- 1State Key Laboratory of Bio-Control, Southern Marine Science and Engineering Guangdong Laboratory (Zhuhai), Guangdong Key Laboratory of Pharmaceutical Functional Genes, School of Life Sciences, Sun Yat-sen University, University City, Guangzhou, China
- 2Laboratory of Freshwater Genetics and Breeding, Shandong Freshwater Fisheries Research Institute, Jinan, China
- 3Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao National Laboratory for Marine Science and Technology, Qingdao, China
Vaccination is an effective measure to prevent infection by pathogens. Live vaccines have higher protective efficacy than inactivated vaccines. However, how live vaccines interact with the host from a metabolic perspective is unknown. The present study aimed to explore whether a live Edwardsiella tarda vaccine regulates host metabolism and whether this regulation is related to the protective efficacy of the vaccine. Therefore, a gas chromatography mass spectrometry (GC-MS)-based metabolomics approach was used to investigate the metabolomic profile of mice serum after vaccination with live E. tarda vaccine. Fructose was identified as a key biomarker that contributes to the immune protection induced by the live vaccine. Moreover, co-administration of exogenous fructose and the live vaccine synergistically promoted survival of mice and fish after bacterial challenge. These results indicate that metabolites, especially fructose, can potentiate the live E. tarda vaccine to increase its protective efficiency.
1 Introduction
Edwardsiella tarda is a Gram-negative bacterial species that causes infectious disease called edwardsiellosis in fish, reptiles, birds, and mammals (1, 2). Symptoms include gastroenteritis, peritonitis, meningitis, wound infections, and septicemia (3, 4). In the aquaculture industry, edwardsiellosis results in great economic losses all over the world (5, 6). Antibiotics are an effective approach to treat edwardsiellosis, but antibiotic overuse and misuse result in frequent isolation of antibiotic-resistant E. tarda (7, 8). These antibiotic-resistant E. tarda strains are insensitive to antibiotics. Therefore, alternative methods are needed to control these bacteria. Among them, vaccination is an effective way to prevent bacterial infection with few side effects (9–11). Therefore, a vaccine approach would be highly valuable.
Among the multiple types of vaccines, whole-cell vaccines were developed first but are still used (12, 13). Whole-cell vaccines include live and inactivated vaccines. As inactivated vaccines are unable to replicate in vivo, this type of vaccine is considered to be safer than live vaccines (14). However, live vaccines have higher protective efficacy than inactivated vaccines as live vaccines can be better recognized by the immune system (15, 16). Thus, improving the efficacy of a live E. tarda vaccine is a key issue for the development of a high-quality live E. tarda vaccine.
Metabolic modulation plays a role in immune response against bacterial pathogens (17–19). Several lines of evidence have shown that metabolites, such as linoleic acid, leucine, N-acetylglucosamine, glucose, malic acid, palmitic acid, and glycine, increase hosts’ survival of Vibrio alginolyticus, Streptococcus iniae, and E. tarda infection (20–27). The protective efficacy of live vaccines against E. tarda infection has been reported to be related to increased biosynthesis of palmitic acid (27). These results motivated us to explore whether metabolites potentiate the protective efficacy of a live E. tarda vaccine.
2 Materials and methods
2.1 Ethics statement
All work was conducted in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the US National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee of Sun Yat-sen University (animal welfare assurance number: 16).
2.2 Bacterial strains and animals
Bacterial strain E. tarda EIB202 was obtained from Prof. Yuanxin Zhang at East China University of Science and Technology. The strain was grown in tryptic soy broth (TSB) medium at 30°C and harvested when the optical density at 600 nm (OD600) reached 1.0. SPF Kunming mice (20.2 ± 1.32g) were provided by the Animal Center of Sun Yat-sen University and fed twice a day with a dry pellet diet along with sterile water. Micropterus salmoides (27.8 ± 2.36g) were purchased from a commercial breeding corporation (Guangzhou Mingfeng Fisheries Co., Ltd, Guangzhou, P.R. China) and maintained in 25-L open-circuit water tanks with aeration. The animals were fed with 3% of their body weight/day. After acclimating for 1 week, the animals were randomly divided into several groups to investigate the effect of the live vaccine.
2.3 Preparation of live vaccine
Vaccines were prepared as previously described (13). In brief, a single colony of E. tarda EIB202 was picked from a TSB plate and cultured overnight in TSB medium at 30°C. The cultures were diluted 1:100 in fresh TSB medium and grown at 30°C. Bacterial cells with an OD600 of 1.0 were harvested by centrifugation at 4,000 g for 15 min and washed three times with saline. The cells were resuspended in sterile saline, and the solution was used as the live E. tarda vaccine.
2.4 Protective efficacy of live vaccine
Twenty mice were divided into two groups, live vaccine and PBS control. The live vaccine was intraperitoneally injected into mice at 106 CFU/mouse (live vaccine group), with PBS being used as the control (PBS control). After two injections at an interval of 7 days, these mice were challenged by 5x108 CFU of EIB202 and observed twice daily for 7 days.
2.5 Administration of vaccine and collection of plasma samples
The live vaccine was administered to mice at 106 CFU/mouse by intraperitoneal injection, with PBS being used as the control. After two injections at an interval of 7 days, blood was drawn from the orbit of the live mice, and sodium citrate was added as an anticoagulant. Plasma was collected by centrifugation at 3,000 g and 4°C for 10 min. Metabolites were extracted from 50 μL plasma with 0.2 mL cold methanol (Sigma, USA) containing 10 μL 0.1 mg/mL ribitol (Sigma, USA) as an analytical internal standard for normalization across samples. After centrifugation at 12,000 g and 4°C for 10 min, 0.1 mL supernatant was collected and dried using a vacuum centrifugation device (LABCONCO, USA). The dried samples were used for gas chromatography mass spectrometry (GC-MS) analysis, involving six biological samples with two technical repeats per group.
2.6 GC-MS analysis
Samples were processed by derivatization involving a two-stage technique, as previously described (28). In brief, 20 μL of 40 mg/L methoxyamine hydrochloride in pyridine (Sigma) was added to the dried samples for 90 min at 37°C. Thereafter, 80 μL N-methyl-N-(trimethylsily)trifluoroacetamide (MSTFA, Sigma, USA) with 1% trimethylchlorosilane (TMCS) was mixed and reacted with the samples for 30 min at 37°C.
GC-MS analysis was carried out using an Agilent 7890A gas chromatograph equipped with an Agilent 5975C VL MSD detector (Agilent Technologies, USA). 1 μL sample was injected into a DB-5MS column (30 m length × 250 μm i.d. × 0.25 μm thickness) with splitless injection and the flow rate of carrier gas (helium) was 1 mL/min. The initial temperature of the gas chromatograph oven was 85°C for 5 min followed by an increase to 270°C at a rate of 15°C/min, and then the temperature was held at 270°C for 5 min. The mass spectrometer was operated in the range of 50–600 m/z.
