
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol., 24 August 2023
Sec. Inflammation
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1235155
This article is part of the Research TopicSystemic Inflammation in Severe Infectious DiseasesView all 21 articles
Background: Peri-implantitis is an infectious/inflammatory disease with similar clinical and radiographic features to periodontitis. Overwhelming evidence confirmed that periodontitis causes elevations in systemic inflammatory mediators; this is unclear for peri-implantitis. Hence, this study aimed to appraise all available evidence linking peri-implantitis with systemic inflammation.
Methods: A systematic review was completed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Eight electronic databases (Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE, Web of Science, Dentistry & Oral Sciences Source, Scopus, LILACS, and China Online), ClinicalTrials.gov, WHO International Clinical Trials Registry Platform (ICTRP), and gray literature were searched up to February 9, 2023. Human studies of randomized controlled trials, non-randomized intervention studies, cohort studies, case–control, and cross-sectional studies were eligible for inclusion. Quantitative analyses were performed using random effects models.
Results: A total of 27 full-text articles were retrieved, and 11 clinical studies were included in the final analyses. All evidence gathered demonstrated a consistent association between peri-implantitis and systemic inflammation. Patients with peri-implantitis exhibited higher levels of serum C-reactive protein (CRP) (standard mean difference (SMD): 4.68, 98.7% CI: 2.12 to 7.25), interleukin-6 (IL-6) (weighted mean difference (WMD): 6.27 pg/mL, 0% CI: 5.01 to 7.54), and white blood cell counts (WMD: 1.16 * 103/μL, 0% CI: 0.61 to 1.70) when compared to participants without peri-implantitis.
Conclusion: Peri-implantitis is associated with higher systemic inflammation as assessed by serum CRP, IL-6, and white blood cell counts. Further research is needed to clarify the nature of this association.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=246837, identifier CRD42021246837.
Over the last three decades, dental implants have been proven to be an effective treatment for replacing teeth. A study reported that the survival rates of dental implants exceeded 85% after 25 years of follow-up (1), but they are not free from complications (2). The prevalence of peri-implant diseases is increasing. Evidence proved that the development and progression of peri-implant disease can result in the eventual loss of dental implants (3), while the definition of peri-implantitis continues to be a controversial issue due to the various case definitions of the disease reported (4). The 2017 International Classification of Periodontal and Peri-Implant Diseases and Conditions defined peri-implantitis as the presence of bleeding and/or suppuration on gentle probing combined with more than 6-mm probing depths and bone loss ≥3 mm (5). Peri-implant diseases display some unique features; the amount of surface area and inflammatory cell composition differentiate peri-implant lesions from periodontal pockets (4, 5).
However, periodontitis not only is linked to local inflammation but also triggers a systemic host response (6). This is usually assessed by serological biomarkers including C-reactive protein (CRP) and interleukin (IL)-6 (7). A raised systemic inflammatory state has been advocated as a potential mechanism linking periodontitis to a variety of systemic diseases including cardiovascular and metabolic diseases (8, 9). Some evidence also suggests that peri-implantitis with its local pathogen and inflammatory burden could be an unrecognized trigger of systemic inflammation (10). With the increasing use of dental implants including in patients with existing co-morbidities, it is therefore unclear what systemic impact peri-implant disease/infection might have.
We therefore aimed to conduct a systematic appraisal of all published evidence evaluating the association between peri-implantitis and its treatment and systemic inflammation.
A systematic review was conducted according to the Cochrane Handbook and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (11), and the protocol was registered in the PROSPERO register (reference no: CRD42021246837). The focused research questions were “Is there an association between peri-implantitis and systemic inflammation in adults?” and “Is there an association between the treatments of peri-implantitis and systemic inflammation in adults?”, using the PECOS/PICOS.
P (Patients): Adult population. Studies that recruited participants who were under 18 years old, pregnant, lactating, or taking antibiotics for purposes other than an intervention to treat peri-implantitis were excluded.
E (Exposure): Diagnosis of peri-implantitis according to respective definitions in their studies.
C (Comparison): Individuals without peri-implantitis.
O (Outcome): Systemic inflammation assessed by
1. serum CRP level (primary), and
2. any other biomarkers (such as white blood cell (WBC) counts, interleukin-6, interleukin-10, necrosis factor-α, interleukin-1β, neutrophils, hemoglobin (Hb), platelets (PLT), and lymphocytes) in peripheral blood (secondary).
S (Study designs): Human studies including randomized controlled trials, non-randomized intervention studies, cohort studies, case–control, and cross-sectional studies. However, case reports and series, reviews, and animal studies were excluded.
P (Patients): Adult population. Studies that recruited participants who were under 18 years old, pregnant, lactating, or taking antibiotics for purposes other than an intervention to treat peri-implantitis were excluded.
I (intervention): Peri-implantitis treatments including non-surgical or surgical implant decontamination with or without other adjunctive therapies.
C (Comparison): No treatment or control intervention (supra-mucosal cleaning, oral hygiene instructions, and/or community dental treatment).
O (Outcome): Systemic inflammation assessed by
1. serum CRP level reduction (primary) at 3 and 6 months of follow-up, and
2. any other biomarkers (such as white blood cell counts, interleukin-6, interleukin-10, necrosis factor-α, interleukin-1β, neutrophils, hemoglobin, platelets, and lymphocytes) in peripheral blood (secondary) at 3 and 6 months of follow-up.
S (Study designs): Human studies including randomized controlled trials.
Broad and inclusive electronic search strategies were designed and conducted to include citations until February 9, 2023. The following electronic databases were searched without language limitation using medical subject headings and free-text terms: Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, Web of Science, Dentistry & Oral Sciences Source, Scopus, LILACS, and China Online (example-Medline search Supplementary Table 1). Further, SIGLE was used to search for gray literature. The following journals were searched by hand since 2002: Journal of Periodontology, Journal of Clinical Periodontology, and Clinical Oral Implants Research. Registered studies were searched from ClinicalTrials.gov and WHO International Clinical Trials Registry Platform (ICTRP). Resulting hits from the application of the search strategies were imported into a reference manager software (EndNote, version 20).
