- 1US Horticultural Research Laboratory, US Department of Agriculture - Agricultural Research Service (USDA-ARS), Fort Pierce, FL, United States
- 2Institute of Food and Agricultural Sciences (IFAS) Gulf Coast Research and Education Center, University of Florida, Horticultural Sciences Department, Wimauma, FL, United States
- 3Daniel K. Inouye U.S. Pacific Basin Agricultural Research Center, USDA-ARS, Hilo, HI, United States
- 4Western Regional Research Center, USDA-ARS, Albany, CA, United States
Introduction: Citrus hybrids with Poncirus trifoliata L. Raf. introgression have gained interest due to their tolerance to Huanglongbing (HLB), a devastating disease for Florida citrus agriculture. While these hybrids inherit disease tolerance from P. trifoliata, they sometimes also suffer from undesirable off-flavors.
Methods: A selection of thirteen genotypes were harvested over the 2020-2021 and 2021-2022 seasons. Their juices were evaluated by a trained sensory panel and were comprehensively analyzed for their chemical makeup, including soluble solids content (SSC), titratable acidity (TA), volatiles, flavonoids and limonoids.
Results & discussion: Overall, along with the commercial orange cultivars ‘Valencia’ and ‘Hamlin’, the HLB-tolerant Poncirus hybrid ‘US SunDragon,’ and the mandarin hybrids Sugar Belle®, FF-5-51-2, and ‘US Superna’ had positive citrus flavor quality. Esters, some sesquiterpenes, along with flavonoids, eriocitrin and quercetin-3-(3R-glucosylrutinoside), were positively correlated with orange flavor while β-ionone and eucalyptol were highly abundant in the mandarins. The flavonoid linarin, was more abundant in Poncirus hybrids with off-flavors than in the Poncirus hybrid ‘US SunDragon’, having high orange flavor. Two mandarin hybrids, FF-5-6-36 and FTP-6-32-67, were not bitter at harvest, but the juice exhibited delayed bitterness after storage at -20°C, which was associated with significant increases of limonin, nomilin, naringenin, and prunin. Interestingly, during freezer storage, a newly identified flavonoid in citrus, tricin-C-hexoside, increased dramatically across all of the genotypes. The identification of disease-tolerant hybrids with satisfactory flavor quality at juicing as well as after storage where delayed bitterness may develop, has great significance for future breeding efforts for fresh fruit or for use in stand-alone juice/juice blends.
1 Introduction
Citrus greening disease, a.k.a. Huanglongbing (HLB), has devastated Florida citrus agriculture and efforts to combat this disease have been challenging thus far. Along with reduced fruit yields, fruit flavor quality is negatively impacted by HLB (Dala-Paula et al., 2018). There have been efforts to identify HLB-tolerant citrus hybrids and their chemical components. Within the USDA, Agricultural Research Service (ARS) citrus breeding program in Florida, dating back to the 1900’s, there are complex Citrus hybrids with Poncirus trifoliata introgression into a largely mandarin-derived pedigree (referred to as Poncirus hybrids herein) (Webber and Swingle, 1905). P. trifoliata is cold tolerant and has shown tolerance to HLB (Albrecht and Bowman, 2011), but the fruit have unacceptable eating quality. Recently, a few advanced Poncirus hybrids with reduced pedigree contributions from P. trifoliata, were reported to have acceptable citrus flavor with balanced sweet and sour taste (Deterre et al., 2023). Sensory descriptors were explained by levels of soluble solids content (SSC), titratable acidity (TA), volatiles and a handful of limonoids and flavonoids (Deterre et al., 2021). No in-depth analysis of flavonoids was performed to explain nuances in taste.
Citrus juice is chemically complex and involves various amounts of organic acids, sugars, volatile components, and other non-volatile components, such as limonoids, flavonoids, and carotenoids. Additionally, synergistic and antagonistic effects between these compounds contribute to the complexity of citrus juice flavor (Spence, 2015). A trained sensory panel provides information for a number of attributes, including both aroma and taste, when a juice sample is analyzed. As the number of compounds in a food matrix increases, the methods for their analysis must also be more sophisticated and often requires multiple analytical techniques. Most publications focus on the most abundant flavonoids and limonoids, but with the selectivity and sensitivity of LC-MS/MS, it is possible and advantageous to widely target chemical components for a full profile (Wang et al., 2021; Zhao et al., 2020). A “flavoromics” approach has recently become an efficient strategy to investigate citrus flavor and to identify key flavor compounds (Charve et al., 2011; Feng et al., 2021). Using this approach, octanal, decanal, ethyl hexanoate, and ethyl octanoate were found to be highly correlated with the citrusy attribute of sweet-orange-like mandarin hybrids while linalool, citronellol, and 1-octanol were correlated with the fruity/floral attribute (Feng et al., 2021).
Citrus fruits are a rich source of flavonoids and limonoids, which have been studied for their nutritional value and diverse biological activities in humans (Tripoli et al., 2007). The chemical structures of flavonoids endogenous to citrus are incredibly diverse and small substitutions to a common backbone structure can change the biological function and taste significantly (Peterson et al., 2006). For example, hesperidin (hesperetin-7-O-rutinoside), which is slightly bitter, differs from neohesperidin (hesperetin-7-O-neohesperidoside), which is extremely bitter, only by the position of attachment of rhamnose to glucose within the disaccharide (Horowitz and Gentili, 1961). While there has been some effort to predict the binding of flavonoids to the 25 human bitter receptors based on chemical structure, this has proven to be a daunting task as there are often exceptions to the rules (Li et al., 2023). For instance, in general flavonoids with an opened C ring, such as dihydrochalcones and chalcones, impart sweetness, except for poncirin dihyrochalcone, which is bitter. Flavonoids are generally known to contribute to bitterness, but can also be sweetness enhancers. A recent study revealed hesperetin to be a sweetness enhancer and eriodictyol to be a bitterness inhibitor (Wang et al., 2022).
Over years of analyzing quality of fruit from the collection of USDA hybrids, it was observed that some of the juice that was not bitter at harvest was then rated highly bitter after storage at -20°C for various periods of time (Raithore et al., 2016). Delayed bitterness has been studied since the 1950’s (Marsh and Cameron, 1950), but questions regarding the biosynthetic mechanisms of the diverse limonoid species in citrus remain unanswered (De La Pena et al., 2023; Zhang et al., 2018). Limonoate A-ring lactone, a tasteless precursor of limonin (Maier and Beverly, 1968), decreases during maturation (Hasegawa et al., 2002) and is converted to bitter limonin upon juicing as it is released into an acidic environment. There are studies analyzing limonoids in orange juice upon storage at room temperature or 5°C (Bai et al., 2010; Raithore et al., 2016; Zhang et al., 2018), but our study gives insight on how frozen storage of juice affects limonoids and other flavonoids, possibly contributing to delayed bitterness.
In this study, different hybrids from the same USDA Citrus breeding program as in the 2021 and 2023 study (Deterre et al., 2023, 2021) were selected due to their HLB-tolerance and quality in the 2020-2021 and 2021-2022 harvest seasons, along with commercial cultivars for reference. Samples in this study are a subset of a larger study initiated in 2016 (Fan et al., 2024) with new data of off-flavor related sensory responses and chemical analyses. GC/MS analyses of juice from many genotypes in that large scale study led to the identification of seven esters responsible for the distinction between orange and mandarin flavors; however, off-flavors were not studied (Fan et al., 2024). While there is a lot of literature focused on determining the compounds contributing to positive attributes of citrus juices, such as orange or mandarin flavors, there was a recent report where “poncirus”-like off-flavor was shown to be due to a combination of higher than typical amounts of sesquiterpene hydrocarbons, monoterpenes, and terpene esters and a lack of aldehydes with typical citrus odor (Deterre et al., 2023). Off-flavors in citrus can range from pumpkin to more negative ones, such as gasoline. Sensory evaluation and comprehensive chemical analyses of hybrids with acceptable citrus flavors, as well as some with off-flavors were performed to fill knowledge gaps in the chemistry of citrus flavor and give insight for future breeding efforts since the identification of these compounds is the first step in determining the genetics involved in their biosynthesis. While the known chemical contributors to citrus flavor were measured herein, such as sugars, acids, and bitter limonoids, we widely profiled volatiles, flavonoids, and limonoids to identify compounds important for citrus and off-flavors. Common techniques, using a refractometer and a titrator, were used to measure sugars and acids, respectively. Additionally, more advanced analytical techniques were used to measure sixty volatile compounds via GC/MS and sixty-one non-volatile compounds via LC-MS/MS.
2 Materials and methods
2.1 Fruit material and juicing
Fruits were harvested from mature trees grown at the USDA, ARS, Whitmore Citrus Research Foundation Farm in Groveland, Lake County, FL or the USDA, ARS Research Farm in Fort Pierce, FL. Along with the named varieties, hybrids from trees with a healthy appearance were harvested at commercial maturity during the 2020-2021 and 2021-2022 seasons. After being washed and sanitized (Deterre et al., 2021), fruits were batched into four biological replicates with equal numbers of fruit, varying from 5 to 10 fruits, depending on fruit size. Juicing was performed manually, utilizing a reamer-type juicer (Vinci™ Hand Free Juicer, Vinci® Housewares, La Mirada, CA, USA). Juice samples were stored at -20°C until sensory panels were conducted at the end of the season or until analyzed for sugars, acids, flavonoids, limonoids, and volatiles. An additional set of juice samples for use in the delayed bitterness experiment was stored at -80°C until analyzed via LC-MS/MS.
