- 1Geno-Immune Medical Institute, Shenzhen, China
- 2Department of Hematology, Beijing Jingdu Children’s Hospital, Beijing, China
- 3Department of Hematology, Zhujiang Hospital, Southern Medical University, Guangzhou, China
- 4Department of Hematology, Peking University People’s Hospital, Beijing, China
- 5Department of Hematology, Aerospace Central Hospital, Beijing, China
- 6Department of Hematology, Beijing Children’s Hospital, Beijing, China
- 7Department of Pediatrics, Nagoya University Graduate School of Medicine, Nagoya, Japan
- 8University of Florida (UF) Shands Children’s Hospital, University of Florida, Gainesville, FL, United States
Introduction: Allogeneic hematopoietic stem cell transplantation (allo-HCT) is a standard treatment for relapsed/refractory B-cell acute lymphoblastic leukemia (r/r B-ALL). However, about 30-40% of patients still relapse after HCT. Chimeric antigen receptor-modified T-cell (CAR-T) therapy has been proven effective in the treatment of relapsed or refractory B-ALL.
Patients and methods: We report a cohort of 30 B-ALL patients, who relapsed after HCT and were enrolled in the 4SCAR2.0 study, receiving CD19 CAR-Ts alone (20 patients), or two types of CAR-Ts targeting CD19, CD22, CD38 or CD123 antigens (10 patients), depending on the tumor antigen expression profile. These patients had extramedullary (EM) relapse or bone marrow (BM) relapse, or both. Based on the GVHD history, donor chimerism, and the available T-cell source, 25 patients received allogeneic donor CAR-Ts, and 5 patients received autologous CAR-T treatment.
Results: All 20 patients receiving a single CD19 CAR-T infusion achieved a minimal residual disease (MRD) remission within 60 days. The remaining 10 patients, due to low CD19 antigen expression profile, received 2 CAR-T products given on the same day, and 9 of 10 achieved complete remission (CR) and one had disease progression within 60 days. After CAR-T infusion, no cytokine release syndrome (CRS) was observed in 14 patients, and 16 patients experienced grade 1 CRS, and there was no neurotoxicity. Seventeen of the 30 patients who achieved remission (57%) remained in continuous remission following CAR-T treatment with a median follow-up period of 2 years and a median duration of remission of 12 months (range: 2.8 months - 67 months). Twelve out of 29 patients (41%) who achieved remission, subsequently relapsed at a median of 6.3 months (range: 2.8 months - 22.3 months) after CAR-T treatment. In summary, 29 patients (97%) achieved MRD negative remission within 60 days of therapy with a single or double CAR-T infusion, and seven patients remained in durable remission (7/30, 23%) after more than 2 years of follow-up.
Discussion: The tumor antigen profile-guided precision 4SCAR2.0 regimen for the treatment of r/r B-ALL after allo-HCT was highly effective with low toxicity. This approach warrants extended follow-up and further studies.
Clinical trial registration: ClinicalTrials.gov, identifier NCT03125577.
Introduction
Chimeric antigen receptor (CAR) gene therapy is a breakthrough technology in the treatment of refractory hematologic malignancies (1–3). Currently, four chimeric antigen receptor-modified T-cell (CAR-T) products have been approved by the U.S. FDA for treating leukemia and lymphoma, all targeting the CD19 antigen. However, antigen escape, disease recurrence, CAR-T exhaustion, long manufacturing time, and high cost have been the major limitations of currently approved CAR-T therapies.
Allogeneic hematopoietic stem cell transplantation (allo-HCT) remains the standard-of-care treatment for relapsed or refractory B-cell acute lymphoblastic leukemia (r/r B-ALL) patients. The outcomes are poor, however, for patients with relapsed disease after HCT, and there is a need for novel treatments for these patients (4). CAR-T therapy has been proposed for treating r/r B-ALL patients after transplantation, yet several important issues remain, including how to choose the CAR-T source (allogeneic vs. autologous), the risk of graft-versus-host disease (GVHD), the best timing for CAR-T therapy, and the persistence of CAR-T in the HCT patients.
GVHD is a common complication after allo-HCT, but it also contributes to graft-versus-leukemia (GVL) effects. Despite GVHD, patients with B-ALL may develop extramedullary (EM) recurrence, such as central nervous system leukemia (CNSL) or testicular leukemia, without residual disease in the bone marrow (BM). Most chemotherapeutic agents and antibodies have limited CNS penetration, so EM B-ALL involving CNS poses a serious challenge. However, numerous reports indicate that CAR-T therapies are effective in patients with CNS-relapse of B-ALL and B-cell lymphomas (5–8).