The titles and abstracts (when available) identified through the search were screened independently by two reviewers (YY and MO) based on the inclusion and exclusion criteria. Any disagreement was resolved by discussion. Full reports were assessed for studies which there was insufficient information in the title and abstract to make a clear decision. The full reports were assessed independently, in duplicate, by the same reviewers to establish eligibility for inclusion. If necessary, a third reviewer was consulted (FD). Data extracted and collated in evidence tables included study characteristics, population, exposure, intervention, outcomes, and author conclusions.
Descriptive analysis was performed to determine the quality of data, checking further for study variations, in terms of study characteristics, and results and assessing suitability for inclusion in meta-analysis. Quality assessment of included studies was undertaken independently by two reviewers (YY and MO). Quality assessment and risk of bias in observational studies, randomized controlled trials, and non-randomized studies of interventions were determined using the Newcastle-Ottawa Scale (NOS) (12), the revised Cochrane tool (RoB 2) (13), and the ROBINS-I tool (14), respectively.
Qualitative analyses for all included studies were completed and reported in evidence tables. Further quantitative analyses were performed on all available evidence retrieved using Stata/MP 17.0 (StataCorp, College Station, TX, USA). Weighted mean differences (WMDs) with a 95% confidence interval (CI) were calculated for parameters reported and assessed using similar methods, while for those assessed and reported with different methods/units, standard mean difference (SMD) was applied.
The heterogeneity of the data was assessed using the c2-based Q-statistic method and considered significant if p < 0.05 and quantified with the I-squared statistic. Pooled estimates were calculated using random effects models when at least two studies with the data were available. Potential inter-study heterogeneity was considered to adopt more conservative analyses. The pooled effect was considered significant if p < 0.05. Publication bias was examined using a funnel plot and Egger’s test (15). Sensitivity analyses were defined a priori to understand the influence of individual studies on the aggregate estimates. Meta-analyses of different inflammatory biomarkers/outcomes were evaluated based on the Grading of Recommendations Assessment, Development, and Evaluations (GRADE) approach (16).
The electronic search identified 1,208 articles of potential relevance. After the removal of duplicates, 827 citations remained. After title and abstract screening, 26 articles were retrieved for full-text assessment from databases and registers, and one record was identified by hand searching. Finally, a total of 11 articles were eligible for inclusion in the review, among which seven were case–control studies (17–23), and four were cross-sectional studies (24–27) (Table 1; Figure 1). Five studies (17, 19, 22, 25, 26) compared participants with healthy implants. Another four studies only mentioned that participants from comparison groups were with healthy oral conditions (18, 20, 21, 24) and did not specify whether dental implants existed or not. Two studies (23, 26) divided the comparison groups into two groups, which were participants with healthy dental implants, or without dental implants but with healthy periodontium. Two studies defined the inclusion criteria in their studies as the presence of at least one implant diagnosed with peri-implantitis (17, 27), and another study divided peri-implantitis patients into different groups based on dental implant numbers and severity of peri-implantitis (24). Other included studies did not clearly illustrate the number of implants that were diagnosed with peri-implantitis. No randomized controlled trials were retrieved. A total of 32 blood parameters were measured to investigate the association between peri-implantitis and systemic inflammation (Supplementary Table 2). After the assessment of available data, one study was not eligible to be included in the meta-analysis due to a lack of standard deviation (20), and another article presented the data only as medians (25). Finally, nine articles were included in the quantitative analysis.
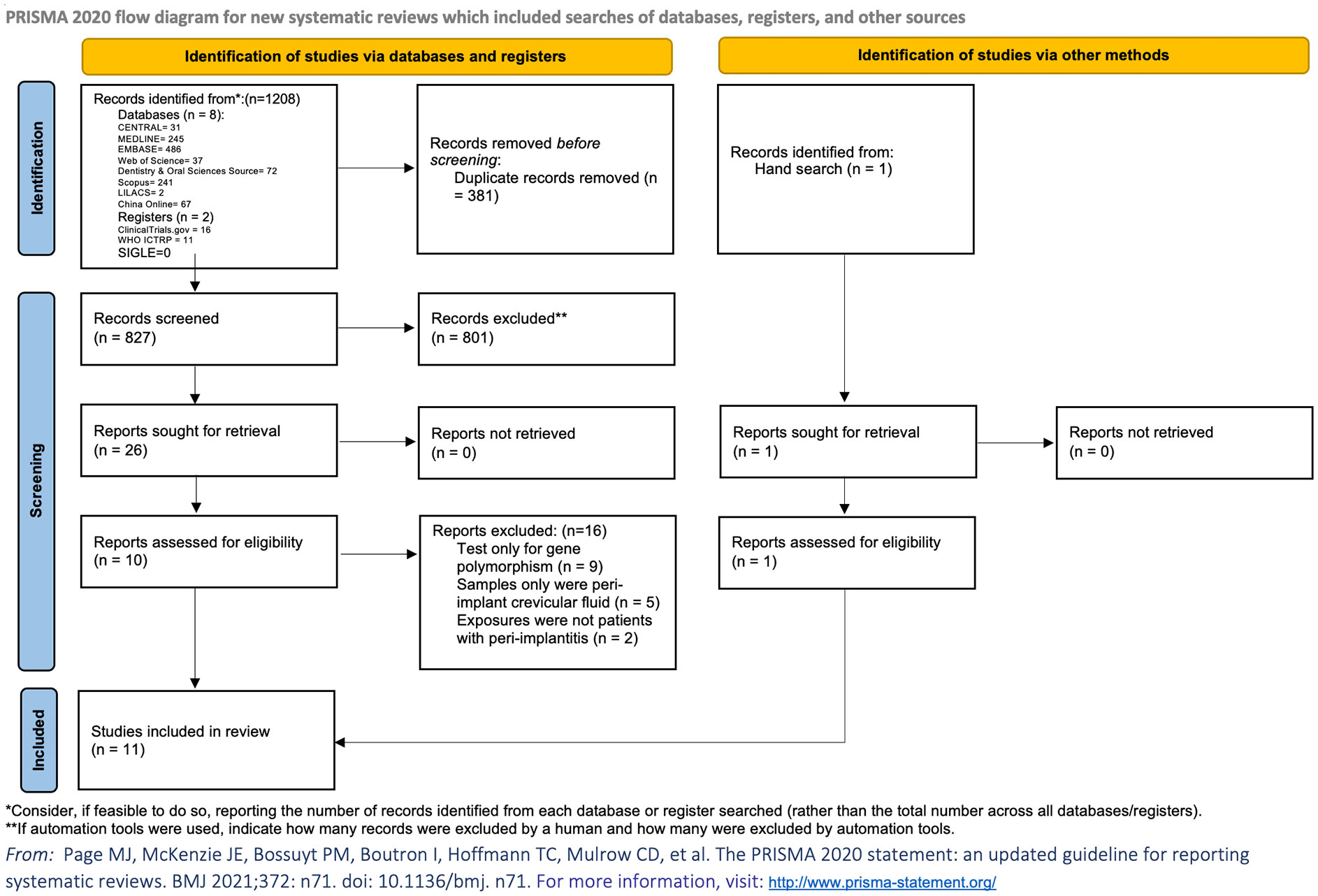
Figure 1 PRISMA flowchart. Flowchart of this study shows the process that we followed to identify and select studies. The original databases and registers retrieved 1,208 records. A total of 827 citations remained after removal of duplicates; 26 articles were retrieved for full-text assessment databases and registers. Another record was identified by hand search. A total of 11 articles were eligible after the application of inclusion and exclusion criteria.
