- 1Department of Targeting Therapy and Immunology, Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 2Division of Thoracic Tumor Multimodality Treatment, Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan, China
Colorectal cancer (CRC) remains a significant cause of cancer-related mortality worldwide. Despite advancements in surgery, chemotherapy, and radiotherapy, the effectiveness of these conventional treatments is limited, particularly in advanced cases. Therefore, transition to novel treatment is urgently needed. Immunotherapy, especially immune checkpoint inhibitors (ICIs), has shown promise in improving outcomes for CRC patients. Notably, patients with deficient mismatch repair (dMMR) or microsatellite instability-high (MSI-H) tumors often benefit from ICIs, while the majority of CRC cases, which exhibit proficient mismatch repair (pMMR) or microsatellite-stable (MSS) status, generally show resistance to this approach. It is assumed that the MSI phenotype cause some changes in the tumor microenvironment (TME), thus triggering antitumor immunity and leading to response to immunotherapy. Understanding these differences in the TME relative to MSI status is essential for developing more effective therapeutic strategies. This review provides an overview of the TME components in CRC and explores current approaches aimed at enhancing ICI efficacy in MSS CRC.
1 Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer, accounting for 10.2% of all cases globally, and it is the second leading cause of cancer mortality, responsible for 9.2% of cancer death (1). Standard treatments for CRC include surgery, chemotherapy, radiotherapy, and targeted therapy, such as epidermal growth factor receptor (EGFR) inhibitors (e.g., cetuximab) and vascular endothelial growth factor receptor (VEGFR) inhibitors (e.g., bevacizumab) (2, 3). Unfortunately, the prognosis for patients with metastatic CRC remains poor, with over 80% of these patients succumbing to the disease within 5 years of diagnosis (4, 5).
Immunotherapy, which harnesses and modulates the patient’s immune system to combat cancer, is a promising treatment avenue. Immune system distinguishes self from non-self through the interaction between T-cell receptors (TCRs) and peptides by major histocompatibility complex (MHC) molecules on the surface of all cells, including tumor cells (6, 7). Co-stimulatory or co-inhibitory ligands can modulate the TCR-MHC signaling pathway, a mechanism often exploited by tumor cells to evade immune surveillance (8, 9). Immune checkpoint inhibitors (ICIs), which target co-inhibitory pathways such as cytotoxic T lymphocyte antigen 4 (CTLA4) and programmed cell death 1 (PD-1) on T cells, as well as programmed death ligand 1 (PD-L1) on tumor and immune cells, can restore T-cell function and enhance antitumor immunity (10). While ICIs have shown impressive efficacy in cancers like lung cancer and melanoma, their success in CRC varies significantly with disease stage and molecular subtype, underscoring the molecular heterogeneity of CRC.
Microsatellites, defined as short series of DNA repeats, are susceptible to occur replication errors typically corrected by the mismatch repair (MMR) system. Patients with the dMMR phenotype often exhibit MSI in tumor microenvironment (TME), a feature present in approximately 15% in CRC cases (11). Dysfunctional MMR systems lead to higher tumor mutational burdens (TMBs) in MSI tumors compared to microsatellite stable (MSS) tumors, generating a more robust antitumor immune response (12). Previous studies have shown that patients with dMMR/MSI-high (MSI-H) CRCs exhibit an objective response rate (ORR) of approximately 60% when treated with ICIs (13, 14), leading the FDA to approve ICIs for dMMR/MSI-H CRC patients (10). In contrast, MSS CRCs—which represent approximately 85% of metastatic CRC cases—demonstrate an ORR of only 5%–10% (10).
Immunophenotyping and antigenome meta-analyses reveal that MSI CRCs have a TME enriched in CD4+ and CD8+ T cells and depleted of myeloid-derived suppressor cells (MDSCs), underscoring the critical role of the TME in determining immunotherapy efficacy (15). However, among MSI tumors, those with mutations in genes involved in antigen processing and presentation, such as B2M, TAP1, TAP2, NLRC5, and RFX5, may still evade immune surveillance and show limited response to immunotherapy, which provides a clue of immunotherapy resistance of MSS phenotype (16–18). The mechanisms underlying immunotherapy resistance in these cases remain unclear and warrant further investigation.
2 Tumor immune microenvironment in CRC
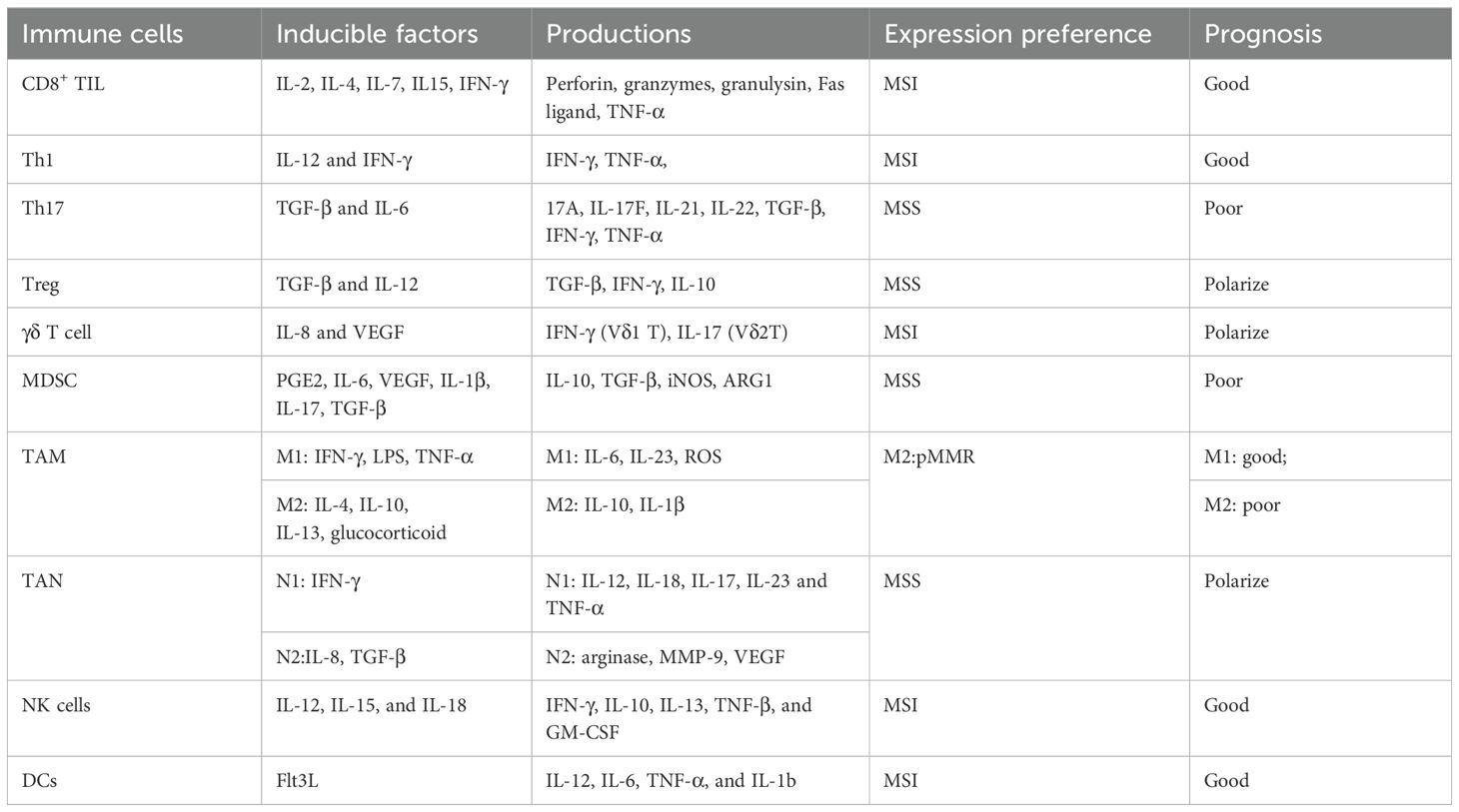
The TME, where tumors reside, is composed of immune cells, blood vessels, extracellular matrix (ECM), fibroblasts, and various signaling molecules (19, 20). Interactions between malignant and nonmalignant cells within TME are crucial for cancer development, progression, metastasis, and therapeutic outcome (21–23). Notably, the composition and spatial distribution of TME differ between MSS and MSI CRCs (10). Table 1 provides a summary of the characteristics of cellular components within the TME.
2.1 T cells
T cells, the most abundant immune cells in TME, are traditionally categorized into two main subsets: CD8+ and CD4+ T cells, each equipped with TCRs composed of an alpha and a beta chain (24). Tumor antigens are initially recognized and processed by antigen-presenting cells (APCs), such as dendritic cells, and subsequently presented on MHC class I and MHC class II molecules to CD8+ T cells and CD4+ T cells, respectively, collectively driving a robust antitumor immune response.
2.1.1 CD8+ T cell
CD8+ cytotoxic T lymphocytes (CTLs) are the primary T-cell subset responsible for directly killing tumor cells in the antitumor immune response. These cells are activated by cytokines such as interleukin-2 (IL-2), IL-4, IL-7, IL-15, and interferon-γ (IFN-γ). In TME, chemokines like CCL5 and CXCL9/10/11 bind to CXCR3 on CD8+ T cells, recruiting them to the TME, where they exert cytotoxic effects by secreting perforin, granzymes, granulysin, Fas ligand, and tumor necrosis factor α (TNF-α) (25–28). CD8+ T-cell infiltration is known to correlate strongly with improved patient survival and reduced recurrence risk, particularly in advanced cases (T4, N1-2), regardless of DNA MMR deficiency, POLE mutation, or chromosomal instability status (29, 30).
In MSS CRC, immune evasion often occurs through reduced CD8+ T-cell infiltration in the tumor center and functional exhaustion of these cells. In CRC, CD8+ T-cell density is generally lower in the tumor center than at the invasive margin, with higher central infiltration levels associated with better overall survival (31). Moreover, while CD8+ T-cell densities in stroma were similar in both MSS and MSI CRCs, as well as that in the tumor glands, MSI-H tumors tend to exhibit greater CD8+ T-cell density in both the tumor core and invasive margin (32). Studies have shown that CD8+ T cells in MSI-H tumors are more abundant and exhibit enhanced cytotoxic activity (33–37).
In MSS CRC, TIM-3+PD-1+CD8+CILs often accumulate with exhaustion phenotype, highlighting the role of co-inhibitory signal in T-cell dysfunction (38). Research has indicated that cholesterol accumulation in TME may exacerbate endoplasmic reticulum (ER) stress, which upregulates inhibitory receptors such as PD-1, 2B4, TIM-3, and LAG-3 on CD8+ T cells. Notably, inhibition of the ER stress sensor XBP1 or reduction of cholesterol has been shown to restore CD8+ T-cell antitumor activity (39). Furthermore, high level of IL-2 in TME can enhance inhibitory receptors’ expression and reduce cytokine and effector molecule production in CD8+ T cells through the activation of the STAT5 pathway (40). TOX, a transcription factor closely associated with T-cell exhaustion, is necessary for T-cell persistence within tumors; TOX deletion impairs T-cell survival, suggesting that T-cell exhaustion may serve as a regulatory mechanism to prevent overactivation and subsequent cell death in chronic immune responses (41).
2.1.2 CD4+ T cell
CD4+ helper T lymphocytes are crucial players in the immune response, not only activating other immune cells like B cells and cytotoxic T cells but also differentiating into subsets with diverse functions, including T helper (TH) 1 cells, TH2 cells, TH17 cells, TH9 cells, follicular helper T (Tfh) cells, and regulatory T (Treg) cells (42). Each of these subsets has distinct surface markers, transcription factors, polarizing cytokines, functions, and cytokine profiles, as detailed in prior comprehensive review (24). Unlike their CD8+ counterparts, CD4+ memory T cells are persistently present, which is vital for a sustained response to tumor antigens (43). Within TME, each CD4+ subset plays unique and sometimes contrasting roles, interacting in complex ways. For example, TH1-derived IFN-γ and TH2-derived IL-4 mutually inhibit each other’s expression (44). TH2 and TH9 lineages, despite sharing transcriptional pathways like STAT6, exert opposing effects within the TME, with TH2 generally supporting tumor progression, whereas TH9 promotes antitumor immunity (44).
TH1 cells are associated with favorable outcomes in CRC, as they can enhance cancer cell apoptosis, inhibit angiogenesis, and recruit toxic CD8+ T cells (45, 46). Compared with the MSS phenotype, MSI CRCs have a higher density of TH1 cells, underscoring their antitumor role within TME (32, 47). TH1 cells have also been reported to reshape the tumor-associated myeloid cell network, promoting interferon-activated antigen presentation and iNOS-expressing tumoricidal effector phenotypes, which together indirectly eradicates interferon-unresponsive and MHC-deficient tumors (48). TH2 cells, traditionally involved in immunity to parasites and allergic diseases, have recently been observed to exhibit antitumor activity through cytokines such as IL-4 and IL-5 (49). However, the TH2 cluster expression does not correlate with CRC patient prognosis (45). The role of TH2 cells in the TME remains complex and warrants further investigation to better understand their potential contributions to CRC outcomes.
TH17 cells play a crucial role in mucosal defense by producing various pro-inflammatory cytokines, including IL-17A, IL-17F, IL-21, IL-22, and TNF-α (50). They are activated through TGF-β and IL-6 via the p-STAT3 and RAR-related orphan receptor-γt (ROR-γt)-dependent pathways, while IL-23 serves as a growth and stabilizing factor (51). Within TME, TH17 cells are recruited by chemokines CCL4, CCL17, CCL20, and CCL22 (52). Research has demonstrated the pro-tumorigenic role of TH17 cells through several mechanisms (1): reducing CD8+ T-cell infiltration by inhibiting the production of chemokines CXCL9 and CXCL10 and downregulating CXCR3 expression via the IL-17A/STAT3 signaling pathway; (2) creating an immunosuppressive TME by recruiting and accumulating MDSCs; (3) impeding antigen presentation by dendritic cells (DCs) to CD4+ T cells through upregulation of nitric oxide (NO) levels; (4) enhancing the immunosuppressive capacity of mesenchymal stem/stromal cells (MSCs) and macrophage, thus facilitating tumor progression; and (5) inducing the expression of immunosuppressive mediators, including IL-6, TGF-β, and CCR6 (53–58). Nearly two-thirds of primary sporadic CRCs exhibit increased levels of TH17 cells, which is associated with poor prognosis (45). Compared to MSI-H tumors, MSS tumors show a higher proportion (approximately 40%) of TH17-cell-infiltrated phenotype (47, 59). Among patients with MSS CRC, those with lower IL-17A+ cell infiltration have shown better tumor control rates following anti-PD-1 immunotherapy (60), making IL-17 a potential target. Interestingly, owing to the presence of different IL-17 subtypes, TH17 cells can exhibit dual roles. Besides a protumor role, IL-17F has been shown to have antitumoral effects, and intraepithelial IL-17+ cell infiltration is positively associated with an improved prognosis (61, 62). Additionally, TH17 lineage can exert antitumorigenic effects by recruiting immune cells such as DCs, CD4+ T cells, and CD8+ T cells (52). This dual functionality might explain the association between tumor-infiltrating TH17 cells and favorable prognosis in cancers like oral squamous cell carcinoma, gastric cancer, and cervical cancer (63–65).
2.1.3 Regulatory T cells
Regulatory T cells (Tregs), characterized by high expression of CD25 and the transcription factor forkhead box protein P3 (FOXP3), play a crucial role in maintaining tolerance to self-antigens and immune homeostasis through production of TGF-β and IFN-γ, as well as direct cell-to-cell contact (66, 67). Within CRC TME, Tregs are induced by TGF-β and IL-12 and recruited by chemokines such as CCL1, CCL3, CCL4, CCL17, CCL20, and CCL22 (68, 69). The conversion of naive and memory conventional T cells to Tregs is facilitated by immature APCs and MDSCs (70). Tregs suppress antitumor immunity by inducing apoptosis in CD8+ T cells, producing IL-10 to inhibit NK cells and conversion of TH17 and TH1 cells, thus creating an immunosuppressive TME that supports CRC progression (71, 72). Elevated levels of Tregs have been detected in peripheral blood, tumor-draining lymph node, and tumor site of CRC patients (68, 71, 73). In particular, intratumor Tregs demonstrate higher suppressive activity (74). Compared to MSI subtypes, higher expression levels of FOXP3 and TGF-β are observed in MSS CRC (75), which is paralleled by the decreased number of CD8+ lymphocytes (76). Moreover, depletion of Tregs related to increased MSI phenotype correlated with T-cell response, which indicates the important role of Tregs to shape a suppressive TME in the MSS phenotype (77).
