- 1Sinopharm Dongfeng General Hospital, Hubei University of Medicine, Hubei Key Laboratory of Wudang Local Chinese Medicine Research (Hubei University of Medicine), Shiyan, China
- 2Department of Rheumatology and Immunology, Tongji Hospital, Tongji Medical College of Huazhong University of Science and Technology, Wuhan, Hubei, China
- 3Department of Cardiology, Tongji Hospital, Huazhong University of Science and Technology, Wuhan, China
Glucagon-like peptide-1 (GLP-1) is a 30-amino acid hormone secreted by L cells in the distal ileum, colon, and pancreatic α cells, which participates in blood sugar regulation by promoting insulin release, reducing glucagon levels, delaying gastric emptying, increasing satiety, and reducing appetite. GLP-1 specifically binds to the glucagon-like peptide-1 receptor (GLP-1R) in the body, directly stimulating the secretion of insulin by pancreatic β-cells, promoting proliferation and differentiation, and inhibiting cell apoptosis, thereby exerting a glycemic lowering effect. The glycemic regulating effect of GLP-1 and its analogues has been well studied in human and murine models in the circumstance of many diseases. Recent studies found that GLP-1 is able to modulate innate immune response in a number of inflammatory diseases. In the present review, we summarize the research progression of GLP-1 and its analogues in immunomodulation and related signal pathways.
1 Introduction
Glucagon-like peptide-1 (GLP-1) is an endogenous incretin secreted by small intestinal L cells, consisting of two bioactive isoforms: GLP-1 (7-37) and GLP-1 (7-36). GLP-1 is rapidly degraded by an enzyme named dipeptidyl peptidase-IV (DPP-4), converting to bioinactive products GLP-1 (9-36) and GLP-1 (9-37) (1–3), which have a very low affinity for GLP-1 receptor (GLP-1R) and have no insulin secretion effect. Endogenous GLP-1 has a short plasma half-life of about 2-3 min (4–6).
GLP-1 exerts its function by binding to GLP-1R and is involved in the development and progression of many diseases (7, 8). GLP-1R is a family of transmembrane G protein-coupled receptor B that was originally found in islet β cells but is widely expressed in extra pancreatic tissues, including the lungs, kidneys, central nervous system, enteric nervous systems, lymphocytes, blood vessels, and kidneys (9–11). Binding of GLP-1 with its receptor enhances glucose-stimulated insulin secretion and reduces glucagon. GLP-1 also delays gastric emptying, increases satiety, and reduces appetite. Therefore, it lowers blood glucose through multiple mechanisms (12, 13). The interaction between GLP-1 and GLP-1R exerts a variety of physiological functions, including promoting insulin synthesis and secretion, inhibiting islet cell production and releasing glucagon, reducing hepatic glycogen output, acting on the central nervous system, increasing satiety, and reducing food intake, by activating different downstream signaling molecules (14–16).
GLP-1R agonists (GLP-1RAs) are a group of GLP-1 analogues that are resistant to DPP4-mediated degradation, working through activating GLP-1R and its downstream signaling (3, 17, 18). GLP-1RA is widely used to treat type 2 diabetes by enhancing insulin production, and they also have the added benefit of suppressing appetite and losing weight (19, 20). GLP-1RAs are also involved in the nervous, cardiovascular, and endocrine systems (21–23).
The expression of GLP-1R remains controversial due to the lack of specific antibody against GLP-1R, with most of the literatures using qPCR to detect mRNA levels (24, 25). There were also a few studies detected the expression of GLP-1R at the protein level using a lineage-tracking animal model (26, 27). Although Glp1r mRNA transcripts have been detected in murine lymphoid tissue, little is known about the role of GLP-1 in the immune system (28). Here, we will discuss in-depth the actions of GLP-1 and its analogues in immunomodulation and related signal pathways in the setting of a number of diseases.
2 GLP-1 in regulating immune system
2.1 The role of GLP-1 in innate immune cells
Recent studies have demonstrated that GLP-1 and its analogues exert regulatory functions in innate immune cells, especially macrophages. It has been shown that GLP-1 analogue exenatide could activate the human monocyte-derived macrophage towards M2 phenotype (29). Lixisenatide, another GLP-1 analogue, has also been reported to decrease atheroma plaque size and instability in animal models, by reprogramming macrophages towards M2 phenotype (10 μg/kg/day) (30). Besides, Exendin-4 could promote the polarization of bone marrow-derived macrophages into M2 subtype (4.2 µg/kg/day) (31). These studies indicate that the GLP-1 and its analogues may act directly on macrophages and polarize the macrophages to M2 phenotype. Some other studies revealed that GLP-1 and its analogues may also indirectly promote M2 polarization by suppressing M1 and enhancing regulatory T cells (Tregs). DPP4 inhibitor alogliptin, which preserves GLP-1, was able to reduce visceral adipose tissue macrophage content in LDLR-/- mice with a concomitant upregulation of M2 marker CD163 (32). Another DPP4 inhibitor Des-fluoro-sitagliptin was also reported to decrease the accumulation of M1 macrophages in Gck+/-mice, a β-cell–specific glucokinase haploinsufficient (Gck+/−) diabetic model (33). Liraglutide, a widely used GLP-1RA in clinic, ameliorated the macrophage accumulation in periodontitis, by decreasing M1 macrophages but not M2 macrophages (30 μg/kg/day) (34). GLP-1 could directly reduce the M1 polarization by modulating the JNK/STAT3 activation in a murine monocyte/macrophage cell line RAW264.7 (35). In addition, GLP-1 administration also enhances the Treg function (36), which may promote M2 polarization (37). In a recent clinical study, we reported that the expression of GLP-1R on macrophages, especially M2 marcrophages, was significantly reduced in patients with coronary heart disease (CHD), suggesting a potential role of macrophage-derived GLP-1R signaling in cardiovascular disease (38). In addition to macrophages, human neutrophils and eosinophils have also been shown to express GLP-1R and GLP-1 signaling significantly decreased the expression of eosinophil-surface activation markers along with a decrease in the production of IL-4, IL-8 and IL-13 (39). Taken together, GLP-1 and its analogues play a crucial role in innate immune cells, especially in macrophage.
2.2 The role of GLP-1 in innate-like T lymphocytes
Invariant Natural Killer T (iNKT) cells are a subpopulation of T lymphocytes that bridge the innate and adaptive immune systems (40). A. E. Hogan, et al (10). reported that GLP-1R on the surface of iNKT could trigger downstream signal transduction cascades. GLP-1 inhibits the secretion of IFN-γ and IL-4 by iNKT cell in a dose-dependent manner. Dermal γδ T cells are another innate-like T lymphocyte subset that evoke innate and adaptive immune responses. They play a very important role in psoriasis patients with type 2 diabetes, and the administration of a GLP-1 analogue improved the psoriasis severity by decreasing the dermal γδ T-cell number and IL-17 expression (41).
