
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 07 January 2025
Sec. Vaccines and Molecular Therapeutics
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1475886
This article is part of the Research TopicNew Insights in Nucleic Acid Approaches for Vaccine and Biologic DeliveryView all 6 articles
 Muhammad Bashir Bello1
Muhammad Bashir Bello1 Ahlam Alsaadi1
Ahlam Alsaadi1 Asif Naeem1
Asif Naeem1 Sarah A. Almahboub1
Sarah A. Almahboub1 Mohammad Bosaeed1,2
Mohammad Bosaeed1,2 Safia S. Aljedani1*
Safia S. Aljedani1*Due to their widespread geographic distribution and frequent outbreaks, mosquito-borne flaviviruses, such as DENV (DENV), Zika virus (ZIKV), Japanese encephalitis virus (JEV), yellow fever virus (YFV), and West Nile virus (WNV), are considered significant global public health threats and contribute to dramatic socioeconomic imbalances worldwide. The global prevalence of these viruses is largely driven by extensive international travels and ecological disruptions that create favorable conditions for the breeding of Aedes and Culex species, the mosquito vectors responsible for the spread of these pathogens. Currently, vaccines are available for only DENV, YFV, and JEV, but these face several challenges, including safety concerns, lengthy production processes, and logistical difficulties in distribution, especially in resource-limited regions, highlighting the urgent need for innovative vaccine approaches. Nucleic acid-based platforms, including DNA and mRNA vaccines, have emerged as promising alternatives due to their ability to elicit strong immune responses, facilitate rapid development, and support scalable manufacturing. This review provides a comprehensive update on the progress of DNA and mRNA vaccine development against mosquito-borne flaviviruses, detailing early efforts and current strategies that have produced candidates with remarkable protective efficacy and strong immunogenicity in preclinical models. Furthermore, we explore future directions for advancing nucleic acid vaccine candidates, which hold transformative potential for enhancing global public health.
Mosquito-borne flaviviruses are a collection of emerging infectious pathogens that constitute huge threats to human health globally (1). These viruses have, in recent times, caused an unprecedented increase in disease outbreaks and a dramatic expansion in their geographic distribution owing to urbanization, climate changes, international trade, and other factors that favor their continuous emergence (2, 3). Annually, not less than 400 million human infections, with several million mortalities, are recorded globally (4). Apart from their direct impact on human health, outbreaks of mosquito-borne flaviviral infections are always associated with disproportionate socio-economic imbalances, especially in developing countries, which are already confronted with other public health-related challenges (2). The most important mosquito-borne flaviviruses include dengue virus (DENV), West Nile virus (WNV), yellow fever virus (YFV), Japanese encephalitis virus (JEV), and Zika virus (ZIKV), which are all single-stranded, positive-sense enveloped RNA viruses that belong to the genus Orthoflavivirus in the Flaviviridae family. Their genome is approximately 10-11 kb in length and is made up of a single open reading frame that is translated into a polyprotein processed by cellular and viral proteases into 10 mature proteins (5). Three of these proteins are structural, namely, capsid (C), pre-membrane (PrM) or membrane and envelope (E), and form the core of the viral particles, while the other seven nonstructural proteins including NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5 are crucial to virus replication following infection in a susceptible host (6).
Mosquito-borne flaviviruses are transmitted by various species of mosquitoes. Aedes aegypti and Ae. albopictus mosquitoes, which are widely distributed across the tropical and subtropical areas, are responsible for the transmission of YFV, DENV, and ZIKV to humans. On the other hand, the dispersal of WNV and JEV is facilitated by Culex species of mosquitoes (7). Generally, Flaviviruses are maintained in an enzootic cycle between mosquitoes and mammals/avians, which act as amplifying hosts (8). Flavivirus infection in mosquitoes occurs when the mosquito ingests a blood meal containing the virus, which replicates in the insect’s midgut epithelial cells and, subsequently, the salivary gland (9), leading to the secretion of the virus in the mosquito’s saliva. Infection of a new host then occurs following mosquito bite which introduces the virus into the host, causing viraemia. While pigs and water birds function as amplifying hosts for specific flaviviruses, humans typically assume the role of dead-end hosts. This is attributed to their general inability to generate adequate viremia for the infection of other hosts, with the exception of instances involving immunocompromised individuals (10). Nevertheless, humans may serve as amplifying hosts for DENV, ZIK and YFV. Transmission may also take place through blood transfusion, sexual contact, and transplacental transmission. This transmission mode can result in abnormal gestational development in the fetus, particularly in the case of ZIKV, (11) and to a certain extent WNV.
Despite the huge public health burden of mosquito-borne flaviviruses globally, to date, no specific drugs are available to treat most of the illnesses caused by those viruses (11, 12). Consequently, vaccines stand to be the most effective countermeasures against the Flaviviruses. At present, no vaccine has been approved for the control of ZIKV and WNV (13). Noteworthy, a few vaccines have been approved to control Yellow fever, Japanese encephalitis and Dengue fever (2, 13). However, these vaccines are mainly conventional live attenuated vaccines or inactivated whole virus vaccines with serious shortcomings that significantly limit their clinical usefulness. For instance, the Dengvaxia (CYD-TDV), a tetravalent chimeric live attenuated vaccine produced by engineering yellow fever virus vaccine strain to vector the PrM and E structural proteins (PrM/E) of DENV from serotypes 1−4 (14, 15), has been shown to significantly increase the risk of cytoplasmic leakage syndrome, especially among those with no prior exposure to DENV (3, 16). As a result, the World Health Organization has discouraged the use of this vaccine in areas where Dengue fever endemicity is low (17). Similarly, 17DD, 17D-213 and 17D204 substrains are live attenuated Yellow fever vaccines developed through extensive cell culture attenuation and large-scale production in embryonated eggs (18). Although they have track records of safety and effectiveness, there are concerns that they could result in some rare side effects, such as neurologic or viscerotropic syndromes or anaphylaxis, particularly in infants, pregnant women, and immunocompromised individuals (19). More so, the development of these conventional vaccines is considerably slow and limited by the requirement of BSL-3 to produce large quantities of the vaccine virus. Thus, in order to address the expanding threats of these and several other newly emerging mosquito-borne flaviviruses, there is an urgent need for novel vaccines with remarkable effectiveness and improved safety.
With the increasing pace of genomic sequencing, reverse vaccinology approaches have evolved to rapidly identify potential protective antigens, thereby accelerating vaccine development against any pathogen at a much-reduced cost compared to the conventional approach (20). Indeed COVID-19 pandemic has shown us that emerging technologies could be used to fast track vaccine development against new public health threats in our modern society (21, 22). Some of the next-generation technologies applied to develop vaccines against mosquito-borne viruses include virus vectors, virus-like particles, engineered peptide subunits, and nucleic acid-based platforms (23). Among these platforms, nucleic acid vaccines (DNA and mRNA) stand out as the most advanced fighters against mosquito-borne flaviviruses, with several of them already advancing to various phases of clinical trials.
Although some excellent reviews on nucleic acid-based vaccines targeting specific mosquito-borne flaviviruses, DENV and ZIKV, have recently been provided (24), comprehensive overviews covering all five major mosquito-borne flaviviruses, including JEV, YFV, and WNV, are lacking scanty in the existing literature. Furthermore, available literature on modern vaccine platforms for these viruses (12, 13) often does not extensively emphasize the progress, challenges, and strategies for improving the future prospects of DNA and mRNA-based vaccines against mosquito-borne flaviviruses. Scientific challenges such as enhancing vaccine immunogenicity, ensuring broad coverage against different strains, and dealing with the complex molecular biology of flavivirus replication have further slowed progress (25, 26). Thus, there is a need for a dedicated review that focuses on the development of nucleic acid-based vaccines against all these significant pathogens, providing a detailed exploration of the past achievements, current advancements, and future directions. Our review aims to fill this gap by providing a comprehensive update on the progress and challenges associated with nucleic acid vaccine development against these rapidly emerging viruses. Additionally, we will discuss future directions for advancing these vaccines to better address global public health challenges.
Nucleic acid vaccines utilize the genetic material of pathogens to selectively elicit a strong immune response against the respective pathogen. These vaccines are engineered with complete instructions for producing protein antigens specific to the pathogen. Upon administration, the body’s protein-synthesizing machinery manufactures the encoded antigen, initiating an immune response. Nucleic acid vaccines may be based on either DNA or mRNA (27).
The initial demonstration that direct in vivo gene delivery could trigger an immune response was by Tang et al. (28), showing an antibody response after delivering the human growth gene into mice skin using a biolistic device. Around the same time, plasmid vaccines encoding Influenza A nucleoproteins were found to elicit protective immune responses in mice, highlighting the potential of DNA vaccines (29). DNA vaccines, capable of inducing cytotoxic T cell responses, became a promising platform due to their safety, stability, and ease of manufacturing. They were developed for various infectious diseases (30, 31) and cancer (32). Plasmid DNA vaccines include key elements for efficient antigen expression, such as viral promoters (commonly the CMV promoter), the Kozak sequence for translation, and codon optimization for host-specific protein expression (33) (Figure 1A). Additionally, polyadenylation signals, such as the SV40 signal, enhance transgene expression. DNA vaccines can be administered through various routes like intramuscular or mucosal, where the plasmid DNA is internalized by cells and triggers immune responses via MHC pathways (34). However, despite their relative stability and ease of production, DNA vaccines generally exhibit lower immunogenicity compared to mRNA vaccines, which may necessitate multiple booster doses or the use of adjuvants to improve efficacy. The reduced immunogenicity of DNA vaccines is primarily due to their reliance on nuclear entry for transcription, an inherently inefficient process. In contrast, mRNA functions directly in the cytoplasm, enabling faster and more robust antigen expression. Safety concerns with DNA vaccines, such as the potential for genomic integration leading to mutations and the risk of inducing anti-DNA autoimmunity, further limit their widespread use (33). Consequently, only a few DNA vaccines have been approved for human use. These issues and strategies for improvement are discussed in detail in recent reviews (35–37).
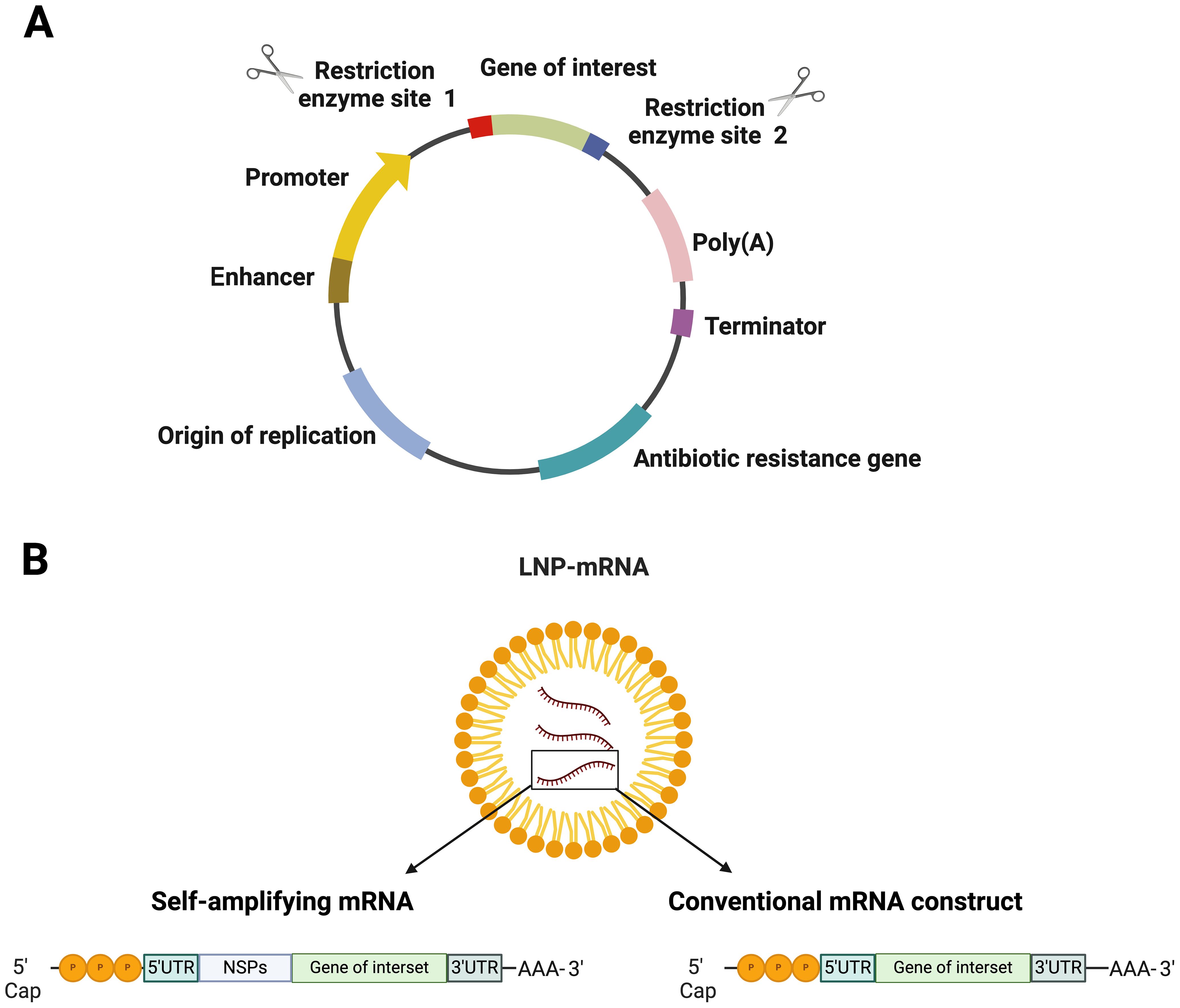
Figure 1. Structural elements in nucleic acid-based vaccines (A) DNA vaccine and (B) various types of mRNA vaccines encapsulated in lipid nanoparticle (LNP). Figures were created using BioRender (https://app.biorender.com).
