
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Hum. Neurosci. , 25 June 2020
Sec. Cognitive Neuroscience
Volume 14 - 2020 | https://doi.org/10.3389/fnhum.2020.00196
Neural synchronization patterns are involved in several complex cognitive functions and constitute a growing trend in neuroscience research. While synchrony patterns in working memory have been extensively discussed, a complete understanding of their role in cognitive control and inhibition is still elusive. Here, we provide an up-to-date review on synchronization patterns underlying behavioral inhibition, extrapolating common grounds, and dissociating features with other inhibitory functions. Moreover, we suggest a schematic conceptual framework and highlight existing gaps in the literature, current methodological challenges, and compelling research questions for future studies.
Investigating the relationship between cognitive function and underlying cerebral activity has been, and still is, one of the greatest neuroscientific challenges. Functional magnetic resonance imaging (fMRI) is a leading imaging method for quantifying and mapping the geographical distribution of metabolic changes associated with brain activity, while resting (Riedl et al., 2016) or actively processing information (Chen and Glover, 2015). Electroencephalography (EEG) is a well-established electrophysiological technique providing a temporally accurate recording of postsynaptic superficial brain activity (Burle et al., 2015), safely and non-invasively (Cohen, 2017), at rest or during task performance (Zani and Proverbio, 2003). Together with magneto-electroencephalography (MEG), EEG has extensively contributed to the understanding of how the brain’s oscillations at different frequencies relate to specific mental states and processes (Benedek et al., 2014). Moreover, it permits to measure local alterations in amplitude, phase, and synchrony, and to explore spatial and temporal distributions associated with specific cognitive functions (Perfetti et al., 2011; Groppe et al., 2013; Roux and Uhlhaas, 2014), such as attention and memory. This article will review the current knowledge of the patterns of focal and large-scale coordination supporting cognitive control and inhibition.
Increased EEG/MEG amplitude, power and event-related synchronization (ERS), or desynchronization (ERD), within local circuits and specific frequencies support distinct cognitive processes, including sensory processing and memory (reviewed in Uhlhaas et al., 2008; Roohi-Azizi et al., 2017). However, functional connectivity reports suggest that more complex cognitive tasks, involving a dynamic combination of cognitive processes, require a fast and adjustable information exchange between brain circuits of large scale (Hampshire et al., 2012). More emphasis is thus now given to investigating coordination processes between long-range neural networks and the underlying neurobiological mechanisms during more demanding cognitive functions (Fries, 2005; Fell and Axmacher, 2011; Kazanovich, 2019; Wang et al., 2019).
Phase-synchronization processes ease the information exchange within distributed brain networks, increasing network efficiency, and facilitating synaptic plasticity (Varela et al., 2001; Fries, 2005, 2015; Womelsdorf et al., 2007; Deco et al., 2011; Fell and Axmacher, 2011; Parkin et al., 2015; Constantinidis and Klingberg, 2016; Violante et al., 2017). The importance of intact long-range synchronization dynamics is evident in clinical contexts, where cognitively impaired Alzheimer’s patients display significantly decreased phase-coordination between most cortical regions in the delta band, relative to controls (Hata et al., 2016). This calls for the need for further investigations and the development of methods (e.g., Pesaran et al., 2018; Widge et al., 2019a) to study synchronization patterns between large-scale networks and the underlying synaptic mechanisms, as well as their alterations in different neuropsychiatric diseases.
The study of large-scale synchronization implies recording neural activity contemporaneously from distributed brain locations before assessing whether the activity at different loci alters in a synchronous fashion (Nowak et al., 2017). Activity within single voxels or region-of-interests is tracked measuring the correlation across them over time series (Harris and Gordon, 2015). Long-range phase-synchronization dynamics between large-scale circuits can be explored within the same, or between a broad span of, different frequencies in EEG/MEG. Phase-coordination in different frequencies is a type of cross-frequency coupling (CFC), called “cross-frequency phase-phase coupling” (Palva et al., 2005). Phase-amplitude coupling is another type of CFC, which describes the synchronization of the phase of a low-frequency rhythm to the amplitude/power of a higher-frequency rhythm (Canolty and Knight, 2010).
Long-range phase-coordination between distributed frontal/executive and sensory networks is associated to increased cognitive demand, as a result of increased sensory-processing (Crespo-Garcia et al., 2013), manipulation of sensory information in working memory (Sauseng et al., 2005), as well as memory encoding and retrieval (Schack and Klimesch, 2002; Sauseng et al., 2004; Schack et al., 2005). The contribution of different network components in a given task is dynamic in time and extent and depends on the specific cognitive requirement.
Cross-frequency phase-phase/amplitude coupling dynamics have been described in working memory processes. However, it is yet to be established how they apply to behavioral inhibition, a complex function that relies on a combination of cognitive processes, including attention, working memory, action selection (Hampshire et al., 2007; Stokes et al., 2013; Provenza et al., 2019; Widge et al., 2019a), and that is likewise distributed across brain networks (Erika-Florence et al., 2014; Hampshire and Sharp, 2015). Existing knowledge and evidence in this regard will be reviewed and elucidated in the following sections.
Executive control is a major cognitive function comprising several sub-functions (Jewsbury et al., 2016; Purpura et al., 2017), including attentional control and working memory. But it encompasses also inhibitory control or inhibition (Jones et al., 2016), which regulates flexible and adaptive overt responses as well as purpose-directed mental processes (Stuphorn and Emeric, 2012). The ability to inhibit an internal process, or to interfere with external information is generally referred to as inhibitory control or, simply, inhibition (Xie et al., 2017). The latter is, in turn, distinguished between behavioral or response inhibition, which refers to the process of suppressing an ongoing motor action, whenever necessary (e.g., to implement an alternative response; Aron, 2007). The most established paradigms used to study behavioral inhibition are summarized in Box 1. Cognitive inhibition (Bari and Robbins, 2013), instead, involves the blockade of a mental process, such as selective attention or memory retrieval (MacLeod, 2007), either intentionally or unconsciously (Harnishfeger, 1995). A schematic representation of the different inhibitory subfunctions is shown in Figure 1.
Box 1. Classical response inhibition tasks
A variety of behavioral tasks have been developed and used to study the neural underpinnings of behavioral inhibition, among which the most established constitute the Go/No-go task (GNGT; e.g., Luijten et al., 2011; Uzefovsky et al., 2016) and stop-signal task (SST; e.g., Jahfari et al., 2010; Leunissen et al., 2016). Alternative paradigms include the antisaccade (e.g., Tervo-Clemmens et al., 2017; Fernandez-Ruiz et al., 2018) and the delayed-gratification tasks (e.g., Jiang et al., 2018).
In the GNGT, the subject is presented with a series of different stimuli (e.g., arrows on a screen) and must respond to those defined as target (e.g., left-pointing arrows) by taking a given action (e.g., button-press) as fast as possible. Upon occurrence of any non-target stimuli (e.g., right-pointing arrows) the participant must instead suppress the response and not press. The task can be implemented using different stimuli, sensory modalities and response effectors. The GNGT performance can be quantified in terms of reaction time to target stimuli (aka “go-trials”) and frequency of correct/incorrect presses and correct/incorrect suppressions, which would define the accuracy.
In the SST, the subject must respond to different stimuli (e.g., left/right-pointing arrows) by selecting the corresponding response option (i.e., left/right button-press based on arrow orientation) and inhibit the response whenever an additional infrequent stimulus (e.g., audio-tone), namely the stop-signal (SS), is presented (Ko et al., 2016). Performance on the SST can be quantitatively modeled as a horse-race model (Logan et al., 1984), where a competition between the “go” and “stop” processes determines behavior, producing an estimation of the SS reaction time (SSRT), which is the time necessary for suppressing the motor response (Band et al., 2003; Boucher et al., 2007). The latter largely depends on the type of effectors chosen for selecting the response, with estimations averaging from 130 ms in the saccade SST (Hanes and Carpenter, 1999) to 250 ms in the manual SST (Boucher et al., 2007). The delay of the SS relative to the antecedent (go) stimulus determines the stop success probability (Logan et al., 1984). In the classical SST, the SS-latency is defined through a staircase design, which enables to adjust the paradigm to the individual performance, narrowing it on to the 50% success probability of making the stop (Erika-Florence et al., 2014). Other relevant outcome measures include direction errors, percentage of successful stops, and RT in go-trials (Stuphorn and Emeric, 2012).