To overcome the limitations of CAR-T therapies, here we report the results of the 4SCAR2.0 trial, which utilized CD19 CAR-T or a combination of CD19 with CD22, CD123, or CD38 CAR-T products based on individual leukemia antigen expression profiles, to target post-allo-HCT BM or extramedullary relapse of B-ALL. Similar to combining different chemotherapy agents to overcome chemotherapy resistance, we have started combining CAR-T therapies targeting different antigens to overcome tumor antigen escape. The fourth-generation safety switch CAR (4SCAR) design improved the safety and effector activity of multiple CAR-T therapies with reduced manufacturing time and costs. This report illustrates a high remission rate and an encouraging efficacy with low toxicity.
Patients and methods
Patient enrollment
The current study was approved by the Institutional Review Board of Geno-immune Medical Institute (GIMI) of Shenzhen, China (GIMI-IRB-17.005) and is registered at ClinicalTrials.gov as NCT03125577. The patients provided informed consent in accordance with institutional guidelines and the Declaration of Helsinki. This is an interim report from an ongoing multicenter trial of the 4SCAR2.0 study of combining multi-CAR-T therapies targeting B-cell malignancies.
Patients between 6 months and 80 years of age, who have had relapse after allo-HCT, did not have any active infections or major organ dysfunctions, and did not have a history of GVHD grade 3 or higher during their transplantation course were eligible for treatment in this study.
4SCAR2.0 study design
The 4SCAR2.0 regimen is designed to apply multiple CAR-T therapies based on target antigens identified in individual tumors. The targets for the CAR-T therapy were identified by flow cytometry or immunohistochemistry analysis of the pathological specimens, including blood, BM, spinal fluid, and EM tumor biopsies. The peripheral blood mononuclear cells (PBMCs) were obtained by apheresis from the patients at relapse with sufficient lymphocyte counts or from the healthy transplant donors, and the T cells were selected by CD3 magnetic beads. After activation, the T cells were transduced with a caspase 9-inducible, safety-engineered lentivector (LV) CAR containing multiple intracellular signaling domains, including a target antigen-specific single-chain antibody, single-chain variable fragment (scFv)/CD28/CD27/CD3z-iCasp9 (4SCAR), targeting the specific surface antigens (CD19, CD22, CD38, and CD123) as described in Nair et al. (9). After immunodepletion with fludarabine and cyclophosphamide, all patients received CD19 CAR-Ts. In addition, patients with a high leukemia burden, EM disease, and/or low CD19 expression in leukemic cells based on flow cytometry analysis received another CAR-T infusion targeting a different antigen (CD22, CD38, or CD123) according to the leukemic antigen profile. Daily adverse event assessments, including cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS), were performed within 14 days after infusion. The CAR-T expansion was measured by qPCR detection of the specific CAR DNA in the blood mononuclear cells after infusion. Long-term therapeutic responses continue to be followed up and evaluated by an oncologist.
Lentiviral CAR engineering and CAR-T preparation
Lentiviral vectors (LVs) were generated based on the NHP/TYF LV system, as previously described (9–11). A fourth-generation CAR, containing antigen-specific scFv fused with CD28-CD27-CD3z signaling domains and an inducible caspase 9 motif, was chemically synthesized and cloned into a pTYF-transducing vector behind a human EF1α promoter as previously described (9, 12). The scFv gene for the CD19 CAR was human codon-optimized based on the hybridoma FMC63 clone (9). The scFv fragments for the CD38 CAR, CD22 CAR, and CD123 CAR were codon-optimized and chemically synthesized by Epoch Life Science (Sugar Land, TX, USA) based on monoclonal antibody clones Hu-Max-CD38, RFB4, and 7G3, respectively (13–15). In addition, a CD19 CAR design based on the IL-15Ra intracellular signaling domain (4SCAR19-153z) was constructed and functionally tested as previously described (9). The final LV-CAR constructs and their target-killing activities were verified by DNA sequencing and target-specific functional analyses. For the preparation of clinical-grade CAR-Ts, a standard operation procedure has been established in compliance with good manufacturing and laboratory practice (GMP) following the regulatory guidelines for cell and gene therapy products.
CAR detection by quantitative PCR
The CAR copy number in blood was determined by real-time qPCR, based on both SYBR™ and TaqMan™ probe methods using primers designed according to the individual CAR scFv gene sequences; therefore, different CAR-Ts could be monitored in patients simultaneously (9, 12, 16). Genomic DNA was harvested from blood cells using a Promega genomic DNA purification kit (Promega Corp., Madison, WI, USA). The qPCR data were collected using a Mx3000P™ system (Stratagene, Agilent Technologies, Santa Clara, CA, USA). The percentage of CAR-Ts in the PBMCs was calculated based on the copy number of a housekeeping gene.