For mRNA vaccines, research dates back decades, with Wolff et al. (38) for the first time demonstrating in vivo protein expression in mice. This was followed by another fantastic study, which showed that diabetes insipidus could be reversed by intrahypothalamic injection of vasopressin mRNA in rats (39) (Figure 1B). However, mRNA technology faced challenges due to its instability and degradation before reaching target cells. Recent advancements in nanobiotechnology, particularly lipid nanoparticles (LNPs), have revolutionized mRNA vaccine delivery, allowing for robust immune responses and making mRNA vaccines critical in combating the COVID-19 pandemic (40). There are two major types of mRNA vaccines: conventional mRNA and self-replicating mRNA. Self-replicating vaccines include viral replication machinery, enabling larger antigen expression at lower doses (41). In general, the synthesis of mRNA vaccines involves the transcription of plasmid DNA templates, followed by purification to remove impurities that could cause immune reactions (42). Delivery remains a significant challenge due to mRNA’s susceptibility to degradation (43). LNPs have emerged as the leading delivery system (44), but innovations are ongoing to enhance endosomal escape and cellular uptake. Once successfully delivered into the cytoplasm of antigen-presenting cells, the mRNA construct is acted upon by the host’s translational machinery to produce the encoded protein. This process is swiftly followed by intracellular antigen processing, which culminates in the maturation of antigen-presenting cells. The matured antigen-presenting cells are characterized by the expression of co-stimulatory molecules, a response triggered by IFN-1 induction (45). Subsequently, these mature antigen-presenting cells migrate to the nearby lymph nodes. Here, they engage in close interactions with CD4+ and CD8+ cells, initiating the activation of humoral and cell-mediated immune responses through relevant major histocompatibility complex pathways. This intricate process has been extensively reviewed elsewhere (46, 47) (Figure 2).
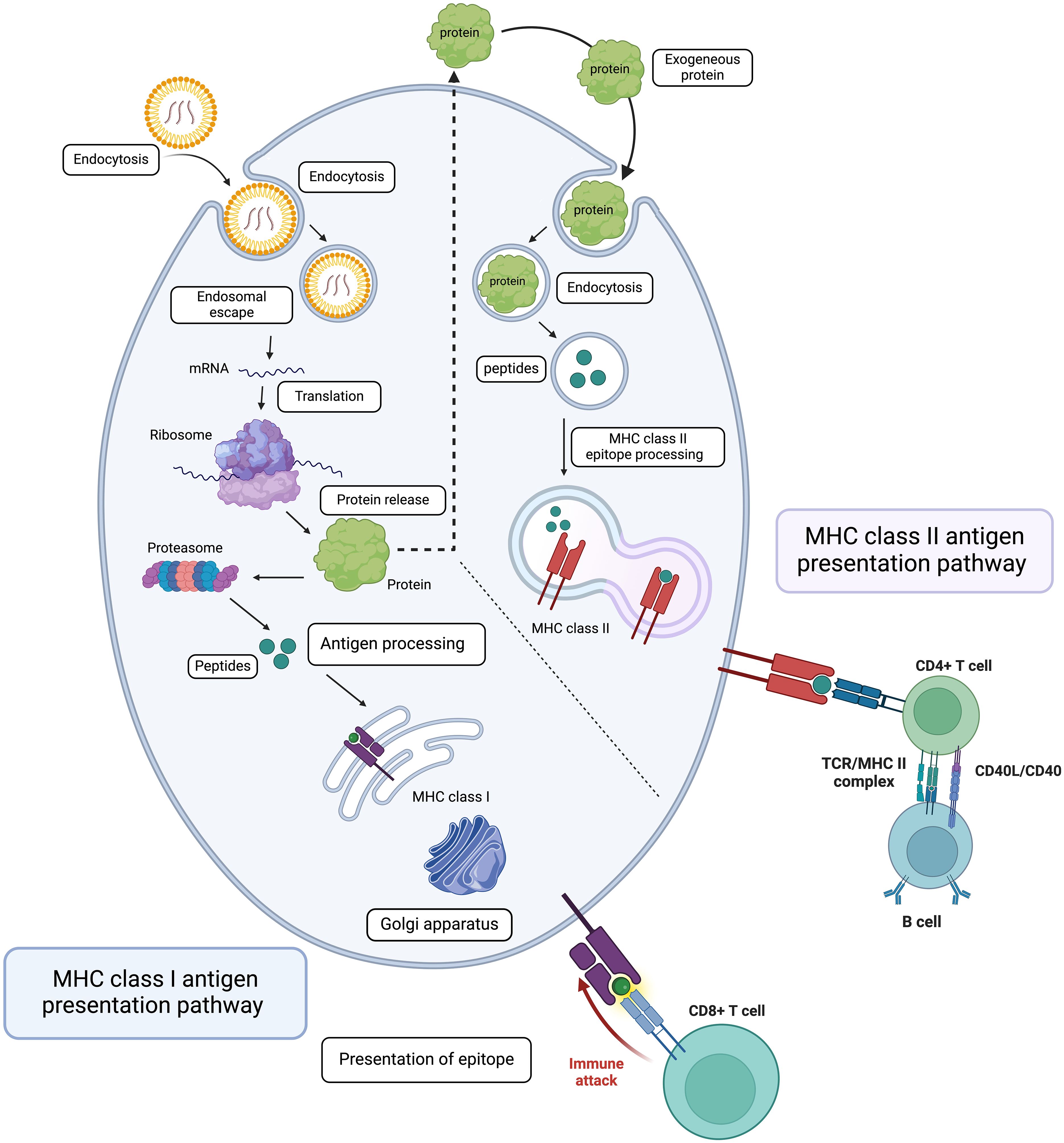
Figure 2. Mechanism of Action of mRNA Vaccines. This diagram illustrates the intracellular processes involved in mRNA vaccine function, starting with the transfection of mRNA into the cell and its endocytosis. The mRNA is then translated into protein by the ribosome. The synthesized proteins are processed and presented via MHC class I and II pathways, leading to the activation of the adaptive immune response. This includes the stimulation of naive CD8+ and CD4+ T lymphocytes, B lymphocytes, and the production of plasma cells, culminating in a targeted immune attack against infected cells. Figures were created using BioRender (https://app.biorender.com).
It is however noteworthy that, although they are more immunogenic than DNA vaccines, mRNA vaccines are more sensitive to degradation, posing challenges in distribution, especially in regions with limited cold-chain infrastructure. While recent advancements in LNPs and nanotechnology have largely addressed these issues, their higher production complexity and cost may limit accessibility in low- and middle-income countries where mosquito-borne flaviviruses are endemic (48). The main safety concerns with mRNA vaccines include potential inflammatory reactions due to immune recognition of the mRNA itself and the risk of unintended immune responses to impurities in the vaccine. However, mRNA does not integrate into the host genome, reducing long-term risks (49).
The Flaviviral genome is organized into a single open reading frame (ORF) flanked by 5’ and 3’ untranslated regions (UTRs). The ORF encodes a single polyprotein, which is cleaved by viral and host proteases into three structural proteins—capsid (C), premembrane/membrane (prM/M), and envelope (E)—and seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, NS5). The structural proteins are essential for viral particle assembly and entry into host cells, while the nonstructural proteins are involved in viral replication, immune evasion, and replication complex assembly (12) (Figure 3A).
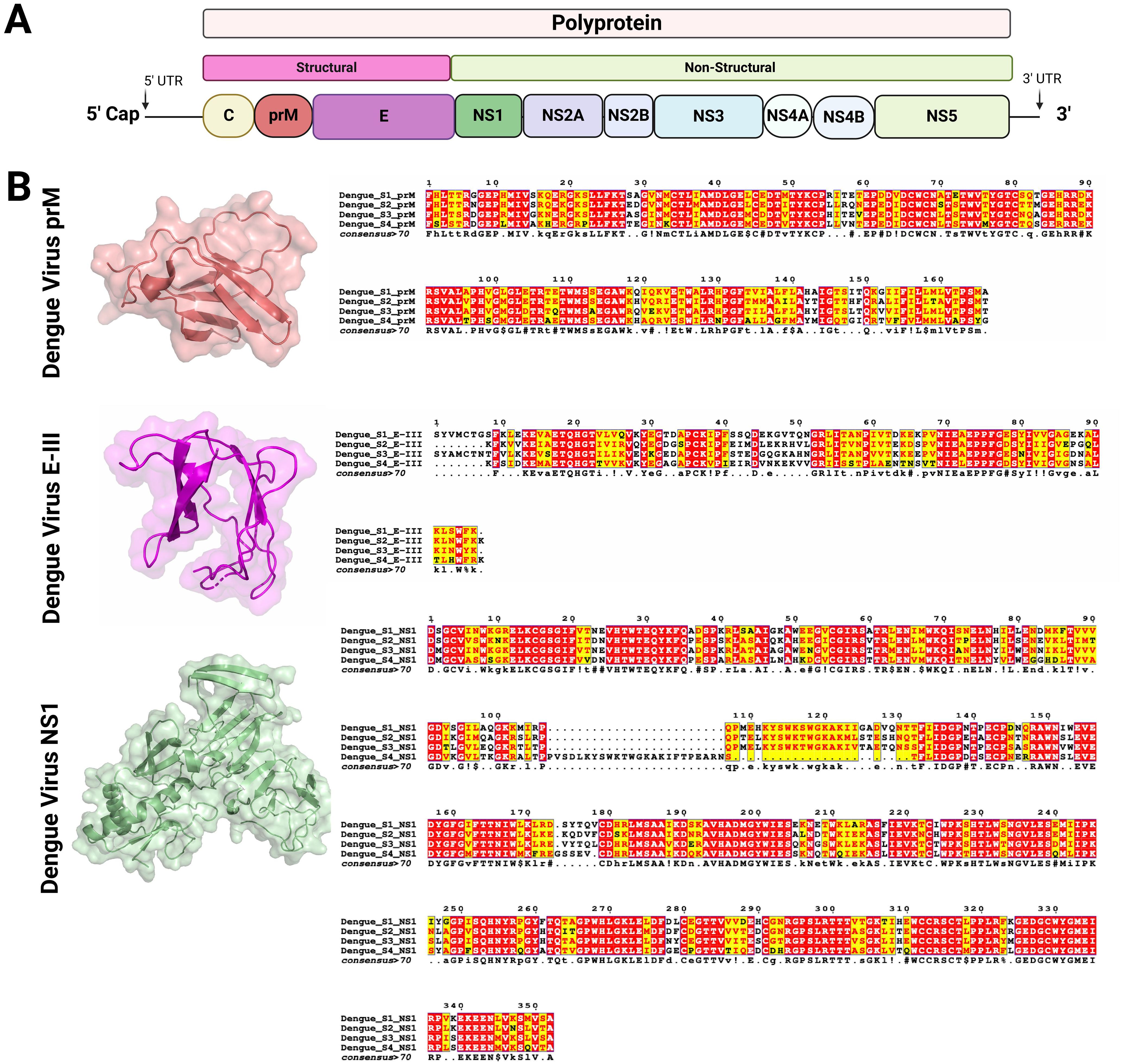
Figure 3. Genome organization of Flavivirus and structure of the key viral proteins used as vaccine antigens (A) Schematic representation of the Flavivirus genome (10-11Kb). The genome encodes a single polyprotein which is post-transcriptionally processed into three structural and seven non-structural proteins. (B) Structural models and sequence alignments were generated for prM, E-III, and NS1 proteins, which are used as targets for vaccine development against DENV and other mosquito-borne flaviviruses. The prM and E-III protein structures were modeled using PDB entry 8fe3 (51), while the NS1 protein structure was based on PDB entry 6weq (52). The sequence alignment highlights conserved residues across different DENV serotypes, with color coding used to indicate the level of conservation (53, 54). Figures were created using BioRender (https://app.biorender.com).
In the development of novel vaccines against flaviviruses, the most commonly targeted proteins are the E, prM, and NS1 proteins (50). Although these proteins are relatively conserved across flaviviruses, their amino acid composition can vary among different viruses and even among serotypes of a single virus, such as DENV (Figure 3B). The E protein is crucial for viral entry into host cells and serves as the primary target for neutralizing antibodies. Structurally, the E glycoprotein consists of three domains (DI, DII, DIII), each with distinct functions (55). Domain II contains a fusion loop that facilitates membrane fusion, allowing viral RNA to enter the host cell cytoplasm. Domain III (EIII), in particular, plays a key role in receptor binding and is frequently targeted in vaccine designs to elicit neutralizing antibodies. Targeting EIII reduces the risk of antibody-dependent enhancement (ADE), a concern with dengue vaccines (56).
The prM protein acts as a chaperone during viral assembly, protecting the E protein from premature fusion before the virus matures. It ensures proper folding of the E protein, and upon maturation, prM is cleaved into the mature membrane (M) protein, making the virion infectious (57). Although prM is generally less immunogenic than the E protein, it still contributes to immune responses by promoting overall viral stability and assisting in the formation of neutralizing antibodies against the E protein. However, Antibodies generated against prM have consistently been shown to trigger ADE (58). This presents a significant challenge in the development of vaccines for dengue and other flavivirus infections, requiring careful evaluation of prM’s inclusion in vaccine formulations. While prM may play a supportive role in immune responses, its critical contribution to ADE makes it a less favorable candidate as a vaccine antigen. Despite its common use in flavivirus vaccine development efforts, we believe prM’s involvement should be reconsidered to minimize ADE-related risks. Ongoing research seeks to clarify prM’s interactions with the immune system to refine its application in vaccine design.