2.7 Data processing and statistical analysis
Mass spectra were analyzed based on the Total ion chromatogram (TIC) by XCalibur 2.1 software (Thermo Fisher Scientific), and compounds were identified using the National Institute of Standards and Technology (NIST) library and NIST MS Search Program 2.0. Peaks from different samples were aligned based on retention time and the mass spectrum. After normalization to ribitol, the data on the peak areas were used for subsequent analysis. The normalized abundances of differential metabolites were used in a Z-score analysis, which was based on the sample value minus the mean and divided by the standard deviation. Principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA) were carried out using SIMCA 12.0 (Umetrics, Umeå, Sweden). Differential metabolites were used for pathway enrichment analysis using MetaboAnalyst 2.0 (29). Interactive Pathways (iPath) analysis was carried out by iPath3.0 (https://pathways.embl.de/).
2.8 Protective efficacy of live vaccine potentiated by fructose
Exogenous fructose was administered as previously described (25). In brief, Kuming mice and Micropterus salmoides were acclimatized at 28°C for 7 days. They were randomly divided into five groups of 30 or 60 per group, respectively. For both the mouse and fish groups, the animals in the five groups were injected twice at an interval of 7 days with 0.1 mL one of the following: (1) live vaccine, (2) live vaccine and 0.18 mg fructose, (3) live vaccine and 1.80 mg fructose, (4) 0.85% sterilized PBS, or (5) 1.80 mg fructose. The live vaccine was used at dosages of 106 CFU/mouse or 4×103 CFU/fish. Thereafter, the mice and fish were challenged by intraperitoneal inoculation of EIB202 (5×108 CFU/mouse and 5×105 CFU/fish) and observed twice daily for 15 and 7 days, respectively.
3 Results
3.1 Plasma metabolomic profiling of mice immunized with live E. tarda vaccine
Mice were immunized twice with live E. tarda vaccine and then challenged using E. tarda EIB202, which led to 50% survival (Figure 1A). Plasma was collected from the live mice to explore the association of metabolites with the protective efficacy of vaccine. A GC-MS-based metabolomics analysis was performed to profile the metabolic signature in the plasma after vaccination, with PBS being used as a control. A total of 130 aligned individual peaks were obtained from each sample (Figure 1B). After removing the artificial peaks, 49 metabolites were identified. Scatter plots of the metabolite abundances in pairs of technical repeats indicated correlation coefficients ranging between 0.993 and 0.999, demonstrating the reliability of the methods (Figure 1C). The metabolites were classified into five metabolic categories: carbohydrates (22.4%), amino acids (18.4%), fatty acids (22.4%), nucleotides (2.0%), and others (34.7%) (Figure 1D). Hierarchical clustering of the 49 metabolites is shown in (Figure 1E). These results indicate that vaccination induced a metabolic shift.
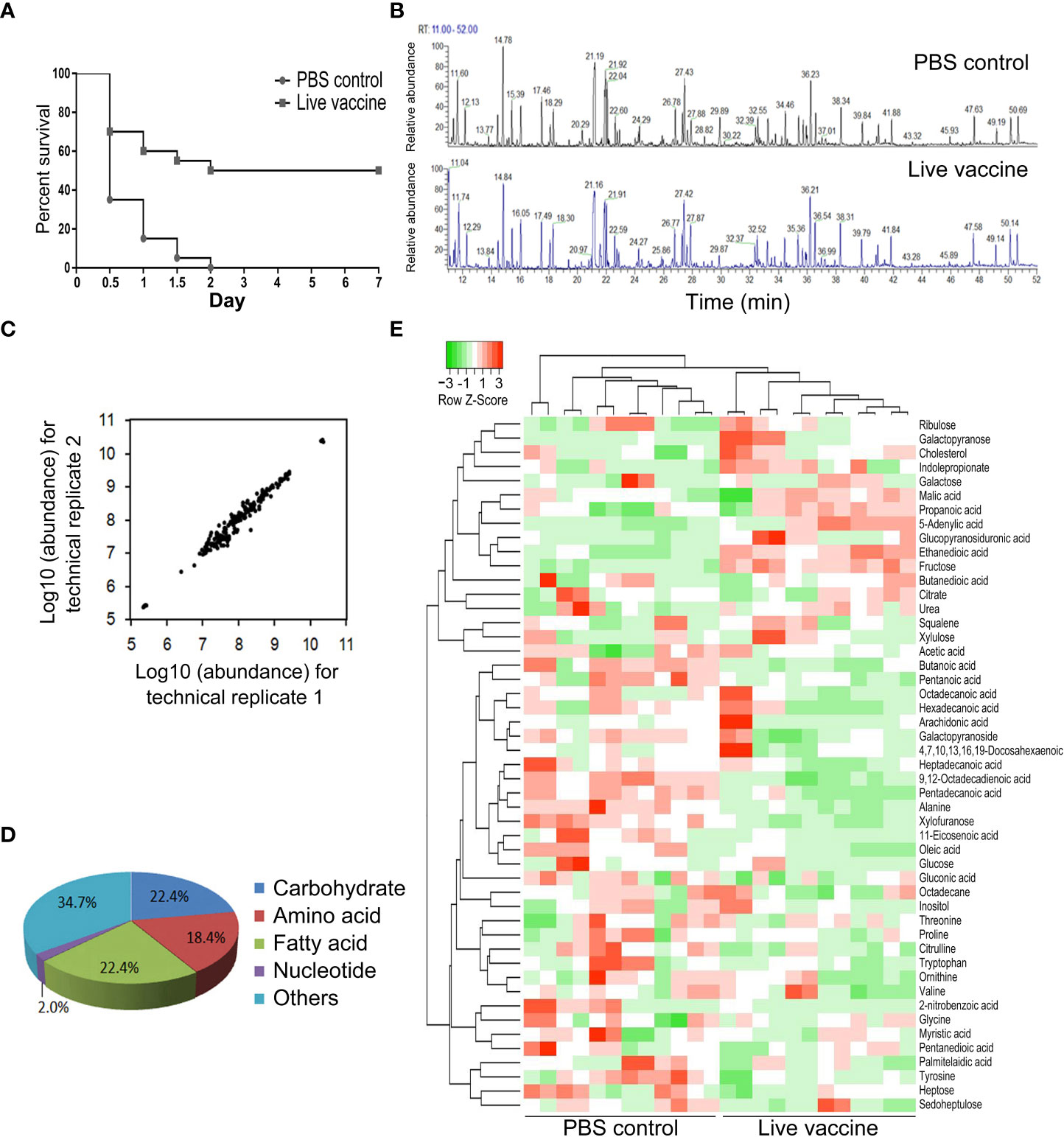
Figure 1 Metabolomic profiling of identified metabolites. (A) Survival of mice immunized by live E. tarda vaccine and then challenged by EIB202. (B) Representative total ion current chromatogram from control and live groups. (C) Reproducibility of metabolomic profiling platform. The correlation coeffcient is shown by two technical replicates of metabolite abundances. (D) Category of the identified metabolites. (E) Cluster analysis of metabolites. Heat map shows differential metabolites. Green and red color indicates decrease and increase of metabolites, respectively (see color scale).
