune responses (33). The ability of tumor-infiltrating dendritic cells (TIDCs) to efficiently process antigens may be suppressed in the TME, but this capability can be restored by exiting this immunosuppressive milieu (34).
Over the past decade, numerous studies have demonstrated the critical role of adaptive immune cells in the antitumor immune response (35–37), with particular emphasis on T lymphocytes, which will be discussed in greater detail later. Similar to tumor-infiltrating T cells, tumor-infiltrating B cells (TIL-Bs) are adaptive immunocytes with diverse functions. In addition to their pro-tumorigenic effects, B cells exhibit antitumor activity (38). In the TME, CD8+ T lymphocytes, CD4+ T lymphocytes, and NKs are activated to block tumor propagation and inhibit immune escape. Conversely, other immunocytes such as DCs, regulatory T cells (Tregs), and TAMs promote tumor growth, progression, invasion, and angiogenesis, thereby inhibiting the antitumor immune response (39).
2.2 The intercellular communication and disease prognosis in cholangiocarcinoma
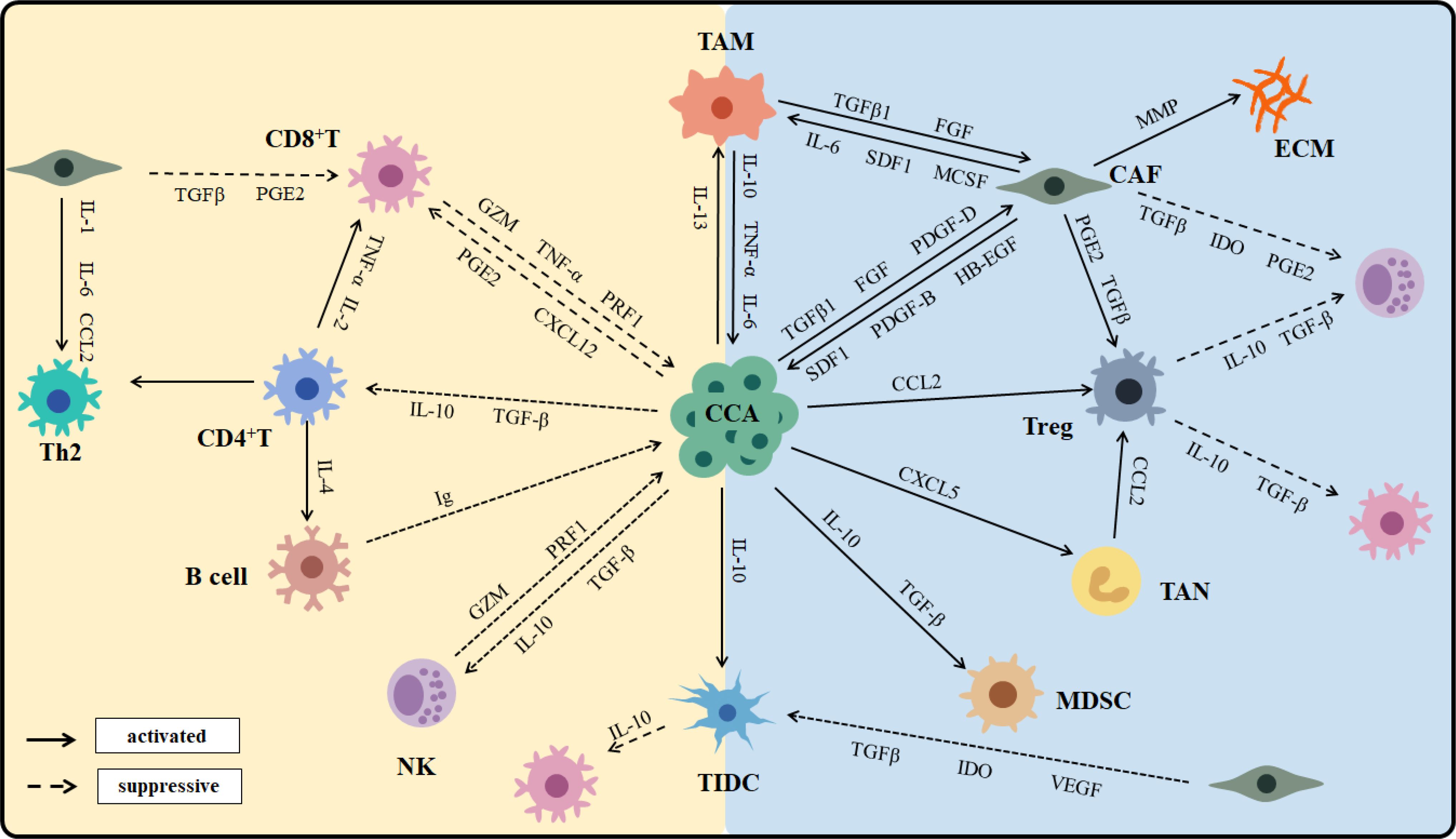
CCA is characterized by a desmoplastic tumor microenvironment that encompasses a complex immunological landscape and a tumor-reactive stroma. The tumor microenvironment of CCA is notably enriched in myeloid cells, particularly TAMs and TANs, along with other immunosuppressive populations (40–42). In contrast, cells that mediate antitumor immunity are markedly diminished (43). The CCA phenotype is shaped not only by epigenetic alterations within the cancer cells but also by extensive crosstalk between malignant cells and their surrounding cellular environment (44). Cell–cell communication in CCA generates and maintains an immunosuppressive environment; tumors typically reprogram the TME to support survival (21) (Figure 2).
CAFs generate an extracellular matrix (ECM) that provides immune barriers, contributing to the highly desmoplastic tumor microenvironment characteristic of CCA (45). CAFs also secrete heparin-binding epidermal growth factor (HB‐EGF), which activates the epidermal growth factor receptor (EGFR) expressed by CCA cells (46). Additionally, CAFs attract DCs and dampen the expression of antigen-presenting molecules, which impair their ability to activate tumor-infiltrating lymphocytes (TILs) and stimulate immunosuppressive functions (47). Among all innate immune cells, TAMs represent the most significant population within the TME (48). CCA cells induce polarization of macrophages toward the M2 phenotype via the STAT3 pathway. TAMs participate in tumor growth and metastasis by releasing TNF-α, TGF‐β, IL6, IL10, and VEGF-A. High infiltration of TAMs is associated with angiogenesis and increased recruitment of Tregs and has been linked to poor prognosis in CCA patients. TANs are predominantly driven by C-X-C motif ligand 5 (CXCL5) and express CCL2 and CCL17, which recruit TAMs and Tregs, ultimately creating an immunosuppressive environment that sustains CCA progression (49). An increased presence of TANs in the TME, along with an elevated preoperative peripheral blood neutrophil-to-lymphocyte ratio, are poor prognostic factors for CCA.
NKs are recruited into the TME by CXCL9, where they utilize death receptor-mediated apoptosis and perforin/granzyme-mediated cytotoxicity to target tumor cells and inhibit primary tumor growth (50). Preclinical and clinical studies have shown that NK cell deficiency or impaired function is associated with an increased incidence of various malignancies. TIL-Bs contribute to limiting tumor growth by secreting immunoglobulins, enhancing T cell responses, and directly destroying cancer cells (51). Although TIL-Bs constitute a minor proportion of TILs in cholangiocarcinoma, a higher density of infiltrating B cells is significantly correlated with longer PFS and OS in CCA patients (52).
2.3 The heterogeneity of the immune microenvironment in cholangiocarcinoma
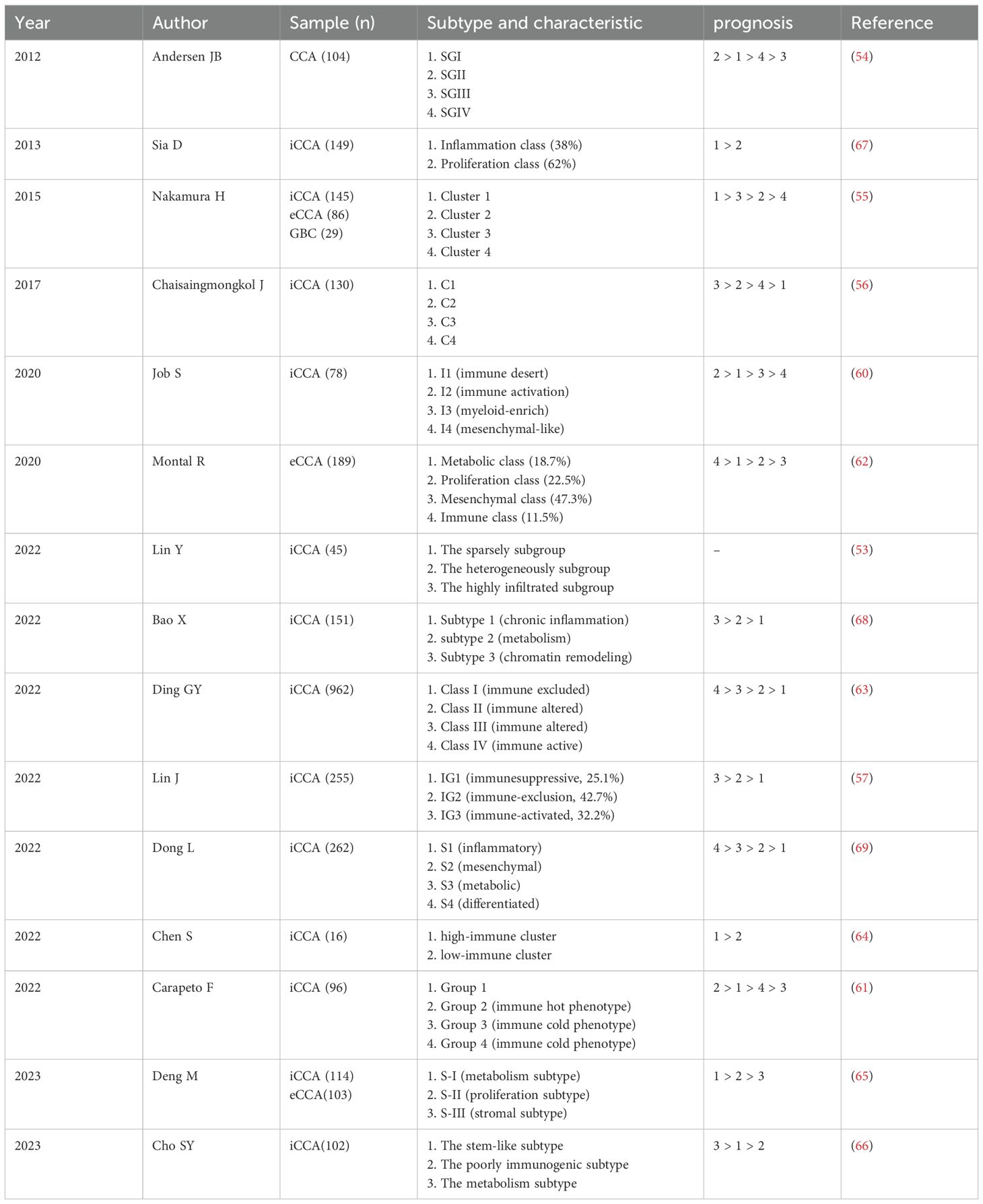
The immune heterogeneity of the TME is a prevalent characteristic of cholangiocarcinoma (53), as demonstrated by variations in the abundance and composition of infiltrating immune cells, along with the diverse activation states observed within individual immune cell subtypes (54–56). A comprehensive understanding of TME heterogeneity is essential for elucidating the molecular and cellular landscape of immune cells in CCA, deciphering the varied responses to anti-tumor therapies among CCA patients, and facilitating the development of personalized immunotherapies tailored to the specific characteristics of the TME (Table 1).
In a study involving 255 human samples of iCCA (57), Lin and colleagues identified three TME-based subtypes: IG1 (immunosuppressive), IG2 (immune-exclusion), and IG3 (immune-activated). Researchers found that IG1 was characterized by excessive infiltration of neutrophils and immature dendritic cells (iDCs), whereas tumor-infiltrating T lymphocytes were predominant in IG3. Furthermore, the immune subgroups exhibited significant differences in OS and recurrence-free survival, with IG1 associated with the worst prognosis and IG3 associated with the best prognosis. Patients exhibiting an enrichment of innate immune cells within the TME may respond positively to myeloid-targeted therapies such as C-X-C motif chemokine receptor 2 (CXCR2) and colony-stimulating factor receptor (CSFR) inhibitors, which aim to deplete or reprogram tumor-associated neutrophils (58, 59). Conversely, patients with a predominance of adaptive immune cells may continue to benefit from ICI treatment. This comprehensive multimodal analysis of the three immune subgroups provides valuable insights into the immune landscape of iCCA, offering potential opportunities for personalized treatment of CCA patients. Job et al. categorized 78 human iCCA samples into four subtypes: I1 (immune desert), I2 (immune activation), I3 (myeloid-enrich), and I4 (mesenchymal-like) (60). Notably, I2 subtype exhibited a high infiltration of immune cells and demonstrated strong activation of inflammatory and immune checkpoint pathways, suggesting the potential effectiveness of immunotherapy targeting this subtype. In contrast, I4 subtype displayed the poorest overall survival, while the other two subtypes exhibited intermediate survival outcomes. This classification highlights the dynamic interaction between tumors and the immune system, aiding the identification of patients who may benefit from effective immunotherapy. Consequently, developing an immune classification method to identify CCA phenotypes characterized by high immune cell infiltration is essential to identify potential candidates for effective immunotherapy. In several other clinical studies of iCCA and eCCA have identified immune microenvironment-based prognostic subtypes, indicating a strong correlation between TILs and favorable patient outcomes (57, 61–66). Comprehensive characterization of these immune subtypes is critical for establishing CCA stratification, which may ultimately facilitate the design of subpopulation-specific immunotherapies. Concurrently, these immune subtypes with more favorable prognoses demonstrate activated inflammatory pathways (67).
Chronic inflammation within the TME may promote tumor progression, with specific immune cells linked to poor prognostic outcomes. A study identified three distinct subtypes: chromatin remodeling, metabolism, and chronic inflammation. Subsequently, Bao and colleagues found that APOE+ C1QB+ macrophage have the ability to reshape the chronic inflammation subtype, which is linked to an unfavorable prognosis in patients with iCCA (68). Another study revealed that the inflammatory subgroup characterized by high expression of inflammatory proteins and dominated by Treg infiltration exhibited a comparatively poor prognosis compared to the metabolic and differentiated subgroups. Furthermore, both inflammatory and stromal responses were found to significantly facilitate the progression of iCCA (69). Although these classifications provide a comprehensive overview of the immune landscape of CCA and suggest potential avenues for personalized treatments, no clinical applications have yet been reported. Therefore, prospective validation of these classifications is essential before they can be integrated into patient care for CCA.