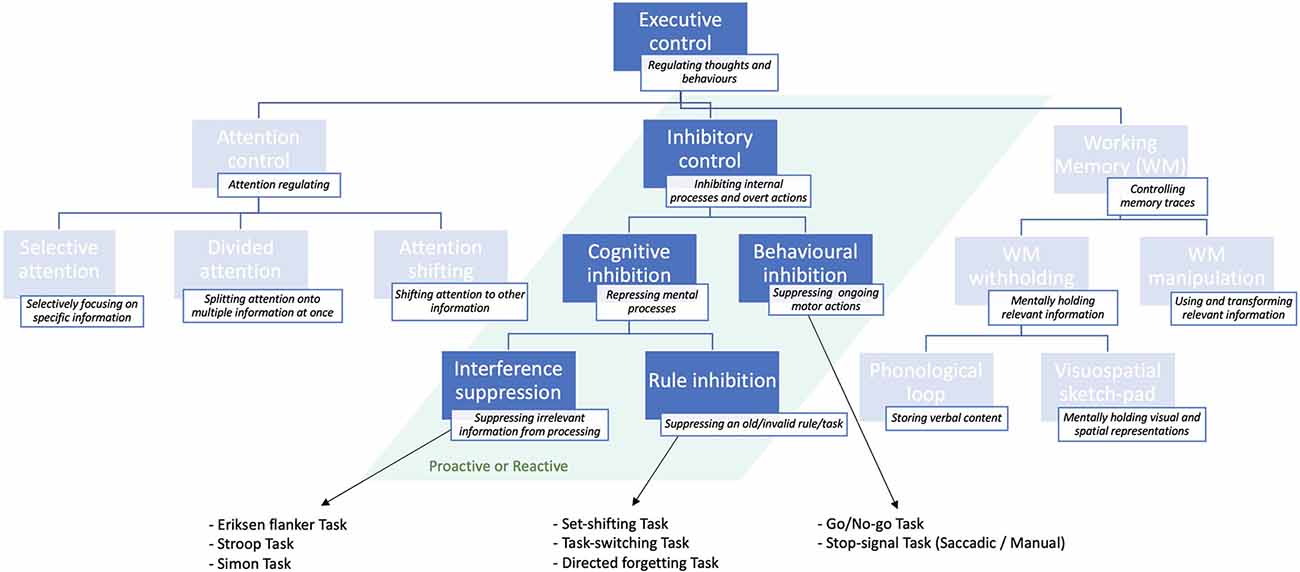
Figure 1. Schematic illustration of the inhibitory sub-functions of executive control presented in descending order of specificity: from more general (top) to more specific (bottom) and classical tasks that are used to study them. For a more exhaustive overview of executive control sub-functions, see Jones et al. (2016).
Cognitive inhibition can be further distinguished into sub-types. The act of preventing irrelevant sensory information from undergoing further processing in working memory (Wilson and Kipp, 1998; Diamond et al., 2013) is known as interference suppression (Nigg, 2000), and can be assessed through the Eriksen flanker task (Eriksen and Eriksen, 1974). This task requires the subject to focus on the target letter in the center, ignoring the neighboring letters (flankers), which are either matching, neutral, or unmatching the central letter concerning a specific feature (e.g., color, shape, size). Such experiments demonstrated that people are generally slower and more inaccurate at responding in target-unmatching, relative to target-matching, trials (Eriksen, 1995). This finding is known as “stimulus-response compatibility” effect (e.g., Richez et al., 2016). Variants to the Eriksen paradigm include the Stroop task (Stroop, 1935), and the Simon task (Simon and Wolf, 1963).
The process of actively suppressing an invalid rule or “task-goal” is instead known as rule inhibition (Xie et al., 2017), and is typically studied with directed forgetting and “task-switching” or “set-shifting” paradigms (Nigg, 2000; Monsell, 2003; Koch et al., 2010). As opposed to interference suppression, rule inhibition involves a working memory component, in that subjects must actively suppress an old (invalid) task-goal or rule, while mentally maintaining and applying the new (valid) task/rule (Xie et al., 2017).
Within the scientific literature, a distinction is often made between the proactive and reactive forms of inhibitory control (Braver et al., 2008; Braver, 2012). Proactive inhibition represents an anticipatory form of selection, by which goal-relevant information is actively and continuously maintained in working memory to direct attentional, perceptual, and motor systems (Miller and Cohen, 2001). In other words, it cues attention according to the current goal, preventing interference, and thus allowing optimal performance (Stuphorn and Emeric, 2012). Reactive inhibition is, instead, a late form of control acting as a corrective mechanism that is transiently implemented when encountering an interfering event (Jacoby et al., 1999). It allows a reformulation of the goal based on episodic associations (i.e., previous experience) or/and interference demands (Stuphorn and Emeric, 2012). The proactive and reactive inhibition mechanisms complement each other in terms of advantages and disadvantages, since the first is less prone to interference, although more cognitively demanding than the second (Braver et al., 2008; Mäki-Marttunen et al., 2019). Proactive and reactive inhibitory control have been probed in both behavioral inhibition (Verbruggen and Logan, 2009; Benis et al., 2014; Castro-Meneses et al., 2015) and task-switching studies (Braver et al., 2008; Karayanidis and Jamadar, 2014).
The neural basis of inhibitory control functions is a research topic that has received conspicuous attention in the cognitive neurosciences in the last few decades. This focus is in part because deficits of response inhibition and cognitive flexibility characterize several neuropsychological conditions, including obsessive-compulsive disorder (OCD; McLaughlin et al., 2016), schizophrenia (Hughes et al., 2012), as well as, post-traumatic-stress-disorder (PTSD; Clausen et al., 2017), depression (Katz et al., 2010), drug-addictions (Morein-Zamir and Robbins, 2015; Wang et al., 2018) and attention-deficit-hyperactivity-disorder (ADHD; Hwang et al., 2019).
Functional neuroimaging studies have provided essential insights into the distribution of the cortical circuits underlying behavioral inhibition. The earliest works have built extensively on a modular view of the inhibitory functions, supporting that the right inferior frontal gyrus (IFG) and the anterior insula (AI) are brain regions specifically devoted to response inhibition (Aron et al., 2003, 2004), primarily based on the association of these areas’ activity with the successful withholding of automated (go-signal) responses in stop-signal tasks (SSTs; Rubia et al., 2001a,b). In support of this, selective disruptions of IFG activity compromise SST performance (Aron et al., 2003). Clinically, dysfunctional activations of the IFG and AI are observed in subjects with impulse-control disorders (Rubia et al., 1999, 2014; Seeley et al., 2009; Jilka et al., 2014). However, this is arguably overly simplistic, since the activation of these two regions is not specific to behavioral inhibition (Shallice et al., 2008; Hampshire et al., 2010; Sharp et al., 2010; Erika-Florence et al., 2014; Hampshire, 2015).
Saccadic and manual SST studies have demonstrated that, additionally to the IFG and AI, a wide circuit of frontoparietal structures, including the supplementary eye fields, the supplementary and pre-supplementary motor cortices (Curtis et al., 2005; Aron and Poldrack, 2006; Li et al., 2006; Aron et al., 2007) and the intraparietal sulcus (Osada et al., 2019), support response inhibition in collaboration with the limbic basal ganglia. Notably, the striatum (Zandbelt and Vink, 2010; Mallet et al., 2016) and the subthalamic nucleus (Brittain et al., 2012; Alegre et al., 2013).
Functional connectivity analyses have probed the co-activation of the IFC and AI with spatially distributed subcortical and frontoparietal structures (Dosenbach et al., 2008; Mostofsky and Simmonds, 2008; Duann et al., 2009; Hampshire et al., 2012; Zhang and Li, 2012; Cai et al., 2019) that compose the Multiple Demand Cortex (MDC; Hampshire and Sharp, 2015), whose contribution in behavioral inhibition differs in the extent, depending on the sensory modality and cognitive demands (Erika-Florence et al., 2014). Relative to the AI, the IFG is more involved in implementing inhibitory control and more strongly connected to the dorsomedial PFC and lateral frontoparietal cortices (Cai et al., 2014). The AI, instead, predominantly deals with the detection of salient inhibitory cues and shows a stronger intrinsic functional connectivity with the ACC (Cai et al., 2017) and the STN (Cai et al., 2019). The latter contributes to proactive and reactive inhibitory processes through distinct EEG spectral patterns (Benis et al., 2014).
Response-inhibition is produced by inhibitory processes that are ubiquitous in the human brain, namely lateral inhibition and top-down potentiation (Desimone and Duncan, 1995; Chelazzi et al., 1998). These are enacted at the level of both local neuronal populations and long-range networks (MacLeod, 2007; Hampshire and Sharp, 2015). Motor responses in the SST are modulated and adjusted online by top-down (or feedforward) control signals originating from the MDC (Hampshire and Sharp, 2015) and by bottom-up (or feedback) processes of lateral-inhibition, occurring at the level of local sensorimotor neuronal populations (Boucher et al., 2007; Schall and Godlove, 2012) that support competing motor programs (Munakata et al., 2011).
While button presses to go-signals are automated responses produced via direct sensorimotor mappings, blocking a routine response is a non-automated process that requires the additional intervention of higher-order frontoparietal circuits (Hampshire and Sharp, 2015). The detection of a stop-signal results in the activation of the MDC (Hampshire et al., 2007; Stokes et al., 2013; Erika-Florence et al., 2014) and the sensorimotor cortex (Hampshire et al., 2007; Erika-Florence et al., 2014). The first would reinforce the motor program for the stop, while downregulating the sensorimotor representations via lateral-inhibition, decelerating the “go-response” and thus producing the stop outcome (Hampshire and Sharp, 2015). Upon training, response-withholding will eventually become automatic (learning), no longer requiring top-down adjustments (Erika-Florence et al., 2014; Widge et al., 2019a).
