- 1Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, Moscow, Russia
- 2National Research Center for Hematology, Russia Federation Ministry of Public Health, Moscow, Russia
- 3Department of Genetics and Molecular Biology, Gamaleya National Research Centre of Epidemiology and Microbiology, Moscow, Russia
- 4The “Russian Clinical Research Center for Gerontology” of the Ministry of Healthcare of the Russian Federation, Pirogov Russian National Research Medical University, Moscow, Russia
Neutrophils play a primary role in protecting our body from pathogens. When confronted with invading bacteria, neutrophils begin to produce leukotriene B4, a potent chemoattractant that, in cooperation with the primary bacterial chemoattractant fMLP, stimulates the formation of swarms of neutrophils surrounding pathogens. Here we describe a complex redox regulation that either stimulates or inhibits fMLP-induced leukotriene synthesis in an experimental model of neutrophils interacting with Salmonella typhimurium. The scavenging of mitochondrial reactive oxygen species by mitochondria-targeted antioxidants MitoQ and SkQ1, as well as inhibition of their production by mitochondrial inhibitors, inhibit the synthesis of leukotrienes regardless of the cessation of oxidative phosphorylation. On the contrary, antioxidants N-acetylcysteine and sodium hydrosulfide promoting reductive shift in the reversible thiol-disulfide system stimulate the synthesis of leukotrienes. Diamide that oxidizes glutathione at high concentrations inhibits leukotriene synthesis, and the glutathione precursor S-adenosyl-L-methionine prevents this inhibition. Diamide-dependent inhibition is also prevented by diphenyleneiodonium, presumably through inhibition of NADPH oxidase and NADPH accumulation. Thus, during bacterial infection, maintaining the reduced state of glutathione in neutrophils plays a decisive role in the synthesis of leukotriene B4. Suppression of excess leukotriene synthesis is an effective strategy for treating various inflammatory pathologies. Our data suggest that the use of mitochondria-targeted antioxidants may be promising for this purpose, whereas known thiol-based antioxidants, such as N-acetylcysteine, may dangerously stimulate leukotriene synthesis by neutrophils during severe pathogenic infection.
1 Introduction
The main effector functions of polymorphonuclear leukocytes (PMNLs, neutrophils) in the fight against pathogens include phagocytosis, oxidative burst, degranulation. They eliminate pathogens through the production of reactive oxygen species (ROS) and releasing azurophilic granules containing antimicrobial proteins such as neutrophil elastase and myeloperoxidase (MPO) (1, 2). Nitric oxide (NO) production in human PMNLs, along with ROS and MPO is important to execute antimicrobial activity (3). In cases where these weapons are not effective enough to kill pathogens, a program of collective behavior known as swarming can be initiated. This program involves the production of leukotriene B4 (LTB4) as a potent chemoattractant and the gathering of neutrophils into dense clusters surrounding pathogens (4). In these clusters, neutrophils activate suicidal production of extracellular chromatin traps (NETs), which enhance pathogen fixation (5). Both swarming and NETs formation (NETosis) programs are subject to complex redox regulation.
Early neutrophil recruitment is initiated by pathogen-associated molecular patterns (PAMPs), including N-formyl peptides, and damage-associated molecular patterns (DAMPs) (6). This interaction of “pioneer” neutrophils with pathogen (or danger signal from damaged tissue) results in leukotriene B4 (LTB4) synthesis, and started the next step, swam attractant release (7), leading to exponential accumulation of neutrophils at infection/damage loci (8). At this stage, LTB4 and the receptors BLT1 for LTB4 coordinate cellular responses by neutrophils with each other, and the swarm is formed. Also, the coordinated transcellular biosynthesis of LTB4 drives swarming responses (9). The initiation of swarming converges on the synthesis of LTB4. During this swarm recruitment NETs could not be observed. On the timeline of major events in neutrophil swarming, the onset of NETs forming proceeds later, on the aggregation phase of the swarming response when neutrophils clusters surrounding pathogens has been formed, and activated cells release chromatin, which is accompanied with the loss of integrity of cellular membrane (5, 10).
Leukotrienes play role also in inhibition of neutrophil swarming (6). ω-OH-LTB4 and ω-COOH-LTB4 compete with LTB4 for BLT1 receptor binding (11) and act as inhibitors of LTB4-mediated responses. When LTB4 is easy transformed to ω-OH-LTB4, this decreases neutrophil swarming. We recently found that stimulation by fMLP of neutrophils after preincubation with bacteria Salmonella typhimurium strongly increased leukotriene synthesis; but when the bacteria:neutrophil ratio increased, the transformation of LTB4 to ω-OH-LTB4 was suppressed (12), which support increased level of LTB4. LTB4 is the strongest chemoattractant and works at sub nanomolar concentrations (13). The increased formation of LTB4 during the interaction of neutrophils with bacteria works as a signal of neutrophils for help with an increase in bacterial load. In this study we explored intervention of redox processes in neutrophil on fMLP-induced leukotriene synthesis in the experimental model of neutrophil interaction with bacteria Salmonella typhimurium.
NADPH-oxidase (NOX2) is the primary source of ROS which is not only responsible for oxidative burst but also involved in phagocytosis (14), degranulation (15) and NETosis (16). ROS were required for PMNLs antimicrobial activity against S. pneumoniae; however the NADPH oxidase was dispensable for that (17). S. pneumoniae infection induced mitochondrial ROS production in PMNLs. And mitochondrial ROS were critical for the ability of PMNLs to kill S.pneumoniae. DPI which inhibits ROS production by the NADPH oxidase, did not blunt the ability of PMNs to kill S. pneumoniae, but MitoTempo did. Dunham-Snary et al. (18) were first who showed that neutrophil mitochondria actively participate in phagocytosis and killing of Staphylococcus aureus, and antimycin and MitoTempo increased bacterial survival (18). Human pathogen Shigella dramatically changed neutrophils toward enhanced microbial recognition and mitochondrial ROS production (19). What is the role of mitochondrial ROS in leukotriene synthesis in infected neutrophils?
Mitochondria are an important source of ROS in various cell types, but their role in ROS production in neutrophils has long been underestimated. Only our recent studies (20–22) using the mitochondria-targeted antioxidant SkQ1 [10-(6’-plastoquinonyl)decyltriphenylphosphonium bromide] (23) have demonstrated the important role of mitochondrial ROS (mtROS) in NADPH oxidase activation, degranulation, extracellular trap formation (NETosis), and leukotriene synthesis. These studies analyzed the activation of neutrophils by the Ca2+ ionophore A23187 and the chemoattractant N-formyl-L-methionyl-L-leucyl-L-phenylalanine (fMLP), while the role of mtROS in the interaction of neutrophils with bacteria remains unknown.
Neutrophils control the infection, in turn, microorganisms affect the functions of neutrophils, controlling phagocytosis, the production of oxidants and the lifespan of neutrophils (24). Pathogens antagonize neutrophils, for example, by secreting catalase to reduce ROS (25, 26). To protect from ROS, the fungal pathogen Histoplasma capsulatum infects both neutrophils and macrophages producing a superoxide dismutase (SOD) (27). Gram-negative pathogen Coxiella burnetii (28) and Pseudomonas aeruginosa (26), as well as E.coli producing enterobactin (29) inhibit NADPH oxidase in human neutrophils. Microbial avoidance strategies can target not only ROS production but also degranulation and synthesis of leukotriene B4 (30). Decreased intracellular GSH correlates with the susceptibility to infections. Glutathione reductase (Gsr) catalyzes the reduction of glutathione disulfide to glutathione using NADPH as an electron donor (31). Glutathione reductase promotes Candida albicans clearance (32). As NADPH is a cofactor required for GSH regeneration from GSSG, the consumption of NADPH affects the regeneration of GSH (33). One can propose that NADPH-oxidase inhibition can support GSH level. These processes certainly affect LT synthesis and neutrophil swarming around pathogens.
5-Lipoxygenase (5-LOX) is a key enzyme in synthesis of LTB4 involved in chemical cell-to-cell communication. LTB4 is critical for enhancing chemotactic responses to primary chemoattractants, such as fMLP (34) increasing clustering and surface mobility of adhesion receptors integrins (35). Thus, LTB4 is not only a chemotactic, but also a neutrophil aggregating substance (36) that promotes local neutrophil interactions during swarming (4). Initial neutrophil–neutrophil contacts are critical to initiate swarming (37). We recently found, that the synthesis of LTB4 in neutrophils in the presence of bacteria and fMLP correlates with the appearance of cell-cell contacts (12), which can serve as a signal conductor to further clustering and swarming.
In the current study, we observed that redox processes can either activate or inhibit fMLP-induced leukotriene synthesis in an experimental model of neutrophil interaction with the bacteria Salmonella typhimurium. Our study demonstrated that mitochondrial ROS are crucial for 5-LOX activation and LT synthesis, and mitochondria-targeted antioxidants inhibited LT synthesis. On the other hand, inhibition of NOX2-dependent ROS supported LTB4 synthesis, and potentiated the stimulating effect of thiol oxidant diamide on LT synthesis.
2 Materials and methods
Hank’s balanced salt solution with calcium and magnesium but without Phenol Red and sodium hydrogen carbonate (HBSS), Dulbecco’s phosphate-buffered saline (PBS) with magnesium but without calcium, N-Formyl-L-methionyl-L-Leucyl-L-Phenylalanine (fMLP), Ellman’s reagent [5,5′-Dithiobis(2-nitrobenzoic acid)], oxythiamine, and fibrinogen from human plasma, were purchased from Sigma (Steinheim, Germany). Dextran T-500 was from Pharmacosmos (Holbæk, Denmark). ROS indicator Carboxy-H2DCFDA and Pierce™ Avidin, Fluorescein (FITC) conjugated were from Thermo Fisher Scientific (Waltham, MA USA). Mitochondrial ToxGlo™ Assay was from Promega Corp. (Madison, WI USA). Biotinylated murine IgG1 antibodies CD11b and CD54 were from Ancell Corp. (Bayport, MN USA). Bacteria (S. typhimurium IE 147 strain) were obtained from the Collection of Gamaleya National Research Center of Epidemiology and Microbiology (Moscow, Russia). Bacteria were grown in Luria–Bertani broth to a concentration of 1 × 109 colony-forming units (CFU)/mL. In this study not opsonized bacteria were used.
2.1 Neutrophil isolation
Human polymorphonuclear leukocytes (PMNL) were isolated from freshly collected blood with citrate anticoagulant. Leukocyte-rich plasma was obtained from donated blood by sedimentation in the presence of dextran T-500. Granulocytes were obtained as described (38). Cell viability was checked by trypan blue exclusion. Control suspension samples were stained in parallel with Hoechst and Romanovsky-Giemsa dyes to assess the homogeneity of the cell population. PMNLs (95–97% purity, 98–99% viability) were stored at room temperature in Dulbecco’s PBS containing 1 mg/mL glucose (no CaCl2) until use.