In recent years, the association between Tregs and prognosis of CRC patients has been controversial (78–81). This paradox may be explained by several factors. Both CD4+CD25+ natural Tregs (nTregs), which primarily suppress self-reactive T cells, and induced Tregs (iTregs), which maintain immune homeostasis, express FOXP3 during maturation (82, 83). Additionally, FOXP3 is not exclusive to Tregs, as some non-regulatory T cells and even tumor cells may also express this marker (84). Besides Foxp3, other surface markers expressed on Tregs, such as CTLA-4, LAG3, GITR, HLA-DR, ICOS, and CD127, are also essential for Treg functionality (85). These markers provide a foundation for novel strategies to improve homogeneous Treg purification. For example, based on CD45RA and HLA-DR expression, Tregs can be classified into three distinct subpopulations: naïve Tregs (CD45RA+, HLA-DR−), memory Tregs (CD45RA−, HLA-DR−), and memory/activated Tregs (CD45RA−, HLA-DR+) (86). Tregs may initially have a protective function by suppressing cancer-associated inflammation in early-stage CRC; however, they may adopt a pro-inflammatory phenotype as the cancer progresses to advanced stages (87). These findings suggest that previous studies focusing solely on FOXP3 expression may not fully capture the role of Tregs in the TME. Identifying additional biomarkers, such as HLA-DR and TGF-β, may help in isolating functionally suppressive Tregs more accurately.
2.1.4 γδ T cell
In addition to the predominant CD4+ and CD8+ T cells expressing αβ TCRs, a subset of primarily CD4/CD8 double-negative T cells with γδ TCRs has garnered attention as a promising immune cell population for next-generation cancer immunotherapy. These γδ T cells can recognize antigens directly on the cell surface, independent of MHC presentation, and are found at lower expression levels in the colonic tissue of CRC patients compared to healthy controls (88–91). In CRC, two main subtypes of γδ T cells are observed: those with variable region 1 of δ chain (Vδ1), which primarily produce IFN-γ, and those expressing Vδ6, which exhibit inflammatory properties through production of IL-17A (90, 92).
In the early stages of CRC, γδ T cells display cytotoxic markers and contribute to tumor surveillance and regression through IFN-γ production. However, as the tumor progresses, tumor-infiltrating γδ T cells can acquire a pro-tumorigenic profile, characterized by IL-17 secretion (90, 93, 94). A study by Sumana et al. identified a positive correlation between the expression levels of perforin and granzymes and MSI-H status, as well as higher levels of activated memory CD4+ T cells, γδ T cells, and M1 macrophages, suggesting a potential antitumor role of γδ T cells in MSI-H CRC (95). Because of the diverse functional roles of γδ T cells throughout tumorigenesis, it is challenging to define their precise role in MSS and MSI CRC without taking into account tumor stage and γδ T-cell subtype. To date, differences in the function and presence of γδ T cells between MSS and MSI CRC remain largely unexplored.
2.1.5 NK T cell
Apart from CD8+ T cell and NK cell, there is another cytotoxic subset, NK T cells, which exhibit characteristics of both conventional T cells and NK cells (96). Owing to the expression of both NK cell- and T cell-associated functional molecules, NKT cells can be activated in a T cell-like manner via recognition of glycolipids in the context of CD1d molecules and in an NK cell-like manner through inhibitory and stimulatory signals (97, 98). In addition to killing target cells directly, they are potent immune regulators by secreting TH1-, TH2-, TH17-, Treg-, and TFH cell-associated cytokines (99). Based on their TCR diversity, NKT cells can be divided into two subtypes: type I and type II NKT cells. Type I NKT cells recognize the glycosphingolipid α-galactosylceramide (α-GalCer) or its synthetic analogs, presented by MHC I-like CD1d molecules, while type II NKT cells recognize non-α-GalCer molecules presented by CD1d molecules (100). So far, it is found that type I NKT cells can be divided into five different functional subsets: TH1-, TH2-, TH17-, Treg-, and TFH-like type I NKT cells, while only two type I NKT cells were identified, TH1- and TH2-like subtypes (96). In TME, type I NKT cells generally play an antitumor role to inhibit tumor development and metastasis, while type II NKT cells associated with immunosuppression and tumor progression (101–103). CRC patients with high numbers of tumor-infiltrating type I NKT cells tend to have relatively favorable clinical outcome (104). However, tumor-infiltrating type I NKT cells exhibit impaired antitumor ability with decreased production of IFN-γ, which indicate that type I NKT cells might switch from a TH1- toward a TH2-like NKT cell subset in TME (105). Currently, owing to their variable population and function, NK T cells become a potential target to reshape TME. However, there is still an urgent need to expand and identify the NK T-cell population and the interaction between NK T cells and TME.
2.2 NK cells
NK cells, the cytotoxic members of the versatile family of innate lymphoid cells (ILCs), are capable of eliciting a robust and immediate antitumor response (106). Activated by cytokines such as IL-12, IL-15, and IL-18 produced by APCs, NK cells exert their antitumor effects in the TME through mechanisms including perforin/granzyme exocytosis, engagement of death receptors (such as Fas-FasL and TRAIL-TRAILR), and secretion of effector cytokines IFN-γ and TNF-α (107). In peripheral blood, nearly 90% of NK cells are characterized as CD56dim, CD16+, perforin+, expressing receptors such as CXCR1, CXCR2, CXCR4, and CX3CR1, which enable antibody-dependent cell-mediated cytotoxicity (ADCC) (108–110). In contrast, CD56bright NK cells, which express CCR7, CXCR3, CXCR4, and CD62L, are known for producing cytokines including IFN-γ, IL-10, IL-13, TNF-β, and GM-CSF, and often exhibit protumorigenic potential (111, 112). In the TME, TGF-β—a key immunosuppressive molecule—alters the balance between NK cell subsets by downregulating chemokines associated with CD56dim NK cells (CXCL1, CXCL2, CXCL1, and CXCL8) and upregulating those linked to CD56bright NK cells (CXCL9, CXCL10, CCL19, and CCL5) [33,34]. Notably, neutralization of TGF-β has been shown to restore the antitumor responses of both T cells and NK cells (113, 114).
Like T cells, NK cell function is regulated by signals from activating or inhibitory receptors (115). Activating receptors include natural killer group 2-C (NKG2C), NKG2D, DNAX accessory molecule-1 (DNAM-1), CD161, and natural cytotoxicity receptors (NCRs), such as NKp30, NKp44, and NKp46. Inhibitory receptors comprise NKG2A and killer cell immunoglobulin (Ig)-like receptors, such as CD158a and CD158b (116). In CRC, NK cells exhibit reduced expression of the natural cytotoxicity receptors NKp44 and NKp46 compared to healthy controls, with functional exhaustion of NK cells correlating with shorter overall survival (116, 117). Moreover, in CRC, higher activation levels of NK cells have been positively associated with MSI status (118).
2.3 MDSC
MDSCs are characterized by their strong immunosuppressive role and related to poor prognosis (119). MDSCs induced by proinflammatory mediators, produced during cancer-related chronic mucosal inflammation, such as prostaglandin E2 (PGE2), IL-6, VEGF, IL-1β, S100A8/A9 proteins, and the complement component 5a (C5a) (120). Local hypoxia, low pH and exosomes secreted by tumor cells also could activate MDSCs (121–126). Moreover, IL-17 plays a crucial role in the induction, expansion, and suppressive function development of MDSCs (127). The suppressive role of MDSCs in cancer mostly contributes to the activation of two enzymes, inducible NO synthase (iNOS) and arginase-1 (ARG1), impeding proliferation and proper functioning of T cells through depletion of L-arginine (128–131). Moreover, MDSCs activate Tregs and anergy of NK cells by secretion of IL-10 and TGF-β, with TGF-β also inducing MDSCs and the epithelial-to-mesenchymal cell transition (EMT) process (132–135). MDSCs can also regulate VEGF bioavailability through inducing high levels of matrix metalloprotease 9 (MMP9) and pro-MMP9 to stimulate tumor growth and metastases in CRC (136, 137). Based on cell surface markers and cell morphology, the MDSC population can be divided into granulocytic/polymorphonuclear MDSCs (G-MDSCs/PMN-MDSCs, CD33+ CD11b+ HLA-DRlowCD15+ cells) settling in the peripheral lymphoid organs, and monocytic MDSCs (M-MDSCs, CD33+ CD11b+ HLA-DRlowCD14+ cells) existing in the tumor bed (138–140). PMN-MDSCs are mainly responsible for reactivating oxygen species (ROS) production, while M-MDSCs have high expression of longer activity iNOS (140, 141). In addition, enhanced infiltration of Tregs and MDSCs was detected in MSS and MSI-L tumors than its counterpart with the MSI-H phenotype (142).
2.4 Tumor-associated macrophages
Macrophages, a key component of the mononuclear phagocytic system (MPS), are essential for maintaining innate immune response, tissue homeostasis, and inflammation (143). Tumor-associated macrophages (TAMs) represent a major population within the immune cell component of the TME, recruited by various chemokines, including CCL2 and CCL5 and colony-stimulating factor 1 (CSF1) (144, 145). TAMs can be broadly divided into two distinct subtypes: classically activated M1 macrophages and alternatively activated M2 macrophages (146–148). M1 macrophages, which are activated by cytokines such as IFN-γ, lipopolysaccharide (LPS), and TNF-α, contribute to antitumor inflammation by promoting a TH1 response and secreting pro-inflammatory mediators, including IL-6, IL-23, and ROS (149, 150). In contrast, M2 macrophages, induced by IL-4, IL-10, IL-13 or glucocorticoids, secrete anti-inflammatory cytokines like IL-10 and IL-1β, and they play a role in promoting angiogenesis, tissue remodeling, injury repair, tumor initiation, and progression (151, 152). During the initial stages of tumor development, TAMs are primarily composed of M1 macrophages, which promote antitumor responses. However, as neoplasia progresses, there is a shift towards a predominance of M2 macrophages in the TME leading to antitumor activation, while after neoplasia formation, M2 macrophages are predominantly recruited in TME (152, 153). In CRC, tumor cells have been shown to increase the population of M2 TAMs through the PI3K/AKT signaling pathway (154, 155). Notably, M1 and M2 macrophages exhibit a degree of plasticity, with the ability to transition between states, suggesting the therapeutic potential of inducing a switch from M2 to M1 phenotypes (156, 157). Consistently, these macrophage subtypes are associated with opposite prognoses in CRC: M1 macrophage infiltration is correlated with favorable outcomes, whereas M2 TAMs are linked to poorer prognosis (158, 159). Compared to dMMR CRC, pMMR CRC shows enhanced M2 macrophage polarization, which is associated with poor survival in these cases (160). Furthermore, a recent multi-omic of MSS patients revealed that a higher density of M2 macrophage is positively correlated with improved response rate to immunotherapy (161).
2.5 Tumor-associated neutrophils
In human peripheral blood, neutrophils comprise 50%–70% of the circulating leukocytes and serve as the primary defense against infection (162, 163). Within TME, neutrophils are recruiting by chemokines secreted by tumor cells, notably through the CXCL1, CXCL2, CXCL5, and CXCL8 signaling axes that interact with CXCR1/2 receptors (164, 165). Tumor-derived GM-CSF can activate neutrophils and induce PD-L1 expression via the JAK/STAT3 signaling pathway (166). Neutrophils in the TME, known as tumor-associated neutrophils (TANs), can be polarized into either antitumor (N1) or protumor (N2) phenotypes, depending on the activating factors (167). N1-TANs, induced by IFN-γ, enhance tumor cytotoxicity and attenuate immunosuppression by producing TNF-α, intercellular adhesion molecule-1 (ICAM-1), ROS, and apoptosis-related factor (Fas). They also reduce the expression of arginase, a contributor to immunosuppression (167, 168). In addition, N1-TANs can release various chemokines and cytokines that stimulate immune cell proliferation and activation, thereby initiating antitumor immune responses (169). For example, TANs can recruit and activate CD8+ T cells by secreting CCL-3, CXCL-10, TNF-α, and IL-12 (170), and can activate NK cells and DCs through IL-18 and TNF-α release, respectively (171, 172). In contrast, N2-TANs, which are induced by TGF-β, promote tumor growth and participate in tumor migration and metastasis by upregulating arginase, MMP-9, VEGF, and additional chemokines (167, 173). As tumors progress, the N1 phenotype can convert into the N2 phenotype (174). In CRC, the prognostic value of TAN infiltration remains debated. For example, a study by Maria et al., using CD66b and myeloperoxidase (MPO) as neutrophil markers, found that higher infiltration of TANs correlated with improved prognosis and favorable response to 5-fluorouracil (5-FU)-based chemotherapy, suggesting a predominantly antitumor role for TANs (175). Conversely, other studies have identified TAN infiltration as an unfavorable prognostic marker, with higher TAN density frequently observed in MSS CRC (176–178). This paradox may be attributable to issues such as inconsistent marker selection, limited sample sizes, and unaddressed confounding factors (163).
2.6 Dendritic cells
DCs, as specialized professional APCs induced by Flt3L, play a pivotal role in initiating, coordinating, and amplifying antitumor immune responses (179–181). DCs encompass two primary subsets: classical/conventional DCs (cDCs) and plasmacytoid DCs (pDCs) (182, 183). The cDC subset is further divided into CD141+ cDC1, which presents antigens on MHC I for CD8 T cells priming by cross-presentation, and CD1c+ cDC2, which presents MHC II to activate CD4 T cells (184). pDCs, capable of producing large amounts of type I interferons (IFNs) during virus infection, play an essential role in maintaining immune tolerance in autoimmune conditions (185, 186). DCs enhance infiltration and toxicity of TH1, NK, and CD8+ T cells by enhancing their migration through CCR7 expression, upregulating co-stimulatory molecules such as CD80, CD83, and CD86, and secreting of pro-inflammatory cytokines like IL-12, IL-6, TNF-α, and IL-1β (187–190). However, within TME, DC function is frequently impaired by immunosuppressive signals from tumor cells. This impairment is a key contributor to immune evasion, tumor growth, metastasis initiation, and treatment resistance in various cancers, including CRC (187, 188, 191–195). In TME, TGF-β, TNF-α, IDO-1, PGE2, IL-6, IL-10, VEGF, and GM-CSF hinder DC migration, antigen presentation, and the effective activation of T cell and NK (196, 197). In CRC specifically, the density of mature DCs ranks lowest in metastatic sites, intermediate in primary tumor sites, and highest in normal mucosa (198, 199). In addition, mature DCs are typically localized in the invasive margin and form clusters with T cells with tertiary lymphoid structures (TLS), whereas immature DCs are more dispersed through the tumor stroma, underscoring the suppressive effects of TME on DC maturation and function (200, 201). Recent findings have also highlighted a deficiency of activated T cells and DCs in MSS CRCs and liver metastasis, where enhanced DC infiltration combined with immune checkpoint blockade (ICB) treatment significantly improved survival in murine models (202).