3.2 Differential abundance of metabolites
There were 19 differential abundance of metabolites between the live vaccine group and PBS control group (Figure 2A). The Z-score plot spanned from −4.72 to 85.0 in the live vaccine group (Figure 2A), with fructose being the metabolite with the most increased abundance. Among the metabolites with differential abundances, 31.58% were carbohydrates, 21.05% were amino acids, 26.32% were fatty acids, 5.26% were nucleotides, and 15.79% were metabolites with unknown function (Figure 2B). There were more decreased amino acids and fatty acids in the live vaccine group than the PBS group, while the number of altered carbohydrates was similar between the two groups (Figure 2C). These results indicate that the live vaccine induced a differential metabolome, with fructose being the most elevated metabolite.
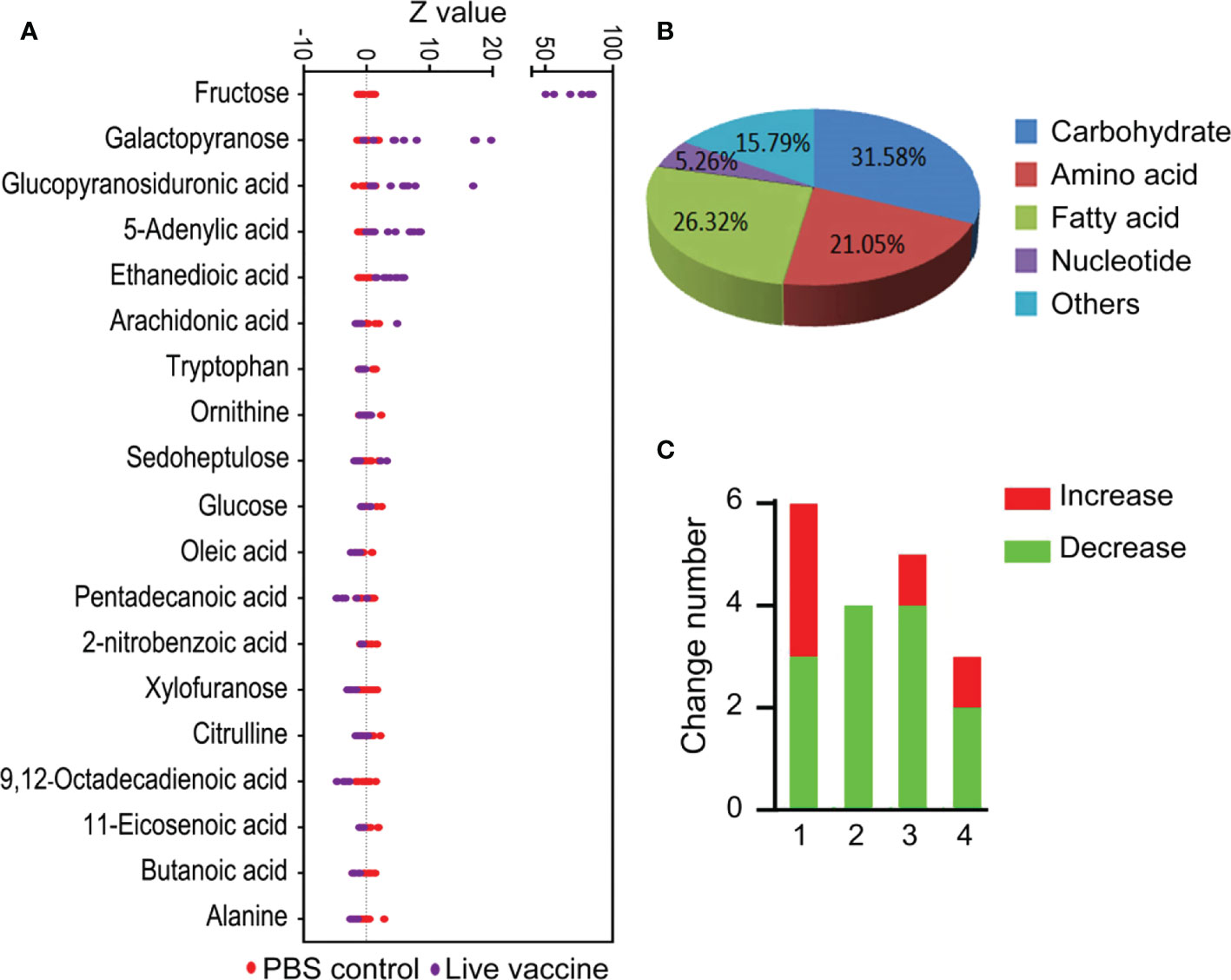
Figure 2 Differential metabolites analysis in response to vaccine stimulation. (A) Z-score plot of differential metabolites immunized with vaccine compared with PBS control. Each point represents one metabolite in one technical repeat and colored by sample types. (B) Category of different metabolites in live bacteria group. (C) Number of differential metabolites in live bacteria group. 1, carbohydrate; 2, amino acid; 3, fatty acid; 4, others.
3.3 Metabolic biomarkers identified using multivariate analysis
OPLS-DA was used to identify potential metabolic biomarkers associated with the protective efficacy of the vaccine. In the PCA, PC1 separated the two groups (Figure 3A). An S-plot was used to identify discriminatory variables, and the following biomarkers were selected from component p[1]: increased fructose and ethanedioic acid, and decreased oleic acid, 5-adenylic acid, 9,12-octadecadienoic acid, butanoic acid, and alanine (Figure 3B). These results confirm that fructose is a key biomarker.