NS1 is a multifunctional nonstructural protein involved in viral replication and immune evasion. It exists in intracellular, membrane-associated, and secreted forms, influencing viral replication and modulating the host immune response, particularly by inhibiting complement activation (5). While NS1-targeted antibodies do not neutralize the virus directly, they can aid in eliminating infected cells and limiting viral spread. Importantly, NS1-based vaccines are less likely to induce ADE, making them an attractive alternative for dengue vaccines (59). However, NS1’s role in dengue pathogenesis must not be underestimated. First, anti NS-1 antibodies may recognize and bind to epitopes on human endothelial cells, contributing to disease pathogenesis (60). Secondly, NS-1 protein is overexpressed in severe dengue infections, functioning as a pathogen-associated molecular pattern that activates Toll-like receptor-4 on peripheral blood mononuclear cells. This activation leads to the production of pro-inflammatory cytokines and contributes to vascular leakage (61). Furthermore, anti-NS1 antibody titers are significantly higher in patients with severe dengue compared to those with mild forms of the disease. The anti-NS1 antibody repertoire also differs between severe and non-severe dengue cases, suggesting a link between specific NS1 epitopes and disease severity (62). Given these complexities, while whole NS1 is frequently used in newer vaccine designs, future strategies should prioritize targeting NS1 regions that elicit protective rather than pathogenic antibody responses to enhance vaccine safety and efficacy.
The first DNA immunization against DENV was by Kochel et al. (63), showing that plasmids encoding truncated E and PrM proteins induced neutralizing antibodies in mice. Interestingly, adding CpG molecules was shown to boost this immunologic response (64). In non-human primates, full-length E and PrM plasmids led to higher antibody titers when administered intradermally (65). Studies also showed stronger immune responses with full-length E and PrM plasmids (66, 67). Moderate protective efficacy was also observed against Denv type-3 following immunization of Aotus monkeys with a plasmid DNA expressing PrM and E protein (68) even though up to 80% survival rate was observed in another study (69). Intriguingly, enhanced immunogenicity and protective efficacy were noted when a plasmid DNA expressing DENV-2 PrM/E was administered in mice via electroporation (70). The same observation was made when a bivalent vaccine consisting of pVAX1-D1ME and pVAX1-D2ME was used to immunize BalbC mice (71).
Simmons et al. (72) explored DENV DNA vaccine immunogenicity through a prime-boost strategy, using a DNA vaccine followed by protein boosters or a combination of both, showing slightly better neutralizing antibodies. Further studies (73–75) confirmed enhanced protection using DNA priming and protein/virus-vector boosting (76) also found increased antibody titers with combined DNA and protein vaccines. Adding lysosome-associated membrane protein sequences or co-administering DNA vaccines with GM-CSF improved immune responses (77, 78). Enhanced efficacy was also noted with plasmids expressing NS1 and tPA (74), though tPA didn’t always boost immunity (79). Co-administration of E and NS1 plasmids showed strong neutralizing immunity (80).
A tetravalent DNA vaccine based on domain III of the E protein or conserved epitopes from all four DENV serotypes induced long-term neutralizing immunity (81, 82) and provided strong protection against DENV type-2 (83, 84). Immunization with a mix of four plasmids expressing PrM/E genes from each serotype led to robust antibody responses post-challenge (85). Electroporation and prime-boost strategies further enhanced tetravalent immunity (86). A PrM/E tetravalent vaccine neutralized all serotypes but offered partial protection upon challenge (87). Synthetic consensus domain III of the E protein also induced strong immunity (88, 89). Combining DNA vaccines with inactivated or protein-based vaccines boosted immunogenicity (90), and priming with DNA followed by a live attenuated DENV vaccine resulted in anamnestic immune responses (Monika 91).
A DENV DNA vaccine combining PrM/E and NS-1 induced strong, long-lasting protection in mice (92), though its suitability in addressing poor immunogenicity remains uncertain. Co-expressing E and NS-1 in a bicistronic vector produced anti-E antibodies but failed to elicit NS-1 antibodies (93). Immunogenicity was lower in a PrM/E DNA vaccine compared to an inactivated DENV-4 vaccine, though 80% of mice were protected (94). Novel adjuvants have shown promise, with a lipid-based adjuvant (Vaxfectin) significantly boosting immune responses in preclinical trials (95). Higher protection was also noted when using NS1-loaded PLGA/PEG microspheres in mice (96). Co-expressing PrM/E, NS-1, and GM-CSF improved neutralizing antibodies and protection against DENV-2 (97), though caution is advised with GM-CSF due to immune suppression risks (98). A chimeric DNA vaccine substituting JEV PrM/E with DENV EDIII also generated high neutralizing antibodies and reduced infection enhancement (99).
Multi-epitope DNA vaccine that encodes conserved immunogenic HLA-restricted cytotoxic T cells epitopes derived from DENV serotype 1 was found to induce strong immune response, suggesting the possibility of using this approach in developing universal DENV vaccine (100). Similarly, Hou et al. (101) reported that that mosaic vaccines comprising of DENV serotype 1 and 2 variant epitopes could stimulate strong and broad immune responses against all four serotypes. Indeed, the strategy of fusing the consensus EDIII for each serotype with a single NSI derived one of the serotypes was proven to be efficacious in eliciting significant protective immunity in preclinical models (56). Other approaches involved the use Plasmids encoding the scFv αDEC205, or an isotype control (scFv ISO), fused to the DENV2 envelope protein domain III (EDIII) to induce neutralizing immune response (102). Furthermore, an innovative approach involving the use of adeno associated vectors to enhance the delivery of DENV DNA vaccine has recently been described (103).
Following the widespread ZIKV outbreaks from 2015 to 2016, Larocca et al. (104) and Dowd et al. (105) reported the development of a recombinant plasmid DNA expressing Zika pre-membrane and envelope proteins. A single administration of this vaccine provided full protection in susceptible mice when challenged with a strain of ZIKV linked to the outbreak in northeast Brazil. Same observation was made in non-human primates (106). Noteworthy, the observed protective efficacy predominantly relied on neutralizing antibody titers, since passive protection was conferred through the adoptive transfer of purified IgG from vaccinated mice and that depletion of CD4 and CD8 T lymphocytes in vaccinated mice did not diminish this protective efficacy. In another study, immunization of IFNAR-/- mice with a novel synthetic ZIKV DNA vaccine expressing PrM/E via electroporation elicited antigen-specific cellular and humoral immunity, along with neutralizing activity. This led to 100% protection of IFNAR-/- mice against infection-induced brain pathology following in vivo viral challenge. Moreover, passive transfer of non-human primate anti-ZIKV immune serum protected IFNAR-/- mice against subsequent viral challenge, further emphasizing the significance of immune responses targeting PrM/E in ZIKV infection (107). In addition, Yi et al. (108) showed that a DNA vaccine encoding the complete ZIKV PrM/E provides robust protection against ZIKV infection in humanized mice. Post-vaccination, the humanized DRAG mice demonstrated seroconversion, producing antibodies targeting ZIKV, as indicated by ELISA and neutralization assays. Indeed subsequent ZIKV challenge revealed markedly reduced viral loads in both human cells and serum of vaccinated animals compared to unvaccinated controls, highlighting the vaccine’s potent antiviral efficacy (108).
Maternal administration of a DNA vaccine candidate has been shown to confer specific protection against post-natal ZIKV infection in immunocompetent BALB/c mice (109). DNA immunization strategy may also offer the potential to deliver highly potent monoclonal antibodies against Zika virus for infection control in non-human primates (110). It has also been shown to reverse ZIKV-induced infertility (111) and improve negative fetal outcomes among ZIKV exposed pregnant macaques (112). Additionally, a heterologous prime-boost vaccination approach, comprising priming with a recombinant DNA vaccine followed by boosting with non-replicating vaccinia virus-based vaccines, shows promise in combatting ZIKV infection (113). In another strategy, IL-36 gamma has been shown to demonstrate excellent adjuvant properties, enhancing the protective efficacy of Zika DNA vaccine following lethal viral challenge in mouse model (114). Surprisingly, the DNA vaccine encoding the Zika EDIII domain could not provide protection against lethal viral challenge (115); despite eliciting a anti E protein antibody mediated response in the vaccinated hosts (116). This contrasts with what is known for other flaviviruses. While this result may signify the unsuitability of EDIII as a vaccine candidate against ZIKV, it is possible that the immunogenic potential of this protein may increase when used in combination with NS-1.
DNA vaccine encoding NS-1 alone was shown to elicit protective immune response in the form of reduced viremia and viral burden in tissues upon ZIKV challenge (117). Indeed, when fused to Herpes Simplex Virus (HSV) glycoprotein D (gD) protein, enhanced immunogenicity and protective efficacy were observed, further demonstrating the relevance of NS-1 protein as a Zik vaccine antigen (118). It is however crucial to highlight that prior exposure to other flaviviruses, whether through vaccination or infection, could influence the effectiveness of DNA immunization against ZIKV. For example, individuals who received the ZIK DNA vaccine (VRC5283), a plasmid encoding ZIK PrM and E, without prior exposure to flaviviruses or vaccination demonstrated limited binding ability towards various viruses in their antibody response. Conversely, those with previous flavivirus exposure displayed varying binding capability of vaccine-induced antibodies, albeit without neutralization of distantly related flaviviruses being observed (119).
Lin et al. (120) demonstrated that plasmid DNA expressing JEV NS1 induces protective immunity in mice as effectively as constructs expressing PrM/E, for the first time suggesting genetic immunization as a strategy for JE infection. Although JEV E protein is key to protective immunity (121, 122), single vaccination with plasmid DNA encoding PrM/E induces low initial antibody titers, which significantly increase after viral challenge, highlighting the role of anamnestic response (123). Additionally, immunity to JEV PrM/E is long-lasting due to virus-specific memory B cells (124).
To improve the immunogenicity of a DNA vaccine expressing JEV NS-1, Chen et al. (125) co-administered it with another plasmid encoding heat shock protein 70. This approach significantly enhanced T cell proliferation and cytotoxicity, although it did not boost humoral responses. Similarly, Ashok and Rangarajan (126) showed protection against JEV challenge despite undetectable antibody titers, emphasizing the role of cell-mediated immunity (127). Other strategies include using colloidal gold particles with plasmid DNA to elicit a strong immune response (128), gene-gun delivery of chitosan-based vaccines (129), and microcapsule-encapsulated DNA vaccines, which improved Th1-Th2 responses. These findings reflect the need for integrating multiple strategies, rather than relying on individual approaches, to dramatically improve the immunogenicity of JEV DNA vaccines by effectively addressing both cellular and humoral immune responses.
A combined approach of plasmid DNA and protein-based JEV vaccines in mice led to a synergistic boost in neutralizing immune responses (130). Gene gun-assisted immunization also enhanced JEV DNA vaccine efficacy (131, 132), and the use of the Vaxfectin adjuvant further improved immunogenicity in both mice and human trials, where it was well tolerated (130, 133). A prime-boost strategy using plasmid DNA priming followed by protein-based boosting proved effective in inducing protective immunity (134, 135). Co-immunization with IL-15 also improved antibody-mediated immunity (89). Multiple strategies, such as co-administering PrM/E and GM-CSF, significantly enhanced protection against lethal JEV challenge (136, 137). However, certain cytokines could negatively impact JEV-specific responses, requiring careful evaluation of their use as adjuvants (138).
Imoto et al. (139) reported the induction of robust neutralizing antibody mediated immunity that protected against fetal mummification in pregnant sows following needle free immunization with a mixture of JEV plasmid DNA and inactivated vaccines. In another studies, immunization with the JEV DNA vaccine construct containing murine ICAM-1 gene (pICAM-1) resulted in a notable increase in the percentage of CD4(+) and T cells, high level of JEV-specific cytotoxic T lymphocyte response, and high production of T helper 1 (Th1)-type cytokines in splenic T cells (140). Thus, the strategies for improving the immunogenicity of JEV DNA vaccines are numerous, involving the rational manipulation of the vaccine antigen, use of novel adjuvants and optimal use of vaccine delivery systems (Figure 4).