3 Tumor-infiltrating T lymphocytes in cholangiocarcinoma
T lymphocytes originate from progenitors in the bone marrow and undergo differentiation in the thymus. Following this process, they migrate to various immune organs and tissues throughout the body via lymphatic vessels, blood, and tissue fluid circulation, where they play a crucial role in adaptive immunity (70). During the immune response, T cells can be activated and proliferate in response to specific antigens expressed by tumor cells, leading to their differentiation into effector T cells. After the immune response concludes, apoptosis occurs in the majority of effector T cells. Within the tumor microenvironment of CCA, T lymphocytes represent the most common inflammatory cell type (71). CCA can be classified into two groups based on the presence of tumor-infiltrating lymphocytes (TILs) in the TME: lymphocyte-infiltrated tumors and non-lymphocyte-infiltrated tumors (72, 73). Tumors that exhibit immune cell invasion are regarded as immune-responsive tumors, and the immune cell population in lymphocyte-infiltrated tumors can either promote or inhibit tumor progression through their immune responses (74).
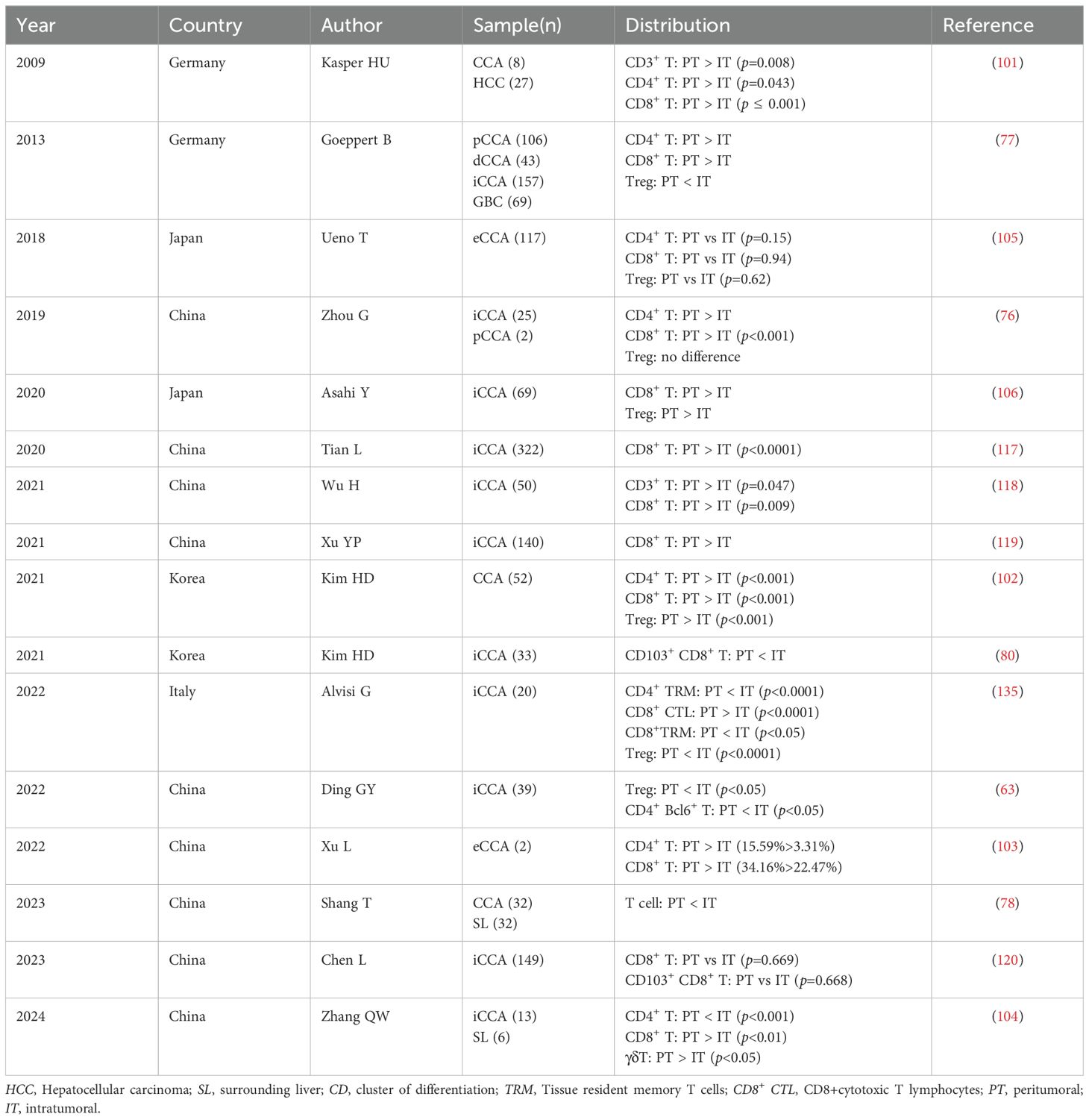
The heterogeneity of tumor-infiltrating T lymphocytes is prevalent in CCA, encompassing both intertumoral and intratumoral heterogeneity (75). Intertumoral heterogeneity is closely associated with the subtype and stage of CCA, characterized by variations in the quantity, proportion, and distribution of T cells among cholangiocarcinoma subtypes (76). Goeppert observed a decreasing trend in the density of CD4+ and CD8+ T cells in the tumor immune microenvironment as bile duct cancer progressed (77). Different subsets of tumor-infiltrating T lymphocytes exert distinct effects on the long-term prognosis of CCA patients (78). Although occasional conflicting findings have been reported, infiltrated CD8+ T and CD4+ T lymphocytes are generally positively correlated with prognosis for CCA patients (71). In contrast, a high number of infiltrated Tregs may be associated with poorer overall survival. The intratumoral heterogeneity of tumor-infiltrating T lymphocytes is primarily related to T cell plasticity and intercellular communication within the TME, manifesting as state transformation and functional changes in T lymphocytes. Numerous studies have demonstrated that the TME of cholangiocarcinoma exhibits distinct T-cell states and potential trajectories for cellular development. Single-cell analyses of CCA have identified multiple subpopulations of TILs (79). A study involving 33 iCCA patients demonstrated that tissue resident memory (TRM)-like CD8+ TILs expressing CD69+ CD103+ showed significantly elevated levels of T cell markers (80). The phenotypic exhaustion of CD4+ T and CD8+ T cells, along with the aberrant activation of Tregs within the TME, has been extensively investigated (81). T cell exhaustion is a distinct state of cell differentiation, accompanied by changes in chromatin conformation and DNA methylation, and associated alterations in gene expression (82). Epigenetic therapy can restore defects in antigen processing and presentation of MHC-1 molecules during tumor immunoediting (83). Research has shown that increased expression of CCL5 by epigenetic treatment could increase T-cell infiltration and promote the memory and effector T-cell phenotypes (84). Additionally, the expression level of CCL5 chemokines is up-regulated, which may further attract CD8+ T cells to infiltrate the TME (85). Zhou and colleagues found that antibodies against TIM-3 or LAG-3 can repair the response of T cells to tumor antigens, and the combination of antibodies shows a superimposed effect (86). Heterogeneous expression patterns are also observed in certain genes both within and between different T cell subtypes. For instance, immune checkpoint molecules such as PD1, CTLA4, LAG3, and TIGIT exhibit differential expression in CD4+ T and CD8+ T cells (61). Jing and colleagues found that the expression frequency of human endogenous retrovirus-H long terminal repeat associated protein 2 (HHLA2) was higher in iCCA than in PDL1. HHLA2 overexpression is associated with a lower density of CD8+ TILs (87). The heterogeneous expression of these genes presents significant challenges in identifying predictors of immunotherapy responses. A comprehensive understanding of the heterogeneity among tumor-infiltrating T lymphocytes will enhance the advancement of immunotherapy.
3.1 CD4+ T lymphocytes in cholangiocarcinoma
CD4+ T lymphocytes play a pivotal role in the regulation of the immune system and the promotion of anti-tumor responses (88). They facilitate B cell activation for antibody production, enhance and sustain CD8+ T cell responses, regulate the immune response to control the strength and persistence of autoimmunity (89). These diverse functions are achieved through the differentiation of native CD4+ T cells upon stimulation by tumor antigens presented by antigen-presenting cells (APCs), leading to their development into effector or memory cells with specialized phenotypes (90, 91). Various subsets of CD4+ T cells, including Th1, Th2, Th17, and follicular T helper cells contribute differently to these processes (92, 93). Additionally, CD4+ T cells possess the ability to directly eliminate tumor cells by releasing cytotoxic particles (94). Recent studies also indicate the appearance of exhausted CD4+ T cells upon persistent antigen stimulation (95).
3.1.1 The CD4+ T lymphocytes interact with other cells in the immune microenvironment
CD4+ T lymphocytes possess the ability to activate monocytes, macrophages, and NKs (96). However, CD4+ T cells in the TME gradually lost the ability to proliferate and recognize tumors (97). Tran and colleagues demonstrated that the progression of CCA was effectively inhibited by the adoptive transfer of T-helper (Th) cells that specifically recognize the tumor-expressed erbb2 mutant protein (98). This finding suggests that Th cell responses may facilitate the regression of late-stage CCA. The release of interleukin-10 (IL-10) by MSDCs and TAMs promotes a Th2 response while disrupting Th1/Th2 balance. Shen and colleagues found that HBV-infected iCCA patients show more Th2 cells within immune landscape (99). Studies have shown that most malignant tumors are skewed towards a Th2 response, but Qiu found that Th1 cytokines such as IFN-γ and IL-2 are mainly expressed in primary liver cancer (100). The accurate identification of T-cell phenotypes in CCA may aid in the development of effective personalized cancer immunotherapies.
3.1.2 The distribution and prognosis of tumor infiltrating CD4+ T cells
The proportion and distribution of tumor-infiltrating CD4+ T lymphocytes in CCA subtypes exhibited significant disparities. Most studies have observed a marked increase in CD4+ T cell infiltration at the periphery of CCA compared to the central region of the tumor (76, 77, 101–103). Conversely, one study indicated that CD4+ T cell infiltration was notably higher at the center of the tumor than at its edge (104). Another investigation found no substantial variance in the distribution of CD4+ T cells surrounding and within the tumor (105). It has been demonstrated that there is a gradient decrease in T cell infiltration from the periphery to the center of the tumor, and that the total number of intraepithelial infiltrating CD4+ T lymphocytes serve as an independent staging and prognostic indicator for CCA (77). Kim et al. found that tumor margins with active infiltration of Foxp3- CD4+ T helper cells exhibited higher expression levels of LAG3 and TIM3, suggesting that the infiltration of Foxp3- CD4+ T helper cells at the tumor margin is a key group associated with clinical outcomes in patients with CCA (102). Ding and colleagues observed a significant increase in follicular helper T (Tfh) cells within the tumor and the elevated levels of Tfh cells potentially indicating a favorable prognosis (63). CCAs demonstrate diverse TILs, with a high density of CD4+ T cells at the tumor margin being associated with improved disease-free survival (DFS) and OS (106). Kasper et al. found that CD4+ T cells predominantly localize at the periphery of the tumor tissue, where they are induced by tumor cells to establish immune tolerance within the TME, thereby adapting to its immunosuppressive milieu (101). The infiltration of CD4+ T cells may signify malignant enhancement (Table 2).
3.2 CD8+ T lymphocytes in cholangiocarcinoma
CD8+ T cells are a subset of lymphocytes developing in the thymus and are committed to detecting antigenic peptides presented by MHC class I molecules expressed by all tumor cell types. DCs cross present the MHC class I molecules to CD8+ T cells to induce the generation of effector CD8+ T cells with cytotoxic capacity, namely CTLs (107). Following CD8+ T cell activation, CTLs migrate to the TME to mount effective responses (108). sustained overexpression of the receptors on CD8+ T cells could promote their dysfunction or exhaustion, leading to impaired efficacy in combating cancer (109). Activated CTLs employ two primary mechanisms to kill tumor cells: granule exocytosis and Fas ligand (FasL)-mediated apoptosis induction. The granule exocytosis pathway is mediated by the release of granzymes (GZM) A and B from CTLs. The released granzymes then enter cancer cells and cleave their intracellular substrates. The second mechanism is that the FasL on CTLs binds to Fas receptors on tumor cells to accelerate apoptosis (110).
3.2.1 The intercellular communication of CD8+ T cells in the tumor microenvironment
CD8+ T lymphocytes play critical roles in interacting with other cells within the TME (111). As cholangiocarcinoma progresses, these interactions become attenuated. CD8+ T lymphocytes positively interact with immunostimulatory cells while negatively interacting with immunosuppressive cells (112). In addition to directly targeting tumor cell elimination, CTLs can also release TNF-α into the TME, inducing apoptosis in cancer cells (113). The production of prostaglandin E2 and adenosine by cholangiocarcinoma cells directly restrains the function and activity of CTLs, further inhibiting CTL-mediated anti-tumor immunity through the overexpression of immune checkpoint ligands such as PD-L1 and B7-H7, or through the downregulation of MHC-I expression on their surface (114). CAFs generate a substantial amount of extracellular matrix, impeding CTL contact with tumor cells, while also secreting the chemokine CXCL12, which inhibits T cell migration toward the tumor (115, 116). Furthermore, the activation of pathways involving TGF-β, B7-H1/PD-1, and Fas/FasL has been observed in the cholangiocarcinoma microenvironment, which hampers the proliferation and activity of CD8+ T lymphocytes (71).
3.2.2 The distribution and prognosis of tumor infiltrating CD8+ T cells
Regardless of iCCA or eCCA, the predominant infiltrating inflammatory cells are CD8+ T lymphocytes (80, 103, 104). Numerous studies have consistently demonstrated that CD8+ T cells are primarily localized in the peritumor area of CCA (76, 77, 101, 102, 106, 117–119). Conversely, it has been reported that there is no significant variance in the distribution of CD8+ T lymphocytes around and within CCA tumors (105, 120). The quantity and positioning of CD8+ T cells at the tumor site are closely associated with clinical diagnosis and prognosis (121). Asahi et al. found a negative correlation between the number of CD8+ T cells and tumor size, suggesting that the count of CD8+ T cells can serve as a prognostic factor for postoperative iCCA patients (106). Immune checkpoints are closely associated with the prognosis of CCA patients. The abundant expression of ICOS, LAG3, OX40, PD-1, and TIM3 at the tumor margin indicates active participation of T cells in the immune response to tumor cells, which can lead to T cell depletion (61). Overexpression of PD-1 by CD8+ T cells result in the depletion of these cells and poor prognosis. Additionally, upregulated expression of the immunosuppressive cytokine IL-10 is observed in activated CD8+ PD-1+ T cells, suggesting that CD8+ PD-1High T cells may acquire the ability to inhibit the immune response to CCA (117) (Table 2).