2.2 Sensory analysis
During each harvest year, 10 to 12 panelists with over 10 years of experience met for training/practicing/refreshing sessions of descriptive sensory evaluation of citrus juices. After refresher training, panelists tasted juice samples from no more than 4 genotypes per day, repeated in 2 daily sessions. The panelists rated each juice using a linear intensity scale with anchor points from 0 to 15 (none to high) for each of the descriptors, including orange flavor and mandarin flavor (Fan et al., 2024), grapefruit flavor, sweetness, sourness, bitterness, off-flavor, and aftertaste. For off-flavor and aftertaste, panelists were instructed to rate the intensity as well as describe any off-flavor or aftertaste. Panelists’ comments were analyzed to determine if the sample had “poncirus” or pumpkin flavors, which were defined with P. trifoliata fruit peel and fresh pumpkin puree, respectively, kept frozen at -80°C. Juice was stored at -20°C, defrosted at 4°C, and then served to the panelists at 14°C. Juice samples were served as 35 mL samples in coded 4 oz (118 mL) plastic soufflé cups covered with clear plastic lids (Solo® Cups Co., Urbana, IL, USA) along with reference standards, which were served as 18 mL samples in 1 oz (30 mL) cups. Each descriptor had a reference standard: orange flavor, unpasteurized orange juice (Al’s Family Farm, Fort Pierce, FL); mandarin flavor, “gourmet pasteurized” (i.e. pasteurized at low temperature) tangerine juice (Natalie’s Orchid Island Juice Company, Fort Pierce, FL); grapefruit flavor, unpasteurized grapefruit juice (Al’s Family Farm, Fort Pierce, FL); sweetness, 8% sucrose in water; sourness, 0.2% citric acid (Sigma-Aldrich) in water; bitterness, 11.5 mg/L quinine hydrochloride (Sigma-Aldrich) in water. Orange, mandarin, and grapefruit reference standards were given an intensity value of 12 while the sweet, sour, and bitter reference standards were given an intensity rating of 7 on the 0 to 15 scale. Crackers and water were provided for each session. Panelists recorded their ratings with Compusense® software (Compusense Inc., Guelph, ON, Canada) and had an option to record general comments for each juice sample. This study was conducted within the guidelines of the human subject exemption as stated in 45 CFR 46.104 (d)(6).
2.3 Volatile analysis
Volatile profiling was performed via headspace-SPME-GC/MS analysis as previously described (Bai et al., 2016; Deterre et al., 2021; Fan et al., 2024). Six milliliters of whole juice were added to a 20 mL vial, crimp-capped, and stored at -20°C until analysis. After a 30-minute incubation at 40°C, a 2 cm tri-phase SPME fiber (50/30 μm DVB/Carboxen/PDMS, Supelco, Bellefonte, PA, USA) was exposed to the headspace for 30 minutes. After exposure, the SPME fiber was inserted into the injector of an Agilent 7890 GC equipped with a DB-5 column (60 m x 0.25 mm i.d., 1.00 µm film thickness, J&W Scientific, Folsom, CA, USA) and coupled with a 5975 MS (Agilent Technologies, Palo Alto, CA, USA). After desorbing for 15 min at 250°C, the column was initially at 40°C and then ramped to 230°C at a rate of 4°C min-1, followed by a ramp to 260°C at a rate of 100°C min-1. Ionization voltage was 70 eV and ions from m/z 30 to 250 were detected. A Kovats mixture of C-5 to C-18 n-alkanes was run at the beginning of each day to calculate retention indices (RIs) and to validate the inter-day reproducibility. The volatile compounds were identified utilizing NIST 14 (http://chemdata.nist.gov) and Adams (Adams, 2017) spectral libraries. As described previously by Deterre et al (Deterre et al., 2021), compound identities were confirmed using authentic standards under the same conditions as the juice samples, as well as with a column of opposite polarity (DB-Wax capillary column; 60 m x 0.25 mm i.d., 0.5 μm film thickness; J&W Scientific) (Molyneux and Schieberle, 2007).
MassHunter Quantitative Analysis software (Agilent, Santa Clara, CA, USA) was used for data analysis and peak areas were reported. Four biological replicates were measured, and their averages were reported.
2.4 Soluble solids content and titratable acidity
Juice samples were centrifuged at 10,000 x g for 15 min and the soluble solids content (SSC) of the supernatant was measured with a refractometer (Atago RX-5000α, Tokyo, Japan). The titratable acidity (TA) of the juice samples was measured by titrating ~5 g of juice supernatant to pH 8.1 with 0.1 N NaOH using a titrator (Dosino model 800, Metrohm, Herisau, Switzerland). Four biological replicates were measured, and their averages were reported.
2.5 Absolute quantification of limonin and nomilin
Quantification of limonin and nomilin in juice was performed via LC-MS/MS analyses. Juice (supernatant) was filtered with a 0.2 µm PES syringe and injected on a 1290 Infinity II UPLC coupled with a 6470 triple quadrupole MS (Agilent, Santa Clara, CA, USA). The ZORBAX RRHD Eclipse Plus C18 column (2.1 x 50 mm, 1.8 µm, Agilent) was held at 40°C. Mobile A consisted of water, 0.1% formic acid and mobile B was acetonitrile, 0.1% formic acid. Initial UPLC conditions were 5% B, 95% A for 1 minute, followed by a linear gradient to 99% B at 5 minutes, held at 99% B for 1 minute, and then increased to 100% B at 7 minutes. After flushing with 100% B for 1 minute, the column was re-equilibrated with initial conditions (5% B, 95% A) for 1 minute. The flow rate was kept constant at 0.3 mL min-1. The Agilent Jet Stream ESI source was operated with a gas temperature of 300°C, gas flow of 10 L min-1, nebulizer pressure of 20 psi, sheath gas temperature of 350°C, sheath gas flow of 11 L min-1, and a positive capillary voltage of 4500 V. The MS was operated in MRM mode and nomilin was detected with a m/z 515.3 precursor ion and a m/z 411.2 product ion with fragmentor and collision energy voltages set to 135 V and 14 V respectively. Limonin was detected with a m/z 471.2 precursor ion and a m/z 425.2 product ion with fragmentor and collision energy voltages set to 135 V and 19 V respectively. Limonoids were identified by the comparison of MRM transitions and retention times with analytical standards. Quantification of limonoids were performed by integrating the area under the chromatographic peak and calculating the amount of each compound based on standard curves (R2 ≥ 0.99 with range 0.006 - 25 mg L-1). Each sample had four biological replicates, which were averaged. MassHunter Quantitative Analysis software (Agilent, Santa Clara, CA, USA) was used for data analysis.
2.6 Relative quantification of flavonoids and limonoids
Relative quantification of flavonoids and limonoids in juice was performed via LC-MS/MS analyses with a 1290 Infinity II UPLC coupled with a 6470 triple quadrupole MS (Agilent, Santa Clara, CA, USA). Whole juice was extracted with 80% methanol and then filtered with a 0.2 µm nylon syringe. Samples were normalized by the addition of an internal standard, mangiferin, at a concentration of 10 µg/mL. The InfinityLab Poroshell 120 EC-C18 column (2.1 x 100mm, 1.9 µm, Agilent) was held at 42.0°C. Mobile A consisted of water, 0.1% formic acid and mobile B was acetonitrile, 0.1% formic acid. Initial UPLC conditions were 5% B, 95% A for 0.2 minutes, followed by a linear gradient to 95% B at 11 minutes, then a linear gradient to 98% B at 15 minutes, held at 98% B for 3 minutes, held at 99% for 3 minutes and then increased to 100% B at 21.2 minutes. After flushing with 100% B for 13.8 minutes, the column was re-equilibrated with initial conditions (5% B, 95% A) for 5 minutes. The flow rate was kept constant at 0.4 mL min-1. The Agilent Jet Stream ESI source was operated with a gas temperature of 300°C, gas flow of 10 L min-1, nebulizer pressure of 45 psi, sheath gas temperature of 350°C, sheath gas flow of 11 L min-1, and positive/negative capillary voltage 3500 V. In dMRM mode, analytes were identified with MRM transitions listed in Supplementary Table S1 in comparison with analytical standards, public databases, and references (Feng et al., 2018; Wang et al., 2021, 2017). Each sample had 3 biological replicates, which were averaged. MassHunter Quantitative Analysis software (Agilent, Santa Clara, CA, USA) was used for data analysis.
2.7 Statistical analyses
Sensory data were analyzed by analyses of variance (ANOVA) using SenPAQ version 5.01 (Qi Statistics, Reading, UK). A mixed model was used, with “panelist” as a random variable and where the main effects (panelist or sample) were tested against their interaction, with the two replications included in the error term (Lawless and Heymann, 1999). Means separation was performed using the least significant difference (LSD) test (p < 0.05). For variables where many samples were rated close to zero and the LSD was greater than the mean, variables were transformed into rank (Lawless and Heymann, 1999). Since data were continuous, the same ranking was assigned to integer values +/- 0.5. A non-parametric Kruskal-Wallis test was performed with the rank data; means separation was performed with the Dunn’s procedure multiple pairwise comparison. Data presented in Table 1 are the non-transformed means. SSC and TA measurements as well as GC/MS analyses of the volatiles were replicated four times using biological replicates of each sample. LC-MS/MS analysis of flavonoids and limonoids were replicated three times using biological replicates of each sample. JMP software Version 16 (JMP Statistical Discovery, Cary, NC, USA) was used to perform Principal Components Analysis (PCA) and to create the biplots. JMP software was used to perform 2-way hierarchical clustering analysis, with variety on the y-axis and non-volatile compounds on the x-axis.