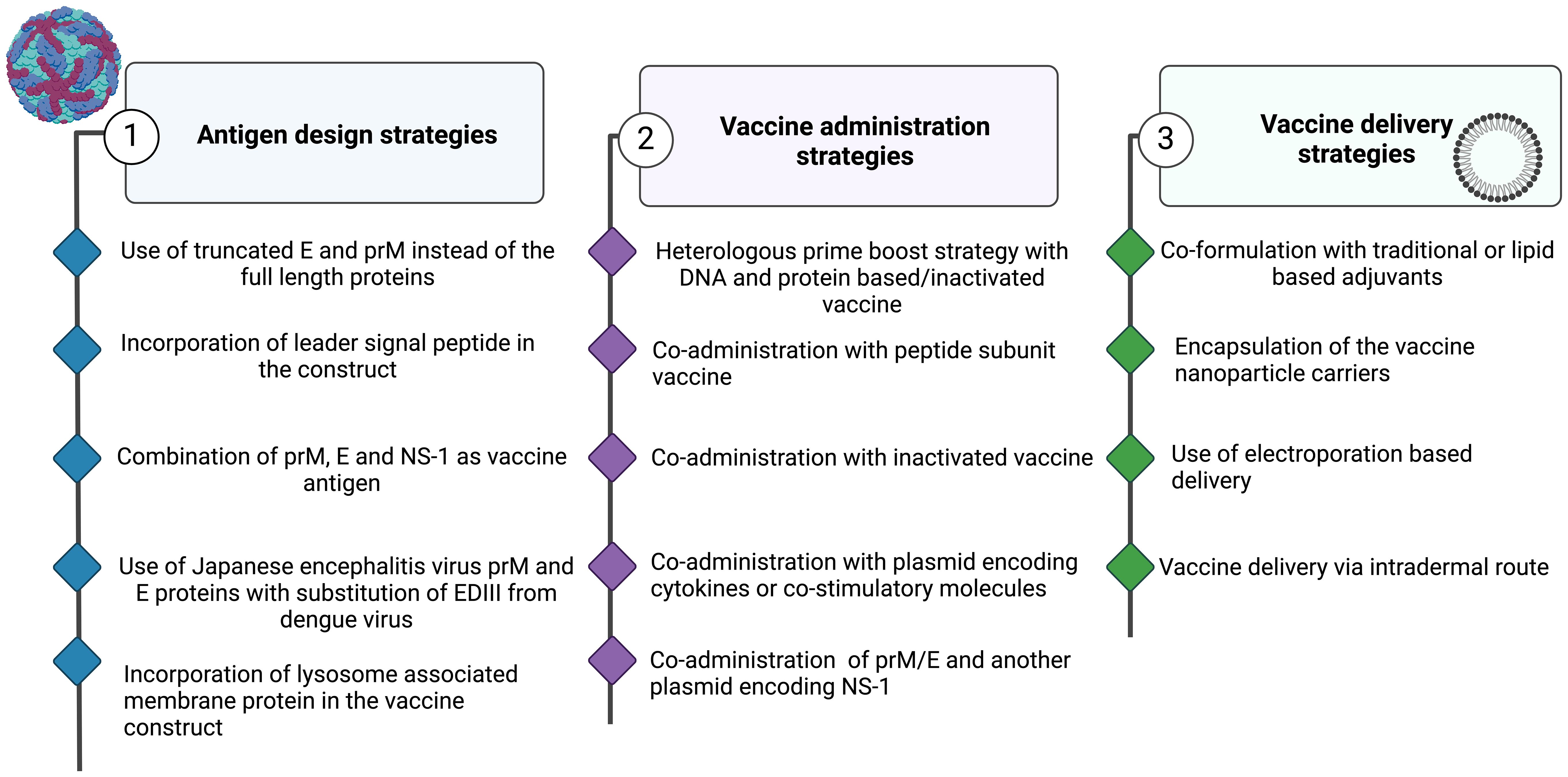
Figure 4. Strategies to Enhance the Immunogenicity of Nucleic Acid-Based Vaccines. Approaches Involving (1) Antigen Design, (2) Vaccine Administration, and (3) Vaccine Delivery. Figures were created using BioRender (https://app.biorender.com).
Although DNA vaccine has been developed against a variety of pathogens more than 3 decades ago, it is only recently that platform was utilized for YFV control. This might not be unconnected with the long-standing success of the existing 17D YFV vaccines. The iDNA vaccine technology represents a newly emerged vaccine strategy that combines the safety of DNA vaccine and the efficacy of live attenuated vaccines. In this approach, the entire genomic RNA of the virus is placed under the control of a eukaryotic promoter, allowing intact viral RNA to be transcribed in vivo following vaccination. This enables limited rounds of virus replication, as demonstrated by (141) in their study on a novel vaccine against Venezuelan equine encephalitis virus. Using this technology, Tretyakova et al. (142) showed that immunization of BalbC mice with a plasmid DNA containing the full-length 17D YFV cDNA under the transcriptional control of cytomegalovirus promoter induced robust seroconversion and virus-specific neutralizing antibodies. It was also shown to be safe in AG129 mice, genetically engineered mice that lack IFN α/β/γ receptors.
In another study, two DNA vaccine candidates encoding the full-length YFV envelope protein or the full-length YFV envelope protein fused to the lysosomal-associated membrane protein signal, LAMP-1 were reported (143). Findings revealed that vaccine constructs elicited robust T cell responses and neutralizing antibodies in C57Bl/6 and BALB/c mice. In particular, the construct with the LAMP-1 signals for enhanced MHC class II presentation generated higher interferon-gamma (IFN-γ) spot-forming cells and stronger epitope-specific responses. Furthermore, the vaccine candidates provided 100% protection against intracerebral YFV challenge in mouse models (143). This indicates that the candidates are promising and may be considered for further development in clinical studies.
Several DNA vaccines have been explored for preventing WNV infection. In 2001, a DNA vaccine expressing WNV PrM proteins and E glycoprotein was developed, which provided 100% protection against WNV in mice and elicited strong neutralizing antibody responses in horses (144). A similar vaccine encoding the E glycoprotein ectodomain protected mice from WNV with a single dose, and subsequent injections of recombinant E domain DIII further boosted immunity (145). A heterologous prime-boost strategy combining DNA and inactivated vaccines led to higher neutralizing antibody levels (146). Another study using DNA priming followed by a protein boost with WNV E glycoprotein significantly improved neutralizing antibody titers and protected mice from lethal WNV challenge, although DNA alone failed to generate a measurable immune response (147).
A limited number of DNA vaccines targeting proteins other than the WNV E glycoprotein have been developed. One such vaccine focused on the C protein of the NY99 WNV strain, demonstrating strong antigen-specific humoral, Th1, and cytotoxic T-cell responses in mice (148). Hall et al. developed a DNA vaccine encoding full-length cDNA of Kunjin virus, a closely related virus to WNV, with mutation in the NS1 protein (Pro-250 to Leu). This vaccine, containing an attenuated Kunjin virus, protected mice from WNV NY99 and Kunjin viral challenges (149). Another group created a replication-defective Kunjin-based DNA vaccine that generated single-cycle viral replication, eliciting strong immune responses and virus-neutralizing antibodies in horses (150). To improve vaccine yield, capsid protein expression was enhanced by using different forms of the C protein and a stronger promoter (151).
Two DNA vaccines for WNV have undergone clinical trials. The first, VRC 302, was developed based on a licensed WNV DNA vaccine for horses and targeted the PrM and E glycoproteins of the NY99 strain. In a phase I trial (NCT00106769), it was shown to be safe, immunogenic, and capable of producing neutralizing antibodies, similar to those protective in horses (152). The second vaccine, VRC 303, an improved version of VRC 302, used a modified promoter (CMV/R) to enhance protein expression. In a phase I trial (NCT00300417), it induced strong neutralizing antibody and T-cell responses in both younger and older adults, with better T-cell responses than VRC 302 (153).
Several factors may have contributed to the lack of approved WNV vaccines despite extensive clinical trials. The unpredictable nature of outbreaks makes it challenging to design efficacy studies, as selecting appropriate geographic areas and obtaining ethical approval before outbreaks occur is logistically complex. Additionally, the disease primarily affects a subset of the population, such as individuals aged 50 or older or those with certain underlying conditions, leading to low case counts and prolonged trial enrollment (154). The high costs of vaccination programs, as indicated by cost-effectiveness models, also pose a significant barrier to large-scale implementation. To address these issues, an age- and incidence-based vaccine program could improve cost-effectiveness and reduce disease burden. Furthermore, by targeting areas with sustained high incidence, vaccination programs could ensure more consistent demand.
Unlike the DENV DNA vaccine candidates widely found in the literature, only a few mRNA vaccines are being developed against DENV. Roth et al. (59) were the pioneers in reporting the utilization of the mRNA vaccine platform against DENV infection. Their method involved the use of viral non-structural proteins, known for their conservation across all viral serotypes or their ability to induce a cross-reactive inter-serotype cell-mediated immune response. Consequently, the immunogen in their vaccine comprised a consensus 540-amino acid long multiepitope string derived from DENV-1 NS3, NS4b, and NS4. Interestingly, employing a prime-boost vaccination strategy with this modified mRNA at a low dosage, administered to human HLA class I transgenic mice, not only resulted in a robust T cell immune response but also provided significant protection against DENV-1 infection, especially when combined with a temporary inhibition of the IFN type I receptor (59). However, while the study has yielded promising data regarding protective efficacy against homologous viral challenge with DENV-1, it remains to be determined whether the immune response elicited by this vaccine is cross protective against all the DENV serotypes.
An alternative approach in the field involves formulating an mRNA vaccine that integrates PrM/E and E80—in addition to NS1 derived from DENV-2 (155). This strategy demonstrates the efficacy of E80-mRNA, whether administered independently or in conjunction with NS1-mRNA to serve as an excellent vaccine antigen. Indeed, this particular combination has been observed to trigger a robust immune response characterized by the production of potent neutralizing antibodies and T cell responses. Critically, these formulated vaccines exhibit a high level of efficacy, providing full protection against DENV-2 challenge. Notably, the inclusion of E80, either alone or in combination with NS1, has proven particularly effective in eliciting protective immunity against the DENV-2. Interestingly, vaccination with NS1-mRNA alone also stimulates antigen-specific T cell responses and the development of antigen specific antibodies, conferring partial protection against the DENV-2 virus in immunocompetent BALB/c mice (155). This suggests that the vaccine, even when focusing solely on NS1-mRNA, holds promise in conferring immunity against DENV-2 viral challenges.
In another strategy, LNP-mRNA encoding PrM and E proteins were used as a immunogen to target DENV-1 (156). The open reading frame of these fused proteins (PrM and E) was inserted downstream of various signal peptides from either Japanese encephalitis virus, IL-2, tissue plasminogen activator, or Gaucia luciferase, all previously shown to increase transgene expression (157). Mice immunization using constructs with JEV signal peptide led to robust antiviral immune responses, characterized by high levels of neutralizing antibody titers and antiviral CD4+ and CD8+ T cells. Indeed, immunocompromised AG129 mice vaccinated with the PrM/E mRNA-LNP vaccine were protected from a lethal DENV-1 challenge, demonstrating the potential of this strategy to trigger strong protective immunity against DENV as earlier asserted by (158).
As a unique approach for stimulating tetravalent immunity and simultaneously avoiding potential Antibody-Dependent Enhancement (ADE), He et al. (159) constructed a modified mRNA vaccine consisting of conserved EDIII and NS-1 from DENV-2. The vaccine antigens were placed immediately downstream of the tPA signal peptide but upstream of the C-terminal vesicular stomatitis virus G protein transmembrane and cytoplasmic domains. Findings revealed that this strategy stimulates a robust antiviral immune response that not only neutralizes all four DENV serotypes but also significantly reduces ADE (159). This demonstrates that the combination of EDIII and a conserved nonstructural protein is an attractive strategy for inducing a safe and effective immune response against DENVes (Figure 4).
Pardi et al. (160) reported that LNP-encapsulated modified mRNA encoding the ZIKV PrM/E elicited robust immune responses in animal models, with vaccinated mice and non-human primates developing high titers of neutralizing antibodies, resulting in complete protection against ZIKV challenge (161). Richner et al. (162) applied a similar approach, demonstrating that a single low-dose vaccination with a ZIKV mRNA vaccine could induce potent neutralizing antibody responses and confer protection against ZIKV infection in mice, showcasing the vaccine’s efficacy. In addition, Jagger et al. (163) reported that ZIKV mRNA vaccine encoding PrM/E elicited higher levels of antigen-specific long-lived plasma cells and memory B cells, while significantly reducing ADE in mice. Similarly, Medina-Magües et al. (164) reported that ZIKV PrM/E mRNA-LNP vaccine candidate elicited protective antibody responses in AG129 mice lacking interferon (IFN) alpha/beta/gamma receptors. More so, two-dose immunization strategy with this vaccine led to the induction of E-specific double-positive IFN-γ and TNF-α T cells in BALB/c mice. This makes it a potential candidate for further development.
Zhong et al. (165) introduced a novel approach by developing a self-amplifying mRNA (SAM) vaccine encoding ZIKV PrM/E antigens. This approach, which allows for prolonged antigen expression, induced strong immune responses in mice, signifying the potential of SAM vaccines to enhance the immunogenicity of mRNA vaccines against ZIKV. 166) explored another innovative strategy by combining mRNA encoding ZIKV PrM/E with mRNA encoding the conserved non-structural protein NS1 with the aim of eliciting both antibody-mediated and cellular immune responses. Interestingly, mice vaccinated with this combination developed high levels of neutralizing antibodies and strong T cell responses, providing significant protection against the ZIKV challenge. This shows that the inclusion of NS1 was particularly effective in enhancing the overall immunogenicity of the ZIKV mRNA vaccine candidate.
More recently, Shin et al. (167) described the development of a ZIKV mRNA vaccine encoding full-length ZIKV PrM/E proteins using porous silica nanoparticles (PPSNs) as a delivery vehicle. The vaccine was shown to elicit strong immune responses, including high levels of neutralizing antibodies and ZIKV-specific T cell responses. Furthermore, a single injection with the vaccine provided complete protection against the ZIKV challenge in C57BL/6 mice (167). This indicates that the vaccine could be a promising candidate for further clinical development and potential application against ZIKV infection. Taken together, the development of mRNA vaccine candidates against ZIKV has significantly progressed in the last few years, demonstrating promising preclinical results on safety and immunogenicity in mice models. Indeed, some candidates have already advanced to clinical trials where they demonstrate tolerability and effectiveness in healthy flavivirus seropositive and seronegative adults (168). This progress suggests a great future for mRNA vaccines as a cornerstone platform for controlling ZIKV infections. However, it is important to consider the implications of anti-ZIKV antibodies on acute DENV infections. A recent study by (Estofolete et al. (169) indicated that prior Zika infection could be a risk factor for severe dengue disease and hospitalization, though not necessarily through the widely recognized mechanism of ADE.
At present, there is only one study on the development of mRNA vaccine against JEV. The vaccine candidate, similar to other mRNA vaccine candidates for flaviviruses, utilized PrM/E as antigen and was encapsulated in LNP. When used to immunize C57BL/6J mice at the dose of 15µg per mouse and boosted 3 weeks later with same dose of the vaccine, a significant increase in neutralizing antibody titer was observed, with PRNT50 reaching approximately 1:200 in vaccinated mice. More so, a strong proliferation of CD8+, but not CD4+, T cells was observed. Most importantly, a 100% protection was observed following the challenge of vaccinated mice with 1 × 106 PFU JEV P3 strains 3 weeks after booster immunization (170). This indicates that mRNA vaccine is a promising platform for the development of safe and effective JEV vaccines.