Results
Patient characteristics
Patient characteristics are summarized in Table 1. The study profile is illustrated in Figure 1. Thirty patients who relapsed after allo-HCT for B-ALL were enrolled in the study. The median age was 14 years (range: 2 years–58 years), and nine patients (30%) had a BCR/ABL (p190) mutation. Before relapse, 10 (33%) patients had experienced grade 2 or lower acute GVHD (aGVHD), and 20 had no history of GVHD. No patients had active GVHD at the time of study enrollment. The relapse sites included the BM (n = 22), CNS relapse alone (n = 5), and combined BM and CNS relapse (n = 3). Bridging therapy prior to CAR-Ts was used in 22 patients (73%), as outlined in Table 1, and patients 14, 18, and 21 had previously received CD19 CAR-Ts from an unknown source. The BM tumor burden before CAR-T infusion ranged from MRD-negative to 91% of blasts, based on flow cytometric analysis before CAR-T therapy. Prior to CAR-T infusion, all patients received standard immunodepletion treatment with cyclophosphamide and fludarabine (8–12).
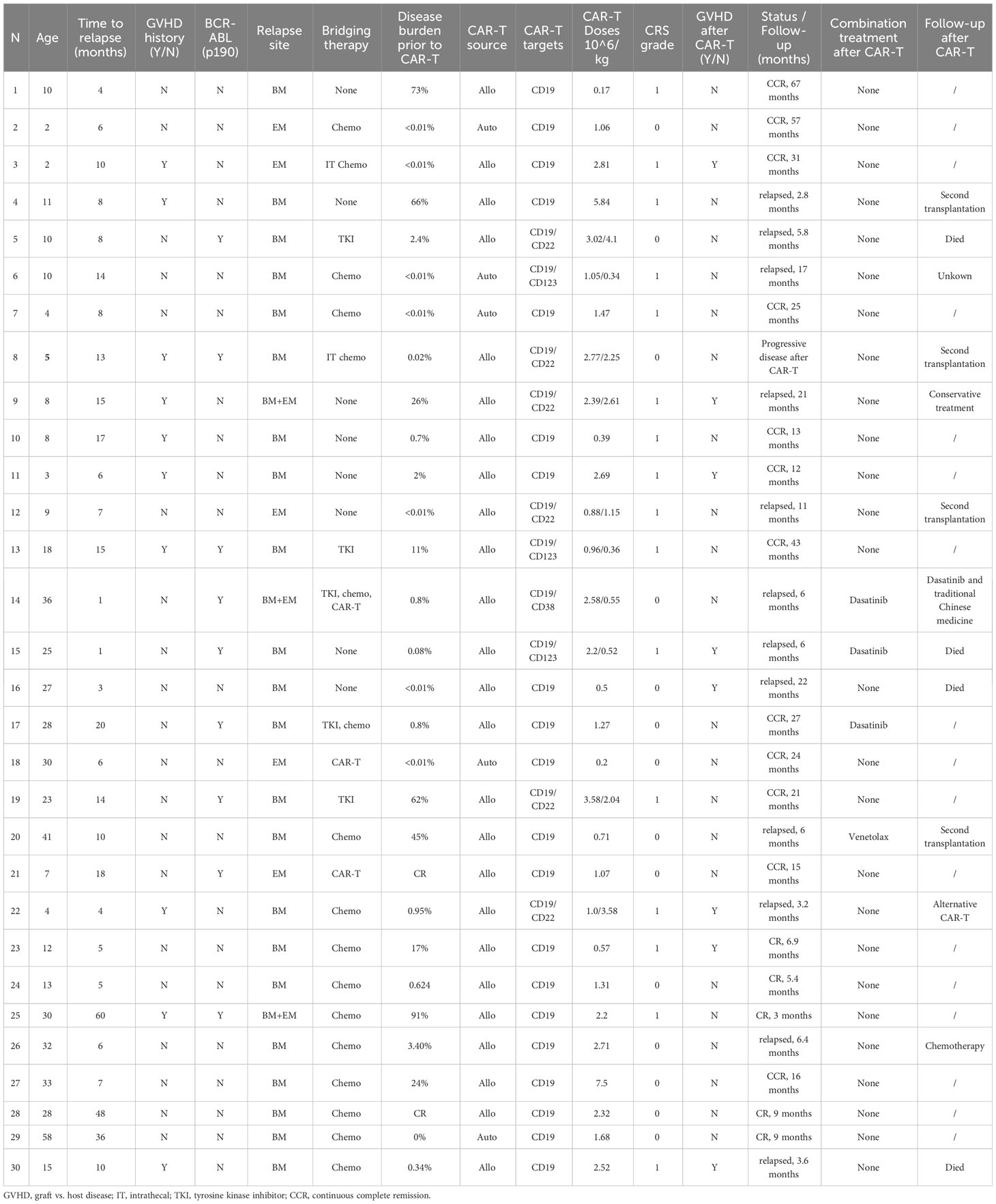
Figure 1 Study profile. The overall study profile, including patient enrollment, target analysis, CAR-T treatment cohorts, and follow-up responses, is illustrated. FACS, flow cytometry analysis; IHC, immunohistochemistry analysis; CR, complete remission; PD, progressive disease.