3 Results and discussion
3.1 Sensory evaluation
A trained descriptive panel evaluated the selected named varieties and hybrids harvested during the 2020-2021 and 2021-2022 seasons. Average intensity ratings from the trained panelists are reported in Table 1 with a scale from 0 (none) to 15 (high) for each sensory attribute. Panelists were encouraged to leave open comments for each sample, describing the flavor, off-flavors, or aftertastes. These comments were compiled and combined from both harvest seasons for each variety in Table 2. In Figure 1, the PCA biplot shows the relationships between sensory attributes and the known chemical contributors (SSC, TA, limonin, and nomilin) to citrus flavor for the genotypes in this study. Principal components (PC) 1 and 2 accounted for 40.4% and 25.7% of the variation, respectively. Orange and mandarin flavors and sweetness described PC1 on the negative side while sourness, bitterness, off-flavor, aftertaste, “poncirus” flavor, and grapefruit flavor described PC1 on the positive side. Sourness correlated with titratable acidity (TA) (r = 0.91), and bitterness, off-flavor and aftertaste correlated with limonin (r = 0.86) and nomilin (r = 0.75), known bitter compounds absolutely quantified in juice supernatant (Supplementary Tables S2, S3). Mandarin flavor, sweetness, and SSC/TA correlated with each other (r = 0.54, 0.40 and 0.41, respectively; Supplementary Table S3), as previously reported (Deterre et al., 2023). The juice from named sweet orange varieties commercially grown in Florida, ‘Valencia’ and ‘Hamlin’, had high orange flavor, moderate mandarin flavor, high sweetness, and minimal off-flavor and aftertaste (Table 1). Overall, the sensory descriptive profiles and basic chemical characteristics (soluble solids content, titratable acidity, and limonoids) (Figure 1) for each genotype varied but were similar between the two harvest seasons for most genotypes. ‘Valencia’, however, was less sweet and more sour and bitter when harvested in 2022 and was accompanied with a significantly higher Candidatus Liberibacter asiaticus titer (data not shown), indicating a higher level of HLB-disease infection (Dala Paula et al., 2018). Some panelists who have participated in orange juice trained panels for many years perceived off flavor described as “typical HLB off-flavor” in the juice from the 2022 harvest (Table 2) (Plotto et al., 2017).
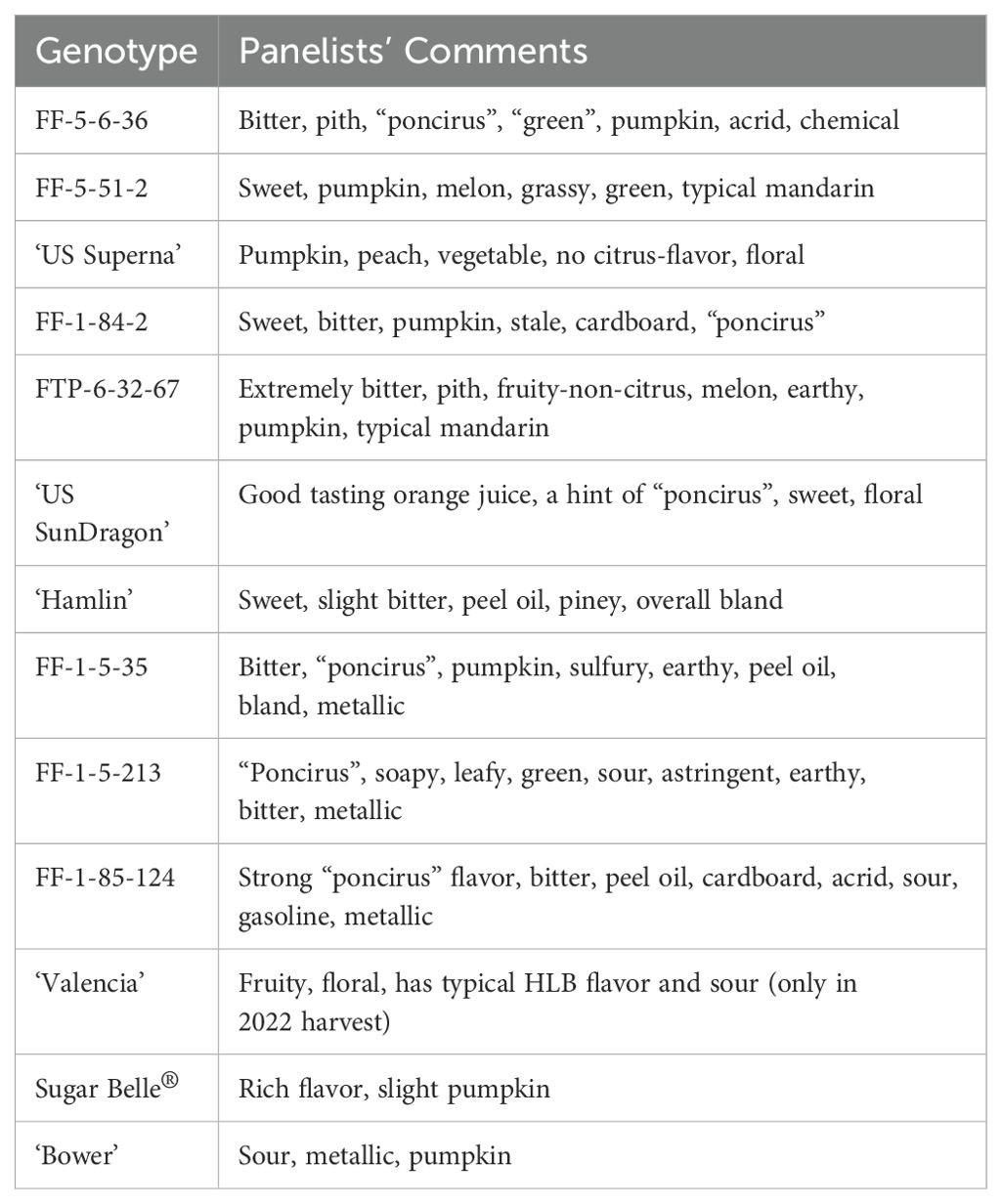
Table 2. Panelists’ descriptive comments of genotypes from combined 2020-2021 and 2021-2022 harvests.
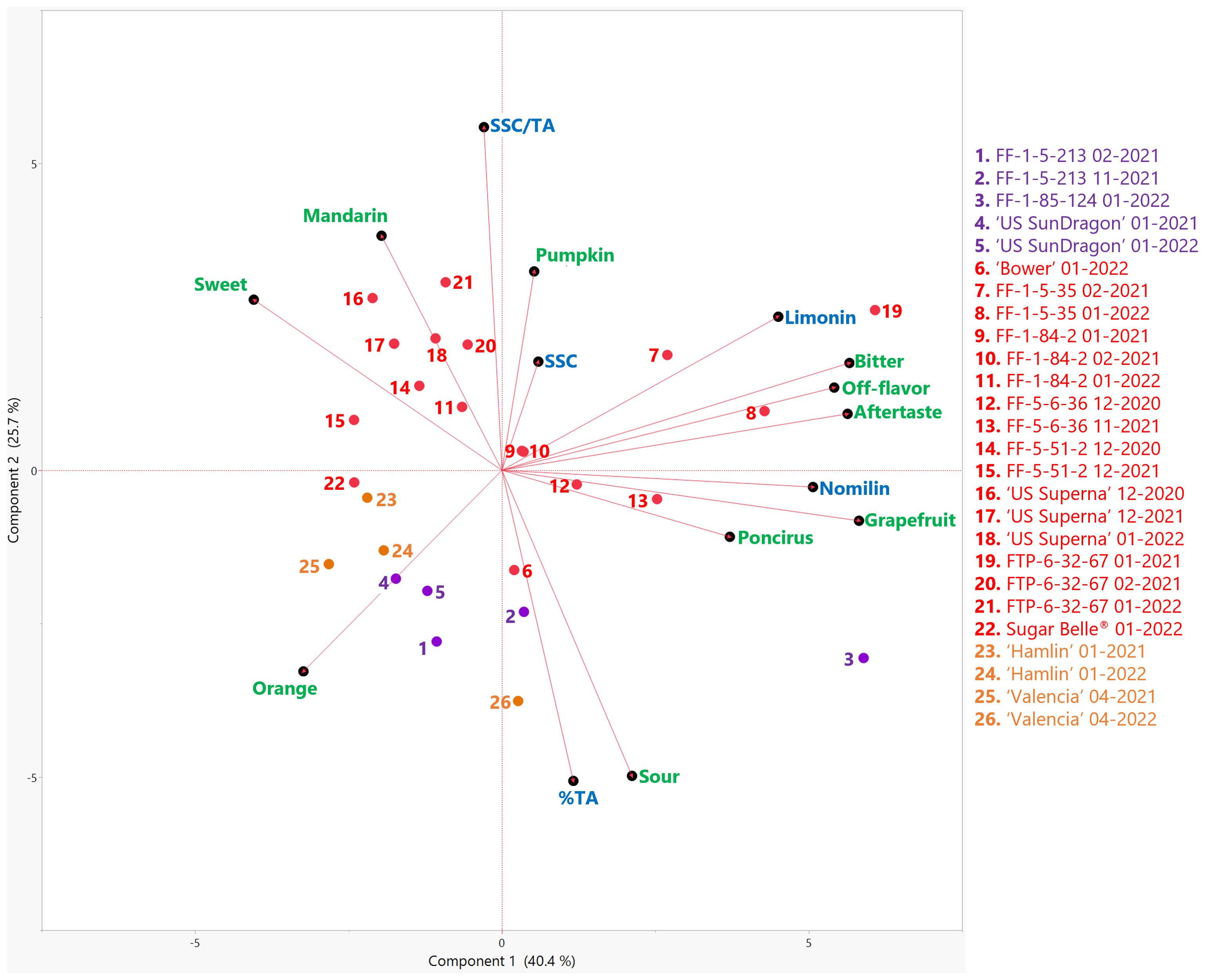
Figure 1. Biplot of the first two principal components (PC) from the principal component analysis (PCA) of sensory attributes (green) and basic chemical components (blue) in genotypes harvested in the 2020-21 and 2021-22 seasons. Mandarin hybrids without Poncirus introgression are red, orange varieties are orange, and Poncirus hybrids are purple. Harvest month and year follow the genotype. SSC, soluble solids content; % TA, titratable acidity.