3 Current therapeutic strategies
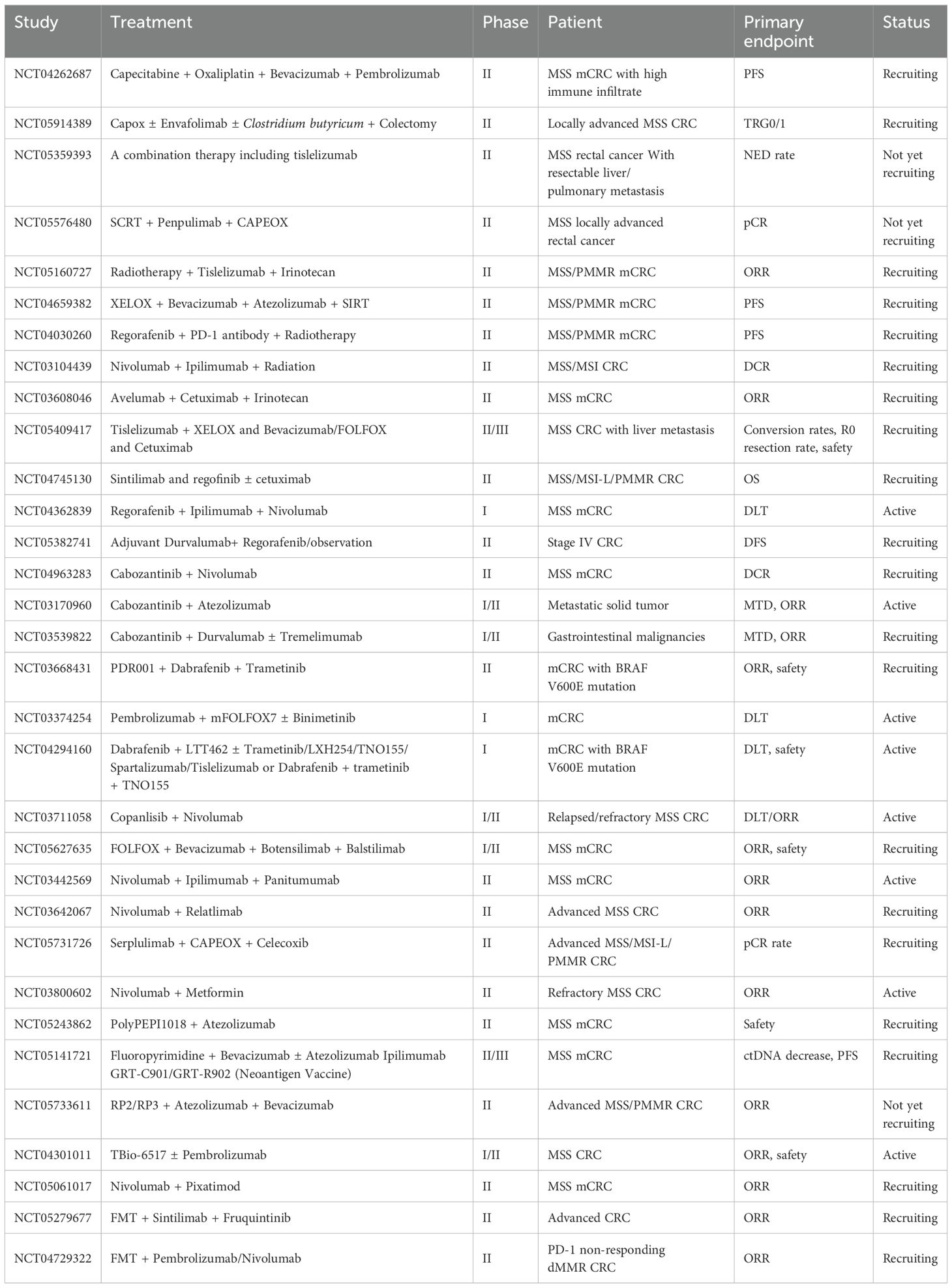
Since initial immunotherapy trials in CRC revealed substantial efficacy differences between MSS and MSI-H subtypes, research efforts have largely pivoted to focus on the MSS subtype. A landmark phase 3 open-label trial, KEYNOTE-177, compared the efficacy of pembrolizumab to standard chemotherapy in patients with MSI-H and dMMR CRC, demonstrating a significantly improved progression-free survival (PFS) in the immunotherapy group (16.5 vs. 8.2 months). This trial marked a milestone in CRC immunotherapy, establishing pembrolizumab as the new first-line treatment for patients with MSI-H/dMMR mCRC (203). Additionally, the combination of CTLA-4 and PD-1 inhibitors has been shown to improve survival in patients with MSI-H/dMMR mCRC and has been approved as a second-line treatment option (204). In contrast, immunotherapy attempts in MSS CRC have yielded unsatisfactory outcomes. As the MSS phenotype represents the majority of CRC cases, numerous strategies are being explored to enhance immunotherapy efficacy in MSS CRC. The current clinical trials investigating these approaches are summarized in Table 2.
3.1 Combination with chemotherapy
Chemotherapy, a cornerstone of cancer treatment, has been shown to impact immune cells directly, in addition to its cytotoxic effects on malignant cells. In the murine model, cyclophosphamide appears to enhance TH1 differentiation and reduce Treg by mediating an IRF1-dependent, IL-12-mediated response (205). Agents like 5-fluorouracil (5-FU) and gemcitabine have been found to selectively decrease the frequency of MDSCs in the spleen and tumor bed, without directly affecting T cells, DCs, NK cells, or B cells. The elimination of MDSCs, in turn, promotes IFN-γ production by infiltrating CD8+ T cells (206). Furthermore, cyclophosphamide, paclitaxel, and doxorubicin can repolarization of macrophages from an M2 to an M1 phenotype, shifting them towards a more pro-inflammatory, antitumor function (207, 208). Chemotherapy also exerts differing effects on TME of primary and metastatic lesions. An analysis of immune profiles in primary tumor and paired metastasis tissue samples from patients with MSS CRCs revealed that increased expression of CD8 and PD-L1 was higher in metastatic sites following neoadjuvant FOLFOX treatment (folinic acid, 5-FU, and oxaliplatin) (209). Beyond MSI CRCs, studies indicate that a subset of MSS CRCs exhibit high CD8+ infiltration, suggesting potential sensitivity to immunotherapy (38). A phase II clinical trial (NCT04262687) is currently investigating the efficacy of PD-1 inhibitors in patients with high immune infiltration. However, for the majority of MSS CRC cases, the combination of PD-1 inhibitors and chemotherapy has shown limited effectiveness. Recent research trends are exploring the potential of this combination in neoadjuvant settings, as seen in clinical trials NCT05914389, NCT05359393, and NCT05576480.
3.2 Combination with radiotherapy
Radiotherapy also plays a pivotal role in remodeling antitumor immunity and enhancing immunotherapy efficacy. Studies have shown that radiation exposure can induce M1 polarization, enhance NK cell infiltration, and reduce TGF-β levels, collectively contributing to an immune-stimulatory environment (210–212). In the VOLTAGE-A phase I b/II study (NCT02948348), neoadjuvant chemoradiotherapy (CRT) followed by nivolumab and radical surgery was tested in patients with MSS locally advanced rectal cancer, with 30% of patients achieving a pathologic complete response (pCR), which show a great potential of this combination (213). Post-surgical pathological analysis revealed that a PD-L1 tumor proportion score (TPS) of ≥1% and a CD8+ T cell-to-effector Treg ratio of ≥2.5 were associated with a higher pCR rate. Current clinical trials are further investigating the efficacy of combining radiotherapy with PD-1 monoclonal antibody therapy in patients with metastatic CRC (NCT05160727, NCT04659382, NCT04030260, and NCT03104439).
3.3 Combination with anti-angiogenic agent
VEGF, known for promoting tumor vessel growth, also plays an immunosuppressive role by increasing the recruitment of Tregs and MDSCs and inhibiting the differentiation and activation of DCs. Antiangiogenic agents targeting the VEGF/VEGFR pathway, such as bevacizumab, can help mitigate VEGF-associated immunosuppression (214). In the phase II AtezoTRIBE trial, patients with metastatic CRC received FOLFOXIRI plus bevacizumab with or without the addition of atezolizumab. In the pMMR subgroup, disease progression was observed in 81% of patients in the control group versus 70% in the atezolizumab group, indicating a potential synergistic effect between atezolizumab and bevacizumab (215). However, another phase II trial comparing mFOLFOX6 plus bevacizumab alone or with avelumab, showed no significant difference in median PFS between experimental and control groups (216).
3.4 Combination with an anti-EGFR agent
Cetuximab, a chimeric IgG1 monoclonal antibody targeting EGFR, is a first-line treatment for advanced CRC. Recent studies have shown that cetuximab administration can increase CD3+ T, CD8+ T, and NK cells, while decreasing Tregs (217). The phase II AVETRIC trial investigated the efficacy and safety of first-line mFOLFOXIRI combined with cetuximab and avelumab in RAS wild-type mCRC patients, reporting an impressive 98% disease control rate (DCR) at the ASCO meeting, highlighting the potential of cetuximab combined with immunotherapy. A phase II study (NCT03608046) is currently evaluating avelumab combined with cetuximab and irinotecan for patients with refractory MSS CRC. Additionally, clinical trials (NCT05409417 and NCT04745130) are comparing the efficacy of cetuximab with anti-angiogenic agents (bevacizumab and regorafenib) in combination with immunotherapy for CRC patients.
3.5 Combination with tyrosine kinase inhibitors
Regorafenib is a multi-targeted small-molecule tyrosine kinase inhibitor approved for the treatment of refractory and mCRC. It achieves antitumor effects by inhibiting various tyrosine kinases involved in tumor growth, angiogenesis, and metastasis. In the phase Ib trial REGONIVO, 25 patients with CRC (24 pMMR-MSS and 1 dMMR-MSI-H) received a combination of nivolumab and regorafenib, achieving an objective response rate (ORR) of 36% and a PFS of 7.9 months (218). However, in a subsequent phase II clinical trial of the same combination in 70 patients with pMMR/MSS CRC, ORR dropped to 7%, with a PFS of just 1.8 months (219). Another phase Ib/II study combining regorafenib and toripalimab reported an improved ORR of 15.2% and a DCR of 36.4% (220). Given the limited efficacy of these combinations, researchers are now exploring regorafenib in conjunction with two ICIs—nivolumab and ipilimumab (NCT04362839)—and as part of adjuvant therapy (NCT05382741) to assess potential improvements in treatment outcomes.
Cabozantinib, a small-molecule multi-tyrosine kinase inhibitor targeting kinases such as VEGFR2, MET/HGF, AXL, MER, and TYRO3, has demonstrated antitumor activity in the preclinical CRC model. It enhances the immune environment by reducing the function of Tregs and increasing cytokine production (221–223). In the CAMILL trial, 17 patients with MSS CRC were treated with a combination of cabozantinib and durvalumab, showing potential efficacy with ORR of 23.5% and a DCR of 88.2%. The median PFS was 4.6 months, and the OS reached 9.6 months (224).
The RAS/BRAF/MEK/ERK pathway, which operates downstream of various growth factor receptors, including EGFR, is often overexpressed and activated in CRC, particularly in cases with dysregulated MAPK pathway signaling (225). Studies have shown that dysregulation in this pathway is linked to reduced T-cell infiltration, activation of MDSCs, DC function, and lower expression of inhibitory molecules such as CTLA4, PD-L1, PD-L2, LAG3, and TIM3, contributing to an immunosuppressive TME (226, 227). These findings suggest a potential synergy between immunotherapy and selective RAS/BRAF/MEK/ERK pathway inhibitors in patients with pMMR/MSS mCRC. However, a phase III trial (COTEZO) evaluating the combination of atezolizumab and MEK inhibitor cobimetinib versus atezolizumab alone or regorafenib in patients with chemo-refractory CRC did not meet its primary endpoint, showing no significant difference in PFS or ORR between the treatment arms (228). Ongoing clinical trials are now assessing the efficacy of PD-1/PD-L1 inhibitors combined with MEK inhibitors (NCT03668431, NCT03374254, and NCT04294160).
3.6 Combination with a PI3K inhibitor
The PI3K/Akt pathway is frequently activated in various cancers and contributes to carcinogenesis by promoting cell proliferation, survival, metabolic reprogramming, invasion, and metastasis, while inhibiting autophagy and senescence. Recent studies have indicated that PI3K/Akt pathway status can significantly influence the immune microenvironment (229). In patients with CRC, mutations in the PI3K/Akt pathway have emerged as an independent predictor of immunotherapy response, correlating to better OS and increased immune cell infiltration, such as M1 macrophages, neutrophils, and NK cells, within TME (230). Copanlisib, a pan-class I PI3K IV inhibitor, has shown promising preclinical antitumor activity in CRC (231). A phase I/II clinical trial (NCT03711058) is currently underway to evaluate the efficacy and safety of combining copanlisib with nivolumab in patients with relapsed/refractory MSS CRC.
3.7 Combination with novel ICIs
CTLA-4, another checkpoint, is expressed on the surface of activated T cells, where it competes with CD28 receptors for binding to B7 ligands on APCs, leading to anergy in T cells (232). CTLA-4 expression is primarily regulated by FOXP3 on Tregs, thereby contributing to an immunosuppressive TME (233). The combined blockade of PD-1 and CTLA-4 has shown synergistic clinical benefit, resulting in regulatory approval for conditions such as metastatic melanoma, advanced renal cell carcinoma, and metastatic CRC with the MMR/MSI-H phenotype (234). The CCTG CO.26 study was the first to demonstrate that anti-PD-L1 combined with anti-CTLA-4 could extend OS in patients with refractory MSS CRC compared to best supportive care (BSC) (235). Ongoing clinical trials are assessing the efficacy of combining PD-1 and CTLA-4 blockade with additional treatments, such as FOLFOX and bevacizumab (NCT05627635) or panitumumab (NCT03442569).
LAG-3 is an inhibitory signaling expressed on both CD4+ and CD8+ T cells upon antigen simulation (236). It is constitutively expressed on FOXP3+ Tregs, resulting in elevated levels of immunoregulatory cytokines IL-10 and TGF-β, which suppress tumor-specific T-cell responses (236). Preclinical studies indicate that dual blockade of LAG-3 and PD-1 can restore T-cell function and reverse anergy. In a phase I clinical trial, the combination of an anti-LAG-3 antibody (favezelimab) and an anti-PD-1 antibody (pembrolizumab) in patients with advanced MSS mCRC demonstrated promising antitumor ability with an OS of 8.3 months and a median PFS of 2.1 months (237). Currently, a phase II study (NCT03642067) is underway to assess the safety and efficacy of nivolumab in combination with relatlimab in patients with advanced MSS CRC.
3.8 Combination with other drugs
Temozolomide (TMZ) is an oral alkylating agent methylating the O6 position of guanine in DNA, providing therapeutic efficacy in several solid tumors such as glioma, glioblastoma, neuroendocrine tumors, melanoma, and sarcomas. O6-methylguanine methyltransferase (MGMT), a DNA repair enzyme encoded by the MGMT gene, plays a crucial role in repairing DNA damage caused by alkylating agents and is associated with the reduced therapeutic efficacy of TMZ (238). Epigenetic silencing of MGMT, primarily through methylation of its promoter region, is observed in approximately 40% of CRC cases (239). The ORR of patients with MGMT-methylated mCRC treated with TMZ was 12% (240). Acquired resistance to TMZ is often linked to hypermutation and the emergence of mutations in MMR genes, particularly MSH6 (239). The ARETHUSA trial (NCT03519412) analyzed tissue and circulating tumor DNA (ctDNA) in patients with MGMT-deficient, MMR-proficient, RAS-mutant mCRC treated with TMZ priming. Findings showed increased TMB and MSH6 mutation in 94% of cases post-TMZ treatment. A subset of patients with TMB > 20 mutations per megabase, subsequently treated with pembrolizumab, achieved disease control (238). These promising results suggest that TMZ could serve as a priming agent for re-sensitizing pMMR/MSS CRCs to ICIs. Supporting these findings, the phase II MAYA trial (NCT03832621) administrated two cycles of TMZ followed by a combination of ipilimumab and nivolumab in patients with chemo-refractory MSS CRC and MGMT silencing, yielding an improved ORR of 45%, an 8-month PFS rate of 36%, and an OS of 18.4 months (239).