Recently, a study by Medina-Magües et al. (171) found that mRNA-based vaccine candidates targeting the Yellow Fever (YF) virus elicited strong immune responses and provided significant protection in a dose dependent manner. Formulated in LNP, the vaccines were designed to express YFV PrM/E and the non-structural protein 1 (NS1). Findings revealed that vaccination with PrM/E mRNA-LNP induced high titers of neutralizing antibodies and robust T cell responses in both mice and non-human primates, leading to protection against experimental YFV challenge. Indeed, vaccinated mice not only showed reduced viral loads and minimal disease symptoms, passive transfer of their serum was able to confer protection in naive mice (171). These findings collectively indicate that mRNA vaccines encoding PrM/E and NS1 antigens have the potential to become effective YF vaccines and overcome the limitations of current vaccines.
The development of recombinant DNA and engineered mRNA vaccines against mosquito-borne flaviviruses has recorded a great deal of progress, yet there remains a considerable journey ahead. While several candidates are currently in clinical trials (Table 1), further research is required to refine various aspects of these vaccines. Future efforts should concentrate on enhancing vaccine design to create more effective and safer candidates by targeting conserved regions of viral proteins, thereby reducing the risk of ADE. In this regard, utilizing a combination of the EDIII domain and various non-structural proteins (not only NS-1) as vaccine antigens could particularly be attractive.
To date, there are excellent candidates targeting single serotypes of DENV with remarkable preclinical efficacy. Consequently, efforts must be directed towards developing more tetravalent DENV vaccines to ensure comprehensive coverage of all circulating strains. Additionally, there is a pressing need for more mRNA vaccine candidates against all mosquito borne flaviviruses. Novel delivery platforms should also be explored to enhance immune responses and reduce dosage requirements. In this context, the development of self-amplifying mRNA vaccines, which have demonstrated enhanced antigen expression at lower doses (41), appears particularly promising. For ZIKV in particular, efforts should be focused on developing safe and effective vaccines, particularly for individuals who are pregnant or may become pregnant and those living in or traveling to Zika-endemic regions. At present, different neutralization methods are used to evaluate ZIKV neutralization titers. There is therefore the need to define universal correlates of protection based on a normalized neutralization assay, for future efficacy studies. Same applies to JEV and WNV where similar challenges in neutralization, antigen design, and correlates of protection must be addressed to improve vaccine efficacy.
It is glaringly evident that nucleic acid-based vaccines represent a promising platform for the control of mosquito-borne flaviviruses. So far, a number of candidates have been reported for DENV and other mosquito-borne flaviviruses, with several candidates in clinical trials. However, most of these candidates are DNA vaccines, with a few mRNA vaccines available for all the viruses. Currently, no mRNA vaccine for DENV is in clinical trials, while only a few are available for ZIKV. In fact, WNV does not even have a single preclinical mRNA vaccine candidate while JEV and YFV each has only a single candidate in preclinical trials. Therefore, there is a need to develop more mRNA vaccines against these viruses since mRNA vaccines have proven to be the cornerstones of modern vaccinology.
Early efforts to develop nucleic acid vaccines focused on using full PrM/E as antigens. However, it is now becoming evident that the whole envelope protein might harbor epitopes, particularly in the EDI and EDII regions, which have the tendency to elicit ADE in immunized individuals. Consequently, recent approaches are concentrating on using the EDIII domain known to be devoid of these motifs, particularly in DENV and ZIKV vaccines. New vaccine candidates are also exploring the addition of non-structural proteins to expand the breadth of the immune response to include cell-mediated immunity, which plays a crucial role in viral clearance. Furthermore, novel adjuvants, including various cytokines, are being explored to improve the immunogenicity of new vaccine candidates. Various delivery systems, including gene gun biolistics, have also been explored. Taken together, to effectively control mosquito-borne flaviviruses using either DNA or mRNA vaccine platforms, there is a need for a combination of multiple strategies encompassing rational antigen design, the use of novel adjuvants, and the careful selection of delivery systems.
MBB: Conceptualization, Data curation, Funding acquisition, Validation, Writing – original draft, Writing – review & editing. AA: Validation, Writing – original draft, Writing – review & editing. AN: Writing – original draft, Writing – review & editing, Validation. SA: Writing – original draft, Writing – review & editing, Validation. MB: Validation, Writing – original draft, Writing – review & editing. SSA: Conceptualization, Data curation, Funding acquisition, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The authors declare that financial support was received from KAIMRC for the research, authorship and/or publication of this article under the projects under the projects NRC23R/531/08 and NRC23R/598/10.].
The authors are grateful for the support from the management and staff of King Abdullah International Medical Research Center (KAIMRC).
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Pandit PS, Doyle MM, Smart KM, Young CCW, Drape GW, Johnson CK. Predicting wildlife reservoirs and global vulnerability to zoonotic flaviviruses. Nat Commun. (2018) 9:5425. doi: 10.1038/s41467-018-07896-2
2. Silva NM, Santos NC, Martins IC. Dengue and zika viruses: epidemiological history, potential therapies, and promising vaccines. Trop Med Infect Dis. (2020) 5:150. doi: 10.3390/tropicalmed5040150
3. Sridhar S, Luedtke A, Langevin E, Zhu M, Bonaparte M, Machabert T, et al. Effect of dengue serostatus on dengue vaccine safety and efficacy. New Engl J Med. (2018) 379:327–40. doi: 10.1056/NEJMoa1800820
4. Pierson TC, Diamond MS. The continued threat of emerging flaviviruses. Nat Microbiol. (2020) 5:796–812. doi: 10.1038/s41564-020-0714-0
5. Murray CL, Jones CT, Rice CM. Architects of assembly: roles of flaviviridae nonstructural proteins in virion morphogenesis. Nat Rev Microbiol. (2008) 6:699–708. doi: 10.1038/nrmicro1928
6. Mazeaud C, Freppel W, Chatel-Chaix L. The multiples fates of the flavivirus RNA genome during pathogenesis. Front Genet. (2023) 9:595. doi: 10.3389/fgene.2018.00595
7. Daep CA, Muñoz-Jordán JL, Eugenin EA. Flaviviruses, an expanding threat in public health: focus on dengue, west nile, and Japanese encephalitis virus. J neurovirology. (2014) 20:539–60. doi: 10.1007/s13365-014-0285-z
8. Chong HY, Leow CY, Majeed ABA, Leow CH. Flavivirus infection—A review of immunopathogenesis, immunological response, and immunodiagnosis. Virus Res. (2019) 274:197770. doi: 10.1016/j.virusres.2019.197770
9. Chen LH, Wilson ME. Update on non-vector transmission of dengue: relevant studies with Zika and other flaviviruses. Trop Dis Travel Med Vaccines. (2016) 2:15. doi: 10.1186/s40794-016-0032-y
10. Weissenböck H, Hubálek Z, Bakonyi T, Nowotny N. Zoonotic mosquito-borne flaviviruses: worldwide presence of agents with proven pathogenicity and potential candidates of future emerging diseases. Veterinary Microbiol. (2010) 140:271–80. doi: 10.1016/j.vetmic.2009.08.025
11. Qureshi AI ed. Chapter 2 - Mosquito-Borne Diseases. In: Zika Virus Disease. Academic Press. p. 27–45. doi: 10.1016/B978-0-12-812365-2.00003-2
12. Qian X, Qi Z. Mosquito-borne flaviviruses and current therapeutic advances. Viruses. (2022) 14:1226. doi: 10.3390/v14061226
13. Kim YC, Reyes-Sandoval A. Recent developments in vaccines against flaviviruses and alphaviruses. Vaccines. (2023) 11:448. doi: 10.3390/vaccines11020448
14. Vannice KS, Wilder-Smith A, Barrett ADT, Carrijo K, Cavaleri M, de Silva A, et al. Clinical development and regulatory points for consideration for second-generation live attenuated dengue vaccines Vaccine. (2018) 36(24):3411–17. doi: 10.1016/j.vaccine.2018.02.062
15. Guy B, Briand O, Lang J, Saville M, Jackson N. Development of the Sanofi Pasteur tetravalent dengue vaccine: One more step forward. Vaccine. (2015) 33(50):7100–11. doi: 10.1016/j.vaccine.2015.09.108
16. Aguiar M, Halstead SB, Stollenwerk N. Consider stopping dengvaxia administration without immunological screening. Expert Rev Vaccines. (2017) 16:301–2. doi: 10.1080/14760584.2017.1276831
17. Wilder-Smith A, Hombach J, Ferguson N, Selgelid M, O’Brien K, Vannice K, et al. Deliberations of the strategic advisory group of experts on immunization on the use of CYD-TDV dengue vaccine. The Lancet Infect Dis. (2019) 19:e31–38. doi: 10.1016/S1473-3099(18)30494-8
18. Collins ND, Barrett ADT. Live attenuated yellow fever 17D vaccine: A legacy vaccine still controlling outbreaks in modern day. Curr Infect Dis Rep. (2017) 19:14. doi: 10.1007/s11908-017-0566-9
19. Barrett ADT. Yellow fever live attenuated vaccine: A very successful live attenuated vaccine but still we have problems controlling the disease. Vaccine. (2017) 35(44):5951–5. doi: 10.1016/j.vaccine.2017.03.032
20. Afrough B, Dowall S, Hewson R. Emerging viruses and current strategies for vaccine intervention. Clin Exp Immunol. (2019) 196(2):157–66. doi: 10.1111/cei.13295
21. Rappuoli R, Gregorio ED, Giudice GD, Phogat S, Pecetta S, Pizza M, et al. Vaccinology in the post–COVID-19 era. Proc Natl Acad Sci. (2021) 118:e2020368118. doi: 10.1073/pnas.2020368118
22. Kim JH. SARS-CoV-2 vaccine development, access, and equity. J Exp Med 2 November. (2020) 217(11):e20201288. doi: 10.1084/jem.20201288
23. Excler J-L, Saville M, Berkley S, Kim JH. Vaccine development for emerging infectious diseases. Nat Med. (2021) 27:591–600. doi: 10.1038/s41591-021-01301-0
24. Taslem Mourosi J, Awe A, Jain S, Batra H. Nucleic acid vaccine platform for DENGUE and ZIKA flaviviruses. Vaccines. (2022) 10:834. doi: 10.3390/vaccines10060834
25. Geerling E, Steffen TL, Brien JD, Pinto AK. Current flavivirus research important for vaccine development. Vaccines. (2020) 8:477. doi: 10.3390/vaccines8030477
26. Wu B, Qi Z, Qian X. Recent advancements in mosquito-borne flavivirus vaccine development. Viruses. (2023) 15:813. doi: 10.3390/v15040813
27. Qin F, Xia F, Chen H, Cui B, Feng Y, Zhang P, et al. A guide to nucleic acid vaccines in the prevention and treatment of infectious diseases and cancers: from basic principles to current applications. Front Cell Dev Biol. (2021) 9:633776. doi: 10.3389/fcell.2021.633776
28. Tang DC, DeVit M, Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature. (1992) 356:152–54. doi: 10.1038/356152a0
29. Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Sci (New York N.Y.). (1993) 259:1745–49. doi: 10.1126/science.8456302
30. Wang B, Ugen KE, Srikantan V, Agadjanyan MG, Dang K, Refaeli Y, et al. Gene inoculation generates immune responses against human immunodeficiency virus type 1. Proc Natl Acad Sci United States America. (1993) 90:4156–60. doi: 10.1073/pnas.90.9.4156
31. Wang R, Doolan DL, Le TP, Hedstrom RC, Coonan KM, Charoenvit Y, et al. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Sci (New York N.Y.). (1998) 282:476–80. doi: 10.1126/science.282.5388.476
