
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
GENERAL COMMENTARY article
Front. Immunol., 11 February 2020
Sec. Inflammation
Volume 11 - 2020 | https://doi.org/10.3389/fimmu.2020.00173
This article is a commentary on:
Triggering Receptor Expressed on Myeloid Cells-1 Inhibitor Targeted to Endothelium Decreases Cell Activation
A Commentary on
Triggering Receptor Expressed on Myeloid Cells-1 Inhibitor Targeted to Endothelium Decreases Cell Activation
by Gibot, S., Jolly, L., Lemarié, J., Carrasco, K., Derive, M., and Boufenzer, A. Front. Immunol. (2019) 10:2314. doi: 10.3389/fimmu.2019.02314
Triggering receptor expressed on myeloid cells-1 (TREM-1), an inflammation amplifier, first reported in 2000 (1) was initially demonstrated to play a role in sepsis (2). Currently, the crucial pathophysiological role of TREM-1 is defined not only in infectious diseases but also in both acute and chronic forms of aseptic inflammation (3) as well as in different types of cancer (4, 5). Examples are ischemia-reperfusion, hemorrhagic shock, pancreatitis, spinal cord injury, inflammatory bowel diseases, rheumatic diseases, retinopathy, liver diseases, atherosclerosis, psoriasis, cystic fibrosis, Parkinson's disease, lung cancer, pancreatic cancer, liver cancer, and colon cancer. This implicates TREM-1 as a new, highly promising multi-indication therapeutic target.
Conventional TREM-1 inhibitors such as either TREM-1 inhibitory peptides LP17 and LR12 first reported in 2006 (6) and 2013 (7), respectively, or an antibody against TREM-1 first reported in 2016 (8), all attempt to block the receptor binding to its ligand(s). However, the actual nature of the TREM-1 ligand(s) is still uncertain, emphasizing the hurdles that need to be overcome before TREM-1-targeted therapy can become a clinical reality.
To address this problem, we applied our model of receptor-mediated transmembrane signaling, the Signaling Chain HOmoOLigomerization (SCHOOL), first published in 2004 (9, 10) to rationally design TREM-1-specific inhibitory peptide sequence(s) (SCHOOL peptides/sequences) that employ a novel, ligand-independent mechanism of TREM-1 inhibition. We then successfully demonstrated high efficacy of these peptides/sequences in a variety of in vitro and in vivo studies (Table 1). Recently, therapeutic efficacy of the SCHOOL peptides/sequences has been independently confirmed in a mouse model of liver cancer [(15); Table 1].
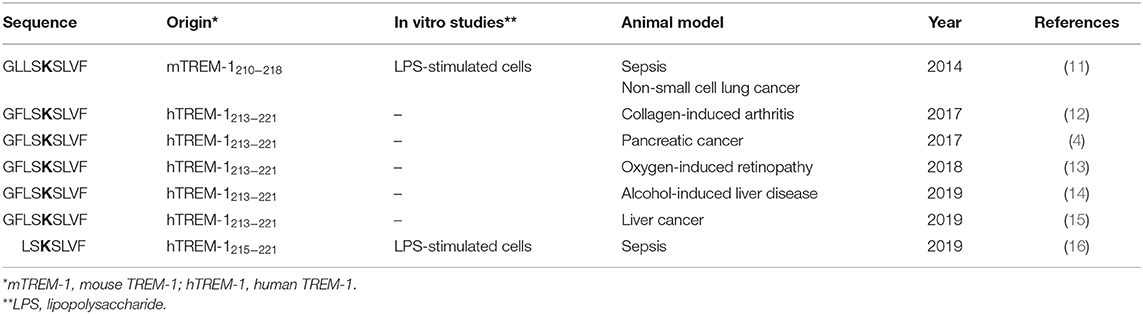
Table 1. Published in vitro and in vivo studies of TREM-1 inhibitory peptide sequences that employ a ligand-independent molecular mechanism of TREM-1 inhibition (SCHOOL inhibitors).
The recently published manuscript by Gibot et al. (16) describes the use of ligand-independent modulation of TREM-1 to reduce lipopolysaccharide (LPS)-induced cell activation and confer protection during experimental sepsis in mice. To inhibit TREM-1 in a ligand-independent manner, the authors used a peptide sequence LSKSLVF (Table 1), which they claimed they rationally designed.
In this regard, we thought it proper to remind the readership of Frontiers in Immunology of our pioneering study of 2014 that demonstrated the therapeutic effect of a first-in-class ligand-independent TREM-1 inhibitory peptide sequence GLLSKSLVF (mouse TREM-1-specific
SCHOOL peptide) in experimental sepsis [(11); Table 1]. In this study, we presented for the first time, direct evidences that GLLSKSLVF suppresses TREM-1-mediated production of proinflammatory cytokines TNF-α, IL-1β, and IL-6 both in vitro (LPS-stimulated cells) and in vivo (LPS-challenged mice) as well as significantly prolongs survival of mice with LPS-induced septic shock (11). We specifically demonstrated that a control peptide (GLLSGSLVF) with single amino acid substitution of functionally important lysine (highlighted in bold type in Table 1) for glycine does not exhibit TREM-1 inhibitory effect, as it has been predicted by the SCHOOL model (10, 11).
We note that the paper by Gibot et al. does not refer to this previously published work (11). It should be also noted that in our another study, one of the cancer studies cited [Shen and Sigalov (4) in the paper by Gibot et al. (16)], we used a ligand-independent human TREM-1 inhibitory peptide GFLSKSLVF (GF9), not peptide LR12, to suppress tumor growth and prolong survival of mice with experimental pancreatic cancer (4).
