- Department of Plant Science and Crop Protection, University of Nairobi, Nairobi, Kenya
Mango (Mangifera indica) is the second most economically important fruit in Kenya for local and export markets. Huge postharvest losses estimated between 30 to 50% characterize the mango value chain due to its climacteric nature and high perishability. These losses are exacerbated during ripening. However, the fruit’s shelf-life can be extended through the application of 1-Methylcyclopropene (1-MCP), an inhibitor of ethylene action. The efficacy of 1-MCP is affected by maturity at harvest, its formulation and concentration, and exposure time. This study sought to establish the effectiveness of the 1-MCP dosing range for the Tommy Atkins' mango variety harvested at two maturity stages defined subjectively based on shoulder elevation and objectively on flesh color as stage 1 (mature green) and stage 2 (advanced maturity). A homogeneous batch of 60 fruits from each maturity stage was exposed to two 1-MCP formulations (SmartFresh™–SmartTabs™ and SmartFresh™–Inbox Sachet). SmartFresh™–SmartTabs™ was applied at 0.5, 1.0, and 2.0 ppm concentrations for 12hrs and 24hrs while SmartFresh™–Inbox Sachet was applied at 0.5, 1.0, 2.0, and 4.0 ppm concentrations for 12hrs only. All treatment combinations were ripened at ambient conditions (25±3°C; 60±5% relative humidity). Samples of three fruits were taken at 3-day intervals for measurement of ethylene evolution, respiration rates, total soluble solids (TSS), color, and firmness. The 1-MCP response was significantly (P≤0.05) affected by maturity stage, 1-MCP treatment concentration, and exposure time. Untreated fruits exhibited higher ethylene peaks of 9.56 μL kg-1 h-1 and 13.29 μL kg-1 h-1 for stages 1 and 2 respectively. Stage 1 fruits subjected to 2.0ppm SmartFresh™–SmartTabs™ recorded a lower ethylene peak of 5.62 μL kg-1 h-1 and 3.62 μL kg-1 h-1 compared to fruits subjected to 1.0 ppm concentration, which recorded an ethylene peak of 5.95 μL kg-1 h-1 and 4.93 μL kg-1 h-1 for 12hrs and 24hrs respectively. Stage 2 fruits subjected to SmartFresh™–SmartTabs™ formulation, also delayed the ethylene peak with 2.0ppm recording ethylene peak of 6.55 μL kg-1 h-1 and 5.32 μL kg-1 h-1 compared to 1.0 ppm, which recorded 7.61 μL kg-1 h-1 and 7.15 μL kg-1 h-1 for 12hrs and 24hrs respectively. Stage 1 fruits subjected to SmartFresh™–Inbox Sachet at 4.0ppm lowered the ethylene peak to 3.89 μL kg-1 h-1. This concentration recorded the lowest peak compared to 5.54 µL kg-1 h-1, 5.12 μL kg-1 h-1, and 4.27 μL kg-1 h-1 for 0.5, 1.0, and 2.0ppm respectively. Interaction of 1-MCP concentration, exposure time, and maturity stage delayed other ripening-related changes including a decrease in hue angle, firmness, and increase in TSS. Results indicated that ripening was delayed by 1-MCP at an early stage of maturity; while the shelf life of treated fruits increased with increasing 1-MCP concentrations. SmartFresh™–SmartTabs™ applied at 2.0ppm for 24 hours achieved an increased shelf life of 12 days compared to the control in stage 1. SmartFresh™–Inbox Sachet applied at 2.0ppm and 4.0ppm significantly extended shelf life to 24 days and 21 days for stages 1 and 2 respectively. Overall, the findings of this study suggest that 2.0ppm of the ‘powder’ formulation and 4.0ppm of the ‘Inbox Sachet’ formulation of SmartFresh™ would offer optimal effects at 24hrs and 12hrs exposure respectively. We, therefore, wish to recommend these two formulations at the reported levels for commercial application in the Tommy Atkins' mango variety to prolong postharvest shelf life and maintain desirable quality attributes.
Introduction
Mango (Mangifera indica) is the second most economically viable fruit in Kenya after banana (Musa spp) produced for local and export markets (HCD, 2020). Mango production contributes to both the Agricultural and National Gross Domestic Product by about 5% and 2% respectively (HCD, 2019). The fruit is highly nutritious with carbohydrates, proteins, fats, minerals, organic acids, and vitamins as part of the nutritional components. The nutritional composition, delicacy, and flavor have led to the mango fruit being termed “the king fruit” (Rajan, 2022). In Kenya, mangoes come to season between November and March, with a peak period between January and February. The seasonality of this fruit creates scarcity during the offseason and wasteful surplus during the glut period (Maloba et al., 2017). Mango fruits suffer immeasurable postharvest losses and have a short life span from harvesting to consumption (Prasantha and Amunogoda, 2013). According to FAO (2020), the magnitude of postharvest losses in fresh mango fruits in Kenya is estimated to be greater than 50% per annum. Postharvest losses in mango fruits can be attributed to fast deterioration after harvest which is accompanied by increased rates of respiration, high ethylene evolution, physiological disorders, and overall senescence (Xu et al., 2017; Sakhale et al., 2018).
Mango is a climacteric fruit that is highly affected by ethylene during ripening (Gao et al., 2020; Manigo and Antibo, 2022). The presence of ethylene in the storage environment and internal evolution by the ripening fruit accelerates the ripening process (Thongkum et al., 2018; Liu et al., 2020; Manigo and Antibo, 2022). Therefore, techniques that can reduce or regulate ethylene production and action along the mango supply chain from the time of harvest until the point of consumption or utilization are important for quality preservation and extension of the fruit’s shelf life. In addition, other complementary practices and technologies including proper harvest practices, packaging, temperature control, proper transportation and storage conditions are required to preserve the fruit quality (Sivakumar et al., 2011; Lobo and Sidhu, 2017).
One of the innovative strategies applied to minimize postharvest losses in fruits has involved the use of ethylene inhibitors to minimize its evolution rate and its action during transportation and storage (Gaikwad et al., 2020; Yuan et al., 2023). The use of 1-Methylcylopropene (1-MCP) as an ethylene inhibitor and suppressor is interrelated with the fruits’ ethylene-sensitive sites and how these endogenous and exogenous factors affect the fruits’ response to ethylene sources. The use of 1-MCP has been reported to slow down the ripening and softening of fruits (Gwanpua et al., 2018; Yuan et al., 2023). A study by Lin et al. (2022) reported that 1-MCP functions to prevent the cascade of reactions leading to the ripening stage and aging of climacteric fruits. Therefore, the postharvest shelf life of mango fruit could be prolonged through the employment of 1-MCP (Dias et al., 2021).
Previous studies have shown that application of 1-MCP can be challenging for fruits such as mango, as the goal is to delay ripening rather than completely inhibit it (Watkins, 2008). This requires careful control of both 1-MCP concentrations and the exposure period. Treatments involving phytohormones such as ethylene, have shown promising outcomes, however, the effectiveness of such treatments varies depending on the cultivar, timing of application, and the concentration of the compounds used (Dias et al., 2021). Determining a 1-MCP concentration that effectively minimizes the occurrence of physiological disorders while ensuring proper ripening is a significant challenge (Dias et al., 2021). The effectiveness of 1-MCP can vary significantly and is influenced by various factors, such as the fruit cultivar (Pan et al., 2016), stage of maturity (Ambuko et al., 2013; Rupavatharam et al., 2015; Satekge and Magwaza, 2022) application concentration (Gaikwad et al., 2020), and exposure duration (Gaikwad et al., 2020; Satekge and Magwaza, 2022). Studies conducted in banana fruit showed the green life of fruits treated with -MCP varied with the stage of maturity. The least mature fruit were shown to have a longer green life compared to the more mature fruits (Harris et al., 2000). Ambuko et al. (2016) reported similar findings in ‘apple mango’ fruits where fruits harvested at earlier maturity were more responsive to -MCP treatment and had a significantly longer shelf life compared to fruits of advanced maturity.
Apart from commodity factors, treatment factors have been shown to affect the efficacy of 1-MCP. Researchers who employed 1-MCP with different delivery methods and on different climacteric fruits reported concentrations ranging from a minimum of 0.5 ppm to a maximum of 2.0ppm (Barthakur, 2017; Gaikwad et al., 2020; Satekge and Magwaza, 2022). Furthermore, studies have also proposed various 1-MCP exposure durations, ranging from 12 to 24 hours, for different climacteric fruits (Barthakur, 2017; Sakhale et al., 2018; Satekge and Magwaza, 2022).
Therefore, selection of the effective 1-MCP treatment combination (concentration and exposure duration) for target fruits should be informed by previous studies in similar fruits. Given the dynamic nature of fruits, and how various factors affect response to ethylene, more specific research studies are required to offer a more tailored approach to determine the optimum dosage rates of applying 1-MCP. The current study sought to determine the response of ‘Tommy Atkins’ mangos to various dosing ranges of two innovative 1-MCP formulations applied on fruits harvested at different stages of maturity.
Materials and methods
Plant material and process
‘Tommy Atkins’ mango fruits were sourced from a commercial orchard in Machakos County in Kenya. Machakos County experiences semi-arid climactic conditions and is found in lower midland agroecological zones IV and V of Kenya. The annual average temperatures range between 22°C- 24°C and receive an annual rainfall ranging between 250-400 mm and 800-900 mm per annum in the lower regions and higher regions respectively (Manzi and Gweyi-Onyango, 2021).
The mango fruits were manually harvested at two stages of maturity – stage 1 and stage 2. The two stages were determined by an accurate record of days after flowering maintained by farmers, along with maturity indices assessed subjectively by shoulder elevation and objectively by flesh color. Stage 1 fruits represented early maturity, occurring 90 to 100 days after the 50% flower set (mature green). The flesh color was predominantly white, with a slight yellow coloration beginning to develop around the seed. Stage 2 consisted of mango fruits at an advanced maturity stage, occurring 110 to 120 days after the 50% flower set. Additionally, full cheeks and a yellow-orange flesh color characterized these fruits. All the harvested fruits were transported to the experimental site in crates lined with dampened newspapers to stimulate evaporative cooling during transit. Upon arrival at the Postharvest Laboratory, the fruits were sorted for uniformity based on size and freedom of damage and allowed to cool. All the graded fruits were washed with a 10% sodium hypochlorite solution and left to air dry before applying the 1-MCP treatments. Fruits of each maturity stage were then divided into homogenous batches containing 60 fruits and exposed to two 1-MCP formulations (SmartFresh™–SmartTabs™ (0.63%) and SmartFresh™–Inbox Sachet (0.14%). SmartFresh™–SmartTabs™ was applied at 0.5, 1.0, and 2.0 ppm concentration for 12hrs and 24hrs in sealed containers while SmartFresh™–Inbox Sachet was applied at 0.5, 1.0, 2.0, and 4.0 ppm concentration for 12hrs only. All the treated fruits alongside the untreated control were allowed to ripen at ambient room conditions (25 ± 3°C; 60 ± 5% relative humidity). The experiment was laid down as a completely randomized design with a factorial arrangement. Samples of three fruits were taken at a three-day interval for measurements of ripening changes including, ethylene evolution, respiration rates, color (peel and pulp), firmness (peel and pulp), and total soluble solids (TSS).
Determination of ripening parameters
Ethylene evolution
Three mangoes from each treatment were incubated for one hour in a two-litre (2L) airtight container that was fitted with a rubber septum. Approximately, 10ml of the gas from the headspace was drawn using a hypodermic syringe and injected into a gas chromatograph (GC- 9A, Shimadzu Corp., Kyoto, Japan) fitted with a flame ionization detector. Ethylene (C2H4) was then expressed as μL kg-1 h-1.
