
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 10 April 2024
Sec. Cancer Immunity and Immunotherapy
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1303259
This article is part of the Research Topic Advances in GI and hepatic cancer mechanisms and therapeutic approaches View all 5 articles
 Mei Li1,2,3†
Mei Li1,2,3† Jun Liao1,2,3†
Jun Liao1,2,3† Li Wang1,2,3†
Li Wang1,2,3† Tianye Lv1,2,3
Tianye Lv1,2,3 Qianfu Sun1,2,3
Qianfu Sun1,2,3 Yan Xu1,2,3
Yan Xu1,2,3 Zhi Guo1,2,3
Zhi Guo1,2,3 Manman Quan1,2,3
Manman Quan1,2,3 Hao Qin1,2,3
Hao Qin1,2,3 Haoyang Yu4
Haoyang Yu4 Kai Zhang5,6*
Kai Zhang5,6* Wenge Xing1,2,3*
Wenge Xing1,2,3* Haipeng Yu1,2,3*
Haipeng Yu1,2,3*Objectives: This study aimed to examine the effectiveness of the best response rate (BRR) as a surrogate for overall survival (OS), using the modified Response Evaluation Criteria in Solid Tumors (mRECIST), in patients with unresectable hepatocellular carcinoma (HCC) undergoing hepatic arterial infusion chemotherapy (HAIC) with fluorouracil, leucovorin, and oxaliplatin (FOLFOX) combined with molecular targeting and immunotherapy.
Methods: This study enrolled 111 consecutive patients who had complete imaging data. The median age of patients was 58 years (IQR 50.5-65.0). Among the patients, those with Barcelona Clinic Liver Cancer (BCLC) stage A, BCLC stage B, and BCLC stage C comprised 6.4%, 19.1%, and 73.6%, respectively. The optimal threshold of BRR can be determined using restricted cubic splines (RCS) and the rank sum statistics of maximum selection. Survival curves of patients in the high rating and low rating groups were plotted. We then used the change-in-estimate (CIE) method to filter out confounders and the inverse probability of treatment weighting (IPTW) to balance confounders between the two groups to assess the robustness of the results.
Results: The median frequency of the combination treatment regimens administered in the overall population was 3 times (IQR 2.0-3.0). The optimal BRR truncation value calculated was −0.2. Based on this value, 77 patients were categorized as the low rating group and 34 as the high rating group. The differences in the OS between the high and low rating groups were statistically significant (7 months [95%CI 6.0-14.0] vs. 30 months [95%CI 30.0-]; p< 0.001). Using the absolute 10% cut-off value, the CIE method was used to screen out the following confounding factors affecting prognosis: successful conversion surgery, baseline tumor size, BCLC stage, serum total bilirubin level, number of interventional treatments, alpha-fetoprotein level, presence of inferior vena cava tumor thrombus, and partial thrombin activation time. The survival curve was then plotted again using IPTW for confounding factors, and it was found that the low rating group continued to have better OS than the high rating group. Finally, the relationship between BRR and baseline factors was analyzed, and inferior vena cava tumor thrombus and baseline tumor size correlated significantly with BRR.
Conclusions: BRR can be used as a surrogate endpoint for OS in unresectable HCC patients undergoing FOLFOX-HAIC in combination with molecular targeting and immunotherapy. Thus, by calculating the BRR, the prognosis of HCC patients after combination therapy can be predicted. Inferior vena cava tumor thrombus and baseline tumor size were closely associated with the BRR.
HCC constitutes approximately 90% of all primary liver cancers and ranks fourth as the leading cause of cancer-related mortality. Although the incidence of virus-associated HCC has potentially reduced through vaccination and antiviral therapy, the incidence of HCC associated with other etiologies has escalated rapidly (1, 2). According to most clinical practice guidelines, patients with early HCC (BCLC stages 0 and A) should undergo excision, thermal ablation, or transplantation, while those with intermediate (BCLC stage B) and advanced (BCLC stage C) HCC should receive transarterial chemoembolization (TACE) or systemic therapy (3, 4). Nonetheless, the effectiveness of TACE for HCC with a substantial tumor burden is suboptimal. High-concentration drug chemotherapy without embolization can be administered through HAIC to treat localized tumors. Mounting evidence suggests that HAIC with fluorouracil, leucovorin, and oxaliplatin yields superior therapeutic outcomes and fewer adverse effects than TACE (5–7). In Asia, HAIC has been extensively employed as an alternative therapy to sorafenib in patients with advanced HCC (8, 9).
The latest developments in systemic therapy for advanced liver cancer have opened up fresh prospects for multimodal treatment of this disease. In the last decade, multi-kinase inhibitors aimed at tumor angiogenesis have been prescribed for advanced HCC (10–12). More recently, immune checkpoint inhibitors (ICI) that target the programmed cell death-1 (PD-1) pathway have emerged as the mainstream option for advanced HCC combination therapy, considering their favorable safety profile and promising objective response (13).
Recent studies have shown that for primary unresectable HCC, HAIC treatment combined with targeted therapy and immunotherapy can achieve a significant surgical conversion rate (14, 15). Although these findings are optimistic for individuals with unresectable HCC, it is important to note that not all patients may benefit from this combination therapy. In the realm of liver cancer research, the primary endpoint remains OS. However, to employ OS as an endpoint, several endpoint events must be documented, which could significantly amplify the intricacy of clinical research. Objective response rate (ORR) is now emerging as a crucial indicator for early evaluation of treatment efficacy (16–18). To our knowledge, no studies have explored whether the BRR can be used as a surrogate endpoint for OS in patients with HCC receiving combination therapy. Within this framework, this study aimed to investigate the effectiveness of BRR as a surrogate for OS based on the modified Response Evaluation Criteria in Solid Tumors (mRECIST) in HCC patients undergoing FOLFOX-HAIC therapy in conjunction with molecular targeting and immunotherapy.
This retrospective study followed the ethical principles of the Declaration of Helsinki and received approval from the Cancer Institute and Hospital Review Committee of Tianjin Medical University (bc2020099). Each participant was assigned a random number, and all identifying information was expunged to ensure anonymity (19). This study retrospectively collected clinicopathologic data and prognostic information of patients who underwent HAIC-FOLFOX combined with molecular targeting and immunotherapy at the Interventional Therapy Department of Tianjin Medical University Cancer Hospital between August 2019 and December 2021.