Cyclooxygenase enzyme-2 (COX-2) expression is significantly elevated in 79% of MSS CRC, compared to only 48% of MSI CRC and is associated with poorer OS (241, 242). Prostaglandin E2 (PGE2), a product of COX-2, can activate MDSCs via STAT3 phosphorylation, leading to suppressed CD8+ T-cell proliferation, reduced cytotoxicity against tumor cells, and impaired macrophage phagocytosis of cancer cells through the EP4-PI3K-Akt-ND-1 signaling pathway (243–250). Preclinical studies suggest that COX inhibitors could serve as effective adjuvant for ICIs, potentially reactivating immunosuppressive TME in MSS CRCs (251). The NICHE study (NCT03026140) explored the neoadjuvant administration of ipilimumab and nivolumab, with or without celecoxib, in patients with pMMR CRC. Results showed that 4 of 15 patients (27%) in the non-celecoxib group experienced pathological responses, whereas 4 of 7 patients (57%) in the celecoxib group achieved pathological responses (252). An ongoing phase II study (NCT05731726) is investigating the role of celecoxib as part of a neoadjuvant treatment regimen combined with PD-1 monoclonal antibody and chemotherapy in locally advanced CRC patients with the pMMR/MSS phenotype.
Accumulating evidence shows that metformin, a medication primarily used to treat type II diabetes, may regulate tumor cell metabolism through reducing their oxygen consumption, alleviating tumor hypoxia, and supporting the activity of cytotoxic T lymphocytes within tumor tissues (253). A phase II clinical trial (NCT03800602) is investigating the efficacy of combining metformin with nivolumab in the treatment of refractory MSS CRC.
3.9 Combination with a bispecific antibody
A bispecific antibody is engineered to recognize two distinct antigens, effectively bridging the tumor cell and the T cell to bolster immune activity against tumor cells (254). Cibisatamab (RO6958688), the most extensively studied bispecific antibody in CRC, targets CEA on colorectal tumor cells and CD3 on T cells, promoting increased T-cell infiltration, activation, and PD-1/PD-L1 upregulation (255). Preclinical studies have demonstrated that combining PD-1/PD-L1 blockade with an anti-CEA/CD3 bispecific antibody can significantly enhance T cell-mediated antitumor activity (256). A phase I study comparing the efficacy of anti-CEA/CD3 bispecific antibody as monotherapy versus in combination with atezolizumab (NCT02650713) in patients with mCRC showed that combination therapy notably improved outcomes, particularly in patients with MSS CRC (257).
3.10 Combination with vaccines
Cancer vaccines aim to stimulate an antitumor response by isolating and presenting tumor-specific antigens. DCs, as pivotal APCs, are considered promising vectors for cancer vaccines. However, two phase II clinical trials assessing the efficacy of an autologous tumor lysate DC vaccine—both as monotherapy and in combination with avelumab—in patients with MSS CRC demonstrated that while the vaccine induced a tumor-specific immune response, it did not yield significant clinical benefits (258, 259).
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is an important growth and differentiation factor for DCs. The GVAX colon vaccine, composed of GM-CSF-expressing irradiated tumor cells, is designed to induce T-cell immunity (260). Preclinical studies have demonstrated that dual blockade of PD-1 and CTLA-4, combined with the GM-CSF gene-transfected GVAX vaccine, effectively induced tumor rejection and restored T-cell activity in CRC murine models (261). In a phase II study (NCT02981524), the efficacy of GVAX combined with pembrolizumab was assessed in patients with pMMR CRC; although it failed to meet its primary objective, with no objective responses observed, biochemical responses (≥30% decline in CEA) were achieved in 7 out of 17 patients (41%) (262).
PolyPEPI1018 is a cancer vaccine consisting of six synthetic peptides derived from seven tumor-associated antigens (TAAs) commonly expressed in CRCs. In OBERTO-101 study, PolyPEPI1018 was administered as an interim therapy between first-line induction and maintenance treatment in 11 previously treated patients with MSS mCRC, resulting in an increased density of tumor-infiltrating lymphocytes (TILs) and a broad antitumor T-cell response (263). Following these promising results, a phase II clinical study (NCT05243862) is currently recruiting to further investigate the safety and efficacy of combining PolyPEPI1018 with atezolizumab.
Neoantigens are tumor-specific antigens produced by somatic mutations, and personalized neoantigen vaccines have demonstrated the ability to induce robust antitumor immune responses across multiple cancer types, including MSS CRC (264). When combined with PD-1 blockade, these vaccines produce a synergistic effect by enhancing neoantigen-specific CD4+ and CD8+ T-cell responses (265, 266). The efficacy of combining personalized neoantigen vaccines with immunotherapy for MSS CRC is currently under investigation in a clinical trial (NCT05141721).
3.11 Combination with an oncolytic virus
Oncolytic viruses (OVs), such as adenovirus, vaccinia virus, and herpes simplex virus, are genetically modified to selectively or preferentially infect cancer cells, resulting in direct lysis of tumor cells and induction of an antitumor immune response (267). Moreover, OVs engineered with cytokines like GM-CSF, IL-15, and TNF superfamily members can enhance this immune response by activating immune cells, including CD4+ and CD8+ T cells, and increasing the expression of perforin and granzyme B in the TME. This process effectively transforms “cold” tumors into “hot” ones (268–270). The combination of OVs with ICIs has shown synergistic effects in preclinical CRC models (271, 272). A phase 1b clinical study of OV (Pexa-Vec) combined with ICIs in patients with CRC demonstrated an acceptable safety profile and achieved a 67% radiographically stable disease rate, resulting in further trails to evaluating the efficacy of Pexa-Vec (273). Two additional clinical studies are ongoing to further evaluate the efficacy of this combination (NCT05733611 and NCT04301011).
3.12 Combination with other therapies
Toll-like receptor 9 (TLR9), a pattern recognition receptor, is expressed on B cells and plasmacytoid DCs in humans (274). Pixatimod, a TLR9 pathway activator, inhibits the infiltration of TAMs, stimulates DCs, and activates NK cells (275). A phase Ib clinical trial evaluated the safety and efficacy of pixatimod combined with nivolumab in 25 participants with microsatellite-stable (MSS) metastatic CRC (mCRC), achieving PR in 3 participants and SD in 8 participants, which meets its primary endpoint and warrants further investigation (276). A phase II clinical trial (NCT05061017) is currently recruiting to further investigate this combination in MSS mCRC.
Fecal microbiota transplantation (FMT) offers a promising approach to modify the gut microbiome with a favorable safety profile. Preclinical studies have shown that microbiome manipulation can enhance the antitumor activity of ICIs, likely due to alterations in glycerophospholipid metabolism and increased expression of IFN-γ and IL-2 in TME (277). Two clinical trials (NCT05279677 and NCT04729322) are currently investigating the efficacy of combining FMT with ICIs.
4 Conclusion
Despite considerable advancements in immunotherapy, specifically ICIs, the efficacy of these treatments remains limited for the majority of CRC patients, especially those with MSS and pMMR tumors. Given that MSS/pMMR CRC represents a substantial proportion of cases, there is an urgent need to develop novel therapeutic strategies. The TME plays a significant role in determining treatment responses, with variations in immune cell composition and activity affecting the efficacy of ICIs in MSS CRC.
As we have discussed, CRCs exhibit distinct TME profiles depending on their MSI status. MSI CRCs tend to have an inflammatory TME, characterized by a significant increase in plasma cells, CD8+ T cells, activated memory CD4+ T cells, follicular T helper cells, NK cells, M1 macrophages, and neutrophils, alongside a marked reduction in Tregs (278). Additionally, the TME of MSI CRCs shows higher levels of inflammatory cytokines, including TNF-α, perforin, granzyme, IL-1, IL-6, and IFN-γ (279). Some components of the TME, such as TH17 cells, Tregs, γδ T cells, TAMs, TANs, and NK T cells, exhibit dual functions depending on the specific context, contributing to the complex network of interactions within the TME (92, 152, 153, 167, 280). Furthermore, the TME is highly dynamic throughout CRC progression and metastasis. For example, TAMs initially exhibit an inflammatory profile dominated by antitumor M1 macrophages, but over time, as the tumor progresses, the TME shifts to a more pro-tumorigenic environment with an increased presence of M2 macrophages (152, 153). The roles and properties of individual TME components can change significantly during tumor progression and in response to treatment. Given the dynamic nature of the TME, comprehensive and functional characterization of its components at different stages of tumor development is crucial. This will help identify CRC patient subgroups most likely to benefit from TME-modulating therapies and determine the optimal timing for these treatments.
Recent studies underscore the potential of combination therapies involving ICIs and traditional treatments such as chemotherapy and radiotherapy, as well as targeted approaches such as tyrosine kinase inhibitors, anti-angiogenic agents, and EGFR inhibitors, to re-sensitize MSS CRC to immunotherapy. Emerging strategies incorporating bispecific antibodies, cancer vaccines, OVs, and fecal microbiota transplantation (FMT) also show promise in enhancing immune responses within the TME, converting it from an immunosuppressive to an immunostimulatory state. Personalized approaches, including neoantigen vaccines and other novel ICIs targeting checkpoints like CTLA-4 and LAG-3, may further optimize treatment efficacy by addressing specific immune evasion mechanisms.
While these approaches hold promise, clinical efficacy and long-term outcomes for MSS/pMMR CRC patients remain to be conclusively determined. Future research and clinical trials will be essential to better understand the complex interactions within the TME and to develop integrated, personalized therapeutic strategies that leverage the latest advancements in immuno-oncology.
Author contributions
QW: Investigation, Methodology, Writing – original draft. MY: Resources, Visualization, Writing – review & editing. SZ: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Feng M, Zhao Z, Yang M, Ji J, Zhu D. T-cell-based immunotherapy in colorectal cancer. Cancer Lett. (2021) 498:201–9. doi: 10.1016/j.canlet.2020.10.040
3. Vacchelli E, Pol J, Bloy N, Eggermont A, Cremer I, Fridman WH, et al. Trial watch: tumor-targeting monoclonal antibodies for oncological indications. Oncoimmunology. (2015) 4:e985940. doi: 10.4161/2162402x.2014.985940
4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. (2019) 69:7–34. doi: 10.3322/caac.21551
5. Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. (2017) 67:177–93. doi: 10.3322/caac.21395
6. Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: immunomodulation, cars and combination immunotherapy. Nat Rev Clin Oncol. (2016) 13:273–90. doi: 10.1038/nrclinonc.2016.65
7. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. (2011) 331:1565–70. doi: 10.1126/science.1203486
8. Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discovery. (2018) 8:1069–86. doi: 10.1158/2159-8290.Cd-18-0367
9. Sharma P, Allison JP. The future of immune checkpoint therapy. Science. (2015) 348:56–61. doi: 10.1126/science.aaa8172
10. Ganesh K, Stadler ZK, Cercek A, Mendelsohn RB, Shia J, Segal NH, et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. (2019) 16:361–75. doi: 10.1038/s41575-019-0126-x
11. Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, et al. A national cancer institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. (1998) 58:5248–57.
12. Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. (2017) 9:34. doi: 10.1186/s13073-017-0424-2
13. Asaoka Y, Ijichi H, Koike K. Pd-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. (2015) 373:1979. doi: 10.1056/NEJMc1510353
14. Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (Checkmate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. (2017) 18:1182–91. doi: 10.1016/s1470-2045(17)30422-9
15. Angelova M, Charoentong P, Hackl H, Fischer ML, Snajder R, Krogsdam AM, et al. Characterization of the immunophenotypes and antigenomes of colorectal cancers reveals distinct tumor escape mechanisms and novel targets for immunotherapy. Genome Biol. (2015) 16:64. doi: 10.1186/s13059-015-0620-6
16. Giannakis M, Mu XJ, Shukla SA, Qian ZR, Cohen O, Nishihara R, et al. Genomic correlates of immune-cell infiltrates in colorectal carcinoma. Cell Rep. (2016) 15:857–65. doi: 10.1016/j.celrep.2016.03.075
17. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to pd-1 blockade. Science. (2017) 357:409–13. doi: 10.1126/science.aan6733
18. Grasso CS, Giannakis M, Wells DK, Hamada T, Mu XJ, Quist M, et al. Genetic mechanisms of immune evasion in colorectal cancer. Cancer Discovery. (2018) 8:730–49. doi: 10.1158/2159-8290.CD-17-1327
19. Spill F, Reynolds DS, Kamm RD, Zaman MH. Impact of the physical microenvironment on tumor progression and metastasis. Curr Opin Biotechnol. (2016) 40:41–8. doi: 10.1016/j.copbio.2016.02.007
20. Del Prete A, Schioppa T, Tiberio L, Stabile H, Sozzani S. Leukocyte trafficking in tumor microenvironment. Curr Opin Pharmacol. (2017) 35:40–7. doi: 10.1016/j.coph.2017.05.004
21. Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci. (2012) 125:5591–6. doi: 10.1242/jcs.116392
22. Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. (2012) 21:309–22. doi: 10.1016/j.ccr.2012.02.022
23. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. (2013) 19:1423–37. doi: 10.1038/nm.3394
24. Zheng Z, Wieder T, Mauerer B, Schäfer L, Kesselring R, Braumüller H. T cells in colorectal cancer: unravelling the function of different T cell subsets in the tumor microenvironment. Int J Mol Sci. (2023) 24:11673. doi: 10.3390/ijms241411673
25. Russell JH, Ley TJ. Lymphocyte-mediated cytotoxicity. Annu Rev Immunol. (2002) 20:323–70. doi: 10.1146/annurev.immunol.20.100201.131730
26. Golstein P, Griffiths GM. An early history of T cell-mediated cytotoxicity. Nat Rev Immunol. (2018) 18:527–35. doi: 10.1038/s41577-018-0009-3
27. Dangaj D, Bruand M, Grimm AJ, Ronet C, Barras D, Duttagupta PA, et al. Cooperation between constitutive and inducible chemokines enables T cell engraftment and immune attack in solid tumors. Cancer Cell. (2019) 35:885–900.e10. doi: 10.1016/j.ccell.2019.05.004
28. Morishima N, Owaki T, Asakawa M, Kamiya S, Mizuguchi J, Yoshimoto T. Augmentation of effector cd8+ T cell generation with enhanced granzyme B expression by il-27. J Immunol. (2005) 175:1686–93. doi: 10.4049/jimmunol.175.3.1686
29. Bruni D, Angell HK, Galon J. The immune contexture and immunoscore in cancer prognosis and therapeutic efficacy. Nat Rev Cancer. (2020) 20:662–80. doi: 10.1038/s41568-020-0285-7