2.2 Determination of 5-LOX product formation in cells
PMNLs [(1.2-1.5)x107/6 ml HBSS with 10 mM HEPES (HBSS/HEPES)] were pre-incubated for 10 min at 37°C, 5% CO2. At this stage, 2-deoxy-D-glucose (2-DG) was added to the samples, in those cases where this was provided for in the experimental protocol. Then, maintaining incubation conditions, S. typhimurium (bacteria per cell ratio ~25:1) and indicated reagents were added for 30 min, followed by 10 min exposure to 0,1 µM fMLP. The treatment was stopped by adding of an equal volume of methanol (-18°C) with 90 ng PGB2 as internal standard. Major 5-LOX metabolites, 5S, 12R-dihydroxy-6,14-cis-8,10-trans-eicosatetraenoic acid (LTB4), iso-LTB4 (5S, 12SR-all-trans-diHETE), ω-OH-LTB4, ω-COOH-LTB4 and 5S-hydroxy-6-trans-8,11,14-cis-eicosatetraenoic acid (5-HETE) were identified as previously described (12).
2.3 ATP assessment
ATP detection component from Mitochondrial ToxGlo™ Assay kit was used. In accordance with the manufacturer’s protocol, the lyophilized enzyme/substrate mixture (ATP Detection Substrate) was reconstituted by lysis buffer (ATP Detection Buffer) to obtain ATP Detection Reagent. Just before the experiment PMNLs were resuspend in HBSS/HEPES, seeded in solid white F-bottom 96-well plates (4 × 105 cells/well), pre-incubated for 10 min at 37°C, 5% CO2. 2-DG was added at this stage if prescribed by the protocol. Then S. typhimurium (bacteria per cell ratio ~25:1) and reagents were added, according to experimental protocol. Samples were incubated for 20 min under the same conditions. After the treatment was complete, plates were equilibrated to room temperature for 5 min followed by adding an equal volume of ATP Detection Reagent to the contents of each well. After 3 min orbital shaking luminescence intensity was measured on a CLARIOstar microplate reader (BMG Labtech, Ortenberg, Germany) and MARS data analysis software package from BMG Labtech was used to process the data obtained.
2.4 Cytosolic ROS assessment
ROS accumulation in the cytosol was quantified by measuring the green fluorescence intensity of 2’,7’-dichlorofluorescein (DCF). According to manufacturer’s recommendation, neutrophils were loaded with 5 μM carboxy-2’,7’-dichlorodihydrofluorescein diacetate (H2DCF-DA) for 60 min at room temperature followed by washing with PBS, suspended in D-PBS and then stored at room temperature in the dark until use. Immediately before the experimental treatment cells were resuspended in HBSS/HEPES, seeded in fibrinogen-coated wells of the 96-well plate (4 × 105 cells/well) and pre-incubated for 10 min at 37°C, 5% CO2. Then S. typhimurium (bacteria per cell ratio ~25:1) and indicated reagents were added for 30 min followed by 0,1 µM fMLP stimulation. Fluorescence intensity at excitation and emission wavelengths of 488 and 525 nm was measured using a CLARIOstar microplate reader.
2.5 Thiol redox state assessment
Ellman’s assay was used for quantitating reduced sulfhydryl groups. Neutrophils were resuspended in HBSS/HEPES (2 × 106 cells/1 mL probe) and pre-incubated for 10 min at 37°C, 5% CO2. After the treating according to experimental protocol cells were centrifuged for 7 min at 600 g, 4°C. Permeabilizing buffer (67 mM Na2HPO4, 35 mM citric acid and 0.1% Triton X-100; 100 µL/probe) was added to packed cell pellets, shaken and kept on ice for 10 min. Lysates were centrifuged at 10000 g, 4°C for 10 min. 50 µL supernatant (in duplicate for each probe) was mixed with 100 µL DTNB solution (0.1 mg/mL in 0.1 M NaH2PO4/Na2HPO4, 1M EDTA, pH 7.8) in 96-well plate. Equal volumes of reduced glutathione solutions from 1 to 500 µM were used to generate standard curve. The samples were allowed to stand for 15 min at room temperature followed by absorbance reading at 412 nm on CLARIOstar microplate reader.
2.6 Adhesion assessment
Spectrophotometric detection of 2,3-diaminophenazine, which is a product of myeloperoxidase-catalyzed oxidation of o-phenylenediamine dihydrochloride (OPD) by hydrogen peroxide, was used to assess neutrophil substrate adhesion (39). PMNLs (2 × 105 cells/sample) were seeded onto fibrinogen-coated 96-well plates containing pre-warmed HBSS/HEPES and agents required by experimental protocol. Samples were incubated at 37°C, 5% CO2 after which the plate was washed twice to remove unattached cells. 4 mM H2O2 and 5.5 mM OPD in permeabilizing buffer (67 mM Na2HPO4, 35 mM citric acid and 0.1% Triton X-100) were added for 5 min. The reaction was stopped with 1 M H2SO4. The percentage of attached neutrophils was determined by measuring the absorption (490 nm) of 2,3-diaminophenazine and comparing the obtained values with the calibration ones.
2.7 Scanning electron microscopy
For scanning electron microscopy, cells were fixed for 30 min in 2.5% glutaraldehyde, postfixed for 15 min with 1% osmium tetroxide in 0.1 M cacodylate (pH 7.3), dehydrated in an acetone series, and processed by conventional scanning electron microscopic techniques, as described (40).
2.8 Cell adhesion molecules expression assessment
CD11b, alpha subunit of the Mac-1 integrin, and intercellular adhesion molecule-1 (CD54) proteins expression were determined by flow cytometry. Neutrophils were resuspended in HBSS/HEPES (106 cells/1 mL probe) and pre-incubated for 5 min at 37°C, 5% CO2. After the treating according to experimental protocol cells were centrifuged while cooled for 10 min at 200 g, 4°C. Then biotinylated mouse CD11b (20 µg/mL HBSS/HEPES) or CD54 (30 µg/mL HBSS/HEPES) antibodies were added for 45 min, on ice. After being washed with cold PBS cells were stained with avidin-FITC (30 µg/mL HBSS/HEPES) for 30 min on ice followed by flow cytometry on Amnis FlowSight Imaging Flow Cytometer (Luminex Corp., Austin, Texas, USA) at 488/525 ex/em filter set. IDEAS Image Data Exploration and Analysis Software (Luminex Corp., ustin, Texas, USA) was used for data analyzing.
2.9 Statistics
Results are presented as mean ± SEM. Analysis of statistical significance for multiple comparisons was performed using GraphPad Prism 10.0.1 software. Differences with P-values <0.05 were considered statistically significant.
3 Results
3.1 Mitochondrial ROS production is critical for fMLP-induced leukotriene synthesis induced in neutrophils stimulated with Salmonella typhimurium
We have previously found that preincubation of human neutrophils with S. typhimurium strongly stimulates fMLP-induced LTB4 synthesis (12). These conditions modeled the collective behavior of neutrophils known as swarming (4). We have shown that S. typhimurium stimulates 5LOX, but the mechanism of the enhancing effect of bacteria upon subsequent addition of fMLP remains unclear. We have recently shown that LTB4 synthesis induced by fMLP (as well as some other stimuli) is dependent on mtROS production (22). However, the effect of fMLP in this study was artificially stimulated by cytochalasin, which disrupts the actin cytoskeleton. In the present study, we analyzed the possible role of mtROS in the stimulation of fMLP-induced LTB4 synthesis in neutrophil interaction with bacteria S. typhimurium. Separate addition of fMLP (Control w/o Salm) or bacteria S. typhimurium (Control w/o fMLP) to neutrophils produced very slight effect on leukotriene synthesis (Supplementary Figure 1).
As shown in Figure 1A, mitochondria-targeted antioxidants SkQ1 and MitoQ inhibit fMLP-induced LTB4 synthesis in neutrophils exposed to S. typhimurium at 50 nM and 200 nM, respectively. The synthesis of the omega-hydroxylation product of LTB4 (ω-LTB4) and total leukotrienes (ΣLT=LTB4+isomers of LTB4+ω-OH-LTB4) were also inhibited by SkQ1 and MitoQ. These leukotrienes are the main 5-LOX products in our experimental model (Supplementary Figure 1).
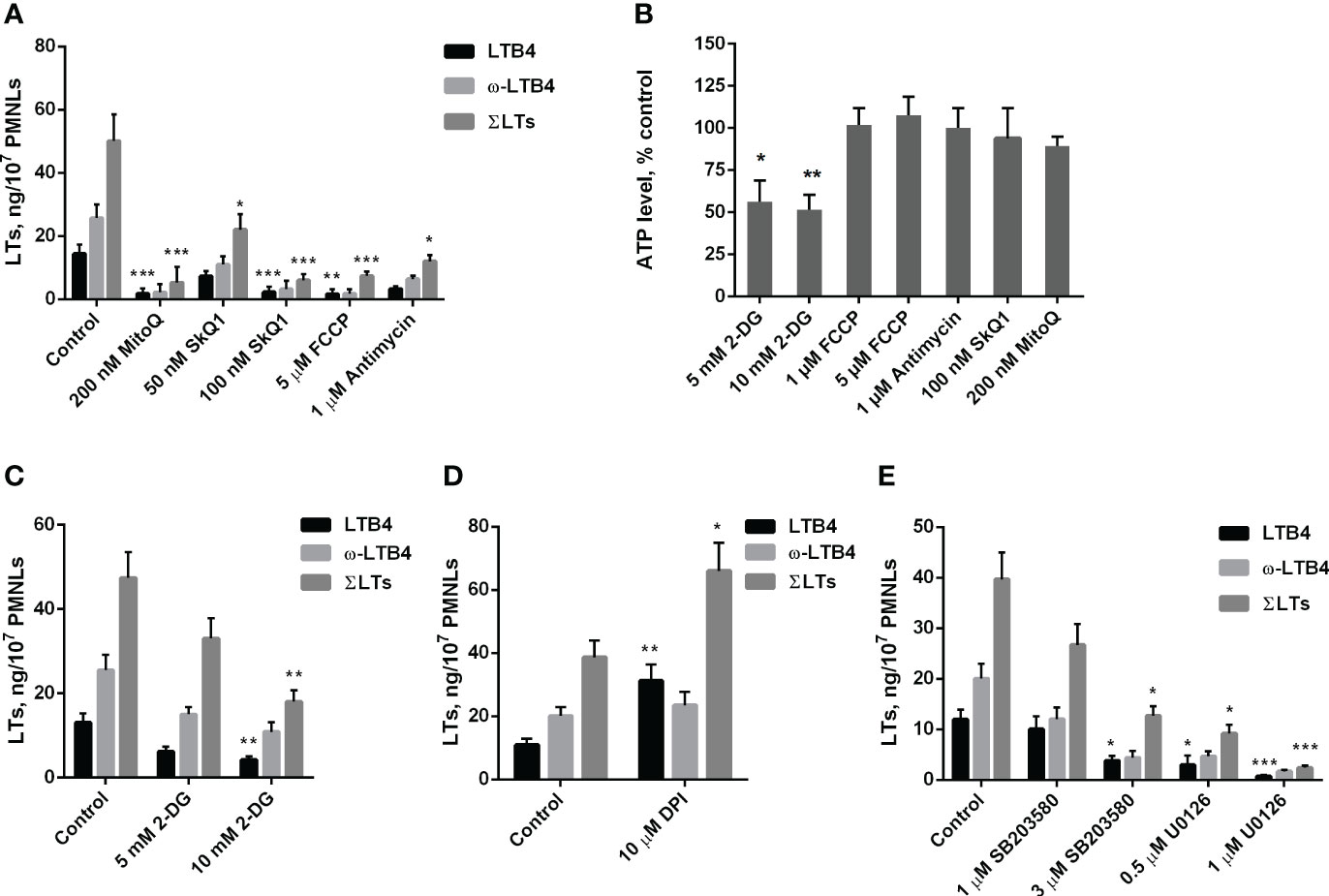
Figure 1 (A–C). Effect of mitochondria-targeted compounds and 2-DG treatment on leukotriene synthesis and ATP generation in neutrophils. (A, C) After 10 min pre-incubation without (A) or in the presence of indicated concentrations of 2-DG (C) PMNLs were exposed for 30 min to either bacteria alone (Control) or bacteria in combination with reagents indicated on X-axis. Then fMLP was added (0.1 µM sample concentration) for 10 min. After the reaction was terminated, the 5-LOX products were analyzed. Presented are absolute values of LTB4, ω-OH-LTB4 and the sum of LTs (ΣLTs) in ng per 107 PMNLs. (B) PMNLs were pre-incubated for 10 min without additives or in the presence of 2-DG (where indicated). Bacteria were then added, either alone (Control, 2-DG probes) or in combination with the indicated stimuli. After 20 min, cells were lysed, and ATP content was determined using the bioluminescent method. Presented are relative values of ATP content in samples as average percentages of control luminescence intensity values (mean ± SEM of luminescence intensity in control samples was 105326 ± 4596 RLU, while 10 µM ATP, used as a positive control, provided 397233± 2187 RLU). (D, E). Effect of NADPH oxidase inhibitors and inhibitors of MAP kinases on leukotriene synthesis in neutrophils. After 10 min pre-incubation, PMNLs were exposed for 30 min to either bacteria alone (Control) or bacteria in combination with reagents indicated on X-axis, followed by 10 min of 0.1 µM fMLP stimulation. When incubations stopped, the 5-LOX products were analyzed. (A–E). Values shown are means ± SEM of three independent experiments performed in duplicate. *p < 0.05, **p < 0.01, ***p < 0.001, for pairs of data compared to corresponding control values as shown by two-way ANOVA with Tukey’s multiple comparison test (A, C–E) or by one-way ANOVA with Dunnett’s multiple comparison test (B).