In summary, we believe that it is important that our novel and clinically relevant ligand-independent approach to modulation of diverse immune receptors including TREM-1 [recently reviewed in Sigalov (17)] attracts more and more attention from the scientific and industrial community.
AS conceived and wrote the manuscript.
This work was supported by grants R43GM128369 from the National Institute of General Medical Sciences (NIGMS), R43EY028779 from the National Eye Institute (NEI), and R44CA217400 from the National Institute of Cancer (NCI). NIGMS, NEI, and NCI are components of the National Institutes of Health (NIH).
AS is employed by SignaBlok, Inc., a company developing ligand-independent TREM-1 inhibitors.
1. Bouchon A, Dietrich J, Colonna M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol. (2000) 164:4991–5. doi: 10.4049/jimmunol.164.10.4991
2. Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. (2001) 410:1103–7. doi: 10.1038/35074114
3. Tammaro A, Derive M, Gibot S, Leemans JC, Florquin S, Dessing MC. TREM-1 and its potential ligands in non-infectious diseases: from biology to clinical perspectives. Pharmacol Ther. (2017) 177:81–95. doi: 10.1016/j.pharmthera.2017.02.043
4. Shen ZT, Sigalov AB. Novel TREM-1 inhibitors attenuate tumor growth and prolong survival in experimental pancreatic cancer. Mol Pharm. (2017) 14:4572–82. doi: 10.1021/acs.molpharmaceut.7b00711
5. Nguyen AH, Berim IG, Agrawal DK. Chronic inflammation and cancer: emerging roles of triggering receptors expressed on myeloid cells. Expert Rev Clin Immunol. (2015) 11:849–57. doi: 10.1586/1744666X.2015.1043893
6. Gibot S, Alauzet C, Massin F, Sennoune N, Faure GC, Bene MC, et al. Modulation of the triggering receptor expressed on myeloid cells-1 pathway during pneumonia in rats. J Infect Dis. (2006) 194:975–83. doi: 10.1086/506950
7. Derive M, Boufenzer A, Bouazza Y, Groubatch F, Alauzet C, Barraud D, et al. Effects of a TREM-like transcript 1-derived peptide during hypodynamic septic shock in pigs. Shock. (2013) 39:176–82. doi: 10.1097/SHK.0b013e31827bcdfb
8. Brynjolfsson SF, Magnusson MK, Kong PL, Jensen T, Kuijper JL, Hakansson K, et al. An antibody against triggering receptor expressed on myeloid cells 1 (TREM-1) dampens proinflammatory cytokine secretion by lamina propria cells from patients with IBD. Inflamm Bowel Dis. (2016) 22:1803–11. doi: 10.1097/MIB.0000000000000822
9. Sigalov AB. Multichain immune recognition receptor signaling: different players, same game? Trends Immunol. (2004) 25:583–9. doi: 10.1016/j.it.2004.08.009
10. Sigalov AB. Immune cell signaling: a novel mechanistic model reveals new therapeutic targets. Trends Pharmacol Sci. (2006) 27:518–24. doi: 10.1016/j.tips.2006.08.004
11. Sigalov AB. A novel ligand-independent peptide inhibitor of TREM-1 suppresses tumor growth in human lung cancer xenografts and prolongs survival of mice with lipopolysaccharide-induced septic shock. Int Immunopharmacol. (2014) 21:208–19. doi: 10.1016/j.intimp.2014.05.001
12. Shen ZT, Sigalov AB. Rationally designed ligand-independent peptide inhibitors of TREM-1 ameliorate collagen-induced arthritis. J Cell Mol Med. (2017) 21:2524–34. doi: 10.1111/jcmm.13173
13. Rojas MA, Shen ZT, Caldwell RB, Sigalov AB. Blockade of TREM-1 prevents vitreoretinal neovascularization in mice with oxygen-induced retinopathy. Biochim Biophys Acta. (2018) 1864:2761–8. doi: 10.1016/j.bbadis.2018.05.001
14. Tornai D, Furi I, Shen ZT, Sigalov AB, Coban S, Szabo G. Inhibition of triggering receptor expressed on myeloid cells 1 ameliorates inflammation and macrophage and neutrophil activation in alcoholic liver disease in mice. Hepatol Commun. (2019) 3:99–115. doi: 10.1002/hep4.1269
15. Wu Q, Zhou W, Yin S, Zhou Y, Chen T, Qian J, et al. Blocking triggering receptor expressed on myeloid cells-1-positive tumor-associated macrophages induced by hypoxia reverses immunosuppression and anti-programmed cell death ligand 1 resistance in liver cancer. Hepatology. (2019) 70:198–214. doi: 10.1002/hep.30593
16. Gibot S, Jolly L, Lemarie J, Carrasco K, Derive M, Boufenzer A. Triggering receptor expressed on myeloid cells-1 inhibitor targeted to endothelium decreases cell activation. Front Immunol. (2019) 10:2314. doi: 10.3389/fimmu.2019.02314
Keywords: TREM-1, sepsis, SCHOOL model of receptor signaling, ligand-independent inhibition, TREM-1 inhibitory SCHOOL peptides
Citation: Sigalov AB (2020) Commentary: Triggering Receptor Expressed on Myeloid Cells-1 Inhibitor Targeted to Endothelium Decreases Cell Activation. Front. Immunol. 11:173. doi: 10.3389/fimmu.2020.00173
Received: 23 November 2019; Accepted: 22 January 2020;
Published: 11 February 2020.
Edited by:
Pietro Ghezzi, Brighton and Sussex Medical School, United KingdomReviewed by:
Markus Philipp Radsak, University Medical Centre, Johannes Gutenberg University Mainz, GermanyCopyright © 2020 Sigalov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexander B. Sigalov, c2lnYWxvdkBzaWduYWJsb2suY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.