The inclusion criteria were as follows (1): diagnosis of initial unresectable liver cancer through multidisciplinary consultation; (2) clinical or pathological diagnosis of HCC; (3) Child-Pugh Grade A, Eastern Cooperative Oncology Group score ≤1, and routine laboratory tests indicating tolerance to the combined regimen; (4) no previous TACE, ablation, or other local treatment; (5) at least one course of HAIC combined with molecular targeting and immunotherapy received; and (6) complete imaging data available for evaluation.
The measurement of the maximum tumor diameter was conducted by two independent imaging doctors using dynamic contrast-enhanced computed tomography or magnetic resonance imaging, with neither examiner possessing any prior knowledge of the patient’s clinical data.
Local anesthesia was induced in the patient, and the femoral artery was punctured by the Seldinger puncture technique. Digital subtraction angiography was used to display the anatomical characteristics of the abdominal cavity, superior Mesentery artery and hepatic artery, and to check the blood supply of the tumor site. A 2.7-F microcatheter is used in HAIC for implantation in the tumor-supplying artery. The FOLFOX regimen comprised oxaliplatin 85 mg/m2 infusion for 4 h, calcium folinate 400 mg/m2 infusion for 2-3 h, fluorouracil 400 mg/m2 injection once, and fluorouracil 1200 mg/m2 infusion for 23 h on the first day of treatment. HAIC is repeated every 4-6 weeks. If toxicity is intolerable, treatment may need to be interrupted or dosage adjusted.
Before or after the first HAIC treatment, the patients were administered anti-PD-1 antibodies intravenously every 3 weeks. For antiangiogenic therapy, the patients were administered 8 mg of lenvatinib orally once daily, sorafenib 200 mg twice daily, and apatinib 250 mg once daily. Please refer to our previously published 001 Research for detailed treatment of patients (14).
The evaluation of individual patient responses involved two independent imaging doctors who were blinded to other clinical data to mitigate potential bias. As per the mRECIST, BRR is characterized by the most substantial percentage decrease in the sum of the diameters of the target lesions as compared to the baseline target lesion diameters following multiple treatments (16, 18). A previous study on TACE therapy for liver cancer showed that ORR was associated with tumor burden. The study used a “6 + 12” score to define tumor burden, the sum of tumor size and number ≤ 6; the sum > 6 but ≤ 12; and the sum > 12 for low/intermediate/high tumor burden, respectively. For patients with low to moderate tumor burden, both the initial ORR and optimal ORR were prognostic indicators, while for patients with high tumor burden, only optimal ORR was prognostic (20). Our study primarily enrolled patients with medium to large liver cancer tumors, leading to the selection of the BRR as a prognostic indicator.
The OS is defined as the duration from the commencement of combination therapy until death from any cause or the date of the final evaluation. The progression-free survival (PFS), on the other hand, is determined by calculating the time from the initiation of combination therapy until either tumor progression is documented or death occurs, whichever comes first. The follow-up period ended in December 2022.
Fisher’s exact test was used to analyze the categorical variables, and the results are expressed as numbers and percentages. Normally distributed data were analyzed using t-tests or variance analysis, while non-normally distributed data were analyzed using the rank sum test; these data are presented as medians and interquartile ranges (IQR). The Kaplan-Meier method was used for the estimation of survival.
In this study, the RCS method and the maximally selected rank statistics of the survminer package of R software were used to establish the optimal threshold of BRR. Restricted cubic splines is one of the common methods to fit the nonlinear relationship between independent and dependent variables (21). The patients were divided into two groups according to the BRR value, and the survival curves were plotted for each group. To reduce potential selection and confounding bias, we first screened out confounders using the CIE method and then balanced the inter-group confounders using the IPTW method to assess the robustness of the relationship between the BRR groups and OS. The CIE method, a data-driven independent variable screening method, is used to reduce the number of independent variables by eliminating the variables that have limited influence on the important independent variable effect in the multifactor regression model (22). IPTW takes the reciprocal probability of each observed value as the weight of the observed value to correct the estimation bias caused by missing data or biased sampling (23, 24). In this study, R software (version 4.2.1) was used for statistical analyses. The R packages used included tidyverse, survival, survminer, ggplot2, rcssci, and chest; p-values <0.05 were considered statistically significant.
This study enrolled 111 consecutive patients with complete imaging data who underwent at least one combination regimen (FOLFOX-HAIC combined with molecular targeting and immunotherapy). The median frequency of the combination treatment regimens administered in the overall population was three times.
The BRR truncation value calculated using the RCS was −0.27 (Figure 1A), while that calculated using the survminer package was −0.17 (Figure 1B). Therefore, we considered the mid-value of the two, namely −0.2, as the final best truncation value of BRR. Those with a BRR <−0.2 were considered to have a high therapeutic response rate and were categorized as the low rating group (n = 77/111), whereas those with a BRR >−0.2 were considered to have a low therapeutic response rate and were categorized as the high rating group (n = 34/111). Table 1 presents the baseline characteristics of the two groups of patients. Overall, the median age of patients was 58 years (IQR 50.5-65.0), with 85.6% being male. The most commonly used targeted drugs were lenvatinib (53.2%) and bevacizumab (38.7%), and most of the patients chose sintilimab (70.3%) as the immunotherapy agent. The primary etiology of HCC was hepatitis B virus (94.6%). The median lesion diameter was 8.9 cm (IQR 6.3-13.0). According to the BCLC system, 6.4%, 19.1%, and 73.6% of the cases in this study were categorized as stages A, B, and C, respectively. The tumor responses of the patients were shown in Table 2. On the basis of mRECIST criteria, the ORR of patients in the low rating group (78.0% vs 0, P < 0.001) was higher than that in the high rating group. The BRR of patients in the low rating group was lower than that in the high rating group (-0.4 vs -0.1, P < 0.001).