2.3 The role of GLP-1 in intestinal epithelial lymphocytes
As a innate like lymphocytes, intestinal epithelial lymphocytes (IELs) patrol the lamina propria and play a critical role in host-bacteria interaction, wound healing, mucosal growth and regeneration, and inflammation (42). Tαβ and Tγδ IELs represent the two major subsets of intestinal IELs, and GLP-1R was expressed in both of these subsets at a higher level than cells isolated from the spleen, lymph nodes, and bone marrow (43). In addition, the activation of GLP-1R by exendin-4 increased cAMP accumulation and reduced cytokine production in IELs. CD4+ CD25+ Tregs are a subpopulation of T cells that are effective in reducing inflammation (44, 45). A significantly lower percentage of peripheral Tregs was detected in naive male GLP-1R-/- mice, while the numbers of CD4+ and CD8+ T cells and B cells in the spleen and lymph nodes had no differences (36). In addition, the activation of GLP-1R can increase Treg frequency and function, as demonstrated by flow cytometry and inhibition assays in diabetic NOD mice, with increased IL-10 expression and enhanced cellular inhibitory function (46). Th1/Th17 cell is a T cell subset which co-expresses IFN-γ and IL-17A (47), driven by IL-12 or IL-23 (47, 48). The development of tissue-infiltrating interferon (IFN)-γ/interleukin (IL)-17A doubly-secreting encephalitogenic CD4-positive T cell subset in the CNS was notably inhibited by dulaglutide (49). CD4-positive T lymphocytes was suppressed by liraglutide, accompanied by improved hepatosteatosis and metabolic function in female mice (200 μg/kg twice daily) (50). In a T-cell-dependent glomerulonephritis model, T-cell proliferation and nephritis was inhibited by liraglutide 200 μg/kg once daily (51). In conclusion, GLP-1 and its analogues have an important immunomodulatory effect in innate like lymphocytes.
3 The role of GLP-1/GLP-R axis in related signal pathways
GLP-1 is known to increase insulin secretion by β cells under hyperglycemic conditions. Although the GLP-1R agonists are used to treat type 2 diabetes in clinic (52, 53), there are direct evidences about the therapeutic actions of GLP-1-based therapies in different healthy conditions in humans, including adipogenesis, osteogenesis, and nociception, with many signaling pathways are involved (54).
3.1 PKA/STAT3 pathway
GLP-1 and its analogues exert their function such as M2 polarization by inducing the activation of the activator of transcription 3 (STAT3) (29). Following the treatment with GLP-1, the phosphorylation of JNK and its signal transduction through the cyclic adenosine monophosphate/protein kinase A (PKA) signaling pathway was attenuated, while the phosphorylation of STAT3 was increased, which further induce the polarization of macrophage towards M2 (35). An ovariectomized and a macrophage-depleted model were employed to investigate the effects of Exendin-4 on macrophages and bone formation. The results showed that Exendin-4 also promoted bone marrow-derived macrophage polarization to M2 phenotye and TGF-β1 secretion via PKA-STAT3 signaling (31). Non-alcoholic fatty liver disease (NAFLD) induced by a high-fat diet was used to investigated the Kupffer cells M2 polarization in the liver, which shows that liraglutide can reverse the negative effects of nonalcoholic fatty liver disease by modulating Kupffer cell M2 polarization via the cAMP-PKA-STAT3 signaling pathway (55).
3.2 MAPK and NF-κB pathway
Mitogen-activated protein kinase (MAPK) pathway was also involved in the signaling of GLP-1R (56–59). Male ob/ob mice were subcutaneously injected with liraglutide daily for 4 weeks, fatty acid synthase (FASN) was down-regulated through the MAPK/ERK and PKA signaling pathways (11). In an in vitro study with peripheral blood mononuclear cells, exendin-4 suppressed inflammatory responses and reduced oxidative stress, which was rmediated by suppressing MAPK signaling pathway (60). The protective effects of GLP-1 on IL-6 production and high glucose-induced endothelial progenitor cells (EPCs) dysfunction also mediated by the MAPK signaling pathway (61, 62). In a partial hepatectomy-induced behavior study using male Sprague-Dawley rats, surgical trauma induced an exacerbated spatial learning and memory impairment, while exendin-4 treatment suppressed the activation of nuclear factor kappa-B (NF-κB) and IL-1β, and thus ameliorated hepatectomy-induced behavioral deficits and inflmmation (63). Similarily, GLP-1R-mediated suppression of NF-κB p65 was also able to modulate neuropathic pain-induced neuroinflammation and improve recognition memory dysfunction (64).
3.3 PI3K/Akt pathway
GLP-1RAs can also function through the Phosphoinositide 3-kinases (PI3K)/AKT pathway (65–67). Microvascular endothelial cells (CMECs) were isolated from neonatal Sprague–Dawley (SD) rat hearts by the enzyme dissociation to induce hypoxia/reoxygenation (H/R) injury, and GLP-1 analogue liraglutide protected cardial microvascular endothelium from H/R injury by activating PI3K/Akt/survivin pathways (68).Liraglutide-induced PI3K activation was also able to enhance keratinocyte migration and promote wound healing in mice (9). MC3T3-E1 cells were incubated with liraglutide, which directly acts on osteoblasts and activates PI3K/AKT signaling, promoting bone formation (69). However, there were also a few reports suggesting that PI3K/Akt pathway was inhibited by GLP-1 and its analogues. Pancreatic cancer cell lines and mouse xenograft models of human pancreatic cancer were used to evaluate the effects of the GLP-1R agonist liraglutide in vitro and vivo. The results demonstrated that GLP-1R activation with liraglutide dose-dependently suppressed Akt activation and tumourigenicity/metastasis in human pancreatic cancer cells both in vitro and in vivo (70).
3.4 Other pathways
In addition to the above mentioned signal pathways, GLP-1/GLP-R axis also also activates a number of other important signal pathways. Chang’s findings suggest that EX-4 inhibited LPS-induced iNOS expression at protein level, but not at transcriptional level, of iNOS gene via a cAMP/PKA dependent mechanism (71). Liraglutide can reduce oxidative stress and fatty degeneration in oxLDL-treated Raw264.7 cells, accompanied by an alteration of AMPK/SREBP1 pathway (72). In consistency, AMPK activation induced by GLP-1 impaired inflammatory signals in keratinocytes and restrained macrophage migration (73).