Risk of bias assessment of observational studies, evaluated using the Newcastle-Ottawa Scale, was based on a) the selection and definition of controls, b) hospital controls rather than community population, c) ambiguous definitions and descriptions of the history of peri-implantitis, and d) non-response rate. The majority of observational studies were considered at low risk of bias (17, 19, 21–27), while only two studies were at high risk of bias (18, 20). The most common sources of bias among included articles were non-response rate and selection of controls (Supplementary Table 3). Publication bias analysis could not be conducted due to the limited number of studies retrieved/available. Due to the limited number of included articles and small sample size, the outcomes could be influenced by inconsistency and imprecision (Supplementary Figure 1). Sensitive analysis was conducted by omitting one article each time (Supplementary Figure 2).
Eight articles assessed CRP levels, while one of the studies lacked standard deviation, and one study only presented CRP as medians in their article. Meta-analysis of six articles confirmed a statistically significant higher level of CRP in patients with peri-implantitis (n = 182) compared to patients without peri-implantitis (n = 318); SMD of 4.68 [2.12, 7.25], with high level of heterogeneity (I2 = 98.7%), was detected, which means higher levels of CRP in patients with peri-implantitis (Supplementary Table 4; Figure 2A).
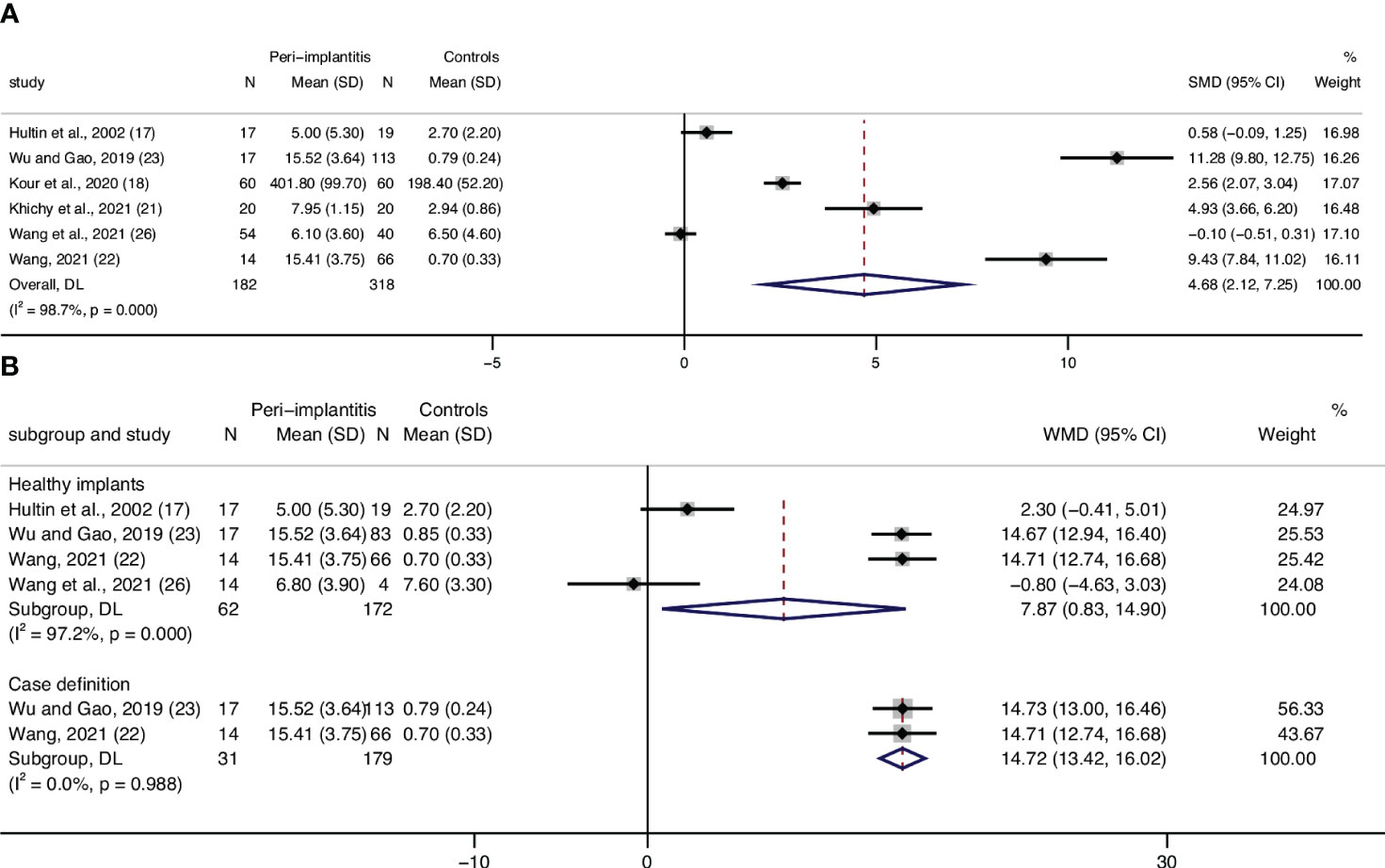
Figure 2 Forest plot for C-reactive protein (CRP) between cases (peri-implantitis) and controls (without peri-implantitis). Forest plot for standard mean difference (SMD) and weight mean difference (WMD) (95% confidence interval [CI]). Random effects model was used. The squares represent the relative effect of studies; the diamond represents the overall effect of all the studies. (A) Peri-implantitis patients (n = 182) showed higher serum CRP levels than controls (n = 318), SMD of 4.68 [2.12, 7.25], with high level of heterogeneity (I2 = 98.4%). (B) Healthy implants: subgroup analysis of patients in control groups of participants with healthy implants. Forest plots indicated higher serum CRP levels in patients with peri-implantitis (n = 62) than the controls (n = 172), WMD of 7.87 mg/L [0.83, 14.9]. (B) Case definition: subgroup analysis of the studies that defined peri-implantitis as gingival crevicular bleeding index >1 and periodontal probing depth ≥5 mm. Results showed significantly higher serum CRP levels in peri-implantitis patients than those without peri-implantitis.