Respiration rate
Headspace gas was collected from the same containers incubated for ethylene extraction following the procedure described above and injected into a gas chromatograph (Models GC-8A, Shimadzu Corp., Kyoto, Japan) fitted with a thermal conductivity detector. The rates of respiration estimated from the CO2 gas produced in µl/g/h (later converted to ml/Kg/hr) were expressed using the formula below:-
Where: -
K = calibration value (µL equivalent to 1 cm peak height on gas chromatography)
R = volume of gas injected for analysis (ml)
H = peak value (cm)
V = volume of incubation container (ml)
W = weight of fruit (g)
T = incubation period (h)
Firmness (peel and flesh)
The penetration force of three randomly selected fruits from all the treatments was determined destructively using a fruit hardness meter (Model FR-5120, Sun Scientific Co. Ltd, Japan) fitted with a 5 mm plunger. The peel penetration force was determined on three unpeeled regions while the pulp firmness was measured on peeled parts along the midsection part of the fruit. The resistance of the flesh to penetration of the plunger was then expressed in Newton (N).
Color (peel and flesh)
The a∗, b∗, and L hue color values of the mango fruits were determined through the use of a portable color meter (Model TES 135A, Taiwan, China) and recorded. The a* and b* of the peel and the flesh were recorded. The color changes were measured at the regions along the midsection of the fruits from three randomly selected fruits in all the treatments and then determined in degrees (H°). The conversion of a* and b* coordinates recorded was done as follows:
Total soluble solids
The total soluble solids (TSS) content of mango fruits was determined using a pocket refractometer (Model 500, Atago, and Tokyo, Japan). During each sampling day, a fruit was randomly picked and the pulp macerated to extract 3ml of juice. The pocket refractometer has a sensor that was zeroed using distilled water then the extracted fruit juice was placed on the glass prism sensor and the reading was recorded. The TSS was indicated as a 0Brix.
The overall shelf-life
The longevity of the storage life was determined by keeping a record of the total days the mango fruits in different treatments took to fully ripen. This was guided by a pre-destined end stage where mango fruits were considered marketable.
Data analysis
The data collected was analyzed using the GenStat 15th Edition statistical program. Analysis of variance (ANOVA) was used to test for significant differences among treatments for each parameter and means separated using Fisher’s protected least significant difference at P ≤0.05.
Results
Ethylene evolution
The rate of ethylene evolution increased gradually to peak levels as the ripening progressed, regardless of the stage of maturity or the 1-MCP treatment applied. Treated fruits generally exhibited lower ethylene evolution with reduced peaks compared to untreated fruits. Ethylene peak for untreated fruits occurred earlier on the 9th and 6th day in stages 1 and 2 respectively. The peak was delayed by 3 to 6 days respectively for 1-MCP-treated fruits. For instance, mangoes exposed to 12-hour treatment of SmartFresh™-SmartTabs™ at 1.0ppm and 2.0ppm achieved ethylene peaks on days 12 and 15 (stage) and on days 9 and 12 (stage 2) respectively. Besides delaying the ethylene peak period, the amount of evolved gas was also significantly lowered with untreated fruits recording higher peak averages of 9.56 μL kg-1 h-1 and 13.29 μL kg-1 h-1 in stages 1 and 2 respectively. The SmartFresh™-SmartTabs™ treated fruits (in stage 1) exposed at 1.0ppm achieved relatively lower peaks of 5.95 μL kg-1 h-1 and 4.93 μL kg-1 h-1 for 12 and 24-hour exposure respectively. In stage 2, (under similar treatment), mean peaks of 7.61 μL kg-1 h-1 and 7.15 μL kg-1 h-1 were recorded for 12 and 24-hour exposure respectively. The second formulation of SmartFresh™-InBox Sachet also revealed a delayed ethylene peak with a delay being achieved in favor of stage 1 fruits compared to stage 2. A SmartFresh™-InBox Sachet exposure at 2.0 ppm extended the ethylene peak period by three days, achieving the peak on day 18 and 15 respectively for stage 1 and 2. A concentration of 4.0 ppm of the same treatments recorded the peak ethylene evolution rate after 21 days of storage in stage 1. Additionally, SmartFresh™-InBox Sachet treatment at 2.0ppm, recorded a peak ethylene evolution of 4.27 μL kg-1 h-1 at stage 1, compared to 5.41 μL kg-1 h-1 recorded in stage 2 (Figure 1). Overall, mangos harvested at stage 1 responded better to the two 1-MCP formulation achieving longer storage period before the outburst of ethylene peaks.
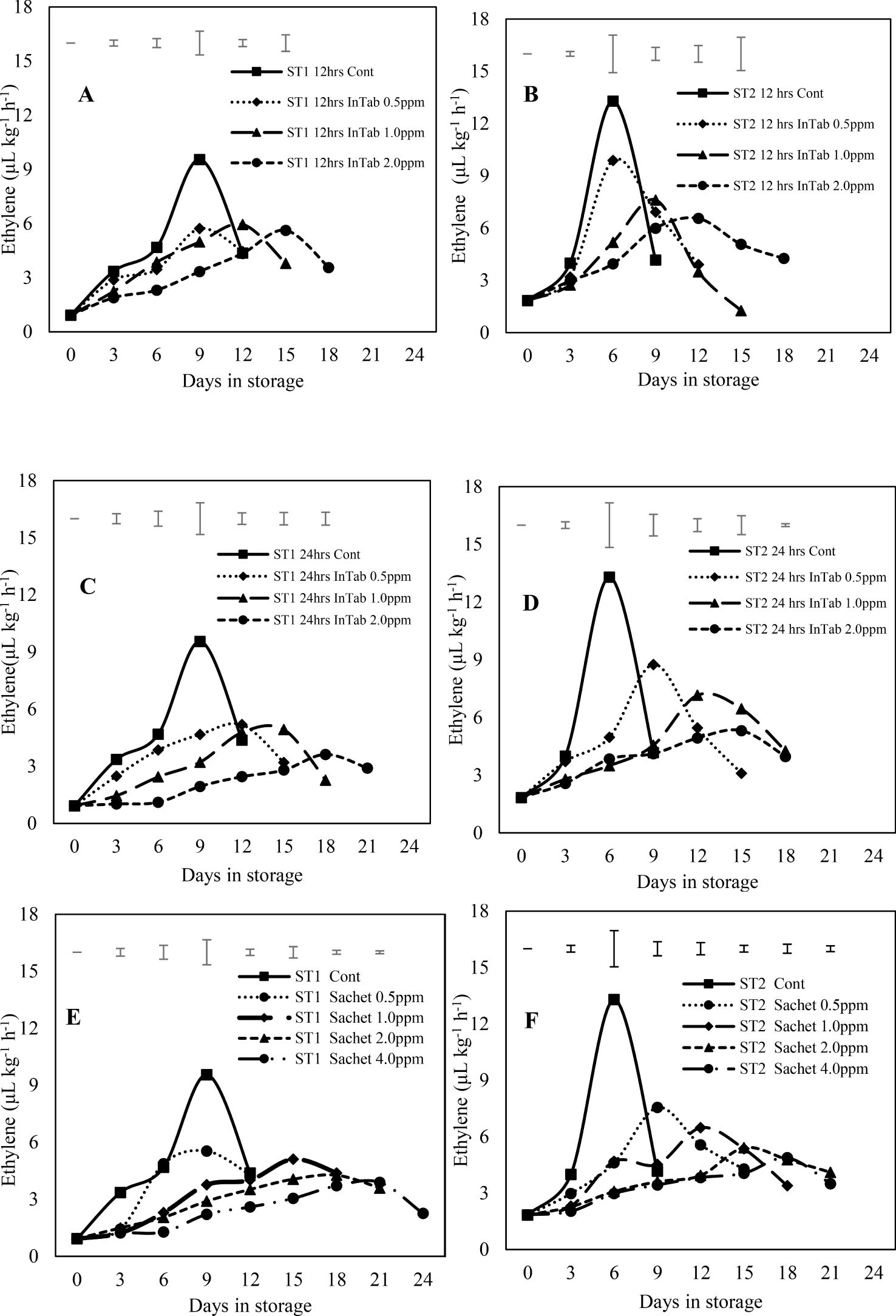
Figure 1. Showing the rates of Ethylene evolution influenced by different 1-MCP formulations. (A–D) represent fruits subjected to SmartFresh™-SmartTabs for 12 and 24-hour durations respectively whereas (E, F) represents fruits subjected to SmartFresh™-Inbox Sachet. The legends show different 1-MCP concentrations (PPM). ST1 and ST2 represent stages 1 and 2 of maturity respectively. Top Bars represent LSD at P ≤ 0.05.
Respiration rates
Respiration gradually increased to peak levels and drastically declined towards the end of the storage period with significantly (P<0.05) lower peaks in all 1-MCP treated fruits. Fruits harvested at stage 1 generally had lower levels of respiration. Stage 1 fruits subjected to SmartFresh™- SmartTabs™ attained peak levels of 54.09, 47.94, and 44.83 mL kg-1 h-1 for 0.5, 1.0, and 2.0ppm concentrations subjected to 12-hour duration respectively while 24 hours of the similar 1-MCP product achieved 50.75, 45.29, and 39.77 mL kg-1 h-1 for 0.5, 1.0, and 2.0ppm concentrations respectively. Untreated stage 1 fruits exhibited a higher peak level of 63.13 mL kg-1 h-1, which was attained on day 9 of the storage period. Stage 2 fruits treated with SmartFresh™- SmartTab™ and exposed to 12-hour duration exhibited a peak level of 59.04, 52.74, and 46.97 mL kg-1 h-1 for 0.5, 1.0, and 2.0ppm respectively which was lower than 69.21 mL kg-1 h-1 recorded on day 6 by the untreated fruits. A similar climacteric pattern was observed in fruits subjected to the SmartFresh™-Inbox Sachet. Stage 1 fruits treated with SmartFresh™-Inbox Sachet had a delayed respiration peak compared to advanced maturity, which exhibited fewer days to respiration peak attainment. Treatment with SmartFresh™-Inbox Sachet recorded lower rates of respiration of 39.24 and 36.89 mL kg-1 h-1 in 2.0 and 4.0ppm respectively for stage 1. Similar treatment recorded peak levels of 43.46 and 39.82 mL kg-1 h-1 for 2.0ppm and 4.0ppm in stage 2 respectively (Figure 2).