‘US SunDragon’, a Poncirus hybrid with proven tolerance to HLB (Stover et al., 2020), had consistent high orange flavor and high sweetness with some sourness, similar to ‘Hamlin’ harvested at the same time. Panelists commented that ‘US SunDragon’ juice was a good-tasting orange juice with a hint of “poncirus” off-flavor. When harvested in February 2021, orange flavor ratings of FF-1-5-213, another Poncirus hybrid, followed that of ‘Hamlin’, with average mandarin flavor, but was rated with higher sourness than ‘Valencia’ or ‘Hamlin’. Panelists described its juice to have “poncirus” and soapy off-flavors (Table 2). Another Poncirus hybrid, FF-1-85-124, had higher grapefruit flavor than most hybrids and was rated with high intensity for sourness and bitterness. Some panelists commented that this juice had strong “poncirus”, peel oil, cardboard, gasoline, and metallic flavors (Table 2).
The hybrids without Poncirus in their pedigree (hereafter described as “mandarin hybrids”, though it should be noted that true mandarin C. reticulata predominates in the pedigree of all material studied even sweet oranges) included in this study have diverse sensory descriptive profiles. While some of them were rated with high mandarin flavor and high sweetness, others were rated high for bitterness, aftertaste, and grapefruit flavor (Table 1). It is to be noted that no true grapefruit was rated in this study. However, since sweet orange and grapefruit have similar complex ancestry (Wu et al., 2018), grapefruit flavor was rated together with orange and mandarin flavors. ‘LB8-9’ Sugar Belle® (hereafter Sugar Belle®), a University of Florida released mandarin-type tolerant to HLB, had a high mandarin flavor (rating 7.0), as demonstrated in the literature (Feng et al., 2018). Some panelists commented that Sugar Belle® had a rich flavor and minimal off-flavor (Tables 1, 2). ‘US Superna’, another recent USDA release, was consistently rated high in sweetness and mandarin flavor. Some panelists’ comments indicated that this juice had pumpkin off-flavor, which has been associated with mandarin flavor (Miyazaki et al., 2012), especially with late harvests (Plotto et al., 2011). ‘Bower’ is a mandarin hybrid tolerant to HLB, but it was rated high in sourness, with slight metallic off-flavor. FF-5-51-2 is another mandarin hybrid with high mandarin flavor, high sweetness and no off-flavor. FF-1-84-2 and FTP-6-32-67 were rated with moderate mandarin flavor and sweetness but were bitter. Bitterness in these juices is an indication of delayed bitterness occurring following storage of juice, as freshly harvested fruit did not exhibit bitterness at all (observations made at the time of juicing). Juice from the FTP-6-32-67 fruit from the January 2021 harvest was extremely bitter (7.7), but the bitterness dropped to 1.9 when harvested a month later in February of 2021, suggesting that the fruit were not mature at their first harvest in 2021. Indeed, significantly lower levels of limonin and nomilin were measured in February than in January of 2021 (Supplementary Table S2). Two mandarins, FF-1-5-35 and FF-5-6-36, were rated higher with grapefruit flavor, bitterness, and off-flavors. Some panelists commented that these juices were bitter and had negative remarks, such as chemical and acrid for FF-5-6-36 and sulfury for FF-1-5-35.
3.2 Volatile profiling
Volatile profiles of genotypes in this study were obtained by headspace GC/MS analysis, and included thirteen sesquiterpenes, fourteen monoterpenes, twelve esters, two ketones, eight aldehydes, five alcohols, four terpene alcohols, one terpene ester, and one terpene ketone. A PCA biplot summarizes the relationships between sensory attributes and volatiles (Figure 2). Component 1 and 2 on Figure 2 accounted for 28.9% and 18.8% of the variation in the PCA biplot, respectively. Orange flavor correlated with most esters, as well as 3-hexen-1-ol (r = 0.71), (E)-2-hexenal (r = 0.69), 1-hexanol (r = 0.69), (E)-2-pentenal (r = 0.67) and some sesquiterpenes (valencene, α-selinene and β-elemene). Mandarin flavor was correlated with SSC and SSC/TA (Figure 1) but only one volatile, β-ionone, was associated with mandarin flavor (Figure 2). This confirms the complexity of mandarin flavor and that it is mostly different from orange flavor due to the lack of or the lower abundances of esters (Fan et al., 2024). Negative descriptors (off-flavor, aftertaste and grapefruit) correlated with bitterness. Bitterness in mandarins was previously reported to be correlated with the volatiles α-copaene, cis-β-farnesene, myrcene, and perilla acetate (Feng et al., 2021). From the genotypes in this study, bitterness correlated with the monoterpenes camphene (r = 0.52), α-thujene (0.45), and terpinen-4-ol (0.42) (Figure 2; Supplementary Table S4). While volatiles may contribute to increased bitterness, the main bitter compounds in mandarin remain to be limonin and nomilin.
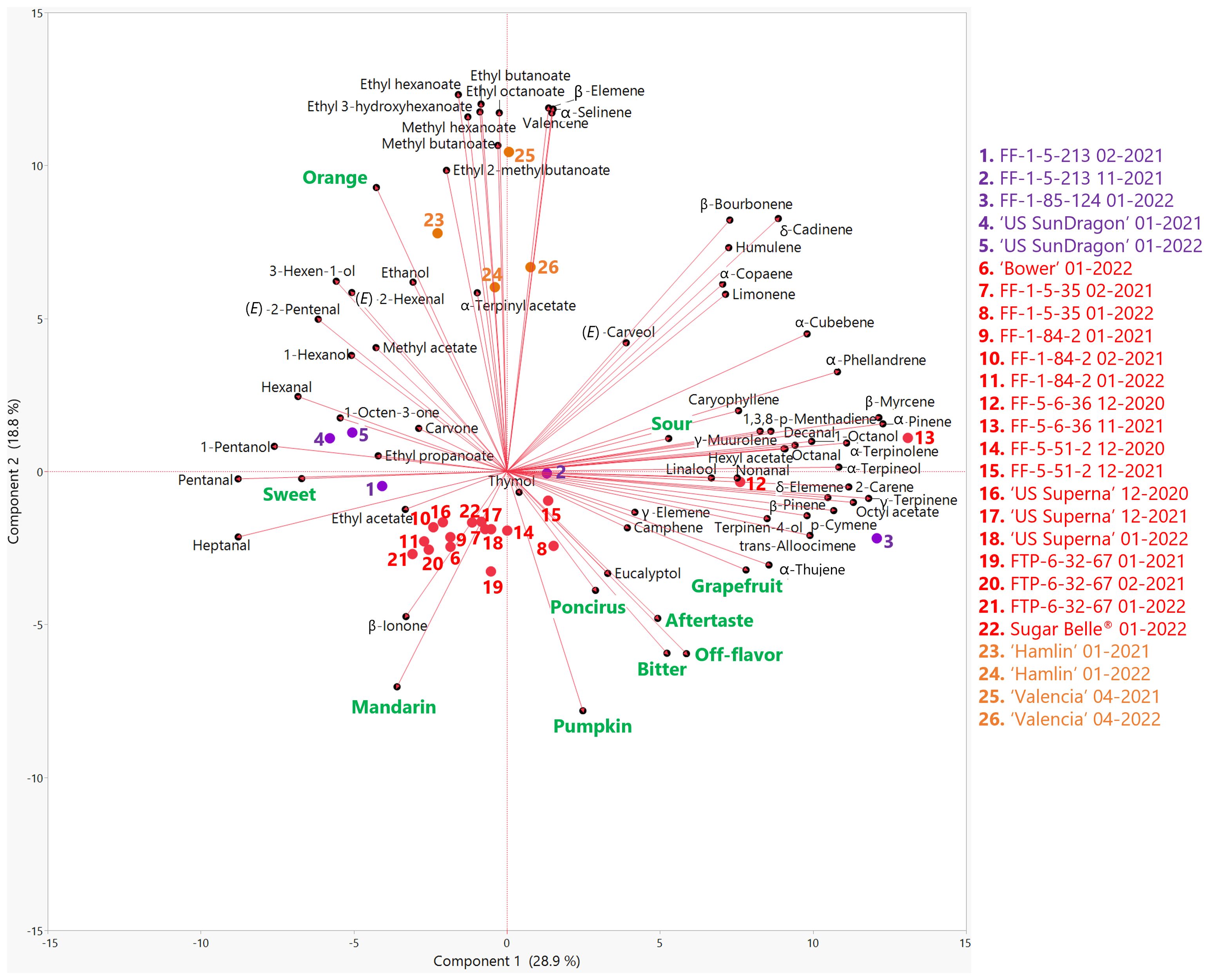
Figure 2. Biplot of the first two principal components from the principal component analysis (PCA) of sensory attributes (green) and volatile compounds (black) in genotypes harvested in the 2020-21 and 2021-22 seasons. Mandarin hybrids without Poncirus introgression are red, orange varieties are orange, and Poncirus hybrids are purple. Harvest month and year follow the listed genotype.
Commercial orange varieties clustered together on the positive side of PC2 and clearly separated from the mandarin hybrids. Recently, it was reported that seven esters, including methyl hexanoate; ethyl hexanoate; ethyl 3-hydroxyhexanoate; ethyl octanoate; methyl butanoate; ethyl butanoate, and ethyl 2-methylbutanoate, were key compounds distinguishing orange from mandarin flavors (Fan et al., 2024). Consistent with those results, mostly esters (fruity) and some sesquiterpenes were highly abundant in ‘Hamlin’ and ‘Valencia’ (Figure 2).