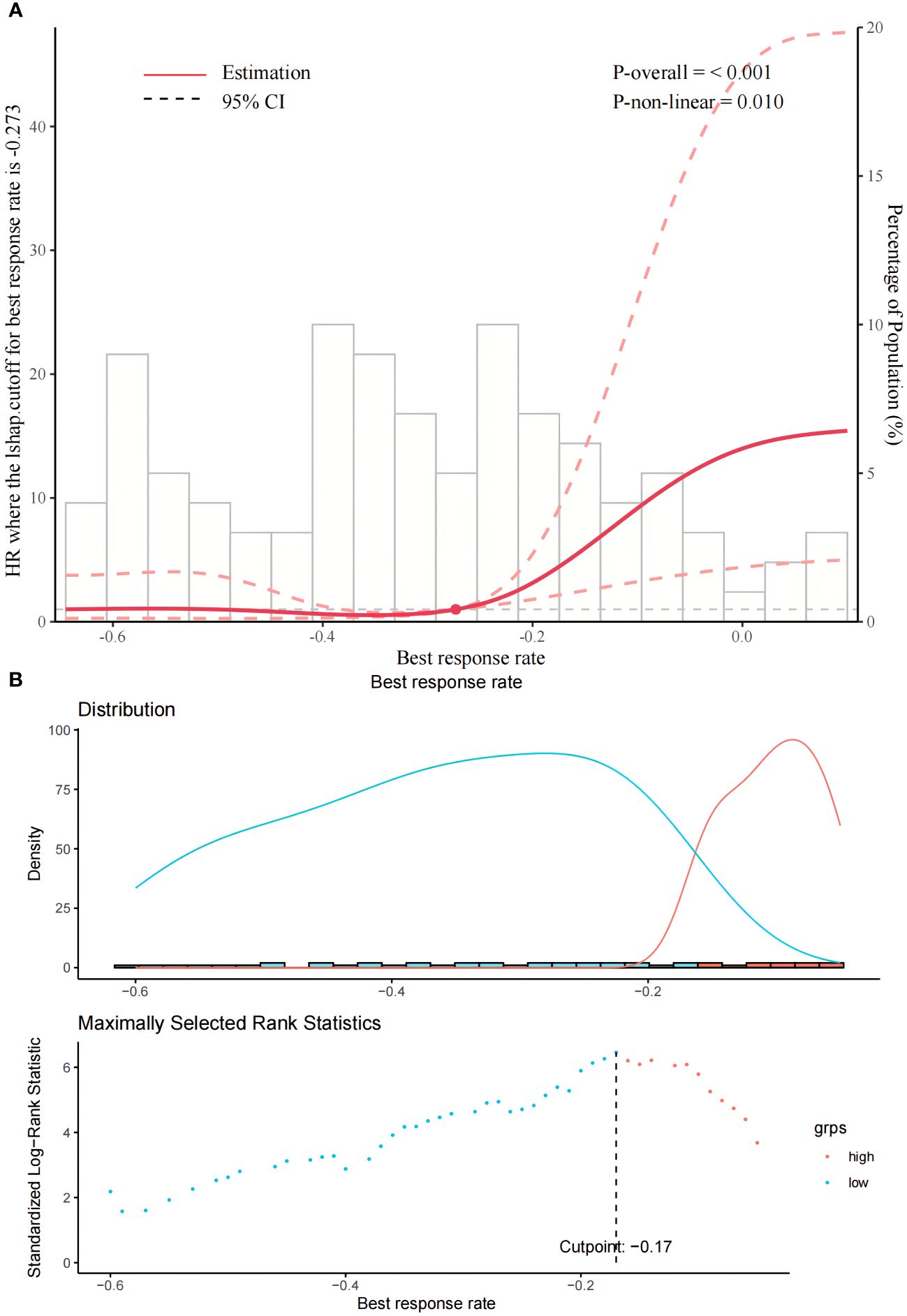
Figure 1 (A, B) present the schematics of the restricted cubic splines and rank sum statistics, respectively, to select the best response rate truncation values.
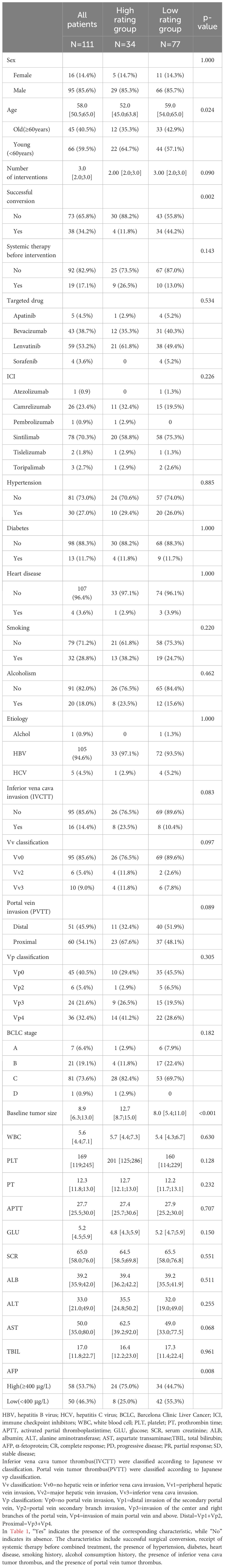
Table 1 Baseline demographic and clinical characteristics of high rating group and low rating group. .
The median OS was 7 months (95% CI 6.0–14.0) in the high rating group and 30 months (95% CI 30.0–) in the low rating group. The median PFS was 3 months (95% CI 3.0–5.0) in the high rating group and 19 months (95% CI 15.0–) in the low rating group. A statistically significant difference in the OS was observed between the two groups (p < 0.001) (Figure 2). Confounding factors screened by the CIE method included successful conversion surgery, baseline tumor size, BCLC stage, serum total bilirubin level, number of interventional treatments, alpha-fetoprotein (AFP) level, presence of inferior vena cava tumor thrombus, and partial thrombin activation time (Figure 3). We used the IPTW method to balance the confounding factors. The survival curves of the two groups were then redrawn, and log-rank tests were performed. The results showed that the low rating group continued to show significant improvements in the OS as compared to the high rating group (Figure 4) (p < 0.01).