Analyses were conducted for the studies that specified that their controls were patients with healthy implants, and results showed WMD of 7.87 mg/L [0.83,14.90], with a heterogeneity of 97.2%, which shows mean CPR levels in patients with peri-implantitis of 7.87 mg/L higher than that of patients without peri-implantitis (Figure 2B, Healthy implants).
Subgroup analysis based on the case definitions was also performed. Included studies have different definitions according to probing depth, bone loss, bleeding on probing, or swelling/suppuration. Two studies (22, 23), which defined peri-implantitis as gingival crevicular bleeding index >1 and periodontal probing depth ≥5 mm, revealed a significantly lower level of CRP in patients without peri-implantitis compared to patients with peri-implantitis (Table 1; Figure 2B, Case definition). Two studies (25, 26) defined peri-implantitis following the guidelines of consensus reports of the 2017 World Workshop. No significant difference in CRP levels was discovered between patients with peri-implantitis and without.
Three articles (17, 19, 27) assessed WBC levels, and data from two articles were eligible to be included in a meta-analysis. Results from the meta-analysis confirmed statistically significant higher leucocyte counts in patients with peri-implantitis (n = 75) compared to those without peri-implantitis (n = 68), with a low level of heterogeneity (0%) (Figure 3A). Although results from Blanco et al. cannot be included in quantitative analysis, they also reported a significantly lower level of WBC in patients without peri-implantitis than in peri-implantitis patients (27).
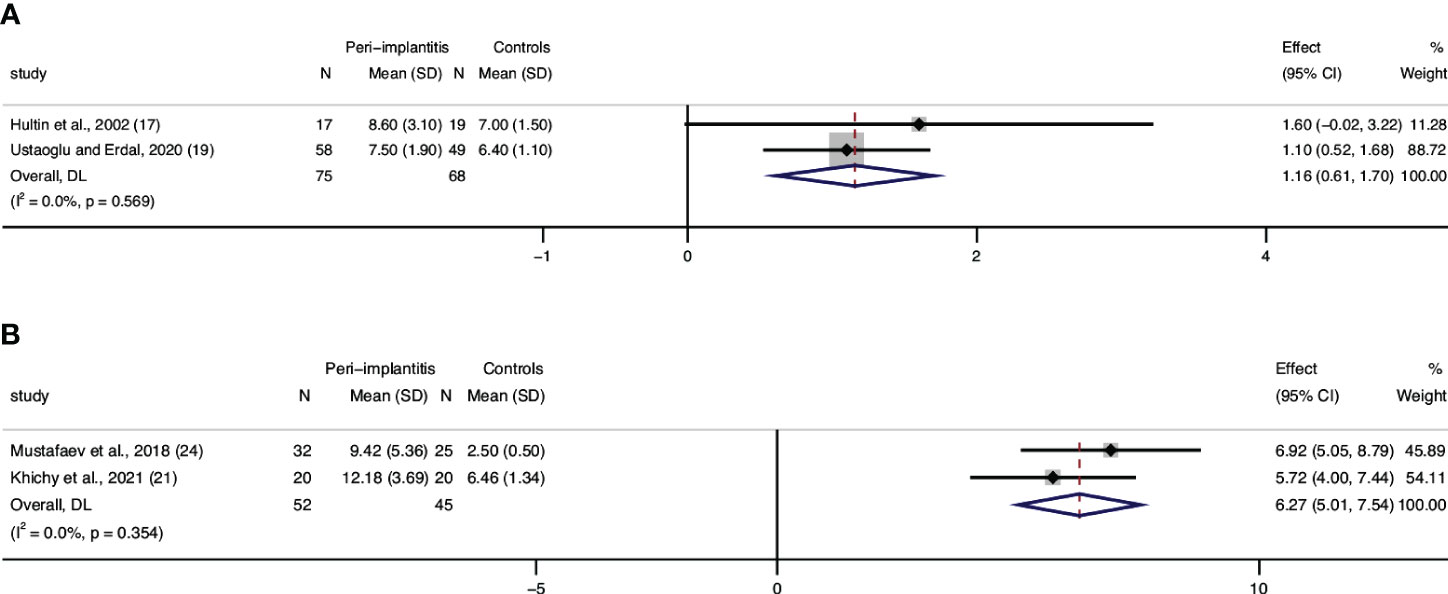
Figure 3 Forest plot for white blood cell (WBC) count interleukin-6 (IL-6) between cases (peri-implantitis) and controls (without peri-implantitis). Forest plot for mean difference (95% confidence interval [CI]). Random effects model was used for weight mean difference (WMD). The squares represent the relative effect of studies; the diamond represents the overall effect of all the studies. Higher levels of WBC (A) and IL-6 (B) in peri-implantitis groups compared with controls were revealed.