We have previously shown that mtROS production in neutrophils can be stimulated by the accumulation of Ca2+ in mitochondria (21). Ca2+ uptake into the mitochondrial matrix is driven by the transmembrane electrical potential at the inner membrane (ΔΨ), which is maintained by the activity of the respiratory chain. In the present model, the respiration inhibitor antimycin A, as well as the oxidative phosphorylation uncoupler FCCP, which dissipates ΔΨ, effectively inhibited leukotriene synthesis (Figure 1A). Inhibition of respiration and dissipation of ΔΨ lead to the cessation of mitochondrial ATP synthesis. Since mitochondria-targeted cationic antioxidants such as SkQ1 and MitoQ have also been shown to dissipate ΔΨ (41), we analyzed the possible role of ATP depletion in the inhibition of leukotriene synthesis. Measurements of ATP content in infected neutrophils revealed no effect of antimycin A or FCCP (Figure 1B). These data are consistent with the leading role of glycolysis in ATP production known for neutrophils and other granulocytes (42, 43). In support of this conclusion, inhibition of glycolysis by 2-deoxy-D-glucose (2-DG) led to a decrease in ATP content (Figure 1B) and inhibition of leukotriene synthesis (Figure 1C).
Stimulation of neutrophils by bacteria and fMLP is accompanied by activation of NADPH oxidase (NOX2) and subsequent massive production of ROS (1, 44). To study the possible role of NOX2 in leukotriene synthesis in our model, we used diphenyleneiodonium (DPI), an effective inhibitor of various flavin enzymes, including NADPH oxidase. DPI stimulated the accumulation of LTB4 and total leukotrienes (Figure 1D). This effect was observed previously when leukotriene synthesis was induced by various stimuli and was attributed to inhibition of LTB4 omega-hydroxylation (22). In fact, the ratio of LTB4 to ω-LTB4 with DPI is higher, than in other treatments (Figures 1, 2). But the sum of LTs is also increased, therefore inhibition of NOX2 contribute to stimulation of leukotriene synthesis. It was demonstrated that chronic granulomatous disease (CGD) neutrophils have an increased LTB4 production (45). But the authors determined only LTB4, so this effect may include as 5-LOX stimulation by downregulation of ROS in CGD neutrophils, as well as inhibition of LTB4 omega-hydroxylation in experiments with DPI.
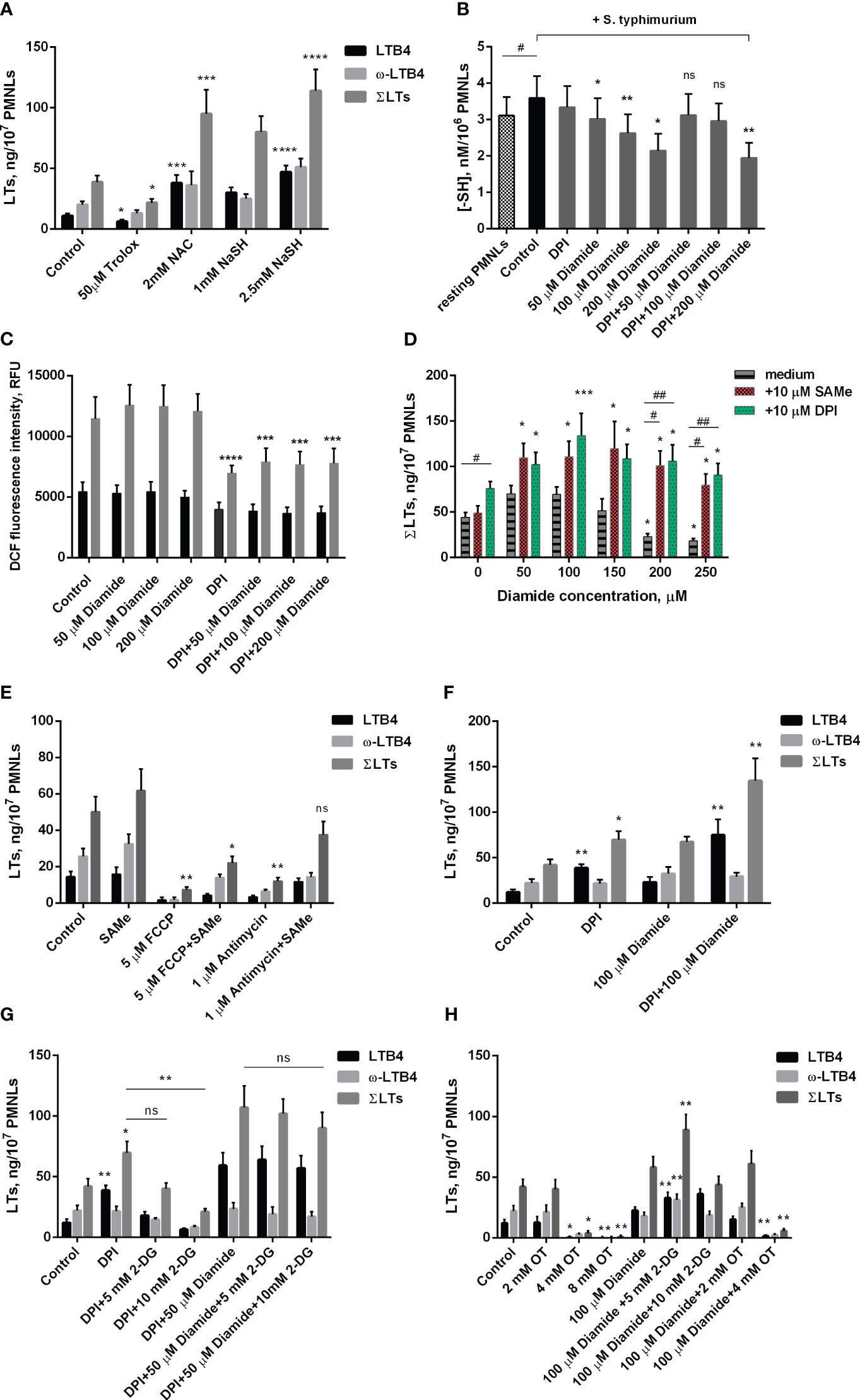
Figure 2 Effect of oxidants and antioxidants on intracellular reduced thiols level, ROS formation and leukotriene synthesis in human neutrophils. (A, D-H). After 10 min pre-incubation without additives or in the presence of indicated concentrations of 2-DG, PMNLs were exposed for 30 min to either bacteria alone (Control) or bacteria in combination with reagents indicated (10 µM DPI and 10 µM SAMe were used), followed by 10 min of 0.1 µM fMLP stimulation. After the reaction was terminated, the 5-LOX products were analyzed. Presented are absolute values of LTB4, ω-OH-LTB4 and the sum of LTs (ΣLTs) in ng per 107 PMNLs. (B) PMNLs were pre-incubated for 10 min, then S. typhimurium alone (Control) or bacteria together with indicated stimuli were added for 20 min; resting PMNLs samples were incubated without any additives. After the exposure time, the cells were pelleted while cooling and assayed for reduced -SH content with Ellman’s reagent. Values are given as the averages of the content of reduced thiols in the samples, nM per 106 PMNLs. (C) PMNLs loaded with H2DCFDA were pre-incubated for 10 min, then either S. typhimurium alone (Control) or bacteria together with indicated stimuli were added. After 30 min 0,1 µM fMLP was added. Presented are the average values of DCF fluorescence intensity measured immediately before (black bars) and 20 minutes after (grey bars) fMLP addition. (A–H). Values shown are means ± SEM of three independent experiments performed in duplicate. ns, not significant; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 for pairs of data compared to corresponding control values; #p < 0.05, ##p < 0.01 for the specified data pairs as shown by one-way ANOVA with Dunnett’s multiple comparison test (B) or two-way ANOVA followed by Tukey’s multiple comparison test (A, C–H).
From these data we can see that mitochondria targeted antioxidant SkQ1 inhibits LT synthesis. We checked the effects of SkQ1 on mtROS production in neutrophils. Measurements of mitochondrial ROS production using the mitochondria-targeted superoxide-sensitive probe MitoSOX (Supplementary Figure 2) showed a significant increase in mtROS upon subsequent stimulation of neutrophils with S. typhimurium and fMLP. This increase in mtROS was inhibited by SkQ1. These data support our conclusion that mtROS production is required for stimulation of fMLP-induced LTB4 synthesis in neutrophil interaction with bacteria S. typhimurium.