While fMRI and connectivity reports have spatially localized the neural correlates of inhibition (Curtis et al., 2005; Aron and Poldrack, 2006; Li et al., 2006; Aron et al., 2007; Zandbelt and Vink, 2010; Brittain et al., 2012; Alegre et al., 2013; Erika-Florence et al., 2014; Hampshire and Sharp, 2015; Mallet et al., 2016), electrophysiological methods can provide precise information about the development across the time of the stop-detection and response-suppression processes, exploiting their high temporal resolution. It would be especially important to explore the chronological dynamics by which the sensorimotor cortices are active, and by which the frontoparietal circuits exert their modulatory (i.e., inhibitory) action over the motor output.
Several EEG/MEG studies of response inhibition have identified characteristic event-related potential (ERP) components (i.e., N2/P2 complex) in association to the stop/no-go, but not the go, trials, in both the SST (Ramautar et al., 2006; González-Villar et al., 2016) and the Go/No-go task (GNGT; Falkenstein et al., 2002; Nieuwenhuis et al., 2004; Johnstone et al., 2007). These consist of a frontomedial negative component arising 200–300 ms after the occurrence of the stop-signal (SS), succeeded, after about 150 ms, by a frontomedial and parietomedial positive deflection. However, as we will discuss in the next paragraphs, these ERP components are not specific to behavioral inhibition, as similar patterns have also been reported in cognitive inhibition processes in Stroop (Liotti et al., 2000; Bruchmann et al., 2010), Flanker (Kopp et al., 1996) and task-switching paradigms (Karayanidis and Jamadar, 2014).
Intracranial stereoelectroencephalography (SEEG) and electrocorticography (ECoG) are invasive electrophysiological recordings of brain activity (Young et al., 2019). Although only applied to clinical populations, the direct recording from the brain tissue allows a relatively superior geographical resolution. The assessment of large-scale LFP synchronization dynamics can provide potential insights into the exact source of top-down inhibitory inputs (Widge et al., 2019a). Increased demands of top-down control, due to conflict (e.g., interference, stop-signal) detection, are indexed by cross-frequency ECoG coupling between prefrontal theta phase and the amplitude of primary motor high-frequency oscillations (Voytek et al., 2015). ECoG theta coupling accompanies information exchange from fronto medial to parietal areas upon error feedback in a Stroop-like paradigm (Smith et al., 2015), likely acting as a modulatory attentional mechanism over motor areas, augmenting the stimulus-detection probability. Theta synchrony circuits during conflict detection also convolve the dorsal cingulate cortex and subcortical structures (Provenza et al., 2019; Smith et al., 2019).
Successful response-inhibition in the GNGT provides SEEG gamma synchrony within the default mode network and the limbic system (Laviolette, 2007; Arnulfo et al., 2018). The affective attribute of response-withholding is further probed by facial electromyography, where the corrugator supercilii, a muscle closely associated with negative affect, shows higher activity in no-go, relative to go, trials (Clancy et al., 2019). This suggests that response-inhibition may negatively affect the emotional/motivational connotation of the response-associated stimulus. This is compatible with the presence of inhibitory deficits in psychiatric conditions, such as major depression and schizophrenia, involving dopaminergic dysregulations in the limbic-prefrontal (mesocortical) projection (Patel et al., 2010; Grace, 2012; Belujon and Grace, 2017). Brain stimulation (Dubreuil-Vall et al., 2019; Widge et al., 2019b) of control circuits can indeed restore both clinical symptoms and cognitive deficits in clinical populations.
Investigating the functional correlates of behavioral inhibition requires the isolation of the mere behavioral act of response-withholding from its cognitive component: conflict detection or interference/stop-expectancy. In this regard, Chikazoe et al. (2009) designed a modified SST that enabled the separation of response inhibition from SS-expectancy or RT slowing, by introducing “certain go-trials” in which the SS never occurs, in addition to “uncertain go-trials” where a SS may occur, as in the classical SST. By comparing RTs to certain and uncertain go-trials, it emerged that slowing of RT to go-trials reflects the subject’s SS-expectancy and proactive inhibition, thus improving the SS reaction time (SSRT).
Stop-expectancy can be quantified trial-by-trial as stop-occurrence-probability from a dynamic Bayesian model (Yu and Cohen, 2009) and behaviorally, it correlates with RT slowing to go-signals. The spectral correlates of stop-expectancy and RT-slowing seem to be inversely related across trials (Chang et al., 2017). Stop-anticipation is accompanied by a pronounced low-theta activity in the supramarginal gyrus (SMG) and anterior SMC preceding, but not after, the occurrence of the go-signal. Slowing of RT is instead negatively associated with IFG and posterior delta-theta activity. The results suggest that stop-expectancy and response-inhibition are processed by distinct frontoparietal networks, in coordination with temporally distinguished theta contributions (Chang et al., 2017). The evidence supports earlier-discussed fMRI findings (Hu et al., 2015a,b; Manza et al., 2016) in that proactive behavioral inhibition does not map onto a specific brain region, but, instead, results from the interaction between distributed frontoparietal MDC networks (Hampshire and Sharp, 2015).
Furthermore, a simultaneous fMRI-EEG SST study (Ko et al., 2016) has shown that beta synchronization in the right medial frontal gyrus (rMFG) after the go-stimuli precedes alpha-beta suppression in the preSMA in the stop-, as opposed to go-, trials. The findings align with Chang et al. (2017) supporting that response inhibition is mediated by beta and theta activity in communication with the same MDC components. In a previous work, Swann et al. (2009) observed a stronger IFG beta (16 Hz) activity occurring 100–250 ms after the SS onset, in successful, compared to unsuccessful stop trials, accompanied by reduced synchronization in the primary motor cortex, possibly reflecting increased GABA-mediated inhibition.
Taken together, the evidence suggests that behavioral inhibition is implemented via IFG beta and preSMC theta activities, in communication with other frontoparietal and basal ganglia circuits, with downstream effects on the M1. Importantly, beta activity during response-inhibition processes shows opposite patterns in different MDC components, decrementing in the preSMC, but increasing in the IFG.
Interference suppression and response-inhibition activate spatially overlapping, yet distinguishable, ERP correlates in combined GNGT and flanker task studies (Johnstone et al., 2009; Brydges et al., 2012, 2013; Van Velzen et al., 2014; Vuillier et al., 2016), supporting a functional dissociation between the two inhibitory subfunctions. Target-matching trials give rise to a stronger N2 component, compared to unmatching ones. However, while the P3 amplitude is higher in congruent trials involving response-suppression compared to those that do not, the N2 component seems to be unaffected (Groom and Cragg, 2015).
In a modified flanker task that allows to contemporaneously assess different inhibitory control sub-functions, Xie et al. (2017) observed that while interference suppression originates a larger frontal N2 compared to non-inhibitory trials, rule inhibition induces higher frontal P3a amplitudes, reflecting the criticality of frontal circuits in cognitive control, via online adjustments of stimulus-response associations. Behavioral inhibition instead shows a more substantial posterior P3b component, presumably indexing motor re-programming. Consistently, time-frequency EEG analyses confirm a fronto medial (Fz) involvement in different inhibitory sub-functions (Cavanagh and Frank, 2014; Cohen, 2014). Increased theta activity predicts slower responses to target-incongruent trials of Simon tasks or variants (Cohen and Donner, 2013; Cohen and Ridderinkhof, 2013; Clayton et al., 2015; Pastötter et al., 2010, 2013; Cohen and Cavanagh, 2011; Nigbur et al., 2011), while frontal alpha activity is instead associated with the suppression of non-relevant sensory stimuli in flanker tasks (Suzuki and Shinoda, 2015).
These findings suggest that different inhibitory sub-functions produce spatially, temporally, and quantitatively distinguishable brain activity patterns, highlighting the importance of maintaining a conceptual separation and the non-generalization of the evidence relating to any sub-functions.
This article reviewed the patterns of neural synchronization underlying cognitive control and behavioral inhibition. So far, electrophysiology research has demonstrated the pivotal role of focal (de)synchronization patterns within specific frequencies in different cognitive processes (reviewed in Uhlhaas et al., 2008; Roohi-Azizi et al., 2017). However, emerging evidence suggests that more complex cognitive functions, in addition to local (de)synchronizations, require the contribution and coordination of brain circuits located distally from the site of primary processing (Schack and Klimesch, 2002; Sauseng et al., 2004, 2005; Schack et al., 2005; Hampshire et al., 2012; Crespo-Garcia et al., 2013).
Phase-synchronization facilitates the communication between distributed neural circuits, by augmenting the transmission efficiency and by promoting synaptic plasticity (Fries, 2015; Parkin et al., 2015; Constantinidis and Klingberg, 2016; Violante et al., 2017). Dysfunctions in long-range synchronization are, not surprisingly, implicated in clinical neurological conditions (Hata et al., 2016), raising the necessity of new methods and research for the study of phase dynamics across distributed brain networks.
Large-scale synchronization can be observed in fMRI and EEG/MEG, where the cooperation between distributed brain regions can occur via phase-synchronization within the same or/and between different frequencies (Palva et al., 2005; Canolty and Knight, 2010). While phase coupling dynamics have been described in working memory (Schack and Klimesch, 2002; Sauseng et al., 2004, 2005; Schack et al., 2005; Crespo-Garcia et al., 2013), the synchronization patterns involved in inhibition, a complex function that is likewise distributed across large-scale circuits, remain elusive.