4 The role of GLP-1/GLP-R in related disease
4.1 Diabetes
Diabetes mellitus is characterized by increased inflammation, reflecting innate immune control disorders, and studies have shown a local intestinal intraepithelial lymphocyte (IEL)-GLP-1 receptor (GLP-1R) signaling network that controls the mucosal immune response (43). GLP-1 agonists, a novel class of anti-diabetic drugs, are an integral part of the management of patients with type 2 diabetes (74–76). GLP-1 agonists bind to GLP-1 receptors on pancreatic β cells, which directly stimulates pancreatic β cells to secrete insulin. They can also promote the proliferation of pancreatic β cells, increase the number of β cells, inhibit apoptosis, and promote insulin synthesis, thus resulting in hypoglycemic effects (77, 78). GLP-1RA has also been reported to promote motor activity and energy expenditure, thus activating the metabolism of brown fat in rodents (20). Weight-loss effect of non-insulin glucose lowering drugs in patients with type 2 diabetes is also a hot spot of current research. A systemic analysis shows that GLP1-RA and Tirzepatide are most effective in inducing weight loss in patients with type 2 diabetes among a variety of anti-diabetic drugs (79). Studies have suggested that in addition to diabetes, GLP-1R agonists may also have a beneficial effect on many other diseases such as cardiovascular disease, central nervous disorder, and tumors (21–23).
4.2 Cardiovascular disease
The latest research has found that GLP-1R is widely expressed in the cardiovascular system and is involved in intracellular metabolism and signal transduction. These metabolites are biologically active, can reduce intravascular oxidative stress, inhibit hepatocyte gluconeogenesis and oxidative stress, increase cardiomyocyte activity, promote vasodilation, protect the cardiovascular system, improve cardiac function, thereby directly or indirectly protecting the cardiovascular system (80–82). For example, a number of large-scale clinical trials have demonstrated that GLP-1RA could reduce the risk of cardiovascular events, which has been well reviewed in elsewhere (83, 84) and we will not further discuss here.
4.3 Nervous system disorder
Recently, a protective effect of the GLP-1/GLP-1R axis on ischemic brain injury has been emphasized. The activation of GLP-1R can reduce the size of cerebral infarction by enhancing cell survival signaling pathways, reducing ischemia-reperfusion injury, promoting brain repair, and inhibiting inflammatory response and oxidative stress (85–88). As we mentioned before, GLP-1R agonism is able to ameliorate neuroinflammation and behavioral deficits induced by hepatectomy or neuropathic pain (63, 64). In addition, activation of GLP-1R in the brain is albe to reduce appetite, lowering the risks for other diseases such as metabolic and cardiovascular disorders (88).
4.4 Tumors
The role of GLP-1RA in tumor remains controversial. Due to the promotive effect of GLP-1R agonism on β-cells proliferation and survival, concerns of tumorigenesis, especially pancreatic cancers, have been raised about incretin-based therapies (89). By analyzing the reported adverse events in the US Food and Drug Administration’s database, Elashoff reported that treatment with DPP4 inhibitor sitagliptin or GLP-1R agonist exenatide increased the risk of pancreatitis and pancreatic cancer as compared with other therapies. However, other types of cancer occurred at a similar level between patients who took sitagliptin and those with other therapies (90). Animal study has also demonstrated that exenatide can promote pancreatic duct hyperplasia, a well-established risk factors for pancreatic cancer (91). However, a meta-analysis including 37 eligible randomized controlled trials failed to identify an association between GLP-1RAs and increased risk of overall cancer. There was even a lower risk of overall cancer in patients treated with albiglutide in the subgroup analysis (92). Another meta-analysis on pancreatic cancer specifically also showed that GLP-1RAs were not associated with increased risk for pancreatic cancer as compared with other treatments (93). Interestingly, GLP-1R has been utilized to mediate tumor specificity for insulinoma that highly expresses GLP-1R during radiotherapy. A single injection of an Ahx-DTPA-(111)In)NH(2) In-labeled GLP-1R agonist markedly reduced tumor volume in a dose-dependent manner in a mouse model of insulinoma (94). Mechanistical studies suggest that GLP-1RAs may reduce tumor growth in prostate (95) and breast cancer (96, 97). Taken together, the exact role of GLP-1R in tumorigenesis remains to be illucidated.
4.5 Asthma
Asthma is a very common chronic lung disease characterized by chronic persistent airway inflammation and airway remodeling, resulting in incompletely reversible airway obstruction, especially in advanced stages (98). Many researchs showed that GLP-1R signaling inhibits the innate immune response in animal models of asthma, by the activation of PKA/NF-κB signaling pathway (99) and the decreased eosinophil production of IL-4, IL-8 and IL-13 (39). GLP-1RA treatment may be a new pharmacologic adjunctive treatment strategy for obese patients with asthma (100, 101).
5 Discussion
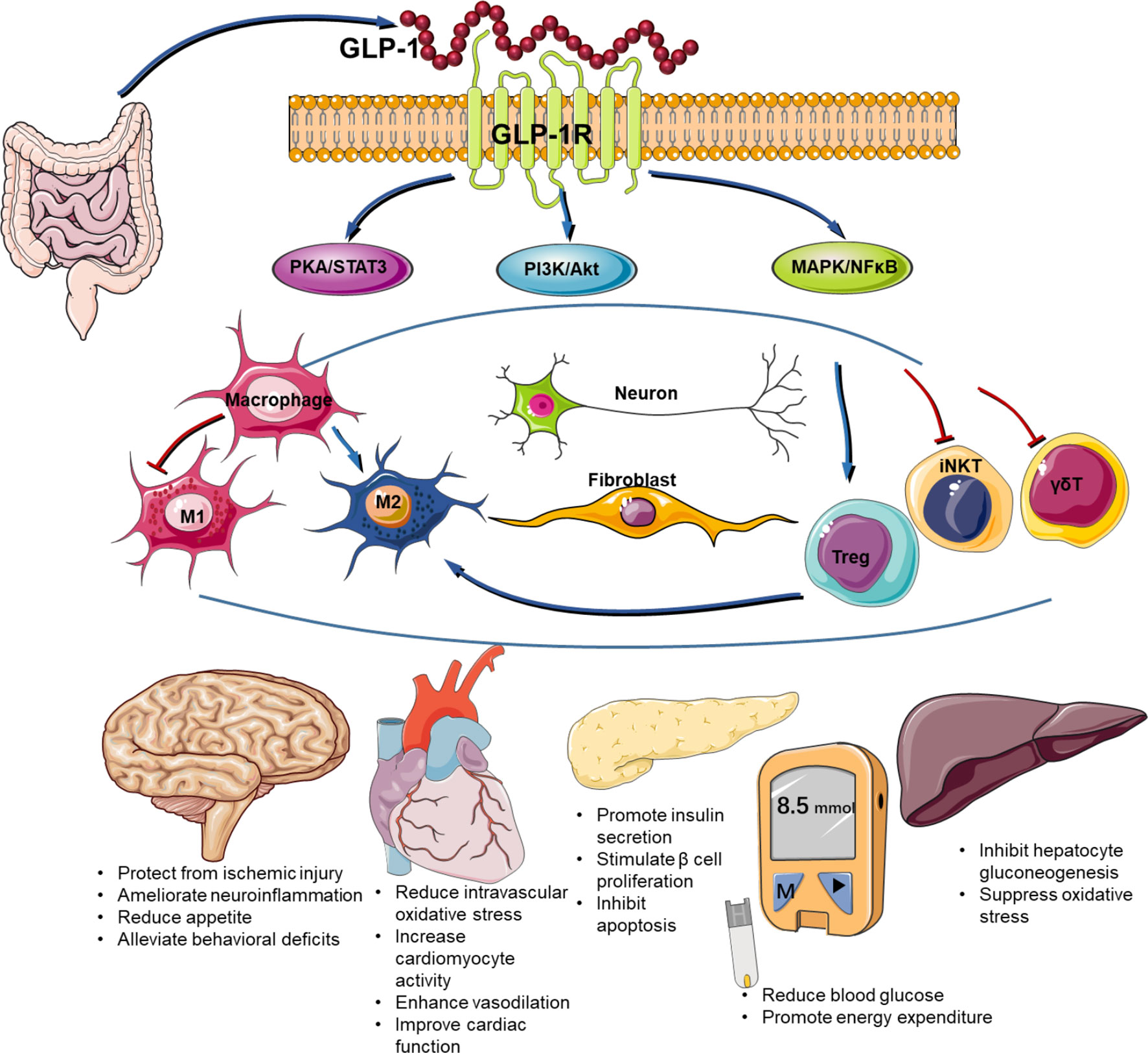