32. Tiptiri-Kourpeti A, Spyridopoulou K, Pappa A, Chlichlia K. DNA vaccines to attack cancer: strategies for improving immunogenicity and efficacy. Pharmacol Ther. (2016) 165:32–49. doi: 10.1016/j.pharmthera.2016.05.004
33. Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nat Rev Genet. (2008) 9:776–88. doi: 10.1038/nrg2432
34. Bai H, Schiralli Lester GM, Petishnok LC, Dean DA. Cytoplasmic transport and nuclear import of plasmid DNA. Bioscience Rep. (2017) 37:BSR20160616. doi: 10.1042/BSR20160616
35. Eusébio D, Neves AR, Costa D, Biswas S, Alves G, Cui Z, et al. Methods to improve the immunogenicity of plasmid DNA vaccines. Drug Discovery Today. (2021) 26:2575–92. doi: 10.1016/j.drudis.2021.06.008
36. Ledesma-Feliciano C, Chapman R, Hooper JW, Elma K, Zehrung D, Brennan MB, et al. Improved DNA vaccine delivery with needle-free injection systems. Vaccines. (2023) 11:280. doi: 10.3390/vaccines11020280
37. Li L, Petrovsky N. Molecular mechanisms for enhanced DNA vaccine immunogenicity. Expert Rev Vaccines. (2016) 15:313–29. doi: 10.1586/14760584.2016.1124762
38. Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, et al. Direct gene transfer into mouse muscle in vivo. Science. (1990) 247:1465–68. doi: 10.1126/science.1690918
39. Jirikowski GF, Sanna PP, Maciejewski-Lenoir D, Bloom FE. Reversal of diabetes insipidus in brattleboro rats: intrahypothalamic injection of vasopressin mRNA. Sci (New York N.Y.). (1992) 255:996–98. doi: 10.1126/science.1546298
40. Fang E, Liu X, Li M, Zhang Z, Song L, Zhu B, et al. Advances in COVID-19 mRNA vaccine development. Signal Transduction Targeted Ther. (2022) 7:1–31. doi: 10.1038/s41392-022-00950-y
41. Bloom K, van den Berg F, Arbuthnot P. Self-amplifying RNA vaccines for infectious diseases. Gene Ther. (2021) 28:117–29. doi: 10.1038/s41434-020-00204-y
42. Whitley J, Zwolinski C, Denis C, Maughan M, Hayles L, Clarke D, et al. Development of mRNA Manufacturing for Vaccines and Therapeutics: mRNA Platform Requirements and Development of a Scalable Production Process to Support Early Phase Clinical Trials. Trans Res. (2022) 242:38–55. doi: 10.1016/j.trsl.2021.11.009
43. Hameed SA, Paul S, Dellosa GKY, Jaraquemada D, Bello MB. Towards the Future Exploration of Mucosal mRNA Vaccines against Emerging Viral Diseases; Lessons from Existing next-Generation Mucosal Vaccine Strategies. NPJ Vaccines. (2022) 7:1–20. doi: 10.1038/s41541-022-00485-x
44. Hou X, Zaks T, Langer R, Dong Y. Lipid nanoparticles for mRNA delivery. Nat Rev Materials. (2021) 6:1078–94. doi: 10.1038/s41578-021-00358-0
45. Salleh MZ, Norazmi MN, Deris ZZ. Immunogenicity Mechanism of mRNA Vaccines and Their Limitations in Promoting Adaptive Protection against SARS-CoV-2. PeerJ. (2022) 10:e13083. doi: 10.7717/peerj.13083
46. Bettini E, Locci M. SARS-coV-2 mRNA vaccines: immunological mechanism and beyond. Vaccines. (2021) 9:147. doi: 10.3390/vaccines9020147
47. Cagigi A, Loré K. Immune responses induced by mRNA vaccination in mice, monkeys and humans. Vaccines. (2021) 9:61. doi: 10.3390/vaccines9010061
48. Gote V, Bolla PK, Kommineni N, Butreddy A, Nukala PK, Palakurthi S Srivatsa, et al. A comprehensive review of mRNA vaccines. Int J Mol Sci. (2023) 24:2700. doi: 10.3390/ijms24032700
49. Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discovery. (2018) 17:261–79. doi: 10.1038/nrd.2017.243
50. Wang W-H, Urbina AN, Lin C-Y, Yang Z-S, Assavalapsakul W, Thitithanyanont A, et al. Targets and strategies for vaccine development against dengue viruses. Biomedicine Pharmacotherapy. (2021) 144:112304. doi: 10.1016/j.biopha.2021.112304
51. Dowd KA, Sirohi D, Speer SD, VanBlargan LA, Chen RE, Mukherjee S, et al. prM-reactive antibodies reveal a role for partially mature virions in dengue virus pathogenesis. Proc Natl Acad Sci. (2023) 120:e2218899120. doi: 10.1073/pnas.2218899120
52. Biering SB, Akey DL, Wong MP, Brown WC, Lo NTN, Puerta-Guardo H, et al. Structural basis for antibody inhibition of flavivirus NS1–triggered endothelial dysfunction. Science. (2021) 371:194–200. doi: 10.1126/science.abc0476
53. Liu H, Zou Q, Xu Y. A novel fast multiple nucleotide sequence alignment method based on FM-index. Briefings Bioinf. (2022) 23:bbab519. doi: 10.1093/bib/bbab519
54. Robert X, Gouet P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. (2014) 42:W320–324. doi: 10.1093/nar/gku316
55. Pitcher TJ, Sarathy VV, Matsui K, Gromowski GD, Huang CY-H, Barrett ADT. Functional analysis of dengue virus (DENV) type 2 envelope protein domain 3 type-specific and DENV complex-reactive critical epitope residues. J Gen Virol. (2015) 96:288–93. doi: 10.1099/vir.0.070813-0
56. Sankaradoss A, Jagtap S, Nazir J, Moula SE, Modak A, Fialho J, et al. Immune profile and responses of a novel dengue DNA vaccine encoding an EDIII-NS1 consensus design based on indo-african sequences. Mol Ther. (2022) 30:2058–77. doi: 10.1016/j.ymthe.2022.01.013
57. Ocazionez Jimenez R, Lopes da Fonseca BA. Recombinant Plasmid Expressing a Truncated Dengue-2 Virus E Protein without Co-Expression of prM Protein Induces Partial Protection in Mice. Vaccine. (2000) 19:648–54. doi: 10.1016/s0264-410x(00)00247-4
58. Dejnirattisai W, Jumnainsong A, Onsirisakul N, Fitton P, Vasanawathana S, Limpitikul W, et al. Cross-reacting antibodies enhance dengue virus infection in humans. Sci (New York N.Y.). (2010) 328:745–48. doi: 10.1126/science.1185181
59. Roth C, Cantaert T, Colas C, Prot M, Casadémont I, Levillayer L, et al. A modified mRNA vaccine targeting immunodominant NS epitopes protects against dengue virus infection in HLA class I transgenic mice. Front Immunol. (2019) 10:1424. https://www.frontiersin.org/articles/10.3389/fimmu.2019.01424.
60. Falconar AK. The dengue virus nonstructural-1 protein (NS1) generates antibodies to common epitopes on human blood clotting, integrin/adhesin proteins and binds to human endothelial cells: potential implications in hemorrhagic fever pathogenesis. Arch Virol. (1997) 142:897–916. doi: 10.1007/s007050050127
61. Modhiran N, Watterson D, Muller DA, Panetta AK, Sester DP, Liu L, et al. Dengue virus NS1 protein activates cells via toll-like receptor 4 and disrupts endothelial cell monolayer integrity. Sci Trans Med. (2015) 7:304ra142. doi: 10.1126/scitranslmed.aaa3863
62. Jayathilaka D, Gomes L, Jeewandara C, Bandara Jayarathna GS, Herath D, Perera PA, et al. Role of NS1 antibodies in the pathogenesis of acute secondary dengue infection. Nat Commun. (2018) 9:5242. doi: 10.1038/s41467-018-07667-z
63. Kochel T, Wu SJ, Raviprakash K, Hobart P, Hoffman S, Porter K, et al. Inoculation of plasmids expressing the dengue-2 envelope gene elicit neutralizing antibodies in mice. Vaccine. (1997) 15:547–52. doi: 10.1016/s0264-410x(97)00215-6
64. Porter KR, Kochel TJ, Wu S-J, Raviprakash K, Phillips I, Hayes CG. Protective efficacy of a dengue 2 DNA vaccine in mice and the effect of cpG immuno-stimulatory motifs on antibody responses. Arch Virol. (1998) 143:997–1003. doi: 10.1007/s007050050348
65. Kochel TJ, Raviprakash K, Hayes CG, Watts DM, Russell KL, Gozalo AS, et al. A dengue virus serotype-1 DNA vaccine induces virus neutralizing antibodies and provides protection from viral challenge in aotus monkeys. Vaccine. (2000) 18:3166–73. doi: 10.1016/S0264-410X(00)00105-5
66. Putri DH, Sudiro TM, Yunita R, Jaya UA, Dewi BE, Sjatha F, et al. Immunogenicity of a candidate DNA vaccine based on the prM/E genes of a dengue type 2 virus cosmopolitan genotype strain. Japanese J Infect Dis. (2015) 68:357–63. doi: 10.7883/yoken.JJID.2014.313
67. Raviprakash K, Kochel TJ, Ewing D, Simmons M, Phillips I, Hayes CG, et al. Immunogenicity of dengue virus type 1 DNA vaccines expressing truncated and full length envelope protein. Vaccine. (2000) 18:2426–34. doi: 10.1016/S0264-410X(99)00570-8
68. Blair PJ, Kochel TJ, Raviprakash K, Guevara C, Salazar M, Wu S-J, et al. Evaluation of immunity and protective efficacy of a dengue-3 pre-membrane and envelope DNA vaccine in aotus nancymae monkeys. Vaccine. (2006) 24:1427–32. doi: 10.1016/j.vaccine.2005.09.032
69. De Paula SO, Lima DM, França RFO, Gomes-Ruiz AC, Fonseca BAd. A DNA Vaccine Candidate Expressing Dengue-3 Virus prM and E Proteins Elicits Neutralizing Antibodies and Protects Mice against Lethal Challenge. Arch Virol. (2008) 153:2215–23. doi: 10.1007/s00705-008-0250-3
70. Chen H, Zheng X, Wang R, Gao N, Sheng Z, Fan D, et al. Immunization with Electroporation Enhances the Protective Effect of a DNA Vaccine Candidate Expressing prME Antigen against Dengue Virus Serotype 2 Infection. Clin Immunol (Orlando Fla.). (2016) 171:41–9. doi: 10.1016/j.clim.2016.08.021
71. Zheng X, Chen H, Wang R, Fan D, Feng K, Gao N, et al. Effective Protection Induced by a Monovalent DNA Vaccine against Dengue Virus (DV) Serotype 1 and a Bivalent DNA Vaccine against DV1 and DV2 in Mice. Front Cell Infection Microbiol. (2017) 7:175. doi: 10.3389/fcimb.2017.00175
72. Simmons M, Murphy GS, Kochel T, Raviprakash K, Hayes CG. Characterization of antibody responses to combinations of a dengue-2 DNA and dengue-2 recombinant subunit vaccine. Am J Trop Med Hygiene. (2001) 65:420–26. doi: 10.4269/ajtmh.2001.65.420
73. Chen L, Ewing D, Subramanian H, Block K, Rayner J, Alterson KD, et al. A heterologous DNA prime-Venezuelan equine encephalitis virus replicon particle boost dengue vaccine regimen affords complete protection from virus challenge in cynomolgus macaques. J Virol. (2007) 81:11634–39. doi: 10.1128/JVI.00996-07
74. Costa SM, Paes MV, Barreto DF, Pinhão AT, Barth OM, Queiroz JLS, et al. Protection against dengue type 2 virus induced in mice immunized with a DNA plasmid encoding the non-structural 1 (NS1) gene fused to the tissue plasminogen activator signal sequence. Vaccine. (2006) 24:195–205. doi: 10.1016/j.vaccine.2005.07.059
75. George JA, Eo SK. Distinct humoral and cellular immunity induced by alternating prime-boost vaccination using plasmid DNA and live viral vector vaccines expressing the E protein of dengue virus type 2. Immune Network. (2011) 11:268–80. doi: 10.4110/in.2011.11.5.268
76. Konishi E, Terazawa A, Imoto J-i. Simultaneous immunization with DNA and protein vaccines against Japanese encephalitis or dengue synergistically increases their own abilities to induce neutralizing antibody in mice. Vaccine. (2003) 21:1826–32. doi: 10.1016/s0264-410x(03)00028-8
77. Raviprakash K, Marques E, Ewing D, Lu Y, Phillips I, Porter KR, et al. Synergistic neutralizing antibody response to a dengue virus type 2 DNA vaccine by incorporation of lysosome-associated membrane protein sequences and use of plasmid expressing GM-CSF. Virology. (2001) 290:74–82. doi: 10.1006/viro.2001.1136
78. Raviprakash K, Ewing D, Simmons M, Porter KR, Jones TR, Hayes CG, et al. Needle-free biojector injection of a dengue virus type 1 DNA vaccine with human immunostimulatory sequences and the GM-CSF gene increases immunogenicity and protection from virus challenge in aotus monkeys. Virology. (2003) 315:345–52. doi: 10.1016/s0042-6822(03)00542-7
79. Pérez-Vélez ME, García-Nieves T, Colón-Sánchez C, Martínez I. Induction of neutralization antibodies in mice by dengue-2 envelope DNA vaccines. Puerto Rico Health Sci J. (2009) 28(3):239–50.
80. Mellado-Sánchez G, García-Cordero J, Luria-Pérez R, Lázaro-Olan L, Santos-Argumedo L, Gutiérrez-Castañeda B, et al. DNA priming E and NS1 constructs–homologous proteins boosting immunization strategy to improve immune response against dengue in mice. Viral Immunol. (2005) 18:709–21. doi: 10.1089/vim.2005.18.709
81. Kulkarni A, Bhat R, Malik M, Sane S, Kothari S, Vaidya S, et al. Neutralizing antibody response and efficacy of novel recombinant tetravalent dengue DNA vaccine comprising envelope domain III in mice. Iranian J Med Sci. (2017) 42(2):152–60.