Coordination patterns between frontoparietal MDC circuits act as a modulatory top-down control mechanism over sensory areas, refining/adjusting the processing, maintenance, retrieval and manipulation of relevant information, to support cognitively demanding tasks (Sauseng et al., 2005; Hampshire et al., 2012; Crespo-Garcia et al., 2013). In behavioral inhibition, specifically, the choice of motor response (press/no-press) depends on given pre-assumptions (i.e., stimulus type), which may incur a change over time (i.e., stop signal), therefore requiring a fast adjustment and correction of the motor command (i.e., “no-press”).
The GNGT and the SST represent two well-established paradigms, by which the cognitive processes and functional neural dynamics underlying behavioral inhibition can be studied. Functional connectivity fMRI studies have been instrumental in demonstrating how response inhibition does not map onto a single dedicated brain area. Still, it is supported by the dynamic coordination between distributed frontoparietal networks, whose specific contribution (i.e., extent and spatial distribution) relates to the contextual demand (Curtis et al., 2005; Aron and Poldrack, 2006; Li et al., 2006; Aron et al., 2007; Zandbelt and Vink, 2010; Brittain et al., 2012; Hampshire et al., 2012; Alegre et al., 2013; Erika-Florence et al., 2014; Hampshire and Sharp, 2015; Mallet et al., 2016).
While fMRI has been fundamental to localize the distributed coordination dynamics during response inhibition spatially, electrophysiology focuses on the dynamic patterns of phase-synchronization over time. Notably, one would define the exact timings at which specific frontoparietal MDC components exert their modulatory action over the sensorimotor cortices. Specific EEG/MEG ERP components have been related to the stop process (Ramautar et al., 2006; González-Villar et al., 2016), although lacking specificity for a given inhibitory sub-function (Kopp et al., 1996; Liotti et al., 2000; Bruchmann et al., 2010; Karayanidis and Jamadar, 2014). Intracranial electrophysiology in clinical populations showed that theta-synchronization within fronto medial, cingulate, and parietal circuits are key components of top-down control. In addition, response-withholding has a motivational attribute and is mediated by gamma synchrony within limbic-prefrontal mesocortical projections.
Electrophysiological investigations showed that response inhibition and the “overlapping” processes of SS-expectancy, RT-slowing are accompanied by oscillatory activity that is temporally and frequency-wise distinguished for different MDC components. This highlights the necessity of experimental designs that allow their separation (e.g., Chikazoe et al., 2009; Swann et al., 2009; Ko et al., 2016; Chang et al., 2017). The evidence is still elusive due to a mismatch between the experimental designs, preventing a close comparison and direct inferences from the results.
Further research is required, and multimodal synchronized EEG-fMRI approaches (e.g., Mizuhara et al., 2005) or the more recent DSI-Hybrid-EEG-fMRI headset (e.g., Hong et al., 2018) have the potential to further elucidate patterns of focal and long-range synchronization. This will be achieved by exploiting the respective advantages of electrophysiological and hemodynamic imaging techniques in terms of temporal and spatial resolution. Neural stimulation techniques, such as transcranial alternating current stimulation (e.g., Violante et al., 2017), can manipulate the modulatory effect of prefrontal networks over sensory areas during inhibitory processes, thus allowing to draw and ascertain conclusions on the topographical and chronological distribution of the causal relations between distributed circuits.
CB and SS: major role in designing and conceptualizing the article, drafted, wrote and revised different versions of the manuscript for intellectual content. IV, AH, and NG: wrote a section of the manuscript and revised a previous version of the manuscript for intellectual content.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Many thanks to Dr. Gregory Scott for his comments and advice on a previous version of the manuscript.
Alegre, M., Lopez-Azcarate, J., Obeso, I., Wilkinson, L., Rodriguez-Oroz, M. C., Valencia, M., et al. (2013). The subthalamic nucleus is involved in successful inhibition in the stop-signal task: a local field potential study in Parkinson’s disease. Exp. Neurol. 239, 1–12. doi: 10.1016/j.expneurol.2012.08.027
Arnulfo, G., Wang, S. H., Toselli, B., Williams, N., Hirvonen, J., Fato, M. M., et al. (2018). Long-range phase synchronization of high-γ activity in human cortex. BioRxiv 442251 [Preprint]. doi: 10.1101/442251
Aron, A. R. (2007). The neural basis of inhibition in cognitive control. Neuroscientist 13, 214–228. doi: 10.1177/1073858407299288
Aron, A. R., Behrens, T. E., Smith, S., Frank, M. J., and Poldrack, R. A. (2007). Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J. Neurosci. 27, 3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007
Aron, A. R., Fletcher, P. C., Bullmore, E. T., Sahakian, B. J., and Robbins, T. W. (2003). Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat. Neurosci. 6, 115–116. doi: 10.1038/nn1003
Aron, A. R., and Poldrack, R. A. (2006). Cortical and subcortical contributions to stop signal response inhibition: role of the subthalamic nucleus. J. Neurosci. 26, 2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006
Aron, A. R., Robbins, T. W., and Poldrack, R. A. (2004). Inhibition and the right inferior frontal cortex. Trends Cogn. Sci. 8, 170–177. doi: 10.1016/j.tics.2004.02.010
Band, G. P. H., van der Molen, M. W., and Logan, G. D. (2003). Horse-race model simulations of the stop-signal procedure. Acta Psychol. 112, 105–142. doi: 10.1016/s0001-6918(02)00079-3
Bari, A., and Robbins, T. W. (2013). Inhibition and impulsivity: behavioral and neural basis of response control. Prog. Neurobiol. 108, 44–79. doi: 10.1016/j.pneurobio.2013.06.005
Belujon, P., and Grace, A. A. (2017). Dopamine system dysregulation in major depressive disorders. Int. J. Neuropsychopharmacol. 20, 1036–1046. doi: 10.1093/ijnp/pyx056
Benedek, M., Schickel, R. J., Jauk, E., Fink, A., and Neubauer, A. C. (2014). α power increases in right parietal cortex reflects focused internal attention. Neuropsychologia 56, 393–400. doi: 10.1016/j.neuropsychologia.2014.02.010
Benis, D., David, O., Lachaux, J. P., Seigneuret, E., Krack, P., Fraix, V., et al. (2014). Subthalamic nucleus activity dissociates proactive and reactive inhibition in patients with Parkinson’s disease. NeuroImage 91, 273–281. doi: 10.1016/j.neuroimage.2013.10.070
Boucher, L., Palmeri, T. J., Logan, G. D., and Schall, J. D. (2007). Inhibitory control in mind and brain: an interactive race model of countermanding saccades. Psychol. Rev. 114, 376–397. doi: 10.1037/0033-295X.114.2.376
Braver, T. S. (2012). The variable nature of cognitive control: a dual mechanisms framework. Trends Cogn. Sci. 16, 106–113. doi: 10.1016/j.tics.2011.12.010
Braver, T. S., Gray, J. R., and Burgess, G. C. (2008). Explaining the many varieties of working memory variation: dual mechanisms of cognitive control. Var. Work. Mem. 75:106. doi: 10.1093/acprof:oso/9780195168648.003.0004
Brittain, J. S., Watkins, K. E., Joundi, R. A., Ray, N. J., Holland, P., Green, A. L., et al. (2012). A role for the subthalamic nucleus in response inhibition during conflict. J. Neurosci. 32, 13396–13401. doi: 10.1523/JNEUROSCI.2259-12.2012
Bruchmann, M., Herper, K., Konrad, C., Pantev, C., and Huster, R. J. (2010). Individualized EEG source reconstruction of Stroop interference with masked color words. NeuroImage 49, 1800–1809. doi: 10.1016/j.neuroimage.2009.09.032
Brydges, C. R., Anderson, M., Reid, C. L., and Fox, A. M. (2013). Maturation of cognitive control: delineating response inhibition and interference suppression. PLoS One 8:e69826. doi: 10.1371/journal.pone.0069826
Brydges, C. R., Clunies-Ross, K., Clohessy, M., Lo, Z. L., Nguyen, A., Rousset, C., et al. (2012). Dissociable components of cognitive control: an event-related potential (ERP) study of response inhibition and interference suppression. PLoS One 7:e34482. doi: 10.1371/journal.pone.0034482
Burle, B., Spieser, L., Roger, C., Casini, L., Hasbroucq, T., and Vidal, F. (2015). Spatial and temporal resolutions of EEG: is it really black and white? A scalp current density view. Int. J. Psychophysiol. 97, 210–220. doi: 10.1016/j.ijpsycho.2015.05.004
Cai, W., Chen, T., Ide, J. S., Li, C. S. R., and Menon, V. (2017). Dissociable fronto-operculum-insula control signals for anticipation and detection of inhibitory sensory cue. Cereb. Cortex 27, 073–4082. doi: 10.1093/cercor/bhw219