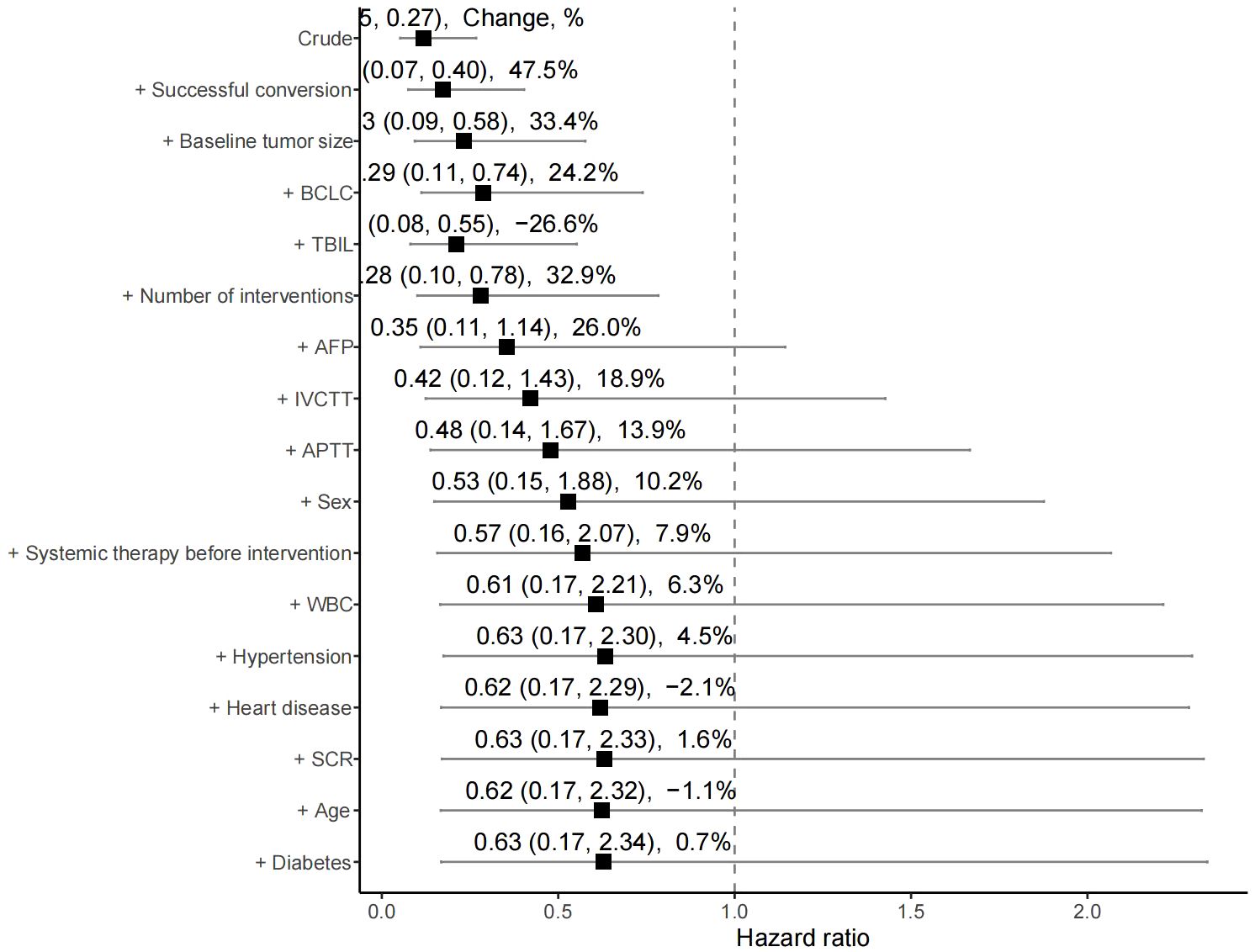
Figure 3 Change-in-estimate method for filtering variables. BCLC, Barcelona Clinic Liver Cancer; TBIL, Total bilirubin; AFP, α-fetoprotein; vv, Inferior vena cava tumor thrombus; APTT, Activated partial thromboplastintime; WBC, White blood cell; SCR, Serum creatinine.
Figure 5 shows the survival curve drawn with BRR as a continuity variable, wherein the greater the response rate, the longer the OS. We analyzed the correlation between BRR and other variables and found that inferior vena cava tumor thrombus (Figure 6) and baseline tumor size (Figure 7) significantly correlated with BRR. Inferior vena cava tumor thrombus negatively correlated with BRR, while baseline tumor size positively correlated with BRR (p < 0.05).