The safety and efficacy of GLP-1RAs in the treatment of type 2 diabetes have been well demonstrated. Recent investigations suggest that it also participates in a number of other diseases by regulating immune response (Figure 1). Both the innate and innate-like cells express GLP-1R. Engagement of GLP-1R and its ligands activates multiple signaling pathways including PKA/STAT, PI3K/Akt, MAPK, and NFκB. Given the importance of inflammation in disease progression, GLP-1RAs have been shown to possess beneficial effects on many other diseases in addition to diabetes. For example, the improvements in cardiovascular outcome have been evidenced in a number of large-scale randomized controlled clinical trials on cardiovascular disease (102). The obvious improvement of skin lesions in patients with psoriasis type 2 diabetes mellitus after liraglutide treatment may be related to inhibition of the expression of inflammatory factors such as IL-23, IL-17, and TNF-α (103). Despite of recent advances in our understanding of the immune regulatory role of GLP-1/GLP-1R, there are many challenges to overcome in this area. First, the expression of GLP-1R remains controversial in many types of cells and tissues due to the lack of specific antibody against GLP-1R. Second, the exact molecular signaling for GLP-1R activation remains elusive. Last, to what extend immune system is involved in the beneficial effects of GLP-1RAs on cardiometabolic and other diseases is not well understood. Therefore, further studies are required to delineate the detailed mechanisms by which GLP-1RAs regulate immune function and chronic inflammatory diseases.
Author contributions
JC, AM, YW, CL, HQ, XM, HY, and LD searched the data, JC, XR, and JZ wrote the main manuscript text. All authors reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (31870906, 81974254, 82170470, and 82270903), Hubei Provincial Natural Science Foundation (2022CFB453), Foundation of Health Commission of Hubei (WJ2021M061, WJ2019F071), the Natural Science Foundation of the Bureau of Science and Technology of Shiyan City (grant no. 21Y71, 19Y89), the Faculty Development Grants from Hubei University of Medicine (2018QDJZR04), and Hubei Key Laboratory of Wudang Local Chinese Medicine Research (Hubei University of Medicine) (Grant No. WDCM2022007), and the Advantages discipline Group (Medicine) Project in Higher Education of Hubei Province (2021–2025) (Grant No. 2022XKQT4).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol (2012) 8(12):728–42. doi: 10.1038/nrendo.2012.140
2. Mahapatra MK, Karuppasamy M, Sahoo BM. Semaglutide, a glucagon like peptide-1 receptor agonist with cardiovascular benefits for management of type 2 diabetes. Rev Endocrine Metab Disord (2022) 23(3):521–39. doi: 10.1007/s11154-021-09699-1
3. Yaribeygi H, Jamialahmadi T, Moallem SA, Sahebkar A. Boosting GLP-1 by natural products. Adv Exp Med Biol (2021) 1328:513–22. doi: 10.1007/978-3-030-73234-9_36
4. Pyke C, Heller RS, Kirk RK, Ørskov C, Reedtz-Runge S, Kaastrup P, et al. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology (2014) 155(4):1280–90. doi: 10.1210/en.2013-1934
5. Chen Y, Xu YN, Ye CY, Feng WB, Zhou QT, Yang DH, et al. GLP-1 mimetics as a potential therapy for nonalcoholic steatohepatitis. Acta Pharmacol Sin (2021) 43(5):1156–66. doi: 10.1038/s41401-021-00836-9
6. Saraiva JFK, Franco D. Oral GLP-1 analogue: perspectives and impact on atherosclerosis in type 2 diabetic patients. Cardiovasc Diabetol (2021) 20(1):235. doi: 10.1186/s12933-021-01417-0
7. Drucker DJ. Biological actions and therapeutic potential of the glucagon-like peptides. Gastroenterology (2002) 122(2):531–44. doi: 10.1053/gast.2002.31068
8. Duan L, Rao X, Braunstein Z, Toomey AC, Zhong J. Role of incretin axis in inflammatory bowel disease. Front Immunol (2017) 8:1734. doi: 10.3389/fimmu.2017.01734
9. Nagae K, Uchi H, Morino-Koga S, Tanaka Y, Oda M, Furue M. Glucagon-like peptide-1 analogue liraglutide facilitates wound healing by activating PI3K/Akt pathway in keratinocytes. Diabetes Res Clin Pract (2018) 146:155–61. doi: 10.1016/j.diabres.2018.10.013
10. Hogan AE, Tobin AM, Ahern T, Corrigan MA, Gaoatswe G, Jackson R, et al. Glucagon-like peptide-1 (GLP-1) and the regulation of human invariant natural killer T cells: lessons from obesity, diabetes and psoriasis. Diabetologia (2011) 54(11):2745–54. doi: 10.1007/s00125-011-2232-3
11. Chen J, Zhao H, Ma X, Zhang Y, Lu S, Wang Y, et al. GLP-1/GLP-1R signaling in regulation of adipocyte differentiation and lipogenesis. Cell Physiol Biochem (2017) 42(3):1165–76. doi: 10.1159/000478872
12. Sisley S, Gutierrez-Aguilar R, Scott M, D'Alessio DA, Sandoval DA, Seeley RJ. Neuronal GLP1R mediates liraglutide's anorectic but not glucose-lowering effect. J Clin Invest (2014) 124(6):2456–63. doi: 10.1172/jci72434
13. Mul JD, Begg DP, Barrera JG, Li B, Matter EK, D'Alessio DA, et al. High-fat diet changes the temporal profile of GLP-1 receptor-mediated hypophagia in rats. Am J Physiol Regul Integr Comp Physiol (2013) 305(1):R68–77. doi: 10.1152/ajpregu.00588.2012
14. Sandoval D. CNS GLP-1 regulation of peripheral glucose homeostasis. Physiol Behav (2008) 94(5):670–4. doi: 10.1016/j.physbeh.2008.04.018
15. Burmeister MA, Brown JD, Ayala JE, Stoffers DA, Sandoval DA, Seeley RJ, et al. The glucagon-like peptide-1 receptor in the ventromedial hypothalamus reduces short-term food intake in male mice by regulating nutrient sensor activity. Am J Physiol Endocrinol Metab (2017) 313(6):E651–e62. doi: 10.1152/ajpendo.00113.2017