3.3 Tregs of tumor microenvironment in cholangiocarcinoma
Regulatory T cells, commonly referred to as Tregs, represent a subset of CD4+ T cells within the immune system characterized by low proliferation capacity and typically expressing phenotypes such as CD4+ CD25+ Foxp3+ and CD4+ CD25+ CD127low (122). Tregs can be categorized into thymus-derived Tregs (tTregs) and peripherally-derived Tregs (pTregs) (123, 124). Tregs are capable of secreting cytokines such as IL-4, IL-10 and TGF-β, which contribute to the maintenance of immune homeostasis by regulating immune responses within the organism. The transcription factor Foxp3, specifically expressed by Tregs, plays an essential role in their maturation and function (125). Recent studies have revealed that Tregs not only participate in immunosuppressive regulation but also play a substantial role in tumor immune evasion (126, 127).
3.3.1 The cross-talk between Tregs and other cells within tumor microenvironment
Tregs in cholangiocarcinoma often work with immunosuppressive cells to promote tumor progression and inhibit CD4+ T and CD8+ T lymphocytes activity in TME in a variety of ways (128). Tumor cells, TAMs, and CAFs release CCL22/CCL17 to bind to CCR4 on the surface of Tregs, and recruit a large number of Tregs to move to the TME (129). CTLA-4 is highly expressed on Tregs and competitively binds to CD80 and CD86 with T cells, leading to a reduction in T cell proliferation and cytokine production. In addition, Tregs down-regulated the function of DCs by competitively binding CD80 (130). Treg produces IL10 and TGF-β, attracts more immunosuppressive cells, transforms DCs into regulatory dendritic cells that produce indoleamine 2, 3-dioxygenase, and blocks the immune system’s rejection of cancer (131). In a study, researchers found that MUC1 interacts with the epidermal growth factor receptor (EGFR) and its downstream carcinogenic pathway EGFR/PI3K/Akt is activated, leading to the accumulation of Tregs. This accumulation enhances the malignant phenotype of CCA cells and ultimately promotes their metastasis and growth (132). Additionally, FoxM1 binds to the Foxp3 promoter region and promotes FoxP3 transcription. The overexpression of FoxM1 enhanced the inhibitory effect of Treg cells on CD8+ T cytotoxicity, promoting immune escape in cholangiocarcinoma (133). Notably, a study demonstrated that knockdown of FoxP3 reduces the proliferation and invasiveness of CCA cells (134).
3.3.2 The distribution and prognosis of tumor infiltrating Tregs in cholangiocarcinoma
There is currently no consensus regarding the spatial distribution of Tregs. Zhou and colleagues reported the presence of Tregs both within the tumor and at the tumor margins in iCCA and pCCA. Their study also noted an enhanced expression of CD69, an activation marker for Tregs, suggesting that CCA has immunosuppressive microenvironment characteristics (76). Similarly, Ueno observed a consistent distribution of Tregs in and around eCCA tumors (105). A study involving 52 patients receiving palliative gemcitabine in combination with cisplatin for BTC showed that the density of Tregs was significantly higher at tumor margins compared to interstitial and core areas. However, Treg density did not correlate with PFS and OS (102). Asahi also reported more Treg infiltration at the tumor margins in iCCA (106). In contrast, Alvisi and colleagues provided a comprehensive analysis of various lymphocyte subsets present in iCCA patients, revealing extensive aggregation of Tregs with a highly immunosuppressive phenotype within tumors (135). Ding (63) and Goeppert (77) similarly noted that higher densities of Tregs in CCA were found in the tumor core region. In addition, Goeppert and colleagues found that a gradual decrease in Tregs was associated with tumor aggressiveness and metastasis. Meanwhile, compared to patients with lower Treg counts in tumor tissue, those with higher Treg counts exhibited a better prognosis, suggesting that the immunosuppressive effect of Tregs may not be a major factor in progression of BTC (77) (Table 2).
4 The immune checkpoints in cholangiocarcinoma
Tumor cells interact with surrounding cells to create a microenvironment that supports their growth and development while evading immune surveillance through various mechanisms, thereby achieving immune escape (136). Traditional treatments for CCA, such as chemotherapy and radiation, often lack specificity, leading to indiscriminate attacks on immune cells and subsequent immune system disorders (137). Immunotherapy aims to enhance or restore the ability of autoimmune cells to recognize and eliminate tumor cells, which is more in line with the anti-tumor mode of the body. Current immunotherapy approaches for CCA primarily focus on T lymphocytes. However, the complexity of the CCA immune microenvironment results in inconsistent responses to anti-tumor therapies, presenting both opportunities and challenges for the development of personalized immunotherapy (138).
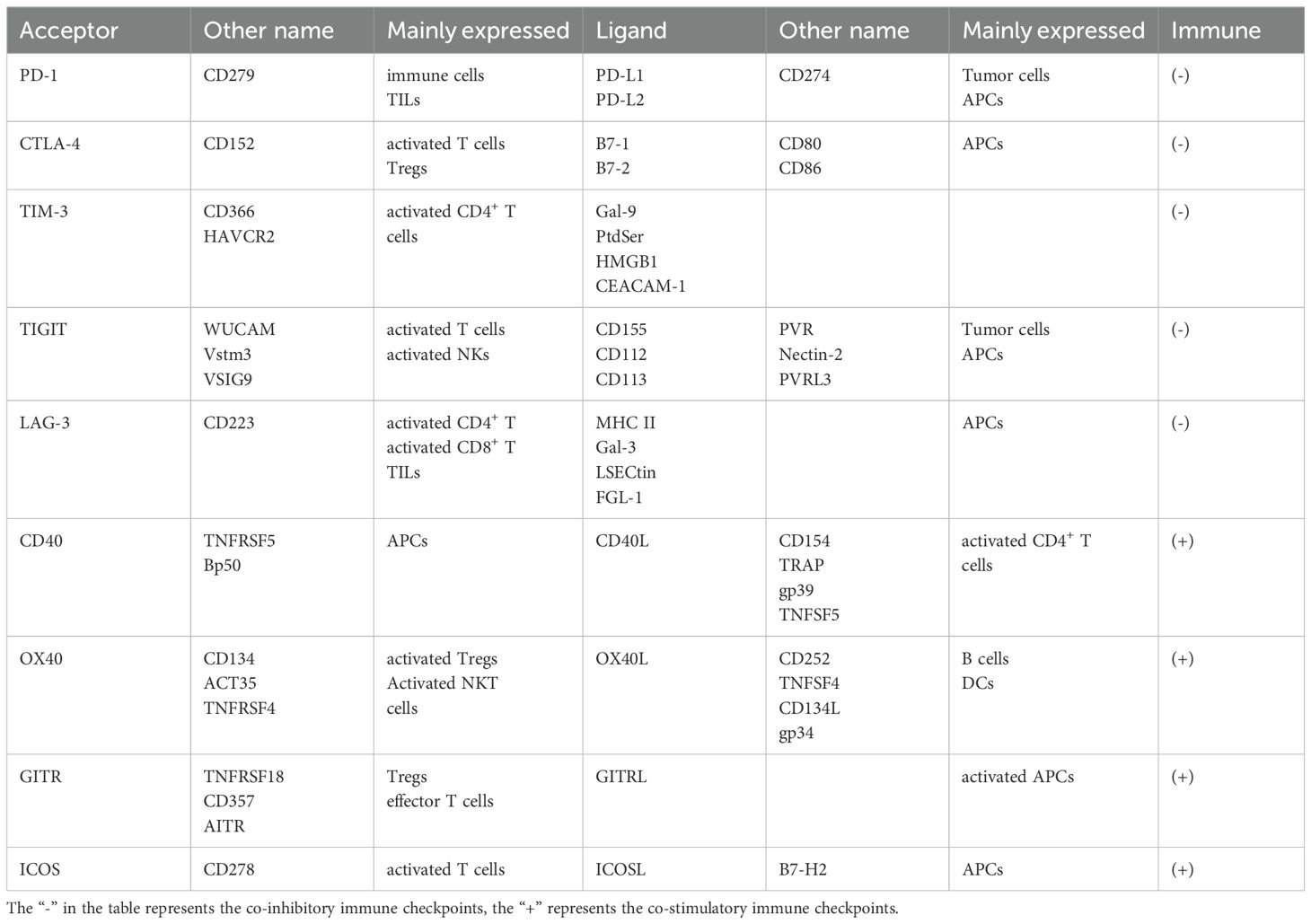
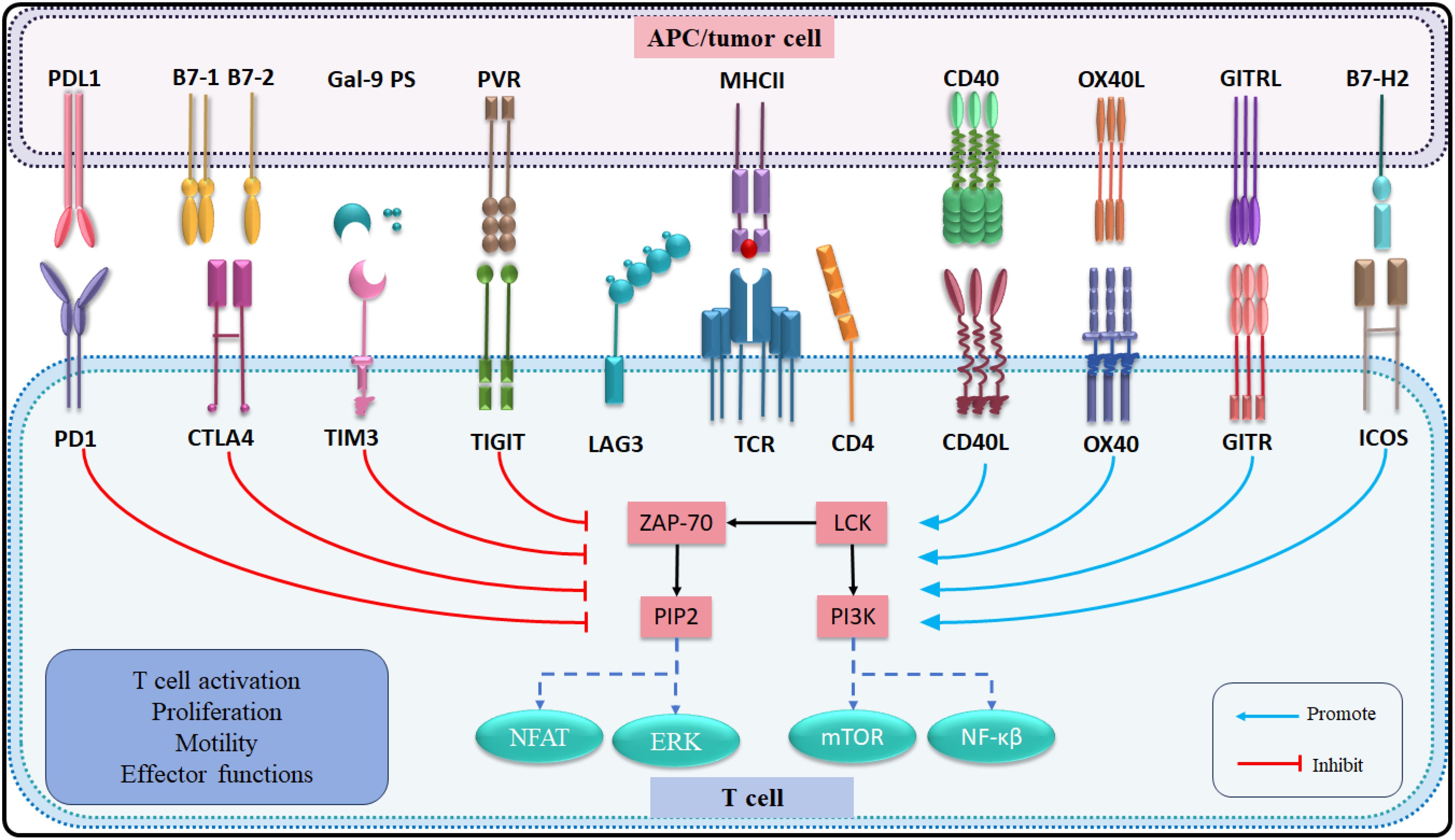
The immune response of T lymphocytes is regulated not only by antigen-specific signals but also by numerous immune checkpoint signaling pathways (139). In recent decades, therapies targeting immune checkpoints have emerged as a promising approach to immunotherapy (140). Co-stimulatory immune checkpoints such as CD40L, OX40, GITR, and ICOS enhance cell activation, whereas co-inhibitory immune checkpoints, including PD-1, CTLA-4, TIM-3, TIGIT, and LAG-3 negatively regulate immune cell activation. The clinical application of blocking co-inhibitory immune checkpoints or activating co-stimulatory immune checkpoints has demonstrated significant potential in the treatment of advanced CCA. The presence of multiple co-expressions on T cells suggests that combination therapy targeting different immune checkpoints may yield more effective therapeutic outcome compared to single immune checkpoint therapies (141) (Table 3; Figure 3).
4.1 PD-1
Programmed cell death protein-1 (PD-1) is a transmembrane protein widely expressed in various activated immune cells. When PD-1 binds to programmed death-ligand 1 (PD-L1) on the surface of tumor cells, the immunoreceptor tyrosine-based inhibitory motif (ITIM) and immunoreceptor tyrosine-based switch motif (ITSM) of PD-1 are phosphorylated (142). Subsequently, Src homology region 2 domain-containing phosphatase (SHP-2) is recruited and activated, inhibiting the phosphorylation of downstream signaling of TCR and CD28. As a result, PD-1 inhibits the activation, proliferation, and cytotoxic secretion of T cells in cancer. The PD-1/PD-L1 pathway plays a crucial role in maintaining immune tolerance within the tumor microenvironment and facilitating the immune escape of tumor cells (143). PD1/PDL1 has emerged as a significant clinical biomarker for prognosticating the effectiveness of immunotherapy in solid tumors (144). At present, antibodies blocking PD-1 or its ligand PD-L1 have been approved to treat various solid and hematologic malignancies (145).