CAR-T cell infusion and doses
Twenty patients received a single CD19 CAR-T infusion, with a mean dose of 1.95 × 106 (± SD 1.85) CAR-T/kg, and all of them achieved an MRD-negative remission by flow cytometry within 60 days of infusion. The remaining 10 patients received two CAR-T infusions given on the same day, including CD19 plus CD22 (n = 6), CD123 (n =3), and CD38 (n = 1) CAR-T based on the leukemic antigen profile (Figure 2A). Nine of the patients achieved CR within 60 days post-infusion, while one patient had a progressive form of the disease. Note that the 4SCAR-T therapy-failed patient had a low disease burden (0.02%) prior to CAR-T infusion and received CD19 plus CD22 double CAR-Ts. Out of the 30 patients, six received a total CAR-T dose lower than 1 × 106 CAR-Ts/kg; the remaining patients received a total CAR-T dose over 1 × 106 cells/kg. The statistics were performed by a paired-sample t-test (p = 1 > 0.01); there were no statistical differential effects on the different CAR-T doses (Figure 2B). This is consistent with previously accumulated and published 4SCAR19 clinical data (8).

Figure 2 Efficacy and safety of the 4SCAR2.0 therapy in post-HCT B-ALL patients. (A) Comparison of single vs. double CAR-T infusions. The responses of patients receiving single (CD19) versus double (CD19 plus a second target) CAR-T infusions and responses evaluated at 60 days are presented. (B) The patient response in relation to the CAR-T infusion doses. The patient response based on CAR-T infusion doses above or below 1 × 106 CAR-T cells/kg is illustrated. CI (CAR Index) refers to millions of CAR-T/kg body weight. (C) Assessment of patients with CRS and GVHD after CAR-T infusion. The number of patients who developed different grades of CRS as well as GVHD after CAR-T treatment was summarized and presented in the bar graph.
GVHD and CAR-T donor selection
After allo-HCT, 10 patients experienced grade 2 or lower aGVHD, and 20 patients had no history of GVHD. None of them had any active GVHD at the time of CAR-T therapy. Based on the GVHD history, donor chimerism, and donor availability, we procured T cells for the preparation of CAR-T products from the original HCT allogeneic donors (n = 25) or the patients themselves (n = 5).
CRS and GVHD after CAR-T treatment
We observed no CRS in 14 patients (47%); the remaining 16 patients (53%) experienced grade 1 CRS, and none of them experienced neurotoxicity using the Penn grading scale (Figure 2C). Among the four patients with a high tumor burden (i.e., > 50% BM blasts), we observed only grade 1 CRS. This is consistent with the low toxicity profile of the 4SCAR design as reported before (8, 9). Seven patients developed both CRS and aGVHD (Table 1). Acute GVHD of grade 2 or lower developed in 8 out of 25 patients (32%) within 1 month of receiving allogeneic CAR-Ts, including rash, liver, lung, and intestinal reactions. All patients with GVHD responded well to treatment with topical corticosteroids or systemic cyclosporine. Five of the eight patients with GVHD had a history of aGVHD following HCT, while the remaining three developed de novo GVHD after CAR-T infusion.
Follow-up after CAR-T therapy
A total of 29 patients achieved MRD-negative remission, and 12 (41%) of them relapsed at a median of 6.3 months (range: 2.8 months–22.3 months) following infusion. With a median follow-up period of 2 years (range: 2.8 months –67 months), 17 patients (57%) remained in continuous remission. Eleven out of the 24 (46%) patients who received allogeneic CAR-Ts and one-fifth (20%) who received autologous CAR-Ts relapsed after an initial MRD-negative response. Ten patients received two CAR-T infusions, including three patients who received CD19 plus CD123 CAR-Ts. While targeting CD123 could induce hematopoietic suppression and pancytopenia, we did not observe such an adverse effect in any of the CD123 CAR-T-treated patients. Three patients received a combination therapy of dasatinib after the CAR-T treatment.