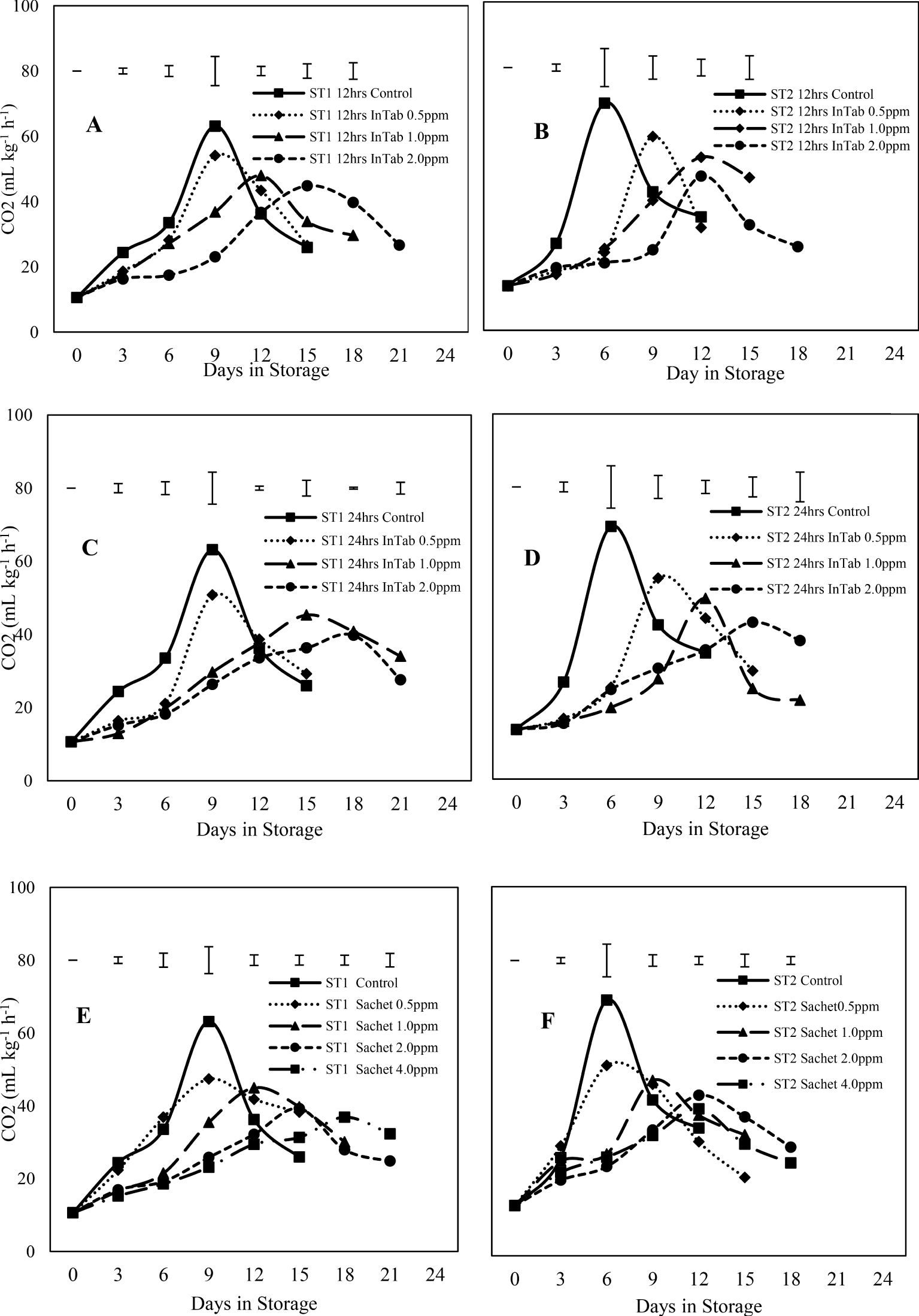
Figure 2. Showing the changes in respiration rate as influenced by different 1-MCP formulations. (A–D) represent fruits subjected to SmartFresh™-SmartTabs for 12 and 24-hour duration respectively whereas (E, F) represents fruits subjected to SmartFresh™-Inbox Sachet. The legends show different 1-MCP concentrations (PPM). ST1 and ST2 represent stages 1 and 2 of maturity respectively. Top Bars represent LSD at P ≤ 0.05.
Changes in peel firmness
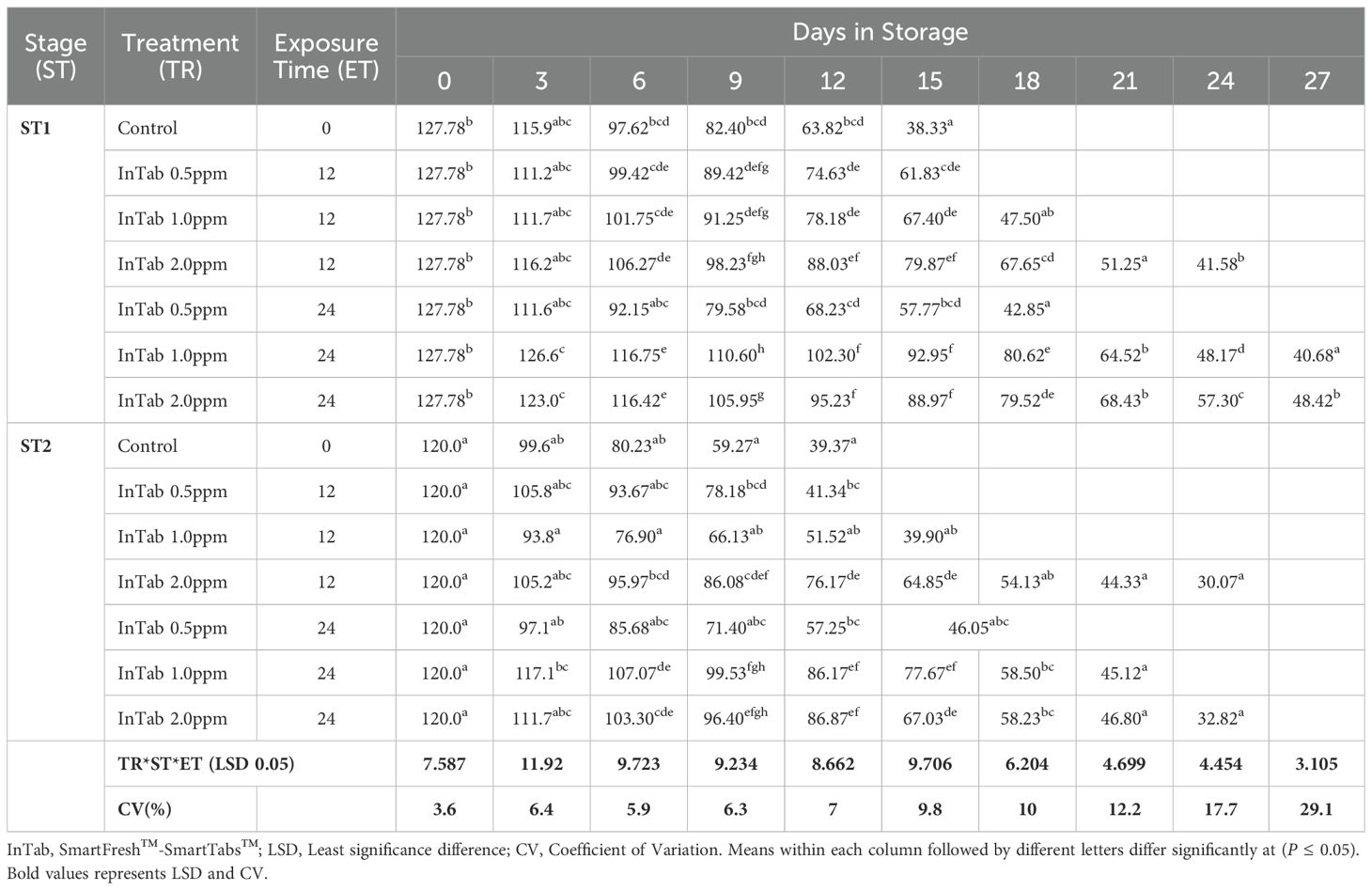
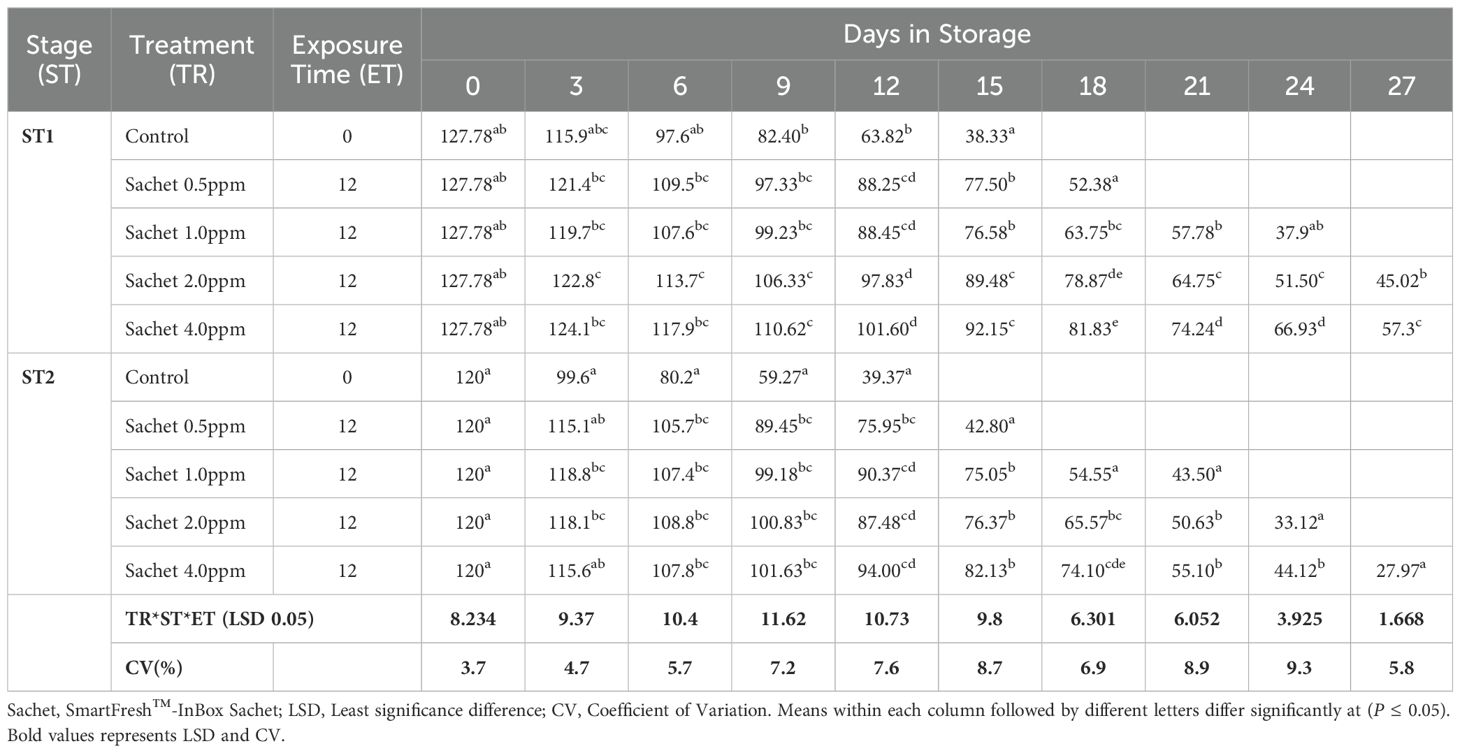
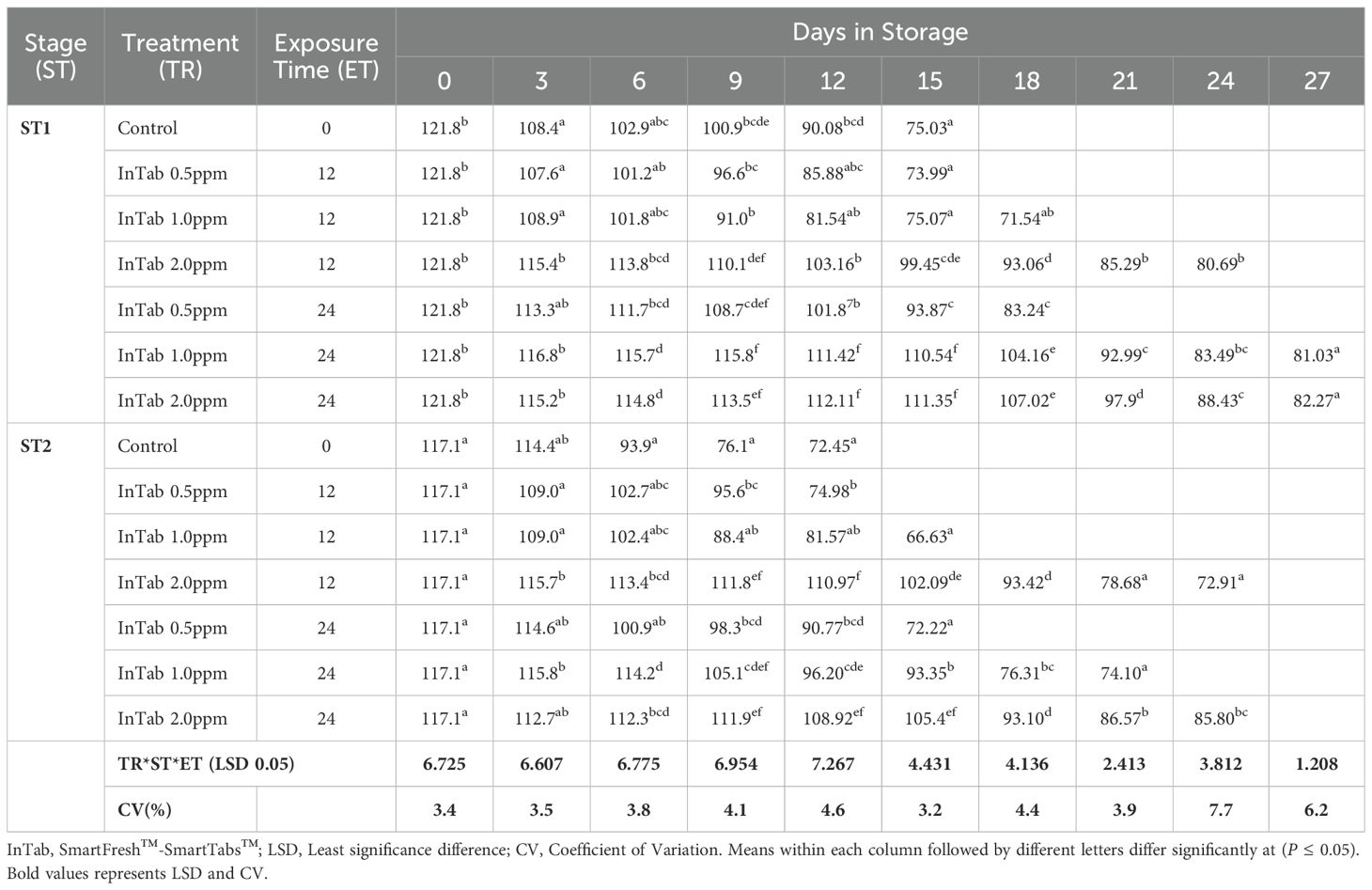
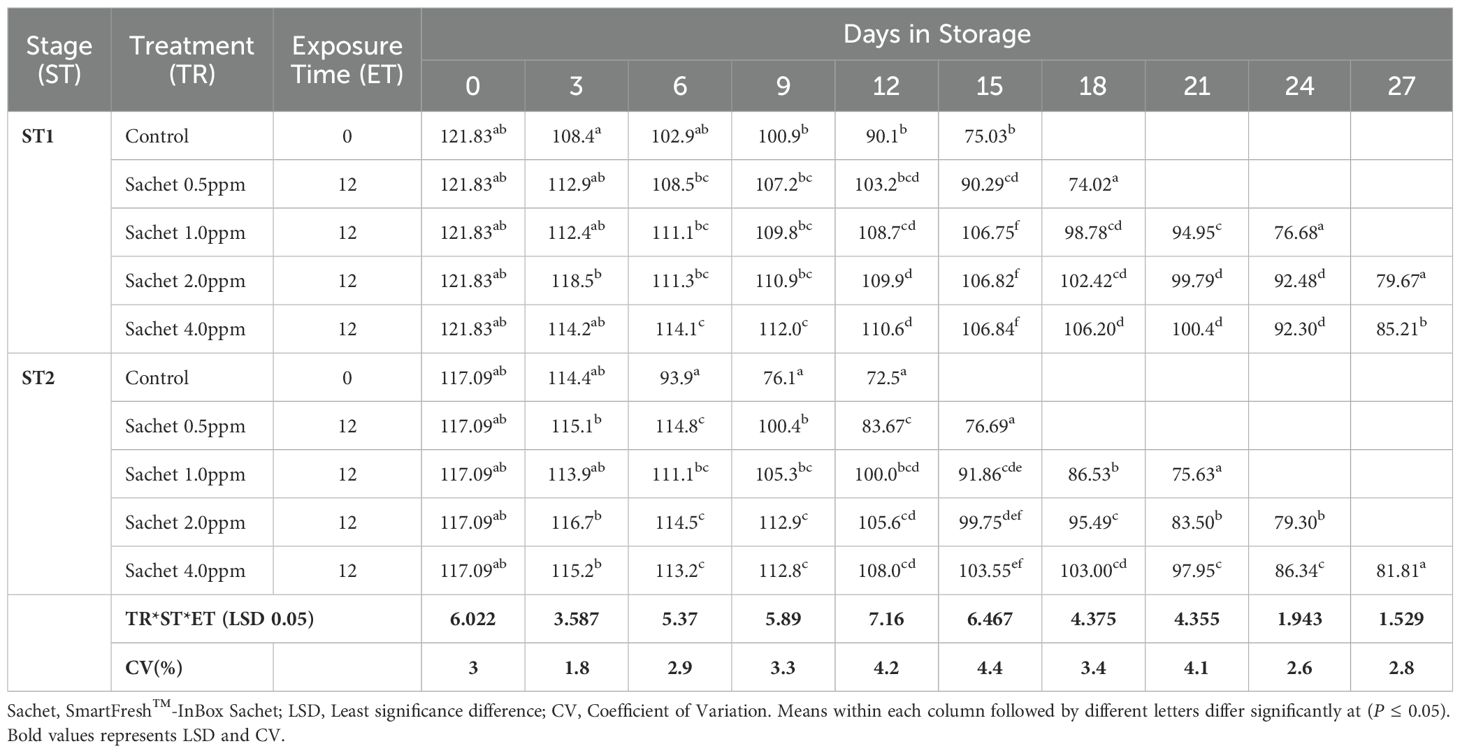
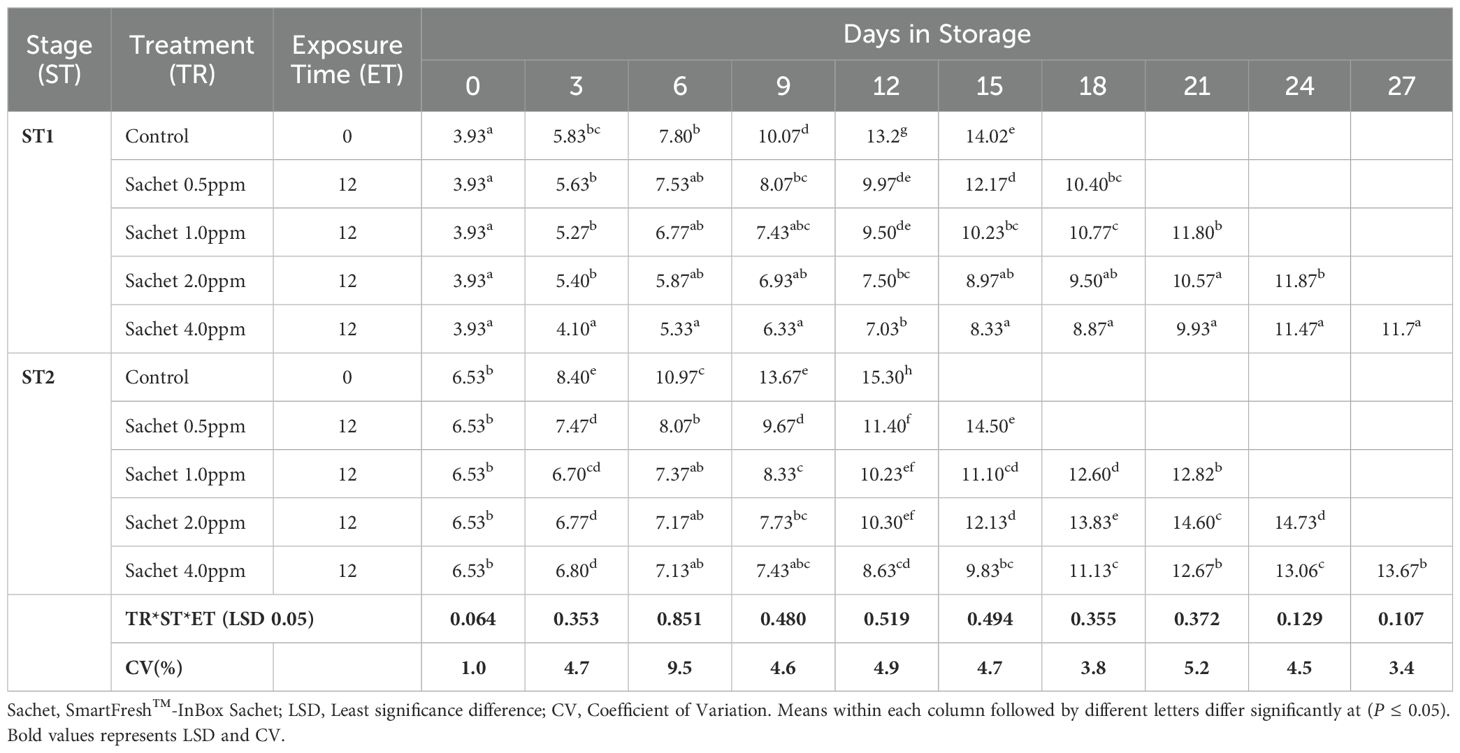
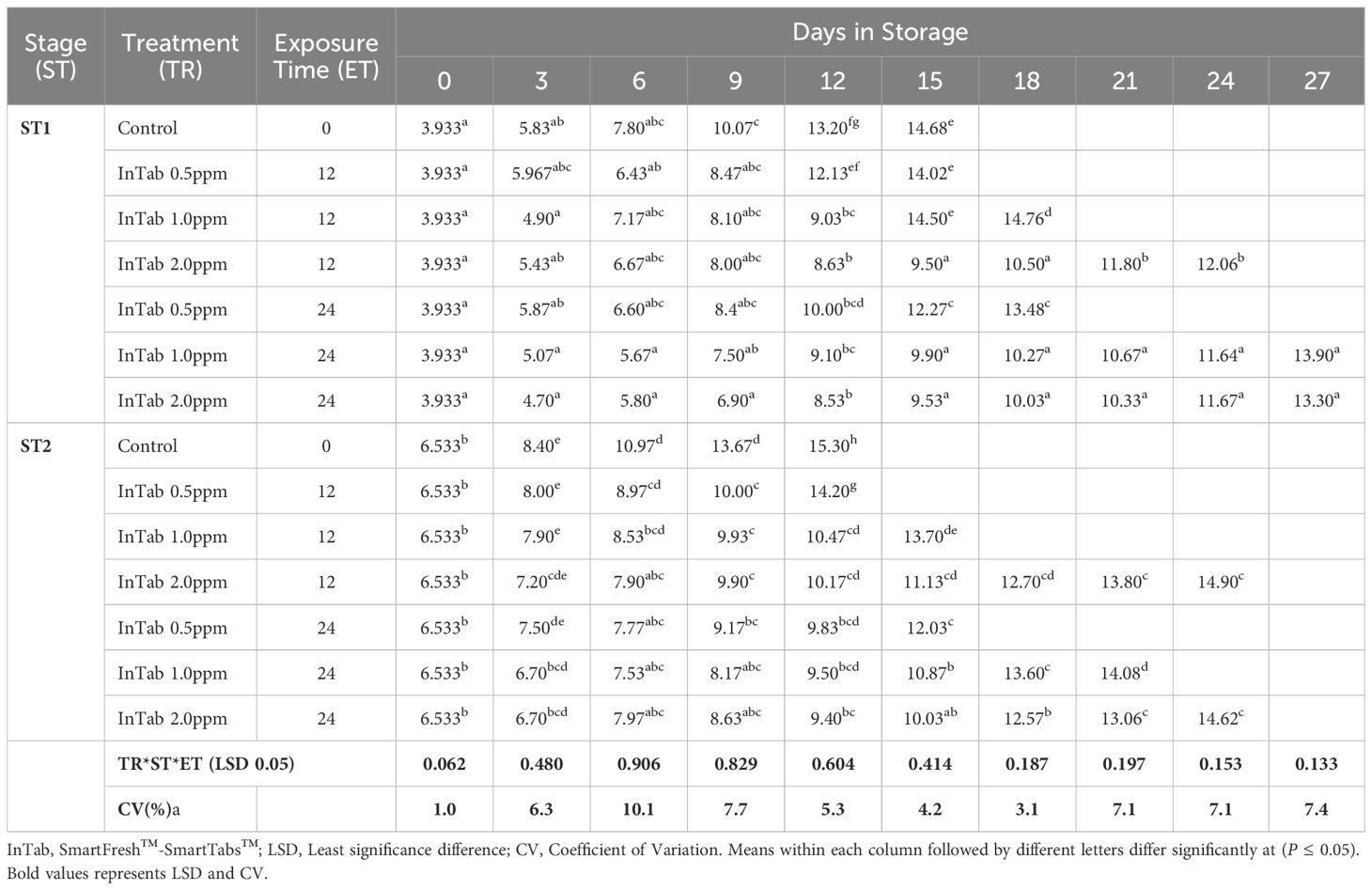
The fruit peel firmness gradually decreased as the fruit ripened in all the treatments. 1-MCP-treated fruits retained much of their initial peel firmness as compared to the controls. The interaction of 1-MCP treatments, stage of maturity, and exposure time significantly (P ≤0.05) affected the peel firmness (N). Treated fruits were generally firmer irrespective of 1-MCP formulation and the concentrations. Stage 1 fruits recorded an average peel firmness of 127.78 N as compared to 120.0 N peel firmness recorded by stage 2 fruits. SmartFresh™- SmartTabs™ 2.0 ppm concentration recorded a firmness of 41.58N and 48.42N for stage 1 at 12 and 24-hour exposure times respectively (Table 1A). Similar treatment was observed to attain a firmness of 30.07N and 32.82N for stage 2 at 12 and 24-hour exposure times respectively at the end of the storage period (Table 1A). In addition, fruits treated with SmartFresh™–Inbox sachet exhibited a gradual decline of peel firmness as the fruits ripen. Fruits exposed to SmartFresh™–Inbox Sachet at 2.0ppm, recorded a firmness of 64.75N on day 21 of storage in stage 1, which was much retained from the initial firmness compared to stage 2, which attained a firmness of 50.63N on the same sampling day (Table 1B).