Tian and colleagues found that iCCA patients exhibiting a high proportion of CD8+ PD-1High T cells had worse postoperative survival compared to those with a low proportion of these cells. Furthermore, a high proportion of tumor-infiltrating CD8+ PD-1High T cells was significantly correlated with advanced TNM stage (117). This finding suggests that a high percentage of CD8+ PD-1High T cells may serve as an independent prognostic factor. However, it is important to note that the data for this study were derived from a single hepatobiliary center, and no prospective studies have been conducted to validate these results. Previous studies have demonstrated the expression of PD-1/PD-L1 in CCA and its correlation with ICI treatment response, the predictive value of PD-L1 in CCA remains uncertain. A clinical trial using pembrolizumab for various advanced cancers included 104 patients with BTC. Although results showed that the objective response rate (ORR) was slightly higher in patients with positive PD-L1 expression than in patients lacking PDL1 expression, significant differences in median PFS or OS were not observed (146). This limited response to monotherapy with an immune checkpoint inhibitor in an unselected cohort of advanced BTC underscores the necessity of identifying specific biomarkers and screening patients who may response from treatment. In another clinical trial that included a cohort of 20 patients with advanced solid tumors, 23 BTC patients with positive PD-L1 expression had a 17% ORR, a median PFS of 1.8 months, and a median OS of 6.2 months after receiving pembrolizumab. The highest response rates were found in patients with elevated tumor mutational burden and inflammatory markers (GEP or PD-L1) (147). These results suggest that a combination of biomarkers may help identify patients most likely to respond to ICIs, while also indicating that it may be feasible to enhance the antitumor response through combination therapy.
4.2 CTLA-4
Cytotoxic T lymphocyte antigen 4 (CTLA-4) is a member of immunoglobulin related receptors family and is predominantly found in intracellular vesicles in Tregs or activated conventional T cells (148). This localization is due to the constitutive endocytosis of the plasma membrane and results in 90% of CTLA-4 being intracellular. CTLA-4 competes with CD28 to bind two different ligands of APC, CD80 and CD86, to regulate adaptive immune responses and inhibit T cell overactivation (149). CTLA-4 tends to have an advantage due to CTLA-4 interacts with both ligands with higher affinity and avidity than CD28. Blocking CTLA-4 is capable of generating an immune response to cancer and self-tissue, and targeting the CD28/CTLA-4 pathway with antibodies has shown considerable promise in the treatment of cancer and autoimmune diseases. Experiments have shown that anti-CTLA-4 therapy combined with Treg consumption is more effective in inducing anti-tumor response than blocking CTLA-4 alone (150), reducing tumor Infiltrating Treg may be an important factor in determining immunotherapy response.
A study found that CTLA-4+ lymphocyte density was elevated in iCCA compared with peritumoral hepatic tissues, and patients with a high density of CTLA-4+ tumor-infiltrating lymphocytes (TILsCTLA-4 High) showed a reduced OS compared with patients with TILsCTLA-4 Low (151). Clinically, the density of CTLA-4+ TIL serves as an independent risk factor for evaluating OS in patients with iCCA. Additionally, the expression of CTLA-4 in TILs is critical in maintaining an inhibitory immune microenvironment in iCCAs. Another study showed a different outcome, the elevated density of CD4+ or CD8+ TILs in patients with high CTLA-4 expression on interstitial lymphocytes or tumor cells, the superior outcomes in the group of high CTLA-4 expression level (152). This study underscores the potential prognostic significance of CTLA-4 expression in eCCA. Notably, the impact of CTLA-4 expression on survival appears to vary depending on the tumor location. This study is not without limitations, its retrospective design and relatively small patient cohort necessitate the acquisition of additional datasets to verify the reliability of the findings. In a study involving 20 patients with advanced BTC, participants received tremelimumab in conjunction with radiofrequency ablation. The results indicated that two patients achieved partial response, and five patients achieved stable disease. Furthermore, an analysis of the cell subsets in these patients post-treatment revealed an increase in the number of activated CD8+ T cells in peripheral blood (153). Although this small study yields limited conclusions, a correlation between immune markers and clinical responses appears to exist.
4.3 TIM-3
T cell immunoglobulin and mucin domain 3 (TIM-3) was originally found to be expressed on the surface of Th1 cells, Expression of TIM-3 on CD8+ T cells in the tumor microenvironment is considered a cardinal sign of T cell dysfunction. Recent studies have shown that TIM-3 is also expressed on other immune cells (154). TIM-3 binding to the ligand galectin-9 to mediate Th1 cell the apoptosis. Tregs highly express TIM-3 and secrete IL-10 to inhibit the function of effector T cells in the TME (155, 156). TIM-3 has emerged as an important checkpoint molecule whose expression correlates with to promote T cell exhaustion in chronic viral infection and cancer (157). A number of clinical trials are under way using blocking monoclonal antibodies directed against TIM-3, however the exact mechanisms underlying the anti-tumor activity of these antibodies are not well understood. In CCA, the highly immunogenic iCCA expressed high levels of TIM-3 (141). Furthermore, TIM-3 is upregulated in infiltrating CD8+ T and infiltrating CD4+ T cells, and high expression of TIM-3 in CD8+ T cells is associated with lymph node metastasis of iCCA (158). A study demonstrated that reducing the expression of several inhibitory molecules, including TIM-3, in CAR T cells resulted in robust immunity against CCA, exhibiting long-term efficacy both in vitro and in vivo (159). However, the safety and efficacy of this approach require further validation through preclinical trials.
4.4 TIGIT
T cell immunoreceptor with immunoglobulin and ITIM domain (TIGIT) is a receptor of the Ig superfamily, expressed by activated CD8+ T and CD4+ T cells in humans, which potently inhibits innate and adaptive immunity through multiple mechanisms (160). TIGIT binds to two ligands, CD155 and CD112, that are expressed by tumor cells and antigen presenting cells in the tumor microenvironment. TIGIT indirectly impedes T cell function by binding to CD155 on DCs. Second, TIGIT exhibits direct immune cell-intrinsic inhibitory effects (161). Dmitrij and colleagues found that TIGIT was upregulated in tumor-infiltrating CD8+ T cells. Furthermore, TIGIT can accurately identify exhausted CD8+ T cells at various stages of differentiation (162). Nicole found that TIGIT is highly expressed by Tregs in the peripheral blood mononuclear cells of healthy donors and cancer patients, and is further upregulated in the TME (163). There is now strong evidence that TIGIT regulates both T-cell-mediated and natural killer cell-mediated tumor recognition in vivo and in vitro. Dual PD-1/TIGIT blocking enhances in vitro expansion and function of tumor antigen-specific CD8+ T cells and promotes tumor rejection in mouse tumor models (164, 165). These findings support the development of ongoing clinical trials of PD-1/TIGIT dual blocking to treat cancer patients.
4.5 LAG-3
Lymphocyte activation gene 3 (LAG-3) is an inhibitory receptor that is highly expressed by exhausted T cells (166). While LAG-3 negatively regulates T cell activation and function, its significance in other cell types remains unclear. LAG-3 is widely expressed by many cell types of both lymphocytic and nonlymphocytic lineage and its expression is a hallmark of exhausted CD4+ T and CD8+ T cells in the context of persistent antigenic stimulation by tumors or chronic viral infections (167)LAG-3 has a higher binding affinity with its typical ligand major histocompatibility complex (MHC) class II than CD4. which can competitively bind MHCII with CD4 and inhibit CD4+ T cell function (168). LAG-3 is a promising immunotherapeutic target, with more than 20 LAG-3-targeting therapeutics in clinical trials (169). The immune profiling analysis of peripheral blood reveals an increased abundance of LAG-3hiPD-1hi memory CD4+ T cell subset in relapsed cholangiocarcinoma patients after gemcitabine plus cisplatin therapy, which provided a basis for the study of immune checkpoint inhibitors for CCA (170). In addition, the study demonstrated that bispecific antibodies targeting LAG-3 and PD-L1 elicit an effective anti-tumor response from immune cells in the tumor microenvironment, although these results further support the potential of targeting LAG-3 as a cancer immunotherapy. However, further research is needed to explore the modulation of tumor-infiltrating T lymphocytes and its translational value.
4.6 OX40
Tumor necrosis factor receptor superfamily member 4 (TNFRSF4), also known as OX40, is a type 1 transmembrane glycoprotein predominantly expressed by activated T lymphocytes (171). The cytoplasmic domain of OX40 is involved in downstream signaling pathways by binding to the tumor necrosis factor receptor-associated factor family (TRAF) of intracellular proteins. Its ligand OX40L, which also belongs to the tumor necrosis factor superfamily, is primarily expressed on APCs. The interaction between OX40 and OX40L has immunomodulatory function on T cell survival and proliferation. Studies have shown that increased OX40 expression in CD8+ T cells with IL-2 via STAT5-mediated signaling in the setting of weak TCR stimulation (172). OX40 signaling also reduces the expression of FOXP3 and CTLA-4, leading to a decline in Tregs function. Furthermore, activated T cells exhibit increased expression of CD28 and enhanced expression of OX40 (173). Giampietri and colleagues found that OX40 was significantly upregulated in 36 cholangiocarcinoma samples compared to 9 normal control samples, suggesting that OX40 may play a potential role in cholangiocarcinoma, as both a diagnostic or prognostic marker and a therapeutic target (174). Another study showed that the frequency of OX40+ nTregs (naïve Tregs) and OX40+ eTregs (effector Tregs) in peripheral blood of CCA patients was significantly higher than that of healthy controls, both before and after surgery (175). This suggests that OX40+ nTregs (naive Tregs) and OX40+ eTregs can be used as biomarkers of therapeutic effect and prognosis of CCA.
4.7 CD40L
Cluster of differentiation 40 ligand (CD40L) is a 39-kDa type II transmembrane protein. The expression of CD40L is typically inducible and primarily restricted to cells of the hematopoietic system (176). CD40 can bind to its ligand CD40L, which activates dendritic cells, enhances antigen presentation, and activate T cells by up-regulating the expression of co-stimulatory molecules while down-regulating immunosuppressive molecules (177). CD40/CD40L immune checkpoint leads to activation of both innate and adaptive immune cells via two-way signaling. CD40/CD40L interaction also participates in regulating thrombosis, tissue inflammation, hematopoiesis and tumor cell fate. In vitro experiments have demonstrated that specific blockade of tumor-secreted IL-10 and TGF-β can lead to the up-regulation of CD40, thereby enhancing the cytolytic activity of effector T cells against CCA cells (178). Current evidence suggests that immunotherapy for CCA holds promise through the activation of CD40/CD40L immune checkpoints (179). An animal demonstrated that combination therapy with a CD40 agonist resulted in a superior effector response compared to anti-PD-1 monotherapy for CCA, accompanied by an increased presence of CD4+ T and CD8+ T cells in tumor-bearing mice (180). Immunotherapy for CCA by activating CD40/CD40L immune checkpoints is a promising approach. Second, strong expression of CD40 was observed in tumor samples from half of patients with cholangiocarcinoma. However, the effects observed in this study were not associated with positive expression of CD40 in tumor cells. It is possible that other factors such as the expression of cytokines IFN-γ or TNF-α are also involved in the process of inducing apoptosis of tumor cells.
4.8 GITR
Glucocorticoid-induced tumor necrosis factor receptor-related protein (GITR) is a member of the TNF receptor superfamily, consistently expressed at high levels on the surfaces of Tregs (181). GITR ligand (GITRL) is mainly expressed in dendritic cells, B cells, macrophages, and endothelial cells. APCs not only constitutively express GITR ligand (GITRL) but also enhance its expression under stimulating conditions (182). The signaling mediated by GITR plays a crucial role in regulating immune responses by providing costimulatory signals that enhance responder T cell functions such as activation, differentiation, survival, and memory formation while simultaneously counteracting the immunosuppressive effects of Tregs (183). Blockade of the GITR/GITRL system has proven beneficial in treating autoimmune diseases and in transplantation, whereas stimulation with an agonistic antibody has reversed immunosuppressive responses in chronic infections and tumors (184). Zhou found that activating GITR on T cells within cholangiocarcinoma tumors increased their production of effector molecules and proliferation, suggesting that targeting GITR could be a potential immunotherapy for CCA patients (76). However, the limited cohort of patients in this study did not correlate immunological data with patient survival, indicating that the clinical application of these findings requires further validation.
4.9 ICOS
Inducible Co-Stimulator (ICOS) is predominantly expressed on activated T cells (185). Its ligand ICOSL is expressed on antigen-presenting cells and somatic cells, including tumor cells in the tumor microenvironment. The expression of both ICOS and ICOSL correlates with the release of cytokines that are induced by immune response activation (186). Together, ICOS and ICOSL facilitate a range of activities across various T cell subsets, encompassing T cell activation, effector functions, and the inhibitory activities mediated by Tregs (187). This dual role in both antitumor and protumor activity makes targeting the ICOS/ICOSL pathway attractive for enhancing antitumor immune responses. A study indicated that ICOS expression is elevated in the TME of cholangiocarcinoma, particularly in regions with increased extracellular matrix distribution, which has significant implications for the stratification of immunotherapy (141). Additionally, Carapeto reported that ICOS expression is greater at the tumor margin compared to the tumor center, and low ICOS expression in iCCA is associated with poor OS (61). These studies also demonstrated the co-expression of checkpoint molecules in CCA, suggesting the necessity for combined therapy targeting different immune checkpoints. However, it remains unclear whether immune cells can be recruited into the immune microenvironment when ICIs are used to treat CCA. Furthermore, investigating the distribution of checkpoint molecules may be crucial in determining the optimal treatment strategies for patients receiving combinations of chemotherapy and immunotherapy.
4.10 Combination therapy for immune checkpoints
Given the limitations of ICI monotherapy, there is significant interest in developing combined immunotherapy strategies. A comprehensive analysis of a database comprising 290 iCCA patients, alongside tumor tissue immunohistochemistry, revealed that CTLA-4+ TILs and PD-L1+ TILs can independently predict tumor recurrence and OS in iCCA patients following surgical resection (151). Consequently, therapies targeting both PD-1/PD-L1 and CTLA-4 may offer potential advantages for the treatment of iCCA patients. The premise of the dual ICI is that blocking a single checkpoint may not be enough to activate CTLs. In a Phase 2 study evaluating the combination of nivolumab and ipilimumab in 39 patients with advanced-stage BTC, the trial reported an overall response rate (ORR) of 23% and a disease control rate of 44% (188). These findings highlight the potential superiority of dual ICI combination therapy compared to monotherapy. Notably, these responses were observed exclusively in patients with iCCA or gallbladder cancer. In developing effective immune-specific therapeutics, understanding the immune landscape characteristics of each CCA subtype will be crucial (189). Although the combination of CTLA-4 and PD-1 blockade improved efficacy, it also increased the incidence of adverse events (AE) in CCA. Results from a clinical study indicated that the early treatment outcomes of durvalumab combined with tremelimumab in patients with hepatocellular carcinoma and BTC were relatively disappointing, particularly among BTC patients. In the BTC cohort, the median progression-free survival was 3.1 months, and the overall survival was 5.5 months. Additionally, multiple grade 3/4 treatment-related adverse events were reported (190). Given the heightened risk of AE and the limited efficacy of PD-1/CTLA-4 blockade, there is considerable interest in exploring alternative combination immunotherapies.