In the search for possible targets of mtROS-dependent regulation of leukotriene synthesis, we previously showed that activation of the MAP kinases Erk1/2 and p38 (associated with their phosphorylation) is prevented by SkQ1 (22). Erk1/2 and p38 kinases are known to phosphorylate 5-LOX and stimulate its translocation to the nuclear envelope, which is required for 5-lipoxygenase activity (46), so they may be critical targets of mtROS. In our experimental model, the Erk1/2 inhibitor U0126 and p38 inhibitor SB203580 suppressed 5-LOX activity (Figure 1E). Thus, it can be assumed that these kinases are important in the mtROS-dependent regulation of leukotriene synthesis induced by the combined action of bacteria and fMLP.
3.2 Redox processes modulate fMLP-induced leukotriene synthesis in neutrophils exposed to bacteria Salmonella typhimurium
To further study the role of the redox balance in the activation of leukotriene synthesis under the combined action of S. typhimurium and fMLP, we used the classical antioxidant Trolox, a water-soluble analogue of vitamin E. It was shown that it inhibits leukotriene synthesis, although at a much higher concentration than SkQ1 and MitoQ (Figure 2A).
Unexpectedly, another known antioxidant N-acetylcysteine (NAC) strongly stimulated fMLP-induced leukotriene synthesis in S. typhimurium-activated neutrophils (Figure 2A). A similar effect was observed in the case of sodium hydrosulfide (NaSH) (Figure 2A), an H2S donor that promotes a reductive shift in the redox balance of various cells (47). We hypothesized that this stimulation may be associated with a shift in the redox equilibrium of the reversible thiol-disulfide system and, first of all, with an increase in the ratio of reduced/oxidized glutathione (GSH/GSSG). In fact, NAC is a direct precursor of GSH, and H2S donors can also increase the GSH/GSSG ratio. To analyze this possibility, we used diamide, which penetrates cell membranes and reacts with thiols to form disulfides (48). Diamide at a concentration of 50-200 μM significantly reduced the level of non-protein thiols (Figure 2B) but did not affect the level of cytosolic ROS measured as the oxidation of 2’,7’-dichlorofluorescein (Figure 2C). 50-150 μM diamide slightly increased fMLP-induced leukotriene synthesis in neutrophils pre-incubated with S. typhimurium, while 200-250 μM diamide, without affecting cell viability (Supplementary Figure 3), significantly inhibited leukotriene synthesis (Figure 2D). These data indicate that a decrease in reduced GSH may limit leukotriene synthesis under our conditions. In support of this assumption, we observed a strong stimulation of leukotriene synthesis by the GSH precursor S-adenosyl-L-methionine (SAMe) (Figure 2D). This effect was especially strong in the presence of an inhibitory concentration of diamide (200-250 μM) and reaches approximately 400%. Interestingly, SAMe also reversed inhibitory effect of FCCP or antimycin A on leukotriene synthesis (Figure 2E). Although in our experimental model we were unable to detect a significant effect of antimycin A and FCCP on GSH/GSSH ratio either at the stage of PMNLs interaction with bacteria or after stimulation with fMLP (Supplementary Figure 4), studies on cell lines indicate that they both can reduce GSH intracellular level (49–51), which can be compensated by SAMe. Measurements of mtROS in stimulated neutrophils (Supplementary Figure 2) showed some increase by diamide, which may be partially responsible for the stimulation of LT synthesis observed at low doses of diamide.
As shown in Figure 2B, under conditions of interaction between neutrophils and bacteria, 50 to 200 μM diamide reduced intracellular -SH amount. The decrease in reduced thiol content caused at 50 and 100 μM diamide was prevented by DPI. Under the same conditions DPI decreased the level of cytosolic ROS, presumably due to inhibition of NOX2, and diamide did not modulate this effect (Figure 2C). These data suggest that the decrease in GSH/GSSG ratio by low doses of diamide may be compensated by the decrease in oxidative stress and increase in NADPH levels caused by inhibition of NADPH oxidase. This effect may explain the DPI stimulation of leukotriene synthesis (Figure 1D), which is especially pronounced in the presence of diamide (Figure 2D), with maximal stimulation at 100 μM diamide (Figure 2F). DPI did not reverse the decrease in reduced thiols at 200 μM diamide but abolished inhibiting effect of diamide on LT synthesis at 200 μM (Figure 2D). We can only propose that effect of DPI is more complex than we determined and need further elucidation.
Biosynthesis of GSH is dependent on ATP, so inhibition of leukotriene synthesis due to 2-DG-dependent ATP depletion (Figure 1B) may be mediated by a decrease in GSH content. 2-DG also inhibits the synthesis of leukotrienes stimulated by DPI alone (Figure 2G). Interestingly, the synthesis of leukotrienes stimulated by DPI in the presence of diamide is practically insensitive to 2-DG (Figure 2G). This may be explained by the known ability of diamide to reduce glucose metabolism through glycolysis in favor of the pentose phosphate pathway (PPP) (52). This pathway is the main source of NADPH (42), which supports the reduction of glutathione. This effect probably underlies the stimulation of leukotriene synthesis observed at low doses of diamide (Figure 2D).
Full pentose cycle, including non-oxidative PPP with enzyme transketolase (TKT), provides maximal NADPH yield (6 NADPH from one molecule of glucose) (53). In our assay TKT inhibitor oxythiamine (OT) suppressed LT synthesis (Figure 2H). The effect was observed with and without diamide in incubations. It is known that blockage of glycolysis can help cells divert more flux into oxPPP under oxidative stress (54, 55). With diamide, LT synthesis was increased in the presence of intermediate concentrations of 2-DG (Figure 2H), and not in control incubations (Figure 1C). We did not observe it in the presence of DPI (Figure 2G).
Under the influence of fMLP, neutrophil adhesion is significantly enhanced (Figure 3A). In addition, this chemotactic peptide induces noticeable morphological changes in both resting and interacting with bacteria neutrophils. In addition to pronounced cellular polarization, special mention should be made to the formation of numerous thread-like cell outgrowths (Figure 3B, control/fMLP, S.typhimurium+fMLP, red arrows). It is known that such outgrowths are a kind of “transport highways” that allow intercellular exchange over long distances (56), and also perform a structural function, promoting cell clustering (57). Mitochondria-targeted antioxidant SkQ1 significantly reduced the substrate adhesion of neutrophils (Figure 3A). Preincubation with SkQ1 not only reduce the pro-adhesive effect of fMLP but also prevents fMLP-induced morphological changes, in particular, it diminishes the formation of cell outgrowths (Figure 3B), in parallel with suppression of leukotriene synthesis (Figure 1A). The addition of LTB4 to samples pretreated with SkQ1 increased the adhesiveness of neutrophils, promoting cell spreading (Figure 3, SkQ1+LTB4). In addition, supplementation of fMLP influence on cells preincubated with SkQ1 and bacteria with exogenous leukotriene B4 restored the ability of neutrophils to form thread-like filaments (Figure 3, SkQ1+LTB4/S.typhimurium+fMLP, red arrows). These data support our earlier suggestion that the emergence of cell-cell contacts is dependent on LTB4 (12). 50 µM diamide also increased adhesiveness of neutrophils (Supplementary Figure 5A). At high diamide concentration, when leukotriene synthesis was inhibited, the cells change morphology to more spherical shape, and the adhesiveness of neutrophils decreased (Supplementary Figure 5).
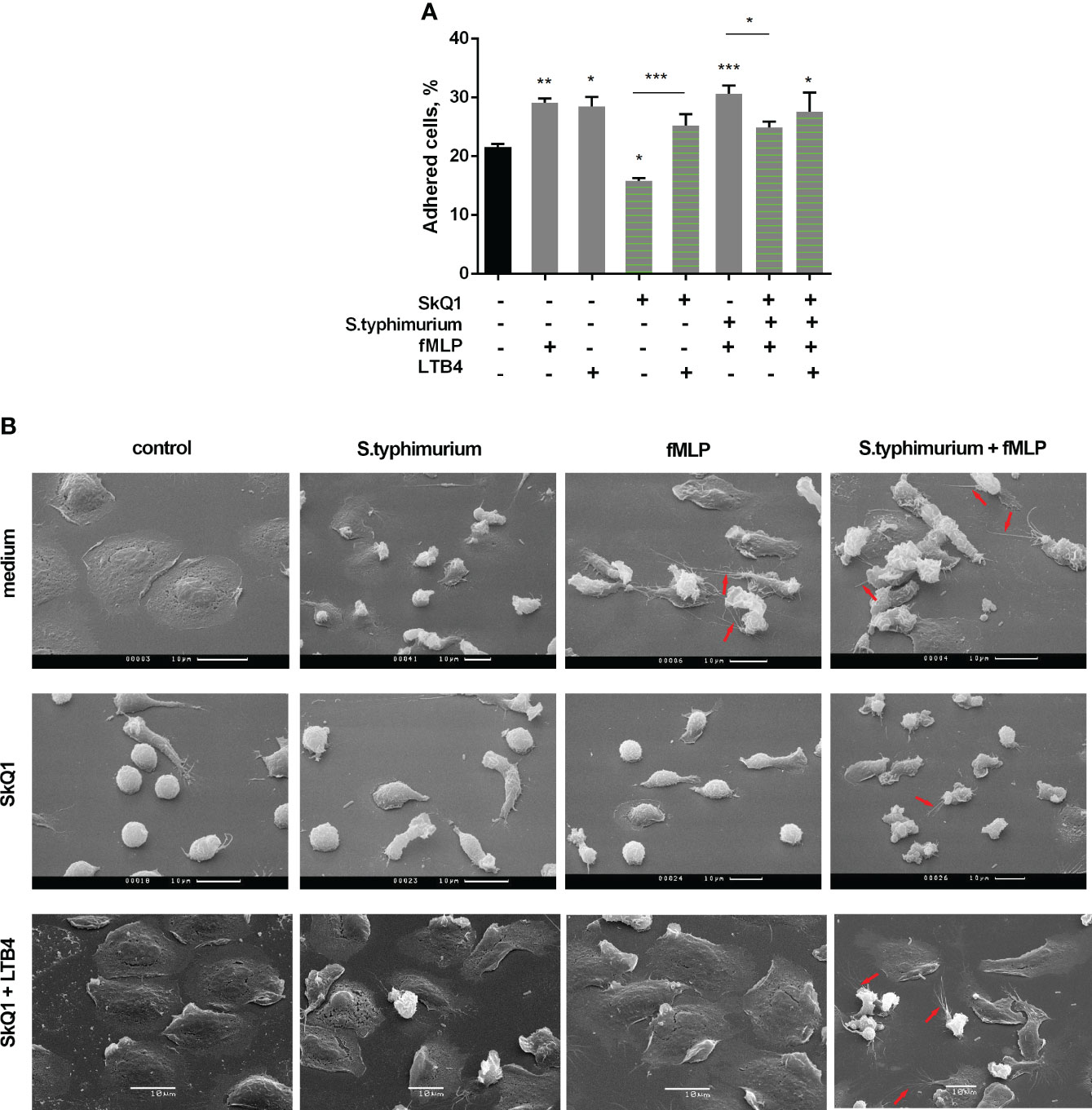
Figure 3 (A) SkQ1 influence on the pro-adhesive effects of fMLP and LTB4. PMNLs (2 × 105 cells/sample) were seeded onto fibrinogen-coated 96-well plates containing pre-warmed HBSS/HEPES without additives, with S. typhimurium (bacteria per cell ratio ~20:1) or S. typhimurium supplemented with 0.1 µM SkQ1. After 30 min incubation at 37°C, 5% CO2, 0.1 µM fMLP or/and 0.1 µM LTB4 were added for next 10min. Values shown means ± SEM of the percentage of cells attached to the substrate obtained from three independent experiments performed in triplicates; *p < 0.05, **p < 0.01, ***p < 0.001 for indicated pairs of data or compared to resting PMNLs sample (black bar) as shown by ordinary one-way ANOVA with Sidak’s multiple comparison test. (B) Effect of SkQ1 and LTB4 on neutrophil morphology upon (co-)stimulation with bacteria S. typhimurium and fMLP. PMNLs (106/ml HBSS/HEPES) were preincubated on coverslips in culture dishes for 10 min and then cultured for 20 min without additives (line medium) or in the presence of 100 nM SkQ1 (lines SkQ1, SkQ1+LTB4) without additional stimulation (control) or with the addition of bacteria (bacteria per cell ratio ~25:1) (S. typhimurium). LTB4 (100 nM sample concentration) was added to (control) and (S. typhimurium) samples for next 5 minutes (line SkQ1+LTB4). Columns fMLP and S. typhimurium+fMLP represent PNMLs incubated for 20 min without additives (line medium) or with 100 nM SkQ1 (lines SkQ1, SkQ1+LTB4) without bacteria (fMLP) or with the addition of bacteria (S. typhimurium+fMLP), followed by fMLP (0.1 µM sample concentration) (lines medium, SkQ1) or fMLP together with LTB4 (100 nM sample concentration of each) (line SkQ1+LTB4) treatment for 5 minutes. At the end of each stage, PMNLs samples were fixed and subsequently visualized using scanning electron microscopy. Thread-like cell outgrowths are marked by red arrows.