Cai, W., Duberg, K., Padmanabhan, A., Rehert, R., Bradley, T., Carrion, V., et al. (2019). Hyperdirect insula-basal-ganglia pathway and adult-like maturity of global brain responses predict inhibitory control in children. Nat. Commun. 10:4798. doi: 10.1038/s41467-019-12756-8
Cai, W., Ryali, S., Chen, T., Li, C. S. R., and Menon, V. (2014). Dissociable roles of right inferior frontal cortex and anterior insula in inhibitory control: evidence from intrinsic and task-related functional parcellation, connectivity, and response profile analyses across multiple datasets. J. Neurosci. 34, .14652–14667. doi: 10.1523/JNEUROSCI.3048-14.2014
Canolty, R. T., and Knight, R. T. (2010). The functional role of cross-frequency coupling. Trends Cogn. Sci. 14, 506–515. doi: 10.1016/j.tics.2010.09.001
Castro-Meneses, L. J., Johnson, B. W., and Sowman, P. F. (2015). The effects of impulsivity and proactive inhibition on reactive inhibition and the go process: insights from vocal and manual stop signal tasks. Front. Hum. Neurosci. 9:529. doi: 10.3389/fnhum.2015.00529
Cavanagh, J. F., and Frank, M. J. (2014). Frontal theta as a mechanism for cognitive control. Trends in cogn. sci. 18, 414–421. doi: 10.1016/j.tics.2014.04.012
Chang, A., Ide, J. S., Li, H.-H., Chen, C. C., and Li, C. S. R. (2017). Proactive control: neural oscillatory correlates of conflict anticipation and response slowing. eNeuro 4:ENEURO.0061–17.2017. doi: 10.1523/eneuro.0061-17.2017
Chelazzi, L., Duncan, J., Miller, E. K., and Desimone, R. (1998). Responses of neurons in inferior temporal cortex during memory-guided visual search. J. Neurophysiol. 80, 2918–2940. doi: 10.1152/jn.1998.80.6.2918
Chen, J. E., and Glover, G. H. (2015). Functional magnetic resonance imaging methods. Neuropsychology review 25, 289–313. doi: 10.1007/s11065-015-9294-9
Chikazoe, J., Jimura, K., Hirose, S., Yamashita, L., Miyashita, Y., and Konishi, S. (2009). Preparation to inhibit a response complements response inhibition during performance of a stop-signal task. J. Neurosci. 29, 15870–15877. doi: 10.1523/JNEUROSCI.3645-09.2009
Clancy, E. M., Fiacconi, C. M., and Fenske, M. J. (2019). Chapter 8—response inhibition immediately elicits negative affect and devalues associated stimuli: evidence from facial electromyography. Prog. Brain Res. 247, 169–191. doi: 10.1016/bs.pbr.2019.03.019
Clausen, A. N., Francisco, A. J., Thelen, J., Bruce, J., Martin, L. E., McDowd, J., et al. (2017). PTSD and cognitive symptoms relate to inhibition-related prefrontal activation and functional connectivity. Depress. Anxiety 34, 427–436. doi: 10.1002/da.22613
Clayton, M. S., Yeung, N., and Cohen Kadosh, R. (2015). The roles of cortical oscillations in sustained attention. Trends Cogn. Sci. 19, 188–195. doi: 10.1016/j.tics.2015.02.004
Cohen, M. X. (2017). Where does EEG come from and what does it mean? Trends Neurosci. 40, 208–218. doi: 10.1016/j.tins.2017.02.004
Cohen, M. X., and Cavanagh, J. F. (2011). Single-trial regression elucidates the role of prefrontal theta oscillations in response conflict. Front. Psychol. 2:30. doi: 10.3389/fpsyg.2011.00030
Cohen, M. X., and Donner, T. H. (2013). Midfrontal conflict-related theta-band power reflects neural oscillations that predict behavior. J. Neurophysiol. 110, 2752–2763. doi: 10.1152/jn.00479.2013
Cohen, M. X., and Ridderinkhof, K. R. (2013). EEG source reconstruction reveals frontal-parietal dynamics of spatial conflict processing. PLoS One 8:e57293. doi: 10.1371/journal.pone.0057293
Constantinidis, C., and Klingberg, T. (2016). The neuroscience of working memory capacity and training. Nat. Rev. Neurosci. 17, 438–449. doi: 10.1038/nrn.2016.43
Crespo-Garcia, M., Pinal, D., Cantero, J. L., Díaz, F., Zurrón, M., and Atienza, M. (2013). Working memory processes are mediated by local and long-range synchronization of α oscillations. J. Cogn. Neurosci. 25, 1343–1357. doi: 10.1162/jocn_a_00379
Curtis, C. E., Cole, M. W., Rao, V. Y., and D’Esposito, M. (2005). Canceling planned action: an fMRI study of countermanding saccades. Cereb. Cortex 15, 1281–1289. doi: 10.1093/cercor/bhi011
Deco, G., Buehlmann, A., Masquelier, T., and Hugues, E. (2011). The role of rhythmic neural synchronization in rest and task conditions. Front. Hum. Neurosci. 5:4. doi: 10.3389/fnhum.2011.00004
Desimone, R., and Duncan, J. (1995). Neural mechanisms of selective visual attention. Annu. Rev. Neurosci. 18, 193–222. doi: 10.1146/annurev.ne.18.030195.001205
Diamond, J. R., Salgia, R., Varella-Garcia, M., Kanteti, R., LoRusso, P. M., Clark, J. W., et al. (2013). Initial clinical sensitivity and acquired resistance to MET inhibition in MET-mutated papillary renal cell carcinoma. J. Clin. Oncol. 31, e254–e258. doi: 10.1200/jco.2012.46.4289
Dosenbach, N. U. F., Fair, D. A., Cohen, A. L., Schlaggar, B. L., and Petersen, S. E. (2008). A dual-networks architecture of top-down control. Trends Cogn. Sci. 12, 99–105. doi: 10.1016/j.tics.2008.01.001
Duann, J. R., Ide, J. S., Luo, X., and Li, C. S. R. (2009). Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. J. Neurosci. 29, 10171–10179. doi: 10.1523/JNEUROSCI.1300-09.2009
Dubreuil-Vall, L., Chau, P., Ruffini, G., Widge, A. S., and Camprodon, J. A. (2019). tDCS to the left DLPFC modulates cognitive and physiological correlates of executive function in a state-dependent manner. Brain Stimul. 12, 1456–1463. doi: 10.1016/j.brs.2019.06.006
Erika-Florence, M., Leech, R., and Hampshire, A. (2014). A functional network perspective on response inhibition and attentional control. Nat. Commun. 5:4073. doi: 10.1038/ncomms5073
Eriksen, C. W. (1995). The flankers task and response competition: a useful tool for investigating a variety of cognitive problems. Vis. Cogn. 2, 101–118. doi: 10.1080/13506289508401726
Eriksen, B. A., and Eriksen, C. W. (1974). Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychophys. 16, 143–149. doi: 10.3758/bf03203267
Falkenstein, M., Hoormann, J., and Hohnsbein, J. (2002). Inhibition-related ERP components: variation with modality, age, and time-on-task. J. Psychophysiol. 16, 167–175. doi: 10.1027//0269-8803.16.3.167
Fell, J., and Axmacher, N. (2011). The role of phase synchronization in memory processes. Nat. Rev. Neurosci. 12, 105–118. doi: 10.1038/nrn2979
Fernandez-Ruiz, J., Peltsch, A., Alahyane, N., Brien, D. C., Coe, B. C., Garcia, A., et al. (2018). Age related prefrontal compensatory mechanisms for inhibitory control in the antisaccade task. NeuroImage 165, 92–101. doi: 10.1016/j.neuroimage.2017.10.001
Fries, P. (2005). A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn. Sci. 9, 474–480. doi: 10.1016/j.tics.2005.08.011
Fries, P. (2015). Rhythms for cognition: communication through coherence. Neuron 88, 220–235. doi: 10.1016/j.neuron.2015.09.034
González-Villar, A. J., Bonilla, F. M., and Carrillo-de-la-Peña, M. T. (2016). When the brain simulates stopping: neural activity recorded during real and imagined stop-signal tasks. Cogn. Affect. Behav. Neurosci. 16, 825–835. doi: 10.3758/s13415-016-0434-3
Grace, A. A. (2012). Dopamine system dysregulation by the hippocampus: implications for the pathophysiology and treatment of schizophrenia. Neuropharmacology 62, 1342–1348. doi: 10.1016/j.neuropharm.2011.05.011
Groom, M. J., and Cragg, L. (2015). Differential modulation of the N2 and P3 event-related potentials by response conflict and inhibition. Brain Cogn. 97, 1–9. doi: 10.1016/j.bandc.2015.04.004
Groppe, D. M., Bickel, S., Keller, C. J., Jain, S. K., Hwang, S. T., Harden, C., et al. (2013). Dominant frequencies of resting human brain activity as measured by the electrocorticogram. NeuroImage 79, 223–233. doi: 10.1016/j.neuroimage.2013.04.044
Hampshire, A. (2015). Putting the brakes on inhibitory models of frontal lobe function. NeuroImage 113, 340–355. doi: 10.1016/j.neuroimage.2015.03.053
Hampshire, A., Chamberlain, S. R., Monti, M. M., Duncan, J., and Owen, A. M. (2010). The role of the right inferior frontal gyrus: inhibition and attentional control. NeuroImage 50, 1313–1319. doi: 10.1016/j.neuroimage.2009.12.109
Hampshire, A., Duncan, J., and Owen, A. M. (2007). Selective tuning of the blood oxygenation level-dependent response during simple target detection dissociates human frontoparietal subregions. J. Neurosci. 27, 6219–6223. doi: 10.1523/JNEUROSCI.0851-07.2007
Hampshire, A., Highfield, R. R., Parkin, B. L., and Owen, A. M. (2012). Fractionating human intelligence. Neuron 76, 1225–1237. doi: 10.1016/j.neuron.2012.06.022
Hampshire, A., and Sharp, D. J. (2015). Contrasting network and modular perspectives on inhibitory control. Trends Cogn. Sci. 19, 445–452. doi: 10.1016/j.tics.2015.06.006
Hanes, D. P., and Carpenter, R. H. (1999). Countermanding saccades in humans. Vision Res. 39, 2777–2791. doi: 10.1016/s0042-6989(99)00011-5
Harnishfeger, K. K. (1995). “The development of cognitive inhibition: theories, definitions and research evidence,” in Interference and Inhibition in Cognition, eds F. N. Dempster and C. J. Brainerd (San Diego, CA: Academic Press), 175–204.