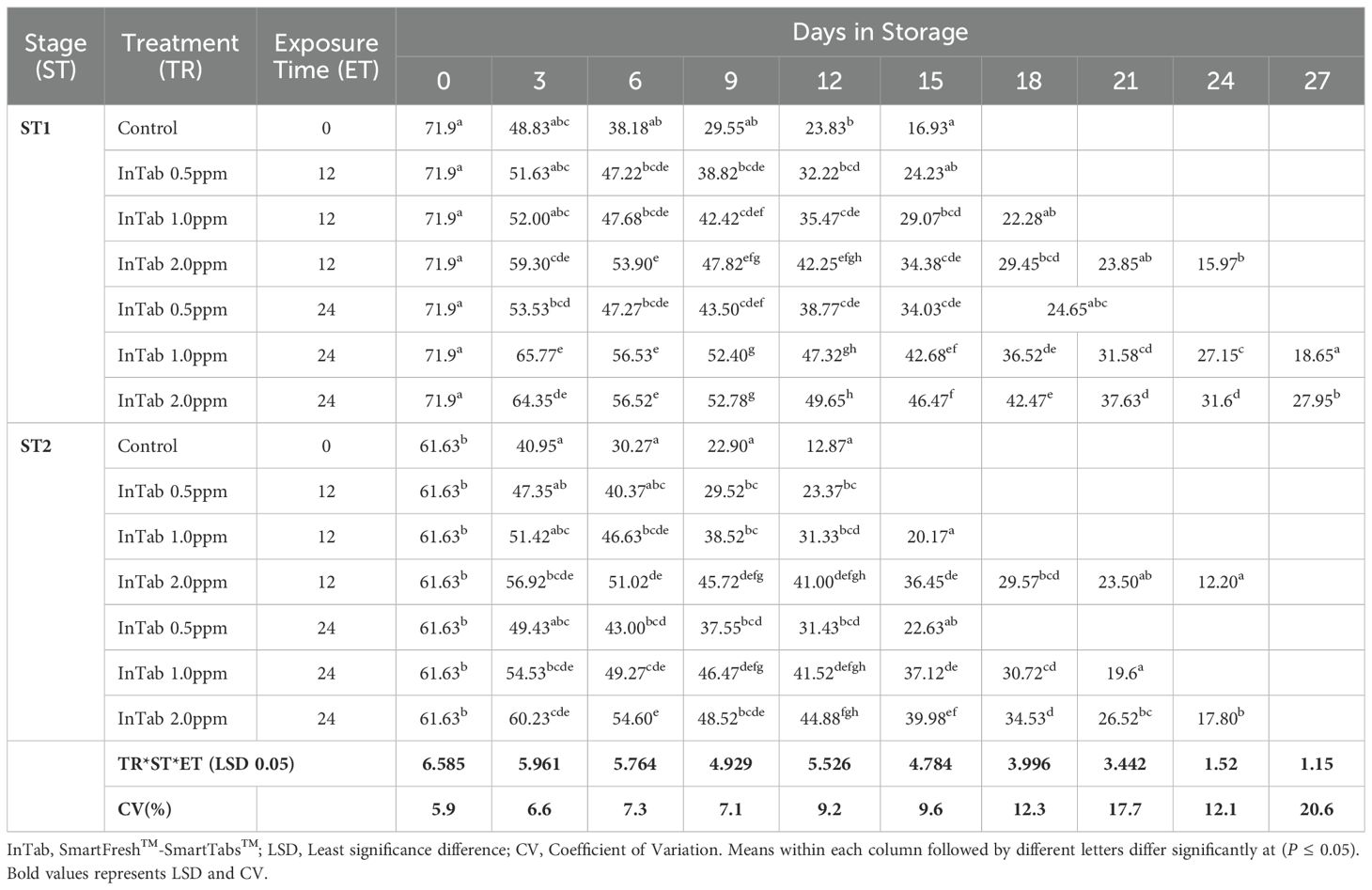
Changes in pulp firmness
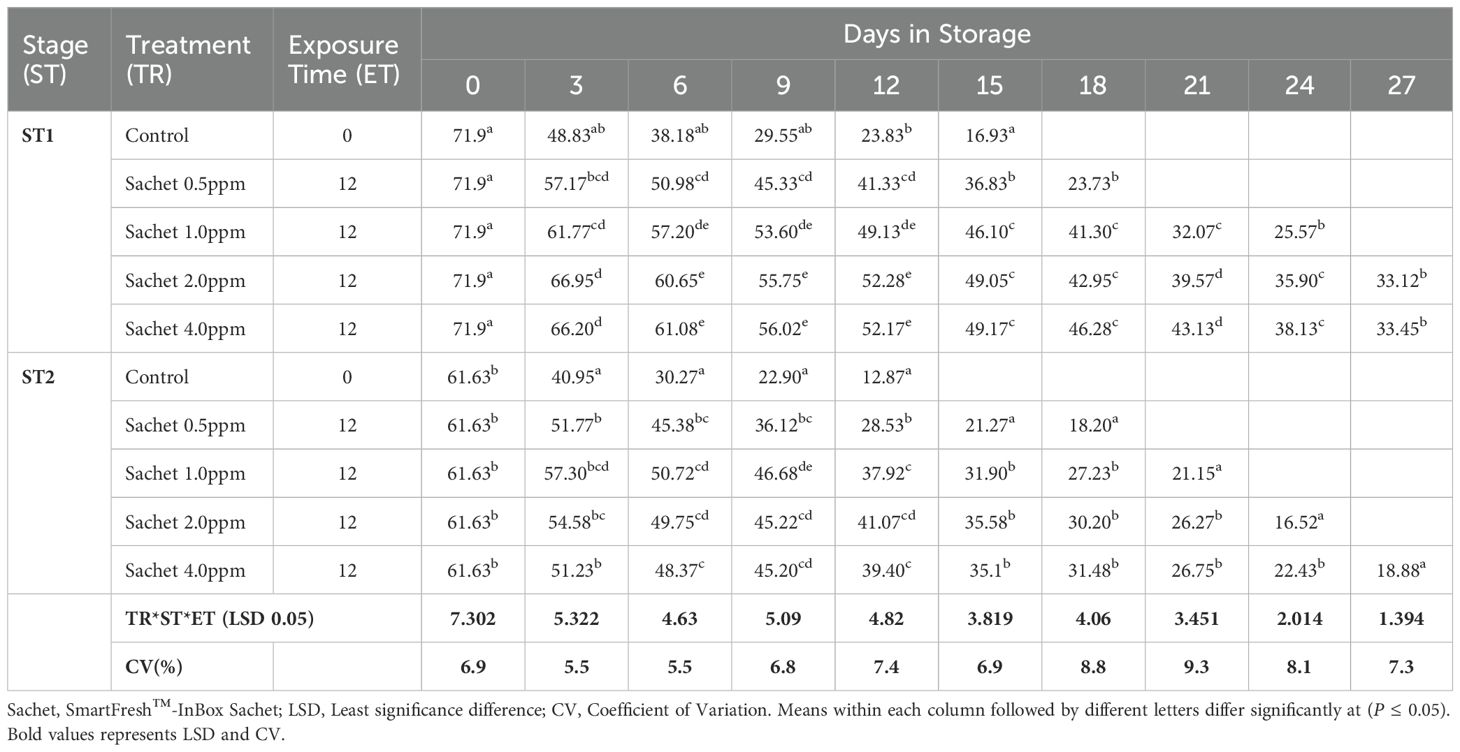
Pulp firmness gradually declined within storage. Generally, stage 1 fruits exhibited a delayed rate of softening compared to stage 2. A longer exposure period of 24 hours had a higher impact in both stages of maturity, with stage 2 fruits displaying a slowed rate of decline of pulp firmness. Stage 1 fruits treated with SmartFresh™- SmartTabs™ for a 24-hour exposure period recorded a firmness of 27.15N and 31.6N on day 24 of storage for 1.0ppm and 2.0ppm respectively (Table 1C). On the other hand, stage 2 fruits had a pulp firmness of 20.17N and 19.6N for the 1.0ppm in 12 and 24-hour exposure time respectively, which was attained at the end of their preservation period (Table 1C). Using SmartFresh™-Inbox Sachet, stage 1 control recorded pulp firmness of 16.93 N at day 15 (end of storage period) compared to the much-retained pulp firmness of 49.05N and 49.17 N for both 2.0ppm and 4.0ppm respectively for stage 1 fruits on the same day (Table 1D). On the other hand, stage 2 untreated fruits had attained pulp firmness of 12.87 N on day 12 as compared to 41.07 N and 39.40 N for fruits treated with SmartFresh™–Inbox Sachet 2.0ppm and 4.0ppm respectively on the same day which was the end stage of untreated stage 2 fruits (Table 1D).
Changes in fruit peel color
Irrespective of the stage of maturity, the peel hue angle reduced gradually as the ripening process advanced. It was observed that Stage 1 fruits exhibited a relatively higher initial peel hue angle of 121.830 compared to 117.090 recorded by Stage 2 fruits. Fruits that were subjected to 1-MCP formulations were observed to exhibit a higher retained peel color compared to untreated fruits, regardless of the stage of maturity, 1-MCP treatments, and exposure period. The peel color was significantly (P ≤0.05) affected by the interaction between stages of maturity, duration of exposure time, and 1-MCP treatments. In stage 1 fruits, treatment with SmartFresh™- SmartTabs™ 2.0 ppm concentration recorded a hue angle of 80.690 and 82.270 for 12 and 24-hour exposure times respectively. Similar maturity stages and formulation at 1.0 ppm recorded 71.540 and 81.030 at the end of their storage period for 12 and 24-hour exposure periods respectively. In stage 2, a similar 1-MCP formulation with a concentration of 1.0ppm was observed to attain a hue angle of 66.630 and 74.100 for 12 and 24-hour exposure time respectively (Table 2A). On the other hand, SmartFresh™–Inbox Sachet 2.0ppm recorded a hue angle of 79.670 and 79.300 for stages 1 and 2 respectively at the end of their storage period (Table 2B). Similarly, at the end of the storage period, a concentration of 4.0ppm achieved a hue angle of 85.210 and 81.810 for stages 1 and 2 respectively (Table 2B).