Two clinical trials are currently underway targeting CD40 (NCT03329950) and OX40 (NCT03071757) as monotherapy or in combination therapy for advanced cancers including BTC (191), Additionally, investigations are underway into combination strategies involving ICIs alongside other treatments for CCA, in addition to ICI immunotherapy administered alone. These strategies include combined local ablation, radiotherapy, intra-tumor injection, and chemotherapy, all of which aim to enhance tumor antigen exposure and thereby increase the likelihood of an immune response when used in conjunction with ICIs (192, 193). The combination of ICIs with anti-angiogenic therapy has the potential to promote immune responses by suppressing immunosuppressive factors or by increasing immune cytokines within the TME (194). Finally, combinations of various immune checkpoint inhibitors with standard chemotherapy have shown an acceptable safety profile in several early-stage clinical trials (195).
5 Conclusions and future directions
CCA is a malignant tumor characterized by its insidious onset and poor prognosis. At present, the main treatment modalities for CCA have not been effective in reducing patient mortality. Immunotherapy, as a novel treatment approach for solid tumors, offers significant hope for CCA patients. However, the application of immunotherapy in CCA encounters substantial challenges. Firstly, CCA is a heterogeneous disease, and the immune microenvironment varies among patients, which affects the efficacy of immunotherapy. Secondly, most CCA patients present with immunosuppressive microenvironments, resulting in low response rates to immunotherapy. Lastly, treatment with a single ICI has shown limited effectiveness, and patients are often prone to developing resistance. These challenges underscore the need for a deep understanding of the immune landscape of CCA, a comprehensive assessment of patient immune status, and the development of personalized combination immunotherapy regimen to address the therapeutic difficulties posed by the immune diversity of CCA. Current immunotherapy strategies primarily target T lymphocytes within the TME, with a particular emphasis on CD8+ T cells. Tumor-infiltrating T cells display various phenotypes and functional states, and the heterogeneity among these T lymphocytes is linked to the malignant progression of CCA as well as the effectiveness of immunotherapy. To achieve more effective and precise treatments, future research should utilize single-cell and multi-omics to explore the complex mechanisms of T-cell interactions with CCA. This could facilitate the development of novel immunomodulators and herald a new era in CCA therapy.
Combination therapy focusing on ICIs has emerged as the first-line treatment for CCA. Dual ICI therapy, which targets different immune checkpoints, has also demonstrated the anticipated synergistic therapeutic effect. However, it is important to note that in clinical practice, most patients exhibit resistance to ICI combination treatment, leading to poor overall prognosis and relapse-free survival rates. Consequently, there is an urgent need to develop a stratified treatment model for CCA patients and to identify biomarkers that can facilitate more accurate and reliable immunotherapy. Additionally, it is essential to evaluate the safety and adverse reactions associated with ICI combination therapy. This necessitates further high-quality, prospective, and randomized controlled trials to ascertain the safety and therapeutic efficacy of various immunization combination regimens. From a clinical perspective, it is vital to consider both standard treatment regimens and the optimal combinations with immunotherapy. On the immunotherapeutic front, ongoing clinical trials are investigating novel immune checkpoint therapies. The study of tumor-infiltrating T cells in CCA has thus far shown promising potential for advancing immunotherapy strategies. With these emerging therapeutic options, patient prognosis in CCA is anticipated to improve.
Author contributions
YD: Conceptualization, Investigation, Supervision, Visualization, Writing – original draft, Writing – review & editing. CD: Conceptualization, Investigation, Supervision, Visualization, Writing – original draft, Writing – review & editing. ZW: Conceptualization, Investigation, Visualization, Writing – review & editing. YZ: Conceptualization, Investigation, Writing – review & editing. YW: Conceptualization, Investigation, Writing – review & editing. YH: Investigation, Writing – review & editing. PC: Investigation, Writing – review & editing. CL: Project administration, Supervision, Writing – review & editing. GL: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by several funding sources: Shanxi Scholarship Council of China (Grant No. 2021-165). Shanxi Province Basic Research Program (Free Exploration) Surface Project (Grant No: 202303021221189). Science and research fund of Shanxi Health Commission (Grant No. 2019059, 2022042, 2022043). Shanxi Province “136 Revitalization Medical Project Construction Funds”. Shanxi Province Science Foundation for Distinguished Young Scholar (Grant No. 201901D211547). National Natural Science Foundation of China for Young Scholars (Grant No. 81201810). The doctor project of Shanxi Cancer Hospital, China (2017A06). Natural Science Foundation of Guangdong Province, China (2015A030313057). Research and Innovation Team Project for Scientific Breakthroughs at Shanxi Bethune Hospital (2024ZHANCHI07). The Science and Education Cultivation Fund of the National Cancer and Regional Medical Center of Shanxi Provincial Cancer Hospital (QH2023027).
Acknowledgments
We express our gratitude to the scientific and clinical professionals worldwide who have dedicated their careers to the investigation of cholangiocarcinoma. Given the numerous recent developments and achievements in cholangiocarcinoma research, we regret that we are not able to encompass all these studies into this review. Additionally, we acknowledge the administrative and technical support provided by Shanxi Bethune Hospital.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhao Y, Yang M, Feng J, Wang X, Liu Y. Advances in immunotherapy for biliary tract cancers. Chin Med J. (2024) 137:524–32. doi: 10.1097/CM9.0000000000002759
2. Kidanemariam S, Gu J, Yoon JH, Challapalli JV, Fruh V, Sax AJ. Cholangiocarcinoma: epidemiology and imaging-based review. Rhode Island Med J. (2024) 107:43–8.
3. Li N, Wang B. Suppressive effects of umbilical cord mesenchymal stem cell-derived exosomal miR-15a-5p on the progression of cholangiocarcinoma by inhibiting CHEK1 expression. Cell Death Discov. (2022) 8:205. doi: 10.1038/s41420-022-00932-7
4. Javle M, Lee S, Azad NS, Borad MJ, Kate Kelley R, Sivaraman S, et al. Temporal changes in cholangiocarcinoma incidence and mortality in the United States from 2001 to 2017. Oncol. (2022) 27:874–83. doi: 10.1093/oncolo/oyac150
5. Vithayathil M, Khan SA. Current epidemiology of cholangiocarcinoma in Western countries. J Hepatol. (2022) 77:1690–8. doi: 10.1016/j.jhep.2022.07.022
6. Bertuccio P, Malvezzi M, Carioli G, Hashim D, Boffetta P, El-Serag HB, et al. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol. (2019) 71:104–14. doi: 10.1016/j.jhep.2019.03.013
7. Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. (2020) 17:557–88. doi: 10.1038/s41575-020-0310-z
8. Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: Epidemiology and risk factors. Liver International: Off J Int Assoc Study Liver. (2019) 39 Suppl 1:19–31. doi: 10.1111/liv.14095
9. Qian MB, Keiser J, Utzinger J, Zhou XN. Clonorchiasis and opisthorchiasis: epidemiology, transmission, clinical features, morbidity, diagnosis, treatment, and control. Clin Microbiol Rev. (2024) 37:e0000923. doi: 10.1128/cmr.00009-23
10. Brown ZJ, Patwardhan S, Bean J, Pawlik TM. Molecular diagnostics and biomarkers in cholangiocarcinoma. Surg Oncol. (2022) 44:101851. doi: 10.1016/j.suronc.2022.101851
11. Izquierdo-Sanchez L, Lamarca A, La Casta A, Buettner S, Utpatel K, Klümpen HJ, et al. Cholangiocarcinoma landscape in Europe: Diagnostic, prognostic and therapeutic insights from the ENSCCA Registry. J Hepatol. (2022) 76:1109–21. doi: 10.1016/j.jhep.2021.12.010
12. Sato K, Glaser S, Alvaro D, Meng F, Francis H, Alpini G. Cholangiocarcinoma: novel therapeutic targets. Expert Opin Ther Targets. (2020) 24:345–57. doi: 10.1080/14728222.2020.1733528
13. Ohaegbulam KC, Koethe Y, Fung A, Mayo SC, Grossberg AJ, Chen EY, et al. The multidisciplinary management of cholangiocarcinoma. Cancer. (2023) 129:184–214. doi: 10.1002/cncr.v129.2
14. Shroff RT, Javle MM, Xiao L, Kaseb AO, Varadhachary GR, Wolff RA, et al. Gemcitabine, cisplatin, and nab-paclitaxel for the treatment of advanced biliary tract cancers: A phase 2 clinical trial. JAMA Oncol. (2019) 5:824–30. doi: 10.1001/jamaoncol.2019.0270
15. Alabraba E, Joshi H, Bird N, Griffin R, Sturgess R, Stern N, et al. Increased multimodality treatment options has improved survival for Hepatocellular carcinoma but poor survival for biliary tract cancers remains unchanged. Eur J Surg Oncol. (2019) 45:1660–7. doi: 10.1016/j.ejso.2019.04.002
16. Cambridge WA, Fairfield C, Powell JJ, Harrison EM, Soreide K, Wigmore SJ, et al. Meta-analysis and meta-regression of survival after liver transplantation for unresectable perihilar cholangiocarcinoma. Ann Surg. (2021) 273:240–50. doi: 10.1097/SLA.0000000000003801
17. Kankeu Fonkoua LA, Serrano Uson Junior PL, Mody K, Mahipal A, Borad MJ, Roberts LR. Novel and emerging targets for cholangiocarcinoma progression: therapeutic implications. Expert Opin Ther Targets. (2022) 26:79–92. doi: 10.1080/14728222.2022.2029412
18. Oh DY, Ruth He A, Qin S, Chen LT, Okusaka T, Vogel A, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. (2022) 1:EVIDoa2200015. doi: 10.1056/EVIDoa2200015
19. Kelley RK, Ueno M, Yoo C, Finn RS, Furuse J, Ren Z, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (London England). (2023) 401:1853–65. doi: 10.1016/S0140-6736(23)00727-4
20. Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. (2019) 19:133–50. doi: 10.1038/s41568-019-0116-x
21. Affo S, Yu LX, Schwabe RF. The role of cancer-associated fibroblasts and fibrosis in liver cancer. Annu Rev Pathol. (2017) 12:153–86. doi: 10.1146/annurev-pathol-052016-100322
22. Desbois M, Wang Y. Cancer-associated fibroblasts: Key players in shaping the tumor immune microenvironment. Immunol Rev. (2021) 302:241–58. doi: 10.1111/imr.v302.1
23. Xiao Y, Yu D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther. (2021) 221:107753. doi: 10.1016/j.pharmthera.2020.107753
24. Gentilini A, Pastore M, Marra F, Raggi C. The role of stroma in cholangiocarcinoma: the intriguing interplay between fibroblastic component, immune cell subsets and tumor epithelium. Int J Mol Sci. (2018) 19(10):2885. doi: 10.3390/ijms19102885
25. Kay EJ, Paterson K, Riera-Domingo C, Sumpton D, Dabritz JHM, Tardito S, et al. Cancer-associated fibroblasts require proline synthesis by PYCR1 for the deposition of pro-tumorigenic extracellular matrix. Nat Metab. (2022) 4:693–710. doi: 10.1038/s42255-022-00582-0
26. Chakravarthy A, Khan L, Bensler NP, Bose P, De Carvalho DD. TGF-beta-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nat Commun. (2018) 9:4692. doi: 10.1038/s41467-018-06654-8
27. Zhang A, Miao K, Sun H, Deng CX. Tumor heterogeneity reshapes the tumor microenvironment to influence drug resistance. Int J Biol Sci. (2022) 18:3019–33. doi: 10.7150/ijbs.72534
28. Atanasov G, Dietel C, Feldbrügge L, Benzing C, Krenzien F, Brandl A, et al. Tumor necrosis and infiltrating macrophages predict survival after curative resection for cholangiocarcinoma. Oncoimmunology. (2017) 6:e1331806. doi: 10.1080/2162402X.2017.1331806
29. Atanasov G, Hau HM, Dietel C, Benzing C, Krenzien F, Brandl A, et al. Prognostic significance of macrophage invasion in hilar cholangiocarcinoma. BMC Cancer. (2015) 15:790. doi: 10.1186/s12885-015-1795-7
30. Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe? Carcinogenesis. (2012) 33:949–55. doi: 10.1093/carcin/bgs123
31. Glasner A, Levi A, Enk J, Isaacson B, Viukov S, Orlanski S, et al. NKp46 receptor-mediated interferon-gamma production by natural killer cells increases fibronectin 1 to alter tumor architecture and control metastasis. Immunity. (2018) 48:396–8. doi: 10.1016/j.immuni.2018.01.010
32. Elsayed I, Li L, Sheahan K, Moran B, Bakheit S, Wang X. Adenoma to carcinoma: A portrait of molecular and immunological profiles of colorectal sporadic tumors. Int Immunopharmacol. (2021) 100:108168. doi: 10.1016/j.intimp.2021.108168
33. Zagorulya M, Spranger S. Once upon a prime: DCs shape cancer immunity. Trends Cancer. (2023) 9:172–84. doi: 10.1016/j.trecan.2022.10.006
34. Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. (2009) 16:183–94. doi: 10.1016/j.ccr.2009.06.017
35. de Miguel M, Calvo E. Clinical challenges of immune checkpoint inhibitors. Cancer Cell. (2020) 38:326–33. doi: 10.1016/j.ccell.2020.07.004
36. Yan Y, Zhang L, Zuo Y, Qian H, Liu C. Immune checkpoint blockade in cancer immunotherapy: mechanisms, clinical outcomes, and safety profiles of PD-1/PD-L1 inhibitors. Arch Immunol Ther Exp (Warsz). (2020) 68:36. doi: 10.1007/s00005-020-00601-6
37. Ye W, Olsson-Brown A, Watson RA, Cheung VTF, Morgan RD, Nassiri I, et al. Checkpoint-blocker-induced autoimmunity is associated with favourable outcome in metastatic melanoma and distinct T-cell expression profiles. Br J Cancer. (2021) 124:1661–9. doi: 10.1038/s41416-021-01310-3