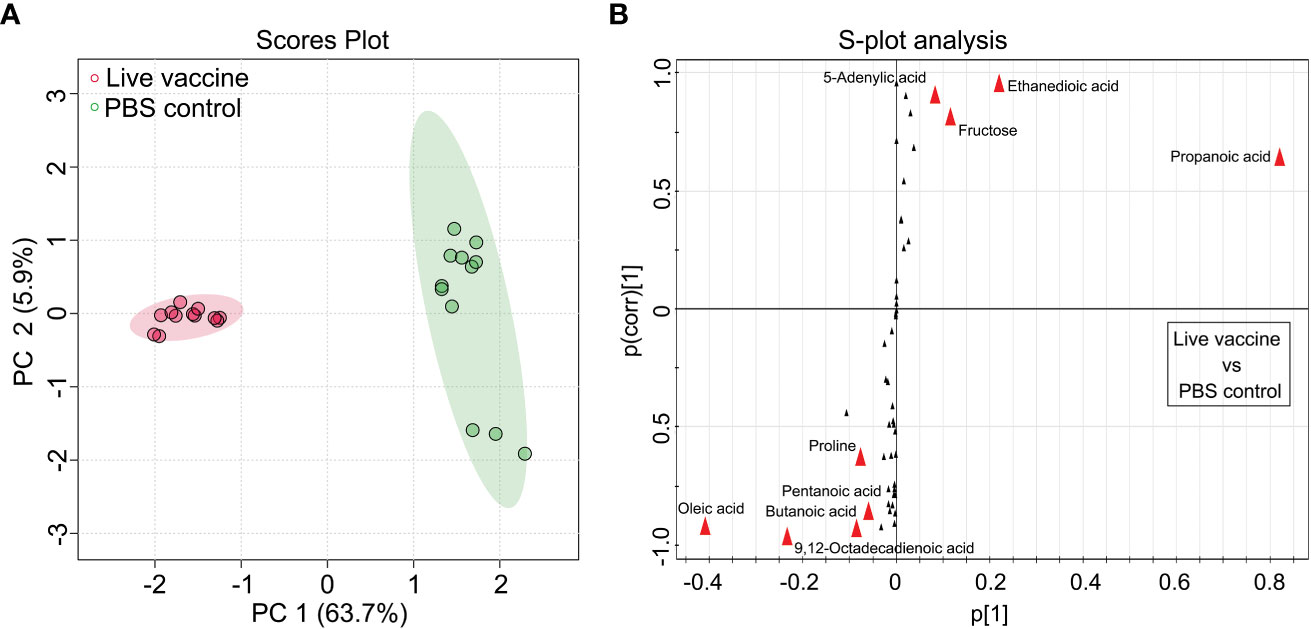
Figure 3 Identification of crucial metabolites. (A) The PCA analysis of the PBS control group and live vaccine group. Each dot represents the technique replicates in the plot. (B) S-plot generated from OPLS-DA. Circle represents individual metabolite, where potential biomarkers are highlighted with red.
3.4 Enrichment of metabolic pathways
Differential abundances of metabolites indicated that metabolic pathways were affected. Identifying the enriched pathways is helpful to identify the altered metabolic pathways. Galactose metabolism was enriched in the live vaccine group compared to the PBS group (Figure 4A). In this pathway, the metabolites with differential abundances included glucose, fructose, and galactopyranose (Figure 4B). Importantly, fructose was the metabolite with the most elevated abundance in the metabolic metabolism (Figures 4B, C). These results indicate that the live vaccine altered galactose metabolism.
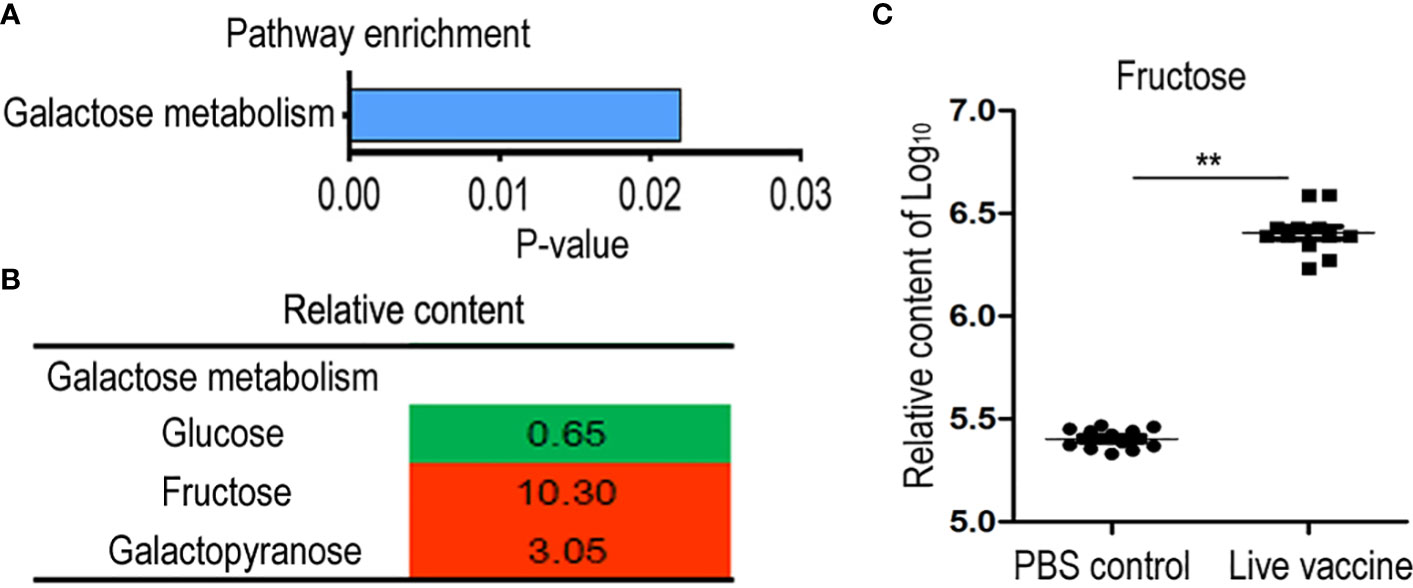
Figure 4 Metabolomics analysis of enriched pathway. (A) Pathway enrichment of different metabolites in live group. (B) Integrative analysis of metabolites in enriched pathways. The value was relative content in the live groups compared with control group. Red color and green color indicate increased and decreased metabolites, respectively. (C) The scatter diagram of different metabolite. The Y-axis is relative content of metabolites. **, P <0.01 using the Chi-square test.
Interactive Pathways Explorer (iPath) was used to provide an overview of the enriched metabolic pathways (Figure 5). Seven pathways were up-regulated in the live vaccine group compared to the PBS group: fructose and mannose metabolism, starch and sucrose metabolism, glycerolipid metabolism, arachidonic acid metabolism, purine metabolism, TCA cycle, and riboflavin metabolism. Two pathways were down-regulated: arginine and proline metabolism and alanine, aspartate, and glutamate metabolism. These results together suggest that the live vaccine altered metabolism to a large degree.