16. Costa A, Ai M, Nunn N, Culotta I, Hunter J, Boudjadja MB, et al. Anorectic and aversive effects of GLP-1 receptor agonism are mediated by brainstem cholecystokinin neurons, and modulated by GIP receptor activation. Mol Metab (2022) 55:101407. doi: 10.1016/j.molmet.2021.101407
17. Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab (2016) 18(3):203–16. doi: 10.1111/dom.12591
18. Zhao Z, Tang Y, Hu Y, Zhu H, Chen X, Zhao B. Hypoglycemia following the use of glucagon-like peptide-1 receptor agonists: a real-world analysis of post-marketing surveillance data. Ann Trans Med (2021) 9(18):1482. doi: 10.21037/atm-21-4162
19. Zeng N, Cutts EJ, Lopez CB, Kaur S, Duran M, Virkus SA, et al. Anatomical and functional characterization of central amygdala glucagon-like peptide 1 receptor expressing neurons. Front Behav Neurosci (2021) 15:724030. doi: 10.3389/fnbeh.2021.724030
20. Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab (2018) 27(4):740–56. doi: 10.1016/j.cmet.2018.03.001
21. Zhao X, Wang M, Wen Z, Lu Z, Cui L, Fu C, et al. GLP-1 receptor agonists: Beyond their pancreatic effects. Front Endocrinol (2021) 12:721135. doi: 10.3389/fendo.2021.721135
22. Webb J, Mount J, von Arx LB, Rachman J, Spanopoulos D, Wood R, et al. Cardiovascular risk profiles: A cross-sectional study evaluating the generalizability of the glucagon-like peptide-1 receptor agonist cardiovascular outcome trials REWIND, LEADER and SUSTAIN-6 to the real-world type 2 diabetes population in the united kingdom. Diabetes Obes Metab (2022) 24(2):289–95. doi: 10.1111/dom.14580
23. Li Y, Glotfelty EJ, Karlsson T, Fortuno LV, Harvey BK, Greig NH. The metabolite GLP-1 (9-36) is neuroprotective and anti-inflammatory in cellular models of neurodegeneration. J Neurochem (2021) 159(5):867–86. doi: 10.1111/jnc.15521
24. Rode AKO, Buus TB, Mraz V, Al-Jaberi FAH, Lopez DV, Ford SL, et al. Induced human regulatory T cells express the glucagon-like peptide-1 receptor. Cells (2022) 11(16):2587. doi: 10.3390/cells11162587
25. Bjornholm KD, Povlsen GK, Ougaard ME, Pyke C, Rakipovski G, Tveden-Nyborg P, et al. Decreased expression of the GLP-1 receptor after segmental artery injury in mice. J Endocrinol (2021) 248(3):289–301. doi: 10.1530/JOE-20-0608
26. Chen J, Mei A, Liu X, Braunstein Z, Wei Y, Wang B, et al. Glucagon-like peptide-1 receptor regulates macrophage migration in monosodium urate-induced peritoneal inflammation. Front Immunol (2022) 13:772446. doi: 10.3389/fimmu.2022.772446
27. Richards P, Parker HE, Adriaenssens AE, Hodgson JM, Cork SC, Trapp S, et al. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes (2014) 63(4):1224–33. doi: 10.2337/db13-1440
28. Hadjiyanni I, Baggio LL, Poussier P, Drucker DJ. Exendin-4 modulates diabetes onset in nonobese diabetic mice. Endocrinology (2008) 149(3):1338–49. doi: 10.1210/en.2007-1137
29. Shiraishi D, Fujiwara Y, Komohara Y, Mizuta H, Takeya M. Glucagon-like peptide-1 (GLP-1) induces M2 polarization of human macrophages via STAT3 activation. Biochem Biophys Res Commun (2012) 425(2):304–8. doi: 10.1016/j.bbrc.2012.07.086
30. Vinué Á, Navarro J, Herrero-Cervera A, García-Cubas M, Andrés-Blasco I, Martínez-Hervás S, et al. The GLP-1 analogue lixisenatide decreases atherosclerosis in insulin-resistant mice by modulating macrophage phenotype. Diabetologia (2017) 60(9):1801–12. doi: 10.1007/s00125-017-4330-3
31. Wang N, Gao J, Jia M, Ma X, Lei Z, Da F, et al. Exendin-4 induces bone marrow stromal cells migration through bone marrow-derived macrophages polarization via PKA-STAT3 signaling pathway. Cell Physiol Biochem (2017) 44(5):1696–714. doi: 10.1159/000485776
32. Shah Z, Kampfrath T, Deiuliis JA, Zhong J, Pineda C, Ying Z, et al. Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation (2011) 124(21):2338–49. doi: 10.1161/CIRCULATIONAHA.111.041418
33. Shirakawa J, Fujii H, Ohnuma K, Sato K, Ito Y, Kaji M, et al. Diet-induced adipose tissue inflammation and liver steatosis are prevented by DPP-4 inhibition in diabetic mice. Diabetes (2011) 60(4):1246–57. doi: 10.2337/db10-1338
34. Sawada N, Adachi K, Nakamura N, Miyabe M, Ito M, Kobayashi S, et al. Glucagon-like peptide-1 receptor agonist liraglutide ameliorates the development of periodontitis. J Diabetes Res (2020) 2020:8843310. doi: 10.1155/2020/8843310
35. Wan S, Sun H. Glucagon-like peptide-1 modulates RAW264.7 macrophage polarization by interfering with the JNK/STAT3 signaling pathway. Exp Ther Med (2019) 17(5):3573–9. doi: 10.3892/etm.2019.7347
36. Hadjiyanni I, Siminovitch KA, Danska JS, Drucker DJ. Glucagon-like peptide-1 receptor signalling selectively regulates murine lymphocyte proliferation and maintenance of peripheral regulatory T cells. Diabetologia (2010) 53(4):730–40. doi: 10.1007/s00125-009-1643-x
37. Liu G, Ma H, Qiu L, Li L, Cao Y, Ma J, et al. Phenotypic and functional switch of macrophages induced by regulatory CD4+CD25+ T cells in mice. Immunol Cell Biol (2011) 89(1):130–42. doi: 10.1038/icb.2010.70
38. Yang L, Chen L, Li D, Xu H, Chen J, Min X, et al. Effect of GLP-1/GLP-1R on the polarization of macrophages in the occurrence and development of atherosclerosis. Mediators Inflamm (2021) 2021:5568159. doi: 10.1155/2021/5568159