38. Wouters MCA, Nelson BH. Prognostic significance of tumor-infiltrating B cells and plasma cells in human cancer. Clin Cancer Res. (2018) 24:6125–35. doi: 10.1158/1078-0432.CCR-18-1481
39. Pansy K, Uhl B, Krstic J, Szmyra M, Fechter K, Santiso A, et al. Immune regulatory processes of the tumor microenvironment under Malignant conditions. Int J Mol Sci. (2021) 22(24):13311. doi: 10.3390/ijms222413311
40. Ma C, Zhang Q, Greten TF. MDSCs in liver cancer: A critical tumor-promoting player and a potential therapeutic target. Cell Immunol. (2021) 361:104295. doi: 10.1016/j.cellimm.2021.104295
41. Zimmer CL, Filipovic I, Cornillet M, O’Rourke CJ, Berglin L, Jansson H, et al. Mucosal-associated invariant T-cell tumor infiltration predicts long-term survival in cholangiocarcinoma. Hepatology. (2022) 75:1154–68. doi: 10.1002/hep.32222
42. Kitano Y, Okabe H, Yamashita YI, Nakagawa S, Saito Y, Umezaki N, et al. Tumour-infiltrating inflammatory and immune cells in patients with extrahepatic cholangiocarcinoma. Br J Cancer. (2018) 118:171–80. doi: 10.1038/bjc.2017.401
43. Highfill SL, Cui Y, Giles AJ, Smith JP, Zhang H, Morse E, et al. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci Trans Med. (2014) 6:237ra67. doi: 10.1126/scitranslmed.3007974
44. Fabris L, Sato K, Alpini G, Strazzabosco M. The tumor microenvironment in cholangiocarcinoma progression. Hepatology. (2021) 73 Suppl 1:75–85. doi: 10.1002/hep.31410
45. Mao X, Xu J, Wang W, Liang C, Hua J, Liu J, et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer. (2021) 20:131. doi: 10.1186/s12943-021-01428-1
46. Caligiuri G, Tuveson DA. Activated fibroblasts in cancer: Perspectives and challenges. Cancer Cell. (2023) 41:434–49. doi: 10.1016/j.ccell.2023.02.015
47. Melchionna R, Trono P, Di Carlo A, Di Modugno F, Nisticò P. Transcription factors in fibroblast plasticity and CAF heterogeneity. J Exp Clin Cancer Res: CR. (2023) 42:347. doi: 10.1186/s13046-023-02934-4
48. Xiang X, Wang J, Lu D, Xu X. Targeting tumor-associated macrophages to synergize tumor immunotherapy. Signal Transduction Targeted Ther. (2021) 6:75. doi: 10.1038/s41392-021-00484-9
49. Zhou SL, Dai Z, Zhou ZJ, Chen Q, Wang Z, Xiao YS, et al. CXCL5 contributes to tumor metastasis and recurrence of intrahepatic cholangiocarcinoma by recruiting infiltrative intratumoral neutrophils. Carcinogenesis. (2014) 35:597–605. doi: 10.1093/carcin/bgt397
50. Fukuda Y, Asaoka T, Eguchi H, Yokota Y, Kubo M, Kinoshita M, et al. Endogenous CXCL9 affects prognosis by regulating tumor-infiltrating natural killer cells in intrahepatic cholangiocarcinoma. Cancer Sci. (2020) 111:323–33. doi: 10.1111/cas.v111.2
51. Wang SS, Liu W, Ly D, Xu H, Qu L, Zhang L. Tumor-infiltrating B cells: their role and application in anti-tumor immunity in lung cancer. Cell Mol Immunol. (2019) 16:6–18. doi: 10.1038/s41423-018-0027-x
52. Huang YH, Zhang CZ, Huang QS, Yeong J, Wang F, Yang X, et al. Clinicopathologic features, tumor immune microenvironment and genomic landscape of Epstein-Barr virus-associated intrahepatic cholangiocarcinoma. J Hepatol. (2021) 74:838–49. doi: 10.1016/j.jhep.2020.10.037
53. Lin Y, Peng L, Dong L, Liu D, Ma J, Lin J, et al. Geospatial immune heterogeneity reflects the diverse tumor-immune interactions in intrahepatic cholangiocarcinoma. Cancer Discov. (2022) 12:2350–71. doi: 10.1158/2159-8290.CD-21-1640
54. Andersen JB, Spee B, Blechacz BR, Avital I, Komuta M, Barbour A, et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology. (2012) 142:1021–31 e15. doi: 10.1053/j.gastro.2011.12.005
55. Nakamura H, Arai Y, Totoki Y, Shirota T, Elzawahry A, Kato M, et al. Genomic spectra of biliary tract cancer. Nat Genet. (2015) 47:1003–10. doi: 10.1038/ng.3375
56. Chaisaingmongkol J, Budhu A, Dang H, Rabibhadana S, Pupacdi B, Kwon SM, et al. Common molecular subtypes among asian hepatocellular carcinoma and cholangiocarcinoma. Cancer Cell. (2017) 32:57–70 e3. doi: 10.1016/j.ccell.2017.05.009
57. Lin J, Dai Y, Sang C, Song G, Xiang B, Zhang M, et al. Multimodule characterization of immune subgroups in intrahepatic cholangiocarcinoma reveals distinct therapeutic vulnerabilities. J Immunother Cancer. (2022) 10(7):e004892. doi: 10.1136/jitc-2022-004892
58. Korbecki J, Kupnicka P, Chlubek M, Gorący J, Gutowska I, Baranowska-Bosiacka I. CXCR2 receptor: regulation of expression, signal transduction, and involvement in cancer. Int J Mol Sci. (2022) 23(4):2168. doi: 10.3390/ijms23042168
59. Bonelli S, Geeraerts X, Bolli E, Keirsse J, Kiss M, Pombo Antunes AR, et al. Beyond the M-CSF receptor - novel therapeutic targets in tumor-associated macrophages. FEBS J. (2018) 285:777–87. doi: 10.1111/febs.2018.285.issue-4
60. Job S, Rapoud D, Dos Santos A, Gonzalez P, Desterke C, Pascal G, et al. Identification of four immune subtypes characterized by distinct composition and functions of tumor microenvironment in intrahepatic cholangiocarcinoma. Hepatology. (2020) 72:965–81. doi: 10.1002/hep.31092
61. Carapeto F, Bozorgui B, Shroff RT, Chagani S, Solis Soto L, Foo WC, et al. The immunogenomic landscape of resected intrahepatic cholangiocarcinoma. Hepatology. (2022) 75:297–308. doi: 10.1002/hep.32150
62. Montal R, Sia D, Montironi C, Leow WQ, Esteban-Fabro R, Pinyol R, et al. Molecular classification and therapeutic targets in extrahepatic cholangiocarcinoma. J Hepatol. (2020) 73:315–27. doi: 10.1016/j.jhep.2020.03.008
63. Ding GY, Ma JQ, Yun JP, Chen X, Ling Y, Zhang S, et al. Distribution and density of tertiary lymphoid structures predict clinical outcome in intrahepatic cholangiocarcinoma. J Hepatol. (2022) 76:608–18. doi: 10.1016/j.jhep.2021.10.030
64. Chen S, Xie Y, Cai Y, Hu H, He M, Liu L, et al. Multiomic analysis reveals comprehensive tumor heterogeneity and distinct immune subtypes in multifocal intrahepatic cholangiocarcinoma. Clin Cancer Res. (2022) 28:1896–910. doi: 10.1158/1078-0432.CCR-21-1157
65. Deng M, Ran P, Chen L, Wang Y, Yu Z, Cai K, et al. Proteogenomic characterization of cholangiocarcinoma. Hepatology. (2023) 77:411–29. doi: 10.1002/hep.32624
66. Cho SY, Hwang H, Kim YH, Yoo BC, Han N, Kong SY, et al. Refining classification of cholangiocarcinoma subtypes via proteogenomic integration reveals new therapeutic prospects. Gastroenterology. (2023) 164:1293–309. doi: 10.1053/j.gastro.2023.02.045
67. Sia D, Hoshida Y, Villanueva A, Roayaie S, Ferrer J, Tabak B, et al. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology. (2013) 144:829–40. doi: 10.1053/j.gastro.2013.01.001
68. Bao X, Li Q, Chen J, Chen D, Ye C, Dai X, et al. Molecular subgroups of intrahepatic cholangiocarcinoma discovered by single-cell RNA sequencing-assisted multiomics analysis. Cancer Immunol Res. (2022) 10:811–28. doi: 10.1158/2326-6066.CIR-21-1101
69. Dong L, Lu D, Chen R, Lin Y, Zhu H, Zhang Z, et al. Proteogenomic characterization identifies clinically relevant subgroups of intrahepatic cholangiocarcinoma. Cancer Cell. (2022) 40:70–87 e15. doi: 10.1016/j.ccell.2021.12.006
70. Kumar BV, Connors TJ, Farber DL. Human T cell development, localization, and function throughout life. Immunity. (2018) 48:202–13. doi: 10.1016/j.immuni.2018.01.007
71. Liu D, Heij LR, Czigany Z, Dahl E, Lang SA, Ulmer TF, et al. The role of tumor-infiltrating lymphocytes in cholangiocarcinoma. J Exp Clin Cancer Res: CR. (2022) 41:127. doi: 10.1186/s13046-022-02340-2
72. Woo SR, Corrales L, Gajewski TF. The STING pathway and the T cell-inflamed tumor microenvironment. Trends Immunol. (2015) 36:250–6. doi: 10.1016/j.it.2015.02.003
73. Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol: Off J Eur Soc Med Oncol. (2015) 26:259–71. doi: 10.1093/annonc/mdu450
74. Mancarella S, Serino G, Coletta S, Armentano R, Dituri F, Ardito F, et al. The tumor microenvironment drives intrahepatic cholangiocarcinoma progression. Int J Mol Sci. (2022) 23(8):4187. doi: 10.3390/ijms23084187
75. Zhang Y, Yan HJ, Wu J. The tumor immune microenvironment plays a key role in driving the progression of cholangiocarcinoma. Curr Cancer Drug Targets. (2024) 24:681–700. doi: 10.2174/0115680096267791231115101107
76. Zhou G, Sprengers D, Mancham S, Erkens R, Boor PPC, van Beek AA, et al. Reduction of immunosuppressive tumor microenvironment in cholangiocarcinoma by ex vivo targeting immune checkpoint molecules. J Hepatol. (2019) 71:753–62. doi: 10.1016/j.jhep.2019.05.026
77. Goeppert B, Frauenschuh L, Zucknick M, Stenzinger A, Andrulis M, Klauschen F, et al. Prognostic impact of tumour-infiltrating immune cells on biliary tract cancer. Br J Cancer. (2013) 109:2665–74. doi: 10.1038/bjc.2013.610
78. Shang T, Jiang T, Lu T, Wang H, Cui X, Pan Y, et al. Tertiary lymphoid structures predict the prognosis and immunotherapy response of cholangiocarcinoma. Front Immunol. (2023) 14:1166497. doi: 10.3389/fimmu.2023.1166497
79. Ma L, Wang L, Khatib SA, Chang CW, Heinrich S, Dominguez DA, et al. Single-cell atlas of tumor cell evolution in response to therapy in hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Hepatol. (2021) 75:1397–408. doi: 10.1016/j.jhep.2021.06.028
80. Kim HD, Jeong S, Park S, Lee YJ, Ju YS, Kim D, et al. Implication of CD69(+) CD103(+) tissue-resident-like CD8(+) T cells as a potential immunotherapeutic target for cholangiocarcinoma. Liver International: Off J Int Assoc Study Liver. (2021) 41:764–76. doi: 10.1111/liv.14814
81. Franco F, Jaccard A, Romero P, Yu YR, Ho PC. Metabolic and epigenetic regulation of T-cell exhaustion. Nat Metab. (2020) 2:1001–12. doi: 10.1038/s42255-020-00280-9
82. Scharer CD, Bally AP, Gandham B, Boss JM. Cutting edge: chromatin accessibility programs CD8 T cell memory. J Immunol. (2017) 198:2238–43. doi: 10.4049/jimmunol.1602086
83. Ritter C, Fan K, Paschen A, Reker Hardrup S, Ferrone S, Nghiem P, et al. Epigenetic priming restores the HLA class-I antigen processing machinery expression in Merkel cell carcinoma. Sci Rep. (2017) 7:2290. doi: 10.1038/s41598-017-02608-0
84. Topper MJ, Vaz M, Chiappinelli KB, DeStefano Shields CE, Niknafs N, Yen RC, et al. Epigenetic therapy ties MYC depletion to reversing immune evasion and treating lung cancer. Cell. (2017) 171:1284–300.e21. doi: 10.1016/j.cell.2017.10.022
85. Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. (2017) 17:559–72. doi: 10.1038/nri.2017.49
86. Zhou G, Sprengers D, Boor PPC, Doukas M, Schutz H, Mancham S, et al. Antibodies against immune checkpoint molecules restore functions of tumor-infiltrating T cells in hepatocellular carcinomas. Gastroenterology. (2017) 153:1107–19.e10. doi: 10.1053/j.gastro.2017.06.017
87. Jing CY, Fu YP, Yi Y, Zhang MX, Zheng SS, Huang JL, et al. HHLA2 in intrahepatic cholangiocarcinoma: an immune checkpoint with prognostic significance and wider expression compared with PD-L1. J Immunother Cancer. (2019) 7:77. doi: 10.1186/s40425-019-0554-8
88. Kravtsov DS, Erbe AK, Sondel PM, Rakhmilevich AL. Roles of CD4+ T cells as mediators of antitumor immunity. Front Immunol. (2022) 13:972021. doi: 10.3389/fimmu.2022.972021
89. Wang B, Hu S, Fu X, Li L. CD4(+) cytotoxic T lymphocytes in cancer immunity and immunotherapy. Advanced Biol. (2023) 7:e2200169. doi: 10.1002/adbi.202200169
90. Preglej T, Ellmeier W. CD4(+) cytotoxic T cells - phenotype, function and transcriptional networks controlling their differentiation pathways. Immunol Lett. (2022) 247:27–42. doi: 10.1016/j.imlet.2022.05.001
91. Li D, Fan H, Dong J, Sun C, Su Y, Liu J, et al. Based on BATMAN-TCM to explore the molecular mechanism of xihuang pill regulating immune function to treat breast precancerous lesions. Breast Cancer (Dove Med Press). (2021) 13:725–42. doi: 10.2147/BCTT.S339607
92. Chraa D, Naim A, Olive D, Badou A. T lymphocyte subsets in cancer immunity: Friends or foes. J Leukoc Biol. (2019) 105:243–55. doi: 10.1002/JLB.MR0318-097R
93. Chang SH. T helper 17 (Th17) cells and interleukin-17 (IL-17) in cancer. Arch Pharmacal Res. (2019) 42:549–59. doi: 10.1007/s12272-019-01146-9
94. Śledzińska A, Vila de Mucha M, Bergerhoff K, Hotblack A, Demane DF, Ghorani E, et al. Regulatory T cells restrain interleukin-2- and blimp-1-dependent acquisition of cytotoxic function by CD4(+) T cells. Immunity. (2020) 52:151–66.e6. doi: 10.1016/j.immuni.2019.12.007
95. Miggelbrink AM, Jackson JD, Lorrey SJ, Srinivasan ES, Waibl-Polania J, Wilkinson DS, et al. CD4 T-cell exhaustion: does it exist and what are its roles in cancer? Clin Cancer Res. (2021) 27:5742–52. doi: 10.1158/1078-0432.CCR-21-0206
96. Venkatesh H, Tracy SI, Farrar MA. Cytotoxic CD4 T cells in the mucosa and in cancer. Front Immunol. (2023) 14:1233261. doi: 10.3389/fimmu.2023.1233261
97. Malyshkina A, Brüggemann A, Paschen A, Dittmer U. Cytotoxic CD4(+) T cells in chronic viral infections and cancer. Front Immunol. (2023) 14:1271236. doi: 10.3389/fimmu.2023.1271236
98. Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Sci (New York NY). (2014) 344:641–5. doi: 10.1126/science.1251102
99. Shen Y, Xu S, Ye C, Li Q, Chen R, Wu W, et al. Proteomic and single-cell landscape reveals novel pathogenic mechanisms of HBV-infected intrahepatic cholangiocarcinoma. iScience. (2023) 26:106003. doi: 10.1016/j.isci.2023.106003
100. Qiu FB, Wu LQ, Lu Y, Zhang S, Zhang BY. Predominant expression of Th1-type cytokines in primary hepatic cancer and adjacent liver tissues. Hepatobiliary Pancreatic Dis International: HBPD Int. (2007) 6:63–6.