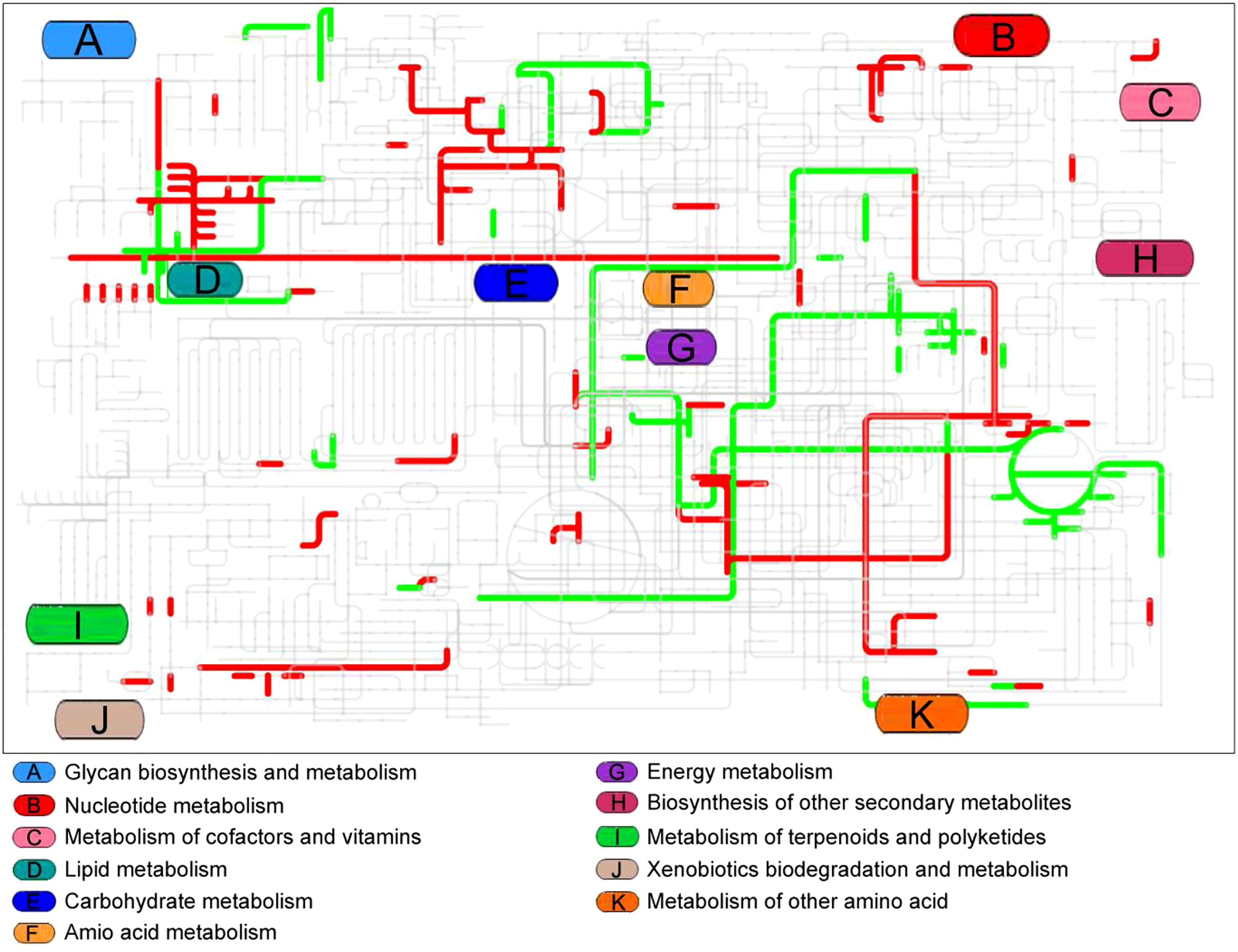
Figure 5 Integrated metabolomics analysis of pathway by iPath. The changed pathway in live group, compared with control group, was shown in color. Red lines represent increase and green lines indicate decrease.
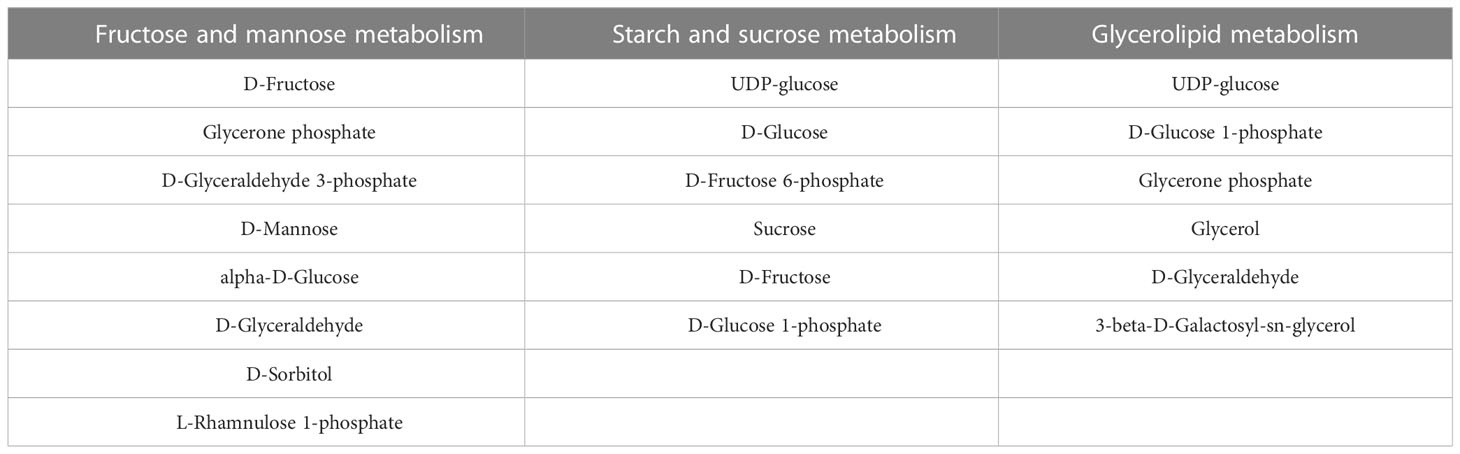
Notably, enrichment of metabolic pathways and iPath are two different analysis methods, which seems no overlapped metabolic pathways between the two identification. However, many metabolites in fructose and mannose metabolism, starch and sucrose metabolism, glycerolipid metabolism, glycerolipid metabolism are included in galactose metabolism (Table 1)(https://www.kegg.jp/kegg/kegg2.html).