A clearly separated cluster of mandarin hybrids on the negative side of PC2 was correlated with mandarin flavor and sweetness. This cluster included hybrids with positive sensory attributes, such as ‘Bower’, FF-5-51-2, ‘US Superna’, FTP-6-32-67, and Sugar Belle®, as well as some hybrids with pronounced off-flavors such as FF-1-5-35 and FF-1-84-2. β-ionone, a product of β-carotene degradation and a potent floral terpene ketone (Winterhalter and Rouseff, 2001) was highly abundant in the juice of the mandarin hybrids clustered together. As shown in Table 2, some panelists commented that some of the mandarins had a pumpkin off-flavor. The only two volatiles that were positively correlated with mandarin flavor and negatively correlated with orange flavor were β-ionone and eucalyptol (Supplementary Table S4). Interestingly, eucalyptol, with a camphor-like or mint-like odor (Klocke et al., 1987; Rychlik et al., 1998), was positively correlated with the previously mentioned negative attributes. It was suggested that eucalyptol could have feeding and ovipositional repellency against the vector of Candidatus Liberibacter asiaticus, Diaphorina citri (Killiny et al., 2018). However, this has yet to be researched.
The Poncirus hybrids displayed volatile profiles very different from each other, spanning PC1. ‘US SunDragon’ clustered in between the mandarin hybrids and oranges, with FF-1-5-213 closer to mandarin hybrids according to their volatile profiles, and with higher C-5 and C-6 alcohols and aldehydes. While it was rated with moderate to high orange flavor, the Poncirus hybrid FF-1-5-213 had some “poncirus” off-flavors as well. FF-1-85-124, with strong “poncirus” off-flavors, separated from the rest of the Poncirus hybrids. Grapefruit flavor, aftertaste, off-flavor, bitterness, and “poncirus” flavor were positively correlated with each other (Figure 2; Supplementary Table S4). “Poncirus” flavor has been shown to be due to a lack of aldehydes with typical citrus odor as well as higher than typical amounts of sesquiterpene hydrocarbons (woody/green), monoterpenes (citrus/pine), and terpene esters (floral) (Deterre et al., 2023). Consistent with these findings, FF-5-6-36, a mandarin hybrid, and FF-1-85-124, a Poncirus hybrid, were clustered together on the positive side of PC1 with a higher abundance of monoterpenes (Figure 2). Specifically, high off-flavor and aftertaste ratings positively correlated with the monoterpene hydrocarbon, camphene, which has a pungent camphor odor and was determined to be a major contributor in the aroma of carrot (Rajkumar et al., 2017). Out of the genotypes in this study, FF-1-5-35, FF-1-85-124, and FF-5-6-36 had the highest abundances of camphene.
3.3 Flavonoid and limonoid profiling
Flavonoid and limonoid profiles of the genotypes in this study were obtained by widely targeted LC-MS/MS analysis. Across the genotypes, there was some variation in the non-volatile profiles (Figure 3) for the identified compounds, which included forty-seven flavonoids, seven limonoids, four coumarins, one phenolic ester, one phenylamine, and one fat-soluble vitamin (Vitamin E) (Supplementary Table S1). Desirable sensory descriptors, orange flavor, mandarin flavor, and sweetness characterized the negative axis of PC1 while less desirable descriptors characterized the positive axis. Many different flavonoid and limonoid compounds correlated with orange and mandarin flavors (Figure 3). Of note, eriocitrin and a rarely reported compound, quercetin 3-(3R-glucosylrutinoside) (Sun et al., 2023), were the most positively correlated flavonoids with orange flavor (Supplementary Table S5). For mandarin flavor, there were significant negative correlations with the abundances of quercetin 3-(3R-glucosylrutinoside), tricin, tricin-5-O-glucoside, and apigenin. Mandarin flavor positively correlated with synephrine, a phenylethylamine, and tetramethoxyisoscutellarein, a polymethoxylated flavone. While not targeted in this study, scutellarein, was correlated with bitterness and was previously reported in mandarins (Feng et al., 2021). Bitterness, off-flavor, aftertaste, and grapefruit flavor strongly correlated with each other and with limonin, nomilin, and limonoate A-ring lactone. Limonoate A-ring lactone is difficult to analyze due to its inherent chemical instability and the lack of a commercially available standard. To the authors’ knowledge, this is the first report of LC-MS/MS MRM transitions to identify and quantitate limonoate A-ring lactone. The limonoid glucosides targeted in this study, obacunone glucoside, limonin glucoside, nomilin glucoside, and nomilinic acid glucoside did not correlate with bitterness. Known bitter compounds, naringin, poncirin, obacunone, and neohesperidin were targeted with LC-MS/MS but were not identified in any of the genotypes in this study. Neoponcirin, shown to be correlated with bitterness in mandarins (Feng et al., 2021), correlated with bitterness among the genotypes in this study. FF-1-5-35 and FF-1-85-124 were the most bitter individuals and were localized on the positive side of PC1 (Figure 3). Along with these two bitter hybrids, FTP-6-32-67 harvested in January 2021 was also on the positive side of PC1. As previously mentioned herein, the juice prematurely harvested in January had a high level of limonin (28.9 mg/L), which dropped to 6.67 mg/L when harvested in the following month (Supplementary Table S2).
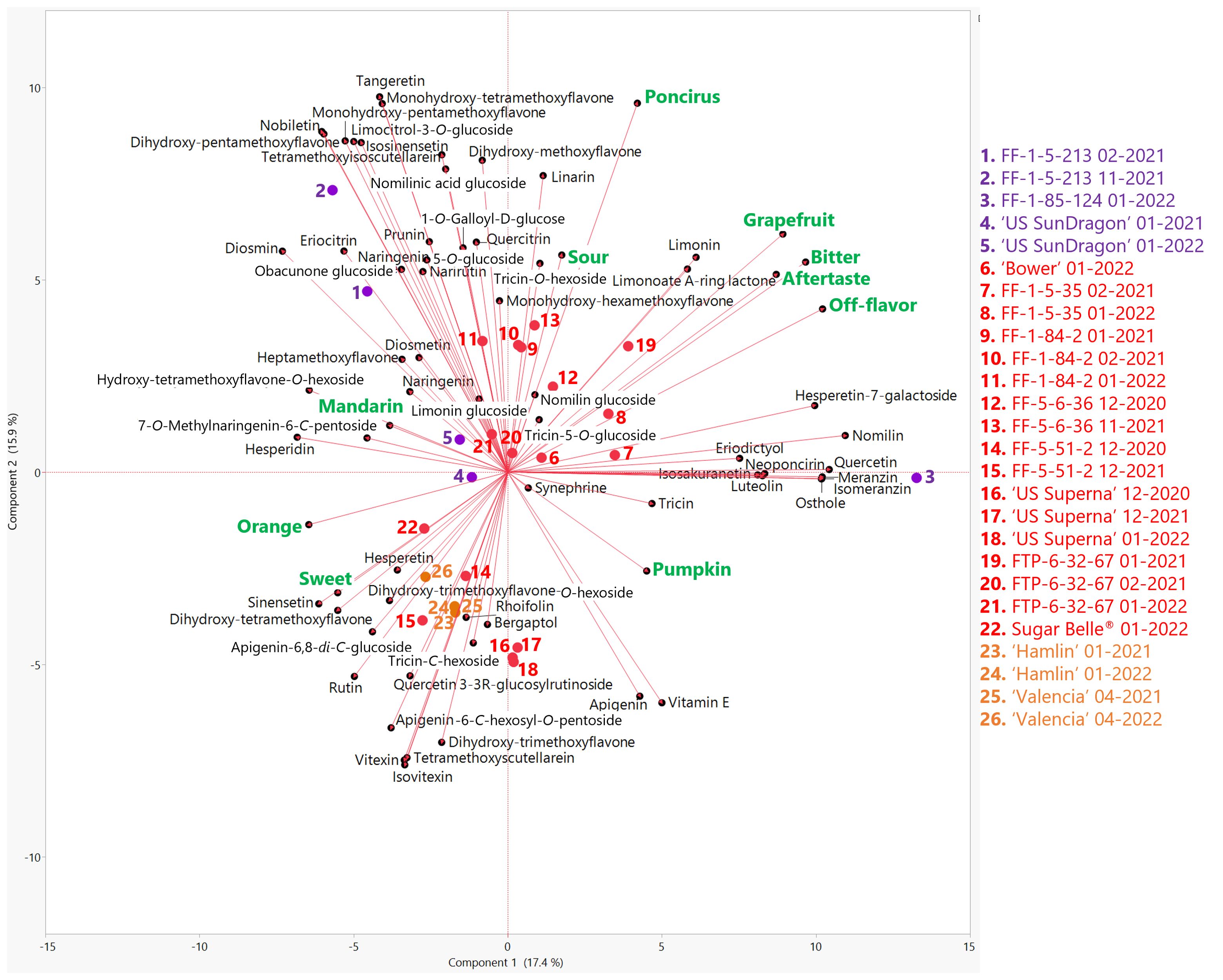
Figure 3. Biplot of the first two principal components from the principal component analysis (PCA) of sensory attributes (green) and flavonoids/limonoids (black) in genotypes harvested in the 2020-21 and 2021-22 seasons. Mandarin hybrids without Poncirus introgression are red, orange varieties are orange, and Poncirus hybrids are purple. Harvest month and year follow the listed genotype.