All eight patients with EM relapses (CNS relapses) achieved CR following the CAR-T treatment. The patients with an EM disease at the time of relapse showed similar toxicity and efficacy as those without an EM disease. After CAR-T infusion, three patients received dasatinib and one received venetoclax as a maintenance treatment, as presented in Table 1. Due to the limited number of patients, the effect of these combination treatments is unknown, and further studies are needed. After CAR-T treatment, four patients received a second transplantation: two of them remained BCR-ABL (p190) positive, and the other two were lost to follow-up.
In vivo expansion and persistence of the CAR-T cells
After infusion, we used a real-time qPCR method to track CAR-Ts in the peripheral blood. We designed CAR-specific primers and probes that could distinguish the specific CAR sequences. From the amplification dynamics of the 4SCAR-Ts in vivo within 30 days, it was determined that the 4SCAR-Ts usually reached their peak in the peripheral blood around 7 (30%) to 14 (45%) days after infusion. We presented the amplification curve of CAR-Ts in the peripheral blood of 20 patients who received a single CD19 CAR-T infusion in Figure 3A. Simultaneous amplification of different CAR-Ts in the blood could be detected in 10 patients who received two CAR-T products, and the different CAR-Ts did not appear to suppress each other in vivo (Figure 3B). The 4SCAR2.0 study also demonstrated that the 4SCAR-Ts can exist in vivo for a prolonged period of time; for example, persistent CAR-Ts were in two patients for more than 1 year, suggesting the establishment of CAR-T memory (Figure 3C).
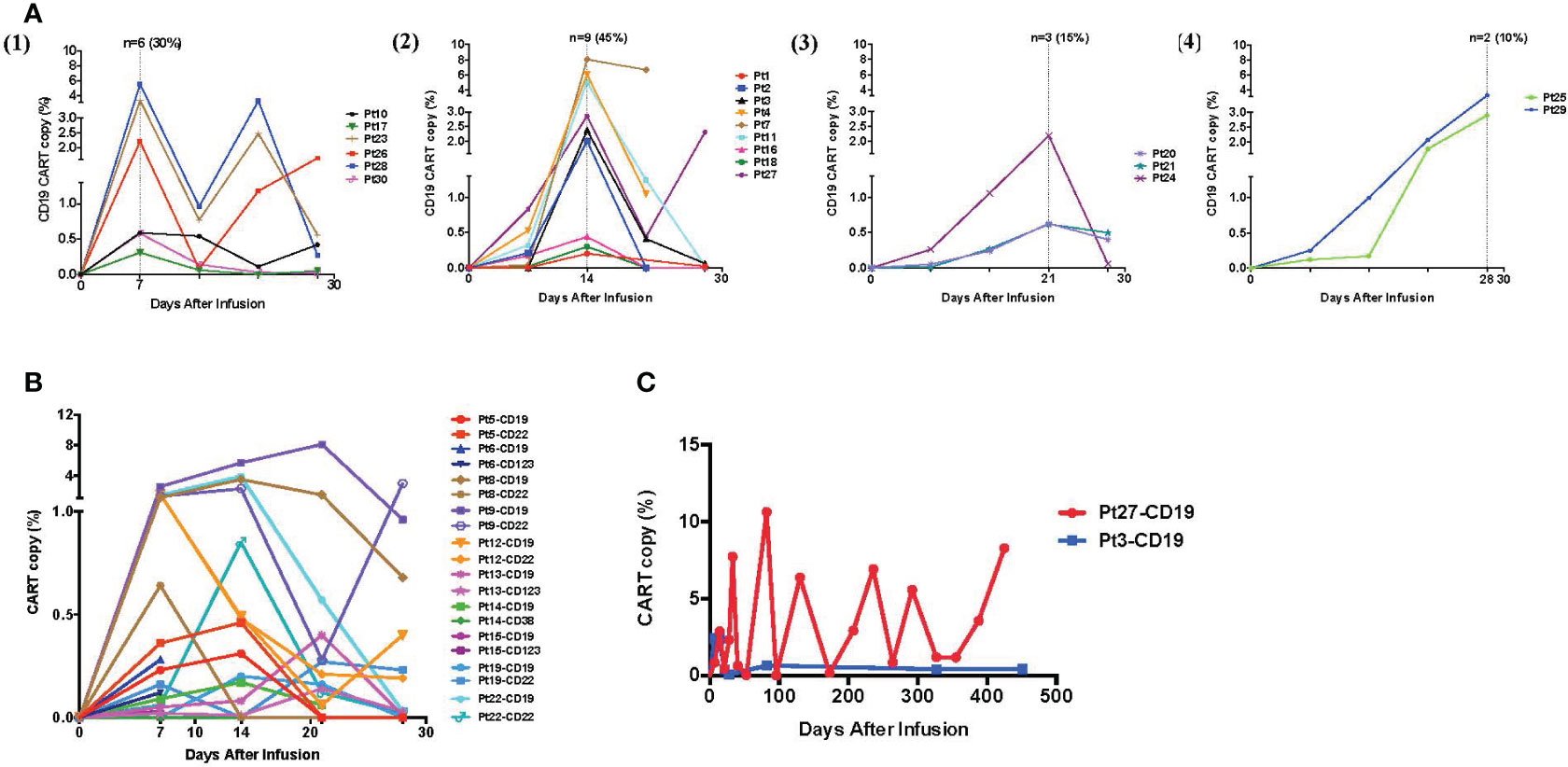
Figure 3 In vivo expansion and persistence of 4SCAR-Ts. (A) The CAR-T kinetics in the peripheral blood within 30 days after treatment. After CAR-T infusion, PBMCs were examined for CAR copy numbers weekly, and the kinetics of CAR-T expansion in the blood of 20 patients who received a single CD19 CAR-T infusion are illustrated. The CAR-T expansion peaks in the peripheral blood of the treated patients were recorded from day 7 to day 28, with day 7, n = 6, 30% (1); day 14, n = 9, 45% (2); day 21, n = 3, 15% (3); and day 28, n = 2, 10% (4). (B) The kinetics of two different CAR-Ts in the peripheral blood within 30 days. The CAR copy number in the peripheral blood was examined weekly, and the kinetics of CAR-T expansion shown as percentages (%) of the total mononuclear cells from the 10 patients who received two CAR-T infusions (targeting CD19/CD22, CD19/CD123, or CD19/CD38) are illustrated. (C) Long-term persistence of the 4SCAR-Ts in vivo. The long-term follow-up of the CD19 CAR-Ts in two patients after infusion for more than 400 days is illustrated.
Discussion