3.5 Fructose improves the protective efficiency of the live vaccine
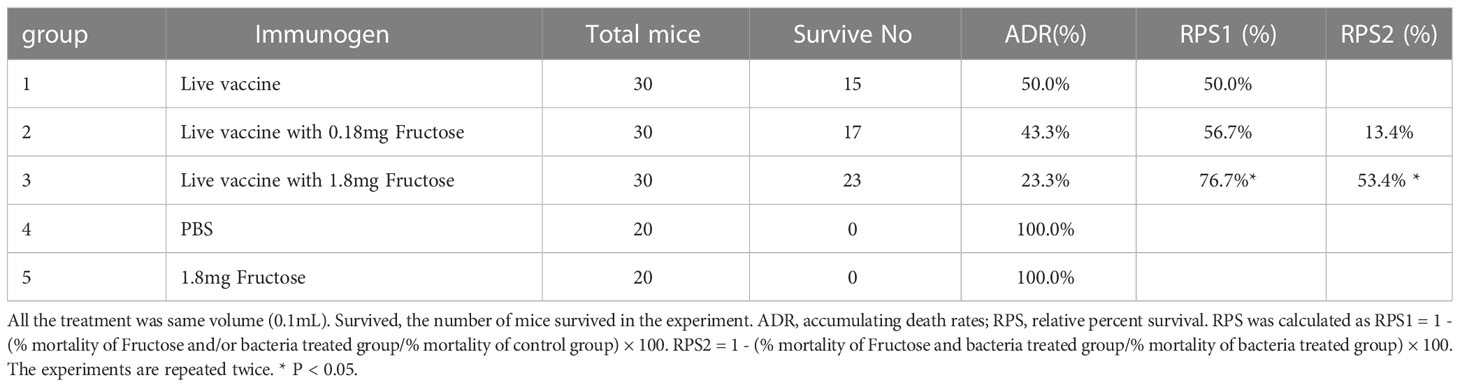
The above results show that fructose was the most altered metabolite. Therefore, increased fructose may contribute to the protective efficacy of the live vaccine. A mouse model was used to confirm the effect of fructose on the vaccine protective efficacy. To do this, mice were injected with live vaccine (group 1), live vaccine and 0.18 or 1.80 mg fructose (groups 2 and 3, respectively), PBS (group 4), or 1.80 mg fructose (group 5). After two injections at an interval of 7 days, the mice were challenged with EIB202 and their survival was monitored for 15 days. All mice in groups 4 and 5 died within 2 days, whereas 50.0%, 56.7% and 76.7% survived in groups 1, 2, and 3, respectively (Table 2). There were significant differences between groups 3 and 4 (RPS1) and groups 3 and 1 (RPS2), suggesting the role of fructose. No significance was found between groups 2 and 3.
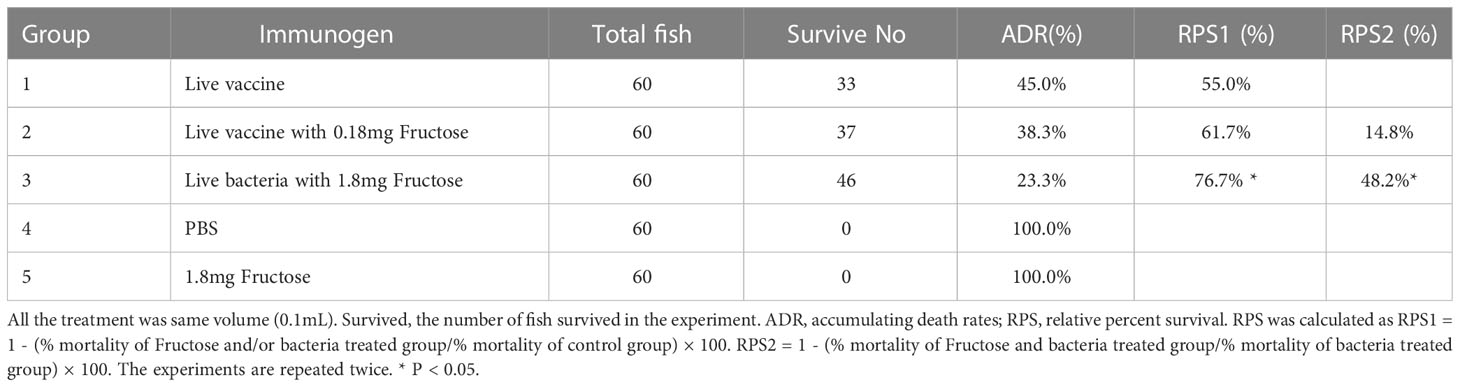
Furthermore, a Micropterus salmoides model was used to test the effect of fructose on the vaccine protective efficacy, using the same groupings as for the mice. The survival of the fish was monitored for 7 days. All fish in groups 4 and 5 died within 4 days, whereas 55.0%, 61.7%, and 76.7% survived in groups 1, 2, and 3, respectively (Table 3). There were significant differences in survival between groups 3 and 4 (RPS1) and groups 3 and 1 (RPS2), confirming the role of fructose. No significance was found between groups 2 and 3.
4 Discussion
The present study explores the role of metabolites in vaccination. Comparing metabolome between the live vaccine group and PBS control group, the abundances of metabolites were significantly changed. Thus, the live vaccine induces a metabolic response, suggesting that the metabolism plays a role in the vaccine-induced immune protection. Pattern recognition analysis identified fructose as the most important biomarker. Furthermore, exogenous fructose increased the live vaccine’s protective efficiency against bacterial challenge in mouse and fish models. These results indicate that metabolites can promote the protective efficiency of live vaccine against bacterial challenge, and fructose was identified as an effective metabolite that potentiates live E. tarda vaccine. To our knowledge, this is the first report on a metabolite promoting a vaccine’s efficiency.
It has been found that a metabolomics approach is effective at identifying biomarkers. To explore which biomarkers are associated with an increase in the protective efficacy of the live E. tarda vaccine, a GC-MS-based metabolomics analysis was performed in the present study. The live E. tarda vaccine induced a differential metabolome, indicating that there is a live vaccine-induced metabolome. This is consistent with the recent findings that, according to their sensitivity to antibiotics, bacteria display either an antibiotic-resistance or antibiotic-sensitive metabolome (28, 30), while hosts exhibit either an anti-infective or infective metabolome based on their ability to combat the infection (21, 31–35). Based on the live vaccine-induced metabolome, fructose was identified as a key biomarker that contributes to the immune protection efficiency of the live vaccine. Indeed, exogenous fructose increased the live vaccine-mediated immune protection against bacterial challenge in mice and fish. Thus, positive feedback was found between the live vaccine and fructose. Specifically, the live vaccine increased fructose and the increased fructose potentiated the ability of the vaccine to stimulate immune protection. Recently, we showed that live and inactivated E. tarda vaccines stimulate the generation of differential abundances of palmitate that contribute to differential innate immunities against bacterial infection (27). These results together indicate that vaccine-induced immune protection is related to the vaccine’s alteration of the metabolome. Therefore, the findings highlight that metabolites could be used as vaccine adjuvants.