Changes in fruit flesh color
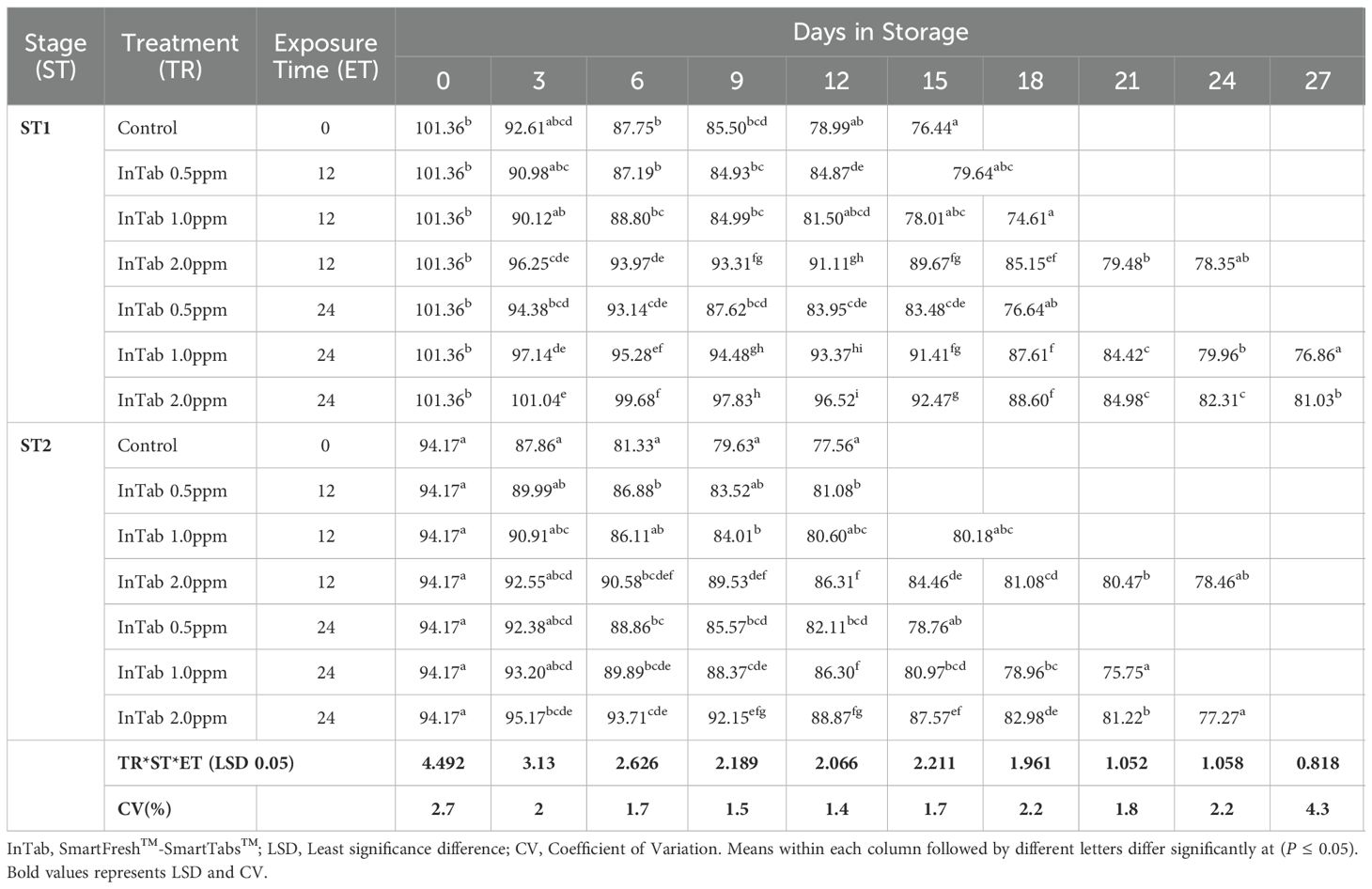
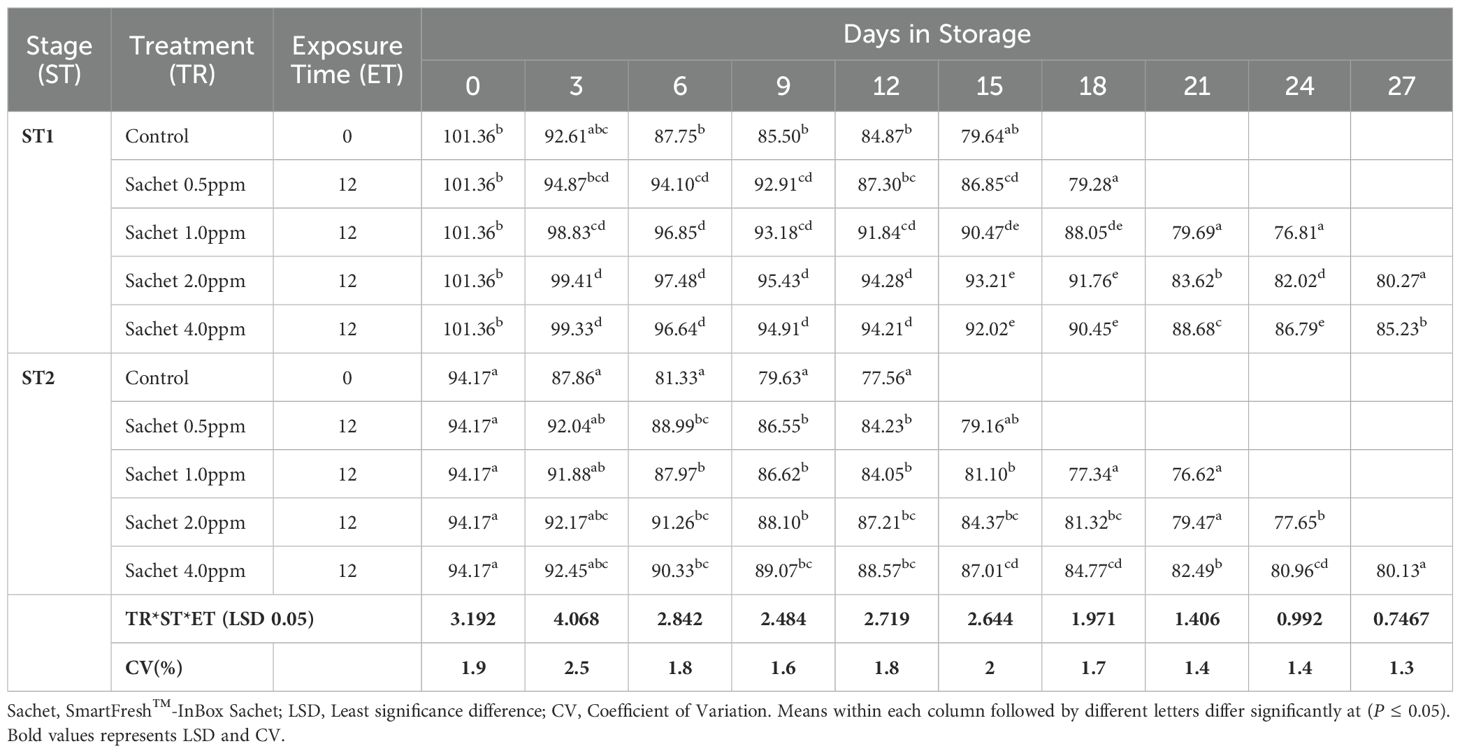
Similarly, the flesh color gradually changed from cream-white to yellow-orange color as the fruits ripened. Irrespective of the stage of maturity, 1-MCP formulations, concentrations, and exposure period, a gradual reduction of flesh hue angle trends was recorded in all fruits under this research. An initial hue angle of 101.360 and 94.170 was recorded for stages 1 and 2 respectively. Stage 1 fruits treated with SmartFresh™-SmartTabs™ at a concentration of 1.0 ppm recorded an average of 74.610 and 76.860 for 12 and 24-hour exposure periods respectively (Table 2C). Treatment with SmartFresh™- Inbox Sachet at 2.0ppm recorded a hue angle of 80.270 and 77.650 for stages 1 and 2 respectively at the end of their storage period compared to 75.030 and 72.500 hue angle for untreated stages 1 and 2 (Table 2D). All fruits that were subjected to 1-MCP formulations were observed to exhibit a higher retained flesh hue angle for a prolonged period compared to untreated fruits regardless of the stage of maturity, 1-MCP treatments, and exposure period. The flesh hue angles were significantly (P ≤0.05) affected by the interaction between stages of maturity, exposure period, and 1-MCP treatments.
Changes in total soluble solids
As the fruits ripened, a gradual rise in the total soluble solid (TSS) content was recorded for all the treatments. The increase in TSS content was slower in treated fruits as compared to untreated fruits. Stage 1 fruits that were subjected to 1-MCP exhibited a much slower rise in the TSS content. Fruits’ TSS content was significantly (P ≤ 0.05) affected by the interaction of 1-MCP treatment, stage of maturity, and time of exposure. In stage 1, treatment using SmartFresh™-Inbox Sachet at a concentration of 4.0ppm, fruits exhibited a rise of up to 11.70 brix at day 27 compared to untreated which had risen to 14.680 brix at day 15 (Table 3A). SmartFresh™-Inbox Sachet at 2.0ppm had a slight difference compared to 4.0ppm and a slower rise of TSS was also observed; the concentration of 2.0ppm achieved TSS levels of 11.870 brix on day 27. In stage 2 where a similar treatment was made, 4.0ppm SmartFresh™-Inbox Sachet achieved a TSS level of 13.670 brix which was slightly higher than 14.730 brix exhibited by 2.0ppm concentration. In stage 2, the control experiment rose to levels of 15.300 brix at day 12 (end of storage period). This shows that irrespective of maturity stage, 4.0ppm, and 2.0ppm extended the shelf life and maintained a gradual increase in TSS level. Using SmartFresh™-SmartTabs™, 24-hour duration and at a concentration of 1.0ppm was observed to exhibit TSS levels of 13.900 brix at day 27 (end of storage period) in Stage 1 while the same concentration in Stage 2 achieved 14.080 brix at day 21, end of storage period (Table 3B).
Overall shelf life
The 1-MCP treated fruits had a significantly (P ≤ 0.05) longer shelf life compared to untreated fruits. Irrespective of 1-MCP products and stages of maturity, 1-MCP action on the ‘Tommy Atkins’ mango variety showed a consistent concentration-dependent response where the higher concentration generally resulted reduced rates of various ripening related changes and subsequently, a longer shelf life. Untreated stage 2 fruits attained a shelf life of 12 days while fruits of the same maturity stage treated with SmartFresh™-SmartTabs™ at a 24-hour exposure period attained a shelf life of 21 and 24 days for 1.0ppm and 2.0ppm respectively. Stage 1 fruits exposed to SmartFresh™-SmartTabs™ at 1.0 ppm for a 24-hour duration had a shelf life of 27 days. Fruits treated with SmartFresh™-Inbox Sachet at 2.0ppm had a shelf life of 27 and 24 days respectively for stage 1 and 2 (Figure 3).

Figure 3. Overall shelf life of Tommy Atkins’ mango fruits subjected to different stages of maturity, 1-MCP treatments, and different exposure periods. InTab represents SmartFresh™-SmartTabs™ while InBox represents SmartFresh™-Inbox Sachet. Top Bars represent the S.E of means.