Harris, A. Z., and Gordon, J. A. (2015). Long-range neural synchrony in behavior. Annu. Rev. Neurosci. 38, 171–194. doi: 10.1146/annurev-neuro-071714-034111
Hata, M., Kazui, H., Tanaka, T., Ishii, R., Canuet, L., Pascual-Marqui, R. D., et al. (2016). Functional connectivity assessed by resting state EEG correlates with cognitive decline of Alzheimer’s disease—an eLORETA study. Clin. Neurophysiol. 127, 1269–1278. doi: 10.1016/j.clinph.2015.10.030
Hong, K. S., Khan, M. J., and Hong, M. J. (2018). Feature extraction and classification methods for hybrid fNIRS-EEG brain-computer interfaces. Front. Hum. Neurosci. 12:246. doi: 10.3389/fnhum.2018.00246
Hu, S., Ide, J. S., Zhang, S., and Li, C. R. (2015a). Anticipating conflict: neural correlates of a Bayesian belief and its motor consequence. NeuroImage 119, 286–295. doi: 10.1016/j.neuroimage.2015.06.032
Hu, S., Ide, J. S., Zhang, S., Sinha, R., and Li, C. R. (2015b). Conflict anticipation in alcohol dependence—a model-based fMRI study of stop signal task. Neuroimage Clin. 8, 39–50. doi: 10.1016/j.nicl.2015.03.008
Hughes, M. E., Fulham, W. R., Johnston, P. J., and Michie, P. T. (2012). Stop-signal response inhibition in schizophrenia: behavioural, event-related potential and functional neuroimaging data. Biol. Psychol. 89, 220–231. doi: 10.1016/j.biopsycho.2011.10.013
Hwang, S., Meffert, H., Parsley, I., Tyler, P. M., Erway, A. K., Botkin, M. L., et al. (2019). Segregating sustained attention from response inhibition in ADHd: an fMRI study. Neuroimage Clin. 21:101677. doi: 10.1016/j.nicl.2019.101677
Jacoby, L. L., Kelley, C. M., and McElree, B. D. (1999). “The role of cognitive control: early selection versus late correction,” in Dual Process Theories in Social Psychology, eds S. Chaiken and Y. Trope (New York, NY: Guildford Press), 383–400.
Jahfari, S., Stinear, C. M., Claffey, M., Verbruggen, F., and Aron, A. R. (2010). Responding with restraint: what are the neurocognitive mechanisms? J. Cogn. Neurosci. 22, 1479–1492. doi: 10.1162/jocn.2009.21307
Jewsbury, P. A., Bowden, S. C., and Strauss, M. E. (2016). Integrating the switching, inhibition, and updating model of executive function with the cattell—horn—carroll model. J. Exp. Psychol. Gen. 145, 220–245. doi: 10.1037/xge0000119
Jiang, X., Liu, L., Ji, H., and Zhu, Y. (2018). Association of affected neurocircuitry with deficit of response inhibition and delayed gratification in attention deficit hyperactivity disorder: a narrative review. Front. Hum. Neurosci. 12:506. doi: 10.3389/fnhum.2018.00506
Jilka, S. R., Scott, G., Ham, T., Pickering, A., Bonnelle, V., Braga, R. M., et al. (2014). Damage to the salience network and interactions with the default mode network. J. Neurosci. 34, 10798–10807. doi: 10.1523/JNEUROSCI.0518-14.2014
Johnstone, S. J., Barry, R. J., Markovska, V., Dimoska, A., and Clarke, A. R. (2009). Response inhibition and interference control in children with AD/HD: a visual ERP investigation. Int. J. Psychophysiol. 72, 145–153. doi: 10.1016/j.ijpsycho.2008.11.007
Johnstone, S. J., Dimoska, A., Smith, J. L., Barry, R. J., Pleffer, C. B., Chiswick, D., et al. (2007). The development of stop-signal and Go/Nogo response inhibition in children aged 7–12 years: performance and event-related potential indices. Int. J. Psychophysiol. 63, 25–38. doi: 10.1016/j.ijpsycho.2006.07.001
Jones, S. M., Bailey, R., Barnes, S. P., and Partee, A. (2016). Executive Function Mapping Project: Untangling the Terms and Skills Related to Executive Function and Self-Regulation in Early Childhood. OPRE Report#2016–88. Washington, DC: Office of Planning, Research and Evaluation, Administration for Children and Families.
Karayanidis, F., and Jamadar, S. D. (2014). “Event-related potentials reveal multiple components of proactive and reactive control in task switching,” in Task Switching and Cognitive Control, eds J. A. Grange and G. Houghton (New York, NY: Oxford University Press), 200–236.
Katz, R., De Sanctis, P., Mahoney, J. R., Sehatpour, P., Murphy, C. F., Gomez-Ramirez, M., et al. (2010). Cognitive control in late-life depression: response inhibition deficits and dysfunction of the anterior cingulate cortex. Am. J. Geriatr. Psychiatry 18, 1017–1025. doi: 10.1097/jgp.0b013e3181d695f2
Kazanovich, Y. (2019). Modeling brain cognitive functions by oscillatory neural networks. Opt. Mem. Neural Netw. 28, 175–184. doi: 10.3103/s1060992x19030044
Ko, L. W., Shih, Y. C., Chikara, R. K., Chuang, Y. T., and Chang, E. C. (2016). Neural mechanisms of inhibitory response in a battlefield scenario: a simultaneous fMRI-EEG study. Front. Hum. Neurosci. 10:185. doi: 10.3389/fnhum.2016.00185
Koch, I., Gade, M., Schuch, S., and Philipp, A. M. (2010). The role of inhibition in task switching: a review. Psychon. Bull. Rev. 17, 1–14. doi: 10.3758/PBR.17.1.1
Kopp, B., Rist, F., and Mattler, U. (1996). N200 in the flanker task as a neurobehavioral tool for investigating executive control. Psychophysiology 33, 282–294. doi: 10.1111/j.1469-8986.1996.tb00425.x
Laviolette, S. R. (2007). Dopamine modulation of emotional processing in cortical and subcortical neural circuits: evidence for a final common pathway in schizophrenia? Schizophr. Bull. 33, 971–981. doi: 10.1093/schbul/sbm048
Leunissen, I., Coxon, J. P., and Swinnen, S. P. (2016). A proactive task set influences how response inhibition is implemented in the basal ganglia. Hum. Brain Mapp. 37, 4706–4717. doi: 10.1002/hbm.23338
Li, C. S. R., Huang, C., Constable, R. T., and Sinha, R. (2006). Imaging response inhibition in a stop-signal task: neural correlates independent of signal monitoring and post-response processing. J. Neurosci. 26, 186–192. doi: 10.1523/JNEUROSCI.3741-05.2006
Liotti, M., Woldorff, M. G., Perez, R., and Mayberg, H. S. (2000). An ERP study of the temporal course of the Stroop color-word interference effect. Neuropsychologia 38, 701–711. doi: 10.1016/s0028-3932(99)00106-2
Logan, G. D., Cowan, W. B., and Davis, K. A. (1984). On the ability to inhibit simple and choice reaction time responses: a model and a method. J. Exp. Psychol. Hum. Percept. Perform. 10, 276–291. doi: 10.1037/0096-1523.10.2.276
Luijten, M., Littel, M., and Franken, I. H. A. (2011). Deficits in inhibitory control in smokers during a Go/NoGo task: an investigation using event-related brain potentials. PLoS One 6:e18898. doi: 10.1371/journal.pone.0018898
MacLeod, C. M. (2007). “The concept of inhibition in cognition,” in Inhibition in Cognition, eds D. S. Gorfein and C. M. MacLeod (Washington, DC: American Psychological Association), 3–23.