Fructose is an important metabolite used for energy supply (36) and is involved in host–pathogen interactions (37, 38). Exogenous fructose decreases bacterial growth and biofilm formation (39). Antibiotic-resistant bacteria have a lower level of fructose than antibiotic-sensitive bacteria, and exogenous fructose has been shown to promote kanamycin-mediated killing (40). Fructose modulates the host’s immune response by increasing the expression of monocyte chemoattractant protein 1 and uric acid (37, 38). Monocyte chemoattractant protein 1 is a chemoattractant for inflammatory monocytes and dendritic cells, and uric acid activates the inflammasome pathway to release interleukin (IL)-1β, which enhances inflammation (41, 42). Furthermore, fructose activates dendritic cells via IL-6 and induces interferon-γ secretion by T cells (43). Therefore, fructose may activate the immune response as an immunologic adjuvant, indicating that fructose could be used as an adjuvant to increase the protection efficiency of vaccines.
5 Conclusion
In summary, this study provides insights into a new strategy to increase the protective efficacy of a live vaccine. This strategy involved using a metabolomics analysis to identify biomarkers that contribute to the protective efficiency of a vaccine and then administering a key biomarker with the vaccine in order to enhance the vaccine’s protective effects. Fructose was identified as the most crucial biomarker and found to be a metabolite that promotes protection against E. tarda infection. These findings highlight a way to identify vaccine adjuvants and how to improve whole-cell vaccines.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee of Sun Yat-sen University (animal welfare assurance number: 16).
Author contributions
HL conceptualized and designed the project. HL and X-XP wrote the manuscript. CW performed experiments. All authors contributed to the article and approved the submitted version.
Funding
This work was financially supported by International Exchanges Scheme (NSFC-RS) (32061133007), NSFC (42276125), Shandong Provincial Natural Science Foundation of China (ZR2018PC028) and Innovation Group Project of Southern Marine Science and Engineering Guangdong Laboratory (Zhuhai; no. 311020006).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Song M, Xie J, Peng XX, Li H. Identifcation of protective immunogens from extracellular secretome of Edwardsiella tarda. Fish Shellfsh Immunol (2013) 35:1932–6. doi: 10.1016/j.fsi.2013.09.033
2. Sun L, Chen H, Lin W, Lin X. Quantitative proteomic analysis of Edwardsiella tarda in response to oxytetracycline stress in biofilm. J Proteomics (2017) 150:141–8. doi: 10.1016/j.jprot.2016.09.006
3. Crosby SN, Snoddy MC, Atkinson CT, Lee DH, Weikert DR. Upper extremity myonecrosis caused by Edwardsiella tarda resulting in transhumeral amputation: case report. J Handb Surg-Am (2013) 38:129–32. doi: 10.1016/j.jhsa.2012.10.009
4. Suezawa C, Yasuda M, Negayama K, Kameyama T, Hirauchi M, Nakai T, et al. Identifcation of genes associated with the penetration activity of the human type of Edwardsiella tarda EdwGII through human colon epithelial cell monolayers. Microb Pathog (2016) 95:148–56. doi: 10.1016/j.micpath.2016.04.007
5. Xu TT, Zhang XH. Edwardsiella tarda: an intriguing problem in aquaculture. Aquaculture (2014) 431:129–35. doi: 10.1016/j.aquaculture.2013.12.001
6. Loch TP, Hawke J, Reichley S, Faisal M, Piero FD, Grifn MJ. Outbreaks of edwardsiellosis caused by Edwardsiella piscicida and Edwardsiella tarda in farmed barramundi (Lates calcarifer). Aquaculture (2017) 481:202–10. doi: 10.1016/j.aquaculture.2017.09.005
7. Yu JE, Cho MY, Kim JW, Kang HY. Large Antibiotic-resistance plasmid of Edwardsiella tarda contributes to virulence in fish. Microb Pathog (2012) 52(5):259–66. doi: 10.1016/j.micpath.2012.01.006
8. Stock I, Wiedemann B. Natural antibiotic usceptibilities of Edwardsiella tarda, e. ictaluri, and E. hoshinae. Antimicrob Agents Chemother (2001) 45(8):2245–55. doi: 10.1128/AAC.45.8.2245-2255.2001
9. Czinn SJ, Blanchard T. Vaccinating against Helicobacter pylori infection. Nat Rev Gastro Hepat (2011) 8:133–40. doi: 10.1038/nrgastro.2011.1
10. Aagaard C, Hoang T, Dietrich J, Cardona PJ, Izzo A, Dolganov G, et al. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat Med (2011) 17:189–94. doi: 10.1038/nm.2285
11. Nguyen HT, Thu Nguyen TT, Tsai MA, Ya-Zhen E, Wang PC, Chen SC. A formalin-inactivated vaccine provides good protection against vibrio harveyi infection in orange-spotted grouper (Epinephelus coioides). Fish Shellfish Immunol (2017) 65:118–26. doi: 10.1016/j.fsi.2017.04.008
12. Svennerholm AM, Lundgren A. Recent progress toward an enterotoxigenic Escherichia coli vaccine. Expert Rev Vaccines (2012) 11:495–507. doi: 10.1586/erv.12.12
13. Wang C, Peng B, Li H, Peng XX. TolC plays a crucial role in immune protection conferred by Edwardsiella tarda whole-cell vaccines. Sci Rep (2016) 6:29488. doi: 10.1038/srep29488
14. Liu X, Yang MJ, Wang SN, Xu D, Li H, Peng XX. Differential antibody responses to outer membrane proteins contribute to differential immune protections between live and inactivated Vibrio parahemolyticus. J Proteome Res (2018) 17(9):2987–94. doi: 10.1021/acs.jproteome.8b00176
15. Yamasaki M, Araki K, Maruyoshi K, Matsumoto M, Nakayasu C, Moritomo T, et al. Comparative analysis of adaptive immune response after vaccine trials using live attenuated and formalin-killed cells of Edwardsiella tarda in ginbuna crucian carp (Carassius auratus langsdorfi). Fish Shellfsh Immunol (2015) 45:437–42. doi: 10.1016/j.fsi.2015.04.038
16. Jeffrey BU, Ulrich V, Rino R. Vaccine manufacturing: challenges and solutions. Nat Biotechnol (2006) 24:1377–83. doi: 10.1038/nbt1261
17. Young T, Alfaro AC. Metabolomic strategies for aquaculture research: a primer. Rev Aquacult (2016) 10:26–56. doi: 10.1111/raq.12146