Commercial orange juices and the mandarin hybrids with desirable attributes (Sugar Belle®, ‘US Superna’, and FF-5-51-2) clustered on the negative side or close to 0 of PC1 characterized by orange flavor, mandarin flavor and sweetness. The remaining mandarins and Poncirus hybrids were distributed in the flavonoids/limonoid space. Of note, ‘US SunDragon’ was positioned near the center of the PCA biplot (Figure 3) and lacked high abundances of a number of polymethoxylated flavonoids. The Poncirus hybrid FF-1-85-124 was an outlier according to its volatile profile (Figure 2) as well as according to its flavonoid and limonoid profiles (Figure 3). This hybrid had the highest abundances of nomilin, quercetin, hesperetin-7-galactoside and the coumarins, osthole, meranzin, and isomeranzin. FF-1-85-124 also had significantly lower abundances of the polymethoxylated flavonoids, tetramethoxyisoscutellarein, tetramethoxyscutellarein, sinensetin, isosinensetin, tangeretin, and nobiletin, than the other genotypes in this study. According to its volatile profile, another Poncirus hybrid, FF-1-5-213, clustered closer to the mandarins (Figure 2), but was an outlier according to its flavonoid and limonoid profile (Figure 3), underscoring the importance of analyzing complete chemical profiles in relation to sensory data for citrus. FF-1-5-213 had the highest abundances of diosmin, nobiletin, a monohydroxy-pentamethoxyflavone, a dihydroxy-pentamethoxyflavone, and limocitrol-3- O-glucoside than the other genotypes in this study. In a previous study, it was suggested that another Poncirus hybrid with strong “poncirus” flavor, ‘US 119’ had an aglycone profile similar to that of P. trifoliata, consisting of mainly isosakuranetin and naringenin and lacking hesperetin (Deterre et al., 2021). Among the Poncirus hybrids in this study, FF-1-5-213 and ‘US SunDragon’ had lower abundances of isosakuranetin while FF-1-85-124 had a higher abundance. Similar to ‘US 119’ (Deterre et al., 2021), naringenin was relatively higher in hybrids with “poncirus” off-flavors, FF-1-85-124 and FF-1-5-213. Interestingly, ‘US SunDragon’ had a lower abundance of the aglycone naringenin, but a higher abundance of its glycoside, narirutin (naringenin-7-O-rutinoside), in relation to the other genotypes. Although the acacetin aglycone was not targeted in this study, FF-1-85-124 and FF-1-5-213 had the highest abundances of linarin (acacetin-7-O-rutinoside) and this compound was negatively correlated with sweetness and positively correlated with sourness. The high abundances of linarin could be due to the Poncirus in their backgrounds, as it was reported to be highly abundant in P. trifoliata (Mou et al., 2021).
The aglycone quercetin and its glycosides (quercitrin, rutin, and quercetin-3-(3R-glucosylrutinoside)) (chemically defined in Supplementary Table S6) differentiated the Poncirus hybrids in this study. FF-1-85-124 had an extremely high abundance of quercetin compared to FF-1-5-213 and ‘US SunDragon’. Quercetin positively correlated with grapefruit and off-flavor ratings and negatively correlated with sweetness (Supplementary Table S5). Quercetin glycosides, quercitrin and rutin, were less abundant in F-1-85-124, and more abundant in the Poncirus hybrids with higher citrus (orange and mandarin) flavors, FF-1-5-213 and ‘US SunDragon’. In a future study, it would be interesting to determine if FF-1-85-124 lacks the glycosyl transferase responsible for the biosynthesis of quercitrin and rutin, leading to the accumulation of quercetin. As previously stated herein, quercetin-3-(3R-glucosylrutinoside) was positively correlated with orange flavor and ‘US SunDragon’ has the highest abundance of this quercetin glycoside of the Poncirus hybrids.
3.4 Delayed bitterness
It was observed that some genotypes in this study had a noticeable increase in bitterness from when the fruit were harvested to when the juice was served in the sensory panels. During this time, the juice was stored at -20°C. It was hypothesized that some enzymatic activity was still ongoing at -20°C but not at -80°C. Therefore, a widely targeted LC-MS/MS analysis was performed on juice samples stored at -20°C and -80°C to understand how flavonoid and limonoid levels change and contribute to this delayed bitterness, assuming enzymatic activity is highly reduced at -80°C. In 2-way hierarchical clustering analysis, the genotypes from the 2021-2022 season are on the y-axis while flavonoids and limonoids are on the x-axis (Figure 4). Each genotype clustered with itself, regardless of storage temperature. Overall, flavonoids increased and decreased with some trends, but there was high variability. The well-studied bitter limonoids, limonin and nomilin, increased 15- 220% and 8- 203%, respectively, across all hybrids during storage at -20°C (Supplementary Table S7). Interestingly, these bitter limonoids did not increase in Sugar Belle® juice, which did not develop bitterness in storage. To a lesser extent, nomilin glucoside decreased and nomilinic acid glucoside increased in all the genotypes in this study (Supplementary Table S8).
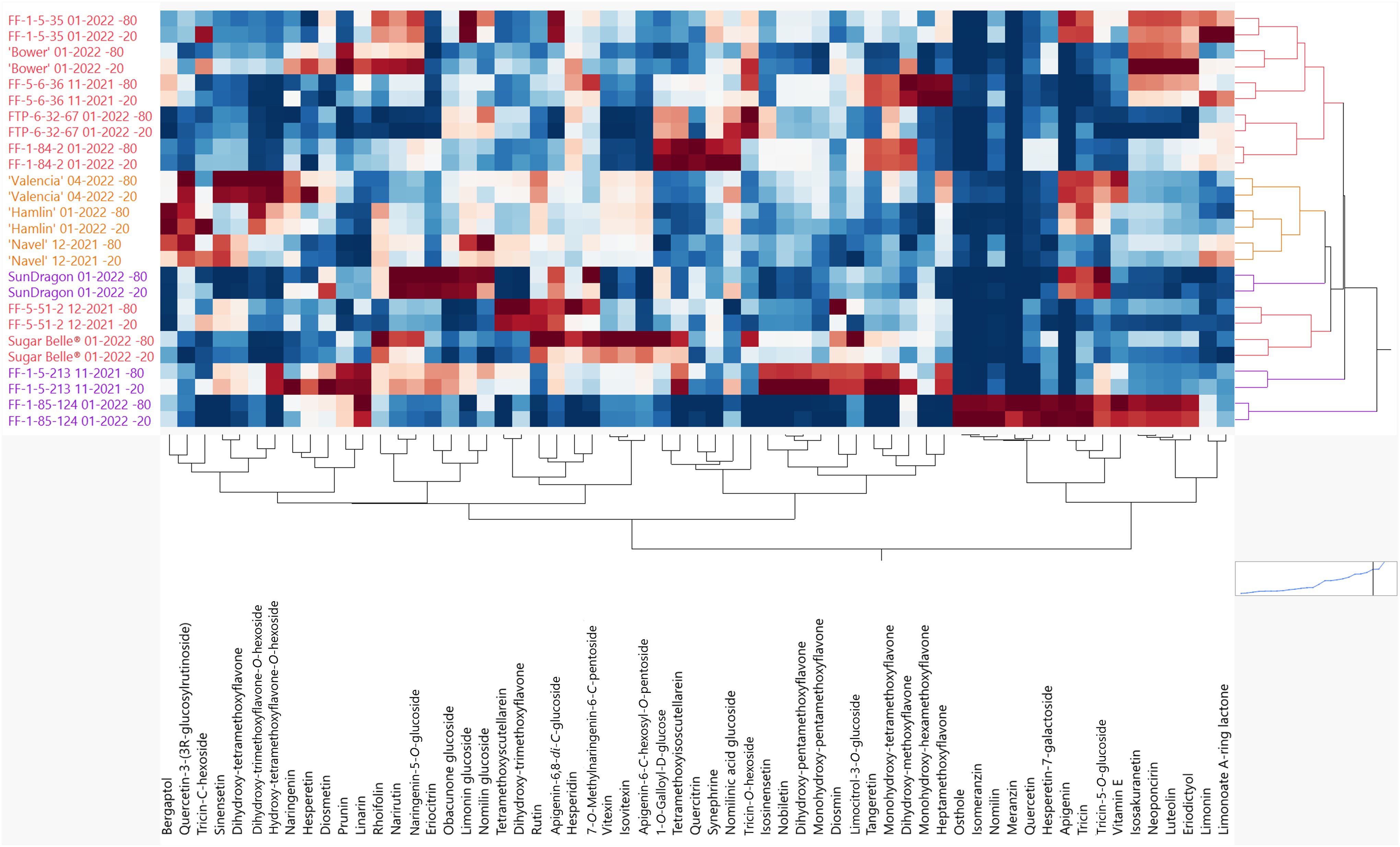
Figure 4. Hierarchical clustering (2-way) of the flavonoids and limonoids in juice stored at -80°C and -20°C. Mandarin hybrids without Poncirus introgression (red), orange varieties (orange), and Poncirus hybrids (purple) are on the y-axis and relative flavonoids and limonoids are on the x-axis. Darker red color indicates higher abundance of each compound and darker blue color indicates lower abundance.