39. Mitchell PD, Salter BM, Oliveria JP, El-Gammal A, Tworek D, Smith SG, et al. Glucagon-like peptide-1 receptor expression on human eosinophils and its regulation of eosinophil activation. Clin Exp Allergy (2017) 47(3):331–8. doi: 10.1111/cea.12860
40. Kamuthachad L, Pisuttimarn P, Kasetthat T, Chetchotisakd P, Anunnatsiri S, Sermswan RW, et al. Invariant natural killer T (iNKT) cells response in human melioidosis. Asian Pac J Allergy Immunol. doi: 10.12932/ap-290821-1217
41. Buysschaert M, Baeck M, Preumont V, Marot L, Hendrickx E, Van Belle A, et al. Improvement of psoriasis during glucagon-like peptide-1 analogue therapy in type 2 diabetes is associated with decreasing dermal γδ T-cell number: a prospective case-series study. Br J Dermatol (2014) 171(1):155–61. doi: 10.1111/bjd.12886
42. Moens E, Veldhoen M. Epithelial barrier biology: good fences make good neighbours. Immunology (2012) 135(1):1–8. doi: 10.1111/j.1365-2567.2011.03506.x
43. Yusta B, Baggio LL, Koehler J, Holland D, Cao X, Pinnell LJ, et al. GLP-1R agonists modulate enteric immune responses through the intestinal intraepithelial lymphocyte GLP-1R. Diabetes (2015) 64(7):2537–49. doi: 10.2337/db14-1577
44. Choi E, Yang J, Ji GE, Park MS, Seong Y, Oh SW, et al. The effect of probiotic supplementation on systemic inflammation in dialysis patients. Kidney Res Clin Pract (2021) 41(1):89–101. doi: 10.23876/j.krcp.21.014
45. Smigiel KS, Srivastava S, Stolley JM, Campbell DJ. Regulatory T-cell homeostasis: steady-state maintenance and modulation during inflammation. Immunol Rev (2014) 259(1):40–59. doi: 10.1111/imr.12170
46. Xue S, Wasserfall CH, Parker M, Brusko TM, McGrail S, McGrail K, et al. Exendin-4 therapy in NOD mice with new-onset diabetes increases regulatory T cell frequency. Ann New York Acad Sci (2008) 1150:152–6. doi: 10.1196/annals.1447.049
47. Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol (2011) 12(3):255–63. doi: 10.1038/ni.1993
48. Harbour SN, Maynard CL, Zindl CL, Schoeb TR, Weaver CT. Th17 cells give rise to Th1 cells that are required for the pathogenesis of colitis. Proc Natl Acad Sci United States America (2015) 112(22):7061–6. doi: 10.1073/pnas.1415675112
49. Chiou HC, Lin MW, Hsiao PJ, Chen CL, Chiao S, Lin TY, et al. Dulaglutide modulates the development of tissue-infiltrating Th1/Th17 cells and the pathogenicity of encephalitogenic Th1 cells in the central nervous system. Int J Mol Sci (2019) 20(7):1584. doi: 10.3390/ijms20071584
50. Itoh A, Irie J, Tagawa H, Kusumoto Y, Kato M, Kobayashi N, et al. GLP-1 receptor agonist, liraglutide, ameliorates hepatosteatosis induced by anti-CD3 antibody in female mice. J Diabetes its Complications (2017) 31(9):1370–5. doi: 10.1016/j.jdiacomp.2017.05.013
51. Moschovaki Filippidou F, Kirsch AH, Thelen M, Kétszeri M, Artinger K, Aringer I, et al. Glucagon-like peptide-1 receptor agonism improves nephrotoxic serum nephritis by inhibiting T-cell proliferation. Am J Pathol (2020) 190(2):400–11. doi: 10.1016/j.ajpath.2019.10.008
52. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology (2007) 132(6):2131–57. doi: 10.1053/j.gastro.2007.03.054
53. Manell E, Puuvuori E, Svensson A, Velikyan I, Hulsart-Billstrom G, Hedenqvist P, et al. Exploring the GLP-1-GLP-1R axis in porcine pancreas and gastrointestinal tract in vivo by ex vivo autoradiography. BMJ Open Diabetes Res Care (2021) 9(1):e002083. doi: 10.1136/bmjdrc-2020-002083
54. Meurot C, Jacques C, Martin C, Sudre L, Breton J, Rattenbach R, et al. Targeting the GLP-1/GLP-1R axis to treat osteoarthritis: A new opportunity? J Orthop Translat (2022) 32:121–9. doi: 10.1016/j.jot.2022.02.001
55. Li Z, Feng PP, Zhao ZB, Zhu W, Gong JP, Du HM. Liraglutide protects against inflammatory stress in non-alcoholic fatty liver by modulating kupffer cells M2 polarization via cAMP-PKA-STAT3 signaling pathway. Biochem Biophys Res Commun (2019) 510(1):20–6. doi: 10.1016/j.bbrc.2018.12.149
56. Li Z, Li S, Wang N, Xue P, Li Y. Liraglutide, a glucagon-like peptide-1 receptor agonist, suppresses osteoclastogenesis through the inhibition of NF-κB and MAPK pathways via GLP-1R. Biomed Pharmacother = Biomed Pharmacother (2020) 130:110523. doi: 10.1016/j.biopha.2020.110523
57. Ye Y, Zhong X, Li N, Pan T. Protective effects of liraglutide on glomerular podocytes in obese mice by inhibiting the inflammatory factor TNF-α-mediated NF-κB and MAPK pathway. Obes Res Clin Pract (2019) 13(4):385–90. doi: 10.1016/j.orcp.2019.03.003
58. Ma J, Shi M, Zhang X, Liu X, Chen J, Zhang R, et al. GLP−1R agonists ameliorate peripheral nerve dysfunction and inflammation via p38 MAPK/NF−κB signaling pathways in streptozotocin−induced diabetic rats. Int J Mol Med (2018) 41(5):2977–85. doi: 10.3892/ijmm.2018.3509
59. Madhu D, Hammad M, Kavalakatt S, Khadir A, Tiss A. GLP-1 analogue, exendin-4, modulates MAPKs activity but not the heat shock response in human HepG2 cells. Proteomics Clin Applications (2018) 12(1). doi: 10.1002/prca.201600169
60. He L, Wong CK, Cheung KK, Yau HC, Fu A, Zhao HL, et al. Anti-inflammatory effects of exendin-4, a glucagon-like peptide-1 analog, on human peripheral lymphocytes in patients with type 2 diabetes. J Diabetes Invest (2013) 4(4):382–92. doi: 10.1111/jdi.12063
61. Zhao YY, Chen LH, Huang L, Li YZ, Yang C, Zhu Y, et al. Cardiovascular protective effects of GLP-1: a focus on the MAPK signaling pathway. Biochem Cell Biol = Biochim Biol Cellulaire (2021) 100(1):9–16. doi: 10.1139/bcb-2021-0365