Activation of neutrophil adhesive properties can be reflected by adhesive receptor expression, first of all CD11b, a component of Mac-1 (CD11b/CD18) β2 integrin (58). In experiments with binding of fluorescently labelled antibodies to CD11b it was shown that both fMLP and LTB4 increased CD11b density on the surface of the cells, and diamide potentiated effect of fMLP. SkQ1 did not influence fMLP-induced expression, but decreased LTB4-induced effects (Figures 4A, B). The surface CD54 (ICAM-1) expression on neutrophils correlates with neutrophil migration, i.e. with cell-cell communication (59); and anti-CD54 monoclonal antibody inhibited neutrophil aggregation and formation of inter-cellular contacts (60). CD54 increased significantly on migrated PMNs; with rather low CD54 expression on adherent neutrophils (61). In our experimental model CD54 plays role as counter-receptor for integrins during homotypic adhesion; and SkQ1 decreased CD54 surface expression on neutrophils, as in the presence of the “first” chemoattractant fMLP, as well as at adding of the “second” chemoattractant LTB4 (Figures 4C, D). These data indicate that SkQ1 affects not only LTB4 synthesis but also LTB4-dependent signaling. The increased adhesiveness of neutrophils induced by LTB4 provided the possibility of forming tight intercellular contacts, which can support swarming and clustering around pathogens.
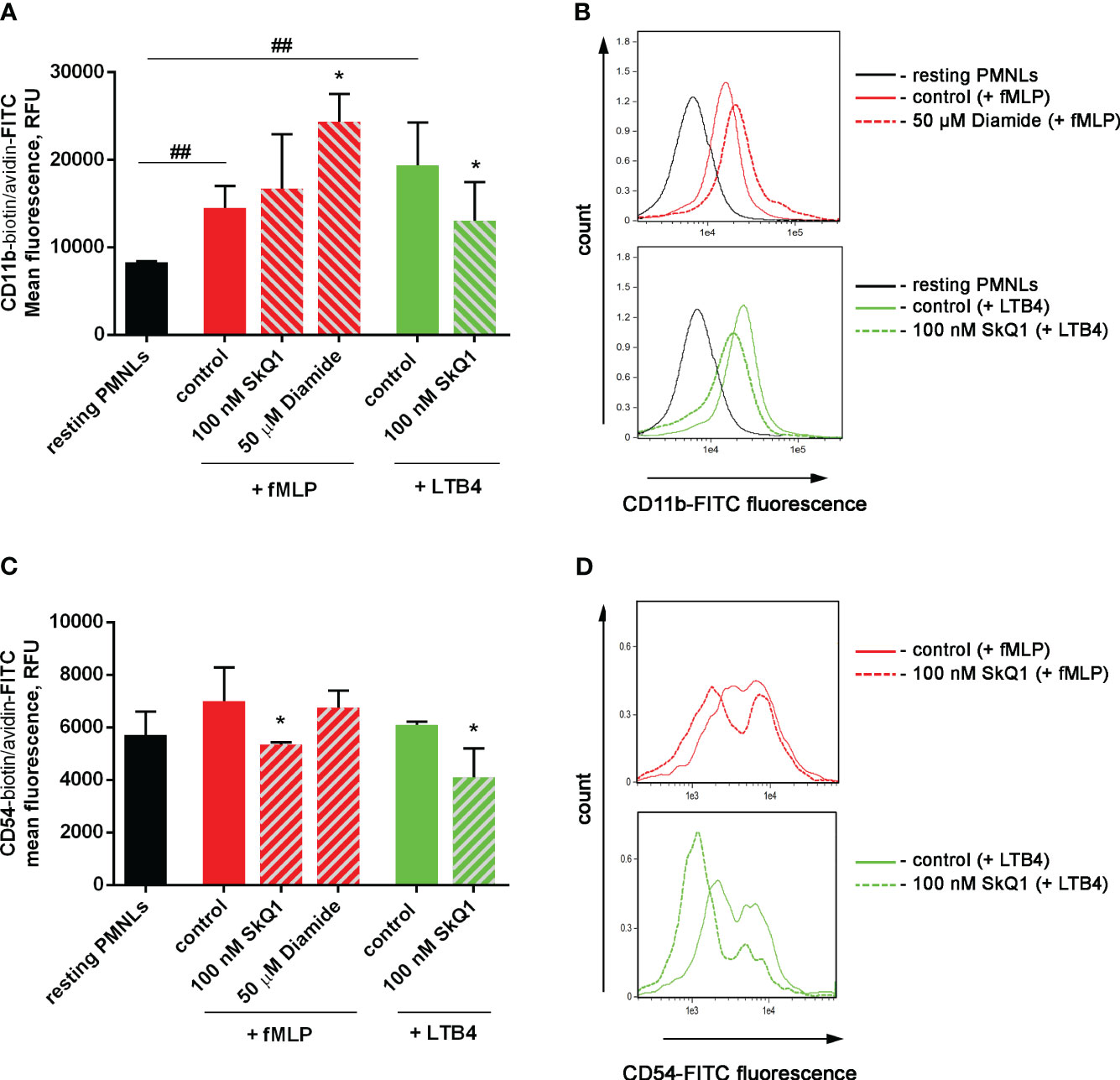
Figure 4 Effect of SkQ1 and diamide on adhesion molecules externalization upon (co-) stimulation with bacteria S. typhimurium and fMLP or LTB4. PMNLs (106/mL HBSS/HEPES) were incubated for 20 min without additives (resting PMNLs), in the presence of bacteria (control) or bacteria in combination with 100 nM SkQ1 or 50 µM diamide (bacteria per cell ratio ~25:1). Then 0.1 µM fMLP (+ fMLP) or 0.1 µM LTB4 (+ LTB4) were added for 10 min followed by staining of surface CD11b and CD54 proteins and flow cytometry. Presented are the average values of three independent experiments (means ± SEM) (A, C) and typical histograms (B, D) of fluorescence for PMNLs stained with avidin-FITC in addition to biotynilated CD11b (A, B) and CD54 (C, D) antibodies. *p < 0.05 for pairs of data compared to corresponding control values; ##p < 0.01 for the specified data pairs as shown by one-way ANOVA with Dunnett’s multiple comparison test.
4 Discussion
Redox regulation plays an important role in the activation of 5-LOX and the regulation of leukotriene synthesis in neutrophils. Specifically, enzymatic activity requires the oxidation of Fe2+ to Fe3+ at the 5-LOX active site. Lipid hydroperoxides are involved in the activation of 5-LOX, at least in part through the oxidation of Fe2+ (62, 63). 5-LOX catalyzes the biosynthesis of leukotrienes using arachidonic acid (AA) as a substrate. Phospholipase A2, which produces AA from phospholipids, can be activated by ROS and lipid hydroperoxides (64), promoting the activation of leukotriene synthesis through oxidative processes. At the same time, excess hydrogen peroxide has been shown to inhibit 5-LOX activity in alveolar macrophages in parallel with ATP depletion (65).
Our previous work (22) using the mitochondria-targeted antioxidant SkQ1 demonstrated the important role of mitochondrial ROS in leukotriene synthesis induced by the Ca2+ ionophore A23187, the chemoattractant fMLP, and the opsonized yeast cell wall components zymosan. Here, we showed (Figure 1) that mitochondria-targeted antioxidants suppress fMLP-induced leukotriene synthesis in neutrophils exposed to S. typhimurium. We also showed that leukotriene synthesis is inhibited by the respiration inhibitor antimycin A and the oxidative phosphorylation uncoupler FCCP, presumably due to inhibition of mtROS production stimulated by voltage-dependent Ca2+ accumulation in mitochondria. Inhibition of respiration or dissipation of membrane potential prevents the synthesis of mitochondrial ATP, but measurements of ATP content did not reveal the effect of antimycin A or FCCP in infected neutrophils (Figure 1B).
Inhibition of glucose metabolism by 2-deoxy-D-glucose (2-DG) led to a decrease in ATP content and, in parallel to inhibition of leukotriene synthesis (Figure 1C) consistently with earlier data on the requirement of energy metabolism for the synthesis of leukotrienes (66). Glucose is catabolized by two fundamental pathways: glycolysis to produce ATP and the oxidative pentose phosphate pathway (PPP) to produce reduced nicotinamide adenine dinucleotide phosphate (NADPH). Very recently, it was shown that activation of the oxidative burst in neutrophils depends on a switch from glycolysis to a unique form of PPP called the “pentose cycle” (53). In this mode, all glucose-6-phosphate is consumed through PPP, while the initial steps of glycolysis are reversed to support pentose phosphates recycling. It has been proposed that this switch is required to maximize the supply of NADPH to fuel NADPH oxidase.
Another important NADPH-dependent enzyme is glutathione reductase, which reduce oxidized glutathione disulfide to sulfhydryl glutathione (67, 68). The main function of glutathione is the detoxification of electrophilic xenobiotics through condensation reactions catalyzed by glutathione S-transferases. Another protective function is mediated by the reduction of hydroperoxides catalyzed by glutathione peroxidase. This process appears to underlie the inhibition of 5-LOX by GSH observed in neutrophil homogenate (69). However, protein S-glutathionylation, activated by oxidative stress, may represent a more important regulatory mechanism.