The mandarin hybrids, FF-5-6-36 and FTP-6-32-67 were identified as having the most noticeable increases in bitterness from harvest to taste panel analysis. These two had significant increases in naringenin, an aglycone known to be the tasteless precursor of the bitter glycoside, naringin (Sinclair, 1972). Naringin (Naringenin 7-O-neohesperidoside) is present in grapefruit and has been shown to be responsible for primary bitterness (Sinclair, 1972). It would be interesting to study if the activated precursors (p-coumaroyl-CoA and malonyl-CoA) (Martin and Liras, 2022) were enzymatically being converted to naringenin upon -20°C storage, or if when the fruit tissue was disrupted during juicing, non-enzymatic de-glycosylation of naringenin glycosides occurred. There was a measured increase in another naringenin glycoside, prunin (Naringenin-7-glucoside) (Horowitz and Gentili, 1961), in these two hybrids (Supplementary Table S7; Figure 4); however, it is likely that another naringenin glycoside not identified in this study could be present in the samples and contributing to bitterness since prunin was reported to be 33% as bitter as naringin (Puri et al., 1996). Untargeted LC/MS analysis of flavonoids could help identify other not previously known compounds. Tricin-C-hexoside, had the most drastic increases from -20°C storage with increases being 48-1,088% across the genotypes, except for FF-1-85-124, in which the compound was not present. Of note, tricin, a polymethoxylated flavone, increased 275% in FTP-6-32-67. Tricin and tricin glycosides are rarely reported in citrus and could play a major role in delayed bitterness, but further research should be performed to validate these findings as there is no data on the taste of these compounds (Li et al., 2016). In a study of healthy versus HLB-affected orange juice, nonvolatile fractions of HLB-affected orange juice were described as bitter. Except for one fraction containing limonoids and polymethoxylated flavones, most compounds were derivatives of hydroxycinnamates (Dala Paula et al., 2018). Further research with more bitter juice should be done to identify specific compounds or groups of compounds, other than limonin and nomilin, contributing to bitterness in citrus juices.
In summary, a selection of oranges, mandarin hybrids, and Poncirus hybrids were comprehensively analyzed for volatiles, flavonoids, limonoids, SSC, TA, and compared with sensory analysis from a trained panel. The flavors of the genotypes in this study ranged from juice that was highly rated in citrus flavors to juice with “poncirus”-like and bitter off-flavors. An important part of understanding acceptable flavors for citrus juice is also to determine which chemical components are contributing to off-flavors and bitterness. From this two-year study, PCA biplots revealed that the juices of the same genotype had similar sensory and chemical profiles between the two harvest years. Of note, along with the commercial cultivars, HLB-tolerant ‘US SunDragon,’ Sugar Belle®, FF-5-51-2, and ‘US Superna’ had acceptable citrus flavor quality, similar to commercial oranges or mandarins. FF-1-5-213, FF-1-84-2, FF-1-85-124, FF-1-5-35 had “poncirus” off-flavors. Volatiles (esters and some sesquiterpenes) along with flavonoids (eriocitrin and quercetin-3-(3R-glucosylrutinoside) were positively correlated with orange flavor while β-ionone and eucalyptol were highly abundant in the mandarins. Previously, “poncirus” flavor was reported to be due to sesquiterpene hydrocarbons, monoterpenes, and terpene esters (Deterre et al., 2023). From this study, some flavonoids were shown to positively correlate with “poncirus’ off-flavors as well. FF-1-85-124, a Poncirus hybrid with strong off-flavors and bitterness, had significantly higher abundances of hesperetin-7-galactoside and the coumarins, osthole, meranzin, and isomeranzin. The flavonoid linarin, was more abundant in Poncirus hybrids with off-flavors than in the Poncirus hybrid ‘US SunDragon’, having high orange flavor. Two mandarin hybrids, FF-5-6-36 and FTP-6-32-67, had decent citrus flavor at harvest, but exhibited delayed bitterness with storage at -20°C, suggesting they should not be used for commercial juice processing, but only as fresh fruit. Some flavonoids and limonoids increased significantly in the stored juice, but further research with more bitter juice would be useful to identify other compounds contributing to bitterness in citrus.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the studies involving humans because This study was conducted within the guidelines of the human subject exemption as stated in 45 CFR 46.104 (d)(6). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements because Participation in tasting orange juice was voluntary.
Author contributions
KJ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. ZF: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – review & editing. XS: Data curation, Investigation, Writing – review & editing. GO: Investigation, Writing – review & editing. WZ: Investigation, Writing – review & editing. MM: Investigation, Resources, Writing – review & editing. ES: Conceptualization, Funding acquisition, Project administration, Resources, Writing – review & editing. EB: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review & editing. JM: Investigation, Resources, Writing – review & editing. AB: Resources, Writing – review & editing. JB: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing. AP: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Visualization, Writing – review & editing, Supervision.
Funding
The author(s) declare that financial support was received for theresearch, authorship, and/or publication of this article. This work is supported by the USDA National Institute of Food and Agriculture grant number 2018–70016-27453.
Acknowledgments
The authors would like to thank sensory panelists for their participation, the Florida Department of Citrus for providing analyte standards for LC-MS/MS analysis, Nancy Owens for GC/MS analysis, and Holly Sisson and David Wood for their help with sugar and acid analyses.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fhort.2024.1425366/full#supplementary-material
Abbreviations
HLB, Huanglongbing; SSC, soluble solids content; TA, titratable acidity; UPLC, ultra performance liquid chromatography; LC-MS/MS, liquid chromatography- tandem mass spectrometry; dMRM, dynamic multiple reaction monitoring; GC/MS, gas chromatography- mass spectrometry; PCA, principal component analysis; ANOVA, analysis of variance; SPME, solid-phase microextraction.
References
Adams R. (2017). Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 5 (Gruver, TX: Texensis Publishing).
Albrecht U., Bowman K. D. (2011). Tolerance of the trifoliate citrus hybrid US-897 (Citrus reticulata Blanco x Poncirus trifoliata L. Raf.) to Huanglongbing. HortScience 46, 16–22. doi: 10.21273/HORTSCI.46.1.16
Bai J., Baldwin E., Hearn J., Driggers R., Stover E. (2016). Volatile and nonvolatile flavor chemical evaluation of USDA orange–mandarin hybrids for comparison to sweet orange and mandarin fruit. J. Amer. Soc Hortic. Sci. 141, 339–350. doi: 10.21273/JASHS.141.4.339
Bai J., Ford B., Manthey J., Baldwin E. (2010). A comparison of processed and fresh squeezed ‘Hamlin’ Orange juice—Nutrients and phytonutrients. Proc. Fla. State Hortic. Soc 123, 207–212.
Charve J., Chen C., Hegeman A. D., Reineccius G. A. (2011). Evaluation of instrumental methods for the untargeted analysis of chemical stimuli of orange juice flavour. Flavour Fragrance J. 26, 429–440. doi: 10.1002/ffj.2078
Dala-Paula B. M., Plotto A., Bai J., Manthey J. A., Baldwin E. A., Ferrarezi R. S., et al. (2018). Effect of huanglongbing or greening disease on orange juice quality, a review. Front. Plant Sci. 9, 1976. doi: 10.3389/fpls.2018.01976
Dala Paula B. M., Raithore S., Manthey J. A., Baldwin E. A., Bai J., Zhao W., et al. (2018). Active taste compounds in juice from oranges symptomatic for Huanglongbing (HLB) citrus greening disease. Lwt 91, 518–525. doi: 10.1016/j.lwt.2018.01.083
De La Pena R., Hodgson H., Liu J. C., Stephenson M. J., Martin A. C., Owen C., et al. (2023). Complex scaffold remodeling in plant triterpene biosynthesis. Science 379, 361–368. doi: 10.1126/science.adf1017
Deterre S. C., Jeffries K. A., McCollum G., Stover E., Leclair C., Manthey J. A., et al. (2023). Sensory quality of Citrus scion hybrids with Poncirus trifoliata in their pedigrees. J. Food Sci. 88, 1684–1699. doi: 10.1111/1750-3841.16499
Deterre S. C., McCollum G., Leclair C., Manthey J. A., Bai J., Baldwin E. A., et al. (2021). Effect of Poncirus trifoliata on the chemical composition of fruits in pedigrees of Citrus scion hybrids. Scientia Hortic. 277, 109816. doi: 10.1016/j.scienta.2020.109816
Fan Z., Jeffries K. A., Sun X., Olmedo G., Zhao W., Mattia M. R., et al. (2024). Chemical and genetic basis of orange flavor. Sci. Adv. 10, eadk2051. doi: 10.1126/sciadv.adk2051
Feng S., Gmitter F. G. Jr., Grosser J. W., Wang Y. (2021). Identification of key flavor compounds in Citrus fruits: A flavoromics approach. ACS Food Sci. Technol. 1, 2076–2085. doi: 10.1021/acsfoodscitech.1c00304
Feng S., Niu L., Suh J. H., Hung W. L., Wang Y. (2018). Comprehensive metabolomics analysis of mandarins (Citrus reticulata) as a tool for variety, rootstock, and grove discrimination. J. Agric. Food Chem. 66, 10317–10326. doi: 10.1021/acs.jafc.8b03877
Hasegawa S., Ou P., Fong C. H., Herman Z., Coggins C. W., Atkin D. R. (2002). Changes in the limonoate A-ring lactone and limonin 17-.beta.-D-glucopyranoside content of navel oranges during fruit growth and maturation. J. Agric. Food Chem. 39, 262–265. doi: 10.1021/jf00002a008
Horowitz R., Gentili B. (1961). Phenolic glycoside of grapefruit: A relation between bitterness and structure. Arch. Biochem. Biophys. 92, 191–192. doi: 10.1016/0003-9861(61)90235-1
Killiny N., Jones S. E., Nehela Y., Hijaz F., Dutt M., Gmitter F. G., et al. (2018). All roads lead to Rome: Towards understanding different avenues of tolerance to huanglongbing in citrus cultivars. Plant Physiol. Biochem. 129, 1–10. doi: 10.1016/j.plaphy.2018.05.005
Klocke J. A., Darlington M. V., Balandrin M. F. (1987). 1,8-cineole (Eucalyptol), A mosquito feeding and ovipositional repellent from volatile oil of hemizonia fitchii (Asteraceae). J. Chem. Ecol. 13, 2131–2141. doi: 10.1007/BF01012562
Lawless H. T., Heymann H. (1999). Sensory Evaluation of Food (New York: Principles and Practices. Kluwer Academic/Plenum Publishers).