62. Yang Y, Zhou Y, Wang Y, Wei X, Wu L, Wang T, et al. Exendin-4 reverses high glucose-induced endothelial progenitor cell dysfunction via SDF-1β/CXCR7-AMPK/p38-MAPK/IL-6 axis. Acta Diabetol (2020) 57(11):1315–26. doi: 10.1007/s00592-020-01551-3
63. Zhou Y, Li Z, Cao X, Ma H, White PF, Xu X, et al. Exendin-4 improves behaviorial deficits via GLP-1/GLP-1R signaling following partial hepatectomy. Brain Res (2019) 1706:116–24. doi: 10.1016/j.brainres.2018.11.007
64. Zhang LQ, Zhang W, Li T, Yang T, Yuan X, Zhou Y, et al. GLP-1R activation ameliorated novel-object recognition memory dysfunction via regulating hippocampal AMPK/NF-κB pathway in neuropathic pain mice. Neurobiol Learn Memory (2021) 182:107463. doi: 10.1016/j.nlm.2021.107463
65. Wang X, Chen J, Rong C, Pan F, Zhao X, Hu Y. GLP-1RA promotes brown adipogenesis of C3H10T1/2 mesenchymal stem cells via the PI3K-AKT-mTOR signaling pathway. Biochem Biophys Res Commun (2018) 506(4):976–82. doi: 10.1016/j.bbrc.2018.10.197
66. Wu X, Li S, Xue P, Li Y. Liraglutide inhibits the apoptosis of MC3T3-E1 cells induced by serum deprivation through cAMP/PKA/β-catenin and PI3K/AKT/GSK3β signaling pathways. Mol Cells (2018) 41(3):234–43. doi: 10.14348/molcells.2018.2340
67. Shi L, Ji Y, Jiang X, Zhou L, Xu Y, Li Y, et al. Liraglutide attenuates high glucose-induced abnormal cell migration, proliferation, and apoptosis of vascular smooth muscle cells by activating the GLP-1 receptor, and inhibiting ERK1/2 and PI3K/Akt signaling pathways. Cardiovasc Diabetol (2015) 14:18. doi: 10.1186/s12933-015-0177-4
68. Zhang Y, Zhou H, Wu W, Shi C, Hu S, Yin T, et al. Liraglutide protects cardiac microvascular endothelial cells against hypoxia/reoxygenation injury through the suppression of the SR-Ca(2+)-XO-ROS axis via activation of the GLP-1R/PI3K/Akt/survivin pathways. Free Radical Biol Med (2016) 95:278–92. doi: 10.1016/j.freeradbiomed.2016.03.035
69. Wu X, Li S, Xue P, Li Y. Liraglutide, a glucagon-like peptide-1 receptor agonist, facilitates osteogenic proliferation and differentiation in MC3T3-E1 cells through phosphoinositide 3-kinase (PI3K)/protein kinase b (AKT), extracellular signal-related kinase (ERK)1/2, and cAMP/protein kinase a (PKA) signaling pathways involving β-catenin. Exp Cell Res (2017) 360(2):281–91. doi: 10.1016/j.yexcr.2017.09.018
70. Zhao H, Wang L, Wei R, Xiu D, Tao M, Ke J, et al. Activation of glucagon-like peptide-1 receptor inhibits tumourigenicity and metastasis of human pancreatic cancer cells via PI3K/Akt pathway. Diabetes Obes Metab (2014) 16(9):850–60. doi: 10.1111/dom.12291
71. Chang SY, Kim DB, Ryu GR, Ko SH, Jeong IK, Ahn YB, et al. Exendin-4 inhibits iNOS expression at the protein level in LPS-stimulated Raw264.7 macrophage by the activation of cAMP/PKA pathway. J Cell Biochem (2013) 114(4):844–53. doi: 10.1002/jcb.24425
72. Wang YG, Yang TL. Liraglutide reduces oxidized LDL-induced oxidative stress and fatty degeneration in raw 264.7 cells involving the AMPK/SREBP1 pathway. J Geriatr Cardiol (2015) 12(4):410–6. doi: 10.11909/j.issn.1671-5411.2015.04.013
73. Yang J, Wang Z, Zhang X. GLP-1 receptor agonist impairs keratinocytes inflammatory signals by activating AMPK. Exp Mol Pathol (2019) 107:124–8. doi: 10.1016/j.yexmp.2019.01.014
74. Maselli DB, Camilleri M. Effects of GLP-1 and its analogs on gastric physiology in diabetes mellitus and obesity. Adv Exp Med Biol (2021) 1307:171–92. doi: 10.1007/5584_2020_496
75. Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Mol Metab (2021) 46:101102. doi: 10.1016/j.molmet.2020.101102
76. Brown E, Cuthbertson DJ, Wilding JP. Newer GLP-1 receptor agonists and obesity-diabetes. Peptides (2018) 100:61–7. doi: 10.1016/j.peptides.2017.12.009
77. Sandoval DA, D'Alessio DA. Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Physiol Rev (2015) 95(2):513–48. doi: 10.1152/physrev.00013.2014
78. Hope DCD, Vincent ML, Tan TMM. Striking the balance: GLP-1/Glucagon Co-agonism as a treatment strategy for obesity. Front Endocrinol (2021) 12:735019. doi: 10.3389/fendo.2021.735019
79. Lazzaroni E, Ben Nasr M, Loretelli C, Pastore I, Plebani L, Lunati ME, et al. Anti-diabetic drugs and weight loss in patients with type 2 diabetes. Pharmacol Res (2021) 171:105782. doi: 10.1016/j.phrs.2021.105782
80. Cantini G, Mannucci E, Luconi M. Perspectives in GLP-1 research: New targets, new receptors. Trends Endocrinol Metabol: TEM (2016) 27(6):427–38. doi: 10.1016/j.tem.2016.03.017
81. Sélley E, Kun S, Szijártó IA, Kertész M, Wittmann I, Molnár GA. Vasodilator effect of glucagon: Receptorial crosstalk among glucagon, GLP-1, and receptor for glucagon and GLP-1. Hormone Metab Res = Hormon- und Stoffwechselforschung = Hormones Metabol (2016) 48(7):476–83. doi: 10.1055/s-0042-101794
82. Bakbak E, Terenzi DC, Trac JZ, Teoh H, Quan A, Glazer SA, et al. Lessons from bariatric surgery: Can increased GLP-1 enhance vascular repair during cardiometabolic-based chronic disease? Rev Endocrine Metab Disord (2021) 22(4):1171–88. doi: 10.1007/s11154-021-09669-7
83. Hirsch IB. The future of the GLP-1 receptor agonists. Jama (2019) 321(15):1457–8. doi: 10.1001/jama.2019.2941
84. Wen S, Nguyen T, Gong M, Yuan X, Wang C, Jin J, et al. An overview of similarities and differences in metabolic actions and effects of central nervous system between glucagon-like peptide-1 receptor agonists (GLP-1RAs) and sodium glucose Co-Transporter-2 inhibitors (SGLT-2is). Diabetes Metab Syndrome Obes Targets Ther (2021) 14:2955–72. doi: 10.2147/dmso.S312527