Mäki-Marttunen, V., Hagen, T., and Espeseth, T. (2019). Proactive and reactive modes of cognitive control can operate independently and simultaneously. Acta Psychol. 199:102891. doi: 10.1016/j.actpsy.2019.102891
Mallet, N., Schmidt, R., Leventhal, D., Chen, F., Amer, N., Boraud, T., et al. (2016). Arkypallidal cells send a stop signal to striatum. Neuron 89, 308–316. doi: 10.1016/j.neuron.2015.12.017
Manza, P., Hu, S., Ide, J. S., Farr, O. M., Zhang, S., Leung, H. C., et al. (2016). The effects of methylphenidate on cerebral responses to conflict anticipation and unsigned prediction error in a stop-signal task. J. Psychopharmacol. 30, 283–293. doi: 10.1177/0269881115625102
McLaughlin, N. C. R., Kirschner, J., Foster, H., O’Connell, C., Rasmussen, S. A., and Greenberg, B. D. (2016). Stop signal reaction time deficits in a lifetime obsessive-compulsive disorder sample. J. Int. Neuropsychol. Soc. 22, 785–789. doi: 10.1017/s1355617716000540
Miller, E. K., and Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 24, 167–202. doi: 10.1146/annurev.neuro.24.1.167
Mizuhara, H., Wang, L. Q., Kobayashi, K., and Yamaguchi, Y. (2005). Long-range EEG phase synchronization during an arithmetic task indexes a coherent cortical network simultaneously measured by fMRI. NeuroImage 27, 553–563. doi: 10.1016/j.neuroimage.2005.04.030
Monsell, S. (2003). Task switching. Trends Cogn. Sci. 7, 134–140. doi: 10.1016/s1364-6613(03)00028-7
Morein-Zamir, S., and Robbins, T. W. (2015). Fronto-striatal circuits in response-inhibition: relevance to addiction. Brain Res. 1628, 117–129. doi: 10.1016/j.brainres.2014.09.012
Mostofsky, S. H., and Simmonds, D. J. (2008). Response inhibition and response selection: two sides of the same coin. J. Cogn. Neurosci. 20, 751–761. doi: 10.1162/jocn.2008.20500
Munakata, Y., Herd, S. A., Chatham, C. H., Depue, B. E., Banich, M. T., and O’Reilly, R. C. (2011). A unified framework for inhibitory control. Trends Cogn. Sci. 15, 453–459. doi: 10.1016/j.tics.2011.07.011
Nieuwenhuis, S., Yeung, N., and Cohen, J. D. (2004). Stimulus modality, perceptual overlap, and the go/no-go N2. Psychophysiology 41, 157–160. doi: 10.1046/j.1469-8986.2003.00128.x
Nigbur, R., Ivanova, G., and Stürmer, B. (2011). Theta power as a marker for cognitive interference. Clin. Neurophysiol. 122, 2185–2194. doi: 10.1016/j.clinph.2011.03.030
Nigg, J. T. (2000). On inhibition/disinhibition in developmental psychopathology: views from cognitive and personality psychology and a working inhibition taxonomy. Psychol. Bull. 126, 220–246. doi: 10.1037/0033-2909.126.2.220
Nowak, A., Vallacher, R. R., Zochowski, M., and Rychwalska, A. (2017). Functional synchronization: The emergence of coordinated activity in human systems. Front. Psychol. 8:945. doi: 10.3389/fpsyg.2017.00945
Osada, T., Ohta, S., Ogawa, A., Tanaka, M., Suda, A., Kamagata, K., et al. (2019). An essential role of the intraparietal sulcus in response inhibition predicted by parcellation-based network. J. Neurosci. 39, 2509–2521. doi: 10.1523/jneurosci.2244-18.2019
Palva, J. M., Palva, S., and Kaila, K. (2005). Phase synchrony among neuronal oscillations in the human cortex. J. Neurosci. 25, 3962–3972. doi: 10.1523/jneurosci.4250-04.2005
Parkin, B. L., Hellyer, P. J., Leech, R., and Hampshire, A. (2015). Dynamic network mechanisms of relational integration. J. Neurosci. 35, 7660–7673. doi: 10.1523/jneurosci.4956-14.2015
Pastötter, B., Hanslmayr, S., and Bäuml, K. H. T. (2010). Conflict processing in the anterior cingulate cortex constrains response priming. NeuroImage 50, 1599–1605. doi: 10.1037/a0017406
Pastötter, B., Dreisbach, G., and Bäuml, K. H. T. (2013). Dynamic adjustments of cognitive control: oscillatory correlates of the conflict adaptation effect. J. Cogn. Neurosci. 25, 2167–2178. doi: 10.1162/jocn_a_00474
Patel, N. H., Vyas, N. S., Puri, B. K., Nijran, K. S., and Al-Nahhas, A. (2010). Positron emission tomography in schizophrenia: a new perspective. J. Nucl. Med. 5, 511–520. doi: 10.2967/jnumed.109.066076
Perfetti, B., Moisello, C., Landsness, E. C., Kvint, S., Pruski, A., Onofrj, M., et al. (2011). Temporal evolution of oscillatory activity predicts performance in a choice-reaction time reaching task. J. Neurophysiol. 105, 18–27. doi: 10.1152/jn.00778.2010
Pesaran, B., Vinck, M., Einevoll, G. T., Sirota, A., Fries, P., Siegel, M., et al. (2018). Investigating large-scale brain dynamics using field potential recordings: analysis and interpretation. Nat. Neurosci. 21, 903–919. doi: 10.1038/s41593-018-0171-8
Provenza, N. R., Paulk, A. C., Peled, N., Restrepo, M. I., Cash, S. S., Dougherty, D. D., et al. (2019). Decoding task engagement from distributed network electrophysiology in humans. J. Neural Eng. 16:056015. doi: 10.1088/1741-2552/ab2c58
Purpura, D. J., Schmitt, S. A., and Ganley, C. M. (2017). Foundations of mathematics and literacy: the role of executive functioning components. J. Exp. Child Psychol. 153, 15–34. doi: 10.1016/j.jecp.2016.08.010
Ramautar, J. R., Kok, A., and Ridderinkhof, K. R. (2006). Effects of stop-signal modality on the N2/P3 complex elicited in the stop-signal paradigm. Biol. Psychol. 72, 96–109. doi: 10.1016/j.biopsycho.2005.08.001
Richez, A., Olivier, G., and Coello, Y. (2016). Stimulus-response compatibility effect in the near-far dimension: a developmental study. Front. Psychol. 7:1169. doi: 10.3389/fpsyg.2016.01169
Riedl, V., Utz, L., Castrillón, G., Grimmer, T., Rauschecker, J. P., Ploner, M., et al. (2016). Metabolic connectivity mapping reveals effective connectivity in the resting human brain. Proc. Natl. Acad. Sci. U S A 113, 428–433. doi: 10.1073/pnas.1513752113
Roohi-Azizi, M., Azimi, L., Heysieattalab, S., and Aamidfar, M. (2017). Changes of the brain’s bioelectrical activity in cognition, consciousness and some mental disorders. Med. J. Islam. Repub. Iran 31:53. doi: 10.14196/mjiri.31.53
Roux, F., and Uhlhaas, P. J. (2014). Working memory and neural oscillations: α-γ versus theta-γ codes for distinct WM information? Trends Cogn. Sci. 18, 16–25. doi: 10.1016/j.tics.2013.10.010
Rubia, K., Alegria, A. A., Cubillo, A. I., Smith, A. B., Brammer, M. J., and Radua, J. (2014). Effects of stimulants on brain function in attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Biol. Psychiatry 76, 616–628. doi: 10.1016/j.biopsych.2013.10.016
Rubia, K., Overmeyer, S., Taylor, E., Brammer, M., Williams, S. C. R., Simmons, A., et al. (1999). Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: a study with functional MRI. Am. J. Psychiatry 156, 891–896. doi: 10.1176/ajp.156.6.891
Rubia, K., Russell, T., Overmeyer, S., Brammer, M. J., Bullmore, E. T., Sharma, T., et al. (2001a). Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. NeuroImage 13, 250–261. doi: 10.1006/nimg.2000.0685
Rubia, K., Smith, A., Lidzba, K., Toone, B., Simmons, A., Williams, S. C. R., et al. (2001b). Neural substrates of successful versus unsuccessful stopping in a cognitively challenging event related stop task. NeuroImage 13:351. doi: 10.1016/s1053-8119(01)91694-5
Sauseng, P., Klimesch, W., Doppelmayr, M., Hanslmayr, S., Schabus, M., and Gruber, W. R. (2004). Theta coupling in the human electroencephalogram during a working memory task. Neurosci. Lett. 354, 123–126. doi: 10.1016/j.neulet.2003.10.002
Sauseng, P., Klimesch, W., Doppelmayr, M., Pecherstorfer, T., Freunberger, R., and Hanslmayr, S. (2005). EEG α synchronization and functional coupling during top-down processing in a working memory task. Hum. Brain Mapp. 26, 148–155. doi: 10.1002/hbm.20150
Schack, B., and Klimesch, W. (2002). Frequency characteristics of evoked and oscillatory electroencephalic activity in a human memory scanning task. Neurosci. Lett. 331, 107–110. doi: 10.1016/s0304-3940(02)00846-7