30. Glaire MA, Domingo E, Sveen A, Bruun J, Nesbakken A, Nicholson G, et al. Tumour-infiltrating cd8(+) lymphocytes and colorectal cancer recurrence by tumour and nodal stage. Br J Cancer. (2019) 121:474–82. doi: 10.1038/s41416-019-0540-4
31. Zou Q, Hu B, Yu HC, Ren DL. Characteristics of cd8+ T cell infiltration in colorectal cancer and their correlation with prognosis. Zhonghua Wei Chang Wai Ke Za Zhi. (2021) 24:1086–92. doi: 10.3760/cma.j.cn441530-20210402-00144
32. Mlecnik B, Bindea G, Angell HK, Maby P, Angelova M, Tougeron D, et al. Integrative analyses of colorectal cancer show immunoscore is a stronger predictor of patient survival than microsatellite instability. Immunity. (2016) 44:698–711. doi: 10.1016/j.immuni.2016.02.025
33. Liu SS, Yang YZ, Jiang C, Quan Q, Xie QK, Wang XP, et al. Comparison of immunological characteristics between paired mismatch repair-proficient and -deficient colorectal cancer patients. J Transl Med. (2018) 16:195. doi: 10.1186/s12967-018-1570-z
34. Le Flahec G, Badic B, Guibourg B, Doucet L, Bail JP, Marcorelles P, et al. Mismatch repair-deficient colorectal cancer: A model of immunogenic and immune cell-rich tumor despite nonsignificant programmed cell death ligand-1 expression in tumor cells. Hum Pathol. (2018) 72:135–43. doi: 10.1016/j.humpath.2017.09.019
35. Michael-Robinson JM, Biemer-Hüttmann A, Purdie DM, Walsh MD, Simms LA, Biden KG, et al. Tumour infiltrating lymphocytes and apoptosis are independent features in colorectal cancer stratified according to microsatellite instability status. Gut. (2001) 48:360–6. doi: 10.1136/gut.48.3.360
36. Phillips SM, Banerjea A, Feakins R, Li SR, Bustin SA, Dorudi S. Tumour-infiltrating lymphocytes in colorectal cancer with microsatellite instability are activated and cytotoxic. Br J Surg. (2004) 91:469–75. doi: 10.1002/bjs.4472
37. Dolcetti R, Viel A, Doglioni C, Russo A, Guidoboni M, Capozzi E, et al. High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am J Pathol. (1999) 154:1805–13. doi: 10.1016/s0002-9440(10)65436-3
38. Klapholz M, Drage MG, Srivastava A, Anderson AC. Presence of tim3(+) and pd-1(+) cd8(+) T cells identifies microsatellite stable colorectal carcinomas with immune exhaustion and distinct clinicopathological features. J Pathol. (2022) 257:186–97. doi: 10.1002/path.5877
39. Ma X, Bi E, Lu Y, Su P, Huang C, Liu L, et al. Cholesterol induces cd8(+) T cell exhaustion in the tumor microenvironment. Cell Metab. (2019) 30:143–56.e5. doi: 10.1016/j.cmet.2019.04.002
40. Liu Y, Zhou N, Zhou L, Wang J, Zhou Y, Zhang T, et al. Il-2 regulates tumor-reactive cd8(+) T cell exhaustion by activating the aryl hydrocarbon receptor. Nat Immunol. (2021) 22:358–69. doi: 10.1038/s41590-020-00850-9
41. Scott AC, Dündar F, Zumbo P, Chandran SS, Klebanoff CA, Shakiba M, et al. Tox is a critical regulator of tumour-specific T cell differentiation. Nature. (2019) 571:270–4. doi: 10.1038/s41586-019-1324-y
42. Bai Z, Zhou Y, Ye Z, Xiong J, Lan H, Wang F. Tumor-infiltrating lymphocytes in colorectal cancer: the fundamental indication and application on immunotherapy. Front Immunol. (2021) 12:808964. doi: 10.3389/fimmu.2021.808964
43. Seder RA, Ahmed R. Similarities and differences in cd4+ and cd8+ Effector and memory T cell generation. Nat Immunol. (2003) 4:835–42. doi: 10.1038/ni969
44. Basu A, Ramamoorthi G, Albert G, Gallen C, Beyer A, Snyder C, et al. Differentiation and regulation of T(H) cells: A balancing act for cancer immunotherapy. Front Immunol. (2021) 12:669474. doi: 10.3389/fimmu.2021.669474
45. Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. (2011) 71:1263–71. doi: 10.1158/0008-5472.Can-10-2907
46. Peng D, Kryczek I, Nagarsheth N, Zhao L, Wei S, Wang W, et al. Epigenetic silencing of th1-type chemokines shapes tumour immunity and immunotherapy. Nature. (2015) 527:249–53. doi: 10.1038/nature15520
47. Zhang L, Yu X, Zheng L, Zhang Y, Li Y, Fang Q, et al. Lineage tracking reveals dynamic relationships of T cells in colorectal cancer. Nature. (2018) 564:268–72. doi: 10.1038/s41586-018-0694-x
48. Kruse B, Buzzai AC, Shridhar N, Braun AD, Gellert S, Knauth K, et al. Cd4(+) T cell-induced inflammatory cell death controls immune-evasive tumours. Nature. (2023) 618:1033–40. doi: 10.1038/s41586-023-06199-x
49. Schreiber S, Hammers CM, Kaasch AJ, Schraven B, Dudeck A, Kahlfuss S. Metabolic interdependency of th2 cell-mediated type 2 immunity and the tumor microenvironment. Front Immunol. (2021) 12:632581. doi: 10.3389/fimmu.2021.632581
50. Karczewski J, Mazur M, Rychlewska-Hańczewska A, Adamski Z. Role of th17 lymphocytes in pathogenesis of colorectal cancer. Postepy Hig Med Dosw (Online). (2014) 68:42–7. doi: 10.5604/17322693.1086074
51. Korn T, Bettelli E, Oukka M, Kuchroo VK. Il-17 and th17 cells. Annu Rev Immunol. (2009) 27:485–517. doi: 10.1146/annurev.immunol.021908.132710
52. Guéry L, Hugues S. Th17 cell plasticity and functions in cancer immunity. BioMed Res Int. (2015) 2015:314620. doi: 10.1155/2015/314620
53. Chen J, Ye X, Pitmon E, Lu M, Wan J, Jellison ER, et al. Il-17 inhibits cxcl9/10-mediated recruitment of cd8(+) cytotoxic T cells and regulatory T cells to colorectal tumors. J Immunother Cancer. (2019) 7:324. doi: 10.1186/s40425-019-0757-z
54. Wang D, Yu W, Lian J, Wu Q, Liu S, Yang L, et al. Th17 cells inhibit cd8(+) T cell migration by systematically downregulating cxcr3 expression via il-17a/stat3 in advanced-stage colorectal cancer patients. J Hematol Oncol. (2020) 13:68. doi: 10.1186/s13045-020-00897-z
55. Song X, Gao H, Lin Y, Yao Y, Zhu S, Wang J, et al. Alterations in the microbiota drive interleukin-17c production from intestinal epithelial cells to promote tumorigenesis. Immunity. (2014) 40:140–52. doi: 10.1016/j.immuni.2013.11.018
56. Markowitz J, Wang J, Vangundy Z, You J, Yildiz V, Yu L, et al. Nitric oxide mediated inhibition of antigen presentation from dcs to cd4(+) T cells in cancer and measurement of stat1 nitration. Sci Rep. (2017) 7:15424. doi: 10.1038/s41598-017-14970-0
57. Ma T, Wang X, Jiao Y, Wang H, Qi Y, Gong H, et al. Interleukin 17 (Il-17)-induced mesenchymal stem cells prolong the survival of allogeneic skin grafts. Ann Transplant. (2018) 23:615–21. doi: 10.12659/aot.909381
58. Yang S, Wang B, Guan C, Wu B, Cai C, Wang M, et al. Foxp3+Il-17+ T cells promote development of cancer-initiating cells in colorectal cancer. J Leukoc Biol. (2011) 89:85–91. doi: 10.1189/jlb.0910506
59. Llosa NJ, Luber B, Tam AJ, Smith KN, Siegel N, Awan AH, et al. Intratumoral adaptive immunosuppression and type 17 immunity in mismatch repair proficient colorectal tumors. Clin Cancer Res. (2019) 25:5250–9. doi: 10.1158/1078-0432.Ccr-19-0114
60. Liu C, Liu R, Wang B, Lian J, Yao Y, Sun H, et al. Blocking il-17a enhances tumor response to anti-pd-1 immunotherapy in microsatellite stable colorectal cancer. J Immunother Cancer. (2021) 9:e001895. doi: 10.1136/jitc-2020-001895
61. Amicarella F, Muraro MG, Hirt C, Cremonesi E, Padovan E, Mele V, et al. Dual role of tumour-infiltrating T helper 17 cells in human colorectal cancer. Gut. (2017) 66:692–704. doi: 10.1136/gutjnl-2015-310016
62. De Simone V, Pallone F, Monteleone G, Stolfi C. Role of T(H)17 cytokines in the control of colorectal cancer. Oncoimmunology. (2013) 2:e26617. doi: 10.4161/onci.26617
63. Zhang S, Wang X, Gupta A, Fang X, Wang L, Zhang C. Expression of il-17 with tumor budding as a prognostic marker in oral squamous cell carcinoma. Am J Transl Res. (2019) 11:1876–83.
64. Chen JG, Xia JC, Liang XT, Pan K, Wang W, Lv L, et al. Intratumoral expression of il-17 and its prognostic role in gastric adenocarcinoma patients. Int J Biol Sci. (2011) 7:53–60. doi: 10.7150/ijbs.7.53
65. Punt S, Fleuren GJ, Kritikou E, Lubberts E, Trimbos JB, Jordanova ES, et al. Angels and demons: th17 cells represent a beneficial response, while neutrophil il-17 is associated with poor prognosis in squamous cervical cancer. Oncoimmunology. (2015) 4:e984539. doi: 10.4161/2162402x.2014.984539
66. Kim KJ, Lee KS, Cho HJ, Kim YH, Yang HK, Kim WH, et al. Prognostic implications of tumor-infiltrating foxp3+ Regulatory T cells and cd8+ Cytotoxic T cells in microsatellite-unstable gastric cancers. Hum Pathol. (2014) 45:285–93. doi: 10.1016/j.humpath.2013.09.004
67. Schmetterer KG, Neunkirchner A, Pickl WF. Naturally occurring regulatory T cells: markers, mechanisms, and manipulation. FASEB J. (2012) 26:2253–76. doi: 10.1096/fj.11-193672
68. Chang LY, Lin YC, Mahalingam J, Huang CT, Chen TW, Kang CW, et al. Tumor-derived chemokine ccl5 enhances tgf-B-mediated killing of cd8(+) T cells in colon cancer by T-regulatory cells. Cancer Res. (2012) 72:1092–102. doi: 10.1158/0008-5472.Can-11-2493
69. Aristin Revilla S, Kranenburg O, Coffer PJ. Colorectal cancer-infiltrating regulatory T cells: functional heterogeneity, metabolic adaptation, and therapeutic targeting. Front Immunol. (2022) 13:903564. doi: 10.3389/fimmu.2022.903564
70. Medina-Echeverz J, Fioravanti J, Zabala M, Ardaiz N, Prieto J, Berraondo P. Successful colon cancer eradication after chemoimmunotherapy is associated with profound phenotypic change of intratumoral myeloid cells. J Immunol. (2011) 186:807–15. doi: 10.4049/jimmunol.1001483
71. Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. (2006) 6:295–307. doi: 10.1038/nri1806
72. Rizzo A, De Mare V, Rocchi C, Stolfi C, Colantoni A, Neurath MF, et al. Smad7 induces plasticity in tumor-infiltrating th17 cells and enables tnf-alpha-mediated killing of colorectal cancer cells. Carcinogenesis. (2014) 35:1536–46. doi: 10.1093/carcin/bgu027
73. Betts G, Jones E, Junaid S, El-Shanawany T, Scurr M, Mizen P, et al. Suppression of tumour-specific cd4+ T cells by regulatory T cells is associated with progression of human colorectal cancer. Gut. (2012) 61:1163–71. doi: 10.1136/gutjnl-2011-300970
74. Szeponik L, Ahlmanner F, Sundström P, Rodin W, Gustavsson B, Bexe Lindskog E, et al. Intratumoral regulatory T cells from colon cancer patients comprise several activated effector populations. BMC Immunol. (2021) 22:58. doi: 10.1186/s12865-021-00449-1
75. Boissière-Michot F, Lazennec G, Frugier H, Jarlier M, Roca L, Duffour J, et al. Characterization of an adaptive immune response in microsatellite-instable colorectal cancer. Oncoimmunology. (2014) 3:e29256. doi: 10.4161/onci.29256
76. Michel S, Benner A, Tariverdian M, Wentzensen N, Hoefler P, Pommerencke T, et al. High density of foxp3-positive T cells infiltrating colorectal cancers with microsatellite instability. Br J Cancer. (2008) 99:1867–73. doi: 10.1038/sj.bjc.6604756
77. Bauer K, Nelius N, Reuschenbach M, Koch M, Weitz J, Steinert G, et al. T cell responses against microsatellite instability-induced frameshift peptides and influence of regulatory T cells in colorectal cancer. Cancer Immunol Immunother. (2013) 62:27–37. doi: 10.1007/s00262-012-1303-8
78. Yaqub S, Henjum K, Mahic M, Jahnsen FL, Aandahl EM, Bjørnbeth BA, et al. Regulatory T cells in colorectal cancer patients suppress anti-tumor immune activity in a cox-2 dependent manner. Cancer Immunol Immunother. (2008) 57:813–21. doi: 10.1007/s00262-007-0417-x
79. Zhuo C, Li Z, Xu Y, Wang Y, Li Q, Peng J, et al. Higher foxp3-tsdr demethylation rates in adjacent normal tissues in patients with colon cancer were associated with worse survival. Mol Cancer. (2014) 13:153. doi: 10.1186/1476-4598-13-153
80. Correale P, Rotundo MS, Del Vecchio MT, Remondo C, Migali C, Ginanneschi C, et al. Regulatory (Foxp3+) T-cell tumor infiltration is a favorable prognostic factor in advanced colon cancer patients undergoing chemo or chemoimmunotherapy. J Immunother. (2010) 33:435–41. doi: 10.1097/CJI.0b013e3181d32f01
81. Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, et al. Tumor-infiltrating foxp3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. (2009) 27:186–92. doi: 10.1200/jco.2008.18.7229
82. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor foxp3. Science. (2003) 299:1057–61. doi: 10.1126/science.1079490
83. Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, et al. Cd4+ Regulatory T cells control th17 responses in a stat3-dependent manner. Science. (2009) 326:986–91. doi: 10.1126/science.1172702
84. Whiteside TL. What are regulatory T cells (Treg) regulating in cancer and why? Semin Cancer Biol. (2012) 22:327–34. doi: 10.1016/j.semcancer.2012.03.004
85. Zhang X, Kelaria S, Kerstetter J, Wang J. The functional and prognostic implications of regulatory T cells in colorectal carcinoma. J Gastrointest Oncol. (2015) 6:307–13. doi: 10.3978/j.issn.2078-6891.2015.017
86. Chevalier MF, Didier C, Petitjean G, Karmochkine M, Girard PM, Barré-Sinoussi F, et al. Phenotype alterations in regulatory T-cell subsets in primary hiv infection and identification of tr1-like cells as the main interleukin 10-producing cd4+ T cells. J Infect Dis. (2015) 211:769–79. doi: 10.1093/infdis/jiu549
87. Khazaie K, Bonertz A, Beckhove P. Current developments with peptide-based human tumor vaccines. Curr Opin Oncol. (2009) 21:524–30. doi: 10.1097/CCO.0b013e328331a78e
88. Meraviglia S, Lo Presti E, Tosolini M, La Mendola C, Orlando V, Todaro M, et al. Distinctive features of tumor-infiltrating Γδ T lymphocytes in human colorectal cancer. Oncoimmunology. (2017) 6:e1347742. doi: 10.1080/2162402x.2017.1347742
89. Noble A, Pring ET, Durant L, Man R, Dilke SM, Hoyles L, et al. Altered immunity to microbiota, B cell activation and depleted Γδ/resident memory T cells in colorectal cancer. Cancer Immunol Immunother. (2022) 71:2619–29. doi: 10.1007/s00262-021-03135-8
90. Silva-Santos B, Mensurado S, Coffelt SB. [amp]]Gamma;δ T cells: pleiotropic immune effectors with therapeutic potential in cancer. Nat Rev Cancer. (2019) 19:392–404. doi: 10.1038/s41568-019-0153-5
91. Sebestyen Z, Prinz I, Déchanet-Merville J, Silva-Santos B, Kuball J. Translating gammadelta (Γδ) T cells and their receptors into cancer cell therapies. Nat Rev Drug Discovery. (2020) 19:169–84. doi: 10.1038/s41573-019-0038-z
92. Bonneville M, O'Brien RL, Born WK. Gammadelta T cell effector functions: A blend of innate programming and acquired plasticity. Nat Rev Immunol. (2010) 10:467–78. doi: 10.1038/nri2781
93. Silva-Santos B, Serre K, Norell H. [amp]]Gamma;δ T cells in cancer. Nat Rev Immunol. (2015) 15:683–91. doi: 10.1038/nri3904
94. Reis BS, Darcy PW, Khan IZ, Moon CS, Kornberg AE, Schneider VS, et al. Tcr-Vγδ Usage distinguishes protumor from antitumor intestinal Γδ T cell subsets. Science. (2022) 377:276–84. doi: 10.1126/science.abj8695
95. Narayanan S, Kawaguchi T, Yan L, Peng X, Qi Q, Takabe K. Cytolytic activity score to assess anticancer immunity in colorectal cancer. Ann Surg Oncol. (2018) 25:2323–31. doi: 10.1245/s10434-018-6506-6
96. Krijgsman D, Hokland M, Kuppen PJK. The role of natural killer T cells in cancer-a phenotypical and functional approach. Front Immunol. (2018) 9:367. doi: 10.3389/fimmu.2018.00367
97. Exley M, Garcia J, Balk SP, Porcelli S. Requirements for cd1d recognition by human invariant valpha24+ Cd4-cd8- T cells. J Exp Med. (1997) 186:109–20. doi: 10.1084/jem.186.1.109
98. Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH. Activating and inhibitory receptors of natural killer cells. Immunol Cell Biol. (2011) 89:216–24. doi: 10.1038/icb.2010.78
99. McEwen-Smith RM, Salio M, Cerundolo V. The regulatory role of invariant nkt cells in tumor immunity. Cancer Immunol Res. (2015) 3:425–35. doi: 10.1158/2326-6066.Cir-15-0062