85. Zhang L, Zhang W, Tian X. The pleiotropic of GLP-1/GLP-1R axis in central nervous system diseases. Int J Neurosci. (2021) 3:1–38. doi: 10.1080/00207454.2021.1924707
86. He W, Wang H, Zhao C, Tian X, Li L, Wang H. Role of liraglutide in brain repair promotion through Sirt1-mediated mitochondrial improvement in stroke. J Cell Physiol (2020) 235(3):2986–3001. doi: 10.1002/jcp.29204
87. Basalay MV, Davidson SM, Yellon DM. Neuroprotection in rats following ischaemia-reperfusion injury by GLP-1 analogues-liraglutide and semaglutide. Cardiovasc Drugs Ther (2019) 33(6):661–7. doi: 10.1007/s10557-019-06915-8
88. Grieco M, Giorgi A, Gentile MC, d'Erme M, Morano S, Maras B, et al. Glucagon-like peptide-1: A focus on neurodegenerative diseases. Front Neurosci (2019) 13:1112. doi: 10.3389/fnins.2019.01112
89. Nauck MA, Friedrich N. Do GLP-1-based therapies increase cancer risk? Diabetes Care (2013) 36 Suppl 2:S245–52. doi: 10.2337/dcS13-2004
90. Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology (2011) 141(1):150–6. doi: 10.1053/j.gastro.2011.02.018
91. Nachnani JS, Bulchandani DG, Nookala A, Herndon B, Molteni A, Pandya P, et al. Biochemical and histological effects of exendin-4 (exenatide) on the rat pancreas. Diabetologia (2010) 53(1):153–9. doi: 10.1007/s00125-009-1515-4
92. Cao C, Yang S, Zhou Z. GLP-1 receptor agonists and risk of cancer in type 2 diabetes: an updated meta-analysis of randomized controlled trials. Endocrine (2019) 66(2):157–65. doi: 10.1007/s12020-019-02055-z
93. Pinto LC, Falcetta MR, Rados DV, Leitao CB, Gross JL. Glucagon-like peptide-1 receptor agonists and pancreatic cancer: a meta-analysis with trial sequential analysis. Sci Rep (2019) 9(1):2375. doi: 10.1038/s41598-019-38956-2
94. Wicki A, Wild D, Storch D, Seemayer C, Gotthardt M, Behe M, et al. [Lys40(Ahx-DTPA-111In)NH2]-Exendin-4 is a highly efficient radiotherapeutic for glucagon-like peptide-1 receptor-targeted therapy for insulinoma. Clin Cancer Res (2007) 13(12):3696–705. doi: 10.1158/1078-0432.CCR-06-2965
95. Nomiyama T, Kawanami T, Irie S, Hamaguchi Y, Terawaki Y, Murase K, et al. Exendin-4, a GLP-1 receptor agonist, attenuates prostate cancer growth. Diabetes (2014) 63(11):3891–905. doi: 10.2337/db13-1169
96. Iwaya C, Nomiyama T, Komatsu S, Kawanami T, Tsutsumi Y, Hamaguchi Y, et al. Exendin-4, a glucagonlike peptide-1 receptor agonist, attenuates breast cancer growth by inhibiting NF-κB activation. Endocrinology (2017) 158(12):4218–32. doi: 10.1210/en.2017-00461
97. Ligumsky H, Wolf I, Israeli S, Haimsohn M, Ferber S, Karasik A, et al. The peptide-hormone glucagon-like peptide-1 activates cAMP and inhibits growth of breast cancer cells. Breast Cancer Res Treat (2012) 132(2):449–61. doi: 10.1007/s10549-011-1585-0
98. Zhu T, Zhang W, Wang DX, Huang NW, Bo H, Deng W, et al. Rosuvastatin attenuates mucus secretion in a murine model of chronic asthma by inhibiting the gamma-aminobutyric acid type a receptor. Chin Med J (Engl) (2012) 125(8):1457–64. doi: 10.3760/cma.j.issn.0366-6999.2012.08.018
99. Zhu T, Wu XL, Zhang W, Xiao M. Glucagon like peptide-1 (GLP-1) modulates OVA-induced airway inflammation and mucus secretion involving a protein kinase a (PKA)-dependent nuclear factor-kappaB (NF-kappaB) signaling pathway in mice. Int J Mol Sci (2015) 16(9):20195–211. doi: 10.3390/ijms160920195
100. Toki S, Newcomb DC, Printz RL, Cahill KN, Boyd KL, Niswender KD, et al. Glucagon-like peptide-1 receptor agonist inhibits aeroallergen-induced activation of ILC2 and neutrophilic airway inflammation in obese mice. Allergy (2021) 76(11):3433–45. doi: 10.1111/all.14879
101. Hur J, Kang JY, Kim YK, Lee SY, Lee HY. Glucagon-like peptide 1 receptor (GLP-1R) agonist relieved asthmatic airway inflammation via suppression of NLRP3 inflammasome activation in obese asthma mice model. Pulm Pharmacol Ther (2021) 67:102003. doi: 10.1016/j.pupt.2021.102003
102. Alkhouri N, Herring R, Kabler H, Kayali Z, Hassanein T, Kohli A, et al. Safety and efficacy of combination therapy with semaglutide, cilofexor and firsocostat in patients with non-alcoholic steatohepatitis: A randomised, open-label phase II trial. J Hepatol (2022) 77(3):607–18. doi: 10.1016/j.jhep.2022.04.003
Keywords: glucagon-like peptide-1, liraglutide, inflammatory diseases, immunomodulation, signal pathways
Citation: Chen J, Mei A, Wei Y, Li C, Qian H, Min X, Yang H, Dong L, Rao X and Zhong J (2022) GLP-1 receptor agonist as a modulator of innate immunity. Front. Immunol. 13:997578. doi: 10.3389/fimmu.2022.997578
Received: 19 July 2022; Accepted: 24 November 2022;
Published: 08 December 2022.
Edited by:
Jorg Hermann Fritz, McGill University, CanadaReviewed by:
Cristian Loretelli, University of Milan, ItalyStokes Peebles, Vanderbilt University Medical Center, United States
Copyright © 2022 Chen, Mei, Wei, Li, Qian, Min, Yang, Dong, Rao and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jixin Zhong, emhvbmdqaXhpbjYyMEAxNjMuY29t; Xiaoquan Rao, eHFyYW9AdGpoLnRqbXUuZWR1LmNu; Lingli Dong, dGpoZG9uZ2xsQDE2My5jb20=
†These authors have contributed equally to this work
 Jun Chen
Jun Chen Aihua Mei1†
Aihua Mei1† Hang Qian
Hang Qian Lingli Dong
Lingli Dong Xiaoquan Rao
Xiaoquan Rao Jixin Zhong
Jixin Zhong