101. Kasper HU, Drebber U, Stippel DL, Dienes HP, Gillessen A. Liver tumor infiltrating lymphocytes: comparison of hepatocellular and cholangiolar carcinoma. World J Gastroenterol. (2009) 15:5053–7. doi: 10.3748/wjg.15.5053
102. Kim HD, Kim JH, Ryu YM, Kim D, Lee S, Shin J, et al. Spatial distribution and prognostic implications of tumor-infiltrating foxP3- CD4+ T cells in biliary tract cancer. Cancer Res Treat. (2021) 53:162–71. doi: 10.4143/crt.2020.704
103. Xu L, Lu Y, Deng Z, Li X, Shi Y, Zhao K, et al. Single-cell landscape of immunocytes in patients with extrahepatic cholangiocarcinoma. J Transl Med. (2022) 20:210. doi: 10.1186/s12967-022-03424-5
104. Zhang QW, Zhu MX, Liu WF, Rui WW, Chen Y, Ding XY, et al. Identification of clinically relevant subsets CD39(+)PD-1(+)CD8(+) T cells and CD39(+) regulatory T cells in intrahepatic cholangiocarcinoma using single-cell CyTOF. Transl Oncol. (2024) 44:101954. doi: 10.1016/j.tranon.2024.101954
105. Ueno T, Tsuchikawa T, Hatanaka KC, Hatanaka Y, Mitsuhashi T, Nakanishi Y, et al. Prognostic impact of programmed cell death ligand 1 (PD-L1) expression and its association with epithelial-mesenchymal transition in extrahepatic cholangiocarcinoma. Oncotarget. (2018) 9:20034–47. doi: 10.18632/oncotarget.25050
106. Asahi Y, Hatanaka KC, Hatanaka Y, Kamiyama T, Orimo T, Shimada S, et al. Prognostic impact of CD8+ T cell distribution and its association with the HLA class I expression in intrahepatic cholangiocarcinoma. Surg Today. (2020) 50:931–40. doi: 10.1007/s00595-020-01967-y
107. Koh CH, Lee S, Kwak M, Kim BS, Chung Y. CD8 T-cell subsets: heterogeneity, functions, and therapeutic potential. Exp Mol Med. (2023) 55:2287–99. doi: 10.1038/s12276-023-01105-x
108. Park J, Hsueh PC, Li Z, Ho PC. Microenvironment-driven metabolic adaptations guiding CD8(+) T cell anti-tumor immunity. Immunity. (2023) 56:32–42. doi: 10.1016/j.immuni.2022.12.008
109. Wang Q, Qin Y, Li B. CD8(+) T cell exhaustion and cancer immunotherapy. Cancer Lett. (2023) 559:216043. doi: 10.1016/j.canlet.2022.216043
110. Spetz J, Presser AG, Sarosiek KA. T cells and regulated cell death: kill or be killed. Int Rev Cell Mol Biol. (2019) 342:27–71. doi: 10.1016/bs.ircmb.2018.07.004
111. Reina-Campos M, Scharping NE, Goldrath AW. CD8(+) T cell metabolism in infection and cancer. Nat Rev Immunol. (2021) 21:718–38. doi: 10.1038/s41577-021-00537-8
112. Cao H, Huang T, Dai M, Kong X, Liu H, Zheng Z, et al. Tumor microenvironment and its implications for antitumor immunity in cholangiocarcinoma: future perspectives for novel therapies. Int J Biol Sci. (2022) 18:5369–90. doi: 10.7150/ijbs.73949
113. Jiang W, He Y, He W, Wu G, Zhou X, Sheng Q, et al. Exhausted CD8+T cells in the tumor immune microenvironment: new pathways to therapy. Front Immunol. (2020) 11:622509. doi: 10.3389/fimmu.2020.622509
114. Wang J, Loeuillard E, Gores GJ, Ilyas SI. Cholangiocarcinoma: what are the most valuable therapeutic targets - cancer-associated fibroblasts, immune cells, or beyond T cells? Expert Opin Ther Targets. (2021) 25:835–45. doi: 10.1080/14728222.2021.2010046
115. Ravichandra A, Bhattacharjee S, Affò S. Cancer-associated fibroblasts in intrahepatic cholangiocarcinoma progression and therapeutic resistance. Adv Cancer Res. (2022) 156:201–26. doi: 10.1016/bs.acr.2022.01.009
116. Ying F, Chan MSM, Lee TKW. Cancer-associated fibroblasts in hepatocellular carcinoma and cholangiocarcinoma. Cell Mol Gastroenterol Hepatol. (2023) 15:985–99. doi: 10.1016/j.jcmgh.2023.01.006
117. Tian L, Ma J, Ma L, Zheng B, Liu L, Song D, et al. PD-1/PD-L1 expression profiles within intrahepatic cholangiocarcinoma predict clinical outcome. World J Surg Oncol. (2020) 18:303. doi: 10.1186/s12957-020-02082-5
118. Wu H, Wei Y, Jian M, Lu H, Song Q, Hao L, et al. Clinicopathological and prognostic significance of immunoscore and PD-L1 in intrahepatic cholangiocarcinoma. Onco Targets Ther. (2021) 14:39–51. doi: 10.2147/OTT.S288982
119. Xu YP, Zhou YQ, Zhao YJ, Zhao Y, Wang F, Huang XY, et al. High level of CD73 predicts poor prognosis of intrahepatic cholangiocarcinoma. J Cancer. (2021) 12:4655–60. doi: 10.7150/jca.51038
120. Chen L, Huang H, Huang Z, Chen J, Liu Y, Wu Y, et al. Prognostic values of tissue-resident CD8(+)T cells in human hepatocellular carcinoma and intrahepatic cholangiocarcinoma. World J Surg Oncol. (2023) 21:124. doi: 10.1186/s12957-023-03009-6
121. Yugawa K, Itoh S, Yoshizumi T, Iseda N, Tomiyama T, Toshima T, et al. Prognostic impact of tumor microvessels in intrahepatic cholangiocarcinoma: association with tumor-infiltrating lymphocytes. Modern Pathol: An Off J United States Can Acad Pathol Inc. (2021) 34:798–807. doi: 10.1038/s41379-020-00702-9
122. Ghobadinezhad F, Ebrahimi N, Mozaffari F, Moradi N, Beiranvand S, Pournazari M, et al. The emerging role of regulatory cell-based therapy in autoimmune disease. Front Immunol. (2022) 13:1075813. doi: 10.3389/fimmu.2022.1075813
123. Hui Z, Zhang J, Zheng Y, Yang L, Yu W, An Y, et al. Single-Cell Sequencing Reveals the Transcriptome and TCR Characteristics of pTregs and in vitro Expanded iTregs. Front Immunol. (2021) 12:619932. doi: 10.3389/fimmu.2021.619932
124. Almeida-Santos J, Bergman ML, Demengeot J. Differentiation of peripheral treg. Methods Mol Biol (Clifton NJ). (2023) 2559:67–77. doi: 10.1007/978-1-0716-2647-4_6
125. Santosh Nirmala S, Kayani K, Gliwiński M, Hu Y, Iwaszkiewicz-Grześ D, Piotrowska-Mieczkowska M, et al. Beyond FOXP3: a 20-year journey unravelling human regulatory T-cell heterogeneity. Front Immunol. (2023) 14:1321228. doi: 10.3389/fimmu.2023.1321228
126. McHugh J. T(reg) cell-inducing nanoparticles show promise for treating OA. Nat Rev Rheumatol. (2023) 19:62. doi: 10.1038/s41584-023-00906-8
127. Stucchi A, Maspes F, Montee-Rodrigues E, Fousteri G. Engineered Treg cells: The heir to the throne of immunotherapy. J Autoimmunity. (2024) 144:102986. doi: 10.1016/j.jaut.2022.102986
128. Adeegbe D, Barbi J, Wing J. Editorial: Regulatory T lymphocytes in cancer immunity. Front Immunol. (2022) 13:1065570. doi: 10.3389/fimmu.2022.1065570
129. Liu Z, Zhou J, Wu S, Chen Z, Wu S, Chen L, et al. Why Treg should be the focus of cancer immunotherapy: The latest thought. BioMed Pharmacother. (2023) 168:115142. doi: 10.1016/j.biopha.2023.115142
130. Guo Y, Xie YQ, Gao M, Zhao Y, Franco F, Wenes M, et al. Metabolic reprogramming of terminally exhausted CD8(+) T cells by IL-10 enhances anti-tumor immunity. Nat Immunol. (2021) 22:746–56. doi: 10.1038/s41590-021-00940-2
131. Disis ML. Immune regulation of cancer. J Clin Oncol: Off J Am Soc Clin Oncol. (2010) 28:4531–8. doi: 10.1200/JCO.2009.27.2146
132. Zhang G, Zheng G, Zhang H, Qiu L. MUC1 induces the accumulation of Foxp3(+) Treg cells in the tumor microenvironment to promote the growth and metastasis of cholangiocarcinoma through the EGFR/PI3K/Akt signaling pathway. Int Immunopharmacol. (2023) 118:110091. doi: 10.1016/j.intimp.2023.110091
133. Ma K, Sun Z, Li X, Guo J, Wang Q, Teng M. Forkhead box M1 recruits FoxP3(+) Treg cells to induce immune escape in hilar cholangiocarcinoma. Immunity Inflammation Dis. (2022) 10:e727. doi: 10.1002/iid3.v10.11
134. Ghidini M, Cascione L, Carotenuto P, Lampis A, Trevisani F, Previdi MC, et al. Characterisation of the immune-related transcriptome in resected biliary tract cancers. Eur J Cancer (Oxford England: 1990). (2017) 86:158–65. doi: 10.1016/j.ejca.2017.09.005
135. Alvisi G, Termanini A, Soldani C, Portale F, Carriero R, Pilipow K, et al. Multimodal single-cell profiling of intrahepatic cholangiocarcinoma defines hyperactivated Tregs as a potential therapeutic target. J Hepatol. (2022) 77:1359–72. doi: 10.1016/j.jhep.2022.05.043
136. Lang F, Schrörs B, Löwer M, Türeci Ö, Sahin U. Identification of neoantigens for individualized therapeutic cancer vaccines. Nat Rev Drug Discov. (2022) 21:261–82. doi: 10.1038/s41573-021-00387-y
137. Ilyas SI, Affo S, Goyal L, Lamarca A, Sapisochin G, Yang JD, et al. Cholangiocarcinoma - novel biological insights and therapeutic strategies. Nat Rev Clin Oncol. (2023) 20:470–86. doi: 10.1038/s41571-023-00770-1
138. Yu X, Zhu L, Wang T, Chen J. Immune microenvironment of cholangiocarcinoma: Biological concepts and treatment strategies. Front Immunol. (2023) 14:1037945. doi: 10.3389/fimmu.2023.1037945
139. Wang SJ, Dougan SK, Dougan M. Immune mechanisms of toxicity from checkpoint inhibitors. Trends Cancer. (2023) 9:543–53. doi: 10.1016/j.trecan.2023.04.002
140. Liu Y, Wang Y, Yang Y, Weng L, Wu Q, Zhang J, et al. Emerging phagocytosis checkpoints in cancer immunotherapy. Signal Transduction Targeted Ther. (2023) 8:104. doi: 10.1038/s41392-023-01365-z
141. Heij L, Bednarsch J, Tan X, Rosin M, Appinger S, Reichel K, et al. Expression of checkpoint molecules in the tumor microenvironment of intrahepatic cholangiocarcinoma: implications for immune checkpoint blockade therapy. Cells. (2023) 12(6):851. doi: 10.3390/cells12060851
142. Marasco M, Berteotti A, Weyershaeuser J, Thorausch N, Sikorska J, Krausze J, et al. Molecular mechanism of SHP2 activation by PD-1 stimulation. Sci Advances. (2020) 6:eaay4458. doi: 10.1126/sciadv.aay4458
143. Li K, Yuan Z, Lyu J, Ahn E, Davis SJ, Ahmed R, et al. PD-1 suppresses TCR-CD8 cooperativity during T-cell antigen recognition. Nat Commun. (2021) 12:2746. doi: 10.1038/s41467-021-22965-9
144. Li G, Jiang Y, Qin Y, Yuan S, Chen X. Comparing development strategies for PD1/PDL1-based immunotherapies. Nat Rev Drug Discov. (2022) 21:484. doi: 10.1038/d41573-022-00003-7
145. Li X, Qu LX, Ren YM, Hu C. Case report: A case report and literature review on severe bullous skin reaction induced by anti-PD-1 immunotherapy in a cervical cancer patient. Front Pharmacol. (2021) 12:707967. doi: 10.3389/fphar.2021.707967
146. Ueno M, Chung H, Nagrial A, Marabelle A, Kelley R, Xu L, et al. Pembrolizumab for advanced biliary adenocarcinoma: Results from the multicohort, phase II KEYNOTE-158 study. Ann Oncol. (2018) 29:viii210. doi: 10.1093/annonc/mdy282.009
147. Ott PA, Bang YJ, Piha-Paul SA, Razak ARA, Bennouna J, Soria JC, et al. T-cell-inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J Clin Oncol: Off J Am Soc Clin Oncol. (2019) 37:318–27. doi: 10.1200/JCO.2018.78.2276
148. Rowshanravan B, Halliday N, Sansom DM. CTLA-4: a moving target in immunotherapy. Blood. (2018) 131:58–67. doi: 10.1182/blood-2017-06-741033
149. Hossen MM, Ma Y, Yin Z, Xia Y, Du J, Huang JY, et al. Current understanding of CTLA-4: from mechanism to autoimmune diseases. Front Immunol. (2023) 14:1198365. doi: 10.3389/fimmu.2023.1198365
150. Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. (2001) 194:823–32. doi: 10.1084/jem.194.6.823
151. Guo XJ, Lu JC, Zeng HY, Zhou R, Sun QM, Yang GH, et al. CTLA-4 synergizes with PD1/PD-L1 in the inhibitory tumor microenvironment of intrahepatic cholangiocarcinoma. Front Immunol. (2021) 12:705378. doi: 10.3389/fimmu.2021.705378