Two studies that assessed serum IL-6 and were included in the meta-analysis showed significant differences between patients with peri-implantitis and without (Figure 3B). Regarding IL-1β, a study by Mustafaev et al. confirmed a significantly lower level of serum IL-1β in patients with healthy periodontium/implants than in patients with peri-implantitis (24). Blanco also announced that patients with peri-implantitis had a significantly higher level of TNF-α than patients with healthy implants (27).
Meta-analyses for neutrophils, hemoglobin, platelets, and lymphocytes were also conducted, while no significant differences were observed in these mediators for patients with peri-implantitis versus those without peri-implantitis (Supplementary Figure 3).
This systematic review and meta-analysis indicates that peri-implantitis is not only associated with local mucosal inflammation but rather accompanied by a systemic host response. It is the first critical appraisal of the limited but consistent evidence suggesting that peri-implantitis could have systemic implications (i.e., patients with existing co-morbidities linked to systemic inflammation).
A recent review discussed the relationship between peri-implantitis and systemic diseases pointing at a possible role of body response to peri-implantitis, but due to the limited evidence, authors urged caution to avoid overinterpretation of the data available (10). Hultin et al. first reported a not statistically significant trend of higher serum CRP levels in patients with peri-implantitis (17). More recently, reports confirmed that higher serum CRP levels are associated with peri-implantitis (18, 20–23, 26). Moreover, two studies revealed that serum CRP significantly decreased after treating peri-implantitis with local metronidazole gel (22, 23). The main limitation of the evidence reported so far, however, is that most of the clinical trials performed were not primarily designed to test the impact of peri-implantitis on systemic inflammation. CRP has been used as a marker of systemic inflammation in these studies, and overwhelming evidence justifies this choice (28). Although CRP can be generated in reconstituted human gingival epithelia (29), which may explain the systemic inflammation induced by pathological periodontal tissues, it can also be released directly by the liver as part of the common acute phase response (30). Systemic inflammatory changes could impact the onset and progression of other systemic disorders (i.e., cardiometabolic diseases), and recent evidence points toward specific immune alterations (trained myelopoiesis) as potential common mechanisms (31, 32). It is plausible that peri-implantitis as periodontitis could represent an enhanced inflammatory stimulus with systemic implications, and we could speculate that the mechanism of CRP release due to periodontal inflammation is similar to that observed in patients with peri-implantitis.
There is plenty of evidence about the role of early inflammatory markers during the development of the host inflammatory response (i.e., cytokines) (33). This review included all reported inflammatory biomarkers rather than CRP, which have been measured in patients with peri-implant disease. As a multifunctional cytokine, IL-6 is secreted by several types of cells and is active in both innate and adaptive immune responses (34). Studies have reported that periodontitis is accompanied by increased production of proinflammatory cytokines (35, 36). Moreover, it has been demonstrated that proinflammatory cytokines in peri-implantitis crevicular fluid are increased (37) and anti-inflammatory cytokines are decreased (38). In this review, two studies (21, 24) also demonstrated higher concentrations of IL-6 in patients with peri-implantitis than without. Among other pro-inflammatory markers, IL-1β, TNF-α, and IL-17 have been extensively studied in the context of the generation of local and systemic inflammation and in particular relevant to periodontal inflammation and later tissue destruction (39, 40). There is currently evidence linking peri-implantitis to increased local production of TNF-α and IL-17 (41, 42). Evidence on the increased levels systemically of these markers in patients with dental implants and related diseases is limited. Higher serum concentrations of IL-1β and TNF-α were detected in patients with peri-implantitis compared to those without peri-implantitis (24, 27). Inconclusive evidence exists on the levels of IL-10 in patients with peri-implantitis (24, 27). Hematological changes were reported to be associated with peri-implantitis. In an experimental animal study, substantial differences in WBC, Hb, red blood cells (RBC), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), PLT, and mean corpuscular volume (MCV) were observed subsequent to the onset of experimental peri-implantitis (43). This review confirmed that peri-implantitis is associated with higher WBC count (a crude measure of systemic inflammation). These results are consistent with the evidence retrieved in patients with periodontitis (44), which reflect the inflammatory response of human body to peri-implantitis.
The exact mechanism behind the systemic impact of peri-implantitis remains unclear. Blanco et al. suggested that peri-implantitis subgingival pathogens induce a local inflammatory response followed by local exaggerated production of pro-inflammatory cytokines and prostaglandins (27). Histological evidence confirmed the greater inflammatory infiltrate within the peri-implant mucosa when peri-implantitis is diagnosed (45). Thus, it is reasonable to hypothesize that peri-implantitis could induce a systemic inflammatory response in a similar fashion to periodontitis (46) (Figure 4). A recent report confirmed indeed that when contrasting the host response of patients with periodontitis and peri-implantitis, the former represented a greater stimulus/trigger (47). This could be explained by the disproportionate number of inflamed sites often identified in patients with periodontitis as opposed to most reports describing the host response in patients with peri-implantitis referring to the single-digit number of implants affected. The main difference between these two conditions would therefore be the relative amount of inflamed surface area. Indeed, it is accepted that the periodontal inflamed surface area could extend up to 40 cm2 (48), while it is unclear how peri-implant inflamed mucosa would differ or be similar. The lack of reliable measures of the amount of gingival inflammation/cellular infiltrate within the peri-implant tissue could influence an accurate evaluation of the local and systemic impact of peri-implantitis.