100. Kumar V, Delovitch TL. Different subsets of natural killer T cells may vary in their roles in health and disease. Immunology. (2014) 142:321–36. doi: 10.1111/imm.12247
101. Gebremeskel S, Clattenburg DR, Slauenwhite D, Lobert L, Johnston B. Natural killer T cell activation overcomes immunosuppression to enhance clearance of postsurgical breast cancer metastasis in mice. Oncoimmunology. (2015) 4:e995562. doi: 10.1080/2162402x.2014.995562
102. Swann JB, Uldrich AP, van Dommelen S, Sharkey J, Murray WK, Godfrey DI, et al. Type I natural killer T cells suppress tumors caused by P53 loss in mice. Blood. (2009) 113:6382–5. doi: 10.1182/blood-2009-01-198564
103. Ambrosino E, Terabe M, Halder RC, Peng J, Takaku S, Miyake S, et al. Cross-regulation between type I and type ii nkt cells in regulating tumor immunity: A new immunoregulatory axis. J Immunol. (2007) 179:5126–36. doi: 10.4049/jimmunol.179.8.5126
104. Tachibana T, Onodera H, Tsuruyama T, Mori A, Nagayama S, Hiai H, et al. Increased intratumor valpha24-positive natural killer T cells: A prognostic factor for primary colorectal carcinomas. Clin Cancer Res. (2005) 11:7322–7. doi: 10.1158/1078-0432.Ccr-05-0877
105. Molling JW, Kölgen W, van der Vliet HJ, Boomsma MF, Kruizenga H, Smorenburg CH, et al. Peripheral blood ifn-gamma-secreting valpha24+Vbeta11+ Nkt cell numbers are decreased in cancer patients independent of tumor type or tumor load. Int J Cancer. (2005) 116:87–93. doi: 10.1002/ijc.20998
106. Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells: 10 years on. Cell. (2018) 174:1054–66. doi: 10.1016/j.cell.2018.07.017
107. Russick J, Torset C, Hemery E, Cremer I. Nk cells in the tumor microenvironment: prognostic and theranostic impact. Recent Adv Trends Semin Immunol. (2020) 48:101407. doi: 10.1016/j.smim.2020.101407
108. Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. (2001) 19:197–223. doi: 10.1146/annurev.immunol.19.1.197
109. Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. (2008) 9:503–10. doi: 10.1038/ni1582
110. Carrega P, Bonaccorsi I, Di Carlo E, Morandi B, Paul P, Rizzello V, et al. Cd56(Bright)Perforin(Low) noncytotoxic human nk cells are abundant in both healthy and neoplastic solid tissues and recirculate to secondary lymphoid organs via afferent lymph. J Immunol. (2014) 192:3805–15. doi: 10.4049/jimmunol.1301889
111. Castriconi R, Carrega P, Dondero A, Bellora F, Casu B, Regis S, et al. Molecular mechanisms directing migration and retention of natural killer cells in human tissues. Front Immunol. (2018) 9:2324. doi: 10.3389/fimmu.2018.02324
112. Wagner JA, Rosario M, Romee R, Berrien-Elliott MM, Schneider SE, Leong JW, et al. Cd56bright nk cells exhibit potent antitumor responses following il-15 priming. J Clin Invest. (2017) 127:4042–58. doi: 10.1172/jci90387
113. Zhang B, Halder SK, Zhang S, Datta PK. Targeting transforming growth factor-beta signaling in liver metastasis of colon cancer. Cancer Lett. (2009) 277:114–20. doi: 10.1016/j.canlet.2008.11.035
114. Villalba M, Evans SR, Vidal-Vanaclocha F, Calvo A. Role of tgf-B in metastatic colon cancer: it is finally time for targeted therapy. Cell Tissue Res. (2017) 370:29–39. doi: 10.1007/s00441-017-2633-9
115. Biassoni R, Cantoni C, Pende D, Sivori S, Parolini S, Vitale M, et al. Human natural killer cell receptors and co-receptors. Immunol Rev. (2001) 181:203–14. doi: 10.1034/j.1600-065x.2001.1810117.x
116. Krijgsman D, de Vries NL, Skovbo A, Andersen MN, Swets M, Bastiaannet E, et al. Nk-, and nkt cell subsets in patients with colorectal cancer: the peripheral blood immune cell profile. Cancer Immunol Immunother. (2019) 68:1011–24. doi: 10.1007/s00262-019-02343-7
117. Sorrentino C, D'Antonio L, Fieni C, Ciummo SL, Di Carlo E. Colorectal cancer-associated immune exhaustion involves T and B lymphocytes and conventional nk cells and correlates with a shorter overall survival. Front Immunol. (2021) 12:778329. doi: 10.3389/fimmu.2021.778329
118. Glaire MA, Ryan NA, Ijsselsteijn ME, Kedzierska K, Obolenski S, Ali R, et al. Discordant prognosis of mismatch repair deficiency in colorectal and endometrial cancer reflects variation in antitumour immune response and immune escape. J Pathol. (2022) 257:340–51. doi: 10.1002/path.5894
119. Al-Mterin MA, Elkord E. Myeloid-derived suppressor cells in colorectal cancer: prognostic biomarkers and therapeutic targets. Explor Target Antitumor Ther. (2022) 3:497–510. doi: 10.37349/etat.2022.00097
120. Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. (2009) 182:4499–506. doi: 10.4049/jimmunol.0802740
121. Chouaib S, Umansky V, Kieda C. The role of hypoxia in shaping the recruitment of proangiogenic and immunosuppressive cells in the tumor microenvironment. Contemp Oncol (Pozn). (2018) 22:7–13. doi: 10.5114/wo.2018.73874
122. Won WJ, Deshane JS, Leavenworth JW, Oliva CR, Griguer CE. Metabolic and functional reprogramming of myeloid-derived suppressor cells and their therapeutic control in glioblastoma. Cell Stress. (2019) 3:47–65. doi: 10.15698/cst2019.02.176
123. Xiang X, Poliakov A, Liu C, Liu Y, Deng ZB, Wang J, et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer. (2009) 124:2621–33. doi: 10.1002/ijc.24249
124. Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, et al. Hif-1α Regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. (2010) 207:2439–53. doi: 10.1084/jem.20100587
125. Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, et al. Pd-L1 is a novel direct target of hif-1α, and its blockade under hypoxia enhanced mdsc-mediated T cell activation. J Exp Med. (2014) 211:781–90. doi: 10.1084/jem.20131916
126. Deng J, Li J, Sarde A, Lines JL, Lee YC, Qian DC, et al. Hypoxia-induced vista promotes the suppressive function of myeloid-derived suppressor cells in the tumor microenvironment. Cancer Immunol Res. (2019) 7:1079–90. doi: 10.1158/2326-6066.Cir-18-0507
127. Zhang Y, Wang J, Wang W, Tian J, Yin K, Tang X, et al. Il-17a produced by peritoneal macrophages promote the accumulation and function of granulocytic myeloid-derived suppressor cells in the development of colitis-associated cancer. Tumour Biol. (2016) 37:15883–91. doi: 10.1007/s13277-016-5414-2
128. Highfill SL, Rodriguez PC, Zhou Q, Goetz CA, Koehn BH, Veenstra R, et al. Bone marrow myeloid-derived suppressor cells (Mdscs) inhibit graft-versus-host disease (Gvhd) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood. (2010) 116:5738–47. doi: 10.1182/blood-2010-06-287839
129. Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: A mechanism of tumor evasion. Cancer Res. (2005) 65:3044–8. doi: 10.1158/0008-5472.Can-04-4505
130. Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. (2005) 5:641–54. doi: 10.1038/nri1668
131. Geiger R, Rieckmann JC, Wolf T, Basso C, Feng Y, Fuhrer T, et al. L-arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell. (2016) 167:829–42.e13. doi: 10.1016/j.cell.2016.09.031
132. Lee CR, Kwak Y, Yang T, Han JH, Park SH, Ye MB, et al. Myeloid-derived suppressor cells are controlled by regulatory T cells via tgf-B During murine colitis. Cell Rep. (2016) 17:3219–32. doi: 10.1016/j.celrep.2016.11.062
133. Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, et al. Abrogation of tgf beta signaling in mammary carcinomas recruits gr-1+Cd11b+ Myeloid cells that promote metastasis. Cancer Cell. (2008) 13:23–35. doi: 10.1016/j.ccr.2007.12.004
134. Toh B, Wang X, Keeble J, Sim WJ, Khoo K, Wong WC, et al. Mesenchymal transition and dissemination of cancer cells is driven by myeloid-derived suppressor cells infiltrating the primary tumor. PloS Biol. (2011) 9:e1001162. doi: 10.1371/journal.pbio.1001162
135. Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of nk cells through membrane-bound tgf-beta 1. J Immunol. (2009) 182:240–9. doi: 10.4049/jimmunol.182.1.240
136. Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, et al. Expansion of myeloid immune suppressor gr+Cd11b+ Cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. (2004) 6:409–21. doi: 10.1016/j.ccr.2004.08.031
137. Mira E, Lacalle RA, Buesa JM, de Buitrago GG, Jiménez-Baranda S, Gómez-Moutón C, et al. Secreted mmp9 promotes angiogenesis more efficiently than constitutive active mmp9 bound to the tumor cell surface. J Cell Sci. (2004) 117:1847–57. doi: 10.1242/jcs.01035
138. Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. (2016) 7:12150. doi: 10.1038/ncomms12150
139. Solito S, Marigo I, Pinton L, Damuzzo V, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity in human cancers. Ann N Y Acad Sci. (2014) 1319:47–65. doi: 10.1111/nyas.12469
140. Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. (2016) 37:208–20. doi: 10.1016/j.it.2016.01.004
141. Raber PL, Thevenot P, Sierra R, Wyczechowska D, Halle D, Ramirez ME, et al. Subpopulations of myeloid-derived suppressor cells impair T cell responses through independent nitric oxide-related pathways. Int J Cancer. (2014) 134:2853–64. doi: 10.1002/ijc.28622
142. Lichtenstern CR, Ngu RK, Shalapour S, Karin M. Immunotherapy, inflammation and colorectal cancer. Cells. (2020) 9:618. doi: 10.3390/cells9030618
143. Liu Y, Cao X. The origin and function of tumor-associated macrophages. Cell Mol Immunol. (2015) 12:1–4. doi: 10.1038/cmi.2014.83
144. Movahedi K, Laoui D, Gysemans C, Baeten M, Stangé G, Van den Bossche J, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from ly6c(High) monocytes. Cancer Res. (2010) 70:5728–39. doi: 10.1158/0008-5472.Can-09-4672
145. Wang H, Tian T, Zhang J. Tumor-associated macrophages (Tams) in colorectal cancer (Crc): from mechanism to therapy and prognosis. Int J Mol Sci. (2021) 22:8470. doi: 10.3390/ijms22168470
146. Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. (2007) 117:1155–66. doi: 10.1172/jci31422
147. Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel). (2014) 6:1670–90. doi: 10.3390/cancers6031670
148. Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. Funct Differentiation Front Immunol. (2014) 5:514. doi: 10.3389/fimmu.2014.00514
149. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. (2012) 122:787–95. doi: 10.1172/jci59643
150. Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. (2017) 14:399–416. doi: 10.1038/nrclinonc.2016.217
151. Ngambenjawong C, Gustafson HH, Pun SH. Progress in tumor-associated macrophage (Tam)-targeted therapeutics. Adv Drug Delivery Rev. (2017) 114:206–21. doi: 10.1016/j.addr.2017.04.010
152. Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. (2012) 12:298–306. doi: 10.1038/nrc3245
153. Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. (2010) 141:39–51. doi: 10.1016/j.cell.2010.03.014
154. Lian G, Chen S, Ouyang M, Li F, Chen L, Yang J. Colon cancer cell secretes egf to promote M2 polarization of tam through egfr/pi3k/akt/mtor pathway. Technol Cancer Res Treat. (2019) 18:1533033819849068. doi: 10.1177/1533033819849068
155. Zhang LL, Zhang LF, Shi YB. Down-regulated paxillin suppresses cell proliferation and invasion by inhibiting M2 macrophage polarization in colon cancer. Biol Chem. (2018) 399:1285–95. doi: 10.1515/hsz-2018-0002
156. Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. (2014) 6:13. doi: 10.12703/p6-13
157. Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. (2014) 41:14–20. doi: 10.1016/j.immuni.2014.06.008
158. Edin S, Wikberg ML, Dahlin AM, Rutegård J, Öberg Å, Oldenborg PA, et al. The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PloS One. (2012) 7:e47045. doi: 10.1371/journal.pone.0047045
159. Lan J, Sun L, Xu F, Liu L, Hu F, Song D, et al. M2 macrophage-derived exosomes promote cell migration and invasion in colon cancer. Cancer Res. (2019) 79:146–58. doi: 10.1158/0008-5472.Can-18-0014
160. Liu R, Han C, Hu J, Zhang B, Luo W, Ling F. Infiltration of apoptotic M2 macrophage subpopulation is negatively correlated with the immunotherapy response in colorectal cancer. Int J Mol Sci. (2022) 23:11014. doi: 10.3390/ijms231911014
161. Takei S, Tanaka Y, Lin YT, Koyama S, Fukuoka S, Hara H, et al. Multiomic molecular characterization of the response to combination immunotherapy in mss/pmmr metastatic colorectal cancer. J Immunother Cancer. (2024) 12:e008210. doi: 10.1136/jitc-2023-008210
162. Ballesteros I, Rubio-Ponce A, Genua M, Lusito E, Kwok I, Fernández-Calvo G, et al. Co-option of neutrophil fates by tissue environments. Cell. (2020) 183:1282–97.e18. doi: 10.1016/j.cell.2020.10.003
163. Zheng W, Wu J, Peng Y, Sun J, Cheng P, Huang Q. Tumor-associated neutrophils in colorectal cancer development, progression and immunotherapy. Cancers (Basel). (2022) 14:4755. doi: 10.3390/cancers14194755
164. Katoh H, Wang D, Daikoku T, Sun H, Dey SK, Dubois RN. Cxcr2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell. (2013) 24:631–44. doi: 10.1016/j.ccr.2013.10.009
165. Faget J, Peters S, Quantin X, Meylan E, Bonnefoy N. Neutrophils in the era of immune checkpoint blockade. J Immunother Cancer. (2021) 9:e002242. doi: 10.1136/jitc-2020-002242
166. Wang TT, Zhao YL, Peng LS, Chen N, Chen W, Lv YP, et al. Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through gm-csf-pd-L1 pathway. Gut. (2017) 66:1900–11. doi: 10.1136/gutjnl-2016-313075
167. Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by tgf-beta: "N1" Versus "N2" Tan. Cancer Cell. (2009) 16:183–94. doi: 10.1016/j.ccr.2009.06.017
168. Andzinski L, Kasnitz N, Stahnke S, Wu CF, Gereke M, von Köckritz-Blickwede M, et al. Type I ifns induce anti-tumor polarization of tumor associated neutrophils in mice and human. Int J Cancer. (2016) 138:1982–93. doi: 10.1002/ijc.29945
169. Riise RE, Bernson E, Aurelius J, Martner A, Pesce S, Della Chiesa M, et al. Tlr-stimulated neutrophils instruct nk cells to trigger dendritic cell maturation and promote adaptive T cell responses. J Immunol. (2015) 195:1121–8. doi: 10.4049/jimmunol.1500709
170. Raftopoulou S, Valadez-Cosmes P, Mihalic ZN, Schicho R, Kargl J. Tumor-mediated neutrophil polarization and therapeutic implications. Int J Mol Sci. (2022) 23:3218. doi: 10.3390/ijms23063218
171. Sun R, Luo J, Li D, Shu Y, Luo C, Wang SS, et al. Neutrophils with protumor potential could efficiently suppress tumor growth after cytokine priming and in presence of normal nk cells. Oncotarget. (2014) 5:12621–34. doi: 10.18632/oncotarget.2181
172. Spiegel A, Brooks MW, Houshyar S, Reinhardt F, Ardolino M, Fessler E, et al. Neutrophils suppress intraluminal nk cell-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discovery. (2016) 6:630–49. doi: 10.1158/2159-8290.Cd-15-1157
173. Jablonska J, Leschner S, Westphal K, Lienenklaus S, Weiss S. Neutrophils responsive to endogenous ifn-beta regulate tumor angiogenesis and growth in a mouse tumor model. J Clin Invest. (2010) 120:1151–64. doi: 10.1172/jci37223
174. Jaillon S, Ponzetta A, Di Mitri D, Santoni A, Bonecchi R, Mantovani A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer. (2020) 20:485–503. doi: 10.1038/s41568-020-0281-y
175. Galdiero MR, Bianchi P, Grizzi F, Di Caro G, Basso G, Ponzetta A, et al. Occurrence and significance of tumor-associated neutrophils in patients with colorectal cancer. Int J Cancer. (2016) 139:446–56. doi: 10.1002/ijc.30076
176. Rao HL, Chen JW, Li M, Xiao YB, Fu J, Zeng YX, et al. Increased intratumoral neutrophil in colorectal carcinomas correlates closely with Malignant phenotype and predicts patients' Adverse prognosis. PloS One. (2012) 7:e30806. doi: 10.1371/journal.pone.0030806