Schack, B., Klimesch, W., and Sauseng, P. (2005). Phase synchronization between theta and upper α oscillations in a working memory task. Int. J. Psychophysiol. 57, 105–114. doi: 10.1016/j.ijpsycho.2005.03.016
Schall, J. D., and Godlove, D. C. (2012). Current advances and pressing problems in studies of stopping. Curr. Opin. Neurobiol. 22, 1012–1021. doi: 10.1016/j.conb.2012.06.002
Seeley, W. W., Crawford, R. K., Zhou, J., Miller, B. L., and Greicius, M. D. (2009). Neurodegenerative diseases target large-scale human brain networks. Neuron 62, 42–52. doi: 10.1016/j.neuron.2009.03.024
Shallice, T., Stuss, D. T., Alexander, M. P., Picton, T. W., and Derkzen, D. (2008). The multiple dimensions of sustained attention. Cortex 44, 794–805. doi: 10.1016/j.cortex.2007.04.002
Sharp, D. J., Bonnelle, V., De Boissezon, X., Beckmann, C. F., James, S. G., Patel, M. C., et al. (2010). Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc. Natl. Acad. Sci. U S A 107, 6106–6111. doi: 10.1073/pnas.1000175107
Simon, J. R., and Wolf, J. D. (1963). Choice reaction time as a function of angular stimulus-response correspondence and age. Ergonomics 6, 99–105. doi: 10.1080/00140136308930679
Smith, E. H., Banks, G. P., Mikell, C. B., Cash, S. S., Patel, S. R., Eskandar, E. N., et al. (2015). Frequency-dependent representation of reinforcement-related information in the human medial and lateral prefrontal cortex. J. Neurosci. 35, 15827–15836. doi: 10.1523/jneurosci.1864-15.2015
Smith, E. H., Horga, G., Yates, M. J., Mikell, C. B., Banks, G. P., Pathak, Y. J., et al. (2019). Widespread temporal coding of cognitive control in the human prefrontal cortex. Nat. Neurosci. 22, 1883–1891. doi: 10.1038/s41593-019-0494-0
Stokes, M. G., Kusunoki, M., Sigala, N., Nili, H., Gaffan, D., and Duncan, J. (2013). Dynamic coding for cognitive control in prefrontal cortex. Neuron 78, 364–375. doi: 10.1016/j.neuron.2013.01.039
Stroop, J. R. (1935). Studies of interference in serial verbal reactions. J. Exp. Psychol. 18, 643–662. doi: 10.1037/h0054651
Stuphorn, V., and Emeric, E. E. (2012). Proactive and reactive control by the medial frontal cortex. Front. Neuroeng. 5:9. doi: 10.3389/fneng.2012.00009
Suzuki, K., and Shinoda, H. (2015). Transition from reactive control to proactive control across conflict adaptation: an sLORETA study. Brain Cogn. 100, 7–14. doi: 10.1016/j.bandc.2015.09.001
Swann, N., Tandon, N., Canolty, R., Ellmore, T. M., McEvoy, L. K., Dreyer, S., et al. (2009). Intracranial EEG reveals a time- and frequency-specific role for the right inferior frontal gyrus and primary motor cortex in stopping initiated responses. J. Neurosci. 29, 12675–12685. doi: 10.1523/jneurosci.3359-09.2009
Tervo-Clemmens, B., Quach, A., Luna, B., Foran, W., Chung, T., De Bellis, M. D., et al. (2017). Neural correlates of rewarded response inhibition in youth at risk for problematic alcohol use. Front. Behav. Neurosci. 11:205. doi: 10.3389/fnbeh.2017.00205
Uhlhaas, P. J., Haenschel, C., Nikolic, D., and Singer, W. (2008). The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr. Bull. 34, 927–943. doi: 10.1093/schbul/sbn062
Uzefovsky, F., Allison, C., Smith, P., and Baron-Cohen, S. (2016). Brief report: the go/no-go task online: inhibitory control deficits in autism in a large sample. J. Autism Dev. Disord. 46, 2774–2779. doi: 10.1007/s10803-016-2788-3
Van Velzen, L. S., Vriend, C., de Wit, S. J., and Van den Heuvel, O. A. (2014). Response inhibition and interference control in obsessive-compulsive spectrum disorders. Front. Hum. Neurosci. 8:419. doi: 10.3389/fnhum.2014.00419
Varela, F., Lachaux, J.-P., Rodriguez, E., and Martinerie, J. (2001). The brainweb: Phase synchronization and large-scale integration. Nat. Rev. Neurosci. 2, 229–239. doi: 10.1038/35067550
Verbruggen, F., and Logan, G. D. (2009). Proactive adjustments of response strategies in the stop-signal paradigm. J. Exp. Psychol. Hum. Percept. Perform. 35, 835–854. doi: 10.1037/a0012726
Violante, I. R., Li, L. M., Carmichael, D. W., Lorenz, R., Leech, R., Hampshire, A., et al. (2017). Externally induced frontoparietal synchronization modulates network dynamics and enhances working memory performance. Elife 6:e22001. doi: 10.7554/elife.22001
Voytek, B., Kayser, A. S., Badre, D., Fegen, D., Chang, E. F., Crone, N. E., et al. (2015). Oscillatory dynamics coordinating human frontal networks in support of goal maintenance. Nat. Neurosci. 18, 1318–1324. doi: 10.1038/nn.4071
Vuillier, L., Bryce, D., Szücs, D., and Whitebread, D. (2016). The maturation of interference suppression and response inhibition: ERP analysis of a cued go/nogo task. PLoS One 11:e0165697. doi: 10.1371/journal.pone.0165697
Wang, P., Goeschl, F., Friese, U., Koenig, P., and Engel, A. K. (2019). Long-range functional coupling predicts performance: oscillatory EEG networks in multisensory processing. NeuroImage 196, 114–125. doi: 10.1016/j.neuroimage.2019.04.001
Wang, W., Worhunsky, P. D., Zhang, S., Le, T. M., Potenza, M. N., and Li, C. S. R. (2018). Response inhibition and fronto-striatal-thalamic circuit dysfunction in cocaine addiction. Drug Alcohol Depend. 192, 137–145. doi: 10.1016/j.drugalcdep.2018.07.037
Widge, A. S., Heilbronner, S. R., and Hayden, B. Y. (2019a). Prefrontal cortex and cognitive control: new insights from human electrophysiology. F1000Res. 8:F1000 Faculty Rev-1696. doi: 10.12688/f1000research.20044.1
Widge, A. S., Zorowitz, S., Basu, I., Paulk, A. C., Cash, S. S., Eskandar, E. N., et al. (2019b). Deep brain stimulation of the internal capsule enhances human cognitive control and prefrontal cortex function. Nat. Commun. 10:1536. doi: 10.1038/s41467-019-09557-4
Wilson, S. P., and Kipp, K. (1998). The development of efficient inhibition: evidence from directed-forgetting tasks. Dev. Rev. 18, 86–123.
Womelsdorf, T., Schoffelen, J. M., Oostenveld, R., Singer, W., Desimone, R., Engel, A. K., et al. (2007). Modulation of neuronal interactions through neuronal synchronization. Science 316, 1609–1612. doi: 10.1126/science.1139597
Xie, L., Ren, M., Cao, B., and Li, F. (2017). Distinct brain responses to different inhibitions: evidence from a modified Flanker Task. Sci. Rep. 7:6657. doi: 10.1038/s41598-017-04907-y
Young, J. J., Friedman, J. S., Panov, F., Camara, D., Yoo, J. Y., Fields, M. C., et al. (2019). Quantitative signal characteristics of electrocorticography and stereoelectroencephalography: the effect of contact depth. J. Clin. Neurophysiol. 36, 195–203. doi: 10.1097/wnp.0000000000000577
Yu, A. J., and Cohen, J. D. (2009). “Sequential effects: superstition or rational behavior?,” in Advances in Neural Information Processing Systems, eds D. Koller, D. Schuurmans, Y. Bengio and L. Bottou (Cambridge: MIT Press), 21, 1873–1880.
Zandbelt, B. B., and Vink, M. (2010). On the role of the striatum in response inhibition. PLoS One 5:e13848. doi: 10.1371/journal.pone.0013848
Zani, A., and Proverbio, A. M. (2003). The Cognitive Electrophysiology of Mind and Brain. Cambridge, MA: Academic Press.
Keywords: neural oscillations, de(synchronization), stop-signal task, Go/No-go task, response inhibition, cognitive inhibition, interference suppression, rule inhibition
Citation: Beppi C, Violante IR, Hampshire A, Grossman N and Sandrone S (2020) Patterns of Focal- and Large-Scale Synchronization in Cognitive Control and Inhibition: A Review. Front. Hum. Neurosci. 14:196. doi: 10.3389/fnhum.2020.00196
Received: 09 March 2020; Accepted: 30 April 2020;
Published: 25 June 2020.
Edited by:
Seiki Konishi, Juntendo University, JapanReviewed by:
Junichi Chikazoe, National Institute for Physiological Sciences (NIPS), JapanCopyright © 2020 Beppi, Violante, Hampshire, Grossman and Sandrone. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carolina Beppi, Y2Fyb2xpbmFiZXBwaUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.