Discussion
Mango fruit ripening is triggered, accelerated, and regulated by ethylene (Lobo and Sidhu, 2017; Gao et al., 2020). Effective ethylene management is crucial for maintaining the quality, freshness and extend the shelf life of mango fruit while contributing to the efforts to reduce postharvest losses throughout the supply chain (Ambuko et al., 2013; Manigo and Antibo, 2022). The use of 1-MCP as a synthetic compound has been applied for horticultural commodities to extend the shelf life and maintain the quality of harvested produce (Li et al., 2020; Valbuena-Tellez et al., 2023). 1-MCP works by inhibiting the action of ethylene, a naturally occurring plant hormone that promotes ripening and senescence (Lin et al., 2022). In this study, the effectiveness of two innovative formulations of -MCP namely SmartFresh™–SmartTabs™ and SmartFresh™–Inbox Sachet was tested in ‘Tommy Atkins’ mango fruits harvested at two stages of maturity (stage 1 and 2). Stage 1 fruits which exhibited lower initial ethylene evolution and respiration rates has a better response to 1-MCP that fruits harvested at an advanced maturity. Application of 1-MCP at an early stage of maturity was reported to inhibit the ethylene action, consequently delaying the onset of ripening, and maintaining the fruit quality for a longer period (Sakhale et al., 2018; Wang et al., 2021).
Fruits harvested at the advanced maturity (stage 2) exhibited higher initials (1.8 μL kg-1 h-1) of ethylene gas levels compared to stage 1 (0.9 μL kg-1 h-1). The high initial ethylene levels implied that ethylene evolution associated with ripening had already kicked in before the 1-MCP treatment. However, applying 1-MCP at this stage still slowed down the overall climacteric peak, and delayed the ripening process. Similar trends were also reported for respiration rates. The reduction of respiratory climacteric pattern in 1-MCP treated fruits as the ripening process advanced may be due to the corresponding slowed ethylene evolution and the associated decline in metabolic activities that drive the ripening process (Silué et al., 2022). A similar reduction in ethylene and respiration evolution with 1-MCP application has been reported in mango (Razzaq et al., 2016; Li et al., 2022), purple passionfruit (Ambuko et al., 2014), plums (Lin et al., 2018), root mustard (Lin et al., 2022), and persimmon (Wang et al., 2021).
Peel and pulp firmness gradually decreased as the fruit ripening process advanced. Untreated and lower concentrations of 1-MCP resulted in a faster rate of peel and pulp softening while those subjected to higher concentrations of 1-MCP retained much of the initial firmness. Ethylene triggers the activity of enzymes such as polygalacturonase, which are responsible for breaking down pectin in the cell walls of fruits, leading to softening (Singh et al., 2019; Lin et al., 2020). The retention of firmness in 1-MCP treated mango fruit is attributed to the inhibition of ethylene perception and action (Chea et al., 2019; Ren et al., 2020). The action of 1-MCP in blocking the ethylene response led to a cascade of activities including reduced degradation by ripening associated enzymes, enhanced preservation of the structural integrity of the cell walls and consequently retaining fruit firmness. The use of 1-MCP not only delays the initial ripening but also inhibits the activities of enzymes such as cellulose and pectinase, which are involved in the degradation of cell wall components resulting in the softening of the fruits (Singh et al., 2019).
Changes in fruit color are the most common subjective judgment and perception of fruit ripening and by consumers (Lauricella et al., 2017; Chen et al., 2022). In the current study, 1-MCP treatments suppressed the skin color changes in Tommy Atkins’ mango fruits as depicted by the different paces of ripening in both stages of maturity. It was evident that higher concentrations of 1-MCP treatment resulted in a slower color (hue angle) change while lower concentrations and untreated controls had a rapid rate of color change. Irrespective of the exposure period, SmartFresh™–SmartTabs™ at 1.0 ppm and 2.0 ppm, and SmartFresh™–Inbox Sachet at 2.0 ppm and 4.0 ppm maintained the low peel and flesh color change while 0.5 ppm in all treatments exhibited a faster color changes. Peel and flesh color changes in mango fruits occur due to the degradation of chlorophyll by the enzyme chlorophyllase and the increased in concentration of carotenoids (beta-carotene) as the ripening process advances (Hu et al., 2021; Chen et al., 2022). Application of 1-MCP affected the rate of degradation and/or concentration of these color pigments in the peel and flesh of mango fruits. The application of 1-MCP has previously been reported to retard the degradation of chlorophyll (Giovannoni et al., 2017; Liu et al., 2020). The effectiveness of 1-MCP in delaying the peel and flesh hue angle was similarly reported by Chen et al. (2022); Manigo and Antibo (2022), and Prabasari et al. (2023).
An increase in TSS content is a natural part of the maturation process in mango fruits and is often used as an indicator of ripeness and sweetness (Watkins, 2008; Hossain et al., 2014). The total soluble solids directly influence the sweetness and overall flavor of mango fruits. TSS levels gradually increased as the mango fruits ripened, irrespective of the maturity stage or 1-MCP treatments. However, the rate of TSS increase was significantly affected by the stage of maturity, 1-MCP concentration, and the exposure period. Untreated fruits and the fruits subjected to low (0.5 ppm) 1-MCP concentrations experienced faster ripening and attained a higher TSS content faster compared to fruits subjected to higher 1-MCP dosage. As the fruit ripens, enzymes break down complex carbohydrates into simple sugars leading to a rise in TSS content (Li et al., 2020). In this study, 1-MCP treatment slowed down the rate of TSS accumulation which could be attributed to slowed activity of the enzymes involved in the breakdown of complex carbohydrates into simple sugars. Reduced accumulation of TSS and sugars following 1-MCP treatments has been previously reported in other fruits including mango (Abu-Bakr et al., 2019; Li et al., 2020), apples (Tomala et al., 2020), pears (Mahajan et al., 2010), plum (Özkaya and Dündar, 2009), peaches (Huan et al., 2018), and tomato (Guillén et al., 2007).
Although ripening was significantly delayed following 1-MCP application in ‘Tommy Atkins’ mango fruits (both stages of maturity), a concentration-dependent response was observed. Thus, the higher concentrations of 1-MCP and longer exposure periods generally resulted in a greater delay in ripening-related changes resulting in a longer shelf life. 1-MCP treated fruits had an extended shelf life of between 9 to 15 days more compared to the untreated fruits. 1-MCP effectively increased the shelf life through inhibition of ethylene perception in the treated fruits. Ethylene triggers a cascade of physiological processes leading to softening and other ripening-related events (Gao et al., 2020). Thus, the prevention of ethylene action through 1-MCP application delayed the ripening-related processes highlighted above. It is essential to note that while 1-MCP is effective in slowing the rate of the ripening process and preserving certain fruit quality attributes, it may not entirely prevent the eventual ripening of the fruit (Prabasari et al., 2023).
Conclusion and recommendations
Various formulations of 1-MCP can be effectively used to suppress ethylene rates in ‘Tommy Atkins’ mango fruits. For optimal effects, a 24-hour exposure at 2.0ppm of SmartFresh™- SmartTabs™ and a 12-hour exposure at 4.0ppm of SmartFresh™- Inbox Sachet are recommended based on the findings of this study. The additional 9 days in storage achieved from the use of 1-MCP could offer significant leverage in slowing down the ripening and senescence of mangos. This will not only reduce the attributed losses through spoilage but also allow longer transit time and increased marketing period while maintaining their quality, appearance, and freshness. These results show that the stage of maturity, the formulation and concentration of 1-MCP, and its exposure period have a significant impact on the effectiveness of 1-MCP to delay ripening and extent the shelf life of mango fruits. Therefore, when selecting 1-MCP treatment options to delay ripening and extend the fruit (mango) shelf life, these parameters must be considered. Effective strategies to delay ripening while maintaining the fruit quality can contribute to the reduction of postharvest losses. In addition, a balance is required based on the use and need to determine when to harvest at an early stage to ensure a longer marketing period and/or to harvest at an advanced stage to ensure enriched taste and other nutritional quality attributes.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
GC: Writing – original draft. JA: Writing – review & editing. CO: Writing – review & editing. JO: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The research was supported by AgroFresh Ltd and the Consortium for Innovation in Post-Harvest Loss & Food Waste Reduction, sponsored by the Foundation for Food and Agriculture Research, FFAR (Subcontract No. 020524).
Conflict of interest
The study received funding from AGROFRESH Ltd and the Foundation for Food and Agriculture Research (FFAR). The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abu-Bakr A., Abu-Goukh M. M. E., Osman. A. (2019). Effect of 1-methylcyclopropene (1-MCP) and waxing on quality and shelf-life of mango fruits. Gezira J. Agric. Sci. 17 (1), 67–87.
Ambuko J., Githiga R. W., Hutchnison M. J., Gemma H., Owino W. O. (2013). Effect of maturity stage and cultivar on the efficacy of 1-MCP treatments in mango fruits. Acta Hortic. 1007, 39–48. doi: 10.17660/actahortic.2013.1007.1
Ambuko J., Onsongo N. K., Hutchinson M. J., Owino W. O. (2016). Response of ‘Apple mango’ Fruit to 1-methylcyclopropene as affected by agro-ecological zone and maturity stage. Acta Hortic. 1111, 347–353. doi: 10.17660/ActaHortic.2016.1111.50
Ambuko J., Yumbya M. P., Shibairo S., Owino W. O. (2014). Efficacy of 1-methylcyclopropene in purple passion fruit (Passiflora edulis Sims) as affected by dosage and maturity stage. Int. J. Postharvest Technol. Innovation 4, 126–137. doi: 10.1504/IJPTI.2014.068707
Barthakur S. V. R. R. R. R. S. S. (2017). Influence of 1-MCP on texture, related enzymes, quality and their relative gene expression in ‘ Amrapali ‘ mango ( Mangifera indica L.) fruits. J. Food Sci. Technol. 54, 4051–4059. doi: 10.1007/s13197-017-2874-3
Chea S., Yu D. J., Park J., Oh H. D., Chung S. W., Lee H. J. (2019). Fruit softening correlates with enzymatic and compositional changes in fruit cell wall during ripening in ‘Bluecrop’ highbush blueberries. Scientia Horticulturae 245(June 2018), 163–170. doi: 10.1016/j.scienta.2018.10.019
Chen M., Gu H., Wang L., Shao Y., Li R., Li W. (2022). Exogenous ethylene promotes peel color transformation by regulating the degradation of chlorophyll and synthesis of anthocyanin in postharvest mango fruit. Front. Nutr. 9. doi: 10.3389/fnut.2022.911542
Dias C., Ribeiro T., Rodrigues A. C., Ferrante A., Vasconcelos M. W., Pintado M. (2021). Improving the ripening process after 1-MCP application: Implications and strategies. Trends Food Sci. Technol. 113, 382–396. doi: 10.1016/j.tifs.2021.05.012
FAO, IFAD, UNICEF, WFP, WHO. (2020). The State of Food Security and Nutrition in the World 2020. Transforming Food Systems for Affordable Healthy Diets. In The State of Food Security and Nutrition in the World 2020.