The 5-lipoxygenase requires activation by fatty acid hydroperoxides (62). Hydroperoxides are inactivated in cells by GSH-dependent reduction by glutathione peroxidase, but diamide has the ability to non-enzymatically oxidize intracellular thiols (48). On this way diamide creates a demand for glutathione reduction by NADPH (70), and DPI provides high NADPH/NADP+ ratio (53) increasing stimulating effect of diamide (Figures 2D, F). The pentose phosphate pathway (PPP) is the major mechanism to maintain high NADPH/NADP+ ratio (71), and it was recently shown that PPP controls ROS production in crowding neutrophils (72). In accordance of the ability of diamide to switch glycolysis-dominant metabolism to pentose phosphate pathway (53), effects of diamide were less sensitive to glucose deprivation (Figures 2G, H). Effects of DPI were inhibited by glucose deprivation (Figure 2G).
Protein S-glutathionylation likely limits leukotriene synthesis in fMLP-activated neutrophils in the presence of S. typhimurium. This may be the reason that the GSH precursor NAC and the H2S donor sodium hydrosulfide, which increase the GSH/GSSG ratio, stimulate the synthesis of leukotrienes, in contrast to some other antioxidants (Figure 2A). In support of this proposal, we observed that thiol-oxidizing diamide (48), which oxidizes GSH, as detected by depletion of non-protein thiols (Figure 2B), at high concentrations (200-250 µM) significantly inhibits leukotriene synthesis (Figure 2D). This inhibition was effectively reversed by the GSH precursor SAMe (Figure 2D). Inhibition of leukotriene synthesis by FCCP or antimycin A may also be due in part to the GSH depletion caused by these agents (49–51). Accordingly, inhibition by FCCP and antimycin A was partially prevented by SAMe (Figure 2E). Importantly, diamide-induced thiol depletion was prevented by DPI (Figure 2B), indicating that inhibition of NADPH oxidase and subsequent decrease of oxidative stress and increase in NADPH levels may compensate for diamide-dependent GSH oxidation. Accordingly, DPI stimulated the synthesis of leukotrienes in the presence of diamide (Figure 2D). Inhibition of leukotriene synthesis by 2-DG-dependent ATP depletion may also be mediated by a decrease in GSH content. This may explain why the synthesis of leukotrienes in the presence of non-inhibitory doses of diamide, which is known to stimulate PPP-dependent NADPH production (52), was not inhibited by 2-DG in the presence of DPI (Figure 2G).
Neutrophils are the first cells in the foci of infection, and they have developed a set of mechanisms to turn on defense very quickly. To potentiate ROS production, they activate NADPH-oxidase, and NADPH is necessary for reduction of oxygen (73). To support NADPH-dependent ROS formation, neutrophils turn on PPP shunt (74). Activation of PPP is extremely important in supplying of NADPH (75). Inhibition of NADPH-oxidase shifted neutrophils from PPP cycle with ultra-high NADPH yield to glycolysis-dominant glucose metabolism (74). At the same time, neutrophil responses to pathogens include activation of glycolysis (76, 77).
Upregulation of PPP during oxidative stress contributes significantly to neutrophil responses, including not only oxidative burst (74) but also NETs formation (78). Redox regulation may involve various signaling pathways, including the MAP kinase-dependent pathway (79). We previously showed that activation of the MAP kinases Erk1/2 and p38, responsible for phosphorylation and activation of 5-LOX (46), is prevented by SkQ1 in neutrophils stimulated with A23187, fMLP, or opsonized zymosan (22). In neutrophils stimulated with S. typhimurium in combination with fMLP, the Erk1/2 inhibitor U0126 and the p38 inhibitor SB203580 inhibited leukotriene synthesis (Figure 1E). It is important to note that the GSH/GSSG ratio may also be involved in the regulation of Erk1/2, as S-glutathionylation, activated by oxidative stress, has been shown to play a key role in regulating MAP kinase kinase (MEKK1), that is an upstream kinase in the cascade of Erk1/2 activation (80). Using MS analysis, the authors demonstrated that oxidative stress induces glutathionylation of Cys1238 in the ATP-binding domain of MEKK1. This modification is easily reversible once the cell’s redox balance is restored. Thus, increasing the GSH/GSSG ratio with NAC or sodium hydrosulfide may improve Erk1/2 activation by preventing MEKK S-glutothionylation in neutrophils exposed to S. typhimurium and fMLP. Thus, redox regulation of Erk1/2 may be an important element in both mtROS-dependent and GSH-dependent regulation of leukotriene synthesis. Moreover, this regulation provides insight into why mitochondria-targeted antioxidants and NAC have opposing effects on leukotriene synthesis.
Our data (Figure 3) showed that Salmonella typhimurium in combination with fMLP stimulates the appearance of intercellular contacts in parallel with the synthesis of LTB4. The mitochondria-targeted antioxidant SkQ1 reduced this intercellular communication, as well as the synthesis of leukotrienes. Thus, during bacterial infection, redox processes in neutrophils play a decisive role in the synthesis of LTB4, ensuring neutrophil swarming - the influx of neutrophils to places of large microbial accumulations.
Excessive synthesis of leukotrienes and especially of LTB4 plays important role in pathogenesis of various inflammatory diseases (81–88). Mitochondria-targeted antioxidants, such as SkQ1, have been proposed as a promising therapy the same range of pathologies (89, 90). SkQ1 demonstrated strong anti-inflammatory activity in acute bacterial infection (91) and in the systemic inflammatory response syndrome (SIRS) model (92). The inhibition of leukotriene synthesis demonstrated above under conditions of pronounced activation of neutrophils can significantly contribute to the therapeutic effect of SkQ1. Importantly, our data indicate that administration of known thiol-based antioxidants such as NAC can dangerously stimulate leukotriene synthesis by neutrophils under the same conditions that mimic severe pathogenic infection.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Experimental and the subject consent procedures were approved by the Bioethics Committee of the Lomonosov Moscow State University, Application # 6-h, version 3, Bioethics Commission meeting # 131-d held on 31.05.2021. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
EG: Writing – original draft, Data curation, Formal analysis, Investigation, Methodology. GV: Investigation, Methodology, Writing – original draft, Funding acquisition. SG: Investigation, Methodology, Writing – original draft, Data curation. NK: Writing – review & editing, Methodology. TG: Conceptualization, Writing – review & editing. YR: Writing – review & editing, Methodology, Resources. KL: Writing – review & editing, Methodology. BC: Writing – review & editing, Conceptualization, Writing – original draft. GS: Conceptualization, Writing – original draft, Writing – review & editing, Project administration, Supervision.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the grant from the Russian Science Foundation, grant number 23-74-01056, https://rscf.ru/project/23-74-01056/.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1295150/full#supplementary-material
References
1. Segal AW. How neutrophils kill microbes. Annu Rev Immunol (2005) 23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653
2. Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev (2007) 219:88–102. doi: 10.1111/j.1600-065X.2007.00550.x
3. Nagarkoti S, Sadaf S, Awasthi D, Chandra T, Jagavelu K, Kumar S, et al. L-Arginine and tetrahydrobiopterin supported nitric oxide production is crucial for the microbicidal activity of neutrophils. Free Radic Res (2019) 53(3):281–92. doi: 10.1080/10715762.2019.1566605
4. Lammermann T, Afonso PV, Angermann BR, Wang JM, Kastenmuller W, Parent CA, et al. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature (2013) 498(7454):371–5. doi: 10.1038/nature12175
5. Isles HM, Loynes CA, Alasmari S, Kon FC, Henry KM, Kadochnikova A, et al. Pioneer neutrophils release chromatin within in vivo swarms. Elife (2021) 10:e68755. doi: 10.7554/eLife.68755
6. Song Z, Bhattacharya S, Clemens RA, Dinauer MC. Molecular regulation of neutrophil swarming in health and disease: Lessons from the phagocyte oxidase. iScience. (2023) 26(10):108034. doi: 10.1016/j.isci.2023.108034
7. Mihlan M, Glaser KM, Epple MW, Lammermann T. Neutrophils: amoeboid migration and swarming dynamics in tissues. Front Cell Dev Biol (2022) 10:871789. doi: 10.3389/fcell.2022.871789
8. Glaser KM, Mihlan M, Lammermann T. Positive feedback amplification in swarming immune cell populations. Curr Opin Cell Biol (2021) 72:156–62. doi: 10.1016/j.ceb.2021.07.009
9. Hopke A, Lin T, Scherer AK, Shay AE, Timmer KD, Wilson-Mifsud B, et al. Transcellular biosynthesis of leukotriene B(4) orchestrates neutrophil swarming to fungi. iScience. (2022) 25(10):105226. doi: 10.1016/j.isci.2022.105226
10. Hopke A, Scherer A, Kreuzburg S, Abers MS, Zerbe CS, Dinauer MC, et al. Neutrophil swarming delays the growth of clusters of pathogenic fungi. Nat Commun (2020) 11(1):2031. doi: 10.1038/s41467-020-15834-4
11. Archambault AS, Poirier S, Lefebvre JS, Robichaud PP, Larose MC, Turcotte C, et al. 20-Hydroxy- and 20-carboxy-leukotriene (LT) B(4) downregulate LTB(4) -mediated responses of human neutrophils and eosinophils. J Leukoc Biol (2019) 105(6):1131–42. doi: 10.1002/JLB.MA0718-306R
12. Golenkina EA, Galkina SI, Pletjushkina O, Chernyak B, Gaponova TV, Romanova YM, et al. Gram-Negative Bacteria Salmonella typhimurium Boost Leukotriene Synthesis Induced by Chemoattractant fMLP to Stimulate Neutrophil Swarming. Front Pharmacol (2021) 12:814113. doi: 10.3389/fphar.2021.814113
13. Borgeat P, Naccache PH. Biosynthesis and biological activity of leukotriene B4. Clin Biochem (1990) 23(5):459–68. doi: 10.1016/0009-9120(90)90272-V
14. Petropoulos M, Karamolegkou G, Rosmaraki E, Tsakas S. Hydrogen peroxide signals E. coli phagocytosis by human polymorphonuclear cells; up-stream and down-stream pathway. Redox Biol (2015) 6:100–5. doi: 10.1016/j.redox.2015.07.004
15. Sheshachalam A, Srivastava N, Mitchell T, Lacy P, Eitzen G. Granule protein processing and regulated secretion in neutrophils. Front Immunol (2014) 5:448. doi: 10.3389/fimmu.2014.00448
16. Vorobjeva NV, Chernyak BV. NETosis: molecular mechanisms, role in physiology and pathology. Biochem (Mosc). (2020) 85(10):1178–90. doi: 10.1134/S0006297920100065
17. Herring SE, Mao S, Bhalla M, Tchalla EYI, Kramer JM, Bou Ghanem EN. Mitochondrial ROS production by neutrophils is required for host antimicrobial function against Streptococcus pneumoniae and is controlled by A2B adenosine receptor signaling. PloS Pathog (2022) 18(11):e1010700. doi: 10.1371/journal.ppat.1010700
18. Dunham-Snary KJ, Surewaard BG, Mewburn JD, Bentley RE, Martin AY, Jones O, et al. Mitochondria in human neutrophils mediate killing of Staphylococcus aureus. Redox Biol (2022) 49:102225. doi: 10.1016/j.redox.2021.102225
19. Gomes MC, Brokatzky D, Bielecka MK, Wardle FC, Mostowy S. Shigella induces epigenetic reprogramming of zebrafish neutrophils. Sci Adv (2023) 9(36):eadf9706. doi: 10.1126/sciadv.adf9706