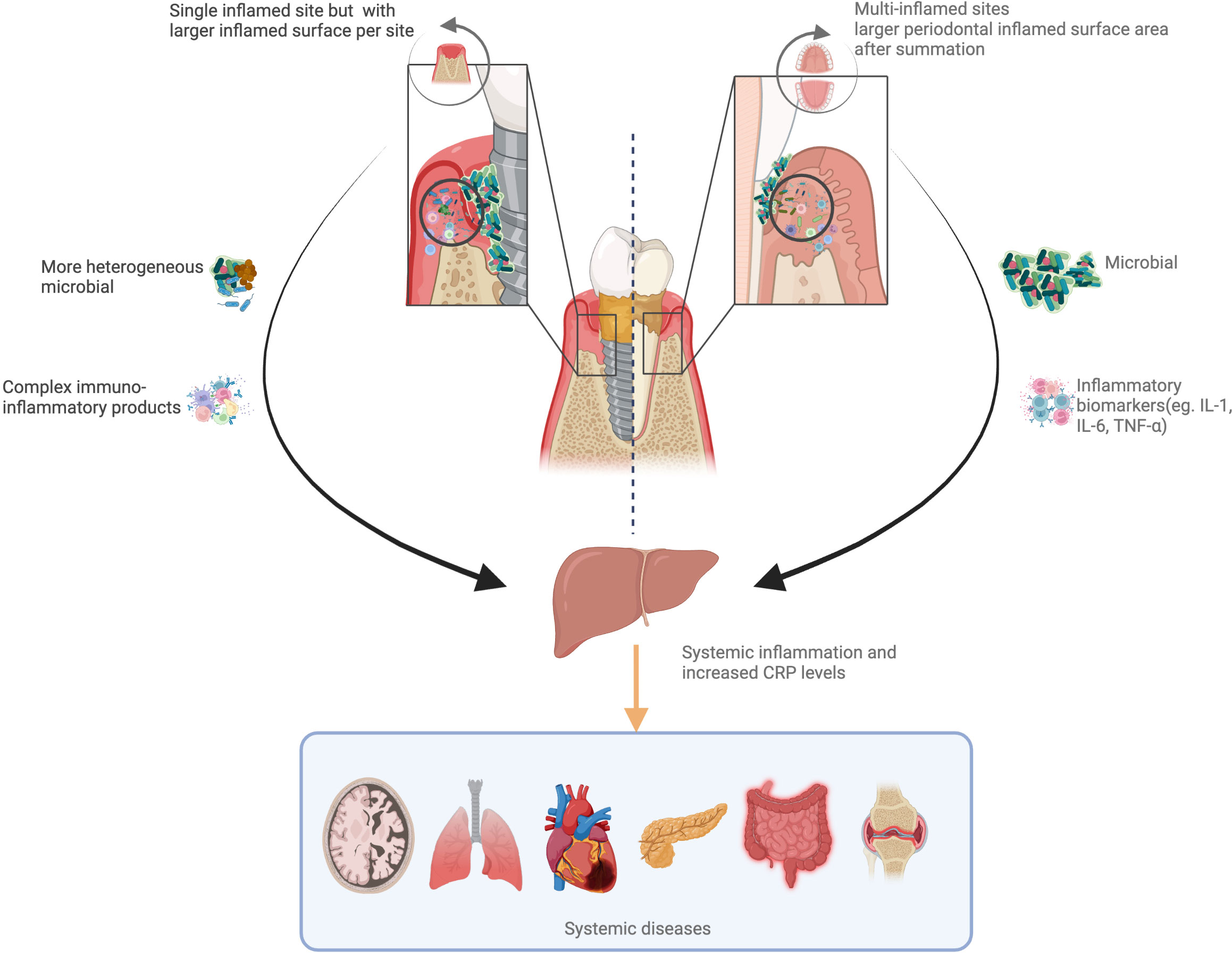
Figure 4 Hypothetical pathways linking peri-implantitis/periodontitis to systemic inflammation. Periodontitis can increase the risk of systemic inflammation and boost the C-reactive protein (CRP) levels with full mouth inflammation. Peri-implantitis always occur as single site, while a more heterogeneous bacterial flora and complex immune-inflammatory products could induce greater systemic inflammation. These two pathways add to systemic health risks.
Finally, there is a lack of randomized controlled trials on the topic. This would represent the highest level of evidence and address the issue of causality when studying the association between peri-implantitis and systemic inflammation/host response. We urge researcher colleagues therefore to focus on designing adequately powered clinical trials to address this question and ascertain the potential systemic implications of peri-implant diseases.
We should acknowledge some limitations of this review such as the high risk of bias of some trials included in our analysis and the differences in the definition of peri-implantitis as well as in the laboratory assessment of CRP. We strongly believe that these factors might have contributed to the high level of heterogeneity detected. Caution in interpreting these data conservatively should be exerted, and further and better-designed observational studies should be reported. In contrast, our systematic review methodology was based on a preregistered protocol, with a strict methodology and detailed process, including all possible confounding factors when assessing the available evidence, and could give confidence when interpreting the quantitative analyses.
In conclusion, our analysis supports an association between peri-implantitis and systemic inflammation as assessed by serum CRP levels. Although the mechanisms behind this association are still unclear, our findings are relevant when exploring potential systemic implications of overlooked sources of systemic inflammation from the oral cavity (i.e., peri-implantitis), especially in patients with other co-morbidities.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
YY contributed to the conception, design, data acquisition, and interpretation, and drafted and critically revised the manuscript. MO contributed to the conception and data acquisition and critically revised the manuscript. JSu contributed to the data interpretation, and drafted and critically revised the manuscript. SH contributed to the statistical analyses. JSm contributed to the search strategy. FD’A contributed to the conception, design, and data interpretation, and drafted and critically revised the manuscript. All authors contributed to the article and approved the submitted version.