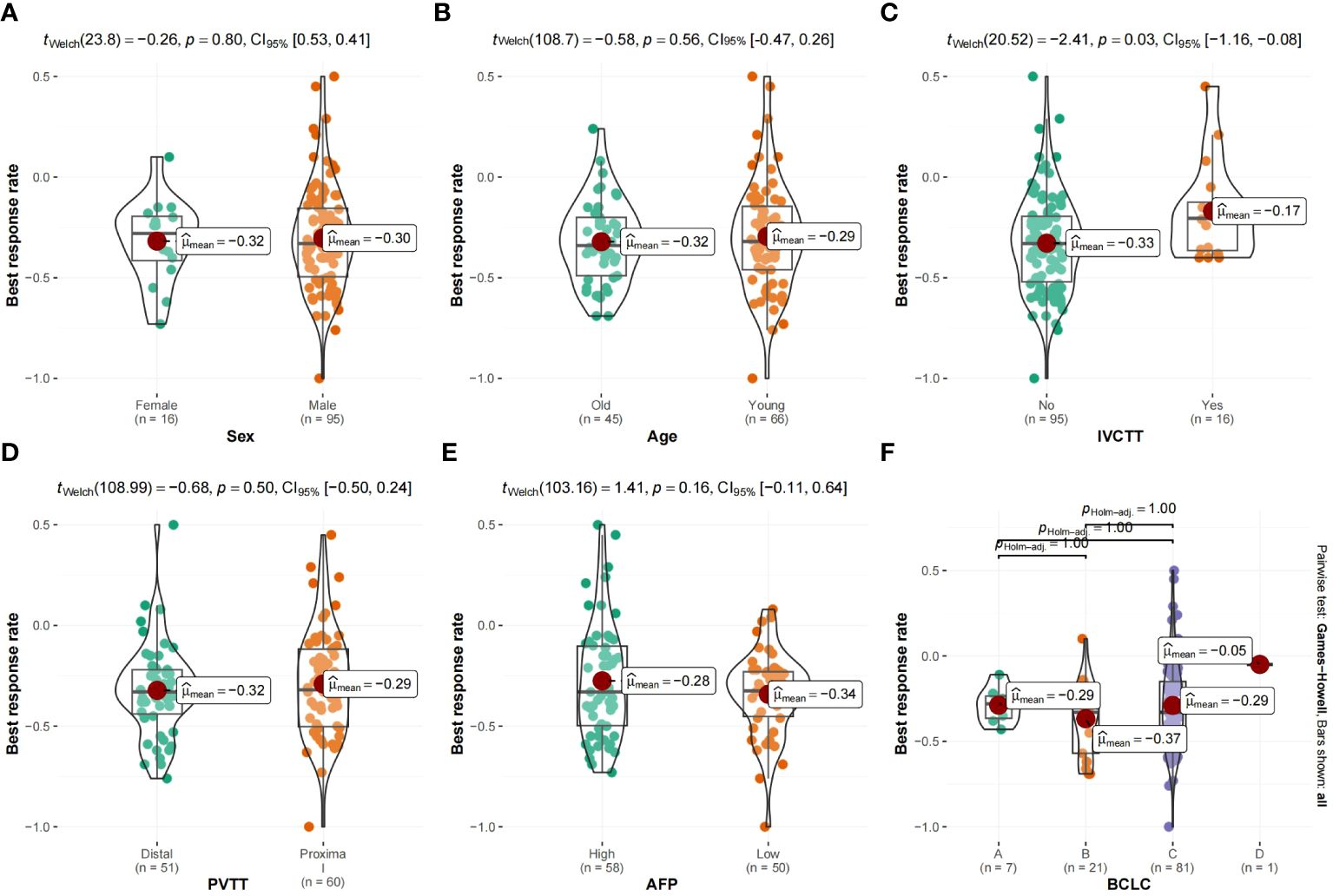
Figure 6 (A–F) Violin plot of categorical variables and best response rate. vv, Inferior vena cava tumor thrombus; vp, Portal vein tumor thrombus; AFP, α-fetoprotein; BCLC, Barcelona Clinic Liver Cancer.
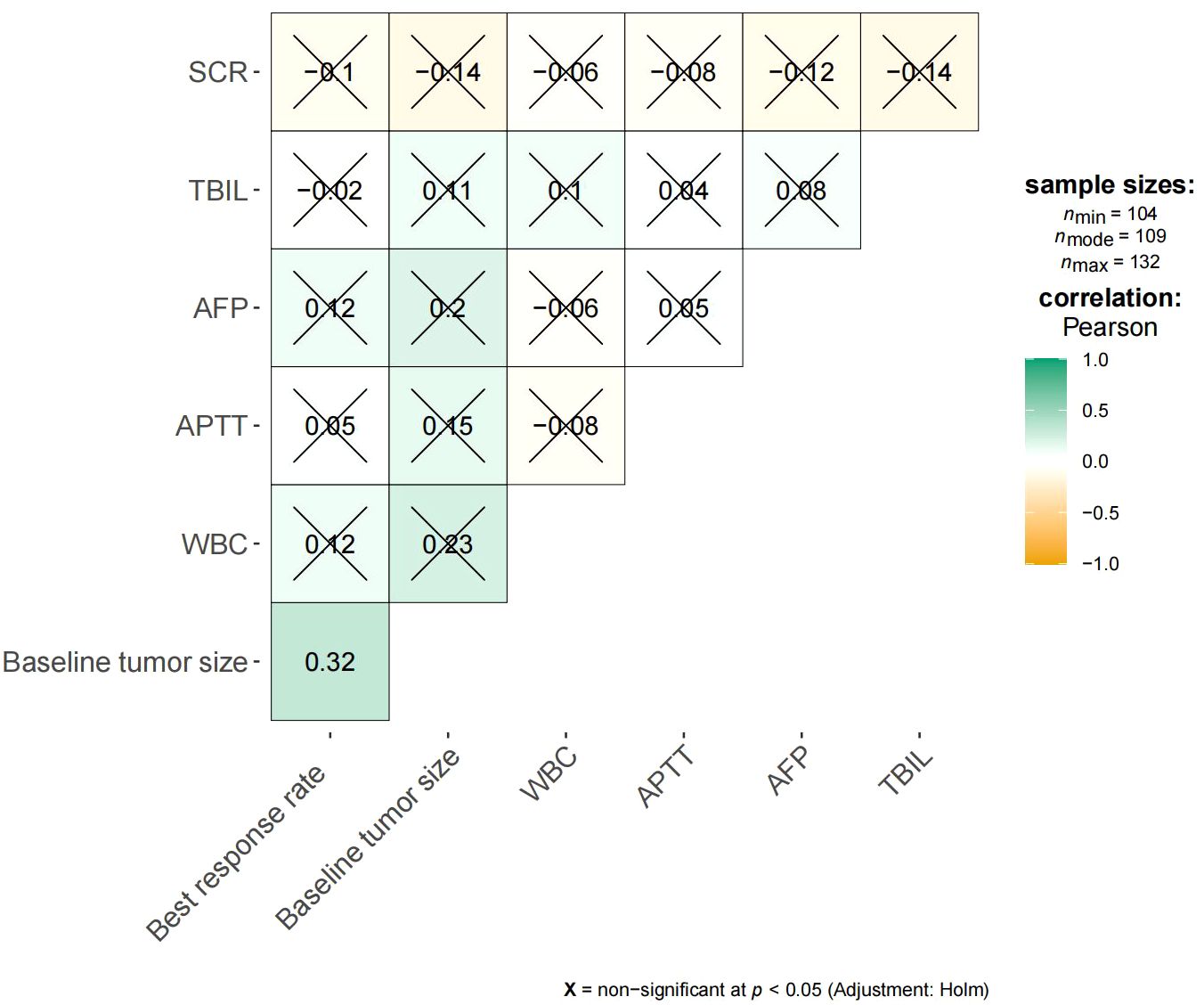
Figure 7 Heat map of continuous variables and best response rate. SCR, Serum creatinine; TBIL, Total bilirubin; AFP, α-fetoprotein; APTT, Activated partial thromboplastintime.