Following the approval of the initial CD19 CAR-T products (Kymriah and Yescarta), many new CAR designs and studies have emerged. However, clinical experiences with CD19 CAR-T therapy have faced many challenges, including high toxicities, frequent disease relapses, and high manufacturing costs. In addition, fast exhaustion of the infused CAR-Ts and CD19 antigen escape could result in disease recurrence within 1 year in more than half of the treated patients (17, 18).
In 2015, we initiated the 4SCAR2.0 study, which allowed for multiple CAR-T infusions targeting different tumor antigens to reduce disease recurrence (19, 20). Under traditional therapies, even with a second HCT, the overall survival of children with B-ALL who relapse after HCT is < 10% (7/97 children) (21). Even with new therapies, the survival of patients relapsing after HCT remains poor. The CAR-T therapy has proven to be effective in r/r B-ALL patients. Considering the good toxicity profile of the 4SCAR2.0 technology, we treated B-ALL patients who relapsed after HCT with this novel regimen, which includes the following: focusing on patients who relapsed after HCT and using allogeneic and individualized combinations of CAR-Ts.
Among the 30 r/r B-ALL patients who relapsed after allo-HCT, 25 received donor source/allogeneic CAR-Ts, and 10 received CAR-Ts of two different specificities. Due to the limited patient number, statistics were performed by a paired-sample t-test (p = 0.33 > 0.01); we did not observe a significant difference between the single CD19 CAR-T arm (n = 20) and the double CAR-T arm (n = 10). The lack of a significant outcome from the double CAR-T arm could also be attributed to the enrollment criteria as well as the leukemia antigen profile, that is, two target antigens chosen due to a low CD19 expression phenotype and/or a high disease risk. Therefore, increased patient numbers and extended follow-up periods are needed to further assess the potential advantage of double or multiple CAR-Ts.
There was also no significant difference in the response to the CAR-T dosages of above or below 1 million CAR-Ts/kg body weight, which was consistent with accumulated 4SCAR-T clinical data (20, 22). The 4SCAR technology applies a quick CAR-T manufacture protocol(5–7 days), which could preserve high levels of T memory stem cells due to the reduced ex vivo expansion time. The CRS was of low grade, even in the allo-CAR-T recipients, including the five patients who had > 50% BM blasts at the time of CAR-T infusion. This is consistent with the low toxicity profile of the 4SCAR design as reported (9, 20, 22, 23). Many post-allo-HCT relapses involve an EM disease. In this study, we showed that all eight patients who had EM relapses prior to CAR-T infusion achieved CR with little to no toxicity. Importantly, many of these patients who had an EM disease at the time of relapse had similar toxicity and efficacy responses as those without an EM disease, suggesting that the 4SCAR-Ts can effectively and safely function in the EM milieu.