20. Vorobjeva N, Prikhodko A, Galkin I, Pletjushkina O, Zinovkin R, Sud'ina G, et al. Mitochondrial reactive oxygen species are involved in chemoattractant-induced oxidative burst and degranulation of human neutrophils in vitro. Eur J Cell Biol (2017) 96(3):254–65. doi: 10.1016/j.ejcb.2017.03.003
21. Vorobjeva N, Galkin I, Pletjushkina O, Golyshev S, Zinovkin R, Prikhodko A, et al. Mitochondrial permeability transition pore is involved in oxidative burst and NETosis of human neutrophils. Biochim Biophys Acta Mol Basis Dis (2020) 1866(5):165664. doi: 10.1016/j.bbadis.2020.165664
22. Sud'ina GF, Golenkina EA, Prikhodko AS, Kondratenko ND, Gaponova TV, Chernyak BV. Mitochondria-targeted antioxidant SkQ1 inhibits leukotriene synthesis in human neutrophils. Front Pharmacol (2022) 13:1023517. doi: 10.3389/fphar.2022.1023517
23. Antonenko YN, Avetisyan AV, Bakeeva LE, Chernyak BV, Chertkov VA, Domnina LV, et al. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 1. Cationic plastoquinone derivatives: synthesis and in vitro studies. Biochem (Mosc). (2008) 73(12):1273–87. doi: 10.1134/s0006297908120018
24. Allen LH, Criss AK. Cell intrinsic functions of neutrophils and their manipulation by pathogens. Curr Opin Immunol (2019) 60:124–9. doi: 10.1016/j.coi.2019.05.004
25. Urban CF, Lourido S, Zychlinsky A. How do microbes evade neutrophil killing? Cell Microbiol (2006) 8(11):1687–96. doi: 10.1111/j.1462-5822.2006.00792.x
26. Vareechon C, Zmina SE, Karmakar M, Pearlman E, Rietsch A. Pseudomonas aeruginosa effector exoS inhibits ROS production in human neutrophils. Cell Host Microbe (2017) 21(5):611–8.e5. doi: 10.1016/j.chom.2017.04.001
27. Youseff BH, Holbrook ED, Smolnycki KA, Rappleye CA. Extracellular superoxide dismutase protects Histoplasma yeast cells from host-derived oxidative stress. PloS Pathog (2012) 8(5):e1002713. doi: 10.1371/journal.ppat.1002713
28. Siemsen DW, Kirpotina LN, Jutila MA, Quinn MT. Inhibition of the human neutrophil NADPH oxidase by Coxiella burnetii. Microbes Infect (2009) 11(6-7):671–9. doi: 10.1016/j.micinf.2009.04.005
29. Saha P, Yeoh BS, Olvera RA, Xiao X, Singh V, Awasthi D, et al. Bacterial siderophores hijack neutrophil functions. J Immunol (2017) 198(11):4293–303. doi: 10.4049/jimmunol.1700261
30. Pulsifer AR, Vashishta A, Reeves SA, Wolfe JK, Palace SG, Proulx MK, et al. Redundant and cooperative roles for yersinia pestis yop effectors in the inhibition of human neutrophil exocytic responses revealed by gain-of-function approach. Infect Immun (2020) 88(3):e00909-19. doi: 10.1128/IAI.00909-19
31. Reed PW. Glutathione and the hexose monophosphate shunt in phagocytizing and hydrogen peroxide-treated rat leukocytes. J Biol Chem (1969) 244(9):2459–64. doi: 10.1016/S0021-9258(19)78244-1
32. Kim VY, Batty A, Li J, Kirk SG, Crowell SA, Jin Y, et al. Glutathione Reductase Promotes Fungal Clearance and Suppresses Inflammation during Systemic Candida albicans Infection in Mice. J Immunol (2019) 203(8):2239–51. doi: 10.4049/jimmunol.1701686
33. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. (2001) 414(6865):813–20. doi: 10.1038/414813a
34. Afonso PV, Janka-Junttila M, Lee YJ, McCann CP, Oliver CM, Aamer KA, et al. LTB4 is a signal-relay molecule during neutrophil chemotaxis. Dev Cell (2012) 22(5):1079–91. doi: 10.1016/j.devcel.2012.02.003
35. Patcha V, Wigren J, Winberg ME, Rasmusson B, Li J, Sarndahl E. Differential inside-out activation of beta2-integrins by leukotriene B4 and fMLP in human neutrophils. Exp Cell Res (2004) 300(2):308–19. doi: 10.1016/j.yexcr.2004.07.015
36. Ford-Hutchinson AW, Bray MA, Doig MV, Shipley ME, Smith MJ. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. (1980) 286(5770):264–5. doi: 10.1038/286264a0
37. Poplimont H, Georgantzoglou A, Boulch M, Walker HA, Coombs C, Papaleonidopoulou F, et al. Neutrophil swarming in damaged tissue is orchestrated by connexins and cooperative calcium alarm signals. Curr Biol (2020) 30(14):2761–76.e7. doi: 10.1016/j.cub.2020.05.030
38. Aleksandrov DA, Zagryagskaya AN, Pushkareva MA, Bachschmid M, Peters-Golden M, Werz O, et al. Cholesterol and its anionic derivatives inhibit 5-lipoxygenase activation in polymorphonuclear leukocytes and MonoMac6 cells. FEBS J (2006) 273(3):548–57. doi: 10.1111/j.1742-4658.2005.05087.x
39. Ngo TT, Lenhoff HM. A sensitive and versatile chromogenic assay for peroxidase and peroxidase-coupled reactions. Anal Biochem (1980) 105(2):389–97. doi: 10.1016/0003-2697(80)90475-3
40. Galkina SI, Fedorova NV, Serebryakova MV, Arifulin EA, Stadnichuk VI, Gaponova TV, et al. Inhibition of the GTPase dynamin or actin depolymerisation initiates outward plasma membrane tubulation/vesiculation (cytoneme formation) in neutrophils. Biol Cell (2015) 107(5):144–58. doi: 10.1111/boc.201400063
41. Severin FF, Severina II, Antonenko YN, Rokitskaya TI, Cherepanov DA, Mokhova EN, et al. Penetrating cation/fatty acid anion pair as a mitochondria-targeted protonophore. Proc Natl Acad Sci U S A. (2010) 107(2):663–8. doi: 10.1073/pnas.0910216107
42. Curi R, Levada-Pires AC, Silva EBD, Poma SO, Zambonatto RF, Domenech P, et al. The critical role of cell metabolism for essential neutrophil functions. Cell Physiol Biochem (2020) 54(4):629–47. doi: 10.33594/000000245
43. Ohms M, Ferreira C, Busch H, Wohlers I, Guerra de Souza AC, Silvestre R, et al. Enhanced glycolysis is required for antileishmanial functions of neutrophils upon infection with leishmania donovani. Front Immunol (2021) 12:632512. doi: 10.3389/fimmu.2021.632512
44. Nguyen GT, Green ER, Mecsas J. Neutrophils to the ROScue: mechanisms of NADPH oxidase activation and bacterial resistance. Front Cell Infect Microbiol (2017) 7:373. doi: 10.3389/fcimb.2017.00373
45. Song Z, Huang G, Chiquetto Paracatu L, Grimes D, Gu J, Luke CJ, et al. NADPH oxidase controls pulmonary neutrophil infiltration in the response to fungal cell walls by limiting LTB4. Blood. (2020) 135(12):891–903. doi: 10.1182/blood.2019003525
46. Radmark O, Werz O, Steinhilber D, Samuelsson B. 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease. Biochim Biophys Acta (2015) 1851(4):331–9. doi: 10.1016/j.bbalip.2014.08.012
47. Olas B. Medical functions of hydrogen sulfide. Adv Clin Chem (2016) 74:195–210. doi: 10.1016/bs.acc.2015.12.007
48. Kosower NS, Kosower EM, Wertheim B, Correa WS. Diamide, a new reagent for the intracellular oxidation of glutathione to the disulfide. Biochem Biophys Res Commun (1969) 37(4):593–6. doi: 10.1016/0006-291X(69)90850-X
49. Park WH, Han YW, Kim SH, Kim SZ. An ROS generator, antimycin A, inhibits the growth of HeLa cells via apoptosis. J Cell Biochem (2007) 102(1):98–109. doi: 10.1002/jcb.21280
50. Gomez-Nino A, Agapito MT, Obeso A, Gonzalez C. Effects of mitochondrial poisons on glutathione redox potential and carotid body chemoreceptor activity. Respir Physiol Neurobiol (2009) 165(1):104–11. doi: 10.1016/j.resp.2008.10.020
51. Mlejnek P, Dolezel P. Loss of mitochondrial transmembrane potential and glutathione depletion are not sufficient to account for induction of apoptosis by carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone in human leukemia K562 cells. Chem Biol Interact (2015) 239:100–10. doi: 10.1016/j.cbi.2015.06.033
52. Hiranruengchok R, Harris C. Diamide-induced alterations of intracellular thiol status and the regulation of glucose metabolism in the developing rat conceptus in vitro. Teratology (1995) 52(4):205–14. doi: 10.1002/tera.1420520406
53. Britt EC, Lika J, Giese MA, Schoen TJ, Seim GL, Huang Z, et al. Switching to the cyclic pentose phosphate pathway powers the oxidative burst in activated neutrophils. Nat Metab (2022) 4(3):389–403. doi: 10.1038/s42255-022-00550-8
54. Stincone A, Prigione A, Cramer T, Wamelink MM, Campbell K, Cheung E, et al. The return of metabolism: biochemistry and physiology of the pentose phosphate pathway. Biol Rev Camb Philos Soc (2015) 90(3):927–63. doi: 10.1111/brv.12140
55. Peralta D, Bronowska AK, Morgan B, Doka E, Van Laer K, Nagy P, et al. A proton relay enhances H2O2 sensitivity of GAPDH to facilitate metabolic adaptation. Nat Chem Biol (2015) 11(2):156–63. doi: 10.1038/nchembio.1720
56. Galkina SI, Fedorova NV, Serebryakova MV, Romanova JM, Golyshev SA, Stadnichuk VI, et al. Proteome analysis identified human neutrophil membrane tubulovesicular extensions (cytonemes, membrane tethers) as bactericide trafficking. Biochim Biophys Acta (2012) 1820(11):1705–14. doi: 10.1016/j.bbagen.2012.06.016
57. Galkina SI, Romanova JM, Bragina EE, Tiganova IG, Stadnichuk VI, Alekseeva NV, et al. Membrane tubules attach Salmonella Typhimurium to eukaryotic cells and bacteria. FEMS Immunol Med Microbiol (2011) 61(1):114–24. doi: 10.1111/j.1574-695X.2010.00754.x
58. Sekheri M, Othman A, Filep JG. beta2 integrin regulation of neutrophil functional plasticity and fate in the resolution of inflammation. Front Immunol (2021) 12:660760. doi: 10.3389/fimmu.2021.660760
59. Grieshaber-Bouyer R, Nigrovic PA. Neutrophil heterogeneity as therapeutic opportunity in immune-mediated disease. Front Immunol (2019) 10:346. doi: 10.3389/fimmu.2019.00346
60. Takashi S, Okubo Y, Horie S. Contribution of CD54 to human eosinophil and neutrophil superoxide production. J Appl Physiol (1985). (2001) 91(2):613–22. doi: 10.1152/jappl.2001.91.2.613
61. Konrad FM, Wohlert J, Gamper-Tsigaras J, Ngamsri KC, Reutershan J. How Adhesion Molecule Patterns Change While Neutrophils Traffic through the Lung during Inflammation. Mediators Inflamm (2019) 2019:1208086. doi: 10.1155/2019/1208086
62. Rouzer CA, Samuelsson B. The importance of hydroperoxide activation for the detection and assay of mammalian 5-lipoxygenase. FEBS Lett (1986) 204(2):293–6. doi: 10.1016/0014-5793(86)80831-6
63. Radmark O. Arachidonate 5-lipoxygenase. Prostaglandins Other Lipid Mediat (2002) 68-69:211–34. doi: 10.1016/S0090-6980(02)00032-1
64. Nanda BL, Nataraju A, Rajesh R, Rangappa KS, Shekar MA, Vishwanath BS. PLA2 mediated arachidonate free radicals: PLA2 inhibition and neutralization of free radicals by anti-oxidants–a new role as anti-inflammatory molecule. Curr Top Med Chem (2007) 7(8):765–77. doi: 10.2174/156802607780487623
65. Sporn PH, Peters-Golden M. Hydrogen peroxide inhibits alveolar macrophage 5-lipoxygenase metabolism in association with depletion of ATP. J Biol Chem (1988) 263(29):14776–83. doi: 10.1016/S0021-9258(18)68105-0
66. Ahnfelt-Ronne I, Olsen UB. Leukotriene production in rat peritoneal leukocytes requires intact energy metabolism. Biochem Pharmacol (1985) 34(17):3095–100. doi: 10.1016/0006-2952(85)90153-4
67. Miller CG, Holmgren A, Arner ESJ, Schmidt EE. NADPH-dependent and -independent disulfide reductase systems. Free Radic Biol Med (2018) 127:248–61. doi: 10.1016/j.freeradbiomed.2018.03.051
68. Hasan AA, Kalinina E, Tatarskiy V, Shtil A. The thioredoxin system of mammalian cells and its modulators. Biomedicines. (2022) 10(7):1757. doi: 10.3390/biomedicines10071757
69. Hatzelmann A, Schatz M, Ullrich V. Involvement of glutathione peroxidase activity in the stimulation of 5-lipoxygenase activity by glutathione-depleting agents in human polymorphonuclear leukocytes. Eur J Biochem (1989) 180(3):527–33. doi: 10.1111/j.1432-1033.1989.tb14678.x
70. Masterson E, Whikehart DR, Chader GJ. Glucose oxidation in the chick cornea: effect of diamide on the pentose shunt. Invest Ophthalmol Vis Sci (1978) 17(5):449–54.