177. Jakubowska K, Koda M, Grudzińska M, Kisielewski W, Lomperta K, Famulski W. Neutrophil infiltration combined with necrosis in the primary tumor is a useful prognostic indicator for three-year disease-free survival time in patients with colorectal cancer. Oncol Lett. (2022) 23:199. doi: 10.3892/ol.2022.13320
178. Rottmann BG, Patel N, Ahmed M, Deng Y, Ciarleglio M, Vyas M, et al. Clinicopathological significance of neutrophil-rich colorectal carcinoma. J Clin Pathol. (2023) 76:34–9. doi: 10.1136/jclinpath-2021-207702
179. Gardner A, Ruffell B. Dendritic cells and cancer immunity. Trends Immunol. (2016) 37:855–65. doi: 10.1016/j.it.2016.09.006
180. Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. (2020) 20:7–24. doi: 10.1038/s41577-019-0210-z
181. Balan S, Saxena M, Bhardwaj N. Dendritic cell subsets and locations. Int Rev Cell Mol Biol. (2019) 348:1–68. doi: 10.1016/bs.ircmb.2019.07.004
182. Naik SH, Sathe P, Park HY, Metcalf D, Proietto AI, Dakic A, et al. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat Immunol. (2007) 8:1217–26. doi: 10.1038/ni1522
183. Anderson DA 3rd, Murphy KM, Briseño CG. Development, diversity, and function of dendritic cells in mouse and human. Cold Spring Harb Perspect Biol. (2018) 10:a028613. doi: 10.1101/cshperspect.a028613
184. Bandola-Simon J, Roche PA. Dysfunction of antigen processing and presentation by dendritic cells in cancer. Mol Immunol. (2019) 113:31–7. doi: 10.1016/j.molimm.2018.03.025
185. Mitchell D, Chintala S, Dey M. Plasmacytoid dendritic cell in immunity and cancer. J Neuroimmunol. (2018) 322:63–73. doi: 10.1016/j.jneuroim.2018.06.012
186. Li S, Wu J, Zhu S, Liu YJ, Chen J. Disease-associated plasmacytoid dendritic cells. Front Immunol. (2017) 8:1268. doi: 10.3389/fimmu.2017.01268
187. Goc J, Germain C, Vo-Bourgais TK, Lupo A, Klein C, Knockaert S, et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating cd8+ T cells. Cancer Res. (2014) 74:705–15. doi: 10.1158/0008-5472.Can-13-1342
188. Headley MB, Bins A, Nip A, Roberts EW, Looney MR, Gerard A, et al. Visualization of immediate immune responses to pioneer metastatic cells in the lung. Nature. (2016) 531:513–7. doi: 10.1038/nature16985
189. Carenza C, Calcaterra F, Oriolo F, Di Vito C, Ubezio M, Della Porta MG, et al. Costimulatory molecules and immune checkpoints are differentially expressed on different subsets of dendritic cells. Front Immunol. (2019) 10:1325. doi: 10.3389/fimmu.2019.01325
190. Kim MK, Kim J. Properties of immature and mature dendritic cells: phenotype, morphology, phagocytosis, and migration. RSC Adv. (2019) 9:11230–8. doi: 10.1039/c9ra00818g
191. Coppola D, Nebozhyn M, Khalil F, Dai H, Yeatman T, Loboda A, et al. Unique ectopic lymph node-like structures present in human primary colorectal carcinoma are identified by immune gene array profiling. Am J Pathol. (2011) 179:37–45. doi: 10.1016/j.ajpath.2011.03.007
192. Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell. (2014) 26:638–52. doi: 10.1016/j.ccell.2014.09.007
193. Tel J, Anguille S, Waterborg CE, Smits EL, Figdor CG, de Vries IJ. Tumoricidal activity of human dendritic cells. Trends Immunol. (2014) 35:38–46. doi: 10.1016/j.it.2013.10.007
194. Vicari AP, Caux C, Trinchieri G. Tumour escape from immune surveillance through dendritic cell inactivation. Semin Cancer Biol. (2002) 12:33–42. doi: 10.1006/scbi.2001.0400
195. Legitimo A, Consolini R, Failli A, Orsini G, Spisni R. Dendritic cell defects in the colorectal cancer. Hum Vaccin Immunother. (2014) 10:3224–35. doi: 10.4161/hv.29857
196. Imai K, Minamiya Y, Koyota S, Ito M, Saito H, Sato Y, et al. Inhibition of dendritic cell migration by transforming growth factor-B1 increases tumor-draining lymph node metastasis. J Exp Clin Cancer Res. (2012) 31:3. doi: 10.1186/1756-9966-31-3
197. Subtil B, Cambi A, Tauriello DVF, de Vries IJM. The therapeutic potential of tackling tumor-induced dendritic cell dysfunction in colorectal cancer. Front Immunol. (2021) 12:724883. doi: 10.3389/fimmu.2021.724883
198. Schwaab T, Weiss JE, Schned AR, Barth RJ Jr. Dendritic cell infiltration in colon cancer. J Immunother (1991). (2001) 24:130–7. doi: 10.1097/00002371-200103000-00007
199. Gulubova M, Manolova I, Ananiev J, Kjurkchiev D, Julianov A, Altunkova I. Relationship of tgf-B1 and smad7 expression with decreased dendritic cell infiltration in liver gastrointestinal cancer metastasis. Apmis. (2013) 121:967–75. doi: 10.1111/apm.12096
200. Suzuki A, Masuda A, Nagata H, Kameoka S, Kikawada Y, Yamakawa M, et al. Mature dendritic cells make clusters with T cells in the invasive margin of colorectal carcinoma. J Pathol. (2002) 196:37–43. doi: 10.1002/path.1018
201. Gulubova M, Manolova I, Cirovski G, Sivrev D. Recruitment of dendritic cells in human liver with metastases. Clin Exp Metastasis. (2008) 25:777–85. doi: 10.1007/s10585-008-9191-1
202. Ho WW, Gomes-Santos IL, Aoki S, Datta M, Kawaguchi K, Talele NP, et al. Dendritic cell paucity in mismatch repair-proficient colorectal cancer liver metastases limits immune checkpoint blockade efficacy. Proc Natl Acad Sci U.S.A. (2021) 118:e2105323118. doi: 10.1073/pnas.2105323118
203. André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med. (2020) 383:2207–18. doi: 10.1056/NEJMoa2017699
204. Leowattana W, Leowattana P, Leowattana T. Systemic treatment for metastatic colorectal cancer. World J Gastroenterol. (2023) 29:1569–88. doi: 10.3748/wjg.v29.i10.1569
205. Buccione C, Fragale A, Polverino F, Ziccheddu G, Aricò E, Belardelli F, et al. Role of interferon regulatory factor 1 in governing treg depletion, th1 polarization, inflammasome activation and antitumor efficacy of cyclophosphamide. Int J Cancer. (2018) 142:976–87. doi: 10.1002/ijc.31083
206. Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, et al. 5-fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. (2010) 70:3052–61. doi: 10.1158/0008-5472.Can-09-3690
207. Wanderley CW, Colón DF, Luiz JPM, Oliveira FF, Viacava PR, Leite CA, et al. Paclitaxel reduces tumor growth by reprogramming tumor-associated macrophages to an M1 profile in a tlr4-dependent manner. Cancer Res. (2018) 78:5891–900. doi: 10.1158/0008-5472.Can-17-3480
208. Buhtoiarov IN, Sondel PM, Wigginton JM, Buhtoiarova TN, Yanke EM, Mahvi DA, et al. Anti-tumour synergy of cytotoxic chemotherapy and anti-cd40 plus cpg-odn immunotherapy through repolarization of tumour-associated macrophages. Immunology. (2011) 132:226–39. doi: 10.1111/j.1365-2567.2010.03357.x
209. Jary M, Liu WW, Yan D, Bai I, Muranyi A, Colle E, et al. Immune microenvironment in patients with mismatch-repair-proficient oligometastatic colorectal cancer exposed to chemotherapy: the randomized mirox gercor cohort study. Mol Oncol. (2022) 16:2260–73. doi: 10.1002/1878-0261.13173
210. Walle T, Kraske JA, Liao B, Lenoir B, Timke C, von Bohlen Und Halbach E, et al. Radiotherapy orchestrates natural killer cell dependent antitumor immune responses through cxcl8. Sci Adv. (2022) 8:eabh4050. doi: 10.1126/sciadv.abh4050
211. Barsoumian HB, Ramapriyan R, Younes AI, Caetano MS, Menon H, Comeaux NI, et al. Low-dose radiation treatment enhances systemic antitumor immune responses by overcoming the inhibitory stroma. J Immunother Cancer. (2020) 8:e000537. doi: 10.1136/jitc-2020-000537
212. Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, et al. Low-dose irradiation programs macrophage differentiation to an inos+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. (2013) 24:589–602. doi: 10.1016/j.ccr.2013.09.014
213. Bando H, Tsukada Y, Inamori K, Togashi Y, Koyama S, Kotani D, et al. Preoperative chemoradiotherapy plus nivolumab before surgery in patients with microsatellite stable and microsatellite instability-high locally advanced rectal cancer. Clin Cancer Res. (2022) 28:1136–46. doi: 10.1158/1078-0432.Ccr-21-3213
214. Yang J, Yan J, Liu B. Targeting vegf/vegfr to modulate antitumor immunity. Front Immunol. (2018) 9:978. doi: 10.3389/fimmu.2018.00978
215. Antoniotti C, Rossini D, Pietrantonio F, Catteau A, Salvatore L, Lonardi S, et al. Upfront folfoxiri plus bevacizumab with or without atezolizumab in the treatment of patients with metastatic colorectal cancer (Atezotribe): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. (2022) 23:876–87. doi: 10.1016/s1470-2045(22)00274-1
216. Redman JM, Tsai YT, Weinberg BA, Donahue RN, Gandhy S, Gatti-Mays ME, et al. A randomized phase ii trial of mfolfox6 + Bevacizumab alone or with adcea vaccine + Avelumab immunotherapy for untreated metastatic colorectal cancer. Oncologist. (2022) 27:198–209. doi: 10.1093/oncolo/oyab046
217. Wang L, Wei Y, Fang W, Lu C, Chen J, Cui G, et al. Cetuximab enhanced the cytotoxic activity of immune cells during treatment of colorectal cancer. Cell Physiol Biochem. (2017) 44:1038–50. doi: 10.1159/000485404
218. Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase ib trial (Regonivo, epoc1603). J Clin Oncol. (2020) 38:2053–61. doi: 10.1200/jco.19.03296
219. Fakih M, Raghav KPS, Chang DZ, Larson T, Cohn AL, Huyck TK, et al. Regorafenib plus nivolumab in patients with mismatch repair-proficient/microsatellite stable metastatic colorectal cancer: A single-arm, open-label, multicentre phase 2 study. EClinicalMedicine. (2023) 58:101917. doi: 10.1016/j.eclinm.2023.101917
220. Wang F, He MM, Yao YC, Zhao X, Wang ZQ, Jin Y, et al. Regorafenib plus toripalimab in patients with metastatic colorectal cancer: A phase ib/ii clinical trial and gut microbiome analysis. Cell Rep Med. (2021) 2:100383. doi: 10.1016/j.xcrm.2021.100383
221. Song EK, Tai WM, Messersmith WA, Bagby S, Purkey A, Quackenbush KS, et al. Potent antitumor activity of cabozantinib, a C-met and vegfr2 inhibitor, in a colorectal cancer patient-derived tumor explant model. Int J Cancer. (2015) 136:1967–75. doi: 10.1002/ijc.29225
222. Kwilas AR, Ardiani A, Donahue RN, Aftab DT, Hodge JW. Dual effects of a targeted small-molecule inhibitor (Cabozantinib) on immune-mediated killing of tumor cells and immune tumor microenvironment permissiveness when combined with a cancer vaccine. J Transl Med. (2014) 12:294. doi: 10.1186/s12967-014-0294-y
223. Scott AJ, Arcaroli JJ, Bagby SM, Yahn R, Huber KM, Serkova NJ, et al. Cabozantinib exhibits potent antitumor activity in colorectal cancer patient-derived tumor xenograft models via autophagy and signaling mechanisms. Mol Cancer Ther. (2018) 17:2112–22. doi: 10.1158/1535-7163.Mct-17-0131
224. Saeed A, Park R, Dai J, Al-Rajabi R, Kasi A, Baranda J, et al. Cabozantinib plus durvalumab in advanced gastroesophageal cancer and other gastrointestinal Malignancies: phase ib camilla trial results. Cell Rep Med. (2023) 4:100916. doi: 10.1016/j.xcrm.2023.100916
225. Fang JY, Richardson BC. The mapk signalling pathways and colorectal cancer. Lancet Oncol. (2005) 6:322–7. doi: 10.1016/s1470-2045(05)70168-6
226. Liao W, Overman MJ, Boutin AT, Shang X, Zhao D, Dey P, et al. Kras-irf2 axis drives immune suppression and immune therapy resistance in colorectal cancer. Cancer Cell. (2019) 35:559–72 e7. doi: 10.1016/j.ccell.2019.02.008
227. Ott PA, Bhardwaj N. Impact of mapk pathway activation in braf(V600) melanoma on T cell and dendritic cell function. Front Immunol. (2013) 4:346. doi: 10.3389/fimmu.2013.00346
228. Eng C, Kim TW, Bendell J, Argilés G, Tebbutt NC, Di Bartolomeo M, et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (Imblaze370): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. (2019) 20:849–61. doi: 10.1016/s1470-2045(19)30027-0
229. O'Donnell JS, Massi D, Teng MWL, Mandala M. Pi3k-akt-mtor inhibition in cancer immunotherapy, redux. Semin Cancer Biol. (2018) 48:91–103. doi: 10.1016/j.semcancer.2017.04.015
230. Lin A, Gu T, Hu X, Zhang J, Luo P. Comprehensive analysis identifies pi3k/akt pathway alternations as an immune-related prognostic biomarker in colon adenocarcinoma patients receiving immune checkpoint inhibitor treatment. J Immunol Res. (2022) 2022:8179799. doi: 10.1155/2022/8179799
231. Yan J, Yang S, Tian H, Zhang Y, Zhao H. Copanlisib promotes growth inhibition and apoptosis by modulating the akt/foxo3a/puma axis in colorectal cancer. Cell Death Dis. (2020) 11:943. doi: 10.1038/s41419-020-03154-w
232. Stamper CC, Zhang Y, Tobin JF, Erbe DV, Ikemizu S, Davis SJ, et al. Crystal structure of the B7-1/ctla-4 complex that inhibits human immune responses. Nature. (2001) 410:608–11. doi: 10.1038/35069118
233. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. (2012) 12:252–64. doi: 10.1038/nrc3239
234. Rotte A. Combination of ctla-4 and pd-1 blockers for treatment of cancer. J Exp Clin Cancer Res. (2019) 38:255. doi: 10.1186/s13046-019-1259-z
235. Chen EX, Jonker DJ, Loree JM, Kennecke HF, Berry SR, Couture F, et al. Effect of combined immune checkpoint inhibition vs best supportive care alone in patients with advanced colorectal cancer: the canadian cancer trials group co.26 study. JAMA Oncol. (2020) 6:831–8. doi: 10.1001/jamaoncol.2020.0910
236. Maruhashi T, Sugiura D, Okazaki IM, Okazaki T. Lag-3: from molecular functions to clinical applications. J Immunother Cancer. (2020) 8:e001014. doi: 10.1136/jitc-2020-001014
237. Garralda E, Sukari A, Lakhani NJ, Patnaik A, Lou Y, Im SA, et al. A first-in-human study of the anti-lag-3 antibody favezelimab plus pembrolizumab in previously treated, advanced microsatellite stable colorectal cancer. ESMO Open. (2022) 7:100639. doi: 10.1016/j.esmoop.2022.100639
238. Crisafulli G, Sartore-Bianchi A, Lazzari L, Pietrantonio F, Amatu A, Macagno M, et al. Temozolomide treatment alters mismatch repair and boosts mutational burden in tumor and blood of colorectal cancer patients. Cancer Discovery. (2022) 12:1656–75. doi: 10.1158/2159-8290.Cd-21-1434
239. Morano F, Raimondi A, Pagani F, Lonardi S, Salvatore L, Cremolini C, et al. Temozolomide followed by combination with low-dose ipilimumab and nivolumab in patients with microsatellite-stable, O(6)-methylguanine-DNA methyltransferase-silenced metastatic colorectal cancer: the maya trial. J Clin Oncol. (2022) 40:1562–73. doi: 10.1200/jco.21.02583
240. Pietrantonio F, Perrone F, de Braud F, Castano A, Maggi C, Bossi I, et al. Activity of temozolomide in patients with advanced chemorefractory colorectal cancer and mgmt promoter methylation. Ann Oncol. (2014) 25:404–8. doi: 10.1093/annonc/mdt547
241. Castells A, Payá A, Alenda C, Rodríguez-Moranta F, Agrelo R, Andreu M, et al. Cyclooxygenase 2 expression in colorectal cancer with DNA mismatch repair deficiency. Clin Cancer Res. (2006) 12:1686–92. doi: 10.1158/1078-0432.Ccr-05-1581
242. Ogino S, Kirkner GJ, Nosho K, Irahara N, Kure S, Shima K, et al. Cyclooxygenase-2 expression is an independent predictor of poor prognosis in colon cancer. Clin Cancer Res. (2008) 14:8221–7. doi: 10.1158/1078-0432.Ccr-08-1841
243. Panni RZ, Sanford DE, Belt BA, Mitchem JB, Worley LA, Goetz BD, et al. Tumor-induced stat3 activation in monocytic myeloid-derived suppressor cells enhances stemness and mesenchymal properties in human pancreatic cancer. Cancer Immunol Immunother. (2014) 63:513–28. doi: 10.1007/s00262-014-1527-x
244. Su YL, Banerjee S, White SV, Kortylewski M. Stat3 in tumor-associated myeloid cells: multitasking to disrupt immunity. Int J Mol Sci. (2018) 19:1803. doi: 10.3390/ijms19061803
245. Burdan F, Chałas A, Szumiło J. Cyclooxygenase and prostanoids–biological implications. Postepy Hig Med Dosw (Online). (2006) 60:129–41.