Besides CRS, GVHD is an obvious concern for allo-HCT patients who receive donor-sourced CAR-Ts. We observed aGVHD of grade 2 or lower develop in eight patients receiving allogeneic HCT within 1 month of a donor source CAR-T infusion, and all of them were manageable with supportive care and/or a cyclosporine infusion. However, since we enrolled only patients with a history of grade 2 or lower aGVHD in this study, the observed safety profile of allogeneic donor CAR-T could apply only to those without a history of severe GVHD.
Conclusions
In conclusion, while the expanded study is ongoing, the results from the 4SCAR2.0 regimen in the treatment of the first 30 patients indicate that the approach is effective and safe in managing post-allo-HCT relapse of r/r B-ALL.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Shenzhen Geno-Immune Medical Institute IRB. The studies were conducted in accordance with local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
L-JC designed the study, engineered the different CAR constructs, and finalized the manuscript. JX, YS, ST, YL, LZ, YC, SX, YZ, BW, HZ, NN, YT, and SK performed the clinical treatments. RZ and YW performed laboratory work and data analysis. RZ drafted the manuscript. BH and L-JC revised the manuscript and participated in the discussion. All authors read and approved the final manuscript. All authors contributed to the article.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank the patients, their families, and the collaborating medical centers for their participation and support. The content of this manuscript has been presented in part at the American Society of Hematology 2020 annual meeting, abstract #162.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author YL declares that they were an editorial board member of Frontiers at the time of submission. This had no impact on the peer-review process or the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fesnak AD, June CH, Levine BL. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat. Rev. Cancer. (2016) 16(9):566–81. doi: 10.1038/nrc.2016.97
2. Davila ML, Sadelain M. Biology and clinical application of CAR T cells for B cell Malignancies. Int. J. hematology. (2016) 104(1):6–17. doi: 10.1007/s12185-016-2039-6
3. Lichtman EI, Dotti G. Chimeric antigen receptor T-cells for B-cell Malignancies. Transl. Res. (2017) 187:59–82. doi: 10.1016/j.trsl.2017.06.011
4. Hong S, Rybicki L, Corrigan D, Hamilton B, Sobecks R, Kalaycio M, et al. Targeted treatment and survival following relapse after allogeneic hematopoietic cell transplantation for acute leukemia and MDS in the contemporary era. Blood (2019) 134. (Supplement_1): 4567. doi: 10.1182/blood-2019-124533
5. Tu SF, Zhou X, Guo ZL, Huang R, Yue CY, He YJ, et al. CD19 and CD70 dual-target chimeric antigen receptor T-cell therapy for the treatment of relapsed and refractory primary central nervous system diffuse large B-cell lymphoma. Front. Oncol. (2019) 9:1350. doi: 10.3389/fonc.2019.01350
6. EI-Galaly TC, Cheah CY, Bendtsen MD, Nowakowski GS, Kansara R, Savage KJ, et al. Treatment strategies, outcomes and prognostic factors in 291 patients with secondary CNS involvement by diffuse large B-cell lymphoma. Eur. J. Cancer. (2018) 93:57–68. doi: 10.1016/j.ejca.2018.01.073
7. Tan Y, Pan J, Deng BP, Ling ZJ, Song WL, Xu JL, et al. Toxicity and effectiveness of CD19 CAR T therapy in children with high-burden central nervous system refractory B-ALL. Cancer Immunol. Immunother. (2021). 70(7): 1979–93. doi: 10.1007/s00262-020-02829-9
8. Jiao C, Zvonkov E, Lai X, Zhang R, Liu YC, Qin Y, et al. 4SCAR2.0: a multi-CAR-T therapy regimen for the treatment of relapsed/refractory B cell lymphomas. Blood Cancer J. (2021) 11(3):59. doi: 10.1038/s41408-021-00455-x
9. Nair S, Wang JB, Tsao ST, Liu Y, Zhu W, Slayton WB, et al. Functional improvement of chimeric antigen receptor through intrinsic interleukin-15Ralpha signaling. Curr. Gene Ther. (2019) 19(1):40–53. doi: 10.2174/1566523218666181116093857
10. Chang L-J, Urlacher V, Iwakuma T, Cui Y, Zucali J. Efficacy and safety analyses of a recombinant human immunodeficiency virus type 1 derived vector system. Gene Ther. (1999) 6:715–28. doi: 10.1038/sj.gt.3300895
11. Chang L-J, Zaiss A-K. Lentiviral vectors. Preparation and use. Methods Mol Med (2002) 69:303–18. doi: 10.1385/1-59259-141-8:303
12. Zhang JP, Zhang R, Tsao ST, Liu YC, Chen X, Lu DP, et al. Sequential allogeneic and autologous CAR-T-cell therapy to treat an immune-compromised leukemic patient. Blood Adv. (2018) 2(14):1691–5. doi: 10.1182/bloodadvances.2018017004
13. Kokhorst HM, Plesner T, Laubach JP, Nahi H, Gimsing P, Hansson M, et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. New Engl. J. Med. (2015) 373(13):1207–19. doi: 10.1056/NEJMoa1506348
14. Krauss J, Arndt MA, Martin AC, Liu H, Rybak SM. Specificity grafting of human antibody frameworks selected from a phage display library: generation of a highly stable humanized anti-CD22 single-chain Fv fragment. Protein Eng. (2003) 16(10):753–9. doi: 10.1093/protein/gzg096
15. Du X, Mitchel H, Pastan I. New immunotoxins targeting CD123, a stem cell antigen on acute myeloid leukemia cells. J. Immunother. (2007) 30:607–13. doi: 10.1097/CJI.0b013e318053ed8e
16. Cui Y, Chang L-J. Detection and selection of lentiviral vector transduced cells. In: Lentivirus gene engineering protocols, vol. 229. Totowa, NJ, United States: Humana Press, Inc (2003). p. 69–85.