Gaikwad S. S., Sakhale B. K., Chavan R. F. (2020). Effect of 1– MCP concentration, exposure time and storage temperature on post-harvest quality of mango fruit cv. Alphanso. Food Res. 4, 746–752. doi: 10.26656/fr.2017.4(3).289
Gao J., Zhang Y., Li Z., Liu M. (2020). Role of ethylene response factors (ERFs) in fruit ripening. Food Qual. Saf. 4, 15–20. doi: 10.1093/fqsafe/fyz042
Giovannoni J., Nguyen C., Ampofo B., Zhong S., Fei Z. (2017). The epigenome and transcriptional dynamics of fruit ripening. Annu. Rev. Plant Biol. 68, 61–84. doi: 10.1146/annurev-arplant-042916-040906
Guillén F., Castillo S., Zapata P. J., Martínez-Romero D., Serrano M., Valero D. (2007). Efficacy of 1-MCP treatment in tomato fruit. 1. Duration and concentration of 1-MCP treatment to gain an effective delay of postharvest ripening. Postharvest Biol. Technol. 43, 23–27. doi: 10.1016/j.postharvbio.2006.07.004
Gwanpua S. G., Jabbar A., Tongonya J., Nicholson S., East A. R. (2018). Measuring ethylene in postharvest biology research using the laser-based ETD-300 ethylene detector. Plant Methods 14, 1–17. doi: 10.1186/s13007-018-0372-x
Harris D. R., Seberry J. A., Wills R. B. H., Spohr L. J. (2000). Effect of fruit maturity on efficiency of 1-methylcyclopropene to delay the ripening of bananas. Postharvest Biol. Technol. 20, 303–308. doi: 10.1016/S0925-5214(00)00150-2
HCD (2019). Horticultural crops directorate.2017-2018 validated horticulture data report. Available online at: http://horticulture.agricultureauthority.go.ke/index.php/statistics/reports. (Accessed June 29, 2022).
Hossain M. A., Rana M. M., Kimura Y., Roslan H. A. (2014). Changes in biochemical characteristics and activities of ripening associated enzymes in mango fruit during the storage at different temperatures. BioMed. Res. Int. 2014, 1–11. doi: 10.1155/2014/232969
Hu X., Khan I., Jiao Q., Zada A., Jia T. (2021). Chlorophyllase, a common plant hydrolase enzyme with a long history, is still a puzzle. Genes 12, 1–6. doi: 10.3390/genes12121871
Huan C., An X., Yu M., Jiang L., Ma R., Tu M., et al. (2018). Effect of combined heat and 1-MCP treatment on the quality and antioxidant level of peach fruit during storage. Postharvest Biol. Technol. 145, 193–202. doi: 10.1016/j.postharvbio.2018.07.013
Lauricella M., Emanuele S., Calvaruso G., Giuliano M., D’Anneo A. (2017). Multifaceted health benefits of Mangifera indica L. (Mango): The inestimable value of orchards recently planted in sicilian rural areas. Nutrients 9, 1–14. doi: 10.3390/nu9050525
Li L., Li C., Sun J., Sheng J., Zhou Z., Xin M., et al. (2020). The effects of 1-methylcyclopropene in the regulation of antioxidative system and softening of mango fruit during storage. J. Food Qual. 2020, 1–11. doi: 10.1155/2020/6090354
Li R., Ma J., Gu H., Jia W., Shao Y., Li W. (2022). 1-Methylcyclopropene counteracts ethylene promotion of fruit softening and roles of MiERF2/8 and MiPG in postharvest mangoes. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.971050
Lin Y., Lin Y., Lin H., Lin M., Li H., Yuan F., et al. (2018). Effects of paper containing 1-MCP postharvest treatment on the disassembly of cell wall polysaccharides and softening in Younai plum fruit during storage. Food Chem. 264, 1–8. doi: 10.1016/j.foodchem.2018.05.031
Lin Y., Lin H., Wang H., Lin M., Chen Y., Fan Z., et al. (2020). Effects of hydrogen peroxide treatment on pulp breakdown, softening, and cell wall polysaccharide metabolism in fresh longan fruit. Carbohydr. Polymers 242, 116427. doi: 10.1016/j.carbpol.2020.116427
Lin W., Liu Y., Di J., Ren G., Wang W., He W., et al. (2022). Effects of 1-MCP treatment on physiology and storage quality of root mustard at ambient temperature. Foods 11, 1–11. doi: 10.3390/foods11192978
Liu S., Huang H., Huber D. J., Pan Y., Shi X., Zhang Z. (2020). Delay of ripening and softening in ‘Guifei’ mango fruit by postharvest application of melatonin. Postharvest Biol. Technol. 163, 111136. doi: 10.1016/j.postharvbio.2020.111136
Lobo M. G., Sidhu J. S. (2017). “Biology, postharvest physiology, and biochemistry of mango,” in Handbook of mango fruit: production, postharvest science, processing technology and nutrition (Hoboken, New Jersey: John Wiley & Sons Ltd), 37–59. doi: 10.1002/9781119014362.ch3
Mahajan B. V. C., Singh K., Dhillon W. S. (2010). Effect of 1-methylcyclopropene (1-MCP) on storage life and quality of pear fruits. J. Food Sci. Technol. 47, 351–354. doi: 10.1007/s13197-010-0058-5
Maloba S., Ambuko J., Hutchinson M., Owino W. (2017). Off-season flower induction in mango fruits using ethephon and potassium nitrate. J. Agric. Sci. 9, 158. doi: 10.5539/jas.v9n9p158
Manigo B., Antibo N. (2022). 1-methylcyclopropene and ethephon effects on “Carabao” Mango fruits harvested at different stages of fruit maturity. Indonesian J. Agric. Res. 5, 11–28. doi: 10.32734/injar.v5i01.7409
Manzi H., Gweyi-Onyango J. P. (2021). Agro-ecological lower midland zones IV and V in Kenya using GIS and remote sensing for climate-smart crop management. Afr. Handb. Climate Change Adaptation: With 610 Figures 361 Tables 49, 965–991. doi: 10.1007/978-3-030-45106-6_35
Özkaya O., Dündar Ö. (2009). Response of 1-methylcyclopropene (1-MCP) treatments on some quality parameters of plum during storage. J. Food Agric. Environ. 7, 233–236.
Pan H., Wang R., Li L., Wang J., Cao J., Jiang W. (2016). Manipulation of ripening progress of different plum cultivars during shelf life by post-storage treatments with ethylene and 1-methylcyclopropene. Scientia Hortic. 198, 176–182. doi: 10.1016/j.scienta.2015.11.007
Prabasari I., Setiawan C. K., Utama N. A., Mabsyuroh M. A. (2023). Application of 1-MCP does not slow down the ripening process of Mango (Mangifera indica L). IOP Conf. Series: Earth Environ. Sci. 1287, 12031. doi: 10.1088/1755-1315/1287/1/012031
Prasantha B. D. R., Amunogoda P. N. R. J. (2013). Moisture adsorption characteristics of solar-dehydrated mango and jackfruit. Food Bioprocess Technol. 6, 1720–1728. doi: 10.1007/s11947-012-0832-7
Rajan S. (2022). The mango genome (Cham, Switzerland: Springer Nature). doi: 10.1007/978-3-030-47829-2
Razzaq K., Singh Z., Khan A. S., Khan S. A. K. U., Ullah S. (2016). Role of 1-MCP in regulating ‘Kensington Pride’ mango fruit softening and ripening. Plant Growth Regul. 78, 401–411. doi: 10.1007/s10725-015-0101-7
Ren Y. Y., Sun P. P., Wang X. X., Zhu Z. Y. (2020). Degradation of cell wall polysaccharides and change of related enzyme activities with fruit softening in Annona squamosa during storage. Postharvest Biol. Technol. 166, 111203. doi: 10.1016/j.postharvbio.2020.111203
Rupavatharam S., East A. R., Heyes J. A. (2015). Re-evaluation of harvest timing in “Unique” feijoa using 1-MCP and exogenous ethylene treatments. Postharvest Biol. Technol. 99, 152–159. doi: 10.1016/j.postharvbio.2014.08.011
Sakhale B. K., Gaikwad S. S., Chavan R. F. (2018). Application of 1-methylcyclopropene on mango fruit (Cv. Kesar): potential for shelf life enhancement and retention of quality. J. Food Sci. Technol. 55, 776–781. doi: 10.1007/s13197-017-2990-0
Satekge T. K., Magwaza L. S. (2022). Postharvest application of 1-methylcyclopropene (1-MCP) on climacteric fruits: factors affecting efficacy. Int. J. Fruit Sci. 22, 595–607. doi: 10.1080/15538362.2022.2085231
Silué Y., Nindjin C., Cissé M., Kouamé K. A., Amani N., Guessan G., et al. (2022). Hexanal application reduces postharvest losses of mango (Mangifera indica L. variety “Kent”) over cold storage whilst maintaining fruit quality. Postharvest Biol. Technol. 189, 1–15. doi: 10.1016/j.postharvbio.2022.111930
Singh V., Jawandha S. K., Gill P. P. S., Gill M. S. (2019). Suppression of fruit softening and extension of shelf life of pear by putrescine application. Scientia Hortic. 256, 108623. doi: 10.1016/j.scienta.2019.108623
Sivakumar D., Jiang Y., Yahia E. M. (2011). Maintaining mango (Mangifera indica L.) fruit quality during the export chain. Food Res. Int. 44, 1254–1263. doi: 10.1016/j.foodres.2010.11.022
Thongkum M., Imsabai W., Burns P., McAtee P. A., Schaffer R. J., Allan A. C., et al. (2018). The effect of 1-methylcyclopropene (1-MCP) on expression of ethylene receptor genes in durian pulp during ripening. Plant Physiol. Biochem. 125, 232–238. doi: 10.1016/j.plaphy.2018.02.004
Tomala K., Małachowska M., Guzek D., Głąbska D., Gutkowska K. (2020). The effects of 1-methylcyclopropene treatment on the fruit quality of ‘idared’ apples during storage and transportation. Agric. (Switzerland) 10, 1–12. doi: 10.3390/agriculture10110490
Valbuena-Tellez E. Y., Patiño-Guio J. E., Balaguera-López H. E. (2023). Effect of applications of 1-MCP and ethylene on the ripening and degreening process of banana fruits cv. Barranquillo. Rev. U.D.C.A Actualidad Divulgacion Cientifica 26, 1–10. doi: 10.31910/rudca.v26.n1.2023.1978
Wang H., Chen G., Shi L., Lin H., Chen Y., Lin Y., et al. (2021). Influences of 1-methylcyclopropene-containing papers on the metabolisms of membrane lipids in Anxi persimmons during storage. Food Qual. Saf. 4, 143–150. doi: 10.1093/FQSAFE/FYAA021
Watkins C. B. (2008). Overview of 1-methylcyclopropene trials and uses for edible horticultural crops. HortScience 43, 86–94. doi: 10.21273/hortsci.43.1.86
Xu X., Lei H., Ma X., Lai T., Song H., Shi X., et al. (2017). Antifungal activity of 1-methylcyclopropene (1-MCP) against anthracnose (Colletotrichum gloeosporioides) in postharvest mango fruit and its possible mechanisms of action. Int. J. Food Microbiol. 241, 1–6. doi: 10.1016/j.ijfoodmicro.2016.10.002
Keywords: 1-methylcyclopropene, 1-MCP, ethylene, mango, shelf life, postharvest
Citation: Chomba G, Ambuko J, Onyango C and Ouko JR (2025) Effect of different 1-methylcyclopropene formulations and dosing on the ripening profile of Tommy Atkins mango fruits. Front. Hortic. 4:1509989. doi: 10.3389/fhort.2025.1509989
Received: 11 October 2024; Accepted: 10 January 2025;
Published: 07 February 2025.
Edited by:
Brian Farneti, Fondazione Edmund Mach, ItalyReviewed by:
Michail Michailidis, Aristotle University of Thessaloniki, GreeceRenu Bhardwaj, Galgotias University, India
Copyright © 2025 Chomba, Ambuko, Onyango and Ouko. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jane Ambuko, amFuZS5hbWJ1a29AdW9uYmkuYWMua2U=; Geoffrey Chomba, Z2VvZmZyZXkuY2hvbWJhQHN0dWRlbnRzLnVvbmJpLmFjLmtl
†ORCID: John Robert Ouko, orcid.org/0000-0002-3131-1923
 Geoffrey Chomba
Geoffrey Chomba Jane Ambuko
Jane Ambuko Cecilia Onyango
Cecilia Onyango