The funder of the study had no role in the study design, data extraction, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
This study was completed at UCL as part of the Biomedical Research Centre funded by NIHR. MO held a NIHR Academic Clinical Lectureship. FD’A received funding from the Biomedical Research Centre. Figure 4 image was created using BioRender software.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1235155/full#supplementary-material
1. Jemt T. Implant survival in the edentulous jaw-30 years of experience. Part I: A retro-prospective multivariate regression analysis of overall implant failure in 4,585 consecutively treated arches. Int J Prosthodont (2018) 31(5):425–35. doi: 10.11607/ijp.5875
2. Aljohani M, Yong SL, Rahmah AB. The effect of surgical regenerative treatment for peri-implantitis: a systematic review. Saudi Dental J (2020) 32(3):109–19. doi: 10.1016/j.sdentj.2019.10.006
3. Schminke B, vom Orde F, Gruber R, Schliephake H, Bürgers R, Miosge N. The pathology of bone tissue during peri-implantitis. J Dental Res (2015) 94(2):354–61. doi: 10.1177/0022034514559128
4. Tarnow DP. Increasing prevalence of peri-implantitis: how will we manage? J Dent Res (2016) 95(1):7–8. doi: 10.1177/0022034515616557
5. Berglundh T, Armitage G, Araujo MG, Avila-Ortiz G, Blanco J, Camargo PM, et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol (2018) 89 Suppl 1:S313–s8. doi: 10.1002/JPER.17-0739
6. Hajishengallis G, Chavakis T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol (2021) 21(7):426–40. doi: 10.1038/s41577-020-00488-6
7. Paz JC, West MP. Acute care handbook for physical therapists e-book. Elsevier Health Sciences. (2019).
8. D’Aiuto F, Sabbah W, Netuveli G, Donos N, Hingorani AD, Deanfield J, et al. Association of the metabolic syndrome with severe periodontitis in a large U.S. Population-based survey. J Clin Endocrinol Metab (2008) 93(10):3989–94. doi: 10.1210/jc.2007-2522
9. Tonetti MS, Van Dyke TE, working group 1 of the joint EFP/AAP workshop. Periodontitis and atherosclerotic cardiovascular disease: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Clin Periodontol (2013) 40:S24–S9. doi: 10.1111/jcpe.12089
10. Radaelli K, Alberti A, Corbella S, Francetti L. The impact of peri-implantitis on systemic diseases and conditions: a review of the literature. Int J Dent (2021) 2021(2021):1–7. doi: 10.1155/2021/5536566
11. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. Bmj (2021) 372:n160. doi: 10.1136/bmj.n160
12. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Oxford, UK: Oxford (2000).
13. Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj (2019) 366:l4898. doi: 10.1136/bmj.l4898
14. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Bmj (2016) 355:i4919. doi: 10.1136/bmj.i4919
15. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
16. Schünemann H, Brożek J, Guyatt G, Oxman A. The GRADE handbook. UK: Cochrane Collaboration London (2013).
17. Hultin M, Gustafsson A, Hallström H, Johansson LÅ, Ekfeldt A, Klinge B. Microbiological findings and host response in patients with peri-implantitis. Clin Oral Implants Res (2002) 13(4):349–58. doi: 10.1034/j.1600-0501.2002.130402.x
18. Kour P, Oswal P, Nainee N, Pawashe Y. Assessment of C Reactive proteins levels in patients with peri-implantitis. J Adv Med Dental Sci Res (2020) 8(6):52–4. doi: 10.21276/jamdsr
19. Ustaoğlu G, Erdal E. Relationship between risk markers for cardiovascular disease and peri-implant diseases. Int J Implant Dent (2020) 6(1):1–7. doi: 10.1186/s40729-020-00273-z
20. Khatavkar D, Rajendra P, Shende D, Sharma DV, Pardeshi DK, Adasul D, et al. Assessment of serum C-reactive level in patients with peri-implantitis-A clinical study. Eur J Mol Clin Med (2021) 8(2):23–7.
21. Khichy A, Khichy R, Singh R, Bali Y, Kaur S, Gill TK. Assessment of levels of C-reactive proteins and interleukin 6 in patients with peri-implantitis: A Case–Control study. J Pharm Bioallied Sci (2021) 13(5):444. doi: 10.4103/jpbs.JPBS_540_20
22. Wang L. Changes of serum crp and urinary dpd levels in patients with peri-implantitis. J Hebei North Univ (Natural Sci Edition) (2021) 37(2):34–5,7. doi: 10.3969/j.issn.1673-1492.2021.02.010
23. Wu P, Gao C. Changes of serum CRP and urinary DPd in patients with dental implants and their correlation with peri-implantitis. Genomics Appl Biol (2019) 38(9):4193–7. doi: 10.13417/j.gab.038.004193
24. Mustafaev MS, Kharaeva ZF, Vissarionov VA, Mustafaeva FM, Zhigunov AK, Atmurzaev MM. Complex prophylaxis and prognosis of inflammatory complications after dental implantation. Asian J Pharm (2017) 11(4 Supplement):S975–S84. doi: 10.22377/ajp.v11i04.1747
25. Ozgur E, Topcu DI, Bayraktar N, Alptekin NO. Peri-implant crevicular fluid and serum levels of soluble ST2 in peri-implant diseases: A pilot study. J Periodontal Res (2023) 58(1):204–11. doi: 10.1111/jre.13082
26. Wang IC, Sugai JV, Majzoub J, Johnston J, Giannobile WV, Wang HL. Pro-inflammatory profiles in cardiovascular disease patients with peri-implantitis. J Periodontol (2021) 93(6):824–36. doi: 10.1002/JPER.21-0419
27. Blanco C, Liñares A, Dopico J, Pico A, Sobrino T, Leira Y, et al. Peri-implantitis, systemic inflammation, and dyslipidemia: a cross-sectional biochemical study. J Periodontal Implant Sci (2020) 51(5):342–51. doi: 10.5051/jpis.2100920046