71. Cracan V, Titov DV, Shen H, Grabarek Z, Mootha VK. A genetically encoded tool for manipulation of NADP(+)/NADPH in living cells. Nat Chem Biol (2017) 13(10):1088–95. doi: 10.1038/nchembio.2454
72. Glaser KM, Doon-Ralls J, Walters N, Rima XY, Rambold AS, Reategui E, et al. Arp2/3 complex and the pentose phosphate pathway regulate late phases of neutrophil swarming. iScience. (2024) 27(1):108656. doi: 10.1016/j.isci.2023.108656
73. Babior BM, Lambeth JD, Nauseef W. The neutrophil NADPH oxidase. Arch Biochem Biophys (2002) 397(2):342–4. doi: 10.1006/abbi.2001.2642
74. Amara N, Cooper MP, Voronkova MA, Webb BA, Lynch EM, Kollman JM, et al. Selective activation of PFKL suppresses the phagocytic oxidative burst. Cell. (2021) 184(17):4480–94.e15. doi: 10.1016/j.cell.2021.07.004
75. Kuehne A, Emmert H, Soehle J, Winnefeld M, Fischer F, Wenck H, et al. Acute activation of oxidative pentose phosphate pathway as first-line response to oxidative stress in human skin cells. Mol Cell (2015) 59(3):359–71. doi: 10.1016/j.molcel.2015.06.017
76. Tan Y, Kagan JC. Innate immune signaling organelles display natural and programmable signaling flexibility. Cell. (2019) 177(2):384–98.e11. doi: 10.1016/j.cell.2019.01.039
77. Kumar S, Dikshit M. Metabolic insight of neutrophils in health and disease. Front Immunol (2019) 10:2099. doi: 10.3389/fimmu.2019.02099
78. Azevedo EP, Rochael NC, Guimaraes-Costa AB, de Souza-Vieira TS, Ganilho J, Saraiva EM, et al. A metabolic shift toward pentose phosphate pathway is necessary for amyloid fibril- and phorbol 12-myristate 13-acetate-induced neutrophil extracellular trap (NET) formation. J Biol Chem (2015) 290(36):22174–83. doi: 10.1074/jbc.M115.640094
79. Runchel C, Matsuzawa A, Ichijo H. Mitogen-activated protein kinases in mammalian oxidative stress responses. Antioxid Redox Signal (2011) 15(1):205–18. doi: 10.1089/ars.2010.3733
80. Cross JV, Templeton DJ. Oxidative stress inhibits MEKK1 by site-specific glutathionylation in the ATP-binding domain. Biochem J (2004) 381(Pt 3):675–83. doi: 10.1042/BJ20040591
81. Gelfand EW. Importance of the leukotriene B4-BLT1 and LTB4-BLT2 pathways in asthma. Semin Immunol (2017) 33:44–51. doi: 10.1016/j.smim.2017.08.005
82. Haeggstrom JZ. Leukotriene biosynthetic enzymes as therapeutic targets. J Clin Invest. (2018) 128(7):2680–90. doi: 10.1172/JCI97945
83. Noiri E, Yokomizo T, Nakao A, Izumi T, Fujita T, Kimura S, et al. An in vivo approach showing the chemotactic activity of leukotriene B(4) in acute renal ischemic-reperfusion injury. Proc Natl Acad Sci U S A. (2000) 97(2):823–8. doi: 10.1073/pnas.97.2.823
84. Dent G, Rabe KF, Magnussen H. Augmentation of human neutrophil and alveolar macrophage LTB4 production by N-acetylcysteine: role of hydrogen peroxide. Br J Pharmacol (1997) 122(4):758–64. doi: 10.1038/sj.bjp.0701428
85. Hirakata T, Matsuda A, Yokomizo T. Leukotriene B(4) receptors as therapeutic targets for ophthalmic diseases. Biochim Biophys Acta Mol Cell Biol Lipids. (2020) 1865(9):158756. doi: 10.1016/j.bbalip.2020.158756
86. Sanchez-Tabernero S, Fajardo-Sanchez J, Weston-Davies W, Parekh M, Kriman J, Kaye S, et al. Dual inhibition of complement component 5 and leukotriene B4 by topical rVA576 in atopic keratoconjunctivis: TRACKER phase 1 clinical trial results. Orphanet J Rare Dis (2021) 16(1):270. doi: 10.1186/s13023-021-01890-6
87. Liao T, Ke Y, Shao WH, Haribabu B, Kaplan HJ, Sun D, et al. Blockade of the interaction of leukotriene b4 with its receptor prevents development of autoimmune uveitis. Invest Ophthalmol Vis Sci (2006) 47(4):1543–9. doi: 10.1167/iovs.05-1238
88. Chistyakov DV, Gancharova OS, Baksheeva VE, Tiulina VV, Goriainov SV, Azbukina NV, et al. Inflammation in dry eye syndrome: identification and targeting of oxylipin-mediated mechanisms. Biomedicines (2020) 8(9):344. doi: 10.3390/biomedicines8090344
89. Zielonka J, Joseph J, Sikora A, Hardy M, Ouari O, Vasquez-Vivar J, et al. Mitochondria-targeted triphenylphosphonium-based compounds: syntheses, mechanisms of action, and therapeutic and diagnostic applications. Chem Rev (2017) 117(15):10043–120. doi: 10.1021/acs.chemrev.7b00042
90. Zinovkin RA, Zamyatnin AA. Mitochondria-targeted drugs. Curr Mol Pharmacol (2019) 12(3):202–14. doi: 10.2174/1874467212666181127151059
91. Plotnikov EY, Morosanova MA, Pevzner IB, Zorova LD, Manskikh VN, Pulkova NV, et al. Protective effect of mitochondria-targeted antioxidants in an acute bacterial infection. Proc Natl Acad Sci U S A. (2013) 110(33):E3100–8. doi: 10.1073/pnas.1307096110
92. Zakharova VV, Pletjushkina OY, Galkin II, Zinovkin RA, Chernyak BV, Krysko DV, et al. Low concentration of uncouplers of oxidative phosphorylation decreases the TNF-induced endothelial permeability and lethality in mice. Biochim Biophys Acta Mol Basis Dis (2017) 1863(4):968–77. doi: 10.1016/j.bbadis.2017.01.024
Keywords: neutrophil, Salmonella typhimurium, leukotriene B4, reactive oxygen species, glutathione, neutrophil swarming
Citation: Golenkina EA, Viryasova GM, Galkina SI, Kondratenko ND, Gaponova TV, Romanova YM, Lyamzaev KG, Chernyak BV and Sud’ina GF (2024) Redox processes are major regulators of leukotriene synthesis in neutrophils exposed to bacteria Salmonella typhimurium; the way to manipulate neutrophil swarming. Front. Immunol. 15:1295150. doi: 10.3389/fimmu.2024.1295150
Received: 15 September 2023; Accepted: 22 January 2024;
Published: 07 February 2024.
Edited by:
Pedro Gonzalez-Menendez, University of Oviedo, SpainReviewed by:
Sophie Dupre-Crochet, Université de Versailles Saint-Quentin-en-Yvelines, FranceJuan Manuel Serrador, Spanish National Research Council (CSIC), Spain
Copyright © 2024 Golenkina, Viryasova, Galkina, Kondratenko, Gaponova, Romanova, Lyamzaev, Chernyak and Sud’ina. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Galina F. Sud’ina, c3VkaW5hQGdlbmViZWUubXN1LnJ1; Boris V. Chernyak, YmNoZXJueWFrMUBnbWFpbC5jb20=
 Ekaterina A. Golenkina
Ekaterina A. Golenkina Galina M. Viryasova
Galina M. Viryasova Svetlana I. Galkina
Svetlana I. Galkina Natalia D. Kondratenko
Natalia D. Kondratenko Tatjana V. Gaponova2
Tatjana V. Gaponova2 Konstantin G. Lyamzaev
Konstantin G. Lyamzaev Boris V. Chernyak
Boris V. Chernyak Galina F. Sud’ina
Galina F. Sud’ina