18. Boothby M, Rickert RC. Metabolic regulation of the immune humoral response. Immunity (2017) 46:743–55. doi: 10.1016/j.immuni.2017.04.009
19. Chen XH, Liu SR, Peng B, Li D, Cheng ZX, Zhu JX, et al. Exogenous l-valine promotes phagocytosis to kill multidrug-resistant bacterial pathogens. Front Immunol (2017) 8:207. doi: 10.3389/fimmu.2017.00207
20. Cheng ZX, Ma YM, Li H, Peng XX. N-acetylglucosamine enhances survival ability of tilapias infected by Streptococcus iniae. Fish Shellfish Immunol (2014) 40:524–30. doi: 10.1016/j.fsi.2014.08.008
21. Peng B, Ma YM, Zhang JY, Li H. Metabolome strategy against Edwardsiella tarda infection through glucose enhanced metabolic modulation in tilapias. Fish Shellfish Immunol (2014) 45:869–76. doi: 10.1016/j.fsi.2015.06.004
22. Zhao XL, Wu CW, Peng XX, Li H. Interferon-2b against microbes through promoting biosynthesis of unsaturated fatty acids. J Proteome Res (2014) 13:4155–63. doi: 10.1021/pr500592x
23. Ma YM, Yang MJ, Wang SY, Li H, Peng XX. Liver functional metabolomics discloses an action of l-leucine against Streptococcus iniae infection in tilapias. Fish Shellfish Immunol (2015) 45:414–21. doi: 10.1016/j.fsi.2015.04.037
24. Zeng ZH, Du CC, Liu SR, Li H, Peng XX, Peng B. Glucose enhances tilapia against Edwardsiella tarda infection through metabolome reprogramming. Fish Shellfish Immunol (2017) 61:34–43. doi: 10.1016/j.fsi.2016.12.010
25. Yang MJ, Cheng ZX, Jiang M, Zeng ZH, Peng B, Peng XX, et al. Boosted TCA cycle enhances survival of zebrafish to Vibrio alginolyticus infection. Virulence (2018) 9(1):634–44. doi: 10.1080/21505594.2017.1423188
26. Cheng ZX, Guo C, Chen ZG, Yang TC, Zhang JY, Wang J, et al. Glycine, serine and threonine metabolism confounds efficacy of complement-mediated killing. Nat Commun (2019) 10:3325. doi: 10.1038/s41467-019-11129-5
27. Xu D, Wang J, Guo C, Peng XX, Li H. Elevated biosynthesis of palmitic acid is required for zebrafish against Edwardsiella tarda infection. Fish Shellfish Immunol (2019) 92:508–18. doi: 10.1016/j.fsi.2019.06.041
28. Peng B, Su YB, Li H, Han Y, Guo C, Tian YM, et al. Exogenous alanine and/or glucose plus kanamycin kills antibiotic-resistant bacteria. Cell Metab (2015) . 21(2):249–62. doi: 10.1016/j.cmet.2015.01.008
29. Xia J, Mandal R, Sinelnikov IV, Broadhurst D, Wishart DS. MetaboAnalyst 2.0-a comprehensive server for metabolomic data analysis. Nucleic Acids Res (2012) 40:127–33. doi: 10.1093/nar/gks374
30. Jiang M, Su YB, Ye JZ, Li H, Kuang SF, Wu JH, et al. Ampicillin-controlled glucose metabolism manipulates the transition from tolerance to resistance in bacteria. Sci Adv (2023) 9:eade8582. doi: 10.1126/sciadv.ade8582
31. Zhao XL, Chen ZG, Yang TC, Jiang M, Wang J, Cheng ZX, et al. Glutamine promotes antibiotic uptake to kill multidrug-resistant uropathogenic bacteria. Sci Transl Med (2021) 13:eabj0716. doi: 10.1126/scitranslmed.abj0716
32. Zhang S, Wang J, Jiang M, Peng XX, Li H. Reduced redox-dependent mechanism and glucose-mediated reversal in gentamicin-resistant Vibrio alginolyticus. Environ Microbiol (2019) 21(12):4724–39. doi: 10.1111/1462-2920.14811
33. Kou TS, Wu JH, Chen XW, Chen ZG, Zheng J, Peng B. Exogenous glycine promotes oxidation of glutathione and restores sensitivity of bacterial pathogens to serum-induced cell death. Redox Biol (2022) 58:102512. doi: 10.1016/j.redox.2022.102512
34. Jiang M, Yang LF, Chen ZG, Lai SS, Zheng J, Peng B. Exogenous maltose enhances zebrafish immunity to levofloxacin-resistant Vibrio alginolyticus. Microb Biotechnol (2020) 13:1213–27. doi: 10.1111/1751-7915.13582
35. Jiang M, Chen ZG, Li H, Zhang TT, Yang MJ, Peng XX, et al. Succinate and inosine coordinate innate immune response to bacterial infection. PloS Pathog (2022) 18(8):e1010796. doi: 10.1371/journal.ppat.1010796
36. Havel PJ. Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev (2005) 63:133–57. doi: 10.1301/nr.2005
37. Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, Glushakova O, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J PhysiolRenal Physiol (2006) 290:625–31. doi: 10.1152/ajprenal.00140.2005
38. Cirillo P, Gersch MS, Mu W, Scherer PM, Kim KM, Gesualdo L, et al. Ketohexokinase-dependent metabolism of fructose induces proinfammatory mediators in proximal tubular cells. J Am Soc Nephrol (2009) 20:545–53. doi: 10.1681/ASN.2008060576
39. Durmus NG, Taylor EN, Inci F, Kummer KM, Tarquinio KM, Webster TJ. Fructose-enhanced reduction of bacterial growth on nanorough surfaces. Int J Nanomed (2012) 7:537–45. doi: 10.2147/IJN.S27957
40. Su YB, Peng B, Han Y, Li H, Peng XX. Fructose restores susceptibility of multidrug-resistant edwardsiella tarda to kanamycin. J Proteome Res (2015) 14(3):1612–20. doi: 10.1021/pr501285f
41. Rock KL, Kataoka H, Lai JJ. Uric acid as a danger signal in gout and its comorbidities. Nat Rev Rheumatol (2013) 9:13–23. doi: 10.1038/nrrheum.2012.143
42. Petrasek J, Iracheta-Vellve A, Saha B, Satishchandran A, Kodys K, Fitzgerald KA, et al. Metabolic danger signals, uric acid and ATP, mediate infammatory cross-talk between hepatocytes and immune cells in alcoholic liver disease. J Leukoc Biol (2015) 98:249–56. doi: 10.1189/jlb.3AB1214-590R
Keywords: Edwardsiella tarda, fructose, whole-cell vaccine, metabolomics, live vaccine
Citation: Wang C, Peng X-x and Li H (2023) Fructose potentiates the protective efficiency of live Edwardsiella tarda cell vaccine. Front. Immunol. 14:1170166. doi: 10.3389/fimmu.2023.1170166
Received: 20 February 2023; Accepted: 14 March 2023;
Published: 30 March 2023.
Edited by:
Jie Yin, Hunan Agricultural University, ChinaReviewed by:
Xiangmin Lin, Fujian Agriculture and Forestry University, ChinaYueling Zhang, Shantou University, China
Copyright © 2023 Wang, Peng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Li, bGlodWkzMkBzeXN1LmVkdS5jbg==
 Chao Wang
Chao Wang Xuan-xian Peng
Xuan-xian Peng Hui Li
Hui Li