28. Du Clos TW. Function of C-reactive protein. Ann Med (2000) 32(4):274–8. doi: 10.3109/07853890009011772
29. Lu Q, Jin L. Human gingiva is another site of C-reactive protein formation. J Clin Periodontol (2010) 37(9):789–96. doi: 10.1111/j.1600-051X.2010.01600.x
30. Tsukasaki M, Komatsu N, Nagashima K, Nitta T, Pluemsakunthai W, Shukunami C, et al. Host defense against oral microbiota by bone-damaging T cells. Nat Commun (2018) 9(1):701. doi: 10.1038/s41467-018-03147-6
31. Chavakis T, Mitroulis I, Hajishengallis G. Hematopoietic progenitor cells as integrative hubs for adaptation to and fine-tuning of inflammation. Nat Immunol (2019) 20(7):802–11. doi: 10.1038/s41590-019-0402-5
32. Netea MG, Joosten LAB, Latz E, Mills KHG, Natoli G, Stunnenberg HG, et al. Trained immunity: A program of innate immune memory in health and disease. Science (2016) 352(6284):aaf1098. doi: 10.1126/science.aaf1098
33. Lenz A, Franklin GA, Cheadle WG. Systemic inflammation after trauma. Injury (2007) 38(12):1336–45. doi: 10.1016/j.injury.2007.10.003
34. Souza JRM, Oliveira RT, Blotta MHS, Coelho OR. Serum levels of interleukin-6 (Il-6), interleukin-18 (Il-18) and C-reactive protein (CRP) in patients with type-2 diabetes and acute coronary syndrome without ST-segment elevation. Arquivos brasileiros Cardiol (2008) 90:94–9. doi: 10.1590/S0066-782X2008000200004
35. D’Aiuto F, Parkar M, Andreou G, Suvan J, Brett PM, Ready D, et al. Periodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markers. J Dental Res (2004) 83(2):156–60. doi: 10.1177/154405910408300214
36. Beklen A, Ainola M, Hukkanen M, Gürgan C, Sorsa T, Konttinen YT. MMPs, IL-1, and TNF are regulated by IL-17 in periodontitis. J Dental Res (2007) 86(4):347–51. doi: 10.1177/154405910708600409
37. Duarte P, Serrão C, MIranda T, Zanatta L, Bastos M, Faveri M, et al. Could cytokine levels in the peri-implant crevicular fluid be used to distinguish between healthy implants and implants with peri-implantitis? A systematic review. J Periodontal Res (2016) 51(6):689–98. doi: 10.1111/jre.12354
38. Casado PL, Canullo L, de Almeida Filardy A, Granjeiro JM, Barboza EP, Duarte MEL. Interleukins 1β and 10 expressions in the periimplant crevicular fluid from patients with untreated periimplant disease. Implant Dent (2013) 22(2):143–50. doi: 10.1097/ID.0b013e3182818792
39. Hardaway AL, Podgorski I. IL-1β, RAGE and FABP4: targeting the dynamic trio in metabolic inflammation and related pathologies. Future Medicinal Chem (2013) 5(10):1089–108. doi: 10.4155/fmc.13.90
40. Orlandi M, Muñoz Aguilera E, Marletta D, Petrie A, Suvan J, D’Aiuto F. Impact of the treatment of periodontitis on systemic health and quality of life: A systematic review. J Clin Periodontol (2021) 49:314–27. doi: 10.1111/jcpe.13554
41. Meyer S, Giannopoulou C, Courvoisier D, Schimmel M, Müller F, Mombelli A. Experimental mucositis and experimental gingivitis in persons aged 70 or over. Clinical and biological responses. Clin Oral Implants Res (2017) 28(8):1005–12. doi: 10.1111/clr.12912
42. Petković A, Matić S, Stamatović N, Vojvodić D, Todorović T, Lazić Z, et al. Proinflammatory cytokines (IL-1β and TNF-α) and chemokines (IL-8 and MIP-1α) as markers of peri-implant tissue condition. Int J Oral Maxillofac Surg (2010) 39(5):478–85. doi: 10.1016/j.ijom.2010.01.014
43. Chaushu L, Tal H, Sculean A, Fernández-Tomé B, Chaushu G. Peri-implant disease affects systemic complete blood count values—an experimental in vivo study. Clin Oral Invest (2020) 24(12):4531–9. doi: 10.1007/s00784-020-03318-0
44. Botelho J, MaChado V, Hussain SB, Zehra SA, Proença L, Orlandi M, et al. Periodontitis and circulating blood cell profiles: a systematic review and meta-analysis. Exp Hematol (2021) 93:1–13. doi: 10.1016/j.exphem.2020.10.001
45. Carcuac O, Berglundh T. Composition of human peri-implantitis and periodontitis lesions. J Dental Res (2014) 93(11):1083–8. doi: 10.1177/0022034514551754
46. Luthra S, Orlandi M, Hussain SB, Leira Y, Botelho J, MaChado V, et al. Treatment of periodontitis and C-reactive protein: A systematic review and meta-analysis of randomized clinical trials. J Clin Periodontol (2022) 50(1):45–60. doi: 10.2139/ssrn.3807963
47. Piero P, Nicola P, Bianca DM, Giorgio P, Antonella P, Claudio L, et al. Association between Subclinical Atherosclerosis and Oral Inflammation. A cross-sectional study. J Periodontol (2022) 94(4):314–27. doi: 10.1002/JPER.22-002
Keywords: systemic inflammation, inflammation, C-reactive protein, peri-implantitis, biomarker, immunity
Citation: Yan Y, Orlandi M, Suvan J, Harden S, Smith J and D’Aiuto F (2023) Association between peri-implantitis and systemic inflammation: a systematic review. Front. Immunol. 14:1235155. doi: 10.3389/fimmu.2023.1235155
Received: 05 June 2023; Accepted: 02 August 2023;
Published: 24 August 2023.
Edited by:
Mukesh Pasupuleti, Central Drug Research Institute (CSIR), IndiaReviewed by:
Aditi Chopra, Manipal College of Dental Sciences, IndiaCopyright © 2023 Yan, Orlandi, Suvan, Harden, Smith and D’Aiuto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesco D’Aiuto, Zi5kYWl1dG9AdWNsLmFjLnVr
†ORCID: Francesco D’Aiuto, orcid.org/0000-0001-8654-935X
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.