152. Lim YJ, Koh J, Kim K, Chie EK, Kim S, Lee KB, et al. Clinical implications of cytotoxic T lymphocyte antigen-4 expression on tumor cells and tumor-infiltrating lymphocytes in extrahepatic bile duct cancer patients undergoing surgery plus adjuvant chemoradiotherapy. Target Oncol. (2017) 12:211–8. doi: 10.1007/s11523-016-0474-1
153. Xie C, Duffy AG, Mabry-Hrones D, Wood B, Levy E, Krishnasamy V, et al. Tremelimumab in combination with microwave ablation in patients with refractory biliary tract cancer. Hepatology. (2019) 69:2048–60. doi: 10.1002/hep.30482
154. Zhao L, Cheng S, Fan L, Zhang B, Xu S. TIM-3: An update on immunotherapy. Int Immunopharmacol. (2021) 99:107933. doi: 10.1016/j.intimp.2021.107933
155. Yang R, Sun L, Li CF, Wang YH, Yao J, Li H, et al. Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat Commun. (2021) 12:832. doi: 10.1038/s41467-021-21099-2
156. Dixon KO, Tabaka M, Schramm MA, Xiao S, Tang R, Dionne D, et al. TIM-3 restrains anti-tumour immunity by regulating inflammasome activation. Nature. (2021) 595:101–6. doi: 10.1038/s41586-021-03626-9
157. Carroll KL, Avery L, Treat BR, Kane LP, Kinchington PR, Hendricks RL, et al. Differential expression of immune checkpoint molecules on CD8(+) T cells specific for immunodominant and subdominant herpes simplex virus 1 epitopes. J Virol. (2020) 94(2):e01132-19. doi: 10.1128/JVI.01132-19
158. Konishi D, Umeda Y, Yoshida K, Shigeyasu K, Yano S, Toji T, et al. Regulatory T cells induce a suppressive immune milieu and promote lymph node metastasis in intrahepatic cholangiocarcinoma. Br J Cancer. (2022) 127:757–65. doi: 10.1038/s41416-022-01838-y
159. Qiao Y, Chen J, Wang X, Yan S, Tan J, Xia B, et al. Enhancement of CAR-T cell activity against cholangiocarcinoma by simultaneous knockdown of six inhibitory membrane proteins. Cancer Commun (Lond). (2023) 43:788–807. doi: 10.1002/cac2.12452
160. Zhang P, Liu X, Gu Z, Jiang Z, Zhao S, Song Y, et al. Targeting TIGIT for cancer immunotherapy: recent advances and future directions. biomark Res. (2024) 12:7. doi: 10.1186/s40364-023-00543-z
161. Guan X, Hu R, Choi Y, Srivats S, Nabet BY, Silva J, et al. Anti-TIGIT antibody improves PD-L1 blockade through myeloid and T(reg) cells. Nature. (2024) 627:646–55. doi: 10.1038/s41586-024-07121-9
162. Ostroumov D, Duong S, Wingerath J, Woller N, Manns MP, Timrott K, et al. Transcriptome profiling identifies TIGIT as a marker of T-cell exhaustion in liver cancer. Hepatology. (2021) 73:1399–418. doi: 10.1002/hep.31466
163. Joller N, Lozano E, Burkett PR, Patel B, Xiao S, Zhu C, et al. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity. (2014) 40:569–81. doi: 10.1016/j.immuni.2014.02.012
164. Johnston RJ, Comps-Agrar L, Hackney J, Yu X, Huseni M, Yang Y, et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell. (2014) 26:923–37. doi: 10.1016/j.ccell.2014.10.018
165. Zhang Q, Bi J, Zheng X, Chen Y, Wang H, Wu W, et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol. (2018) 19:723–32. doi: 10.1038/s41590-018-0132-0
166. Aggarwal V, Workman CJ, Vignali DAA. LAG-3 as the third checkpoint inhibitor. Nat Immunol. (2023) 24:1415–22. doi: 10.1038/s41590-023-01569-z
167. Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. (2012) 72:917–27. doi: 10.1158/0008-5472.CAN-11-1620
168. Maruhashi T, Sugiura D, Okazaki IM, Shimizu K, Maeda TK, Ikubo J, et al. Binding of LAG-3 to stable peptide-MHC class II limits T cell function and suppresses autoimmunity and anti-cancer immunity. Immunity. (2022) 55:912–24 e8. doi: 10.1016/j.immuni.2022.03.013
169. Chocarro L, Blanco E, Arasanz H, Fernández-Rubio L, Bocanegra A, Echaide M, et al. Clinical landscape of LAG-3-targeted therapy. Immuno-oncol Technol. (2022) 14:100079. doi: 10.1016/j.iotech.2022.100079
170. Sung E, Ko M, Won JY, Jo Y, Park E, Kim H, et al. LAG-3xPD-L1 bispecific antibody potentiates antitumor responses of T cells through dendritic cell activation. Mol Ther. (2022) 30:2800–16. doi: 10.1016/j.ymthe.2022.05.003
171. Diab A, Hamid O, Thompson JA, Ros W, Eskens F, Doi T, et al. Open-label, dose-escalation study of the OX40 agonist ivuxolimab in patients with locally advanced or metastatic cancers. Clin Cancer Res. (2022) 28:71–83. doi: 10.1158/1078-0432.CCR-21-0845
172. Verdeil G, Puthier D, Nguyen C, Schmitt-Verhulst AM, Auphan-Anezin N. STAT5-mediated signals sustain a TCR-initiated gene expression program toward differentiation of CD8 T cell effectors. J Immunol. (2006) 176:4834–42. doi: 10.4049/jimmunol.176.8.4834
173. Thapa B, Kato S, Nishizaki D, Miyashita H, Lee S, Nesline MK, et al. OX40/OX40 ligand and its role in precision immune oncology. Cancer Metastasis Rev. (2024) 43:1001–13. doi: 10.1007/s10555-024-10184-9
174. Giampietri C, Scatozza F, Crecca E, Vigiano Benedetti V, Natali PG, Facchiano A. Analysis of gene expression levels and their impact on survival in 31 cancer-types patients identifies novel prognostic markers and suggests unexplored immunotherapy treatment options in a wide range of Malignancies. J Transl Med. (2022) 20:467. doi: 10.1186/s12967-022-03670-7
175. Kida A, Mizukoshi E, Kido H, Toyama T, Terashima T, Arai K, et al. The characteristics of the immune cell profiles in peripheral blood in cholangiocarcinoma patients. Hepatol Int. (2021) 15:695–706. doi: 10.1007/s12072-021-10177-8
176. Richards DM, Sefrin JP, Gieffers C, Hill O, Merz C. Concepts for agonistic targeting of CD40 in immuno-oncology. Hum Vaccin Immunother. (2020) 16:377–87. doi: 10.1080/21645515.2019.1653744
177. Tang T, Cheng X, Truong B, Sun L, Yang X, Wang H. Molecular basis and therapeutic implications of CD40/CD40L immune checkpoint. Pharmacol Ther. (2021) 219:107709. doi: 10.1016/j.pharmthera.2020.107709
178. Thepmalee C, Panya A, Junking M, Chieochansin T, Yenchitsomanus PT. Inhibition of IL-10 and TGF-beta receptors on dendritic cells enhances activation of effector T-cells to kill cholangiocarcinoma cells. Hum Vaccin Immunother. (2018) 14:1423–31. doi: 10.1080/21645515.2018.1431598
179. Sadeghlar F, Vogt A, Mohr RU, Mahn R, van Beekum K, Kornek M, et al. Induction of cytotoxic effector cells towards cholangiocellular, pancreatic, and colorectal tumor cells by activation of the immune checkpoint CD40/CD40L on dendritic cells. Cancer Immunol Immunother. (2021) 70:1451–64. doi: 10.1007/s00262-020-02746-x
180. Diggs LP, Ruf B, Ma C, Heinrich B, Cui L, Zhang Q, et al. CD40-mediated immune cell activation enhances response to anti-PD-1 in murine intrahepatic cholangiocarcinoma. J Hepatol. (2021) 74:1145–54. doi: 10.1016/j.jhep.2020.11.037
181. Tian J, Zhang B, Rui K, Wang S. The role of GITR/GITRL interaction in autoimmune diseases. Front Immunol. (2020) 11:588682. doi: 10.3389/fimmu.2020.588682
182. Riccardi C, Ronchetti S, Nocentini G. Glucocorticoid-induced TNFR-related gene (GITR) as a therapeutic target for immunotherapy. Expert Opin Ther Targets. (2018) 22:783–97. doi: 10.1080/14728222.2018.1512588
183. Fu Z, Wang S, Li J, Zhang Y, Li H, Li G, et al. Biological role of GITR/GITRL in attributes and immune responses of macrophage. J Leukoc Biol. (2020) 107:309–21. doi: 10.1002/JLB.3A0919-387RR
184. Ray A, Basu S, Williams CB, Salzman NH, Dittel BN. A novel IL-10-independent regulatory role for B cells in suppressing autoimmunity by maintenance of regulatory T cells via GITR ligand. J Immunol. (2012) 188:3188–98. doi: 10.4049/jimmunol.1103354
185. Solinas C, Gu-Trantien C, Willard-Gallo K. The rationale behind targeting the ICOS-ICOS ligand costimulatory pathway in cancer immunotherapy. ESMO Open. (2020) 5(1):e000544. doi: 10.1136/esmoopen-2019-000544
186. Park SL, Christo SN, Mackay LK. ICOS-play: dressing T cells for residency. Trends Immunol. (2022) 43:280–2. doi: 10.1016/j.it.2022.02.007
187. Panneton V, Mindt BC, Bouklouch Y, Bouchard A, Mohammaei S, Chang J, et al. ICOS costimulation is indispensable for the differentiation of T follicular regulatory cells. Life Sci Alliance. (2023) 6(4):e202201615. doi: 10.26508/lsa.202201615
188. Klein O, Kee D, Nagrial A, Markman B, Underhill C, Michael M, et al. Evaluation of combination nivolumab and ipilimumab immunotherapy in patients with advanced biliary tract cancers: subgroup analysis of a phase 2 nonrandomized clinical trial. JAMA Oncol. (2020) 6:1405–9. doi: 10.1001/jamaoncol.2020.2814
189. Wang M, Chen Z, Guo P, Wang Y, Chen G. Therapy for advanced cholangiocarcinoma: Current knowledge and future potential. J Cell Mol Med. (2021) 25:618–28. doi: 10.1111/jcmm.16151
190. Floudas CS, Xie C, Brar G, Morelli MP, Fioravanti S, Walker M, et al. Combined immune checkpoint inhibition (ICI) with tremelimumab and durvalumab in patients with advanced hepatocellular carcinoma (HCC) or biliary tract carcinomas (BTC). Am Soc Clin Oncol. (2019) 37:336. doi: 10.1200/JCO.2019.37.4_suppl.336
191. Fiste O, Ntanasis-Stathopoulos I, Gavriatopoulou M, Liontos M, Koutsoukos K, Dimopoulos MA, et al. The emerging role of immunotherapy in intrahepatic cholangiocarcinoma. Vaccines. (2021) 9(5):422. doi: 10.3390/vaccines9050422
192. Ott PA, Hodi FS, Kaufman HL, Wigginton JM, Wolchok JD. Combination immunotherapy: a road map. J Immunother Cancer. (2017) 5:16. doi: 10.1186/s40425-017-0218-5
193. Yuan J, Khilnani A, Brody J, Andtbacka RHI, Hu-Lieskovan S, Luke JJ, et al. Current strategies for intratumoural immunotherapy - Beyond immune checkpoint inhibition. Eur J Cancer (Oxford England: 1990). (2021) 157:493–510. doi: 10.1016/j.ejca.2021.08.004
194. Rahma OE, Hodi FS. The intersection between tumor angiogenesis and immune suppression. Clin Cancer Res. (2019) 25:5449–57. doi: 10.1158/1078-0432.CCR-18-1543
Keywords: tumor-infiltrating T lymphocytes, cholangiocarcinoma, tumor microenvironment, immune checkpoints, immunotherapy
Citation: Dai Y, Dong C, Wang Z, Zhou Y, Wang Y, Hao Y, Chen P, Liang C and Li G (2025) Infiltrating T lymphocytes and tumor microenvironment within cholangiocarcinoma: immune heterogeneity, intercellular communication, immune checkpoints. Front. Immunol. 15:1482291. doi: 10.3389/fimmu.2024.1482291
Received: 18 August 2024; Accepted: 17 December 2024;
Published: 08 January 2025.
Edited by:
Jun Zhang, Kumamoto University, JapanReviewed by:
Paul E. Love, National Institutes of Health (NIH), United StatesYuquan Chen, Monash University, Australia
Xiaofei Liu, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, China
Copyright © 2025 Dai, Dong, Wang, Zhou, Wang, Hao, Chen, Liang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chaojie Liang, bGlhbmdjaGFvamllOEAxMjYuY29t; Gaopeng Li, bWFsb25lMjAwMUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Yunyan Dai
Yunyan Dai Chenyang Dong
Chenyang Dong Zhiming Wang
Zhiming Wang Yunpeng Zhou1
Yunpeng Zhou1 Chaojie Liang
Chaojie Liang Gaopeng Li
Gaopeng Li