We gathered data on adverse events that occurred among patients receiving medications and HAIC therapy. All patients were evaluated for treatment-related adverse events using the CTCAE 4.0 grading system. Both groups exhibited similar frequencies of overall grade 1-2 and grade 3-4 adverse events (AEs), as reflected in Table 3. Specifically, the most common grade 1-2 AEs were pain, fatigue, and abnormal liver function, while the most frequent grade 3-4 AEs were fatigue, pain, and fever. Importantly, no patient died due to adverse events.
This study is the first to explore whether BRR can be used as an alternative endpoint to assess the efficacy of FOLFOX-HAIC therapy combined with molecular targeting and immunotherapy in patients with HCC. Both inferior vena cava tumor thrombus and baseline tumor size were important influencing factors for BRR.
Currently, the recommended initial therapy for advanced liver cancer involves administering a blend of molecular targeted drugs and ICI (25, 26). Targeted therapy combined with immunotherapy, as the mainstream treatment, has achieved a good local control rate and survival benefits in patients with advanced liver cancer; however, the ORR and OS remain unsatisfactory. Hence, researchers are trying to explore more effective combination treatment options (27, 28). Two studies conducted at our center suggested that patients with primary liver cancer undergoing HAIC in conjunction with immunotherapy and molecular targeted therapy exhibited a greater likelihood of undergoing surgical conversion and a notable survival advantage following surgery (14, 15).
Early and precise evaluation of tumor response to therapy is imperative for the optimal treatment of HCC (29). HCC can be diagnosed and monitored by imaging. The only validated non-invasive prognostic markers for HCC are tumor staging and AFP levels, and both have significant limitations in approximating tumor biology (30). Hence, formulating efficacious radiological criteria is crucial to identify HCC patients who would benefit from combination therapy. The criteria of the European Society for the Study of the Liver suggest ORR as a suitable alternative endpoint for assessing the efficacy of topical therapy (31–33). Several studies have demonstrated a correlation between objective response according to mRECIST and OS in patients (34, 35). Hence, it is a justifiable inference that the rate of tumor remission is associated with the prognosis. ORR is characterized by a decline of over 30% in the sum of the maximum diameters of the target lesion (The enhanced portion). The definition of BRR in this investigation is akin to that of ORR, albeit with a threshold of 20%, which may be relevant. The OS remains the principal outcome measure in clinical investigations on oncology and HCC. Nevertheless, it is imperative to ascertain a dependable secondary endpoint that can anticipate the OS. This study aimed to investigate the feasibility of utilizing BRR as a plausible substitute endpoint for assessing the efficacy of combination therapy in HCC. In clinical research, surrogate endpoints are employed as early indicators of treatment effectiveness instead of OS (36). Our study revealed noteworthy disparities in the OS between the high and low rating groups categorized based on the BRR truncation values. Notably, the majority of patients can undergo assessment for BRR after their third HAIC treatment session. However, it is imperative to acknowledge that a few patients may require supplementary therapy to attain a more significant tumor response. Nonetheless, the potential for hepatic injury linked to repeated surgeries renders this approach advantageous for only a restricted cohort of patients. Consequently, it is advisable that patients transition to an alternative treatment protocol if the optimal BRR is not attained following the third HAIC session. During the immunotherapy course with ICI, patients may exhibit atypical reaction patterns, including false progression, reaction separation, and delayed reaction. Notably, objective responses have been documented in non-small cell lung cancer patients up to 2 years following ICI treatment (37, 38). The median apparent time for FOLFOX-HAIC is approximately 3–4 treatment cycles (8, 39). This may explain why most patients achieve the BRR after undergoing three HAIC treatment sessions.
We screened and weighted factors that may affect the prognosis of HCC patients to verify the robustness of the relationship between BRR and OS. We included measures such as tumor characteristics (baseline tumor size, AFP level, and vascular invasion), serum bilirubin levels, blood-clotting parameters, and other liver function indicators. Additionally, age, underlying disease, sex, treatment, laboratory test values, and other indicators reflecting the basic state of the patient’s body were considered (40).
We finally analyzed the correlation between BRR and baseline features and found that BRR was associated with baseline tumor size and inferior vena cava tumor thrombus. The prognosis of HCC patients with inferior vena cava tumor thrombus is poor, and most of them eventually develop liver failure or cancer thrombus detachment shortly and die from pulmonary embolism or cardiac tamponade. There is no international consensus for treating HCC patients with inferior vena cava tumor thrombus (41, 42). Large liver cancer tumors at baseline imply late-stage cancers and poor liver function with a poor prognosis (25). Therefore, combination therapy should be recommended cautiously for HCC patients with large tumors or inferior vena cava tumor thrombus, as they may not benefit from this therapy.
The incidence of adverse events was comparable between the two groups, likely attributed to the absence of a significant disparity in the number of HAIC treatments administered. Nearly all patients encountered at least one adverse event. Specifically, 74% (82/111) of patients experienced at least one grade 3-4 adverse event, representing a notably higher incidence compared to previous studies (12, 15). This elevated frequency may be attributed to the inclusion of adverse events related to the perfusion therapy process, including fever and pain. These adverse events were manageable and did not significantly influence the prognosis of patients.
This retrospective study has certain limitations. First, its retrospective design resulted in inherent bias, including selection bias in patient inclusion and information bias in imaging data evaluation. Second, its retrospective and single-center nature may limit the generalizability of the findings to other cancer research centers. Thus, a prospective multicenter study is warranted to validate the results of this study.