82. Slon Campos JL, Poggianella M, Burrone OR. Long-term stability of antibody responses elicited by dengue virus envelope DIII-based DNA vaccines. J Gen Virol. (2018) 99:1078–85. doi: 10.1099/jgv.0.001094
83. Apt D, Raviprakash K, Brinkman A, Semyonov A, Yang S, Skinner C, et al. Tetravalent neutralizing antibody response against four dengue serotypes by a single chimeric dengue envelope antigen. Vaccine. (2006) 24:335–44. doi: 10.1016/j.vaccine.2005.07.100
84. Mota J, Acosta M, Argotte R, Figueroa R, Méndez A, Ramos C. Induction of protective antibodies against dengue virus by tetravalent DNA immunization of mice with domain III of the envelope protein. Vaccine. (2005) 23:3469–76. doi: 10.1016/j.vaccine.2004.12.028
85. Konishi E, Kosugi S, Imoto J-i. Dengue tetravalent DNA vaccine inducing neutralizing antibody and anamnestic responses to four serotypes in mice. Vaccine. (2006) 24:2200–7. doi: 10.1016/j.vaccine.2005.11.002
86. Prompetchara E, Ketloy C, Keelapang P, Sittisombut N, Ruxrungtham K. Induction of neutralizing antibody response against four dengue viruses in mice by intramuscular electroporation of tetravalent DNA vaccines. PloS One. (2014) 9:e92643. doi: 10.1371/journal.pone.0092643
87. Raviprakash K, Apt D, Brinkman A, Skinner C, Yang S, Dawes G, et al. A chimeric tetravalent dengue DNA vaccine elicits neutralizing antibody to all four virus serotypes in rhesus macaques. Virology. (2006) 353:166–73. doi: 10.1016/j.virol.2006.05.005
88. Poggianella M, Campos JLS, Chan KR, Tan HC, Bestagno M, Ooi EE, et al. Dengue E protein domain III-based DNA immunization induces strong antibody responses to all four viral serotypes. PloS Negl Trop Dis. (2015) 9:e0003947. doi: 10.1371/journal.pntd.0003947
89. Ramanathan MP, Kutzler MA, Kuo Y-C, Yan J, Liu H, Shah V, et al. Coimmunization with an optimized IL15 plasmid adjuvant enhances humoral immunity via stimulating B cells induced by genetically engineered DNA vaccines expressing consensus JEV and WNV E DIII. Vaccine. (2009) 27:4370–80. doi: 10.1016/j.vaccine.2009.01.137
90. Imoto J-I, Konishi E. Dengue tetravalent DNA vaccine increases its immunogenicity in mice when mixed with a dengue type 2 subunit vaccine or an inactivated Japanese encephalitis vaccine. Vaccine. (2007) 25:1076–84. doi: 10.1016/j.vaccine.2006.09.059
91. Simmons M, Burgess T, Lynch J, Putnak R. Protection against dengue virus by non-replicating and live attenuated vaccines used together in a prime boost vaccination strategy. Virology. (2010) 396:280–88. doi: 10.1016/j.virol.2009.10.023
92. Zheng Q, Fan D, Gao N, Chen H, Wang J, Ming Y, et al. Evaluation of a DNA Vaccine Candidate Expressing prM-E-NS1 Antigens of Dengue Virus Serotype 1 with or without Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) in Immunogenicity and Protection. Vaccine. (2011) 29:763–71. doi: 10.1016/j.vaccine.2010.11.014
93. Pinto PBA, Barros TAC, Lima LM, Pacheco AR, Assis ML, Pereira BAS, et al. Combination of E- and NS1-derived DNA vaccines: the immune response and protection elicited in mice against DENV2. Viruses. (2022) 14:1452. doi: 10.3390/v14071452
94. Lima DM, de Paula SO, França RFdO, Palma PVB, Morais FR, Gomes-Ruiz AC, et al. A DNA Vaccine Candidate Encoding the Structural prM/E Proteins Elicits a Strong Immune Response and Protects Mice against Dengue-4 Virus Infection. Vaccine. (2011) 29:831–38. doi: 10.1016/j.vaccine.2010.10.078
95. Porter KR, Ewing D, Chen L, Wu S-J, Hayes CG, Ferrari M, et al. Immunogenicity and protective efficacy of a vaxfectin-adjuvanted tetravalent dengue DNA vaccine. Vaccine. (2012) 30:336–41. doi: 10.1016/j.vaccine.2011.10.085
96. Huang SS, Li IH, Hong PD, Yeh MK. Evaluation of protective efficacy using a nonstructural protein NS1 in DNA vaccine-loaded microspheres against dengue 2 virus. Int J Nanomedicine. (2013) 8:3161–9. doi: 10.2147/IJN.S49972
97. Lu H, Xu X-F, Gao N, Fan D-Y, Wang J, An J. Preliminary evaluation of DNA vaccine candidates encoding dengue-2 prM/E and NS1: their immunity and protective efficacy in mice. Mol Immunol. (2013) 54:109–14. doi: 10.1016/j.molimm.2012.11.007
98. Chen H, Gao N, Wu J, Zheng X, Li J, Fan D, et al. Variable effects of the co-administration of a GM-CSF-expressing plasmid on the immune response to flavivirus DNA vaccines in mice. Immunol Lett. (2014) 162:140–48. doi: 10.1016/j.imlet.2014.08.005
99. Sjatha F, Kuwahara M, Sudiro TM, Kameoka M, Konishi E. Evaluation of chimeric DNA vaccines consisting of premembrane and envelope genes of Japanese encephalitis and dengue viruses as a strategy for reducing induction of dengue virus infection-enhancing antibody response. Microbiol Immunol. (2014) 58:126–34. doi: 10.1111/1348-0421.12125
100. Chen XY, Li DZ, Zhong XZ, Chen B, Duan ZL, Wen JS. Induction of multiple cytotoxic T lymphocyte responses in mice by a multiepitope DNA vaccine against dengue virus serotype 1. Microbiol Immunol. (2016) 60:835–45. doi: 10.1111/1348-0421.12457
101. Hou J, Shrivastava S, Fraser CC, Loo HL, Wong LH, Ho V, et al. Dengue mosaic vaccines enhance cellular immunity and expand the breadth of neutralizing antibody against all four serotypes of dengue viruses in mice. Front Immunol. (2019) 10:1429. doi: 10.3389/fimmu.2019.01429
102. Zaneti AB, Yamamoto MM, Sulczewski FB, Almeida BdS, Souza HFS, Ferreira NS, et al. Dendritic cell targeting using a DNA vaccine induces specific antibodies and CD4+ T cells to the dengue virus envelope protein domain III. Front Immunol. (2019) 10:59. doi: 10.3389/fimmu.2019.00059
103. Slon-Campos JL, Poggianella M, Zentilin L, Burrone OR. Use of adeno-associated viral vectors to improve delivery of a DNA vaccine against dengue virus. J Gen Virol. (2020) 101:73–8. doi: 10.1099/jgv.0.001351
104. Larocca RA, Abbink P, Peron JPS, Zanotto PMdA, Iampietro MJ, Badamchi-Zadeh A, et al. Vaccine protection against zika virus from Brazil. Nature. (2016) 536:474–78. doi: 10.1038/nature18952
105. Dowd KA, Ko S-Y, Morabito KM, Yang ES, Pelc RS, DeMaso CR, et al. Rapid development of a DNA vaccine for zika virus. Sci (New York N.Y.). (2016) 354:237–40. doi: 10.1126/science.aai9137
106. Abbink P, Larocca RA, de la Barrera RA, Bricault CA, Moseley ET, Boyd M, et al. Protective efficacy of multiple vaccine platforms against zika virus challenge in rhesus monkeys. Sci (New York N.Y.). (2016) 353:1129–32. doi: 10.1126/science.aah6157
107. Muthumani K, Griffin BD, Agarwal S, Kudchodkar SB, Reuschel EL, Choi H, et al. In Vivo Protection against ZIKV Infection and Pathogenesis through Passive Antibody Transfer and Active Immunization with a prMEnv DNA Vaccine. NPJ Vaccines. (2016) 1:16021. doi: 10.1038/npjvaccines.2016.21
108. Yi G, Xu X, Abraham S, Petersen S, Guo H, Ortega N, et al. A DNA vaccine protects human immune cells against zika virus infection in humanized mice. EBioMedicine. (2017) 25:87–94. doi: 10.1016/j.ebiom.2017.10.006
109. Wang R, Liao X, Fan D, Wang L, Song J, Feng K, et al. Maternal immunization with a DNA vaccine candidate elicits specific passive protection against post-natal zika virus infection in immunocompetent BALB/c mice. Vaccine. (2018) 36:3522–32. doi: 10.1016/j.vaccine.2018.04.051
110. Esquivel RN, Patel A, Kudchodkar SB, Park DH, Stettler K, Beltramello M, et al. In vivo delivery of a DNA-encoded monoclonal antibody protects non-human primates against zika virus. Mol Therapy: J Am Soc Gene Ther. (2019) 27:974–85. doi: 10.1016/j.ymthe.2019.03.005
111. de La Vega M-A, Piret J, Griffin BD, Rhéaume C, Venable M-C, Carbonneau J, et al. Zika-induced male infertility in mice is potentially reversible and preventable by deoxyribonucleic acid immunization. J Infect Dis. (2019) 219:365–74. doi: 10.1093/infdis/jiy336
112. Van Rompay KKA, Keesler RI, Ardeshir A, Watanabe J, Usachenko J, Singapuri A, et al. DNA Vaccination before Conception Protects Zika Virus-Exposed Pregnant Macaques against Prolonged Viremia and Improves Fetal Outcomes. Sci Trans Med. (2019) 11:eaay2736. doi: 10.1126/scitranslmed.aay2736
113. Zhan Y, Deng Y, Huang B, Song Q, Wang W, Yang Y, et al. Humoral and cellular immunity against both ZIKV and poxvirus is elicited by a two-dose regimen using DNA and non-replicating vaccinia virus-based vaccine candidates. Vaccine. (2019) 37:2122–30. doi: 10.1016/j.vaccine.2019.02.063
114. Louis L, Wise MC, Choi H, Villarreal DO, Muthumani K, Weiner DB. Designed DNA-encoded IL-36 gamma acts as a potent molecular adjuvant enhancing zika synthetic DNA vaccine-induced immunity and protection in a lethal challenge model. Vaccines. (2019) 7:42. doi: 10.3390/vaccines7020042
115. López-Camacho C, Lorenzo GD, Slon-Campos JL, Dowall S, Abbink P, Larocca RA, et al. Immunogenicity and efficacy of zika virus envelope domain III in DNA, protein, and chAdOx1 adenoviral-vectored vaccines. Vaccines. (2020) 8:307. doi: 10.3390/vaccines8020307
116. In HJ, Lee YH, Jang S, Lim HJ, Kim MY, Kim JA, et al. Enhanced effect of modified zika virus E antigen on the immunogenicity of DNA vaccine. Virology. (2020) 549:25–31. doi: 10.1016/j.virol.2020.07.014
117. Zhan Y, Pang Z, Du Y, Wang W, Yang Y, Wang W, et al. NS1-based DNA vaccination confers mouse protective immunity against ZIKV challenge. Infection Genet Evolution: J Mol Epidemiol Evolutionary Genet Infect Dis. (2020) 85:104521. doi: 10.1016/j.meegid.2020.104521
118. Pereira LR, Alves RPDS, Sales NS, Andreata-Santos R, Venceslau-Carvalho AA, Pereira SS, et al. Enhanced immune responses and protective immunity to zika virus induced by a DNA vaccine encoding a chimeric NS1 fused with type 1 herpes virus gD protein. Front Med Technol. (2020) 2:604160. doi: 10.3389/fmedt.2020.604160
119. Burgomaster KE, Foreman BM, Aleshnick MA, Larman BC, Gordon DN, Maciejewski S, et al. Limited flavivirus cross-reactive antibody responses elicited by a zika virus deoxyribonucleic acid vaccine candidate in humans. J Infect Dis. (2021) 224:1550–55. doi: 10.1093/infdis/jiab185
120. Lin YL, Chen LK, Liao CL, Yeh CT, Ma SH, Chen JL, et al. DNA immunization with Japanese encephalitis virus nonstructural protein NS1 elicits protective immunity in mice. J Virol. (1998) 72:191–200. doi: 10.1128/JVI.72.1.191-200.1998
121. Chen HW, Pan CH, Liau MY, Jou R, Tsai CJ, Wu HJ, et al. Screening of protective antigens of Japanese encephalitis virus by DNA immunization: A comparative study with conventional viral vaccines. J Virol. (1999) 73:10137–45. doi: 10.1128/JVI.73.12.10137-10145.1999
122. Kaur R, Sachdeva G, Vrati S. Plasmid DNA immunization against Japanese encephalitis virus: immunogenicity of membrane-anchored and secretory envelope protein. J Infect Dis. (2002) 185:1–12. doi: 10.1086/338015
123. Konishi E, Yamaoka M, Khin-Sane-Win, Kurane I, Takada K, Mason PW. The anamnestic neutralizing antibody response is critical for protection of mice from challenge following vaccination with a plasmid encoding the Japanese encephalitis virus premembrane and envelope genes. J Virol. (1999) 73:5527–34. doi: 10.1128/JVI.73.7.5527-5534.1999
124. Konishi E, Yamaoka M, Kurane I, Mason PW. Japanese encephalitis DNA vaccine candidates expressing premembrane and envelope genes induce virus-specific memory B cells and long-lasting antibodies in swine. Virology. (2000) 268:49–55. doi: 10.1006/viro.1999.0142
125. Chen W, Lin Y, Liao C, Hsieh S. Modulatory effects of the human heat shock protein 70 on DNA vaccination. J Biomed Sci. (2000) 7:412–19. doi: 10.1007/BF02255816
126. Ashok MS, Rangarajan PN. Immunization with Plasmid DNA Encoding the Envelope Glycoprotein of Japanese Encephalitis Virus Confers Significant Protection against Intracerebral Viral Challenge without Inducing Detectable Antiviral Antibodies. Vaccine. (1999) 18:68–75. doi: 10.1016/s0264-410x(99)00180-2
127. Ashok MS, Rangarajan PN. Protective efficacy of a plasmid DNA encoding Japanese encephalitis virus envelope protein fused to tissue plasminogen activator signal sequences: studies in a murine intracerebral virus challenge model. Vaccine. (2002) 20:1563–70. doi: 10.1016/s0264-410x(01)00492-3