17. Hao L, Li TT, Chang L-J, Chen XC. Adoptive immunotherapy for B-cell Malignancies using CD19- targeted chimeric antigen receptor T-cells: a systematic review of efficacy and safety. Curr. Medicinal Chem. (2019) 26(17):3068–79. doi: 10.2174/0929867324666170801101842
18. Hu LH, Charwudzi A, Li Q, Zhu WW, Tao QS, Xiong SD, et al. Anti-CD19 CAR-T cell therapy bridge to HSCT decreases the relapse rate and improves the long-term survival of R/R B-ALL patients: a systematic review and meta-analysis. Ann. Hematol. (2021) 100(4):1003–12. doi: 10.1007/s00277-021-04451-w
19. Chang L-J, Dong L, Zhu J, Ying Z, Kuo H-H, Liu Y, et al. 4SCAR19 chimeric antigen receptor-modified T cells as a breakthrough therapy for highly chemotherapy-resistant late-stage B cell lymphoma patients with bulky tumor mass. Blood. (2015) 126(23):264. doi: 10.1182/blood.V126.23.264.264
20. Chang L-J, Dong L, Liu Y-C, Tsao S-T, Li Y-C, Liu L, et al. Safety and efficacy evaluation fo 4SCAR19 chimeric antigen receptor-modified T cells targeting B cell acute lymphoblastic leukemia - three-year follow-up of a multicenter phase I/II study. Blood (2016) 128(22):587.
21. Dahlberg A, Leisenring W, Bleakley M, Meshinchi S, Baker KS, Summers C, et al. Prognosis of relapse after hematopoietic cell transplant (HCT) for treatment of leukemia or myelodysplastic syndrome (MDS) in children. Bone Marrow Transplant. (2019) 54(8):1337–45. doi: 10.1038/s41409-019-0438-z
22. Dong L, Chang L-J, Gao Z, Lu D-P, Zhang J-P, Wang J-B, et al. Chimeric antigen receptor 4SCAR19-modified T cells in acute lymphoid leukemia: a phase II multi-center clinical trial in China. Blood. (2015) 126(23):3774. doi: 10.1182/blood.V126.23.3774.3774
Keywords: CAR-T, B-ALL, transplantation, CD19, GvHD
Citation: Zhang R, Xiao J, Sun Y, Tu S, Li Y, Zhang L, Cheng Y, Xue S, Zhang Y, Wang B, Zheng H, Nishio N, Takahashi Y, Kojima S, Wang Y, Horn B and Chang L-J (2023) 4SCAR2.0 therapy for the management of post-transplantation relapse of B-cell acute lymphoblastic leukemia. Front. Hematol. 2:1251622. doi: 10.3389/frhem.2023.1251622
Received: 02 July 2023; Accepted: 23 October 2023;
Published: 09 November 2023.
Edited by:
Zhongbo Hu, St. Jude Children’s Research Hospital, United StatesReviewed by:
Natalie Grover, University of North Carolina at Chapel Hill, United StatesJeffrey J. Pu, Harvard Medical School, United States
Smitha Hosahalli Vasanna, Case Western Reserve University, United States
Copyright © 2023 Zhang, Xiao, Sun, Tu, Li, Zhang, Cheng, Xue, Zhang, Wang, Zheng, Nishio, Takahashi, Kojima, Wang, Horn and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lung-Ji Chang, Y0BzemdpbWkub3Jn
†These authors share first authorship
 Rui Zhang
Rui Zhang Juan Xiao2†
Juan Xiao2† Sanfang Tu
Sanfang Tu Yuhua Li
Yuhua Li Yoshiyuki Takahashi
Yoshiyuki Takahashi Biljana Horn
Biljana Horn Lung-Ji Chang
Lung-Ji Chang