In conclusion, this study shows that integrating FOLFOX-HAIC therapy with molecular targeting and immunotherapy can diminish the tumor burden effectively and expeditiously in several patients, leading to enhanced survival results. After three sessions of HAIC, patients with liver cancer undergoing combination therapy can assess their BRR to ascertain the potential benefits of the treatment.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Tianjin Cancer Hospital Medical Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
ML: Writing – original draft, Conceptualization, Data curation. JL: Writing – review & editing. LW: Writing – review & editing. TL: Conceptualization, Data curation, Writing – review & editing. QS: Investigation, Software, Writing – review & editing. YX: Software, Conceptualization, Writing – review & editing. ZG: Supervision, Data curation, Writing – review & editing. MQ: Validation, Methodology, Writing – review & editing. HQ: Project administration, Methodology, Writing – review & editing. KZ: Data curation, Supervision, Writing – review & editing. WX: Resources, Funding acquisition, Writing – review & editing. HPY: Funding acquisition, Resources, Writing – review & editing. HYY: Data curation, Conceptualization, Formal analysis, Validation, Investigation, Funding acquisition, Software, Writing – original draft.
The author(s) declared financial support was received for the research, authorship, and/or publication of this article. This work was supported by the China Health Promotion Foundation (No: XM-2018-011-0006-01), Beijing Health Prevention and Therapy Association (No: IZ 2021-1001), China Health and Medical Development Foundation (No: GQ-KK- 2021-001), and Tianjin Medical University Cancer Institute and Hospital (No: Pharmacology Spontaneous 2021-02).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. (2020) 70:7–30. doi: 10.3322/caac.21590
3. Wang K, Wang C, Jiang H, Zhang Y, Lin W, Mo J, et al. Combination of ablation and immunotherapy for hepatocellular carcinoma: where we are and where to go. Front Immunol. (2021) 12:792781. doi: 10.3389/fimmu.2021.792781
4. Brown ZJ, Hewitt DB, Pawlik TM. Combination therapies plus transarterial chemoembolization in hepatocellular carcinoma: a snapshot of clinical trial progress. Expert Opin Investig Drugs. (2022) 31:379–91. doi: 10.1080/13543784.2022.2008355
5. Li QJ, He MK, Chen HW, Fang WQ, Zhou YM, Xu L, et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus transarterial chemoembolization for large hepatocellular carcinoma: A randomized phase III trial. J Clin Oncol. (2022) 40:150–60. doi: 10.1200/JCO.21.00608
6. He MK, Le Y, Li QJ, Yu ZS, Li SH, Wei W, et al. Hepatic artery infusion chemotherapy using mFOLFOX versus transarterial chemoembolization for massive unresectable hepatocellular carcinoma: a prospective non-randomized study. Chin J Cancer. (2017) 36:83. doi: 10.1186/s40880-017-0251-2
7. Gourd K, Lai C, Reeves C. ESMO virtual congress 2020. Lancet Oncol. (2020) 21:1403–4. doi: 10.1016/S1470-2045(20)30585-4
8. Lyu N, Kong Y, Mu L, Lin Y, Li J, Liu Y, et al. Hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin vs. sorafenib for advanced hepatocellular carcinoma. J Hepatol. (2018) 69:60–9. doi: 10.1016/j.jhep.2018.02.008
9. Liu M, Shi J, Mou T, Wang Y, Wu Z, Shen A. Systematic review of hepatic arterial infusion chemotherapy versus sorafenib in patients with hepatocellular carcinoma with portal vein tumor thrombosis. J Gastroenterol Hepatol. (2020) 35:1277–87. doi: 10.1111/jgh.15010
10. Lai Z, He M, Bu X, Xu Y, Huang Y, Wen D, et al. Lenvatinib, toripalimab plus hepatic arterial infusion chemotherapy in patients with high-risk advanced hepatocellular carcinoma: A biomolecular exploratory, phase II trial. Eur J Cancer. (2022) 174:68–77. doi: 10.1016/j.ejca.2022.07.005
11. Liu BJ, Gao S, Zhu X, Xu YJ, Chen HW, Zhou YM, et al. Real-world study of hepatic artery infusion chemotherapy combined with anti-PD-1 immunotherapy and tyrosine kinase inhibitors for advanced hepatocellular carcinoma. Immunotherapy. (2021) 13:1395–405. doi: 10.2217/imt-2021-0192
12. He MK, Liang RB, Zhao Y, Xu YJ, Chen HW, Zhou YM, et al. Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma. Ther Adv Med Oncol. (2021) 13:1395–405. doi: 10.1177/17588359211002720
13. Dong Y, Liu TH, Yau T, Hsu C. Novel systemic therapy for hepatocellular carcinoma. Hepatol Int. (2020) 14:638–51. doi: 10.1007/s12072-020-10073-7
14. Zhang W, Zhang K, Liu C, Gao W, Si T, Zou Q, et al. Hepatic arterial infusion chemotherapy combined with anti-PD-1/PD-L1 immunotherapy and molecularly targeted agents for advanced hepatocellular carcinoma: a real world study. Front Immunol. (2023) 14:1127349. doi: 10.3389/fimmu.2023.1127349
15. Zhang J, Zhang X, Mu H, Yu G, Xing W, Wang L, et al. Surgical conversion for initially unresectable locally advanced hepatocellular carcinoma using a triple combination of angiogenesis inhibitors, anti-PD-1 antibodies, and hepatic arterial infusion chemotherapy: A retrospective study. Front Oncol. (2021) 11:729764. doi: 10.3389/fonc.2021.729764
16. Llovet JM, Lencioni R. mRECIST for HCC: Performance and novel refinements. J Hepatol. (2020) 72:288–306. doi: 10.1016/j.jhep.2019.09.026
17. Lencioni R, Montal R, Torres F, Park JW, Decaens T, Raoul JL, et al. Objective response by mRECIST as a predictor and potential surrogate end-point of overall survival in advanced HCC. J Hepatol. (2017) 66:1166–72. doi: 10.1016/j.jhep.2017.01.012
18. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. (2010) 30:52–60. doi: 10.1055/s-0030-1247132