Li L., Yan X., Chen F., Zheng L., Hu Y., He F., et al. (2023). A comprehensive review of the metabolism of citrus flavonoids and their binding to bitter taste receptors. Compr. Rev. Food Sci. Food Saf. 22, 1763–1793. doi: 10.1111/1541-4337.13129
Li M., Pu Y., Yoo C. G., Ragauskas A. J. (2016). The occurrence of tricin and its derivatives in plants. Green Chem. 18, 1439–1454. doi: 10.1039/C5GC03062E
Maier V., Beverly G. (1968). Limonin monolactone, the nonbitter precursor responsible for delayed bitterness in certain citrus juices. J. Food Sci. 33, 488–492. doi: 10.1111/j.1365-2621.1968.tb03661.x
Marsh G., Cameron S. (1950). Navel Orange Juice Bitterness: rootstock determines amount of bitterness in juice of Washington Navel oranges investigations reveal. California Agirculture 4, 7–12. doi: 10.3733/ca.v004n06p7
Martin J. F., Liras P. (2022). Comparative molecular mechanisms of biosynthesis of naringenin and related chalcones in actinobacteria and plants: relevance for the obtention of potent bioactive metabolites. Antibiotics (Basel) 11, 82. doi: 10.3390/antibiotics11010082
Miyazaki T., Plotto A., Baldwin E. A., Reyes-De-Corcuera J. I., Gmitter F. G. Jr. (2012). Aroma characterization of tangerine hybrids by gas-chromatography-olfactometry and sensory evaluation. J. Sci. Food Agric. 92, 727–735. doi: 10.1002/jsfa.4663
Molyneux R. J., Schieberle P. (2007). Compound identification: A journal of agricultural and food chemistry perspective. J. Agric. Food Chem. 55, 4625–4629. doi: 10.1021/jf070242j
Mou J., Zhang Z., Qiu H., Lu Y., Zhu X., Fan Z., et al. (2021). Multiomics-based dissection of citrus flavonoid metabolism using a Citrus reticulata x Poncirus trifoliata population. Hortic. Res. 8, 56. doi: 10.1038/s41438-021-00472-8
Peterson J. J., Dwyer J. T., Beecher G. R., Bhagwat S. A., Gebhardt S. E., Haytowitz D. B., et al. (2006). Flavanones in oranges, tangerines (mandarins), tangors, and tangelos: a compilation and review of the data from the analytical literature. J. Food Composition Anal. 19, S66–S73. doi: 10.1016/j.jfca.2005.12.006
Plotto A., Baldwin E., Bai J., Manthey J., Raithore S., Deterre S., et al. (2017). Effect of vector control and foliar nutrition on the quality of orange juice affected by huanglongbing: sensory evaluation. HortScience 52, 1092–1099. doi: 10.21273/HORTSCI12002-17
Plotto A., Baldwin E., McCollum G., Gmitter F. G. J. (2011). Sensory evaluation of tangerine hybrids at multiple harvests. Proc. Fla. State Hortic. Soc. 124, 260–263.
Puri M., Marwaha S. S., Kothari R. M., Kennedy J. F. (1996). Biochemical basis of bitterness in citrus fruit juices and biotech approaches for debittering. Crit. Rev. Biotechnol. 16, 145–155. doi: 10.3109/07388559609147419
Raithore S., Dea S., McCollum G., Manthey J. A., Bai J., Leclair C., et al. (2016). Development of delayed bitterness and effect of harvest date in stored juice from two complex citrus hybrids. J. Sci. Food Agric. 96, 422–429. doi: 10.1002/jsfa.7105
Rajkumar G., Shanmugam S., Galvâo M., d. S., Leite Neta M. T. S., Dutra Sandes R. D., et al. (2017). Comparative evaluation of physical properties and aroma profile of carrot slices subjected to hot air and freeze drying. Drying Technol. 35, 699–708. doi: 10.1080/07373937.2016.1206925
Rychlik M., Schieberle P., Grosch W. (1998). Compilation of odor thresholds, odor qualities and retention indices of key food odorants (Garching, Germany: Institut für Lebensmittelchemie der Technischen Universität München and Deutsche Forschungsanstalt für Lebensmittelchemie).
Sinclair W. (1972). The grapefruit: its composition, physiology, and products (Berkeley, CA: University of California, Division of Agricultural Sciences).
Stover E., Gmitter F. G. Jr., Grosser J., Baldwin E., Wu G. A., Bai J., et al. (2020). Rationale for reconsidering current regulations restricting use of hybrids in orange juice. Hortic. Res. 7, 38. doi: 10.1038/s41438-020-0277-5
Sun R., Xing R., Zhang J., Deng T., Ge Y., Zhang W., et al. (2023). Quality changes of HHP orange juice during storage: Metabolomic data integration analyses. Food Chem. 404, 134612. doi: 10.1016/j.foodchem.2022.134612
Tripoli E., Guardia M. L., Giammanco S., Majo D. D., Giammanco M. (2007). Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 104, 466–479. doi: 10.1016/j.foodchem.2006.11.054
Wang F., Chen L., Chen S., Chen H., Liu Y. (2021). Characterization of two closely related citrus cultivars using UPLC-ESI-MS/MS-based widely targeted metabolomics. PloS One 16, e0254759. doi: 10.1371/journal.pone.0254759
Wang Z., Gmitter F. G. Jr., Grosser J. W., Wang Y. (2022). Natural sweeteners and sweetness-enhancing compounds identified in citrus using an efficient metabolomics-based screening strategy. J. Agric. Food Chem. 70, 10593–10603. doi: 10.1021/acs.jafc.2c03515
Wang S., Yang C., Tu H., Zhou J., Liu X., Cheng Y., et al. (2017). Characterization and metabolic diversity of flavonoids in citrus species. Sci. Rep. 7, 10549. doi: 10.1038/s41598-017-10970-2
Webber H. J., Swingle W. T. (1905). New citrus creations of the Department of Agriculture Yearbook of the United States Department of Agriculture United States Department of Agriculture. (United States Department of Agriculture (USDA)), 221–240.
Winterhalter P., Rouseff R. (2001). Carotenoid-Derived Aroma Compounds: An Introduction. (American Chemical Society, Chapter 1), 1-17. doi: 10.1021/bk-2002-0802.ch001
Wu G. A., Terol J., Ibanez V., Lopez-Garcia A., Perez-Roman E., Borreda C., et al. (2018). Genomics of the origin and evolution of Citrus. Nature 554, 311–316. doi: 10.1038/nature25447
Zhang J., Yang Z., Liang Y., Zhang L., Ling W., Guo C., et al. (2018). Effects of Postharvest Time, Heat Treatment, pH and Filtration on the Limonin Content in Newhall Navel Orange (Citrus sinensis Osbeck cv. Newhall) Juice. Molecules 23, 2691. doi: 10.3390/molecules23102691
Zhao X. J., Guo P. M., Pang W. H., Zhang Y. H., Zhao Q. Y., Jiao B. N., et al. (2020). A rapid UHPLC-QqQ-MS/MS method for the simultaneous qualitation and quantitation of coumarins, furocoumarins, flavonoids, phenolic acids in pummelo fruits. Food Chem. 325, 126835. doi: 10.1016/j.foodchem.2020.126835
Keywords: Citrus ×P. trifoliata, juice flavor, flavonoids, LC-MS/MS, GC-MS, sensory, HLB
Citation: Jeffries KA, Fan Z, Sun X, Olmedo GM, Zhao W, Mattia M, Stover E, Baldwin E, Manthey JA, Breksa A, Bai J and Plotto A (2024) New insights in the flavor and chemistry of Huanglongbing tolerant citrus hybrids with/without Poncirus trifoliata in their pedigree. Front. Hortic. 3:1425366. doi: 10.3389/fhort.2024.1425366
Received: 29 April 2024; Accepted: 16 August 2024;
Published: 10 September 2024.
Edited by:
Vasileios Ziogas, Institute of Olive Tree, Subtropical Plants and Viticulture, Hellenic Agricultural Organization DEMETER, GreeceReviewed by:
Jinyun Li, University of Florida, United StatesSukhvinder Pal (SP) Singh, NSW Government, Australia
At least a portion of this work is authored by Kristen A. Jeffries, Xiuxiu Sun, Gabriela M. Olmedo, Wei Zhao, Matthew Mattia, Ed Stover, Elizabeth Baldwin, John A. Manthey, Andrew Breksa, Jinhe Bai, and Anne Plotto on behalf of the U.S. Government and as regards Kristen A. Jeffries, Xiuxiu Sun, Gabriela M. Olmedo, Wei Zhao, Matthew Mattia, Ed Stover, Elizabeth Baldwin, John A. Manthey, Andrew Breksa, Jinhe Bai, Anne Plotto, and the U.S. Government, is not subject to copyright protection in the United States. Foreign and other copyrights may apply. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinhe Bai, SmluaGUuQmFpQHVzZGEuZ292; Anne Plotto, QW5uZS5QbG90dG9AdXNkYS5nb3Y=
†These authors have contributed equally to this work and share senior authorship
 Kristen A. Jeffries
Kristen A. Jeffries Zhen Fan
Zhen Fan Xiuxiu Sun
Xiuxiu Sun Gabriela M. Olmedo
Gabriela M. Olmedo Wei Zhao
Wei Zhao Matthew Mattia1
Matthew Mattia1 Andrew Breksa
Andrew Breksa Jinhe Bai
Jinhe Bai Anne Plotto
Anne Plotto