128. Zhao Z, Wakita T, Yasui K. Inoculation of plasmids encoding Japanese encephalitis virus prM-E proteins with colloidal gold elicits a protective immune response in BALB/c mice. J Virol. (2003) 77:4248–60. doi: 10.1128/jvi.77.7.4248-4260.2003
129. Huang H-N, Li T-L, Chan Y-L, Chen C-L, Wu C-J. Transdermal immunization with low-pressure-gene-gun mediated chitosan-based DNA vaccines against Japanese encephalitis virus. Biomaterials. (2009) 30:6017–25. doi: 10.1016/j.biomaterials.2009.07.029
130. Konishi E, Ajiro N, Nukuzuma C, Mason PW, Kurane I. Comparison of protective efficacies of plasmid DNAs encoding Japanese encephalitis virus proteins that induce neutralizing antibody or cytotoxic T lymphocytes in mice. Vaccine. (2003) 21:3675–83. doi: 10.1016/s0264-410x(03)00382-7
131. Bharati K, Rani R, Vrati S. Evaluation of Japanese encephalitis virus DNA vaccine candidates in rhesus monkeys [Macaca mulatta. Vaccine. (2009) 27:10–6. doi: 10.1016/j.vaccine.2008.10.050
132. Tanabayashi K, Mukai R, Yamada A, Takasaki T, Kurane I, Yamaoka M, et al. Immunogenicity of a Japanese encephalitis DNA vaccine candidate in cynomolgus monkeys. Vaccine. (2003) 21:2338–45. doi: 10.1016/s0264-410x(03)00079-3
133. Danko JR, Kochel T, Teneza-Mora N, Luke TC, Raviprakash K, Sun P, et al. Safety and immunogenicity of a tetravalent dengue DNA vaccine administered with a cationic lipid-based adjuvant in a phase 1 clinical trial. Am J Trop Med Hygiene. (2018) 98:849–56. doi: 10.4269/ajtmh.17-0416
134. Li P, Cao R-B, Zheng Q-S, Liu J-J, Li Y, Wang E-X, et al. Enhancement of humoral and cellular immunity in mice against Japanese encephalitis virus using a DNA prime-protein boost vaccine strategy. Veterinary J (London England: 1997). (2010) 183:210–16. doi: 10.1016/j.tvjl.2008.09.019
135. Wu HH, Chen CT, Lin Y-L, Lee ST. Sub-fragments of the envelope gene are highly protective against the Japanese encephalitis virus lethal infection in DNA priming–protein boosting immunization strategies. Vaccine. (2004) 22:793–800. doi: 10.1016/j.vaccine.2003.02.001
136. Bharati K, Appaiahgari MB, Vrati S. Effect of cytokine-encoding plasmid delivery on immune response to Japanese encephalitis virus DNA vaccine in mice. Microbiol Immunol. (2005) 49:349–53. doi: 10.1111/j.1348-0421.2005.tb03739.x
137. Zhai Y-z, Li X-m, Zhou Y, Ma L, Feng G-h. Intramuscular Immunization with a Plasmid DNA Vaccine Encoding prM-E Protein from Japanese Encephalitis Virus: Enhanced Immunogenicity by Co-Administration of GM-CSF Gene and Genetic Fusions of prM-E Protein and GM-CSF. Intervirology. (2009) 52:152–63. doi: 10.1159/000224643
138. Chen HW, Pan CH, Huan HW, Liau MY, Chiang JR, Tao MH. Suppression of immune response and protective immunity to a Japanese encephalitis virus DNA vaccine by coadministration of an IL-12-expressing plasmid. J Immunol (Baltimore Md: 1950). (2001) 166:7419–26. doi: 10.4049/jimmunol.166.12.7419
139. Imoto J-i, Ishikawa T, Yamanaka A, Konishi M, Murakami K, Shibahara T, et al. Needle-free jet injection of small doses of Japanese encephalitis DNA and inactivated vaccine mixture induces neutralizing antibodies in miniature pigs and protects against fetal death and mummification in pregnant sows. Vaccine. (2010) 28:7373–80. doi: 10.1016/j.vaccine.2010.09.008
140. Zhai Y-Z, Zhou Y, Ma L, Feng G-H. The dominant roles of ICAM-1-encoding gene in DNA vaccination against Japanese encephalitis virus are the activation of dendritic cells and enhancement of cellular immunity. Cell Immunol. (2013) 281:1–10. doi: 10.1016/j.cellimm.2013.01.005
141. Tretyakova I, Lukashevich IS, Glass P, Wang E, Weaver S, Pushko P. Novel vaccine against Venezuelan equine encephalitis combines advantages of DNA immunization and a live attenuated vaccine. Vaccine. (2013) 31:1019–25. doi: 10.1016/j.vaccine.2012.12.050
142. Tretyakova I, Nickols B, Hidajat R, Jokinen J, Lukashevich IS, Pushko P. Plasmid DNA initiates replication of yellow fever vaccine in vitro and elicits virus-specific immune response in mice. Virology. (2014) 468–470:28–35. doi: 10.1016/j.virol.2014.07.050
143. Maciel M, Cruz FdSP, Cordeiro MT, Motta MAd, Cassemiro KMSd, Maia RdCC, et al. A DNA vaccine against yellow fever virus: development and evaluation. PloS Negl Trop Dis. (2015) 9:e0003693. doi: 10.1371/journal.pntd.0003693
144. Davis EH, Beck AS, Li L, Mellodee M, Greenberg MB, et al. Japanese encephalitis virus live attenuated vaccine strains display altered immunogenicity, virulence and genetic diversity. NPJ Vaccines. (2021) 6:1–14. doi: 10.1038/s41541-021-00371-y
145. Schneeweiss A, Chabierski S, Salomo M, Delaroque N, Al-Robaiy S, Grunwald T, et al. A DNA vaccine encoding the E protein of west nile virus is protective and can be boosted by recombinant domain DIII. Vaccine. (2011) 29:6352–57. doi: 10.1016/j.vaccine.2011.04.116
146. Ishikawa T, Takasaki T, Kurane I, Nukuzuma S, Kondo T, Konishi E. Co-immunization with west nile DNA and inactivated vaccines provides synergistic increases in their immunogenicities in mice. Microbes Infection. (2007) 9:1089–95. doi: 10.1016/j.micinf.2007.05.013
147. Filette MD, Soehle S, Ulbert S, Richner J, Diamond MS, Sinigaglia A, et al. Vaccination of mice using the west nile virus E-protein in a DNA prime-protein boost strategy stimulates cell-mediated immunity and protects mice against a lethal challenge. PloS One. (2014) 9:e87837. doi: 10.1371/journal.pone.0087837
148. Yang JS, Kim JJ, Hwang D, Choo AY, Dang K, Maguire H, et al. Induction of potent th1-type immune responses from a novel DNA vaccine for west nile virus new york isolate (WNV-NY1999). J Infect Dis. (2001) 184:809–16. doi: 10.1086/323395
149. Hall RA, Nisbet DJ, Pham KB, Pyke AT, Smith GA, Khromykh AA. DNA vaccine coding for the full-length infectious kunjin virus RNA protects mice against the new york strain of west nile virus. Proc Natl Acad Sci. (2003) 100:10460–64. doi: 10.1073/pnas.1834270100
150. Chang DC, Liu WJ, Anraku I, Clark DC, Pollitt CC, Suhrbier A, et al. Single-round infectious particles enhance immunogenicity of a DNA vaccine against west nile virus. Nat Biotechnol. (2008) 26:571–77. doi: 10.1038/nbt1400
151. Roby JA, Bielefeldt-Ohmann H, Prow NA, Chang DC, Hall RA, Khromykh AA. Increased expression of capsid protein in trans enhances production of single-round infectious particles by west nile virus DNA vaccine candidate. J Gen Virol. (2014) 95:2176–91. doi: 10.1099/vir.0.064121-0
152. Martin JE, Pierson TC, Hubka S, Rucker S, Gordon IJ, Enama ME, et al. A west nile virus DNA vaccine induces neutralizing antibody in healthy adults during a phase 1 clinical trial. J Infect Dis. (2007) 196:1732–40. doi: 10.1086/523650
153. Ledgerwood JE, Pierson TC, Hubka SA, Desai N, Rucker S, Gordon IJ, et al. A west nile virus DNA vaccine utilizing a modified promoter induces neutralizing antibody in younger and older healthy adults in a phase I clinical trial. J Infect Dis. (2011) 203:1396–404. doi: 10.1093/infdis/jir054
154. Gould CV, Staples JE, Huang CY-H, Brault AC, Nett RJ. Combating west nile virus disease — Time to revisit vaccination. New Engl J Med. (2023) 388:1633–6. doi: 10.1056/NEJMp2301816
155. Zhang M, Sun J, Li M, Jin X. Modified mRNA-LNP Vaccines Confer Protection against Experimental DENV-2 Infection in Mice. Mol Ther - Methods Clin Dev. (2020) 18:702–12. doi: 10.1016/j.omtm.2020.07.013
156. Wollner CJ, Richner M, Hassert MA, Pinto AK, Brien JD, Richner JM. A dengue virus serotype 1 mRNA-LNP vaccine elicits protective immune responses. J Virol. (2021) 95:e02482–20. doi: 10.1128/JVI.02482-20
157. Owji H, Nezafat N, Negahdaripour M, Hajiebrahimi A, Ghasemi Y. A comprehensive review of signal peptides: structure, roles, and applications. Eur J Cell Biol. (2018) 97:422–41. doi: 10.1016/j.ejcb.2018.06.003
158. Wollner CJ, Richner JM. mRNA vaccines against flaviviruses. Vaccines. (2021) 9:148. doi: 10.3390/vaccines9020148
159. He L, Sun W, Yang L, Liu W, Li J. A Multiple-Target mRNA-LNP Vaccine Induces Protective Immunity against Experimental Multi-Serotype DENV in Mice. Virologica Sin. (2022) 37:746–57. doi: 10.1016/j.virs.2022.07.003
160. Pardi N, Hogan MJ, Pelc RS, Muramatsu H, Andersen H, DeMaso CR, et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature. (2017) 543:248–51. doi: 10.1038/nature21428
161. Bollman B, Nunna N, Bahl K, Hsiao CJ, Bennett H, Butler S, et al. An optimized messenger RNA vaccine candidate protects non-human primates from zika virus infection. NPJ Vaccines. (2023) 8:58. doi: 10.1038/s41541-023-00656-4
162. Richner JM, Himansu S, Dowd KA, Butler SL, Salazar V, Fox JM, et al. Modified mRNA Vaccines Protect against Zika Virus Infection. Cell. (2017) 168:1114–1125.e10. doi: 10.1016/j.cell.2017.02.017
163. Jagger BW, Dowd KA, Chen RE, Desai P, Foreman B, Burgomaster KE, et al. Protective efficacy of nucleic acid vaccines against transmission of zika virus during pregnancy in mice. J Infect Dis. (2019) 220:1577–88. doi: 10.1093/infdis/jiz338
164. Medina-Magües LG, Gergen J, Jasny E, Petsch B, Lopera-Madrid J, Medina-Magües ES, et al. mRNA vaccine protects against zika virus. Vaccines. (2021) 9:1464. doi: 10.3390/vaccines9121464
165. Zhong Z, Catani JPP, Cafferty SMc, Couck L, Broeck WVD, Gorlé N, et al. Immunogenicity and protection efficacy of a naked self-replicating mRNA-based zika virus vaccine. Vaccines. (2019) 7:96. doi: 10.3390/vaccines7030096
166. Li A, Yu J, Lu M, Ma Y, Attia Z, Shan C, et al. A zika virus vaccine expressing premembrane-envelope-NS1 polyprotein. Nat Commun. (2018) 9:3067. doi: 10.1038/s41467-018-05276-4
167. Shin H, Kang S, Won C, Min D-H. A single-dose mRNA vaccine employing porous silica nanoparticles induces robust immune responses against the zika virus. Advanced Sci (Weinheim Baden-Wurttemberg Germany). (2024) 11(35):e2404590. doi: 10.1002/advs.202404590
168. Essink B, Chu L, Seger W, Barranco E, Cam NL, Bennett H, et al. The safety and immunogenicity of two zika virus mRNA vaccine candidates in healthy flavivirus baseline seropositive and seronegative adults: the results of two randomized, placebo-controlled, dose-ranging, phase 1 clinical trials. The Lancet Infect Dis. (2023) 23:621–33. doi: 10.1016/S1473-3099(22)00764-2
169. Estofolete CF, Versiani AF, Dourado FS, Milhim BHGA, Pacca CC, Silva GCD, et al. Influence of previous zika virus infection on acute dengue episode. PloS Negl Trop Dis. (2023) 17:e0011710. doi: 10.1371/journal.pntd.0011710
170. Chen T, Zhu S, Wei N, Zhao Z, Niu J, Si Y, et al. Protective Immune Responses Induced by an mRNA-LNP Vaccine Encoding prM-E Proteins against Japanese Encephalitis Virus Infection. Viruses. (2022) 14:1121. doi: 10.3390/v14061121
171. Medina-Magües LG, Mühe J, Jasny E, Medina-Magües ES, Roth N, Lopera-Madrid J, et al. Immunogenicity and Protective Activity of mRNA Vaccine Candidates against Yellow Fever Virus in Animal Models. NPJ Vaccines. (2023) 8:31. doi: 10.1038/s41541-023-00629-7
172. Beckett CG, Tjaden J, Burgess T, Danko JR, Tamminga C, Simmons M, et al. Evaluation of a prototype dengue-1 DNA vaccine in a Phase 1 clinical trial. Vaccine. (2011) 29:960–8. doi: 10.1016/j.vaccine.2010.11.050
173. Gaudinski MR, Houser KV, Morabito KM, Hu Z, Yamshchikov G, Rothwell RS, et al. Safety, tolerability, and immunogenicity of two Zika virus DNA vaccine candidates in healthy adults: randomised, open-label, phase 1 clinical trials. Lancet. (2018) 391(10120):552–62. doi: 10.1016/S0140-6736(17)33105-7
Keywords: denv, mosquito borne-flaviviruses, DNA vaccine, mRNA vaccine, nucleic acid based vaccines
Citation: Bello MB, Alsaadi A, Naeem A, Almahboub SA, Bosaeed M and Aljedani SS (2025) Development of nucleic acid-based vaccines against dengue and other mosquito-borne flaviviruses: the past, present, and future. Front. Immunol. 15:1475886. doi: 10.3389/fimmu.2024.1475886
Received: 04 August 2024; Accepted: 06 December 2024;
Published: 07 January 2025.
Edited by:
Ebony Gary, Wistar Institute, United StatesReviewed by:
Sivaram Gunisetty, Emory University, United StatesCopyright © 2025 Bello, Alsaadi, Naeem, Almahboub, Bosaeed and Aljedani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Safia S. Aljedani, YWxqZWRhbmlzQGthaW1yYy5lZHUuc2E=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.