19. Malik AY, Foster C. The revised Declaration of Helsinki: cosmetic or real change. J R Soc Med. (2016) 109:184–9. doi: 10.1177/0141076816643332
20. Xia D, Wang Q, Bai W, Wang E, Wang Z, Mu W, et al. Optimal time point of response assessment for predicting survival is associated with tumor burden in hepatocellular carcinoma receiving repeated transarterial chemoembolization. Eur Radiol. (2022) 32:5799–810. doi: 10.1007/s00330-022-08716-4
21. Bhaskaran K, Dos-Santos-Silva I, Leon DA, Douglas IJ, Smeeth L. Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3·6 million adults in the UK. Lancet Diabetes Endocrinol. (2018) 6:944–53. doi: 10.1016/S2213-8587(18)30288-2
22. Talbot D, Diop A, Lavigne-Robichaud M, Brisson C. The change in estimate method for selecting confounders: A simulation study. Stat Methods Med Res. (2021) 30:2032–44. doi: 10.1177/09622802211034219
23. Miyazaki Y, Nakano K, Nakayamada S, Kubo S, Inoue Y, Fujino Y, et al. Efficacy and safety of tofacitinib versus baricitinib in patients with rheumatoid arthritis in real clinical practice: analyses with propensity score-based inverse probability of treatment weighting. Ann Rheum Dis. (2021) 80:1130–6. doi: 10.1136/annrheumdis-2020-219699
24. Wang MT, Lai JH, Huang YL, Liou JT, Cheng SH, Lin CW, et al. Comparative effectiveness and safety of different types of inhaled long-acting β(2)-agonist plus inhaled long-acting muscarinic antagonist vs inhaled long-acting β(2)-agonist plus inhaled corticosteroid fixed-dose combinations in COPD A propensity score-inverse probability of treatment weighting cohort study. Chest. (2021) 160:1255–70. doi: 10.1016/j.chest.2021.05.025
25. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. (2022) 76:681–93. doi: 10.1016/j.jhep.2021.11.018
26. Li M, Zhang K, He J, Zhang W, Lv T, Wang L, et al. Hepatic arterial infusion chemotherapy in hepatocellular carcinoma: A bibliometric and knowledge-map analysis. Front Oncol. (2022) 12:1071860. doi: 10.3389/fonc.2022.1071860
27. Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. (2022) 76:862–73. doi: 10.1016/j.jhep.2021.11.030
28. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. (2020) 382:1894–905. doi: 10.1056/NEJMoa1915745
29. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. (2012) 56:908–43. doi: 10.1016/j.jhep.2011.12.001
30. Harding-Theobald E, Louissaint J, Maraj B, Cuaresma E, Townsend W, Mendiratta-Lala M, et al. Systematic review: radiomics for the diagnosis and prognosis of hepatocellular carcinoma. Aliment Pharmacol Ther. (2021) 54:890–901. doi: 10.1111/apt.16563
31. Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. (2002) 359:1734–9. doi: 10.1016/S0140-6736(02)08649-X
32. Sala M, Llovet JM, Vilana R, Bianchi L, Solé M, Ayuso C, et al. Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma. Hepatology. (2004) 40:1352–60. doi: 10.1002/hep.20465
33. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. (2008) 359:378–90. doi: 10.1056/NEJMoa0708857
34. Vincenzi B, Di Maio M, Silletta M, D'Onofrio L, Spoto C, Piccirillo MC, et al. Prognostic relevance of objective response according to EASL criteria and mRECIST criteria in hepatocellular carcinoma patients treated with loco-regional therapies: A literature-based meta-analysis. PloS One. (2015) 10:e0133488. doi: 10.1371/journal.pone.0133488
35. Kim BK, Kim SU, Kim KA, Chung YE, Kim MJ, Park MS, et al. Complete response at first chemoembolization is still the most robust predictor for favorable outcome in hepatocellular carcinoma. J Hepatol. (2015) 62:1304–10. doi: 10.1016/j.jhep.2015.01.022
36. Wilson MK, Karakasis K, Oza AM. Outcomes and endpoints in trials of cancer treatment: the past, present, and future. Lancet Oncol. (2015) 16:e32–42. doi: 10.1016/S1470-2045(14)70375-4
37. Borcoman E, Kanjanapan Y, Champiat S, Kato S, Servois V, Kurzrock R, et al. Novel patterns of response under immunotherapy. Ann Oncol. (2019) 30:385–96. doi: 10.1093/annonc/mdz003
38. Guan Y, Feng D, Yin B, Li K, Wang J. Immune-related dissociated response as a specific atypical response pattern in solid tumors with immune checkpoint blockade. Ther Adv Med Oncol. (2022) 14:17588359221096877. doi: 10.1177/17588359221096877
39. Qin S, Bai Y, Lim HY, Thongprasert S, Chao Y, Fan J, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. (2013) 31:3501–8. doi: 10.1200/JCO.2012.44.5643
40. Rich NE, Murphy CC, Yopp AC, Tiro J, Marrero JA, Singal AG. Sex disparities in presentation and prognosis of 1110 patients with hepatocellular carcinoma. Aliment Pharmacol Ther. (2020) 52:701–9. doi: 10.1111/apt.15917
41. Xia Y, Zhang J, Ni X. Diagnosis, treatment and prognosis of hepatocellular carcinoma with inferior vena cava/right atrium tumor thrombus. Oncol Lett. (2020) 20:101. doi: 10.3892/ol
Keywords: targeted therapy, immunotherapy, FOLFOX-HAIC, surrogate endpoint, combination therapy, overall survival
Citation: Li M, Liao J, Wang L, Lv T, Sun Q, Xu Y, Guo Z, Quan M, Qin H, Yu H, Zhang K, Xing W and Yu H (2024) A preliminary study of optimal treatment response rates in patients undergoing hepatic arterial infusion chemotherapy combined with molecular targeting and immunotherapy. Front. Immunol. 15:1303259. doi: 10.3389/fimmu.2024.1303259
Received: 27 September 2023; Accepted: 25 March 2024;
Published: 10 April 2024.
Edited by:
Utpreksha Vaish, University of Alabama at Birmingham, United StatesReviewed by:
Sagnik Giri, University of Arizona, United StatesCopyright © 2024 Li, Liao, Wang, Lv, Sun, Xu, Guo, Quan, Qin, Yu, Zhang, Xing and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kai Zhang, emhhbmdrYWljckBmb3htYWlsLmNvbQ==; Wenge Xing, eGluZ3dlbmdlQHRqbXVjaC5jb20=; Haipeng Yu, amllcnVrZUAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.