246. Obermajer N, Muthuswamy R, Lesnock J, Edwards RP, Kalinski P. Positive feedback between pge2 and cox2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood. (2011) 118:5498–505. doi: 10.1182/blood-2011-07-365825
247. Han C, Demetris AJ, Stolz DB, Xu L, Lim K, Wu T. Modulation of stat3 activation by the cytosolic phospholipase A2alpha and cyclooxygenase-2-controlled prostaglandin E2 signaling pathway. J Biol Chem. (2006) 281:24831–46. doi: 10.1074/jbc.M602201200
248. Corvinus FM, Orth C, Moriggl R, Tsareva SA, Wagner S, Pfitzner EB, et al. Persistent stat3 activation in colon cancer is associated with enhanced cell proliferation and tumor growth. Neoplasia. (2005) 7:545–55. doi: 10.1593/neo.04571
249. Lee H, Herrmann A, Deng JH, Kujawski M, Niu G, Li Z, et al. Persistently activated stat3 maintains constitutive nf-kappab activity in tumors. Cancer Cell. (2009) 15:283–93. doi: 10.1016/j.ccr.2009.02.015
250. Wei J, Zhang J, Wang D, Cen B, Lang JD, DuBois RN. The cox-2-pge2 pathway promotes tumor evasion in colorectal adenomas. Cancer Prev Res (Phila). (2022) 15:285–96. doi: 10.1158/1940-6207.Capr-21-0572
251. Zelenay S, van der Veen AG, Böttcher JP, Snelgrove KJ, Rogers N, Acton SE, et al. Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell. (2015) 162:1257–70. doi: 10.1016/j.cell.2015.08.015
252. Chalabi M, Fanchi LF, Dijkstra KK, Van den Berg JG, Aalbers AG, Sikorska K, et al. Neoadjuvant immunotherapy leads to pathological responses in mmr-proficient and mmr-deficient early-stage colon cancers. Nat Med. (2020) 26:566–76. doi: 10.1038/s41591-020-0805-8
253. Song L, Hao Y, Wang C, Han Y, Zhu Y, Feng L, et al. Liposomal oxaliplatin prodrugs loaded with metformin potentiate immunotherapy for colorectal cancer. J Control Release. (2022) 350:922–32. doi: 10.1016/j.jconrel.2022.09.013
254. Labrijn AF, Janmaat ML, Reichert JM, Parren P. Bispecific antibodies: A mechanistic review of the pipeline. Nat Rev Drug Discovery. (2019) 18:585–608. doi: 10.1038/s41573-019-0028-1
255. Bacac M, Fauti T, Sam J, Colombetti S, Weinzierl T, Ouaret D, et al. A novel carcinoembryonic antigen T-cell bispecific antibody (Cea tcb) for the treatment of solid tumors. Clin Cancer Res. (2016) 22:3286–97. doi: 10.1158/1078-0432.Ccr-15-1696
256. Osada T, Patel SP, Hammond SA, Osada K, Morse MA, Lyerly HK. Cea/cd3-bispecific T cell-engaging (Bite) antibody-mediated T lymphocyte cytotoxicity maximized by inhibition of both pd1 and pd-L1. Cancer Immunol Immunother. (2015) 64:677–88. doi: 10.1007/s00262-015-1671-y
257. Tabernero J, Melero I, Ros W, Argiles G, Marabelle A, Rodriguez-Ruiz ME, et al. Phase ia and ib studies of the novel carcinoembryonic antigen (Cea) T-cell bispecific (Cea cd3 tcb) antibody as a single agent and in combination with atezolizumab: preliminary efficacy and safety in patients with metastatic colorectal cancer (Mcrc). J Clin Oncol. (2017) 35:3002. doi: 10.1200/JCO.2017.35.15_suppl.3002
258. Caballero-Baños M, Benitez-Ribas D, Tabera J, Varea S, Vilana R, Bianchi L, et al. Phase Ii Randomised Trial of Autologous Tumour Lysate Dendritic Cell plus Best Supportive Care compared with Best Supportive Care in Pre-Treated Advanced Colorectal Cancer Patients. Eur J Cancer. (2016) 64:167–74. doi: 10.1016/j.ejca.2016.06.008
259. Español-Rego M, Fernández-Martos C, Elez E, Foguet C, Pedrosa L, Rodríguez N, et al. A phase I-ii multicenter trial with avelumab plus autologous dendritic cell vaccine in pre-treated mismatch repair-proficient (Mss) metastatic colorectal cancer patients; gemcad 1602 study. Cancer Immunol Immunother. (2023) 72:827–40. doi: 10.1007/s00262-022-03283-5
260. Zheng L, Edil BH, Soares KC, El-Shami K, Uram JN, Judkins C, et al. A safety and feasibility study of an allogeneic colon cancer cell vaccine administered with a granulocyte-macrophage colony stimulating factor-producing bystander cell line in patients with metastatic colorectal cancer. Ann Surg Oncol. (2014) 21:3931–7. doi: 10.1245/s10434-014-3844-x
261. Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual blockade of pd-1 and ctla-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res. (2013) 73:3591–603. doi: 10.1158/0008-5472.Can-12-4100
262. Yarchoan M, Huang CY, Zhu Q, Ferguson AK, Durham JN, Anders RA, et al. A phase 2 study of gvax colon vaccine with cyclophosphamide and pembrolizumab in patients with mismatch repair proficient advanced colorectal cancer. Cancer Med. (2020) 9:1485–94. doi: 10.1002/cam4.2763
263. Hubbard JM, Tőke ER, Moretto R, Graham RP, Youssoufian H, Lőrincz O, et al. Safety and activity of polypepi1018 combined with maintenance therapy in metastatic colorectal cancer: an open-label, multicenter, phase ib study. Clin Cancer Res. (2022) 28:2818–29. doi: 10.1158/1078-0432.Ccr-22-0112
264. Yu YJ, Shan N, Li LY, Zhu YS, Lin LM, Mao CC, et al. Preliminary clinical study of personalized neoantigen vaccine therapy for microsatellite stability (Mss)-advanced colorectal cancer. Cancer Immunol Immunother. (2023) 72:2045–56. doi: 10.1007/s00262-023-03386-7
265. Ott PA, Hu-Lieskovan S, Chmielowski B, Govindan R, Naing A, Bhardwaj N, et al. A phase ib trial of personalized neoantigen therapy plus anti-pd-1 in patients with advanced melanoma, non-small cell lung cancer, or bladder cancer. Cell. (2020) 183:347–62.e24. doi: 10.1016/j.cell.2020.08.053
266. Chen H, Li Z, Qiu L, Dong X, Chen G, Shi Y, et al. Personalized neoantigen vaccine combined with pd-1 blockade increases cd8(+) tissue-resident memory T-cell infiltration in preclinical hepatocellular carcinoma models. J Immunother Cancer. (2022) 10:e004389. doi: 10.1136/jitc-2021-004389
267. Kana SI, Essani K. Immuno-oncolytic viruses: emerging options in the treatment of colorectal cancer. Mol Diagn Ther. (2021) 25:301–13. doi: 10.1007/s40291-021-00517-7
268. Liu Z, Yang Y, Zhang X, Wang H, Xu W, Wang H, et al. An oncolytic adenovirus encoding decorin and granulocyte macrophage colony stimulating factor inhibits tumor growth in a colorectal tumor model by targeting pro-tumorigenic signals and via immune activation. Hum Gene Ther. (2017) 28:667–80. doi: 10.1089/hum.2017.033
269. Stephenson KB, Barra NG, Davies E, Ashkar AA, Lichty BD. Expressing human interleukin-15 from oncolytic vesicular stomatitis virus improves survival in a murine metastatic colon adenocarcinoma model through the enhancement of anti-tumor immunity. Cancer Gene Ther. (2012) 19:238–46. doi: 10.1038/cgt.2011.81
270. Croft M. The role of tnf superfamily members in T-cell function and diseases. Nat Rev Immunol. (2009) 9:271–85. doi: 10.1038/nri2526
271. Heinrich B, Goepfert K, Delic M, Galle PR, Moehler M. Influence of the oncolytic parvovirus H-1, ctla-4 antibody tremelimumab and cytostatic drugs on the human immune system in a human in vitro model of colorectal cancer cells. Onco Targets Ther. (2013) 6:1119–27. doi: 10.2147/ott.S49371
272. Rojas JJ, Sampath P, Hou W, Thorne SH. Defining effective combinations of immune checkpoint blockade and oncolytic virotherapy. Clin Cancer Res. (2015) 21:5543–51. doi: 10.1158/1078-0432.Ccr-14-2009
273. Park SH, Breitbach CJ, Lee J, Park JO, Lim HY, Kang WK, et al. Phase 1b trial of biweekly intravenous pexa-vec (Jx-594), an oncolytic and immunotherapeutic vaccinia virus in colorectal cancer. Mol Ther. (2015) 23:1532–40. doi: 10.1038/mt.2015.109
274. Krieg AM. Development of tlr9 agonists for cancer therapy. J Clin Invest. (2007) 117:1184–94. doi: 10.1172/jci31414
275. Hammond E, Haynes NM, Cullinane C, Brennan TV, Bampton D, Handley P, et al. Immunomodulatory activities of pixatimod: emerging nonclinical and clinical data, and its potential utility in combination with pd-1 inhibitors. J Immunother Cancer. (2018) 6:54. doi: 10.1186/s40425-018-0363-5
276. Lemech C, Dredge K, Bampton D, Hammond E, Clouston A, Waterhouse NJ, et al. Phase ib open-label, multicenter study of pixatimod, an activator of tlr9, in combination with nivolumab in subjects with microsatellite-stable metastatic colorectal cancer, metastatic pancreatic ductal adenocarcinoma and other solid tumors. J Immunother Cancer. (2023) 11:e006136. doi: 10.1136/jitc-2022-006136
277. Xu X, Lv J, Guo F, Li J, Jia Y, Jiang D, et al. Gut microbiome influences the efficacy of pd-1 antibody immunotherapy on mss-type colorectal cancer via metabolic pathway. Front Microbiol. (2020) 11:814. doi: 10.3389/fmicb.2020.00814
278. Lin A, Zhang J, Luo P. Crosstalk between the msi status and tumor microenvironment in colorectal cancer. Front Immunol. (2020) 11:2039. doi: 10.3389/fimmu.2020.02039
279. Kloor M, Staffa L, Ahadova A, von Knebel Doeberitz M. Clinical significance of microsatellite instability in colorectal cancer. Langenbecks Arch Surg. (2014) 399:23–31. doi: 10.1007/s00423-013-1112-3
Keywords: colorectal cancer, tumor microenvironment, immune checkpoint inhibitors, deficient mismatch repair, proficient mismatch repair, microsatellite instable, microsatellite stable
Citation: Wang Q, Yu M and Zhang S (2025) The characteristics of the tumor immune microenvironment in colorectal cancer with different MSI status and current therapeutic strategies. Front. Immunol. 15:1440830. doi: 10.3389/fimmu.2024.1440830
Received: 30 May 2024; Accepted: 16 December 2024;
Published: 14 January 2025.
Edited by:
Zong Sheng Guo, University at Buffalo, United StatesReviewed by:
Youtao Lu, University of Pennsylvania, United StatesWenQing Yang, ClinBridge Biotech Co. Ltd, China
Copyright © 2025 Wang, Yu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuang Zhang, c2h1YW5nLnpoYW5nQHNjdS5lZHUuY24=
†These authors have contributed equally to this work
 Qingzhe Wang
Qingzhe Wang Min Yu
Min Yu Shuang Zhang
Shuang Zhang